Notes
Editorial note
There is a more recent Cochrane review on this topic: https://doi.org/10.1002/14651858.CD013668.pub2
Abstract
Background
Self‐harm (SH; intentional self‐poisoning or self‐injury) is common, often repeated, and associated with suicide. This is an update of a broader Cochrane review first published in 1998, previously updated in 1999, and now split into three separate reviews. This review focuses on psychosocial interventions in adults who engage in self‐harm.
Objectives
To assess the effects of specific psychosocial treatments versus treatment as usual, enhanced usual care or other forms of psychological therapy, in adults following SH.
Search methods
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) trials coordinator searched the CCDAN Clinical Trials Register (to 29 April 2015). This register includes relevant randomised controlled trials (RCTs) from: the Cochrane Library (all years), MEDLINE (1950 to date), EMBASE (1974 to date), and PsycINFO (1967 to date).
Selection criteria
We included RCTs comparing psychosocial treatments with treatment as usual (TAU), enhanced usual care (EUC) or alternative treatments in adults with a recent (within six months) episode of SH resulting in presentation to clinical services.
Data collection and analysis
We used Cochrane's standard methodological procedures.
Main results
We included 55 trials, with a total of 17,699 participants. Eighteen trials investigated cognitive‐behavioural‐based psychotherapy (CBT‐based psychotherapy; comprising cognitive‐behavioural, problem‐solving therapy or both). Nine investigated interventions for multiple repetition of SH/probable personality disorder, comprising emotion‐regulation group‐based psychotherapy, mentalisation, and dialectical behaviour therapy (DBT). Four investigated case management, and 11 examined remote contact interventions (postcards, emergency cards, telephone contact). Most other interventions were evaluated in only single small trials of moderate to very low quality.
There was a significant treatment effect for CBT‐based psychotherapy compared to TAU at final follow‐up in terms of fewer participants repeating SH (odds ratio (OR) 0.70, 95% confidence interval (CI) 0.55 to 0.88; number of studies k = 17; N = 2665; GRADE: low quality evidence), but with no reduction in frequency of SH (mean difference (MD) ‐0.21, 95% CI ‐0.68 to 0.26; k = 6; N = 594; GRADE: low quality).
For interventions typically delivered to individuals with a history of multiple episodes of SH/probable personality disorder, group‐based emotion‐regulation psychotherapy and mentalisation were associated with significantly reduced repetition when compared to TAU: group‐based emotion‐regulation psychotherapy (OR 0.34, 95% CI 0.13 to 0.88; k = 2; N = 83; GRADE: low quality), mentalisation (OR 0.35, 95% CI 0.17 to 0.73; k = 1; N = 134; GRADE: moderate quality). Compared with TAU, dialectical behaviour therapy (DBT) showed a significant reduction in frequency of SH at final follow‐up (MD ‐18.82, 95% CI ‐36.68 to ‐0.95; k = 3; N = 292; GRADE: low quality) but not in the proportion of individuals repeating SH (OR 0.57, 95% CI 0.21 to 1.59, k = 3; N = 247; GRADE: low quality). Compared with an alternative form of psychological therapy, DBT‐oriented therapy was also associated with a significant treatment effect for repetition of SH at final follow‐up (OR 0.05, 95% CI 0.00 to 0.49; k = 1; N = 24; GRADE: low quality). However, neither DBT vs 'treatment by expert' (OR 1.18, 95% CI 0.35 to 3.95; k = 1; N = 97; GRADE: very low quality) nor prolonged exposure DBT vs standard exposure DBT (OR 0.67, 95% CI 0.08 to 5.68; k = 1; N =18; GRADE: low quality) were associated with a significant reduction in repetition of SH.
Case management was not associated with a significant reduction in repetition of SH at post intervention compared to either TAU or enhanced usual care (OR 0.78, 95% CI 0.47 to 1.30; k = 4; N = 1608; GRADE: moderate quality). Continuity of care by the same therapist vs a different therapist was also not associated with a significant treatment effect for repetition (OR 0.28, 95% CI 0.07 to 1.10; k = 1; N = 136; GRADE: very low quality). None of the following remote contact interventions were associated with fewer participants repeating SH compared with TAU: adherence enhancement (OR 0.57, 95% CI 0.32 to 1.02; k = 1; N = 391; GRADE: low quality), mixed multimodal interventions (comprising psychological therapy and remote contact‐based interventions) (OR 0.98, 95% CI 0.68 to 1.43; k = 1 study; N = 684; GRADE: low quality), including a culturally adapted form of this intervention (OR 0.83, 95% CI 0.44 to 1.55; k = 1; N = 167; GRADE: low quality), postcards (OR 0.87, 95% CI 0.62 to 1.23; k = 4; N = 3277; GRADE: very low quality), emergency cards (OR 0.82, 95% CI 0.31 to 2.14; k = 2; N = 1039; GRADE: low quality), general practitioner's letter (OR 1.15, 95% CI 0.93 to 1.44; k = 1; N = 1932; GRADE: moderate quality), telephone contact (OR 0.74, 95% CI 0.42 to 1.32; k = 3; N = 840; GRADE: very low quality), and mobile telephone‐based psychological therapy (OR not estimable due to zero cell counts; GRADE: low quality).
None of the following mixed interventions were associated with reduced repetition of SH compared to either alternative forms of psychological therapy: interpersonal problem‐solving skills training, behaviour therapy, home‐based problem‐solving therapy, long‐term psychotherapy; or to TAU: provision of information and support, treatment for alcohol misuse, intensive inpatient and community treatment, general hospital admission, or intensive outpatient treatment.
We had only limited evidence on whether the intervention had different effects in men and women. Data on adverse effects, other than planned outcomes relating to suicidal behaviour, were not reported.
Authors' conclusions
CBT‐based psychological therapy can result in fewer individuals repeating SH; however, the quality of this evidence, assessed using GRADE criteria, ranged between moderate and low. Dialectical behaviour therapy for people with multiple episodes of SH/probable personality disorder may lead to a reduction in frequency of SH, but this finding is based on low quality evidence. Case management and remote contact interventions did not appear to have any benefits in terms of reducing repetition of SH. Other therapeutic approaches were mostly evaluated in single trials of moderate to very low quality such that the evidence relating to these interventions is inconclusive.
Plain language summary
Psychosocial interventions for self‐harm in adults
Why is this review important?
Self harm (SH), which includes non‐fatal intentional self‐poisoning/overdose and self‐injury, is a major problem in many countries and is linked to risk of future suicide. It is distressing for both patients and their families and friends, and places large demands on clinical services. It is therefore important to assess the evidence on treatments for SH patients.
Who will be interested in this review?
Clinicians working with people who engage in SH, policy makers, people who themselves have engaged in SH or may be at risk of doing so, and their families and relatives.
What questions does this review aim to answer?
This review is an update of a previous Cochrane review from 1999, which found little evidence of beneficial effects of psychosocial treatments on repetition of SH. This update aims to further evaluate the evidence for the effectiveness of psychosocial treatments for patients with SH with a broader range of outcomes.
Which studies were included in the review?
To be included in the review, studies had to be randomised controlled trials of psychosocial interventions for adults who had recently engaged in SH. We searched electronic databases to find all such trials published up until 29 April 2015, and found 55 that met our inclusion criteria.
What does the evidence from the review tell us?
There have now been a number of investigations of psychosocial treatments for SH in adults, with greater representation in recent years of low‐ and middle‐income countries such as China, Iran, Pakistan, and Sri Lanka.
Some moderate quality evidence shows that cognitive‐behavioural‐based (CBT‐based) psychotherapy (a psychotherapy intended to change unhelpful thinking, emotions and behaviour) may help prevent repetition of SH, although it did not reduce overall frequency of SH. There were encouraging results (from small trials of moderate to very low quality) for other interventions aimed at reducing the frequency of SH in people with probable personality disorder, including group‐based emotion‐regulation psychotherapy, mentalisation (a psychosocial therapy intended to increase a person’s understanding of their own and others' mental state), and dialectical behaviour therapies (DBT; psychosocial therapies intended to assist with identification of triggers that lead to reactive behaviours and to provide individuals with emotional coping skills to avoid these reactions). Whilst DBT was not associated with a significant reduction in repetition of SH at final follow‐up as compared to usual treatment, there was evidence of low quality suggesting a reduction in frequency of SH.
There was no clear evidence supporting the effectiveness of prolonged exposure to DBT, case management, approaches to improve treatment adherence, mixed multimodal interventions (comprising both psychological therapy and remote contact‐based interventions), remote contact interventions (postcards, emergency cards, and telephone contact), interpersonal problem‐solving skills training, behaviour therapy, provision of information and support, treatment for alcohol misuse, home‐based problem‐solving therapy, intensive inpatient and community treatment, general hospital admission, intensive outpatient treatment, or long‐term psychotherapy.
We had only limited evidence from a subset of the studies relating to whether the intervention had different effects in men and women. The trials did not report on side effects other than suicidal behaviour.
What should happen next?
The promising results for CBT‐based psychological therapy and dialectical behaviour therapy warrant further investigation to understand which patients benefit from these types of interventions. There were only a few, generally small trials on most other types of psychosocial therapies, providing little evidence of beneficial effects; however, these cannot be ruled out. There is a need for more information about whether psychosocial interventions might work differently between men and women.
Summary of findings
Background
Description of the condition
The term 'self‐harm' is used to describe all non‐fatal intentional acts of self‐poisoning or self‐injury, irrespective of degree of suicidal intent or other types of motivation (Hawton 2003a). Thus it includes acts intended to result in death ('attempted suicide'), those without suicidal intent (e.g., to communicate distress, to temporarily reduce unpleasant feelings), and those with mixed motivation (Hjelmeland 2002; Scoliers 2009). The term 'parasuicide' was introduced by Kreitman 1969 to include the same range of behaviour. However, clinicians in the USA have used 'parasuicide' to refer specifically to acts of self‐harm without suicidal intent (Linehan 1991), and the term has largely fallen into disuse in the UK and other countries. The Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM‐5) includes two types of self‐harming behaviour as conditions for further study, namely non‐suicidal self‐injury (NSSI) and suicidal behaviour disorder (SBD). Many researchers and clinicians, however, believe this to be an artificial and somewhat misleading categorisation (Kapur 2013). We have therefore used the approach favoured in the UK and some other countries where all intentional self‐harm is conceptualised in a single category, namely self‐harm (SH). Within this category, suicidal intent is regarded as a dimensional rather than a categorical concept. Readers more familiar with the NSSI and SBD distinction may regard SH as an umbrella term for these two behaviours (although it should be noted that neither NSSI nor SBD include non‐fatal self‐poisoning).
SH has been a growing problem in most countries over the past 40 years. In the UK, researchers estimate that there are now more than 200,000 presentations to general hospitals per year (Hawton 2007). In addition, self‐harm often occurs in adults in the community and does not come to the attention of clinical services or other helping agencies (Borges 2011). SH consumes considerable hospital resources in both developed and developing countries (Carter 2005; Claassen 2006; Fleischmann 2005; Gibbs 2004; Kinyanda 2005; Parkar 2006; Schmidtke 1996; Schmidtke 2004).
Unlike suicide, in most countries SH usually occurs more commonly in females than males, although this gap decreases over the life cycle (Hawton 2008). It has also decreased in recent years (Perry 2012). SH predominantly occurs in young people, with 60% to 70% of individuals in many studies aged under 35 years. In females, rates tend to be particularly high among those aged 15 to 24, whereas in males the highest rates are usually among those in their late 20s and early 30s. SH is also less common in older people but then tends to be associated with high suicidal intent (Hawton 2008), with consequent greater risk of future suicide (Murphy 2012).
Many people who engage in SH are facing acute life problems, often in the context of longer‐term difficulties (Hawton 2003b). Common problems include disrupted relationships, employment difficulties, financial and housing problems, and social isolation. Alcohol abuse and, to a lesser extent, drug misuse are often present. There may be a history of adverse experiences, such as physical abuse, sexual abuse, or both. In older people, physical health problems, bereavement, and threatened loss of independence become increasingly important.
Many patients who present to hospital following SH have psychiatric disorders, especially depression, anxiety, and substance misuse (Hawton 2013). These disorders frequently occur in combination with personality disorder (Haw 2001).
Both psychological and biological factors appear to increase vulnerability to SH. Psychological factors include difficulties in problem‐solving and a tendency to show black and white (or all or none) thinking patterns, low self‐esteem, impulsivity, vulnerability to having pessimistic thoughts about the future (i.e., hopelessness) and a sense of entrapment ( O'Connor 2012: Williams 2000; Williams 2005). Biological factors include disturbances in the serotonergic and stress‐response systems (Van Heeringen 2014).
SH is often repeated, with 15% to 25% of individuals who present to hospital with SH returning to the same hospital following a repeat episode within a year (Carroll 2014; Owens 2002). Studies from Asia suggest a lower risk of repetition (Carroll 2014). There may be other repeat episodes that do not result in hospital presentation.
The risk of death by suicide within one year amongst people who attend hospital with SH varies across different studies, from nearly 1% to over 3% (Carroll 2014; Owens 2002). This variation reflects differences in the characteristics of the SH population and background national suicide rates. During the first year after a SH episode, the risk in the UK is 50 to 100 times that of the general population (Cooper 2005; Hawton 1988; Hawton 2003b). Of people who die by suicide, over half will have a history of SH (Foster 1997), and at least 15% will have presented to hospital with SH in the preceding year (Gairin 2003). A history of SH is the strongest risk factor for suicide across a range of psychiatric disorders (Sakinofsky 2000). Repetition of SH further increases the risk of suicide (Zahl 2004).
Description of the intervention
Psychosocial interventions include a wide variety of treatments, for example cognitive behavioural therapy (CBT), problem‐solving therapy, behaviour therapy, and dialectical behaviour therapy (DBT). Treatments may vary in relation to the initial management; the location of treatment; the continuity, intensity or frequency of contact with a therapist; and the mode of delivery (individual or group‐based). We included treatments that focused on specific subgroups of SH patients in this review. These subgroups may be defined in terms of age, psychological characteristics or psychiatric diagnoses, substance misuse, and history of repetition of self‐harm. We also included studies of strategies to maintain contact with patients, such as visits to patients with poor therapeutic adherence, and contact by telephone, post and electronic means.
How the intervention might work
The mechanisms of action of psychosocial interventions might include helping people improve their coping skills and self‐esteem, tackle specific problems, overcome psychiatric disorders, increase their sense of social connectedness, and reduce impulsivity, aggression and unhelpful reactions to distressing situations.
CBT‐based psychotherapy
CBT aims to help patients identify and critically evaluate the way in which they interpret and evaluate disturbing emotional experiences and events and then change the way they deal with problems (Westbrook 2011). The therapy has three steps. First, therapists help patients change the way in which they interpret and evaluate distressing emotions. Secondly, patients learn strategies to help them change the way they think about the meaning and consequences of these emotions. Lastly, with the benefit of modified interpretation of emotions and events, the therapy helps patients to change their behaviour and especially to develop positive functional behaviour (Jones 2012).
Problem‐solving therapy, which is an integral part of CBT, assumes that psychopathological processes such as SH are ineffective and maladaptive coping behaviours. Patients might overcome them by learning skills to actively, constructively and effectively solve the problems they face in their daily life (Nezu 2010). Therapists might achieve this by encouraging patients to consciously and rationally appraise problems, reduce or modify the negative emotions generated by problems, and develop a range of possible solutions to address their problems (D'Zurilla 2010). Treatment goals include helping patients to develop a positive problem‐solving orientation, use rational problem‐solving strategies, reduce the tendency to avoid problem‐solving, and reduce the use of impulsive problem‐solving strategies (Washburn 2012).
Our rationale for including CBT and problem‐solving therapy approaches in a single category of CBT‐based psychotherapy in this review is that they share common elements. For example, problem‐solving therapy incorporates other elements of behaviour therapy and constitutes a key part of cognitive behavioural therapy; also, cognitive‐behavioural strategies are important for effective problem‐solving therapy (Hawton 1989; Westbrook 2011)
Interventions for multiple repetition of SH/probable personality disorder
The goal of emotion‐regulation training is to help patients find adaptive ways to respond to distress instead of trying to control, suppress or otherwise avoid experiencing these emotions through behaviours such as SH (Gratz 2007). Emotion‐regulation training therefore helps patients in four stages: first, to become more aware and accepting of their emotional experiences; second, to engage in goal‐directed behaviours whilst inhibiting the expression of impulsive ones; third, to use appropriate strategies to moderate the intensity and duration of their emotional responses; and fourth, to become more accepting of negative emotional experiences within their daily life (Gratz 2004).
Dialectical behaviour therapy (DBT) combines problem‐solving training, skills training, cognitive modification training and mindfulness techniques to encourage patients to accept their thoughts, feelings, and behaviours without necessarily attempting to change, suppress, or avoid these experiences (Lynch 2006; Washburn 2012). Within this framework, the aim of DBT is to help patients better regulate their emotions, achieve a sense of interpersonal effectiveness, become more tolerant of distressing thoughts and feelings, and become better at managing their own thoughts and behaviours (Linehan 1993b; Linehan 2007). The primary treatment goals of DBT are therefore threefold: to reduce SH, to reduce behaviours that interfere with treatment success (e.g., treatment non‐adherence), and to reduce any other factors that may adversely affect the patient's quality of life (e.g., frequency and duration of psychiatric hospitalisations) (Linehan 1993b). As the aim of DBT is to help patients change or adjust to significant personality characteristics, treatment is intensive and relatively prolonged.
Mentalisation refers to the ability to understand the actions of both the self and of others as meaningful given knowledge of the desires, beliefs, feelings, emotions, and motivations that underscore their behaviour (Bateman 2004; Choi‐Kain 2008). During times of interpersonal stress, however, individuals may fail to represent experiences in terms of mental states and instead become overwhelmed with negative thoughts and feelings about the self (Rossouw 2013). Behaviours such as SH may then represent an escape from these negative self‐evaluations. Mentalisation therapy aims to improve patients' ability to empathise with others in order to develop the ability to see how their own behaviours may have an impact on the feelings of others, and to regulate their own emotions more effectively (Rossouw 2013).
Case management
Case management in mental health services has mainly been developed for more severely ill patients. "In its simplest form . . . case management is a means of co‐ordinating services. Each. . . person is assigned a 'case manager' who is expected to assess that person's needs; develop a care plan; arrange for suitable care to be provided; monitor the quality of the care provided; and maintain contact with the person (Holloway 1991)" (Marshall 2000a, p. 2). Case management might have a significant role in the aftercare of self‐harm patients because of the recognised problem of poor treatment adherence in many patients and the heterogeneous nature of the problems patients are often facing (Hawton 2003b; Lizardi 2010). It has included, for example, provision of a care manager, crisis intervention, problem solving, assistance with getting to clinical appointments, and assertive outreach, each provided according to individual patient need (Morthorst 2012).
Treatment adherence enhancement approaches
These approaches include specific efforts to maintain contact with patients, such as following up patients in the community who fail to attend outpatient appointments (Van Heeringen 1995). It also includes strategies to encourage adherence with treatment (Hvid 2011).
Having the same clinician who assessed a patient initially also providing any aftercare intervention may increase treatment adherence and may also have an advantage in that the clinician is already acquainted with the patient's problems and needs.
Remote contact interventions
Remote contact interventions typically involve sending regular letters or postcards to patients. Patients may view this kind of intervention as a 'gesture of caring' that may help to counteract the sense of social isolation many SH patients experience (Cooper 2011). This sense of "social connectedness" may, in turn, have a stabilising emotional effect (Motto 2001).
Another type of remote contact intervention involves the use of emergency cards, which may encourage patients to seek help when they feel distressed as well as offering provision for on‐demand emergency contact with psychiatric services (Kapur 2010).
The fact that in many countries most individuals have their own general practitioner (GP) can also facilitate provision of care directly following SH. Interventions may include guidance for GPs on treating and managing problems commonly experienced by SH patients (e.g., depression, substance misuse, life problems). Such advice may also include advising GPs on referral of SH patients to local community services (Bennewith 2002).
Telephone contact with patients following discharge from hospital can also help to ensure a continuing sense of contact with the service and be used to provide advice and possibly psychotherapy.
The immediacy that psychotherapy by mobile telephone can achieve, when compared with standard clinic‐based psychotherapy, may help with crisis management in times of distress (Marasinghe 2012).
Why it is important to do this review
SH is a major social and healthcare problem. It is responsible for significant morbidity, is often repeated, and has strong links to suicide. It also leads to substantial healthcare costs (Sinclair 2011). Many countries now have suicide prevention strategies (WHO 2014), which include a focus on improved management of patients presenting with SH due to their greatly elevated suicide risk and their high levels of psychopathology and distress. The National Suicide Prevention Strategy for England (Her Majesty's Government Department of Health 2012) and the US suicide prevention strategy (Office of the Surgeon General 2012), for example, highlight SH patients as a high risk group for special attention.
In recent years there has been considerable focus on improving the standards of general hospital care for SH patients. The Royal College of Psychiatrists published consensus guidelines for such services in 1994 and a further guideline in 2004 (Royal College of Psychiatrists 1994; Royal College of Psychiatrists 2004). While these guidelines focus particularly on organisation of services and assessment of patients, there clearly also need to be effective treatments for SH patients. These may include both psychosocial and pharmacological interventions. In 2004 the National Institute for Clinical Excellence (NICE) produced a guideline on self‐harm, which focused on its short‐term physical and psychological management (NCCMH 2004). More recently, NICE produced a second guide focused particularly on long‐term management, using some interim data from the present review as the evidence base for therapeutic interventions (NICE 2011). A similar guideline was produced in Australia and New Zealand (Boyce 2003). We had previously conducted a systematic review of treatment interventions for SH patients in terms of reducing repetition of SH (including suicide); this review highlighted the paucity of evidence for effective treatments, at least in terms of this outcome (Hawton 1998; Hawton 1999). The first NICE guideline essentially reinforced this conclusion (NCCMH 2004). However, there was emerging evidence for beneficial effects of short‐term psychological therapy on other outcomes (depression, hopelessness, and problem resolution) (Townsend 2001). Using interim data from the present review, the second NICE guideline concluded that there was evidence showing clinical benefit of CBT‐based psychotherapeutic interventions in reducing repetition of self‐harm, compared with routine care (NICE 2011).
We have now fully updated our original review in order to provide current evidence to guide clinical policy and practice. Previous versions of this review included SH patients of any age and both psychosocial and pharmacological interventions. We have now divided this research into three separate reviews, one of interventions in children and adolescents (Hawton 2015a), another of pharmacological interventions in adults (Hawton 2015b), and this, the third, focused on psychosocial interventions in adults. We have also now included data on treatment adherence, depression, hopelessness, problem‐solving, and suicidal ideation.
Objectives
To assess the effects of specific psychosocial treatments versus either treatment as usual, enhanced usual care or other forms of alternative psychotherapy, in adults following SH.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials, including cluster‐randomised, multi‐arm, and cross‐over trials of specific psychosocial interventions versus any comparator (e.g., treatment as usual [TAU]/enhanced usual care [EUC]/other alternative forms of psychotherapy) in the treatment of adult SH patients.
Types of participants
Participant characteristics
Participants were adult men and women (aged 18 and over) of all ethnicities. We also included trials where there were a small minority (< 15%) of adolescent participants. However, we undertook sensitivity analyses to assess the effect of including such studies.
Diagnosis
We included participants who had engaged in any type of non‐fatal intentional self‐poisoning or self‐injury in the six months prior to trial entry resulting in presentation to clinical services. There were no restrictions on the frequency with which patients engaged in SH; thus, for example, we included trials where participants had frequently repeated SH (e.g., those with self‐harming behaviour associated with borderline personality disorder).
We defined SH as any non‐fatal intentional act of self‐poisoning or self‐injury, irrespective of degree of suicidal intent or other types of motivation. Thus we included acts intended to result in death (attempted suicide), those without suicidal intent (e.g., to communicate distress, to temporarily reduce unpleasant feelings), and those with mixed motivation. Self poisoning includes both overdoses of medicinal drugs and ingestion of substances not intended for consumption (e.g., pesticides). Self‐injury includes acts such as self‐cutting, self‐mutilation, attempted hanging, and jumping in front of moving vehicles. We only included trials where participants presented to clinical services as a result of SH.
Co‐morbidities
There were no restrictions on included patients in terms of whether or not they had psychiatric disorders nor with regard to the nature of those disorders, with the exception of intellectual disability, as any SH behaviour is likely to be repetitive (e.g., head banging), and the purpose of this behaviour is usually different from that involved in SH (NICE 2011).
Setting
Interventions delivered in inpatient or outpatient settings were eligible for inclusion, as were trials from any country.
Subset data
We did not include trials in which only some participants had engaged in SH or trials in people with psychiatric disorders where SH was an outcome variable but not an inclusion criterion for entry into the trial.
Types of interventions
Comparisons included in this review were between any psychosocial intervention and any comparator (e.g., TAU/EUC/other alternative forms of psychotherapy, or placebo). As the trials included in this review assessed a wide variety of interventions, we developed categories or groups of interventions. This categorisation was based on consensus discussions within the review team and included decisions about combining trials in which there were superficial differences between treatments but the key methodologies between trials were similar. In some cases we sought more details of the therapy from authors to assist this process. Categorisation reflected both prior views on types of psychotherapy and the types of interventions that were identified as a result of the systematic review of the literature.
Experimental interventions
These included:
CBT‐based psychotherapy;
interventions for multiple repetition of SH/probable personality disorder;
case management;
treatment adherence enhancement approaches;
mixed multimodal interventions;
remote contact interventions;
other mixed interventions.
Comparator interventions
Treatment as usual
As treatment as usual (TAU) is likely to vary widely between settings, following previous work we defined TAU as any care that patient would receive had they not been included in the trial (i.e., routine care) (Hunt 2013).
Enhanced usual care
Enhanced usual care (EUC) refers to TAU that has in some way been supplemented, for example through the provision of psycho‐education, assertive outreach, or more regular contacts with case managers.
Treatment by expert
This typically consists of a treatment by a widely recognised authority with significant experience in treating individuals following SH.
Other alternative forms of psychotherapy
This included other forms of psychotherapy designed to be of lower duration or intensity than the experimental intervention and could include:
brief or short‐term psychotherapy;
standard case management;
standard dialectical behaviour therapy (DBT).
Types of outcome measures
Primary outcomes
The primary outcome measure in this review was the occurrence of repeated SH (defined above) over a maximum follow‐up period of two years. Repetition was identified through at least one of the following: self‐report, collateral report, clinical records, or research monitoring systems. As we wished to incorporate the maximum amount of data from each trial, we included both self‐reported and hospital records of SH where available. We also assessed frequency of repetition of SH.
Secondary outcomes
1. Treatment adherence
This was assessed using a range of measures of adherence, including the proportion of participants that both started and completed treatment, pill counts, and changes in blood measures.
2. Depression
This was assessed either continuously, as scores on psychometric measures of depression symptoms (for example total scores on Beck Depression Inventory (BDI; Beck 1961) or scores on the depression sub‐scale of the Hospital Anxiety and Depression Scale (HADS; Zigmond 1983)), or dichotomously, as the proportion of patients reaching defined diagnostic criteria for depression. We included both patient‐ and clinician‐reported instruments.
3. Hopelessness
This was assessed as scores on psychometric measures of hopelessness, for example total scores on the Beck Hopelessness Scale (BHS; Beck 1974). We included both patient‐ and clinician‐reported instruments.
4. Suicidal ideation
This was assessed suicidal ideation either continuously, as scores on psychometric measures (for example total scores on the Beck Scale for Suicidal Ideation (BSSI; Beck 1988)), or dichotomously, as the proportion of patients reaching a defined cut‐off for ideation. We included both patient‐ and clinician‐reported instruments.
5. Problem solving
Problem solving ability was assessed either continuously, as scores on psychometric measures (for example total scores on the Problem‐Solving Inventory (PSI; Heppner 1988)), or dichotomously, as the proportion of patients with improved problems. We included both patient‐ and clinician‐reported instruments.
6. Suicide
This included both register‐recorded deaths and reports from collateral informants such as family or neighbours.
Timing of the outcome assessment
We reported outcomes for the following time points.
At the conclusion of the treatment period.
Between 0 and 6 months after the conclusion of the treatment period.
Between 6 and 12 months after the conclusion of the treatment period.
Between 12 and 24 months after the conclusion of the treatment period.
Hierarchy of outcome assessment
Where a trial measured the same outcome (e.g., depression) in two or more ways, we used the most common measure across trials in any meta‐analysis, but we also reported scores from the other measure in the text of the review.
Search methods for identification of studies
Electronic searches
1. The Cochrane Depression, Anxiety and Neurosis Review Group Specialised Register (CCDANCTR)
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintains two clinical trials registers at their editorial base in Bristol, UK: a references register and a studies‐based register. The CCDANCTR‐References Register contains over 39,500 reports of randomised controlled trials on depression, anxiety and neurosis. Approximately 60% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies register and records are linked between the two registers through the use of unique study ID tags. Coding of trials is based on the EU‐PSI Coding Manual. Please contact the CCDAN Trials Search Coordinator for further details.
Reports of trials for inclusion in the group's registers are collated from weekly generic searches of MEDLINE (1950 to date), EMBASE (1974 to date) and PsycINFO (1967 to date), as well as quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL).
We searched the CCDANCTR (Studies and References) database on 29 April 2015 using terms for self‐harm (condition only), as outlined in Appendix 1.
We applied no restrictions on date, language, or publication status to the search.
2. Additional electronic database searches
Sarah Stockton, librarian at the University of Oxford, conducted earlier searches (1998 to October 2013) of MEDLINE, EMBASE, PsycINFO and CENTRAL (The Cochrane Library), following the search strategy outlined in Appendix 2. As the CCDANCTR already contains relevant reports of RCTs from these databases, it was unnecessary to re‐search these. Additionally, KW searched the Australian Suicide Prevention RCT Database (Christensen 2014). KW also conducted electronic searches of ClinicalTrials.gov and the ISRCTN registry using the keywords random* AND suicide attempt* OR self$harm* to identify relevant ongoing trials.
Both the original version of this review as well as an unpublished version also incorporated searches of the following databases: SIGLE (1980 to March 2005) and SocioFile (1963 to July 2006).
We updated the search of ClinicalTrials.gov and the ISRCTN registry to 29 April 2015.
Searching other resources
Handsearching
For the original version of this review the authors hand‐searched 10 specialised journals within the fields of psychology and psychiatry, including all English language suicidology journals, as outlined in Appendix 3. As these journals are now indexed in major electronic databases, we did not repeat hand‐searching for this update.
Reference lists
We checked the reference lists of all relevant papers known to our review team as well as the reference lists of major reviews that include a focus on interventions for SH patients (Baldessarini 2003; Baldessarini 2006; Beasley 1991; Brausch 2012; Burns 2000; Cipriani 2005; Cipriani 2013; Comtois 2006; Crawford 2007a; Crawford 2007b; Daigle 2011; Daniel 2009; Dew 1987; Gould 2003; Gray 2001; Gunnell 1994; Hawton 1998; Hawton 1999; Hawton 2012; Hennen 2005; Hepp 2004; Hirsch 1982; Kapur 2010; Kliem 2010; Lester 1994; Links 2003b; Lorillard 2011a; Lorillard 2011b; Luxton 2013; Mann 2005; McMain 2007b; Milner 2015; Möller 1989; Möller, 1992; Montgomery 1995; Muehlenkamp 2006; Müller‐Oerlinghausen 2005; Nock 2007; Ougrin 2011; Ougrin 2015; Tarrier 2008b; Tondo 1997; Tondo 2000; Tondo 2001; Townsend 2001; Van der Sande 1997b).
Correspondence
We consulted trial authors and other experts in the field of suicidal behaviour to find out if they were aware of any ongoing or unpublished RCTs concerning the use of psychosocial interventions for adult SH patients.
Data collection and analysis
For details of the data collection and analysis methods used in the original version of this review, see Appendix 4.
Selection of studies
For this review update all review authors independently assessed the titles of trials identified by the systematic search for eligibility. A distinction was made between:
eligible trials that compared any psychosocial intervention with a control (e.g., treatment as usual (TAU), enhanced usual care (EUC), or other alternative forms of psychotherapy);
general treatment trials (without any control treatment).
All trials identified as potentially eligible for inclusion underwent a second screening. Pairs of review authors, working independently from one another, screened the full text of relevant trials to identify whether the trials met our inclusion criteria.
We resolved disagreements following consultation with KH. Where we could not resolve disagreements based on the information reported within the trial, or where it was unclear whether the trial satisfied our inclusion criteria, we contacted authors to provide additional clarification.
Data extraction and management
In the current update, KW and one other author (TTS, EA, DG, PH, ET or KvH) independently extracted data from included trials using a standardised extraction form. In case of disagreement, authors resolved them through consensus discussions with KH.
Data extracted from each eligible trial included participant demographics, details of the treatment and control interventions, and information on the outcome measures used to evaluate the efficacy of the intervention. We contacted study authors to obtain raw data for outcomes that were not reported in the full text of included trials.
We extracted both dichotomous and continuous outcome data from eligible trials. As the use of non‐validated psychometric scales is associated with bias, we extracted continuous data only if the psychometric scale used to measure the outcome of interest had been previously published in a peer‐reviewed journal and was not subjected to item, scoring, or other modification by the trial authors (Marshall 2000b).
We planned the following main comparisons.
CBT‐based psychotherapy versus TAU or other alternative forms of psychotherapy.
Interventions for multiple repetition of SH/probable personality disorder versus TAU or other alternative forms of psychotherapy.
Case management versus TAU or other alternative forms of psychotherapy.
Treatment adherence enhancement approaches versus TAU or other alternative forms of psychotherapy.
Mixed multimodal interventions versus TAU or other alternative forms of psychotherapy.
Remote contact interventions versus TAU or other alternative forms of psychotherapy.
Other mixed interventions versus TAU or other alternative forms of psychotherapy.
Assessment of risk of bias in included studies
Given that highly biased trials are more likely to overestimate treatment effectiveness (Moher 1998), KW and one of TTS, EA, DG, PH, ET or KvH independently evaluated the quality of included trials by using the criteria described in Higgins 2008a. This tool encourages consideration of the following domains:
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We judged each source of potential bias as conferring low, high or unclear risk of bias, and we incorporated a supporting quotation from the report to justify this judgment. Where the original report provided inadequate details of the randomisation, blinding, or outcome assessment procedures, we contacted authors for clarification. We resolved disagreements through discussion with KH and reported risk of bias for each included trial in the text of the review. For cluster‐randomised and cross‐over trials, we used appropriate methods of assessing bias as outlined in Higgins 2011, sections 16.3.2 and 16.4.3.
Measures of treatment effect
Dichotomous outcomes
We summarised dichotomous outcomes, such as the number of participants engaging in a repeat SH episode and the number of deaths by suicide, using summary odds ratios (OR) and the accompanying 95% confidence interval (CI), as the OR is the most appropriate effect size statistic for summarising associations between two dichotomous groups (Fleiss 1994).
Continuous outcomes
For outcomes reported on a continuous scale, we used mean differences (MD) and accompanying 95% CI where trials employed the same outcome measure. Where studies used different scales to assess a given outcome, we used the standardised mean difference (SMD) and its accompanying 95% CI.
We only aggregated trials for the purposes of meta‐analysis if treatments were sufficiently similar. For trials that could not be included in a meta‐analysis, we have instead provided narrative descriptions of the results.
Unit of analysis issues
Zelen design trials
Trials in this area increasingly employ Zelen's method, in which investigators obtain consent after randomisation and treatment allocation. This design may lead to bias if, for example, participants allocated to one particular arm of the trial disproportionally refuse to provide consent for participation or, alternatively, if participants only provide consent provided they are allowed to cross over to the active treatment arm (Torgerson 2004). We included four trials that employed Zelen's method in this review (Carter 2005; Hatcher 2011; Hatcher 2016a; Hatcher 2015). Given the uncertainty of whether to use data for the primary outcome based on all those randomised to the trial, or only those who consented to participation, we extracted data for the primary outcome measure using both sources where possible. We also conducted sensitivity analyses by excluding these trials to investigate what impact, if any, their inclusion had on the pooled estimate of treatment effectiveness.
Cluster‐randomised trials
Cluster randomisation, for example by clinician or general practice, can lead to overestimation of the significance of a treatment effect, resulting in an inflation of the nominal type I error rate, unless there is appropriate adjustment for the effects of clustering (Donner 2002; Kerry 1998). We planned to statistically adjust for the effects of clustering following the guidance outlined in Higgins 2008b, section 16.3.4. Clustering was an issue in one included study (Bennewith 2002); however, we were unable to adjust for the effects of clustering in subsequent analyses as the study authors could not provide us with either the intercluster coefficient or the design effect.
Cross‐over trials
A primary concern with cross‐over trials is the 'carry‐over' effect, in which the effect of the intervention treatment (e.g., pharmacological, physiological, or psychological) influences the participant's response to the subsequent control condition (Elbourne 2002). As a consequence, on entry to the second phase of the trial participants may differ systematically from their initial state despite a wash‐out phase. This, in turn, may result in a concomitant underestimation of the effectiveness of the treatment intervention (Curtin 2002a; Curtin 2002b). One trial included in the current update used cross‐over methodology (i.e., Marasinghe 2012). To protect against the carry‐over effect, we only extracted data from the first phase of this trial, prior to cross‐over.
Studies with multiple treatment groups
Two trials in the current review included multiple treatment groups (Stewart 2009; Wei 2013). As both intervention arms in the Stewart 2009 trial investigated the effectiveness of CBT‐based psychotherapy, we combined dichotomous data from these two arms and compared them with data from the TAU arm. For outcomes reported on a continuous scale, we combined data using the formula given in Higgins 2011, section 7.7.3.8. Wei 2013 compared two different psychotherapies with TAU, namely CBT‐based psychotherapy and telephone contact. Therefore we included this trial in both categories of intervention using the data from the relevant experimental arm. As we did not combine these interventions in any meta‐analysis, we used the same TAU data for both analyses.
Studies with adjusted effect size estimates
None of the trials included in the current update calculated adjusted effect sizes. In future updates of this review, however, where trials report both unadjusted and adjusted effect sizes, we will include only unadjusted effect sizes.
Dealing with missing data
We as review authors did not impute missing data, as we considered that the bias that would be introduced by doing this would have outweighed any benefit (in terms of increased statistical power) that may have been gained by the inclusion of imputed data. However, where authors omitted standard deviations (SD) for continuous measures, we estimated these using the method described in Townsend 2001.
Dichotomous data
Although many authors conducted their own intention‐to‐treat analyses, few presented intention‐to‐treat analyses as defined by Higgins 2008b. Therefore, we based outcome analyses for both dichotomous and continuous data on all information available on trial participants. For dichotomous outcomes, we included data on only those participants whose results were known, using as the denominator the total number of participants with data for the particular outcome of interest, as recommended (Higgins 2008b).
Continuous data
For continuous outcomes, we included data only on observed cases.
Missing data
Where data on outcomes of interest were incomplete or excluded from the text of the trial, we contacted authors to request further information.
Assessment of heterogeneity
It is possible to assess between‐study heterogeneity using either the Chi2 or I2 statistic. In this review, however, we used only the I2 statistic to determine heterogeneity, as Higgins 2003 considers this to be more reliable. The I2 statistic indicates the percentage of between‐study variation due to chance and can take any value from 0% to 100% (Higgins 2003). We used the following values to denote unimportant, moderate, substantial, and considerable heterogeneity, respectively: 0% to 40%, 30% to 60%, 50% to 90%, and 75% to 100% as per the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2008, section 9.5.2). Where we found substantial levels of heterogeneity (i.e., ≥ 75%), we explored possible reasons. We also planned to investigate heterogeneity even when the I2 statistic was lower than 75% but either the direction or magnitude of a trial effect size was clearly discrepant from that of other trials included in the meta‐analysis (see Subgroup analysis and investigation of heterogeneity section for further information on these analyses).
We also report heterogeneity in the results section but only when we observed substantial levels, as indicated by an I2 statistic of 75% or greater.
Assessment of reporting biases
Reporting bias occurs when the direction and significance of a particular trial's results influence the decision to publish a report on it (Egger 1997). Research suggests, for example, that trials with statistically significant findings are more likely to be submitted and subsequently accepted for publication (Hopewell 2009), leading to possible overestimation of the true treatment effect. To assess whether trials included in any meta‐analysis were affected by reporting bias, we entered data into a funnel plot but only, as recommended, when a meta‐analysis included results of at least 10 trials. Where evidence of any small‐study effects were identified, we explored reasons for funnel plot asymmetry, including the presence of publication bias (Egger 1997).
Data synthesis
For the purposes of meta‐analysis, we calculated the pooled OR and accompanying 95% CI using the random‐effects model, as this is the most appropriate model for incorporating heterogeneity between trials (Deeks 2008, section 9.5.4). Specifically, we used the Mantel‐Haenszel method for dichotomous data and the inverted variance method for continuous data. However, we also undertook a fixed‐effect analysis to investigate the potential effect of method choice on the estimates of treatment effect. We descriptively report any material differences in ORs between these two methods in the text of the review. All analyses were conducted in Review Manager, version 5 (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses
In the original version of this review, we planned to undertake subgroup analyses by repeater status (i.e., history of multiple episodes of SH vs first known episode of SH) and gender but found there were insufficient data. Consequently, in this update we only undertook a priori subgroup analyses by sex or repeater status where there were sufficient data to do so.
Investigation of heterogeneity
Where meta‐analyses were associated with substantial levels of between‐study heterogeneity (i.e., as indicated by an I2 statistic ≥ 75%), KH and KW first independently triple‐checked the data to ensure that the review team had correctly entered the data. Assuming this was the case, we investigated the source of heterogeneity by visually inspecting the forest plot and removing each trial that had a very different result from the general pattern of the others until homogeneity was restored as indicted by an I2 statistic < 75%. We have reported the results of this sensitivity analysis in the text of the review alongside hypotheses regarding the likely causes of heterogeneity.
Sensitivity analysis
We undertook sensitivity analyses, where appropriate, as outlined below.
Trial/s that used Zelen's method of randomisation (see Unit of analysis issues section).
Trial/s that contributed substantial levels of between‐study heterogeneity (see Subgroup analysis and investigation of heterogeneity section).
Trial/s that included some adolescent participants.
Trial/s that specifically recruited individuals diagnosed with borderline personality disorder.
Summary of findings table
We prepared a 'Summary of findings' table for the primary outcome measure, repetition of SH, following recommendations outlined in Schünemann 2008a, section 11.5. This table provides information concerning the overall quality of evidence from each included trial. We prepared the 'Summary of findings' table using GRADEpro software (GRADE profiler). We assessed quality of the evidence following recommendations in Schünemann 2008b, section 12.2.
Results
Description of studies
Results of the search
In this update, the search strategy outlined in Appendix 1 and Appendix 2 yielded a total of 23,725 citations. We identified a further 10 trials that were ongoing at the time of the systematic search through correspondence and discussion with researchers in the field. All have subsequently been published.
In consultation with CCDAN, we divided the original review (Hawton 1998; Hawton 1999) into three separate reviews: the present review focuses on psychosocial interventions for adults, a second review deals with pharmacological interventions for adults (Hawton 2015b), and the third assesses interventions for children and adolescents (Hawton 2015a). Nine of the additional 10 trials evaluated psychosocial interventions for adults, and were therefore included in the present update. The remaining trial evaluated an intervention for children and adolescents; this is included in the related relevant review (i.e., Hawton 2015a).
After deduplication, the initial number of citations decreased to 16,700. Of these, we excluded 16,459 after screening the titles and abstracts and a further 217 after reviewing the full texts (Figure 1).
1.
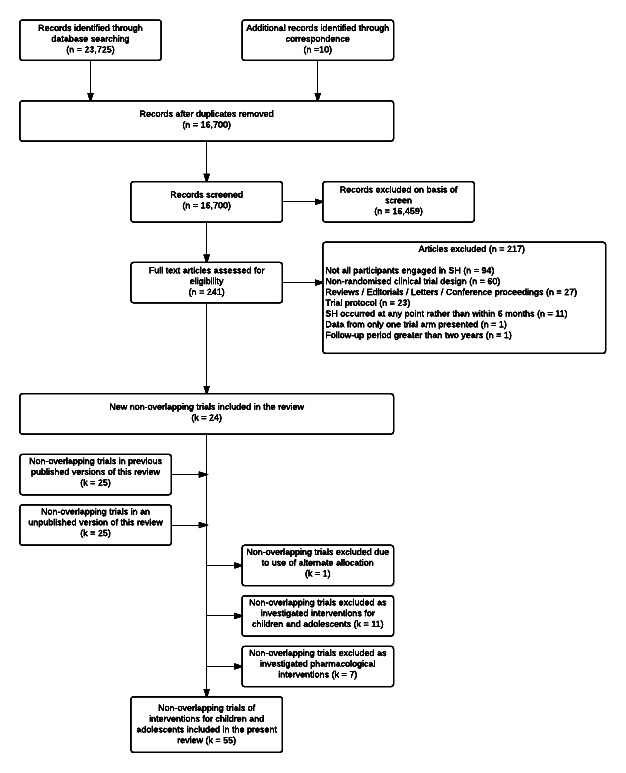
Search flow diagram of included and excluded studies for the 2014 update.
Included studies
In the previous versions of this review (Hawton 1998; Hawton 1999; NICE 2011), we included 36 trials of psychosocial interventions for adults following SH (Allard 1992; Bateman 2009; Bennewith 2002; Brown 2005; Carter 2005; Cedereke 2002; Clarke 2002; Dubois 1999; Evans 1999a; Evans 1999b; Fleischmann 2008; Gibbons 1978; Gratz 2006; Guthrie 2001; Hawton 1981; Hawton 1987a; Liberman 1981; Linehan 1991; Linehan 2006; McLeavey 1994; McMain 2009; Morgan 1993; Patsiokas 1985; Salkovskis 1990; Slee 2008; Stewart 2009; Torhorst 1987; Torhorst 1988; Turner 2000; Tyrer 2003; Vaiva 2006; Van der Sande 1997a; Van Heeringen 1995; Waterhouse 1990; Weinberg 2006; Welu 1977). The present update included information from an additional 19 trials (Beautrais 2010; Crawford 2010; Davidson 2014; Gratz 2014; Harned 2014; Hassanian‐Moghaddam 2011; Hatcher 2016a; Hatcher 2015; Hatcher 2011; Husain 2014; Hvid 2011; Kapur 2013a; Kawanishi 2014; Marasinghe 2012; McAuliffe 2014; Morthorst 2012; Priebe 2012; Tapolaa 2010; Wei 2013). The present review therefore included 55 non‐overlapping trials. Five further follow‐up studies (i.e., Bertolote 2010; Hassanzadeh 2010; McMain 2012, Vijayakumar 2011 and Xu 2012) provide additional data for two of these trials (Fleischmann 2008; McMain 2009).
We obtained unpublished data from corresponding authors from a further 36 trials (Bateman 2009; Beautrais 2010; Bennewith 2002; Brown 2005; Carter 2005; Cedereke 2002; Clarke 2002; Crawford 2010; Davidson 2014; Dubois 1999; Fleischmann 2008; Gratz 2006; Gratz 2014; Guthrie 2001; Harned 2014; Hassanian‐Moghaddam 2011; Hatcher 2016a; Hatcher 2015; Hatcher 2011; Husain 2014; Hvid 2011; Linehan 1991; Linehan 2006; Marasinghe 2012; McAuliffe 2014; McMain 2009; Patsiokas 1985; Priebe 2012; Slee 2008; Stewart 2009; Tapolaa 2010; Turner 2000; Tyrer 2003; Vaiva 2006; Wei 2013; Weinberg 2006).
We also identified 16 ongoing trials of psychosocial interventions following SH in adults (see Characteristics of ongoing studies section for further information on these trials).
Design
Study authors described all 55 independent trials as randomised controlled trials (RCTs). Most (number of studies k = 49; 89.1%) employed a simple randomisation procedure based on individual allocation to the intervention and control groups. Zelen's post‐randomisation consent design was used in four trials (Carter 2005; Hatcher 2016a; Hatcher 2015; Hatcher 2011), a cluster‐randomisation procedure in one (Bennewith 2002), and a matched pair randomisation procedure in another (Cedereke 2002).
Participants
The included trials comprised a total of 17,699 participants. All had engaged in at least one episode of SH in the six months prior to randomisation.
Participant characteristics
In the 39 trials that recorded information on age, the average age of participants at randomisation was 30.9 years (SD 4.6). Twenty trials included a small number of adolescent participants (i.e., under 18 years of age at randomisation) (Carter 2005; Dubois 1999; Evans 1999b; Gibbons 1978; Hassanian‐Moghaddam 2011; Hatcher 2016a; Hatcher 2015; Hatcher 2011; Hawton 1987a; Husain 2014; Hvid 2011; Marasinghe 2012; McLeavey 1994; Morthorst 2012; Priebe 2012; Salkovskis 1990; Slee 2008; Van der Sande 1997a; Van Heeringen 1995; Wei 2013). However, investigators did not record the precise number in any of them. As the majority of participants in these trials were adults, we included them in the present review rather than in the related review specific to interventions for children and adolescents (i.e., Hawton 2015a). The majority of the sample was female in the 46 trials that recorded information on sex (k = 46; mean 69.2%), reflecting the typical pattern for SH (Hawton 2008).
Diagnosis
In all trials, a recent episode of SH was a requirement for trial entry. SH includes intentional acts of self‐harm (i.e., self‐poisoning or self‐injury) and not acts such as recreational use of drugs that may result in accidental harm.
A history of multiple episodes of SH was a requirement for participation in 13 trials (Evans 1999b; Gratz 2006; Gratz 2014; Harned 2014; Liberman 1981; Linehan 1991; Linehan 2006; McMain 2009; Priebe 2012; Salkovskis 1990; Torhorst 1988; Tyrer 2003; Weinberg 2006). For an additional 28 trials, over half the sample had a history of multiple episodes of SH (Allard 1992; Bateman 2009; Beautrais 2010; Bennewith 2002; Brown 2005; Carter 2005; Cedereke 2002; Crawford 2010; Davidson 2014; Dubois 1999; Gibbons 1978; Guthrie 2001; Hatcher 2016a; Hatcher 2015; Hatcher 2011; Husain 2014; Kapur 2013a; Marasinghe 2012; McAuliffe 2014; McLeavey 1994; Morthorst 2012; Patsiokas 1985; Slee 2008; Stewart 2009; Tapolaa 2010; Turner 2000; Wei 2013; Welu 1977). In four further trials, just under half of the sample had a history of multiple episodes of SH (Clarke 2002: 47.0%; Evans 1999a: 47.6%; Kawanishi 2014: 49.2%; Van der Sande 1997a: 46.3%). We present the proportion with a prior history of SH in the remaining eight trials in Table 8. Morgan 1993 excluded those with a history of multiple episodes of SH from participation, whilst Torhorst 1987 provided no information on the proportion of the sample with a history of multiple episodes of SH.
1. Proportion of the sample with a history of self‐harm prior to the index attempt.
| Reference |
History of SH prior to index episode (%) |
| Fleischmann 2008 | 21.1 |
| Hawton 1981 | 32.3 |
| Hawton 1987a | 31.2 |
| Hassanian‐Moghaddam 2011 | 34.2 |
| Hvid 2011 | 38.3 |
| Vaiva 2006 | 8.9a |
| Van Heeringen 1995 | 29.8 |
| Waterhouse 1990 | 36.4 |
aProportion with more than four previous episodes of SH over the three‐year period preceding trial entry.
In around half of the included trials (k = 25; 45.4%), the authors stated either within the trial report or through correspondence that they included participants irrespective of whether or not the episode of SH involved suicidal intent (Bateman 2009; Beautrais 2010; Bennewith 2002; Clarke 2002; Davidson 2014; Fleischmann 2008; Harned 2014; Hatcher 2016a; Hatcher 2015; Hatcher 2011; Hawton 1981; Hawton 1987a; Hvid 2011; Kapur 2013a; Liberman 1981; Linehan 2006; McAuliffe 2014; McMain 2009; Patsiokas 1985; Slee 2008; Tapolaa 2010; Torhorst 1987; Turner 2000; Tyrer 2003; Van Heeringen 1995; Waterhouse 1990). Seven trials included participants following a 'suicide attempt' (i.e., suggestive of evidence of suicidal intent) (Allard 1992; Cedereke 2002; Morthorst 2012; Salkovskis 1990; Torhorst 1988; Van der Sande 1997a; Wei 2013). A further five trials included participants only if there had been evidence of suicidal intent (Brown 2005; Kawanishi 2014; Marasinghe 2012; Stewart 2009; Welu 1977), whilst in an additional two, the majority of participants (76.5% and 74.0% respectively) expressed a wish to die (Guthrie 2001; Husain 2014). Gratz 2014 included participants only if there was no evidence of suicidal intent, whilst only 2.0% in one trial expressed a wish to die (Vaiva 2006). Thirteen trials did not report information on suicidal intent (Carter 2005; Crawford 2010; Dubois 1999; Evans 1999a; Evans 1999b; Gibbons 1978; Gratz 2006; Hassanian‐Moghaddam 2011; Linehan 1991; McLeavey 1994; Morgan 1993; Priebe 2012; Weinberg 2006).
Twenty‐five trials did not report information on the methods of SH for the index episode (Allard 1992; Bateman 2009; Cedereke 2002; Davidson 2014; Dubois 1999; Evans 1999b; Fleischmann 2008; Gratz 2006; Gratz 2014; Hvid 2011; Kapur 2013a; Linehan 1991; Linehan 2006; Marasinghe 2012; McMain 2009; Morthorst 2012; Patsiokas 1985; Priebe 2012; Salkovskis 1990; Stewart 2009; Tapolaa 2010; Turner 2000; Tyrer 2003; Wei 2013; Weinberg 2006). One trial provided information on the methods used in all episodes of SH (including the index episode) in the two years preceding trial entry (Liberman 1981). A total of 55.7% of these episodes involved self‐poisoning and 44.3% involved self‐injury. One additional trial provided information on methods used in the three months prior to trial entry with a total of 61 (67.8%) participants in this trial engaging in self‐injury at least once over this period; however, investigators did not specify the methods that the remaining 29 participants used for SH (Slee 2008). We present methods of SH for the remaining 28 trials in Table 9. In these trials, the majority of participants had engaged in self‐poisoning (k = 28; 79.9%). Two trials included only those who engaged in self‐injury (Harned 2014; Welu 1977), whilst in a further trial the majority of participants (75.6%) had engaged in self‐poisoning using pesticides (Husain 2014).
2. Methods used for the index episode of self‐harm in included studies.
| Reference | Methoda | ||||
|
Self poisoning (any) n (%) |
Self poisoning (pesticides) n (%) |
Self injury (any) n (%) |
Combined self‐poisoning and self‐injury n (%) |
Unspecified n (%) |
|
| Beautrais 2010b | 250 (76.7) | — | 64 (19.6) | — | 15 (4.6) |
| Bennewith 2002 | 7,733 (89.7) | — | 158 (8.2) | — | 41 (2.1) |
| Brown 2005 | 70 (58.3) | — | 33 (27.5) | — | 17 (14.2) |
| Carter 2005 | 772 (100) | — | — | — | — |
| Clarke 2002b | 442 (94.6) | — | 25 (5.3) | 8 (1.7) | — |
| Crawford 2010c | 74 (71.8) | — | 25 (24.3) | — | — |
| Evans 1999a | 808 (97.7) | — | — | — | 19 (2.3) |
| Gibbons 1978 | 400 (100) | — | — | — | — |
| Guthrie 2001 | 119 (100) | — | — | — | — |
| Harned 2014 | — | — | 26 (100) | — | — |
| Hassanian‐Moghaddam 2011 | 2300 (100) | — | — | — | — |
| Hatcher 2011 | 471 (85.3) | — | 81 (14.7) | — | — |
| Hatcher 2015 | 532 (77.8) | — | 125 (18.3) | 27 (3.9) | — |
| Hatcher 2016a | 115 (68.9) | — | 41 (24.5) | 11 (6.6) | — |
| Hawton 1981 | 96 (100) | — | — | — | |
| Hawton 1987a | 80 (100) | — | — | — | |
| Husain 2014b | 65 (29.4) | 167 (75.6) | 4 (1.8) | — | — |
| Kawanishi 2014b | 707 (77.3) | — | 332 (36.3) | — | 42 (4.6) |
| McAuliffe 2014d | 161 (37.2) | — | 57 (13.2) | — | 4 (0.9) |
| Morgan 1993 | 207 (97.6) | — | — | — | 5 (2.4) |
| McLeavey 1994 | 39 (100) | — | — | — | — |
| Torhorst 1987 | 141 (100) | — | — | — | — |
| Torhorst 1988 | 80 (100) | — | — | — | — |
| Vaiva 2006 | 605 (100) | — | — | — | — |
| Van der Sande 1997a | 232 (84.7) | — | — | — | 42 (15.3) |
| Van Heeringen 1995 | 463 (89.7) | — | — | — | 53 (10.3) |
| Waterhouse 1990 | 77 (100) | — | — | — | — |
| Welu 1977 | — | 120 (100) | — | — | |
aRefers to the methods used for the index episode. b Percentages are greater than 100% because participants may have used multiple methods. c The remaining four (3.9%) participants used multiple, unspecified methods. d Methods of self‐harm for the remaining 211 (48.7%) participants were not provided.
Comorbidities
We present information on current psychiatric diagnoses for all 55 trials in Table 10. Eight trials focused specifically on participants diagnosed with borderline personality disorder (Bateman 2009; Gratz 2006; Gratz 2014; Linehan 1991; Linehan 2006; McMain 2009; Turner 2000; Weinberg 2006), three focused on participants diagnosed with any personality disorder (Davidson 2014; Evans 1999b; Priebe 2012), one focused specifically on participants diagnosed with alcohol use (Crawford 2010), and one focused on participants with comorbid post‐traumatic stress disorder and borderline personality disorder (Harned 2014).
3. Major categories of psychiatric diagnoses in included studies.
| Reference | Psychiatric diagnosisa | |||||||||||
|
Major depression n (%) |
Any other mood disorder n (%) |
Any anxiety disorder n (%) |
Any psychotic disorder n (%) |
Post‐traumatic stress n (%) |
Any eating disorder n (%) |
Alcohol use disorder/dependence n (%) |
Drug use disorder/dependence n (%) |
Substance use disorder/dependence n (%) |
Adjustment disorder n (%) |
Borderline personality disorder n (%) |
Any other personality disorder n (%) | |
| Allard 1992 | 130(86.7) | — | — | — | — | — | — | — | 79 (52.7) | — | — | 68 (45.3) |
| Bateman 2009 | 75 (56.0) | 103 (76.9) | 82 (61.2) | 19 (14.2) | 37 (27.6) | — | — | 72 (53.7) | — | 134 (100) | b | |
| Beautrais 2010 | No information on psychiatric diagnosis reported | |||||||||||
| Bennewith 2002 | No information on psychiatric diagnosis reported | |||||||||||
| Brown 2005 | 92 (77.0) | — | — | — | — | — | 36 (30.0) | 48 (40.0) | 82 (68.0) | — | — | — |
| Carter 2005 | No information on specific categories of psychiatric diagnosis reportedc | |||||||||||
| Cedereke 2002d | — | 91 (42.1) | — | — | — | — | — | — | — | 62 (28.7) | — | — |
| Clarke 2002 | — | 98 (56.0)e | 60 (34.0)e | 12 (3.0) | — | — | 26 (41.0)f | — | — | — | — | |
| Crawford 2010 | No information on psychiatric diagnosis reported | |||||||||||
| Davidson 2014 | — | — | — | — | — | — | — | — | — | — | 17 (85.0) | 20 (100) |
| Dubois 1999 | — | — | — | 43 (42.1) | — | — | — | — | 13 (12.7) | — | — | — |
| Evans 1999a | 707/827 (85.5) diagnosed with any major psychiatric disorder | |||||||||||
| Evans 1999b | No information on psychiatric diagnosis reported | |||||||||||
| Fleischmann 2008 | No information on psychiatric diagnosis reported | |||||||||||
| Gibbons 1978 | No information on psychiatric diagnosis reported | |||||||||||
| Gratz 2006 | — | — | — | — | — | — | — | — | — | — | 22 (100) | — |
| Gratz 2014 | — | 31 (50.0) | 38 (61.3) | — | 22 (35.5) | 8 (12.9) | — | — | 1 (1.6) | — | 62 (100) | b |
| Guthrie 2001 | No information on psychiatric diagnosis reported | |||||||||||
| Harned 2014 | — | 22 (83.3) | 23 (87.5) | — | — | 3 (12.5) | — | — | 11 (41.7) | — | 26 (100) | 16 (62.5) |
| Hassanian‐Moghaddam 2011 | No information on psychiatric diagnosis reported | |||||||||||
| Hatcher 2011 | No information on psychiatric diagnosis reported | |||||||||||
| Hatcher 2015 | No information on psychiatric diagnosis reported | |||||||||||
| Hatcher 2016a | No information on psychiatric diagnosis reported | |||||||||||
| Hawton 1981 | No information on psychiatric diagnosis reported | |||||||||||
| Hawton 1987a | No information on psychiatric diagnosis reported | |||||||||||
| Husain 2014 | No information on psychiatric diagnosis reported | |||||||||||
| Hvid 2011 | No information on specific categories of psychiatric diagnosis reported | |||||||||||
| Kapur 2013a | No information on psychiatric diagnosis reported | |||||||||||
| Kawanishi 2014g | — | 425(46.5) | — | 179(19.6) | — | — | — | — | 45 (4.9) | 191 (20.9) | — | — |
| Liberman 1981 | — | 24 (100) | — | — | — | — | — | — | — | — | — | h |
| Linehan 1991 | — | — | — | — | — | — | — | — | — | — | 44 (100) | — |
| Linehan 2006 | 73 (72.3) | — | 79 (78.2) | — | 50 (49.5) | 24 (23.8) | — | — | 30 (29.7) | — | 101(100) | b |
| Marasinghe 2012 | No information on psychiatric diagnosis reported | |||||||||||
| McAuliffe 2014 | No information on psychiatric diagnosis reported | |||||||||||
| McLeavey 1994 | — | 9 (23.1) | 1 (2.5) | — | — | — | 5 (12.8) | — | — | — | — | 6 (15.4) |
| McMain 2009 | 88 (48.9) | — | 135 (75.0) | — | 71 (37.4) | 24 (13.3) | — | — | 17 (9.4) | — | 180(100) | b |
| Morgan 1993 | — | 53 (25.0) | — | — | — | — | — | — | — | — | — | |
| Morthorst 2012 | No information on psychiatric diagnosis reportedi | |||||||||||
| Patsiokas 1985 | No information on specific categories of psychiatric diagnosis reported | |||||||||||
| Priebe 2012j | — | — | — | — | — | — | — | — | — | — | — | 80 (100) |
| Salkovskis 1990 | No information on psychiatric diagnosis reported | |||||||||||
| Slee 2008 | — | 80 (88.9) | 50 (55.6) | — | — | 15 (16.7) | — | — | 15 (16.7) | — | — | — |
| Stewart 2009 | No information on psychiatric diagnosis reported | |||||||||||
| Tapolaa 2010 | No information on psychiatric diagnosis reported | |||||||||||
| Torhorst 1987 | No information on psychiatric diagnosis reported | |||||||||||
| Torhorst 1988 | No information on psychiatric diagnosis reported | |||||||||||
| Turner 2000 | — | — | — | — | — | — | — | — | — | — | 24 (100) | — |
| Tyrer 2003 | — | — | — | — | — | — | — | — | — | — | — | 471(98.1) |
| Vaiva 2006 | No information on specific categories of psychiatric diagnosis reportedk | |||||||||||
| Van der Sande 1997a | — | 86 (31.4) | — | — | — | — | — | — | — | 40 (14.6) | — | — |
| Van Heeringen 1995 | — | 76 (14.7) | 14 (2.7) | — | — | — | — | — | — | — | — | — |
| Waterhouse 1990 | No information on psychiatric diagnosis reported | |||||||||||
| Wei 2013 | No information on psychiatric diagnosis reportedl | |||||||||||
| Weinberg 2006 | — | — | — | — | — | — | — | — | — | — | 30 (100) | — |
| Welu 1977 | No information on psychiatric diagnosis reported | |||||||||||
a All diagnoses represent current rather than lifetime diagnoses. b As participants could be diagnosed with more than one axis II diagnosis, the absolute number of participants diagnosed with any other personality disorder in this trial is unclear. c Median number (interquartile range) of psychiatric diagnoses in the both the intervention and control groups was 2 (1‐3). Information on specific categories of psychiatric diagnosis; however, were not reported. d A total of 47/216 (21.7%) of the sample were diagnosed with any psychiatric disorder other than a mood or adjustment disorder. e Diagnosed with a possible psychiatric disorder according to cut‐off scores on the Hamilton Anxiety and Depression Scale (HADS). Out of a total of 176 participants with complete ratings on this instrument. f Diagnosed with problematic alcohol use according to cut‐off scores on the Alcohol Use Disorders Identification Test (AUDIT). Out of a total of 63 participants with complete ratings on this instrument. g An additional 73/914 (8.0%) were diagnosed with any other major psychiatric disorder. h The authors state that "[m]ost patients would have been given personality disorder designations. . . including histrionic, narcissistic, borderline, avoidant, and dependent types" (p.1127). The absolute number of participants diagnosed with any one of these personality disorders in this trial is, however, unclear. i A total of 14/243 (5.8%) participants had been admitted to a psychiatric inpatient ward in the four weeks prior to the index suicide attempt. These patients were therefore likely to have been diagnosed with a current major psychiatric illness. j Mean (standard deviation (SD)) number of axis I psychiatric disorders was 8.0 (3.1) (n = 63) and mean (SD) number of axis II diagnoses was 3.5 (1.6) (n = 80). k A total of 100/459 (21.8%) of participants had, however, been referred for psychiatric treatment at the time of the index suicide attempt. These patients were therefore likely to have been diagnosed with a current major psychiatric illness. l A total of 166/239 (69.4%) were, however, diagnosed with a major psychiatric illness according to DSM‐IV‐TR criteria.
Details on comorbid diagnoses were not reported in the majority of trials (k = 49; 89.1%) (Allard 1992; Bateman 2009; Beautrais 2010; Bennewith 2002; Cedereke 2002; Clarke 2002; Crawford 2010; Dubois 1999; Evans 1999a; Evans 1999b; Fleischmann 2008; Gibbons 1978; Gratz 2006; Guthrie 2001; Harned 2014; Hassanian‐Moghaddam 2011; Hatcher 2016a; Hatcher 2015; Hatcher 2011; Hawton 1981; Hawton 1987a; Husain 2014; Hvid 2011; Kapur 2013a; Kawanishi 2014; Liberman 1981; Linehan 1991; Linehan 2006; Marasinghe 2012; McAuliffe 2014; McLeavey 1994; Morgan 1993; Morthorst 2012; Patsiokas 1985; Priebe 2012; Salkovskis 1990; Stewart 2009; Tapolaa 2010; Torhorst 1987; Torhorst 1988; Turner 2000; Tyrer 2003; Vaiva 2006; Van der Sande 1997a; Van Heeringen 1995; Waterhouse 1990; Wei 2013; Weinberg 2006; Welu 1977). In Brown 2005, most participants (85.0%) were diagnosed with more than one psychiatric disorder; however, the authors did not provide information on specific diagnoses. In an additional three trials (Carter 2005; McMain 2009; Slee 2008), the median number of psychiatric diagnoses was greater than two, suggesting that participants in these trials were also diagnosed with more than one psychiatric disorder; however, none of the three reported further information on specific diagnoses. In one trial, 45.0% of the participants were diagnosed with comorbid personality disorder and substance misuse (Davidson 2014), whilst in another, just under half of the sample (45.9%) had a comorbid personality diagnosis (Gratz 2014).
Setting
Of the 55 independent RCTs included in this review, most took place in the United Kingdom (k = 17) or the United States (k = 12), followed by New Zealand (k = 4), Australia (k = 2), Canada (k = 2), Denmark (k = 2), France (k = 2), Germany (k = 2), the Netherlands (k = 2), and one each from Belgium, China, Finland, Iran, Japan, Pakistan, the Republic of Ireland, Sri Lanka, and Sweden. One trial was a multicentre study conducted in a number of countries.
Interventions
The trials included in this review investigated the effectiveness of various forms of psychosocial intervention.
CBT‐based psychotherapy versus TAU (k = 18: Brown 2005; Davidson 2014; Dubois 1999; Evans 1999b; Gibbons 1978; Guthrie 2001; Hatcher 2011; Hawton 1987a; Husain 2014; McAuliffe 2014; Patsiokas 1985; Salkovskis 1990; Slee 2008; Stewart 2009; Tapolaa 2010; Tyrer 2003; Wei 2013; Weinberg 2006).
Interventions for multiple repetition of SH versus TAU (k = 6: Bateman 2009; Gratz 2006; Gratz 2014; Linehan 1991; McMain 2009; Priebe 2012) or other alternative forms of psychotherapy (k = 3: Harned 2014; Linehan 2006; Turner 2000).
Case management versus TAU (k = 4: Clarke 2002; Hvid 2011; Kawanishi 2014; Morthorst 2012).
Treatment adherence enhancement approaches versus TAU (k = 1: Van Heeringen 1995) or other alternative forms of psychotherapy (k = 1: Torhorst 1987).
Mixed multimodal interventions versus TAU (k = 2: Hatcher 2016a; Hatcher 2015).
Remote contact interventions versus TAU (k = 11: Beautrais 2010; Bennewith 2002; Carter 2005; Cedereke 2002; Evans 1999a; Hassanian‐Moghaddam 2011; Kapur 2013a; Marasinghe 2012; Morgan 1993; Vaiva 2006; Wei 2013).
Other mixed interventions versus TAU (k = 5: Allard 1992; Crawford 2010; Fleischmann 2008; Van der Sande 1997a; Welu 1977) or other alternative forms of psychotherapy (k = 5: Hawton 1981; Liberman 1981; McLeavey 1994; Torhorst 1988; Waterhouse 1990).
Outcomes
Information on the primary outcome, repetition of SH, was available for all but one of the included trials (Patsiokas 1985). In the case of four trials, we obtained information on this outcome following correspondence with authors (Marasinghe 2012McAuliffe 2014McMain 2009Tapolaa 2010). For around half of the trials (k = 24; 43.6%), information on repetition of SH was based on self‐report (Brown 2005; Cedereke 2002; Davidson 2014; Evans 1999b; Gratz 2006; Gratz 2014; Harned 2014; Hassanian‐Moghaddam 2011; Hawton 1981; Hawton 1987a; Husain 2014; Liberman 1981; Linehan 1991; Linehan 2006; McMain 2009; Priebe 2012; Slee 2008; Tapolaa 2010; Torhorst 1988; Turner 2000; Vaiva 2006; Van Heeringen 1995; Wei 2013; Weinberg 2006), whilst in a further eight trials, collateral reports, hospital/clinical records, or both supplemented the self‐reported information (Allard 1992; Bateman 2009; Bennewith 2002; Fleischmann 2008; Guthrie 2001; Hvid 2011; Morthorst 2012; Tyrer 2003). In 11 trials, information on repetition of SH was based on hospital re‐presentations (Beautrais 2010; Carter 2005; Clarke 2002; Crawford 2010; Evans 1999a; Hatcher 2016a; Hatcher 2015; Hatcher 2011; Kapur 2013a; Waterhouse 1990; Welu 1977), whilst in three others, investigators obtained the information from hospital or medical records (Gibbons 1978; Morgan 1993; Van der Sande 1997a). McLeavey 1994 used collateral records supplemented by hospital records to determine repetition of SH. McAuliffe 2014 used mixed methods to determine repetition of SH, using self‐reported information at the post‐intervention and six‐month follow‐up assessments and data on hospital re‐presentations at the 12 month follow‐up assessment. Patsiokas 1985 did not report information on repetition of SH, and our review team was unable to obtain this information through correspondence. The remaining seven trials provided no details about the source of the data on repetition of SH (Dubois 1999; Kawanishi 2014; Marasinghe 2012; Salkovskis 1990; Stewart 2009; Torhorst 1987).
The 19 trials that recorded information on treatment adherence assessed this using a variety of methods, including: the proportion of participants who completed the full course of treatment (Bateman 2009; Harned 2014; Husain 2014; Linehan 1991; McMain 2009; Priebe 2012; Slee 2008; Torhorst 1987; Turner 2000), the proportion of participants who attended at least one treatment session (Bennewith 2002; Cedereke 2002; Hawton 1981), and the number of treatment sessions attended (Brown 2005; Evans 1999b; Van Heeringen 1995). Three trials assessed treatment adherence using both the proportion of participants that completed the full course of treatment and the total number of treatment sessions attended (McAuliffe 2014; McLeavey 1994; Torhorst 1988), whilst the remaining trial assessed treatment adherence using both the proportion of participants who attended at least one treatment session and the total number of treatment sessions attended (Van der Sande 1997a).
Investigators assessed depression using the BDI in 13 trials (Bateman 2009; Fleischmann 2008; Gibbons 1978; Guthrie 2001; Hawton 1987a; Husain 2014; Linehan 1991; Marasinghe 2012; McAuliffe 2014; McMain 2009; Salkovskis 1990; Slee 2008; Tapolaa 2010), the Hamilton Rating Scale for Depression (HRSD; Hamilton 1960) in 3 trials (Harned 2014; Linehan 2006; Wei 2013), both the BDI and HRSD in 1 trial (Turner 2000), the Depression Anxiety Stress Scales (DASS; Lovibond 1995) in 2 trials (Gratz 2006; Gratz 2014), the Montgomery‐Åsberg Depression Rating Scale (MADRS; Montgomery 1979) in 1 trial (Van der Sande 1997a), the depression sub‐scale of the HADS in 6 trials (Davidson 2014; Evans 1999b; Hatcher 2016a; Hatcher 2015; Hatcher 2011; Tyrer 2003), both the BDI and the HRSD in 1 trial (Brown 2005), and both the BDI and the Zung Self‐Rating Depression Scale (ZSRDS; Zung 1965) in a further trial (Liberman 1981). In one trial it was not clear what scale the researchers used to assess depression (Torhorst 1988).
All 14 trials that recorded information on hopelessness assessed this using the BHS (Brown 2005; Hatcher 2016a; Hatcher 2015; Hatcher 2011; Husain 2014; Kawanishi 2014; Linehan 1991; McAuliffe 2014; McLeavey 1994; Patsiokas 1985; Salkovskis 1990; Stewart 2009; Van der Sande 1997a; Waterhouse 1990).
Ten trials assessed suicidal ideation using the BSSI (Cedereke 2002; Davidson 2014; Guthrie 2001; Hatcher 2011; Husain 2014; Marasinghe 2012; McAuliffe 2014; Patsiokas 1985; Stewart 2009; Turner 2000), two trials used the Suicide Behaviors Questionnaire (SBQ; Linehan 1981) (Linehan 2006; Weinberg 2006), one trial used the Scale for Suicidal Ideators (SSI; Schotte 1982) (Linehan 1991), and one the suicidal ideation sub‐scale of the Psychiatric Status Schedule (PSS; Spitzer 1970) (Waterhouse 1990). Three trials measured suicidal ideation dichotomously as the proportion with self‐reported suicidal ideation (Hassanian‐Moghaddam 2011; Liberman 1981; Wei 2013).
Hatcher 2011 assessed problem‐solving using the Social Problem‐Solving Inventory‐Revised (SPSI‐R; D'Zurilla 1996); Husain 2014 used the Coping Resource Inventory (CRI; Marting 1988); Patsiokas 1985, the Means‐Ends Problem Solving test (MEPS; Maydeu‐Olivares 1996); Salkovskis 1990, the Personal Questionnaire Rapid Scaling Technique (PQRST; Mulhall 1977); McAuliffe 2014 and McLeavey 1994, both the MEPS and Self‐Rated Problem Solving Scale (SRPSS; McLeavey 1987); Slee 2008, the oriented coping subscale of the Coping Inventory for Stressful Situations (CISS; Endler 1994); and Fleischmann 2008 (at one site of the World Health Organization's (WHO) multisite trial SUPRE‐MISS, as reported in Xu 2012), the problem‐solving sub‐scale of an idiosyncratic problem‐solving questionnaire. Gibbons 1978 and Hawton 1987a measured problem‐solving dichotomously as the proportion of participants self‐reporting improved problems at follow‐up.
In about half of the 44 trials (k = 24; 54.5%) that recorded information on completed suicide, the method used to assess this outcome was unclear (Bateman 2009; Beautrais 2010; Brown 2005; Clarke 2002; Davidson 2014; Dubois 1999; Gratz 2006; Gratz 2014; Guthrie 2001; Harned 2014; Husain 2014; Kapur 2013a; Linehan 1991; Linehan 2006; Marasinghe 2012; McMain 2009; McLeavey 1994; Priebe 2012; Salkovskis 1990; Slee 2008; Stewart 2009; Tapolaa 2010; Torhorst 1987; Weinberg 2006). In the 20 remaining trials, investigators used a variety of methods to assess completed suicide, including: collateral reports (Crawford 2010; Fleischmann 2008; Hawton 1987a; Wei 2013), Coroner's records (Hatcher 2016a; Hatcher 2015; Hatcher 2011; Hvid 2011; Tyrer 2003), hospital records, medical records or both (McAuliffe 2014; Morthorst 2012), mortality statistics (Carter 2005; Cedereke 2002; Kawanishi 2014; Van Heeringen 1995), collateral reports supplemented by medical records or Coroner's records (Allard 1992; Hassanian‐Moghaddam 2011), collateral report supplemented by mortality statistics (Van der Sande 1997a), hospital records, medical records or both, supplemented by mortality statistics (Vaiva 2006), or mortality statistics supplemented by Coroner's records (Evans 1999a).
Excluded studies
We excluded a total of 217 articles from this update: 94 in which not all participants engaged in SH; 60 that used a non‐randomised clinical trial design; 27 that were reviews, editorials, letters to the editor, or conference proceedings; 23 that were trial protocols; 11 where SH could have occurred at any point rather than within six months of randomisation; one that only presented data from one trial arm (although a related publication that presented data for both the intervention and control arms was eligible for inclusion), and one that reported data reported for a period beyond two years (although articles reporting data for earlier follow‐up periods for this trial were eligible for inclusion).
We excluded one trial that had been included in the original version of this review following advice from CCDAN due to bias in the method used to randomise participants to the intervention and control groups (Chowdhury 1973). We also had to exclude one further trial that otherwise met inclusion criteria for this review as correspondence with authors revealed that information on non‐fatal SH could not be disaggregated from information on completed suicide (Chen 2013).
We provide details on the reasons for excluding 52 trials clearly related to psychosocial interventions for suicidality in the Characteristics of excluded studies section.
Ongoing studies
We identified a total of 20 ongoing trials of psychosocial interventions for SH in adults. We provide full details of these trials in the Characteristics of ongoing studies section. We note that one of these trials has subsequently been terminated owing to the resignation of key clinical staff and a lack of ongoing funding (Agyapong 2013).
Studies awaiting classification
Four potentially relevant trials are currently awaiting assessment (see Characteristics of studies awaiting classification table), two of DBT (Andreasson 2016; Linehan 2015), one of a mixed multimodal intervention combining face‐to‐face psychosocial therapy with a remote contact intervention (Gysin‐Maillart 2016) and one trial testing the effectiveness of implementation intentions (volitional help sheet) in reducing suicidal ideation and behaviour (Armitage 2016).
Risk of bias in included studies
We present summaries of the overall risk of bias for the included trials in Figure 2 and Figure 3. Risk of bias for each included trial is also considered within the text of the review.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
All of the 55 independent trials used random allocation. We considered the majority (k = 40; 72.7%) to have a low risk of bias for this item. In most trials a computer‐generated randomisation sequence was used to allocate adults to the experimental and control groups (Beautrais 2010; Brown 2005; Carter 2005; Guthrie 2001; Hatcher 2016a; Hatcher 2015; Hatcher 2011; Husain 2014; Kapur 2013a; Kawanishi 2014; Linehan 1991; Linehan 2006; McAuliffe 2014; Morthorst 2012; Priebe 2012; Slee 2008; Tyrer 2003; Vaiva 2006; Van der Sande 1997a; Wei 2013). In two trials a minimisation algorithm was used to allocate participants to the experimental and control groups (Bateman 2009; Harned 2014) whilst in one a pre‐generated block randomisation procedure was used (McMain 2009). In the remaining trials a variety of other randomisation procedures were used, including: a random numbers table (Bennewith 2002; Clarke 2002; Crawford 2010; Davidson 2014; Fleischmann 2008; Hassanian‐Moghaddam 2011; Hawton 1981; Hawton 1987a; Welu 1977), shuffled envelopes (Gibbons 1978; Morgan 1993; Salkovskis 1990; Waterhouse 1990; Weinberg 2006), numbers drawn from a hat (Stewart 2009), and coin tossing (Tapolaa 2010). In one trial details on the method used to allocate participants to the invention and control groups were not provided, but the authors undertook post hoc analyses to investigate the distribution of various pre‐treatment factors and found no significant difference in the distribution of these factors between the two groups, suggesting that the randomisation procedure used was unbiased (Turner 2000). We therefore also rated this trial as having low risk of bias for this item.
We rated 13 trials (23.6%) as having unclear risk of bias for sequence generation, as study authors provided no information on the method used to allocate participants to the experimental and control groups (Cedereke 2002; Dubois 1999; Gratz 2006; Gratz 2014; Hvid 2011; Liberman 1981; Marasinghe 2012; Patsiokas 1985; Torhorst 1987; Torhorst 1988). In an additional three trials opaque, sealed envelopes were used, but it was unclear whether these were shuffled to ensure random sequence generation (Allard 1992; Evans 1999a; Evans 1999b). We rated the two remaining trials as having high risk of bias for this item, as investigators used an open numbers table to allocate participants to the experimental and control groups (McLeavey 1994; Van Heeringen 1995).
Allocation concealment (selection bias)
We considered just over half of the included trials to be at low risk of bias for allocation concealment (k = 31; 56.4%). A third‐party researcher working independently of the trial team handled allocation in nine trials (Beautrais 2010; Bennewith 2002; Clarke 2002; Guthrie 2001; Harned 2014; Hassanian‐Moghaddam 2011; Hvid 2011; Morthorst 2012; Vaiva 2006), 14 trials used opaque, sealed envelopes (Allard 1992; Cedereke 2002; Crawford 2010; Evans 1999a; Evans 1999b; Gibbons 1978; Hawton 1981; Hawton 1987a; McMain 2009; Morgan 1993; Salkovskis 1990; Waterhouse 1990; Weinberg 2006; Van der Sande 1997a), and a remote/offsite researcher allocated participants in six trials (Bateman 2009; Fleischmann 2008; Husain 2014; Kawanishi 2014; Slee 2008; Tyrer 2003). In the one remaining trial information was not provided on the method used to conceal allocation, but correspondence with authors confirmed that they had adequately concealed allocation (Linehan 1991).
We rated a total of 18 trials (32.7%) as having unclear risk of bias for this item, as they provided no information on the method used to conceal allocation (Brown 2005; Davidson 2014; Dubois 1999; Gratz 2006; Gratz 2014; Kapur 2013a; Liberman 1981; Linehan 2006; Marasinghe 2012; Patsiokas 1985; Priebe 2012; Stewart 2009; Tapolaa 2010; Torhorst 1987; Torhorst 1988; Turner 2000; Wei 2013; Welu 1977). We also rated six trials as having a high risk of bias for this item. For four trials this was because the Zelen's design, in which participant consent is obtained after randomisation, was used (Carter 2005; Hatcher 2016a; Hatcher 2015; Hatcher 2011), whilst for the remaining two trials, this was because randomisation was via an open numbers table (McLeavey 1994; Van Heeringen 1995).
Blinding
Blinding was assessed separately for participants, clinical personnel, and outcome assessors.
Blinding of participants
Overall, we classified the majority of trials (k = 53; 96.4%) as having high risk of bias for blinding of participants, as it is generally not possible to blind participants to psychological therapy. In Harned 2014, correspondence with study authors clarified that allocation was concealed from participants until their first therapy session, at which point the therapist informed participants as to which treatment condition they had been allocated, confirming that participants were not blind to treatment allocation. We rated one trial as having low risk of bias for this item, as the authors asserted that "[t]he subjects were blinded as to their assignment" (Fleischmann 2008, p. 704). We rated the remaining trial as having an unclear risk of bias for this item; although authors did not provide any information on participant blinding, treatments were so similar that participants might have been blind to which treatment they were receiving (Liberman 1981).
Blinding of personnel
We classified the majority of trials (k = 52; 94.5%) as having high risk of bias for blinding of clinical personnel, as it is not possible to blind clinicians to the psychological therapy they are delivering. We rated one trial as having an unclear risk of bias for this item as, although GPs received a copy of the green (emergency) card given to participants randomised to the experimental group, it is unclear whether GPs were aware which of their patients received this card (Morgan 1993). We rated the two remaining trials as having a low risk of bias for this item, as clinicians were masked to allocation status (Beautrais 2010; Hassanian‐Moghaddam 2011).
Blinding of outcome assessors
As outcome assessors were blind to treatment allocation in 30 (54.5%) of the trials included in this review, we rated the majority of trials as having low risk of bias for blinding of outcome assessment. We rated 10 trials as having a high risk of bias for this item, as outcome assessor blinding was not possible: in four trials this was due to reliance on self‐reported information from participants who were not blind to treatment allocation (Guthrie 2001; Slee 2008; Torhorst 1987; Van Heeringen 1995), and in six it was due to issues related to feasibility, implementation, or both (Allard 1992; Brown 2005; Gratz 2006; Hassanian‐Moghaddam 2011; Stewart 2009; Waterhouse 1990). We rated the remaining 15 trials as having an unclear risk of bias for this item as they did not provide information on outcome assessor blinding (Bennewith 2002; Cedereke 2002; Dubois 1999; Fleischmann 2008; Liberman 1981; Morgan 1993; Morthorst 2012; Patsiokas 1985; Salkovskis 1990; Tapolaa 2010; Torhorst 1988; Tyrer 2003; Van der Sande 1997a; Wei 2013; Welu 1977).
Incomplete outcome data
For most trials the authors reported having conducted analyses on an intention‐to‐treat basis, earning them a rating of low risk for this item (k = 33; 60.0%), although the method used to conduct these analyses was not clear for the majority of these trials (Allard 1992; Bateman 2009; Beautrais 2010; Carter 2005; Cedereke 2002; Crawford 2010; Davidson 2014; Evans 1999a; Guthrie 2001; Hassanian‐Moghaddam 2011; Hawton 1987a; Husain 2014; Hvid 2011; Kapur 2013a; Kawanishi 2014; Marasinghe 2012; Morgan 1993; Morthorst 2012; Salkovskis 1990; Turner 2000; Vaiva 2006; Van der Sande 1997a; Wei 2013; Weinberg 2006). Three used regression methods (Bennewith 2002; Clarke 2002; Priebe 2012), one used longitudinal modelling (Brown 2005), and one used Bayesian Markov chain Monte Carlo simulation (Gratz 2014). The remaining three trials combined intention‐to‐treat with per protocol analyses (Harned 2014; Hatcher 2011; McMain 2009; Slee 2008), so we also rated them as having low risk of bias for this item. We rated seven trials as having unclear risk, as insufficient information was provided to confirm whether intention‐to‐treat or per protocol analyses had been undertaken (Hawton 1981; Patsiokas 1985; Torhorst 1987; Torhorst 1988; Tyrer 2003; Waterhouse 1990; Van Heeringen 1995). We classified the remaining 15 trials as having high risk of bias because per protocol analyses were undertaken (Dubois 1999; Evans 1999b; Fleischmann 2008; Gibbons 1978; Gratz 2006; Hatcher 2015; Hatcher 2016a; Liberman 1981; Linehan 1991; Linehan 2006; McAuliffe 2014; McLeavey 1994; Stewart 2009; Tapolaa 2010; Welu 1977).
Selective reporting
As the review authors did not have access to trial protocols for the trials included in this review, it is difficult to assess the extent to which selective outcome reporting could have occurred. Consequently, we classified the majority of trials as having an unclear risk of bias for this item (k = 52; 94.5%). We rated the remaining three trials as having high risk of bias for this outcome because data on pre‐specified outcomes were not reported in the text (Kawanishi 2014; McLeavey 1994; Torhorst 1987).
Other potential sources of bias
We classified most trials as having low risk of bias for this item as no evidence of other bias was apparent (k = 47; 85.4%). We rated seven trials as at high risk of bias for this item. Three had used Zelen's post‐consent randomisation procedure to allocate participants to the experimental and control groups (Hatcher 2011; Hatcher 2016a; Hatcher 2015). In an additional three there were substantial imbalances between the experimental and control groups on a number of putative risk factors for repetition of SH, despite randomisation (Beautrais 2010; Davidson 2014; Torhorst 1987). We rated the remaining trial as at high risk of other bias because a small number of participants in the control group (n = 20; 5.1%) mistakenly received the intervention treatment and yet were included in the control group for all subsequent analyses (Carter 2005). We rated one further trial as having unclear risk of bias for this item, as the participants were biased towards more compliant patients who were willing and able to attend a psycho‐education session at the commencement of treatment and were able to attend hospital regularly for case management sessions and follow‐up face‐to‐face interviews. Additionally, this trial excluded individuals who had engaged in non‐suicidal SH from participation (Kawanishi 2014).
Few trials used systematic means to investigate whether participants were able to guess if they had been allocated to the experimental or control arm.
Thirteen trials did not indicate the source of funding (Allard 1992; Dubois 1999; Hatcher 2016a; Hatcher 2015; McLeavey 1994; Morgan 1993; Patsiokas 1985; Salkovskis 1990; Stewart 2009; Tapolaa 2010; Torhorst 1987; Turner 2000; Waterhouse 1990). Fifteen trials received funding from national medical associations, research organisations, or both (Brown 2005; Gratz 2014; Harned 2014; Hawton 1987a; Kapur 2013a; Liberman 1981; Linehan 1991; Linehan 2006; McMain 2009; Priebe 2012; Slee 2008; Tyrer 2003; Van Heeringen 1995; Wei 2013; Welu 1977). An additional nine trials received funding from a health organisation (Bennewith 2002; Carter 2005; Clarke 2002; Davidson 2014; Evans 1999b; Fleischmann 2008; Gratz 2006; Guthrie 2001; Vaiva 2006). The remaining trials received funding from a variety of sources, including: research organisations associated with specific diagnostic groups (Bateman 2009; Weinberg 2006), charitable trusts (Crawford 2010), private research foundations (Cedereke 2002), accident compensation organisations (Hatcher 2011), health insurance companies (Van der Sande 1997a), government departments (Evans 1999a; Hawton 1981; Kawanishi 2014; Torhorst 1988), and university sources (Marasinghe 2012). A number of trials also received joint funding, including from health and accident compensation organisations (Beautrais 2010), health organisations and legal charities (Hassanian‐Moghaddam 2011), university sources and educational institutes (Husain 2014), government departments and health organisations (Gibbons 1978), government departments and health insurance companies (Hvid 2011), government departments and charitable trusts (Morthorst 2012), and health organisations and national research organisations (McAuliffe 2014).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings 1. Comparison 1: CBT‐based psychotherapy vs treatment as usual.
| CBT‐based psychotherapy vs treatment as usual for self‐harm in adults | ||||||
| Patient or population: adults who engage in SH Settings: outpatients Intervention: CBT‐based psychotherapy Comparison: treatment as usual (TAU) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU | CBT‐based psychotherapy | |||||
| Repetition of SH at post‐intervention | Study population | OR 0.66 (0.36 to 1.21) | 313 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | We downgraded quality as, due to the nature of the intervention, it is unlikely participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality as the confidence interval for the treatment effect size is wide. | |
| 190 per 1000 | 134 per 1000 (78 to 221) | |||||
| Repetition of SH at 6 months | Study population | OR 0.54 (0.34 to 0.85) | 1317 (12 RCTs) | ⊕⊕⊕⊝ Moderatea | We downgraded quality as, due to the nature of the intervention, it is unlikely participants and clinical personnel would have been blind to treatment allocation. For some trials, additionally, participants were also not blinded to treatment allocation. | |
| 280 per 1000 | 173 per 1000 (117 to 248) | |||||
| Repetition of SH at 12 months | Study population | OR 0.80 (0.65 to 0.98) | 2232 (10 RCTs) | ⊕⊕⊕⊝ Moderatea | We downgraded quality as, due to the nature of the intervention, it is unlikely participants and clinical personnel would have been blind to treatment allocation. For some trials, additionally, participants were also not blinded to treatment allocation. | |
| 272 per 1000 | 230 per 1000 (196 to 268) | |||||
| Repetition of SH at 24 months | Study population | OR 0.31 (0.14 to 0.69) | 105 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | We downgraded quality as, due to the nature of the intervention, it is unlikely participants and clinical personnel would have been blind to treatment allocation. For 1 trial, additionally, participants were also not blinded to treatment allocation. | |
| 563 per 1000 | 285 per 1000 (153 to 470) | |||||
| Repetition of SH at final follow‐up | Study population | OR 0.70 (0.55 to 0.88) | 2665 (17 RCTs) | ⊕⊕⊝⊝ Lowa,c | We downgraded quality as, due to the nature of the intervention, it is unlikely participants and clinical personnel would have been blind to treatment allocation. For 1 trial, additionally, participants were also not blinded to treatment allocation. We further downgraded quality due to the inconsistency in the magnitude of the effect size estimates across trials. | |
| 262 per 1000 | 199 per 1000 (163 to 238) | |||||
| Frequency of SH at final follow‐up | The mean frequency of episodes of SH in the experimental group was, on average, 0.21 lower (0.68 lower to 0.26 higher) | ‐ | 597 (6 RCTs) | ⊕⊕⊝⊝ Lowa,c | We downgraded quality as, due to the nature of the intervention, it is unlikely participants and clinical personnel would have been blind to treatment allocation. For 1 trial, additionally, participants were also not blinded to treatment allocation. We further downgraded quality due to the inconsistency in the magnitude of the effect size estimates across trials. | |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CBT: cognitive behavioural therapy; CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial: SH: self‐harm; TAU: treatment as usual. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a We rated risk of bias as SERIOUS as the nature of the intervention means that clinical personnel could not have remained blind to treatment allocation. Additionally, for some trials, participants were not blinded to treatment allocation. Performance and detection bias therefore may have been present.
b Imprecision was rated as SERIOUS as the confidence interval is wide c We rated inconsistency as SERIOUS due to notable differences in the magnitude of the effect size estimates between trials on visual inspection of the forest plot.
Summary of findings 2. Comparison 2: Interventions for multiple repetition of SH/probable personality disorder vs treatment as usual or other alternative forms of psychotherapy.
| Interventions for multiple repetition of SH/probable personality disorder vs treatment as usual or other alternative forms of psychotherapy | |||||||
| Patient or population: adults who engage in SH Settings: outpatients Intervention: interventions for multiple repetition of SH/probable personality disorder Comparison: treatment as usual (TAU) or other alternative forms of psychotherapy | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| TAU/other alternative forms of psychotherapy | Interventions for multiple repetition of SH/probable personality disorder | ||||||
| Emotion‐regulation group‐based psychotherapy vs TAU | |||||||
| Repetition of SH at post‐intervention | Study population | OR 0.34 (0.13 to 0.88) | 83 (2 RCTs) | ⊕⊕⊝⊝ Lowa | We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. Additionally, for 1 trial, outcome assessors were also not blind to treatment allocation. We further downgraded quality as study investigators did not adequately describe details on sequence generation and allocation concealment. | ||
| 775 per 1000 | 539 per 1000 (309 to 752) | ||||||
| Frequency of SH at post‐intervention | Study population | — | 83 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. Study investigators also did not adequately describe details on sequence generation and allocation concealment. Additionally, for 1 trial, outcome assessors were also not blind to treatment allocation As the confidence interval for the treatment effect size is wide, we further downgraded quality due to imprecision. | ||
| The mean frequency of episodes of SH in the experimental group was, on average,12.76 lower (34.92 lower to 9.40 higher) | |||||||
| Mentalisation vs TAU | |||||||
| Repetition of SH at post‐intervention | Study population | OR 0.35 (0.17 to 0.73) | 134 (1 RCT) | ⊕⊕⊕⊝ Moderateb | We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. | ||
| 492 per 1000 | 253 per 1000 (141 to 414) | ||||||
| Frequency of SH at post‐intervention | Study population | — | 133 (1 RCT) | ⊕⊕⊕⊝ Moderateb | We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. Additionally, as the confidence interval for the treatment effect size is wide, we further downgraded quality. | ||
| The mean frequency of episodes of SH in the experimental group was, on average,1.28 lower (2.01 lower to 0.55 lower) | |||||||
| DBT‐oriented therapy vs Alternative forms of psychotherapy | |||||||
| Repetition of SH at post‐intervention | Study population |
OR 0.05 (0.00 to 0.49) |
24 (1 RCT) | ⊕⊕⊝⊝ Lowb,c |
We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality as the sample size is small. | ||
| 667 per 1000 | 91 per 1000 (0 to 495) | ||||||
| Frequency of SH at post‐intervention | Study population | — | 24 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality as the sample size is small. | ||
| The mean frequency of episodes of SH in the experimental group was, on average,4.83 lower (7.90 lower to 1.76 lower) | |||||||
| DBT vs TAU | |||||||
| Repetition of SH at post‐intervention | Study population |
OR 0.59 (0.16 to 2.15) |
267 (3 RCTs) | ⊕⊕⊝⊝ Lowb,c |
We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality due to notable differences in the magnitude of the effect size estimates between trials on visual inspection of the forest plot. | ||
| 667 per 1000 | 541 per 1000 (242 to 811) | ||||||
| Repetition of SH at 12 months' follow‐up | Study population |
OR 0.36 (0.05 to 2.47) |
172 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c |
We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality due to notable differences in the magnitude of the effect size estimates between trials on visual inspection of the forest plot. | ||
| 495 per 1000 | 260 per 1000 (47 to 707) | ||||||
| Repetition of SH at final follow‐up | Study population |
OR 0.57 (0.21 to 1.59) |
247 (3 RCTs) | ⊕⊕⊝⊝ Lowb,c |
We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality due to notable differences in the magnitude of the effect size estimates between trials on visual inspection of the forest plot. | ||
| 620 per 1000 | 482 per 1000 (255 to 722) | ||||||
| Frequency of SH at post‐intervention | Study population | — | 292 (3 RCTs) | ⊕⊕⊝⊝ Lowb,c | We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality due to imprecision of the effect size estimate. | ||
| The mean frequency of episodes of SH in the experimental group was, on average,18.82 lower (36.68 lower to 0.95 lower) | |||||||
| DBT vs treatment by expert | |||||||
| Repetition of SH at post‐intervention | Study population |
OR 1.66 (0.53 to 5.20) |
97 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c |
We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. Additionally, study authors did not adequately describe details on allocation concealment. Lastly, as the confidence interval for the treatment effect size is wide, we further downgraded quality. | ||
| 822 per 1000 | 885 per 1000 (710 to 960) | ||||||
| Repetition of SH at 12 months | Study population |
OR 1.18 (0.35 to 3.95) |
97 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c |
We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. Study authors did not adequately describe details on allocation concealment. Lastly, as the confidence interval for the treatment effect size is wide, we further downgraded quality. | ||
| 867 per 1000 | 885 per 1000 (695 to 963) | ||||||
| Frequency of SH at post‐intervention | Study population | — | 97 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c |
We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. Study authors did not adequately describe details on allocation concealment. Lastly, as the confidence interval for the treatment effect size is wide, we further downgraded quality. | ||
| The mean frequency of episodes of SH in the experimental group was, on average,14.85 lower (37.64 lower to 7.94 higher) | |||||||
| DBT prolonged exposure vs DBT standard exposure | |||||||
| Repetition of SH at post‐intervention | Study population |
OR 0.67 (0.08 to 5.68) |
18 (1 RCT) |
⊕⊕⊝⊝ Lowb,c | We downgraded quality as details on participant and clinical personnel blinding were not adequately described. However, given the similarity between the intervention and control treatment in this trial, it is possible that blinding could have been achieved. We further downgraded quality as the confidence interval for the treatment effect size is wide. | ||
| 333 per 1000 |
251 per 1000 (38 to 740) |
||||||
| Repetition of SH at 6 months' follow‐up | Study population |
OR 0.67 (0.08 to 5.68) |
18 (1 RCT) |
⊕⊕⊝⊝ Lowb,c | We downgraded quality as details on participant and clinical personnel blinding were not adequately described. However, given the similarity between the intervention and control treatment in this trial, it is possible that blinding could have been achieved. We further downgraded quality as the confidence interval for the treatment effect size is wide. | ||
| 333 per 1000 |
251 per 1000 (38 to 740) |
||||||
| Frequency of SH at post‐intervention | Study population | — | 18 (1 RCT) |
⊕⊕⊝⊝ Low b,c | We downgraded quality as details on participant and clinical personnel blinding were not adequately described. However, given the similarity between the intervention and control treatment in this trial, it is possible that blinding could have been achieved. We further downgraded quality as the confidence interval for the treatment effect size is wide. | ||
| The mean frequency of episodes of SH in the experimental group was, on average,0.25 lower (2.47 lower to 1.97 higher) | |||||||
| Frequency of SH at 6 months' follow‐up | Study population | — | 18 (1 RCT) |
⊕⊕⊝⊝ Lowb,c | We downgraded quality as details on participant and clinical personnel blinding were not adequately described. However, given the similarity between the intervention and control treatment in this trial, it is possible that blinding could have been achieved. We further downgraded quality as the confidence interval for the treatment effect size is wide. | ||
| The mean frequency of episodes of SH in the experimental group was, on average,0.34 higher (0.61 lower to 1.29 higher) | |||||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial: SH: self‐harm; TAU: treatment as usual. | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
a Risk of bias was rated as VERY SERIOUS as the nature of the intervention means that participants and clinical personnel could not have remained blind to treatment allocation, suggesting that performance and detection bias may have been present. For 1 trial, outcome assessors were not blind to treatment allocation. Additionally, as details on sequence generation and allocation concealment were not adequately described, selection bias may have been present. b Risk of bias was rated as SERIOUS as the nature of the intervention means that participants and clinical personnel could not have remained blind to treatment allocation suggesting that performance and detection bias may have been present. c Imprecision was rated as SERIOUS as the confidence interval is wide or there are notable differences in the magnitude of the effect size between trials on visual inspection of the forest plot.
Summary of findings 3. Comparison 3: Case management vs treatment as usual or other alternative forms of psychotherapy.
| Case management vs treatment as usual or other alternative forms of psychotherapy | ||||||
|
Patient or population: adults who engage in SH
Settings: outpatients
Intervention: case management Comparison: treatment as usual (TAU) or other alternative forms of psychotherapy. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU/other alternative forms of psychotherapy | Case management | |||||
| Repetition of SH at post‐intervention | Study population |
OR 0.78 (0.47 to 1.30) |
1608 (4 RCTs) |
⊕⊕⊕⊝ Moderatea | We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. | |
| 114 per 1000 |
91 per 1000 (57 to 143) |
|||||
| Multiple readmissions for SH at post‐intervention | Study population |
OR 5.23 (1.12 to 24.45) |
469 (1 RCT) |
⊕⊕⊕⊝ Moderatea | We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. | |
| 8 per 1000 |
41 per 1000 (9 to 166) |
|||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial: SH: self‐harm; TAU: treatment as usual. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a Risk of bias was rated as SERIOUS as the nature of the intervention means that participants and clinical personnel could not have remained blind to treatment allocation.
Summary of findings 4. Comparison 4: Adherence enhancement approaches vs treatment as usual or other alternative forms of psychotherapy.
| Adherence enhancement approaches vs treatment as usual or other alternative forms of psychotherapy | ||||||
|
Patient or population: adults who engage in SH
Settings: outpatients Intervention: Adherence enhancement approaches Comparison: treatment as usual (TAU) or other alternative forms of psychotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU/other alternative forms of psychotherapy | Adherence enhancement approaches | |||||
| Compliance enhancement vs TAU | ||||||
| Repetition of SH at 12 months' follow‐up | Study population | OR 0.57 (0.32 to 1.02) | 391 (1 RCT) | ⊕⊕⊝⊝ Lowa | We downgraded quality as an open random numbers table was used to generate the allocation sequence and, as allocation was not concealed, there is possible selection bias. We further downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. | |
| 174 per 1000 | 107 per 1000 (63 to 177) | |||||
| Continuity of care by the same therapist vs other alternative forms of psychotherapy (i.e., care by a different therapist) | ||||||
| Repetition of SH at 12 months' follow‐up | Study population |
OR 0.28 (0.07 to 1.10) |
136 (1 RCT) |
⊕⊝⊝⊝ Very lowb,c | We downgraded quality as neither participants, clinical personnel, nor outcome assessors were blind to treatment allocation. We further downgraded quality as study authors did not specify the method used to allocate participants to the experimental and control groups, nor did they report details on allocation concealment. Finally, we downgraded quality three grades, as there was significant imbalance between the experimental and control group for some putative risk factors for repetition of SH despite randomisation. | |
| 136 per 1000 |
42 per 1000 (11 to 148) |
|||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial: SH: self‐harm; TAU: treatment as usual. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a Risk of bias was rated as VERY SERIOUS as the nature of the intervention means that participants and clinical personnel could not have remained blind to treatment allocation, suggesting that performance and detection bias may have been present. As an open numbers table was used the generate the allocation sequence, and as allocation was not concealed, selection bias also may have been present. b Risk of bias was rated as VERY SERIOUS as the nature of the intervention means that participants and clinical personnel could not have remained blind to treatment allocation, suggesting that performance and detection bias may have been present. Additionally, as no details on the method used to allocate participants to the intervention and control groups or on allocation concealment were reported, selection bias also may have been present. c There was significant imbalance between the intervention and control groups for a number of putative risk factors for repetition of SH despite randomisation.
Summary of findings 5. Comparison 5: Mixed multimodal interventions vs treatment as usual.
| Mixed multimodal interventions vs treatment as usual | ||||||
| Patient or population: adults who engage in SH Settings: outpatients Intervention: mixed multimodal interventions Comparison: treatment as usual (TAU) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU | Mixed multimodal Interventions | |||||
| Mixed multimodal interventions vs TAU | ||||||
| Repetition of SH at post‐intervention | Study population | OR 0.98 (0.68 to 1.43) | 684 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | We downgraded quality as, due to the nature of the intervention, it is unlikely participants and clinical personnel would have been blind to treatment allocation. Additionally, use of Zelen's post‐consent design would indicate that participants were also not blind to treatment allocation. We further downgraded quality as the confidence interval for the treatment effect size is wide. | |
| 204 per 1000 | 201 per 1000 (149 to 269) | |||||
| Culturally‐adapted mixed multimodal interventions vs TAU | ||||||
| Repetition of SH at 12 months | Study population |
OR 0.83 (0.44 to 1.55) |
167 (1 RCT) |
⊕⊕⊝⊝ Lowa,b |
We downgraded quality as, due to the nature of the intervention, it is unlikely participants and clinical personnel would have been blind to treatment allocation. Additionally, use of Zelen's post‐consent design would indicate that participants were also not blind to treatment allocation. We further downgraded quality as the confidence interval for the treatment effect size is wide. | |
| 403 per 1000 |
359 per 1000 (229 to 511) |
|||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial: SH: self‐harm; TAU: treatment as usual. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a Risk of bias was rated as SERIOUS, as the nature of the intervention means that participants and clinical personnel could not have remained blind to treatment allocation. Additionally, the use of Zelen's post‐consent design indicates that participants would not have been blind to treatment allocation. Performance and detection bias therefore may have been present.
b Imprecision was rated as SERIOUS as the confidence interval is wide.
Summary of findings 6. Comparison 6: Remote contact interventions vs treatment as usual.
| Remote contact interventions vs treatment as usual | ||||||
| Patient or population: adults who engage in SH Settings: outpatients Intervention: remote contact interventions Comparison: treatment as usual | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU | Remote contact interventions | |||||
| Postcards vs TAU | ||||||
| Repetition of SH at post‐intervention | Study population | OR 0.87 (0.62 to 1.23) | 3277 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,b | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality due to significant differences in the direction of the effect size estimate between trials on visual inspection of the forest plot. | |
| 132 per 1000 | 117 per 1000 (86 to 157) | |||||
| Repetition of SH at 12 months | Study population | OR 0.76 (0.57 to 1.02) | 2885 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. | |
| 175 per 1000 | 139 per 1000 (108 to 178) | |||||
| Repetition of SH at final follow‐up | Study population | OR 0.88 (0.62 to 1.25) | 3277 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,b | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality due to significant differences in the direction of the effect size estimate between trials on visual inspection of the forest plot. | |
| 185 per 1000 | 167 per 1000 (123 to 221) | |||||
| Frequency of SH at post‐intervention | Study population | — | 1097 (3 RCTs) |
⊕⊝⊝⊝ Very lowa,b | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality due to significant differences in the direction of the effect size estimate between trials on visual inspection of the forest plot. | |
| The mean frequency of episodes of SH in the experimental group was, on average, 0.07 lower (0.32 lower to 0.18 higher) | ||||||
| Frequency of SH at 12 months | Study population | — | 984 (2 RCTs) |
⊕⊝⊝⊝ Very lowa,b | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality due to significant differences in the direction of the effect size estimate between trials on visual inspection of the forest plot. | |
| The mean frequency of episodes of SH in the experimental group was, on average, 0.19 lower (0.58 lower to 0.20 higher) | ||||||
| Frequency of SH at 24 months | Study population | — | 472 (1 RCT) |
⊕⊕⊕⊝ Moderatea | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. | |
| The mean frequency of episodes of SH in the experimental group was, on average, 0.03 lower (0.16 lower to 0.10 higher) | ||||||
| Emergency cards vs TAU | ||||||
| Repetition of SH at post‐intervention | Study population |
OR 0.82 (0.31 to 2.14) |
1039 (2 RCTs) |
⊕⊕⊝⊝ Lowa,d | We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel were bind to treatment allocation. Additionally, quality was further downgraded due to notable differences in the direction of the effect size estimate between trials on visual inspection of the forest plot. | |
| 171 per 1000 |
145 per 1000 (60 to 306) |
|||||
| Repetition of SH at 12 months' follow‐up | Study population |
OR 1.19 (0.85 to 1.67) |
827 (1 RCT) |
⊕⊕⊕⊝ Moderate a | We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel were bind to treatment allocation. | |
| 188 per 1000 |
216 per 1000 (164 to 279) |
|||||
| General practitioner's (GP) letter vsTAU | ||||||
| Repetition of SH at post‐intervention | Study population |
OR 1.15 (0.93 to 1.44) |
1932 (1 RCT) |
⊕⊕⊕⊝ Moderatea | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. | |
| 195 per 1000 |
218 per 1000 (184 to 259) |
|||||
| Telephone contact vs TAU | ||||||
| Repetition of SH at 6 months' follow‐up | Study population |
OR 0.23 (0.02 to 2.11) |
81 (1 RCT) |
⊕⊕⊝⊝ Lowa,e | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality as the confidence interval for the treatment effect size is wide. | |
| 100 per 1000 |
25 per 1000 (2 to 190) |
|||||
| Repetition of SH at 12 months' follow‐up | Study population |
OR 1.00 (0.45 to 2.23) |
172 (1 RCT) |
⊕⊕⊝⊝ Low a,e | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality as the confidence interval for the treatment effect size is wide. | |
| 169 per 1000 |
169 per 1000 (84 to 311) |
|||||
| Repetition of SH at 24 months' follow‐up | Study population |
OR 0.76 (0.49 to 1.16) |
605 (1 RCT) |
⊕⊕⊕⊝ Lowa,e | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality as the confidence interval for the treatment effect size is wide. | |
| 189 per 1000 |
151 per 1000 (103 to 213) |
|||||
| Repetition of SH at final follow‐up | Study population |
OR 0.74 (0.42 to 1.32) |
840 (3 RCTs) |
⊕⊝⊝⊝ Very lowa,b | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality due to significant differences in the direction of the effect size estimate between trials on visual inspection of the forest plot. | |
| 185 per 1000 |
143 per 1000 (87 to 230) |
|||||
| Mobile telephone‐based psychotherapy vs TAU | ||||||
| Repetition of SH at post‐intervention | Study population | Not estimable | 68 (1 RCT) |
⊕⊕⊝⊝ Lowa,e | We downgraded quality as the nature of this intervention means it is unlikely that participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality as the sample size is small. | |
| 0 per 1000 |
0 per 1000 (0 to 0) |
|||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial: SH: self‐harm; TAU: treatment as usual. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a Risk of bias was rated as SERIOUS as the nature of the intervention means that participants and clinical personnel could not have remained blind to treatment allocation. Additionally, for some trials, no details on outcome assessor blinding were reported. Performance and detection bias therefore may have been present. b Inconsistency was rated as VERY SERIOUS as the confidence interval is wide or there are significant differences in the magnitude of the effect size between trials on visual inspection of the forest plot. c Risk of bias was rated as VERY SERIOUS as the nature of the intervention means that participants and clinical personnel could not have remained blind to treatment allocation. Additionally, for some trials, no details on outcome assessor blinding were reported. Performance and detection bias therefore cannot be ruled out. Additionally, as a number of participants randomised to the control group mistakenly received the intervention, and yet were included in the control group for all subsequent analyses, other bias may have been present. d Inconsistency was rated as SERIOUS as the confidence interval is wide or there are notable differences in the magnitude of the effect size between trials on visual inspection of the forest plot.
e Imprecision was rated as SERIOUS as the confidence interval is wide and/or the sample size is small.
Summary of findings 7. Comparison 7: Other mixed interventions vs treatment as usual or other alternative form of psychotherapy.
| Heterogeneous other interventions vs treatment as usual or other alternative forms of psychotherapy | ||||||
|
Patient or population: adults who engage in SH Settings: mixture of in‐ and outpatients Intervention: other mixed interventions Comparison: treatment as usual or other alternative forms of psychotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU or other alternative forms of psychotherapy | Heterogenous other interventions | |||||
| Interpersonal problem‐solving skills training vs other alternative forms of psychotherapy | ||||||
| Repetition of SH at 12 months | Study population |
OR 0.40 (0.06 to 2.57) |
33 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | We downgraded quality as the nature of this intervention means it is unlikely participants and clinical personnel would have been blind to treatment allocation. We further downgraded quality as an open random numbers table was used to generate the allocation sequence and, as allocation was not concealed, there is possible selection bias. We further downgraded quality as the sample size is small. | |
| 250 per 1000 |
118 per 1000 (20 to 461) |
|||||
| Behaviour therapy vs other alternative forms of psychotherapy | ||||||
| Repetition of SH at 12 months | Study population |
OR 0.60 (0.08 to 4.45) |
24 (1 RCT) |
⊕⊕⊝⊝ Lowb,c | We downgraded quality as clinical personnel were not blind to treatment allocation. Additionally, details on sequence generation, allocation concealment, participant blinding, and outcome assessor blinding were not adequately described. Lastly, as the confidence interval for the treatment effect size is wide, we further downgraded quality. | |
| 250 per 1000 |
167 per 1000 (26 to 597) |
|||||
| Information and support vs TAU | ||||||
| Repetition of SH at final follow‐up for the overall cohort | Study population | OR 1.02 (0.71 to 1.47) | 1663 (1 RCT) | ⊕⊕⊝⊝ Lowd | We downgraded quality as the nature of the intervention means it is unlikely that clinical personnel would have been blind to treatment allocation. We further downgraded quality as attrition bias may have been present. | |
| 75 per 1000 | 76 per 1000 (54 to 106) | |||||
| Repetition of SH at final follow‐up for the Campinas, Brazil site | Study population | OR 2.27 (0.97 to 5.28) | 135 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | We downgraded quality as the nature of the intervention means it is unlikely that clinical personnel would have been blind to treatment allocation. We further downgraded quality as attrition bias may have been present. We downgraded quality three grades for this site as the confidence interval for the treatment effect size is wide. | |
| 156 per 1000 | 296 per 1000 (152 to 494) | |||||
| Repetition of SH at final follow‐up for the Colombo, Sri Lanka site | Study population | OR 0.55 (0.13 to 2.34) | 251 (1 RCT) | ⊕⊝⊝⊝ Very lowb,d | We downgraded quality as the nature of the intervention means it is unlikely that clinical personnel would have been blind to treatment allocation. We further downgraded quality as attrition bias may have been present. We further downgraded quality for this site as the confidence interval for the treatment effect size is wide. | |
| 41 per 1000 | 23 per 1000 (6 to 92) | |||||
| Repetition of SH at final follow‐up for the Karaj, Iran site | Study population | OR 1.18 (0.69 to 2) | 601 (1 RCT) | ⊕⊕⊝⊝ Lowd | We downgraded quality as the nature of the intervention means it is unlikely that clinical personnel would have been blind to treatment allocation. We further downgraded quality as attrition bias may have been present. | |
| 94 per 1000 | 109 per 1000 (67 to 172) | |||||
| Repetition of SH at final follow‐up for the Yuncheng, China site | Study population | OR 2.01 (0.08 to 50.6) | 96 (1 RCT) | ⊕⊝⊝⊝ Very lowb,d | We downgraded quality as the nature of the intervention means it is unlikely that clinical personnel would have been blind to treatment allocation. We further downgraded quality as attrition bias may have been present. We further downgraded quality for this site as the confidence interval for the treatment effect size is wide. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Repetition of SH at final follow‐up for the Chennai, India site | Study population | OR 0.39 (0.17 to 0.92) | 561 (1 RCT) | ⊕⊕⊝⊝ Lowd | We downgraded quality as the nature of the intervention means it is unlikely that clinical personnel would have been blind to treatment allocation. We further downgraded quality as attrition bias may have been present. | |
| 65 per 1000 | 27 per 1000 (12 to 60) | |||||
| Frequency of SH at final follow‐up for the Karaj, Iran site | The frequency of episodes of SH for the Karaj, Iran site in the experimental group was, on average, 0.46 higher (0.32 higher to 0.32 higher) | — | 629 (1 RCT) | ⊕⊕⊝⊝ Lowd | We downgraded quality as the nature of the intervention means it is unlikely that clinical personnel would have been blind to treatment allocation. We further downgraded quality as attrition bias may have been present. | |
| Treatment for alcohol misuse vs TAU | ||||||
| Repetition of SH at 6 months | Study population |
OR 0.57 (0.20 to 1.60) |
103 (1 RCT) |
⊕⊕⊝⊝ Lowb,c | We downgraded quality as the nature of this intervention means it is unlikely clinical personnel would have been blind to treatment allocation. We further downgraded quality as the confidence interval for the treatment effect size is wide. | |
| 216 per 1000 |
136 per 1000 (52 to 306) |
|||||
| Home‐based problem‐solving therapy vs other alternative forms of psychotherapy | ||||||
| Repetition of SH at 12 months | Study population |
OR 0.68 (0.20 to 2.32) |
96 (1 RCT) |
⊕⊕⊝⊝ Lowb,c | We downgraded quality as the nature of this intervention means it is unlikely clinical personnel would have been blind to treatment allocation. We further downgraded quality as the confidence interval for the treatment effect size is wide. | |
| 146 per 1000 |
104 per 1000 (33 to 284) |
|||||
| Intensive inpatient and community treatment vs TAU | ||||||
| Repetition of SH at 12 months | Study population |
OR 1.18 (0.62 to 2.25) |
274 (1 RCT) |
⊕⊕⊝⊝ Low b,c | We downgraded quality as the nature of this intervention means it is unlikely clinical personnel would have been blind to treatment allocation. We further downgraded quality as the confidence interval for the treatment effect size is wide. | |
| 149 per 1000 |
172 per 1000 (98 to 283) |
|||||
| Frequency of SH at 12 months | Study population | — | 274 (1 RCT) |
⊕⊕⊕⊝ Moderatec | We downgraded quality as the nature of this intervention means it is unlikely clinical personnel would have been blind to treatment allocation. | |
| The mean frequency of SH at 12 months in the control group was 0.23 episodes | The mean frequency of SH at 12 months in the experimental group was 0 higher (0.17 lower to 0.17 higher | |||||
| General hospital admission vs other alternative forms of psychotherapy | ||||||
| Repetition of SH at post‐intervention | Study population |
OR 1.03 (0.14 to 7.69) |
77 (1 RCT) |
⊕⊕⊝⊝ Lowb,c | We downgraded quality as the nature of this intervention means it is unlikely clinical personnel would have been blind to treatment allocation. Lastly, as the confidence interval for the treatment effect size is wide, quality was further downgraded. | |
| 51 per 1000 |
53 per 1000 (8 to 294) |
|||||
| Repetition of SH at 6 months' follow‐up | Study population |
OR 0.75 (0.16 to 3.60) |
77 (1 RCT) |
⊕⊕⊝⊝ Lowb,c | We downgraded quality as the nature of this intervention means it is unlikely clinical personnel would have been blind to treatment allocation. Lastly, as the confidence interval for the treatment effect size is wide, quality was further downgraded. | |
| 103 per 1000 |
79 per 1000 (18 to 291) |
|||||
| Intensive outpatient intervention vs TAU | ||||||
| Repetition of SH at post‐intervention | Study population |
OR 0.27 (0.07 to 1.06) |
119 (1 RCT) |
⊕⊕⊕⊝ Low b,c |
We downgraded quality as the nature of this intervention means it is unlikely clinical personnel would have been blind to treatment allocation. Lastly, as the confidence interval for the treatment effect size is wide, quality was further downgraded. | |
| 158 per 1000 |
48 per 1000 (13 to 166) |
|||||
| Repetition of SH at 24 months | Study population |
OR 1.24 (0.59 to 2.62) |
126 (1 RCT) |
⊕⊕⊝⊝ Low b,c | We downgraded quality as the nature of this intervention means it is unlikely clinical personnel would have been blind to treatment allocation. Lastly, as the confidence interval for the treatment effect size is wide, quality was further downgraded. | |
| 302 per 1000 |
349 per 1000 (203 to 531) |
|||||
| Repetition of SH at final follow‐up | Study population |
OR 0.65 (0.15 to 2.85) |
245 (2 RCTs) |
⊕⊝⊝⊝ Very lowb,e | We downgraded quality as the nature of this intervention means it is unlikely clinical personnel could have been blind to treatment allocation. Additionally, for 1 trial, participants also were not blind to treatment allocation. We further downgraded quality due to significant differences in the direction of the effect size estimate between trials on visual inspection of the forest plot. | |
| 233 per 1000 |
165 per 1000 (44 to 464) |
|||||
| Long term vs other alternative forms of psychotherapy | ||||||
| Repetition of SH at 12 months | Study population |
OR 1.00 (0.35 to 2.86) |
80 (1 RCT) |
⊕⊕⊝⊝ Low b,c | We downgraded quality as the nature of this intervention means it is unlikely clinical personnel would have been blind to treatment allocation, additionally, the method used to allocate participants to the treatment and interventions groups was not specified and as no details on allocation concealment was reported. We further downgraded quality as the sample size was small and the confidence interval for the treatment effect size is wide. | |
| 225 per 1000 |
225 per 1000 (92 to 454) |
|||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial: SH: self‐harm; TAU: treatment as usual. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a Risk of bias was rated as VERY SERIOUS as the nature of the intervention means that participants and clinical personnel could not have remained blind to treatment allocation, suggesting that performance and detection bias may have been present. As an open numbers table was used the generate the allocation sequence, and as allocation was not concealed, selection bias also may have been present.
b Imprecision was rated as SERIOUS as the confidence interval is wide and/or the sample size is small. c Risk of bias was rated as SERIOUS as clinical personnel were not blind to treatment allocation, suggesting that performance and detection bias may have been present. Additionally, although details on participant blinding and outcome assessor blinding were not adequately described, the nature of the intervention means that participants could not have remained blind to treatment allocation. Finally, authors of some studies did not adequately describe details on sequence generation and allocation concealment. Selection bias therefore may also have been present. d Risk of bias was rated as VERY SERIOUS as the nature of the intervention means that participants and clinical personnel could not have remained blind to treatment allocation, suggesting that performance and detection bias may have been present. Additionally, attrition bias may have been present. e Inconsistency was rated as VERY SERIOUS due to significant differences in the magnitude of the effect size between trials on visual inspection of the forest plot.
Comparison 1: CBT‐based psychotherapy vs treatment as usual (TAU)
Eighteen trials assessed the effectiveness of CBT‐based psychotherapy, in which participants in the experimental group were offered some form of specific psychological therapy, such as cognitive behavioural therapy or problem‐solving therapy (Brown 2005, N = 120; Davidson 2014, N = 20; Dubois 1999, N = 102; Evans 1999b, N = 32; Gibbons 1978, N = 400; Guthrie 2001, N = 119; Hatcher 2011, N = 1094; Hawton 1987a, N = 80; Husain 2014, N = 221; McAuliffe 2014, N = 433; Patsiokas 1985, N = 15; Salkovskis 1990, N = 20; Slee 2008, N = 82; Stewart 2009, N = 32; Tapolaa 2010, N = 16; Tyrer 2003, N = 480; Wei 2013, N = 162; Weinberg 2006; N = 30). One of these used Zelen's design (Hatcher 2011). In most trials, therapy was typically very brief (i.e., less than 10 sessions), and it was delivered on an individual basis in all but one (McAuliffe 2014). One trial included only patients with borderline personality disorder (Weinberg 2006). In Stewart 2009, there were separate treatment arms for cognitive behavioural therapy and problem‐solving therapy. We therefore combined data from these two conditions using the formula outlined in section 7.7.3.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Primary outcome
1.1 Repetition of SH
There was no evidence of a significant treatment effect for CBT‐based psychotherapy on repetition of SH by post‐intervention in McAuliffe 2014 (23/171 vs 27/142; OR 0.66, 95% CI 0.36 to 1.21; k = 1; N = 313; GRADE: low quality).
By the six‐month follow‐up assessment, however, on the basis of data from 12 trials there was evidence of a significant treatment effect for CBT‐based psychotherapy on repetition of SH (Analysis 1.1; OR 0.54, 95% CI 0.34 to 0.85; k = 12; N = 1317), with moderate quality of evidence (see Table 1). Omitting Weinberg 2006, which included only participants diagnosed with borderline personality disorder, did not materially affect this result. There was, however, evidence of a significant difference by modality (Analysis 1.1; test for subgroup differences: Chi2= 7.32, degrees of freedom (df) = 1, P = 0.007, I2 = 86.3%). Specifically, although individual CBT‐based psychotherapy was associated with a significant treatment effect on repetition of SH by the six‐month follow‐up assessment (OR 0.52, 95% CI 0.36 to 0.75; k = 11; N = 1083), a group‐based approach was not associated with a significant treatment effect for this outcome in one trial (OR 1.35, 95% CI 0.75 to 2.41; k = 1; N = 234; McAuliffe 2014).
1.1. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 1: Repetition of SH at 6 months
There was also evidence of a significant treatment effect for CBT‐based psychotherapy by the 12‐month follow‐up assessment in 10 trials (Analysis 1.2; OR 0.80, 95% CI 0.65 to 0.98; k = 10; N = 2142), again with a moderate quality of evidence (see Table 1). Once again, omitting Weinberg 2006 did not materially affect this result, nor did omitting Hatcher 2011, which used Zelen's design. Hatcher 2011 also reported numbers of participants self‐reporting an episode of SH rather than those admitted to hospital following an episode of SH. Using these data, however, did not materially affect this result. There was no evidence of a significant difference by modality for this outcome (Analysis 1.2; test for subgroup differences: Chi2 = 1.68, df = 1, P = 0.19, I2 = 40.6%).
1.2. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 2: Repetition of SH at 12 months
In two trials data on repetition of SH from 12 to 24 months were reported. A significant treatment effect for this outcome was found (Analysis 1.3; OR 0.31, 95% CI 0.14 to 0.69; k = 2; N = 105; GRADE: moderate quality) (see Table 1).
1.3. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 3: Repetition of SH at 24 months
Including all 17 trials that reported information on repetition of SH suggested a significant treatment effect for CBT‐based psychotherapy by the final follow‐up assessment (i.e., including data for the last follow‐up assessment available in each trial) (Analysis 1.4; OR 0.70, 95% CI 0.55 to 0.88; k = 17; N = 2665). Excluding Hatcher 2011 or Weinberg 2006 did not materially affect these results. Once again, using data on self‐reported incidents of SH for Hatcher 2011 did not materially affect this result, nor did using data for the randomised rather than consenting group. There was also no evidence of a significant difference by modality for this outcome (Analysis 1.4; test for subgroup differences: Chi2 = 3.08, df = 1, P = 0.08, I2 = 67.5%). However, quality of evidence, as assessed by the GRADE criteria, was low for this outcome (see Table 1).
1.4. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 4: Repetition of SH at final follow‐up
With respect to frequency of SH, data from six trials indicated no significant treatment effect for CBT‐based psychotherapy on frequency of repetition of SH by final follow‐up (Analysis 1.5; k = 6; N = 594). Excluding Weinberg 2006 did not materially affect this result. There was no evidence of a significant difference by modality (Analysis 1.5; test for subgroup differences: Chi2 = 1.17, df = 1, P = 0.28, I2 = 14.2%). However, this outcome was associated with low quality of evidence (see Table 1). One trial reported information on median, rather than mean, number of episodes of SH at six months. However, the authors found that although "[t]he rate of self‐harm episodes was lower in the [experimental] group. . . [it was not] significantly so" (Evans 1999b, p.22). A further trial reported information on median number of episodes of SH at 12 months' follow‐up, finding that "[t]he median number of self‐harm episodes was two in both [the experimental and TAU] groups" (Tyrer 2003, p. 972).
1.5. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 5: Frequency of SH at final follow‐up
Secondary outcomes
1.2 Treatment adherence
Data on adherence was reported for both the experimental and control groups in one trial in which a significant treatment effect for CBT‐based psychotherapy on the proportion of participants who completed all 12 sessions of therapy in addition to the three follow‐up appointments was found (40/40 vs 33/42; OR 22.97, 95% CI 1.29 to 409.37; k = 1; N = 82; Slee 2008).
Four trials reported adherence data for the experimental group only (Brown 2005; Evans 1999b; Husain 2014; McAuliffe 2014). Brown 2005 found that "participants in the cognitive therapy (CT) group participated in a mean (SD) of 8.92 (5.97) CT sessions (range 0‐24). Thirty participants (50%) received ten or more CT sessions" (p. 568). In Evans 1999b, five participants in the experimental group did not have specific sessions of manual‐assisted cognitive‐behaviour therapy (MACT) and received almost all input from the booklet component of CBT alone. Overall, 17 of the 18 participants in the experimental group received the booklets. Husain 2014 found that "more than half of the (intervention) group attended all six sessions (n = 56)" (p. 466).
McAuliffe 2014, in which a group‐based approach was used, likewise found that "almost half of those assigned to [problem‐solving therapy] (103, 46.4%) attended all 6 therapy sessions" (p. 4).
1.3 Depression
There was no evidence of a significant treatment effect for CBT‐based psychotherapy on depression scores at the post‐intervention assessment in McAuliffe 2014 (mean 18.20, SD 14.80, n = 171 vs mean 20.60, SD 16.0, n = 142; MD ‐2.40, 95% CI ‐5.84 to 1.04; k = 1; N = 313).
Data on depression scores at six months' follow‐up suggested a significant treatment effect for CBT‐based psychotherapy on depression scores (Analysis 1.6; SMD ‐0.30, 95% CI ‐0.50 to ‐0.10; k = 11; N = 1668). Omitting Hatcher 2011, which used Zelen's design, did not materially affect this result. There was also no evidence of a significant difference by modality (Analysis 1.6; test for subgroup differences: Chi2 = 1.38, df = 1, P = 0.24, I2 = 27.3%).
1.6. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 6: Depression scores at 6 months
Seven trials reported data on depression scores at 12 months, suggesting evidence of a significant treatment effect for psychological therapy (Analysis 1.7; SMD ‐0.36, 95% CI ‐0.64 to ‐0.07; k = 7; N = 1130; I2 = 76%). This outcome was associated with substantial levels of heterogeneity (I2= 76%). Omitting Hatcher 2011 did not materially affect the result nor the heterogeneity. Visual inspection of the forest plot did not clearly indicate which trial/s contributed to this substantial level of heterogeneity.
1.7. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 7: Depression scores at 12 months
Only two trials reported depression scores between 12 and 24 months' follow‐up; however, there was no evidence of a significant treatment effect for CBT‐based psychotherapy (Analysis 1.8; k = 2; N = 225). One trial also reported data on depression scores at 6 and 12 months' follow‐up (Hawton 1987a); however, there was not enough information to enable calculation of the SD. Nevertheless, the authors reported that there were no significant differences between groups in BDI scores at any time point.
1.8. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 8: Depression scores at 24 months
Analysis of all 14 trials at final follow‐up indicated a significant treatment effect for CBT‐based psychotherapy on depression (Analysis 1.9; SMD ‐0.31, 95% CI ‐0.48 to ‐0.14; k = 14; N = 1859). Omitting Hatcher 2011 did not materially affect this result.
1.9. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 9: Depression scores at final follow‐up
1.4 Hopelessness
There was no evidence of a significant treatment effect for CBT‐based psychotherapy on hopelessness at the post‐intervention assessment in three trials, regardless of treatment modality (Analysis 1.10; test for subgroup differences: Chi2 = 2.06, df = 1, P = 0.15, I2 = 51.5%).
1.10. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 10: Hopelessness scores at post‐intervention
However, by the six‐month follow‐up assessment, CBT‐based psychotherapy was associated with a significant treatment effect in four trials (Analysis 1.11; SMD ‐0.36, 95% CI ‐0.58 to ‐0.13; k = 4; N = 968). There was evidence of a difference by treatment modality for this outcome, however (Analysis 1.11; test for subgroup differences: Chi2 = 8.11, df = 1, P = 0.004, I2 = 87.7%). A group‐based CBT‐based psychotherapy approach was not associated with a significant treatment effect on hopelessness scores at six months in one trial (MD ‐0.30, 95% CI ‐1.89 to 1.29; k = 1; N = 234; McAuliffe 2014).
1.11. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 11: Hopelessness scores at 6 months
Three trials reported data on hopelessness scores at 12 months, again showing evidence of a significant treatment effect for CBT‐based psychological therapy (Analysis 1.12; MD ‐1.89, 95% CI ‐2.97 to ‐0.81; k = 3; N = 539). Omitting Hatcher 2011, which used Zelen's design, did not materially affect these results.
1.12. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 12: Hopelessness scores at 12 months
Analyses of all seven trials also suggested evidence of a significant treatment effect at final follow‐up (Analysis 1.13, SMD ‐0.31, 95% CI ‐0.51 to ‐0.10; k = 7; N = 1017). Omitting Hatcher 2011 did not materially affect this result. There was also no evidence of a significant treatment difference by modality for this outcome (Analysis 1.13; test for subgroup differences: Chi2 = 3.65, df = 1, P = 0.06, I2 = 72.6%).
1.13. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 13: Hopelessness scores at final follow‐up
1.5 Suicidal ideation
There was no evidence of a significant treatment effect for CBT‐based psychotherapy in three trials at the post‐intervention assessment, regardless of treatment modality (Analysis 1.14; k = 3; N = 360; test for subgroup differences: Chi2 = 1.84, df = 1, P = 0.17, I2 = 45.8%). However, sensitivity analyses using the fixed‐effect, rather than random‐effects model, suggested evidence of a significant treatment effect for CBT‐based psychotherapy on this outcome (fixed: MD ‐1.93, 95% CI ‐3.83 to ‐0.04).
1.14. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 14: Suicidal ideation scores at post‐intervention
By the six‐month follow‐up assessment, CBT‐based psychotherapy was associated with a significant treatment effect for suicidal ideation scores (Analysis 1.15; SMD ‐0.32, 95% CI ‐0.51 to ‐0.13; k = 6; N = 1011). Omitting Hatcher 2011, which used Zelen's design, did not materially affect this result, nor did omitting Weinberg 2006, in which participants had been diagnosed with personality disorders. There was evidence of a significant difference by treatment modality, however (Analysis 1.15; test for subgroup differences: Chi2 = 6.69, df = 1, P = 0.010, I2 = 85.1%), with a group‐based CBT‐based psychotherapy approach not appearing to be associated with a significant treatment effect for this outcome (Analysis 1.15; MD ‐0.20, 95% CI ‐2.49 to 2.09; McAuliffe 2014).
1.15. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 15: Suicidal ideation scores at 6 months
Data from Hatcher 2011 suggested that CBT‐based psychotherapy was not associated with a significant treatment effect for suicidal ideation scores at 12 months (mean 3.70, SD 6.70, n = 187 vs mean 4.80, SD 7.40, n = 231; MD ‐1.10, 95% CI ‐2.45 to 0.25; k = 1; N = 418).
Including all eight trials suggested evidence for a significant treatment effect for psychological therapy on suicidal ideation scores at final follow‐up (Analysis 1.16; SMD ‐0.32, 95% CI ‐0.53 to ‐0.11; k = 8; N = 1129). Omitting Hatcher 2011 or Weinberg 2006 did not materially affect this result. However, once again there was evidence of a significant difference by treatment modality (Analysis 1.16; test for subgroup differences: Chi2 = 4.61, df = 1, P = 0.03, I2 = 78.3%), with a group‐based psychotherapy approach not associated with a significant treatment effect for this outcome (Analysis 1.16; MD ‐0.02, 95% CI ‐0.24 to 0.20; McAuliffe 2014).
1.16. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 16: Suicidal ideation scores at final follow‐up
Wei 2013 recorded data on the proportion of participants self‐reporting suicidal ideation. There was no evidence of a significant treatment effect for CBT‐based psychotherapy in this trial at the six‐month follow‐up assessment (30/35 vs 32/40; OR 1.50, 95% CI 0.44 to 5.10; k = 1; N = 75). Although data were also available for the 12‐month follow‐up period, authors reported a greater number of participants in the CBT arm who self‐reported suicidal ideation (n = 30) than were reported to have been followed‐up by this point (n = 25). As we were unable to clarify these numbers with the authors, we have excluded this analysis from the review.
1.6 Problem solving
Two trials recorded dichotomous data on problem‐solving as the proportion of participants reporting improvement in problems. There was evidence of a significant treatment effect for CBT‐based psychotherapy on problem‐solving in these trial trials at the six‐month follow‐up assessment (Analysis 1.17; OR 2.81, 95% CI 1.50 to 5.24; k = 2; N = 231). However, for the same dichotomous outcome, there was no indication of any apparent treatment effect at the 12‐month follow‐up assessment in Hawton 1987a (24/30 vs 26/35; OR 1.38, 95% CI 0.43 to 4.47; k = 1; N = 65). Gibbons 1978 reported data for problem‐solving at the 24‐month follow‐up assessment, with evidence of a significant treatment effect for CBT‐based psychotherapy (64/73 vs 40/73; OR 5.87, 95% CI 2.54 to 13.54; k = 1; N = 146). Analysis of both these trials at the final follow‐up assessment, however, suggested no overall evidence of a significant treatment effect for CBT‐based psychotherapy on problem‐solving (Analysis 1.18; k = 2; N = 211). On the other hand, there was a significant difference using the fixed‐effect model (fixed: OR 3.66, 95% CI 1.88 to 7.09).
1.17. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 17: Proportion with improved problems at 6 months
1.18. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 18: Proportion with improved problems at final follow‐up
Data on problem‐solving scores at post‐intervention indicated no evidence of a significant treatment effect for CBT‐based psychotherapy with no evidence of a significant difference by treatment modality (Analysis 1.19; test for subgroup differences: Chi2 = 0.07, df = 1, P = 0.79, I2 = 0%). By the six‐month follow‐up, however, there was evidence of a significant treatment effect for CBT‐based psychotherapy for this outcome (Analysis 1.20; SMD 0.33, 95% CI 0.08 to 0.58; k = 4; N = 949). Omitting Hatcher 2011, which used Zelen's design, caused this effect to become non‐significant (MD 0.24, 95% CI ‐0.03 to 0.51). There was also evidence of a significant difference by treatment modality (Analysis 1.20; test for subgroup differences: Chi2 = 8.11, df = 1, P = 0.004, I2 = 87.7%), with a single trial of group‐based psychotherapy indicating no evidence of a significant treatment effect for this outcome (MD 0.30, 95% CI ‐3.55 to 4.15; McAuliffe 2014). There was no apparent benefit for CBT‐based psychotherapy in a single trial at 12 months (mean 92.2, SD 18.1, n = 190 vs mean 90.5, SD 18.9, n = 233; MD 1.70, 95% CI ‐1.84 to 5.24; k = 1; N = 423; Hatcher 2011). Combining all five trials at final follow‐up suggested evidence of a significant treatment effect for problem solving at the final follow‐up assessment (Analysis 1.21; SMD 0.26, 95% CI 0.02 to 0.50; k = 5; N = 958). Omitting Hatcher 2011 caused the association to become non‐significant (SMD 0.35, 95% CI ‐0.00. to 0.69; k = 4; N = 535).
1.19. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 19: Problem‐solving scores at post‐intervention
1.20. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 20: Problem‐solving scores at 6 months
1.21. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 21: Problem‐solving scores at final follow‐up
Finally, Salkovskis 1990 reported the severity of participants' three main problems using the Personal Questionnaire Rapid Scaling Technique (PQRST) at one week, one month, three months, six months and one year following entry to treatment. The authors reported that "the problem‐solving therapy group showed significantly better overall results on their three main problems when compared with the group who received 'treatment as usual' " (Salkovskis 1990, p. 873).
1.7 Suicide
Fifteen trials reported data on suicides during follow‐up; however, there was no evidence of a significant treatment effect for CBT‐based psychotherapy on suicides by final follow‐up (Analysis 1.22; k = 15; N = 2354). In Tyrer 2003, there was one death in the experimental group that medical staff considered to be a suicide, although the coroner did not record a suicide verdict in this case. Including this death as a suicide did not materially change the overall result.
1.22. Analysis.

Comparison 1: Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU), Outcome 22: Suicide at final follow‐up
Comparison 2: Interventions for multiple episodes of SH/probable personality disorder vs TAU or other alternative forms of psychotherapy
A number of trials investigated provision of a specialised treatment for patients with multiple episodes of SH and/ or probable personality disorder, including: group‐based emotion‐regulation psychotherapy (two trials; Gratz 2006; Gratz 2014), mentalisation‐based therapy (MBT; one trial; Bateman 2009), DBT‐oriented therapy (one trial; Turner 2000), DBT (four trials; Linehan 1991; Linehan 2006; McMain 2009; Priebe 2012), and DBT prolonged exposure protocol (one trial; Harned 2014).
Group‐based emotion‐regulation psychotherapy vs TAU
Two trials assessed the effectiveness of group‐based emotion‐regulation psychotherapy in women diagnosed with borderline personality referred for outpatient treatment as a result of recurrent SH (Gratz 2006, N = 22; Gratz 2014, N = 61). Correspondence with authors suggested that this treatment did not require participants to abstain from SH behaviour. Instead, participants were encouraged to work on resisting urges to engage in SH and, when SH occurred, to learn from the reasons for it.
Primary outcome
2.1 Repetition of SH
Group‐based emotion‐regulation psychotherapy was associated with a significant treatment effect by the post‐intervention assessment (Analysis 2.1; OR 0.34, 95% CI 0.13 to 0.88; k = 2; N = 83). Quality of evidence for this outcome, however, was low (see Table 2).
2.1. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 1: Repetition of SH at post‐intervention
With respect to frequency of repetition of SH, there was no evidence of a significant treatment effect for group‐based emotion‐regulation psychotherapy by the post‐intervention assessment (Analysis 2.1; k = 2; N = 83). Once again, this was associated with a low quality of evidence (see Table 2).
Secondary outcomes
2.2 Treatment adherence
No data available.
2.3 Depression
There was evidence of a significant treatment effect for group‐based emotion‐regulation therapy on depression scores at the post‐intervention assessment (Analysis 2.8; MD ‐9.59, 95% CI ‐13.43 to ‐5.75; k = 2; N = 83).
2.8. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 8: Depression scores at post‐intervention
2.4 Hopelessness
No data available.
2.5 Suicidal ideation
No data available.
2.6 Problem solving
No data available.
2.7 Suicide
There were no suicides in either group for either trial.
Mentalisation vs TAU
Bateman 2009 (N = 134) assessed the effectiveness of mentalisation‐based therapy in adults diagnosed with borderline personality disorder referred to a specialist personality disorder treatment service following an attempted suicide or an episode of life‐threatening SH in the six months prior to trial entry.
Primary outcome
2.8 Repetition of SH
There was a significant treatment effect for mentalisation‐based therapy by the conclusion of the 18‐month treatment period, with fewer participants in the experimental group engaging in SH based on data obtained by correspondence (Analysis 2.1; 18/71 vs 31/63; OR 0.35, 95% CI 0.17 to 0.73; k = 1; N = 134). However, quality of evidence was moderate (see Table 2).
There was also evidence of a significant treatment effect for mentalisation‐based therapy on frequency of SH episodes by the post‐intervention assessment according to data obtained by correspondence (Analysis 2.5; mean 0.38, SD 0.83, n = 71 vs mean 1.66, SD 2.87, n = 63; MD ‐1.28, 95% CI ‐2.01 to ‐0.55; k = 1; N = 134). Once again, quality of evidence was moderate (see Table 2).
2.5. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 5: Frequency of repetition of SH at post‐intervention
Secondary outcomes
2.9 Treatment adherence
There was no evidence of a significant treatment effect for mentalisation‐based therapy on the proportion of participants who completed the full course of treatment (Analysis 2.7; k = 1; N = 134).
2.7. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 7: Number completing full course of treatment
2.10 Depression
Mentalisation‐based therapy was associated with a significant treatment effect for depression at the post‐intervention assessment (Analysis 2.8; mean 14.80, SD 8.55, n = 71 vs mean 18.68, SD 8.76, n = 63; MD ‐3.88, 95% CI ‐6.82 to ‐0.94; k = 1; N = 134).
2.11 Hopelessness
No data available.
2.12 Suicidal ideation
No data available.
2.13 Problem solving
No data available.
2.14 Suicide
There had been no suicides in either treatment arm by the time of the post‐treatment assessment.
Dialectical behaviour‐oriented psychotherapy vs other alternative forms of psychotherapy
One small trial in participants diagnosed with borderline personality disorder and referred to outpatient services following a suicide attempt assessed the effectiveness of a DBT‐oriented therapy versus client‐oriented therapy over a 12‐month follow‐up (Turner 2000, N = 24).
Primary outcome
2.15 Repetition of SH
There was evidence of a significant treatment effect for DBT‐oriented therapy for repetition of SH by post‐treatment assessment (Analysis 2.1; 1/12 vs 8/12; OR 0.05, 95% CI 0.00 to 0.49; k = 1; N = 24). This result had low quality evidence (see Table 2).
Data on number of repeat episodes of SH, obtained by correspondence, also suggest a significant treatment effect for DBT‐oriented therapy by the post‐treatment assessment (Analysis 2.5; mean 0.75, SD 1.23, n = 12 vs mean 5.58, SD 5.28, n = 12; MD ‐4.83, 95% CI ‐7.90 to ‐1.76; k = 1; N = 24). Once again, however, a low quality of evidence was associated with this outcome (see Table 2).
Secondary outcomes
2.16 Treatment adherence
There was no evidence of a significant treatment effect for DBT‐oriented therapy on the number of participants who completed the full course of treatment (Analysis 2.7; k = 1; N = 24).
2.17 Depression
DBT‐oriented therapy was associated with a significant treatment effect for depression scores according to both the BDI and the HRSD by the post‐treatment assessment (BDI: Analysis 2.8; mean 14.92, SD 8.26, n = 12 vs mean 24.08, SD 5.55, n = 12; MD ‐9.16, 95% CI ‐14.79 to ‐3.53; k = 1; N = 24; HRSD: mean 7.50, SD 5.96, n = 12 vs mean 12.58, SD 3.90, n = 12; MD ‐5.08, 95% CI ‐9.11 to ‐1.05; k = 1; N = 24).
2.18 Hopelessness
No data available.
2.19 Suicidal ideation
There was also evidence of a significant treatment effect for suicidal ideation at the post‐treatment assessment (Analysis 2.11; mean 3.83, SD 8.03, n = 12 vs mean 11.58, SD 9.21, n = 12; MD ‐7.75, 95% CI ‐14.66 to ‐0.84; k = 1; N = 24).
2.11. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 11: Suicide ideation scores at post‐intervention
2.20 Problem solving
No data available.
2.21 Suicide
No data available.
Dialectical behaviour therapy (DBT) vs TAU
Three trials investigated the effectiveness of dialectical behaviour therapy (DBT) in adults diagnosed with personality disorders, typically borderline personality disorder, referred to specialist DBT services owing to recurrent SH (Linehan 1991, N = 63; McMain 2009, N = 180; Priebe 2012, N = 80).
Primary outcome
2.22 Repetition of SH
We obtained data on repetition of SH through correspondence for all three trials. There was no clear evidence of a significant treatment effect for DBT compared to TAU in terms of the proportion of patients repeating SH in three trials by the post‐intervention assessment (Analysis 2.1; k = 3; N = 267). Similarly, there was no evidence of a significant treatment effect for DBT by the 12‐month follow‐up assessment in two trials (Analysis 2.3; k = 2; N = 172). Combining data from all three trials by the final assessment period suggested no evidence of a significant treatment effect for DBT versus TAU (Analysis 2.4; k = 3; N = 247). Quality of evidence for these three outcomes was low, however (see Table 2).
2.3. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 3: Repetition of SH at 12 months
2.4. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 4: Repetition of SH at final follow‐up
There was evidence of a significant treatment effect for DBT as compared to TAU on frequency of SH by the post‐intervention assessment (Analysis 2.5; MD ‐18.82, 95% CI ‐36.68 to ‐0.95; k = 3; N = 292). Once again quality of evidence for this outcome was low (see Table 2).
Following a "naturalistic" follow‐up period, data from Linehan 1993a (N = 39) indicated that the effectiveness of DBT in the Linehan 1991 trial was maintained at 24 months; however, this outcome was only investigated for a proportion of the original participants (61.9%) who the researchers were able to contact at 24 months. Results have therefore not been reproduced in the present review.
Secondary Outcomes
2.23 Treatment adherence
There was no evidence of a significant treatment effect for DBT for the number of participants completing the full course of treatment in one trial (McMain 2009) (55/90 vs 56/90; OR 0.95, 95% CI 0.52 to 1.74; k = 1; N = 180). Numbers completing treatment in the control group for Priebe 2012 were not provided. Therefore we could not incorporate the results of this trial in a meta‐analysis.
Although Linehan 1991 did not provide numerical data on treatment adherence, the authors did report that participants allocated to the DBT group were ". . . significantly more likely to start individual therapy. . . (100% versus 73%)" (Linehan 1991, p. 1062).
2.24 Depression
There was no evidence of a significant treatment effect for DBT as compared to TAU on depression scores at the post‐intervention assessment (Analysis 2.8; k = 2; N = 198).
Data from McMain 2012 furthermore suggested there was no evidence of a significant treatment effect for DBT on depression at the 24‐month assessment (mean 22.24, SD 16.40, n = 90 vs mean 21.67, SD 14.82, n = 90; MD 0.57, 95% CI ‐4.00 to 5.14; k = 1; N = 180).
2.25 Hopelessness
We obtained data on hopelessness by correspondence for one trial (Linehan 1991). There was no evidence of a significant treatment effect for DBT at the 24‐month follow‐up assessment (mean 10.86, SD 6.04, n = 7 vs mean 10.69, SD 6.18, n = 11; MD 0.17, 95% CI ‐5.61 to 5.95; k = 1; N = 18).
2.26 Suicidal ideation
One trial reported data on suicidal ideation (Linehan 1991). Again, there was no significant treatment effect for DBT at the post‐intervention assessment (mean 24.01, SD 19.80, n = 46 vs mean 31.92, SD 26.80, n = 35; MD ‐7.91, SD ‐18.47 to 2.65; k = 1; N = 81).
2.27 Problem solving
No data available.
2.28 Suicide
Although a suicide occurred in the DBT arm of Linehan 1991 before the post‐intervention assessment, there were no suicides in Priebe 2012 or in McMain 2009. There was therefore no evidence of a significant treatment effect for this outcome (Analysis 2.13; k = 3; N = 317). There were no suicides in Priebe 2012 or in McMain 2009 by the 24‐month follow‐up assessment.
2.13. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 13: Suicide at post‐intervention
Dialectical behaviour therapy vs other alternative forms of psychotherapy
One trial compared the effectiveness of DBT versus psychological treatment by 'experts' (CBT‐E) for women diagnosed with borderline personality disorder and referred to a specialist DBT service owing to recurrent SH (Linehan 2006, N = 101). Community mental health leaders (such as heads of inpatient psychiatric units and clinical directors of mental health agencies) nominated professionals who they considered experts in treating difficult clients. These therapists described themselves as "eclectic but non behavioral" or "mostly psychodynamic" in their treatment approach. No therapists with experience of delivering cognitive behavioural therapy were included, however.
Primary outcome
2.29 Repetition of SH
There was no evidence of a treatment effect for DBT versus treatment by expert on repetition of SH by either the post‐intervention assessment (Analysis 2.1; k = 1; N = 97) or by the 12‐month follow‐up period (Analysis 2.3; k = 1; N = 97). Quality of evidence for this outcome at both time points, as assessed by the GRADE criteria, was very low (see Table 2).
Study authors did, however, state that those allocated to the DBT group had " . . . half the rate of suicide attempts compared with the CTB‐E group (23.1% vs 46%. . . hazard ratio, 2.66, P = 0.005)" (Linehan 2006, p. 761). Nevertheless, correspondence with authors regarding the total number of parasuicidal acts across the 12‐month follow‐up period revealed no evidence of a significant treatment effect for DBT (mean 8.79, SD 10.81, n = 52 vs mean 23.64, SD 77.34, n = 45; MD ‐14.85, 95% CI ‐37.64 to 7.94; k = 1; N = 97). Quality of evidence for this outcome was also very low (see Table 2).
Secondary outcomes
2.30 Treatment adherence
No data available.
2.31 Depression
There was no evidence of a significant treatment effect on depression scores either at the post‐intervention assessment (Analysis 2.8; k = 1; N = 89) or at the 12‐month follow‐up assessment (Analysis 2.10; k = 1 ; N = 81) in this trial.
2.10. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 10: Depression scores at 12 months
2.32 Hopelessness
No data available.
2.33 Suicidal ideation
There was also no evidence of a significant treatment effect for DBT for suicidal ideation scores at either the post‐intervention (Analysis 2.11; k = 1; N = 89) or 12‐month follow‐up assessments (Analysis 2.12; k = 1; N = 81).
2.12. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 12: Suicide ideation scores at 12 months
2.34 Problem solving
No data available.
2.35 Suicide
There were no suicides in either treatment arm by the end of the 12‐month follow‐up period.
Dialectical behaviour therapy prolonged exposure vs other alternative forms of psychotherapy
The effectiveness of two forms of DBT were compared over a three‐month follow‐up period in one small trial of women with comorbid borderline personality disorder and post‐traumatic stress disorder referred to clinical services due to recurrent SH (Harned 2014; N = 26). In the experimental arm, participants received, in addition to the standard DBT protocol, additional weekly therapy sessions involving in vivo and imaginal exposure to previously traumatic experiences.
Primary outcome
2.36 Repetition of SH
Data obtained by correspondence suggested there was no evidence of a significant treatment effect for the DBT prolonged protocol on repetition of SH either by the post‐treatment assessment (Analysis 2.1; k = 1; N = 18) or by the three‐month follow‐up (Analysis 2.2; k = 1; N = 18). Quality of evidence for both these time points was low, however (see Table 2).
2.2. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 2: Repetition of SH at 6 months
Data on frequency of SH, obtained following correspondence with authors, also suggested no apparent benefit of the DBT prolonged exposure protocol by either the post‐treatment (Analysis 2.5; k = 1; N = 18) or three‐month follow‐up (Analysis 2.6; k = 1; N = 18) assessments. Quality of evidence, as assessed by the GRADE criteria, was low (see Table 2). Data on frequency of suicide re‐attempts could not be analysed as there were no repeat suicide attempts in the control group by the final three‐month follow‐up assessment.
2.6. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 6: Frequency of repetition of SH at 6 months
Secondary outcomes
2.37 Treatment adherence
There was no significant difference between the experimental and control groups regarding the number of participants who attended the full one‐year course of treatment (Analysis 2.7; k = 1; N = 26). According to the authors, ". . . one therapist. . . was not adherent to DBT and had a 100% dropout rate" (p. 12). Excluding the four participants treated by this therapist did not, however, materially affect this result.
2.38 Depression
There was no evidence of a significant treatment effect for the DBT prolonged exposure protocol for depression scores at either the post‐treatment (Analysis 2.8; k = 1; N = 18) or three‐month follow‐up (Analysis 2.9; k = 1; N = 18) assessments.
2.9. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 9: Depression scores at 6 months
2.39 Hopelessness
No data available.
2.40 Suicidal ideation
No data available.
2.41 Problem solving
No data available.
2.42 Suicide
There was no evidence of a significant treatment effect on death by suicide by the three‐month follow‐up assessment (Analysis 2.14: 0/17 vs 1/9; OR 0.16, 95% CI 0.01 to 4.41; k = 1; N = 26) in this trial.
2.14. Analysis.

Comparison 2: Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 14: Suicide at 6 months
Comparison 3: Case management vs TAU
Four trials investigated the provision of case management for the prevention of SH either compared to either treatment as usual (TAU; Clarke 2002, N = 467; Hvid 2011, N = 133; Morthorst 2012, N = 243) or to enhanced usual care (EUC; Kawanishi 2014, N = 914). Although the intervention in Hvid 2011 and Morthorst 2012 also included aspects of problem‐solving psychotherapy, this component was not the primary or only element of the case management strategy adopted in these trials, so we felt these trials were sufficiently similar to justify pooling within a meta‐analysis.
Primary outcome
3.1 Repetition of SH
There was no evidence of a significant treatment effect for case management on repetition of SH by the post‐intervention assessment (Analysis 3.1; k = 4; N = 1608). Supplementing hospital‐recorded episodes of SH with self‐reported data for Morthorst 2012 did not materially affect this result. There was also no indication of a significant difference by comparator condition (i.e., TAU vs EUC) for this outcome (Analysis 3.1; test for subgroup differences: Chi2 = 0.20, df = 1, P = 0.66, I2 = 0%). Quality, as assessed using the GRADE criteria, was moderate for this outcome (see Table 3).
3.1. Analysis.

Comparison 3: Case management vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 1: Repetition of SH at post‐intervention
One trial disaggregated data on repetition of SH by sex (Hvid 2011). Although there was no evidence of a significant treatment effect for males in this trial (4/20 vs 4/18; OR 0.88, 95% CI 0.18 to 4.17; k = 1; N = 38), case management was associated with a significant reduction in repetition of SH in females (2/49 vs 10/46; OR 0.15, 95% CI 0.03 to 0.74; k = 1; N = 95).
Multiple readmissions for SH were, however, significantly more common in the case management group than in the control group over the treatment period in one trial (Clarke 2002: 9/220 vs 2/247; OR 5.23, 95% CI 1.12 to 24.45; k = 1; N = 467). Quality of evidence for this outcome was moderate (see Table 3).
Secondary outcomes
3.2 Treatment adherence
The authors of one trial reported that "11 participants in the assertive case management group did not receive the intervention" (Kawanishi 2014, p. 197). However, as corresponding numbers for the enhanced usual care group were not reported, we were unable to analyse the effect of assertive case management on treatment adherence for this trial.
3.3 Depression
No data available.
3.4 Hopelessness
Although the Beck Hopelessness Scale was administered to participants throughout the follow‐up period in one trial (Kawanishi 2014), the authors did not report data on this outcome. Correspondence, however, revealed that they are currently analysing these data and will present them in a future report.
3.5 Suicidal ideation
No data available.
3.6 Problem solving
No data available.
3.7 Suicide
There was no evidence of a significant treatment effect on suicide by the post‐intervention assessment (Analysis 3.2; k = 4; N = 1757), nor was there evidence of a significant difference by comparator condition (i.e., TAU vs EUC) for this outcome (Analysis 3.2; test for subgroup differences: Chi2 = 0.67, df = 1, P = 0.41, I2 = 0%).
3.2. Analysis.

Comparison 3: Case management vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 2: Suicide at post‐intervention
Comparison 4: Treatment adherence enhancement approaches vs TAU or other alternative forms of psychotherapy
Two trials investigated the effectiveness of treatment adherence enhancement approaches compared either to TAU (Van Heeringen 1995) or to other alternative forms of psychotherapy (Torhorst 1987) in patients admitted to hospital following an episode of SH.
Treatment adherence enhancement vs TAU
Van Heeringen 1995 (N = 516) investigated the effectiveness of adherence enhancement, involving home visits by a nurse for those patients who failed to attend outpatient appointments, over a 12‐month follow‐up period in patients referred to accident and emergency departments following an episode of SH, irrespective of suicidal intent.
Primary outcome
4.1 Repetition of SH
There was no evidence of a significant treatment effect on repetition of SH by the 12‐month follow‐up assessment, although the difference in repetition between groups was fairly marked (Analysis 4.1; k = 1; N = 391). Quality of evidence for this outcome, as assessed by the GRADE criteria, was low (see Table 4).
4.1. Analysis.

Comparison 4: Treatment adherence enhancement approaches vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 1: Repetition of SH at 12 months
Secondary outcomes
4.2 Treatment adherence
There was, however, a significant treatment effect for adherence with outpatient aftercare appointments in this trial (129/252 vs 102/256; OR 1.58, 95% CI 1.11 to 2.25; k = 1; N = 508).
4.3 Depression
No data available.
4.4 Hopelessness
No data available.
4.5 Suicidal ideation
No data available.
4.6 Problem solving
No data available.
4.7 Suicide
There was no evidence of a significant treatment effect for the number of participants who died by suicide over the 12‐month follow‐up period (Analysis 4.3; k = 1; N = 391) in this trial.
4.3. Analysis.

Comparison 4: Treatment adherence enhancement approaches vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 3: Suicide at 12 months
Continuity of care by the same therapist vs other alternative forms of psychotherapy
One trial investigated the effectiveness of continuing aftercare with the same therapist (defined as continued therapeutic contact with the original hospital therapist in an outpatient setting) versus changing to a different therapist (defined as receiving therapy in a specialised suicide prevention centre, which involved changing both therapist and institution) over a 12‐month follow‐up period in adults admitted to hospital following an episode of self‐poisoning (Torhorst 1987, N = 141).
Primary outcome
4.8 Repetition of SH
There was no evidence of a significant treatment effect for receiving continued therapeutic contact with the original hospital therapist on repetition of SH by the 12‐month follow‐up assessment (Analysis 4.1; k = 1; N = 136). A very low quality of evidence was associated with this outcome (see Table 4).
Secondary outcomes
4.9 Treatment adherence
There was evidence of a significant treatment effect for treatment adherence, favouring the same‐therapist group (49/68 vs 36/73; OR 2.65, 95% CI 1.32 to 5.34; k = 1; N = 141).
4.10 Depression
Depression scores did not differ significantly between groups at the 12‐month follow‐up assessment (Analysis 4.2: mean 6.20, SD 6.90, n = 65 vs mean 7.60, SD 9.20, n = 62; MD ‐1.40, 95% CI ‐4.24 to 1.44; k = 1; N = 127).
4.2. Analysis.

Comparison 4: Treatment adherence enhancement approaches vs treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 2: Depression scores at 12 months
4.11 Hopelessness
No data available.
4.12 Suicidal ideation
No data available.
4.13 Problem solving
No data available.
4.14 Suicide
There was no evidence of a significant treatment effect for receiving continued therapeutic contact with the original hospital therapist on suicide by the 12‐month follow‐up assessment (Analysis 4.3; k = 1; N = 136).
Comparison 5: Mixed multimodal interventions vs TAU
Two trials investigated the effectiveness of a package of interventions, including problem‐solving psychotherapy, postcards, and a GP voucher entitling participants to one free visit to their GP in adults admitted to emergency departments following an episode of SH, irrespective of intent (Hatcher 2016a: Hatcher 2015).
Mixed multimodal interventions vs TAU
One large trial using Zelen's post‐randomisation consent design investigated the effectiveness of a package of mixed multimodal interventions in adults admitted to emergency departments following an episode of SH irrespective of intent over a 12‐month period (Hatcher 2015; N = 1474).
Primary outcome
5.1 Repetition of SH
There was no evidence of a significant treatment effect for this package of interventions in terms of hospital‐recorded episodes of SH by 12‐month post‐intervention assessment (66/327 vs 73/357; OR 0.98, 95% CI 0.68 to 1.43; k = 1; N = 684). This outcome had a low quality of evidence (see Table 5). Using data from the randomised (including both patients who, following treatment allocation, subsequently consented to participation and those who did not), rather than consenting, sample did not materially affect these results.
Investigators also presented data on repetition of SH by repeater status at trial entry. However, there was no evidence of a significant treatment effect for those with no history of SH prior to the index attempt compared to those with a history of multiple SH episodes by the post‐intervention assessment (those with a history of multiple episodes of SH: 47/176 vs 56/194; OR 0.90, 95% CI 0.57 to 1.42; k = 1; N = 370; those without a history of multiple episodes of SH: 19/151 vs 17/163; OR 1.24, 95% CI 0.62 to 2.48; k = 1; N = 314).
With respect to frequency of SH, the authors reported that "[a]lthough there were 20% fewer episodes in the intervention group, the difference was not statistically significant" (Hatcher 2015, p. 17).
Secondary outcomes
5.2 Treatment adherence
No data available.
5.3 Depression
There was no significant treatment effect for this intervention on depression scores at the 12‐month post‐intervention assessment (mean 6.8, SD 4.9, n = 211 vs mean 6.5, SD 5.1, n = 234; MD 0.30, 95% CI ‐0.63 to 1.23; k = 1; N = 445).
5.4 Hopelessness
There was also no apparent treatment effect for this intervention on hopelessness scores at the 12‐month post‐intervention assessment (mean 8.3, SD 6.3, n = 210 vs mean 8.4, SD 6.4, n = 233; MD ‐0.10, 95% CI ‐1.28 to 1.08; k = 1; N = 443).
5.5 Suicidal ideation
No data available.
5.6 Problem solving
No data available.
5.7 Suicide
Correspondence with authors revealed there was no significant treatment effect for this intervention package on suicides by the 12‐month post‐intervention assessment (1/327 vs 2/357; OR 0.54, 95% CI 0.05 to 6.03; k = 1; N = 684). One death in the experimental and one in the control group were due to uncertain causes as they had yet to be investigated by the Coroner. However, assuming these deaths were attributable to suicide did not materially affect this result.
Culturally adapted multi‐model interventions vs TAU
The effectiveness of a culturally‐adapted mixed multimodal intervention was investigated over a 12‐month follow‐up period in one trial using Zelen's post‐randomisation consent design in adults admitted to emergency departments following SH and who identify themselves as of Māori ethnicity (Hatcher 2016a; N = 365).
Primary outcome
5.8 Repetition of SH
There was no evidence of a significant treatment effect for this intervention on re‐presentation to hospital following an episode of SH by the time of the post‐intervention assessment (34/95 vs 29/72; OR 0.83, 95% CI 0.44 to 1.55; k = 1; N = 167). Using data from the randomised sample only (including both patients who, following treatment allocation, subsequently consented to participation and those who did not) did not materially affect these results. Both outcomes had a low quality of evidence, as assessed using the GRADE criteria (see Table 5).
Investigators presented information on repetition of SH by repeater status; however, there was no significant difference in repetition of SH between groups for either those with a history of multiple episodes of SH (24/60 vs 21/40; OR 0.60, 95% CI 0.27 to 1.35; k = 1; N = 100) or for those without a history of multiple episodes of SH (10/35 vs 8/32; OR 1.20, 95% CI 0.41 to 3.55; k = 1; N = 67).
Secondary outcomes
5.9 Treatment adherence
No data available.
5.10 Depression
There was no treatment effect for this intervention on depression scores at the 12‐month post‐intervention assessment (mean 5.80, SD 4.50, n = 66 vs mean 6.30, SD 4.30, n = 48; MD ‐0.50, 95% CI ‐2.13 to 1.13; k = 1; N = 114).
5.10 Hopelessness
There was also no apparent treatment effect for this intervention on hopelessness scores at the post‐intervention assessment (mean 5.00, SD 4.40, n = 66 vs mean 5.70, SD 4.80, n = 47; MD ‐0.70, 95% CI ‐2.43 to 1.03; k = 1; N = 113).
However, the authors note that "whilst there was a greater change in [hopelessness] scores at . . . 12 months [i.e., the post‐intervention assessment] in the intervention group, the group had a significantly lower baseline score . . . because of the significant differences in baseline scores and missing follow up data we. . . used a mixed linear model to estimate the differences in scores at [the post‐intervention assessment]." Using this model the authors found "there was a decrease in [h]opelessness scores in the treatment group compared to the usual care group but this was statistically non‐significant" (Hatcher 2016a, ePub version, p. 5).
5.11 Suicide ideation
No data available.
5.12 Problem solving
No data available.
5.13 Suicide
Correspondence with authors revealed there was no significant treatment effect for this intervention on suicides by the time of the post‐intervention assessment (0/72 vs 1/95; OR 0.43, 95% CI 0.02 to 10.82; k = 1; N = 167).
Comparison 6: Remote contact interventions vs TAU
A number of trials investigated the effectiveness of remote contact interventions, including, postcards (Beautrais 2010; Carter 2005; Hassanian‐Moghaddam 2011; Kapur 2013a), emergency cards (Evans 1999a; Morgan 1993), general practitioner's (GP) letter (Bennewith 2002), telephone contact (Cedereke 2002; Wei 2013; Vaiva 2006, or mobile telephone‐based psychotherapy (Marasinghe 2012) for the prevention of repetition of SH.
Postcards vs TAU
Four trials assessed the effectiveness of sending postcards to patients on a regular basis over a 12‐month follow‐up period (Beautrais 2010, N = 327; Hassanian‐Moghaddam 2011, N = 2113; Kapur 2013a, N = 66), including one that used Zelen's post‐randomisation consent design and reported data only for the randomised sample (Carter 2005, N = 772). The trials of Beautrais 2010, Carter 2005, and Kapur 2013a took place in three high‐income countries, whilst the Hassanian‐Moghaddam 2011 trial was in a low income country (i.e., Iran).
Primary outcome
6.1 Repetition of SH
Due to the definition of SH used in the report for one trial of postcards, we obtained data for this trial on repetition of SH through correspondence (Hassanian‐Moghaddam 2011).
Overall, there was no evidence of a significant treatment effect on the proportion of patients repeating SH by the post‐intervention assessment (Analysis 5.1; k = 4; N = 3277). Excluding Carter 2005, which used Zelen's design, did not materially affect this result. Quality of evidence, as assessed using the GRADE criteria, was very low for this outcome (see Table 6). Visual examination of the forest plot suggested that the result for Kapur 2013a may have been an outlier. Removing this trial reduced heterogeneity to 0% and suggested a significant treatment effect for postcards on repetition of SH (OR 0.78, 95% CI 0.62 to 0.97; k = 3; N = 3212).
5.1. Analysis.

Comparison 5: Remote contact interventions vs treatment as usual (TAU), Outcome 1: Repetition of SH at post‐intervention
There was no evidence of a significant treatment effect for postcards on repetition of SH by the 12‐month follow‐up assessment in two trials (Analysis 5.2; k = 2; N = 2885). The quality of evidence for this outcome was moderate (see Table 6). Excluding Carter 2005, however, caused this result to become significant (OR 0.67, 95% CI 0.52 to 0.86; k = 1; N = 2113) as did a sensitivity analysis using the fixed‐effect rather than random‐effects model (fixed: OR 0.75, 95% CI 0.61 to 0.91).
5.2. Analysis.

Comparison 5: Remote contact interventions vs treatment as usual (TAU), Outcome 2: Repetition of SH at 12 months
Combining data from both time points indicated no overall significant effect for postcards by the final follow‐up assessment (Analysis 5.3; k = 4; N = 3277). Excluding Carter 2005 did not materially affect this result. However, as before, excluding Kapur 2013a caused this result to become significant (OR 0.77, 95% CI 0.63 to 0.95) as did a sensitivity analysis using the fixed‐effect model (fixed: OR 0.79, 95% CI 0.66 to 0.95), quality of evidence was again very low for this outcome (see Table 6).
5.3. Analysis.

Comparison 5: Remote contact interventions vs treatment as usual (TAU), Outcome 3: Repetition of SH at final follow‐up
Data on repetition of SH by the post‐intervention assessment and 12‐month follow‐up were available by sex in one trial (Carter 2005; Carter 2007); however, there was no evidence of a significant treatment effect for postcards in either sex by either time point (post‐intervention: males 20/145 vs 16/102; OR 0.86, 95% CI 0.42 to 1.75; k = 1; N = 247 versus females 37/233 vs 51/291; OR 0.89, 95% CI 0.56 to 1.41; k = 1; N = 524; 12 months' follow‐up: males 26/145 vs 19/102; OR 0.95, 95% CI 0.50; k = 1; N = 247 versus females 54/233 vs 59/291; OR 1.19, 95% CI 0.78 to 1.80; k = 1; N = 524).
With respect to frequency of SH, we obtained data on mean number of repeat SH episodes by correspondence for three of the four trials of postcards (Carter 2005; Hassanian‐Moghaddam 2011; Kapur 2013a). For the one remaining trial (Beautrais 2010), the available data indicated a reduced mean number of SH episodes for the experimental group (0.57 vs 0.78); however, as no information on SDs, t‐test or F statistics were reported, we were unable to impute SDs using the method outlined in Townsend 2001 to calculate the mean difference in frequency of SH episodes between groups. Overall, there was no evidence of a significant treatment effect for postcards on frequency of repetition of SH by the post‐intervention assessment (Analysis 5.4; k = 3; N = 1,097). Quality of evidence for this outcome was very low (see Table 6).
5.4. Analysis.

Comparison 5: Remote contact interventions vs treatment as usual (TAU), Outcome 4: Frequency of SH at post‐intervention
By the 12‐month follow‐up assessment, there was similarly no evidence of a significant treatment effect for postcards in two trials (Analysis 5.5; k = 2; N = 984). One trial also provided data for the 24‐month follow‐up period; however, no evidence of a significant treatment effect was apparent (mean 0.21, SD 0.75, n = 217 vs mean 0.24, SD 0.68, n = 255; MD ‐0.03, 95% CI ‐0.16 to 0.10; k = 1; N = 472; Hassanian‐Moghaddam 2011). Quality of evidence was very low to moderate for these outcomes (see Table 6).
5.5. Analysis.

Comparison 5: Remote contact interventions vs treatment as usual (TAU), Outcome 5: Frequency of SH at 12 months
Through correspondence, we were also able to obtain post hoc data on frequency of repetition of SH by the post‐intervention assessment by both sex and repeater status for three trials (Carter 2005; Hassanian‐Moghaddam 2011; Kapur 2013a). There was no evidence of a significant treatment effect for postcards on frequency of repetition by the post‐intervention assessment for either sex (males: Analysis 5.4; k = 3; N = 401; females: Analysis 5.4; k = 3; N = 695), those with a history of multiple episodes of SH (Analysis 5.4; k = 3; N = 339), or those without a history of multiple episodes of SH (Analysis 5.4; k = 3; N = 758). There was also no evidence of a significant treatment effect for postcards on frequency of repetition for males (Analysis 5.5; k = 2; N = 336), females (Analysis 5.5; k = 2; N = 647), those with a history of multiple episodes of SH (Analysis 5.5; k = 2; N = 296), or those without a history of multiple episodes of SH (Analysis 5.5; k = 2; N = 688) by the 12‐month follow‐up assessment in two trials. Correspondence with study authors revealed no significant treatment effect for postcards on frequency of repetition in either males (mean 0.33, SD 1.07, n = 116 vs mean 0.29, SD 0.82, n = 104; MD 0.04, 95% CI ‐0.21 to 0.29; k = 1; N = 220), females (mean 0.14, SD 0.52, n = 101 vs mean 0.21, SD 0.59, n = 151; MD ‐0.07, 95% CI ‐0.21 to 0.07, k = 1; N = 252), those with a history of multiple episodes of SH (mean 0.42, SD 1.06, n = 155 vs mean 0.51, SD 0.96, n = 183; MD ‐0.09, 95% CI ‐0.31 to 0.13; k = 1; N = 338), or those without a history of multiple episodes of SH (mean 0.09, SD 0.47, n = 62 vs mean 0.10, SD 0.41, n = 72; MD ‐0.01, 95% CI ‐0.16 to 0.14; k = 1; N = 134) by the 24‐month follow‐up assessment in one of these trials (Hassanian‐Moghaddam 2011).
Secondary outcomes
6.2 Treatment adherence
No data available.
6.3 Depression
No data available.
6.4 Hopelessness
No data available.
6.5 Suicidal ideation
One trial recorded information on suicidal ideation at both the post‐intervention assessment and 12‐months' follow‐up (Hassanian‐Moghaddam 2011; Hassanian‐Moghaddam 2015). There was a significant treatment effect for the number of people reporting suicidal ideation at the post‐intervention assessment (302/1043 vs 446/1070; OR 0.57, 95% CI 0.48 to 0.68; k = 1; N = 2113). Data reported by the trial authors in a subsequent follow‐up paper suggested that this effect was maintained at the 12‐month follow‐up assessment (465/997 vs 588/1004; OR 0.62, 95% CI 0.52 to 0.74; k = 1; N = 2001; Hassanian‐Moghaddam 2015).
6.6 Problem solving
No data available.
6.7 Suicide
There was no evidence of a significant treatment effect for postcards on suicide by the post‐intervention assessment (Analysis 5.6; k = 4; N = 3464). Excluding Carter 2005, however, suggested a harmful effect of postcards on suicides (OR 3.74, 95% CI 1.04 to 13.51; k = 3; N = 2692).
5.6. Analysis.

Comparison 5: Remote contact interventions vs treatment as usual (TAU), Outcome 6: Suicide at post‐intervention
Data on suicides by the 12‐month follow‐up assessment were available for one trial (i.e., Carter 2005); however, no significant treatment effect was found (Analysis 5.7; k = 1; N = 772).
5.7. Analysis.

Comparison 5: Remote contact interventions vs treatment as usual (TAU), Outcome 7: Suicide at 12 months
Emergency cards vs TAU
Two trials investigated the effectiveness of providing an emergency contact card ('green card') providing 24‐hour access to emergency advice from a psychiatrist in addition to TAU in adults admitted to general hospitals following an episode of SH, most frequently self‐poisoning (Evans 1999a, N = 827; Morgan 1993, N = 212). Evans 1999a reported data on repetition of SH in a secondary trial publication (Evans 2005).
Primary outcome
6.8 Repetition of SH
There was no evidence of a significant treatment effect for emergency cards on repetition of SH by the post‐intervention assessment (Analysis 5.1; k = 2; N = 1039). Quality of evidence for this outcome was low (see Table 6). There was also no evidence of a significant treatment effect for emergency cards by the time of the 12‐month follow‐up assessment in Evans 1999a (Analysis 5.2; k = 1; N = 827). For this outcome, quality of evidence as assessed by the GRADE criteria was moderate (see Table 6).
Evans 1999a disaggregated data on repetition of SH by repeater status (i.e., those without a history of multiple episodes of SH versus those with a history of multiple episodes of SH) in post hoc analyses. Whilst there was no evidence of a significant treatment effect for emergency cards on repetition of SH in those without a history of multiple episodes of SH (18/221 vs 25/206; OR 0.64, 95% CI 0.34 to 1.22; k = 1; N = 427), emergency cards were associated with a significantly increased risk of repetition of SH in those with a history of multiple episodes of SH (52/194 vs 33/200; OR 1.85, 95% CI 1.14 to 3.03; k = 1; N = 394) in this trial.
Evans 1999a also reported data on frequency of repetition of SH as the proportion with no episodes at follow‐up, the proportion with a single episode at follow‐up, and the proportion of two or more repeat episodes of SH by the 12‐month follow‐up assessment. There was no significant difference between groups in the number of participants who had none (347/417 vs 351/410; OR 0.83, 95% CI 0.57 to 1.21; k = 1; N = 827), one (46/417 vs 32/410; OR 1.46, 95% CI 0.91 to 2.35; k = 1; N = 827), or two or more (24/417 vs 27/410; OR 0.87, 95% CI 0.49 to 1.53; k = 1; N = 827) episodes of SH over the six‐month follow‐up period.
This authors also presented data on frequency of repetition of SH by repeater status in post hoc analyses. For those without a history of multiple episodes of SH, there was no significant difference between groups in the number of participants who had none (203/221 vs 181/206; OR 1.56, 95% CI 0.82 to 2.95; k = 1; N = 427), one (13/221 vs 16/206; OR 0.74, 95% CI 0.35 to 1.58; k = 1; N = 427), or two or more (5/221 vs 9/206; OR 0.51, 95% CI 0.17 to 1.54; k = 1; N = 427) repeat episodes of SH. For those with a history of multiple episodes of SH, however, receipt of an emergency card was associated with a significant reduction in the number of participants with no further episodes of SH (142/194 vs 167/200; OR 0.54, 95% CI 0.33 to 0.88; k = 1; N = 394) coupled with a significant increase in the number of participants with one repeat episode of SH (33/194 vs 15/200; OR 2.53, 95% CI 1.33 to 4.82; k = 1; N = 394). There was no significant difference between the experimental and control groups with respect to the number of participants with or two or more subsequent episodes of SH for those with a history of multiple episodes of SH, however (19/194 vs 18/200; OR 1.10, 95% CI 0.56 to 2.16; k = 1; N = 394).
Secondary outcomes
6.9 Treatment adherence
No data available.
6.10 Depression
No data available.
6.11 Hopelessness
No data available.
6.12 Suicidal ideation
No data available.
6.13 Problem solving
No data available.
6.14 Suicide
Data on suicides were reported in only one trial (Evans 1999a). There was no evidence of a significant treatment effect for emergency cards on suicide by the time of the six‐month follow‐up assessment (2/417 vs 1/410; OR 1.97, 95% CI 0.18 to 21.82; k = 1; N = 827).
General practitioner's letter vs TAU
A single, cluster‐randomised controlled trial compared the effectiveness of a letter from patients' general practitioners following discharge from hospital care offering an appointment and advice on patient management versus TAU over a 12‐month follow‐up period (Bennewith 2002, clusters = 98 practices, N = 1932).
We were unable to adjust for the effects of clustering in this analysis, as the study authors could not provide us with either the intercluster coefficient or the design effect. Therefore the effects we report for this intervention should be interpreted with caution.
Primary outcome
6.15 Repetition of SH
There was no evidence of a significant treatment effect for a letter from patients' general practitioners on repetition of SH by the 12‐month follow‐up assessment (211/964 vs 189/968; OR 1.15, 95% CI 0.93 to 1.44; k = 1; N = 1932). A moderate quality of evidence was associated with this outcome (see Table 6).
A post hoc analysis by sex, however, suggested that whilst there was no significant treatment effect for males (82/383 vs 84/413; OR 1.07, 95% CI 0.76 to 1.50; k = 1; N = 796), a GP letter was associated with a significant treatment effect on repetition of SH for females (30/581 vs 105/555; OR 0.23, 95% CI 0.15 to 0.36; k = 1; N = 1136).
In a second post hoc analysis, the authors also analysed repetition of SH by repeater status at trial entry and concluded that "[t]he odds ratio for the effect of the intervention in patients with a history of self‐harm was 0.57 (0.33 to 0.98), indicating a beneficial effect, and in those with no history was 1.32 (1.02 to 1.70), indicating a harmful effect" (Bennewith 2002, p. 1258). As the raw data on which these sub‐group results were based is not reported, we were unable to reproduce these results in this review.
Secondary outcomes
6.16 Treatment adherence
There was no significant treatment effect for the number of participants with at least one contact with treatment services by the time of the 12‐month follow‐up assessment (351/599 vs 387/681; OR 1.08, 95% CI 0.86 to 1.34; k = 1; N = 1280).
6.17 Depression
No data available.
6.18 Hopelessness
No data available.
6.19 Suicidal ideation
No data available.
6.20 Problem solving
No data available.
6.21 Suicide
No data available.
Telephone contact vs TAU
Three trials investigated the effectiveness of telephone contact in adults admitted to emergency departments following a 'suicide attempt' (i.e., suggestive of suicidal intent) (Cedereke 2002, N = 216; Vaiva 2006, N = 605; Wei 2013, N = 157).
Primary outcome
6.22 Repetition of SH
There was no evidence of a significant treatment effect for telephone contact on repetition of SH at the six‐month follow‐up assessment in Wei 2013 (1/41 vs 4/40; OR 0.23, 95% CI 0.02 to 2.11; k = 1; N = 81), by the 12‐month follow‐up period in Cedereke 2002(14/83 vs 15/89; OR 1.00, 95% CI 0.45 to 2.23; k = 1; N = 172), or by the 24‐month follow‐up period in Vaiva 2006 (44/293 vs 59/312; OR 0.76, 95% CI 0.49 to 1.16; k = 1; N = 605). Combining data for these three time points indicated no significant treatment effect for telephone contact by the final follow‐up point (Analysis 5.3; k = 3; N = 840). Quality of evidence for these three time points was very low to low (see Table 6).
With respect to frequency of repetition of SH, the mean number of episodes of SH was similar between treatment groups in both Cedereke 2002 (0.31 vs 0.30) and Vaiva 2006 (0.15 vs 0.19). However, as study authors did not report information on SDs, t‐test or F statistics, we were unable to impute SDs using the method outlined in Townsend 2001 to calculate the mean difference in the number of repeat episodes of SH between groups in these two trials.
Secondary outcomes
6.23 Treatment adherence
There was no evidence of a significant treatment effect on the number of patients attending treatment at least once by 12‐month follow‐up assessment in one trial (60/83 vs 58/89, OR 1.39, 95% CI 0.73 to 2.67; k = 1; N = 172; Cedereke 2002).
6.24 Depression
There was no evidence of a significant treatment effect for telephone contact on depression at either the six‐month (mean 6.01, SD 8.87, n = 41 vs mean 5.85, SD 8.16, n = 40; MD 0.16, 95% CI ‐3.55 to 3.87; k = 1; N = 81) or the 12‐month (mean 5.73, SD 8.71, n = 36 vs mean 5.84, SD 8.23, n = 27; MD ‐0.11, 95% CI ‐4.32 to 4.10; k = 1; N = 63) follow‐up assessments in the only trial of telephone contact to report data on depression scores (Wei 2013).
6.25 Hopelessness
No data available.
6.26 Suicidal ideation
Suicidal ideation was recorded continuously in Cedereke 2002, whereas Wei 2013 recorded data on suicidal ideation dichotomously as the proportion self‐reporting an episode of suicidal ideation.
In Cedereke 2002, telephone contact was not associated with a significant treatment effect on suicidal ideation scores by the 12‐month follow‐up assessment (mean 5.80, SD 7.80, N = 5 vs mean 4.00, SD 6.20, N = 8; MD 1.80, 95% CI ‐6.27 to 9.87; k = 1; N = 13).
Telephone contact was also not associated with a significant treatment effect on the proportion of participants reporting suicidal ideation by the six‐month follow‐up assessment in Wei 2013 (26/41 vs 24/40; OR 1.16, 95% CI 0.47 to 2.83; k = 1; N = 81). By the 12‐month follow‐up assessment, however, telephone contact was associated with a significant treatment effect on the proportion of participants reporting suicidal ideation in this trial (24/36 vs 25/27; OR 0.16, 95% CI 0.03 to 0.79; k = 1; N = 63).
6.27 Problem solving
No data available.
6.28 Suicide
There was no evidence of a significant treatment effect for telephone contact on suicides by either the 12‐month (1/107 vs 1/109; OR 1.02, 95% CI 0.06 to 16.50; k = 1; N = 216; Cedereke 2002) or 24‐month (1/293 vs 2/312; OR 0.52, 95% CI 0.05 to 5.89; k = 1; N = 605; Vaiva 2006) follow‐up assessments. Combining data from these trials suggested no evidence of a significant treatment effect by the time of the final follow‐up assessment (Analysis 5.8; k = 2; N = 821).
5.8. Analysis.

Comparison 5: Remote contact interventions vs treatment as usual (TAU), Outcome 8: Suicide at final follow‐up
Mobile telephone‐based psychotherapy vs TAU
One trial assessed the effectiveness of psychotherapy, including elements of training in problem‐solving therapy, meditation and social support, delivered by mobile telephone over a six‐month follow‐up period in adults admitted to general hospitals following an episode of SH with significant suicidal intent (Marasinghe 2012; N = 68). As this trial used a cross‐over design, we report only data from the post‐intervention assessment (i.e., prior to cross‐over) in this review.
Primary outcome
6.29 Repetition of SH
Data obtained by correspondence from the authors indicated there were no repeat episodes of SH in either the treatment or control groups by the post‐intervention assessment. It was therefore not possible to calculate the pooled odds ratio and accompanying 95% confidence interval owing to zero cell counts (Analysis 5.1; k = 1; N = 68). A low quality of evidence was apparent for this outcome (see Table 6).
Secondary outcomes
6.30 Treatment adherence
No data available.
6.31 Depression
There was evidence of a significant treatment effect for mobile telephone‐based psychotherapy for depression at the post‐intervention assessment (mean 7.00, SD 5.00, n = 34 vs mean 14.60, SD 10.40, n = 34; MD ‐7.60, 95% CI ‐11.48 to ‐3.72; k = 1; N = 68).
However, a priori analyses suggested that this effect varied by sex. Mobile telephone‐based psychotherapy was associated with a significant treatment effect for depression at the post‐intervention assessment in males (mean 5.90, SD 2.40, n = 17 vs mean 13.30, SD 6.10, n = 17; MD ‐7.40, 95% CI ‐10.52 to ‐4.28; k = 1; N = 34) but not in females (mean 8.10, SD 6.30, n = 17 vs mean 11.60, SD 6.50, n = 17; MD ‐3.50, 95% CI ‐7.80 to 0.80; k = 1; N = 34).
6.32 Hopelessness
No data available.
6.34 Suicidal ideation
There was evidence of a significant treatment effect for mobile telephone‐based psychotherapy for suicidal ideation at the post‐intervention assessment (mean 3.60, SD 1.60, n = 34 vs mean 7.30, SD 5.50, n = 34; MD ‐3.70, 95% CI ‐5.63 to ‐1.77; k = 1; N = 68) in this trial.
When we analysed results separately by sex, however, there was no evidence of a significant treatment effect for mobile telephone‐based psychotherapy for suicidal ideation at the post‐intervention assessment in males (mean 3.50, SD 1.80, n = 17 vs mean 6.20, SD 5.50, n = 17; MD ‐2.70, 95% CI ‐5.45 to 0.05; k = 1; N = 34). There was, however, a significant treatment effect for females (mean 3.80, SD 1.40, n = 17 vs mean 8.90, SD 6.20, n = 17; MD ‐5.10, 95% CI ‐8.12 to ‐2.08; k = 1; N = 34).
6.35 Problem solving
No data available.
6.36 Suicide
Information obtained by correspondence indicated that there was one suicide in the experimental group by the time of the post‐intervention assessment and none in the control group. Mobile telephone‐based psychotherapy was not associated with a significant treatment effect for suicide by this time point (Analysis 5.6; k = 1; N = 68).
Comparison 7: Other mixed interventions vs TAU or other alternative forms of psychotherapy
A number of single, small trials investigated the effectiveness of other types of heterogeneous interventions, including: interpersonal problem‐solving skills training (vs TAU; McLeavey 1994), behaviour therapy (vs other alternative forms of psychotherapy; Liberman 1981), provision of information and support (vs TAU; Fleischmann 2008), treatment for alcohol misuse (vs TAU; Crawford 2010), home‐based problem‐solving therapy (vs other alternative forms of psychotherapy; Hawton 1981), intensive inpatient and community treatment (vs TAU; Van der Sande 1997a, general hospital admission (vs other alternative forms of psychotherapy; Waterhouse 1990, intensive outpatient treatment (vs TAU; Allard 1992; Welu 1977), and long‐term therapy (vs other alternative forms of psychotherapy; Torhorst 1988).
Interpersonal problem‐solving skills training vs other alternative forms of psychotherapy
One small trial compared the effectiveness of interpersonal problem‐solving skills training (IPSST) with brief problem‐oriented therapy in adults admitted to accident and emergency facilities following an episode of self‐poisoning (McLeavey 1994; N = 39).
Primary outcome
7.1 Repetition of SH
There was no evidence of a significant treatment effect for repetition of SH, defined as a 'self‐poisoning act', within the 12‐month follow‐up period (2/17 vs 4/16; OR 0.40, 95% CI 0.06 to 2.57; k = 1; N = 33) in this trial. A very low quality of evidence was associated with this outcome (see Table 7).
Secondary outcomes
7.2 Treatment adherence
There was no evidence of a significant treatment effect on the number of participants who completed the full course of treatment (2/19 vs 3/20; OR = 0.67, 95% CI 0.10 to 4.51; k = 1; N = 39).There was, however, evidence for a significant treatment effect in terms of the number of treatment sessions attended (mean 5.30, SD 0.48, n = 17 vs mean 4.20, SD 1.32, n = 16; MD 1.10, 95% CI 0.41 to 1.79; k = 1; N = 33).
7.3 Depression
No data available.
7.4 Hopelessness
There was no evidence of a significant treatment effect for hopelessness at the six‐month follow‐up assessment (mean 6.12, SD 4.61, n = 19 vs mean 4.35, SD 4.39, n = 20; MD 1.77, 95% CI ‐1.06 to 4.60; k = 1; N = 39).
7.5 Suicidal ideation
No data available.
7.6 Problem solving
Analysis of means estimated by the review authors from graphics in the original report suggests that, at the post‐intervention assessment, participants allocated to the experimental group had scores within the normal range whilst those allocated to the control group remained impaired according to both the Means‐Ends Problem‐Solving Scale (estimated means 6.4 vs 2.9) and the Self‐Rated Problem Solving Scale (estimated means 89.8 vs 78.0). Additionally, participants in the experimental group reported feeling more confident in solving problems post‐treatment according to scores on the Perceived Ability to Solve Current Problems scale (estimated means 0.9 vs 1.9). Lastly, both groups reported a reduction in self‐reported number of problems (estimated means 1.1 vs 1.5).
Results reported by the trial authors suggest an equal benefit of both treatments in reducing the "number of presenting problems. . ." (McLeavey 1994, p. 382). However, the authors conclude that IPSST was "significantly more effective . . . as determined by other outcome measures. . ." including measures of interpersonal cognitive problem‐solving, self‐rated personal problem‐solving ability, and perceived ability to cope with ongoing problems (McLeavey 1994, p.382).
7.7 Suicide
There were no suicides in either group during the 12‐month follow‐up period.
Behaviour therapy vs other alternative forms of psychotherapy
One small trial compared the effectiveness of behaviour therapy versus insight‐oriented therapy in adults referred for inpatient treatment following a suicide attempt (Liberman 1981; N = 24).
Primary outcome
7.8 Repetition of SH
There was no evidence of a significant treatment effect with regards to the number of patients repeating SH by the 24‐month follow‐up period (2/12 vs 3/12; OR 0.60, 95% CI 0.08 to 4.45; k = 1; N = 24). This outcome was associated with a low quality of evidence (see Table 7)
Secondary outcomes
7.9 Treatment adherence
No data available.
7.10 Depression
Depression was measured using both the BDI and the ZSRDS in this trial. There was evidence of a significant treatment effect for behaviour therapy at the post‐treatment assessment according to both measures (BDI: mean 4.00, SD 4.00, n = 12 vs mean 14.00, SD 12.00, n = 12; MD ‐10.00, 95% CI ‐17.16 to ‐2.84; k = 1; N = 24; ZSRDS: mean 32.00, SD 8.00, n = 12 vs mean 43.00, SD 14.00, n = 12; MD ‐11.00, 95% CI ‐20.12 to ‐1.88; k = 1; N = 24).
At the six‐month (24‐week) follow‐up assessment, although there was no significant treatment effect for depression according to the ZSRDS (mean 34.00, SD 8.00, n = 12 vs mean 41.00, SD 13.00, n = 12; MD ‐7.00, 95% CI ‐15.64 to 1.64; k = 1; N = 24), BDI scores did show an effect (mean 4.00, SD 6.00, n = 12 vs mean 13.00, SD 11.00, n = 12; MD ‐9.00, 95% CI ‐16.09 to ‐1.91; k = 1; N = 24).
7.11 Hopelessness
No data available.
7.12 Suicidal ideation
There was no evidence of a significant effect for behaviour therapy on the number of patients reporting suicidal ideation at the 24‐month follow‐up assessment (5/12 vs9/12; OR 0.24, 95% CI 0.04 to 1.36; k = 1; N = 24).
7.13 Problem solving
No data available.
7.14 Suicide
No data available.
Provision of information and support vs TAU
The effectiveness of providing a one‐off hospital‐based information session combined with regular home visits and/or telephone contact in addition to TAU over an 18 month follow‐up period was investigated in one multicentre trial (SUPRE‐MISS) conducted in ten countries, although data from only five of these countries are reported in Bertolote 2010 (N = 1,663) and Fleischmann 2008 (N = 1,699). Data from three of the individual countries (Hassanzadeh 2010, N = 632; Vijayakumar 2011, N = 680; Xu 2012, N = 111) were also included for some outcomes.
Correspondence with authors indicted that the term 'attempted suicide' in this trial was used to refer to SH both with and without suicidal intent.
Primary outcome
7.15 Repetition of SH
For the overall SUPRE‐MISS cohort, data from Bertolote 2010 indicated there was no evidence for a significant treatment effect for information and support on repetition of SH by the 18‐month follow‐up assessment (66/863 vs 60/800; OR 1.02, 95% CI 0.71 to 1.47; k = 1; N = 1663). This outcome was associated with low quality of evidence according to the GRADE criteria (see Table 7).
Data on repetition of SH were also available for males and females separately. Overall, across all five sites, there was no evidence of a significant treatment effect on repetition of SH by the 18‐month follow‐up assessment in either males (30/349 vs 27/340; OR 1.09, 95% CI 0.63 to 1.88; k = 1; N = 689) or females (36/514 vs 33/460; OR 0.97, 95% CI 0.60 to 1.59; k = 1; N = 974).
Data on repetition of SH by the 18‐month follow‐up assessment were also available for each of the five countries separately. Although there was no significant difference between groups for the individual sites in Campinas, Brazil (21/71 vs 10/64; OR 2.27, 95% CI 0.97 to 5.28; k = 1; N = 135), Colombo, Sri Lanka (3/130 vs 5/121; OR 0.55, 95% CI 0.13 to 2.34; k = 1; N = 251), Karaj, Iran (33/303 vs 28/298; OR 1.18, 95% CI 0.69 to 2.00; k = 1; N = 601), and Yuncheng, China (1/58 vs 0/38; OR 2.01, 95% CI 0.08 to 50.60; k = 1; N = 96), significantly fewer participants in the experimental group had repeated SH by the 18‐month follow‐up period at the Chennai, India site (8/301 vs 17/260; OR 0.39, 95% CI 0.17 to 0.92; k = 1; N = 561). Quality of evidence for these five sites varied from very low to low (see Table 7).
Breaking results down by gender revealed no significant effect for information and support on repetition of SH by the 18‐month follow‐up assessment for either gender at either of the five study sites [Campinas, Brazil: males 4/21 vs. 3/25; OR 1.73, 95% CI 0.34 to 8.76; k = 1; N = 46 versus females 17/50 vs. 7/39; OR 2.35, 95% CI 0.86 to 6.44; k = 1; N = 89; Chennai, India: males 5/148 vs. 7/125; OR 0.59, 95% CI 0.18 to 1.91; k = 1; N = 273 versus females 3/153 vs. 10/153; OR 0.29, 95% CI 0.08 to 1.06; k = 1; N = 306; Colombo, Sri Lanka: males 1/54 vs. 3/53; OR 0.31, 95% CI 0.03 to 3.12; k = 1; N = 107 versus females 2/76 vs. 2/68; OR 0.89, 95% CI 0.12 to 6.51; k = 1; N = 144; Karaj, Islamic Republic of Iran: males 19/109 vs. 14/118; OR 1.57, 95% CI 0.74 to 3.31; k = 1; N = 227 versus females 14/194 vs. 14/180; OR 0.92, 95% CI 0.43 to 1.99; k = 1; N = 374; Yuncheng, China: males: 1/17 vs. 0/19; OR 3.55, 95% CI 0.14 to 93.01; k = 1; N = 36 versus females 0/41 vs. 0/38; OR not calculable; k = 1; N = 79].
Hassanzadeh 2010 reported data on frequency of SH for one sub‐sample at the six‐month follow‐up assessment in Karaj, Iran. In this sample, there was evidence of a significant increase in frequency of repetition of SH in the information and support group relative to the TAU group (mean 1.63, SD 1.19, n = 319 vs mean 1.17, SD 0.38, n = 310; MD 0.46, 95% CI 0.32 to 0.60; k = 1; N = 629). Quality of evidence for this outcome was low (see Table 7).
Secondary outcomes
7.16 Treatment adherence
No data available.
7.17 Depression
Correspondence with authors revealed that information on depression was recorded at one site only: Yuncheng, China (reported by Xu 2012). Information and support was associated with a significant treatment effect for depression scores at this site by the 18‐month follow‐up assessment (mean 2.51, SD 3.25, n = 57 vs mean 5.60, SD 9.25, n = 54; MD ‐3.09, 95% CI ‐5.70 to ‐0.48; k = 1; N = 111).
7.18 Hopelessness
No data available.
7.19 Suicidal ideation
No data available.
7.20 Problem solving
Correspondence with authors revealed that information on problem solving was reported for one site only: Yuncheng, China (reported in Xu 2012). There was evidence of a significant treatment effect for information and support at this site by the 18‐month follow‐up assessment (mean 0.64, SD 0.29, n = 57 vs mean 0.52, SD 0.30, n = 54; MD 0.12, 95% CI 0.01 to 0.23; k = 1; N = 111).
7.21 Suicide
In the overall SUPRE‐MISS cohort, as reported in the primary study reference (Fleischmann 2008), there was evidence of a significant treatment effect for information and support on suicide by the 18‐month follow‐up period (2/872 vs 18/827; OR 0.10, 95% CI 0.02 to 0.45; k = 1; N = 1699).
Data on suicides were also available for three of the five study sites in related publications: Vijayakumar 2011 reported data from Chennai, India, Hassanzadeh 2010 from Karaj, Iran, and Xu 2012 from Yuncheng, China. There was evidence of a significant treatment effect for information and support on suicides by the 18‐month follow‐up assessment at the Chennai, India site (1/302 vs 9/320; OR 0.11, 95% CI 0.01 to 0.91; k = 1; N = 622) but not at either the Karaj, Iran (2/319 vs 2/310; OR 0.97, 95% CI 0.14 to 6.94; k = 1; N = 629) or the Yuncheng, China (0/57 vs 2/54; OR 0.18, 95% CI 0.01 to 3.89; k = 1; N = 111) sites.
Notably, the number of completed suicides in the experimental group reported for these three subsamples is greater than the number reported for the overall SUPRE‐MISS cohort in the primary study reference (i.e., Fleischmann 2008). We were unable to confirm the correct number of completed suicides in the experimental group with the authors. Including the one additional suicide for the experimental group identified from the three subsample publications with the data reported in the primary study reference, however, did not materially affect the result obtained for the overall SUPRE‐MISS cohort.
Treatment for alcohol misuse vs TAU
One trial investigated the effectiveness of a brief intervention for alcohol misuse on repetition of SH over a six‐month follow‐up period in adults who were misusing alcohol and were admitted to emergency departments following an episode of SH (Crawford 2010; N = 103).
Primary outcome
7.22 Repetition of SH
There was no evidence of a significant treatment effect for treatment for alcohol misuse on repetition of SH by the six‐month follow‐up period (7/52 vs11/51; OR 0.57, 95% CI 0.20 to 1.60; k = 1; N = 103). This was associated with a moderate quality of evidence (see Table 7).
Secondary outcomes
7.23 Treatment adherence
The study authors report that only 47.1% of those randomised to the experimental group attended the brief alcohol treatment session (Crawford 2010, p.1826). However, as corresponding numbers were not available for the control group, who did not receive an invitation to a brief alcohol treatment session, we could not calculate treatment effect sizes for this outcome.
7.24 Depression
No data available.
7.25 Hopelessness
No data available.
7.26 Suicidal ideation
No data available.
7.27 Problem solving
No data available.
7.28 Suicide
Correspondence with authors confirmed that no participants died by suicide in either group over the course of the six‐month follow‐up period. However, the authors warn that as they were unable to track participants via their National Health Service (NHS) identity numbers, they were unable to confirm numbers of suicides from national mortality data. Thus, there may have been suicides amongst those participants whom the authors were unable to contact by the six‐month follow‐up assessment.
Home‐based problem‐solving therapy vs other alternative forms of psychotherapy
Hawton 1981 (N = 96) investigated the effectiveness of brief problem‐oriented counselling delivered in two different ways, namely as a flexibly‐timed home‐based therapy, combined with open access via telephone services to the general hospital psychiatric service, versus treatment in weekly outpatient clinics, in adults referred to the psychiatric department of a general hospital following admission for self‐poisoning, irrespective of intent.
Primary outcome
7.29 Repetition of SH
There was no evidence of a significant treatment effect for home‐based problem‐solving therapy on repetition of SH by the 12‐month follow‐up assessment (5/48 vs 7/48; OR 0.68; 95% CI 0.20 to 2.32; k = 1; N = 96). Quality of evidence, as assessed using the GRADE criteria, was moderate for this outcome (see Table 7).
Secondary outcomes
7.30 Treatment adherence
There was, however, a significant treatment effect for home‐based problem‐solving therapy on the number of participants who attended at least one treatment session over the course of the 12‐month follow‐up period (45/48 vs 35/48; OR 5.57, 95% CI 1.47 to 21.08; k = 1; N = 96).
7.31 Depression
Although this trial included data on depression, the authors modified the scale used (Lorr and McNair Mood Scale; McNair 1964; Lorr 1967), thereby precluding inclusion of this data in this review.
7.32 Hopelessness
No data available.
7.33 Suicidal Ideation
Data obtained by correspondence suggested there was no significant treatment effect for suicidal ideation at either the post‐treatment assessment (Mann‐Whitney U = 984, P = 0.29) or six‐month follow‐up (Mann‐Whitney U = 726, P = 0.14). As only median, rather than mean, scores were available for this outcome, we were unable to reproduce the mean difference in suicidal ideation scores between the experimental and control groups in this review.
7.34 Problem solving
No data available.
7.35 Suicide
No data available.
Intensive inpatient and community treatment vs TAU
One trial compared the effectiveness of brief psychiatric inpatient admission followed by regular outpatient appointments and 24‐hour access to the psychiatric unit with TAU over a 12‐month follow‐up period in adults admitted to a general hospital following a 'suicide attempt' (i.e., suggestive of suicidal intent) (Van der Sande 1997a, N = 274).
Primary outcome
7.36 Repetition of SH
There was no evidence of a significant treatment effect for intensive inpatient and community treatment on repetition of SH by the 12‐month follow‐up (24/140 vs 20/134; OR 1.18, 95% CI 0.62 to 2.25; k = 1; N = 274). Quality of evidence, according to the GRADE criteria, was low for this outcome (see Table 7).
With respect to frequency of repetition of SH, there was also no evidence of a significant treatment effect for intensive inpatient and community treatment (mean 0.23, SD 0.57, n = 140 vs mean 0.23, SD 0.81, n = 134; MD 0.00, 95% CI ‐0.17 to 0.17, k = 1; N = 274). Quality of evidence for this outcome was also low (see Table 7).
Secondary outcomes
7.37 Treatment adherence
There was a significant treatment effect for treatment adherence. More patients in the experimental group attended at least one outpatient treatment session by the 12‐month follow‐up assessment (119/140 vs 64/134; OR 6.99, 95% CI 3.69 to 12.36; k = 1; N = 274). However, there was no difference in the total number of treatment sessions attended (mean 14.30, SD 24.20, n = 140 vs mean 11.40, SD 27.70, n = 134; MD 2.90, 95% CI ‐3.27 to 9.07; k = 1; N = 274).
7.38 Depression
There was no significant treatment effect for intensive inpatient and community treatment on depression scores by the 12‐month follow‐up assessment (mean 30.80, SD 15.90, n = 94 vs mean 35.80, SD 16.20, n = 50; MD ‐5.00, 95% CI ‐10.52 to 0.52; k = 1; N = 144).
7.39 Hopelessness
There was no significant treatment effect for intensive inpatient and community treatment on hopelessness scores by the 12‐month follow‐up assessment (mean 6.10, SD 5.00, n = 94 vs mean 7.50, SD 5.90, n = 50; MD ‐1.40, 95% CI ‐3.32 to 0.52; k = 1; N = 144).
7.40 Suicidal ideation
No data available.
7.41 Problem solving
No data available.
7.42 Suicide
There was also no evidence of a significant treatment effect for suicide by the 12‐month follow‐up assessment (1/140 vs 2/134; OR 0.47, 95% CI 0.04 to 5.30; k = 1; N = 274).
General hospital admission vs other alternative forms of psychotherapy
One trial investigated the effectiveness of general hospital admission versus non‐admission over a four‐month follow‐up period in a group of adults attending an emergency room following an episode of self‐poisoning, who had no immediate medical or psychiatric treatment needs (Waterhouse 1990, N = 77). In this trial, admission was described as consisting of little more than admission to an inpatient bed. The investigators did not attempt to influence referral to psychiatric or other treatment services. The median length of admission for those allocated to the experimental group was 17 hours.
Primary outcome
7.43 Repetition of SH
There was no evidence of a significant treatment effect for hospital admission on repetition of SH at the post‐intervention assessment (2/38 vs 2/39; OR 1.03, 95% CI 0.14 to 7.69; k = 1; N = 77) or by the four‐month follow‐up assessment (3/38 vs 4/39; OR 0.75, 95% CI 0.16 to 3.60; k = 1; N = 77). Quality of evidence for these time points was low (see Table 7).
Secondary outcomes
7.44 Treatment adherence
No data available.
7.45 Depression
No data available.
7.46 Hopelessness
The authors state that there was no significant difference in hopelessness scores at the post‐intervention assessment (mean 10.29, SD 5.68 vs mean 10.21, SD 4.97); however, they did not provide the numbers of patients in each group, thus precluding calculation of the MD and its associated 95% CI.
7.47 Suicidal ideation
There was no evidence of a significant treatment effect for hospital admission on suicidal ideation scores by the four‐month follow‐up assessment (mean 0.22, SD 0.85, n = 27 vs mean 0.04, SD 0.20, n = 25; MD 0.18, 95% CI ‐0.15 to 0.51; k = 1; N = 52).
7.48 Problem solving
No data available.
7.49 Suicide
No data available.
Intensive outpatient treatment vs TAU
Two trials compared the effectiveness of intensive outreach interventions with standard outpatient care in adults admitted to emergency departments following a 'suicide attempt' (i.e., suggestive of suicidal intent) (Allard 1992, N = 150; Welu 1977, N = 119). Allard 1992 compared an intensive intervention, involving psychiatrists and a social worker, a schedule of visits including at least one home visit, therapy provided where needed, reminders (telephone or written), and home visits with treatment by regular personnel in the same hospital over a 12‐month treatment period. Therapies in the experimental group varied, and drug therapy was also an option. Welu 1977 compared a specialist, intensive outreach programme in which a community mental health team contacted participants immediately after discharge and arranged home visits and weekly or bi‐weekly contact with therapists alongside routine psychiatric consultation.
Primary outcome
7.50 Repetition of SH
There was no evidence of a significant treatment effect for intensive intervention on repetition of SH by either the four‐month (Welu 1977: 3/62 vs 9/57; OR 0.27, 95% CI 0.07 to 1.06; k = 1; N = 119) or 24‐month (Allard 1992: 22/63 vs 19/63; OR 1.24, 95% CI 0.59 to 2.62; k = 1; N = 126) follow‐up assessments. For both follow‐up periods, quality of evidence was low(see Table 7). We combined the results of these two trials, and again there was no evidence of a significant treatment effect for intensive outpatient intervention by the final follow‐up point (Analysis 6.1; k = 2; N = 245). The quality of evidence was very low for this outcome (see Table 7).
6.1. Analysis.

Comparison 6: Other mixed interventions versus treatment as usual (TAU) or other alternative forms of psychotherapy, Outcome 1: Repetition of SH at final follow‐up
In the one trial that reported information on frequency of SH over the course of the 24‐month follow‐up period (i.e., Allard 1992), "the experimental subjects did not make fewer attempts than the comparison subjects" (Allard 1992, p. 310).
Secondary outcomes
7.51 Treatment adherence
Data on treatment adherence were only available for Allard 1992. However, as the authors did not report information on SDs, t‐test or F statistics we were unable to impute SDs using the method outlined in Townsend 2001 to calculate the mean difference in the number of treatment sessions attended. Nevertheless, the authors themselves report that "[t]he mean numbers of encounters with psychiatrists were 12.35 versus 1.54 (P < 0.001) in the first year and 2.11 versus 0.64 (P = 0.071) in the second year" (Allard 1992, p. 311).
7.52 Depression
No data available.
7.53 Hopelessness
No data available.
7.54 Suicidal ideation
No data available.
7.55 Problem solving
No data available.
7.56 Suicide
Allard 1992 reported data on suicide during follow‐up. There was no evidence of a significant treatment effect for the intensive outpatient intervention on suicides, however, by the 24‐month follow‐up assessment (3/76 vs 1/74; OR 3.00, 95% CI 0.30 to 29.52; k = 1; N = 150) in this trial.
Long‐term psychotherapy vs other alternative forms of psychotherapy
One trial investigated the effectiveness of long‐term (one session per month over 12 months) versus short‐term (one session per week over 12 weeks) outpatient psychotherapy on repetition of SH over a 12‐month follow‐up period in adults admitted to hospital due to repeated episodes of self‐poisoning (Torhorst 1988, N = 80). The content of therapy was not specified in this trial, however.
Primary outcome
7.57 Repetition of SH
There was no evidence of a significant treatment effect for long‐term therapy on repetition of SH by the post‐treatment assessment (9/40 vs 9/40; OR 1.00, 95% CI 0.35 to 2.86; k = 1; N = 80). A low quality of evidence was associated with this outcome (see Table 7).
Secondary outcomes
7.58 Treatment adherence
The authors did not provide numerical data on treatment adherence, although they state, "[a]ttendance at the first session was about equal for both groups (about 60%)" (Torhorst 1988, p. 420). However, the authors further state that "participation of the 12‐month (long‐term therapy) group dropped drastically by the second session to under 40%, while the participation of the patients in the 3‐month (intensive short‐term therapy) program remained higher" (Torhorst 1988, p. 420). It is unclear whether this difference was significant.
Overall adherence also appears to have been very low in both groups as the "average number of sessions was 3.9 (out of a possible 12 sessions) in the three‐month group and 2.6 (out of a possible 12 sessions) for the 12‐month group" (Torhorst 1988, p.420). Again, it is unclear whether this difference is significant. Additionally, as neither SDs, nor t‐test, nor F statistics were reported, we were unable to impute SDs using the method outlined in Townsend 2001 to calculate the mean difference in the number of treatment sessions attended by the experimental and control groups.
7.59 Depression
Although numerical data on depression scores were not available, means estimated by the review authors from a graph in the original report suggest there was little difference in depression scores between those allocated to long‐term therapy and those allocated to short‐term therapy by the 12‐month follow‐up assessment (estimated means 9.3 vs 6.7).
The study authors, however, stated that "self‐evaluated depressivity. . . improved considerably more for the patients of the three‐month program than for those of the 12‐month program" (Torhorst 1988, p. 421). This improvement was described by the authors as significant.
7.60 Hopelessness
No data provided.
7.61 Suicidal ideation
No data provided.
7.62 Problem solving
No data provided.
7.63 Suicide
No data provided.
Discussion
This systematic review represents an update of previous versions (Hawton 1998; Hawton 1999; NICE 2011). Whilst those versions included psychosocial and pharmacological interventions as well as data for adults, children and adolescents who engage in SH, this update focused solely on psychosocial treatments for adults. In the previous versions of this review, we only focused on a limited number of clinical outcomes, namely repetition of SH and suicide. In this update we have considerably expanded the range of clinically relevant outcomes examined to include treatment adherence, depression, hopelessness, problem‐solving, and suicidal ideation where available. We also reported frequency of SH where data on this outcome were available. For the primary outcome of SH, we have included SH episodes with any type of motivation, including suicidal. Where we clarified suicidal intent with study authors a number reported including all episodes of SH irrespective of suicidal intent despite inclusion criteria suggesting that only those indicating intent to die were eligible to participate, highlighting the problems in attempting to ascertain suicidal intent.
Recently there has been a considerable increase in the number of trials conducted in this field and in the types of interventions evaluated, reflecting the international concern about self‐harm, the increased attention to suicide prevention in particular, and the involvement of newer countries in this research, especially in Asia.
Previously we commented on the fact that the majority of trials included either patients who had all taken overdoses, or samples where the majority had, reflecting the types of patients who present to general hospitals following SH (Hawton 2007). However, there are other important patient subgroups, especially those who engage in self‐mutilation. Some of the more recent trials in this review included such participants, particularly those that focused on patients who had a history of multiple episodes of SH at trial entry (e.g., Gratz 2006; Gratz 2014; Harned 2014; Linehan 1991; Linehan 2006; McMain 2009; Priebe 2012; Weinberg 2006). It should be noted that people who repeat SH may change the methods they use (Owens 2015; Lilley 2008). It is also important to note that multiple repetition of SH is associated with increased suicide risk (Zahl 2004).
None of the trials included information on adverse effects of these interventions, other than further suicidal behaviour.
We have used the intention‐to‐treat method where data allowed. This was usually possible when examining the outcomes of repetition of SH and suicide. Where outcomes relied on patient interview, this was generally not possible and we have instead used all available case data.
Summary of main results
CBT‐based psychotherapy
There were 18 trials that compared CBT‐based psychotherapy, comprising cognitive behavioural therapy, problem‐solving therapy, or both, versus treatment as usual (TAU) (Brown 2005; Davidson 2014; Dubois 1999; Evans 1999b; Gibbons 1978; Guthrie 2001; Hatcher 2011; Hawton 1987a; Husain 2014; McAuliffe 2014; Patsiokas 1985; Salkovskis 1990; Slee 2008; Stewart 2009; Tapolaa 2010; Tyrer 2003; Wei 2013; Weinberg 2006). Meta‐analysis of these trials provided evidence to suggest a reduction in repetition of SH at both 6 and 12 months after trial entry and at final follow‐up. However, we did not find any significant treatment effect for CBT‐based psychotherapy on the frequency of SH at final follow‐up. On the basis of data from 15 trials, there was no evidence of a significant effect of psychological therapy on suicides, although relatively few events (i.e., 24) were recorded. We also found beneficial effects for depression and hopelessness at 6 and 12 months as well as at final follow‐up. Few trials assessed suicidal ideation, although there was an apparent benefit for CBT‐based psychotherapy at three months, six months, and at final follow‐up. Relatively few trials reported findings for treatment adherence, and they did so in different ways, so we cannot draw firm conclusions for this outcome.
Interventions for multiple repetition of SH/probable personality disorder
Group‐based emotion‐regulation psychotherapy
On the basis of two trials, conducted by the same research group, emotion‐regulation therapy for patients with borderline personality disorder provided in a group‐based setting was associated with a reduction in the proportion of patients repeating SH in the final two months of the initial treatment period, but not with an overall reduction in the frequency of SH over the whole treatment period (Gratz 2006; Gratz 2014). There was also no effect for this type of therapy on depression.
Mentalisation
In a single trial, mentalisation therapy for patients diagnosed with borderline personality disorder was associated with fewer participants repeating both SH and suicide attempts by the post‐intervention assessment (Bateman 2009). There were also beneficial effects for frequency of repetition of suicide attempts and for depression scores.
Dialectical behaviour therapy
Three trials compared DBT with TAU in patients diagnosed with borderline personality disorder, with no apparent overall effect on the proportion of patients repeating SH at 12 and 24 months following trial entry (Linehan 1991; McMain 2009; Priebe 2012). There was, however, a significant treatment effect for DBT on frequency of repetition of SH.
A single trial compared DBT versus psychological treatment by 'experts' (CBT‐E; Linehan 2006). There was no evidence of differences in outcomes for patients in the two groups in terms of the proportion repeating SH or in the total number for 'parasuicidal' acts, although the authors stated that there was a beneficial effect for DBT on suicide re‐attempts. There were no differences between the DBT and CBT‐E groups for depression and suicidal ideation, however.
There was no difference in terms of repetition of SH, depression, or treatment adherence in a single small trial between two forms of DBT, an experimental one in which participants were given significantly longer cognitive exposure to stressful events coupled with the standard DBT protocol and a control one in which participants received the standard DBT protocol as devised by Linehan 1991 (Harned 2014).
Dialectical behaviour‐oriented therapy
In a single small trial (Turner 2000), DBT‐oriented therapy appeared to be more effective than client‐oriented therapy in terms of the proportion of patients repeating SH and the frequency of SH. There were also benefits for depression and suicidal ideation.
Case management
In a single trial comparing case management versus TAU, there was no effect for the experimental treatment on the proportion of participants repeating SH, but it was associated with fewer multiple admissions (Clarke 2002).
Two trials compared case management with added assertive outreach versus TAU (Hvid 2011; Morthorst 2012). There was no evidence of a significant treatment effect for repetition of SH, but there was a reduction in the proportion of females repeating SH in the experimental group in one of these trials (Hvid 2011).
A single large trial compared case management plus assertive outreach versus enhanced usual care (Kawanishi 2014). There was no difference between groups for repetition of SH or for suicide by the 24‐month follow‐up period, although the study authors state that are undertaking further analyses and also data for hopelessness.
Treatment adherence enhancement approaches
Adherence enhancement
In a single trial that made efforts to improve adherence with treatment by having nurses make home visits to participants who had not attended initial outpatient appointments, there were increased rates of attendance at the outpatient clinic in the group receiving the experimental intervention. However, in spite of a marked reduction in subsequent repetition of SH in this group, the difference was non‐significant (Van Heeringen 1995).
Continuity of care by the same therapist
In a single trial, continuity of care (i.e., where the clinician who assessed each participant in hospital also provided aftercare for them) resulted in better treatment attendance than where different therapists treated participants (Torhorst 1987). However, there was no beneficial effect on repetition of SH.
Mixed multimodal interventions
Mixed multimodal interventions
A single large trial compared a package of interventions including problem‐solving therapy, postcards, and vouchers entitling participants to GP care versus TAU (Hatcher 2015). However, there was no beneficial effect for the experimental treatment on repetition of SH, depression, or hopelessness.
Culturally‐adapted mixed multimodal interventions
Hatcher 2016a adapted the treatment package developed by Hatcher 2015 for participants self‐identifying as of Māori ethnicity. There were no apparent benefits of this intervention in terms of repetition of SH (including in subgroups of those with only a single episode of SH prior to trial entry and those with a history of multiple episodes of SH), depression, or hopelessness.
Remote contact interventions
Postcards
Four trials compared the effectiveness of postcards sent on a regular basis over a 12‐month period with TAU (Beautrais 2010; Carter 2005; Hassanian‐Moghaddam 2011; Kapur 2013a). However, there was no benefit for this intervention in terms of proportion of patients repeating SH. Substantial heterogeneity was associated with this analysis, and removing Kapur 2013a (a small pilot trial which may have been an outlier) resulted in a significant treatment effect. The single largest trial of this intervention did find fewer patients repeating SH in the experimental group (Hassanian‐Moghaddam 2011). This result is notable because the control treatment used in this trial would have consisted of little more than discharge because of the paucity of psychiatric services in Iran as compared to Australia, New Zealand, and the UK, which have well‐developed services. This raises the possibility that such an intervention may be more effective in such settings. Additionally, the postcards used in this trial included religious and philosophical messages in addition to general support, which may also explain their apparent efficacy in reducing suicidal behaviour.
There was also no evidence of a significant treatment effect for postcards on frequency of repetition of SH at either post‐intervention or at 12 or 24 months' follow‐up in three of the four trials of postcards (Carter 2005; Hassanian‐Moghaddam 2011; Kapur 2013a). It should be noted that the positive effect on frequency of repetition reported in Carter 2005 was, according to the study's author, largely accounted for by difference in repetition in a small subsample (less than 3% of the total sample) of women with a history of three or more episodes of SH prior to trial entry.
Emergency cards
On the basis of two trials, there was no evidence that provision of an emergency contact card allowing emergency access to a psychiatrist on demand had an impact on repetition of SH (Evans 1999a; Morgan 1993). In the original report, however, a post hoc subgroup analysis indicated that receipt of the emergency card was associated with an increased risk of repetition of SH in those with a history of multiple episodes of SH prior to the index episode (Evans 1999a).
General practitioner's letter
In a single trial in which general practitioners sent a letter to participants following their discharge from hospital after SH offering an appointment coupled with specialist advice on the management of SH patients (Bennewith 2002), there was no apparent beneficial effect on repetition of SH, although there was evidence of a substantial beneficial effect in females. When analysed by repeater status, furthermore, there appeared to be a beneficial effect in those with a history of multiple episodes of SH at trial entry, but the reverse was true in those with only a single episode of SH at trial entry.
Telephone contact
In three trials telephone contact with patients after discharge from hospital did not produce any apparent benefits in terms of repetition of SH compared with standard care (Cedereke 2002; Vaiva 2006; Wei 2013). There was also no evidence of any impact of telephone contact on depression, suicidal ideation, or on the proportion of participants attending at least one treatment session during the 12‐month follow‐up period.
Mobile telephone‐based psychotherapy
A single trial delivered psychotherapy, which included elements of problem‐solving therapy, meditation, and social support, by mobile telephone (Marasinghe 2012), but there was no effect of this intervention on repetition of SH. In the mobile phone psychotherapy group, however, depression improved more in males compared to those assigned to the wait list control group, whereas suicidal ideation improved more in females.
Other mixed interventions
Interpersonal problem‐solving skills training
In a single trial, interpersonal problem‐solving skills training (IPSST) was no better than brief problem‐oriented therapy in terms of repetition of SH, suicide and hopelessness (McLeavey 1994), although patients in the IPSST group may have had better scores after treatment with regard to measures of problem‐solving. Treatment adherence also did not differ between groups, although patients in the IPSST group did attend more treatment sessions.
Behaviour therapy
In a single trial, although behaviour therapy appeared to lead to significant reductions in depression at the 10‐week post‐treatment assessment, by the nine‐month follow‐up assessment there was no apparent benefit for behaviour therapy compared to insight‐oriented therapy (Liberman 1981). Behaviour therapy was also associated with mixed findings with respect to suicidal ideation. There was no apparent effect of behaviour therapy in reducing the proportion of participants reporting suicidal ideation at the two‐year follow‐up period, although there was some evidence of a significant treatment effect for shorter follow‐up periods. Behaviour therapy was also not associated with a significant treatment effect for repetition of SH over a two‐year follow‐up period.
Provision of information and support
In a single trial conducted in five countries, a hospital‐based information service combined with regular home support, telephone support, or both, appeared to have no extra benefit compared to TAU in terms of repetition of SH (Bertolote 2010). However, when results were analysed separately by site, although no apparent benefit for information and support was found for four of the five sites, this intervention package was associated with a significant reduction in repetition of SH at the Chennai, India site. There was evidence of significant reduction in suicide for the overall cohort (Fleischmann 2008) and for the Chennai, India site (Vijayakumar 2011), but not for the remaining two sites for which data on suicides were available (Karaj, Iran: Hassanzadeh 2010 and Yuncheng, China: Xu 2012). We have noted that there is a discrepancy between the findings for the overall cohort and those reported from the individual sites, in that the number of suicides reported for the overall cohort for the experimental group is less than that presented in three local site reports (i.e., two vs three, respectively).
Treatment for alcohol misuse
Evaluation in a single trial of a brief intervention for alcohol misuse in SH patients showed no significant effect for this intervention on repetition of SH, although the proportion of participants repeating SH was somewhat lower in the experimental group (Crawford 2010). However, only 47.1% of those randomised to the experimental group attended the alcohol treatment session. The original trial report did, however, observe a non‐significant trend towards reduced alcohol consumption per drinking day in those allocated to the experimental arm.
Home‐based problem‐solving psychotherapy
In a single trial, home‐based problem‐solving psychotherapy resulted in better treatment adherence than outpatient problem‐solving therapy, but there was no difference in repetition of SH, depression, or suicidal ideation between the groups (Hawton 1981).
Intensive inpatient and community treatment
A single trial compared an intervention involving brief psychiatric admission followed by regular outpatient appointments plus 24‐hour access to a treatment service versus TAU (Van der Sande 1997a). There was no difference between the two groups in repetition of SH, depression, or hopelessness, although more patients in the experimental groups attended at least one outpatient appointment.
General hospital admission
In a single trial, there was no beneficial effect of general hospital admission on repetition of SH or on hopelessness compared with discharge from hospital (Waterhouse 1990). However, as this trial was limited to low‐risk participants, only around 15% of the presenting patients were included.
Intensive outpatient intervention
Two trials compared a combination of intensive therapies, including psychotherapy, behaviour therapy and family therapy, versus standard outpatient care (Allard 1992; Welu 1977). There was no effect of this treatment package on repetition of SH.
Long‐term psychotherapy
In a single trial that compared long‐term therapy (the nature of which was unspecified) versus short‐term intensive therapy, there was no difference in repetition of SH (Torhorst 1988). Estimates of scores from graphs also suggests little difference in depression scores.
Overall completeness and applicability of evidence
Completeness of evidence
There have now been a considerable number of trials of psychosocial interventions for adult SH patients (we identified 55 independent trials). There have been multiple trials of CBT‐based psychotherapy, dialectical behaviour therapy, case management, and postcards. Investigators have also evaluated a wide range of other types of interventions, including attempts to increase adherence with treatment and specific aftercare interventions; however, many of these evaluations have been limited to single trials.
It is important to note that we identified no trials of psychosocial interventions for older (> 60/65 years) adults.
Most trials evaluated a range of relevant primary and secondary outcomes (e.g., repetition of SH, hopelessness, depression). However, they infrequently reported information on suicide, and we had to request it from many authors. In 11 trials, information on repetition of SH was based only on hospital re‐presentations, whereas in a large number of trials this information came from self‐reported data, which in some cases was supplemented by information from clinical and other sources. More episodes of SH will be identified through self‐report compared with information from clinical records, as much SH occurs in the community and does not result in presentation to clinical services (Borges 2011). However, these differences in the recording of SH would not have affected the overall results, as whatever approach was used in the individual trials would have affected the experimental and control arms equally. Also, some trials only assessed repetition during the period in which participants received therapy (e.g., Bateman 2009), whereas for most trials there were further post‐treatment follow‐up assessments.
Acceptability of evidence
The proportions of participants from the two sexes in these trials appears to be in accord with SH patients more generally (Hawton 2007). A number of trials focused on those with a history of multiple episodes of SH, including patients diagnosed with borderline personality disorder; this focus is welcome given that a history of multiple episodes of SH is associated with a particularly high risk of subsequent suicide (Zahl 2004). A number of trials did not record information on suicidal intent of participants (e.g., Carter 2005; Crawford 2010; Dubois 1999; Evans 1999a; Evans 1999b; Gibbons 1978; Gratz 2006; Hassanian‐Moghaddam 2011; Linehan 1991; McLeavey 1994; Morgan 1993; Priebe 2012; Weinberg 2006), which is surprising given the association of SH with future risk of suicide (Carroll 2014; Owens 2002).
Compared to previous versions of this review (Hawton 1998; Hawton 1999; NICE 2011), there is now a greater representation of trials from low‐ to middle‐income countries, including China (Wei 2013; Xu 2012), India (Vijayakumar 2011), Iran (Hassanian‐Moghaddam 2011; Hassanzadeh 2010), Pakistan (Husain 2014), and Sri Lanka (Marasinghe 2012).
It is worth noting that this review focused exclusively on patients who had previously engaged in SH. As a result, we excluded patients with conditions such as borderline personality disorder who had not engaged in SH and mixed trials of patients with either SH or suicidal ideation in the absence of actual SH.
Quality of the evidence
Apart from trials of CBT‐based psychotherapy (18 trials), group‐based emotion‐regulation psychotherapy (two trials), dialectical behaviour therapy (three trials in which DBT was compared with TAU), case management (four trials) and postcards (four trials), all the included trials compared specific interventions, thus limiting the robustness of possible conclusions about their effectiveness compared with routine care (TAU). Also, many trials were too small to detect significant differences in proportions of patients experiencing the primary outcome, namely repetition of SH. Additionally, quality of evidence, as assessed using the GRADE approach, was generally low to moderate, suggesting that further research is likely to have an important impact on our confidence in the estimate of treatment effectiveness and may, in some cases, change the estimates.
Limitations in design and implementation
All 55 included trials were rated as at high risk of bias in relation to at least one aspect of trial design, especially with respect to blinding of both participants and clinical personnel. In part this may reflect the fact that the focus of the present review was on the effectiveness of psychological interventions, and we believe it is generally not possible to blind participants or clinical personnel to psychological therapy. Nevertheless, we cannot rule out performance or detection bias.
Indirectness of evidence
Repetition of SH was measured using either self‐reported information, medical records, or re‐presentation to hospital in all 55 trials included in this review. It is possible that self‐reported information might over‐ or underestimate the real recurrence of SH. On the other hand, use of medical records, hospital presentations, or both may underestimate the real recurrence of SH, as many episodes of SH occur in the community and do not result in presentation to clinical services (Borges 2011). However, these differences in the recording of SH would not have affected the overall results, as whatever approach was used in the individual trials would have affected the experimental and control arms equally.
Trials assessed secondary outcomes using widely validated psychometric measures (e.g., BDI, BHS), which authors did not typically modify in scoring.
Unexplained heterogeneity or inconsistency of results
One meta‐analysis included in the review, effectiveness of CBT‐based psychotherapy on depression scores at 12 months, was associated with substantial levels of heterogeneity (I2= 76%). Excluding Hatcher 2011, which used Zelen's method of randomisation, did not materially affect heterogeneity.
We also conducted sensitivity analyses where visual inspection of the forest plot indicated that one or more trials may have been outliers. For this reason, we excluded the postcard trial by Kapur 2013a from meta‐analyses for repetition of SH both at post‐intervention and at 12‐month follow‐up. In both cases, exclusion of this trial caused the overall estimate of treatment effectiveness for postcard‐based interventions to obtain significance. This could be due to the fact that Kapur 2013a was a small pilot investigation.
We also undertook sensitivity analyses where one or more trials included adolescent participants. Excluding trials for this reason, however, did not appear to systematically explain heterogeneity.
Imprecision of results
Results of the individual trials included in this review were associated with a high level of imprecision as indicated by the wide confidence intervals around the effect size estimates for many of the outcomes reported in this review.
Probability of publication bias
We could only formally evaluate the presence of publication bias for CBT‐based psychotherapy with respect to repetition of SH at six months (Figure 4), 12 months (Figure 5), and final follow‐up (Figure 6), and for depression scores at final follow‐up (Figure 7). In all four cases, some funnel plot asymmetry was evident and particularly seemed to affect the right side of the plot. It is therefore possible that there are unpublished trials in which experimental treatment was ineffective. However, it should also be noted that funnel plot asymmetry could also be due to high levels of heterogeneity.
4.

Funnel plot of comparison 1: CBT‐based psychotherapy vs treatment as usual for repetition of SH at six months
5.

Funnel plot of comparison 1: CBT‐based psychotherapy vs treatment as usual for repetition of SH at 12 months
6.

Funnel plot of comparison 1: CBT‐based psychotherapy vs treatment as usual for repetition of SH at final follow‐up
7.

Funnel plot of comparison 1: CBT‐based psychotherapy vs Treatment as usual for depression scores at final follow‐up.
For one trial of provision of information and support, investigators have not published data from two of the seven study sites (Fleischmann 2008; Bertolote 2010). Therefore we cannot rule out publication bias for this intervention. We are also aware of one further unpublished trial of CBT‐based psychotherapy for which we were unable to obtain the results from the authors. All other trials included in this review have been published in full in peer‐reviewed journals. We therefore believe that publication bias is unlikely to have been a major cause of heterogeneity. Instead, we believe either poor methodological design or true heterogeneity between trials may be responsible for this result (Sterne 2011).
Potential biases in the review process
We have no reason to believe we have not identified all relevant published trials of psychosocial interventions for SH in adults. Nevertheless, by using the random‐effects model in all analyses our results possess greater generalisability than if we had used the fixed‐effect model (Erez 1996). However, because our review criteria included trials in people who had all engaged in SH and presented to clinical services in the preceding six months, we excluded trials where only some of the participants had engaged in SH and also trials where SH was an outcome measured in general psychosocial interventions for patients with psychiatric disorders. Data on repetition of SH were available for all but one of the included trials (Patsiokas 1985). Information on suicides was available for 43 (78.2%) of the trials included in this review, although for most trials this information had to be obtained via correspondence with authors.
Owing to uncertainties about the impact of using Zelen's design, for trials using this approach we analysed data for the primary outcome of repetition of SH using data for both the randomised sample (including both those patients who, following treatment allocation, subsequently consented to participation and those who did not) and the consenting sample only (see Unit of analysis issues section). This typically had little impact on the pattern of results observed.
Due to the large number and varied nature of the interventions included in the earlier versions of this review (Hawton 1998; Hawton 1999) we decided, with agreement of the editors, to divide the review into three (the others reviews being of pharmacological interventions in adults (Hawton 2015b) and both pharmacological and psychosocial interventions for children and adolescents (Hawton 2015a)). Because of the fact that we approached this review with a view to identifying the types of psychosocial interventions that had been evaluated to date in this clinical population, we used a consensus approach to grouping the interventions. This process might have been subject to bias, but in general, there was very good agreement between members of the review group, who have considerable experience in research and clinical practice in relation to SH in adults.
Risk of bias for selective outcome reporting was based on the analyses undertaken by the study authors. As we were unable to include data that had been statistically adjusted for missingness in the present review, we believe it would be unfair to rate trials that made use of statistical adjustments to account for missing data at follow‐up as having high risk of bias for this outcome simply because of our choice of outcome.
Agreements and disagreements with other studies or reviews
There have been several reviews of the efficacy of psychosocial interventions for adult SH patients. None of those that used systematic review methodology to identify all relevant treatment interventions also present meta‐analyses of treatment efficacy (Comtois 2006; Crawford 2007a; Daigle 2011; Hepp 2004; Van der Sande 1997b), aside from Inagaki 2015 and NICE 2011; however, this latter review was conducted using data supplied by our review team during a previous update of the present review. Of two further meta‐analyses, one specifically focused on cognitive behavioural interventions (Tarrier 2008b) and one examined remote contact‐based interventions (Milner 2015).
Inagaki 2015 combined trials of case management, treatment adherence enhancement, and remote contact interventions into one category which the authors termed "active contact and follow‐up" interventions. They concluded that these interventions show promise in reducing repetition at 12 months' follow‐up but not at 24 months' follow‐up. Combining trials of such different treatment approaches, however, is potentially misleading given their very different mechanisms of action. Crawford 2007a also combined different treatment approaches (e.g., CBT‐based psychotherapy, DBT, and adherence enhancement), as well as interventions specifically developed for children and adolescents (e.g., Spirito 2002Wood 2001). The authors concluded there was no evidence of a preventive effect of psychosocial interventions for the prevention of suicide. However, they only assessed efficacy with respect to completed suicide, which few trials are adequately powered to evaluate. Repetition of SH, on the other hand, is a much more common outcome for which a greater number of trials are powered to evaluate.
There is general agreement amongst these reviews concerning the efficacy of CBT‐based psychotherapy. Comtois 2006, for example, concluded there were positive effects for psychotherapy and outreach interventions. However, conclusions concerning the latter type of intervention in this review were particularly influenced by the findings of a single trial in which regular letters were mailed to suicidal patients discharged from psychiatric inpatient care (Motto 2001). As not all participants included in this trial had engaged in SH behaviour, this trial did not meet inclusion criteria for the present review. Tarrier 2008b, moreover, concluded there was evidence for the effectiveness of CBT‐based psychotherapy but only when compared against TAU rather than another form of active psychosocial therapy. Benefits of psychological therapy have recently been reported by findings of a national, non‐randomised naturalistic study in Denmark (Erlangsen 2015).
A recent meta‐analysis of contact‐based interventions found no significant reduction in terms of repetition of SH (both proportion and number of episodes per person) for these interventions (Milner 2015). Surprisingly, however, the review pooled together several different types of contact‐based intervention, including letters, emergency cards, and postcards. Additionally, despite the stated focus of the review being on interventions following SH, it also included trials in which not all participants had engaged in SH prior to inclusion (e.g., Motto 2001; Robinson 2012). Also, for one of the trials included in this review, our correspondence with the original trial authors revealed that data on non‐fatal repetition of SH could not be disaggregated from information on completed suicide (e.g., Chen 2013).
Other reviews have focused specifically on interventions for patients with personality disorder, particularly borderline personality disorder (McMain 2007b; Kliem 2010). They, along with the review by Comtois 2006, concluded that dialectical behaviour therapy was effective for the prevention of SH, although these reviews included some trials in which not all participants had engaged in SH prior to trial entry. Further reviews by Luxton 2013 and Kapur 2010 of postcard, telephone, emergency card, and face‐to‐face interventions concluded they may be effective in preventing suicidal behaviour, although again, these reviews included some trials in which not all participants had engaged in SH at trial entry. The inclusion of these participants means that the focus of the intervention in such trials, and hence the specificity of the findings for SH patients and planning of clinical services for these patients, will be unclear.
Authors' conclusions
Implications for practice.
Evidence drawn from an earlier version of this review has been incorporated in guidance for commissioners of clinical services in the United Kingdom, which states that brief CBT‐based psychotherapy should be available in self‐harm services (NICE 2011). Our updated findings would reinforce the view that there is some evidence to suggest that CBT‐based psychotherapy is effective in reducing repetition of SH compared with treatment as usual (TAU). There is a lack of evidence with respect to the prevention of suicide, however, although there was a relatively small number of such events in these trials, precluding a firm conclusion.
In most trials CBT‐based psychotherapy was typically very brief (i.e., less than 10 sessions). It was delivered on an individual basis in all trials except one (i.e., McAuliffe 2014).
While dialectical behaviour therapy (DBT) did not reduce the proportion of participants with borderline personality disorder who engaged in a repeated episode of SH as compared with TAU, it did appear to reduce the frequency of repetition. Arguably, where patients are multiple repeaters of SH and much of their SH will have included acts such as superficial self‐cutting, reduction in the frequency of SH could be viewed as a key outcome.
Less intensive remote contact‐based interventions, such as sending regular postcards to patients in the year following an episode of SH, did not appear to reduce the proportion of patients repeating SH. However, these interventions may hold promise in settings where there are very limited psychiatric services (where the alternative may be no provision of little or no aftercare). Case management approaches also did not appear to be effective in reducing the proportion of patients who repeat SH.
Other interventions have mostly been evaluated in single trials. In a trial of DBT‐oriented therapy, for example, there was a significant reduction in both the number of patients repeating SH and the total number of repeat episodes, as well as significant reductions in depression and suicidal ideation. Mentalisation therapy was associated with reduced repetition of SH and depression in the latter stages of follow‐up in patients with borderline personality disorder. Group‐based emotion‐regulation psychotherapy was associated with a reduction in the proportion of patients repeating SH in the final two months of the treatment period, but there was no apparent effect on depression. Provision of information and support was associated with reduced numbers of completed suicides in a single multicentre trial conducted in five low‐ and middle‐income countries, but not with reduced repetition of SH. There is, however, some inconsistency in the reporting of numbers of suicides in the experimental group for the overall cohort as reported in Fleischmann 2008 and those from three of the five individual study sites (in Karaj, Iran (Hassanzadeh 2010); Chennai, India (Vijayakumar 2011); and Yuncheng, China (Xu 2012)). Home‐based problem‐solving psychotherapy and continuity of treatment by the same therapist from assessment to aftercare appeared to improve treatment adherence, but without clear benefit in terms of repetition of SH.
Where possible, we analysed results separately by sex. Whilst one trial indicated reduced benefit for the intervention on depression in females as compared to males (Marasinghe 2012), in the few trials where a subgroup analysis by sex was possible, the majority suggested a significant treatment effect of psychosocial interventions for females but not males.
In terms of repeater status, it appears that some more limited interventions (emergency cards) may have negative effects in patients with a history of multiple episodes of SH. However, trials of some more intensive interventions (e.g., group‐based emotion‐regulation psychotherapy, mentalisation, DBT) appear to have positive benefits on repetition of SH, and particularly for participants who were multiple repeaters of SH at trial entry, which would mostly have included participants diagnosed with borderline personality disorder.
Implications for research.
Given the apparent positive benefits of CBT‐based psychotherapy and some other treatment approaches, future trials should identify which types of patients are most likely to benefit from these interventions. Although we were only able to undertake subgroup analyses by sex in five trials (Bennewith 2002; Carter 2005; Fleischmann 2008; Hvid 2011; Marasinghe 2012), evidence from the present review would suggest that some psychosocial interventions, particularly remote contact‐based interventions and case management, tend to be of greater benefit for female patients. There should therefore be a greater focus on sex‐specific interventions, especially to identify treatments most likely to benefit male SH patients.
We could only undertake subgroup analyses by repeater status (i.e., with or without a history of multiple episodes of SH) in five trials (Bennewith 2002; Evans 1999a; Hatcher 2011; Hatcher 2016a; Hatcher 2015). Nevertheless, there is limited evidence that whilst some forms of psychosocial interventions may be more effective in those with a history of multiple episodes of SH (e.g., CBT‐based psychotherapy) other forms of contact‐based interventions, such as emergency cards, may lead to an increased risk of repetition of SH in those with a history of multiple episodes of SH prior to trial entry. For these reasons, history of prior SH should be clearly identified in future trials, with stratified randomisation according to repeater status being desirable.
Researchers investigating psychosocial treatments should endeavour to investigate whether the intervention results in changes in the psychological or social mechanisms that are the targets of treatment (e.g., problem‐solving, emotion‐regulation, interpersonal skills) and the extent to which such changes relate to positive outcomes (Arensman 2001). Such knowledge will help clarify the mediators of treatment efficacy and allow therapists to modify interventions so that they may be more effective.
In view of the apparent effectiveness of CBT‐based psychotherapy in reducing repetition of SH and the development of online therapy for a range of psychological problems (Andersson 2014; Griffiths 2006), and the introduction online means of providing this therapy should be a priority, particularly given the findings of some short‐term benefits of online self‐help for suicidal thoughts (Van Spijker 2014) and behaviours (Franklin 2016), although these effects may not be maintained at longer‐term follow‐ups. The longer term effectiveness of these interventions is therefore yet to be determined.
What's new
| Date | Event | Description |
|---|---|---|
| 13 September 2021 | Amended | There is a more recent Cochrane review on this topic: https://doi.org/10.1002/14651858.CD013668.pub2 An editorial note has been added to redirect readers. |
History
Review first published: Issue 5, 2016
| Date | Event | Description |
|---|---|---|
| 26 February 2016 | New search has been performed | Original review CD001764 was split into three and the searches and methodology updated. |
Notes
Acknowledgements
We thank the following for providing us with unpublished data and other information: Anthony Bateman, Ryan Barnhart, Kirsten Barnicot, Annette Beautrais, Olive Bennewith, Greg Carter, Marie Cedereke, Tom Clarke, Mike Crawford, Kate Davidson, Elspeth Guthrie, Tony Fitzgerald, Alexandra Fleischmann, Peter Fonagy, Kim Gratz, Melanie Harned, Simon Hatcher, Nusrat Husain, Nav Kapur, Chiaki Kawanishi, Marsha Linehan, Rohana Marasinghe, Shelley McMain, Stefan Priebe, Nadja Slee, Carmen Stewart, Vojna Tapolaa, Barbara Tomenson, Peter Tyrer, Guillaume Vaiva, Lakshmi Vijayakumar, August Wang, Igor Weinberg, and Dong Xu.
We also wish to thank Andrea Cipriani, Jane Dennis, Jessica Sharp, and Catroina Shatford for advice on data extraction and management issues. We also wish to thank Thorsten Barnhofer, Zheng Chang, Carolyn Guillo, and Ka Liu for translating articles.
This project has previously had support from the National Co‐ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO). KH is funded by Oxford Health NHS Foundation Trust. He is a National Institute for Health (NIHR) Senior Investigator, and personal funding from NIHR helped support this update. The opinions expressed are solely those of the authors.
Appendices
Appendix 1. CCDANCTR Search Strategy
CCDANCTR Date range searched: 01.01.56 to 29.04.15
1. ((deliberat* or self*) NEXT (destruct* or harm* or injur* or mutilat* or poison*)):ab,ti,kw,ky,emt,mh,mc 2. DSH:ab 3. (parasuicid* or "para suicid*") 4. (suicid* NEAR2 (attempt* or episod* or frequen* or future or histor* or multiple or previous* or recur* or repeat* or repetition)):ab,ti,kw,ky,emt,mh,mc 5. "post suicid*" 6. (suicid* and (BPD or "borderline personality disorder")) 7. (overdos* or "over dos*") 8. ((crisis or suicid*) NEAR (emergenc* or hospital or outpatient or "repeat* attend*" or "frequent* attend*" )): ab,ti,kw,ky,emt,mh,mc 9. (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8) [ab:abstract; ti:title; kw:keywords; ky:additional keywords; emt:EMTREE headings; mh:MeSH headings; mc:MeSH checkwords]
Appendix 2. EMBASE, MEDLINE, PreMEDLINE, PsycINFO and CENTRAL Search Strategies
Search Strategy 2012 to 2013:
EMBASE, MEDLINE, PreMEDLINE, PsycINFO (OVID SP interface)
Date range searched: 01.01.1998 to 13.10.2014.
1. automutilation/ or drug overdose/ or exp suicidal behavior/ 2. 1 use emez 3. overdose/ or self‐injurious behavior/ or self‐mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ 4. 3 use mesz, prem 5. drug overdoses/ or self destructive behavior/ or exp self injurious behavior/ or attempted suicide/ or suicidal ideation/ or suicide/ or suicide prevention/ or suicide prevention centers/ or suicidology/ 6. 5 use psyh 7. (auto mutilat$ or automutilat$ or cutt$ or head bang$ or head bang$ or overdos$ or (self adj2 cut$) or self destruct$ or selfdestruct$ or self‐harm$ or selfharm$ or self immolat$ or selfimmolat$ or self inflict$ or selfinflict$ or self injur$ or selfinjur$ or selfmutilat$ or self mutilat$ or self poison$ or selfpoison$ or suicid$).ti,ab. 8. or/2,4,6‐7 9. exp "clinical trial (topic)"/ or exp clinical trial/ or crossover procedure/ or double blind procedure/ or placebo/ or randomization/ or random sample/ or single blind procedure/ 10. 9 use emez 11. exp clinical trial/ or exp "clinical trials as topic"/ or cross‐over studies/ or double‐blind method/ or placebos/ or random allocation/ or single‐blind method/ 12. 11 use mesz, prem 13. (clinical trials or placebo or random sampling).sh,id. 14. 13 use psyh 15. (clinical adj2 trial$).ti,ab. 16. (crossover or cross over).ti,ab. 17. (((single$ or doubl$ or trebl$ or tripl$) adj2 blind$) or mask$ or dummy or doubleblind$ or singleblind$ or trebleblind$ or tripleblind$).ti,ab. 18. (placebo$ or random$).ti,ab. 19. treatment outcome$.md. use psyh 20. animals/ not human$.mp. use emez 21. animal$/ not human$/ use mesz 22. (animal not human).po. use psyh 23. (or/10,12,14‐19) not (or/20‐22) 24. 8 and 23
CENTRAL (Wiley interface)
Date range searched: 01.01.1998 to 13.10.2014.
#1. MeSH descriptor: [Drug Overdose], this term only #2. MeSH descriptor: [Self‐Injurious Behavior], this term only #3. MeSH descriptor: [Self Mutilation], this term only #4. MeSH descriptor: [Suicide], this term only #5. MeSH descriptor: [Suicide, Attempted], this term only #6. MeSH descriptor: [Suicidal Ideation], this term only #7. auto mutilat*" or automutilat* or cutt* or "head bang*" or headbang* or overdos* or "self destruct*" or selfdestruct* or "self‐harm*" or selfharm* or "self immolat*" or selfimmolat* or "self inflict*" or selfinflict* or "self injur*" or selfinjur* or selfmutilat* or "self mutilat*" or "self poison*" or selfpoison* or suicid*:ti #8. "auto mutilat*" or automutilat* or cutt* or "head bang*" or "head bang*" or overdos* or "self destruct*" or selfdestruct* or "self‐harm*" or selfharm* or "self immolat*" or selfimmolat* or "self inflict*" or selfinflict* or "self injur*" or selfinjur* or selfmutilat* or "self mutilat*" or "self poison*" or selfpoison* or suicid*:ab #9. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
Appendix 3. Journals hand‐searched for relevant literature in the original version of this review
1. Archives of Suicide Research (1995‐1998); 2. Crisis (1980‐1998); 3. Suicide & Life‐Threatening Behavior (1971‐1998); 4. Der Nervenarzt (1950‐1979); 5. Journal of Adolescence (1978‐1996); 6. Journal of Affective Disorders (1994‐1996); 7. Journal of the American Academy of Child and Adolescent Psychiatry (1978‐1996); 8. Journal of Clinical Psychiatry (1978‐1996); 9. Journal of Psychiatric Research (1961‐1972) and (1985‐1996); 10. Social Psychiatry (1966‐1987), and 11. Social Psychiatry & Psychiatric Epidemiology (1988‐1996).
Appendix 4. Data collection and analysis methods used for the original review
Selection of studies
In the original version of this review, Sarah Stockton, Librarian at the University of Oxford, conducted the systematic search for trials. Two out of TTS, EA, ET, and KH then independently screened the titles of identified trials for relevancy. A distinction was made between:
1. eligible studies, in which any psychological and/or psychopharmacological treatment was compared with a control (e.g., standard or less intensive types of aftercare or medication); and
2. general treatment studies, without any control treatment.
A second screening was then undertaken in which two of TTS, EA, ET, and KH independently screened the full text of relevant studies with reference to the following inclusion criteria:
1. All participants must have engaged in SH (self‐poisoning or self‐injury) shortly prior to randomisation.
2. Studies must have reported the number of participants engaging in a repeat episode of SH as an outcome measure.
3. Study participants must have been randomised to the treatment and control groups.
Data extraction and management
Data extraction was carried out by EA and second member of the review group (TTS, ET, or KH) using a standardised data extraction form. Members of the review team extracted data independently from one another. Disputes were resolved through consensus discussions with a third member of the review group with assistance from the CCDAN editorial base.
We extracted data from each eligible trial concerning the characteristics of patients, the details of the interventions used, and information on the number of participants engaging in a repeat episode of SH during the follow‐up period. Where these details were unclear, corresponding authors were contacted to provide additional clarification.
Assessment of risk of bias
For the original version of this review, three independent review authors (EA and ET plus another member of the review group) rated the quality of the studies. Review authors were blind to authorship according to the recommended Cochrane criteria for quality assessment (Sackett 1997).
Given that the quality of allocation concealment can affect the results of trials (Schulz 1995), studies were assigned a quality of concealment rating ranging from C (poor quality) to A (high quality). Trials rated as inadequately concealed, for example via reference to an open random number table, were given a rating of C. Trials that did not provide adequate details about how the randomisation procedure was carried out were given a rating of B, and trials that were deemed to have taken adequate measures to conceal allocation, for example through the use of serially numbered, opaque, sealed envelopes; numbered or coded bottles or containers, were rated as A quality. Where the concealment of allocation was not clearly reported (i.e., where trials were initially in category B), we contacted corresponding authors for more information. Where raters disagreed as to the category to which a trial had been allocated, the final rating was made by consensus discussion in consultation with TS, KH, and a third member of the review group.
Measures of treatment effect
RevMan, version 3.0, was used to calculate summary odds ratios and accompanying 95% CIs for the number of participants engaging in a repeat episode of SH during the follow‐up period.
Unit of analysis issues
1. Cluster trials
Clustering was an issue in one included study (Bennewith 2002); however, as the authors reported adjusting for the effects of clustering in their primary analyses, we reproduced the data from this study as if it came from a non‐cluster randomised study.
2. Studies with multiple treatment groups
One included study presented data for multiple treatment groups (Hirsch 1982). As both treatment groups were prescribed antidepressants in this study, we combined the data from these two treatment arms.
Dealing with missing data
Where data on the primary outcome measure were incomplete or excluded from the study, corresponding author(s) were contacted to obtain further information. Some authors used intention‐to‐treat analyses to account for missing data using a variety of different methods which will be discussed within the 'Risk of bias' tables. We as review authors did not attempt to impute data for those studies in which intention‐to‐treat analyses had not been conducted, however. Instead, the effects of missing data will be discussed in the text of the review.
Assessment of heterogeneity
Clinical heterogeneity was examined using the Chi2 statistic. Where this statistic was significant, we investigated potential causes of heterogeneity as outlined in the 'Subgroup analysis and investigation of heterogeneity' section below.
Assessment of reporting biases
To assess whether any meta‐analysis reported in this review are affected by reporting bias, we planned to construct funnel plots to investigate the likelihood that the results of our meta‐analysis were affected by reporting bias. We were unable to undertake these analyses, however, due to the very small number of trials included in our meta‐analyses.
Data synthesis
The Mantel‐Haenszel fixed‐effect method was used to calculate pooled summary ORs and accompanying 95% CIs.
Subgroup analysis and investigation of heterogeneity
In analyses resulting in significant heterogeneity, as indicated by the Chi2 statistic, an investigation into the source of this heterogeneity was conducted. We had planned to conduct subgroup analyses by repeater status and sex; however, there were insufficient studies with appropriate data to enable these analyses to be undertaken.
Sensitivity analysis
Sensitivity analyses were undertaken where appropriate (e.g., in relation to risk of bias of included trials in the relative intensity of treatment).
Data and analyses
Comparison 1. Cognitive behavioural therapy (CBT)‐based psychotherapy vs. treatment as usual (TAU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Repetition of SH at 6 months | 12 | 1317 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.85] |
| 1.1.1 Individual psychotherapy | 11 | 1083 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.36, 0.75] |
| 1.1.2 Group‐based psychotherapy | 1 | 234 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.75, 2.41] |
| 1.2 Repetition of SH at 12 months | 10 | 2232 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.65, 0.98] |
| 1.2.1 Individual psychotherapy | 9 | 1799 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.94] |
| 1.2.2 Group‐based psychotherapy | 1 | 433 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.67, 1.61] |
| 1.3 Repetition of SH at 24 months | 2 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.14, 0.69] |
| 1.3.1 Indivdual psychotherapy | 2 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.14, 0.69] |
| 1.4 Repetition of SH at final follow‐up | 17 | 2665 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.55, 0.88] |
| 1.4.1 Individual psychotherapy | 16 | 2232 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.53, 0.84] |
| 1.4.2 Group‐based psychotherapy | 1 | 433 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.67, 1.61] |
| 1.5 Frequency of SH at final follow‐up | 6 | 594 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.68, 0.26] |
| 1.5.1 Individual psychotherapy | 5 | 161 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.71, 0.40] |
| 1.5.2 Group‐based psychotherapy | 1 | 433 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.32, 0.20] |
| 1.6 Depression scores at 6 months | 11 | 1668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.50, ‐0.10] |
| 1.6.1 Individual psychotherapy | 10 | 1434 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.56, ‐0.11] |
| 1.6.2 Group‐based psychotherapy | 1 | 234 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.39, 0.13] |
| 1.7 Depression scores at 12 months | 7 | 1130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.64, ‐0.07] |
| 1.7.1 Individual psychotherapy | 7 | 1130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.64, ‐0.07] |
| 1.8 Depression scores at 24 months | 2 | 225 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.48, 0.05] |
| 1.8.1 Individual psychotherapy | 2 | 225 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.48, 0.05] |
| 1.9 Depression scores at final follow‐up | 14 | 1859 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.48, ‐0.14] |
| 1.9.1 Individual psychotherapy | 13 | 1625 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.54, ‐0.16] |
| 1.9.2 Group‐based psychotherapy | 1 | 234 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.39, 0.13] |
| 1.10 Hopelessness scores at post‐intervention | 3 | 360 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐3.62, 0.61] |
| 1.10.1 Individual psychotherapy | 2 | 47 | Mean Difference (IV, Random, 95% CI) | ‐4.23 [‐8.71, 0.25] |
| 1.10.2 Group‐based psychotherapy | 1 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.17, 0.57] |
| 1.11 Hopelessness scores at 6 months | 4 | 968 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.58, ‐0.13] |
| 1.11.1 Individual psychotherapy | 3 | 734 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.63, ‐0.33] |
| 1.11.2 Group‐based psychotherapy | 1 | 234 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.31, 0.21] |
| 1.12 Hopelessness scores at 12 months | 3 | 539 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐2.97, ‐0.81] |
| 1.12.1 Individual psychotherapy | 3 | 539 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐2.97, ‐0.81] |
| 1.13 Hopelessness scores at final follow‐up | 7 | 1017 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.51, ‐0.10] |
| 1.13.1 Individual psychotherapy | 6 | 783 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.60, ‐0.16] |
| 1.13.2 Group‐based psychotherapy | 1 | 234 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.31, 0.21] |
| 1.14 Suicidal ideation scores at post‐intervention | 3 | 360 | Mean Difference (IV, Random, 95% CI) | ‐2.52 [‐5.60, 0.56] |
| 1.14.1 Individual psychotherapy | 2 | 47 | Mean Difference (IV, Random, 95% CI) | ‐5.92 [‐11.98, 0.14] |
| 1.14.2 Group‐based psychotherapy | 1 | 313 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐3.50, 0.50] |
| 1.15 Suicidal ideation scores at 6 months | 6 | 1011 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.51, ‐0.13] |
| 1.15.1 Individual psychotherapy | 5 | 777 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.55, ‐0.27] |
| 1.15.2 Group‐based psychotherapy | 1 | 234 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.28, 0.24] |
| 1.16 Suicidal ideation scores at final follow‐up | 8 | 1131 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.47, ‐0.09] |
| 1.16.1 Individual psychotherapy | 7 | 818 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.55, ‐0.15] |
| 1.16.2 Group‐based psychotherapy | 1 | 313 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.24, 0.20] |
| 1.17 Proportion with improved problems at 6 months | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 2.81 [1.50, 5.24] |
| 1.17.1 Individual psychotherapy | 2 | 231 | Odds Ratio (M‐H, Random, 95% CI) | 2.81 [1.50, 5.24] |
| 1.18 Proportion with improved problems at final follow‐up | 2 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 3.03 [0.74, 12.41] |
| 1.18.1 Individual psychotherapy | 2 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 3.03 [0.74, 12.41] |
| 1.19 Problem‐solving scores at post‐intervention | 2 | 328 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.07, 0.36] |
| 1.19.1 Individual psychotherapy | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.79, 1.37] |
| 1.19.2 Group‐based psychotherapy | 1 | 313 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.08, 0.36] |
| 1.20 Problem‐solving scores at 6 months | 4 | 949 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.08, 0.58] |
| 1.20.1 Individual psychotherapy | 3 | 715 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.30, 0.60] |
| 1.20.2 Group‐based psychotherapy | 1 | 234 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.24, 0.28] |
| 1.21 Problem‐solving scores at final follow‐up | 5 | 958 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.02, 0.50] |
| 1.21.1 Individual psychotherapy | 4 | 724 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.04, 0.66] |
| 1.21.2 Group‐based psychotherapy | 1 | 234 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.24, 0.28] |
| 1.22 Suicide at final follow‐up | 15 | 2354 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.29, 1.51] |
| 1.22.1 Individual psychotherapy | 14 | 1921 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.29, 1.67] |
| 1.22.2 Group‐based psychotherapy | 1 | 433 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.25] |
Comparison 2. Interventions for multiple repetition of self‐harm (SH)/probable personality disorder vs treatment as usual (TAU) or other alternative forms of psychotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Repetition of SH at post‐intervention | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 Group‐based emotion‐regulation psychotherapy vs TAU | 2 | 83 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.13, 0.88] |
| 2.1.2 Mentalisation vs TAU | 1 | 134 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.17, 0.73] |
| 2.1.3 DBT‐oriented therapy vs Alternative forms of psychotherapy | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.49] |
| 2.1.4 DBT vs TAU | 3 | 267 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.16, 2.15] |
| 2.1.5 DBT vs treatment by expert | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [0.53, 5.20] |
| 2.1.6 DBT prolonged exposure vs DBT standard exposure | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.08, 5.68] |
| 2.2 Repetition of SH at 6 months | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2.1 DBT prolonged exposure vs DBT standard exposure | 1 | 18 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.08, 5.68] |
| 2.3 Repetition of SH at 12 months | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 DBT vs. TAU | 2 | 172 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.05, 2.47] |
| 2.3.2 DBT vs treatment by expert | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.35, 3.95] |
| 2.4 Repetition of SH at final follow‐up | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 DBT vs TAU | 3 | 247 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.21, 1.59] |
| 2.5 Frequency of repetition of SH at post‐intervention | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 Group‐based emotion‐regulation psychotherapy vs TAU | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐12.76 [‐34.92, 9.40] |
| 2.5.2 Mentalisaiton vs TAU | 1 | 134 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐2.01, ‐0.55] |
| 2.5.3 DBT‐oriented therapy vs Alternative forms of psychotherapy | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐4.83 [‐7.90, ‐1.76] |
| 2.5.4 DBT vs TAU | 3 | 292 | Mean Difference (IV, Random, 95% CI) | ‐18.82 [‐36.68, ‐0.95] |
| 2.5.5 DBT vs treatment by expert | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐14.85 [‐37.64, 7.94] |
| 2.5.6 DBT prolonged exposure vs DBT standard exposure | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐2.47, 1.97] |
| 2.6 Frequency of repetition of SH at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.6.1 DBT prolonged exposure vs DBT standard exposure | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.61, 1.29] |
| 2.7 Number completing full course of treatment | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.7.1 Mentalisation vs TAU | 1 | 134 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.43, 2.02] |
| 2.7.2 DBT‐oriented therapy vs TAU | 1 | 24 | Odds Ratio (M‐H, Random, 95% CI) | 3.00 [0.53, 16.90] |
| 2.7.3 DBT prolonged exposure vs DBT standard exposure | 1 | 26 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.22, 5.84] |
| 2.8 Depression scores at post‐intervention | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.8.1 Group‐based emotion‐regulation psychotherapy vs TAU | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐9.59 [‐13.43, ‐5.75] |
| 2.8.2 Mentalisaiton vs TAU | 1 | 134 | Mean Difference (IV, Random, 95% CI) | ‐3.88 [‐6.82, ‐0.94] |
| 2.8.3 DBT‐oriented therapy vs Alternative forms of psychotherapy | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐9.16 [‐14.79, ‐3.53] |
| 2.8.4 DBT vs TAU | 2 | 198 | Mean Difference (IV, Random, 95% CI) | ‐2.37 [‐6.52, 1.78] |
| 2.8.5 DBT vs treatment by expert | 1 | 89 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐6.27, 0.27] |
| 2.8.6 DBT prolonged exposure vs DBT standard exposure | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐10.59, 3.19] |
| 2.9 Depression scores at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.9.1 DBT prolonged exposure vs. DBT standard exposure | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐9.68, 1.08] |
| 2.10 Depression scores at 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.10.1 DBT vs treatment by expert | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐5.40, 1.80] |
| 2.11 Suicide ideation scores at post‐intervention | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.11.1 DBT‐oriented therapy vs Alternative forms of psychotherapy | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐7.75 [‐14.66, ‐0.84] |
| 2.11.2 DBT vs treatment by expert | 1 | 89 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐13.69, 7.69] |
| 2.12 Suicide ideation scores at 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.12.1 DBT vs treatment by expert | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐7.82 [‐18.38, 2.74] |
| 2.13 Suicide at post‐intervention | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.13.1 DBT vs TAU | 3 | 317 | Odds Ratio (M‐H, Random, 95% CI) | 3.00 [0.12, 76.49] |
| 2.14 Suicide at 6 months | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.14.1 DBT prolonged exposure vs DBT standard exposure | 1 | 26 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.01, 4.41] |
Comparison 3. Case management vs treatment as usual (TAU) or other alternative forms of psychotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Repetition of SH at post‐intervention | 4 | 1608 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.47, 1.30] |
| 3.1.1 Case management plus assertive outreach vs TAU | 3 | 843 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.38, 1.78] |
| 3.1.2 Case management plus assertive outreach vs enhanced usual care | 1 | 765 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.40, 1.10] |
| 3.2 Suicide at post‐intervention | 4 | 1757 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.57, 1.57] |
| 3.2.1 Case management plus assertive outreach vs TAU | 3 | 843 | Odds Ratio (M‐H, Random, 95% CI) | 1.77 [0.36, 8.68] |
| 3.2.2 Case management plus assertive outreach vs enhanced usual care | 1 | 914 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.52, 1.51] |
Comparison 4. Treatment adherence enhancement approaches vs treatment as usual (TAU) or other alternative forms of psychotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Repetition of SH at 12 months | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1.1 Adherence enhancement vs TAU | 1 | 391 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.32, 1.02] |
| 4.1.2 Continuity of care by the same therapist vs other alternative forms of psychotherapy | 1 | 136 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.07, 1.10] |
| 4.2 Depression scores at 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.2.1 Continuity of care by the same therapist vs other alternative forms of psychotherapy | 1 | 127 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐4.24, 1.44] |
| 4.3 Suicide at 12 months | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.3.1 Adherence enhancement vs TAU | 1 | 391 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.28, 2.57] |
| 4.3.2 Continuity of care by the same therapist vs other alternative forms of psychotherapy | 1 | 136 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.10, 3.82] |
Comparison 5. Remote contact interventions vs treatment as usual (TAU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Repetition of SH at post‐intervention | 8 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1.1 Postcards vs TAU | 4 | 3277 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.62, 1.23] |
| 5.1.2 Emergency cards vs TAU | 2 | 1039 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.31, 2.14] |
| 5.1.3 GP letter vs TAU | 1 | 1932 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.93, 1.44] |
| 5.1.4 Mobile telephone‐based psychotherapy vs TAU | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
| 5.2 Repetition of SH at 12 months | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.2.1 Postcards vs TAU | 2 | 2885 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.02] |
| 5.2.2 Emergency cards vs TAU | 1 | 827 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.85, 1.67] |
| 5.2.3 Telephone contact vs TAU | 1 | 172 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.45, 2.23] |
| 5.3 Repetition of SH at final follow‐up | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.3.1 Postcards vs TAU | 4 | 3277 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.62, 1.25] |
| 5.3.2 Telephone contact vs TAU | 3 | 840 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.32] |
| 5.4 Frequency of SH at post‐intervention | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.4.1 Postcards vs TAU | 3 | 1097 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.32, 0.18] |
| 5.4.2 Postcards vs TAU (males only) | 3 | 401 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.13, 0.12] |
| 5.4.3 Postcards vs TAU (females only) | 3 | 695 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.29, 0.20] |
| 5.4.4 Postcards vs TAU (history of prior SH) | 3 | 339 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.68, 0.51] |
| 5.4.5 Postcards vs TAU (no history of prior SH) | 3 | 758 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.32, 0.77] |
| 5.5 Frequency of SH at 12 months | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.5.1 Postcards vs TAU | 2 | 984 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.58, 0.20] |
| 5.5.2 Postcards vs TAU (males only) | 2 | 336 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.11, 0.16] |
| 5.5.3 Postcards vs TAU (females only) | 2 | 647 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.62, 0.18] |
| 5.5.4 Postcards vs TAU (history of prior SH) | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐2.07, 0.80] |
| 5.5.5 Postcards vs TAU (no history of prior SH) | 2 | 688 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.22, 0.09] |
| 5.6 Suicide at post‐intervention | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.6.1 Postcards vs TAU | 4 | 3464 | Odds Ratio (M‐H, Random, 95% CI) | 1.86 [0.61, 5.72] |
| 5.6.2 Mobile telephone‐based psychotherapy vs TAU | 1 | 68 | Odds Ratio (M‐H, Random, 95% CI) | 3.09 [0.12, 78.55] |
| 5.7 Suicide at 12 months | 1 | 772 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.08, 2.15] |
| 5.7.1 Postcards vs TAU | 1 | 772 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.08, 2.15] |
| 5.8 Suicide at final follow‐up | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.8.1 Telephone contact vs TAU | 2 | 821 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.11, 4.33] |
Comparison 6. Other mixed interventions versus treatment as usual (TAU) or other alternative forms of psychotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Repetition of SH at final follow‐up | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1.1 Intensive outpatient intervention vs TAU | 2 | 245 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.15, 2.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allard 1992.
| Study characteristics | ||
| Methods |
Allocation: random assignment using sealed and numbered envelopes Follow‐up period: 24 months after trial entry N lost to follow‐up: 24/150 (16%) for repetition data |
|
| Participants |
Inclusion criteria: i) resident in catchment area of hospital; ii) able to speak French or English; iii) no physical handicap preventing attendance; iv) not already in institutional care; v) capacity to give informed consent; vi) not sociopathic; vii) suicide attempt was made within one week prior to trial entry Exclusion criteria: i) no fixed address; ii) expecting to move out of the catchment area; iii) in the care of an institution that ensures follow‐up after all suicide attempts; iv) diagnosed with a physical disability that would prevent attendance at follow‐up sessions; v) unable to provide informed consent; vi) diagnosed with sociopathy and presents a physical threat to hospital personnel; vii) suicide attempt occurred a week or more prior to randomisation Numbers: Of the 150 participants, 76 were allocated to the experimental arm and 74 to the control arm. Profile: 55% (n = 83) were female. 50% (n = 75) were repeaters. 87% (n = 131) had diagnosis of depression, 53% (n = 80) substance abuse diagnosis, and 45% (n = 68) were diagnosed with a personality disorder. Source of participants: patients presenting to hospital following a suicide attempt Location: Montreal, Canada |
|
| Interventions |
Experimental: intensive intervention involving a schedule of visits, including at least one home visit. Therapy provided where needed. Reminders (telephone or written) and home visits were made in case of missed appointments. Control: treatment by the regular personnel within the same hospital Therapist: 1 social worker Type of therapy offered: various interventions offered to participants in the experimental arm, including: psychoanalytic psychotherapy, psychosocial, drug therapy, behavioural therapy, or a combination of these Length of treatment: 12 months |
|
| Outcomes |
Included: i) repetition of SH according to hospital records, Coroner's office records, interviews with participants or collateral informants, or a combination of these; ii) suicide; iii) compliance measured as encounters with therapist Excluded: none |
|
| Notes |
Sources of funding: no details on funding are provided Declaration of author interests: no details on author interests are provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Subjects were randomly assigned either to the intensive intervention group or to the comparison group, using sealed and numbered envelopes" (p. 306) Comment: No mention of how the sequence was generated or how envelopes were numbered. It is therefore unclear if the allocation sequence was adequately generated. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Subjects were randomly assigned either to the intensive intervention group or to the comparison group, using sealed and numbered envelopes" (p. 306) Comment: No mention of whether the envelopes were opaque or not, although they probably were. |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "If in the experimental group, subjects. . . were put under the care of the project team (two staff psychiatrists and a social worker); if in the comparison group, they were treated by other personnel" (p. 306) Comment: It is not known whether the participants were aware they were being treated by a different team or not, although the nature of the intervention means it is likely participants were aware of which treatment group they had been assigned to. |
| Blinding (performance bias and detection bias) Of personnel | High risk |
Quote: "If in the experimental group, subjects. . . were put under the care of the project team (two staff psychiatrists and a social worker); if in the comparison group, they were treated by other personnel" (p. 306) Comment: Personnel would have been aware of which team they had been assigned to. |
| Blinding (performance bias and detection bias) Of outcome assessors | High risk |
Quote: "For most of the study, patients in the experimental group were interviewed at 12 months by their psychiatrist (instead of the research assistant)" (p. 307) Comment: If personnel were also acting as outcome assessors they would not have been blinded to allocation for the above reason. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "All comparisons between groups were made on an intention‐to‐treat basis" (p. 307) Comment: Within the section on 'Losses to follow‐up', the authors state that follow‐up information was not available for 24 participants. No reasons for dropouts were provided in this section. The authors do, however, assert these losses were unlikely to introduce bias and "unlikely to affect the comparisons between the two groups" (pp. 308‐309) as dropouts (who shared a similar demographic profile) were "equally distributed between groups". |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not reported; however, in the absence of the trial protocol this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Bateman 2009.
| Study characteristics | ||
| Methods |
Allocation: offsite random assignment made using a stochastic minimisation programme (MINIM) balanced for age (18‐25, 26‐30, and > 30 years), sex, and diagnosis of antisocial personality disorder Follow‐up period: 6, 12, and 18 months N lost to follow‐up: 8/134 (6.0%) at the 18‐month follow‐up period |
|
| Participants |
Inclusion criteria: i) diagnosed with borderline personality disorder; ii) made a suicide attempt or an episode of SH within 6 months prior to randomisation; iii) aged 18‐65 years Exclusion criteria: i) currently in long‐term psychotherapeutic treatment; ii) met DSM‐IV criteria for any psychosis or bipolar I disorder; iii) dependent on any opiate to such a degree that specialist treatment was required; iv) presence of a mental impairment or evidence of an organic brain disorder Numbers: Of the 134 participants, 71 were allocated to the experimental arm and 63 to the control arm. Profile: 80% (n = 107) were female,100% (n = 134) were multiple repeaters, 56% (n = 75) were diagnosed with major depression, 77% (n = 103) were diagnosed with a milder depressive disorder such as dysthymia, 14% (n = 19) were diagnosed with post‐traumatic stress disorder, 61% (n = 82) were diagnosed with an anxiety disorder, 54% (n = 72) were diagnosed with a substance use disorder, 28% (n = 37) were diagnosed with an eating disorder, 13% (n = 17) were diagnosed with somatoform disorder, and 28% (n = 37) were diagnosed with comorbid antisocial personality disorder Source of participants: consecutive referrals to 1 of 2 community outpatient psychiatric facilities, 1 of which provides specific treatment for personality disorder Location: London, UK |
|
| Interventions |
Experimental: mentalisation‐based treatment involving weekly individual and group sessions of psychotherapy. Participants were also prescribed medication (e.g., antidepressants, antipsychotics, mood stabilisers, minor tranquillizers) as needed. Control: structured case management involving 3 monthly individual and group sessions based on a counselling model resembling a supportive approach combined with case management, advocacy support, and problem‐solving psychotherapy. Participants were also prescribed any medication (e.g., antidepressants, antipsychotics, mood stabilisers, minor tranquillizers) as needed. Therapist: 2 psychotherapists Type of therapy offered: mentalisation‐based psychotherapy Length of treatment: 18 months |
|
| Outcomes |
Included: i) repetition of SH, ii) repetition of suicide attempts; iii) suicides; iv). compliance; v) depression scores. Excluded: i) psychiatric readmissions; ii) length of psychiatric readmissions; iii) medication use; iv) social functioning scores; v) symptom distress scores; vi) social adjustment scores; vii) interpersonal functioning scores |
|
| Notes |
Sources of funding: "Supported by a grant from the Borderline Personality Disorder Research Foundation" (p. 1363). Declaration of author interests: none stated Other: repetition data for SH, suicide attempts, and completed suicides was obtained through correspondence with Dr Fonagy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Treatment allocation was made offsite via telephone randomisation using a stochastic minimization program (MINIM)" (p. 1356). Comment: Use of a computerised algorithm is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Treatment allocation was made offsite" (p. 1356). Comment: Use of offsite allocation is likely to have ensured allocation was adequately concealed. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: no mention of participant blinding; however, the nature of the trial means it is likely participants were aware of which treatment group they had been assigned to |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: No mention of personnel blinding is made; however, the nature of the trial means it is likely that therapists knew which treatment they were providing |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Independent evaluators blind to treatment allocation conducted assessments." (p. 1355). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All results were analysed using an intention‐to‐treat analysis based on treatment assignment" (p. 1359). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not reported; however, in the absence of the trial protocol this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Beautrais 2010.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a predetermined computer‐generated random number procedure Follow‐up period: 12 months N lost to follow‐up: 0/327 (0%) for repetition of SH |
|
| Participants |
Inclusion criteria: i) aged 16 or older; ii) admitted to a psychiatric emergency service following an episode of SH or attempted suicide; iii) resident in New Zealand; iv) able to understand English well enough to provide informed consent Exclusion criteria: none stated Numbers: Of the 327 participants, 153 were allocated to the experimental arm and 174 were allocated to the control arm. Profile: 66.0% (n = 216) were female, 17.7% (n = 58) were multiple repeaters Source of participants: patients admitted to a psychiatric emergency service following an episode of SH or attempted suicide Location: Christchurch, New Zealand |
|
| Interventions |
Experimental: postcards mailed at 2 and 6 weeks and 3, 6, 9, and 12 months after discharge in addition to usual care Control: TAU involving crisis assessment and referral to inpatient community‐based mental health services as required Therapist: none Type of therapy offered: outreach through the mailing of frequent postcards encouraging participants to make contact with the service Length of treatment: 12 months |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide Excluded: i) number of re‐presentations to psychiatric emergency services |
|
| Notes |
Source of funding: "This study was supported by grants from the Canterbury District Health Board and the Accident Compensation Corporation (ACC). S.J.G. was supported by a University of Otago Postgraduate Publishing Bursary" (p. 59). Declaration of author interests: none stated. Other: Data on repetition of SH were obtained from psychiatric emergency service records, hospital medical records, or both. Data on suicides were obtained following correspondence with authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Participants were randomised 1:1 . . . using predetermined computer‐generated random numbers. . . The number sequence was computer‐generated in SAS 9.1 for Windows using a uniform distribution to generate a sequence of random numbers between 0 and 1. Numbers of 0.5 or above were classified as the intervention group; numbers below 0.5 were classified as the control group." (p. 56). Comment: Use of a computer‐generated list is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed. . . by research staff who were not involved in the recruitment or clinical care of participants" (p.56). |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | Low risk | Quote: "Participants' randomisation status was not conveyed to clinical. . . staff" (p. 56). |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Participants' randomisation status was not conveyed to. . . data‐collection staff" (p. 56). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: ". . . results of the trial were analysed using the intention‐to‐treat design" (p. 56). |
| Selective reporting (reporting bias) | Unclear risk | Comment: Data on suicides had to be requested from authors, suggesting that selective reporting bias may have been present. In the absence of the trial protocol; however, the degree of selective reporting cannot be ascertained. |
| Other bias | High risk | Quote: "[T]here was a significant difference between the groups in the number of prior attendances for self‐harm in the [12 months prior to randomisation] with the number of prior attendances being lower in the intervention than in the control group . . . the reduced number of re‐presentations for self‐harm in the intervention group . . . may reflect a pre‐existing tendency for those in the intervention group to have lower numbers of prior hospital attendances for self‐harm. . . Adjusting for the number of prior hospital visits for self‐harm reduced, and in many cases removed, the effect of the intervention on re‐presentation for self‐harm" (pp. 57‐58). |
Bennewith 2002.
| Study characteristics | ||
| Methods |
Allocation: Primary care practices were stratified into 4 groups according to rate of SH. Practices were divided again into 2 groups (8 groups total) according to practice size. Allocation was then made using a random numbers table. Follow‐up period: 12 months N lost to follow‐up: 0/1932 (0%) for repetition data |
|
| Participants |
Inclusion criteria: for primary care practices: i) based in geographical area whose patients lived in catchment area of 4 general hospitals. For participants: i) found in general hospital case register for SH; ii) recruitment data collected weekly from hospitals' A&E sites; iii) not an alcohol (taken alone) or illicit drug overdose, except where casualty officer felt purpose was SH or suicide; iv) aged 16 years and older; v) of fixed residence Exclusion criteria: i) imprisoned; ii) made a request that no one be informed of SH episode; iii) SH occurred in direct response to a hallucination or delusion; iv) SH episode managed entirely in primary care Numbers: Of the 98 primary care practices, 49 were assigned to the experimental arm and 49 to the control arm. Of the 1932 participants, 964 were assigned to the experimental arm, and 968 to the control arm. Profile: 59% (n = 1140) female, 12.6% (n = 244) were repeaters (based on case register information) Source of participants: patients presenting to hospital following an episode of SH and who are also registered with one of the participating primary care practices Location: Avon, Wiltshire, and Somerset, UK |
|
| Interventions |
Experimental: letter from GP inviting patient to a consultation with GP (provided with management guideline) Control: usual care involving GP, psychiatric or other referral Therapist: GPs Type of therapy offered: one‐off consultation in GP practice Length of treatment: one‐off consultation |
|
| Outcomes |
Included: i) repetition of SH according to hospital case registers; ii) contact with services Excluded: i) initiation of contact from GP; ii) days to first repeat SH episode |
|
| Notes |
Sources of funding: "National Health Service South West Research and Development Directorate" (p. 1260). Decalartion of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "98 general practices were assigned in equal numbers to an intervention or a control group" (p. 1254) Comment: correspondence with authors confirmed that a random numbers table had been used to generate the allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Comment: Correspondence with authors confirmed that primary care practices were stratified into 4 groups according to rate of SH, were divided again into 2 groups (8 groups total) according to practice size, and were then allocated using random numbers tables by individuals blind to identity of practices. It is likely this process was adequately concealed. |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "The intervention comprised a letter from the general practitioner inviting the patient to consult" (p. 1254) Comment: It is therefore likely that participants aware of their allocation to the intervention arm. |
| Blinding (performance bias and detection bias) Of personnel | High risk |
Quote: "The intervention comprised a letter from the general practitioner inviting the patient to consult" (p. 1254) Comment: It is therefore likely that GPs were aware of which arm a participant had been assigned. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details on blinding of outcome assessors were provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "For the primary analysis, which compared the intervention and control groups on an intention to treat basis, we carried out a logistic regression analysis with repeat episodes of deliberate self‐harm within 12 months of the index event as the outcome variable. This analysis controlled for practice size (two categories) and quartile of rates of deliberate self‐harm by practice at baseline and allowed for clustering by practice, using random effects logistic regression. We used a Poisson regression analysis to compare the intervention and control groups in terms of differences in the number of repeat episodes. We used Cox's proportional hazards regression for time (in days) to first repeat episode. Clustering was taken into account for both of these (intention‐to‐treat) analyses" (p. 1255) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Brown 2005.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a computer randomisation sequence programmed to prohibit more than 7 consecutive assignments in the same treatment group Follow‐up period: 18 months N lost to follow‐up: 35/120 (29%) for repetition data |
|
| Participants |
Inclusion criteria: i) attempted suicide and received medical/psychiatric evaluation within 48 hours of attempt; ii) able to provide at least 2 verifiable contacts; iii) 16 years or older; iv) able to speak English; v) able to complete baseline assessment; vi) able to provide informed consent Exclusion criteria: i) diagnosed with any medical disorder that would prevent participation in an outpatient clinical trial Numbers: Of the 120 participants, 60 were allocated to the experimental arm, and 60 to the control arm. Profile: 61% (n = 73) female. 74% (n = 89) were repeaters. 68% (n = 82) were diagnosed with substance abuse, and 77% (n = 92) were diagnosed with major depressive disorder Source of participants: patients presenting to hospital after suicide attempt Location: Pennsylvania, USA |
|
| Interventions |
Experimental: 10 sessions of cognitive therapy in addition to treatment as usual Control: TAU Therapist: outpatient sessions were delivered by trial therapists Type of therapy offered: cognitive therapy Length of treatment: 10‐20 weeks |
|
| Outcomes |
Included: i) repetition of SH according to self‐report; ii) suicide; iii) suicidal ideation scores; iv) depression scores; v) hopelessness scores Excluded: i) adherence (due to nature of data reported) |
|
| Notes |
Sources of funding: "This research was supported by grants R01 MH60915 and P20 MH71905 from the National Institute of Mental Health and grant R37 CCR316866 from the Centers for Disease Control and prevention" (p. 570). Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "A computerized randomization sequence programmed to prohibit more than 7 consecutive assignments in either treatment group was used" (p. 564) Comment: Use of a computerised algorithm is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment provided |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "Participants. . . were randomly assigned to cognitive therapy or usual care" (p. 564) Comment: Due to the nature of the intervention treatment, it is unlikely participants could have been blinded to which treatment they had been assigned. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: Due to the nature of the intervention treatment, it is unlikely therapists could have been blinded to which treatment they were delivering. |
| Blinding (performance bias and detection bias) Of outcome assessors | High risk | Quote: "Although blinded assessments were conducted at baseline, blinded follow‐up evaluations were not possible" (p.5 64) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "All effectiveness analyses were conducted using the intent‐to‐treat (ITT) principle" (p. 565) Additionally, "[t]ests and estimates of ITT differences for both continuous and binary outcomes were based on longitudinal models with random effects" (p. 566) Comment: of the 120 randomised participants, 2 dropped out during the intervention and 35 were lost to follow‐up at 18 months. Reasons were given for dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias. Care was taken to test for differences between groups with respect to other care, including psychotropic medications and treatments for substance misuse, but no significant differences were found. |
Carter 2005.
| Study characteristics | ||
| Methods |
Allocation: randomisation based on Zelen's method using a computer generated randomisation schedule Follow‐up period: 24 months N lost to follow‐up: 0/772 (0%) for repetition data |
|
| Participants |
Inclusion criteria: i) aged over 16 years; ii) presented to a toxicology service with deliberate self‐poisoning; iii) able to provide informed consent; iv) fixed address; v) sufficient English; vi) did not pose a potential threat to the interviewer Numbers: Of the 772 participants, 378 were allocated to the experimental arm, and 394 to the control arm. Profile: 68% (n = 525) female, 17% (n = 131) were repeaters. 43% (n = 333) were diagnosed with any affective disorder, 13% (n = 104) with alcohol abuse or dependence, 40% (n = 311) with other substance‐related disorders, and 22% (n = 169) with any personality disorder Source of participants: patients presenting to hospital toxicology service Location: Hunter Valley, NSW, Australia |
|
| Interventions |
Experimental: postcards mailed at 1, 2, 3, 4, 6, 8, 10, and 12 months after discharge in addition to usual care Control: usual care Therapist: none Type of therapy offered: outreach through the mailing of frequent postcards encouraging participants to make contact with the service Length of treatment: 12 months |
|
| Outcomes |
Included: i) repetition of SH according to hospital databases; ii) suicide Excluded: none |
|
| Notes |
Sources of funding: "KC is funded by the NSW Health, Burdekin Mental Health Enhancement Strategy" (p. 4). Declaration of author interests: none stated Other: data on suicides obtained following correspondence with the authors. 20 control group participants received intervention due to clerical errors but were included by the authors in the control group for all intention‐to‐treat analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomisation was by database (HanDBase, version 2.0, DDH Softwards, FL, USA) on a personal digital assistant (Palm III, Palm, CA, USA) that was populated with a pre‐generated randomisation schedule (in blocks of 10) . . . " (p. 2). Comment: Use of a computerised program is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | High risk | Comment: As consent was obtained using Zelen's method, participants were given the option to change treatment arms following allocation. Therefore, allocation cannot have been concealed. |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "We used a randomised consent design, using the single consent version (Zelen's design). This design is a variation on the standard randomised controlled experimental design, where participants are randomised to control or intervention before consent is sought. In the single consent version, written informed consent to receive the intervention (eight non‐obligatory postcards) was sought from participants randomised to the intervention." (p. 2). Comment: As participants were required to give consent to the treatment they were receiving, blinding to allocation status could not have been maintained. |
| Blinding (performance bias and detection bias) Of personnel | High risk |
Quote: " [The] secretary responsible for managing the mailing database and postcards [was] not blind to allocation status" (p. 4). Comment: As personnel knew whether or not a postcard had been sent, they could not have been blinded to allocation status. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "All other. . . research staff remained blinded to allocation." (p. 2) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "We assessed the outcomes by an intention to treat analysis on the basis of allocation" (p. 2). |
| Selective reporting (reporting bias) | Unclear risk | Comment: Data on completed suicides had to be obtained through correspondence with the authors. In the absence of the trial protocol, the degree of selective reporting cannot be ascertained. |
| Other bias | High risk |
Quote: "Twenty participants in the control group received the intervention due to clerical errors but were included in the control group for the intention to treat analyses." (p. 2). Comment: The inclusion of participants who received the treatment intervention within the control group may lead to bias in the estimation of the treatment effect. |
Cedereke 2002.
| Study characteristics | ||
| Methods |
Allocation: randomisation in groups of 2 or 4 using sealed envelopes Follow‐up period: 12 months N lost to follow‐up: 44/216 (20%) for repetition data |
|
| Participants |
Inclusion criteria: i) individuals treated after suicide attempt Numbers: Of the 216 participants, 107 were allocated to the experimental arm and 109 to the control arm. Profile: 66% (n = 143) were female, 52% (n = 112) were repeaters, and 91% (n = 197) were diagnosed with a mood disorder Source of participants: patients treated in hospital after suicide attempt Location: Lund, Sweden |
|
| Interventions |
Experimental: telephone contact (20‐45 minutes) at 4 and 8 months to increase motivation in addition to usual care Control: usual care Therapist: therapists with at least 10 years' experience working with suicidal individuals Type of therapy offered: motivational therapy Length of treatment: 8 months |
|
| Outcomes |
Included: i) repetition of SH according to self‐report checked against both patient records and admission charts; ii) suicide; iii) suicidal ideation scores; iv) compliance Excluded: i) global functioning scores; ii) psychiatric symptoms scores |
|
| Notes |
Sources of funding: "This study was supported by grants from The Axson Johnsons foundation and from the Vardal Foundation (V97 341)" (p. 90) Declaration of author interests: No details on author interests are provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Random allocation" and "personal (sic) . . . performed the randomisation" (p. 83). Comment: The trialists appear to have conducted randomisation using the matched pair design, as blocks of 2 or 4 patients were randomised. Although it is likely the random sequence was adequately generated, without further information on the method used, we cannot ascertain this. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Random allocation (sealed envelope)" (p. 83) Comment: no mention of whether the envelopes were opaque or not, although they probably were |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "The patients did not know whether they would be contacted at 4 and 8 months or not" (p. 83) Comment: The nature of this trial means that participants could have known to which group they had been allocated when they received the telephone call. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: As telephone interventions were made by therapists, personnel could not be blinded to treatment allocation. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk |
Quote: "[A]ll study participants were interviewed again after 12 months at a personal meeting" (p.84). Comment: It is unclear if outcome assessors conducted these meetings and, if so, whether they were blinded to treatment allocation or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "An intent‐to‐treat analysis was performed on all patients who were followed up (n = 178) and the results were the same as in those 172 patients who got at least one intervention" (p.86). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Clarke 2002.
| Study characteristics | ||
| Methods |
Allocation: random numbered lists stratified for sex and admitting hospital; constructed independently of research team; administrator provided clinician with allocation by telephone after patient details given Follow‐up period: 12 months N lost to follow‐up: 0/467 (0%) for repetition data |
|
| Participants |
Inclusion criteria: i) resident in catchment area; ii) aged 16 years or older; iii) not aged 16‐19 years and still in full‐time secondary education; iv) overdoses did not include recreational or problematic substance use Exclusion criteria: i) aged less than 16 years; ii) aged between 16‐19 years and still enrolled in full‐time secondary education; iii) overdose episode occurred as the result of recreational or problematic substance use. Numbers: Of the 467 participants, 220 were allocated to the experimental arm and 247 to the control arm. Profile: 56% (n = 263) were female, 47% (n = 104) were repeaters, 17% (n = 80) had a history of psychiatric treatment, 13% (n = 60) had alcohol problems, and 3% (n = 12) were diagnosed with schizoaffective disorder Source of participants: patients presenting to hospital for SH Location: East London and Essex, UK |
|
| Interventions |
Experimental: case management involving psychosocial assessment, a negotiated care plan, and 'open access' to case manager who helped patient identify and access suitable services in addition to usual care Control: usual care involving triage, and medical and psychosocial assessment and treatment as required Therapists: assessing researchers, case managers or both Type of therapy offered: case management Length of treatment: up to 6 months, reviewable |
|
| Outcomes |
Included: i) repetition of SH according to hospital admission records; ii) suicide Excluded: none |
|
| Notes |
Sources of funding: "Funded by the participating health authority" (p. 167) Declaration of author interests: no details on author interests provided Other: data on suicides obtained following correspondence with authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomisation was conducted using random numbered lists, stratified for sex and admitting hospital. . . The researchers were required to telephone an administrator with possible candidates' details and were then informed of the treatment group" (p. 169) Comment: Use of a random numbers table is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "The random number lists were constructed independently of the research team and they did not have sight of them." "The researchers were required to telephone an administrator with possible candidates' details and were then informed of the treatment group" (p. 169) |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "Case management as deployed in the trial comprised a psychosocial assessment, a negotiated care plan and 'open access' to the assessing researcher (the case manager) via a dedicated (mobile) telephone contact number" (p. 169) Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk |
Quote: "Case management as deployed in the trial comprised a psychosocial assessment, a negotiated care plan and 'open access' to the assessing researcher (the case manager) via a dedicated (mobile) telephone contact number" (p. 169) Comment: As the intervention was delivered by therapists, personnel could not be blinded to treatment allocation. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Comment: Authors report no details on blinding of outcome assessors. However, as readmission rates were the primary outcome and other adverse outcomes during follow‐up were assessed from A&E records, it would not appear that blinding of outcome assessors would have been problematic. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Data analysis proceeded on an intention to treat basis using the unpaired t test procedure, Yates corrected chi‐square and univariate and multivariate logistic regression. The analysis was carried out with SPSS 9 for Windows" (p. 170) Comment: In addition, the trial profile provided on p. 171 does not suggest there were any dropouts, as all patients were followed up at 12 months via A&E records. |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to request data on suicides from authors, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Crawford 2010.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a random numbers table and delivered using prepared, sealed, opaque envelopes Follow‐up period: 6 months N lost to follow‐up: 0/103 (0%) for repetition of SH; 65/103 (63.1%) for secondary outcomes not included in this review (e.g., alcohol consumption, mental health problems, and satisfaction with treatment) |
|
| Participants |
Inclusion criteria: i) aged 18 or older; ii) admitted to an emergency department following an episode of SH; iii) diagnosed with alcohol misuse according to scores on the Paddington Alcohol Test Exclusion criteria: i) unwilling to provide informed consent; ii) unable to provide informed consent (e.g., due to an inability to communicate in English or impaired consciousness); iii) no fixed address in the greater London area; iv) already receiving treatment from alcohol misuse services; v) made a specific request to receive treatment from alcohol misuse services at index presentation Numbers: Of the 103 participants, 51 were allocated to the experimental arm and 52 were allocated to the control arm. Profile: 48.5% (n = 50) were female, 100% (n = 103) were diagnosed with alcohol misuse Source of participants: consecutive admissions to an emergency department following an episode of SH and who were diagnosed with alcohol misuse according to scores on the Paddington Alcohol Test Location: London, UK |
|
| Interventions |
Experimental: a one‐off appointment with an alcohol nurse specialist involving assessment and discussion of both current and previous drinking behaviours delivered according to the FRAMES approach in addition to a health information leaflet advising on the damaging effects of excessive alcohol consumption, recommended limits of alcohol consumption, and the contact details of nationally‐based alcohol misuse help lines (Miller 1993). Participants could also be referred by the alcohol nurse specialist to individual alcohol counselling or detoxification services as required. Control: TAU involving a health information leaflet advising on the damaging effects of excessive alcohol consumption, recommended limits of alcohol consumption, and the contact details of nationally‐based alcohol misuse help lines Therapist: 1 alcohol nurse specialist Type of therapy offered: alcohol‐specific therapy Length of treatment: approximately 30 minutes |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide Excluded: i) alcohol consumption; ii) alcohol involved in SH episode; iii) diagnosis of probably personality disorder; iv) satisfaction with treatment |
|
| Notes |
Sources of funding: "This study was funded by St Mary's Paddington Charitable Trust" (p. 1827). Declaration of author interests: none stated Other: Data on suicides were obtained following correspondence with authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "We used simple randomization with an experimental to control treatment ratio of 1:1. . . using random numbers tables" (p. 1822). Comment: Use of a random numbers table is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Sealed opaque envelopes" (p. 1822) Comment: Use of sealed opaque envelope containing either an appointment card or a blank piece of card would ensure adequate allocation concealment. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: This was a single‐blind study and only "researchers collecting follow‐up data were masked to allocation status" (p. 1825), suggesting that participants were not blind to allocation status. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: This was a single‐blind trial and only "researchers collecting follow‐up data were masked to allocation status" (p. 1825), suggesting that personnel were not blind to allocation status. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "[R]esearchers collecting follow‐up data were masked to allocation status" (p. 1825). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Our primary analysis [repetition of SH] was conducted using an intention‐to‐treat principle" (p.1823). |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to request data on suicides from authors, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Davidson 2014.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a random numbers table Follow‐up period: 3 months N lost to follow‐up: 6/20 (30.0%) at the 3 month follow‐up period |
|
| Participants |
Inclusion criteria: i) aged 18‐65 years; ii) diagnosed with any personality disorder according to the SCID‐II; iii) score 3 or greater on the Standardised Assessment of Personality Abbreviated Scale Exclusion criteria: i) unable to provide informed consent Numbers: Of the 20 participants, 14 were allocated to the experimental arm and 6 to the control arm. Profile: 100% (n = 20) were diagnosed with a personality disorder; 45.0% (n = 9) were diagnosed with comorbid substance misuse Source of participants: patients admitted to the medical receiving ward of the A&E department following an episode of SH Location: Glasgow, UK |
|
| Interventions |
Experimental: manualised cognitive therapy involving psycho‐education to help participants understand SH, potential alternatives to resolving problems, and referral to appropriate mental health services where required Control: TAU involving referral to community mental health teams, appointments with psychiatrists and a community psychiatric nurse, and inpatient psychiatric treatment as required Therapist: 2 therapists: 1 doctoral‐level clinical psychologist and 1 psychiatrist who received weekly training in manualised cognitive therapy Type of therapy offered: cognitive behavioural therapy Length of treatment: no details on length of treatment were provided |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide; iii) suicidal ideation; iv) depression Excluded: i) alcohol use; ii) anxiety and depression severity |
|
| Notes |
Source of funding: "This work was supported by NHS Greater Glasgow and the Scottish Mental Health Research Network" (p. 4). Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Patients were randomised. . . using a random numbers table with an allocation of 2:1 in favour of [the intervention]" (p.2). Comment: Use of a random numbers table is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation sequence provided |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "The research assistant, who assessed patients at baseline and outcome, remained masked to treatment allocation throughout the study" (p. 2). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[T]he intention‐to‐treat principle [was] applied, i.e. analyses [were] based on the initial treatment intent, not on the treatment eventually administered" (p. 2). |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to request data on repetition of SH and depression from authors, suggesting that selective reporting bias may have been present. |
| Other bias | High risk | Comment: This was a very small trial with substantial imbalances between intervention and control groups with respect to levels of non‐suicidal self‐harm, anxiety, and depression at baseline. Follow‐up analyses did not adjust for these baseline imbalances. This is likely to result in exaggerated treatment effects as, in all cases, the control group had higher levels of self‐harm, anxiety, and depression. |
Dubois 1999.
| Study characteristics | ||
| Methods |
Allocation: random assignment using an unknown method Follow‐up period: 12 months N lost to follow‐up: 18/102 (17.6%) for repetition of SH data |
|
| Participants |
Inclusion criteria: i) attended emergency department following a suicide attempt; ii) aged 15‐34 years Exclusion criteria: i) hospitalised for more than 24 hours; ii) currently being treated by a psychiatrist Numbers: Of the 102 participants, 51 were randomised to the experimental arm and 51 to the control arm. Profile: 80% (n = 82) female Source of participants: patients attending emergency department Location: Bohars, France |
|
| Interventions |
Experimental: Brief psychotherapy involving 5 sessions during first month following the index episode. These sessions followed a specific therapeutic model. Control: TAU involving an assessment by a clinical psychiatrist. Upon leaving, these participants were followed‐up by a psychiatrist or psychologist. Therapists: participants continued to receive treatment from the same therapist who initially saw them at hospital Type of therapy offered: brief psychotherapy Length of treatment: 1 month |
|
| Outcomes |
Included: i) repetition of SH according to unknown source; ii) suicide Excluded: i) compliance |
|
| Notes |
Source of funding: no details on funding provided Declaration of author interests: no details provided Other: As compliance data were not reported for the control group, this outcome had to be excluded from subsequent analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Two groups, with 51 patients each, [were] distributed by randomisation" (p. 557). Comment: Although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details on allocation concealment are reported. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of this trial means that personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Quote: "Patients were evaluated by a clinician different to their therapist (translation)" (p. 558). Comment: However, it is not stated whether this clinician was blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Of the 70 participants, 34 refused to attend follow‐up and 12 were lost to follow‐up (could not be found). No further reasons for dropouts given. The authors, in addition, note that less than 2/3 of patients attended all 3 appointments. Despite this, they did not attempt ITT analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Evans 1999a.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a sealed envelope which contained either an emergency green card or a 'dummy' card Follow‐up period: 12 months N lost to follow‐up: 0/827 (0%) for repetition data |
|
| Participants |
Inclusion criteria: i) admitted to emergency departments following an episode of SH; ii) referred for routine psychiatric evaluation; iii) resident in catchment area; iv) judged likely to use intervention appropriately; v) made contact with and used mental health services; vi) acceptable level of aggressive behaviour Exclusion criteria: i) inappropriate substance abuse leading to repetitive presentation in which the participant was aggressive or unable to engage in treatment Numbers: Of the 827 participants, 417 were allocated to the experimental arm and 410 to the control arm. Profile: 55.4% (n = 458) female, 42% (n = 349) were multiple repeaters Source of participants: patients admitted to general hospital following SH episode Location: Bristol, UK |
|
| Interventions |
Experimental: emergency card in addition to TAU. Participants were provided with an emergency card offering 24‐hour service for crisis telephone consultation with an on‐call psychiatrist. Control: TAU Therapist: on‐duty trainee psychiatrist Type of therapy offered: emergency card offering 24‐hour service for crisis telephone consultation with an on‐call psychiatrist in addition to TAU Length of treatment: 6 months |
|
| Outcomes |
Included: i) repetition of SH according to A&E and hospital admissions records; ii) suicide Excluded: none |
|
| Notes | Sources of funding: "Funding was provided by a grant from the Department of Health" (p. 23) Declaration of author interests: none stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Randomised on a 1:1 basis. . . using the sealed envelope technique" (p. 23) Comment: although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomised. . . using the sealed envelope technique, ensuring that it was impossible to tell from feeling or looking at the envelopes whether they contained a green card or a 'dummy card' (which was not given out)" (p. 23) Comment: Use of opaque sealed envelope containing either a green card or a 'dummy' card would ensure adequate allocation concealment. |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "Those randomised to receive a green card were offered the card immediately after the psychiatric assessment" (p. 23) Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of this trial means that personnel (e.g., GPs and psychiatrists on telephone duty) are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "All subjects' repeat hospital attendances for SH within 6 months of randomisation were monitored (blind to their study group) by means of a computerised case register based on routine accident and emergency admission data" (p. 24) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: ". . . all analyses were conducted on an intention‐to‐treat basis." (p. 24) |
| Selective reporting (reporting bias) | Unclear risk | Comment: No reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Evans 1999b.
| Study characteristics | ||
| Methods |
Allocation: randomised using opaque sealed envelopes opened sequentially Follow‐up period: 6 months N lost to follow‐up: 2/34 (6%) for repetition data |
|
| Participants |
Inclusion criteria: i) personality disturbance (antisocial, dissocial, impulsive or borderline); ii) at least 1 episode of SH in 12 months preceding entry to trial Exclusion criteria: i) diagnosed with alcohol or drug dependence, schizophrenia, or organic psychiatric disorder Numbers: Of the 34 participants, 18 were allocated to the experimental arm and 16 to the control arm. Profile: 62% (n = 21) were female, 100% (n = 34) were repeaters, 100% (n = 34) had a diagnosis of a personality disorder Source of participants: patients admitted after an episode of SH to 1 of 2 hospitals in the London area (Paddington and Chelsea, Westminster) Location: London, UK |
|
| Interventions |
Experimental: 2‐6 sessions of manual assisted cognitive behavioural therapy including basic cognitive techniques, problem‐solving, techniques for managing emotions and thoughts, and relapse prevention plans in individuals with personality disorders Control: TAU. 5 participants had contact with a psychiatrist, 3 saw a community mental health team, 4 saw a specialist social worker, and 2 saw no mental health professional Therapist: 1 psychiatrist, 2 nurses, and 2 social workers. The type of therapy recieved by the remaining 2 participants in the control group was not specified. Type of therapy offered: cognitive behavioural therapy Length of treatment: varied |
|
| Outcomes |
Included: i) repetition of SH according to self‐report and hospital records; ii) depression; iii) compliance Excluded: i) time to repetition of SH; ii) cost of care; iii) social functioning; iv) anxiety |
|
| Notes |
Sources of funding: "This work was supported by a grant from the North Thames Regional Health Authority" (p. 24)
Declaration of author interests: no details on author interests provided Other: 5 participants in the experimental group did not see a therapist and instead received therapy from the booklets. 1 participant in the experimental group did not receive any intervention. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients were allocated by opening opaque sealed envelopes sequentially at each centre" (p. 20). Comment: Although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Patients were allocated by opening opaque sealed envelopes sequentially at each centre" (p. 20) Comment: Use of opaque sealed envelope would have ensured adequate allocation concealment. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of this trial means that personnel (a psychiatrist, 2 nurses and 2 social workers) would not have been blinded to the type of treatment they were giving. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Baseline assessments, before randomization, and follow‐up assessments, at 6 months, were completed by an independent assessor, who had no contact with the clinical teams during the trial and made assessments without any knowledge of treatment received" (p. 20). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Of the 34 participants, 2 dropped out after initial assessment and randomisation but "prior to knowledge of treatment allocation". They were subsequently excluded from all analyses, which the authors felt was appropriate, as no service had been provided to them following the initial assessment. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Fleischmann 2008.
| Study characteristics | ||
| Methods |
Allocation: random number table using opaque sealed envelopes Follow‐up period: 18 months N lost to follow‐up: 204/1867 (11%) for repetition data |
|
| Participants |
Inclusion criteria: i) diagnosis of self‐harm/self‐poisoning by medical staff Exclusion criteria: i) died on the ward; ii) presence of clinical conditions which would disallow interview; iii) left hospital against medical order; iv) resident in a different catchment area; v) had language difficulties Numbers: Of the 1867 participants, 922 were allocated to the experimental arm and 945 to the control arm. Profile: 58% (n = 1086) were female Source of participants: patients presenting to emergency care settings following an episode of self‐harm/self‐poisoning within a defined catchment area with a population of at least 250,000 Location: Brazil, India, Sri Lanka, Iran, and China |
|
| Interventions |
Experimental: brief cognitive behavioural intervention involving "information about suicidal behaviour as a sign of psychological and/or social distress, risk and protective factors, basic epidemiology, repetition, alternatives to suicidal behaviours, and referral options" (p. 705) and contact via telephone or home visits to provide referral support in addition to TAU Control: TAU "according to the norms prevailing in the respective emergency departments" (p. 704). This typically involved only acute treatment for somatic problems only. Therapist: clinician (e.g., psychiatrist, nurse, doctor) Type of therapy offered: information and support Length of treatment: 18 months |
|
| Outcomes |
Included: i) repetition; ii) suicide Excluded: i) compliance; ii) depression; iii) hopelessness; iv) impulsiveness; v) social support; vi) suicidal intent; vii) anger; viii) well‐being |
|
| Notes |
Sources of funding: "The study was funded by the Department of Mental Health and Substance Abuse, WHO. Some field research sites obtained additional funding from the following agencies: Campinas: Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant no 02/08288‐9, São Paulo, Brazil; Durban: Medical Research Council (MRC), Tygerberg, Cape Town, South Africa; Karaj: Tehran Psychiatric Institute, Mental Health Research Centre (IUMS), Tehran, Iran; Tallinn: Estonian Health Insurance Fund, Tallinn, Estonia; the Swedish National and Stockholm County Centre for Suicide Research and Prevention of Mental Ill‐Health (NASP), WHO Colloborating Centre for Research and Training in Suicide Prevention, Department of Public Health Sciences, Karolinska Institute, Stockholm, Sweden" (p. 708). Declaration of author interests: none stated Other: We obtained data on repetition of SH and suicides following correspondence with authors. Excluded outcomes are taken from the trial protocol. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "An allocation sequence based on a random‐number table was used to randomly assign all enrolled subjects" (p. 704) Comment: Use of a random numbers table is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: ". . . the allocation sequence was maintained in a separate location to prevent clinician bias" (p. 704) |
| Blinding (performance bias and detection bias) Of participants | Low risk | Quote: "The subjects were blinded as to their assignment" (p. 704) |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: No details on personnel blinding are provided; however, the nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: No details on outcome assessor blinding are provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Authors report the number of participants lost to follow‐up; however, they did not provide reasons for dropout, nor did they attempt to use intention‐to‐treat analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Authors collected additional outcome information, including adherence, depression, hopelessness, impulsiveness, social support, suicidal intent, anger, and well‐being. They did not reportit, but they report some of these outcomes in related trials (i.e., Hassanzadeh 2010; Vijayakumar 2011; Xu 2012). |
| Other bias | Low risk | Comment: no apparent sources of other bias |
Gibbons 1978.
| Study characteristics | ||
| Methods |
Allocation: Correspondence with authors confirmed that participants were randomly assigned using sequentially numbered, sealed, opaque envelopes. Follow‐up period: 12 months N lost to follow‐up: 0/400 (0%) for repetition data |
|
| Participants |
Inclusion criteria: i) over 17 years old Exclusion criteria: i) immediate suicide risk; ii) no formal psychiatric illness Numbers: Of the 400 participants, 200 were allocated to the experimental arm and 200 to the control arm. Profile: Self poisoning patients, including both multiple repeaters and first‐timers. 71% (n = 284) were female, 44% (n = 176) were diagnosed with depressive neurosis, 2% (n = 8) with phobic neurosis, 2% (n = 8) with affective psychosis, and 1% (n = 4) with schizophrenia Source of participants: patients presenting to an A&E department following an episode of deliberate self‐poisoning Location: Southampton, UK |
|
| Interventions |
Experimental: crisis‐oriented, time‐limited, task‐centred social work provided at home, which included problem‐solving intervention for personal relationships, emotional distress, practical problems, etc. Control: TAU. 54% (n = 108) were referred to their GP, 33% (n = 66) received a psychiatric referral, and 13% (n = 26) received an unspecified referral. Therapist: 2 social workers Type of therapy offered: task‐centred case management alongside problem‐solving therapy Length of treatment: 3 months |
|
| Outcomes |
Included: i) repetition of SH according to hospital records; ii) depression; iii) social problems Excluded: i) satisfaction with service |
|
| Notes |
Sources of funding: "The study was supported by the Department of Health and Social Security, and the Wessex Regional Health Authority" (p. 117) Declaration of author interests: no details on author interests were provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Correspondence with authors confirmed that participants were randomly assigned using sequentially numbered, sealed, opaque envelopes. |
| Allocation concealment (selection bias) | Low risk | Comment: Correspondence with authors confirmed that participants were randomly assigned using sequentially numbered, sealed, opaque envelopes. Use of opaque sealed envelope would have ensured adequate allocation concealment. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: No details on personnel blinding are provided; however, the nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk |
Quote: "The follow‐up interviews were carried out by three experienced interviewers. . . [who] had had no connection with the project and did not know what treatment patients had received" (pp. 113‐114) Comment: Additionally, reliability between outcome assessors was also assessed on p. 116. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: ". . . there were no differences in age and sex distribution between the interviewed sample and the missing cases" (p. 114). Comment: Given there were no difference in age and sex distribution between the interviewed and missing cases, missing data were unlikely to have affected the outcome. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Gratz 2006.
| Study characteristics | ||
| Methods |
Allocation: random assignment using an unknown method Follow‐up period: 14 weeks N lost to follow‐up: 2/24 (8%) for repetition data |
|
| Participants |
Inclusion criteria: i) diagnosis of borderline personality disorder; ii) history of deliberate self‐harm, with at least 1 episode in the past 6 months; iii) have an individual therapist; iv) aged 18 to 60 years; v) female Exclusion criteria: i) diagnosis of a psychotic disorder, bipolar I disorder, or substance dependence; ii) suicide attempt rated as having a 'high' risk of death or greater within past 6 months; iii) at risk of attempting suicide within the next year; iv) received dialectical behaviour therapy in the past 6 months Numbers: Of the 24 participants, 13 were allocated to the experimental arm and 11 to the control arm. Profile: 100% (n = 24) were female, 100% (n = 24) were multiple repeaters Source of participants: clinician referrals and self referrals from advertisements posted at a hospital and on 2 websites Location: Boston, MA, USA |
|
| Interventions |
Experimental: weekly emotion regulation group intervention and individual therapy sessions in addition to TAU Control: TAU, including individual therapy sessions Therapists: group and individual emotion regulation therapists Type of therapy offered: emotion regulation group intervention Length of treatment: 14 weeks |
|
| Outcomes |
Included: i) repetition of SH according to self report; ii) depression Excluded: i) emotion regulation; ii) emotional avoidance; iii) impairment due to BPD; iv) anxiety; v) stress |
|
| Notes |
Sources of funding: "This research was supported by the Psychosocial Fellowship of McLean Hospital, awarded to the first author" (p. 25). Declaration of author interests: Although no details on author interests were provided, Prof Gratz developed emotion regulation group therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: ""Random assignment" (p. 30) Comment: Although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details on allocation concealment were provided. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | High risk | Quote: "Research team members were not blind to condition" (p. 30). |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "Two participants dropped out of the study (one from each condition)" (p. 27) Comment: Despite this, authors did not attempt ITT analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Gratz 2014.
| Study characteristics | ||
| Methods |
Allocation: stratified randomisation procedure matching for i) emotion dysregulation; ii) number of lifetime episodes of SH; iii) Global Assessment of Functioning scores; iv) age Follow‐up period: 3 and 9 months N lost to follow‐up: 12/61 (23.5%) |
|
| Participants |
Inclusion criteria: i) females; ii) aged 18‐60 years; iii) diagnosed with threshold or subthreshold borderline personality disorder; iv) history of repeated SH with at least 1 episode in the past 6 months; v) have one or more of the following: individual therapist, psychiatrist, case manager Exclusion criteria: i) diagnosed with psychosis or bipolar I disorder; ii) current (past month) substance use Numbers: Of the 61 participants, 31 were allocated to the intervention arm and 30 to the control arm. Profile: 100% (n = 61) were female; 100% (n = 61) were multiple repeaters; 62.3% (n = 38) had previously made a suicide attempt; 50.0% (n = 31) were diagnosed with any mood disorder; 62.3% (n = 38) were diagnosed with an anxiety disorder; 36.0% (n = 22) were diagnosed with PTSD; 13.3% (n = 8) were diagnosed with an eating disorder; 1.6% (n = 1) were diagnosed with substance use disorder. Source of participants: referrals from clinicians to the emotion regulation group therapy and from self referrals in response to an advertisement posed both online and in the community Location: Jackson, MS, USA |
|
| Interventions |
Experimental: emotion‐regulation group therapy involving psycho‐education to develop awareness, understanding, and acceptance of emotions, the ability to engage in goal‐directed behavior whilst inhibiting impulsive behaviours without experiencing negative emotions, use of situationally appropriate strategies to moderate either the intensity or duration of emotions, and the willingness to experience some negative emotions as a consequence of daily life Control: TAU involving outpatient treatment with individual therapists. Some participants also received group therapy as part of TAU, although this was not emotion‐regulation group therapy. Therapist: 2 doctoral‐level therapists who received at least 4 months of training in delivering emotion‐regulation group therapy Type of therapy offered: emotion‐regulation group therapy Length of treatment: 14 weeks |
|
| Outcomes | Included: i) repetition of SH; ii) depression Excluded: i) non‐acceptance of emotions; ii) impulsiveness with respect to emotions; iii) goal‐directed emotions; iv) awareness of emotions; v) emotion strategies; vi) emotional clarity; vii) acceptance and action; viii) borderline personality disorder severity; ix) interpersonal problems; x) anxiety; xi) stress; xii) disability severity; xiii) quality of life | |
| Notes |
Sources of funding: "This research was supported by National Institute of Mental Health Grant R34 MH079248, awarded to Dr. Gratz" (p. 2110). Declaration of author interests: Although no details on author interests were provided, Prof Gratz developed emotion‐regulation group therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Participants . . . were matched on four prognostic variables [emotion dysregulation, number of lifetime incidents of SH, global assessment of functioning (GAF) scores, and age] and randomly assigned. . . using a stratified randomization procedure" (p. 2100). Comment: although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details on allocation concealment were provided. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "All assessments were conducted by trained assessors masked to participant condition" (p. 2103). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "We adopted a Bayesian approach . . . using the Markov chain Monte Carlo routines . . . This approach implements a multiple imputation strategy to handle missing data. . . enabling an analysis of the intent‐to‐treat (ITT) sample" (p. 2104). |
| Selective reporting (reporting bias) | Unclear risk | Comment: No reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Guthrie 2001.
| Study characteristics | ||
| Methods |
Allocation: After consent, recruiting member of research team referred to an allocation sequence provided by the trial statistician and based on a computer‐generated list of random numbers to assign participants. Follow‐up period: 6 months N lost to follow‐up: 0/119 (0%) for repetition data |
|
| Participants |
Inclusion criteria: i) aged 18‐65 years; ii) presenting with episode of deliberate self‐poisoning; iii) able to read and write English; iv) living in the catchment area of the hospital; v) registered with a GP Exclusion criteria: i) requiring psychiatric treatment Numbers: Of the 119 participants, 58 were allocated to the experimental arm and 61 to the control arm. Profile: 55.5% (n = 66) were female, 60% (n = 71) were multiple repeaters, 55% (n = 65) had a history of psychiatric treatment. Source of participants: patients presenting to hospital after deliberate self‐poisoning Location: Manchester, UK |
|
| Interventions |
Experimental: weekly 50‐minute sessions of an individual home‐based psychodynamic interpersonal therapy involving identification of personal difficulties. Participants were left to resolve interpersonal difficulties causing distress through a conversational approach focused on the identification of feelings and relating these to problems and relationships to develop shared understanding and approaches to family problems. Control: TAU. In most cases this involved assessment by doctor in the emergency department and referral to psychiatry outpatient treatment, addiction services, or GP Therapists: nurse therapists Type of therapy offered: psychodynamic interpersonal therapy Length of treatment: 4 weeks |
|
| Outcomes |
Included: i) repetition of SH according to self report and hospital records; ii) suicide; iii) suicidal ideation; iv) depression Excluded: i) patient satisfaction |
|
| Notes |
Sources of funding: "North West Regional Health Authority and the NHS Research and Development Levy" (p. 4). Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "block randomised design" (p. 5). Comment: Correspondence with the authors further clarified that after consent, recruiting member of research team referred to an allocation sequence, provided by the trial statistician and based on a computer‐generated list of random numbers to assign participants in groups of 12 participants (stratified according to whether or not participants had a history of SH). Use of a random numbers table is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Comment: Correspondence with authors clarified that allocation was concealed from the recruiting member of the research team. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel (e.g., nurse therapists, GPs) are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | High risk |
Quote: "Follow up assessments were conducted by one of two research assistants, who were blind to treatment groups" (p. 2) Comment: Data on repetition of SH, however, were obtained from participant self report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: " [W]e included in the analysis all patients who completed the assessments at the end of treatment or at six month follow up assessments. Comparisons between groups were made on an intention to treat basis" (p. 2). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Harned 2014.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a minimisation procedure matched for: i) number of suicide attempts in the past year; ii) number of episodes of NSSI in the past year; iii) PTSD symptom severity; iv) dissociation symptom severity; v) current use of any SSRI medication Follow‐up period: 3 months N lost to follow‐up: 8/26 (30.8%) |
|
| Participants |
Inclusion criteria: i) female; ii) aged 18‐60 years; iii) diagnosed with borderline personality disorder; iv) diagnosed with post‐traumatic stress disorder (PTSD); v) satisfactory recall of at least part of the index trauma; vi) recent and recurrent engagement in SH (at least 2 suicide attempts or episodes of NSSI in the previous 5 years with at least 1 occurring within the past 8 weeks); vii) lives within commuting distance of the specialist clinic Exclusion criteria: i) diagnosed with psychosis, bipolar disorder, or mental retardation; ii) receiving treatment under a legal mandate; iii) require treatment for another life‐threatening condition (e.g., anorexia nervosa) Numbers: Of the 26 participants, 19 were allocated to the intervention arm and 7 were allocated to the control arm. Profile: 100% (n = 26) were female; 100% (n = 26) were diagnosed with borderline personality disorder; 100% (n = 26) were diagnosed with PTSD. Source of participants: patients seeking treatment from a specialist treatment service for suicidal individuals with comorbid borderline personality disorder and PTSD, flyers, and from outreach services within the catchment area. Location: Seattle, WA, USA |
|
| Interventions |
Experimental: dialectical behaviour therapy with the prolonged exposure protocol involving individual psychotherapy, group skills training, phone consultations as required, and weekly therapist consultation sessions. The prolonged exposure protocol enabled participants to receive longer individual therapy sessions per week. Control: dialectical behaviour therapy involving individual psychotherapy, group skills training, phone consultations as required, and weekly therapist consultation sessions Therapists: masters' level clinicians with an average of 2 years of clinical experience. Most were doctoral‐level students in training (52.6%), followed by licensed professionals (36.8%), and postdoctoral fellows (10.5%). Clinicians had received training in DBT for at least 1 day. Type of therapy offered: dialectical behaviour therapy with the prolonged exposure protocol Length of treatment: 12 months |
|
| Outcomes | Included: i) repetition of SH; ii) suicides; iii) suicide attempts; iv) depression; v) compliance Excluded: i) repetition of SH and suicide attempts combined; ii) treatment sessions attended; iii) adjunct skills sessions attended; iv) PTSD symptom severity; v) dissociation symptom severity; vi) trauma‐related guilt cognitions severity; vii) shame severity; viii) anxiety; ix) global symptomatology | |
| Notes |
Source of funding: "This work was supported by grant R34MH082143 from the National Institute of Mental Health" (p. 16). Declaration of author interests: "Drs. Harned, Korslund, and Linehan are trainers and consultants for Behavioral Tech, LLC" (p. 16). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "A minimization randomization procedure was used to match participants on the five primary prognostic variables: (1) number of suicide attempts in the last year; (2) number of NSSI episodes in the last year; (3) PTSD severity; (4) dissociation severity; and (5) current use of SSRI medication" (pp. 8‐9). Comment: Use of a minimisation randomisation algorithm is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Comment: Correspondence with authors clarified that allocation was concealed from assessors as randomisation was completed by a staff member not involved in assessments. Furthermore, there was no way to foresee the outcome of the randomisation algorithm. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: Correspondence with authors clarified that allocation was concealed from participants until their first therapy session, at which point their therapist informed them as to which treatment condition they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: Correspondence with authors clarified that allocation was concealed from participants until their first therapy session, at which point their therapist informed them as to which treatment condition they had been allocated, suggesting that personnel were aware of which participant had been allocated to which treatment condition. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "[A]ssessments were conducted by independent clinical assessors who were blind to treatment condition" (p. 9). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: both intention‐to‐treat and per protocol analyses provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Hassanian‐Moghaddam 2011.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a block randomisation procedure using a random digit table. Follow‐up period: 12 months N lost to follow‐up: 187/2300 (8.1%) for repetition of SH at 12 months |
|
| Participants |
Inclusion criteria: i) aged 12 or older; ii) admitted or transferred to a specialist hospital for the treatment of poisoning following an episode of deliberate self‐poisoning Exclusion criteria: i) treated in the emergency department of a regular hospital; ii) diagnosed with psychosis; iii) unable to provide informed consent (e.g., unable to communicate in Farsi); iv) of no fixed address; v) potential threat to interviewers; vi) episode of self‐poisoning was classified by the attending toxicologist as recreational, habitual, accidental, or iatrogenic. Numbers: Of the 2300 participants, 1150 were allocated to the experimental arm and 1150 to the control arm. Profile: 66.4% (n = 1402) were female, 31.4% (n = 723) were multiple repeaters. Source of participants: patients admitted or transferred to a specialist hospital for the treatment of poisoning following an episode of deliberate self‐poisoning Location: Tehran, Iran |
|
| Interventions |
Experimental: postcards mailed at 1, 2, 3, 4, 6, 8, 10, and 12 months after discharge in addition to TAU Control: TAU. Although no specific details are provided, the authors note that "[f]ollow‐up care for self‐poisoning in Tehran is generally poor. . . Contact is mainly hospital‐ or office‐based, and community‐based programs are almost non‐existent. Psychiatric beds are often at 100% occupancy, with short admissions and frequent readmissions." (pp. 310‐311). Therapist: none Type of therapy offered: outreach through the mailing of frequent postcards encouraging participants to make contact with the service Length of treatment: 12 months |
|
| Outcomes |
Included: i) repetition of SH according to self report; ii) suicide; iii) suicide attempts according to self report cross‐validated against hospital records; iv) suicidal ideation Excluded: ii) number receiving postcard; ii) number finding postcard helpful in the prevention of SH; iii) death from any cause |
|
| Notes |
Sources of funding: "This study was supported by a grant from the Legal Medicine Organization of Iran and the Loghman‐Hakim Research Development Unit, Shahid Beheshti Medical University" (p. 315). Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Block randomisation (blocks of 100) was undertaken using a random digit table" (p. 310). Comment: the authors note that although " . . . this older form of randomisation is potentially liable to interference. . . no imbalances at baseline suggest that the randomisation was likely to have been successful" (p. 314). |
| Allocation concealment (selection bias) | Low risk | Quote: "To maintain masking to allocation, randomisation was not revealed to the recruiting toxicologist until all information was entered and eligibility determine" (p. 310). |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | Low risk | Quote: "Other staff were masked to allocation status during hospital treatment" (p. 310). |
| Blinding (performance bias and detection bias) Of outcome assessors | High risk | Quote: "The research psychologist was not masked to allocation status at follow‐up" (p. 310). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All outcomes were analysed on randomisation status at baseline for 12‐month follow‐up" (p. 311). |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to obtain data on suicides following correspondence with authors, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Hatcher 2011.
| Study characteristics | ||
| Methods |
Allocation: randomisation based on Zelen's method using a computer‐generated numbers list Follow‐up period: 12 months for primary outcome (i.e., repetition of SH) and 3 and 12 months for secondary outcomes (i.e., suicidal ideation, depression, hopelessness, and problem‐solving) N lost to follow‐up: 158/1094 (14.4%) by the 1‐year follow‐up period |
|
| Participants |
Inclusion criteria: i) 16 years or older; ii) admitted to hospital following an episode of SH Exclusion criteria: i) still enrolled full time in school; ii) currently receiving dialectical behaviour therapy for the treatment of borderline personality disorder; iii) had a treatment management plan which precluded receiving short‐term therapy; iv) cognitively impaired; v) admitted to a psychiatric care unit following the index episode of SH for a minimum period of 48 h Numbers: Of the 552 participants who provided informed consent, 253 were allocated to the experimental arm and 299 were allocated to the control arm. Profile: 68.8% (n = 380) were female, 44.7% (n = 247) were multiple repeaters Source: patients admitted to hospital following an episode of SH Location: Auckland and Wellington, New Zealand |
|
| Interventions |
Experimental: problem‐solving therapy based on D'Zurilla 1971 involving problem orientation, problem listing, definition, brainstorming of alternative solutions, devising an action plan, and reviewing the plan in addition to TAU Control: TAU involving a one‐off psychosocial assessment by a mental health professional Therapist: clinicians without extensive clinical experience working in the mental health care setting who received 1 week of training in problem‐solving therapy Type of therapy offered: problem‐solving therapy Length of treatment: 3 months |
|
| Outcomes |
Included: i) repetition of SH according to hospital records; ii) suicide; iii) suicidal ideation; iv) depression; v) hopelessness; vi) problem‐solving Excluded: i) anxiety |
|
| Notes |
Sources of funding: "This study was funded by the Accident Compensation Corporation of New Zealand" (p. 316). Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "[P]atients were randomised (1:1) using computer‐generated random numbers. . . " (p. 311) Comment: Use of a computer‐generated list is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | High risk |
Quote: "patients were randomised (1:1) using computer‐generated random numbers (from an independent statistician) contained in sealed envelopes" (p. 311) Comment: Use of opaque sealed envelope could have ensured that allocation was concealed; however, use of Zelen's design makes it unlikely that participants and clinical personnel would have remained unaware of allocation. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: As consent was obtained using Zelen's method, participants were given the option to change treatment arms following allocation. Therefore, participants cannot have been blinded as to treatment allocation. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Researchers masked to treatment allocation subsequently interviewed consenting participants by telephone" (p. 311) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "For the primary outcome we could obtain information on repetition of self‐harm for everyone who was randomised, so the analysis . . . is a true intention‐to treat analysis. . . For the analysis of secondary outcomes we used data from just those people who consented to take part in the study and we have called this a per protocol analysis" (p. 312). Comment: mixture of intention‐to‐treat and per protocol analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data on suicides had to be requested from authors, suggesting that selective reporting bias may have been present. |
| Other bias | High risk | Comment: Use of Zelen's design may have led to bias. |
Hatcher 2015.
| Study characteristics | ||
| Methods |
Allocation: randomisation based on Zelen's method using a centrally generated randomisation sequence. A stratified minimisation procedure was also used to ensure balance in key prognostic factors (i.e., history of SH and method of SH) between the 4 sites Follow‐up period: 12 months for primary outcome (i.e., hospital recorded repetition of SH) and 3 and 12 months for secondary outcomes (i.e., self reported repetition of SH, depression, and hopelessness) N lost to follow‐up: 0/1474 (0%) for the primary outcome measure of hospital re‐presentations for SH. |
|
| Participants |
Inclusion criteria: i) presented to the emergency department at 1 of the 4 participating hospitals following an episode of SH Exclusion criteria: i) aged less than 17 years; ii) still enrolled full‐time in school; iii) unable to provide informed consent; iv) self identified as Māori (these participants were instead invited to participate in the Hatcher 2016a trial). Numbers: Of the 684 participants who provided informed consent, 327 were allocated to the experimental arm and 357 were allocated to the control arm. Profile: Of those who consented to participation 67.8% (n = 464) were female, 54.1% (n = 370) were multiple repeaters. Source: patients admitted to hospital following an episode of SH Location: Waitemata, Manukau, Northland, and Waikato regions, New Zealand |
|
| Interventions |
Experimental: 4‐6 sessions of problem‐solving therapy in the 4 weeks following the index SH episode; postcards mailed at 1,2,3,4,6,8,10 and 12 months following the index SH episode; 1‐2 face‐to‐face or telephone patient support sessions over the 2‐week period following discharge from hospital to ensure patients were adhering to their agreed discharge plan; improved access to primary care via the provision of a voucher that could be used to access 1 free GP consultation; development of a risk management strategy; and a cultural assessment focused on identifying patients' sense of belonging and identification with their ethnic group Control: TAU involving referral to multidisciplinary teams for psychiatric/psychological assessment, intervention, or both; referral to crisis teams; or referral to community‐based drug or alcohol treatment teams as necessary Therapist: research clinicians (no further details on qualifications or experience were provided), mental health crisis and community mental health clinicians (no further details on qualifications or experience were provided), GPs, and substance misuse counsellors (no further details on qualifications or experience were provided) Type of therapy offered: mixture of brief psychosocial therapy, telephone contact, and postal intervention Length of treatment: 12 months |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide; iv) depression; v) hopelessness Excluded: i) anxiety; ii) quality of life; iii) sense of belonging; iv) ethnic identification |
|
| Notes |
Sources of funding: none stated Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "All eligible participants were allocated randomly to the intervention or usual care groups using a central computerised randomisation system at the Clinical Trials Research Unit (subsequently the National Institute for Health Innovation) . . . Stratified minimisation randomization was used to ensure a balance in key prognostic factors between the study groups" (p. 6 of the manuscript). Comment: Use of a computerised randomisation procedure is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | High risk |
Quote: "[P]articipants were allocated randomly. . . using a central computerised randomisation system at the Clinical Trials Research Unit (subsequently the National Institute for Health Innovation)" (p. 6 of the manuscript). Comment: Use of offsite randomisation could have ensured that allocation was concealed; however, use of Zelen's design makes it unlikely that participants and clinical personnel would have remained unaware of allocation. |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: " [T]he introduction to the study differed depending on whether [the participant was] randomised to the control or intervention group" (p. 3 of the manuscript). Comment: As consent was obtained using Zelen's method, participants were given the option to change treatment arms following allocation. Therefore, participants cannot have been blinded as to treatment allocation. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "The research assistants were blind to treatment allocation" (p. 6 of the manuscript). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Quote: "Analysis of the primary outcome was in everyone who was randomised. . . The secondary outcomes were analysed only in those people who had consented to be in the study" (p. 6 of the manuscript). Comment: mixture of intention‐to‐treat and per protocol analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to request data on suicides from authors, suggesting that selective reporting bias may have been present. |
| Other bias | High risk | Comment: Use of Zelen's design may have led to bias. |
Hatcher 2016a.
| Study characteristics | ||
| Methods |
Allocation: randomisation based on Zelen's method using a centrally‐generated randomisation sequence. A stratified minimisation procedure was also used to ensure balance in key prognostic factors (i.e., history of SH and method of SH) between the 3 sites. Follow‐up period: 12 months for primary outcome (i.e., hospital recorded repetition of SH) and 3 and 12 months for secondary outcomes (i.e., self reported repetition of SH, depression, and hopelessness) N lost to follow‐up: 0/365 (0%) for hospital re‐presentations for SH. |
|
| Participants |
Inclusion criteria: i) presented to the emergency department at 1 of the 3 participating hospitals following an episode of SH; ii) self identified as Māori; iii) able to communicate effectively in Te Reo Maori (Māori language) Exclusion criteria: i) aged less than 17 years; ii) still enrolled full‐time in school; iii) unable to provide informed consent Numbers: Of the 167 participants who provided informed consent, 95 were allocated to the experimental arm and 72 were allocated to the control arm. Profile: Of those who consented to participation 65.3% (n = 109) were female, 59.9% (n = 100) were multiple repeaters. Source: patients admitted to hospital following an episode of SH Location: Waitemata, Manukau, and Northland regions, New Zealand |
|
| Interventions |
Experimental: a culturally sensitive treatment framework consisting of 4‐6 sessions of problem‐solving therapy in the 4 weeks following the index SH episode; postcards mailed at 1,2,3,4,6,8,10 and 12 months following the index SH episode, 1‐2 face‐to‐face or telephone patient support sessions over the 2 week period following discharge from hospital to ensure patients were adhering to their agreed discharge plan, improved access to primary care via the provision of a voucher that could be used to access 1 free GP consultation, development of a risk management strategy, and a cultural assessment focused on identifying patients' sense of belonging and identification with Māori culture Control: TAU involving referral to multi‐disciplinary teams for psychiatric/psychological assessment, intervention, or both; referral to crisis teams; or referral to community‐based drug or alcohol treatment teams as necessary Therapist: research clinicians (no further details on qualifications or experience were provided), mental health crisis and community mental health clinicians (no further details on qualifications or experience were provided), GPs, and addiction counsellors (no further details on qualifications or experience were provided) Type of therapy offered: mixture of brief psychosocial therapy, telephone contact, and postal intervention Length of treatment: 12 months |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide; iv) depression; v) hopelessness Excluded: i) anxiety; ii) quality of life; iii) sense of belonging; iv) ethnic identification; v) cultural impact |
|
| Notes |
Sources of funding: none stated Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "All eligible participants were allocated randomly to the intervention or usual care groups using a central computerised randomization system at the Clinical Trials Research Unit (subsequently the National Institute for Health Innovation) . . . Stratified minimisation randomization was used to ensure a balance in key prognostic factors between the study groups" (p. 7 of the manuscript). Comment: Use of a computerised randomisation procedure is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | High risk |
Quote: " [P]articipants were allocated randomly using a central computerised randomisation system at the Clinical Trials Research Unit (subsequently the National Institute for Health Innovation) . . . " (p. 7 of the manuscript). Comment: Use of offsite randomisation could have ensured that allocation was concealed; however, use of Zelen's design makes it unlikely that participants and clinical personnel would have remained unaware of allocation. |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "[T]he introduction to the study differed depending on which arm of the trial [the participant was] randomised to" (p. 3 of the manuscript). Comment: As consent was obtained using Zelen's method, participants were given the option to change treatment arms following allocation. Therefore, participants cannot have been blinded as to treatment allocation. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "The research assistants were blind to treatment allocation" (p. 6 of the manuscript). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: mixture of intention‐to‐treat and per protocol analyses. Hospital‐recorded episodes of repeated SH, for example, were available for all 365 participants who were enrolled, whereas data on outcomes measured on a continuous scale (e.g., depression, hopelessness) are available for the 167 participants who provided informed consent. |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to request data on suicides from authors, suggesting that selective reporting bias may have been present. |
| Other bias | High risk | Comment: Use of Zelen's design may have led to bias. |
Hawton 1981.
| Study characteristics | ||
| Methods |
Allocation: random number method using sealed, opaque envelopes Follow‐up period: 12 months N lost to follow‐up: 0/96 (0%) for repetition of SH data |
|
| Participants |
Inclusion criteria: i) aged over 16 years; ii) suitable for randomisation (e.g., fixed abode) Exclusion criteria: i) in psychiatric care; ii) residing outside of catchment area; iii) requiring treatment for alcohol or dug addiction; iv) in need of inpatient psychiatric care Numbers: Of the 96 participants, 48 were allocated to the experimental arm and 48 to the control arm. Profile: 70% (n = 67) were female, 32% (n = 31) were multiple repeaters Source of participants: patients admitted to a general hospital following an episode of deliberate self‐poisoning Location: Oxford, UK |
|
| Interventions |
Experimental: domiciliary (home‐based) therapy, where the frequency of treatment sessions was flexible according to therapists' 'assessment of needs'. Open telephone access to the general hospital service was also available. Control: outpatient therapy once a week in an outpatient clinic in a general hospital Therapist: 2 junior psychiatrists, 1 psychiatric nurse, and 1 social worker Type of therapy offered: brief problem‐oriented psychological therapy Length of treatment: up to 3 months |
|
| Outcomes |
Included: i) repetition of SH according to hospital records, self report, and from a GP questionnaire; ii) compliance; iii) improvement in problems; iv) suicidal ideation Excluded: i) mood; ii) social adjustment; iii) GP questionnaire |
|
| Notes |
Sources of funding: "The project was supported by a grant from the Department of Health and Social Security" (p. 177). Declaration of author interests: no details on author interests provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "A random number method was used to select subjects" and "each patient was then allocated to 1 of the 2 treatment conditions by a randomized procedure" (p. 172) Comment: Use of a random numbers method is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Comment: Correspondence with authors clarified that sealed, opaque envelopes were used to conceal allocation. Use of opaque sealed envelope would ensure adequate allocation concealment. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "The assessor remained blind to the treatment offered" (p. 172). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 6% of patients were not available for post‐treatment assessment and 15% were not available for 6‐month assessment. No further details on whether intention‐to‐treat analyses were undertaken are provided, however. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Hawton 1987a.
| Study characteristics | ||
| Methods |
Allocation: randomisation using opaque envelopes according to a random number table in blocks of 8 with equal allocation to the experimental and control arms Follow‐up period: 12 months N lost to follow‐up: 0/80 (0%) for repetition of SH data |
|
| Participants |
Inclusion criteria: i) aged over 16; ii) registered with a general practitioner; iii) living up to 15 miles away from hospital; iv) suitable for outpatient counselling; iv) willing to accept aftercare offered Exclusion criteria: i) in need of psychiatric care (day‐patient or inpatient); ii) currently in psychiatric care Numbers: Of the 80 participants, 41 were allocated to the experimental arm and 39 to the control arm. Profile: 66% (n = 53) were female, 31% (n = 25) were multiple repeaters Source of participants: patients admitted to a general hospital following an episode of self‐poisoning Location: Oxford, UK |
|
| Interventions |
Experimental: up to 8 sessions, each lasting on average 54 minutes, of outpatient problem‐solving therapy delivered by non‐medical clinicians Control: GP care including individual support, marriage counselling, psychiatric referral, etc. Therapist: 5 counsellors from clinical team in the general hospital psychiatric service Type of therapy offered: problem‐solving therapy Length of treatment: not stated |
|
| Outcomes |
Included: i) repetition of SH according to hospital records, self report, collateral informant report, or from interviews with the participants' GP; ii) suicide; iii) depression; iv) improvement in problems Excluded: i) social adjustment; ii) attitudes to treatment; iii) General Health Questionnaire; iv) GP interview; v) compliance |
|
| Notes |
Sources of funding: "This study was supported by a grant from the Medical Research Council" (p. 760). Declaration of author interests: no details on author interests provided Other: As compliance data were not reported for the control group, this outcome had to be excluded from subsequent analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Patients were allocated by a randomized procedure" (p. 752). Comment: Correspondence with authors clarified that the allocation sequence was generated using a random number table in blocks or 8 with equal allocation to the experimental and control groups. Use of a random numbers table is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Comment: Correspondence with authors clarified that sealed, opaque envelopes were used to conceal allocation. Use of opaque sealed envelope would ensure adequate allocation concealment. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Assessment interviews were conducted by research interviewers, who, until towards the end of the second follow‐up, remained blind to which treatment group the patients had been allocated" (p. 753). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: combined use of hospital records and GP reports would enable information on all clinically treated SH episodes to be obtained, suggesting intention‐to‐treat analyses were undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Husain 2014.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a computer‐generated allocation sequence Follow‐up period: 3 and 6 months N lost to follow‐up: 4/221 (1.8%) by the 3‐month follow‐up period; 8/221 (3.6%) by the 6‐month follow‐up period |
|
| Participants |
Inclusion criteria: i) aged 16‐64 years; ii) living within the catchment area of 1 of the 3 participating university hospitals Exclusion criteria: i) requiring inpatient psychiatric treatment; ii) temporarily resident in the catchment area of 1 of the 3 participating university hospitals; iii) diagnosed with a mental disorder due to a general medical condition, substance misuse, dementia, delirium, substance dependence, schizophrenia, bipolar disorder, or an intellectual disability according to DSM‐IV criteria. Numbers: Of the 221 participants, 108 were allocated to the intervention arm and 113 were allocated to the control arm. Profile: 68.8% (n = 152) were female; 4.1% (n = 9) were multiple repeaters Source of participants: patients admitted to the medical unit a university hospitals following an episode of SH Location: Karachi, Sindh province, Pakistan |
|
| Interventions |
Experimental: manualised culturally adapted problem‐solving therapy based on principles of cognitive behavioural therapy involving an evaluation of the SH attempt, development of crisis management skills, use of problem‐solving and cognitive‐behavioural techniques to improve emotion regulation skills, negative thinking, interpersonal relationships, and to improve relapse prevention strategies. Control: TAU. The authors further clarify that "[p]atients are not routinely referred to psychiatric or psychological services" (p. 464). Therapist: qualified, Masters‐level psychologists with a minimum of 3 years postqualification clinical experience. Clinicians also received training in delivering the intervention treatment. Type of therapy offered: problem‐solving therapy Length of treatment: 3 months |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide; iii) suicidal ideation; iv) depression; v) hopelessness; vi) problem‐solving; vii) compliance Excluded: i) quality of life; ii) help‐seeking behaviours; iii) days spent in inpatient treatment; iv) attendances at outpatient clinics; v) GP consultations; vi) consultations with any other doctors; vii) consultations with non‐medical religious healers; viii) consultations with non‐medical homeopathic healers |
|
| Notes |
Source of funding: "This study was jointly funded by the University of Manchester and Pakistan Institute of Learning and Living" (p. 469). Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: " [A]n allocation sequence . . . was based on a computer‐generated list of random numbers. . . Randomisation was performed using www.randomization.com. Participants meeting the entry criteria were randomly allocated to each condition in a 1:1 ratio using permuted blocks of 6" (p.463). Comment: Use of a computer‐generated list is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: " [A]n allocation sequence. . . was provided by the off‐site statistician (independent of the research team)" (p. 463). |
| Blinding (performance bias and detection bias) Of participants | High risk | Quote: "It was not possible to keep the. . . participants themselves masked to the group allocation" (p. 463). |
| Blinding (performance bias and detection bias) Of personnel | High risk | Quote: "It was not possible to keep the clinicians at participating centres. . . masked to the group allocation" (p.463). |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Research assistants [were] masked to treatment allocation" (p. 463). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Comparisons between groups were made on an intention‐to‐treat basis" (p.465). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Hvid 2011.
| Study characteristics | ||
| Methods |
Allocation: randomisation stratifying for: i) history of multiple suicide attempts; ii) history of previous psychiatric treatment; iii) use of alcohol during the index suicide attempt Follow‐up period: 6 months N lost to follow‐up: 8/133 (6.0%) for repetition of SH |
|
| Participants |
Inclusion criteria: i) admitted to an emergency department or clinical department following an episode of SH Exclusion criteria: i) less than 12 years old; ii) diagnosed with any major psychiatric illness, including: schizophrenia, other psychoses, bipolar disorder, major depression, psychotic depression, mental retardation, and severe dementia; iii) unable to communicate in Danish without an interpreter Numbers: Of the 133 participants, 69 were allocated to the experimental arm and 64 to the control arm. Profile: 71.4% (n = 95) were female, 38.3% (n = 51) were multiple attempters Source of participants: patients admitted to an emergency or clinical department following an episode of SH. Location: Amager, Denmark |
|
| Interventions |
Experimental: assertive outreach delivered according to the Baerum model involving assertive outreach via home visits, telephone calls, email messages, and text messages, solution‐focused problem‐solving therapy, adherence therapy, and treatment continuity as participants were contacted by the same psychiatric nurse (as far as practical) throughout the course of treatment (Dieserud 2000). Control: TAU involving encouraging participants to contact their GP who could, where required, refer the participant on to further psychiatric or psychological treatment. Therapist: 1 consultant‐level psychiatrist and 2 psychiatric nurses Type of therapy offered: assertive outreach and compliance enhancement Length of treatment: maximum period of 6 months |
|
| Outcomes |
Included: i) repetition of SH according to hospital records; ii) suicide according to coroner's records Excluded: none |
|
| Notes |
Sources of funding: "The trial has been funded by a grant from the Danish Ministry of Social Affairs, the Lundbeck Foundation and the Health Insurance Foundation" (p. 297). Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "We applied a stratified randomization procedure . . . this stratified randomization procedure. . . created eight categories and randomization was performed for each independently" (p. 293). Comment: Although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed by an independent office" (p. 293). |
| Blinding (performance bias and detection bias) Of participants | High risk | Quote: "The patient. . . knew who was a case and who was a control" (p. 293). |
| Blinding (performance bias and detection bias) Of personnel | High risk | Quote: "[I]ntervention staff knew who was a case and who was a control" (p. 293). |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: " [I]ndependent assessors (three psychiatrists) who reviewed all incidents did not have this information" on who had been allocated to the experimental or control arms (p. 294). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Outcomes were measured by an intent‐to‐treat design in which all patients were followed until the end of the trial, irrespective of whether the patient was still receiving or complying with the assigned treatment" (p. 294). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Kapur 2013a.
| Study characteristics | ||
| Methods |
Allocation: randomisation using web‐based randomisation software Follow‐up period: 12 months N lost to follow‐up: 0/66 (0%) by the 12‐month follow‐up period |
|
| Participants |
Inclusion criteria: i) aged 18 or older; ii) resident in Manchester, UK; iii) admitted to emergency departments following an episode of SH Exclusion criteria: i) required admission to a psychiatric unit; ii) not in possession of a telephone; iii) required admission to a general hospital for a period of greater than 7 days; iv) lived outside of the catchment area; v) experienced deterioration in psychosis symptoms; vi) denied having engaged in SH; vii) declined to participate Numbers: Of the 66 participants, 33 were allocated to the intervention arm and 33 to the control arm. Profile: no details are provided, although the authors note "[i]ntervention and usual treatment groups were similar in terms of age, gender" (p. 73). Source of participants: admissions to emergency departments following an episode of SH Location: Manchester, UK |
|
| Interventions |
Experimental: outreach involving mailing of an information leaflet listing both local and national sources of support, 2 semi‐structured telephone calls, and a series of letters mailed at 1, 2, 4, 6, 8, and 12 months designed to facilitate referral to appropriate specialist treatment as required Control: TAU involving referral to mental health services, social services, or voluntary‐sector services as required Therapist: clinical researchers. No other details on qualifications or experience were provided. Type of therapy offered: outreach through telephone contact and the mailing of frequent letters encouraging participants to make contact with the service Length of treatment: 12 months |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide Excluded: i) number of emergency department attendances; ii) number of days on a medical inpatient ward; iii) number of face‐to‐face contacts with mental health services; iv) number of admissions to psychiatric inpatient services |
|
| Notes |
Source of funding: "commissioned by the National Institute for Health Research (NIHR) under its Program Grants for Applied Research scheme (RP‐PG‐0606‐1247)" (p. 74). Declaration of author interest: "N.K. chaired the Naitonal Institute for Health and Clinical Excellence (NICE) guideline development group and evidence for the longer‐term management of self‐harm. N.K., D.G., K.H. are members of the National Suicide Prevention Strategy Advisory Group" (p. 73). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomisation was conducted via a remote Internet‐based service (www.sealedenvelope.com)" (p. 73). Comment: Use of computer‐based randomisation software is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment provided |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "All outcome data were collected by researchers masked to allocation status" (p. 73). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Primary analysis was on an intention‐to‐treat basis" (p. 73). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Kawanishi 2014.
| Study characteristics | ||
| Methods |
Allocation: randomisation using web‐based randomisation software using minimisation to ensure balance between the treatment and control groups with respect to site, sex, age, and history of episodes of SH prior to the index episode Follow‐up period: 18 months to 5 years N lost to follow‐up: 0/914 (0%) by the 18‐month follow‐up period |
|
| Participants |
Inclusion criteria: i) aged 20 years or older; ii) admitted to the emergency department at 1 of 17 hospitals following a suicide attempt; iii) have at least 2 prior suicide attempts rated as having definite suicidal intent as determined by scores on the Suicide Intent Scale; iv) diagnosed with any axis I psychiatric disorder according to DSM‐IV‐TR criteria; v) able to understand the trial procedure; vi) provide informed consent; vii) attend a face‐to‐face interview; viii) attend a psychoeducation session during their hospital admission Exclusion criteria: i) diagnosed with any psychiatric disorder which did not meet DSM‐IV‐TR criteria Numbers: Of the 914 participants, 460 were allocated to the intervention arm and 454 to the control arm. Profile: 56.2% (n = 514) were females, 49.2% (n = 450) had multiple episodes of attempted suicide Source of participants: admissions to emergency departments following a suicide attempt Location: various locations around Japan |
|
| Interventions |
Experimental: assertive outreach and case management involving contact with patients at week 1 and 1, 2, 3, 6, 12, and 18 months after the index suicide attempt with a view to collecting information about the participant's treatment status and any problems that could interfere with treatment adherence, providing encouragement to remain adherent with treatment, coordination and referral to appointments with psychiatrists and any other primary care physicians, outreach for those who had dropped out of treatment, referral to social services and other support organisations as needed, psycho‐education, and access to a dedicated website designed to provide participants with information and resources Control: enhanced usual care. No further details on treatment content provided Therapist: mixture of psychiatrists, nurses, social workers, and clinical psychologists Type of therapy offered: assertive outreach Length of treatment: 18 months |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide Excluded: none |
|
| Notes |
Source of funding: "This study was funded by the Ministry of Health, Labour, and Welfare of Japan" (p. 200) Declaration of author interest: no conflicts of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Participants were randomly assigned (1:1) by and Internet‐based system . . . to either the intervention group (assertive case management) or the control group (enhanced usual care). Assignment was by the minimisation method, with four factors: participating hospital, sex, age. . . and history of previous suicide attempts before the current episode. We regarded these as factors that could affect the study outcomes." (p. 194) Comment: Use of computer‐based randomisation software is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Participants were randomly assigned. . . by an Internet‐based system operated by a central, independent data centre" (p. 194) Comment: use of offsite randomisation would have ensured that allocation was concealed. |
| Blinding (performance bias and detection bias) Of participants | High risk | Quote: "Outcome assessors were masked to group assignment, but patients. . . were not" (p. 194). |
| Blinding (performance bias and detection bias) Of personnel | High risk | Quote: "Outcome assessors were masked to group assignment, but. . . case managers who provided the interventions were not" (p. 194). |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Outcome assessors were masked to group assignment. . . The assessors did not know the participants' assigned groups, the status of implementation of the intervention or information about events obtained by other on‐site staff" (p. 194). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analyses were done in accordance with the intention‐to‐treat principle" (p. 196) |
| Selective reporting (reporting bias) | High risk | Comment: outcomes determined ad hoc. In addition, data on some protocol‐specified outcomes (e.g., number of repeat SH episodes, hopelessness) are yet to be published. |
| Other bias | Unclear risk | Comment: sample was biased towards more compliant patients who were willing and able to attend a psycho‐education session seminar at the commencement of treatment and were able to attend hospital regularly for face‐to‐face interviews and case management sessions. Additionally, those individuals who had engaged in non‐suicidal SH were excluded from participation. |
Liberman 1981.
| Study characteristics | ||
| Methods |
Allocation: random assignment Follow‐up period: 24 months N lost to follow‐up: 0/24 (0%) for repetition data |
|
| Participants |
Inclusion criteria: i) at least 1 previous suicide attempt Exclusion criteria: i) diagnosed with psychosis; ii) addicted to drugs and alcohol; ii) diagnosed with organic brain syndrome. Numbers: Of the 24 participants, 12 were assigned to the experimental arm and 12 to the control arm. Profile: 16 (67%) were female, 24 (100%) were multiple repeaters, 24 (100%) were diagnosed with depressive neurosis, most met criteria for personality disorder Source of participants: patients referred by psychiatric emergency services or hospital A&E departments following an episode of SH. Location: Los Angeles, CA, USA |
|
| Interventions |
Experimental: inpatient treatment involving behaviour therapy. Treatment consisted of social skills training, anxiety management, and family therapy. A therapeutic milieu with a token economy was also established. Aftercare at a community mental health centre or with a private therapist was also used as required. Control: inpatient treatment involving insight oriented therapy. Treatment consisted of individual therapy, group therapy and psychodrama, and family therapy. A therapeutic milieu with a token economy was also established. Aftercare at a community mental health centre or with a private therapist was also used as required. Therapist: 1 psychologist assisted by 2 bachelor level technicians Type of therapy offered: behavioural therapy Length of treatment: 10 days |
|
| Outcomes |
Included: i) repetition of SH according to self report; ii) suicidal ideation; iii) depression Excluded: i) reinforcement; ii) assertiveness; iii) fear |
|
| Notes |
Source of funding: "The project was made possible by grant MH 22804 from the Clinical Research Branch of the National Institute of Mental Helath to Michael Serber, MD, and R.P.L., the co‐principal investigators." (p. 1130). Declaration of author interests: no details on author interests provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients were randomly assigned" (p. 1127). Comment: Although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details on allocation concealment were provided. |
| Blinding (performance bias and detection bias) Of participants | Unclear risk | Comment: No information on participant blinding was provided. However, both treatments were so similar that it is possible participants were unaware of which treatment they were receiving. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details on outcome assessor blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 4 participants dropped out during the early stages of the trial (2 in each arm) and were not included in any subsequent analyses, suggesting researchers undertook per protocol analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Linehan 1991.
| Study characteristics | ||
| Methods |
Allocation: randomised allocation via computer programme Follow‐up period: 24 months N lost to follow‐up: 24/63 (38.1%) participants were deliberately not included in the 24‐month follow‐up. |
|
| Participants |
Inclusion criteria: i) female; ii) diagnosed with borderline personality disorder; iii) at least 2 suicide attempts in the last 5 years, with at least 1 in the previous 8 weeks; iv) aged 18‐45 years; v) agree to trial conditions Exclusion criteria: Numbers: Of the 63 participants, 32 were allocated to the experimental arm and 31 to the control arm. Profile: 63 (100%) were female, 63 (100%) were multiple repeaters with multiple episodes of SH each and who were at high risk of further episodes of SH, 63 (100%) were diagnosed with borderline personality disorder Source of participants: clinically referred patients who had at least 1 episode of SH in the last 8 weeks Location: Seattle, WA, USA |
|
| Interventions |
Experimental: dialectical behaviour therapy involving cognitive behavioural treatment developed specifically for the treatment of for suicidal patients with borderline personality disorder (see Linehan 1993a), which targets increasing behavioural capabilities and motivation for treatment whilst also reinforcing functional behaviour. The manualised treatment consisted of 1 h per week of individual psychotherapy, 2.5 h per week of group skills training, telephone consultation as required (within each therapists' limitations), and weekly therapist team meetings. Control: TAU involving referral to alternative therapy Therapist: 5 psychologists, 1 clinical psychology graduate, and 1 psychiatrist Type of therapy offered: dialectical behaviour therapy Length of treatment: 12 months |
|
| Outcomes |
Included: i) repetition of SH according to self report; ii) suicide; iii) compliance; iv) depression; v) suicidal ideation; vi) hopelessness Excluded: i) psychiatric admissions; ii) reasons for living |
|
| Notes |
Sources of funding: "This research was supported by grant MH34486 from the National Institute of Mental Health, Bethesda, Md (Dr Linehan). Declaration of author interests: Although no details on author interests were provided, Dr. Linehan was developed dialectical behaviour therapy. Other: Half (50%) of the self reported episodes of SH were checked against medical records, therapist records, and observer/nurse/physician ratings. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "randomized" (p. 1060; 1991 article). Comment: Correspondence with authors clarified that they used a computer programme to generate the random sequence. Use of a computerised randomisation sequence is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Comment: Correpondence with authors clarified that allocation had been concealed. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Every effort was made to keep the assessors blind about treatment condition" (p. 1061). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: subsequent analyses appear to be based on those participants with available information at each follow‐up period, suggesting investigators undertook per protocol analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Linehan 2006.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a computerised adaptive minimisation procedure whereby participants were matched using 5 primary prognostic variables: i) number of lifetime suicide attempts or non‐suicidal self injuries combined; ii) number of psychiatric hospitalisations; iii) history of only suicide attempts, only non‐suicidal self‐injury, or both; iv) age; v) Beck Depression Inventory score > 30 or a Global Assessment of Functioning score < 45 for any comorbid condition Follow‐up period: 24 months N lost to follow‐up: 0/101 (0%) for repetition data |
|
| Participants |
Inclusion criteria: i) 18‐45 years; ii) female; iii) met criteria for borderline personality disorder; iv) at least 2 suicide attempts or episodes of SH in the past 5 years, with at least 1 in the past 8 weeks Exclusion criteria: i) lifetime diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, psychotic disorder not otherwise specified, or mental retardation; ii) seizure disorder requiring medication; iii) mandate to treatment; iv) requiring primary treatment for another debilitating condition Numbers: Of the 101 participants 52 were allocated to the experimental arm and 49 to the control arm. Profile: 101 (100%) were female, 97 (96%) had a lifetime diagnosis of a depressive disorder, 73 (72.3%) were diagnosed with major depression, and 30 (29.7%) had substance abuse. Source of participants: clinical referrals and individuals attending inpatient units, emergency rooms and outpatient clinics Setting: Seattle, WA, USA |
|
| Interventions |
Experimental: dialectical behavior therapy involving cognitive behavioural treatment developed specifically for the treatment of for suicidal patients with borderline personality disorder (see Linehan 1993a), which targets increasing behavioural capabilities and motivation for treatment whilst also reinforcing functional behaviour. The manualised treatment consisted of 1 h weekly individual psychotherapy, 2.5 h weekly group skills training, telephone consultation as required (within each therapists' limitations), and weekly therapist team meetings. Control: community treatment by experts specifically designed for the trial to control for factors previously uncontrolled in DBT trials. Whilst similar to TAU, as therapists were free to decide on type and dose of therapy they believed was most suited to the patient (minimum of 1 scheduled individual session per week), the characteristics of therapists were controlled via selection of therapists and supervisory arrangements. Therapists: specially trained to provide either experimental or control therapy Type of therapy offered: dialectical behaviour therapy Length of treatment: 1 year |
|
| Outcomes |
Included: i) repetition of SH according to the Suicide Attempt Self‐Injury Interview; ii) suicide; iii) suicidal ideation; iv) depression; v) compliance Excluded: i) severity of SH episode; ii) importance of reasons for living; iii) use of additional service (e.g., re‐presenting to A&E) |
|
| Notes |
Sources of funding: "This study was supported by grants MH34486 and MH01593 from the National Institute of Mental Health" (p. 765). Declaration of author interests: although no details on author interests were provided, Dr Linehan developed dialectical behaviour therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Computerized adaptive minimization randomization procedure" (p. 758) Comment: Use of a computerised randomisation sequence is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details on allocation concealment provided |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "Initial assessments were done before informing subjects of treatment assignment" (p. 758). Comment: suggests participants were subsequently informed of treatment allocation |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Assessments were conducted by blinded independent clinical assessors" (p. 758). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "[W]e examined the effects of differential missing data and treatment dropout on each of our major outcome variables and found no evidence that the findings were biased by these differences" (p. 760). Comment: 111 participants were randomised, 10 were pilot cases (not analysed), 20 were lost to follow‐up, and 21 discontinued interventions. 101 were analysed overall. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Marasinghe 2012.
| Study characteristics | ||
| Methods |
Allocation: randomisation using an unknown method Follow‐up period: as this was a cross‐over trial, only data from the first follow‐up period at 6 months was extracted N lost to follow‐up: 0/68 (0%) for the 6‐month follow‐up period |
|
| Participants |
Inclusion criteria: i) aged 15‐74 years; ii) admitted to hospital following an episode of SH; iii) episode of SH was associated with significant suicidal intent as reported either at the intake interview or according to scores on Beck's Scale for Suicidal Ideation; iv) considered likely to be discharged from hospital within 2 days or able to be re‐approached if admitted for longer than 2 days; v) able to provide informed consent Exclusion criteria: i) currently receiving ongoing psychiatric treatment; ii) diagnosed with psychosis; iii) diagnosed with dementia Numbers: Of the 68 participants, 34 were allocated to the intervention arm and 34 to the control arm Profile: 50.0% (n = 34) were female Source of participants: patients admitted to hospital following an episode of SH Location: Colombo, Sri Lanka |
|
| Interventions |
Experimental: brief mobile treatment involving an assessment of mental health, meditation, problem‐solving therapy, interventions to increase social support, interventions to address alcohol or other substance misuse problems, a series of 10 telephone calls to reaffirm techniques learnt during treatment, the ability to access telephone messages to reaffirm techniques learnt during treatment, and a series of up to 26 text messages to encourage the participant to practice meditation techniques, problem‐solving skills, to seek social support, to avoid alcohol and other drugs, and to use the telephone helpline to get individual support in times of crisis Control: wait list Therapist: no details on qualifications or experience provided Type of therapy offered: brief problem‐solving treatment via mobile telephone Length of treatment: up to 26 weeks |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide reattempts; iii) suicide; iv) suicidal ideation; v) depression Excluded: i) medical outcomes; ii) alcohol use; iii) drug use; iv) substance use severity |
|
| Notes |
Sources of funding: "We are grateful for funding from the Improving Relevance and Quality of Undergraduate Education (IRQUE) project of the University of Jeyewardenepura, Sri Lanka" (p. 155). Declaration of author interests: no details on author interests provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The participants were randomly allocated" (p. 152). Comment: Although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment provided |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "The assessor was blind to the treatment" (p. 152). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Intention to treat analyses. . . " (p. 152). |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to request data on repetition of SH, suicide reattempts, and suicide from authors, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
McAuliffe 2014.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a computer‐generated sequence of numbers stratified by: i) sex; ii) repeater status; iii) site Follow‐up period: 6 and 12 months N lost to follow‐up: 107/433 (24.7%) by the 6‐month follow‐up period |
|
| Participants |
Inclusion criteria: i) aged 18‐64 years; ii) engaged in SH in the previous 3 days Exclusion criteria: i) diagnosed with psychosis, intellectual disability, sensory disability, or an organic cognitive impairment; ii) currently substance dependent according to scores on the Short Alcohol Dependent Data questionnaire; iii) imprisoned; iv) of no fixed abode Numbers: Of the 433 participants, 222 were allocated to the experimental arm and 211 to the control arm. Profile: 64.4% (n = 279) were female; 29.3% (n = 127) were multiple repeaters Source of participants: admissions to the emergency department following an episode of SH, or patients engaging in SH on acute psychiatric facilities even if this did not necessitate admission to the emergency department Location: Cork and Limerick, Republic of Ireland |
|
| Interventions |
Experimental: problem‐solving skills training involving manualised, group‐therapy sessions of interpersonal problem‐solving skills training Control: TAU involving assessment by mental health professional staff and by crisis staff, and referral to acute mental health or community‐based services, psychotherapy, and pharmacotherapy as necessary Therapist: 1 therapist and 1 co‐therapist who received training in the delivery of problem‐solving skills training Type of therapy offered: problem‐solving group therapy Length of treatment: 6 weeks |
|
| Outcomes |
Included: i) repetition of SH; ii) suicides; iii) suicidal ideation; iv) depression; v) hopelessness; vi) problem‐solving; vii) compliance Excluded: i) anxiety; ii) impulsiveness; iii) generalised self efficacy; iv) social life confiding/emotions skills; v) social life practical support skills; vi) social life negative skills |
|
| Notes |
Source of funding: "This work was supported by funding from the Health Service Executive (HSE) South, HSE Mid‐West, the HSE National Office for Suicide Prevention, the Health Research Board and Pobal‐Dormant Accounts Fund in Ireland" (p. 389). Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "[P]articipants were randomly assigned to treatment conditions on the basis of a computer generated sequence of numbers" (p. 384). Comment: Use of a computerised randomisation sequence is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was concealed using sealed opaque envelopes" |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: Correspondence with authors confirmed that participants were not blinded to treatment allocation. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: Correspondence with authors confirmed that personnel were not blinded to treatment allocation. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: " [R]esearchers [were] masked to participant treatment allocation" (p. 384). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: subsequent analyses appear to be based on those participants with available information at each follow‐up period, suggesting per protocol analyses were undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
McLeavey 1994.
| Study characteristics | ||
| Methods |
Allocation: randomisation using an open random number table Follow‐up period: 12 months N lost to follow‐up: 6/39 (15.4%) for repetition data |
|
| Participants |
Inclusion criteria: i) aged 15‐45 years Exclusion criteria: i) history of psychosis, mental retardation, or organic cognitive impairment; ii) requiring psychiatric treatment (day care or inpatient) Numbers: Of the 39 participants, 19 were allocated to the experimental arm and 20 to the control arm. Profile: 29 (74%) were female, 14 (35.6%) were multiple repeaters, 9 (23%) were diagnosed with dysthymia, 6 (15%) had dependent personality disorder, and 5 (13%) had alcohol abuse Source of participants: patients admitted to an A&E department following an episode of self‐poisoning Location: Cork, Republic of Ireland |
|
| Interventions |
Experimental: interpersonal problem‐solving skills training involving a manualised training regimen including instruction, active discussion, reflective listening, modelling, coping strategy, role playing, sentence completion, and prompting Control: brief problem‐solving therapy involving therapy focused on patients' current problems and prevention by helping patients gain insight into problems. No specific skills training Therapist: clinical psychologists and psychiatry registrars Type of therapy offered: interpersonal problem‐solving therapy Length of treatment: 5 weeks |
|
| Outcomes |
Included: i) repetition of SH according to hospital records and a GP questionnaire; ii) suicide; iii) compliance; iv) hopelessness; v) problem‐solving; vi) number of problems Excluded: i) self perception; ii) Optional Thinking Test; iii) awareness of consequences |
|
| Notes |
Sources of funding: no details on funding provided Declaration of author interests: Although no details on author interests were provided, Dr McLeavey was the developer of interpersonal problem‐solving skills training. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: " [P]articipants were assigned on a random basis to the two treatment groups using an open random number table" (p. 384). Comment: As the numbers table was open, it is possible there may have been bias in the generation of the random sequence. |
| Allocation concealment (selection bias) | High risk | Comment: As an open numbers table was used, it is possible there was bias in allocation. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "An independent assessor, blind to the treatment conditions in which the patients had participated, administered both pretreatment and post‐treatment measures" (p. 385). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Of the 50 randomised participants, 5 dropped out of treatment before completion and 6 were lost to follow‐up. Only the 39 participants that completed the trial were included in all subsequent analyses, however, suggesting that analyses were per protocol. |
| Selective reporting (reporting bias) | High risk | Comment: Numerical data on problem‐solving were not reported, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
McMain 2009.
| Study characteristics | ||
| Methods |
Allocation: randomisation using pre‐generated block procedure Follow‐up period: outcomes after 1 year of active treatment are reported in this review N lost to follow‐up: unclear |
|
| Participants |
Inclusion criteria: i) met DSM‐IV criteria for borderline personality disorder;ii) 18‐60 years old; iii) at least 2 episodes of suicidal or non‐suicidal self injurious acts in the past 5 years with at least 1 in the 3 months proceeding enrolment Exclusion criteria: i) meeting DSM‐IV criteria for a psychotic disorder, bipolar I disorder, delirium, dementia, or mental retardation; ii) diagnosed with substance dependence in the preceding 30 days; iii) live outside of a 40‐mile radius of Toronto; iv) have a serious medical condition likely to require hospitalisation within the next year (e.g., cancer); v) have plans to leave the province of Ontario within the next 2 years Numbers: of the 180 participants, 90 were allocated to the experimental arm and 90 to the control arm. Profile: 155 (86.1%) were female, 180 (100%) were multiple repeaters, 180 (100%) met criteria for Borderline Personality Disorder, 135 (75%) had a current diagnosis of any anxiety disorder, 17 (9.4)% had a current diagnosis of substance abuse, 88 (48.9%) had a current diagnosis of major depression, 39 (21.7%) had a current diagnosis of panic disorder, and 71 (37.4%) had a current diagnosis of PTSD Source of participants: patients attending a specialised Centre for Addiction and Mental Health, hospital or both Location: Toronto, ON, Canada |
|
| Interventions |
Experimental: manualised dialectical behaviour therapy involving 1 h weekly sessions of individual therapy, 2 h weekly sessions of skills group training, and 2 h weekly of telephone‐based coaching aimed at providing psycho‐education about borderline personality disorder, improving personal relationships, and providing validation and empathy, within a 'here and now' focus on the prevention of self‐harm and suicidal behaviour. Additionally, therapists' attended weekly therapist team meetings. Control: general psychiatric management involving 1 h weekly sessions of individual therapy focused on improving medication management through the use of a structured drug algorithm. Participants also received psycho‐education about borderline personality disorder, improving personal relationships, and providing validation and empathy, within a 'here and now' focus. Additionally, therapists' attended weekly therapist team meetings. Therapists: 7 doctoral‐level clinicians and 1 board‐certified psychiatrist Type of therapy offered: dialectical behaviour therapy Length of treatment: 12 months |
|
| Outcomes | Included: i) repetition of SH; ii) suicide; iii) depression; iv) number completing full 1 year course of treatment Excluded: i) repetition of NSSI |
|
| Notes |
Sources of funding: "Supported by grant 200204MCT‐101123 from the Canadian Institutes for Health Research" (p. 1373). Declaration of author interests: "Dr. Links has received an unrestricted educational grant from Eli Lilly Canada Inc. All other authors report no competing interests" (p. 1373). Other: data on hospital admissions for self‐harm obtained from self report following clinician interview |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Eligible participants were randomly assigned to treatment arms using a pre‐generated block randomization scheme developed and held by the statistician" (p. 1366) Comment: Use of a pre‐generated block randomisation sequence is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "[T]he statistician, who prepared 45 sealed envelopes, each containing the group allocations in random order for four participants" (p. 1366) Comment: although no details on whether the envelopes were opaque is not provided, it is likely they were thereby ensuring adequate allocation concealment. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: As this was a single blind trial, participants were aware of the treatment group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk |
Quote: "The study coordinator… was not blind to treatment assignment" (p. 1366) Comment: As this was a single blind trial, all personnel, not just the trial coordinator, are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Assessors . . . were. . . blind to treatment assignment" (p. 1366). Additionally, "Assessors were polled after the treatment phase to ascertain whether they could correctly guess participants' treatment assignment; they did not know treatment assignment for 86% of the cases, suggesting that blinding was largely maintained" (p. 1366). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All results were analysed using an intent‐to‐treat analysis (n = 180). We also conducted a per‐protocol analysis based on 'treated' participants, defined as those who were in treatment for at least 8 weeks from initial session to last session. This included a total of 167 patients (dialectical behavior therapy, n = 85; general psychiatric management, n = 82)" (p. 1370). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Morgan 1993.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a supply of sealed envelopes, half of which contained an emergency green card Follow‐up period: 12 months N lost to follow‐up: 0/212 (0%) for repetition data |
|
| Participants |
Inclusion criteria: i) no previous episode of SH; ii) resident within healthcare trust catchment area Exclusion criteria: none stated Numbers: of the 212 participants, 101 were allocated to the experimental arm and 111 to the control arm Profile: 25% (n = 53) were diagnosed with any depressive disorder, 100% (n = 212) were non‐repeaters Source of participants: patients admitted to hospital following first episode of SH Location: Bristol, UK |
|
| Interventions |
Experimental: emergency green card in addition to TAU. The green card outlined that a doctor was available by telephone and how to contact them. Control: TAU involving referral to the primary healthcare team, and psychiatric or inpatient admissions if required Therapist: telephone contact, face‐to‐face interviews, or both conducted by a doctor on‐call Type of therapy offered: emergency green card Length of treatment: 12 months |
|
| Outcomes |
Included: i) repetition of SH according to hospital, psychiatric, and GP records Excluded: i) use of the green card; ii) admission to psychiatric hospital; iii) use of psychiatric services |
|
| Notes |
Source of funding: no details on funding were provided Declaration of author interests: no details on author interests provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Allocation to experimental or control group was carried out by random selection from a supply of closed envelopes, half of which contained the green card" (p. 111) Comment: Randomisation using sealed envelopes is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk |
Quote: "closed envelopes" (p. 111) Comment: Although authors provide no details on whether the envelopes were opaque, it is likely they were, thereby ensuring adequate allocation concealment. |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "[Patients] receive[ed] the green card" (p. 111) Comment: suggests participants would have known to which treatment arm they had been allocated |
| Blinding (performance bias and detection bias) Of personnel | Unclear risk |
Quote: "GPs were also sent copies of the green card" (p. 111) Comment: It is unclear if they knew which of their patients received the intervention. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details on outcome assessor blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "Data concerning outcome were obtained for all patients included in the study" (p. 111). Comment: Subsequent analyses include all those randomised to the experimental and control groups, suggesting intention‐to‐treat analyses were undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Morthorst 2012.
| Study characteristics | ||
| Methods |
Allocation: randomisation using computer‐based software and stratified by: i) history of suicide attempts; ii) history of psychiatric treatment or hospitalisation; iii) alcohol consumption at the time of the index suicide attempt Follow‐up period: 12 months N lost to follow‐up: 0/243 for primary outcomes (suicide reattempts, suicide). 74/243 (30.4%) for secondary outcomes (depression, compliance) |
|
| Participants |
Inclusion criteria: i) 12 years or older; ii) admitted to acute emergency units, intensive care units, paediatric units, or psychiatric emergency room units following a suicide attempt Exclusion criteria: i) living in an institution; ii) admitted to a psychiatric unit for more than 14 days; iii) diagnosed with a schizophrenia‐spectrum disorder; iv) diagnosed with severe depression, bipolar disorder, or dementia; v) currently receiving outreach services from social service agencies Numbers: Of the 243 participants, 123 were allocated to the intervention arm and 120 to the control arm. Profile: 75.7% (n = 184) were female; 53.5% (n = 130) were multiple repeaters Source of participants: patients admitted to acute emergency units, intensive care units, paediatric units, or psychiatric emergency room units following a suicide attempt Location: Copenhagen, Denmark |
|
| Interventions |
Experimental: assertive intervention involving case management, crisis intervention as required, problem‐solving therapy, and assertive outreach based on motivational support to encourage patients to attend treatment sessions, assist patients to attend these sessions, and to improve adherence to after‐treatment in addition to TAU Control: TAU involving referral to a range of different treatments depending on diagnosis, clinical, and social needs. Treatment included a psychiatric assessment and may also incorporate substance abuse treatment, psychological therapy, and GP referral as required. Pharmacological treatment was also provided where necessary. Therapist: psychiatric nurses who had received training in suicidology Type of therapy offered: assertive outreach Length of treatment: 6 months |
|
| Outcomes |
Included: i) suicide reattempts; ii) suicide; iii) depression; iv) compliance Excluded: none |
|
| Notes |
Sources of funding: "This study received funding from the Ministry of Health and Internal Affairs, Denmark, the National Board of Social Services, and independent subdivision of The Ministry of Social Affairs and Integration, TrygFoden, and Aase og Ejnar Danielsens Foundation" (p. 6). Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer randomisation was done. . . stratified by whether the patient had previously attempted suicide (first attempt v previous attempt), previous psychiatric contacts or hospitalisations (none v previous contacts), and alcohol consumption at the time of suicide attempt (none v alcohol consumption . . . The randomisation procedure ensured adequate sequence generation. . . " (p. 3). Comment: Use of a computer‐based randomisation procedure is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer randomisation was done by an independent research assistant . . . The randomisation procedure ensured adequate. . . allocation concealment" (p. 3) |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "[P]articipants were immediately informed of the outcome [i.e., allocation]" (p. 3). Comment: suggests participants were not blind as to treatment allocation |
| Blinding (performance bias and detection bias) Of personnel | High risk | Quote: "Owing to the nature of the study design, the intervention staff were not blinded" (p. 3). |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Quote: "An external medical evaluation committee conducted a blinded outcome assessment using medical records" (p. 3). However, authors later state that: "The researcher conducting the analyses on self‐reported outcomes was. . . not blinded." (p. 3). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All participants were included in the analysis regardless of subsequent adherence to treatment, according to the intention to treat principle" (p. 3). |
| Selective reporting (reporting bias) | Unclear risk | Comment: No reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Patsiokas 1985.
| Study characteristics | ||
| Methods |
Allocation: random allocation Follow‐up period: 3 weeks N lost to follow‐up: no details provided |
|
| Participants |
Inclusion criteria: i) admitted to a psychiatric ward following a suicide attempt Exclusion criteria: ii) diagnosed with psychosis; ii) diagnosed with substance abuse Numbers: Of the 15 participants, 10 were allocated to the experimental arms (5 to the cognitive restructuring arm and 5 to the problem‐solving arm), and 5 were allocated to the control arm. Profile: no details provided Source of participants: patients admitted to a psychiatric ward following a suicide attempt Location: Charleston, SC, USA |
|
| Interventions |
Experimental: 10 one‐hour sessions of cognitive restructuring with a focus on suicidal ideation or problem‐solving Control: non‐directive therapy involving open discussions about suicidal behaviour, problems, and daily life Therapist: The same therapist conducted therapy sessions for all 3 arms. Type of therapy offered: i) cognitive therapy; ii) problem‐solving therapy Length of treatment: 3 weeks |
|
| Outcomes |
Included: i) repetition of SH according to an unknown source; ii) suicidal ideation (measured in 2 ways); iii) hopelessness; iv) problem‐solving; v) problem‐solving skills Excluded: i) flexibility of thinking |
|
| Notes |
Sources of funding: no details provided Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Subjects were randomly assigned" (p. 282). Comment: Although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment provided |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: As the same therapist provided therapy for all 3 arms, personnel would have known which participant was receiving which treatment |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details on outcome assessor blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no details provided on whether intention‐to‐treat analyses were conducted |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Priebe 2012.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a computer‐generated algorithm Follow‐up period: 12 months N lost to follow‐up: 10/80 (12.5%) by the 12‐month follow‐up period |
|
| Participants |
Inclusion criteria: i) aged 16 years or older; ii) engaged in SH on 5 or more days in the year prior to randomisation; iii) diagnosed with at least 1 personality disorder Exclusion criteria: i) diagnosed with a severe learning disability that would interfere with the ability to benefit from DBT; ii) unable to read or write in English Numbers: Of the 80 participants, 40 were allocated to the intervention arm and 40 to the control arm. Profile: 87.5% (n = 70) were female Source of participants: referrals to a specialist DBT service Location: London, UK |
|
| Interventions |
Experimental: dialectical behaviour therapy delivered according to Linehan (i.e., Linehan 1993b) involving both individual and group‐based cognitive behavioural therapy, mindfulness, validation, supportive therapeutic techniques, and skills training. Out‐of‐hours telephone skills training was also available as required. Control: TAU involving referral back to the referee agency where the participant was encouraged to engage with any treatment other than DBT, including psychotherapy, referral to psychiatrists, mental health teams, counsellors, GPs, or other user‐run support services Therapist: no details on qualifications or clinical experience reported Type of therapy offered: dialectical behaviour therapy Length of treatment: 12 months |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide; iii) compliance. Excluded: i) days with SH; ii) borderline personality disorder symptom severity; iii) psychiatric disorder symptom severity; iv) quality of life |
|
| Notes | Sources of funding: "This paper. . . [was] funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit Programme (grant reference No. PB‐PG‐0906‐10540). All authors were funded by this grant with the exception of K.B. whose contribution was funded by the NIHR Doctoral Research Fellowship Scheme" (p. 364). Declaration of author interests: no author interests provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomisation was computer generated with a 1:1 allocation. . . using 6 blocks of 12 randomly permuted treatment allocation sequences, with a final block of 8." (p. 358). Comment: Use of a computerised randomisation sequence is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment provided |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "[T]he data analyst remained masked throughout the study period" (p. 358). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "[M]issing covariate values were . . . [estimated using] maximum likelihood estimation [to] ensure . . . unbiased parameter estimates. We. . . [also] conducted a sensitivity analysis with last observation carried forward." (p. 358). |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to obtain data on repetition of SH and suicides from authors, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Salkovskis 1990.
| Study characteristics | ||
| Methods |
Allocation: predetermined random allocation using sampling without replacement and sealed envelopes Follow‐up period: 12 months N lost to follow‐up: 0/20 (0%) for repetition data |
|
| Participants |
Inclusion criteria: i) aged 16‐65 years; ii) of fixed abode and living within Health Authority boundary; iii) antidepressants were taken as part of the self‐poisoning episode; iv) a history of 2 or more previous suicide attempts; v) Buglass and Horton Risk of Repetition Scale score of at least 4. Participants had to fulfil at least 2 criteria to be included. Exclusion criteria: i) not requiring immediate psychiatric treatment; ii) diagnosed with psychosis; iii) diagnosed with a serious organic illness. Numbers: of the 20 participants, 12 were allocated to the experimental arm and 8 to the control arm. Profile: 10 (50%) were female, 20 (100%) were multiple repeaters with a high risk of further repetition Source of participants: patients referred by the duty psychiatrist following an episode of self‐poisoning using antidepressant and assessed in an A&E department Setting: Leeds, UK |
|
| Interventions |
Experimental: 5 one‐hour sessions of domiciliary (home‐based) cognitive‐behavioural problem‐solving treatment Control: TAU Therapist: community psychiatric nurse Type of therapy offered: problem‐solving therapy Length of treatment: 1 month |
|
| Outcomes |
Included: i) repetition of SH according to hospital records; ii) depression; iii) hopelessness; iv) suicide; v) suicidal ideation (measured in 2 ways); vi) severity of 3 main problems; vii) problem‐solving Excluded: i) mood |
|
| Notes |
Sources of funding: no details provided Declaration of author interests: no details provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Predetermined random allocation" (p. 872) Comment: Correspondence with authors clarified that the method used was "sampling without replacement using envelopes". |
| Allocation concealment (selection bias) | Low risk | Comment: Correspondence with authors clarified that sealed envelopes were used to conceal allocation. Use of sealed envelopes would ensure adequate allocation concealment. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: Although it is possible that outcome assessors could have rated data on repetition of self‐poisoning from hospital records blind, other assessments were gathered by the same psychiatric nurse who delivered the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There were no treatment drop outs" (p. 872) |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to request data on suicides from authors, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Slee 2008.
| Study characteristics | ||
| Methods |
Allocation: randomisation by computer and random number generator Follow‐up period: 3 months, 6 months and 9 months N lost to follow‐up: 8/90 (21%) for repetition of SH data |
|
| Participants |
Inclusion criteria: i) recently engaged in self‐harm; ii) aged 15‐35; iii) Dutch‐speaking; iv) live in the Leiden region Exclusion criteria: i) diagnosed with a psychiatric disorder requiring intensive inpatient treatment; ii) diagnosed with a cognitive impairment Numbers: Of the 90 participants, 48 were allocated to the experimental arm and 42 to the control arm. Profile: Of the 82 participants who received the intervention, 77 (93.9%) were female. Source of participants: patients presenting to hospital/mental health centre following an episode of self‐harm Setting: Leiden, the Netherlands |
|
| Interventions |
Experimental: 12 sessions of CBT in addition to TAU Control: TAU involving psychotropic medication, psychotherapy, or hospitalisation as required Therapist: experienced CBT practitioners Type of therapy offered: cognitive‐behavioural therapy Length of treatment: 5.5 months |
|
| Outcomes |
Included: i) repetition of SH from self report; ii) suicide; iii) depression; iv) compliance; iv) problem‐solving Excluded: i) anxiety; ii) self esteem; iii) suicidal cognition; iv) use of psychological and psychiatric services |
|
| Notes |
Sources of funding: "Support for the study was provided by The Netherlands Organisation for Health Research and Development (AonMw) (contract grant number: 2100.0068)" (p. 210).
Declaration of author interests: none stated Other: Repetition data provided by participants was subjected to reliability analysis by comparing self reports to hospital records and information from treatment sessions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomisation to treatment was accomplished using a computer program and a random‐number generator provided by an independent investigator" (p. 203) Comment: use of a random‐number generator is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Comment: Correspondence with authors clarified that computerised, central allocation had been used to conceal allocation. |
| Blinding (performance bias and detection bias) Of participants | High risk | Quote: "Masking of follow up assessments was not possible because participants were asked about their use of healthcare services at each assessment" (p. 203). |
| Blinding (performance bias and detection bias) Of personnel | High risk |
Quote: "Masking of follow up assessments was not possible because participants were asked about their use of healthcare services at each assessment" (p. 203) Comment: As personnel were required to question participants about their use of healthcare services, this would suggest that personnel would have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | High risk | Comment: all measures were self reports. Participants were not blinded at follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: of the 90 participants randomised, 8 did not receive their allocated intervention and 9 were lost to follow‐up. Analyses are conducted both including and excluding these participants, suggesting a combination of per protocol and intention‐to‐treat analyses (using the LOCF method). |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to request data on suicides from authors, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Stewart 2009.
| Study characteristics | ||
| Methods |
Allocation: randomisation using the method of drawing names from a hat Follow‐up period: 2 months N lost to follow‐up: unknown as no apparent attempt was made to follow‐up patients who did not complete treatment |
|
| Participants |
Inclusion criteria: i) suicide attempt with self reported suicide intent; ii) admitted to 1 of the 2 participating hospitals Exclusion criteria: i) diagnosed with an intellectual disability; ii) current diagnosis of mania, psychosis, or both; iii) under 18 years. Correspondence with authors further clarified that 1 participant was subsequently excluded after randomisation due to being a frequent repeater of SH, possibly due to borderline personality disorder. Numbers: Of the 32 participants, 11 were allocated to the CBT arm, 12 were allocated to the PST arm, and 9 were allocated to the control arm. Profile: 53.1% (n = 17) were female Source of participants: patients admitted to 1 of 2 participating hospitals following a suicide attempt Location: Brisbane (QLD), Australia |
|
| Interventions |
Experimental: 4 weekly individual sessions of cognitive‐behavioural therapy or 7 weekly individual sessions of problem‐solving therapy. Cognitive‐behavioural therapy was offered as a manualised treatment involving elements of both Beck's cognitive behaviour therapy and Ellis' theory of rational emotive therapy (Ellis 1986; Ellis 1996). Problem‐solving therapy was also manualised and was based on the 6‐step model of D'Zurilla 1971. Control: TAU involving treatment by the hospital acute care team Therapist: treatment was provided by "the researcher" (p. 542). No further details on qualifications, training, or experience provided Type of therapy offered: i) cognitive‐behavioural therapy; ii) problem‐solving therapy Length of treatment: 2 months |
|
| Outcomes |
Included: i) suicide reattempts; ii) suicides; iii) suicidal ideation; iv) hopelessness; v) problem‐solving; vi) compliance. Excluded: i) satisfaction with treatment |
|
| Notes |
Sources of funding: no details provided Declaration of author interests: no details provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Correspondence with authors clarified that "[n]ames of treatment groups were drawn from a container and participants were allocated to a treatment group." |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment were provided |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: correspondence with authors clarified that the "treatment condition was offered to the client via a phone call", suggesting that participants would have known to which treatment arm they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: Correspondence with authors clarified that the therapist running the research was aware of which treatment condition the participant was being offered. |
| Blinding (performance bias and detection bias) Of outcome assessors | High risk | Comment: Correspondence with authors clarified that the "therapist collected outcome data via self‐report measures and chart audits." Neither participants nor personnel were blinded as to which treatment arm participants had been allocated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Correspondence with authors clarified that 10 participants dropped out of the TAU arm, 12 dropped out of the CBT arm, and 11 dropped out of the PST arm. It would appear that data were only collected on patients who completed treatment and that no intention‐to‐treat analyses were attempted. |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to obtain data on suicidal ideation, hopelessness, problem‐solving (for TAU arm), repetition of suicide attempts (for TAU and PST arms), and suicides (for TAU, CBT, and PST arms) following correspondence with authors, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Tapolaa 2010.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a coin toss Follow‐up period: 4 and 6 months N lost to follow‐up: 3/16 (18.7%) for incidence of SH during the 6‐month follow‐up period. |
|
| Participants |
Inclusion criteria: i) aged 18‐65 years; ii) able to communicate effectively in Finnish, including reading and writing; iii) living within the hospital catchment area Exclusion criteria: none stated Numbers: Of the 16 participants, 9 were allocated to the experimental arm and 7 were allocated to the control arm. Profile: 100% (n = 16) were female Source of participants: admissions to an emergency department following a episode of SH Location: Jyväskylä, Finland |
|
| Interventions |
Experimental: acceptance commitment therapy and solution‐focused brief therapy involving meditation, identification of problems, strategies to solve these problems, reflection on alternative methods of problem‐solving, providing motivation to solve these problems, frustration tolerance exercises, and identity assimilation exercises. Control: Correspondence with authors clarified that TAU involved psychiatric outpatient treatment in the form of supportive sessions with a mental health nurse in addition to pharmacological treatment as required. Therapist: advanced level psychology students who received 36 h of training in acceptance and commitment therapy and solution‐focused brief therapy Type of therapy offered: brief psychological therapy Length of treatment: 4 weeks |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide; iii) depression Excluded: i) anxiety; ii) health‐related quality of life; iii) action and acceptance; iv) difficulties in emotion regulation |
|
| Notes |
Source of funding: no details provided Declaration of author interests: no details provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Correspondence with authors clarified that randomisation was with a simple coin toss. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Quote: "The assessor was not blind to conditions; however, all outcome measures were self‐reported, and there was limited interaction between participants and the assessor." (p. 97). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: participants who did not receive treatment appear to have been excluded from all subsequent analyses, suggesting that investigators undertook per protocol analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to request data on repetition of SH and suicides from authors, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Torhorst 1987.
| Study characteristics | ||
| Methods |
Allocation: randomisation using an unknown method Follow‐up period: 12 months N lost to follow‐up: 11/141 (5.7%) for repetition of SH data |
|
| Participants |
Inclusion criteria: i) admitted to the toxicology department of a hospital following a suicide attempt by acute intoxication Exclusion criteria: i) diagnosed with psychosis Numbers: Of the 141 participants, 68 were allocated to the experimental arm and 73 to the control arm. Profile: 63.1% (n = 89) were female, 48.2% (n = 68) were multiple repeaters, 100% (n = 141) had engaged in self‐poisoning Source of participants: patients hospitalised following a suicide attempt Location: Munich, Germany |
|
| Interventions |
Experimental: short crisis intervention during hospital stay followed by a fixed outpatient appointment with the same therapist. Treatment involved a motivational interview, as well as a letter and assessment of motivation towards therapy. Control: short crisis intervention during hospital stay followed by a fixed outpatient appointment with a different therapist. Treatment involved a motivational interview, as well as a letter and assessment of motivation towards therapy. Therapist: 3 therapists trained in psychotherapy and 1 therapist trained in behaviour therapy Type of therapy offered: compliance enhancement plus therapy delivered by the same therapist as in hospital. Length of treatment: 3 months |
|
| Outcomes |
Included: i) repetition of SH according to self report; ii) suicide; iii) compliance; iv) depression Excluded: none |
|
| Notes |
Sources of funding: no details provided Declaration of author interests: no details provided Other: in the first phase of this trial, the efficacy of standard care was assessed in terms of compliance. 85 participants were not randomly assigned to this group but were instead "referred routinely" (p. 53). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients were randomly offered [intervention or control treatment]" (p.54) Comment: Although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment provided |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | High risk | Comment: no details on outcome assessor blinding provided. However, most outcome measures, with the exception of data on suicides, were self reported. Given the nature of this trial, participants could have known to which group they had been allocated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 11 participants were lost to follow‐up. A greater number of participants in the control arm (n = 7) dropped out compared to number in the experimental arm (n = 4). No details on whether intention‐to‐treat analyses were conducted was provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | High risk | Quote: "There is some evidence that patients of the experimental group . . . had more risk factors for further suicidal behavior than did patients of the control group . . . despite randomization. In the experimental group there were more older patients . . . more men . . . more divorced persons . . . and more had been hospitalised in psychiatry in the past . . . Also, there were more parasuicides in the 12 months before index parasuicide. . . Most differences did not reach statistical significance; nevertheless, they can indicate some unequal distribution of risk factors between treatment groups" (p. 56). |
Torhorst 1988.
| Study characteristics | ||
| Methods |
Allocation: randomisation using an unknown method Follow‐upperiod: 12 months N lost to follow‐up: 0/80 (0%) for repetition of SH data |
|
| Participants |
Inclusion criteria: i) able to understand German; ii) living within travelling distance of research centre; iii) previous episodes of SH Exclusion criteria: i) diagnosed with endogenous psychosis; ii) already in psychotherapeutic treatment; iii) already in inpatient psychiatric treatment; iv) overdose involved use of illicit drugs Numbers: Of the 80 participants, 40 were allocated to the experimental arm and 40 to the control arm. Profile: 100% (n = 80) were multiple repeaters Source of participants: patients who were hospitalised following an episode of deliberate self‐poisoning and who were referred to the liaison service of toxicological ward Location: Munich, Germany |
|
| Interventions |
Experimental: long‐term therapy involving 1 therapy session per month over a period of 12 months in addition to a brief crisis intervention delivered 3 days after admission. Control: short‐term therapy involving 12 weekly therapy sessions over a period of 3 months in addition to a brief crisis intervention delivered 3 days after admission. Therapist: 3 psychiatric attendants. Type of therapy offered: no further details on the content of therapy sessions provided Length of treatment: for the experimental arm, 12 months; for the control arm, 3 months |
|
| Outcomes |
Included: i) repetition of SH according to an unknown source; ii) compliance; iii) depression Excluded: i) complaints; ii) psychopathology |
|
| Notes |
Sources of funding: "Supported by a grant from the FRG Ministry for Research and Technology" (p. 419) Declaration of author interests: no details provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Randomly assigned" (p. 419) Comment: Although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment were provided. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details on outcome assessor blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Of the 80 participants, data on 50‐67% were available at 3 months, and data on 97.5% were available at 12 months. Self and experts' ratings data from personal follow‐up were available for 85% of participants. No details provided on whether intention‐to‐treat analyses were conducted |
| Selective reporting (reporting bias) | High risk | Comment: numerical data on depression scores not reported, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Turner 2000.
| Study characteristics | ||
| Methods |
Allocation: randomised using an unknown method Follow‐up period: 12 months N lost to follow‐up: 0/24 (0%) for repetition of SH at the 6‐ and 12‐month follow‐up assessments |
|
| Participants |
Inclusion criteria: i) diagnosed with borderline personality disorder according to both the Diagnostic Interview for Borderlines and the Personality Disorders Examination criteria ; ii) admitted to hospital following a suicide attempt; iii) able to provide written informed consent; iv) consent to randomised assignment Exclusion criteria: i) diagnosed with schizophrenia, schizoaffective disorder, bipolar disorder, organic mental disorder, mental retardation Numbers: Of the 24 participants, 12 were allocated to the intervention arm and 12 were allocated to the control arm. Profile: 79.2% (n = 19) were female, 95.8% (n = 23) met criteria for a comorbid Axis I disorder, including: dysthymia with a comorbid generalised anxiety disorder (n = 17), major depression (n = 3), and dysthymia (n = 3). 95.8% (n = 23) met criteria for a comorbid Axis II disorder, including: dependent personality disorder (n = 9), histrionic personality disorder (n = 6), narcissistic personality disorder (n = 6), schizotypal personality disorder (n = 3), antisocial personality disorder (n = 2), paranoid personality disorder (n = 2), and compulsive personality disorder (n = 1). 75.0% (n = 18) had alcohol misuse, 83.3% (n = 20) had substance misuse. Source of participants: patients admitted to hospital following a suicide attempt Location: Philadelphia, PA, USA |
|
| Interventions |
Experimental: dialectical behaviour therapy involving elements of Linehan's manualised DBT protocol (see Linehan 1993a) but modified to include: i) psychodynamic techniques to conceptualise patients' behavioural, emotional, and relationship schema. Additionally, no group skills training sessions were provided. Instead, skills training occurred during individual therapy. The 6 sessions intended to be used as group skills training sessions were instead used for interpersonal skills training focusing on the identification of significant persons in the participants' environment, including problems in relationships, with family, etc. Control: client‐centred therapy based on Carkhuff's model involving emphatic understanding of the patients' sense of aloneness and the provision of a supportive atmosphere to enable individuation (Carkhuff 1969; Carkhoff 1976). Carkhuff's manual provides directions for increasing the therapeutic relationship through emphatic and supportive elements. The primary focus of treatment was to provide support to enable participants to deal with everyday stress and prevent relapse. Participants also received 6 sessions of interpersonal skills training focusing on the identification of significant persons in the participants' environment, including problems in relationships, with family, etc. Treatment also included the creation and signing of a contract by the therapist and patient stipulating that the patient would not engage in SH or make a suicide attempt during the 12‐month treatment period. Therapist: 4 therapists with an average of 22 years' clinical experience in family systems, client‐centred, and psychodynamic treatment therapies. All therapists also received 12 sessions of training in dialectical behaviour therapy delivered over a 3‐month period prior to randomisation. Type of therapy offered: dialectical behavioural therapy Length of treatment: 12 months |
|
| Outcomes |
Included: i) suicide reattempts according to self report; ii) suicides; iii) suicidal ideation according to self report; iv) depression according to self report Excluded: i) impulsiveness; ii) anger; iii) anxiety; iv) psychiatric symptomatology; v) days in hospital |
|
| Notes |
Sources of funding: no details provided Declaration of author interests: no details provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Participants [were] randomly assigned. . . " (p. 414). Comment: the authors further note that "To determine if the random assignment procedure worked, we examined the pretreatment values of the dependent variables for each . . . outcome. . . there were no significant differences between the groups" (pp. 416‐417), suggesting that the random sequence generation was unbiased. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment provided |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "The independent assessor was unaware of the patients' treatment condition. . . " (p. 415). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "All 24 patients participated in the 6 month and 12 month assessments and composed the intention‐to‐treat sample for the analyses" (p. 414). Comment: no additional details provided on the method used to perform intention‐to‐treat analyses |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Tyrer 2003.
| Study characteristics | ||
| Methods |
Allocation: central independent telephone randomisation system using a computer allocation sequence composed of randomly permuted blocks of sizes 2, 4, and 6 in a non‐systematic sequence. Randomisation stratified by hospital and parasuicide risk Follow‐up period: 12 months N lost to follow‐up: 50/480 (10%) for repetition of SH data |
|
| Participants |
Inclusion criteria: i) aged 16‐65 years; ii) previous history of SH; iii) able to provide informed consent; iv) sufficient English to provide informed consent; v) live in the catchment area; vi) likely to be available for follow‐up Exclusion criteria: ii) have an ICD‐10 diagnosis within the organic, alcohol and drug dependence, schizophrenia or bipolar affective disorder group of codes; viii) psychiatric hospitalisation required. Numbers: Of the 480 participants, 239 were allocated to the experimental arm and 241 to the control arm. Profile: 67.9% (n = 326) were female, 42.1% (n = 202) were diagnosed with a personality disorder Source of participants: patients presenting to hospital following an episode of SH Location: Glasgow, Edinburgh, Nottingham, West London, and South London, UK |
|
| Interventions |
Experimental: manual‐assisted cognitive‐behavioural therapy involving an evaluation of the most recent suicide attempt, crisis skills problem‐solving therapy, cognitive techniques for emotional, and negative thinking management, and the development of relapse prevention strategies Control: TAU involving psychiatric assessment, outpatient care, occasional day‐patient care, referral to GP, or a combination of these Therapist: therapists from the existing services Type of therapy offered: cognitive‐behavioural therapy Length of treatment: 3 to 6 months |
|
| Outcomes |
Included: i) repetition of SH according to self report and verified by GP notes, hospital records, or both; ii) suicide; iii) depression Excluded: i) anxiety; ii) social functioning; iii) quality of life; iv) global functioning; v) future thinking |
|
| Notes |
Sources of funding: "The POPMACT study is funded by the Medical Research Council of the United Kingdom" (p. 67). Declaration of author interests: no details provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "After initial research assessments, participants were randomised to either MACT or TAU using a central independent telephone randomising system so that patients could be allocated to treatment immediately. . . Stata software was used to generate allocation using randomly permuted blocks of sizes two, four and six in a non‐systematic sequence. Random allocation was stratified by participating hospital and parasuicide risk status (high versus low)" (p. 60) Comment: Use of a computerised randomisation sequence is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk |
Quote: "After initial research assessments, participants were randomised to either MACT or TAU using a central independent telephone randomising system. . . " (p. 60) Comment: Use of central allocation means that allocation was probably concealed. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details on outcome assessor blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: of the 480 participants randomised, we could not obtain 12‐month data for 78 (16.2%) for the following reasons: i) could not be traced (n = 27); ii) refused follow‐up assessment (n = 19); iii) did not attend follow‐up assessment (n = 9); iv) died (n = 8); v) withdrew (n = 4); vi) other reasons (n = 11). No details provided on whether intention‐to‐treat analyses were conducted. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Vaiva 2006.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a computer‐generated list of pseudo‐random numbers in opaque sealed envelopes Follow‐up period: 13 months N lost to follow‐up: 0/605 (0%) for suicide reattempts data |
|
| Participants |
Inclusion criteria: i) aged 18‐65 years; ii) hospitalised following a suicide attempt by drug overdose; iii) examined by a psychiatrist who agreed to patients' discharge; iv) able to provide name of GP; v) able to be be contacted by phone; vi) able to provide written consent Exclusion criteria: i) homeless; ii) addicted to illicit drugs Numbers: of the 605 participants, 293 were allocated to the experimental arm and 312 to the control arm. Profile: 72.9% (n = 441) were female, 9% (n = 54) had history of more than 4 suicide attempts in the past 3 years, 49% (n = 296) had experienced a stressful life event in past 6 months. Source of participants: patients presenting to hospital following a drug overdose Setting: Lille, France |
|
| Interventions |
Experimental: telephone contact involving a review of the emergency department recommended treatment in addition to TAU. Where participants found the the treatment recommended during their hospitalisation too difficult to follow, a new regimen was suggested. For those at high risk of suicide, an urgent appointment was made at the emergency department where the patient initially received treatment. No therapy other than support was provided. Control: TAU typically involving referral to the participants' GP. Therapist: psychiatrists with at least 5 years of experience in managing suicidal crises Type of therapy offered: supportive therapy by telephone Length of treatment: 1 telephone call at 1 or 3 months postdischarge |
|
| Outcomes |
Included: i) suicide reattempts according to both self report and hospital records; ii) suicide Excluded: none |
|
| Notes |
Sources of funding: "This study was funded by a hospital clinical research grant (PHRC98), a state region contract plan, a subsidy from the regional hospitalization agency" (p. 1245). Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Patients were randomised. . . on the basis of a computer generated list of pseudo‐random numbers. We used two strata for the randomisation process: one for patients who had attempted fewer than four suicides in the past three years and one for those who had attempted more than four suicides in the past three years. For each stratum the patients were assigned by random allocation" (pp. 1241‐1242) Comment: use of a computerised randomisation sequence is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk |
Quote: " [P]atients were allocated to a group according to the number in an opaque, sealed envelope. The allocation sequence was provided by a statistician uninvolved in the assessment of patients" (p. 1241). Study authors further note that "The allocation list was stored in tamper proof envelopes in a locked cabinet, accessible only to authorised staff" (p. 1242). Comment: Use of sealed, tamper‐proof envelopes stored in a locked cabinet would ensure adequate allocation concealment. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "A specially trained research psychologist, blind to allocation group, assessed the outcome by telephone" (p. 1242). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "At the end of the 13 month follow‐up period we assessed all the included participants, regardless of whether their assigned telephone intervention had taken place" (p. 1243). Comment: of the 605 participants, 89 (14.7%) did not complete the intervention, and 121 (20.0%) were lost to follow‐up at 13 months for the following reasons: i) died; ii) unstated reasons. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Van der Sande 1997a.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a computer generated series of random numbers Follow‐up period: 12 months N lost to follow‐up: 0/274 (0%) for repetition data |
|
| Participants |
Inclusion criteria: i) admitted to hospital following an attempted suicide; ii) able to understand and write Dutch; iii) living in the hospital catchment area Exclusion criteria: i) engaged in habitual wrist cutting of minor severity; ii) currently admitted as a psychiatric inpatient; iii) currently in prison; iv) diagnosed with an substance addiction; v) requires recurrent consultations with a liaison psychiatrist during a stay of more than 2 days on a somatic ward Numbers: Of the 274 participants, 140 were allocated to the experimental arm and 134 were allocated to the control arm. Profile: 57.7% (n = 158) were female, 63.9% (n = 175) were multiple repeaters, 28.1% (n = 77) were diagnosed with a mood disorder. Source of participants: patients admitted to hospital following a suicide attempt Location: Utrecht, the Netherlands |
|
| Interventions |
Experimental: brief psychiatric unit admission to a specialist unit for the treatment of suicide attempters for a period of 1‐4 days. Participants were then offered outpatient treatment based on Hawton and Catalan's problem‐solving approach (Hawton 1987b). Treatment specifically focused on encouraging participants to: i) discuss the reasons behind the current suicide attempt; ii) discuss these reasons with family, partner, or both if required; iii) contact the unit on discharge in the case of a suicidal crisis; iv) change their ability to cope with future problems. 24‐hour emergency access to unit was offered throughout the duration of outpatient treatment. Control: TAU. For around 25% (n = 34) this involved admission to an inpatient unit, whilst for the remaining 75% (n = 100), this involved referral to outpatient services. Therapists: 1 psychiatrist, 2 community psychiatric nurses, and 9 psychiatric nurses Type of therapy offered: problem‐solving therapy Length of treatment: not specified |
|
| Outcomes |
Included: i) suicide reattempts according to self report, hospital records, or both; ii) suicide; iii) compliance; iv) depression; v) hopelessness Excluded: i) anxiety; ii) sleep disorder; iii) psychiatric hospitalisation; iv) phobic anxiety; v) somatisation; vi) obsession‐compulsion; vii) interpersonal sensitivity; viii) hostility |
|
| Notes |
Sources of funding: "This study was supported by grant OG 92‐023 of the National Health Insurance Council (Ziekenfonds‐Raad)" (p. 40). Declaration of author interests: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Envelope[s] contained a number obtained from a list of random numbers generated by computer" (p. 36) Comment: Use of a computerised randomisation sequence is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk |
Quote: "The nurse on duty in the experimental ward performed the randomisation by opening the next from a series of sealed and opaque envelopes" (p. 36) Comment: Use of sealed, tamper‐proof envelopes stored in a locked cabinet would ensure adequate allocation concealment from all except the nurse on duty. |
| Blinding (performance bias and detection bias) Of participants | High risk |
Quote: "Patients assigned to the experimental treatment were informed about the experiment" (p. 36). Additionally, "patients in the control group were sent written information about the experiment" (p. 36) Comment: As patients were aware of the trial, it is likely they were also aware of which treatment arm they had been allocated to. |
| Blinding (performance bias and detection bias) Of personnel | High risk |
Quote: "The nurse on duty in the experimental ward performed the randomisation by opening the next from a series of sealed and opaque envelopes" (p. 36) Comment: suggests that nurses were aware of allocation. However, no details on blinding of other personnel blinding were provided. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details on outcome assessor blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All comparisons were made on an 'intention to treat' basis, regardless of how long (or even whether) patients had received the treatment assigned" (p. 37) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Van Heeringen 1995.
| Study characteristics | ||
| Methods |
Allocation: randomisation using an open randomisation list Follow‐up period: 12 months N lost to follow‐up: 125/516 (24%) for repetition of SH data |
|
| Participants |
Inclusion criteria: i) over 15 years old; ii) resident in catchment area Exclusion criteria: i) currently receiving inpatient medical treatment Numbers: of the 516 participants, 258 were allocated to the experimental arm and 258 were allocated to the control arm Profile: 43% (n = 222) were female, 30% (n = 155) were multiple repeaters, 15% (n = 77) were diagnosed with mood disorder, 2.7% (n = 14) were diagnosed with anxiety disorder Source of participants: patients treated in an A&E department following a suicide attempt Location: Ghent, Belgium |
|
| Interventions |
Experimental: compliance enhancement involving home visits to those participants who did not keep to scheduled outpatient appointments in addition to TAU. Reasons for not attending appointments were discussed and the patient was encouraged to attend future treatment sessions. Control: outpatient appointments only. Non‐compliant participants did not receive home visits. Therapist: community nurse. Type of therapy offered: assertive outreach and compliance enhancement. Length of treatment: not specified. |
|
| Outcomes |
Included: i) repetition of SH according to self report, with collateral report from GPs, relatives or both, if the participant could not be contacted; ii) suicide; iii) compliance Excluded: none |
|
| Notes |
Sources of funding: "This study was supported by a grant from the National Fund for Scientific Research (NFWO, grant no. 3.0061.86)" (p. 969). Declaration of author interests: no details provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: "Patients were randomly allocated. . . using a randomization list" (p. 964) Comment: As the numbers table was open, it is possible there may have been bias in the generation of the random sequence. |
| Allocation concealment (selection bias) | High risk | Comment: As the numbers table was open, it is possible there may have been bias in the concealment of allocation. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | High risk | Comment: no details on outcome assessor blinding were provided. However, most outcome measures, were either self reported or reported by relatives, GPs or both. Given the nature of this trial, participants, relatives, and GPs could have known group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Of the 516 participants, 125 (24.2%) were lost to follow‐up. Reasons given for dropouts included: i) refused follow‐up assessment (n = 97); ii) moved from catchment area without leaving a forwarding address (n = 22); iii) death following a somatic illness (n = 2); iv) admitted to hospital with a terminal illness (n = 2); v) imprisoned (n = 2). No details on whether intention‐to‐treat analyses were conducted was provided, however. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Waterhouse 1990.
| Study characteristics | ||
| Methods |
Allocation: randomisation using sequentially numbered sealed envelopes Follow‐up period: 16 weeks N lost to follow‐up: 0/77 (0%) for repetition of SH data |
|
| Participants |
Inclusion criteria: i) aged over 16 years old Exclusion criteria: i) immediate medical or psychiatric treatment needs Numbers: Of the 77 participants, 38 were allocated to the experimental arm and 39 were allocated to the control arm. Profile: 62% (n = 48) female. 36% (n = 28) were repeaters. Mean age of 30 years. Source of participants: patients admitted to an A&E department for SH Location: York, UK |
|
| Interventions |
Experimental: general hospital admission excluding additional treatment or counselling. "Hospital admission consisted of little more than a bed, without further referral to other helping agencies" (p. 238). Control: discharge from hospital Therapist: none Type of therapy offered: hospital admission Length of treatment: median length of admission was 17 hours |
|
| Outcomes |
Included: i) repetition of SH according to GP interview, hospital records, or both; ii) suicidal ideation; iii) hopelessness Excluded: i) depression; ii) psychiatric admission; iii) time off work; iv) social isolation; v) somatic concerns; vi) daily routine; vii) social behaviour assessment schedule; viii) GP questionnaire |
|
| Notes |
Sources of funding: no specific sources of funding were provided for this trial. Declaration of author interests: "John Waterhouse was in receipt of a research grant from the Yorkshire Regional Health Authority" (p. 241). Other: As depression data had been combined with anxiety data, this outcome was not included in the present review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomisation took place. . . using sequentially numbered sealed envelopes" (p. 237) Comment: Although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Randomisation took place. . . using sequentially numbered sealed envelopes" (p. 237) Comment: No mention of whether the envelopes were opaque or not, although they probably were. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means that personnel (e.g., hospital staff, GPs) are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | High risk | Quote: "Follow up interviews . . . were performed one week after the attempt by one of the authors. . . who was not blind to the patient's treatment group" (p. 237) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Of the 77 participants, 4 (5.2%) dropped out after 1 week and a further 21 (28.4%) dropped out by 16 weeks. No details provided on whether intention‐to‐treat analyses were conducted, however. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Wei 2013.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a computerised randomisation programme Follow‐up period: 3, 6, and 12 months N lost to follow‐up: 77/239 (32.2%) at 3‐month follow‐up; 123/239 (51.5%) at the 6‐month follow‐up; 151/239 (63.2%) at the 12‐month follow‐up |
|
| Participants |
Inclusion criteria: i) older than 15 years; ii) admitted to emergency departments following a suicide attempt; iii) have at least 1 contact person to provide collateral reports on suicidal behaviour, etc; iv) able to understand the trial procedures; v) able to provide written informed consent Exclusion criteria: none stated Numbers: Of the 239 participants, 82 were allocated to the cognitive therapy intervention arm, 80 were allocated to the telephone intervention, and 77 were allocated to the control arm. Profile: 76.1% (n = 182) were female; 45.2% (n = 108) were diagnosed with any psychiatric disorder Source of participants: patients admitted to emergency departments following a suicide attempt Location: Shenyang, Liaoning Province, China |
|
| Interventions |
Experimental: there were 2 experimental arms in this trial: i) cognitive therapy, and ii) telephone intervention. Cognitive therapy involved sessions of cognitive therapy as well as supporting patients to reconnect with family and friends. The telephone intervention involved psychological support based on reassurance and emphatic reasoning, and collaborative problem‐solving therapy. Control: " [P]atients in the control group did not receive any interventions" (p. 109). Therapist: Therapists had more than 5 years clinical work experience. Type of therapy offered: i) cognitive‐behavioural therapy; ii) telephone contact Length of treatment: 3 months |
|
| Outcomes |
Included: i) repetition of SH; ii) suicide; iii) suicidal ideation; iv) depression Excluded: i) quality of life |
|
| Notes |
Sources of funding: "This project was part of the 'Small Grants Program to Improve the Quality and Implementation of Suicide Research in China' which was supported by the China Medical Board of New York (grant number 05‐813)" (p. 113). Declaration of author interests: no details provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "[P]articipants . . . were randomly assigned. . . using a computerized randomization program" (p. 109). Comment: Use of a computerised randomisation sequence is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment provided |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details on blinding of outcome assessors provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All analyses were conducted using the intent‐to‐treat (ITT) principle. . . " (p. 110). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Weinberg 2006.
| Study characteristics | ||
| Methods |
Allocation: randomisation by asking participants to choose between 2 similar envelopes Follow‐up period: 8 months N lost to follow‐up: 0/30 (0%) for repetition of SH data |
|
| Participants |
Inclusion criteria: i) female; ii) aged 18‐40 years; iii) diagnosed with borderline personality disorder; iv) history of repetitive SH with at least 1 episode during the month before enrolment Exclusion criteria: i) diagnosed with comorbid psychosis; ii) judged to be at an elevated risk of suicide; iii) diagnosed with substance abuse; iv) history of attempted suicide (only those engaging in repetitive SH were eligible for inclusion in this trial) Numbers: Of the 30 participants, 15 were allocated to the experimental arm and 15 to the control arm. Profile: 100% (n = 30) were female Source of participants: recruited from the community via advertisements in local newspapers, clinical services at a hospital, and from individuals participating in a longitudinal study Location: Boston, MA, USA |
|
| Interventions |
Experimental: manual assisted cognitive treatment involving of 6 sessions aimed at evaluation an attempt, developing crisis skills problem‐solving skills, developing cognitive techniques for emotional, and negative thinking management, and outlining relapse prevention strategies Control: TAU Therapists: primary investigator acted as the therapist Type of therapy offered: cognitive behavioural therapy Length of treatment: 2 months |
|
| Outcomes |
Included: i) repetition of SH according to self report; ii) suicidal ideation; iii) suicide Excluded: i) severity of SH |
|
| Notes |
Souces of funding: "This study was supported by a Young Investigator Aware from the Borderline Personality Disorder Research Foundation (I.W.)" (p. 482). Declaration of author interests: no details provided Other: All participants were also simultaneously participating in additional treatment throughout the duration of this trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Subjects were randomly assigned" (p. 485) Comment: Correspondence with authors further clarified that "subjects were asked to choose between 2 similar envelopes containing either manual assisted cognitive behaviour therapy or non‐manual assisted cognitive behaviour therapy." |
| Allocation concealment (selection bias) | Low risk | Comment: correspondence with authors clarified that subjects choose between 2 similar envelopes. |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: ". . . interviewers were blind to baseline ratings and to participants' group allocation at post‐treatment assessments and 6‐month follow‐up" (p. 487). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: Although "[a]ll MACT participants completed 6 sessions of MACT. Two TAU group participants were not available for the post‐treatment assessments (p. 485). Nevertheles, "[a]ll participants were interviewed at the 6 months follow up" (p.485), suggesting that intention‐to‐treat analyses were undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: We had to request data on suicides from authors, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
Welu 1977.
| Study characteristics | ||
| Methods |
Allocation: randomisation using a table of random numbers Follow‐up period: 4 months N lost to follow‐up: 1/120 (1%) for repetition of SH data |
|
| Participants |
Inclusion criteria: i) over 16 years old Exclusion criteria: i) student living in university accommodation; ii) resident in a caregiving institution or institutionalised at the time of the index episode of SH Numbers: Of the 120 participants, 63 were allocated to the experimental arm and 57 to the control arm. Profile: 60% (n = 72) were multiple repeaters Source of participants: patients admitted to an A&E department following an episode of SH Location: Pittsburgh, PA, USA |
|
| Interventions |
Experimental: special outreach programme involving a community mental health team contacting participants immediately after discharge to arrange weekly/bi‐weekly home visits Control: TAU involving a psychiatric consultation at request of the treating physician. Participants were also given a next day appointment for evaluation at the community mental health team centre. Any further contact after discharge was at the participant's request. Therapist: 4 nurses, 3 social workers, and 2 community workers Type of therapy offered: special outreach involving a variety of treatments Length of treatment: 4 months |
|
| Outcomes |
Included: i) repetition of SH according to one or more of: self report, hospital records, collateral informant report Excluded: i) extent of follow‐up coverage; ii) type and frequency of contacts; iii) purposive accidents; iv) excessive use of alcohol; v) drug misuse |
|
| Notes |
Sources of funding: "This investigation was supported by Research Grant MH19491 from the National Institute of Mental Health" (p. 17). Declaration of author interests: no details provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Random assignment was worked out in advance from a table of random numbers" (p. 20) Comment: Use of a random numbers table is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment provided |
| Blinding (performance bias and detection bias) Of participants | High risk | Comment: The nature of this trial means that participants could have known to which group they had been allocated. |
| Blinding (performance bias and detection bias) Of personnel | High risk | Comment: The nature of the trial means personnel are likely to have known which participant was receiving which treatment. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details provided on outcome assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: of the 120 participants, 6 (9.5%) in the experimental arm and 26 (45.6%) in the control arm were lost to follow‐up for unstated reasons. Intention‐to‐treat analyses were not attempted, however. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured; however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no other apparent sources of bias |
A&E: accident and emergency; BPD: borderline personality disorder; CBT: cognitive behavioural therapy; DBT: dialectical behavioural therapy; DSM‐IV (TR): Diagnostic and Statistical Manual of Mental Disorders, fourth edition (text revision); ITT: intention‐to‐treat; MACT: manual‐assisted cognitive therapy; NSSI: non‐suicidal self‐injury; PST: problem‐solving therapy; PTSD: post‐traumatic stress disorder; SCID‐II: Structured Clinical Interview for DSM‐IV Axis II Personality Disorders; SH: self‐harm; SSRI: selective serotonin reuptake inhibitors; TAU: treatment as usual.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almeida 2012 | Participants were not required to have engaged in SH prior to trial entry. |
| Aoun 1999 | Non‐randomised clinical trial |
| Bannan 2010 | Correspondence with authors suggested bias in both allocation and allocation concealment to the intervention and control groups. |
| Bartman 1979 | Method of allocation to intervention and control groups unclear |
| Bateson 1989 | Non‐randomised clinical trial |
| Berrino 2011 | Non‐randomised clinical trial |
| Carter 2013 | Reports on 5‐year outcomes, rather than within the 2‐year time frame |
| Cebrià 2013 | Non‐randomised clinical trial |
| Chen 2013 | Correspondence with authors revealed that information on non‐fatal repetition of SH could not be disaggregated from information on completed suicide. Additionally, the study did not collect data on the secondary outcomes included in this review. |
| Chowdhury 1973 | Correspondence with authors revealed that participants were alternately allocated to the intervention and control groups. |
| Christensen 2014 | Database of RCTs |
| Comtois 2011 | Correspondence with authors confirmed that not all participants engaged in self‐harm in the 6 months prior to randomisation. |
| Crawford 1998 | Non‐randomised clinical trial |
| Currier 2010 | Participants were not required to have engaged in SH prior to trial entry |
| Davidson 2006 | Participants could have engaged in SH at any point within 1 year prior to trial entry, rather than within 6 months. |
| De Leo 2007 | Participants could have engaged in SH at any point, rather than within 6 months. |
| Evans 1998 | Conference proceedings |
| George 2014 | Participants not required to have engaged in SH prior to trial entry |
| Ghahramanlou‐Holloway 2012 | Review |
| Gunnarsdottir 2010 | Non‐randomised clinical trial |
| Harned 2010 | RCT of a psychosocial intervention for SH patients that only presents data from the intervention arm |
| Hatcher 2005 | Non‐randomised clinical trial |
| Hellerstein 2003 | Conference proceedings |
| Horrocks 2002 | Letter to the editor |
| Kapur 2013b | Non‐randomised clinical trial |
| Lamprecht 2007 | Non‐randomised clinical trial |
| Liberman 2001 | Letter to the editor |
| Links 1999 | Non‐randomised clinical trial |
| Links 2003a | Non‐randomised clinical trial |
| Low 2001 | Non‐randomised clinical trial |
| Martin 2013 | Non‐randomised clinical trial |
| McMain 2007a | Non‐randomised clinical trial |
| McQuillan 2005 | Non‐randomised clinical trial |
| Montgomery 1983 | RCT of a pharmacological intervention for SH patients |
| Morley 2014 | Participants were not required to have engaged in SH prior to trial entry |
| Ono 2008 | Non‐randomised clinical trial |
| Pham‐Scottez 2010 | Non‐randomised clinical trial |
| Raj 2001 | Non‐randomised clinical trial |
| Razzaque 2013 | Non‐randomised clinical trial in which only 3 participants were enrolled |
| Ruchlewska 2013 | Participants not required to have engaged in SH prior to trial entry |
| Sáiz 2014 | Correspondence with authors confirmed that not all participants were randomised to the intervention or control groups; some chose to receive the intervention treatment. |
| Sambrook 2007 | Non‐randomised clinical trial |
| Strum 2012 | Correspondence with authors confirmed that not all participants engaged in SH in the 6 months prior to randomisation. |
| Tarrier 2008a | Review |
| Termansen 1975 | Non‐randomised clinical trial |
| Trembley 2013 | Review |
| Van Spijker 2010 | Participants were not required to have engaged in SH prior to trial entry |
| Vitiello 2009 | Not all participants were randomised to the intervention or control groups; some chose to receive the intervention treatment. |
| Warren 2004 | Participants were not required to have engaged in SH prior to trial entry |
| Winter 2007 | Non‐randomised clinical trial |
| Wullimier 1979 | Non‐randomised clinical trial |
| Zhang 2013 | Participants could have engaged in SH at any point within 1 year of trial entry, rather than within 6 months. |
RCT: randomised controlled trial; SH: self‐harm.
Characteristics of studies awaiting classification [ordered by study ID]
Andreasson 2016.
| Methods |
Allocation: 2‐arm, parallel group randomisation Design: single centre (outpatient psychiatric clinic) Setting: community Follow‐up period: 52 weeks Location: Copenhagen, Denmark |
| Participants | Males and females, 18‐65 years of age, meeting 2 or more criteria for a diagnosis of borderline personality disorder according to the DSM‐IV, who made a suicide attempt within 1 month of inclusion into the trial, and are able to provide informed consent |
| Interventions | Participants randomised to the experimental group will receive either 16 weeks of dialectical behaviour therapy (DBT) or 16 weeks of Collaborative Assessment and Management (CAMS) of suicidality alongside CAMS‐informed supportive psychotherapy |
| Outcomes |
Primary outcome: number of subsequent episodes of self‐harm and suicide attempts at 17, 28, and 52 weeks follow‐up Secondary outcomes: scores on the Hamiliton Depression Rating Scale (HDRS), BDI, BSSI, the Suicide Attempt Self Injury Interview (SASII), Beck Hopelessness Scale (BHI), Barratt Impulsivity Scale (BIS), the Zanarini Borderline Personality Scale (ZBPS), the State Trait Anger Scale (STAS), and Rosenberg's Self Esteem Scale measured at 16, 28, and 52 weeks follow‐up |
| Notes | Dr. Kate Andreasson very kindly provided unpublished information relating to this trial. |
Armitage 2016.
| Methods |
Allocation: individual randomisation Design: single centre Setting: hospital Location: Kuala Lumpur, Malaysia |
| Participants | Males and females admitted to Kuala Lumpur Hospital following an episode of self‐harm (ICD‐10 X60–X84, intentional self‐harm13) between 1 March 2010 and 28 February 2011. |
| Interventions | Implementation intentions to reduce suicidal ideation and behaviour. All participants were initially presented with a brief statement designed to encourage them to plan not to self‐harm: ‘We want you to plan not to self‐harm. Research shows that you are much more likely to be successful in your intention not to self‐harm if you can identify critical situations and appropriate responses’. Following this statement, participants were randomised to one of three groups: (i) volitional help sheet with implementation intentions (11 critical situations and 11 appropriate responses) (N=75); (ii) self‐generating implementation intentions, without help (N=78); (iii) a control condition (the volitional help sheet, but no instruction on how to form implementation intentions, participants were simply asked to identify critical situations and appropriate responses that might be useful to them) (N=73). |
| Outcomes | Primary outcomes: suicidal ideation and behaviour (revised Suicidal Behaviours Questionnaire); depression (BDI‐II); motivation to avoid self‐harm. |
| Notes | Personal communication between KH and Rory O'Connor |
Gysin‐Maillart 2016.
| Methods |
Allocation: individual randomisation using shuffled sealed envelopes. Design: single centre (hospital‐based recruitment). Setting: emergency unit of a university general hospital. Location: Bern, Switzerland. |
| Participants | Males and females, 18 years of age and older, admitted to the emergency unit of a university general hospital following a suicide attempt for which there is evidence of an intent to die. Those with a history of multiple episodes of self‐harm (i.e., indicative of probable borderline personality disorder pathology), serious cognitive impairment, psychosis, insufficient ability to communicate in German, or those resident outside of the hospital catchment area will be excluded from participation. |
| Interventions | Individuals randomised to the intervention group will receive between three and four weekly sessions of between 60‐90 minutes in length of face‐to‐face psychosocial therapy delivered according to the ASSIP manual (Gysin‐Maillart 2013; Michel 2015) involving a narrative interview, cognitive restructuring, and crisis safety planning. Additionally, participants in the intervention group will receive one letter every three months for a total of 24 months reminding them of the importance of safety planning during times of crisis. |
| Outcomes |
Primary outcomes: suicide reattempts according to hospital records during the 24 month follow‐up period. Secondary outcomes: scores on the 11‐item Penn Helping Alliance Questionnaire, the Beck Depression Inventory, and the Beck Scale for Suicidal Ideation during the 24 month follow‐up period. |
| Notes |
Linehan 2015.
| Methods |
Allocation: randomisation using a computerised adaptive minimisation procedure whereby participants were matched using 5 primary prognostic variables: i) age; ii) number of prior 'suicide attempts'; iii) number of prior NSSI episodes; iv) number of psychiatric hospitalisations within the past year; v) depression severity Follow‐up period: 12 months N lost to follow‐up: 0/99 (0%) for repetition of NSSI and 'suicide attempts' |
| Participants |
Inclusion criteria: i) 18‐60 years; ii) female; iii) met criteria for borderline personality disorder; iv) at least 2 'suicide attempts' or episodes of NSSI over the past 5 years; v) at least 1 'suicide attempt' or episode of NSSI within the 8 weeks prior to entering the study; vi) at least 1 'suicide attempt' in the previous year. Due to difficulties in reaching recruitment targets, the authors relaxed inclusion criteria towards the end of the recruitment period to include 1 participant who had engaged in an episode of NSSI in the 8‐week period prior to entering the study but who did not have a prior history of NSSI and 5 participants who did not have a history of repeated episodes of NSSI but who did make a 'suicide attempt' within the past year. Exclusion criteria: i) met criteria for a current psychotic or bipolar disorder; ii) diagnosed with a seizure disorder requiring the use of medication; iii) currently undergoing treatment for another life‐threatening condition (e.g., anorexia nervosa); iv) an IQ score of less than 70 on the Peabody Picture Vocabulary Test‐Revised Numbers: of the 99 participants 33 were allocated to the DBT + skills training arm, 33 were allocated to the DBT + individual therapy arm, and 33 were allocated to the DBT standard protocol arm. Profile: 99 (100%) were female, 95 (95.9%) had a lifetime diagnosis of major depression, 87 (87.9%) had a lifetime diagnosis of any anxiety disorder, and 69 (69.7%) had a lifetime diagnosis of a substance use disorder. Source of participants: healthcare practitioners Setting: Seattle, WA, USA |
| Interventions |
Experimental: This trial involved 2 experimental arms. The first, 'DBT + skills training', incorporated a manualised standard case management protocol, group‐based skills training sessions, and telephone coaching as required; it was designed to resemble the DBT standard protocol with the omission of all sessions of individual‐based psychotherapy. The second, 'DBT + individual therapy', incorporated individual‐based therapy, an activity‐based support group, and telephone coaching as required; it was designed to resemble the DBT standard protocol with the omission of all sessions of group‐based skills training. Control: DBT standard protocol incorporating sessions of individual‐based psychotherapy, group‐based skills training, and telephone coaching as required Therapists: specially trained to provide either experimental or control therapy Type of therapy offered: dialectical behaviour therapy with skills training and dialectical behaviour therapy with individual‐based psychotherapy Length of treatment: 1 year |
| Outcomes |
To be included: i) repetition of SH (requires correspondence from study authors as to whether it is possible to aggregate episodes of NSSI and 'suicide reattempts'); ii) depression; iii) suicidal ideation; iv) adherence with treatment Excluded: i) anxiety; ii) importance of reasons for living |
| Notes |
BDI: Beck Depression Inventory; BSSI: Beck Scale for Suicidal Ideation; DBT: dialectical behaviour therapy; MINI: Mini International Neuropsychiatric Interview; NSSI: non‐suicidal self‐injury; TAU: treatment as usual.
Characteristics of ongoing studies [ordered by study ID]
Agyapong 2013.
| Study name | Text message intervention to reduce repeat self‐harm. Trial registration number: NCT01823120. |
| Methods |
Allocation: single blind, parallel assignment, randomisation Design: single centre Setting: community Location: Dublin, Republic of Ireland Follow‐up period: 3 months |
| Participants |
Inclusion criteria: males and females, 18 years of age and older, presenting to the emergency department following an episode of self‐harm, with a mobile phone and familiar with text messaging Exclusion criteria: those who do not provide consent to participate, who do not have a mobile phone or are unfamiliar with text messaging, who are admitted to psychiatric inpatient facilities following assessment in the emergency department, who require admission to a medical ward for more than 48 h, or who are unavailable at any point during the 3‐month follow‐up period |
| Interventions | Those randomised to the intervention arm will receive daily text messages for 1 month, followed by 1 message every 2 days for the second month, followed by 1 message per week for the third month following discharge from the emergency department. Text messages will target the relief of mood symptoms and will provide advice on strategies for coping with suicidal thoughts. Messages will also provide patients with a mobile phone number for the Samaritans. All messages will encourage participants to contact the Samaritans in times of crisis. |
| Outcomes |
Primary outcome measures: proportion of patients repeating self‐harm and scores on the Suicide Behaviors Questionnaire Secondary outcome measures: number of repeat episodes of self‐harm per person, scores on the Modified Scale for Suicidal Ideation, scores on the Positive and Negative Suicide Ideation Inventory, scores on the Beck Hopelessness Scale, and scores on the Global Assessment of Functioning |
| Starting date | March 2013. End date: March 2014. |
| Contact information |
Name: Dr Vincent Agyapong. Affiliation: Department of Psychiatry, Trinity College Dublin, Republic of Ireland email: israelhans@hotmail.com |
| Notes | We made three attempts to contact Dr Agyapong to confirm these details; however, we received no response. We therefore extracted information for this trial from the ClincialTrials.gov record. |
Andover 2008.
| Study name | Treatment for Non‐Suicidal Self‐Injury in Young Adults (T‐SIB). Trial registration number: NCT01018433. |
| Methods |
Allocation: Single‐blind, parallel assignment, randomised Design: single centre Setting: outpatient clinic Location: Bronx, NY, USA Follow‐up period: 3 months |
| Participants |
Inclusion criteria: males and females, aged 18‐29, with a history of engaging in NSSI (with or without an urge to self injure) within the month prior to randomisation Exclusion criteria: those with psychotic symptomatology or severe suicidal ideation |
| Interventions | Participants randomised to the experimental group will receive 9 sessions of therapy to reduce both the frequency and severity of non‐suicidal self‐injury. |
| Outcomes |
Primary outcome measures: frequency and severity of NSSI Secondary outcome measures: scores on the Beck Depression Inventory (BDI), the Beck Anxiety Inventory (BAI), the McLean Screening Instrument for Borderline Personality Disorder, the College Student Inventory, the Social Problem Solving Inventory‐Revised (SPSI‐R), the Symptom Checklist‐90‐Revised (SCL‐90‐R), and the University of Rhode Island Change Assessment (URICA) |
| Starting date | September, 2008.End date: July, 2013. |
| Contact information | Name: Prof. Margaret Andover.Affiliation: Department of Psychology, Fordham University.email:moodbehavior@fordham.edu |
| Notes | We made three attempts to contact Prof Andover to confirm these details; however, we received no response and so were unable to confirm whether all participants either engaged in deliberate self‐harm or made a suicide attempt within six months prior to randomisation. Additionally, we were unable to confirm whether the trial was ongoing. We extracted information for this trial from the ClinicalTrials.gov record. |
Berrouiquet 2015.
| Study name | SIAM: Suicide Intervention Assisted by Messages. Trial registration number: NCT02106949. |
| Methods |
Allocation: randomised. Design: multicentre. Setting: recruitment from hospital settings, treatment provided in the community. Location: Brest, Rennes, Nates, Lille, Angers, Tours, and Vannes, France. Follow‐up period: 6 and 13 months. |
| Participants | Inclusion criteria: males and females, 18 years of age or older, who attempt suicide and are admitted to the emergency department and/or psychiatric unit of one of seven participating hospitals, who are hospitalised for no more than 7 days, and are able to be contacted by mobile telephone. |
| Interventions | Those randomised to the intervention group will receive 9 text messages, one within 48 hours of discharge, one a days 8 and 15, and one at months 1,2,3,4,5, and 6. Content of these messages will address validation, recall of the discharge treatment agreement, and outreach via a continuing care intervention program. Messages will also provide participants with information on their treating doctor's name and contact information (GP or psychiatrist as appropriate), as well as dates of scheduled appointments (as applicable). Information on a crisis telephone number, available 24/7, will also be included. |
| Outcomes |
Primary outcomes: number of subsequent suicide attempts at the 6 month follow‐up assessment. Secondary outcomes: number of subsequent suicide attempts at the 13 month follow‐up assessment, number of deaths by suicide at the 6 and 13 month follow‐up assessments, and the number self‐reporting suicidal ideation at the 6 and 13 month follow‐up assessments. |
| Starting date | June, 2014. Anticipated end date: not specified. |
| Contact information |
Name: Dr Sofian Berrouiguet (Principal Investigator) Affiliation: Hôspital Cavale Blanche, Brest, France. email:sofian.berrouiguet@chu-brest.fr |
| Notes |
Brimes 2007.
| Study name | Effectiveness of Standard Emergency Department Psychiatric Treatment Associated With Treatment Delivery by a Suicide Prevention Center Trial registration number: NCT00641498. |
| Methods |
Allocation: randomised Design: multicentre Setting: dedicated outpatient suicide prevention centres Location: Toulouse, France Follow‐up period: 2 years |
| Participants |
Inclusion criteria: males and females, 18 years of age and older, who have made a suicide attempt by self‐poisoning, who have a Glasgow score of 15, and who are currently receiving standard psychiatric treatment Exclusion criteria: unable to speak French; admitted to inpatient facilities will be excluded from participation |
| Interventions | Participants randomised to the intervention arm will receive sessions of individual supportive psychotherapy delivered in a dedicated outpatient suicide prevention centre. No further details on the content, number, or duration of these sessions is reported. Proposed N= 405 |
| Outcomes | Outcome measures: frequency of subsequent suicidal behaviour and death by suicide during the 2‐year follow‐up period |
| Starting date | March, 2007. End date: October, 2011. |
| Contact information |
Name: Dr. Philippe Birmes. Affiliation: University Hospital, Toulouse, France. email:birmes.p@chu-toulouse.fr |
| Notes | We made 3 attempts to contact Dr Birmes to confirm these details; however, we received no response and so were unable to confirm whether all participants either engaged in deliberate self‐harm or made a suicide attempt within 6 months prior to randomisation. Additionally, we were unable to confirm whether the trial was ongoing. We extracted information for this trial was extracted from the ClinicalTrials.gov record. |
Brown 2014.
| Study name | Community‐based cognitive therapy for suicide attempters Trial registration number: NCT00081367. |
| Methods |
Allocation: randomised Design: multicentre Setting: recruitment from hospital settings, treatment provided in community mental health clinics Location: Philadelphia, PA, USA Follow‐up period: unclear |
| Participants | Inclusion criteria: males and females, 16 years of age or older, who attempt suicide within 48 h of presenting to an emergency department or trauma care unit, who are able to speak English, and are able to understand the nature of the trial, and who provide written informed consent |
| Interventions | Those randomised to the intervention group will receive 10 weekly sessions of cognitive therapy in addition to enhanced usual care. |
| Outcomes | Primary outcomes: number of subsequent suicide attempts, scores on a measure of suicidal ideation, scores on a measure of depression, and scores on a measure of hopelessness |
| Starting date | April, 2004 Data collection completed: September 2009 |
| Contact information |
Name: Prof Gregory Brown (Principal Investigator) Affiliation: Department of Psychiatry, University of Pennsylvania email: gregbrow@mail.med.upenn.edu |
| Notes | Prof Gregory Brown very kindly provided unpublished information relating to this trial. |
Collinson 2014.
| Study name | MIDSHIPS: Multicentre Intervention Designed for Self‐Harm using Interpersonal Problem‐Solving. Trial registration number: ISRCTN54036115. |
| Methods |
Allocation: stratified block random allocation with minimisation Design: single‐centre (hospital‐based recruitment by a specialist Self Harm Assessment Team within the Leeds and York NHS Trust) Setting: clinic rooms, GP practices, or both Location: Leeds and York, UK |
| Participants | Males and females, 18 years of age and older, who present to hospital following an episode of self‐harm are eligible to participate in this trial. Both first time self‐harmers and those with more extensive self‐harming histories will be included. Individuals diagnosed with any psychiatric disorder are also eligible to participate. N = 60 |
| Interventions | Individuals randomised to the intervention group will receive 4‐6 one‐hour weekly problem‐solving therapy sessions aimed at helping patients to identify problems and to provide them with strategies for resolving these and future problems more constructively. |
| Outcomes | Primary outcomes: repetition of self‐harm necessitating hospital admission within 6 months of randomisation, attendance at therapy sessions as measured by the Health and Social Care Information Centre, scores on the General Health Questionnaire and the EuroQol‐5D, and other health economics data |
| Starting date | January, 2012. Completed: July, 2015. |
| Contact information |
Name: Dr David Owens (Principal Investigator) Affiliation: University of Leeds. email:d.w.owens@leeds.ac.uk |
| Notes | Dr David Owens very kindly provided unpublished information relating to this trial. Additionally, Dr Owens provided the following notes pertaining to the this trial: "Funded by the National Institutes of Health Research (NIHR) Research for Patient Benefits (RfPB) program. The planned application for the full (multicentre) trial (will be made) to the Health Technology Assessment (HTA) (program) in late 2014." |
Davidson 2009.
| Study name | ENGAGE ‐ Meeting mental health needs of complex comorbid patients attending A&E following a suicide attempt. A pilot study Trial registration number: NCT00980824. |
| Methods |
Allocation: single blind randomisation Design: single‐centre (community) Setting: postdischarge patients followed up in the community Follow‐up period: 3 months Location: Glasgow, UK |
| Participants |
Inclusion criteria: males and females, 18 years of age and older, who were admitted to a general hospital following an episode of self‐harm or a suicide attempt, and who score above the threshold for personality disorder using the SAPAS will be included in this trial. Those with substance misuse, defined as scoring above the threshold for substance misuse according to the AUDIT or the DAST, will not be excluded from participation. N= 20 |
| Interventions | Those randomised to the experimental group will receive 6 sessions of Manual‐Assisted Cognitive Therapy (MACT), a brief focused therapy to address self‐harm and to promote engagement with services. ENGAGE is designed to help patients to identify problems that lead to self‐harming behaviour or attempted suicide and to assist patients in using problem‐solving therapy to resolve these problems. Emphasis will also be placed on encouraging engagement and on facilitating contact with specialist substance misuse, personality disorder treatment, or both, as appropriate. |
| Outcomes | Primary outcomes: scores on measures of depressed mood, anxiety, and suicidality at baseline and after 3 months of follow‐up |
| Starting date | November 2009 End date: December 2010 |
| Contact information |
Name: Prof Kate Davidson (Principal investigator) Affiliation: University of Glasgow email: kate.davidson@glasgow.ac.uk |
| Notes | Prof Davidson kindly very kindly provided unpublished information relating to this trial. Additionally, Prof Davidson provided the following notes pertaining to this trial: "Pilot study to assess feasibility to recruit a sample of these complex patients to a randomised controlled trial of MACT following an index episode of self‐harm. There is preliminary support that MACT could be an acceptable and effective intervention in patients with personality disorder and substance misuse." |
Hatcher 2016b.
| Study name | Ottawa Suicide Prevention in men pilot study (OSSUPilot): A cluster‐randomised trial of a smart phone assisted problem‐solving therapy in men who present to hospital with intentional self‐harm. Trial Registration Number: NCT02718248. |
| Methods |
Allocation: cluster randomisation with emergency departments being the unit of randomisation Design: multicentre (hospital emergency department facilities). Setting: postdischarge patients followed up in the community. Follow‐up period: 1 year. Location: Ontario, Canada. |
| Participants |
Inclusion criteria: males, 18 years of age and older, presenting to hospital‐based emergency department facilities following an episode of intentional self‐harm Exclusion criteria: females, and those younger than 18 years N expected: 1200 participants: 600 in each arm |
| Interventions | Participants randomised to the intervention group will receive 6 sessions of face‐to‐face problem‐solving therapy, 1 additional follow‐up session, and smartphone assisted problem‐solving therapy embedded in a quality improvement programme (CHESS app) over an approximate 2‐month follow‐up period. |
| Outcomes |
Primary outcome: re‐presentation to hospital for any reason over a 1‐year follow‐up period Secondary outcomes: re‐presentation to hospital for intentional self‐harm over a 1‐year follow‐up period, suicide |
| Starting date | April 2016 Proposed End Date: September 2018 |
| Contact information |
Name: Dr Simon Hatcher (Principal investigator) Affiliation: University of Ottawa email: shatcher@uottawa.ca |
| Notes | Dr Hatcher very kindly provided unpublished information relating to this trial. |
Huang 2013.
| Study name | Efficacy of dialectical behavior therapy in patients with borderline personality disorder. Trial registration number: NCT01952405. |
| Methods |
Allocation: randomised Design: single centre (hospital‐based) Setting: hospital Location: Taipei, Taiwan |
| Participants |
Inclusion criteria: males and females, aged 18‐60, meeting DSM‐IV criteria for borderline personality disorder, and who engaged in at least 2 episodes of suicidal or non‐suicidal self injurious behaviour in the past 5 years with at least 1 episode occurring in the 3 months preceding randomisation Exclusion criteria: those diagnosed with bipolar I disorder, delirium, dementia, mental retardation, or a diagnosis of substance dependence within the preceding 30 days |
| Interventions | Participants randomised to the intervention group will receive sessions of dialectical behaviour therapy over a 12‐month follow‐up period. |
| Outcomes |
Primary outcome: frequency of suicide attempts as measured by the Suicide Attempt Self Injury Interview at 4, 8, and 12 months Secondary outcomes: scores on the Borderline Symptom Checklist (BSL‐23), the Patient Health Questionnaire (PHQ‐9), the SCL‐90‐R, BSSI, BHS, Quality of Life Enjoyment and Satisfaction Questionnaire‐Short Form (Q‐LES‐Q SF), the Clinical Global Impressions‐Severity (CGI‐S) and Improvement (CGI‐I), and the Brief Disability Questionnaire (BDQ) at 4, 8, and 12 months |
| Starting date | September 2013. Proposed End Date: August 2016. |
| Contact information |
Name: Hui‐Chun Huang (Assistant Investigator) Affiliation: Mackay Memorial Hospital email: aihch@yahoo.com.tw |
| Notes | Hui‐Chun Huang very kindly provided unpublished information relating to this trial. |
Leybman 2014.
| Study name | Commitment and Motivation in a Brief DBT Intervention for Self Harm. Trial registration number: NCT02354183. |
| Methods |
Allocation: single blind randomisation Design: single‐centre Setting: centre for addiction and mental health Location: Canada |
| Participants |
Inclusion criteria: males and females, with borderline personality disorder, 18‐80 years of age, with at least 3 self‐harm episodes (either suicidal or non‐suicidal) in the past 5 years, including at least 1 in the past eight weeks. N expected: 120 Exclusion criteria: evidence of organic brain syndrome or mental retardation |
| Interventions | A 1‐hour orientation session consisting of DBT commitment strategies plus psychoeducation. Therapists will also use commitment strategies to discuss goals related to self‐harm. The psychoeducation will consist of information about DBT's biosocial theory and about why people self‐harm. All participants will complete a DBT skills training group after their orientation. |
| Outcomes |
Primary outcome: change in autonomous and controlled motivation (Autonomous and Controlled Motivation for Treatment Questionnaire) Secondary outcomes: change in frequency and severity of self‐harm behaviour (Deliberate Self‐Harm Inventory) |
| Starting date | April 2015 Proposed End Date: April 2016 |
| Contact information |
Name: Michelle Leybman Affiliation: Centre for Addiction & Mental Health, Canada Email: michelle.leybman@camh.ca |
| Notes |
Liu 2007.
| Study name | Effect of Psychosocial Treatment by the Case Manager in Patients After a Suicide Attempt. Trial registration number: NCT00664872. |
| Methods |
Allocation: single blind randomisation Design: single centre, hospital‐based intervention Setting: hospital Follow‐up period: 6 and 12 months Location: Taipei, Taiwan |
| Participants | Inclusion criteria: males and females, 18 years of age and older, who engaged in at least 1 episode of self‐harm within 6 months prior to randomisation |
| Interventions | Participants randomised to the intervention group will receive 6 sessions of a proactive psychosocial intervention for a 4‐month period. Each session will last approximately 30 min and will consist of telephone or face‐to‐face contact with the case manager at regular, scheduled intervals or when clinically necessary. Psychotherapy will consist of both cognitive‐behavioural and problem‐solving therapy and will be delivered by trained psychologists. |
| Outcomes |
Primary outcomes: the proportion of patients who self‐report a subsequent episode of self‐harm or a suicide attempt at the 6‐ and 12‐month follow‐up and the number of suicides at the 6‐ and 12‐month follow‐up Secondary outcomes: treatment attendance and adherence at the 6‐ and 12‐month follow‐up periods, types and number of contacts with healthcare services at the 6‐ and 12‐month follow‐up periods, scores on suicidal ideation as measured by the BSSI at the 6‐month follow‐up period, scores on depression as measured by the HRSD, and the 21‐item BDI over the 6‐month follow‐up period, and patient satisfaction with treatment at the 6‐ and 12‐month follow‐up assessments |
| Starting date | August, 2007. End date: July, 2008. |
| Contact information |
Name: Dr. Shen‐Ing Liu. Affiliation: Department of Psychiatry, Mackay Memorial Hospital, Taipei, Taiwan. email:maryliuyip@gmail.com |
| Notes | Dr Liu very kindly provided unpublished information relating to this trial. |
McMain 2015.
| Study name | DBT for Chronically Self‐harming Individuals With BPD: Evaluating the Clinical &Cost Effectiveness of a 6 mo. Treatment (FASTER‐DBT). Trial registration number: NCT02387736. |
| Methods |
Allocation: single blind randomisation (outcomes assessor) Location: Greater Toronto or Vancouver area, Canada |
| Participants |
Inclusion criteria: males and females, aged 18‐40 years, diagnosed with borderline personality disorder, with at least 2 self‐harm episodes (either suicidal or non‐suicidal) in the past 5 years, including at least 1 in the past 8 weeks; with absence of 8 or more standard weeks of DBT in the past year. Exclusion criteria: meets the DSM‐IV criteria for a psychotic disorder; with an IQ of less than 70; with chronic or serious physical health problem requiring hospitalization within the next year. N expected: 240 |
| Interventions | Compare 6 months v 12 months of DBT. |
| Outcomes |
Primary outcome: change in frequency and severity of suicide and self‐harm behaviours over time as measured by the Suicide Attempt Self‐Injury Interview (SASII) Secondary outcomes: changes in health care use as measured by the Treatment History Interview‐2 (THI‐2); general functioning as measured by the Euroqol‐5D; BPD symptoms as measured by the Borderline Symptom List‐23 (BSL‐23); general psychopathology and symptoms, as measures by the Symptom Checklist 90 Revised (SCL‐90R); anger as measured by the State‐Trait Anger Expression Inventory‐2 (STAXI‐2); depression as measured by the Beck Depression Inventory‐II (BDI‐II); interpersonal functioning as measured by the Inventory of Interpersonal Problems‐64 (IIP‐64) |
| Starting date | February 2015 End date: March 2019 |
| Contact information |
Name: Shelly McMain Affiliation: Centre for Addiction and Mental Health, Simon Fraser University email: not given – contact: mariana.mendozaalvarez@camh.ca |
| Notes |
O'Connor 2011.
| Study name | Improving Care Provided to Patients Treated in a Level 1 Trauma Center Post‐suicide Attempt. Trial registration number: NCT01355848. |
| Methods |
Allocation: single blind randomisation Design: single centre (hospital‐based), pre‐post design Setting: acute inpatient medical setting Location: Seattle, WA, USA |
| Participants | Inclusion criteria: males and females, of any age, who are admitted to a medical or surgical ward following a suicide attempt. Those with psychiatric diagnoses will not be excluded from participation. |
| Interventions | Those randomised to the experimental group will receive a brief intervention consisting of a stepped care protocol, including building rapport, functional analysis of suicidal behavior, and crisis planning for medically admitted suicide attempt survivors in addition to usual care. |
| Outcomes |
Primary outcome: scores on the Patient Satisfaction Questionnaire post‐intervention Secondary outcomes: scores on the BSSI, Self Injury, Readiness to Change, and Reasons for Living scales post‐intervention |
| Starting date | May, 2011. End date: June, 2013. |
| Contact information |
Name: Prof. Stephen O'Connor (PI). Affiliation: Western Kentucky University. email:stephen.oconnor@wku.edu |
| Notes | Prof Stephen O'Connor very kindly provided unpublished information relating to this trial. |
O'Connor 2012.
| Study name | A help sheet to reduce self‐harm among people admitted to hospital for self‐harm. Trial registration number: ISRCTN99488269. |
| Methods |
Allocation: randomised Design: single centre (hospital based) Setting: hospital Location: Edinburgh, Scotland |
| Participants |
Inclusion criteria: males and females, 16 years or older, admitted to the Royal Infirmary of Edinburgh with self‐harm, who have a history of prior self‐harm including both hospital‐treated and non‐hospital‐treated episodes, and those with suicidal intent associated with the present attempt necessitating admission to the Royal Infirmary of Edinburgh. Exclusion criteria: those under 16 years of age, with no history of self‐harming behaviour prior to the present episode, those with no reported suicidal intent associated with the present episode, those unfit for interview, those unable to provide informed consent, those for whom English is not their first language, those participating in other research at the Royal Infirmary of Edinburgh, and those who present to the emergency department but who are subsequently discharged without hospital admission N = 518: 259 in each arm of the trial |
| Interventions | Individuals randomised to the experimental group will receive TAU in addition to completing the Volitional Help Sheet with the assistance of a research assistant. A carbon copy of this sheet will also be produced so that participants can take home a copy of the Volitional Help Sheet to refer to as necessary. Approximately 2 months post‐baseline, individuals randomised to the experimental group will receive a similar "booster" help sheet and a covering letter explaining that the Volitional Help Sheet can be completed again if required. |
| Outcomes |
Primary outcomes: repetition of self‐harm necessitating hospital admission to any hospital in Scotland during the 6‐month follow‐up period, number of re‐presentations to hospital for self‐harm during the 6‐month follow‐up period, and cost‐effectiveness of the Volitional Help Sheet, as measured by the estimated incremental cost per episode of self‐harm or suicide averted Secondary outcomes: Time to re‐presentation to any hospital in Scotland with self‐harm during the 6‐month follow‐up period measured in weeks, months, or both |
| Starting date | April, 2012. Proposed End Date: April, 2015. |
| Contact information | Name: Prof. Rory O'Connor (PI).Affiliation: The University of Glasgow.Email:Rory.OConnor@glasgow.ac.uk |
| Notes | Prof Rory O'Connor very kindly provided unpublished information relating to this trial. Additionally, Prof O'Connor provided the following to describe the theoretical basis, content, and purpose of the Volitional Help Sheet: "The intervention takes the form of a help sheet (Armitage 2008), which is developed from three well established theoretical perspectives and previous research: Gollwitzer's concept of Implementation Interventions (Gollwitzer 1993), Prochaska and DiClemente's trans‐theoretical model (Prochaska 1983), Integrated Motivational‐Volitional Model of suicidal behaviour (IMV; O'Connor 2011) and previous work on self‐harm (e.g., O'Connor 2006; O'Connor 2009; Hawton 2006). In essence, the help sheet is a behavioural change technique which encourages participants to link critical situations in which they are tempted to self‐harm with alternative responses/solutions." |
O'Connor 2014.
| Study name | Pilot study of a brief intervention for medically hospitalised suicide attempt survivors Trial registration number: NCT02414763. |
| Methods |
Allocation: single blind randomisation Design: single centre (hospital‐based), longitudinal design Setting: level 1 trauma centre Location: Nashville, TN, USA |
| Participants | Inclusion criteria: males and females over 17 years of age, who are admitted to a medical or surgical ward following a suicide attempt. Those with psychiatric diagnoses will not be excluded from participation. |
| Interventions | Those randomised to the intervention group will receive a brief intervention of a stepped care protocol, including building rapport, functional analysis of suicidal behavior, and crisis planning for medically admitted suicide attempt survivors in addition to usual care. |
| Outcomes | Primary outcomes: Scores on the Patient Satisfaction Questionnaire, the Readiness to Change, Reasons for Living, Perceived Burdensomeness, Thwarted Belongingness, Acquired Capability, and Scale for Suicidal Ideation scales post‐intervention. In addition, investigators will assess repetition of suicide attempts and non‐suicidal self‐injury post‐intervention. |
| Starting date |
Proposed start date: October 2014 Proposed end date: September 2016 |
| Contact information |
Name: Prof Stephen O'Connor (Principal investigator) Affiliation: Western Kentucky University email: stephen.oconnor@wku.edu |
| Notes | Prof Stephen O'Connor very kindly provided unpublished information relating to this trial. |
Pham‐Scottez 2009.
| Study name | Effectiveness of a 24 hour phone line on the rate of suicide attempts in borderline patients. Trial registration number: NCT00603421. |
| Methods |
Allocation: single blind, parallel assignment, randomisation Design: multicentre. Setting: in‐ and outpatient clinics Location: various locations around Paris, France Follow‐up period: 1 year |
| Participants |
Inclusion criteria: males and females, aged 18‐40 years, diagnosed with borderline personality disorder, treated as in‐ or outpatients at 1 of the trial recruiting centres (Hôpital St Anne and Hôpital Cichin Centre de Recherche Clinque Paris), and able to provide written informed consent Exclusion criteria: those below 18 or over 40 years of age, those diagnosed with schizophrenia or a severe somatic disorder, those who refuse consent to participate, and those already participating in another intervention trial |
| Interventions | Participants randomised to the intervention arm will receive 1 year of access to a 24 h crisis phone line monitored by a team of psychiatrists with experience treating borderline personality disorder patients in addition to TAU |
| Outcomes |
Primary outcome measure: annualised rate of suicide attempts Secondary outcome measure: annualised rate of self injurious behaviour |
| Starting date | February 2009. Estimated end date: September 2014 |
| Contact information |
Name: Dr Alexandra Pham‐Scottez Affiliation: Centre Hôspitalier Sainte Anne email: a.pham@ch-sainte-anne.fr |
| Notes | We made 3 attempts to contact Dr Pham‐Scottez to confirm these details; however, we received no response and so were unable to confirm whether all participants either engaged in deliberate self‐harm or made a suicide attempt within 6 months prior to randomisation. We extracted information for this trial from the ClinicalTrials.gov record. |
Sayal 2015.
| Study name | RCT of the Clinical and Cost Effectiveness of Cognitive Behaviour Therapy (CBT) Delivered Remotely Versus Treatment as Usual in Adolescents and Young Adults With Depression Who Repeatedly Self‐harm (eDASH). Trial registration number: NCT02377011. |
| Methods |
Allocation: single‐blind randomisation Design: single centre Setting: hospital Location: Chesterfield Royal Hospital NHS Foundation Trust |
| Participants |
Inclusion criteria: males and females, aged 16‐30 years; within 96 hours of last self‐harm presentation (self‐harm as defined by NICE criteria); with >2 self‐harm episodes; with high levels of unipolar depressive symptoms (BDI‐2 score of 17 or more). Exclusion criteria: clinical judgement of high level of suicide risk, other risk to self or others requiring other urgent approaches; other severe mental illness; currently receiving structured psychological therapy. N expected:120 |
| Interventions | Problem solving cognitive behaviour therapy (PS CBT) will be delivered remotely by means of telephone or video calling by a cognitive behaviour therapist in addition to their usual care. |
| Outcomes |
Primary outcome measure: Beck Depression Inventory ((BDI‐II) at 6 months Secondary outcome measures: Beck Depression Inventory (V2); Patient Health Questionnaire 9 (PHQ‐9); Beck hopelessness scale; Columbia Suicide Severity Rating Scale (CSSRS); social functioning (Work and Social Adjustment Scale (WSAS)); quality of life (EQ‐5D); cost effectiveness (modified version of the CSRI); qualitative interviews. All measured at 12 months. |
| Starting date | January 2014. Estimated end date: December 2017 |
| Contact information | Kapil.sayal@nottingham.ac.uk |
| Notes |
Vaiva 2011.
| Study name | ALGOS. Trial registration number: NCT01123174. |
| Methods |
Allocation: single‐blind randomisation Design: multicentre. Setting: 23 community mental health centres Follow‐up period: 6 and 14 months Location: various locations around France |
| Participants |
Inclusion criteria: males and females, over 18 years of age, who present to emergency departments following an episode of attempted suicide Exclusion criteria: multiple repeaters, those with 4 or more suicide attempts in the preceding 3 years Expected N: 900 |
| Interventions | Using the ALGOS algorithm, a decision tree concerning the type of contact a participant should receive based on his or her number of previous suicide attempts, first‐time attempters randomised to the experimental group will receive a crisis card. Those with 1‐3 previous suicide attempts in the preceding 3 years, on the other hand, will receive telephone contact on the 10th and 21st day following the most recent suicide attempt, and postcard contact for 5 months. |
| Outcomes |
Primary outcome: number of participants who subsequently make a suicide attempt during the follow‐up period Secondary outcomes: number of deaths by suicide, scores on the BSSI, psychopathology as assessed by scores on the MINI, number of health care contacts, and a medico‐economic assessment of the costs of ALGOS |
| Starting date | February, 2010. Proposed end date: April, 2014. |
| Contact information |
Name: Prof. Guillaume Vaiva (PI). Affiliation: Centre Hospitalier Régional Universitaire de Lille. email:guillaume.vaiva@chru-lille.fr |
| Notes | Prof Guillaume Vaiva very kindly provided unpublished information relating to this trial. |
van den Bosch 2013.
| Study name | Intensified, Inpatient Adaptation of Dialectical Behavior Therapy (DBT) REDBT. Trial registration number: NCT01904227. |
| Methods |
Allocation: randomised, open label Design: single centre Setting: inpatient and outpatient (different arms of trial ‐ Jelgersma Treatment Centre, or the outpatient DBT programs of Rivierduinen) Location: Netherlands |
| Participants |
Inclusion criteria: males and females, aged 18 to 40 years, with severe borderline personality disorder( > 24 on the BPDSI), admitted to hospital with suicidal and/or self‐harming behavior in the year preceding the start of DBT treatment, including the last month preceding baseline measurement. Exclusion criteria: IQ < 80; a chronic psychotic condition; bipolar disorder; hard drug abuse that requires inpatient detoxification; forced treatment framework; DBT in the year preceding intake. Expected N: 150 |
| Interventions | Inpatient DBT v outpatient DBT |
| Outcomes |
Primary outcome: change in number of suicide attempts/self‐harming acts. Secondary outcomes: change in severity of borderline symptomatology (BPDSI). |
| Starting date | February 2012 Proposed end date: April 2015 (no longer recruiting) |
| Contact information |
Name: Louisa M van den Bosch Affiliations: Rivierduinen, Centre for Personality disorders Jelgersma Email: n/a |
| Notes |
Walker 2012.
| Study name | Women Offenders Repeat Self‐Harm Intervention Pilot II. Trial registration number: ISRCTN18761534. |
| Methods |
Allocation: randomisation by minimisation Design: multicentre (3 closed‐category prisons) Setting: closed‐category prisons housing a mixture of remand and sentenced prisoners Follow‐up period: 3 and 6 months Location: Cheshire, Derby, and Yorkshire, UK |
| Participants |
Inclusion criteria: female prisoners, 18 years or older, remanded or sentenced to any 1 of 3 prisons for any offence, who have a history of repeated self‐harming behaviour with at least 1 incident within the month prior to randomisation, and are currently on an Assessment, Care in Custody and Teamwork (ACCT). As the trial does not discriminate between the severity and frequency of self‐harming behaviour, previous self‐harming behaviour can range from superficial cuts to ligaturing. Expected N: 120 |
| Interventions | Participants randomised to the experimental group will receive weekly sessions of psychodynamic interpersonal therapy for a minimum of 6 weeks. Those randomised to the control group will receive active control comprising a weekly session of time out of their cell to play card games, read magazines, listen to music, or to discuss practical topics (e.g., developing financial management skills) with research assistants. Women randomised to this group are specifically instructed that they cannot discuss emotive topics with research assistants. |
| Outcomes |
Primary outcome: scores on Beck's Scale for Suicidal Ideation immediately post‐treatment (3 months) and at 6 months Secondary outcomes: scores on Beck's Depression Inventory and Beck's Hopelessness Inventory immediately post‐treatment (3 months) and at 6 months. Additionally, information on both the frequency and severity of self‐harm and thoughts of self‐harm immediately post‐treatment (3 months) and at 6 months will be measured using the Self Harm Incidents Questionnaire. Lastly, information on satisfaction with treatment will be assessed immediately post‐treatment (3 months) using the Intervention Satisfaction Questionnaire. |
| Starting date | June, 2013. Proposed end date: June, 2015. |
| Contact information |
Name: Dr. Tammi Walker. Affiliations: Institute of Brain, Behaviour and Mental Health (University of Manchester) and School of Social and International Studies (University of Bradford). |
| Notes | Dr Tammi Walker very kindly provided information relating to this trial. |
AUDIT: Alcohol Use Disorders Identification Test; BHS: Beck Hopelessness Scale; BSSI: Beck scale for suicidal ideation; DAST: drug abuse screening test; DSM‐IV: Diagnostic and Statistical Manual for Mental Disorders, fourth edition; MACT: manual‐assisted cognitive therapy; SAPAS: Standardised Assessment of Personality: Abbreviated Scale; SCL‐90‐R: Symptom Checklist‐90‐Revised.
Differences between protocol and review
In the original protocol for this review we planned to assess dichotomous outcome data (i.e., repetition of self‐harm and suicide) using the Peto odds ratio. Following revisions to iterations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2003) and new statistical advice, however, we have instead used the Mantel Haenzel method in this update. For this version of the review we have been able to add data for the previously stated outcomes of interest: depression, hopelessness, problem‐solving, and suicidal ideation. We have also used the I2 statistic, rather than the Chi2 test, to summarise between‐study heterogeneity in this version in light of revisions to Higgins 2003.
We also planned to assess methodological quality of included trials by the means recommended by the contemporary version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2003). For this version of the review, we have therefore created 'Risk of bias' and 'Summary of findings' tables as per current recommendations. We have also refined the unit of analysis section, as per current recommendations, to include Zelen designed trials and trials that report adjusted effect sizes.
We have also added four sensitivity analyses, one for trials that employed Zelen's method of randomisation; one for trials that contributed substantial (> 75%) levels of heterogeneity; one for trials that specifically recruited individuals diagnosed with borderline personality disorder; and a fourth for trials that included a small minority (< 15%) of adolescent participants.
Contributions of authors
KH had the idea for the review. All authors extracted data and assessed risk of bias for included trials. Both TTS and KW conducted the statistical analyses. KH, TTS, and KW wrote the initial version of the report and all authors contributed to the writing of drafts. All authors also approved the final version of the review for publication.
Sources of support
Internal sources
University Department of Psychiatry, Warneford Hospital, Oxford, UK
Oxford Health NHS Foundation Trust, Oxford, UK
External sources
NHS Executive Anglia and Oxford Research and Development Program, UK
NIHR Service Delivery and Organisation programme, UK
Declarations of interest
KH and DG each authored three of the trials included in the review, EA authored two trials, and KvH is the author of one of the trials.
Edited (no change to conclusions)
References
References to studies included in this review
Allard 1992 {published data only}
- Allard R, Marshall M, Plante MC. Intensive follow-up does not decrease the risk of repeat suicide attempts. Suicide and Life-Threatening Behavior 1992;22(3):303-14. [PubMed] [Google Scholar]
Bateman 2009 {published and unpublished data}27660668
- Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. American Journal of Psychiatry 2009;166(12):1355-64. [DOI] [PubMed] [Google Scholar]
Beautrais 2010 {published and unpublished data}
- Beautrais AL, Gibb SJ, Faulkner A, Fergusson DM, Mulder RT. Postcard intervention for repeat self-harm: randomised controlled trial. British Journal of Psychiatry 2010;197(1):55-60. [DOI] [PubMed] [Google Scholar]
Bennewith 2002 {published data only}
- Bennewith O, Stocks N, Gunnell D, Peters TJ, Evans MO, Sharp DJ. General practice based intervention to prevent repeat episodes of deliberate self-harm: cluster randomised controlled trial. British Medical Journal 2002;324(7348):1254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2005 {published data only}
- Brown GK, Ten Have T, Henriques GR, Xie SX, Hollander JE, Beck AT. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. Journal of the American Medical Association 2005;294(5):563-70. [DOI] [PubMed] [Google Scholar]
- Ghahramanlou-Holloway M, Bhar SS, Brown GK, Olsen C, Beck AT. Changes in problem-solving appraisal after cognitive therapy for the prevention of suicide. Psychological Medicine 2012;42(6):1185-93. [DOI] [PubMed] [Google Scholar]
- Stirman SW, Brown GK, Ghahramanlou-Holloway M, Fox AJ, Chohan MZ, Beck AT. Participation bias among suicidal adults in a randomized controlled trial. Suicide and Life Threatening Behavior 2011;41(2):203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2005 {published and unpublished data}
- Carter GL, Clover K, Whyte IM, Dawson AH, D'este C. Postcards from the EDge: 24-month outcomes of a randomised controlled trial for hospital-treatment self-poisoning. British Journal of Psychiatry 2007;191:548-53. [DOI] [PubMed] [Google Scholar]
- Carter GL, Clover K, Whyte IM, Dawson AH, D'este C. Postcards from the EDge project: randomised controlled trial of an intervention using postcards to reduce repetition of hospital treated deliberate self-poisoning. British Medical Journal 2005;331(7520):805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cedereke 2002 {published data only}
- Cedereke M, Monti K, Ojehagen A. Telephone contact with patients in the year after a suicide attempt: Does it affect treatment attendance and outcome? A randomised controlled study. European Psychiatry 2002;17(2):82-91. [DOI] [PubMed] [Google Scholar]
Clarke 2002 {published data only}
- Clarke T, Baker P, Watts CJ, Williams K, Feldman RA, Sherr L. Self-harm in adults: A randomised controlled trial of nurse-led case management versus routine care only. Journal of Mental Health 2002;11(2):167-76. [Google Scholar]
Crawford 2010 {published and unpublished data}
- Crawford MJ, Csipke E, Brown A, Reid S, Nilsen K, Redhead J, Touquet R. The effect of referral for brief intervention for alcohol misuse on repetition of deliberate self-harm: an exploratory randomized controlled trial. Psychological Medicine 2010;40(11):1821-8. [DOI] [PubMed] [Google Scholar]
Davidson 2014 {published and unpublished data}
- Davidson KM, Brown TM, James V, Kirk J, Richardson J. Manual-assisted cognitive therapy for self-harm in personality disorder and substance misuse: a feasibility trial. The Psychiatric Bulletin 2014;38(3):108-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dubois 1999 {published data only}
- Dubois L, Walter M, Bleton L, Genest P, Lemonnier E, Lachevre G. Evaluation of a comparative and prospective protocol for suicidal youth: analysis of psychiatric diagnosis, therapeutic compliance and rate of recurrence over one year (preliminary results) [Evaluation comparative et prospective d'un protocole de prise en charge specifique de jeunes suicidants: Analyse du diagnostic psychiatrique initial, de l'observance therapeutique et du taux de recidive a un an (resultats preliminaires)]. Annales Medico-Psychologiques 1999;157:557-61. [Google Scholar]
Evans 1999a {published data only}
- Evans J, Evans M, Morgan HG, Hayward A, Gunnell, D. Crisis card following self-harm: 12-month follow-up of a randomised controlled trial. British Journal of Psychiatry 2005;187:186-187. [DOI] [PubMed] [Google Scholar]
- Evans MO, Morgan HG, Hayward A, Gunnell DJ. Crisis telephone consultation for deliberate self-harm patients: effects on repetition. British Journal of Psychiatry 1999;175:23-7. [DOI] [PubMed] [Google Scholar]
Evans 1999b {published data only}
- Evans K, Tyrer P, Catalan J, Schmidt U, Davidson K, Dent J, Tata P, Thornton S, Barber J, Thompson S. Manual-assisted cognitive-behaviour therapy (MACT): A randomized controlled trial of a brief intervention with bibliotherapy in the treatment of recurrent deliberate self-harm. Psychological Medicine 1999;29(1):19-25. [DOI] [PubMed] [Google Scholar]
Fleischmann 2008 {published and unpublished data}
- Bertolote JM, Fleischmann A, De Leo D, Phillips MR, Botega NJ, Vijayakumar L, De Silva D, Schlebusch L, Nguyen VT, Sisask M, Bolhari J, Wasserman D. Repetition of suicide attempts: Data from five culturally different low- and middle-income country emergency care settings participating in the WHO SUPRE-MISS study. Crisis: The Journal of CrisisIntervention and Suicide Prevention 2010;31(4):194-201. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Bertolote JM, Wasserman D, De Leo D, Bolhari J, Botega NJ, De Silva D, Phillips M, Vijayakumar L, Värnik A, Schlebusch L, Thanh HT. Effectiveness of brief intervention and contact for suicide attempters: A randomized controlled trial in five countries. Bulletin of the World Health Organisation 2008;86(9):703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanzadeh M, Khajeddin N, Nojomi M, Fleischmann A, Eshrati T. Brief intervention and contact after deliberate self-harm: an Iranian randomised controlled trial. Iranian Journal of Psychiatry and Behavioral Sciences 2010;4(2):5-12. [Google Scholar]
- Vijayakumar L, Umamaheswari C, Shujaath Ali ZS, Devaraj P, Kesavan K. Intervention for suicide attempters: A randomized controlled study. Indian Journal of Psychiatry 2011;53(3):244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Zhang X-L, Li X-Y, Niu Y-J, Zhang Y-P, Wang S-L, et al. Effectiveness of 18-month psychosocial intervention for suicide attempters. Zhongguo Xinli Weisheng Zazhi [Chinese Mental Health Journal] 2012;26:24-9. [Google Scholar]
Gibbons 1978 {published data only}
- Gibbons JS, Butler J, Urwin P, Gibbons JL. Evaluation of a social work service for self-poisoning patients. British Journal of Psychiatry 1978;133:111-118. [DOI] [PubMed] [Google Scholar]
Gratz 2006 {published and unpublished data}
- Gratz KL, Gunderson JG. Preliminary data on an acceptance-based emotion regulation group intervention for deliberate self-harm among women with borderline personality disorder. Behavior Therapy 2006;37(1):25-35. [DOI] [PubMed] [Google Scholar]
Gratz 2014 {published and unpublished data}
- Gratz KL, Bardeen JR, Levy R, Dixon-Gordon KL, Tull MT. Mechanisms of change in an emotion regulation group therapy for deliberate self-harm among women with borderline personality disorder. Behaviour Research and Therapy 2015;65:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Dixon-Gordon KL, Tull MT. Predictors of treatment response to an adjunctive emotion regulation group therapy for deliberate self-harm among women with borderline personality disorder. Personality disorders: theory, research, & treatment 2014;5(1):97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, Tull MT, Levy R. Randomized controlled trial and uncontrolled 9-month follow-up of an adjunctive emotion regulation group therapy for deliberate self-harm among women with borderline personality disorder. Psychological Medicine 2013;44(10):2099-122. [DOI] [PubMed] [Google Scholar]
Guthrie 2001 {published data only}
- Guthrie E, Kapur N, Kway-Jones K, Chew-Graham C, Moorey J, Mendel E, Marino-Francis F, Sanderson S, Turpin C, Boddy G, Tomenson B. Randomised controlled trial of brief psychological intervention after deliberate self-poisoning. British Medical Journal 2001;323(7305):135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harned 2014 {published and unpublished data}
- Harned MS, Korslund KE, Linehan MM. A pilot randomized controlled trial of dialectical behavior therapy with and without the dialectical behavior therapy prolonged exposure protocol for suicidal and self-injuring women with borderline personality disorder and PTSD. Behaviour Research and Therapy 2014;55:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hassanian‐Moghaddam 2011 {published and unpublished data}
- Hassanian-Moghaddam H, Sarjami S, Kolahi A-A, Carter GL. Postcards in Persia: Randomised controlled trial to reduce suicidal behaviours 12 months after hospital-treated self-poisoning. British Journal of Psychiatry 2011;198(4):309-16. [DOI] [PubMed] [Google Scholar]
- Hassanian-Moghaddam H, Sarjami S, Kolahi A-A, Lewin T, Carter G. Postcards in Persia: A 12-24 month follow-up of a randomised controlled trial for hospital treated deliberate self-poisoning. Archives of Suicide Research 2015;Epub ahead of print:DOI: 10.1080/13811118.2015.1004473. [DOI] [PubMed]
Hatcher 2011 {published and unpublished data}
- Hatcher S, Sharon C, Parag V, Collins N. Problem-solving therapy for people who present to hospital with self-harm: Zelen randomised controlled trial. British Journal of Psychiatry 2011;199(4):310-6. [DOI] [PubMed] [Google Scholar]
Hatcher 2015 {published and unpublished data}
- Hatcher S, Sharon C, House A, Collins N, Collings S, Pillai A. The ACCESS study: Zelen randomised controlled trial of a package of care for people presenting to hospital after self-harm. British Journal of Psychiatry 2015;206(3):229-36. [DOI] [PubMed] [Google Scholar]
Hatcher 2016a {published and unpublished data}
- Hatcher S, Coupe N, Wikirwhi K, Durie M, Pillai A. Te Ira Tangaga: A Zelen randomised controlled trial of A culturally informed treatment compared to treatment as usual in Maori who present to hospital after self-harm. Social Psychiatry and Psychiatric Epidemiology 2016;Epub ahead of print:DOI: 10.1007/s00127-016-1194-7. [DOI] [PubMed] [Google Scholar]
Hawton 1981 {published and unpublished data}
- Hawton K, Bancroft J, Catalan J, Kingston B, Stedeford A. Domiciliary and out-patient treatment of self-poisoning patients by medical and non-medical staff. Psychological Medicine 1981;11(1):169-77. [DOI] [PubMed] [Google Scholar]
Hawton 1987a {published and unpublished data}
- Hawton K, McKeown S, Day A, Martin P, O'Connor M, Yule J. Evaluation of out-patient counselling compared with general practitioner care following overdoses. Psychological Medicine 1987;17(3):751-61. [DOI] [PubMed] [Google Scholar]
Husain 2014 {published and unpublished data}
- Husain N, Afsar S, Ara J, Fayyaz H, Ur Rahman R, Tomenson B, et al. Brief psychological intervention after self-harm: randomised controlled trial from Pakistan. British Journal of Psychiatry 2014;204(6):462-70. [DOI] [PubMed] [Google Scholar]
Hvid 2011 {published and unpublished data}
- Hvid M, Vangborg K, Sørensen HJ, Nielsen IK, Stenborg JM, Wang AG. Preventing repetition of attempted suicide - II: The Amager Project, a randomized controlled trial. Nordic Journal of Psychiatry 2011;65(5):292-8. [DOI] [PubMed] [Google Scholar]
Kapur 2013a {published data only}65171515
- Kapur N, Gunnell D, Hawton K, Nadeem S, Khalil S, Longson D, Jordan R, Donaldson I, Emsley R, Cooper J. Messages from Manchester: Pilot randomised controlled trial following self-harm. British Journal of Psychiatry 2013;203(1):73-4. [DOI] [PubMed] [Google Scholar]
Kawanishi 2014 {published and unpublished data}
- Kawanishi C, Aruga T, Ishizuka N, Yonemoto N, Otsuka K, Kamijo Y, Okubo Y, Ikeshita K, Sakai A, Miyaoka H, Hitomi Y, Iwakuma A, Kinoshita T, Akiyoshi J, Horikawa N, Hirotsune H, Eto N, Iwata N, Kohno M, Iwanami A, Mimura M, Asada T, Hirayasu Y on behalf of the ACTION-J Group. Assertive case management versus enhanced usual care for people with mental health problems who had attempted suicide and were admitted to hospital emergency departments in Japan (ACTION-J): A multicentre, randomised controlled trial. The Lancet Psychiatry 2014;1(3):193-201. [DOI] [PubMed] [Google Scholar]
Liberman 1981 {published data only}
- Liberman RP, Eckman T. Behavior therapy vs insight-oriented therapy for repeated suicide attempters. Archives of General Psychiatry 1981;38(10):1126-30. [DOI] [PubMed] [Google Scholar]
Linehan 1991 {published data only}
- Linehan MM, Armstrong HE, Suarez A, Allmon D, Heard HL. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Archives of General Psychiatry 1991;48(12):1060-4. [DOI] [PubMed] [Google Scholar]
- Linehan MM, Heard HL, Armstrong HE. Naturalistic follow-up of a behavioral treatment for chronically parasuicidal borderline patients. Archives of General Psychiatry 1993;50(12):971-4. [DOI] [PubMed] [Google Scholar]
Linehan 2006 {published and unpublished data}
- Bedics JD, Atkins DC, Comtois JA, Linehan MM. Treatment differences in the therapeutic relationship and introject during a 2-year randomized controlled trial of dialectical behavior therapy versus non-behavioral psychotherapy experts for borderline personality disorder. Journal of Consulting and Clinical Psychology 2012;80(1):66-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedics JD, Atkins DC, Harned MS, Linehan MM. The therapeutic alliance as a predictor of outcome in dialectical behavior therapy versus nonbehavioral psychotherapy by experts for borderline personality disorder. Psychotherapy: Theory, Research and Practice 2015;52(1):67-77. [DOI] [PubMed] [Google Scholar]
- Harned MS, Chapman AL, Dexter-Mazza ET, Murray A, Comtois KA, Linehan MM. Treating co-occurring axis I disorders in recurrently suicidal women with borderline personality disorder: A 2-year randomized trial of dialectical behavior therapy versus community treatment by experts. Journal of Consulting and Clinical Psychology 2008;76(6):1068-75. [DOI] [PubMed] [Google Scholar]
- Harned MS, Chapman AL, Dexter-Mazza ET, Murray A, Comtois KA, Linehan MM. Treating co-occurring axis I disorders in recurrently suicidal women with borderline personality disorder: A 2-year randomized trial of dialectical behavior therapy versus community treatment by experts. Personality Disorders: Theory, Research and Treatment 2009;5:35-45. [DOI] [PubMed] [Google Scholar]
- Linehan MM, Comtois KA, Murray AM, Brown MZ, Gallop RJ, Heard HL, et al. Two-year randomized controlled trial and follow-up of dialectical behavior therapy vs therapy by experts for suicidal behaviors and borderline personality disorder. Archives of General Psychiatry 2006;63(7):757-66. [DOI] [PubMed] [Google Scholar]
- Neacsiu AD, Lungu A, Harned MS, Rizvi SL, Linehan MM. Impact of dialectical behavior therapy versus community treatment by experts on emotional experience, expression, and acceptance in borderline personality disorder. Behaviour Research and Therapy 2014;53:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marasinghe 2012 {published and unpublished data}
- Marasinghe RB, Edirippulige S, Kavanagh D, Smith A, Jiffry MTM. Effect of mobile phone-based psychotherapy in suicide prevention: A randomized controlled trial in Sri Lanka. Journal of Telemedicine and Telecare 2012;18(3):151-5. [DOI] [PubMed] [Google Scholar]
- Marasinghe RB. Evaluation of a Brief Inpatient and Community Intervention to Address Suicide Risk in Sri Lanka Using Mobile Phones [PhD thesis]. Brisbane, Australia: The Univerity of Queensland, School of Medicine, 2012. [Google Scholar]
McAuliffe 2014 {published and unpublished data}
- McAuliffe C, McLeavey BC, Fitzgerald T, Corcoran P, Carroll B, Ryan L, O'Keeffe B, Fitzgerald E, Hickey P, O'Regan M, Mulqueen J, Arensman E. Group problem-solving skills training for self-harm: Randomised controlled trial. British Journal of Psychiatry 2014;204:383-90. [DOI] [PubMed] [Google Scholar]
McLeavey 1994 {published data only}
- McLeavey B, Daly R, Ludgate J, Murray C. Interpersonal problem-solving skills training in the treatment of self-poisoning patients. Suicide and Life-Threatening Behavior 1994;24(4):382-94. [PubMed] [Google Scholar]
McMain 2009 {published and unpublished data}
- Case BG. Dialectical behavior therapy versus general psychiatric management in the treatment of borderline personality disorder. American Journal of Psychiatry 2010;167(4):475. [DOI] [PubMed] [Google Scholar]
- McMain SF, Guimond T, Streiner DL, Cardish RJ, Links PS. Dialectical behavior therapy compared with general psychiatric management for borderline personality disorder: Clinical outcomes and functioning over a 2-year follow-up. American Journal of Psychiatry 2012;169(6):650-61. [DOI] [PubMed] [Google Scholar]
- McMain SF, Links PS, Gnam WH, Guimond T, Cardish RJ, Korman L, Streiner DL. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. American Journal of Psychiatry 2009;166(12):1365-74. [DOI] [PubMed] [Google Scholar]
Morgan 1993 {published data only}
- Morgan HG, Jones EM, Owen JH. Secondary prevention of non-fatal deliberate self-harm. The green card study. British Journal of Psychiatry 1993;163:111-2. [DOI] [PubMed] [Google Scholar]
Morthorst 2012 {published data only}
- Morthorst B, Krogh J, Erlangsen A, Alberdi F, Nordentoft M. Effect of assertive outreach after suicide attempt in the AID (Assertive Intervention for Deliberate self-harm) trial: randomised controlled trial. British Medical Journal 2012;345:e4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patsiokas 1985 {published data only}
- Patsiokas AT, Clum GA. Effects of psychotherapeutic strategies in the treatment of suicide attempters. Psychotherapy: Theory, Research and Practice 1985;22(2):281-90. [Google Scholar]
Priebe 2012 {published and unpublished data}
- Priebe S, Bhatti N, Barnicot K, Bremner S, Gaglia A, Katsakou C, Molosankwe I, McCrone P, Zinkler M. Effectiveness and cost-effectiveness of dialectical behaviour therapy for self-harming patients with personality disorder: A pragmatic randomised controlled trial. Psychotherapy and Psychosomatics 2012;81(6):356-65. [DOI] [PubMed] [Google Scholar]
Salkovskis 1990 {published data only}
- Salkovskis PM, Atha C, Storer D. Cognitive-behavioural problem solving in the treatment of patients who repeatedly attempt suicide. A controlled trial. British Journal of Psychiatry 1990;157:871-876. [DOI] [PubMed] [Google Scholar]
Slee 2008 {published and unpublished data}
- Slee N, Garnefski N, Van der Leeden R, Arensman E, Spinhoven P. Cognitive-behavioural intervention for self-harm: Randomised controlled trial. British Journal of Psychiatry 2008;192:202-11. [DOI] [PubMed] [Google Scholar]
Stewart 2009 {published and unpublished data}
- Stewart CD, Quinn A, Plever S, Emmerson B. Comparing cognitive behavior therapy, problem solving therapy, and treatment as usual in a high risk population. Suicide and Life-Threatening Behavior 2009;39(5):538-47. [DOI] [PubMed] [Google Scholar]
Tapolaa 2010 {published and unpublished data}
- Tapolaa V, Lappalainen R, Wahlström J. Brief intervention for deliberate self-harm: An exploratory study. Suicidology Online 2010;1:95-108. [Google Scholar]
Torhorst 1987 {published data only}
- Möller HJ. Efficacy of different strategies of aftercare for patients who have attempted suicide. Journal of the Royal Society of Medicine 1989;82(11):643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torhorst A, Möller HJ, Bürk F, Kurz A, Wächtler C, Lauter H. The psychiatric management of parasuicide patients: A controlled clinical study comparing different strategies of outpatient treatment. Crisis 1987;8(1):53-61. [PubMed] [Google Scholar]
Torhorst 1988 {published data only}
- Torhorst A, Möller HJ, Kurz A, Schmid-Bode W, Lauter H. Comparing a 3-month and a 12-month-outpatient aftercare program for parasuicide repeaters. In: Möller HJ, Schmidtke A, Welz R, editors(s). Current Issues of Suicidology. Berlin, Germany: Springer-Verlag, 1988:419-24. [Google Scholar]
Turner 2000 {published data only}
- Turner RM. Naturalistic evaluation of dialectical behavior therapy-oriented treatment for borderline personality disorder. Cognitive and Behavioral Practice 2000;7(4):413-9. [Google Scholar]
Tyrer 2003 {published data only}
- Tyrer P, Thompson S, Schmidt U, Jones V, Knapp M, Davidson K, Catalan J, Airlie J, Baxter S, Byford S, Byrne G, Cameron S, Caplan R, Cooper S, Ferguson B, Freeman C, Frost S, Godley J, Greenshields J, Henderson J, Holden N, Keech P, Kim L, Logan K, Manley C, MacLeod A, Murphy R, Patience L, Ramsay L, De Munroz S, Scott J, Seivewright H, Sivakumar K, Tata P, Thornton S, Ukoumunne OC, Wessely S. Randomized controlled trial of brief cognitive behaviour therapy versus treatment as usual in recurrent deliberate self-harm: The POPMACT study. Psychological Medicine 2003;33(6):969-76. [DOI] [PubMed] [Google Scholar]
Vaiva 2006 {published data only}
- Vaiva G, Ducrocq F, Meyer P, Mathieu D, Philippe A, Libersa C, Goudemand M. Effect of telephone contact on further suicide attempts in patients discharged from an emergency department: Randomised controlled study. British Medical Journal 2006;332(7552):1241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Sande 1997a {published data only}
- Van der Sande R, Buskens E, Van der Graaf Y, Van Rooijen E, Allart E, Van Engeland H. No measurable effect from general socio-psychiatric care for those attempting suicide: A randomized experiment [Geen meetbaar effect van algemene sociaal-psychiatrische nazorg voor suicidepogers; een gerandomiseerd experiment]. Nederlands Tijdschrift voor de Geneeskunde 1998;142:2356. [Google Scholar]
- Van der Sande R, Van Rooijen L, Buskens E, Allart E, Hawton K, Van der Graaf Y, Van Engeland H. Intensive in-patient and community intervention versus routine care after attempted suicide. A randomised controlled intervention study. British Journal of Psychiatry 1997;171:35-41. [DOI] [PubMed] [Google Scholar]
Van Heeringen 1995 {published data only}
- Van Heeringen C, Jannes S, Buylaert W, Hendrick H, De Bacquer D, Van Remoortel J. The management of non-compliance with referral to out-patient after-care among attempted suicide patients: A controlled intervention study. Psychological Medicine 1995;25(5):963-70. [DOI] [PubMed] [Google Scholar]
Waterhouse 1990 {published data only}
- Waterhouse J, Platt S. General hospital admission in the management of parasuicide. A randomised controlled trial. British Journal of Psychiatry 1990;156:236-242. [DOI] [PubMed] [Google Scholar]
Wei 2013 {published data only}
- Wei S, Liu L, Bi B, Li H, Hou J, Tan S, Chen X, Chen W, Jia X, Dong G, Qin X, Liu Y. An intervention and follow-up study following a suicide attempt in the emergency departments of four general hospitals in Shenyang, China. Crisis 2013;34(2):107-15. [DOI] [PubMed] [Google Scholar]
Weinberg 2006 {published data only}
- Weinberg I, Gunderson JG, Hennen J, Cutter CJ. Manual assisted cognitive treatment for deliberate self-harm in borderline personality disorder patients. Journal of Personality Disorders 2006;20(5):482-92. [DOI] [PubMed] [Google Scholar]
Welu 1977 {published data only}
- Welu T. A follow-up program for suicide attempters: Evaluation of effectiveness. Suicide and Life-Threatening Behavior 1977;7(1):17-30. [PubMed] [Google Scholar]
References to studies excluded from this review
Almeida 2012 {published data only}
- Almeida OP, Pirkis J, Kerse N, Sim M, Flicker L, Snowdon J, Draper B, Byrne G, Goldney R, Lautenschlager NT, Stocks N, Alfonso H, Pfaff JJ. A randomized trial to reduce the prevalence of depression and self-harm behavior in older primary care patients. Annals of Family Medicine 2012;10(4):347-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aoun 1999 {published data only}
- Aoun S. Deliberate self-harm in rural Western Australia: Results of an intervention study. Australian and New Zealand Journal of Mental Health Nursing 1999;8(2):65-73. [DOI] [PubMed] [Google Scholar]
Bannan 2010 {published and unpublished data}
- Bannan, N. Group-based problem-solving therapy in self-poisoning females: A pilot study. Counselling and Psychotherapy Research 2010;10(3):201-13. [Google Scholar]
Bartman 1979 {published data only}
- Bartman ER. Assertive training with hospitalized suicide attempters [PhD thesis]. Washington DC: Catholic University of America, 1979. [Google Scholar]
Bateson 1989 {published data only}
- Bateson M, Oliver JPJ, Goldberg DP. A comparative study of the management of cases of deliberate self-harm in a district general hospital. British Journal of Social Work 1989;19(1):461-78. [Google Scholar]
Berrino 2011 {published data only}
- Berrino A, Ohlendorf P, Duriaux S, Burnand Y, Lorillard S, Andreoli A. Crisis intervention at the general hospital: An appropriate treatment choice for acutely suicidal borderline patients. Psychiatry Research 2011;186(2-3):287-92. [DOI] [PubMed] [Google Scholar]
Carter 2013 {published data only}
- Carter GL, Clover K, Whyte IM, Dawson AH, D'este C. Postcards from the EDge: 5-year outcomes of a randomised controlled trial for hospital-treated self-poisoning. British Journal of Psychiatry 2013;202:372-80. [DOI] [PubMed] [Google Scholar]
Cebrià 2013 {published data only}
- Cebrià AI, Parra I, Pàmias M, Escayola A, García-Parés G, Puntí J, Laredo A, Vallès V, Cavero M, Oliva JC, Hegerl U, Pérez-Solà V, Palao DJ. Effectiveness of a telephone management programme for patients discharged from an emergency department after a suicide attempt: Controlled study in a Spanish population. Journal of Affective Disorders 2013;147(1-3):269-76. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published and unpublished data}
- Chen W-J, Ho C-K, Shyu S-S, Chen C-C, Lin G-G, Chou L-S, Fang YJ, Yeh PY, Chung TC, Chou FH. Employing crisis postcards with case management in Kaohsiung, Taiwan: 6-month outcomes of a randomised controlled trial for suicide attempters. BMC Psychiatry 2013;13(191):191-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chowdhury 1973 {published data only}
- Chowdhury N, Hicks R, Kreitman N. Evaluation of an after-care service for parasuicide (attempted suicide) patients. Social Psychiatry 1973;8:67-81. [Google Scholar]
Christensen 2014 {published data only}
- Christensen H, Calear AL, Van Spijker B, Gosling J, Petrie K, Donker T, Fenton K. Psychosocial interventions for suicidal ideation, plans, and attempts: A database of randomised controlled trials. BMC Psychiatry 2014;14:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Comtois 2011 {published and unpublished data}
- Comtois KA, Jobes DA, O'Connor SS, Atkins DC, Janis K, Chessen CE, Landes SJ, Holen A, Yuodelis-Flores C. Colloborative assessment and management of suicidality (CAMS): Feasibility trial for next-day appointment services. Depression and Anxiety 2011;28(11):963-72. [DOI] [PubMed] [Google Scholar]
- Ellis TE, Green KL, Allen JG, Jobes DA, Nadorff MR. Collaborative assessment and management of suicidality in an inpatient setting: Results of a pilot study. Psychotherapy 2012;49(1):72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobes DA. The Collaborative Assessment and Management of Suicidality (CAMS): An evolving evidence-based clinical approach to suicidal risk. Suicide and Life-Threatening Behavior 2012;42(6):640-53. [DOI] [PubMed] [Google Scholar]
Crawford 1998 {published data only}
- Crawford MJ, Turnbull G, Wessely S. Deliberate self-harm assessment by accident and emergency staff--an intervention study. Journal of Accident and Emergency Medicine 1998;15(1):18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Currier 2010 {published data only}
- Currier GW, Fisher SG, Caine ED. Mobile crisis team intervention to enhance linkage of discharged suicidal emergency department patients to outpatient psychiatric services: A randomized controlled trial. Academic Emergency Medicine 2010;17(1):35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidson 2006 {published data only}
- Davidson K, Norrie J, Tyrer P, Gumley A, Tata P, Murray H, Palmer S. The effectiveness of cognitive behavior therapy for borderline personality disorder: Results from the borderline personality disorder study of cognitive therapy (BOSCOT) trial. Journal of Personality Disorders 2006;20(5):450-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrie J, Davidson K, Tata P, Gumley A. Influence of therapist competence and quantity of cognitive behavioural therapy on suicidal behaviour and inpatient hospitalisation in a randomised controlled trial in borderline personality disorder: Further analyses of treatment effects in the BOSCOT study. Psychology and Psychotherapy: Theory, Research and Practice 2013;86:280-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Leo 2007 {published data only}
- De Leo D, Heller T. Intensive case management in suicide attempters following discharge from psychiatric care. Australian Journal of Primary Health 2007;13(3):49-58. [Google Scholar]
Evans 1998 {published and unpublished data}
- Evans J, Sheard T. A pilot study of a three session treatment package beginning during admission for deliberate self-harm. In: South West Research and Development Directorate Conference. 1998.
George 2014 {published data only}
- George MS, Raman R, Benedek DM, Pelic CG, Crammer GG, Stokes KT, Schmidt M, Spiegel C, Dealmeida N, Beaver KL, Borckardt JJ, Sun X, Jain S, Stein MB. A two-site pilot randomized 3 day trial of high dose left prefrontal repetitiveTranscranial Magnetic Stimulation (rTMS) for suicidal inpatients. Brain Stimulation 2014;7(3):421-31. [DOI] [PubMed] [Google Scholar]
Ghahramanlou‐Holloway 2012 {published data only}
- Ghahramanlou-Holloway M, Crox DW, Greene FN. Post-admission cognitive therapy: A brief intervention for psychiatric inpatients admitted after a suicide attempt. Cognitive and Behavioral Practice 2012;19(2):233-44. [Google Scholar]
Gunnarsdottir 2010 {published data only}
- Gunnarsdottir OS, Rafnsson V. Risk of suicide and fatal drug poisoning after discharge from the emergency department: A nested case-control study. Emergency Medicine Journal 2010;27(2):93-6. [DOI] [PubMed] [Google Scholar]
Harned 2010 {published data only}
- Harned MS, Jackson SC, Comtois KA, Linehan MM. Dialectical behavior therapy as a precursor to PTSD treatment for suicidal and/or self-injuring women with borderline personality disorder. Journal of Traumatic Stress 2010;23(4):421-9. [DOI] [PubMed] [Google Scholar]
Hatcher 2005 {published data only}
- Hatcher S, Sharon C, Fontanella I, Healey C. A patient preference trial of problem solving therapy after attempted suicide: Work in progress. Australian and New Zealand Journal of Psychiatry 2005;39:A121-A122. [Google Scholar]
Hellerstein 2003 {published data only}
- Hellerstein DJ, Aviram R, Gerson, Stanley B. Supportive therapy for BPD patients with self-injurious behavior. In: Proceedings of the 156th Annual Meeting of the American Psychiatric Association. San Francisco, CA. 2003:10.
Horrocks 2002 {published data only}
- Horrocks J, Owens D, House A. General practice based interventions to prevent repeat episodes of deliberate self-harm. Pictures of self-injury misrepresent published trial. British Journal of Medicine 2002;325(7358):281. [PubMed] [Google Scholar]
Kapur 2013b {published data only}
- Kapur N, Steeg S, Webb R, Haigh M, Bergen H, Hawton K, Ness J, Waters K, Cooper J. Does clinical management improve outcomes following self-harm? Results from the multicentre study of self-harm in England. PLoS One 2013;8(8):e70434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamprecht 2007 {published data only}
- Lamprecht H, Laydon C, McQuillan C, Wiseman S, Williams L, Gash A, Reilly J. Single-session solution-focused brief therapy and self-harm: a pilot study. Journal of Psychiatric and Mental Health Nursing 2007;14(6):601-2. [DOI] [PubMed] [Google Scholar]
Liberman 2001 {published data only}
- Liberman RP. Follow-up for parasuicidal patients. Psychiatric Services 2001;52(9):1254. [DOI] [PubMed] [Google Scholar]
Links 1999 {published data only}
- Links PS, Balchand K, Dawe I, Watson WJ. Preventing recurrent suicidal behaviour. Canadian Family Physician 1999;45:2656-60. [PMC free article] [PubMed] [Google Scholar]
Links 2003a {published data only}
- Links PS, Bergmans Y, Cook M. Psychotherapeutic interventions to prevent repeated suicidal behavior. Brief Treatment and Crisis Intervention 2003;3:445-64. [Google Scholar]
Low 2001 {published data only}
- Low G, Jones D, Duggan C, Power M, MacLeod A. The treatment of deliberate self-harm in borderline personality disorder using dialectical behaviour therapy: A pilot study in a high security hospital. Behavioural and Cognitive Psychotherapy 2001;29(1):85-92. [Google Scholar]
Martin 2013 {published and unpublished data}
- Martin S, Martin G, Lequertier B, Swannell S, Follent A, Choe F. Voice movement therapy: Evaluation of a group-based expressive arts therapy for nonsuicidal self-injury in young adults. Music and Medicine 2013;5(1):31-8. [Google Scholar]
McMain 2007a {published data only}
- McMain S. Effectiveness of psychosocial treatments on suicidality in personality disorders. Canadian Journal of Psychiatry/Revue Canadienne de Psychiatrie 2007;52(6 Suppl 1):s103-s114. [PubMed] [Google Scholar]
McQuillan 2005 {published data only}
- McQuillan A, Nicastro R, Guenot F, Girard M, Lissner C, Ferrero F. Intensive dialectical behavior therapy for outpatients with borderline personality disorder who are in crisis. Psychiatric Services 2005;56(2):193-7. [DOI] [PubMed] [Google Scholar]
Montgomery 1983 {published data only}
- Montgomery S, Roy D, Montgomery D. The prevention of recurrent suicidal acts. British Journal of Clinical Pharmacology 1983;15(Suppl 2):s183s-s188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morley 2014 {published data only}
- Morley KC, Sitharthan G, Haber PS, Tucker P, Sitharthan T. The efficacy of an Opportunistic Cognitive Behavioral Intervention Package (OCB) on substance use and comorbid suicide risk: A multisite randomized controlled trial. Journal of Consulting and Clinical Psychology 2014;82(1):130-40. [DOI] [PubMed] [Google Scholar]
Ono 2008 {published data only}
- Inagaki M, Yamada M, Tonemoto N, Takahashi K on behalf of the J-MISP group. NOCOMIT-J: A community intervention trial of multi-modal suicide prevention program in Japan. European Psychiatry 2012;27:s1. [Google Scholar]
- Ono Y, Awata S, Iida H, Ishida Y, Ishizuka N, Iwasa H, Kamei Y, Motohashi Y, Nakagawa A, Nakamura J, Nishi N, Otsuka K, Oyama H, Sakai A, Sakai H, Suzuki Y, Tajima M, Tanaka E, Uda H, Yonemoto N, Yotsumoto T, Watanabe N. A community intervention trial of multimodal suicide prevention program in Japan: A novel multimodal community intervention program to prevent suicide and suicide attempt in Japan, NOCOMIT-J. BMC Public Health 2008;8:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pham‐Scottez 2010 {published data only}
- Pham-Scottez A. Impact of a 24/24 phone permanency on suicide attempts of borderline patients [Évaluation de l'efficacité d'une permanence téléphonique sur l'incidencedes tentatives de suicide des patients borderline]. Annales Medico-Psychologiques 2010;168(2):141-4. [Google Scholar]
Raj 2001 {published data only}
- Raj MAJ, Kumaraiah V, Bhide AV. Cognitive-behavioural intervention in deliberate self-harm. Acta Psychiatrica Scandinavica 2001;104(5):340-5. [DOI] [PubMed] [Google Scholar]
Razzaque 2013 {published data only}
- Razzaque R. An acceptance and commitment therapy based protocol for the management of acute self-harm and violence in severe mental illness. Journal of Psychiatric Intensive Care 2013;9:72-6. [Google Scholar]
Ruchlewska 2013 {published data only}
- Ruchlewska A, Wierdsma AI, Kamperman AM, Gaag M, Smulders R, Roosenschoon B-J, Mulder CL. Effect of crisis plans on admissions and emergency visits: A randomized controlled trial. PLoS One 2014;9(3):e91882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sáiz 2014 {published and unpublished data}
- Sáiz PA, Rodríguez-Revuelta J, González-Blanco L, Burón P, Al-Halabí S, Garrido M, García-Alvarez L, García-Portilla P, Bobes J. Study protocol of a prevention of recurrent suicidal behaviour program based on case management (PSyMAC) [Protocolo de estudio de un programa para la prevención de la recurrencia del comportamiento suicida basado en el manejo de casos (PSyMAC)]. Revista de Psiquiatría y Salud Mental 2014;7(3):131-8. [DOI] [PubMed] [Google Scholar]
Sambrook 2007 {published data only}
- Sambrook S, Abba N, Chadwick P. Evaluation of DBT emotional coping skills groups for people with parasuicidal behaviours. Behavioural and Cognitive Psychotherapy 2007;35(2):241-4. [Google Scholar]
Strum 2012 {published and unpublished data}
- Sturm J, Plöderl M, Fartacek C, Kralovec K, Neunhäuserer D, Niederseer D, Hitzl W, Niebauer J, Schiepek G, Fartacek R. Physical exercise through mountain hiking in high-risk suicide patients. A randomized crossover trial. Acta Psychiatrica Scandinavica 2012;126(6):467-75. [DOI] [PubMed] [Google Scholar]
Tarrier 2008a {published data only}
- Tarrier N, Taylor K, Gooding P. Cognitive-behavioral interventions to reduce suicide behavior: A systematic review and meta-analysis. Behavior Modification 2008;32(1):77-108. [DOI] [PubMed] [Google Scholar]
Termansen 1975 {published data only}
- Termansen PE, Bywater C. S.A.F.E.R.: A follow-up service for attempted suicide in Vancouver. Canadian Psychiatric Association Journal 1975;20(1):29-34. [DOI] [PubMed] [Google Scholar]
Trembley 2013 {published data only}
- Trembley AL, Page D. Staying off the ledge: Effectiveness of follow-up postcards to suicidal patients. Journal of Emergency Medical Services 2010;38:26-7. [Google Scholar]
Van Spijker 2010 {published data only}
- Van Spijker BAJ, Van Straten A, Kerkhof AJFM. The effectiveness of a web-based self-help intervention to reduce suicidal thoughts: A randomized controlled trial. Trials 2010;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vitiello 2009 {published data only}
- Stanley B, Brown G, Brent DA, Wells K, Poling K, Curry J, Kennard BD, Wagner A, Cwik MF, Klomek AB, Goldstein T, Vitiello B, Barnett S, Daniel S, Hughes J. Cognitive-behavioral therapy for suicide prevention (CBT-SP): Treatment model, feasibility, and acceptability. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(10):1005-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello B, Brent DA, Greenhill LL, Emslie G, Wells K, Walkup JT, Stanley B, Bukstein O, Kennard BD, Compton S, Coffey B, Cwik MF, Posner K, Wagner A, March JS, Riddle M, Goldstein T, Curry J, Capasso L, Mayes T, Shen S, Gugga SS, Turner JB, Barnett S, Zelazny J. Depressive symptoms and clinical status during the Treatment of Adolescent Suicide Attempters (TASA) study. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(10):997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warren 2004 {published data only}
- Warren F, Evans C, Dolan B, Norton K. Impulsivity and self-damaging behaviour in severe personality disorder: The impact of democratic therapeutic community treatment. Therapeutic Communities 2004;25(1):55-71. [Google Scholar]
Winter 2007 {published data only}
- Winter D, Sireling L, Riley T, Metcalfe C, Quaite A, Bhandari S. A controlled trial of personal construct psychotherapy for deliberate self-harm. Psychology and Psychotherapy 2007;80(Pt 1):23-37. [DOI] [PubMed] [Google Scholar]
Wullimier 1979 {published data only}
- Wullimier F, Bovet J, Meylan D. Comparative study of two intervention modes on suicide attempters hospitalized in the general hospital. In: Proceedings of the 9th International Conference on Suicide Prevention. Helsinki, Finland, 1977.
- Wullimier F, Bovet J, Meylan D. The future of suicidal patients admitted to a general hospital. Comparative study of two methods of preventing recurrence and suicides. Sozial-und Praventivmedizin 1979;24(1):73-88. [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang H, Neelarambam K, Schwenke TJ, Rhodes MN, Pittman DM, Kaslow NJ. Mediators of a culturally-sensitive intervention for suicidal African American women. Journal of Clinical Psychology in Medical Settings 2013;20(4):401-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Andreasson 2016 {published and unpublished data}
- Andreasson K, Krogh J, Rosenbaum B, Gluud C, Jobes DA, Nordentoft M. The DiaS trial: dialectical behavior therapy versus collaborative assessment and management of suicidality on self-harm in patients with a recent suicide attempt and borderline personality disorder traits - study protocol for a randomized controlled trial. Trials 2014;15:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson K, Krogh J, Wenneberg C, Jessen HK, Krakauer K, Gluud C, Thomsen RR, Randers L, Nordentoft M. Effectiveness of dialectical behavior therapy versus collaborative assessment and management of suicidality treatment for reduction of self-harm in adults with borderline personality traits and disorder - a randomized observer-blinded clinical trial. Depression and Anxiety 2016:DOI: 10.1002/da.22472. [DOI] [PubMed] [Google Scholar]
Armitage 2016 {published and unpublished data}
- Armitage CJ, Rahim WA, Rowe R, O'Connor RC. An exploratory randomised trial of a simple, brief psychological intervention to reduce subsequentsuicidal ideation and behaviour in patients admitted to hospital for self-harm. British Journal of Psychiatry 2016;208(5):470-6. [DOI] [PubMed] [Google Scholar]
Gysin‐Maillart 2016 {published data only}
- Gysin-Maillart A, Schwab S, Soravia L, Megert M, Michel K. A novel brief therapy for patients who attempt suicide: A 24-months follow-up randomized controlled study of the Attempted Suicide Short Intervention Program (ASSIP). PLoS Medicine 2016;13:e1001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linehan 2015 {published data only (unpublished sought but not used)}
- Linehan MM, Korslund KE, Harned MS, Gallop RJ, Lungu A, Neacsiu AD, McDavid J, Comtois KA, Murray-Gregory AM. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder: a randomized clinical trial and component analysis. Journal of the American Medical Association 2015;72(5):475-82. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Agyapong 2013 {published data only (unpublished sought but not used)}
- Agyapong VIO, Buckmaster R, McKeever P, O'Raghallaigh JW, Houlihan P, MacHale S. Text message intervention to reduce repeat self-harm in patients presenting to the emergency department - a study protocol. British Journal of Medicine and Medical Research 2013;3(4):2222-3. [Google Scholar]
- NCT01823120. Text message intervention to reduce repeat self-harm in patients presenting to the emergency department. https://clinicaltrials.gov/ct2/show/NCT01823120 (accessed 4 December 2015).
Andover 2008 {published data only (unpublished sought but not used)}
- NCT01018433. Development of an intervention for non-suicidal self-injury in young adults. https://clinicaltrials.gov/ct2/show/NCT01018433 (accessed 4 December 2015).
Berrouiquet 2015 {published data only}
- Berrouiguet S, Alavi Z, Vaiva G, Courtet P, Baca-García E, Vidailhet P, Gravey M, Guillodo E, Brandt S, Walter M. SIAM (Suicide Intervention Assisted by Messages): The development of a post-acute crisis text messaging outreach for suicide prevention. BMC Psychiatry 2014;14:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrouiguet SB, Alavi AZ, Vaiva GV, Courtet PC, Baca-García EBG, Brandt SB, Walter MW. SIAM (Suicide Intervention Assisted by Messages): The development of a post-acute crisis text messaging outreach for suicide prevention. European Psychiatry, Abstracts of the 23rd European Congress of Psychiatry; 2015; Mar 28-31; Vienna, Austria 2015;30:957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brimes 2007 {published data only (unpublished sought but not used)}
- NCT00641498. Effectiveness of standard emergency department psychiatric treatment compared with effectiveness of standard emergency department psychiatric treatment associated with treatment delivery by a suicide prevention center. https://clinicaltrials.gov/ct2/show/NCT00641498 (accessed 4 December 2015).
Brown 2014 {unpublished data only}
- NCT00081367. Community-based cognitive therapy Community-Based Cognitive Therapy for suicide attemptersSuicide Attempters. https://clinicaltrials.gov/ct2/show/NCT00081367 (accessed 4 December 2015). [Google Scholar]
- Stirman SW, Brown GK, Ghahramanlou-Holloway M, Fox AJ, Chohan MZ, Beck AT. Participation bias among suicidal adults in a randomized controlled trial. Suicide and Life-Threatening Behavior 2011;41(2):203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collinson 2014 {published and unpublished data}54036115
- Collinson M, Owens D, Blenkiron P, Burton K, Graham L, Hatcher S, House A, Martin K, Pembroke L, Protheroe D, Tubeuf S, Farrin A. MIDSHIPS: Multicentre Intervention Designed for Self-Harm using Interpersonal Problem-Solving. Protocol for a randomised controlled feasibility study. Trials 2014;15:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN54036115. MIDSHIPS: Multicentre Intervention Designed for Self-Harm using Interpersonal Problem Solving: a feasibility study. http://isrctn.com/ISRCTN54036115 (accessed 4 December 2015). [DOI] [PMC free article] [PubMed]
Davidson 2009 {published and unpublished data}
- NCT00980824. ENGAGE - Meeting mental health needs of complex comorbid patients attending A&E following a suicide attempt. A pilot study.. https://clinicaltrials.gov/ct2/show/NCT00980824 (accessed 4 December 2015).
Hatcher 2016b {published and unpublished data}
- NCT02718248 Ottawa Suicide Prevention in Men Pilot Study (OSSUPilot). https://clinicaltrials.gov/ct2/show/NCT02718248 (accessed 21 April 2016).
Huang 2013 {unpublished data only}
- NCT01952405. Efficacy of dialectical behavior therapy in patients with borderline personality disorder: a controlled trial in Taiwan. https://clinicaltrials.gov/ct2/show/NCT01952405 (accessed 4 December 2015).
Leybman 2014 {unpublished data only}
- NCT02354183. Commitment and Motivation in a Brief DBT Intervention for Self Harm. https://clinicaltrials.gov/ct2/show/NCT02354183 2014 (accessed 29 April 2015). [Google Scholar]
Liu 2007 {unpublished data only}
- NCT00664872. Effect of proactive psychosocial treatment by the case manager in patients after a suicide attempt: a randomised controlled trial. https://clinicaltrials.gov/ct2/show/NCT00664872 (accessed 4 December 2015).
McMain 2015 {unpublished data only}
- NCT02387736. Dialectical Behaviour Therapy for Chronically Self-harming Individuals With BPD: Evaluating the Clinical and Cost Effectiveness of a 6-month Treatment. https://clinicaltrials.gov/ct2/show/NCT02387736 2015 (accessed 29 April 2015). [Google Scholar]
O'Connor 2011 {unpublished data only}
- NCT01355848. Improving care provided to patients treated in a level 1 trauma center post-suicide attempt. https://clinicaltrials.gov/ct2/show/NCT01355848 (accessed 4 December 2015).
O'Connor 2012 {unpublished data only}
- ISRCTN99488269. A volitional help sheet to reduce self-harm among people admitted to hospital for self-harm: A randomised controlled trial. http://isrctn.com/ISRCTN99488269 (accessed 4 December 2015).
O'Connor 2014 {unpublished data only}
- NCT02414763. Pilot Study of a Brief Intervention for Medically Hospitalized Suicide Attempt Survivors. https://clinicaltrials.gov/ct2/show/NCT02414763 (accessed 28 January 2016). [Google Scholar]
Pham‐Scottez 2009 {published data only (unpublished sought but not used)}
- NCT00603421. Effectiveness of a 24 hour phone line on the rate of suicide attempts in borderline patients. https://clinicaltrials.gov/ct2/show/NCT00603421 (accessed 4 December 2015).
Sayal 2015 {unpublished data only}
- NCT02377011. Randomised Controlled Trial of the Clinical and Cost Effectiveness of NICE Recommended Problem Solving Cognitive Behaviour Therapy (PS CBT) Delivered Remotely Versus Treatment as Usual in Adolescents and Young Adults With Depression Who Repeatedly Self-harm. https://clinicaltrials.gov/ct2/show/NCT02377011 2015 (accessed 29 April 2015). [Google Scholar]
Vaiva 2011 {published and unpublished data}
- NCT01123174. Effectiveness of a "case management algorithm" after a suicide attempt in terms of repetition of the suicidal behaviors and medico-economic impact. https://clinicaltrials.gov/ct2/show/NCT01123174 (accessed 4 December 2015).
- Vaiva G, Walter M, Al Arab AS, Courtet P, Bellivier F, Demarty AL, Duhem S, Ducrocq F, Goldstein P, Libersa C. ALGOS: The development of a randomized controlled trial testing a case management algorithm designed to reduce suicide risk among suicide attempters. BMC Psychiatry 2011;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
van den Bosch 2013 {unpublished data only}
- NCT01904227. A Randomized Controlled Study of the Efficacy of an Intensified, Inpatient Adaptation of Dialectical Behavior Therapy (DBT) for a Population of Borderline Patients (Young Adults/Adults: 18 - 40), Compared With Standard Outpatient DBT. https://clinicaltrials.gov/ct2/show/NCT01904227 2013 (accessed 29 April 2015). [Google Scholar]
Walker 2012 {unpublished data only}18761534
- ISRCTN18761534. Women offenders repeat self-harm intervention pilot II. http://isrctn.com/ISRCTN18761534 (accessed 4 December 2015).
Additional references
Andersson 2014
- Andersson G, Cuijpers P, Carlbring P, Riper H, Hedman E. Guided Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: A systematic review and meta-analysis. World Psychiatry 2014;13(3):288-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arensman 2001
- Arensman E, Townsend E, Hawton K, Bremner S, Feldman E, Goldney R, Gunnell D, Hazell P, Heeringen K, House A, Owens D, Sakinofsky I, Träskman-Bendz L. Psychosicial and pharmacological treatment of patients following deliberate self-harm: the methodological issues involved in evaluating effectiveness. Suicide and Life-Threatening Behavior 2001;31(2):169-80. [DOI] [PubMed] [Google Scholar]
Armitage 2008
- Armitage CJ. A volitional help sheet to encourage smoking cessation: A randomized exploratory trial. Health Psychology 2008;27(5):557-66. [DOI] [PubMed] [Google Scholar]
Baldessarini 2003
- Baldessarini RJ, Tondo L, Hennen J. Lithium treatment and suicide risk in major affective disorders: Update and new findings. Journal of Clinical Psychiatry 2003;64(Suppl 5):s44-s52. [PubMed] [Google Scholar]
Baldessarini 2006
- Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long-term lithium treatment: A meta-analytic review. Bipolar Disorders 2006;8(5 Pt 2):625-39. [DOI] [PubMed] [Google Scholar]
Bateman 2004
- Bateman A, Fonagy P. Mentalization-based treatment of BDP. In: Psychotherapy for Borderline Personality Disorder: Mentalization-Based Treatment. Oxford, UK: Oxford University Press, 2004. [Google Scholar]
Beasley 1991
- Beasley CM, Dornseif BE, Bosomworth JC, Sayler ME, Rampey AH Jr, Heiligenstein JH, Thompson VL, Murphy DJ, Masica DN. Fluoxetine and suicide: A meta-analysis of controlled trials of treatment for depression. British Medical Journal 1991;303(6804):685-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561. [DOI] [PubMed] [Google Scholar]
Beck 1974
- Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The Hopelessness Scale. Journal of Consulting and Clinical Psychology 1974;42(6):861-5. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Steer RA, Ranieri WF. Scale for Suicide Ideation: Psychometric properties of a self-report version. Journal of Clinical Psychology 1988;44(4):499-505. [DOI] [PubMed] [Google Scholar]
Bertolote 2010
- Bertolote JM, Fleischmann A, Leo D, Phillips MR, Botega NJ, Vijayakumar L, Silva D, Schlebusch L, Nguyen VT, Sisask M, Bolhari J, Wasserman D. Repetition of suicide attempts: Data from five culturally different low- and middle-income country emergency care settings participating in the WHO SUPRE-MISS study. Crisis: The Journal of Crisis Intervention and Suicide Prevention 2010;31:194-201. [DOI] [PubMed] [Google Scholar]
Borges 2011
- Borges G, Nock MK, Abad JMH, Hwang I, Sampson NA, Alonso J, Andrade LH, Angermeyer MC, Beautrais A, Bromet E, Bruffaerts R, Girolamo G, Florescu S, Gureje O, Hu C, Karam EG, Kovess-Masfety V, Lee S, Levinson D, Medina-Mora ME, Ormel J, Posada-Villa J, Sagar R, Tomov T, Uda H, Williams DR, Kessler RC. Twelve month prevalence of and risk factors for suicide attempts in the WHO World Mental Health Surveys. Journal of Clinical Psychiatry 2010;71(12):1617-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyce 2003
- Boyce P, Carter G, Penrose-Wall J, Wilhelm K, Goldney R. Summary Australian and New Zealand clinical practice guideline for the management of adult deliberate self-harm (2003). Australasian Psychiatry 2003;38(11-12):150-5. [Google Scholar]
Brausch 2012
- Brausch AM, Girresch SK. A review of empirical treatment studies for adolescent nonsuicidal self-injury. Journal of Cognitive Psychotherapy: An International Quarterly 2012;26(1):3-18. [Google Scholar]
Burns 2000
- Burns JM, Patton GC. Preventive interventions for youth suicide: a risk factor-based approach. Australian and New Zealand Journal of Psychiatry 2000;34(3):388-407. [DOI] [PubMed] [Google Scholar]
Carroll 2014
- Carroll R, Metcalfe C, Gunnell D. Hospital presenting self-harm and risk of fatal and non-fatal repetition: Systematic review and meta-analysis. PLoS One 2014;9(2):e89944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2007
- Carter GL, Clover K, Whyte IM, Dawson AH, D'este C. Postcards from the EDge: 24-month outcomes of a randomised controlled trial for hospital-treatment self-poisoning. British Journal of Psychiatry 2007;191:548-53. [DOI] [PubMed] [Google Scholar]
Choi‐Kain 2008
- Choi-Kain LW, Gunderson JG. Mentalization: Ontogeny, assessment, and application in the treatment of borderline personality disorder. American Journal of Psychiatry 2008;165(9):1127-35. [DOI] [PubMed] [Google Scholar]
Christensen 2014
- Christensen H, Calear AL, Van Spijker B, Gosling J, Petrie K, Donker T, Fenton K. Psychosocial interventions for suicidal ideation, plans, and attempts: A database of randomised controlled trials. BMC Psychiatry 2014;14:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2005
- Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicide behavior and all-cause mortality in patients with mood disorders: A systematic review of randomized trials. American Journal of Psychiatry 2005;162(10):1805-19. [DOI] [PubMed] [Google Scholar]
Cipriani 2013
- Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: Updated systematic review and meta-analysis. British Medical Journal 2013;346:f3646. [DOI] [PubMed] [Google Scholar]
Claassen 2006
- Claassen CA, Trivedi MH, Shimizu I, Stewart S, Larkin GL, Litovitz T. Epidemiology of nonfatal deliberate self-harm in the United States as described in three medical databases. Suicide and Life-Threatening Behavior 2006;36(2):192-212. [DOI] [PubMed] [Google Scholar]
Comtois 2006
- Comtois KA, Linehan MM. Psychosocial treatments of suicidal behaviors: a practice-friendly review. Journal of Clinical Psychology in Session 2006;62(2):161-70. [DOI] [PubMed] [Google Scholar]
Cooper 2005
- Cooper J, Kapur N, Webb R, Lawlor M, Guthrie E, Mackway-Jones K, Appleby L. Suicide after deliberate self-harm: A 4-year cohort study. American Journal of Psychiatry 2005;162(2):297-303. [DOI] [PubMed] [Google Scholar]
Cooper 2011
- Cooper J, Hunter C, Owen-Smith A, Gunnell D, Donovan J, Hawton K, Kapur N. “Well it's like someone at the other end cares about you.” A qualitative study exploring the views of users and providers of care of contact-based interventions following self-harm. General Hospital Psychiatry 2011;33:166-176. [DOI] [PubMed] [Google Scholar]
Crawford 2007a
- Crawford MJ, Thomas O, Khan N, Kulinskaya E. Psychosocial interventions following self-harm: systematic review of their efficacy in preventing suicide. British Journal of Psychiatry 2007;190:11-17. [DOI] [PubMed] [Google Scholar]
Crawford 2007b
- Crawford MJ, Kumar P. Intervention following deliberate self-harm: Enough evidence to act? Evidence-Based Mental Health 2007b;10(2):37-9. [DOI] [PubMed] [Google Scholar]
Curtin 2002a
- Curtin F, Elbourne D, Altman DG. Meta-analysis combining parallel and cross-over clinical trials III: The issue of carry-over. Statistics in Medicine 2002a;21(15):2161-73. [DOI] [PubMed] [Google Scholar]
Curtin 2002b
- Curtin F, Altman DG, Elbourne D. Meta-analysis combining parallel and cross-over clinical trials I: Continuous outcomes. Statistics in Medicine 2002b;21(15):2131-44. [DOI] [PubMed] [Google Scholar]
D'Zurilla 1996
- D'Zurilla TJ, Nezu AM, Maydeu-Olivares A. Manual for the Social Problem-Solving Inventory - Revised (SPSI-R). Toronto, ON: Multi-Health Systems, 1996. [Google Scholar]
D'Zurilla 2010
- D'Zurilla TJ, Nezu AM. Problem-solving therapy. In: Dobson KS, editors(s). Handbook of Cognitive-Behavioral Therapies. 3rd edition. New York, NY: The Guilford Press, 2010:197-225. [Google Scholar]
Daigle 2011
- Daigle MS, Pouliot L, Chagnon F, Greenfield B, Mishara B. Suicide attempts: Prevention of repetition. Canadian Journal of Psychiatry 2011;56(10):621-9. [DOI] [PubMed] [Google Scholar]
Daniel 2009
- Daniel SS, Goldston DB. Interventions for suicidal youth: A review of the literature and developmental considerations. Suicide and Life-Threatening Behavior 2009;39(3):252-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking analyses. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Dew 1987
- Dew MA, Bromet EJ, Brent D. A quantitative literature review of the effectiveness of suicide prevention centers. Journal of Consulting and Clinical Psychology 1987;55(2):239-44. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine 2002;21(19):2971-80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: Methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Endler 1994
- Endler NS, Parker JDA. Coping Inventory for Stressful Situations. Toronto, ON: Multi-Health Systems, 1990. [Google Scholar]
Erez 1996
- Erez A, Bloom MC, Wells MT. Using random rather than fixed effects models in meta-analysis: Implications for situation specificity and validity generalization. Personnel Psychology 1996;49(2):275-306. [Google Scholar]
Erlangsen 2015
- Erlangsen A, Lind BD, Stuart EA, Qin P, Stenager E, Larsen KJ, Wang AG, Hvid M, Nielsen AC, Pedersen CM, Winsløv JH, Langhoff C, Mühlmann C, Nordentoft M. Short-term and long-term effects of psychosocial therapy for people after deliberate self-harm: a register-based, nationwide multicentre study using propensity score matching. The Lancet Psychiatry 2015;2(1):49-58. [DOI] [PubMed] [Google Scholar]
Evans 2005
- Evans J, Evans M, Morgan HG, Hayward A, Gunnell, D. Crisis card following self-harm: 12-month follow-up of a randomised controlled trial. British Journal of Psychiatry 2005;187:186-187. [DOI] [PubMed] [Google Scholar]
Fleischmann 2005
- Fleischmann A, Bertolote JM, De Leo D, Botega N, Phillips M, Sisask M, Vijayakumar L, Malakouti K, Schlebusch L, De Silva D, Nguyen VT, Wasserman D. Characteristics of attempted suicides seen in emergency-care settings of general hospitals in eight low- and middle-income countries. Psychological Medicine 2005;35(10):1467-74. [DOI] [PubMed] [Google Scholar]
Fleiss 1994
- Fleiss JL. Measures of effect size for categorical data. In: Cooper H, Hedges LV, editors(s). The Handbook of Research Synthesis. New York, NY: Russell Sage Foundation, 1994:245-60. [Google Scholar]
Foster 1997
- Foster T, Gillespie K, McClelland R. Mental disorders and suicide in Northern Ireland. British Journal of Psychiatry 1997;170:447-52. [DOI] [PubMed] [Google Scholar]
Franklin 2016
- Franklin JC, Fox KR, Franklin CR, Kleiman EM, Ribeiro JD, Jaroszewski AC, Hooley JM, Nock MK. A brief mobile app reduces nonsuicidal and suicidal self-injury: Evidence from three randomized controlled trials. Journal of Consulting and Clinical Psychology 2016;Epub ahead of print:DOI: 10.1037/ccp0000093. [DOI] [PubMed] [Google Scholar]
Gairin 2003
- Gairin I, House A, Owens D. Attendance at the accident and emergency department in the year before suicide: Retrospective study. British Journal of Psychiatry 2003;183:28-33. [DOI] [PubMed] [Google Scholar]
Gibbs 2004
- Gibbs S, Beautrais A. Epidemiology of attempted suicide in Canterbury Province, New Zealand (1993-2002). New Zealand Medical Journal 2004;117(1205):U1141. [PubMed] [Google Scholar]
Gollwitzer 1993
- Gollwitzer PM. Goal achievement: The role of intentions. European Review of Social Psychology 1993;4(1):141-85. [Google Scholar]
Gould 2003
- Gould MS, Greenberg T, Velting DM, Shaffer D. Youth suicide risk and preventive interventions: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(4):386-405. [DOI] [PubMed] [Google Scholar]
GRADE profiler [Computer program]
- Cochrane Informatics and Knowledge Management Department. Accessed 10 December, 2014 GRADE profiler. Cochrane Informatics and Knowledge Management Department. Accessed 10 December, 2014.
Gratz 2004
- Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment 2004;26(1):41-54. [Google Scholar]
Gratz 2007
- Gratz KL. Targeting emotion dysregulation in the treatment of self-injury. Journal of Clinical Psychology 2007;63(11):1091-103. [DOI] [PubMed] [Google Scholar]
Gray 2001
- Gray SM, Otto MW. Psychosocial approaches to suicide prevention: Applications to patients with bipolar disorder. Journal of Clinical Psychiatry 2001;62(Suppl 25):s56-s64. [PubMed] [Google Scholar]
Griffiths 2006
- Griffiths KM, Christensen H. Review of randomised controlled trials of Internet interventions for mental disorders and related conditions. Clinical Psychologist 2006;10(1):16-29. [Google Scholar]
Gunnell 1994
- Gunnell D, Frankel S. Prevention of suicide: Aspirations and evidence. British Medical Journal 1994;308(6938):1227-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gysin‐Maillart 2013
- Gysin-Maillart A, Michel K. Kurztherapie nach Suizidversuch: ASSIP - Attempted Suicide Short Intervention Program. Bern, Switzerland: Hans Huber, 2013. [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hassanian‐Moghaddam 2015
- Hassanian-Moghaddam H, Sarjami S, Kolahi A-A, Lewin T, Carter G. Postcards in Persia: A 12-24 month follow-up of a randomised controlled trial for hospital treated deliberate self-poisoning. Archives of Suicide Research 2015 Mar 16 [Epub ahead of print]:DOI: 10.1080/13811118.2015.1004473. [STUDY: Hassanian-Moghaddam 2011] [DOI] [PubMed]
Hassanzadeh 2010
- Hassanzadeh M, Khajeddin N, Nojomi M, Fleischmann A, Eshrati T. Brief intervention and contact after deliberate self-harm: An Iranian randomised controlled trial. Iranian Journal of Psychiatry and Behavioral Sciences 2010;4(2):5-12. [Google Scholar]
Haw 2001
- Haw C, Hawton K, Houston K, Townsend E. Psychiatric and personality disorders in deliberate self-harm patients. British Journal of Psychiatry 2001;178:48-54. [DOI] [PubMed] [Google Scholar]
Hawton 1987b
- Hawton K, Catalan J. Attempted Suicide: A Practical Guide to its Nature and Management. Oxford, UK: Oxford University Press, 1987. [Google Scholar]
Hawton 1988
- Hawton K, Fagg J. Suicide, and other causes of death, following attempted suicide. British Journal of Psychiatry 1988;152:359-66. [DOI] [PubMed] [Google Scholar]
Hawton 1989
- Hawton K, Kirk J. Problem-solving. In: Hawton K, Salkovskis PM, Kirk J, Clark DM, editors(s). Cognitive Behaviour Therapy for Psychiatric Problems: A Practical Guide. Oxford, UK: Oxford University Press, 1989:406-26. [Google Scholar]
Hawton 2003a
- Hawton K, Harriss L, Hall S, Simkin S, Bale E, Bond A. Deliberate self-harm in Oxford, 1990-2000: A time of change in patient characteristics. Psychological Medicine 2003;33(6):987-95. [DOI] [PubMed] [Google Scholar]
Hawton 2003b
- Hawton K, Zahl D, Weatherall R. Suicide following deliberate self-harm: long-term follow-up of patients who presented to a general hospital. British Journal of Psychiatry 2003;182:537-42. [DOI] [PubMed] [Google Scholar]
Hawton 2006
- Hawton K, Rodham K. By their Own Hand. Deliberate Self-harm and Suicidal Ideas in Adolescents. London, UK: Jessica Kingsley Publishers, 2006. [Google Scholar]
Hawton 2007
- Hawton K, Bergen H, Casey D, Simkin S, Palmer B, Cooper J, Kapur N, Horrocks J, House A, Lilley R, Noble R, Owens D. Self-harm in England: A tale of three cities. Multicentre study of self-harm. Social Psychiatry and Psychiatric Epidemiology 2007;42(7):513-21. [DOI] [PubMed] [Google Scholar]
Hawton 2008
- Hawton K, Harriss L. The changing gender ratio in occurrence of deliberate self-harm across the life-cycle. Crisis 2008;29(1):4-10. [DOI] [PubMed] [Google Scholar]
Hawton 2012
- Hawton K, Saunders KEA, O'Connor R. Self-harm and suicide in adolescents. The Lancet 2012;379(9834):2373-82. [DOI] [PubMed] [Google Scholar]
Hawton 2013
- Hawton K, Saunders KEA, Topiwala A, Haw C. Psychiatric disorders in patients presenting to hospital following self-harm: A systematic review. Journal of Affective Disorders 2013;151(13):821-30. [DOI] [PubMed] [Google Scholar]
Hawton 2015a
- Hawton K, Witt KG, Taylor Salisbury TL, Arensman E, Gunnell D, Townsend E, Van Heeringen K, Hazell P. Interventions for self-harm in children and adolescents. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD012013. [DOI: 10.1002/14651858.CD012013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawton 2015b
- Hawton K, Witt KG, Taylor Salisbury TL, Arensman E, Gunnell D, Hazell P, Townsend E, Van Heeringen K. Pharmacological interventions for self-harm in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD011777. [DOI: 10.1002/14651858.CD011777] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hennen 2005
- Hennen J, Baldessarini RJ. Suicidal risk during treatment with clozapine: A meta-analysis. Schizophrenia Research 2005;73(2-3):139-45. [DOI] [PubMed] [Google Scholar]
Hepp 2004
- Hepp U, Wittman L, Schnyder U, Michel K. Psychological and psychosocial interventions after attempted suicide: An overview of treatment studies. Crisis 2004;25(3):108-17. [DOI] [PubMed] [Google Scholar]
Heppner 1988
- Heppner, P. The Problem-Solving Inventory. Palo Alto, CA: Consulting Psychologist Press, 1988. [Google Scholar]
Her Majesty's Government Department of Health 2012
- Her Majesty's Government and Department of Health. Preventing Suicide in England: A Cross-Government Outcomes Strategy to Save Lives. London, UK: Her Majesty's Government and Department of Health, 2012. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008a
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for SystematicReviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Higgins 2008b
- Higgins JPT, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group. Special topics in statistics. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org..
Hirsch 1982
- Hirsch S, Walsh C, Draper R. Parasuicide: A review of treatment interventions. Journal of Affective Disorders 1982;4(4):299-311. [DOI] [PubMed] [Google Scholar]
Hjelmeland 2002
- Hjelmeland H, Hawton K, Nordvik H, Bille-Brahe U, De Leo D, Fekete S, Grad O, Haring C, Kerkhof JF, Lönnqvist J, Michel K, Renberg ES, Schmidtke A, Van Heeringen K, Wasserman D. Why people engage in parasuicide: A cross-cultural study of intentions. Suicide and Life-Threatening Behavior 2002;32(4):380-93. [DOI] [PubMed] [Google Scholar]
Holloway 1991
- Holloway F. Case management for the mentally ill: Looking at the evidence. International Journal of Social Psychology 1991;37(1):2-13. [DOI] [PubMed] [Google Scholar]
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009;1:MR000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunt 2013
- Hunt GE, Siegfried N, Morley K, Sitharthan T, Cleary M. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database of Systematic Reviews 2013;10:CD001088. [DOI] [PubMed] [Google Scholar]
Inagaki 2015
- Inagaki M, Kawashima Y, Kawanishi C, Yonemoto N, Sugimoto T, Furuno T, Ikeshita K, Eto N, Tachikawa H, Shiraishi Y, Yamada M. Interventions to prevent repeat suicidal behavior in patients admitted to an emergency department for a suicide attempt: A meta-analysis. Journal of Affective Disorders 2015;175:66-78. [DOI] [PubMed] [Google Scholar]
Jones 2012
- Jones C, Hacker D, Cormac I, Meaden A, Irving CB. Cognitive behavioural therapy versus other psychosocial treatments for schizophrenia. Cochrane Database of Systematic Reviews 2012;5:SBS080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kapur 2010
- Kapur N, Cooper J, Bennewith O, Gunnell D, Hawton K. Postcards, green cards and telephone calls: Therapeutic contact with individuals following self-harm. British Journal of Psychiatry 2010;197:5-7. [DOI] [PubMed] [Google Scholar]
Kapur 2013
- Kapur N, Cooper J, O'Connor RC, Hawton K. Non-suicidal self-injury v. attempted suicide: New diagnosis or false dichotomy? British Journal of Psychiatry 2013;202:326-8. [DOI] [PubMed] [Google Scholar]
Kerry 1998
- Kerry SM, Bland JM. Analysis of a trial randomised in clusters. British Medical Journal 1998;316(7124):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinyanda 2005
- Kinyanda E, Hjelmeland H, Musisi S. Psychological factors in deliberate self-harm as seen in an urban African population in Uganda: A case-control study. Suicide & Life-Threatening Behavior 2005;35:468-77. [DOI] [PubMed] [Google Scholar]
Kliem 2010
- Kliem S, Kröger C, Kosfelder J. Dialectical behavior therapy for borderline personality disorder: A meta-analysis using mixed-effects modeling. Journal of Consulting and Clinical Psychology 2010;78(6):936-51. [DOI] [PubMed] [Google Scholar]
Kreitman 1969
- Kreitman N, Philip AE, Greer S, Bagley CR. Parasuicide. British Journal of Psychiatry 1969;115:746-7. [DOI] [PubMed] [Google Scholar]
Lester 1994
- Lester D. The effectiveness of centres for the prevention of suicide [L'efficacité des centres de prévention du suicide]. Santé Mentale au Québec 1994;19:15-24. [PubMed] [Google Scholar]
Lilley 2008
- Lilley R, Owens D, Horrocks J, House A, Noble R, Bergen H, Hawton K, Casey D, Simkin S, Murphy E, Cooper J, Kapur N. Hospital care and repetition following self-harm: Multicentre comparison of self-poisoning and self-injury. British Journal of Psychiatry 2008;192:440-5. [DOI] [PubMed] [Google Scholar]
Linehan 1981
- Linehan MM, Nielsen SL. Assessment of suicide ideation and parasuicide: Hopelessness and social desirability. Journal of Consulting and Clinical Psychology 1981;49(5):773-5. [DOI] [PubMed] [Google Scholar]
Linehan 1993a
- Linehan MM, Heard HL, Armstrong HE. Naturalistic follow-up of a behavioral treatment for chronically parasuicidal borderline patients. Archives of General Psychiatry 1993;50(12):971-4. [DOI] [PubMed] [Google Scholar]
Linehan 1993b
- Linehan MM. Skills Training Manual for Treating Borderline Personality Disorder. New York, NY: Guildford Press, 1993. [Google Scholar]
Linehan 2007
- Linehan MM, Bohus M, Lynch TR. Dialectical behavior therapy for pervasive emotion dysregulation: theoretical and practical underpinnings. In: Gross J, editors(s). Handbook of Emotion Regulation. New York, NY: Guilford Press, 2007:581-605. [Google Scholar]
Links 2003b
- Links PS, Bergmans Y, Cook M. Psychotherapeutic interventions to prevent repeated suicidal behavior. Brief Treatment and Crisis Intervention 2003;3:445-64. [Google Scholar]
Lizardi 2010
- Lizardi D, Stanley B. Treatment engagement: a neglected aspect in the psychiatric care of suicidal patients. Psychiatric Services 2010;61(12):1183-91. [DOI] [PubMed] [Google Scholar]
Lorillard 2011a
- Lorillard, S, Schmitt, L, Andreoli, A. How to treat deliberate self-harm from clinical research to effective treatment choice? Part 1: An update on treatment efficacy among unselected patients referred to emergency room with deliberate self-harm [Comment traiter la tentative de suicide? 1 re partie: Efficacité des interventions psychosociales chez des patients suicidants à la sortie des urgences]. Annales Médico-Psychologiques 2011;169:211-28. [Google Scholar]
Lorillard 2011b
- Lorillard S, Schmitt L, Andreoli A. How to treat suicide attempt? Part 2: A review of treatments and the efficiency among borderline personality disorder patients [Comment traiter la tentative de suicide? Seconde partie: Une revue des traitements et de leur efficacité chez de patients borderline]. Annales Médico-Psychologiques 2011;169:229-36. [Google Scholar]
Lorr 1967
- Lorr M, Daston P, Smith IR. An analysis of mood states. Educational and Psychological Measurement 1967;27(1):89-96. [Google Scholar]
Lovibond 1995
- Lovibond PF, Lovibond SH. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy 1995;33(3):335-42. [DOI] [PubMed] [Google Scholar]
Luxton 2013
- Luxton DD, June JD, Comtois KA. Can postdischarge follow-up contacts prevent suicide and suicidal behavior? A review of the evidence. Crisis 2013;34(1):32-41. [DOI] [PubMed] [Google Scholar]
Lynch 2006
- Lynch TR, Chapman AL, Rosenthal MZ, Kuo JR, Linehan MM. Mechanisms of change in dialectical behavior therapy: Theoretical and empirical observations. Journal of Clinical Psychology 2006;62(4):459-80. [DOI] [PubMed] [Google Scholar]
Mann 2005
- Mann JJ, Apter A, Bertolote J, Beautrais A, Currier D, Haas A, Hegerl U, Lonnqvist J, Malone K, Marusic A, Mehlum L, Patton G, Phillips M, Rutz W, Rihmer Z, Schmidtke A, Shaffer D, Silverman M, Takahashi Y, Varnik A, Wasserman D, Yip P, Hendin H. Suicide prevention strategies: A systematic review. Journal of the American Medical Association 2005;294(16):2064-74. [DOI] [PubMed] [Google Scholar]
Marshall 2000a
- Marshall M, Gray A, Lockwood A, Green R. Case management for people with severe mental disorders. Cochrane Database of Systematic Reviews 2000;2:CD000050. [DOI] [PubMed] [Google Scholar]
Marshall 2000b
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249-52. [DOI] [PubMed] [Google Scholar]
Marting 1988
- Marting MS, Hammer AL. Coping Resources Inventory. Mind Garden, Inc, 1988. [http://www.mindgarden.com]. [Google Scholar]
Maydeu‐Olivares 1996
- Maydeu-Olivares A, D'Zurilla TJ. A factor analytic study of the Social Problem-Solving Inventory: an integration of theory and data. Cognitive Therapy and Research 1996;20(2):115-33. [Google Scholar]
McLeavey 1987
- McLeavey BC, Daly RJ, Murray CM, O'Riordan J, Taylor M. Interpersonal problem-solving deficits in self-poisoning patients. Suicide and Life-Threatening Behavior 1987;17:33-49. [DOI] [PubMed] [Google Scholar]
McMain 2007b
- McMain S. Effectiveness of psychosocial treatments on suicidality in personality disorders. Canadian Journal of Psychiatry 2007;52(6 Suppl 1):s103-s114. [PubMed] [Google Scholar]
McMain 2012
- McMain SF, Guimond T, Streiner DL, Cardish RJ, Links PS. Dialectical behavior therapy compared with general psychiatric management for borderline personality disorder: Clinical outcomes and functioning over a 2-year follow-up. American Journal of Psychiatry 2012;169(6):650-61. [DOI] [PubMed] [Google Scholar]
McNair 1964
- McNair DM, Lorr M. An analysis of mood in neurotics. Journal of Abnormal and Social Psychology 1964;69(6):620-7. [DOI] [PubMed] [Google Scholar]
Michel 2015
- Michel K, Gysin-Maillart A. ASSIP - Attempted Suicide Short Intervention Program: A manual for clinicians. Göttingen, Germany: Hogrefe Publishing, 2015. [Google Scholar]
Milner 2015
- Milner AJ, Carter G, Pirkis J, Robinson J, Spittal MJ. Letters, green cards, telephone calls and postcards: Systematic review and meta-analytic review of brief contact interventions for reducing self-harm, suicide attempts and suicide. British Journal of Psychiatry 2015;206:184-90. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? The Lancet 1998;352(9128):609-13. [DOI] [PubMed] [Google Scholar]
Möller, 1992
- Möller HJ. Attempted suicide: efficacy of different aftercare strategies. International Clinical Psychopharmacology 1992;6(Suppl 6):s58-s69. [PubMed] [Google Scholar]
Möller 1989
- Möller HJ. Efficacy of different strategies of aftercare for patients who have attempted suicide. Journal of the Royal Society of Medicine 1989;82(11):643-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382-9. [DOI] [PubMed] [Google Scholar]
Montgomery 1995
- Montgomery SA, Dunner DL, Dunbar GC. Reduction of suicidal thoughts with paroxetine in comparison with reference antidepressants and placebo. European Neuropsychopharmacology 1995;5(1):5-13. [DOI] [PubMed] [Google Scholar]
Motto 2001
- Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatric Services 2001;52(6):828-33. [DOI] [PubMed] [Google Scholar]
Muehlenkamp 2006
- Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. Journal of Mental Health Counseling 2006;28(2):166-85. [Google Scholar]
Mulhall 1977
- Mulhall D. Manual for the Personal Questionnaire Rapid Scaling Technique. Windsor, UK: National Foundation for Education Research, 1977. [Google Scholar]
Müller‐Oerlinghausen 2005
- Müller-Oerlinghausen B, Felber W, Berghöfer A, Lauterbach E, Ahrens B. The impact of lithium long-term medication of suicidal behavior and mortality of bipolar patients. Archives of Suicide Research 2005;9(3):307-319. [DOI] [PubMed] [Google Scholar]
Murphy 2012
- Murphy E, Kapur N, Webb R, Purandare N, Hawton K, Bergen H, Waters K, Cooper J. Risk factors for repetition and suicide following self-harm in older adults: Multicentre cohort study. British Journal of Psychiatry 2012;200:399-404. [DOI] [PubMed] [Google Scholar]
NCCMH 2004
- National Collaborating Centre for Mental Health. Clinical Guideline 16. Self-harm: The Short-term Physical and Psychological Management and Secondary Prevention of Self-harm in Primary and Secondary Care. London, UK: National Institute for Clinical Excellence, 2004. [Google Scholar]
Nezu 2010
- Nezu AM, Nezu CM, D'Zurilla TJ. Problem-solving therapy. In: Kazantzis N, Reinecke MA, Freeman A, editors(s). Cognitive and Behavioral Theories in Clinical Practice. New York, NY: Guilford Press, 2010:76-114. [Google Scholar]
Nock 2007
- Nock MK, Teper R, Hollander M. Psychological treatment of self-injury among adolescents. Journal of Clinical Psychology 2007;63(11):1081-9. [DOI] [PubMed] [Google Scholar]
O'Connor 2006
- O'Connor RC, Armitage CJ, Gray L. The role of clinical and social cognitive variables in parasuicide. British Journal of Clinical Psychology 2006;45(Pt 4):465-81. [DOI] [PubMed] [Google Scholar]
O'Connor 2009
- O'Connor RC, Rasmussen S, Miles J, Hawton K. Self-harm in adolescents: Self-report survey in schools in Scotland. British Journal of Psychiatry 2009;194:68-72. [DOI] [PubMed] [Google Scholar]
O'Connor 2011
- O'Connor RC. Towards an integrated motivational-volitional model of suicidal behaviour. In: O'Connor RC, Platt S, Gordon J, editors(s). International Handbook of Suicide Prevention: Research, Policy and Practice. Chichester, UK: Wiley-Blackwell, 2011:181-98. [Google Scholar]
O'Connor 2012
- O'Connor RC, Rasmussen S, Hawton K. Distinguishing adolescents who think about self-harm from those who engage in self-harm. British Journal of Psychiatry 2012;200:330-5. [DOI] [PubMed] [Google Scholar]
Office of the Surgeon General 2012
- Office of the Surgeon General/National Action Alliance for Suicide Prevention (US) 2012. 2012 National Strategy for Suicide Prevention: Goals and Objects for Action. A Report of the U.S. Surgeon General and of the National Action Alliance for Suicide Prevention. Washington, DC: US Department of Health and Human Services, 2012. [PubMed] [Google Scholar]
Ougrin 2011
- Ougrin D, Latif S. Specific psychological treatment versus treatment as usual in adolescents with self-harm: Systematic review and meta-analysis. Crisis 2011;32(2):74-80. [DOI] [PubMed] [Google Scholar]
Ougrin 2015
- Ougrin D, Tranah T, Stahl D, Moran P, Asarnow JR. Therapeutic interventions for suicide attempts and self-harm in adolescents: Systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry 2014;54(2):97-107. [DOI] [PubMed] [Google Scholar]
Owens 2002
- Owens D, Horrocks J, House A. Fatal and non-fatal repetition of self-harm. Systematic review. British Journal of Psychiatry 2002;181:193-9. [DOI] [PubMed] [Google Scholar]
Owens 2015
- Owens D, Kelley R, Munyombwe T, Bergen H, Hawton K, Cooper J, Ness J, Waters K, West R, Kapur K. Switching methods of self-harm at repeat episodes: Findings from a multinational cohort study. Journal of Affective Disorders 2015;180:44-51. [DOI] [PubMed] [Google Scholar]
Parkar 2006
- Parkar SR, Dawani V, Weiss MG. Clinical diagnostic and sociocultural dimensions of deliberate self-harm in Mumbai, India. Suicide and Life-Threatening Behavior 2006;36(2):223-38. [DOI] [PubMed] [Google Scholar]
Perry 2012
- Perry IJ, Corcoran P, Fitzgerald AP, Keeley HS, Reulbach U, Arensman E. The incidence and repetition of hospital-treated deliberate self-harm: Findings from the world's first national registry. PLoS One 2012;7(2):e31663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prochaska 1983
- Prochaska JO, Di Clemente CC. Stages and processes of self-change of smoking: Towards an integrated model of change. Journal of Consulting and Clinical Psychology 1983;51(3):390-5. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2012
- Robinson J, Yuen HP, Gook S, Hughes A, Cosgrave E, Killackey E, Baker K, Jorm A, McGorry P, Yung A. Can receipt of a regular postcard reduce suicide-related behaviour in young help seekers? A randomized controlled trial. Early Intervention in Psychiatry 2012;6(2):145-52. [DOI] [PubMed] [Google Scholar]
Rossouw 2013
- Rossouw TI. Mentalization-based treatment: Can it be translated into practice in clinical settings and teams? Journal of the American Academy of Child and Adolescent Psychiatry 2013;52(3):220-2. [DOI] [PubMed] [Google Scholar]
Royal College of Psychiatrists 1994
- Royal College of Psychiatrists. The General Hospital Management of Adult Deliberate Self-Harm. Council Report CR32. London, UK: Royal College of Psychiatrists, 1994. [Google Scholar]
Royal College of Psychiatrists 2004
- Royal College of Psychiatrists. Assessment Following Self-Harm in Adults. Council Report CR122. London, UK: Royal College of Psychiatrists, 2004. [Google Scholar]
Sakinofsky 2000
- Sakinofsky I. Repetition of suicidal behaviour. In: Hawton K, Van Heeringen K, editors(s). The International Handbook of Suicide and Attempted Suicide. Chichester, UK: Wiley, 2000:385-404. [Google Scholar]
Schmidtke 1996
- Schmidtke A, Bille Brahe U, De Leo D, Kerkhof A, Bjerke T, Crepet P, Haring C, Hawton K, Lönnqvist J, Michel K, Pommereau X, Querejeta I, Phillipe I, Salander-Renberg E, Temesváry B, Wasserman D, Fricke S, Weinacker B, Sampaio-Faria JG. Attempted suicide in Europe: rates, trends and sociodemographic characteristics of suicide attempters during the period 1989-1992. Results of the WHO/EURO multicentre study on parasuicide. Acta Psychiatrica Scandinavica 1996;93(5):327-38. [DOI] [PubMed] [Google Scholar]
Schmidtke 2004
- Schmidtke A, Weinacker B, Lähr C, Bille-Brahe U, De Leo D, Kerkhof A. Suicide and suicide attempts in Europe - an overview. In: Schmidtke A, Bille-Brahe U, De Leo D, Kerkhof A, editors(s). Suicidal Behaviour in Europe: Results from the WHO/EURO Multicentre Study on Suicidal Behaviour. Göttingen, Germany: Hogrefe & Huber Publishers, 2004:15-28. [Google Scholar]
Schotte 1982
- Schotte DE, Clum GA. Suicide ideation in a college population. Journal of Consulting and Clinical Psychology 1982;50(5):690-6. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF. Subverting randomization in controlled trials. Journal of the American Medical Association 1995;274(18):1456-8. [PubMed] [Google Scholar]
Schünemann 2008a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'summary of findings’ tables. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Schünemann 2008b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Scoliers 2009
- Scoliers G, Portzky G, Madge N, Hewitt A, Hawton K, De Wilde EJ, Ystgaard M, Arensman E, De Leo D, Fekete S, Van Heeringen K. Reasons for adolescent deliberate self-harm: A cry of pain and/or a cry for help? Findings from the child and adolescent self-harm in Europe (CASE) study. Social Psychiatry and Psychiatric Epidemiology 2009;44(8):601-7. [DOI] [PubMed] [Google Scholar]
Sinclair 2011
- Sinclair JMA, Gray A, Rivero-Arias O, Saunders KEA, Hawton K. Healthcare and social services resource use and costs of self-harm patients. Social Psychiatry and Psychiatric Epidemiology 2011;46(4):263-71. [DOI] [PubMed] [Google Scholar]
Spirito 2002
- Spirito A, Boergers J, Donaldson D, Bishop D, Lewander W. An intervention trial to improve adherence to community treatment by adolescents after a suicide attempt. Journal of Child and Adolescent Psychiatry 2002;41(4):435-42. [DOI] [PubMed] [Google Scholar]
Spitzer 1970
- Spitzer R, Endicott J, Fleiss JL, Cohen J. The Psychiatric Status Schedule: A technique for evaluating psychopathology and impairment in role functioning. Archives of General Psychiatry 1970;23(1):41-55. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. British Medical Journal 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Tarrier 2008b
- Tarrier N, Taylor K, Gooding P. Cognitive-behavioral interventions to reduce suicide behavior: A systematic review and meta-analysis. Behavior Modification 2008;32(1):77-108. [DOI] [PubMed] [Google Scholar]
Tondo 1997
- Tondo L, Jamison KR, Baldessarini RJ. Effect of lithium maintenance on suicidal behavior in major mood disorders. Annals of the New York Academy of Science 1997;836:339-51. [DOI] [PubMed] [Google Scholar]
Tondo 2000
- Tondo L, Baldessarini RJ. Reduced suicide risk during lithium maintenance treatment. Journal of Clinical Psychiatry 2000;61(Suppl 9):s97-s104. [PubMed] [Google Scholar]
Tondo 2001
- Tondo L, Hennen J, Baldessarini RJ. Lower suicide risk with long-term lithium treatment in major affective illness: A meta-analysis. Acta Psychiatrica Scandinavica 2001;104:163-172. [DOI] [PubMed] [Google Scholar]
Torgerson 2004
- Torgerson D. The use of Zelen's design in randomised trials. British Journal of Obstetrics and Gynaecology: An International Journal of Obstetrics and Gynaecology 2004;111(1):2. [DOI] [PubMed] [Google Scholar]
Townsend 2001
- Townsend E, Hawton K, Altman D, Arensman E, Gunnell D, Hazell P, House A, Van Heeringen K. The efficacy of problem-solving treatments after deliberate self-harm: Meta-analysis of randomized controlled trials with respect to depression, hopelessness and improvement in problems. Psychological Medicine 2001;31(6):979-88. [DOI] [PubMed] [Google Scholar]
Van der Sande 1997b
- Van der Sande R, Buskens E, Allart E, Van der Graaf Y, Van Engeland H. Psychosocial intervention following suicide attempt: A systematic review of treatment interventions. Acta Psychiatrica Scandinavica 1997;96(1):43-50. [DOI] [PubMed] [Google Scholar]
Van Heeringen 2014
- Van Heeringen K, Mann JJ. The neurobiology of suicide. The Lancet Psychiatry 2014;1(1):63-72. [DOI] [PubMed] [Google Scholar]
Van Spijker 2014
- Van Spijker BA, Van Straten A, Kerkhof AJFM. Effectiveness of online self-help for suicidal thoughts: Results of a randomised controlled trial. PLoS One 2014;9(2):e90118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vijayakumar 2011
- Vijayakumar L, Umamaheswari C, Shujaath Ali ZS, Devaraj P, Kesavan K. Intervention for suicide attempters: A randomized controlled study. Indian Journal of Psychiatry 2011;53(3):244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Washburn 2012
- Washburn JJ, Richardt SL, Styer DM, Gebhardt M, Juzwin KR, Yourek A, Aldridge D. Psychotherapeutic approaches to non-suicidal self-injury in adolescents. Child and Adolescent Psychiatry and Mental Health 2012;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westbrook 2011
- Westbrook D, Kennerley H, Kirk J. An Introduction to Cognitive Behaviour Therapy: Skills and Applications. 2nd edition. London, UK: SAGE Publications Ltd, 2011. [Google Scholar]
WHO 2014
- World Health Organization. Preventing Suicide: A Global Imperative. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
Williams 2000
- Williams JMG, Pollock LR. The psychology of suicidal behaviour. In: Hawton K, Van Heeringen K, editors(s). The International Handbook of Suicide and Attempted Suicide. Chichester, UK: Wiley, 2000. [Google Scholar]
Williams 2005
- Williams JMG, Crane C, Barnhofer T, Duggan D. Psychology and suicidal behaviour: Elaborating the entrapment model. In: Hawton K, editors(s). Prevention and Treatment of Suicidal Behaviour: From Science to Practice. Oxford, UK: Oxford University Press, 2005. [Google Scholar]
Wood 2001
- Wood A, Trainor G, Rothwell J, Moore A, Harrington R. Randomized trial of group therapy for repeated deliberate self-harm in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(11):1246-53. [DOI] [PubMed] [Google Scholar]
Xu 2012
- Xu D, Zhang X-L, Li X-Y, Niu Y-J, Zhang Y-P, Wang S-L. Effectiveness of 18-month psychosocial intervention for suicide attempters. Zhongguo Xinli Weisheng Zazhi [Chinese Mental Health Journal] 2012;26:24-9. [Google Scholar]
Zahl 2004
- Zahl D, Hawton K. Repetition of deliberate self-harm and subsequent suicide risk: long-term follow-up study in 11,583 patients. British Journal of Psychiatry 2004;185:70-5. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]
Zung 1965
- Zung WWK. A self-rating depression scale. Archives of General Psychiatry 1965;12:63-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hawton 1998
- Hawton K, Arensman E, Townsend E, Bremner S, Feldman E, Goldney R, Gunnell D, Hazell P, Van Heeringen K, House A, Owens D, Sakinsfsky I, Träskman-Bendz L. Deliberate self-harm: Systematic review of efficacy of psychosocial and pharmacological treatments in preventing repetition. British Medical Journal 1998;317(7156):441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawton 1999
- Hawton K, Townsend E, Arensman E, Gunnell D, Hazell P, House A, Van Heeringen K. Psychosocial and pharmacological treatments for deliberate self-harm. Cochrane Database of Systematic Reviews 1999;4:CD001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence. Clinical Guideline 133. Self-harm: Longer-term Management. London, UK: National Institute for Health and Clinical Excellence, 2011. [PubMed] [Google Scholar]


