Abstract
Oxidation of succinate by mitochondria can generate a higher protonmotive force (pmf) than can oxidation of NADH-linked substrates. Fundamentally, this is because of differences in redox potentials and gearing. Biology adds kinetic constraints that tune the oxidation of NADH and succinate to ensure that the resulting mitochondrial pmf is suitable for meeting cellular needs without triggering pathology. Tuning within an optimal range is used, for example, to shift ATP consumption between different consumers. Conditions that overcome these constraints and allow succinate oxidation to drive pmf too high can cause pathological generation of reactive oxygen species. We discuss the thermodynamic properties that allow succinate oxidation to drive pmf higher than NADH oxidation, and discuss the evidence for kinetic tuning of ATP production and for pathologies resulting from substantial succinate oxidation in vivo.
Keywords: bioenergetics, ischaemia-reperfusion injury, membrane potential, mitochondria, reactive oxygen species, thermodynamics
Introduction
The operation of a biological system is like the operation of a factory: each machine (enzyme) in the factory performs one or more linked tasks. At steady-state, the rate of each reaction in the system is the same. This rate is not, as is sometimes stated, set by the capacity of the slowest machine (the ‘rate-limiting step'). Instead, it is dynamic and responsive; adjustment of the concentrations of the intermediates by the system coordinates the rate of each machine with the rates of all the others linked to it. This coordination helps to avoid the accumulation of too much of one thing or the depletion of another and keeps the factory running smoothly.
Seeing the molecular machines present, and what each one does, is clearly necessary to understanding the system. However, you can only understand how the factory operates when the machines are all running and material is flowing between them. In steady-state, all the machines run at a rate that keeps all intermediates at constant and appropriate levels, and the factory is supported by the supply of raw materials and meets economic demand for the product.
Mitochondrial energetics is an excellent model factory, whose primary function is to transform energy released from substrate oxidation into the transferable currency of ATP. Standard textbook descriptions of this ‘energetic factory' carefully present the architecture of the molecular machines but tend to minimize the factory's moving parts — the thermodynamic and kinetic properties that drive these machines. The goal of this short review is to discuss why and how understanding the moving parts is crucial to understanding the system, and to examine the kinetic tools used by biology to manage the thermodynamic challenges specific to succinate oxidation.
With this lens, we examine why different substrates (NADH and succinate) with different tendencies to donate electrons (redox potential) behave differently from one another. Specifically, why does the less-powerful reductant, succinate, deliver a higher resting (i.e. non-phosphorylating, State IV) mitochondrial protonmotive force (pmf, Δp, the sum of the electrical and pH potentials across the mitochondrial inner membrane) [1]? What biological or pathological implications does high pmf have? Since pmf is linked to both cellular phosphorylation potential (tendency to drive phosphate transfer) and production of reactive oxygen species (ROS: here, superoxide, O2•−, and hydrogen peroxide, H2O2), it is an indirect modulator of any process sensitive to ATP/ADP or to ROS. Pmf itself may also drive cell signaling-related responses (such as parkin-dependent mitophagy [2,3])
Why succinate? As an intermediate in the tricarboxylic acid (TCA) cycle, succinate is a small component of normal cellular metabolism. However, there is abundant evidence that succinate accumulates during tissue ischemia [4,5], and that succinate oxidation upon reperfusion causes ROS-dependent damage (e.g. [6]). Succinate accumulation is associated with diverse pathologies, including epilepsy [7], cancer [8–10], and intestinal inflammation [11]. This review illustrates the fundamental bioenergetics underlying the more complicated pathophysiologies of these diseases. In principle, other pathways (such as oxidation of glycerol 3-phosphate and acyl-CoA dehydrogenases within β-oxidation of fatty acids) that feed electrons into the ubiquinone (Q) pool have the same ability as succinate to drive high pmf, but they don't appear to do so in vivo, perhaps due to different (and stronger) upstream kinetic constraints.
In the classic complete tricarboxylic acid cycle oxidizing pyruvate, the ratio of NADH to succinate production is fixed at 4 : 1, predicting pmf generation >80% by NADH oxidation. However, by diverting carbon into and out of the cycle, biology can shift reducing equivalent generation entirely to NADH oxidation (e.g. by running pyruvate to citrate and using the citrate for fatty acid synthesis) or strongly to succinate oxidation (e.g. running glutamine to lactate, or oxidizing a pool of succinate generated during prior anoxia). In this way, pmf, phosphorylation potential, and superoxide and hydrogen peroxide production will change with the proportion that each substrate contributes to total oxidation. We discuss how such changes would result from systems oxidizing NADH or succinate.
Thermodynamic gearing of succinate oxidation drives high mitochondrial pmf
Mitochondrial protonmotive force is established through proton (H+) pumping by Complexes I, III, and IV of the electron transport chain to generate the electrical and chemical separation of H+ across the mitochondrial inner membrane. The energy to pump these H+ derives from electron transfer from an initial donor through the chain of redox centers in the respiratory complexes, ending with reduction in O2 to H2O. For an electron pair (2e−) to enter the respiratory chain, the electron donor must be more reducing than the initial acceptor.
The two electron donor couples generally presented together are NADH2/NAD (2e− enter at Complex I) and FADH2/FAD (2e− enter at succinate dehydrogenase/Complex II). However, they are not exactly analogous — whereas NADH is an intermediate 2e− carrier between substrates (e.g. pyruvate) and Complex I, FADH2/FAD is embedded within succinate dehydrogenase and receives its 2e− directly from succinate oxidation to fumarate — making the FADH2/FAD couple more analogous to the FMN moiety of Complex I than to NADH. Therefore, succinate/fumarate is the more appropriate redox couple to compare with NADH2/NAD.
The NADH2/NAD couple is more reducing than the succinate/fumarate couple, yet succinate oxidation can drive pmf higher than NADH2 oxidation can [1]. The thermodynamic ‘gearing ratio' of energy available to work done makes it clear why. The energy available is the redox potential difference (ΔE) between the donor couple and O2, multiplied by number of e− transferred (here, [2]). The work done is the number of protons (n) pumped across the mitochondrial inner membrane multiplied by the pmf they are pumped against. The higher the ΔE/n ratio, the higher the pmf.
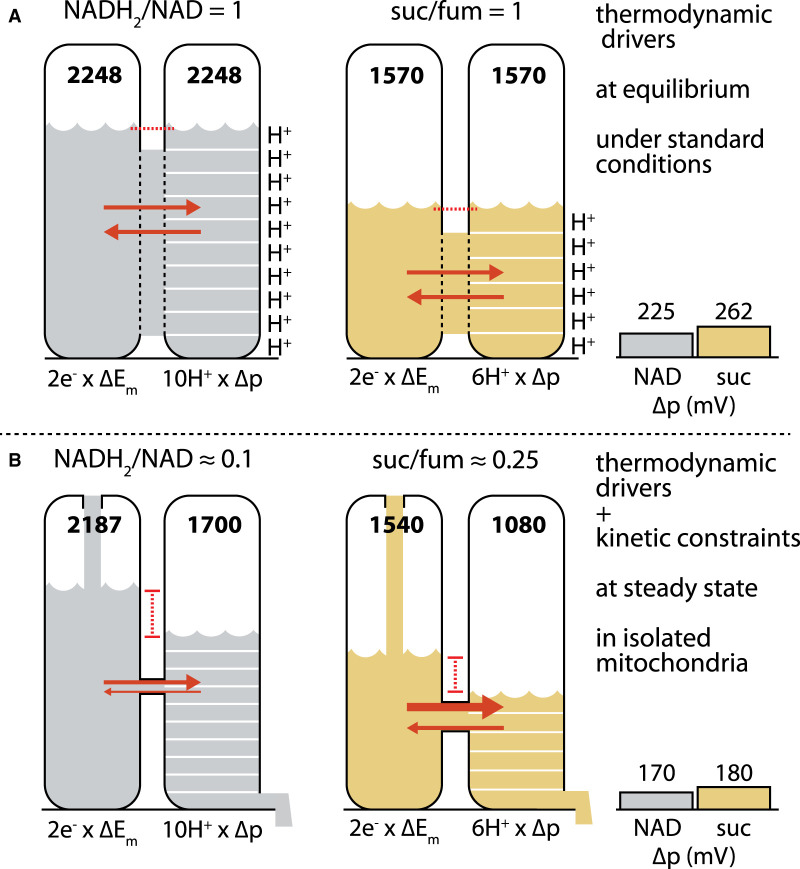
This situation is illustrated in Figure 1A, where pools of potential energy (‘potential') are represented as liquid in a tank, and energy transduction as flow through a connecting pipe into a linked tank. The left tank in each system (NADH oxidation in gray; succinate in tan) represents redox potential, summed over the two electrons transferred. The right tank represents the potential of the pmf, summed over the n protons pumped. For a hypothetical system at equilibrium under standard conditions, flow between connected tanks is unrestricted and each of the donor redox couples is 50% reduced (i.e. the ratio of reduced to oxidized species equals 1). The redox potential (derived from standard reduction potentials measured in electrochemical half cells, e.g. [12]) under these conditions is Em, the midpoint potential. Under these conditions at pH 7.4, Em7.4 values for NADH2/NAD and H2O/O2 are about −332 mV and +791 mV, respectively (derived from [13]). The ΔEm between them is therefore 1124 mV. Since 2e− are transferred, the total potential is 2 × 1124 = 2248 mV. For succinate/fumarate, Em is about +6 mV, so ΔEm is 785 mV and the total potential is 2 × 785 = 1570 mV.
Figure 1. Thermodynamic and kinetic determinants of protonmotive force (pmf, Δp) in (A) isolated mitochondria under standard conditions at equilibrium and (B) liver mitochondria oxidizing excess substrates (succinate or pyruvate/malate) in steady-state resting conditions.
(A) Under standard equilibrium conditions (50% reduction in redox couples, pH 7.4) at equilibrium, 2ΔEm for NADH2 oxidation is 2248 mV, supporting an nΔp of 2248 mV (n is the gearing: the number of protons pumped as 2e− flow to O2). 2ΔEm for succinate oxidation is 1570 mV, supporting an nΔp of 1570 mV. Since n is 10 for NADH2 oxidation and 6 for succinate oxidation, Δp values of 225 mV (from NADH2 oxidation) and 262 mV (from succinate oxidation) result. (B) Kinetics of supply and demand in liver mitochondria under non-standard conditions and steady-state resting respiration yield lower 2ΔEh than under standard conditions because redox couples are more oxidized: for NADH2, 2ΔEh is 2187 mV; for succinate, 2ΔEh is 1540 mV. Net flow between 2ΔEh and nΔp requires that nΔp < 2ΔEh. The diameter of the connecting pipe reflects the restriction of flow between the two pools that contributes to displacement between 2ΔEh and nΔp. Succinate oxidation generally operates faster, and closer to equilibrium than NADH2 oxidation, shown by a wider pipe diameter and smaller difference in pool levels. These constraints operating on thermodynamic drivers result in Δp of ∼170 mV (NADH2) and 180 mV (succinate). Extensive kinetic controls on succinate dehydrogenase/Complex II activity (which alter the diameter of the connecting pipe in this analogy) may serve to keep the pmf supported by succinate oxidation within acceptable limits in cells (see text). Suc/fum: succinate/fumarate. ‘NAD' and ‘suc' in plots at right refer to the NADH2/NAD couple and succinate/fumarate couple, respectively.
Because the systems in Figure 1A are at equilibrium, each of the tanks in a paired system has the same potential. In the right tank in each system representing pmf, the potential is divided into units to show its distribution over all n protons pumped; for NADH oxidation through Complex I, n = 10. Since succinate-derived electrons are not reducing enough to enter at Complex I (electrons, like water, can't be poured uphill), they enter at Complex II and therefore drive proton pumping only from Complexes III and IV, so n = 6 [13,14].
The pmf generated by proton pumping can increase to thermodynamic equilibrium with ΔE, as described by the equation
| 1 |
which is usefully rearranged to
| 2 |
For NADH2, pmf solves to (2 × 1124)/10 = 225 mV. For succinate it solves to (2 × 785)/6 = 262 mV. Thus, even though succinate is a weaker reductant than NADH, its available ΔEm is divided by fewer protons pumped (different gearing), so the resulting pmf is higher. A good analogy is pressure (force distributed over surface area). Compared with succinate oxidation, NAD-linked oxidation is ‘heavier' (greater ΔEm) but is distributed over a larger ‘area' (H+ pumped), resulting in a smaller ‘pressure' (pmf). Consider how lying on a bed of vertical nails is preferable to lying on one nail.
Biology imposes kinetic constraints to safely contain thermodynamic drivers of mitochondrial pmf
Although standard equilibrium conditions (Figure 1A) clarify the thermodynamics at work here, biology does not operate this way. First, the kinetics of the supply and demand reactions drive the donor couples relatively oxidized, with the mitochondrial NADH2/NAD pool ∼10% reduced [15,16], and the succinate/fumarate pool ∼25% reduced [12,17]. This relative oxidation decreases the actual redox potential (Eh) from the midpoint potential Em. In Figure 1B, this is reflected by lower potentials in the left tank of each system relative to Figure 1A. Second, these systems operate in non-equilibrium steady state, indicated by flow into the left tank and out from the right. Potential in the upstream tank must exceed that in the downstream tank, otherwise no energy flow occurs.
Connecting pipes between the left and right tanks are different diameters in the two systems. A smaller pipe diameter represents greater kinetic constraint imposed by the enzyme machinery, holding flow between tanks further from equilibrium. Figure 1B illustrates that, by comparing the electron transfer potential of the NADH2/NAD and succinate/fumarate pools to the pmf sustained by each, NADH oxidation operates further from equilibrium than succinate oxidation, further limiting the pmf established by NAD-linked flux. For succinate-linked flux, the pipe is wider, flux is higher (e.g. by more than 2-fold in isolated liver mitochondria, while between 1- and 2-fold in isolated muscle mitochondria) and the potentials therefore are closer to equilibrium [21–23].
What empirical evidence (aside from the redox and pmf measurements that underlie the values in Figure 1B) supports and explains these differences in pipe diameter, i.e. in the magnitude of kinetic constraints at Complex I and Complex II? It is helpful to reiterate that the pipe diameter in Figure 1B represents overall enzyme activity and therefore the kinetics, the abundance, and the regulation of the respective enzymes. The following lines of evidence support slower Complex I flux:
In isolated mitochondria with saturating substrates, the observed rate of uncoupled NADH-linked respiration (via Complex I) is about half the rate of succinate-linked respiration (via Complex II) [21–23], and about one third when compared at identical, non-zero pmf [1].
The turnover number (moles substrate transformed per second) for isolated Complex I is not much faster, and may be slower, than Complex II. There are few valid literature comparisons — the turnover numbers of succinate dehydrogenase (SDH) and Complex I have been rarely determined side by side, and experimental conditions (enzyme purification, electron acceptors used) varied between laboratories. Ackrell and colleagues reported a 2-fold higher turnover number for Complex I than for Complex II using the same electron acceptor [24]. Conversely, a 5-fold lower turnover number was found for Complex I in [25].
Complex II is more abundant than Complex I. In mammalian mitochondria SDH was 1.2–2 fold more abundant than Complex I, based on electron microscopic [26,27], spectrophotometric [24] and electrophoretic methods [28,29].
Low control of pathway flux is associated with near-equilibrium operation [30]. The control of flux across the respiratory chain has been studied using different approaches of metabolic control analysis. In general, control was distributed across the components of the electron transport chain, and SDH did not have particularly low flux control. Direct control analysis [31–38] indicated similar rate control by Complexes I and II, while calculation from elasticities showed slightly smaller flux control by SDH [1,39,40]. Thus, metabolic control analysis provides little support for particularly near-equilibrium operation of SDH.
Altogether, considering the turnover number and stoichiometry of NADH to succinate oxidation (4 : 1 per complete turn of the TCA cycle oxidizing pyruvate), SDH activity is in excess (parity being a 4-fold lower rate). The relative abundance of Complex II activity may help it operate closer to equilibrium, allowing the generation of high pmf [41].
So, what is the net effect on pmf when these biological kinetic constraints modulate the underlying thermodynamics? As Figure 1B illustrates, in isolated mammalian liver mitochondria, succinate oxidation can sustain a pmf up to ∼180 mV, while NADH2 (derived from oxidation of glutamate/malate, pyruvate/malate, and oxoglutarate/malate, in different experiments) oxidation can only sustain a pmf up to 170 mV under the same conditions [1,42]. Thus, isolated mitochondria assayed in vitro can sustain a higher pmf on succinate, by ∼10 mV. In mitochondria isolated from cultured cells, the electrical portion (Δψm) differed by ∼15 mV [51,56]. In mitochondria isolated from mammalian skeletal muscle, pmf values differed less, ∼4 mV [57,58]. While all comparable measurements to date show succinate driving pmf higher than NADH2, the variation in these differences may at least partially reflect tissue specificity.
The same principle applies when different sites of 2e− entry are compared, demonstrating how predominantly the thermodynamics of the mitochondrial machine predict its operation. Brown et al. found that in isolated liver mitochondria, feeding 2e− at Complex IV (via sulfite oxidation) gave 2ΔEh of 987 mV, ∼65% of the value for succinate oxidation, yet drove a resting pmf of 187 mV, ∼10 mV higher than the 176 mV driven by succinate oxidation [59].
Kinetic constraints are highly tunable by extensive biological regulation
Biological regulation of Complex II activity in vivo is extensive, and why so much regulation exists on this enzyme has remained an open question. A tentative model, drawing from the thermodynamic properties above, is that such regulation exists to rein in this powerful machine in cells, by decreasing its activity (narrowing the pipes) when ATP demand slackens and preventing pmf from being driven too high. Multiple regulatory mechanisms are summarized below.
Complex II/SDH exhibits conformational changes through interaction with many metabolites, including the TCA intermediates succinate, malate and oxaloacetate, ATP, reduced ubiquinone, protons (i.e. low pH), and certain anions [48]. These changes modulate enzyme activity. The mechanism of conformational activation/inhibition remains unknown. However, we speculate that its physiological role is to restrict pmf generation to an optimal range, while retaining the ability to drive high pmf when these restrictions are eased (and also allow pmf generation when NADH oxidation is blocked).
Three endogenous metabolites competitively inhibit SDH: oxaloacetate, itaconate, and malonate. The keto-tautomeric form of oxaloacetate produced by malate dehydrogenase (MDH), is a tightly binding competitive inhibitor [49]. Since MDH is fully reversible, keto-oxaloacetate levels are mostly dependent on mass action; higher NADH2/NAD will increase malate/oxaloacetate ratio, decreasing oxaloacetate concentration. This interaction may exist to increase SDH activity when Complex I is unable to oxidize NADH, e.g. at very high pmf [50], though activation by ATP may also explain this observation [51]. Oxaloacetate can also be produced in its enol- form by SDH itself oxidizing malate [61,64,65]. This high-affinity, slow-dissociating tautomer can spontaneously interconvert with its keto- form but too slowly to prevent SDH inactivation; oxaloacetate tautomerase activity rescues SDH activity [66,67]. Itaconate, another competitive inhibitor of SDH, is derived through decarboxylation of citrate by the immune-responsive gene 1 product (IRG1) [56]. Itaconate suppresses respiration in activated macrophages [57,58]. Malonate, mostly thought of as an experimental inhibitor, has been detected in brain [59]. Endogenous malonate is also implied by the presence of a malonyl-CoA synthase (ACSF3) [60].
SDH is also subject to posttranslational phosphorylation and succinylation. Mitochondrial Fgr tyrosine kinase phosphorylates SDH on the A subunit and activates the enzyme [61]. Phosphorylation is triggered by H2O2, but not O2•−. A corresponding matrix-localized phosphatase, PTPMT1, is reported [62]. Succinylation may be non-enzymatic when succinyl-CoA is high, but succinyl-CoA synthases such as α-KGDH may also succinylate SDH and other proteins, at least in the nucleus [63]. Desuccinylation can occur through SIRT5 activity in the mitochondrial matrix [64].
Complex I also undergoes reversible conformation changes (activation/deactivation A/D transition). One putative function of deactivation is to mitigate reperfusion injury during anoxia [65]. In tissues, there is an equilibrium between A- and D-forms, influenced by a range of factors: SH-oxidation, divalent cations and fatty acids [65]. Furthermore, multiple phosphorylation sites have been proposed; possibly phosphorylation increases Complex I activity by bolstering assembly [66].
Portioning of the cellular ATP budget is tied to pmf
How cells consume ATP is partly dependent on cellular phosphorylation potential [67]. This form of potential energy is close to equilibrium with pmf, with higher pmf sustaining a higher phosphorylation potential and ATP/ADP ratio. The pmf therefore determines the supply side of the ATP/ADP ratio, a key piece of metabolic information ‘read' by every ATP-binding protein in the cell [13,68]. As pmf changes, the ATP/ADP ratio also changes, which tunes consumption by different energetic processes. This reflects a sensible underlying biological goal: to maintain essential functions while making less-essential functions dependent on the available potential. This was demonstrated by titrating pmf with mitochondrial inhibitors, revealing that as pmf declines, protein synthesis decreases the most, while ion cycling decreases the least [69,70].
As an analogy, when your paycheck is high, you might spend more on luxuries than when times are lean. However, your rent stays about the same. Some categories of your spending (luxuries) are more dependent on your income than others (rent). In biology, the implication is that sustained high pmf through oxidation of succinate drives proportionally more protein synthesis. Pmf is therefore not just an intermediate to be charged and discharged; in the light of an ATP consumption hierarchy, pmf becomes an important director of cellular activity.
As an aside, a common oversimplification is that high pmf is ‘functional', and low pmf is ‘dysfunctional'. A model that better fits the available data is that both extremes carry the risk of dysfunction; too low a pmf leaves a cell under-powered and unable to run essential functions, whereas too high a pmf drives high rates of superoxide production and leads to damage and pathology (section 6). What defines ‘extreme' remains to be determined but it likely cell type-specific. Plausibly, pmf values between these extremes are neither strictly functional nor dysfunctional; the biological outcome depends on how pmf supports cellular functions and viability in the environment occupied by the biological system (defined as narrowly or as broadly as appropriate). For example, in both neurons and pancreatic β-cells the pmf can change by up to 50 mV as a part of apparently normal function [71,72]
Mitochondrial superoxide and hydrogen peroxide production rises with pmf
As well as driving high ATP/ADP, high pmf also slows electron flow, reducing redox centers, which drives faster mitochondrial production of superoxide and hydrogen peroxide. Succinate oxidation can therefore drive faster mitochondrial O2•−/H2O2 production than NADH oxidation [73–75]. Like water overflowing a clogged sink, mitochondrial O2•−/H2O2 production rate increases at high pmf when the ubiquinone pool is highly reduced (sink full of water) and single electrons more readily reduce O2 to form O2•− (water pooling on the floor). In isolated mitochondria O2•−/H2O2 production increases non-linearly with respect to pmf; between pmf values of ∼180 (achieved by NADH oxidation) and 220 mV (achieved by succinate oxidation) O2•−/H2O2 production can increase 5- to 10-fold [76–79].
Considering the framework presented above, isolated mitochondria can be exposed to conditions (such as exclusive oxidation of abundant succinate) that bypass the biological constraints that would presumably be present in vivo to prevent damage and pathology. Are there conditions in cells and in vivo that approximate the unconstrained state achievable in vitro, and are they pathological?
First, can high pmf drive O2•−/H2O2 production when mitochondria are within cells or in vivo? Yes — mitochondria are a major contributor to cellular H2O2 production, with the majority emanating from site IQ of Complex I and site IIIQo of Complex III, the sites are routinely driven in isolated mitochondria by the oxidation of excess added succinate [80–83]. O2•−/H2O2 production by sites IQ and IIIQo is not restricted to cell models; substantial pathological effects of H2O2 production from sites IQ and IIIQo can also be demonstrated in vivo when endogenous mitochondrial superoxide dismutase is knocked out [84]. Under conditions of low succinate and high pmf, Complex II is also a significant contributor to ROS production, which could be biologically relevant [85,93], but would contribute less to overall ROS production as succinate accumulates [85].
Second, can succinate levels rise high enough in vivo that succinate oxidation could drive high rates of O2•−/H2O2 production? The answer is yes, with the best-studied model being ischemia/reperfusion (I/R) injury [86]. Estimated intramitochondrial succinate levels in normal rat skeletal muscle are 200–300 µM [80], in the range of the KM of SDH for succinate (100–400 µM [58,87] in mammalian mitochondria). In contrast, succinate levels can accumulate 3- to 20-fold during ischemia [6]. Reperfusion drives rapid oxidation of the accumulated succinate, high pmf, a reduced ubiquinone pool, and high rates of O2•−/H2O2 production. Succinate accumulation appears to amplify the HIF-1-dependent hypoxic response [88], suggesting that succinate accumulation is a common feature of hypoxia more broadly.
Two plausible models explain why succinate accumulates under ischemia. The absence of sufficient O2 can lead cellular reductants to drive SDH in reverse, pulling fumarate from different sources and reducing it to succinate. Alternatively, succinate may accumulate through forward SDH activity, supported partially by aminotransferase anaplerosis [4,6].
Having evidence that O2•−/H2O2 production from sites IQ and IIIQo occurs in vivo, and that succinate can accumulate in vivo, it needs to be demonstrated that oxidation of accumulated succinate is associated with high rates of O2•−/H2O2 production from these sites. Strong evidence comes from experiments showing that tissue damage due to I/R is attenuated when different elements required for succinate oxidation-associated O2•−/H2O2 production are disrupted, by inhibiting succinate oxidation [89,90], dissipating pmf (and oxidizing the QH2/Q pool) using chemical uncouplers [91,92], inhibiting electron transport through site IQ [93,94], or by suppressing O2•−/H2O2 production at site IQ using SIQELs, which impedes electron leak to O2•−/H2O2 but not electron flux to H2O [95,96]. Supporting the idea that O2•−/H2O2 derived from site IQ has broad biological effects, S1QELs modulate many different physiological and pathological conditions in vivo [74,95].
Conclusion
Oxidation of succinate supports a higher pmf than oxidation of NADH. This is not because of some specific molecular property of succinate or NADH, but because of the thermodynamics (redox potentials), gearing (numbers of protons pumped) and kinetics of the components of the pathways by which energy from NADH and succinate oxidation is captured as pmf. In the absence of kinetic constraints, thermodynamics enables succinate to drive a pmf high enough to cause significant biological damage. This may explain the extensive regulation of SDH: biology has responded to the thermodynamic hazard created by succinate oxidation by tightly controlling SDH activity to maintain pmf within a range that allows optimal ATP production and signaling (by ATP/ADP, O2•−/H2O2, metabolite levels) but minimizes oxidative damage by O2•−/H2O2. If these controls are compromised, pathology can follow.
Perspectives
We discuss the importance of thermodynamic and kinetic properties in explaining the mitochondrial protonmotive forces that result when different substrates are oxidized.
Current thinking is complicated when the molecular properties of different substrates and substrate oxidation pathways are emphasized; models of mitochondrial function can be unified and simplified by considering these thermodynamic and kinetic properties.
Predicting how mitochondrial oxidation of succinate may contribute to physiology and to pathology can be simplified by applying these considerations.
Abbreviations
- MDH
malate dehydrogenase
- ROS
reactive oxygen species
- SDH
succinate dehydrogenase
- TCA
tricarboxylic acid
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors acknowledge funding from Touro University California (S.A.M.) and Buck Institute for Research on Aging (M.D.B.).
Author contributions
Article idea was developed by S.M. and M.D.B. S.M., A.A.G., and M.A.W. wrote the manuscript. S.M. generated the figure. All authors edited and finalized the manuscript.
References
- 1.Brand, M.D., Harper, M.E. and Taylor, H.C. (1993) Control of the effective P/O ratio of oxidative phosphorylation in liver mitochondria and hepatocytes. Biochem. J. 291, 739–748 10.1042/bj2910739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin, S.M., Lazarou, M., Wang, C., Kane, L.A., Narendra, D.P. and Youle, R.J. (2010) Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 191, 933–942 10.1083/jcb.201008084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narendra, D., Tanaka, A., Suen, D.F. and Youle, R.J. (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang, J., Wang, Y.T., Miller, J.H., Day, M.M., Munger, J.C. and Brookes, P.S. (2018) Accumulation of succinate in cardiac ischemia primarily occurs via canonical Krebs cycle activity. Cell Rep. 23, 2617–2628 10.1016/j.celrep.2018.04.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin, J.L., Costa, A.S.H., Gruszczyk, A.V., Beach, T.E., Allen, F.M., Prag, H.A.et al. (2019) Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 1, 966–974 10.1038/s42255-019-0115-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chouchani, E.T., Pell, V.R., Gaude, E., Aksentijević, D., Sundier, S.Y., Robb, E.L.et al. (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 10.1038/nature13909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang, Y., Zhang, M., Zhu, W., Yu, J., Wang, Q., Zhang, J.et al. (2020) Succinate accumulation induces mitochondrial reactive oxygen species generation and promotes status epilepticus in the kainic acid rat model. Redox Biol. 28, 101365 10.1016/j.redox.2019.101365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardella, C., Olivero, M., Lorenzato, A., Geuna, M., Adam, J., O'Flaherty, L.et al. (2012) Cells lacking the fumarase tumor suppressor are protected from apoptosis through a hypoxia-inducible factor-independent, AMPK-dependent mechanism. Mol. Cell. Biol. 32, 3081–3094 10.1128/MCB.06160-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores, R.E., Brown, A.K., Taus, L., Khoury, J., Glover, F., Kami, K.et al. (2018) Mycoplasma infection and hypoxia initiate succinate accumulation and release in the VM-M3 cancer cells. Biochim. Biophys. Acta Bioenergetics 1859, 975–983 10.1016/j.bbabio.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 10.Weber, A., Klocker, H., Oberacher, H., Gnaiger, E., Neuwirt, H., Sampson, N.et al. (2018) Succinate accumulation is associated with a shift of mitochondrial respiratory control and HIF-1α upregulation in PTEN negative prostate cancer cells. Int. J. Mol. Sci. 19, 2129 10.3390/ijms19072129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh, Y., Trautwein, C., Romani, J., Salker, M.S., Neckel, P.H., Fraccaroli, I.et al. (2020) Overexpression of human alpha-synuclein leads to dysregulated microbiome/metabolites with ageing in a rat model of Parkinson disease. bioRxiv 10.1101/2020.12.23.424226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrett, R.H. and Grisham, C.M. (2016) Biochemistry, 6th ed, Cengage Learning, p.71 [Google Scholar]
- 13.Nicholls, D.G. and Ferguson, S.J. (2013) Bioenergetics, 4th ed, Elsevier, p.40 [Google Scholar]
- 14.Mookerjee, S.A., Gerencser, A.A., Nicholls, D.G. and Brand, M.D. (2017) Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. J. Biol. Chem. 292, 7189–7207 10.1074/jbc.AAC118.004855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veech, R.L., Guynn, R. and Veloso, D. (1972) The time-course of the effects of ethanol on the redox and phosphorylation states of rat liver. Biochem. J. 127, 387–397 10.1042/bj1270387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williamson, D.H., Lund, P. and Krebs, H.A. (1967) The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem. J. 103, 514–527 10.1042/bj1030514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lendvai, N., Pawlosky, R., Bullova, P., Eisenhofer, G., Patocs, A., Veech, R.L.et al. (2014) Succinate-to-fumarate ratio as a new metabolic marker to detect the presence of sdhb/d-related paraganglioma: initial experimental and ex vivo findings. Endocrinology 155, 27–32 10.1210/en.2013-1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medja, F., Allouche, S., Frachon, P., Jardel, C., Malgat, M., de Camaret, B.M., et al. (2009) Development and implementation of standardized respiratory chain spectrophotometric assays for clinical diagnosis. Mitochondrion. 9, 331–339 10.1016/j.mito.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 19.Taylor, R.W., Birch-Machin, M.A., Bartlett, K., Turnbull, D.M. (1993) Succinate-cytochrome c reductase: assessment of its value in the investigation of defects of the respiratory chain. Biochim. Biophys. Acta. 1181, 261–265 10.1016/0925-4439(93)90030-5 [DOI] [PubMed] [Google Scholar]
- 20.Taylor, R.W., Birch-Machin, M.A., Bartlett, K., Lowerson, S.A., Turnbull, D.M. (1994) The control of mitochondrial oxidations by complex III in rat muscle and liver mitochondria. Implications for our understanding of mitochondrial cytopathies in man. J. Biol. Chem. 269, 3523–3528 PMID: 8106394 [PubMed] [Google Scholar]
- 21.Acin-Perez, R., Benador, I.Y., Petcherski, A., Veliova, M., Benavides, G.A., Lagarrigue, S.et al. (2020) A novel approach to measure mitochondrial respiration in frozen biological samples. EMBO J. 39, e104073 10.15252/embj.2019104073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers, G.W., Brand, M.D., Petrosyan, S., Ashok, D., Elorza, A.A., Ferrick, D.A.et al. (2011) High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS ONE 6, e21746 10.1371/journal.pone.0021746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das, K.C. and Muniyappa, H. (2013) Age-dependent mitochondrial energy dynamics in the mice heart: role of superoxide dismutase-2. Exp. Gerontol. 48, 947–959 10.1016/j.exger.2013.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackrell, B.A.C., Maguire, J.J., Dallman, P.R. and Kearney, E.B. (1984) Effect of iron deficiency on succinate- and NADH-ubiquinone oxidoreductases in skeletal muscle mitochondria. J. Biol. Chem. 259, 10053–9 10.1016/S0021-9258(18)90926-9 [DOI] [PubMed] [Google Scholar]
- 25.Grivennikova, V.G., Kapustin, A.N. and Vinogradov, A.D. (2001) Catalytic activity of NADH-ubiquinone oxidoreductase (Complex I) in intact mitochondria: evidence for the slow active/inactive transition. J. Biol. Chem. 276, 9038–9044 10.1074/jbc.M009661200 [DOI] [PubMed] [Google Scholar]
- 26.Schwerzmann, K., Hoppeler, H., Kayar, S.R. and Weibel, E.R. (1989) Oxidative capacity of muscle and mitochondria: correlation of physiological, biochemical, and morphometric characteristics. Proc. Natl Acad. Sci. U.S.A. 86, 1583–1587 10.1073/pnas.86.5.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwerzmann, K., Cruz-Orive, L.M., Eggman, R., Sänger, A. and Weibel, E.R. (1986) Molecular architecture of the inner membrane of mitochondria from rat liver: a combined biochemical and stereological study. J. Cell Biol. 102, 97–103 10.1083/jcb.102.1.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schägger, H. and Pfeiffer, K. (2001) The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J. Biol. Chem. 276, 37861–7 10.1074/jbc.M106474200 [DOI] [PubMed] [Google Scholar]
- 29.Capaldi, R.A., Halphen, D.G., Zhang, Y.Z. and Yanamura, W. (1988) Complexity and tissue specificity of the mitochondrial respiratory chain. J. Bioenerg. Biomembr. 20, 290–311 10.1007/BF00769634 [DOI] [PubMed] [Google Scholar]
- 30.Crabtree, B. and Newsholme, E.A. (1978) Sensitivity of a near-equilibrium reaction in a metabolic pathway to changes in substrate concentration. Eur. J. Biochem. 89, 19–22 10.1111/j.1432-1033.1978.tb20891.x [DOI] [PubMed] [Google Scholar]
- 31.Moreno-Sánchez, R., Bravo, C. and Westerhoff, H.V. (1999) Determining and understanding the control of flux: an illustration in submitochondrial particles of how to validate schemes of metabolic control. Eur. J. Biochem. 264, 427–433 10.1046/j.1432-1327.1999.00621.x [DOI] [PubMed] [Google Scholar]
- 32.Kaambre, T., Chekulayev, V., Shevchuk, I., Karu-Varikmaa, M., Timohhina, N., Tepp, K.et al. (2012) Metabolic control analysis of cellular respiration in situ in intraoperational samples of human breast cancer. J. Bioenerg. Biomembr. 44, 539–558 10.1007/s10863-012-9457-9 [DOI] [PubMed] [Google Scholar]
- 33.Fritzen, A.J., Grunnet, N. and Quistorff, B. (2007) Flux control analysis of mitochondrial oxidative phosphorylation in rat skeletal muscle: pyruvate and palmitoyl-carnitine as substrates give different control patterns. Eur. J. Appl. Physiol. 101, 679–689 10.1007/s00421-007-0544-2 [DOI] [PubMed] [Google Scholar]
- 34.Gellerich, F.N., Kunz, W.S. and Bohnensack, R. (1990) Estimation of flux control coefficients from inhibitor titrations by non-linear regression. FEBS Lett. 274, 167–170 10.1016/0014-5793(90)81355-R [DOI] [PubMed] [Google Scholar]
- 35.Groen, A.K., Wanders, R.J., Westerhoff, H.V., van der Meer, R. and Tager, J.M. (1982) Quantification of the contribution of various steps to the control of mitochondrial respiration. J. Biol. Chem. 257, 2754–2757 10.1016/S0021-9258(19)81026-8 [DOI] [PubMed] [Google Scholar]
- 36.Small, J.R. (1993) Flux control coefficients determined by inhibitor titration: the design and analysis of experiments to minimize errors. Biochem. J. 296, 423–433 10.1042/bj2960423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanchi, C., Genova, M.L., Castelli, G.P. and Lenaz, G. (2004) The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J. Biol. Chem. 279, 36562–9 10.1074/jbc.M405135200 [DOI] [PubMed] [Google Scholar]
- 38.Ventura, B., Genova, M.L., Bovina, C., Formiggini, G. and Lenaz, G. (2002) Control of oxidative phosphorylation by complex I in rat liver mitochondria: implications for aging. Biochim. Biophys. Acta Bioenergetics 1553, 249–260 10.1016/S0005-2728(01)00246-8 [DOI] [PubMed] [Google Scholar]
- 39.Lionetti, L., Iossa, S., Brand, M.D. and Liverini, G. (1996) Relationship between membrane potential and respiration rate in isolated liver mitochondria from rats fed an energy dense diet. Mol. Cell. Biochem. 158, 133–138 10.1007/BF00225839 [DOI] [PubMed] [Google Scholar]
- 40.Mildaziene, V., Nauciene, Z., Baniene, R. and Grigiene, J. (2002) Multiple effects of 2,2′,5,5′-tetrachlorobiphenyl on oxidative phosphorylation in rat liver mitochondria. Toxicol. Sci. 65, 220–227 10.1093/toxsci/65.2.220 [DOI] [PubMed] [Google Scholar]
- 41.Heinrich, R. and Rapoport, T.A. (1974) A linear steady-state treatment of enzymatic chains: general properties, control and effector strength. Eur. J. Biochem. 42, 89–95 10.1111/j.1432-1033.1974.tb03318.x [DOI] [PubMed] [Google Scholar]
- 42.Brand, M.D., Harper, W.G., Nicholls, D.G. and Ingledew, W.J. (1978) Unequal charge separation by different coupling spans of the mitochondrial electron transport chain. FEBS Lett. 95, 125–129 10.1016/0014-5793(78)80066-0 [DOI] [PubMed] [Google Scholar]
- 43.Amo, T., Yadava, N., Oh, R., Nicholls, D.G., Brand, M.D. (2008) Experimental assessment of bioenergetic differences caused by the common European mitochondrial DNA haplogroups H and T. Gene. 411, 69–76 10.1016/j.gene.2008.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amo, T., Brand, M.D. (2007) Were inefficient mitochondrial haplogroups selected during migrations of modern humans? A test using modular kinetic analysis of coupling in mitochondria from cybrid cell lines. Biochem. J. 404, 345–351 10.1042/bj20061609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambert, A.J., Brand, M.D. (2004) Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem. J. 382, 511–517 10.1042/bj20040485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert, A.J., Brand, M.D. (2004) Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J. Biol. Chem. 279, 39414–39420 10.1074/jbc.m406576200 [DOI] [PubMed] [Google Scholar]
- 47.Brown, G.C., Brand, M.D. (1988) Proton/electron stoichiometry of mitochondrial complex I estimated 472 from the equilibrium thermodynamic force ratio. Biochem. J. 252, 473–479 10.1042/bj2520473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singer, T.P., Kearney, E.B. and Ackrell, B.A.C. (1973) Newer Knowledge of the Regulatory Properties of Succinate Dehydrogenase. In Mechanisms in Bioenergetics (Azzone, G.F., Ernster, L., Papa, S., Quagliariello, E. and Siliprandi, N., eds), pp. 485–498, Academic Press, New York [Google Scholar]
- 49.Ackrell, B.A.C., Kearney, E.B. and Mayr, M. (1974) Role of oxalacetate in the regulation of mammalian succinate dehydrogenase. J. Biol. Chem. 249, 2021–2027 10.1016/S0021-9258(19)42790-7 [DOI] [PubMed] [Google Scholar]
- 50.Fink, B.D., Bai, F., Yu, L., Sheldon, R.D., Sharma, A., Taylor, E.B.et al. (2018) Oxaloacetic acid mediates ADP-dependent inhibition of mitochondrial complex II-driven respiration. J. Biol. Chem. 293, 19932–19941 10.1074/jbc.RA118.005144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutman, M., Kearney, E.B. and Singer, T.P. (1971) Multiple control mechanisms for succinate dehydrogenase in mitochondria. Biochem. Biophys. Res. Commun. 44, 526–532 10.1016/S0006-291X(71)80114-6 [DOI] [PubMed] [Google Scholar]
- 52.Panchenko, M.V., Vinogradov, A.D. (1991) Direct demonstration of enol-oxaloacetate as an immediate product of malate oxidation by the mammalian succinate dehydrogenase. FEBS Lett. 286, 76–78 10.1016/0014-5793(91)80944-x [DOI] [PubMed] [Google Scholar]
- 53.Belikova, Y.O., Kotlyar, A.B., Vinogradov, A.D. (1988) Oxidation of malate by the mitochondrial succinate-ubiquinone reductase. Biochim. Biophys. Acta. 936, 1–9 10.1016/0005-2728(88)90245-9 [DOI] [PubMed] [Google Scholar]
- 54.Vinogradov, A.D., Kotlyar, A.B., Burov, V.I., Belikova, Y.O. (1989) Regulation of succinate dehydrogenase and tautomerization of oxaloacetate. Adv. Enzyme Regul. 28, 271–280 10.1016/0065-2571(89)90076-9 [DOI] [PubMed] [Google Scholar]
- 55.Niehaus, T.D., Hillmann, K.B. (2020) Enzyme, promiscuity, metabolite, damage, and metabolite damage control systems of the tricarboxylic acid cycle. FEBS J. 287, 1343–1358 10.1111/febs.15284 [DOI] [PubMed] [Google Scholar]
- 56.Michelucci, A., Cordes, T., Ghelfi, J., Pailot, A., Reiling, N., Goldmann, O.et al. (2013) Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl Acad. Sci. U.S.A. 110, 7820–7825 10.1073/pnas.1218599110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi, Z., Deng, M., Scott, M.J., Fu, G., Loughran, P.A., Lei, Z.et al. (2020) IRG1/itaconate activates Nrf2 in hepatocytes to protect against liver ischemia–reperfusion injury. Hepatology 72, 1394–1411 10.1002/hep.31147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lampropoulou, V., Sergushichev, A., Bambouskova, M., Nair, S., Vincent, E.E., Loginicheva, E.et al. (2016) Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 24, 158–166 10.1016/j.cmet.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riley, K.M., Dickson, A.C. and Koeppen, A.H. (1991) The origin of free brain malonate. Neurochem. Res. 16, 117–122 10.1007/BF00965698 [DOI] [PubMed] [Google Scholar]
- 60.Witkowski, A., Thweatt, J. and Smith, S. (2011) Mammalian ACSF3 protein is a malonyl-CoA synthetase that supplies the chain extender units for mitochondrial fatty acid synthesis. J. Biol. Chem. 286, 33729–33736 10.1074/jbc.M111.291591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acín-Pérez, R., Carrascoso, I., Baixauli, F., Roche-Molina, M., Latorre-Pellicer, A., Fernández-Silva, P.et al. (2014) ROS-triggered phosphorylation of complex II by Fgr kinase regulates cellular adaptation to fuel use. Cell Metab. 19, 1020–1033 10.1016/j.cmet.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nath, A.K., Ryu, J.H., Jin, Y.N., Roberts, L.D., Dejam, A., Gerszten, R.E.et al. (2015) PTPMT1 inhibition lowers glucose through succinate dehydrogenase phosphorylation. Cell Rep. 10, 694–701 10.1016/j.celrep.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang, Y. and Gibson, G.E. (2019) Succinylation links metabolism to protein functions. Neurochem. Res. 44, 2346–2359 10.1007/s11064-019-02780-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu, L., Wang, Q., Zhao, B., Wu, Q. and Wang, P. (2019) Exogenous nicotinamide adenine dinucleotide administration alleviates ischemia/reperfusion-induced oxidative injury in isolated rat hearts via Sirt5-SDH-succinate pathway. Eur. J. Pharmacol. 858, 172520 10.1016/j.ejphar.2019.172520 [DOI] [PubMed] [Google Scholar]
- 65.Babot, M., Birch, A., Labarbuta, P. and Galkin, A. (2014) Characterisation of the active/de-active transition of mitochondrial complex I. Biochim. Biophys. Acta Bioenergetics 1837, 1083–1092 10.1016/j.bbabio.2014.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valsecchi, F., Ramos-Espiritu, L.S., Buck, J., Levin, L.R. and Manfredi, G. (2013) cAMP and mitochondria. Physiology 28, 199–209 10.1152/physiol.00004.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manchester, K. (1980) Free energy ATP hydrolysis and phosphorylation potential. Biochem. Educ. 8, 70–72 10.1016/0307-4412(80)90043-6 [DOI] [Google Scholar]
- 68.Nicholls, D.G. (2004) Mitochondrial membrane potential and aging. Aging Cell 3, 35–40 10.1111/j.1474-9728.2003.00079.x [DOI] [PubMed] [Google Scholar]
- 69.Buttgereit, F. and Brand, M.D. (1995) A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J. 312, 163–167 10.1042/bj3120163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wieser, W. and Krumschnabel, G. (2001) Hierarchies of ATP-consuming processes: direct compared with indirect measurements, and comparative aspects. Biochem. J. 355, 389–395 10.1042/bj3550389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gerencser, A.A. (2018) Metabolic activation-driven mitochondrial hyperpolarization predicts insulin secretion in human pancreatic beta-cells. Biochim. Biophys. Acta Bioenergetics 1859, 817–828 10.1016/j.bbabio.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerencser, A.A., Chinopoulos, C., Birket, M.J., Jastroch, M., Vitelli, C., Nicholls, D.G.et al. (2012) Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. J. Physiol. 590, 2845–2871 10.1113/jphysiol.2012.228387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quinlan, C.L., Perevoshchikova, I., Hey-Mogensen, M., Orr, A.L. and Brand, M.D. (2013) Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 1, 304–312 10.1016/j.redox.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brand, M.D. (2020) Riding the tiger–physiological and pathological effects of superoxide and hydrogen peroxide generated in the mitochondrial matrix. Crit. Rev. Biochem. Mol. Biol. 55, 592–661 10.1080/10409238.2020.1828258 [DOI] [PubMed] [Google Scholar]
- 75.Brand, M. (2016) Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 100, 14–31 10.1016/j.freeradbiomed.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 76.Brand, M.D., Buckingham, J.A., Esteves, T.C., Green, K., Lambert, A.J., Miwa, S.et al. (2004) Mitochondrial superoxide and aging: uncoupling-protein activity and superoxide production. Biochem. Soc. Symp. 71, 203–213 10.1042/bss0710203 [DOI] [PubMed] [Google Scholar]
- 77.Miwa, S. and Brand, M.D. (2003) Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem. Soc. Trans. 31, 1300–1301 10.1042/bst0311300 [DOI] [PubMed] [Google Scholar]
- 78.Liu, S.S. (1997) Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci. Rep. 17, 259–272 10.1023/A:1027328510931 [DOI] [PubMed] [Google Scholar]
- 79.Kushnareva, Y., Murphy, A.N. and Andreyev, A. (2002) Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem. J. 368, 545–553 10.1042/bj20021121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goncalves, R.L.S., Quinlan, C.L., Perevoshchikova, I., Hey-Mogensen, M. and Brand, M.D. (2015) Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem. 290, 209–227 10.1074/jbc.M114.619072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goncalves, R.L.S., Watson, M.A., Wong, H.S., Orr, A.L. and Brand, M.D. (2020) The use of site-specific suppressors to measure the relative contributions of different mitochondrial sites to skeletal muscle superoxide and hydrogen peroxide production. Redox Biol. 28, 101341 10.1016/j.redox.2019.101341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong, H.S., Benoit, B. and Brand, M.D. (2019) Mitochondrial and cytosolic sources of hydrogen peroxide in resting C2C12 myoblasts. Free Radic. Biol. Med. 130, 140–150 10.1016/j.freeradbiomed.2018.10.448 [DOI] [PubMed] [Google Scholar]
- 83.Fang, J., Wong, H.S. and Brand, M.D. (2020) Production of superoxide and hydrogen peroxide in the mitochondrial matrix is dominated by site IQ of complex I in diverse cell lines. Redox Biol. 37, 101722 10.1016/j.redox.2020.101722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong, H.S., Mezera, V., Dighe, P., Melov, S., Gerencser, A.A., Sweis, R.F.et al. (2021) Superoxide produced by mitochondrial site IQ inactivates cardiac succinate dehydrogenase and induces hepatic steatosis in Sod2 knockout mice. Free Radic. Biol. Med. 164, 223–232 10.1016/j.freeradbiomed.2020.12.447 [DOI] [PubMed] [Google Scholar]
- 85.Quinlan, C.L., Orr, A.L., Perevoshchikova, I.V., Treberg, J.R., Ackrell, B.A., Brand, M.D. (2012) Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 287, 27255–27264 10.1074/jbc.m112.374629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chouchani, E.T., Pell, V.R., James, A.M., Work, L.M., Saeb-Parsy, K., Frezza, C.et al. (2016) A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 23, 254–263 10.1016/j.cmet.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 87.Grivennikova, V.G., Gavrikova, E.V., Timoshin, A.A. and Vinogradov, A.D. (1993) Fumarate reductase activity of bovine heart succinate-ubiquinone reductase. New assay system and overall properties of the reaction. Biochim. Biophys. Acta Bioenergetics 1140, 282–292 10.1016/0005-2728(93)90067-P [DOI] [PubMed] [Google Scholar]
- 88.Selak, M.A., Armour, S.M., MacKenzie, E.D., Boulahbel, H., Watson, D.G., Mansfield, K.D.et al. (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7, 77–85 10.1016/j.ccr.2004.11.022 [DOI] [PubMed] [Google Scholar]
- 89.Dröse, S., Bleier, L. and Brandt, U. (2011) A common mechanism links differently acting complex II inhibitors to cardioprotection: modulation of mitochondrial reactive oxygen species production. Mol. Pharmacol. 79, 814–822 10.1124/mol.110.070342 [DOI] [PubMed] [Google Scholar]
- 90.Valls-Lacalle, L., Barba, I., Miró-Casas, E., Alburquerque-Béjar, J.J., Ruiz-Meana, M., Fuertes-Agudo, M.et al. (2016) Succinate dehydrogenase inhibition with malonate during reperfusion reduces infarct size by preventing mitochondrial permeability transition. Cardiovasc. Res. 109, 374–384 10.1093/cvr/cvv279 [DOI] [PubMed] [Google Scholar]
- 91.Korde, A.S., Pettigrew, L.C., Craddock, S.D. and Maragos, W.F. (2005) The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J. Neurochem. 94, 1676–1684 10.1111/j.1471-4159.2005.03328.x [DOI] [PubMed] [Google Scholar]
- 92.Hoerter, J., Gonzalez-Barroso, M.-M., Couplan, E., Mateo, P., Gelly, C., Cassard-Doulcier, A.-M.et al. (2004) Mitochondrial uncoupling protein 1 expressed in the heart of transgenic mice protects against ischemic-reperfusion damage. Circulation 110, 528–533 10.1161/01.CIR.0000137824.30476.0E [DOI] [PubMed] [Google Scholar]
- 93.Lesnefsky, E.J., Chen, Q., Moghaddas, S., Hassan, M.O., Tandler, B. and Hoppel, C.L. (2004) Blockade of electron transport during ischemia protects cardiac mitochondria. J. Biol. Chem. 279, 47961–7 10.1074/jbc.M409720200 [DOI] [PubMed] [Google Scholar]
- 94.Chen, Q., Hoppel, C.L. and Lesnefsky, E.J. (2005) Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J. Pharmacol. Exp. Ther. 316, 200–207 10.1124/jpet.105.091702 [DOI] [PubMed] [Google Scholar]
- 95.Watson, M.A., Wong, H.-S. and Brand, M.D. (2019) Use of S1QELs and S3QELs to link mitochondrial sites of superoxide and hydrogen peroxide generation to physiological and pathological outcomes. Biochem. Soc. Trans. 47, 1461–1469 10.1042/BST20190305 [DOI] [PubMed] [Google Scholar]
- 96.Brand, M.D., Goncalves, R.L.S., Orr, A.L., Vargas, L., Gerencser, A.A., Borch Jensen, M.et al. (2016) Suppressors of superoxide-H2O2 production at site IQ of mitochondrial complex I protect against stem cell hyperplasia and ischemia-reperfusion injury. Cell Metab. 24, 582–592 10.1016/j.cmet.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]