Abstract
Metabolic disorders, such as obesity, type 2 diabetes mellitus, and nonalcoholic fatty liver disease, are characterized by chronic low-grade tissue and systemic inflammation. During obesity, the adipose tissue undergoes immunometabolic and functional transformation. Adipose tissue inflammation is driven by innate and adaptive immune cells and instigates insulin resistance. Here, we discuss the role of innate immune cells, that is, macrophages, neutrophils, eosinophils, natural killer cells, innate lymphoid type 2 cells, dendritic cells, and mast cells, in the adipose tissue in the healthy (lean) and diseased (obese) state and describe how their function is shaped by the obesogenic microenvironment, and humoral, paracrine, and cellular interactions. Moreover, we particularly outline the role of hypoxia as a central regulator in adipose tissue inflammation. Finally, we discuss the long-lasting effects of adipose tissue inflammation and its potential reversibility through drugs, caloric restriction, or exercise training.
Keywords: Adipose tissue, Obesity, Chronic inflammation, Innate immune cells, Hypoxia, Endothelium, Physical exercise
Introduction
Immune mechanisms and metabolism are inextricably connected and mutually co-regulate each other [1, 2]. Immune responses require metabolic adaptation at the cellular and organismal level [1, 3, 4], while metabolic dysregulation, for example, in obesity, leads to immune activation [1, 2, 5, 6]. The adipose tissue comprises immune cells, which shape its function in health and disease [1, 2, 5, 6, 7]. In the healthy state, immune mechanisms contribute to maintenance of tissue homeostasis [1, 2, 5, 6, 7]. In obesity, the immune profile of the adipose tissue changes, shifting to a chronic low-grade inflammatory state, which gradually becomes systemic and drives insulin resistance and metabolic disease [1, 2, 5, 6, 7, 8, 9, 10]. Type 2 diabetes mellitus, cardiovascular disease, fatty liver disease, and several cancers are linked to obesity [10]. Recently, it became evident that obesity also predisposes to coronavirus disease 2019 (COVID-19) severity [11, 12]. Hence, especially but not limited to the times of the COVID-19 pandemic, obesity constitutes an important public health issue [10]. Gaining a better understanding of the immune mechanisms implicated in obesity will be key in developing therapeutic strategies to prevent or restrain associated metabolic and immune disturbances.
Here, we describe the innate immune networks in the adipose tissue and how these change in obesity. We also discuss the interplay of innate immune cells with the endothelium and particularly focus on the role of hypoxia in this context. We highlight recent knowledge on the molecular and metabolic pathways regulating the function of innate immune cells in the adipose tissue and their interaction with their surrounding tissue. Finally, we discuss the long-lasting character and potential reversibility of adipose tissue inflammation.
The Adipose Tissue Niche
The white and brown adipose tissue are two different types of adipose tissue, which are functionally and developmentally distinct [7, 13]. The brown adipose tissue is located in specific depots, as the interscapular region, and is responsible for thermogenesis. Functional brown adipose tissue is found primarily in mice and human newborns but is also present in human adults inversely correlating with BMI [7, 13, 14, 15]. The white adipose tissue can be present in almost every nonnervous tissue, and although it can also produce to some extent heat upon cold exposure, it mainly functions as an energy storage tissue, which also exerts multiple immune, metabolic, and endocrine functions [7, 13]. For the purposes of the present review, we focus on the immunological milieu of the white adipose tissue.
The white adipose tissue is organized in anatomically distinct depots, which can be broadly categorized into subcutaneous and intra-abdominal/visceral depots [16]. The subcutaneous and visceral adipose tissue differ in their growing and adipogenic capacity, as well as their metabolic, endocrine, and immune functions [16, 17, 18]. The adipose tissue niche is composed of mature adipocytes, fibroblast-like cell populations including adipocyte precursors, the vasculature, and immune cells, which all spatially and functionally interact [2, 6, 19]. The vasculature runs through the adipose tissue, providing oxygen and nutrients to tissue cells [20]. Adipocyte progenitors reside mainly in the perivascular space and exhibit adipogenic capacity [16, 21, 22]. Fibroblast-like cells generate connective tissue and can be pro-fibrotic and pro-inflammatory [16, 23, 24, 25]. Essentially, the adipose tissue also comprises a broad spectrum of immune cells. Innate immune cells present in the adipose tissue are macrophages, neutrophils, eosinophils, dendritic cells (DCs), mast cells, innate lymphoid cells (ILCs), and natural killer (NK) cells; adaptive immune cells are T and B lymphocytes [2, 6, 26, 27, 28]. While in the lean, that is, healthy, adipose tissue these components harmonically co-function, upon overnutrition, metabolic pressure disrupts homeostasis, leading to an oversized inflamed, hypoxic and fibrotic adipose tissue [16, 19, 29, 30, 31].
Immune Reprogramming of the Adipose Tissue in Obesity
The adipose tissue has the capacity to expand at a remarkable extent through adipocyte size increase (hypertrophy) and formation of new adipocytes (hyperplasia) [18, 19, 32, 33, 34]. Both visceral and subcutaneous adipose tissue display hyperplastic capacity in a gender- and age-dependent manner [16, 17, 18, 19, 33]. Evolutionarily, the capability of the adipose tissue to store lipids and expand in size at the cellular and tissue level was developed as a mechanism of energy storage during periods of nutritional excess, while during less propitious times, the adipose tissue confers energy supply [19]. This relies on the ability of adipocytes to synthesize triglycerides during times of excess of food supply (lipogenesis) and liberate free fatty acids from triglycerides through lipolysis, when food is scarce [19, 35]. In addition, sequestration of lipids inside the adipocytes protects other tissues, such as the liver, muscle and heart, from lipotoxicity [19].
However, overnutrition extended over long periods of time in combination with physical inactivity leads to obesity [19]. The expansion of adipocytes outpaces the growing capacity of the vasculature, resulting in inadequate vascularization and hypoxia in the obese adipose tissue [20, 36]. Adipocyte expansion also creates mechanical stress due to contact with the surrounding matrix and cells [19, 37]. Hypoxia and mechanical stress on lipid-overloaded adipocytes are associated with increased adipocyte cell death, leading to recruitment of pro-inflammatory macrophages and fibrotic tissue deposition [16, 19, 29, 30, 31, 38, 39]. Fibrosis develops through adipocyte progenitor-driven extracellular matrix remodeling and disturbs adipose tissue plasticity and metabolic function [23].
Adipocytes secrete a broad spectrum of factors, including proteins, termed as adipokines, through which they influence their neighboring cells, as well as establish intra-organ communication [40]. Altered adipokine secretion and function contributes to development of obesity and associated complications [40, 41]. Essentially, hypertrophic adipocytes contribute to the establishment of adipose tissue and systemic chronic inflammation through secretion of cytokines and pro-inflammatory adipokines [40, 41]. Hypertrophic adipocytes secrete increased amounts of tumor necrosis factor (TNF), interleukin (IL)-6, monocyte chemoattractant protein 1 (MCP-1), IL-1 and IL-8, thereby activating and attracting immune cells [42, 43]. Although secretion of many cytokines, such as IL-1, IL-6 and TNF, is shared by adipocytes and immune cells, the cellular origin of the cytokines might determine their effects; for instance, while myeloid cell-derived IL-6 restrains macrophage accumulation in the adipose tissue, adipocyte-derived IL-6 has the opposite effect [43, 44]. Leptin is a pro-inflammatory adipokine, the serum levels of which increase proportionally to the adipose tissue mass [45]. Besides its role in the regulation of food intake and energy expenditure [41, 46], it also triggers pro-inflammatory responses in immune and endothelial cells and promotes insulin resistance [45, 47]. Other adipokines such as resistin and chemerin also display proinflammatory properties, while others such as adiponectin, omentin, C1q/TNF-related proteins, and secreted frizzled-related protein 5 have anti-inflammatory effects [47]. Out of these, the best described is adiponectin, which inhibits leptin-induced TNF production in macrophages [48].
Moreover, adipose tissue is a significant source of microRNAs, which are secreted in exosomes and regulate gene expression in distant tissues, such as fibroblast growth factor 21 expression in the liver [49]. Circulating exosomal miRNAs of obese animals promote insulin tolerance, adipose tissue inflammation, and hepatic steatosis in lean animals [50]. Moreover, exosomal miRNAs might mediate communication of adipocytes with adipose tissue macrophages (ATM); for instance, miR-34a is secreted by adipocytes and inhibits alternative activation (M2-like) macrophage [51]. Adipocytes also release lipid-filled vesicles, which transport lipids to local ATM determining their differentiation and function [52]. On the other hand, ATMs in the obese adipose tissue also secrete exosomes loaded with miRNAs, such as miR-155, promoting insulin resistance in the liver and muscle [53].
The lean adipose tissue predominantly contains noninflammatory cells, including alternatively activated macrophages, eosinophils, regulatory T cells (Tregs) and ILC2 cells [2, 54, 55, 56, 57, 58, 59, 60]. Cytokines such as IL-4, IL-13, IL-5 and the alarmin IL-33 mediate type 2 immune responses in the adipose tissue [2, 54, 55, 56, 57, 58, 59, 60]. Type 2 immunity is thought to mitigate inflammation and may support metabolic health through the effects of IL-4, IL-13 and IL-10 [2, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67]. In obesity, the immune profile of the adipose tissue shifts to a pro-inflammatory state through recruitment of macrophages, neutrophils and cytotoxic CD8+ T cells [5, 6, 26, 28, 68, 69, 70, 71]. Adipocyte-derived pro-inflammatory factors, such as MCP-1, IL-6, TNF and leptin, hypoxia and adipocyte cell death stimulate activation and recruitment of immune cells [6, 20, 42, 43, 44, 45, 47, 72, 73, 74, 75, 76]. Although inflammatory signals are required for proper adipose tissue remodeling and expansion and are therefore an adaptation that allows nutrient storage [27, 77], obesity-associated low-grade chronic inflammation in the adipose tissue and other organs, such as the liver, muscle and colon, is linked to metabolic disorders and is therefore termed “meta-inflammation” [2, 5, 7, 26, 78, 79]. In obese patients and mice, inflammation exemplified by macrophage abundance is greater in omental compared to subcutaneous adipose tissue [80, 81, 82]. In general, increased subcutaneous relative to visceral adiposity is associated with a favorable metabolic state [16, 32]. Indeed, “metabolically healthy” obese individuals may have lower visceral adiposity, display greater insulin sensitivity and have a lower risk for development of type 2 diabetes mellitus and cardiovascular disease [83, 84].
In essence, adipose tissue chronic inflammation significantly contributes to the development of insulin resistance [1, 5, 6, 26, 85]. Obese subjects display hyperinsulinemia and insulin resistance and are predisposed to the development of type 2 diabetes mellitus [86]. Specifically, IL-6 and TNF perpetuate insulin resistance [85, 87, 88, 89, 90]. Mechanistically, the c-Jun N-terminal kinase (JNK) signaling pathway, a central mediator of inflammatory responses in obesity and type 2 diabetes, is activated in adipose tissue of obese humans and mice and promotes insulin resistance through phosphorylation of insulin receptor substrate (IRS) [87, 91, 92, 93]. In accordance with this, genetic deficiency or pharmacological inhibition of JNK confers metabolic protection [91, 92, 94]. Moreover, IκBα kinase Komplexes (IKK), the upstream activator of nuclear factor “kappa-light-chain-enhancer” of activated B cells (NF-κB), phosphorylates IRS-1 and thereby blocks insulin signaling [95]. Obese mice display elevated levels of IKKε in the liver, adipocytes and ATM, while IKKε deficiency protects against diet-induced obesity, chronic inflammation, hepatic steatosis and insulin resistance [96]. Treatment of obese diabetic patients with an inhibitor of IKKε can improve blood glucose levels and increase energy expenditure [97]. Additionally, suppressor of cytokine signaling (SOCS) proteins, which are upregulated during inflammation, induce proteolytic degradation of IRS proteins, thereby contributing to insulin resistance [98, 99, 100].
Adipose tissue chronic inflammation and insulin resistance can drive the development of nonalcoholic steatohepatitis and cardiovascular disease [101, 102]. JNK1 deletion in the adipose tissue of mice protects from high-fat diet-induced insulin resistance and liver steatosis [87]. IL-6 secretion from the visceral adipose tissue can directly target the liver through the portal circulation and induce SOCS3 protein expression in the liver, which mediates insulin resistance [87]. IL-6 may also induce hepatic production of C-reactive protein (CRP) and serum amyloid A (SAA) [103, 104, 105, 106]. Both CRP and SAA are associated with development of insulin resistance and cardiovascular disease [84]. However, mice with liver-specific IL-6 receptor deficiency display greater hepatic inflammation and insulin resistance, suggesting that IL-6 may also have protective functions in the liver [107]. Innate immune cells populating the adipose tissue and playing a role in its chronic inflammation are macrophages, neutrophils, NK cells, eosinophils, ILC2, DCs, and mast cells (Fig. 1, 2).
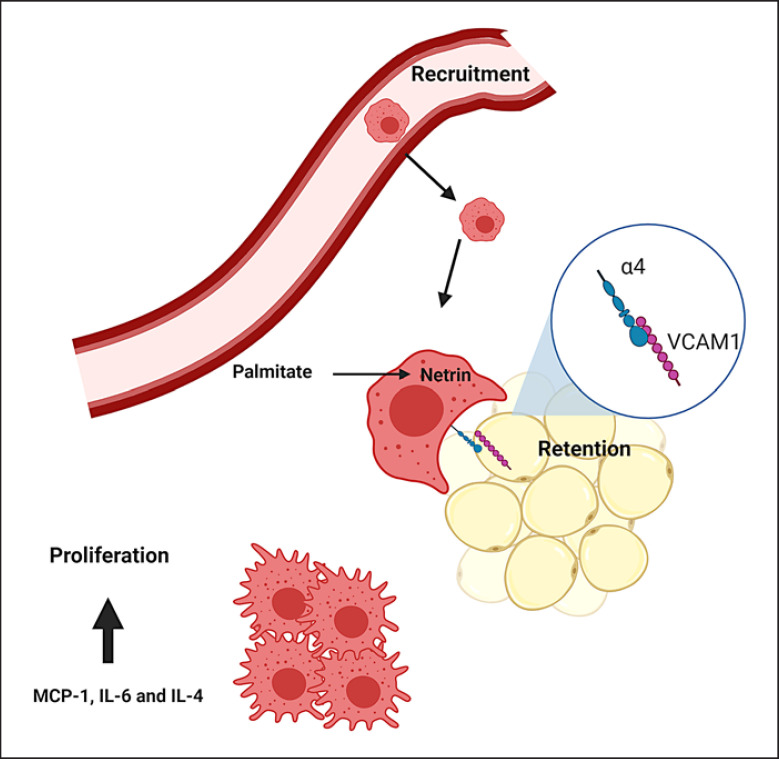
Fig. 1.
ATM accumulation through proliferation, recruitment, and retention. Macrophages accumulate in the adipose tissue through cell division, recruitment and retention. Recruitment can be driven by MCP-1 [112, 117]. Local proliferation occurs especially in the early stage of obesity [111, 113, 114] and is induced by IL-4, IL-6 and MCP-1 [111, 113, 128]. Retention is mediated by direct adhesion of macrophages to adipocytes through the interaction of α4 on macrophages with VCAM-1 on adipocytes [115, 116] and netrin-1, the expression of which is induced in macrophages by palmitate [115]. ATM, adipose tissue macrophage; MCP-1, monocyte chemoattractant protein 1; IL, interleukin; VCAM-1, vascular cell adhesion molecule-1.
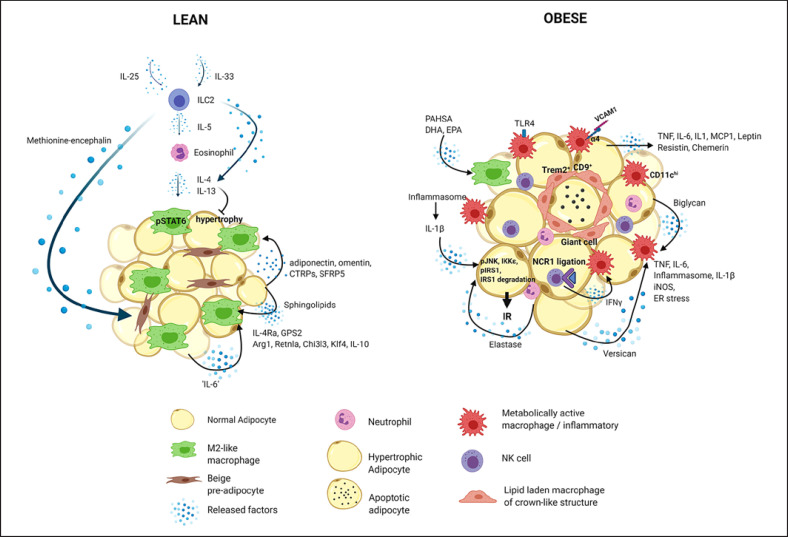
Fig. 2.
Innate immune cells in the lean and obese adipose tissue. The lean adipose tissue predominantly contains non-inflammatory cells, including alternatively activated (M2-like) macrophages, eosinophils, regulatory T cells and ILC2 cells, and type 2 cytokines, such as IL-4, IL-13, IL-5 and IL-33 [2, 54, 55, 56, 57, 58, 59, 60]. ILC2 are maintained in the adipose tissue by IL-33 and IL-25 [56, 58, 59, 60, 215] and produce IL-5, which is a key cytokine for maintenance of eosinophil and consequently M2-like macrophage populations [54, 56, 57, 59]. ILC2 also produce IL-13, which induces macrophage alternative activation [215]. Moreover, they secrete methionine-encephalin, which promotes adipose tissue thermogenesis (beiging) [60]. Eosinophils are a major cell source of IL-4 in the adipose tissue [57]. IL-4 maintains M2-like macrophages through STAT6 signaling and attenuates adipocyte hypertrophy [55, 56, 57]. IL-6 upregulates the expression of the IL-4 receptor, thereby supporting macrophage alternative activation [128, 171]. Adipokines, such as adiponectin, omentin, CTRPs and SFRP5, and sphingolipids also have anti-inflammatory effects [47, 179]. Hallmarks of M2-like ATM are KLF4, arginase 1, Retnla and Chi3l3 and GPS2 expression [170, 174]. In obesity, the immune profile of the adipose tissue shifts to a pro-inflammatory state through recruitment of macrophages, neutrophils and NK cells [5, 6, 26, 28, 68, 69, 70, 71]. Adipocyte-derived pro-inflammatory factors, such as MCP-1, IL-6, TNF and leptin, chemerin and resistin, stimulate activation and recruitment of immune cells [6, 20, 42, 43, 44, 45, 47, 72, 73, 74, 75, 76]. Extracellular matrix components, such as versican and biglycan derived from adipocytes and macrophages, respectively, promote macrophage pro-inflammatory activation [143, 144]. NCR1 ligation on NK cells triggers IFN-γ release, which also instigates macrophage activation [71]. Macrophages are retained in the adipose tissue through the interaction of α4 with VCAM-1 [116]. Pro-inflammatory macrophages are featured by increased expression of TNF, IL-6, iNOS, inflammasome activation, IL-1β production, and enhanced ER stress [68, 132, 133, 134, 135, 136, 149, 150, 151, 152, 153, 154, 156, 157, 158, 159, 163, 164]. Macrophages within crown-like structures surrounding dying adipocytes are CD9+, highly phagocytic, lipid-laden, Trem2+ and can form multinucleated giant cells through cell fusion [76, 181, 191, 193]. ATMs outside of crown-like structures are pro-inflammatory and marked by CD11c [191]. Pro-inflammatory macrophages via IL-1β and neutrophils through elastase perpetuate insulin resistance [69, 149, 150, 151, 152, 153, 154, 159, 166, 167, 230]. In contrast, PAHSA, DHA, and EPA decrease macrophage-mediated adipose tissue inflammation and promote insulin sensitivity [187, 188]. IL, interleukin; ILC, innate lymphoid cell; CTRP, C1q/TNF-related protein; NK, natural killer; MCP-1, monocyte chemoattractant protein 1; SFRP5, secreted frizzled-related protein 5; VCAM-1, vascular cell adhesion molecule-1; TNF, tumor necrosis factor; iNOS, inducible nitric oxide synthase; ATM, adipose tissue macrophage; KLF4, Krüppel-like factor 4; GPS2, G protein pathway suppressor 2; ER, endoplasmic reticulum; Trem2, triggering receptor expressed on myeloid cells 2; PAHSA, palmitic acid-9-hydroxystearic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Macrophages
Macrophages constitute an abundant cell population in the adipose tissue, which expands in obesity [5, 6, 39, 68, 85, 108]. Due to their abundance as well as their functional flexibility, they are major determinants of adipose tissue inflammation in metabolic disease. Resident macrophages are already present in the adipose tissue of young mice and are thought to play a role in adipose tissue development [109, 110]. During obesity, their numbers increase through recruitment, proliferation and retention [39, 68, 108, 111, 112, 113, 114, 115, 116, 117] (Fig. 1). Recruitment was shown to be driven by myeloid C-C motif chemokine receptor-2 (CCR2), the receptor of MCP-1 [112, 117], although other studies reported MCP-1-independent macrophage infiltration [118, 119]. This discrepancy could rely on the fact that MCP-1 is not the sole ligand of CCR2, which also binds other chemokines such as MCP-2 (CCL2) and MCP-3 (CCL7), and CCR2 is not the only receptor for MCP-1 [119, 120]. Interestingly, MCP-3 expression increases in the adipose tissue of obese mice in an MCP-1-dependent manner [119]. CD8+ T cells can also promote macrophage infiltration in the adipose tissue during obesity, most probably through secretion of factors that induce macrophage migration, such as interferon-inducible protein-10, MCP-1, and MCP-3 [108]. Also CCL5 (RANTES) was suggested to promote monocyte recruitment in the human adipose tissue [121]. In accordance with this, CCR5−/− mice have reduced ATM numbers and are protected against insulin resistance when fed a high-fat diet [122]. Other adipocyte-derived factors produced in high amounts in obesity, such as SAA3 and hyaluronic acid, might contribute to monocyte recruitment and retention [123, 124, 125]. Formyl peptide receptor 2 expression was shown to be increased in the obese adipose tissue, and its deletion restrained ATM abundance and activation, obesity and insulin resistance, and enhanced energy expenditure [126]. Vascular endothelial growth factor (VEGF) mediates adipose tissue angiogenesis and promotes macrophage infiltration and M2-like polarization in mice under high-fat diet [127]. Moreover, ATM undergo local cell division, especially in the early stage of obesity [111, 113, 114]. ATM proliferation is promoted by IL-4, IL-13, IL-6, GM-CSF and MCP-1 [111, 113, 128]. Essentially, blood monocyte depletion does not reduce ATM proliferation in the adipose tissue of obese mice, indicating that in situ proliferation significantly contributes to the expansion of the ATM population, especially during the early stages of obesity [111]. However, as obesity develops, the role of monocyte recruitment in the adipose tissue becomes more significant [113].
While ATM recruitment has been extensively explored, their retention was only recently studied [115, 116]. Chronic macrophage retention significantly contributes to adipose tissue inflammation and involves direct adhesion of macrophages to adipocytes mediated by the interaction of the α4 integrin of macrophages with its counter-receptor vascular cell adhesion molecule-1 (VCAM-1) on adipocytes. Accordingly, VCAM-1 expression increases in the adipose tissue in obesity. Mechanistically, TNF increases VCAM-1 expression in adipocytes and their progenitors. In essence, blockade of α4β1 integrin with the monoclonal antibody natalizumab improves the metabolic profile of mice fed a high-fat diet [116]. Macrophage retention is also mediated by netrin-1, the expression of which is induced in macrophages by palmitate [115]. Netrin-1 restrains macrophage migration and favors macrophage retention in the adipose tissue, thereby contributing to development of insulin resistance [115]. However, not only ATM numbers but also their tissue localization changes in obesity. Characteristically, in the visceral more prominently than in the subcutaneous adipose tissue, ATM form crown-like clusters surrounding dying adipocytes [81, 85, 112, 113, 114, 117, 129, 130]. The exact signals guiding ATM attraction to dying adipocytes to form crown-like structures are not clear. Recently, it was postulated that estrogen receptor β (ERβ) might play a role in this context, since ERβ-deficient mice have increased numbers of crown-like structures in both the subcutaneous and visceral adipose tissue, which consists of ATM highly expressing osteopontin, while treatment with an ERβ-selective agonist reduces crown-like structure numbers [130].
During the course of obesity, ATM acquire pro-inflammatory features [68, 112, 131]. For instance, macrophages in the obese adipose tissue express higher CD11c, inducible nitric oxide synthase, TNF, IL-6 and IL-1β, along with lower IL-10, arginase 1, CD206 and macrophage galactose-type lectin-1 (MGL1) levels, compared to macrophages in the lean adipose tissue [68, 132, 133, 134, 135, 136]. Similar to other macrophages, ATM also undergo metabolic rewiring upon activation: ATM from obese mice display enhanced glycolysis, succinate levels and hypoxia-inducible factor (HIF)-1α activation correlating with elevated IL-1β production [137, 138]. The pro-inflammatory activation of ATM is thought to be driven by different extracellular signals including cytokines, lipids and hormones such as leptin, finally resulting in activation of pro-inflammatory signaling pathways, like the NF-κB and JNK pathways, which mediate inflammatory gene transcriptional programs [5, 6, 39]. Also gut microbiome-derived factors, which increase in the circulation of obese humans and mice, could instigate ATM-mediated inflammation [139, 140]. Obesity is associated with profound alterations in the gut microbiome, referred as dysbiosis and an impaired intestinal barrier, leading to influx of microbes and their circulating components, such as endotoxins [139, 141, 142]. ATM could be affected by these components, as genetic depletion of NOD1, a bacterial peptidoglycan receptor, in hematopoietic cells rendered ATM less inflammatory [140]. Furthermore, extracellular matrix components such as versican and biglycan derived from adipocytes and macrophages, respectively, promote macrophage accumulation and pro-inflammatory activation [143, 144]. Extracellular matrix deposition increases in the adipose tissue during the course of obesity, leading to fibrotic tissue formation [145, 146, 147, 148].
Several lines of evidence suggest that the inflammasome plays a central role in the development of obesity-associated insulin resistance [149, 150, 151, 152, 153, 154]. The production of IL-1β and IL-18 in macrophages is mediated by inflammasome activation [149, 150, 151, 155]. Specifically, the inflammasome component NLR family pyrin domain containing 3 (NLRP3) is highly expressed in macrophages of crown-like structures and activated by palmitate, which is abundantly present in the serum of high-fat diet-fed mice, in a Toll-like receptor (TLR)-dependent manner [152, 156, 157, 158]. Consistently, NLRP3 deficiency blocks inflammasome activation and prevents systemic inflammation and insulin resistance in obese mice [149, 150, 151, 152, 153, 154, 159].
Chronic endoplasmic reticulum (ER) stress is induced in the adipose tissue of obese mice and humans and promotes inflammation [160, 161, 162]. ER stress causes aberrant JNK phosphorylation, which blocks insulin signaling through IRS1 phosphorylation [162]. In accordance with this, genetic ablation of X-box-binding protein-1 (XBP-1), a transcription factor that modulates the ER stress, leads to development of insulin resistance in mice fed a high-fat diet [162]. In accordance with this, inositol-requiring enzyme 1α, a key mediator of ER stress, supports inflammatory activation of ATM, while its myeloid cell-specific deletion in mice restrains diet-induced obesity and insulin resistance [163, 164]. Moreover, C/EBP homologous protein, a downstream component of ER stress, is upregulated in adipocytes of mice fed a high-fat diet, while C/EBP homologous protein ablation is associated with alternative activation of ATM and improvement of insulin resistance [165].
The accumulation of macrophages in the adipose tissue and the switch to a pro-inflammatory macrophage phenotype is tightly linked to the development of insulin resistance [5, 6, 39, 85, 133]. Cytokines secreted by macrophages promote insulin resistance in the adipose tissue, as well as the liver and skeletal muscles [5, 6, 26, 85, 112, 114, 133]. IL-1β suppresses insulin signaling and glucose uptake through inhibition of glucose transporter type 4 translocation to the plasma membrane [166, 167]. Macrophage-secreted factors impair adipocyte insulin sensitivity through downregulation of glucose transporter type 4 translocation to the plasma membrane and reduction of IRS-1 expression, effects which are partially reversed by TNF neutralization [133]. Interestingly, it was recently shown that myeloid-derived IL-6 suppresses insulin resistance in obese mice, in contrast to adipocyte-derived IL-6, which promotes it [44]. Macrophages also produce the lectin galectin-3, which is elevated in obese humans and mice [168]. Galectin-3 enhances macrophage chemotaxis and inhibits insulin signaling through direct binding to the insulin receptor [168]. Accordingly, pharmacologic inhibition or genetic deletion of galectin-3 improves adipose tissue inflammation and insulin resistance [168, 169].
In contrast to the obesity-driven pro-inflammatory phenotype of ATM, an M2-like macrophage phenotype is considered to be protective [5, 6]. Krüppel-like factor 4 (KLF4), a factor mediating M2-like macrophage gene transcription, including Arginase 1, Retnla, and Chi3l3 expression, was found to protect mice against insulin resistance in diet-induced obesity [170]. Moreover, despite its many pro-inflammatory functions, IL-6 was found to promote macrophage alternative activation through upregulation of the IL-4 receptor and thereby ameliorate insulin sensitivity in obese mice [128, 171]. Adiponectin might also shift ATM toward M2-like polarization [172, 173]. Also, the macrophage component of the co-repressor complex G protein pathway suppressor 2 (GPS2) supports insulin sensitivity: its expression in macrophages negatively correlates with systemic and adipose tissue inflammation and diabetes, while GPS2-deficient mice fed a high-fat diet display exaggerated systemic inflammation and impaired glucose intolerance [174].
The inflammatory activation of ATM can be driven by lipids deriving from adipocyte lipolysis and death [39, 76, 175, 176]. Although serum fatty acids also induce inflammatory activation of macrophages through TLR4 signaling, plasma lipids may play a less significant role in ATM activation [177, 178]. Adipocyte-derived sphingolipids were shown to regulate ATM accumulation in obesity, evidenced by increased numbers of crown-like structures in the subcutaneous adipose tissue of mice with adipocyte-specific deletion of serine palmitoyltransferase, the rate-limiting enzyme of sphingolipid biosynthesis [179]. Along the same line, inflammatory ATM of obese mice display greater lipid content and particularly relatively more short-chain saturated lipid species compared to their M2-like counterparts [180]. It is thought that ingestion of adipocyte debris leads to lipid accumulation in ATM, justified by the fact that macrophages in crown-like structures are lipid-laden [74, 76, 176, 181]. Mechanistically, fatty acids bind fetuin-1, which directly interacts with TLR4, triggering its activation [182, 183]. In accordance with this, very-long-chain-GM3 gangliosides, the serum levels of which are elevated in obesity, were recently found to regulate TLR4 activation [184]. However, reduced lipid storage in ATM as a result of myeloid cell-specific deletion of HILPDA (hypoxia-inducible lipid droplet associated), a physiological inhibitor of ATGL (adipose triglyceride lipase)-mediated lipolysis, does not affect adipose tissue inflammation [185]. Moreover, fatty acid-mediated inflammatory macrophage activation is restrained by acyl CoA: diacylglycerol acyltransferase 1 (DGAT1), which increases the capacity of macrophages for triglyceride storage, as shown in mice overexpressing DGAT1 in macrophages and adipocytes [186]. On the other hand, several lipids may also negatively regulate ATM activation [1]. For instance, branched fatty acid esters of hydroxy fatty acid isomers, such as palmitic acid-9-hydroxystearic acid (9-PAHSA), which are endogenously synthesized, increase in obesity and correlate with insulin sensitivity [187]. PAHSA administration to mice mitigates adipose tissue inflammation in obesity, lowering the abundance of TNF+ and IL-1β+ ATM [187]. Also treatment of mice with the ω-3 fatty acids docosahexa-enoic acid and eicosapentaenoic acid restrains ATM inflammatory activation and insulin resistance [188]. Both, PAHSA and ω-3 fatty acids mediate their effects through the G protein-coupled receptor 120 (GPR120) [187, 188]. In accordance with this, GPR120 deficiency in mice fed a high-fat diet aggravates obesity, liver steatosis, insulin resistance and ATM accumulation, while presence of a GPR120 mutation in humans correlates with development of obesity [189]. Additionally, PAHSA activates GPR40, which also mediates its protective effects against insulin resistance [190].
ATM are not a uniform cell population but consist of distinct cell populations, as shown by single-cell transcriptomic analyses [181, 191, 192]. Macrophages within crown-like structures are CD9+ and lipid-laden and can form multinucleated giant cells through cell fusion [76, 181, 191, 193]. ATM outside of crown-like structures are pro-inflammatory and marked by CD11c [191]. Depletion of CD11c+ cells rapidly improves adipose tissue inflammation and insulin resistance [194]. Interferon regulatory factor 5 (IRF5) is expressed in CD11c+ cells and ATM in obesity, mediating pro-inflammatory macrophage activation [195, 196, 197]. IRF5 depletion favors alternative activation of ATM and reduces adipocyte hypertrophy and adipose tissue fibrosis in obese mice [195].
Although exhibiting pro-inflammatory features, macrophages in the obese adipose tissue clearly differ from classically activated (M1-like) macrophages, which are activated upon acute inflammation [74, 198, 199, 200]. Instead, ATM get rather metabolically activated [199]. ATM in obese humans present upregulation of peroxisome proliferator-activated receptor gamma (PPARγ) and p62-driven lipid metabolism, exemplified by increased ABCA1 and CD36 expression [198]. Lipid-associated ATM are positive for triggering receptor expressed on myeloid cells 2 (TREM2), a key membrane protein for phagocytosis and lipid uptake [181]. TREM2-deficient mice display adipocyte hypertrophy, systemic hypercholesterolemia, and glucose intolerance [181]. Accordingly, lipid-associated macrophages show increased transcriptional programs related to phagocytosis and endocytosis, lysosome function, PPARγ signaling and oxidative phosphorylation [181]. Moreover, in the obese adipose tissue, macrophages are featured by enhanced lysosome biogenesis associated with lipid catabolism, while inhibition of macrophage lysosomal function interferes with their lipid metabolism, resulting in increased lipid accumulation in macrophages [201]. Lysosomal exocytosis was found to be required for clearance of dead adipocytes by macrophages, a process which is mediated by NADPH oxidase 2 [74].
Finally, ATM were demonstrated to regulate the thermogenic capacity of white and brown adipose tissue and thereby control whole body energy expenditure and insulin resistance [54, 55, 116, 202, 203]. Mechanistically, the adhesive interaction of macrophages and adipocytes through α4 and VCAM-1 was found to downregulate UCP1 expression in adipocytes in an extracellular signal-regulated kinase (ERK) 1/2-dependent manner, while genetic deletion or pharmacological inhibition of α4 in mice resulted in increased UCP1 expression in the subcutaneous adipose tissue [116]. Moreover, cold exposure promotes macrophage alternative activation in an eosinophil-IL-4-dependent manner [202]. The cold exposure-induced M2-like macrophage accumulation and beige adipogenesis could be adiponectin-dependent [203]. Meteorin-like (Metrnl), a circulating factor released by muscles after exercise and adipose tissue upon cold exposure, was also shown to promote M2-like polarization of ATM via stimulation of the eosinophil-IL-4 axis [61]. M2-like ATMs were actually suggested to promote thermogenesis via their production of catecholamines [54, 55, 202], although these findings were debated by other studies [204, 205].
In conclusion, ATM determine adipose tissue function in health and disease. They present heterogeneity and exert a wide spectrum of functions, ranging from lipid scavenging, phagocytosis and cytokine production to regulation of thermogenesis [2, 26, 39, 78]. Overall, they may exhibit both beneficial and harmful functions depending on their type of activation, location and the stage of the metabolic disease [74, 192] (Fig. 2). Exploring the mechanisms governing their function will be key for the development of therapeutic strategies against obesity-related inflammation. To this end, changes in their metabolic signature, phagocytic function and inflammatory activation should be analyzed in close conjunction ideally using single-cell techniques.
Eosinophils
Eosinophils are innate immune cells mediating type 2 responses, such as allergies and responses to parasitic infections [206, 207]. In the adipose tissue, their relative numbers are low and even more reduced in obesity [57]. High-fat diet feeding rapidly reduces chemotactic signals for eosinophils, and eosinophil numbers drop first in the visceral followed by the subcutaneous adipose tissue [208, 209]. IL-5 plays a crucial role in the maintenance and expansion of eosinophil cell populations, and its overproduction in transgenic mice leads to eosinophil accumulation in the adipose tissue [57, 59, 210]. ILC2 are important producers of IL-5 in several tissues, including the adipose tissue, evidenced by the fact that deletion of ILC2 leads to decreased eosinophil numbers in the adipose tissue and attenuates their increase after parasitic infection [59, 210].
Adipose tissue eosinophils play a critical role in the maintenance of tissue homeostasis [57, 59, 211]. They are a major source of IL-4 and thereby support ATM alternative activation [54, 57, 59] (Fig. 2). Eosinophil-deficient mice fed a high-fat diet develop more severe adiposity, glucose intolerance, and adipose tissue inflammation [57]. Conversely, high-fat diet-fed mice with helminth-induced hypereosinophilia display reduced obesity and an improved metabolic phenotype [57, 212, 213]. Also honeybee pollen extract-induced hypereosinophilia in mesenteric and gonadal adipose tissue restrains insulin resistance in ob/ob mice [214]. Along the same line, treatment of obese mice with IL-25 induces infiltration of ILC2, eosinophils, and M2-like macrophages in the visceral adipose tissue; improves glucose tolerance; and induces weight loss [215]. Moreover, mice overexpressing eotaxin, an eosinophil chemoattractant, in the adipose tissue display reduced adipose tissue mass and improved glucose tolerance [216]. In accordance with this, application of exogenous IL-4 increases insulin sensitivity and attenuates weight gain and adipose tissue expansion in mice fed with a high-fat diet [62, 63]. In contrast, deficiency of STAT6, the transcription factor mediating the majority of the effects of IL-4, decreases insulin sensitivity in mice with diet-induced obesity [62]. Mechanistically, IL-4 inhibits adipogenesis by downregulating the expression of PPARγ and CCAAT/enhancer-binding protein α and favoring lipolysis by enhancing the activity and translocation of hormone-sensitive lipase in mature adipocytes [217, 218]. Moreover, IL-4 production by eosinophils was reported to promote thermogenesis (beiging) in the white adipose tissue and thereby increase energy expenditure and glucose tolerance in mice [54, 56]. Although abundance of eosinophils in the adipose tissue was shown by many studies to correlate with beiging [54, 56, 213, 219], the involved mechanisms, such as the implication of M2-like macrophages, have been debated [204, 205]. Moreover, restoring eosinophil numbers in obese mice to physiological levels through treatment with recombinant IL-5 did not improve glucose tolerance, adiposity, or beiging [220]. In contrast, in humans, circulating and subcutaneous adipose tissue eosinophil counts positively correlate with obesity and associated metabolic disturbances [221, 222, 223].
Finally, type 2 immunity can also have detrimental effects owing to promotion of fibrosis [31, 61, 224]. Progression of nonalcoholic fatty liver disease (NAFLD) may be associated with increased eosinophilic type 2 inflammation in humans and mice, and IL-10/IL-4 deficiency or inhibition of TGF-β and IL-13 signaling confers resistance to NAFLD in mice [225]. In the white adipose tissue, interstitial fibrosis is destructive for adipose tissue elasticity and adipocyte function, and the frequency of profibrotic adipocyte progenitors in the adipose correlates with insulin resistance [24, 31].
Neutrophils
Neutrophils accumulate in the adipose tissue within the first days upon start of a high-fat diet feeding in mice [69, 226, 227]. Metabolically activated macrophages might attract neutrophils through nucleotide release [228]. Neutrophil recruitment in the adipose tissue is also dependent on phospholipase A2α (cPLA2α) and neutrophil elastase [69, 229]. Increased neutrophil elastase activity in the adipose tissue and decreased serum levels of its inhibitor, serpinA1, were reported in obese humans and mice [69, 230]. Elastase mediates several pro-inflammatory effects of neutrophils in the adipose tissue [69, 230]. Its genetic deletion reduces neutrophil and macrophage accumulation in the adipose tissue, reduces body weight gain and improves glucose tolerance and insulin sensitivity in mice fed with a high-fat diet [69, 230]. Neutrophil elastase-deficient mice also display enhanced fatty acid oxidation in the liver and improved liver steatosis [230]. In accordance with this, pharmacological inhibition of neutrophil elastase ameliorates insulin resistance and reduces obesity [230]. Mechanistically, elastase was shown to impair insulin signaling via degradation of IRS1 [69, 230]. Circulating neutrophils of obese subjects also show inflammatory traits, such as enhanced release of myeloperoxidase (MPO), MMP9, and CCL2 (IL-8) [231]. In accordance with this, MPO levels and activity increase in gonadal adipose tissue of mice fed with a high-fat diet [227]. MPO deficiency halts obesity, macrophage infiltration, and adipose tissue inflammation and improves insulin sensitivity [227]. Hence, although only few studies have investigated the role of neutrophils in obesity, there is a clear consensus that they contribute to adipose tissue inflammation (Fig. 2).
Dendritic Cells
DC instruct adaptive immunity through antigen presentation [232]. They share many surface markers with macrophages such as F4/80, CD11b, MHCII, C-X3-C motif chemokine receptor 1, and CD11c [233, 234], although in contrast to macrophages, they do not express CD64 [209, 235]. Due to their similarities to ATM, their function in the adipose tissue has been hard to distinguish from that of ATM [233, 234]. Adipose tissue DC can be conventional DC (cDC, CD11b−CD11c+) or plasmacytoid DC (pDC, CD11b−CD11c+B220+) [209, 235, 236, 237, 238]. Although initially their numbers in adipose tissue were suggested to increase with obesity [234, 235, 236, 238], a later report showed that DC numbers normalized to adipose tissue mass are not altered in obesity, but they rather decrease as a proportion of all stromal-vascular fraction cells due to the massive expansion of other immune cell populations [209]. DC preferentially accumulate in the perinodal adipose tissue, where they detect antigens traveling from the adipose tissue to the draining lymph node and thereby coordinate immunity in the adipose tissue [239, 240]. MHCII depletion in CD11c-expressing cells leads to reduced T cell receptor expression in CD4+ T cells, less CD4+ and more CD8+ cells in the adipose tissue [241].
CD11c+ cell depletion protects against chronic inflammation and insulin resistance in mice with diet-induced obesity; however, these effects could be attributed to the loss of CD11c+ inflammatory macrophages [194, 234, 236]. In accordance with this, high-fat diet-fed mice deficient for FMS-like tyrosine kinase 3 ligand, which display low DC levels, have decreased adipose tissue mass and liver steatosis, improved insulin sensitivity and glucose tolerance, and reduced macrophage infiltration in adipose tissue and the liver [236, 242]. CCR7-deficient mice also have decreased DC numbers in the adipose tissue and present reduced adipose tissue inflammation and insulin resistance [235]. The adipokine chemerin, which is upregulated in the circulation of obese compared to lean individuals, attracts pDC to the visceral adipose tissue and pDC deficiency reduces diet-induced obesity [238, 243, 244].
In contrast, GM-CSF-deficient mice, also presenting stark reduction in DC numbers, show increased whole body adiposity [245]. Moreover, steady-state conventional DC in the visceral adipose tissue were shown to have protective functions delaying the onset of obesity and adipose tissue inflammation [246]. Specifically, activation of the Wnt/β-catenin in CD11chiMHCII+CD11b− cells (termed as cDC1) induces IL-10 production and activation of the PPARγ pathway in CD11chiMHCII+CD11b+ cells (cDC2) suppresses their proinflammatory activation [246]. In combination, these effects lead to a delay in the onset of inflammation and insulin resistance in mice fed with a high-fat, high-sugar (Western) diet [246]. However, this control mechanism is bypassed after long-term overnutrition through inhibition of the β-catenin and PPARγ pathways in DC [246]. Taken together, the role of DC in adipose tissue inflammation is so far unclear.
Innate Lymphoid Cell 2
ILC2 play a central role in type 2 immunity [58, 210, 247, 248, 249, 250, 251, 252]. They react in response to IL-33 or IL-25 secreting IL-4, IL-5, and IL-13 [56, 58, 59, 247, 248, 249, 250, 251, 252] (Fig. 2). They reside in the subcutaneous and visceral adipose tissue and are thought to preserve tissue homeostasis [56, 58, 59, 60]. For instance, helminth infection triggers ILC2 activation in the mesenteric adipose tissue, which is required for goblet cell hyperplasia and helminth expulsion [58, 59]. Maintenance and expansion of their population in the adipose tissue are driven by IL-33 [56, 58, 59, 60]. In obese humans and mice, the function of ILC2 in the adipose tissue is severely compromised due to reduced IL-33 receptor expression [60]. Moreover, IL-33 serum and adipose tissue levels drop in obese mice [253]. In accordance with this, IL-33-deficient mice have decreased ILC2 numbers per gram of adipose tissue and present greater adiposity when fed a high-fat diet [60]. Along the same line, treatment of obese mice with IL-25 leads to accumulation of ILC2 in the visceral adipose tissue, weight loss and improvement of glucose tolerance [215]. In contrast, depletion of ILC2 in obese Rag1-deficient mice leads to exacerbated weight gain and glucose intolerance, while adoptive transfer of ILC2 has the opposite effects [215]. Mechanistically, IL-33 induces the expression of death receptor 3 (DR3) in ILC2, which triggers their stimulation through activation of the NF-κB pathway [254]. On the other hand, TNF and IL-33 increase programmed cell death protein 1 (PD-1) expression in ILC2 in the adipose tissue of obese mice [255]. PD-1 on ILC2 interacts with PD-L1 on macrophages, promoting inflammatory activation of the latter [255]. In contrast, PD-1 inhibition partially restores eosinophil and M2-like macrophage numbers in the adipose tissue, thereby improving glucose tolerance [255].
ILC2 are important producers of IL-5, which is a key cytokine for maintenance of eosinophil and consequently M2-like macrophage populations in the adipose tissue [54, 56, 57, 59]. Moreover, they produce IL-13, which promotes macrophage alternative activation [215]. ILC2 accumulation in response to IL-25 correlates with increased numbers of adipose tissue eosinophils and M2-like macrophages [215]. IL-33 treatment also increases eosinophil numbers in the adipose tissue [56]. Hence, the ILC2-IL-5 axis was suggested to combat adipose tissue inflammation and insulin resistance through supporting the function of eosinophils and M2-like macrophages [57, 59, 215] (Fig. 2).
The protective functions of ILC2 also involve induction of beige adipogenesis [56, 60]. IL-33-deficient mice fed a high-fat diet display reduced energy expenditure and beiging, while IL-33 treatment or transfer or IL-33-elicited ILC2 have the opposite effects [56, 60, 253]. Whether eosinophils and IL-4/IL-13 signaling are required for ILC2-induced beiging is a matter of debate [56, 60]. Lee et al. [56] showed that IL-4 receptor deficiency abrogates the IL-33-induced beiging. Moreover, they showed that IL-4 and IL-13 produced by ILC2 and eosinophils target adipocyte progenitors, inducing their proliferation and metabolic reprogramming into thermogenic (UCP-1-expressing) cells [56]. However, Brestoff et al. [60] suggested that ILC2-induced beiging is eosinophil- and IL-4-independent and, instead, is mediated by methionine-encephalin, which is produced by ILC2 and directly promotes beiging.
ILC2 are also required for accumulation of regulatory T cells (Treg) in the adipose tissue in response to IL-33 or helminth infection [256, 257]. ILC2 and Treg cells colocalize and interact via association of ICOS with ICOSL rather than through type 2 cytokines [256]. Moreover, ILC2 can interact with Treg through the co-stimulatory molecule OX40L [258]. IL-33 induces upregulation of OX40L in adipose tissue ILC2 and consequently Treg expansion, while ILC2-specific OX40L deletion blunts Treg expansion in response to IL-33, parasite infection or allergy [258]. Transgenic mice overexpressing IFN-γ present reduced ILC2, eosinophil and Treg cell numbers in the adipose tissue [256], thereby providing a potential explanation of the reduction in eosinophil and Treg numbers in the obese adipose tissue [57, 259]. In accordance, CD8+ T cells, being important producers of IFN-γ, promote shrinkage of ILC2 and eosinophil adipose tissue populations [256, 260, 261].
NK Cells
NK cells are innate immune cells with lymphoid origin [262, 263]. They are activated by IL-12, IL-18 and IFN-γ and promote type 1 immune responses [262]. Their numbers are more pronounced in the visceral compared to the subcutaneous adipose tissue and, essentially, increase in obesity, indicating that NK cells are involved in aggravated adipose tissue inflammation [264, 265, 266]. Moreover, NK numbers in the circulation of obese subjects correlate with insulin resistance [264]. In mice with diet-induced obesity, NK numbers and activation are elevated in the gonadal but not in the subcutaneous adipose tissue [71, 267, 268]. In accordance with this, expression of IL-15, a cytokine promoting NK cell proliferation and activation, is upregulated in visceral ATMs in obese mice [71, 267]. Plasma IL-15 levels also correlate with visceral adipose tissue mass in aged humans [269].
The cross talk between NK cells and ATM plays a critical role in adipose tissue inflammation (Fig. 2). NK cell ablation with neutralizing antibodies or genetic deletion restrains macrophage accumulation in the visceral adipose tissue, while macrophage infiltration in the subcutaneous adipose tissue and spleen is not affected [71, 267, 268]. This is associated with amelioration of insulin sensitivity in mice with diet-induced obesity [268]. Conversely, IL-15 administration leading to increased NK cell populations or their reconstitution in NK cell-deficient mice, lacking E4 promoter-binding protein 4 (E4bp4), increases adipose tissue inflammation and exacerbates obesity-induced insulin resistance [267]. Mechanistically, it was shown that adipocyte-mediated production of ligands of the NK cell-activating receptor (NCR1) stimulates NK cell proliferation and IFN-γ release, which promotes ATM activation and insulin resistance in obesity [71]. NCR1 or IFN-γ deficiency abrogates ATM accumulation in the visceral adipose tissue and improves insulin sensitivity [71]. Interestingly, NK cells in the subcutaneous adipose tissue of obese individuals display lower expression of the signaling molecules NKp30 and NKp44, which might suggest that they acquire a phenotype less efficient in the defense against neoplastic cells [270].
Mast Cells
Mast cells mediate IgE-driven type 2 immune responses [271, 272], while broad pro-inflammatory functions were also ascribed to them [273, 274]. The adipose tissue contains precursors, which can differentiate to mature mast cells [275, 276]. Mast cells accumulate in the white adipose tissue of obese humans and mice [277, 278, 279, 280]. Accordingly, tryptase and chymase levels are increased in the adipose tissue of obese compared to lean individuals and more tryptase- and chymase-positive mast cells are found in both the subcutaneous and omental adipose tissue in obese diabetic patients [280, 281]. Moreover, mast cell accumulation in subcutaneous adipose tissue positively correlates with serum glucose, leptin, IL-6, triglycerides and homeostatic model of assessment-insulin resistance (HOMA-IR) in patients with metabolic syndrome [280]. In contrast to these findings, obese individuals with high mast cell accumulation in visceral or subcutaneous adipose tissue were shown to be metabolically healthier, displaying a better weight loss response following bariatric surgery [282].
Macrophages promote the accumulation of mast cells in the adipose tissue [283]. In turn, mast cells were suggested to produce more IL-6 and MCP-1 in obesity, thereby contributing to adipose tissue inflammation [281]. Moreover, thermoneutrality also favors mast cell accumulation in adipose tissue [284]. Genetic ablation of mast cells or pharmacological inhibition of their degranulation was suggested to decrease macrophage infiltration and activation, attenuate adipose tissue and systemic inflammation, reduce body weight gain and ameliorate glucose tolerance and energy expenditure in mice fed with a Western diet [277, 279, 283]. These effects were reversed by reconstitution of mast cells and dependent on IL-6 and IFN-γ expression in mast cells [277, 279, 285]. The protective effects of blockage of mast cell degranulation were dependent on high dietary amounts of cholesterol present in the Western diet [286].
Mast cells were also suggested to promote adipogenesis, adipose tissue fibrosis and adipocyte senescence [278, 279, 283]. Mast cells in the lean adipose tissue express negligible amounts of leptin, while its expression is increased in mast cells in the obese adipose tissue. Adoptive transfer of leptin-deficient mast cells expanded ex vivo restrained obesity and insulin resistance in obese mice. Mechanistically, leptin-deficient mast cells were found to promote alternative activation of macrophages [287]. Additionally, mast cells were demonstrated to negatively impact adipose tissue thermogenesis through serotonin synthesis [277, 284, 288], while adoptive transfer of mast cells deficient in tryptophan hydroxylase 1, the rate-limiting enzyme regulating peripheral serotonin synthesis, attenuates adiposity and insulin resistance and increases energy expenditure and beiging [284, 288].
However, most of these findings were based on the use of Kit mutant mast cell-deficient mice, which have several hematopoietic perturbations [277, 279, 289] and could not be reproduced in 2 other mast cell-deficient mouse lines, which express normal levels of functional Kit: the Cpa3Cre/+ mice and mice generated by crossing Mcpt5-Cre to the R-DTA mouse line [290, 291]. Hence, mast cell deficiency, in the absence of Kit mutations, might actually not play any role in the regulation of adiposity or insulin resistance [290, 291]. Overall, although mast cell numbers unequivocally rise in the obese adipose tissue, their role in the context of metabolic diseases remains elusive [277, 278, 279, 280, 281].
In conclusion, innate immune cells in the adipose tissue communicate with each other and their surrounding tissue through complex cytokine and other humoral factor networks, as well as through cell-to-cell contact. Pro-inflammatory macrophages, neutrophils, and NK cells perpetuate adipose tissue inflammation, while alternatively activated macrophages, eosinophils, and ILC2 mediate type 2 immune responses and sustain metabolic health (Fig. 2). Although the mechanisms mediating the crosstalk between ATM-NK cells and ILC2-eosinophil-alternatively activated ATM have been intensively investigated, less is known on whether and how pro- and anti-inflammatory innate immune cells suppress one another. For instance, TNF produced by macrophages, neutrophils or adipocytes could inhibit the production of IL-4 by eosinophils and ILC2 or directly impede M2-like macrophage polarization [292, 293]. Such regulatory mechanisms could orchestrate the progression of adipose tissue inflammation and merit further investigation.
A Balancing Act: Oxygen Availability, Endothelium and Immune Cells
Oxygen availability in the adipose tissue is a key determinant of its metabolic profile [294], with the vasculature being critical in maintaining oxygen homeostasis [295, 296]. Adipose tissue hypoxia is the result of (a) imbalance of the hypertrophic adipocytes and a compromised adipose vasculature [295, 297] and (b) elevated oxygen consumption in adipocytes during early stages of adipose tissue expansion, the latter has been termed “relative adipocyte hypoxia” [298]. Interdependence between hypoxia and inflammatory diseases, including obesity, is widely documented [294, 299].
Oxygen Availability, HIFs and Immune Cells in Obesity
In response to hypoxia, the master regulators of this adaptive response, the HIFs are activated [300]. HIFs are basic helix-loop-helix transcription factors and consist of a constitutively expressed β subunit (HIF-1β) and an oxygen-dependent HIF-α subunit [300]. HIF-1α is ubiquitously expressed in all tissues [301], whereas HIF-2α is predominantly expressed in endothelial cells in highly vascularized tissues [302]. At normal oxygen levels, HIF-α is negatively regulated by HIF-prolyl hydroxylases (PHD1, PHD2, and PHD3). All three hydroxylate two proline residues (Pro402, Pro564 in HIF-1 and Pro405, Pro531 in HIF-2) in the oxygen-dependent degradation domain of HIF-α [303], allowing proteasomal degradation of HIF-α [304, 305]. Under hypoxic conditions, HIF-α hydroxylation is inhibited, leading to its stabilization [304, 305, 306] and upregulation of a plethora of genes involved in glucose and lipid metabolism, angiogenesis and inflammation [307].
Although the mechanisms by which HIFs regulate immune cell function are not fully understood, it is well accepted that HIFs act as a regulatory hub that link metabolic activity with immune responses (immunometabolism) [308]. HIF-1-dependent increased glycolysis is associated with the activation of macrophages, neutrophils, DC, NK cells and cells of the adaptive immunity [308, 309]. For example, in neutrophils, HIF-1 increases their life span and bactericidal capacity [310] by a time-dependent induction of key glycolytic enzymes glyceraldehyde 3-phosphate dehydrogenase and triosephosphate isomerase [311] or induction of glycogen synthase [312]. The seminal work of Cramer and colleagues [313] demonstrated that HIF-1 deletion in myeloid cells causes depletion in the ATP cellular pool, impairs myeloid cell motility and induces infiltration in models of experimental arthritis. Interestingly, opposite to HIF-1, HIF-2 deficiency does not alter cellular ATP production [314]. Myeloid cells can undergo metabolic reprogramming to adapt to changes in their local microenvironment. For example, when macrophages are exposed to LPS, the levels of the tricarboxylic acid cycle intermediate succinate are elevated. Succinate induction further stabilizes HIF-1, leading to increased IL-1β production [138]. Opposing roles of HIF-1 and HIF-2 are also reported for their regulation on eosinophil chemotaxis, with HIF-1-deletion reducing chemotaxis and with HIF-2-deletion increasing chemotaxis [315]. DC-specific HIF-1 deletion leads to significantly higher body weight loss, increased pro-inflammatory cytokines production, and severe intestinal inflammation in a model of experimental colitis compared to HIF-1 proficient DC [316]. In ILC2 cells, the VHL-HIF-1-glycolysis pathway acts as a checkpoint for their terminal differentiation [317]. Activation of HIF-1 (via VHL deletion) elevates the glycolytic enzyme pyruvate kinase M2, which in turn downregulates the IL-33-ST2 pathway, thus directly impacting ILC-2 maturation [317]. Finally, in obese mice, it was recently demonstrated that palmitate upregulates ATM glycolysis and HIF-1 activation and induces IL-1β in macrophages [318]. Thus, enhanced glycolysis and HIF-1α activation in ATM could be partially driving the low-grade inflammation in obesity.
The role of HIFs in adipose tissue inflammation was demonstrated in several studies using mouse models of targeted HIF modulation in adipocytes (Fabp4Cre) but with contrasting results. Briefly, deletion of HIF-1, in most studies, reduces adipose tissue inflammation, whereas HIF-1 overexpression increases macrophage infiltration in white adipose tissue and leads to insulin resistance [38, 319]. In contrast, deletion of HIF-2 in adipocytes leads to both white and brown adipose tissue inflammation (higher numbers of F4/80+ macrophages) and consequently negatively impacts systemic insulin resistance in obesity [129]. Stabilization of both isoforms (via deletion of their negative regulator, PHD2) in adipose tissue provides metabolic flexibility and does not affect immune cell population infiltration under basal conditions [320] but reduces macrophage infiltration in high-fat diet-fed mice [321].
Fewer studies addressed the role of HIFs in myeloid cell populations (LysMCre) in the context of diet-induced obesity, again with contrasting results. Myeloid cell-specific HIF-1 deletion protects against adipose tissue inflammation and reduces macrophage crown-like structure formation [318, 322]. However the role in glucose and insulin tolerance is controversial, with one study showing that myeloid HIF-1 deletion protected from the development of systemic insulin resistance in obese mice [322], whereas the second study showed no effect [318]. In another study, however, deletion of HIF-1 in myeloid cells had no effect on the inflammatory state of adipose tissue after a shorter (8 weeks) high-fat-feeding protocol, and the transgenic mice were heavier with slightly elevated glucose levels [323]. Deletion of HIF-2 in myeloid cells has no impact on diet-induced obesity and associated metabolic dysregulation [129]. In vitro, HIF-2 adenoviral overexpression in peritoneal macrophages facilitated the M2-like polarization by increasing the expression of arginase 1, suppressing NO production and reducing TNF, IL-6, and IL-1β expression [324]. Coculture of adipocytes with HIF-2-overexpressing macrophages reduces IL-6 expression in adipocytes and restores the insulin-stimulated glucose ability in adipocytes [324]. In contrast, high-fat-fed haplodeficient HIF-2 (Hif2a+/−) mice show increased number of crown-like structure in adipose tissue and systemic insulin resistance [324]. However, in the latter study, it cannot be concluded that macrophage HIF-2 is responsible for the impaired insulin response as HIF-2 levels are universally reduced. These findings suggest that HIF-2 could be a potential regulator of the cross talk between macrophages and adipocytes and are consistent with the notion that HIF-1 is required for M1 polarization of macrophages, whereas HIF-2 for M2-like polarization [325]. More recently, it was shown that deletion of PHD2 in myeloid cells increases ATM infiltration and leads to insulin resistance in high-fat-fed mice via enhanced interleukin-1 receptor associated kinase-M (Irak-M) expression [326]. The authors concluded that the observed effects were due to HIF-1 activation, although only elevated HIF-1a mRNA, but not protein, levels were reported and the role of HIF-2 was not addressed. Together, these studies raise the divergent roles of HIF-1 and HIF-2 in adipose tissue inflammation. This divergence has also been documented by a number of studies in the context of developmental processes, inflammatory diseases, or cancer [327]. Given that advanced PHD inhibitors, which do not pose HIF isoform specificity, are now under consideration for FDA approval for the treatment of renal anemia [328], it is important to carefully evaluate systemic and off-target effects, especially in the context of obese adipose tissue and its inflammatory chronicity.
Adipose Endothelium and Innate Immune Cells in Obesity
The endothelium, apart from its central role in supplying nutrients and oxygen to the tissues, facilitates the extravasation of leukocytes form the blood stream into the tissue via expression of selectins, such as E- and P-selectins, and adhesion molecules, such as intercellular adhesion molecule 1 and VCAM-1, enabling rolling and adhesion of immune cells [329]. In mouse models of obesity, it is well documented that there is increased leukocyte-endothelial cell-platelet interaction in the microcirculation of visceral adipose [117]. Moreover, in the obese adipose tissue, increased P-selectin expression and formation of monocyte-platelet conjugates suggests platelet activation [117]. However, changes in the leukocyte adhesion cascade in the obese adipose tissue have been little investigated.
Pericytes, another less-studied cell-type of the vasculature, was shown to regulate some aspects of the immune response. Pericytes are present outside of capillaries, and they are identified by expression of the growth factor receptor PDGFRβ and the proteoglycan, neuron-glial antigen 2 (NG2), which is a PDGF co-receptor [330]. NG2+ pericytes express inflammation-sensing receptors, such as TLR4 and TLR2, which allow them to sense inflammatory cues and release chemoattractants such as CXCL1, MIF and CCL2, thereby promoting leukocyte recruitment and survival [331]. In turn, ATM inhibit pericyte detachment from blood vessels, which results in less vascularization [332].
Recently, a new type of macrophages was described in epididymal adipose tissue, named vasculature-associated ATM (VAM) [209]. VAM are tissue-resident macrophages that are tightly associated with blood vessels, displaying very high endocytic capacity. VAM appears to be very sensitive to different environmental changes (high-fat diet, fasting, and inflammatory stimuli) [209]. Both nutritional and inflammatory acute stresses cause rapid reduction of VAM numbers, yet VAM maintain capacity for rapid recovery [209]. Open questions remain such as whether VAM are regulated by hypoxic stress and whether VAM depletion affects the interactions with other resident immune cells in the adipose tissue.
A number of studies showed that during diet-induced obesity upregulation of the vascular response directly via VEGF-A overexpression [127, 333] or indirectly via activation of the hypoxia response [320, 321] has advantageous metabolic outcomes. These studies also point to the distinct roles of HIF-1 and HIF-2 in the vascularization process. For example, HIF-1α regulates mainly endothelial cell proliferation, migration and sprouting, whereas HIF-2α controls vascular morphogenesis [334]. Finally, failure to induce a vascular response in adipose tissue hypoxia can lead to HIF-1-dependent fibrogenesis by inducing the expression of ECM-modifying factors (i.e., TIMP-1 and PAI-1), connective tissue growth factor, and lysyl oxidase genes [38]. Scar formation may further restrict blood supply and oxygen availability, spawning a pathological cycle that disrupts further adipose tissue plasticity and promotes adipose tissue inflammation.
Adipose Tissue Inflammation Is Long-Lasting, but Is It Reversible?
The chronicity of inflammation in obesity is associated with development of life-threatening diseases, such as cardiovascular complications, and might even predict COVID-19 severity [84, 335, 336]. As outlined above, in obesity, continuous presence of inflammatory stimuli, such as cytokines, adipokines, circulating lipids, and endotoxins, drive chronic inflammation of innate immune and nonimmune cells (e.g., endothelial cells and adipocytes). However, inflammation may also have long-lasting effects through induction of “trained immunity” [337, 338, 339, 340, 341]. Trained immunity refers to the capacity of innate immune cells, such as monocytes, macrophages and NK cells, to “remember” inflammatory encounters with pathogens and respond in a more sensitized manner upon reencountering pathogens [337, 338, 339, 340, 341]. Its long-lasting effects largely depend on epigenetic and metabolic cellular reprogramming [154, 337, 338, 339, 340]. It is possible that also obesity-associated inflammation guides myeloid cell training [338, 341]. This is evidenced by studies showing that switching from a Western to a chow diet in Ldlr-deficient mice does not reverse myeloid cell inflammatory responses although it reduces systemic inflammation [154]. Adipose tissue, and particularly the visceral rather than the subcutaneous adipose tissue, might also possess immunological memory and thereby still display an inflammatory profile after returning from a high- to a low-calorie diet, despite weight reduction, improvement of glucose intolerance and amelioration of other metabolic parameters [342]. ATM, adipocytes, adipocyte precursors and endothelial cells might all undergo “immunological training” during obesity [341, 343]. Essentially, induction of trained immunity in obesity might have important clinical implications. In people who were previously obese, trained immunity could instigate stronger inflammation upon weight regain. Moreover, trained immunity could promote an excessive inflammatory response upon infection in obese subjects, as in COVID-19 [11, 12, 336].
Essentially, trained immunity also involves adaptive responses of hematopoietic progenitor cells in the bone marrow [338]. Obesity and diabetes are associated with altered hematopoiesis, resulting in neutrophilia and monocytosis due to increased proliferation and expansion of bone marrow myeloid progenitors [158, 338, 344, 345]. TLR4 and its downstream molecules MyD88 (myeloid differentiation primary response 88) and TRIF (TIR domain-containing adapter protein-inducing interferon-β) are essential mediators of myelopoiesis in obese mice [346]. High-fat diet feeding-associated changes in the gut microbiome were shown to skew hematopoietic stem cell differentiation toward myelopoiesis through reshaping the bone marrow niche [347]. Increased production of S100A8/S100A9 by neutrophils mediates enhanced myelopoiesis through activation of receptor for advanced glycation end products (RAGE) in common myeloid progenitor cells [344]. Moreover, inflammasome-dependent IL-1β production in ATM also contributes to hematopoietic stem cell reprogramming [158]. In turn, increased myelopoiesis contributes to ATM accumulation and sustains chronic inflammation, thereby promoting metabolic disease [341, 345, 346]. Along these lines, it was suggested that trained immunity might link obesity with the development of atherosclerosis and cardiovascular disease [341]. However, only recently, the importance of trained immunity in obesity was recognized, hence so far it has been barely studied.
Despite the long-lasting effects of inflammation in metabolic disease, several lines of evidence suggest that it could be manageable by pharmaceutical interventions [348]. Several drugs against obesity were developed and clinically administered, but many were withdrawn due to occurrence of side effects and reduced efficacy [349, 350]. Currently approved drugs include a blocker of pancreatic lipase inhibiting fat absorption (orlistat), appetite suppressors (phentermine/topiramate, lorcaserin, and naltrexone/bupropion), and a glucagon-like peptide-1 agonist (liraglutide) [349, 350]. Antidiabetic agents, such thiazolidinediones, metformin, and dipeptidyl peptidase-4 inhibitors can reduce inflammation [348]. Reduction of hyperglycemia restrains monocytosis in obese mice [344]. Moreover, treatment of obese diabetic patients with an inhibitor of IKKε and TBK1 (amlexanox) reduces to some extent inflammatory gene expression in the subcutaneous adipose tissue and ameliorates insulin sensitivity and hepatic steatosis [97]. Furthermore, anakinra (a recombinant human IL-1 receptor antagonist) was found to mitigate systemic inflammation in type 2 diabetes mellitus patients [351, 352]. However, systemic treatments with anti-inflammatory drugs can have harmful side effects [353]. Recently, targeting visceral ATM through intraperitoneally injected conjugates of dexamethasone (a potent anti-inflammatory drug) with dextran, which were efficiently and specifically taken up by macrophages, strongly reduced adipose tissue inflammation in obese mice [354]. Finally, nutritional adaptations involving, for instance, intake of more mono- and polyunsaturated and less saturated fatty acids could also protect against development of obesity-associated inflammation [355]. Also, several natural anti-inflammatory products were suggested to reduce adipose tissue inflammation and glucose intolerance [356, 357].
However, lifestyle interventions, that is, weight loss through caloric restriction and exercise, could be the most applicable way to combat obesity and associated inflammation and metabolic dysregulation [358]. Weight loss in humans after bariatric surgery and mice is associated with reduced monocytosis and neutrophilia [158]. Although adiposity, hepatic inflammation and systemic glucose homeostasis are improved after weight loss in both humans and mice, features of visceral adipose tissue inflammation, such as mRNA expression levels of inflammatory cytokines, number of crown-like structures, pro-inflammatory macrophages, ILC2, CD8+ T cells and Tregs, remain rather unaffected [342, 359, 360, 361, 362]. In fact, fasting or weight loss due to switch to a low-calorie diet induces ATM accumulation and upregulation of inflammatory pathways in the adipose tissue, accompanied by metabolic reprogramming involving downregulation of glycolysis, oxidative phosphorylation, lipogenesis, canonical lipolysis and upregulation of lysosomal lipid digestion [176, 363, 364, 365]. The initial induction of ATM recruitment upon caloric restriction is followed by a decline in ATM numbers in the case of a prolonged period of weight loss in both visceral and subcutaneous adipose tissue [176]. ATM recruitment coincides with elevated circulating free fatty acid levels, suggesting that adipose tissue lipolysis induced by caloric restriction might drive ATM accumulation [176].
Inadequate physical activity is a major contributor to the development of obesity, while regular exercise can prevent, delay or even reverse metabolic disease [358, 366, 367, 368]. The protective effects of exercise are thought to be mediated by both exercise-associated weight loss and its anti-inflammatory effects [358, 369]. Several studies reported a reduction of adipose tissue inflammation, as evidenced by attenuation of pro-inflammatory macrophages, neutrophils, CD8+ T cells, proinflammatory cytokines, intercellular adhesion molecule 1 and leptin by exercise in humans and mice, especially when combined with dietary changes [370, 371, 372, 373, 374, 375, 376, 377, 378]. Exercise reduces the percentage of CD14+CD16+ monocytes and endotoxin-induced TNF production in humans [379, 380] and elevates IL-10 and IL-6 secretion [379, 381]. Daily exercise and a hypocaloric diet for 15 weeks in severely obese subjects reduced body weight, insulin resistance, and plasma levels of CRP, IL-6, IL-8, and MCP-1, increased adiponectin and decreased the ATM content [375]. Moreover, physically active elderly subjects had decreased inflammatory and oxidative stress marker expression and fewer CD36+ macrophages in the subcutaneous adipose tissue compared to sedentary subjects [376]. Chronic exercise training correlated with increased expression of the M2-like macrophage marker CD163 [373]. In accordance with this, in a recent clinical trial (PREDIMED-plus), physical activity in combination with energy-restricted Mediterranean diet reduced insulin resistance and circulating levels of leptin, IL-18 and MCP-1 in overweight or obese subjects [382]. Aerobic exercise training for 4–16 weeks also reduced liver steatosis and increased the liver content in polyunsaturated lipids [383, 384, 385]. In mice fed a high-fat-high-fructose diet, which induces NAFLD, exercise attenuated macrophage infiltration, TNF expression and fibrosis in the liver [386]. Accordingly, exercise was reported to reduce adipose tissue fibrosis, liver inflammation and steatosis in obesity [358, 371, 374].
The precise mechanisms mediating the protective effects of exercise are not fully understood. Exercise training reduces the adipose tissue mass due to lipolysis, thereby attenuating the production of proinflammatory adipokines [387]. In rodents, it increases mitochondrial biogenesis and the thermogenic capacity of the subcutaneous and visceral adipose tissue [388]. However, in humans, 10 weeks of aerobic exercise or endurance training was not found to be associated with subcutaneous adipose tissue browning [389, 390]. Exercise also increases the mitochondrial content and capacity of skeletal muscle for β-oxidation [391]. Increased fatty acid oxidation in the muscle could reduce circulating levels of free fatty acids, thereby attenuating fatty acid-mediated activation of TLR4 [178, 182, 183, 383]. In addition, acute and chronic exercise are associated with reduced TLR4 expression in monocytes [369, 392, 393, 394]. Moreover, exercise decreases circulating fetuin-A levels, thereby possibly even more restraining fatty acid-mediated TLR4 activation [380]. Along the same line, acute and chronic exercise inhibit TLR4 signaling through JNK and NF-κB and improve insulin signaling in the adipose tissue, liver and muscle [395]. Hence, attenuation of TLR signaling could present an important mechanism mediating the anti-inflammatory effects of exercise in insulin-sensitive tissues, such as the adipose tissue, liver and muscle [358]. Moreover, exercise-induced secretion of IL-6 in muscles could mediate some of the protective effects of exercise [396]. In a recent clinical trial, aerobic exercise reduced cardiac fat through an IL-6 receptor-dependent mechanism in abdominally obese subjects [397]. The effects could be mediated by increasing the levels of IL-10 and IL-1ra, the latter functioning as an inhibitor of IL-1 signaling [398]. Moreover, IL-6 promotes M2-like polarization by increasing the expression of IL-4 receptor α [171] and restrains inflammation and insulin resistance in the liver [107]. However, the role of IL-6 as a mediator of the effects of exercise on immunometabolism is complex, since it is a pleiotropic cytokine, which also exerts important pro-inflammatory actions [44, 399]. Interestingly, endurance and resistance training could have different effects in adipose tissue inflammation: for instance, resistance training reduced NLRP3, while endurance training attenuated TNF and IL-18 expression in the adipose tissue and more effectively improved glucose tolerance [377]. Overall, exercise combined or not with dietary adaptations ameliorates the meta-inflammation in the adipose tissue. Although the exact mechanisms mediating the effects of exercise remain to be elucidated, physical activity has undoubtedly protective effects against chronic inflammation and is keystone for metabolic health.
Conclusion
In obesity, adipose tissue inflammation develops as a result of functional reprogramming, proliferation and retention of resident immune cells, recruitment of circulating immune cells, and reprogramming of hematopoietic stem cell and progenitor cells. A complex interplay between innate and adaptive immune cells, hypertrophic adipocytes, endothelium dysfunction and hypoxia shapes the immunometabolic profile of the adipose tissue. Consequently, adipose tissue inflammation perpetuates development of insulin resistance and fibrosis in the adipose tissue and contributes to systemic inflammation, NAFLD, cardiovascular disease, or other obesity-related disease. The chronicity and long-lasting effects of adipose tissue inflammation render it difficult to treat. Integrated approaches studying the complex microenvironment of the adipose tissue and interorgan communication are required in order to reveal the mechanisms governing immunometabolic dysregulation. Single-cell approaches are already implemented and will provide important information for the better understanding of these dynamic circuits. Identification of the key immunometabolic perturbations leading to unhealthy obesity will pave the way for novel therapeutic strategies.
Several parameters need to be taken into consideration for the development and application of such interventions. First, continuous and long-term treatments are required, which increases the risk of complications and abstinence. Moreover, interindividual differences in patients, that is, BMI, type of obesity (visceral vs. subcutaneous adiposity), age, gender, metabolic disease (insulin resistance, fatty liver disease, etc.), the inflammatory profile (as revealed by immunological screenings), lifestyle (physical activity, smoking, and stress) and genetic background, should be considered for the application of therapeutic treatments. Along these lines, individualized treatment could increase the therapeutic outcome in obese patients. For instance, patients with visceral obesity and high levels of inflammatory markers would benefit most from anti-inflammatory treatments. Furthermore, specific cell-type targeting could increase drug efficiency and minimize side effects. ATM are promising targets due to their significant role in adipose tissue chronic inflammation as well as their capacity to phagocytose nanoparticles [400]. Nanoparticle-mediated delivery of inflammatory pathway blockers might therefore efficiently restrain adipose tissue inflammation. However, adjustment of the physicochemical properties of the applied nanoparticles would be required to improve their delivery to the adipose tissue [400]. Finally, personalized treatments, new-age therapeutic strategies and prevention will be all needed to combat metabolic disease-associated chronic inflammation.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by funds from the Deutsche Forschungsgemeinschaft (SFB-TRR 205 to V.I.A.) and the British Heart Foundation Research Excellence Award (RE/13/3/30183 and RE/18/5/34216 to Z.M.).
Author Contributions
Z.M. wrote and revised the manuscript, M.G.-S. wrote the manuscript and made the figures, and V.I.A. wrote and revised the manuscript.
References
- 1.Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47((3)):406–20. doi: 10.1016/j.immuni.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161((1)):146–60. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16((9)):553–65. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troha K, Ayres JS. Metabolic adaptations to infections at the organismal level. Trends Immunol. 2020;41((2)):113–25. doi: 10.1016/j.it.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chmelar J, Chung KJ, Chavakis T. The role of innate immune cells in obese adipose tissue inflammation and development of insulin resistance. Thromb Haemost. 2013;109((3)):399–406. doi: 10.1160/TH12-09-0703. [DOI] [PubMed] [Google Scholar]
- 6.Chung K-J, Nati M, Chavakis T, Chatzigeorgiou A. Innate immune cells in the adipose tissue. Rev Endocr Metab Disord. 2018;19((4)):283–92. doi: 10.1007/s11154-018-9451-6. [DOI] [PubMed] [Google Scholar]
- 7.Alexaki VI, Chavakis T. The role of innate immunity in the regulation of brown and beige adipogenesis. Rev Endocr Metab Disord. 2016;17((1)):41–9. doi: 10.1007/s11154-016-9342-7. [DOI] [PubMed] [Google Scholar]
- 8.Man K, Kutyavin VI, Chawla A. Tissue immunometabolism: development, physiology, and pathobiology. Cell Metab. 2017;25((1)):11–26. doi: 10.1016/j.cmet.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makowski L, Chaib M, Rathmell JC. Immunometabolism: from basic mechanisms to translation. Immunol Rev. 2020;295((1)):5–14. doi: 10.1111/imr.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15((5)):288–98. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 11.Hamer M, Gale CR, Kivimäki M, Batty GD. Overweight, obesity, and risk of hospitalization for COVID-19: a community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci U S A. 2020;117((35)):21011–3. doi: 10.1073/pnas.2011086117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 2020;28((7)):1200–4. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19((10)):1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 14.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360((15)):1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360((15)):1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 16.Vishvanath L, Gupta RK. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest. 2019;129((10)):4022–31. doi: 10.1172/JCI129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra-Peña R, et al. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. 2016;24((1)):142–50. doi: 10.1016/j.cmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17((4)):376–85. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20((4)):242–58. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- 20.Michailidou Z. Fundamental roles for hypoxia signalling in adipose tissue metabolism and inflammation in obesity. Curr Opin Physiol. 2019;12:39–43. [Google Scholar]
- 21.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322((5901)):583–6. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15((2)):230–9. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcelin G, Silveira ALM, Martins LB, Ferreira AV, Clément K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J Clin Invest. 2019;129((10)):4032–40. doi: 10.1172/JCI129192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcelin G, Ferreira A, Liu Y, Atlan M, Aron-Wisnewsky J, Pelloux V, et al. A PDGFRalpha-mediated switch toward CD9(high) adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell Metab. 2017;25((3)):673–85. doi: 10.1016/j.cmet.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Hepler C, Shan B, Zhang Q, Henry GH, Shao M, Vishvanath L, et al. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. Elife. 2018;7:e39636. doi: 10.7554/eLife.39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017;127((1)):5–13. doi: 10.1172/JCI88876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18((3)):363–74. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 28.Chatzigeorgiou A, Karalis KP, Bornstein SR, Chavakis T. Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia. 2012;55((10)):2583–92. doi: 10.1007/s00125-012-2607-0. [DOI] [PubMed] [Google Scholar]
- 29.Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15((4)):277–87. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- 30.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121((6)):2094–101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18((4)):470–7. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab. 2014;19((1)):8–20. doi: 10.1016/j.cmet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19((10)):1338–44. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453((7196)):783–7. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 35.Symonds M. Adipose tissue biology. 2017. [Google Scholar]
- 36.Michailidou Z, Turban S, Miller E, Zou X, Schrader J, Ratcliffe PJ, et al. Increased angiogenesis protects against adipose hypoxia and fibrosis in metabolic disease-resistant 11beta-hydroxysteroid dehydrogenase type 1 (HSD1)-deficient mice. J Biol Chem. 2012;287((6)):4188–97. doi: 10.1074/jbc.M111.259325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K, Kuo CK. The mechanobiology of obesity and related diseases. 2013. Extracellular matrix remodeling and mechanical stresses as modulators of adipose tissue metabolism and inflammation; pp. p. 105–22. [Google Scholar]
- 38.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29((16)):4467–83. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev. 2014;262((1)):134–52. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36((7)):461–70. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89((6)):2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 42.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11((2)):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han MS, White A, Perry RJ, Camporez JP, Hidalgo J, Shulman GI, et al. Regulation of adipose tissue inflammation by interleukin 6. Proc Natl Acad Sci U S A. 2020;117((6)):2751–60. doi: 10.1073/pnas.1920004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4((5)):371–9. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 46.Farr OM, Gavrieli A, Mantzoros CS. Leptin applications in 2015: what have we learned about leptin and obesity? Curr Opin Endocrinol Diabetes Obes. 2015;22((5)):353–9. doi: 10.1097/MED.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016;5:47–56. doi: 10.2147/ITT.S73223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao T, Hou M, Xia M, Wang Q, Zhu H, Xiao Y, et al. Globular adiponectin decreases leptin-induced tumor necrosis factor-alpha expression by murine macrophages: involvement of cAMP-PKA and MAPK pathways. Cell Immunol. 2005;238((1)):19–30. doi: 10.1016/j.cellimm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542((7642)):450–5. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castano C, Kalko S, Novials A, Parrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A. 2018;115((48)):12158–63. doi: 10.1073/pnas.1808855115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan Y, Hui X, Hoo RLC, Ye D, Chan CYC, Feng T, et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129((2)):834–49. doi: 10.1172/JCI123069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flaherty SE, 3rd, Grijalva A, Xu X, Ables E, Nomani A, Ferrante AW., Jr A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363((6430)):989–93. doi: 10.1126/science.aaw2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, et al. Adipose tissue macrophage-derived exosomal mirnas can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171((2)):372–84.e12. doi: 10.1016/j.cell.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 54.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157((6)):1292–308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157((6)):1279–91. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160((1–2)):74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332((6026)):243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463((7280)):540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 59.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210((3)):535–49. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519((7542)):242–6. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lloyd CM, Snelgrove RJ. Type 2 immunity: expanding our view. Sci Immunol. 2018;3((25)):eaat1604. doi: 10.1126/sciimmunol.aat1604. [DOI] [PubMed] [Google Scholar]
- 62.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107((52)):22617–22. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang YH, Ho KT, Lu SH, Huang CN, Shiau MY. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int J Obes. 2012;36((7)):993–8. doi: 10.1038/ijo.2011.168. [DOI] [PubMed] [Google Scholar]
- 64.Cintra DE, Pauli JR, Araújo EP, Moraes JC, de Souza CT, Milanski M, et al. Interleukin-10 is a protective factor against diet-induced insulin resistance in liver. J Hepatol. 2008;48((4)):628–37. doi: 10.1016/j.jhep.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 65.Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, et al. Differential effects of interleukin-6 and-10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53((4)):1060–7. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 66.Darkhal P, Gao M, Ma Y, Liu D. Blocking high-fat diet-induced obesity, insulin resistance and fatty liver by overexpression of Il-13 gene in mice. Int J Obes. 2015;39((8)):1292–9. doi: 10.1038/ijo.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, et al. Direct control of hepatic glucose production by interleukin-13 in mice. J Clin Invest. 2013;123((1)):261–71. doi: 10.1172/JCI64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117((1)):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18((9)):1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zlotnikov-Klionsky Y, Nathansohn-Levi B, Shezen E, Rosen C, Kagan S, Bar-On L, et al. Perforin-positive dendritic cells exhibit an immuno-regulatory role in metabolic syndrome and autoimmunity. Immunity. 2015;43((4)):776–87. doi: 10.1016/j.immuni.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 71.Wensveen FM, Jelenčić V, Valentić S, Šestan M, Wensveen TT, Theurich S, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16((4)):376–85. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez FJ, Xie C, Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol. 2018;15((1)):21–32. doi: 10.1038/s41574-018-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Engin A. Adipose tissue hypoxia in obesity and its impact on preadipocytes and macrophages: hypoxia hypothesis. Adv Exp Med Biol. 2017;960:305–26. doi: 10.1007/978-3-319-48382-5_13. [DOI] [PubMed] [Google Scholar]
- 74.Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, Peris E, Cui C, Hoffman A, et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 2017;20((13)):3149–61. doi: 10.1016/j.celrep.2017.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56((12)):2910–8. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 76.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46((11)):2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 77.Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20((1)):103–18. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li C, Xu MM, Wang K, Adler AJ, Vella AT, Zhou B. Macrophage polarization and meta-inflammation. Transl Res. 2018;191:29–44. doi: 10.1016/j.trsl.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chouchani ET, Kajimura S. Metabolic adaptation and maladaptation in adipose tissue. Nat Metab. 2019;1((2)):189–200. doi: 10.1038/s42255-018-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55((6)):1554–61. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 81.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49((7)):1562–8. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Harman-Boehm I, Blüher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92((6)):2240–7. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- 83.Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1((2)):152–62. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 84.Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22. doi: 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112((12)):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23((7)):804–14. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322((5907)):1539–43. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389((6651)):610–4. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 89.Hoene M, Weigert C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes Rev. 2008;9((1)):20–9. doi: 10.1111/j.1467-789X.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 90.Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245((6)):621–5. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 91.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420((6913)):333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 92.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103((28)):10741–6. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277((2)):1531–7. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 94.Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10((10)):1128–32. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- 95.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277((50)):48115–21. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 96.Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138((5)):961–75. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oral EA, Reilly SM, Gomez AV, Meral R, Butz L, Ajluni N, et al. Inhibition of IKKɛ and TBK1 improves glucose control in a subset of patients with type 2 diabetes. Cell Metab. 2017;26((1)):157–7.e7. doi: 10.1016/j.cmet.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stoiber D, Kovarik P, Cohney S, Johnston JA, Steinlein P, Decker T. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-gamma. J Immunol. 1999;163((5)):2640–7. [PubMed] [Google Scholar]
- 99.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277((44)):42394–8. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 100.Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17((9)):365–71. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 101.Bieghs V, Rensen PC, Hofker MH, Shiri-Sverdlov R. NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis. 2012;220((2)):287–93. doi: 10.1016/j.atherosclerosis.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 102.Girard J, Lafontan M. Impact of visceral adipose tissue on liver metabolism and insulin resistance. Part II: visceral adipose tissue production and liver metabolism. Diabetes Metab. 2008;34((5)):439–45. doi: 10.1016/j.diabet.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 103.Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res. 2001;24((2)):163–76. doi: 10.1385/IR:24:2:163. [DOI] [PubMed] [Google Scholar]
- 104.Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64((6)):1403–15. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 105.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282((22)):2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 106.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3((6)):e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wunderlich FT, Ströhle P, Könner AC, Gruber S, Tovar S, Brönneke HS, et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 2010;12((3)):237–49. doi: 10.1016/j.cmet.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 108.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15((8)):914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 109.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56((6)):1517–26. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 110.Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res. 2007;100((4)):e47–57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 111.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19((1)):162–71. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112((12)):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng C, Yang Q, Cao J, Xie N, Liu K, Shou P, et al. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis. 2016;7:e2167. doi: 10.1038/cddis.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haase J, Weyer U, Immig K, Klöting N, Blüher M, Eilers J, et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014;57((3)):562–71. doi: 10.1007/s00125-013-3139-y. [DOI] [PubMed] [Google Scholar]
- 115.Ramkhelawon B, Hennessy EJ, Ménager M, Ray TD, Sheedy FJ, Hutchison S, et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014;20((4)):377–84. doi: 10.1038/nm.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chung KJ, Chatzigeorgiou A, Economopoulou M, Garcia-Martin R, Alexaki VI, Mitroulis I, et al. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat Immunol. 2017;18((6)):654–64. doi: 10.1038/ni.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118((2)):710–21. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56((9)):2242–50. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- 119.Kirk EA, Sagawa ZK, McDonald TO, O'Brien KD, Heinecke JW. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected] Diabetes. 2008;57((5)):1254–61. doi: 10.2337/db07-1061. [DOI] [PubMed] [Google Scholar]
- 120.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354((6)):610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 121.Keophiphath M, Rouault C, Divoux A, Clément K, Lacasa D. CCL5 promotes macrophage recruitment and survival in human adipose tissue. Arterioscler Thromb Vasc Biol. 2010;30((1)):39–45. doi: 10.1161/ATVBAHA.109.197442. [DOI] [PubMed] [Google Scholar]
- 122.Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61((7)):1680–90. doi: 10.2337/db11-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han CY, Subramanian S, Chan CK, Omer M, Chiba T, Wight TN, et al. Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 2007;56((9)):2260–73. doi: 10.2337/db07-0218. [DOI] [PubMed] [Google Scholar]
- 124.Sanada Y, Yamamoto T, Satake R, Yamashita A, Kanai S, Kato N, et al. Serum amyloid A3 gene expression in adipocytes is an indicator of the interaction with macrophages. Sci Rep. 2016;6:38697. doi: 10.1038/srep38697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.den Hartigh LJ, Wang S, Goodspeed L, Ding Y, Averill M, Subramanian S, et al. Deletion of serum amyloid A3 improves high fat high sucrose diet-induced adipose tissue inflammation and hyperlipidemia in female mice. PLoS One. 2014;9((9)):e108564. doi: 10.1371/journal.pone.0108564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen X, Zhuo S, Zhu T, Yao P, Yang M, Mei H, et al. Fpr2 deficiency alleviates diet-induced insulin resistance through reducing body weight gain and inhibiting inflammation mediated by macrophage chemotaxis and M1 polarization. Diabetes. 2019;68((6)):1130–42. doi: 10.2337/db18-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elias I, Franckhauser S, Ferré T, Vilà L, Tafuro S, Muñoz S, et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61((7)):1801–13. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Braune J, Weyer U, Hobusch C, Mauer J, Brüning JC, Bechmann I, et al. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J Immunol. 2017;198((7)):2927–34. doi: 10.4049/jimmunol.1600476. [DOI] [PubMed] [Google Scholar]
- 129.Garcia-Martin R, Alexaki VI, Qin N, Rubin de Celis MF, Economopoulou M, Ziogas A, et al. Adipocyte-specific hypoxia-inducible factor 2alpha deficiency exacerbates obesity-induced brown adipose tissue dysfunction and metabolic dysregulation. Mol Cell Biol. 2016;36((3)):376–93. doi: 10.1128/MCB.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang L, Zhao RP, Song XY, Wu WF. Targeting ERβ in macrophage reduces crown-like structures in adipose tissue by inhibiting osteopontin and HIF-1α. Sci Rep. 2019;9((1)):15762. doi: 10.1038/s41598-019-52265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boulenouar S, Michelet X, Duquette D, Alvarez D, Hogan AE, Dold C, et al. Adipose type one innate lymphoid cells regulate macrophage homeostasis through targeted cytotoxicity. Immunity. 2017;46((2)):273–86. doi: 10.1016/j.immuni.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 132.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59((7)):1648–56. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292((1)):E166–74. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, et al. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206((13)):3143–56. doi: 10.1084/jem.20091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57((12)):3239–46. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao D, Madi M, Ding C, Fok M, Steele T, Ford C, et al. Interleukin-1β mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am J Physiol Endocrinol Metab. 2014;307((3)):E289–304. doi: 10.1152/ajpendo.00430.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sharma M, Boytard L, Hadi T, Koelwyn G, Simon R, Ouimet M, et al. Enhanced glycolysis and HIF-1α activation in adipose tissue macrophages sustains local and systemic interleukin-1β production in obesity. Sci Rep. 2020;10((1)):5555. doi: 10.1038/s41598-020-62272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496((7444)):238–42. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020;20((1)):40–54. doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- 140.Chan KL, Tam TH, Boroumand P, Prescott D, Costford SR, Escalante NK, et al. Circulating NOD1 activators and hematopoietic NOD1 contribute to metabolic inflammation and insulin resistance. Cell Rep. 2017;18((10)):2415–26. doi: 10.1016/j.celrep.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 141.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17((4)):219–32. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 142.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability: a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roedig H, Nastase MV, Wygrecka M, Schaefer L. Breaking down chronic inflammatory diseases: the role of biglycan in promoting a switch between inflammation and autophagy. FEBS J. 2019;286((15)):2965–79. doi: 10.1111/febs.14791. [DOI] [PubMed] [Google Scholar]
- 144.Han CY, Kang I, Harten IA, Gebe JA, Chan CK, Omer M, et al. Adipocyte-derived versican and macrophage-derived biglycan control adipose tissue inflammation in obesity. Cell Rep. 2020;31((13)):107818. doi: 10.1016/j.celrep.2020.107818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jones JEC, Rabhi N, Orofino J, Gamini R, Perissi V, Vernochet C, et al. The adipocyte acquires a fibroblast-like transcriptional signature in response to a high fat diet. Sci Rep. 2020;10((1)):2380. doi: 10.1038/s41598-020-59284-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59((11)):2817–25. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29((6)):1575–91. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE, Jr, Peterson CA, et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96((12)):E1990–8. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327((5963)):296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 150.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108((37)):15324–9. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metab. 2012;15((1)):10–8. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 152.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17((2)):179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12((6)):593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. 2018;172((1–2)):162–75.e14. doi: 10.1016/j.cell.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10((2)):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 156.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12((5)):408–15. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Snodgrass RG, Huang S, Choi IW, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J Immunol. 2013;191((8)):4337–47. doi: 10.4049/jimmunol.1300298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19((5)):821–35. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11((2)):136–40. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 160.Kawasaki N, Asada R, Saito A, Kanemoto S, Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2:799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57((9)):2438–44. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306((5695)):457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 163.Shan B, Wang X, Wu Y, Xu C, Xia Z, Dai J, et al. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol. 2017;18((5)):519–29. doi: 10.1038/ni.3709. [DOI] [PubMed] [Google Scholar]
- 164.Garfinkel BP, Hotamisligil GS. ER stress promotes inflammation through Re-wIREd macrophages in obesity. Mol Cell. 2017;66((6)):731–3. doi: 10.1016/j.molcel.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 165.Suzuki T, Gao J, Ishigaki Y, Kondo K, Sawada S, Izumi T, et al. ER stress protein CHOP mediates insulin resistance by modulating adipose tissue macrophage polarity. Cell Rep. 2017;18((8)):2045–57. doi: 10.1016/j.celrep.2017.01.076. [DOI] [PubMed] [Google Scholar]
- 166.Jager J, Grémeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148((1)):241–51. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lagathu C, Yvan-Charvet L, Bastard JP, Maachi M, Quignard-Boulangé A, Capeau J, et al. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia. 2006;49((9)):2162–73. doi: 10.1007/s00125-006-0335-z. [DOI] [PubMed] [Google Scholar]
- 168.Li P, Liu S, Lu M, Bandyopadhyay G, Oh D, Imamura T, et al. Hematopoietic-derived galectin-3 causes cellular and systemic insulin resistance. Cell. 2016;167((4)):973–e12. doi: 10.1016/j.cell.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pejnovic NN, Pantic JM, Jovanovic IP, Radosavljevic GD, Milovanovic MZ, Nikolic IG, et al. Galectin-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets. Diabetes. 2013;62((6)):1932–44. doi: 10.2337/db12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, et al. Krüppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121((7)):2736–49. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15((5)):423–30. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286((15)):13460–9. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, et al. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299((3)):H656–63. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fan R, Toubal A, Goñi S, Drareni K, Huang Z, Alzaid F, et al. Loss of the co-repressor GPS2 sensitizes macrophage activation upon metabolic stress induced by obesity and type 2 diabetes. Nat Med. 2016;22((7)):780–91. doi: 10.1038/nm.4114. [DOI] [PubMed] [Google Scholar]
- 175.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27((1)):84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 176.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120((10)):3466–79. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56((3)):564–73. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- 178.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276((20)):16683–9. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 179.Chaurasia B, Kaddai VA, Lancaster GI, Henstridge DC, Sriram S, Galam DL, et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 2016;24((6)):820–34. doi: 10.1016/j.cmet.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 180.Prieur X, Mok CY, Velagapudi VR, Núñez V, Fuentes L, Montaner D, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60((3)):797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell. 2019;178((3)):686–98.e14. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18((8)):1279–85. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 183.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29((11)):1944–9. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 184.Kanoh H, Nitta T, Go S, Inamori KI, Veillon L, Nihei W, et al. Homeostatic and pathogenic roles of GM3 ganglioside molecular species in TLR4 signaling in obesity. EMBO J. 2020;39((12)):e101732. doi: 10.15252/embj.2019101732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.van Dierendonck XAMH, de la Rosa Rodriguez MA, Georgiadi A, Mattijssen F, Dijk W, van Weeghel M, et al. HILPDA uncouples lipid droplet accumulation in adipose tissue macrophages from inflammation and metabolic dysregulation. Cell Rep. 2020;30((6)):1811–22.e6. doi: 10.1016/j.celrep.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 186.Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, Terayama K, et al. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest. 2010;120((3)):756–67. doi: 10.1172/JCI36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159((2)):318–32. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142((5)):687–98. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483((7389)):350–4. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 190.Syed I, Lee J, Moraes-Vieira PM, Donaldson CJ, Sontheimer A, Aryal P, et al. Palmitic acid hydroxystearic acids activate GPR40, which is involved in their beneficial effects on glucose homeostasis. Cell Metab. 2018;27((2)):419–27.e4. doi: 10.1016/j.cmet.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc Natl Acad Sci U S A. 2018;115((22)):E5096–105. doi: 10.1073/pnas.1802611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brykczynska U, Geigges M, Wiedemann SJ, Dror E, Böni-Schnetzler M, Hess C, et al. Distinct transcriptional responses across tissue-resident macrophages to short-term and long-term metabolic challenge. Cell Rep. 2020;30((5)):1627–e7. doi: 10.1016/j.celrep.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 193.McNally AK, Anderson JM. Macrophage fusion and multinucleated giant cells of inflammation. Adv Exp Med Biol. 2011;713:97–111. doi: 10.1007/978-94-007-0763-4_7. [DOI] [PubMed] [Google Scholar]
- 194.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8((4)):301–9. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dalmas E, Toubal A, Alzaid F, Blazek K, Eames HL, Lebozec K, et al. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat Med. 2015;21((6)):610–8. doi: 10.1038/nm.3829. [DOI] [PubMed] [Google Scholar]
- 196.Sindhu S, Thomas R, Kochumon S, Wilson A, Abu-Farha M, Bennakhi A, et al. Increased adipose tissue expression of interferon regulatory factor (IRF)-5 in obesity: association with metabolic inflammation. Cells. 2019;8((11)):1418. doi: 10.3390/cells8111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Weiss M, Byrne AJ, Blazek K, Saliba DG, Pease JE, Perocheau D, et al. IRF5 controls both acute and chronic inflammation. Proc Natl Acad Sci U S A. 2015;112((35)):11001–6. doi: 10.1073/pnas.1506254112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20((4)):614–25. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13((11)):633–43. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 200.Tiwari P, Blank A, Cui C, Schoenfelt KQ, Zhou G, Xu Y, et al. Metabolically activated adipose tissue macrophages link obesity to triple-negative breast cancer. J Exp Med. 2019;216((6)):1345–58. doi: 10.1084/jem.20181616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18((6)):816–30. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480((7375)):104–8. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, et al. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. 2015;22((2)):279–90. doi: 10.1016/j.cmet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 204.Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med. 2017;23((5)):623–30. doi: 10.1038/nm.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Spadaro O, Camell CD, Bosurgi L, Nguyen KY, Youm YH, Rothlin CV, et al. IGF1 shapes macrophage activation in response to immunometabolic challenge. Cell Rep. 2017;19((2)):225–34. doi: 10.1016/j.celrep.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rankin SM, Conroy DM, Williams TJ. Eotaxin and eosinophil recruitment: implications for human disease. Mol Med Today. 2000;6((1)):20–7. doi: 10.1016/s1357-4310(99)01635-4. [DOI] [PubMed] [Google Scholar]
- 207.Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242((1)):161–77. doi: 10.1111/j.1600-065X.2011.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.van den Berg SM, van Dam AD, Kusters PJH, Beckers L, den Toom M, van der Velden S, et al. Helminth antigens counteract a rapid high-fat diet-induced decrease in adipose tissue eosinophils. J Mol Endocrinol. 2017;59((3)):245–55. doi: 10.1530/JME-17-0112. [DOI] [PubMed] [Google Scholar]
- 209.Silva HM, Báfica A, Rodrigues-Luiz GF, Chi J, Santos PDA, Reis BS, et al. Vasculature-associated fat macrophages readily adapt to inflammatory and metabolic challenges. J Exp Med. 2019;216((4)):786–806. doi: 10.1084/jem.20181049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502((7470)):245–8. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lee EH, Itan M, Jang J, Gu HJ, Rozenberg P, Mingler MK, et al. Eosinophils support adipocyte maturation and promote glucose tolerance in obesity. Sci Rep. 2018;8((1)):9894. doi: 10.1038/s41598-018-28371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hussaarts L, García-Tardón N, van Beek L, Heemskerk MM, Haeberlein S, van der Zon GC, et al. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015;29((7)):3027–39. doi: 10.1096/fj.14-266239. [DOI] [PubMed] [Google Scholar]
- 213.Hams E, Bermingham R, Wurlod FA, Hogan AE, O'Shea D, Preston RJ, et al. The helminth T2 RNase ω1 promotes metabolic homeostasis in an IL-33- and group 2 innate lymphoid cell-dependent mechanism. FASEB J. 2016;30((2)):824–35. doi: 10.1096/fj.15-277822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kitamura H, Naoe Y, Kimura S, Miyamoto T, Okamoto S, Toda C, et al. Beneficial effects of Brazilian propolis on type 2 diabetes in ob/ob mice: possible involvement of immune cells in mesenteric adipose tissue. Adipocyte. 2013;2((4)):227–36. doi: 10.4161/adip.25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191((11)):5349–53. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.DeFilippis EM, Kumbhakar R, Pereira MR, Marboe CC, Maurer MS. Eosinophils, lymphocytes, and myocytes, oh my: HIV-associated myocarditis. Am J Med. 2020;133((1)):52–5. doi: 10.1016/j.amjmed.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 217.Tsao CH, Shiau MY, Chuang PH, Chang YH, Hwang J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J Lipid Res. 2014;55((3)):385–97. doi: 10.1194/jlr.M041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shiau MY, Chuang PH, Yang CP, Hsiao CW, Chang SW, Chang KY, et al. Mechanism of interleukin-4 reducing lipid deposit by regulating hormone-sensitive lipase. Sci Rep. 2019;9((1)):11974. doi: 10.1038/s41598-019-47908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fabbiano S, Suárez-Zamorano N, Rigo D, Veyrat-Durebex C, Stevanovic Dokic A, Colin DJ, et al. Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab. 2016;24((3)):434–46. doi: 10.1016/j.cmet.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 220.Bolus WR, Peterson KR, Hubler MJ, Kennedy AJ, Gruen ML, Hasty AH. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments. Mol Metab. 2018;8:86–95. doi: 10.1016/j.molmet.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shim WS, Kim HJ, Kang ES, Ahn CW, Lim SK, Lee HC, et al. The association of total and differential white blood cell count with metabolic syndrome in type 2 diabetic patients. Diabetes Res Clin Pract. 2006;73((3)):284–91. doi: 10.1016/j.diabres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 222.Sunadome H, Matsumoto H, Izuhara Y, Nagasaki T, Kanemitsu Y, Ishiyama Y, et al. Correlation between eosinophil count, its genetic background and body mass index: the Nagahama study. Allergol Int. 2020;69((1)):46–52. doi: 10.1016/j.alit.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 223.Moussa K, Gurung P, Adams-Huet B, Devaraj S, Jialal I. Increased eosinophils in adipose tissue of metabolic syndrome. J Diabetes Complications. 2019;33((8)):535–8. doi: 10.1016/j.jdiacomp.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 224.Gieseck RL, 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18((1)):62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 225.Hart KM, Fabre T, Sciurba JC, Gieseck RL, 3rd, Borthwick LA, Vannella KM, et al. Type 2 immunity is protective in metabolic disease but exacerbates NAFLD collaboratively with TGF-β. Sci Transl Med. 2017;9((396)):eaal3694. doi: 10.1126/scitranslmed.aal3694. [DOI] [PubMed] [Google Scholar]
- 226.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49((9)):1894–903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 227.Wang Q, Xie Z, Zhang W, Zhou J, Wu Y, Zhang M, et al. Myeloperoxidase deletion prevents high-fat diet-induced obesity and insulin resistance. Diabetes. 2014;63((12)):4172–85. doi: 10.2337/db14-0026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 228.Tam TH, Chan KL, Boroumand P, Liu Z, Brozinick JT, Bui HH, et al. Nucleotides released from palmitate-activated murine macrophages attract neutrophils. J Biol Chem. 2020;295((15)):4902–11. doi: 10.1074/jbc.RA119.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hadad N, Burgazliev O, Elgazar-Carmon V, Solomonov Y, Wueest S, Item F, et al. Induction of cytosolic phospholipase a2α is required for adipose neutrophil infiltration and hepatic insulin resistance early in the course of high-fat feeding. Diabetes. 2013;62((9)):3053–63. doi: 10.2337/db12-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mansuy-Aubert V, Zhou QL, Xie X, Gong Z, Huang JY, Khan AR, et al. Imbalance between neutrophil elastase and its inhibitor α1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 2013;17((4)):534–48. doi: 10.1016/j.cmet.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rouault C, Pellegrinelli V, Schilch R, Cotillard A, Poitou C, Tordjman J, et al. Roles of chemokine ligand-2 (CXCL2) and neutrophils in influencing endothelial cell function and inflammation of human adipose tissue. Endocrinology. 2013;154((3)):1069–79. doi: 10.1210/en.2012-1415. [DOI] [PubMed] [Google Scholar]
- 232.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen Y, Tian J, Tian X, Tang X, Rui K, Tong J, et al. Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells. PLoS One. 2014;9((3)):e92450. doi: 10.1371/journal.pone.0092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61((9)):2238–47. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, et al. Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. J Immunol. 2016;197((9)):3650–61. doi: 10.4049/jimmunol.1600820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Stefanovic-Racic M, Yang X, Turner MS, Mantell BS, Stolz DB, Sumpter TL, et al. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes. 2012;61((9)):2330–9. doi: 10.2337/db11-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26((6)):741–50. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 238.Hannibal TD, Schmidt-Christensen A, Nilsson J, Fransén-Pettersson N, Hansen L, Holmberg D. Deficiency in plasmacytoid dendritic cells and type I interferon signalling prevents diet-induced obesity and insulin resistance in mice. Diabetologia. 2017;60((10)):2033–41. doi: 10.1007/s00125-017-4341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mattacks CA, Sadler D, Pond CM. The effects of dietary lipids on dendritic cells in perinodal adipose tissue during chronic mild inflammation. Br J Nutr. 2004;91((6)):883–92. doi: 10.1079/BJN20041147. [DOI] [PubMed] [Google Scholar]
- 240.Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, et al. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol. 2015;194((11)):5200–10. doi: 10.4049/jimmunol.1500221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Porsche CE, Delproposto JB, Patrick E, Zamarron BF, Lumeng CN. Adipose tissue dendritic cell signals are required to maintain T cell homeostasis and obesity-induced expansion. Mol Cell Endocrinol. 2020;505:110740. doi: 10.1016/j.mce.2020.110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95((11)):3489–97. [PubMed] [Google Scholar]
- 243.Ghosh AR, Bhattacharya R, Bhattacharya S, Nargis T, Rahaman O, Duttagupta P, et al. Adipose recruitment and activation of plasmacytoid dendritic cells fuel metaflammation. Diabetes. 2016;65((11)):3440–52. doi: 10.2337/db16-0331. [DOI] [PubMed] [Google Scholar]
- 244.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010;21((11)):660–7. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 245.Pamir N, Liu NC, Irwin A, Becker L, Peng Y, Ronsein GE, et al. Granulocyte/macrophage colony-stimulating factor-dependent dendritic cells restrain lean adipose tissue expansion. J Biol Chem. 2015;290((23)):14656–67. doi: 10.1074/jbc.M115.645820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Macdougall CE, Wood EG, Loschko J, Scagliotti V, Cassidy FC, Robinson ME, et al. Visceral adipose tissue immune homeostasis is regulated by the crosstalk between adipocytes and dendritic cell subsets. Cell Metab. 2018;27((3)):588–e4. doi: 10.1016/j.cmet.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169((1)):443–53. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 248.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15((6)):985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 249.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12((1)):21–7. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 250.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464((7293)):1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203((4)):1105–16. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464((7293)):1362–6. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ding X, Luo Y, Zhang X, Zheng H, Yang X, Yang X, et al. IL-33-driven ILC2/eosinophil axis in fat is induced by sympathetic tone and suppressed by obesity. J Endocrinol. 2016;231((1)):35–48. doi: 10.1530/JOE-16-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shafiei-Jahani P, Hurrell BP, Galle-Treger L, Helou DG, Howard E, Painter J, et al. DR3 stimulation of adipose resident ILC2s ameliorates type 2 diabetes mellitus. Nat Commun. 2020;11((1)):4718. doi: 10.1038/s41467-020-18601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Oldenhove G, Boucquey E, Taquin A, Acolty V, Bonetti L, Ryffel B, et al. PD-1 is involved in the dysregulation of type 2 innate lymphoid cells in a murine model of obesity. Cell Rep. 2018;25((8)):2053–e4. doi: 10.1016/j.celrep.2018.10.091. [DOI] [PubMed] [Google Scholar]
- 256.Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, et al. Interleukin-33 and interferon-γ counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity. 2015;43((1)):161–74. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16((3)):276–85. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- 258.Halim TYF, Rana BMJ, Walker JA, Kerscher B, Knolle MD, Jolin HE, et al. Tissue-restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity. 2018;48((6)):1195–e6. doi: 10.1016/j.immuni.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cipolletta D, Kolodin D, Benoist C, Mathis D. Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol. 2011;23((6)):431–7. doi: 10.1016/j.smim.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 260.Moysidou M, Karaliota S, Kodela E, Salagianni M, Koutmani Y, Katsouda A, et al. CD8+ T cells in beige adipogenesis and energy homeostasis. JCI Insight. 2018;3((5)):e95456. doi: 10.1172/jci.insight.95456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25((1)):19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 262.O'Sullivan TE, Sun JC, Lanier LL. Natural killer cell memory. Immunity. 2015;43((4)):634–45. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kamimura Y, Lanier LL. Homeostatic control of memory cell progenitors in the natural killer cell lineage. Cell Rep. 2015;10((2)):280–91. doi: 10.1016/j.celrep.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ballesteros-Pomar MD, Calleja S, Díez-Rodríguez R, Calleja-Fernández A, Vidal-Casariego A, Nuñez-Alonso A, et al. Inflammatory status is different in relationship to insulin resistance in severely obese people and changes after bariatric surgery or diet-induced weight loss. Exp Clin Endocrinol Diabetes. 2014;122((10)):592–6. doi: 10.1055/s-0034-1382035. [DOI] [PubMed] [Google Scholar]
- 265.O'Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes. 2009;33((9)):978–90. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Guo H, Xu B, Gao L, Sun X, Qu X, Li X, et al. High frequency of activated natural killer and natural killer T-cells in patients with new onset of type 2 diabetes mellitus. Exp Biol Med. 2012;237((5)):556–62. doi: 10.1258/ebm.2012.011272. [DOI] [PubMed] [Google Scholar]
- 267.Lee BC, Kim MS, Pae M, Yamamoto Y, Eberlé D, Shimada T, et al. Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity. Cell Metab. 2016;23((4)):685–98. doi: 10.1016/j.cmet.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.O'Rourke RW, Meyer KA, Neeley CK, Gaston GD, Sekhri P, Szumowski M, et al. Systemic NK cell ablation attenuates intra-abdominal adipose tissue macrophage infiltration in murine obesity. Obesity. 2014;22((10)):2109–14. doi: 10.1002/oby.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Al-Attar A, Presnell SR, Clasey JL, Long DE, Walton RG, Sexton M, et al. Human body composition and immunity: visceral adipose tissue produces IL-15 and muscle strength inversely correlates with NK cell function in elderly humans. Front Immunol. 2018;9:440. doi: 10.3389/fimmu.2018.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Shoae-Hassani A, Behfar M, Mortazavi-Tabatabaei SA, Ai J, Mohseni R, Hamidieh AA. Natural killer cells from the subcutaneous adipose tissue underexpress the NKp30 and NKp44 in obese persons and are less active against major histocompatibility complex class I non-expressing neoplastic cells. Front Immunol. 2017;8:1486. doi: 10.3389/fimmu.2017.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hepworth MR, Maurer M, Hartmann S. Regulation of type 2 immunity to helminths by mast cells. Gut Microbes. 2012;3((5)):476–81. doi: 10.4161/gmic.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18((5)):693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4((10)):787–99. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 274.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10((6)):440–52. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Poglio S, De Toni-Costes F, Arnaud E, Laharrague P, Espinosa E, Casteilla L, et al. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells. 2010;28((11)):2065–72. doi: 10.1002/stem.523. [DOI] [PubMed] [Google Scholar]
- 276.Li Z, Liu S, Xu J, Zhang X, Han D, Liu J, et al. Adult connective tissue-resident mast cells originate from late erythro-myeloid progenitors. Immunity. 2018;49((4)):640–53.e5. doi: 10.1016/j.immuni.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 277.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15((8)):940–5. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hirai S, Ohyane C, Kim YI, Lin S, Goto T, Takahashi N, et al. Involvement of mast cells in adipose tissue fibrosis. Am J Physiol Endocrinol Metab. 2014;306((3)):E247–55. doi: 10.1152/ajpendo.00056.2013. [DOI] [PubMed] [Google Scholar]
- 279.Tanaka A, Nomura Y, Matsuda A, Ohmori K, Matsuda H. Mast cells function as an alternative modulator of adipogenesis through 15-deoxy-delta-12, 14-prostaglandin J2. Am J Physiol Cell Physiol. 2011;301((6)):C1360–7. doi: 10.1152/ajpcell.00514.2010. [DOI] [PubMed] [Google Scholar]
- 280.Gurung P, Moussa K, Adams-Huet B, Devaraj S, Jialal I. Increased mast cell abundance in adipose tissue of metabolic syndrome: relevance to the proinflammatory state and increased adipose tissue fibrosis. Am J Physiol Endocrinol Metab. 2019;316((3)):E504–9. doi: 10.1152/ajpendo.00462.2018. [DOI] [PubMed] [Google Scholar]
- 281.Divoux A, Moutel S, Poitou C, Lacasa D, Veyrie N, Aissat A, et al. Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metab. 2012;97((9)):E1677–85. doi: 10.1210/jc.2012-1532. [DOI] [PubMed] [Google Scholar]
- 282.Goldstein N, Kezerle Y, Gepner Y, Haim Y, Pecht T, Gazit R, et al. Higher mast cell accumulation in human adipose tissues defines clinically favorable obesity sub-phenotypes. Cells. 2020;9((6)):1508. doi: 10.3390/cells9061508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kumar D, Pandya SK, Varshney S, Shankar K, Rajan S, Srivastava A, et al. Temporal immmunometabolic profiling of adipose tissue in HFD-induced obesity: manifestations of mast cells in fibrosis and senescence. Int J Obes. 2019;43((6)):1281–94. doi: 10.1038/s41366-018-0228-5. [DOI] [PubMed] [Google Scholar]
- 284.Yabut JM, Desjardins EM, Chan EJ, Day EA, Leroux JM, Wang B, et al. Genetic deletion of mast cell serotonin synthesis prevents the development of obesity and insulin resistance. Nat Commun. 2020;11((1)):463. doi: 10.1038/s41467-019-14080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zelechowska P, Agier J, Kozlowska E, Brzezinska-Blaszczyk E. Mast cells participate in chronic low-grade inflammation within adipose tissue. Obes Rev. 2018;19((5)):686–97. doi: 10.1111/obr.12670. [DOI] [PubMed] [Google Scholar]
- 286.Zhang X, Huang Q, Wang X, Deng Z, Li J, Yan X, et al. Dietary cholesterol is essential to mast cell activation and associated obesity and diabetes in mice. Biochim Biophys Acta Mol Basis Dis. 2019;1865((6)):1690–700. doi: 10.1016/j.bbadis.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 287.Zhou Y, Yu X, Chen H, Sjöberg S, Roux J, Zhang L, et al. Leptin deficiency shifts mast cells toward anti-inflammatory actions and protects mice from obesity and diabetes by polarizing M2 macrophages. Cell Metab. 2015;22((6)):1045–58. doi: 10.1016/j.cmet.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhang X, Wang X, Yin H, Zhang L, Feng A, Zhang QX, et al. Functional inactivation of mast cells enhances subcutaneous adipose tissue browning in mice. Cell Rep. 2019;28((3)):792–803.e4. doi: 10.1016/j.celrep.2019.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: fact or fiction? Immunity. 2012;37((1)):13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 290.Gutierrez DA, Muralidhar S, Feyerabend TB, Herzig S, Rodewald HR. Hematopoietic kit deficiency, rather than lack of mast cells, protects mice from obesity and insulin resistance. Cell Metab. 2015;21((5)):678–91. doi: 10.1016/j.cmet.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 291.Chmelar J, Chatzigeorgiou A, Chung KJ, Prucnal M, Voehringer D, Roers A, et al. No role for mast cells in obesity-related metabolic dysregulation. Front Immunol. 2016;7:524. doi: 10.3389/fimmu.2016.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 293.Schleicher U, Paduch K, Debus A, Obermeyer S, König T, Kling JC, et al. TNF-mediated restriction of arginase 1 expression in myeloid cells triggers type 2 NO synthase activity at the site of infection. Cell Rep. 2016;15((5)):1062–75. doi: 10.1016/j.celrep.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93((1)):1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 295.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58((3)):718–25. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124((1)):67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 297.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56((4)):901–11. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 298.Lee YS, Kim JW, Osborne O, Oh DY, Sasik R, Schenk S, et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014;157((6)):1339–52. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41((4)):518–28. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92((12)):5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1996;225((2)):485–8. doi: 10.1006/bbrc.1996.1199. [DOI] [PubMed] [Google Scholar]
- 302.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11((1)):72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 303.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107((1)):43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 304.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292((5516)):468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 305.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292((5516)):464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 306.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399((6733)):271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 307.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148((3)):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Corcoran SE, O'Neill LA. HIF1α and metabolic reprogramming in inflammation. J Clin Invest. 2016;126((10)):3699–707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Semba H, Takeda N, Isagawa T, Sugiura Y, Honda K, Wake M, et al. HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat Commun. 2016;7:11635. doi: 10.1038/ncomms11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Harris AJ, Thompson AR, Whyte MK, Walmsley SR. HIF-mediated innate immune responses: cell signaling and therapeutic implications. Hypoxia. 2014;2:47–58. doi: 10.2147/HP.S50269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201((1)):105–15. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sadiku P, Willson JA, Dickinson RS, Murphy F, Harris AJ, Lewis A, et al. Prolyl hydroxylase 2 inactivation enhances glycogen storage and promotes excessive neutrophilic responses. J Clin Invest. 2017;127((9)):3407–20. doi: 10.1172/JCI90848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112((5)):645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120((8)):2699–714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Crotty Alexander LE, Akong-Moore K, Feldstein S, Johansson P, Nguyen A, McEachern EK, et al. Myeloid cell HIF-1α regulates asthma airway resistance and eosinophil function. J Mol Med. 2013;91((5)):637–44. doi: 10.1007/s00109-012-0986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Fluck K, Breves G, Fandrey J, Winning S. Hypoxia-inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol. 2016;9((2)):379–90. doi: 10.1038/mi.2015.67. [DOI] [PubMed] [Google Scholar]
- 317.Li Q, Li D, Zhang X, Wan Q, Zhang W, Zheng M, et al. E3 ligase VHL promotes group 2 innate lymphoid cell maturation and function via glycolysis inhibition and induction of interleukin-33 receptor. Immunity. 2018;48((2)):258–e5. doi: 10.1016/j.immuni.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Onogi Y, Wada T, Okekawa A, Matsuzawa T, Watanabe E, Ikeda K, et al. Pro-inflammatory macrophages coupled with glycolysis remodel adipose vasculature by producing platelet-derived growth factor-B in obesity. Sci Rep. 2020;10((1)):670. doi: 10.1038/s41598-019-57368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhang X, Lam KS, Ye H, Chung SK, Zhou M, Wang Y, et al. Adipose tissue-specific inhibition of hypoxia-inducible factor 1{alpha} induces obesity and glucose intolerance by impeding energy expenditure in mice. J Biol Chem. 2010;285((43)):32869–77. doi: 10.1074/jbc.M110.135509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Michailidou Z, Morton NM, Moreno Navarrete JM, West CC, Stewart KJ, Fernández-Real JM, et al. Adipocyte pseudohypoxia suppresses lipolysis and facilitates benign adipose tissue expansion. Diabetes. 2015;64((3)):733–45. doi: 10.2337/db14-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Matsuura H, Ichiki T, Inoue E, Nomura M, Miyazaki R, Hashimoto T, et al. Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation. 2013;127((21)):2078–87. doi: 10.1161/CIRCULATIONAHA.113.001742. [DOI] [PubMed] [Google Scholar]
- 322.Takikawa A, Mahmood A, Nawaz A, Kado T, Okabe K, Yamamoto S, et al. HIF-1α in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes. 2016;65((12)):3649–59. doi: 10.2337/db16-0012. [DOI] [PubMed] [Google Scholar]
- 323.Boutens L, Hooiveld GJ, Dhingra S, Cramer RA, Netea MG, Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia. 2018;61((4)):942–53. doi: 10.1007/s00125-017-4526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Choe SS, Shin KC, Ka S, Lee YK, Chun JS, Kim JB. Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes. 2014;63((10)):3359–71. doi: 10.2337/db13-1965. [DOI] [PubMed] [Google Scholar]
- 325.Takeda N, O'Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C, et al. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24((5)):491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Poblete JMS, Ballinger MN, Bao S, Alghothani M, Nevado JB, Jr, Eubank TD, et al. Macrophage HIF-1α mediates obesity-related adipose tissue dysfunction via interleukin-1 receptor-associated kinase M. Am J Physiol Endocrinol Metab. 2020;318((5)):E689–700. doi: 10.1152/ajpendo.00174.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12((1)):9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Sanghani NS, Haase VH. Hypoxia-inducible factor activators in renal anemia: current clinical experience. Adv Chronic Kidney Dis. 2019;26((4)):253–66. doi: 10.1053/j.ackd.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Mitroulis I, Alexaki VI, Kourtzelis I, Ziogas A, Hajishengallis G, Chavakis T. Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther. 2015;147:123–35. doi: 10.1016/j.pharmthera.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods Mol Biol. 2011;686:49–68. doi: 10.1007/978-1-60761-938-3_2. [DOI] [PubMed] [Google Scholar]
- 331.Stark K, Pekayvaz K, Massberg S. Role of pericytes in vascular immunosurveillance. Front Biosci. 2018;23:767–81. doi: 10.2741/4615. [DOI] [PubMed] [Google Scholar]
- 332.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119((12)):3637–51. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci U S A. 2012;109((15)):5874–9. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell. 2009;16((2)):167–79. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 335.Karastergiou K, Fried SK. Multiple adipose depots increase cardiovascular risk via local and systemic effects. Curr Atheroscler Rep. 2013;15((10)):361. doi: 10.1007/s11883-013-0361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Chiappetta S, Sharma AM, Bottino V, Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes. 2020;44((8)):1790–2. doi: 10.1038/s41366-020-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20((6)):375–88. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Chavakis T, Mitroulis I, Hajishengallis G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat Immunol. 2019;20((7)):802–11. doi: 10.1038/s41590-019-0402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Penkov S, Mitroulis I, Hajishengallis G, Chavakis T. Immunometabolic crosstalk: an ancestral principle of trained immunity? Trends Immunol. 2019;40((1)):1–11. doi: 10.1016/j.it.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352((6284)):aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Bekkering S, Saner C, Riksen NP, Netea MG, Sabin MA, Saffery R, et al. Trained immunity: linking obesity and cardiovascular disease across the life-course? Trends Endocrinol Metab. 2020;31((5)):378–89. doi: 10.1016/j.tem.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 342.Fischer IP, Irmler M, Meyer CW, Sachs SJ, Neff F, Hrabě de Angelis M, et al. A history of obesity leaves an inflammatory fingerprint in liver and adipose tissue. Int J Obes. 2018;42((3)):507–17. doi: 10.1038/ijo.2017.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Hamada A, Torre C, Drancourt M, Ghigo E. Trained immunity carried by non-immune cells. Front Microbiol. 2018;9:3225. doi: 10.3389/fmicb.2018.03225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17((5)):695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Singer K, DelProposto J, Morris DL, Zamarron B, Mergian T, Maley N, et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3((6)):664–75. doi: 10.1016/j.molmet.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Griffin C, Eter L, Lanzetta N, Abrishami S, Varghese M, McKernan K, et al. TLR4, TRIF, and MyD88 are essential for myelopoiesis and CD11c(+) adipose tissue macrophage production in obese mice. J Biol Chem. 2018;293((23)):8775–86. doi: 10.1074/jbc.RA117.001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Luo Y, Chen GL, Hannemann N, Ipseiz N, Krönke G, Bäuerle T, et al. Microbiota from obese mice regulate hematopoietic stem cell differentiation by altering the bone niche. Cell Metab. 2015;22((5)):886–94. doi: 10.1016/j.cmet.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 348.Kothari V, Galdo JA, Mathews ST. Hypoglycemic agents and potential anti-inflammatory activity. J Inflamm Res. 2016;9:27–38. doi: 10.2147/JIR.S86917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Valsamakis G, Konstantakou P, Mastorakos G. New targets for drug treatment of obesity. Annu Rev Pharmacol Toxicol. 2017;57:585–605. doi: 10.1146/annurev-pharmtox-010716-104735. [DOI] [PubMed] [Google Scholar]
- 350.Williams DM, Nawaz A, Evans M. Drug therapy in obesity: a review of current and emerging treatments. Diabetes Ther. 2020;11((6)):1199–216. doi: 10.1007/s13300-020-00816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356((15)):1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 352.Ruscitti P, Masedu F, Alvaro S, Airò P, Battafarano N, Cantarini L, et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): a multicentre, open-label, randomised controlled trial. PLoS Med. 2019;16((9)):e1002901. doi: 10.1371/journal.pmed.1002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Buttgereit F, Burmester GR, Lipworth BJ. Optimised glucocorticoid therapy: the sharpening of an old spear. Lancet. 2005;365((9461)):801–3. doi: 10.1016/S0140-6736(05)17989-6. [DOI] [PubMed] [Google Scholar]
- 354.Prabhu S, Deng H, Cross TL, Shahoei SH, Konopka CJ, Gonzalez Medina N, et al. Nanocarriers targeting adipose macrophages increase glucocorticoid anti-inflammatory potency to ameliorate metabolic dysfunction. Biomater Sci. 2021 Jan 21;9((2)):506–18. doi: 10.1039/d0bm01142h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Charles-Messance H, Mitchelson KAJ, De Marco Castro E, Sheedy FJ, Roche HM. Regulating metabolic inflammation by nutritional modulation. J Allergy Clin Immunol. 2020;146((4)):706–20. doi: 10.1016/j.jaci.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 356.Alsaggar M, Bdour S, Ababneh Q, El-Elimat T, Qinna N, Alzoubi KH. Silibinin attenuates adipose tissue inflammation and reverses obesity and its complications in diet-induced obesity model in mice. BMC Pharmacol Toxicol. 2020;21((1)):8. doi: 10.1186/s40360-020-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Li D, Zhang T, Lu J, Peng C, Lin L. Natural constituents from food sources as therapeutic agents for obesity and metabolic diseases targeting adipose tissue inflammation. Crit Rev Food Sci Nutr. 2020 May 28;:1–19. doi: 10.1080/10408398.2020.1768044. [DOI] [PubMed] [Google Scholar]
- 358.Lancaster GI, Febbraio MA. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014;35((6)):262–9. doi: 10.1016/j.it.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 359.Schmitz J, Evers N, Awazawa M, Nicholls HT, Brönneke HS, Dietrich A, et al. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol Metab. 2016;5((5)):328–39. doi: 10.1016/j.molmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Surendar J, Karunakaran I, Frohberger SJ, Koschel M, Hoerauf A, Hübner MP. Macrophages mediate increased CD8 T cell inflammation during weight loss in formerly obese mice. Front Endocrinol. 2020;11:257. doi: 10.3389/fendo.2020.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Blaszczak AM, Bernier M, Wright VP, Gebhardt G, Anandani K, Liu J, et al. Obesogenic memory maintains adipose tissue inflammation and insulin resistance. Immunometabolism. 2020;2((3)):e200023. doi: 10.20900/immunometab20200023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Jung DY, Ko HJ, Lichtman EI, Lee E, Lawton E, Ong H, et al. Short-term weight loss attenuates local tissue inflammation and improves insulin sensitivity without affecting adipose inflammation in obese mice. Am J Physiol Endocrinol Metab. 2013;304((9)):E964–76. doi: 10.1152/ajpendo.00462.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Fazeli PK, Zhang Y, O'Keefe J, Pesaresi T, Lun M, Lawney B, et al. Prolonged fasting drives a program of metabolic inflammation in human adipose tissue. Mol Metab. 2020;42:101082. doi: 10.1016/j.molmet.2020.101082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Tareen SHK, Kutmon M, de Kok TM, Mariman ECM, van Baak MA, Evelo CT, et al. Stratifying cellular metabolism during weight loss: an interplay of metabolism, metabolic flexibility and inflammation. Sci Rep. 2020;10((1)):1651. doi: 10.1038/s41598-020-58358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Aleman JO, Iyengar NM, Walker JM, Milne GL, Da Rosa JC, Liang Y, et al. Effects of rapid weight loss on systemic and adipose tissue inflammation and metabolism in obese postmenopausal women. J Endocr Soc. 2017;1((6)):625–37. doi: 10.1210/js.2017-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2((2)):1143–211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Thyfault JP, Booth FW. Lack of regular physical exercise or too much inactivity. Curr Opin Clin Nutr Metab Care. 2011;14((4)):374–8. doi: 10.1097/MCO.0b013e3283468e69. [DOI] [PubMed] [Google Scholar]
- 368.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346((6)):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11((9)):607–15. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 370.Kawanishi N, Mizokami T, Yano H, Suzuki K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med Sci Sports Exerc. 2013;45((9)):1684–93. doi: 10.1249/MSS.0b013e31828ff9c6. [DOI] [PubMed] [Google Scholar]
- 371.Kawanishi N, Niihara H, Mizokami T, Yano H, Suzuki K. Exercise training attenuates adipose tissue fibrosis in diet-induced obese mice. Biochem Biophys Res Commun. 2013;440((4)):774–9. doi: 10.1016/j.bbrc.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 372.Kawanishi N, Niihara H, Mizokami T, Yada K, Suzuki K. Exercise training attenuates neutrophil infiltration and elastase expression in adipose tissue of high-fat-diet-induced obese mice. Physiol Rep. 2015;3((9)):e12534. doi: 10.14814/phy2.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–18. [PubMed] [Google Scholar]
- 374.Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol Endocrinol Metab. 2009;296((5)):E1164–71. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290((5)):E961–7. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 376.Cizkova T, Stepan M, Dadova K, Ondrujova B, Sontakova L, Krauzova E, et al. Exercise training reduces inflammation of adipose tissue in the elderly: cross-sectional and randomized interventional trial. J Clin Endocrinol Metab. 2020;105((12)):dgaa63. doi: 10.1210/clinem/dgaa630. [DOI] [PubMed] [Google Scholar]
- 377.Mardare C, Krüger K, Liebisch G, Seimetz M, Couturier A, Ringseis R, et al. Endurance and resistance training affect high fat diet-induced increase of ceramides, inflammasome expression, and systemic inflammation in mice. J Diabetes Res. 2016;2016:4536470. doi: 10.1155/2016/4536470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Silveira LS, Biondo LA, de Souza Teixeira AA, de Lima Junior EA, Castoldi A, Camara NOS, et al. Macrophage immunophenotype but not anti-inflammatory profile is modulated by peroxisome proliferator-activated receptor gamma (PPARgamma) in exercised obese mice. Exerc Immunol Rev. 2020;26:10–22. [PubMed] [Google Scholar]
- 379.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17((8)):884–6. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 380.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84((5)):1271–8. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 381.Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999;515((Pt 1)):287–91. doi: 10.1111/j.1469-7793.1999.287ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Salas-Salvado J, Diaz-Lopez A, Ruiz-Canela M, Basora J, Fito M, Corella D, et al. Effect of a lifestyle intervention program with energy-restricted mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-plus trial. Diabetes Care. 2019;42((5)):777–88. doi: 10.2337/dc18-0836. [DOI] [PubMed] [Google Scholar]
- 383.Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50((4)):1105–12. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 384.Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55((6)):1738–45. doi: 10.1002/hep.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Haus JM, Solomon TP, Kelly KR, Fealy CE, Kullman EL, Scelsi AR, et al. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2013;98((7)):E1181–8. doi: 10.1210/jc.2013-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Kawanishi N, Yano H, Mizokami T, Takahashi M, Oyanagi E, Suzuki K. Exercise training attenuates hepatic inflammation, fibrosis and macrophage infiltration during diet induced-obesity in mice. Brain Behav Immun. 2012;26((6)):931–41. doi: 10.1016/j.bbi.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 387.Lehnig AC, Stanford KI. Exercise-induced adaptations to white and brown adipose tissue. J Exp Biol. 2018;221((Pt Suppl 1)) doi: 10.1242/jeb.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes. 2015;64((7)):2361–8. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Riis S, Christensen B, Nellemann B, Møller AB, Husted AS, Pedersen SB, et al. Molecular adaptations in human subcutaneous adipose tissue after ten weeks of endurance exercise training in healthy males. J Appl Physiol (1985) 2019;126((3)):569–577. doi: 10.1152/japplphysiol.00989.2018. [DOI] [PubMed] [Google Scholar]
- 390.Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EB, van der Lans AA, et al. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes. 2015;39((12)):1696–702. doi: 10.1038/ijo.2015.130. [DOI] [PubMed] [Google Scholar]
- 391.Sparks LM, Johannsen NM, Church TS, Earnest CP, Moonen-Kornips E, Moro C, et al. Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J Clin Endocrinol Metab. 2013;98((4)):1694–702. doi: 10.1210/jc.2012-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, McFarlin BK, et al. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun. 2005;19((5)):389–97. doi: 10.1016/j.bbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 393.Lancaster GI, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, et al. The physiological regulation of toll-like receptor expression and function in humans. J Physiol. 2005;563((Pt 3)):945–55. doi: 10.1113/jphysiol.2004.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Flynn MG, McFarlin BK, Phillips MD, Stewart LK, Timmerman KL. Toll-like receptor 4 and CD14 mRNA expression are lower in resistive exercise-trained elderly women. J Appl Physiol. 2003;95((5)):1833–42. doi: 10.1152/japplphysiol.00359.2003. [DOI] [PubMed] [Google Scholar]
- 395.Oliveira AG, Carvalho BM, Tobar N, Ropelle ER, Pauli JR, Bagarolli RA, et al. Statement of Retraction. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes. 2011;60:784–796. doi: 10.2337/db09-1907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 396.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8((8)):457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 397.Christensen RH, Lehrskov LL, Wedell-Neergaard AS, Legaard GE, Ried-Larsen M, Karstoft K, et al. Aerobic exercise induces cardiac fat loss and alters cardiac muscle mass through an interleukin-6 receptor-dependent mechanism: cardiac analysis of a double-blind randomized controlled clinical trial in abdominally obese humans. Circulation. 2019;140((20)):1684–6. doi: 10.1161/CIRCULATIONAHA.119.042287. [DOI] [PubMed] [Google Scholar]
- 398.Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285((2)):E433–7. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 399.Wueest S, Konrad D. The controversial role of IL-6 in adipose tissue on obesity-induced dysregulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2020;319((3)):E607–E13. doi: 10.1152/ajpendo.00306.2020. [DOI] [PubMed] [Google Scholar]
- 400.Zhao Z, Ukidve A, Kim J, Mitragotri S. Targeting strategies for tissue-specific drug delivery. Cell. 2020;181((1)):151–67. doi: 10.1016/j.cell.2020.02.001. [DOI] [PubMed] [Google Scholar]