Abstract
Aims
This study aimed to assess the sex‐specific distribution of heart failure (HF) with preserved, mid‐range, and reduced ejection fraction across three health care settings.
Methods and results
In this descriptive observational study, we retrieved the distribution of HF types [with reduced ejection fraction (HFrEF), mid‐range ejection fraction (HFmrEF), and preserved ejection fraction (HFpEF)] for men and women between 65 and 79 years of age in three health care settings from a single country: (i) patients with screening‐detected HF in the high‐risk community (i.e. those with shortness of breath, frailty, diabetes mellitus, and chronic obstructive pulmonary disease) from four screening studies, (ii) patients with confirmed HF from primary care derived from a single observational study, and (iii) patients with confirmed HF from outpatient cardiology clinics participating in a registry. Among 1407 patients from the high‐risk community, 288 had screen‐detected HF (15% HFrEF, 12% HFmrEF, 74% HFpEF), and 51% of the screen‐detected HF patients were women. In both women (82%) and men (65%), HFpEF was the most prevalent HF type. In the routine general practice population (30 practices, 70 000 individuals), among the 160 confirmed HF cases, 35% had HFrEF, 23% HFmrEF, and 43% HFpEF, and in total, 43% were women. In women, HFpEF was the most prevalent HF type (52%), while in men, this was HFrEF (41%). In outpatient cardiology clinics (n = 34), of the 4742 HF patients (66% HFrEF, 15% HFmrEF, 20% HFpEF), 36% were women. In both women (56%) and men (71%), HFrEF was the most prevalent HF type.
Conclusions
Both HF types and sex distribution vary considerably in HF patients of 65–79 years of age among health care settings. From the high‐risk community through to general practice to the cardiology outpatient setting, there is a shift in HF type from HFpEF to HFrEF and a decrease in the proportion of HF patients that are women.
Keywords: Heart failure, Sex differences, HFrEF, HFmrEF, HFrEF, Screening
Introduction
The landscape of heart failure (HF) has changed dramatically over the last few decades. HF with preserved ejection fraction (HFpEF) has now become a major health care challenge, as despite its high prevalence and growing incidence in the older population, there is a distinct lack of evidence‐based prognostic therapies. 1 , 2
In the last two decades, evidence‐based disease‐modifying drugs and multidisciplinary HF outpatient programmes have greatly improved the care of patients with HF, which is particularly true for those with reduced ejection fraction (HFrEF). 3 , 4 , 5 , 6 By transition of key elements of such programmes, near home management of stable HFrEF patients by general practitioners, preferably supported by HF nurses, and/or by eHealth, seems feasible and can lead to reduction of use of resources and achieve ‘best care on the right place’. 4 , 5 , 7 , 8 , 9
Most patients with HF are diagnosed in primary care. 6 , 10 In general practice, the diagnosis of HF is typically based on symptoms and signs, followed by electrocardiography (ECG), and preferably by the addition of natriuretic peptide measurements. If abnormal, referral should follow for echocardiography to confirm the diagnosis and differentiate between the three main HF types, that is, HFpEF, HF with mid‐range ejection fraction (HFmrEF), and HFrEF, and to identify correctable abnormalities. These cases are nearly always of slow onset; acute onset HF is generally diagnosed in the hospital, sometimes preceded by an initial period with complaints unrecognized as HF symptoms.
Previous studies reported that HFpEF mainly affects older women, whereas HFrEF affects younger men. 1 , 2 , 11 Despite these observations, there is a lack of comparison on the distribution of sex and different types of HF over different health care settings: community, general practice, and cardiology hospital‐based setting. This information may contribute to early diagnosis of HF and tailored management of HF patients in the specific health care setting.
We therefore assessed the distribution of sex and HF types (HFrEF, HFmrEF, and HFpEF) in an older population (65 to 79 years) among community‐dwelling high‐risk men and women with screening‐detected HF, in HF patients from primary care, and in HF patients in the cardiology outpatient setting.
Methods
Study design, study population, and outcome definition
In this descriptive study, we included six studies: four cross‐sectional screening studies among older high‐risk community people (463 HF patients), one study from general practice (434 HF patients), and a large registry study of outpatient cardiology departments containing data from 10 910 HF patients (Table 1 ). The studies were conducted between 2001 and 2016 in the Netherlands and are described in detail elsewhere. 12 , 13 , 14 , 15 , 16 , 17
Table 1.
Study characteristics and diagnostic criteria to define heart failure
| Domain | High‐risk community | General practice | Cardiology OPC | ||||
|---|---|---|---|---|---|---|---|
| van Riet | van Mourik | Boonman‐de Winter | Rutten | Valk | Brugts | ||
| Design | Cross‐sectional | Cross‐sectional | Cross‐sectional | Cross‐sectional | Cross‐sectional | Cross‐sectional | |
| Time window | 2010–2012 | 2010–2012 | 2009–2010 | 2001–2003 | 2011 | 2013–2016 | |
| Study population | ≥65 years old. No previous diagnosis of heart failure. Presented in primary care with shortness of breath on exertion. | ≥65 years old. No previous diagnosis of heart failure. Classified as frail (≥ chronic or vitality threatening diseases and/or using ≥5 prescribed drugs daily during the last year) and exercise intolerance and/or dyspnoea. | ≥60 years old. No previous diagnosis of heart failure. Diagnosis of diabetes mellitus type 2. | ≥65 years old. No previous diagnosis of heart failure. General practitioner's diagnosis of COPD based on the ICPC codes R91 or R95. | Community‐dwelling individuals registered in primary care with a heart failure diagnosis (International Classification of Primary Care code K77) during at least two encounters. | ≥18 years old. Diagnosed with chronic heart failure and treated at Dutch outpatient heart failure clinics. | |
| Heart failure diagnosis and definition | Expert panel decision. Signs and symptoms of heart failure and structural or functional echocardiographic evidence of cardiac dysfunction at rest. | Expert panel decision. Signs and symptoms of heart failure and structural or functional echocardiographic evidence of cardiac dysfunction at rest. | Expert panel decision. Signs and symptoms of heart failure and structural or functional echocardiographic evidence of cardiac dysfunction at rest. | Expert panel decision. Signs and symptoms of heart failure and structural or functional echocardiographic evidence of cardiac dysfunction at rest. | Expert panel decision. Signs and symptoms of heart failure and structural or functional echocardiographic evidence of cardiac dysfunction at rest. | Cardiologists at individual centres. Signs and symptoms of heart failure and structural or functional echocardiographic evidence of cardiac dysfunction at rest. | |
| Participants without a previous heart failure diagnosis (n) | 585 | 370 | 581 | 405 | Participants (n) | 683 | 10 910 |
| Prevalence of newly detected heart failure (%) | 16% | 35% | 28% | 21% | Prevalence of confirmed heart failure (%) | 64% | 100% |
COPD, chronic obstructive pulmonary disease; OPC, outpatient clinic.
Screening studies in high‐risk community patients
Between 2010 and 2012, van Riet and co‐workers screened for HF in 585 patients aged ≥65 years presenting to general practice with shortness of breath (SOB) on exertion in the previous 12 months and unknown with a history of established HF. 12 All participants underwent history taking, physical examination, ECG, and a blood test for measurement of N‐terminal pro B‐type natriuretic peptide (NT‐proBNP). Only those with an abnormal electrocardiogram or NT‐proBNP level exceeding the exclusionary cut‐point for non‐acute onset HF of >125 pg/mL underwent echocardiography. An expert panel established presence or absence of HF according to the criteria of the 2012 European Society of Cardiology (ESC) HF guidelines. 18
Also between 2010 and 2012, van Mourik and co‐workers screened for HF in 570 community‐dwelling frail persons aged ≥65 years, unknown with a history of HF with a two‐step screening strategy. 13 First, they received a questionnaire about SOB and exercise tolerance. Those with exercise intolerance and/or SOB were invited to visit the general practice for a local screening programme, similarly as in the study of van Riet et al. Again, the final diagnosis was determined by a panel of experts based on all available diagnostic data, and presence or absence of HF established according to the criteria of the 2008 ESC HF guidelines, which are similar to the 2012 guidelines on HF. 19
Boonman‐de Winter and co‐workers screened 581 patients aged 60 years or over with type 2 diabetes (T2D) and without a history of HF. 14 Between February 2009 and March 2010, these patients underwent a similar standardized diagnostic work‐up, and an expert panel decided on presence or absence of HF according to the criteria of the 2008 ESC HF guidelines. 19
Between 2001 and 2003, Rutten and co‐workers screened 405 participants aged 65 years of over with a general practitioner's diagnosis of chronic obstructive pulmonary disease (COPD) and unknown with a history of HF. 15 Also in this study, a similar diagnostic work‐up including echocardiography and an expert panel was used to establish presence or absence of HF according to the diagnostic criteria of the 2001 ESC HF guidelines, which are similar to the 2012 guidelines on HF. 20
Routine general practice care
Valk and co‐workers conducted a cross‐sectional study among 683 patients from 30 general practices (70 000 individuals enlisted) with a general practitioner's diagnosis of HF between June and November 2011. 16 Information on the diagnosis, medical history, medication use, and laboratory tests were collected from electronic medical record. An expert panel consisting of two cardiologists and an experienced general practitioner used all available diagnostic information and adjudicated the presence or absence of HF according to the criteria of the 2012 ESC HF guidelines. 18 In 434 patients (63.5%), HF was established by the panel and these patients were included in the current study.
Outpatient cardiology clinics
Brugts and co‐workers studied 10 910 patients with established HF receiving routine care at 34 Dutch cardiology outpatient centres in the period 2013–2016 and who were enrolled in the CHECK‐HF registry. 17 HF was diagnosed, similarly as in the screening studies and primary care study, that is, signs and symptoms suggestive of HF plus structural and/or functional cardiac abnormalities with echocardiography, and in accordance with the 2012 ESC guidelines. 18
Outcome definition
The objective of this study is to assess the distribution of HF type (i) in those with screening‐detected HF in the high‐risk community, (ii) in patients with confirmed HF from primary care, and (iii) in those with confirmed HF in the outpatient cardiology clinics. For the current study, we reclassified the types of HF in the six studies according to the 2016 ESC guidelines on HF into the following: HFrEF if left ventricular ejection fraction (LVEF) < 40%, HFmrEF if LVEF 40–49%, and HFpEF if LVEF ≥ 50%. 6 An overview of the studies with patient characteristics and the diagnostic criteria used to define HF is given in Table 1 .
Ethical approval
The studies conformed to the principles outlined in the Declaration of Helsinki. 22 The studies of van Riet, van Mourik, and Rutten were approved by the Medical Ethics Committee of the University Medical Center Utrecht, the Netherlands; all participants gave written informed consent. The study of Boonman‐de Winter was approved by the institutional review board of the University Medical Center Utrecht and the Admiraal de Ruyter Hospital in Goes, the Netherlands; all participants gave written informed consent. The study of Valk was approved by the Regional Medical Ethics Committee (Verenigde Commissies Mensgebonden Onderzoek—VCMO) of four hospitals in the Utrecht region, including the Meander Medical Center in Amersfoort, the Netherlands. The CHECK‐HF registry study was approved by the ethics committee of Maastricht University Medical Center 2017, Maastricht, the Netherlands.
Statistical analysis
For men and women, we extracted age‐specific data regarding HFrEF, HFmrEF, and HFpEF diagnosis in strata of 5 years (65–69; 70–74; 75–79) from the three health care settings and for each outpatient cardiology centre separately. As age categories such as <65 and >80 years hamper comparability of prevalence estimates between studies due to the unknown age range and distribution, we restricted ourselves to the 5 year strata between 65 and 79 years because all studies provided these age‐specific categories. Results are presented in absolute numbers and percentages.
Patient characteristics are presented as counts and percentages per health care setting for the following comorbidities: ischaemic heart disease, atrial fibrillation, diabetes, renal dysfunction, COPD, hypertension, hyperlipidaemia, peripheral artery disease, stroke, or transient ischaemic attack. If (i) studies used different definitions and (ii) if more than 10% of values were missing, the information was not presented.
All visualizations were performed in R statistical software Version 4.3, Foundation for Statistical Computing, Vienna, Austria. 23
Results
In the high‐risk community, among 1407 screened patients between 65 and 79 years old (703 women and 704 men), 288 (20.5%) had screen‐detected HF. In the routine general practice, there were 160 confirmed cases of HF in patients between 65 and 79 years old. In the outpatient cardiology clinics, there were 4742 HF patients between 65 and 79 years old with numerically known LVEF.
Sex and heart failure type distribution
In the high‐risk community, 146 of the 288 screening‐detected HF patients were women (51%). Of the 288 patients, 15% had HFrEF, 12% HFmrEF, and 74% HFpEF. HFpEF was the most prevalent screening‐detected type of HF for both men and women, being present in 82% of women and in 65% of men with HF (Figure 1 , Table 2 ).
Figure 1.
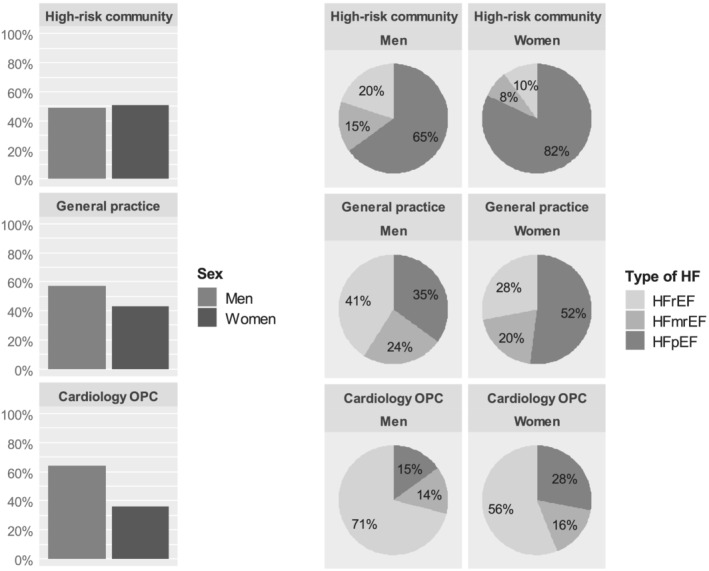
Distribution of sex and HFrEF, HFmrEF, and HFpEF in the high‐risk community, general practice, and cardiology outpatient clinics. HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; OPC, outpatient clinic.
Table 2.
Age‐specific and sex‐specific number of patients with heart failure with reduced, mid‐range, and preserved ejection fraction in the high‐risk community, general practice, and outpatient cardiology clinics
| High‐risk community | |||||||
|---|---|---|---|---|---|---|---|
| Age (years) | Men (n = 142) | Women (n = 146) | |||||
| HFrEF | HFmrEF | HFpEF | HFrEF | HFmrEF | HFpEF | Total | |
| 65–69 | 9 | 11 | 26 | 3 | 1 | 16 | 66 (23%) |
| 70–74 | 12 | 7 | 28 | 6 | 3 | 53 | 109 (38%) |
| 75–79 | 7 | 4 | 38 | 5 | 8 | 51 | 113 (39%) |
| Total | 28 (10%) | 22 (8%) | 92 (32%) | 14 (5%) | 12 (4%) | 120 (42%) | 288 (100%) |
| General practice | |||||||
|---|---|---|---|---|---|---|---|
| Age (years) | Men (n = 91) | Women (n = 69) | |||||
| HFrEF | HFmrEF | HFpEF | HFrEF | HFmrEF | HFpEF | Total | |
| 65–69 | 7 | 3 | 3 | 4 | 2 | 5 | 24 (15%) |
| 70–74 | 9 | 9 | 11 | 6 | 8 | 8 | 51 (32%) |
| 75–79 | 21 | 10 | 18 | 9 | 4 | 23 | 85 (53%) |
| Total | 37 (23%) | 22 (14%) | 32 (20%) | 19 (12%) | 14 (9%) | 36 (23%) | 160 (100%) |
| Cardiology OPC | |||||||
|---|---|---|---|---|---|---|---|
| Age (years) | Men (n = 3050) | Women (n = 1692) | |||||
| HFrEF | HFmrEF | HFpEF | HFrEF | HFmrEF | HFpEF | Total | |
| 65–69 | 652 | 116 | 144 | 243 | 73 | 103 | 1331 (28%) |
| 70–74 | 738 | 142 | 149 | 311 | 78 | 163 | 1581 (33%) |
| 75–79 | 771 | 167 | 171 | 402 | 117 | 202 | 1830 (39%) |
| Total | 2161 (46%) | 425 (9%) | 464 (10%) | 956 (20%) | 268 (6%) | 468 (10%) | 4742 (100%) |
HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; OPC, outpatient clinic.
In the routine general practice, 69 of the 160 confirmed HF patients were women (43%). Of the 160 patients, 35% had HFrEF, 23% HFmrEF, and 43% HFpEF. In women, HFpEF was the most prevalent HF type (52%), while in men, this was HFrEF (41%).
In the outpatient cardiology clinics, 1692 of the 4742 HF patients were women (36%). Of the 4742 patients, 66% had HFrEF, 15% HFmrEF, and 20% HFpEF. HFrEF was the most prevalent HF type in both women (56%) and men (71%). The distribution of HFrEF, HFmrEF, and HFpEF varied considerably between the 34 outpatient cardiology centres, ranging from 32% to 91% for HFrEF, 0% to 29% for HFmrEF, and 1% to 40% for HFpEF.
Comorbidities
In all three domains, patients with HFrEF and HFmrEF more often had a history of ischaemic heart disease than patients with HFpEF. In addition, in the general practice and the outpatient cardiology centres, patients with HFpEF more often had a history of atrial fibrillation and hypertension (Table 3 ). Although renal dysfunction is important in HF, unfortunately information on that topic was insufficient for presentation due to differences between studies in definition, and missing data (20%) from routine care.
Table 3.
Baseline characteristics of included patient populations: patients with heart failure between 65 and 79 years old
| High‐risk community | ||||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| HFrEF (n = 28) | HFmrEF (n = 22) | HFpEF (n = 92) | HFrEF (n = 14) | HFmrEF (n = 12) | HFpEF (n = 120) | |
| Ischaemic heart disease, n (%) | 9 (32) | 12 (55) | 38 (41) | 6 (43) | 5 (42) | 28 (23) |
| Atrial fibrillation, n (%) | 3 (11) | 2 (9) | 13 (14) | 2 (14) | 4 (33) | 14 (12) |
| Diabetes, n (%) a | 1 (5) | 5 (24) | 24 (38) | 3 (27) | 0 (0) | 24 (34) |
| COPD, n (%) b | 4 (36) | 5 (63) | 16 (20) | 3 (33) | 1 (13) | 15 (14) |
| Hypertension, n (%) | 13 (46) | 10 (46) | 66 (72) | 10 (71) | 8 (67) | 94 (78) |
| Hypercholesterolaemia, n (%) c | 10 (36) | 8 (36) | 51 (55) | 8 (57) | 8 (67) | 68 (57) |
| Peripheral artery disease, n (%) | 3 (11) | 4 (18) | 11 (12) | 1 (7) | 1 (8) | 10 (8) |
| Stroke or TIA, n (%) | 5 (18) | 2 (9) | 13 (14) | 0 (0) | 2 (17) | 17 (14) |
| General practice | ||||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| HFrEF (n = 37) | HFmrEF (n = 22) | HFpEF (n = 32) | HFrEF (n = 19) | HFmrEF (n = 14) | HFpEF (n = 36) | |
| Ischaemic heart disease, n (%) | 25 (68) | 10 (46) | 10 (31) | 11 (58) | 5 (36) | 9 (25) |
| Atrial fibrillation, n (%) | 14 (38) | 12 (54) | 22 (69) | 3 (16) | 7 (50) | 22 (61) |
| Diabetes, n (%) | 13 (35) | 7 (32) | 17 (53) | 8 (42) | 6 (43) | 8 (22) |
| COPD, n (%) | 4 (11) | 7 (32) | 4 (13) | 1 (5) | 6 (43) | 11 (31) |
| Hypertension, n (%) | 17 (46) | 8 (36) | 24 (75) | 9 (47) | 7 (50) | 23 (64) |
| Hypercholesterolaemia, n (%) c | 23 (62) | 14 (64) | 19 (59) | 11 (58) | 8 (57) | 20 (56) |
| Peripheral artery disease, n (%) | NA | NA | NA | NA | NA | NA |
| Stroke or TIA, n (%) | 2 (5) | 1 (5) | 8 (25) | 2 (11) | 3 (21) | 5 (14) |
| Cardiology OPC | ||||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| HFrEF (n = 2161) | HFmrEF (n = 425) | HFpEF (n = 464) | HFrEF (n = 956) | HFmrEF (n = 268) | HFpEF (n = 468) | |
| Ischaemic heart disease, n (%) | 1323 (63) | 237 (58) | 203 (45) | 405 (44) | 101 (39) | 102 (23) |
| Atrial fibrillation, n (%) | 553 (26) | 137 (33) | 175 (38) | 184 (20) | 92 (35) | 164 (35) |
| Diabetes, n (%) | 615 (32) | 130 (34) | 162 (37) | 281 (32) | 69 (28) | 184 (42) |
| COPD, n (%) | 396 (20) | 90 (24) | 104 (24) | 176 (20) | 47 (19) | 87 (20) |
| Hypertension, n (%) | 763 (40) | 173 (45) | 217 (50) | 371 (43) | 113 (47) | 250 (57) |
| Hypercholesterolaemia, n (%) | 302 (16) | 44 (12) | 49 (11) | 117 (14) | 35 (14) | 67 (15) |
| Peripheral artery disease, n (%) | 158 (8) | 19 (5) | 16 (4) | 45 (5) | 13 (5) | 20 (5) |
| Stroke or TIA, n (%) | NA | NA | NA | NA | NA | NA |
COPD, chronic obstructive pulmonary disease; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NA, not applicable; OPC, outpatient clinic; TIA, transient ischaemic attack.
Boonman‐de Winter excluded: inclusion criterion was diabetes.
Rutten et al. excluded: inclusion criterion was COPD.
Defined as using a lipid lowering drug.
Discussion
This study showed that in 65‐ to 79‐year‐old high‐risk community men and women, the most common screening‐detected HF type was HFpEF. In primary care, HFrEF was the most prevalent HF type in men and HFpEF was most prevalent in women. In similarly aged patients managed at the cardiology outpatient setting, HFrEF was the most encountered HF type for both men and women. HF was somewhat more prevalent in women than in men in the high‐risk community. In primary care, the HF population consisted of more men than women, and the vast majority of HF patients managed in the cardiology outpatient setting were men.
Clinical interpretation and implication
It is difficult to diagnose HF in the community, and screening studies in high‐risk populations (i.e. aged above 60–65 years with COPD, T2D, frail, or with SOB) showed that unrecognized HF, notably HFpEF, is very common. 12 , 13 , 14 , 15 Symptoms of HF (both HFrEF, HFmrEF, and HFpEF) are non‐specific and can easily be attributed to age, deconditioning, or to comorbidities such as COPD and T2D. Some studies suggest that comorbidities are more prevalent in patients with HFpEF, which complicates diagnosis in patients with this specific HF type even more and might therefore be a possible explanation for the relatively more HFpEF than HFrEF cases detected through screening in our high‐risk population. 23 , 24 However, in the current study, comorbidities were not more prevalent in HFpEF patients detected through screening compared with those already in routine general practice care, suggesting that number of comorbidities is not the only explanation. The differences in sex and type of HF distribution can mainly be explained by the fact that patients with HFrEF are predominantly managed in the cardiology setting, with some outpatient cardiology centres seeing hardly any HFpEF patients (this study), presumably because of the lack of treatment options for this HF type. Thus, patients with HFpEF are mainly managed in general practice with especially HFpEF remaining unnoticed or being misclassified as another disorder, for example, COPD. Management of patients with HFrEF requires strict medication adherence, up‐titration of disease‐modifying drugs along with extensive lifestyle changes including salt and fluid restriction. However, for HFpEF patients, treatment is based on restricting episodes of fluid overload and blood pressure management, which is typically performed by general practitioners together with practice nurses. In regard to HFmrEF patients, they seem to benefit from therapies that have shown to improve outcomes in patients with HFrEF. 25 , 26 , 27 These differences in management underline the importance of early diagnosis, accurate distinction between HF types and subsequently appropriate care in the right place. A second explanation for the differences we found in sex and type of HF distribution are the not yet completely unravelled biological differences between women and men, with HFpEF being more common among older women and HFrEF among younger men.
Other factors influencing the setting where HF care is delivered include severity of disease, other patient characteristics, for example, number and severity of comorbidities or patient preferences, and governmental policy. The Dutch governmental policy is to encourage the management of chronic care from hospital to primary care setting, which includes stable HF, notably, when patients are on optimal doses of medication and given the appropriate education. 8 , 28 Two trials, the Danish NorthStar trial and the Dutch Comparative Study on Guideline Adherence and Patient Compliance in Heart Failure Patients (COACH‐2) study, showed that stable HFrEF patients can be safely referred back to primary care after initial management and medical optimization in the HF outpatient clinic. 8 , 9
Caregivers need to be aware of these considerable differences in sex and HF type distribution between health care settings. Participants in the HF drug randomized controlled trials often do not reflect the HF population as seen in primary care; especially, women and older patients are much less represented and less studies have been done in HFpEF patients. 11 , 29 , 30 Thus, the vast majority of HF drug trials were performed in relatively young and predominantly male HFrEF patients with a scarcity on comorbidities in clear contrast to real life practice. 2 , 6 Given the difference in distribution of HFpEF in the community and general practice compared with the cardiology setting, these patient populations differ in severity and comorbidities. This fits with the knowledge that there is large heterogeneity among HFpEF patients, with distinct clusters of patients. 31 , 32 Importantly however, therapeutic studies in screening‐detected HFpEF patients are lacking. So findings in HFpEF patients seen in the hospital may not be applicable to this screening‐detected HFpEF population.
Comparison with other studies
Comparison with other studies is limited due to a lack of data on screening in routine general practice care with details on HF types. A retrospective cohort study focusing on gender differences in utilization of HF clinics in Canada reported that 35.5% (314 out of 884 patients) of the participants were women, which is similar to our results seen in the cardiology outpatient setting. 34 Another Canadian prospective cohort study showed that among 549 patients who were diagnosed with HF at the emergency department, men had HFrEF more often than women (58.7% in men and 39.8% in women), and these men were more likely to be referred to an HF clinic. 35 Similar to this study, a cohort study used medical record data from primary care clinics in Massachusetts, USA, to examine the difference in ethnicity, location of care (hospital or community based), and sex in regard to referral to a cardiologist. In the 4444 patients with reported HF, they found that women were less likely to be referred to a cardiologist than men; however, this study did not provide information on the type of HF. 36 When investigating sex differences in HF care, it is important to consider the type of HF, which may at least partly explain the observed differences. In our study, we did not assess referral rates, but compared the ratios of HFpEF/HFmrEF/HFrEF and women/men across the primary and secondary care, and showed similar data regarding disparities in sex. However, we can, in contrast to the aforementioned studies, show that part of this disparity was related to the type of HF.
Strength and limitations
One strength of our analysis is the use of the same reference for the diagnosis of HF, all based on signs and symptoms suggestive of HF and structural and/or functional abnormalities with echocardiography. In addition, a panel of clinical experts was used in the four community and single general practice study. All studies used the criteria of the ESC HF guidelines and were executed in a single country. In the Netherlands, everybody has a health insurance and access to the health care system, and therefore, differences in percentages we observed were not due to limitations in access to health care services or differences in re‐imbursement.
A limitation is the relatively small sample size of the single general practice study; this may affect precision of the estimates, but it has no effect on the directions of effects of our findings. Secondly, we could not compare the quality of care provided or extract details on the severity of HF. Thirdly, because the location of HF care is influenced by the structure of national health care, governmental policy, and population structure and composition, the distribution may differ for other countries or ethnicities.
In conclusion, the types of HF and sex distribution greatly vary in HF patients of 65–79 years of age among three different health care settings. From the high‐risk community through to general practice to the cardiology outpatient setting, there is a shift in HF type from HFpEF to HFrEF and a decrease in the proportion of HF patients that are women.
Conflict of interest
H.B.R.L.R. reports grants and personal fees from Novartis, grants and personal fees from Vifor, grants and personal fees from Roche Diagnostics, grants and personal fees from Boehringer Ingelheim, and personal fees from AstraZeneca, outside the submitted work. J.B. reports grants and personal fees from Abbott, outside the submitted work.
Funding
This work was supported by several grants from the Dutch Heart Foundation: Cardiovascular Disease in the Netherlands (Hartstichting) (grant Facts and Figures to A.R.B. and I.V. and grant numbers CVON 2014‐11 RECONNECT to H.R. and F.R., CVON 2013T084 Queen of Hearts to H.R. and F.R.) and ZonMw (grant number 849100003, Reviews en Kennissynthese Gender en Gezondheid to H.R. and F.R.).
Servier, the Netherlands, funded the inclusion of data and software programme for CHECK‐HF. The steering committee of CHECK‐HF (J.B., G.L., A.H., and H.B.R.L.R.) received no funding for this project. This combined analysis was initiated by the authors and was designed, conducted, interpreted, and reported independently of the sponsor. The current study had no other funding source or any with a participating role in outcome assessment, or writing of the manuscript.
de Boer, A. R. , Vaartjes, I. , Gohar, A. , Valk, M. J. M. , Brugts, J. J. , Boonman‐de Winter, L. J. M. , van Riet, E. E. , van Mourik, Y. , Brunner‐La Rocca, H.‐P. , Linssen, G. C. M. , Hoes, A. W. , Bots, M. L. , den Ruijter, H. M. , and Rutten, F. H. (2022) Heart failure with preserved, mid‐range, and reduced ejection fraction across health care settings: an observational study. ESC Heart Failure, 9: 363–372. 10.1002/ehf2.13742.
Institution where work was performed: Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands.
References
- 1. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail 2020; 22: 1342–1356 Published online ahead of print 1 June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dunlay SH, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017; 14: 591–602. [DOI] [PubMed] [Google Scholar]
- 3. Jaarsma T, Tan B, Bos RJ, van Veldhuisen DJ. Heart failure clinics in the Netherlands in 2003. Eur J Cardiovasc Nurs 2004; 3: 271–274. [DOI] [PubMed] [Google Scholar]
- 4. Liljeroos M, Stromberg A. Introducing nurse‐led HF clinics in Swedish primary care settings. Eur J Heart Fail 2019; 21: 103–109. [DOI] [PubMed] [Google Scholar]
- 5. Van Spall HGC, Rahman T, Mytton O, Ramasundarahettige C, Ibrahim Q, Kabali C, Coppens M, Brian Haynes R, Connolly S. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta‐analysis. Eur J Heart Fail 2017; 19: 1427–1443. [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 7. Wagenaar KP, Broekhuizen BDL, Jaarsma T, Kok I, Mosterd A, Willems FF, Linssen GCM, Agema WRP, Anneveldt S, Lucas CMHB, Mannaerts HFJ, Wajon EMCJ, Dickstein K, Cramer MJ, Landman MAJ, Hoes AW, Rutten FH. Effectiveness of the European Society of Cardiology/Heart Failure Association website ‘heartfailurematters.org’ and an e‐health adjusted care pathway in patients with stable heart failure: results of the ‘e‐Vita HF’ randomized controlled trial. Eur J Heart Fail 2019; 21: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luttik MLA, Jaarsma T, van Geel PP, Brons M, Hillege HL, Hoes AW, de Jong R, Linssen G, Lok DJA, Berge M, van Veldhuisen DJ. Long‐term follow‐up in optimally treated and stable heart failure patients: primary care vs. heart failure clinic. Results of the COACH‐2 study. Eur J Heart Fail 2014; 16: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 9. Schou M, Gislason G, Videbaek L, Kober L, Tuxen C, Torp‐Pedersen C, Hildebrandt PR, Gustafsson F, NorthStar Investigators . Effect of extended follow‐up in a specialized heart failure clinic on adherence to guideline recommended therapy: NorthStar Adherence Study. Eur J Heart Fail 2014; 16: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 10. Hobbs FD, Korewicki J, Cleland JG, Eastaugh J, Freemantle N, IMPROVEMENT Investigators . The diagnosis of heart failure in European primary care: the IMPROVEMENT Programme survey of perception and practice. Eur J Heart Fail 2005. Aug; 7: 768–779. [DOI] [PubMed] [Google Scholar]
- 11. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker‐Kleiner D, Kaye DM, Ky B, Santema BT, Sliwa K, Voors AA. Sex differences in heart failure. Eur Heart J 2019; 40: 3859–3868. [DOI] [PubMed] [Google Scholar]
- 12. van Riet EES, Hoes AW, Limburg A, Landman MAJ, van der Hoeven H, Rutten FH. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur J Heart Fail 2014; 16: 772–777. [DOI] [PubMed] [Google Scholar]
- 13. van Mourik Y, Bertens LCM, Cramer MJM, Lammers JW, Reitsma JB, Moons KGM, Hoes AW, Rutten FH. Unrecognized heart failure and chronic obstructive pulmonary disease (COPD) in frail elderly detected through a near‐home targeted screening strategy. J Am Board Fam Med 2014; 27: 811–821. [DOI] [PubMed] [Google Scholar]
- 14. Boonman‐de Winter LJM, Rutten FH, Cramer MJM, Landman MJ, Liem AH, Rutten GEHM, Hoes AW. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 2012; 55: 2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rutten FH, Cramer MJM, Grobbee DE, Sachs APE, Kirkels JH, Lammers JWJ, Hoes AW. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J 2005; 26: 1887–1894. [DOI] [PubMed] [Google Scholar]
- 16. Valk MJ, Mosterd A, Broekhuizen BDL, Zuithoff NP, Landman MA, Hoes AW, Rutten FH. Overdiagnosis of heart failure in primary care: a cross‐sectional study. Br J Gen Pract 2016; 66: e587–e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brugts JJ, Linssen GCM, Hoes AW, Brunner‐La Rocca HP, CHECK‐HF investigators . Real‐world heart failure management in 10,910 patients with chronic heart failure in the Netherlands: design and rationale of the Chronic Heart failure ESC guideline‐based Cardiology practice Quality project (CHECK‐HF) registry. Neth Heart J 2018; 26: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology , Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Almenar Bonet L, Avraamides P, A Ben Lamin H, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowki P, ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 19. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole‐Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, ESC Committee for Practice Guidelines (CPG) . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 2008; 10: 933–989. [DOI] [PubMed] [Google Scholar]
- 20. Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001; 22: 1527–1560. [DOI] [PubMed] [Google Scholar]
- 21. World Medical Association . Declaration of Helsinki. Br Med J 1964; ii: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. Accessed 15 November 2020 https://www.R‐project.org/
- 23. Chamberlain AM, St Sauver JL, Gerber Y, Manemann SM, Boyd CM, Dunlay SM, Rocca WA, Finney Rutten LJ, Jiang R, Weston SA, Roger VL. Multimorbidity in heart failure: a community perspective. Am J Med 2015; 128: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Streng KW, Nauta JF, Hillege HL, Anker SD, Cleland JG, Dickstein K, Filippatos G, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Zannad F, Damman K, van der Meer P, Voors AA. Non‐cardiac comorbidities in heart failure with reduced, mid‐range and preserved ejection fraction. Int J Cardiol 2018; 271: 132–139. [DOI] [PubMed] [Google Scholar]
- 25. Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, Shiroto T, Takahashi J, Miyata S, Shimokawa H, CHART‐2 Investigators . Characterization of heart failure patients with mid‐range left ventricular ejection fraction—a report from the CHART‐2 study. Eur J Heart Fail 2017; 19: 1258–1269. [DOI] [PubMed] [Google Scholar]
- 26. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA, TOPCAT Investigators . Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016; 37: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, Swedberg K, Yusuf S, Granger CB, Pfeffer MA, McMurray JJV, Solomon SD. Heart failure with mid‐range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018; 20: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 28. Rutten FH, Gallagher J. What the general practitioner needs to know about their chronic heart failure patient. Card Fail Rev 2016; 2: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tahhan AS, Vaduganathan M, Greene SJ, Fonarow GC, Fiuzat M, Jessup M, Lindenfeld J, O'Connor CM, Butler J. Enrollment of older patients, women, and racial and ethnic minorities in comtemporary heart failure clinical trials. A systematic review. JAMA Cardiol 2018; 3: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 30. Gollop ND, Ford J, Mackeith P, Thurlow C, Wakelin R, Steel N, Fleetcroft R. Are patients in heart failure trials representative of primary care populations? A systematic review. BJGP Open 2018; 2: bjgpopen18X101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015; 131: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJV, Lindenfeld J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail 2015; 17: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abrahamyan L, Sahakyan Y, Wijeysundera HC, Krahn M, Rac VE. Gender differences in utilization of specialized heart failure clinics. J Womens Health 2018; 27: 623–629. [DOI] [PubMed] [Google Scholar]
- 34. Feldman DE, Huynh T, Lauriers JD, Giannetti N, Frenette M, Grondin F, Michel C, Sheppard R, Montigny M, Lepage S, Nguyen V, Behlouli H, Pilote L. Gender and other disparities in referral to specialized heart failure clinics following emergency department visits. J Womens Health 2013; 22: 526–531. [DOI] [PubMed] [Google Scholar]
- 35. Cook NL, Ayanian JZ, Orav EJ, Hicks LS. Differences in specialist consultations for cardiovascular disease by race, ethnicity, gender, insurance status, and site of primary care. Circulation 2009; 119: 2463–2470. [DOI] [PubMed] [Google Scholar]


