Abstract
Aims
The relationship between insulin resistance (IR) and glucose intolerance with pulmonary hypertension (PH) has been suggested in recent investigations. In the present study, we aimed to show the prevalence of IR and its correlation with haemodynamic variables as well as its prognostic significance in this group of patients.
Methods and results
Among 100 new and returning patients with PH, scheduled for right heart catheterization (RHC), 59 non‐diabetic patients were enrolled. The homeostasis model assessment of insulin resistance (HOMA‐IR) was used to assess IR. The study population were followed up for a median (interquartile range) of 48 (23–48) months for all‐cause mortalities. Most of the study population [mean (standard deviation) age of 45.9 (17.3)] were classified as class I of PH classification (47.5%). Overall, 27% of our study population had IR considering the Iranian cut points of HOMA‐IR. The prevalence of IR in non‐diabetic, non‐metabolic syndrome patients with precapillary PH (PAH) was 34.2%, which was higher than the prevalence of IR in non‐diabetic, non‐metabolic syndrome Iranian population (24.1%). There was no difference between IR and insulin sensitive (IS) groups regarding demographic and clinical findings, 6 min walk test, and laboratory and haemodynamic data in univariable and multivariable analyses. The mortality rate in the follow‐up period was 44.1%. The survival of patient with IR was slightly lower than IS patients; however, IR was not an independent predictor of death.
Conclusions
The glucose metabolism is dysregulated in patients with PH, and IR may increase the risk of adverse events among these patients.
Keywords: Pulmonary hypertension, Insulin resistance, Glucose intolerance
Introduction
Pulmonary arterial hypertension (PAH) is a disease of pulmonary vascular bed in which the remodelling, vasoconstriction, and increased thrombotic state in pulmonary vasculature lead to a progressive increase in pulmonary vascular resistance, right ventricular failure, and death. 1
The pathophysiological mechanisms involved in the development and progression of PAH are complex and the role of many genetic and environmental factors have been suggested. 2 , 3
One of the recent advances in describing the pathophysiology of PAH is the presence of an association between insulin resistance (IR) and PAH. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11
Our previous study 6 indicated that the incidence of glucose intolerance and IR could be higher in patients with PAH. However, our study population was only composed of patients with class I of PAH. In addition, we did not directly assess IR and used triglyceride (TG) to high‐density lipoprotein cholesterol (HDL‐C) ratio (TG/HDL‐C) as a surrogate of IR in our study population.
The homeostasis model assessment‐estimated insulin resistance (HOMA‐IR), developed by Matthews et al., is a convenient method for estimation of IR and has been widely used in clinical and epidemiological researches. 12 , 13
In the present study, we aimed to assess HOMA‐IR in patients with pulmonary hypertension (PH) in all PH classes [without a history of diabetes mellitus (DM)] and its prognostic significance in this group of patients.
Methods
Patient selection
The study population was selected among patients scheduled for right heart catheterization (RHC) between March 2015 and March 2016 for evaluation of PH for the first time or at follow‐up according to the following inclusion/exclusion criteria.
Inclusion criteria:
-
1‐
Presence of PH based on updated clinical classification of PH; a mean of pulmonary artery pressure (PAP) ≥ 25 mmHg in RHC
-
2‐
Age ≥ 18 years
Exclusion criteria:
-
1‐
Established DM
-
2‐
Under treatment for hyperlipidaemia
-
3‐
Presence of glucose intolerance [glycosylated haemoglobin (HbA1c) > 6%] or high blood sugar in favour of undiagnosed DM [fasting blood sugar (FBS) > 126 mg/dL or 2 h plasma glucose ≥ 200 mg/dL] in laboratory evaluations preceded RHC
-
4‐
A history of prednisolone intake or other corticosteroids
-
5‐
Decompensated right ventricular failure and/or overt fluid overload
-
6‐
Inability to perform 6 min walk test (6MWT)
-
7‐
Isolated postcapillary PH
Patients' evaluation and follow‐up
Clinical history and a thorough physical examination were obtained from all the patients. The New York Heart Association (NYHA) functional class of all study population was assessed by the same investigator.
Body weight of the study population was measured with the subject wearing a hospital gown and their height was measured without shoes. Waist circumference was measured midway between the iliac crest and the lower rib margin.
Body mass index (BMI) was calculated by the weight divided by the square of the height.
The 6MWT was performed according to the protocol of Guyatt et al. 14
All the blood analyses were performed at our laboratory on the day of RHC. Blood samples were collected from all study population after 12 h of overnight fasting. FBS was measured using enzymatic colorimetric method with glucose oxidase. HbA1c was assayed using high performance liquid chromatography (HPLC) by standardized laboratory protocol using a method certified by the National Glycohemoglobin Standardization Program. Fasting insulin was assayed by radioimmunoassay (Immunotech, Prague, Czech Republic) with a sensitivity of 0.5 μU/mL.
HOMA‐IR was calculated by the following formula:
Fasting insulin (U/L) × FBS (mg/dL)/405, as described by Matthews et al. 12 , 13
Total cholesterol was measured using enzymatic colorimetric method with cholesterol esterase and cholesterol oxidase. HDL‐C was measured after precipitation of the apolipoprotein B‐containing lipoproteins with phosphotungstic acid. Serum TG level was measured using an enzymatic colorimetric method with glycerol phosphate oxidase.
Patients were defined to have IR or insulin sensitivity (IS) based on the HOMA‐IR. The proposed value of HOMA‐IR for distinction between IS and IR was considered according to what described in an Iranian study by Esteghamati et al. 12 Accordingly, the HOMA‐IR ≥ 1.775 was considered as IR in non‐diabetic people. The cut point of HOMA‐IR for IR in those who have metabolic syndrome features is 4.325.
As our previous study, 6 we also checked the ratio of TG/HDL‐C. We defined an individual as IS when TG/HDL‐C ratio was <2.0 and IR when TG/HDL‐C ratio was >3.0.
We also defined the metabolic syndrome in our study population according to the International Diabetes Federation (IDF). 15
According to IDF definition, a person defined as having metabolic syndrome should have central obesity plus any two of the following: (i) fasting plasma glucose ≥ 100 mg/dL (or diabetes); (ii) TG ≥ 150 mg/dL; (iii) HDL < 40 mg/dL for men and <50 mg/dL for women; and (iv) systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg.
The central obesity, based on cut‐off points of the Iranian population, is defined as waist circumference > 90 cm in males and females. 15
Using HOMA‐IR cut‐off, the prevalence of IR in those without and with IDF defined‐metabolic syndrome is considered 24.1% and 42.5%, respectively, in Iranian population. 12
Right heart catheterization was performed by standard method in all patients in catheterization laboratory. The following variables were measured for each patient: mean right atrial pressure (RAP); systolic and end‐diastolic right ventricular pressure; systolic, diastolic, and mean PAP; pulmonary capillary wedge pressure (PCWP); and mixed venous oxygen saturation and cardiac output (CO) by the Fick method. The cardiac index (CI) was calculated by dividing CO to body surface area (BSA). The European Society of Cardiology 2015 guideline in diagnosis and management of PH was considered to define the presence of PH and determine the haemodynamic category (precapillary vs. postcapillary) as well as the PH World Health Organization (WHO) group of each patient. 1
All selected patients were followed up until the end of 2019 for all causes of death by reviewing the hospital records or by contacting them on the phone.
This study was approved by the research and ethics committee of Rajaie Cardiovascular Medical and Research Center, and written informed consent was obtained from all patients.
Mortality risk assessment
For the mortality risk assessment of study participant, the simplified risk assessment tool as described by Boucly et al. was used. 16
Four variables, namely, NYHA functional class, 6MWT distance, RAP, and CI, were considered for this risk stratification. The study participants were divided into low and high risk according to these criteria. The patients were categorized as low risk if they had three to four of four low‐risk criteria.
Low‐risk criteria:
-
1‐
NYHA class I–II
-
2‐
6MWT distance > 440 m
-
3‐
RAP < 8 mmHg
-
4‐
CI ≥ 2.5 L/min/m2
Study endpoints
The primary endpoint of the study was to determine the prevalence of IR in patients with PH and its different subgroups.
The secondary endpoint was the prevalence and the predictors of all causes of mortality and its relationship with IR.
Statistical analysis
IBM SPSS Statistics 19 (IBM SPSS Statistics for Windows, Version 19.0, Armonk, NY, USA: IBM Corp.) was used for all statistical analyses. The normality of distribution for all variables was verified by the one‐sample Kolmogorov–Smirnov test. Categorical variables were reported as number (percentage), and quantitative variables were expressed as mean (standard deviation) or median (interquartile range, IQR) as appropriate. The Student's t‐test, χ 2 test, and Mann–Whitney test were used for comparisons and associations, as appropriate. Relationships were assessed using the Pearson or Spearman correlation coefficient depending on their distribution. P value < 0.05 was considered significant.
Results
Among 100 new and returning patients with PH scheduled for RHC between 2015 and 2016, a total of 59 patients (54.2% female) were enrolled according to our inclusion/exclusion criteria.
Table 1 depicts clinical, laboratory, and haemodynamic characteristics of all study participants, IR, and IS subgroups.
Table 1.
Clinical, laboratory, and haemodynamic characteristics of all study participants, insulin resistance, and insulin sensitive subgroups, n = 59
| Variables | All study participants N = 59 | Insulin resistance N = 16 | Insulin sensitive N = 43 | P value a |
|---|---|---|---|---|
| Sex (female), number (%) | 32 (54.9) | 9 (57) | 23 (53) | 0.02 |
| Age, years, mean (SD) | 45.9 (17.3) | 46.2 (19.9) | 45.8 (16.5) | 0.1 |
| Body mass index, kg/m2, mean (SD) | 23.9 (4.9) | 24.3 (5.8) | 23.8 (4.6) | 0.01 |
| Waist circumference, cm, mean (SD) | 83.8 (17.6) | 84.7 (17) | 82 (16) | 0.4 |
| History of hypertension, number (%) | 10 (17) | 2 (12.5) | 8 (18.6) | 0.5 |
| Central obesity, number (%) | 22 (37) | 5 (31.2) | 17 (39.5) | 0.5 |
| Metabolic syndrome, number (%) | 6 (10.2) | 2 (12.5) | 4 (9.3) | 0.7 |
| Pulmonary hypertension class, number (%) | ||||
| Class I | 28 (47.5) | 11 (69) | 17 (39.5) | 0.05 b |
| Class II | 17 (28.8) | 1 (6.3) | 16 (37.2) | |
| Class III | 3 (5.1) | 1 (6.3) | 2 (4.7) | |
| Class IV | 10 (16.9) | 3 (19) | 7 (16.3) | |
| Class V | 1 (1.7) | 0 | 1 (2.3) | |
| Pulmonary hypertension haemodynamic type | 0.02 | |||
| Precapillary | 42 (71.2) | 15 (25.4) | 27 (45.7) | |
| Combined postcapillary | 17 (28.8) | 1 (1.7) | 16 (27.1) | |
| NYHA functional class | 0.5 | |||
| I | 1 (1.6) | 0 | 1 (2.3) | |
| II | 26 (44) | 8 (50) | 18 (42) | |
| III | 29 (49.1) | 8 (50) | 21 (49) | |
| IV | 3 (0.5) | 0 | 3 (7) | |
| 6MWT, m, median (IQR) | 330 (290–400) | 375 (300–403) | 330 (280–390) | 0.3 |
| FBS, mg/dL, mean (SD) | 91.7 (10.7) | 91.7 (10.4) | 91.7 (10.9) | 0.9 |
| TG, mg/dL, mean (SD) | 111 (42) | 124 (50) | 106 (38) | 0.1 |
| HDL, mg/dL, mean (SD) | 39 (10) | 38 (9.5) | 40 (10) | 0.6 |
| Total cholesterol, mg/dL, mean (SD) | 145 (41) | 152 (42) | 143 (41) | 0.4 |
| HbA1c, %, median (IQR) | 5.0 (4.9–5.8) | 5.02 (4.9–5.8) | 5.2 (4.9–5.2) | 0.9 |
| TG/HDL, mg/dL, mean (SD) | 2.8 (1.07) | 3.2 (1.3) | 2.7 (0.9) | 0.1 |
| Insulin level, mIU/L, median (IQR) | 42.9 (20.9–104.9) | 142 (117–207) | 31 (20–51) | <0.0001 |
| HOMA‐IR, median (IQR) | 0.8 (0.4–1.9) | 2.5 (2.2–3.7) | 0.6 (0.4–1) | <0.0001 |
| Cardiac index, L/min/m2, mean (SD) | 2.4 (0.6) | 2.6 (0.6) | 2.3 (0.6) | 0.3 |
| Right atrial pressure, mmHg, median (IQR) | 10 (8–14) | 8.5 (7–13) | 11 (8–16) | 0.1 |
| Mean pulmonary artery pressure, mmHg, median (IQR) | 40 (30–50) | 33 (27–51) | 42 (36–50) | 0.2 |
| High‐risk criteria, number (%) | 49 (83.1) | 13 (81.3) | 36 (83.7) | 0.8 |
| Mortality rate, number (%) | 26 (44.1) | 7 (43.8) | 19 (44.2) | 0.9 |
6MWT, 6 min walk test; FBS, fasting blood sugar; HbA1c, glycosylated haemoglobin; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; IQR, interquartile range; NYHA, New York Heart Association; SD, standard deviation; TG, triglyceride.
The comparison is between insulin sensitivity and insulin resistance.
Comparison between the different pulmonary hypertension groups by Fisher's exact test.
The mean (standard deviation) of age was 45.9 (17.3) years. The majority of patients had NYHA class of II and III and considering the WHO PH classification, 47.5% were in class I (PAH), 28.8% class II (PH due to left heart), 5.1% class III (PH due to lung diseases), 16.9% class IV (chronic thromboembolic PH), and 1.7% class V (multifactorial PH including patients with haemolytic anaemias).
Regarding the haemodynamic classification of PH, 42 (71.2%) and 17 (28.8%) patients had precapillary PH and combined postcapillary PH, respectively.
The aetiological diagnoses in patients with class I of PH were idiopathic PAH and Eisenmenger syndrome in 11 and 17 patients, respectively. Ten (35%) patients had been evaluated as naïve PH and the rest of them were on specific PH therapy including phosphodiesterase 5‐inhibitors (PDE5‐I) and/or bosentan.
The most common aetiology for the patients in WHO PH group of II was dilated (ischaemic or non‐ischaemic) cardiomyopathy.
Insulin resistance
As shown in Table 1 , 16 (27.1%) patients had criteria of IR. Except one case who had combined postcapillary PH, and the rest of IR patients were in precapillary PH group. So we decided to analyse the significance of IR only in patients with precapillary PH.
Table 2 depicts the comparison of clinical, laboratory, and haemodynamic characteristics of IR and IS patients with precapillary PH (PAH).
Table 2.
Comparison of clinical, laboratory, and haemodynamic characteristics of insulin resistance and insulin sensitive patients with precapillary pulmonary hypertension (PAH), n = 42
| Variables | All patients with precapillary PH N = 42 | Insulin resistance N = 15 | Insulin sensitive N = 27 | P value |
|---|---|---|---|---|
| Sex (female), number (%) | 25 (59.5) | 9 (59) | 16 (60) | 0.9 |
| Age, years, mean (SD) | 45.9 (17.3) | 46.3 (20.6) | 42.6 (16) | 0.5 |
| Body mass index, kg/m2, mean (SD) | 23.9 (4.9) | 24.2 (6) | 23.1 (4.4) | 0.4 |
| Waist circumference, cm, mean (SD) | 81 (17) | 80 (18) | 81 (16) | 0.8 |
| History of hypertension, number (%) | 5 (11.9) | 1 (6.7) | 4 (14.8) | 0.4 |
| Central obesity, number (%) | 12 (30) | 4 (27) | 8 (29) | 0.8 |
| Metabolic syndrome, number (%) | 4 (9.5) | 2 (13.3) | 2 (7.4) | 0.5 |
| NYHA class | 0.6 | |||
| I | 1 (2.4) | 0 | 1 (3.7) | |
| II | 24 (57.1) | 8 (53) | 16 (59) | |
| III | 17 (40.5) | 7 (47) | 10 (37) | |
| IV | 0 | 0 | 0 | |
| 6MWT, m, median (IQR) | 330 (290–400) | 390 (300–405) | 380 (310–410) | 0.8 |
| FBS, mg/dL, mean (SD) | 91.7 (10.7) | 92 (9.7) | 89 (9.4) | 0.5 |
| TG, mg/dL, mean (SD) | 113.7 (41) | 128 (50) | 105 (33) | 0.09 |
| HDL, mg/dL, mean (SD) | 40 (10) | 39 (9.5) | 41 (11) | 0.6 |
| Total cholesterol, mg/dL, mean (SD) | 146 (38) | 156 (40) | 141 (37) | 0.2 |
| HbA1c, %, median (IQR) | 5.0 (4.9–5.8) | 5.0 (4.9–5.8) | 5.1 (4.8–5.9) | 0.5 |
| TG/HDL, mg/dL, mean (SD) | 2.8 (1.07) | 3.3 (1.3) | 2.7 (0.9) | 0.09 |
| Insulin level, mIU/L, median (IQR) | 42.9 (20.9–104.9) | 148.8 (121–239) | 31.3 (21–60) | <0.0001 |
| HOMA‐IR, median (IQR) | 0.8 (0.4–1.9) | 3.2 (2.4–3.7) | 0.6 (0.1–1.1) | <0.0001 |
| Cardiac index, L/min/m2, mean (SD) | 2.4 (0.6) | 2.6 (0.5) | 2.5 (0.5) | 0.6 |
| Right atrial pressure, mmHg, median (IQR) | 10 (8–14) | 8 (7–13) | 10 (8–12) | 0.6 |
| Mean pulmonary artery pressure, mmHg, median (IQR) | 40 (30–50) | 34 (27–52) | 45 (30–55) | 0.4 |
| High‐risk criteria, number (%) | 33 (78.6) | 12 (80) | 21 (78) | 0.8 |
| Mortality rate, number (%) | 14 (33.3) | 7 (47) | 7 (26) | 0.1 |
6MWT, 6 min walk test; FBS, fasting blood sugar; HbA1c, glycosylated haemoglobin; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; IQR, interquartile range; NYHA, New York Heart Association; PH, pulmonary hypertension; SD, standard deviation; TG, triglyceride.
Among 42 patients with precapillary PH, 4 patients showed the criteria of metabolic syndrome. As the patients with high fasting glucose level were excluded before enrolment, these 4 patients had central obesity plus a history of systemic hypertension or TG ≥ 150 mg/dL or HDL < 40 mg/dL for men and <50 mg/dL for women.
Thirteen of the remaining 38 patients had the criterion of IR, which means that the prevalence of IR (34.2%) in non‐diabetic, non‐metabolic syndrome PAH patients might be higher than Iranian general population.
As shown in Table 2 , there was no statistically significant difference between the groups of IR and IS in terms of demographic, clinical, BMI, features of metabolic syndrome, and haemodynamic data.
The central obesity was observed in 30% of PAH group and according to the IDF, four (9.5%) of them fulfilled the criteria for the metabolic syndrome in which 50% had IR.
As HOMA‐IR had a non‐normal distribution, Spearman's rank correlation was used to show whether there was any correlation between HOMA‐IR and TG/HDL ratio. We could not find any correlation between these two variables (Spearman's rho = 0.2, P value = 0.1); however, the TG/HDL ratio was higher in IR group and all the patients with metabolic syndrome features had a TG/HDL ratio more than 3. On the other hand, despite the fact that the difference was not statistically significant, both serum insulin levels [median (IQR) = 76 (25–149) mIU/L in those with TG/HDL > 3 vs. 56 (20–110) mIU/L in those with TG/HDL < 3, P = 0.5] and HOMA‐IR [median (IQR) = 1.4 (0.5–2.8) in those with TG/HDL > 3 vs. 1 (0.4–1.9) in those with TG/HDL < 3, P = 0.5] were higher in those with a TG/HDL ratio more than 3.
There was also no statistically significant correlation between fasting insulin, HOMA‐IR, and BMI or 6MWT distance.
Study outcomes
Mortality risk assessment
Considering the simplified mortality risk assessment tool, 49 of 59 patients could be categorized as elevated risk.
In precapillary PH group, 33 of 42 patients were categorized as elevated risk.
There was no difference between IR and IS groups in terms of the risk category (P = 0.8). However, although the HOMA‐IR level was not different in elevated risk and low‐risk groups [the median (IQR) of HOMA‐IR in elevated risk and low‐risk groups was 0.9 (0.5–2) vs. 0.8 (0.4–1.9), respectively, P = 0.8], the TG/HDL ratio was significantly higher in elevated risk group (3.05 ± 0.15 vs. 2.1 ± 0.18 mg/dL in elevated risk and low‐risk groups, respectively, P = 0.01).
Patient follow‐up
There was no missed follow‐up and we could find the destiny of all patients.
The mortality rate during a median (IQR) follow‐up period of 48 (23–48) months was 44.1%. Nine patients (five precapillary) died within a year after the RHC. The survival rate was much better in patients with precapillary PH [48 (28.5–48) months] than combined postcapillary PH [24 (18–48) months] (P = 0.002).
Survival analysis in patients with precapillary pulmonary hypertension
As mentioned above and because of the low prevalence of IR in patients with combined postcapillary PH, the prognostic importance of IR in patients with precapillary PH was separately analysed.
At the end of the follow‐up period, the mortality rate in this group of patients was 33.3%. There were 14 deaths in precapillary group, in which 6 of them was in class I and the rest were in other classes of PH.
The median (IQR) of survival in IR and IS patients was 48 (20–48) and 48 (36–48) months, respectively (P = 0.1).
Although the survival was not statistically different between the two IR and IS groups, the Kaplan–Meier curve of survival analysis showed less life span in IR patients (Figure 1 ).
Figure 1.
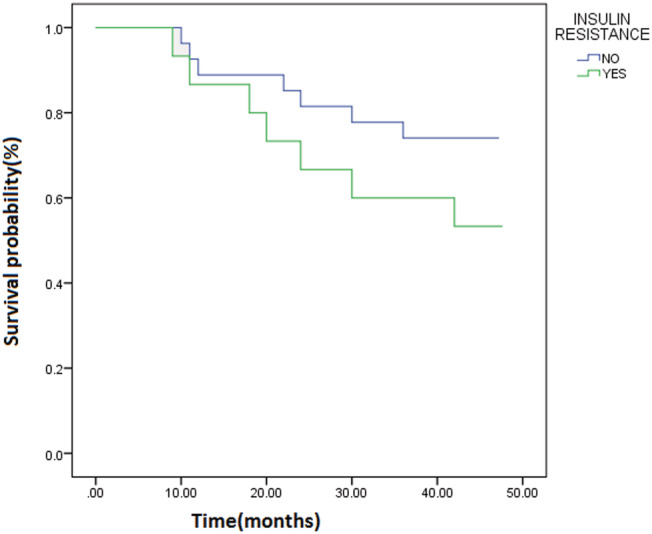
The Kaplan–Meier curve of survival analysis in patients with insulin resistance compared with insulin sensitive patients.
Table 3 depicts univariate and multivariate analyses for predictors of mortality in patients with precapillary PH.
Table 3.
Univariate and multivariate analyses for predictors of mortality in patients with precapillary pulmonary hypertension, n = 42
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Beta | P value | OR (95% CI) | Beta | P value | OR (95% CI) | |
| Age, years | 0.094 | 0.001 | 1.1 (1.03–1.16) | 0.15 | 0.005 | 1.1 (1.04–1.4) |
| Sex, male | −1.5 | 0.03 | 0.2 (0.06–0.87) | −3.3 | 0.03 | 0.04 (0.002–0.8) |
| Body mass index, kg/m2 | 0.18 | 0.03 | 1.2 (1.02–1.4) | 0.35 | 0.02 | 1.4 (1.05–1.9) |
| NYHA class | 0.64 | 0.3 | 1.9 (0.5–6.5) | |||
| Pulmonary hypertension class | 0.5 | 0.04 | 1.6 (1.01–2.6) | |||
| 6MWT, m | −0.008 | 0.07 | 0.98 (0.99–1.001) | |||
| FBS, mg/dL | −0.01 | 0.7 | 0.99 (0.92–1.05) | |||
| HbA1c | 0.19 | 0.7 | 0.8 (0.3–2.4) | |||
| TG/HDL, mg/dL | 0.23 | 0.4 | 1.2 (0.7–2.2) | |||
| Insulin level, mIU/L | 0.008 | 0.08 | 1 (0.99–1.02) | |||
| HOMA‐IR | 0.4 | 0.07 | 1.5 (0.9–2.4) | |||
| IR, yes | 0.9 | 0.1 | 2.5 (0.7–9.5) | |||
| Cardiac index, L/min/m2 | −0.4 | 0.4 | 0.6 (0.2–2.2) | 2.5 | 0.04 | 11.7 (1.02–133.9) |
| Right atrial pressure, mmHg | 0.05 | 0.5 | 1 (0.9–1.2) | |||
| Mean pulmonary artery pressure, mmHg | 0.002 | 0.9 | 1 (0.9–1.03) | |||
| High‐risk criteria | 0.6 | 0.4 | 2 (0.35–11) | |||
6MWT, 6 min walk test; CI, confidence interval; FBS, fasting blood sugar; HbA1c, glycosylated haemoglobin; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; IR, insulin resistance; NYHA, New York Heart Association; OR, odds ratio; TG, triglyceride.
Mortality was correlated with older age, male gender [9 (64.3%) males vs. 5 (35.7%) females], lower BMI, and the class of PH in univariate analyses.
People with lower 6MWT and higher HOMA‐IR had higher, but not statistically significant, mortality rate.
The multivariable analysis showed that age, male sex, BMI, and CI are independent predictors of mortality.
The IR was not correlated with all‐cause mortality in univariate and multivariate analyses.
Discussion
In the present study, we evaluated the prevalence of IR in patients with PH in all PH classes by HOMA‐IR method; a more robust and validated index for IR showed that IR is more prevalent in those with isolated precapillary PH or PAH.
More than one‐third of patients with PAH had IR in this study, which was higher than the prevalence of IR in the Iranian general population. Surprisingly, although we could show a trend towards higher mortality rate in patients with IR, despite a relatively high prevalence of IR, a significant difference in PAH aetiology, NYHA functional classification, 6MWT distance, and haemodynamic findings was not found between IS and IR PAH groups. The IR in our study population was also independent of age, sex, BMI, and features of metabolic syndrome including the TG/HDL ratio.
The subgroup analyses showed a lower prevalence of IR in combined pre–postcapillary group. Although the small number of patients in this group can be a possible explanation for this finding, a different pathophysiologic pathway rather than those related to the IR may be suggested for vascular remodelling and adding a precapillary component to a postcapillary PH.
Furthermore, IR was more prevalent in those with precapillary PH, suggesting the involvement of IR in chronic inflammatory pathways responsible for pan arteritis in precapillary PH.
Among recent advances in the underlying pathogenesis of PH, one of the most interesting aspects of research is the apparent association between PAH and IR. The main aim of these investigations would be finding the potential for novel therapeutic targets. 4 , 5 , 6 , 8 , 9 , 10 , 17 , 18 , 19 , 20
The recent investigations have shown that different classes of PH may have similar structural and functional changes in vascular bed. 3 , 17 , 21 , 22 , 23 , 24 These findings, as well as favourable clinical response to pulmonary vasodilator therapies in PH classes rather than PAH, suggest a common pathophysiologic feature. Therefore, IR as a common finding in different types of PH may contribute to similar pulmonary vascular abnormalities.
Animal models and clinical studies suggest that a variety of chemokines and pro‐inflammatory and inflammatory cytokines including receptor for advanced glycation end products (RAGE), interleukin‐6 (IL‐6), interleukin (IL‐8), tumour necrotizing factor‐alpha (TNF‐α), chemokine CXC ligand10, 13 (CXCL), interferon‐γ‐induced protein 10 (IP‐10), peroxisome proliferator‐activated receptor‐gamma (PPAR‐γ), and adiponectin contribute directly to endothelial dysfunction, ongoing inflammation, and proliferation of pulmonary artery smooth muscle cell. Many of these pro‐inflammatory and anti‐inflammatory cytokines and chemokines have been known to induce failure of pancreatic beta cells and be involved in IR process in many clinical conditions including obesity. 2 , 3 , 4 , 8 , 9 , 10 , 19 , 20 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 Mitochondrial dysfunction is an emerging concept, which has been suggested as a major pathogenic mechanism in both IR and PH. 33 , 34
Although many clinical investigations have shown the correlation between IR and PH, there are some limitations for these studies. First of all, most of them have considered the indirect evidences of IR such as TG/HDL ratio and HbA1c, which makes the results less conclusive because of the high prevalence of features of the metabolic syndrome in the general population. 5 , 6 , 11
On the other hand, the data regarding the accuracy of TG/HDL ratio in assessment of IR have been conflicting in different studies and study groups, and a strong and significant relationship could not be found in many studies such as our present study. 35 , 36 , 37
To overcome the problems regarding indirect measures for IR, Heresi et al. 19 assessed metabolic phenotyping of patients with IPAH by measuring plasma insulin and glucose and estimated IR by two validated indices of IR (the Stumvoll index 38 and HOMA‐IR). They also assessed pancreatic beta cell function in this group of patients.
They compared 14 patients with IPAH with 14 healthy subjects matched for sex, age, blood pressure, and BMI and showed a distinct pattern of metabolic abnormalities in patients with IPAH similar to those with type 2 DM suggesting an abnormal pancreatic beta cell function. 19
Association between insulin resistance and prognostic factors of pulmonary hypertension
There are conflicting data regarding the correlation between IR and PH prognostic factors and/or adverse outcomes.
Although in the current study, the patients with IR had less survival, like previous studies, no correlation between IR and clinical, 6MWT distance, haemodynamics, prognostic factors, as well as the risk of mortality was found.
However, a significant number of our study population (78.5% of patients in precapillary PH group) were categorized as high risk for mortality, which would be an explanation for lack of relationship between IR and the risk of mortality.
In a study by Zamanian et al., patients with IR had worse 6 month event‐free survival but no relationship was found between IR and NYHA class, 6MWT distance, and haemodynamic measures. 5
In a cohort by Pugh et al., there was no difference between IR and IS patients in terms of survival and other PH‐related variables including NYHA class, 6MWT distance, and haemodynamic measures. 11 However, the same study group showed significant improvement in haemodynamic measures of PH including mean PAP and PVR after 20% weight reduction following bariatric surgery in an obese female with IPAH, which was associated with a significant decrease in IR (2.6 to 1.2) as measured by the HOMA‐IR. 18
There were also no significant associations between the IR and NYHA class, echocardiography data, and haemodynamics variables in the Heresi et al. study. Albeit, this study group could show a modest correlation between IS and 6MWT distance (r = 0.55, P = 0.05). No death was reported during the following period (26 months) in this study. They have also found no correlation between IR and increased risk of hospitalization as well. 19
Study strength and limitations
The careful selection of patients among all PH classes, excluding the patients with high HbA1c level, using the Iranian cut points of HOMA‐IR for interpretation and a relatively prolonged follow‐up period (40 months) were the strength of the study.
One of the most important limitations of our study and similar studies would be using surrogates for IR including the TG/HDL ratio and HOMA‐IR.
The hyperinsulinaemic–euglycaemic clamp technique 39 , 40 is the gold standard method for assessing IS, but this method is a difficult and expensive procedure.
In conclusion, all of these clinical and basic investigations including our study suggest that the glucose metabolism is dysregulated in patient with PH and IR may be considerably prevalent in this group of patients independent of the metabolic syndrome features. The IR is involved in inflammatory process responsible for development and progression of PH and may increase the risk of adverse events among these patients.
There are many published and ongoing clinical trials targeting IR in the medical therapies of PH 18 , 41 , 42 ; however, further studies using more precise methods for defining IR are needed to clarify the prognostic significance of this finding in this group of patients.
Conflict of interest
None declared.
Acknowledgements
We would like to thank our friend Dr. Nick Austin for the language editing of the manuscript.
Zare, E. , Kafshbani, P. , Chenaghlou, M. , Noori, M. , Ghaemmaghami, Z. , Amin, A. , Taghavi, S. , and Naderi, N. (2022) Prognostic significance of insulin resistance in pulmonary hypertension. ESC Heart Failure, 9: 318–326. 10.1002/ehf2.13752.
References
- 1. Galiè N, Humbert M, Vachiery J‐L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 2. Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: S20–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JXJ, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009; 54: S10–S19. [DOI] [PubMed] [Google Scholar]
- 4. Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation 2015; 131: 1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M, Doyle RL. Insulin resistance in pulmonary arterial hypertension. Eur Respir J 2009; 33: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naderi N, Boobejame P, Bakhshandeh H, Amin A, Taghavi S, Maleki M. Insulin resistance in pulmonary arterial hypertension, is it a novel disease modifier? Res Cardiovasc Med 2014; 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. West J, Niswender KD, Johnson JA, Pugh ME, Gleaves L, Fessel JP, Hemnes AR. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J 2013; 41: 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moral‐Sanz J, Menendez C, Moreno L, Moreno E, Cogolludo A, Perez‐Vizcaino F. Pulmonary arterial dysfunction in insulin resistant obese Zucker rats. Respir Res 2011; 12: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradley EA, Bradley D. Pulmonary arterial hypertension and insulin resistance. J Mol Genet Med 2014; 2 015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trammell AW, Talati M, Blackwell TR, Fortune NL, Niswender KD, Fessel JP, Newman JH, West JD, Hemnes AR. Pulmonary vascular effect of insulin in a rodent model of pulmonary arterial hypertension. Pulm circulation 2017; 7: 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pugh M. E., Robbins I. M., Rice T.W., West J., Newman J. H., Hemnes A. R. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. The Journal of Heart and Lung Transplantation. 2011; 30(8): 904–911. 10.1016/j.healun.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esteghamati A, Ashraf H, Khalilzadeh O, Zandieh A, Nakhjavani M, Rashidi A, Haghazali M, Asgari F. Optimal cut‐off of homeostasis model assessment of insulin resistance (HOMA‐IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non‐communicable diseases in Iran (SuRFNCD‐2007). Nutr Metab (Lond) 2010; 7: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 14. Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6‐minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985; 132: 919–923. [PMC free article] [PubMed] [Google Scholar]
- 15. Esteghamati A, Abbasi M, Rashidi A, Meysamie A, Khalilzadeh O, Haghazali M, Asgari F, Nakhjavani M. Optimal waist circumference cut‐offs for the diagnosis of metabolic syndrome in Iranian adults: results of the third national survey of risk factors of non‐communicable diseases (SuRFNCD‐2007). Diabet Med 2009; 26: 745–746. [DOI] [PubMed] [Google Scholar]
- 16. Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, Picard F, de Groote P, Jevnikar M, Bergot E, Chaouat A, Chabanne C, Bourdin A, Parent F, Montani D, Simonneau G, Humbert M, Sitbon O. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. [DOI] [PubMed] [Google Scholar]
- 17. Moral‐Sanz J, Lopez‐Lopez JG, Menendez C, Moreno E, Barreira B, Morales‐Cano D, Escolano L, Fernandez‐Segoviano P, Villamor E, Cogolludo A, Perez‐Vizcaino F, Moreno L. Different patterns of pulmonary vascular disease induced by type 1 diabetes and moderate hypoxia in rats. Exp Physiol 2012; 97: 676–686. [DOI] [PubMed] [Google Scholar]
- 18. Pugh ME, Newman JH, Williams DB, Brittain E, Robbins IM, Hemnes AR. Hemodynamic improvement of pulmonary arterial hypertension after bariatric surgery: potential role for metabolic regulation. Diabetes Care 2013; 36: e32–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heresi GA, Malin SK, Barnes JW, Tian L, Kirwan JP, Dweik RA. Abnormal glucose metabolism and high‐energy expenditure in idiopathic pulmonary arterial hypertension. Ann Am Thorac Soc 2017; 14: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grinnan D, Farr G, Fox A, Sweeney L. The role of hyperglycemia and insulin resistance in the development and progression of pulmonary arterial hypertension. J Diabetes Res 2016; 2016: 2481659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim NH, Delcroix M, Jais X, Madani MM, Matsubara H, Mayer E, Ogo T, Tapson VF, Ghofrani HA, Jenkins DP. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1801915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perros F, Montani D, Dorfmüller P, Durand‐Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, Fadel E, Mussot S, Mercier O, Hervé P. Platelet‐derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2008; 178: 81–88. [DOI] [PubMed] [Google Scholar]
- 23. Ulrich S, Fischler M, Speich R, Popov V, Maggiorini M. Chronic thromboembolic and pulmonary arterial hypertension share acute vasoreactivity properties. Chest 2006; 130: 841–846. [DOI] [PubMed] [Google Scholar]
- 24. Zhang M, Zhang Y, Pang W, Zhai Z, Wang C. Circulating biomarkers in chronic thromboembolic pulmonary hypertension. Pulmon Circ 2019; 9 2045894019844480‐204589401984448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett 2008; 582: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Draznin B. Molecular mechanisms of insulin resistance. In Zeitler P. S., Nadeau K. J., eds. Insulin Resistance: Childhood Precursors of Adult Disease. Cham: Springer International Publishing; 2020. p 55–66. [Google Scholar]
- 27. Gutiérrez‐Rodelo C, Roura‐Guiberna A, Olivares‐Reyes JA. Molecular mechanisms of insulin resistance: an update. Gac Med Mex 2017; 153: 214–228. [PubMed] [Google Scholar]
- 28. Hansmann G, Zamanian RT. PPARγ activation: a potential treatment for pulmonary hypertension. Sci Transl Med 2009; 1: 12ps14. [DOI] [PubMed] [Google Scholar]
- 29. Huertas A, Tu L, Gambaryan N, Girerd B, Perros F, Montani D, Fabre D, Fadel E, Eddahibi S, Cohen‐Kaminsky S, Guignabert C, Humbert M. Leptin and regulatory T‐lymphocytes in idiopathic pulmonary arterial hypertension. Eur Respir J 2012; 40: 895–904. [DOI] [PubMed] [Google Scholar]
- 30. Kochetkova EA, Ugai LG, Maistrovskaia YV, Nevzorova VA. Adipokines: a possible contribution to vascular and bone remodeling in idiopathic pulmonary arterial hypertension. Calcif Tissue Int 2017; 100: 325–331. [DOI] [PubMed] [Google Scholar]
- 31. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rabinovitch M. PPARγ and the pathobiology of pulmonary arterial hypertension. Membrane receptors, channels and transporters in pulmonary circulation. Springer; 2010. p 447–458. [Google Scholar]
- 33. Culley MK, Chan SY. Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains. J Clin Invest 2018; 128: 3704–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fazakerley DJ, Minard AY, Krycer JR, Thomas KC, Stöckli J, Harney DJ, Burchfield JG, Maghzal GJ, Caldwell ST, Hartley RC, Stocker R, Murphy MP, James DE. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J Biol Chem 2018; 293: 7315–7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MoradiBinabaj M, Namjoo M, Nejabat M, Joshaghani H. Association of HDL/TG ratio as an insulin resistance marker with various levels of fasting blood glucose. Med Lab J 2016; 10: 50–55. [Google Scholar]
- 36. Yeh W‐C, Tsao Y‐C, Li W‐C, Tzeng IS, Chen L‐S, Chen J‐Y. Elevated triglyceride‐to‐HDL cholesterol ratio is an indicator for insulin resistance in middle‐aged and elderly Taiwanese population: a cross‐sectional study. Lipids Health Dis 2019; 18: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ren X, Chen ZA, Zheng S, Han T, Li Y, Liu W, Hu Y. Association between triglyceride to HDL‐C ratio (TG/HDL‐C) and insulin resistance in Chinese patients with newly diagnosed type 2 diabetes mellitus. PLoS ONE 2016; 11: e0154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stumvoll M, Van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 2001; 24: 796–797. [DOI] [PubMed] [Google Scholar]
- 39. Kim JK. Hyperinsulinemic–euglycemic clamp to assess insulin sensitivity in vivo. In Stocker C., ed. Type 2 Diabetes: Methods and Protocols. Totowa, NJ: Humana Press; 2009. p 221–238. [DOI] [PubMed] [Google Scholar]
- 40. Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic‐euglycemic clamps. Diabetes Care 2012; 35: 1605–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dean A, Nilsen M, Loughlin L, Salt IP, MacLean MR. Metformin reverses development of pulmonary hypertension via aromatase inhibition. Hypertension 2016; 68: 446–454. [DOI] [PubMed] [Google Scholar]
- 42. Agard C, Rolli‐Derkinderen M, Dumas‐de‐La‐Roque E, Rio M, Sagan C, Savineau JP, Loirand G, Pacaud P. Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br J Pharmacol 2009; 158: 1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]


