Graphical abstract
Keywords: Nanomaterials, Zn, COVID-19, Detection and diagnosis, Biosensors, Prophylaxis, Respiratory viruses
Abstract
Respiratory viruses represent a severe public health risk worldwide, and the research contribution to tackle the current pandemic caused by the SARS-CoV-2 is one of the main targets among the scientific community. In this regard, experts from different fields have gathered to confront this catastrophic pandemic. This review illustrates how nanotechnology intervention could be valuable in solving this difficult situation, and the state of the art of Zn-based nanostructures are discussed in detail. For virus detection, learning from the experience of other respiratory viruses such as influenza, the potential use of Zn nanomaterials as suitable sensing platforms to recognize the S1 spike protein in SARS-CoV-2 are shown. Furthermore, a discussion about the antiviral mechanisms reported for ZnO nanostructures is included, which can help develop surface disinfectants and protective coatings. At the same time, the properties of Zn-based materials as supplements for reducing viral activity and the recovery of infected patients are illustrated. Within the scope of noble adjuvants to improve the immune response, the ZnO NPs properties as immunomodulators are explained, and potential prototypes of nanoengineered particles with metallic cations (like Zn2+) are suggested. Therefore, using Zn-associated nanomaterials from detection to disinfection, supplementation, and immunomodulation opens a wide area of opportunities to combat these emerging respiratory viruses. Finally, the attractive properties of these nanomaterials can be extrapolated to new clinical challenges.
1. Introduction: COVID-19 and its emergence
According to the World Health Organization, the current coronavirus outbreak (COVID-19) has spread worldwide, giving rise to a pandemic with roughly 5 million deaths reported so far. Unfortunately, the pandemic continues to expand and remains without proper treatment. In addition, the accelerated surge of new viral strains such as the so-called Delta variant has led to new records in hospitalization and deceased cases [1]. The etiological agent of COVID-19 is the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). This is an enveloped virus, which means that the second layer of glycoproteins surrounds the capsid that contains its single-stranded RNA genome. Its structure comprises the Spike protein (S) (S1 and S2 domains), membrane protein M, envelop protein E, and the nucleocapsid protein N. The viral recognition occurs through the interaction between the host cell receptor Angiotensin-Converting Enzyme 2 (ACE2) and the S protein, then followed by endocytosis and subsequent viral replication (Fig. 1 (a-b)) [2].
Fig. 1.
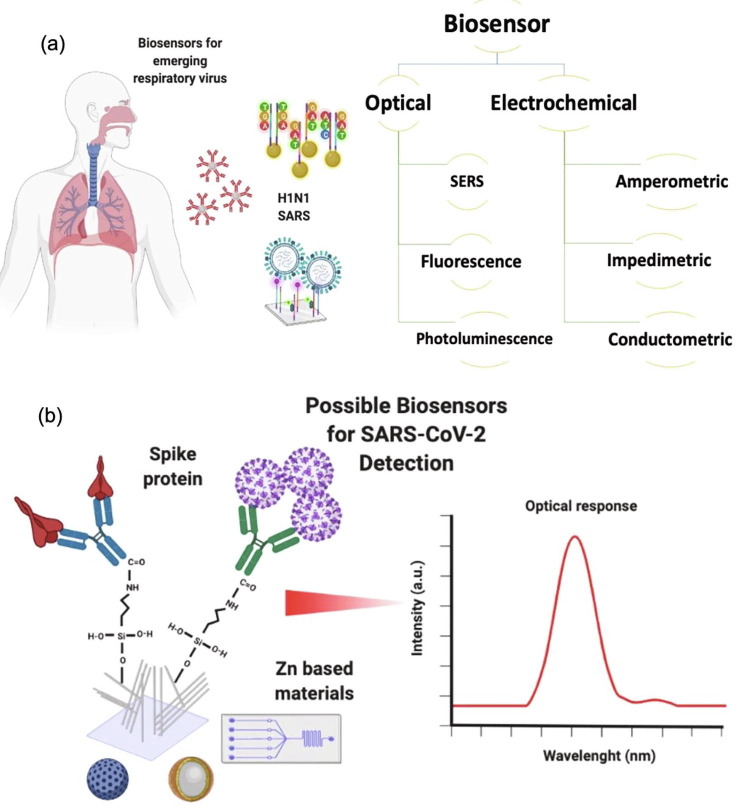
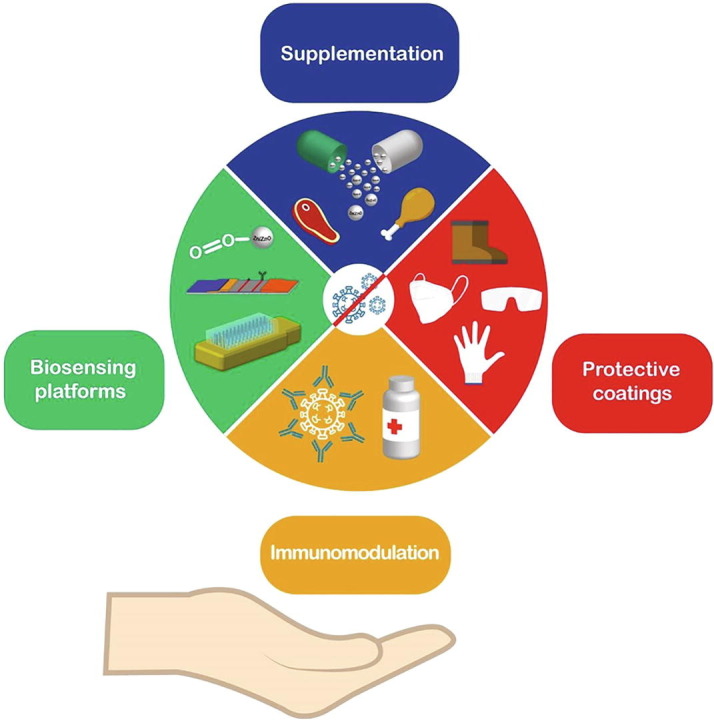
Nanotechnological approaches to combat SARS-CoV-2.(a) Representation of SARS-CoV-2 structure. (b) SARS-CoV-2 binds to the host cell via interactions with the ACE2 receptor. Up to now, the spike protein stands out as the main target in the development of novel detection and treatment strategies. (c) Schematic regarding the enveloped and non-enveloped structure of respiratory viruses such as influenza, rhinovirus, and syncytial virus. (d) Patients with comorbidities like obesity, hypertension, cardiovascular diseases, and diabetes show decreased Zn levels. (e) Different biosensing techniques are employed for virus detection using Zn-based nanostructures. (f) Possible Zn (salts and nanostructures) applications to combat the current pandemic. Figure created with BioRender.com.
Apart from the novel SARS-CoV-2, there are other viruses responsible for causing respiratory diseases such as SARS-CoV, MERS coronavirus (MERS-CoV), syncytial virus, rhinovirus, and influenza virus, whose contagion capacity have caused former pandemic outbreaks (e.g., SARS-CoV, 2003 [3], H1N1, 2009 [4] and MERS, 2012 [5]). Compared with the SARS-CoV-2, the SARS-CoV possess 79.5% of identity and MERS 40%, both being part of the same β-coronavirus class [6]. On the other hand, the influenza virus resembles coronaviruses in its spherical morphology, nanometric size (80–120 nm), and rapid mutagenic capacity. The influenza viruses can be divided into four genera A, B, C, and D, where the principal human infection occurs due to the A and B subtypes. It is also an enveloped virus surrounded by hemagglutinin and neuraminidase glycoproteins, being the first one responsible for host cell recognition [7]. In the case of Human rhinovirus (HRVs), its size is smaller (∼30 nm) and, unlike the coronavirus, it has a non-enveloped structure and icosahedral morphology ( Fig. 1 (c)). HRVs cause most acute respiratory infections worldwide. Thus, understanding how these viruses enter the human cell and their replication mechanisms allow us to extrapolate previous strategies to fight back the ongoing pandemic.
The different mutations that the respiratory viruses undergo can lead to continuous seasonal outbreaks where prophylaxis and early detections play a determining role. The above-mentioned leads to the ongoing necessity of developing novel materials that help us mitigate current and future pandemics. In this regard, the nanomaterials have been used to deal with the SARS-CoV-2 and other viruses, among which we can highlight drug delivery [8], immunomodulatory responses [9], disinfecting [10], [11], and biosensing applications [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. For biosensing, several platforms are based on Au, Se, Fe, Graphene, and Zn (for further details, see Table 1 in Section 2). However, one of the elements that have been studied for biosensor platforms is zinc (Zn) due to its excellent optical and electronic properties, with the additional advantage of being cheap and abundant in nature. A variety of Zn-based nanostructures (e.g., ZnO, CdZnSeS/ZnSeS, CdSe/CdS/ZnS, C-ZnO) continue to be extensively investigated for detecting respiratory viruses. For example, in work developed by Thevasahayam et al. [23], ZnO NRs have been functionalized with 1-ethyl-3-(3-dimethyl aminopropyl) (EDC) (linker) and the ACE2 protein to form the Zn-ZnO-EDC-ACE2 species that allows rapid identification and quantification of the virus in 18–60 s [24]. ZnO nanostructures have also been used for the selective detection of other emerging viruses, for instance, H1N1, H5N1, and H7N9 influenza viruses (Section 2b, Table 2 ); as can be observed, the detection limit for Zn-based nanomaterials is comparable with the reported materials up to date.
Table 1.
Different reported nanobiosensing platforms for SARS-CoV-2 detection.
| Nanostructure | Interaction mechanism | Response signal | Analyte | Bioselective layer | Synthesis technique | Limit of detection/ Linear response range | Reference |
|---|---|---|---|---|---|---|---|
| Au NIs | DNA hybridization | PPT* & LSPR* | RdRp-COVID, ORF1ab-COVID and E genes from SARS-CoV-2 | Hybridization probe (ssDNA) | Self-assembly thermal dewetted of Au thin film | 0.22 pM/ 5–1 pM | [67] |
| Au NPS | DNA hybridization | Optical absorbance | SARS-CoV-2 nucleocapsid phosphoprotein (N-gene). | Oligonucleotide probe (ssDNA) | Modified citrate reduction | 0.18 ng mL−1 / 0.2–3.0 ng mL−1 | [12] |
| AuNPs-graphene (Paper-based platform) | DNA hybridization | Electrochemical | SARS-CoV-2 nucleocapsid phosphoprotein (N-gene). | Oligonucleotide probe (ssDNA) | Modified citrate reduction/ Spin coating | 6.9 copies μL−1 | [63] |
| FTO electrode-AuNPs | Antigen-antibody interaction | Electrochemical: Amperometric | Covid-19 spike antigen | Covid-19 monoclonal antibody | Modified citrate reduction | 10 fM / 1fM- 1 µM | [271] |
| Graphene sheet | Protein-antibody interaction | Field-effect transistor | SARS-CoV-2 spike protein | Antibody against SARS-CoV-2 spike protein | Wet-transfer method | Culture: 1.6 × 101 pfu mL−1 /clinicsamples: 2.42 × 102 copies mL−1 | [70] |
| rGO nanoflakes | Protein-antibody interaction | Electrochemical: Impedance | RDB SARS-CoV-2 spike protein | Antibody against SARS-CoV-2 spike S1 protein | 3D nanoprinting/ drop-casting process | 16.9 × 10–19 M | [71] |
| CdSe QDs | DNA hybridization | Fluorescence | SARS-CoV-2 nucleocapsid phosphoprotein (N-gene). | RNA aptamer | Commercially available CdSe QDs. Invitrogen Corp (QD605). | 0.1 pgmL−1 | [75] |
| Fe3O MNPs* | Transcription | RT-PCR & magnetic response | SARS-CoV-2 viral sequence | Carboxylizedpoly(amino ester) | Co-precipitation | 10 copies / 10–105 copies | [21] |
| Iron oxide NPs | DNA hybridization | C2CA-optomagnetic detection | RdRp SARS-CoV-2 sequence | Biotinylated detection probe | CommerciallyavailableMNPs. MicromodPartikeltechnologieGmbH. | 0.4 fM | [79] |
| Chemiresistance | Antibody-based | Electrochemical: capacitance change | SARS-CoV-2 spike protein | Zinc containing metalloenzyme receptor | Hydrothermal | __ | Patent application number IN2020-41034067 [23] |
Au NIs: Gold Nanoislands; Au NPs: Gold nanoparticles; FTO: Fluorine doped thin oxide; RGO: reduced graphene oxide; Lanthanide-doped polystyrene nanoparticles; MNPs: Magnetic Nanoparticles; C2CA: Circle-to-circle amplification; QDs: Quantum dots; VOCs: volatile organic compounds; PPT: Plasmonic Photothermal Effect; RBD: Receptor Binding Domain; LSPR: Localized Surface Plasmon Resonance; SPR: Surface Plasmon Resonance; ssDNA: single-strand DNA.
*MNPs application to enhance PCR amplification efficiency.
Table 2.
Use of Zn-based nanomaterials for emerging respiratory virus detection by immunosensors.
| Material | Target Virus | Biological recognition element (antibodies) | Signal response | Reference |
|---|---|---|---|---|
| Zinc oxide nanowires (ZnO NWs) | SARS-CoV-2 | IgG (CR3022) | Electrochemical impedance sensing (EIS) | [101] |
| Patterned zinc oxide nanorod networks | Influenza H1N1 | Anti-H1N1 | Cyclic Voltammetry (CV) | [96] |
| CdSe/CdS/ZnS quantum dot (QD) fluorescent | Influenza H1N1 and H3N2 | Anti-influenza A 7307 monoclonal | Fluorescence | [97] |
| Graphene/Zinc oxide nanocomposite | Influenza H5 | Antifluorescein antibody | Cyclic voltammetry (CV) and amperometry | [98] |
| ZnO NRs in Polydimethylsiloxane (PDMS) | Influenza H1N1, H5N1 and H7N9 | H1N1, H5N1, and H7N9 antibodies | Electrical response | [99] |
| CdZnSeS/ZnSeS quantum dots (QDs) | Influenza A/H1N1 | Anti-influenza virus A/H1N1 | Fluorescence | [100] |
| CdSe/ZnS Quantum dot nanobeads (QDNBs) | Influenza A (H7N9) | Anti-influenza A (H7N9) Monoclonal | Fluorescence | [272] |
On the other hand, one of the principal concerns about the SARS-CoV-2 is its high contagiousness, increasing the mortality rate worldwide. The main transmission, in this case, is through expelled droplets from infected individuals or indirectly by contact with contaminated surfaces. For this reason, Zn/ZnO nanoparticles (NPs) are also used as disinfectants and have been incorporated in commercial products, e.g., in food packing, owing to their low cytotoxicity and high antimicrobial activity [25], [26], [27]. Furthermore, the use of nano Zn as disinfectant agents could restrict the SARS-CoV-2 transmission by developing self-sterilized coatings since the virus can remain active over plastic and stainless steel surfaces [28].
Further, COVID-19 symptomatology varies from mild (fever, cough) to severe (pneumonia or acute respiratory distress syndrome (ARDS)) to fatality. Casualties are most common in patients with comorbidities, like obesity [29], [30], cardiovascular disease [31], diabetes mellitus [32], [33], hypertension [34], and cancer [35] due to inflammatory and immune response complications. In this sense, recent medical research has highlighted Zn as an essential element in this viral infection; e.g., the diseases mentioned above (diabetes and hypertension) are associated with a Zn deficiency [36], [37]. Likewise, anosmia and dysfunctional taste are also related to low levels of Zn [38], [39]. Additionally, it has been found that Zn can improve innate and adaptive immunity in the course of a viral infection. Furthermore, Zn supplementation (based on different nano Zn compounds) can boost the recovery of SARS-CoV-2 patients [40], [41], [42], [43], [44] (Fig. 1 (d)). Notably, Zn is involved in multiple human body responses, including the immune system [45], and this explains why it has been employed as a treatment for other pathologies such as pneumonia and diarrhea by rhinovirus [46], [47], stunted growth in children [48], [49], Wilsons disease[50], and acrodermatitis enteropathica [51] to mention a few.
As in the case of bacteria, viruses are also susceptible to developing resistance to specific antiviral treatments. In this sense, given the remarkable properties of Zn, Zn-based nanomaterials have also been tested against several respiratory viruses, thus demonstrating their potential application as a treatment. The commonly proposed antiviral mechanism suggest the delivery of Zn2+ ions, which are active against the SARS-CoV-2 due to its capacity to reduce the inflammatory responses by (i) inhibition of NF-κB and modulation of T-cell [52], (ii) decreasing the activity of ACE2, (iii) avoiding the entry into the host cell, and (iv) regulating the interferon α production (INFα) [53]. For instance, a combination of ZnO NPs-aspartic acid reduces the duration of the common flu symptoms caused by rhinovirus and increases the number of CD4+T in individuals infected with the Human Immunodeficiency Virus (patent application number PCT/US01/20579C [54]). The former immunomodulatory properties also suggest using Zn-based nanomaterials as adjuvants to enhance the release of inflammatory cytokines, activating the immune cell.
Different databases such as Web of Science, PubMed, and Journal of the American Medical Association (JAMA) have been used to compile information. To identify the relevant literature, multiple keywords and term combinations were used, including “SARS-CoV-2”, “COVID-19”, “Zn,” “studies” “biosensor,” “treatment,” “completed studies,” “respiratory virus,” “supplement,” and “nano” keywords with filters. The results show an increase in the extent of nanotechnology research compared with 2018, where almost no results were found for vaccination. As shown in Fig. 2 (a), there is an uprising trend in the number of publications linked to the use of nano Zn in supplementation applications or directly associated with the treatment of viruses, thus reflecting the relevance of this material in health-related applications. However, only a tiny fraction of the publications mentioned focus on using this material in a nanostructured form, which demands further research and development in this field. Fig. 2 (b–d) shows tree diagrams of the research fields obtained using the Web of Science analysis tool for various keyword combinations. These graphs show the multidisciplinary nature of the bionanotechnological applications and visualize the necessity of an interdisciplinary approach. Finally, the interrelation between the fundamental concepts of the systematic search is outlined in Fig. 2 (e), employing a Venn diagram. It is possible to observe that the intersection between nano Zn and COVID-19 is small and yet to be explored in detail.
Fig. 2.
Bibliographic search results using multiple databases. (a) Bar graph displaying the number of publications per year for Zn, virus, supplementation, and nano keywords on the Web of Science (Web of Knowledge) database. (b-d)Treemap graphs displaying the various Web of Science Categories for the multiple keywords and research concept combinations. To include most of the published works, boolean (AND, OR, NOT) and wildcard operators (*, $, ?) were used in the title/theme search options. A subsequent discrimination process was carried out to discard unwanted material. Created with webofknowledge.com.(e) Venn diagram of the key concepts used for the bibliographic review in various databases (Web of Science, PubMed, among others).
The present review highlights the state-of-the-art nanostructured Zn-based materials as biosensing platforms, antimicrobial coatings, supplements in the diet, and potential adjuvants in immunomodulation, which are key points to handle the current pandemic and future events related to respiratory virus infections (Fig. 1 (f)). We aim to encourage the implementation of nano Zn materials into future treatments and the development of point-of-care devices, which also demands further research. The results found in the present work point them out as promising candidates to be employed in health emergencies such as emerging respiratory viruses.
2. A summary of nanomaterials for the detection of COVID-19
As stated by many authors [10], [15], [55], [56], [57], one of the main axes of the transversal efforts to confront this global health emergency lies in the efficient, rapid, economic, and scalable development of biosensing platforms for the precise and selective detection of the SARS-CoV-2. Therefore, the fast and accurate detection of infected individuals in the early stages of the disease is vital as it allows prompt treatment and prevents further dissemination.
Currently, the most used techniques for the detection of human coronaviruses include nucleic acid amplification (e.g., reverse transcriptase-polymerase chain reaction (RT-PCR)) and sequencing, lung visualization through computer tomography, chest radiography or ultrasound, cultures, and antibody-antigen immunoassays tests (serological assays) [58]. However, as the magnitude of the pandemic continues to rise, these methods have not been able to cope due to limitations such as time-consuming, expensive instruments required, elaborate sample preparation, and false-positive results [59], [60].
Consequently, incorporating specific nanomaterials into emerging technologies such as point-of-care tests and optical, electrochemical, or magnetic biosensors can contribute to the efficient surveillance of SARS-CoV-2.
a. An update on different nanomaterials employed as nanobiosensors
Viruses are a typical example of recurrent nanostructured materials in nature; for instance, in the case of the SARS-CoV-2, it is commonly described as a spherical-like particle with a diameter from 120 to 160 nm [61]. Consequently, the detection of such small biomarkers has been enhanced by implementing nanostructured transducing materials into multiple biosensing platforms. The improvement is mainly attributed to the high surface-to-volume ratio of the nanomaterials that increases the interaction with the biological analyte and results in selective and sensitive detection.
Real-time fluorescence quantitative RT-PCR plus viral gene sequencing are the gold standards for diagnosing SARS-CoV-2 infection [62]. However, the need for quick detection methods is one of the most critical control strategies. Currently, different works have been published in the field of biosensors to detect the new COVID-19.
Among the various nanomaterials used to detect human viruses, Au nanostructures, e.g., NPs and nanorods (NRs), have been used as bio-transducing systems to detect COVID-19 employing different types of bioreceptors [12], [13], [18], [63], [64], thereby allowing an accelerated transition of these systems to COVID-19 diagnostic devices ( Fig. 3 (a)). Wen et al. [65] reports the implementation of Au NPs coated with IgG antibodies against SARS-CoV-2 into a lateral flow immunoassay strip (LFIAs) with reasonable specificity and sensitivity. The system allows to detect immune response, customarily manifested two weeks after contracting the infection [66]. On the other hand, a hybridization probe detection approach is reported by Qui et al. to detect the SARS-CoV-2 sequence [67]. A thiol bond attached the bioreceptor to the surface of the Au nanoislands. Then, the Localized Surface Plasmon Resonance (LSPR) Plasmonic Photothermal (PPT) responses were used to detect the targeted viral sequences with a LOD of 0.22 pM. Discrimination tests performed in multigene mixtures (SARS-CoV/SARS-CoV-2) showed good selectivity towards the SARS-CoV-2 virus due to the localized thermal enhancement of the signal. Other reported detection systems include optical biosensors [18] ( Fig. 3 (b)), SERS (Surface Enhanced Raman Spectroscopy) platforms [60] (Fig. 3 (c)), and Colorimetric [12] sensors, allowing the fabrication of detection kits that enable the user to know the response with the naked eye. Therefore, the development of this kind of Point of Care (PoC) test could offer a viable solution to detect the virus where no infrastructure for molecular tests is available, for example, in distant or marginalized communities.
Fig. 3.
COVID-19 nanostructured biosensors. (a) Schematic representation of various bioselective layers developed to detect the most common biomarkers of SARS-CoV-2 using nanostructured sensing materials. Copyrights Springer 2020 [77]. (b) Plasmonic-based model for the optical detection of SARS-CoV-2 spike protein using gold nanorods. Copyrights Wiley 2020 [18]. (c) SERS responsive materials implementation into Personal Protective Equipment (PPE) to detect infectious agents (colorimetric sensor). Copyrights MDPI 2020 [60].
On the other hand, various carbon nanostructures have proved to be effective biocompatible materials for detecting human viruses [68], [69] and specifically the SARS-CoV-2 virus [68], [70], [71]. A microfluidic electrochemical biosensor for detecting SARS-CoV-2 spike S1 protein employing reduced-graphene-oxide (rGO) nanoflakes as electrode covering material is reported by Azahar et al. [71]. They reported a LOD of 16.9 × 10−15 M, good reusability, and no cross-reaction with other antibodies or interfering proteins (Fig. 4 (a–e)). Similarly, the novel coronavirus S protein was targeted as a biomarker in the field-effect transistor (FET) based biosensing device reported by Seo et. al. [70], where the authors used antibody biofunctionalized graphene sheets as sensing elements achieving LOD of 2.24 x102 copies/mL in clinical samples (Fig. 4 (f)).
Fig. 4.
Biosensing platforms for the detection of SARS-CoV-2. (a-e) A microfluidic electrochemical platform for SARS-CoV-2 spike protein antibody. Reduced graphene nanoflakes were used as an electrode material, achieving a detection limit of 2.8 × 10−15 M. The device uses a cellphone interface to display the test results. Copyrights Wiley 2020 [71]. (f) Graphene-based FET device for the detection of SARS-CoV-2 spike protein. The system shows a LOD of 242 copies/mL for nasopharyngeal swap clinical samples. Copyrights ACS 2020 [70]. (g) Proposed nanostructured gas sensing platform for possible Point of Care detection of COVID-19 patients. Copyrights ACS 2020 [74].
Other semiconducting nanostructures such as Si, CdSe, and lanthanide materials have also been reported to detect SARS-CoV-2 proteins or antibodies [72], [73], [74], [75], [76]. Shan et al. [74] reported the label-free detection of volatile organic compounds (VOCs) in exhaled breath of patients using nanostructured platinum-silicon electrodes to distinguish those affected by COVID-19 from other common lung conditions (Fig. 4 (g)). Due to the rapid response time of the device, it can be used for testing large populations if an adequate sterilization process is added to the platform. Sensitive electro-transducing materials such as In2O3 nanowires (NWs) could also be surface engineered to detect the N protein of SARS-CoV-2 [77]. LFIAs tests have also been developed using lanthanide-doped nanoparticles by Chen et. al. [76]. The sensor allows the detection of IgG antibodies against SARS-CoV-2 in human serum samples with a response time of about 10 min.
In addition to the optoelectronic biosensor response, some authors have reported the use of magnetic nanoparticles (MNPs) (e.g., iron oxide nanoparticles, zinc ferrite) to enhance the RNA extraction process for boosting conventional PCR test efficiency or to detect the specific sequences of SARS-CoV-2 [14], [21], [78]. Therefore, this kind of system offers a practical alternative to the traditional nucleic material extraction process as well as sensitive and specific optomagnetic based sensors that use the response of the MNPs to detect the presence of the virus analyte in the femtomolar range [79]. In the case of Mexico, until now, more than 20 investigation projects to confront the COVID-19 pandemic have been developed by different National Universities and Research Institutes, where the role of magnetic hybridization biosensing probes and fluorescence microfluidic platforms stand out as cost-effective and time-reliable alternatives [80], [81], [82], [83], [84], [85]. Additionally, Rodriguez-Moncayo et al. [85] reported the performance of a fluorescence microfluidic platform to detect IgG and IgM antibodies against spike (RBD, and S1 subunit proteins) and nucleoplasmic, showing a specificity in the range of 90–100%. The device is made with PDMS (polydimethylsiloxane) and has a response time of approximately 2 h, and it can be considered mutually cost-effective and time reliable. These characteristics highlighted the biosensor as both a selective and mass scalable opportunity. The state of the art of different nanobiosensing platforms is shown in Table 1 [12], [21], [23], [63], [65], [67], [70], [71], [74], [75], [76], [79].
Nevertheless, ZnO nanostructures are highlighted as promising biosensing candidates due to their structural, optical, and electric properties [86], [87], [88]. These materials could be surface engineered for the detection of the SARS-CoV-2 virus, and as mentioned earlier, evidence of this is the pending patent of Thevasahayam et al. [23]. This work presents the application of ZnO NRs for the electrochemical detection of SARS-CoV-2 spike protein by an antibody-based approximation. However, developing a biosensing platform to tackle the current global health emergency through the available nanomaterials and different bioselective strategies remains an ongoing challenge.
b. Zn as a suitable tool for detection of emerging respiratory viruses: Challenges and perspectives
As stated before, different materials are studied to establish their sensing performance towards the emerging respiratory viruses (SARS, Influenza, SARS-CoV-2). Hence, the need for the development of complementary techniques to the existing traditional methods is constantly increasing. The actual pandemic situation around the world has shown the lack of infrastructure and trained personnel to solve the demand for tests; that represents a window of opportunity for developing biosensing devices.
Biosensors can be divided into various groups according to their transduction system, such as optical, piezoelectric, and electrochemical [89]. F. Narita et al. [90] reported different materials that can be used as piezoelectric biosensors ( Fig. 5 (a–c)) for the detection of human papilloma, vaccinia, dengue, Ebola, influenza A, human immunodeficiency, and hepatitis B viruses. Diverse types of used strategies for pathogenic detection are based on the antibody-antigen reaction; as a result, different immobilization processes have been developed on materials such as gold electrodes, platinum, and graphite, as well as the use of highly crystalline materials, is promoted [91]. However, these materials are expensive, and their processing at a large scale is a challenge for the generation of single-use devices. Therefore, it is desirable to use low-cost and biocompatible materials with a sensitive response signal. In this sense, the platforms based on Zn are a viable alternative to overcome these limitations. Additionally, implementing plasmonic ZnO-based nanocomposites for biological detection could reduce operational costs without losing the optical advantages offered by the material, and the same benefits can also be fetched by utilizing biosensors based on Surface Enhanced Raman Spectroscopy (SERS) substrates.
Fig. 5.
The basic concept of virus detection using piezoelectric material. (a) operation principle of a piezoelectric biosensor; (b,c) schematics of voltage to time (b) and amplitude to frequency (c) during detection. Copyrights Wiley Advanced Materials 2020 [90]. SEM images of the ZnO nanowires (d) random oriented ZnO NWs (e) High ordered orientation of ZnO NWs Copyrights Elsevier Materials Letters 2019 [87]. ZnO-based biosensors for various viral disease detection. (f) EIS-Nanobiosensor fabrication. 1. Paper substrate, 2. A layer of carbon ink to the substrate for conductivity, 3. Growing ZnO NWs on the carbon ink, 4. Immobilizing probe and blocking proteins to the surface of Wes, 5. WEs to capture and measure target protein [101].
Numerous researchers worldwide are currently working on ZnO biosensing maturing projects. In our group, different approaches have been studied recently to observe the influence of morphological properties of these nanostructures for various applications, including bio and gas sensors [87], [88], [92], [93] catalysis [94], among others. A. Galdamez et al. [87] reported the orientations of the Au orchestrated ZnO nanowires (ZnO/Au NWs) on the detection sensitivity of DNA using optical and morphological studies (Fig. 5 (d & e)). Due to the knowledge generated from detecting different viruses, Zn-based platforms have an important future projection [95].
In the area of detection of respiratory viruses, Jang et al. [96] reported the fabrication of an immunosensor based on patterned ZnO nanorods (NRs) networks for influenza (H1N1 SIV) detection. Although an Au electrode was used in the device, ZnO played a fundamental role in the immobilization process of the captured antibody. Nguyen et al. [97] developed a CdSe/CdS/ZnS quantum dot (QD) fluorescent dye to detect H1N1 and H3N2 influenza A virus. Even though it is not a complete Zn-based system, the use of this material is essential for bioconjugation (discussed earlier), which allows the detection of the target analyte ( Fig. 6 (a)). Low et al. [98] reported an electrochemical sensor platform for detecting Avian Influenza H5 (H5N1) based on graphene/ZnO nanocomposite. This work has an interesting approach, as the results obtained from the amperometric study were compared with the efficiency of a conventional agarose gel electrophoresis, finally suggesting a robust validation for the use of these types of biosensors. Further, Ji-Hoon Han et al.[99] reported a nano-flow immunosensor based on ZnO NRs grown on a PDMS sensor for H1N1, H5N1, and H7N9 influenza virus detection using an electrochemical method. The ZnO NRs allowed the immobilization of different antibodies, as well as an increase insensitivity. Nasrin et al. [100] employed ZnO as a precursor in the development of fluorescent CdZnSeS/ZnSeS quantum dots (QDs) and gold nanoparticles (AuNPs) for the detection of influenza virus A/H1N1 (Fig. 6 (b)). Table 2 presents a summary of the works involving the use of Zn-based nanomaterials to detect emerging respiratory virus diseases.
Fig. 6.
(a)Schematic view of a fluorescent test showing the application of the conjugate and sample to rapid diagnostic strips. Copyrights Elsevier 2020 [97]. (b) Diagram for the preparation of CdZnSeS/ZnSeS QD-peptide-AuNP nanocomposite and influenza virus detection. Copyrights Elsevier 2020 [100].
From these works, a retrospective is opened about Zn-based nanomaterials and their potential use in SARS-CoV-2 detection. These promising devices could develop trials to involve specific antibodies against the well-known spike protein S1. Xiao Li et al. [101] reported an experimental approach to enhance the biosensing performance of paper-based electrochemical impedance sensing (EIS) nanobiosensors with working electrodes (WEs) decorated with vertically grown ZnO NWs. These nanobiosensors can differentiate the concentrations (blank, 10 ng ml−1, 100 ng ml−1, and 1 μg ml−1) of IgG antibody (CR3022) to SARS-CoV-2 in human serum samples ( Fig. 5 (f)). Furthermore, Thevasahayam et al. [23], also reported the rapid detection of SARS-CoV-2 by employing ACE2 functionalized ZnO NWs.
As discussed, to detect different respiratory viruses, Zn presents a wide range of characteristics in the various platforms. In some cases, as a part of a nanocomposite or in other cases, it is even used in the union of functional groups to recognize biomolecules ( Fig. 7 (a)). Hence, it is possible to take advantage of signal transduction strategies to develop different types of optical or electrochemical biosensors. The development of immunosensors is based on the specificity of the antigen–antibody reaction. COVID-19 disease is reported to induce acute antibody (IgM, IgG) response, and the levels of antiviral antibodies are plateaued within 6-days after seroconversion [102]. The spike protein S1 protein is one of the main references for COVID-19 detection. This could function as an indicator of the virus even in the incubation period and for asymptomatic cases [103], [104], [105]. Nevertheless, it is suggested that other envelope or membrane proteins can be used to identify different SARS-CoV-2 variants. This could allow the generation of more selective biosensors based on the knowledge, response signals, and characterized behavior of devices based on nano Zn and related compounds. For this reason, ZnO can also be used as a highly sensitive platform for the immobilization of antibodies; consequently, a “serology” test can be performed to measure antiviral antibodies in the blood [106]. In addition, the current knowledge about nano Zn handling and its advances for detecting low analyte concentrations could reduce the analysis times in different population sectors.
Fig. 7.
Prospective biosensor for the early detection of SARS-CoV-2. (a) Schematical view of different biosensors employed for emerging respiratory viruses. (b) Possible applications for Zn-based materials.
According to the previous explanations, some of the functionalization strategies based on silane group immobilization can be employed suitably to use merely nano Zn and its compounds as a prospective biosensor platform. ZnO is one of the materials used for biofunctionalization [88]; since it is an oxide, the generation of OH– groups is favored compared to other metallic materials that sometimes require more complex treatments. Hence, in this case, silanization represents an advantage in performing functionalization strategies, resulting in more OH– groups available for binding with the biological recognition element. For instance, Allen et al. [107] showed the analysis of treatments using triethoxysilane on ZnO thin sol–gel grown films, emphasizing the role of silane in this material. Corso et al. [108] reported the use of two (3-glycidyloxypropyl)-trimethoxysilane (GPS) and (3-mercaptopropyl)-trimethoxysilane (MTS) for the immobilization of IgG antibodies on ZnO surfaces.
Moreover, García et al. [109] evaluated the effects of silanization with amino-propyldiethoxymethylsilane (APDEMS) on hydroxylated sidewalls of ZnO-NWs, highlighting that some studies only focus on the binding of a biomolecule; however, just a few studies address the effective surface functionalization of ZnO. The reported functionalization strategies show a proposed method for their adaptation on Zn-based biosensing devices ( Fig. 7 (b)), which leads to the chemical modification of a surface for the antibody immobilization [93] and an optical transduction response. Therefore, it represents an affordable alternative for research groups that have developed biosensors through functionalization standards using silanes that could be adapted to immobilising recognition agents against the spike S1 protein (SARS-CoV-2).
In addition, it is noteworthy to mention that the preparation of biosensors faces diverse challenges and offers a comprehensive perspective and versatility in the assembly. For example, the strategies for antibody immobilization using crosslinkers improve the orientation in different materials giving a better sensibility; however, it raises the overall cost. Another challenge is the coupling of response signals of biosensors with electronic and portable devices that are subjected to international quality standards for daily use. Lastly, the validation of detection limits and its comparison with molecular reference methods is still an issue to be further solved. On the other hand, random immobilization strategies can also affect the sensitivity of these devices. The increasing knowledge in this health emergency proposes the investigation of improved sensor surfaces from different nanomaterials such as Zn and its compounds to achieve fast detection response and high selectivity and sensitivity. Already gained information with Zn-based biosensing platforms for other respiratory viruses could be a surplus and could act as a reference guide to design these new potential biosensors for early detection of diseases. According to the databases consulted, there is a limited number of works related to the detection of COVID-19 with nano Zn and its compounds, which opens the panorama for future research and studies.
3. Antimicrobial activity of Zn-based materials for COVID-19 disinfection
Apart from the above-mentioned biosensing platforms, another essential method to constraint COVID-19 is the development of self-sterilizing materials. Up to this moment, it has been accepted that the primary source of transmission of SARS-CoV-2 as well as other respiratory viruses occurs through liquid droplets and aerosols coming from the exhalation or sneeze of infected persons [110]. While the use of personal protective equipment (PPE) such as face masks considerably reduces the probability of contagion, continuous and prolonged use of this type of equipment requires meticulous subsequent disinfection processes to assure reusability and/or responsible disposal [111].
Likewise, public space facilities such as hospitals, schools, and transports are also recommended to be sanitized regularly as the indirect contagious by fomites could lead to a viral spread in the community [112]. Thus, the development of effective viral disinfectant materials is of great importance, and a complementary approach that can tackle the current pandemic and possible outbreaks of emerging viruses is of high interest (Fig. 8 (a)). In this regard, metallic antiviral nanoparticles are the most reported and studied systems due to their inherent broad range of antimicrobial activities and effectiveness at a much lower dosage than their bulk counterparts [10], [113]. Nevertheless, Zn-based nanomaterials have proven to be efficient antimicrobial materials that offer diverse valuable photocatalytic, surface, and morphological properties to inhibit and deactivate pathogens. In other words, ZnO nanostructures exhibit tunable antibacterial, antifungal, and antiviral capacities.
Fig. 8.

Antimicrobial activity of Zn-based nanostructures. (a) Development of self-sterilized coatings for implementation in protective personal equipment PPE (masks, face shield, gloves) and public space surface (hospitals, schools, transport) disinfection. ZnO nanostructures can be integrated into both soft fabric-like and large infrastructure surfaces. (b) Proposed bactericidal and cytotoxic mechanisms induced by ZnO internalization or membrane interaction. Figure created with BioRender.com. (c) ROS oxidative stress effects and ions cellular internalization antibacterial mechanisms reported for nanoparticles. Copyrights Elsevier 2014 [131].
In addition, cost-effectiveness and low cytotoxicity to various human cell lines have led to the practical implementation of nanostructured Zn-based materials into food packaging applications to maintain the proper hygiene and prolong the quality of intake products [27], [114], [115]. ZnO NPs have also been implemented in the development of water purification systems [116]. In the work of Munnawar et al. [117], the obtention of ZnO NPs loaded with chitosan and their cytotoxic activity towards fungi and bacteria is presented. Even when chitosan biopolymers exhibit inherent antibacterial properties, the hydrophilicity and porosity of the membrane were further improved by incorporating ZnO NPs. The authors reported a population reduction in the treated samples with a 15% loading of ZnO NPs. Thus, the synergic effect between organic matrices and inorganic reinforcement phases can be further explored for more disinfectant applications, as is also pointed out by Mizielinska et al. [115].
The properties mentioned above illustrate the cytotoxic mechanisms that ZnO exhibits on various cellular components, and it has shown high effectivity against a wide variety of Gram-positive (Staphylococcus epidermidis, Streptococcus pneumoniae, Staphylococcus aureus), Gram-negative (Klebsiella pneumonia, Camphylobacterjejuni, Escherichia coli) bacteria and fungi (Candida albicans, Aspergillus Niger) [118], [119], [120], [121], [122], [123]. The antimicrobial and antifungal mechanisms are strongly influenced by parameters such as the nanostructures concentration, size, morphology, and surface functionalization [27], [124], which can be precisely controlled with the different deposition techniques. For instance, Cha et al. [125] described the effect of different morphologies of ZnO nanostructures (spherical and pyramidal) over the antibacterial and enzyme inactivation activity. The authors reported a biomimetic design of pyramidal NPs to act against super-resistant bacteria (methicilllinc-resistant Staphylococcus aureus), which is presented in most hospitals. Another experiment comparing the antibacterial activity of different morphologies of ZnO nanostructures is presented in the work of Lopez et al. [126]. In this case, the authors observed an enhancement in the microbial inhibition of E. coli and S. aureus by ZnO nanotubes (ZnO NTs) in comparison to the one displayed by commercial ZnO NPs. The ZnO NTs morphology aids in an increase in the surface area and also reduces the aggregation in the system, thus favoring antibacterial activity.
Even though the precise mechanisms of the antibacterial/antifungal activity of ZnO nanostructures remain under debate, cellular membrane-nanoparticle electrostatic interaction, reactive oxygen species (ROS) generation [123], [127], [128], and intracellular Zn2+ release [129], [130] are expected to be responsible for reducing cell viability (Fig. 8 (b)). As pointed out by Sirelkhatim et al. [27], the generation of H2O2, O2 •- and •OH ROS species by ZnO nanomaterials plays a significant role in localized cell wall damage by oxidative stress, in some cases leading to cell death. Other proposed mechanisms include the penetration of neutral ROS into the cell as the cellular membrane repelling the negative species. Then the chemical oxidation process could interrupt the respiratory activity of the cell, thereby producing DNA damage or protein denaturalization [114], [131] (Fig. 8 (c)). A comparative study of the ROS generation for bacteria and/or fungal control applications of several metal oxides [132]. They compared the performance of ZnO with other typical binary semiconductors, corroborating the potential and relevance of the material in this research field. In the current COVID-19 pandemic, the antibacterial properties of ZnO materials could be targeted towards opportunistic diseases linked with SARS-CoV-2 infection, such as pneumonia (caused by bacterial or fungal pathogens) which is responsible in some cases for fatal complications in the patient [133].
Although most of the publications found in the literature are focused on the antibacterial/ antifungal properties of nanostructured ZnO materials, some authors also report the application of these nano-systems as antiviral agents against viruses such as Herpes simplex virus (HSV-1), H1N1 influenza virus, and recently against the SARS-CoV-2 virus.
Among the first works to study the effect of nanostructured ZnO as an agent to reduce the infectious capacity of a virus is the one reported by Mishra et al. [134]. The authors reported the obtention of ZnO's micro-nano structures (MNSs) with a morphology that bio-mimics the cellular membrane receptors that the HSV-1 virus typically targets. The viral inhibition mechanism is based on the electrostatic interaction between the negatively charged surface of the ZnO nanostructures and the positively charged protein surface of the virus. As a result, the ZnO MNSs are found to function as anchor points for HSV-1 virus particles and thus preventing them from reaching the target cell gD-receptors. The former mechanism could be used to develop ZnO nanostructures that target SARS-CoV-2 surface proteins (Fig. 9 (a)).
Fig. 9.
Possible mechanisms of antiviral activity of ZnO against SARS-CoV-2. (a) Design of ZnO nanostructures for the possible anchoring of SARS-CoV-2 virons, thus inhibiting interaction with host cell receptors. (b) Internalization of ZnO nanostructures for the inhibition of early stages of the viral replication cycle. (c) Ion release as a surface attack mechanism to disrupt the plasmid and RNA virus integrity. (d) Photocatalytic generation of reactive oxygen species for the possible degradation of the lipid, protein, and nucleic structure of SARS-CoV-2. Figure created with BioRender.com.
In the work of Tavakoli et al. [135], a more recent study about the antiviral properties of ZnO NPs against the HSV-1 virus is presented. Herein, a surface functionalization approach using polyethylene glycol (PEG) was developed to increase the antiviral response and reduce the cytotoxicity of the NPs. A similar ZnO-PEG functionalized NPs approach is reported by Ghaffari et al. to perform against the H1N1 influenza virus [136]. Cytotoxic analysis revealed that implementing a PEG layer significantly enhances the biocompatibility of ZnO NPs towards Madin-Darby canine kidney MDCK culture cells. The smaller the size of NPs, efficient is the cellular absorption. The authors propose that the antiviral activity of the material is carried out when the NPs are internalized in the host cells (Fig. 9 (b)).
Some recent publications have been made over the direct antiviral properties of ZnO nanomaterials against the current SARS-CoV-2 virus. In the work of El-Megharbel et al. [137] According to the proposed mechanism, ZnO NPs are tested towards Vero-E6 cells infected with SARS-CoV-2, resulting in the virus inactivation by Zn2+ release and ROS formation (Fig. 9 (c)). The authors pointed out that the same mechanisms for antibacterial activity are also responsible for damaging the lipid membrane and RNA and thereby inactivating the virus. However, the cytotoxic studies reflect healthy cell apoptosis at relatively low concentration levels of ZnO NPs, suggesting the need to improve their biocompatibility.
Furthermore, another molecular docking analysis about the interaction between the adhesion proteins of the SARS-CoV-2 virus and ZnO nanostructures is presented by Adhikari et al. [138]. In this work, the authors determined that the interaction between the spike protein of SARS-CoV-2 and the ACE-2 receptor is comparable in energy values, with that experienced between the spike adhesion protein of the virus and the surface of both 0D spherical NPs and the 〈1 0 0〉, 〈1 0 1〉 and 〈0 0 2〉 crystal faces of 2D ZnO nanostructures. The computational study also reflects the viral denaturalization posterior to the adsorption in the semiconducting surface. This feature makes the material a suitable option to be applied in face mask manufacturing. This application was also explored by Adhikari et al. when manufacturing ZnO nanoflowers (NFs) on cotton fabrics substrates using a low-temperature hydrothermal approach. The implementation of antiviral organic compounds has previously been reported; however, the thermal and chemical stability of the latter is still among the main challenges to be overcome. This is one of the reasons why obtaining antimicrobial materials at low temperatures is of great interest for their incorporation into fabrics. As a proof of concept, the authors studied the antimicrobial activity against the model bacteria Pseudomonas aeruginosa. The pathogen was targeted due to its LecA membrane protein which confers its mimicry with the spike protein of SARS-CoV-2. The results showed an antibacterial effect through the rupture of the cell membrane, a behavior that was maintained even after washing the fabric 50 times. Scanning electron microscopy (SEM) images also showed minimal fiber deterioration even after several washes. This suggests the effective implementation of functionalized fabrics with ZnO nanostructures in washable antimicrobial clothing and personal protective equipment.
Furthermore, the photocatalytic properties of ZnO nanostructures can also be used to develop photoinduced self-cleaning coatings, where good adhesion to the substrate, improved broadband photoabsorption, and innocuous biodegradability of the photocatalytic platforms are among the main goals to be achieved [55]. In fact, during the health emergency of COVID-19, street sterilization in Italy using TiO2/Ag nanomaterials has already been carried out [139]. Therefore, the implementation of other UV-active semiconductors such as ZnO in metalized composites (for example, ZnO NWs with Au NPs on the tip obtained by the VLS process) for disinfection applications seems feasible and can be attributed to ROS damaging the virus membrane, proteins, or RNA structure ( Fig. 9 (d)).
However, a debate remains whether the environmental and ecological consequences of high amounts of nanomaterials in a specific media might play a two-edged sword. For this reason, among other things, the cytotoxic effects of ZnO nanostructures over a broad spectrum of cell lines must be further studied. More details about the cytotoxicity of this material are discussed in the subsequent sections.
Bearing the above in mind, Zn/ZnO nanoplatforms can be used to avoid the propagation of the SARS-CoV-2 virus by developing surface disinfectants and novel protective equipment. Thus far, implementing these nanosystems into mass scalable and commercially available products is limited due to the lack of cost-effective materials. The implementation of nano-based personal protective equipment could be justified for medical personnel and patients on the front lines of the battle against these infections, such as intensive care units. Likewise, the development of disinfectant coating using Zn/ZnO nanomaterials requires further investigation of the possible cytotoxic and eco-toxic effects on the biosphere during the disposal process of these types of nano-based products. The next section will discuss recent studies regarding the in vitro and in vivo trials for viral treatment (including SARS-CoV-2) using Zn-based nanomaterials.
4. Zn as an essential element in emerging virus mediated-respiratory diseases
It has been found that the emergent lethal respiratory viruses acquire newer survival tactics to live inside host cells and trick the host's immune system. For instance, novel coronavirus and drug-resistant species of influenza viruses call for an urgent requirement of novel treatment strategies [140], [141]. Antiviral agents directly act on the virus or intracellular paths for viral replication by several mechanisms such as attacking target proteins, acting as ligand analogs, and disturbing the viral replication cycle. Nevertheless, most available antiviral agents exhibit disadvantages, such as low solubility, minor stability, high potential of drug-drug interaction, and high toxicity, which should be further improved. Therefore, this clearly remarks the need for further research in this respective area [7], [140]. Nanomedicine has come to be one of the most promising technologies because of its ability to combat viral infections facing the restrictions of conventional therapies along with nanoformulations, including solid lipid NPs, polymeric NPs, liposomes, dendrimers, micelles, selfassembled nanoemulsions, and cyclodextrins [7], [56]. Metallic NPs exhibit inherent antiviral activity and are extensively studied for this purpose; among them, Zn has been found efficacious against respiratory infection-causing viruses [7]. The role of Zn-associated compounds, Zn conjugates, and Zn ionophores have been studied against respiratory viruses [142], [143], [144]. In the present COVID-19 pandemic situation, specific treatment for this infection has not yet been determined; however, the therapeutic options are mainly aimed at improving respiratory function by Oxygenation and Ventilation and controlling the advanced symptoms through diverse Pharmacologic Interventions [145]. In this context, medical professionals are starting to consider the responses through supplements such as vitamin D, vitamin B12, probiotics, and Zn [146], [147], [148]. Likewise, clinical trials have been performed to determine the effect of Zn supplementation on virus-mediated-respiratory diseases, which will be analyzed below, along with the potential relevance of their nanostructured form, toxicity, and role as a supportive treatment and prophylaxis of emerging respiratory infections.
a. Zn supplementation as a supporting treatment
Zn is a vital mineral, and it regulates numerous enzymes and proteins that participate in necessary cellular events, mostly related to inflammatory system regulation [147], [149]. Zn in its bulk form is registered safe by the United States Food and Drug Administration (FDA) (21CFR182.8991). The standard route to obtain this element is through the ingestion of enriched food or nutritional supplements, while the Recommended Daily Intake (RDI) values are in between 3 mg and 16 mg; having an oral Lethal Dose (LD50) close to 3 g/kg per body weight [150]. The main Zn compounds used for supplements are zinc gluconate (C12H22O14Zn), zinc citrate (C12H10O14Zn3), zinc sulfate (ZnSO4), bis-glycinate(C4H8N2O4Zn), zinc acetate (C4H6O4Zn), zinc picolinate (C12H8N2O4Zn), zinc oxide (ZnO), zinc chloride (ZnCl₂), and zinc aspartate (C8H12N2O8Zn), among others [151], [152]. Depending on the Zn compound, its functionality could vary for their use as supplementation; for instance, zinc acetate (C4H6O4Zn) has been more effective than zinc gluconate (C12H22O14Zn), and lastly, zinc citrate (C12H10O14Zn3) for the common cold [153]. Notably, it has been found that zinc gluconate (C12H22O14Zn), zinc citrate (C12H10O14Zn3), and zinc sulfate (ZnSO4) are absorbed better in healthy people, while ZnO is least absorbed (Fig. 10 (a)) [154], [155]. However, in a different study, it was found that along with additional food supplementation, ZnO absorption was equivalent to that of ZnSO4 [156]. Furthermore, Zn can also be used as a food fortifier, and in that case, bioavailability will be affected by interactions with different food components or through proper process handling. Therefore, it is essential to study the compound solubility, charge density, reduction potential, pH, complex formation, and processing impact before manufacturing [156]. However, Zn performance depends on its ion availability from different salts, for example, zinc chloride (ZnCl2), ZnSO4, and zinc acetate (Zn(OAc)2), as well as the target virus [157].
Fig. 10.
Prospective role of Zn as a supplement for respiratory viral infections. (a) Fractional absorption of Zn from zinc citrate, zinc gluconate, and ZnO supplements consumed with water. Copyright Oxford Academic 2014. (b) Effects of Zn excess and deficiency by malnutrition or medical conditions Copyright MDPI 2010 [161]. (c) Serum Zn levels in patients with SARS-COV-2 and healthy controls Copyright Elsevier B.V. 2020 [165]. (d) Illustration of antiviral and immunomodulatory properties of Zn in SARS-COV Copyright Elsevier B.V. 2020 [165]. (e) Counts of clinical trials worldwide related to Zn and viral infections, from 2019 to 2021 [198]. (f) Counts of clinical trials completed worldwide related to Zn respiratory viral infections from 2019 to 2021 [198].
On the other hand, previously reported studies established that nearly two billion people in the world may have prolonged Zn deficiency, thus showing related clinical problems like growth retardation, cognitive impairment, liver, and renal diseases, chronic inflammation, and triggering oxidative stress, among others [158]. The studies have also demonstrated that plasma Zn levels are markedly reduced through infections, and there is a correlation of around 16% of the world’s severe respiratory infections and bacterial or fungal co-infections [159], [160], [161], [151]. A representation of different before-mentioned diseases is shown in Fig. 10 (b). The association among how Zn deficiency affects the severity of respiratory infections is also under investigation. Till now, several studies have shown that Zn deficiency predisposes patients to viral infections such as the common cold and boosts lung inflammation gastrointestinal episodes related to SARS-CoV-1 [158], [162], [163]. Likewise, clinical trials have been performed to determine the effect of Zn supplementation over the group of so-called “common cold viruses.” For instance, Rerksuppaphol et al. [164] reported a reduction in the duration of the disease and the appearance of symptoms for rhinovirus infection using Zn salt supplementation. Fig. 10 (c) illustrates serum Zn levels (µg/dL) in COVID-19 patients compared with healthy controls, indicating that patients had lower levels; therefore, Zn deficiency in COVID-19 patients may not be just a mere coincidence [165]. Various studies also showed that Zn is crucial in regulating inflammatory cytokines by reducing IL-6 levels and pro-inflammatory responses via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), controlling oxidative stress avoiding host-tissue damage by inflammation [166], [167]. Furthermore, Zn homeostasis is crucial for wound healing and tissue recovery when it is linked to inflammation or mechanical damage. Also, Zn has been associated with ROS production in platelets, indicating a decrease in thrombus formation and inhibiting other complications by respiratory virus infections [151]. Furthermore, it has been studied that Zn affects the IFN-λ3 binding to IFNL receptor 1 (Lambda interferons), ensuing in reduced antiviral activity (Influenza virus) in vitro, in which the mechanism of Zn-mediated inhibition could occur in an extracellular manner through degradation of viral RNA, and thereby abrogate viral infectivity [168]. Consequently, there is a suggestion to monitor Zn levels in the high-risk population (elderly and patients with comorbidities) to achieve the most beneficial therapeutic value [146], [169], [151]. Furthermore, Fig. 10 (d) exemplified Zn’s in vitro evidence of antiviral properties in emergent respiratory virus infections [165]. State-of-art in vitro evidence of possible Zn mechanisms for antiviral action by supplementation is described in Table 3 [52], [53], [151], [157], [161], [167], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185], [186], [187], [188], [158], [189], [190] .
Table 3.
Proposed biological mechanisms of Zn against viral infections.
| Type of intervention of Zinc against SARS-COV infection | Suggested Antiviral mechanism | Reference |
|---|---|---|
| Zn inhibits RNA-dependent RNA polymerase (RdRp) of SARS-CoV | Decreases the rate of virus transcription | [52] |
| Zn may induce inhibition of S. pneumoniae growth | Modulation of coinfection of bacterial Mn (II) dependent homeostasis | [188] |
| Zn binds to the SARS-COV enzyme catalytic residues | Inhibition of SARS-CoV activity | [53], [158] |
| Zinc may lower plasma C-Reactive Protein (CRP) concentration | Downregulating the response to inflammation via CRP | [161] |
| Zn exposure may decrease recombinant human ACE-2 activity and expression | Modulating effect on SARS-CoV-2/ACE2 interaction | [189], [190] |
| Regulate tight junction proteins ZO-1 and claudin-1 | Controlling Antioxidant and Anti-inflammatory activity of respiratory epithelium | [171], [172] |
| Control of IFNα production and intensifying its antiviral activity through IFNα-induced JAK1/STAT1 signaling | Increasing antiviral activity of cytokines | [173], [174] |
| Inhibition of caspases-3, 6, and 9, and an increase of the Bcl-2/Bax ratio expression | Enhances cells resistance to apoptosis | [175] |
| Zn exerts anti-inflammatory activity through inhibition of IKK activity and subsequent NF-κB and modulation of regulatory T-cell functions. | Down-regulation of pro-inflammatory cytokine production and increases the cytotoxicity of NK and T cells | [167], [176], [177], [178], [179] |
| Inhibition of ADAM 17 enzyme at the Zn cofactor site inhibits the enzyme, causing downregulation of inflammation | Downregulation inflammation via of ADAM enzyme | [180] |
| Zn supplementation improves mucociliary clearance, aid with the removal of bacteria and viruses and, diminishes inflammatory reaction and lung damage | Improving defense of the airways and bronchial epithelium | [170], [171], [151] |
| Zn can counteract virus fusion with the host membrane, impairs protein translation and processing, blocks viral particle release, and destabilize the viral envelope. | Improving effects on host cell metabolism to prevent damage from infection | [181], [152] |
| Zn supplementation can reverse lymphocytopenia, Attenuated lipopolysaccharide-induced hyperactivation, recruitment, and formation of neutrophil extracellular traps | Improving Immune system cells response | [182], [183] |
| Zn supplementation might reduce the harmful microbes and increase the helpful ones in a dose-dependent manner | Prophylaxis based on gut microbiota profile to improve immunity | [184], [185], [186], [187] |
Moreover, it can be suggested that Zn use as a supplement may confer various benefits associated with treating emerging respiratory virus infection, considering that its use is safe at controlled doses, has a positive role in inflammatory response modulation and antiviral activity. Likewise, it has been revealed that Zn treatment diminished the length of common cold signs in healthy persons [191], can restrict influenza virus infections [153], had antiviral action in rhinovirus and respiratory syncytial virus (RSV) infection, as well as influenza-MRSA bacterial superinfection [53], [192]. According to the COVID-19 Treatment Guidelines of the National Institute of Health U.S.A. [193], there is still insufficient data to recommend either for or against the use of Zn to treat respiratory infection viruses. However, few studies were recently registered worldwide in the National Library of Medicine of the United States and in the European Union Clinical Trials to assess Zn efficacy in treatment combined with drugs or prophylaxis against complications of SARS-CoV-2 and Influence (flu) infection [194], [195]. In the analyzed databases, the use of ZnSO4 and C12H22O14Zn in concentrations between 50 and 220 mg can be understood (Fig. 10 (e & f)). Nevertheless, no conclusive results have been reported so far, and this is a topic of keen attention among different research groups [196], [197], [198], [199], [200], [201], [202], [203], [204], [205]. It should be highlighted that the outcomes that can be modified in virus infection with the use of Zn are too diverse and require a separate analysis in each case (from the reduction of infections, hospitalizations, and intubations to the decrease in deaths). Also, it is crucial to evaluate the effect of Zn supplementation in infected patients and to identify if they have a preceding deficiency of this mineral or not before initiating treatment.
Moreover, many investigators recently have pointed out the importance of nanomaterial-based technological resolutions to combat emerging respiratory virus infections [206], [207]. As revealed, nanomaterials as supplements have a better capacity than conventional sources due to their form, easier uptake by the GI tract, and enhanced effectiveness at lower doses [208]. The following section discusses the state-of-the-art of Zn NPs studies against the emerging respiratory viruses.
b. Potential of nano-Zn compounds against respiratory viruses
This recent coronavirus pandemic has shown promising results, seeking complementary therapy to support prevention, treatment, and recovery. In this context, a few studies have investigated the properties of metal NPs to augment their selectivity and efficacy against respiratory viruses since these NPs can bind with the viral proteins (via Van der Waals forces) and can help to conduce its inactivation [7], [209]. Correspondingly, in double-stranded RNA viruses, Ag NPs have shown that after interaction with the viral genome, they inhibit viral replication; likewise, Cu NPs inhibit virus-cell binding and attachment and destroy the viral genome. Whereas Fe NPs bind to the virus to prevent it from attaching to the host cells. Though, Se NPs guard from apoptosis caused by the infection of the virus [210]. In the case of Zn NPs, it has been observed that it interferes with viral DNA polymerase activity and binds to virions resulting in inhibition in viral replication and entry [7], [210], [211]. Although the exact mechanism for Zn nanomaterials to act as agents for the treatment of the recurrent respiratory virus is still under investigation, there is evidence showing that the performance of Zn depends on the ion availability from different composites. It further depends on NPs morphological aspects such as particle size, shape, concentration, agglomeration, colloidal formulation, and media pH [7], [209]. In this context, PEGylated ZnO NPs have better antiviral activity than bare ZnO NPs, significantly reducing H1NI virus titer post-infection [135], [136], [158]. Likewise, the antiviral activity of ZnO-PEG-NPs is dose-dependent compared with an antiviral drug, noticing the greatest H1N1 antiviral influence at 75 μg/mL with an inhibition rate of 52.2% (Fig. 11 (a)) [136]. In antiviral therapy, ZnO NPs offer advantages and attempt to defy the occurrence of drug resistance. In this context, an investigation employing ZnSO4 (1.5 mg/mL) and Ag NPs showed comparable potentiated antiviral action with epigallocatechin gallate (EGCG) (50 μM) against the H5N1 avian flu virus in embryonated SPF eggs, further helping to limit the infection resistance and without been affected by virus mutations (Fig. 11 (b)) [212]. Another study with a composite of Au/Ag/ZnO-NP (TPNT1) showed inhibition of different strains of SARS-CoV-2, human H1N1, and avian H5N1 influenza viruses, including the oseltamivir-resistant strains, proposing it as a prophylactic agent against SARS-CoV-2 and opportunistic infections by oral gargling, nasal spray, nebulized inhalation or even systemic use after an appropriate clinical trial (Fig. 11 (c)) [141]. Also, it has been observed that utilizing Ni/ZnO and MnZnS NPs there is a 2 or 3-log order inhibition of β-galactosidase enzyme respectively, in comparison to ZnO NP or Ag NP controls [213]. Moreover, docking molecular studies have been performed to determine the interaction between ZnO NPs and the common SARS-CoV-2 targets. One of the stududies speculated the possible interaction among ZnO NPs and ACE2 receptor, COVID-19 RNA-dependent RNA polymerase, and the main protease, putting forward the attention towards the binding of ZnO NPs with the tested targets and observing an enhanced dose-dependent cellular uptake (Fig. 11 (d)) [158]. In the work performed by Hamdi et al. [214] it was found that ZnO could mimic the inhibition behavior of remdesivir and saquinavir drugs by hydrogen bond interactions with the Arg555, Ser759 amino acid and U10, U20 uracil bases, respectively. The role of hydrogen bond and other interactions as π-cation and π-π are essential in the ligand–protein stabilization indicating the ZnO NPs as a possible treatment to COVID-19. Furthermore, Fig. 12 exemplified the in vitro evidence of Zn NPs antiviral properties in emergent respiratory virus infections.
Fig. 11.
Antiviral activity of Zn nanomaterials. (a) The inhibitory rates of compounds against the H1N1 influenza virus by Real-Time PCR assay regarding the post-exposure antiviral activity. Copyright © BioMed Central Ltd 2019 [136]. (b) Plaque assay method for antiviral effects against the HAV virus. A: Vero cells control, B: HAV-infected Vero cells treated with ZnO NPs, C: HAV-infected Vero cells treated with hesperidin, D: HAV-infected Vero cells [212]. Copyright European Review for Medical and Pharmacological Sciences. (c) Inhibition of SARS-CoV-2 entry by TPNT1, Immunofluorescence assay. (pretreat + infection), infection-only and post-infection experiments delineate the stage where TPNT1 was added to the viruses or the cells. (Scale bars: 100 µm) [141]. Copyright Springer Nature Limited. (d) Structural alignment between SARS-CoV (pink) and COVID-19 (cyan) of the RdRp. (A and B) The second zinc-binding site and (C) overall structural alignment. The white box indicates the area where zinc binds based on the overall structure [158]. Copyright © Spandidos Publications.
Fig. 12.
Findings of the potential role of Zn NPs as a supplement against emerging respiratory infections. Left, mechanism of Zn NPs after administration in the body. Right, mechanism of Zn NPs against the respiratory virus.
It should be noted the aforementioned respiratory viruses, even despite using different cellular pathways, share the existence of the ACE2 protein at the beginning of the digestive system and upper respiratory tract (discussed earlier) [215], which represents an excellent motivation for the proposed therapy with Zn NPs. The results of nano Zn compounds in various models studies for antiviral action against the emerging respiratory virus are summed up in Table 4 [136], [141], [158], [212], [213], [216], [217], [218].
Table 4.
Antiviral activity by ZnNPs against respiratory virus.
| Nano Zn Compound | Species/Study | Effects | Remarks | Ref |
|---|---|---|---|---|
| ZnO-NPs and PEGylated ZnONPs | Influenza A/Puerto Rico/8/34 (H1N1; PR8) propagated in Madin-Darby canine kidney (MDCK)-SIAT1 cells | PEGylated and unPEGylated ZnO-NPs led to inhibition rates of 94.6% and 52.2%, respectively of H1N1 virus | A proposal as an antiviral agent against H1N1 influenza virus infection | [136] |
| Zn-based physiometacomposites (PMCs) (Mn, Fe, Ni and Co-doped, ZnO, ZnS or ZnSe | 3D organoid in β-Galactosidase enzyme activity and PRR virus infection. | Inhibition of β-Galactosidase enzyme activity and PRR virus infection. | Imaging and delivery targeting cancer and infectious disease. | [213] |
| ZnSO4 (zinc II) NPs | Influenza A Virus (H9N2) in embryonated Specific-Pathogen-Free hen's eggs | ZnSO4 (1.5 mg/mL) and Ag NPs comparable antiviral action with epigallocatechin gallate EGCG (50 μM) against the H5N1 avian flu virus | Potentiated antiviral activity of EGCG by co-administering it with zinc II and AgNPs | [212] |
| ZnO NPs | BALB/c mice injected with M. pneumoniae | Reduced total protein, inflammatory cells, inflammatory cytokines (IL-1, IL-6, IL-8, TNF-a and TGF). | Potential effects against upper and lower respiratory tract infections | [216] |
| ZnO/ Berberine (BER) complex | VeroE6 toxicity, anti-COVID-19 activity | ZnO/BER complex inhibits spike protein binding with angiotensin-converting enzyme II (ACE II), PL pro activity, spike protein and E protein levels, and expression of both E-gene and RNA dependent RNA polymerase (RdRp) | It acts as an anti-COVID-19 and could be used to treat a second bacterial infection | [217] |
| Au-NP/Ag-NP/ZnO-NP and ClO2 nanocomposite (TPNT1) | SARS-CoV-2 strains in vitro cell-based assay | Block viral entry by inhibiting the binding of spike proteins to ACE2 receptor and to interfere with the syncytium formation | Effective concentration within the range to be used as food additives provide prophylactic effects against both SARS-CoV-2 | [273] |
| Au-NP/Ag-NP/ZnO-NP and ClO2 nanocomposite (TPNT1) | (H1N1) and avian influenza A virus (H5N1) cell-based assays | Reduced the cytopathic effects induced by human H1N1 and avian H5N1 influenza viruses, including oseltamivir-resistant virus isolates | Inhibit or prevent viral infectionand opportunistic infections | [273] |
| ZnO NPs | COVID-19 targets include the ACE2 receptor, COVID-19 RNA-dependent RNA polymerase in humans in lung fibroblast cells and in silico molecular docking | The binding of ZnO NPs with the three tested COVID-19 targets via hydrogen bond formation was detected. | Infer further biological and therapeutic studies | [158] |
| EGCG-AgNPs/ZnSO4 | Influenza A Virus (H9N2) in embryonated Specific-Pathogen-Free hen's eggs | Co-treatment with ZnSO4 (1.3 mg/mL) increased the EGCG antiviral effect | May prevent virus transmission, inhibit virus replication and inhibit microbial resistance | [218] |
The actual pandemic situation has emphasized the urgent need for new antiviral therapeutics. From the above discussion, it can be seen that considerable efforts have focused on developing Zn NPs, and as a result, these materials have demonstrated promising properties as prophylactic agents, acting against viral DNA polymerase activity and viral replication, helping to limit the infection resistance processed by virus mutations and binding to target proteins to reduce complications[215]. Therefore, scientific evidence gained through additional research can add valuable foundations to treat viral diseases. However, some remaining concerns about Zn toxicity in nano-dimensions will be discussed further in the next section [206], [219], [220], [221].
c. Analysis of toxicity of oral administration of nano-Zn for respiratory infections
In humans, the main studied routes of Zn toxicity are gastrointestinal, dermal, and respiratory, where the majority of cases occur by ZnCl2, ZnO, ZnSO4, and zinc sulfide(ZnS) compounds [222]. In gastrointestinal exposure, toxicity appears to develop until the ingestions exceed 1 to 2 g of Zn [222]. For dermal toxicity, the small quantity of information available for Zn for the bulk is not correlated with significant adverse events; however, it has been observed that ZnO NPS can be overused through makeup, sunscreen, and ointments, having an LD50 greater than 2000 mg/kg per body weight [223], [224], [225], [226]. Inhalational toxicity can vary reliant on the compound involved and the duration of the exposure; for example, individuals who breathe Zn-containing smoke (ZnCl2 and/or ZnO), mostly from industrial processes or military activities, can ultimately experience an acute condition of ARDS-like [227]. The permissible exposure limit in the workplace is 5 mg/m3 for ZnO [228]. Similarly, chronic Zn toxicity, which occurs more frequently, happens after long-term high intakes of Zn (150 mg to 450 mg per day)[161], and some of the warning signs comprise lethargy, compromised immunity, and health problems associated with obesity [229]. Excessive Zn intake is related to alterations in Cu and Fe homeostasis at the cellular level, leading to anemia and damage to the nervous system [147]. Some reports indicate that the impact of Zn on apoptosis (programmed cell death) is still under discussion. Zn can either be a pro- or anti-apoptotic element and equally, its deprivation and excess can induce apoptosis. The apoptotic effects are related to regulating the proteins Bcl-2-like, Bax-like, and cytochrome-c in the mitochondria and its intervention in the pathway of Akt/ERK proteins. The anti-apoptotic activities have been recognized by suppressing signaling pathways that regulate apoptosis mainly through caspases and TPEN protein. However, the variables in this system need further research effort [161].
As mentioned above, the leakage of free Zn2+ ions or Zn OH– from ZnO NPs has been suggested to justify their cytotoxicity; nevertheless, as mentioned earlier, some reports indicated that ROS species could be the ones that can trigger their antimicrobial activity [27], [114], [123], [127], [128], [129], [130]. Also, it has been observed that ZnO NPs toxicity should be associated with the release of Zn2+ ions rather than with the presence of NPs since they can be easily dissolved in natural aqueous media with a duration expectancy smaller than 90 min, facilitated by the water chemistry, and the presence of dissolved organic matter. Similarly, dissolution rates increased with the addition of strong chelating agents, such as EDTA and L-cysteine. In contrast, a decrease was observed in the presence of polymeric organic matter such as sodium alginate [168], [230]. ZnO NPs toxicity is mainly determined based on the direct measurement of released Zn2+ ion concentrations under different environmental conditions such as pH, temperature, ionic strength, and chemical composition [231]. Likewise, it has been shown that there is a specificity of biomolecular interactions of Zn nanocomposites with proteins, observing a change in spectral signature when proteins such as ribonuclease A (RNase A) interact with ZnO NPs. It was found that when RNase A was bound to Mg/ZnO, the intensity was quenched, while it was magnified when torula yeast RNA was bound to RNase A and Mg/Zn [232].
In a human inhalation study, it was found that healthy adults inhaled approximately 500 g/m3 of ultrafine ZnO, fine ZnO, and filtered air while at rest for 2 h. It has shown no differences between exposure conditions in leukocyte surface markers, hemostasis, and cardiac electrophysiology conducted 24 h post-exposure [233]. In studies in invertebrates have shown that the toxic responses of ZnO NPs are due to their interactions and/or uptake by the blood cells within the insect’s body, and the toxic reactions may diminish once the insects excrete these NPs [234]. In another study that explored the role of the group of proteins that bind to the NPs surface (protein corona) for biokinetic and toxicology propose, they found that the protein corona formed on silica-coated ZnO NPs had higher amounts of plasma proteins, mostly albumin and, transferrin, compared to the NPs without the coat, showing that surface modification with amorphous silica alters the protein corona, agglomerate size, and zeta potential of ZnO NPs, which in turn influences ZnO biokinetic behavior in the circulation. This emphasizes the critical role of the protein corona in the biokinetics and toxicology of ZnO NPs [235]. An investigation described that corona formation occurs in the alveolar lining fluid and comprises plasma proteins, a surface-active phospholipid (PL) protein mixture, and a layer of aqueous hypo phase. In this scenario, ZnO NPs incubated in rat lung lining fluid in vitro binds mainly with albumin, transferrin, and α-1 antitrypsin. A more extensive database of corona composition of a varied NP library should be developed to help predict the effects and biokinetics of inhaled or ingested NPs [236].
The influence of serum Zn concentrations is the primary health outcome for clinical applications of Zn-based biomaterials. In an earlier study, elevated serum Zn levels were significantly associated with an increase in total spine and femur bone mineral density; also, each 10 μg/dL increase was associated with an increase in the risk of diabetes mellitus, cardiovascular diseases, and coronary heart disease in participants with serum Zn levels ≥ 100 μg/dL. However, it had no significant associations with the risk of fractures, congestive heart failure, heart attack, thyroid disease, arthritis, osteoarthritis, rheumatoid arthritis, dyslipidemia, and cancer [237]. Furthermore, the main problem that has been reported regarding the interface among Zn NPs and diet components is that it might affect nutrient absorption, could modify the expression of nutrient-related genes, or, as mentioned earlier, could induce oxidative stress[136]. Although, it has been reported that most of the observed toxic effects by ingestion of ZnO NPs are reversible after vitamin C or Quercetin supplementation, among other antioxidants[238], [239]. The treatment for Zn toxicity is directed to overcome the symptoms and restore Cu or Fe levels, though severe cases may also require chelation therapy and specialized medical attention[222]. However, discontinuing Zn supplementation may suffice and resolve the symptoms[161], [240].
In this regard, toxicity investigations with ZnO NPs, have shown that the oral administration of ZnO had no impact on organ weight, intestinal microbiota, and other mineral concentrations whereas, ZnSO4 appeared to incite further toxicity[241]. It has been found that ZnO and ZnO NPs at applied concentrations do not affect the growth performance and composition of blood minerals in Markhoz goats [242]. Zn-deficient rat models were supplemented orally with nano ZnO fortified Biscuits (13.5 ppm, 27 ppm, and 54 ppm) and were compared with ZnO from the bulk. Results showed an enhancement of body growth rate, appetite, and hair growth, without mortality and neither histopathological abnormalities in the Liver, Kidneys, and Testis. Recommending 13.5 ppm nano Zn fortified biscuits for managing mild Zn deficiency and the dose of 27 ppm for more severe conditions [243]. Likewise, considerable improvements were observed in the health status and birds' immunity supplemented with nano Zn to broiler diets at 0.06 mg/kg compared with the conventional dose of 15 mg/kg of organic and inorganic Zn with the basal diet [208]. In newly-weaned piglets, replacing high-dose dietary ZnO with low-dose porous and nano ZnO had a similar effect on improving growth performance and intestinal morphology, reducing diarrhea and intestinal inflammation [243]. In a virus mitigation assay, no cytotoxicity of MARC-145 cells was observed for any of the MnZnS NPs concentrations tested (100 μg/ml, 50 μg/ml, 20 μg/ml, 10 μg/ml) [213]. An in vitro pulmonary toxicity test of ZnO NPs in human lung cell lines showed a toxic effect at concentrations between 10 and 50 μg ml−1. Also, 2D cell model submerged exposure revealed toxicity for ZnO at 10 μg ml−1 for huAEC and hAELVi cell lines, where A549 were only significantly affected at 50 μg ml−1. In contrast, the tight junctions were not affected by the NP (Fig. 13 (a)) [244].
Fig. 13.
Potential toxicological mechanisms of administration of Zn nanomaterials. (a) Metabolic activity (A), LDH (cytotoxicity) (B), and release and ROS generation (C) after ZnO NP exposure in different lung epithelia cells [244]. Copyright Royal Society of Chemistry. (b) Metal oxide nanoparticles have efficient antimicrobial activity, but many biological challenges have restricted their application and hurdles to scale‐up manufacture for the clinic [247]. Copyright John Wiley & Sons 2019. (c) Effect of food on orally ingested nanoparticle behaviors in the simulated digestive tract. Copyright Europe PMC 2020 [245].
Moreover, a study employing the interaction between different food matrices and TiO2 and ZnO NPs (Fig. 13 (b)) found that depending on the nanomaterial and the food matrix, it can change the following parameters such as intermolecular interactions and the size of the nanomaterial through the GI tract. These relations could finally modify the toxicological responses induced by NPs after oral exposure [243], [245]. It is essential to mention that the doses administered in most studies were excessive compared to what is required in regular human daily consumption. In vitro studies utilizing NPs for oral consumption might lead to uncertain information of toxicity of NPs, and it is necessary to assess the synergistic effects of NPs in a complex system [246].
As mentioned, Zn-associated NPs are in their early life in mineral nutrition, and additional research is required. Further research should be directed to find the optimum levels of Zn NPs that can present better performance and benefits. Evaluation of toxical effects needs a more significant number of studies and the most prolonged period to standardize both the positive and possible adverse effects. In addition, the financial side and stability of the produced mineral NPs beneath normal storage conditions should also be extensively studied. The biological challenges of utilizing metal oxide NPs for efficient antimicrobial activity and scale-up manufacturing for clinical applications are shown in Fig. 13(c) [247]. Henceforth, it is vital to mention that a careful study is required to determine the exact relationship between morphological properties of these nanomaterials and their dissolution, transport, metabolism, oxidative impacts, biotoxicity, and other biological effects. Further experiments should be conducted to characterize the differences between nanosized Zn behavior in animal models and human GI tracts (or different routes of administration). Also, it is imperative to understand the implications of its effect on the gut microbiota and its possible outcome in respiratory virus infection.
5. Immunomodulatory properties of the Zn nanomaterials
Nanotechnology strategies have contributed as advanced resolutions for the treatment against emergent viruses like H1N1, MERS, and SARS-CoV-2 [7]. Apart from the attractive applications mentioned earlier in this report, such as diagnosis, disinfection, and supplementation, ZnO NPs have attractive immunomodulatory properties suggesting their potential use as an adjuvant.
First of all, the immune system is interconnected between innate and adaptative responses. The innate response includes macrophages that engulf pathogens and natural killer (NK) cells that induce cell death in infected host cells. For the adaptative immune system, pathogens or antigens move to immune tissues, like lymph nodes (LNs), where antigen-presenting cells (APCs), like dendritic cells (DCs), process and present the antigen on their surface by displaying specialized proteins called major histocompatibility complexes (MHCs) (Fig. 14 ) [248]. NPs can participate in immunomodulation through the release of various cytokines and growth factors [249]. Specifically, ZnO NPs can induce the expression of immunoregulatory cytokines, which is also a relevant consideration for potential use in biomedical applications, as many current treatments for human disease function by manipulating and controlling components of the immune response [250]. The immunomodulatory response has been associated with systemic deposition of ZnO NPs in the tissues that can cause classic pulmonary oxidative inflammatory responses [251]. For instance, Saptarshi et al. [252] have reported the potential of ZnO NPs to cause pulmonary immunomodulation when exposed via intranasal instillation, highlighting that the cytotoxicity depends on NPs concentration, size, and time of exposition. As mentioned earlier, ZnO NP cytotoxicity has been reported in vitro test systems, including immune cells, lung epithelial cells, etc. [253], [254], [255], [256]. The reports suggest that ZnO NP cytotoxicity is mainly associated with ROS generation [256], [257], [258]. In a few studies, ZnO NPs are shown to increase the expression of inflammatory markers (ICAM-1, IL-8, and MCP-1) [259] and to enhance the production of cytokines (IFN-γ, TNF, and IL-12) [250].
Fig. 14.
Activation of the immune cells by ZnO NPs exposure. (a) Types of immunity cells: Innate immunity include macrophages, dendritic cell, and natural killer which are involved in the first-line defense against pathogens and particles. Adaptative immunity requires antigen recognition along with trigger signals to produce specific antibodies. (b) B) ZnO NPs immunization via the respiratory activating the adaptative and innate immune responses.
On the other hand, Poon et al. [260] studied nano- and bulk-sized ZnO NPs, showing their effect on cell metabolisms and gene expression through transcriptomic analyses associated with exposure to the maximum sub-lethal dose to characterize the early immune signaling and material-specific adaptative responses. They found that exposure to ZnO NPs induced the expression of several innate and adaptative immunity pathways.
According to the above discussion, the search for effective adjuvants for improving the immune response is vital (as a form of contention) against emergent respiratory diseases that have not been controlled. Several reports indicate that ZnO NPs have adjuvant properties [249], [261], [262], [263], [264] and describe the mechanisms involved in adjuvant response induced in cell lines [261]. It is mentioned before, the stimulatory effects are mediated by intracellular Zn2+ dissolution in the lysosomes and ROS generation, resulting in the release of inflammatory cytokines and cellular activation of immune cells [264]. Then, ZnO NPs could participate as either a delivery system to enhance antigen processing or an immune-stimulating adjuvant. For instance, ZnO NPs have also been found to show adjuvant effect via activating the innate immune system and recruiting APCs (CD19+ MHC class-II+ B cells and activated macrophages), lymphocytes, eosinophils, mast cells, and neutrophils [249]. However, these NPs boost the secondary immune response leading to the exacerbation of inflammation characterized by enhanced expression of Cox2, MMP 9, and PGE2 [261].
To explore ZnO NPs intrinsic adjuvant-like properties and immunomodulatory functions, Afroz et al. [262] designed a novel mesoporous ZnO nanocapsule (mZnO) to investigate its immunomodulatory properties by using Ova loaded mZnOnanocapsules [mZnO(Ova)] in a mice model. Their findings showed that mZnO(Ova) administration steered the enhanced expansion of antigen-specific T-cells and induction of IFN-γ producing effector CD4+ and CD8+ T-cells. Also, antigen-specific IgG levels are enriched in both the serum and lymph nodes of mZnO(Ova) immunized mice. Further, they noticed a substantial increase in serum IgG2a or IgG2b levels and IFN-γ secretion in Ova restimulated splenocytes from mZnO(Ova) immunized mice. Given these features in combination with its immunomodulatory characteristics reinforces the idea that ZnO NPs could be used as an effective antigen-adjuvant platform for developing novel nano-based vaccines against multiple diseases, including respiratory viruses.
Mucosal immunization via the respiratory tract mimics the natural entry of antigens or viruses, such as the case of respiratory viruses like SARS-CoV-2, that usually have the first contact with the nasal or pulmonary mucosa when entering the human body. An earlier study using different spray-dried ZnO formulations to test the aerodynamic performances and immunological response, shown promising properties about mucosal vaccination via the respiratory tract, via increasing the expression of CD80, CD86, and MHC-II, which indicated an activation of the dendritic cells, proposing the use of ZnO for respiratory vaccination [265].
Despite the properties mentioned above, the utilization of Zn NPs or Zn2+ in the vaccine development against virus-like H1N1, H5N1, MERS, or SARS-CoV-2 has not been fully explored even when the nanotechnology has been valuable in the COVID-19 pandemic. Until now, the greatest approach in this sense could be the utilization of Zn in the stabilization of liposomes, taking into account that they are essential components in some of the authorized vaccines (Moderna [266] and Biotech/Pfizer [266].
Notably, the use of lipid nanoparticles (LNPs) is one of the most promising vaccine platforms, which are highly efficient in encapsulating DNA- or RNA- based immunogens or antibodies[55]. The new generation of nano vaccines coupling LNPs and metal ions, like Zn2+, Mn2+, Co2+, by functionalizing PoP-bilayers or another biomolecule is a promising alternative. For instance, Federizon et al. (2021) reported that Co2+ incorporation into liposomes could increase the immune response, proposing it as a new vaccine candidate that warrants further investigation since attaching polypeptides to lipid bilayers is often indirect, ineffective, and can represent a substantial bottleneck in forming functionalized lipid-based materials [267], [268], [269]. Consequently, novel prototypes of nanoengineered particles such as the LNPs complex with metallic cations like Zn2+, with its intrinsic viricidal activity [157], could be demanding (Fig. 15 ). More investigation is required to clarify how this novel nanosystem could increase human immune response in the same way as previously observed in animal models [148]. The main limitation of nano vaccines coupling LNPs as a delivery system is their poor stability in gastric juice, high manufacturing cost, and inactivation of phospholipid membrane integrity [270]. However, after coupling with metal ions, their stability can be further improved, and it could open the pathway for their mass production, providing an excellent choice of delivering vaccines. Therefore, nano vaccines research is inherently a field in which more challenges demand our attention. It could help develop more efficient and safer vaccines, mainly directed to people with specific comorbidities.
Fig. 15.
Zinc participation for stabilizing liposomes as a strategy in the nanovaccines development against SARS-CoV-2. Schematic representation showing the coupling of metallic ions with liposome nanoparticles (LNP) and surface functionalization using SARS-CoV-2 Spike protein (PDB code 6XS6), which may be useful in a novel development of nanovaccines.
6. Conclusions and future perspectives
The use of nanomaterials as Zn and its compounds has provided an essential contribution to the management of COVID-19. Some of the significant conclusions from the present study and the future challenges for applying nanostructured Zn and its compounds to combat respiratory viruses are highlighted in Fig. 16 . One of the main concerns about COVID-19 is the diagnosis and detection of infected patients. Detection strategies are focused on molecular techniques, which are expensive and time-consuming; however, the need for rapid detection methods is one of the most critical control strategies. The development of nanomaterials as sensor platforms can reduce detection time, reagents, and infrastructure. The use of Zn-based nanostructures to detect the principal biomarkers of the SARS-CoV-2 remains an ongoing challenge, where the development of selective and sensitive devices is the primary goal. The former application of ZnO nanostructures for the effective detection of multiple biomarkers, including viruses, suggests the potential use of these systems for developing electrochemical and optical biosensing platforms for COVID-19 surveillance. However, it is crucial to consider that the main limitations are the costs of fabrication and mass-scale production of these biosensors based on nanotechnology. Additionally, for the successful implementation of biosensors, different analytes must be conserved and transported on distant, isolated zones, which could significantly hinder the implication of these upcoming technologies.
Fig. 16.
Future perspectives of nano Zn and its compounds for COVID-19 detection and treatment methods.
To impede virus propagation, the increasing demand for self-sterilized coating requires alternative materials to fulfill this issue. In this respect, the use of Zn/ZnO nanostructures has exhibited significant antiviral activity against several viruses such as influenza and coronaviruses. As illustrated in Fig. 16 , the cytotoxic study of this material, along with the potential scalability, is the leftover task. On the other hand, the role of Zn as a supplement has shown benefits such as modulation of the immune response and antiviral activity against SARS-CoV-2 and other respiratory viruses, although more studies are still needed. Therefore, it is necessary to determine the relationship between nano Zn materials and their biotoxicity, dissolution, transport, metabolism, oxidative impacts, and other biological effects in the human body. Until now, ZnO NPs have not been used to develop vaccines; however, their potential adjuvant properties have been explored, producing several innate and adaptative immunity responses. Taking this into consideration, the addition of metal ions to stabilized liposomes could be a way to develop novel platforms that may include Zn or ZnO NPs, since the global research efforts converge on the development of specific vaccines. This could help develop more efficient and safer vaccines coupling LNP and metal ions, even though its final effect on the immune response is still under study. It is noteworthy to mention that this materials appealing properties can be extrapolated to new clinical challenges and can also be used in emergency health situations like the COVID-19 pandemic. Overall, the implementation of Zn-based nanomaterials could impact technological development, thus contributing to well-being and economic growth.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors wish to thank the parental institutes for providing the necessary facilities to accomplish this work. A.G.M acknowledges CONACyT Scholarship CVU 860916. E.A.J acknowledges CONACyT Scholarship CVU 770372. G.S. gratefully acknowledges the CONACyT project PN 4797 and IG100320. S.A acknowledges CONACyT Postdoctoral Scholarship CVU 253744. A.D. thanks the DGAPA project PAPIIT IA101321. V.K.T. would also like to thank the research support provided by the UKRI via Grant No. EP/T024607/1, Royal Academy of Engineering (IAPP18-19∖295), and UKIERI (DST/INT/UK/P-164/2017). Walaa F. Alsanie acknowledges Taif University for support No. TURSP (2020/53).
The authors also acknowledge José Antonio Cera Ramos and Selene Mendoza Morales for creating/editing images.
References
- 1.C.D. (COVID-19), WHO, (2021). https://covid19.who.int/?gclid=CjwKCAjwnK36BRBVEiwAsMT8WJ3y00_BUzvrLsvbl3uthuoTH_Occ45gyEUbpYRyEqAzll3aZB6TYxoCcM0QAvD_BwE.
- 2.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 3.J.W. LeDuc, M.A. Barry, SARS, the First Pandemic of the 21st Century, Emerg. Infect. Dis. 10 (2004) e26. 10.3201/eid1011.040797_02.
- 4.Jain S., Finelli L., Shaw M.W., Lindstrom S., Garten R.J., Gubareva L.V., Xu X., Bridges C.B., Uyeki T.M. Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. N. Engl. J. Med. 2009;360:2605–2615. doi: 10.1056/nejmoa0903810. [DOI] [PubMed] [Google Scholar]
- 5.Middle East respiratory syndrome coronavirus (MERS-CoV), (n.d.).
- 6.Hu T., Liu Y., Zhao M., Zhuang Q., Xu L., He Q. A comparison of COVID-19, SARS and MERS. PeerJ. 2020;8:1–30. doi: 10.7717/peerj.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawre S., Maru S. Human respiratory viral infections: current status and future prospects of nanotechnology-based approaches for prophylaxis and treatment. Life Sci. 2021;278 doi: 10.1016/J.LFS.2021.119561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu M., Wu J., Shi J., Farokhzad O.C. Nanotechnology for protein delivery: overview and perspectives. J. Control. Release. 2016;240:24–37. doi: 10.1016/j.jconrel.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W.C., Zhou S., He X., Chiem K., Mabrouk M.T., Nissly R.H., Bird I.M., Strauss M., Sambhara S., Ortega J., Wohlfert E.A., Martinez-Sobrido L., Kuchipudi S.V., Davidson B.A., Lovell J.F. SARS-CoV-2 RBD neutralizing antibody induction is enhanced by particulate vaccination. Adv. Mater. 2020;2005637:1–11. doi: 10.1002/adma.202005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talebian S., Wallace G.G., Schroeder A., Stellacci F., Conde J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020;15:618–621. doi: 10.1038/s41565-020-0751-0. [DOI] [PubMed] [Google Scholar]
- 11.Shah K.W., Huseien G.F. Inorganic nanomaterials for fighting surface and airborne pathogens and viruses. Nano Express. 2020;1 doi: 10.1088/2632-959x/abc706. [DOI] [Google Scholar]
- 12.Moitra P., Alafeef M., Alafeef M., Alafeef M., Dighe K., Frieman M.B., Pan D., Pan D., Pan D. Selective naked-eye detection of SARS-CoV-2 Mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano. 2020;14:7617–7627. doi: 10.1021/acsnano.0c03822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., Pan W., Zhan Z., Chen L., Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H., Liu F., Xie W., Zhou T.C., OuYang J., Jin L., Li H., Zhao C.Y., Zhang L., Wei J., Zhang Y.P., Li C.P. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators, B Chem. 2021;327 doi: 10.1016/j.snb.2020.128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campos E.V.R., Pereira A.E.S., De Oliveira J.L., Carvalho L.B., Guilger-Casagrande M., De Lima R., Fraceto L.F. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J. Nanobiotechnol. 2020;18:1–23. doi: 10.1186/s12951-020-00685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw A.M., Hyde C., Merrick B., James-Pemberton P., Squires B.K., Olkhov R.V., Batra R., Patel A., Bisnauthsing K., Nebbia G., Macmahon E., Douthwaite S., Malim M., Neil S., Martinez Nunez R., Doores K., Mark T.K.I., Signell A.W., Betancor G., Wilson H.D., Galão R.P., Pickering S., Edgeworth J.D. Real-world evaluation of a novel technology for quantitative simultaneous antibody detection against multiple SARS-CoV-2 antigens in a cohort of patients presenting with COVID-19 syndrome. Analyst. 2020;145:5638–5646. doi: 10.1039/d0an01066a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karami A., Hasani M., Azizi Jalilian F., Ezati R. Conventional PCR assisted single-component assembly of spherical nucleic acids for simple colorimetric detection of SARS-CoV-2. Sens. Actuators B Chem. 2021;328 doi: 10.1016/j.snb.2020.128971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das C.M., Guo Y., Yang G., Kang L., Xu G., Ho H.P., Yong K.T. Gold nanorod assisted enhanced plasmonic detection scheme of COVID-19 SARS-CoV-2 spike protein. Adv. Theory Simul. 2020;3:1–8. doi: 10.1002/adts.202000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D., Zhang X., Ma R., Deng S., Wang X., Wang X., Zhang X., Huang X., Liu Y., Li G., Qu J., Zhu Y., Li J. Ultra-fast and onsite interrogation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in waters via surface enhanced Raman scattering (SERS) Water Res. 2021;200 doi: 10.1016/j.watres.2021.117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C., Yang X., Gu B., Liu H., Zhou Z., Shi L., Cheng X., Wang S., Wang C., Gu B., Wang S. Sensitive and simultaneous detection of SARS-CoV-2-Specific IgM/IgG using lateral flow immunoassay based on dual-mode quantum dot nanobeads. Anal. Chem. 2020;92:15542–15549. doi: 10.1021/acs.analchem.0c03484. [DOI] [PubMed] [Google Scholar]
- 21.Z. Zhao, H. Cui, W. Song, X. Ru, W. Zhou, X. Yu, A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2, 518055 (2020). 10.1101/2020.02.22.961268.
- 22.Gorshkov K., Susumu K., Chen J., Xu M., Pradhan M., Zhu W., Hu X., Breger J.C., Wolak M., Oh E. Quantum dot-conjugated SARS-CoV-2 spike pseudo-virions enable tracking of angiotensin converting enzyme 2 binding and endocytosis. ACS Nano. 2020;14:12234–12247. doi: 10.1021/acsnano.0c05975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A. Thevasahayam, Diagnosis and qualification of COVID-19 employing LAB-ON-CHIP Technology, IN202041034067, 2020. 202041034067.
- 24.F. Thevasahayam, A. By, P. Agent, B. Deepa, Figure 1 Dated this 26th day of September 2020 For THEVASAHAYAM AROCKIADOSS By his Patent Agent, (2020).
- 25.Espitia P.J.P., Soares N.de F.F., Coimbra J.S.dos R., de Andrade N.J., Cruz R.S., Medeiros E.A.A. Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012;5:1447–1464. doi: 10.1007/s11947-012-0797-6. [DOI] [Google Scholar]
- 26.Pasquet J., Chevalier Y., Pelletier J., Couval E., Bouvier D., Bolzinger M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surfaces A Physicochem. Eng. Asp. 2014;457:263–274. doi: 10.1016/j.colsurfa.2014.05.057. [DOI] [Google Scholar]
- 27.Sirelkhatim A., Mahmud S., Seeni A., Kaus N.H.M., Ann L.C., Bakhori S.K.M., Hasan H., Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015;7:219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patients L., Taylor D., Lindsay A.C., Halcox J.P. Correspondence Niacin compared with ezetimibe. N. Engl. J. Med. 2020;0–3 [Google Scholar]
- 29.Cardoso V.M.de O., Moreira B.J., Comparetti E.J., Sampaio I., Ferreira L.M.B., Lins P.M.P., Zucolotto V. Is nanotechnology helping in the fight against COVID-19? Front. Nanotechnol. 2020;2 doi: 10.3389/fnano.2020.588915. [DOI] [Google Scholar]
- 30.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S., Southern W.N., Mantzoros C.S. Severe obesity is associated with higher in-hospital mortality in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2019;2020:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 32.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., Boehm B., Amiel S., Holt R.I., Skyler J.S., DeVries J.H., Renard E., Eckel R.H., Zimmet P., Alberti K.G., Vidal J., Geloneze B., Chan J.C., Ji L., Ludwig B. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargas-Vázquez A., Bello-Chavolla O.Y., Ortiz-Brizuela E., Campos-Muñoz A., Mehta R., Villanueva-Reza M., Bahena-López J.P., Antonio-Villa N.E., González-Lara M.F., Ponce de León A., Sifuentes-Osornio J., Aguilar-Salinas C.A. Impact of undiagnosed type 2 diabetes and pre-diabetes on severity and mortality for SARS-CoV-2 infection. BMJ Open Diabetes Res. Care. 2021;9 doi: 10.1136/bmjdrc-2020-002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21 doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Luis D.A., Pacheco D., Izaola O., Terroba M.C., Cuellar L., Cabezas G. Micronutrient status in morbidly obese women before bariatric surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2013;9:323–327. doi: 10.1016/j.soard.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Williams C.R., Mistry M., Cheriyan A.M., Williams J.M., Naraine M.K., Ellis C.L., Mallick R., Mistry A.C., Gooch J.L., Ko B., Cai H., Hoover R.S. Zinc deficiency induces hypertension by promoting renal Na+ reabsorption. Am. J. Physiol. - Ren. Physiol. 2019;316:F646–F653. doi: 10.1152/ajprenal.00487.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pisano M., Hilas O. Zinc and taste disturbances in older adults: a review of the literature. Consult. Pharm. J. Am. Soc Consult. Pharm. 2016;31:267–270. doi: 10.4140/TCP.n.2016.267. [DOI] [PubMed] [Google Scholar]
- 39.Gonçalves L.F., Gonzáles A.I., Paiva K.M., Patatt F.S.A., Stolz J.V., Haas P. Smell and taste alterations in COVID-19 patients: a systematic review. Rev. Assoc. Med. Bras. 2021;66:1602–1608. doi: 10.1590/1806-9282.66.11.1602. [DOI] [PubMed] [Google Scholar]
- 40.Rahman M.T., Idid S.Z. Can Zn be a critical element in COVID-19 treatment? Biol. Trace Elem. Res. 2021;199:550–558. doi: 10.1007/s12011-020-02194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar A., Kubota Y., Chernov M., Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Razzaque M.S. COVID-19 pandemic: can maintaining optimal zinc balance enhance host resistance? Tohoku J. Exp. Med. 2020;251:175–181. doi: 10.1620/tjem.251.175. [DOI] [PubMed] [Google Scholar]
- 43.Carlucci P.M., Ahuja T., Petrilli C., Rajagopalan H., Jones S., Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J. Med. Microbiol. 2020;69:1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Derwand R., Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win todays battle against COVID-19? Med. Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasad A.S. Zinc in human health: effect of zinc on immune cells. Mol. Med. 2008;14:353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhutta Z.A., Black R.E., Brown K.H., Meeks Gardner J., Gore S., Hidayat A., Khatun F., Martorell R., Ninb N.X., Penny M.E., Rosado J.L., Roy S.K., Ruel M., Sazawal S., Shankar A. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J. Pediatr. 1999;135:689–697. doi: 10.1016/S0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 47.Turner R.B., Cetnarowski W.E. Effect of treatment with zinc gluconate or zinc acetate on experimental and natural colds. Clin. Infect. Dis. 2000;31:1202–1208. doi: 10.1086/317437. [DOI] [PubMed] [Google Scholar]
- 48.Hamza R.T., Hamed A.I., Sallam M.T. Effect of zinc supplementation on growth Hormone Insulin growth factor axis in short Egyptian children with zinc deficiency. Ital. J. Pediatr. 2012;38:1–7. doi: 10.1186/1824-7288-38-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collipp P.J., Castro-Magana M., Petrovic M., Thomas J., Cheruvanky T., Chen S.-Y., Sussman H. Zinc deficiency: improvement in growth and growth hormone levels with oral zinc therapy. Ann. Nutr. Metab. 1982;26:287–290. doi: 10.1159/000176575. [DOI] [PubMed] [Google Scholar]
- 50.Hoogenraad T.U. Paradigm shift in treatment of Wilson’s disease: zinc therapy now treatment of choice. Brain Dev. 2006;28:141–146. doi: 10.1016/j.braindev.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 51.S.M. Bonanome, Andrea; Grundy, The New England Journal of Medicine Downloaded from nejm.org at MOUNT SINAI SCHOOL OF MEDICINE on October 26, 2014. For personal use only. No other uses without permission. From the NEJM Archive. Copyright © 2010 Massachusetts Medical Society. All rights, N. Engl. J. Med. (1988) 1244–1248.
- 52.te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., Svistunov A.A., Petrakis D., Spandidos D.A., Aaseth J., Tsatsakis A., Tinkov A.A. Zinc and respiratory tract infections: perspectives for CoviD’19 (Review) Int. J. Mol. Med. 2020;46:17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.W.O. A, Wo 02/02130 a1 (10), 1 (2002).
- 55.Ruiz-Hitzky E., Darder M., Wicklein B., Ruiz-Garcia C., Martín-Sampedro R., del Real G., Aranda P. Nanotechnology responses to COVID-19. Adv. Healthc. Mater. 2020;9:1–26. doi: 10.1002/adhm.202000979. [DOI] [PubMed] [Google Scholar]
- 56.W. Abdul, A. Muhammad, K. Atta Ullah, A. Asmat, B. Abdul, Role of nanotechnology in diagnosing and treating COVID-19 during the Pandemi, Int. J. Clin. Virol. 4 (2020) 065–070. 10.29328/journal.ijcv.1001017.
- 57.Adhikari S., Adhikari U., Mishra A., Guragain B.S. Nanomaterials for diagnostic, treatment and prevention of COVID-19. Appl. Sci. Technol. Ann. 2020;1:155–164. doi: 10.3126/asta.v1i1.30295. [DOI] [Google Scholar]
- 58.Yan Y., Chang L., Wang L. Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): current status, challenges, and countermeasures. Rev. Med. Virol. 2020;30:1–14. doi: 10.1002/rmv.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rai M., Bonde S., Yadav A., Plekhanova Y., Reshetilov A., Gupta I., Golińska P., Pandit R., Ingle A.P. Nanotechnology-based promising strategies for the management of COVID-19: current development and constraints. Expert Rev. Anti. Infect. Ther. 2020 doi: 10.1080/14787210.2021.1836961. [DOI] [PubMed] [Google Scholar]
- 60.Rabiee N., Bagherzadeh M., Ghasemi A., Zare H., Ahmadi S., Fatahi Y., Dinarvand R., Rabiee M., Ramakrishna S., Shokouhimehr M., Varma R.S. Point-of-use rapid detection of sars-cov-2: nanotechnology-enabled solutions for the covid-19 pandemic. Int. J. Mol. Sci. 2020;21:1–23. doi: 10.3390/ijms21145126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharifi M., Hasan A., Haghighat S., Taghizadeh A., Attar F., Bloukh S.H., Edis Z., Xue M., Khan S., Falahati M. Rapid diagnostics of coronavirus disease 2019 in early stages using nanobiosensors: challenges and opportunities. Talanta. 2021;223 doi: 10.1016/j.talanta.2020.121704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu J., Liu J., Li S., Peng Z., Xiao Z., Wang X., Yan R., Luo J. Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med. Infect. Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alafeef M., Dighe K., Moitra P., Pan D. Rapid, ultrasensitive, and quantitative detection of sars-cov-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano. 2020 doi: 10.1021/acsnano.0c06392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.V. Kumar, S. Mishra, R. Sharma, J. Agarwal, U. Ghoshal, T. Khanna, L. Sharma, S.K. Verma, P. Mishra, S. Tiwari, Development of RNA-based assay for rapid detection of SARS-CoV-2 in clinical samples, (2020). 10.1101/2020.06.30.172833. [DOI] [PMC free article] [PubMed]
- 65.Wen T., Huang C., Shi F.J., Zeng X.Y., Lu T., Ding S.N., Jiao Y.J. Development of a lateral flow immunoassay strip for rapid detection of IgG antibody against SARS-CoV-2 virus. Analyst. 2020;145:5345–5352. doi: 10.1039/d0an00629g. [DOI] [PubMed] [Google Scholar]
- 66.T. Liang, Handbook of COVID-19 Prevention and Treatment, 2020.
- 67.Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14:5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 68.Ehtesabi H. Application of carbon nanomaterials in human virus detection. J. Sci. Adv. Mater. Devices. 2020;5:436–450. doi: 10.1016/j.jsamd.2020.09.005. [DOI] [Google Scholar]
- 69.Demeke Teklemariam A., Samaddar M., Alharbi M.G., Al-Hindi R.R., Bhunia A.K. Biosensor and molecular-based methods for the detection of human coronaviruses: a review. Mol. Cell. Probes. 2020;54 doi: 10.1016/j.mcp.2020.101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seo G., Lee G., Kim M.J., Baek S.H., Choi M., Ku K.B., Lee C.S., Jun S., Park D., Kim H.G., Kim S.I.S.J., Lee J.O., Kim B.T., Park E.C., Kim S.I.S.J. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 71.Ali M.A., Hu C., Jahan S., Yuan B., Saleh M.S., Ju E., Gao S.J., Panat R. Sensing of COVID-19 antibodies in seconds via aerosol jet nanoprinted reduced-graphene-oxide-coated 3D electrodes. Adv. Mater. 2020;2006647:1–15. doi: 10.1002/adma.202006647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ivanov Y.D., Malsagova K.A., Pleshakova T.O., Galiullin R.A., Kozlov A.F., Shumov I.D., Ivanova I.A., Archakov A.I., Popov V.P., Latyshev A.V., Rudenko K.V., Glukhov A.V. Ultrasensitive detection of 2,4-dinitrophenol using nanowire biosensor. J. Nanotechnol. 2018;2018 doi: 10.1155/2018/9549853. [DOI] [Google Scholar]
- 73.Soler M., Estevez M.C., Cardenosa-Rubio M., Astua A., Lechuga L.M. How nanophotonic label-free biosensors can contribute to rapid and massive diagnostics of respiratory virus infections: COVID-19 case. ACS Sensors. 2020;5:2663–2678. doi: 10.1021/acssensors.0c01180. [DOI] [PubMed] [Google Scholar]
- 74.Shan B., Broza Y.Y., Li W., Wang Y., Wu S., Liu Z., Wang J., Gui S., Wang L., Zhang Z., Liu W., Zhou S., Jin W., Zhang Q., Hu D., Lin L., Zhang Q., Li W., Wang J., Liu H., Pan Y., Haick H. Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano. 2020;14:12125–12132. doi: 10.1021/acsnano.0c05657. [DOI] [PubMed] [Google Scholar]
- 75.Roh C., Jo S.K. Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots-conjugated RNA aptamer on chip. J. Chem. Technol. Biotechnol. 2011;86:1475–1479. doi: 10.1002/jctb.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Z., Zhang Z., Zhai X., Li Y., Lin L., Zhao H., Bian L., Li P., Yu L., Wu Y., Lin G. Rapid and SENSITIVE DETECTION OF Anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Anal. Chem. 2020;92:7226–7231. doi: 10.1021/acs.analchem.0c00784. [DOI] [PubMed] [Google Scholar]
- 77.Antiochia R. Nanobiosensors as new diagnostic tools for SARS, MERS and COVID-19: from past to perspectives. Microchim. Acta. 2020;187 doi: 10.1007/s00604-020-04615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Somvanshi S.B., Kharat P.B., Saraf T.S., Somwanshi S.B., Shejul S.B., Jadhav K.M. Multifunctional nano-magnetic particles assisted viral RNA-extraction protocol for potential detection of COVID-19. Mater. Res. Innov. 2021;25:169–174. doi: 10.1080/14328917.2020.1769350. [DOI] [Google Scholar]
- 79.Tian B., Gao F., Fock J., Dufva M., Hansen M.F. Homogeneous circle-to-circle amplification for real-time optomagnetic detection of SARS-CoV-2 RdRp coding sequence. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112356. [DOI] [PubMed] [Google Scholar]
- 80.Bañuelos C., Orozco E. La biotecnología en la diplomacia científica mexicana y la lucha contra la COVID-19. Rev. La Soc. Mex. Biotecnol. y Bioingeniería. 2020;24:11–28. [Google Scholar]
- 81.Desarrollan en la UNAM biosensor para detección rápida de COVID-19, (2020).
- 82.Biosensores Covid19|Facultad de Ciencias, (2020).
- 83.Listo primer microdispositivo mexicano para prueba de anticuerpos y detectar nuevo coronavirus, 2020. (n.d.).
- 84.Diseñan dispositivo portátil para detección rápida de SARS-CoV-2, 2020. (n.d.).
- 85.Rodriguez-Moncayo R., Cedillo-Alcantar D.F., Guevara-Pantoja P.E., Chavez-Pineda O.G., Hernandez-Ortiz J.A., Amador-Hernandez J.U., Rojas-Velasco G., Sanchez-Muñoz F., Manzur-Sandoval D., Patino-Lopez L.D., May-Arrioja D.A., Posadas-Sanchez R., Vargas-Alarcon G., Garcia-Cordero J.L. A high-throughput multiplexed microfluidic device for COVID-19 serology assays. Lab Chip. 2021;21:93–104. doi: 10.1039/d0lc01068e. [DOI] [PubMed] [Google Scholar]
- 86.Tereshchenko A., Bechelany M., Viter R., Khranovskyy V., Smyntyna V., Starodub N., Yakimova R. Optical biosensors based on ZnO nanostructures: advantages and perspectives. A review. Sens. Actuators, B Chem. 2016 doi: 10.1016/j.snb.2016.01.099. [DOI] [Google Scholar]
- 87.A. Galdamez, A. Serrano, G. Santana, N. Arjona, L.G. Arriaga, J. Tapia Ramirez, G. Oza, A. Dutt, DNA probe functionalization on different morphologies of ZnO/Au nanowire for bio-sensing applications, Mater. Lett. 235 (2019) 250–253. 10.1016/j.matlet.2018.10.026.
- 88.Salinas Domínguez R.A., Domínguez Jiménez M.Á., Orduña Díaz A. Antibody immobilization in zinc oxide thin films as an easy-handle strategy for Escherichia coli detection, ACS. Omega. 2020;5:20473–20480. doi: 10.1021/acsomega.0c02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu M., Wang R., Li Y. Electrochemical biosensors for rapid detection of Escherichia coli O157:H7. Talanta. 2017;162:511–522. doi: 10.1016/j.talanta.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 90.Narita F., Wang Z., Kurita H., Li Z., Shi Y., Jia Y., Soutis C. A review of piezoelectric and magnetostrictive biosensor materials for detection of COVID-19 and other viruses. Adv. Mater. 2021;33 doi: 10.1002/adma.202005448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vashist S.K., Lam E., Hrapovic S., Male K.B., Luong J.H.T. Immobilization of antibodies and enzymes on platforms for biosensors and diagnostics. Chem. Rev. 2014;114:11083–11130. doi: 10.1021/cr5000943. [DOI] [PubMed] [Google Scholar]
- 92.González-Garnica M., Galdámez-Martínez A., Malagón F., Ramos C.D., Santana G., Abolhassani R., Kumar Panda P., Kaushik A., Mishra Y.K., Karthik T.V.K., Dutt A. One dimensional Au-ZnO hybrid nanostructures based CO2 detection: growth mechanism and role of the seed layer on sensing performance. Sens. Actuators, B Chem. 2021;337 doi: 10.1016/j.snb.2021.129765. [DOI] [Google Scholar]
- 93.J.F. Malagón G, R.A. Salinas, A. Galdámez, A. Orduña-Díaz, A. Dutt, Functionalization of 3-aminopropyltrimethoxysilane self-assembled monolayers on ZnO/Au nanowires: role of the seed layer, Mater. Lett. 290 (2021) 129452. 10.1016/j.matlet.2021.129452.
- 94.Galdámez-Martínez A., Bai Y., Santana G., Sprick R.S., Dutt A. Photocatalytic hydrogen production performance of 1-D ZnO nanostructures: Role of structural properties. Int. J. Hydrogen Energy. 2020;45:1–10. doi: 10.1016/j.ijhydene.2020.08.247. [DOI] [Google Scholar]
- 95.Faria A.M., Mazon T. Early diagnosis of Zika infection using a ZnO nanostructures-based rapid electrochemical biosensor. Talanta. 2019;203:153–160. doi: 10.1016/j.talanta.2019.04.080. [DOI] [PubMed] [Google Scholar]
- 96.Jang Y., Park J., Pak Y.K., Pak J.J. Immunosensor based on the ZnO nanorod networks for the detection of H1N1 swine influenza virus. J. Nanosci. Nanotechnol. 2012;12:5173–5177. doi: 10.1166/jnn.2012.6361. [DOI] [PubMed] [Google Scholar]
- 97.Nguyen A.V.T., Dao T.D., Trinh T.T.T., Choi D.Y., Yu S.T., Park H., Yeo S.J. Sensitive detection of influenza a virus based on a CdSe/CdS/ZnS quantum dot-linked rapid fluorescent immunochromatographic test. Biosens. Bioelectron. 2020;155 doi: 10.1016/j.bios.2020.112090. [DOI] [PubMed] [Google Scholar]
- 98.Low S.S., Tan M.T.T., Loh H.S., Khiew P.S., Chiu W.S. Facile hydrothermal growth graphene/ZnO nanocomposite for development of enhanced biosensor. Anal. Chim. Acta. 2016;903:131–141. doi: 10.1016/j.aca.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 99.Han J.H., Lee D., Chew C.H.C., Kim T., Pak J.J. A multi-virus detectable microfluidic electrochemical immunosensor for simultaneous detection of H1N1, H5N1, and H7N9 virus using ZnO nanorods for sensitivity enhancement. Sens. Actuators, B Chem. 2016;228:36–42. doi: 10.1016/j.snb.2015.07.068. [DOI] [Google Scholar]
- 100.Nasrin F., Chowdhury A.D., Takemura K., Kozaki I., Honda H., Adegoke O., Park E.Y. Fluorometric virus detection platform using quantum dots-gold nanocomposites optimizing the linker length variation. Anal. Chim. Acta. 2020;1109:148–157. doi: 10.1016/j.aca.2020.02.039. [DOI] [PubMed] [Google Scholar]
- 101.Li X., Qin Z., Fu H., Li T., Peng R., Li Z., Rini J.M., Liu X. Enhancing the performance of paper-based electrochemical impedance spectroscopy nanobiosensors: an experimental approach. Biosens. Bioelectron. 2021;177 doi: 10.1016/j.bios.2020.112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., Wang D.Q., Hu Y., Ren J.H., Tang N., Xu Y.Y., Yu L.H., Mo Z., Gong F., Zhang X.L., Tian W.G., Hu L., Zhang X.X., Xiang J.L., Du H.X., Liu H.W., Lang C.H., Luo X.H., Wu S.B., Cui X.P., Zhou Z., Zhu M.M., Wang J., Xue C.J., Li X.F., Wang L., Li Z.J., Wang K., Niu C.C., Yang Q.J., Tang X.J., Zhang Y., Liu X.M., Li J.J., Zhang D.C., Zhang F., Liu P., Yuan J., Li Q., Hu J.L., Chen J., Huang A.L. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 103.Tan Y.J., Goh P.Y., Fielding B.C., Shen S., Chou C.F., Fu J.L., Leong H.N., Leo Y.S., Ooi E.E., Ling A.E., Lim S.G., Hong W. Profiles of antibody responses against severe acute respiratory syndrome coronavirus recombinant proteins and their potential use as diagnostic markers. Clin. Diagn. Lab. Immunol. 2004;11:362–371. doi: 10.1128/CDLI.11.2.362-371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu L., Manopo I., Leung B.P., Chng H.H., Ling A.E., Chee L.L., Ooi E.E., Chan S.W., Kwang J. Immunological characterization of the spike protein of the severe acute respiratory syndrome coronavirus. J. Clin. Microbiol. 2004;42:1570–1576. doi: 10.1128/JCM.42.4.1570-1576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qiu M., Shi Y., Guo Z., Chen Z., He R., Chen R., Zhou D., Dai E., Wang X., Si B., Song Y., Li J., Yang L., Wang J., Wang H., Pang X., Zhai J., Du Z., Liu Y., Zhang Y., Li L., Wang J., Sun B., Yang R. Antibody responses to individual proteins of SARS coronavirus and their neutralization activities. Microbes Infect. 2005;7:882–889. doi: 10.1016/j.micinf.2005.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J., Bermudez-Gonzalez M., Kleiner G., Aydillo T., Miorin L., Fierer D., Lugo L.A., Kojic E.M., Stoever J., Liu S.T.H., Cunningham-Rundles C., Felgner P.L., Moran T., Garcia-Sastre A., Caplivski D., Cheng A., Kedzierska K., Vapalahti O., Hepojoki J.M., Simon V., Krammer F. A serological assay to detect SARS-CoV-2 seroconversion in humans. MedRxiv. 2020;26 doi: 10.1101/2020.03.17.20037713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Allen C.G., Baker D.J., Albin J.M., Oertli H.E., Gillaspie D.T., Olson D.C., Furtak T.E., Collins R.T. Surface modification of zno using triethoxysilane-based molecules. Langmuir. 2008;24:13393–13398. doi: 10.1021/la802621n. [DOI] [PubMed] [Google Scholar]
- 108.Corso C.D., Dickherber A., Hunt W.D. An investigation of antibody immobilization methods employing organosilanes on planar ZnO surfaces for biosensor applications. Biosens. Bioelectron. 2008;24:805–811. doi: 10.1016/j.bios.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 109.García Núñez C., Sachsenhauser M., Blashcke B., García Marín A., Garrido J.A., Pau J.L. Effects of hydroxylation and silanization on the surface properties of ZnO nanowires. ACS Appl. Mater. Interfaces. 2015;7:5331–5337. doi: 10.1021/am508752m. [DOI] [PubMed] [Google Scholar]
- 110.Shirvanimoghaddam K., Akbari M.K., Yadav R., Al-Tamimi A.K., Naebe M. Fight against COVID-19: the case of antiviral surfaces. APL Mater. 2021;9 doi: 10.1063/5.0043009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gopal V., Nilsson-Payant B.E., French H., Siegers J.Y., Yung W.S., Hardwick M., Te Velthuis A.J.W. Zinc-embedded polyamide fabrics inactivate SARS-CoV-2 and influenza A virus. ACS Appl. Mater. Interfaces. 2021;13:30317–30325. doi: 10.1021/acsami.1c04412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rakowska P.D., Tiddia M., Faruqui N., Bankier C., Pei Y., Pollard A.J., Zhang J., Gilmore I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021;2 doi: 10.1038/s43246-021-00153-y. [DOI] [Google Scholar]
- 113.Shahzadi S., Zafar N., Sharif R. Antibacterial activity of metallic nanoparticles. Bact. Pathog. Antibact. Control. 2018 doi: 10.5772/intechopen.72526. [DOI] [Google Scholar]
- 114.Yemmireddy V.K., Hung Y.C. Using photocatalyst metal oxides as antimicrobial surface coatings to ensure food safety—Opportunities and challenges. Compr. Rev. Food Sci. Food Saf. 2017;16:617–631. doi: 10.1111/1541-4337.12267. [DOI] [PubMed] [Google Scholar]
- 115.Mizielińska M., Nawrotek P., Stachurska X., Ordon M., Bartkowiak A. Packaging covered with antiviral and antibacterial coatings based on ZnO nanoparticles supplemented with geraniol and carvacrol. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22041717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mukherjee M., De S. Antibacterial polymeric membranes: a short review. Environ. Sci. Water Res. Technol. 2018;4:1078–1104. doi: 10.1039/c8ew00206a. [DOI] [Google Scholar]
- 117.Munnawar I., Iqbal S.S., Anwar M.N., Batool M., Tariq S., Faitma N., Khan A.L., Khan A.U., Nazar U., Jamil T., Ahmad N.M. Synergistic effect of Chitosan-Zinc Oxide Hybrid Nanoparticles on antibiofouling and water disinfection of mixed matrix polyethersulfone nanocomposite membranes. Carbohydr. Polym. 2017;175:661–670. doi: 10.1016/j.carbpol.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 118.Shaban M., Mohamed F., Abdallah S. Production and characterization of superhydrophobic and antibacterial coated fabrics utilizing ZnO nanocatalyst. Sci. Rep. 2018;8:1–15. doi: 10.1038/s41598-018-22324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Siddiqi K.S., ur Rahman A., Tajuddin, Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018;13:1–13. doi: 10.1186/s11671-018-2532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Janaki A.C., Sailatha E., Gunasekaran S. Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2015;144:17–22. doi: 10.1016/j.saa.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 121.De Souza R.C., Haberbeck L.U., Riella H.G., Ribeiro D.H.B., Carciofi B.A.M. Antibacterial activity of zinc oxide nanoparticles synthesized by solochemical process. Brazilian J Chem. Eng. 2019;36:885–893. doi: 10.1590/0104-6632.20190362s20180027. [DOI] [Google Scholar]
- 122.Dimapilis E.A.S., Hsu C.S., Mendoza R.M.O., Lu M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018;28:47–56. doi: 10.1016/j.serj.2017.10.001. [DOI] [Google Scholar]
- 123.Xie Y., He Y., Irwin P.L., Jin T., Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011;77:2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sharmin S., Rahaman M.M., Sarkar C., Atolani O., Islam M.T., Adeyemi O.S. Nanoparticles as antimicrobial and antiviral agents: a literature-based perspective study. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cha S.H., Hong J., McGuffie M., Yeom B., Vanepps J.S., Kotov N.A. Shape-dependent biomimetic inhibition of enzyme by nanoparticles and their antibacterial activity. ACS Nano. 2015;9:9097–9105. doi: 10.1021/acsnano.5b03247. [DOI] [PubMed] [Google Scholar]
- 126.de Dicastillo C.L., Vidal C.P., Falcó I., Sánchez G., Márquez P., Escrig J. Antimicrobial bilayer nanocomposites based on the incorporation of as-synthetized hollow zinc oxide nanotubes. Nanomaterials. 2020;10:1–14. doi: 10.3390/nano10030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jalal R., Goharshadi E.K., Abareshi M., Moosavi M., Yousefi A., Nancarrow P. ZnO nanofluids: green synthesis, characterization, and antibacterial activity. Mater. Chem. Phys. 2010;121:198–201. doi: 10.1016/j.matchemphys.2010.01.020. [DOI] [Google Scholar]
- 128.Lipovsky A., Nitzan Y., Gedanken A., Lubart R. Antifungal activity of ZnO nanoparticles-the role of ROS mediated cell injury. Nanotechnology. 2011;22 doi: 10.1088/0957-4484/22/10/105101. [DOI] [PubMed] [Google Scholar]
- 129.Premanathan M., Karthikeyan K., Jeyasubramanian K., Manivannan G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation, nanomedicine nanotechnology. Biol. Med. 2011;7:184–192. doi: 10.1016/j.nano.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 130.Sevinç B.A., Hanley L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. - Part B Appl. Biomater. 2010;94:22–31. doi: 10.1002/jbm.b.31620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dizaj S.M., Lotfipour F., Barzegar-Jalali M., Zarrintan M.H., Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 132.Li Y., Zhang W., Niu J., Chen Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano. 2012;6:5164–5173. doi: 10.1021/nn300934k. [DOI] [PubMed] [Google Scholar]
- 133.Chong W.H., Saha B.K., Ananthakrishnan Ramani A., Chopra, State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection. 2021 doi: 10.1007/s15010-021-01602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mishra Y.K., Adelung R., Röhl C., Shukla D., Spors F., Tiwari V. Virostatic potential of micro-nano filopodia-like ZnO structures against herpes simplex virus-1. Antiviral Res. 2011;92:305–312. doi: 10.1016/j.antiviral.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tavakoli A., Ataei-Pirkooh A., Mm Sadeghi G., Bokharaei-Salim F., Sahrapour P., Kiani S.J., Moghoofei M., Farahmand M., Javanmard D., Monavari S.H. Polyethylene glycol-coated zinc oxide nanoparticle: an efficient nanoweapon to fight against herpes simplex virus type 1. Nanomedicine. 2018;13 doi: 10.2217/nnm-2018-0089. [DOI] [PubMed] [Google Scholar]
- 136.Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M., Farahmand M., Javanmard D., Kiani S.J., Esghaei M., Pirhajati-Mahabadi V., Ataei-Pirkooh A., Monavari S.H. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J. Biomed. Sci. 2019;26:70. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.El-Megharbel S.M., Alsawat M., Al-Salmi F.A., Hamza R.Z. Utilizing of (Zinc oxide nano-spray) for disinfection against “sars-cov-2” and testing its biological effectiveness on some biochemical parameters during (covid-19 pandemic)—”zno nanoparticles have antiviral activity against (sars-cov-2)”. Coatings. 2021;11 doi: 10.3390/coatings11040388. [DOI] [Google Scholar]
- 138.Adhikari A., Pal U., Bayan S., Mondal S., Ghosh R., Darbar S., Saha-Dasgupta T., Ray S.K., Pal S.K. Nanoceutical fabric prevents COVID-19 spread through expelled respiratory droplets: a combined computational, spectroscopic, and antimicrobial study. ACS Appl. Bio Mater. 2021;4:5471–5484. doi: 10.1021/acsabm.1c00238. [DOI] [PubMed] [Google Scholar]
- 139.Coronavirus: Nanotech Surface Sanitizes Milan with Nanomaterials Remaining Self-sterilized for Years|STATNANO, (n.d.).
- 140.S. Chatterjee, S. Mishra, K.D. Chowdhury, C.K. Ghosh, K. Das Saha, Various theranostics and immunization strategies based on nanotechnology against Covid-19 pandemic: an interdisciplinary view, Life Sci. 278 (2021) 119580. 10.1016/j.lfs.2021.119580. [DOI] [PMC free article] [PubMed]
- 141.S.-Y. Chang, K.-Y. Huang, T.-L. Chao, H.-C. Kao, Y.-H. Pang, L. Lu, C.-L. Chiu, H.-C. Huang, T.-J.R. Cheng, J.-M. Fang, P.-C. Yang, Nanoparticle composite TPNT1 is effective against SARS-CoV-2 and influenza viruses, Sci. Rep. 2021 111. 11 (2021) 1–13. 10.1038/s41598-021-87254-3. [DOI] [PMC free article] [PubMed]
- 142.Barocas J.A., So-Armah K., Cheng D.M., Lioznov D., Baum M., Gallagher K., Fuster D., Gnatienko N., Krupitsky E., Freiberg M.S., Samet J.H. Zinc deficiency and advanced liver fibrosis among HIV and hepatitis C co-infected anti-retroviral naïve persons with alcohol use in Russia. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kar M., Khan N.A., Panwar A., Bais S.S., Basak S., Goel R., Sopory S., Medigeshi G.R. Zinc chelation specifically inhibits early stages of dengue virus replication by activation of NF-κB and induction of antiviral response in epithelial cells. Front. Immunol. 2019;10:2347. doi: 10.3389/fimmu.2019.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oyagbemi A.A., Ajibade T.O., Aboua Y.G., Gbadamosi I.T., Adedapo A.D.A., Aro A.O., Adejumobi O.A., Thamahane-Katengua E., Omobowale T.O., Falayi O.O., Oyagbemi T.O., Ogunpolu B.S., Hassan F.O., Ogunmiluyi I.O., Ola-Davies O.E., Saba A.B., Adedapo A.A., Nkadimeng S.M., McGaw L.J., Kayoka-Kabongo P.N., Oguntibeju O.O., Yakubu M.A. Potential health benefits of zinc supplementation for the management of COVID-19 pandemic. J. Food Biochem. 2021;45:1–12. doi: 10.1111/jfbc.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.N. 2021 N.I. of H. National Institutes of Health, 2021, COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health., Accessed Febr. 22,2021. (2021) https://www.covid19treatmentguidelines.nih.gov/. [PubMed]
- 146.Mehta S. Nutritional status and COVID-19: an opportunity for lasting change? Clin. Med. J. R. Coll. Physicians London. 2020;20:270. doi: 10.7861/clinmed.2020-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pal A., Squitti R., Picozza M., Pawar A., Rongioletti M., Dutta A.K., Sahoo S., Goswami K., Sharma P., Prasad R. Zinc and COVID-19: basis of current clinical trials. Biol. Trace Elem. Res. 2020:1. doi: 10.1007/s12011-020-02437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Han H.J., Nwagwu C., Anyim O., Ekweremadu C., Kim S. COVID-19 and cancer: From basic mechanisms to vaccine development using nanotechnology. Int. Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rani I., Goyal A., Bhatnagar M., Manhas S., Goel P., Pal A., Prasad R. Potential molecular mechanisms of zinc- and copper-mediated antiviral activity on COVID-19. Nutr. Res. 2021;92:109–128. doi: 10.1016/J.NUTRES.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wintergerst E.S., Maggini S., Hornig D.H. Immune-enhancing role of Vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006;50:85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 151.Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front. Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harri H. Zinc lozenges may shorten the duration of colds: a systematic review. Open Respir Med. J. 2011;5:51–58. doi: 10.2174/1874306401105010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Siepmann M., Spank S., Kluge A., Schappach A., Kirch W. The pharmacokinetics of zinc from zinc gluconate: a comparison with zinc oxide in healthy men. Int. J. Clin. Pharmacol. Ther. 2005;43:562–565. doi: 10.5414/CPP43562. [DOI] [PubMed] [Google Scholar]
- 155.Wegmüller R., Tay F., Zeder C., Brnić M., Hurrell R.F. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J. Nutr. 2014;144:132–136. doi: 10.3945/jn.113.181487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.J.L. Rosado, Zinc and copper: Proposed fortification levels and recommended zinc compounds, in: J. Nutr., American Institute of Nutrition, 2003: pp. 2985S-2989S. 10.1093/jn/133.9.2985s. [DOI] [PubMed]
- 157.Scott A Read, Peran Seng dalam Kekebalan Antiviral, Univ. Sydney Westmead Hosp. Westmead, New South Wales, Aust. (2019) 696–710.
- 158.Pormohammad A., Monych N.K., Turner R.J., Pormohammad A. Zinc and SARS-CoV-2: a molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes. Int. J. Mol. Med. 2021;47:326–334. doi: 10.3892/ijmm.2020.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Provinciali M., Montenovo A., di Stefano G., Colombo M., Daghetta L., Cairati M., Veroni C., Cassino R., Torre F.D., Fabris N. Effect of zinc or zinc plus arginine supplementation on antibody titre and lymphocyte subsets after influenza vaccination in elderly subjects: a randomized controlled trial. Age Ageing. 1998;27:715–722. doi: 10.1093/AGEING/27.6.715. [DOI] [PubMed] [Google Scholar]
- 160.International news, Midwifery. 19 (2003) 72–74. 10.1054/midw.2002.0343.
- 161.Plum L.M., Rink L., Hajo H. The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Public Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Samad N., Sodunke T.E., Abubakar A.R., Jahan I., Sharma P., Islam S., Dutta S., Haque M. The implications of zinc therapy in combating the COVID-19 global pandemic. J. Inflamm. Res. 2021;14:527. doi: 10.2147/JIR.S295377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Knoell D.L., Smith D.A., Sapkota M., Heires A.J., Hanson C.K., Smith L.M., Poole J.A., Wyatt T.A., Romberger D.J. Insufficient zinc intake enhances lung inflammation in response to agricultural organic dust exposure. J. Nutr. Biochem. 2019;70:56–64. doi: 10.1016/j.jnutbio.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rerksuppaphol S., Rerksuppaphol L. A randomized controlled trial of chelated zinc for prevention of the common cold in thai school children. Paediatr. Int. Child Health. 2013;33:145–150. doi: 10.1179/2046905513Y.0000000064. [DOI] [PubMed] [Google Scholar]
- 165.Jothimani D., Kailasam E., Danielraj S., Nallathambi B., Ramachandran H., Sekar P., Manoharan S., Ramani V., Narasimhan G., Kaliamoorthy I., Rela M. COVID-19: poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gammoh N.Z., Rink L. Zinc in infection and inflammation. Nutrients. 2017;9:624. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jarosz M., Olbert M., Wyszogrodzka G., Młyniec K., Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.M.T. Rahman, Review on The Role of Zn 2+ Ions in Viral Pathogenesis and the Effect of Zn 2+ Ions for Host Cell-Virus Growth I Related papers Can Zn Be a Critical Element in COVID-19 Treatment ?, (n.d.). 10.34297/AJBSR.2019.02.000566.
- 169.Alexander J., Tinkov A., Strand T.A., Alehagen U., Skalny A., Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12:1–12. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.A. Darma, A.F. Athiyyah, R.G. Ranuh, W. Merbawani, R.A. Setyoningrum, B. Hidajat, S.N. Hidayati, A. Endaryanto, S.M. Sudarmo, Zinc supplementation effect on the bronchial cilia length, the number of cilia, and the number of intact bronchial cell in zinc deficiency rats, Indones. Biomed. J. 12(2020) 78–84. 10.18585/INABJ.V12I1.998.
- 171.Woodworth B.A., Zhang S., Tamashiro E., Bhargave G., Palmer J.N., Cohen N.A. Zinc increases ciliary beat frequency in a calcium-dependent manner. Am. J. Rhinol. Allergy. 2010;24:6–10. doi: 10.2500/ajra.2010.24.3379. [DOI] [PubMed] [Google Scholar]
- 172.Wittekindt O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch. Eur. J. Physiol. 2017;469:135–147. doi: 10.1007/s00424-016-1917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Berg K., Bolt G., Andersen H., Owen T.C. Zinc potentiates the antiviral action of human IFN-α tenfold. J. Interf. Cytokine Res. 2001;21:471–474. doi: 10.1089/10799900152434330. [DOI] [PubMed] [Google Scholar]
- 174.Cakman I., Kirchner H., Rink L. Zinc supplementation reconstitutes the production of interferon-α by leukocytes from elderly persons. J. Interf. Cytokine Res. 1997;17:469–472. doi: 10.1089/jir.1997.17.469. [DOI] [PubMed] [Google Scholar]
- 175.Perry D.K., Smyth M.J., Stennicke H.R., Salvesen G.S., Duriez P., Poirier G.G., Hannun Y.A. Zinc is a potent inhibitor of the apoptotic protease, caspase-3: a novel target for zinc in the inhibition of apoptosis. J. Biol. Chem. 1997;272:18530–18533. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 176.Foster M., Samman S. Zinc and Regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4:676–694. doi: 10.3390/nu4070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rolles B., Maywald M., Rink L. Influence of zinc deficiency and supplementation on NK cell cytotoxicity. J. Funct. Foods. 2018;48:322–328. doi: 10.1016/j.jff.2018.07.027. [DOI] [Google Scholar]
- 178.Rosenkranz E., Metz C.H.D., Maywald M., Hilgers R.D., Weßels I., Senff T., Haase H., Jager M., Ott M., Aspinall R., Plumakers B., Rink L. Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Mol. Nutr. Food Res. 2016;60:661–671. doi: 10.1002/mnfr.201500524. [DOI] [PubMed] [Google Scholar]
- 179.Wessels I., Haase H., Engelhardt G., Rink L., Uciechowski P. Zinc deficiency induces production of the proinflammatory cytokines IL-1β and TNFα in promyeloid cells via epigenetic and redox-dependent mechanisms. J. Nutr. Biochem. 2013;24:289–297. doi: 10.1016/j.jnutbio.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 180.Henry B.M., Aggarwal G., Wong J., Benoit S., Vikse J., Plebani M., Lippi G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am. J. Emerg. Med. 2020;38:1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pasternak C.A. A novel form of host defence: membrane protection by Ca2+ and Zn2+ Biosci. Rep. 1987;7:81–91. doi: 10.1007/BF01121871. [DOI] [PubMed] [Google Scholar]
- 182.Honscheid A., Rink L., Haase H. T-Lymphocytes: a target for stimulatory and inhibitory effects of zinc ions, endocrine. Metab. Immune Disord. - Drug Targets. 2009;9:132–144. doi: 10.2174/187153009788452390. [DOI] [PubMed] [Google Scholar]
- 183.Hasan R., Rink L., Haase H. Chelation of free Zn2+ impairs chemotaxis, phagocytosis, oxidative burst, degranulation, and cytokine production by neutrophil granulocytes. Biol. Trace Elem. Res. 2016;171:79–88. doi: 10.1007/s12011-015-0515-0. [DOI] [PubMed] [Google Scholar]
- 184.Rishi P., Thakur K., Vij S., Rishi L., Singh A., Kaur I.P., Patel S.K.S., Lee J.K., Kalia V.C. Diet, gut microbiota and COVID-19. Indian J. Microbiol. 2020;60:420–429. doi: 10.1007/s12088-020-00908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zackular J.P., Moore J.L., Jordan A.T., Juttukonda L.J., Noto M.J., Nicholson M.R., Crews J.D., Semler M.W., Zhang Y., Ware L.B., Washington M.K., Chazin W.J., Caprioli R.M., Skaar E.P. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat. Med. 2016;22:1330–1334. doi: 10.1038/nm.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Reed S., Neuman H., Moscovich S., Glahn R., Koren O., Tako E. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients. 2015;7:9768–9784. doi: 10.3390/nu7125497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dhar D., Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Osendarp S.J.M., Prabhakar H., Fuchs G.J., van Raaij J.M.A., Mahmud H., Tofail F., Santosham M., Black R.E. Immunization with the heptavalent pneumococcal conjugate vaccine in Bangladeshi infants and effects of zinc supplementation. Vaccine. 2007;25:3347–3354. doi: 10.1016/j.vaccine.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 189.J. wen Cao, S. yu Duan, H. xin Zhang, Y. Chen, M. Guo, Zinc Deficiency Promoted Fibrosis via ROS and TIMP/MMPs in the Myocardium of Mice, Biol. Trace Elem. Res. 196 (2020) 145–152. 10.1007/s12011-019-01902-4. [DOI] [PubMed]
- 190.Chilvers M.A., McKean M., Rutman A., Myint B.S., Silverman M., O’Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur. Respir. J. 2001;18:965–970. doi: 10.1183/09031936.01.00093001. [DOI] [PubMed] [Google Scholar]
- 191.Singh M., Das R.R. Zinc for the common cold. Cochrane Database Syst. Rev. 2013;2013 doi: 10.1002/14651858.CD001364.pub4. [DOI] [PubMed] [Google Scholar]
- 192.Oyagbemi A.A., Ajibade T.O., Aboua Y.G., Gbadamosi I.T., Adedapo A.D.A., Aro A.O., Adejumobi O.A., Thamahane-Katengua E., Omobowale T.O., Falayi O.O., Oyagbemi T.O., Ogunpolu B.S., Hassan F.O., Ogunmiluyi I.O., Ola-Davies O.E., Saba A.B., Adedapo A.A., Nkadimeng S.M., McGaw L.J., Kayoka-Kabongo P.N., Oguntibeju O.O., Yakubu M.A. Potential health benefits of zinc supplementation for the management of COVID-19 pandemic. J. Food Biochem. 2021;45 doi: 10.1111/jfbc.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.U.S. government An official website of the National Institutes of Health, COVID-19 Treatment Guidelines, Zinc Suppl. COVID-19. (2021). https://www.covid19treatmentguidelines.nih.gov/supplements/zinc/#:∼:text=The recommended dietary allowance for elemental zinc is 11 mg,8 mg for nonpregnant women.&text=The doses used in registered,of elemental zinc) twice daily. (accessed March 15, 2021).
- 194.Abd-Elsalam S., Soliman S., Esmail E.S., Khalaf M., Mostafa E.F., Medhat M.A., Ahmed O.A., El Ghafar M.S.A., Alboraie M., Hassany S.M. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine?: a randomized, multicenter trial. Biol. Trace Elem. Res. 2020:1–5. doi: 10.1007/s12011-020-02512-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 195.The European Union Clinical Trials Register, (2021) https://www.clinicaltrialsregister.eu/ctr-search/s.
- 196.S. Thomas, D. Patel, B. Bittel, K. Wolski, Q. Wang, A. Kumar, Z.J. Il’Giovine, R. Mehra, C. McWilliams, S.E. Nissen, M.Y. Desai, Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial, JAMA Netw. Open. 4 (2021) e210369–e210369. 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed]
- 197.JAMA Network, American Medical Association, (2021).
- 198.N.L. of Medicine, ClinicalTrials.gov, (2021).
- 199.R. S, R. L, A randomized controlled trial of zinc supplementation in the treatment of acute respiratory tract infection in Thai children, Pediatr. Rep. 11 (2019) 15–20. 10.4081/PR.2019.7954. [DOI] [PMC free article] [PubMed]
- 200.Mahalanabis D., Lahiri M., Paul D., Gupta S., Gupta A., Wahed M.A., Khaled M.A. Randomized, double-blind, placebo-controlled clinical trial of the efficacy of treatment with zinc or vitamin A in infants and young children with severe acute lower respiratory infection. Am. J. Clin. Nutr. 2004;79:430–436. doi: 10.1093/AJCN/79.3.430. [DOI] [PubMed] [Google Scholar]
- 201.Shah U.H., Abu-Shaheen A.K., Malik M.A., Alam S., Riaz M., Al-Tannir M.A. The efficacy of zinc supplementation in young children with acute lower respiratory infections: a randomized double-blind controlled trial. Clin. Nutr. 2013;32:193–199. doi: 10.1016/J.CLNU.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 202.Hulisz D. Efficacy of zinc against common cold viruses: an overview. J. Am. Pharm. Assoc. 2003;44(2004):594–603. doi: 10.1331/1544-3191.44.5.594.HULISZ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.H. H, Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage, JRSM Open. 8 (2017) 205427041769429. 10.1177/2054270417694291. [DOI] [PMC free article] [PubMed]
- 204.Abdulhamid I., Beck F.W.J., Millard S., Chen X., Prasad A. Effect of zinc supplementation on respiratory tract infections in children with cystic fibrosis. Pediatr. Pulmonol. 2008;43:281–287. doi: 10.1002/PPUL.20771. [DOI] [PubMed] [Google Scholar]
- 205.Malik A., Taneja D.K., Devasenapathy N., Rajeshwari K. Zinc supplementation for prevention of acute respiratory infections in infants: a randomized controlled trial. Indian Pediatr. 2014;51:780–784. doi: 10.1007/S13312-014-0503-Z. [DOI] [PubMed] [Google Scholar]
- 206.Sportelli M.C., Izzi M., Kukushkina E.A., Hossain S.I., Picca R.A., Ditaranto N., Cioff N. Can nanotechnology and materials science help the fight against sars-cov-2? Nanomaterials. 2020;10 doi: 10.3390/nano10040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Search of: zinc | Respiratory Disease - List Results - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/results?cond=Respiratory+Disease&term=zinc&cntry=&state=&city=&dist=&Search=Search (accessed September 29, 2021).
- 208.Swain P.S., Rao S.B.N., Rajendran D., Dominic G., Selvaraju S. Nano zinc, an alternative to conventional zinc as animal feed supplement: a review. Anim. Nutr. 2016;2:134–141. doi: 10.1016/j.aninu.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.K.S. Siddiqi, A. ur Rahman, Tajuddin, A. Husen, Properties of zinc oxide nanoparticles and their activity against microbes, Nanoscale Res. Lett. 2018 131. 13 (2018) 1–13. 10.1186/S11671-018-2532-3. [DOI] [PMC free article] [PubMed]
- 210.Maduray K., Parboosing R. Metal nanoparticles: a promising treatment for viral and arboviral infections. Biol. Trace Elem. Res. 2020;199:3159–3176. doi: 10.1007/S12011-020-02414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Attia G.H., Moemen Y.S., Youns M., Ibrahim A.M., Abdou R., El Raey M.A. Antiviral zinc oxide nanoparticles mediated by hesperidin and in silico comparison study between antiviral phenolics as anti-SARS-CoV-2. Colloids Surfaces B Biointerfaces. 2021;203 doi: 10.1016/J.COLSURFB.2021.111724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Inhibitory effects of epigallocatechin gallate (EGCG) combined with zinc sulfate and silver nanoparticles on avian influenza A virus subtype H5N1, (n.d.). [DOI] [PubMed]
- 213.DeLong R.K., Cheng Y.H., Pearson P., Lin Z., Coffee C., Mathew E.N., Hoffman A., Wouda R.M., Higginbotham M.L. Zn-based physiometacomposite nanoparticles: distribution, tolerance, imaging, and antiviral and anticancer activity. Nanomedicine (Lond) 2021;16:1857–1872. doi: 10.2217/NNM-2021-0179. [DOI] [PubMed] [Google Scholar]
- 214.Hamdi M., Abdel-Bar H.M., Elmowafy E., El-Khouly A., Mansour M., Awad G.A.S. Investigating the internalization and COVID-19 antiviral computational analysis of optimized nanoscale zinc oxide. ACS Omega. 2021;6:6848–6860. doi: 10.1021/acsomega.0c06046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.S.W. Mcpherson, J.E. Keunen, A.C. Bird, E.Y. Chew, F.J. Van Kuijk, EDITORIAL investigate oral zinc as a prophylactic treatment for those at risk for COVID-19, (2020). 10.1016/j.ajo.2020.04.028. [DOI] [PMC free article] [PubMed]
- 216.Yu L., Lu M., Zhang W., Alarfaj A.A., Hirad A.H., Zhang H. Ameliorative effect of Albizia chinensis synthesized ZnO-NPs on Mycoplasma pneumoniae infected pneumonia mice model. Microb. Pathog. 2020;141 doi: 10.1016/J.MICPATH.2019.103960. [DOI] [PubMed] [Google Scholar]
- 217.Ghareeb D.A., Saleh S.R., Seadawy M.G., Nofal M.S., Abdulmalek S.A., Hassan S.F., Khedr S.M., AbdElwahab M.G., Sobhy A.A., Saber A., Abdel-Hamid A., Yassin A.M., Elmoneam A.A.A., Masoud A.A., Kaddah M.M.Y., El-Zahaby S.A., Al-mahallawi A.M., El-Gharbawy A.M., Zaki A., Seif I.K., Kenawy M.Y., Amin M., Amer K., El Demellawy M.A. Nanoparticles of ZnO/Berberine complex contract COVID-19 and respiratory co-bacterial infection in addition to elimination of hydroxychloroquine toxicity. J. Pharm. Investig. 2021:1. doi: 10.1007/S40005-021-00544-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Saadh M.J., Aggag M.M., Alboghdadly A., Kharshid A.M., Aldalaen S.M., Abdelrazek M.A. Silver nanoparticles with epigallocatechingallate and zinc sulphate significantly inhibits avian influenza A virus H9N2. Microb. Pathog. 2021;158 doi: 10.1016/J.MICPATH.2021.105071. [DOI] [PubMed] [Google Scholar]
- 219.Syama S., Sreekanth P.J., Varma H.K., Mohanan P.V. Zinc oxide nanoparticles induced oxidative stress in mouse bone marrow mesenchymal stem cells. Toxicol. Mech. Methods. 2014;24:644–653. doi: 10.3109/15376516.2014.956914. [DOI] [PubMed] [Google Scholar]
- 220.Reddy S.T., Van Der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O’Neil C.P., Lee L.K., Swartz M.A., Hubbell J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 221.Kumar A., Najafzadeh M., Jacob B.K., Dhawan A., Anderson D. Zinc oxide nanoparticles affect the expression of p53, Ras p21 and JNKs: an ex vivo/in vitro exposure study in respiratory disease patients. Mutagenesis. 2015;30:237–245. doi: 10.1093/MUTAGE/GEU064. [DOI] [PubMed] [Google Scholar]
- 222.U.M. Agnew, T.L. Slesinger, Zinc Toxicity, StatPearls Publishing, 2021. http://www.ncbi.nlm.nih.gov/pubmed/32119435 (accessed June 14, 2021). [PubMed]
- 223.Zinc oxide - Registration Dossier - ECHA, (n.d.). https://echa.europa.eu/registration-dossier/-/registered-dossier/16139/7/3/1 (accessed June 21, 2021).
- 224.Subramaniam V.D., Prasad S.V., Banerjee A., Gopinath M., Murugesan R., Marotta F., Sun X.F., Pathak S. Health hazards of nanoparticles: understanding the toxicity mechanism of nanosized ZnO in cosmetic products. Drug Chem. Toxicol. 2019;42:84–93. doi: 10.1080/01480545.2018.1491987. [DOI] [PubMed] [Google Scholar]
- 225.Osmond-Mcleod M.J., Oytam Y., Kirby J.K., Gomez-Fernandez L., Baxter B., McCall M.J. Dermal absorption and short-term biological impact in hairless mice from sunscreens containing zinc oxide nano- or larger particles. Nanotoxicology. 2014;8:72–84. doi: 10.3109/17435390.2013.855832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Vinardell M.P., Llanas H., Marics L., Mitjans M. In vitro comparative skin irritation induced by nano and non-nano zinc oxide. Nanomaterials. 2017;7:56. doi: 10.3390/nano7030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.El Idrissi A., van Berkel L., Bonekamp N.E., Dalemans D.J.Z., van der Heyden M.A.G. The toxicology of zinc chloride smoke producing bombs and screens. Clin. Toxicol. 2017;55:167–174. doi: 10.1080/15563650.2016.1271125. [DOI] [PubMed] [Google Scholar]
- 228.O.S. and H.A. (OSHA), Occupational Safety and Health Standards, Toxic and Hazardous Substances, Code Fed. Regul. (1998). https://ci.nii.ac.jp/naid/80015434389 (accessed June 15, 2021).
- 229.Uwitonze A.M., Ojeh N., Murererehe J., Atfi A., Razzaque M.S. Zinc adequacy is essential for the maintenance of optimal oral health. Nutrients. 2020;12:949. doi: 10.3390/nu12040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang N., Tong T., Xie M., Gaillard J.F. Lifetime and dissolution kinetics of zinc oxide nanoparticles in aqueous media. Nanotechnology. 2016;27 doi: 10.1088/0957-4484/27/32/324001. [DOI] [PubMed] [Google Scholar]
- 231.Yang Z., Xie C. Zn2+ release from zinc and zinc oxide particles in simulated uterine solution. Colloids Surfaces B Biointerfaces. 2006;47:140–145. doi: 10.1016/j.colsurfb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 232.H. A, W. X, W. J, B. A, T. R, T. R, H. H, G. GP, D. M, W. A, D. RK, Two-dimensional fluorescence difference spectroscopy of ZnO and Mg composites in the detection of physiological protein and RNA interactions, Mater. (Basel, Switzerland). 10 (2017). 10.3390/MA10121430. [DOI] [PMC free article] [PubMed]
- 233.B. WS, C. DF, P.-B. A, S. DM, S. JC, F. MW, U. MJ, H. LS, C. C, Z. W, O. G, Comparing inhaled ultrafine versus fine zinc oxide particles in healthy adults: a human inhalation study, Am. J. Respir. Crit. Care Med. 171 (2005) 1129–1135. 10.1164/RCCM.200406-837OC. [DOI] [PMC free article] [PubMed]
- 234.Mir A.H., Qamar A., Qadir I., Naqvi A.H., Begum R. Accumulation and trafficking of zinc oxide nanoparticles in an invertebrate model, Bombyx mori, with insights on their effects on immuno-competent cells. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-58526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.K. NV, M. KM, S. A, J. RJ, D. TC, D. P, B. JD, M. RM, Surface modification of zinc oxide nanoparticles with amorphous silica alters their fate in the circulation, Nanotoxicology 10 (2016) 720–727. 10.3109/17435390.2015.1113322. [DOI] [PMC free article] [PubMed]
- 236.Konduru N.V., Molina R.M., Swami A., Damiani F., Pyrgiotakis G., Lin P., Andreozzi P., Donaghey T.C., Demokritou P., Krol S., Kreyling W., Brain J.D. Protein corona: Implications for nanoparticle interactions with pulmonary cells. Part. Fibre Toxicol. 2017;14 doi: 10.1186/S12989-017-0223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Qu X., Yang H., Yu Z., Jia B., Qiao H., Zheng Y., Dai K. Serum zinc levels and multiple health outcomes: Implications for zinc-based biomaterials. Bioact. Mater. 2020;5:410–422. doi: 10.1016/J.BIOACTMAT.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shalini D., Senthilkumar S., Rajaguru P. Effect of size and shape on toxicity of zinc oxide (ZnO) nanomaterials in human peripheral blood lymphocytes. Toxicol. Mech. Methods. 2018;28:87–94. doi: 10.1080/15376516.2017.1366609. [DOI] [PubMed] [Google Scholar]
- 239.McCracken C., Dutta P.K., Waldman W.J. Critical assessment of toxicological effects of ingested nanoparticles. Environ. Sci. Nano. 2016;3:256–282. doi: 10.1039/c5en00242g. [DOI] [Google Scholar]
- 240.Tatineni V., An J.Y., Leffew M.R., Mahesh S.A. Anemia from A to zinc: hypocupremia in the setting of gastric bypass and zinc excess. Clin. Case Reports. 2020;8:745–750. doi: 10.1002/ccr3.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang C., Lu J., Zhou L., Li J., Xu J., Li W., Zhang L., Zhong X., Wang T. Effects of long-term exposure to zinc oxide nanoparticles on development, zinc metabolism and biodistribution of minerals (Zn, Fe, Cu, Mn) in mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wiering F.T., Berger J., Dijkhuizen M.A. COMBINED IRON AND ZINC SUPPLEMENTATION IN INFANTS IMPROVED IRON AND ZINC STATUS, BUT INTERACTIONS REDUCED EFFICACY IN A MULTICOUNTRY TRIAL IN SOUTHEAST ASIA. J. Nutr. 2007;137:466–471. doi: 10.1093/jn/137.2.466. [DOI] [PubMed] [Google Scholar]
- 243.Long L., Chen J., Zhang Y., Liang X., Ni H., Zhang B., Yin Y. Comparison of porous and nano zinc oxide for replacing high-dose dietary regular zinc oxide in weaning piglets. PLoS One. 2017;12 doi: 10.1371/JOURNAL.PONE.0182550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Leibrock L., Wagener S., Singh A.V., Laux P., Luch A. Nanoparticle induced barrier function assessment at liquid-liquid and air-liquid interface in novel human lung epithelia cell lines. Toxicol. Res. (Camb) 2019;8:1016–1027. doi: 10.1039/C9TX00179D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhou P., Guo M., Cui X. Effect of food on orally-ingested titanium dioxide and zinc oxide nanoparticle behaviors in simulated digestive tract. Chemosphere. 2020 doi: 10.1016/j.chemosphere.2020.128843. [DOI] [PubMed] [Google Scholar]
- 246.Cao Y., Li J., Liu F., Li X., Jiang Q., Cheng S., Gu Y. Consideration of interaction between nanoparticles and food components for the safety assessment of nanoparticles following oral exposure: a review. Environ. Toxicol. Pharmacol. 2016;46:206–210. doi: 10.1016/j.etap.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 247.Abo-zeid Y., Williams G.R. The potential anti-infective applications of metal oxide nanoparticles: a systematic review. WIREs Nanomed. Nanobiotechnol. 2020;12 doi: 10.1002/wnan.1592. [DOI] [PubMed] [Google Scholar]
- 248.Hess K.L., Medintz I.L., Jewell C.M. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today. 2019;27:73–98. doi: 10.1016/j.nantod.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Roy R., Das M., Dwivedi P.D. Toxicological mode of action of ZnO nanoparticles: impact on immune cells. Mol. Immunol. 2015;63:184–192. doi: 10.1016/j.molimm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 250.Hanley C., Thurber A., Hanna C., Punnoose A., Zhang J., Wingett D.G. The influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Res. Lett. 2009;4:1409–1420. doi: 10.1007/s11671-009-9413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang H., Wingett D., Engelhard M.H., Feris K., Reddy K.M., Turner P., Layne J., Hanley C., Bell J., Tenne D., Wang C., Punnoose A. Fluorescent dye encapsulated ZnO particles with cell-specific toxicity for potential use in biomedical applications. J. Mater. Sci. Mater. Med. 2009;20:11–22. doi: 10.1007/s10856-008-3541-z. [DOI] [PubMed] [Google Scholar]
- 252.Saptarshi S.R., Feltis B.N., Wright P.F.A., Lopata A.L. Investigating the immunomodulatory nature of zinc oxide nanoparticles at sub-cytotoxic levels in vitro and after intranasal instillation in vivo. J. Nanobiotechnol. 2015;13:1–11. doi: 10.1186/s12951-015-0067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lin W., Xu Y., Huang C.-C., Ma Y., Shannon K.B., Chen D.-R., Huang Y.-W. Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. J. Nanoparticle Res. 2009;11:25–39. doi: 10.1007/s11051-008-9419-7. [DOI] [Google Scholar]
- 254.Feltis B.N., O’Keefe S.J., Harford A.J., Piva T.J., Turney T.W., Wright P.F.A. Independent cytotoxic and inflammatory responses to zinc oxide nanoparticles in human monocytes and macrophages. Nanotoxicology. 2012;6:757–765. doi: 10.3109/17435390.2011.620718. [DOI] [PubMed] [Google Scholar]
- 255.Kang T., Guan R., Chen X., Song Y., Jiang H., Zhao J. In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells. Nanoscale Res. Lett. 2013;8:496. doi: 10.1186/1556-276X-8-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Prach M., Stone V., Proudfoot L. Zinc oxide nanoparticles and monocytes: Impact of size, charge and solubility on activation status. Toxicol. Appl. Pharmacol. 2013;266:19–26. doi: 10.1016/j.taap.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 257.Guo D., Bi H., Liu B., Wu Q., Wang D., Cui Y. Reactive oxygen species-induced cytotoxic effects of zinc oxide nanoparticles in rat retinal ganglion cells. Toxicol. Vitr. 2013;27:731–738. doi: 10.1016/j.tiv.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 258.Yang H., Liu C., Yang D., Zhang H., Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J. Appl. Toxicol. 2009;29:69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- 259.Gojova A., Guo B., Kota R.S., Rutledge J.C., Kennedy I.M., Barakat A.I. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ. Health Perspect. 2007;115:403–409. doi: 10.1289/ehp.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Poon W.L., Alenius H., Ndika J., Fortino V., Kolhinen V., Meščeriakovas A., Wang M., Greco D., Lähde A., Jokiniemi J., Lee J.C.Y., El-Nezami H., Karisola P. Nano-sized zinc oxide and silver, but not titanium dioxide, induce innate and adaptive immunity and antiviral response in differentiated THP-1 cells. Nanotoxicology. 2017;11:936–951. doi: 10.1080/17435390.2017.1382600. [DOI] [PubMed] [Google Scholar]
- 261.Roy R., Kumar D., Sharma A., Gupta P., Chaudhari B.P., Tripathi A., Das M., Dwivedi P.D. ZnO nanoparticles induced adjuvant effect via toll-like receptors and Src signaling in Balb/c mice. Toxicol. Lett. 2014;230:421–433. doi: 10.1016/j.toxlet.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 262.Afroz S., Medhi H., Maity S., Minhas G., Battu S., Giddaluru J., Kumar K., Paik P., Khan N. Mesoporous ZnO nanocapsules for the induction of enhanced antigen-specific immunological responses. Nanoscale. 2017;9:14641–14653. doi: 10.1039/c7nr03697c. [DOI] [PubMed] [Google Scholar]
- 263.Roy R., Kumar S., Verma A.K., Sharma A., Chaudhari B.P., Tripathi A., Das M., Dwivedi P.D. Zinc oxide nanoparticles provide an adjuvant effect to ovalbumin via a th2 response in Balb/c mice. Int. Immunol. 2014;26:159–172. doi: 10.1093/intimm/dxt053. [DOI] [PubMed] [Google Scholar]
- 264.Sharma P., Jang N.Y., Lee J.W., Park B.C., Kim Y.K., Cho N.H. Application of ZnO-based nanocomposites for vaccines and cancer immunotherapy. Pharmaceutics. 2019;11:6–10. doi: 10.3390/pharmaceutics11100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.M.G. Hellfritzsch, Z INC OXIDE IN FORMULATION, 2021.
- 266.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O’Dell S., Schmidt S.D., Swanson P.A., Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/nejmoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Huang W.C., Deng B., Seffouh A., Ortega J., Long C.A., Suresh R.V., He X., Miura K., Lee S.M., Wu Y., Lovell J.F. Antibody response of a particle-inducing, liposome vaccine adjuvant admixed with a Pfs230 fragment. NPJ Vaccines. 2020;5:1–9. doi: 10.1038/s41541-020-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Federizon J., Feugmo C.G.T., Huang W.C., He X., Miura K., Razi A., Ortega J., Karttunen M., Lovell J.F. Experimental and computational observations of immunogenic cobalt porphyrin lipid bilayers: nanodomain-enhanced antigen association. Pharmaceutics. 2021;13:1–17. doi: 10.3390/pharmaceutics13010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Shao S., Geng J., Yi H., Gogia S., Neelameghan S., Jacobs A., Lovell J. Functionalization of cobalt porphyrin-phospholipid bilayers with His-tagged ligands and antigens. Nat Chem. 2015;7:438–446. doi: 10.1038/nchem.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.K. Khosravi-Darani, M.R. Mozafari, Nanoliposome Potentials in Nanotherapy: A Concise Overview, n.d.
- 271.Mahari S., Roberts A., Shahdeo D., Gandhi S. eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19 antigen, a spike protein domain 1 of SARS-CoV-2. BioRxiv. 2020 doi: 10.1101/2020.04.24.059204. [DOI] [Google Scholar]
- 272.Xiao M., Huang L., Dong X., Xie K., Shen H., Huang C., Xiao W., Jin M., Tang Y. Integration of a 3D-printed read-out platform with a quantum dot-based immunoassay for detection of the avian influenza A (H7N9) virus. Analyst. 2019;144:2594–2603. doi: 10.1039/c8an02336k. [DOI] [PubMed] [Google Scholar]
- 273.Chang S.Y., Huang K.Y., Chao T.L., Kao H.C., Pang Y.H., Lu L., Chiu C.L., Huang H.C., Cheng T.J.R., Fang J.M., Yang P.C. Nanoparticle composite TPNT1 is effective against SARS-CoV-2 and influenza viruses. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-021-87254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]