Abstract
Hox genes play key roles in axial patterning and regulating the regional identity of cells and tissues in a wide variety of animals from invertebrates to vertebrates. Nested domains of Hox expression generate a combinatorial code that provides a molecular framework for specifying the properties of tissues along the A–P axis. Hence, it is important to understand the regulatory mechanisms that coordinately control the precise patterns of the transcription of clustered Hox genes required for their roles in development. New insights are emerging about the dynamics and molecular mechanisms governing transcriptional regulation, and there is interest in understanding how these may play a role in contributing to the regulation of the expression of the clustered Hox genes. In this review, we summarize some of the recent findings, ideas and emerging mechanisms underlying the regulation of transcription in general and consider how they may be relevant to understanding the transcriptional regulation of Hox genes.
Keywords: Hox genes, transcriptional regulation, transcription factors, nascent transcripts, gene regulation, coordinate regulation, enhancers
1. Introduction
Animals display remarkable variety in their body plans and there is great interest in understanding the degree to which conserved and distinct mechanisms underlie this diversity in the formation and elaboration of basic body plans in animal evolution. In chordate evolution, there is emerging evidence for a deeply conserved regulatory network, involving transcription factors (TFs) and signaling pathways, that governs patterning along the anterior–posterior (A–P) body axis [1,2,3,4,5,6]. Remarkably, despite very different morphologies among chordates, many key TFs and components of major signaling pathways (e.g., Wnts and FGFs), known to regulate developmental processes, have been shown to be similarly aligned along the A–P axis. This suggests that regulatory interactions between signaling pathways and core TFs set up a conserved gene regulatory network (GRN) that guides the formation of the basic body plan and patterning of the A–P axis. However, the question of how TFs are coupled to these ancient signaling pathways and how they integrate responses to signaling gradients is not fully understood.
The highly conserved HOX family of TFs are an example of TFs that are coupled to this ancient GRN. Hox genes are known to play key roles in axial patterning and regulating the regional identity of cells and tissues in a wide variety of animals from invertebrates to vertebrates [7,8,9,10,11]. The clustered Hox genes exhibit an interesting property known as collinearity [12,13,14,15,16]. Genes in the four mammalian Hox clusters are all transcribed in the same 5′ to 3′ direction with respect to transcription, and the order of Hox genes in each cluster on a chromosome corelates with their temporal and spatial expression domains and functions along the A–P axis of developing embryos (Figure 1). These nested domains of expression generate a combinatorial Hox code, which provides a molecular framework that serves as a key regulatory step in specifying regional identities and properties of tissues along the A–P axis. A wide variety of studies in different species and cell culture models have revealed that the nested domains of Hox expression along the A–P axis arise in part through the ability of Hox clusters to integrate and respond to opposing signaling gradients, such as those of Retinoic acid (RA), Fibroblast growth factors (Fgfs) and Wingless related integration sites (WNTs) [5,17,18,19,20,21,22,23,24,25,26,27,28,29]. Hence, it is important to understand the regulatory mechanisms through which signaling pathways are able to coordinately control the precise patterns of the transcription of the clustered Hox genes required for their roles in specifying diverse morphologic features along the A–P axis.
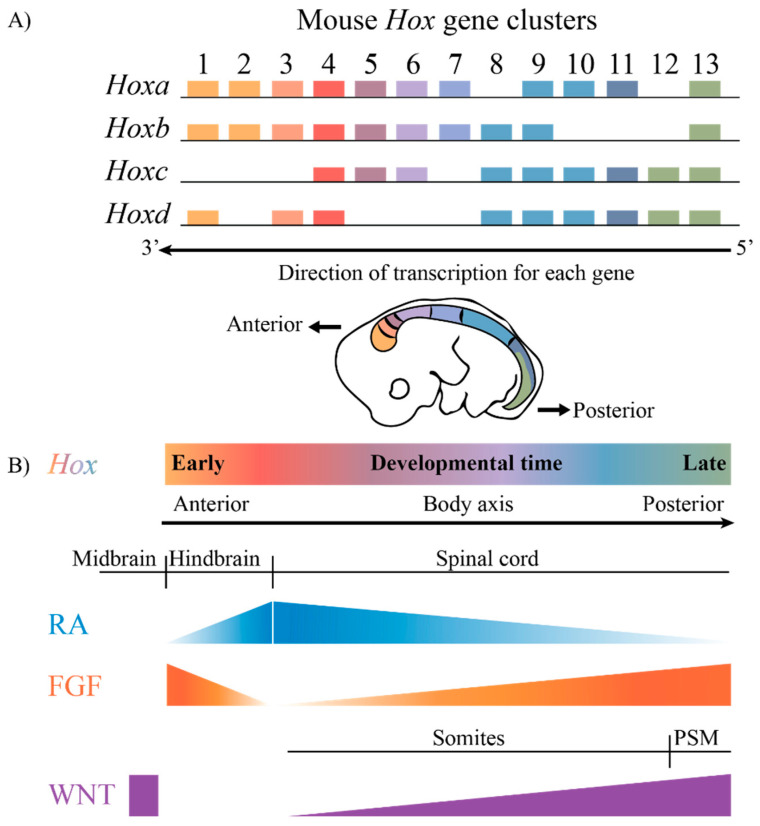
Figure 1.
The mammalian Hox gene clusters and the conserved signaling pathways that play a role in defining the Hox gene expression profiles. (A) In mammals, there are four clusters of Hox genes, each on different chromosomes. They exhibit spatial and temporal collinearity, such that 3′ Hox genes are expressed early in development as well as more anteriorly in an embryo generating nested domains of expression as depicted in the drawing of an E10 mouse embryo. (B) The restricted domains of Hox expression arise through an integration of signaling molecules such as RA, FGF and WNT, which are expressed in gradients along the embryonic axis. PSM, presomitic mesoderm.
In the case of RA signaling, Hox genes are direct transcriptional targets of retinoids, and their response to RA signaling involves retinoic acid response elements (RAREs) embedded within and adjacent to the Hox clusters [18,30,31,32]. These RAREs are cis-regulatory components of RA-dependent enhancers that provide regulatory inputs both locally on adjacent Hox genes and over a long range to coordinately regulate multiple genes in a Hox cluster [33,34,35,36,37,38]. This tightly clustered organization of cis-regulatory elements and the Hox genes they control raises interesting questions with respect to roles for chromosome topology, epigenetic modifications, dynamics of transcription and the underlying transcriptional mechanisms for how enhancers display selectivity or competition between genes, and they may be shared by multiple genes in a cluster [39]. It is important that these diverse aspects of transcriptional regulation are properly coordinated to ensure the right spatial and temporal patterns and appropriate levels of expression needed for their roles in axial patterning.
The advent of new technologies for investigating the dynamics of interactions that underlie the activation of transcription are generating surprising findings. These observations challenge the widely postulated role of stable long-term enhancer promoter interactions and the notion of a single RNA polymerase with a small number of components regulating transcription [40,41,42,43,44,45,46,47,48,49]. New models suggest that dynamic condensates and mechanisms involving a series of rapid and complex interactions underlie the activation of transcription and the regulation of gene expression. It will be interesting and important to understand how this newly emerging picture of the dynamic molecular mechanisms governing transcription plays a role in modulating the inputs controlling the coordinated expression of the clustered Hox genes. In this review, we summarize some of the recent findings, ideas and emerging mechanisms underlying the regulation of transcription in general and consider how they are relevant to the transcriptional regulation of Hox genes.
2. Regulatory Features
2.1. Enhancers
Enhancers were first discovered in simian virus 40 (SV40), where it was found that they function in an orientation-independent manner to stimulate transcription on heterologous genes [50]. Since then, a variety of analyses have revealed that animal genomes contain a large number of putative enhancers, out numbering coding genes [51,52]. It is challenging to identify cis-regulatory elements, such as enhancers, encoded in the genome through sequence analyses and computational methods alone [53]. Major efforts have been made to find ways of identifying and characterizing enhancers and their properties on a genome-wide and individual basis, which is important to facilitate our ability to decode regulatory information embedded in the genome [54,55,56,57,58,59,60]. While many development specific enhancers, including some of those discovered in the Hox clusters, are evolutionarily conserved [35,61,62,63], many adult or tissue-specific enhancers can be highly variable across species [64,65]. Even when enhancers are highly conserved, it can be challenging to understand the information content and the critical arrangements of the cis-elements that govern their ability to regulate expression [54,59,60]. Furthermore, highly conserved patterns of gene expression can arise through enhancers that display divergence [65]. Enhancers serve to stimulate transcription by integrating a variety of different regulatory inputs and binding sites for TFs to confer precise temporal, spatial and cell-type specific gene expression programs. Precise regulatory outputs from enhancers do not require that upstream factors have highly restricted domains of expression and can arise through the cumulative integration of weak, imprecise or wide-spread inputs by TFs [66,67]. The convergence of inputs can result in the integration of disparate and very broad patterns of regulatory signals into robust and tightly controlled specific outputs. Similarly, clusters of weak enhancers can synergize to serve as super enhancers to robustly regulate gene expression [68].
Enhancers can be located directly upstream of a gene or up to over a megabase away from its target gene promoter [69,70]. They frequently reside within introns of genes, even in ones they do not regulate, [70], and there is evidence for enhancers and cis-regulatory elements embedded in coding exons, including those of Hox genes [71,72,73]. Studies have shown that enhancer regions are themselves transcriptionally active. Several groups have demonstrated that non-coding enhancer RNAs are more than just transcriptional noise or byproducts of the transcriptional machinery, but are useful indicators in predicting active enhancers [74].
A challenge in identifying the targets of enhancer activity is that they can function independently of their orientation with respect to target genes and can make long-range enhancer–promoter contacts to more than the near adjacent genes [75]. Typically, an average vertebrate enhancer can be ~5 to 50 kb away from target promoters and ~1 to 10 kb away in the more compact Drosophila genome [39]. Intriguingly, the proximity of an enhancer to its target promoter required for functional activity is variable. Hence, there are no clear rules on how close enhancers should be positioned relative to the promoters they activate. Some studies have shown through proximity-dependent ligation techniques, such as 3C (Chromatin conformation capture), that enhancers physically come into contact with promoters, resulting in the activation of gene transcription [76]. Imaging approaches have shown that following the activation of genes, the distance between the enhancers and their target promoter tends to increase, suggesting a change in their interactions dependent upon their activity state [77]. This raises fundamental questions, such as, how do enhancers locate and distinguish between the target genes they activate; what confers enhancer–promoter specificity; and what degree of proximity is essential for the enhancer interactions required for gene regulation [39,55,69,75,78,79,80]?
These questions are relevant to understanding the regulation of the Hox clusters because of the high gene density and compact nature of the clusters. The enhancers embedded within and flanking an individual Hox cluster can display selective preferences, competition between genes and can regulate both near adjacent genes or act more globally on other genes in the complex. For example, in the mouse Hoxb complex, there are three RAREs in the middle of the cluster, two upstream and one downstream of Hoxb4 (Figure 2A), which participate in mediating its response to RA by regulating multiple coding and long non-coding (lncRNAs) transcripts [33,35,37,81]. One of these RAREs (DE-RARE) is an essential cis element of an RA-dependent enhancer, which undergoes epigenetic modifications, and is required to coordinate the global regulation of Hoxb genes in hematopoietic stems cells [34]. This functional role for an enhancer raises many questions regarding the mechanisms through which the DE-RARE participates in regulating so many transcripts, how targets are selected and the dynamics of the process. Why, in contrast, do other enhancers embedded in the Hoxb cluster only appear to work on a single near adjacent gene [37,62,82,83,84]?
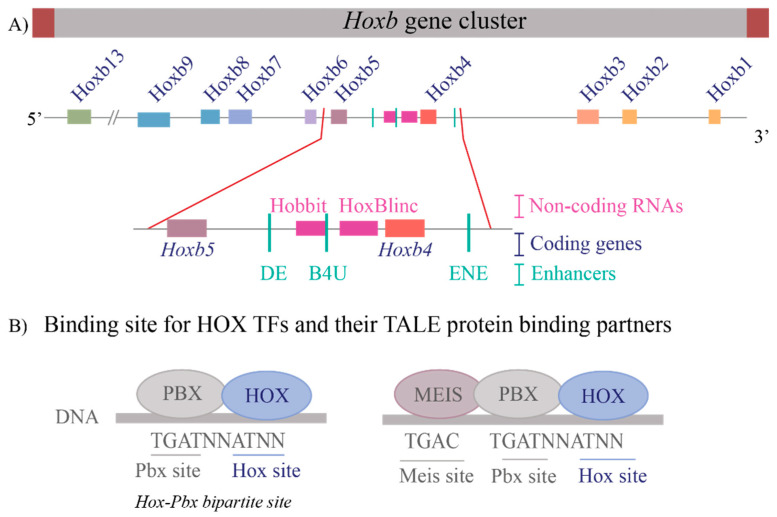
Figure 2.
Transcriptional complexity of the Hoxb gene cluster and binding of HOX Transcription factors to DNA (A) A drawing of the Hoxb gene cluster to illustrate that non-coding RNAs as well as enhancers that contain RAREs (Retinoic Acid Response Elements) are interspersed within the coding Hox genes. The enlargement of the Hoxb4-Hoxb5 region shows the complexity within the region that contains three RAREs, two present upstream of Hoxb4 and one present downstream of Hoxb4 and two non-coding RNAs, Hobbit and HoxBlinc. Brown boxes flank the cluster depict boundary elements, colored squares are different Hox genes, pink boxes are non-coding RNAs, and green lines represent RARE enhancers. (B) Depicts the consensus DNA binding sites for HOX proteins and their binding partners, the TALE proteins PBX and MEIS. HOX proteins can bind on Hox-Pbx bipartite sites, or they can bind on DNA in ternary complexes along with both PBX and MEIS. Blue ovals are HOX proteins, and grey ovals are TALE protein binding partners.
2.2. Transcription Factors and Their Impact on Enhancer Activity
One reason it is hard to identify and predict the functions of enhancers is related to our level of understanding of TFs and their properties [55]. TFs do not bind in isolation, and their binding specificities or other properties will depend on cofactors, interacting proteins and epigenetic states [85,86]. The DNA binding domains of the families of TFs generally recognize 6–12 base pair consensus motifs identified in vitro, and these TFs can activate and/or repress transcription [87,88,89,90]. However, it can be experimentally challenging to characterize the rules and basis for the context-specific activities of TFs in vivo. The Hox genes encode TFs that are thought to play highly conserved roles across species and the studies on their properties serve to illustrate some of the complex issues for understanding how TFs contribute to enhancer activity and function.
In vitro HOX proteins display very similar DNA binding properties [87,88,89,91,92,93,94,95]. However, their in vivo binding properties and functions can be distinctly different [96,97]. Some insights into the basis for these differences have come from experimental studies that have shown that the Hox genes have many auto- and cross-regulatory inputs in controlling their expression. The characterization of endogenous HOX-response elements has enabled insights into their in vivo properties and interactions with co-factors [38,83,85,98,99,100]. The binding specificity of HOX proteins arises not only from the homeodomain’s ability to recognize a short AT-rich motif (ATTA) defined in vitro, but in concert with other domains adjacent to the homeodomain (hexapeptide) and in different regions of HOX proteins, which can impact its specificity and ability to bind DNA [85,97,101,102,103]. The nature of this altered specificity for HOX proteins on target sequences is, to a large degree, related to their ability to interact with cofactors, in particular with members of the TALE family, PBX and MEIS (Figure 2B) [85,86,97,99,103,104,105,106,107]. HOX proteins interact with PBX proteins through multiple domains, which alters their target site preferences. HOX-PBX dimers bind to a bipartite HOX-PBC motif and can form a ternary complex with MEIS proteins [108,109,110]. This bipartite HOX-PBC motif is highly enriched in genome-wide analyses of downstream HOX target regions identified using chromatin immune precipitation (ChIP) assays [96,97,99,111,112,113], underscoring the important role TALE proteins play as cofactors in potentiating HOX functions.
While the clustered Hox gene family and its organizational features are highly conserved in animals, what about the functional roles of HOX proteins? Genetic analyses of Hox paralogous group members reveal many overlapping roles [114,115,116,117,118], but there is also evidence for specific transcriptional functions in vivo [97,119] and specific binding preferences that vary with cell-type specific [67,95]. Furthermore, it is unknown whether there is a conserved ancestral role for the duplicated and diverged paralogous Hox genes in vertebrates or if have they evolved new functions. Recent cross-species functional studies on the mouse HOX1 paralogs, which are related to labial in Drosophila, have revealed that despite an only 35% amino acid identity between the proteins, mouse HOXA1 has retained the ancestral activities of Labial [97]. The other mouse HOX1 paralogs, HOXB1 and HOXD1, display altered DNA binding properties and they have diversified to take on new functional roles. The basis for the retention of ancestral activity versus new functional properties maps to a small number of subtle amino acid changes in domains outside of the homeodomain that appear to alter interactions with TALE proteins [97]. Contributions of small, previously uncharacterized domains in other HOX proteins have also been found to alter binding properties though interactions with TALE cofactors [102], indicating that this may be a general mechanism used in evolution for modulating the activities and interactions of HOX proteins and other TFs. This illustrates the challenges in predicting the binding properties and functional roles of closely related TF proteins and may help to explain how they have diverse impacts on the enhancer activity.
Recently, HOX proteins bound to specific enhancers have been shown to confer tissue-specific transcriptional outputs by altering the activity of TALE proteins [120]. There is evidence that HOX proteins have pioneer activity, such that the HOX TFs of posterior Hox genes have different affinities for compacted chromatin [121] or can alter the accessibility to chromatin [90]. Therefore, for a deep level of understanding of how Hox-responsive enhancers work, it is important to understand how many and what kinds of TFs bind to an enhancer, the rules or syntax of their spacing and organization, the positions of nearby motifs and the identity of motifs that can serve to synergize or antagonize their activities [54]. Furthermore, the modifications of epigenetic states can alter these relationships to aid or prevent binding and activity. For a mechanistic insight into the coordinate regulation of Hox genes, we need to understand the enhancer “grammar” that dictates TFs binding to the DNA and their correlation with functional outputs.
2.3. Genome Organization and Its Impact on Enhancer Activity
Chromatin is spatially organized in the nucleus and several studies have shown that distinct organized chromatin domains facilitate processes such as the regulation of gene expression, DNA replication and repair and chromosome compaction [122]. The mechanisms underlying how the dynamics of chromosome arrangements impact function is still unclear [123]. Technological advances, especially chromatin capture assays (e.g., Hi-C), have shown that genomes are organized into Topologically Associated Domains (TADs) [124,125,126]. TADs physically confine segments of the chromatin, and there is evidence suggesting that enhancers and their target genes are often within the same TAD [39,127]. Enhancers can interact with other enhancers in a TAD and generally do not appear to interact with and activate genes outside the TAD [124,125,126]. With respect to Hox genes, analyses have shown that long range enhancer–promoter contacts are restricted within the TAD containing the Hoxa cluster [128]. Interestingly, TAD boundaries are not fixed and can vary. Elegant genetic and molecular studies from the Duboule group have shown that TAD boundaries in the Hoxd cluster can shift over developmental time, altering the ability of a long-range limb enhancer to regulate expression in embryos and digits [124,129,130,131,132]. Enhancer promoter interactions within a TAD appear to correlate with gene expression levels, and enhancer interactions outside of a TAD may be inhibited by the boundary elements present on the edges of TAD [131,133,134]. These boundary elements contain insulator proteins such as CTCF, and this TAD-based organization of chromatin is thought to help prevent unwanted enhancer interactions and effects on gene expression [133]. The importance of CTCF sites in regulating genes in the Hoxa cluster have been shown in cells and embryos [128,131,135]. Hence, the disruption of TAD boundaries can be associated with diseased states [127,136,137,138].
The regulation of genes by cis elements within TADs implies that there must be features and mechanisms that influence stable and/or dynamic contacts among the regulatory elements and their target promoters to facilitate precise transcriptional activity. Models, such as linking [139] and tracking [140], have been proposed for how enhancers can activate genes, but new data indicating very rapid dynamics make it challenging to explain or predict enhancer activity with these existing models [39]. A mechanism that currently seems plausible is based on the presence of chromatin loops that can be formed using a loop-extrusion model [141,142]. The loop-extrusion model postulates that DNA is squeezed out into a loop aided by Structural Maintenance of Chromosome (SMC) proteins, such as cohesin and condensin. They clasp DNA and these proteins stop moving DNA through the loop when they reach properly oriented CTCF sites [122,143]. This mechanism also allows for loops to form within the TADs, and dynamic enhancer contacts can thus be envisioned as the formation of different loop conformations to activate or inhibit transcription. It has been shown for Hoxb genes that active genes loop out of chromosome territories, and this looping out corelates with transcriptional activity [144]. The whole Hoxb cluster is in one TAD [124], and it can serve as an example to postulate different loop confirmations associated with previously identified enhancers involved in activating specific Hoxb genes (Figure 3A) [18,33,34].
Figure 3.
Schematic of how the chromatin may loop to activate genes within the transcriptionally complex Hoxb gene cluster (A) Different loop confirmations envisioned for activation of specific genes within the Hoxb cluster, which contains several enhancer elements (such as DE, B4U and ENE) as well as coding and non-coding genes. (B) Inference [41,44] for how nascent Hoxb transcripts may promote condensate formation to increase nascent transcription, and subsequently, how an increased number of nascent transcripts may inhibit transcription by promoting condensate dissolution. Brown boxes at cluster edges depict boundary elements, blue and pink colored ovals depict coding genes and non-coding RNAs, respectively, and green ovals are RARE enhancers.
TADs have been shown to enable regulatory contacts that take place within their boundaries [145], and therefore appear critical for the maintenance of gene regulation. Intriguingly, however, mutations in CTCF binding sites [125,146] and of cohesion [147], do not appear to disrupt genome compartmentalization or significantly impact gene expression even though they disrupt TADs [148]. This indicates that there is not an absolute correlation between TAD formation and the proper regulation of gene expression. It could be that the formation of a TAD does not necessarily restrict or promote all the enhancer contacts, it biases them [149]. Indeed, for early developmental enhancers, it has been observed that they may already be near their target promoters even before TADs are formed and the respective genes are expressed [150]. Furthermore, it has been shown that multiple cis-regulatory modules can compete with each other while present in the same TAD or regulatory hub, and that these regulatory hubs are made before there is an activation of transcription [151]. Hence, it appears that the formation of TADs and gene activation do not always need to be sequential.
Adding to this complexity, is the fact that enhancer–promoter distances and transcriptional states do not follow consistent correlations. While some studies have shown that with a decreasing enhancer–promoter proximity or the formation of loops by force there is an increase in gene transcription [79,152,153,154], other groups have shown that active transcriptional states correlate with an increase in enhancer–promoter distances [77,155]. Furthermore, regulatory analyses have shown that that multiple seemingly redundant or shadow enhancers work together to fine tune the robustness of gene expression [35,156,157]. Recently, it has also been shown that shadow enhancers can help to reduce the transcriptional noise that arises due to the fluctuating levels of TFs [158]. This raises a question as to how shadow enhancers and their counterparts coordinate interactions with promoters to modulate the activity and robustness of gene expression.
In eukaryotes, gene transcription has been observed to take place in bursts [153,159,160,161]. In the regulation of transcriptional bursting, it has been suggested that the events occur within condensates that contain all of the required regulatory components [40,42,43,45,162]. It has been proposed that the activation of transcription is a two-step process [39]. Initially, enhancers have to attain a “ready-to-go state”, evidenced by the presence of paused Pol II and coupled with being in close proximity to target promoters [150,163]. Secondly, in response to a cue, topological and spatial changes occur, leading to the activation of transcription [39]. If transcriptional activation occurs within the boundary of a condensate, then the mediator complexes, pre-initiation complexes and Pol II present within the condensate can act as bridges or connectors between enhancers and promoters [164]. In this model, strict physical enhancer–promoter contacts are not required, and a proximity range of 100–300 nm between enhancers and promoters may be sufficient to activate transcription [165]. To enhance the understanding of enhancer–promoter dynamics, high resolution imaging and, preferably, live imaging of transcriptional events alongside the visualization of allelic locations is required. This will give measurements that can address how enhancers dynamically interact either locally or globally and whether they do so on a single promoter one at a time or simultaneously on multiple promoters. These approaches can also give insight into how transcriptional activation corelates with changes in the chromatin structure. With respect to the Hox clusters, these approaches will be relevant for understanding how shared enhancers can coordinately regulate multiple genes and why some enhancers have selective preferences for some genes but not others.
2.4. Deciphering Enhancer Activity through Nascent Expression
It has been observed that enhancer contacts with Hox promoters can be made when enhancers are active but are also established irrespective of enhancer activity [166,167,168]. Enhancer contacts have been mapped through chromatin capture techniques, but their resolution has not been good enough to infer contacts with elements that are within a few kilobases of each other, which is the case in the vertebrate Hox clusters. However, there have been new techniques (Hi-M and Hi-CO) and improved Hi-C protocols that show a better resolution for inferring genomic contacts [169,170,171,172]. These techniques will aid in identifying and characterizing the proximal enhancer contacts that are relevant to transcriptional regulation. One way to map enhancer activity is to measure the transcriptional readout. Enhancer RNAs provide a means to infer active or inactive states of enhancers [74], while newly synthesized RNA or nascent transcripts of target genes can serve as a means of observing the transcriptional activation of genes.
Toward this goal, the development of several imaging and high throughput sequencing techniques has made it possible to identify nascent transcripts. Imaging methods are able to detect nascent transcripts via a single molecule fluorescent in situ hybridization (smFISH) [173,174], a Hybridization Chain Reaction (HCR) [175] and in a time lapse through the MS2/MCP stem loop system [176,177]. By combining MS2/MCP to monitor nascent transcripts and llama antibody tags to detect the corresponding proteins, it is possible to correlate the dynamics of rates of transcription with protein expression [178,179]. While these approaches have been extremely useful for insight into Drosophila development, to date it has been challenging to scale up these imaging methods beyond cell culture in different species in a high through-put manner. To infer the sequence of transcriptional activation by enhancers and bursting activities, it will be important to expand this to more diverse developmental contexts and species. There is a need to obtain live visualizations of the dynamics of nascent transcripts with cellular resolution, have a record of transcriptional activity from both alleles and to monitor the relative positions of enhancers regulating these patterns in the endogenous loci.
In genome wide analysis, with the advent of new sequencing technologies and computational programs, methods have been developed to detect or infer the presence of nascent transcripts. These methods employ the detection of RNA that is associated with chromatin, the enrichment of RNA that is associated with Pol II, or the detection of new RNA with Biotin or metabolic labelling [180]. These techniques enable the bulk detection of nascent transcripts in different tissues of interest. While each of these imaging and sequencing techniques are a step forward in the field, currently, each one has its own caveats and there is room to improve the detection of nascent transcripts in conjunction with genome organization.
Progress has been made in the detection of transcriptional hubs containing a mediator complex using high resolution microscopy [43,164]. Recently, it has been observed that nascent transcripts themselves are in a feedback loop with the formation of transcriptional condensates; while nascent transcripts initially promote condensate formation, once they increase in numbers, they promote the dissolution of condensates [41,44]. This is relevant to the regulation of clustered Hox genes as it can be envisioned that enhancers and multiple gene promoters may reside within the same transcriptional hub or condensate for the coordinate regulation of their transcription. The gene promoters excluded from this hub would not be activated. Feedback from Hoxb nascent transcripts could, for example, promote transcription or lead to condensate dissolution (Figure 3B), renewing the cycle of transcriptional activation. Moreover, it has been shown that some TFs, through their activation domains, can form condensates with a mediator to activate genes [181] and some mediator subunits are known to interact with HOX proteins [182]. Further work needs to be undertaken to understand whether condensate formation and dissolution directly correlate with changes in enhancer contacts, and whether TFs such as HOX proteins have a direct role in affecting the enhancer contacts that result in gene activation or repression.
2.5. Additional Features in Regulation of Transcription of Hox Genes
Histone modifications along the Hox gene clusters and the 3D genome conformations within and around the clusters play a significant role in defining which Hox genes are going to be transcriptionally active [81,183,184,185]. This activation of Hox genes may be regulated by elements within the cluster, but it has also been seen that they can be regulated by sequences outside the cluster [35,167,186,187,188,189]. In addition, Polycomb Repressive Complexes (PRC1 and PRC2), which are involved in the silencing of Hox genes, effect chromatin compaction by post-translationally modifying histones [190] and, in turn, effect the transcriptional activation of Hox genes. Another layer of complexity for Hox genes is the presence of several non-coding RNAs within the gene clusters [191]. Non-coding RNAs can be widely expressed, display differential tissue expression patterns, have unique subcellular localizations or can participate in specific DNA, RNA and protein interactions to regulate chromatin [192]. Thus, the Hox cluster-specific non-coding RNAs may exhibit their own transcriptional signatures and inputs toward how Hox genes are transcriptionally activated [193].
In summary, exciting new ideas and insights are emerging on the regulatory mechanisms that govern the control of transcription. These offer new ways of thinking about how transcriptional events and processes are regulated in cell and developmental contexts. The Hox genes present a challenging and interesting system to understand the nuances of transcriptional control for gene activation [194]. The unique features of the Hox gene clusters (collinearity, high density of genes and regulatory elements, shifting TADs, etc.) and their important and conserved functional roles in development, disease and evolution provide an important paradigm for investigating transcriptional regulation in a deeper and more detailed mechanistic manner. Furthermore, from an evolutionary perspective, it will be interesting to explore whether conserved GRNs that involve Hox genes, such as the one for hindbrain segmentation [19,29,195,196], utilize the same or different transcriptional mechanisms in the regulatory circuits modulating conserved programs of expression. With the advent of new imaging and sequencing technologies, gaining insight into how enhancers function in the complex dynamics of the transcriptional regulation of Hox genes is beginning to enter an exciting new phase.
Acknowledgments
The authors thank all other members of the Krumlauf laboratory as well as Viraj Doddihal and Vivekanandan Ramalingam for helpful feedback and discussions on the manuscript. This work was conducted to fulfill, in part, the requirements for Z.A.’s degree as a PhD student with the Kansas University Medical Center.
Author Contributions
Conceptualization, Z.A. and R.K.; manuscript writing, review and editing, Z.A. and R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds from the Stowers Institute for Medical Research (grant no: 1001) to R.K.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data and results referred to in this review have been previously published and cited with the appropriate reference.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Swalla B.J. Building divergent body plans with similar genetic pathways. Heredity. 2006;97:235–243. doi: 10.1038/sj.hdy.6800872. [DOI] [PubMed] [Google Scholar]
- 2.Lowe C.J., Clarke D.N., Medeiros D.M., Rokhsar D.S., Gerhart J. The deuterostome context of chordate origins. Nature. 2015;520:456–465. doi: 10.1038/nature14434. [DOI] [PubMed] [Google Scholar]
- 3.Lowe C.J., Wu M., Salic A., Evans L., Lander E., Stange-Thomann N., Gruber C.E., Gerhart J., Kirschner M. Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell. 2003;113:853–865. doi: 10.1016/S0092-8674(03)00469-0. [DOI] [PubMed] [Google Scholar]
- 4.Pani A.M., Mullarkey E.E., Aronowicz J., Assimacopoulos S., Grove E.A., Lowe C.J. Ancient deuterostome origins of vertebrate brain signalling centres. Nature. 2012;483:289–294. doi: 10.1038/nature10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darras S., Fritzenwanker J.H., Uhlinger K.R., Farrelly E., Pani A.M., Hurley I.A., Norris R.P., Osovitz M., Terasaki M., Wu M., et al. Anteroposterior axis patterning by early canonical Wnt signaling during hemichordate development. PLoS Biol. 2018;16:e2003698. doi: 10.1371/journal.pbio.2003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao Y., Minor P.J., Zhao Y.T., Jeong Y., Pani A.M., King A.N., Symmons O., Gan L., Cardoso W.V., Spitz F., et al. Cis-regulatory architecture of a brain signaling center predates the origin of chordates. Nat. Genet. 2016;48:575–580. doi: 10.1038/ng.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll S.B., Weatherbee S.D., Langeland J.A. Homeotic genes and the regulation and evolution of insect wing number. Nature. 1995;375:58–61. doi: 10.1038/375058a0. [DOI] [PubMed] [Google Scholar]
- 8.Carroll S.B. Homeotic genes and the evolution of arthropods and chordates. Nature. 1995;376:479–485. doi: 10.1038/376479a0. [DOI] [PubMed] [Google Scholar]
- 9.Mallo M., Wellik D.M., Deschamps J. Hox genes and regional patterning of the vertebrate body plan. Dev. Biol. 2010;344:7–15. doi: 10.1016/j.ydbio.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-N. [DOI] [PubMed] [Google Scholar]
- 11.Arendt D. Hox genes and body segmentation. Science. 2018;361:1310–1311. doi: 10.1126/science.aav0692. [DOI] [PubMed] [Google Scholar]
- 12.Duboule D., Dolle P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989;8:1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaunt S.J., Sharpe P.T., Duboule D. Spatially restricted domains of homeo-gene transcripts in mouse embryos: Relation to a segmented body plan. Development. 1988;104:169–181. doi: 10.1242/dev.104.Supplement.169. [DOI] [Google Scholar]
- 14.Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989;57:367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- 15.Lewis E.B. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 16.Dollé P., Izpisùa-Belmonte J.C., Falkenstein H., Renucci A., Duboule D. Co-ordinate expression of the murine Hox-5 complex homeobox-containing genes during limb pattern formation. Nature. 1989;342:767–772. doi: 10.1038/342767a0. [DOI] [PubMed] [Google Scholar]
- 17.Mallo M., Alonso C.R. The regulation of Hox gene expression during animal development. Development. 2013;140:3951–3963. doi: 10.1242/dev.068346. [DOI] [PubMed] [Google Scholar]
- 18.Nolte C., De Kumar B., Krumlauf R. Hox genes: Downstream “effectors” of retinoic acid signaling in vertebrate embryogenesis. Genesis. 2019;57:e23306. doi: 10.1002/dvg.23306. [DOI] [PubMed] [Google Scholar]
- 19.Parker H.J., Krumlauf R. Segmental arithmetic: Summing up the Hox gene regulatory network for hindbrain development in chordates. Wiley Interdiscip. Rev. Dev. Biol. 2017;6:e286. doi: 10.1002/wdev.286. [DOI] [PubMed] [Google Scholar]
- 20.Frank D., Sela-Donenfeld D. Hindbrain induction and patterning during early vertebrate development. Cell. Mol. Life Sci. 2019;76:941–960. doi: 10.1007/s00018-018-2974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deschamps J., van Nes J. Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development. 2005;132:2931–2942. doi: 10.1242/dev.01897. [DOI] [PubMed] [Google Scholar]
- 22.Deschamps J., Duboule D. Embryonic timing, axial stem cells, chromatin dynamics, and the Hox clock. Genes Dev. 2017;31:1406–1416. doi: 10.1101/gad.303123.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diez del Corral R., Storey K.G. Opposing FGF and retinoid pathways: A signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis. Bioessays. 2004;26:857–869. doi: 10.1002/bies.20080. [DOI] [PubMed] [Google Scholar]
- 24.Wilson V., Olivera-Martinez I., Storey K.G. Stem cells, signals and vertebrate body axis extension. Development. 2009;136:1591–1604. doi: 10.1242/dev.021246. [DOI] [PubMed] [Google Scholar]
- 25.Young T., Rowland J.E., van de Ven C., Bialecka M., Novoa A., Carapuco M., van Nes J., de Graaff W., Duluc I., Freund J.N., et al. Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev. Cell. 2009;17:516–526. doi: 10.1016/j.devcel.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Simeone A., Acampora D., Arcioni L., Andrews P.W., Boncinelli E., Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990;346:763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- 27.Schilling T.F., Nie Q., Lander A.D. Dynamics and precision in retinoic acid morphogen gradients. Curr. Opin. Genet. Dev. 2012;22:562–569. doi: 10.1016/j.gde.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedois A.M.H., Parker H.J., Krumlauf R. Retinoic Acid Signaling in Vertebrate Hindbrain Segmentation: Evolution and Diversification. Diversity. 2021;13:398. doi: 10.3390/d13080398. [DOI] [Google Scholar]
- 29.Krumlauf R., Wilkinson D.G. Segmentation and patterning of the vertebrate hindbrain. Development. 2021;148:dev186460. doi: 10.1242/dev.186460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangelsdorf D.J., Thummel C., Beato M., Herrlich P., Schutz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., et al. The nuclear receptor superfamily: The second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhinn M., Dolle P. Retinoic acid signalling during development. Development. 2012;139:843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 32.Niederreither K., Dolle P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 33.Ahn Y., Mullan H.E., Krumlauf R. Long-range regulation by shared retinoic acid response elements modulates dynamic expression of posterior Hoxb genes in CNS development. Dev. Biol. 2014;388:134–144. doi: 10.1016/j.ydbio.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Qian P., De Kumar B., He X.C., Nolte C., Gogol M., Ahn Y., Chen S., Li Z., Xu H., Perry J.M., et al. Retinoid-Sensitive Epigenetic Regulation of the Hoxb Cluster Maintains Normal Hematopoiesis and Inhibits Leukemogenesis. Cell Stem Cell. 2018;22:740–754.e7. doi: 10.1016/j.stem.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Nolte C., Jinks T., Wang X., Martinez Pastor M.T., Krumlauf R. Shadow enhancers flanking the HoxB cluster direct dynamic Hox expression in early heart and endoderm development. Dev. Biol. 2013;383:158–173. doi: 10.1016/j.ydbio.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Oosterveen T., Niederreither K., Dolle P., Chambon P., Meijlink F., Deschamps J. Retinoids regulate the anterior expression boundaries of 5’ Hoxb genes in posterior hindbrain. EMBO J. 2003;22:262–269. doi: 10.1093/emboj/cdg029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharpe J., Nonchev S., Gould A., Whiting J., Krumlauf R. Selectivity, sharing and competitive interactions in the regulation of Hoxb genes. EMBO J. 1998;17:1788–1798. doi: 10.1093/emboj/17.6.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gould A., Morrison A., Sproat G., White R.A., Krumlauf R. Positive cross-regulation and enhancer sharing: Two mechanisms for specifying overlapping Hox expression patterns. Genes Dev. 1997;11:900–913. doi: 10.1101/gad.11.7.900. [DOI] [PubMed] [Google Scholar]
- 39.Furlong E.E.M., Levine M. Developmental enhancers and chromosome topology. Science. 2018;361:1341–1345. doi: 10.1126/science.aau0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narayanan A., Meriin A., Andrews J.O., Spille J.H., Sherman M.Y., Cisse I.I. A first order phase transition mechanism underlies protein aggregation in mammalian cells. eLife. 2019;8:e39695. doi: 10.7554/eLife.39695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henninger J.E., Oksuz O., Shrinivas K., Sagi I., LeRoy G., Zheng M.M., Andrews J.O., Zamudio A.V., Lazaris C., Hannett N.M., et al. RNA-Mediated Feedback Control of Transcriptional Condensates. Cell. 2021;184:207–225.e24. doi: 10.1016/j.cell.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y.E., Manteiga J.C., Henninger J.E., Sabari B.R., Dall’Agnese A., Hannett N.M., Spille J.H., Afeyan L.K., Zamudio A.V., Shrinivas K., et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature. 2019;572:543–548. doi: 10.1038/s41586-019-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamudio A.V., Dall’Agnese A., Henninger J.E., Manteiga J.C., Afeyan L.K., Hannett N.M., Coffey E.L., Li C.H., Oksuz O., Sabari B.R., et al. Mediator Condensates Localize Signaling Factors to Key Cell Identity Genes. Mol. Cell. 2019;76:753–766.e6. doi: 10.1016/j.molcel.2019.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharp P.A., Chakraborty A.K., Henninger J.E., Young R.A. RNA in formation and regulation of transcriptional condensates. RNA. 2021;28:52–57. doi: 10.1261/rna.078997.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mir M., Stadler M.R., Ortiz S.A., Hannon C.E., Harrison M.M., Darzacq X., Eisen M.B. Dynamic multifactor hubs interact transiently with sites of active transcription in Drosophila embryos. eLife. 2018;7:e40497. doi: 10.7554/eLife.40497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berrocal A., Lammers N.C., Garcia H.G., Eisen M.B. Kinetic sculpting of the seven stripes of the Drosophila even-skipped gene. eLife. 2020;9:e61635. doi: 10.7554/eLife.61635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chong S., Dugast-Darzacq C., Liu Z., Dong P., Dailey G.M., Cattoglio C., Heckert A., Banala S., Lavis L., Darzacq X., et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science. 2018;361:aar2555. doi: 10.1126/science.aar2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z., Tjian R. Visualizing transcription factor dynamics in living cells. J. Cell Biol. 2018;217:1181–1191. doi: 10.1083/jcb.201710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z., Tjian R. Measuring dynamics of eukaryotic transcription initiation: Challenges, insights and opportunities. Transcription. 2018;9:159–165. doi: 10.1080/21541264.2017.1363017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981;27:299–308. doi: 10.1016/0092-8674(81)90413-X. [DOI] [PubMed] [Google Scholar]
- 51.Shen Y., Yue F., McCleary D.F., Ye Z., Edsall L., Kuan S., Wagner U., Dixon J., Lee L., Lobanenkov V.V., et al. A map of the cis-regulatory sequences in the mouse genome. Nature. 2012;488:116–120. doi: 10.1038/nature11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Consortium E.P. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pennacchio L.A., Bickmore W., Dean A., Nobrega M.A., Bejerano G. Enhancers: Five essential questions. Nat. Rev. Genet. 2013;14:288–295. doi: 10.1038/nrg3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avsec Z., Weilert M., Shrikumar A., Krueger S., Alexandari A., Dalal K., Fropf R., McAnany C., Gagneur J., Kundaje A., et al. Base-resolution models of transcription-factor binding reveal soft motif syntax. Nat. Genet. 2021;53:354–366. doi: 10.1038/s41588-021-00782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeitlinger J. Seven myths of how transcription factors read the cis-regulatory code. Curr. Opin. Syst. Biol. 2020;23:22–31. doi: 10.1016/j.coisb.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He Q., Johnston J., Zeitlinger J. ChIP-nexus enables improved detection of in vivo transcription factor binding footprints. Nat. Biotechnol. 2015;33:395–401. doi: 10.1038/nbt.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Girskis K.M., Stergachis A.B., DeGennaro E.M., Doan R.N., Qian X., Johnson M.B., Wang P.P., Sejourne G.M., Nagy M.A., Pollina E.A., et al. Rewiring of human neurodevelopmental gene regulatory programs by human accelerated regions. Neuron. 2021;109:3239–3251.e7. doi: 10.1016/j.neuron.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snetkova V., Pennacchio L.A., Visel A., Dickel D.E. Perfect and imperfect views of ultraconserved sequences. Nat. Rev. Genet. 2021 doi: 10.1038/s41576-021-00424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snetkova V., Ypsilanti A.R., Akiyama J.A., Mannion B.J., Plajzer-Frick I., Novak C.S., Harrington A.N., Pham Q.T., Kato M., Zhu Y., et al. Ultraconserved enhancer function does not require perfect sequence conservation. Nat. Genet. 2021;53:521–528. doi: 10.1038/s41588-021-00812-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuqua T., Jordan J., van Breugel M.E., Halavatyi A., Tischer C., Polidoro P., Abe N., Tsai A., Mann R.S., Stern D.L., et al. Dense and pleiotropic regulatory information in a developmental enhancer. Nature. 2020;587:235–239. doi: 10.1038/s41586-020-2816-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marshall H., Studer M., Popperl H., Aparicio S., Kuroiwa A., Brenner S., Krumlauf R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature. 1994;370:567–571. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- 62.Parker H.J., De Kumar B., Green S.A., Prummel K.D., Hess C., Kaufman C.K., Mosimann C., Wiedemann L.M., Bronner M.E., Krumlauf R. A Hox-TALE regulatory circuit for neural crest patterning is conserved across vertebrates. Nat. Commun. 2019;10:1189. doi: 10.1038/s41467-019-09197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tümpel S., Maconochie M., Wiedemann L.M., Krumlauf R. Conservation and diversity in the cis-regulatory networks that integrate information controlling expression of Hoxa2 in hindbrain and cranial neural crest cells in vertebrates. Dev. Biol. 2002;246:45–56. doi: 10.1006/dbio.2002.0665. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt D., Wilson M.D., Ballester B., Schwalie P.C., Brown G.D., Marshall A., Kutter C., Watt S., Martinez-Jimenez C.P., Mackay S., et al. Five-vertebrate ChIP-seq reveals the evolutionary dynamics of transcription factor binding. Science. 2010;328:1036–1040. doi: 10.1126/science.1186176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paris M., Kaplan T., Li X.Y., Villalta J.E., Lott S.E., Eisen M.B. Extensive divergence of transcription factor binding in Drosophila embryos with highly conserved gene expression. PLoS Genet. 2013;9:e1003748. doi: 10.1371/journal.pgen.1003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farley E.K., Olson K.M., Zhang W., Brandt A.J., Rokhsar D.S., Levine M.S. Suboptimization of developmental enhancers. Science. 2015;350:325–328. doi: 10.1126/science.aac6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crocker J., Abe N., Rinaldi L., McGregor A.P., Frankel N., Wang S., Alsawadi A., Valenti P., Plaza S., Payre F., et al. Low affinity binding site clusters confer hox specificity and regulatory robustness. Cell. 2015;160:191–203. doi: 10.1016/j.cell.2014.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi J., Lysakovskaia K., Stik G., Demel C., Söding J., Tian T.V., Graf T., Cramer P. Evidence for additive and synergistic action of mammalian enhancers during cell fate determination. eLife. 2021;10:e65381. doi: 10.7554/eLife.65381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marsman J., Horsfield J.A. Long distance relationships: Enhancer-promoter communication and dynamic gene transcription. Biochim. Biophys. Acta. 2012;1819:1217–1227. doi: 10.1016/j.bbagrm.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Lettice L.A., Heaney S.J., Purdie L.A., Li L., de Beer P., Oostra B.A., Goode D., Elgar G., Hill R.E., de Graaff E. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum. Mol. Genet. 2003;12:1725–1735. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- 71.Tümpel S., Cambronero F., Sims C., Krumlauf R., Wiedemann L.M. A regulatory module embedded in the coding region of Hoxa2 controls expression in rhombomere 2. Proc. Natl. Acad. Sci. USA. 2008;105:20077–20082. doi: 10.1073/pnas.0806360105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lampe X., Samad O.A., Guiguen A., Matis C., Remacle S., Picard J.J., Rijli F.M., Rezsohazy R. An ultraconserved Hox-Pbx responsive element resides in the coding sequence of Hoxa2 and is active in rhombomere 4. Nucleic Acids Res. 2008;36:3214–3225. doi: 10.1093/nar/gkn148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin M.F., Kheradpour P., Washietl S., Parker B.J., Pedersen J.S., Kellis M. Locating protein-coding sequences under selection for additional, overlapping functions in 29 mammalian genomes. Genome Res. 2011;21:1916–1928. doi: 10.1101/gr.108753.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arnold P.R., Wells A.D., Li X.C. Diversity and Emerging Roles of Enhancer RNA in Regulation of Gene Expression and Cell Fate. Front. Cell Dev. Biol. 2020;7:377. doi: 10.3389/fcell.2019.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schoenfelder S., Fraser P. Long-range enhancer-promoter contacts in gene expression control. Nat. Rev. Genet. 2019;20:437–455. doi: 10.1038/s41576-019-0128-0. [DOI] [PubMed] [Google Scholar]
- 76.Lai F., Gardini A., Zhang A., Shiekhattar R. Integrator mediates the biogenesis of enhancer RNAs. Nature. 2015;525:399–403. doi: 10.1038/nature14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benabdallah N.S., Williamson I., Illingworth R.S., Kane L., Boyle S., Sengupta D., Grimes G.R., Therizols P., Bickmore W.A. Decreased Enhancer-Promoter Proximity Accompanying Enhancer Activation. Mol. Cell. 2019;76:473–484.e7. doi: 10.1016/j.molcel.2019.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haberle V., Stark A. Eukaryotic core promoters and the functional basis of transcription initiation. Nat. Rev. Mol. Cell Biol. 2018;19:621–637. doi: 10.1038/s41580-018-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanyal A., Lajoie B.R., Jain G., Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489:109–113. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Long H.K., Prescott S.L., Wysocka J. Ever-Changing Landscapes: Transcriptional Enhancers in Development and Evolution. Cell. 2016;167:1170–1187. doi: 10.1016/j.cell.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Kumar B., Parrish M.E., Slaughter B.D., Unruh J.R., Gogol M., Seidel C., Paulson A., Li H., Gaudenz K., Peak A., et al. Analysis of dynamic changes in retinoid-induced transcription and epigenetic profiles of murine Hox clusters in ES cells. Genome Res. 2015;25:1229–1243. doi: 10.1101/gr.184978.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maconochie M.K., Nonchev S., Manzanares M., Marshall H., Krumlauf R. Differences in Krox20-dependent regulation of Hoxa2 and Hoxb2 during hindbrain development. Dev. Biol. 2001;233:468–481. doi: 10.1006/dbio.2001.0197. [DOI] [PubMed] [Google Scholar]
- 83.Popperl H., Bienz M., Studer M., Chan S.K., Aparicio S., Brenner S., Mann R.S., Krumlauf R. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell. 1995;81:1031–1042. doi: 10.1016/S0092-8674(05)80008-X. [DOI] [PubMed] [Google Scholar]
- 84.Manzanares M., Cordes S., Kwan C.-T., Sham M.-H., Barsh G., Krumlauf R. Segmental regulation of Hoxb3 by kreisler. Nature. 1997;387:191–195. doi: 10.1038/387191a0. [DOI] [PubMed] [Google Scholar]
- 85.Merabet S., Mann R.S. To Be Specific or Not: The Critical Relationship Between Hox And TALE Proteins. Trends Genet. 2016;32:334–347. doi: 10.1016/j.tig.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mann R.S., Lelli K.M., Joshi R. Hox specificity unique roles for cofactors and collaborators. Curr. Top. Dev. Biol. 2009;88:63–101. doi: 10.1016/S0070-2153(09)88003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berger M.F., Badis G., Gehrke A.R., Talukder S., Philippakis A.A., Pena-Castillo L., Alleyne T.M., Mnaimneh S., Botvinnik O.B., Chan E.T., et al. Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell. 2008;133:1266–1276. doi: 10.1016/j.cell.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noyes M.B., Christensen R.G., Wakabayashi A., Stormo G.D., Brodsky M.H., Wolfe S.A. Analysis of homeodomain specificities allows the family-wide prediction of preferred recognition sites. Cell. 2008;133:1277–1289. doi: 10.1016/j.cell.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nitta K.R., Jolma A., Yin Y., Morgunova E., Kivioja T., Akhtar J., Hens K., Toivonen J., Deplancke B., Furlong E.E., et al. Conservation of transcription factor binding specificities across 600 million years of bilateria evolution. eLife. 2015;4:e04837. doi: 10.7554/eLife.04837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loker R., Sanner J.E., Mann R.S. Cell-type-specific Hox regulatory strategies orchestrate tissue identity. Curr. Biol. 2021;31:4246–4255.e4. doi: 10.1016/j.cub.2021.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carroll S.B. Evolution at two levels: On genes and form. PLoS Biol. 2005;3:e245. doi: 10.1371/journal.pbio.0030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gehring W., Muller M., Affolter M., Percival-Smith A., Billeter M., Qian Y., Otting G., Wuthrich K. The structure of the homeodomain and its functional implications. Trends Genet. 1990;6:323–329. doi: 10.1016/0168-9525(90)90253-3. [DOI] [PubMed] [Google Scholar]
- 93.Jabet C., Gitti R., Summers M.F., Wolberger C. NMR studies of the pbx1 TALE homeodomain protein free in solution and bound to DNA: Proposal for a mechanism of HoxB1-Pbx1-DNA complex assembly. J. Mol. Biol. 1999;291:521–530. doi: 10.1006/jmbi.1999.2983. [DOI] [PubMed] [Google Scholar]
- 94.Kissinger C., Liu B., Martin-Blanco E., Kronberg T., Pabo C. Crystal structure of an engrailed homeodomain-DNA complex at 2.8A resolution: A framework for understanding homeodomain-DNA interactions. Cell. 1990;63:579–590. doi: 10.1016/0092-8674(90)90453-L. [DOI] [PubMed] [Google Scholar]
- 95.Slattery M., Riley T., Liu P., Abe N., Gomez-Alcala P., Dror I., Zhou T., Rohs R., Honig B., Bussemaker H.J., et al. Cofactor binding evokes latent differences in DNA binding specificity between Hox proteins. Cell. 2011;147:1270–1282. doi: 10.1016/j.cell.2011.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh N.P., De Kumar B., Paulson A., Parrish M.E., Scott C., Zhang Y., Florens L., Krumlauf R. Genome-Wide Binding Analyses of HOXB1 Revealed a Novel DNA Binding Motif Associated with Gene Repression. J. Dev. Biol. 2021;9:6. doi: 10.3390/jdb9010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh N.P., De Kumar B., Paulson A., Parrish M.E., Zhang Y., Florens L., Conaway J.W., Si K., Krumlauf R. A six-amino-acid motif is a major determinant in functional evolution of HOX1 proteins. Genes Dev. 2020;34:1680–1696. doi: 10.1101/gad.342329.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Studer M., Gavalas A., Marshall H., Ariza-McNaughton L., Rijli F.M., Chambon P., Krumlauf R. Genetic interactions between Hoxa1 and Hoxb1 reveal new roles in regulation of early hindbrain patterning. Development. 1998;125:1025–1036. doi: 10.1242/dev.125.6.1025. [DOI] [PubMed] [Google Scholar]
- 99.De Kumar B., Parker H.J., Paulson A., Parrish M.E., Pushel I., Singh N.P., Zhang Y., Slaughter B.D., Unruh J.R., Florens L., et al. HOXA1 and TALE proteins display cross-regulatory interactions and form a combinatorial binding code on HOXA1 targets. Genome Res. 2017;27:1501–1512. doi: 10.1101/gr.219386.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manzanares M., Bel-Vialer S., Ariza-McNaughton L., Ferretti E., Marshall H., Maconochie M.K., Blasi F., Krumlauf R. Independent regulation of initiation and maintenance phases of Hoxa3 expression in the vertebrate hindbrain involves auto and cross-regulatory mechanisms. Development. 2001;128:3595–3607. doi: 10.1242/dev.128.18.3595. [DOI] [PubMed] [Google Scholar]
- 101.Merabet S., Kambris Z., Capovilla M., Berenger H., Pradel J., Graba Y. The hexapeptide and linker regions of the AbdA Hox protein regulate its activating and repressive functions. Dev. Cell. 2003;4:761–768. doi: 10.1016/S1534-5807(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 102.Dard A., Reboulet J., Jia Y., Bleicher F., Duffraisse M., Vanaker J.M., Forcet C., Merabet S. Human HOX Proteins Use Diverse and Context-Dependent Motifs to Interact with TALE Class Cofactors. Cell Rep. 2018;22:3058–3071. doi: 10.1016/j.celrep.2018.02.070. [DOI] [PubMed] [Google Scholar]
- 103.Merabet S., Litim-Mecheri I., Karlsson D., Dixit R., Saadaoui M., Monier B., Brun C., Thor S., Vijayraghavan K., Perrin L., et al. Insights into Hox protein function from a large scale combinatorial analysis of protein domains. PLoS Genet. 2011;7:e1002302. doi: 10.1371/journal.pgen.1002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zandvakili A., Gebelein B. Mechanisms of Specificity for Hox Factor Activity. J. Dev. Biol. 2016;4:16. doi: 10.3390/jdb4020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeiske T., Baburajendran N., Kaczynska A., Brasch J., Palmer A.G., 3rd, Shapiro L., Honig B., Mann R.S. Intrinsic DNA Shape Accounts for Affinity Differences between Hox-Cofactor Binding Sites. Cell Rep. 2018;24:2221–2230. doi: 10.1016/j.celrep.2018.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Merabet S., Hudry B., Saadaoui M., Graba Y. Classification of sequence signatures: A guide to Hox protein function. Bioessays. 2009;31:500–511. doi: 10.1002/bies.200800229. [DOI] [PubMed] [Google Scholar]
- 107.Rivas M.L., Espinosa-Vazquez J.M., Sambrani N., Greig S., Merabet S., Graba Y., Hombria J.C. Antagonism versus cooperativity with TALE cofactors at the base of the functional diversification of Hox protein function. PLoS Genet. 2013;9:e1003252. doi: 10.1371/journal.pgen.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berthelsen J., Zappavigna V., Ferretti E., Mavilio F., Blasi F. The novel homeoprotein Prep1 modulates Pbx-Hox protein cooperativity. EMBO J. 1998;17:1434–1445. doi: 10.1093/emboj/17.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ferretti E., Cambronero F., Tümpel S., Longobardi E., Wiedemann L.M., Blasi F., Krumlauf R. Hoxb1 enhancer and control of rhombomere 4 expression: Complex interplay between PREP1-PBX1-HOXB1 binding sites. Mol. Cell. Biol. 2005;25:8541–8552. doi: 10.1128/MCB.25.19.8541-8552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Longobardi E., Penkov D., Mateos D., De Florian G., Torres M., Blasi F. Biochemistry of the tale transcription factors PREP, MEIS, and PBX in vertebrates. Dev. Dyn. 2014;243:59–75. doi: 10.1002/dvdy.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Penkov D., Tanaka S., Di Rocco G., Berthelsen J., Blasi F., Ramirez F. Cooperative interactions between PBX, PREP, and HOX proteins modulate the activity of the alpha 2(V) collagen (COL5A2) promoter. J. Biol. Chem. 2000;275:16681–16689. doi: 10.1074/jbc.M909345199. [DOI] [PubMed] [Google Scholar]
- 112.De Kumar B., Parker H.J., Parrish M.E., Lange J.J., Slaughter B.D., Unruh J.R., Paulson A., Krumlauf R. Dynamic regulation of Nanog and stem cell-signaling pathways by Hoxa1 during early neuro-ectodermal differentiation of ES cells. Proc. Natl. Acad. Sci. USA. 2017;114:5838–5845. doi: 10.1073/pnas.1610612114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Kumar B., Parker H.J., Paulson A., Parrish M.E., Zeitlinger J., Krumlauf R. Hoxa1 targets signaling pathways during neural differentiation of ES cells and mouse embryogenesis. Dev. Biol. 2017;432:151–164. doi: 10.1016/j.ydbio.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 114.Gavalas A., Trainor P., Ariza-McNaughton L., Krumlauf R. Synergy between Hoxa1 and Hoxb1: The relationship between arch patterning and the generation of cranial neural crest. Development. 2001;128:3017–3027. doi: 10.1242/dev.128.15.3017. [DOI] [PubMed] [Google Scholar]
- 115.Manley N.R., Capecchi M.R. Hox group 3 paralogous genes act synergistically in the formation of somitic and neural crest-derived structures. Dev. Biol. 1997;192:274–288. doi: 10.1006/dbio.1997.8765. [DOI] [PubMed] [Google Scholar]
- 116.Rossel M., Capecchi M.R. Mice mutant for both Hoxa1 and Hoxb1 show extensive remodeling of the hindbrain and defects in craniofacial development. Development. 1999;126:5027–5040. doi: 10.1242/dev.126.22.5027. [DOI] [PubMed] [Google Scholar]
- 117.Wellik D.M., Capecchi M.R. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301:363–367. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- 118.Horan G.S., Ramirez-Solis R., Featherstone M.S., Wolgemuth D.J., Bradley A., Behringer R.R. Compound mutants for the paralogous Hoxa-4, Hoxb-4, and Hoxd-4 genes show more complete homeotic transformations and a dose-dependent increase in the number of vertebrae transformed. Genes Dev. 1995;9:1667–1677. doi: 10.1101/gad.9.13.1667. [DOI] [PubMed] [Google Scholar]
- 119.Svingen T., Tonissen K.F. Hox transcription factors and their elusive mammalian gene targets. Heredity. 2006;97:88–96. doi: 10.1038/sj.hdy.6800847. [DOI] [PubMed] [Google Scholar]
- 120.Bridoux L., Zarrineh P., Mallen J., Phuycharoen M., Latorre V., Ladam F., Losa M., Baker S.M., Sagerstrom C., Mace K.A., et al. HOX paralogs selectively convert binding of ubiquitous transcription factors into tissue-specific patterns of enhancer activation. PLoS Genet. 2020;16:e1009162. doi: 10.1371/journal.pgen.1009162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bulajić M., Srivastava D., Dasen J.S., Wichterle H., Mahony S., Mazzoni E.O. Differential abilities to engage inaccessible chromatin diversify vertebrate Hox binding patterns. Development. 2020;147:dev194761. doi: 10.1242/dev.194761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Banigan E.J., Mirny L.A. Loop extrusion: Theory meets single-molecule experiments. Curr. Opin. Cell Biol. 2020;64:124–138. doi: 10.1016/j.ceb.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 123.Mirny L., Dekker J. Mechanisms of Chromosome Folding and Nuclear Organization: Their Interplay and Open Questions. Cold Spring Harb. Perspect. Biol. 2021 doi: 10.1101/cshperspect.a040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dixon J.R., Selvaraj S., Yue F., Kim A., Li Y., Shen Y., Hu M., Liu J.S., Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nora E.P., Lajoie B.R., Schulz E.G., Giorgetti L., Okamoto I., Servant N., Piolot T., van Berkum N.L., Meisig J., Sedat J., et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sexton T., Yaffe E., Kenigsberg E., Bantignies F., Leblanc B., Hoichman M., Parrinello H., Tanay A., Cavalli G. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell. 2012;148:458–472. doi: 10.1016/j.cell.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 127.Lee T.I., Young R.A. Transcriptional regulation and its misregulation in disease. Cell. 2013;152:1237–1251. doi: 10.1016/j.cell.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Narendra V., Bulajić M., Dekker J., Mazzoni E.O., Reinberg D. CTCF-mediated topological boundaries during development foster appropriate gene regulation. Genes Dev. 2016;30:2657–2662. doi: 10.1101/gad.288324.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim T.H., Abdullaev Z.K., Smith A.D., Ching K.A., Loukinov D.I., Green R.D., Zhang M.Q., Lobanenkov V.V., Ren B. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dostie J., Richmond T.A., Arnaout R.A., Selzer R.R., Lee W.L., Honan T.A., Rubio E.D., Krumm A., Lamb J., Nusbaum C., et al. Chromosome Conformation Capture Carbon Copy (5C): A massively parallel solution for mapping interactions between genomic elements. Genome Res. 2006;16:1299–1309. doi: 10.1101/gr.5571506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Narendra V., Rocha P.P., An D., Raviram R., Skok J.A., Mazzoni E.O., Reinberg D. CTCF establishes discrete functional chromatin domains at the Hox clusters during differentiation. Science. 2015;347:1017–1021. doi: 10.1126/science.1262088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bolt C.C., Duboule D. The regulatory landscapes of developmental genes. Development. 2020;147:dev171736. doi: 10.1242/dev.171736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lupiáñez D.G., Kraft K., Heinrich V., Krawitz P., Brancati F., Klopocki E., Horn D., Kayserili H., Opitz J.M., Laxova R., et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161:1012–1025. doi: 10.1016/j.cell.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guo Y., Xu Q., Canzio D., Shou J., Li J., Gorkin D.U., Jung I., Wu H., Zhai Y., Tang Y., et al. CRISPR Inversion of CTCF Sites Alters Genome Topology and Enhancer/Promoter Function. Cell. 2015;162:900–910. doi: 10.1016/j.cell.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oh J.H., Kim C.Y., Lee J.Y., Kim M.H. Retinoic acid and CTCF play key roles in inducing the collinear expression of the Hoxa cluster. Acta Biochim. Biophys. Sin. 2018;50:555–559. doi: 10.1093/abbs/gmy039. [DOI] [PubMed] [Google Scholar]
- 136.Luo H., Wang F., Zha J., Li H., Yan B., Du Q., Yang F., Sobh A., Vulpe C., Drusbosky L., et al. CTCF boundary remodels chromatin domain and drives aberrant HOX gene transcription in acute myeloid leukemia. Blood. 2018;132:837–848. doi: 10.1182/blood-2017-11-814319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li Y., Liao Z., Luo H., Benyoucef A., Kang Y., Lai Q., Dovat S., Miller B., Chepelev I., Li Y., et al. Alteration of CTCF-associated chromatin neighborhood inhibits TAL1-driven oncogenic transcription program and leukemogenesis. Nucleic Acids Res. 2020;48:3119–3133. doi: 10.1093/nar/gkaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qiu Y., Huang S. CTCF-mediated genome organization and leukemogenesis. Leukemia. 2020;34:2295–2304. doi: 10.1038/s41375-020-0906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morcillo P., Rosen C., Baylies M.K., Dorsett D. Chip, a widely expressed chromosomal protein required for segmentation and activity of a remote wing margin enhancer in Drosophila. Genes Dev. 1997;11:2729–2740. doi: 10.1101/gad.11.20.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kong S., Bohl D., Li C., Tuan D. Transcription of the HS2 enhancer toward a cis-linked gene is independent of the orientation, position, and distance of the enhancer relative to the gene. Mol. Cell. Biol. 1997;17:3955–3965. doi: 10.1128/MCB.17.7.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Deng W., Lee J., Wang H., Miller J., Reik A., Gregory P.D., Dean A., Blobel G.A. Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell. 2012;149:1233–1244. doi: 10.1016/j.cell.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dunn T.M., Hahn S., Ogden S., Schleif R.F. An operator at -280 base pairs that is required for repression of araBAD operon promoter: Addition of DNA helical turns between the operator and promoter cyclically hinders repression. Proc. Natl. Acad. Sci. USA. 1984;81:5017–5020. doi: 10.1073/pnas.81.16.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takaki R., Dey A., Shi G., Thirumalai D. Theory and simulations of condensin mediated loop extrusion in DNA. Nat. Commun. 2021;12:5865. doi: 10.1038/s41467-021-26167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chambeyron S., Bickmore W.A. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev. 2004;18:1119–1130. doi: 10.1101/gad.292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Symmons O., Uslu V.V., Tsujimura T., Ruf S., Nassari S., Schwarzer W., Ettwiller L., Spitz F. Functional and topological characteristics of mammalian regulatory domains. Genome Res. 2014;24:390–400. doi: 10.1101/gr.163519.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gambetta M.C., Furlong E.E.M. The Insulator Protein CTCF Is Required for Correct. Genetics. 2018;210:129–136. doi: 10.1534/genetics.118.301350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rao S.S.P., Huang S.C., Glenn St Hilaire B., Engreitz J.M., Perez E.M., Kieffer-Kwon K.R., Sanborn A.L., Johnstone S.E., Bascom G.D., Bochkov I.D., et al. Cohesin Loss Eliminates All Loop Domains. Cell. 2017;171:305–320.e24. doi: 10.1016/j.cell.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.de Wit E., Vos E.S., Holwerda S.J., Valdes-Quezada C., Verstegen M.J., Teunissen H., Splinter E., Wijchers P.J., Krijger P.H., de Laat W. CTCF Binding Polarity Determines Chromatin Looping. Mol. Cell. 2015;60:676–684. doi: 10.1016/j.molcel.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 149.Schwarzer W., Abdennur N., Goloborodko A., Pekowska A., Fudenberg G., Loe-Mie Y., Fonseca N.A., Huber W., Haering C.H., Mirny L., et al. Two independent modes of chromatin organization revealed by cohesin removal. Nature. 2017;551:51–56. doi: 10.1038/nature24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ghavi-Helm Y., Klein F.A., Pakozdi T., Ciglar L., Noordermeer D., Huber W., Furlong E.E. Enhancer loops appear stable during development and are associated with paused polymerase. Nature. 2014;512:96–100. doi: 10.1038/nature13417. [DOI] [PubMed] [Google Scholar]
- 151.Espinola S.M., Götz M., Bellec M., Messina O., Fiche J.-B., Houbron C., Dejean M., Reim I., Cardozo Gizzi A.M., Lagha M., et al. Cis-regulatory chromatin loops arise before TADs and gene activation, and are independent of cell fate during early Drosophila development. Nat. Genet. 2021;53:477–486. doi: 10.1038/s41588-021-00816-z. [DOI] [PubMed] [Google Scholar]
- 152.Chen H., Levo M., Barinov L., Fujioka M., Jaynes J.B., Gregor T. Dynamic interplay between enhancer-promoter topology and gene activity. Nat. Genet. 2018;50:1296–1303. doi: 10.1038/s41588-018-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bartman C.R., Hsu S.C., Hsiung C.C., Raj A., Blobel G.A. Enhancer Regulation of Transcriptional Bursting Parameters Revealed by Forced Chromatin Looping. Mol. Cell. 2016;62:237–247. doi: 10.1016/j.molcel.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Larsson A.J.M., Johnsson P., Hagemann-Jensen M., Hartmanis L., Faridani O.R., Reinius B., Segerstolpe Å., Rivera C.M., Ren B., Sandberg R. Genomic encoding of transcriptional burst kinetics. Nature. 2019;565:251–254. doi: 10.1038/s41586-018-0836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alexander J.M., Guan J., Li B., Maliskova L., Song M., Shen Y., Huang B., Lomvardas S., Weiner O.D. Live-cell imaging reveals enhancer-dependent Sox2 transcription in the absence of enhancer proximity. eLife. 2019;8:e41769. doi: 10.7554/eLife.41769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hong J.W., Hendrix D.A., Levine M.S. Shadow enhancers as a source of evolutionary novelty. Science. 2008;321:1314. doi: 10.1126/science.1160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Perry M.W., Boettiger A.N., Bothma J.P., Levine M. Shadow enhancers foster robustness of Drosophila gastrulation. Curr. Biol. 2010;20:1562–1567. doi: 10.1016/j.cub.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Waymack R., Fletcher A., Enciso G., Wunderlich Z. Shadow enhancers can suppress input transcription factor noise through distinct regulatory logic. eLife. 2020;9:e59351. doi: 10.7554/eLife.59351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fukaya T., Lim B., Levine M. Enhancer Control of Transcriptional Bursting. Cell. 2016;166:358–368. doi: 10.1016/j.cell.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wan Y., Anastasakis D.G., Rodriguez J., Palangat M., Gudla P., Zaki G., Tandon M., Pegoraro G., Chow C.C., Hafner M., et al. Dynamic imaging of nascent RNA reveals general principles of transcription dynamics and stochastic splice site selection. Cell. 2021;184:2878–2895.e20. doi: 10.1016/j.cell.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bothma J.P., Garcia H.G., Esposito E., Schlissel G., Gregor T., Levine M. Dynamic regulation of eve stripe 2 expression reveals transcriptional bursts in living Drosophila embryos. Proc. Natl. Acad. Sci. USA. 2014;111:10598–10603. doi: 10.1073/pnas.1410022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hnisz D., Shrinivas K., Young R.A., Chakraborty A.K., Sharp P.A. A Phase Separation Model for Transcriptional Control. Cell. 2017;169:13–23. doi: 10.1016/j.cell.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shao W., Zeitlinger J. Paused RNA polymerase II inhibits new transcriptional initiation. Nat. Genet. 2017;49:1045–1051. doi: 10.1038/ng.3867. [DOI] [PubMed] [Google Scholar]
- 164.Cho W.K., Spille J.H., Hecht M., Lee C., Li C., Grube V., Cisse I.I. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science. 2018;361:412–415. doi: 10.1126/science.aar4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lim B., Heist T., Levine M., Fukaya T. Visualization of Transvection in Living Drosophila Embryos. Mol. Cell. 2018;70:287–296.e6. doi: 10.1016/j.molcel.2018.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Delpretti S., Montavon T., Leleu M., Joye E., Tzika A., Milinkovitch M., Duboule D. Multiple enhancers regulate Hoxd genes and the Hotdog LncRNA during cecum budding. Cell Rep. 2013;5:137–150. doi: 10.1016/j.celrep.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 167.Andrey G., Montavon T., Mascrez B., Gonzalez F., Noordermeer D., Leleu M., Trono D., Spitz F., Duboule D. A switch between topological domains underlies HoxD genes collinearity in mouse limbs. Science. 2013;340:1234167. doi: 10.1126/science.1234167. [DOI] [PubMed] [Google Scholar]
- 168.Bonev B., Mendelson Cohen N., Szabo Q., Fritsch L., Papadopoulos G.L., Lubling Y., Xu X., Lv X., Hugnot J.P., Tanay A., et al. Multiscale 3D Genome Rewiring during Mouse Neural Development. Cell. 2017;171:557–572.e24. doi: 10.1016/j.cell.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cardozo Gizzi A.M., Cattoni D.I., Fiche J.B., Espinola S.M., Gurgo J., Messina O., Houbron C., Ogiyama Y., Papadopoulos G.L., Cavalli G., et al. Microscopy-Based Chromosome Conformation Capture Enables Simultaneous Visualization of Genome Organization and Transcription in Intact Organisms. Mol. Cell. 2019;74:212–222.e5. doi: 10.1016/j.molcel.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 170.Ohno M., Ando T., Priest D.G., Taniguchi Y. Hi-CO: 3D genome structure analysis with nucleosome resolution. Nat. Protoc. 2021;16:3439–3469. doi: 10.1038/s41596-021-00543-z. [DOI] [PubMed] [Google Scholar]
- 171.Akgol Oksuz B., Yang L., Abraham S., Venev S.V., Krietenstein N., Parsi K.M., Ozadam H., Oomen M.E., Nand A., Mao H., et al. Systematic evaluation of chromosome conformation capture assays. Nat. Methods. 2021;18:1046–1055. doi: 10.1038/s41592-021-01248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mateo L.J., Murphy S.E., Hafner A., Cinquini I.S., Walker C.A., Boettiger A.N. Visualizing DNA folding and RNA in embryos at single-cell resolution. Nature. 2019;568:49–54. doi: 10.1038/s41586-019-1035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Raj A., Tyagi S. Detection of individual endogenous RNA transcripts in situ using multiple singly labeled probes. Methods Enzymol. 2010;472:365–386. doi: 10.1016/S0076-6879(10)72004-8. [DOI] [PubMed] [Google Scholar]
- 174.Shaffer S.M., Wu M.T., Levesque M.J., Raj A. Turbo FISH: A method for rapid single molecule RNA FISH. PLoS ONE. 2013;8:e75120. doi: 10.1371/journal.pone.0075120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Choi H.M.T., Schwarzkopf M., Fornace M.E., Acharya A., Artavanis G., Stegmaier J., Cunha A., Pierce N.A. Third-generation in situ hybridization chain reaction: Multiplexed, quantitative, sensitive, versatile, robust. Development. 2018;145:dev165753. doi: 10.1242/dev.165753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Garcia H.G., Gregor T. Live Imaging of mRNA Synthesis in Drosophila. Methods Mol. Biol. 2018;1649:349–357. doi: 10.1007/978-1-4939-7213-5_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lim B., Fukaya T., Heist T., Levine M. Temporal dynamics of pair-rule stripes in living Drosophila embryos. Proc. Natl. Acad. Sci. USA. 2018;115:8376–8381. doi: 10.1073/pnas.1810430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bothma J.P., Garcia H.G., Ng S., Perry M.W., Gregor T., Levine M. Enhancer additivity and non-additivity are determined by enhancer strength in the Drosophila embryo. eLife. 2015;4:e07956. doi: 10.7554/eLife.07956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bothma J.P., Norstad M.R., Alamos S., Garcia H.G. LlamaTags: A Versatile Tool to Image Transcription Factor Dynamics in Live Embryos. Cell. 2018;173:1810–1822.e16. doi: 10.1016/j.cell.2018.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wissink E.M., Vihervaara A., Tippens N.D., Lis J.T. Nascent RNA analyses: Tracking transcription and its regulation. Nat. Rev. Genet. 2019;20:705–723. doi: 10.1038/s41576-019-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Boija A., Klein I.A., Sabari B.R., Dall’Agnese A., Coffey E.L., Zamudio A.V., Li C.H., Shrinivas K., Manteiga J.C., Hannett N.M., et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell. 2018;175:1842–1855.e16. doi: 10.1016/j.cell.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Boube M., Hudry B., Immarigeon C., Carrier Y., Bernat-Fabre S., Merabet S., Graba Y., Bourbon H.M., Cribbs D.L. Drosophila melanogaster Hox transcription factors access the RNA polymerase II machinery through direct homeodomain binding to a conserved motif of mediator subunit Med19. PLoS Genet. 2014;10:e1004303. doi: 10.1371/journal.pgen.1004303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Soshnikova N., Duboule D. Epigenetic temporal control of mouse Hox genes in vivo. Science. 2009;324:1320–1323. doi: 10.1126/science.1171468. [DOI] [PubMed] [Google Scholar]
- 184.Noordermeer D., Leleu M., Schorderet P., Joye E., Chabaud F., Duboule D. Temporal dynamics and developmental memory of 3D chromatin architecture at Hox gene loci. eLife. 2014;3:e02557. doi: 10.7554/eLife.02557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rodríguez-Carballo E., Lopez-Delisle L., Yakushiji-Kaminatsui N., Ullate-Agote A., Duboule D. Impact of genome architecture on the functional activation and repression of Hox regulatory landscapes. BMC Biol. 2019;17:55. doi: 10.1186/s12915-019-0677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Spitz F., Gonzalez F., Duboule D. A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell. 2003;113:405–417. doi: 10.1016/S0092-8674(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 187.Gonzalez F., Duboule D., Spitz F. Transgenic analysis of Hoxd gene regulation during digit development. Dev. Biol. 2007;306:847–859. doi: 10.1016/j.ydbio.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 188.Neijts R., Amin S., van Rooijen C., Tan S., Creyghton M.P., de Laat W., Deschamps J. Polarized regulatory landscape and Wnt responsiveness underlie Hox activation in embryos. Genes Dev. 2016;30:1937–1942. doi: 10.1101/gad.285767.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Berlivet S., Paquette D., Dumouchel A., Langlais D., Dostie J., Kmita M. Clustering of tissue-specific sub-TADs accompanies the regulation of HoxA genes in developing limbs. PLoS Genet. 2013;9:e1004018. doi: 10.1371/journal.pgen.1004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Eskeland R., Freyer E., Leeb M., Wutz A., Bickmore W.A. Histone acetylation and the maintenance of chromatin compaction by Polycomb repressive complexes. Cold Spring Harb. Symp. Quant. Biol. 2010;75:71–78. doi: 10.1101/sqb.2010.75.053. [DOI] [PubMed] [Google Scholar]
- 191.De Kumar B., Krumlauf R. HOXs and lincRNAs: Two sides of the same coin. Sci. Adv. 2016;2:e1501402. doi: 10.1126/sciadv.1501402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Statello L., Guo C.J., Chen L.L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Deng C., Li Y., Zhou L., Cho J., Patel B., Terada N., Li Y., Bungert J., Qiu Y., Huang S. HoxBlinc RNA Recruits Set1/MLL Complexes to Activate Hox Gene Expression Patterns and Mesoderm Lineage Development. Cell Rep. 2016;14:103–114. doi: 10.1016/j.celrep.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gentile C., Kmita M. The remote transcriptional control of Hox genes. Int. J. Dev. Biol. 2018;62:685–692. doi: 10.1387/ijdb.180198mk. [DOI] [PubMed] [Google Scholar]
- 195.Parker H.J., Bronner M.E., Krumlauf R. The vertebrate Hox gene regulatory network for hindbrain segmentation: Evolution and diversification: Coupling of a Hox gene regulatory network to hindbrain segmentation is an ancient trait originating at the base of vertebrates. Bioessays. 2016;38:526–538. doi: 10.1002/bies.201600010. [DOI] [PubMed] [Google Scholar]
- 196.Parker H.J., Krumlauf R. A Hox gene regulatory network for hindbrain segmentation. Curr. Top. Dev. Biol. 2020;139:169–203. doi: 10.1016/bs.ctdb.2020.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and results referred to in this review have been previously published and cited with the appropriate reference.