Abstract
This study analyzed the effects of male age and abstinence time on semen quality and explored the best abstinence time for Chinese males among different age groups. Semen parameters, including sperm kinetics, morphology, and DNA fragmentation index (DFI), were reviewed from 2952 men. Samples were divided into six age groups (≤25 years, 26–30 years, 31–35 years, 36–40 years, 41–45 years, and >45 years) and were divided into six groups according to different abstinence time (2 days, 3 days, 4 days, 5 days, 6 days, and 7 days). The differences in semen quality between the groups were compared, and the effect of age and abstinence time on semen quality was analyzed. Significant differences were observed in semen volume, progressive motility (PR), and DFI among the age groups (all P < 0.05), and no significant differences were observed in sperm morphological parameters (all P > 0.05). There were significant differences in semen volume, PR, and DFI among different abstinence time groups (all P < 0.05) and no significant differences in sperm morphological parameters (all P > 0.05). Pearson analysis showed that male age and abstinence time were both significantly correlated with sperm kinetics and DFI (both P < 0.05), while no significant correlation was found with sperm morphological parameters (all P > 0.05). The box plots and histograms of men's age, abstinence time, and semen quality show that most semen quality parameters differ significantly between the 2 days and 7 days abstinence groups and other groups at different ages. Except for the sperm morphology parameters, sperm kinetic parameters and sperm DFI are linearly related to male age and abstinence time.
Keywords: abstinence time, age, DNA fragmentation index, sperm kinetics, sperm morphology
INTRODUCTION
Although couples are allowed to have three children, China is still struggling with a series of problems, such as population aging, declining birth rate, and the decline of male semen quality.1 How to improve male fertility has become an urgent problem. Studies have shown that male fertility levels have shown a downward trend worldwide and decrease with advancing age.2,3,4 The current male fertility assessments are mainly based on the Human Semen Examination and Processing Laboratory Manual, published by the World Health Organization (WHO). According to the manual, males are required to stay celibate for 2–7 days before having a semen test.5 On the contrary, some studies have shown that abstinence of less than one day or even continuous ejaculation is beneficial to obtain higher-quality sperm, leading to the increase of the pregnancy rate of intrauterine insemination (IUI).6,7 The article focused on evaluating the effect of male age and abstinence time on sperm quality by analyzing a large sample size, with the aim to explore the best abstinence time for different age groups.
PARTICIPANTS AND METHODS
Study population
Semen parameters (sperm kinetics, morphology, and DNA fragmentation index [DFI] parameters) from 2952 men who visited Fujian Maternity and Child Health Hospital (Fuzhou, China) from June 1, 2018, to July 31, 2020, were examined for effects of male age and abstinence time on semen quality, where the kinetic parameters include semen volume, sperm concentration, sperm progressive motility percentage (PR), and sperm non-progressive motility percentage (NP). The morphological parameters include normal morphology rate of sperm, deformity rate of the sperm head, deformity rate of the sperm neck and middle region, and malformation rate of the sperm main segment. Samples without all three test results were not involved in this study. This study was approved by the Ethics Committee of Fujian Provincial Maternity and Children Hospital (Approval No. YCXM2019-105).
Semen collection
The semen samples were collected by masturbation, abstinence for 2–7 days accordingly. The semen volume was measured using the weighing method,5 the sample was then stored at 35°C, and the degree of liquefaction was observed every 10 min. After liquidation, the semen quality test was performed by two skilled technicians who had passed the internal (quality control products are provided by Shanghai Beion Medical Technology Co., Ltd., Shanghai, China) and external (the Human Sperm Bank Technology Training Base of China Health and Family Planning Commission, Guangzhou, China) quality control assessment standards. The semen volume (ml) = weight (g)/density (g ml−1).
For age-factor assessment, the study participants were divided into six groups according to the age: ≤25 years, 26–30 years, 31–35 years, 36–40 years, 41–45 years, and >45 years. According to the length of abstinence time (2 days to 7 days), the subjects were also divided into six groups.
Sperm analysis
Sperm concentration was assessed by adding a diluted fixative (NaHCO3 + formaldehyde) to the semen samples, followed by manual counting with a Makler counting chamber (Sefi Medical Instruments, Haifa, Israel). Details are as follows: (1) mix the semen and diluent at a ratio of 1:5 (1 + 4); (2) pipette 10 ul of semen into the Makler counting chamber and cover it with a cover glass; and (3) count the sperm in a strip of 10 squares in Makler, after 5 min of incubation. Concentration = sperm number in 10 squares × 5 × 106 ml−1.
Semen samples with high concentrations were diluted with phosphate-buffered saline (PBS) before the kinetic test. After that, 10 ul of semen was pipetted onto the glass slide, covered with the cover glass, and let stand for 1 min. Under the microscope (Zeiss Primo Star, Oberkochen, Germany), at least 200 sperms (at least 400 sperms for low sperm count samples) for at least 5 random examination views were assessed, calculating the level of movement of each sperm (PR stands for forward-moving sperm, NP stands for nonforward-moving sperm, and IM stands for immobile sperm). PR = total PR/total sperm; NP = total NP/total sperm.
Diff-Quik staining kit (Anke Biotechnology Co., Ltd., Hefei, China) was used for cytological staining. In accordance with the reagent instructions, more than 200 sperms were observed via microscope. The rate of normal morphology, the rate of head deformity, the rate of the neck and mid-segment deformity, and the rate of main-segment deformity were all evaluated. The relevant evaluation standards for the morphology analysis of each part of the sperm refer to the fifth edition of the World Health Organization standard.5
Sperm chromatin diffusion8,9 was used for sperm staining (Anke Biotechnology Co., Ltd.). More than 500 sperms were observed and evaluated under the microscope. The DFI of the sperm was calculated (DFI = the number of sperm without a halo and with a small halo/the total number of observed sperm × 100%).
Statistical analyses
Statistical analysis was performed by SPSS 26.0 (IBM, Armonk, NY, USA). The measurement data were represented as mean ± standard deviation (s.d.), and the one-way analysis of variance analysis (ANOVA-LSD) was used to determine the significant difference of means among the groups. Data were controlled for parametric by Kolmogorov–Smirnov test. The effect of age and abstinence time on sperm quality was analyzed using the Pearson test, and P < 0.05 indicates a significant difference.
RESULTS
The statistical results regarding semen parameters
The age (mean ± s.d.) of 2952 patients was 32.3 ± 5.0 years, the abstinence time was 4.2 ± 1.2 days, the semen volume was 3.3 ± 1.3 ml, the sperm concentration was 45.3 × 106 ± 29.3 × 106 ml−1, the sperm PR was 38.3% ± 16.5%, the normal morphology rate was 7.5% ± 4.3%, and the sperm DFI was 14.0% ± 8.0%. The results are within the normal reference range recommended by the WHO guidelines.5
Effect of age on sperm quality
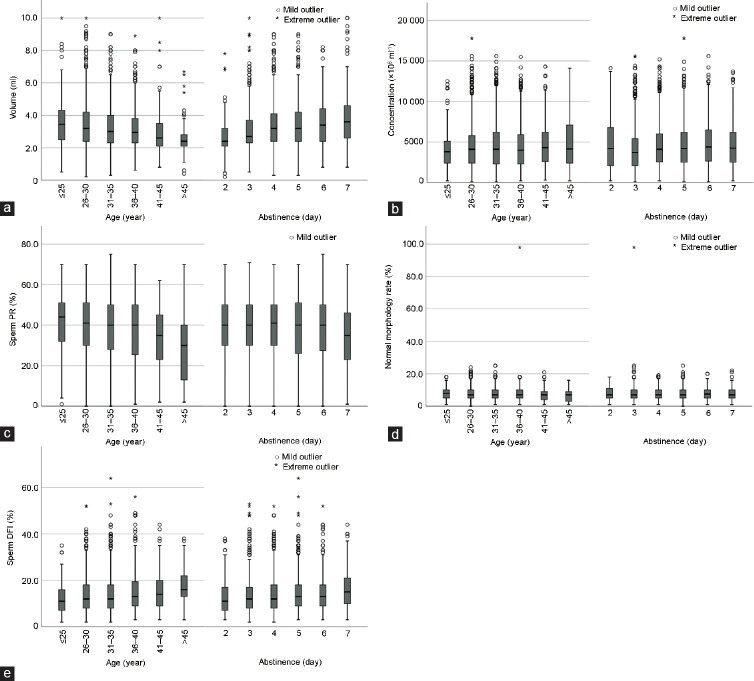
There were statistical differences in semen volume, sperm PR, and sperm DFI among the groups (all P < 0.05). The negative correlations have been found among volume, PR of sperm, and age. The positive correlations have been found between DFI of sperm and age. There were no statistically significant differences in sperm concentration, sperm NP, and sperm morphological parameters among the groups (all P > 0.05; Table 1 and Figure 1).
Table 1.
Comparison of sperm quality in different age (n=2952)
| Age (year) | Participant (n) | Volume (ml), mean±s.d. | Sperm concentration (×106 ml-1), mean±s.d. | Sperm PR (%), mean±s.d. | Sperm NP (%), mean±s.d. | Normal form rate (%), mean±s.d. | Head deformity rate (%), mean±s.d. | Middle section deformity rate (%), mean±s.d. | Primary segment deformity rate (%), mean±s.d. | Sperm DFI (%), mean±s.d. |
|---|---|---|---|---|---|---|---|---|---|---|
| ≤25 | 138 | 3.6±1.5 | 40.9±24.9 | 41.2±15.7 | 4.6±1.8 | 7.7±3.6 | 89.6±5.2 | 18.9±5.7 | 6.5±4.0 | 12.3±6.4 |
| 26–30 | 1074 | 3.4±1.4 | 44.5±28.6 | 39.7±16.1 | 4.8±1.9 | 7.6±4.0 | 89.8±5.6 | 19.1±6.2 | 6.8±4.3 | 13.4±7.5 |
| 31–35 | 1079 | 3.2±1.3 | 46.2±30.1 | 38.4±16.6 | 4.9±1.9 | 7.6±4.0 | 89.7±5.7 | 19.2±8.5 | 6.8±4.3 | 13.8±8.0 |
| 36–40 | 440 | 3.2±1.3 | 45.0±29.4 | 36.8±16.9 | 4.7±2.5 | 7.6±5.8 | 90.0±6.4 | 20.3±11.3 | 7.2±4.6 | 15.5±9.1 |
| 41–45 | 171 | 2.9±1.3 | 48.7±30.3 | 33.7±15.6 | 4.7±1.8 | 7.1±3.8 | 90.4±5.7 | 20.2±5.9 | 7.1±4.5 | 15.5±8.0 |
| >45 | 50 | 2.6±1.3 | 48.7±32.6 | 28.5±17.3 | 4.6±1.6 | 6.8±4.1 | 90.6±5.6 | 20.0±5.5 | 7.6±4.6 | 17.4±8.4 |
| F-distribution | - | 11.490 | 1.574 | 9.389 | 0.886 | 0.682 | 0.804 | 2.043 | 0.963 | 8.276 |
| P | - | <0.001 | 0.164 | <0.001 | 0.490 | 0.637 | 0.547 | 0.070 | 0.439 | <0.001 |
s.d.: standard deviation; PR: progressive motility percentage; NP: non-progressive motility percentage; -: no data
Figure 1.
The relationship between the sperm quality and age and abstinence time. (a) The relationship between the semen volume and age and abstinence time. (b) The relationship between the sperm concentration and age and abstinence time. (c) The relationship between the sperm PR and age and abstinence time. (d) The relationship between the normal sperm morphology and age and abstinence time. (e) The relationship between the sperm DFI and age and abstinence time. PR: progressive motility percentage; DFI: DNA fragmentation index.
Effect of the abstinence period on sperm quality
The semen volume, sperm concentration, sperm PR, and sperm DFI were statistically different among the groups (all P < 0.05). Both semen volume and sperm DFI showed an upward trend with the increase of abstinence time. There were no statistically significant differences in sperm NP and sperm morphological parameters among the groups (all P > 0.05; Table 2 and Figure 1).
Table 2.
Comparison of sperm quality in different abstinence time (n=2952)
| Abstinence time (day) | Participat (n) | Volume (ml), mean±s.d. | Sperm concentration (×106 ml-1), mean±s.d. | Sperm PR (%), mean±s.d. | Sperm NP (%), mean±s.d. | Normal form rate (%), mean±s.d. | Head deformity rate (%), mean±s.d. | Middle section deformity rate (%), mean±s.d. | Primary segment deformity rate (%), mean±s.d. | Sperm DFI (%), mean±s.d. |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 157 | 2.6±1.1 | 48.6±33.7 | 37.7±16.9 | 4.8±2.1 | 7.6±4.1 | 89.3±6.2 | 18.8±6.8 | 6.7±4.1 | 12.9±7.9 |
| 3 | 810 | 3.0±1.2 | 41.2±28.1 | 38.6±16.3 | 4.9±1.8 | 7.6±5.1 | 89.7±6.1 | 19.0±9.0 | 6.6±4.3 | 13.3±7.7 |
| 4 | 836 | 3.3±1.3 | 45.5±28.0 | 39.5±16.5 | 4.8±1.9 | 7.6±3.8 | 89.8±5.5 | 19.7±9.1 | 6.9±4.3 | 13.6±7.8 |
| 5 | 689 | 3.4±1.3 | 47.3±29.8 | 37.9±17.0 | 4.7±2.4 | 7.5±4.0 | 90.0±5.7 | 19.2±6.3 | 7.0±4.4 | 14.7±8.2 |
| 6 | 256 | 3.5±1.4 | 49.1±31.1 | 37.9±16.2 | 4.7±1.8 | 7.7±4.1 | 89.6±5.8 | 19.4±6.4 | 6.9±4.5 | 15.1±8.3 |
| 7 | 204 | 3.8±1.7 | 46.7±30.0 | 34.7±15.7 | 4.8±1.8 | 7.1±3.9 | 90.4±5.4 | 20.1±5.9 | 7.5±4.5 | 16.1±8.1 |
| F-distribution | - | 23.572 | 5.163 | 2.943 | 0.731 | 0.633 | 0.878 | 1.128 | 1.907 | 7.152 |
| P | - | <0.001 | <0.001 | 0.012 | 0.600 | 0.675 | 0.495 | 0.343 | 0.090 | <0.001 |
s.d.: standard deviation; PR: progressive motility percentage; NP: non-progressive motility percentage; -: no data
Correlations among age, abstinence time, and sperm quality
There were significantly positive correlations between paternal ages and sperm concentrations, sperm neck and mid-segment deformity rate, sperm primary segment deformity rate, and sperm DFI (all P < 0.05). A significant negative correlation was observed among paternal age and semen volume and sperm PR (both P < 0.05). There were no correlations between male ages and sperm NP and sperm morphological parameters (all P > 0.05). There was a strongly positive correlation among abstinence time and semen volume, sperm concentration, sperm malformation rate, and sperm DFI (all P < 0.05). Moreover, sperm PR was inversely proportional to sperm NP (P < 0.05). However, there was no correlation among the normal sperm morphology rate, the sperm head deformity rate, and the sperm neck and middle deformity rate (all P > 0.05). The volume of semen is positively correlated with male age and abstinence time. The sperm DNA integrity is significantly negatively correlated with male age and abstinence time. In addition, there was a significant correlation between sperm DFI and sperm kinetics and morphological parameters (all P < 0.05; Table 3 and Figure 1).
Table 3.
Correlation among age, abtinence time, and sperm quality (n=2952)
| Sperm parameter | Value (mean±s.d.) | Abstinence (day)a | Age (year)b | Sperm DFI (%)c | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| r | P | r | P | r | P | ||
| Volume (ml) | 3.3±1.3 | 0.184 | <0.001 | −0.136 | <0.001 | −0.001 | 0.952 |
| Sperm concentration (×106 ml−1) | 45.3±29.3 | 0.058 | 0.002 | 0.042 | 0.023 | −0.271 | <0.001 |
| Sperm PR (%) | 38.3±16.5 | −0.043 | 0.019 | −0.124 | <0.001 | −0.646 | <0.001 |
| Sperm NP (%) | 4.8±2.0 | −0.022 | 0.225 | −0.007 | 0.698 | −0.162 | <0.001 |
| Normal form rate (%) | 7.5±4.3 | −0.018 | 0.324 | −0.030 | 0.102 | −0.433 | <0.001 |
| Head deformity rate (%) | 89.8±5.7 | 0.027 | 0.150 | 0.031 | 0.091 | 0.472 | <0.001 |
| Middle deformity rate (%) | 19.4±8.0 | 0.027 | 0.137 | 0.045 | 0.015 | 0.299 | <0.001 |
| Primary deformity rate (%) | 6.9±4.4 | 0.050 | 0.007 | 0.041 | 0.025 | 0.405 | <0.001 |
| Sperm DFI (%) | 14.0±8.0 | 0.107 | <0.001 | 0.108 | <0.001 | 1.000 | 1.000 |
aThe mean±s.d. of total patients’ abstinence time is 4.2±1.2 days; bthe mean±s.d. of total patients’ age is 32.3±5.0 years; cthe mean±s.d. of total patients’ sperm DFI is 14.0%±8.0%. PR: progressive motility percentage; NP: non-progressive motility percentage; DFI: DNA fragmentation index; s.d.: standard deviation
The best abstinence time for different age groups
The sperm quality parameters of men of different ages are not entirely the same under different periods of abstinence. The semen volume and sperm DFI of men aged 26 years to 40 years increased with abstinence time. Men of all ages had the smallest semen volume and sperm DFI value for 2 days of abstinence. The rate of normal sperm morphology of men aged ≤35 years is less affected by the duration of abstinence, and the rate of normal sperm morphology of men aged >45 years of abstinence for 6–7 days is significantly higher. Men of all ages had the lowest sperm PR at 7 days of abstinence (Figure 2).
Figure 2.
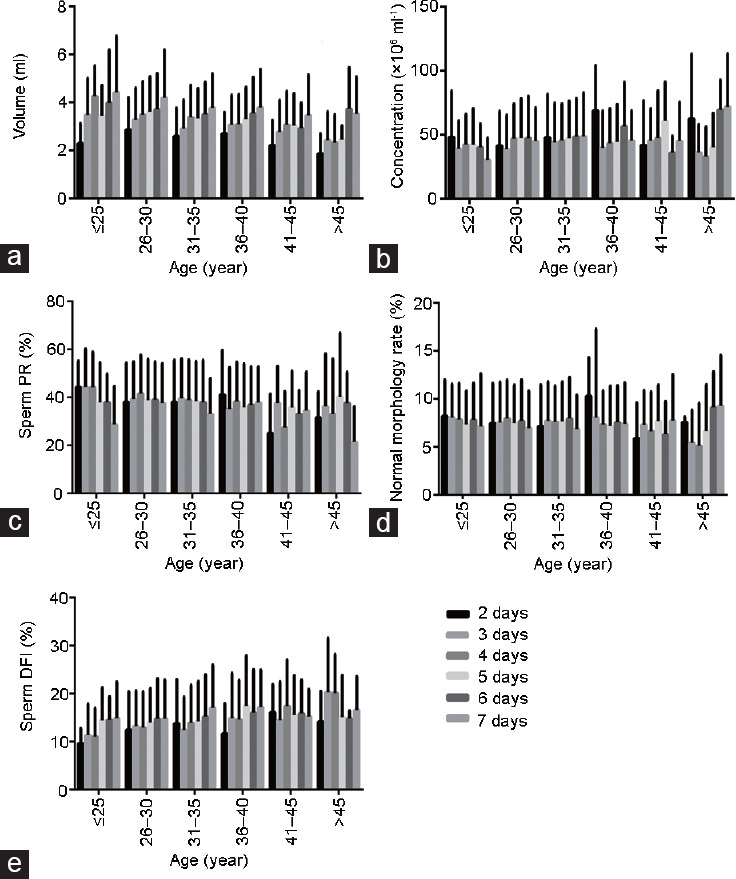
The best abstinence time for different age groups. (a) The effect of abstinence time in different age groups on semen volume. (b) The effect of abstinence time in different age groups on semen concentration. (c) The effect of abstinence time in different age groups on sperm PR. (d) The effect of abstinence time in different age groups on normal sperm morphology. (e) The effect of abstinence time in different age groups on sperm DFI. PR: progressive motility percentage; DFI: DNA fragmentation index.
DISCUSSION
Semen analysis is the cornerstone for the assessment of the male partner in a subfertile couple to assess testicular function and patency of the seminal ducts. Before semen analysis, it is important to administer a questionnaire with important questions to the patients to ensure the accuracy of the test. In particular, before semen testing, it is necessary to know the age of the man, the duration of abstinence, the method of semen collection, the integrity of the semen collection sample, and the mental state of the patient. These parameters can help us better understand the patient's condition. At present, the semen evaluation operation manual published by the WHO is used in many countries as the reference standard to evaluate semen quality. However, some studies have shown that sperm quality decreases with the increase of male age.10,11,12 This article further elaborates on this research.
Regarding the relationship between male age and sperm quality, our results showed that, with the increase of age, semen volume, and sperm concentration, and motility showed a downward trend, while sperm DFI showed an upward trend. This may be related to a possible decline in testosterone levels. There was little correlation between male age and sperm morphology. The above results are roughly the same as related reports.11,12 Regarding the relationship between male abstinence time and sperm quality, our results showed that, with the increase of abstinence time, semen volume and sperm DFI showed an upward trend. Our study showed that abstinence time had small correlations with sperm kinetics and morphology, but there were significant positive correlations with semen volume, concentration, and DFI. In particular, the integrity of sperm DNA showed significant linear changes with age and abstinence time (Figure 1). Our findings are basically consistent with some reported results13,14,15 but differ from others.16
Regarding the analysis of the optimal abstinence time, our results show that the semen quality parameters of men of different age groups under different abstinence time are not entirely the same (Figure 2). For example, statistical analysis shows that the sperm quality parameters in the age group <35 years are the best after 3–4 days of abstinence, while ages >36 years have better sperm quality parameters after 5–6 days of abstinence. Furthermore, the sperm quality parameters of the 2-day and 7-day abstinence groups were significantly different from those of other groups, and some of those differences were statistically significant (P < 0.05; Table 2).
In recent years, DFI has been used as a new parameter standard for evaluating sperm quality. Its diagnostic value for assessing male fertility is higher than traditional semen analysis methods.17 The increase in sperm DFI is significantly correlated with male infertility, recurrent miscarriage, genetic diseases, fetal malformations, and assisted reproductive technology pregnancy rates.18 This study found that, when analyzing the effects of male age and abstinence time on sperm quality only from the perspective of sperm kinetics and morphology, only a few of the parameters are correlated among the three parameters. The sperm morphology parameters are less relevant to age and abstinence time. However, after including DFI in the scope of the study, it is evident that there are significant correlations between male age and abstinence time and sperm DNA integrity (all P < 0.05). This result illustrates the importance of sperm DFI detection and also confirms the accuracy of previous research results.17 The DNA integrity in the sperm nucleus decreases with age, and DFI increases with the increase in abstinence time. The reason for this is that, in sperm formation, the histones in the nucleus are converted into protamine. The chromatin is highly folded under the action of protamine to form a stable and complete DNA double-strand. The conversion of histones to protamine mediates the folding and concentration of genetic material, which is regulated by various mechanisms. As men age, sperm DNA, and histones are more likely to undergo epigenetic changes, such as methylation and acetylation, DFI increases.19 With an increase in abstinence time, reactive oxygen species (ROS) in the testes and epididymis increase, then excessive ROS will cause the DNA chain of sperm to break, and DFI will increase. At the same time, with the increase of age and abstinence time, the abnormal sperm apoptosis caused by the external environment can also cause the increase of DFI.18,20
Sperm are produced in the seminiferous tubules of the testis, and the produced sperm are stored in the epididymis. Sperm production is affected by many factors, including genetic factors (such as Y chromosome microdeletion), endocrine factors (such as testosterone levels), immune factors (such as interleukin and tumor necrosis factor), and external environment (such as temperature, radiation, reactive oxygen species, sexual function, and varicocele).21,22 The reason male age affects sperm quality is that the increase of male age leads to the decline of physiological function, the decline of sexual function, the decline of androgen levels in the body, and the decline of immunity. Aging of the body and an increase in abstinence lead to the appearance of chromatin methylation and other changes in epigenetic factors. These changes lead to the mismatch of genetic material during sperm production, the abnormal structure of microfilament and microtubule, and the abnormal adenosine triphosphate (ATP) level in mitochondria. As abstinence time increases, the damage caused by the external environment to the sperm in the epididymis also increases. The increases in age and abstinence time eventually lead to changes in the kinetics, morphology, and DNA integrity of sperm.
This research is a retrospective study in which the single-factor data analysis was involved. Other factors that affect semen quality (such as occupational factors, living habits, body mass index, and female factors) were not included in the scope of the study. The sperm morphology count is only based on 200 sperms, and sperm kinetic analysis uses the Makler counting chamber instead of the Neubauer counting chamber recommended by the WHO guideline. This may lead to some inherent deviations in the results of these studies. Nevertheless, the conclusions of this study are in good agreement with the results of previous studies. The amount of data in this study is large, and the analysis of the results is reasonable and reliable. Studying the relationships between age, abstinence time, and semen quality and summarizing the best abstinence time for men of different ages will help establish relevant reference standards suitable for males. It will help reflect the actual situation of male fertility and help diagnose and treat male diseases.
AUTHOR CONTRIBUTIONS
GXC designed the research, performed the research, analyzed the data, and wrote the manuscript. HYL and YHL collected the data. HS, LY, YBF, ZQH, PYH, and LCD searched the literature. BHZ finished language editing, project designed and guided the whole process. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by funding from the Fujian Provincial Maternity and Children Hospital (No. YCXM19-29), the Fujian Natural Science Foundation (No. 2019J01511 and No. 2020Y0067), and the Health Research Project of the Department of Finance (Fujian finance refers to [2019] No. 827).
REFERENCES
- 1.Li HT, Xue M, Hellerstein S, Cai Y, Gao Y, et al. Association of China's universal two child policy with changes in births and birth related health factors: national, descriptive comparative study. BMJ. 2019;366:l4680. doi: 10.1136/bmj.l4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng JB, Liang QF, Li JH, Zhang SC, Yu XH, et al. Longitudinal trends of AMS and IIEF-5 scores in randomly-selected community men 40 to 80 years old: preliminary results. J Sex Med. 2019;16:1567–73. doi: 10.1016/j.jsxm.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Cooper TG, Noonan E, Von Eckardstein S, Auger J, Gordon Baker HW, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Odoom A, Brown CA, Adjei DN. Level of male infertility in the Ghanaian city of Tema. J Obstet Gynaecol. 2015;35:825–8. doi: 10.3109/01443615.2015.1009876. [DOI] [PubMed] [Google Scholar]
- 5.WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: WHO; 2010. pp. 49–98. [Google Scholar]
- 6.Bahadur G, Almossawi O, Zeirideen ZR, Ilahibuccus A, Al-Habib A, et al. Semen characteristics in consecutive ejaculates with short abstinence in subfertile males. Reprod Biomed Online. 2016;32:323–8. doi: 10.1016/j.rbmo.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Bahadur G, Homburg R, Bosmans JE, Huirne , JA , Hinstridge P, et al. Observational retrospective study of UK national success, risks and costs for 319,105 IVF/ICSI and 30,669 IUI treatment cycles. BMJ Open. 2020;16:e03456. doi: 10.1136/bmjopen-2019-034566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Sun J, Wang L, Gao X, Lu X, et al. Assessment of density gradient centrifugation (DGC) and sperm chromatin dispersion (SCD) measurements in couples with male factor infertility undergoing ICSI. J Assist Reprod Genet. 2014;31:1655–63. doi: 10.1007/s10815-014-0339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez JL, Muriel L, Goyanes V, Segrelles E, Gosalvez J, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84:833–42. doi: 10.1016/j.fertnstert.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 10.Kidd SA, Eskenazi B, Andrew J. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237–48. doi: 10.1016/s0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 11.Stone BA, Alex A, Werlin LB, Marrs RP. Age thresholds for changes in semen parameters in men. Fertil Steril. 2013;100:952–8. doi: 10.1016/j.fertnstert.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 12.Li WN, Jia MM, Peng YQ, Ding R, Fan LQ, et al. Semen quality pattern and age threshold: a retrospective cross-sectional study of 71,623 infertile men in China, between 2011 and 2017. Reprod Biol Endocrinol. 2019;17:107–15. doi: 10.1186/s12958-019-0551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorostghoal M, Kazeminejad SR, Shahbazian N, Pourmehdi M, Jabbari A. Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia. 2017;49:1–9. doi: 10.1111/and.12762. [DOI] [PubMed] [Google Scholar]
- 14.Sørensen MB, Bergdahl IA, Hjøllund NH, Bonde JP, Stoltenberg M. Zinc, magnesium and calcium in human seminal fluid: relations to other semen parameters and fertility. Mol Hum Reprod. 1999;5:331–7. doi: 10.1093/molehr/5.4.331. [DOI] [PubMed] [Google Scholar]
- 15.Lipshultz LT, Howards SS, Niederberger CS. Infeitility in the Male. Lambridge: Cambridge University Press; 2009. pp. 117–8. [Google Scholar]
- 16.Keihani S, Craig JR, Zhang C, Presson AP, Myers JB, et al. Impacts of abstinence time on semen parameters in a large population-based cohort of subfertile men. Urology. 2017;108:90–5. doi: 10.1016/j.urology.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 17.Wiweko B, Utami P. Predictive value of sperm deoxyribonucleic acid (DNA) fragmentation index in male infertility. Basic Clin Androl. 2017;27:1–7. doi: 10.1186/s12610-016-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panner Selvam MK, Agarwal A. A systematic review on sperm DNA fragmentation in male factor infertility: laboratory assessment. Arab J Urol. 2018;16:65–76. doi: 10.1016/j.aju.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaughan DA, Tirado E, Garcia D, Datta V, Sakkas D. DNA fragmentation of sperm: a radical examination of the contribution of oxidative stress and age in 16945 semen samples. Hum Reprod. 2020;35:2188–96. doi: 10.1093/humrep/deaa159. [DOI] [PubMed] [Google Scholar]
- 20.Zandieh Z, Vatannejad A, Doosti M, Zabihzadeh S, Haddadi M, et al. Comparing reactive oxygen species and DNA fragmentation in semen samples of unexplained infertile and healthy fertile men. Ir J Med Sci. 2018;187:657–62. doi: 10.1007/s11845-017-1708-7. [DOI] [PubMed] [Google Scholar]
- 21.Kretser DM, Loveland KL, Meinhardt A, Simorangkir D, Wreford N. Spermatogenesis. Hum Reprod. 1998;13:1–8. doi: 10.1093/humrep/13.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 22.Shehzad B. Male hypogonadism. Lancet. 2014;383:1250–63. doi: 10.1016/S0140-6736(13)61126-5. [DOI] [PubMed] [Google Scholar]