ABSTRACT
A variety of eubacteria, plants, and protozoa can modify membrane lipids by cyclopropanation, which is reported to modulate membrane permeability and fluidity. The ability to cyclopropanate membrane lipids has been associated with resistance to oxidative stress in Mycobacterium tuberculosis, organic solvent stress in Escherichia coli, and acid stress in E. coli and Salmonella. In bacteria, the cfa gene encoding cyclopropane fatty acid (CFA) synthase is induced during the stationary phase of growth. In the present study, we constructed a cfa mutant of Salmonella enterica serovar Typhimurium 14028s (S. Typhimurium) and determined the contribution of CFA-modified lipids to stress resistance and virulence in mice. Cyclopropane fatty acid content was quantified in wild-type and cfa mutant S. Typhimurium. CFA levels in the cfa mutant were greatly reduced compared to CFA levels in the wild type, indicating that CFA synthase is the major enzyme responsible for cyclopropane modification of lipids in Salmonella. S. Typhimurium cfa mutants were more sensitive to extreme acid pH, the protonophore CCCP, and hydrogen peroxide compared to the wild type. In addition, cfa mutants exhibited reduced viability in murine macrophages and could be rescued by the addition of the NADPH phagocyte oxidase inhibitor diphenyleneiodonium (DPI) chloride. S. Typhimurium lacking cfa was also attenuated for virulence in mice. These observations indicate that CFA modification of lipids makes an important contribution to Salmonella virulence.
KEYWORDS: cyclopropane fatty acid, Salmonella, virulence
INTRODUCTION
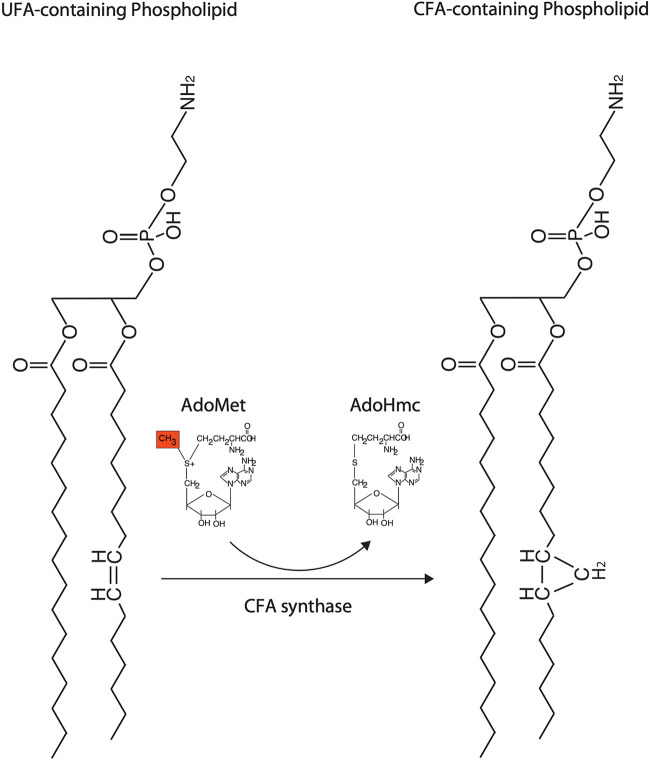
Cyclopropanation of unsaturated fatty acids has been described in a variety of eubacteria, plants, and protozoa (reviewed in references 1 and 2). An S-adenosylmethionine-dependent methyltransferase called cyclopropane fatty acid (CFA) synthase (encoded by the cfa gene) mediates the formation of cyclopropane rings in lipid bilayers (2, 3). The reaction catalyzed by CFA synthase requires S-adenosylmethione (SAM or AdoMet) to donate a methylene group to the double bond of a membrane unsaturated fatty acid (UFA), creating a cyclopropane ring on the alkyl chain of the membrane phospholipid (Fig. 1) (2). Although cyclopropanated fatty acids in bacteria were discovered more than 50 years ago, their role in acid stress resistance and bacterial virulence has been established relatively recently (4–12).
FIG 1.
Biosynthesis of cyclopropane fatty acids. Phospholipids in the membrane serve as substrates for the reaction. A methylene group from S-adenosylmethione (AdoMet) is transferred to the double bond of an unsaturated fatty acid in the phospholipid to create a cyclopropane ring on the alkyl chain. A cellular enzyme, S-adenosylmethionine-dependent methyltransferase, known as CFA synthase, catalyzes the reaction.
Bacteria can alter the composition of their membrane lipids in response to environmental stress conditions (reviewed in references 13 and 14). Lipopolysaccharide modifications in the outer membranes of Gram-negative bacteria render them more resistant to antimicrobial peptides and to host innate immunity (reviewed in reference 15). Nutrient deprivation or changes in growth phase can also affect lipid composition and modification (16, 17). Bacteria can remodel phospholipid composition in membranes, as well as induce modifications in phospholipids, by desaturation, cis-trans isomerization, or cyclopropanation in response to stresses from heat, cold, osmolarity, pH, or organic solvent exposure (18–24).
Various growth and environmental conditions such as diverse carbon sources, pH, oxygenation, temperature, pressure, Mg2+ concentration, and exposure to acetate, organic solvents, terpenes, or fullerenes, can modulate CFA levels (21, 25–29). The expression of cfa is RpoS (σS)-dependent, which is consistent with the higher proportion of CFA-modified lipids in E. coli and Salmonella during stationary phase (8, 21, 30). The activity of CFA synthase during stationary phase is also controlled by RpoH (σH)-dependent protease activity (31). As a result of this additional level of regulation, the greatest quantities of CFA are observed during early stationary phase (31).
CFA membrane modification in bacteria has been associated with stress-related phenotypes. An E. coli cfa mutant exhibits reduced viability after exposure to ethanol, repeated freeze-thaw cycles, or extreme acidic conditions (5, 6, 11, 32). Some data suggest that E. coli survival in acidified minimal medium is partly due to the reduced membrane proton permeability which results from increased CFA levels (12). Both pathogenic and nonpathogenic strains of E. coli exhibit increased CFA in their cell membranes following acid adaptation, suggesting that CFA lipid modification is a conserved survival response (5).
The role of cyclopropanated lipids in other pathogenic bacteria has also been examined. At least five different S-adenosylmethionine-dependent methyltransferases able to cyclopropanate mycolic acids unique to mycobacteria have been identified (7, 33–36). Cyclopropanated mycolic acids are more widespread in pathogenic mycobacterial species (37). Cyclopropanation of mycolic acids by the S-adenosylmethionine-dependent methyltransferase Cma1 has been found to play a role in resistance to oxidative stress (36). Moreover, the cyclopropanation of mycolic acids can affect the virulence of M. tuberculosis in infection models (7, 9, 10, 34, 38). Recently, cyclopropane fatty acid synthase has been found to be required for acid resistance, macrophage survival, and gastric colonization in Helicobacter pylori (39).
This study investigated whether cfa-dependent lipid cyclopropanation is important for stress resistance in S. Typhimurium. We found that cfa mutant S. Typhimurium is more sensitive to oxidative and extreme acid stress compared to the wild type. Enhanced susceptibility to the protonophore CCCP (carbonyl cyanide 3-chlorophenylhydrazone) suggests that CFA-modified lipids may help to maintain proton motive force (PMF) during stationary phase. In addition, a cfa mutant strain exhibited reduced survival in cultured macrophages and attenuated virulence in mice, compared to the wild-type strain.
RESULTS
An S. Typhimurium cfa mutant has an altered fatty acid profile and is defective for survival at extreme acid pH.
To determine the effect of a cfa mutation on fatty acid composition, wild-type and isogenic cfa mutant S. Typhimurium ATCC14028s were grown in liquid culture to stationary phase, after which lipids were extracted and analyzed by gas chromatography/mass spectrometry (see Materials and Methods). The fatty acid composition of wild-type S. Typhimurium was comparable to previously published values (Table 1) (40). The total CFA levels in the S. Typhimurium cfa mutant were reduced to 1.1% of wild-type levels in stationary phase (Table 1). The CFA precursors 16:1 and 18:1 UFA were elevated 10- and 2.5-fold, respectively, in the cfa mutant, confirming the absence of CFA synthase activity (Table 1). S. Typhimurium ATCC14028s had elevated percent conversion values of 17:cyclopropane and 19:cyclopropane fatty acid (89.5% and 64.8%) compared to previously reported values for S. Typhimurium strain UK-1 (81.3 and 47.2) (Table 1) (8). The percent conversion of the sum of both types of CFA was approximately 15% higher in S. Typhimurium strain ATCC14028s compared to strain UK-1 (Table 1) (8).
TABLE 1.
Fatty acids of stationary-phase S. Typhimurium ATCC14028s wild-type and cfa mutant strains
| Fatty acida | No. of carbons:no. of double bonds | Wild type |
cfa mutant |
||
|---|---|---|---|---|---|
| Fatty acid leveld | Conversion of CFA (%)e | Fatty acid leveld | Conversion of CFA (%)e | ||
| SFA | 12:0 | 1.3 | 0.88 | ||
| SFA | 14:0 | 4.6 | 6.2 | ||
| SFA | 15:0 | 4.4 | 6.0 | ||
| SFA | 16:0 | 29.0 | 27.7 | ||
| UFA | 16:1b | 3.2 | 30.0 | ||
| SFA | 17:0 | 3.2 | 4.4 | ||
| CFA | 17:cyclopropane | 27.4 | 89.5 | 1.1 | 3.5 |
| SFA | 18:0 | 0.36 | 1.4 | ||
| UFA | 18:1c | 9.3 | 23.0 | ||
| CFA | 19:cyclopropane | 17.1 | 64.8 | 0 | 0 |
Saturated fatty acid (SFA), unsaturated fatty acid (UFA), cyclopropane fatty acid (CFA).
6:1 UFA precursor of 17:cyclopropane.
18:1, UFA precursor of 19:cyclopropane.
Fatty acid levels in %weight = ([peak area/sum of all peaks] × 100).
%Conversion of CFA = (%CFA/[%UFA + %CFA]/100).
A cfa mutant derivative of S. Typhimurium strain UK-1 was previously shown to be defective for survival at extreme acid pH (8). To confirm that a cfa mutation conferred a similar phenotype in strain 14028s, the mutant was tested for viability after acid stress. Strains grown in LB medium experienced lower survival compared to the wild type following exposure to pH 3, relative to cells grown in M9 minimal medium (compare Fig. 2A and B). In addition, the cfa mutants experienced higher mortality at pH 3 compared to the wild type (Fig. 2A and B). These observations confirm earlier findings that CFA contributes to the survival of S. Typhimurium 14028s during extreme acid stress.
FIG 2.
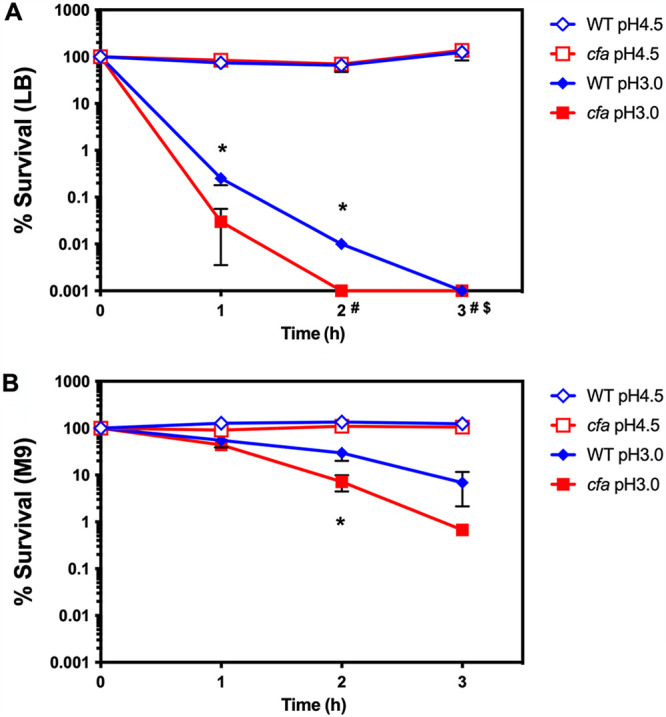
Cyclopropane fatty acids are required for survival at extreme acid pH. Stationary-phase S. Typhimurium strains were grown in either LB medium (A) or in M9 containing 0.4% glucose (B) and were diluted 1:1,000 into fresh media adjusted to pH 4.5 (wild-type, blue open diamonds; cfa mutant, red open squares) or pH 3.0 (wild-type, blue closed diamonds; cfa mutant, red closed squares). The CFU of surviving cells were determined at 0, 1, 2 and 3 h post-acid shock treatment. $The wild type and #cfa mutant were below the theoretical limit of detection at these time points. A Student’s t test was performed between wild type and mutant. The asterisk (*) indicates P < 0.05.
Cyclopropane fatty acids are required for Salmonella resistance to the protonophore CCCP.
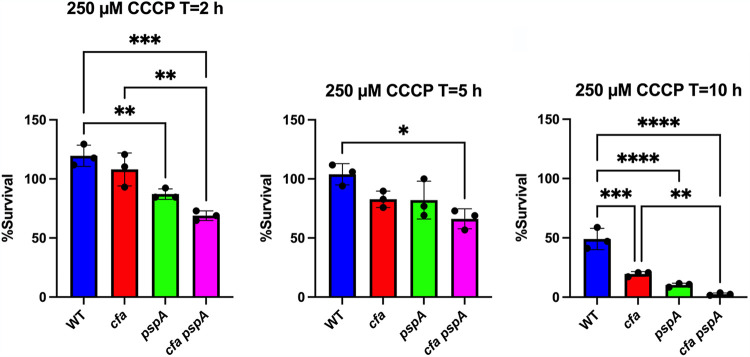
Environmental stresses such as extreme acid pH and nutrient deprivation can affect membrane potential Δψ (41, 42). As bacteria enter stationary phase, nutrients are exhausted and energy utilization diminishes, resulting in decreased proton motive force (PMF) (43). Levels of the phage shock protein PspA increase during stationary phase and play a role in PMF maintenance (43, 44). Because CFA synthesis increases during stationary phase, we sought to determine whether CFA membrane modification contributes to the maintenance of PMF. S. Typhimurium strains were treated with the protonophore CCCP (carbonyl cyanide 3-chlorophenylhydrazone), which disrupts PMF (46). Stationary-phase cells were treated with 250 μM CCCP, and viability was determined by plating of serial dilutions. A pspA mutant previously shown to be CCCP-sensitive was included as a positive control (Fig. 3) (44). The S. Typhimurium cfa mutant was found to be more sensitive to CCCP than the wild type (Fig. 3). Moreover, a cfa pspA double mutant was found to have increased sensitivity to CCCP when compared to a strain carrying a single cfa mutation (Fig. 3). These observations suggest that cyclopropane fatty acids play a role in the maintenance of PMF in Salmonella that is independent of the phage shock response.
FIG 3.
Cyclopropane fatty acids are needed for survival upon exposure to protonophore carbonyl cyanide 3-chlorophenylhydrazone (CCCP). Stationary-phase S. Typhimurium wild type (WT), cfa mutant, pspA mutant, and cfa pspA double mutant strains were grown in LB, diluted to 105 CFU per ml in LB with 250 μM CCCP, and incubated for 10 h at 37°C. CFU of surviving cells were determined at T = 2 h, T = 5 h, and T = 10 h. Means and standard deviations from three independent experiments are shown. A one-way ANOVA with Tukey’s multiple comparison test was used to compare WT and mutant strains at 2, 5, and 10 h. Asterisks indicate the P value as follows. (*) P = 0.0332, (**) P = 0.0021, (***) P = 0.0002, and (****) P < 0.0001.
Cyclopropane fatty acids are important for Salmonella resistance to oxidative stress.
The role of CFA in protection from oxidative stress has been previously demonstrated in Pseudomonas and Mycobacterium spp. Highly reactive singlet oxygen generated by fullerenes has been shown to increase CFA levels in P. putida (27). Overexpression of the M. tuberculosis CFA synthase homolog Cma1, an S-adenosylmethionine-dependent methyltransferase, confers increased resistance to hydrogen peroxide in M. smegmatis (36). However, whether CFA contributes to oxidative stress resistance in enteric bacteria has previously been unclear. An E. coli K-12 cfa mutant derivative exhibited wild-type resistance levels following exposure to singlet oxygen generated by illuminated eosin Y (32). To determine whether CFA contributes to oxidative stress resistance in S. Typhimurium, we monitored the growth of wild-type and isogenic cfa mutant strains in the presence of hydrogen peroxide (H2O2) (see Materials and Methods). A dps mutant strain previously shown to exhibit enhanced H2O2-susceptibility was included as a positive control (47). Treatment with 1 mM H2O2 prolonged the lag phase of wild-type S. Typhimurium by 5.5 h, and by 8.5 h in the isogenic cfa mutant (Fig. 4A). The dps mutant was more profoundly inhibited by 1 mM H2O2, with a growth delay of 15.5 h (Fig. 4A).
FIG 4.
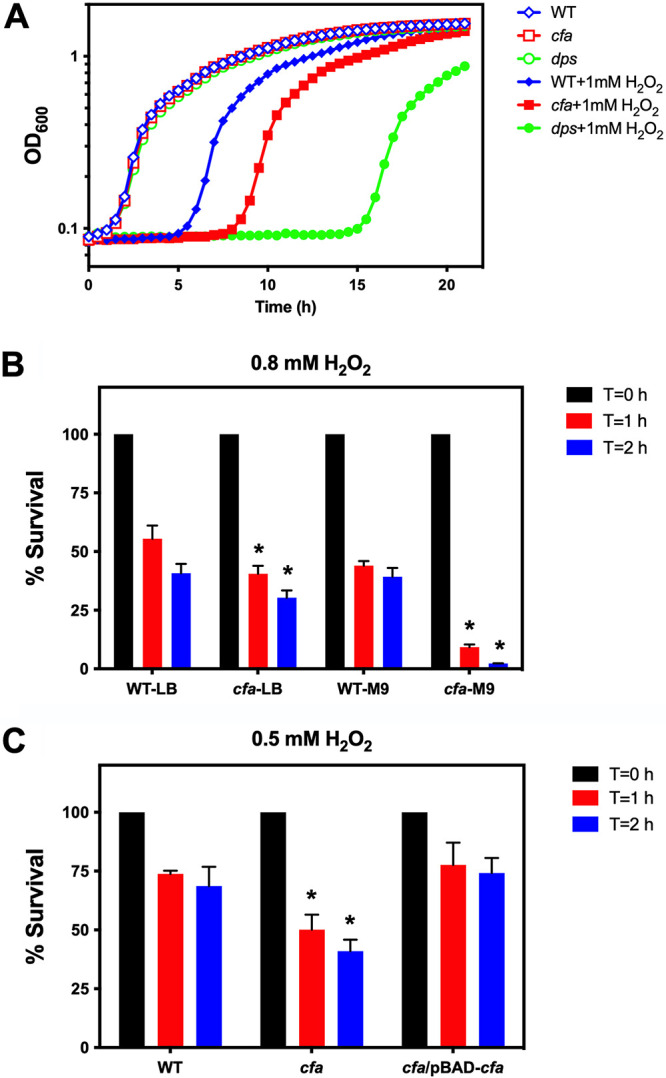
Cyclopropane fatty acids are important for are important for protection against hydrogen peroxide exposure. (A) S. Typhimurium strains that lack cyclopropane fatty acids are susceptible to growth inhibition by hydrogen peroxide. Stationary-phase S. Typhimurium strains were diluted to a final optical density (OD600) of 0.002 in LB medium (LB), and cell growth was monitored by OD600 at 37°C for 21 h (wild type, open blue diamonds; cfa mutant, open red squares; dps mutant, open green circles). Strains were also monitored for cell growth in LB medium with the addition of 1 mM hydrogen peroxide (H2O2) (wild-type, blue closed diamonds; cfa mutant, red closed squares; dps mutant, green closed circles). (B) Cyclopropane fatty acids are important for survival in hydrogen peroxide. Stationary-phase S. Typhimurium strains (wild-type and cfa mutant) were grown in LB medium or in M9 medium (M9) with 0.4% glucose and diluted in PBS, containing 0.8 mM H2O2, for 2 h at 37°C. CFU of surviving cells were determined post-H2O2 challenge at T = 0 (black bars), T = 1 h (red bars), and T = 2 h (blue bars). A Student’s t test was performed between the wild type and the cfa mutant at each time point. An asterisk (*) indicates P < 0.05. (C) The plasmid pBAD-cfa could complement a cfa mutant for survival in hydrogen peroxide. Stationary-phase S. Typhimurium strains (wild-type, cfa mutant, and cfa/pBAD-cfa) were grown in LB medium with 0.2% arabinose and incubated in PBS, containing 0.5 mM H2O2, for 2 h at 37°C. CFU of surviving cells were determined post-H2O2 challenge at T = 0 (black bars), T = 1 h (red bars) and T = 2 h (blue bars). A Student’s t test was performed between wild-type and cfa mutant or cfa/pBAD-cfa strains at each time point. An asterisk (*) indicates P < 0.05.
Stationary-phase wild-type and cfa mutant S. Typhimurium strains were also tested for survival after treatment with H2O2. Cells grown in LB were more resistant to H2O2 compared to strains grown in M9 minimal medium (Fig. 4B). The cfa mutant was more sensitive to H2O2 regardless of the growth medium used (Fig. 4B). The enhanced H2O2-susceptibility of a cfa mutant was restored to wild-type levels using a plasmid containing a copy of the cfa gene (Fig. 4C). Taken together, these observations demonstrate that CFA contributes to oxidative stress resistance in S. Typhimurium.
Cyclopropane fatty acids are required for Salmonella survival in macrophages and virulence in mice.
Previous studies have demonstrated the role of lipid cyclopropanation in M. tuberculosis virulence (7, 9, 10, 34, 38). Here, we determined if cyclopropane-modified fatty acids contribute to Salmonella pathogenesis. A cfa mutant S. Typhimurium strain was assayed for its ability to survive in murine macrophages. Murine sodium peroidate-elicted peritoneal macrophages were isolated from C3H/HeN (ItyR) mice and activated with IFN-γ prior to infection. The S. Typhimurium cfa mutant was more vulnerable to macrophages than the wild type at 2 and 4 h postinfection (Fig. 5). The macrophage defect of the cfa mutant could be repaired by treatment using DPI (diphenyleneiodinium) (Fig. 5) to inhibit both the Nox2 NADPH phagocyte oxidase and the Nos2 inducible nitric oxide synthase, which are required for the production of antimicrobial reactive oxygen and nitrogen species, respectively (48). S. Typhimurium strains were also tested for sensitivity to the cathelicidin antimicrobial peptide CRAMP, produced by murine macrophages (49). We observed no difference in resistance to CRAMP in cfa mutant S. Typhimurium compared to the wild type (Fig. S1). In addition, an S. Typhimurium cfa mutant survived as well as the wild type at a pH of 4.5, corresponding to the pH of an acidified macrophage vacuole (Fig. 2A and B). Collectively, these studies suggest that CFA promotes intra-macrophage survival by conferring resistance to reactive species generated by activated macrophages.
FIG 5.
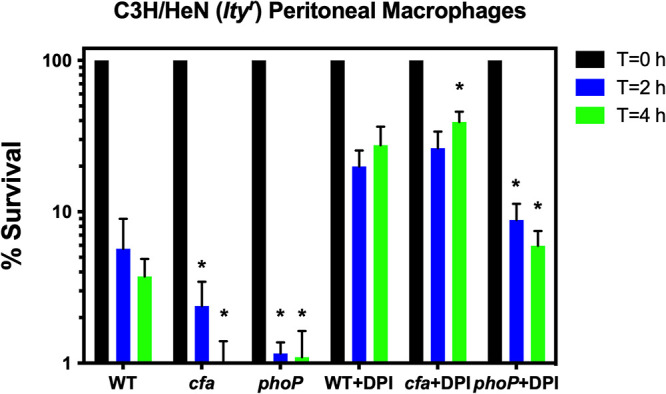
Cyclopropane fatty acids are important for survival in murine peritoneal macrophages. Peritoneal macrophages isolated from mice C3H/HeN (ItyR) were infected with an MOI of 10:1 with S. Typhimurium wild-type, cfa mutant, and phoP mutant strains, and the CFU were determined postinfection at T = 0 (black bars), T = 2 h (blue bars), and T = 4 h (green bars). In addition, peritoneal macrophages were treated with 25 μM diphenyleneiodonium (DPI) chloride to inhibit both NADPH phagocyte oxidase and inducible nitric oxide synthase prior to infection. A Student’s t test was performed between the wild-type, cfa mutant, and phoP mutant strains at T = 2 and T = 4 h within each group (with and without DPI). An asterisk (*) indicates P < 0.05.
Wild-type S. Typhimurium, an isogenic cfa mutant, and a complemented cfa mutant carrying a low-copy-number plasmid with cfa and its native promoter (see Materials and Methods) were assayed for virulence in C3H/HeN (ItyR) mice. Mice were infected intraperitoneally with 3,000 CFU and monitored daily, and moribund mice were euthanized. Comparisons of survival between the wild type, the cfa mutant, and the complemented cfa mutant revealed that the cfa mutant strain is detective for virulence in mice (Fig. 6A). Survival analysis by a Gehan-Breslow-Wilcoxon test showed significant differences between the cfa mutant and the complemented cfa mutant (P = 0.0463) while a trend toward reduced survival of mice infected with wild-type Salmonella compared to those infected with cfa mutant strains did not achieve statistical significance (P = 0.1085). A competitive survival assay was performed by intraperitoneally infecting mice with a 1:1 mixture of cfa mutant and wild-type strains, euthanizing mice at time intervals ranging from 4 to 17 days postinfection. Livers and spleens were harvested and homogenized prior to plating and enumeration of CFU, and competitive indices of wild-type and cfa mutant strains were determined (see Materials and Methods). An S. Typhimurium strain lacking cfa exhibited reduced competitive fitness beginning on day 7, with a median CI of 0.6 and 0.83, respectively (Fig. 6B). By day 17, the median CI of cfa mutant versus wild type in livers and spleens was 0.48 and 0.41, respectively (Fig. 6B). CI data from livers and spleens from days 4 to 17 were combined, and a Wilcoxon Signed Rank test showed a survival defect of the cfa mutant compared to the wild type (P < 0.0001) (Fig. 6C). These observations demonstrate that CFA contributes to Salmonella survival in macrophages and virulence in mice.
FIG 6.
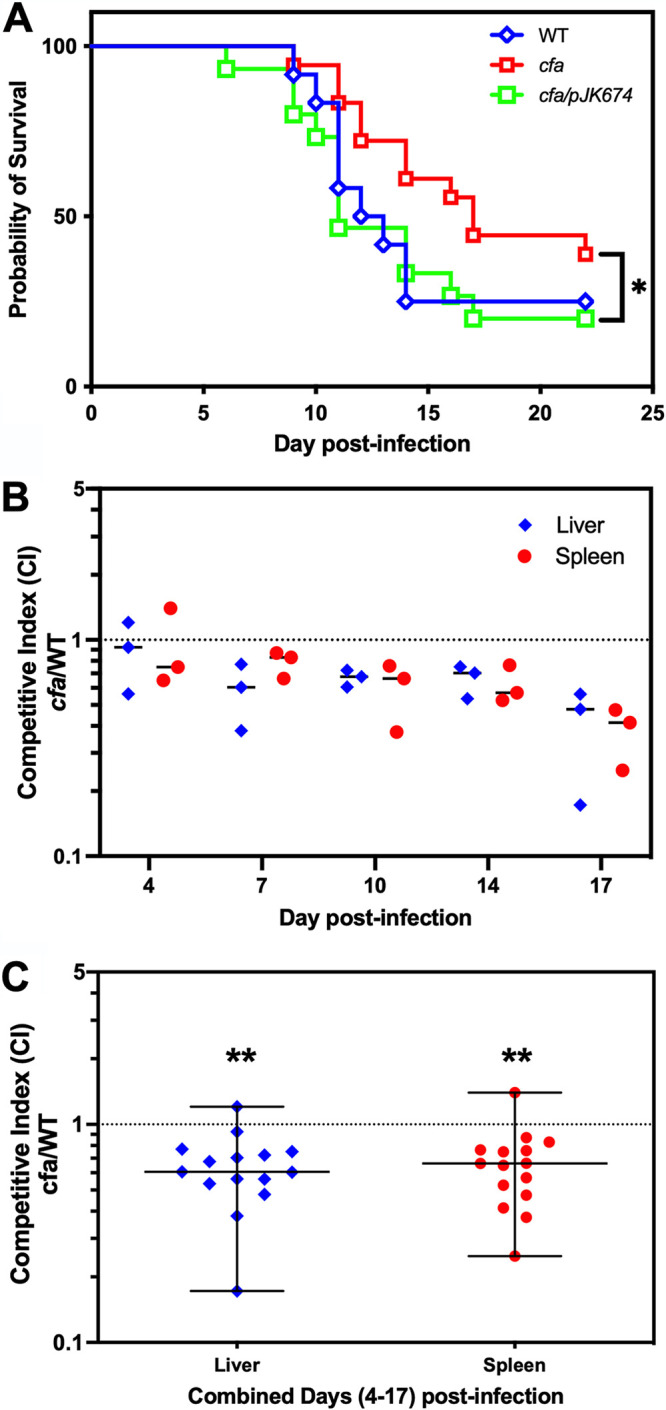
Cyclopropane fatty acids are important for virulence in mice. (A) C3H/HeN (ItyR) mice were infected intraperitoneally with S. Typhimurium strains wild-type (blue open diamonds), cfa mutant (red open squares), and cfa/pJK674 (green open squares) with 3000 CFU. Mice were monitored daily, and moribund mice were euthanized. *, P = 0.0463 between cfa mutant and cfa mutant with complementing plasmid pJK674 using Gehan-Breslow-Wilcoxon test. (B) Competitive survival experiment of cfa mutant versus wild type in C3H/HeN (ItyR). Mice were inoculated intraperitoneally with a mixture of cfa mutant and wild-type S. Typhimurium strains. Three independent mice were assayed for each time period postinfection. The competitive index (CI) of the cfa mutant versus the wild type was determined for both the liver (blue closed diamonds) and the spleen (red closed circles). A CI of 1 indicates that both strains grew equally well in vivo. (C) The competitive index data from panel B were combined from days 4 to 17 postinfection and a Wilcoxon signed rank test was performed. A double asterisk (**) indicates P < 0.0001.
DISCUSSION
S. Typhimurium experiences diverse stress conditions in both external and host environments and has developed strategies to withstand these stresses (reviewed in references 50 and 51). Previous studies have shown that the cyclopropanation of bacterial membrane unsaturated fatty acids occurs in response to a diverse range of environmental stresses (19, 21). In the present study, we have demonstrated the importance of cyclopropane modification of membrane lipids in S. Typhimurium in resistance to CCCP treatment and oxidative stress, as well as its importance for virulence in mice.
The proton motive force (PMF) represents the sum of electrical and chemical gradients across the cell membrane, which can be profoundly influenced by environmental stresses and by the metabolic status of the cell. When E. coli enters the stationary phase of growth, PMF begins to decrease as a consequence of diminishing nutrient availability and energy utilization (43). Recent studies have shown that E. coli exhibits increased membrane proton permeability when subjected to organic solvents, such as ethanol (52). Furthermore, E. coli cfa mutants are less viable than the wild type following exposure to ethanol (32), suggesting a role for CFA in PMF maintenance. Extreme acid conditions can enhance the transmembrane proton gradient, affecting intracellular pH homeostasis and membrane potential (53, 54). In E. coli, CFAs reduce membrane proton permeability and enhance bacterial survival in acidified minimal media (12). We found that S. Typhimurium that lack CFAs are defective for survival following exposure to extreme acid stress or treatment with the protonophore CCCP (Fig. 2 and 3). CCCP collapses the PMF by uncoupling the transport of protons across the membrane (46). The phage shock protein PspA has been proposed to play a role in PMF maintenance, and suppresses membrane proton permeability (44, 52, 55). S. Typhimurium pspA and cfa mutants are both defective for survival when treated with CCCP, compared to the wild type (Fig. 3). Furthermore, a pspA cfa double mutant is more sensitive to CCCP than a strain carrying a cfa mutation alone (Fig. 3). Taken together, these results suggest that the CFA modification of membranes contributes to the maintenance of PMF under stress conditions by reducing proton permeability.
The role of CFA-modified membranes in oxidative stress resistance has not been well characterized. Previous observations found no difference in survival between wild-type and cfa mutant E. coli following exposure to singlet oxygen (32). In contrast, we have found that S. Typhimurium cfa mutants are more susceptible to hydrogen peroxide (Fig. 4), indicating that fatty acid cyclopropanation confers resistance to oxidative stress. The difference between earlier results with singlet oxygen and the present study with hydrogen peroxide may suggest that the membrane poses a greater diffusion barrier for hydrogen peroxide than for singlet oxygen, and that this barrier function is sensitive to fatty acid cyclopropanation, which can influence packing within lipid bilayers (56). Alternatively, CFA-modified membranes may have greater integrity or enhance membrane protein function during oxidative stress. For example, changes in lipid composition have been shown to modulate the activity of osmoregulated uptake systems in Corynebacterium (57).
We observed no difference between S. Typhimurium wild-type and cfa mutant strains in their susceptibility to growth inhibition by the nitric oxide donor SperNO (Fig. S2). However, it is intriguing to note that E. coli flavohemoglobin (HMP), an important nitric oxide scavenger, preferentially binds to unsaturated (UFA) and cyclopropanated fatty acids (CFA), but not to saturated fatty acids (58). The binding of UFA or CFA to HMP augments HMP heme iron-binding properties in vitro (58). It is not presently known whether UFA or CFA influences HMP activity in vitro or in vivo.
The S. Typhimurium cfa mutant was found to be defective for survival in peritoneal murine macrophages (Fig. 5). The survival defect of the cfa mutant could be repaired by treating macrophages with DPI, which inhibits both the phagocyte NADPH oxidase and inducible nitric oxide synthase. These results suggest that lipid CFAs confer resistance to antimicrobial reactive species produced by activated macrophages, and support the in vitro finding that CFAs confer resistance to hydrogen peroxide. Finally, S. Typhimurium cfa mutants were also found to be deficient for virulence in mice, with a cfa mutant strain exhibiting a competitive defect compared to the wild type (Fig. 6B and C). A role for cyclopropanated lipids in pathogenic bacteria has recently been described in M. tuberculosis and Helicobacter pylori. M. tuberculosis has at least five putative cyclopropane synthase homologs that make at least three major types of cyclopropanated mycolic acids (7, 33–36). Some of these cyclopropanated mycolic acid derivatives have been found to be involved in hydrogen peroxide resistance, virulence, and long-term survival in mice, as well as influencing host inflammatory responses (7, 9, 10, 34, 38). Future studies will determine how CFA modification promotes Salmonella virulence and whether this includes modulation of the host response.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
Bacterial strains, plasmids, and primers used in this study are listed in Table 2.
TABLE 2.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Genotype or sequence (5′–3′) | Source or reference |
|---|---|---|
| Strains | ||
| 14028s | 14028s wild type | ATCC |
| AF441 | 14028s cfa::frt-cat-frt | This study |
| LB133 | 14028s pspA::kan | (44) |
| AF453 | 14028s cfa::frt-cat-frt pspA::kan | This study |
| JV104 | 14028s dps::cat | (67) |
| CS015 | 14028s phoP102::Tn10dCm | S. Miller |
| JK811 | 14028s cfa::frt-cat-frt/pJK674 | This study |
| Plasmids | ||
| pTP223 | (61) | |
| pKD3 | bla frt-cat-frt PS1PS2 ori R6K | (60) |
| pBAD30 | bla araC araBAD promoter ori p15A | (62) |
| pRB3-273C | bla par RK2 stable low-copy-no. cloning vector | (63) |
| pBR322 | bla tet ori colE1 | (64) |
| pBAD::cfa | bla araC araBAD promoter::cfa ori p15A | This study |
| pJK650 | pBR322-(-389bp from ATG and coding region of cfa) | This study |
| pJK674 | pRB3-(-389bp from ATG and coding region of cfa) | This study |
| Primers | ||
| 5’cfa | ATGGAGAAACTATGAGTTCATCGTGTATAGAAGAAGTCAGCGTACCGGATGATAACTGGTGTGTAGGCTGGAGCTGCTTC | |
| 3’cfa | CACCAGCCATCCATGTAACTCTCACCTAACCCCAACGATCCTTCCTGAAGGACGCGTTTACATATGAATATCCTCCTTAG | |
| cfa1 | AGCCAAAAAAGCGTCTACGC | |
| cfa2 | TCAGGCCGACATTGGCTAAC | |
| cfa3 | GAATTCCAGTGATGGAGAAACTAT | |
| cfa4 | CTAACGGAAAATAAGATTCCCCCGC | |
| JKP149 | GGGGGAATTCGTTGTTCTGGCGGCGTTAGG | |
| JKP152 | GGGGCTGCAGTAGAACGCGTACTGGCTGCG | |
Reagents and standard genetic and molecular techniques.
Bacteria were grown in LB medium, minimal M9 medium supplemented with 0.4% glucose, or brain heart infusion (Difco Laboratories, Sparks, MD), at 37°C with aeration, unless otherwise indicated. Restriction and modifying enzymes were purchased from New England Biolabs (Ipswich, MA). Chemicals were purchased from Sigma (St. Louis, MO). Taq DNA polymerase was purchased from Promega (Madison, WI). Phage P22 transduction methods were performed as described (59).
Construction of cfa mutant.
A chromosomal mutation in cfa was constructed by λ-Red-mediated recombination (60). The cfa gene was deleted from codons 16 to 47, which were replaced with the chloramphenicol resistance (cat) gene from pKD3 (60) as follows: the chloramphenicol resistance gene with flanking sequences from cfa was PCR-amplified using primers 5’cfa, 3’cfa, and plasmid DNA pKD3. The 1,100-bp PCR-amplified fragment was purified using a Qiagen PCR purification kit (Valencia, CA) and electroporated into S. Typhimurium containing the λ-Red-expressing plasmid pTP223 (61). Cells were plated onto LB agar containing 20 μg ml−1 chloramphenicol and incubated at 37°C. Chloramphenicol-resistant colonies were screened by PCR, with primers cfa1 and cfa2, to confirm that the chloramphenicol-resistance cassette was inserted in cfa. The cfa: frt-cat-frt allele was reintroduced into a new S. Typhimurium background by P22 transduction. Quantitative RT-PCR was performed on the cfa::frt-cat-frt S. Typhimurium strain to verify the lack of cfa expression in log and stationary-phase cells.
Construction of cfa plasmid constructs.
The cfa gene was cloned under the control of an arabinose-inducible promoter in the low-copy plasmid pBAD30 (62) as follows: the cfa gene was PCR-amplified with primers cfa3 and cfa4 using the genomic DNA template isolated from S. Typhimurium ATCC14028s. The 1,148-bp PCR-amplified fragment was digested with EcoRI, then gel-purified with a Qiagen gel purification kit (Valencia, CA). The cfa-containing PCR fragment was subsequently ligated, using T4 DNA polymerase, into pBAD30 digested with EcoRI and SmaI. The pBAD::cfa construct was confirmed by DNA sequencing. The promoter and coding region of cfa were cloned into the stable low-copy plasmid pRB3-273C (63), designated pJK674, as follows: primers JKP149 and JKP152 were used to PCR-amplify a 1637-bp fragment from S. Typhimurium 14028s which included both the promoter and the coding region of cfa. The fragment was digested with EcoRI and PstI, then ligated into plasmid pBR322 (64), digested with EcoRI and PstI, to create pJK650. The plasmid pJK650 was subsequently digested with EcoRI and PstI, and the 1,637-bp fragment containing the promoter and coding region of cfa was blunt ended with T4 DNA polymerase, then ligated into pRB3-273C digested with SmaI to create pJK674.
Isolation and analysis of fatty acid composition in S. Typhimurium.
Wild-type S. Typhimurium and the isogenic cfa mutant derivative were grown overnight in brain heart infusion broth at 37°C with agitation. Cells were harvested by centrifugation at 3000 × g for 15 min. The resulting cell pellets were washed once with ice-cold 20 mM morpholinepropanesulfonic acid (pH 7.2) and stored frozen at −20°C. Lipids were extracted by the Kates modification of the method of Bligh and Dyer (65). The lower chloroform layer was washed once with 0.1 M KCl and filtered through a phase-separating filter (Whatman).
Alkaline methanolysis was performed as follows: solvent was evaporated under a stream of N2 and the lipids suspended in 0.2 ml chloroform and 0.3 ml methanol, to which 0.2 M NaOH in methanol was added. The sample was mixed by vortexing and allowed to stand for 20 min at room temperature. Chloroform (0.8 ml), methanol (0.2 ml), and water (0.9 ml) were then added before the sample was mixed and centrifuged at 1,200 rpm for 4 min. The lower layer was washed once with methanol/water at a ratio of 10:9 (vol/vol) and twice filtered through a phase-separating filter (65).
The resulting fatty acid methyl esters were subjected to gas chromatography/mass spectrometry by standard methods. The mass spectra were compared to those of pure standards of saturated and unsaturated fatty acids. Cyclopropane fatty acids were confirmed to have the expected mass.
Hydrogen peroxide bioscreen and killing assays.
A bioscreen assay to test strains for susceptibility to hydrogen peroxide (H2O2) was performed. Stationary-phase cells grown in LB were diluted to OD600 0.002 with or without the addition of 1 mM H2O2. Cells were subsequently grown aerobically at 37°C with agitation and cell density monitored at OD600 with a Labsystems Bioscreen C microplate reader (Helsinki, Finland). Hydrogen peroxide killing assays were performed as follows: stationary-phase cells grown in LB or M9 containing 0.4% glucose were washed once in phosphate-buffered saline (PBS), diluted to 2 × 106 in PBS with or without 0.8 mM H2O2, and then incubated at 37°C for up to 2 h. Viable cells were determined by plating diluted aliquots onto LB agar. Percent survival was determined by calculating the CFU after H2O2 treatment divided by the CFU of untreated cells at each time interval.
Acid stress survival assays.
Stationary-phase cells grown in LB or M9 broth containing 0.4% glucose were diluted 1:1,000 in fresh medium, with pH adjusted to 3 or 4.5 using HCl. Cells were subsequently grown aerobically at 37°C for up to 3 h. Viable cells were determined by plating diluted aliquots onto LB agar. Percent survival was determined by calculating the CFU of acid-stressed cells divided by the CFU of untreated cells.
CCCP survival assays.
Stationary-phase cells grown in LB were diluted to 1 × 105 CFU in LB containing 250 μM carbonyl cyanide 3-chlorophenylhydrazone (CCCP) and incubated at 37°C for 10 h. Viable cells were determined by plating diluted aliquots onto LB agar. Percent survival was determined by calculating the CFU of CCCP-treated cells divided by the CFU of untreated cells.
Macrophage survival and mouse experiments.
The animal experiments in this study were approved by the University of Washington Institutional Animal Care and Use Committee and performed as described in protocol 3373-01. Macrophage survival assays were performed using periodate-elicited macrophages isolated from 7-week-old female C3H/HeN (Ityr) mice (Charles River Laboratories, Wilmington, MA) as previously described (66). To inhibit the NADPH phagocyte oxidase (Nox2), 25 μM diphenyleneiodonium (DPI) chloride (Sigma, Saint Louis, MO) was added 24 h prior to infection as indicated.
Virulence assays were performed as follows: 7-week-old female C3H/HeN (Ityr) mice were infected intraperitoneally with 3000 CFU of wild-type, cfa::frt-cat-frt mutant, or complemented cfa::frt-cat-frt/pJK674 S. Typhimurium. The actual inocula were measured by plating serial dilutions onto LB agar. Mice were monitored daily for the duration of the experiment, and moribund mice were euthanized. Statistical analysis of the survival curves was determined using a Gehan-Breslow-Wilcoxon test with Prism 6.0c (GraphPad Software, Inc.).
Competitive index assays were performed as follows: 7-week-old female C3H/HeN (Ityr) mice were infected as described above, but with an equal mixture of wild-type and cfa::frt-cat-frt mutant strains. At designated time intervals, the mice were euthanized, livers and spleens were harvested, organs homogenized in PBS, and viable counts determined by plating serial dilutions onto LB agar, with or without the addition of 20 μg ml−1 chloramphenicol. The competitive index of the cfa::frt-cat-frt mutant versus the wild type was determined as the ratio of (mutant/wild-type)output to (mutant/wild-type)input. Statistical significance was determined using the Wilcoxon Signed Rank test with Prism 6.0c (GraphPad Software, Inc.).
ACKNOWLEDGMENTS
We thank Michael J. Bennett (Children’s Hospital of Philadelphia) for the analysis of the fatty acids, and we thank Margaret Nartea for her expert technical assistance.
This work was supported by the National Institutes of Health (AI044486, AI118962, AI160130).
Footnotes
Supplemental material is available online only.
Contributor Information
Ferric C. Fang, Email: fcfang@uw.edu.
Manuela Raffatellu, University of California San Diego School of Medicine.
REFERENCES
- 1.Grogan DW, Cronan JE. 1997. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol Mol Biol Rev 61:429–441. 10.1128/mmbr.61.4.429-441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Law J. 1971. Biosynthesis of cyclopropane rings. Acc Chem Res 4:199–203. 10.1021/ar50042a002. [DOI] [Google Scholar]
- 3.Grogan DW, Cronan JE. 1984. Cloning and manipulation of the Escherichia coli cyclopropane fatty acid synthase gene: physiological aspects of enzyme overproduction. J Bacteriol 158:286–295. 10.1128/jb.158.1.286-295.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Ordóñez A, Fernández A, López M, Arenas R, Bernardo A. 2008. Modifications in membrane fatty acid composition of Salmonella Typhimurium in response to growth conditions and their effect on heat resistance. Int J Food Microbiol 123:212–219. 10.1016/j.ijfoodmicro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Brown JL, Ross T, McMeekin TA, Nichols PD. 1997. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int J Food Microbiol 37:163–173. 10.1016/S0168-1605(97)00068-8. [DOI] [PubMed] [Google Scholar]
- 6.Chang YY, Cronan JE. 1999. Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol Microbiol 33:249–259. 10.1046/j.1365-2958.1999.01456.x. [DOI] [PubMed] [Google Scholar]
- 7.Glickman MS, Cox JS, Jacobs WR. 2000. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol Cell 5:717–727. 10.1016/S1097-2765(00)80250-6. [DOI] [PubMed] [Google Scholar]
- 8.Kim BH, Kim S, Kim HG, Lee J, Lee IS, Park YK. 2005. The formation of cyclopropane fatty acids in Salmonella enterica serovar Typhimurium. Microbiology (Reading) 151:209–218. 10.1099/mic.0.27265-0. [DOI] [PubMed] [Google Scholar]
- 9.Rao V, Fujiwara N, Porcelli SA, Glickman MS. 2005. Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule. J Exp Med 201:535–543. 10.1084/jem.20041668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao V, Gao F, Chen B, Jacobs WR, Glickman MS. 2006. Trans-cyclopropanation of mycolic acids on trehalose dimycolate suppresses Mycobacterium tuberculosis-induced inflammation and virulence. J Clin Invest 116:1660–1667. 10.1172/JCI27335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riggins DP, Narvaez MJ, Martinez KA, Harden MM, Slonczewski JL. 2013. Escherichia coli K-12 survives anaerobic exposure at pH 2 without RpoS, Gad, or hydrogenases, but shows sensitivity to autoclaved broth products. PLoS One 8:e56796. 10.1371/journal.pone.0056796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shabala L, Ross T. 2008. Cyclopropane fatty acids improve Escherichia coli survival in acidified minimal media by reducing membrane permeability to H+ and enhanced ability to extrude H+. Res Microbiol 159:458–461. 10.1016/j.resmic.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Denich TJ, Beaudette LA, Lee H, Trevors JT. 2003. Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J Microbiol Methods 52:149–182. 10.1016/S0167-7012(02)00155-0. [DOI] [PubMed] [Google Scholar]
- 14.Sajbidor J. 1997. Effect of some environmental factors on the content and composition of microbial membrane lipids. Crit Rev Biotechnol 17:87–103. 10.3109/07388559709146608. [DOI] [PubMed] [Google Scholar]
- 15.Dixon DR, Darveau RP. 2005. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J Dent Res 84:584–595. 10.1177/154405910508400702. [DOI] [PubMed] [Google Scholar]
- 16.Cronan JE. 1968. Phospholipid alterations during growth of Escherichia coli. J Bacteriol 95:2054–2061. 10.1128/jb.95.6.2054-2061.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guckert JB, Hood MA, White DC. 1986. Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae: increases in the trans/cis ratio and proportions of cyclopropyl fatty acids. Appl Environ Microbiol 52:794–801. 10.1128/aem.52.4.794-801.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguilar PS, de Mendoza D. 2006. Control of fatty acid desaturation: a mechanism conserved from bacteria to humans. Mol Microbiol 62:1507–1514. 10.1111/j.1365-2958.2006.05484.x. [DOI] [PubMed] [Google Scholar]
- 19.Cronan JE. 2002. Phospholipid modifications in bacteria. Curr Opin Microbiol 5:202–205. 10.1016/S1369-5274(02)00297-7. [DOI] [PubMed] [Google Scholar]
- 20.Guerzoni ME, Lanciotti R, Cocconcelli PS. 2001. Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiology (Reading) 147:2255–2264. 10.1099/00221287-147-8-2255. [DOI] [PubMed] [Google Scholar]
- 21.Knivett VA, Cullen J. 1965. Some factors affecting cyclopropane acid formation in Escherichia coli. Biochem J 96:771–776. 10.1042/bj0960771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos JL, Duque E, Gallegos MT, Godoy P, Ramos-Gonzalez MI, Rojas A, Teran W, Segura A. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu Rev Microbiol 56:743–768. 10.1146/annurev.micro.56.012302.161038. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto T, Murata N. 2002. Regulation of the desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr Opin Microbiol 5:208–210. 10.1016/S1369-5274(02)00306-5. [DOI] [PubMed] [Google Scholar]
- 24.Asakura H, Ekawa T, Sugimoto N, Momose Y, Kawamoto K, Makino S, Igimi S, Yamamoto S. 2012. Membrane topology of Salmonella invasion protein SipB confers osmotolerance. Biochem Biophys Res Commun 426:654–658. 10.1016/j.bbrc.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Charoenwong D, Andrews S, Mackey B. 2011. Role of rpoS in the development of cell envelope resilience and pressure resistance in stationary-phase Escherichia coli. Appl Environ Microbiol 77:5220–5229. 10.1128/AEM.00648-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Golden DA, Critzer FJ. 2014. Salmonella survival and differential expression of fatty acid biosynthesis-associated genes in a low-water-activity food. Lett Appl Microbiol 59:133–138. 10.1111/lam.12253. [DOI] [PubMed] [Google Scholar]
- 27.Fang J, Lyon DY, Wiesner MR, Dong J, Alvarez PJ. 2007. Effect of a fullerene water suspension on bacterial phospholipids and membrane phase behavior. Environ Sci Technol 41:2636–2642. 10.1021/es062181w. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal AZ, Kim Y, Gralla JD. 2008. Regulation of transcription by acetate in Escherichia coli: in vivo and in vitro comparisons. Mol Microbiol 68:907–917. 10.1111/j.1365-2958.2008.06186.x. [DOI] [PubMed] [Google Scholar]
- 29.Ye B, He S, Zhou X, Cui Y, Zhou M, Shi X. 2019. Response to acid adaptation in Salmonella enterica serovar Enteritidis. J Food Sci 84:599–605. 10.1111/1750-3841.14465. [DOI] [PubMed] [Google Scholar]
- 30.Wang AY, Cronan JE. 1994. The growth phase-dependent synthesis of cyclopropane fatty acids in Escherichia coli is the result of an RpoS(KatF)-dependent promoter plus enzyme instability. Mol Microbiol 11:1009–1017. 10.1111/j.1365-2958.1994.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 31.Chang YY, Eichel J, Cronan JE. 2000. Metabolic instability of Escherichia coli cyclopropane fatty acid synthase is due to RpoH-dependent proteolysis. J Bacteriol 182:4288–4294. 10.1128/JB.182.15.4288-4294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grogan DW, Cronan JE. 1986. Characterization of Escherichia coli mutants completely defective in synthesis of cyclopropane fatty acids. J Bacteriol 166:872–877. 10.1128/jb.166.3.872-877.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barkan D, Rao V, Sukenick GD, Glickman MS. 2010. Redundant function of cmaA2 and mmaA2 in Mycobacterium tuberculosis cis cyclopropanation of oxygenated mycolates. J Bacteriol 192:3661–3668. 10.1128/JB.00312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glickman MS, Cahill SM, Jacobs WR. 2001. The Mycobacterium tuberculosis cmaA2 gene encodes a mycolic acid trans-cyclopropane synthetase. J Biol Chem 276:2228–2233. 10.1074/jbc.C000652200. [DOI] [PubMed] [Google Scholar]
- 35.Glickman MS. 2003. The mmaA2 gene of Mycobacterium tuberculosis encodes the distal cyclopropane synthase of the alpha-mycolic acid. J Biol Chem 278:7844–7849. 10.1074/jbc.M212458200. [DOI] [PubMed] [Google Scholar]
- 36.Yuan Y, Lee RE, Besra GS, Belisle JT, Barry CE. 1995. Identification of a gene involved in the biosynthesis of cyclopropanated mycolic acids in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 92:6630–6634. 10.1073/pnas.92.14.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minnikin DE. 1982. Lipids: complex lipids, their chemistry, biosynthesis and roles, p 95–184. In C. Ratledge, and S. J. (ed), The biology of the mycobacteria, vol. 1. Academic Press, London, United Kingdom. [Google Scholar]
- 38.Barkan D, Hedhli D, Yan HG, Huygen K, Glickman MS. 2012. Mycobacterium tuberculosis lacking all mycolic acid cyclopropanation is viable but highly attenuated and hyperinflammatory in mice. Infect Immun 80:1958–1968. 10.1128/IAI.00021-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang X, Duan Y, Zhou B, Guo Q, Wang H, Hang X, Zeng L, Jia J, Bi H. 2019. The cyclopropane fatty acid synthase mediates antibiotic resistance and gastric colonization of Helicobacter pylori. J Bacteriol 201. 10.1128/JB.00374-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkinson SG. 1988. Gram-negative bacteria. Academic Press, London, United Kingdom. [Google Scholar]
- 41.Richard H, Foster JW. 2004. Escherichia coli glutamate- and arginine-dependent acid resistance systems increase internal pH and reverse transmembrane potential. J Bacteriol 186:6032–6041. 10.1128/JB.186.18.6032-6041.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rezaeinejad S, Ivanov V. 2011. Heterogeneity of Escherichia coli population by respiratory activity and membrane potential of cells during growth and long-term starvation. Microbiol Res 166:129–135. 10.1016/j.micres.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Weiner L, Model P. 1994. Role of an Escherichia coli stress-response operon in stationary-phase survival. Proc Natl Acad Sci USA 91:2191–2195. 10.1073/pnas.91.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker LA, Bang IS, Crouch ML, Fang FC. 2005. Compensatory role of PspA, a member of the phage shock protein operon, in rpoE mutant Salmonella enterica serovar Typhimurium. Mol Microbiol 56:1004–1016. 10.1111/j.1365-2958.2005.04604.x. [DOI] [PubMed] [Google Scholar]
- 45.Reference deleted. [Google Scholar]
- 46.McLaughlin SG, Dilger JP. 1980. Transport of protons across membranes by weak acids. Physiol Rev 60:825–863. 10.1152/physrev.1980.60.3.825. [DOI] [PubMed] [Google Scholar]
- 47.Halsey TA, Vazquez-Torres A, Gravdahl DJ, Fang FC, Libby SJ. 2004. The ferritin-like Dps protein is required for Salmonella enterica serovar Typhimurium oxidative stress resistance and virulence. Infect Immun 72:1155–1158. 10.1128/IAI.72.2.1155-1158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vazquez-Torres A, Fang FC. 2001. Salmonella evasion of the NADPH phagocyte oxidase. Microbes Infect 3:1313–1320. 10.1016/S1286-4579(01)01492-7. [DOI] [PubMed] [Google Scholar]
- 49.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R. 1997. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem 272:13088–13093. 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 50.Foster JW, Spector MP. 1995. How Salmonella survive against the odds. Annu Rev Microbiol 49:145–174. 10.1146/annurev.mi.49.100195.001045. [DOI] [PubMed] [Google Scholar]
- 51.Rychlik I, Barrow PA. 2005. Salmonella stress management and its relevance to behaviour during intestinal colonisation and infection. FEMS Microbiol Rev 29:1021–1040. 10.1016/j.femsre.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi R, Suzuki T, Yoshida M. 2007. Escherichia coli phage-shock protein A (PspA) binds to membrane phospholipids and repairs proton leakage of the damaged membranes. Mol Microbiol 66:100–109. 10.1111/j.1365-2958.2007.05893.x. [DOI] [PubMed] [Google Scholar]
- 53.Setty OH, Hendler RW, Shrager RI. 1983. Simultaneous measurements of proton motive force, delta pH, membrane potential, and H+/O ratios in intact Escherichia coli. Biophys J 43:371–381. 10.1016/S0006-3495(83)84360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zilberstein D, Agmon V, Schuldiner S, Padan E. 1984. Escherichia coli intracellular pH, membrane potential, and cell growth. J Bacteriol 158:246–252. 10.1128/jb.158.1.246-252.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleerebezem M, Crielaard W, Tommassen J. 1996. Involvement of stress protein PspA (phage shock protein A) of Escherichia coli in maintenance of the protonmotive force under stress conditions. EMBO J 15:162–171. 10.1002/j.1460-2075.1996.tb00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poger D, Mark AE. 2015. A ring to rule them all: the effect of cyclopropane fatty acids on the fluidity of lipid bilayers. J Phys Chem B 119:5487–5495. 10.1021/acs.jpcb.5b00958. [DOI] [PubMed] [Google Scholar]
- 57.Ozcan N, Ejsing CS, Shevchenko A, Lipski A, Morbach S, Krämer R. 2007. Osmolality, temperature, and membrane lipid composition modulate the activity of betaine transporter BetP in Corynebacterium glutamicum. J Bacteriol 189:7485–7496. 10.1128/JB.00986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonamore A, Farina A, Gattoni M, Schininà ME, Bellelli A, Boffi A. 2003. Interaction with membrane lipids and heme ligand binding properties of Escherichia coli flavohemoglobin. Biochemistry 42:5792–5801. 10.1021/bi0206311. [DOI] [PubMed] [Google Scholar]
- 59.Davis RW, Botstein D, Roth JR, Wanner BL. 1980. A manual for genetic engineering: advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 60.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645. 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poteete AR, Fenton AC. 1984. Lambda red-dependent growth and recombination of phage P22. Virology 134:161–167. 10.1016/0042-6822(84)90281-2. [DOI] [PubMed] [Google Scholar]
- 62.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177:4121–4130. 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berggren RE, Wunderlich A, Ziegler E, Schleicher M, Duke RC, Looney D, Fang FC. 1995. HIV gp120-specific cell-mediated immune responses in mice after oral immunization with recombinant Salmonella. J Acquir Immune Defic Syndr Hum Retrovirol 10:489–495. 10.1097/00042560-199510050-00001. [DOI] [PubMed] [Google Scholar]
- 64.Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW, Crosa JH, Falkow S. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95–113. 10.1016/0378-1119(77)90000-2. [DOI] [PubMed] [Google Scholar]
- 65.Kates M. 1986. Techniques of lipidology: isolation, analysis, and identification of lipids. North-Holland Publishing Company, Amsterdam. [Google Scholar]
- 66.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med 192:227–236. 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Velayudhan J, Castor M, Richardson A, Main-Hester KL, Fang FC. 2007. The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron-sulphur cluster repair and virulence. Mol Microbiol 63:1495–1507. 10.1111/j.1365-2958.2007.05600.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download IAI.00479-21-s0001.pdf, PDF file, 0.1 MB (129.2KB, pdf)