Abstract
The epidermis forms an essential barrier against a variety of insults. The overall goal of this study was to shed light not only on the effects of accidental epidermal injury, but also on the mechanisms that support laser skin resurfacing with intra-epidermal focal laser-induced photodamage, a widespread medical practice used to treat a range of skin conditions. To this end, we selectively photodamaged a single keratinocyte with intense, focused and pulsed laser radiation, triggering Ca2+ waves in the epidermis of live anesthetized mice with ubiquitous expression of a genetically encoded Ca2+ indicator. Waves expanded radially and rapidly, reaching up to eight orders of bystander cells that remained activated for tens of minutes, without displaying oscillations of the cytosolic free Ca2+ concentration (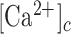 ). By combining in vivo pharmacological dissection with mathematical modeling, we demonstrate that Ca2+ wave propagation depended primarily on the release of ATP, a prime damage-associated molecular patterns (DAMPs), from the hit cell. Increments of the
). By combining in vivo pharmacological dissection with mathematical modeling, we demonstrate that Ca2+ wave propagation depended primarily on the release of ATP, a prime damage-associated molecular patterns (DAMPs), from the hit cell. Increments of the 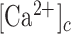 in bystander cells were chiefly due to Ca2+ release from the endoplasmic reticulum (ER), downstream of ATP binding to P2Y purinoceptors. ATP-dependent ATP release though connexin hemichannels (HCs) affected wave propagation at larger distances, where the extracellular ATP concentration was reduced by the combined effect of passive diffusion and hydrolysis due to the action of ectonucleotidases, whereas pannexin channels had no role. Bifurcation analysis suggests basal keratinocytes have too few P2Y receptors (P2YRs) and/or phospholipase C (PLC) to transduce elevated extracellular ATP levels into inositol trisphosphate (IP3) production rates sufficiently large to sustain
in bystander cells were chiefly due to Ca2+ release from the endoplasmic reticulum (ER), downstream of ATP binding to P2Y purinoceptors. ATP-dependent ATP release though connexin hemichannels (HCs) affected wave propagation at larger distances, where the extracellular ATP concentration was reduced by the combined effect of passive diffusion and hydrolysis due to the action of ectonucleotidases, whereas pannexin channels had no role. Bifurcation analysis suggests basal keratinocytes have too few P2Y receptors (P2YRs) and/or phospholipase C (PLC) to transduce elevated extracellular ATP levels into inositol trisphosphate (IP3) production rates sufficiently large to sustain 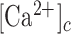 oscillations.
oscillations.
Keywords: Skin, laser-induced intra-epidermal photodamage, wound healing, purinergic signaling, connexins, pannexins, reaction-diffusion equations, mathematical model
Graphical Abstract
Graphical Abstract.
Introduction
The epidermis forms an essential barrier against dehydration and pathogens, as well as light, chemical and mechanical injury.1,2 It is a stratified epithelium largely composed of keratinocytes (95%), melanocytes (that donate pigment to the keratinocytes), Langerhans’ cells, which have immunological functions, and Merkel cells, also known as Merkel-Ranvier cells or tactile epithelial cells.2 It is well established that epidermis’ continuous renewal is sustained by stem cells contained in the stratum basale.3–8 The differentiating keratinocytes are gradually displaced in the outward direction, from stratum basale through stratum spinosum to stratum corneum, where corneocytes are continually shed from the skin surface.1,2 In different epithelia, including the epidermis, damage triggers perturbations of the 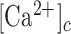 that spread from cell to cell, known as (intercellular) Ca2+ waves (see e.g. Refs.9–14) and considered a fundamental mechanism for coordinating multicellular responses.15 Ca2+ signaling is at the core of epidermal homeostasis,16–18 however, the mechanisms underlying Ca2+ wave propagation and their significance in the damaged epidermis are incompletely understood. A gradient of Ca2+ across the epidermis is key for keratinocyte differentiation and formation of the epidermal permeability barrier,16–18 maintenance of which relies on a delicate balance between proliferation and differentiation, two Ca2+-dependent cellular processes.2,8,19 Defects of epidermal Ca2+ homeostasis cause skin pathologies, such as Darier's disease for which ATP2A2 has been identified as the defective gene, indicating that the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA)2 plays a key role.20
that spread from cell to cell, known as (intercellular) Ca2+ waves (see e.g. Refs.9–14) and considered a fundamental mechanism for coordinating multicellular responses.15 Ca2+ signaling is at the core of epidermal homeostasis,16–18 however, the mechanisms underlying Ca2+ wave propagation and their significance in the damaged epidermis are incompletely understood. A gradient of Ca2+ across the epidermis is key for keratinocyte differentiation and formation of the epidermal permeability barrier,16–18 maintenance of which relies on a delicate balance between proliferation and differentiation, two Ca2+-dependent cellular processes.2,8,19 Defects of epidermal Ca2+ homeostasis cause skin pathologies, such as Darier's disease for which ATP2A2 has been identified as the defective gene, indicating that the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA)2 plays a key role.20
ATP, which is present at mM concentration in the cytosol whereas its normal concentration in the extracellular environment is in low nM range, is released by most cells as an extracellular signaling molecule.21,22 ATP release was detected in cultured human neonatal keratinocytes exposed to air,23 as well as from injured mouse epidermal keratinocytes,24 normal human epidermal keratinocytes (NHEKs) co-cultured with mouse dorsal root ganglion (DRG) neurons,25 and HaCaT cells26 (a spontaneously transformed aneuploid immortal keratinocyte cell line from adult human skin, with high capacity to differentiate and proliferate in vitro27). Extracellular ATP can affect the 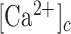 by activating Ca2+-permeable P2XRs28 and/or by promoting Ca2+-release from the endoplasmic reticulum (ER) via G-protein coupled P2YRs29,30 on the surface of keratinocytes and other epidermal cells types.31 Therefore, ATP release and purinergic signaling have the potential to interfere with epidermal homeostasis and have been implicated in a host of processes, including pain, inflammation and wound healing.31
by activating Ca2+-permeable P2XRs28 and/or by promoting Ca2+-release from the endoplasmic reticulum (ER) via G-protein coupled P2YRs29,30 on the surface of keratinocytes and other epidermal cells types.31 Therefore, ATP release and purinergic signaling have the potential to interfere with epidermal homeostasis and have been implicated in a host of processes, including pain, inflammation and wound healing.31
In this study, we evoked Ca2+ waves by selectively photodamaging a single keratinocyte of the epidermal basal layer of the earlobe skin in live anesthetized mice. Our experimental model is related not only to accidental injury, but also to the medical practice of skin resurfacing/rejuvenation based on intra-epidermal focal laser-induced photodamage that is used to treat numerous conditions such as photodamage and acne scars, hidradenitis suppurativa and posttraumatic scarring from basal cell carcinoma excision.32–35 As detailed in the following Methods and Results sections, we used intravital multiphoton microscopy to visualize Ca2+ waves in mice expressing GCaMP6s, a sensitive and selective genetically encoded Ca2+ indicator.36 To dissect the molecular components contributing to Ca2+ wave propagation, we performed in vivo pharmacological interference experiments by intradermal microinjection of different drugs. With the insight gained from data analysis, we formulated a mathematical model that accounts for the observed Ca2+ dynamics based on ATP release and diffusion, and activation of P2YRs. The significance of our findings for wound healing and skin therapy is discussed in relation to Ca2+ signaling pathway activation in the epidermis.
Methods
Animals
Mice were bred and genotyped at the National Research Council-Institute of Biochemistry and Cell Biology (CNR-IBBC), Infrafrontier/ESFRI-European Mouse Mutant Archive (EMMA), Specific Pathogen-Free (SPF) barrier unit (Monterotondo Scalo, Rome). All the experimental procedures were agreed upon, reviewed and approved by local animals welfare oversight bodies and were performed with the approval and direct supervision of the CNR-IBBC/Infrafrontier—Animal Welfare and Ethical Review Body (AWERB), in accordance with general guidelines regarding animal experimentation, approved by the Italian Ministry of Health, in compliance with the Legislative Decree 26/2014 (ref. Project license 603/2018-PR), transposing the 2010/63/EU Directive on protection of animals used in research). In addition, all animal experimentation was conducted in adherence to the NIH Guide for the Care and Use of Laboratory Animals and recommendations from both ARRIVE and PREPARE guidelines.37,38 Mice were housed in individually ventilated caging systems (Tecniplast, Gazzada, Italy) at a temperature (T) of 21 ± 2°C, relative humidity (RH) of 55 ± 15% with 50–70 air changes per hour (ACH) and under controlled (12 : 12 hour) light–dark cycles (7 am–7 pm). Mice had ad libitum access to water and a standard rodent diet (Emma 23, Mucedola, Settimo Milanese, Italy).
Adult transgenic mice, both male and female, aged between 5 and 40 weeks and ubiquitously expressing the Ca2+ biosensor GCaMP6s36 were used for in vivo Ca2+ imaging experiments. These mice were generated in the animal facility of the laboratory by crossing the Jackson Laboratory strain #024106 (STOCK-Gt(ROSA)26Sortm96(CAG−GCaMP6s)Hze/J) with the European Mouse Mutant Archive (EM) Cre-deleter strain EM:01135 (B6.C-Tg(CMVcre) 1Cgn/CgnCnrm). Double mutant mice were identified amplifying tail genomic DNA by means of PCR. The presence of the CAG-GCamp6 insertion in the Rosa26 locus and the wild type allele were detected using the primers:
5’-ACG-AGT-CGG-ATC-TCC-CTT-TG-3’, 5'- AAG-GGA-GCT-GCA-GTG-GAG-TA-3'
and
5’-CCG-AAA-ATC-TGT-GGG-AAG-TC-3’.
The CRE-deleter transgene was detected using the primer pair
5’-CGA-GTG-ATG-AGG-TTC-GCA-AG -3’
and
5’-TGA-GTG-AAC-GAA-CCT-GGT-CG -3’.
PCR products were run on 2% agarose gels, visualized with ethidium bromide and photographed. Expected band sizes were 450 bp for the CAG-GCamp6 and 297 bp for the wt allele and 390bp for the Cre transgene.
Multiphoton microscopy
System description
We used a custom-made multiphoton system (Figure S1) based on a Bergamo II architecture (Thorlabs Imaging System, Sterling, VI, USA), as previously described.12 The system was equipped with two scanning heads, one with resonant-galvo (RG) mirrors and the other with galvo-galvo (GG) mirrors, and was coupled to a mode-locked titanium-sapphire (Ti:Sa) fs pulsed laser (Chameleon Vision II Laser, Coherent, Inc., Santa Clara, CA, USA) (Figure S2). The RG scanner was used for imaging, whereas the GG scanner was used to focally photodamage a pre-defined spot in the field of view (FOV) by focusing the collimated laser beam onto the sample through a 25× water- immersion objective (XLPLN25XWMP2, NA 1.05, Olympus Corporation, Tokyo, Japan; the same objective was also used for imaging).
Multiphoton excitation of GCamp6s was performed at 920 nm, whereas its emission signal was filtered by a single band-pass filter (Cat. No. FF02-525/40-25, Semrock/IDEX, Rochester, NY, USA) placed in front of a non-descanned GaAsP detector (Cat. No. H7422–50, Hamamatsu Photonics K.K., Shizuoka, Japan). Electro-optical modulators (EOM) and mechanical ultra-fast shutters were used to control both photodamage and imaging light exposure using the ThorImage LS 3.1 software (Thorlabs). Parameters used for photodamage and image acquisition are listed in Table 1. Laser excitation intensity and frame averaging were adjusted to minimize photobleaching and phototoxicity, while achieving enough signal to noise ratio and temporal resolution.
Table 1.
Photodamage and image acquisition parameters
| Parameter | Value |
|---|---|
| Excitation and photodamage wavelength | 920 nm |
| Photodamage optical power in the focal plane | 118 mW |
| Photodamage duration | 500 ms |
| Average focal plane optical power used for imaging | 25 mW |
| Frame size (pixels) | 512 × 512 |
| Pixel dwell time | 81.2 ns |
| Raw frame rate | 45.7 fps |
| Number of averaged frames | 9 |
| Effective frame rate | 2.5 fps |
| Resolution | 0.62 µm/pixel |
Intravital multiphoton microscopy and drug delivery
Mice were anesthetized with an intraperitoneal (i.p.) injection of physiological solution containing 90 mg/kg ketamine and 0.5 mg/kg medetomidine. If the experiment duration was longer than 2 hours, half-dose of the anesthesia was reinjected to avoid mouse awaking. Mice were positioned on the stage of the multiphoton microscope equipped with a heated pad kept at 35°C for homeothermic control. Ca2+ signals were recorded from the basal keratinocytes of the mouse pinna (earlobe) skin, fixed under the objective with double-sided tape. For optical coupling, a drop of phosphate buffered saline (PBS) solution was placed between the skin and the 25× water-immersion objective lens. The PBS solution was composed of 10 mM Phosphate (as sodium phosphates), 2.68 mM KCl and 140 mM NaCl.
After positioning the animal under the microscope objective lens, we waited 10 minutes to allow intracellular Ca2+ (mobilized during earlobe manipulation) to return to baseline (as judged by low levels of GCaMP6s fluorescence). For each mouse, control photodamage experiments were repeated n ≥ 3 times with similar results in different positions of the earlobe. Next, to block or enhance a specific pathway, the ear surface was gently cleaned and dried and a 4 µl solution containing a selected drug (Table 2) diluted in PBS was microinjected in the earlobe skin using a 10 µl NANOFIL syringe (World Precision Instruments Inc., Sarasota, FL, USA) equipped with a gauge 33 needle. The microinjected PBS solution also contained 1.78 µM of fluorescent Dextran Texas Red (molecular weight, MW = 70,000 Da; single-photon Excitation/Emission wavelengths = 595/615 nm; two-photon excitation wavelength = 920 nm) to confirm that the drug-containing fluid reached the imaged area. Thereafter, the same photodamage protocol used in the previous control recording was used to acquire image sequences in the presence of the selected drug. A dedicated series of experiments was performed to verify that photodamage-evoked Ca2+ waves were insensitive to the presence of the microinjected fluorescent Dextran Texas Red. Due to its large MW, Dextran diffused slowly through the tight layers of keratinocytes and accumulated mainly in the extracellular spaces below the stratum basale. The investigator was not blinded during administration of treatments or result assessment. No samples were excluded from analyses. To minimize animal stress and avoid cross-interaction between different drugs, no more than one drug was injected in the same earlobe.
Table 2.
Details of drugs used to dissect components of Ca2+ wave propagation mechanisms. The last column reports the serial number of the mice used for the corresponding experiments.
| Reagent | Concentration | Part number | Supplier | Mouse # |
|---|---|---|---|---|
| Dextran Texas RedTM | 1.78 µM | D1830 | ThemoFisher Scientific | 1–18 |
| Apyrase | 500 U/ml | A2230 | Merck & Co. | 3, 4 |
| PPADS | 625 µM | P178 | Merck & Co. | 5, 6 |
| ARL 67156 | 400 µM | A265 | Merck & Co. | 7, 8 |
| EGTA | 5 mM | E3889 | Merck & Co. | 9, 10 |
| Thapsigargin | 400 nM | T9033 | Merck & Co. | 11, 12 |
| CBX | 400 µM | C4790 | Merck & Co. | 13, 14 |
| Probenecid | 4 mM | P8761 | Merck & Co. | 15, 16 |
| TAT-Gap19 | 200 µM | A230580 | GenoSphere | 17, 18 |
Images acquired with different PMTs of the multiphoton microscope are shown in Figure S3 together with the corresponding composite images. In general, a given focal plane intercepts furrows and bulges, exposing a multitude of cell populations and structures while performing intravital microscopy in the skin. Cells corresponding to different epidermal layers can be distinguished morphologically: smaller polygonal cells are keratinocytes of the stratum basale, while increasingly larger cells correspond to the suprabasal layers. Second-harmonic generation (SHG) images were acquired at 460/50 nm emission wavelength, allowing the imaging of collagen fibers (Figure S3 A, E). GCaMP6s fluorescence (and keratinocytes autofluorescence) contributed to the signal detected in the 525/40 nm emission channel (Figure S3 B, F). Images in Figure S3 C, G were acquired in the 647/70 nm emission channel respectively before and after the microinjection of fluorescent Dextran Texas Red.
Image processing and data analysis
To establish a relationship between the 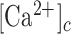 and GCaMP6s fluorescence emission F, we treated GCaMP6s as a Ca2+ buffer (denoted as G) that is present in the cytosol at a concentration [G]. Chelation of Ca2+ by buffer G to form a complex CaG is described by the reaction:
and GCaMP6s fluorescence emission F, we treated GCaMP6s as a Ca2+ buffer (denoted as G) that is present in the cytosol at a concentration [G]. Chelation of Ca2+ by buffer G to form a complex CaG is described by the reaction:
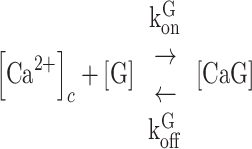 |
and the corresponding kinetic equation:
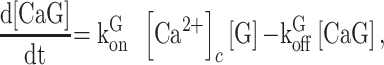 |
(E1) |
where square brackets are used to indicate concentration, 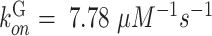 is the rate constant for Ca2+ binding to G and
is the rate constant for Ca2+ binding to G and 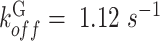 is the rate constant for Ca2+ dissociation.36
is the rate constant for Ca2+ dissociation.36
At chemical equilibrium  , therefore
, therefore
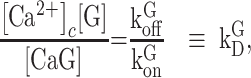 |
(E2) |
where 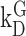 is the equilibrium or dissociation constant, thus
is the equilibrium or dissociation constant, thus
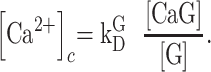 |
(E3) |
Assuming that [G] is low enough, F can be written as the following linear combination:
 |
(E4) |
where the proportionality constant Sf and Sb lump all factors such as the illumination intensity, the efficiency of fluorescence emission, the molar absorption coefficient, and the length of the absorbing medium traversed by the illuminating beam. If cT denotes the total (constant) fluorophore concentration, the mass balance equation is:
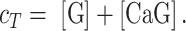 |
(E5) |
Defining the emission under Ca2+-saturating and Ca2+-free conditions respectively as 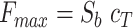 and
and 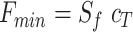 , the expression for F can be re-written as
, the expression for F can be re-written as
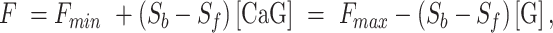 |
(E6) |
yielding
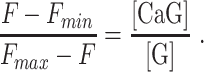 |
(E7) |
Combining equations E3 and E7, we conclude that
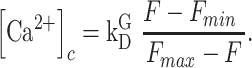 |
(E8) |
Equation E8 can be used to estimate the change in the 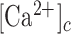 ,
,
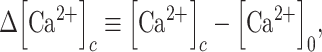 |
(E9) |
where 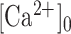 is the pre-stimulus (baseline) concentration. For changes that fall within the approximately linear region of the input/output relation, we may write:
is the pre-stimulus (baseline) concentration. For changes that fall within the approximately linear region of the input/output relation, we may write:
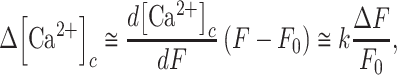 |
(E10) |
where  is pre-stimulus fluorescence and
is pre-stimulus fluorescence and
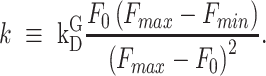 |
(E11) |
Thus, if imaging experiments are performed in such a way that photobleaching is negligible, and as long cT and the optical path length do not change during the measurement, the pixel-by-pixel ratio
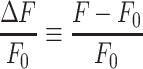 |
(E12) |
is a unique function of the stimulus-induced 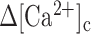 .39
.39
On this ground, image processing and data analysis used to relate 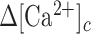 and
and 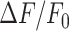 were carried out using the open source ImageJ software, the MATLAB programming environment (R2015a, The MathWorks, Inc., Natick, MA, USA) and Vimmaging, a custom-made software developed under the MATLAB environment by Catalin D. Ciubotaru and Fabio Mammano. To automatically select cell boundaries in an image obtained by averaging all frames in a calcium wave, we used roiSelection, a Matlab graphics user interface (GUI) developed by Catalin D. Ciubotaru.
were carried out using the open source ImageJ software, the MATLAB programming environment (R2015a, The MathWorks, Inc., Natick, MA, USA) and Vimmaging, a custom-made software developed under the MATLAB environment by Catalin D. Ciubotaru and Fabio Mammano. To automatically select cell boundaries in an image obtained by averaging all frames in a calcium wave, we used roiSelection, a Matlab graphics user interface (GUI) developed by Catalin D. Ciubotaru.
To eliminate contributions independent of GCaMP6s expression, all image analyses were preceded by background subtraction. To define the background-subtraction procedure, four images of basal layer keratinocytes were recorded from a wild type mouse (not expressing GCaMP6s, Figure S4) under the same conditions used for all other experiments (see Table 1). Histograms of pixel intensity  were extracted from regions of interest (ROIs) of 76×76 pixels with ImageJ and fitted with a Gaussian distribution
were extracted from regions of interest (ROIs) of 76×76 pixels with ImageJ and fitted with a Gaussian distribution
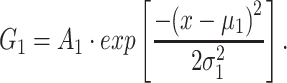 |
(E13) |
The fitting parameters for wild type mouse epidermis were μ1 = 440 ± 1 and σ1 = 31 ± 4.
Next, we examined basal layer keratinocytes in a cohort of n = 15 GCaMP6s-expressing mice. The distribution of baseline pixel intensity values in ROIs composed of 76×76 pixels, averaged over the first 5 frames preceding the photodamage stimulus (that occurred at frame 6), followed a bimodal distribution that was well fitted by the sum of two Gaussians, G1 + G2, where
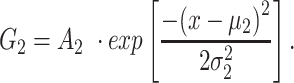 |
(E14) |
The parameters μ1 = 440 ± 2 and σ1 = 30 ±1 (mean ± s.e.m.) of G1 for the GCaMP6s samples were not significantly different from those of the wild type control (p = 0.98 for μ1; p = 0.5 for σ1; t-test). In contrast, the parameters μ2 = 697 ± 9 and σ2 = 206 ± 5 (mean ± s.e.m.) of G2 differed significantly from those of the wild type control (P = 2×10–10 for μ1; P = 2×10–12 for σ1; t-test). Therefore, we attributed the pixel intensity distributions corresponding to G1 to the sum of instrument noise/offset plus keratinocyte autofluorescence, and the distributions corresponding to G2 to GCaMP6s pre-stimulus (baseline) fluorescence. Based on this analysis, the intersection between the two fitting curves (Figure S5, vertical dash-dotted lines), was selected as the threshold value to be subtracted from each image of the corresponding sequence. All pixel values below threshold were set to 1, thus eliminating autofluorescence and the instrumental offset. Thereafter, Ca2+ signals evoked by photodamage were quantified as the time-dependent background-subtracted pixel-by-pixel relative changes in fluorescence, 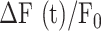 , where F0 is the pre-stimulus fluorescence obtained by averaging over the first 5 (pre-stimulus) frames.
, where F0 is the pre-stimulus fluorescence obtained by averaging over the first 5 (pre-stimulus) frames.
Statistical analysis
Mean values are quoted ± standard error of the mean (s.e.m.). Comparisons of means for non-Gaussian sample distributions were made using the Wilcoxon Rank sum test (as implemented in the Matlab function ranksum). For samples that had Gaussian distribution, the two-sample t-test (as implemented in the Matlab function ttest2) was adopted; P = P-value < 0.05 was assumed as statistically significant.
Immunofluorescence
Adult mice were euthanized by cervical dislocation. Hair was gently removed from earlobe skin by applying hair removal cream for 3 min. Thereafter the cream was removed with cotton buds and the skin surface was rinsed with PBS. Earlobes were then excised and immersed in PBS.
For whole mount preparations, skin (dermis and epidermis) was mechanically detached from the underlining cartilage and transferred in a Petri dish containing ice-cold PBS. Dermal fat was gently peeled off with a scalpel. The skin sample was then divided in about 3 × 3 mm2 portions, immersed in ice-cold PFA (4%) and shaken gently for 1 hour at 4°C.
For slice preparations, ∼1 mm-thick slices were obtained from the earlobe sample by free-hand cutting with a scalpel. Slices were then immersed in ice-cold PFA (4%) and shaken gently for 1 hour at 4°C.
After fixation as described above, whole mount and slice preparations were washed 3 times for 5 min in 1 mL of PBS and incubated for 30 min at room temperature in blocking buffer [PBS complemented with 10% heat-inactivated fetal bovine serum (FBS, 10% v/v, Cat. No. 10270-106, Thermo Fisher Scientific) and 0.2% Tryton X-100 (TX-100, #X100, Sigma)]. Samples were then incubated overnight in a humidified chamber at 4°C with primary anti-P2Y1R or anti-P2Y2R antibodies (#APR-009 and #APR-010, respectively, Polyclonal, knock out-validated, Alomone Labs, Jerusalem, Israel) diluted 1:100 in blocking buffer. The next day, samples were washed 3 times for 15 min at room temperature with washing buffer (2% FBS diluted in PBS with 0.2% TX-100) and incubated with cross-adsorbed Alexa Fluor 488 secondary antibody (1:500, #A-11008, Thermo Fisher Scientific) for 1 h at room temperature. Then, samples were washed 3 times for 15 minutes in washing buffer at room temperature and stained with DAPI (5 µM in washing buffer for 5 min). Samples were finally mounted in a liquid anti-fade medium (Mowiol 4–88) between a microscope slide and a coverslip. After 2 hours, coverslips were sealed with nail polish.
Fluorescence images acquisition was performed with a confocal TCS SP5 microscope (Leica) equipped with a 63× oil immersion objective (HC PL Apo, UV optimized, NA 1.4, Leica). DAPI, Alexa 488 and autofluorescence signal were excited with 405 nm, with 488 nm and with 543 nm laser light, respectively. DAPI fluorescence was collected between 415 nm and 480 nm; Alexa Fluor 488 fluorescence was collected between 500 nm and 540 nm; skin autofluorescence was collected between 625 nm and 700 nm. Images were acquired by averaging each line 32 times in a line-by-line sequential protocol (total pixel dwell time about 2.5 ms; pixel size, 120 nm).
Mathematical model
The model we developed is based on radial ATP diffusion from the damaged cell, signaling via P2YRs, PLC and IP3Rs, and the following geometrical arrangement. The photodamaged cell was modeled as a cylinder with radius 5 µm, height 10 µm and center at r = 0 µm. The upper surface of the cell hit by laser radiation was assumed to burst open at time t = 0 seconds, so that its cytosolic ATP content diffused first in the space just above the cell, then radially in the thin (20 nm) clefts between the tightly packed sheets of epidermal keratinocytes.40 We assumed 2-dimensional radial symmetry and discretized the inter-sheet space into 20 rings of radial length 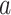 = 10 µm, corresponding to the cellular diameter. ATP in each ring stimulated IP3 production (via P2YRs and PLC) in the adjacent cells above and below the ring, and – vice versa – these cells were allowed to release ATP via connexin HCs into the inter-sheet clefts. The equations for ATP dynamics are41
= 10 µm, corresponding to the cellular diameter. ATP in each ring stimulated IP3 production (via P2YRs and PLC) in the adjacent cells above and below the ring, and – vice versa – these cells were allowed to release ATP via connexin HCs into the inter-sheet clefts. The equations for ATP dynamics are41
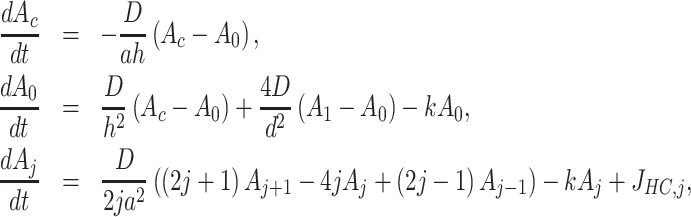 |
(M1) |
where Ac is the ATP concentration in the photodamaged cell, A0 is the ATP concentration in the disk above the damaged cell, and Aj is the ATP level in ring number j of the inter-sheet space, D is the effective diffusion constant of ATP, h = 0.02 µm is the height of the space between cellular sheets,40 k is the degradation rate of ATP, and JHC,j is the flux of ATP via HCs, which is assumed to be a steep Hill-function of the intracellular calcium concentration (Ca) in cell j,42
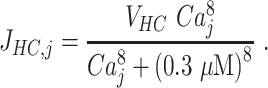 |
(M2) |
ATP binding to P2YRs activates PLC, which produces IP3 from PIP2. All isoforms of PLC depend on Ca2+,43 and in particular PLC-δ, which is expressed in keratinocytes44 and is activated by intracellular Ca2+.45–47 We therefore introduced a feedback term 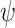 from Ca2+ onto the rate of IP3 production. IP3 dynamics in cell j was described by the equations
from Ca2+ onto the rate of IP3 production. IP3 dynamics in cell j was described by the equations
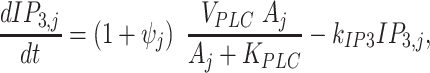 |
(M3) |
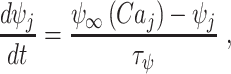 |
(M4) |
where  and
and 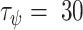 s.
s.
Finally, we modelled cytosolic Ca2+ dynamics by treating the cell cytoplasm as a well stirred closed compartment. Hence, the equation for cytosolic free Ca2+ in cell j reads
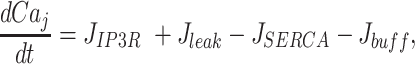 |
(M5) |
where JIP3R is IP3-dependent Ca2+ flux from the ER to cytosol through IP3Rs, which we modelled in accord with the Li-Rinzel model48 (see below),
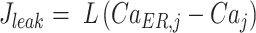 |
(M6) |
represents IP3-independent Ca2+ flux from the ER to cytosol, and
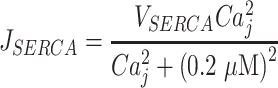 |
(M7) |
represents pumping from the cytosol to the ER by sarco/endo-plasmic reticulum Ca2+-ATPases (SERCA pumps).
The free Ca2+ concentration in the ER is linked to the 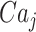 by the conservation equation
by the conservation equation
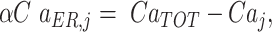 |
(M8) |
where α denotes the ratio of ER to cytosol volume and CaTOT stands for total Ca2+ present within the j-th cell (part in the cytosol, part in the ER). Further, Jbuff is used to model Ca2+ buffering, where, for simplicity, endogenous buffers are assumed to have total concentration twice the concentration of GCaMP6 and to have the same kinetics as GCaMP6. These assumptions lead to
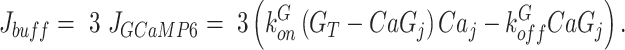 |
(M9) |
The Li-Rinzel model for IP3 receptors48 assumes that Ca2+ and IP3 rapidly and independently activate the channels, whereas Ca2+ more slowly inactivates the channels. The Ca2+ flux from ER to cytosol through the IP3Rs in cell j was modeled as
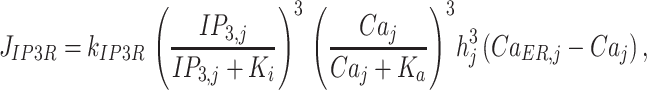 |
(M10) |
where the inactivation variable h follows Hodgkin-Huxley-like dynamics,
 |
(M11) |
To relate model and experimental GCaMP6 signals, we used the following equation representing binding/unbinding of cytosolic Ca2+ to GCaMP6s,
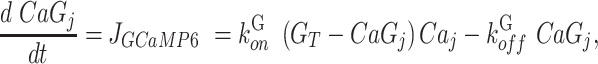 |
(M12) |
normalized the simulated CaG traces within the interval [0,1] and compared them to the corresponding normalized experimental traces.
The differential equations listed above were solved by using the stiff ode15s solver in MATLABTM version 2019a (The MathWorks Inc., Natick, MA, USA). Model parameters in Table 3 were chosen to give reasonable fits, both quantitatively and qualitatively, to a series of experiments.
Table 3.
List of model parameters
| Parameter | Unit | Value | Description |
|---|---|---|---|
| D | µm2/s | 65 | Effective ATP diffusion constant |
| k | s–1 | 0.27 | ATP degradation constant |
| a | µm | 10 | Cell diameter (and radial discretization step size) |
| h | µm | 0.02 | Height of space between adjacent cellular sheets |
| VHC | µM/s | 1200 | Maximal ATP release rate though connexin HCs |
| VPLC | µM/s | 1.85 | Maximal rate of PLC production |
| KPLC | µM | 1.05 | ATP EC50 value for PLC production |
| kIP 3 | s–1 | 15 | IP3 degradation rate |
| ψmax | - | 0.6 | Maximal feedback strength from Ca2+ onto PLC |
| τψ | s | 30 | Time-constant of feedback from Ca2+ onto PLC |
| L | s–1 | 0.5 | Leak rate |
| VSERCA | µM/s | 65 | Maximal SERCA pump rate |
| α | - | 0.1 | Ratio between cytosolic and ER volumes |
| CaTOT | µM | 2 | Total amount of free cellular Ca2+ |
| kIP 3 R | µM/s | 42625 | Maximal IP3R release rate |
| Ki | µM | 1 | Li-Rinzel model parameter for IP3 receptor activation by IP3 |
| Ka | µM | 0.4 | Li-Rinzel model parameter for IP3 receptor activation by Ca2+ |
| Kd | µM | 0.4 | Li-Rinzel model parameter for IP3 receptor inactivation by Ca2+ |
| A | µM–1 s–1 | 5 | Li-Rinzel model parameter for IP3 receptor inactivation by Ca2+ |
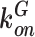
|
µM–1 s–1 | 7.78 | Ca2+-GCaMP6s association constant129 |
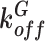
|
s–1 | 1.12 | Ca2+-CaGCaMP6s dissociation constant129 |
| GT | µM | 7.4 | Total GCaMP6s concentration130 |
Results
Dynamics of Ca2+ waves elicited by focal intradermal photodamage in the earlobe skin of live anesthetized mice
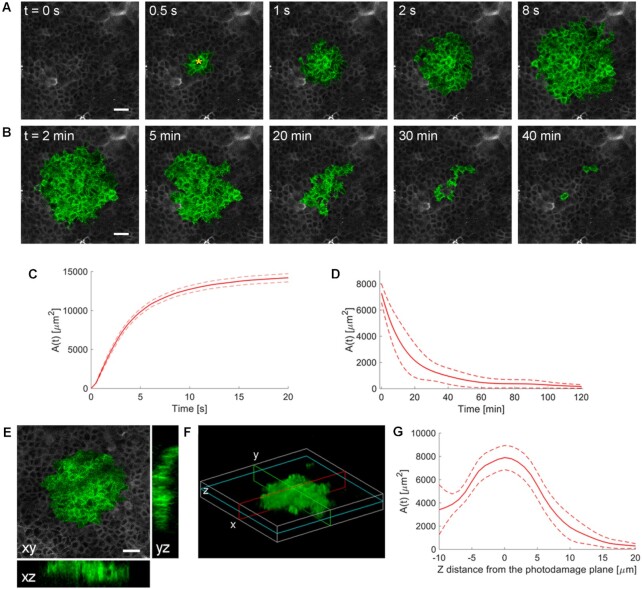
To photodamage a single keratinocyte in the basal epidermal layer of earlobe skin in live anesthetized mice expressing the GCaMP6s Ca2+ indicator, we used the second scanning head of the multiphoton microscope (GG branch of the optical path, see Methods). Ca2+ waves propagated radially from the damaged to the surrounding/bystander epidermal cells (referred to as expansion phase, Figure 1 A). Fluorescence signals persisted for several minutes in keratinocytes invaded by the Ca2+ wave, and slowly returned to basal levels (referred to as waning phase, Figure 1 B). Mean ± s.e.m. of the area A(t) invaded by the Ca2+ wave are shown for both expansion and waning phases in Figure 1 C, D. A(t) was computed by counting all pixels in which 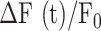 (see Methods) exceeded an arbitrary threshold, corresponding to 30% of the maximum signal achieved as a result of photodamage in the given image sequence.
(see Methods) exceeded an arbitrary threshold, corresponding to 30% of the maximum signal achieved as a result of photodamage in the given image sequence.
Figure 1.
Intravital multiphoton microscopy of Ca2+ waves elicited by focal photodamage in the epidermal basal layer of earlobe skin in live anesthetized mice. (A, B) Representative sequences of GCaMP6s fluorescence images acquired at shown time points from the instant preceding the beginning of the photodamage (t = 0 s) showing (A) Ca2+ wave expansion from the photodamage site (yellow asterisk in the 0.5 s image) and (B) subsequent wave waning. (C, D) Invaded area vs. time, A(t), during Ca2+ wave expansion (C) and waning (D). (C) Mean (solid line) ± s.e.m. (dashed lines) of n = 60 experiments in 14 mice. (D) mean (solid line) ± s.e.m. (dashed lines) of n = 3 experiments in 1 mouse. (E, F) Volume of tissue invaded by the Ca2+ wave after focal photodamage. (E) Views of the wave in the xy plane (focal plane) and two orthogonal (xz and yz) planes. (F) 3D rendering of the volume invaded by the wave; cyan, red and green lines indicate the xy, xz and yz planes of panel (E), respectively. (G) Invaded area vs. axial coordinate (z, along the optical axis of the objective lens); data are mean (solid line) ± s.e.m. (dashed lines) of n = 7 experiments in 1 mouse. Scale bars in A, B, E: 25 µm.
The volume of tissue invaded by the Ca2+ wave at the time of maximal expansion (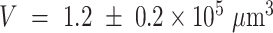 ) was visualized by acquiring through-focus image sequences (also known as z-stacks) at 2 µm increments along the optical axis (z) of the objective lens (Figure 1 E, F). The graph
) was visualized by acquiring through-focus image sequences (also known as z-stacks) at 2 µm increments along the optical axis (z) of the objective lens (Figure 1 E, F). The graph 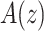 of the invaded area vs. depth (Figure 1 G) shows that Ca2+ waves extended up to ∼10 µm above the photodamage plane, and occasionally stimulated cells with the typical morphology of Langerhans cells, reaching the surface of the thin earlobe mouse skin (corneocyte layer). In the opposite, dermal direction, Ca2+ waves reached down to ∼20 µm below the photodamage plane invading the fibroblast-populated collagen matrix. Given the approximate radial symmetry of Ca2+ waves during the expansion phase in the focal plane, the equivalent radius of the invaded area was computed as
of the invaded area vs. depth (Figure 1 G) shows that Ca2+ waves extended up to ∼10 µm above the photodamage plane, and occasionally stimulated cells with the typical morphology of Langerhans cells, reaching the surface of the thin earlobe mouse skin (corneocyte layer). In the opposite, dermal direction, Ca2+ waves reached down to ∼20 µm below the photodamage plane invading the fibroblast-populated collagen matrix. Given the approximate radial symmetry of Ca2+ waves during the expansion phase in the focal plane, the equivalent radius of the invaded area was computed as
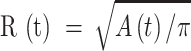 |
(R1) |
(Figure 2 A). The maximal value Rmax = max[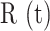 ] averaged over the control experiments that gave the largest waves was 67 ± 1 µm (mean ± s.e.m.; n = 21 experiments in 6 mice).
] averaged over the control experiments that gave the largest waves was 67 ± 1 µm (mean ± s.e.m.; n = 21 experiments in 6 mice).
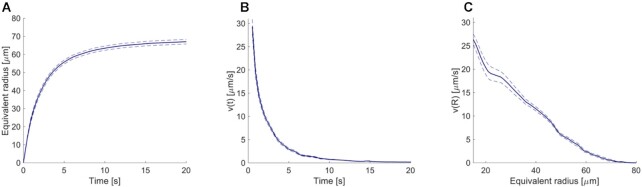
Figure 2.
Detailed analysis of the expansion phase. (A) Equivalent radius of the area invaded during Ca2+ wave expansion. (B) Propagation velocity of the Ca2+ wave as function of time and (C) of the equivalent radius. Mean (blue solid lines) ± s.e.m. (blue dashed lines) of n = 21 experiments in 6 mice.
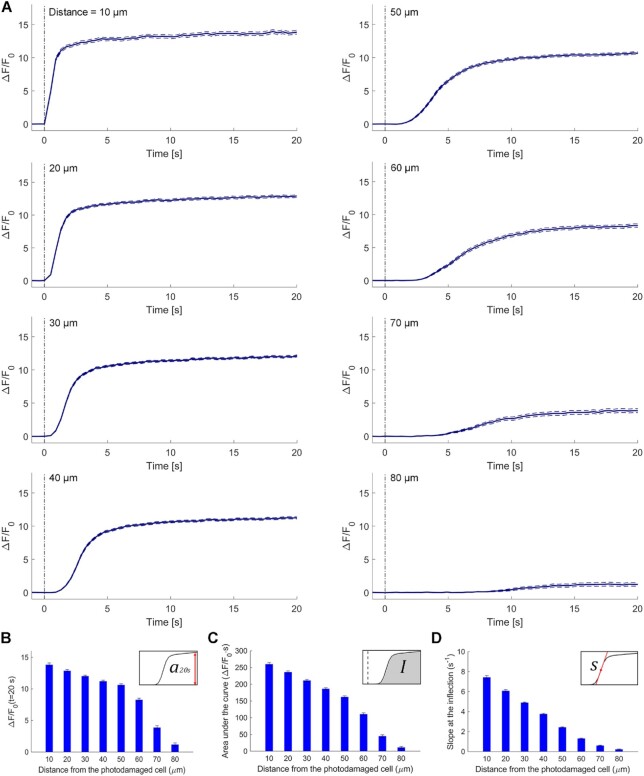
To further characterize the expansion phase, we computed whole-cell 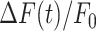 responses by spatially averaging pixel signals over individual bystander keratinocytes. Data from different keratinocytes in different experiments were grouped and averaged based on the distance d of the cell centroid from the photodamage site in the range from 10 ± 3 µm to 80 ± 3 µm (Figure 3 A). Note that this range exceeds the average value of Rmax given above. The discrepancy is due to the 30% threshold criterion used to select pixels that contributed to the estimate of the area A(t) invaded by the Ca2+ wave. In general
responses by spatially averaging pixel signals over individual bystander keratinocytes. Data from different keratinocytes in different experiments were grouped and averaged based on the distance d of the cell centroid from the photodamage site in the range from 10 ± 3 µm to 80 ± 3 µm (Figure 3 A). Note that this range exceeds the average value of Rmax given above. The discrepancy is due to the 30% threshold criterion used to select pixels that contributed to the estimate of the area A(t) invaded by the Ca2+ wave. In general 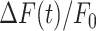 responses at 70 ± 3 µm and 80 ± 3 µm remained subthreshold, and therefore did not contribute to A(t).
responses at 70 ± 3 µm and 80 ± 3 µm remained subthreshold, and therefore did not contribute to A(t).
Figure 3.
Analysis of GCaMP6s fluorescence changes in bystander keratinocytes at increasing distance from the photodamage site. (A) In each panel, the vertical black dash-dotted line at 0 s marks the end of the 0.5 s photodamage time interval. Data are mean (solid line) ± s.e.m. (dashed lines) vs. time for n = 60 experiments in 15 mice. (B) Amplitude (a20s) of the 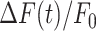 signal at time t = 20 s. (C) Area (I) under the
signal at time t = 20 s. (C) Area (I) under the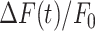 trace, computed between 0 and 20 s. (D) Slope (s) of the
trace, computed between 0 and 20 s. (D) Slope (s) of the 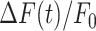 trace at the inflection point (mean ± s.e.m). Data in (B-D) are mean ± s.e.m. vs. bystander cell distance from the photodamage site.
trace at the inflection point (mean ± s.e.m). Data in (B-D) are mean ± s.e.m. vs. bystander cell distance from the photodamage site.
From the analysis of 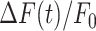 traces, we extracted 3 parameters for each order of bystander keratinocytes. The first parameter is the amplitude reached at time t = 20 s,
traces, we extracted 3 parameters for each order of bystander keratinocytes. The first parameter is the amplitude reached at time t = 20 s,
 |
(R2) |
which decreased slowly with d, up to a critical value dstop (typically comprised between 70 µm and 80 µm) beyond which wave propagation ceased abruptly and a20s approached zero (Figure 3 B).
The second parameter is the Ca2+ load imparted on the cell by the Ca2+ wave, computed as the integral
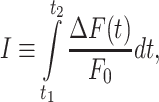 |
(R3) |
where t1 = 0 and t2 = 20 s. I increases if the amplitude of 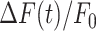 increases and/or the velocity of wave propagation increases (due to the fixed limits of the integration interval). For the same reason, due to the progressively delayed rise of the Ca2+ response, I decreases with d (Figure 3 C).
increases and/or the velocity of wave propagation increases (due to the fixed limits of the integration interval). For the same reason, due to the progressively delayed rise of the Ca2+ response, I decreases with d (Figure 3 C).
The third parameter is the response speed of the cell
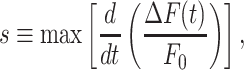 |
(R4) |
computed as the slope of the trace around the inflection point of its rising phase. Clearly, s depends both on the velocity of wave propagation and the kinetics of the signal transduction chain that promotes the 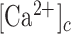 increase in the given cell. Altogether, a cohort of 17 control mice were used to generate the data presented in Figures 1-3. The results show that s was a monotonically decreasing function of d (Figure 3 D).
increase in the given cell. Altogether, a cohort of 17 control mice were used to generate the data presented in Figures 1-3. The results show that s was a monotonically decreasing function of d (Figure 3 D).
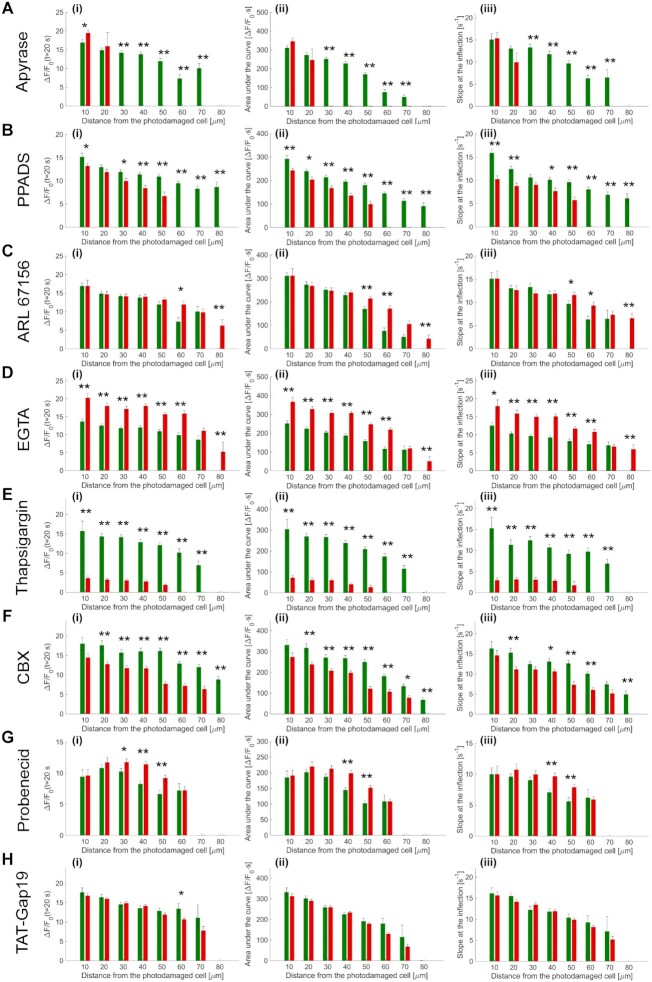
To explore the mechanisms underlying the propagation of Ca2+ waves, we used different drugs (Table 2) that interfere (more or less specifically) with candidate pathways. A different cohort of 18 mice were used for these in vivo pharmacological interference experiments, whose results are summarized graphically in Figure 4 and described in detail hereafter and in the Figures S6-S14.
Figure 4.
Summary of pharmacological interference experiments results. (i) Amplitude (a20s) of the 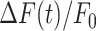 signal at time t = 20 s. (ii) Area (I) under the
signal at time t = 20 s. (ii) Area (I) under the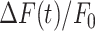 trace, computed between 0 and 20 s. (iii) Slope (s) of the
trace, computed between 0 and 20 s. (iii) Slope (s) of the 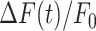 trace at the inflection point (mean ± s.e.m). Data are mean ± s.e.m. vs. bystander cell distance from the photodamage site in control conditions (green) and after VS microinjection (red) containing one of the following drugs: apyrase 500 U/ml (A), PPADS 625 µM (B), ARL 400 µM (C), EGTA 5 mM (D), thapsigargin 400 nM (E), carbenoxolone 200 µM (CBX, F), probenecid 4 mM (G), TAT-Gap19 200 µM (H). Significant differences are shown above each pair of bars (two-sample t-test): * = P-value < 0.05; ** = P-value < 0.005.
trace at the inflection point (mean ± s.e.m). Data are mean ± s.e.m. vs. bystander cell distance from the photodamage site in control conditions (green) and after VS microinjection (red) containing one of the following drugs: apyrase 500 U/ml (A), PPADS 625 µM (B), ARL 400 µM (C), EGTA 5 mM (D), thapsigargin 400 nM (E), carbenoxolone 200 µM (CBX, F), probenecid 4 mM (G), TAT-Gap19 200 µM (H). Significant differences are shown above each pair of bars (two-sample t-test): * = P-value < 0.05; ** = P-value < 0.005.
Initially, a dedicated set of experiments were conducted to ascertain the effects of microinjecting solely vehicle solution (VS = PBS supplemented with Dextran Texas Red, 1.78 μM; see Methods) in the earlobe skin proximal to the photodamage site. Statistical analyses of the results indicate that the parameters used to characterize wave expansion after photodamage were largely unaffected by VS microinjection (Figure S6). Because VS injection had no significant influence on the parameters used to characterize quantitatively the expansion phase of Ca2+ waves, all successive experiments were performed in the following way: (1) a certain number of waves (used as reference) were evoked in n ≥ 3 non-overlapping areas of the naïve mouse earlobe epidermis; thereafter, (2) different areas of the same earlobe were microinjected with the drug of interest dissolved in VS and a (3) second set of waves were evoked (in n ≥ 3 non-overlapping areas reached by Dextran Texas Red), (4) recorded and (5) compared with the previously acquired reference waves.
Based on co-injected Dextran Texas Red fluorescence, microinjected drugs diffused in an area with a diameter of about 3 mm, wide enough to contain more than 20 non-overlapping fields of view (FOVs). In each non-overlapping FOV, a single photodamage experiment was carried out generating a wave that remained completely confined within the FOV (Figure S15).
Critical role of ATP release in photodamage-induced Ca2+ waves
ATP in the extracellular milieu may couple to P2 purinoceptors on the cell plasma membrane to develop Ca2+ waves with or without involvement of IP3 diffusion through intercellular gap junction channels (IGJCs).15 To test the hypothesis that photodamage triggered Ca2+ waves by promoting ATP release from the photodamaged keratinocyte, we injected 4 µl of VS containing apyrase (500 U/mL), an enzyme that catalyzes ATP hydrolysis.49 The presence of microinjected apyrase in the extracellular milieu correlated with a highly significant reduction of the invaded area 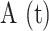 , therefore also of the equivalent Ca2+ wave radius
, therefore also of the equivalent Ca2+ wave radius 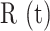 (Figure S7 A, A’). From the same experiments, we derived and analyzed
(Figure S7 A, A’). From the same experiments, we derived and analyzed  traces from bystander keratinocytes and confirmed that apyrase severely hampered Ca2+ wave propagation, such that keratinocytes at distances d > 20 μm from the photodamage site failed to respond (Figure S7 D, D’). Consequently, all three critical parameters used to characterize these responses (a20s, I and s) were null at d > 20 μm (Figure 4A, Figure S7 E-G, E’-G’).
traces from bystander keratinocytes and confirmed that apyrase severely hampered Ca2+ wave propagation, such that keratinocytes at distances d > 20 μm from the photodamage site failed to respond (Figure S7 D, D’). Consequently, all three critical parameters used to characterize these responses (a20s, I and s) were null at d > 20 μm (Figure 4A, Figure S7 E-G, E’-G’).
To determine whether P2 purinoceptors contributed to photodamage-evoked Ca2+ waves, we verified P2YR expression in keratinocytes of basal layer of the earlobe epidermis by immunofluorescence analysis with specific knock-out validated antibodies against P2Y1R and P2Y2R (Figure S16). Next, we microinjected 4 µl of VS containing pyridoxal phosphate-6-azo (benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPADS, 625 µM), a P2 purinoceptor antagonist.31,50 PPADS reduced significantly the area invaded by Ca2+ waves following focal photodamage (Figure S8 A, A’) and slowed down significantly 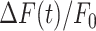 responses in all bystander keratinocytes (Figure S8 B-D, B’-D’). The parameters a20s, I and s were significantly reduced in PPADS compared to controls at d > 30 μm, and the arrest distance dstop decreased from 80 μm (control) to 50 μm (PPADS) (Figure 4 B, Figure S8 E-G, E’-G’).
responses in all bystander keratinocytes (Figure S8 B-D, B’-D’). The parameters a20s, I and s were significantly reduced in PPADS compared to controls at d > 30 μm, and the arrest distance dstop decreased from 80 μm (control) to 50 μm (PPADS) (Figure 4 B, Figure S8 E-G, E’-G’).
Once released in the extracellular space, ATP is rapidly hydrolyzed by cell surface-located ectonucleotidases, in particular by members of the ecto-nucleoside triphosphate diphosphohydrolase family (NTPDase1,2,3, and 8).51–53 Therefore, we predicted that interfering with ectonucleotidase function should influence Ca2+ wave propagation. To test this hypothesis, we injected 4 µl of VS containing ARL 67156 trisodium salt hydrate (shortened as ARL, 400 µM), a widely used NTPDase inhibitor.54–56 The presence of microinject ARL in the extracellular milieu correlated with a significant increase of the area 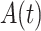 invaded by Ca2+ waves, hence of
invaded by Ca2+ waves, hence of 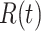 (Figure S9 A, A’). ARL increased also the velocity
(Figure S9 A, A’). ARL increased also the velocity 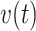 of Ca2+ wave propagation (Figure S9 B, C, B’, C’), confirmed by the significantly reduced lag with which
of Ca2+ wave propagation (Figure S9 B, C, B’, C’), confirmed by the significantly reduced lag with which 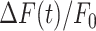 traces grew after photodamage in bystander keratinocytes at d > 20 μm (Figure S9 D, D’). In addition, ARL increased significantly the parameters a20s, I and s at d > 40 μm (Figure 4C, Figure S9 E-G, E’-G’).
traces grew after photodamage in bystander keratinocytes at d > 20 μm (Figure S9 D, D’). In addition, ARL increased significantly the parameters a20s, I and s at d > 40 μm (Figure 4C, Figure S9 E-G, E’-G’).
Together, the results in Figures S7-S9 indicate that (ii) extracellular ATP is a key signaling molecule underlying Ca2+ wave propagation in the photodamaged epidermis; (ii) the area 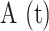 invaded by the Ca2+ wave and its velocity of propagation
invaded by the Ca2+ wave and its velocity of propagation 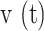 depend on degradation of extracellular ATP by NTPDases expressed by epidermal keratinocytes (which can be partially inhibited by ARL).
depend on degradation of extracellular ATP by NTPDases expressed by epidermal keratinocytes (which can be partially inhibited by ARL).
Role of extracellular Ca2+
To determine the role of extracellular Ca2+ in the dynamics of photodamage-evoked Ca2+ waves, we microinjected 4 µl of VS containing 5 mM EGTA, a widely used Ca2+ chelator.57 Reduction of the extracellular free Ca2+ concentration ([Ca2+]ex) with EGTA caused a paradoxical enhancement of the invaded area, gauged by the effective radius 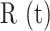 (Figure S10 A, A’), paralleled by a significant increase of
(Figure S10 A, A’), paralleled by a significant increase of 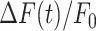 bystander responses at all distances within 60 μm from the photodamaged cell (Figure S10 D, D’). The parameters a20s, I and s were significantly increased in EGTA compared to controls, and the arrest distance dstop increased from 70 μm (control) to 80 μm (EGTA) (Figure 4D, Figure S10 E-G, E’-G’).
bystander responses at all distances within 60 μm from the photodamaged cell (Figure S10 D, D’). The parameters a20s, I and s were significantly increased in EGTA compared to controls, and the arrest distance dstop increased from 70 μm (control) to 80 μm (EGTA) (Figure 4D, Figure S10 E-G, E’-G’).
Because EGTA increased Ca2+ responses, Ca2+ influx through plasma membrane channels (including P2XRs28) did not to contribute significantly to the observed increments of the 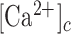 . Hence, the latter should be attributed chiefly to signal transduction through P2YRs, via the coupling to G proteins, activation of PLC, leading to the formation of IP3 and release of Ca2+ from intracellular stores.29–31,58
. Hence, the latter should be attributed chiefly to signal transduction through P2YRs, via the coupling to G proteins, activation of PLC, leading to the formation of IP3 and release of Ca2+ from intracellular stores.29–31,58
Role of Ca2+ release from the ER
To corroborate the involvement of Ca2+ release from the ER downstream of P2YRs activation by extracellular ATP, we microinjected 4 µl of VS containing 400 nM of thapsigargin (previously dissolved in DMSO), a potent, cell-permeable, selective and irreversible inhibitor of the sarcoplasmic and endoplasmic reticulum Ca2+-ATPase family (SERCA pumps).59 As expected, thapsigargin caused a significant increase in the basal level of GCaMP6s fluorescence, 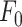 , from
, from 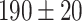 gray levels (GLs) in control conditions to
gray levels (GLs) in control conditions to 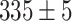 GLs in thapsigargin (P = 0.01, two-sample t-test) due to uncompensated passive leakage of Ca2+ from the ER. Thapsigargin caused Ca2+ waves to propagate over smaller distances, significantly reducing the equivalent radius
GLs in thapsigargin (P = 0.01, two-sample t-test) due to uncompensated passive leakage of Ca2+ from the ER. Thapsigargin caused Ca2+ waves to propagate over smaller distances, significantly reducing the equivalent radius  of the invaded area (Figure S11 A, A’). Moreover, the increased
of the invaded area (Figure S11 A, A’). Moreover, the increased 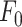 values caused an evident reduction of
values caused an evident reduction of 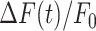 bystander responses at any order of distance and consequent significantly reduced a20s (Figure S11 D, D’, Figure 4 E(i), Figure S11 E, E’). Also the parameters I and s were significantly reduced in thapsigargin vs. controls at all distances d from the photodamage site (Figure 4 E(ii,iii), Figure S11 F, G, F’, G’).
bystander responses at any order of distance and consequent significantly reduced a20s (Figure S11 D, D’, Figure 4 E(i), Figure S11 E, E’). Also the parameters I and s were significantly reduced in thapsigargin vs. controls at all distances d from the photodamage site (Figure 4 E(ii,iii), Figure S11 F, G, F’, G’).
Role connexin HCs and pannexin 1 channels
Open connexin HCs mediate the diffusive release of paracrine messengers, most importantly ATP.60–64 Also pannexin 1 (Panx1) channels65 are permeable to ATP,66 expressed in the epidermis and important for differentiation of keratinocytes and wound healing.67 In addition, Panx1 channels can be activated by extracellular ATP acting through purinergic receptors of the P2Y group, as well as by cytoplasmic Ca2+.68 Therefore, a positive-feedback loop involving the control by cytoplasmic Ca2+ of Panx168 and/or connexin HCs42,69,70 may lead to an overall augmented ATP-induced ATP-release68,71 that would increase both the area invaded by Ca2+ waves and the 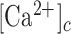 increments in individual keratinocytes. Testing these hypotheses required further pharmacological interventions, as summarized hereafter.
increments in individual keratinocytes. Testing these hypotheses required further pharmacological interventions, as summarized hereafter.
Carbenoxolone (CBX) is a broad-spectrum inhibitor of connexin-made channel (i.e. HCs and IGJCs)72 that inhibits also Panx1 channels65,66 and IP3-dependent Ca2+ release from the ER.73 We injected 4 µl of VS containing CBX (400 µM), which reduced significantly both the area 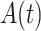 invaded by Ca2+ waves following focal photodamage (Figure S12 A, A’) and the
invaded by Ca2+ waves following focal photodamage (Figure S12 A, A’) and the 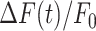 responses in all bystander keratinocytes, except those adjected to the photodamage site (Figure S12 D, D’). The parameters a20s, I and s were significantly reduced by CBX compared to controls at d > 10 μm, and the arrest distance dstop decreased by 20 μm with respect to control conditions (Figure 4F, Figure S12 E-G, E’-G’).
responses in all bystander keratinocytes, except those adjected to the photodamage site (Figure S12 D, D’). The parameters a20s, I and s were significantly reduced by CBX compared to controls at d > 10 μm, and the arrest distance dstop decreased by 20 μm with respect to control conditions (Figure 4F, Figure S12 E-G, E’-G’).
To discriminate between Panx1 and connexin-made channels, we microinjected 4 µl of VS containing 4 mM of probenecid, which affects primarily Panx1 channels.74 Probenecid modified slightly the time course of the equivalent radius 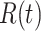 without affecting significantly the area invaded by the Ca2+ waves at their maximal expansion (Figure S13 A, A’). In addition,
without affecting significantly the area invaded by the Ca2+ waves at their maximal expansion (Figure S13 A, A’). In addition, 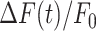 signals were largely unaffected, with the exception of bystander keratinocytes located at 40 μm ≤ d ≤ 50 μm where responses appeared increased rather than decreased by the drug (Figure S13 D, D’). Only in this range of distances, the parameters a20s, I and s were significantly larger in probenecid compared to controls, and the arrest distance dstop was not affected (Figure 4G, Figure S13 E-G, E’-G’). These experiments lead us to conclude that (i) Panx1 channels were not involved in the Ca2+ signaling triggered by focal photodamage and (ii) the effect of CBX may reflect inhibition of connexin-made channel72 and/or IP3-dependent Ca2+ release from the ER.73
signals were largely unaffected, with the exception of bystander keratinocytes located at 40 μm ≤ d ≤ 50 μm where responses appeared increased rather than decreased by the drug (Figure S13 D, D’). Only in this range of distances, the parameters a20s, I and s were significantly larger in probenecid compared to controls, and the arrest distance dstop was not affected (Figure 4G, Figure S13 E-G, E’-G’). These experiments lead us to conclude that (i) Panx1 channels were not involved in the Ca2+ signaling triggered by focal photodamage and (ii) the effect of CBX may reflect inhibition of connexin-made channel72 and/or IP3-dependent Ca2+ release from the ER.73
Various expression profiles of connexins through epidermis layers are reported in the literature, the majority of them agreeing in the predominance of Cx43 in the basal layer of the epidermis.75–86 To discriminate between connexin HCs and IGJCs, we microinjected 4 µl of VS containing 200 µM TAT-Gap19, a peptide that selectively inhibits Cx43 HCs.87 TAT-Gap19 did not change significantly the area invaded by Ca2+ waves (Figure S14 A, A’), whereas 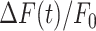 signals in bystander keratinocytes were attenuated at larger distances (60, 70 µm) (Figure S14 D, D’) compared to controls. The parameters a20s, I and s were significantly reduced by Tat-Gap19 compared to controls at d > 50 μm, but the arrest distance dstop was not affected (Figure 4H, Figure S14 E-G, E’-G’).
signals in bystander keratinocytes were attenuated at larger distances (60, 70 µm) (Figure S14 D, D’) compared to controls. The parameters a20s, I and s were significantly reduced by Tat-Gap19 compared to controls at d > 50 μm, but the arrest distance dstop was not affected (Figure 4H, Figure S14 E-G, E’-G’).
Comparison of experimental results with mathematical model
To overcome the limitations of pharmacological dissection, gain further mechanistic insight into these photodamage-evoked Ca2+ waves and determine whether the conclusions drawn from the experimental results can be reproduced coherently, we formulated a mathematical model (see Methods). To solve the system of model differential equations numerically, we assumed radial symmetry and reduced the problem to one dimension (see Methods). Considering the inter-animal variation (Figures S6-S14) the model with the chosen parameters (Table 3) reproduced well the experimental traces averaged over all control experiments (Figure 5A).
Figure 5.
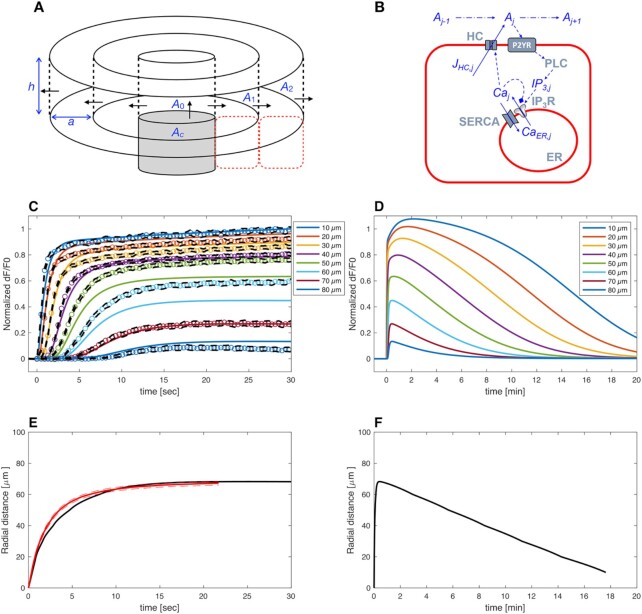
Model simulations for GCaMP6s fluorescence responses and equivalent radius of the area invaded by Ca2+ waves under control condition. (A) Schematic representation of model geometry. ATP dynamics is simulated as being released from the photodamaged cell (grey cylinder; Ac) into a thin disc above the cell (A0). ATP diffusion in the intercellular space is simulated by discretizing in the radial direction assuming radial symmetry. The ATP concentration in each ring (Aj) communicates with the cells above and below (indicated by the red dashed rectangles). (B) Cartoon representing main actors of Ca2+ signaling in this model. HC, connexin hemichannels; SERCA, sarco-endoplasmic reticulum Ca2+ ATPases; IP3R, inositol (1,4,5) triphosphate receptors; PLC, phospholipases C; IP3,j, flux of IP3; Caj, flux of ionized calcium out of endoplasmic reticulum (ER) through IP3R; Aj, extracellular ATP concentration; JHC,j, flux of ATP via connexin HCs; CaER,j, flux of ionized calcium into the ER through SERCA; solid arrows represent net fluxes; dash-dotted arrows represent extracellular diffusion down the concentration gradient; dashed lines represent interactions: arrowheads for stimulatory interactions, diamond for inhibitory interaction. Aj stimulates IP3 production via PLCβ. IP3 in turn stimulates Ca2+ release from the ER via IP3Rs. Intracellular Ca2+ is assumed to stimulate ATP release via HC and to stimulate PLC. In addition, Ca2+ feeds back onto IP3R, and is pumped into the ER via SERCA pumps. (C) Simulated GCaMP6s 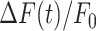 responses at increasing distances, in 10 µm steps, from the center of the damaged cell. Circles show the experimental results at the same distance as indicated by identical colors and specified in the legend. The black dashed curves show s.e.m of the experimental data points. (D) The simulation from panel C is shown here on an extended time scale to include the waning phase of the Ca2+ wave (note change of units from seconds to minutes in the abscissae). (E) Radius of the equivalent Ca2+ wave vs. time computed as the distance at which the response is 30% of the simulated response in panel C (black). The red curve shows corresponding experimental data with s.e.m. indicated by the dashed curves. (F) The simulation from panel E is shown here on an extended time scale to include the waning phase of the Ca2+ wave.
responses at increasing distances, in 10 µm steps, from the center of the damaged cell. Circles show the experimental results at the same distance as indicated by identical colors and specified in the legend. The black dashed curves show s.e.m of the experimental data points. (D) The simulation from panel C is shown here on an extended time scale to include the waning phase of the Ca2+ wave (note change of units from seconds to minutes in the abscissae). (E) Radius of the equivalent Ca2+ wave vs. time computed as the distance at which the response is 30% of the simulated response in panel C (black). The red curve shows corresponding experimental data with s.e.m. indicated by the dashed curves. (F) The simulation from panel E is shown here on an extended time scale to include the waning phase of the Ca2+ wave.
In the model, the simulated 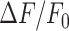 signal raised rapidly close to the damaged cell, followed by gradually more shallow and delayed responses at greater distances. The maximum model responses were achieved within 1–2 minutes after photodamage, thereafter
signal raised rapidly close to the damaged cell, followed by gradually more shallow and delayed responses at greater distances. The maximum model responses were achieved within 1–2 minutes after photodamage, thereafter 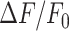 signals slowly returned towards baseline on a timescale of more than 20 minutes for the cells closest to the damage site (Figure 5B). In other words, the simulations show a propagating wave that halts at approximately 80 µm from the damaged cell (Figure 5A) in agreement with the experiments, and with an equivalent radius (i.e., location of 30% response) as a function of time that mimics the experimental behavior (Figure 5C), in particular making allowance for the variability between different experimental animals (Figure S17). The model wave then slowly dissipated so that the equivalent radius reduced to 10 μm after about 18 min (Figure 5D).
signals slowly returned towards baseline on a timescale of more than 20 minutes for the cells closest to the damage site (Figure 5B). In other words, the simulations show a propagating wave that halts at approximately 80 µm from the damaged cell (Figure 5A) in agreement with the experiments, and with an equivalent radius (i.e., location of 30% response) as a function of time that mimics the experimental behavior (Figure 5C), in particular making allowance for the variability between different experimental animals (Figure S17). The model wave then slowly dissipated so that the equivalent radius reduced to 10 μm after about 18 min (Figure 5D).
After photodamage, the relatively large amounts of ATP present in the cytosol of the damaged cell diffused into the tight intercellular space. Near the damaged cells, the model ATP concentration reached tens to hundreds of µM (Figure 6A, B), hence the IP3 producing machinery was completely saturated by ATP (the EC50 parameter in the model is KPLC = 1.05 µM). As a result of the saturation of IP3 production near the damaged cell, steady-state IP3 levels are very similar at d <50 µm (Figure 6C). The long-lived responses with a duration of tens of minutes (Figure 5B) are due to the high ATP concentrations, which decrease only slowly over time due to the modest degradation rate and relatively low effective diffusion constant, D. We used D = 65 µm2/s, which is substantially lower than the diffusion constant of ATP in solution (350–400 µm2/s).88 However, the extracellular space between adjacent cells is about 20 nm,40 i.e., only an order of magnitude larger than an ATP molecule, and is likely a crowded environment, which may slow down diffusion of ATP.89 Indeed, the intracellular diffusion coefficient of ATP is ∼3.5 times lower than the extracellular value.90 Further, ATP is buffered as it binds to e.g. ectonucleotidases, P2YRs and P2XRs on the cell membranes, and it is well-known that fixed buffers can reduce the effective diffusion constant substantially.91
Figure 6.

Model simulations for the concentrations of extracellular ATP and intracellular IP3 under control conditions. (A) Simulated extracellular ATP responses at increasing distances, in 10 µm steps, from the center of the damaged cell as indicated by the color codes in the legend. (B) First 30 sec of the traces plotted in panel A. (C) Simulated intracellular IP3 concentrations at increasing distances in the first 30 sec post damage and in 10 µm steps from the center of the damaged cell (traces color coded as in panel A).
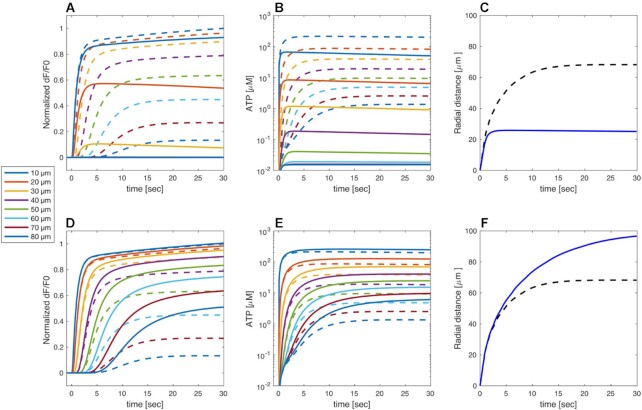
The slowly increasing Ca2+ concentrations seen in the experiments even at very low distances are reproduced by the model because of slow feedback from cytosolic Ca2+ on PLC activity.43–47 Note that this behavior cannot be explained by increased ATP levels, for example due to release from HCs, since any further increase in ATP beyond saturation would not lead to a raise in IP3 and downstream 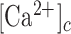 levels. Simulating increased ATP degradation, as in the apyrase experiments, reduced the
levels. Simulating increased ATP degradation, as in the apyrase experiments, reduced the  model responses substantially at d >10 µm, and response were basically suppressed at d >30 µm (Figure 7A). The lack of model Ca2+ responses follows from the greatly reduced ATP levels at all distances (Figure 7B). Consequently, the waves stopped at 20–30 µm from the damaged cell (Figure 7C). In contrast, reducing the rate of ATP consumption to mimic the ARL experiments yielded larger
model responses substantially at d >10 µm, and response were basically suppressed at d >30 µm (Figure 7A). The lack of model Ca2+ responses follows from the greatly reduced ATP levels at all distances (Figure 7B). Consequently, the waves stopped at 20–30 µm from the damaged cell (Figure 7C). In contrast, reducing the rate of ATP consumption to mimic the ARL experiments yielded larger 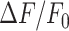 model responses, in particular at larger distances (Figure 7D). The increased model ATP levels seen at all distances (Figure 7E) affected the cells at greater distances more, since their IP3 production was far from saturation. Quantifying, the simulation corresponding to the ARL experiments shows a greater equivalent radius than the control simulation, corresponding to waves that terminate at ∼100 µm (Figure 7F). Together, these model results show that the effects of apyrase and ARL can be explained entirely by interference with extracellular ATP degradation, which controls the extent of extracellular ATP levels.
model responses, in particular at larger distances (Figure 7D). The increased model ATP levels seen at all distances (Figure 7E) affected the cells at greater distances more, since their IP3 production was far from saturation. Quantifying, the simulation corresponding to the ARL experiments shows a greater equivalent radius than the control simulation, corresponding to waves that terminate at ∼100 µm (Figure 7F). Together, these model results show that the effects of apyrase and ARL can be explained entirely by interference with extracellular ATP degradation, which controls the extent of extracellular ATP levels.
Figure 7.
Model simulation of interference with extracellular ATP handling. (A-C) Simulated apyrase experiment performed by increasing 10-fold the ATP degradation rate k (full curves). The simulations under control conditions (from Figures 5 and 6) are shown for comparison as dashed curves with the same color code. (A) Simulated normalized GCaMP6s fluorescence responses, as indicated by the color codes in the legend. (B) Model extracellular ATP levels. (C) Equivalent radii from apyrase (blue solid line) and control (black dashed line, same as black solid line in Figure 5E) simulations. (D-F) Simulated ARL experiment performed by decreasing the ATP degradation rate k by 50% (solid lines). Legends as in panels A-C. Traces in panels A, B, D, E were computed at increasing distances, in 10 µm steps, from the center of the damaged cell.
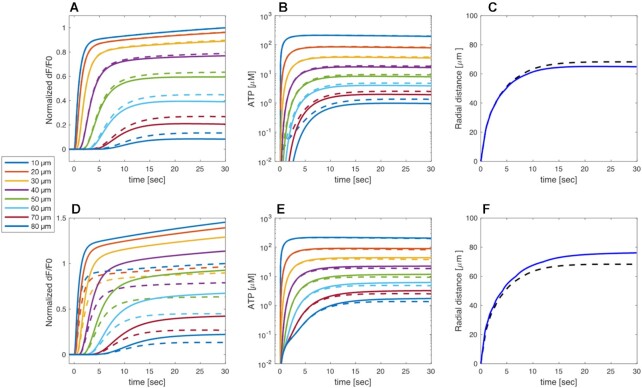
Inhibition of connexin HCs, mimicking the TAT-Gap19 experiments, reduced the model responses at larger distances compared to the control scenario (Figure 8A), in agreement with experiments. Model cells with closed HCs did not release ATP, hence the extracellular ATP levels decreased (Figure 8B) causing a drop in IP3 production and Ca2+ release from the ER. In control conditions, ATP release from HCs was larger close to the damaged cell since the 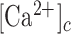 was higher there than further away. However, the extracellular ATP was already saturating even without the contribution from the HCs, so blocking them had virtually no effect on
was higher there than further away. However, the extracellular ATP was already saturating even without the contribution from the HCs, so blocking them had virtually no effect on 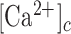 variations at short distances from the photodamaged cell. On the contrary, at larger distances the contribution to extracellular ATP from HCs was relatively important compared to the ATP diffusion from the site of damage. Consequently, inhibiting HCs has a substantial effect on the simulated response. This explains why HCs blockade lowered the extent of wave propagation in the model (Figure 8C). Increasing the maximal rate of IP3 production, VPLC, yielded simulated responses that resembled the experimental data with zero extracellular Ca2+ and EGTA. At all distances, importantly also at the shortest distances from the damaged cell, the
variations at short distances from the photodamaged cell. On the contrary, at larger distances the contribution to extracellular ATP from HCs was relatively important compared to the ATP diffusion from the site of damage. Consequently, inhibiting HCs has a substantial effect on the simulated response. This explains why HCs blockade lowered the extent of wave propagation in the model (Figure 8C). Increasing the maximal rate of IP3 production, VPLC, yielded simulated responses that resembled the experimental data with zero extracellular Ca2+ and EGTA. At all distances, importantly also at the shortest distances from the damaged cell, the 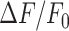 signals increased (Figure 8D) partly because of the positive feedback from Ca2+ on ATP release from HCs, which increased ATP levels at the larger distances (Figure 8E). Consequently, the wave propagated ∼10 µm further than in control simulations (Figure 8F).
signals increased (Figure 8D) partly because of the positive feedback from Ca2+ on ATP release from HCs, which increased ATP levels at the larger distances (Figure 8E). Consequently, the wave propagated ∼10 µm further than in control simulations (Figure 8F).
Figure 8.
Model simulation of connexin HCs inhibition and extracellular Ca 2+ chelation. (A-C) Simulated TAT-Gap19 experiment performed by decreasing the rate of ATP release through connexin HCs (full curves). The simulations under control conditions (from Figures 5 and 6) are shown for comparison as dashed curves with the same color code. (A) Simulated normalized GCaMP6s fluorescence responses, as indicated by the color codes in the legend. (B) Model extracellular ATP levels. (C) Equivalent radii from TAT-Gap19 (blue solid line) and control (black dashed line, same as black solid line in Figure 5E) simulations. (D-F) Simulated EGTA experiment performed by increasing the maximal rate of IP3 production VPLC by 7% (see main text). Legends as in panels A-C. Traces in panels A, B, D, E were computed at increasing distances, in 10 µm steps, from the center of the damaged cell.
Discussion
We have shown here that ATP is released from epidermal keratinocytes of live anesthetized mice following intradermal photodamage caused by intense pulsed IR laser radiation. Kumamoto et al. obtained results compatible with those reported here using point laser stimulation of keratinocytes in ex vivo human epidermis. In particular, they observed different responses in the various layers of the epidermis after stimulating a keratinocyte of the stratum granulosum, with higher responses in the basal layer.13
Insight provided by in vivo pharmacological dissection experiments
The largest effects were caused by drugs that interfere with degradation of extracellular ATP or P2 purinoceptors, suggesting that Ca2+ waves in the photodamaged epidermis are primarily due to release of ATP from the target cell whose plasma membrane integrity was compromised by laser irradiation. As 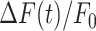 signals in bystander keratinocytes were augmented by exposure to the Ca2+ chelator EGTA in the extracellular medium, the corresponding transient increments of the
signals in bystander keratinocytes were augmented by exposure to the Ca2+ chelator EGTA in the extracellular medium, the corresponding transient increments of the 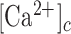 should be ascribed primarily to Ca2+ release from the ER, downstream of ATP binding to P2Y purinoceptors with Ca2+ entry through plasma membrane channels playing a comparatively negligible role. The effect of thapsigargin supports this conclusion.
should be ascribed primarily to Ca2+ release from the ER, downstream of ATP binding to P2Y purinoceptors with Ca2+ entry through plasma membrane channels playing a comparatively negligible role. The effect of thapsigargin supports this conclusion.
PPADS antagonizes P2Y1,92 P2Y4,93 P2Y694 and P2Y1395 receptors (see also ref.29). In contrast, P2Y2Rs are PPADS-insensitive.29,93 Published work on P2Y mRNA expression in murine keratinocytes identified P2Y1R, P2Y2R, P2Y4R and P2Y6R subtypes,96 therefore P2Y2Rs are likely responsible for the incomplete effect of PPADS administration.
The ineffectiveness of probenecid suggests pannexin channels had no role, whereas the limited effect of TAT-Gap19 suggests ATP-dependent ATP release though connexin HCs affected Ca2+ wave propagation only at larger distances, where the concentration of ATP released from the photodamaged cell was reduced by the combined effect of passive diffusion and hydrolysis due to the action of ecto-ATPases. The fact that CBX exerted a stronger inhibitory action compared to TAT-Gap19 may suggest that IGJCs played a more prevalent role than Cx43 HCs in Ca2+ wave propagation. However, CBX has been recently reported to interference also with IP3Rs,97 which would instead lend further support to a fundamental role played by the P2Y-dependent pathway and IP3-dependent Ca2+ release from intracellular stores.
Insight provided by the mathematical model
Our mathematical model reproduced the experimental observations by assuming that extracellular ATP diffusion carried the signal from the damaged to bystander cells, where it activated PLC and increased IP3 levels, which in turn triggered Ca2+ release via IP3Rs. Ca2+ feedback onto PLC caused the slowly rising response during the expansion phase of the Ca2+ waves. Close to the damaged cell, ATP concentrations were saturating for the P2YRs/PLC machinery, whereas the wave stopped further away where degradation and dilution of ATP lowered the ATP concentration to levels insufficient to cause substantial Ca2+ release. Changes in the model degradation rate reproduced experiments with apyrase and ARL. ATP release through connexin HCs contributed comparatively more at longer distances, where the flux of ATP from cytosol raised the extracellular ATP levels sufficiently to yield an intracellular Ca2+ response. However, HCs contribution was eclipsed in the proximity of the damage site by the far preponderant contribution from photodamage.
We also propose a plausible explanation for how chelation of extracellular Ca2+ with EGTA might increase PLC activity. In keratinocytes, extracellular Ca2+ partly activates PKC,98 thus removal of extracellular Ca2+ may ensue in a reduced PKC activity. Since PKC inhibits PLC,44,99,100 PKC deactivation would remove the brake on the rate of IP3 production. Note that alternative explanations invoking an increase in extracellular ATP levels following chelation of extracellular Ca2+, for example because of a direct effect of low Ca2+ on connexin HCs, are incompatible with the saturating ATP levels near the damaged cell.
In the proposed model, we neglected intercellular Ca2+ and IP3 diffusion via inter-cellular gap junction channels since our experiments suggested that extracellular ATP diffusion is the major route of signal propagation, in contrast to other physiological systems (e.g. Refs101–103). Thus, in our model, the waves are propagating with no communication between the bystander cells, except from a near-negligible contribution due to ATP diffusion through hemichannels.
The mechanism for wave block of the simulated abortive waves that we studied here differs from – but also has similarities to – other systems. For example, in gap-junction coupled pancreatic beta-cells, an externally imposed glucose gradient causes abortive, repetitive Ca2+ waves,104,105 which have been proposed to stop because of the gradual reduction in excitability of the cells,106,107 resembling the ATP gradient observed here, although the waves between beta-cells are driven by electrical communication via gap junctions. Heterogeneity in cellular and gap-junction properties in combination with discretization effects may also lead to propagation failure between coupled cells.108–111 Such heterogeneity was not considered here, and the model waves stop simply when the concentration of diffusing ATP becomes too low to stimulate sufficient IP3 production to increase the intracellular Ca2+ concentration.
The observed near-steady and long-lived 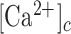 elevations were quantitatively different from the
elevations were quantitatively different from the 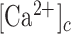 oscillations often observed, for example, in the sensory epithelium of the cochlea following photodamage11,12 or IP3 uncaging.71,112–114 Why are such oscillations not seen in the photodamaged skin? Also in this case, the mathematical model provides a possible explanation. The core of the Ca2+ signaling part of the model is the Li-Rinzel model,48 which is well-known to sustain oscillations, but only for a range of IP3 concentrations. We therefore investigated how the single-cell model depends on the IP3 production rate VPLC (assuming that ATP levels were saturating). The bifurcation diagram in Figure 9 shows that the model has a single stable equilibrium at low VPLC values, which becomes unstable due to a subcritical Hopf bifurcation at VPLC = 2.02. The equilibrium becomes stable again for values above VPLC = 2.44, where another subcritical Hopf bifurcation occurs. The unstable limit cycles born in the Hopf bifurcations become stable at saddle-node of limit cycles bifurcations, thus the model generates stable oscillations for values of VPLC between 2.0 and 2.45 (approximately). Hence, the default value of VPLC = 1.85, which we used to reproduce the experiments, is too low to sustain oscillations. Biologically, this explanation corresponds to the hypothesis that keratinocytes have too few P2YRs and/or amounts of PLC to transduce the high ATP levels into sufficiently high rates of IP3 production to cause
oscillations often observed, for example, in the sensory epithelium of the cochlea following photodamage11,12 or IP3 uncaging.71,112–114 Why are such oscillations not seen in the photodamaged skin? Also in this case, the mathematical model provides a possible explanation. The core of the Ca2+ signaling part of the model is the Li-Rinzel model,48 which is well-known to sustain oscillations, but only for a range of IP3 concentrations. We therefore investigated how the single-cell model depends on the IP3 production rate VPLC (assuming that ATP levels were saturating). The bifurcation diagram in Figure 9 shows that the model has a single stable equilibrium at low VPLC values, which becomes unstable due to a subcritical Hopf bifurcation at VPLC = 2.02. The equilibrium becomes stable again for values above VPLC = 2.44, where another subcritical Hopf bifurcation occurs. The unstable limit cycles born in the Hopf bifurcations become stable at saddle-node of limit cycles bifurcations, thus the model generates stable oscillations for values of VPLC between 2.0 and 2.45 (approximately). Hence, the default value of VPLC = 1.85, which we used to reproduce the experiments, is too low to sustain oscillations. Biologically, this explanation corresponds to the hypothesis that keratinocytes have too few P2YRs and/or amounts of PLC to transduce the high ATP levels into sufficiently high rates of IP3 production to cause 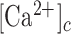 oscillations.
oscillations.
Figure 9.
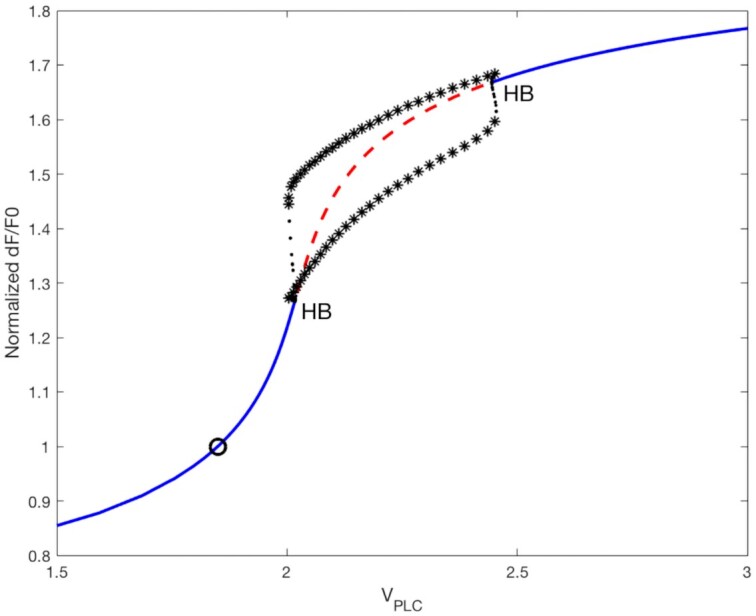
Bifurcation diagram of the single-cell model. The steady-state 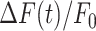 response, normalized to the response at the default value of VPLC= 1.85 µM/s, is shown as a function of the maximal rate of IP3 production, VPLC, under the assumption of saturating ATP levels. At low VPLC values, the steady-state is stable (blue solid line), but loses stability in a Hopf bifurcation (HB) at VPLC≈2.02. The unstable fixpoint (red dashed line) regains stability in another HB at VPLC≈2.44. Unstable limit cycles (black asterisks) are born at both HBs, but due to saddle-node-of-limit-cycles bifurcations at VPLC≈2.0 and VPLC≈ 2.45, stable limit cycles are present between these values. The black circle indicates the default value of VPLC used in the model, which is located below the interval where the system may oscillate.
response, normalized to the response at the default value of VPLC= 1.85 µM/s, is shown as a function of the maximal rate of IP3 production, VPLC, under the assumption of saturating ATP levels. At low VPLC values, the steady-state is stable (blue solid line), but loses stability in a Hopf bifurcation (HB) at VPLC≈2.02. The unstable fixpoint (red dashed line) regains stability in another HB at VPLC≈2.44. Unstable limit cycles (black asterisks) are born at both HBs, but due to saddle-node-of-limit-cycles bifurcations at VPLC≈2.0 and VPLC≈ 2.45, stable limit cycles are present between these values. The black circle indicates the default value of VPLC used in the model, which is located below the interval where the system may oscillate.
Significance of the present findings for skin therapy and wound healing
In laser skin resurfacing with intra-epidermal focal laser-induced photodamage, the targeted micro-regions are thought to promote formation of new dermal collagen and elastin during the repair process,32–35 but the underlying mechanisms were poorly understood before this study. Our results clearly indicate that P2YRs are activated by ATP downstream of photodamage, and P2YRs regulate cell proliferation, differentiation and migration,115–117 besides being involved in wound healing.31 A prime target of the associated signaling activity is likely to be the extracellular matrix (ECM). In cultured keratinocytes, ATP caused a rapid and strong but transient activation of hyaluronan synthase 2 (HAS2) expression (via PKC-, CaMKII-, MAPK- and CREB-dependent pathways) by activating P2Y2Rs.118 Hyaluronan, also named hyaluronic acid, is a major component of the ECM, and its synthesis is rapidly upregulated after tissue wounding.119 Thus, epidermal insults are associated with extracellular ATP release, as well as hyaluronan synthesis, and the two phenomena are linked.118
In addition, ATP is well known as one of the DAMPs that, once released from injured cells, activate the immune system.120 Besides alerting the body about danger and initiate the immune response, DAMPs also promote regeneration processes.120 P2YRs are important also in this context, because they regulate many aspects of immune cell function, including phagocytosis and killing of pathogens, antigen presentation, chemotaxis, degranulation, cytokine production, and lymphocyte activation.121 In the epidermis, Langerhans cells, characterized by the expression of Langerin (CD207) and CD1a, are considered to be macrophages that retain the function of dendritic cells, playing a clear role of antigen-presenting cells in skin inflammation, triggering a series of immune responses by migrating from the epidermis to the lymph nodes and presenting antigens to T-regulatory cells.122 A Langerhans cell line (XS106) was shown to express mRNA for P2X1, P2X7, P2Y1, P2Y2, P2Y4, and P2Y11 receptors,123 which is consistent with the increase in GCaMP6s fluorescence in Langerhans cells we observed in some of our in vivo experiments. Therefore, it would be interesting, in future studies, to trace the movements of the Langerhans cells invested by damage-evoked Ca2+ waves.
In our experimental model, Ca2+ waves reached into the fibroblast-populated collagen matrix, and this event may activate and coordinate the keratinocyte-fibroblast interaction that is key to wound healing and skin remodeling.124,125 Indeed, fibroblasts are well known not only for being the major producers of extracellular matrix, but also as a cell type actively involved in synthesis of inflammatory mediators and tissue repair. ATP or UTP increased the levels of ECM-related proteins through the activation of P2Y2Rs in mouse fibroblasts.126 Therefore, it is tempting to speculate that response coordination after accidental injury or laser treatment occurs via propagation of the ATP-dependent Ca2+ waves we visualized and analyzed in this article.
Concluding remarks
Numerous other molecular players contribute to epidermal Ca2+ homeostasis, therefore Ca2+ dynamics may involve a variety of other processes we did not take into account, including Ca2+-sensing receptor, transient receptor potential channels, store-operated calcium entry channel Orai1, endoplasmic Ca2+ depletion sensor (stromal interaction molecule 1 [STIM1]), and voltage-gated calcium channels such as L-type calcium channels.18,127 In addition, further experiments are required to better characterize the dynamics of ATP after it is released into the extracellular space and is degraded rapidly to ADP, AMP, and further to adenosine,64 which (with the exception of AMP) activate specific receptors even in a self-sustaining fashion.128
Data and code availability
Data were archived in a repository handled by the University of Padova and can be accessed at: http://researchdata.cab.unipd.it/id/eprint/547.
The model code is available in the repository managed by the University of Padova at http://researchdata.cab.unipd.it/id/eprint/559 and at http://www.dei.unipd.it/∼pedersen.
The roiSelection GUI code is available on GitHub at https://github.com/DonatiViola/ROISELECTION.git.
Supplementary Material
Contributor Information
Viola Donati, Department of Physics and Astronomy “G. Galilei”, University of Padova, 35131 Padova, Italy; Institute of Biochemistry and Cell Biology (IBBC)-CNR, 00015 Monterotondo (RM), Italy.
Chiara Peres, Institute of Biochemistry and Cell Biology (IBBC)-CNR, 00015 Monterotondo (RM), Italy.
Chiara Nardin, Institute of Biochemistry and Cell Biology (IBBC)-CNR, 00015 Monterotondo (RM), Italy.
Ferdinando Scavizzi, Institute of Biochemistry and Cell Biology (IBBC)-CNR, 00015 Monterotondo (RM), Italy.
Marcello Raspa, Institute of Biochemistry and Cell Biology (IBBC)-CNR, 00015 Monterotondo (RM), Italy.
Catalin D Ciubotaru, IOM-CNR, Institute of Materials, Area Science, 34149 Basovizza (TS), Italy.
Mario Bortolozzi, Department of Physics and Astronomy “G. Galilei”, University of Padova, 35131 Padova, Italy; Institute of Biochemistry and Cell Biology (IBBC)-CNR, 00015 Monterotondo (RM), Italy; Foundation for Advanced Biomedical Research, Veneto Institute of Molecular Medicine (VIMM), 35129 Padova (PD), Italy.
Morten Gram Pedersen, Department of Information Engineering, University of Padova, 35131 Padova (PD), Italy; Department of Mathematics “Tullio Levi-Civita”, University of Padova, 35121 Padova (PD), Italy.
Fabio Mammano, Department of Physics and Astronomy “G. Galilei”, University of Padova, 35131 Padova, Italy; Institute of Biochemistry and Cell Biology (IBBC)-CNR, 00015 Monterotondo (RM), Italy.
Author contributions
Conceptualization: F.M. Data Curation: V.D., C.P. Formal Analysis: V.D., C.P. Funding Acquisition: F.M. Investigation: V.D., C.P., C.N. Methodology: M.B., M.G.P. Project Administration: F.M. Resources: F.M., F.S., M.R. Software: F.M., C.D.C., V.D., C.P., M.B., M.G.P. Supervision: F.M., M.B.
Validation: V.D., C.P. C.N. Visualization: V.D., C.P., M.B., M.G.P. Writing - Original Draft Preparation: F.M. Writing - Review and Editing: F.M., V.D., C.P., C.N., M.B., M.G.P.
Funding
This work was supported by the University of Padova (Grant No. BIRD187130 to FM) and Fondazione Telethon (Grant No. GGP19148 to FM). The funders had no role in study design, data collection, data analysis, interpretation or writing of the report.
Conflict of interest statement. The authors declare no competing interests.
References
- 1. McGrath JA, Eady RAJ, Pope FM. Anatomy and Organization of Human Skin. Rook's Textbook of Dermatology, 2004. doi:0.1002/9780470750520.ch3.
- 2. Koster MI. Making an epidermis. Ann N Y Acad Sci. 2009;1170(1):7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watt FM. Stem cell fate and patterning in mammalian epidermis. Curr Opin Genet Dev. 2001;11:410–417. [DOI] [PubMed] [Google Scholar]
- 4. Alonso L, Fuchs E. Stem cells of the skin epithelium. Proc Natl Acad Sci. 2003;100(Supplement 1):11830–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118(5):635–648. [DOI] [PubMed] [Google Scholar]
- 6. Horsley V et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126(3):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nature reviews. Molecular cell biology. 2009;10:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuchs E. In Current Topics in Developmental BiologyVol. 116, ed Wassarman P M. 357–374. Academic Press, Cambridge, MA. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258(5080):292–295. [DOI] [PubMed] [Google Scholar]
- 10. Klepeis VE, Cornell-Bell A, Trinkaus-Randall V. Growth factors but not gap junctions play a role in injury-induced Ca2+ waves in epithelial cells. J Cell Sci. 2001;114(23):4185–4195. [DOI] [PubMed] [Google Scholar]
- 11. Gale JE, Piazza V, Ciubotaru CD, Mammano F. A mechanism for sensing noise damage in the inner ear. Curr Biol. 2004;14(6):526–529. [DOI] [PubMed] [Google Scholar]
- 12. Mazzarda F et al. Organ-on-chip model shows that ATP release through connexin hemichannels drives spontaneous Ca(2+) signaling in non-sensory cells of the greater epithelial ridge in the developing cochlea. Lab Chip. 2020;20(16):3011–3023. [DOI] [PubMed] [Google Scholar]
- 13. Kumamoto J, Goto M, Nagayama M, Denda M. Real-time imaging of human epidermal calcium dynamics in response to point laser stimulation. J Dermatol Sci. 2017;86(1):13–20. [DOI] [PubMed] [Google Scholar]
- 14. Handly LN, Wollman R. Wound-induced Ca(2+) wave propagates through a simple release and diffusion mechanism. Mol Biol Cell. 2017;28(11):1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leybaert L, Sanderson MJ. Intercellular Ca2+ waves: mechanisms and function. Physiol Rev. 2012;92(3):1359–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elias P, Ahn S, Brown B, Crumrine D, Feingold KR. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol. 2002;119(6):1269–1274. [DOI] [PubMed] [Google Scholar]
- 17. Elias PM et al. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J Invest Dermatol. 2002;119(5):1128–1136. [DOI] [PubMed] [Google Scholar]
- 18. Lee SE, Lee SH. Skin Barrier and Calcium. Annals of dermatology. 2018;30(3):265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michaletti A et al. Multi-omics profiling of calcium-induced human keratinocytes differentiation reveals modulation of unfolded protein response signaling pathways. Cell Cycle. 2019;18(17):2124–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dhitavat J et al. Mutations in the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase isoform cause Darier's disease. J Invest Dermatol. 2003;121(3):486–489. [DOI] [PubMed] [Google Scholar]
- 21. Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 22. Giuliani AL, Sarti AC, Di Virgilio F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol Lett. 2019;205:16–24. [DOI] [PubMed] [Google Scholar]
- 23. Barr TP et al. Air-stimulated ATP release from keratinocytes occurs through connexin hemichannels. PLoS One. 2013;8(2):e56744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takahashi T et al. In vivo imaging demonstrates ATP release from murine keratinocytes and its involvement in cutaneous inflammation after tape stripping. J Invest Dermatol. 2013;133(10):2407–2415. [DOI] [PubMed] [Google Scholar]
- 25. Koizumi S et al. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J. 2004;380(2):329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takada H, Furuya K, Sokabe M. Mechanosensitive ATP release from hemichannels and Ca(2)(+) influx through TRPC6 accelerate wound closure in keratinocytes. J Cell Sci. 2014;127:4159–4171. [DOI] [PubMed] [Google Scholar]
- 27. Boukamp P et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106(3):761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442(7102):527–532. [DOI] [PubMed] [Google Scholar]
- 29. Abbracchio MP et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58(3):281–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Erb L, Weisman GA. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling. 2012;1(6):789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burnstock G, Knight GE, Greig AV. Purinergic signaling in healthy and diseased skin. J Invest Dermatol. 2012;132(3):526–546. [DOI] [PubMed] [Google Scholar]
- 32. Orringer JS et al. Intraepidermal erbium:YAG laser resurfacing: impact on the dermal matrix. J Am Acad Dermatol. 2011;64(1):119–128. [DOI] [PubMed] [Google Scholar]
- 33. Tanghetti EA. The histology of skin treated with a picosecond alexandrite laser and a fractional lens array. Lasers Surg Med. 2016;48(7):646–652. [DOI] [PubMed] [Google Scholar]
- 34. Balu M et al. In vivo multiphoton-microscopy of picosecond-laser-induced optical breakdown in human skin. Lasers Surg Med. 2017;49(6):555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Connor KO, Cho SB, Chung HJ. Wound Healing Profile After 1064- and 532-nm Picosecond Lasers With Microlens Array of In Vivo Human Skin. Lasers Surg Med, 2021. doi:10.1002/lsm.23390. [DOI] [PubMed] [Google Scholar]
- 36. Chen T-W et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Osteoarthritis Cartilage. 2012;20(4):256–260. [DOI] [PubMed] [Google Scholar]
- 38. Smith AJ, Clutton RE, Lilley E, Hansen KEA, Brattelid T. PREPARE: guidelines for planning animal research and testing. Lab Anim. 2018;52(2):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mammano F, Bortolozzi M. In Calcium Measurement MethodsVol. 43, eds 57–80. Humana Press, Totowa, New Jersey. 2010. [Google Scholar]
- 40. Wolff K, Wolff-Schreiner EC. Trends in electron microscopy of skin. J Invest Dermatol. 1976;67(1):39–57. [DOI] [PubMed] [Google Scholar]
- 41. Crank J. The Mathematics of Diffusion. second edn, Oxford, UK, Oxford University Press, 1975. [Google Scholar]
- 42. De Vuyst E et al. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suh PG et al. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB reports. 2008;41(6):415–434. [DOI] [PubMed] [Google Scholar]
- 44. Punnonen K et al. Keratinocyte differentiation is associated with changes in the expression and regulation of phospholipase C isoenzymes. J Invest Dermatol. 1993;101(5):719–726. [DOI] [PubMed] [Google Scholar]
- 45. Allen V, Swigart P, Cheung R, Cockcroft S, Katan M. Regulation of inositol lipid-specific phospholipase cdelta by changes in Ca2+ ion concentrations. Biochem J. 1997;327(2):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim YH et al. Phospholipase C-delta1 is activated by capacitative calcium entry that follows phospholipase C-beta activation upon bradykinin stimulation. J Biol Chem. 1999;274(37):26127–26134. [DOI] [PubMed] [Google Scholar]
- 47. Thore S, Dyachok O, Gylfe E, Tengholm A. Feedback activation of phospholipase C via intracellular mobilization and store-operated influx of Ca2+ in insulin-secreting beta-cells. J Cell Sci. 2005;118(19):4463–4471. [DOI] [PubMed] [Google Scholar]
- 48. Li YX, Rinzel J. Equations for InsP3 receptor-mediated [Ca2+]i oscillations derived from a detailed kinetic model: a Hodgkin-Huxley like formalism. J Theor Biol. 1994;166(4):461–473. [DOI] [PubMed] [Google Scholar]
- 49. Handa M, Guidotti G. Purification and cloning of a soluble ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum). Biochem Biophys Res Commun. 1996;218(3):916–923. [DOI] [PubMed] [Google Scholar]
- 50. Lambrecht G. Design and pharmacology of selective P2-purinoceptor antagonists. J Auton Pharmacol. 1996;16(6):341–344. [DOI] [PubMed] [Google Scholar]
- 51. Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2(2):409–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zuo P et al. Mathematical model of nucleotide regulation on airway epithelia. Implications for airway homeostasis. J Biol Chem. 2008;283(39):26805–26819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kukulski F et al. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic signalling. 2005;1(2):193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Levesque SA, Lavoie EG, Lecka J, Bigonnesse F, Sevigny J. Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br J Pharmacol. 2007;152(1):141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Westfall TD, Kennedy C, Sneddon P. Enhancement of sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens by the novel ecto-ATPase inhibitor ARL 67156. Br J Pharmacol. 1996;117(5):867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baqi Y. Ecto-nucleotidase inhibitors: recent developments in drug discovery. Mini Rev Med Chem. 2015;15(1):21–33. [DOI] [PubMed] [Google Scholar]
- 57. Raaflaub J. Applications of Metal Buffers and Metal Indicators in Biochemistry. Methods Biochem Anal, 1956;301–325. [DOI] [PubMed] [Google Scholar]
- 58. Lee WK et al. Purinoceptor-mediated calcium mobilization and proliferation in HaCaT keratinocytes. J Dermatol Sci. 2001;25(2):97–105. [DOI] [PubMed] [Google Scholar]
- 59. Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266(26):17067–17071. [PubMed] [Google Scholar]
- 60. Toma I et al. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2008;294(6):R1769–R1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cotrina ML et al. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci. 1998;95(26):15735–15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277(12):10482–10488. [DOI] [PubMed] [Google Scholar]
- 63. Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46(5):731–744. [DOI] [PubMed] [Google Scholar]
- 64. Ho CL, Yang CY, Lin WJ, Lin CH. Ecto-nucleoside triphosphate diphosphohydrolase 2 modulates local ATP-induced calcium signaling in human HaCaT keratinocytes. PLoS One. 2013;8(3):e57666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci. 2003;100(23):13644–13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ruan Z, Orozco IJ, Du J, Lu W. Structures of human pannexin 1 reveal ion pathways and mechanism of gating. Nature,584,(7822):646–651., 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Penuela S et al. Panx1 regulates cellular properties of keratinocytes and dermal fibroblasts in skin development and wound healing. J Invest Dermatol. 2014;134(7):2026–2035. [DOI] [PubMed] [Google Scholar]
- 68. Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580(1):239–244. [DOI] [PubMed] [Google Scholar]
- 69. Carrer A et al. Cx32 hemichannel opening by cytosolic Ca2+ is inhibited by the R220X mutation that causes Charcot-Marie-Tooth disease. Hum Mol Genet. 2018;27(1):80–94. [DOI] [PubMed] [Google Scholar]
- 70. De Vuyst E et al. Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium. 2009;46(3):176–187. [DOI] [PubMed] [Google Scholar]
- 71. Ceriani F, Pozzan T, Mammano F. Critical role of ATP-induced ATP release for Ca2+ signaling in nonsensory cell networks of the developing cochlea. Proc Natl Acad Sci. 2016;113(46):E7194–E7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Connors BW. Tales of a dirty drug: carbenoxolone, gap junctions, and seizures. Epilepsy currents. 2012;12(2):66–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Buckley C, Zhang X, Wilson C, McCarron JG. Carbenoxolone and 18beta-Glycyrrhetinic acid inhibit IP3 -mediated endothelial cell calcium signalling and depolarise mitochondria. Br J Pharmacol, 2020. doi:10.1111/bph.15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lohman AW, Isakson BE. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 2014;588(8):1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Garcia-Vega L, O'Shaughnessy EM, Albuloushi A, Martin PE. Connexins and the epithelial tissue barrier: a focus on connexin 26. Biology. 2021;10(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mese G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol. 2007;127(11):2516–2524. [DOI] [PubMed] [Google Scholar]
- 77. Martin PE, Easton JA, Hodgins MB, Wright CS. Connexins: sensors of epidermal integrity that are therapeutic targets. FEBS Lett. 2014;588(8):1304–1314. [DOI] [PubMed] [Google Scholar]
- 78. Churko JM, Laird DW. Gap junction remodeling in skin repair following wounding and disease. Physiology. 2013;28(3):190–198. [DOI] [PubMed] [Google Scholar]
- 79. Wong P et al. The role of connexins in wound healing and repair: novel therapeutic approaches. Frontiers in physiology. 2016;7. doi:10.3389/fphys.2016.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang X, Ramirez A, Budunova I. Overexpression of connexin26 in the basal keratinocytes reduces sensitivity to tumor promoter TPA. Exp Dermatol. 2010;19(7):633–640. [DOI] [PubMed] [Google Scholar]
- 81. Goliger JA, Paul DL. Expression of gap junction proteins Cx26, Cx31.1, Cx37, and Cx43 in developing and mature rat epidermis. Dev Dyn. 1994;200(1):1–13. [DOI] [PubMed] [Google Scholar]
- 82. Delmar M et al. Connexins and disease. Cold Spring Harb Perspect Biol, 2017. doi:10.1101/cshperspect.a029348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Faniku C, Wright CS, Martin PE. Connexins and pannexins in the integumentary system: The skin and appendages. Cell Mol Life Sci. 2015;72(15):2937–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kandyba EE, Hodgins MB, Martin PE. A murine living skin equivalent amenable to live-cell imaging: analysis of the roles of connexins in the epidermis. J Invest Dermatol. 2008;128(4):1039–1049. [DOI] [PubMed] [Google Scholar]
- 85. Kretz M et al. Altered connexin expression and wound healing in the epidermis of connexin-deficient mice. J Cell Sci. 2003;116(16):3443–3452. [DOI] [PubMed] [Google Scholar]
- 86. Maher AC et al. Rat epidermal keratinocytes as an organotypic model for examining the role of Cx43 and Cx26 in skin differentiation. Cell Commun Adhes. 2005;12:219–230. [DOI] [PubMed] [Google Scholar]
- 87. Abudara V et al. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Frontiers in cellular neuroscience. 2014;8:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hubley MJ, Locke BR, Moerland TS. The effects of temperature, pH, and magnesium on the diffusion coefficient of ATP in solutions of physiological ionic strength. Biochimica et biophysica acta. 1996;1291(2):115–121. [DOI] [PubMed] [Google Scholar]
- 89. Dix JA, Verkman AS. Crowding effects on diffusion in solutions and cells. Annu Rev Biophys. 2008;37(1):247–263. [DOI] [PubMed] [Google Scholar]
- 90. Vendelin M, Birkedal R. Anisotropic diffusion of fluorescently labeled ATP in rat cardiomyocytes determined by raster image correlation spectroscopy. American journal of physiology. Cell physiology. 2008;295(5):C1302–C1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Smith GD, Pearson JE, Keizer JE. in Computational Cell Biologyeds Fall C P., Marland E S., Wagner J M., Tyson J J. 198–229. Springer: New York: 2002. [Google Scholar]
- 92. Lambrecht G et al. Structure-activity relationships of suramin and pyridoxal-5'-phosphate derivatives as P2 receptor antagonists. Curr Pharm Des. 2002;8(26):2371–2399. [DOI] [PubMed] [Google Scholar]
- 93. Charlton SJ et al. PPADS and suramin as antagonists at cloned P2Y- and P2U-purinoceptors. Br J Pharmacol. 1996;118(3):704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Robaye B, Boeynaems JM, Communi D. Slow desensitization of the human P2Y6 receptor. Eur J Pharmacol. 1997;329(2–3):231–236. [PubMed] [Google Scholar]
- 95. Marteau F et al. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64(1):104–112. [DOI] [PubMed] [Google Scholar]
- 96. Braun M, Lelieur K, Kietzmann M. Purinergic substances promote murine keratinocyte proliferation and enhance impaired wound healing in mice. Wound Repair Regen. 2006;14(2):152–161. [DOI] [PubMed] [Google Scholar]
- 97. Buckley C, Zhang X, Wilson C, McCarron JG. Carbenoxolone and 18β-glycyrrhetinic acid inhibit inositol 1, 4, 5-trisphosphate-mediated endothelial cell calcium signalling and depolarise mitochondria. 2020. [DOI] [PMC free article] [PubMed]
- 98. Denning MF et al. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1995;6:149–157. [PubMed] [Google Scholar]
- 99. Ryu SH et al. Feedback regulation of phospholipase C-beta by protein kinase C. J Biol Chem. 1990;265(29):17941–17945. [PubMed] [Google Scholar]
- 100. Yue C, Ku CY, Liu M, Simon MI, Sanborn BM. Molecular mechanism of the inhibition of phospholipase C beta 3 by protein kinase C. J Biol Chem. 2000;275(39):30220–30225. [DOI] [PubMed] [Google Scholar]
- 101. Hofer T, Politi A, Heinrich R. Intercellular Ca2+ wave propagation through gap-junctional Ca2+ diffusion: a theoretical study. Biophys J. 2001;80(1):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Edwards JR, Gibson WG. A model for Ca2+ waves in networks of glial cells incorporating both intercellular and extracellular communication pathways. J Theor Biol. 2010;263(1):45–58. [DOI] [PubMed] [Google Scholar]
- 103. Sneyd J, Wetton BT, Charles AC, Sanderson MJ. Intercellular calcium waves mediated by diffusion of inositol trisphosphate: a two-dimensional model. American Journal of Physiology-Cell Physiology. 1995;268(6):C1537–C1545. [DOI] [PubMed] [Google Scholar]
- 104. Rocheleau JV, Walker GM, Head WS, McGuinness OP, Piston DW. Microfluidic glucose stimulation reveals limited coordination of intracellular Ca2+ activity oscillations in pancreatic islets. Proc Natl Acad Sci. 2004;101(35):12899–12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Benninger RK, Zhang M, Head WS, Satin LS, Piston DW. Gap junction coupling and calcium waves in the pancreatic islet. Biophys J. 2008;95(11):5048–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pedersen MG, Sorensen MP. Wave-block due to a threshold gradient underlies limited coordination in pancreatic islets. J Biol Phys. 2008;34(3–4):425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bulai IM, Pedersen MG. Stopping waves: geometric analysis of coupled bursters in an asymmetric excitation field. Nonlinear Dyn. 2019;96(3):1927–1937. [Google Scholar]
- 108. Keener JP. Propagation and its failure in coupled systems of discrete excitable cells. SIAM J Appl Math. 1987;47(3):556–572. [Google Scholar]
- 109. Keener JP. The effects of discrete gap junction coupling on propagation in myocardium. J Theor Biol. 1991;148(1):49–82. [DOI] [PubMed] [Google Scholar]
- 110. Pedersen MG. Homogenization of heterogeneously coupled bistable ODE's-applied to excitation waves in pancreatic islets of langerhans. J Biol Phys. 2004;30(3):285–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cappon G, Pedersen MG. Heterogeneity and nearest-neighbor coupling can explain small-worldness and wave properties in pancreatic islets. Chaos. 2016;26(5):053103. [DOI] [PubMed] [Google Scholar]
- 112. Anselmi F et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci. 2008;105(48):18770–18775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Majumder P et al. ATP-mediated cell-cell signaling in the organ of Corti: the role of connexin channels. Purinergic Signal. 2010;6(2):167–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rodriguez L et al. Reduced phosphatidylinositol 4,5-bisphosphate synthesis impairs inner ear Ca2+ signaling and high-frequency hearing acquisition. Proc Natl Acad Sci. 2012;109(35):14013–14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol. 1998;78:113–145. [DOI] [PubMed] [Google Scholar]
- 116. Neary JT et al. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. The Journal of Neuroscience. 1999;19(11):4211–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Klepeis VE, Weinger I, Kaczmarek E, Trinkaus-Randall V. P2Y receptors play a critical role in epithelial cell communication and migration. J Cell Biochem. 2004;93(6):1115–1133. [DOI] [PubMed] [Google Scholar]
- 118. Rauhala L et al. Extracellular ATP activates hyaluronan synthase 2 (HAS2) in epidermal keratinocytes via P2Y2, Ca(2+) signaling, and MAPK pathways. Biochem J. 2018;475(10):1755–1772. [DOI] [PubMed] [Google Scholar]
- 119. Tammi R, Pasonen-Seppänen S, Kolehmainen E, Tammi M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Dermatol. 2005;124(5):898–905. [DOI] [PubMed] [Google Scholar]
- 120. Hato T, Dagher PC. How the innate immune system senses trouble and causes trouble. Clinical Journal of the American Society of Nephrology. 2015;10(8):1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lovaszi M, Branco Haas C, Antonioli L, Pacher P, Hasko G. The role of P2Y receptors in regulating immunity and metabolism. Biochem Pharmacol, 2021;187:114419. [DOI] [PubMed] [Google Scholar]
- 122. Collin M, Milne P. Langerhans cell origin and regulation. Curr Opin Hematol. 2016;23(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Granstein RD et al. Augmentation of cutaneous immune responses by ATP gamma S: purinergic agonists define a novel class of immunologic adjuvants. J Immunol. 2005;174(12):7725–7731. [DOI] [PubMed] [Google Scholar]
- 124. Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998–1008. [DOI] [PubMed] [Google Scholar]
- 125. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jin H et al. P2Y2 R activation by nucleotides promotes skin wound-healing process. Exp Dermatol. 2014;23(7):480–485. [DOI] [PubMed] [Google Scholar]
- 127. Elsholz F, Harteneck C, Muller W, Friedland K. Calcium–a central regulator of keratinocyte differentiation in health and disease. European journal of dermatology : EJD. 2014;24:650–661. [DOI] [PubMed] [Google Scholar]
- 128. Pellegatti P, Falzoni S, Pinton P, Rizzuto R, Di Virgilio F. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell. 2005;16(8):3659–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen TW et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Greenberg DS, Kay-Michael Voit DJW, Wuertenberger S et al. Accurate action potential inference from a calcium sensor protein through biophysical modeling. bioRxiv preprint, 2018;1–84. doi:10.1101/479055.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were archived in a repository handled by the University of Padova and can be accessed at: http://researchdata.cab.unipd.it/id/eprint/547.
The model code is available in the repository managed by the University of Padova at http://researchdata.cab.unipd.it/id/eprint/559 and at http://www.dei.unipd.it/∼pedersen.
The roiSelection GUI code is available on GitHub at https://github.com/DonatiViola/ROISELECTION.git.