Abstract
During meiosis, DNA double-strand breaks (DSBs) are formed at high frequency at special chromosomal sites, called DSB hotspots, to generate crossovers that aid proper chromosome segregation. Multiple chromosomal features affect hotspot formation. In the fission yeast S. pombe the linear element proteins Rec25, Rec27 and Mug20 are hotspot determinants – they bind hotspots with high specificity and are necessary for nearly all DSBs at hotspots. To assess whether they are also sufficient for hotspot determination, we localized each linear element protein to a novel chromosomal site (ade6 with lacO substitutions) by fusion to the Escherichia coli LacI repressor. The Mug20-LacI plus lacO combination, but not the two separate lac elements, produced a strong ade6 DSB hotspot, comparable to strong endogenous DSB hotspots. This hotspot had unexpectedly low ade6 recombinant frequency and negligible DSB hotspot competition, although like endogenous hotspots it manifested DSB interference. We infer that linear element proteins must be properly placed by endogenous functions to impose hotspot competition and proper partner choice for DSB repair. Our results support and expand our previously proposed DSB hotspot-clustering model for local control of meiotic recombination.
INTRODUCTION
The formation of viable haploid gametes from diploid precursor cells occurs during the two specialized nuclear divisions called meiosis. A critical event is the segregation of the parental centromeres, with their attached chromosomal arms, on the two replicated chromosomes (homologs) at the first division. Successful segregation requires in most species physical connection of the homologs by one or more crossovers in the arms (1,2). In conjunction with sister chromatid cohesion, a crossover provides tension during meiosis I to allow proper segregation; tension signals that the centromeres are going to opposite poles of the cell (3), as required for successful meiosis. Crossovers are formed by homologous genetic recombination between parental chromosomes. Crossing-over also generates genetic diversity among the progeny and thus aids evolution. These dual roles of crossing-over likely account for the nearly universal occurrence of recombination at high level during meiosis. Understanding meiotic recombination requires knowledge of its molecular determinants and their functions, the subject here.
DNA double-strand breaks (DSBs) occur at high frequency during meiosis, and their repair produces the crossovers critical for meiosis (4). DSBs are not uniformly distributed across the chromosomes. Rather, there are sites, called DSB hotspots, at which DSBs occur preferentially (5). In the fission yeast Schizosaccharomyces pombe, studied here, DSBs occur at hotspots up to 200 times more frequently than the genome median (6,7). Hotspots have also been characterized in the distantly related budding yeast Saccharomyces cerevisiae, which have hotspots up to 200 times the genome median (8) and in mice (up to 500 times the genome median (9) (reviewed in (5)). The determinants of DSB hotspots are not completely known in any case, but multiple factors, both DNA sequence and chromosome-bound proteins, contribute. The binding of certain transcription factors is a major determinant in some cases, but the individual transcription factors tested appear to account for only a small minority of hotspots across the genome (8,10). More general aspects of chromatin structure are also important.
Hotspots show a preference for nucleosome-depleted regions in S. cerevisiae (8,11,12) but less so in S. pombe (7,13). Histone modifications, such as histone H3 lysine 4 methylation (H3 K4Me), are correlated with DSB formation at most hotspots in S. cerevisiae and mice (14–16). In S. pombe acetylation of histone H3 lysine 9 (H3 K9Ac) is elevated at DSB hotspots (17), and the histone variant H2A.Z is needed to localize DSB-forming proteins to hotspots (18). Thus, a complex interplay of protein binding and chromatin structure appears to determine the distribution of DSBs across the genome.
DSB hotspots do not act independently—they interact with neighboring hotspots. Introduction of a novel hotspot reduces DSB frequency at nearby hotspots. This feature, called DSB competition, acts primarily along one homolog (‘in cis’) and over ∼200 kb regions in S. pombe (19) and ∼70 kb in S. cerevisiae (20–23). In addition, the frequency of two DSBs on the same chromatid, one at each hotspot, is less than that expected from independence (i.e., the product of the individual frequencies). This feature, called DSB interference, also acts over ∼200 kb in S. pombe (19) and ∼70 kb in S. cerevisiae (24). A model for hotspot competition and DSB interference embodying clustering of nearby hotspots has been proposed and supported by a variation of the chromosome conformation capture (3C) method, which demonstrated 3D clustering of DSB hotspots over ∼200 kb regions (19). These authors proposed that DSB hotspot competition and interference might result from the same mechanism. Results reported here indicate that they are separable features, in agreement with DSB interference being dependent on the Tel1 DNA damage response protein kinase in S. cerevisiae (24), whereas competition is independent of Tel1, although its strength varies between hotspots (25). DSB competition has been proposed to be independent of DSB formation per se (26) and to result from competitive loading of factors for DSB formation (20,23,27,28).
A clear case of DSB hotspot determinants acting across the whole genome is provided by the linear element (LinE) proteins of S. pombe. LinE proteins are meiosis–specific and bind along the chromosomes and form long lines (LinE structures) visible by light and electron microscopy (6,29–31). LinE structures resemble the axial element precursors to the meiosis–specific synaptonemal complex (SC) of other species (32), which forms a regular structure along and between homologous chromosome pairs from one end to the other of the many species investigated (33). Four S. pombe LinE proteins have been identified by genetics and microscopy—Rec10, Rec25, Rec27 and Mug20 (34–36). These proteins appear to act as a complex, since where tested deletion of any one renders foci of the other LinE proteins undetectable by light microscopy, except for Rec10, which remains diffusely visible in the nucleus (31,34,35,37). Rec10 has limited amino acid similarity to S. cerevisiae Red1 (36), and Rec27 has limited amino acid similarity to the Caenorhabditis elegans SC protein SYP-2 (6). Mug20 appears similar to DDL-1, an SYP-2-interacting protein, although DDL-1 has not to our knowledge been reported to be in the SC (38). Both SC and LinE structures are dissociated by the chaotropic agent 1,6-hexanediol (31,39) [but see (40)]. Thus, the S. pombe LinEs have several functional properties of the SC of other species.
Three LinE proteins—Rec25, Rec27 and Mug20—are determinants of DSB hotspots in S. pombe. Whole-genome analysis shows that these proteins bind to hotspots with high specificity and abundance: at hotspots there is up to 80 times the genome-median protein density (6). LinE protein abundance at hotspots is highly correlated with the DSB frequency at hotspots (Pearson's correlation coefficient r = 0.79–0.88). In the absence of any one of these three proteins, DSB frequency at most hotspots is strongly reduced or undetectable. DSBs apparently remain in ‘cold regions’ between hotspots, because there is significant residual meiotic recombination in mutants lacking any one (or tested pair) of these proteins (34,35). By contrast, in the absence of Rec10, DSBs and recombination are not detectable above background levels (6,41). Rec10 also localizes to DSB hotspots (up to ∼3 times the genome median), but with less specificity as there are other sites of localization (6,42). Rec10 interacts with the other LinE proteins, cohesin, and the DSB-forming complex (the Spo11 homolog Rec12 and its half-dozen essential partners) (42–44).
Although these three LinE proteins bind DSB hotspots with high specificity, no simple DNA sequence is discernible within the hotspot region that might account for LinE protein specificity for binding hotspots. Their preferential binding may depend on a complex set of factors which in turn bind a complex set of DNA sequences. This outcome has precluded testing whether LinE protein binding to a chromosomal site is sufficient to create a DSB hotspot.
Consequently, we have used a different experimental approach to show that binding of a LinE protein indeed is sufficient to create a DSB hotspot. In addition, our results reported here reveal unexpected complexities in the behavior of a novel set of LinE protein-dependent DSB hotspots, which sheds light on the molecular mechanisms controlling DSB hotspot activity and recombination dependent upon DSBs at hotspots and their repair (19,35).
MATERIALS AND METHODS
Strains and genetic methods
Genotypes and origins of S. pombe strains are in Supplementary Table S1. The notation ‘::’ indicates a substitution (deletion and insertion) of part or all of the gene to the left of the symbol with the gene to the right of the symbol; ‘:’ indicates a simple insertion (without deletion) near the gene to the left of the symbol. Growth media were described previously (45). Transformation of strains to introduce an allele into the chromosome used the lithium acetate method for homology-directed integration of polymerase chain reaction (PCR) products containing ∼80 bp of homology with the targeted gene (46); new chromosomal alleles were confirmed by sequencing a PCR product generated from the transformant. Oligonucleotides are in Supplementary Table S2, and plasmids are in Supplementary Tables S3 and S4. Genetic crosses to determine recombinant frequencies were done at 25°C on supplemented SPA medium (45). Intra- and inter-genic recombinant frequencies were determined by random spore analysis. Differential plating for total and recombinant (prototrophic) types was used to assay intragenic recombination between heterozygous lacO substitutions in ade6, such as ade6-3101, and ade6-52 (primarily gene conversion); analysis of individual spore colonies by picking to grids and replica-plating was used to assay intergenic recombination between ade6 and arg1 (primarily crossing over) (see Figure 1).
Figure 1.
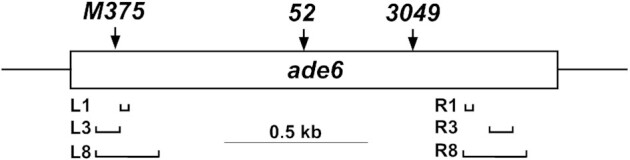
Location of ade6 mutations. lacO operator arrays (horizontal brackets) of 1, 3 or 8 copies were substituted for an identical size interval of the ade6 open reading frame (open box; 1659 bp) on the left (L) at the ade6-M375 position (single bp control mutation G133T, three bp from the ade6-M26 recombination hotspot with ATGACGT bound by the transcription factor Atf1-Pcr1), or on the right (R). The ade6-52 single bp mutation (G796A), between the L and R arrays, was crossed with the arrays to determine intragenic recombinant frequencies. ade6-3049 (C1214T) creates another ATGACGT heptamer and a strong DSB hotspot. arg1, 300 kb to the right, was used to determine intergenic recombinant frequencies. bub1-243, 0.65 kb to the left of ade6, and vtc4-1104, 1.49 kb to the right, were used for DNA analyses of recombinants and intermediates (Figure 6).
Generation of LinE-LacI fusion proteins
A Gibson Assembly Kit (New England Biolabs, Ipswich, MA) was used to create plasmids expressing the LinE-LacI fusion proteins. Two separate plasmids were used as templates for DNA synthesis; one contained the full-length LinE protein-coding DNA (including the 5′ and 3′ untranslated regions), and another (pRS406-CMV-LacI-NLS-3FLAG) contained the lacI gene followed by DNA encoding a nuclear localization signal (NLS; PKKKRKV) and three copies of the FLAG epitope (DYKDDDDK) for immunoprecipitation (47). To fuse LacI to a LinE’s C-terminus, the LinE-containing plasmid was linearized between its coding sequence and its translational stop codon. This procedure used inverse PCR with a forward primer that starts immediately after the stop codon and a reverse primer that starts immediately before the stop codon. In a parallel PCR, a linear fragment containing lacI-nls-3FLAG and its stop codon was generated with ∼20–25 extra nucleotides on both sides that were homologous to the region where lacI was to be inserted, adjacent to the LinE protein-coding sequence. Using Gibson Assembly, the two PCR products were assembled into a circular plasmid expressing LinE-LacI. To fuse LacI to a LinE’s N-terminus, the LinE protein-coding plasmid was linearized immediately before the start codon and combined, using Gibson Assembly, with a lacI fragment (without its stop codon) that had extra nucleotides homologous to the region of its insertion. Note that NLS-3FLAG is not present in LacI-Mug20 (mug20-252) or in Mug20-LacI' (mug20-254). DNA encoding a LinE fusion to LacI was placed on the chromosome by transformation to fluoro-orotic acid (FOA)-resistance of a ura4 deletion strain containing the ura4+ gene in place of the corresponding LinE protein-coding sequence at its endogenous locus (48,49).
Generation of lacO arrays
ade6 alleles with a single lacO were generated with the NEB Q5 mutagenesis kit and oligonucleotides containing lacO flanked by ∼20–25 ade6 nucleotides separated by the size of the lacO insertion (27 bp), to form an exact substitution. ade6 alleles with a short array of lacO were generated using Gibson Assembly from a plasmid containing ade6 (such as pJC1 or pJC13) and a plasmid containing 3 or 8 copies of lacO repeats (pUC-TALO3 or pUC-TALO8, respectively) (47); each lacO repeat had the 27-bp lacO sequence 5′ ACTAGCAATTGTGAGCGGATAACAATT 3′. The ade6 plasmid was linearized by inverse PCR, excluding the nucleotides in ade6 to be substituted with lacO repeats. A parallel PCR generated a linear fragment containing the repeated lacO array and an additional 15 and 23 bp (L3 and L8) or 15 and 25 bp (R3 and R8) plus 15–27 ade6 bp flanking the substitution site for homologous integration. The number of bp inserted equaled the number of ade6 bp deleted, to maintain wt spacing of DNA flanking the lacO array. Using Gibson Assembly, the two PCR products were assembled into a circular plasmid containing ade6 with lacO arrays. These alleles were placed on the chromosome by transformation to FOA-resistance of strain GP6104 (ade6-3095::ura4+, a substitution of the entire ade6 ORF with ura4+). The nucleotide sequences of the ade6 gene, wt and with each of the L1, L3, L8, R1, R3 and R8 substitutions, are in Supplementary Data following Supplementary Table S4. To couple the ade6::lacO substitutions with the flanking restriction site polymorphisms bub1-243 (L) and vtc4-1104 (R), strains GP8897 and GP8898 were similarly transformed. These strains, with flanking bub1 or vtc4 mutations, contain the ade6-3103::ura4+ substitution (1.8 kb of ura4+ DNA replacing the ade6 ORF plus 201 bp 5′ and 78 bp 3′ of the ORF).
Construction of ura1::hphMX6, tel1::natMX6 and mde2::hphMX6
A DSB hotspot was introduced into the ura1 gene by substituting bp –80–1607 of the ura1 coding sequence with 1767 bp of the hphMX6 hygromycin-resistance determinant in plasmid PCR2.1-hph (46,50). The PCR product using oligos OL4418 and OL4419 was used to transform strain GP9901 to hygromycin-resistance and uracil auxotrophy. The substitution mde2::hphMX6 was made by generating a PCR product from plasmid PCR2.1-hph with OL4461 and OL4462 and transforming strain GP8875 to hygromycin-resistance. Strain GP8985 (containing the substitution tel1::kanMX6) was transformed to nourseothricin-resistance and kanamycin-sensitivity with a PCR product using oligos MD1 and MD2 and plasmid PCR2.1-nat (50).
Meiotic induction and DNA preparation
Meiotic induction and DNA preparation were performed as described (51). Briefly, strains with pat1-114 (temperature-sensitive) or pat1-as1 (ATP analog-sensitive) were used to induce synchronous meiosis after nitrogen starvation (which produces G1-arrested cells) by shifting the temperature to 34°C for pat1-114, or by adding 3-MB-PP1 (Toronto Research Chemicals) to 25 μM at 25°C or 34°C for pat1-as1, and adding NH4Cl as a nitrogen source. All DNA analyses were done with cultures induced at 34°C except for those in Figure 4B at 25°C. Cells were harvested at the specified times after induction, embedded in agarose plugs, and digested with lytic enzymes to break the cells. The plugs were further treated with proteinase K, subsequently inactivated with phenylmethylsulfonyl fluoride (PMSF), and washed thoroughly with TE buffer.
Figure 4.
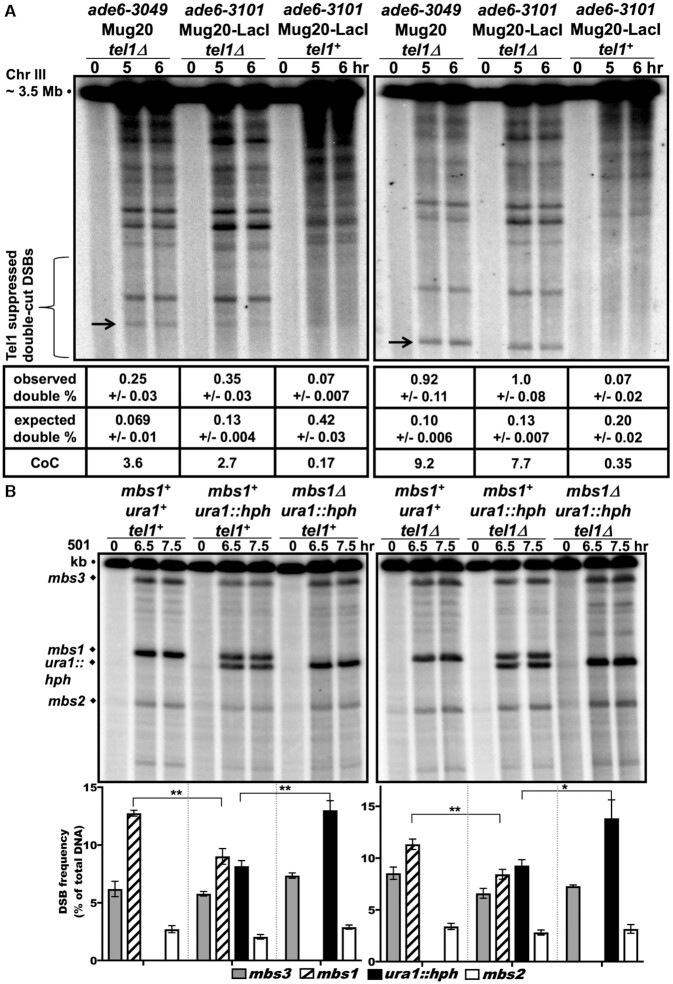
The ade6-3101 hotspot manifests Tel1-dependent DSB interference; Tel1-independent DSB competition indicates separate mechanisms. rad50S strains were induced for meiosis, and DNA analyzed as in Figure 3, except meiosis was at 25°C in (B). (A) Both ade6-3049 and ade6-3101 manifest Tel1-dependent DSB interference. DNA was digested with NotI and analyzed on four to six blots with a probe between ade6 and the 75R DSB hotspot (left panel) or between ade6 and the tel1L hotspot near tel1 (right panel). Double-cut DSBs (black arrows; 75 kb left and 40 kb right) were evident in tel1Δ (left and middle lane sets) but not in tel1+ (right lane set). Coefficients of coincidence (CoC; mean ± SEM) show positive DSB interference (1 - CoC) in tel1+ and negative interference in tel1Δ. Single-cut DSBs and frequencies are visible in Supplementary Figure S3 using a different radioactive probe. (B) DSB competition at mbs1 is Tel1-independent. DNA was digested with NotI and analyzed on three to seven blots with a probe at the left end of the 501 kb NotI fragment J. DSBs at both mbs1+ and ura1::hph hotspots were reduced in the presence of the other hotspot (compare the double hotspot in the middle lane set to either single hotspot; **P = 0.007 for mbs1+ and **P = 0.0034 for ura1::hph), indicating mutual DSB competition. This competition was also present without Tel1 (right panel; **P = 0.002 for mbs1+ and *P = 0.018 for ura1::hph).
Induction of meiosis was confirmed by flow cytometry to determine pre-meiotic DNA replication, which typically began at 2 h and was completed by 3 h at 34°C (3.5 and 5.5 h, respectively, at 25°C).
DSB analysis
DNA in agarose plugs was digested with appropriate restriction enzymes and analyzed either by standard agarose gel electrophoresis (for fragments shorter than 20 kb) or pulsed-field gel electrophoresis (for fragments longer than 20 kb). Southern blot hybridization was performed as previously described (51). For the 150.5 kb SacII fragment with ade6, DNA probes, made by PCR using oligos OL4416 and OL4417 and chromosomal DNA, correspond to positions 1427–1428 kb of chromosome III. Previously described were probes for the 11.8 kb BsrGI fragment and the double-cut DNA fragment between ade6 and a hotspot ∼75 kb to its right (49); for the 74.2 kb PmeI fragment containing ade6 (6); and for the 501 kb NotI J fragment and the 64.4 kb PmeI fragment, both containing mbs1 (52). See Supplementary Figure S1 for positions of restriction sites and probes. Signals were detected using a Typhoon Odyssey PhosphorImager system (GE Healthcare) and quantified using ImageQuant TL (GE Healthcare) software.
DNA joint molecule and crossover analysis
DNA in agarose plugs was digested with BsrGI and analyzed by two-dimensional (2D) gel electrophoresis to separate the branched DNA intermediates (joint molecules, such as Holliday junctions) at the ade6 locus (51). Determination of inter-sister and inter-homolog Holliday junctions and crossover DNA fragments used heterozygous restriction sites for ScaI (bub1-243) and PmlI (vtc4-1104) flanking ade6 and Southern blot hybridization using a probe for ade6 previously described (53). Signals were detected as above.
Statistics
Intragenic recombinant frequencies and DNA fragment data were expressed as mean ± standard error of the mean (SEM), based on n repeats and calculated using GraphPad software. Intergenic recombinant fractions were used to estimate one standard deviation (SD) based on the Poisson distribution (GraphPad); fractions were converted to centiMorgans (cM) using Haldane's relation (54). Two-sided unpaired t tests were used to evaluate statistical significance.
RESULTS
Chromosomal localization of Mug20-LacI fusion generates a strong recombination hotspot
Does forced localization of a LinE protein at a site in a non-hotspot region of the chromosome create a DSB hotspot; i.e., is LinE protein localization sufficient for hotspot determination? To answer this question, we used the E. coli lacO-LacI localization elements to target LinE-LacI fusion proteins to a lacO array introduced into the S. pombe ade6 gene, which lacks noticeable DSB hotspots and has low-level recombination (55). We inserted lacI-nls-3flag at the C-terminus of the rec10, mug20, rec25 and rec27 coding sequences and determined the intragenic recombinant frequency between ade6-52 and ade6-3101. The ade6-3101 allele contains 8 copies of lacO substituted for an equal length of ade6 near its left (5′) end, about 400 bp from ade6-52; this substitution covers the position of ade6-M375 used as a non-hotspot control (Figure 1). In the absence of any fusion protein, the recombinant frequency was 89 Ade+/106 viable spores (Table 1). There was an ∼2-fold or ∼1.5-fold increase in recombinant frequency when homozygous Rec10-LacI or Rec27-LacI, respectively, was present, and ∼40% decrease with homozygous Rec25-LacI. Strikingly, the Mug20-LacI fusion (mug20-231) gave an impressive 5- to 6-fold increase in recombinant frequency (to 530 Ade+/106 viable spores) compared to wild type. Subsequent experiments used fusions of Mug20 and LacI.
Table 1.
Mug20-LacI fusion is most active in creating a meiotic recombination hotspot
| ade6 allele | No fusion | Rec10-LacI (rec10-233) | Mug20-LacI (mug20-231) | Rec25-LacI (rec25-230) | Rec27-LacI (rec27-232) |
|---|---|---|---|---|---|
| 3101 | 89 ± 13 (8) | 209 + 28 (5) | 529 ± 27 (8) | 60 (1)* | 158 ± 27 (5) |
Intragenic recombinant frequency (Ade+ per 106 viable spores) was assayed with ade6-3101 and ade6-52 in either the absence or presence of different LinE-LacI fusion proteins. ade6-3101 contains 8 copies of the lacO operator substituted for part of the ade6 gene (Figure 1). Data are mean ± SEM from (n) crosses. See Table S5 for data with additional ade6::lacO alleles.
*In independent experiments, the frequency was 70 ± 5 (n = 6) with Rec25-LacI and 119 ± 10 (n = 8) with Rec25.
In an attempt to get an even stronger recombination hotspot, we modified the organization of the lacO array by altering the number of lacO repeat units (1, 3 or 8) and by generating symmetrical LacI localization with direct repeat of the arrays on either the ‘left’ (5′ or ‘L’) or ‘right’ (3′ or ‘R’) side of ade6, or both (Figure 1). These double alleles are designated L3, R3; L3, R8; L8, R3 and L8, R8, where 3 and 8 indicate the number of direct lacO repeats (Supplementary Table S4). However, the largest increase among single-site substitutions was still obtained with the ade6-3101 (L8) allele containing eight lacO copies on the left (Table 2). Double-site substitutions (e.g., L3 R3) produced a low frequency of recombinants (double exchange events), making them less useful to work with.
Table 2.
Meiotic recombination hotspot requires both lacO and Mug20-LacI fusion
| ade6 allele (lacO operators) | No fusion (mug20+) | Mug20-LacI (mug20-231) | Fold increase by LacI fusion |
|---|---|---|---|
| 3098 (L1) | 185 ± 35 (5) | 369 ± 8 (3) | 2.0 |
| 3102 (L3) | 121 ± 18 (5) | 502 ± 82 (5) | 4.1 |
| 3101 (L8) | 89 ± 13 (8) | 529 ± 27 (8) | 5.9 |
| 3099 (R1) | 87 ± 11 (5) | 220 ± 4 (2) | 2.5 |
| 3111 (R8) | 52 (1) | 257 (1) | 4.9 |
| 3106 (L3, R3) | 11 ± 1 (5) | 97 ± 4 (5) | 8.8 |
| 3107 (L3, R8) | 9 ± 1 (6) | 52 ± 6 (6) | 5.8 |
| 3108 (L8, R3) | 7 ± 1 (5) | 68 ± 5 (5) | 9.7 |
| 3109 (L8, R8) | 6 ± 1 (4) | 31 ± 4 (4) | 5.2 |
| M375 (none) | 205 ± 9 (4) | 208 ± 7 (4) | 1.0 |
Intragenic recombinant frequency (Ade+ per 106 viable spores) was assayed with ade6 alleles bearing the indicated lacO array (Figure 1) and ade6-52 in either the absence (mug20+) or presence of the Mug20-LacI fusion (mug20-231). ‘L’ and ‘R’ indicate left and right sides of ade6, where the lacO operators (number per array indicated) were positioned; ade6-M375 is a single bp mutation near the position of the L arrays. Note that with double lacO substitutions, such as L3 R3, Ade+ recombinants require double exchanges. Data are mean ± SEM from (n) crosses, or value or range for n = 1 or 2.
We also varied the position of the LacI fusion on the Mug20 protein. We tested LacI fusions on the N-terminus (LacI-Mug20; mug20-252) or the C-terminus (Mug20-LacI'; mug20-254) without the NLS (nuclear localization signal) and FLAG (immunoprecipitable) components in the mug20-231 allele used above. There were modest increases in the recombinant frequency with the N-terminal fusion (LacI-Mug20; 8-fold higher than mug20+) or a C-terminal fusion without the NLS or 3FLAG (Mug20-LacI'; 6.5-fold), compared to Mug20-LacI (mug20-231; 6-fold) (Table 3). We used mug20-252 (designated as LacI-Mug20 fusion) or mug20-231 (Mug20-LacI fusion) and ade6-3101 (L8 lacO array) for the remaining analyses.
Table 3.
LacI fusion to the N-terminus of Mug20 creates the most active recombination hotspot
| ade6 allele | No fusion (mug20+) | Mug20-LacI (mug20-231) | LacI-Mug20 (mug20-252) | Mug20-LacI’ (mug20-254) |
|---|---|---|---|---|
| 3101 | 89 ± 13 (8) | 529 ± 27 (8) | 713 ± 107 (5) | 576 ± 106 (4) |
Intragenic recombinant frequency (Ade+ per 106 viable spores) was assayed with ade6-3101 and ade6-52 in either the absence or presence of the indicated LacI fusions with Mug20. ade6-3101 contains 8 copies of the lacO operator substituted for part of the ade6 gene (Figure 1).
Data are mean ± SEM from (n) crosses. See Table S6 for data with additional ade6::lacO alleles.
We further tested the specificity of recombination observed with ade6-3101 and either the LacI-Mug20 or Mug20-LacI fusion by addition of isopropylthiogalactoside (IPTG) to disrupt the lacO-LacI interaction. Addition of IPTG during meiosis reduced the ade6 recombinant frequency significantly only with the combination of ade6-3101 and LacI-Mug20 fusion, from 820 to 94 Ade+ per million viable spores (Supplementary Tables 4A and S6). Intergenic recombination between ade6 and arg1 was not significantly different with or without IPTG (Table 4) but was reduced by a factor of ∼5 relative to that in mug20+ (with or without ade6-3101). By contrast, the Mug20-LacI fusion promoted ade6 – arg1 recombination at wild-type frequency and ade-3101 recombination at enhanced level but was insensitive to IPTG (Supplementary Tables 4B and S6). Thus, in Mug20-LacI, Mug20 has enhanced activity at ade6-3101 and retains wild-type activity elsewhere, but LacI has lost IPTG-sensitivity although it apparently retains lacO binding activity. In LacI-Mug20, Mug20 has enhanced activity at ade6-3101 but has reduced activity elsewhere, and LacI apparently binds lacO with IPTG-sensitivity. These unexpected phenotypes presumably reflect complex interactions between fused Mug20 and LacI, which may in turn reflect complex interactions among the native LinE proteins. A similar, unexpected phenotype is also reported in mice: the Gal4-BD-Spo11 fusion has reduced DSB frequency at many endogenous DSB hotspots, although it creates DSB hotspots at other sites devoid of detectable DSBs in wild type (56).
Table 4.
IPTG differentially inactivates LacI fusions with Mug20
| Ade+ per 106 spores | ade6 – arg1 (cM) | |||||||
|---|---|---|---|---|---|---|---|---|
| Allele x ade6-52 | No fusion (mug20+) | LacI-Mug20 (mug20-252) | No fusion (mug20+) | LacI-Mug20 (mug20-252) | ||||
| (A) | − IPTG | + IPTG | − IPTG | + IPTG | − IPTG | + IPTG | − IPTG | + IPTG |
| ade6-M375 | 369 ± 26 | 272 ± 44 | 23 ± 7 | 43 ± 18 | 76 ± 14 | 71 ± 22 | 16 ± 3 | 16 ± 7 |
| ade6-3101 | 311 ± 66 | 272 ± 64 | 821 ± 93 | 94 ± 18 | 71 ± 17 | 46 ± 10 | 14 ± 4 | 14 ± 4 |
| No fusion (mug20+) | Mug20-LacI (mug20-231) | No fusion (mug20+) | Mug20-LacI (mug20-231) | |||||
| (B) | − IPTG | + IPTG | − IPTG | + IPTG | − IPTG | + IPTG | − IPTG | + IPTG |
| ade6-M375 | 250, 310 | 240, 260 | 350, 390 | 360, 180 | 71 ± 17 | 80 ± 21 | 86 ± 24 | 57 ± 12 |
| ade6-3101 | 190, 230 | 300, 210 | 1400, 1400 | 1600, 1200 | 64 ± 14 | 64 ± 14 | 76 ± 19 | 71 ± 17 |
Intragenic (Ade+ per 106 viable spores) and intergenic (cM) recombinant frequencies were assayed in either the absence (mug20+) or presence of the LacI-Mug20 (mug20-252) or Mug20-LacI (mug20-231) fusion and with or without IPTG (10 mM). Data for ade6 intragenic recombination are mean ± SEM from five crosses in (A); data from crosses on two separate days are shown for crosses in (B). Data for ade6 – arg1 intergenic recombination are from pooled (homogeneous) data from crosses on separate days; SD is estimated from observed fractions of recombinants. See Supplementary Table S6 for additional data with other Mug20-LacI fusions.
Recombination at the ade6-3101 hotspot is similar to that in wild type
To determine the other molecular requirements for recombination at and near the novel ade6-3101 hotspot, we tested the requirement for other proteins involved in DSB formation – the cohesin subunits (Rec8 and Rec11), the other LinE proteins (Rec10, Rec25 and Rec27), the DSB-forming protein (Rec12; Spo11 homolog), and one of its essential partners (Mde2) (6,35,41,57,58). These tests used both LacI-Mug20 and Mug20-LacI. As expected, both intra- and inter-genic recombination at or near the ade6-3101 hotspot were essentially completely dependent on Rec10, Rec12 and Mde2 (Table 5), as they are in wild-type (mug20+) strains. Absence of Rec8 and Rec11 reduced recombination by factors of 3–10 for both LacI-Mug20 and Mug20-LacI; for mug20+ the reductions were much greater, by factors ≥100. Interestingly, both Rec25 and Rec27 were also partially required with LacI-Mug20 (reductions by factors of ∼2–10 in their absence) but much more stringently required with Mug20-LacI and Mug20+ (reductions by factors of ∼25–100 in their absence) (Table 5) (35,49). As noted above (Tables 3 and 4), this difference in phenotype suggests that placement of the LacI protein on Mug20 affects function of the LinE complex, possibly through Mug20’s interaction with Rec25-Rec27. Nevertheless, the entire LinE protein complex can function at this hotspot, as at wild-type hotspots (6,34,35).
Table 5.
ade6-3101 hotspot recombination requires Rec proteins required for wild-type recombination
| LacI-Mug20 | Mug20-LacI | Mug20a | ||||
|---|---|---|---|---|---|---|
| rec gene deletion | ade6-3101 x ade6-52 (intragenic) | ade6 – arg1 (intergenic) | ade6-3101 x ade6-52 (intragenic) | ade6 – arg1 (intergenic) | ade6-M26 x ade6-52 (intragenic) | ade6 – arg1 (intergenic) |
| + | 518 ± 54 (5) | 15 ± 4 (3) | 718 ± 39 (8) | 49 | 3800 ± 700 | 73 |
| rec8Δ | 115 ± 16 (5) | 1.3 (2) | 103 ± 18 (4) | <5.9 | 5 ± 0.3 | 0.8 |
| rec11Δ | 201 ± 10 (5) | 3.6 (2) | NDb | ND | 7 ± 1.7 | 0.7 |
| rec10Δ | 2.5 ± 1.7 (4) | <2 (2) | 5.0 ± 2.0 (4) | <2.4 | <8 | <0.4 |
| rec12Δ | 2.4 ± 0.9 (5) | <3 (2) | 3.9 ± 1.7 (4) | <2 | <5 | 0.2 |
| rec25Δ | 233 ± 49 (5) | <3 (2) | ND | ND | 34 ± 1.6 | 3.3 ± 0.4 |
| rec27Δ | 112 ± 6 (5) | 1.4 (2) | 11.9 ± 1.8 (4) | 1 | 39 ± 2 | 2.9 ± 0.6 |
| mde2Δ | ND | ND | 5.8 ± 1.2 (4) | <2 | 31c | 0.4d |
Intragenic (Ade+ per 106 spores) and intergenic (cM) recombinant frequencies were assayed with the LacI-Mug20 fusion (mug20-252), the Mug20-LacI fusion (mug20-231), or wild-type Mug20 in the presence and absence of the indicated rec genes involved in meiotic recombination. Data are mean ± SEM from (n) crosses. For crosses with either zero or one recombinant, recombinant frequency was calculated at the upper 95% confidence interval based on the Poisson distribution.
aData for rec25Δ and rec27Δ are from (49), and mde2 data are from (58); other data are from (41) except for ade6 – arg1 in rec12Δ from (82).
bND, not determined.
cData are for ade6-M26 x ade6-469. mde2+ gave 8850 Ade+/106 viable spores.
dData are for leu2-120 x lys7-2. mde2+ gave 14.6 cM.
Double-strand breaks are strongly induced by Mug20-LacI around the ade6-3101 lacO array
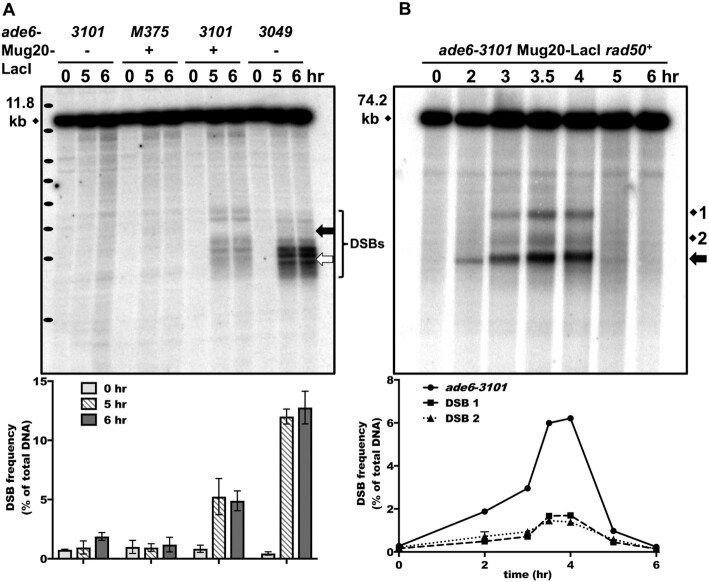
We tested the induction of meiotic DSBs at the lacO hotspot in the presence of either ade6-3101 or the Mug20-LacI fusion protein alone or both together. We observed distinct, meiosis–specific DSBs only when both ade6-3101 and Mug20-LacI were present (Figure 2A), as expected from recombination enhancement requiring both elements (Tables 2 and 4). As at wild-type DSB hotspots, DSBs were maximal at 4 h after induction of meiosis in rad50+ and accumulated as expected in the rad50S (K81I) mutant, in which DSBs are repaired very slowly (52) (Figure 2). In a rad50+ strain DSBs at ade6-3101 (Figure 2B, arrow) were detectable as early as 2 h after induction, when DNA replication was beginning (Supplementary Figure S2A). In contrast, DSBs at nearby endogenous DSB sites 1 and 2 and mbs1 on another chromosome (Figure 2B and Supplementary Figure S2B) appeared only after replication was completed at 3 h, as previously observed (52,55). To test if these early DSBs were formed before replication, we tested an mde2Δ strain, in which endogenous DSBs and meiotic recombination are severely reduced (58), and the expression of Mde2 is blocked when replication is blocked (42), showing that DSBs are dependent on replication. Recombination at ade6-3101 with Mug20-LacI was very strongly reduced, to the same level as in rec10Δ and rec12Δ (Table 5). This demonstrates that the hotspot is dependent on Mde2 and the early DSBs observed must be formed immediately after replication. This result suggests that Mug20-LacI can bind to the ade6-3101 lacO array before and independent of normal (wt) LinE loading (see Discussion, Implications for the mechanism of DSB hotspot competition and interference). All DSBs were repaired about the same time as in wild type (mug20+). On a short (11.8 kb) BsrGI fragment, we observed clear bands corresponding to DSBs flanking a DSB-free region at the lacO array in ade6-3101 (Figure 2A). This indicates that the Mug20-LacI fusion binds to the lacO array and induces breaks to both sides flanking the array. The DSB hotspot allele ade6-3049, analyzed for comparison, also showed DSBs flanking the binding site of its hotspot-determinant, the transcription factor Atf-Pcr1 (55).
Figure 2.
Linear element fusion protein Mug20-LacI induces abundant DSBs at the ade6-3101 hotspot. (A) Formation and accumulation of DSBs in rad50S strains with ade6-3101 containing 8 lacO operators (at the thick black arrow) alone, with Mug20-LacI alone (mug20-231), or with both to generate the ade6-3101 hotspot. ade6-3049 (thick white arrow) is a non-LacI-lacO DSB hotspot control. Bracket indicates the ∼2 kb region of DSB formation in the two strains. Cells were induced for meiosis and harvested at the indicated times. DNA was digested with BsrGI and analyzed by electrophoresis and Southern blot hybridization using a probe at the right end of the 11.8 kb fragment with ade6. Black ovals on the left margin indicate a DNA ladder (1 kb Plus, Invitrogen; from the top 15, 10, 8, 7, 6, 5, 4, and 3 kb). Quantification is based on 2 or 3 blots from two independent inductions; error bars indicate the range or SEM. (B) Early formation and timely repair of DSBs in a rad50+ strain with ade6-3101 and Mug20-LacI (mug20-231). DNA was analyzed as in (A) after digestion with PmeI using a probe at the right end of the 74.2 kb fragment with ade6. DSBs at ade6-3101 are indicated by the thick black arrow (∼20 kb fragment); endogenous DSB sites 1 and 2 are 15 and 5 kb from ade6. Quantification is based on two independent inductions; error bars (some invisible) indicate the range. Note that DSBs at ade6-3101 are visible before replication is complete at 3 h, but DSBs at endogenous site 1, site 2, and mbs1 are not (see also Supplementary Figure S1).
DSBs induced by Mug20-LacI have negligible competition with neighboring DSB hotspots but retain DSB interference
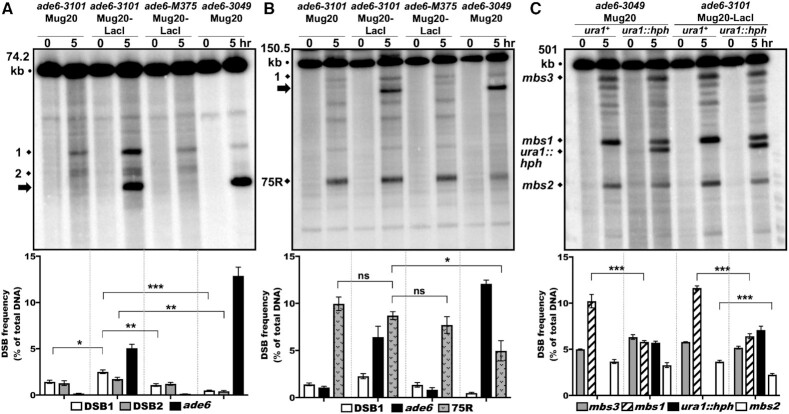
In cells with wild-type LinEs, DSBs show both competition and interference (19). Competition is the reduction of DSB frequency upon introduction of a nearby hotspot (or increase upon deletion of one hotspot of a pair). Interference is the occurrence of two nearby DSBs on one DNA molecule less frequently than the product of the individual DSB frequencies (as expected from DSB independence). These features act locally, over ∼200 kb regions. We tested these features at the ade6-3101 hotspot with Mug20-LacI. Remarkably, we observed three distinct bands corresponding to weak endogenous DSB hotspots about 15 and 5 kb from ade6, whether ade6-3101 and Mug20-LacI were present or not (Figure 3A, marked by 1 and 2, a doublet). A strong DSB hotspot ∼75 kb to the right of ade6 and weaker DSB hotspots in the intervening interval also were not competed by the ade6-3101 hotspot (75R, Figure 3B). These DSBs were, however, competed by ade6-3049 as previously observed (19), indicating that the lack of DSB competition is a special feature of the ade6-3101 hotspot. Interestingly, not only does DSB competition appear lost, but the endogenous DSB hotspot 15 kb away (marked by 1) was stimulated in the presence of Mug20-LacI and ade6-3101. DSBs at site 1 were ∼2-fold more frequent in this strain than in strains with only Mug20-LacI or ade6-3101 (1.43% and 1.08%, respectively, in the single mutants and 2.5% in the double mutant; P = 0.01 and 0.0004, respectively); the more distant 75 kb DSB was not detectably stimulated (Figure 3A and B).
Figure 3.
The Mug20-LacI fusion protein lacks DSB hotspot competition at the ade6-3101 hotspot but retains competition at the endogenous mbs1 hotspot. rad50S strains were induced for meiosis, and DNA analyzed as in Figure 2. In each panel, quantification is based on n blots from two independent inductions; error bars indicate SEM (range for n = 2). (A) ade6-3049 but not ade6-3101 plus Mug20-LacI (mug20-231) competes with close hotspots; ade6-M375 is a non-hotspot control. DNA was digested with PmeI and analyzed on three to five blots with a probe at the right end of the 74.2 kb fragment containing ade6. DSBs at sites 1 and 2 were significantly less frequent with ade6-3049 than with ade6-M375 (***P < 0.0001 for site 1 and **P = 0.0004 for site 2) but were not less frequent with the ade6-3101 hotspot (thick black arrow, ∼20 kb). Rather, DSBs at site 1 were moderately stimulated by ade6-3101 plus Mug20-LacI (mug20-231) versus ade6-3101 alone or M375: *P = 0.011 or **0.004, respectively). DSBs at site 2 were not significantly different (P = 0.18 or 0.054, respectively). (B) ade6-3049 but not the ade6-3101 hotspot (DSBs indicated by thick black arrow, ∼110 kb) competes with a distant hotspot. DNA was digested with SacII and analyzed on three or four blots with a probe at the right end of the 150.5 kb fragment with ade6. Only ade6-3049 competed with the strong DSB hotspot 75 kb to the right of ade6 (75R) (*P = 0.015) or with weaker DSBs in between. (C) Mug20-LacI manifests DSB competition on another chromosome. DNA was digested with NotI and analyzed on four to seven blots with a probe at the left end of the 501 kb NotI fragment J. mbs1 was competed by the artificial hotspot ura1::hph in both Mug20 and Mug20-LacI strains (***P < 0.0001). mbs2, an endogenous hotspot 100 kb to the left of mbs1, was also competed by ura1::hph in Mug20-LacI strains (***P < 0.0001). DSBs at mbs3, 200 kb to the right of mbs1, did not differ significantly in these strains. Strains with no black bar (first and third from the left) are ura1+.
We did, however, observe weak DSB competition between ade6-3101 and a DSB hotspot created by substitution of the tel1 ORF with the natMX6 drug-resistance determinant. Insertion of a drug-resistance determinant often forms a strong DSB hotspot, and these hotspots compete and interfere with endogenous hotspots (19). The DSBs at ade6-3101 were reduced from 5.42% to 3.56% – a reduction of 1/3 – when the tel1::natMX6 hotspot was introduced (Supplementary Figure S3A). This reduction was, however, markedly less than that observed at ade6-3049 (4-fold reduction) or at the 75R DSB (2-fold reduction; Supplementary Figure S3A). The DSB hotspot created by the tel1::natMX6 substitution may have different properties than the other endogenous DSBs nearby.
To test whether the Mug20-LacI fusion protein was functionally defective for DSB competition, perhaps by interfering with LinE complex assembly or activity, we investigated DSB hotspot competition at the mbs1 hotspot on chromosome I (ade6 is on chromosome 3). DSB hotspots - including mbs1 - are dependent on the LinE proteins (6,41). A substitution of the hygromycin-resistance determinant (hphMX6) in ura1 15–20 kb from the strong endogenous hotspot mbs1 created a strong DSB hotspot that competed with mbs1, reducing its DSB frequency by a factor of 1.8 (from 10.2 to 5.8%; Figure 3C). The same factor of reduction (1.8; from 11.6 to 6.3%) occurred in a strain with the Mug20-LacI fusion (Figure 3C), indicating that the lack of competition at ade6-3101 is not due to lack of competitive activity by the Mug20-LacI fusion. We infer that DSB competition depends on the manner of LinE protein loading onto the DSB hotspot sites (see Discussion, Implications for the mechanism of DSB hotspot competition and interference).
We next assayed DSB interference between ade6-3101 and an endogenous DSB hotspot ∼75 kb away (Figure 4A). DSB interference requires the DNA damage-response protein kinase Tel1 (ATM homolog) (19,24). The double-cut fragment was readily seen in a tel1Δ strain with mug20+ and the ade6-3049 hotspot, as well as in a tel1Δ strain with Mug20-LacI and the ade6-3101 DSB hotspot. It was much more frequent than expected from independence, as measured by the coefficient of coincidence (CoC; the frequency of observed double-cut DNA divided by the product of the frequency of each single-cut DNA) (Supplementary Figure S3A and B). A CoC < 1 indicates positive interference (I = 1 - CoC). The CoC was 3.6 in mug20+ade6-3049 tel1Δ and 2.7 in Mug20-LacI ade6-3101 tel1Δ. In contrast, very little double-cut fragment was detected in the isogenic tel1+ strain with Mug20-LacI and ade6-3101 (Figure 4A, left blot; CoC = 0.17). Similar results were observed between ade6 and a DSB hotspot 40 kb to the opposite side, near tel1. Here, the CoC was 9.2 in mug20+ade6-3049 tel1Δ and 7.7 in Mug20-LacI ade6-3101 tel1Δ. Again, very little double-cut fragment was detected in the isogenic tel1+ strain with Mug20-LacI and ade6-3101 (Figure 4A, right blot; CoC = 0.35). These data agree with previous data of endogenous DSB hotspot pairs (19) and indicate Tel1-dependent DSB interference with Mug20-LacI and the ade6-3101 hotspot.
In S. cerevisiae, DSB interference but not competition depends on Tel1 (24,25); we thus measured DSB competition in a tel1Δ strain at mbs1, as assays of competition at ade6 are complicated by its proximity to the tel1 locus. In both tel1+ and tel1Δ strains, we observed mutual competition between mbs1 and ura1::hph—the DSB frequency of each hotspot was reduced in the presence of the other (Figure 4B). The results of these experiments at 25°C were similar to those at 34°C (Figure 3C), indicating temperature-independence of DSB competition. These observations confirm that DSB competition is Tel1-independent and agree with DSB competition and interference being separable at ade6-3101 with Mug20-LacI. They are consistent with DSB competition arising during the loading of LinE proteins at DSB hotspots before DSB formation and DSB interference arising by action of Tel1 after the first DSB has been made (25,28) (see Discussion, Implications for the mechanism of DSB hotspot competition and interference).
Recombination intermediates at the ade6-3101 hotspot are similar to those at the stronger ade6-3049 DSB hotspot but show less frequent interhomolog DSB repair
Our analyses showed a discrepancy between the DSB and recombinant frequencies when comparing the ade6-3101 hotspot and the Atf1-Pcr1-dependent hotspots ade6-M26 and ade6-3049 (Figure 1). DSBs at the ade6-3101 hotspot (Figures 2 and 3A) were 4-fold more frequent than those at the ade6-M26 hotspot (55). However, the recombinant frequency with ade6-3101 was 5 times less frequent than that with ade6-M26: in crosses with ade6-52, ade6-3101 produces at most 1400 Ade+/106 viable spores (Tables 1–5), but ade6-M26 produces ∼4000 Ade+/106 viable spores (59). Thus, the recombinant:DSB ratio is ∼20-fold lower for ade6-3101 than for ade6-M26, even though they are at the same place in the ade6 gene (Figure 1). One possible explanation for this discrepancy is frequent repair of DSBs at the ade6-3101 hotspot without formation of joint molecules between the broken and intact homolog. An alternative is frequent DSB repair with the sister chromatid, which cannot yield recombinants.
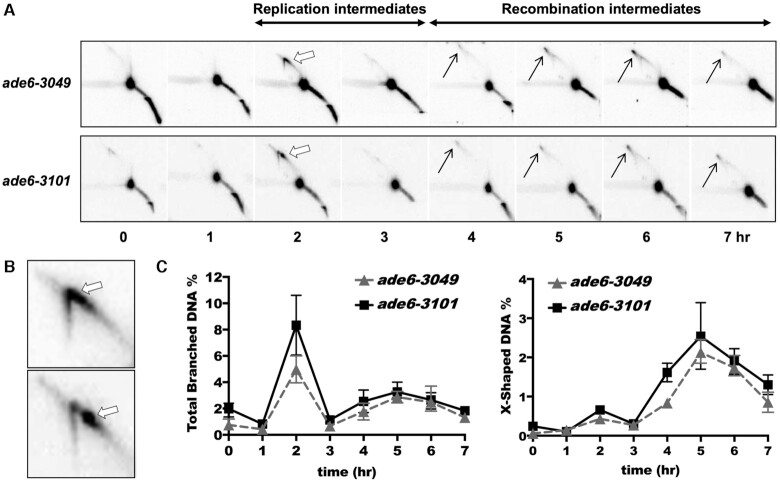
To examine these possibilities, we determined the total amount of homologous recombination intermediates (X-shaped joint molecules, black arrows in Figure 5) generated at each hotspot locus during meiosis. Two hours after induction of meiosis, we observed at the two loci similar levels of replication intermediates (Y-shaped branched molecules, white arrows), which disappeared by 3 h (Figure 5A). One distinct difference was a prominent spot on the Y- arc with the ade6-3101 hotspot (Figure 5B). Since DSBs were initiated as replication was beginning (Figure 2B and Supplementary Figure S1A), replication may pause at ade6-3101 bound by Mug20-LacI; this view suggests that loading, but not DSB formation, precedes replication, as noted above.
Figure 5.
Joint DNA molecules arise at similar time and frequency at the ade6-3101 and ade6-3049 DSB hotspots. mus81Δ strains were induced for meiosis, and DNA, digested with BsrGI, was analyzed by two-dimensional gel electrophoresis and Southern blot hybridization using a probe near the right end of the 11.8 kb fragment with ade6. (A) Branched DNA molecules, predominantly replication intermediates (Y-shaped; thick white arrows), arose at 2 h, and recombination intermediates (X-shaped Holliday junctions; thin black arrows) appeared at 4–6 h. The prominent spot is the parental DNA fragment. (B) Expanded view of replication arc at 2 h, showing a prominent pause or DSB site in the ade6-3101 strain (bottom panel) but not in the ade6-3049 strain (upper panel). (C) For quantification, branched DNA (structures above the linear DNA arc) was normalized to total DNA. Quantification is based on two or three blots from two independent inductions; error bars indicate the range or SEM.
This spot was transient, and meiotic progression was not impeded. Recombination intermediates started to appear at 4 h and accumulated until 7 h, due to absence of the Mus81-Eme1 Holliday junction (HJ)-resolving factor in the mus81Δ strain used (60) (Figure 5A). Remarkably, the frequency of the X-shaped recombination intermediates was similar at both ade6-3101 and ade6-3049 (Figure 5C), even though the DSB frequency differed by a factor of 2.5 (Figures 2 and 3A). The total (X- plus Y-shaped) intermediates were also similar in frequency at both loci (Figure 5C). This result shows that homologous recombination intermediates (HJs) were readily formed at ade6-3101 but leaves unexplained its low recombinant frequency.
DSB repair can occur by joint molecule formation with the homolog, which can generate a genetic recombinant, or with the sister chromatid, which cannot. Preferential repair with the sister could explain the low recombinant frequency with the ade6-3101 hotspot. We thus compared the ratio of intersister to interhomolog (IS:IH) X-shaped recombination intermediates (single HJs) at these hotspots. Heterozygous restriction sites flanking the hotspots allowed IS vs. IH distinction (53) (Figure 6A). For ade6-3049, the IS:IH ratio was 2.3, close to that reported previously (53). For the ade6-3101 hotspot with Mug20-LacI the IS:IH ratio was 6.5, or 3 times higher than that with ade6-3049. The total HJ frequency was nearly the same (2.3% and 2.2%) for each hotspot, as noted in the previous experiments (Figure 5). Thus, preferential repair of DSBs at the ade6-3101 hotspot with the sister can account for some but not all of the difference in recombinant frequency (see Discussion, Alterations in partner choice for DSB repair).
Figure 6.
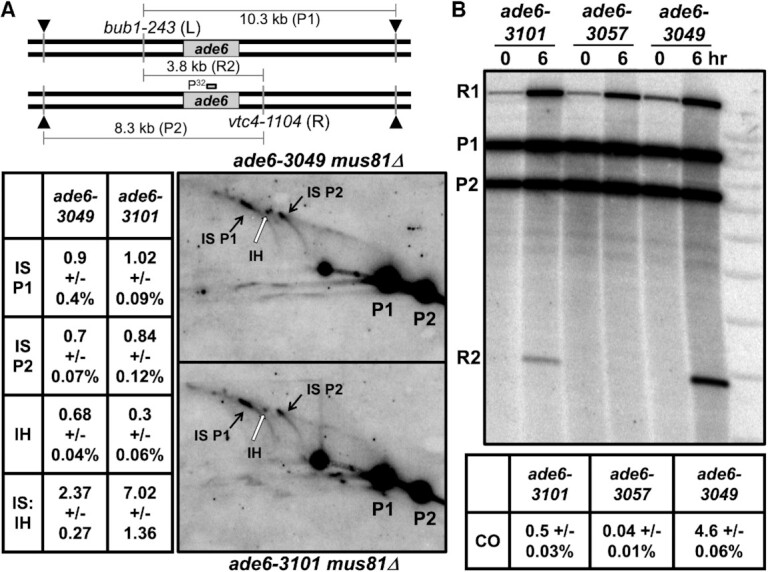
Less interhomolog DSB repair occurs at the ade6-3101 hotspot than at the ade6-3049 hotspot, and crossover DNA is strongly reduced.Diploid strains heterozygous for bub1-243 (L) and vtc4-1104 (R), flanking ade6 were used to distinguish intersister (IS) and interhomolog (IH) Holliday junctions (HJs) in (A) and crossover DNA in (B) (53). (A) mus81Δ strains were induced for meiosis and harvested at 5 h. DNA was digested with PmlI and ScaI and analyzed as in Figure 5 using a probe near the middle of ade6. IS HJs (black arrows) and IH HJs (white arrows) were quantified from three (ade6-3049) or six (ade6-3101 mug20-231) blots from two independent inductions; data are IS L/P1, IS R/P2, and IH/[(P1 + P2)/2], each as % ± SEM, where P1 and P2 are parental DNAs 1 and 2, respectively. The IS to IH ratio of HJs is indicated. (B) Strains with the indicated homozygous ade6 alleles were induced for meiosis and harvested at 5 hr; DNA was analyzed as in (A), except electrophoresis was in only one dimension. ade6-3057 is a non-hotspot control (Figure 1). R1 and R2 are reciprocal recombinant fragments. The fraction of crossover fragment, 2 × R2/(P1 + P2) because R1 can also arise from a partial restriction digestion, is based on three to five blots from two independent meiotic inductions; error bars indicate the SEM.
Physical assay of crossover DNA also reveals low recombinant frequency at ade6-3101 hotspot
It remained possible that the low recombinant frequency with the ade6-3101 hotspot in genetic assays (Tables 1–5) reflects incomplete recovery of recombinants, e.g., inviability of spores after DSB formation and repair at the ade6-3101 hotspot. Alternatively, the heterologous sequence created by the lacO substitution might impede strand invasion and recombinant formation. (Note that the lacO substitution was homozygous in the physical HJ assays above but heterozygous in the genetic recombination assays.) To test these possibilities, we assayed before spore formation total recombinant DNA with a physical assay employing the heterozygous restriction sites flanking ade6 used in Figure 6A in a strain homozygous for ade6-3101 (i.e., no large heterology present). Recombinant DNA bearing both restriction cut-sites was assayed by gel electrophoresis and Southern blot hybridization (Figure 6B). The ade6-3101 with Mug20-LacI produced 0.50% recombinant DNA fragment, 9 times less than ade6-3049 produced (4.6%); the non-hotspot control ade6-3057 (nine bp from ade6-3049) produced even less (0.04%). Thus, these physical assays of recombinants parallel the genetic assays (Tables 1–5). Below, we discuss possible explanations of these seemingly disparate data for recombination intermediates (DSBs and HJs) and final recombinants (genetic and physical).
DISCUSSION
The results presented here show that localization of meiotic linear element (LinE) proteins to a chromosomal site is sufficient to generate a DSB and recombination hotspot, complementing the necessity of LinE proteins reported previously (6). Here, we discuss these results, which provide further evidence for the DSB hotspot-clustering model previously proposed to aid solving two long-standing problems in meiosis – determining the molecular mechanisms of DSB hotspot competition and DSB and crossover interference (19).
Fusion proteins lead to DSB hotspots
In earlier, related research, S. cerevisiae Spo11 protein, with the active site for DSB formation, was localized to a chromosomal site by fusing Spo11 to the DNA-binding domain (BD) of the Gal4 transcription activator protein; this fusion led to a DSB and recombination hotspot at a Gal4-binding site in the GAL2 promoter and multiple other DSB hotspots (23,27,61). Fusion of Gal4-BD to any of seven proteins in the Spo11 complex also leads to new DSB hotspots at GAL2 (62). Fusion of Spo11 to Gal4-BD stimulates DSB formation at >200 sites in mice (56), and fusion of Spo11 to other DNA site-specific binding proteins, such as Cas9–sgRNA and zinc fingers, results in novel DSB and crossover hotspots in S. cerevisiae (63). In an alternative approach, introduction of the DNA sequence for binding of each of three transcription factors into the S. pombe ade6 gene results in recombination hotspots dependent on the respective endogenous (unfused) transcription factor (10,64). Similar to previous work in S. cerevisiae that tethered Ssp1, a subunit of the histone-methylating COMPASS complex, to Gal4-BD to create DSB hotspots (65,66), we have created hotspots using the LinE proteins that determine endogenous meiotic hotspots and act before DSB formation. Thus, proteins in addition to Spo11 and its partners can be used for genetic engineering of meiotic recombination (67).
DSBs are not formed precisely at the localization site; rather, DSBs occur to the sides of the site, spread over as much as ∼1 kb regions to both sides in S. pombe. This is true for the ade6-3101 hotspot described here (Figure 2A) and for the Atf1-Pcr1-dependent hotspots ade6-M26, ade6-3049 and other ade6 alleles (55) (Figure 2A). We infer that the LinE complex binds to special chromosomal sites and then directs the Rec12-complex to cut the DNA to either side but not where the localization factors (LinE proteins or Atf1-Pcr1) are bound. This result and the strict Rec10- and Rec12-dependence of recombination (Table 5) suggest that, although the LinE-LacI fusion protein is loaded onto its bound hotspot site differently, subsequent DSB formation proceeds in a manner similar to that in wild type.
Implications for the mechanism of DSB hotspot competition and interference
The introduced DSB hotspots in S. cerevisiae mentioned above all compete with endogenous DSB hotspots, a distinct difference from the ade6-3101 DSB hotspot studied here. In S. cerevisiae hotspot competition is, however, not always observed (68,69). The ade6-3101 hotspot also differs from the endogenous S. pombe hotspots examined to date, which manifest both hotspot competition and DSB interference (19). In S. pombe, introduction of a hotspot such as ade6-3049 without any fusion protein reduces DSB or recombination frequency at nearby hotspots (within ∼200 kb) (Figure 3) (19,70). When introduced with the Mug20-LacI fusion protein, the ade6-3101 DSB hotspot, however, did not compete with endogenous DSB hotspots on either side of the lacO array, even as close as 5 kb or as far as 75 kb (Figure 3). DSBs at the ade6-3101 hotspot do, however, manifest Tel1-dependent DSB interference, just like DSBs at endogenous hotspots (Figure 4) (19). As in S. cerevisiae (25), DSB competition is largely independent of Tel1 (Figure 4). These observations confirm that DSB competition and DSB interference are separable phenomena and invite discussion of their molecular mechanisms.
We have proposed that DSB hotspot competition and DSB interference reflect the clustering of a limited number of DSB hotspots, perhaps only two, over chromosomal regions up to ∼200 kb and the formation of a limited number of DSBs, perhaps only one, in each cluster (19). The absence of competition by the ade6-3101 hotspot suggests that competition occurs during loading of the hotspot-determinant proteins (LinEs), preceding DSB formation as previously proposed (20,23,26,27). We suggest that some factor, such as cohesin or condensin, loads LinEs onto sites with DSB hotspot potential. As the factor moves along the chromosome, it loads additional LinEs onto subsequently encountered potential hotspots on that chromosome or the sister chromatid (i.e., in cis) and clusters these LinE-bound hotspots together (Figure 7). At some point, the moving factor ceases loading, limiting potential hotspots, which manifests as competition of the DSB sites. Loading likely does not occur at all potential hotspots; some have greater potential for loading than others, thus accounting for the variation in DSB hotspot strength (6,7). Introduction of a strong potential hotspot would reduce the frequency of loading at sites subsequently encountered by the loader; deletion of a hotspot would have the opposite effect, thus accounting for localized hotspot competition in cis (19). Cohesin and condensin separately form topologically associated domains (TADs) over ∼80 kb and ∼300 kb, respectively, in S. pombe mitotic cells (71), similar to the distance (∼200 kb) over which competition and interference occur in S. pombe (19). In support of this view, the hotspot at ade6-3101 with Mug20-LacI is much less dependent on cohesin subunits Rec8 and Rec11 than are endogenous hotspots (Table 5), suggesting that cohesin's role in loading of LinEs has been at least partially bypassed; condensin has yet to be tested.
Figure 7.
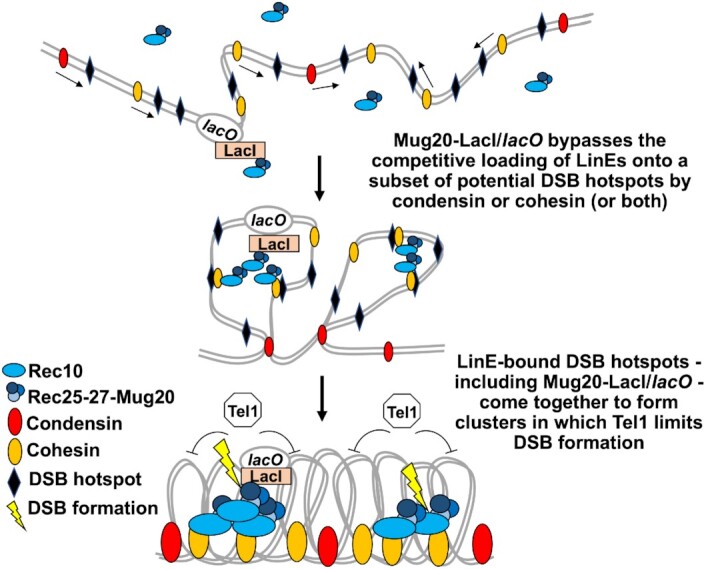
Model for DSB competition arising from the competitive loading of LinE complexes onto DSB hotspots. A loader, such as cohesin or condensin, moves along paired sister chromatids (thin black arrows) and loads LinE complexes (blue circles and ovals) onto a limited number of potential DSB hotspot sites. This prevents other sites in this traversed interval from being bound by LinEs and therefore limits DSBs to only the LinE-loaded sites (DSB competition). The ade6-3101 hotspot with a lacO array allows independent loading of Mug20-LacI and thus lacks DSB competition. The loader (cohesin or condensin) groups the LinE-hotspot complexes, including the Mug20-LacI-bound site, into a cluster, in which a DSB is formed. This DSB activates Tel1 protein kinase to prevent further DSB formation in that cluster (DSB interference).
This model may have general features that apply to other species. Cohesin is necessary for proper DSB regulation in many species (43,72–75). Studies of meiotic chromosome organization in mammals and S. cerevisiae have revealed similar dynamic compaction and loop extrusion (76–79). In S. cerevisiae, this meiotic chromosome structure depends on the cohesin subunit Rec8, and chromosome compaction also depends on proteins of the synaptonemal complex (SC) (77). Interestingly, in mammals TADs are diminished during meiosis (76,78,79), a change dependent on the SC (79). These data show that meiotic chromosome structure is unique and dynamic, and that loading of recombination factors and bringing these factors together through changes in chromosomal domains may be a conserved feature in meiotic recombination. They also highlight differences, and further investigation of the structure of S. pombe meiotic chromosomes will be of interest.
In this scenario, a hotspot at which the LinE was artificially loaded, such as by the Mug20-LacI fusion protein at ade6-3101, would not be loaded by the normal loader (e.g., cohesin or condensin) and would not compete with neighboring hotspots (Figure 7). Alternative scenarios, such as self-loading of DSB-promoting proteins limited by localized diffusion and self- enhanced binding (28), are also possible. In these alternative scenarios, however, it is not clear how DSB competition occurs only in cis. In either loading scenario, once the proteins are localized at potential DSB hotspot sites, the neighboring LinE proteins may assemble as condensates to form a higher-order regulatory cluster, as observed for proteins of the S. cerevisiae DSB-forming complex (28).
DSB interference is proposed to arise from the formation of a limited number of DSBs in a cluster, i.e., after formation of the hotspot cluster (19). Once one DSB is formed in a cluster, some factor, such as the Tel1 DNA damage-response protein kinase, is activated and prevents further DSB formation; Tel1 is required for DSB and crossover interference (19,24,80). Once the LinE complex is loaded onto the hotspot, even by its own action, the ade6-3101 hotspot could enter the surrounding cluster and be subject to Tel1’s limitation of DSB formation. This scenario accounts for the Tel1-dependent DSB interference observed with the ade6-3101 hotspot (Figure 4). These observations support the clustering model (19) and encourage further investigation of the mechanism of LinE loading and a search for a loader, such as cohesin or condensin.
Alterations in partner choice for DSB repair
While the combination of LinE-LacI and lacO array clearly created both a DSB hotspot and a recombination hotspot, we were surprised that the DSB frequency was so high (Figures 2–4), given the modest frequency of recombinants at the lacO site in ade6-3101 (Tables 1–5). This discrepancy between DSB and recombinant frequencies may have multiple sources. One source may be the large heterology imparted by the lacO array: recombinant frequencies in the absence of a LinE-LacI fusion protein steadily decreased as the length of heterology increased (Table 2). But the lacO array was homozygous (hence, no heterology) in the physical recombinant assays, which showed ∼10 times fewer recombinants with ade6-3101 than with ade6-3049 (Figure 6B). But only some of this reduction is due to the 2-fold lower DSBs at ade6-3101 than at ade6-3049 (Figures 2 and 3). Interhomolog Holliday junctions (HJs), which unlike intersister HJs can be converted into recombinants, also were 2-fold less abundant with ade6-3101 than with ade6-3049 (Figure 6A), but these factors still do not fully account for the paucity of recombinants. In addition to their established role in DSB formation (6), LinEs may have a role in directing DSB repair, as proposed from LinE structures evolving from early to late meiosis (31,36,40). In particular, the manner of loading LinEs at hotspots, self-loading vs. cohesin- or condensin-mediated loading for example, may influence both partner choice for HJ-formation and crossover vs. non-crossover preference during DSB repair. The 3-fold higher IS:IH ratio of HJs with Mug20-LacI than with ade6-3049 (Figure 6A) may be related to the previously observed crossover invariance – more uniform crossover frequency than DSB frequency (53). The mechanism for invariance has been unknown, but DSBs at hotspots with high IS:IH ratio are more dependent on the histone variant H2A.Z than are DSBs in cold regions or at weak hotspots (18,81). It was proposed that H2A.Z promotes LinE binding to chromatin-bound cohesin; this view suggests that crossover invariance is directly related to LinE function and loading.
The LinE-LacI–lacO DSB and recombination hotspots studied here support the hotspot-clustering model for DSB competition and interference. They also reveal new features of meiotic DSB hotspots and their activating proteins. Additional investigations of LinE-LacI–lacO hotspots should help understand further the molecular mechanism of meiotic recombination.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Toshi Tsukiyama for plasmids and advice; Mai-Chi Nguyen for data on rec25-230; and Sue Amundsen, Yu-Chien Chuang and anonymous reviewers for helpful comments on the manuscript.
Notes
Present address: Joshua D. Cho, Dept. of Pathology, Columbia University, New York, NY 10032, USA.
Present address: Mridula Nambiar, Department of Biology, Indian Institute of Science Education and Research, Pune 411008, India.
Contributor Information
Randy W Hyppa, Division of Basic Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA.
Joshua D Cho, Division of Basic Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA.
Mridula Nambiar, Division of Basic Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA.
Gerald R Smith, Division of Basic Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Institutes of General Medical Sciences [R01 GM031693 and R35 GM118120 to G.R.S.]; National Cancer Institute [P30 CA015704 to the Fred Hutchinson Cancer Research Center]. Funding for open access charge: NIH [R35 GM118120].
Conflict of interest statement. None declared.
REFERENCES
- 1. Zickler D., Kleckner N.. Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 1999; 33:603–754. [DOI] [PubMed] [Google Scholar]
- 2. Bhalla N., Dernburg A.F.. Prelude to a division. Annu. Rev. Cell Dev. Biol. 2008; 24:397–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nicklas R.B., Koch C.A.. Chromosome micromanipulation. 3. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J. Cell Biol. 1969; 43:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lam I., Keeney S.. Mechanism and regulation of meiotic recombination initiation. Cold Spring Harb. Perspect. Biol. 2015; 7:a016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nambiar M., Chuang Y.C., Smith G.R.. Distributing meiotic crossovers for optimal fertility and evolution. DNA Repair (Amst.). 2019; 81:102648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fowler K.R., Gutiérrez-Velasco S., Martín-Castellanos C., Smith G.R.. Protein determinants of meiotic DNA break hotspots. Mol. Cell. 2013; 49:983–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fowler K.R., Sasaki M., Milman N., Keeney S., Smith G.R.. Evolutionarily diverse determinants of meiotic DNA break and recombination landscapes across the genome. Genome Res. 2014; 24:1650–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan J., Sasaki M., Kniewel R., Murakami H., Blitzblau H.G., Tischfield S.E., Zhu X., Neale M.J., Jasin M., Socci N.D.et al.. A hierarchical combination of factors shapes the genome-wide topography of yeast meiotic recombination initiation. Cell. 2011; 144:719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lange J., Yamada S., Tischfield S.E., Pan J., Kim S., Zhu X., Socci N.D., Jasin M., Keeney S.. The landscape of mouse meiotic double-strand break formation, processing, and repair. Cell. 2016; 167:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mukiza T.O., Protacio R.U., Davidson M.K., Steiner W.W., Wahls W.P.. Diverse DNA sequence motifs activate meiotic recombination hotspots through a common chromatin remodeling pathway. Genetics. 2019; 213:789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohta K., Shibata T., Nicolas A.. Changes in chromatin structure at recombination initiation sites during yeast meiosis. EMBO J. 1994; 13:5754–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu T.C., Lichten M.. Meiosis-induced double-strand break sites determined by yeast chromatin structure. Science. 1994; 263:515–518. [DOI] [PubMed] [Google Scholar]
- 13. de Castro E., Soriano I., Marin L., Serrano R., Quintales L., Antequera F.. Nucleosomal organization of replication origins and meiotic recombination hotspots in fission yeast. EMBO J. 2011; 31:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borde V., Robine N., Lin W., Bonfils S., Geli V., Nicolas A.. Histone H3 lysine 4 trimethylation marks meiotic recombination initiation sites. EMBO J. 2009; 28:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grey C., Barthes P., Chauveau-Le Friec G., Langa F., Baudat F., de Massy B. Mouse PRDM9 DNA-binding specificity determines sites of histone H3 lysine 4 trimethylation for initiation of meiotic recombination. PLoS Biol. 2011; 9:e1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smagulova F., Gregoretti I.V., Brick K., Khil P., Camerini-Otero R.D., Petukhova G.V.. Genome-wide analysis reveals novel molecular features of mouse recombination hotspots. Nature. 2011; 472:375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamada T., Ohta K.. Initiation of meiotic recombination in chromatin structure. J. Biochem. (Tokyo). 2013; 154:107–114. [DOI] [PubMed] [Google Scholar]
- 18. Yamada S., Kugou K., Ding D.Q., Fujita Y., Hiraoka Y., Murakami H., Ohta K., Yamada T.. The histone variant H2A.Z promotes initiation of meiotic recombination in fission yeast. Nucleic Acids Res. 2018; 46:609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fowler K.R., Hyppa R.W., Cromie G.A., Smith G.R.. Physical basis for long-distance communication along meiotic chromosomes. Proc. Natl. Acad. Sci. U.S.A. 2018; 115:E9333–E9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu T.C., Lichten M.. Factors that affect the location and frequency of meiosis- induced double-strand breaks in Saccharomyces cerevisiae. Genetics. 1995; 140:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu L., Kleckner N.. Sequence non-specific double-strand breaks and interhomolog interactions prior to double-strand break formation at a meiotic recombination hot spot in yeast. EMBO J. 1995; 14:5115–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jessop L., Allers T., Lichten M.. Infrequent co-conversion of markers flanking a meiotic recombination initiation site in Saccharomyces cerevisiae. Genetics. 2005; 169:1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robine N., Uematsu N., Amiot F., Gidrol X., Barillot E., Nicolas A., Borde V.. Genome-wide redistribution of meiotic double-strand breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 2007; 27:1868–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia V., Gray S., Allison R.M., Cooper T.J., Neale M.J.. Tel1(ATM)-mediated interference suppresses clustered meiotic double-strand-break formation. Nature. 2015; 520:114, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohibullah N., Keeney S.. Numerical and spatial patterning of yeast meiotic DNA breaks by Tel1. Genome Res. 2017; 27:278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cooper T.J., Garcia V., Neale M.J.. Meiotic DSB patterning: A multifaceted process. Cell Cycle. 2016; 15:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fukuda T., Kugou K., Sasanuma H., Shibata T., Ohta K.. Targeted induction of meiotic double-strand breaks reveals chromosomal domain-dependent regulation of Spo11 and interactions among potential sites of meiotic recombination. Nucleic Acids Res. 2008; 36:984, 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Claeys Bouuaert C., Pu S., Wang J., Oger C., Daccache D., Xie W., Patel D.J., Keeney S. DNA-driven condensation assembles the meiotic DNA break machinery. Nature. 2021; 592:144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bähler J., Wyler T., Loidl J., Kohli J.. Unusual nuclear structures in meiotic prophase of fission yeast: a cytological analysis. J. Cell Biol. 1993; 121:241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lorenz A., Estreicher A., Kohli J., Loidl J.. Meiotic recombination proteins localize to linear elements in Schizosaccharomyces pombe. Chromosoma. 2006; 115:330–340. [DOI] [PubMed] [Google Scholar]
- 31. Chuang Y.C., Smith G.R.. Dynamic configurations of meiotic hotspot determinants. 2020; bioRxiv doi:27 June 2020, preprint: not peer reviewed 10.1101/2020.06.26.167775. [DOI] [PMC free article] [PubMed]
- 32. Loidl J. S. pombe linear elements: the modest cousins of synaptonemal complexes. Chromosoma. 2006; 115:260–271. [DOI] [PubMed] [Google Scholar]
- 33. Page S.L., Hawley R.S.. The genetics and molecular biology of the synaptonemal complex. Annu. Rev. Cell Dev. Biol. 2004; 20:525–558. [DOI] [PubMed] [Google Scholar]
- 34. Estreicher A., Lorenz A., Loidl J.. Mug20, a novel protein associated with linear elements in fission yeast meiosis. Curr. Genet. 2012; 58:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davis L., Rozalén A.E., Moreno S., Smith G.R., Martín-Castellanos C.. Rec25 and Rec27, novel components of meiotic linear elements, link cohesin to DNA breakage and recombination in fission yeast. Curr. Biol. 2008; 18:849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lorenz A., Wells J.L., Pryce D.W., Novatchkova F.E., Eisenhaber F., McFarlane R.J., Loidl J.. S. pombe meiotic linear elements contain proteins related to synaptonemal complex components. J. Cell Sci. 2004; 117:3343–3351. [DOI] [PubMed] [Google Scholar]
- 37. Wintrebert M., Nguyen M.-C., Smith G.R.. Activation of meiotic recombination by nuclear import of the DNA break hotspot-determining complex in fission yeast. J. Cell Sci. 2021; 134:jcs253518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simonis N., Rual J.F., Carvunis A.R., Tasan M., Lemmens I., Hirozane-Kishikawa T., Hao T., Sahalie J.M., Venkatesan K., Gebreab F.et al.. Empirically controlled mapping of the Caenorhabditis elegans protein-protein interactome network. Nat. Methods. 2009; 6:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rog O., Kohler S., Dernburg A.F.. The synaptonemal complex has liquid crystalline properties and spatially regulates meiotic recombination factors. eLife. 2017; 6:e21455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ding D.Q., Matsuda A., Okamasa K., Hiraoka Y.. Linear elements are stable structures along the chromosome axis in fission yeast meiosis. Chromosoma. 2021; 130:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ellermeier C., Smith G.R.. Cohesins are required for meiotic DNA breakage and recombination in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. U.S.A. 2005; 102:10952, 10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miyoshi T., Ito M., Kugou K., Yamada S., Furuichi M., Oda A., Yamada T., Hirota K., Masai H., Ohta K.. A central coupler for recombination initiation linking chromosome architecture to S phase checkpoint. Mol. Cell. 2012; 47:722–733. [DOI] [PubMed] [Google Scholar]
- 43. Sakuno T., Watanabe Y.. Phosphorylation of cohesin Rec11/SA3 by casein kinase 1 promotes homologous recombination by assembling the meiotic chromosome axis. Dev. Cell. 2015; 32:220–230. [DOI] [PubMed] [Google Scholar]
- 44. Kariyazono R., Oda A., Yamada T., Ohta K.. Conserved HORMA domain-containing protein Hop1 stabilizes interaction between proteins of meiotic DNA break hotspots and chromosome axis. Nucleic Acids Res. 2019; 47:10166–10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith G.R. Keeney S. Genetic analysis of meiotic recombination in Schizosaccharomyces pombe. Meiosis. 2009; Totowa, NJ: Humana Press; 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bähler J., Wu J.-Q., Longtine M.S., Shah N.G., McKenzie A. III, Steever A.B., Wach A., Philippsen P., Pringle J.R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998; 14:943–951. [DOI] [PubMed] [Google Scholar]
- 47. Unnikrishnan A., Akiyoshi B., Biggins S., Tsukiyama T.. An efficient purification system for native minichromosome from Saccharomyces cerevisiae. Methods Mol. Biol. 2012; 833:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grimm C., Kohli J.. Observations on integrative transformation in Schizosaccharomyces pombe. Mol. Gen. Genet. 1988; 215:87–93. [DOI] [PubMed] [Google Scholar]
- 49. Ma L., Fowler K.R., Martín-Castellanos C., Smith G.R.. Functional organization of protein determinants of meiotic DNA break hotspots. Sci. Rep. 2017; 7:1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sato M., Dhut S., Toda T.. New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast. 2005; 22:583–591. [DOI] [PubMed] [Google Scholar]
- 51. Hyppa R.W., Smith G.R.. Keeney S. Using Schizosaccharomyces pombe meiosis to analyze DNA recombination intermediates. Meiosis. 2009; Totowa, NJ: Humana Press; 235–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Young J.A., Schreckhise R.W., Steiner W.W., Smith G.R.. Meiotic recombination remote from prominent DNA break sites in S. pombe. Mol. Cell. 2002; 9:253–263. [DOI] [PubMed] [Google Scholar]
- 53. Hyppa R.W., Smith G.R.. Crossover invariance determined by partner choice for meiotic DNA break repair. Cell. 2010; 142:243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Haldane J.B.S. The combination of linkage values, and the calculation of distances between the loci of linked factors. J. Genet. 1919; 8:299–309. [Google Scholar]
- 55. Steiner W.W., Schreckhise R.W., Smith G.R.. Meiotic DNA breaks at the S. pombe recombination hotspot M26. Mol. Cell. 2002; 9:847–855. [DOI] [PubMed] [Google Scholar]
- 56. Smagulova F., Brick K., Pu Y., Sengupta U., Camerini-Otero R.D., Petukhova G.V.. Suppression of genetic recombination in the pseudoautosomal region and at subtelomeres in mice with a hypomorphic Spo11 allele. BMC Genomics. 2013; 14:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cervantes M.D., Farah J.A., Smith G.R.. Meiotic DNA breaks associated with recombination in S. pombe. Mol. Cell. 2000; 5:883–888. [DOI] [PubMed] [Google Scholar]
- 58. Gregan J., Rabitsch P.K., Sakem B., Csutak O., Latypov V., Lehmann E., Kohli J., Nasmyth K.. Novel genes required for meiotic chromosome segregation are identified by a high-throughput knockout screen in fission yeast. Curr. Biol. 2005; 15:1663–1669. [DOI] [PubMed] [Google Scholar]
- 59. Ponticelli A.S., Smith G.R.. Meiotic recombination-deficient mutants of Schizosaccharomyces pombe. Genetics. 1989; 123:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cromie G.A., Hyppa R.W., Taylor A.F., Zakharyevich K., Hunter N., Smith G.R.. Single Holliday junctions are intermediates of meiotic recombination. Cell. 2006; 127:1167, 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pecina A., Smith K.N., Mezard C., Murakami H., Ohta K., Nicolas A.. Targeted stimulation of meiotic recombination. Cell. 2002; 111:173–184. [DOI] [PubMed] [Google Scholar]
- 62. Koehn D.R., Haring S.J., Williams J.M., Malone R.E.. Tethering recombination initiation proteins in Saccharomyces cerevisiae promotes double strand break formation. Genetics. 2009; 182:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sarno R., Vicq Y., Uematsu N., Luka M., Lapierre C., Carroll D., Bastianelli G., Serero A., Nicolas A.. Programming sites of meiotic crossovers using Spo11 fusion proteins. Nucleic Acids Res. 2017; 45:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steiner W.W., Steiner E.M., Grivin A.R., Plewik L.E.. Novel nucleotide sequence motifs that produce hotspots of meiotic recombination in Schizosaccharomyces pombe. Genetics. 2009; 182:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Acquaviva L., Szekvolgyi L., Dichtl B., Dichtl B.S., de La Roche Saint Andre C., Nicolas A., Geli V.. The COMPASS subunit Spp1 links histone methylation to initiation of meiotic recombination. Science. 2013; 339:215–218. [DOI] [PubMed] [Google Scholar]
- 66. Sommermeyer V., Beneut C., Chaplais E., Serrentino M.E., Borde V.. Spp1, a member of the Set1 complex, promotes meiotic DSB formation in promoters by tethering histone H3K4 methylation sites to chromosome axes. Mol. Cell. 2013; 49:43–54. [DOI] [PubMed] [Google Scholar]
- 67. Fayos I., Mieulet D., Petit J., Meunier A.C., Perin C., Nicolas A., Guiderdoni E.. Engineering meiotic recombination pathways in rice. Plant Biotechnol. J. 2019; 17:2062, 2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bullard S.A., Kim S., Galbraith A.M., Malone R.E.. Double strand breaks at the HIS2 recombination hot spot in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 1996; 93:13054–13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhu X., Keeney S.. High-resolution global analysis of the influences of Bas1 and Ino4 transcription factors on meiotic DNA break distributions in Saccharomyces cerevisiae. Genetics. 2015; 201:525–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zahn-Zabal M., Lehmann E., Kohli J.. Hot spots of recombination in fission yeast: inactivation of the M26 hot spot by deletion of the ade6 promoter and the novel hotspot ura4-aim. Genetics. 1995; 140:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim K.D., Tanizawa H., Iwasaki O., Noma K.. Transcription factors mediate condensin recruitment and global chromosomal organization in fission yeast. Nat. Genet. 2016; 48:1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Panizza S., Mendoza M.A., Berlinger M., Huang L., Nicolas A., Shirahige K., Klein F.. Spo11-accessory proteins link double-strand break sites to the chromosome axis in early meiotic recombination. Cell. 2011; 146:372–383. [DOI] [PubMed] [Google Scholar]
- 73. Bhattacharyya T., Walker M., Powers N.R., Brunton C., Fine A.D., Petkov P.M., Handel M.A.. Prdm9 and meiotic cohesin proteins cooperatively promote DNA double-strand break formation in mammalian spermatocytes. Curr. Biol. 2019; 29:1002–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Castellano-Pozo M., Pacheco S., Sioutas G., Jaso-Tamame A.L., Dore M.H., Karimi M.M., Martinez-Perez E.. Surveillance of cohesin-supported chromosome structure controls meiotic progression. Nat. Commun. 2020; 11:4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kumar R., Ghyselinck N., Ishiguro K., Watanabe Y., Kouznetsova A., Hoog C., Strong E., Schimenti J., Daniel K., Toth A.et al.. MEI4 - a central player in the regulation of meiotic DNA double-strand break formation in the mouse. J. Cell Sci. 2015; 128:1800–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Patel L., Kang R., Rosenberg S.C., Qiu Y., Raviram R., Chee S., Hu R., Ren B., Cole F., Corbett K.D.. Dynamic reorganization of the genome shapes the recombination landscape in meiotic prophase. Nat. Struct. Mol. Biol. 2019; 26:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schalbetter S.A., Fudenberg G., Baxter J., Pollard K.S., Neale M.J.. Principles of meiotic chromosome assembly revealed in S. cerevisiae. Nat. Commun. 2019; 10:4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Alavattam K.G., Maezawa S., Sakashita A., Khoury H., Barski A., Kaplan N., Namekawa S.H.. Attenuated chromatin compartmentalization in meiosis and its maturation in sperm development. Nat. Struct. Mol. Biol. 2019; 26:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang Y., Wang H., Zhang Y., Du Z., Si W., Fan S., Qin D., Wang M., Duan Y., Li L.et al.. Reprogramming of meiotic chromatin architecture during spermatogenesis. Mol. Cell. 2019; 73:547–561. [DOI] [PubMed] [Google Scholar]
- 80. Anderson C.M., Oke A., Yam P., Zhuge T., Fung J.C.. Reduced crossover interference and increased ZMM-independent recombination in the absence of Tel1/ATM. PLoS Genet. 2015; 11:e1005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yamada T., Yamada S., Ding D.Q., Fujita Y., Takaya E., Hiraoka Y., Murakami H., Ohta K.. Maintenance of meiotic crossover against reduced double-strand break formation in fission yeast lacking histone H2A.Z. Gene. 2020; 743:144615. [DOI] [PubMed] [Google Scholar]
- 82. Farah J.A., Cromie G., Davis L., Steiner W.W., Smith G.R.. Activation of an alternative, Rec12 (Spo11)-independent pathway of fission yeast meiotic recombination in the absence of a DNA flap endonuclease. Genetics. 2005; 171:1499–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.