Abstract
Background:
The renin-angiotensin-aldosterone system (RAAS) is an important driver of BP but the association of the RAAS with ambulatory blood pressure (ABP) and ABPM phenotypes among African Americans (AA) has not been assessed.
Methods:
ABP and ABPM phenotypes were assessed in 912 Jackson Heart Study participants with aldosterone and plasma renin activity (PRA). Multivariable linear and logistic regression analysis were used to analyze the association of aldosterone, and PRA with clinic, awake and asleep systolic blood pressure (SBP) and diastolic blood pressure (DBP) and ABPM phenotypes, adjusting for important confounders.
Results:
The mean age of participants was 59 ±11 years and 69% were female. In fully adjusted models, lower log-PRA was associated with higher clinic, awake, and asleep SBP and DBP (all p<0.05). A higher log-aldosterone was associated with higher clinic, awake, and asleep DBP (all p<0.05). A 1-unit higher log-PRA was associated with lower odds of daytime hypertension (OR: 0.59, 95%CI: 0.49, 0.71), nocturnal hypertension (OR: 0.68, 95%CI: 0.58, 0.79), daytime and nocturnal hypertension (OR: 0.59, 95%CI: 0.48, 0.71), sustained hypertension (OR: 0.52, 95%CI: 0.39, 0.70) and masked hypertension (OR 0.75, 95%CI: 0.62, 0.90). A 1-unit higher log-aldosterone was associated with higher odds of nocturnal hypertension (OR: 1.38, 95%CI: 1.05, 1.81). Neither PRA nor aldosterone were associated with percent dipping, non-dipping BP pattern, or white-coat hypertension. Patterns for aldosterone:renin ratio were similar to PRA.
Conclusions:
Suppressed renin activity and higher aldosterone:renin ratios were associated with both higher SBP and DBP in the office and during the awake and asleep periods as evidenced by ABPM. Higher aldosterone levels were associated with higher DBP, but not SBP, in the clinic and during the awake and asleep periods. Further clinical investigation of novel and approved medications that target low renin physiology such as epithelial sodium channel inhibitors and mineralocorticoid receptor antagonists may be paramount in improving hypertension control in AAs.
Keywords: African Americans, Ambulatory Blood Pressure, Plasma Renin Activity, Aldosterone, Jackson Heart Study, Hypertension
Introduction:
Cardiovascular disease (CVD) is the leading cause of death in the United States and contributes substantially to morbidity in the United States (US).1–3 CVD disproportionately impacts African Americans (AA) with younger age at diagnoses and lower long-term survival compared to non-Hispanic whites (NHW).4,5 This trend may in part be the result of the disproportionate prevalence of CVD risk factors, particularly hypertension, in AAs.5 Hypertension diagnosed based on clinic measured blood pressure (BP) is a major risk factor for CVD,6 but clinic based BP measurements are prone to variation due to random error, systematic error, and a patient’s response to other daytime physiologic variables,7–9 complicating the diagnosis and treatment of hypertension. Ambulatory blood pressure monitoring (ABPM) improves on office measurements by measuring blood pressure in a natural environment and is therefore potentially more reliable in the diagnosis and long-term prognosis of hypertension.10 Higher ambulatory blood pressures (ABP) have been associated with an increased risk for CVD events and all-cause mortality independent of clinic BP among AAs and in diverse cohorts.10–12 Several ABPM phenotypes are associated with an increased risk for CVD including sustained hypertension, masked hypertension, nocturnal hypertension, nocturnal non-dipping status and white-coat hypertension.8,9,11,12,10,13
BP homeostasis is influenced by the renin-angiotensin-aldosterone system (RAAS) via regulation of fluids, electrolytes, and vasoconstriction. Increased activity of downstream RAAS mediators, angiotensin II and aldosterone, have been implicated in the higher rates of hypertension and related complications in AAs contributing to poorer health outcomes compared to other racial/ethnic groups.5,14 However, the associations of the RAAS system including aldosterone and plasma renin activity (PRA) with clinic and ABP have been mixed and vary by race/ethnicity. Higher aldosterone was associated with higher clinic BP among Hispanic Americans but not among Whites, Blacks or Chinese Americans in The Multi-Ethnic Study of Atherosclerosis (MESA).15
However, previous studies have not systematically investigated the associations of aldosterone and PRA with BP in a large cohort of AAs. Because ABPM may provide more reliable data than BP measured in the clinic, we investigated the cross-sectional association of aldosterone, PRA and aldosterone:renin ratio (ARR) with clinic and ABPs and ABPM phenotypes among AAs in the JHS. AA have high rates of low PRA and salt sensitive hypertension;14,15 thus, we hypothesized that higher aldosterone and ARR, and lower PRA would be associated with higher clinic BP, ABPs and higher odds of ABPM hypertension phenotypes. Additionally, we explored: 1) The association of renin phenotypes (suppressed, indeterminate and unsuppressed) with ABP and ABPM phenotypes; and 2) The association of aldosterone with ABP and ABPM phenotypes within the suppressed, indeterminate and unsuppressed renin phenotype groups to investigate the potential drivers of underlying RAAS pathophysiology (Figure I in the Supplement).
Methods:
The JHS is a prospective community-based cohort study of the development and progression of CVD in 5,306 AA adults aged 21–94 years within the tri-county area (Hinds, Madison, Rankin) of the Jackson, Mississippi metropolitan area. The baseline examination was performed between 2000–2004. The design of the study has been described elsewhere.16 The study was approved by the institutional review boards of the University of Mississippi Medical Center, Jackson State University, and Tougaloo College. All participants provided informed consent. The data, analytic methods, and study materials are available to other researchers for purposes of reproducing the results or replicating the procedure by following the JHS publication procedures and data use agreements guidelines.17
All participants were offered ABPM at the baseline visit and the current analysis involved 1148 individuals who completed ABPM between 2000 and 2004.18 In the aldosterone analyses participants were excluded for missing data on aldosterone (n=15), clinic systolic BP (SBP) and diastolic BP (DBP) (n=5), awake SBP and DBP (n=3), asleep SBP and DBP (n=68), and covariates at the initial exam including: education (n=8), smoking (n=7), alcohol (n=3), diabetes status (n=2), waist circumference (n=2), estimated glomerular filtration rate [eGFR] (n=2), low-density lipoprotein [LDL] (n=77), and antihypertensive medications (n=21). Further, in accordance with previous investigations, participants without 10+ daytime measurements or 5+ nighttime BP measurements were excluded (n=23).19 Age, sex, occupation, physical activity, or hormone replacement therapy (HRT) were all also included in the analysis and there were no additional exclusions of individuals due to missing data for these variables. The PRA analyses were performed using the same data set with additional exclusion of individuals that lacked PRA data (n=266). The final analytic cohort included 912 in aldosterone analyses and 646 in PRA analyses. Characteristics of the excluded versus included participants are presented in Table I in the Supplement.
Exposures: Serum Aldosterone, PRA and Aldosterone:Renin Ratio
Serum aldosterone and PRA were collected at Exam 1 (2000–2004). Fasting blood samples were collected with participants in the supine position and processed using a standardized protocol. Plasma and serum samples were centrifuged within 2 hours of blood collection, stored at −70°C, and sent to central laboratories (University of Minnesota).16,18,20 Serum aldosterone was measured using radioimmunoassay (Coat-a-count aldosterone, Siemens, Munich, Germany) and the intra-assay coefficients of variation were 8.7% and 6.2% for low and high concentrations, respectively. PRA was measured at baseline using immunoradiometric assays in ng/ml/hr with an intra-assay coefficient of variation of 8.0%. ARR was calculated by dividing the aldosterone by PRA. The respective contribution of aldosterone and PRA to the ARR is presented in Figure II in the Supplement, showing that the majority of higher ARR values were due to lower PRA.
Outcomes: Clinic Blood Pressure, Ambulatory Blood Pressure and Ambulatory Blood Pressure Monitoring Phenotypes
Resting seated BP was measured twice at 5-minute intervals using an appropriately sized cuff with standard Hawksley random-zero instruments and measurements were averaged for analysis. The random zero sphygmomanometer measurements were calibrated to oscillometric measurements for clinic SBP and DBP using the following equations: Systolic blood pressure (calibrated oscillometric) = 11.02 + 0.92 x systolic blood pressure (random zero sphygmomanometer measured); and diastolic blood pressure (calibrated oscillometric) = 10.36 + 0.83 x diastolic blood pressure (random zero sphygmomanometer measured).21 Ambulatory blood pressures were recorded using an ABPM device (Spacelabs 90207, Spacelabs) on the non-dominant arm of participants with an appropriately sized blood pressure cuff for 24 hours.22 Measurements were taken every 20 minutes and mean SBP and DBP values were determined. After 24 hours, the device was removed, and data were downloaded onto a computer and processed with Medifacts International’s Medicom software International Database on ABPM. The International Database of Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) criteria were used to define a complete ABPM measurement, defined as ≥10 daytime (10:00–20:00) and ≥5 nighttime (00:00–06:00) SBP and DBP measurements.19 Mean SBP and DBP levels were calculated for daytime, nighttime, and 24-hour periods.22 Clinic hypertension was defined as clinic SBP ≥140 mmHg or DBP ≥90 mmHg. Daytime hypertension was defined as daytime SBP at least 135 mmHg or daytime DBP at least 85 mmHg. Nocturnal hypertension was defined as nighttime SBP at least 120 mmHg or DBP at least 70 mmHg. Daytime and nocturnal hypertension was defined as the combination of daytime and nocturnal hypertension as described above. Sustained hypertension was defined as the combination of clinic and daytime hypertension. Non-dipping blood pressure pattern was defined as < 10% decrease in mean daytime vs mean nighttime SBP. White coat hypertension was defined as not having daytime hypertension among participants with clinic hypertension. Masked hypertension was defined as daytime hypertension among participants without clinic hypertension (Table II in the Supplement).22,23 Sensitivity analyses were performed using the clinic 130/80 mmHg cutoff from the AHA/ACC 2017 guidelines and the corresponding ABPM phenotype cutoffs (daytime hypertension defined as daytime SBP at least 130 mmHg or daytime DBP at least 80 mmHg and nocturnal hypertension is defined as night-time SBP at least 110 mmHg or DBP at least 65 mmHg).23
Covariates
The covariates included demographics (age [continuous], sex [male/female]), level of education (≥ Bachelor’s degree versus < Bachelor’s degree), occupation (management/professional versus not), tobacco use (current smoking versus not), alcohol use (in the past 12 months versus not), medical conditions (history of CVD [coronary heart disease, stroke or heart failure]) and current prescription medication usage (HRT, anti-hypertensive therapy). Waist circumference was measured around the umbilicus. Physical activity was categorized according to the American Heart Association 2020 Cardiovascular health guidelines as poor, intermediate or ideal health, as described previously.24,25 Glucose and glycosylated hemoglobin (A1c) concentrations were measured as previously described.25 Diabetes was defined based on 2010 American Diabetes Association guidelines (A1c ≥ 6.5%, fasting glucose ≥ 126 mg/dL, taking diabetes medications or self-reported physician diagnosis).26 Fasting serum LDL (mg/dL) was assayed using standard techniques.18 EGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (ml/min per 1.73 m2).27
Statistical Analysis:
Baseline characteristics were presented by quartiles of aldosterone: Q1: 1.9 to 2.7 ng/dL (n=237); Q2: 2.8 to 4.5 ng/dL (n=231); Q3: 4.6 to 7.5 ng/dL (n=228); Q4: 7.6 to 61.1 ng/dL (n=216). Quartiles were compared using one-way analysis of variance for normally distributed continuous variables, Kruskal-Wallis test for non-normally distributed continuous variables and the Chi-square test for categorical variables. Aldosterone and PRA were log-transformed due to non-normality. Multivariable linear regressions were performed to examine the association of aldosterone, PRA, and ARR with SBP and DBP in clinic, awake, and asleep settings. Multivariable logistic regression analysis was performed to determine associations with the ABPM phenotypes. For both linear and logistic regression, sequential multivariable adjustments were performed for regressions as follows: Model 1 included age, sex, education, occupation, smoking, waist circumference, physical activity, LDL, history of CVD, alcohol, diabetes, HRT and eGFR; Model 2 was Model 1 + PRA or aldosterone in a mutually adjusted model. Initially antihypertensive medication status (yes/no) was included in the models. However, the significance and magnitudes of associations were similar and antihypertensive medication status was not statistically significant in the models. Thus, antihypertensive medication status was removed from the primary models. Models including anti-hypertensive medication status as a covariate are displayed in Tables III and IV in the Supplement.
To confirm the robustness of the findings and explore potential physiological relationships, we performed exploratory secondary analyses examining: 1) The association of renin phenotypes with clinic, daytime, and nighttime SBP and DBP, percent dipping, and ABPM phenotypes. PRA was categorized as ≤0.50, 0.51 to 0.99, and ≥1.0 ng/ml/hr, to characterize “suppressed renin phenotype” (a state of autonomous aldosterone production), “indeterminate renin phenotype,” and “unsuppressed renin phenotype” (a state of potentially appropriate mineralocorticoid receptor activation in the setting of physiologic renin-dependent secretion of aldosterone);28 and 2) The association of log-aldosterone assessed among participants in the 3 renin phenotype categories with clinic and ABP and ABPM phenotypes. In all analyses, effect modification was tested by age, sex, diabetes, and antihypertensive medication use by inserting multiplicative interaction term models and using the likelihood ratio test. Statistical significance was defined as two-sided alpha <0.05 in the main analysis and <0.10 for interactions.29 All analyses were performed with the use of SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results:
Participant characteristics are described in Table 1. Among the 912 participants, 69% were women with a mean age was 59.2 years. The mean aldosterone and PRA were 4.5 ng/dL and 0.5 ng/ml/hr, respectively. Participants in the highest quartile of aldosterone had higher waist circumference, clinic SBP, clinic DBP, asleep SBP, asleep DBP, clinic hypertension, nocturnal hypertension, sustained hypertension, usage of anti-hypertensives and lower eGFR compared to the lowest quartile of aldosterone (all p<0.05). PRA levels increased across aldosterone quartiles (p<0.001), with quartile 1 having the highest percentage of participants with a “suppressed PRA phenotype” at 63% (p<0.001).
Table 1:
Demographic Summary by Quartiles of Aldosterone
| Variable* | Total (n=912) | Quartiles of Aldosterone | P-value | |||
|---|---|---|---|---|---|---|
| Q1: 1.9 to 2.7 (ng/dL) (n=237) | Q2: 2.8 to 4.5 (ng/dL) (n=231) | Q3: 4.6 to 7.5 (ng/dL) (n=228) | Q4: 7.6 to 61.1 (ng/dL) (n=216) | |||
| Age (years) | 59.2 (11) | 59.6 (11.6) | 58.8 (11.2) | 58.4 (10.6) | 60.3 (10.6) | 0.2473 |
| Waist circumference(cm) | 99.7 (15.6) | 97.8 (16.4) | 98.5 (13.8) | 100.4 (17.1) | 102.4 (14.7) | 0.0075 |
| Low-density lipoprotein (mg/dL) | 125.8 (35.9) | 124.5 (34.7) | 123.2 (37.3) | 126.7 (37) | 129.3 (34.4) | 0.2872 |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | 84.4 (18.6) | 89.8 (19.7) | 85.9 (15.3) | 84.5 (16.4) | 76.8 (20.1) | <.0001 |
| Sodium (mmol/L) | 140.6 (2.3) | 140.8 (2.2) | 140.7 (2.4) | 140.2 (2.3) | 140.5 (2.3) | 0.0476 |
| Potassium (mmol/L) | 4.23 (0.4) | 4.26 (0.4) | 4.25 (0.4) | 4.25 (0.4) | 4.15 (0.5) | 0.0216 |
| Chloride (mmol/L) | 103.7 (3.4) | 104.4 (2.8) | 104.3 (4.3) | 103.1 (2.9) | 102.9 (3.3) | <.0001 |
| Sodium intake (g) (n=848) | 3.49 (2.39, 4.83) | 3.59 (2.43, 4.95) | 3.74 (2.62, 4.89) | 3.50 (2.34, 4.85) | 3.24 (2.12, 4.63) | 0.0405 |
| Potassium intake (g) (n=848) | 2.34 (1.82, 3.12) | 2.31 (1.83, 3.02) | 2.46 (1.87, 3.12) | 2.40 (1.77, 3.19) | 2.25 (1.77, 3.04) | 0.4397 |
| Systolic blood pressure (mmHg) | 128 (15.9) | 127.8 (17) | 126.4 (14.5) | 126.8 (15) | 131.2 (16.7) | 0.0058 |
| Diastolic blood pressure (mmHg) | 74.5 (8.3) | 73.6 (8.4) | 74.6 (8.1) | 74 (7.8) | 76 (8.6) | 0.0105 |
| Awake systolic blood pressure | 129.2 (13.3) | 128.8 (13.8) | 128 (12.5) | 129.1 (12.6) | 131.2 (14.3) | 0.0697 |
| Awake diastolic blood pressure | 77.9 (9.2) | 77.2 (8.4) | 77.6 (9.2) | 78.5 (9.2) | 78.3 (9.8) | 0.3679 |
| Asleep systolic blood pressure | 120.4 (15.3) | 119.4 (15.9) | 118.8 (14.3) | 120.1 (14.1) | 123.6 (16.7) | 0.0047 |
| Asleep diastolic blood pressure | 68.1 (9.6) | 66.9 (9.3) | 67.4 (9.4) | 69.1 (9.9) | 69.3 (9.7) | 0.0123 |
| Percent dipping | 6.8 (7.7) | 7.3 (7.5) | 7.1 (7.4) | 6.9 (7.3) | 5.7 (8.4) | 0.1326 |
| Plasma aldosterone (ng/dL)* | 4.5 (3, 7.3) | 1.9 (2, 2.2) | 3.6 (3, 4) | 5.8 (5, 6.5) | 10.5 (9, 13.4) | <.0001 |
| Plasma renin activity (PRA) (ng/mL/hr)* (n=646) | 0.5 (0.2, 1.1) | 0.3 (0.2, 0.9) | 0.4 (0.2, 0.8) | 0.5 (0.3, 1.1) | 0.8 (0.3, 2) | <.0001 |
| Log-aldosterone | 1.5 (0.99, 1.98) | 0.64 (0.64, 0.79) | 1.28 (1.16, 1.39) | 1.76 (1.63, 1.87) | 2.35 (2.19, 2.59) | <.0001 |
| Log-PRA (n=646) | −0.69 (−1.61, 0.1) | −1.2 (−1.66, −0.11) | −0.92 (−1.66, −0.22) | −0.69 (−1.2, 0.1) | −0.29 (−1.2, 0.69) | <.0001 |
| Log-Aldosterone:Renin ratio (n=646) | 2.25 (1.4, 2.89) | 1.85 (0.95, 2.3) | 2.05 (1.42, 2.86) | 2.3 (1.61, 3.18) | 2.78 (1.81, 3.69) | <.0001 |
| Plasma renin activity phenotypes (n=646) | ||||||
| Suppressed (≤ 0.50 ng/mL/hr) | 348 (53.9%) | 103 (62.8%) | 99 (60.7%) | 86 (50.3%) | 60 (40.5%) | 0.0006 |
| Indeterminate (0.51 – 0.99 ng/mL/hr) | 113 (17.5%) | 23 (14.0%) | 27 (16.6%) | 36 (21.1%) | 27 (18.2%) | |
| Unsuppressed (≥ 1.0 ng/mL/hr) | 185 (28.6%) | 38 (23.2%) | 37 (22.7%) | 49 (28.7%) | 61 (41.2%) | |
| Sex | ||||||
| Female | 629 (69%) | 172 (73%) | 148 (64%) | 148 (65%) | 161 (75%) | 0.0305 |
| Male | 283 (31%) | 65 (27%) | 83 (36%) | 80 (35%) | 55 (25%) | |
| Education | ||||||
| < High school education | 165 (18%) | 51 (22%) | 32 (14%) | 40 (18%) | 42 (19%) | 0.1724 |
| ≥ High school education | 747 (82%) | 186 (78%) | 199 (86%) | 188 (82%) | 174 (81%) | |
| Occupation | ||||||
| Management/ Professional | 393 (43%) | 99 (42%) | 100 (43%) | 102 (45%) | 92 (43%) | 0.9307 |
| Other | 519 (57%) | 138 (58%) | 131 (57%) | 126 (55%) | 124 (57%) | |
| Current Smoking | 86 (9%) | 23 (10%) | 24 (10%) | 23 (10%) | 16 (7%) | 0.7008 |
| AHA Physical activity | ||||||
| Poor Health | 435 (48%) | 118 (50%) | 105 (45%) | 103 (45%) | 109 (50%) | 0.1003 |
| Intermediate Health | 287 (31%) | 78 (33%) | 81 (35%) | 62 (27%) | 66 (31%) | |
| Ideal Health | 190 (21%) | 41 (17%) | 45 (19%) | 63 (28%) | 41 (19%) | |
| Any alcohol use | 403 (44%) | 91 (38%) | 117 (51%) | 114 (50%) | 81 (38%) | 0.0027 |
| Diabetes | 191 (21%) | 49 (21%) | 41 (18%) | 50 (22%) | 51 (24%) | 0.4759 |
| Cardiovascular disease | 80 (9%) | 25 (11%) | 18 (8%) | 18 (8%) | 19 (9%) | 0.6982 |
| Use of antihypertensive medications | 525 (58%) | 97 (41%) | 114 (49%) | 143 (63%) | 171 (79%) | <.0001 |
| Angiotensin Converting Enzyme Inhibitor Use | 186 (20%) | 38 (16%) | 50 (22%) | 47 (21%) | 51 (24%) | 0.2231 |
| Angiotensin Receptor Blocker Use | 66 (7%) | 17 (7%) | 15 (6%) | 18 (8%) | 16 (7%) | 0.9508 |
| Mineralocorticoid Receptor Antagonist Use | 12 (1%) | 0 (0%) | 0 (0%) | 1 (0.4%) | 11 (5%) | <.0001 |
| Beta Blocker Use | 103 (11%) | 18 (8%) | 21 (9%) | 28 (12%) | 36 (17%) | 0.0129 |
| Calcium Channel Blocker Use | 188 (21%) | 26 (11%) | 37 (16%) | 49 (21%) | 76 (35%) | <.0001 |
| Diuretic Use | 351 (38%) | 41 (17%) | 72 (31%) | 100 (44%) | 138 (64%) | <.0001 |
| Use of hormone replacement therapy | 199 (22%) | 56 (24%) | 54 (23%) | 44 (19%) | 45 (21%) | 0.6246 |
| Clinic hypertension | 181 (19.9%) | 46 (19.4%) | 39 (16.9%) | 36 (15.8%) | 60 (27.8%) | 0.0067 |
| Daytime hypertension | 346 (38%) | 85 (36%) | 84 (36%) | 84 (37%) | 93 (43%) | 0.3626 |
| Nocturnal hypertension | 505 (55%) | 119 (50%) | 118 (51%) | 129 (57%) | 139 (64%) | 0.0095 |
| Daytime and Nocturnal Hypertension | 307 (34%) | 73 (31%) | 73 (32%) | 76 (33%) | 85 (39%) | 0.2168 |
| Sustained hypertension | 128 (14%) | 34 (14.4%) | 23 (10%) | 25 (11%) | 46 (21.3%) | 0.0024 |
| Non-dipping pattern | 598 (66%) | 154 (65%) | 147 (64%) | 147 (64%) | 150 (69%) | 0.5772 |
| White coat hypertension | 53 (6%) | 12 (5%) | 16 (7%) | 11(5%) | 14 (6%) | 0.7173 |
| Masked hypertension | 218 (24%) | 51 (22%) | 61 (26%) | 59 (26%) | 47 (22%) | 0.4626 |
Values are mean +/− (SD) or median (interquartile range) or %.
P-values were calculated using chi-square (categorical variables), ANOVA (parametric continuous variables), and Kruskal-Wallis tests (non-parametric continuous variables).
CKD-EPI (Chronic Kidney Disease Epidemiology collaboration equation); AHA, American Heart Association, AHA physical activity was defined by AHA “2020” guidelines. Physical activity was considered as poor health (0 min of moderate and vigorous activity), intermediate health (> 0 min but < 150 min of moderate activity),>0 min but<75 min of vigorous activity, or > 0 min but < 150 min of combined moderate and vigorous activity), and ideal health (≥ 150 min of moderate activity, ≥ 75 min of vigorous activity, or ≥ 150 min of combined moderate and vigorous activity).
Clinic Hypertension = clinic SBP ≥140 mmHg or clinic DBP ≥90 mmHg; Daytime Hypertension = daytime SBP ≥ 135 mmHg or daytime DBP ≥85 mmHg, Nocturnal Hypertension = night-time SBP ≥ 120 mmHg or night-time DBP ≥ 70 mmHg; Daytime & Nocturnal hypertension = combination of Daytime and Nocturnal Hypertension; Sustained hypertension = combination of Clinic and Daytime Hypertension; Non-Dipping Pattern = <10% decrease in mean awake vs mean asleep SBP; White Coat Hypertension = absence of Daytime Hypertension with presence of Clinic Hypertension, Masked Hypertension = presence of Daytime Hypertension with absence of Clinic Hypertension.
Daily sodium and potassium intake in milligrams were evaluated using a food frequency questionnaire.30
Higher log-aldosterone was associated with higher clinic DBP, awake SBP and DBP, and asleep SBP and DBP after adjusting for all covariates except PRA (all p<0.05) (Table V in the Supplement - Model 1). An association with clinic, awake, and asleep DBPs remained after additional adjustment for log-PRA (all p<0.05) (Figure 1, Table V in the Supplement - Model 2). A higher log-PRA was associated with lower clinic, awake and asleep SBP and DBP in all models including the fully adjusted model with log-aldosterone (all p<0.05). Many of the SBP associations were greater for PRA. For instance, a 100% higher PRA and aldosterone were associated with 2.25 mmHg lower asleep SBP (p<0.0001) and 0.98 mmHg higher asleep SBP (p=0.1017), respectively. A higher ARR was associated with higher clinic, awake and asleep SBP and DBP (all p<0.05). There was no association of any of the RAAS exposures with percent dipping.
Figure 1.
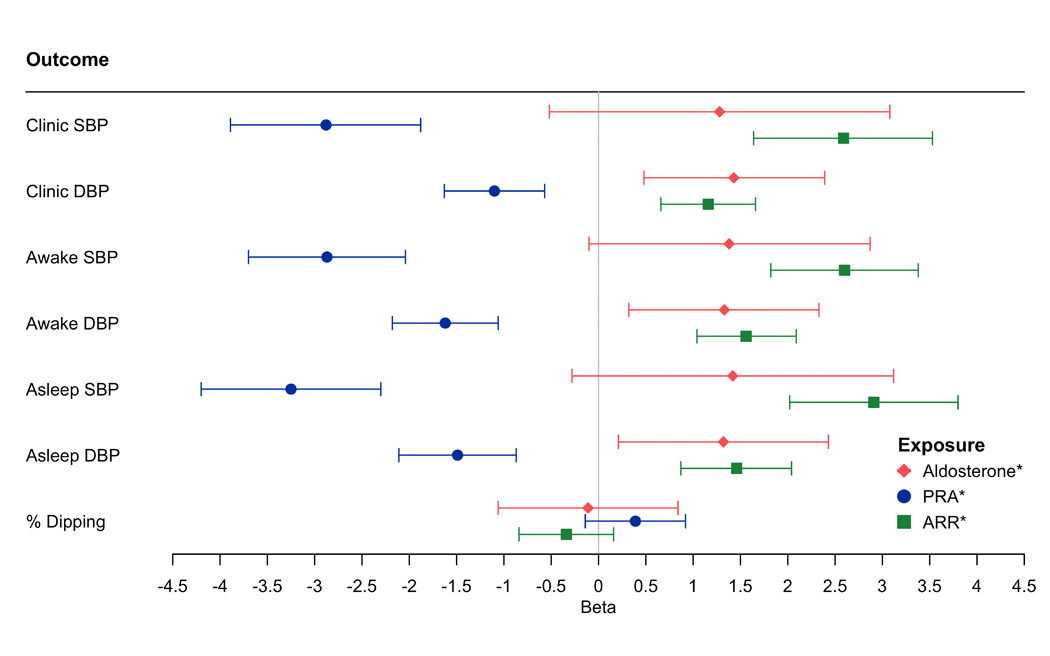
The Association of Log-Aldosterone, Log-Plasma Renin Activity and Log-Aldosterone: Renin Ratio with Clinic, Daytime, and Nighttime Systolic Blood Pressure, Diastolic Blood Pressure and Percent Dipping
Model adjusted for age, sex, education and occupation, smoking, waist circumference, physical activity, low-density lipoprotein, history of cardiovascular disease, alcohol, diabetes, hormone replacement therapy, estimated glomerular filtration rate, and PRA or aldosterone (Table V in the Supplement, Model 2)
* indicates log-transformed variable
Abbreviations: SBP = systolic blood pressure; DBP = diastolic blood pressure, PRA = plasma renin activity; ARR = aldosterone: renin ratio.
Beta to mmHg Conversion: Beta*ln(2)
A 1-unit higher log-aldosterone was associated with 38% higher odds of nocturnal hypertension after adjusting for all covariates including PRA (OR: 1.38, 95%CI: 1.05, 1.81) (Figure 2, Table VI in the Supplement – Model 2). A 1-unit higher log-PRA was associated with lower odds of clinic hypertension (OR: 0.64, 95% CI: 0.51, 0.80), daytime hypertension (OR: 0.59, 95%CI: 0.49, 0.71), nocturnal hypertension (OR: 0.68, 95%CI: 0.58, 0.79), daytime and nocturnal hypertension (OR: 0.59, 95%CI: 0.48, 0.71), sustained hypertension (OR: 0.52, 95%CI: 0.39, 0.70) and masked hypertension (OR: 0.75, 95% CI: 0.62, 0.90) in fully adjusted analyses. There were no associations of PRA with non-dipping blood pressure pattern or white coat hypertension. A 1-unit increase in ARR was associated with higher odds of clinic hypertension (OR:1.47, 95%CI: 1.21, 1.79), daytime hypertension (OR: 1.57, 95%CI: 1.34, 1.84), nocturnal hypertension (OR: 1.46, 95%CI: 1.26, 1.69), daytime and nocturnal hypertension (OR: 1.57, 95%CI: 1.33, 1.85), sustained hypertension (OR:1.69, 95%CI:1.33, 2.14), and masked hypertension (OR: 1.28, 95%CI:1.08, 1.51). Using BP thresholds for hypertension from the 2017 AHA/ACC hypertension guidelines,23 findings were similar except nocturnal hypertension was non-significant for log-aldosterone and masked hypertension was non-significant for PRA and ARR (Table VII in the Supplement).
Figure 2.
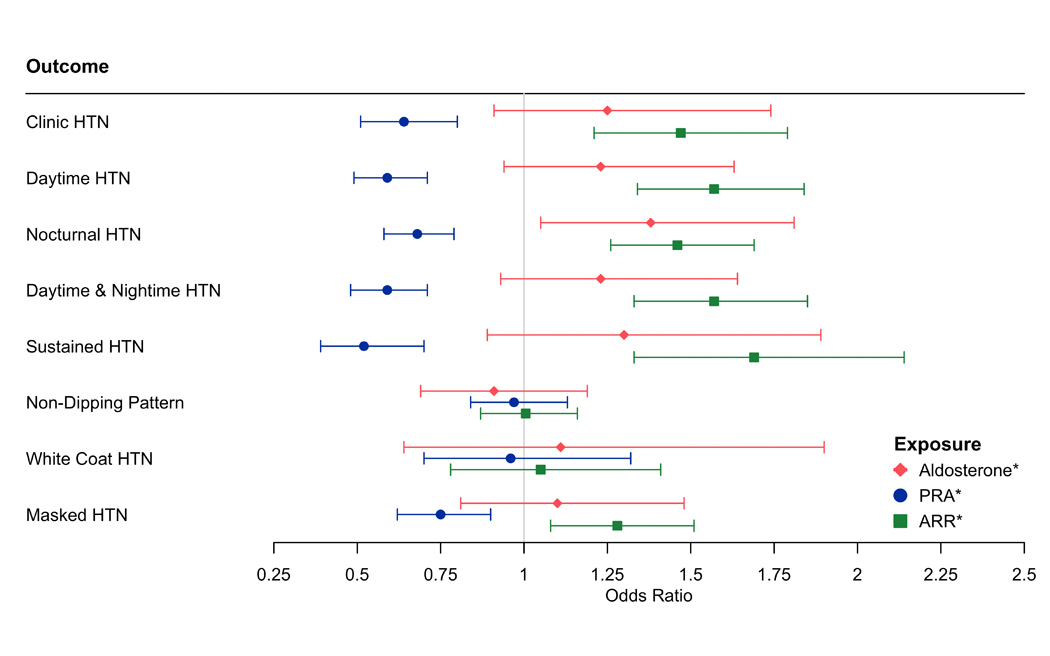
The Association of Log-Aldosterone, Log-Plasma Renin Activity and Log-Aldosterone: Renin Ratio with Ambulatory Blood Pressure Phenotypes
Model adjusted for age, sex, education and occupation, smoking, waist circumference, physical activity, low-density lipoprotein, history of cardiovascular disease, alcohol, diabetes, hormone replacement therapy, estimated glomerular filtration rate, and PRA or aldosterone (Table VI in the Supplement, Model 2)
* indicates log-transformed variable
Abbreviations: HTN = hypertension; PRA = plasma renin activity; ARR = aldosterone: renin ratio
Clinic Hypertension = clinic SBP ≥140 mmHg or clinic DBP ≥90 mmHg; Daytime Hypertension = daytime SBP ≥ 135 mmHg or daytime DBP ≥85 mmHg, Nocturnal Hypertension = night-time SBP ≥ 120 mmHg or night-time DBP ≥ 70 mmHg; Daytime & Nocturnal hypertension = combination of Daytime and Nocturnal Hypertension; Sustained hypertension = combination of Clinic and Daytime Hypertension; Non-Dipping Pattern = <10% decrease in mean awake vs mean asleep SBP; White Coat Hypertension = absence of Daytime Hypertension with presence of Clinic Hypertension, Masked Hypertension = presence of Daytime Hypertension with absence of Clinic Hypertension.
In fully adjusted analyses, the suppressed renin phenotype (PRA ≤0.50 ng/ml/hr) compared to the unsuppressed renin phenotype (defined as PRA ≥1.0 ng/ml/hr) was associated with higher clinic SBP (6.03 mmHg) and DBP (2.45 mmHg), awake SBP (5.93 mmHg) and DBP (3.03 mmHg), and asleep SBP (7.54 mmHg) and DBP (3.24 mmHg) (all p<0.01) (Figure 3, Table VIII in the Supplement – Model 2). The suppressed renin phenotype compared to the unsuppressed renin phenotype was associated with higher odds of clinic hypertension (OR:2.89, 95%CI: 1.66, 5.05), daytime hypertension (OR: 3.01, 95%CI: 1.94, 4.67) nocturnal hypertension (OR: 2.3, 95%CI: 1.53, 3.45), daytime and nocturnal hypertension (OR: 2.93, 95%CI:1.85, 4.62), sustained hypertension (OR: 4.59, 95%CI: 2.25, 8.37), and masked hypertension (OR: 1.66, 95%CI: 1.04, 2.64) (Figure 4, Table IX in the Supplement – Model 2).
Figure 3:
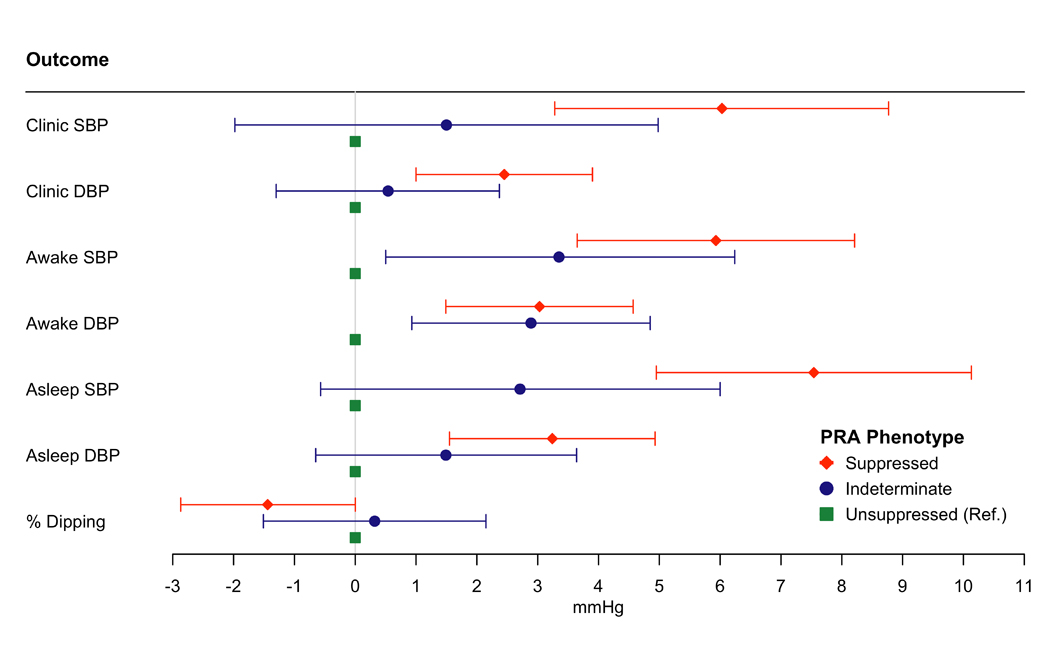
The Association of Plasma Renin Activity Phenotypes with Clinic, Daytime, and Nighttime Systolic Blood Pressure, Diastolic Blood Pressure and Percent Dipping
Model adjusted for age, sex, education and occupation, smoking, waist circumference, physical activity, low-density lipoprotein, history of cardiovascular disease, alcohol, diabetes, hormone replacement therapy, estimated glomerular filtration rate, and aldosterone (Table X in the Supplement, Model 1)
Suppressed Renin Phenotype is defined as plasma renin activity ≤ 0.50 ng/ml/hr; Indeterminate Renin Phenotype is defined as plasma renin activity between 0.51 and 0.99 ng/ml/hr; Unsuppressed Renin Phenotype is defined as plasma renin activity ≥1.0 ng/ml/hr.
Abbreviations: SBP = systolic blood pressure; DBP = diastolic blood pressure; PRA = plasma renin activity
Figure 4:
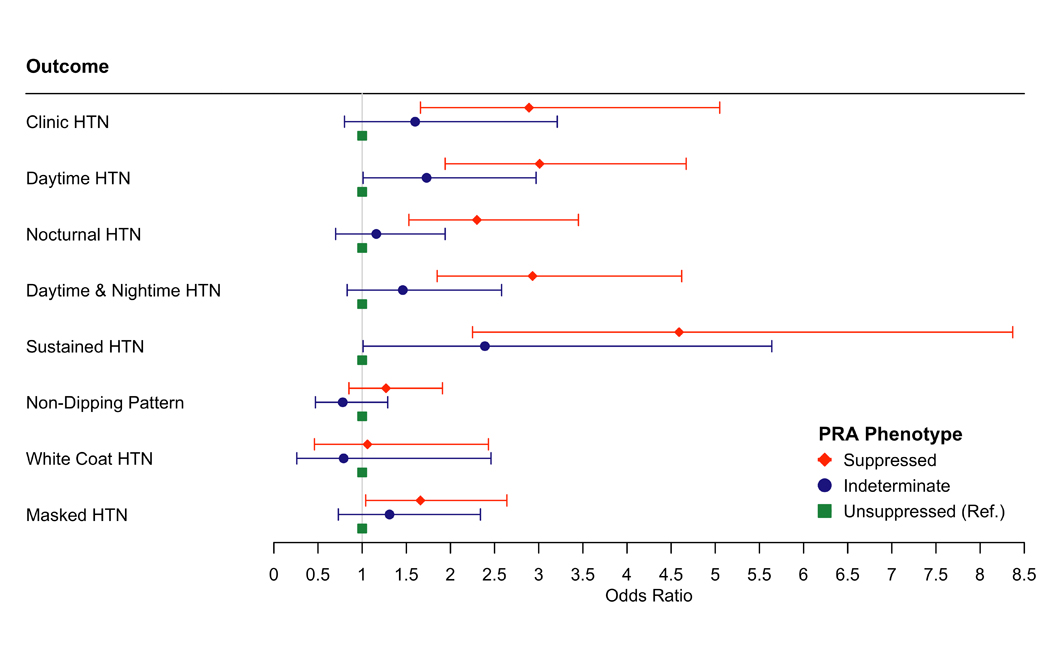
The Association of Plasma Renin Activity Phenotypes with Ambulatory Blood Pressure Phenotypes
Model adjusted for age, sex, education and occupation, smoking, waist circumference, physical activity, low-density lipoprotein, history of cardiovascular disease, alcohol, diabetes, hormone replacement therapy, estimated glomerular filtration rate, and aldosterone (Table XI in the Supplement, Model 1)
Suppressed Renin Phenotype is defined as plasma renin activity ≤ 0.50 ng/ml/hr; Indeterminate Renin Phenotype is defined as plasma renin activity between 0.51 and 0.99 ng/ml/hr; Unsuppressed Renin Phenotype is defined as plasma renin activity ≥1.0 ng/ml/hr.
Abbreviations: HTN = hypertension; PRA = plasma renin activity
Clinic Hypertension = clinic SBP ≥140 mmHg or clinic DBP ≥90 mmHg; Daytime Hypertension = daytime SBP ≥ 135 mmHg or daytime DBP ≥85 mmHg, Nocturnal Hypertension = night-time SBP ≥ 120 mmHg or night-time DBP ≥ 70 mmHg; Daytime & Nocturnal hypertension = combination of Daytime and Nocturnal Hypertension; Sustained hypertension = combination of Clinic and Daytime Hypertension; Non-Dipping Pattern = <10% decrease in mean awake vs mean asleep SBP; White Coat Hypertension = absence of Daytime Hypertension with presence of Clinic Hypertension, Masked Hypertension = presence of Daytime Hypertension with absence of Clinic Hypertension.
The association of aldosterone with clinic, daytime and nighttime SBP and DBP, percent dipping stratified by renin phenotypes is presented in Figure III in the Supplement and Table X in the Supplement. After adjustment, in the suppressed renin phenotype group (PRA<0.50 ng/ml/hr), a 100% increase in aldosterone was associated with a 1.84 mmHg higher clinic SBP, 1.13 mmHg higher clinic DBP, 1.57 mmHg higher awake SBP, and 1.15 mmHg higher awake DBP. Figure IV in the Supplement demonstrates similar findings with predicted increased SBP values associated with higher log-aldosterone within the suppressed renin phenotype using a spline analysis. The association of aldosterone with ABP and ABPM phenotypes was non-significant in the indeterminate and unsuppressed renin phenotypes (Figure V in the Supplement, Table XI in the Supplement – Model 1).
In the suppressed renin phenotype group there was a trend towards higher dietary sodium intake compared to the unsuppressed renin phenotype (p=0.0878; Table XII in the Supplement). Adjusting for dietary sodium did not significantly alter the associations between components of the RAAS and ABP metrics and phenotypes (Table XIII in the Supplement and Table XIV in the Supplement). There was no effect modification in the analyses by age, sex, antihypertensive medications or diabetes status.
Discussion:
In this study of AA adults, a higher log-PRA was associated with lower clinic, awake and asleep SBP and DBP and lower odds of clinic, daytime, nocturnal, daytime and nocturnal hypertension, sustained hypertension and masked hypertension. Similar findings in the inverse direction were seen for ARR with higher ARR being associated with higher BPs and higher odds of outcomes. Higher ARRs were driven by low PRA. Aldosterone associations were limited to positive associations with DBP (clinic, awake and asleep), nocturnal hypertension, as well as clinic and awake SBP and DBP in the suppressed PRA phenotype. The negative association of PRA with BP and ABPM phenotypes, and the importance of low PRA in elevated ARR, suggest that low PRA may be the predominant driver of the clinic, ambulatory awake and asleep BP measurements and ABPM phenotypes in AAs. To further elucidate this observation, we examined renin phenotypes. The suppressed renin phenotype was associated with higher clinic and ambulatory BPs, and higher odds of ABPM hypertension phenotypes (except non-dipping pattern and white coat hypertension), suggesting that PRA levels below 0.50 ng/mL/hr are of particular importance in the association of PRA with ambulatory BP in AAs. Notably, in quartile 1 of aldosterone (low aldosterone) 63% of participants had a suppressed PRA, compared to 41% in quartile 4 (high aldosterone), suggesting there were high degrees of both low aldosterone/low PRA and high aldosterone/low PRA in the suppressed PRA phenotype.
Previous investigations support the importance of low PRA in clinic hypertension. In the HyperPATH cohort, the lowest PRA quartiles were associated with increased BP across racial and ethnic groups.31 In the Multi-Ethnic Study of Atherosclerosis, suppressed PRA phenotype was associated with an increased risk of incident clinic hypertension.32 While others have shown weak or no associations of low PRA states with clinic and 24-hour ambulatory hypertension in Blacks.15,33,34 The findings from this study, which represents the largest cohort of African Americans, provide novel findings by revealing that PRA is inversely associated with multiple ABPM phenotypes, including daytime hypertension, nocturnal hypertension, sustained hypertension and masked hypertension in AAs. These findings are critical given the improved risk prediction of ambulatory BP compared to clinic BP for CVD and mortality and the potential implications for antihypertensive therapy guidelines.9–12
The suppressed renin phenotype implicated in the ABPM phenotypes described, has previously been shown to be more prevalent among AAs.35,36 Further, it has been shown that the low renin phenotype is associated with a non-normal, bimodal distribution of aldosterone, suggestive of multiple mechanisms underlying the low renin phenotype.37 One mechanism postulates that increased aldosterone from pathophysiological processes (i.e. primary aldosteronism, atypical congenital hyperplasia, etc.) results in negative feedback inhibiting the production of renin.38 Another hypothesis centers on a reduced rate of renin secretion through genetic factors or volume expansion and results in both low PRA and aldosterone levels (Liddle phenotype).35 In support of higher aldosterone suppressing PRA, the association of log-aldosterone with BP in the suppressed renin phenotype was significant for clinic and awake BPs and there were additional trends towards associations of log-aldosterone with ABPM phenotypes in the suppressed renin phenotypes. A spline analysis performed to evaluate the influence of aldosterone on BP within the suppressed renin phenotype showed similar findings. In the MESA cohort, among participants with a suppressed renin phenotype, those with higher levels of aldosterone had a dose-dependent higher incidence of hypertension as evidenced by office BP measurement.32 Our findings are consistent with this observation, as only among the participants with suppressed renin phenotype was a higher aldosterone associated with clinic and awake ABP. Brown et al. proposed that among the suppressed renin phenotype, those with low aldosterone may have lower BPs due to an appropriate dampening of the RAAS in response to salt loading. In contrast, those in the suppressed renin phenotype with elevated aldosterone may have elevated BPs due to decoupling of renin and aldosterone regulation.32 This study extends those findings, supporting the potential decoupling of renin and aldosterone regulation as one potential mechanism driving ABP and ABPM phenotypes in AAs.
Conversely, in support of low renin production or secretion, in Figure II in the Supplement, the majority of individuals with suppressed PRA demonstrated levels of aldosterone < 15 ng/dl. Second, the association of PRA with ambulatory BP and ABPM phenotypes was not attenuated by adjustment for aldosterone. Third, the suppressed renin phenotype compared to unsuppressed renin phenotypes was associated with much higher BPs and the lowest aldosterone quartile (aldosterone 1.9 to 2.7 ng/dL) had the highest prevalence of suppressed renin phenotype at 63% (Liddle’s phenotype) compared to 41% in quartile 4 (aldosterone 7.6 to 61.1 ng/dl) (hyperaldosteronism phenotype).
AAs have lower PRA and aldosterone levels compared to other racial or ethnic groups.15 Some of the mechanisms driving aldosterone-independent lower PRA levels are high sodium intake, low renin secretion, and ENaC mutations leading to increased ENaC activity. First, sodium intake is known to reduce renin secretion and thus aldosterone, establishing a mechanism for steady state sodium handling in the setting of variation in sodium intake.39,40 Some AAs have reduced ability to excrete sodium, and are consequently prone to salt sensitive blood volume increases and hypertension, potentially contributing to the observed reduction in renin secretion.41–43 Further, AAs, have greater reductions in BP through reduced dietary sodium compared to other ethnicities.44 In this analysis, those with the suppressed renin phenotype demonstrated a non-significant trend towards higher dietary sodium intake than those with unsuppressed or indeterminate renin phenotypes (Table XII in the Supplement). The increased salt-sensitivity among AAs is of particular importance in the JHS, as the average sodium intake among JHS participants is 3.5 grams per day, well above recommended levels.45 Indeed, the difference in handling of salt and the contribution of sodium excretion to hypertension among AAs is reflected in the current difference in hypertension management guidelines between AAs and other ethnicities with increased emphasis placed on thiazide diuretics in AAs.23 We hypothesize that within this cohort of AAs, the relationship between increased salt sensitivity, salt intake, and resultant suppression of renin secretion may be a key contributor to the risk of BP and ABPM phenotypes and is a key avenue for further research.
Second, low renin secretion may be heritable among AAs35 and there is evidence that AAs with low PRA have increased epithelial sodium channel activity.46 Among AAs with low PRA, amiloride, an epithelial sodium channel antagonist, as well as mineralocorticoid antagonists, have been shown to reduce blood pressures, with greater responses for epithelial sodium channel antagonists.47 Given the continued poor attainment of hypertension control among AAs compared to other racial/ethnic groups, and the previously underrecognized role that low PRA may play in ABP and ABPM phenotypes among AAs, further large-scale prospective studies are needed to evaluate the effectiveness of physiologically individualized therapies based on aldosterone and renin such as amiloride and mineralocorticoid antagonists.48,49
Strengths and limitations:
The strengths of our study include a large, contemporary, community-based cohort that obtained ABPM data throughout the day. Further, the racial composition of the JHS allowed us to specifically investigate the association of the RAAS with clinic, ABP and ABPM phenotypes among AAs, a population that maintains disproportionate burden of hypertension and CVD. Despite these and many other strengths, our findings should be viewed in light of some limitations. First, this analysis was cross-sectional, so causation and temporality are unable to be evaluated. Second, this analysis was completed in the Southeastern United States, so the results may not be generalizable to other populations of AAs. Third, ABPM was performed over a single 24-hour period, which may not be representative of long-term ABPs. Finally, the statistical associations were interpreted without correction for multiple comparisons. Typical multiple comparison corrections assume that tests are independent and are too conservative for correlated hypotheses as we have here. Bonferroni correction accounting for predictors and outcomes in Tables V, VI, VIII, and IX in the Supplement resulted in Bonferroni adjusted p-values ranging from 0.0031 to 0.0071. Using the Bonferroni adjusted p-value there was limited impact on the significance of the findings for PRA and ARR. Aldosterone was no longer significantly associated with awake diastolic blood pressure, asleep diastolic blood pressure, and nocturnal hypertension. Hence, some caution is warranted in the interpretation of our study results with aldosterone as the predictor.
Conclusion:
In this novel study in AAs, PRA was negatively and ARR was positively associated with clinic, awake and asleep SBP and DBP and ABPM phenotypes. Further, the suppressed plasma renin phenotype was associated with higher odds of multiple ABPM phenotypes compared to unsuppressed renin phenotype. While both high aldosterone/low PRA and low aldosterone/low PRA are plausible mechanisms for suppression of PRA, in this AA cohort, the critical aspect driving the ABP and ABPM phenotype results appears to be the suppression of PRA. These findings are important given the increased risk of CVD associated with ABPM phenotypes compared to clinic BP phenotypes.9–12 Further investigation of the RAAS and BP among AAs is paramount to identify novel targets to modulate low renin phenotypes in AAs and to increase the utilization of approved medications that target either high aldosterone/low renin phenotype such as mineralocorticoid receptor antagonists and low aldosterone/low renin phenotype such as ENaC inhibitors. Novel approaches using physiologically individualized therapies represent one essential precision medicine-based pathway to advance cardiovascular health equity in the United States.
Supplementary Material
Clinical Perspective.
What is new?
In African Americans, plasma renin activity (PRA) was negatively associated and aldosterone:renin ratio (ARR) was positively associated with clinic, awake and asleep SBP and DBP and multiple ambulatory blood pressure monitoring (ABPM) phenotypes
Suppression of renin appears to be critical, as the suppressed renin phenotype (PRA ≤ 0.50 ng/ml/hr) was associated with higher BPs and odds of multiple ABPM phenotypes compared to unsuppressed renin phenotype (PRA ≥ 1.0 ng/ml/hr)
What are the clinical implications?
Providers should be aware of the importance of low PRA in multiple ABPM phenotypes including daytime, nocturnal, daytime and nocturnal and sustained hypertension in African Americans
The investigation and utilization of therapeutics that act on either high aldosterone/low renin phenotypes such as mineralocorticoid receptor antagonists or low aldosterone/low renin phenotypes such as epithelial sodium channel inhibitors may represent one pathway to reduce health disparities in African Americans
Acknowledgements:
The authors wish to thank the staff and participants of the JHS. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Funding Sources:
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I/HHSN26800001) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). Preparation of this manuscript was supported by The Robert Wood Johnson Foundation (Harold Amos Medical Faculty Development Program ID# 76236) and the National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK117041, JJJ) of the National Institutes of Health and the Roessler Research Scholarship through the Ohio State College of Medicine (NKP) as well as 18AFMDP34380732 from the American Heart Association and from NIH/NHLBI (K23 HL141682-01A1, MA).
Abbreviations:
- A1c
hemoglobin A1c
- AA
African American
- ABP
ambulatory blood pressure
- ABPM
ambulatory blood pressure monitoring
- ACC
American College of Cardiology
- AHA
American Heart Association
- ARR
aldosterone:renin ratio
- DBP
diastolic blood pressure
- IDACO
International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes
- JHS
Jackson Heart Study
- MESA
Multi-Ethnic Study of Atherosclerosis
- NHW
non-Hispanic white
- RAAS
renin-angiotensin-aldosterone system
- SBP
systolic blood pressure
Footnotes
The authors have no conflicts of interest to declare.
References:
- 1.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of Major Diseases to Disparities in Mortality. New England Journal of Medicine. 2002;347:1585–1592. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 3.Murphy SL, Xu J, Kochanek KD, Arias E. Mortality in the United States, 2017. NCHS Data Brief. 2018;National Center for Health Statistics:8. [PubMed] [Google Scholar]
- 4.Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial Differences in Risks for First Cardiovascular Events and Noncardiovascular Death: The Atherosclerosis Risk in Communities Study, the Cardiovascular Health Study, and the Multi-Ethnic Study of Atherosclerosis. Circulation. 2012;126:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas KL, Honeycutt E, Shaw LK, Peterson ED. Racial differences in long-term survival among patients with coronary artery disease. American Heart Journal. 2010;160:744–751. [DOI] [PubMed] [Google Scholar]
- 6.Atlas SA. The Renin-Angiotensin Aldosterone System: Pathophysiological Role and Pharmacologic Inhibition. Journal of Managed Care Pharmacy. 2007;13:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parati G, Omboni S, Bilo G. Why Is Out-of-Office Blood Pressure Measurement Needed?: Home Blood Pressure Measurements Will Increasingly Replace Ambulatory Blood Pressure Monitoring in the Diagnosis and Management of Hypertension. Hypertension. 2009;54:181–187. [DOI] [PubMed] [Google Scholar]
- 8.Peacock J, Diaz KM, Viera AJ, Schwartz JE, Shimbo D. Unmasking masked hypertension: prevalence, clinical implications, diagnosis, correlates and future directions. Journal of Human Hypertension. 2014;28:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Wang J-G. Isolated Nocturnal Hypertension: A Disease Masked in the Dark. Hypertension. 2013;61:278–283. [DOI] [PubMed] [Google Scholar]
- 10.Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, Ruiz-Hurtado G, Segura J, Rodríguez-Artalejo F, Williams B. Relationship between Clinic and Ambulatory Blood-Pressure Measurements and Mortality. N Engl J Med. 2018;378:1509–1520. [DOI] [PubMed] [Google Scholar]
- 11.Yang W-Y, Melgarejo JD, Thijs L, Zhang Z-Y, Boggia J, Wei F-F, Hansen TW, Asayama K, Ohkubo T, Jeppesen J, et al. Association of Office and Ambulatory Blood Pressure With Mortality and Cardiovascular Outcomes. JAMA. 2019;322:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yano Y, Tanner RM, Sakhuja S, Jaeger BC, Booth JN, Abdalla M, Pugliese D, Seals SR, Ogedegbe G, Jones DW, et al. Association of Daytime and Nighttime Blood Pressure With Cardiovascular Disease Events Among African American Individuals. JAMA Cardiol. 2019;4:910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahabala C, Kamath P, Bhaskaran U, Pai ND, Pai AU. Antihypertensive therapy: nocturnal dippers and nondippers. Do we treat them differently? Vasc Health Risk Manag. 2013;9:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michel FS, Norton GR, Majane OHI, Badenhorst M, Vengethasamy L, Paiker J, Maseko MJ, Sareli P, Woodiwiss AJ. Contribution of Circulating Angiotensinogen Concentrations to Variations in Aldosterone and Blood Pressure in a Group of African Ancestry Depends on Salt Intake. Hypertension. 2012;59:62–69. [DOI] [PubMed] [Google Scholar]
- 15.Rifkin DE, Khaki AR, Jenny NS, McClelland RL, Budoff M, Watson K, Ix JH, Allison MA. Association of Renin and Aldosterone With Ethnicity and Blood Pressure: The Multi-Ethnic Study of Atherosclerosis. American Journal of Hypertension. 2014;27:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: Design and methods of the Jackson Heart Study. Ethnicity and Disease. 2005;15:S6-4–S6-17. [PubMed] [Google Scholar]
- 17.Jackson Heart Study. JHS data access [Internet]. [cited 2020 Dec 12];Available from: https://www.jacksonheartstudy.org/Research/Study-Data/Data-Access
- 18.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. [DOI] [PubMed] [Google Scholar]
- 19.Thijs L, Hansen T, Kikuya M, Björklund-Bodegård K, Li Y, Dolan E, Tikhonoff V, Seidlerová J, Kuznetsova T, Stolarz K, et al. The International Database of Ambulatory blood pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Pressure Monitoring. 2007;12:255–262. [DOI] [PubMed] [Google Scholar]
- 20.Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, Yeh H-C, Bertoni AG, Effoe VS, Casanova R, Sims M, Wu W-C, Wand GS, et al. Aldosterone, Renin, Cardiovascular Events, and All-Cause Mortality Among African Americans: The Jackson Heart Study. JACC Heart Fail. 2017;5:642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seals SR, Colantonio LD, Tingle JV, Shimbo D, Correa A, Griswold ME, Muntner P. Calibration of blood pressure measurements in the Jackson Heart Study. Blood Pressure Monitoring. 2019;24:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravenell J, Shimbo D, Booth JN, Sarpong DF, Agyemang C, Beatty Moody DL, Abdalla M, Spruill TM, Shallcross AJ, Bress AP, et al. Thresholds for Ambulatory Blood Pressure Among African Americans in the Jackson Heart Study. Circulation. 2017;135:2470–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. , on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association’s Strategic Impact Goal Through 2020 and Beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 25.Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, Yeh H-C, Bertoni AG, Effoe VS, Casanova R, Sims M, Correa A, Wu W-C, et al. Aldosterone, Renin, and Diabetes Mellitus in African Americans: The Jackson Heart Study. The Journal of Clinical Endocrinology & Metabolism. 2016;101:1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33:S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Young BA, Fülöp T, de Boer IH, Boulware LE, Katz R, Correa A, Griswold ME. Effects of Serum Creatinine Calibration on Estimated Renal Function in African Americans: The Jackson Heart Study. The American journal of the medical sciences. 2015;349:379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown JM, Robinson-Cohen C, Fernandez MAL, Allison MA, Baudrand R, Ix JH, Kestenbaum B, de Boer IH, Vaidya A. The Spectrum of Subclinical Primary Aldosteronism and Incident Hypertension: A Cohort Study. Ann Intern Med. 2017;167:630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph JJ, Echouffo-Tcheugui JB, Talegawkar SA, Effoe VS, Okhomina V, Carnethon MR, Hsueh WA, Golden SH. Modifiable Lifestyle Risk Factors and Incident Diabetes in African Americans. Am J Prev Med. 2017;53:e165–e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carithers T, Dubbert PM, Crook E, Davy B, Wyatt SB, Bogle ML, Taylor HA, Tucker KL. Dietary assessment in African Americans: methods used in the Jackson Heart Study. Ethn Dis. 2005;15:S6–49–55. [PubMed] [Google Scholar]
- 31.Hundemer GL, Baudrand R, Brown JM, Curhan G, Williams GH, Vaidya A. Renin Phenotypes Characterize Vascular Disease, Autonomous Aldosteronism, and Mineralocorticoid Receptor Activity. J Clin Endocrinol Metab. 2017;102:1835–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown JM, Robinson-Cohen C, Luque-Fernandez MA, Allison MA, Baudrand R, Ix JH, Kestenbaum B, de Boer IH, Vaidya A. The Spectrum of Subclinical Primary Aldosteronism and Incident Hypertension: A Cohort Study. Ann Intern Med. 2017;167:630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grim CE, Cowley AW, Hamet P, Gaudet D, Kaldunski ML, Kotchen JM, Krishnaswami S, Pausova Z, Roman R, Tremblay J, et al. Hyperaldosteronism and Hypertension: Ethnic Differences. Hypertension. 2005;45:766–772. [DOI] [PubMed] [Google Scholar]
- 34.Kidambi S, Kotchen JM, Grim CE, Raff H, Mao J, Singh RJ, Kotchen TA. Association of Adrenal Steroids With Hypertension and the Metabolic Syndrome in Blacks. Hypertension. 2007;49:704–711. [DOI] [PubMed] [Google Scholar]
- 35.Sagnella GA. Why is plasma renin activity lower in populations of African origin? J Hum Hypertens. 2001;15:17–25. [DOI] [PubMed] [Google Scholar]
- 36.Fisher ND, Hurwitz S, Ferri C, Jeunemaitre X, Hollenberg NK, Williams GH. Altered adrenal sensitivity to angiotensin II in low-renin essential hypertension. Hypertension. 1999;34:388–394. [DOI] [PubMed] [Google Scholar]
- 37.Adlin EV, Braitman LE, Vasan RS. Bimodal Aldosterone Distribution in Low-Renin Hypertension. Am J Hypertens. 2013;26:1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baudrand R, Vaidya A. The Low-Renin Hypertension Phenotype: Genetics and the Role of the Mineralocorticoid Receptor. International Journal of Molecular Sciences. 2018;19:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, et al. Salt Sensitivity of Blood Pressure. Hypertension. 2016;68:e7–e46. [DOI] [PubMed] [Google Scholar]
- 40.Satoh M, Kikuya M, Hara A, Ohkubo T, Mori T, Metoki H, Utsugi MT, Hirose T, Obara T, Inoue R, et al. Aldosterone-to-renin ratio and home blood pressure in subjects with higher and lower sodium intake: the Ohasama Study. Hypertension Research. 2011;34:361–366. [DOI] [PubMed] [Google Scholar]
- 41.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8(6 pt 2):II127–II134. [DOI] [PubMed] [Google Scholar]
- 42.Svetkey LP, McKeown SP, Wilson AF. Heritability of Salt Sensitivity in Black Americans. Hypertension. 1996;28:854–858. [DOI] [PubMed] [Google Scholar]
- 43.Luft FC, Grim CE, Fineberg N, Weinberger MC. Effects of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Circulation. 1979;59:643–650. [DOI] [PubMed] [Google Scholar]
- 44.Svetkey LP. Effects of Dietary Patterns on Blood Pressure: Subgroup Analysis of the Dietary Approaches to Stop Hypertension (DASH) Randomized Clinical Trial. Archives of Internal Medicine. 1999;159:285–293. [DOI] [PubMed] [Google Scholar]
- 45.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 8th Edition. December 2015 [Accessed 2019 Jul 31]; Available from: https://health.gov/dietaryguidelines/2015/guidelines/ [Google Scholar]
- 46.Laffer CL, Elijovich F, Eckert GJ, Tu W, Pratt JH, Brown NJ. GENETIC VARIATION IN CYP4A11 AND BLOOD PRESSURE RESPONSE TO MINERALOCORTICOID RECEPTOR ANTAGONISM OR ENAC INHIBITION: AN EXPLORATORY PILOT STUDY IN AFRICAN AMERICANS. J Am Soc Hypertens. 2014;8:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saha C, Eckert GJ, Ambrosius WT, Chun TY, Wagner MA, Zhao Q, Pratt JH. Improvement in Blood Pressure With Inhibition of the Epithelial Sodium Channel in Blacks With Hypertension. Hypertension. 2005;46:481–487. [DOI] [PubMed] [Google Scholar]
- 48.Lackland DT. Racial Differences in Hypertension: Implications for High Blood Pressure Management. Am J Med Sci. 2014;348:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spence JD, Rayner BL. Hypertension in Blacks: Individualized Therapy Based on Renin/Aldosterone Phenotyping. Hypertension. 2018;72:263–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


