Visual Abstract
Keywords: chronic kidney disease, adverse outcomes, CKD, ESKD, functional assessments, IADL, MMSE, SPPB
Key Points
Association of performance-based functional measures with adverse outcomes pertinent to patients with CKD has not been fully evaluated.
Physical function assessments have the strongest association with CKD adverse outcomes, including death, ESKD, and decline in GFR.
Low-tech, inexpensive, performance-based functional assessments offer providers a tool to categorize risk in CKD.
Abstract
Background
The comparative utility of performance-based functional assessments in predicting adverse outcomes in CKD is unknown. To examine their relative utility, we examined three performance-based functional assessments in an observational cohort of patients with CKD.
Methods
We recruited 350 participants with stage II–V, predialysis CKD. Participants were administered three performance-based functional assessments: Short Physical Performance Battery (SPPB), Modified Mini-Mental Status Exam (M3SE), and Lawton Instrumental Activities of Daily Living (IADL). Scores were dichotomized on the basis of the median and combined into a summary score. Outcomes included 50% GFR reduction, ESKD, and death. We used Cox proportional hazards to assess the association of performance-based functional assessments with outcomes.
Results
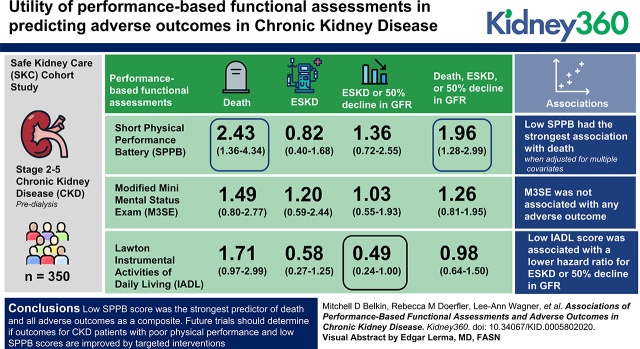
Compared with high performers, low SPPB performers had the highest adjusted rate of death, ESKD, or 50% reduction in GFR (HR, 1.96; 95% CI, 1.28 to 2.99). Low SPPB had the strongest association with death when adjusted for multiple covariates (HR, 2.43; 95% CI, 1.36 to 4.34). M3SE performance was not associated with any adverse outcome. None of the performance-based functional assessments were associated with ESKD, but a low IADL score was associated with a lower hazard ratio for ESKD or 50% decline GFR (HR, 0.49; 95% CI, 0.24 to 1.00).
Conclusions
Low SPPB score was the strongest predictor of death and all adverse outcomes as a composite. Future trials should determine if outcomes for patients with CKD who have poor physical performance and low SPPB scores are improved by targeted interventions.
Clinical Trial registry name and registration number:
Safe Kidney Care Cohort Study, NCT01407367
Introduction
CKD affects approximately 37 million Americans (1), is characterized by a reduced GFR, and contributes to outcomes such as ESKD and death (2,3). Because CKD occurs without prototypical signs or symptoms, it is often undetected and, hence, undertreated and referred late for specialty care (4). Better prediction of which patients with CKD are most vulnerable to adverse outcomes would enable providers to target care to those most at risk for excess morbidity and mortality.
Patients with CKD have reduced performance on a range of performance-based functional assessments, including physical and cognitive function and functional independence (5–9). However, performance-based functional assessments to assess CKD outcomes have been inadequately studied (10). Previous reports indicate performance on many performance-based functional assessments is linked to survival in numerous disease populations (11,12), but, to our knowledge, the association of performance-based functional assessments with outcomes (including ESKD and death) in patients with CKD have not been fully evaluated.
In this study, we report on three performance-based functional assessments in a CKD cohort, the concordance among performance-based functional assessments, and whether the measures were associated with adverse outcomes. We compared the adverse outcomes of high- and low-functioning participants across the three assessments to understand their potential predictive value.
Materials and Methods
The Safe Kidney Care (SKC) Cohort Study was a prospective, outpatient, observational cohort of 350 participants with stage II–V, predialysis CKD completed in 2017. The study tracked patients with CKD longitudinally, with annual visits, to ascertain the following adverse events: 50% reduction in GFR, ESKD, or death. Participants were evaluated using three performance-based functional assessments designed to independently assess physical function, cognitive function, and functional independence. The Short Physical Performance Battery (SPPB) was chosen as the measure of physical function. The Modified Mini-Mental Status Exam (M3SE) was selected to assess cognitive function. The Lawton Instrumental Activities of Daily Living (IADL) was used to assess self-perceived independent-living skills.
The study was conducted in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by the University of Maryland Baltimore Institutional Review Board and the Baltimore Veterans Affairs Medical Center Research and Development Committee. All participants were recruited from nephrology clinics at either the University of Maryland Medical Center or the Baltimore Veterans Affairs Medical Center. Participants were >21 years old and provided written informed consent. Study eligibility criteria for all participants included the presence of CKD, defined by a GFR (estimated with the Modified Diet in Renal Disease [MDRD] equation) of <60 ml/min per 1.73 m2 (13). The GFR values used for eligibility determination were measured on two outpatient occasions at least 90 days apart and no more than 18 months before study enrollment. Participants were excluded if it was anticipated they would develop ESKD or die within 12 months of study enrollment.
Procedures
Participants were tracked prospectively for up to 6 years, or until the onset of ESKD or death. The first consenting patient was enrolled on March 18, 2011. All participants had annual in-center visits, and telephone calls every 6 months between visits, with the final in-center visit on June 29, 2016. At the baseline visit, participants were administered all performance-based functional assessments: SPPB, M3SE, and IADL. At both telephone and annual in-center visits, the data collected included medical events, onset of ESKD, and death (as reported by next of kin). At annual visits, participants had phlebotomy for serum creatinine and physical measures. GFR was estimated using serum creatinine and the MDRD equation, the prevailing GFR-estimating equation at the time of the study (13). To track outcomes, additional follow-up by telephone contact with study participants or next of kin continued until June 30, 2017.
Statistical Methods
Given their non-normal distribution, each performance-based functional assessment was dichotomized into high- and low-performing groups on the basis of the median score of the study group. In addition to each independent score, we constructed a summary score, which was determined by grouping participants into the high-performing category when scoring above the median on two or more performance-based functional assessments, or the low-performing category when scoring above the median on only one or none of the measures.
For descriptive characteristics, continuous variables were summarized as mean±SD and compared using the t test. Dichotomous and categoric variables were summarized as N (%) and compared with the chi-squared test.
To compare key outcomes across performance-based functional assessments in high- and low-performance groups, we used time-to-event analyses from baseline to outcome, loss to follow-up, or end of study. Kaplan–Meier curves depict survival time in each group for key outcomes: death, ESKD, 50% reduction in GFR or ESKD, and any adverse outcome. Cox proportional hazards models were used to estimate an adjusted hazard ratio (HR) of low-performance versus high-performance groups in multivariate analyses. To account for the competing risks between ESKD and pre-ESKD death, we used the Fine and Gray proportional subdistribution hazards models to examine the associations of death with performance measures while considering the competing risk of ESKD. Similar proportional subdistribution hazards models were fitted for the composite renal end point and the competing risk of death. The subdistribution hazards are the hazards directly linked to the cause-specific failure of each event. Subdistribution HRs and 95% confidence intervals were reported. Analyses were performed using SAS 9.4 (SAS institute Inc., Cary, NC) (14). The covariates measured at baseline included age, sex, race, baseline GFR, presence of diabetes, cardiovascular disease, and cancer. Additional covariates included baseline body mass index, mean arterial pressure, level of education, and income.
To assess the concordance of the performance measures, we used contingency tables categorizing individuals as to whether they were concordant or discordant in their performance on each measure (high or low) and we used the Cohen κ statistic to assess interinstrument correlation (agreement). To evaluate the independent contribution of each performance measure in predicting study outcomes, we added each dichotomized performance measure to the multivariate model and assessed the incremental value of each using the −2 log likelihood parameter.
Results
Baseline Characteristics
The 350 SKC cohort participants were followed for a median (interquartile range [IQR]) of 42 (31.0–50.0) months. The median (IQR) length of follow-up in the high- and low-performing SPPB, M3SE, and IADL groups was 46 (35.0–54.5) and 37 (27.5–48.0), 46 (33.5–52.0) and 39 (28.0–48.5), and 45.0 (33.8–52.0) and 38 (23.0–48.8) months, respectively. The number of participants who withdrew or were lost to follow-up was not statistically significant for the SKC participants in the high- and low-performing SPPB and M3SE groups, but was significantly different for the SKC participants classified into high- and low-performing groups on the basis of the IADL instrument: 10 (4%) and 11 (11%) participants, respectively (P=0.02).
Table 1 depicts the demographic characteristics of each of the three performance-based functional assessments. Participants in the high-performing groups for SPPB, IADL, and M3SE were younger and better educated than their low-performing study counterparts. The high-performing SPPB and IADL groups had significantly higher baseline GFR and lower rates of cardiovascular disease as compared with their respective counterparts. Those in the high-performing SPPB group included fewer people with diabetes. Participants in the high-performing IADL group were more likely to be female and to have higher mean arterial pressure, whereas the SPPB and M3SE groups showed no significant difference on these metrics as compared with their peers. Those in the high-performing M3SE group were less likely to be Black as compared with low performers.
Table 1.
Baseline demographic characteristics of participants by study group
| Characteristics | SPPB | IADL | M3SE | |||
| High | Low | High | Low | High | Low | |
| Participants, n (%)a | 197 (56) | 153 (44) | 246 (70) | 104 (30) | 177 (51) | 173 (49) |
| Age | ||||||
| Mean±SD | 64.1±10.9 | 68.5±10.2 | 64.9±10.2 | 68.9±11.7 | 64.1±10.1 | 68.1±11.1 |
| ≥65 yr, n (%) | 105 (53) | 99 (65) | 110 (45) | 36 (35) | 90 (51) | 114 (66) |
| <65 yr, n (%) | 92 (47) | 54 (35) | 136 (55) | 68 (65) | 87 (49) | 59 (34) |
| Sex, n (%) | ||||||
| Male | 142 (72) | 109 (71) | 168 (68) | 83 (80) | 121 (68) | 130 (75) |
| Female | 55 (28) | 44 (29) | 78 (32) | 21 (20) | 56 (32) | 43 (25) |
| Black, n (%) | ||||||
| Yes | 135 (69) | 108 (71) | 173 (70) | 70 (67) | 109 (62) | 134 (77) |
| No | 62 (31) | 45 (29) | 73 (30) | 34 (33) | 68 (38) | 39 (23) |
| BMI (kg/m2), mean±SD | 32.0±7.0 | 34.7±7.7 | 32.9±7.2 | 33.9±7.9 | 33.7±7.4 | 32.7±7.4 |
| GFR (ml/min per 1.73 m2), mean±SD | 47.1±13.9 | 42.2±15.2 | 46.1±14.8 | 42.2±14.1 | 45.3±14.3 | 44.6±15.1 |
| CKD stage, n (%) | ||||||
| Stage 2 (60–89 ml/min) | 35 (18) | 19 (12) | 42 (17) | 12 (12) | 27 (15) | 27 (16) |
| Stage 3A (45–60 ml/min) | 73 (37) | 49 (32) | 93 (38) | 29 (28) | 65 (37) | 57 (33) |
| Stage 3B (30–45 ml/min) | 71 (36) | 47 (31) | 74 (30) | 44 (42) | 60 (34) | 58 (34) |
| Stage 4 (15–30 ml/min) | 13 (7) | 33 (22) | 31 (13) | 15 (14) | 19 (11) | 27 (16) |
| Stage 5 (<15 ml/min) | 5 (3) | 5 (3) | 6 (2) | 4 (4) | 6 (3) | 4 (2) |
| Mean arterial pressure (mm Hg), mean±SDb | 88.8±12.3 | 87.2±14.3 | 89.0±12.6 | 85.9±14.3 | 87.8±12.7 | 88.4±13.7 |
| Cardiovascular disease, n (%) c | ||||||
| Yes | 98 (50) | 99 (65) | 125 (51) | 72 (69) | 98 (55) | 99 (57) |
| No | 99 (50) | 54 (35) | 121 (49) | 32 (31) | 79 (45) | 74 (43) |
| Cancer, n (%) d | ||||||
| Yes | 50 (25) | 27 (18) | 56 (23) | 21 (20) | 43 (24) | 34 (20) |
| No | 147 (75) | 126 (82) | 190 (77) | 83 (80) | 134 (76) | 139 (80) |
| Diabetes, n (%) | ||||||
| Yes | 114 (58) | 105 (69) | 154 (63) | 65 (63) | 117 (66) | 102 (59) |
| No | 83 (42) | 48 (31) | 92 (37) | 39 (37) | 60 (34) | 71 (41) |
| Medical alert device, n (%) | ||||||
| Yes | 62 (31) | 46 (30) | 78 (32) | 30 (29) | 50 (28) | 58 (34) |
| No | 135 (69) | 107 (70) | 168 (68) | 74 (71) | 127 (72) | 115 (66) |
| Education, n (%) | ||||||
| No high-school diploma | 23 (12) | 39 (25) | 37 (15) | 25 (24) | 8 (5) | 54 (31) |
| High school or greater | 174 (88) | 114 (75) | 209 (85) | 79 (76) | 169 (95) | 119 (69) |
| Income, n (%) e | ||||||
| ≥$50,000 | 64 (32) | 30 (20) | 70 (28) | 24 (23) | 69 (39) | 25 (14) |
| <$50,000 | 133 (68) | 123 (80) | 176 (72) | 80 (77) | 108 (61) | 148 (86) |
SPPB, Short Physical Performance Battery; IADL, Lawton Instrumental Activities of Daily Living; M3SE, Modified Mini-Mental Status Exam; BMI, body mass index.
One participant who did not have an SPPB baseline score was included in the low-performance group on the basis of a later score.
Data were missing from three participants.
Cardiovascular disease includes coronary artery disease (heart attack, chest pain, angina), heart failure, atrial fibrillation, atrial flutter, stroke, transient ischemic attack, peripheral vascular disease, carotid artery disease, and thoracic or abdominal aneurysm.
One participant who refused to answer was included in the no-cancer group.
The 13 participants who refused to answer were included in the income <$50,000 group.
Outcomes
Over the study duration, 41 participants developed ESKD, 52 had a 50% reduction in GFR or developed ESKD, and 56 died. Table 2 shows adverse outcomes of participants on the basis of dichotomized performance measures (i.e., high versus low groups). There was a statistically lower number of deaths in all high-performance groups. The high-performing SPPB group had 20 (10%) deaths compared with the low SPPB group, which had 36 (24%) deaths (P=0.001). Similarly, the high-performing SPPB group had fewer of the composite outcome of 50% reduction in GFR, ESKD, or death as compared with the low-performance group (21% versus 43%, respectively; P≤0.001). The high-performing M3SE group had 20 (11%) deaths compared with the low-performing M3SE group, which had 36 (21%) deaths (P=0.02). Likewise, there were fewer of the composite outcome in the high-performing M3SE group as compared with the low-performance group (25% versus 35%, respectively; P<0.05). The high-performing IADL group had 32 (13%) deaths compared with the low-performing IADL group, which had 24 (23%) deaths (P=0.02). A similar trend was seen when dichotomizing participants on the basis of their summary score, with fewer deaths in those who were high performers versus those who were low performers (24% versus 39%, respectively; P≤0.001), and fewer composite outcomes.
Table 2.
Adverse outcomes of high and low performance-based functional assessments groups and dichotomous summary score in the Safe Kidney Care cohort
| Outcome | Total (N) | n (%) | |||||||
| High SPPB | Low SPPB | High IADL | Low IADL | High M3SE | Low M3SE | High Summary Score | Low Summary Score | ||
| Participants | 350 | 197 (56) | 153 (44) | 246 (70) | 104 (30) | 177 (51) | 173 (49) | 213 (61) | 137 (39) |
| ESKD | 41 | 20 (10) | 21 (14) | 32 (13) | 9 (9) | 21 (12) | 20 (12) | 24 (11) | 17 (12) |
| Death | 56 | 20 (10) | 36 (24)a | 32 (13) | 24 (23)b | 20 (11) | 36 (21)b | 23 (11) | 33 (24)a |
| ESKD or 50% decline GFR | 52 | 21 (11) | 31 (20)b | 42 (17) | 10 (10) | 26 (15) | 26 (15) | 30 (14) | 22 (16) |
| ESKD, 50% decline GFR, or death | 106 | 41 (21) | 65 (43)c | 72 (29) | 34 (33) | 45 (25) | 61 (35)b | 52 (24) | 54 (39)a |
P values compared two groups listed side by side. SPPB, Short Physical Performance Battery; IADL, Lawton Instrumental Activities of Daily Living; M3SE, Modified Mini-Mental Status Exam.
P≤0.001.
P≤0.05.
P≤0.0001.
Also depicted in Table 2, only the SPPB had a statistically different number of the kidney composite outcome of ESKD and/or 50% GFR reduction events between the high and low groups. The high-performing SPPB group had 21 (11%) ESKD or 50% reduced GFR events compared with the low-performing SPPB group, which had 31 (20%; P=0.01). In the combined outcome assessment of death, ESKD, or reduced GFR, the SPPB and M3SE had significant differences between high- and low-performing groups.
Figures 1 and 2 display the Kaplan–Meier curves for death (Figure 1) and a composite of all outcomes (Figure 2) for each performance measure independently, along with the summary score of all three performance measures. Supplemental Figures 1 and 2 display Kaplan–Meier curves for the other outcomes: ESKD and ESKD or 50% reduction in GFR. High performance on each measure was associated with lower rates of death, as compared with low performance. Similarly, a summary score of all three measures showed a significant difference between high and low performers. Classifying participants into high and low performers on each measure, along with a dichotomous summary score for all three, revealed no difference in the incidence of ESKD. Only the SPPB dichotomized into high- and low-performing groups demonstrated a significant difference in 50% reduction in GFR or ESKD. When using a composite of ESKD, 50% reduction in GFR, or death as the outcome of interest, all three instruments showed significantly lower incidence of each outcome when dichotomized into high and low performers. Similarly, the summary score demonstrated a significant difference between high and low performers.
Figure 1.
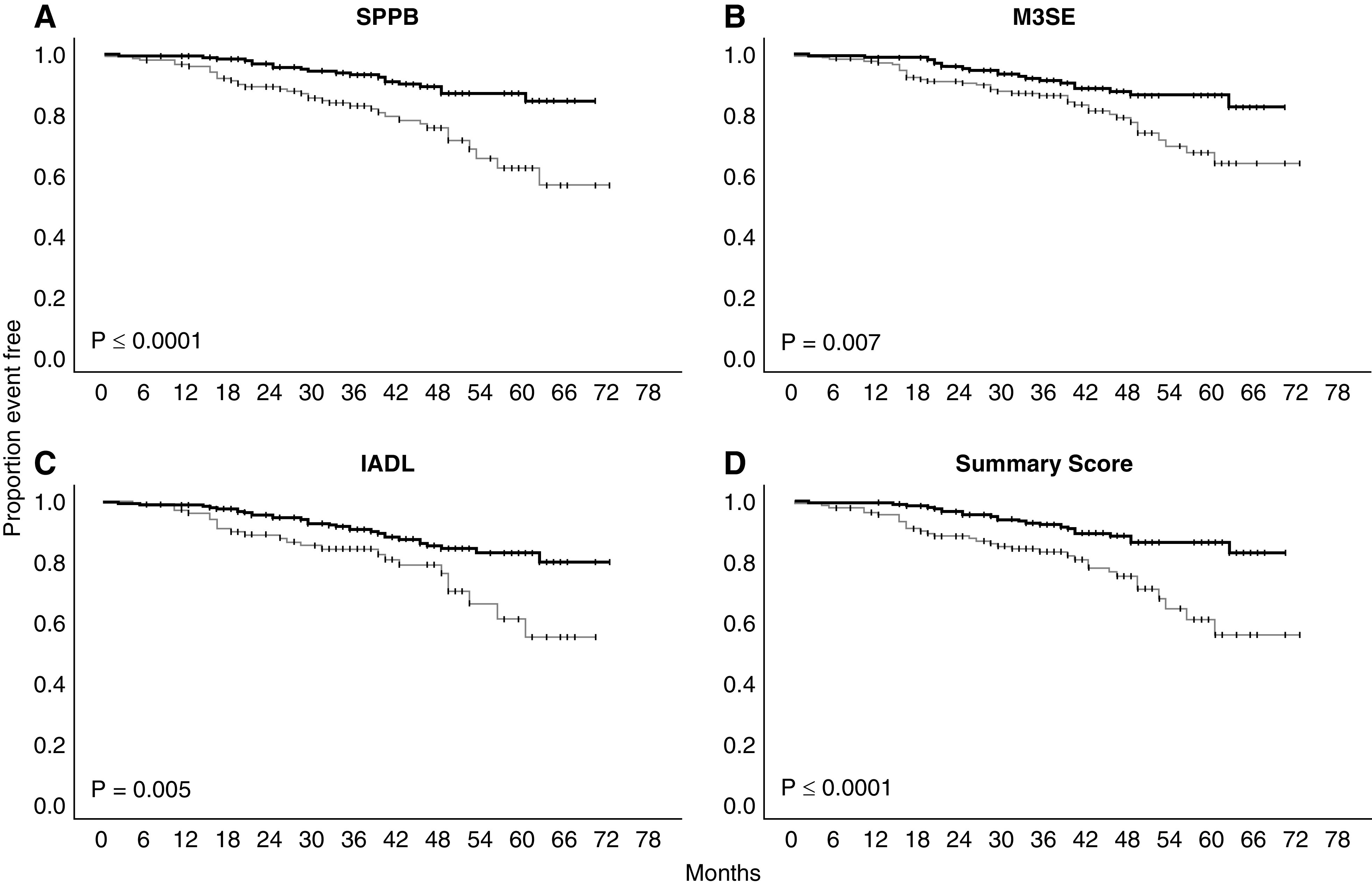
Kaplan–Meier plots of high (thick line) versus low-performance (thin line) groups for each performance-based functional assessment and a summary of all with the outcome of death. Log-rank statistic P value. IADL, Lawton Instrumental Activities of Daily Living; M3SE, Modified Mini-Mental Status Exam; SPPB, Short Physical Performance Battery.
Figure 2.
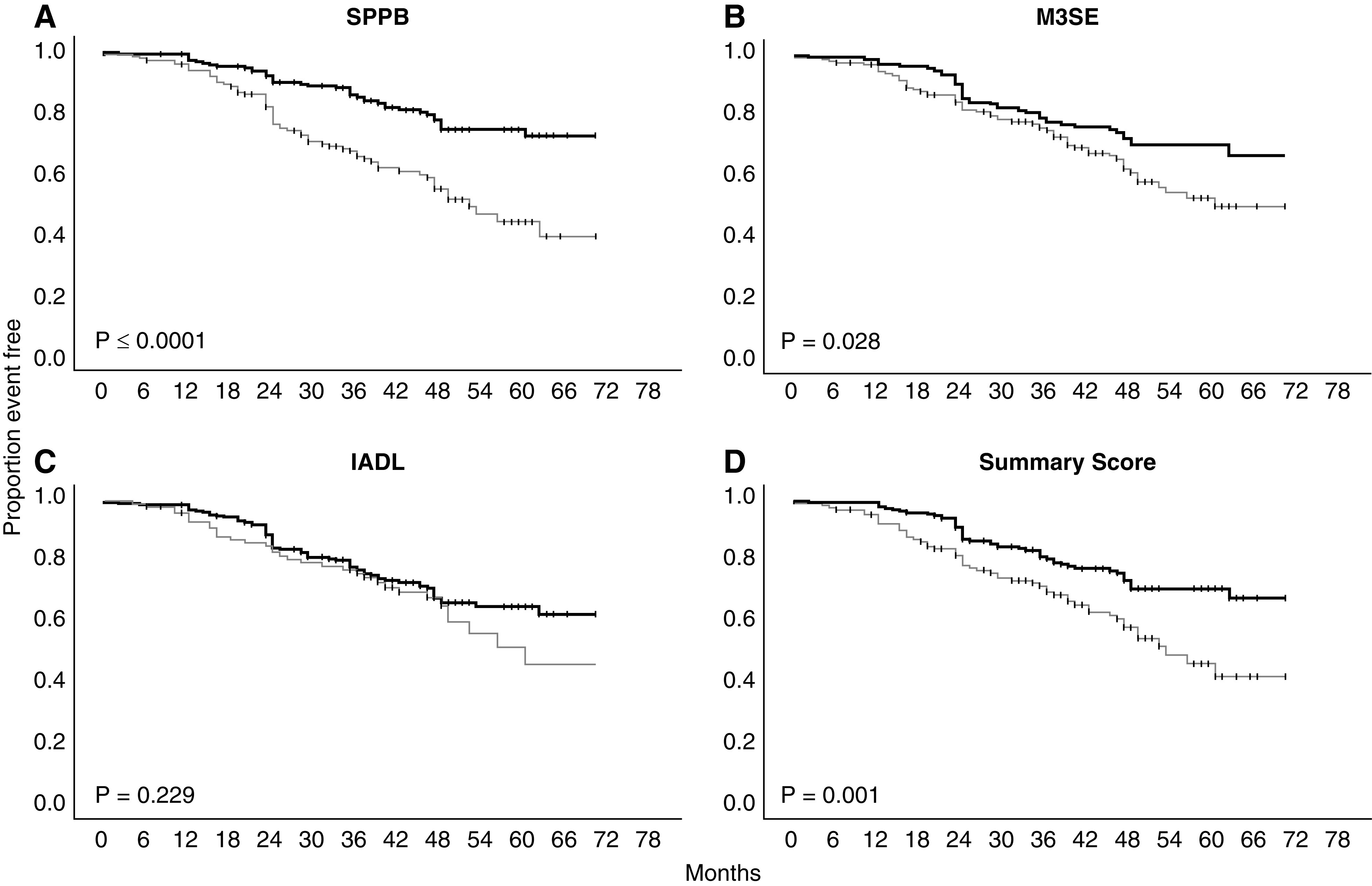
Kaplan–Meier plots of high (thick line) versus low-performance (thin line) groups for each performance-based functional assessment and a summary of all with the composite outcome. Log-rank statistic P value. IADL, Lawton Instrumental Activities of Daily Living; M3SE, Modified Mini-Mental Status Exam; SPPB, Short Physical Performance Battery.
Performance Measure Concordance
Table 3 depicts the concordance of each of the three performance instruments using the Cohen κ statistic. With respect to high versus low group assignment for each of the three pairings, there was only slight concordance; that is, participants with a high functional score in one measure and a high score in the other, or a low functional score in one measure and a low score in the other. The largest proportion of study participants with discordant performance-based functional assessments were those with discordant IADL and M3SE scores, which had a corresponding Cohen κ value of 0.179, indicating slight agreement of the two measures. The highest concordance was between the SPPB and IADL scores, with a Cohen κ value of 0.295, indicating fair agreement in measures. Supplemental Table 1 exhibits detailed results of the concordance/discordance of the pairing instruments and their outcomes.
Table 3.
Concordance of performance-based functional assessments using the Cohen (κ) coefficient statistic
| Assessment 1 | Assessment 2 (κ) | ||
| SPPB | M3SE | IADL | |
| SPPB | 1.00 | 0.187 | 0.295 |
| M3SE | 0.187 | 1.00 | 0.179 |
| IADL | 0.295 | 0.179 | 1.00 |
SPPB, Short Physical Performance Battery; IADL, Lawton Instrumental Activities of Daily Living; M3SE, Modified Mini-Mental Status Exam.
Outcome Assessment
Table 4 depicts the adjusted relative hazard for each end point, comparing high and low performance-based functional assessments groups for each outcome individually and then as a composite. The risk estimates are adjusted for demographic factors, case mix, baseline GFR, and other characteristics. The adjusted risk of death was significantly higher for SPPB and summary score when comparing low-performing groups to their high-performing peers (SPPB, HR, 2.43; 95% CI, 1.36 to 4.34; P=0.003; summary score, HR, 2.07; 95% CI, 1.16 to 3.69; P=0.01). The adjusted risk for the composite of death, ESKD, and 50% reduction in GFR was only statistically significant for the SPPB (SPPB, HR, 1.96; 95% CI, 1.28 to 2.99; P=0.002). The risk of ESKD alone was not associated with any of the performance-based functional assessments. Of note, IADL was associated with a reduced adjusted risk of ESKD or 50% reduction in GFR (HR, 0.49; 95% CI, 0.24 to 1.00; P=0.05).
Table 4.
Cox regression analysis displaying individual hazard ratio for each performance-based functional assessment and the summary score associated with each outcome and the incremental reduction in variance for each assessment in the model.
| Model | Death | ESKD | ESKD or 50% Decline in GFR | Death, ESKD, or 50% Decline in GFR | ||||||||||||
| P Value | HR (95% CI) | −2 Log Likelihood | Chi-Squared Score | P Value | HR (95% CI) | −2 Log Likelihood | Chi-Squared Score | P Value | HR (95% CI) | −2 Log Likelihood | Chi-Squared Score | P Value | HR (95% CI) | −2 Log Likelihood | Chi-Squared Score | |
| Unadjusted model | 591.40 | 420.63 | 561.16 | 1127.03 | ||||||||||||
| Adjusted model | 569.53 | 21.87 | 352.74 | 67.89 | 487.10 | 74.07 | 1063.14 | 63.89 | ||||||||
| SPPB | 0.003 | 2.43 (1.36 to 4.34) | 560.12 | 9.41 | 0.42 | 0.82 (0.40 to 1.68) | 352.09 | 0.65 | 0.34 | 1.36 (0.72 to 2.55) | 486.19 | 0.91 | 0.002 | 1.96 (1.28 to 2.99) | 1053.26 | 9.88 |
| M3SE | 0.21 | 1.49 (0.80 to 2.77) | 567.93 | 1.61 | 0.62 | 1.20 (0.59 to 2.44) | 352.49 | 0.25 | 0.92 | 1.03 (0.55 to 1.93) | 487.09 | 0.01 | 0.30 | 1.26 (0.81 to 1.95) | 1062.05 | 1.09 |
| IADL | 0.06 | 1.71 (0.97 to 2.99) | 566.18 | 3.35 | 0.17 | 0.58 (0.27 to 1.25) | 350.65 | 2.08 | 0.05 | 0.49 (0.24 to 1.00) | 482.77 | 4.33 | 0.93 | 0.98 (0.64 to 1.50) | 1063.13 | 0.01 |
| Summary score | 0.01 | 2.07 (1.16 to 3.69) | 563.34 | 6.20 | 0.56 | 0.81 (0.39 to 1.66) | 352.40 | 0.34 | 0.62 | 0.85 (0.46 to 1.59) | 486.85 | 0.26 | 0.13 | 1.39 (0.91 to 2.10) | 1060.81 | 2.33 |
Adjusted model includes baseline values for age, sex, race, body mass index, mean arterial pressure, baseline GFR, diabetes, cardiovascular disease, cancer, education, and income. HR, hazard ratio; SPPB, Short Physical Performance Battery; M3SE, Modified Mini-Mental Status Exam; IADL, Lawton Instrumental Activities of Daily Living.
Supplemental Table 2 displays data for a competing-risk analysis of the composite of ESKD and 50% decline in GFR and death as outcomes, with death and ESKD as the corresponding subdistribution hazards, respectively. In this competing-risk survival analysis, there were no substantial changes in our reported findings. The aforementioned association of IADL with reduced adjusted risk of ESKD or 50% reduction in GFR was sustained (adjusted HR, 0.43; 95% CI, 0.198 to 0.918).
Table 4 also demonstrates the independent contribution of each dichotomized performance measure to the strength of association between the baseline clinical factors and outcomes, as measured by decrement in −2 log likelihood score and change indicated by the chi-squared score. The SPPB augmented the association between predictive factors and death more than any other of the performance measures, as indicated by the largest chi-squared contribution and decrement in −2 log likelihood score versus a summary of all three scores. Furthermore, addition of the other performance measures did not contribute any incremental value to the model versus the SPPB alone.
Supplemental Table 3 depicts the additive effect of each performance score to the model, starting with the model adjusted for clinical covariate and then the stepwise addition of the first SPPB (with the largest chi-squared score), followed by M3SE, and then IADL, which made nonsignificant improvements in the model. The final model contrasts the −2 log likelihood and chi-squared score with the model including all three performance measures added independently, and reveals there was no benefit to the summary score over stepwise addition of each performance measure, but the addition of SPPB is still more significant alone than the summary measure.
Discussion
Clinical presentations of CKD are nonspecific, without any signature disease findings that indicate poor disease outcomes (4). Laboratory abnormalities, which are key attributes of CKD, are often underappreciated by physicians (4). Patients with CKD are frequently unaware of their condition, and this awareness is only marginally improved when their disease has clinical manifestations (15). Knowledge of CKD does not guarantee appropriate monitoring and care (16). Failure to identify patients with CKD and to stratify their risk is a lost opportunity to minimize morbidity and mortality. Better prediction of which patient's CKD may progress, including to ESKD or death, would enable provision of appropriate therapies to those most at risk.
Functional assessments offer a low-tech, inexpensive means to categorize patient risk. Various screening tools have been evaluated for their ability to predict CKD progression. The M3SE, a modified version of the Mini-Mental Status Exam, consists of 17 questions on memory, recall, awareness of today's date, and ability to follow directions and name objects (17). Previous studies have examined the relationship between M3SE and CKD progression and determined no direct relationship when controlling for covariates (7). The IADL is a self-reported questionnaire with eight questions: ability to use a telephone, shop, prepare food, perform housekeeping work, do laundry, maintain finances, take medications, and use transportation (18). Evaluation of the IADL as a screening tool for frailty and disability in predialysis CKD has been limited (8,9). The SPPB assesses lower-extremity physical performance status as determined in three timed tasks: standing balance, walking speed, and chair stand tests. In a meta-analysis, SPPB scores predicted all-cause mortality independent of follow-up length, geographic area, and age (11). Low functional capacity has been correlated with poor outcomes among patients with ESKD, but this correlation has not been well demonstrated in the CKD population (19). Gait speed and hand-grip strength have been correlated with all-cause mortality among patients with CKD, although use of this more comprehensive, lower-extremity functionality was not assessed (20).
The comparative advantage of one measure over others on the basis of their association with adverse outcomes in patients with CKD has not been well defined. To our knowledge, this is the first United States–based study comparing the associations of these performance-based functional assessments with outcomes in a predialysis CKD population. This study demonstrates that different dimensions of functional status, including physical, cognitive, and functional independence, are not highly correlated in CKD. However, the SPPB physical-function measure appeared superior to the other performance assessments in its association with CKD outcomes, most prominently with death, but also for kidney outcomes when combined with death into a single composite outcome. A summary score of all three performance-based functional assessments appears to track the performance of the SPPB, but does not increase the predictive value of the independent constituent measures.
These study findings should be interpreted with certain limitations. The study was not a randomized trial, and the potential for a sampling bias cannot be dismissed. Baseline characteristics differed between high and low performance-based functional assessments groups, potentially on variables that were not measured, including baseline proteinuria (which was unavailable). Notably, some participants who were found eligible on the basis of screening had improved baseline kidney function. Inclusion of participants with stage II CKD likely increases the generalizability of the study findings. The relatively small sample size possibly hinders the assessment of time-varying exposures. Moreover, the findings may be subject to a type-1 error given the number of hypothesis tests (multiple comparisons). Nevertheless, the risk estimates are substantial and relatively stable with multivariate adjustment.
There have been numerous clinical trials of exercise in patients with CKD. A meta-analysis including several randomized controlled trials of exercise in patients with CKD demonstrated that exercise improved health outcomes, health-related quality of life, BP, and physical performance, including aerobic capacity and muscle function (21). One 12-month study found strength training led to significant decreases in albuminuria; however, long-term trials are lacking (22). To the extent that exercise reflects physical function, physical-function assessments—especially the SPPB—act as a useful tool to assess strength and activity as a means to categorize risk in CKD.
When selecting instruments to assess exercise capacity in a clinical setting, the SPPB stands out as a cost-effective instrument to probe strength and endurance in a disease population. SPPB may also serve as a behavior prompt to direct patients to resources on best practices in self-care, disease management, and exercise. Others have examined whether exercise counseling could improve SPPB score, and the relationship of SPPB to frailty and physical function in patients with CKD (5,6). This exploratory analysis points to informative instruments other than clinical parameters to use in the clinic when assessing predictors of key outcomes. Moreover, the findings call for a randomized trial to evaluate which specific interventions for patients with low physical function could improve outcomes in CKD.
Disclosures
All authors have nothing to disclose.
Funding
The study in this paper was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK084017, University of Maryland, Baltimore, Institute for Clinical & Translational Research (ICTR) and the National Center for Advancing Translational Sciences (NCATS) Clinical Translational Science Award (CTSA) grant number 1UL1TR003098, and University of Maryland School of Medicine, Summer Program in Obesity, Diabetes, and Nutrition Research Training (UMSOM SPORT) grant T35-DK095737.
Acknowledgments
A special thanks to Ms. Elizabeth A. Mitgang for her assistance with the tables and figures.
Author Contributions
M.D. Belkin was responsible for investigation, project administration, and software; M.D. Belkin and J.C. Fink conceptualized the study; M.D. Belkin, J.C. Fink, and L.-A. Wagner reviewed and edited the manuscript; M.D. Belkin and J.C. Fink wrote the original draft; M.D. Belkin, L.-A. Wagner, and M. Zhan were responsible for formal analysis; M.D. Belkin and M. Zhan were responsible for methodology; R.M. Doerfler was responsible for data curation and validation; and J.C. Fink provided supervision and was responsible for funding acquisition and resources.
Footnotes
See related articles, “Functional Status in CKD: What Measures to Use?” on pages 608–610.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0005802020/-/DCSupplemental.
Association of concordant and discordant performance-based functional assessments and outcomes. Download Supplemental Table 1, PDF file, 1.13 MB (467.4KB, pdf)
Performance-based functional assessment hazard ratios accounting for competing risk of death, ESKD or 50% decline GFR. Download Supplemental Table 2, PDF file, 1.13 MB (467.4KB, pdf)
Comparative utility of performance-based functional assessments and summary score using Cox-regression analysis. Download Supplemental Table 3, PDF file, 1.13 MB (467.4KB, pdf)
Kaplan–Meier plots of high (thick line) versus low-performance (thin line) groups for each performance-based functional assessment and a summary of all with the outcome of ESKD. Download Supplemental Figure 1, PDF file, 1.13 MB (467.4KB, pdf)
Kaplan–Meier plots of high (thick line) versus low-performance (thin line) groups for each performance-based functional assessment and a summary of all with the outcome of ESKD or 50% decline in GFR. Download Supplemental Figure 2, PDF file, 1.13 MB (467.4KB, pdf)
References
- 1.US Department of Health and Human Services, Centers for Disease Control and Prevention. Chronic kidney disease in the United States, 2019. Atlanta, GA, US Department of Health and Human Services, Centers for Disease Control and Prevention, 2019. Available at: https://www.cdc.gov/kidneydisease/publications-resources/2019-national-facts.html. Accessed November 13, 2019 [Google Scholar]
- 2.Fink JC, Brown J, Hsu VD, Seliger SL, Walker L, Zhan M: CKD as an underrecognized threat to patient safety. Am J Kidney Dis 53: 681–688, 2009. 10.1053/j.ajkd.2008.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner L-A, Fink JC: Ensuring patient safety during the transition to ESRD. Semin Nephrol 37: 194–208, 2017. 10.1016/j.semnephrol.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 4.Stevens LA, Fares G, Fleming J, Martin D, Murthy K, Qiu J, Stark PC, Uhlig K, Van Lente F, Levey AS: Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: Evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol 16: 2439–2448, 2005. 10.1681/ASN.2005020192 [DOI] [PubMed] [Google Scholar]
- 5.Bohm CJ, Storsley LJ, Hiebert BM, Nelko S, Tangri N, Cheskin LJ, McAdams-DeMarco MA, Rigatto C: Impact of exercise counseling on physical function in chronic kidney disease: An observational study. Can J kidney Heal Dis 5: 2054358117753615, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiner DE, Seliger SL: Cognitive and physical function in chronic kidney disease. Curr Opin Nephrol Hypertens 23: 291–297, 2014. 10.1097/01.mnh.0000444821.87873.7b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurella Tamura M, Yaffe K, Hsu C-Y, Yang J, Sozio S, Fischer M, Chen J, Ojo A, DeLuca J, Xie D, Vittinghoff E, Go AS; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Cognitive impairment and progression of CKD. Am J Kidney Dis 68: 77–83, 2016. 10.1053/j.ajkd.2016.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baddour NA, Robinson-Cohen C, Lipworth L, Bian A, Stewart TG, Jhamb M, Siew ED, Abdel-Kader K: The surprise question and self-rated health are useful screens for frailty and disability in older adults with chronic kidney disease. J Palliat Med 22: 1522–1529, 2019. 10.1089/jpm.2019.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H-M, Hsiao S-M, Kuo M-C, Lo Y-C, Huang M-F, Yeh Y-C, Yen CF, Chen CS: Identifying early decline of daily function and its association with physical function in chronic kidney disease: Performance-based and self-reported measures. PeerJ 6: e5286, 2018. 10.7717/peerj.5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira Á, Álvarez G, Russo F, San-José B, Sánchez-Tomero JA, Barril G: Is SPPB useful as a screening method of functional capacity in patients with advanced chronic kidney disease? Nefrologia 39: 489–496, 2019. 10.1016/j.nefro.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 11.Pavasini R, Guralnik J, Brown JC, di Bari M, Cesari M, Landi F, Vaes B, Legrand D, Verghese J, Wang C, Stenholm S, Ferrucci L, Lai JC, Bartes AA, Espaulella J, Ferrer M, Lim JY, Ensrud KE, Cawthon P, Turusheva A, Frolova E, Rolland Y, Lauwers V, Corsonello A, Kirk GD, Ferrari R, Volpato S, Campo G: Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med 14: 215, 2016. 10.1186/s12916-016-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu SK, Ward M, Montgomery J, Mecchella JN, Masutani R, Bartels SJ, Batsis JA: Association of hospital admission risk profile score with mortality in hospitalized older adults. Innov Aging 1: igx007, 2017. 10.1093/geroni/igx007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS: A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 11: A0828, 2000 [Google Scholar]
- 14.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 15.Plantinga LC, Boulware LE, Coresh J, Stevens LA, Miller ER 3rd, Saran R, Messer KL, Levey AS, Powe NR: Patient awareness of chronic kidney disease: Trends and predictors. Arch Intern Med 168: 2268–2275, 2008. 10.1001/archinte.168.20.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClellan WM, Ramirez SPB, Jurkovitz C: Screening for chronic kidney disease: Unresolved issues. J Am Soc Nephrol 14[Suppl 2]: S81–S87, 2003. 10.1097/01.ASN.0000070144.86024.04 [DOI] [PubMed] [Google Scholar]
- 17.Foster R, Walker S, Brar R, Hiebert B, Komenda P, Rigatto C, Storsley L, Prasad B, Bohm C, Tangri N: Cognitive impairment in advanced chronic kidney disease: The Canadian frailty observation and interventions trial. Am J Nephrol 44: 473–480, 2016. 10.1159/000450837 [DOI] [PubMed] [Google Scholar]
- 18.Vittengl JR, White CN, McGovern RJ, Morton BJ: Comparative validity of seven scoring systems for the instrumental activities of daily living scale in rural elders. Aging Ment Health 10: 40–47, 2006. 10.1080/13607860500307944 [DOI] [PubMed] [Google Scholar]
- 19.Stack AG, Molony DA, Rives T, Tyson J, Murthy BVR: Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis 45: 690–701, 2005. 10.1053/j.ajkd.2004.12.013 [DOI] [PubMed] [Google Scholar]
- 20.Roshanravan B, Robinson-Cohen C, Patel KV, Ayers E, Littman AJ, de Boer IH, Ikizler TA, Himmelfarb J, Katzel LI, Kestenbaum B, Seliger S: Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 24: 822–830, 2013. 10.1681/ASN.2012070702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heiwe S, Jacobson SH: Exercise training in adults with CKD: A systematic review and meta-analysis. Am J Kidney Dis 64: 383–393, 2014. 10.1053/j.ajkd.2014.03.020 [DOI] [PubMed] [Google Scholar]
- 22.Hellberg M, Höglund P, Svensson P, Clyne N: Randomized controlled trial of exercise in CKD—the RENEXC study. Kidney Int Rep 4: 963–976, 2019. 10.1016/j.ekir.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association of concordant and discordant performance-based functional assessments and outcomes. Download Supplemental Table 1, PDF file, 1.13 MB (467.4KB, pdf)
Performance-based functional assessment hazard ratios accounting for competing risk of death, ESKD or 50% decline GFR. Download Supplemental Table 2, PDF file, 1.13 MB (467.4KB, pdf)
Comparative utility of performance-based functional assessments and summary score using Cox-regression analysis. Download Supplemental Table 3, PDF file, 1.13 MB (467.4KB, pdf)
Kaplan–Meier plots of high (thick line) versus low-performance (thin line) groups for each performance-based functional assessment and a summary of all with the outcome of ESKD. Download Supplemental Figure 1, PDF file, 1.13 MB (467.4KB, pdf)
Kaplan–Meier plots of high (thick line) versus low-performance (thin line) groups for each performance-based functional assessment and a summary of all with the outcome of ESKD or 50% decline in GFR. Download Supplemental Figure 2, PDF file, 1.13 MB (467.4KB, pdf)