Abstract
Olfactory receptors (ORs) represent the largest gene family in the human genome. Despite their name, functions exist for these receptors outside of the nose. Among the tissues known to take advantage of OR signaling is the kidney. From mouse to man, the list of renal ORs continues to expand, and they have now been linked to a variety of processes involved in the maintenance of renal homeostasis, including the modulation of blood pressure, response to acidemia, and the development of diabetes. In this review, we highlight the recent progress made on the growing appreciation for renal ORs in physiology and pathophysiology.
Keywords: renal physiology, acidemia, basic science, blood pressure, diabetes, GPCR, odorant receptors, olfactory receptor
Introduction
G protein-coupled receptors (GPCRs) are the largest gene family in the genome and are involved in almost every aspect of physiology, ranging from hormonal regulation to eyesight. These receptors also happen to represent the largest class of “druggable” proteins; in fact, 20–30% of all FDA-approved drugs target GPCRs (1,2). However, only a small subset of this protein family is being actively studied, leaving behind an extensive list of underappreciated GPCRs. Included in this list are the “sensory” GPCRs, which consist of taste receptors, opsins, and olfactory receptors (ORs).
Although ORs are known to govern one’s sense of smell, it has become clear these receptors are expressed in extranasal tissues including sperm, muscle, skin, adipose, and the gastrointestinal tract, with functions ranging from cell migration and motility to hormone release (3–7). The kidney in particular is a “sensory organ,” the tubular epithelial cells and renal vasculature are tasked with monitoring the composition of the blood and ultrafiltrate to adjust filtration, reabsorption, and secretion accordingly. Given this, it is no wonder that chemosensory ORs have emerged as major factors in the maintenance of renal homeostasis. Although the list of renal ORs is constantly expanding, most of these receptors remain orphan receptors with no known functions and/or localization (8–10) (Figure 1; Table 1). Nonetheless, several renal ORs have emerged as key contributors to kidney physiology. This review focuses on the established and emerging roles of renal ORs in both health and disease and highlights the clinically relevant future directions.
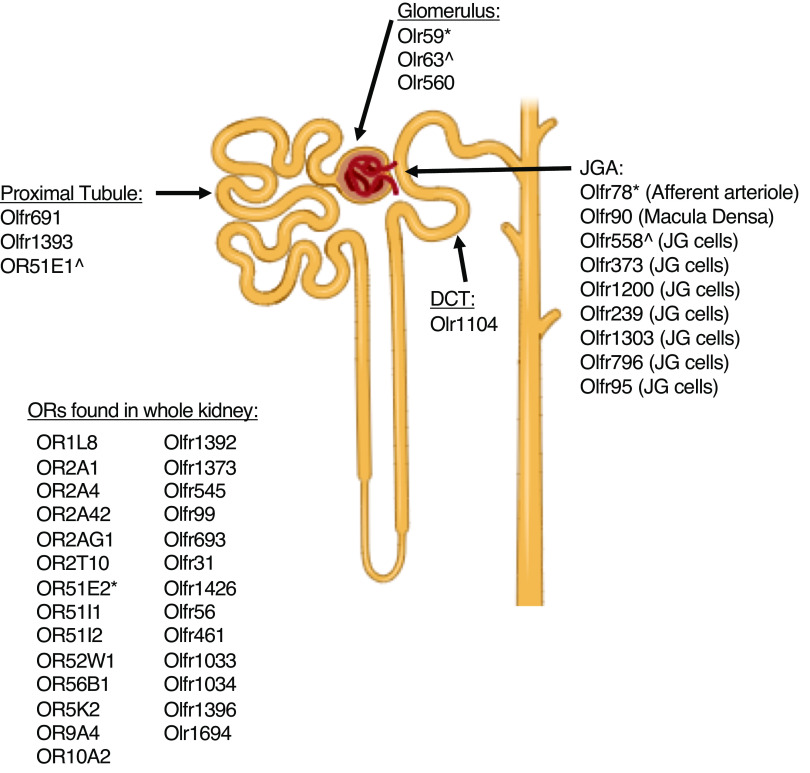
Figure 1.
Renal olfactory receptors. The mouse, rat, and human kidneys are known to express a growing list of olfactory receptors (ORs). Although the localization of most remains unknown (list on the left), several of these have been identified in distinct parts of the nephron. As listed, human ORs begin with the prefix “OR,” murine ORs begin with “Olfr,” and rat ORs start with “Olr.” *Murine Olfr78, rat Olr59, and human OR51E2 are functional orthologs. ^Murine Olfr558, rat Olr63, and human OR51E1 are functional orthologs.
Table 1.
Confirmed renal olfactory receptors
| Human Olfactory Receptors | Mouse Olfactory Receptors | Rat Olfactory Receptors | Known Ligands | References |
| OR51E2 | Olfr78 | Olr59 | Acetate, propionate, β-ionone, steroid hormones | (11,12,13) |
| OR51E1 | Olfr558 | Olr63 | Valeric acid, isovaleric acid, methyl valeric acid, cyclobutene-carboxylic acid, norbonene-2-carboxylic acid, methylbutyric acid, hexanoic acid, heptanoic acid, decanoic acid, nonanoic acid, octanoic acid, methyl-nonanoic acid, decanoic acid, propionate, butyrate | (14,15,16,17) |
| OR2A4 | Cyclohexyl salicylate | |||
| OR2AG1 | Amylbutyrate | (12) | ||
| OR2T10 | Maltyl isobutyrate, cinnamaldehyde, vanillin, terpinyl acetate, α-damascone | (18) | ||
| OR2A42 | α-pinene, farnesol | (19) | ||
| OR1L8 | Unknown | |||
| OR2A1 | Unknown | |||
| OR51I1 | Unknown | |||
| OR52W1 | Unknown | |||
| OR56B1 | Unknown | |||
| OR5K2 | Unknown | |||
| OR9A4 | Unknown | |||
| OR10A2 | Unknown | |||
| OR51I2 | Unknown | |||
| Olfr691 | Short and medium chain fatty acids | (20,21) | ||
| Olfr1393 | Cycloheptanol, cycloheptanone, cyclooctenone, cyclohexanone, 4,4 dimethylcyclohexanone, nopinone, norcamphor, 4-tertbutylcyclohexanone | (22) | ||
| Olfr90 | 2-methyl-4-propyl-1,3-oxathiane, 1-octen-3-ol, 3-octanol, 2-octanol, 2-octanone, amyl acetate, linalool, 2-pentylfuran, 3-octanone, benzyl cyanide, 1-octanol, 2-octen-1–0l, allylbenzene, cinnamaldehyde | (14) | ||
| Olfr1200 | Citris accord (mixture of limonene, γ-terpinene, citral) | (23) | ||
| Olfr545 | Sebacid acid (conflicting reports) | (20,24) | ||
| Olfr1392 | Unknown | |||
| Olfr1373 | Unknown | |||
| Olfr99 | Unknown | |||
| Olfr693 | Unknown | |||
| Olfr31 | Unknown | |||
| Olfr1426 | Unknown | |||
| Olfr56 | Unknown | |||
| Olfr461 | Unknown | |||
| Olfr1033 | Unknown | |||
| Olfr1034 | Unknown | |||
| Olfr1396 | Unknown | |||
| Olfr1694 | Unknown | |||
| Olfr95 | Unknown | |||
| Olfr796 | Unknown | |||
| Olfr1303 | Unknown | |||
| Olfr239 | Unknown | |||
| Olfr373 | Unknown | |||
| Olr560 | Unknown | |||
| Olr1104 | Unknown | |||
| Olr1694 | Unknown |
OR Signaling in the Nose and Kidney
ORs are members of the Class A Rhodopsin GPCR family. They are also the largest single class of proteins, making up nearly 0.1% of the human genome (25). Although there are more than 350 human ORs, there are more than 1000 within the mouse and rat, making the identification of functional orthologs a challenge (26,27). Throughout this review, references will be made to human, mouse, and rat ORs, and all three species have slightly different naming conventions (26,28,29). Human ORs are grouped into gene families and subfamilies on the basis of phylogenetic classification. They begin with the prefix “OR” followed by a family-subfamily-individual gene classification (e.g., OR51E2 is a human OR in gene family 51, subfamily E, gene 2). For mouse ORs, two naming systems dominate. They are either grouped by subfamilies using the prefix “MOR” or on the basis of their location within the genome using the prefix “Olfr” (e.g., MOR256–24 = Olfr1393). Finally, for rat ORs, the prefix “Olr” is used followed by a gene number on the basis of chromosome localization (e.g., Olr59).
Despite thousands of gene polymorphisms that modulate an individual’s sense of smell, these receptors are extremely similar at the molecular level and all signal through a conserved downstream signaling cascade (30–32). In the nose, ORs are localized to the cilia on the olfactory sensory neurons, which ensures they are exposed to a wide variety of volatile odorants. On odorant binding, the trimeric G protein (Golfactory or Golf) dissociates into active α and βγ subunits, with the α subunit triggering activation of adenylate cyclase 3 (AC3) (30–32). As the field of OR biology expands, these conserved signaling proteins have been found in other tissues as well. Although it is unclear if extranasal ORs can couple to other G proteins outside of the olfactory epithelium, a role for this signaling cascade exists within the kidney (Figure 2). In fact, studies using the AC3 knockout (KO) mouse were the first to indicate that ORs and OR signaling have important contributions to renal homeostasis (11).
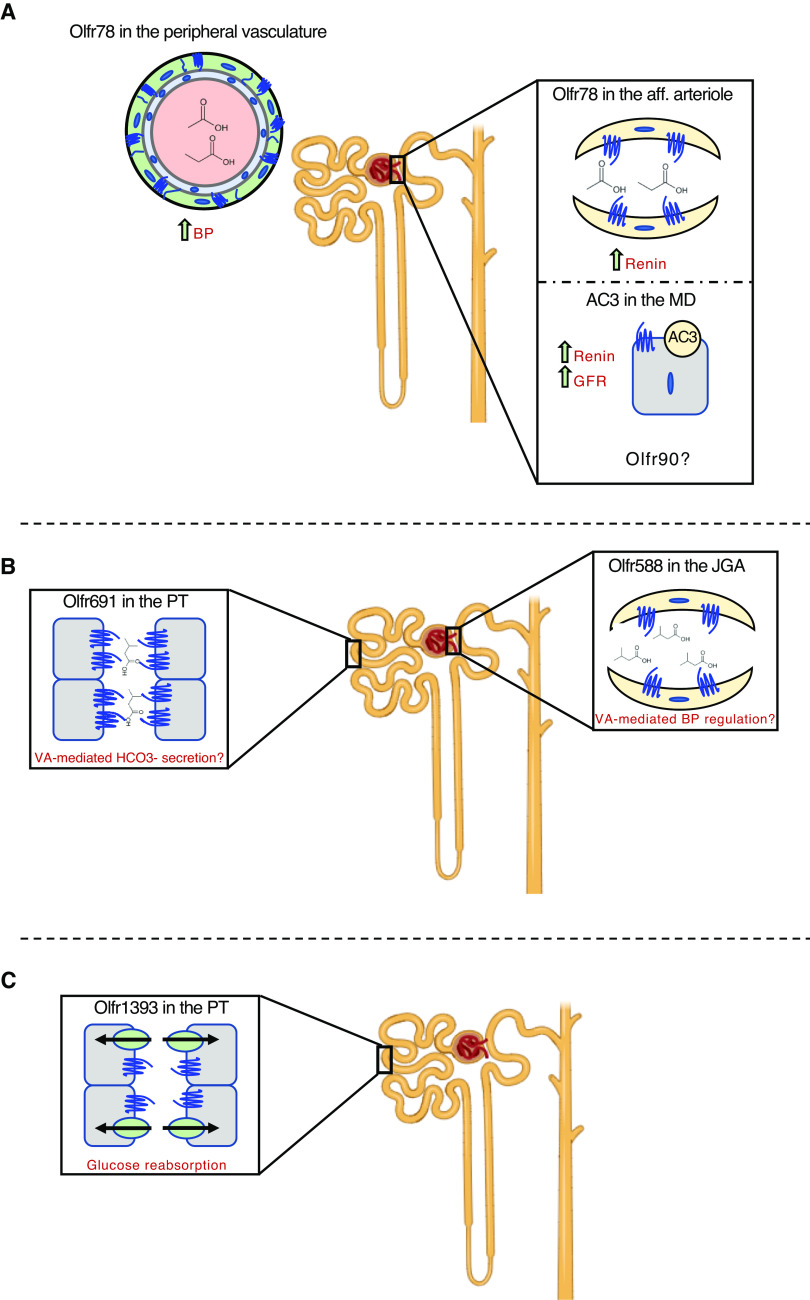
Figure 2.
Proposed functions of select renal olfactory receptors. (A) Olfr78 responds to short chain fatty acids in the peripheral vasculature and the afferent (aff) arteriole to increase BP and promote renin release. Within the macula densa (MD), adenylate cyclase 3 (AC3) has also been linked to an increase in renin release and GFR. Olfr90 has been localized to a MD cell line and may be involved in these processes. (B) Olfr691, localized within the proximal tubule (PT) and Olfr558 (localized to the juxtaglomerular apparatus; JGA) both respond to isovaleric acid (IVA). Under conditions of isovaleric acidemia, these receptors may be involved in bicarbonate secretion and BP regulation. (C) Olfr1393 has been localized within the PT where it contributes to the maintenance of glucose homeostasis via the sodium glucose co-transporters. Its function is exacerbated under conditions of type 1 or type 2 diabetes.
ORs and Blood Pressure Control
Both AC3 and Golf have been shown to be expressed in the human and murine kidney at both the transcript and protein level (11). Antibody labeling places murine AC3 specifically within the macula densa (MD) and Golf primarily within the distal convoluted tubule (both at the MD and surrounding epithelial cells) (11). Given the role of the MD in monitoring tubular fluid to control glomerular filtration and renin secretion, it is tempting to speculate that renal ORs are involved in these processes. Indeed, AC3 KO mice have a decreased GFR and lowered plasma renin levels (11). This correlates with a reduction in potassium excretion and an upregulation of COX-2 and nNOS activity that is likely a result of a feedback mechanism. Although the exact OR responsible for these processes is unknown, full-length gene expression of Olfr90 (MOR256–21) was found in an MD cell line (11). An orphan receptor at the time, it has since been deorphanized and determined to respond to a wide range of compounds that are closely aligned with fungal metabolism (the human ortholog is unknown) (14). Although fungi represent a minority of the host microbiome, they do exist in the body as both commensals and toxins, and it is possible the Olfr90-AC3 signaling cascade could be modulating GFR in response to changing levels of circulating fungal metabolites (Figure 2A). Clearly, future work is needed to examine this possible pathway.
The MD is part of the juxtaglomerular apparatus (JGA), which is a specialized structure formed by both the distal convoluted tubule and the afferent arteriole. It is responsible for synthesizing and secreting renin to regulate blood pressure and GFR, and is home to several renal ORs. Perhaps the most “famous” of these is Olfr78 (MOR18–2; functional orthologs: OR51E2 and Olr59) (11,33–36, 12,13). Olfr78 responds to short chain fatty acids (SCFAs), including acetate and propionate (13). These compounds are bacterial-derived metabolites that are produced by fermentation of dietary fiber by intestinal microbes, and have been linked to a myriad of host functions, including the maintenance of blood pressure (37,38). Using reporter mice and isolated glomeruli, Olfr78 has been localized to the smooth muscle of the major branches of the renal artery and the afferent arteriole (13). Propionate administration promotes renin release from the JGA and leads to an acute drop in blood pressure (13). In Olfr78 KO mice, propionate-induced renin release was all but lost, and the mice were hypersensitive to a drop in blood pressure (13). This is likely due to another SCFA-responsive GPCR, which is found within the renal vasculature that mediates the hypotensive response (GPR41) (39). Collectively, these data indicate that on activation by SCFAs, Olfr78 mediates a hypertensive response. It does so by increasing renin release from the JGA and by changing peripheral vascular resistance (Figure 2A). In addition to Olfr78, the JGA expresses several additional ORs. Microarray analysis of isolated juxtaglomerular cells revealed a total of seven additional murine ORs, whose expression is enriched within this population of cells (34). Among these is Olfr558 (MOR18–1), a carboxylic acid responsive receptor. Along with its rat (Olr63) (36) and human (OR51E1) (15) orthologs, these ORs are activated by butyrate, another SCFA (14–16). Although it remains to be seen if Olfr558 coordinates with Olfr78 to regulate blood pressure, it is certainly notable the JGA expresses ORs that can respond to all three SCFAs, which are known to play protective roles in the kidney via the host microbiome.
ORs and Acidemia
As alluded to above, the host microbiome produces a number of metabolites that contribute to kidney function and many of these activate ORs (40,41). Microbially derived byproducts have been linked to renal protection, including a mitigation of inflammation, decreased reactive oxygen species, and improved renal outcomes after ischemia/reperfusion injury (40–42). In support of this, dysbiosis is associated with a myriad of renal pathogenesis, including inflammation, hypertension, IgA nephropathy, and kidney stone formation (40,41).
Apart from the SCFA butyrate, Olfr588 (found in the JGA) can also respond to several other fatty acids including isovaleric acid (14). This same branched-chain fatty acid activates Olfr588’s human ortholog (OR51E1 [15,17,43]; found within human proximal tubule cells) and Olfr691 (MOR31–6), which is localized to the S1 and S3 segments of the renal proximal tubule (20). Isovaleric acid is produced during leucine metabolism and is a known bacterial metabolite (44). Point mutations in the mitochondrial enzyme isovaleryl CoA dehydrogenase prevents the conversion of isovaleryl-CoA to 3-methylcrotonyl CoA, leading to the accumulation of isovaleric acid in the blood and urine, resulting in the metabolic disorder, isovaleric acidemia (45). This acid buildup can lead to life-threatening metabolic acidosis and is cleared via the kidney, where it has potential to activate Olfr588/OR51E1 and Olfr691. It is tempting to speculate that these receptors may sense the excess acid load, and modulate blood pressure (Olfr588) and alter bicarbonate secretion (Olfr691) as a result (Figure 2B). Clearly, these hypotheses require further testing to determine if renal ORs may serve as therapeutic targets for those suffering from organic acidosis and acidemia.
ORs and Diabetes
Recently, several ORs have been linked to adiposity and metabolic balance (7,46–49). The kidney is responsible for filtering all blood glucose and quickly returning it to circulation via reabsorption in the proximal tubule. This task is accomplished in a sodium-dependent manner via two glucose transporters, sodium glucose co-transporter 1 and 2 (SGLT1 and SGLT2, respectively) (50–52). Under euglycemic conditions, this process is seamless; little to no glucose appears in the final urine. However, when glucose concentrations exceed the transport maximum of SGLT1 and SGLT2, glycosuria occurs. Olfr1393 (MOR256–24; human ortholog unknown) is found in all three segments of the renal proximal tubule (via gene expression analysis on hand-dissected nephron segments), where it localizes to the apical membrane when overexpressed in polarized epithelial cells (22). Loss of renal Olfr1393 leads to mild glycosuria and improved glucose tolerance despite euglycemia and normal plasma and urinary electrolytes (22). Coupled with this phenotype was the observation that SGLT1, the transporter responsible for reabsorbing approximately 10% of glucose under normal conditions, was mislocalized in Olfr1393 KO mice (22) (Figure 2C). Given the emerging roles for these transporters in the treatment of diabetes (SGLT2 inhibitors are extensively reviewed elsewhere) (53–57), we wondered if Olfr1393 KO mice may exhibit an altered diabetic phenotype. Using a high-fat diet to induce obesity and early stages of type 2 diabetes (58), we observed that Olfr1393 KO mice had improved glucose tolerance and an attenuation in diabetes-induced hyperfiltration. Similar findings are observed in Olfr1393 KO mice challenged with streptozotocin to induce pancreatic beta cell depletion and type 1 diabetes. It is worth noting that much of these findings with Olfr1393 KO mice were found to be sex dependent, shedding new light on sex differences with glucose homeostasis (58,59). Moreover, Olfr1393 was found to be activated by small cyclic compounds, and the physiologic relevance of these is still under investigation (Table 1). Identification of physiologic ligands and the functional human ortholog for Olfr1393 will be necessary to appreciate the translational potential for this receptor.
Anosmia and the Kidney?
Although the sense of smell is often taken for granted, those suffering from certain diseases and illnesses (60) know all too well how important functional OR signaling is. The appreciation of this affliction has been heightened by the recent emergence of temporary anosmia as a symptom of SARS-CoV-2 infection (61–63). Does the loss of smell imply a connection between the nose and extranasal ORs? Although it has been postulated that SARS-CoV-2 infection has potential to alter OR signaling throughout the body (64) (including the kidney and bladder), experimental studies in this area are lacking. Despite this, there is a known connection between olfactory and renal function (65,66). In particular, patients suffering from various ciliopathies that are characterized by progressive development of fluid-filled renal cysts, often present with anosmia. Cystic diseases often arise from mutations in select ciliary proteins (e.g., nephrocystin 6, Bardet-Biedl Syndrome proteins), and it has been shown these mutations lead to an altered organization of the olfactory epithelium, including the motile cilia on which ORs reside (66). Future research in this field is clearly needed to appreciate the role that ciliopathies and other anosmia-inducing diseases affect renal OR signaling.
The Future of Renal ORs
To date, 23 murine, five rat, and 15 human ORs have been found to be expressed in the kidney (Figure 1; Table 1). However, the vast majority of these receptors have not been studied in a molecular or physiologic context. This research is hindered by the lack of available reagents for this class of proteins (10) (reliable antibodies are rare, most are orphan receptors, ortholog identification is challenging). Given the physiologic relevance of the few renal ORs that have been characterized thus far (Figure 2), the future is ripe for renal OR research. In fact, expression of these receptors is not confined to the kidney, as there is evidence that the entire urinary system uses OR signaling as a means for maintaining function. Both human (OR10H1) and murine (Olfr895, Olfr544, Olfr1392, Olfr181) ORs have been found in the bladder, and it is notable that some of these are the same as those found in the kidney (67,68). In particular, evidence has emerged that some of these receptors may be highly expressed in bladder cancers (renal carcinomas have not been examined) and activation of these receptors may represent a novel therapeutic treatment option (69). These recent findings align with previous studies showing that several ORs are known biomarkers for various cancers (renal ORs OR51E1 and OR51E2 are highly expressed in prostate cancer) (69). As this field expands, increased identification and characterization of ORs throughout the urinary system will undoubtedly expand our current appreciation for this signaling pathway in both health and disease.
Disclosures
B. Shepard reports having patents and inventions with Firmenich.
Funding
This work was supported by the National Institutes of Health, Office of Extramural Research grants K01-DK106400 and R03-DK123546.
Author Contributions
B Shepard was responsible for the funding acquisition and investigation, wrote the original draft, and reviewed and edited the manuscript.
References
- 1.Lundstrom K: An overview on GPCRs and drug discovery: Structure-based drug design and structural biology on GPCRs. Methods Mol Biol 552: 51–66, 2009. 10.1007/978-1-60327-317-6_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wacker D, Stevens RC, Roth BL: How ligands illuminate GPCR molecular pharmacology. Cell 170: 414–427, 2017. 10.1016/j.cell.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldmesser E, Olender T, Khen M, Yanai I, Ophir R, Lancet D: Widespread ectopic expression of olfactory receptor genes. BMC Genomics 7: 121, 2006. 10.1186/1471-2164-7-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuda N, Yomogida K, Okabe M, Touhara K: Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J Cell Sci 117: 5835–5845, 2004. 10.1242/jcs.01507 [DOI] [PubMed] [Google Scholar]
- 5.Griffin CA, Kafadar KA, Pavlath GK: MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell 17: 649–661, 2009. 10.1016/j.devcel.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H: Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299: 2054–2058, 2003. 10.1126/science.1080376 [DOI] [PubMed] [Google Scholar]
- 7.Wu C, Hwang SH, Jia Y, Choi J, Kim YJ, Choi D, Pathiraja D, Choi IG, Koo SH, Lee SJ: Olfactory receptor 544 reduces adiposity by steering fuel preference toward fats. J Clin Invest 127: 4118–4123, 2017. 10.1172/JCI89344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu M, Echeverri F, Moyer BD: Endoplasmic reticulum retention, degradation, and aggregation of olfactory G-protein coupled receptors. Traffic 4: 416–433, 2003. 10.1034/j.1600-0854.2003.00097.x [DOI] [PubMed] [Google Scholar]
- 9.McClintock TS, Sammeta N: Trafficking prerogatives of olfactory receptors. Neuroreport 14: 1547–1552, 2003. 10.1097/00001756-200308260-00001 [DOI] [PubMed] [Google Scholar]
- 10.Shepard BD, Pluznick JL: How does your kidney smell? Emerging roles for olfactory receptors in renal function. Pediatr Nephrol 31: 715–723, 2015. 10.1007/s00467-015-3181-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ: Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA 106: 2059–2064, 2009. 10.1073/pnas.0812859106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuhaus EM, Zhang W, Gelis L, Deng Y, Noldus J, Hatt H: Activation of an olfactory receptor inhibits proliferation of prostate cancer cells. J Biol Chem 284: 16218–16225, 2009. 10.1074/jbc.M109.012096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ: Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. 10.1073/pnas.1215927110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halperin Kuhns VL, Sanchez J, Sarver DC, Khalil Z, Rajkumar P, Marr KA, Pluznick JL: Characterizing novel olfactory receptors expressed in the murine renal cortex. Am J Physiol Renal Physiol 317: F172–F186, 2019. 10.1152/ajprenal.00624.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalbe B, Schlimm M, Wojcik S, Philippou S, Maßberg D, Jansen F, Scholz P, Luebbert H, Ubrig B, Osterloh S, Hatt H: Olfactory signaling components and olfactory receptors are expressed in tubule cells of the human kidney. Arch Biochem Biophys 610: 8–15, 2016. 10.1016/j.abb.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 16.Adipietro KA, Mainland JD, Matsunami H: Functional evolution of mammalian odorant receptors. PLoS Genet 8: e1002821, 2012. 10.1371/journal.pgen.1002821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita Y, Takahashi T, Suzuki A, Kawashima K, Nara F, Koishi R: Deorphanization of Dresden G protein-coupled receptor for an odorant receptor. J Recept Signal Transduct Res 27: 323–334, 2007. 10.1080/10799890701534180 [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Kristeller DC, do Nascimento JB, Galante PA, Malnic B: Identification of agonists for a group of human odorant receptors. Front Pharmacol 6: 35, 2015. 10.3389/fphar.2015.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasi EA, Eisen SL, Wang H, Sugianto W, Minniefield AR, Hoover KA, Branham PJ, Peralta-Yahya P: Rapid deorphanization of human olfactory receptors in yeast. Biochemistry 58: 2160–2166, 2019. 10.1021/acs.biochem.8b01208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajkumar P, Aisenberg WH, Acres OW, Protzko RJ, Pluznick JL: Identification and characterization of novel renal sensory receptors. PLoS One 9: e111053, 2014. 10.1371/journal.pone.0111053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD: Odor coding by a Mammalian receptor repertoire. Sci Signal 2: ra9, 2009. 10.1126/scisignal.2000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepard BD, Cheval L, Peterlin Z, Firestein S, Koepsell H, Doucet A, Pluznick JL: A renal olfactory receptor aids in kidney glucose handling. Sci Rep 6: 35215, 2016. 10.1038/srep35215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClintock TS, Wang Q, Sengoku T, Titlow WB, Breheny P: Mixture and concentration effects on odorant receptor response patterns in vivo. Chem Senses 45: 429–438, 2020. 10.1093/chemse/bjaa032 [DOI] [PubMed] [Google Scholar]
- 24.Abaffy T, Matsunami H, Luetje CW: Functional analysis of a mammalian odorant receptor subfamily. J Neurochem 97: 1506–1518, 2006. 10.1111/j.1471-4159.2006.03859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buck, LB: Unraveling the sense of smell (Nobel lecture). Angew Chem Int Ed Engl 44: 6128–6140, 2005. 10.1002/anie.200501120 [DOI] [PubMed] [Google Scholar]
- 26.Glusman G, Yanai I, Rubin I, Lancet D: The complete human olfactory subgenome. Genome Res 11: 685–702, 2001. 10.1101/gr.171001 [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Firestein S: The olfactory receptor gene superfamily of the mouse. Nat Neurosci 5: 124–133, 2002. 10.1038/nn800 [DOI] [PubMed] [Google Scholar]
- 28.Glusman G, Bahar A, Sharon D, Pilpel Y, White J, Lancet D: The olfactory receptor gene superfamily: Data mining, classification, and nomenclature. Mamm Genome 11: 1016–1023, 2000. 10.1007/s003350010196 [DOI] [PubMed] [Google Scholar]
- 29.Godfrey PA, Malnic B, Buck LB: The mouse olfactory receptor gene family. Proc Natl Acad Sci USA 101: 2156–2161, 2004. 10.1073/pnas.0308051100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belluscio L, Gold GH, Nemes A, Axel R: Mice deficient in G(olf) are anosmic. Neuron 20: 69–81, 1998. 10.1016/s0896-6273(00)80435-3 [DOI] [PubMed] [Google Scholar]
- 31.Lowe G, Nakamura T, Gold GH: Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proc Natl Acad Sci U S A 86: 5641–5645, 1989. 10.1073/pnas.86.14.5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong ST, Trinh K, Hacker B, Xia Z, Gold GH, Storm DR: Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27: 487–497, 2000. 10.1016/S0896-6273(00)00060-X [DOI] [PubMed] [Google Scholar]
- 33.Abaffy T, Malhotra A, Luetje CW: The molecular basis for ligand specificity in a mouse olfactory receptor: A network of functionally important residues. J Biol Chem 282: 1216–1224, 2007. 10.1074/jbc.M609355200 [DOI] [PubMed] [Google Scholar]
- 34.Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA: Genes that confer the identity of the renin cell. J Am Soc Nephrol 22: 2213–2225, 2011. 10.1681/ASN.2011040401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flegel C, Manteniotis S, Osthold S, Hatt H, Gisselmann G: Expression profile of ectopic olfactory receptors determined by deep sequencing. PLoS One 8: e55368, 2013. 10.1371/journal.pone.0055368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. 10.1681/ASN.2014111067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bugaut M: Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B 86: 439–472, 1987. 10.1016/0305-0491(87)90433-0 [DOI] [PubMed] [Google Scholar]
- 38.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ: Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20: 159–166, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL: Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics 48: 826–834, 2016. 10.1152/physiolgenomics.00089.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colombo I, Aiello-Battan F, Elena R, Ruiz A, Petraglia L, Musso CG: Kidney-gut crosstalk in renal disease [published online ahead of print November 20, 2020]. Ir J Med Sci 10.1007/s11845-020-02437-7 [DOI] [PubMed] [Google Scholar]
- 41.Mahmoodpoor F, Rahbar Saadat Y, Barzegari A, Ardalan M, Zununi Vahed S: The impact of gut microbiota on kidney function and pathogenesis. Biomed Pharmacother 93: 412–419, 2017. 10.1016/j.biopha.2017.06.066 [DOI] [PubMed] [Google Scholar]
- 42.Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, Bassi EJ, Moraes-Vieira PM, Hiyane MI, Rodas AC, Peron JP, Aguiar CF, Reis MA, Ribeiro WR, Valduga CJ, Curi R, Vinolo MA, Ferreira CM, Câmara NO: Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol 26: 1877–1888, 2015. 10.1681/ASN.2014030288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mainland JD, Li YR, Zhou T, Liu WL, Matsunami H: Human olfactory receptor responses to odorants. Sci Data 2: 150002, 2015. 10.1038/sdata.2015.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Britz ML, Wilkinson RG: Leucine dissimilation to isovaleric and isocaproic acids by cell suspensions of amino acid fermenting anaerobes: The Stickland reaction revisited. Can J Microbiol 28: 291–300, 1982. 10.1139/m82-043 [DOI] [PubMed] [Google Scholar]
- 45.Vockley J, Ensenauer R: Isovaleric acidemia: New aspects of genetic and phenotypic heterogeneity. Am J Med Genet C Semin Med Genet 142C: 95–103, 2006. 10.1002/ajmg.c.30089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim KS, Lee IS, Kim KH, Park J, Kim Y, Choi JH, Choi JS, Jang HJ: Activation of intestinal olfactory receptor stimulates glucagon-like peptide-1 secretion in enteroendocrine cells and attenuates hyperglycemia in type 2 diabetic mice. Sci Rep 7: 13978, 2017. 10.1038/s41598-017-14086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li E, Shan H, Chen L, Long A, Zhang Y, Liu Y, Jia L, Wei F, Han J, Li T, Liu X, Deng H, Wang Y: OLFR734 mediates glucose metabolism as a receptor of asprosin. Cell Metab 30: 319–328.e8, 2019. 10.1016/j.cmet.2019.05.022 [DOI] [PubMed] [Google Scholar]
- 48.Tong T, Ryu SE, Min Y, de March CA, Bushdid C, Golebiowski J, Moon C, Park T: Olfactory receptor 10J5 responding to α-cedrene regulates hepatic steatosis via the cAMP-PKA pathway. Sci Rep 7: 9471, 2017. 10.1038/s41598-017-10379-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C, Thach TT, Kim YJ, Lee SJ: Olfactory receptor 43 reduces hepatic lipid accumulation and adiposity in mice. Biochim Biophys Acta Mol Cell Biol Lipids 1864: 489–499, 2019. 10.1016/j.bbalip.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 50.Gerich JE: Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet Med 27: 136–142, 2010. 10.1111/j.1464-5491.2009.02894.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mather A, Pollock C: Glucose handling by the kidney. Kidney Int Suppl 79: S1–S6, 2011. 10.1038/ki.2010.509 [DOI] [PubMed] [Google Scholar]
- 52.Shepard BD, Pluznick JL: Saving the sweetness: Renal glucose handling in health and disease. Am J Physiol Renal Physiol 313: F55–F61, 2017. 10.1152/ajprenal.00046.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertero E, Prates Roma L, Ameri P, Maack C: Cardiac effects of SGLT2 inhibitors: The sodium hypothesis. Cardiovasc Res 114: 12–18, 2018. 10.1093/cvr/cvx149 [DOI] [PubMed] [Google Scholar]
- 54.Cefalo CMA, Cinti F, Moffa S, Impronta F, Sorice GP, Mezza T, Pontecorvi A, Giaccari A: Sotagliflozin, the first dual SGLT inhibitor: Current outlook and perspectives. Cardiovasc Diabetol 18: 20, 2019. 10.1186/s12933-019-0828-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallo LA, Wright EM, Vallon V: Probing SGLT2 as a therapeutic target for diabetes: Basic physiology and consequences. Diab Vasc Dis Res 12: 78–89, 2015. 10.1177/1479164114561992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabino-Silva R, Mori RC, David-Silva A, Okamoto MM, Freitas HS, Machado UF: The Na(+)/glucose cotransporters: From genes to therapy. Braz J Med Biol Res 43: 1019–1026, 2010. 10.1590/s0100-879x2010007500115 [DOI] [PubMed] [Google Scholar]
- 57.Zeni L, Norden AGW, Cancarini G, Unwin RJ: A more tubulocentric view of diabetic kidney disease. J Nephrol 30: 701–717, 2017. 10.1007/s40620-017-0423-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shepard BD, Koepsell H, Pluznick JL: Renal olfactory receptor 1393 contributes to the progression of type 2 diabetes in a diet-induced obesity model. Am J Physiol Renal Physiol 316: F372–F381, 2019. 10.1152/ajprenal.00069.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shepard BD: Sex differences in diabetes and kidney disease: Mechanisms and consequences. Am J Physiol Renal Physiol 317: F456–F462, 2019. 10.1152/ajprenal.00249.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boesveldt S, Postma EM, Boak D, Welge-Luessen A, Schöpf V, Mainland JD, Martens J, Ngai J, Duffy VB: Anosmia: A clinical review. Chem Senses 42: 513–523, 2017. 10.1093/chemse/bjx025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.da Silva Júnior PR, Gomes ALOR, Coelho LEA, Morais MA, de Almeida PVFC, Neri WJR, Mascena GV, de Farias Leal AA: Anosmia and COVID-19: Perspectives on its association and the pathophysiological mechanisms involved. Egypt J Neurol Psychiat Neurosurg 57: 8, 2021. 10.1186/s41983-020-00266-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rebholz H, Braun RJ, Ladage D, Knoll W, Kleber C, Hassel AW: Loss of olfactory function-early indicator for Covid-19, other viral infections and neurodegenerative disorders. Front Neurol 11: 569333, 2020. 10.3389/fneur.2020.569333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santos REA, da Silva MG, do Monte Silva MCB, Barbosa DAM, Gomes ALDV, Galindo LCM, da Silva Aragão R, Ferraz-Pereira KN: Onset and duration of symptoms of loss of smell/taste in patients with COVID-19: A systematic review. Am J Otolaryngol 42: 102889, 2021. 10.1016/j.amjoto.2020.102889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerslake R, Hall M, Randeva HS, Spandidos DA, Chatha K, Kyrou I, Karteris E: Co-expression of peripheral olfactory receptors with SARS-CoV-2 infection mediators: Potential implications beyond loss of smell as a COVID-19 symptom. Int J Mol Med 46: 949–956, 2020. 10.3892/ijmm.2020.4646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee SP, Wu WY, Hsiao JK, Zhou JH, Chang HH, Chien CT: Aromatherapy: Activating olfactory calcium-sensing receptors impairs renal hemodynamics via sympathetic nerve-mediated vasoconstriction. Acta Physiol (Oxf) 225: e13157, 2019. 10.1111/apha.13157 [DOI] [PubMed] [Google Scholar]
- 66.Pluznick JL, Rodriguez-Gil DJ, Hull M, Mistry K, Gattone V, Johnson CA, Weatherbee S, Greer CA, Caplan MJ: Renal cystic disease proteins play critical roles in the organization of the olfactory epithelium. PLoS One 6: e19694, 2011. 10.1371/journal.pone.0019694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park BB, Lee N, Kim Y, Jae Y, Choi S, Kang N, Hong YR, Ok K, Cho J, Jeon YH, Lee EH, Byun Y, Koo J: Analogues of dehydroacetic acid as selective and potent agonists of an ectopic odorant receptor through a combination of hydrophilic and hydrophobic interactions. ChemMedChem 12: 477–482, 2017. 10.1002/cmdc.201600612 [DOI] [PubMed] [Google Scholar]
- 68.Weber L, Schulz WA, Philippou S, Eckardt J, Ubrig B, Hoffmann MJ, Tannapfel A, Kalbe B, Gisselmann G, Hatt H: Characterization of the olfactory receptor OR10H1 in human urinary bladder cancer. Front Physiol 9: 456, 2018. 10.3389/fphys.2018.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Weng J, Cai Y, Penland R, Liu M, Ittmann M: The prostate-specific G-protein coupled receptors PSGR and PSGR2 are prostate cancer biomarkers that are complementary to alpha-methylacyl-CoA racemase. Prostate 66: 847–857, 2006. 10.1002/pros.20389 [DOI] [PubMed] [Google Scholar]