Sodium-glucose cotransporter 2 inhibitors (SGLT2is) have emerged as an effective class of medications to treat CKD and heart failure. SGLT2is improve cardiovascular outcomes in patients with type 2 diabetes mellitus (1–3) and in those with heart failure with reduced ejection fraction (4–7). Moreover, they have been shown to attenuate kidney disease progression in patients with proteinuric CKD, irrespective of diabetes status (8,9). The rapid uptake of SGLT2is into practice necessitates a careful understanding of their risks. Furthermore, clinicians need to know what to expect when prescribing these agents, including an early decline in eGFR after initiation. We aim to put this observation into context, while providing clinicians with a practical approach to managing this scenario.
The suggested mechanism of action of the SGLT2is has previously been described (10). These drugs inhibit sodium and glucose reabsorption in the proximal tubule, leading to increased sodium and chloride delivery to the macula densa. This results in afferent arteriolar vasoconstriction secondary to adenosine-mediated myogenic activation, leading to a reduction in the intraglomerular pressure and the GFR (10). Therefore, it is not surprising that the major SGLT2i outcome trials have reported an early decline in eGFR (around 3–6 ml/min per 1.73 m2) shortly after initiating these drugs compared with placebo controls (5,8,9,11,12). These early declines or dips were typically observed at 2–4 weeks after initiation of the SGLT2i, with subsequent partial recovery of the eGFR curve by week 12, and, ultimately, followed by an attenuation of the slope of eGFR decline compared with placebo controls after 52 weeks (Table 1).
Table 1.
Randomized controlled trials reporting an initial dip of eGFR
| Trial Name | Agent Studied | Primary Outcomes | Observed Early Drop in eGFR |
| CREDENCE (8) | Canagliflozin | Reduction in the composite risk of ESKD, doubling serum creatinine level, or death from renal or cardiovascular causes (HR, 0.70; 95% CI, 0.59 to 0.82), compared with placebo. | 5 ml/min per 1.73 m2 |
| DAPA-CKD (9) | Dapagliflozin | Reduction in the risk of 50% eGFR decline, ESKD, or death from renal or cardiovascular causes (HR, 0.61; 95% CI, 0.51 to 0.72), compared with placebo. | 4 ml/min per 1.73 m2 |
| EMPEROR-Reduced (5) | Empagliflozin | Reduction of the risk of cardiovascular death or hospitalization for worsening heart failure (HR, 0.75; 95% CI, 0.65 to 0.86), compared with placebo. | 4 ml/min per 1.73 m2 |
| EMPA-REG Outcome (11) | Empagliflozin | Canagliflozin decreased the risk of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (HR, 0.86; 95% CI, 0.74 to 0.99), compared with placebo. | 3–4 ml/min per 1.73 m2 |
| CANTATA-SU (12) | Canagliflozin | Canagliflozin slowed the progression of kidney disease compared with glimepiride in patients with type 2 DM (P<0.01 for each canagliflozin group versus glimepiride). | 3–6 ml/min per 1.73 m2 |
CREDENCE, Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; HR, hazard ratio; DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes in CKD; EMPEROR-Reduced, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction; EMPA-REG Outcome, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; CANTATA-SU, Canagliflozin Treatment and Trial Analysis–Sulfonylurea; DM, diabetes mellitus.
How can we reconcile early dips in eGFR with long-term nephroprotection? Maladaptive glomerular hemodynamics play a central role in kidney disease progression (13). Single nephron hyperfiltration and increased glomerular capillary pressure occur in response to a diminished number of functional nephrons, regardless of the cause. The resulting proteinuria and glomerulosclerosis is the common final pathway for various kidney diseases (13). Hyperfiltration is frequently seen in diabetic kidney disease, and often precedes the development of overt albuminuria (14,15). Therefore, mitigation of pathologic hyperfiltration may be therapeutic, even at the cost of an acute eGFR decline. These data lend credence to the somewhat counterintuitive notion that agents that cause initial decrements in eGFR have long-term therapeutic benefits for patients with proteinuric kidney disease.
In clinical practice, SGLT2i prescribers, who will frequently be non-nephrologists (16), may be uncomfortable watching the serum creatinine rise in response to initiating these agents. This anxiety may have been inadvertently heightened by a sensitivity to “causing” AKI. The Kidney Disease Improving Global Outcomes Clinical Practice Guidelines (KDIGO) define AKI as an acute rise in serum creatinine from baseline of ≥0.3 mg/dl in 48 hours, or ≥1.5× within 1 week (17). Therefore, it is conceivable that a newly started SGLT2i could potentially induce a rise in serum creatinine that would meet AKI criteria. However, one has to question whether a SGLT2i-driven drop in eGFR is genuinely representative of AKI. Contemporary thresholds for defining AKI were guided by data from hospitalized patients in whom relatively modest increments in serum creatinine were found to be associated with adverse outcomes (18). Serum creatinine rises of similar magnitude are unlikely to have the same significance in an otherwise stable outpatient who initiated an SGLT2i.
In a post hoc analysis of the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG Outcome) study, which showed that empagliflozin reduced the risk of major adverse cardiovascular events in patients with type 2 diabetes mellitus, Kraus et al. (19) performed a granular analysis of eGFR decrements after the initiation of study drugs. A postinitiation eGFR decline of ≥10% was considered significant “dipping,” and was observed in 28% of participants in the empagliflozin arm versus 13% in the placebo arm. Reassuringly, the patients with an early dip in eGFR partially recovered some of their “lost” GFR by week 12, with a net eGFR decline of 4–6 ml/min per 1.73 m2. However, it is unclear if this ostensible recovery resulted from medication adjustments in response to the initial dip. Most importantly, the presence of a post–drug initiation eGFR dip did not seem to diminish the protective effect of empagliflozin on cardiovascular outcomes. It remains to be seen if these findings will extend to patients with lower baseline eGFR and significant albuminuria, such as those enrolled in the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) and Dapagliflozin and Prevention of Adverse Outcomes in CKD trials (DAPA-CKD) (8,9). A theoretic extrapolation of the CREDENCE trial data would suggest that ESKD in a typical trial participant may be delayed by 15 years, even when accounting for the initial eGFR dip (Figure 1).
Figure 1.
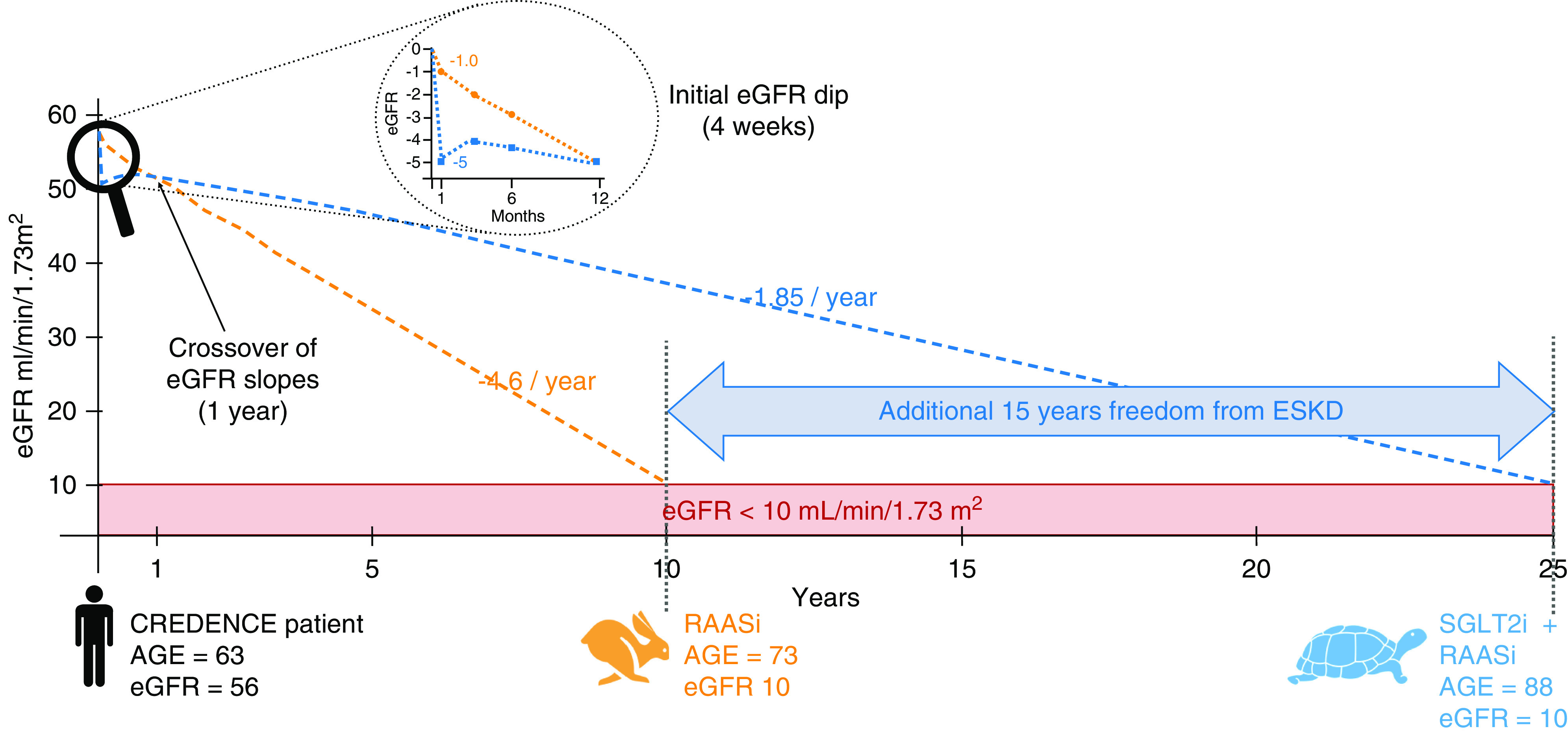
SGLT2is may delay ESKD by 15 years. A typical patient included in CREDENCE would lose 4.6 ml/min per year of eGFR if treated with RAASi only, reaching ESKD in 10 years. However, if canagliflozin is added to his treatment, he would only lose 1.85 ml/min per year of eGFR, delaying ESKD by 15 years. RAASi, renin-angiotensin-aldosterone system inhibitors; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
The seemingly paradoxic coexistence of eGFR declines and long-term clinical benefits has precedent in nephrology. This phenomenon is well described with renin-angiotensin-aldosterone system inhibitors (20,21). A reanalysis of the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation trial showed that acute increases in serum creatinine after commencing perindopril-indapamide were associated with more significant short-term risks of major macrovascular events, new or worsening nephropathy, and all-cause mortality. However, perindopril’s continuation reduced the long-term risk of major clinical outcomes, irrespective of the acute increase in serum creatinine, compared with patients who stopped the drug (22).
In a post hoc analysis of the Systolic BP Intervention Trial, 10% of patients randomized to the intensive BP control arm had an early decline (first 6 months) in eGFR of ≥20%. This decline did not have a negative effect on the overall benefits of intensive BP control (23). In the recently published Effect of Finerenone on CKD Outcomes in Type 2 Diabetes trial, patients randomized to finerenone experienced a steeper decline in eGFR that persisted until month 24, when the slope of eGFR crossed over that of the placebo group. At the end of follow-up, patients in the finerenone group had better kidney outcomes (for the composite outcome of kidney failure, a sustained decrease of at least 40% in the eGFR from baseline, or death from renal causes; hazard ratio, 0.82; 95% CI, 0.73 to 0.93) (24).
Conversely, maneuvers that increase kidney blood flow and eGFR have not been associated with better outcomes. A vivid example is bardoxolone, where an improvement in eGFR occurred, but this did not translate into a reduction in the risk of ESKD or death from cardiovascular causes (25,26). Collectively, these data support the notion of “permissive hypercreatinemia,” which broadly highlights the need to consciously accept a modest decline in eGFR as the cost of initiating and maintaining medications that have long-term benefits that are meaningful for patients (27).
How does a clinician respond when faced with an abrupt rise in serum creatinine after initiation of an SGLT2i? Although the data cited above would suggest that most such dips are merely expected hemodynamic changes of limited clinical relevance, it is essential to recognize that, in some cases, this may signal systemic illness (i.e., infection, occult bleeding) with bona fide kidney injury. Traditionally, a 30% increase in serum creatinine has prompted clinicians to re-evaluate renin-angiotensin-aldosterone system blockade, and we believe the same approach may be reasonable for SGLT2is (28,29). A significant increase in serum creatinine (>30% from baseline) should prompt a detailed clinical review to verify if the patient has suffered a volume-contracting illness (which may justify temporarily holding the SGLT2i and other medications that affect kidney hemodynamics), initiated new medications that may affect kidney function, or has another reason for AKI. In Figure 2, we propose an approach to navigating abrupt eGFR declines in a patient commencing an SGLT2i. The overarching goal is to maintain patients on therapy by addressing non-SGLT2i–related factors that may have precipitated the acute eGFR decline, and by adjusting the cardiorenal drug regimen to enable the safe, continued use of the SGLT2is and other therapies that have an established effect on patient-relevant outcomes.
Figure 2.
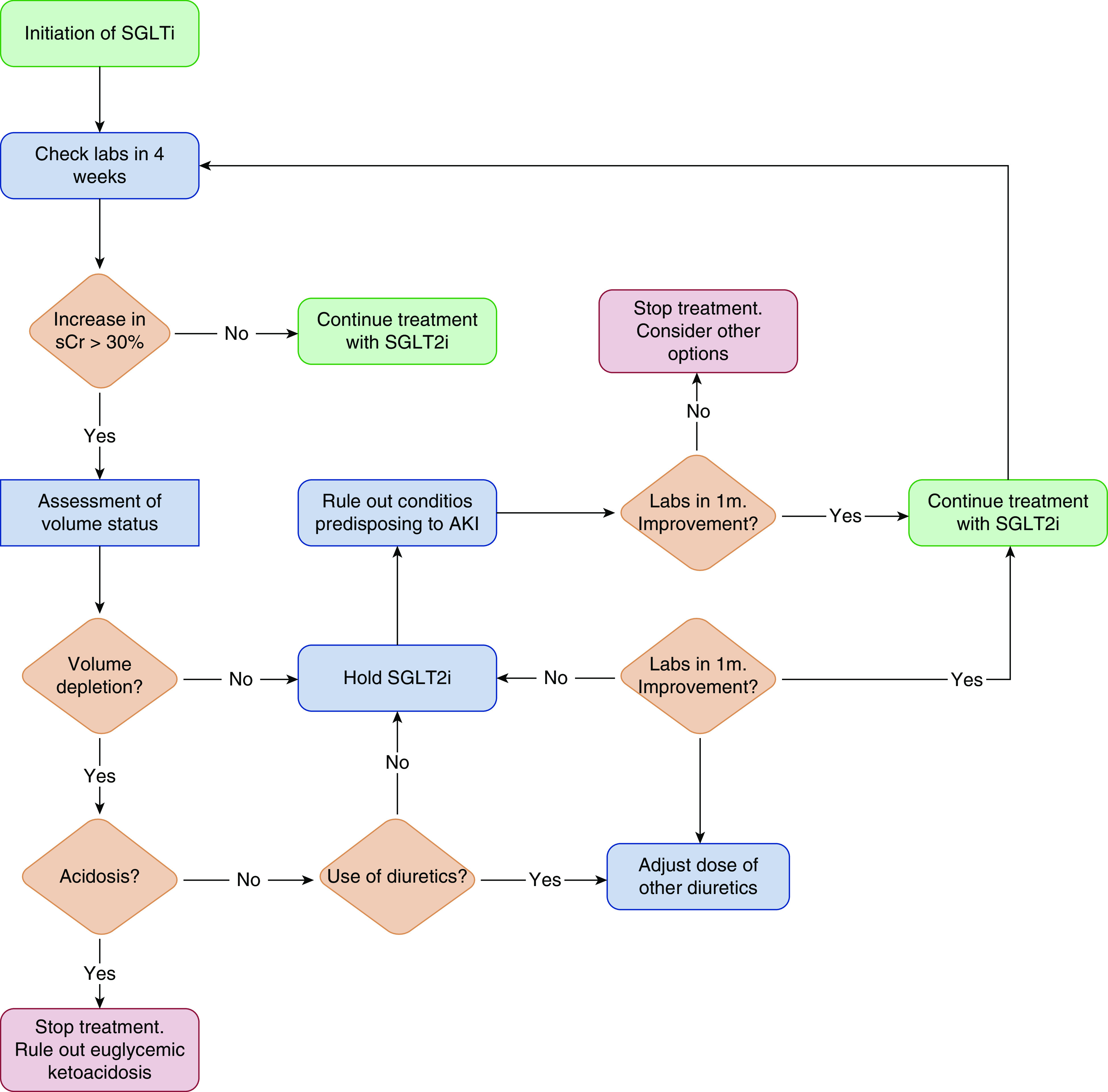
Proposed algorithm for initiation and surveillance of treatment with SGLT2is. SCr, serum creatinine.
The Tortoise and the Hare is perhaps the most famous of Aesop’s fables. It tells the story of a tenacious tortoise who defeated an overconfident hare in a race, demonstrating that enthusiasm and perseverance can prevail over hastiness and overconfidence. The race against ESKD is a marathon, not a sprint. We ought to be patient and persistent, much like the tortoise in Aesop’s fable, and set our eyes on the critical clinical outcomes, most notably, preventing ESKD and preventing cardiovascular events. We thus advocate resisting the urge of stopping SGLT2is when faced with an early modest dip in eGFR. More often than not, this acute dip will be mild and, even if not reversible, clinicians should avoid the urge to discontinue the SGLT2i. Ultimately, the prevention of kidney failure and cardiovascular events should take precedence over excursions in serum creatinine.
Disclosures
R. Wald reports receiving research funding from Baxter; serving on the editorial board of CJASN, Kidney360, and Kidney Medicine; and having other interests in/relationships with UpToDate as a contributor. J. Weinstein reports having consultancy agreements with, and receiving honoraria from, Amgen, AstraZeneca, Boehringer Ingelheim, Janssen, and Lilly. The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
We would like to thank Dr. Steven Coca for inviting our group to contribute to Kidney360 and to Dr. Aldo R. Jimenez for his help in the conceptualization of Figures 1 and 2. The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Author Contributions
A.Y. Meraz-Muñoz conceptualized the study, was responsible for data curation, and wrote the original draft; A.Y. Meraz-Muñoz, R. Wald, and J. Weinstein reviewed and edited the manuscript; and R. Wald and J. Weinstein provided supervision.
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators: Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 2.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group: Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators: Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347–357, 2019. 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators: Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381: 1995–2008, 2019. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 5.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators: Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 383: 1413–1424, 2020. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 6.Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, Cherney DZI, Dwyer JP, Scirica BM, Bailey CJ, Díaz R, Ray KK, Udell JA, Lopes RD, Lapuerta P, Steg PG; SCORED Investigators: Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 384: 129–139, 2021. 10.1056/NEJMoa2030186 [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST-WHF Trial Investigators: Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 384: 117–128, 2021. 10.1056/NEJMoa2030183 [DOI] [PubMed] [Google Scholar]
- 8.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 9.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators: Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 10.Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZI: Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134: 752–772, 2016. 10.1161/CIRCULATIONAHA.116.021887 [DOI] [PubMed] [Google Scholar]
- 11.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators: Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016. 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 12.Heerspink HJL, Desai M, Jardine M, Balis D, Meininger G, Perkovic V: Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 28: 368–375, 2017. 10.1681/ASN.2016030278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner BM, Lawler EV, Mackenzie HS: The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int 49: 1774–1777, 1996. 10.1038/ki.1996.265 [DOI] [PubMed] [Google Scholar]
- 14.Mogensen CE: Early glomerular hyperfiltration in insulin-dependent diabetics and late nephropathy. Scand J Clin Lab Invest 46: 201–206, 1986. 10.3109/00365518609083660 [DOI] [PubMed] [Google Scholar]
- 15.Alicic RZ, Rooney MT, Tuttle KR: Diabetic kidney disease: Challenges, progress, and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017. 10.2215/CJN.11491116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaduganathan M, Sathiyakumar V, Singh A, McCarthy CP, Qamar A, Januzzi JL Jr, Scirica BM, Butler J, Cannon CP, Bhatt DL: Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol 72: 3370–3372, 2018. 10.1016/j.jacc.2018.08.2202 [DOI] [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Inter 2[Suppl]: 1–138, 2012 [Google Scholar]
- 18.Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM: Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol 13: 1350–1357, 2002. 10.1097/01.ASN.0000014692.19351.52 [DOI] [PubMed] [Google Scholar]
- 19.Kraus BJ, Weir MR, Bakris GL, Mattheus M, Cherney DZI, Sattar N, Heerspink HJL, Ritter I, von Eynatten M, Zinman B, Inzucchi SE, Wanner C, Koitka-Weber A: Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int 99: 750–762, 2021. 10.1016/j.kint.2020.10.031 [DOI] [PubMed] [Google Scholar]
- 20.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001. 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 21.Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ; ACCOMPLISH Trial Investigators: Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 359: 2417–2428, 2008. 10.1056/NEJMoa0806182 [DOI] [PubMed] [Google Scholar]
- 22.Ohkuma T, Jun M, Rodgers A, Cooper ME, Glasziou P, Hamet P, Harrap S, Mancia G, Marre M, Neal B, Perkovic V, Poulter N, Williams B, Zoungas S, Chalmers J, Woodward M; ADVANCE Collaborative Group: Acute increases in serum creatinine after starting angiotensin-converting enzyme inhibitor-based therapy and effects of its continuation on major clinical outcomes in type 2 diabetes mellitus. Hypertension 73: 84–91, 2019. 10.1161/HYPERTENSIONAHA.118.12060 [DOI] [PubMed] [Google Scholar]
- 23.Beddhu S, Shen J, Cheung AK, Kimmel PL, Chertow GM, Wei G, Boucher RE, Chonchol M, Arman F, Campbell RC, Contreras G, Dwyer JP, Freedman BI, Ix JH, Kirchner K, Papademetriou V, Pisoni R, Rocco MV, Whelton PK, Greene T: Implications of early decline in eGFR due to intensive BP control for cardiovascular outcomes in SPRINT. J Am Soc Nephrol 30: 1523–1533, 2019. 10.1681/ASN.2018121261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators: Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 383: 2219–2229, 2020. 10.1056/NEJMoa2025845 [DOI] [PubMed] [Google Scholar]
- 25.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG; BEAM Study Investigators: Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 365: 327–336, 2011. 10.1056/NEJMoa1105351 [DOI] [PubMed] [Google Scholar]
- 26.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM; BEACON Trial Investigators: Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369: 2492–2503, 2013. 10.1056/NEJMoa1306033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coca SG: Ptolemy and copernicus revisited: The complex interplay between the kidneys and heart failure. Clin J Am Soc Nephrol 13: 825–828, 2018. 10.2215/CJN.05090418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakris GL, Weir MR: Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: Is this a cause for concern? Arch Intern Med 160: 685–693, 2000. 10.1001/archinte.160.5.685 [DOI] [PubMed] [Google Scholar]
- 29.Bakris GL, Agarwal R: Creatinine bump following antihypertensive therapy. Hypertension 72: 1274–1276, 2018. 10.1161/HYPERTENSIONAHA.118.12051 [DOI] [PubMed] [Google Scholar]


