Abstract
Objective
Medicinal plants and essentials oils are well known for diverse biological activities including antidiabetic potential. This study was designed to isolate essential oils from the leaves of Persicaria hydropiper L. (P. hydropiper), perform its phytochemical analysis, and explore its in vitro antidiabetic effects.
Materials and Methods
P. hydropiper leaves essential oils (Ph.Los) were extracted using a hydrodistillation apparatus and were subjected to phytochemical analysis using the gas chromatography mass spectrometry (GC-MS) technique. Ph.Lo was tested against two vital enzymes including α-glucosidase and α-amylase which are important targets in type-2 diabetes. The identified compounds were tested using in silico approaches for their binding affinities against the enzyme targets using MOE-Dock software.
Results
GC-MS analysis revealed the presence of 141 compounds among which dihydro-alpha-ionone, cis-geranylacetone, α-bulnesene, nerolidol, β-caryophyllene epoxide, and decahydronaphthalene were the most abundant compounds. Ph.Lo exhibited considerable inhibitory potential against α-glucosidase enzyme with 70% inhibition at 1000 μg mL−1 which was the highest tested concentration. The inhibitory activity of positive control acarbose was 77.30 ± 0.61% at the same tested concentration. Ph.Lo and acarbose exhibited IC50 of 170 and 18 µg mL−1 correspondingly. Furthermore, dose-dependent inhibitions were observed for Ph.Lo against α-amylase enzyme with an IC50 of 890 μg mL−1. The top-ranked docking conformation was observed for β-caryophyllene epoxide with a docking score of -8.3182 against α-glucosidase, and it has established seven hydrogen bonds and one H-pi interaction at the active site residues (Phe 177, Glu 276, Arg 312, Asp 349, Gln 350, Asp 408, and Arg 439). Majority of the identified compounds fit well in the binding pocket of Tyr 62, Asp 197, Glu 233, Asp 300, His 305, and Ala 307 active residues of α-amylase. β-Caryophyllene epoxide was found to be the most active inhibitor with a docking score of -8.3050 and formed five hydrogen bonds at the active site residues of α-amylase. Asp 197, Glu 233, and Asp 300 active residues were observed to be making polar interactions with the ligand.
Conclusions
The current study revealed that Ph.Lo is rich in bioactive metabolites which might contribute to its enzyme inhibitory potential. Inhibition of these enzymes is the key target in reducing postprandial hyperglycemia. However, further detailed in vivo studies are required for their biological and therapeutic activities.
1. Introduction
Diabetes mellitus (DM) is a metabolic syndrome associated with hyperglycemia due to the body's inability to produce sufficient amount of insulin or abnormalities in its secretion or tissue resistance to its action [1, 2]. Hyperglycemia in DM may also occur due to defects in the metabolic processes involved in processing carbohydrates, proteins, and fats [3, 4]. This results in development of some classical symptoms including polyuria, polydipsia, and polyphagia [5]. These metabolic abnormalities are due to low insulin level or resistance of target tissues (adipose tissue, skeletal muscles, and liver) to insulin at the level of signal transduction, insulin receptors, genes, or effecter enzymes [6]. In DM, elevated level of blood glucose for a long time is associated with a number of acute or chronic complications [7]. Globally, it has been estimated that the occurrence of diabetes has increased from 4% in 1995 to 5.4% by the year 2025 [8]. The overall prevalence as reported by the International Diabetes Federation (IDF) in 2011 was increased to 366 million people and is supposed to increase up to 552 million people by the year 2030 [9]. Furthermore, it has also been reported that 450 million people have been suffering from DM globally and the prevalence is expected to rise to 690 million by the year 2044 [10].
Regarding type-2 diabetes, targeting enzymes involved in processing dietary carbohydrates in the intestinal tract is among the vital targets. Among these, α-amylase and α-glucosidase enzymes are of high pharmacological interest and are used to control elevated glucose level in T2DM. These enzymes cause metabolic breakdown of complex dietary carbohydrates to simple sugars which are subsequently absorbed [11]. Long-chain carbohydrates are broken down into glucose by alpha-amylase enzyme, whereas α-glucosidase is responsible for the breakdown of disaccharides and starch into simpler monosaccharide glucose, resulting in hyperglycemia [12].
The use of medicinal plants and natural products is still a major source of therapy in the developing countries [13–15]. The discovery of modern analytical techniques has further eased the process of ethnomedicinal drug discovery to identify, isolate, and characterize target molecules [16–18]. Approximately more than four hundred plants are identified having antidiabetic potential, but only few of these plants have received medical and scientific evaluation [19]. A large number of α-amylase and α-glucosidase inhibitors are produced by different microorganisms and plants to regulate the activities of these enzymes [20]. The natural α-glucosidase inhibitors from plant sources, whose α-glucosidase inhibitory activities have been reported previously, include alkaloids, flavonoids, anthocyanins, terpenoids, curcuminoids, and phenolic compounds [21]. Miglitol, voglibose, and acarbose are the only three α-glucosidase inhibitors which are in clinical practice presently for the treatment of patients with T2DM [22].
Persicaria hydropiper L. belongs to the family Polygonaceae (smartweed family) which consists of about fifty genera and twelve hundred species. It is ethnopharmacologically famous for its use as a diuretic, anti-inflammatory agent, stomachic, central nervous system (CNS) stimulant, and natural remedy in other gastrointestinal disorders [23]. P. hydropiper contains flavonoids, chalcone derivatives, phenylpropanoid derivatives, phenolic compounds, anthraquinone, isocoumarin, terpenoids, and steroids [24]. Previously, crude extracts and isolated compounds were reported for neuroprotective [25, 26], cytotoxic [27, 28], antimicrobial [29], gastroprotective [30], and toxicological potential [23, 31]. The current study aimed to isolate essential oils from the leaves of P. hydropiper and evaluate its detailed composition via gas chromatography mass spectrometry (GC-MS). Also, the study analyses the essential oils against two important targets of the type-2 diabetes, α-glucosidase and α-amylase and dock the identified compounds against these enzymes.
2. Materials and Methods
2.1. Plant Collection and Extraction of Essential Oils from Leaves
Fresh leaves from the plant were collected in 2014 from the village of Talash (Dir), KP Pakistan, and authenticated via a botanical taxonomist and curator at the botanical garden in the University of Malakand. For preservation, dried compressed leaves were submitted to the herbarium with reference no H.UOM.BG.107. Fresh leaves were then carefully rinsed using distilled water and were processed via a Clevenger apparatus to isolate essential oils [32]. In brief, leaves were macerated followed by hydrodistillation in a Clevenger apparatus coupled with a condenser. Hydrodistillation was continued for three days at 100°C until a sufficient amount of essential oil was collected. Yellowish oil was collected in air-tight glass bottles and was refrigerated before being used for analysis and other assays.
2.2. GC-MS Analysis
GC-MS analysis of essential oils was performed via an Agilent USB-393752 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) having a HHP-5MS 5% phenylmethylsiloxane capillary column (Restek, Bellefonte, PA) with 30 m × 0.25 mm × 0.25 μm film thickness and coupled with a mass spectrometer. Initially, oven temperature was sustained at 70°C for one minute, gradually increased to 180°C (at 6°C/min increase), and finally maintained at 280°C for twenty minutes. Temperatures of both the injector and detector were set at 220°C and 290°C, respectively. Helium was used as the carrier gas with a flow rate of 1 ml/min, and diluted Ph.Lo samples (1/1000 in n-pentane, v/v) were injected in the split-less mode. Components of the Ph.Lo were identified via comparison of their retention time (RT) with already reported spectral data in NIST, NIH, and Wiley libraries [33]. Moreover, comparison of the fragmentation pattern of mass spectra was done with the published literature [34].
2.3. α-Glucosidase Inhibitory Studies
Enzyme inhibitory potential of Ph.Lo was obtained according to the previously reported standard protocol [35]. Baker's yeast alpha-glucosidase, substrate (P-nitrophenyl-α-D-glucopyranoside), and control (acarbose) were acquired from authentic sources of Sigma Aldrich (USA). Enzyme solution (100 mM) was prepared using phosphate buffer of pH 6.8. Ph.Lo solutions were prepared using a small amount of surfactants (31.25–1000 μg mL−1) in 320 μl of 100 mM phosphate buffer and were kept for five minutes at 30°C. Subsequently, 3 ml (50 mM) of NaOH solution was mixed with it, and using a spectrophotometer, absorbency rates were recorded at 410 nm. Control solution consisted of all ingredients except the inhibitor (sample). Positive control was acarbose. Percent enzyme inhibitions were derived from the data using the given formula.
| (1) |
2.4. α-Amylase Inhibitory Studies
Likewise, α-amylase inhibitory studies were performed following the already established procedure [12]. In brief, 20 μl enzyme was mixed in 200 μl of 0.02 M sodium phosphate buffer mixed with the plant extracts (test compounds) of varying concentration ranges of 31.25–1000 μg m L−1. The assay mixtures were then maintained at 25 ± 3°C for about ten minutes, and 200 μl of starch was added to it. To terminate the reaction, 400 μl of DNS reagent (dinitrosalicylic acid) was transferred to the mixture. The resultant solution was kept in a boiling water bath for five minutes and cooled. After cooling, 15 ml of distilled water was added to dilute the mixture and the absorbance was noted at 540 nm. Standard drug was acarbose, and enzyme inhibition was determined via the formula.
2.5. Molecular Docking Studies
The identified compounds were docked for their binding capacity in the enzymes protein pocket via MOE-Dock tool in molecular operating environment (MOE) (http://www.chemcomp.com) [36, 37]. Due to unavailability of α-glucosidase crystal structure, a previously reported homology model was used [38], whereas the α-amylase (4W93) 3D crystal structure was obtained from the Protein Databank (PDB). Before starting the docking process, the water molecules and ions present in crystal structures were removed via MOE. Thereafter, protein structures were added to hydrogen atoms via 3D protonation with subsequent minimization of energy via MOE default parameters including the gradient of 0.05 and Force Field Amber99.
Target compound structures were generated in MOE, and using the software default parameters, the energy was minimized. The selected enzymes including α-glucosidase and α-amylase subjected to docking with the identified compounds via the MOE parameters including Placement: Triangle Matcher, Rescoring: London dG. At least 10 confirmations were generated for every ligand. Subsequently, for each compound, top-ranked confirmations were developed and were subjected to further analysis. Finally, those docking results having comparatively good poses with polar, arene-arene, H-pi, and pi-H interactions were analyzed via Pymol software.
2.6. Statistical Analysis
Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Dunnett's test. The results are presented as the means ± SEM of triplicate observations. P values < 0.05 were considered as statistically significant. GraphPad Prism software (version 5) (USA) was used for the data analysis and figure creation.
3. Results and Discussion
3.1. GC-MS Analysis
In the GC-MS study, 141 compounds were recognized (Table S1), among which the most abundant compounds (File S1) were β-elemene (RT: 14.359, height%: 39.24, area%: 17.79, m/z: 81.1), dihydro-alpha-ionone (RT: 14.822, height%: 8.68, area%: 3.52, m/z: 43.1), cis-geranylacetone (RT: 15.505, height%: 21.89, area%: 9.7, m/z: 43.1), alpha-bulnesene (RT: 16.382, height%: 14.39, area%: 6.67, m/z: 93.1), bicyclo[4.1.0]heptane,-3-cyclopropyl,-7-hydroxymethyl, trans (RT: 17.722, height%: 12.08, area%: 7.4, m/z: 79.1), nerolidol (RT: 17.838, height%: 13.14, area%: 5.17, m/z: 69.1), bicyclo[2.2.2]oct-2-ene, 1,2,3,6-tetramethyl (RT: 18.449, height%: 94.65, area%: 94.88, m/z: 79.1), (1R,5S,8R,9 R)-4,4,8-trimethyltricyclo [6.3.1.0(1,5)] dodeca-2-en-9-ol (RT: 18.482, height%: 17.96, area%: 2.3, m/z: 161.1), β-caryophyllene epoxide (RT: 18.663, height%: 16.02, area%: 7.02, m/z: 83), and decahydronaphthalene (RT: 18.951, height%: 100, area%: 100, m/z:109.1) (Figure 1).
Figure 1.
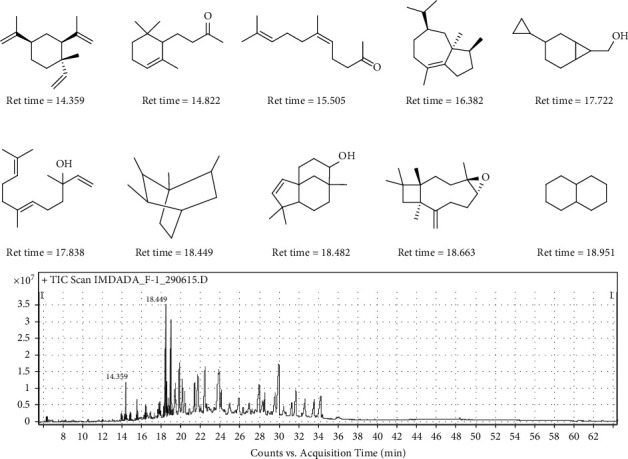
Representative image for the most abundant identified compounds.
3.2. Enzyme Inhibition Studies
3.2.1. Ph.Lo Exhibited Concentration-Dependent α-Glucosidase Inhibition
In the present study, Ph.Lo was found to be highly active against α-glucosidase enzyme as shown in Figure 2. Ph.Lo showed inhibition rates of 70.00 ± 0.00, 63.66 ± 1.20, 59.16 ± 0.60, 53.00 ± 1.15, 47.37 ± 0.65, and 41.33 ± 1.30% at selected doses of 1000, 500, 250, 125, 62.50, and 31.25 μg mL−1 correspondingly. The standard drug acarbose inhibitory activity showed 77.30 ± 0.61, 73.00 ± 0.00, 69.00 ± 0.00, 55.50 ± 1.04, 49.83 ± 0.44, and 41.00 ± 0.00% using the abovementioned doses, respectively. For test (Ph.Lo) and control (acarbose), IC50 of 170 and 18 μg mL−1 was calculated.
Figure 2.
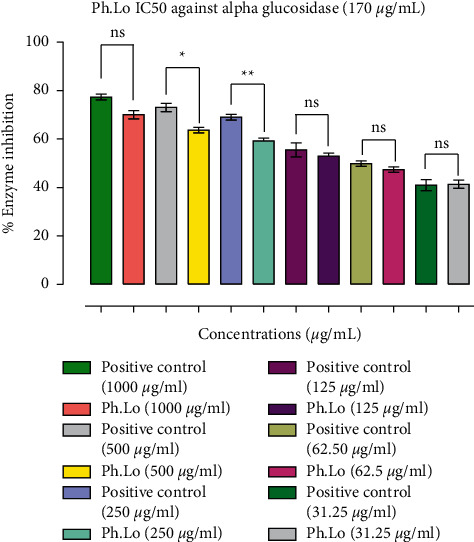
Results of α-glucosidase inhibition study. Data bars represent results from three independent experimental observations. Data are presented as means ± SEM. Values are significantly different (∗p < 0.05, ∗∗p < 0.01) when compared with positive control at the same tested concentrations. ns represents data groups not significantly different when compared with positive control.
3.2.2. Ph.Lo Exhibits Concentration-Dependent Inhibition against α-Amylase Enzyme
Results of alpha-amylase inhibitory potential of Ph.Lo are summarized in Figure 3. Enzyme inhibitory activity of the Ph.Lo was 70.36% at 1000 µg mL−1, 51.91% at 500 μg mL−1, 42.66% at 250 μg mL−1, 32.00% at 125 μg mL−1, 24.00% at 62.50 μg mL−1, and 14.50% at 31.25 μg mL−1. Positive control showed inhibition rates of 77.3% at 1000 μg mL−1, 73.00% at 500 μg mL−1, 69.00% at 250 μg mL−1, 55.50% at 125 μg mL−1, 49.83% at 62.50 μg mL−1, and 41.00% at 31.25 μg mL−1. Overall, concentration-dependent amylase inhibitory activities were observed for Ph.Lo as shown in Figure 3 at an IC50 of 890 μg mL−1.
Figure 3.
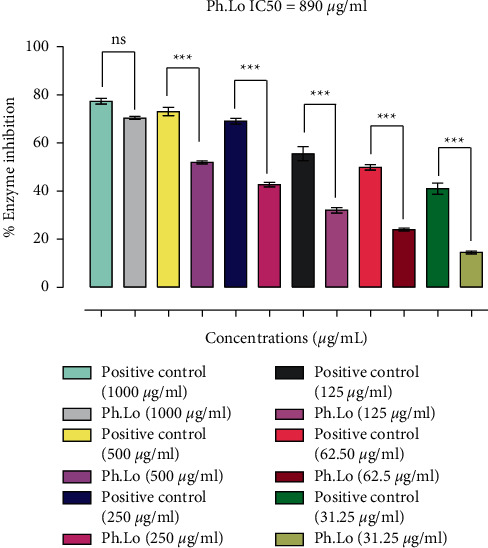
Results of α-amylase inhibition study. Data bars represent results from three independent experimental observations. Data are presented as means ± SEM. Values are significantly different (∗∗∗p < 0.001) when compared with positive control at the same tested concentrations. ns represents data groups not significantly different when compared with positive control.
In GC-MS characterization, 141 phytochemicals were identified, among which dihydro-alpha-ionone, cis-geranylacetone, alpha-bulnesene, nerolidol, β-caryophyllene epoxide, and decahydronaphthalene were the most abundant compounds. It has been suggested by Jabeen et al. that, in a molecule, the presence of lipophilic side chain is responsible for the inhibition of alpha-glucosidase enzymes [39]. Inhibitory potential of both glucosidase and amylase enzymes has been reported previously for various volatile oils including Eruca vesicaria subsp. longirostris. Here, erucin was suggested to inhibit alpha-glucosidase. Apart from erucin, it was also reported that β-elemene may inhibit alpha-glucosidase activity [40]. In essential oils, the presence of monoterpenes and sesquiterpenes may contribute to inhibition of both selected enzymes [41]. Alpha-pinene, germacrene D, drimenin, and drimane-type sesquiterpene lactone are the compounds in Hertia cheirifolia essential oils obtained from its leaves and flowers and were suggested to contribute α-amylase inhibitory activity [42]. Recently, it has also been reported by Majouli et al. that H. cheirifolia volatile oils possess inhibitory potential against α-glucosidase enzyme [43]. Apart from this, inhibitory activities against both selected enzymes were reported for Nepeta curviflora volatile oils [44]. In these essential oils, the major phytochemical constituents include caryophyllene oxide, 1,6-dimethyl spiro-decane, and β-caryophyllene which are suggested for their antiamylase and antiglucosidase activities. These compounds in addition to other bioactive metabolites were identified in Ph.Lo analysis and might contribute to the overall enzyme inhibitory potential.
3.2.3. Molecular Docking Studies against α-Amylase Enzyme
Binding of the selected compounds in the binding pocket was observed (Tyr 62, Asp 197, Glu 233, Asp 300, His 305, and Ala 307 active residues) for the α-amylase enzyme. Docking studies revealed that the β-caryophyllene epoxide is the most active inhibitor with a docking score of -8.3050 and formed five hydrogen bonds with the active site residues of α-amylase. Asp 197, Glu 233, and Asp 300 active residues were observed to be making polar interactions with the ligand (Figure 4).
Figure 4.
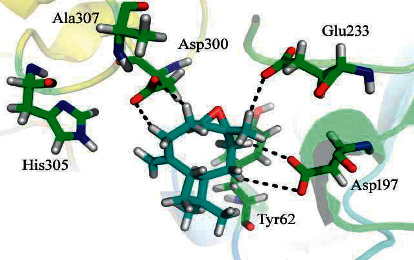
Docking conformation of β-caryophyllene epoxide with α-amylase.
Enzyme inhibition properties of the phytochemicals might be attributed to electron-donating group (-CH3) on the identified compound. The oxygen atom of the ligand might be implicated in the considerable in silico performance of the compound. Interaction reports of the remaining inhibitors are given in Table 1.
Table 1.
Results of the docking studies against α-amylase.
| Compounds | Ligand | Receptor | Interaction | Distance | E (kcal/mol) | Docking scores | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4-Thujanol | O | 28 | O | TYR | 62 | (A) | Hydrogen-donor | 2.98 | −1.3 | −7.1947 |
| O | 28 | NE2 | HIS | 101 | (A) | Hydrogen-acceptor | 3.01 | −1.5 | ||
| Alpha-bulnesene | C | 3 | OD1 | ASP | 197 | (A) | Hydrogen-donor | 3.93 | −0.1 | −7.3019 |
| C | 14 | OE1 | GLU | 233 | (A) | Hydrogen-donor | 3.63 | −0.1 | ||
| C | 33 | OD1 | ASP | 300 | (A) | Hydrogen-donor | 3.79 | −0.1 | ||
| Alpha-muurolene | C | 32 | 5-ring | HIS | 101 | (A) | Hydrogen-pi | 4.77 | −0.4 | −6.7334 |
| C | 36 | 5-ring | HIS | 299 | (A) | Hydrogen-pi | 4.59 | −0.3 | ||
| Beta-elemene | C | 24 | OD2 | ASP | 300 | (A) | Hydrogen-donor | 3.91 | −0.1 | −6.0798 |
| C | 24 | 5-ring | HIS | 305 | (A) | Hydrogen-pi | 4.74 | −0.1 | ||
| Beta-ocimene | C | 11 | O | TYR | 62 | (A) | Hydrogen-donor | 3.75 | −0.1 | −6.5892 |
| C | 11 | 5-ring | HIS | 101 | (A) | Hydrogen-pi | 4.03 | −0.1 | ||
| C | 19 | 5-ring | TRP | 59 | (A) | Hydrogen-pi | 4.66 | −0.3 | ||
| Bornyl acetate | C | 6 | O | TYR | 62 | (A) | Hydrogen-donor | 3.61 | −0.1 | −8.0205 |
| C | 6 | OD2 | ASP | 197 | (A) | Hydrogen-donor | 3.3 | −0.1 | ||
| C | 25 | OD1 | ASP | 300 | (A) | Hydrogen-donor | 3.75 | −0.1 | ||
| C | 25 | OD2 | ASP | 300 | (A) | Hydrogen-donor | 4.12 | −0.1 | ||
| O | 35 | NE2 | HIS | 299 | (A) | Hydrogen-acceptor | 2.96 | −1.1 | ||
| C | 1 | 5-ring | HIS | 101 | (A) | Hydrogen-pi | 4.52 | −0.2 | ||
| C | 16 | 6-ring | TYR | 62 | (A) | Hydrogen-pi | 4.75 | −0.1 | ||
| Campherenone | C | 1 | OD1 | ASP | 197 | (A) | Hydrogen-donor | 3.86 | −0.1 | −5.9272 |
| O | 23 | CZ3 | TRP | 58 | (A) | Hydrogen-acceptor | 3.69 | −0.1 | ||
| O | 23 | NE2 | HIS | 299 | (A) | Hydrogen-acceptor | 3.3 | −1.9 | ||
| C | 16 | 6-ring | TRP | 58 | (A) | Hydrogen-pi | 4.87 | −0.2 | ||
| Caprylic acid | O | 1 | OD1 | ASP | 197 | (A) | Hydrogen-donor | 3.04 | −4.5 | −7.5270 |
| O | 1 | OD2 | ASP | 197 | (A) | Hydrogen-donor | 2.95 | −1.1 | ||
| C | 16 | OD2 | ASP | 300 | (A) | Hydrogen-donor | 3.51 | −0.1 | ||
| C | 19 | OD1 | ASP | 300 | (A) | Hydrogen-donor | 3.85 | −0.1 | ||
| Fenchol | C | 21 | OD1 | ASP | 197 | (A) | Hydrogen-donor | 3.71 | −0.1 | −6.4744 |
| C | 25 | OD2 | ASP | 300 | (A) | Hydrogen-donor | 3.7 | −0.1 | ||
| O | 29 | CZ3 | TRP | 58 | (A) | Hydrogen-acceptor | 3.42 | −0.1 | ||
| Fixol | C | 23 | OD1 | ASP | 197 | (A) | Hydrogen-donor | 3.66 | −0.1 | −6.3792 |
| O | 1 | NE2 | GLN | 63 | (A) | Hydrogen-acceptor | 3.08 | −0.4 | ||
| O | 1 | 5-ring | TRP | 59 | (A) | Hydrogen-pi | 3.62 | −0.1 | ||
| Isocaryophyllene | C | 13 | OD1 | ASP | 300 | (A) | Hydrogen-donor | 3.74 | −0.1 | −7.2755 |
| C | 13 | OD2 | ASP | 300 | (A) | Hydrogen-donor | 3.48 | −0.1 | ||
| C | 18 | OD2 | ASP | 300 | (A) | Hydrogen-donor | 3.5 | −0.1 | ||
| C | 29 | OE1 | GLU | 233 | (A) | Hydrogen-donor | 3.34 | −0.1 | ||
| Limonene | C | 5 | 6-ring | TYR | 62 | (A) | Hydrogen-pi | 4.63 | −0.4 | −6.7494 |
| C | 15 | 5-ring | HIS | 299 | (A) | Hydrogen-pi | 4.56 | −0.3 | ||
| Myrcene | C | 1 | OE1 | GLU | 233 | (A) | Hydrogen-donor | 3.46 | −0.1 | −6.1922 |
| C | 1 | OD2 | ASP | 300 | (A) | Hydrogen-donor | 3.85 | −0.1 | ||
| Nerolidol | C | 17 | OD2 | ASP | 300 | (A) | Hydrogen-donor | 3.68 | −0.1 | −7.0590 |
| C | 20 | OD2 | ASP | 300 | (A) | Hydrogen-donor | 3.82 | −0.1 | ||
| C | 27 | OD1 | ASP | 197 | (A) | Hydrogen-donor | 3.47 | −0.1 | ||
| C | 20 | 5-ring | HIS | 299 | (A) | Hydrogen-pi | 4.64 | −0.2 | ||
| Octylcyclopropane | C | 15 | OD2 | ASP | 197 | (A) | Hydrogen-donor | 4.11 | −0.1 | −6.9528 |
| C | 24 | OE1 | GLU | 233 | (A) | Hydrogen-donor | 3.93 | −0.1 | ||
| Sativene | C | 3 | O | TYR | 62 | (A) | Hydrogen-donor | 3.57 | −0.1 | −6.9528 |
| C | 6 | O | TYR | 62 | (A) | Hydrogen-donor | 3.45 | −0.1 | ||
| β-Caryophyllene epoxide | C | 9 | OD2 | ASP | 300 | (A) | Hydrogen-donor | 3.65 | −0.1 | −8.3050 |
| C | 12 | OD1 | ASP | 197 | (A) | Hydrogen-donor | 3.57 | −0.1 | ||
| C | 21 | OD1 | ASP | 197 | (A) | Hydrogen-donor | 3.67 | −0.1 | ||
| C | 21 | OE1 | GLU | 233 | (A) | Hydrogen-donor | 3.61 | −0.1 | ||
| C | 38 | OD1 | ASP | 300 | (A) | Hydrogen-donor | 3.88 | −0.1 | ||
| Terpineol | C | 9 | OD1 | ASP | 197 | (A) | Hydrogen-donor | 3.76 | −0.1 | −7.4857 |
| C | 12 | O | TYR | 62 | (A) | Hydrogen-donor | 3.6 | −0.1 | ||
| C | 20 | OE1 | GLU | 233 | (A) | Hydrogen-donor | 4.15 | −0.1 | ||
| C | 24 | OD1 | ASP | 197 | (A) | Hydrogen-donor | 3.58 | −0.1 | ||
| O | 28 | NH2 | ARG | 195 | (A) | Hydrogen-acceptor | 3 | −0.4 | ||
| O | 28 | NE2 | HIS | 299 | (A) | Hydrogen-acceptor | 3.17 | −1.9 | ||
| O | 28 | 6-ring | TYR | 62 | (A) | Hydrogen-pi | 3.87 | −0.1 | ||
3.2.4. Docking with α-Glucosidase Enzyme
Our simulation studies revealed that the selected phytochemicals preferentially bind with the α-glucosidase receptor active sites. Considerable docking conformations were observed for ß-caryophyllene epoxide with a docking score of −8.3182 which indicates that the compound established seven hydrogen bonds and one H-pi interaction with the residues of active sites (Glu 276, Phe 177, Arg 312, Asp349, Arg 439, Gln 350, and Asp 408) (Figure 5).
Figure 5.
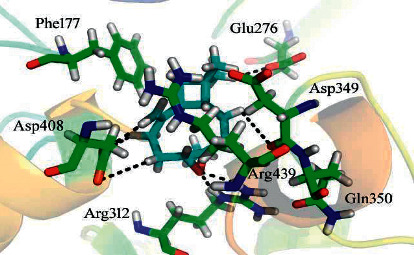
Docking conformation of β-caryophyllene epoxide in the active site of α-glucosidase.
A considerably high inhibitory potential of the identified metabolite might be attributed to the existence of the two methyl moieties and oxygen atom attached to the (S)-2-methyloxirane moiety of the ligand (Table 2).
Table 2.
Results of the docking study against α-glucosidase.
| Compounds | Ligand | Receptor | Interaction | Distance | E (kcal/mol) | Docking scores | |||
|---|---|---|---|---|---|---|---|---|---|
| 4-Thujanol | C | 1 | OD2 | ASP | 68 | Hydrogen-donor | 3.26 | −0.1 | −8.0694 |
| C | 1 | OD2 | ASP | 349 | Hydrogen-donor | 3.76 | −0.2 | ||
| C | 18 | OD1 | ASP | 214 | Hydrogen-donor | 3.48 | −0.1 | ||
| O | 28 | OD2 | ASP | 68 | Hydrogen-donor | 2.91 | −1.8 | ||
| O | 28 | NH1 | ARG | 439 | Hydrogen-acceptor | 3.06 | −3.4 | ||
| Alpha-bulnesene | C | 1 | O | ASP | 349 | Hydrogen-donor | 3.67 | −0.1 | −7.6718 |
| C | 17 | O | ASP | 349 | Hydrogen-donor | 3.57 | −0.1 | ||
| C | 21 | OE1 | GLU | 276 | Hydrogen-donor | 3.84 | −0.1 | ||
| C | 21 | OE2 | GLU | 276 | Hydrogen-donor | 3.5 | −0.1 | ||
| C | 25 | OD2 | ASP | 408 | Hydrogen-donor | 3.76 | −0.1 | ||
| C | 3 | 6-ring | PHE | 300 | Hydrogen-pi | 4.61 | −0.1 | ||
| C | 30 | 6-ring | PHE | 177 | Hydrogen-pi | 4.23 | −0.1 | ||
| Alpha-muurolene | C | 22 | OD2 | ASP | 349 | Hydrogen-donor | 4.14 | −0.1 | −7.5763 |
| C | 28 | OE1 | GLU | 276 | Hydrogen-donor | 3.56 | −0.1 | ||
| C | 28 | OE2 | GLU | 276 | Hydrogen-donor | 3.62 | −0.1 | ||
| C | 32 | OD1 | ASN | 347 | Hydrogen-donor | 3.79 | −0.1 | ||
| C | 14 | 6-ring | PHE | 300 | Hydrogen-pi | 4.55 | −0.1 | ||
| C | 32 | 6-ring | PHE | 300 | Hydrogen-pi | 4.04 | −0.3 | ||
| Beta-elemene | C | 21 | OE1 | GLN | 350 | Hydrogen-donor | 3.57 | −0.1 | −7.7074 |
| C | 33 | OE2 | GLU | 276 | Hydrogen-donor | 3.38 | −0.1 | ||
| C | 36 | OD2 | ASP | 349 | Hydrogen-donor | 3.81 | −0.1 | ||
| C | 33 | 5-ring | HIS | 348 | Hydrogen-pi | 4.26 | −0.1 | ||
| Beta-ocimene | C | 7 | OD2 | ASP | 408 | Hydrogen-donor | 3.76 | −0.1 | −7.1334 |
| C | 13 | O | ASP | 349 | Hydrogen-donor | 3.94 | −0.1 | ||
| C | 19 | OE1 | GLN | 350 | Hydrogen-donor | 3.86 | −0.1 | ||
| C | 23 | OD1 | ASN | 347 | Hydrogen-donor | 3.55 | −0.1 | ||
| C | 23 | O | ASP | 349 | Hydrogen-donor | 3.41 | −0.1 | ||
| C | 19 | 6-ring | PHE | 300 | Hydrogen-pi | 3.53 | −0.3 | ||
| C | 23 | 6-ring | PHE | 300 | Hydrogen-pi | 4.55 | −0.1 | ||
| Bornyl acetate | C | 1 | 6-ring | PHE | 177 | Hydrogen-pi | 4.06 | −0.7 | −7.2826 |
| Campherenone | O | 23 | NH1 | ARG | 439 | Hydrogen-acceptor | 2.95 | −1 | −6.5621 |
| Caprylic acid | O | 1 | O | ASP | 349 | Hydrogen-donor | 2.97 | −1.8 | −8.0814 |
| C | 19 | OD2 | ASP | 349 | Hydrogen-donor | 3.48 | −0.1 | ||
| O | 26 | NE | ARG | 312 | Hydrogen-acceptor | 3.02 | −0.2 | ||
| O | 26 | CE1 | TYR | 313 | Hydrogen-acceptor | 3.37 | −0.1 | ||
| Fenchol | C | 6 | OD2 | ASP | 408 | Hydrogen-donor | 3.67 | −0.1 | −7.3643 |
| O | 29 | O | ASP | 349 | Hydrogen-donor | 2.99 | −1.3 | ||
| C | 21 | 6-ring | PHE | 300 | Hydrogen-pi | 4.5 | −0.1 | ||
| C | 25 | 6-ring | PHE | 300 | Hydrogen-pi | 4.31 | −0.4 | ||
| Fixol | O | 1 | OD2 | ASP | 68 | Hydrogen-donor | 2.94 | −2.3 | −7.6468 |
| C | 4 | OD1 | ASP | 214 | Hydrogen-donor | 3.87 | −0.1 | ||
| C | 8 | OD2 | ASP | 349 | Hydrogen-donor | 3.58 | −0.1 | ||
| C | 21 | OD2 | ASP | 408 | Hydrogen-donor | 3.98 | −0.1 | ||
| O | 1 | NH1 | ARG | 439 | Hydrogen-acceptor | 3.11 | −3.2 | ||
| C | 12 | 6-ring | PHE | 177 | Hydrogen-pi | 3.98 | −0.2 | ||
| Isocaryophyllene | C | 1 | OD2 | ASP | 349 | Hydrogen-donor | 3.75 | −0.1 | −7.0742 |
| C | 4 | O | ASP | 349 | Hydrogen-donor | 3.41 | −0.1 | ||
| C | 25 | OD2 | ASP | 349 | Hydrogen-donor | 3.83 | −0.1 | ||
| C | 33 | OD1 | ASP | 214 | Hydrogen-donor | 4.11 | −0.1 | ||
| C | 33 | OE1 | GLU | 276 | Hydrogen-donor | 3.3 | −0.1 | ||
| C | 25 | 5-ring | HIS | 348 | Hydrogen-pi | 4.65 | −0.1 | ||
| Limonene | C | 2 | OE1 | GLN | 350 | Hydrogen-donor | 3.96 | −0.1 | −7.1971 |
| C | 5 | O | ASP | 349 | Hydrogen-donor | 3.51 | −0.1 | ||
| C | 15 | O | VAL | 303 | Hydrogen-donor | 3.82 | −0.1 | ||
| C | 15 | OE1 | GLN | 350 | Hydrogen-donor | 3.77 | −0.1 | ||
| C | 24 | OD2 | ASP | 408 | Hydrogen-donor | 3.75 | −0.1 | ||
| Myrcene | C | 23 | O | VAL | 303 | Hydrogen-donor | 3.35 | −0.1 | −7.8979 |
| C | 23 | OE1 | GLN | 350 | Hydrogen-donor | 3.4 | −0.1 | ||
| Nerolidol | C | 2 | OE1 | GLN | 350 | Hydrogen-donor | 3.7 | −0.1 | −8.2988 |
| C | 6 | O | ASP | 349 | Hydrogen-donor | 3.53 | −0.1 | ||
| C | 33 | OD2 | ASP | 68 | Hydrogen-donor | 3.31 | −0.1 | ||
| C | 37 | OD2 | ASP | 68 | Hydrogen-donor | 3.8 | −0.1 | ||
| C | 37 | OD2 | ASP | 349 | Hydrogen-donor | 3.59 | −0.1 | ||
| C | 24 | 6-ring | PHE | 177 | Hydrogen-pi | 4.78 | −0.2 | ||
| C | 30 | 6-ring | PHE | 177 | Hydrogen-pi | 4 | −0.8 | ||
| C | 33 | 6-ring | PHE | 177 | Hydrogen-pi | 4.57 | −0.3 | ||
| O | 41 | 6-ring | PHE | 300 | Hydrogen-pi | 3.96 | −0.1 | ||
| Octylcyclopropane | C | 9 | OD1 | ASP | 214 | Hydrogen-donor | 3.69 | −0.1 | −7.5104 |
| C | 12 | OE2 | GLU | 276 | Hydrogen-donor | 4.17 | −0.1 | ||
| C | 15 | OD2 | ASP | 349 | Hydrogen-donor | 3.51 | −0.1 | ||
| C | 21 | O | ASP | 349 | Hydrogen-donor | 3.96 | −0.1 | ||
| C | 27 | O | ASP | 349 | Hydrogen-donor | 4.04 | −0.1 | ||
| C | 30 | OE1 | GLN | 350 | Hydrogen-donor | 3.72 | −0.1 | ||
| C | 27 | 6-ring | PHE | 300 | Hydrogen-pi | 3.88 | −0.1 | ||
| Sativene | C | 3 | OE1 | GLN | 350 | Hydrogen-donor | 3.81 | −0.1 | −7.6121 |
| C | 35 | OD1 | ASP | 214 | Hydrogen-donor | 3.78 | −0.1 | ||
| ß-Caryophyllene epoxide | C | 9 | OD2 | ASP | 408 | Hydrogen-donor | 3.75 | −0.1 | −8.3182 |
| C | 12 | O | ASP | 349 | Hydrogen-donor | 3.52 | −0.1 | ||
| C | 16 | OD2 | ASP | 408 | Hydrogen-donor | 3.66 | −0.1 | ||
| C | 30 | OE1 | GLU | 276 | Hydrogen-donor | 3.6 | −0.1 | ||
| C | 38 | OD2 | ASP | 408 | Hydrogen-donor | 3.66 | −0.1 | ||
| O | 25 | NE | ARG | 312 | Hydrogen-acceptor | 2.83 | −3.7 | ||
| O | 25 | NH2 | ARG | 312 | Hydrogen-acceptor | 2.98 | −2.8 | ||
| C | 27 | 6-ring | PHE | 177 | Hydrogen-pi | 3.97 | −0.2 | ||
| Terpineol | C | 7 | OD1 | ASP | 214 | Hydrogen-donor | 3.91 | −0.1 | −7.8178 |
| C | 20 | OD1 | ASP | 214 | Hydrogen-donor | 3.68 | −0.1 | ||
| C | 24 | OE1 | GLN | 181 | Hydrogen-donor | 4.12 | −0.1 | ||
| O | 28 | OD2 | ASP | 68 | Hydrogen-donor | 2.93 | −2.2 | ||
| O | 28 | NH1 | ARG | 439 | Hydrogen-acceptor | 3.35 | −1.6 | ||
| C | 4 | 6-ring | PHE | 177 | Hydrogen-pi | 4.62 | −0.5 | ||
| C | 24 | 6-ring | PHE | 177 | Hydrogen-pi | 3.49 | −0.1 | ||
4. Conclusions
In summary, findings of this study showed that Ph.Lo is rich in bioactive phytochemicals which might contribute to the antidiabetic and health-promoting potentials of the oils. The test samples exhibited concentration-dependent inhibition of the vital enzymes implicated in the gastrointestinal absorption of postprandial glucose and thus might help in reducing the hyperglycemia in type-2 diabetes. The binding mode and energies of the identified phytochemicals against the target enzymes using the molecular docking approach further supported our claim regarding the antidiabetic potential of our test samples. Nevertheless, we suggest that, in future, in vivo studies be performed for the therapeutic and beneficial effects of these compounds in metabolism-associated disorders.
Acknowledgments
The authors would like to acknowledge the support of the Deputy for Research and Innovation, Ministry of Education, Kingdom of Saudi Arabia, for this research through a grant (NU-IF/INT/01/006) under the Institutional Funding Committee at Najran University, Kingdom of Saudi Arabia.
Data Availability
The experimental data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
All authors contributed equally towards the project design, experimental work, and manuscript writeup. All authors read and approved the manuscript for publication.
Supplementary Materials
Supplementary file S1: data related to identified compounds re provided as File S1 and Table S1 containing the list of identified compounds and their details.
References
- 1.Ovais M., Ayaz M., Khalil A. T., et al. HPLC-DAD finger printing, antioxidant, cholinesterase, and α-glucosidase inhibitory potentials of a novel plant Olax nana. BMC Complementary and Alternative Medicine . 2018;18(1):1–13. doi: 10.1186/s12906-017-2057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahnashi M. H., Alqahtani Y. S., Alqarni A. O., et al. Crude extract and isolated bioactive compounds from Notholirion thomsonianum (Royale) Stapf as multitargets antidiabetic agents: in-vitro and molecular docking approaches. BMC Complementary Medicine and Therapies . 2021;21(1):270–313. doi: 10.1186/s12906-021-03443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arky R. Clinical Correlates of Metabolic Derangements of Diabetes Mellitus . Philadelphia, PA, USA: Complications of Diabetes Mellitus WB Saunders; 1982. pp. 16–20. [Google Scholar]
- 4.Booth G., Lipscombe L., Butalia S., et al. Pharmacologic management of type 2 diabetes: 2016 interim update. Canadian Journal of Diabetes . 2016;40(6):484–486. doi: 10.1016/j.jcjd.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed F., Urooj A. Antihyperglycemic activity of Ficus glomerata stem bark in streptozotocin-induced diabetic rats. Global Journal of Pharmacology . 2008;2(3):41–45. [Google Scholar]
- 6.Kharroubi A. T., Darwish H. M. Diabetes mellitus: the epidemic of the century. World Journal of Diabetes . 2015;6(6):p. 850. doi: 10.4239/wjd.v6.i6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care . 1993;16(1):329–334. [PubMed] [Google Scholar]
- 8.Kumar A., Ilavarasan R., Jayach T., et al. Anti-diabetic activity of Syzygium cumini and its isolated compound against streptozotocin-induced diabetic rats. Journal of Medicinal Plants Research . 2013;2(9):246–249. [Google Scholar]
- 9.Whiting D. R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Research and Clinical Practice . 2011;94(3):311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Cho N. H., Shaw J. E., Karuranga S., et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice . 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Gin H., Rigalleau V. Post-prandial hyperglycemia. post-prandial hyperglycemia and diabetes. Diabetes & Metabolism . 2000;26(4):265–272. [PubMed] [Google Scholar]
- 12.Nair S. S., Kavrekar V., Mishra A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. European Journal of Experimental Biology . 2013;3(1):128–132. [Google Scholar]
- 13.Ayaz M., Ullah F., Sadiq A., Kim M. O., Ali T. Editorial: natural products-based drugs: potential therapeutics against alzheimer’s disease and other neurological disorders. Frontiers in Pharmacology . 2019;10:p. 1417. doi: 10.3389/fphar.2019.01417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ullah I., Subhan F., Alam J., Shahid M., Ayaz M. Suppression of cisplatin-induced vomiting by cannabis sativa in pigeons: neurochemical evidences. Frontiers in Pharmacology . 2018;9:p. 231. doi: 10.3389/fphar.2018.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saleem U., Akhtar R., Anwar F., et al. Neuroprotective potential of Malva neglecta is mediated via down-regulation of cholinesterase and modulation of oxidative stress markers. Metabolic Brain Disease . 2021;36(5):1–12. doi: 10.1007/s11011-021-00683-x. [DOI] [PubMed] [Google Scholar]
- 16.Petrovska B. Historical review of medicinal plants’ usage. Pharmacognosy Reviews . 2012;6(11):p. 1. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil A. T., Ovais M., Iqbal J., et al. Seminars in Cancer Biology . Amsterdam, Netherlands: Elsevier; 2021. Microbes-mediated synthesis strategies of metal nanoparticles and their potential role in cancer therapeutics. [DOI] [PubMed] [Google Scholar]
- 18.Saleem U., Khalid S., Zaib S., et al. Phytochemical analysis and wound healing studies on ethnomedicinally important plant Malva neglecta Wallr. Journal of Ethnopharmacology . 2020;249 doi: 10.1016/j.jep.2019.112401.112401 [DOI] [PubMed] [Google Scholar]
- 19.Bailey C. J., Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care . 1989;12(8):553–564. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 20.Choudhury A., Maeda K., Murayama R., DiMagno E. Character of a wheat amylase inhibitor preparation and effects on fasting human pancreaticobiliary secretions and hormones. Gastroenterology . 1996;111(5):1313–1320. doi: 10.1053/gast.1996.v111.pm8898646. [DOI] [PubMed] [Google Scholar]
- 21.Kumar V., Prakash O., Kumar S., Narwal S. α-glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacognosy Reviews . 2011;5(9):p. 19. doi: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dirir A. M., Daou M., Yousef A. F., Yousef L. F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochemistry Reviews . 2021 doi: 10.1007/s11101-021-09773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayaz M., Junaid M., Subhan F., et al. Heavy metals analysis, phytochemical, phytotoxic and anthelmintic investigations of crude methanolic extract, subsequent fractions and crude saponins from Polygonum hydropiper L. BMC Complementary and Alternative Medicine . 2014;14(1):p. 465. doi: 10.1186/1472-6882-14-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayaz M., Ahmad I., Sadiq A., et al. Persicaria hydropiper (L.) Delarbre: a review on traditional uses, bioactive chemical constituents and pharmacological and toxicological activities. Journal of Ethnopharmacology . 2020;251 doi: 10.1016/j.jep.2019.112516.112516 [DOI] [PubMed] [Google Scholar]
- 25.Tong X., Li X., Ayaz M., et al. Neuroprotective studies on Polygonum hydropiper L. essential oils using transgenic animal models. Frontiers in Pharmacology . 2021;11 doi: 10.3389/fphar.2020.58006937.580069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayaz M., Junaid M., Ullah F., et al. Anti-alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Frontiers in Pharmacology . 2017;8:p. 697. doi: 10.3389/fphar.2017.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayaz M., Sadiq A., Wadood A., Junaid M., Ullah F., Zaman Khan N. Cytotoxicity and molecular docking studies on phytosterols isolated from Polygonum hydropiper L. Steroids . 2019;141:30–35. doi: 10.1016/j.steroids.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Mahnashi M. H., Alqahtani Y. S., Alyami B. A., et al. Cytotoxicity, anti-angiogenic, anti-tumor and molecular docking studies on phytochemicals isolated from Polygonum hydropiper L. BMC Complementary Medicine and Therapies . 2021;21(1):1–14. doi: 10.1186/s12906-021-03411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayaz M., Junaid M., Ullah F., et al. Chemical profiling, antimicrobial and insecticidal evaluations of Polygonum hydropiper L. BMC Complementary and Alternative Medicine . 2016;16(1):502–514. doi: 10.1186/s12906-016-1491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayaz M., Junaid M., Ullah F., et al. GC-MS analysis and gastroprotective evaluations of crude extracts, isolated saponins, and essential oil from Polygonum hydropiper L. Frontiers of Chemistry . 2017;5:p. 58. doi: 10.3389/fchem.2017.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayaz M., Junaid M., Ullah F., et al. Molecularly characterized solvent extracts and saponins from Polygonum hydropiper L. show high anti-angiogenic, anti-tumor, brine shrimp, and fibroblast NIH/3T3 cell line cytotoxicity. Frontiers in Pharmacology . 2016;7:p. 74. doi: 10.3389/fphar.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayaz M., Junaid M., Ullah F., et al. Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum hydropiper L: a preliminary anti-alzheimer’s study. Lipids in Health and Disease . 2015;14(1):1–12. doi: 10.1186/s12944-015-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein S., Mirokhin D., Tchekhovskoi D., et al. The NIST Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectra Library . Gaithersburg, MD, USA: Standard Reference Data Program of the National Institute of Standards and Technology Gaithersburg; 2002. [Google Scholar]
- 34.Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry . Vol. 456. Carol Stream, IL, USA: Allured Publishing Corporation; 2007. [Google Scholar]
- 35.McCue P., Kwon Y.-I., Shetty K. Anti-amylase, anti-glucosidase and anti-angiotensin I-converting enzyme potential of selected foods. Journal of Food Biochemistry . 2005;29(3):278–294. doi: 10.1111/j.1745-4514.2005.00020.x. [DOI] [Google Scholar]
- 36.Ghufran M., Rehman A. U., Shah M., Ayaz M., Ng H. L., Wadood A. In-silico design of peptide inhibitors of K-Ras target in cancer disease. Journal of Biomolecular Structure and Dynamics . 2020;38(18):5488–5499. doi: 10.1080/07391102.2019.1704880. [DOI] [PubMed] [Google Scholar]
- 37.Ayaz M., Wadood A., Sadiq A., Ullah F., Anichkina O., Ghufran M. In-silico evaluations of the isolated phytosterols from Polygonum hydropiper L against BACE1 and MAO drug targets. Journal of Biomolecular Structure and Dynamics . 2021:1–9. doi: 10.1080/07391102.2021.1940286. [DOI] [PubMed] [Google Scholar]
- 38.Liu M., Zhang W., Wei J., Lin X. Synthesis and α-glucosidase inhibitory mechanisms of bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, a potential marine bromophenol α-glucosidase inhibitor. Marine Drugs . 2011;9(9):1554–1565. doi: 10.3390/md9091554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jabeen F., Oliferenko P. V., Oliferenko A. A., et al. Dual inhibition of the α-glucosidase and butyrylcholinesterase studied by molecular Field topology analysis. European Journal of Medicinal Chemistry . 2014;80:228–242. doi: 10.1016/j.ejmech.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Hichri F., Omri A., Hossan A. S. M., Ben Jannet H. Alpha-glucosidase and amylase inhibitory effects of Eruca vesicaria subsp. longirostris essential oils: synthesis of new 1,2,4-triazole-thiol derivatives and 1,3,4-thiadiazole with potential inhibitory activity. Pharmaceutical Biology . 2019;57(1):564–570. doi: 10.1080/13880209.2019.1642363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oboh G., Akinbola I. A., Ademosun A. O., et al. Essential oil from clove bud (Eugenia aromatica Kuntze) inhibit key enzymes relevant to the management of type-2 diabetes and some pro-oxidant induced lipid peroxidation in rats pancreas in vitro. Journal of Oleo Science . 2015;64(7):775–782. doi: 10.5650/jos.ess14274. [DOI] [PubMed] [Google Scholar]
- 42.Rahali N., Mehdi S., Younsi F., Boussaid M., Messaoud C. Antioxidant, α-amylase, and acetylcholinesterase inhibitory activities of Hertia cheirifolia essential oils: influence of plant organs and seasonal variation. International Journal of Food Properties . 2017;20(2):1637–1651. doi: 10.1080/10942912.2017.1352597. [DOI] [Google Scholar]
- 43.Majouli K., Besbes Hlila M., Hamdi A., Flamini G., Ben Jannet H., Kenani A. Antioxidant activity and α-glucosidase inhibition by essential oils from Hertia cheirifolia (L.) Industrial Crops and Products . 2016;82:23–28. doi: 10.1016/j.indcrop.2015.12.015. [DOI] [Google Scholar]
- 44.Jaradat N., Al-Maharik N., Abdallah S., Shawahna R., Mousa A., Qtishat A. Nepeta curviflora essential oil: phytochemical composition, antioxidant, anti-proliferative and anti-migratory efficacy against cervical cancer cells, and α-glucosidase, α-amylase and porcine pancreatic lipase inhibitory activities. Industrial Crops and Products . 2020;158 doi: 10.1016/j.indcrop.2020.112946.112946 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file S1: data related to identified compounds re provided as File S1 and Table S1 containing the list of identified compounds and their details.
Data Availability Statement
The experimental data used to support the findings of this study are available from the corresponding author upon request.


