Abstract
The use of a variety of RNA molecules, including messenger RNA, small interfering RNA, and microRNA, has shown great potential for prevention and therapy of many pathologies. However, this therapeutic promise has historically been limited by short in vivo half-life, lack of targeted delivery, and safety issues. Nanoparticle (NP)-mediated delivery has been a successful platform to overcome these limitations, with multiple formulations already in clinical trials and approved by the FDA. Although there is a diversity of NPs in terms of material formulation, size, shape, and charge that have been proposed for biomedical applications, specific modifications are required to facilitate sufficient RNA delivery and adequate therapeutic effect. This includes optimization of (i) RNA incorporation into NPs, (ii) specific cell targeting, (iii) cellular uptake and (iv) endosomal escape ability. In this review, we summarize the methods by which NPs can be modified for RNA delivery to achieve optimal therapeutic effects.
Keywords: RNA therapy, drug delivery, nanomedicine, endosomal escape, targeting, cellular uptake
1. Introduction
Ribonucleic acid (RNA) exists in multiple forms with various nucleotide chain lengths and functions that have essential roles in both health and disease (Kim, 2020). Messenger RNA (mRNA; responsible for protein translation), antisense RNAs (asRNA; block protein translation) including microRNAs (miRNA; regulate post-transcriptional gene expression), and small interfering RNA (siRNA; silence gene expression) have all been explored and successfully applied as vaccines, protein replacement therapies, and immunomodulatory agents in many diseases, including cancer and atherosclerosis (Lin et al., 2020). Considering that only ~15% of proteins are considered targetable for therapy using small molecule drugs, RNA therapy represents an alternative treatment option with several advantages (Dammes & Peer, 2021). RNA is easier to design and scale up than protein-based drugs (Guevara et al., 2019; Lin et al., 2020), and the recent explosion of RNA research has informed novel methods to chemically modify RNA, leading to decreased costs associated with synthesis (Jasinski et al., 2017).
However, naked RNA molecules have shown a lack of therapeutic efficacy for many reasons. RNA has a short half-life due to nuclease degradation (Orlandini von Niessen et al., 2019) and reticuloendothelial clearance (Valle et al., 2020). They also show limited cellular internalization due to their negative charge (Wadhwa et al., 2020) and/or potentially long nucleotide chain length depending on the sequence used (Park et al., 2021) (Fig. 1). Furthermore, dose-limiting toxicity can preclude RNA for therapeutic use as it can induce an immune response via stimulation of proinflammatory cytokine production (Freund et al., 2019). Finally, the lack of RNA thermal stability hinders clinical translation potential (Park et al., 2021).
Figure 1.
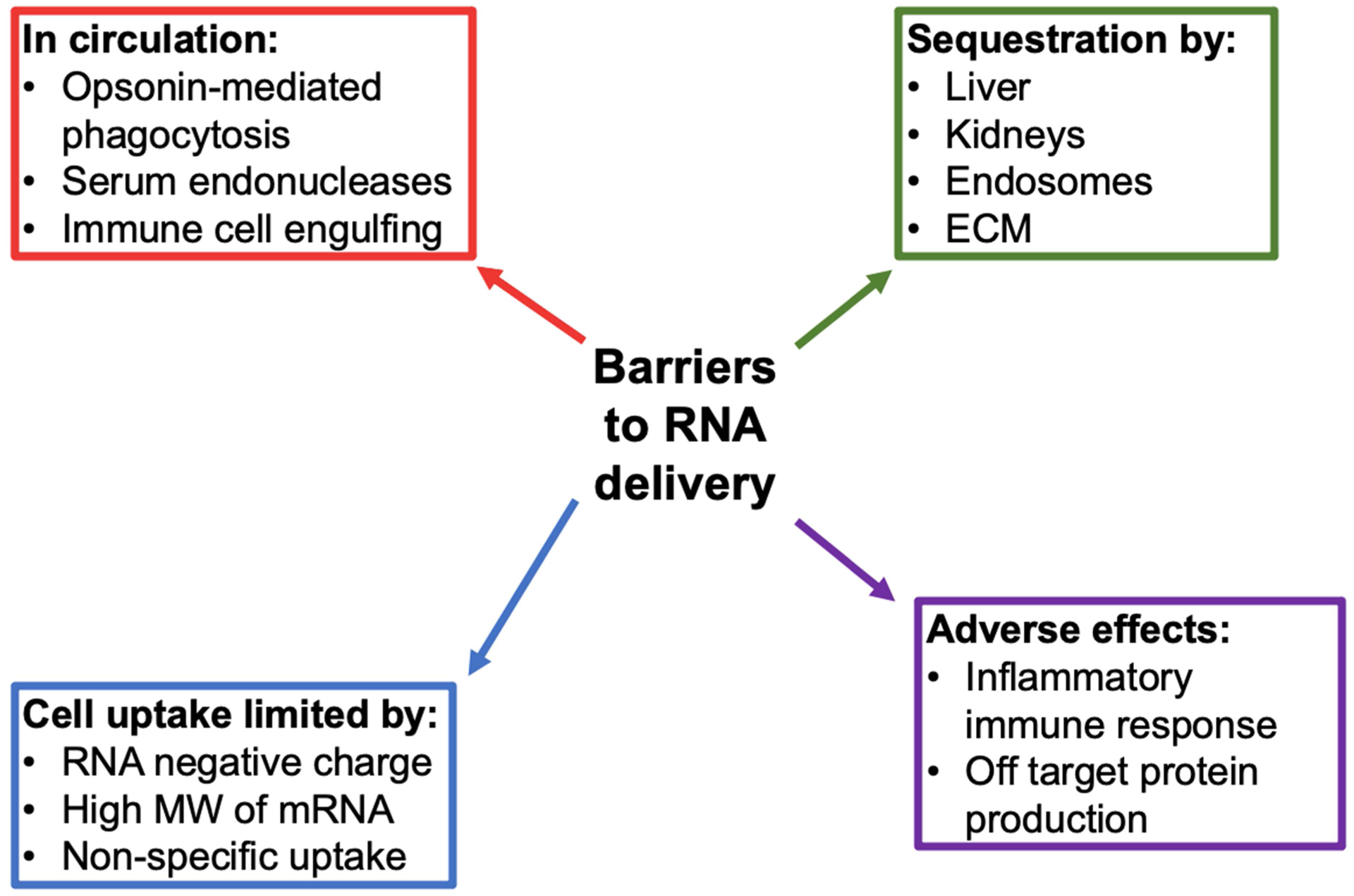
Barriers to RNA delivery for therapeutic use. ECM: extracellular matrix, MW: molecular weight.
Additionally, even upon cell uptake, RNA can be sequestered by endosomes, posing a significant barrier to inducing a therapeutic response. For example, only 1–2% of siRNAs that enter cells escape the endosome into the cytosol where they can induce mRNA degradation or block translation (Kim, 2020). Although some of these issues can be solved by direct chemical modification of RNA, specific and effective delivery to the pathological site is still lacking (Wadhwa et al., 2020). Insufficient targeting results in RNA accumulation in indiscriminate cell types, thereby decreasing therapeutic efficacy, increasing the dose required to induce an adequate response, and potentially inducing adverse side effects at off-target sites (Kim et al., 2020).
In order to overcome these limitations, nanoparticles (NPs) have been extensively used as delivery tools (Lei et al., 2020), with some formulations approved for use by the United States Food and Drug Administration (FDA). NP use for RNA delivery has been remarkably successful in recent years, with dozens of formulations currently in clinical trials, and two effective vaccines combatting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection currently under Emergency Authorization Use (EUA) in the United States and worldwide (Chung et al., 2020; Park et al., 2021). There are multiple reasons for this recent success: 1) Non-toxic and biodegradable components that show low immunogenicity can be used to create biologically compatible NPs for clinical use and drug delivery. 2) NPs have the distinct advantage of multifunctionalization, which is the ability to incorporate multiple therapeutic and/or diagnostic moieties on one particle, due to their large volume-to-surface area ratio (Ho et al., 2021). This can allow for combination therapy using multiple small molecule drugs, antibodies, and/or nucleic acids, along with specific cell targeting using peptides or proteins. The inclusion of labelling moieties, e.g., fluorescent markers or radioactive molecules, is particularly useful when completing cell uptake studies in vitro and biodistribution studies in vivo (Y. Sato et al., 2020). 3) NPs can also be scaled up efficiently and relatively cheaply with less potential for contamination than viral vectors (Wadhwa et al., 2020). 4) NP incorporation of RNA can prolong its half-life, facilitating increased circulation time in vivo (Yoshinaga et al., 2019) and increase its safety profile by shielding it from biological interactions until it is delivered to the intended site of action (Halbur et al., 2019). 5) Careful modification of NP size can also bypass macrophage clearance and enhance cellular uptake of RNA (Xu et al., 2018). 6) Furthermore, NPs have been shown to improve RNA stability. For example, when mRNA was loaded in lipid-like NPs incorporating sucrose, mannitol or trehalose, delivery efficiency was maintained in vitro and in vivo following storage for at least three months (Zhao et al., 2020). 7) Additionally, functional mRNA delivery with NPs can be effective, inducing protein expression within one hour of administration (Li et al., 2019) and continuing for up to 48 hours (Yasar et al., 2018).
Nanomedicine is a broad field encompassing NPs of vastly different size, shape, and material composition, and there is no “one size fits all approach” when it comes to NP use for RNA delivery (Formicola et al., 2019). Many reviews have discussed the various types of NPs proposed to deliver RNA for therapy (Ickenstein & Garidel, 2019; Schlich et al., 2021; Shen et al., 2019) or detailed the clinical applications of specific RNA NP combinations (Charbe et al., 2020; Li et al., 2019; Lin et al., 2020). However, there is a lack of discussion regarding the methods and approaches that can be used to incorporate RNA into NPs for successful delivery. Here, we detail the major physicochemical modifications that are required to optimize NPs for RNA delivery. This includes an analysis of the stability and efficiency of different techniques for RNA incorporation into NPs. We also examine targeting strategies that prevent off-target accumulation of RNA NPs, thereby increasing drug delivery and subsequent therapeutic efficacy and reducing side effects. Finally, we present methods to create RNA NPs that are efficiently taken up by cells and subsequently released from endosomes for therapy.
2. NP materials and methods of RNA incorporation
The most common materials used for NP formulation in RNA delivery are lipids and polymers (Uchida et al., 2020). Considering the wide variety of materials available, NP formulations should be chosen and altered based on the RNA type to be delivered. For example, NPs containing higher levels of phospholipids and polyethylene glycol (PEG) and lower levels of cholesterol and ionizable lipids have been shown to be more effective for mRNA release than for siRNA release (Hajj & Whitehead, 2017). For mRNA delivery, lipid NPs with a lamellar lipid phase, and faceted and multilamellar structures exhibit increased gene transfection (Eygeris et al., 2020). For siRNA delivery, the replacement of cholesterol with sphingomyelin in solid lipid NPs decreased NP size to 22 nm and enhanced gene silencing activity (Yusuke Sato et al., 2020). In a similar characterization study, a library of cationic multivalent peptide-functionalized polymers was synthesized by simple step-growth polymerization of L-lysine and cystine using 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC) as a coupling reagent. These polymers were tested for maximal RNA delivery following attachment via electrostatic interactions. Numerous factors improved the nanoformulation, including the incorporation of triethylene glycol (TEG) for increased stability and removal of aliphatic side chains which are trapped by endosomes (Yang et al., 2020).
Alongside the NP formulation itself, the method of RNA incorporation can affect the therapeutic outcome. As such, in this next section, we discuss the main methods used: encapsulation within the NP, adsorption to the surface of pre-formed NPs (Fig. 2), and a combination of these methods using the “layer-by-layer” (LbL) approach. Regardless of the method used, free, unincorporated RNA molecules should be removed, although this step is often omitted from the methods in the literature.
Figure 2.
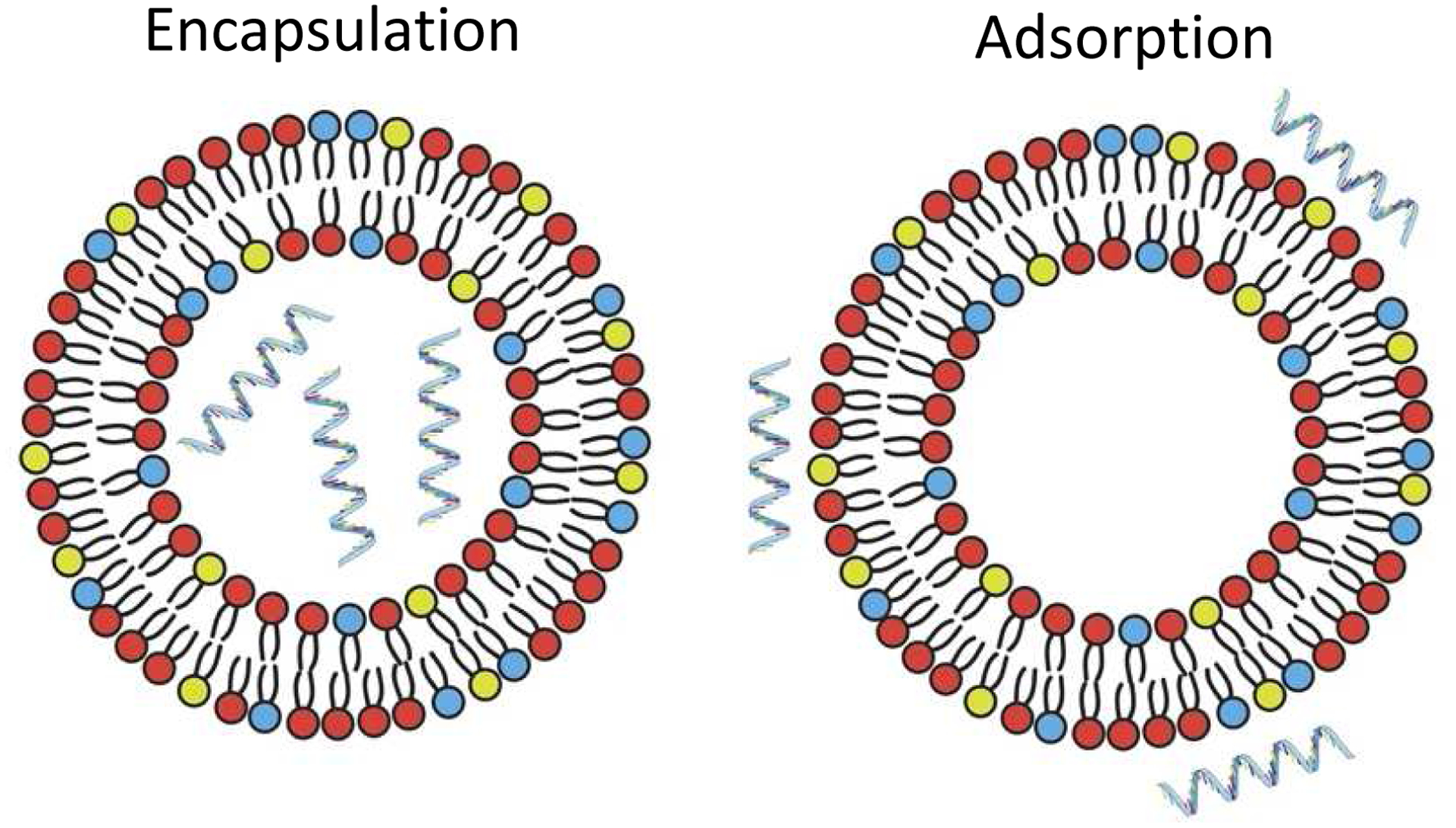
Schematic of RNA that is encapsulated within NPs (left) vs. adsorbed on the surface of NPs (right), as shown with the example of liposomes. These methods can be used with NPs consisting of a variety of materials, including lipids and polymers. Adapted from (Blakney et al., 2019) under the terms of the Creative Commons CC BY license.
2.1. Encapsulation
Similar to the FDA-approved liposome Doxil® (Zhao et al., 2018), RNA molecules are often embedded in lipid- and/or polymer-based NPs. This method, known as encapsulation, can be achieved by multiple methods, most commonly nanoprecipitation or thin lipid film hydration (TLFH) (Fig. 3). The use of cationic components can enhance complexation and improve encapsulation efficiency (Lilavivat et al., 2012; Pattni et al., 2015). In nanoprecipitation, the NP components (lipid and/or polymer) are dissolved in an organic solvent and added to RNA in an acidic aqueous solution while stirring using a magnetic stir bar and stir plate. Hydrophobic and electrostatic interactions between the polar, water soluble, and hydrophilic domains drive the formation of NPs containing RNA in their core which remain suspended in the aqueous solution following evaporation of the organic solvent (Cullis & Hope, 2017; Salvage et al., 2015). TLFH involves dissolving lipids in an organic solvent, which is then evaporated under argon or nitrogen. A lipid film forms, which can be further dried under vacuum and subsequently rehydrated in an aqueous solution containing the RNA molecule, most commonly water or phosphate buffered saline (PBS) (Magro et al., 2018; J. Wang et al., 2020). Notably, vortexing and sonication during rehydration can produce heterogeneous, large multilamellar vesicles (Evers et al., 2018). As such, many researchers use extrusion to control NP size polydispersity (Chen et al., 2018; Szoka & Papahadjopoulos, 1980). However, extrusion is not typically appropriate for NPs with a monolayer, as it can alter their conformation, making them rod-shaped instead of spherical (Chen et al., 2019).
Figure 3.
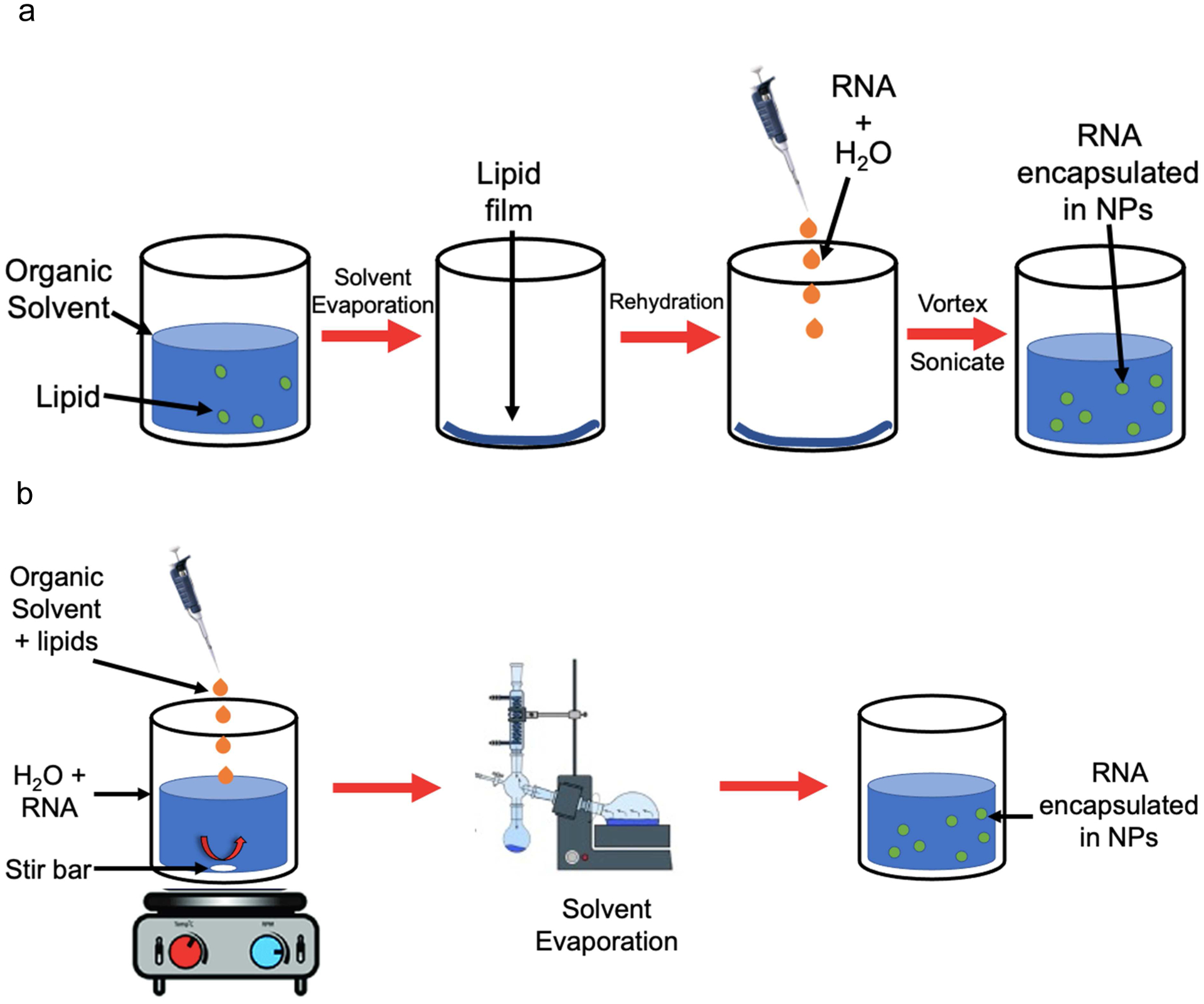
Schemes showing common methods of RNA encapsulation in lipid- and/or polymer-based NPs. a) Thin lipid film hydration. Lipids are suspended in an organic solvent, which is then evaporated under nitrogen flow. Further evaporation can be carried out under vacuum to ensure solvent evaporation. The resulting lipid film is rehydrated with an aqueous solution containing RNA, and then vortexed and sonicated to produce NPs containing RNA in their core. b) Nanoprecipitation. An organic solvent containing NP components (lipids and/or polymers) is added dropwise to an aqueous solution containing RNA while stirring. The solvent is evaporated under nitrogen or by using a Rotavapor, leaving an aqueous solution with NPs containing RNA in their core.
The main advantage of NP encapsulation is the physical protection of RNA from degradation in the serum, thereby extending their half-life in vivo (Thomas et al., 2018). NP encapsulation can also condense RNA molecules, thereby overcoming the limited cellular uptake associated with larger molecules, and increase their stability by protecting them from degradation in vivo (Workman & Flynn, 2009). Furthermore, siRNA has been shown to remain encapsulated within PEGylated liposomes following incubation in human serum, indicating the stability of this delivery method (Buyens et al., 2009). Examples of successful applications of RNA encapsulation within NPs, are detailed below.
2.1.1. Examples of NPs encapsulating therapeutic RNA
Encapsulation has been used for RNA delivery for multiple therapeutic applications, including siRNA treatment of cancer (Wang, Xu, et al., 2013; Xu et al., 2014) and mRNA vaccines against Zika virus (Pardi, Hogan, et al., 2017), human immunodeficiency virus (HIV)-1 (Pardi, Secreto, et al., 2017), H1N1 influenza, Ebola, and Toxoplasma gondii (Chahal et al., 2016). Notably, the only RNA NP formulations that are FDA-approved, or have EUA, use encapsulation to incorporate RNA into lipid-based NPs. Alnylam’s Onpattro®, FDA-approved in 2018, is used to treat polyneuropathy associated with hereditary transthyretin amyloidosis (hATTR) (Urits et al., 2020). The formulation consists of the pH sensitive ionizable lipid DLin-MC3-DMA (MC3), cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), and a PEG lipid encapsulating siRNA targeting transthyretin, a protein that is predominantly generated by the liver and can be mutated to cause hATTR (Agency, 2018; Buschmann et al., 2021).
In 2020, two vaccines against coronavirus disease 2019 (COVID-19) were produced by Moderna Therapeutics and Pfizer-BioNTech and authorized by the FDA for emergency use. The Moderna vaccine consists of lipid NPs with the ionizable lipid sphingomyelin-102 (SM-102), cholesterol (for stabilization), PEG (to reduce non-specific interactions) and phospholipids (for structure and promotion of intracellular release) that can deliver mRNA-1273 encoding the spike protein of SARS-CoV-2, the pathological virus that causes COVID-19, enabling an effective immune response against the disease (Park et al., 2021). Pfizer-BioNTech uses a similar formulation for their vaccine, replacing SM-102 with the ionizable lipid ALC-0315 from Acuitas and incorporating the mRNA BNT162b2 also encoding for the SARS-CoV-2 spike protein but stabilized by two prolines (Buschmann et al., 2021; Khurana et al., 2021). The benefits of NP-mediated delivery of encapsulated RNA, by protecting RNA from degradation and facilitating cellular uptake, is highlighted in these widely used formulations.
Despite the advantages of RNA encapsulation within NPs, RNA encapsulation can induce large increases in NP diameter which can reduce cellular uptake (Zhang et al., 2021). Certain NP components can also limit the effectiveness of RNA encapsulation. For example, it has been shown that if PEG is present on the inner surface of the inner layer of the liposome bilayer, siRNA encapsulation can be physically inhibited as there is no space remaining for the RNA to exist. To avoid this without excluding PEG usage, post-insertion of PEG-lipids can be carried out by slowly adding a PEG-lipid solution to liposomes encapsulating RNA (Nosova et al., 2019). Furthermore, RNA release can be a challenge in these systems as the encapsulated RNA is not released from NPs that do not readily disassemble at the target site (Yasar et al., 2018).
2.2. Adsorption
Given the disadvantages of encapsulated RNA in terms of limited release and potential size increases, RNA incorporation into NPs by other methods has been explored. The most common alternative to encapsulation is RNA adsorption to the NP surface. RNA is strongly negatively charged, and hence, RNA can be adsorbed onto the surface of preformed cationic NPs through electrostatic interactions or combined with NP components to form lipoplexes or polyplexes in a relatively facile manner. Adsorption is normally carried out via room temperature or 4°C incubation of pre-formed NPs with RNA while shaking (Chen et al., 2018). Incubation time varies according to publication but one study based on asRNA binding to PLGA NPs showed that a length of incubation up to 60 minutes did not affect the size or zeta potential of the NPs, possibly due to maximal absorption rapidly occurring with the first 15 minutes of incubation (Nafee et al., 2007). However, differences in adsorption rates between different RNA and NP types should be considered when establishing a protocol for formulation, in order to optimize the length of incubation time for optimal RNA adsorption. For example, double stranded RNA cannot uncoil and expose its bases which led to slower adsorption rates to gold NPs compared to single-stranded molecules (Li & Rothberg, 2005). This study also showed that long single-stranded RNA adsorbed more slowly, possibly due to secondary structure formation or limitations in flexibility related to increased length. Adsorption ability has also been related to NP size, with the amount of adsorbed RNA decreasing with increased mesoporous silica (MPS) NP size, from 249 μg/mg of MPS on 50 nm NPs, to 93.2 μg/mg of MPS on 300 nm NPs due to differences in surface area-volume ratio (Hikosaka et al., 2018).
A major advantage of surface adsorption of RNA is that it can reduce the overall charge of cationic NPs, which are known to induce cytotoxicity. Adsorption of both single and double-stranded RNA to gold NPs decreased their net charge density and prevented NP aggregation (Li & Rothberg, 2005). In a direct comparison, self-amplifying RNA decreased the positive charge of lipid NPs when adsorbed to, but not encapsulated in, the NPs (Blakney et al., 2019). This cation quenching is used as an indication of successful RNA adsorption to the NP surface.
Another advantage of NP RNA adsorption is that it does not significantly change the NP size as demonstrated with asRNA and chitosan-coated PLGA NPs (Nafee et al., 2007), and ribosomal RNA and copolymer-based NPs (Hernández et al., 2019). In fact, mRNA adsorption has actually been shown to reduce the size of cationic or ionizable lipid/polymer based NPs due to compaction and condensation following electrostatic interactions between the RNA molecule and flexible NPs (Blakney et al., 2019).
2.2.1. NP materials to facilitate RNA adsorption
The electrostatic mechanism of adsorption limits the materials that can be used for NP formulation, precluding those that are anionic at physiological pH, such as PLGA and phosphatidylserine (Kimura et al., 2021). Cationic lipids, including 1,2-dioleoyl-3-trimethylammonium propane (DOTAP), dimethyldioctadecylammonium (DDA), N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA), and DC-cholesterol are commonly used to facilitate RNA adsorption (Guevara et al., 2020; Gómez-Aguado et al., 2020). Although DOTAP NPs have a similar charge to DDA NPs, the former are larger, possibly due to their less rigid conformation, and are therefore less likely to be taken up by cells (Anderluzzi et al., 2020). Such changes in size can limit the use of certain cationic elements in NP formulation and requires investigation of alternative methods to increase the cationic charge of NPs for RNA attachment, as detailed below.
NPs with insufficient positive charge can be coated with cationic elements, such as polyethylenimine (PEI) to promote electrostatic interactions. Cationic lipids have been used to coat calcium phosphate NPs to successfully deliver siRNA against programmed cell death protein (PD)-1 and programmed death ligand (PD-L)1 in order to increase the toxicity of tumor-infiltrating lymphocytes (Wu et al., 2019). Dimethyl-di-octadecyl-346 ammonium (DDAB), a cationic surfactant widely used in NP formulation, has been incorporated into liposomes using TLFH to increase their positive charge and deliver anti-miRNA 155 delivery in vivo to treat acute kidney injury (Chen et al., 2018).
Chitosan, a cationic linear polysaccharide derived from the exoskeleton of shellfish, has been used to coat PLGA NPs in order to increase their zeta potential from −10 mV to 17.1 mV (Nafee et al., 2007). In this study, Nafee et al. showed that increased chitosan concentration on the NP surface directly correlated with increased zeta potential measurements. The coating enabled successful adsorption of an asRNA inhibiting telomerase to the NP surface and subsequent uptake by A549 human lung carcinoma cells. However, it should be noted that the chitosan coating also increased the NP size, a factor which may influence the extent of cellular uptake.
Notably, RNA adsorption onto an anionic molecule has also been attempted using human serum albumin (HSA) NPs (Wen et al., 2017). HSA is anionic at physiological pH due to its isoelectric point (pI) of 4.7. However, Wen et al. adjusted an HSA solution to pH 4.0, under which conditions the plasma protein becomes cationic. The HSA was then complexed with RNA and heated at 75 °C for 15 minutes. This method led to the formation of 110 nm NPs incorporating RNA that showed high uptake in HeLa cells.
In addition to polymers and proteins, cationic peptides with arginine-rich sequences (Lee et al., 2015), including the protamine family derived from fish sperm, have been extensively used to promote RNA cell uptake via co-incubation in vitro (Amos, 1961) and by incorporation into NPs to enhance RNA attachment. Aside from lipid based NPs, protamine-based complexes are the most used delivery tools for mRNA applied in clinical trials, in part due to their added benefit of protecting RNA from degradation via condensation (Gómez-Aguado et al., 2020), and have also been incorporated into liposomes for enhanced RNA attachment (Hoerr et al., 2000). For example, Wang et al. incubated protamine with mRNA encoding herpes simplex virus1-thymidine kinase, an anti-cancer suicide gene. This complex was then incubated with preformed DOTAP/cholesterol liposomes, resulting in efficient mRNA loading (7.6 μg mRNA/μmol liposome). Systemic administration of this formulation significantly inhibited tumor growth in a H460 xenograft mouse model of lung carcinoma, indicating that protamine complexation on NPs is an effective way to deliver functional mRNA (Wang, Su, et al., 2013).
2.2.2. Examples of NPs with adsorbed therapeutic RNA
Adsorption to many NP types has consistently been shown effective at delivering siRNA (Cui et al., 2017; Huang et al., 2018; Leng et al., 2020; Li et al., 2016; Xie et al., 2016), mRNA (Grabbe et al., 2016; Leng et al., 2020) and miRNA (Chen et al., 2018), and inducing a therapeutic effect in vivo. For example, multifunctional polymeric NPs incorporating PEG, chitosan-polyamine and lipoic acid produced by nanoprecipitation was used to adsorb siRNA targeting Enhancer of Zeste Homologue 2 (EZH2). In vitro, these NPs exhibited intracellular drug release in luc-A549 cells and protected therapeutic siRNA from degradation by nucleases in serum for 4 hours. In vivo, these NPs were administered intravenously (IV) five times every other day to an orthotopic lung tumor mouse model using luc-A549 cells. This treatment led to successful downregulation of EZH2 mRNA and protein in the tumor, thereby inhibiting tumor growth and metastasis development compared to PBS control (Yuan et al., 2017). Similarly, cationic liposomes consisting of DDAB, cholesterol and DSPE-PEG were created using TLFH. Pre-made liposomes were then incubated with anti-miRNA-155 for 12 hours at 4°C to facilitate adsorption, and centrifugation was used to remove free RNA. Mice with acute lipopolysaccharide-induced kidney injury were treated with one dose of the NP-RNA formulation or PBS and sacrificed after 12 hours. Compared to the control, the NP-RNA formulation significantly decreased miRNA-155 expression and inflammatory cell infiltration in the kidneys (Chen et al., 2018). These studies demonstrate that adsorption is a feasible approach for RNA attachment to NPs and subsequent administration in vivo for therapy.
Though adsorption has been shown to overcome some of the pitfalls of encapsulation as an RNA incorporation approach, there are still limitations to this approach. Most notably, NP material choices are limited as a cationic charge is essential to facilitate electrostatic interactions with anionic RNA. Potential materials that can be used to overcome this obstacle and successfully facilitate RNA adsorption to NPs are discussed in section 2.5.
2.3. Layer-by-layer approach
Some formulations exploit electrostatic adsorption in order to encapsulate RNA molecules using a LbL approach, where sequential immobilization of RNA and cationic lipids are used to create a multi-layered NP. A NP core is usually incubated with the desired component to create another layer. After centrifugation to remove any free molecules, incubation is repeated with another component until multilayer particles are produced. This method combines the ease of simple adsorption with the benefit of using multiple layers to optimize and increase RNA loading to the NP, while also protecting the RNA from degradation (Gu et al., 2017). This method has been successfully used to attach siRNA to gold NPs surrounded by layers of PEI (Fig. 4) (Elbakry et al., 2009). LbL NPs also successfully prevented both luciferase and green fluorescent protein (GFP) mRNA degradation, which are common models used to assess mRNA delivery capability. These NPs had low toxicity and high transfection rates in vitro in dendritic cells (DCs) (Lacroix et al., 2020). Two types of LbL NPs have been used to deliver miRNA-34a to triple-negative breast cancer cells. Spherical PLGA cores surrounded by alternating layers of poly-L-lysine (PLL) and miRNA-34a successfully suppressed four target genes in vitro, resulting in reduced cell proliferation (Kapadia et al., 2020). Negatively-charged gold NPs were also coated with alternating layers of PLL and miRNA-34a and exhibited cellular uptake, target gene inhibition and suppression of cell proliferation (Goyal et al., 2018). Furthermore, this LbL formulation was more effectively internalized than simple NPs consisting of the miRNA bound to PLL. These studies highlight the wide variety of NP types and surface layers that can be exploited for RNA incorporation and therapeutic delivery.
Figure 4.
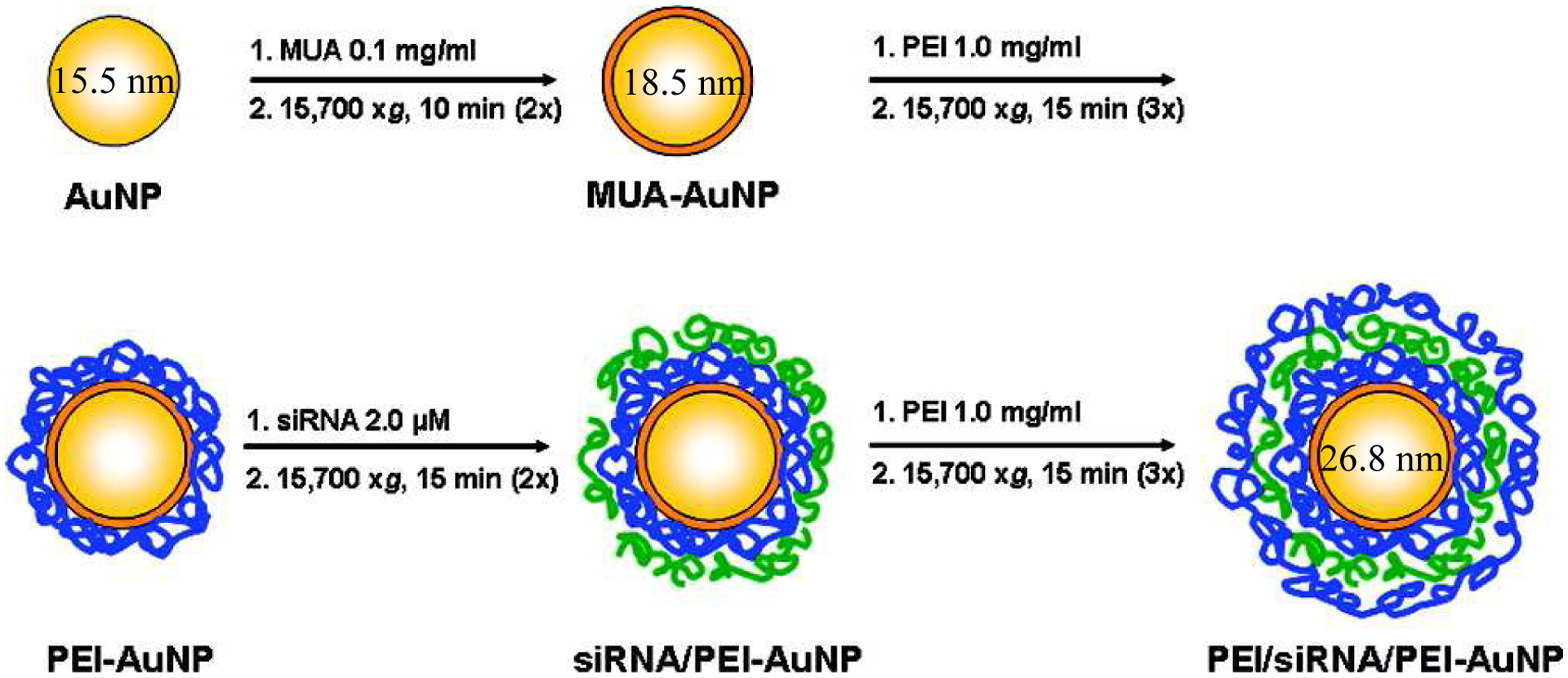
Scheme indicating the layer-by-layer formation of NPs for siRNA delivery, adapted with permission from (Elbakry et al., 2009). Copyright 2009 American Chemical Society. Gold NPs (AuNP) were coated with 11-mercaptoundecanoic acid (MUA) to facilitate binding of the subsequent layers. Poly(ethylene imine) (PEI; molecular weight = 25 kDa) was then added to create a positive surface for siRNA to be attached. Finally, an outer layer of PEI was added to protect the siRNA from premature release or degradation in the serum. The hydrodynamic diameter of the NPs at some steps are indicated in nanometers. 1. indicates the compound and its concentration when added to form the next layer, and 2. indicates the centrifugation settings used to remove any unbound material after each 30 min incubation.
The major disadvantage of the LbL approach is its complex methodology compared to simple encapsulation or adsorption. Furthermore, a stable NP core that can be centrifuged is essential to ensure removal of free molecules at each step of layer attachment. Additionally, the material choice for each layer must be precise to enable electrostatic binding of RNA. This complexity may limit the translatability and reproducibility of this approach.
2.4. Other approaches for RNA attachment
A slightly different encapsulation approach was achieved in a study using peptide amphiphile micelles by incorporating covalent binding of miRNA (D. Chin et al., 2020). Thiolated miRNA-145 was conjugated to DSPE-PEG(2000)-maleimide and the resulting conjugates were used to form micelles via TLFH. This covalent bond protected miRNA from nuclease degradation but was readily cleaved at cytosolic glutathione levels after cell internalization in vitro. This system was successfully applied to a mouse model of atherosclerosis, where lesion growth was reduced by 49%. Similar approaches have been used to create polymer conjugates via terminal nucleotide modification of siRNA to facilitate coupling to PEG, poly(lactic-co-glycolic acid) (PLGA), hyaluronic acid (HA), and dextran, among others, which are then used to make NPs (Hong & Nam, 2014). Thiolation of RNA molecules, including siRNA targeting the bicoid gene, for attachment to silver or gold NPs has also been indicated as an effective way to facilitate stable RNA loading and subsequent delivery (Aali et al., 2020; Yamankurt et al., 2020).
2.5. Choosing the best method for RNA incorporation
The decision to use a specific RNA loading method must consider the biological barriers that the NP will encounter. For example, encapsulation may not be the optimal choice for inhalable formulations if the overall charge of the final product is positive. The negatively charged airway surface liquid may interact with such positively charged NPs, causing them to disassemble or aggregate over time (Yıldız-Peköz & Ehrhardt, 2020). Additionally cationic NPs tend to be cytotoxic, though the overall positive charge may be shielded by the attached RNA (Lin et al., 2020). Differences in RNA loading, in terms of physical clustering on the NP surface or in terms of absolute quantity of RNA incorporated, may be achieved depending on which method is used and should be taken into account when designing the NP delivery vehicle (Corti et al., 2020; Cox et al., 2019).
Multiple studies have been carried out comparing the efficacy of RNA delivery when adsorbed to or encapsulated within NPs. siRNA adsorbed to preformed PLGA NPs was shown to be more effective than encapsulation by producing NPs that were more monodisperse, smaller than 100 nm, and with high RNA association (Ceylan et al., 2020). Furthermore, the adsorbed formulation was able to silence the target GPR-87 gene in HEK 293 T cells >40 times more effectively than the encapsulated siRNA, possibly due to the limited release of encapsulated RNA. In a study directly comparing a LbL NP incorporating mRNA and mRNA adsorbed to pre-made NPs, both formulations showed low toxicity in vitro in DCs, and complete protection of mRNA degradation in serum. Although both formulations showed successful transfection of model GFP and luciferase mRNA, the LbL approach had superior efficiency, emphasizing the importance of comparing multiple approaches for each RNA type (Lacroix et al., 2020). However, it is important to note that adsorption alone is often a simpler and faster process than LbL or encapsulation, so the advantages and disadvantages must be carefully weighed before choosing an approach for any given NP-RNA combination.
Conversely, although adsorption has been extensively shown to stabilize and protect RNA from degradation, one study found that surface bound siRNA degraded rapidly (1 hour) in serum compared to siRNA encapsulated in dendrimer-based NPs, which was stable for >24 hours (Raval et al., 2019). Additionally, a recent study directly compared four cationic NP types (liposomes, solid lipid NPs, polymeric NPs, and nanoemulsions) in their ability to deliver an mRNA vaccine when the mRNA was either encapsulated or adsorbed in order to evaluate which incorporation method was best for in vivo applications (Anderluzzi et al., 2020). All formulations contained DOTAP or DDA. The authors show that mRNA encapsulated within DOTAP polymeric NPs and liposomes along with DDA liposomes, evoked the highest antigen expression in vitro in BHK cells compared to NPs with mRNA adsorbed to the surface, potentially by more effectively preventing RNA degradation by nucleases, though this hypothesis was not further tested by the authors. The superior efficacy of encapsulated RNA was verified in vivo, with mRNA encapsulated in DOTAP polymeric NPs showing the highest efficacy in BALB/c mice. Similarly, self-amplifying RNA encoding HIV-1 Env gp140 as a model antigen was either adsorbed or encapsulated in solid lipid NPs (Blakney et al., 2019). The authors found that RNA was equally protected from RNAse degradation in vitro and both methods resulted in functional delivery and antibody protection in vitro and in vivo. Based on the literature, the differences in efficacy of adsorption vs. encapsulation as RNA incorporation methods into NPs vary broadly depending on the combination of RNA type and NP formulation used, indicating that multiple approaches should be attempted in the initial characterization of such delivery vehicles to optimize each formulation for RNA therapy.
Examples of methods used for RNA loading into NPs are provided in Table 1. However, it should be noted that many publications do not include sufficient information to determine initial RNA concentrations or final RNA loading amount. Specifically, many studies report the initial amount of RNA used for loading but do not consider the unincorporated RNA in the final nanoformulation. The discrepancy between actual vs. reported RNA amount can hinder translational potential and must be more precisely reported for reproducibility. In addition, the terms “encapsulation” and “adsorption” are often misused in the literature. Many authors describe RNA as being encapsulated in NPs, but their methods and characterization results indicate that they are in fact adsorbed on the surface following incubation with pre-formed NPs. When reporting techniques and methods of RNA incorporation in NPs and characterization of the resulting complexes, nomenclature clarity along with standardized methods of RNA loading will be essential to apply these technologies toward the clinic.
Table 1.
Examples of NPs and methods used for RNA loading.
| NP Components | Type of RNA | μg of RNA/mg of NP | Method of Incorporation | Reference |
|---|---|---|---|---|
| Gold | siRNA | 7×108 | Adsorption | Yamankurt et al., 2020 |
| CTAB | HIV TAR RNA, U4 snRNA | 55.5 | Encapsulation | Workman & Flynn, 2009 |
| DOTAP, cholesterol (1:1 ratio), DSPEPEG, DSPE-PEG-AA | siRNA | 103.4 | Encapsulation | Wang, Xu, et al., 2013, Xu et al., 2014 |
| ionizable cationic lipid (proprietary to Acuitas), phosphatidylcholine, cholesterol, PEG-lipid (50:10:38.5:1.5 ratio) | mRNA | 50 | Encapsulation | Pardi, Hogan, et al., 2017, Pardi, Secreto, et al., 2017 |
| Stöber silica | RNA | 333 | Adsorption | Hikosaka et al., 2018 |
| DOTAP, DOPC, cholesterol (2:1:3 ratio) | siRNA | 92.6 | Adsorption | Wu et al., 2019 |
| Human serum albumin | RNA | 33 | Adsorption | Wen et al., 2017 |
| DOTAP, cholesterol (1:1 ratio), DSPEPEG, DSPE-PEG-AA | mRNA | 215.5 | Adsorption | Wang, Su, et al., 2013 |
| mPEG-bPEI-PAsp(DIP-BzA), simvastatin | siRNA | 92.6 | Adsorption | Huang et al., 2018 |
| DSPC, POPG, Cholesterol) (7:2:1 ratio) | siRNA/miRNA (combined together) | 66.2 | LbL | Gu et al., 2017 |
| Gold, PEI | siRNA | 24.4 | LbL | Elbakry et al., 2009 |
| PLGA, PLL | miRNA | 56.6 | LbL | Kapadia et al., 2020 |
| Gold, silica, PLL | miRNA | 35.4 | LbL | Goyal et al., 2018 |
| PLGA | siRNA | 8.5×10−4 | Encapsulation | Ceylan et al., 2020 |
| DDA bromide, DOTAP | Self-amplifying RNA | 1.2×104 | Encapsulation and adsorption | Blakney et al., 2019 |
3. Targeting
The most successful NP-based therapies used in the clinic, Doxil® and the COVID-19 vaccines from Pfizer-BioNTech and Moderna Therapeutics, do not use active targeting strategies. Doxil® relies on the enhanced permeability and retention (EPR) effect, a hypothesis postulating that NPs tend to accumulate in tumor tissue due to alterations in blood and lymph flow and dysregulation of the endothelial lining of tumor cells (Shi et al., 2020). In addition, current COVID-19 vaccines under EUA do not target a specific cell type. Instead, mRNA translation occurs in local cells, including epithelial cells, adipocytes, myocytes, fibroblasts, keratinocytes, and immune cells, at the intramuscular injection site (Blakney et al., 2021; Iavarone et al., 2017; Tan et al., 2020) or are cleared to the liver, where translation occurs. The resulting proteins are then released into the circulation to interact with immune cells that produce antibodies (Guevara et al., 2020). Generally, vaccines do not require targeting, as mRNA translation of antigens can occur in any cell type and subsequently induce antibody production in local immune cells.
Most RNA therapies, however, will require delivery to a specific cell type for therapeutic effect, particularly for protein replacement therapies. Based on this requirement, a staggering number of non-targeted RNA NPs in clinical trials have failed due to insufficient delivery to the target site (Herrera et al., 2018). Therefore, NP modification to enable specific targeting should be considered for RNA NP formulations. (Chu et al., 2016; Huang et al., 2016; Tietjen et al., 2017). It has been established that nonspecific expression/suppression of protein production by RNA can have detrimental effects, as shown with factor VII, factor IX, and interleukin-12 (Dammes & Peer, 2021). For this reason, targeted NPs have been employed to ensure precise delivery of RNA therapeutics to the desired cell type in a variety of disease states, e.g., to tumor cells for cancer treatment (Fig. 5) (Ho et al., 2021). It is important to note that targeting can be affected by both the disease and age states, due to dysregulation of specific receptors and/or biological barriers (Magro et al., 2018). Therefore, it is essential to use in vitro and in vivo models incorporating as many relevant variables as possible, including pathology and aging, when validating targeted RNA nanotherapeutics. Here we describe some targeting moieties that have been used for specific delivery of NPs incorporating RNA therapeutics, along with modifications in NP structure and administration route for targeted RNA delivery. Although the list of potential targeting agents and modifications is extensive, we highlight some of the most common strategies used, along with recent, novel, and promising contributions to the field of RNA NP targeted delivery primarily developed in the last five years.
Figure 5.
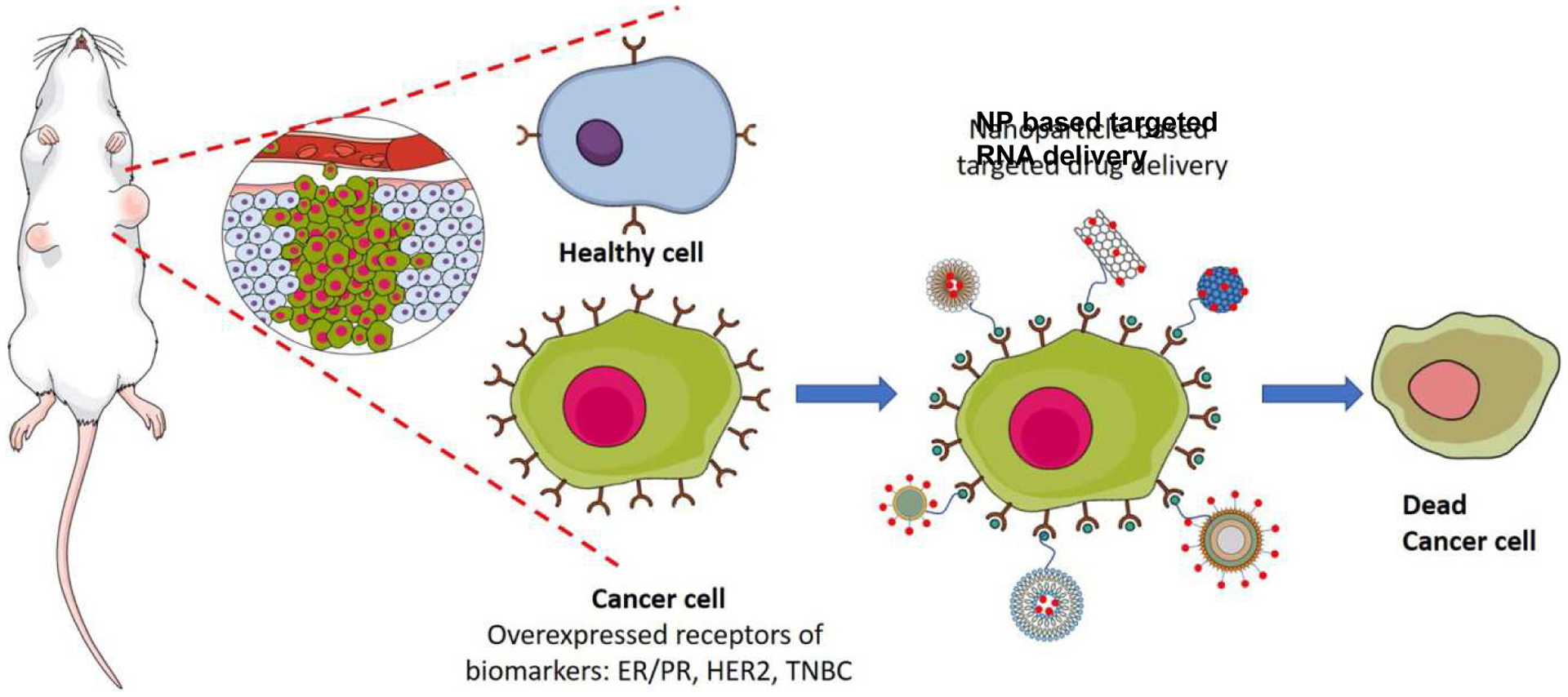
Scheme showing specific targeting of cancer cells by NPs, adapted with permission from (Liyanage et al., 2019). Cancer cells can be specifically targeted as they overexpress receptors such as the estrogen receptor and progesterone receptor (ER/PR) and human epidermal growth factor receptor (HER2) compared to healthy cells. Ligands for these receptors can be attached to NPs containing RNA, facilitating their preferential uptake and therapeutic effects in cancer cells vs. healthy cells.
3.1. Types of targeting moieties
3.1.1. Peptides as targeting moieties
Targeting is commonly achieved via incorporation of peptides that specifically bind to a receptor that is highly expressed on the target cell compared to off-target cells. The most common method for peptide incorporation into RNA delivery vehicles exploits maleimide-thiol reactions via conjugation of lipids/polymers or NPs with an attached maleimide to peptides containing a free cysteine. This covalent binding is generally accepted to be stable in vitro and in vivo (Wang et al., 2017). For example, two peptide sequences (REKA, VHPKQHR) targeting fibrin and vascular cell adhesion protein (VCAM-1) respectively were incorporated into micelles for targeted delivery of anti-miRNA to treat atherosclerosis. This peptide-based targeting successfully promoted specific uptake by macrophages and inflamed endothelial cells, and facilitated the functional inhibition of miRNA-33a and miRNA-92a (Kuo et al., 2014). Angiopep-2 peptide was effectively used to target low-density lipoprotein receptor-related protein (LRP), which is overexpressed on both the blood brain barrier (BBB) and glioblastoma multiforme (GBM) cells. This dual targeting by a single peptide promoted BBB crossing and specific tumor delivery of TGF-ß siRNA (Qiao et al., 2018). Tumor-associated macrophages have been targeted using a-peptide, a scavenger receptor B type 1 targeting peptide, and M2pep, an M2 macrophage binding peptide, to deliver siRNA against anti-colony stimulating factor-1 receptor. This formulation eliminated macrophages by 52% from melanoma in mice (Qian et al., 2017). The same M2pep was used to deliver gold NPs with anti-vascular endothelial growth factor (VEGF) siRNA to treat lung cancer in mice (Conde et al., 2015). Vascular smooth muscle cell plaque targeting has been achieved using micelles incorporating CCR2-binding peptides for delivery of miRNA-145 (D. D. Chin et al., 2020). This platform prevented lesion growth by ~50% in a mouse model of early-stage atherosclerosis. The wide variety of biological peptides identified, along with the extensive use of phage display technology to identify synthetic peptides that preferentially bind to specific receptors, makes peptide incorporation a versatile and comprehensive targeting strategy.
3.1.2. Proteins as targeting moieties
Whole proteins, including antibodies, can also be applied for cell targeting, although their effective use is limited by their size. The transferrin receptor (Tfr) is commonly used for specific delivery due to its upregulation in multiple disease states. For example, transferrin was conjugated to PEI for selective siRNA delivery to activated T cells in the asthmatic lungs of mice (Xie et al., 2016). Apolipoprotein A-I (ApoA-I) has been used for liver targeting of siRNA to treat hepatitis B-infected mice (Kim et al., 2007).
Monoclonal antibody incorporation on the NP surface has been used for targeted delivery of RNA to leukocytes (Peer et al., 2008), DCs (Zheng et al., 2009), and CD4+ T cells (Ramishetti et al., 2015). Peer et al. covalently attached anti-ß7 integrin mAbs to hyaluronan on unilamellar vesicles which encapsulated protamine-condensed siRNA targeting cyclin D1. This antibody incorporation facilitated ß7 integrin specific leukocyte uptake of NPs, thereby inducing an anti-inflammatory effect in mice with dextran sodium sulfate-induced colitis (Peer et al., 2008). siRNA has also been embedded in polyelectrolyte layers coating a polymeric core, with an outer layer of CD20 antibodies conjugated to HA to facilitate dual targeting of blood cancer cells (Choi et al., 2019). This system successfully suppressed B-cell lymphoma (BCL)2 protein expression and cell proliferation in vitro and in vivo. These studies demonstrate that whole proteins can be used for targeting, despite their larger size compared to peptides.
3.1.3. Other targeting moieties
Other targeting moieties have shown some success in vitro and in vivo. Dual targeted chitosan NPs were developed to deliver siRNA targeting P21-activated kinase 1, which is overexpressed in many cancers, to hepatocellular carcinoma cells (Zheng et al., 2020). The targeting ligands used were: 1) Lactobionic acid, the galactose of which binds the asialoglycoprotein receptor. This receptor is overexpressed on the hepatocellular carcinoma cell membrane. 2) Glycyrrhetinic acid, which binds to the glycyrrhetinic acid receptor. This receptor is overexpressed on hepatocytes, particularly hematoma cells. These NPs enhanced both targeting and cell uptake in BALB/c nude mice bearing Hep3B tumors compared to non-targeted NPs. Folate conjugation to NPs has also been used to deliver functional siRNA to GBM, as folate receptors are commonly overexpressed in cancer cells in order to promote DNA replication (Yoo et al., 2021). Additionally, receptors for mannose, a sugar monomer, on DCs have been targeted in vivo in the development of anti-cancer vaccines (Mockey et al., 2007; Perche et al., 2011; Pichon & Midoux, 2013), as such targeting has been shown to increase internalization into the target cell (Pei et al., 2021).
3.2. Modifying NP structure for targeting
Targeting can also be achieved by altering the NP structure itself. For example, lipid NPs incorporating the helper lipid 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) were shown to preferentially interact with ApoE and thereby accumulated in the liver, while those with DSPC targeted the spleen (Zhang et al., 2020). The DOPE formulation doubled firefly luciferase mRNA delivery to the liver and increased liver transfection three-fold in mice. The DSPC-based NPs showed a similar increase in delivery of the same mRNA as well as Cy3-siRNA. Interestingly, in vaccine development, it has been shown that a cationic charge is essential to retain NPs at the injection site, prolonging interaction with innate immune cells and subsequent success of mRNA transfection and translation (Henriksen-Lacey et al., 2010). This knowledge can be used to develop more effective vaccines by using encapsulation to maintain a cationic surface charge on NPs.
3.3. Administration routes for targeting
NP administration route, which has often been shown to determine NP localization, can also be used to optimize RNA therapeutic targeting. RNA NPs are most frequently injected IV in preclinical studies, with injection site varying from the tail vein in mice for systemic administration to subretinal injection for targeted treatment of monogenic retinal degeneration (S. Patel et al., 2019) or glaucoma (Dillinger et al., 2018). In a comprehensive study, the most efficient renal localization occurred when NPs were administered IV in the tail vein, compared to retro-orbital vein injection, oral gavage or injection in the subcutaneous flank or intraperitoneal regions of mice (Williams et al., 2017). The same study showed that dosage affected targeting ability, with the administration of a decreased NP dose corresponding to decreased non-specific organ accumulation. The use of alternative administration routes not only offers hope for more targeted and effective RNA delivery, but could also allow for safer, more convenient treatment and better patient compliance (Eek et al., 2016).
For lung diseases, intranasal and inhalable RNA NPs have been exploited to treat respiratory conditions including asthma, chronic obstructive pulmonary disease (COPD), and lung cancer (Gómez-Aguado et al., 2020; Kankala et al., 2018; A. K. Patel et al., 2019; Xu et al., 2018; Xu et al., 2021). Lung cancer treatment has been investigated using NPs that co-deliver the anti-cancer drugs doxorubicin and cisplatin, and two types of siRNA to suppress multidrug resistance-associated protein (MRP)-1 and BCL2 mRNA (Taratula et al., 2011). Inhalation of this formulation led to preferential accumulation in the mouse lungs, with minimal escape into the systemic circulation and limited deposition in off-target organs. Encapsulation is particularly useful when creating inhalable formulations, as this method can protect the integrity of the RNA molecule during the drying process (Xu et al., 2018). For example, encapsulation within solid lipid NPs protected the structure and function of tumor necrosis factor (TNF)-α siRNA following thin-film freeze-drying to create aerosolizable dry powders for lung delivery to treat a variety of respiratory illnesses including asthma (Wang et al., 2021). Furthermore, mannitol can be used for inhalable RNA NP optimization, as it has been shown to enhance stability of siRNA-lipid-polymer NP complexes, possibly by replacing water as a hydrogen-bonding agent during lyophilization and acting as a barrier via matrix formation to prevent aggregation (Dormenval et al., 2019).
Transdermal delivery of NPs containing RNA has been achieved using multiple methods. Mesoporous silica NPs were coated with siRNA targeting transforming growth factor-ß receptor-1 (TGFßR-1) and PLL, mixed with moisturizer and topically applied on a squamous cell carcinoma tumor in a mouse xenograft model (Lio et al., 2019). Following treatment every three days for 18 days, tumor growth was not significant. This was compared to the application of a scrambled siRNA sequence which resulted in a three-fold increase in tumor size. Similarly, gold NPs with covalently attached siRNA designed to knock out epidermal growth factor receptor (EGFR), were mixed with the petrolatum-based moisturizer Aquaphor® and topically applied on SKH1-E hairless and C57BL/6J mice. This siRNA was chosen as a model due to its importance in epidermal cell proliferation, and its potential for use in cancer, where it is often overexpressed. The attached siRNA could penetrate human epidermal keratinocytes in vitro, and reduced EGFR mRNA levels by 52% after three week treatment (three applications per week) (Zheng et al., 2012). Alternatively, microneedle patches consisting of a HA matrix with mesoporous silica-coated NPs have been used for TGFßR-1 siRNA delivery through the stratum corneum (M. Wang et al., 2020).
Along with non-invasive transdermal delivery, oral delivery of RNA has been investigated using many NP platforms, including mannose-modified trimethyl chitosan-cysteine (MTC) polymers (He et al., 2020) and lipidoid NPs (Ball et al., 2018). Iqbal et al. demonstrated the importance of surface charge when administering RNA within NPs by developing PLGA-PEG NPs coated with amine group-containing lipids with varying surface charges to deliver TNF-α siRNA. The aminated NPs, but not carboxylated or neutrally-charged ones, significantly accumulated in the inflamed colons of mice and suppressed mRNA expression and TNF-α secretion (Iqbal et al., 2018).
Although incorporation of active targeting moieties and optimization for administration route can increase the amount of RNA delivered in NPs to the target site, modifications are still required to ensure adequate cell uptake. Subsequent endosomal escape is also essential for NPs to reach the cytosol where protein translation/silencing occurs. Below, modifications to enhance both cellular uptake and endosomal escape are discussed.
4. Cellular uptake and endosomal escape
Intracellular delivery is vital for successful protein translation or silencing, and NP modifications can ensure effective penetration of the cell membrane. There are various mechanisms of cellular uptake of RNA lipid NPs, with clathrin-dependent endocytosis and micropinocytosis being the most common. NPs taken up by the latter pathway show higher efficacy (Evers et al., 2018; Gilleron et al., 2013; Wang & Huang, 2013). Clathrin- and caveolin-dependent endocytosis are both receptor-mediated, thereby allowing targeting and promotion of cellular uptake via appropriate ligand incorporation on the NP surface (Donahue et al., 2019). Alternatively, NPs can be internalized by immune cells such as macrophages, neutrophils, and DCs by phagocytosis (Ahmad et al., 2019; Donahue et al., 2019). In this case, NPs must bind to a phagocytic cell surface receptor, e.g., mannose receptor, before being internalized in phagosome vesicles (Donahue et al., 2019).
Along with poor cellular uptake, a major factor limiting the efficacy of NP-based RNA therapies is insufficient endosomal escape into the cytoplasm. Following internalization, only NPs that can escape the endosome are able to exert their intended therapeutic effects, making it an essential aspect of NP formulation (Donahue et al., 2019; Martens et al., 2014). In this section, we will discuss the incorporation of cell penetrating peptides (CPPs) on NPs to enhance cellular uptake, along with methods to promote endosomal escape and increase the efficacy of RNA therapeutics.
4.1. Cell penetrating peptides
Along with targeting, peptides are commonly used to promote cellular uptake. CPPs can disrupt the lipid bilayer, or enhance micropinocytosis by triggering the grouping of anionic glycosaminoglycans on the cell surface (Vallazza et al., 2015). As most CPPs are positively charged, they have the added benefit of condensing mRNA, allowing for more effective delivery. The HIV-1 transactivator of transcription derived (TAT) peptide is a well characterized and widely used CPP. When conjugated to lipid NPs, siRNA uptake increased in the mouse lung in vivo, knocking down p38 MAP kinase mRNA by 30–45% after six hours (Moschos et al., 2007). The CPP Hph1 was conjugated to PEG-siRNA with KALA peptides to increase cellular uptake and subsequent gene silencing in vitro (Choi et al., 2010). KL4 can mediate siRNA and mRNA cell transfection in vitro in A549 human lung epithelial cells and BEAS-2B human bronchial epithelial cells (Qiu et al., 2017; Qiu et al., 2020; Qiu et al., 2019). The cationic CPPs RALA, LAH4 and LAH4-L1 have been used to facilitate mRNA adsorption to PLA NPs and to promote cellular uptake in DCs in vitro (Coolen et al., 2019). The amphipathic RALA peptide has also been used in vaccine formulation to condense mRNA into nanocomplexes capable of escaping endosomes and inducing an immune response (Udhayakumar et al., 2017). If insufficient cellular uptake of RNA NPs is observed in in vitro studies, incorporation of a well-established CPP should be considered.
4.2. NP modifications to promote endosomal escape
After NPs are internalized through endocytosis into an early endosome, the endosome can mature from an early to late stage endosome, during which acidification can occur (Donahue et al., 2019; Martens et al., 2014). During acidification, ATP-dependent proton pumps transport hydrogen ions into the endosomes, thus lowering the pH inside the vesicle. Due to the relatively negative charge of the phospholipids comprising the outer layer of the endosomal membrane, interaction with the sudden influx of positively charged hydrogen ions causes the endosomal membrane to destabilize (Martens et al., 2014). During this destabilization period, the NP may be able to escape from the endosome or the endosome may fuse with a lysosome (Donahue et al., 2019; Martens et al., 2014). NPs and their internal constructs may become degraded in these lysosomes, recycled in the perinuclear region, or exocytozed from the cell. Those that are capable of escaping the lysosome are then able to exert their intended therapeutic effects.
Many researchers have added surface modifications that can destabilize the endosomal membrane or have taken advantage of pH changes during endosomal maturation to enhance endosomal escape of NPs containing therapeutic RNA (Fig. 6). Such modifications are described below.
Figure 6:
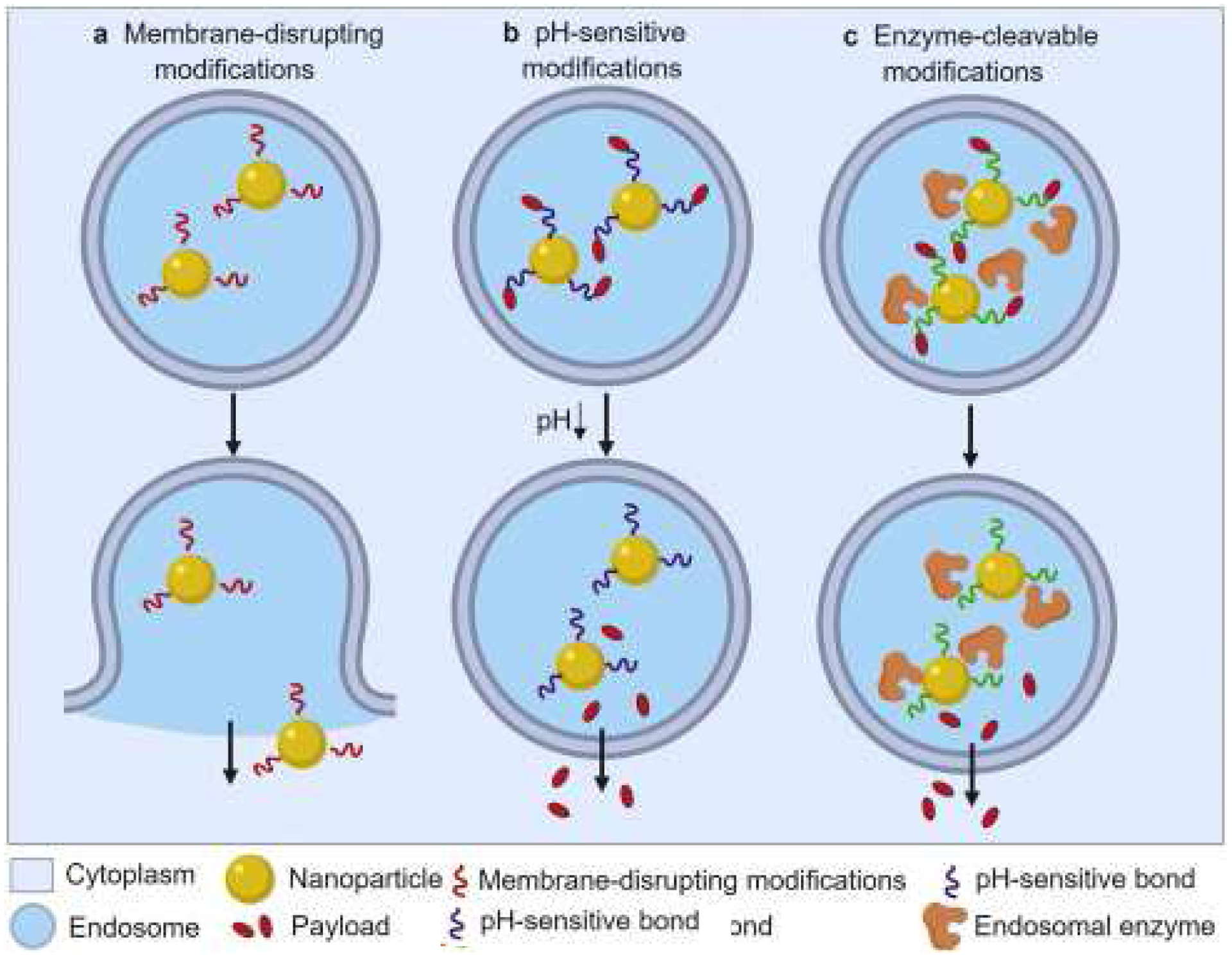
Scheme showing NP modifications to enhance endosomal escape, modified with permission from (Donahue et al., 2019). a) Endosomal membrane-disrupting modifications on the NP can form openings by which the NP can 1) escape with its RNA cargo or 2) promote NP fusion to the endosomal membrane and release RNA directly into the cytoplasm. b) pH-sensitive modifications. The acidic environment in endosomes weakens the bonds that bind the NPs to the RNA cargo, thus allowing the RNA to escape the endosome
4.2.1. Membrane disrupting modifications
Membrane destabilizing modifications target the transformation stage from a late endosome to a lysosome. At this stage, the membrane is naturally unstable, and the endosomal membrane can be disrupted through pore formation, rupture, or fusion (Fig. 7). Late endosome instability can be exacerbated through intelligent NP design, most commonly with the use of cationic polymers and lipids to form pores or rupture the endosomal membrane (Martens et al., 2014). For example, PEI has a large number of protonable amine groups and can act as a “proton sponge” with a high buffering capacity (Boussif et al., 1995). The abundant binding of cations to the surface of PEI leads to an influx of chloride ions and water into the endosome, inducing endosome swelling and rupture (Martens et al., 2014). Through this mechanism, PEI NPs successfully delivered siRNA to treat GBM in vitro as well as miRNA to treat prostate cancer both in vitro and in vivo (Melamed et al., 2018; Zhang et al., 2015). While PEI can efficiently disrupt the endosome and allow RNA NP endosomal escape, it has high cellular toxicity. This has been combatted through conjugation with other polymers such as chitosan (Mainini & Eccles, 2020).
Figure 7.
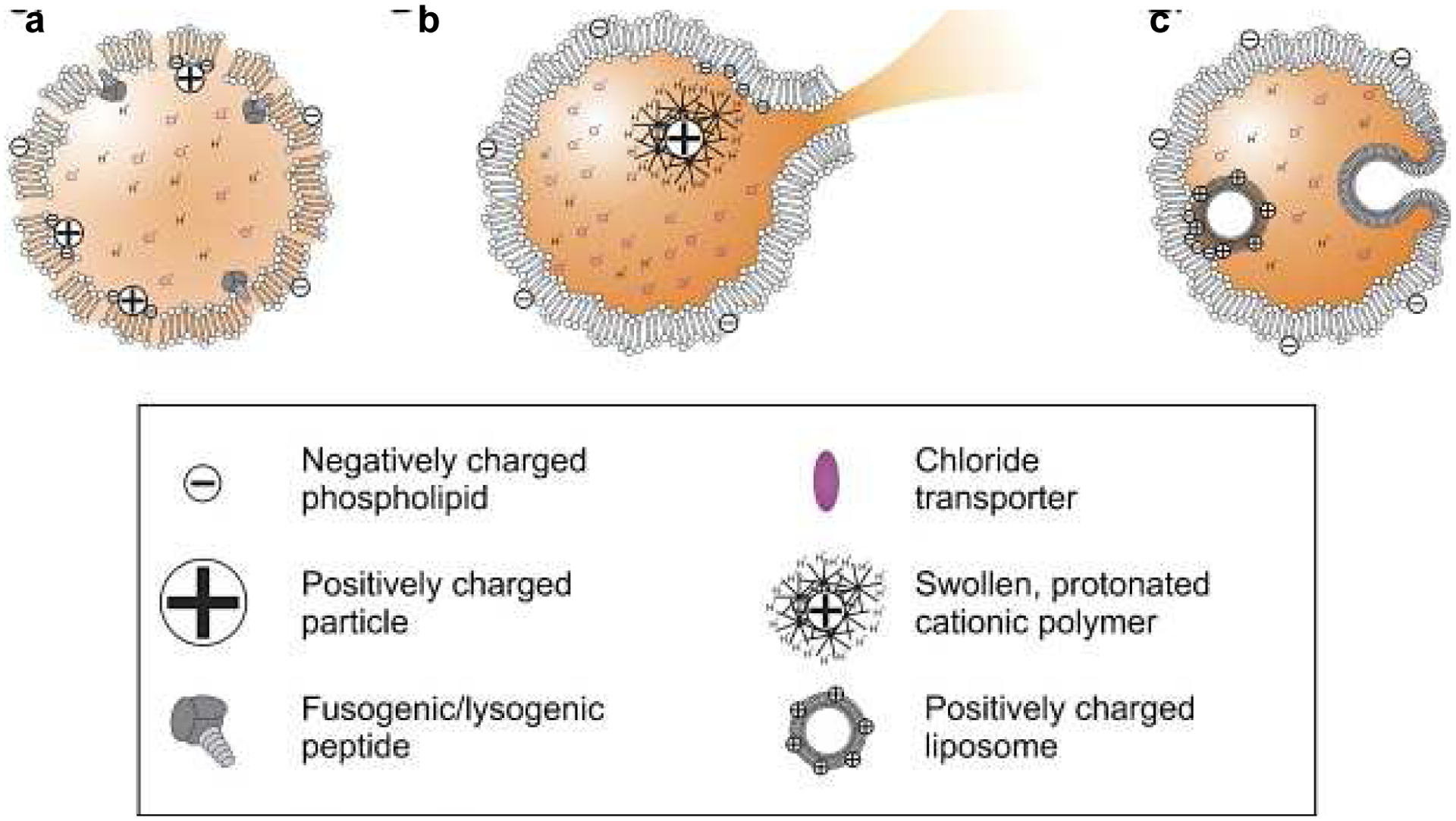
Scheme displaying different types of membrane destabilization that can lead to endosomal escape, reprinted with permission from (Martens et al., 2014). a) Pore formation on the endosomal membrane due to cationic molecules or fusogenic peptides. b) Endosomal rupture due to osmotic swelling caused by an increased cationic charge. c) Fusion of the endosomal membrane and the NP (e.g., positively charged liposome) allowing for RNA release from the NP into the cytoplasm.
Along with their role in enhancing cellular uptake, CPPs can be used to enhance endosomal release (Ahmad et al., 2019; Donahue et al., 2019; Remaut et al., 2007). CPPs generally form a secondary amphipathic alpha-helical structure that displaces the endosomal lipid membrane in response to the low pH in the endosome/lysosome. This conformational change facilitates NP fusion to the endosome and RNA release into the cytosol (Ahmad et al., 2019; Liang & Lam, 2012). This concept was successfully applied when hemagglutinin-based NPs showed increased lysosomal escape of siRNA and increased cytotoxicity towards MCF-7 breast cancer cells (Guo et al., 2021).
4.2.2. pH-sensitive disruption
The acidic environment of the late endosome/lysosome can also be exploited by incorporating pH-sensitive polymers and amino acids into the NP membrane for RNA delivery. For example, poly(2-(diisopropylamino ethylmethacrylate) (PDPA) is a pH-sensitive polymer that is capable of self-assembly into NPs at neutral pH but disassemble after protonation at pH 6.51–6.85 (Jäger et al., 2015; Xu et al., 2016). Therefore, upon endosome acidification, the NPs disassemble, induce membrane instability, and release siRNA in vitro and in vivo (Xu et al., 2016). Alternatively, incorporation of arginine and lysine, which both protonate at an acidic pH, can promote endosomal escape (Mainini & Eccles, 2020). For example, decoration of apoferritin nanocages with lysine improved the anti-tumor effect exerted by siRNA delivery in 4T1 breast cancer mouse models compared to those lacking lysine (Huang et al., 2020). Similarly, arginine was added to PEI-based NPs to deliver siRNA to breast cancer cells (Lu et al., 2019). While PEI itself is usually sufficient to induce endosomal escape, it’s toxicity could be mitigated by the addition of arginine.
5. Conclusion
NPs have proven themselves effective and as safe delivery tools for RNA molecules in vitro, in vivo, and in clinical settings. Furthermore, they have shown impressive versatility, being applied in a wide range of pathological states as therapeutics and in preventative medicine through vaccination. As seen with the rapid development of lipid NP-based mRNA vaccines for COVID-19, RNA NP technology can be translatable and effective, and mass production of such therapies is feasible. The continued success of RNA NP therapy should continue to build on the strengths of previous successful formulations and the knowledge gathered to date.
In general, translatable RNA NP formulations should include (i) effective and stable RNA incorporation methods, (ii) appropriate targeting, (iii) sufficient cellular uptake and (iv) endosomal escape. The ease of NP multifunctionalization makes it possible to incorporate all these characteristics. The choice of NP material and modification should be optimized based on the RNA molecule to be delivered and the target site, as both factors can impact the efficacy of RNA delivery. This optimization is particularly important as the RNA construct in the NP can be easily exchanged for another, thus allowing for easy technology transfer to produce therapies for a variety of diseases. Initially, the stability and efficiency of RNA incorporation into NPs should be evaluated before application in vitro to identify if the specific nanocarrier chosen is appropriate for the RNA to be delivered. Furthermore, comprehensive characterization of the extent of cellular uptake at the target site and subsequent endosomal escape in vitro should be completed for each RNA NP complex to identify what specific modifications are essential for optimal delivery in vitro and in vivo.
As RNA molecules continue to be characterized as causative agents of disease or potential targets for therapy, NPs will offer a safe and efficient way to deliver them. The rapid increase in knowledge emerging related to NP processing in vivo will facilitate more advanced modifications. Utilization and optimization of the wide array of NP surface modifications available, individually or in combination, presents a great opportunity to enhance NP delivery efficacy.
Acknowledgements
The authors would like to acknowledge Madelynn Tung for their help with this article. This work was supported by the University of Southern California, New Innovator Award (NIH, DP2-DK121328), NSF EAGER from DMR BMAT 2132744 and WISE Major Support Award granted to E.J.C, and by the PKD Foundation postdoctoral fellowship 839636 to A.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests’ statement: The authors declare no competing interests.
References
- Aali E, Shokuhi Rad A, & Esfahanian M (2020). Computational investigation of the strategy of DNA/RNA stabilization through the study of the conjugation of an oligonucleotide with silver and gold nanoparticles [https://doi.org/10.1002/aoc.5690]. Applied Organometallic Chemistry, 34(8), e5690. 10.1002/aoc.5690 [DOI] [Google Scholar]
- Agency, E. M. (2018). Assessment report for Onpattro. https://www.ema.europa.eu/en/documents/assessment-report/onpattro-epar-public-assessment-report_.pdf
- Ahmad A, Khan JM, & Haque S (2019). Strategies in the design of endosomolytic agents for facilitating endosomal escape in nanoparticles. Biochimie, 160, 61–75. 10.1016/j.biochi.2019.02.012 [DOI] [PubMed] [Google Scholar]
- Amos H (1961). Protamine enhancement of RNA uptake by cultured chick cells. Biochemical and Biophysical Research Communications, 5(1), 1–4. [Google Scholar]
- Anderluzzi G, Lou G, Gallorini S, Brazzoli M, Johnson R, O’Hagan DT, … Perrie Y (2020). Investigating the Impact of Delivery System Design on the Efficacy of Self-Amplifying RNA Vaccines. Vaccines, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball RL, Bajaj P, & Whitehead KA (2018). Oral delivery of siRNA lipid nanoparticles: Fate in the GI tract. Sci Rep, 8(1), 2178. 10.1038/s41598-018-20632-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakney AK, Deletic P, McKay PF, Bouton CR, Ashford M, Shattock RJ, & Sabirsh A (2021). Effect of complexing lipids on cellular uptake and expression of messenger RNA in human skin explants. Journal of Controlled Release, 330, 1250–1261. 10.1016/j.jconrel.2020.11.033 [DOI] [PubMed] [Google Scholar]
- Blakney AK, McKay PF, Yus BI, Aldon Y, & Shattock RJ (2019). Inside out: optimization of lipid nanoparticle formulations for exterior complexation and in vivo delivery of saRNA. Gene Ther, 26(9), 363–372. 10.1038/s41434-019-0095-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussif O, Lezoualc h, F., Zanta MA, Mergny MD, Scherman D, … Behr JP (1995). A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences, 92(16), 7297. 10.1073/pnas.92.16.7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann MD, Carrasco MJ, Alishetty S, Paige M, Alameh MG, & Weissman D (2021). Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines, 9(1). 10.3390/vaccines9010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyens K, Demeester J, De Smedt SS, & Sanders NN (2009). Elucidating the encapsulation of short interfering RNA in PEGylated cationic liposomes. Langmuir, 25(9), 4886–4891. 10.1021/la803973p [DOI] [PubMed] [Google Scholar]
- Ceylan S, Bahadori F, & Akbas F (2020). Engineering of siRNA loaded PLGA Nano-Particles for highly efficient silencing of GPR87 gene as a target for pancreatic cancer treatment. Pharm Dev Technol, 25(7), 855–864. 10.1080/10837450.2020.1745232 [DOI] [PubMed] [Google Scholar]
- Chahal JS, Khan OF, Cooper CL, McPartlan JS, Tsosie JK, Tilley LD, … Anderson DG (2016). Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc Natl Acad Sci U S A, 113(29), E4133–4142. 10.1073/pnas.1600299113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbe NB, Amnerkar ND, Ramesh B, Tambuwala MM, Bakshi HA, Aljabali AAA, … Zacconi FC (2020). Small interfering RNA for cancer treatment: overcoming hurdles in delivery. Acta Pharm Sin B, 10(11), 2075–2109. 10.1016/j.apsb.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Zhang X, & Zhang H (2019). Fusion and clustering of spherical micelles by extruding through a cylindrical channel [10.1039/C9RA05146E]. RSC Advances, 9(42), 24394–24400. 10.1039/C9RA05146E [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Shan J, Niu W, Lin F, Liu S, Wu P, … Jiang G (2018). Micro RNA-155 inhibitor as a potential therapeutic strategy for the treatment of acute kidney injury (AKI): a nanomedicine perspective [10.1039/C7RA13440A]. RSC Advances, 8(29), 15890–15896. 10.1039/C7RA13440A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D, Poon C, Wang J, Joo J, Ong V, Jiang Z, … Chung EJ (2020). Nanoparticle-mediated microRNA-145 Delivery for Vascular Smooth Muscle Cell Phenotype Modulation and Atherosclerosis Treatment. bioRxiv. [Google Scholar]
- Chin DD, Poon C, Wang J, Joo J, Ong V, Jiang Z, … Chung EJ (2020). Nanoparticle-mediated microRNA-145 Delivery for Vascular Smooth Muscle Cell Phenotype Modulation and Atherosclerosis Treatment. bioRxiv, 2020.2009.2009.290361. 10.1101/2020.09.09.290361 [DOI] [Google Scholar]
- Choi KY, Correa S, Min J, Li J, Roy S, Laccetti KH, … Hammond PT (2019). Binary Targeting of siRNA to Hematologic Cancer Cells In Vivo Using Layer-by-Layer Nanoparticles [https://doi.org/10.1002/adfm.201900018]. Advanced Functional Materials, 29(20), 1900018. 10.1002/adfm.201900018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Lee SH, Mok H, & Park TG (2010). Multifunctional siRNA delivery system: polyelectrolyte complex micelles of six-arm PEG conjugate of siRNA and cell penetrating peptide with crosslinked fusogenic peptide. Biotechnol Prog, 26(1), 57–63. 10.1002/btpr.310 [DOI] [PubMed] [Google Scholar]
- Chu D, Zhao Q, Yu J, Zhang F, Zhang H, & Wang Z (2016). Nanoparticle Targeting of Neutrophils for Improved Cancer Immunotherapy. Advanced Healthcare Materials, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Beiss V, Fiering SN, & Steinmetz NF (2020). COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano, 14(10), 12522–12537. 10.1021/acsnano.0c07197 [DOI] [PubMed] [Google Scholar]
- Conde J, Bao C, Tan Y, Cui D, Edelman ER, Azevedo HS, … Tian F (2015). Dual targeted immunotherapy via. Adv Funct Mater, 25(27), 4183–4194. 10.1002/adfm.201501283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen AL, Lacroix C, Mercier-Gouy P, Delaune E, Monge C, Exposito JY, & Verrier B (2019). Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials, 195, 23–37. 10.1016/j.biomaterials.2018.12.019 [DOI] [PubMed] [Google Scholar]
- Corti R, Cox A, Cassina V, Nardo L, Salerno D, Marrano CA, … Mantegazza F (2020). The Clustering of mApoE Anti-Amyloidogenic Peptide on Nanoparticle Surface Does Not Alter Its Performance in Controlling Beta-Amyloid Aggregation. Int J Mol Sci, 21(3). 10.3390/ijms21031066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A, Vinciguerra D, Re F, Magro RD, Mura S, Masserini M, … Nicolas J (2019). Protein-functionalized nanoparticles derived from end-functional polymers and polymer prodrugs for crossing the blood-brain barrier. Eur J Pharm Biopharm, 142, 70–82. 10.1016/j.ejpb.2019.06.004 [DOI] [PubMed] [Google Scholar]
- Cui ZK, Sun JA, Baljon JJ, Fan J, Kim S, Wu BM, … Lee M (2017). Simultaneous delivery of hydrophobic small molecules and siRNA using Sterosomes to direct mesenchymal stem cell differentiation for bone repair. Acta Biomater, 58, 214–224. 10.1016/j.actbio.2017.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis PR, & Hope MJ (2017). Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol Ther, 25(7), 1467–1475. 10.1016/j.ymthe.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammes N, & Peer D (2021). Paving the Road for RNA Therapeutics. Trends in Pharmacological Sciences, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillinger AE, Guter M, Froemel F, Weber GR, Perkumas K, Stamer WD, … Breunig M (2018). Intracameral Delivery of Layer-by-Layer Coated siRNA Nanoparticles for Glaucoma Therapy [https://doi.org/10.1002/smll.201803239]. Small, 14(50), 1803239. 10.1002/smll.201803239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue ND, Acar H, & Wilhelm S (2019). Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev, 143, 68–96. 10.1016/j.addr.2019.04.008 [DOI] [PubMed] [Google Scholar]
- Dormenval C, Lokras A, Cano-Garcia G, Wadhwa A, Thanki K, Rose F, … Foged C (2019). Identification of Factors of Importance for Spray Drying of Small Interfering RNA-Loaded Lipidoid-Polymer Hybrid Nanoparticles for Inhalation. Pharm Res, 36(10), 142. 10.1007/s11095-019-2663-y [DOI] [PubMed] [Google Scholar]
- Eek D, Krohe M, Mazar I, Horsfield A, Pompilus F, Friebe R, & Shields AL (2016). Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer Adherence, 10, 1609–1621. 10.2147/PPA.S106629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbakry A, Zaky A, Liebl R, Rachel R, Goepferich A, & Breunig M (2009). Layer-by-layer assembled gold nanoparticles for siRNA delivery. Nano Lett, 9(5), 2059–2064. 10.1021/nl9003865 [DOI] [PubMed] [Google Scholar]
- Evers MJW, Kulkarni JA, van der Meel R, Cullis PR, Vader P, & Schiffelers RM (2018). State-of-the-Art Design and Rapid-Mixing Production Techniques of Lipid Nanoparticles for Nucleic Acid Delivery [https://doi.org/10.1002/smtd.201700375]. Small Methods, 2(9), 1700375. 10.1002/smtd.201700375 [DOI] [Google Scholar]
- Eygeris Y, Patel S, Jozic A, & Sahay G (2020). Deconvoluting Lipid Nanoparticle Structure for Messenger RNA Delivery. Nano Lett, 20(6), 4543–4549. 10.1021/acs.nanolett.0c01386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formicola B, Cox A, Dal Magro R, Masserini M, & Re F (2019). Nanomedicine for the Treatment of Alzheimer’s Disease. J Biomed Nanotechnol, 15(10), 1997–2024. 10.1166/jbn.2019.2837 [DOI] [PubMed] [Google Scholar]
- Freund I, Eigenbrod T, Helm M, & Dalpke AH (2019). RNA Modifications Modulate Activation of Innate Toll-Like Receptors. Genes (Basel), 10(2). 10.3390/genes10020092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, … Zerial M (2013). Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol, 31(7), 638–646. 10.1038/nbt.2612 [DOI] [PubMed] [Google Scholar]
- Goyal R, Kapadia CH, Melamed JR, Riley RS, & Day ES (2018). Layer-by-layer assembled gold nanoshells for the intracellular delivery of miR-34a. Cell Mol Bioeng, 11(5), 383–396. 10.1007/s12195-018-0535-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe S, Haas H, Diken M, Kranz LM, Langguth P, & Sahin U (2016). Translating nanoparticulate-personalized cancer vaccines into clinical applications: case study with RNA-lipoplexes for the treatment of melanoma. Nanomedicine (Lond), 11(20), 2723–2734. 10.2217/nnm-2016-0275 [DOI] [PubMed] [Google Scholar]
- Gu L, Deng ZJ, Roy S, & Hammond PT (2017). A Combination RNAi-Chemotherapy Layer-by-Layer Nanoparticle for Systemic Targeting of KRAS/P53 with Cisplatin to Treat Non–Small Cell Lung Cancer. Clinical Cancer Research, 23(23), 7312. 10.1158/1078-0432.CCR-16-2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara ML, Persano F, & Persano S (2020). Advances in Lipid Nanoparticles for mRNA-Based Cancer Immunotherapy. Front Chem, 8, 589959. 10.3389/fchem.2020.589959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara ML, Persano S, & Persano F (2019). Lipid-Based Vectors for Therapeutic mRNA-Based Anti-Cancer Vaccines. Curr Pharm Des, 25(13), 1443–1454. 10.2174/1381612825666190619150221 [DOI] [PubMed] [Google Scholar]
- Guo Y, Hu Y, Zheng X, Cao X, Li Q, Wei Z, … Zhang S (2021). Self-assembled peptide nanoparticles with endosome escaping permits for co-drug delivery. Talanta, 221, 121572. 10.1016/j.talanta.2020.121572 [DOI] [PubMed] [Google Scholar]
- Gómez-Aguado I, Rodríguez-Castejón J, Vicente-Pascual M, Rodríguez-Gascón A, Solinís M, & Del Pozo-Rodríguez A (2020). Nanomedicines to Deliver mRNA: State of the Art and Future Perspectives. Nanomaterials (Basel), 10(2). 10.3390/nano10020364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajj KA, & Whitehead KA (2017). Tools for translation: non-viral materials for therapeutic mRNA delivery. Nature Reviews Materials, 2(10), 1–17. [Google Scholar]
- Halbur C, Choudhury N, Chen M, Kim JH, & Chung EJ (2019). siRNA-Conjugated Nanoparticles to Treat Ovarian Cancer. SLAS Technol, 24(2), 137–150. 10.1177/2472630318816668 [DOI] [PubMed] [Google Scholar]
- He C, Yue H, Xu L, Liu Y, Song Y, Tang C, & Yin C (2020). siRNA release kinetics from polymeric nanoparticles correlate with RNAi efficiency and inflammation therapy via oral delivery. Acta Biomater, 103, 213–222. 10.1016/j.actbio.2019.12.005 [DOI] [PubMed] [Google Scholar]
- Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrøm T, Agger EM, Andersen P, & Perrie Y (2010). Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J Control Release, 145(2), 102–108. 10.1016/j.jconrel.2010.03.027 [DOI] [PubMed] [Google Scholar]
- Hernández M, Leyva G, Magaña JJ, Guzmán-Vargas A, Felipe C, Lara V, & Lima E (2019). New copolymers as hosts of ribosomal RNA. BMC Chem, 13(1), 33. 10.1186/s13065-019-0555-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera VL, Colby AH, Ruiz-Opazo N, Coleman DG, & Grinstaff MW (2018). Nucleic acid nanomedicines in Phase II/III clinical trials: translation of nucleic acid therapies for reprogramming cells. Nanomedicine (London, England), 13(16), 2083–2098. 10.2217/nnm-2018-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka R, Nagata F, Tomita M, & Kato K (2018). Elucidating the effect of different amino-functionalized spherical mesoporous silica characteristics on ribonucleic acid selectivity and adsorption capacity. Journal of Asian Ceramic Societies, 6(1), 70–81. 10.1080/21870764.2018.1443755 [DOI] [Google Scholar]
- Ho W, Gao M, Li F, Li Z, Zhang XQ, & Xu X (2021). Next-Generation Vaccines: Nanoparticle-Mediated DNA and mRNA Delivery. Adv Healthc Mater, e2001812. 10.1002/adhm.202001812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerr I, Obst R, Rammensee HG, & Jung G (2000). In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol, 30(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Hong CA, & Nam YS (2014). Functional nanostructures for effective delivery of small interfering RNA therapeutics. Theranostics, 4(12), 1211–1232. 10.7150/thno.8491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Yuan S, Ma Z, Ji P, Ma X, Wu Z, & Qi X (2020). Genetic recombination of poly(l-lysine) functionalized apoferritin nanocages that resemble viral capsid nanometer-sized platforms for gene therapy [10.1039/C9BM01822K]. Biomaterials Science, 8(6), 1759–1770. 10.1039/C9BM01822K [DOI] [PubMed] [Google Scholar]
- Huang J, Lin C, Fang J, Li X, Wang J, Deng S, … Shuai X (2018). pH-Sensitive Nanocarrier-Mediated Codelivery of Simvastatin and Noggin siRNA for Synergistic Enhancement of Osteogenesis. ACS Appl Mater Interfaces, 10(34), 28471–28482. 10.1021/acsami.8b10521 [DOI] [PubMed] [Google Scholar]
- Huang W-Y, Lin J-N, Hsieh J-T, Chou S-C, Lai C-H, Yun E-J, … Lin Y-H (2016). Nanoparticle Targeting CD44-Positive Cancer Cells for Site-Specific Drug Delivery in Prostate Cancer Therapy. ACS Appl. Mater. Interfaces, 8. [DOI] [PubMed] [Google Scholar]
- Iavarone C, O’hagan DT, Yu D, Delahaye NF, & Ulmer JB (2017). Mechanism of action of mRNA-based vaccines. Expert Rev Vaccines, 16(9), 871–881. 10.1080/14760584.2017.1355245 [DOI] [PubMed] [Google Scholar]
- Ickenstein LM, & Garidel P (2019). Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin Drug Deliv, 16(11), 1205–1226. 10.1080/17425247.2019.1669558 [DOI] [PubMed] [Google Scholar]
- Iqbal S, Du X, Wang J, Li H, Yuan Y, & Wang J (2018). Surface charge tunable nanoparticles for TNF-α siRNA oral delivery for treating ulcerative colitis. Nano Research, 11(5), 2872–2884. 10.1007/s12274-017-1918-3 [DOI] [Google Scholar]
- Jasinski D, Haque F, Binzel DW, & Guo P (2017). Advancement of the Emerging Field of RNA Nanotechnology. ACS Nano, 11(2), 1142–1164. 10.1021/acsnano.6b05737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger A, Jäger E, Surman F, Höcherl A, Angelov B, Ulbrich K, … Štěpánek P (2015). Nanoparticles of the poly([N-(2-hydroxypropyl)]methacrylamide)-b-poly[2-(diisopropylamino)ethyl methacrylate] diblock copolymer for pH-triggered release of paclitaxel [10.1039/C5PY00567A]. Polymer Chemistry, 6(27), 4946–4954. 10.1039/C5PY00567A [DOI] [Google Scholar]
- Kankala RK, Lin XF, Song HF, Wang SB, Yang DY, Zhang YS, & Chen AZ (2018). Supercritical Fluid-Assisted Decoration of Nanoparticles on Porous Microcontainers for Codelivery of Therapeutics and Inhalation Therapy of Diabetes. ACS Biomater Sci Eng, 4(12), 4225–4235. 10.1021/acsbiomaterials.8b00992 [DOI] [PubMed] [Google Scholar]
- Kapadia CH, Ioele SA, & Day ES (2020). Layer-by-layer assembled PLGA nanoparticles carrying miR-34a cargo inhibit the proliferation and cell cycle progression of triple-negative breast cancer cells. J Biomed Mater Res A, 108(3), 601–613. 10.1002/jbm.a.36840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana A, Allawadhi P, Khurana I, Allwadhi S, Weiskirchen R, Banothu AK, … Bharani KK (2021). Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today, 101142. 10.1016/j.nantod.2021.101142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SI, Shin D, Choi TH, Lee JC, Cheon GJ, Kim KY, … Kim M (2007). Systemic and specific delivery of small interfering RNAs to the liver mediated by apolipoprotein A-I. Mol Ther, 15(6), 1145–1152. 10.1038/sj.mt.6300168 [DOI] [PubMed] [Google Scholar]
- Kim T, Hyun HN, Heo R, Nam K, Yang K, Kim YM, … Roh YH (2020). Dual-targeting RNA nanoparticles for efficient delivery of polymeric siRNA to cancer cells. Chem Commun (Camb), 56(49), 6624–6627. 10.1039/d0cc01848a [DOI] [PubMed] [Google Scholar]
- Kim Y-K (2020). RNA Therapy: Current Status and Future Potential. Chonnam Medical Journal, 56(2), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Maeki M, Ishida A, Tani H, & Tokeshi M (2021). One-Step Production Using a Microfluidic Device of Highly Biocompatible Size-Controlled Noncationic Exosome-like Nanoparticles for RNA Delivery. ACS Applied Bio Materials, 4(2), 1783–1793. 10.1021/acsabm.0c01519 [DOI] [PubMed] [Google Scholar]
- Kuo CH, Leon L, Chung EJ, Huang RT, Sontag TJ, Reardon CA, … Fang Y (2014). Inhibition of atherosclerosis-promoting microRNAs via targeted polyelectrolyte complex micelles. J Mater Chem B, 2(46), 8142–8153. 10.1039/C4TB00977K [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix C, Humanes A, Coiffier C, Gigmes D, Verrier B, & Trimaille T (2020). Polylactide-Based Reactive Micelles as a Robust Platform for mRNA Delivery. Pharm Res, 37(2), 30. 10.1007/s11095-019-2749-6 [DOI] [PubMed] [Google Scholar]
- Lee K, Yu P, Lingampalli N, Kim HJ, Tang R, & Murthy N (2015). Peptide-enhanced mRNA transfection in cultured mouse cardiac fibroblasts and direct reprogramming towards cardiomyocyte-like cells. Int J Nanomedicine, 10, 1841–1854. 10.2147/IJN.S75124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, Zhang X, Li J, Gao Y, Wu J, Duan X, & Men K (2020). Current Progress in Messenger RNA-Based Gene Therapy. J Biomed Nanotechnol, 16(7), 1018–1044. 10.1166/jbn.2020.2961 [DOI] [PubMed] [Google Scholar]
- Leng Q, Chen L, & Lv Y (2020). RNA-based scaffolds for bone regeneration: application and mechanisms of mRNA, miRNA and siRNA. Theranostics, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Zhang X, & Dong Y (2019). Nanoscale platforms for messenger RNA delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 11(2), e1530. 10.1002/wnan.1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, & Rothberg L (2005). Detection of specific sequences in RNA using differential adsorption of single-stranded oligonucleotides on gold nanoparticles. Anal Chem, 77(19), 6229–6233. 10.1021/ac050921y [DOI] [PubMed] [Google Scholar]
- Li J, Lee WY, Wu T, Xu J, Zhang K, Li G, … Bian L (2016). Multifunctional Quantum Dot Nanoparticles for Effective Differentiation and Long-Term Tracking of Human Mesenchymal Stem Cells In Vitro and In Vivo. Adv Healthc Mater, 5(9), 1049–1057. 10.1002/adhm.201500879 [DOI] [PubMed] [Google Scholar]
- Liang W, & Lam JKW (2012). Endosomal Escape Pathways for Non-viral Nucleic Acid Delivery Systems, Molecular Regulation of Endocytosis (Ceresa B, Ed.). InTech. [Google Scholar]
- Lilavivat S, Sardar D, Jana S, Thomas GC, & Woycechowsky KJ (2012). In vivo encapsulation of nucleic acids using an engineered nonviral protein capsid. J Am Chem Soc, 134(32), 13152–13155. 10.1021/ja302743g [DOI] [PubMed] [Google Scholar]
- Lin YX, Wang Y, Blake S, Yu M, Mei L, Wang H, & Shi J (2020). RNA Nanotechnology-Mediated Cancer Immunotherapy. Theranostics, 10(1), 281–299. 10.7150/thno.35568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio DCS, Liu C, Oo MMS, Wiraja C, Teo MHY, Zheng M, … Xu C (2019). Transdermal delivery of small interfering RNAs with topically applied mesoporous silica nanoparticles for facile skin cancer treatment. Nanoscale, 11(36), 17041–17051. 10.1039/c9nr06303j [DOI] [PubMed] [Google Scholar]
- Liyanage PY, Hettiarachchi SD, Zhou Y, Ouhtit A, Seven ES, Oztan CY, … Leblanc RM (2019). Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim Biophys Acta Rev Cancer, 1871(2), 419–433. 10.1016/j.bbcan.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Morris VB, & Labhasetwar V (2019). Effectiveness of Small Interfering RNA Delivery via Arginine-Rich Polyethylenimine-Based Polyplex in Metastatic and Doxorubicin-Resistant Breast Cancer Cells. The Journal of pharmacology and experimental therapeutics, 370(3), 902–910. 10.1124/jpet.119.256909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro RD, Cox A, Zambelli V, Mancini S, Masserini M, & Re F (2018). The ability of liposomes, tailored for blood-brain barrier targeting, to reach the brain is dramatically affected by the disease state. Nanomedicine (Lond), 13(6), 585–594. 10.2217/nnm-2017-0317 [DOI] [PubMed] [Google Scholar]
- Mainini F, & Eccles MR (2020). Lipid and Polymer-Based Nanoparticle siRNA Delivery Systems for Cancer Therapy. Molecules (Basel, Switzerland), 25(11), 2692. 10.3390/molecules25112692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens TF, Remaut K, Demeester J, De Smedt SC, & Braeckmans K (2014). Intracellular delivery of nanomaterials: How to catch endosomal escape in the act. Nano Today, 9(3), 344–364. 10.1016/j.nantod.2014.04.011 [DOI] [Google Scholar]
- Melamed JR, Ioele SA, Hannum AJ, Ullman VM, & Day ES (2018). Polyethylenimine–Spherical Nucleic Acid Nanoparticles against Gli1 Reduce the Chemoresistance and Stemness of Glioblastoma Cells. Molecular Pharmaceutics, 15(11), 5135–5145. 10.1021/acs.molpharmaceut.8b00707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockey M, Bourseau E, Chandrashekhar V, Chaudhuri A, Lafosse S, Le Cam E, … Midoux P (2007). mRNA-based cancer vaccine: prevention of B16 melanoma progression and metastasis by systemic injection of MART1 mRNA histidylated lipopolyplexes. Cancer Gene Ther, 14(9), 802–814. 10.1038/sj.cgt.7701072 [DOI] [PubMed] [Google Scholar]
- Moschos SA, Jones SW, Perry MM, Williams AE, Erjefalt JS, Turner JJ, … Lindsay MA (2007). Lung delivery studies using siRNA conjugated to TAT(48–60) and penetratin reveal peptide induced reduction in gene expression and induction of innate immunity. Bioconjug Chem, 18(5), 1450–1459. 10.1021/bc070077d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafee N, Taetz S, Schneider M, Schaefer UF, & Lehr CM (2007). Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomedicine, 3(3), 173–183. 10.1016/j.nano.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Nosova AS, Koloskova OO, Nikonova AA, Simonova VA, Smirnov VV, Kudlay D, & Khaitov MR (2019). Diversity of PEGylation methods of liposomes and their influence on RNA delivery. Medchemcomm, 10(3), 369–377. 10.1039/c8md00515j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandini von Niessen AG, Poleganov MA, Rechner C, Plaschke A, Kranz LM, Fesser S, … Sahin U (2019). Improving mRNA-Based Therapeutic Gene Delivery by Expression-Augmenting 3’ UTRs Identified by Cellular Library Screening. Mol Ther, 27(4), 824–836. 10.1016/j.ymthe.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, … Weissman D (2017). Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature, 543(7644), 248–251. 10.1038/nature21428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Secreto AJ, Shan X, Debonera F, Glover J, Yi Y, … Weissman D (2017). Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat Commun, 8, 14630. 10.1038/ncomms14630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Sun X, Aikins ME, & Moon JJ (2021). Non-viral COVID-19 vaccine delivery systems. Adv Drug Deliv Rev, 169, 137–151. 10.1016/j.addr.2020.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AK, Kaczmarek JC, Bose S, Kauffman KJ, Mir F, Heartlein MW, … Anderson DG (2019). Inhaled Nanoformulated mRNA Polyplexes for Protein Production in Lung Epithelium. Adv Mater, 31(8), e1805116. 10.1002/adma.201805116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Ryals RC, Weller KK, Pennesi ME, & Sahay G (2019). Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J Control Release, 303, 91–100. 10.1016/j.jconrel.2019.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattni BS, Chupin VV, & Torchilin VP (2015). New Developments in Liposomal Drug Delivery. Chem Rev, 115(19), 10938–10966. 10.1021/acs.chemrev.5b00046 [DOI] [PubMed] [Google Scholar]
- Peer D, Park EJ, Morishita Y, Carman CV, & Shimaoka M (2008). Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science, 319(5863), 627–630. 10.1126/science.1149859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei M, Xu R, Zhang C, Wang X, Li C, & Hu Y (2021). Mannose-functionalized antigen nanoparticles for targeted dendritic cells, accelerated endosomal escape and enhanced MHC-I antigen presentation. Colloids and Surfaces B: Biointerfaces, 197, 111378. 10.1016/j.colsurfb.2020.111378 [DOI] [PubMed] [Google Scholar]
- Perche F, Benvegnu T, Berchel M, Lebegue L, Pichon C, Jaffrès PA, & Midoux P (2011). Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine, 7(4), 445–453. 10.1016/j.nano.2010.12.010 [DOI] [PubMed] [Google Scholar]
- Pichon C, & Midoux P (2013). Mannosylated and histidylated LPR technology for vaccination with tumor antigen mRNA. Methods Mol Biol, 969, 247–274. 10.1007/978-1-62703-260-5_16 [DOI] [PubMed] [Google Scholar]
- Qian Y, Qiao S, Dai Y, Xu G, Dai B, Lu L, … Zhang Z (2017). Molecular-Targeted Immunotherapeutic Strategy for Melanoma via Dual-Targeting Nanoparticles Delivering Small Interfering RNA to Tumor-Associated Macrophages. ACS Nano, 11(9), 9536–9549. 10.1021/acsnano.7b05465 [DOI] [PubMed] [Google Scholar]
- Qiao C, Yang J, Shen Q, Liu R, Li Y, Shi Y, … Zhang X (2018). Traceable Nanoparticles with Dual Targeting and ROS Response for RNAi-Based Immunochemotherapy of Intracranial Glioblastoma Treatment. Adv Mater, 30(18), e1705054. 10.1002/adma.201705054 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Chow MYT, Liang W, Chung WWY, Mak JCW, & Lam JKW (2017). From Pulmonary Surfactant, Synthetic KL4 Peptide as Effective siRNA Delivery Vector for Pulmonary Delivery. Mol Pharm, 14(12), 4606–4617. 10.1021/acs.molpharmaceut.7b00725 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Lo JCK, Kwok KCW, Mason AJ, & Lam JKW (2020). Modification of KL4 Peptide Revealed the Importance of Alpha-Helical Structure for Efficient Small Interfering RNA Delivery. Nucleic Acid Ther. 10.1089/nat.2020.0855 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Man RCH, Liao Q, Kung KLK, Chow MYT, & Lam JKW (2019). Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J Control Release, 314, 102–115. 10.1016/j.jconrel.2019.10.026 [DOI] [PubMed] [Google Scholar]
- Ramishetti S, Kedmi R, Goldsmith M, Leonard F, Sprague AG, Godin B, … Peer D (2015). Systemic Gene Silencing in Primary T Lymphocytes Using Targeted Lipid Nanoparticles. ACS Nano, 9(7), 6706–6716. 10.1021/acsnano.5b02796 [DOI] [PubMed] [Google Scholar]
- Raval N, Jogi H, Gondaliya P, Kalia K, & Tekade RK (2019). Method and its Composition for encapsulation, stabilization, and delivery of siRNA in Anionic polymeric nanoplex: An In vitro- In vivo Assessment. Sci Rep, 9(1), 16047. 10.1038/s41598-019-52390-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut K, Sanders NN, De Geest BG, Braeckmans K, Demeester J, & De Smedt SC (2007). Nucleic acid delivery: Where material sciences and biosciences meet. Materials Science and Engineering: R: Reports, 58(3), 117–161. 10.1016/j.mser.2007.06.001 [DOI] [Google Scholar]
- Salvage JP, Thom C, Lewis AL, Phillips GJ, & Lloyd AW (2015). Nanoprecipitation of polymeric nanoparticle micelles based on 2-methacryloyloxyethyl phosphorylcholine (MPC) with 2-(diisopropylamino)ethyl methacrylate (DPA), for intracellular delivery applications. Journal of Materials Science: Materials in Medicine, 26(3), 150. 10.1007/s10856-015-5480-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Nakamura T, Yamada Y, & Harashima H (2020). The nanomedicine rush: New strategies for unmet medical needs based on innovative nano DDS. J Control Release, 330, 305–316. 10.1016/j.jconrel.2020.12.032 [DOI] [PubMed] [Google Scholar]
- Sato Y, Okabe N, Note Y, Hashiba K, Maeki M, Tokeshi M, & Harashima H (2020). Hydrophobic scaffolds of pH-sensitive cationic lipids contribute to miscibility with phospholipids and improve the efficiency of delivering short interfering RNA by small-sized lipid nanoparticles. Acta Biomaterialia, 102. [DOI] [PubMed] [Google Scholar]
- Schlich M, Palomba R, Costabile G, Mizrahy S, Pannuzzo M, Peer D, & Decuzzi P (2021). Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles [https://doi.org/10.1002/btm2.10213]. Bioengineering & Translational Medicine, 6(2), e10213. 10.1002/btm2.10213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Huang X, Min J, Le S, Wang Q, Wang X, … Xiao J (2019). Nanoparticle Delivery Systems for DNA/RNA and their Potential Applications in Nanomedicine. Curr Top Med Chem, 19(27), 2507–2523. 10.2174/1568026619666191024170212 [DOI] [PubMed] [Google Scholar]
- Shi Y, van der Meel R, Chen X, & Lammers T (2020). The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics, 10(17), 7921–7924. 10.7150/thno.49577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoka F, & Papahadjopoulos D (1980). Comparative Properties and Methods of Preparation of Lipid Vesicles (Liposomes). Annual Review of Biophysics and Bioengineering, 9(1), 467–508. 10.1146/annurev.bb.09.060180.002343 [DOI] [PubMed] [Google Scholar]
- Tan L, Zheng T, Li M, Zhong X, Tang Y, Qin M, & Sun X (2020). Optimization of an mRNA vaccine assisted with cyclodextrin-polyethyleneimine conjugates. Drug Deliv Transl Res, 10(3), 678–689. 10.1007/s13346-020-00725-4 [DOI] [PubMed] [Google Scholar]
- Taratula O, Garbuzenko OB, Chen AM, & Minko T (2011). Innovative strategy for treatment of lung cancer: targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. J Drug Target, 19(10), 900–914. 10.3109/1061186X.2011.622404 [DOI] [PubMed] [Google Scholar]
- Thomas A, M Garg S, De Souza RAG, Ouellet E, Tharmarajah G, Reichert D, … Ramsay EC (2018). Microfluidic Production and Application of Lipid Nanoparticles for Nucleic Acid Transfection. Methods Mol Biol, 1792, 193–203. 10.1007/978-1-4939-7865-6_14 [DOI] [PubMed] [Google Scholar]
- Tietjen GT, Hosgood SA, DiRito J, Cui J, Deep D, Song E, … Kirkiles-Smith NC (2017). Nanoparticle targeting to the endothelium during normothermic machine perfusion of human kidneys. Science Translational Medicine, 9(418). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Perche F, Pichon C, & Cabral H (2020). Nanomedicine-Based Approaches for mRNA Delivery. Mol Pharm, 17(10), 3654–3684. 10.1021/acs.molpharmaceut.0c00618 [DOI] [PubMed] [Google Scholar]
- Udhayakumar VK, De Beuckelaer A, McCaffrey J, McCrudden CM, Kirschman JL, Vanover D, … De Koker S (2017). Arginine-Rich Peptide-Based mRNA Nanocomplexes Efficiently Instigate Cytotoxic T Cell Immunity Dependent on the Amphipathic Organization of the Peptide. Adv Healthc Mater, 6(13). 10.1002/adhm.201601412 [DOI] [PubMed] [Google Scholar]
- Urits I, Swanson D, Swett MC, Patel A, Berardino K, Amgalan A, … Viswanath O (2020). A Review of Patisiran (ONPATTRO®) for the Treatment of Polyneuropathy in People with Hereditary Transthyretin Amyloidosis. Neurol Ther, 9(2), 301–315. 10.1007/s40120-020-00208-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallazza B, Petri S, Poleganov MA, Eberle F, Kuhn AN, & Sahin U (2015). Recombinant messenger RNA technology and its application in cancer immunotherapy, transcript replacement therapies, pluripotent stem cell induction, and beyond. Wiley Interdiscip Rev RNA, 6(5), 471–499. 10.1002/wrna.1288 [DOI] [PubMed] [Google Scholar]
- Valle A, Gualberto A, & Pittella F (2020). Short Interfering RNA (siRNA) based Medicines and the Future of RNAi Therapy: A Mini Review. Current Trends in Biomedical Engineering & Biosciences, 19. [Google Scholar]
- Wadhwa A, Aljabbari A, Lokras A, Foged C, & Thakur A (2020). Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics, 12(2). 10.3390/pharmaceutics12020102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chin D, Poon C, Mancino V, Pham J, Li H, … Chung EJ (2020). Oral delivery of metformin by chitosan nanoparticles for polycystic kidney disease. Journal of Controlled Release. 10.1016/j.jconrel.2020.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Masehi-Lano JJ, & Chung EJ (2017). Peptide and antibody ligands for renal targeting: nanomedicine strategies for kidney disease. Biomater Sci, 5(8), 1450–1459. 10.1039/c7bm00271h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Hanafy MS, Xu H, Leal J, Zhai Y, Ghosh D, … Cui Z (2021). Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int J Pharm, 596, 120215. 10.1016/j.ijpharm.2021.120215 [DOI] [PubMed] [Google Scholar]
- Wang M, Han Y, Yu X, Liang L, Chang H, Yeo DC, … Xu C (2020). Upconversion Nanoparticle Powered Microneedle Patches for Transdermal Delivery of siRNA. Adv Healthc Mater, 9(2), e1900635. 10.1002/adhm.201900635 [DOI] [PubMed] [Google Scholar]
- Wang Y, & Huang L (2013). A window onto siRNA delivery. Nat Biotechnol, 31(7), 611–612. 10.1038/nbt.2634 [DOI] [PubMed] [Google Scholar]
- Wang Y, Su HH, Yang Y, Hu Y, Zhang L, Blancafort P, & Huang L (2013). Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Mol Ther, 21(2), 358–367. 10.1038/mt.2012.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu Z, Guo S, Zhang L, Sharma A, Robertson GP, & Huang L (2013). Intravenous delivery of siRNA targeting CD47 effectively inhibits melanoma tumor growth and lung metastasis. Mol Ther, 21(10), 1919–1929. 10.1038/mt.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Yin Y, Huang C, Pan W, & Liang D (2017). Encapsulation of RNA by negatively charged human serum albumin via physical interactions. Science China Chemistry, 60(1), 130–135. 10.1007/s11426-016-0094-8 [DOI] [Google Scholar]
- Williams RM, Shah J, Tian HS, Chen X, Geissmann F, Jaimes EA, & Heller DA (2017). Selective Nanoparticle Targeting of the Renal Tubules. HYPERTENSION, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman H, & Flynn PF (2009). Stabilization of RNA oligomers through reverse micelle encapsulation. J Am Chem Soc, 131(11), 3806–3807. 10.1021/ja8084753 [DOI] [PubMed] [Google Scholar]
- Wu Y, Gu W, Li J, Chen C, & Xu ZP (2019). Silencing PD-1 and PD-L1 with nanoparticle-delivered small interfering RNA increases cytotoxicity of tumor-infiltrating lymphocytes. Nanomedicine (Lond), 14(8), 955–967. 10.2217/nnm-2018-0237 [DOI] [PubMed] [Google Scholar]
- Xie Y, Kim NH, Nadithe V, Schalk D, Thakur A, Kılıç A, … Merkel OM (2016). Targeted delivery of siRNA to activated T cells via transferrin-polyethylenimine (Tf-PEI) as a potential therapy of asthma. J Control Release, 229, 120–129. 10.1016/j.jconrel.2016.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PY, Kankala RK, Pan YJ, Yuan H, Wang SB, & Chen AZ (2018). Overcoming multidrug resistance through inhalable siRNA nanoparticles-decorated porous microparticles based on supercritical fluid technology. Int J Nanomedicine, 13, 4685–4698. 10.2147/IJN.S169399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wu J, Liu Y, Yu M, Zhao L, Zhu X, … Farokhzad OC (2016). Ultra-pH-Responsive and Tumor-Penetrating Nanoplatform for Targeted siRNA Delivery with Robust Anti-Cancer Efficacy [https://doi.org/10.1002/anie.201601273]. Angewandte Chemie International Edition, 55(25), 7091–7094. 10.1002/anie.201601273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Thakur A, Zhang Y, & Foged C (2021). Inhaled RNA Therapeutics for Obstructive Airway Diseases: Recent Advances and Future Prospects. Pharmaceutics, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wang Y, Zhang L, & Huang L (2014). Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano, 8(4), 3636–3645. 10.1021/nn500216y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamankurt G, Stawicki RJ, Posadas DM, Nguyen JQ, Carthew RW, & Mirkin CA (2020). The effector mechanism of siRNA spherical nucleic acids. Proceedings of the National Academy of Sciences of the United States of America, 117(3), 1312–1320. 10.1073/pnas.1915907117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D-C, Eldredge AC, Hickey JC, Muradyan H, & Guan Z (2020). Multivalent Peptide-Functionalized Bioreducible Polymers for Cellular Delivery of Various RNAs. Biomacromolecules, 21(4), 1613–1624. 10.1021/acs.biomac.0c00211 [DOI] [PubMed] [Google Scholar]
- Yasar H, Biehl A, De Rossi C, Koch M, Murgia X, Loretz B, & Lehr CM (2018). Kinetics of mRNA delivery and protein translation in dendritic cells using lipid-coated PLGA nanoparticles. J Nanobiotechnology, 16(1), 72. 10.1186/s12951-018-0401-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yıldız-Peköz A, & Ehrhardt C (2020). Advances in Pulmonary Drug Delivery. Pharmaceutics, 12(10). 10.3390/pharmaceutics12100911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JY, Yeh M, Kaur B, & Lee TJ (2021). Targeted delivery of small noncoding RNA for glioblastoma. Cancer Letters, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga N, Cho E, Koji K, Mochida Y, Naito M, Osada K, … Uchida S (2019). Bundling mRNA Strands to Prepare Nano-Assemblies with Enhanced Stability Towards RNase for In Vivo Delivery. Angew Chem Int Ed Engl, 58(33), 11360–11363. 10.1002/anie.201905203 [DOI] [PubMed] [Google Scholar]
- Yuan ZQ, Chen WL, You BG, Liu Y, Yang SD, Li JZ, … Zhang XN (2017). Multifunctional nanoparticles co-delivering EZH2 siRNA and etoposide for synergistic therapy of orthotopic non-small-cell lung tumor. J Control Release, 268, 198–211. 10.1016/j.jconrel.2017.10.025 [DOI] [PubMed] [Google Scholar]
- Zhang R, El-Mayta R, Murdoch TJ, Warzecha CC, Billingsley MM, Shepherd SJ, … Mitchell MJ (2020). Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver. Biomaterials Science, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Xue X, He D, & Hsieh J-T (2015). A prostate cancer-targeted polyarginine-disulfide linked PEI nanocarrier for delivery of microRNA. Cancer Letters, 365(2), 156–165. 10.1016/j.canlet.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xie F, Yin Y, Zhang Q, Jin H, Wu Y, … Gao J (2021). Immunotherapy of Tumor RNA-Loaded Lipid Nanoparticles Against Hepatocellular Carcinoma. Int J Nanomedicine, 16, 1553–1564. 10.2147/IJN.S291421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Woodle MC, & Mixson AJ (2018). Advances in delivery systems for doxorubicin. J Nanomed Nanotechnol, 9(5). 10.4172/2157-7439.1000519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Hou X, Yan J, Du S, Xue Y, Li W, … Dong Y (2020). Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact Mater, 5(2), 358–363. 10.1016/j.bioactmat.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Giljohann DA, Chen DL, Massich MD, Wang X-Q, Iordanov H, … Paller AS (2012). Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proceedings of the National Academy of Sciences of the United States of America, 109(30), 11975–11980. 10.1073/pnas.1118425109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QC, Jiang S, Wu YZ, Shang D, Zhang Y, Hu SB, … Li M (2020). Dual-Targeting Nanoparticle-Mediated Gene Therapy Strategy for Hepatocellular Carcinoma by Delivering Small Interfering RNA. Frontiers in Bioengineering and Biotechnology, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Vladau C, Zhang X, Suzuki M, Ichim TE, Zhang ZX, … Min WP (2009). A novel in vivo siRNA delivery system specifically targeting dendritic cells and silencing CD40 genes for immunomodulation. Blood, 113(12), 2646–2654. 10.1182/blood-2008-04-151191 [DOI] [PubMed] [Google Scholar]


