Abstract
Introduction: Uropathogenic Escherichia coli (UPECs) are a significant cause of urinary tract infections (UTIs). In Kenya, UTIs are typically treated with β-lactam antibiotics without antibiotic susceptibility testing, which could accelerate antibiotic resistance among UPEC strains.
Aim: This study determined the occurrence of UPEC producing extended-spectrum β-lactamases (ESBLs), the genes conferring resistance to β-lactams, and the phylogenetic groups associated with ESBLs in Kenyan UPECs.
Methodology: Ninety-five UPEC isolates from six Kenyan hospitals were tested for ESBL and plasmid-mediated AmpC β-lactamase (pAmpC) production by combined disk diffusion and disk approximation tests, respectively. Real-time and conventional polymerase chain reactions (PCRs) were used to detect three ESBL and six pAmpC genes, respectively, and phylogenetic groups were assigned by a quadruplex PCR method.
Results: Twenty-four percent UPEC isolates were ESBL producers with blaCTX-M (95.6%), blaTEM (95.6%), and blaSHV (21.7%) genes detected. Sixteen isolates had blaCTX-M/TEM, whereas five had blaTEM/CTX-M/SHV. A total of 5/23 ESBLs were cefoxitin resistant, but no AmpC genes were detected. The UPECs belonged predominantly to phylogenetic groups B2 (31/95; 32.6%) and D (30/95; 31.6%), while groups B2 and A had the most ESBL producers.
Conclusions: β-Lactam antibiotics have reduced utility for treating UTIs as a quarter of UPECs were ESBL producing. Single or multiple ESBL genes were present in UPECs, belonging primarily to phylogenetic groups B2 and A.
Keywords: phenotypic, genotypic, ESBL, pAmpC, uropathogenic Escherichia coli (UPEC), antimicrobial resistance, urinary tract infections
Introduction
Globally, an estimated 150 million people contract urinary tract infections (UTIs) annually and account for most adult outpatient visits.1 The prevalence of UTIs ranges from 6% to 37% in developing nations.2 UTIs result from the ascent of bacteria from the periurethral area to the urethra, bladder, and upper urinary tract.3 Colonization of the periurethral area with uropathogenic bacteria is a critical factor in causing UTIs.3 Women are more likely to suffer from UTIs than males due to their shorter urethral distance and proximity of the urethral tract to the anus.4 Among women, the prevalence is higher in pregnant women.5 A recent study in Kenya among pregnant women indicated a prevalence of 15.7% UTIs.6 The severity of UTI depends upon the virulence of bacteria and the host's susceptibility.5 Uropathogenic Escherichia coli (UPEC) is the primary etiological agent of UTIs.7 β-Lactam antibiotics are the main class of drugs used to treat hospital and community-acquired UPEC infections.8 The emergence and spread of bacterial resistance to β-lactam leading to treatment failure and recurrent infections are of clinical concern.9 In E. coli, the main mechanism of resistance to β-lactam antibiotics10,11 is the production of extended-spectrum β-lactamases (ESBLs) and plasmid-mediated β-lactamases (pAmpCs).
E. coli ESBL producers' occurrence is geographically variable and is correlated with the overuse of antibiotics.12 ESBL producers have been reported in several African countries: Egypt, Morocco, Tunisia, Senegal, South Africa, and Nigeria, with prevalence rates ranging from 5% to 44.3%.12 Studies of ESBL producers among E. coli isolates from fecal samples in Kenya show prevalence rates ranging from 39% in malnourished children13 to 3.4% in healthy adults.14 Few studies have focused on determining the prevalence among UPEC strains, but both ESBL- and pAmpC-producing bacteria have been reported in UPECs in Kenya.15 The genes conferring resistance to ESBLs often coexist on the same plasmid with genes conferring resistance to other antibiotics.9,11 These plasmids facilitate the horizontal transmission of resistance to multiple antibiotics used to treat bacterial infections.16 Therefore, ESBL production by UPEC can hinder the effective management of UTIs.17 Multidrug-resistant (MDR) UPEC limits therapeutic options, leading to increased morbidity and health care costs.12,18 The ESBL genes that have been associated with E. coli isolated from enteric, environmental, and wound samples in Kenya are predominantly blaCTX-M and blaTEM genes.13,15,19
The AmpC β-lactamases confer resistance to penicillins, cephems, and monobactams, but not carbapenems.9,11 These enzymes are not inhibited by β-lactamase inhibitors such as clavulanate, tazobactam, and sulbactam,9,11 but are inhibited by boronic acid and cloxacillin.10 In E. coli, AmpC β-lactamases are encoded by genes located either on the chromosome or plasmids.20 Plasmid-mediated AmpC β-lactamases (pAmpCs) have origins from chromosomally encoded AmpCs of multiple genera within the Enterobacteriaceae family and display structural and functional similarities.21 The most commonly detected plasmid-mediated AmpC is the CMY type,15 of which there are 64 plasmid-mediated variants.20 Plasmid-mediated AmpC β-lactamase production in bacteria is associated with multidrug resistance due to the carriage of resistance determinants for other antibiotic classes on the same plasmids.11 Chromosomally mediated AmpC genes do not cause resistance to cephalosporins unless they are overproduced.22
β-Lactams are often used for the empirical treatment of UTI. Despite the changing epidemiology of ESBL genes, there are insufficient data on the occurrence of β-lactam genes in E. coli isolates from UTI patients in Kenya. Therefore, continuous surveillance to provide knowledge of genetic determinants of resistance to commonly prescribed antibiotics in different regions is essential. Surveillance outcomes can guide the selection of effective antibiotic therapy and implementation of infection control strategies to minimize the spread of resistant bacteria. Determination of E. coli phylogenetic groups is of epidemiological significance as isolates belonging predominantly to phylogenetic group B2 are associated with virulence of extraintestinal strains.23 Therefore, this study aimed to determine the occurrence of ESBL producers, ESBL genes (blaTEM, blaSHV, and blaCTX-M), and pAmpC genes (blaMOX, blaCIT, blaDHA, blaACC, blaEBC, and blaFOX) and the phylogenetic groups of UPEC in isolates from patients with UTI from several hospitals in Kenya.
Methods
Bacterial isolation and culture
The Department of Emerging Infectious Diseases (DEID) of the United States Army Medical Research Directorate-Africa, Kenya (USAMRD-A, Kenya), based in the Kenya Medical Research Institute (KEMRI), Nairobi, has been conducting antimicrobial resistance surveillance in Kenya since April 2015 (KEMRI SERU#2767/WRAIR IRB#2089). This study utilized 95 archived UPEC isolates collected between April 2015 and August 2018 in that study. Urine specimens had been collected from individuals older than 2 months with UTI symptoms, at both in- and outpatient departments, who gave informed consent. For individuals <18 years old, the parent or guardian provided informed consent before participating in the parent protocol. These participants were enrolled from six public health care facilities in five counties: Kisumu, Kericho, Kilifi, Kisii, and Nairobi. One of the following three methods was used to obtained urine samples: midstream clean-catch urine collection, pediatric urine bags from infants and young children, or sterile needles and syringes to collect fresh urine from existing catheter tubing. The urine samples were transferred in boric acid and transported at room temperature within 48 hours to the Centre for Microbiology Research laboratories in KEMRI for testing.
The urine samples were cultured in CLED and MacConkey media to detect bacterial pathogens and bacteria were identified on the VITEK 2 (bioMerieux) platform. Antimicrobial susceptibility testing (AST) was performed using the GN83 and AST-XN05 VITEK 2 (bioMerieux) panels, which collectively test a panel of 27 antibiotics (cefoxitin, ceftazidime, cefotaxime, cefuroxime, cefixime, ceftriaxone, ciprofloxacin, clindamycin, aztreonam, azithromycin, tetracycline, oxacillin, gentamicin, erythromycin, levofloxacin, clarithromycin, minocycline, imipenem, amoxicillin/clavulanic acid, ampicillin/sulbactam, amikacin, trimethoprim, colistin, tigecycline, chloramphenicol, and moxifloxacin). Results were interpreted using the Clinical and Laboratory Standards Institute (CLSI) guidelines.24 Isolate identification and antibiotic susceptibility results were recorded in the study database. MDR isolates were defined as nonsusceptible to at least one antimicrobial agent in three or more antimicrobial classes.25 Extensively drug-resistant isolates were defined as bacterial isolates susceptible to only one or two antimicrobial classes.25 Pandrug-resistant isolates were defined as nonsusceptible to all agents in all antimicrobial classes.25 The bacterial isolates were archived in glycerol stocks at −80°C for future studies. All isolates of UPEC collected from April 2015 to August 2018 and viable on subculture on Muller–Hinton agar (MHA; Becton Dickinson) were included in the study.
Phenotypic confirmatory test for ESBL production
Combination Disk Diffusion Test
Based on the VITEK 2 results, the UPEC isolates that showed a minimum inhibition concentration (MIC) ≥16 μg/mL for ceftazidime, according to CLSI guidelines, were subjected to the combination disk diffusion test (CDDT) as an ESBL confirmatory test. In brief, a ceftazidime 30-μg disk (Becton Dickinson) and combination ceftazidime+clavulanic acid 30 + 10-μg disk (Becton Dickinson) were placed 60 mm away from each other and incubated at 37°C for 24 hours. A difference in zone size of >5 mm between the ceftazidime+clavulanic acid disk and the ceftazidime disk is confirmatory for ESBL-producing strains as per CLSI guidelines because ESBL producers are inhibited by clavulanic acid. Ceftazidime (a third-generation cephalosporin) resistance is indicative of an ESBL-producing isolate. E. coli ATCC 25922 was the ESBL-negative control, and Klebsiella pneumoniae ATCC 700603 was the ESBL-positive control.
AmpC screening using the disk diffusion method
The UPEC isolates that were found to be positive for ESBL production were also screened for presumptive AmpC β-lactamase production by the disk diffusion method using a cefoxitin disk of 30 μg (Becton Dickinson). AmpC producers, unlike ESBL isolates producing other enzymes, are resistant to cephamycins, such as cefoxitin. In brief, a cefoxitin disk of 30 μg (Becton Dickinson) was placed at the center of a plate inoculated with the bacterial isolate and incubated at 37°C for 24 hours before reading the results. Isolates with zone diameters <18 mm, indicating resistance as per CLSI guidelines, were presumed positive for AmpC β-lactamase screening and selected for confirmatory AmpC production testing.26,27
AmpC phenotypic confirmatory test
A disk approximation test was used to detect inducible AmpC production. In brief, the cefotaxime 30-μg disk and cefoxitin 30-μg disk (Becton Dickinson) were placed 20 mm apart on a plate inoculated with the test isolate and incubated overnight at 37°C. Distortion of the zone of inhibition adjacent to the cefoxitin 30-μg disk indicated the production of pAmpC. A negative result was the absence of distortion near the cefoxitin 30-μg disk.27,28
Genotyping of ESBL-producing isolates
Detection of ESBL genes using a multiplex, real-time polymerase chain reaction
The DNA was extracted from isolates confirmed to be ESBL positive using a standard boiling method.29 DNA extracts were screened for the presence of blaTEM, blaSHV, and blaCTX-M genes by fluorescent probe-based, multiplex, real-time polymerase chain reaction (PCR) using the MIC PCR instrument (Bio Molecular Systems) using published primers and probes for blaTEM, blaSHV, and blaCTX-M (New England Biolabs).29 The positive controls used in this assay were in-house controls expressing the blaTEM and blaCTX-M genes and E. coli MRSN#489100 to detect blaSHV genes. A DNA-free PCR and nuclease-free water taken through the DNA extraction process were the negative controls.
Multiplex PCR for detection of pAmpC β-lactamase genes
Multiplex PCR for detection of pAmpC genes (blaMOX, blaCIT, blaDHA, blaACC, blaEBC, and blaFOX) was performed using published primers (New England Biolabs).21 The positive control strain used was CMY-2-positive E. coli (MRSN#570581), and a DNA-free PCR and nuclease-free water taken through the DNA extraction process were the negative controls.
Phylogenetic grouping of uropathogenic E. coli isolates
The new Clermont phylogenetic grouping method30 improves the specificity of phylogenetic assignments using the presence or absence of two or three markers to assign E. coli isolates into one of seven phylogenetic groups. The banding patterns observed from the PCR are used to determine the genotype and the isolate assigned to a phylogenetic group. Isolates with ambiguous groupings are tested using D or C group-specific primers (New England Biolabs).31 The positive control strain used was E. coli (ATCC 25922), and a DNA-free PCR and extracted nuclease-free water taken through the DNA extraction process were the negative controls.
DNA from the UPEC isolates was amplified by a published quadruplex PCR assay, using primers that target three markers: chuA, yjaA, and TspE4.C2.30 The assay also amplifies arpA, which acts as an international control for DNA quality and distinguishes the phylogenetic group F, formally mistaken as phylogenetic group D.31
Data analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS), version 20. Descriptive statistics were used to compute the frequencies and percentages for the occurrence of ESBLs and pAmpC β-lactamases plus phylogenetic groups in UPEC isolates. The statistical significance of the distribution of ESBLs within the phylogenetic groups was determined using Fisher's exact test.
Results
Confirmation of ESBL and AmpC production using CLSI-approved phenotypic tests
A total of 23 (24.2%) of the 95 UPEC isolates collected between April 2015 and August 2018 screened positive for ESBL production by MIC (≥16 μg/mL) using ceftazidime (Table 1). These isolates were further confirmed to be ESBL producers by the CDDT. Five of the 23 ESBL producers were identified as cefoxitin resistant using the AmpC screening test, which is indicative of an ESBL phenotype conferred not by the typical genes, but by an AmpC gene. However, none of the five UPEC isolates showed a flattening of the inhibition zone and were classified as negative AmpC producers using the disk approximation test.
Table 1.
Antimicrobial Susceptibility Test Results of Uropathogenic Escherichia coli Isolates to Ceftazidime and Cefoxitin Cephalosporins to Confirm Extended-Spectrum β-Lactamase and AmpC Production
| |
ESBL detection |
ESBL confirmation |
AmpC confirmation |
|||||
|---|---|---|---|---|---|---|---|---|
| Isolate ID | A: Ceftazidime MIC > 16 μg/mL | Inter. | B: Difference in zone size > 5 mm | Inter. | C: Cefoxitin disk zone < 18 mm) | Inter. | D: Disk approximation | Inter. |
| UPEC 1 | >16 | POS | 21 | POS | 20 | NEG | NEG | NEG |
| UPEC 2 | >16 | POS | 12 | POS | 15 | POS | NEG | NEG |
| UPEC 3 | >16 | POS | 14 | POS | 26 | NEG | NEG | NEG |
| UPEC 4 | >16 | POS | 14 | POS | 20 | NEG | NEG | NEG |
| UPEC 5 | >16 | POS | 14 | POS | 22 | NEG | NEG | NEG |
| UPEC 6 | >16 | POS | 17 | POS | 24 | NEG | NEG | NEG |
| UPEC 7 | >16 | POS | 15 | POS | 23 | NEG | NEG | NEG |
| UPEC 8 | >16 | POS | 17 | POS | 20 | NEG | NEG | NEG |
| UPEC 9 | >16 | POS | 17 | POS | 12 | POS | NEG | NEG |
| UPEC 10 | >16 | POS | 17 | POS | 20 | NEG | NEG | NEG |
| UPEC 11 | >16 | POS | 16 | POS | 22 | NEG | NEG | NEG |
| UPEC 12 | >16 | POS | 8 | POS | 20 | NEG | NEG | NEG |
| UPEC 13 | >16 | POS | 10 | POS | 17 | POS | NEG | NEG |
| UPEC 14 | >16 | POS | 15 | POS | 24 | NEG | NEG | NEG |
| UPEC 15 | >16 | POS | 21 | POS | 25 | NEG | NEG | NEG |
| UPEC 16 | >16 | POS | 16 | POS | 21 | NEG | NEG | NEG |
| UPEC 17 | >16 | POS | 10 | POS | 20 | NEG | NEG | NEG |
| UPEC 18 | >16 | POS | 15 | POS | 13 | POS | NEG | NEG |
| UPEC 19 | >16 | POS | 17 | POS | 23 | NEG | NEG | NEG |
| UPEC 20 | >16 | POS | 13 | POS | 17 | POS | NEG | NEG |
| UPEC 21 | >16 | POS | 13 | POS | 23 | NEG | NEG | NEG |
| UPEC 22 | >16 | POS | 19 | POS | 17 | POS | NEG | NEG |
| UPEC 23 | >16 | POS | 8 | POS | 23 | NEG | NEG | NEG |
| Klebsiella pneumoniae (ATCC 700603) | — | — | 10 | POS | — | — | — | — |
| E. coli CMY-2 (MRSN#570581) | — | — | — | — | 6 | POS | NEG | NEG |
| E. coli (ATCC#25922) | — | — | 0 | NEG | 22 | NEG | NEG | NEG |
ESBL and AmpC production were confirmed using CLSI-approved phenotypic tests. A: Ceftazidime minimum inhibitory concentration (MIC) of >16 μg/mL. B: Difference in zone size >5 mm for the ceftazidime (30 μg) + clavulanic acid (10 μg) disk compared with the ceftazidime (30 μg) disc (ESBL POS). C: AmpC screening test using the cefoxitin disc (30 μg). Inhibition zone size <18 mm, AmpC screen POS. D: Disk approximation confirmatory test for AmpC production using the cefoxitin disk (30 μg) and cefotaxime disk (30 μg).
CLSI, Clinical and Laboratory Standards Institute; ESBL, extended-spectrum β-lactamase; Inter., interpretation of the test result; MIC, minimum inhibition concentration; MRSN, Multidrug-Resistant Organism Repository and Surveillance Network; NEG, no flattening of the zone of inhibition toward cefoxitin (30 μg); POS, flattening of the zone of inhibition toward cefoxitin (30 μg); UPEC, uropathogenic Escherichia coli.
Molecular detection of ESBL genes
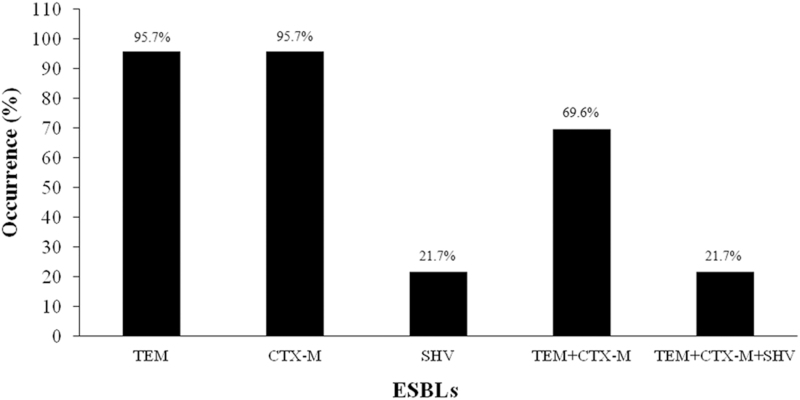
All the 23 phenotypically confirmed, ESBL-producing UPEC isolates carried at least one ESBL gene (Table 2). The results indicated that blaTEM and blaCTX-M were the predominant ESBL genes, each present in 22/23 (95.6%) isolates, followed by blaSHV in 5/23 (21.7%) isolates. Five of 23 (21.7%) isolates carried 3 ESBL genes (blaTEM, blaCTX-M, and blaSHV), while 16/23 (69.5%) isolates had both blaCTX-M and blaTEM genes only (Fig. 1). One of the 23 isolates encoded blaTEM and another isolate encoded blaCTX-M only (Table 2).
Table 2.
The Occurrence of the Three Extended-Spectrum β-Lactamase Genes, Temoneira, Sulfhydryl Variable, and Cefotaximase-Munich, Their Phylogenetic Groups, and the Multidrug Resistance Status Among the 23 Uropathogenic Escherichia coli Extended-Spectrum β-Lactamase-Producing Isolates
| Isolate ID | Resistance status | blaTEM | blaSHV | blaCTX-M | Phylogenetic group |
|---|---|---|---|---|---|
| UPEC 1 | MDR | + | − | + | B2 |
| UPEC 2 | MDR | + | − | + | A |
| UPEC 3 | MDR | + | − | + | B2 |
| UPEC 4 | MDR | + | − | − | B2 |
| UPEC 5 | MDR | + | − | + | B2 |
| UPEC 6 | MDR | + | − | + | D |
| UPEC 7 | MDR | + | − | + | A |
| UPEC 8 | MDR | + | + | + | D |
| UPEC 9 | MDR | + | − | + | B2 |
| UPEC 10 | MDR | + | − | + | B2 |
| UPEC 11 | MDR | + | − | + | B2 |
| UPEC 12 | MDR | + | − | + | B2 |
| UPEC 13 | MDR | + | − | + | B2 |
| UPEC 14 | MDR | + | + | + | A |
| UPEC 15 | MDR | − | − | + | Ungrouped |
| UPEC 16 | MDR | + | − | + | A |
| UPEC 17 | MDR | + | + | + | F |
| UPEC 18 | MDR | + | + | + | A |
| UPEC 19 | MDR | + | + | + | B1 |
| UPEC 20 | MDR | + | − | + | B2 |
| UPEC 21 | MDR | + | − | + | D |
| UPEC 22 | MDR | + | − | + | B2 |
| UPEC 23 | MDR | + | − | + | B2 |
| MRSN/SHV/489100 | − | + | − | ND | |
| CTX control | − | − | + | ND | |
| TEM control | + | − | − | ND | |
| Nuclease-free water | − | − | − | ND |
Phylogenetic groups are based on the new Clermont quadruplex method (Derakhshandeh et al.30).
CTX-M, cefotaximase-Munich; MDR, multidrug-resistant; ND, not done; SHV, sulfhydryl variable; TEM, temoneira.
FIG. 1.
Occurrence of ESBL genes among UPEC isolates indicating the presence of single-gene and multiple-gene combinations of the four ESBL genes: TEM, SHV, and CTX-M among the 23/95 (24.2%) UPEC isolates. CTX-M, cefotaximase-Munich; ESBL, extended-spectrum β-lactamase; SHV, sulfhydryl variable; TEM, temoneira; UPEC, uropathogenic Escherichia coli.
Multiplex PCR for detection of pAmpC β-lactamase genes
None of the six known pAmpC genes (blaMOX, blaCIT, blaDHA, blaACC, blaEBC, and blaFOX) were detected by PCR in the cefoxitin-resistant isolates. The E. coli CMY-2 (MRSN#570581) strain was used as the positive control, while a DNA-free PCR and nuclease-free water taken through the DNA extraction process were the negative controls.
Phylogenetic distribution of UPEC isolates
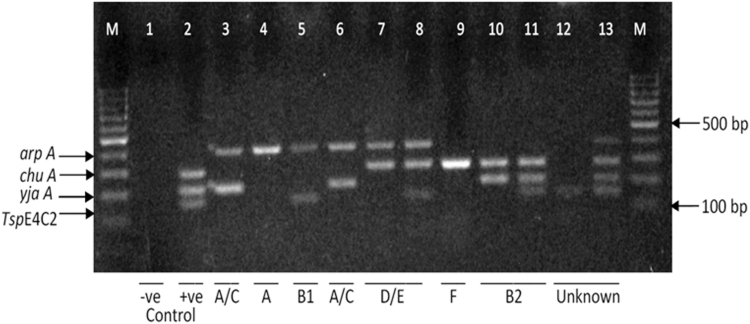
A total of 93 of 95 UPEC isolates were assigned to 5 of the 8 phylogenetic groups using the extended quadruplex PCR method (Fig. 2). Two isolates, 2/95 (2.1%), could not be assigned to a phylogenetic group by this method. The phylogenetic groups assigned to the isolates were A, Bı, B2, D, and F (Table 2). Group B2 was the predominant phylogenetic group, 31/95 (32.6%), followed by group D 30/95 (31.6%); group A 18/95 (18.9%); group Bı 11/95 (11.6%); and finally group F 3/95 (3.2%).
FIG. 2.
Gel electrophoresis images for the extended quadruplex PCR profile of a few UPEC isolates. The isolates were assigned to a phylo group according to the presence or absence of the following genes in the order arpA, chuA, yjaA, and TspE4C2, where group A is (+ − − −), group A/C is (+ − + −), group B1 is (+ − − +), group B2 is (− + + −), group D or E is (+ + − −) or (+ + − +), group F is (− + − −), and other combinations represent unknown groups (− + + +), (− − − +), and (+ + + +). PCR, polymerase chain reaction.
Discussion
Clinical management of UTIs is a major global challenge due to the increasing cases of MDR UPECs. A study on the contribution of UTI to the burden of febrile illnesses in young children in rural Kenya showed a prevalence of 11.9% UTIs.32 Another study in Kenya among pregnant women indicated a prevalence of 15.7% UTIs.6 UPEC has acquired resistance to the β-lactam antibiotics, commonly used antimicrobial agents for UTI treatment.21 Previous studies have reported that the production of ESBL and pAmpC β-lactamases is the leading cause of β-lactam resistance and that the resistance genes are related to phylogenetic grouping.12,23 This study aimed to determine the production of ESBL and pAmpC β-lactamases among the archived Kenyan UPEC isolates, screen for the genes associated with resistance, identify their phylogenetic groups, and determine the phylogenetic groups associated with β-lactamase resistance.
The study showed that 24.2% of the UPEC isolates were ESBL producers and multidrug resistant. They were resistant to third-generation cephalosporins, which are often used to treat UTIs. All the 23 ESBL-positive E. coli isolates possessed at least 1 of 3 genes found to confer resistance to β-lactams in the Kenyan isolates: blaTEM, blaCTX-M, and blaSHV. These three ESBL genes are prevalent among UPEC isolates in the East African regions as they have been detected in Kenya15 and its neighbor, Uganda.33 The ESBL genotypes appear ubiquitous among E. coli isolates from different sources and are not unique to UPEC isolates.
Although this study showed that 5/23 (21.7%) ESBL-positive isolates were cefoxitin resistant, suggesting AmpC production, none of the known transferable AmpC β-lactamases were detected. These findings were unexpected given previous reports in Kenya15 of 10% pAmpC-producing E. coli isolated from different clinical samples and 37% AmpC β-lactamases among Enterobacteriaceae from Uganda, with 30 isolates having more than 1 gene coding for AmpC-mediated resistance.33 Since the cefoxitin-resistant isolates were not AmpC β-lactamase producers as observed in other studies,20,26 it suggests that the isolates in this study may have other enzymatic mechanisms for cefoxitin resistance such as production of different ESBLs or nonenzymatic mechanisms such as decreased porin production,21 warranting further investigation.
Determination of E. coli phylogenetic groups is of epidemiological importance as several studies indicate that phylogenetic groups could be related to the severity of disease.34 These studies demonstrated a relationship between the phylogenetic group and virulence, with more virulent extraintestinal strains belonging to phylogenetic groups B2 and D.35 In contrast, most commensals belonged to phylogenetic group A and group B1.
In this study, the predominant phylogenetic group was B2 (32.6%), followed by D (31.6%), group A (18.9%), group B1 (11.6%), group F, and finally the ungrouped group (2.1%). These were the main phylogenetic groups detected among the isolates studied from diverse parts of the country and could reflect the phylogenetic groups circulating in Kenya. Predominance of groups B2 and D among the Kenyan UPEC isolates indicates virulent strains in the population. The study showed that most ESBL-producing isolates belonged to phylogenetic group B2 and group A (Table 2). The isolates in phylogenetic group B2 were more likely to be ESBL producers compared with other phylogenetic groups (p < 0.05) (Table 3). This study has demonstrated a link between the B2 phylogenetic group and penicillin/cephalosporin resistance, combining virulence and antibiotic resistance traits. Tracking of these phylogenetic groups can give an indication of the burden of ESBL producers among UPECs.
Table 3.
Distribution of Extended-Spectrum β-Lactamase Producers Versus Nonextended-Spectrum β-Lactamase Producers Among the Phylogenetic Groups
| Phylogenetic group | ESBL producers, n (%) | Non-ESBL producers, n (%) | p |
|---|---|---|---|
| A | |||
| Positive | 5 (27.8) | 18 (19.0) | 0.5224 |
| Negative | 13 (72.2) | 77 (81.0) | |
| B1 | |||
| Positive | 2 (18.2) | 22 (20.7) | >0.9999 |
| Negative | 9 (81.8) | 84 (79.3) | |
| B2 | |||
| Positive | 12 (38.7) | 11 (14.7) | 0.0095 |
| Negative | 19 (61.3) | 64 (85.3) | |
| D | |||
| Positive | 3 (10.0) | 20 (23.5) | 0.1826 |
| Negative | 27 (90.0) | 65 (76.5) | |
| F | |||
| Positive | 1 (33.3) | 22 (19.3) | 0.4847 |
| Negative | 2 (66.7) | 92 (80.7) | |
| Ungrouped | |||
| Positive | 1 (50.0) | 22 (19.0) | 0.3532 |
| Negative | 1 (50.0) | 94 (81.0) | |
Isolates in phylogenetic group B2 were more likely to be ESBL producers compared with other phylogenetic groups (p = 0.0095).
The presence of ESBL producers among the commensal groups Bı, A, and F shows the potential horizontal transfer of antimicrobial resistance genes from the phylogenetic groups associated with resistance to the typically non-ESBL-producing phylogenetic groups. Isolates of groups C, E, and Clade 1 were not detected in this study. A more extensive UPEC study would be needed to determine if they are absent or just rare in Kenya, as they have been reported in UPEC isolates from Australia and Iran.31,35 In our study, two isolates could not be assigned to any of the eight recognized phylogenetic groups using the new extended quadruplex method. This inability to assign isolates to a phylogenetic group confirms the assay's known limitation, the use of primers specific to currently known gene groups, which may not detect minor or novel phylogenetic groups.31 New strains could emerge due to large-scale recombination between two or more different phylogenetic groups or by the gain or loss of genes in the E. coli genome.31 These ungrouped isolates could be other phylotypes of UPEC and are worth investigating in further studies.
Conclusions
UTIs are the leading cause of outpatient visits, so understanding the epidemiology of UPEC can contribute to better treatment and reduced morbidity of UTIs. This study has determined that ESBL-producing UPEC isolates are present in Kenyan hospitals, with 24.2% of the isolates being resistant to the commonly prescribed cephalosporin drugs for UTIs. This resistance level indicates possible antibiotic selection pressure in Kenyan hospitals and community settings, driving resistance to these widely prescribed drugs. The blaCTX-M and blaTEM genes predominated in this study, with the coexistence of multiple genes in single isolates indicating increased transmission of genetic determinants and the likely increase of ESBL pathogens in Kenyan hospitals. However, the AmpC genes typically associated with cefoxitin resistance were not observed in this study, a possible indication of other cefoxitin-resistant mechanisms that warrant further investigation. The phylogenetic groups B2 and D predominated in this study, while phylogenetic groups B2 and A had the greatest number of ESBL-producing UPECs. Although the study tested only 95 UPEC isolates, the isolates were from diverse regions within Kenya and provided some indication of the resistance patterns and genes among UPEC isolates. The characterization of UPEC phylogenetic groups has contributed to the understanding of the epidemiology of UPEC isolates in Kenya. Identification of the new uncharacterized phylogenetic groups emerging on the landscape opens up new avenues to study these important pathogens using more robust whole-genome approaches.
Ethical Approval
This study was approved by the KEMRI/Scientific Ethics Review Unit (SERU), (KEMRI/SERU/CCR/0088/3609), and the Walter Reed Army Institute of Research (WRAIR) Institutional Review Board (IRB; WRAIR No. 2089B).
Acknowledgments
The authors appreciate the support and contribution of the Center Director, Laboratory Manager, and all members of the Centre for Microbiology Research in KEMRI, Nairobi. Special appreciation is extended to Beth Mutai and the entire USAMRD-A, Kenya, Basic Science Laboratory staff in Kisumu for assisting with laboratory work. The control strain, E. coli CMY-2 (MRSN#570581), was provided by the Multidrug-Resistant Organism Repository and Surveillance Network (MRSN) at the WRAIR.
Authors' Contributions
C.W.M., L.M., and L.A.O. conceived and designed the experiments. C.W.M., D.M., E.O., and C.K. retrieved the archived isolates. C.W.M., D.M., C.M., and C.K. performed the experiments. C.W.M. and C.M. analyzed the descriptive data. C.W.M., C.M., E.O., C.K., D.M., L.A.O., and L.M. wrote and reviewed the manuscript.
Disclosure Statement
Material has been reviewed by the WRAIR. There is no objection to its presentation and publication. The opinions or assertions contained herein are the private views of the authors. They are not to be construed as official or as reflecting true views of the Army or the Department of Defense. The investigators have adhered to the policies for protecting human subjects, as prescribed in AR 70–25. This work has been published with the permission of the Director of KEMRI. The authors declare that there are no conflicts of interest.
Funding Information
This study was funded by the Armed Forces Health Surveillance Division (AFHSD) and its Global Emerging Infections Surveillance (GEIS) Branch under project P0136_19_KY_07.06 FY19.
References
- 1. Koshesh, M., Mansouri S., Hashemizadeh Z., and Kalantar-Neyestanaki D.. 2016. Identification of extended-spectrum β-lactamase genes and AmpC-β-lactamase in clinical isolates of Escherichia coli recovered from patients with urinary tract infections in Kerman, Iran. Arch. Pediatr. Infect. Dis. 5:e3; 7968. [Google Scholar]
- 2. Uwaezuoke, S. 2016. The prevalence of urinary tract infection in children with severe acute malnutrition: a narrative review. Pediatr. Heal. Med. Ther. 7:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leung, A.K.C., A.H.C. Wong, A.A.M. Leung, and Hon K.L.. 2019. Urinary tract infection in children recent patents on inflammation & allergy drug discovery. Recent Pat. Inflamm. Allergy Drug Discov. 13:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scribano, D., Sarshar M., Prezioso C., et al. . 2020. d-Mannose treatment neither affects uropathogenic Escherichia coli properties nor induces stable fimh modifications. Molecules 25:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nabbugodi, W.F., J.W. Gichuhi, and Mugo N.W.. 2015. Prevalence of urinary tract infection, microbial aetiology, and antibiotic sensitivity pattern among antenatal women presenting with lower abdominal pains at Kenyatta National Hospital, Nairobi, Kenya. Open Access J. Sci. Technol. 3:1–6. [Google Scholar]
- 6. Onyango, H.A., Ngugi C., Maina J., and Kiiru J.. 2018. Urinary tract infection among pregnant women at Pumwani Maternity Hospital, Nairobi, Kenya: bacterial etiologic agents, antimicrobial susceptibility profiles and associated risk factors. Adv. Microbiol. 8:175–187. [Google Scholar]
- 7. Al-Jamei, S.A., A.Y. Albsoul, F.G. Bakri, and Al-Bakri A.G.. 2019. Extended-spectrum β-lactamase producing E. coli in urinary tract infections: a two-center, cross-sectional study of prevalence, genotypes and risk factors in Amman, Jordan.. J. Infect. Public Health 12:21–25. [DOI] [PubMed] [Google Scholar]
- 8. Dasgupta, A., Majumdar T., and Bhowmik R.. 2018. Identification and molecular characterisation of ESBL producing uropathogenic Escherichia coli strains isolated from a tertiary care hospital of Tripura. J. Evol. Med. Dent. Sci. 7:2829–2833. [Google Scholar]
- 9. Gajamer, V.R., Bhattacharjee A., Paul D., et al. . 2020. High prevalence of carbapenemase, AmpC β-lactamase and aminoglycoside resistance genes in extended-spectrum β-lactamase-positive uropathogens from Northern India. J. Glob. Antimicrob. Resist. 20:197–203. [DOI] [PubMed] [Google Scholar]
- 10. Ghonaim, R.A., and Moaety H.A.. 2018. Comparison between multiplex PCR and phenotypic detection methods for identifying AmpC B-lactamases among clinical isolates of Enterobacteriaceae in Zagazig University Hospitals, Egypt. Clin. Microbiol. 7:3. [Google Scholar]
- 11. Gupta, V., Rani H., Singla N., Kaistha N., and Chander J.. 2013. Determination of extended-spectrum β-lactamases and AmpC production in uropathogenic isolates of Escherichia coli and susceptibility to fosfomycin. J. Lab. Physicians 5:90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giwa, F., Ige O., Haruna D., Yaqub Y., Lamido T., and Usman S.. 2018. Extended-spectrum beta-lactamase production and antimicrobial susceptibility pattern of uropathogens in a tertiary hospital in Northwestern Nigeria. Ann. Trop. Pathol. 9:11. [Google Scholar]
- 13. Njoroge, S., Kiiru J., and Kikuvi G.. 2014. Broad spectrum β-lactam resistance in faecal Escherichia coli isolated from severely malnourished and nourished children attending Mbagathi district hospital, Nairobi: a case-control study. Chronicles Young Sci. 5:39. [Google Scholar]
- 14. Juma, A.O., Ngugi C., and Kiiru J.. 2017. Antimicrobial susceptibility profiles and prevalence of ESBLs among E. coli isolates recovered from people working in hospitality industry within Nairobi, Kenya.. East Afr. Med. J. 94:445–448. [Google Scholar]
- 15. Kiiru, J., Kariuki S., B.M. Goddeeris, and Butaye P.. 2012. Analysis of β-lactamase phenotypes and carriage of selected β-lactamase genes among Escherichia coli strains obtained from Kenyan patients during an 18-year period. BMC Microbiol. 12:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruppé, É., P.L. Woerther, and Barbier F.. 2015. Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ann. Intensive Care 5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Hoek, A.H.A.M., Mevius D., Guerra B., Mullany P., A.P. Roberts, and H.J.M. Aarts. 2011. Acquired antibiotic resistance genes: an overview. Front. Microbiol. 2:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desai, S., R.A. Kagal, and Bharadwaj R.. 2013. Virulence factors in uropathogenic Escherichia coli (UPEC) causing urinary tract infections. Indian J. Basic Appl. Med. Res. 2:886–896. [Google Scholar]
- 19. Maina, D., Makau P., Nyerere A., and Revathi G.. 2013. Antimicrobial resistance patterns in extended-spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae isolates in a private tertiary hospital, Kenya. Microbiol. Discov. DOI: 10.7243/2052-6180-1-5. [DOI] [Google Scholar]
- 20. Helmy, M.M., and Wasfi R.. 2014. Phenotypic and molecular characterization of plasmid mediated AmpC β-lactamases among Escherichia coli, Klebsiella spp., and Proteus mirabilis isolated from urinary tract infections in Egyptian hospitals. Biomed Res. Int. 2014:171548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khari, F.I.M., Karunakaran R., Rosli R., and Tay S.T.. 2016. Genotypic and phenotypic detection of AmpC β-lactamases in Enterobacter spp. isolated from a teaching hospital in Malaysia. PLoS One 11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ingti, B., Paul D., A.P. Maurya, et al. . 2017. Occurrence of bla DHA-1 mediated cephalosporin resistance in Escherichia coli and their transcriptional response against cephalosporin stress: a report from India. Ann. Clin. Microbiol. Antimicrob. 16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saralaya, V., Shenoy S., Baliga S., Hegde A., Adhikari P., and Chakraborty A.. 2015. Characterization of Escherichia coli phylogenetic groups associated with extraintestinal infections in South Indian population. Ann. Med. Health Sci. Res. 5:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing. 27th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 25. Basak, S., Singh P., and Rajurkar M.. 2016. Multidrug resistant and extensively drug resistant bacteria: a study. J. Pathog. 2016:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madhumati, B., Rani L., C.Y. Ranjini, and Rajendran R.. 2015. Prevalence of AMPC beta lactamases among gram negative bacterial isolates in a tertiary care hospital. Int. J. Curr. Microbiol. App. Sci. 4:219–227. [Google Scholar]
- 27. Upadhyay, S., Hussain A., Mishra S., A.P. Maurya, Bhattacharjee A., and Joshi S.R.. 2015. Genetic environment of plasmid mediated CTX-M-15 extended spectrum beta-lactamases from clinical and food borne bacteria in north-eastern India. PLoS One 10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El-Hady, S.A., and Adel L.A.. 2015. Occurrence and detection of AmpC β-lactamases among Enterobacteriaceae isolates from patients at Ain Shams University Hospital. Egypt J. Med. Hum. Genet. 16:239–244. [Google Scholar]
- 29. Roschanski, N., Fischer J., Guerra B., and Roesler U.. 2014. Development of a multiplex real-time PCR for the rapid detection of the predominant beta-lactamase genes CTX-M, SHV, TEM and CIT-type ampcs in enterobacteriaceae. PLoS One 9:e100956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Derakhshandeh, A., Firouzi R., Moatamedifar M., Motamedi A., Bahadori M., and Naziri Z.. 2013. Phylogenetic analysis of Escherichia coli strains isolated from human samples. Mol. Biol. Res. Commun. 2:143–149. [Google Scholar]
- 31. Clermont, O., J.K. Christenson, Denamur E., and Gordon D.M.. 2013. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 5:58–65. [DOI] [PubMed] [Google Scholar]
- 32. Masika, W.G., W.P. O'Meara, T.L. Holland, and Armstrong J.. 2017. Contribution of urinary tract infection to the burden of febrile illnesses in young children in rural Kenya. PLoS One 12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakaye, M., Bwanga F., Itabangi H., I.J. Stanley, Bashir M., and Bazira J.. 2014. AmpC-BETA lactamases among Enterobacteriaceae isolated at a tertiary hospital, South Western Uganda. Br. Biotechnol. J. 4:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abd, A.R. 2015. Genotyping of Escherichia coli isolated from clinical and hospitals environment. Univ. Thi-Qar. J. Sci. 5:3–13. [Google Scholar]
- 35. Iranpour, D., Hassanpour M., Ansari H., Tajbakhsh S., Khamisipour G., and Najafi A.. 2015. Phylogenetic groups of Escherichia coli strains from patients with urinary tract infection in Iran based on the new Clermont phylotyping method. Biomed Res. Int. 2015:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]