Abstract
Background and Objectives
In patients with severe coronavirus disease 2019 (COVID-19), disorders of consciousness (DoC) have emerged as a serious complication. The prognosis and pathophysiology of COVID-DoC remain unclear, complicating decisions about continuing life-sustaining treatment. We describe the natural history of COVID-DoC and investigate its associated brain connectivity profile.
Methods
In a prospective longitudinal study, we screened consecutive patients with COVID-19 at our institution. We enrolled critically ill adult patients with a DoC unexplained by sedation or structural brain injury and who were planned to undergo a brain MRI. We performed resting-state fMRI and diffusion MRI to evaluate functional and structural connectivity compared to healthy controls and patients with DoC resulting from severe traumatic brain injury (TBI). We assessed the recovery of consciousness (command following) and functional outcomes (Glasgow Outcome Scale Extended [GOSE] and the Disability Rating Scale [DRS]) at hospital discharge and 3 and 6 months after discharge. We also explored whether clinical variables were associated with recovery from COVID-DoC.
Results
After screening 1,105 patients with COVID-19, we enrolled 12 with COVID-DoC. The median age was 63.5 years (interquartile range 55–76.3 years). After the exclusion of 1 patient who died shortly after enrollment, all of the remaining 11 patients recovered consciousness 0 to 25 days (median 7 [5–14.5] days) after the cessation of continuous IV sedation. At discharge, all surviving patients remained dependent: median GOSE score 3 (1–3) and median DRS score 23 (16–30). Ultimately, however, except for 2 patients with severe polyneuropathy, all returned home with normal cognition and minimal disability: at 3 months, median GOSE score 3 (3–3) and median DRS score 7 (5–13); at 6 months, median GOSE score 4 (4–5), median DRS score 3 (3–5). Ten patients with COVID-DoC underwent advanced neuroimaging; functional and structural brain connectivity in those with COVID-DoC was diminished compared to healthy controls, and structural connectivity was comparable to that in patients with severe TBI.
Discussion
Patients who survived invariably recovered consciousness after COVID-DoC. Although disability was common after hospitalization, functional status improved over the ensuing months. While future research is necessary, these prospective findings inform the prognosis and pathophysiology of COVID-DoC.
Trial Registration Information
ClinicalTrials.gov identifier: NCT04476589.
Months into the coronavirus disease 2019 (COVID-19) pandemic, neurologic manifestations of the disease were recognized.1,2 Impaired consciousness was observed in 1% to 20% of patients with COVID-19, mostly in patients with severe infection and comorbid conditions.1,3-5 It soon became evident that these disorders of consciousness (DoC) in severe COVID-19 may be prolonged, carrying an unclear prognosis for neurologic recovery.6 This uncertainty has had profound implications. For families and surrogates already unsure about whether to continue intensive medical care for their loved ones, COVID-DoC has often prompted discussions about withdrawing life-sustaining treatment. To assist in these challenging decisions, some institutions have built dedicated services for deliberating the probability of neurologic recovery (i.e., coma boards7) and have used ethics consultations.8 However, the uncertain prognosis of COVID-DoC raises the alarming possibility of error in decisions about life-sustaining treatment: continuation for patients with little chance of meaningful recovery or withdrawal for patients who would have otherwise recovered.
Data on recovery from prolonged COVID-DoC are slowly emerging. In July 2020, we described a patient with COVID-DoC who recovered consciousness ∼40 days after sedation was discontinued.9 In November 2020, another group reported a patient with COVID-DoC who recovered consciousness after 2 months.10 A case series in December 2020 subsequently described 6 patients with COVID-DoC who similarly recovered consciousness after 8 to 31 days.11 While these reports indicate that recovery of consciousness after COVID-DoC is possible, their susceptibility to selection bias makes the likelihood of recovery uncertain. Recovery from COVID-DoC has not been evaluated in a prospective cohort, and longer-term functional outcomes, which are crucial to guiding discussions of prognosis, have not been assessed.
The pathophysiology of COVID-DoC remains similarly unclear. DoC caused by other etiologies of brain injury, whether traumatic, anoxic, or cerebrovascular, are characterized by diminished neural connectivity, including functional connectivity as measured with resting-state fMRI (rs-fMRI)12-14 and structural connectivity as measured with diffusion MRI (d-MRI).15-18 Whether COVID-DoC is characterized by similar connectivity disruptions is unknown. We reported intact functional connectivity in 1 patient with COVID-DoC, but these findings may not be generalizable.9 Preliminary d-MRI studies have found diminished white matter integrity in patients with COVID-19.10,19 Investigating brain connectivity in a larger cohort of patients with COVID-DoC and comparing their connectivity to that of patients with other types of DoC may shed light on the pathophysiology of this condition.
We launched a prospective, longitudinal, multimodal study to characterize long-term recovery from COVID-DoC and to evaluate its brain connectivity profile (ClinicalTrials.gov NCT04476589). Given the urgent need for information to guide discussions of prognosis and decisions about life-sustaining treatment, we provide data from an initial patient cohort, additionally addressing short-term recovery. We aim (1) to identify the demographic and clinical features of patients with COVID-DoC; (2) to describe the recovery of consciousness and longer-term function after COVID-DoC; (3) to evaluate functional network connectivity in patients with COVID-DoC compared to healthy controls; (4) to evaluate structural connectivity in COVID-DoC compared to healthy controls and patients with DoC due to severe traumatic brain injury (TBI), a condition known to disrupt white matter tracts15,16,18; and (5) to explore variables potentially associated with recovery from COVID-DoC.
Methods
Patients
Between July 2020 and March 2021, we consecutively screened all patients admitted or transferred to any intensive care unit at Massachusetts General Hospital (given that reported instances of COVID-DoC occurred in critically ill patients). Inclusion criteria were (1) age ≥18 years, (2) positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) assay, (3) DoC (coma, vegetative state or minimally conscious state established with behavioral examinations in the medical record, per the Aspen Neurobehavioral Workgroup criteria20), and (4) the treating team's intention to investigate the etiology of the patient's DoC with a clinical brain MRI to which research sequences could be appended. Patients were excluded for doses of sedation or structural brain injury on CT sufficient to explain the patient's DoC, as determined by both the treating team and a team of investigators trained in neurocritical care (D.F. and B.L.E.). Patients were not excluded for neuroimaging findings associated with COVID-19 (specifically, microhemorrhages and leukoencephalopathy21,22), given their uncertain effects on consciousness.
Once patients were stable for transport, clinical MRI sequences, rs-fMRI, and d-MRI were acquired. Although sedation during the scan was minimized when possible, some patients received sedatives for safety, comfort, and immobility. Although the clinical applications of functional fMRI remain uncertain, guidelines have begun to suggest its judicious use in DoC diagnosis and prognostication.23,24 Therefore, rs-fMRI data were rapidly processed and reported to the clinical teams and patient families in accordance with an Institutional Review Board–approved protocol.
Neuroimaging
MRI data were acquired with a 32-channel head coil on a 3T Skyra MRI scanner (Siemens Healthcare, Erlangen, Germany). The neuroradiologists' interpretation of the clinical sequences was reviewed. Reports of microhemorrhages (or microthrombi, which are often indistinguishable) and leukoencephalopathy, neuroimaging findings associated with COVID-19,21,22 were included in the analyses below.
rs-fMRI measures spontaneous fluctuations in the blood oxygen level–dependent signal, a proxy for neuronal activity, and evaluates whether such fluctuations correlate across spatially disparate brain regions, a proxy for network connectivity.25 The rs-fMRI sequence was obtained with an echo time of 30.3 milliseconds, a repetition time of 1,250 milliseconds, and simultaneous multislice acquisition (n = 4) to optimize spatial and temporal resolution (parameters and analytic code provided at www.github.com/ComaRecoveryLab/COVID-19_rsfMRI).9 Images were normalized to Montreal Neurological Institute space, denoised, and processed with the CONN toolbox software26 using previously described parameters.27
Functional network connectivity was quantified in 2 ways. Given its well-established association with DoC and neurologic recovery, we first assessed connectivity between nodes of the default mode network (DMN)—that is, intranetwork connectivity—measured as the average correlation between each pair of nodes, as done previously.13,14,28 Because a negative correlation between resting-state networks may similarly carry prognostic value,14 we also assessed connectivity between the DMN and salience network (SN)—that is, internetwork connectivity—measured as the average correlation between each DMN node and each SN node. Previously described DMN and SN nodes29 were used as regions of interest, including 10 DMN nodes and 23 SN nodes (10-mm spheres for cortical nodes and 4-mm spheres for subcortical nodes). For qualitative visualization in figures, rs-fMRI data are presented as seed-to-voxel maps, with seeds defined as 4 principal nodes of the DMN—the medial prefrontal cortex, the posterior cingulate cortex, and the bilateral inferior parietal lobules—as done previously.9,27
d-MRI evaluates the integrity of axonal pathways by measuring the diffusion of water along white matter tracts. We acquired high-angular-resolution diffusion imaging, which uses a multidirectional model to optimize resolution of crossing and branching axons,15,30 and processed images with FSL (FMRIB, UK) with parameters described previously.31 Fractional anisotropy (FA), which represents the directionality of water diffusion in each voxel of the brain (with higher values suggestive of greater white matter integrity or structural connectivity), has been associated with neurologic recovery after brain injury.17 We therefore first measured the average FA of white matter across the whole brain. Second, given evidence of brainstem dysfunction in COVID-DoC,19,32 we measured the average FA within the brainstem (eMethods, links.lww.com/WNL/B660, provides details).
The imaging analysis was conducted after clinical data were abstracted from the medical record to avoid the possibility that the connectivity results could bias the interpretation of the clinical data. Although the investigators conducting the imaging analysis were not blinded to the clinical data, the analysis procedure described above was uniform across participants and required no manual adjustment or subjective interpretation.
We compared functional and structural connectivity of patients with COVID-DoC to that of 14 healthy controls, recruited and scanned with an identical MRI protocol as part of a separate research study approved by the Institutional Review Board (ClinicalTrials.gov NCT03504709). Healthy controls had no history of neurologic/psychiatric disease, diabetes, hypertension, heart disease, or kidney disease. One healthy control did not complete the d-MRI sequence. To determine whether patients with COVID-DoC exhibit connectivity impairments similar to those of other etiologies of DoC, we compared FA values from the COVID-DoC cohort to those of 18 patients with DoC due to severe TBI, recruited and scanned with an identical d-MRI sequence as part of a separate study (eTable 1, links.lww.com/WNL/B660).33 Functional connectivity was not compared due to differences in the rs-fMRI sequences. The ages of the healthy controls ranged from 22 to 52 years (median 32.5 years, interquartile range [IQR] 28.3–37.3 years), and the ages of the TBI cohort ranged from 19 to 51 years (median 27 years, IQR 22.5–32.8 years).
Outcome Assessment
The primary outcome measures established on ClinicalTrials.gov (NCT04476589) were the Glasgow Outcome Scale Extended (GOSE; lower values indicate higher levels of disability) and the Disability Rating Scale (DRS; higher values indicate higher levels of disability).34,35 These measures were assessed at hospital discharge through evaluations by physical and occupational therapists (either measured directly by therapists or, in cases when therapists did not complete these measures, estimated from documentation of patient function). These measures were assessed again prospectively at 3 and 6 months (±4 days) after hospital discharge according to patient interviews, with an interpreter when necessary. If patients were still admitted to an inpatient rehabilitation facility at the time of these postdischarge assessments, scores were also informed by records from physical and occupational therapists at the facility.
Here, we also focus on earlier metrics of recovery. We evaluated whether consciousness was recovered (as indicated by chart documentation of command following after COVID-DoC), time to recover consciousness (TTRC; measured as the number of days from the cessation of continuous IV sedatives [propofol, midazolam, hydromorphone, fentanyl, or ketamine] to the first documented report of command following), hospital length of stay, discharge disposition, and mortality. Of note, because the criteria for enrollment included the minimally conscious state, 2 patients already demonstrated intermittent command following by the time of enrollment; for these 2 patients, the day of consciousness recovery occurred before enrollment.
Primary Analysis
We used 2-sample t tests to compare functional and structural connectivity measures between patients with COVID-DoC and healthy controls. Given that patients tended to be older than controls and that connectivity metrics may change with age,36,37 we also used a linear regression model with terms for condition (patient vs control) and age to determine whether the main effect for condition remained statistically significant after controlling for age. We used 2-sample t tests to compare the d-MRI measures between patients with COVID-DoC and patients with TBI. We applied a Bonferroni correction to account for multiple comparisons. To ensure that t test results were not driven by outliers, all comparisons were repeated with nonparametric Mann-Whitney U tests.
Exploratory Analyses
We conducted exploratory analyses to determine whether any clinical factors or biomarkers were associated with recovery of consciousness. Due to low variability in whether consciousness was recovered, we instead used TTRC as the outcome metric for these analyses. A Spearman correlation coefficient was computed between TTRC and several clinical variables, including age, medical comorbid conditions, COVID-19 treatment, degree of hypoxemia, renal dysfunction, liver dysfunction, and presence of microhemorrhages or leukoencephalopathy (see eMethods, links.lww.com/WNL/B660).
Although patients were excluded for the ongoing administration of sedatives sufficient to explain the DoC, the cumulative effect of previous sedatives may contribute to COVID-DoC. Thus, a Spearman correlation coefficient was computed between TTRC and the cumulative amounts of midazolam equivalents, morphine equivalents, propofol, ketamine, and dexmedetomidine administered from intubation to cessation of continuous IV sedation, and from cessation of continuous IV sedation to recovery of consciousness (for patients who received IV sedatives before transfer to our institution, values were estimated from available reports). For each patient, we also evaluated whether the recovery of consciousness occurred before or after the estimated elimination of sedative medications (see eMethods, links.lww.com/WNL/B660).
We computed the Spearman correlation coefficients between the rs-fMRI/d-MRI metrics and TTRC and between the rs-fMRI metrics and time from rs-fMRI to recovery of consciousness. For these neuroimaging metrics, we used 2-sample t tests to assess whether they differed between patients with and without microhemorrhages and between patients with and without leukoencephalopathy. Given the exploratory nature of these analyses and our limited sample size, we did not correct them for multiple comparisons.
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was approved by the Mass General Brigham Institutional Review Board. We obtained informed consent for research from all participants or surrogate decision-makers for participants in this study. Study information can be found at ClinicalTrials.gov (NCT04476589).
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Patients and Outcomes
We screened 1,105 consecutive patients admitted to the hospital with COVID-19. Patients who died in the hospital (n = 98, 8.8%), had a negative SARS-CoV-2 test on confirmatory testing (n = 22, 2.0%), were <18 years old (n = 9, 0.8%), or were not admitted to an intensive care unit (n = 880, 79.6%) were excluded. Of the remaining 185 adult patients with COVID-19 admitted to any intensive care unit, we identified 33 (17.8%) with a DoC unexplained by sedative medications. Of those 33, 4 (12.1%) had a brain injury sufficient to explain the DoC (1 with anoxia caused by cardiac arrest, 2 with large strokes and malignant edema, and 1 with aneurysmal subarachnoid hemorrhage and hydrocephalus), 9 (27.3%) recovered from the DoC before enrollment, 4 (12.1%) were too medically unstable for enrollment, 3 (9.1%) had a DoC attributed to renal failure and thus were not planned to undergo a brain MRI, and 1 was eligible but not enrolled.
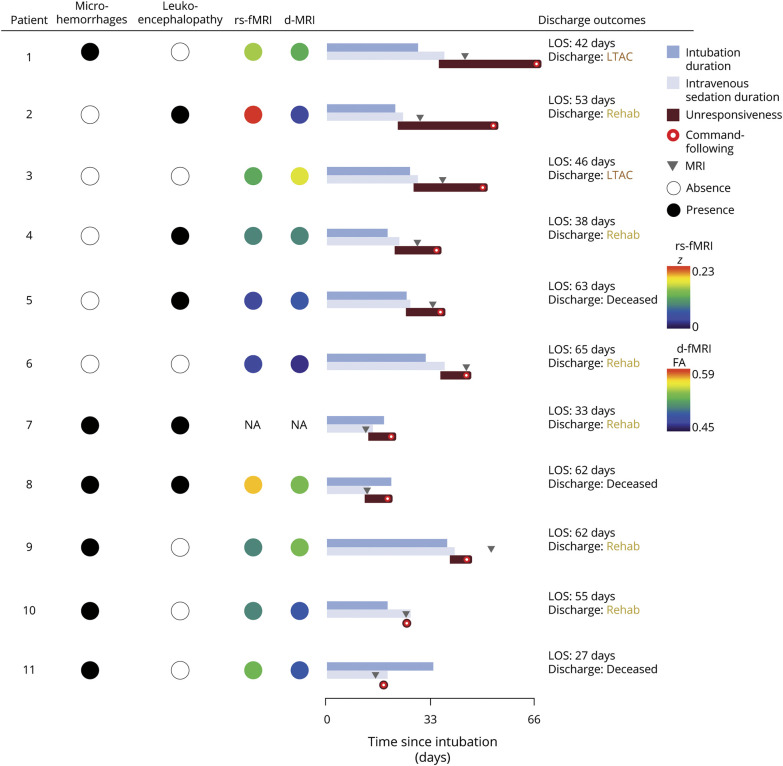
We prospectively enrolled the remaining 12 patients (eTable 2, links.lww.com/WNL/B660). Patient ages ranged from 33 to 82 years (median 63.5 years, IQR 55–76.3 years). Five were male (42%). The distribution of race and ethnicity paralleled that of other patients hospitalized with COVID-19 across the United States.38 At the time of enrollment, 2 patients were comatose (17%), 8 were in a vegetative state (67%), and 2 were in a minimally conscious state (17%). At admission, 11 carried a diagnosis of hypertension (92%), 8 of obesity (67%), 8 of diabetes (67%), 7 of hyperlipidemia (58%), and 5 of asthma (42%). All patients were intubated for acute respiratory distress syndrome due to COVID-19 (9 severe [75%], 2 moderate [17%], 1 mild [8%])39 and remained hospitalized for 27 to 65 days (median 49.5 days, IQR 36.8–62 days). Four patients had microhemorrhages; another 3 had leukoencephalopathy; and another 2 had both. Of the 6 patients with microhemorrhages, 3 had a mild burden (<5 microhemorrhages; patients 8, 10, and 11) and 3 had a severe burden (>100 microhemorrhages; patients 1, 7, and 9) (Figure 1 and eFigure 1, links.lww.com/WNL/B660). Eleven patients underwent a clinical EEG. All demonstrated slowing; 4 demonstrated generalized rhythmic delta activity; and 1 demonstrated seizures (generalized periodic discharges up to 3 Hz) that resolved before enrollment.
Figure 1. Neuroimaging Features and Recovery of Patients With COVID-DoC.
Patients are listed by descending duration of disorders of consciousness in coronavirus disease 2019 (COVID-DoC). The patient who died shortly after enrollment (patient 12) is not depicted. Patients with microhemorrhages or leukoencephalopathy on a structural brain MRI are marked with a black circle; those without findings are marked with a white circle. Intranetwork functional connectivity of the default mode network, measured with resting-state fMRI (rs-fMRI), is represented by colored circles; red represents the average intranetwork default mode network connectivity of healthy controls (z = 0.23), and purple represents absent connectivity (z = 0). White matter integrity, measured as whole-brain fractional anisotropy (FA) with diffusion MRI (d-MRI), is represented by colored circles; red represents the average whole-brain FA of healthy controls (0.59), and purple represents the lowest FA measured among patients (0.45). Recovery of consciousness is depicted relative to the day of intubation. Duration of intubation is depicted in dark blue (terminating with tracheostomy or extubation), and the duration of IV sedation (including propofol, midazolam, hydromorphone, or ketamine) is depicted in light blue. Duration of unresponsiveness, starting with the cessation of IV sedation and ending with the first documentation of command following (represented by a red circle), is depicted in red. Timing of the brain MRI is depicted as gray arrows. Outcomes at hospital discharge are depicted in the right-hand column, including acute hospitalization length of stay (LOS) and disposition. LTAC = long-term acute care; Rehab = rehabilitation facility.
Of the 152 patients in an intensive care unit with COVID-19 but without a DoC, we identified 12 who were age matched to the patients with COVID-DoC (±5 years) and regained command following within 24 hours of the cessation of continuous IV sedation. eTable 3, links.lww.com/WNL/B660, provides details. This age-matched cohort had similar comorbid conditions and acute respiratory distress syndrome severity. Only 1 patient underwent a clinical brain MRI (to assess for anoxia after a brief cardiac arrest), which was normal, without evidence of microhemorrhages or leukoencephalopathy. A post hoc 2-sample t test showed that patients with COVID-DoC spent more days under IV sedation than patients with severe COVID-19 but no DoC (COVID-DoC 21.6 days [SD 7.3 days], COVID-no-DoC 12 days [SD 5.5 days], t [22] = 3.65, p < 0.005).
Of the 12 patients with COVID-DoC, 1 died of medical complications of COVID-19 shortly after enrollment, before the MRI scan was obtained. Another patient improved neurologically shortly after enrollment, and research sequences were not obtained. For the remaining 10 patients who underwent an MRI, 9 required sedation at the time of the scan to facilitate safety and comfort: 2 were on a low-dose sedative infusion; 2 received a single dose of IV sedative before the scan; and the remaining 5 were on an enteral regimen of intermittent sedatives to prevent iatrogenic opioid or benzodiazepine withdrawal. eTable 2, links.lww.com/WNL/B660, gives details of sedation at enrollment and during the MRI.
A summary of patient outcomes is presented in Figure 1 and eFigure 1 and eTable 4, links.lww.com/WNL/B660. Excluding the patient who died shortly after enrollment, all of the remaining 11 patients recovered consciousness. Ten patients recovered consciousness while still hospitalized, and 1 patient recovered consciousness after discharge. The TTRC after the cessation of continuous sedation ranged from 0 to 25 days (median 7 days, IQR 5–14.5 days). Four patients died during hospitalization, all due to medical complications of COVID-19; no deaths were attributable to the withdrawal of life-sustaining treatment based on a poor neurologic prognosis. Two were discharged to a long-term acute care facility and 6 to an inpatient rehabilitation facility.
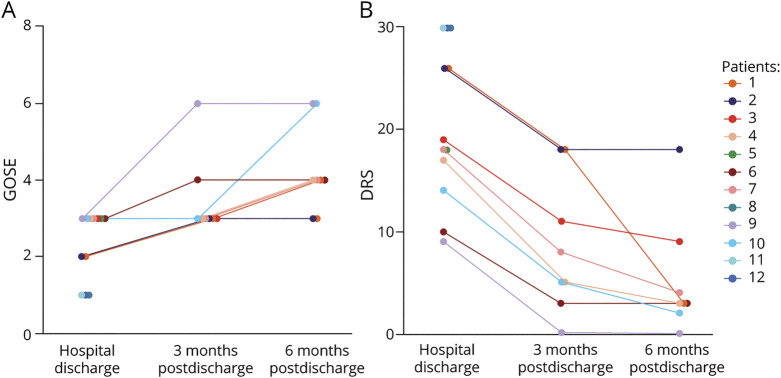
A summary of neurologic outcomes is presented in Figure 2. By discharge, GOSE scores ranged from 1 to 3 (median 3, IQR 1–3), and DRS scores ranged from 9 to 30 (median 23, IQR 16–30); all patients who survived demonstrated cognitive impairment and required significant assistance with activities of daily living at hospital discharge. All patients who survived to discharge (8) were evaluated 3 months later, when GOSE scores ranged from 3 to 6 (median 3, IQR 3–3) and DRS scores ranged from 0 to 18 (median 7, IQR 5–13). By 3 months, 3 patients (those with the longest DoC) remained in an inpatient rehabilitation facility, requiring supervision and support for weakness and cognitive impairment, and 5 returned home with normal or nearly normal cognition and mild disability due to weakness and pain. All 8 patients were again evaluated at 6 months after discharge; 6 lived at home with normal cognition and minimal or no disability, and 2, whose recovery was complicated by severe polyneuropathy, remained in an inpatient living facility for more constant support. Their GOSE scores ranged from 3 to 6 (median 4, IQR 4–5) and DRS scores ranged from 0 to18 (median 3, IQR 3–5). eTable 4, links.lww.com/WNL/B660, provides for details.
Figure 2. Longer-term Functional Outcomes for Patients With COVID-DoC.
Glasgow Outcome Scale Extended (GOSE; A) and Disability Rating Scale (DRS; B) scores for each patient are shown (higher scores on GOSE and lower scores on DRS reflect less disability). Functional outcomes are depicted at hospital discharge and 3 and 6 months after discharge. COVID-DoC = disorders of consciousness in coronavirus disease 2019.
Neuroimaging Results
Compared to healthy controls, patients with COVID-DoC exhibited significantly reduced (i.e., less positive) intranetwork connectivity within the DMN (COVID-DoC 0.12 [SD 0.06], healthy control 0.23 [SD 0.07], t [22] = 3.88, p < 0.001) and significantly reduced (i.e., less negative) internetwork connectivity between the DMN and SN (COVID-DoC 0.04 [SD 0.03], healthy control −0.003 [SD = 0.04], t [22] = 2.86, p < 0.01) (Figure 3). Both findings remained significant after Bonferroni correction (p < 0.025) and with Mann-Whitney U tests. After controlling for age, COVID-DoC remained significantly associated with reduced intranetwork (b = −0.15, p < 0.01) and internetwork (b = 0.06, p < 0.05) connectivity.
Figure 3. Resting-State Functional Connectivity for Patients With COVID-DoC and Healthy Controls.
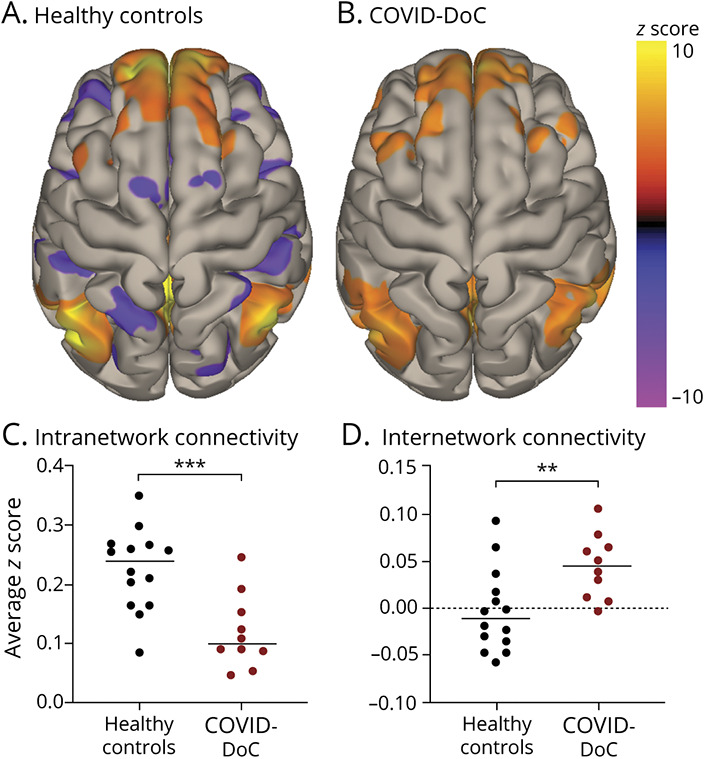
Connectivity of the default mode network is depicted as group-wise seed-to-voxel maps, generated with seeds at 4 nodes—the medial prefrontal cortex, posterior cingulate cortex, and bilateral inferior parietal lobules—for healthy controls (A) and patients with disorders of consciousness in coronavirus disease 2019 (COVID-DoC; B). Warmer colors represent positive correlations (reflecting intranetwork connectivity); cooler colors represent negative correlations (reflecting internetwork connectivity). Patients with COVID-DoC demonstrated less positive intranetwork connectivity (C) and less negative internetwork connectivity (D) than healthy controls. **p < 0.01. ***p < 0.001.
Compared to healthy controls, patients with COVID-DoC exhibited reduced whole-brain FA (COVID-DoC 0.51 [SD 0.02], healthy control 0.59 [SD 0.02], t [21] = 9.06, p < 1 x 10-8) and brainstem FA (COVID-DoC 0.53 [SD 0.04], healthy control 0.59 [SD 0.02], t [21] = 4.27, p < 0.001) (Figure 4). Both findings remained significant after Bonferroni correction (p < 0.0125) and with Mann-Whitney U tests. After controlling for age, COVID-DoC remained significantly associated with reduced whole-brain FA (b = −0.09, p < 0.05) and brainstem FA (b = −0.06, p < 0.005). Whole-brain FA and brainstem FA in COVID-DoC were comparable to those of patients with severe TBI (p > 0.05). eTable 5, links.lww.com/WNL/B660, provides details, and eTable 6 gives FA within specific white matter tracts. Although the limited sample and heterogenous sedation regimens precluded formal statistical testing, there was no appreciable trend toward lower connectivity values in patients on higher amounts of sedation during the MRI (eFigures 2 and 3).
Figure 4. Diffusion MRI Metrics for Patients With COVID-DoC and Healthy Controls.
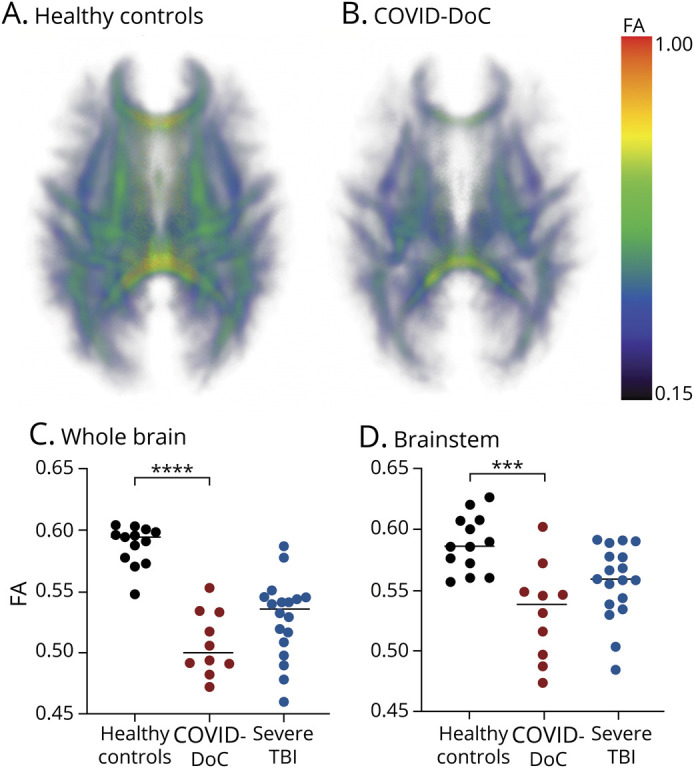
White matter integrity within a white matter skeleton is depicted as a 3-dimensional group-wise fractional anisotropy (FA) map, with warmer colors representing higher FA and thus higher white matter integrity, for healthy controls (A) and patients with disorders of consciousness in coronavirus disease 2019 (COVID-DoC; B). Whole-brain (C) and brainstem (D) FA values of patients with COVID-DoC were low compared to those of healthy controls but comparable to those of patients with severe traumatic brain injury (TBI). ***p < 0.001. ****p < 1 × 10-8.
Exploratory Results
The clinical variables assessed did not demonstrate a significant correlation with TTRC. Of them, the presence of renal dysfunction at the time of IV sedation cessation demonstrated the strongest trend, whereby patients with renal dysfunction trended toward a longer TTRC (Spearman r [9] = 0.61, p = 0.06). Intranetwork functional connectivity, internetwork functional connectivity, whole-brain FA, and brainstem FA did not correlate with TTRC or time from MRI to recovery of consciousness (p > 0.05). These neuroimaging metrics did not differ between patients with (n = 6) and without (n = 5) microhemorrhages or between patients with (n = 5) and without (n = 6) leukoencephalopathy (p > 0.05), nor were they correlated with the degree of hypoxemia (p > 0.05). Patients with a severe burden of microhemorrhages trended toward a longer TTRC (26, 6, and 4 days) compared to patients with a mild burden of microhemorrhages (6, 0, and 0 days).
Of the 11 patients who recovered consciousness, 7 did so while feasibly still under the effect of sedation, according to the timing and estimated half-lives of sedatives they received. The other 4 patients, even with conservative half-life estimates for critically ill patients with prolonged exposures, did not recover consciousness for 2 to 20 days after the elimination of all sedatives. eTable 7, links.lww.com/WNL/B660, gives details of cumulative patient sedation.
Discussion
Here we report prospective findings from a cohort of patients with COVID-DoC. Notably, all patients who survived severe COVID-19 recovered consciousness. This observation corroborates prior reports that consciousness recovery from COVID-DoC is possible9-11 and indicates that such recovery is highly likely. Moreover, enrolling patients prospectively, soon after patients developed an unexplained DoC, revealed that the weeks- or months-long DoC described in previous reports are not common in COVID-DoC, with 50% of patients regaining consciousness within a week after continuous sedation was discontinued.
While patients were typically of advanced age, with comorbid conditions such as hypertension, diabetes, and asthma, such demographics characterize patients susceptible to severe COVID-19 in general40 and may not indicate a susceptibility to COVID-DoC in particular (an age-matched cohort of patients with severe COVID-19 but no DoC had similar comorbid conditions). Similarly, while there was a high prevalence of structural neuroimaging findings associated with COVID-19—82% of patients demonstrated microhemorrhages or leukoencephalopathy—it is unclear whether these findings cause or predispose to COVID-DoC. Given that the prevalence of these findings in severe COVID-19 is uncertain and that these findings may be attributable to alternative etiologies (e.g., cerebral amyloid angiopathy, coagulopathy from critical illness, or leukoaraiosis), it is challenging to confirm their association with COVID-DoC. We note that the severity of microhemorrhages and leukoencephalopathy varied between patients, and while patients with numerous microhemorrhages tended to demonstrate a longer TTRC than patients with few microhemorrhages, there was no clear link between severity and recovery: some patients with severe findings had brief COVID-DoC with favorable outcomes (e.g., patient 7 in eFigure 1, links.lww.com/WNL/B660), while other patients with no such findings had prolonged COVID-DoC with protracted disability (e.g., patient 3 in eFigure 1). Ultimately, given that some patients with COVID-DoC exhibited neither microhemorrhages nor leukoencephalopathy, we can conclude that such findings are not necessary for COVID-DoC.
Some have speculated that COVID-DoC may result from the high doses of sedation required to treat severe COVID-19.6 Patients with COVID-DoC were indeed sedated longer than those with severe COVID-19 but no DoC, but sedatives alone may not necessarily explain the differences between these 2 small cohorts, given potential confounds such as illness severity. While some patients with COVID-DoC may have been affected by lingering sedatives, others remained unresponsive long after sedatives were likely eliminated. Thus, while sedation may contribute to COVID-DoC, our findings suggest that sedation alone is not sufficient to explain all instances of COVID-DoC.
The heterogeneity of clinical characteristics observed in this patient cohort suggests that the etiology of COVID-DoC is likely multifactorial. Structural brain injury, medical causes of encephalopathy, systemic inflammation (as suggested by a recent study41), hypoxemia, and the lingering effect of sedation may all play a role, to varying degrees, in COVID-DoC. Although we found no variables consistently associated with TTRC, we note that there was a trend toward an association with renal failure, a known complication of COVID-19 that can cause encephalopathy.42
Functional connectivity, both within and between networks, was reduced in patients with COVID-DoC compared to healthy controls. White matter integrity was also reduced in individuals with COVID-DoC compared to healthy controls. Moreover, white matter integrity was comparable between patients with COVID-DoC and patients with DoC caused by severe TBI, a condition known to cause axonal injury.15,16,18 Just as diminished functional and structural connectivity has been observed in other DoC, disruption of brain network integrity may similarly explain impaired consciousness in COVID-DoC.
The cause of diminished brain connectivity in COVID-DoC is unknown. The fact that microhemorrhages and leukoencephalopathy were observed in many patients raises the possibility that these findings represent overt manifestations of neurologic injury present across all patients with COVID-DoC. Whether such neurologic injury reflects systemic inflammation, toxic-metabolic insults, the effects of prolonged intubation and intensive care, direct viral infection, hypoxemia, or a combination of these and other factors remains a question for future research.
Although the recovery of consciousness is a critical consideration in prognostication, the prospects for longer-term functional recovery are similarly important. All patients who survived the hospitalization required constant support at discharge, but by 3 months after discharge, >60% had returned home. By 6 months, 75% lived at home with normal cognition and minimal disability (due to persistent weakness or pain); the other 25% (2 patients) remained in inpatient facilities due to ongoing medical illness.
This study has several limitations. Although among the largest to date, this cohort of patients with COVID-DoC remains small due to the challenges of enrolling and obtaining advanced neuroimaging for critically ill patients with COVID-19. Therefore, it is possible that we failed to capture the full spectrum of potential neurologic outcomes. Similarly, while we found no significant association between TTRC and several clinical variables, this study may have been underpowered for detecting such associations. The clinical judgment inherent to the definition of COVID-DoC may lead to heterogeneity within this patient cohort; future studies are needed to develop uniform and objective diagnostic criteria. It is possible that sedatives reduced functional connectivity in patients with COVID-DoC, although the low-dose sedatives that patients received have a limited impact on functional connectivity,43 and sedation and connectivity were not appreciably associated. Because the controls were enrolled in a separate study, we were unable to ensure that they were matched to the patients with COVID-DoC, raising the possibility of confounds; all comparisons, however, remained significant after accounting for differences in age. Comparing the MRI characteristics of patients with COVID-DoC to patients with COVID-19 but no DoC may be an informative topic of future investigation; given the potential risks to patients and staff, at the time of this study, it was not feasible to obtain research scans on patients with severe COVID-19 who did not otherwise warrant the scans clinically. Finally, clinical teams were not blinded to the neuroimaging findings; while we cannot exclude the possibility that patient care was subtly influenced, life-sustaining treatment was never withdrawn for a poor neurologic prognosis, despite diminished brain connectivity. Ultimately, larger prospective cohorts are necessary to further elucidate the natural history of COVID-DoC, to more precisely characterize its neural connectivity profile, and to identify prognostic biomarkers.
Among the countless tragedies of the COVID-19 pandemic, COVID-DoC has presented unique and profound challenges. Rendering patients unable to communicate, and with uncertain prospects for recovery, COVID-DoC has forced families and clinicians to decide whether to continue life-sustaining treatment with little data available for guidance. This study indicates that patients who survive the medical complications of severe COVID-19 are highly likely to regain consciousness. Although rates of disability are high immediately after hospital discharge, such disability is due mostly to the sequelae of prolonged medical illness, and patients frequently improve substantially in the ensuing months. Moreover, COVID-DoC appears to be associated with loss of functional and structural brain connectivity, findings common to other DoC caused by brain injury. While future research is necessary to further characterize COVID-DoC, these findings help inform the prognosis and pathophysiology of COVID-DoC, a condition fraught with uncertainty.
Glossary
- COVID-19
coronavirus disease 2019
- d-MRI
diffusion MRI
- DMN
default mode network
- DoC
disorders of consciousness
- DRS
Disability Rating Scale
- FA
fractional anisotropy
- GOSE
Glasgow Outcome Scale Extended
- IQR
interquartile range
- rs-fMRI
resting-state fMRI
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SN
salience network
- TBI
traumatic brain injury
- TTRC
time to recover consciousness
Appendix. Authors
Study Funding
This study was supported by the NIH National Institute of Neurologic Disorders and Stroke (R25NS06574309, R21NS109627), the James S. McDonnell Foundation COVID-19 Recovery of Consciousness Consortium, the NIH Director's Office (DP2HD101400), and the Tiny Blue Dot Foundation.
Disclosure
D. Fischer, S.B. Snider, M.E. Barra, W.R. Sanders, O. Rapalino, P. Schaefer, A.S. Foulkes, Y.G. Bodien, and B.L. Edlow reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambrecq V, Hanin A, Munoz-Musat E, et al. Association of clinical, biological, and brain magnetic resonance imaging findings with electroencephalographic findings for patients with COVID-19. JAMA Netw Open. 2021;4(3):e211489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinzon RT, Wijaya VO, Buana RB, Al Jody A, Nunsio PN. Neurologic characteristics in coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Front Neurol. 2020;11:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, et al. . Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060-e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edlow BL, Claassen J, Victor JD, Brown EN, Schiff ND. Delayed reemergence of consciousness in survivors of severe COVID-19. Neurocrit Care. 2020;33(3):627-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldman GJ, Thakur KT, Der Nigoghossian C, et al. Multidisciplinary guidance to manage comatose patients with severe COVID-19. Ann Neurol. 2020;88(4):653-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huberman BJ, Mukherjee D, Gabbay E, et al. Phases of a pandemic surge: the experience of an ethics service in New York City during COVID-19. J Clin Ethics. 2020;31(3):219-227. [PubMed] [Google Scholar]
- 9.Fischer D, Threlkeld ZD, Bodien YG, et al. Intact brain network function in an unresponsive patient with COVID-19. Ann Neurol. 2020;88(4):851-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sangare A, Dong A, Valente M, et al. Neuroprognostication of consciousness recovery in a patient with COVID-19 related encephalitis: preliminary findings from a multimodal approach. Brain Sci. 2020;10(11):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdo WF, Broerse CI, Grady BP, et al. Prolonged unconsciousness following severe COVID-19. Neurology. 2021;96(10):e1437-e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva S, de Pasquale F, Vuillaume C, et al. Disruption of posteromedial large-scale neural communication predicts recovery from coma. Neurology. 2015;85(23):2036-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song M, Yang Y, He J, et al. Prognostication of chronic disorders of consciousness using brain functional networks and clinical characteristics. Elife. 2018;7:e36173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sair HI, Hannawi Y, Li S, et al. Early functional connectome integrity and 1-year recovery in comatose survivors of cardiac arrest. Radiology. 2018;287(1):247-255. [DOI] [PubMed] [Google Scholar]
- 15.Snider SB, Bodien YG, Bianciardi M, Brown EN, Wu O, Edlow BL. Disruption of the ascending arousal network in acute traumatic disorders of consciousness. Neurology. 2019;93(13):e1281-e1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng ZS, Reggente N, Lutkenhoff E, Owen AM, Monti MM. Disentangling disorders of consciousness: insights from diffusion tensor imaging and machine learning. Hum Brain Mapp. 2017;38(1):431-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velly L, Perlbarg V, Boulier T, et al. Use of brain diffusion tensor imaging for the prediction of long-term neurological outcomes in patients after cardiac arrest: a multicentre, international, prospective, observational, cohort study. Lancet Neurol. 2018;17(4):317-326. [DOI] [PubMed] [Google Scholar]
- 18.Jolly AE, Bălăeţ M, Azor A, et al. Detecting axonal injury in individual patients after traumatic brain injury. Brain. 2021;144(1):92-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newcombe VFJ, Spindler LRB, Das T, et al. Neuroanatomical substrates of generalized brain dysfunction in COVID-19. Intensive Care Med. 2021;47(1):116-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58(3):349-353. [DOI] [PubMed] [Google Scholar]
- 21.Edlow BL, Boly M, Chou SH, et al. Common data elements for COVID-19 neuroimaging: a GCS-neuroCOVID proposal. Neurocrit Care. 2021;34(2):365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal S, Jain R, Dogra S, et al. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke. 2020;51(9):2649-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondziella D, Bender A, Diserens K, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020;27(5):741-756. [DOI] [PubMed] [Google Scholar]
- 24.Giacino JT, Katz DI, Schiff ND, et al. . Comprehensive systematic review update summary: disorders of consciousness. Neurology. 2018;91(10):461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673-9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125-141. [DOI] [PubMed] [Google Scholar]
- 27.Threlkeld ZD, Bodien YG, Rosenthal ES, et al. Functional networks reemerge during recovery of consciousness after acute severe traumatic brain injury. Cortex. 2018;106:299-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y, Meng F, Zhang L, et al. . A multi-domain prognostic model of disorder of consciousness using resting-state fMRI and laboratory parameters. Brain Imaging Behav. 2021;15(4):1966-1976. [DOI] [PubMed] [Google Scholar]
- 29.Demertzi A, Antonopoulos G, Heine L, et al. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain. 2015;138(pt 9):2619-2631. [DOI] [PubMed] [Google Scholar]
- 30.Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48(4):577-582. [DOI] [PubMed] [Google Scholar]
- 31.Snider SB, Bodien YG, Frau-Pascual A, Bianciardi M, Foulkes AS, Edlow BL. Ascending arousal network connectivity during recovery from traumatic coma. Neuroimage Clin. 2020;28:102503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yong SJ. Persistent brainstem dysfunction in long-COVID: a hypothesis. ACS Chem Neurosci. 2021;12(4):573-580. [DOI] [PubMed] [Google Scholar]
- 33.Edlow BL, Chatelle C, Spencer CA, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain. 2017;140(9):2399-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573-585. [DOI] [PubMed] [Google Scholar]
- 35.Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability Rating Scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63(3):118-123. [PubMed] [Google Scholar]
- 36.Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A brain-wide study of age-related changes in functional connectivity. Cereb Cortex. 2015;25(7):1987-1999. [DOI] [PubMed] [Google Scholar]
- 37.Bisdas S, Bohning DE, Besenski N, Nicholas JS, Rumboldt Z. Reproducibility, interrater agreement, and age-related changes of fractional anisotropy measures at 3T in healthy subjects: effect of the applied b-value. AJNR Am J Neuroradiol. 2008;29(6):1128-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karaca-Mandic P, Georgiou A, Sen S. Assessment of COVID-19 hospitalizations by race/ethnicity in 12 states. JAMA Intern Med. 2021;181(1):131-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573-1582. [DOI] [PubMed] [Google Scholar]
- 40.Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65(5):533-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehme AK, Doyle K, Thakur KT, et al. . Disorders of consciousness in hospitalized patients with COVID-19: the role of the systemic inflammatory response syndrome. Neurocrit Care. Epub 2021 Jun 28. [DOI] [PMC free article] [PubMed]
- 42.Raza A, Estepa A, Chan V, Jafar MS. Acute renal failure in critically ill COVID-19 patients with a focus on the role of renal replacement therapy: a review of what we know so far. Cureus. 2020;12(6):e8429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greicius MD, Kiviniemi V, Tervonen O, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29(7):839-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.