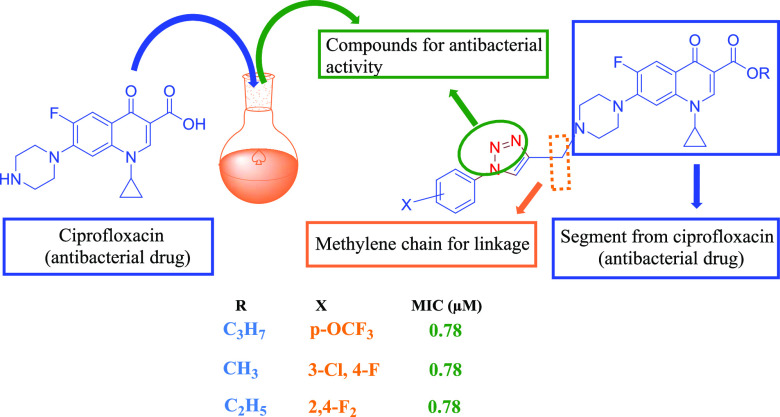
Abstract
A newer ciprofloxacin series containing 1,2,3-triazole conjugates of ciprofloxacin was designed, synthesized, and well characterized using modern analytical techniques by reacting diversified anilines with ciprofloxacin obtained from ciprofloxacin hydrochloride. The newer conjugates were evaluated for their antimicrobial activity against various strains, viz. Staphylococcus aureus (ATCC25923), Enterococcus faecalis (clinical isolate), Staphylococcus epidermidis (ATCC3594), Escherichia coli (ATCC25922), Pseudomonas aeruginosa (ATCC27853), Salmonella typhi (clinical isolate), Salmonella typhimurium (clinical isolate), Acinetobacter baumannii (ATCC19606), Aeromonas hydrophila (ATCC7966), Plesiomonas shigelloides (ATCC14029), and Sphingo biumpaucimobilis (MTCC6362) in vitro. Interestingly, some of the conjugates showed superior antimicrobial activity as compared to the control drug ciprofloxacin. The three compounds 4i, 4j, and 4n showed strong activity with minimum inhibitory concentration (MIC) 0.78 μM, while the compound 4g showed MIC 1.56 μM against S. typhi (clinical). The compound 4a showed good efficacy against S. aureus (ATCC25923) and S. typhi (clinical) with MIC 3.12 μM, while the compound 4b exhibited efficacy with MIC 3.12 μM against S. aureus (ATCC25923) and the control drug ciprofloxacin showed MIC 6.25 μM. Among all of the synthesized compounds, 4e, 4f, 4g, 4h, 4p, 4q, 4t, and 4u displayed less than 20% hemolysis, while the rest of the compounds showed hemolysis in the range of 21–48%. Moreover, the structure of compound 4b was also established by single-crystal X-ray diffraction studies.
1. Introduction
Quinolones are synthetic antibacterial agents that inhibit DNA gyrase and topoisomerase enzymes in bacteria.1 Since their discovery back in 1970, quinolones and their various synthetic analogs continue to be the most effective and widely used common antibiotics.2 Ciprofloxacin, a second-generation fluoroquinolone, displays broad-spectrum antibiotic activity. Acute uncomplicated infections such as urinary tract infections, cystitis, shigellosis, acute sinusitis disorder, and chronic bacterial prostatitis are generally treated by ciprofloxacin and its analogs.3,4 Ciprofloxacin functions by expressing ternary complexes with enzyme-DNA, thus restricting the repair of bacterial DNA and transcription of RNA.1,2 The remarkable antibacterial activity, efficient pharmacokinetics, and insignificant side effects of ciprofloxacin have led to its extended use as a fluoroquinolone antibiotic. However, excessive use of ciprofloxacin in recent times has led to emergence of bacterial resistance,5 and thus, it is no longer useful to treat various bacterial infections. Therefore, there is an urgent need to find an alternative drug with high efficacy, negligible toxicity, and cost effectiveness. Further, the complexation of quinolone antibiotics with metals is also reported to enhance their biological activity by decreasing the bacterial resistance of the drug due to increase of liposolubility, thereby leading to greater intracellular accumulation. This is another approach that would add to the already existing batteries of antibiotic arsenal in derivatization of the previously effective existing drugs.
The most common and rational strategy for the modification of ciprofloxacin is the introduction of a substituent at position C-7.6 Previous studies have demonstrated that a large group at this position does not affect the drug permeability across the bacterial membrane and concurrently modifies its strength and spectrum.7,8 This strategy has delivered a wide variety of newer conjugates containing 1,2,3-triazole,9−11 carboxamide,12 poly(2-oxazoline) and poly(ethylene glycol),13 amino-glycoside,14 nitrofuran,15 nitroimidazole,16 antitumor,17 1,3,4-thiadiazole,18 isatin,19 bromothiophene,20 acyl group21 etc.
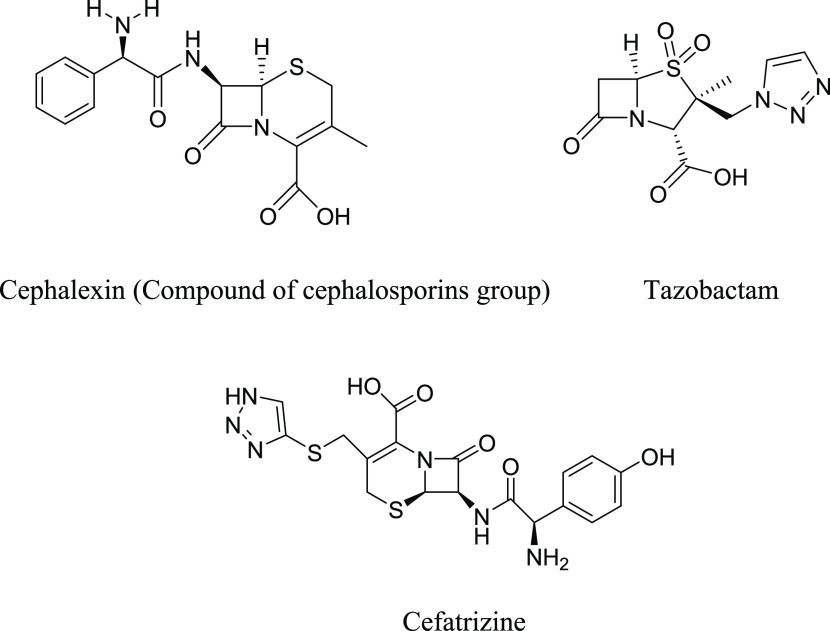
Huisgen 1,3-dipolar cycloaddition reactions (1,3-DCRs) serve as an extremely versatile and significant approach for the production of pharmacologically active compounds with one or more five-membered heterocyclic rings. The synthesis approach chosen for 1,3-DCRs might fulfill the stringent criteria that define click chemistry reactions: the reaction must be modular, render good yields, produce only harmless byproducts that can be separated by non-chromatographic processes, and be stereospecific but not necessarily enantioselective in nature.22−24 Click chemistry is capable of generating novel pharmacophores that represent a wide range of functional organic molecules.10 The advantages of click chemistry include simple and efficient reaction conditions, readily obtainable starting materials and reagents, reaction under solvent-free conditions, including use of benign solvents such as water or alcohol, and easy and simple workup procedure for isolation of the final title compound.22 Further, 1,3-DCRs have emerged as an alternative method to obtain 1,2,3-triazole compounds for screening of their broad-spectrum biological activity, including antibacterial,24 antitubercular,25 antitumor,26 anti-HIV,27 antifungal, and antiphytopathogenic activities. Moreover, the 1,2,3-triazole scaffold is also an integral component of the current extensively used clinical antibacterial drugs, such as cephalexin, tazobactam, and cefatrizine, as shown in Figure 1.
Figure 1.
Marketed antibacterial drugs.
Encouraged by our previous studies,28 we aimed to design and synthesize a newer efficacious series of ciprofloxacin conjugates containing 1,2,3-triazole for antimicrobial studies (Scheme 1).
Scheme 1. Synthesis of Desired Ciprofloxacin–Triazole Conjugates and Their Design Strategy.
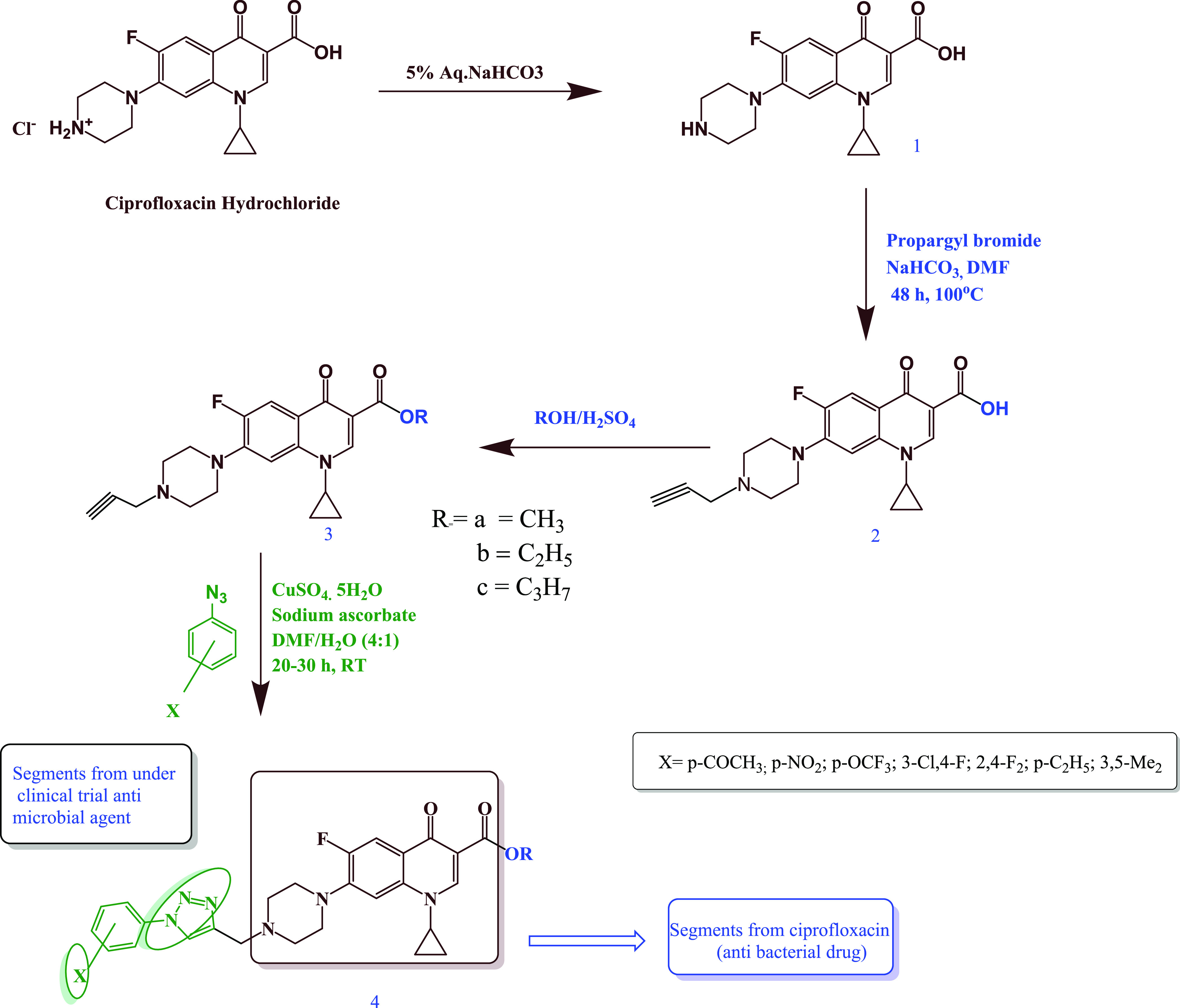
These ciprofloxacin analogues were screened against Gram-positive and Gram-negative bacterial strains, and their toxicity was also evaluated against human red blood cells (hRBCs).
There are a few reports in literature on ciprofloxacin–triazole hybrids exhibiting synergistic biological activities.28 Keeping these facts in mind, we have synthesized a series of ciprofloxacin-tethered triazoles with ester on one side and triazole ring on the other side of ciprofloxacin and evaluated their antibacterial and hemolytic activities.
2. Results and Discussion
2.1. Chemistry
New ciprofloxacin–triazole compounds (4a–u) were synthesized in three simple steps starting from ciprofloxacin hydrochloride as summarized in Scheme 1. Briefly, the free ciprofloxacin (1) was achieved by dissolving ciprofloxacin hydrochloride in a surplus of 5% aqueous solution of sodium bicarbonate after the usual workup procedure as reported elsewhere.29 Further, free ciprofloxacin was treated with propargyl bromide and NaHCO3 at 100 °C to obtain a propargylated ciprofloxacin (2).30 Furthermore, propargylated ciprofloxacin (2) was dissolved in three different solvents separately, viz. methanol, ethanol, and propanol, for esterification of the COOH group. Subsequently, concentrated H2SO4 was added to this mixture and the reaction mixture was refluxed for 7 h to obtain the corresponding esters (3). The compounds (3a–c) containing the propargyl group were used as substrate to further generate the 1,4-disubstituted 1,2,3-triazole library of 21 compounds (4a–u) by reacting various substituted aromatic azides by click chemistry. The structure of compound 4b was also confirmed by X-ray crystallographic studies to validate the design criteria, and the crystal data of compound 4b is presented in Figure 2.
Figure 2.
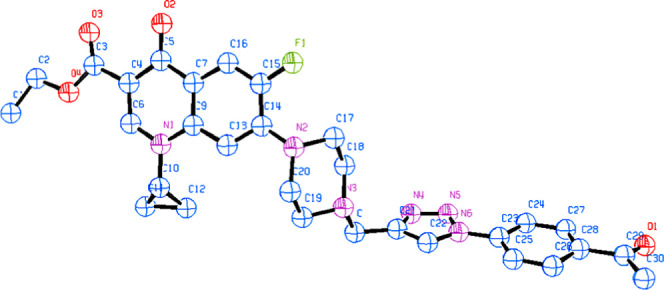
X-ray structure of 1,2,3-triazole-linked ciprofloxacin conjugate (4b).
2.2. Biological Activity
2.2.1. Antibacterial Activity
A study on 21 compounds (4a–u) containing ciprofloxacin with 1,2,3-triazole on one side and ester with varying alkyl groups on the other side was done via propargylation, followed by esterification and copper-catalyzed azide–alkyne (3 + 2) cycloaddition. All of the synthesized chemical compounds (4a–u) were evaluated for their antibacterial activity and minimum inhibitory concentration (MIC) against a broad spectrum of bacterial strains. The antibacterial activity of the compounds was examined against three strains of Gram-positive bacteria, i.e., Staphylococcus aureus (ATCC25923), Enterococcus faecalis (clinical isolate), Staphylococcus epidermidis (ATCC3594), and eight strains of Gram-negative bacteria, i.e., Escherichia coli (ATCC25922), Pseudomonas aeruginosa (ATCC27853), Salmonella typhi (clinical isolate), Salmonella typhimurium (clinical isolate), Acinetobacter baumannii (ATCC19606), Aeromonas hydrophila (ATCC7966), Plesiomonas shigelloides (ATCC14029), and Sphingo biumpaucimobilis (MTCC6362). The MIC results support the fact that the nature and place of the substituent as well as their isomeric consequences on phenyl rings have a dramatic effect on the antibacterial activity of the moiety (Table 1).
Table 1. Antibacterial Activity and % Hemolysis of Compounds (4a–u).
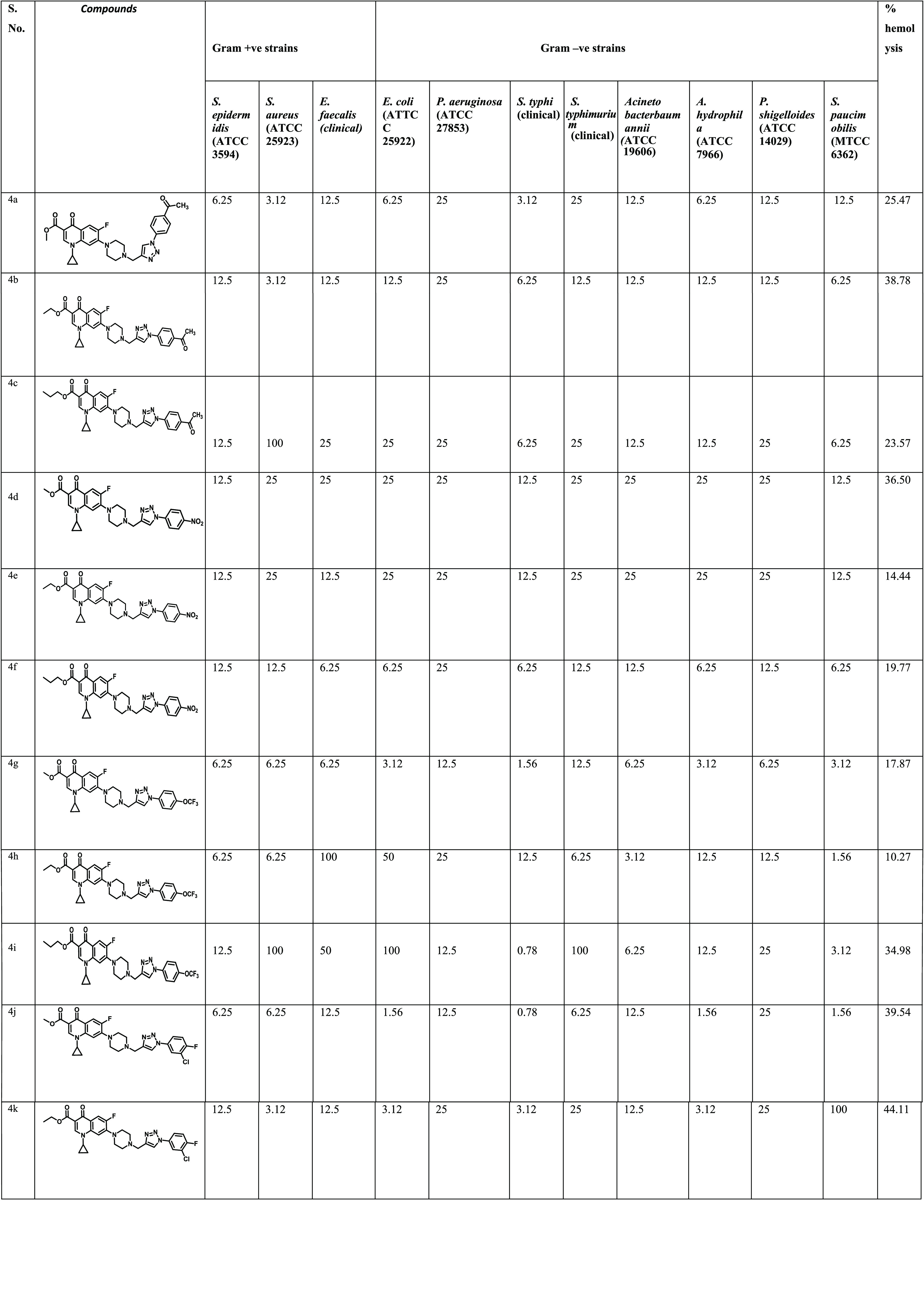
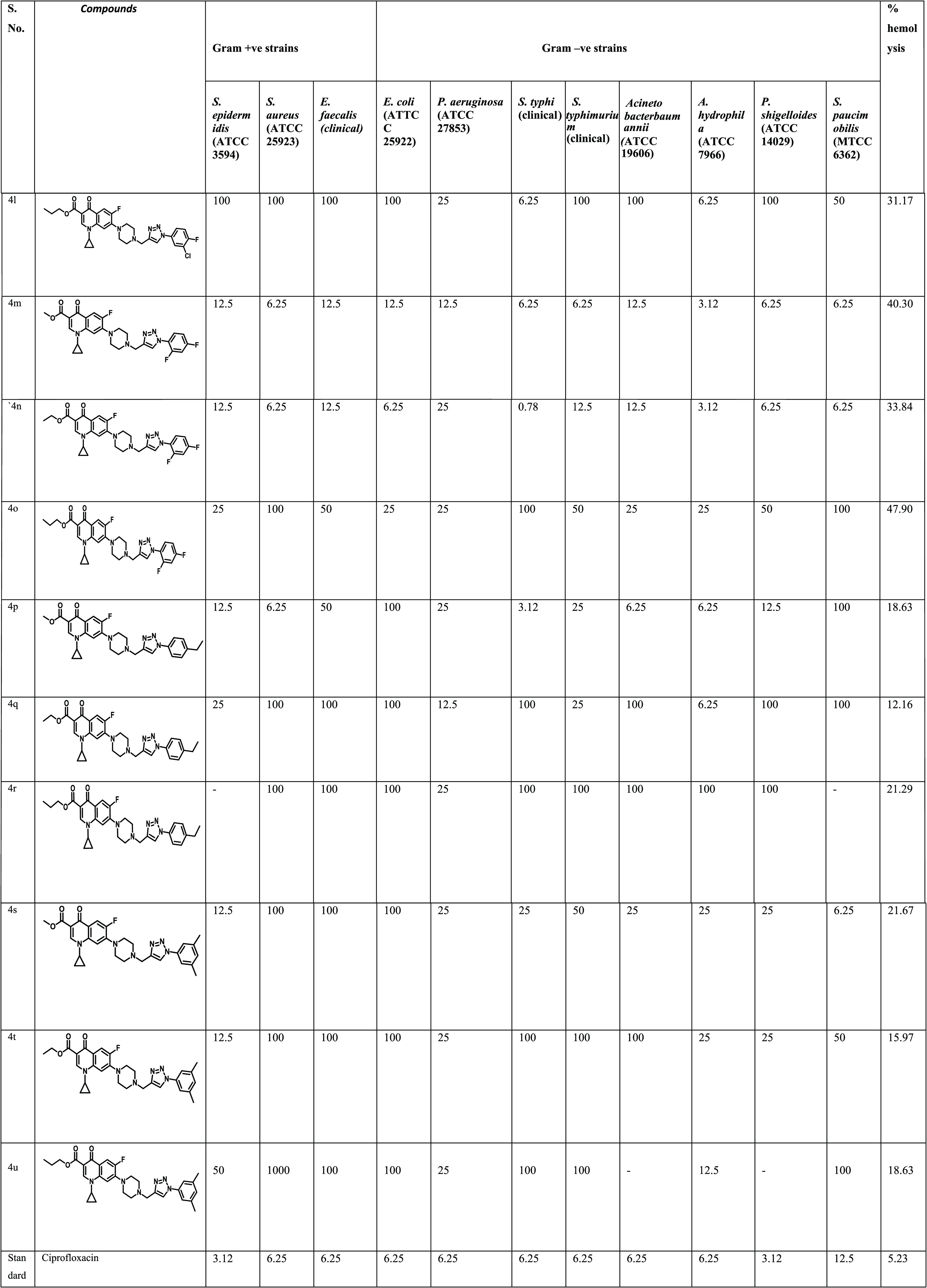
It is evident from Table 1 that the synthesized compounds, viz. 4a, 4b, 4g, 4h, 4i, 4j, 4k, 4m, 4n, and 4p were more potent, with either less or equal MIC as compared to the standard drug ciprofloxacin.
Compound 4a, with a methyl ester and p-acetyl substituent on triazole, showed very good efficacy against S. aureus (ATCC25923) and S. typhi (clinical) with MIC 3.12 μM, and also against E. coli (ATCC25922) and A. hydrophila (ATCC7966) with MIC 6.25 μM, while the control drug showed MIC 6.25 μM. Compound 4b with ethyl ester and p-COCH3-substituted benzene showed the potent activity against S. aureus (ATCC25923) with MIC 3.12 μM, while this compound was also found active against S. typhi (clinical) and S. paucimobilis (MTCC6362) with MIC 6.25 μM similar to the control drug. Compound 4c with propyl ester and p-acetyl group on trizole was found active only against two strains, viz. S. typhi (clinical) and S. paucimobilis (MTCC6362), with MIC 6.25 μM, while it showed moderate to weak activity against the rest of the strains. Compound 4d with methyl ester and 4e with ethyl ester and p-NO2 group on a benzene ring in both compounds showed weak activity against all tested strains with the MIC in the range from 12.5 to 25 μM. Compound 4f with the propyl ester group and p-nitrobenzene on triazole was found active against five strains, viz. E. coli (clinical), E. faecalis (clinical), S. typhi (clinical), A. hydrophila (ATCC7966), and S. paucimobilis (MTCC6362) with MIC 6.25 μM, which is comparable to the control drug. Compound 4g with methyl ester and the p-trifluoromethoxy group on benzene was found most potent against S. typhi (clinical) with MIC 1.56 μM and against A. hydrophila (ATCC7966), S. paucimobilis (MTCC6362), and E. coli (clinical) with MIC 3.12 μM. Further, the compound 4g also showed promising activity against five strains, including S. epidermidis (ATCC3594), S. aureus (ATCC25923), A. baumannii (ATCC19606), P. shigelloides (ATCC14029), and E. faecalis (clinical), with MIC 6.25 μM similar to the standard drug ciprofloxacin. Compound 4h with ethyl ester having p-OCF3 substituted on the benzene ring showed excellent activity against strains S. paucimobilis (MTCC6362) and A. baumannii (ATCC19606), having MIC values of 1.56 and 3.12 μM, respectively, while it showed moderate potency against S. aureus (clinical), S. epidermidis (ATCC3594), and S. typhimurium (clinical) with MIC values comparable to that of ciprofloxacin (6.25 μM). Compound 4i with propyl ester and the p-OCF3 group on benzene showed excellent potency against S. typhi (clinical) with MIC 0.78 μM, which indicates approximately 8-fold higher activity than that of the control drug ciprofloxacin with MIC 6.25 μM, while the same compound exhibited potent activity against S. paucimobilis (MTCC6362) and A. baumannii (ATCC19606) with MIC values of 3.12 and 6.25 μM, respectively. Compound 4j having 3-chloro, 4-fluoro substitution on the benzene in triazole along with methyl ester showed excellent activity against S. typhi (clinical) with MIC 0.78 μM, while E. coli (clinical), S. paucimobilis (MTCC6362), and A. hydrophila (ATCC7966) showed MIC 1.56 μM. The same compound showed good activity against three strains, namely S. epidermidis (ATCC3594), S. aureus (ATCC25923), and S. typhimurium (clinical), with MIC 6.25 μM, which is comparable to the control drug. Further, compound 4k with ethyl ester and 3-chloro, 4-fluoro substitution on benzene showed potent activity against S. aureus (clinical), A. hydrophila (ATCC7966), E. coli (clinical), and S. typhi (clinical) with MIC 3.12 μM each. Compound 4l having propyl ester and 3-chloro, 4-fluoro group substitution on benzene showed activity against S. typhi (clinical) and A. hydrophila (ATCC7966) with MIC 6.25 μM, whereas compound 4m with methyl ester and 2,4-difluoro on triazole showed activity against A. hydrophila (ATCC7966) with MIC 3.12 μM, while in S. aureus (clinical), S. typhi (clinical), S. typhimurium (clinical), and P. shigelloides (ATCC14029), and S. paucimobilis (MTCC6362) it showed MIC 6.25 μM, which is similar to that of the reference drug ciprofloxacin. Compound 4n with 2,4-difluoro substitution on the benzene ring and ethyl ester showed excellent activity against S. typhi (Clinical) and A. hydrophila (ATCC7966) with MIC values of 0.78 and 3.12 μM, respectively, while the same compound showed good activity against S. aureus (ATCC25923), S. paucimobilis (MTCC6362), P. shigelloides (ATCC14029), and E. coli (clinical) with MIC 6.25 μM each, comparable to the control drug. Compound 4o with propyl ester and a 2,4-difluoro-substituted benzene ring did not show any reasonable activity against any of the strains under the study. Compound 4p having an ethyl group on the benzene ring and methyl ester was found most potent against S. typhi (clinical) with MIC 3.12 μM, while it showed good activity against three strains, viz. S. aureus (ATCC25923), A. baumannii (ATCC19606), and A. hydrophila (ATCC7966), with MIC 6.25 μM, again comparable to the control drug. Compound 4q having an ethyl group on benzene and ethyl ester showed MIC 6.25 μM against the Gram-negative bacteria A. hydrophila (ATCC7966), which is similar to the MIC of the control drug. No activity was seen in other strains. Compound 4r having an ethyl group on benzene and propyl ester did not show any activity against any strain selected for study. Compound 4s having methyl ester and a 3,5-dimethyl-substituted benzene ring exhibited potent activity against S. paucimobilis (MTCC6362) with MIC 6.25 μM, which is just half of the MIC of the standard drug, while no activity was seen against any of the strains. Again, compounds 4t and 4u having a 3,5-dimethyl benzene ring and ethyl and propyl esters, respectively, did not show any activity against any of the tested Gram strains.
Our findings indicate that the nature of the groups present in the substituent has a strong role in antimicrobial activity. Therefore, functional groups such as strong electron-withdrawing groups, e.g. trifluoromethoxy, nitro, and acetyl groups having electron-withdrawing nature at position 4 in the benzene ring, strongly affect the antibacterial activity. This hypothesis is supported by the higher activity of compounds 4f, 4g, 4j, 4k, 4m, and 4n against all of the Gram-positive and Gram-negative bacteria screened in the present study. Further, the ester group on the opposite side of the phenyl ring also plays a critical role in governing the activity. Thus, we can conclude from this study that position 4 is critical for determining the antibacterial activity against both Gram-positive and Gram-negative bacteria. The presence of strong electron-withdrawing groups has a major effect on the potency of the compounds. The sensitivity of the bacterial strain is another noteworthy observation. For example, S. typhi (clinical) showed a higher sensitivity to most of the synthesized compounds than the parent drug ciprofloxacin, except the compounds bearing electron-donating groups. Interestingly, compounds 4i, 4j, and 4n were found to be most active against S. typhi (clinical). Thus, it is clear from this study that there is a need of second-generation synthesis based on activity data, and structure–activity relationship (SAR) should be done on lead molecules to get meaningful results.
2.2.2. Hemolytic Activity
The hemolytic activity of drugs is executed by several mechanisms, including increase in the permeability of cell membranes to absolute cell lysis. It is observed that latent damage is caused by the drugs present in oils to the membranes of erythrocytes, which undergo lysis and subsequently liberate hemoglobin. The cause underlying this hemolytic movement is to optimize the % hemolysis of the compounds against red blood cells, which fills in as an extra criterion to analyze the significance of the incorporated compounds and may likewise guide the development of such compounds as medications. While determining the toxicity profile of whole synthesized compounds, their hemolytic activities were evaluated by following the route originated by Nielson et al.31 at a stable concentration of 100 μM on human RBCs. The results showed that these compounds caused 10–48% hemolysis. Interestingly, most of the synthesized compounds (4e, 4f, 4g, 4h, 4p, 4q, 4t, and 4u) displayed less than 20% hemolysis, while the standard drug ciprofloxacin showed 5.23% hemolysis. The outcomes of the hemolytic experiment of the tested compounds are represented in Table 1 in conjunction with the antibacterial activity results.
3. Conclusions
Briefly, twenty-one newer synthetic ciprofloxacin analogues were screened against various antibacterial strains. From a small library pool, three compounds, viz. 4i, 4j, and 4n, showed excellent activity against S. typhi (clinical) strains with MIC 0.78 μM, while two compounds, viz. 4h and 4j, showed excellent activity against S. paucimobilis (MTCC6362) and A. hydrophila (ATCC7966) with MIC 1.56 μM, respectively. Moreover, nine compounds, viz. 4a, 4b, 4g, 4h, 4i, 4k, 4m, 4n, and 4p, showed very good activity with MIC 3.12 μM against various strains, whereas the control drug ciprofloxacin showed MIC 6.25 μM. Moreover, the electron-withdrawing groups such as −OCF3, −NO2, and −COCH3, at para-position in the benzene ring, have a strong influence on determining the antibacterial activity. The study of hemotoxicity revealed a negligible toxicity profile for all of the compounds. Thus, there is need to study the structure–activity relationship (SAR) of lead molecules to generate second-generation compounds for antimicrobial studies. Moreover, the lead compounds from second generation may be combined with existing clinical drugs to generate multifunctional hybrids in order to establish meaningful structure–activity relationship (SAR), aimed toward lowering their further effective concentrations and higher antimicrobial activity. This idea may solve to some extent the drug resistance problem in bacteria, and work in this direction is progressing well. Moreover, single-crystal X-rays of the compound validate the structure of our designed compound.
4. Experimental Section
All chemicals and solvents used in this study were purchased from Sigma-Aldrich and E. Merck (India). The reactions during synthesis were monitored by thin-layer chromatography (TLC) on precoated silica gel 60 F254 (mesh), and spots were visualized with UV light. Silica gel (60–120 mesh) was used for column chromatography. The melting points of all synthesized compounds were determined using the open capillary method and may be uncorrected. The structural assignments of the synthesized products were based on 1H, 13C NMR, MS, and single-crystal X-ray diffraction (XRD). NMR data were collected using a 400 MHz, JEOL JNM-ECS spectrometer in CDCl3 with TMS as internal standard, and data were processed with its Delta software. The following abbreviations were used in reporting the spectra: s = singlet, bs = broad singlet, d = doublet, dd = doublet of doublets, t = triplet, m = multiple. Mass data were generated using Bruker Compass spectrometer. X-ray analysis was performed using an Oxford Diffraction Xcalibur four-circle diffractometer with an Eos CCD detector using graphite monochromatized Mo Kα radiation (λ = 0.71073 Å).
4.1. Regeneration of Free 1-Cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic Acid (Ciprofloxacin 1)
Ciprofloxacin hydrochloride (5.0 g, 13.59 mmol) was dissolved in water (30 mL) to get a clear solution. This solution was treated with an excess of 5% aqueous sodium bicarbonate solution, resulting in the formation of a white precipitate. The precipitate was filtered off and left to dry as a free 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic acid (ciprofloxacin 1, 4.3 g, 12.98 mmol). The free ciprofloxacin was pure enough and used as starting material for propargylation reaction without purification. White solid, yield 4.3 g (96%), MP 253–255 °C. 1H NMR (DMSO-d6) δ: 8.63 (s, 1H, Ar-H), 7.87–7.84 (d, J = 13.72 Hz, 1H, Ar-H), 7.51–7.50 (d, J = 4.56 Hz, 1H, Ar-H), 3.79 (bs, 1H, −CH), 3.21 (bs, 4H, 2-CH2), 2.90 (bs, 4H, 2-CH2), 1.29–1.27 (m, 2H, −CH2), 1.15 (bs, 2H, −CH2).
4.2. Propargylation of 1-Cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic Acid (2)
To the solution of 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic acid (1 mmol) in dimethylformamide (15 mL), 25 mL of NaHCO3 solution (1.2 mmol) and 1.2 mmol of propargyl bromide were added under vigorous stirring. The mixture was allowed to react at 100 °C for 48 h. The completion of the reaction was monitored by TLC. After evaporating the solvent, the residue was purified by column chromatography using a CH2Cl2/MeOH (98:2) mixture as eluent. Light yellow solid, yield 1.9 g (73%), MP 225–227 °C. 1H NMR (CDCl3) δ: 8.71 (s, 1H, Ar-H), 7.97 (d, J = 13.08 Hz, 1H, Ar-H), 7.33 (d, J = 7.42 Hz, 1H, Ar-H), 7.25 (s, 1H, Ar-H), 3.53 (m, 1H), 3.37 (t, 4H), 2.92 (s, 1H), 2.78 (t, 4H), 2.29 (s, 2H), 1.36 (d, J = 6.91 Hz, 2H), 1.18 (d, J = 6.2 Hz, 2H); 13C NMR (CDCl3) δ: 173.2 (−CO), 166.5 (−CO), 154.6, 148.3, 144.5, 138.0, 113.4, 110.4, 104.7, 73.6, 52.1, 51.5, 49.8, 46.8, 34.4, 8.1. Chemical formula: C20H20FN3O3; MS m/z (ES, +ve, CH3OH) 370.1579 [M + H]+.
4.3. General Procedure for Synthesis of 1-Cyclopropyl-6-fluoro-4-oxo-7-(4-prop-2-ynyl-piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic Acid Methyl/Ethyl/Propyl Ester (3a–c)
The compound 2 was dissolved separately in methanol, ethanol, and propanol (25 mL each). Concentrated H2SO4 (3 mL) was added to this mixture and the mixture was refluxed for 7 h. The completion of the reaction was monitored by TLC. After completion of the reaction as seen by TLC, the reaction mixture was cooled at room temperature; ice was added to the mixture and neutralized by aqueous sodium bicarbonate solution. The reaction mixture was extracted with chloroform. The organic layer was washed with water and dried over anhydrous sodium sulfate to get the corresponding ester.
4.3.1. 1-Cyclopropyl-6-fluoro-4-oxo-7-(4-prop-2-ynyl-piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic Acid Methyl Ester (3a)
Yield 0.9 g (88.5%), MP 208–210 °C. 1H NMR (CDCl3) δ: 8.51 (s, 1H, Ar-H), 8.01 (s, 1H, Ar-H), 7.25 (s, 1H, Ar-H), 3.8 (s, 2H), 3.77–3.29 (m, 8H), 2.77 (t, 4H), 2.28 (s, 1H), 1.32–1.09 (m, 4H); 13C NMR (CDCl3) δ: 177.2 (−CO), 167.0 (−CO), 147.4, 145.6, 139.3, 119.9, 112.9, 108.0, 104.8, 73.7, 51.4, 49.6, 46.8, 35.2, 8.2. Chemical formula: C21H22FN3O3; MS m/z (ES, +ve, CH3OH) 384.1736 [M + H]+.
4.3.2. 1-Cyclopropyl-6-fluoro-4-oxo-7-(4-prop-2-ynyl-piperazin-1-yl)-1,4,dihydroquinoline-3-carboxylic Acid Ethyl Ester (3b)
Yield 1 g (96%), MP 194–196 °C. 1H NMR (CDCl3) δ: 8.73 (s, 1H, Ar-H), 7.99 (s, 1H, Ar-H), 7.33 (s, 1H, Ar-H), 3.54 (m, 1H), 3.39–3.35 (m, 7H), 2.80–2.77 (m, 7H), 2.29 (s, 1H), 1.68 (s, 1H), 1.39 (d, J = 7.65 Hz, 2H), 1.17 (t, 2H); 13C NMR (CDCl3) δ: 173.1 (−CO), 166.0 (−CO), 148.1, 144.4, 137.8, 113.6, 110.4, 104.3, 73.6, 51.5, 49.8, 46.8, 34.4, 29.3, 14.4, 8.1. Chemical formula: C22H24FN3O3: MS m/z (ES, +ve, CH3OH) 398.1856 [M + H]+.
4.3.3. 1-Cyclopropyl-6-fluoro-4-oxo-7-(4-prop-2-ynyl-piperazin-1-yl)-1,4-dihydro-quinoline-3-carboxylic Acid Propyl Ester (3c)
Yield 1 g (98.2%), MP 176–178 °C. 1H NMR (CDCl3) δ: 8.45 (s, 1H, Ar-H), 7.96 (d, 1H, J = 14.11 Hz), 7.19 (s, 1H, Ar-H), 4.20 (t, 2H), 3.34–3.24 (m, 7H), 2.74 (t, 4H), 2.25 (s, 1H), 1.74–1.73 (m, 2H), 1.26–0.94 (m, 7H); 13C NMR (CDCl3) δ: 173.2 (−CO), 165.6 (−CO), 148.0, 144.1, 138.0, 113.3, 110.8, 104.7, 66.4, 51.5, 49.8, 46.8, 34.4, 22.1, 10.5, 8.1. Chemical formula: C23H26FN3O3; MS m/z (ES, +ve, CH3OH) 412.2041 [M + H]+.
4.4. General Procedure for the Synthesis of Azides
The synthesis of azides was carried out according to the published procedure.32 Briefly, aniline (1 equiv) was dissolved in 6 N HCl solution (10 mL/mmol of aniline) at room temperature and cooled upto 0 °C, followed by addition of aqueous NaNO2 (1 equiv) solution under stirring. After 10 min of stirring at the same temperature, sodium azide (1.2 equiv) was added to the reaction mixture. This mixture was further stirred at room temperature for 2–3 h. After the completion of the reaction as seen by TLC, the reaction was worked up by extraction with chloroform. The organic layer was washed with brine solution and dried over anhydrous Na2SO4. After evaporation of the solvent, the crude product was pure enough for further reactions. All of the synthesized azides were stored at −20 °C.
4.5. General Procedure for the Synthesis of 1,2,3-Triazole Scaffolds (4a–u)
The synthesis of triazoles was carried out according to the published procedure.33 Briefly,1-cyclopropyl-6-fluoro-4-oxo-7-(4-prop-2-ynyl-piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid methyl/ethyl/propyl ester (3.1 equiv) and substituted aromatic azide (1.2 equiv) were suspended in N,N-dimethylformamide (25 mL/mmol of alkyne). The solution of sodium ascorbate (0.4 equiv in minimum water) was added, followed by copper(II) sulfate pentahydrate solution (0.2 equiv in minimum water). The heterogeneous mixture was stirred vigorously at room temperature until consumption of alkyne as monitored by TLC. After completion of the reaction, the mixture was poured onto ice water and the precipitate was collected by filtration. The desired products were purified by column chromatography using an eluent of 3% methanol in chloroform.
4.5.1. Methyl-7-(4-((1-(4-acetylphenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4a)
Light yellow solid, yield 0.11 g (77.5%), MP 216–218 °C. 1H NMR (CDCl3) δ: 8.50 (s, 1H, Ar-H), 8.12–7.86 (m, 6H, Ar-H), 3.87–3.85 (m, 3H), 3.28 (t, 3H), 2.79 (t, 4H), 2.60 (s, 3H, −COCH3), 2.64 (s, 3H, −OCH3), 1.98 (m, 2H) 1.29 (t, 2H), 1.10 (t, 2H); 13C NMR (CDCl3) δ: 196.6 (−CO), 172.8 (−CO), 166.7 (−CO), 154.4, 148.3, 139.9, 137.9, 136.7, 130.0, 120.7, 119.8, 113.4, 109.8, 104.8, 52.9, 52.0, 49.7, 34.4, 26.6, 8.0. Chemical formula: C29H29FN6O4; MS m/z (ES, +ve, CH3OH) 545.2302 [M + H]+.
4.5.2. Ethyl-7-(4-((1-(4-acetylphenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4b)
Light yellow compound, yield 0.11 g (78%), MP 208–210 °C. 1H NMR (CDCl3) δ: 8.48 (s, 1H, Ar-H), 8.12–8.07 (m, 5H), 7.99 (d, 2H, J = 13.80 Hz), 7.89 (d, 2H, J = 9.10 Hz), 4.35 (q, 2H, −CH2CH3), 3.85 (s, 2H), 3.39 (m, 1H), 3.28 (t, 4H), 2.78 (t, 4H), 2.64 (s, 3H, −OCH3), 1.37 (t, 2H), 1.22 (m, 1H), 1.10 (t, 2H); 13C NMR (CDCl3) δ: 196.5 (−CO), 173.0 (−CO), 165.7 (−CO), 154.3, 152.1, 148.0, 145.5, 144.5, 140.1, 137.8, 136.7, 130.0, 120.7, 119.8, 113.3, 110.0, 104.7, 60.8, 52.9, 52.6, 49.7, 34.4, 26.6, 14.3, 8.0. Chemical formula: C30H31FN6O4; MS m/z (ES, +ve, CH3OH) 559.2459 [M + H]+.
4.5.3. Propyl-7-(4-((1-(4-acetylphenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4c)
Light yellow solid, yield 0.13 g (93%), MP 198–200 °C. 1H NMR (CDCl3) δ: 8.47 (s, 2H, Ar-H), 8.10–7.87 (m, 5H), 7.25 (s, 1H), 4.25 (t, 2H), 3.85 (s, 2H), 3.46 (s, 1H), 3.28 (t, 4H), 2.79 (s, 4H), 2.64 (s, 3H), 1.78 (m, 2H), 1.28 (d, J = 7.34 Hz, 2H), 0.98 (m, 5H); 13C NMR (CDCl3) δ: 196.5 (−CO), 173.2 (−CO), 165.8 (−CO), 152.1, 148.0, 144.4, 139.7, 138.4, 136.7, 130.0, 120.6, 119.8, 113.3, 110.0, 104.7, 66.3, 52.6, 49.7, 34.4, 26.6, 22.0, 10.5, 8.0. Chemical formula: C31H33FN6O4; MS m/z (ES, +ve, CH3OH) 573.2643 [M + H]+.
4.5.4. Methyl-1-cyclopropyl-6-fluoro-7-(4-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-4-oxo-1,4-dihydroqinoline-3-carboxylate (4d)
Light pink solid, yield 0.11 g (77%), MP 211–213 °C. 1H NMR (CDCl3) δ: 8.53 (s, 1H, Ar-H), 8.42 (d, 2H, J = 9.30), 8.12–7.98 (m, 5H), 7.25 (s, 2H), 3.89 (m, 4H), 3.31 (m, 1H), 3.29 (m, 3H), 2.81 (m, 2H), 1.74 (t, 2H), 1.29 (t, 2H), 1.10 (t, 2H); 13C NMR (CDCl3) δ: 173.0 (−CO), 166.4 (−CO), 148.3, 146.9, 145.6, 140.8, 138.2, 125.5, 120.7, 120.3, 113.2, 109.4, 104.1, 52.9, 52.6, 52.0, 49.7, 34.4, 8.1. Chemical formula: C27H26FN7O5; MS m/z (ES, +ve, CH3OH) 548.2041 [M + H]+.
4.5.5. Ethyl-1-cyclopropyl-6-fluoro-7-(4-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (4e)
Light pink solid, yield 0.12 g (85%), MP 205–207 °C. 1H NMR (CDCl3) δ: 8.44 (s, 1H, Ar-H), 8.34 (d, 2H, J = 8.46 Hz), 8.09 (s, 1H), 7.99 (d, 2H, J = 8.45 Hz), 7.19 (s, 1H), 4.32 (q, 2H, −CH2CH3), 3.81 (s, 2 H), 3.36 (m, 1H), 3.23 (t, 4H), 2.74 (t, 4H), 1.34–1.22 (m, 6H), 1.06 (t, 2H); 13C NMR (CDCl3) δ: 173.11 (−CO), 165.61 (−CO), 148.12, 147.03, 141.10, 137.93, 125.5, 120.35, 113.14, 110.20, 104.79, 60.78, 52,60, 49.66, 34.46, 29.35, 14.36, 8.08. Chemical formula: C28H28FN7O5; MS m/z (ES, +ve, CH3OH) 562.2207 [M + H]+.
4.5.6. Propyl-1-cyclopropyl-6-fluoro-7-(4-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (4f)
Pink solid, yield 0.13 g (93%), MP 181–183 °C. 1H NMR (CDCl3) δ: 8.43 (s, 1H, Ar-H), 8.37 (d, 3H, J = 9.4 Hz), 8.16 (s, 1H), 7.98 (d, 2H, J = 8.59 Hz), 7.24 (s, 1H), 4.21 (t, 2H, −CH2CH2CH3), 3.83 (s, 2H), 3.39–3.38 (m, 1H), 3.25 (t, 4H), 2.75 (t, 4H), 1.76–1.72 (m, 2H, −CH2CH2CH3), 1.27–1.20 (m, 2H), 0.97 (t, 5H, −CH2CH2CH3); 13C NMR (CDCl3) δ: 173.0 (−CO), 165.4 (−CO), 147.9, 147.0, 144.2, 141.0, 137.8, 125.4, 120.9, 120.3, 113.2, 110.1, 104.7, 52.8, 52.5, 49.7, 34.4, 22.0, 10.4, 8.0. Chemical formula: C29H30FN7O5; MS m/z (ES, +ve, CH3OH) 576.2342 [M + H]+.
4.5.7. Methyl-1-cyclopropyl-6-fluoro-4-oxo-7-(4-((1-(4-(trifluoromethoxy)phenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (4g)
Light yellow solid, yield 0.14 g (92%), MP 196–198 °C. 1H NMR (CDCl3) δ: 8.52 (s, 1H, Ar-H), 8.01 (m, 2H), 7.81 (d, 2H, J = 8.60 Hz), 7.39 (d, 2H, J = 9.13 Hz), 7.25 (s, 1H), 3.92 (m, 4H), 3.41 (m, 1H), 3.32 (t, 4H), 2.87 (t, 3H), 1.32 (t, 3H), 1.09 (t, 3H); 13C NMR (CDCl3) δ: 173.2 (−CO), 166.5 (−CO), 149.2, 148.2, 143.9, 137.6, 122.3, 121.8, 113.1, 110.0, 104.7, 52.5, 52.0, 49.4, 34.5, 8.1. Chemical formula: C28H26F4N6O4; MS m/z (ES, +ve, CH3OH) 587.2032 [M + H]+.
4.5.8. Ethyl-1-cyclopropyl-6-fluoro-4-oxo-7-(4-((1-(4-(trifluoromethoxy)phenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (4h)
White solid, yield 0.11 g (72%), MP 166–168 °C. 1H NMR (CDCl3) δ: 8.48 (s, 1H, Ar-H), 7.97 (m, 2H), 7.80 (d, 2H, J = 8.83 Hz), 7.38 (d, 2H, J = 8.83 Hz), 7.24 (s, 1H), 4.35 (q, 2H, −CH2CH3), 3.84 (s, 2H), 3.39 (m, 3H), 3.28 (t, 4H), 2.78 (t, 4H), 1.37 (3H, −CH2CH3), 1.09 (t, 2H); 13C NMR (CDCl3) δ: 173.3 (−CO), 165.8 (−CO), 154.5, 152.2, 148.9, 148.1, 145.2, 144.3, 138.1, 135.3, 122.3, 121.8, 113.3, 110.5, 104.7, 60.8, 53.0, 52.6, 49.7, 34.4, 14.3, 8.0. Chemical formula: C29H28F4N6O4; MS m/z (ES, +ve, CH3OH) 601.2188 [M + H]+.
4.5.9. Propyl-1-cyclopropyl-6-fluoro-4-oxo-7-(4-((1-4-(trifluoromethoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate (4i)
Brown solid, yield 0.13 g (87%), MP 161–163 °C. 1H NMR (CDCl3) δ: 8.47 (s, 1H, Ar-H), 7.98–7.96 (m, 2H), 7.80 (d, 2H, J = 8.54 Hz), 7.38 (d, 2H, J = 8.63 Hz), 7.25 (s, 1H, Ar-H), 4.24 (t, 2H, −CH2CH2CH3), 3.84 (s, 2H), 3.39 (m, 6H), 2.79 (t, 4H), 1.76 (m, 2H, −CH2CH2CH3), 1.09 (t, 3H), 0.98 (t, 3H); 13C NMR (CDCl3) δ: 173.2 (−CO), 165.8 (−CO), 154.8, 152.1, 148.0, 144.5, 137.8, 135.5, 122.2, 121.8, 113.2, 110.4, 104.7, 66.3, 52.6, 49.7, 34.4, 22.0, 10.5, 8.0. Chemical formula: C30H30F4N6O4; MS m/z (ES, +ve, CH3OH) 615.2359 [M + H]+.
4.5.10. Methyl-7-(4-((1-(3-chloro-4-fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4j)
Light yellow solid, yield 0.14 g (97%), MP 190–192 °C. 1H NMR (CDCl3) δ: 8.52 (s, 1H, Ar-H), 7.85 (s, 1H), 7.33 (m, 3H), 3.41 (m, 5H), 3.30 (m, 5H), 2.79 (t, 4H), 1.32–1.24 (m, 3H), 1.13–1.09 (m, 2H); 13C NMR (CDCl3) δ: 173.2 (−CO), 165.4 (−CO), 154, 148.1, 144.5, 137.8, 135.5, 122.9, 121.8, 113.2, 110.4, 104.7, 52.6, 49.7, 34.4, 8.1. Chemical formula: C27H25ClF2N6O3; MS m/z (ES, +ve, CH3OH) 555.1691 [M + H]+.
4.5.11. Ethyl-7-(4-((1-(3-chloro-4-fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4k)
Light yellow solid, yield 0.13 g (91%), MP 115–117 °C. 1H NMR (CDCl3) δ: 8.48 (s, 1H, Ar-H), 7.95–7.83 (m, 3H), 7.77 (m, 1H), 7.32–7.22 (m, 1H), 4.37–4.32 (q, 2H, −CH2CH3), 3.83 (s, 2H), 3.393.28 (m, 5H), 2.77 (t, 4H), 1.92 (t, 2H), 1.37 (t, 5H), 1.10 (t, 2H); 13C NMR (CDCl3) δ: 172.8 (−CO), 166.0 (−CO), 148.1, 145.2, 144.3, 138.2, 133.5, 122.9, 120.9, 120.1, 117.7, 109.5, 104.9, 60.8, 52.9, 52.6, 49.7, 34.4, 14.3, 8.1. Chemical formula: C28H27Cl F2N6O3; MS m/z (ES, +ve, CH3OH) 569.1845 [M + H]+.
4.5.12. Propyl-7-(4-((1-(3-chloro-4-fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4l)
Brown solid, yield 0.13 g (92%), MP 103–105 °C. 1H NMR (CDCl3) δ: 8.47 (s, 1H), 7.96–7.84 (m, 3H), 7.66 (m, 1H), 7.32–7.21 (m, 2H), 4.26 (m, 2H), 3.79 (s, 2H), 3.40 (m, 1H), 3.28 (t, 4H), 2.79 (t, 4H), 1.80–1.78 (m, 2H), 1.29 (d, J = 6.41 Hz, 2H), 1.1.0–0.98 (m, 5H); 13C NMR (CDCl3) δ: 173.0 (−CO), 165.6 (−CO), 156.4, 151.7, 148.0, 144.2, 137.9, 133.6, 122.8, 120.1, 120.0, 117.7, 112.7, 104.7,66.3, 52.9, 52.4, 49.7, 34.4, 22.0, 10.4, 8.0. Chemical formula: C29H29ClF2N6 O; MS m/z (ES, +ve, CH3OH) 583.2034 [M + H]+.
4.5.13. Methyl-1-cyclopropyl-7-(4-((1-(2,4-difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4m)
Light yellow solid, yield 0.07 g (50%), MP 172–174 °C. 1H NMR (CDCl3) δ: 8.49 (s, 1H, Ar-H), 7.99–7.94 (m, 3H), 7.25–7.22 (m, 1H), 7.08–7.03 (m, 2H), 3.87 (s, 4H), 3.40 (m, 1H), 3.28 (t, 4H), 2.77 (t, 4H), 2.00 (s, 1H), 1.29 (d, J = 6.92 Hz, 2H), 1.09 (t, 2H); 13C NMR (CDCl3) δ: 173.2 (−CO), 166.3 (−CO), 154.9, 152.3, 148.3, 144.5, 137.9, 126.1, 126.0, 113.2, 113.0, 109.9, 104.7, 52.9, 52.5, 52.0, 49.7, 34.4, 8.0. Chemical formula: C27H25F3N6O3; MS m/z (ES, +ve, CH3OH) 539.2016 [M + H]+.
4.5.14. Ethyl-1-cyclopropyl-7-(4-((1-(2,4-difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4n)
Light yellow solid, yield 0.05 g (36%), MP 161–163 °C. 1H NMR (CDCl3) δ: 8.50 (s, 1H), 8.03–7.95 (m, 3H), 7.25 (d, J = 6.55 Hz, 1H), 7.09–7.05 (m, 2H), 4.37 (q, 2H, −CH2CH3), 3.86 (s, 2H), 3.30 (s, 4H), 2.79 (t, 4H), 1.78 (s, 1H), 1.40–1.26 (m, 5H), 1.13 (t, 2H); 13C NMR (CDCl3) δ: 165.89 (−CO), 148.1, 144.5, 137.8, 125.8, 113.4, 110.8, 105.1, 60.8, 52.6, 49.7, 34.4, 14.4, 8.1. Chemical formula: C28H27F3N6O3; MS m/z (ES, +ve, CH3OH) 553.2148 [M + H]+.
4.5.15. Propyl-1-cyclopropyl-7-(4-((1-(2,4-difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4o)
Brown solid, yield 0.09 g (65%), MP 153–155 °C. 1H NMR (CDCl3) δ: 8.47 (s, 1H), 8.00–7.90 (m, 3H), 7.24 (s, 1H), 7.07–7.02 (m, 2H), 4.24 (t, 2H), 3.85 (s, 2H), 3.39 (m, 1H), 3.28 (t, 4H), 2.78 (t, 4H), 1.79 (q, 2H), 1.28–0.98 (m, 7H); 13C NMR (CDCl3) δ: 173.2 (−CO), 165.8 (−CO), 154.8, 148.0, 144.9, 137.4, 125.8, 110.2, 105.2, 66.4, 52.9, 52.5, 49.7, 34.4, 22.0, 10.5, 8.1. Chemical formula: C29H29F3N6O3; MS m/z (ES, +ve, CH3OH) 567.2311 [M + H]+.
4.5.16. Methyl-1-cyclopropyl-7-(4-((1-(4-ethylphenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4p)
Dark brown solid, yield 0.12 g (87%) MP 172–174 °C. 1H NMR (CDCl3) δ: 8.52 (s, 1H, Ar-H), 8.01–7.96 (d, J = 13.32 Hz, 2H), 7.64 (d, J = 8.69 Hz, 2H), 7.34 (d, J = 8.93 Hz, 2H), 7.26 (s, 1H), 3.82 (s, 2H), 3.41 (m, 1H), 3.31 (t, 4H), 2.82 (s, 3H), 2.70 (q, 2H), 1.30–1.12 (m, 11H); 13C NMR (CDCl3) δ: 178.39 (−CO), 159.92, 148.50, 145.08, 137.46, 129.08, 120.14, 113.28, 109.85, 52.74, 49.67, 34.07, 29.91, 15.41, 8.08. Chemical formula: C29H31FN6O3; MS m/z (ES, +ve, CH3OH) 531.2499 [M + H]+.
4.5.17. Ethyl-1-cyclopropyl-7-(4-((1-(4-ethylphenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4q)
Dark brown solid, yield 0.11 g (80%), MP 103–105 °C. 1H NMR (CDCl3) δ: 8.50 (s, 1H), 7.98 (s, 2H), 7.63 (m, 3H), 7.33 (m, 4H,), 4.35 (q, 2H), 3.87 (d, 4H), 3.39 (m, 2H), 3.87 (m, 3H), 2.80 (s, 2H), 1.38–1.23 (m, 10H); 13C NMR (CDCl3) δ: 173.2 (−CO), 165.9 (−CO), 148.0, 145.6, 138.0, 129.0, 120.4, 113.4, 104.1, 61.1, 52.5, 49.6, 34.4, 28.4, 15.4, 14.3, 8.0. Chemical formula: C30H33FN6O3; MS m/z (ES, +ve, CH3OH) 545.2658 [M + H]+.
4.5.18. Propyl-1-cyclopropyl-7-(4-((1-(4-ethylphenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4r)
Dark brown solid, yield 0.11 g (81%), MP 88–90 °C. 1H NMR (CDCl3) δ: 8.49 (s, 1H), 8.03–8.00 (m, 2H), 7.64 (d, 2H, J = 8.8 Hz), 7.34 (d, 3H, J = 8.6 Hz), 4.27 (t, 2H, −CH2CH2CH3), 3.40 (m, 1H), 3.31 (m, 4H), 2.82–2.68 (m, 5H), 1.81 (q, 2H), 1.29–1.01 (m, 13H); 13C NMR (CDCl3) δ: 165.8 (−CO), 147.9, 144.8, 137.6, 129.1, 120.2, 104.3, 52.1, 48.9, 34.1, 28.4, 22.0, 15.4, 10.5, 8.0. Chemical formula: C31H35FN6O3; MS m/z (ES, +ve, CH3OH) 559.2832 [M + H]+.
4.5.19. Methyl-1-cyclopropyl-7-(4-((1-(3,5-dimethylphenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4s)
Dark brown solid, yield 0.11 g (83%), MP 108–110 °C. 1H NMR (CDCl3) δ: 8.52 (s, 1H), 7.99 (s, 2H), 7.25 (d, 2H, J = 8.68 Hz), 7.05 (s, 1H), 6.50 (s, 1H), 3.8 (t, 3H), 3.46 (m, 7H), 2.38 (s, 10H), 1.26 (m, 4H); 13C NMR (CDCl3) δ: 166.6 (−CO), 148.3, 139.5, 138.0, 130.4, 122.0, 118.2, 113.0, 104.7, 52.5, 50.7, 33.7, 21.2, 8.0. Chemical formula: C29H31FN6O3; MS m/z (ES, +ve, CH3OH) 531.2510 [M + H]+.
4.5.20. Ethyl-1-cyclopropyl-7-(4-((1-(3,5-dimethylphenyl)-1H-1,2,3-triazol-4-4-yl)methyl)piperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (4t)
Dark brown solid, yield 0.12 g (88%), MP 96–98 °C. 1H NMR (CDCl3) δ: 8.50 (s, 1H, Ar-H), 7.99 (s, 2H), 7.33–7.22 (m, 3H), 7.05 (s, 1H), 4.35 (q, 2H, −CH2CH3), 3.87 (d, J = 14.7 Hz, 4H), 3.40 (m, 1H), 3.29 (t, 4H), 2.79 (s, 2H), 2.37 (m, 6H), 1.37–1.22 (m, 7H); 13C NMR (CDCl3) δ: 166.0 (−CO), 148.3, 139.6, 138.0, 136.9, 130.3, 118.2, 113.0, 105.4, 61.5, 52.5, 49.7, 33.1, 29.3, 14.3, 8.0. Chemical formula: C30H33FN6O3; MS m/z (ES, +ve, CH3OH) 545.2655 [M + H]+.
4.5.21. Propyl-1-cyclopropyl-7-(4-((1-(3,5-dimethylphenyl)-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)-6-fluoro-oxo-1,4-dihydroquinoline-3-carboxylate (4u)
Dark brown solid, yield 0.11 g (82%), MP 90–92 °C. 1H NMR (CDCl3) δ: 8.49 (s, 1H, Ar-H), 8.02–7.96 (m, 2H), 7.34–7.24 (m, 3H), 7.06 (s, 1H), 4.27 (t, 2H, −CH2CH2CH3), 3.89 (d, J = 13.09 Hz, 2H), 3.38 (m, 1H), 3.31 (t, 4H), 2.81 (t, 4H), 2.39 (s, 6H), 1.79 (q, 2H), 1.32–1.00 (m, 7H); 13C NMR (CDCl3) δ: 165.8 (−CO), 148.3, 139.7, 138.0, 130.3, 118.2, 113.1, 104.9, 66.4, 52.5, 49.9, 34.3, 22.0, 21.2, 10.5, 8.1. Chemical formula: C31H35FN6O3; MS m/z (ES, +ve, CH3OH) 559.4488 [M + H]+.
4.6. X-ray Crystallographic Analysis
The structure of the compound (4b) was determined by X-ray analysis. A good-quality single crystal of (4b) was obtained by slow evaporation at room temperature. The selected crystal was mounted on a glass fiber and used for data collection. The data was gathered at 20° C by the X-ray scan technique using an Oxford Diffraction Xcalibur four-circle diffractometer with graphite monochromatized Mo Kα radiation (λ = 0.71073 Å). The crystal structure was solved by direct methods using the program SHELXS-86 and refined by the full-matrix least-squares technique on F2 by SHELXL-86. The thermal ellipsoid plot was prepared using ORTEP-3 as shown in Figure 2.
The crystallographic data, data collection, and structure refinement details are given in Table 2.
Table 2. Crystal Data, Data Collection, and Structure Refinement Details for (4b).
| CCDC | 1885454 |
| molecular formula | C30H31FN6O4 |
| molecular weight | 558.24 |
| temperature | 293 K |
| wavelength | 0.71073 |
| crystal system | triclinic |
| space group | p1̅ |
| unit cell dimensions | a = 14.5024(7) Å; α = 87.207(5)° |
| b = 14.5614(11)Å; β = 89.583(4)° | |
| c = 24.1403(12)Å; ϒ = 85.244(5)° | |
| volume | 5074.2(5) |
| Z | 14; Z′ = 7 |
| density | 0.33 g/cm3 |
| absorption coefficient | 0.024 mm–1 |
| F(000) | 522.3 |
| theta range for data collection | 6.08–58.7 |
| Rint | 0.0709 |
| index ranges | –19 ≤ h ≤ 19, |
| –18 ≤ k ≤ 19, | |
| –32 ≤ l ≤ 33 | |
| reflections collected | 72 381 |
| completeness to theta | 100% |
| absorption correction | multi-scan |
| refinement method | full-matrix |
| least-squares on F2 | |
| goodness-of-fit on F2 | 4.323 |
| final R indices | R1 = 0.9813 |
| [I > 2Σ(I)] | R2 = 0.9996 |
4.7. Biological Assays
4.7.1. Determination of Antibacterial Activity
The in vitro antibacterial studies employed the bacterial strains S. aureus (ATCC25923), S. epidermidis (ATCC35984), E. faecalis (clinical isolate), E. coli (ATCC25922), P. aeruginosa (ATCC27853), P. aeruginosa (clinical isolate), A. hydrophila (ATCC7966), S. typhi (clinical isolate), S. typhimurium (clinical isolate), S. paucimobilis (MTCC6362), and P. shigelloides (ATCC14029). The cultures used in the study were preserved at the Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. All cultures were obtained from American Type Culture Collection (ATCC) or Microbial Type Culture Collection (MTCC), or were clinical strains. The fresh microbial broth cultures were prepared in normal saline before the screening procedure. Ciprofloxacin was used as the reference drug for antibacterial evaluation. Minimum inhibitory concentration (MIC) was determined using the micro-dilution method using a series of dilutions (10-fold) of all compounds. The various concentrations of the compounds were serially diluted in a microtiter plate. Specifically, 10 mL of standardized inoculum (1–2 × 107cfu/mL) was added in each tube of the microtiter plate. The plates were incubated aerobically at 37 °C for 18–24 h. The lowest concentration of the compounds at which they exhibited no visible bacterial growth and no turbidity in the solution when compared with the wells containing the control was regarded as the MIC.
4.7.2. Determination of the Hemolytic Activity of the Compounds on hRBC
The hemolytic activity was investigated by following the process published by Nielson et al.31 Briefly, fresh human blood was collected from the hospital and washed thrice in sterile phosphate-buffered saline (PBS) solution. After each washing, the cells were centrifuged at 3000 rpm for 7 min at room temperature and the supernatant was discarded. The RBCs were resuspended in PBS and adjusted to a final concentration of 5 × 108 RBCs/mL. An aliquot (10 mL) of the cell suspension was added to 100 mL of buffer solution containing 100 mM of the test compounds in 1% v/v dimethyl sulfoxide (DMSO) in PBS. Further, controls were also taken as 1% v/v DMSO in PBS and sterile water. The cell suspensions were incubated at 37 °C for 1 h with constant shaking. After 1 h, the solution was centrifuged at 1300 rpm for 5 min at room temperature and absorbance was recorded at 540 nm. The UV–vis absorbance values of the test compounds were expressed as % of the absorbance of sterile water (equivalent to 100% hemolysis) to obtain the % hemolysis results.
Acknowledgments
A.A., A.M., and G.N. are grateful to Banaras Hindu University, Varanasi, India, for the financial assistance. P.S. is thankful to UGC, New Delhi, India, for providing the SRF. U.K.P. is thankful to CSIR, HRDG New Delhi, India, for providing the JRF, and A.S. is thankful to CSIR-UGC, New Delhi, India, for providing the JRF.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c05303.
Spectra of synthesized compounds; CCDC No. 1885454 for compound (4b) contain the supplementary crystallographic data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Harine A.-G.; Sumitra M.; Chitra V. Consummated review on prostatitis. Asian J. Pharm. Clin. Res. 2019, 12, 1–7. 10.22159/ajpcr.2019.v12i1.28326. [DOI] [Google Scholar]
- Mitscher L.-A. Bacterial topoisomerase inhibitors: quinolone and pyridone antibacterial agents. Chem. Rev. 2005, 105, 559–592. 10.1021/cr030101q. [DOI] [PubMed] [Google Scholar]
- Drlica K.; Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 1997, 61, 377–392. 10.1128/.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Z.; Yushe Y.; Ji R.; Zhang S. Synthesis and antibacterial activity of novel fluoroquinolones containing substituted piperidines. Bioorg. Med. Chem. Lett. 2007, 17, 4523–4526. 10.1016/j.bmcl.2007.05.093. [DOI] [PubMed] [Google Scholar]
- Plech T.; Barbara K.; Agata P.; Urszula K.; Anna M.; Aleksandra S.; Paweł S.; Łukasz Ś.; Barbara R.; Małgorzata P.-D. Determination of the primary molecular target of 1, 2, 4-triazole-ciprofloxacin hybrids. Molecules 2015, 20, 6254–6272. 10.3390/molecules20046254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana N.; Muhammad S. A.; Syeda B. S. R.; Urooj H. Synthesis, characterization and biological evaluations of ciprofloxacin carboxamide analogues. Bull. Korean Chem. Soc. 2011, 32, 483–488. 10.5012/bkcs.2011.32.2.483. [DOI] [Google Scholar]
- Schmidt M.; Harmuth S.; Barth E.-R.; Wurm E.; Fobbe R.; Sickmann A.; Krumm C.; Tiller J.-C. Conjugation of ciprofloxacin with poly(2-oxazoline)s and polyethylene glycol via end groups. Bioconjugate Chem. 2015, 26, 1950–1962. 10.1021/acs.bioconjchem.5b00393. [DOI] [PubMed] [Google Scholar]
- Pokrovskaya V.; Valery B.; Mariana H.; Sima Y.; Timor B. Design, synthesis, and evaluation of novel fluoroquinolone aminoglycoside hybrid antibiotics. J. Med. Chem. 2009, 52, 2243–2254. 10.1021/jm900028n. [DOI] [PubMed] [Google Scholar]
- Foroumadi A.; Adibi H.; Ardestani S.-K.; Shirooie S.; Bozorgomid A.; Jafari A. Synthesis and Leishmanicidal Activity of 1-[5-(5-nitrofuran-2-yl)-1, 3, 4-thiadiazole-2-yl]-4-benzoylepiperazines. Iran. J. Pharm. Res. 2017, 16, 904–909. [PMC free article] [PubMed] [Google Scholar]
- Fallah-Tafti A.; Tahmineh A.; Parastoo S.; Farideh S.; Abbas S.; Alireza F. Synthesis and anti-Helicobacter pylori activity of (4-nitro-1-imidazolylmethyl)-1, 2, 4-triazoles, 1, 3, 4-thiadiazoles, and 1, 3, 4-oxadiazoles. Turk. J. Chem. 2011, 35, 307–316. [Google Scholar]
- Sadat-Eebrahimi S.-E. M.; Tabatabaei Z. M.; Arani M.-A.; Jafari-Ashtiani S.; Hashemian M.; Foroumadi P.; Yahya-Meymandi A.; Moghimi S.; Moshafi M.-H.; Norouzi P.; Ardestani S.-K.; Foroumadi A. Novel 5-(nitrothiophene-2-yl)-1, 3, 4-Thiadiazole Derivatives: Synthesis and Antileishmanial Activity against promastigote stage of Leishmania major. Iran. J. Pharm. Res. 2019, 18, 1816–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroumadi A.; Emami S.; Hassanzadeh A.; Rajaee M.; Sokhanvar K.; Moshafi M.-H.; Shafiee A. Synthesis and antibacterial activity of N-(5-benzylthio-1, 3, 4-thiadiazol-2-yl) and N-(5-benzylsulfonyl-1, 3, 4-thiadiazol-2-yl) piperazinyl quinolone derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 4488–4492. 10.1016/j.bmcl.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Feng L.-S.; Liu M.-L.; Zhang S.; Chai Y.; Wang B.; Zhang Y.-B.; Lv K.; Guan Y.; Guo H.-Y.; Xiao C.-L. Synthesis and in vitro antimycobacterial activity of 8-OCH3 ciprofloxacin methylene and ethylene isatin derivatives. Eur. J. Med. Chem. 2011, 46, 341–348. 10.1016/j.ejmech.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Foroumadi A.; Emami S.; Mehni M.; Moshafi M.-H.; Shafiee A. Synthesis and antibacterial activity of N-[2-(5-bromothiophen-2-yl)-2-oxoethyl] and N-[(2-5-bromothiophen-2-yl)-2-oximinoethyl] derivatives of piperazinyl quinolones. Bioorg. Med. Chem. Lett. 2005, 15, 4536–4539. 10.1016/j.bmcl.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Rabbani M.-G.; Islam M.-R.; Ahmad M.; Hossion A.-M. Synthesis of some NH-derivatives of ciprofloxacin as antibacterial and antifungal agents. Bangladesh J. Pharmacol. 2011, 6, 6–13. 10.3329/bjp.v6i1.7720. [DOI] [Google Scholar]
- Tăbăcaru A.; Furdui B.; Ghinea I.-O.; Carac G.; Dinică R.-M. Recent advances in click chemistry reactions mediated by transition metal based systems. Inorg. Chim. Acta 2017, 455, 329–349. 10.1016/j.ica.2016.07.029. [DOI] [Google Scholar]
- Hameed P.-S.; Bharatham N.; Katagihallimath N.; Sharma S.; Nandishaiah R.; Shanbhag A.-P.; Thomas T.; Narjari R.; Sarma M.; Bhowmik P.; Amar P.; Ravishankar R.; Jayaraman R.; Muthan K.; Subbiah R.; Ramachandran V.; et al. Nitrothiophene carboxamides, a novel narrow spectrum antibacterial series: Mechanism of action and Efficacy. Sci. Rep. 2018, 8, 7263 10.1038/s41598-018-25407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal K.; Yadav P.; Kumar A.; Kumar A.; Paul A.-K. Design, synthesis, characterization, antimicrobial evaluation and molecular modeling studies of some dehydroacetic acid-chalcone-1, 2, 3-triazole hybrids. Bioorg. Chem. 2018, 77, 236–244. 10.1016/j.bioorg.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Agalave S.-G.; Maujan S.-R.; Pore V.-S. Click chemistry: 1, 2, 3-triazoles as pharmacophores. Chem. - Asian J. 2011, 6, 2696–2718. 10.1002/asia.201100432. [DOI] [PubMed] [Google Scholar]
- Karthick K.-K.; Seenivasan S.-P.; Kumar V.; Das T.-M. Synthesis of quinoline coupled [1, 2, 3]-triazoles as a promising class of anti-tuberculosis agents. Carbohydr. Res. 2011, 346, 2084–2090. 10.1016/j.carres.2011.06.028. [DOI] [PubMed] [Google Scholar]
- Al-Masoudi N. A.; Al-Soud Y.-A. New glycosyl-(carboxamide)-1,2,3-triazole-N-nucleosides: synthesis and antitumor activity. Nucleosides, Nucleotides Nucleic Acids 2002, 21, 361–375. 10.1081/NCN-120006830. [DOI] [PubMed] [Google Scholar]
- Da Silva F.-D.; De Souza M.-C.; Castro H.-C.; Silmara L.-D.; De Souza T.-M.; Rodrigues D.-Q.; Souza A.-M.; Abreu P.-A.; Passamani F.; Rodrigues C.-R.; et al. Synthesis, HIV-RT inhibitory activity and SAR of 1-benzyl-1H-1, 2, 3-triazole derivatives of carbohydrates. Eur. J. Med. Chem. 2009, 44, 373–383. 10.1016/j.ejmech.2008.02.047. [DOI] [PubMed] [Google Scholar]
- Costa A. V.; Oliveira M.-V.; Pinto R.-T.; Moreira L.-C.; Gomes E.-M.; Alves T.-D.; Pinheiro P.-F.; Queiroz V.-T.; Vieira L.-F.; Teixeira R.-R.; Júnior W.-C. Synthesis of novel glycerol-derived 1, 2, 3-triazoles and evaluation of their fungicide, phytotoxic and cytotoxic activities. Molecules 2017, 22, 1666 10.3390/molecules22101666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Dai Z.-C.; Chen Y.-F.; Cao L.-L.; Yan W.; Li S.-K.; Wang J. X.; Zhang Z.-G.; Ye Y.-H. Synthesis of 1, 2, 3-triazole hydrazide derivatives exhibiting anti-phytopathogenic activity. Eur. J. Med. Chem. 2017, 126, 171–182. 10.1016/j.ejmech.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Sayes C.-M.; Sayes C.-M.; Reed K.-L.; Warheit D.-B. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol. Sci. 2007, 97, 163–180. 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- Yuan Q.; Liu Z.; Xiong C.; Wu L.; Wang J.; Ruan J. A novel, broad-spectrum antitumor compound containing the 1-hydroxycyclohexa-2, 5-dien-4-one group: the disclosure of a new antitumor pharmacophore in protoapigenone 1. Bioorg. Med. Chem. Lett. 2011, 21, 3427–3430. 10.1016/j.bmcl.2011.03.108. [DOI] [PubMed] [Google Scholar]
- Miyazaki C. M. S.; Hirota B. C. K.; Delima C.-P.; Dos Santos M.-C.; Paula C.-D.; Chaves S.-C.; Pavan P. M. N.; Miguel M.-D.; Miguel O.-G. Coumarin isolation and comparative study of biological activities of Pterocaulon alopecuroides DC and Pterocaulon lorentzii Malme. Int. J. Phytomed. 2013, 5, 298. [Google Scholar]
- Kant R.; Singh V.; Nath G.; Awasthi S.-K.; Agarwal A. Design, synthesis and biological evaluation of ciprofloxacin tethered bis-1, 2, 3-triazole conjugates as potent antibacterial agents. Eur. J. Med. Chem. 2016, 124, 218–228. 10.1016/j.ejmech.2016.08.031. [DOI] [PubMed] [Google Scholar]
- Thakre Y. M.; Choudhary M.-D. Synthesis and spectral studies of noval benzyl derivative of ciprofloxacin and their Cu (II) and Co (II) complexes. J. Chem. Pharm. Res. 2012, 4, 1048–1051. [Google Scholar]
- McPherson J.-C. III; Runner R.; Buxton T.-B.; Hartmann J.-F.; Farcasiu D.; Bereczki I.; Roth E.; Tollas S.; Ostorházi E.; Rozgonyi F.; Herczegh P. Synthesis of osteotropic hydroxybisphosphonate derivatives of fluoroquinolone antibacterials. Eur. J. Med. Chem. 2012, 47, 615–618. 10.1016/j.ejmech.2011.10.049. [DOI] [PubMed] [Google Scholar]
- Nielsen S. F.; Larsen M.; Boesen T.; Schønning K.; Kromann H. Cationic chalcone antibiotics. Design, synthesis, and mechanism of action. J. Med. Chem. 2005, 48, 2667–2677. 10.1021/jm049424k. [DOI] [PubMed] [Google Scholar]
- Hu M.; Li J.; Yao S.-Q. In situ “click” assembly of small molecule matrix metalloprotease inhibitors containing zinc-chelating groups. Org. Lett. 2008, 10, 5529–5531. 10.1021/ol802286g. [DOI] [PubMed] [Google Scholar]
- Kolb H.-C.; Finn M.-G.; Sharpless K.-B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.