Abstract
Background and Objectives
The neurologic deficits of neonatal post-hemorrhagic hydrocephalus (PHH) have been linked to periventricular white matter injury. To improve understanding of PHH-related injury, diffusion basis spectrum imaging (DBSI) was applied in neonates, modeling axonal and myelin integrity, fiber density, and extrafiber pathologies. Objectives included characterizing DBSI measures in periventricular tracts, associating measures with ventricular size, and examining MRI findings in the context of postmortem white matter histology from similar cases.
Methods
A prospective cohort of infants born very preterm underwent term equivalent MRI, including infants with PHH, high-grade intraventricular hemorrhage without hydrocephalus (IVH), and controls (very preterm [VPT]). DBSI metrics extracted from the corpus callosum, corticospinal tracts, and optic radiations included fiber axial diffusivity, fiber radial diffusivity, fiber fractional anisotropy, fiber fraction (fiber density), restricted fractions (cellular infiltration), and nonrestricted fractions (vasogenic edema). Measures were compared across groups and correlated with ventricular size. Corpus callosum postmortem immunohistochemistry in infants with and without PHH assessed intra- and extrafiber pathologies.
Results
Ninety-five infants born very preterm were assessed (68 VPT, 15 IVH, 12 PHH). Infants with PHH had the most severe white matter abnormalities and there were no consistent differences in measures between IVH and VPT groups. Key tract-specific white matter injury patterns in PHH included reduced fiber fraction in the setting of axonal or myelin injury, increased cellular infiltration, vasogenic edema, and inflammation. Specifically, measures of axonal injury were highest in the corpus callosum; both axonal and myelin injury were observed in the corticospinal tracts; and axonal and myelin integrity were preserved in the setting of increased extrafiber cellular infiltration and edema in the optic radiations. Increasing ventricular size correlated with worse DBSI metrics across groups. On histology, infants with PHH had high cellularity, variable cytoplasmic vacuolation, and low synaptophysin marker intensity.
Discussion
PHH was associated with diffuse white matter injury, including tract-specific patterns of axonal and myelin injury, fiber loss, cellular infiltration, and inflammation. Larger ventricular size was associated with greater disruption. Postmortem immunohistochemistry confirmed MRI findings. These results demonstrate DBSI provides an innovative approach extending beyond conventional diffusion MRI for investigating neuropathologic effects of PHH on neonatal brain development.
Approximately 20% of infants who are born very preterm (VPT; ≤32 weeks' gestation) sustain spontaneous intraventricular hemorrhage (IVH).1-3 High-grade IVH, defined as blood filling more than 50% of the ventricular volume with or without intraparenchymal involvement,4 often results in disruption of CSF dynamics, CSF accumulation, and raised intracranial pressure, leading to post-hemorrhagic ventricular dilatation (PHVD). PHVD progresses in some cases to post-hemorrhagic hydrocephalus (PHH) that requires long-term hydrocephalus treatment.5 Critically, PHH is associated with cognitive deficits and cerebral palsy in affected infants and is a leading cause of epilepsy and hearing, visual, and speech impairments.1,6
Many of the neurologic deficits in infants with PHH are attributed to injury of critical periventricular white matter (PVWM) structures, including the corpus callosum (CC), corticospinal tracts (CST), and optic radiations (OPRA).7,8 Diffusion tensor imaging (DTI) has been widely used to demonstrate PVWM injury in PHH and across forms of neonatal hydrocephalus.8,9 DTI findings in PHH include reduced fractional anisotropy suggesting white matter disruption, reduced axial diffusivity reflecting axonal injury, and increased radial diffusivity reflecting myelin injury. These abnormal DTI measures in PHH correlate with poor neurodevelopmental outcomes.8 Nevertheless, DTI has inherent limitations in its ability to delineate complex white matter pathologies with adequate sensitivity and specificity.10,11 Critically, DTI is not capable of resolving diffusivities of crossing fibers,12 quantifying axonal density, or accurately assessing myelin integrity in the mixed presence of myelinated, dysmyelinated, and unmyelinated axons.12,13 Further, neuroinflammation, a hallmark of PHH pathophysiology, is associated with multiple extrafiber water compartments, varying cell densities, edema, and axonal loss. DTI's inability to accurately differentiate these pathologies limits its efficacy for investigating PHH-related injury.14
Diffusion basis spectrum imaging (DBSI), a multitensor diffusion MRI (dMRI) analysis approach, addresses these key DTI limitations. In DBSI, water displacements are represented as multiple diffusion tensors reflecting different water compartments. This allows for separation of crossing fibers and provides measures of crossing angles, directional diffusivity of individual crossing fibers, and axonal loss.15 Furthermore, in addition to anisotropic diffusion compartments, the DBSI model includes isotropic tensors reflecting water compartments accounting for inflammatory cells and edema.16 DBSI has been validated in preclinical models of white matter injury16,17 and utilized in adults to investigate clinical populations with varied white matter pathologies.15,18
Leveraging DBSI's unique strengths, we evaluated white matter integrity in a prospectively acquired, case–control cohort of infants born VPT with and without high-grade IVH or PHH. The aims of the study were to 1) compare DBSI measures in the CC, CST, and OPRA of infants with PHH to infants born VPT with and without IVH; 2) associate DBSI measures with ventricular size and determine whether the associations differed by group; and 3) compare postmortem histology between infants with PHH and those born VPT with and without IVH to confirm the MRI findings in a similar cohort. We hypothesized that, compared to controls, the PVWM in infants with PHH has increased cellularity, reduced fiber fraction (FF), and disrupted fiber integrity, and that larger ventricular size is associated with greater magnitude of PVWM disruption.
Methods
Participants
Infants born VPT who were screened for IVH via serial head ultrasounds obtained on day of life 3 and 7–10 as part of routine clinical care were prospectively recruited between 2007 and 2016 from a level IV neonatal intensive care unit. Based on cranial ultrasound findings and clinical course, infants were categorized into 3 groups: VPT, IVH, and PHH. The VPT group had no identifiable brain injury or low-grade IVH (Papile grade I or II19). The IVH group comprised infants born VPT who sustained high-grade IVH (Papile grade III or IV19) but did not develop hydrocephalus. As part of routine clinical care to monitor for development of PHH, cranial ultrasounds were at minimum repeated twice weekly for 21 days following diagnosis of IVH.
Hydrocephalus Clinical Research Network (HCRN) consensus criteria20 were used to identify the PHH group, which included infants with IVH who developed PHVD and required surgical treatment. Inclusion criteria were high-grade IVH; >72-hour life expectancy; frontal-occipital horn ratio (FOHR) ≥0.55; and 2 of the following: bulging fontanel (above level of surrounding bone), split sagittal sutures (≥2 mm in mid-parietal region), or ≥3 episodes of documented bradycardia over 24 hours. Treatment of infants with PHH involved 2 distinct phases: a temporizing phase to permit ventricular decompression prior to term equivalent postmenstrual age (PMA) and a permanent phase to enable long-term CSF diversion with a ventriculoperitoneal shunt or endoscopic third ventriculostomy (ETV). The decision to perform a permanent CSF diversionary procedure was made using HCRN consensus criteria20 (eAppendix 1, links.lww.com/WNL/B665). Choroid plexus cauterization (CPC) was not performed because the infants in this cohort who underwent ETV were treated prior to CPC's adaptation as common practice at our institution.
Standard Protocol Approvals, Registrations, and Patient Consents
Parental informed written consent was provided prior to each patient's enrollment in the study. All protocols and procedures were approved in advance by the Washington University Human Research Protection Office.
Perinatal Clinical Factors
To minimize confounding factors, all infants who had congenital infections (e.g., cytomegalovirus, toxoplasmosis) or chromosomal abnormalities were excluded. CSF microbiological analyses of infants with PHH throughout their hospital course did not suggest any of them had a CNS infection. Clinical status was assessed across patients using an established clinical risk index score21 that assigned a 1 or 0 for presence or absence of 10 discrete clinical factors (eAppendix 1, links.lww.com/WNL/B665). Scores from the 10 factors were added to obtain a composite risk index score.21
Image Acquisition
All infants were scanned without sedation following institutional guidelines22 at or near term equivalent (35–43 weeks PMA) on a 3T Siemens Trio MRI system using an infant-specific quadrature head coil (Advanced Imaging Research). Anatomical images including T2W sequences (repetition time [TR] 8,210 ms, echo time [TE] 161 ms, 1.0 × 1.0 × 1.0 mm3 spatial resolution) and dMRI data (TR 13,300 ms, TE 112 ms, 1,266 Hz/Px bandwidth, 128 mm field of view, 1.2 × 1.2 × 1.2 mm3 spatial resolution) with 25–48 b-directions and amplitudes ranging from 0 to 1,200 s/mm2 were obtained.
dMRI Processing
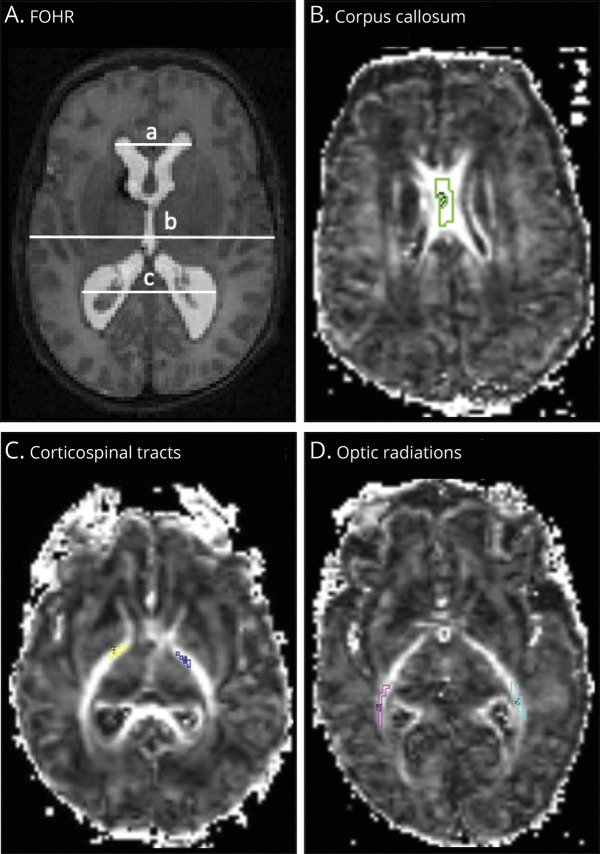
dMRI data were preprocessed using established methods23,24 (eAppendix 1, links.lww.com/WNL/B665). The CC, bilateral posterior limbs of the internal capsules, designated CST, and bilateral OPRA were selected a priori as regions of interest (ROIs), as they have been shown to demonstrate improvement in dMRI indices following hydrocephalus treatment and relate to PHH-related neurodevelopmental outcomes.8,25 Each ROI was segmented on 3 contiguous axial dMRI slices to minimize through-slice partial volume averaging using Analyze 10.0 (AnalyzeDirect, Inc.) (Figure 1). The preprocessed dMRI images were analyzed via the DBSI pipeline16 in MATLAB (MathWorks) (eAppendix 1, links.lww.com/WNL/B665).
Figure 1. Representative Diffusion MRI.
Axial T2-weighted MRI scan (A) and contiguous axial fractional anisotropy maps (B–D) of infants born very preterm. Ventricular size was determined by the frontal-occipital horn ratio (FOHR), which measures ventricular size as the sum of the distance between the widest lateral walls of the frontal and occipital horns, divided by twice the widest biparietal diameter at the level of the foramen of Monro. (A) FOHR = (a+c)/2b. On the fractional anisotropy maps (B-D), segments of the white matter bundles of interest within the corpus callosum, bilateral corticospinal tracts, and bilateral optic radiations are demarcated.
Division of isotropic components was established using an apparent diffusion coefficient (ADC) cutoff of ≤0.3 µm2/ms representing restricted diffusion, 0.3–2.5 µm2/ms representing hindered diffusion, and ≥2.5 µm2/ms representing free diffusion.15-18 Following DBSI analysis, ROI-specific metrics were obtained via voxel-based computations. DBSI metrics included fiber-specific fractional anisotropy (FFA; measuring directional dependence of water diffusivity), axial diffusivity (FAD; measuring rate of water diffusion parallel to axons), and radial diffusivity (FRD; measuring rate of water diffusion perpendicular to axons); FF (measuring fiber density); and markers of inflammation reflected as nonfiber-related restricted fraction (RF; measuring cellular infiltration) and nonrestricted fraction (NRF; measuring vasogenic edema). Conventional DTI measures, including fractional anisotropy, axial diffusivity, radial diffusivity, and mean diffusivity, were also obtained (eTable 1, links.lww.com/WNL/B665).
Ventricular Size Assessment
Ventricular size was measured by 2 independent raters on axial T2W and coronal cranial ultrasound images using the FOHR approach,26 given its wide use in clinical practice and availability of patient outcome data in neonates.27 Reproducibility of these measures had been validated previously.28 Ventricular size was assessed for all patients on their term equivalent scans. However, to track progression of ventriculomegaly in the PHH group, ventricular size was also measured using the FOHR method26 on serial coronal head ultrasound images obtained at initial routine cranial ultrasound, diagnosis of IVH, and diagnosis of PHH requiring surgical intervention. The largest ventricular size measured for each participant on either cranial ultrasound or term equivalent MRI was recorded. The concordance between coronal cranial ultrasound and axial MRI FOHR measures has been validated previously.29
Immunohistochemistry
Neurocytology was performed on formalin-fixed postmortem brain specimens from 12 infants who were not able to undergo MRI due to critical illness. This included 5 infants born VPT without injury, 3 with IVH, 2 with PHH (medical records supported a PHH diagnosis based on HCRN criteria20 prior to death), and 2 born full-term who died of non-neurologic causes. Paraffin-embedded 5-µm sections of all brains containing the CC were stained with hematoxylin & eosin to assess cellular structure, edema, and cellularity. In addition, immunostaining for synaptophysin and GFAP (1:300, catalog #7260, Abcam) was performed to better assess neuropil and astrocytes, respectively. Qualitative comparisons were performed by a neuropathologist.
Statistical Analysis
All statistical analyses were performed with SAS version 9.4. Numeric values were tested for normality using the Shapiro-Wilk test and box plots for visual examination, suggesting the use of parametric and nonparametric tests when applicable. Voxel-based DBSI measures in the right and left CST and OPRA were combined for primary analyses. Multiple comparisons with Kruskal-Wallis and Wilcoxon rank-sum tests with Bonferroni adjustments were performed between VPT, IVH, and PHH groups for all DBSI measures. To ensure appropriate comparability across groups, an ordinary linear regression was fit using a general linear model to compare group differences while adjusting for the following covariates: (1) gestational age at birth, (2) PMA at MRI scan, (3) number of diffusion-encoding directions, (4) clinical composite risk index, and (5) sex. The association of FOHR with DBSI variables was described by Pearson correlation coefficients with locally weighted smoothing curves. Bonferroni-corrected p values < 0.05 were considered significant.
Data Availability
Data supporting the findings of this study are available within the article. Anonymized data not provided in the article because of space limitations, but can be made available by request to qualified investigators through the study authors.
Results
Participants
A total of 130 infants (VPT = 91, IVH = 20, PHH = 19) with dMRI data were screened for inclusion. Reasons for exclusion across groups included positive maternal urine drug screen (VPT = 1, IVH = 1) and atypical pattern of brain injury/abnormal MRI finding (VPT = 2). DBSI modeling could not be accurately performed in 9 infants whose abnormal anatomy due to severe brain injury prevented accurate delineation of ROIs or FOHR definition. These included infants with severe cystic periventricular leukomalacia, hypoxic-ischemic brain injury, and congenital ventricular asymmetry causing distortion of the white matter or ventricular morphology. There were no infants included in the study with confounding brain abnormalities including perinatal stroke, hypoxic-ischemic encephalopathy, and congenital malformations. Scans of 7 infants could not be fit with DBSI due to having less than 25 diffusion-encoding directions or b-values. Among 95 infants (48 male, 47 female) included in the final analyses, there were 68 VPT, 15 IVH, and 12 PHH infants by group (eFigure 1).
The median gestational age at birth and PMA at MRI acquisition across groups were 27 ± 2 and 38 ± 2 weeks, respectively. There were no between-group differences in median birthweight (910 ± 390 g, p = 0.4). Median of the composite clinical risk scores were similar among infants with IVH (3; interquartile range [IQR] 2) and PHH (3; IQR 4), whereas that those for the infants born VPT were lower (1; IQR 3) (Table 1).
Table 1.
Baseline Clinical Characteristics of the Study Cohort
On serial cranial ultrasound, the median FOHR of the PHH group on their initial routine clinical scan, at IVH diagnosis, and at PHH diagnosis prompting intervention for hydrocephalus were 0.45 (IQR 0.05), 0.49 (IQR 0.16), and 0.67 (IQR 0.07), respectively (Figure 2). Prior to MRI, all 12 infants with PHH had undergone temporary ventricular access device placement, including 10 and 2 who had undergone ventriculoperitoneal shunt placement and ETV only to treat their hydrocephalus, respectively. On term equivalent MRI, the infants with PHH had larger ventricles than the VPT and IVH groups (median FOHR 0.52 ± 0.10 vs 0.41 ± 0.03 and 0.41 ± 0.04, respectively). On subgroup analysis, there were no differences between the shunt- and ETV-treated infants on clinical characteristics and DBSI measures. There were also no differences in dMRI measures in all tracts between infants with unilateral vs bilateral intraparenchymal hemorrhage (eTable 2, links.lww.com/WNL/B665). Among infants who experienced unilateral parenchymal hemorrhage, there were no differences in pairwise comparisons of dMRI measures for the CST and OPRA in hemispheres ipsilateral vs contralateral to the hemorrhage.
Figure 2. Ventricular Size Measures.
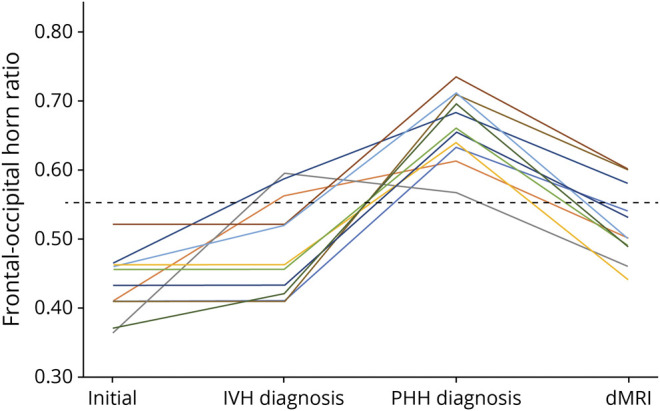
Frontal-occipital horn ratio (FOHR) of 12 infants born very preterm on their initial routine cranial ultrasound, at time of diagnosis with intraventricular hemorrhage (IVH), and at diagnosis of post-hemorrhagic hydrocephalus (PHH) prompting surgical treatment of their hydrocephalus. At term equivalent age, all infants underwent diffusion MRI (dMRI), demonstrating a reduction in their median FOHR following treatment.
Corpus Callosum
In the CC, the PHH group had the lowest FAD (p < 0.001) and there was no difference between IVH and VPT groups. There were no between-group differences for FRD. The PHH group also had the lowest FFA (p < 0.001), with no difference between IVH and VPT groups. FFs in the PHH group were 55% (p < 0.001) and 37% (p = 0.004) lower than the VPT and IVH groups, respectively. The IVH group had 28% lower FF than the VPT group (p = 0.021). Whereas the PHH group had higher RF than the VPT group (p = 0.002), there was no difference between PHH and IVH or IVH and VPT groups. There were no between-group differences in NRF (Figure 3).
Figure 3. Diffusion MRI Measures.
Diffusion basis spectrum imaging measures across 3 critical white matter tracts in infants born very preterm without brain injury (VPT), with high-grade intraventricular hemorrhage (IVH), and with post-hemorrhagic hydrocephalus (PHH) requiring treatment. While the tract-specific patterns were variable, in comparison to VPT and IVH groups, infants with PHH had the most severe white matter disruption, marked by the lowest fiber fraction (FF), fiber fractional anisotropy (FFA), and fiber axial diffusivity (FAD, μm2/s), as well as the highest measures of fiber radial diffusivity (FRD, μm2/s) and markers of inflammation including cellular infiltration and vasogenic edema reflected as restricted fraction (RF) and nonrestricted fractions (NRF), respectively. All comparisons are Bonferroni corrected with ***, **, and * representing p values <0.001, <0.01, and <0.05, respectively, between the PHH and corresponding group.
Corticospinal Tracts
In the CST, fiber-specific metrics demonstrated that infants with PHH had the lowest FAD (p < 0.001) and highest FRD (p < 0.001), with no difference between IVH and VPT groups across both measures. Although the PHH group had lower FFA than the VPT group (p = 0.010), there were no differences between PHH and IVH (p = 0.1) or IVH and VPT (p = 0.8) groups. FF analyses showed that the PHH group had 21% (p = 0.003) and 17% (p = 0.018) less FF than the VPT and IVH groups, respectively. There was no difference in FF between IVH and VPT groups. Isotropic spectrum analyses demonstrated that the PHH group had higher RF (p = 0.002) and NRF (0.012) than the VPT group; however, there was no difference between PHH and IVH groups or between IVH and VPT groups on both measures (Figure 3).
Optic Radiations
In the OPRA, fiber level analysis showed that the PHH group had the highest FAD (p < 0.001) with no difference between IVH and VPT groups. No between-group differences were found for FRD and FFA. FF analysis showed that the PHH group had 29% lower FF than the VPT group (p < 0.001) and 26% lower FF than the IVH group (p < 0.001), with no difference between the IVH and VPT groups. Isotropic analyses showed that the PHH group had the highest RF and NRF. However, there was no difference between IVH and VPT groups on RF measures, as well as no difference between the PHH and IVH groups (p = 0.1) or the VPT and IVH groups (p = 0.6) (Figure 3).
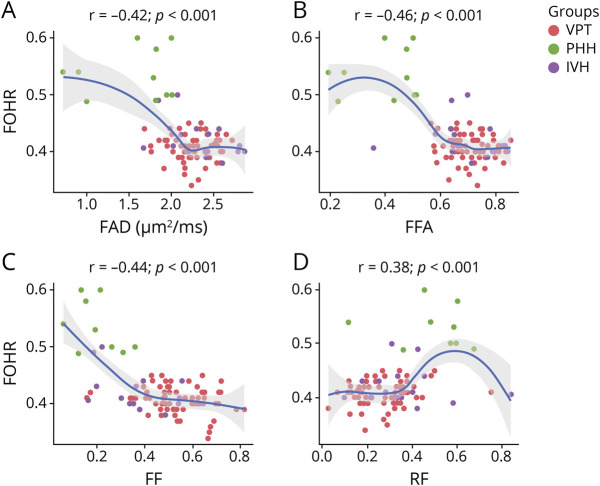
Ventricular Size–DBSI Correlations
Across all 3 white matter tracts, greater ventricular size on the term equivalent dMRI scans had fair to moderate correlations with greater magnitude of abnormality in DBSI measures. In the CC, FOHR related negatively with FAD (p < 0.001), FFA (p < 0.001), and FF (p < 0.001) and positively with RF (p < 0.001). There was no correlation between FOHR and FRD or NRF in the CC. Similar to the CC, FOHR related negatively to both FAD (p < 0.001) and FFA (p < 0.001) in the CST. Positive correlations were observed between FOHR and NRF (p = 0.011) and FRD (p < 0.001) in the CST. There were no correlations between FOHR and FF or RF in the CST. Finally, in the OPRA, FOHR related positively with FAD (p < 0.001) and NRF (p = 0.006) and negatively with FF (p = 0.031). No correlations were found between FOHR and FRD, FFA, or RF in the OPRA (Figure 4) (eTable 3, links.lww.com/WNL/B665). Correlations between dMRI measures and the largest FOHRs were stronger than with FOHRs on term equivalent MRI, with additional correlations identified between the largest FOHR measures and NRF in the CC, FF, and RF in the CST and FRD in the OPRA (eTable 3).
Figure 4. Ventricular Size–Diffusion MRI Correlations.
(A–D) Increase in ventricular size correlates with poorer diffusion MRI measures in the corpus callosum. As ventricular size increases, a decrease in white matter fiber axial diffusivity (FAD), fiber fractional anisotropy (FFA), and fiber density (fiber fraction, FF) and an increase in cellular infiltration (restricted fraction, RF) were observed. Ventricular size was obtained using the frontal occipital horn ratio approach (FOHR). IVH = intraventricular hemorrhage; PHH = post-hemorrhagic hydrocephalus; VPT = very preterm.
Postmortem Tissue Analyses
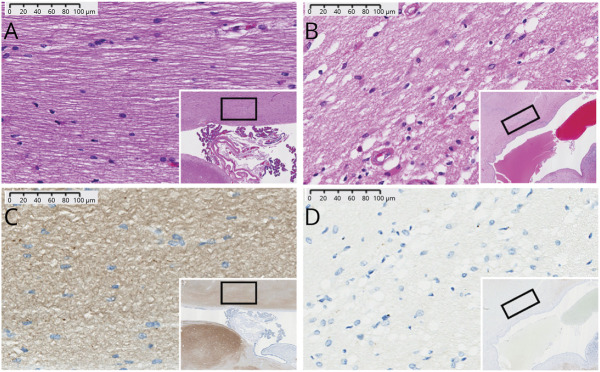
In comparison to the full-term controls, VPT, and IVH groups, infants with PHH had decreased synaptophysin staining, variable cytoplasmic vacuolation, and the highest white matter cellularity (Figure 5).
Figure 5. Postmortem Brain Immunohistochemistry.
Representative histologic staining of postmortem tissue from corpus callosum (CC) of preterm infants. Hematoxylin & eosin (A, B) and synaptophysin (C, D) of preterm infants without (A, C) or with (B, D) post-hemorrhagic hydrocephalus (PHH). Infants with PHH had relatively increased cellular infiltration (B, D) and vacuolation of their cytoplasm to suggest edema (B). Decreased axonal staining in PHH (D) suggests a relatively decreased fiber fraction in comparison to controls (C). The CC is not myelinated at term equivalent.
Discussion
In comparison to IVH and VPT groups, infants with PHH demonstrated the most severe white matter abnormalities across DBSI measures. There were no consistent differences in measures between the IVH and VPT groups, a finding that indicates the deviations observed in the PHH group were associated with hydrocephalus rather than the antecedent hemorrhage or preterm birth. Key tract-specific white matter injury patterns in PHH included reduced FF in the setting of axonal or myelin injury, increased cellular infiltration, vasogenic edema, and inflammation. Loss of white matter fibers occurred across all 3 tracts in PHH, and all tracts demonstrated evidence of high cellular infiltration. Measures of axonal injury were highest in the unmyelinated CC. In the myelinated CST, both axonal and myelin injury were observed. In the OPRA, axonal and myelin integrity were preserved in the setting of increased extrafiber cellular infiltration (RF) and edema (NRF), indicating there was increased extracellular space due to fiber loss and transependymal CSF migration28 (Figure 6). Of note, IVH independently had a detrimental effect on FF in the CC, likely due to injury to the germinal matrix or periventricular region resulting in degeneration of fibers, or potentially from the direct effects of blood breakdown products in the CSF. This reflects that more severe IVH is more likely to progress to PHH. Increasing ventricular size correlated with worse DBSI metrics, providing objective, quantifiable data that can be incorporated into long-standing discussions of the role of ventriculomegaly in PHH-related neurologic disability.30 Finally, DBSI findings of decreased FF, sparser architecture, increased cellular infiltration, edema, and inflammation in periventricular white matter tracts were consistent with immunohistochemistry in postmortem specimens.
Figure 6. Postulated Pathology Underlying White Matter Injury in Post-hemorrhagic Hydrocephalus.
Schematic rendering of pathology underlying the differences in white matter bundles detected with diffusion MRI across the 3 tracts. MRI voxels from healthy myelinated white matter (A) and the corpus callosum (B), corticospinal tracts (C), and optic radiations (D) of infants with PHH are drawn to the same scale to demonstrate relative fiber fraction, inflammatory cells, and estimated interaxonal spacing. The corpus callosum is not myelinated at this developmental stage. Relative to healthy white matter, there was reduced fiber fraction in all tracts, which was most prominent in the corpus callosum. The striated axons of the corpus callosum and corticospinal tracts indicate axonal injury. In the corticospinal tract, increased fiber radial diffusivity suggests myelin injury, which is represented as red sleeves around the axons. In the isotropic spectrum, there was increased cellularity across all 3 tracts.
Whereas this study is the first to utilize DBSI to investigate white matter microstructure in pediatric hydrocephalus, abnormalities in conventional DTI measures in these tracts have been associated with impaired neuromotor and cognitive development among infants with PHH in other studies.8,9 Lean et al.8 demonstrated that IVH/PHH-related aberrant dMRI measures were associated with poorer motor, cognitive, and language scores at 2 years compared to infants born VPT. In addition, infants with PHH specifically had white matter disruptions in the CST that related to poorer motor outcomes at age 2 years. These relationships likely result from disturbances in the complex sequence of events that underlie early white matter development due to IVH/PHH, engendering local and widespread effects through direct cellular mechanisms and disruption of subsequent cerebral development.31 This sequence of events begins in the fetal ventricular and subventricular zones (VZ/SVZ), where neural stem cells differentiate into oligodendrocytes, astrocytes, neurons, and mature ependymal cells.32 In humans, these processes of neurogliogenesis continue into the newborn period, and thus are at risk precisely when IVH and PHH most commonly occur.33 In the setting of IVH and PHH, differentiating neural stem cells are disrupted and often cleaved and released into CSF. Elevated levels of neurodevelopment-related proteins are differentially elevated in IVH/PHH compared with healthy infants.34 Evidence for widespread VZ/SVZ disruption has also been reported in human postmortem tissues of preterm infants with IVH/PHH.35 Collectively, the loss of progenitor cells and impaired neuroblast migration,36 further affected by pressure-related structural deformation, ischemia, inflammation, and other phenomena,37 have brain-wide effects, altering both cortical and subcortical regions and the development of white matter tracts.32 As demonstrated in this investigation, DBSI provides a unique mechanism to noninvasively study these critical alterations in cerebral development that extends beyond conventional DTI measures.
The variability in typical tract-specific patterns of white matter development must be taken into consideration when interpreting dMRI data. The data in this study were acquired at term equivalent PMA when some, but not all, white matter tracts are expected to be myelinated. Myelination begins and ends at variable ages among different white matter tracts. In general, myelination in the CST and OPRA start at 36 and 40 weeks’ gestation, respectively, whereas myelination in the CC does not begin until after 2 months postnatally.38 Therefore, our findings are interpreted in the context of whether the assessed white matter is largely myelinated or unmyelinated. For myelinated tracts, FRD is largely taken to reflect myelin health. In unmyelinated tracts, FRD is challenging to interpret, perhaps reflecting the extent of fiber packing. However, in our study, despite having between-group differences in FF, we did not detect differences in FRD of the unmyelinated CC. In contrast, the myelinated CST demonstrated higher FRD in the PHH than IVH and VPT groups, suggesting myelin disruption/injury in this tract. For the myelinated OPRA, FRD was not different between groups, suggesting that myelin was preserved. Note also that FF was lower and FAD higher in the PHH group in the OPRA. These values are consistent with findings of transependymal flow injury reported in infants with PHH28,39 that may be associated with the tissue edema, hypercellularity, and inflammation.
Although ventricular size may be a risk factor for neurodevelopmental impairment in PHH,40-42 little is known regarding the link between ventricular size and white matter integrity. This underscores the importance of utilizing DBSI to evaluate the effects of ventricular size on white matter injury, providing a potential noninvasive marker for therapeutic responsiveness following surgical treatment. Larger ventricles were associated with axonal injury across all tracts, correlated negatively with FF in the CC and OPRA, and were associated with myelin injury in the CST, consistent with the literature suggesting a positive correlation between larger ventricular size and greater disruptions in white matter.28 This study identified increases in ventricular size correlated with increased hypercellularity in the CC and edema in the CST and OPRA. These patterns may have been secondary to reactive cellular infiltration and inflammation, respectively, due to detrimental effects of the expanding ventricles on white matter.
It has been shown that in PHH, the mechanical pressure from the large ventricles distorts PVWM fiber organization, decreases cerebral perfusion, and initiates an inflammatory cascade, all of which culminates in injury and axonal loss.37,43 In this cohort, the larger the ventricular size, the worse the DBSI metrics of axonal loss, edema, and hypercellularity, which all relate to neuroinflammation. These findings have critical clinical implications. Although controversial and an area of active investigation, several studies have shown that progressive ventricular distension in PHH is associated with higher rates of neurologic disability,9,30 and infants who undergo early intervention for ventriculomegaly have better cognitive and neuromotor outcomes.40,44 Thus, the findings of this study and the existing literature underscore the value of utilizing DBSI to evaluate the role of PHH and ventricular size—a readily modifiable factor—on white matter integrity, brain development, and neurodevelopmental disability associated with PHH.
In this study, the DBSI findings of increased RF, representative of cellular infiltration, in the white matter of infants with PHH were consistent with histologic findings on postmortem brains from infants with PHH who were not able to undergo MRI (indicative of the challenges of imaging neonates who are critically ill). The increased number of cells in the white matter could be attributed, at least in part, to inflammatory processes such as gliosis and astrocytic proliferation, which have been shown to occur in the white matter of hydrocephalic human and animal tissues.35,45 Edema typically occurs in white matter in the setting of inflammation, which has been associated with the transependymal CSF flow observed in PHH.28,39 While variable amounts of edema were seen in the CC of infants with PHH, the DBSI correlate, NRF, did not differ between groups; this is presumably because NRF reflects interstitial or extracellular edema. Fiber loss, expressed as reduced FF, was demonstrated on immunohistochemistry as decreased synaptophysin, which suggested decreased neuropil and synaptic processes. White matter neuropil damage has been reported in both humans and experimental models of hydrocephalus and has been attributed to factors such as inflammation and fiber loss from mechanical compression of PVWM tracts by the expanding ventricles.46,47
The diffusion-weighted data used for DBSI analyses are similar to those required for conventional multi-shell DTI and other computational approaches. However, DBSI differs in its signal modeling approach. Specifically, DBSI models diffusion-weighted image signals as a linear combination of discrete anisotropic and a spectrum of isotropic diffusion tensors. Signals within each diffusion-weighted image voxel are modeled independently to derive a mean ADC for isotropic diffusion tensors and axial and radial diffusivity for anisotropic diffusion tensors. Based on the modeled diffusion tensors, DBSI estimates the extent of individual tensors within each image voxel, offering greater pathologic and structural detail. Of note, while acquisition durations are longer than clinical diffusion sequences (typically on the order of 3–4 minutes), the diffusion data used in these analyses can be collected in as little as 5 minutes, which is less time than commonly employed multi-shell research DTI sequences.
A notable limitation of this study is the relatively small sample size of the PHH and IVH groups, which may have limited our ability to observe subtle DBSI differences between groups. In addition, there is a potential for sex bias as all 12 patients with PHH were male; however, covarying for sex in our analyses did not affect the results. While chromosomal differences and dimorphic sex differences in neural protection pathways have been implicated, it remains unclear why IVH tends to affect more males than females,48 also likely reflected in the higher incidence of PHH in male than female preterm infants. Finally, to minimize the effect of age on brain development, all scans were acquired cross-sectionally at term-equivalent PMA, which precludes assessment of the relationships between dMRI measures and brain development over time.
DBSI analyses of dMRI data collected at term equivalent age in infants born VPT demonstrated that PHH, and to a lesser magnitude IVH, were associated with PVWM disruption. Whereas axonal fiber loss and hypercellularity were major underlying pathologies evident in all fiber tracts assessed, the OPRA and CST demonstrated evidence of edema, likely related to transependymal CSF migration. DBSI uniquely distinguished direct effects on white matter from changes in the extracellular milieu. Representative histologic analysis of postmortem tissue was consistent with the DBSI findings in the CC. Ventricular size correlated with DBSI measures in that larger ventricle size was associated with greater disruptions in the PVWM. These findings suggest that similar to other neurologic conditions in which DBSI has been successfully employed to study white matter injury, there is potential for DBSI to be established as a biomarker for clinical management and comparative effectiveness research in PHH. In addition, novel insights into the pathophysiology of PHH gained through DBSI may enable investigation of new treatment strategies to minimize the developmental disability of this devastating disease. Future studies are necessary to examine the responsiveness of DBSI measures to varying management approaches (e.g., timing of ventricular decompression) or emerging neurosurgical treatments such as endoscopic lavage or ETV with or without choroid plexus cauterization and to link DBSI measures to long-term developmental outcomes to elucidate the cellular mechanisms underlying impairment in high-risk infants born VPT.
Acknowledgment
The authors thank the IDDRC at Washington University for assistance with data collection; Tara Smyser for review of the manuscript; the children and their families for participating in the study; and the funding agencies for support.
Glossary
- ADC
apparent diffusion coefficient
- CC
corpus callosum
- CPC
choroid plexus cauterization
- CST
corticospinal tract
- DBSI
diffusion basis spectrum imaging
- dMRI
diffusion MRI
- DTI
diffusion tensor imaging
- ETV
endoscopic third ventriculostomy
- FAD
fiber-specific axial diffusivity
- FF
fiber fraction
- FFA
fiber-specific fractional anisotropy
- FOHR
frontal-occipital horn ratio
- FRD
fiber-specific radial diffusivity
- GA
gestational age
- HCRN
Hydrocephalus Clinical Research Network
- IQR
interquartile range
- IVH
intraventricular hemorrhage
- NRF
nonrestricted fraction
- OPRA
optic radiation
- PHH
post-hemorrhagic hydrocephalus
- PMA
postmenstrual age
- PHVD
post-hemorrhagic ventricular dilatation
- PMA
postmenstrual age
- PVWM
periventricular white matter
- RF
restricted fraction
- ROI
region of interest
- TE
echo time
- TR
repetition time
- VPT
very preterm
- VZ/SVZ
ventricular and subventricular zones
Appendix. Authors
Study Funding
Vanier Canada Graduate Scholarship (grant number 396212); NIH (grant numbers K02 NS089852, K23 NS075151, K23 MH105179, TL1 TR002344, P30 NS098577, R01 MH113570, R01 HD061619, R01 HD057098, R01 NS047592, and U01 EY025500); Eunice Kennedy Shriver National Institute of Child Health & Human Development (grant number P50 HD103525); Child Neurology Foundation; Cerebral Palsy International Research Foundation; The Dana Foundation; March of Dimes Prematurity Research Center at Washington University; The Doris Duke Charitable Foundation; and Orion Pharma Research Foundation.
Disclosure
D.D. Limbrick and J.P. McAllister have received research funds or research equipment for unrelated projects from Medtronic, Inc. and Microbot Medical, Inc. The authors have no personal, financial, or institutional interest in any of the materials or devices described in this article. No other authors have any relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inder TE, Perlman JM, Volpe JJ. Preterm intraventricular hemorrhage/posthemorrhagic hydrocephalus. In: Volpe JJ, ed. Volpe's Neurology of the Newborn, 6 ed. Elsevier Inc.; 2018:637-698.e621. [Google Scholar]
- 3.Yeo KT, Thomas R, Chow SS, et al. . Improving incidence trends of severe intraventricular haemorrhages in preterm infants <32 weeks gestation: a cohort study. Arch Dis Child Fetal Neonatal Ed. 2020;105(2):145-150. [DOI] [PubMed] [Google Scholar]
- 4.Ancel PY, Livinec F, Larroque B, et al. . Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: the EPIPAGE cohort study. Pediatrics. 2006;117(3):828-835. [DOI] [PubMed] [Google Scholar]
- 5.Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC. Hydrocephalus in children. Lancet. 2016;387(10020):788-799. [DOI] [PubMed] [Google Scholar]
- 6.Limbrick DD Jr, Mathur A, Johnston JM, et al. . Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: a 10-year single-institution study. J Neurosurg Pediatr. 2010;6(3):224-230. [DOI] [PubMed] [Google Scholar]
- 7.Robinson S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr. 2012;9(3):242-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lean RE, Han RH, Smyser TA, et al. . Altered neonatal white and gray matter microstructure is associated with neurodevelopmental impairments in very preterm infants with high-grade brain injury. Pediatr Res. 2019;86(3):365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangano FT, Altaye M, McKinstry RC, et al. . Diffusion tensor imaging study of pediatric patients with congenital hydrocephalus: 1-year postsurgical outcomes. J Neurosurg Pediatr. 2016;18(3):306-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 2014;103:411-426. [DOI] [PubMed] [Google Scholar]
- 11.Jones DK, Alexander DC, Bowtell R, et al. . Microstructural imaging of the human brain with a “super-scanner”: 10 key advantages of ultra-strong gradients for diffusion MRI. Neuroimage. 2018;182:8-38. [DOI] [PubMed] [Google Scholar]
- 12.Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239-254. [DOI] [PubMed] [Google Scholar]
- 13.Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed. 2002;15(7-8):435-455. [DOI] [PubMed] [Google Scholar]
- 14.Habiyaremye G, Morales DM, Morgan CD, et al. . Chemokine and cytokine levels in the lumbar cerebrospinal fluid of preterm infants with post-hemorrhagic hydrocephalus. Fluids Barriers CNS. 2017;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Sun P, Wang Q, et al. . Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain. 2015;138(pt 5):1223-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Cusick MF, Wang Y, et al. . Diffusion basis spectrum imaging detects and distinguishes coexisting subclinical inflammation, demyelination and axonal injury in experimental autoimmune encephalomyelitis mice. NMR Biomed. 2014;27(7):843-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin TH, Sun P, Hallman M, et al. . Noninvasive quantification of axonal loss in the presence of tissue swelling in traumatic spinal cord injury mice. J Neurotrauma. 2019;36(15):2308-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun P, George A, Perantie DC, et al. . Diffusion basis spectrum imaging provides insights into MS pathology. Neurol Neuroimmunol Neuroinflamm. 2020;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. [DOI] [PubMed] [Google Scholar]
- 20.Wellons JC III, Shannon CN, Holubkov R, et al. . Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. J Neurosurg Pediatr. 2017;20(1):19-29. [DOI] [PubMed] [Google Scholar]
- 21.Rogers CE, Smyser T, Smyser CD, Shimony J, Inder TE, Neil JJ. Regional white matter development in very preterm infants: perinatal predictors and early developmental outcomes. Pediatr Res. 2016;79(1-1):87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008;38(3):260-264. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke CD, Bretthorst GL, Inder TE, Neil JJ. Modeling water diffusion anisotropy within fixed newborn primate brain using Bayesian probability theory. Magn Reson Med. 2006;55(1):187-197. [DOI] [PubMed] [Google Scholar]
- 24.Woolrich MW, Jbabdi S, Patenaude B, et al. . Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 suppl):S173-S186. [DOI] [PubMed] [Google Scholar]
- 25.Thompson DK, Inder TE, Faggian N, et al. . Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. NeuroImage. 2012;59(4):3571-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29(5):245-249. [DOI] [PubMed] [Google Scholar]
- 27.El-Dib M, Limbrick DD Jr, Inder T, et al. . Management of post-hemorrhagic ventricular dilatation in the infant born preterm. J Pediatr 2020;226:16-27.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isaacs AM, Smyser CD, Lean RE, et al. . MR diffusion changes in the perimeter of the lateral ventricles demonstrate periventricular injury in post-hemorrhagic hydrocephalus of prematurity. Neuroimage Clin. 2019;24:102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radhakrishnan R, Brown BP, Kralik SF, et al. . Frontal occipital and frontal temporal horn ratios: comparison and validation of head ultrasound-derived indexes with MRI and ventricular volumes in infantile ventriculomegaly. Am J Roentgenol. 2019;213(4):925-931. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasakumar P, Limbrick D, Munro R, et al. . Posthemorrhagic ventricular dilatation-impact on early neurodevelopmental outcome. Am J Perinatol. 2013;30(3):207-214. [DOI] [PubMed] [Google Scholar]
- 31.Woodward LJ, Clark CA, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS One. 2012;7(12):e51879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malik S, Vinukonda G, Vose LR, et al. . Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 2013;33(2):411-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morales DM, Townsend RR, Malone JP, et al. . Alterations in protein regulators of neurodevelopment in the cerebrospinal fluid of infants with posthemorrhagic hydrocephalus of prematurity. Mol Cell Proteomics. 2012;11(6):M111.011973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAllister JP, Guerra MM, Ruiz LC, et al. . Ventricular zone disruption in human neonates with intraventricular hemorrhage. J Neuropathol Exp Neurol. 2017;76(5):358-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castaneyra-Ruiz L, Morales DM, McAllister JP, et al. . Blood exposure causes ventricular zone disruption and glial activation in vitro. J Neuropathol Exp Neurol. 2018;77(9):803-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Bigio MR. Neuropathology and structural changes in hydrocephalus. Dev Disabil Res Rev. 2010;16(1):16-22. [DOI] [PubMed] [Google Scholar]
- 38.Kinney HC, Karthigasan J, Borenshteyn NI, Flax JD, Kirschner DA. Myelination in the developing human brain: biochemical correlates. Neurochem Res. 1994;19(8):983-996. [DOI] [PubMed] [Google Scholar]
- 39.Pena A, Bolton MD, Whitehouse H, Pickard JD. Effects of brain ventricular shape on periventricular biomechanics: a finite-element analysis. Neurosurgery. 1999;45(1):107-116. [DOI] [PubMed] [Google Scholar]
- 40.Cizmeci MN, Groenendaal F, Liem KD, et al. . Randomized controlled early versus late ventricular intervention study in posthemorrhagic ventricular dilatation: outcome at 2 years. J Pediatr. 2020;226:28-35.e3. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy CR, Ayers S, Campbell MJ, Elbourne D, Hope P, Johnson A. Randomized, controlled trial of acetazolamide and furosemide in posthemorrhagic ventricular dilation in infancy: follow-up at 1 year. Pediatrics. 2001;108(3):597-607. [DOI] [PubMed] [Google Scholar]
- 42.Whitelaw A, Evans D, Carter M, et al. . Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics. 2007;119(5):e1071-1078. [DOI] [PubMed] [Google Scholar]
- 43.Cherian S, Whitelaw A, Thoresen M, Love S. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 2004;14(3):305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leijser LM, Miller SP, van Wezel-Meijler G, et al. . Posthemorrhagic ventricular dilatation in preterm infants: when best to intervene? Neurology. 2018;90(8):e698-e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAllister JP II, Chovan P. Neonatal hydrocephalus: mechanisms and consequences. Neurosurg Clin N Am. 1998;9(1):73-93. [PubMed] [Google Scholar]
- 46.Aoyama Y, Kinoshita Y, Yokota A, Hamada T. Neuronal damage in hydrocephalus and its restoration by shunt insertion in experimental hydrocephalus: a study involving the neurofilament-immunostaining method. J Neurosurg. 2006;104(5 suppl):332-339. [DOI] [PubMed] [Google Scholar]
- 47.Jinkins JR. The callosal impingement syndrome of hydrocephalus. In: du Boulay G, Molyneux A, Moseley I, eds. Proceedings of the XIV Symposium Neuroradiologicum. Springer Berlin Heidelberg; 1991:448-450. [Google Scholar]
- 48.Cuestas E, Bas J, Pautasso J. Sex differences in intraventricular hemorrhage rates among very low birth weight newborns. Gend Med. 2009;6(2):376-382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available within the article. Anonymized data not provided in the article because of space limitations, but can be made available by request to qualified investigators through the study authors.