Abstract
Alcohol is one of the most widely used recreational substances worldwide, with drinking frequently initiated during adolescence. The developmental state of the adolescent brain makes it vulnerable to initiating alcohol use, often in high doses, and particularly susceptible to alcohol-induced brain changes. Microglia, the brain parenchymal macrophages, have been implicated in mediating some of these effects, though the role that these cells play in the progression from alcohol drinking to dependence remains unclear. Microglia are uniquely positioned to sense and respond to central nervous system insult, and are now understood to exhibit innate immune memory, or “priming,” altering their future functional responses based on prior exposures. In alcohol use disorders (AUDs), the role of microglia is debated. Whereas microglial activation can be pathogenic, contributing to neuroinflammation, tissue damage, and behavioral changes, or protective, it can also engage protective functions, providing support and mediating the resolution of damage. Understanding the role of microglia in adolescent AUDs is complicated by the fact that microglia are thought to be involved in developmental processes such as synaptic refinement and myelination, which underlie the functional maturation of multiple brain systems in adolescence. Thus, the role microglia play in the impact of alcohol use in adolescence is likely multifaceted. Long-term sequelae may be due to a failure to recover from EtOH-induced tissue damage, altered neurodevelopmental trajectories, and/or persistent changes to microglial responsivity and function. Here, we review critically the literature surrounding the effects of alcohol on microglia in models of adolescent alcohol misuse. We attempt to disentangle what is known about microglia from other neuroimmune effectors, to which we apply recent discoveries on the role of microglia in development and plasticity. Considered altogether, these studies challenge assumptions that proinflammatory microglia drive addiction. Alcohol priming microglia and there by perturbing their homeostatic roles in neurodevelopment, especially during critical periods of plasticity such as adolescence, may have more serious implications for the neuropathogenesis of AUDs in adolescents.
Keywords: adolescent, alcohol, EtOH, microglia, neuroimmune
INTRODUCTION
Alcohol remains the most commonly used drug among adolescents in the United States where drinking rates in the Americas are the second highest in the world at 38.2% (Johnston et al., 2020; SAMHSA, 2018; World Health Organization, 2018). Adolescents (10 to 19 years, or 25 for some measures) drink less frequently than adults but consume similar quantities when they do drink (Deas et al., 2000; SAMHSA, 2018). Often, alcohol is consumed in “binges” defined as 4+ (females) or 5+ (males) standard drinks consumed within 2 h and achieving a blood alcohol level (BAL) topping 0.08% (>80 mg/dl; NIAAA, 2004). In 2018, 4.7% of adolescents aged 12–17 and 34.9% aged 18–25 were current binge drinkers in the United States (SAMHSA, 2019), while rates in Europe were even higher (Chung et al., 2018). Strikingly, approximately 10% of adolescents report extreme binge drinking of 10+ drinks per occasion with 5% reporting over 15 drinks in the past 2 weeks alone (Johnston et al., 2020; Patrick et al., 2013). Males remain overrepresented in the extreme binge drinking category (Patrick & Terry-McElrath, 2019), though the gender gap is narrowing in adolescents (Johnston et al., 2020).
Unfortunately, these surveys likely underestimate binge drinking in adolescents due to the definition of a binge, which is based on BALs in adults (NIAAA, 2004), and that teens underestimate the amount of alcohol in a standard drink (White et al., 2005). The smaller size and body weight of adolescents and therefore higher BAL per standard drink force reconsideration of these definitions for adolescents as they may hit the >0.08 mg% threshold with as little as 1–2 drinks within a 2-h period (Chung et al., 2018; Donovan, 2009). Such hazardous drinking patterns increase the risk for developing an alcohol use disorder (AUD), for which 1.6% of young adolescents aged 12–17 meet criteria, while older adolescents are similar to adult rates (~14%; Clark et al., 2002, 2016; Grant et al., 2015; SAMHSA, 2019). Adolescent drinking also predicts later alcohol misuse and/or dependence in adulthood (DeWit et al., 2000; Grant & Dawson, 1997).
Disentangling the factors that underlie this vulnerability toward excessive alcohol drinking in adulthood from those that are a consequence of adolescent drinking remains a challenge (Crum & Hulvershorn, 2020). Microglia have emerged as an interesting target due to not only their role in brain development and homeostasis but also their dual roles in response to insult both proinflammatory and reparative/resolving of inflammation. This critical review gathers and integrates findings on the effect of adolescent alcohol exposure on microglia and considers them in relation to processes that occur during adolescence and those that underlie vulnerability for addiction. Microglial activation states are outlined, and the concept of priming, a type of innate immune memory, is introduced. Finally, we highlight evidence of microglial priming following adolescent alcohol exposure and identify several gaps in our understanding of the role of microglia and their activation in adolescent susceptibility to the causes and consequences of AUD pathogenesis.
ADOLESCENT VULNERABILITY FOR EXCESS ALCOHOL CONSUMPTION
Adolescence, typically 10 to 19 years of age, is a critical time for brain and behavioral maturation with neurodevelopmental changes occurring up to age 25 (Brenhouse & Schwarz, 2016; Lees et al., 2020; Spear, 2000). In rodents, adolescence ranges between postnatal days (PND) 28 and 42, with 43 to 55 considered late adolescence, especially in males (Brenhouse & Andersen, 2011; Spear & Brake, 1983). During this time, white matter volume increases remarkably from ongoing myelination, while gray matter volume decreases due to synaptic pruning and refinement (Giedd, 2004; Lees et al., 2020; Pfefferbaum et al., 2018). The relative developmental immaturity of the adolescent brain and behavior coupled with specific effects of alcohol in adolescents favor the development of alcohol misuse (Crews & Boettiger, 2009; Crews et al., 2016; Spear, 2000, 2018). Exploration, risk-taking, and sensation seeking are behaviors associated with adolescence and correspond to relatively immature regions and circuits in the adolescent brain (Spear, 2018). The brain's “go system,” including structures such as the mesolimbic nucleus accumbens (NAc) that are involved in reward and motivation, are functionally more mature at this time, whereas the “stop system” inclusive of the prefrontal cortices, involved in judgment and reasoning, develops later into adolescence (Gulley & Juraska, 2013; Markham et al., 2007; Sowell et al., 1999; Spear, 2018). Thus, age-typical impulsive behavior during adolescence likely results from hyperresponsivity to reward-based stimuli (Spear & Varlinskaya, 2005), while the underdeveloped top-down control systems hinder the ability of the frontal cortex to assess risk or exhibit impulse control (Gulley & Juraska, 2013; Townshend & Duka, 2005). Impulsive behavior, a hallmark of adolescence, often manifests as risk-taking or sensation seeking (Romer & Moreno, 2017), which are thought to play major roles in the initiation of alcohol use (Bardo et al., 2013; Crum & Hulvershorn, 2020; Hamilton et al., 2019). In preclinical models, measures of impulsivity are predictive of both increased sensitivity to drug reward (Yates et al., 2012) and dose-related aspects of subjective reinforcement (Marusich & Bardo, 2009).
In addition to an immature “stop system,” adolescents are more prone to excess consumption. Adolescents demonstrate enhanced sensitivity to the positive reinforcing action of alcohol with reduced sensitivity to sedation and motor impairment that normally help to regulate consumption (Gee et al., 2018; Li et al., 2003; Little et al., 1996; Spear, 2011, 2018). For example, rats consume more saccharin-sweetened ethanol (EtOH) solution during adolescence compared with adulthood, while consumption induced a greater tachycardic effect, which is associated with enhanced EtOH reward (Doremus et al., 2005; Ristuccia & Spear, 2008; Spear & Varlinskaya, 2005). Enhanced sensitivity to reward may be due to changes in the striatal dopaminergic system, which demonstrates high plasticity during adolescence, with evidence of increased activity and responsivity of mesolimbic and mesocortical projections (Gulley & Juraska, 2013; Lees et al., 2020; Silverman et al., 2015; Sowell et al., 1999; Spear, 2018; Wahlstrom et al., 2010). Indeed, bidirectional, dopaminergic projections between prefrontal cortex (PFC) and hippocampus - regions important for modulating reward processing, attention, working memory, and episodic memory - are still maturing (Gee et al., 2018). Developmental differences in alcohol's effects on GABAergic neurotransmission may underlie the reduced sensitivity of adolescents to regulators of consumption, including the sedative/motor impairing effects of alcohol, compared with adults (Fleming et al., 2007; Li et al., 2003; Little et al., 1996). Several animal model studies, however, suggest faster EtOH metabolism in adolescents, which would also influence the sedative and motor impairing effects of EtOH (Walker & Ehlers, 2009; Watson et al., 2020). The literature varies widely, discussed below, and it remains a significant gap as to whether EtOH pharmacokinetic differences occur in human adolescents versus adults as well.
ADOLESCENT VULNERABILITY TO NEGATIVE CONSEQUENCES OF EXCESS CONSUMPTION
The adolescent's enhanced vulnerability to alcohol-induced neurotoxicity, particularly in regions still maturing, profoundly impacts development (Crews et al., 2000, 2016; Spear, 2018). These damaging effects occur within 2 domains: (1) alterations in brain structure/function through damage and/or derangement of physiology and (2) disruptions of the brain's developmental trajectory. Negative impact from either domain may persist into adulthood and drive and/or be exacerbated by continued alcohol misuse.
Imaging studies have revealed that immature regions such as the PFC are targets of alcohol toxicity (Gulley & Juraska, 2013; Jacobus & Tapert, 2013; Luciana et al., 2013; Pfefferbaum et al., 2018; Silveri et al., 2016; Squeglia et al., 2014). Seminal cross-sectional studies have shown that heavy drinking adolescents have smaller frontal and temporal gray and white matter volumes than low to no drinking teens (Alba-Ferrara et al., 2016; De Bellis et al., 2000, 2005; Fein et al., 2013; Jones et al., 2018; Medina et al., 2008; Silveri et al., 2016). However, the cross-sectional designs of this early work are marred by the limitations that predisposition (e.g., family history of AUD or psychiatric disorders) or existing structural deficits cannot be ruled out. Thus, results from preclinical models coupled with recent human, longitudinal data have been essential to our understanding of how the course of adolescent brain development is impacted by heavy drinking (Luciana et al., 2013; Pfefferbaum et al., 2018; Squeglia et al., 2014, 2015). Specifically, age-typical decreases in cortical gray matter volume were exacerbated, while expected increases in white matter volume were attenuated in heavy drinking adolescents (Luciana et al., 2013; Pfefferbaum et al., 2018; Squeglia et al., 2014, 2015). In addition, subcortical regions involved in the development of AUDs such as the hippocampus and amygdala also show volume reductions in adolescents with an AUD (De Bellis et al., 2000, 2005; Nagel et al., 2005). Preclinical studies are consistent with many of these deficits described in human adolescents as rodent models show that alcohol exposure leads to death of neurons and glia across cortical regions and hippocampus (Crews et al., 2000, 2016, 2019; Hu et al., 2020; Marshall et al., 2020; Morris et al., 2010a; Risher et al., 2015; Swartzwelder et al., 2019).
The increased risk for development of alcohol dependence or AUDs in adulthood after adolescent alcohol consumption is associated with long-term damage or impact on the developmental trajectory of brain structures essential for normal, adaptive behaviors (DeWit et al., 2000; Grant & Dawson, 1997). However, it is difficult to extricate factors that underlie the propensity to drink to excess from the consequences of excessive alcohol drinking. Although alcohol-induced damage and neuroimmune effects in the adolescent are more widespread, we focus on 3 brain areas or circuits involved in addiction processes that are also vulnerable to alcohol damage and neuroimmune effects: the PFC, mesolimbic reward system, and hippocampus. Evidence from human imaging, behavior, and preclinical models converges for these 3 areas, though we acknowledge that this overlooks exciting new discoveries in regions underappreciated for their role in addiction, including especially, the cerebellum (Sullivan et al., 2020). We briefly review each area as a foundation for understanding the effects of innate immune system dysregulation by alcohol on these key regions that contribute to the pathogenesis of AUDs in adolescence.
Dysregulation of prefrontal cortical behavioral control systems is involved as both cause and consequence of adolescent alcohol drinking. Impaired behavioral control, or impulsivity, contributes to exploratory alcohol use during adolescence (Fernie et al., 2013); however, it also results from excess alcohol use and therefore plays a role in the persistence of this maladaptive behavior (Ivanov et al., 2021; Lopez-Caneda et al., 2014). Impulsivity or risky choice behavior has been observed in human heavy drinkers and preclinical models of AUD (Sanchez-Roige, Baro, et al., 2014; Sanchez-Roige, Pena-Oliver, et al., 2014). For example, emerging adult binge drinkers (aged 18 to 30) displayed greater motor impulsivity in a visual search matching task than nonbinge drinkers (Townshend & Duka, 2005). In adolescent males with higher self- and parental-reported impulsivity ratings, increased impulsive behavior was found to be a consequence of heavy drinking, an effect not seen in individuals with low a priori impulsive behavior ratings (White et al., 2011). Additionally, baseline measures of impulsivity in adolescents during the monetary incentive delay task were predictive of subsequent drinking rates, while heavy drinking predicted increases in impulsivity upon follow-up (Ivanov et al., 2021). In this cohort, blunted activity of the medial orbitofrontal cortex (OFC) in response to reward at baseline also predicted greater alcohol use (Ivanov et al., 2021). These behavioral studies parallel preclinical evidence that alcohol damages behavioral control systems, including the OFC required for response inhibition (Spinella, 2004), or alters neurotransmitter systems such as serotonin, dopamine, and acetylcholine, which are associated with heightened impulsivity or increased risky choice (Boutros et al., 2015; Leamy et al., 2016). For example, rats with the highest voluntary drinking in adolescence displayed greater risk preference in a probability discounting task (Nasrallah et al., 2009), while chronic adolescent alcohol exposure results in more prevalent risky choice in probability discounting tasks measured in adulthood, whether voluntary (Nasrallah et al., 2009; Schindler et al., 2014), or forced exposure to alcohol was used (Boutros et al., 2015). Rodent studies also implicate the OFC in both impulsive action and choice, where decreased OFC DAT function was associated with enhanced impulsive action (Yates et al., 2016), while enhanced OFC SERT function was associated with greater impulsive choice (Darna et al., 2015).
While the development of “top-down” control of the mesolimbic reward system emerges during adolescence, alcohol exposure during this time can derange the reward system, leading to an increased incidence of addictive-like behaviors in adulthood. For example, in rats, 2-bottle choice alcohol consumption during adolescence increased appetitive and consummatory operant behaviors toward alcohol as adults (Amodeo et al., 2017). Interestingly, compared with controls, rats exposed to intermittent EtOH in adolescence displayed reduced frontolimbic functional connectivity following acute EtOH administration as adults, and showed decreased baseline resting-state connectivity between subregions of the PFC and between the PFC and striatum (Broadwater et al., 2018). Mechanistically, evidence from both animal and human studies supports that adolescent alcohol exposure persistently changes brain dopaminergic systems that may lead to enhanced vulnerability to developing an AUD in adulthood. Whole-cell patch clamp recordings in PFC slices from adult rats exposed to intermittent EtOH vapor during adolescence display a loss of D1 receptor modulation over intrinsic excitability and synaptic transmission between the mPFC and NAc core, and the mPFC and basolateral amygdala (Trantham-Davidson et al., 2017).
In rats, basal dopamine levels in the NAc septi reach adult levels by PND 35 and peak in late adolescence (PND 45) before returning to adult levels around PND 60 with levels of the dopamine metabolite, DOPAC, consistent from PND 35 forward (Philpot et al., 2009). However, there is a developmental transition in the rat's ability to adapt to repeated EtOH exposure between PND 35 and 45: Peak dopamine release upon acute EtOH challenge is blunted in younger (PND 25 and 35) versus older (PND 45 and 60) rats with a history of prior EtOH exposure. These data may reflect an enhanced susceptibility for addiction vulnerability during early adolescence (Philpot et al., 2009). Rats exposed intermittently to alcohol during adolescence consumed more alcohol in a 2-bottle choice test, which was driven potentially by enhanced motivation for EtOH in a self-administration paradigm, effects that were correlated with reduced c-Fos reactivity in the NAc (Alaux-Cantin et al., 2013). Alcohol-induced disruptions in dopamine signaling come about through a variety of mechanisms (Nixon & McClain, 2010). In humans, young adults who were either high reward-seekers, behaviorally disinhibited, or low responders to the effects of alcohol showed increased dopamine release in the striatum post oral alcohol administration versus placebo (Setiawan et al., 2014). Even moderate amounts of alcohol in adolescence enhanced a reward predictive cue through increasing phasic dopamine release during Pavlovian conditioned approach in adulthood (Spoelder et al., 2015). Polymorphisms in the D1 receptor have been associated with changes in signaling (BOLD response) in the medial OFC after an alcohol reward anticipation cue (Baker et al., 2019). Other neurotransmitter systems affected directly by alcohol also influence dopaminergic signaling. For example, increased GABAergic transmission in the ventral tegmental area leads to decreased basal dopamine, an effect thought to underlie maladaptive decision making as a consequence of adolescent alcohol drinking (Schindler et al., 2016).
Finally, hippocampal systems, which are well known for their canonical role in learning and memory, also contribute to behavioral control (Abela et al., 2013; Nixon et al., 2011; Rubin et al., 2017). Although this region has the least direct role in controlling consumption, it is a primary target of alcohol toxicity during adolescence, impacting PFC and mesolimbic functions that rely on interaction with the hippocampus (White & Swartzwelder, 2004). Adolescent binge drinkers consistently demonstrate learning and memory deficits across a variety of hippocampal-dependent tasks (Jacobus & Tapert, 2013; Mahmood et al., 2010; Schweinsburg et al., 2010; Vinader-Caerols, Duque, et al., 2017; Vinader-Caerols, Talk, et al., 2017). These findings in humans are paralleled by several studies in preclinical models where alcohol exposure impairs learning and memory through accelerated forgetting, decreased retention, or other impairments on hippocampal-dependent tasks such as the Morris water maze or radial arm maze (Ji et al., 2018; Schulteis et al., 2008; Seemiller & Gould, 2020; Sircar et al., 2009; Sircar & Sircar, 2005; Swartzwelder et al., 2014). Indeed, impairments on the Morris water maze persisted longer in adolescent than in adult rats (Sircar & Sircar, 2005), which may reflect alcohol's more damaging effects on limbic circuitry in adolescents (Broadwater et al., 2014; Crews et al., 2000; Markwiese et al., 1998; Morris et al., 2010a). Following binge treatment, adolescent rats also displayed greater sensitivity to EtOH's memory impairing effects in a radial arm maze (Risher et al., 2013, 2015; White et al., 2000). For hippocampus, the role of adult neurogenesis must also be considered. Neurogenesis occurs throughout the lifetime of the organism, and alcohol impacts multiple aspects of this process, particularly in adolescence (Broadwater et al., 2014; Crews et al., 2006; Macht et al., 2019; McClain et al., 2014; Morris et al., 2010a; Sakharkar et al., 2016; Vetreno & Crews, 2015; reviewed in Wooden et al., 2021). Thus, both cell death and cell birth mechanisms contribute to the enhanced sensitivity of the adolescent brain to alcohol's damaging effects.
MECHANISMS OF ADOLESCENT VULNERABILITY TO ALCOHOL: MICROGLIA
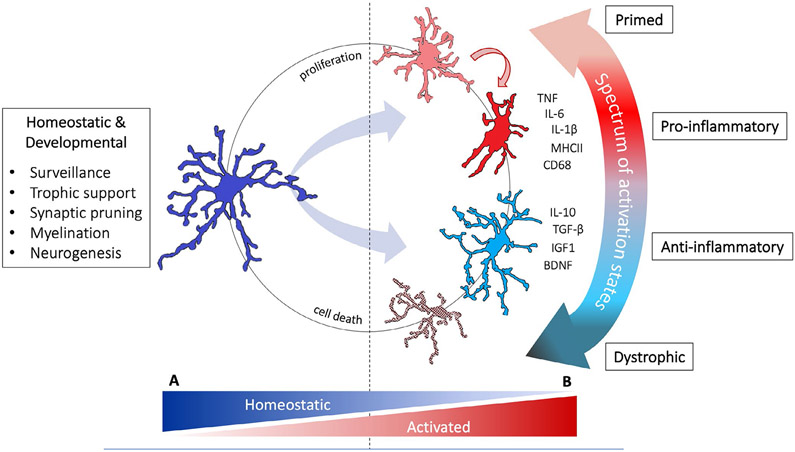
Activation of the innate immune system is implicated in the causes and consequences of adult AUD (Crews et al., 2017; Erickson et al., 2019; Melbourne et al., 2019) but much less is known for adolescence. Growing evidence supports that innate immune system activation impacts adolescent vulnerability to developing AUDs at multiple levels, from influencing cell signaling to roles in synaptic and structural plasticity both during normal development and in response to insult. This review focuses on microglia, the hallmark cell of the brain's innate immune system. An overview of microglial functions in development and homeostasis is described in Figure 1A.
FIGURE 1.
Microglial phenotypes and functions. (A) Healthy adolescent brain. Microglia maintain CNS homeostasis and contribute to ongoing neurodevelopmental fine-tuning by surveying their environment through frequent contacts with synapses and other glia (Nimmerjahn et al., 2005; Tremblay et al., 2010; Wu et al., 2015). This active surveillance accompanied by the microglial sensome, the wide array of receptors these cells use to sample their environment, makes these cells exceptionally sensitive to changes in the CNS milieu (Hickman et al., 2013). Microglia have an even wider range of functions than previously recognized (Hammond et al., 2018); not only do they act as primary responders to CNS injury, microglia are intricately involved in nervous system development and homeostasis (Prinz et al., 2019). (B) Adolescent brain exposed to alcohol. Because microglia can respond to activation by releasing high levels of proinflammatory mediators and reactive oxygen species leading to tissue damage and cell death, they are often the first culprit implicated in neuronal damage. When accompanied by secretion of high levels of proinflammatory cytokines and reactive oxygen species, microglia are frequently termed “classical” or “M1” activated (Kreutzberg, 1996; Ransohoff, 2016). However, activation of these cells can also elicit protective functions that limit tissue damage and facilitate repair. The “alternative” or “M2” state is associated with increased secretion of anti-inflammatory cytokines and growth factors, and may explain why ablating microglia is associated with poorer recovery or outcomes in some conditions (e.g., Lalancette-Hebert et al., 2007; Szalay et al., 2016). With advances in single-cell sequencing, mass cytometry, and microglial manipulation techniques, current designations of microglial phenotypes are an oversimplification (Ransohoff, 2016; Salter & Stevens, 2017). Microglial responses in each disease state have distinct profiles that are not easily classified into classical/M1 or alternative/M2 phenotypes. Instead, specific signaling systems and functions are engaged in a context-dependent manner, with beneficial versus detrimental effects similarly contextually determined. For example, microglial phagocytosis may be detrimental in one context, such as in the over-pruning of synapses in Alzheimer's disease, but beneficial in another, such as in the necessary clearance of cellular debris after injury (Salter & Stevens, 2017). Thus, following EtOH exposure microglia respond in a context-dependent manner and may adopt a spectrum of phenotypes. These phenotypes reflect the net response to insult and namely degeneration and recovery events, but each of these phenotypes impacts ongoing developmental processes and synaptic plasticity
MICROGLIAL RESPONSE TO INSULT: PHENOTYPES AND PRIMING
Microglia were originally investigated in the context of illness and injury, where tissue examined histologically was noted to contain “reactive” microglia with enlarged cell bodies and retracted processes (Prinz et al., 2019). This marked response is now understood to reflect an extreme phenotype along a continuum of states of cellular activation (see Figure 1B for an overview of microglial activation; Dubbelaar et al., 2018; Kreutzberg, 1996). The microglial response to a particular stimulus appears to be highly dependent on the history of that cell's previous systemic or microenvironmental exposures, further complicating the picture. When sensitized, macrophages and microglia are referred to as primed, essentially a form of innate immune memory (Haley et al., 2018; Locati et al., 2013; Neher & Cunningham, 2019; Perry & Holmes, 2014; Ransohoff & Perry, 2009; Wendeln et al., 2018). Primed microglia, according to models of aging, stress, and neurodegeneration, do not exhibit a fully proinflammatory phenotype; they show an exaggerated or heightened response to an inflammatory stimulus compared with that observed in stimulus-naïve microglia (Fonken et al., 2016; Neher & Cunningham, 2019; Perry & Holmes, 2014; Rosczyk et al., 2008). Identifying primed microglia remains challenging as unique markers have not been identified, though changes in morphology and up-regulation of cell surface antigens occurs (Perry & Holmes, 2014).
Numerous endogenous and exogenous stimuli have been shown to prime microglia. Of particular relevance to AUDs are pathogen-associated molecular patterns and endogenous damage-associated molecular patterns (DAMPs), or sterile insult signals that are released from neurons during cellular stress or neurotoxicity and are detected by microglial pattern recognition receptors (Haley et al., 2018). Molecules expressed in the degenerating or injured brain, such as colony-stimulating factor (Chapoval et al., 1998) and C-C motif chemokine ligand 2/monocyte chemotactic protein 1 (CCL2/MCP-1; Rankine et al., 2006), also prime microglia. The DAMP, high mobility group box protein 1 (HMGB1), has been implicated as a key factor mediating priming in stress, aging, and most recently in substance misuse including adolescent susceptibility to developing an AUD (Coleman et al., 2018; Crews et al., 2013; Kang et al., 2014; Wang et al., 2015; Weber et al., 2015; Yang & Tracey, 2010). Though the exact mechanisms of microglial priming remain poorly understood, evidence suggests that shifts in the microglial epigenetic landscape underlie these changes to microglial responsivity (Martins-Ferreira et al., 2020).
The priming phenomenon is pertinent to adolescence and AUD development as microglia are long-lived compared with their peripheral counterparts and therefore have a greater propensity to “remember” prior insults (Eyo & Wu, 2019; Neher & Cunningham, 2019). Activation of previously primed microglia results in a stronger inflammatory response and may exacerbate damage or disease progression. Evidence of priming in alcohol studies has been observed in repeated binge models in adult rats where a sensitized microglial response was observed (Marshall et al., 2016) or in a fetal alcohol spectrum disorder model (Chastain et al., 2019). The shift in cellular phenotype that occurs in primed microglia leads to a decreased expression of homeostatic genes. Studies in primed peripheral macrophages demonstrate an accumulation of permissive histone modifications at proinflammatory gene promoters and repressive modifications at genes that underlie homeostatic and reparative functions (Kang et al., 2017). Therefore, priming in adolescence may result in microglia that are redirected from their homeostatic, protective, and developmental functions. This persistent shift in microglial set point following perturbation provides a framework for understanding how cumulative insults, particularly those experienced during developmentally sensitive windows, could have a lasting impact on brain function later in life.
THE ROLE OF MICROGLIA IN NEURODEVELOPMENT
Across different stages of life, from embryogenesis to adolescence and adulthood, microglia display distinct morphologies, transcrip tomic and proteomic signatures (Masuda et al., 2019; Prinz et al., 2019). Though research demonstrates a critical role of these cells in sculpting early brain development, far less is known about their function in adolescence. As discussed above, the brain continues to mature throughout adolescence, with widespread fine-tuning of synaptic connections, and ongoing myelination and neurogenesis, all processes in which microglia are thought to play an important role. Factors that affect the immune system during adolescence not only impact existing circuity but also perturb developmental trajectories, affecting development of brain circuits and behaviors, such as decision making, impulsivity and cognition, that occur during this time.
In the early developing brain, microglia induce normal programmed cell death, phagocytose dying neurons, and contribute to synapse formation and elimination, shaping the architecture and wiring of the developing brain (Frost & Schafer, 2016; Konishi et al., 2019; Prinz et al., 2019; Schafer et al., 2012; Schafer & Stevens, 2015; Squarzoni et al., 2014). Microglial trophic support is critical for neuronal survival and the development and homeostasis of oligodendrocytes and their precursors (Ueno et al., 2013; Wlodarczyk et al., 2017). During early postnatal development, microglia are perhaps best appreciated for their role in synaptic pruning (reviewed in Salter & Stevens, 2017), mediated through an experience- and complement-dependent mechanism (Schafer et al., 2012; Stevens et al., 2007; Wu et al., 2015). Conversely, a complement-independent mechanism has been demonstrated involving the ligand fractalkine and the microglial fractalkine receptor CX3CR1 (Gunner et al., 2019). Knockout of CX3CR1 decreases synaptic pruning and hippocampal–frontal functional connectivity postnatally (Paolicelli et al., 2011; Zhan et al., 2014). When microglial TREM2 receptors were knocked out, mice showed similar deficits in synaptic pruning and connectivity, with alterations predominantly in hippocampus (Filipello et al., 2018). Thus, microglia have critical roles in normal brain developmental processes.
Less is known about the contribution of microglia to adolescent neurodevelopment. As adolescence is a critical period for synaptic pruning, refinement, and myelination—events involving microglia directly—perturbing microglia likely impacts these events (see Figure 1; Schafer & Stevens, 2015). Microglial engulfment of dendritic spines and glutamatergic presynaptic terminals in the PFC is elevated following the peak in spine density in adolescent rats (Mallya et al., 2019), and depletion of microglia during early adolescence (P30) altered glutamatergic spine plasticity via microglia-derived BDNF, impairing learning (Parkhurst et al., 2013). Microglia, through complement-dependent mechanisms, mediated down-regulation of dopamine receptors in the NAc during adolescence, which affected social play behavior in male, but not female, rats (Kopec et al., 2018). This exciting finding aligns with the hypothesis that factors that impact the neuroimmune system could have widespread effects on systems that are being developmentally fine-tuned during this critical period.
In adult animals, microglia are necessary for myelin homeostasis, promoting survival of oligodendrocyte precursor cells and phagocytosing myelin (Hagemeyer et al., 2017; Li & Barres, 2018). However, the precise nature of microglia–oligodendrocyte interactions during the significant myelination that takes place throughout adolescence remain to be elucidated (Giedd et al., 1999). Another developmental process modulated by microglia is adult neurogenesis, levels of which begin to asymptote in adolescence (Ekdahl et al., 2009; He & Crews, 2007; Wooden et al., 2021). In adult animals, microglial effects on adult neurogenesis depend upon their phenotypic state (Ekdahl et al., 2009): Inflammatory microglia reduce adult neurogenesis (Ekdahl et al., 2003; Monje et al., 2003), while noninflammatory microglia are beneficial (Butovsky et al., 2006; Kreisel et al., 2019; Sierra et al., 2010, 2014). Finally, recent data indicate that microglia have important homeostatic function in regulating neuronal activity, acting as a negative feedback mechanism by detecting and suppressing excess neuronal activation in the healthy brain and following insult (Badimon et al., 2020; Cserep et al., 2020; Pfeiffer & Attwell, 2020).
THE EFFECT OF ALCOHOL ON MICROGLIA IN ADOLESCENT MODELS: A REVIEW OF THE EVIDENCE
It is generally well accepted that alcohol affects the neuroimmune system; however, the precise nature and mechanisms of the effect are debated (summarized in Coleman & Crews, 2018; Erickson et al., 2019; Guerri & Pascual, 2019a,b; Melbourne et al., 2019). The most convincing evidence that the neuroimmune response is relevant for AUDs is that bacterial or viral mimetics escalate alcohol consumption in rodents (Blednov et al., 2011), an effect reversed by the microglial “inhibitor” minocycline (Agrawal et al., 2011), in addition to the observation of an activated microglial morphology in the brains of humans with AUDs (He & Crews, 2008). Activation of the neuroimmune system, including microglia, is apparent in adult animals: Subtle to moderate changes in morphology, up-regulation of cell surface proteins, and changes in cytokine and/or growth factor expression have been observed (reviewed in Melbourne et al., 2019). The nature of immune and microglial alterations, however, varies according to the species, duration and pattern of alcohol administration, and outcome measure (e.g., Bell-Temin et al., 2013; Doremus-Fitzwater et al., 2014, 2015; Gano et al., 2016; Guergues et al., 2020; Marshall et al., 2013; Qin et al., 2008; Rath et al., 2020; Zahr et al., 2010). Furthermore, many of these studies have failed to consider microglial phenotype (Melbourne et al., 2019). Based on the presence of cytokines and DAMPs, several groups hypothesize that proinflammatory microglial activation produces brain volume loss (Crews et al., 2016; Vetreno et al., 2018). However, these immune signals are not specific to microglia nor are they a robust indicator of an inflammatory state. For example, some extent of increase in NF-κB activation or even TNF-α levels are necessary aspects of the immune response, whether that response is pro- or anti-inflammatory (Nennig & Schank, 2017; Scherbel et al., 1999).
Even less is known about the effects of adolescent drinking on the neuroimmune system, specifically microglia. Excessive alcohol use in adolescence is associated with even greater structural changes and damage to the brain than seen in adults (Crews et al., 2000). The loss of brain mass may be due to cell death, altered myelination or synaptic connectivity, and/or impaired neurogenesis (Crews & Nixon, 2009), all of which could involve microglial activation (Crews et al., 2019). Given the sensitivity of the adolescent brain to the damaging effects of alcohol and the potential life-long impact from microglia altering developmental trajectories, this gap is striking. Therefore, to better elucidate converging results, we summarize existing literature for effects of adolescent alcohol exposure on (a) microglia-specific markers and cell morphology, (b) induction of immune molecules related to microglia, and (c) evidence that microglia are primed by alcohol.
Markers and morphology
In adolescent models of alcohol misuse, morphological changes and increased cell surface marker expression signifying microglial activation are consistent, but the activation phenotype is not clear. As detailed in Table 1, increased binding of translocator protein 18 kD ligands using PET imaging was observed after EtOH exposure in adolescent nonhuman primates and rats, indicating microglial activation but not phenotype specificity (Marshall et al., 2013; Saba et al., 2018; Tournier et al., 2020). Increased immunoreactivity or immunopositive (+) cell numbers using Iba1, the calcium binding protein specific to microglia, are observed in regions such as the cortex, hippocampus, amygdala, and substantia nigra in multiple adolescent rat alcohol models (Table 1). In mice, however, Iba1 immunoreactivity is mixed, while microglial numbers are decreased (Table 1). These discrepancies may be due to the time point examined. As one rat model shows, microglial number initially declines with 2 days of binge exposure (Marshall et al., 2020) followed by proliferation of microglia in withdrawal, a hallmark of activation (McClain et al., 2011a; Nixon et al., 2008). Further, immunoreactivity could reflect increased protein expression independent of changes in microglial number, which highlights the importance of quantifying cell number. Another hallmark of activation, elevated complement receptor 3 immunoreactivity (CD11b + IR), is observed in frontal cortex and limbic regions after chronic intermittent, 4-day binge, but not acute, EtOH exposure in adolescent rats, persisting into adulthood with chronic intermittent exposure (Table 1).
TABLE 1.
Microglia-specific and other neuroimmune measures in models of adolescent alcohol exposure
| Study | Model | Exposure | Intox/WD/Abs | Mean BAL | Marker | Result |
|---|---|---|---|---|---|---|
| Microglial markers—histology | ||||||
| Ward et al. (2009a) | Rat | 2 to 3 g/kg/day × 21 day | 5 days of abs | – | MHCII | ⇧ + IR |
| McClain et al. (2011b) | Rat | 4-day binge | WD 7, 30 days of abs | ~305 to 410 mg/dl | Iba1, BrdU, ED1, MHC11 | ⇧Iba1 + BrdU-Prolif ⇔MHCII ⇔ED1 |
| Walter et al. (2017) | Rat | AIE 5 g/kg × 30 days | Intox | ~130 to 170 mg/dl | CD11b | ⇧⇔+IR |
| Vetreno et al. (2017) | Rat | AIE 5 g/kg × 30 days | 25 days of abs | ~180 mg/dl | Iba1, CD11 | ⇧ Iba1# ⇧ CD11+IR ⇔ED1 IR |
| Ji et al. (2018) | Rat | 4-day binge; 9 to 11 g/kg/day | Intox | – | Iba1 | ⇧ # |
| Sanchez-Alavez et al. (2019) | Rat | CIE vapor × 36 days | Intox/24-h WD/28 days of abs | ~170 to 185 mg/dl | Iba1 | ⇧+IR Intox ⇔↓ + IR |
| Li et al. (2019) | Rat | 10% EtOH; 9.7 g/kg/days × 21 days | Intox | ~34 to 105 mg/dl | Iba1, ED1 | ⇧ Iba1# ED1+ (no stats) |
| Grifasi et al. (2019) | Mouse | DID | Intox | ~70 to 100 mg/dl | Iba1 | ⇧⇔ + IR ↓Iba1# |
| Hu et al. (2020) | Mouse | 3.5 g/kg – acute AIE 3.5 g/kg/d × 14 days | Intox 21 days of abs | ~120 to 150 mg/dl | Iba1, BrdU | ⇧Iba1+BrdU-Prolif ↓Iba1# |
| Marshall and McClain et al. (2020) | Rat | 2-day binge (9 to 11 g/kg/day) | Intox | ~300 mg/dl | Iba1 | ↓ Iba1# ⇔MHCII ⇔ED1 |
| Peng and Nixon (2021) | Rat | 4-day binge | Intox, WD | ~404 mg/dl | CD11b, MHCII, CD32, CD86, CD206, cytokines, growth factors | ⇧⇔ ⇧⇔ ↓ |
| Microglial markers-PET | ||||||
| Saba et al. (2018) | Baboon | 0.6 g/kg + 0.1 g/kg/h—acute | Intox 7–12 months of abs | ~100 mg/dl | 18F-DPA-714 (TSPO ligand) | ⇧ ⇧ |
| Tournier et al. (2020) | Rat | AIE 3 g/kg × 14 days | Intox | 210–270 mg/dl | 18F-DPA-714 (TSPO ligand) | ⇧ |
| Other neuroimmune measures | ||||||
| McClain et al. (2011b) | Rat | 4-day binge | WD 7, 30 days of abs | ~305 to 410 mg/dl | TNF-α | ⇔ |
| Vetreno et al. (2013) | Rat | AIE 5 g/kg × 30 days | Intox 24 days of abs | ~135 to 165 mg/dl | HMGB1, TLR4 | ⇧ + IR ⇧ + IR |
| Montesinos et al. (2015) | Mouse | AIE 3 g/kg × 14 days | Intox 22 days of abs | ~180 mg/dl | NF-κB, HMGB1, cytokines, chemokines, other | ⇧ |
| Kane et al. (2014) | Mouse | 6g/kg/days × 10 days | Intox | ~220 mg/dl | Cytokines, CCL2 | ⇔ |
| Montesinos et al. (2016) | Mouse | AIE 3 g/kg × 14 days | Intox 22 days of abs |
~180 mg/dl | NF-κB, MHCII, CD11b | ⇧ ⇧ |
| Pascual et al. (2016) | Mouse | AIE 3 g/kg × 14 days | Intox | ~165 to 170 mg/dl | Cytokines, chemokines, TLR4, NF-κB | ⇧⇔ |
| Doremus-Fitzwater et al. (2015) | Rat | 4 g/kg—acute | Intox | ~150 to 370 mg/dl | mRNA: cytokines | ⇧⇔ ↓ |
| Vetreno et al. (2017) | Rat | AIE 5 g/kg × 30 days | 25 days of abs | ~180 mg/dl | NF-κB | ⇧+IR |
| Vetreno et al. (2018) | Rat | AIE 5 g/kg × 30 days | 25 days of abs | ~170 to 180 mg/dl | NF-κB mRNA: TLRs, HMGB1, cytokines |
⇧+IR ⇧⇔ |
| Vetreno and Crews (2018) | Rat | AIE 5 g/kg × 30 days | 25 days of abs | ~170 to 180 mg/dl | NF-κB | ⇧+IR |
| Swartzwelder et al. (2019) | Rat | AIE 5 g/kg × 16 days | 25 days of abs | – | HMGB1, NF-κB | ⇧+IR |
| Sanchez-Alavez et al. (2019) | Rat | CIE vapor × 36 days | Intox/24-h WD/28 days of abs | ~170 to 185 mg/dl | Cytokines, chemokines, other | ⇧⇔ |
| Li et al. (2019) | Rat | 10% EtOH; 9.7 g/kg/days × 21 days | Intox | ~34 to 105 mg/dl | TLR4, NF-κB, cytokines | ⇧⇔ |
| Vore et al. (2021) | Rat | AIE 4 g/kg × 20 days | 21 to 28 days of abs | – | Cytokines, NF-κB | ⇧⇔ ↓ |
Abbreviations: +IR, immunoreactivity; Abs, abstinence; AIE, adolescent intermittent EtOH; CIE, chronic intermittent EtOH; DID, drinking in the dark; Intox, intoxication; WD, withdrawal.
Notably, in all of these studies microglial processes remained ramified, though thickened compared with controls, consistent with low level activation (Beynon & Walker, 2012; McClain et al., 2011b; Walter et al., 2017). Furthermore, several studies in rodents report microglial loss, which may be more detrimental for synaptic plasticity and development than activation (Grifasi et al., 2019; Hu et al., 2020; Marshall et al., 2020). Only limited studies have reported M1-like markers after alcohol exposure in adolescent rats (Table 1). Flow cytometric analysis of microglia showed increased expression of both M1 and M2 markers after binge exposure (Peng & Nixon, 2021). Some MHCII-positive cells were noted in the dentate gyrus after binge-like exposure and CD68 was detected in hippocampus after chronic alcohol drinking, but both showed ramified morphologies (e.g., see Figure 2 in Li et al., 2019) inconsistent with a proinflammatory phenotype.
Immune molecules
A number of studies in adolescents report EtOH-induced increases in mRNA or tissue protein expression of cytokines, chemokines, and other inflammatory mediators in the PFC and hippocampus (Table 1). While these results support the interpretation that neuroimmune activation is a consequence of alcohol exposure in adolescence, gene expression changes do not necessarily predict changes in protein. In contrast, others have observed a blunted cytokine response to acute EtOH in adolescence versus adults (Table 1). This observation has been interpreted as a relative immaturity of neuroimmune function (Doremus-Fitzwater et al., 2015), but the specific role of microglia is not clear. Indeed, for all of these reports on cytokine effects after alcohol exposure, the source of the cytokine or growth factor proteins is not clear as whole tissue homogenates are used. This point includes the hallmark proinflammatory cytokine TNF-α, the basis of the neuroimmune hypothesis of addiction (Crews et al., 2011). As detailed in Table 1, more studies report a lack of effect in robust measures of TNF-α, such as protein or microglia-specific TNF-α gene expression.
Similarly, a role for the DAMP, HMGB1, in AUD development is compelling but not specific to microglia nor necessarily proinflammatory. Adults with a history of adolescent-onset AUD have increased HMGB1, an effect not only mimicked in adolescent alcohol models but also observed in comorbid conditions such as stress or depression (Coleman et al., 2018; Franklin et al., 2018; Swartzwelder et al., 2019; Vetreno & Crews, 2012; Vetreno et al., 2013; Weber et al., 2015). HMGB1 is released from dying neurons (Scaffidi et al., 2002) as observed in a rat AUD model (Wang et al., 2015). As a DAMP, it activates neuroimmune signaling and may activate or prime microglia, but outcomes may be pro- or anti-inflammatory. Thus, increased expression of cytokines and other innate immune molecules implicate neuroimmune activation in response to alcohol, but a causal role of these factors in adolescence remains unclear.
Microglial phenotypes and priming
Demonstration of microglial activation is not sufficient to infer that these cells produce cell death and structural damage (Marshall et al., 2013). M2 “anti-inflammatory” microglia also up-regulate cell surface markers, and morphology becomes hyperramified (Beynon & Walker, 2012). Secondary waves of cell death, as would be predicted from M1-like microglia, have not been reported in alcohol models (e.g., Crews et al., 2000; Kelso et al., 2011). Furthermore, the pattern of cell surface and phenotypic markers and gene expression changes in microglia isolated from adolescent rats after alcohol dependence support that microglia are M2-like, consistent with being primed (Peng & Nixon, 2021).
Observation of a heightened microglial response to subsequent challenge—the definition of priming—confirms this hypothesis (Perry & Holmes, 2014). In an adult rat model, microglia show a heightened response upon a double versus single binge, though adolescents have not been reported (Marshall et al., 2016). Chronic alcohol exposure in adolescence resulted in a heightened microglial response (increased CD11b + IR) to restraint stress in adulthood, indicating microglial priming (Walter et al., 2017). Preliminary observations of microglial hyperresponse to LPS stimulation following an alcohol binge (Peng and Nixon, 2018) correspond to reports of increased presence of priming factors such as the DAMP, HMGB1, in rodent models and human AUD (Coleman et al., 2018; Swartzwelder et al., 2019; Vetreno & Crews, 2012; Vetreno et al., 2013). EtOH applied directly to neurons in culture increases release of HMGB1 (Wang et al., 2015), and its expression is increased in human postmortem OFC in subjects with AUD (Crews et al., 2013; Crews & Vetreno, 2014; Flatscher-Bader et al., 2005; Lewohl et al., 2000). Thus, alcohol may prime microglia through neuronal damage producing extracellular DAMPs such as HMGB1.
ARE ADOLESCENTS MORE SUSCEPTIBLE TO MICROGLIA-BASED EFFECTS?
Critical developmental processes occur during adolescence that increase the susceptibility of the brain to the consequences of EtOH (Crews et al., 2000, 2007; White & Swartzwelder, 2004). One consequence is damage, which is distinct in the adolescent brain when compared directly to adults (Crews et al., 2000). The hippocampus is a particular target of alcohol toxicity via effects on cell death and birth (De Bellis et al., 2000; McClain et al., 2014; Morris et al., 2010a; White & Swartzwelder, 2004), both mechanisms of which are modulated by microglia (Ekdahl et al., 2009). Whether microglia are impacted by alcohol differently in adolescents versus adults is not well described. There are few direct comparisons, work from our own laboratories included, though there are a few studies of adolescents or adults by the same investigators with identical approaches that allow for strong comparisons. Furthermore, valid comparisons between ages requires comparable BALs. There is some evidence of faster rates of EtOH clearance in adolescent animals, but the presence of significant pharmacokinetic differences varies widely across routes of administration, species, strains, and sex (Walker & Ehlers, 2009; Watson et al., 2020, but see Carrara-Nascimento et al., 2017; Fleming et al., 2019; Lacaille et al., 2015; Morris et al., 2010b; Silveri & Spear, 2000; Vore et al., 2021). Importantly, where EtOH metabolism differences were observed, adolescents typically showed quicker clearance and therefore lower BALs despite greater pharmacodynamic effects. Thus, the slight metabolic differences may not underlie their susceptibility in brain outcomes. Regardless, BALs are critically necessary for rigorous valid comparisons of age differences. In addition, drinking-based models are not good comparisons as adolescent rats consume more alcohol than adults, which produces different BALs and confounds interpretations (e.g., Chung et al., 2008 versus Morris et al., 2010b). Titrated binge models or vapor exposure can overcome slight pharmacokinetic differences between ages as observed in males (Morris et al., 2010b; Varlinskaya & Spear, 2004; Walker & Ehlers, 2009).
For microglia, adults and adolescents seem similar prima facie. Two recent papers show similar microglial dystrophy and loss in the hippocampus in adolescents and adults or in adolescents versus aging rats after days of binge-like exposure (Grifasi et al., 2019; Marshall et al., 2020). Another pair of articles described subtle differences in cytokine expression but increased microglial reactivity—more regions and timepoints had increased Iba1 + IR in adults (Sanchez-Alavez et al., 2019b) versus adolescents (Sanchez-Alavez et al., 2019a) despite similar BALs during 35 days of EtOH vapor. For microglial phenotype, both ages show mixed phenotypes with a distinct increase in M2-like microglia (Peng et al., 2017; Peng & Nixon, 2021). The consequences of these effects in adolescents, however, may be where adolescents have greater vulnerabilities. Effects may be more severe and persistent by diverting microglia from their homeostatic or developmental roles, impacting brain structure and function long term.
EMERGING EVIDENCE FOR A ROLE OF MICROGLIA IN ADDICTION BEHAVIOR
The implications of microglial activation on adolescent development of addiction behavior remain unclear. In adults, depleting microglia prevented some, but not all, of the neuroimmune responses to alcohol, preventing the escalation of drinking after alcohol dependence, but not reducing voluntary drinking (Walter & Crews, 2017; Warden et al., 2020). These results point to a role of microglia on a symptom of addiction, escalation of intake, which is similar to what occurs after adolescent exposure. Speculating on how microglia influence increased adult drinking after adolescent exposure requires us to revisit the factors in adolescent development that impact consumption and/or predict future addiction behaviors.
Impairments in PFC-related behavior are well established to increase risk for addiction (Koob & Volkow, 2016), and adolescent alcohol impairs PFC-dependent functions such as impulse control, behavioral flexibility, delay discounting, and working memory (Boutros et al., 2015; Seemiller & Gould, 2020; Sey et al., 2019). Little is known about the role of microglia in these behaviors, and most evidence related to immune signaling is not microglia-specific. Enhanced immune signaling in the PFC after adolescent EtOH exposure was associated with reversal learning deficits and increased perseverative behavior in adult rats tested in the Barnes maze (Vetreno & Crews, 2012). Increased expression of immune markers was seen in the PFC of human postmortem AUD and associated with drinking onset in adolescence, an effect mimicked in animal models after AIE exposure (Crews et al., 2013; Vetreno et al., 2013). Interestingly, adolescent rodents were protected from effects of alcohol, such as neuroimmune activation, cell death, and increased drinking, in TLR4−/− mice or by treatment with the anti-inflammatory indomethacin (Montesinos et al., 2015, 2016; Vetreno & Crews, 2018). However, the role of TLR4 in alcohol drinking was discounted in a comprehensive study in adult mice (Harris et al., 2017) and whether any of these effects involve microglia specifically is not established. Thus, major gaps remain in our understanding of the role of microglia in PFC-controlled behaviors in AUD development.
Alterations in mesolimbic reward circuity that influences reward processing may underlie the adolescent's increased susceptibility for developing alcohol problems in adulthood (Hill et al., 2009; Stice et al., 2013). While there is evidence for differential sensitivity to alcohol-induced neuroadaptations in the mesolimbic system in adolescents versus adults (Alaux-Cantin et al., 2013; Jacobsen et al., 2018; Liu & Crews, 2015; Nees et al., 2015), how microglia influence these changes is difficult to disentangle from the few neuroimmune effects reported. For example, nalmefene, an opioid receptor and TLR4 antagonist approved for reduction in alcohol consumption in patients with AUD, reduced microglial activation and other neuroimmune effects in the striatum and NAc, but astrocytes may be the site of action (Montesinos et al., 2017; Tournier et al., 2020). A role for BDNF has been described in reward processing in adolescents, with specific gene polymorphisms differentially impacting reward processing and striatal function and associated with adolescent drinking onset (Nees et al., 2015). Microglia release BDNF and other growth factors depending on their phenotype, functions that are affected by alcohol exposure in adolescence (Marshall et al., 2020; Peng & Nixon, 2021). Loss of microglia-derived BDNF would have huge implications for striatal function, for example increasing alcohol drinking (Jeanblanc et al., 2009), which is consistent with alcohol preferring rats having lower BDNF levels (Yan et al., 2005).
The hippocampus plays a broad role in behavior (Abela et al., 2013; Rubin et al., 2017), and accordingly, alcohol's neurotoxic effects in hippocampus relate to broad impairments in behavior (De Bellis et al., 2000; Jacobus & Tapert, 2013; Nixon et al., 2011; White & Swartzwelder, 2004). Examples of the relationship between neuroimmune activation and impairments in hippocampal integrity are numerous in the literature including human (Ward et al., 2009b) and animal models (Ji et al., 2018; Ward et al., 2008), though again not necessarily specific to microglia. The loss of microglia and its role in hippocampal development and function could be more detrimental, but this has not been tested mechanistically (Hu et al., 2020; Marshall et al., 2020). A number of groups report alcohol effects on microglia and adult neurogenesis in adolescence, but mechanistic ties between microglia and neurogenesis specifically are lacking (Ji et al., 2018; Liu & Crews, 2017; Morris et al., 2010a; Vetreno & Crews, 2015; Vetreno et al., 2018; Zou & Crews, 2012). These interactions are actively being pursed in adult and adolescent AUD models (Alvarez Cooper et al., 2020; Nixon et al., 2008).
CONCLUSION
We critically review how adolescent alcohol exposure effects microglia and the implications for AUD pathogenesis. We focused on microglia not only because of their varied roles in homeostatic processes but also because of their dual response to infection and insult, that is, phenotypes, that have been overlooked in the alcohol field (Prinz & Priller, 2017). We predict that microglia have a greater impact on adolescents due not only to relatively subtle effects on addiction relevant behaviors but perhaps more extensive effects on developmental trajectories. However, much remains to be studied. It is difficult to disentangle effects of neuroimmune signaling in general with that of microglia in particular due to a lack of specific tools to manipulate microglia, though these are evolving. Further, few studies make direct, valid comparisons between adult and adolescent animals with BALs controlled. We describe microglial priming, essentially innate immune memory, as a means by which these early insults have long-lasting effects on the brain. There is strong evidence that adolescent alcohol exposure primes microglia persistently. Given the role of microglia in neurodevelopment and in maintaining CNS homeostasis, perturbations of these cells, especially during critical periods of plasticity, have serious implications for brain development, function, and AUD pathogenesis.
ACKNOWLEDGMENT
The authors thank the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, the National Institute of Neurological Disorders and Stroke for support of R01AA025591 (KN), R21AA025563 (HP/KN), R61NS111081 (JP), T32AA007471 (JKM), and T32DA35200 (CC), and the University of Kentucky College of Pharmacy for funding various works described herein.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Abela AR, Dougherty SD, Fagen ED, Hill CJ & Chudasama Y (2013) Inhibitory control deficits in rats with ventral hippocampal lesions. Cerebral Cortex, 23, 1396–1409. [DOI] [PubMed] [Google Scholar]
- Agrawal RG, Hewetson A, George CM, Syapin PJ & Bergeson SE (2011) Minocycline reduces ethanol drinking. Brain, Behavior, and Immunity, 25(Suppl. 1), S165–S169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C et al. (2013) Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology, 67, 521–531. [DOI] [PubMed] [Google Scholar]
- Alba-Ferrara L, Muller-Oehring EM, Sullivan EV, Pfefferbaum A & Schulte T (2016) Brain responses to emotional salience and reward in alcohol use disorder. Brain Imaging and Behavior, 10, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Cooper I, Beecher K, Chehrehasa F, Belmer A & Bartlett SE (2020) Tumour necrosis factor in neuroplasticity, neurogenesis and alcohol use disorder. Brain Plasticity, 6, 47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo LR, Kneiber D, Wills DN & Ehlers CL (2017) Alcohol drinking during adolescence increases consumptive responses to alcohol in adulthood in Wistar rats. Alcohol, 59, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon A, Strasburger HJ, Ayata P, Chen X, Nair A, Ikegami A et al. (2020) Negative feedback control of neuronal activity by microglia. Nature, 586, 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TE, Castellanos-Ryan N, Schumann G, Cattrell A, Flor H, Nees F et al. (2019) Modulation of orbitofrontal-striatal reward activity by dopaminergic functional polymorphisms contributes to a predisposition to alcohol misuse in early adolescence. Psychological Medicine, 49, 801–810. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL & Kelly TH (2013) Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacological Reviews, 65, 255–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Temin H, Zhang P, Chaput D, King MA, You M, Liu B et al. (2013) Quantitative proteomic characterization of ethanol-responsive pathways in rat microglial cells. Journal of Proteome Research, 12, 2067–2077. [DOI] [PubMed] [Google Scholar]
- Beynon SB & Walker FR (2012) Microglial activation in the injured and healthy brain: what are we really talking about? Practical and theoretical issues associated with the measurement of changes in microglial morphology. Neuroscience, 225, 162–171. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H & Harris RA (2011) Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain, Behavior, and Immunity, 25(Suppl. 1), S92–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Liu W, Crews FT & Markou A (2015) Adolescent intermittent ethanol exposure is associated with increased risky choice and decreased dopaminergic and cholinergic neuron markers in adult rats. International Journal of Neuropsychopharmacology, 18, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC & Andersen SL (2011) Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neuroscience and Biobehavioral Reviews, 35, 1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC & Schwarz JM (2016) Immunoadolescence: neuroimmune development and adolescent behavior. Neuroscience and Biobehavioral Reviews, 70, 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater MA, Lee SH, Yu Y, Zhu H, Crews FT, Robinson DL et al. (2018) Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addiction Biology, 23, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater MA, Liu W, Crews FT & Spear LP (2014) Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Developmental Neuroscience, 36, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S et al. (2006) Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Molecular and Cellular Neurosciences, 31, 149–160. [DOI] [PubMed] [Google Scholar]
- Carrara-Nascimento PF, Hoffmann LB, Contó MB, Marcourakis T & Camarini R (2017) Ethanol Sensitization during adolescence or adulthood induces different patterns of ethanol consumption without affecting ethanol metabolism. Frontiers in Behavioral Neuroscience, 11, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapoval AI, Kamdar SJ, Kremlev SG & Evans R (1998) CSF-1 (M-CSF) differentially sensitizes mononuclear phagocyte subpopulations to endotoxin in vivo: a potential pathway that regulates the severity of gram-negative infections. Journal of Leukocyte Biology, 63, 245–252. [DOI] [PubMed] [Google Scholar]
- Chastain LG, Franklin T, Gangisetty O, Cabrera MA, Mukherjee S, Shrivastava P et al. (2019) Early life alcohol exposure primes hypothalamic microglia to later-life hypersensitivity to immune stress: possible epigenetic mechanism. Neuropsychopharmacology, 44, 1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CS, Wang J, Wehman M & Rhoads DE (2008) Severity of alcohol withdrawal symptoms depends on developmental stage of Long-Evans rats. Pharmacology, Biochemistry and Behavior, 89,137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Creswell KG, Bachrach R, Clark DB & Martin CS (2018) Adolescent binge drinking. Alcohol Research, 39, 5–15. [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Bukstein O & Cornelius J (2002) Alcohol use disorders in adolescents: epidemiology, diagnosis, psychosocial interventions, and pharmacological treatment. Paediatric Drugs, 4, 493–502. [DOI] [PubMed] [Google Scholar]
- Clark DB, Martin CS, Chung T, Gordon AJ, Fiorentino L, Tootell M & et al. (2016) Screening for underage drinking and diagnostic and statistical manual of mental disorders, 5th edition alcohol use disorder in rural primary care practice. The Journal of Pediatrics, 173, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman L SCrews FT (2018) Innate immune signaling and alcohol use disorders. Handbook of Experimental Pharmacology, 248, 369–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., Zou J, Qin L & Crews FT (2018) HMGB1/IL-1beta complexes regulate neuroimmune responses in alcoholism. Brain, Behavior, and Immunity, 72, 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT & Boettiger CA (2009) Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry and Behavior, 93, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC III & Knapp DJ (2000) Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism, Clinical and Experimental Research, 24, 1712–1723. [PubMed] [Google Scholar]
- Crews F, He J & Hodge C (2007) Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology, Biochemistry and Behavior, 86, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ & Coleman LG Jr. (2017) The role of neuroimmune signaling in alcoholism. Neuropharmacology, 122, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J & Nixon K (2006) Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience, 137, 437–445. [DOI] [PubMed] [Google Scholar]
- Crews FT & Nixon K (2009) Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and Alcoholism, 44, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP & Zou J (2013) High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biological Psychiatry, 73, 602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC et al. (2019) Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings. Alcoholism, Clinical and Experimental Research, 43, 1806–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT & Vetreno RP (2014) Neuroimmune basis of alcoholic brain damage. International Review of Neurobiology, 118, 315–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA & Robinson DL (2016) Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacological Reviews, 68, 1074–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J & Qin L (2011) Induction of innate immune genes in brain create the neurobiology of addiction. Brain, Behavior, and Immunity, 25(Suppl. 1), S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum K & Hulvershorn L (2020) Editorial: heavy adolescent alcohol use: an accelerant of impulsivity? Journal of the American Academy of Child and Adolescent Psychiatry, 60, 575–576. [DOI] [PubMed] [Google Scholar]
- Cserep C, Posfai B, Lenart N, Fekete R, Laszlo ZI, Lele Z et al. (2020) Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science, 367, 528–537. [DOI] [PubMed] [Google Scholar]
- Darna M, Chow JJ, Yates JR, Charnigo RJ, Beckmann JS, Bardo MT et al. (2015) Role of serotonin transporter function in rat orbitofrontal cortex in impulsive choice. Behavioral Brain Research, 293, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J et al. (2000) Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry, 157, 737–744. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P & Clark DB (2005) Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism, Clinical and Experimental Research, 29, 1590–1600. [DOI] [PubMed] [Google Scholar]
- Deas D, Riggs P, Langenbucher J, Goldman M & Brown S (2000) Adolescents are not adults: developmental considerations in alcohol users. Alcoholism, Clinical and Experimental Research, 24, 232–237. [PubMed] [Google Scholar]
- Dewit DJ, Adlaf EM, Offord DR & Ogborne AC (2000) Age at first alcohol use: a risk factor for the development of alcohol disorders. American Journal of Psychiatry, 157, 745–750. [DOI] [PubMed] [Google Scholar]
- Donovan JE (2009) Estimated blood alcohol concentrations for child and adolescent drinking and their implications for screening instruments. Pediatrics, 123, e975–e981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P & Spear LP (2005) Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism, Clinical and Experimental Research, 29, 1796–1808. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME & Deak T (2014) Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcoholism, Clinical and Experimental Research, 38, 2186–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Gano A, Paniccia JE & Deak T (2015) Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiology & Behavior, 148, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbelaar ML, Kracht L, Eggen BJL & Boddeke E (2018) The kaleidoscope of microglial phenotypes. Frontiers in Immunology, 9, 1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z & Lindvall O (2003) Inflammation is detrimental for neurogenesis in adult brain. Proceedings of the National Academy of Sciences USA, 100, 13632–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z & Lindvall O (2009) Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience, 158, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Erickson EK, Grantham EK, Warden AS & Harris RA (2019) Neuroimmune signaling in alcohol use disorder. Pharmacology, Biochemistry and Behavior, 177, 34–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB & Wu LJ. (2019) Microglia: lifelong patrolling immune cells of the brain. Progress in Neurobiology, 179, 101614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Greenstein D, Cardenas VA, Cuzen NL, Fouche JP, Ferrett H et al. (2013) Cortical and subcortical volumes in adolescents with alcohol dependence but without substance or psychiatric comorbidities. Psychiatry Research, 214, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie G, Peeters M, Gullo MJ, Christiansen P, Cole JC, Sumnall H et al. (2013) Multiple behavioural impulsivity tasks predict prospective alcohol involvement in adolescents. Addiction, 108, 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipello F, Morini R, Corradini I, Zerbi V, Canzi A, Michalski B et al. (2018) The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity, 48, 979–991 e8. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, Van Der Brug M, Hwang JW, Gochee PA, Matsumoto I, Niwa S et al. (2005) Alcohol-responsive genes in the frontal cortex and nucleus accumbens of human alcoholics. Journal of Neurochemistry, 93, 359–370. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Wilson WA & Swartzwelder HS (2007) Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. Journal of Neurophysiology, 97, 3806–3811. [DOI] [PubMed] [Google Scholar]
- Fleming W, Jones Q, Chandra U, Saini A, Walker D, Francis R et al. (2019) Withdrawal from brief repeated alcohol treatment in adolescent and adult male and female rats. Alcoholism, Clinical and Experimental Research, 43, 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, D'angelo HM, Norden DM, Weber MD et al. (2016) The alarmin HMGB1 mediates age-induced neuroinflammatory priming. Journal of Neuroscience, 36, 7946–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TC, Xu C & Duman RS (2018) Depression and sterile inflammation: essential role of danger associated molecular patterns. Brain, Behavior, and Immunity, 72, 2–13. [DOI] [PubMed] [Google Scholar]
- Frost JL & Schafer DP (2016) Microglia: architects of the developing nervous system. Trends in Cell Biology, 26, 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano A, Doremus-Fitzwater TL & Deak T (2016) Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Research, 1646, 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Bath KG, Johnson CM, Meyer HC, Murty VP, Van Den Bos W et al. (2018) Neurocognitive development of motivated behavior: dynamic changes across childhood and adolescence. Journal of Neuroscience, 38, 9433–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN (2004) Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences, 1021, 77–85. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A et al. (1999) Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience, 2, 861–863. [DOI] [PubMed] [Google Scholar]
- Grant BF & Dawson DA (1997) Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse, 9, 103–110. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H et al. (2015) Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry, 72, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifasi IR, Evans WA, Rexha AD, Sako LW & Marshall SA (2019) A comparison of hippocampal microglial responses in aged and young rodents following dependent and non-dependent binge drinking. International Review of Neurobiology, 148, 305–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guergues J, Wohlfahrt J, Zhang P, Liu B & Stevens SM Jr. (2020) Deep proteome profiling reveals novel pathways associated with pro-inflammatory and alcohol-induced microglial activation phenotypes. Journal of Proteomics, 220, 103753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C & Pascual M (2019a) Chapter nine - role of neuroinflammation in ethanol neurotoxicity. In: Aschner M & Costa LG (Eds.) Advances in neurotoxicology (vol. 3, pp. 259–294). Academic Press. [Google Scholar]
- Guerri C & Pascual M (2019b) Impact of neuroimmune activation induced by alcohol or drug abuse on adolescent brain development. International Journal of Developmental Neuroscience, 77, 89–98. [DOI] [PubMed] [Google Scholar]
- Gulley JM & Juraska JM (2013) The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience, 249, 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunner G, Cheadle L, Johnson KM, Ayata P, Badimon A, Mondo E et al. (2019) Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nature Neuroscience, 22, 1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeyer N, Hanft KM, Akriditou MA, Unger N, Park ES, Stanley ER et al. (2017) Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathologica, 134, 441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley MJ, Brough D,Quintin J & Allan SM(2018) Microglial priming as trained immunity in the brain. Neuroscience, 405, 47–54. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Felton JW, Goncalves SF, Tasheuras ON, Yoon M & Lejuez CW (2019) Trait impulsivity during early adolescence predicts steepness of alcohol use escalation across adolescence. Addictive Behaviors, 98, 106017. [DOI] [PubMed] [Google Scholar]
- Hammond TR, Robinton D & Stevens B (2018) Microglia and the brain: complementary partners in development and disease. Annual Review of Cell and Developmental Biology, 34, 523–544. [DOI] [PubMed] [Google Scholar]
- Harris RA, Bajo M, Bell RL, Blednov YA, Varodayan FP, Truitt JM et al. (2017) Genetic and pharmacologic manipulation of TLR4 has minimal impact on ethanol consumption in rodents. Journal of Neuroscience, 37, 1139–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J & Crews FT (2007) Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacology, Biochemistry and Behavior, 86, 327–333. [DOI] [PubMed] [Google Scholar]
- He J & Crews FT (2008) Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental Neurology, 210, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang L-C, Means TK et al. (2013) The microglial sensome revealed by direct RNA sequencing. Nature Neuroscience, 16, 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, Mcdermott M et al. (2009) Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biological Psychiatry, 65, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Wang D, Zhang Y, Cai Z, Ye T, Tong L et al. (2020) Apoptosis-triggered decline in hippocampal microglia mediates adolescent intermittent alcohol exposure-induced depression-like behaviors in mice. Neuropharmacology, 170, 108054. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Parvaz MA, Velthorst E, Shaik RB, Sandin S, Gan G et al. (2021) Substance use initiation, particularly alcohol, in drug naïve adolescents: possible predictors and consequences from a large cohort naturalistic study. Journal of the American Academy of Child and Adolescent Psychiatry, 60, 623–636. [DOI] [PubMed] [Google Scholar]
- Jacobsen JHW, Buisman-Pijlman FT, Mustafa S, Rice KC & Hutchinson MR (2018) Antagonising TLR4-TRIF signalling before or after a low-dose alcohol binge during adolescence prevents alcohol drinking but not seeking behaviour in adulthood. Neuropharmacology, 128, 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J & Tapert SF (2013) Neurotoxic effects of alcohol in adolescence. Annual Review of Clinical Psychology, 9, 703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH & Ron D (2009) Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. Journal of Neuroscience, 29, 13494–13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z, Yuan L, Lu X, Ding H, Luo J & Ke ZJ (2018) Binge alcohol exposure causes neurobehavioral deficits and gsk3beta activation in the hippocampus of adolescent rats. Scientific Reports, 8, 3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’malley PM, Bachman JG, Schulenberg JE & Patrick ME (2020) Monitoring the Future national survey results on drug use 1975-2019: overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research: University of Michigan. [Google Scholar]
- Jones SA, Lueras JM & Nagel BJ (2018) Effects of binge drinking on the developing brain. Alcohol Research, 39, 87–96. [PMC free article] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J et al. (2014) Effects of ethanol on immune response in the brain: region-specific changes in adolescentversus adult mice. Alcoholism, Clinical and Experimental Research, 38, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Park SH, Chen J, Qiao Y, Giannopoulou E, Berg K et al. (2017) Interferon-gamma represses M2 gene expression in human macrophages by disassembling enhancers bound by the transcription factor MAF. Immunity, 47, 235–250 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Chen R, Zhang Q, Hou W,Wu S, Cao L et al. (2014) HMGB1 in health and disease. Molecular Aspects of Medicine, 40, 1–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, Liput DJ, Eaves DW & Nixon K (2011) Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience, 197, 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Kiyama H & Ueno M (2019) Dual functions of microglia in the formation and refinement of neural circuits during development. International Journal of Developmental Neuroscience, 77, 18–25. [DOI] [PubMed] [Google Scholar]
- Koob GF & Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry, 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec AM, Smith CJ, Ayre NR, Sweat SC & Bilbo SD (2018) Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nature Communications, 9, 3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T, Wolf B, Keshet E & Licht T (2019) Unique role for dentate gyrus microglia in neuroblast survival and in VEGF-induced activation. Glia, 67, 594–618. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends in Neurosciences, 19, 312–318. [DOI] [PubMed] [Google Scholar]
- Lacaille H, Duterte-Boucher D, Liot D, Vaudry H, Naassila M & Vaudry D (2015) Comparison of the deleterious effects of binge drinking-like alcohol exposure in adolescent and adult mice. Journal of Neurochemistry, 132, 629–641. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC & Kriz J (2007) Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. Journal of Neuroscience, 27, 2596–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamy TE, Connor JP, Voisey J, Young RM & Gullo MJ (2016) Alcohol misuse in emerging adulthood: association of dopamine and serotonin receptor genes with impulsivity-related cognition. Addictive Behaviors, 63, 29–36. [DOI] [PubMed] [Google Scholar]
- Lees B, Meredith LR, Kirkland AE, Bryant BE & Squeglia LM (2020) Effect of alcohol use on the adolescent brain and behavior. Pharmacology, Biochemistry and Behavior, 192, 172906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR & Harris RA (2000) Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcoholism, Clinical and Experimental Research, 24, 1873–1882. [PubMed] [Google Scholar]
- Li Q & Barres BA (2018) Microglia and macrophages in brain homeostasis and disease. Nature Reviews Immunology, 18, 225–242. [DOI] [PubMed] [Google Scholar]
- Li Q, Liu D, Pan F, Ho CSH & Ho RCM (2019) Ethanol exposure induces microglia activation and neuroinflammation through TLR4 activation and SENP6 modulation in the adolescent rat hippocampus. Neural Plasticity, 2019, 1648736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wilson WA & Swartzwelder HS (2003) Developmental differences in the sensitivity of hippocampal GABAA receptor-mediated IPSCS to ethanol. Alcoholism, Clinical and Experimental Research, 27, 2017–2022. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA & Swartzwelder HS (1996) Differential effects of ethanol in adolescent and adult rats. Alcoholism, Clinical and Experimental Research, 20, 1346–1351. [DOI] [PubMed] [Google Scholar]
- Liu W & Crews FT (2015) Adolescent intermittent ethanol exposure enhances ethanol activation of the nucleus accumbens while blunting the prefrontal cortex responses in adult rat. Neuroscience, 293, 92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W & Crews FT (2017) Persistent decreases in adult subventricular and hippocampal neurogenesis following adolescent intermittent ethanol exposure. Frontiers in Behavioural Neurosciences, 11, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locati M, Mantovani A & Sica A (2013) Macrophage activation and polarization as an adaptive component of innate immunity. Advances in Immunology, 120, 163–184. [DOI] [PubMed] [Google Scholar]
- Lopez-Caneda E, Rodriguez Holguin S, Cadaveira F, Corral M & Doallo S (2014) Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol and Alcoholism, 49, 173–181. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Muetzel RL & Lim KO (2013) Effects of alcohol use initiation on brain structure in typically developing adolescents. American Journal of Drug and Alcohol Abuse, 39, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht V, Crews FT & Vetreno RP (2019) Neuroimmune and epigenetic mechanisms underlying persistent loss of hippocampal neurogenesis following adolescent intermittent ethanol exposure. Current Opinion in Pharmacology, 50, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood OM, Jacobus J, Bava S, Scarlett A & Tapert SF (2010) Learning and memory performances in adolescent users of alcohol and marijuana: interactive effects. Journal of Studies on Alcohol and Drugs, 71, 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallya AP, Wang HD, Lee HNR & Deutch AY (2019) Microglial pruning of synapses in the prefrontal cortex during adolescence. Cerebral Cortex, 29, 1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Morris JR & Juraska JM (2007) Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience, 144, 961–968. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA & Swartzwelder HS (1998) Differential effects of ethanol on memory in adolescent and adult rats. Alcoholism, Clinical and Experimental Research, 22, 416–421. [PubMed] [Google Scholar]
- Marshall SA, Geil CR & Nixon K (2016) Prior binge ethanol exposure potentiates the microglial response in a model of alcohol-induced neurodegeneration. Brain Sciences, 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Mcclain JA, Kelso ML, Hopkins DM, Pauly JR & Nixon K (2013) Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: the importance of microglia phenotype. Neurobiology of Diseases, 54, 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Mcclain JA, Wooden JI & Nixon K (2020) Microglia dystrophy following binge-like alcohol exposure in adolescent and adult male rats. Frontiers in Neuroanatomy, 14, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Ferreira R, Leal B, Costa PP & Ballestar E (2020) Microglial innate memory and epigenetic reprogramming in neurological disorders. Progress in Neurobiology, 200, 101971. [DOI] [PubMed] [Google Scholar]
- Marusich JA & Bardo MT (2009) Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behavioural Pharmacology, 20, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Scheiwe C et al. (2019) Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature, 566, 388–392. [DOI] [PubMed] [Google Scholar]
- McClain JA, Hayes DM, Morris SA & Nixon K (2011a) Adolescent binge alcohol exposure alters hippocampal progenitor cell proliferation in rats: effects on cell cycle kinetics. The Journal of Comparative Neurology, 519, 2697–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM et al. (2011b) Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain, Behavior, and Immunity, 25(Suppl. 1), S120–S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Marshall SA & Nixon K (2014) Ectopic hippocampal neurogenesis in adolescent male rats following alcohol dependence. Addiction Biology, 19, 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Mcqueeny T, Nagel BJ, Hanson KL, Schweinsburg AD & Tapert SF (2008) Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcoholism, Clinical and Experimental Research, 32, 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melbourne JK, Thompson KR, Peng H & Nixon K (2019) Its complicated: the relationship between alcohol and microglia in the search for novel pharmacotherapeutic targets for alcohol use disorders. Progress in Molecular Biology and Translational Science, 167, 179–221. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H & Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science, 302, 1760–1765. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Gil A & Guerri C (2017) Nalmefene prevents alcohol-induced neuroinflammation and alcohol drinking preference in adolescent female mice: role of TLR4. Alcoholism, Clinical and Experimental Research, 41, 1257–1270. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Pla A, Maldonado C, Rodriguez-Arias M, Minarro J et al. (2015) TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain, Behavior, and Immunity, 45, 233–244. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Rodriguez-Arias M, Minarro J & Guerri C (2016) Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain, Behavior, and Immunity, 53, 159–171. [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR & Nixon K (2010a) Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus, 20, 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA & Nixon K (2010b) Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol, 44, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V & Tapert SF (2005) Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research, 139, 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TW & Bernstein IL (2009) Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proceedings of the National Academy of Sciences USA, 106, 17600–17604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism. (2004) NIAAA Council approves definition of binge drinking. NIAAA Newsletter. [Google Scholar]
- Nees F, Witt SH, Dinu-Biringer R, Lourdusamy A, Tzschoppe J, Vollstadt-Klein S et al. (2015) BDNF Val66Met and reward-related brain function in adolescents: role for early alcohol consumption. Alcohol, 49, 103–110. [DOI] [PubMed] [Google Scholar]
- Neher JJ & Cunningham C (2019) Priming microglia for innate immune memory in the brain. Trends in Immunology, 40, 358–374. [DOI] [PubMed] [Google Scholar]
- Nennig SE & Schank JR (2017) The role of NFkB in drug addiction: beyond inflammation. Alcohol and Alcoholism, 52, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F & Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science, 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J & Crews FT (2008) Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiology of Diseases, 31, 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K & Mcclain JA (2010) Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Current Opinion in Psychiatry, 23, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Pauly JR & Hayes DM (2011) Alcohol, nicotine, and adult neurogenesis. In: Olive MF (Ed.) Drug addiction and adult neurogenesis. Kerala, India: Research Signpost. [Google Scholar]
- Paolicelli R, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P et al. (2011) Synaptic pruning by microglia is necessary for normal brain development. Science, 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ et al. (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell, 155, 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Marcos M, Torres JL, Costa-Alba P, Garcia-Garcia F et al. (2016) Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict Biol, 22, 1829–1841. [DOI] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’malley PM & Johnston LD (2013) Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. JAMA Pediatrics, 167, 1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME & Terry-Mcelrath YM (2019) Prevalence of high-intensity drinking from adolescence through young adulthood: national data from 2016–2017. Substance Abuse: Research and Treatment, 13, 1178221818822976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Geil Nickell CR, Chen KY, Mcclain JA & Nixon K (2017) Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol, 62, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H & Nixon K (2018) Two days of binge-like alcohol exposure during adolescence induces long-lasting effects on rat microglia. Alcoholism, Clinical and Experimental Research, 4(Suppl. 1), 23A. [Google Scholar]
- Peng H & Nixon K (2021) Microglia phenotypes following the induction of alcohol dependence in adolescent rats. Alcoholism, Clinical and Experimental Research, 45, 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH & Holmes C (2014) Microglial priming in neurodegenerative disease. Nature Reviews Neurology, 10, 217–224. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF et al. (2018) Altered brain developmental trajectories in adolescents after initiating drinking. American Journal of Psychiatry, 175, 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer T & Attwell D (2020) Brain's immune cells put the brakes on neurons. Nature, 586, 366–367. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Wecker L & Kirstein CL (2009) Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. International Journal of Developmental Neuroscience, 27, 805–815. [DOI] [PubMed] [Google Scholar]
- Prinz M, Jung S & Priller J (2019) Microglia biology: one century of evolving concepts. Cell, 179, 292–311. [DOI] [PubMed] [Google Scholar]
- Prinz M & Priller J (2017) The role of peripheral immune cells in the CNS in steady state and disease. Nature Neuroscience, 20, 136–144. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS & Crews FT (2008) Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of Neuroinflammation, 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankine EL, Hughes PM, Botham MS, Perry VH & Felton LM (2006) Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1-deficient mice prior to leucocyte recruitment. European Journal of Neuroscience, 24, 77–86. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nature Neuroscience, 19, 987–991. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM & Perry VH (2009) Microglial physiology: unique stimuli, specialized responses. Annual Review of Immunology, 27, 119–145. [DOI] [PubMed] [Google Scholar]
- Rath M, Guergues J, Pinho JPC, Zhang P, Nguyen TG, Macfadyen KA et al. (2020) Chronic voluntary binge ethanol consumption causes sex-specific differences in microglial signaling pathways and withdrawal-associated behaviors in mice. Alcoholism, Clinical and Experimental Research, 44, 1791–1806. [DOI] [PubMed] [Google Scholar]
- Risher ML, Fleming RL, Boutros N, Semenova S, Wilson WA, Levin ED et al. (2013) Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: radial-arm maze performance and operant food reinforced responding. PLoS One, 8, e62940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher ML, Fleming RL, Risher WC, Miller KM, Klein RC, Wills T et al. (2015) Adolescent intermittent alcohol exposure: persistence of structural and functional hippocampal abnormalities into adulthood. Alcoholism, Clinical and Experimental Research, 39, 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristuccia RC & Spear LP (2008) Adolescent and adult heart rate responses to self-administered ethanol. Alcoholism: Clinical and Experimental Research, 32, 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D & Moreno M (2017) Digital media and risks for adolescent substance abuse and problematic gambling. Pediatrics, 140, S102–S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosczyk HA,Sparkman NL&Johnson RW(2008) Neuroinflammation and cognitive function in aged mice following minor surgery. Experimental Gerontology, 43, 840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RD, Schwarb H, Lucas HD, Dulas MR & Cohen NJ. (2017) Dynamic hippocampal and prefrontal contributions to memory processes and representations blur the boundaries of traditional cognitive domains. Brain Sciences, 7, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba W, Goutal S, Auvity S, Kuhnast B, Coulon C, Kouyoumdjian V et al. (2018) Imaging the neuroimmune response to alcohol exposure in adolescent baboons: a TSPO PET study using (18) F-DPA-714. Addiction Biology, 23, 1000–1009. [DOI] [PubMed] [Google Scholar]
- Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT & Pandey SC (2016) A role for histone acetylation mechanisms in adolescentalcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Structure and Function, 221, 4691–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW & Stevens B (2017) Microglia emerge as central players in brain disease. Nature Medicine, 23, 1018–1027. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Nguyen W, Mori S, Wills DN, Otero D, Aguirre CA et al. (2019a) Time course of blood and brain cytokine/chemokine levels following adolescent alcohol exposure and withdrawal in rats. Alcoholism, Clinical and Experimental Research, 43, 2547–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Nguyen W, Mori S, Wills DN, Otero D, Ehlers CL et al. (2019b) Time course of microglia activation and brain and blood cytokine/chemokine levels following chronic ethanol exposure and protracted withdrawal in rats. Alcohol, 76, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S, Baro V, Trick L, Pena-Oliver Y, Stephens DN & Duka T (2014) Exaggerated waiting impulsivity associated with human binge drinking, and high alcohol consumption in mice. Neuropsychopharm, 39, 2919–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S, Pena-Oliver Y, Ripley TL & Stephens DN (2014) Repeated ethanol exposure during early and late adolescence: double dissociation of effects on waiting and choice impulsivity. Alcoholism, Clinical and Experimental Research, 38, 2579–2589. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T & Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature, 418, 191–195. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R et al. (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP & Stevens B (2015) Microglia function in central nervous system development and plasticity. Cold Spring Harbor Perspectives in Biology, 7, a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbel U, Raghupathi R, Nakamura M, Saatman KE, Trojanowski JQ, Neugebauer E et al. (1999) Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proceedings of the National Academy of Sciences USA, 96, 8721–8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Soden ME, Zweifel LS & Clark JJ (2016) Reversal of alcohol-induced dysregulation in dopamine network dynamics may rescue maladaptive decision-making. Journal of Neuroscience, 36, 3698–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Tsutsui KT & Clark JJ (2014) Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcoholism, Clinical and Experimental Research, 38, 1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Archer C, Tapert SF & Frank LR (2008) Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol, 42, 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Mcqueeny T, Nagel BJ, Eyler LT & Tapert SF (2010) A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol, 44, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemiller LR & Gould TJ (2020) The effects of adolescent alcohol exposure on learning and related neurobiology in humans and rodents. Neurobiology of Learning and Memory, 172, 107234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Dagher A, Schlagintweit H, Casey KF, Benkelfat C et al. (2014) Differential striatal dopamine responses following oral alcohol in individuals at varying risk for dependence. Alcoholism, Clinical and Experimental Research, 38, 126–134. [DOI] [PubMed] [Google Scholar]
- Sey NYA, Gomez AA, Madayag AC, Boettiger CA & Robinson DL (2019) Adolescent intermittent ethanol impairs behavioral flexibility in a rat foraging task in adulthood. Behavioral Brain Research, 373, 112085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJP, Chancey JH, Enikolopov G, Overstreet-Wadiche LS et al. (2010) Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell, 7, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Tremblay ME & Wake H (2014) Never-resting microglia: physiological roles in the healthy brain and pathological implications. Frontiers in Cellular Neuroscience, 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Dager AD, Cohen-Gilbert JE & Sneider JT (2016) Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neuroscience and Biobehavioral Reviews, 70, 244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM & Spear LP (2000) Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol, 20, 45–53. [DOI] [PubMed] [Google Scholar]
- Silverman MH, Jedd K & Luciana M (2015) Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. Neuroimage, 122, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar R, Basak AK & Sircar D (2009) Repeated ethanol exposure affects the acquisition of spatial memory in adolescent female rats. Behavioural Brain Research, 202, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar R & Sircar D (2005) Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcoholism, Clinical and Experimental Research, 29, 1402–1410. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL &Toga AW (1999) In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience, 2, 859–861. [DOI] [PubMed] [Google Scholar]
- Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews, 24, 417–463. [DOI] [PubMed] [Google Scholar]
- Spear LP (2011) Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: setting the stage for alcohol use disorders? Child Development Perspectives, 5, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2018) Effects of adolescent alcohol consumption on the brain and behaviour. Nature Reviews Neuroscience, 19, 197–214. [DOI] [PubMed] [Google Scholar]
- Spear LP & Brake SC (1983) Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Developmental Psychobiology, 16, 83–109. [DOI] [PubMed] [Google Scholar]
- Spear LP & Varlinskaya EI (2005) Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Developments in Alcoholism, 17, 143–159. [PubMed] [Google Scholar]
- Spinella M (2004) Neurobehavioral correlates of impulsivity: evidence of prefrontal involvement. International Journal of Neuroscience, 114, 95–104. [DOI] [PubMed] [Google Scholar]
- Spoelder M, Tsutsui KT, Lesscher HM, Vanderschuren LJ & Clark JJ (2015) Adolescent alcohol exposure amplifies the incentive value of reward-predictive cues through potentiation of phasic dopamine signaling. Neuropsychopharm, 40, 2873–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D et al. (2014) Microglia modulate wiring of the embryonic forebrain. Cell Reports, 8, 1271–1279. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM et al. (2014) Brain volume reductions in adolescent heavy drinkers. Developmental Cognitive Neuroscience, 9, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T et al. (2015) Brain development in heavy-drinking adolescents. American Journal of Psychiatry, 172, 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N et al. (2007) The classical complement cascade mediates CNS synapse elimination. Cell, 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S & Burger KS (2013) Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biological Psychiatry, 73, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse Mental Health Services Administration (SAMHSA) (2018) Report to congress on the prevention and reduction of underage drinking. Washington, DC: SAMHSA, U.S. Department of Health and Human Services. [Google Scholar]
- Substance Abuse Mental Health Services Administration (SAMHSA) (2019) Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Sullivan EV, Brumback T, Tapert SF, Brown SA, Baker FC, Colrain IM et al. (2020) Disturbed cerebellar growth trajectories in adolescents who initiate alcohol drinking. Biological Psychiatry, 87, 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Healey KL, Liu W, Dubester K, Miller KM & Crews FT (2019) Changes in neuroimmune and neuronal death markers after adolescent alcohol exposure in rats are reversed by donepezil. Scientific Reports, 9, 12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Hogan A, Risher ML, Swartzwelder RA, Wilson WA & Acheson SK (2014) Effect of sub-chronic intermittent ethanol exposure on spatial learning and ethanol sensitivity in adolescent and adult rats. Alcohol, 48, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L et al. (2016) Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nature Communications, 7, 11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier N, Pottier G, Caille F, Coulon C, Goislard M, Jego B et al. (2020) Nalmefene alleviates the neuroimmune response to repeated binge-like ethanol exposure: a TSPO PET imaging study in adolescent rats. Addiction Biology, 26, e12962. [DOI] [PubMed] [Google Scholar]
- Townshend JM & Duka T (2005) Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcoholism, Clinical and Experimental Research, 29, 317–325. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT et al. (2017) Binge-like alcohol exposure during adolescence disrupts dopaminergic neurotransmission in the adult prelimbic cortex. Neuropsychopharmacology, 42, 1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL & Majewska AK (2010) Microglial interactions with synapses are modulated by visual experience. PLoS Biology, 8, e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Fujita Y,Tanaka T, Nakamura Y, Kikuta J, Ishii M et al. (2013) Layer V cortical neurons require microglial support for survival during postnatal development. Nature Neuroscience, 16, 543–551. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI & Spear LP (2004) Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcoholism, Clinical and Experimental Research, 28, 40–50. [DOI] [PubMed] [Google Scholar]
- Vetreno RP & Crews FT (2012) Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience, 226, 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP & Crews FT (2015) Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Frontiers in Neuroscience, 9, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP & Crews FT (2018) Adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons and neuroimmune activation are prevented by exercise and indomethacin. PLoS One, 13, e0204500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Lawrimore CJ, Rowsey PJ & Crews FT (2018) Persistent adult neuroimmune activation and loss of hippocampal neurogenesis following adolescent ethanol exposure: blockade by exercise and the anti-inflammatory drug indomethacin. Frontiers in Neuroscience, 12, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Patel Y, Patel U, Walter TJ & Crews FT (2017) Adolescent intermittent ethanol reduces serotonin expression in the adult raphe nucleus and upregulates innate immune expression that is prevented by exercise. Brain, Behavior, and Immunity, 60, 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Qin L & Crews FT (2013) Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiology of Diseases, 59, 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinader-Caerols C, Duque A, Montanes A & Monleon S (2017a) Blood alcohol concentration-related lower performance in immediate visual memory and working memory in adolescent binge drinkers. Frontiers in Psychology, 8, 1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinader-Caerols C, Talk A, Montanes A, Duque A & Monleon S (2017b) Differential effects of alcohol on memory performance in adolescent men and women with a binge drinking history. Alcohol and Alcoholism, 52, 610–616. [DOI] [PubMed] [Google Scholar]
- Vore AS, Barney TM, Gano A, Varlinskaya EI & Deak T (2021) Adolescent intermittent ethanol (AIE) produces sex specific alterations in adult neuroimmune gene expression and ethanol sensitivity that are independent of ethanol metabolism. Neuropharmacology, 195, 108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T & Luciana M (2010) Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and Biobehavioral Reviews, 34, 631–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM & Ehlers CL (2009) Age-related differences in the blood alcohol levels of Wistar rats. Pharmacology, Biochemistry and Behavior, 91, 560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ & Crews FT (2017) Microglial depletion alters the brain neuroimmune response to acute binge ethanol withdrawal. Journal of Neuroinflammation, 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter TJ, Vetreno RP & Crews FT (2017) Alcohol and stress activation of microglia and neurons: brain regional effects. Alcoholism, Clinical and Experimental Research, 41, 2066–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chu G, Yang Z, Sun Y, Zhou H, Li M et al. (2015) Ethanol directly induced HMGB1 release through NOX2/NLRP1 inflammasome in neuronal cells. Toxicology, 334, 104–110. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, De Witte P et al. (2009a) Neuro-inflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. Journal of Neurochemistry, 111, 1119–1128. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Lallemand F, De Witte P & Dexter DT(2008) Neurochemical pathways involved in the protective effects of nicotine and ethanol in preventing the development of Parkinson's disease: potential targets for the development of new therapeutic agents. Progress in Neurobiology, 85, 135–147. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Lallemand F & De Witte P (2009b) Biochemical and neurotransmitter changes implicated in alcohol-induced brain damage in chronic or ‘binge drinking’ alcohol abuse. Alcohol and Alcoholism, 44, 128–135. [DOI] [PubMed] [Google Scholar]
- Warden AS, Wolfe SA, Khom S, Varodayan FP, Patel RR, Steinman MQ et al. (2020) Microglia control escalation of drinking in alcohol-dependent mice: genomic and synaptic drivers. Biological Psychiatry, 88, 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MR, James K, Mittleman G & Matthews DB (2020) Impact of acute ethanol exposure on body temperatures in aged, adult and adolescent male rats. Alcohol, 82, 81–89. [DOI] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Tracey KJ, Watkins LR & Maier SF (2015) Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. Journal of Neuroscience, 35, 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeln AC, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G et al. (2018) Innate immune memory in the brain shapes neurological disease hallmarks. Nature, 556, 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED & Swartzwelder HS (2000) Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcoholism, Clinical and Experimental Research, 24, 1251–1256. [PubMed] [Google Scholar]
- White AM, Kraus CL, Flom JD, Kestenbaum LA, Mitchell JR, Shah K et al. (2005) College students lack knowledge of standard drink volumes: implications for definitions of risky drinking based on survey data. Alcoholism, Clinical and Experimental Research, 29, 631–638. [DOI] [PubMed] [Google Scholar]
- White AM & Swartzwelder HS (2004) Hippocampal function during adolescence: a unique target of ethanol effects. Annals of the New York Academy of Sciences, 1021, 206–220. [DOI] [PubMed] [Google Scholar]
- White HR, Marmorstein NR, Crews FT, Bates ME, Mun EY & Loeber R (2011) Associations between heavy drinking and changes in impulsive behavior among adolescent boys. Alcoholism, Clinical and Experimental Research, 35, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R et al. (2017) A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO Journal, 36, 3292–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooden JI Thompson KR Guerin SP Nawarawong NN & Nixon K (2021) Consequences ofadolescent alcohol use on adult hippocampal neurogenesis and hippocampal integrity. In: Bell RL & Rahman S (Eds.) Effects of peri-Adolescent Licit and Illicit Drug Use on the Developing CNS, International Review of Neurobiology. https://www.sciencedirect.com/bookseries/international-review-of-neurobiology/chapters-in-press. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2018) Global status report on alcohol and health 2018: executive summary. Geneva: World Health Organization. [Google Scholar]
- Wu Y, Dissing-Olesen L, Macvicar BA & Stevens B (2015) Microglia: dynamic mediators of synapse development and plasticity. Trends in Immunology, 36, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QS, Feng MJ & Yan SE (2005) Different expression of brain-derived neurotrophic factor in the nucleus accumbens of alcohol-preferring (P) and -nonpreferring (NP) rats. Brain Research, 1035, 215–218. [DOI] [PubMed] [Google Scholar]
- Yang H & Tracey KJ (2010) Targeting HMGB1 in inflammation. Biochimica et Biophysica Acta, 1799, 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Darna M, Beckmann JS, Dwoskin LP & Bardo MT (2016) Individual differences in impulsive action and dopamine transporter function in rat orbitofrontal cortex. Neuroscience, 313, 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Marusich JA, Gipson CD, Beckmann JS & Bardo MT (2012) High impulsivity in rats predicts amphetamine conditioned place preference. Pharmacology, Biochemistry and Behavior, 100, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Luong R, Sullivan EV & Pfefferbaum A (2010) Measurement of serum, liver, and brain cytokine induction, thiamine levels, and hepatopathology in rats exposed to a 4-day alcohol binge protocol. Alcoholism, Clinical and Experimental Research, 34, 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F et al. (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature Neuroscience, 17, 400–406. [DOI] [PubMed] [Google Scholar]
- Zou J & Crews FT (2012) Inflammasome-IL-1beta signaling mediates ethanol inhibition of hippocampal neurogenesis. Frontiers in Neuroscience, 6, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]