Abstract
Current guidelines recommend restricting acetaminophen (APAP) use in patients with cirrhosis, but evidence to support that recommendation is lacking. Prior studies focused on pharmacokinetics (PK) of APAP in cirrhosis but did not rigorously examine clinical outcomes, sensitive biomarkers of liver damage, or serum APAP‐protein adducts, which are a specific marker of toxic bioactivation. Hence, the goal of this pilot study was to test the effects of regularly scheduled APAP dosing in a well‐defined compensated cirrhosis group compared to control subjects without cirrhosis, using the abovementioned outcomes. After a 2‐week washout, 12 subjects with and 12 subjects without cirrhosis received 650 mg APAP twice per day (1.3 g/day) for 4 days, followed by 650 mg on the morning of day 5. Patients were assessed in‐person at study initiation (day 1) and on days 3 and 5. APAP‐protein adducts and both conventional (alanine aminotransferase) and sensitive (glutamate dehydrogenase [GLDH], full‐length keratin 18 [K18], and total high‐mobility group box 1 protein) biomarkers of liver injury were measured in serum on the mornings of days 1, 3, and 5, with detailed PK analysis of APAP, metabolites, and APAP‐protein adducts throughout day 5. No subject experienced adverse clinical outcomes. GLDH and K18 were significantly different at baseline but did not change in either group during APAP administration. In contrast, clearance of APAP‐protein adducts was dramatically delayed in the cirrhosis group. Minor differences for other APAP metabolites were also detected. Conclusion: Short‐term administration of low‐dose APAP (650 mg twice per day, <1 week) is likely safe in patients with compensated cirrhosis. These data provide a foundation for future studies to test higher doses, longer treatment, and subjects who are decompensated, especially in light of the remarkably delayed adduct clearance in subjects with cirrhosis.
The safety of acetaminophen (APAP) use in cirrhosis is highly controversial. In this study, we compared novel, sensitive biomarkers of liver injury and pharmacokinetics between non‐cirrhotic and cirrhotic subjects with repeated APAP use over 5 days. There was no APAP‐induced liver damage in either group, but there was evidence of greater APAP‐protein binding and reduced APAP‐protein adduct clearance in the cirrhosis group that warrants caution and further study.
Abbreviations
- ALF
acute liver failure
- ALT
alanine aminotransferase
- APAP
acetaminophen
- APAP‐Gluc
acetaminophen‐glucuronide
- APAP‐Sulf
APAP‐sulfate
- AUC
area under the curve
- BMI
body mass index
- CAP
controlled attenuation parameter
- CLD
chronic liver disease
- Cmax
serum concentration maximum
- E
elastic modulus
- ELISA
enzyme‐linked immunosorbent assay
- GLDH
glutamate dehydrogenase
- GSH
glutathione
- HMGB1
high‐mobility group box 1 protein
- HPLC‐EC
high‐pressure liquid chromatography with electrochemical detection
- K18
keratin 18
- LC‐MS/MS
liquid chromatography with tandem mass spectrometry
- NAPQI
N‐acetyl‐p‐benzoquinone imine
- PK
pharmacokinetics
- t1/2
terminal‐phase elimination serum half‐life
- Tmax
time to maximum serum concentration
- UMAS
University of Arkansas for Medical Sciences
Acetaminophen (APAP) is the most commonly used analgesic in the United States and throughout much of the world. However, overdose of APAP causes massive centrilobular hepatocyte necrosis that can lead to acute liver failure (ALF) and death. It is overwhelmingly the most common cause of drug‐induced ALF in the United States and similarly developed countries, accounting for at least 45%‐65% of all cases( 1 ) and possibly even more that go undiagnosed.( 2 ) Although mortality is generally lower in APAP‐induced ALF compared to other etiologies, the large number of cases makes APAP overdose the most common cause of ALF‐related deaths as well.
Chronic liver disease (CLD) and cirrhosis are enormous global public health problems. Roughly 1.5 billion people suffer from some form of CLD.( 3 , 4 ) Determining the prevalence of cirrhosis specifically is more challenging due to limited data from many countries, but estimates from North America and Europe range from 270 to 1,100 per 100,000 population or roughly 15 million to 60 million when extrapolated to all adults worldwide.( 3 ) Pain is a frequent complaint among patients with CLD and cirrhosis,( 5 , 6 , 7 ) and managing their pain is challenging due to unique adverse events associated with analgesics in patients with cirrhosis. Nonsteroidal anti‐inflammatory drugs can compromise renal function and thereby worsen portal hypertension, leading to complications of cirrhosis, such as variceal and mucosal bleeding,( 8 , 9 ) while opioids can precipitate or exacerbate hepatic encephalopathy.( 10 , 11 ) APAP is the last major option, but many clinicians worry that patients with underlying liver disease are more susceptible to APAP hepatotoxicity. As a result, published recommendations suggest reducing the dose of APAP given to patients with cirrhosis.( 12 , 13 , 14 , 15 ) Consistent with that recommendation, survey studies have revealed that the vast majority (>80%) of physicians recommend reducing APAP dose in patients with compensated cirrhosis and completely avoiding it in the context of decompensated cirrhosis.( 16 )
Although the practice of limiting APAP use in patients with CLD or cirrhosis is widespread, it is highly controversial. Most studies to date have focused on the pharmacokinetics (PK) of APAP in patients with CLD or cirrhosis after a single dose.( 17 ) These studies have revealed delayed APAP clearance in patients with CLD that may lead to accumulation of the drug and greater risk of hepatotoxicity. On the other hand, administration of APAP to patients with cirrhosis over a 13‐day period did not result in alteration of aminotransferases in one seminal study that has become a classic in the field.( 18 )
We undertook a pilot study to begin to address this discrepancy. We performed detailed analyses of advanced, sensitive, liver‐injury biomarkers as well as the PK of APAP, the major phase II APAP metabolites, and APAP‐protein adducts (a highly specific and sensitive marker of phase I oxidative APAP metabolism) in patients with compensated cirrhosis and in noncirrhotic controls. These two groups were administered therapeutic doses of APAP over a 5‐day period. We included both conventional (alanine aminotransferase [ALT]) and more sensitive (keratin 18 [K18], total high mobility group box 1 protein [HMGB1], and glutamate dehydrogenase [GLDH]) serum biomarkers of liver injury.
Patients and Methods
Patient Selection and Safety
This was a prospective, parallel, controlled study to assess the safety of short‐term low‐dose APAP use by patients with cirrhosis. Patients with compensated cirrhosis were recruited from the outpatient Gastroenterology Clinic at the University of Arkansas for Medical Sciences (UAMS) in Little Rock, AR. Inclusion criteria were individuals 18‐65 years of age, a body mass index (BMI) of 18 to 45, and a diagnosis of cirrhosis with confirmation by biopsy or transient elastography within the past 2 years. Those with evidence of decompensation or severe portal hypertension (jaundice, ascites, variceal bleeding, or hepatic encephalopathy) were excluded. Noncirrhotic control volunteers were recruited from the UAMS community using the same inclusion criteria except that those with a history of CLD and/or evidence of advanced (metavir stage ≥3) fibrosis were excluded. All subjects were informed of the study purpose and risks and signed a consent form before participation. For each subject, a brief history was obtained and a physical examination was performed to collect data regarding age, sex, race, ethnicity, BMI, and current medication use. The study was approved by the UAMS Institutional Review Board and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
APAP Administration Protocol
The study protocol is shown as a schematic in Fig. 1. Subjects were required to abstain from APAP use for 2 weeks before study initiation and to abstain from alcohol use for the duration of the study. On the morning of study day 1, subjects received a childproof medication bottle containing 18 APAP tablets (325 mg each) and a medication diary and were instructed to take two tablets in the morning and two in the evening (approximately 12 hours apart) each day for 4 days. The total daily dose was 1.3 g. Most published recommendations recommend limiting APAP dose to 2‐3 g/day in patients with cirrhosis,( 12 , 13 , 14 ) but some recommend ≤2 g/day.( 15 ) We selected 1.3 g/day for this pilot study to maintain consistency with these recommendations and to minimize risk to our subjects. The subjects were required to record the time of dosing in a medication diary, and dosing compliance was verified by review of medication diaries and pill counts at each study visit. A blood sample was collected before the first APAP dose on day 1 and again before dosing on the mornings of days 3 and 5 to measure liver injury biomarkers. On the morning of day 5 after an overnight fast, subjects returned to the study site where a peripheral venous line was placed for serial blood sampling and two 325‐mg APAP tablets were administered orally under the supervision of study personnel. Blood was collected in red‐top serum tubes immediately before dosing (0 hours) and at 0.25, 0.5, 1, 2, 4, 8, 12, and 24 hours after APAP for detailed PK analysis. The samples were allowed to clot before centrifugation at 1,500g and 4°C for 5 minutes to separate serum from cells. Twelve‐hour total urine specimens were also collected for measurement of major APAP metabolites, and total urine volume was recorded.
FIG. 1.
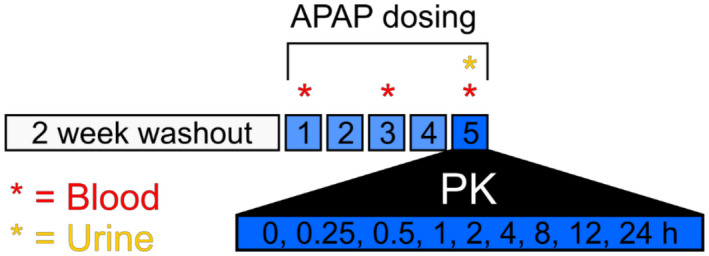
Study protocol. Schematic of the study design. Further details can be found in the Patients and Methods section.
Biomarkers of Liver Injury
All samples were collected and analyzed as a batch for measurement of liver‐injury biomarkers. ALT was measured using reagents from MedTest Dx (Canton, MI). Full‐length K18 was measured using an enzyme‐linked immunosorbent assay (ELISA) kit from Diapharma (West Chester, OH). HMGB1 was measured using an ELISA kit from G‐Biosciences (St. Louis, MO). GLDH was measured using a kinetic assay, as described.( 19 ) All biomarkers were measured according to the manufacturer’s instructions, using a 96‐well ultraviolet/visible plate reader from Molecular Devices (San Jose, CA).
APAP‐Protein Adducts
Protein‐derived APAP‐cysteine was measured by high‐pressure liquid chromatography with electrochemical detection (HPLC‐EC), as described,( 20 ) with modifications. Briefly, 100 μL of serum was filtered through a size‐exclusion column to remove APAP, free APAP‐cysteine, and other small molecules with potential to interfere in the assay. The filtrate was then incubated with a mixture of proteases at 50°C for 1 hour to liberate APAP‐cysteine. The proteases were then precipitated by mixing the sample 1:1 with 40% trichloroacetic acid, and the precipitate was pelleted by centrifugation. The supernatant was then filtered through a 0.22‐μm‐cut‐off membrane to ensure complete removal of the protease protein pellet. Finally, APAP‐cysteine was separated by HPLC with a reverse‐phase C18 column and detected by oxidation at 155 and 280 mV.
APAP and Major APAP Metabolites
APAP, APAP‐glucuronide (APAP‐Gluc), and APAP‐sulfate (APAP‐Sulf) were measured using HPLC with detection by triple quadrupole tandem mass spectrometry (LC‐MS/MS). Briefly, standards and controls were prepared in charcoal‐stripped human plasma with certified reference solutions. Calibration curves were established over the range 250‐10,000 ng/mL (APAP, APAP‐Sulf) and 250‐35,000 ng/mL (APAP‐Gluc), with N‐(4‐hydroxyphenyl‐2,3,5,6‐d4)‐acetamide (D4‐APAP) used as the internal standard. We extracted 50‐µL aliquots of standards, controls, and samples by a liquid–liquid protocol. All reagents were prepared with type 1 water and LC‐MS‐grade chemicals and solvents, as appropriate. Analysis was performed on an Agilent 6920 Triple Quadrupole LC/MS, and data analysis was accomplished with Agilent MassHunter software, version B.08.00 (Santa Clara, CA).
PK Analysis
APAP and APAP metabolites serum concentration versus time data from each patient were analyzed by noncompartmental analysis in WinNonlin Phoenix software (version 8.3; Certara, Princeton, NJ), and PK parameters (e.g., serum concentration maximum [Cmax], time to Cmax [Tmax], area under the curve [AUC], total body serum clearance, terminal‐phase elimination serum half‐life [t1/2], and volume of distribution [Vd]) were determined.
Statistical Methods
Medians and interquartile ranges were used to summarize continuous demographic, PK, and daily measures of the study groups. Wilcoxon rank‐sum tests were used to compare the demographics, PK parameters, and daily biomarker data among the cirrhotic and noncirrhotic groups. Biomarker time profiles were compared using nonparametric longitudinal data analysis methods as described by Brunner et al.( 21 ) Strengths of association between PK parameters and other values were estimated using Kendall’s tau correlation coefficient. Because this was a pilot study, no adjustments for multiple comparisons were performed. We considered P < 0.05 indicative of associations or differences worthy of further study.
Results
Patient Demographics
Patient demographics, clinical laboratory values, and concurrent medication use are shown in Tables 1, 2, 3. All subjects in the compensated cirrhosis group had a current diagnosis with recent confirmation by biopsy or transient elastography and no evidence of decompensation, while the noncirrhotic group consisted of healthy volunteers with no history of liver disease. The most common etiology of cirrhosis was nonalcoholic steatohepatitis (42%), followed by primary biliary cholangitis (33%) (Table 2). Two subjects (17%) had a history of hepatitis C virus infection (1 with a concomitant history of alcohol abuse), and 1 (8%) had a history of alcohol abuse only. All subjects were required to abstain from alcohol use for the duration of the study. There were no significant differences in demographics or polypharmacy rates between the noncirrhotic and cirrhotic groups, except that mean age was higher (P = 0.012) in the subjects with cirrhosis compared to the noncirrhotic controls (Table 1).
TABLE 1.
Subject demographics
| Group | n | Age, years (Median, Range) | Sex (F/M) | Race | BMI (Median, Range) |
|---|---|---|---|---|---|
| No cirrhosis | 12 | 40, 22‐61 | 7/5 | White, non‐Hispanic 10 | 29.5, 22.5‐41.3 |
| White, Hispanic 0 | |||||
| Black 2 | |||||
| Cirrhosis | 12 | 60, 44‐ 66 | 6/6 | White, non‐Hispanic 11 | 33.3, 24.6‐44.6 |
| White, Hispanic 1, | |||||
| Black 0 |
Abbreviations: F, female; M, male.
TABLE 2.
Detailed information for subjects with cirrhosis
| Subject | Etiology | CTP | MELD‐Na | ALT (U/L) | TBili (mg/dL) | Serum Cre (mg/dL) |
|---|---|---|---|---|---|---|
| 1 | NASH | 5 (A) | 10 | 28 | 2.0 | 0.8 |
| 2 | NASH | 6 (A) | 11 | 25 | 1.1 | 1.7 |
| 3 | NASH | 5 (A) | 7 | 15 | 0.6 | 1.5 |
| 4 | HCV/alcohol | 5 (A) | 8 | 16 | 0.8 | 1.1 |
| 5 | NASH | 7 (B) | 14 | 23 | 1.1 | 1.1 |
| 6 | AIH/PBC | 5 (A) | 8 | 11 | 0.8 | 0.9 |
| 7 | Alcohol | 6 (A) | 12 | 35 | 1.1 | 1.0 |
| 8 | HCV | 5 (A) | 7 | 17 | 0.7 | 1.1 |
| 9 | PBC | 5 (A) | 8 | 21 | 0.9 | 0.9 |
| 10 | NASH | 5 (A) | 7 | 20 | 1.6 | 0.8 |
| 11 | PBC | 5 (A) | 6 | 38 | 0.5 | 1.3 |
| 12 | PBC | 6 (A) | 7 | 25 | 0.6 | 1.2 |
Abbreviations: AIH, autoimmune hepatitis; CTP, Child‐Turcotte‐Pugh; HCV, hepatitis C virus; MELD‐Na, Model for End‐Stage Liver Disease with sodium; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cholangitis; Serum Cre, serum creatinine; TBili, total bilirubin.
TABLE 3.
Prevalence of concurrent medication use by class
| Drug or Class | No Cirrhosis | Cirrhosis |
|---|---|---|
| ACE‐I | 0/12 | 2/12 |
| Alpha‐blocker | 0/12 | 2/12 |
| Analgesic (non‐APAP) | 1/12 | 2/12 |
| Antibiotic | 1/12 | 0/12 |
| Antihistamine | 1/12 | 2/12 |
| ARB | 1/12 | 2/12 |
| Beta‐blocker | 1/12 | 1/12 |
| Ca2+ channel blocker | 1/12 | 2/12 |
| Immunosuppressant | 0/12 | 1/12 |
| Levothyroxine | 0/12 | 3/12 |
| Metformin | 0/12 | 3/12 |
| Proton‐pump inhibitor | 0/12 | 1/12 |
| Sulfonylurea | 0/12 | 2/12 |
| SSRI/SNRI | 5/12 | 3/12 |
| Statin | 1/12 | 5/12 |
| Ursodiol | 0/12 | 4/12 |
| Vitamin supplement | 2/12 | 3/12 |
| % taking other medications | 58% | 100% |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; SNRI, serotonin‐norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Effect of APAP on Conventional and Advanced Biomarkers of Liver Injury
To explore the safety of regularly dosed APAP in patients with cirrhosis, we administered 1.3 g APAP per day to the subjects with cirrhosis and the noncirrhotic controls and measured both conventional (ALT) and more sensitive (full‐length K18, total HMGB1, GLDH) serum biomarkers of liver injury before (day 1) and during (days 3 and 5) APAP use. The latter biomarkers were chosen because they have been shown to be earlier more sensitive markers of drug‐induced liver injury than ALT.( 22 , 23 ) Importantly, while baseline ALT values were similar between subjects with cirrhosis and noncirrhotic controls overall (Fig. 2A), both GLDH and K18 were consistently higher in the cirrhosis group (Fig. 2B,C). These data are consistent with earlier work showing improved sensitivity of these novel biomarkers to detect liver damage compared to ALT. However, no changes were detected over time during APAP dosing for any of the biomarkers in either group (Fig. 2A‐D), confirming that short‐term repeated dosing with APAP does not result in biomarker evidence of liver injury in subjects with cirrhosis.
FIG. 2.
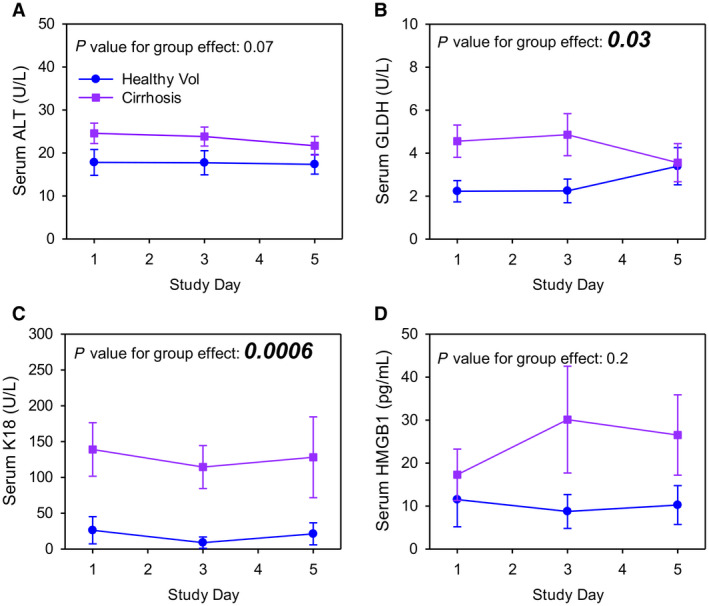
Liver injury biomarkers. ALT, GLDH, K18, and HMGB1 were measured in serum from the morning of days 1, 3, and 5 by kinetics or ELISA. (A) Serum ALT. (B) Serum GLDH. (C) Serum K18. (D) Serum HMGB1. Data are expressed as mean ± standard error for n = 12 per group. P values for group effects are shown in each panel. No significant differences were found within groups over time. Abbreviation: Vol, volunteer.
PK of APAP in Subjects With Cirrhosis and Noncirrhotic Controls
To determine if there were major differences between groups in the PK of APAP and the major phase II metabolites APAP‐Gluc and APAP‐Sulf, we performed a detailed PK analysis after the final dose of APAP on day 5. The mean time course data are shown in Fig. 3, and comparisons of the resulting PK parameters are displayed in Table 4. The median time to peak APAP‐Gluc (Tmax) was delayed 2 hours in the cirrhosis group compared to the controls, while the t1/2 of APAP‐Sulf was prolonged 0.23 hours. APAP‐Gluc t1/2 also displayed a trend toward prolongation. No other differences in PK parameters for these metabolites were observed. Overall, these data indicate moderately impaired formation and clearance of phase II metabolites in the cirrhosis group. Despite this, there were no differences in overall phase II metabolite distribution in 12‐hour urine samples between the cirrhotic and noncirrhotic groups (Fig. 3E,F).
FIG. 3.
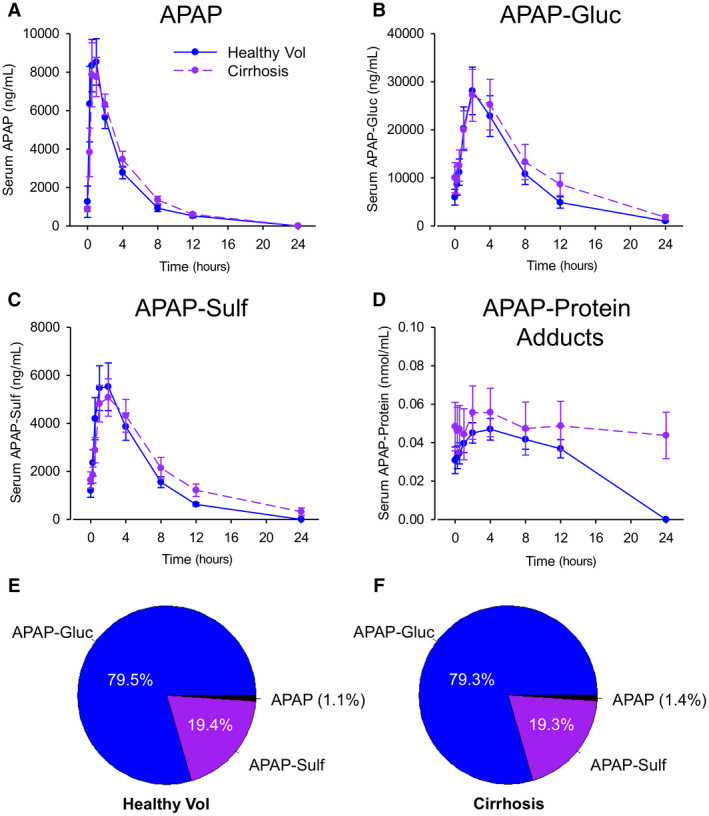
PK and excretion of APAP, major APAP metabolites, and APAP‐protein adducts. Parent APAP, APAP‐Gluc, APAP‐Sulf, and APAP‐protein adducts were measured in serial blood samples after the final dose of APAP on day 5 by LC‐MS/MS or HPLC‐EC. (A) Serum APAP. (B) Serum APAP‐Gluc. (C) Serum APAP‐Sulf. (D) Serum APAP‐protein adducts. (E) Fractional urinary excretion of APAP, APAP‐Gluc, and APAP‐Sulf in control subjects without cirrhosis. (F) Fractional urinary excretion of APAP, APAP‐Gluc, and APAP‐Sulf in subjects with cirrhosis. Data are expressed as mean ± standard error for n = 12 per group. PK parameters with P values are displayed in Table 4. Abbreviation: Vol, volunteer.
TABLE 4.
Summary of PK data for APAP and APAP metabolites
| Variable | PK Parameter | Controls | Cirrhosis | P Value |
|---|---|---|---|---|
| Median (IQR) | Median (IQR) | |||
| APAP | t1/2 (hours) | 2.69 (2.45, 3.08) | 2.88 (2.68, 3.15) | 0.3405 |
| AUC (×103) (hours × ng/mL) | 30.55 (25.31, 35.48) | 35.53 (26.14, 44.20) | 0.4428 | |
| Cmax (×103) (ng/mL) | 8.97 (7.71, 13.24) | 9.22 (7.78, 11.03) | 1.0000 | |
| Tmax (hours) | 0.75 (0.50, 1.00) | 1.00 (0.50, 1.00) | 0.5170 | |
| APAP‐Gluc | t1/2 (hours) | 3.99 (3.31, 4.42) | 4.77 (4.19, 7.47) | 0.0804 |
| AUC (×103) (hours × ng/mL) | 224.86 (72.30, 351.94) | 245.89 (104.71, 428.30) | 0.1432 | |
| Cmax (×103) (ng/mL) | 27.65 (12.89, 37.65) | 29.18 (9.97, 40.26) | 0.9323 | |
| Tmax | 2.00 (2.00, 2.50) | 4.00 (2.00, 4.00) | 0.0336* | |
| APAP‐Sulf | t1/2 (hours) | 3.40 (3.03, 3.74) | 4.03 (3.54, 4.54) | 0.0215* |
| AUC (×103) (hours × ng/mL) | 0.62 (0.49, 0.75) | 0.67 (0.49, 0.92) | 0.7508 | |
| Cmax (×103) (ng/mL) | 5.17 (3.75, 7.70) | 4.59 (3.26, 7.08) | 0.7125 | |
| Tmax (hours) | 1.50 (1.00, 2.00) | 2.00 (1.00, 2.00) | 0.3018 |
Statistically significant.
Abbreviation: IQR, interquartile range.
In contrast to the phase II metabolites, there was a striking difference in serum APAP‐protein adducts between groups (Fig. 3D). Not all subjects had detectable APAP‐protein adducts in serum during the PK study. This between‐ and within‐subject variation in APAP‐protein adducts was expected as prior studies have demonstrated considerable variation in serum adducts at therapeutic doses, even when the APAP dose was 3‐fold greater than what we used.( 24 , 25 ) Only 7‐8 subjects in each group had detectable APAP‐protein adducts in serum on the morning of day 5, before the PK study. More subjects (6‐10 per group) had detectable adducts in a sufficient number of samples to accurately estimate t1/2 and AUC, and even more (9‐11 per group) had detectable adducts in a sufficient number of samples to estimate Cmax. The results for each individual subject are displayed in Fig. 4, with analysis of the subjects with detectable adducts in Fig. 5. The latter revealed that morning day‐5 adduct levels (before the start of the PK study) and adduct Cmax values were greater in subjects with cirrhosis (Fig. 5A,B) and that clearance was dramatically delayed in the subjects with cirrhosis compared to the noncirrhotic controls (Fig. 5C,D). This is concerning because recent data from rodent studies have revealed that the accumulation of these adducted proteins in hepatocytes due to impaired autophagy or multiple dosing contributes to the pathophysiology of APAP hepatotoxicity.( 26 , 27 ) Glutathione (GSH) is an antioxidant and nucleophile that has a critical function as a scavenger of N‐acetyl‐p‐benzoquinone imine (NAPQI), the reactive metabolite of APAP, to prevent it from binding to proteins, and there is evidence that systemic GSH concentrations decrease with increasing age.( 28 , 29 , 30 ) Thus, to determine if the increase in day‐5 adducts and Cmax could be a result of reduced GSH levels in the older cirrhosis group, we tested for correlations between day‐5 adducts or Cmax and age. Neither day‐5 adducts nor Cmax correlated significantly with age for all subjects (r 2 = 0.02 and −0.40 for day‐5 adducts and Cmax, respectively; P > 0.05) or in the cirrhosis group alone (r 2 = 0.11 and 0.00037, respectively; P > 0.05).
FIG. 4.
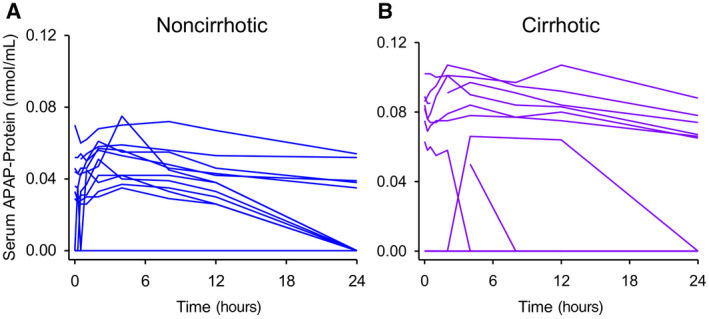
Time courses of serum APAP‐protein adducts in individual subjects. Serum APAP‐protein adducts were measured in serial blood samples collected after the final dose of APAP on day 5 using HPLC‐EC. Each line represents 1 individual. (A) Control subjects without cirrhosis. (B) Subjects with cirrhosis.
FIG. 5.
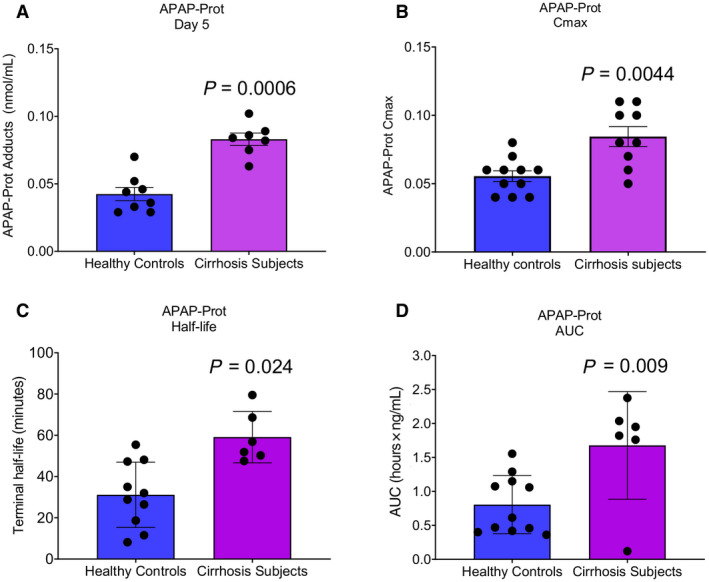
PK of serum APAP‐protein adducts in subjects with cirrhosis and noncirrhotic controls. Serum APAP‐protein adducts were measured in serial blood samples collected after the final dose of APAP on day 5 using HPLC‐EC. (A) Starting day‐5 APAP‐protein adducts in serum. (B) APAP‐protein adducts Cmax on day 5. (C) Half‐life of APAP‐protein adducts. (D) AUC of APAP‐protein adducts. Data are expressed as mean ± SE. Dots show individual data points as described in the text. *P < 0.05 versus noncirrhotic controls.
To identify other factors that may be associated with altered APAP metabolism, especially delayed adduct clearance, in patients with cirrhosis, we performed a secondary analysis of correlations between PK parameters, age, BMI, and concomitant medication use as well as elastic modulus (E) and controlled attenuation parameter (CAP) from transient elastography (Table 5). E and CAP are well‐validated and widely used measures of liver stiffness/fibrosis and hepatic fat content, respectively.( 31 , 32 , 33 ) Due to our relatively small sample size, only correlation coefficients >|0.6| were considered to be of interest for future investigation. Based on that cutoff, no strong correlations were observed between PK parameters for APAP, APAP‐Gluc, and APAP‐Sulf and either age or BMI, nor were there any clear relationships with concurrent medication use. However, APAP Tmax increased with increasing liver stiffness, while Tmax for both APAP and APAP‐Sulf decreased with increasing liver fat. Only 3 patients had both detectable adducts and available transient elastography data, which prevented correlation analysis of those factors. However, there was a modest positive correlation (0.69) between age and adduct clearance, indicating that age could be a contributor to the prolonged APAP‐protein t1/2 observed in the cirrhosis group.
TABLE 5.
Kendall’s tau correlation coefficients
| Variable | Age* | BMI† | E † | CAP † |
|---|---|---|---|---|
| APAP t1/2 | 0.1563 | 0.0909 | 0.6000 | −0.3333 |
| APAP AUC | 0.0625 | −0.5636 | 0.3333 | −0.3333 |
| APAP Cmax | −0.0313 | −0.3091 | −0.3333 | 0.3333 |
| APAP Tmax | 0.1601 | −0.3252 | 0.7877 ‡ | −0.9309 ‡ |
| APAP‐Gluc t1/2 | 0.0920 | 0.2222 | 0.6000 | −0.2000 |
| APAP‐Gluc AUC | 0.2189 | −0.0182 | −0.4667 | 0.2000 |
| APAP‐Gluc Cmax | 0.0313 | −0.2000 | −0.3333 | 0.0667 |
| APAP‐Gluc Tmax | 0.2644 | −0.2180 | 0.7006 | −0.3892 |
| APAP‐Sulf t1/2 | 0.2832 | −0.2000 | 0.0667 | 0.2000 |
| APAP‐Sulf AUC | 0.0158 | 0.0545 | −0.4667 | 0.2000 |
| APAP‐Sulf Cmax | 0.1563 | −0.2727 | −0.6000 | 0.3333 |
| APAP‐Sulf Tmax | 0.3218 | 0.1140 | 0.6025 ‡ | −0.7746 ‡ |
| APAP‐Prot t1/2 | 0.6901 ‡ | 0.0667 | NA | NA |
| APAP‐Prot AUC | −0.4140 | −0.2000 | NA | NA |
| APAP‐Prot Cmax | −0.3706 | −0.3581 | NA | NA |
| APAP‐Prot Tmax | −0.1612 | 0.2335 | NA | NA |
n = 12.
n = 7.
Statistically significant.
Abbreviations: APAP‐Prot, acetaminophen‐protein adducts; NA, not available.
Discussion
The safety of APAP in cirrhosis is highly controversial. On one hand, most physicians recommend that their patients with CLD and cirrhosis either restrict APAP use or avoid it altogether.( 16 ) These recommendations are based in part on reports of transient nonprogressive ALT elevations in healthy volunteers taking APAP,( 34 ) the rationale being that this mild liver damage may be worse or may itself worsen the underlying disease in patients with preexisting CLD. On the other hand, expression of the cytochromes P450 that convert APAP to NAPQI, a necessary step for the initiation of APAP toxicity,( 35 , 36 ) appears to be suppressed in some patients with CLD.( 37 , 38 ) Furthermore, the few studies of APAP safety in CLD that have been done to date have failed to find evidence of increased susceptibility to APAP toxicity.( 17 ) Unfortunately, most of those studies suffer from significant methodological limitations.
Most prior studies of the safety of APAP in CLD or cirrhosis have focused on APAP PK after a single dose, with the major analytes being serum and urine APAP and phase II metabolites.( 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ) Only three have included repeated dosing with monitoring of liver injury using ALT and liver function tests (e.g., prothrombin time and serum albumin), and only two of those have been published.( 18 , 39 ) In all three studies, no longitudinal changes in ALT or other test values were observed during the treatment period. Since these studies were done, however, more sensitive biomarkers of APAP hepatotoxicity have become available. For example, Dear et al.( 22 ) recently reported that total HMGB1, full‐length K18, and GLDH are elevated in patients with early presenting APAP overdose before an increase in ALT and that they predict later liver injury. To further test if cirrhosis increases susceptibility to APAP‐induced liver injury during short‐term scheduled dosing, we included those biomarkers in our study. Although we observed higher baseline values for GLDH and K18 in the patients with cirrhosis, consistent with the greater sensitivity of these biomarkers for liver damage, there was no increase over time during the APAP dosing period. Overall, these data are consistent with the earlier studies indicating that short‐term use of low‐dose APAP is safe for patients with cirrhosis. In addition, the elevated GLDH in cirrhosis may indicate that mitochondrial dysfunction occurs in patients with cirrhosis as there is evidence that serum GLDH is specific for mitochondrial damage.( 19 , 49 )
The most common PK findings from the prior single‐dose studies in patients with CLD have been prolonged t1/2 and greater AUC for the parent drug,( 39 , 43 , 44 , 46 , 47 ) indicating reduced overall metabolism and clearance of APAP in liver disease. Consistent with that, several studies have also found that the proportion of APAP excreted unchanged is increased in patients with CLD.( 40 , 43 , 46 ) Although we could not detect a difference in APAP t1/2 or excreted APAP, we did observe minor changes in the PK of both APAP‐Gluc and APAP‐Sulf. The former may reflect less severe disease in our CLD cohort compared to earlier publications as we included only patients with compensated cirrhosis. Consistent with that, another study found that APAP t1/2 was unaffected by mild CLD despite being significantly prolonged in patients with severe CLD.( 43 ) The differences in PK in our study compared to earlier reports may also be due to differences in etiology of the liver injury. Prior studies have included mostly patients with CLD secondary to viral hepatitis or alcohol abuse, while the dominant etiology of cirrhosis in our patients was nonalcoholic/metabolism‐associated fatty liver disease. On the other hand, the differences that we did observe (extended Tmax for APAP‐Gluc and prolonged t1/2 for APAP‐Sulf) may still reflect mildly impaired metabolism in our cirrhosis group. In addition, the positive correlation between liver stiffness and APAP t1/2 that we observed is consistent with prolonged t1/2 with more advanced disease. Overall, it is clear from our study and from prior work that phase II metabolism and clearance of APAP is moderately impaired in patients with CLD and cirrhosis, although the clinical consequences of those changes are less clear.
The most striking differences between the subjects with and without cirrhosis in this study were the elevated APAP‐protein adducts on day 5 and the dramatically delayed clearance of adducts in the subjects with cirrhosis. Only a few studies of APAP in CLD or cirrhosis have included measurement of APAP‐thiol conjugates as a marker of phase I oxidative metabolism, and none of these studies have detected significant changes.( 45 , 47 ) However, those studies included only free APAP‐cysteine and APAP‐mercapturate, measured only in urine. This is the first study to include measurement of serum APAP‐protein adducts, which form from the reactive metabolite of APAP (NAPQI) binding to free thiol groups on cysteine residues in proteins. The possibility of greater NAPQI formation in subjects with cirrhosis indicated by the higher levels in the first sample from day 5 is worrisome because NAPQI initiates APAP toxicity.( 35 ) Our observation that the t1/2 of serum APAP‐protein adducts was delayed in the cirrhosis group is also concerning. Not only does APAP‐protein binding initiate the hepatotoxicity of APAP by damaging proteins( 35 ) but the adducted proteins themselves seem to contribute to the toxicity. Recent studies have demonstrated that APAP‐protein adducts are cleared by autophagic degradation within hepatocytes and that blocking or enhancing autophagy increases or decreases, respectively, both hepatic and serum adduct levels.( 26 ) In addition, accumulation of protein adducts, either due to reduced autophagic clearance or repeated dosing, leads to greater injury.( 26 , 27 ) While it seems likely that Kupffer cells also contribute to clearance of adducts from serum, there is currently no evidence to support that hypothesis. Furthermore, although one might expect elimination by urine to be an additional route of adduct clearance at least for low molecular weight adducts, that does not appear to be the case. APAP‐protein adducts were undetectable in 70% of urine samples with matched serum containing high levels of adducts, and the urine adducts in the remaining 30% were likely derived from renal proximal tubule cells postglomerulus.( 50 ) This is consistent with the fact that glomerular filtration retains most serum proteins. Thus, degradation in the liver likely represents the major route of adduct clearance. We also observed a positive correlation between age and adduct t1/2 that requires further investigation. Although that association could partially explain the difference in adducts between our groups, considering the somewhat older age of the subjects with cirrhosis, this seems unlikely for two reasons. First, although our data set was limited, there was no correlation between age and either day‐5 morning APAP‐protein adducts or day‐5 Cmax for adducts. Second, prior studies have failed to find a positive association between age and oxidative APAP metabolism.( 51 , 52 ) Importantly, the differences in adducts between groups is probably not due to differences in comedications either as none of the reported comedications are metabolized by cytochrome P450 2E1 (CYP2E1; Supporting Table S1), which is the primary enzyme responsible for bioactivation of APAP to NAPQI. Taken together, our data indicate that while short‐term APAP use is likely safe in the context of cirrhosis, longer term use, especially at higher doses, may carry a greater risk of toxicity in patients with cirrhosis or possibly even advanced age. This needs to be carefully explored in future larger studies.
Overall, these pilot results provide a starting point toward a better understanding of the safe use of APAP in patients with cirrhosis. Based only on these data, short‐term use of low‐dose APAP in patients with compensated cirrhosis appears to be acceptable. However, no conclusions can be drawn yet regarding use of the standard full dose of APAP or long‐term APAP use in such patients, especially considering the delayed adduct clearance that we observed. Moreover, no conclusions can be drawn regarding use of any dose of APAP in patients with decompensated cirrhosis based on these data alone. We intend to explore those questions in the future. Because tools to better answer them, such as more sensitive liver injury biomarkers and methods to measure serum APAP‐protein adducts, have recently become available, the imperative to address them in future studies is high.
Supporting information
Table S1
Acknowledgment
We are grateful to Cynthia L. Witkowski, R.N., and Heather L. Moody, R.N., of the UAMS Translational Research Institute for critical assistance with study subjects.
Present address for Davis P. Fleming is Department of Psychiatry and Behavioral Sciences, School of Medicine, University of Louisville, Louisville, KY.
Supported in part by the American Association for the Study of Liver Diseases Foundation (Pinnacle Research Award to M.R.M.), University of Arkansas for Medical Sciences Medical Research Endowment Fund (pilot award to M.R.M., L.P.J., J.A.D.), and National Institutes of Health (grants R42 DK079387 to L.P.J., UG1 OD024945 to L.P.J., UL1 TR003107 to L.P.J., and KL2 TR003108 to S.K.M.).
Potential conflict of interest: Dr. James is part owner of Acetaminophen Toxicity Diagnostics, LLC, which has a patent on a rapid assay for measuring acetaminophen adducts. Dr. McGill consults for Acetaminophen Toxicity Diagnostics, LLC, GlaxoSmithKline, and Alkermes Pharmaceuticals. Dr. Moran owns stock in and is employed by PinPoint Testing LLC. The other authors have nothing to report.
Contributor Information
Mitchell R. McGill, Email: mmcgill@uams.edu.
Jonathan A. Dranoff, Email: jadranoff@uams.edu.
References
Author names in bold designate shared co‐first authorship.
- 1. Stravitz RT, Lee WM. Acute liver failure. Lancet 2019;394:869‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ganger DR, Rule J, Rakela J, Bass N, Reuben A, Stravitz R, et al.; Acute Liver Failure Study Group . Acute liver failure of indeterminate etiology: a comprehensive systematic approach by an expert committee to establish causality. Am J Gastroenterol 2018;113:1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol 2020;18:2650‐2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2017 Cirrhosis Collaborators . The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:245‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whitehead AJ, Dobscha SK, Morasco BJ, Ruimy S, Bussell C, Hauser P. Pain, substance use disorders and opioid analgesic prescription patterns in veterans with hepatitis C. J Pain Symptom Manage 2008;36:39‐45. [DOI] [PubMed] [Google Scholar]
- 6. Rogal SS, Winger D, Bielefeldt K, Szigethy E. Pain and opioid use in chronic liver disease. Dig Dis Sci 2013;58:2976‐2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng JK, Hepgul N, Higginson IJ, Gao W. Symptom prevalence and quality of life of patients with end‐stage liver disease: a systematic review and meta‐analysis. Palliat Med 2019;33:24‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Lédinghen V, Mannant PR, Foucher J, Perault MC, Barrioz T, Ingrand P, et al. Non‐steroidal anti‐inflammatory drugs and variceal bleeding: a case‐control study. J Hepatol 1996;24:570‐573. [DOI] [PubMed] [Google Scholar]
- 9. Lee YC, Chang CH, Lin JW, Chen HC, Lin MS, Lai MS. Non‐steroidal anti‐inflammatory drugs use and risk of upper gastrointestinal adverse events in cirrhotic patients. Liver Int 2012;32:859‐866. [DOI] [PubMed] [Google Scholar]
- 10. Tapper EB, Henderson JB, Parikh ND, Ioannou GN, Lok AS. Incidence of and risk factors for hepatic encephalopathy in a population‐based cohort of Americans with cirrhosis. Hepatol Commun 2019;3:1510‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moon AM, Jiang Y, Rogal SS, Tapper EB, Lieber SR, Barritt AS 4th. Opioid prescriptions are associated with hepatic encephalopathy in a national cohort of patients with compensated cirrhosis. Aliment Pharmacol Ther 2020;51:652‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chandok N, Watt KDS. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc 2010;85:451‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dwyer JP, Jayasekera C, Nicoll A. Analgesia for the cirrhotic patient: a literature review and recommendations. J Gastroenterol Hepatol 2014;29:1356‐1360. [DOI] [PubMed] [Google Scholar]
- 14. Imani F, Motavaf M, Safari S, Alavian SM. The therapeutic use of analgesics in patients with liver cirrhosis: a literature review and evidence‐based recommendations. Hepat Mon 2014;14:e23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rakoski M, Goyal P, Spencer‐Safier M, Weissman J, Mohr G, Volk M. Pain management in patients with cirrhosis. Clin Liver Dis (Hoboken) 2018;11:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rossi S, Assis DN, Awsare M, Brunner M, Skole K, Rai J, et al. Use of over‐the‐counter analgesics in patients with chronic liver disease: physicians’ recommendations. Drug Saf 2008;31:261‐270. [DOI] [PubMed] [Google Scholar]
- 17. Schweighardt AE, Juba KM. A systematic review of the evidence behind use of reduced doses of acetaminophen in chronic liver disease. J Pain Palliat Care Pharmacother 2018;32:226‐239. [DOI] [PubMed] [Google Scholar]
- 18. Benson GD. Acetaminophen in chronic liver disease. Clin Pharmacol Ther 1983;33:95‐101. [DOI] [PubMed] [Google Scholar]
- 19. McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen‐induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest 2012;122:1574‐1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, et al. Determination of acetaminophen‐protein adducts in mouse liver and serum and human serum after hepatotoxlc doses of acetaminophen using high‐performance liquid chromatography with electrochemical detection. Drug Metab Dispos 2002;30:446‐451. [DOI] [PubMed] [Google Scholar]
- 21. Brunner E, Domhof S, Langer F, eds. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. New York, NY: J. Wiley; 2002. [Google Scholar]
- 22. Dear JW, Clarke JI, Francis B, Allen L, Wraight J, Shen J, et al. Risk stratification after paracetamol overdose using mechanistic biomarkers: results from two prospective cohort studies. Lancet Gastroenterol Hepatol 2018;3:104‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rupprechter SAE, Sloan DJ, Oosthuyzen W, Bachmann TT, Hill AT, Dhaliwal K, et al. MicroRNA‐122 and cytokeratin‐18 have potential as a biomarkers of drug‐induced liver injury in European and African patients on treatment for mycobacterial infection. Br J Clin Pharmacol 2021;87:3206‐3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heard K, Anderson VE, Lavonas EJ, Dart RC, Green JL. Serum paracetamol‐protein adducts in ambulatory subjects: relationship to recent reported paracetamol use. Biomarkers 2018;23:288‐292. [DOI] [PubMed] [Google Scholar]
- 25. Heard K, Green JL, Anderson V, Bucher‐Bartelson B, Dart RC. Paracetamol (acetaminophen) protein adduct concentrations during therapeutic dosing. Br J Clin Pharmacol 2016;81:562‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ni HM, McGill MR, Chao X, Du K, Williams JA, Xie Y, et al. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen‐induced liver injury in mice. J Hepatol 2016;65:354‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen NT, Akakpo JY, Weemhoff JL, Ramachandran A, Ding WX, Jaeschke H. Impaired protein adduct removal following repeat administration of subtoxic doses of acetaminophen enhances liver injury in fed mice. Arch Toxicol 2021;95:1463‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med 2000;28:625‐635. [DOI] [PubMed] [Google Scholar]
- 29. Giustarini D, Dalle‐Donne I, Lorenzini S, Milzani A, Rossi R. Age‐related influence on thiol, disulfide, and protein‐mixed disulfide levels in human plasma. J Gerontol A biol Sci Med Sci 2006;61:1030‐1038. [DOI] [PubMed] [Google Scholar]
- 30. Paredes J, Jones DP, Wilson ME, Herndon JG. Age‐related alterations of plasma glutathione and oxidation of redox potentials in chimpanzee (Pan troglodytes) and rhesus monkey (Macaca mulatta). Age (Dordr) 2014;36:719‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006;55:403‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTETM guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 2010;36:1825‐1835. [DOI] [PubMed] [Google Scholar]
- 33. de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non‐invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int 2012;32:911‐918. [DOI] [PubMed] [Google Scholar]
- 34. Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA 2006;296:87‐93. [DOI] [PubMed] [Google Scholar]
- 35. McGill MR, Hinson JA. The development and hepatotoxicity of acetaminophen: reviewing over a century of progress. Drug Metab Rev 2020;52:472‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther 1973;187:195‐202. [PubMed] [Google Scholar]
- 37. Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. Am J Ther 2005;12:133‐141. [DOI] [PubMed] [Google Scholar]
- 38. George J, Murray M, Byth K, Farrell GC. Differential alterations of cytochrome P450 proteins in livers from patients with severe chronic liver disease. Hepatology 1995;21:120‐128. [PubMed] [Google Scholar]
- 39. Andreasen PB, Hutters L. Paracetamol (acetaminophen) clearance in patients with cirrhosis of the liver. Acta Med Scand Suppl 1979;624:99‐105. [DOI] [PubMed] [Google Scholar]
- 40. Fevery J, de Groote J. Conjugation of N‐acetyl‐p‐aminophenol (N.A.P.A.) in adult liver patients. Acta Hepatosplenol 1969;16:11‐18. [PubMed] [Google Scholar]
- 41. Forrest JA, Finlayson ND, Adjepon‐Yamoah KK, Prescott LF. Proceedings: antipyrine, lingocaine and paracetamol metabolism in chronic liver disease. Gut 1975;16:828‐829. [PubMed] [Google Scholar]
- 42. Forrest JA, Finlayson ND, Adjepon‐Yamoah KK, Prescott LF. Antipyrine, paracetamol, and lignocaine elimination in chronic liver disease. Br Med J 1977;1:1384‐1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Forrest JA, Adriaenssens P, Finlayson ND, Prescott LF. Paracetamol metabolism in chronic liver disease. Eur J Clin Pharmacol 1979;15:427‐431. [DOI] [PubMed] [Google Scholar]
- 44. Arnman R, Olsson R. Elimination of paracetamol in chronic liver disease. Acta Hepatogastroenterol (Stuttg) 1978;25:283‐286. [PubMed] [Google Scholar]
- 45. Leung NW, Critchley JA. Increased oxidative metabolism of paracetamol in patients with hepatocellular carcinoma. Cancer Lett 1991;57:45‐48. [DOI] [PubMed] [Google Scholar]
- 46. El‐Azab G, Youssef MK, Higashi Y, Murakami T, Yata N. Acetaminophen plasma level after oral administration in liver cirrhotic patients suffering from schistosomal infection. Int J Clin Pharmacol Ther 1996;34:299‐303. [PubMed] [Google Scholar]
- 47. Zapater P, Lasso De La Vega MC, Horga JF, Such J, Frances R, Esteban A, et al. Pharmacokinetic variations of acetaminophen according to liver dysfunction and portal hypertension status. Aliment Pharmacol Ther 2004;20:29‐36. [DOI] [PubMed] [Google Scholar]
- 48. Cormack CRH, Sudan S, Addison R, Keating J, Sherwood RA, Ashley EMC. The pharmacokinetics of a single rectal dose of paracetamol (40 mg x kg‐1) in children with liver disease. Paediatr Anaesth 2006;16:417‐423. Erratum in: Paediatr Anaesth 2006;16:708. [DOI] [PubMed] [Google Scholar]
- 49. McGill MR, Jaeschke H. Biomarkers of mitotoxicity after acute liver injury: further insights into the interpretation of glutamate dehydrogenase. J. Clin Transl Res 2021;7:61‐65. [PMC free article] [PubMed] [Google Scholar]
- 50. Curry SC, Padilla‐Jones A, O’Connor AD, Ruha AM, Bikin DS, Wilkins DG, et al.; Acetaminophen Adduct Study Group . Prolonged acetaminophen‐protein adduct elimination during renal failure, lack of adduct removal by hemodiafiltration, and urinary adduct concentrations after acetaminophen overdose. J Med Toxicol 2015;11:169‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Miners JO, Penhall R, Robson RA, Birkett DJ. Comparison of paracetamol metabolism in young adult and elderly males. Eur J Clin Pharmacol 1988;35:157‐160. [DOI] [PubMed] [Google Scholar]
- 52. Pickering G, Schneider E, Papet I, Pujos‐Guillot E, Pereira B, Simen E, et al. Acetaminophen metabolism after major surgery: a greater challenge with increasing age. Clin Pharmacol Ther 2011;90:707‐711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


