ABSTRACT
Vitamin B5 (panthotenic acid), the precursor of coenzyme A (CoA), is contained in most food items and is produced by the intestinal microbiota. A recent study published in Cell Metabolism reports that vitamin B5 and CoA favor the differentiation of CD8+ cytotoxic T cells into interleukin-22 (IL-22)-producing Tc22 cells, likely through fueling mitochondrial metabolism. Importantly, in a small cohort of melanoma patients, the plasma levels of vitamin B5 positively correlate with responses to PD-1-targeted immunotherapy. Moreover, in mice, supplementation with vitamin B5 increases the efficacy of PD-L1-targeted cancer immunotherapy, and in vitro culture of T cells with CoA enhances their antitumor activity upon adoptive transfer into mice. These finding suggest that vitamin B5 is yet another B vitamin that stimulates anti-cancer immunosurveillance.
KEYWORDS: Acetyl coenzyme A, immune checkpoint inhibitor, nicotinamide, microbiome, vitamin B3
Main text
Considerable evidence argues in favor of the hypothesis that the host microbiota reinforces anti-cancer immune responses elicited by immune checkpoint inhibition (ICI) and that a healthy microbiota may be indispensable for the positive clinical outcome of immunotherapies.1–3 Numerous reports have described microbial species that boosted the effectiveness of ICI in melanoma cancer, as exemplified by Akkermancia muciniphila and others.4–6 Some of these beneficial effects of the intestinal microbiota have been attributed to immunostimulatory metabolites generated in the host intestine (e.g., short-chain fatty acids).7
Recently, several B vitamins, all of which are produced at least in part by the microbiota, have been shown to play a major role in the immunoregulatory function of the gut microflora.8 For example, vitamin B3 (niacin, also known as nicotinic acid) attenuates the development of colon cancers in mice, likely through its anti-inflammatory effects.9,10 Clinical trials employing a vitamin B3 derivative, nicotinamide (NAM), supported the idea that vitamin B3 mediates effective chemoprevention against non-melanoma skin cancer.11–13 A recent study dissected the mechanisms through which NAM can delay the manifestation and the progression of luminal B breast cancer in mice, showing that NAM stimulates the activity of NK and T cells involved in immunosurveillance.14,15 Of note, NAM could be advantageously combined with anthracycline-based immunogenic chemotherapy and gemcitabine against preclinical models of breast cancer and pancreatic cancer, respectively.14,16 Similarly, NAM can be combined with gemcitabine for the treatment of murine pancreatic cancers to deplete myeloid-derived suppressor cells, to enhance local infiltration by T lymphocytes and to achieve superior tumor growth control.16
Yet another example is provided by vitamin B6 (pyridoxine), the metabolism of which is linked to prognosis in non-small cell lung cancer (NSCLC). Thus, low levels of pyridoxal kinase (PDXK), the enzyme that generates the active vitamin B6, correlate with poor responses to cisplatin-based chemotherapy in mouse models and NSCLC patients,17 as well as with an infiltration of NSCLC by activated dendritic cells (DCs) expressing lysosomal associated membrane glycoprotein (DC-LAMP).18 Similarly, in patients with locally advanced cervical carcinoma, a positive correlation between PDXK expression and tumor infiltration by DC-LAMP+ cells has been observed.18 Conversely, supplementation of vitamin B6, if combined with cisplatin-based chemotherapy, stimulates anticancer immune responses by enhancing immunogenic stress and death of NSCLC cells.19 Altogether, these results support the idea that vitamin B6 stimulates anticancer immunosurveillance.
Vitamin B5 (pantothenic acid) has recently joined the club of immunostimulatory B vitamins. Vitamin B5 is a precursor of coenzyme A (CoA), an essential cofactor for energy metabolism and fatty acid oxidation.20 CoA can be conjugated to acetate to form acetyl-CoA thioester, which plays a central role in the intersection between amino acid catabolism, glycolysis, fatty acid metabolism, as well as a donor of acetyl groups for acetylation reactions,21 and longer acyl-CoA derivatives, which serve as “activated” fatty acids to participate in intracellular fatty acid transport and lipid biosynthesis.22,23 Of note, a protective effect has been ascribed to vitamin B5 in the context of infection by Plasmodium falciparum, the pathogen responsible for malaria.24 Similarly, vitamin B5 supplementation of mice can afford protection against Mycobacterium tuberculosis, the infectious agent causing tuberculosis, through improved T cell-mediated immunity.25
A very recent study reinforces the idea of vitamin B5-mediated immunostimulatory effects in the context of cancer immunotherapy.26 When characterizing the function of antitumor T cells in immunotherapy, the authors first evaluated the metabolic profiles of several effector CD8+ T cell subpopulations that can be distinguished according to their cytokine profile into Tc1 (that produce interferon-γ and interleukin [IL]-2), Tc17 (that produce IL-17) and Tc22 cells (that produce IL-2 and IL-22). Tc22 cells, which are particularly efficient as antitumor effectors, require for their differentiation a process of metabolic reprogramming toward oxidative phosphorylation and hence mitochondrial ATP generation. To identify the metabolic drivers of Tc22 polarization, mass spectrometric metabolomic analyses were performed on mouse Tc1, Tc17 and Tc22 T cells differentiated in vitro. These analyses, revealed that vitamin B5 and CoA are particularly abundant in Tc22 cells.26 Moreover, the in vitro differentiation of Tc22 in the presence of exogenous CoA gave rise to further elevation of glycolysis with incorporation of glucose-derived13C into tricyclic acid cycle (TCA) metabolites, increased oxidative phosphorylation, mitochondrial production of reactive oxygen species (ROS), higher cellular ATP levels and enhanced IL-2 and IL-22 production. Mechanistically, the increase in IL-22 production was linked to the activation of two transcription factors, hypoxia inducible factor (HIF)-1α (which is sensitive to the TCA metabolites succinate) and aryl hydrocarbon receptor (AhR, which is sensitive to ROS).26 Importantly, when tumor antigen-specific T cells were activated in vitro in the presence of CoA, and then injected into transgenic mice expressing this antigen in pancreatic islet cancers, they acquired superior tumor growth-reducing capabilities. In addition, injection of vitamin B5 into mice enhanced the response of subcutaneously implanted MC38 cells to immunotherapy with a PD-L1-specific antibody. In a final twist, St. Paul et al. demonstrated that vitamin B5 (panthotenic acid) levels were more elevated in the plasma from patients with melanoma that responded to PD-1 blockade (n = 21) than in non-responder patients (n = 21). Patients in the highest tertile of plasma B5 levels exhibited the highest survival with respect to time to next treatment as compared to patients with intermediate and low B5 levels. Moreover, in another, independent cohort of melanoma patients, high IL-22 mRNA levels in tumor biopsies were associated with immunotherapy responses.26
The aforementioned data support the idea that vitamin B5 and CoA may have important immunostimulatory functions that ultimately determine anticancer immunosurveillance (Figure 1). For this, however, it will be important to confirm the elevation of circulating vitamin B5 levels (and the expected increase of intracellular CoA levels affecting specific T lymphocyte subpopulations) in large cohorts of patients with melanoma and other cancers under immunotherapy. Moreover, a number of confounding factors must be considered before definitive conclusions can be reached. Indeed, in the first place, high levels of vitamin B5 might simply reflect a healthy diet and microbiota required for a state of general health or “fitness” that predisposes to efficient immune responses against pathogens or malignant cells.27,28 Moreover, the fecal microbiota efficiently generates vitamin B5 from the dietary fiber component inulin,29 and the abundance of dietary fiber has a positive impact on the outcome of immunotherapy in melanoma patients.30 Although it is well possible that enhanced vitamin B5 levels explain this correlation, it should be noted that the microbiota may affect anticancer immunosurveillance through multiple additional effects,7 calling for further mechanistic studies.
Figure 1.
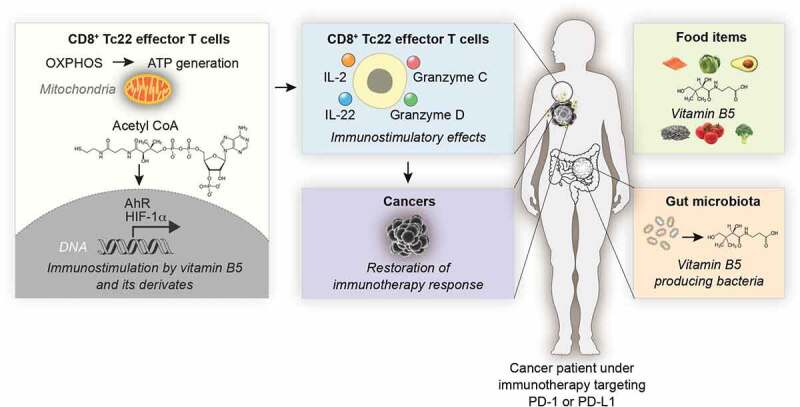
Immunostimulatory effects of vitamin B5 in anticancer immunotherapy. Schematic overview on the role of vitamin B5 and coenzyme A (CoA) on cancer immunotherapy targeting the PD-1/PD-L1 interaction. For details see main text.
Abbreviations: AhR, aryl hydrocarbonreceptor; HIF, hypoxia inducible factor; IL-22, interleukin-22: Tc22, cytotoxic T cells producing interleukin-22;
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); Gustave Roussy Odyssea, the European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); the Leducq Foundation; a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Torino Lumière; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001.
Disclosure statement
OK is a scientific co-founder of Samsara. GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Samsara, Sanofi, Sotio, Vascage and Vasculox/Tioma. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Samsara Therapeutics and Therafast Bio. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders. MB has no relevant conflicts of interest.
References
- 1.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–4. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 2.Boesch M, et al. Local tumor microbial signatures and response to checkpoint blockade in non-small cell lung cancer. Oncoimmunology. 2021;10(1):1988403. doi: 10.1080/2162402X.2021.1988403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang D, Wang X, Zhou X, Zhao J, Yang H, Wang S, Morse MA, Wu J, Yuan Y, Li S, et al. Blood microbiota diversity determines response of advanced colorectal cancer to chemotherapy combined with adoptive T cell immunotherapy. Oncoimmunology. 2021;10(1):1976953. doi: 10.1080/2162402X.2021.1976953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkrief A, El Raichani L, Richard C, Messaoudene M, Belkaid W, Malo J, Belanger K, Miller W, Jamal R, Letarte N, et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology. 2019;8(4):e1568812. doi: 10.1080/2162402X.2019.1568812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Li Y, Wang Y, Xu L, Guo Y, Wang Y, Wang L, Guo C.. Oral administration of Bifidobacterium breve promotes antitumor efficacy via dendritic cells-derived interleukin 12. Oncoimmunology. 2021;10(1):1868122. doi: 10.1080/2162402X.2020.1868122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derosa L, et al. Microbiota-centered interventions: the next breakthrough in immuno-oncology? Cancer Discov. 2021;11(10):2396–2412. doi: 10.1158/2159-8290.CD-21-0236. [DOI] [PubMed] [Google Scholar]
- 8.Peterson CT, Rodionov DA, Osterman AL, Peterson SN. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients. 2020;12(11):3380. doi: 10.3390/nu12113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad P, Manicassamy S, Munn D, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr. 2019;6:48. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, Scolyer RA, Dhillon HM, Vardy JL, Kricker A, et al. A phase 3 randomized Trial of Nicotinamide for skin-cancer Chemoprevention. N Engl J Med. 2015;373(17):1618–1626. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 12.Minocha R, Martin AJ, Chen AC, Scolyer RA, Lyons JG, McKenzie CA, Madore J, Halliday GM, Damian DL. A Reduction in Inflammatory Macrophages May Contribute to Skin Cancer Chemoprevention by Nicotinamide. J Invest Dermatol. 2019;139(2):467–469. doi: 10.1016/j.jid.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Giacalone S, Spigariolo CB, Bortoluzzi P, Nazzaro G. Oral nicotinamide: the role in skin cancer chemoprevention. Dermatol Ther. 2021;34(3):e14892. doi: 10.1111/dth.14892. [DOI] [PubMed] [Google Scholar]
- 14.Buque A, et al. Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat Commun. 2020;11(1):3819. doi: 10.1038/s41467-020-17644-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buque A, Bloy N, Petroni G, Kroemer G, Galluzzi L. NK cells beat T cells at early breast cancer control. Oncoimmunology. 2020;9(1):1806010. doi: 10.1080/2162402X.2020.1806010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvanesan BC, Meena K, Beck A, Meheus L, Lara O, Rooman I, Gravekamp C. Nicotinamide combined with gemcitabine is an immunomodulatory therapy that restrains pancreatic cancer in mice. J Immunother Cancer. 2020;8(2):e001250. doi: 10.1136/jitc-2020-001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galluzzi L, et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Rep. 2012;2(2):257–269. doi: 10.1016/j.celrep.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Joseph A, Juncheng P, Mondini M, Labaied N, Loi M, Adam J, Lafarge A, Astesana V, Obrist F, Klein C, et al. Metabolic features of cancer cells impact immunosurveillance. J Immunother Cancer. 2021;9(6):e002362. doi: 10.1136/jitc-2021-002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aranda F, Bloy N, Pesquet J, Petit B, Chaba K, Sauvat A, Kepp O, Khadra N, Enot D, Pfirschke C, et al. Immune-dependent antineoplastic effects of cisplatin plus pyridoxine in non-small-cell lung cancer. Oncogene. 2015;34(23):3053–3062. doi: 10.1038/onc.2014.234. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan B, Baratashvili M, van der Zwaag M, Kanon B, Colombelli C, Lambrechts RA, Schaap O, Nollen EA, Podgoršek A, Kosec G, et al. Extracellular 4’-phosphopantetheine is a source for intracellular coenzyme A synthesis. Nat Chem Biol. 2015;11(10):784–792. doi: 10.1038/nchembio.1906. [DOI] [PubMed] [Google Scholar]
- 21.Pietrocola F, Galluzzi L, Bravo-San Pedro JM, Madeo F, Kroemer G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 2015;21(6):805–821. doi: 10.1016/j.cmet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Pedro JMB, Sica V, Madeo F, Kroemer G. Acyl-CoA-binding protein (ACBP): the elusive ‘hunger factor’ linking autophagy to food intake. Cell Stress. 2019;3(10):312–318. doi: 10.15698/cst2019.10.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trefely S, Lovell CD, Snyder NW, Wellen KE. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol Metab. 2020;38:100941. doi: 10.1016/j.molmet.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naquet P, Kerr EW, Vickers SD, Leonardi R. Regulation of coenzyme A levels by degradation: the ‘Ins and Outs’. Prog Lipid Res. 2020;78:101028. doi: 10.1016/j.plipres.2020.101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, Hu S, Du X, Wen Q, Zhong X-P, Zhou X, Zhou C, Xiong W, Gao Y, Zhang S, et al. Vitamin B5 Reduces Bacterial Growth via Regulating Innate Immunity and Adaptive Immunity in Mice Infected with Mycobacterium tuberculosis. Front Immunol. 2018;9:365. doi: 10.3389/fimmu.2018.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St Paul M, Saibil SD, Han S, Israni-Winger K, Lien SC, Laister RC, Sayad A, Penny S, Amaria RN, Haydu LE, et al. Coenzyme A fuels T cell anti-tumor immunity. Cell Metab. 2021;33(12):2415–2427 e2416. doi: 10.1016/j.cmet.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15(6):382–396. doi: 10.1038/s41571-018-0006-2. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Otin C, Kroemer G. Hallmarks of health. Cell. 2021;184(7):1929–1939. doi: 10.1016/j.cell.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 29.Deng P, Valentino T, Flythe MD, Moseley HNB, Leachman JR, Morris AJ, Hennig B. Untargeted Stable Isotope Probing of the Gut Microbiota Metabolome Using 13 C-Labeled Dietary Fibers. J Proteome Res. 2021;20(5):2904–2913. doi: 10.1021/acs.jproteome.1c00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG, Peterson CB, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]


