Significance
Adverse childhood experiences (ACEs) are extreme stressors that have a profound impact on cognitive development. Using an explore/exploit foraging paradigm, we demonstrate that ACEs are associated with reduced exploration, leading these individuals to accumulate fewer rewards from their environment. Using computational modeling, we identify that reduced exploration is associated with ACE-exposed individuals underweighting reward feedback, which highlights a cognitive mechanism that may link childhood trauma to the onset and maintenance of psychopathology.
Keywords: adverse childhood experiences, trauma, exploration, reward, learning
Abstract
Adverse childhood experiences (ACEs) are extreme stressors that lead to negative psychosocial outcomes in adulthood. Nonhuman animals explore less after exposure to early stress. Therefore, in this preregistered study, we hypothesized that reduced exploration following ACEs would also be evident in human adults. Further, we predicted that adults with ACEs, in a foraging task, would adopt a decision-making policy that relies on the most-recent reward feedback, a rational strategy for unstable environments. We analyzed data from 145 adult participants, 47 with four or more ACEs and 98 with fewer than four ACEs. In the foraging task, participants evaluated the trade-off between exploiting a known patch with diminishing rewards and exploring a novel one with a fresh distribution of rewards. Using computational modeling, we quantified the degree to which participants’ decisions weighted recent feedback. As predicted, participants with ACEs explored less. However, contrary to our hypothesis, they underweighted recent feedback. These unexpected findings indicate that early adversity may dampen reward sensitivity. Our results may help to identify cognitive mechanisms that link childhood trauma to the onset of psychopathology.
Across animal species, an organism’s survival depends on its ability to adapt to the conditions of its environment (1). In humans, experiences in childhood and adolescence provoke strategies of decision-making that are adaptive in those environmental conditions and that can persist into adulthood (2). Early experiences of adversity have been associated with significant negative psychosocial outcomes (3). However, these outcomes may result from strategies of decision-making, which individuals adopt to cope with their early caregiving environments (4). In the present study, we examine how exposure to early adversity affects exploration and sensitivity to reward feedback while foraging.
Adverse childhood experiences (ACEs) are events that are extreme stressors experienced by an individual during development (ages 0 to 18 y old) (3). ACEs can be categorized into three broad groups: threatening events, which are directly experienced by the individual (e.g., physical abuse), neglect (e.g., emotional neglect), or household adversity, which refers to circumstances in the individuals’ environment that can cause high levels of stress (e.g., parental divorce) (5). Notably, higher rates of ACEs are associated with poorer health and social outcomes in adulthood, such as substance misuse and antisocial behavior (6, 7).
A theoretical account known as life history theory proposes that adverse rearing conditions direct individuals to adopt strategies that maximize short-term benefits (8). This behavior may manifest through reduced exploration and greater delay discounting, both of which indicate a preference for rewards that are immediately available, compared to greater but more-delayed rewards. Behaviorally, greater delay discounting is negatively correlated with exploration, suggesting that these preferences both reflect cognitive processes related to the individual’s temporal horizon (9). Consistent with life history theory, adolescent rats exposed to early stress explore their environment less compared to controls (10). Moreover, in human adolescents, adversity related to childhood poverty is associated with a preference for immediate rather than delayed rewards in delay discounting paradigms (11). These findings are notable as decision-making that prioritizes short-term rewards can lead to poorer socioeconomic outcomes (12) and has also been linked to problematic health behaviors (e.g., substance misuse) (13). While individuals might adapt to their surroundings in their formative years by adopting this decision-making strategy, its continued use can lead to poorer psychosocial outcomes later in life.
The adoption of a decision-making strategy that is focused on exploiting immediate rewards from the environment may be beneficial in resource-scarce conditions, which are characteristic of the rearing context of ACE-exposed individuals (14). This benefit has been demonstrated in adolescents with experience of early life stress (specifically events leading to institutionalization) using a variant of the Balloon Analog Risk Task (BART) (15). In a study by Humphreys and colleagues (4), adolescents who had experienced institutionalization explored less when the environment favored less exploration, meaning they collected more rewards in this condition compared to adolescents with more-stable upbringings (4). Furthermore, in this same study, adolescents with experience of institutionalization explored less when the optimal decision-making strategy was to explore more, meaning that they collected fewer rewards in this condition compared to adolescents from stable backgrounds. Yet, a potential limitation associated with the variant of the BART used by Humphreys and colleagues (4) is that choosing to explore can lead the participant to lose any unbanked points they have accumulated if they pump beyond the balloon’s limit. This may be problematic as early adversity is associated with a heightened sensitivity toward negative feedback (16), and therefore, previous research demonstrating reduced exploration following early stress may be confounded by heightened loss aversion in this population (17). Moreover, while the effects of early adversity on exploration have been investigated in adolescents, it is unknown whether ACEs in humans lead to reduced levels of exploration in adulthood.
This previous literature poses new hypotheses that we tested here. First, we examined whether forms of early stress other than institutionalization led to a reduction in exploration in adulthood. Humphreys and colleagues’ (4) focus on institutionalization parallels the rodent literature, which has manipulated early stress through mother–infant separation. Here, we adopted a wider definition of early adversity that encompasses experiences that are more common in the general human population (18, 19). Identifying how ACEs affect exploration can contribute toward understanding how more prevalent forms of early stress affect decision-making across the lifespan. In addition, we examined whether adults who had experienced early adversity also adopted exploration strategies that are optimal in environments that favor exploitation.
A canonical paradigm for studying how organisms explore their environment is patch-foraging. When foraging, an agent decides whether to remain with a known patch to exploit rewards from it or to explore a novel patch that has a fresh distribution of rewards (20, 21). Whether the organism exploits a current patch or explores a novel one should depend on the richness of the environment, which refers to the average number of rewards accumulated while foraging (22). This is formalized in a computational account of foraging known as Marginal Value Theorem (MVT). The theorem proves that to maximize reward intake, the forager should opt to explore when the rewards expected from exploiting the present patch fall below the average reward rate for the environment (20). Previous research has found that human adults adjust their foraging strategies according to qualities of the environment so as to maximize rewards (23). Nevertheless, adults explore less than an optimal foraging strategy would dictate (24). Here, we examine whether this tendency to under-explore is particularly pronounced in ACE-exposed individuals.
If early adversity indeed reduces exploration later in life, such an adaptive decision-making strategy might arise from the computational mechanisms that ACE-exposed individuals use to learn from reward feedback. Specifically, the rate at which individuals learn associations between stimuli and outcomes may provide insight into why ACE-exposed individuals prefer to exploit immediate rewards rather than potentially delaying reward by exploring. In adverse household conditions, there is inconsistency in caregiver behavior and the individual learns that positive and negative behaviors occur at random (25). Indeed, a recent paper has characterized early adversity as a violation of environmental predictability, which has profound consequences for sensitive periods of development (26). On this account, it is argued that experiences that the child should expect, such as parental care, are either unreliable or atypical in adverse households. For example, adverse households can involve frequent and unpredictable threats to survival, such as instances of physical assault (3). It has been argued that such conditions of adversity can lead to schemas of unpredictability, in which the world is perceived as unstable (27). Consistent with this view, individuals exposed to childhood adversity develop neurobiological and behavioral adaptations to navigate changeable environments, such as rapidly shifting attention (28). Together, this evidence suggests that individuals who have been exposed to ACEs perceive the environment as relatively volatile, which can impact neurobiological and behavioral outcomes (3).
In rapidly changeable or volatile environments, knowledge of the more distant history of reward feedback has less utility in predicting future outcomes than recent feedback (29). As such, decisions in volatile environments should be based on more recent feedback about whether actions will be rewarded (30). As a real-world example, during the COVID-19 pandemic, information regarding health behaviors rapidly changed in response to the spread of the virus, and as such, adaptive decision-making involved utilizing the most-recent information to make health-related choices (31). The utility of relying on recent feedback in volatile environments has been demonstrated in instrumental learning paradigms, in which participants learn the likelihood of receiving rewards from sampling stimuli with probabilistic reward schedules. In environments where the probabilities that relate stimuli, actions, and reward to each other are volatile, the decision-maker should update knowledge quickly in response to recent feedback (29). This ability—the “learning rate”—is quantified in most traditional, formal reinforcement-learning models by a parameter known as alpha (in the specific implementation we use here, the learning rate equals 1 − alpha). The learning rate measures how highly individuals weight recent feedback relative to more historic feedback, with higher rates indicating greater emphasis on recent events (32). By contrast, in environments where the probability of receiving rewards from each action remains stable, the optimal learning strategy is to utilize a wider range of historic experience during decision-making to avoid overweighting rare events (i.e., use a lower learning rate) (29).
To our knowledge, no research to date has examined whether early life stress is associated with differences in how highly individuals weight recent feedback (i.e., how high their learning rate is). Certain disorders can lead individuals to overestimate the volatility of stable environments, which can lead to heightened emphasis placed on recent events (e.g., Autism Spectrum Disorder) (33). Recently, it has been suggested that adverse experiences might lead to atypical learning strategies, which could explain why early adversity is linked to the onset of emotional disorders such as anxiety and depression (34). Neuroimaging research has demonstrated that ACEs lead to reduced volume of the anterior cingulate cortex (ACC) (35), a region that has been implicated in tracking environmental volatility (29) and the value of exploring new patches while foraging (36, 37) (however, refer to ref. 38). As such, we hypothesized that ACEs would impact ACC-mediated learning mechanisms capable of adjusting learning rates to the (in)stability of the environment. Specifically, as exposure to ACEs disposes the individual to perceive the environment as unstable (3), we predicted that in adulthood, these experiences will be associated with overestimating the volatility of stable environments, reflected by a higher learning rate.
The current study investigated how early experiences of adversity impact decision-making. We measured exploration behavior (leaving thresholds) on a patch-foraging task in individuals with more or fewer ACEs and fit a reinforcement-learning model (23) to their behavior to estimate their rate of learning from reward feedback. We preregistered three hypotheses. Our first hypothesis had two parts: Hypothesis 1a was that participants with a high number of ACEs would explore less (i.e., exploit patches for longer) compared to those in the low-ACE group. Hypothesis 1b was that participants with high rates of ACEs would weight recent evidence higher (as represented by their learning rates) than participants with fewer ACEs. Hypothesis 1b also predicted that higher learning rates would be associated with lower leaving thresholds in patches (i.e., less exploration). Our second hypothesis (Hypothesis 2) was that ACE-related decision strategies would lead to real-world problematic outcomes in the form of a positive relationship between ACEs and self-reported risk-taking.
As adults exposed to ACEs are expected to explore less (Hypothesis 1a), they should be closer to optimal in conditions where exploitation garners greater rewards (4). As such, the first part of our third hypothesis (3a) was that participants who reported higher levels of ACEs would demonstrate more optimal exploration in the “poorer” task environment, where the better strategy is to explore less, compared to participants with lower levels of ACEs. Complementing this, the second part of the third hypothesis (3b) was that participants with lower levels of ACEs would demonstrate more optimal exploration in the “richer” task environment, where the better strategy was to explore more, compared to participants with higher levels of ACEs. Addressing these questions can inform our understanding of the computational mechanisms underlying different decision-making strategies associated with early adversity and their relationship with risk-taking behaviors.
Results
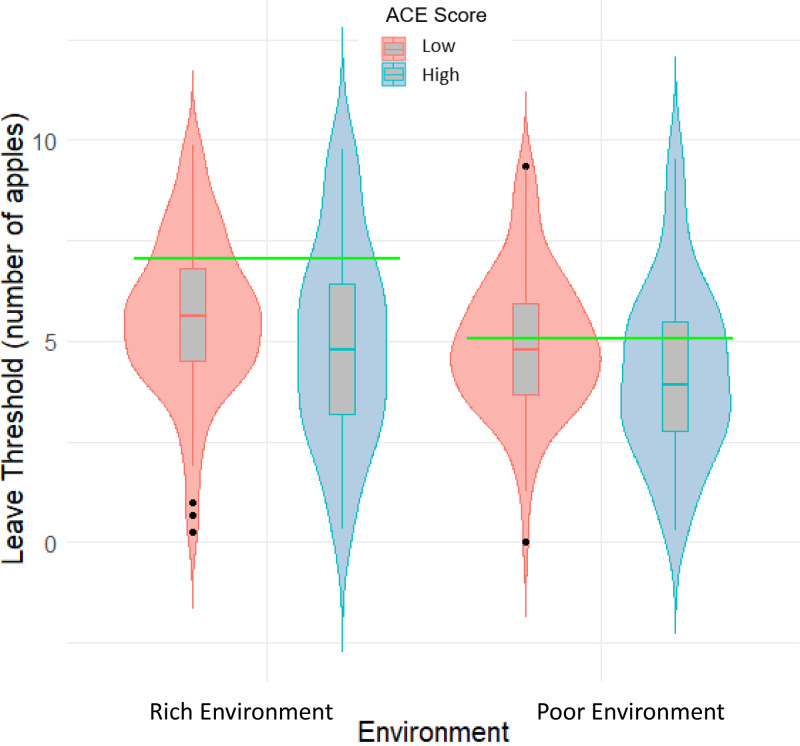
To test Hypothesis 1a that individuals with a high number of ACEs would explore less (i.e., would have a lower leaving threshold) compared to individuals with a low number of ACEs, we ran a mixed ANOVA with the foraging environment (rich or poor) as the within-subject factor and ACE exposure (high or low) as the between-subject factor. We replicated findings (23, 24) that in the rich environment, participants had a higher leaving threshold than in the poor-quality environment [F(1,137) = 28.26, P < 0.001, and η2 = 0.03]. Furthermore, participants in the high-ACE group remained in patches significantly longer (i.e., explored less) than participants with less exposure to ACEs [F(1,137) = 4.46, P = 0.037, and η2 = 0.03] (Fig. 1). There was no interaction between environment type and ACE exposure [F(1,137) = 0.63, P = 0.429, and η2 < 0.001]. These effects were robust to the addition of gender as a covariate, an analysis designed to account for the overrepresentation of women in the high-ACE group (SI Appendix).
Fig. 1.
Violin and boxplots demonstrating the leaving thresholds for each environment. The central line in each boxplot refers to the mean. Upper and Lower lines reflect the upper and lower quartiles, respectively. Black dots identify outliers that are greater than 1.5 times the interquartile range from the upper and lower quartiles. Horizontal green bars indicate the optimal leaving thresholds for each environment. Higher leaving threshold indicates more exploration and less exploitation.
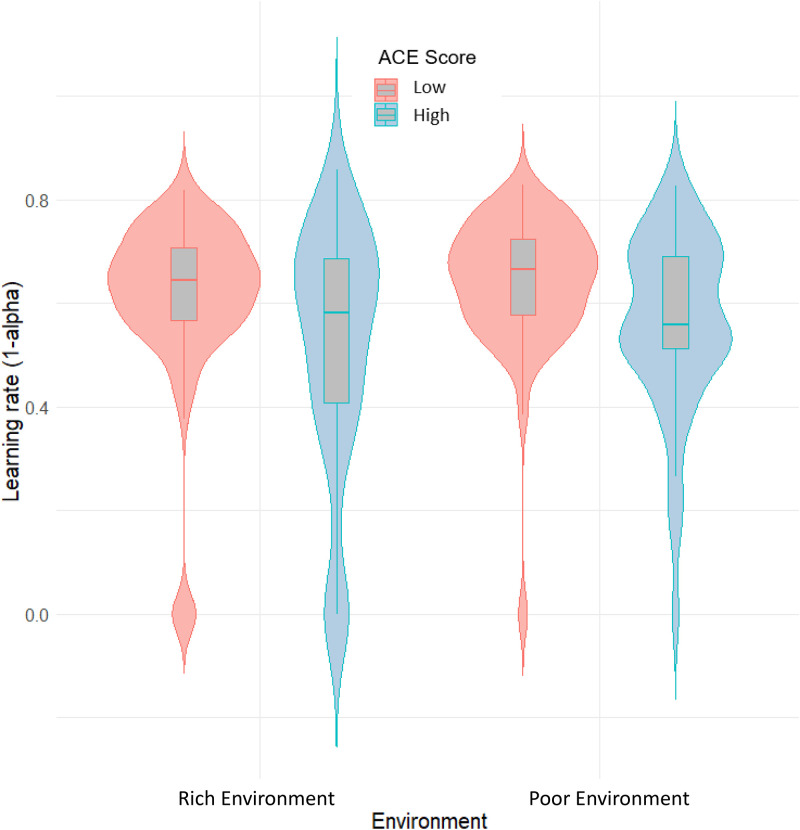
Examining Hypothesis 1b, a mixed ANOVA demonstrated that individuals in the high-ACE group had a lower mean learning rate across the two environments compared to the low-ACE group [F(1,137) = 8.92, P = 0.003, and η2 = 0.05]. This finding was in the opposite direction to our hypothesis and suggested that those in the high-ACE group weighted recent feedback lower than those in the low-ACE group (Fig. 2). This analysis also revealed that participants adjusted their learning rate between the two environments [F(1,137) = 9.63, P = 0.002, and η2 = 0.01], as participants utilized a lower learning rate in the rich environment compared to the poor environment. However, there was no interaction between environment and ACE score with respect to participants’ learning rate [F(1,137) = 0.40, P = 0.527, and η2 < 0.001]. This finding was also robust to the addition of gender as a covariate. We found no significant differences between the ACE groups on the other two free parameters, associated with the reinforcement-learning model, including the beta parameter [F(1,137) = 0.25, P = 0.621, and η2 < 0.001] and the intercept parameter c [F(1,137) = 0.76, P = 0.377, and η2 = 0.003].
Fig. 2.
Violin plot demonstrating the difference in mean learning rate (y-axis) between the two environments (x-axis) for both the high-ACE and low-ACE groups. The central line in each boxplot refers to the mean. Upper and Lower lines reflect the upper and lower quartiles, respectively.
Inconsistent with the second part of Hypothesis 1b, there was a significant negative correlation between the alpha parameter and leaving thresholds in both the rich-quality environment [r(149) = −0.91, P < 0.001] and the poor-quality environment [r(149) = −0.71, P < 0.001]. As the learning rate was equal to 1 − alpha, this suggested that in both environments, weighting recent feedback higher was associated with higher rates of exploration. We also simulated data using the mean parameter estimate from the high and low-ACE groups to examine whether we could recapitulate the trends observed in participants’ data. These simulations demonstrated that the free parameters estimated from participants’ behavior were able to reproduce the differences in leaving thresholds that we observed between the high- and low-ACE groups as well as between environments (SI Appendix).
To examine whether ACEs are associated with heightened risk-taking (Hypothesis 2), we conducted several regression analyses. Each subscale of the Domain-Specific Risk-Taking scale (DOSPERT) was entered as an outcome variable, and ACE score, gender, and age were entered as predictors. We did not find support for the hypothesis that ACEs were associated with more risk-taking. However, across all risk domains (with the exception of the social domain), being male was significantly positively associated with risk-taking (refer to SI Appendix for full model statistics).
Our third set of hypotheses predicted that because of their unstable backgrounds, individuals with high levels of ACEs would adopt closer to optimal leaving thresholds in the poorer environment, where the optimal strategy was to explore less than participants with low levels of ACEs (Hypothesis 3a). In contrast, we predicted that the high-ACE group would be less optimal than the low-ACE group in the rich environment (Hypothesis 3b). Inconsistent with our predictions, individuals with high ACE scores were further from the optimal leaving threshold in both the rich-quality (M = 2.04, SD = 2.46) and poor-quality environments (M = 0.79, SD = 2.16) compared to individuals with low ACE scores [Mrich = 1.43, SDrich = 1.94, Mpoor = 0.03, SDpoor = 1.68, F(1,137) = 4.46, P = 0.037, and η2 = 0.03]. Participants across the board exhibited more optimal behavior in the poor-quality environment than in the rich-quality one [F(1,137) = 107.31, P < 0.001, and η2 = 0.09]. There was no interaction between ACE score and environment [F(1,137) = 0.63, P = 0.429, and η2 < 0.001].
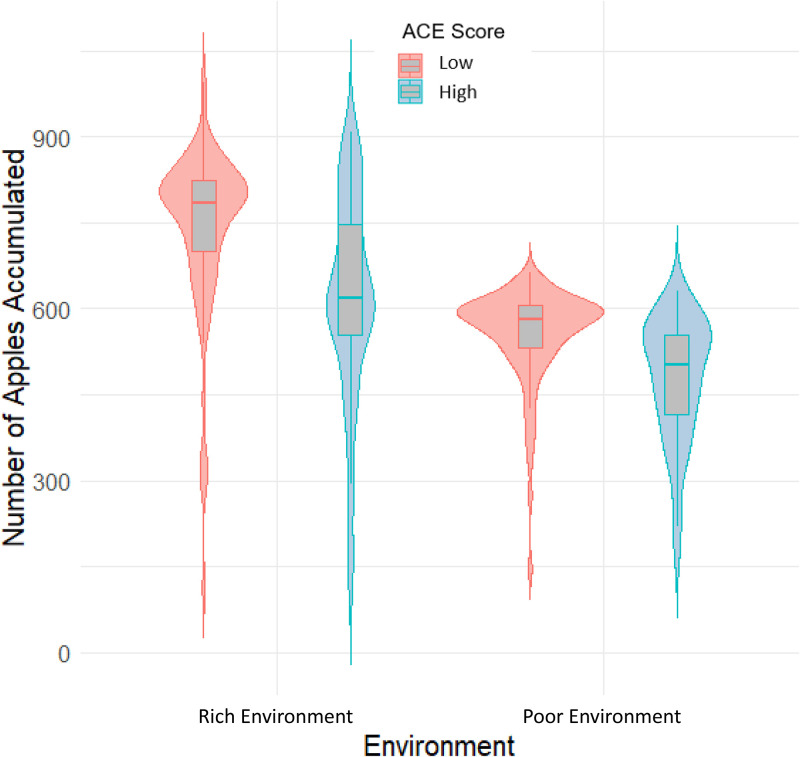
We conducted exploratory analyses to examine whether ACEs affected the number of rewards accumulated during the task (i.e., the number of apples they harvested in each environment). Results of a mixed ANOVA demonstrated that the high-ACE group collected fewer apples and, hence, were less optimal foragers than the low-ACE group [F(1,137) = 24.39, P < 0.001, and η2 = 0.09; Fig. 3]. Further, participants accumulated more points in the rich environment compared to the poor environment, as demonstrated by a main effect of environment type [F(1,137) = 187.14, P < 0.001, and η2 = 0.24]. There was not a significant interaction between environment type and ACE group [F(1,137) = 3.79, P = 0.053, and η2 = 0.01].
Fig. 3.
Apples accumulated in each environment by ACE group. The central line in each boxplot refers to the mean. Lines above and below the mean reflect the upper and lower quartiles, respectively.
Discussion
The present study tested whether ACEs are associated with reduced exploration and with the degree to which individuals weight recent feedback, as measured by participants’ learning rate. Consistent with our preregistered predictions, individuals with more ACEs explored their environment significantly less (had lower leaving thresholds) than individuals who reported fewer ACEs. However, contrary to our hypotheses, ACEs were associated with a lower learning rate, meaning that participants who reported these experiences integrated recent feedback less in their decision-making. We also found no associations between ACEs and self-reported risk-taking. While these results were not entirely in line with our predictions, they are consistent with evidence that for individuals who are exposed to them, ACEs introduce patterns into decision-making, which have deleterious outcomes that last into adulthood.
Using an explore/exploit foraging paradigm, our findings demonstrate that early adversity is associated with reduced exploration of one’s surroundings. Our findings are consistent with a previous study, which found that rats exposed to early stress demonstrated reduced exploration (10). In addition, these findings build on important work with human participants that has investigated the impact of early stress in adolescence (e.g., ref. 4) by demonstrating how an alternative set of stressors, ACEs, impact behavioral outcomes in adulthood.
While foraging, the decision-maker must compare the value of the current patch to the average reward rate for the environment when evaluating whether to explore or exploit (20, 36). Participants exposed to ACEs appeared poorer at evaluating this trade-off and were more likely to select the option that yielded an immediate reward (i.e., exploitation). These findings are consistent with empirical and theoretical work that suggests that experience of instability during childhood directs the individual later in life toward decision-making strategies that maximize short-term rewards (8, 11). Results of our computational modeling indicated that the preference for immediate rewards in ACE-exposed individuals was driven by a reduced sensitivity to reward feedback. Specifically, upon arriving at a new patch with a large initial harvest, participants who have a lower learning rate integrate this reward feedback less into their estimate of the average reward rate compared to participants who have a higher learning rate. As such, participants with a lower learning rate may underweight the larger bounty of rewards that can be gained through exploration relative to participants with a higher learning rate, leading to less-frequent exploration. Furthermore, our findings demonstrate that reduced exploration exhibited by ACE-exposed individuals led them to accumulate fewer rewards from the environment. These findings highlight how a preference for immediate reward can prevent individuals from taking advantage of the full panoply of rewards available in an environment. This is important, as the preference for immediate rewards has been causally linked to poorer socioeconomic outcomes (12), so our results inform our understanding of the link between childhood trauma and adult poverty (39).
We did not find evidence that individuals with higher levels of ACE exposure were more optimal in a foraging environment that favored less exploration (the poor-quality environment). However, several studies have found that adults typically exploit patches for longer than is optimal (23, 40), suggesting that even typical adults’ foraging behavior is already somewhat suited to environments that are poorer in quality. As such, future research should seek to recruit populations that do not demonstrate this bias to overexploit, such as adolescents (24). Examining how ACEs affect exploration in adolescence would also be important for theoretical reasons, as this is a period during which exploration serves a developmental purpose, providing this age group with the experiential knowledge necessary for adult independence (41). As the present foraging task lends itself to formal modeling techniques, this paradigm can be used to identify mechanistic explanations for reduced exploration in adolescents who experience early adversity (4). A failure to engage in typical levels of exploration during adolescence can have long-term psychosocial impacts (42), highlighting the need to understand environmental factors that lead to reduced rates of exploration at this point in the lifespan.
Unexpectedly, we found that ACEs were associated with lower learning rates, meaning that individuals with high numbers of ACEs weighted recent feedback lower than individuals with fewer of these experiences. Our original prediction was based on evidence that ACEs dispose the individual to perceive the environment as unstable (3) and on the predictions of theories such as life history theory that these early experiences lead the individual to adopt decision-making strategies to suit their environment (43). We therefore hypothesized that ACE-exposed individuals would utilize decision-making strategies that were adapted to the unstable reward availability in their formative environments, leading them to adopt a strategy that prioritizes recent feedback.
While we did not find support for this hypothesis, this inconsistency might be reconciled if one considers the evidence that individuals with high numbers of ACEs underweight recent feedback about stimulus–outcome contingencies due to their difficulty utilizing positive feedback (44). For example, women who had experienced childhood sexual abuse demonstrated a poorer ability to utilize positive-reward feedback to guide future decisions compared to participants without these experiences (45). This insensitivity to reward feedback might arise from hypoactive neural responses to rewards. Indeed, individuals with experience of trauma exhibit less activation in the ventral striatum upon receiving reward feedback compared to controls (44, 46). As the striatum encodes stimulus–outcome contingencies for gains (but not losses) (47), it is a prime candidate for the region where recent reward feedback might be underweighted in individuals with high numbers of ACEs. However, this hypothesis would need to be tested empirically (36) in future research.
Along with being associated with hypoactive striatal responses to rewards (48), ACEs have also been associated with hyperactive responses to punishment (16). This poses a further testable hypothesis, which could explain why our findings with respect to the learning rate were in the opposite direction to our predictions, as the reward feedback used in the current paradigm was positively valenced, with participants making choices to accumulate rewards rather than avoid punishment. Future research should compare how participants with ACEs weight feedback in response to both rewards and punishments. Based on our findings, we predict that in adulthood, ACEs will lead to overweighting feedback to avoid punishment and underweighting feedback to accumulate rewards compared to individuals without these experiences. This asymmetry in learning from reward and punishment could yield important insights into how childhood trauma is associated with the development and maintenance of psychopathology across the lifespan (25).
Our study has several limitations that are important to consider. We did not control for rates of stress, which mediate the association between ACEs and adult psychopathology (49). State and trait stress have been associated with decreased exploration in a foraging paradigm (50), which could explain some of the differences observed in foraging behavior between our high- and low-ACE groups. Indeed, it could be interesting for future research to consider whether stress mediates the relationship between ACE exposure and foraging behavior. Moreover, the ACE measure utilized in the present study includes a wide range of experiences, which may provide a less-specific measure of participants’ exposure to adverse events compared to previous operationalizations of early stress (4). For example, the ACE questionnaire does not ask about the frequency of each experience. A final limitation is that we manipulated both the travel time and depletion rate between foraging environments, meaning that we cannot separately examine whether participants have greater sensitivity to changes in the depletion rate or to travel time and whether these sensitivities differed by ACE exposure. Future research could address this limitation by comparing environments with long and short travel times, while independently manipulating fast and slow depletion rates (e.g., ref. 24). Administering environments more than once (e.g., ref. 50) might further enhance the effect of environment quality on foraging behavior that we observed in the current study.
In sum, this study has demonstrated that ACEs are associated with reduced exploration and with underweighting positive-reward feedback in a patch-foraging paradigm. These findings demonstrate the negative impacts on reward-processing that are associated with adversity in childhood, further highlighting the need for children to be protected from these experiences. Our findings identify a computational component of decision-making that is impacted by ACEs: learning rate. This can provide direction for future work examining how reward-based decision-making is affected by ACEs and how this contributes to the heightened rates of psychopathology observed in this population.
Materials and Methods
Participants.
To selectively recruit participants who had been exposed to ACEs, we advertised among four international charities and support groups for adult survivors of childhood trauma. These were the following: Survivors South West Yorkshire, the National Association for People Abused in Childhood (NAPAC), The Survivor’s Trust, and one anonymous support group. Control participants were recruited from a recruitment platform (Sona Systems; https://www.sona-systems.com/) hosted by a United Kingdom–based university and through Prolific (https://www.prolific.co/). The Prolific sample was recruited from the same regions that the charities were based in the United Kingdom and Europe. We recruited a total of 151 participants (Mage = 38.91, SDage = 11.09), with a mean of 2.66 ACEs (SD = 2.76). For group-level analyses, we categorized participants as having experienced a high number of ACEs if they reported ≥ 4 ACEs. This threshold was determined based on previous research (e.g., refs. 5 and 19). Six participants did not provide answers for the ACE questionnaire and were excluded from the analyses. Of the final sample, 47 participants met the threshold for the high-ACE group, and 98 were included in the low-ACE group. Age did not significantly differ between the two groups, t(143) = 1.28, P = 0.202, nor did level of education, t(141) = 0.01, P = 0.991. Because there were more females in the high-ACE group compared to the control group Χ2 (1, n = 143) = 13.10, P < 0.001, we controlled for gender in the analyses. Ethical approval for this study was received from Royal Holloway, University of London’s ethical review board (reference: Full-Review-2128-2020-04-07-13-13-PFJT001).
Materials.
ACEs.
The Adverse Childhood Events Scale (51) is a self-report measure of the number of ACEs that an individual has experienced. The scale details 10 items referring to different categories of ACEs, such as physical abuse, neglect, and parental imprisonment. For each category of ACE, the participant reported whether they experienced this during childhood (between the ages of 0 and 18), which is coded as a binary option (yes/no). Total scores on the measure range from 0 to 10, with higher values denoting the individual has been exposed to more ACEs (Table 1).
Table 1.
Prevalence of each ACE in the current sample
| ACE | Percentage of total sample |
| Threatening events | |
| Emotional abuse | 44.08 |
| Physical abuse | 30.26 |
| Sexual abuse | 36.25 |
| Neglect | |
| Emotional neglect | 44.08 |
| Physical neglect | 14.47 |
| Family adversity | |
| Divorce | 35.52 |
| Witnessing domestic abuse | 15.13 |
| Substance abuse within the household | 20.39 |
| Mental illness within the household | 35.52 |
| Incarcerated relative | 7.24 |
Risk-taking.
The DOSPERT (52) is a 30-item self-report measure assessing an individual’s risk-taking propensity. The scale measures five domains of risk-taking the following: financial, health/safety, recreational, ethical, and social risks. Each domain is measured by six items ranging from 1 to 7, in which 1 denotes that the individual would be highly unlikely to engage in that behavior and 7 denotes that the individual is highly likely to engage in that behavior. In the current sample, mean scores for each domain of risk-taking ranged from 1 to 6. The reliability of the scales computed from the current sample were as follows: social α = 0.56, recreational α = 0.85, financial α = 0.80, health/safety α = 0.66, and ethical α = 0.67.
Patch-foraging.
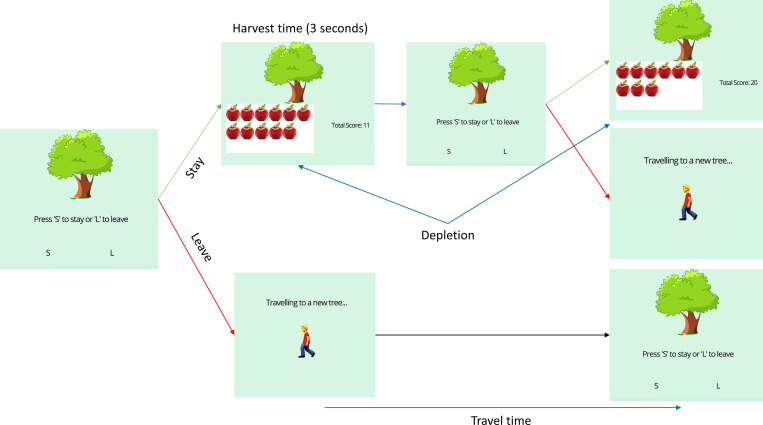
We used a patch-foraging task (Fig. 4) in which participants harvested apples (rewards) from trees (patches) (23). In this task, the decision-maker must decide between exploiting a known patch that gradually yields fewer rewards or exploring a novel patch with a fresh distribution of rewards. We designed the paradigm such that the time it took to exploit apples (i.e., the harvest time) was always 3 s, regardless of participants’ reaction times. The 3 s included participants’ reaction time and the presentation of rewards on that trial. This design feature was implemented to ensure that faster reaction times did not impact how quickly participants could accumulate rewards. For example, a participant who responded in 0.6 s would be presented with their score for 2.4 s. Participants had up to 2 s to make a decision before they were presented with a timeout screen. Timeout trials were subsequently excluded from further analyses.
Fig. 4.
Outline of the patch-foraging paradigm. The leftmost screen is presented to participants when they first enter the environment. They decide between staying or leaving the current patch. If participants choose to stay (Top screen), they are presented with the number of apples they have harvested and their cumulative score. They are then returned to the patch to decide whether to stay or leave. If participants choose to leave (Bottom screen), they wait for an environment-specific interval before reaching the new patch.
We presented participants with two foraging environments, which differed in the number of rewards obtainable. In the “poor-quality” environment, the optimal forager should explore less and exploit each patch more (i.e., use a lower leaving threshold) relative to the “rich-quality” environment, where the optimal forager should explore more and exploit each patch less (i.e., use a higher leaving threshold). To maximize the difference between optimal leaving thresholds in the rich and poor foraging environments, we manipulated environment quality both by varying the rate at which rewards exponentially depleted from individual patches and by varying the travel time between patches. Both manipulations were based on previous research (23, 24). The depletion rate was applied using the following formula: , where s refers to the reward experienced on each trial (i), and Ni refers to a value drawn on each trial from a Gaussian distribution. In the rich-quality environment, depletion rates were drawn from a Gaussian with a mean of 0.94 (SD = 0.07) and a travel time of 6 s between patches. In the poor-quality environment, depletion rates were drawn from a Gaussian with a mean of 0.88 (SD = 0.07) and a 12-s travel time between patches. In both environments, the initial distribution of rewards on each patch (s0) was drawn from a Gaussian distribution with a mean of 10 (SD = 1). Participants completed each patch-foraging environment for 7 min in a counterbalanced order.
We utilized two behavioral variables from this task in addition to the learning-rate parameter derived from our computational model (see Computational Modeling below). The first was the participants’ leaving thresholds for each environment. As in previous research (23), we assume that participants select an expected value of apples as their leaving threshold and only leave the patch when they expect fewer apples than this value in future harvests. As such, participants who choose to explore earlier will leave patches when there is a relatively high number of apples still expected from the next exploit decision, whereas participants who choose to exploit more will persist in the same patch until the expectation of apples is relatively low. Higher values of leaving threshold denote greater exploration, and lower values denote greater exploitation. We took the average number of apples from the last two harvests when calculating this variable, as in previous research (23). The second dependent variable measured how well participants performed: the difference between participants’ leaving threshold and the optimal leaving threshold. Negative values of this variable suggest that the participant remained in patches for less time than was optimal, whereas positive values suggest that participants remained for longer than was optimal. Following previous research (24, 50), we determined the optimal leave threshold by running a grid search across leaving thresholds between 1 and 10 in increments of 0.001 and summing the total number of apples accrued. The simulation was run for both the rich and poor environments, allowing us to identify the leaving threshold that yielded the highest number of apples and was therefore optimal in that environment. The optimal leaving threshold in the rich-quality environment was 7.04 apples, and in the poor-quality environment, the optimal leaving threshold was 5.07 apples (refer to the green horizontal lines in Fig. 1).
Procedure.
Participants who chose to take part were provided with a link to the study (or were transferred automatically by Prolific), which was hosted on Gorilla.sc, an online behavioral study platform (53). Participants completed a consent form, which informed them that they would be asked to complete a computerized task. They were also informed that they would be asked questions about their childhood, which might be stressful. Participants were recommended not to take part if answering such questions might cause them a high level of distress. Contact details for the researcher were provided to ensure that participants had the opportunity to ask questions about the study. Participants were paid a base rate of £3 (or equivalent) but were also informed that they could earn a performance-based bonus (up to an additional £2 or the equivalent amount in the participant’s currency). Participants were then directed to a page in which they filled out demographic information. Following this, participants were provided with the task instructions and completed a 2-min practice of the patch-foraging task. The depletion rate of the practice environment was drawn from a Gaussian distribution with a mean of 0.90 (SD = 0.07), and the travel time was set at 9 s. As such, the parameters of the practice were different to those used in the main task.
After completing the practice, participants completed both the rich- and poor-quality foraging environments. Once both environments were completed, participants were taken to a break screen, informing them they were about to be asked questions that were sensitive in nature, and were reminded they were free to omit any questions they did not wish to answer. Participants then completed the ACE questionnaire and DOSPERT. Finally, participants were provided with a debrief, which included information about support pages for survivors of childhood trauma.
Computational Modeling.
According to a prominent theory of foraging behavior (MVT) (20), the rational agent aiming to maximize reward intake should leave the current patch when the reward expected from staying within that patch falls below the average reward rate for that environment. However, as such an agent does not know the state of the environment a priori, this must be learned.
To model participants’ learning rate, we used an equation developed by Constantino and Daw (23). The model explains how participants estimate the average reward rate (pi) in each environment. Crucially, this estimate depends on a free parameter (α), which varies across individuals (32). This parameter captures the degree to which participants weight recent feedback to guide their decision-making:
| [1] |
Although traditional reinforcement-learning models directly equate the alpha parameter with the learning rate, it can be seen from Eq. 1 that the model developed by Constantino and Daw (23) parameterizes alpha as the complement of the learning rate. Thus, lower values of alpha mean higher values of (1 − α) and, hence, a higher learning rate. Participants’ estimate of the average reward rate is also subject to the time cost (Ti) associated with the participant’s explore or exploit decision and the reward experienced on each trial (si). Participants’ estimate of the average reward rate is then entered into a Softmax function, which produces the probability that the participant will stay on each trial:
| [2] |
This Softmax function [2] contains a further two free parameters. The first, β, captures stochasticity in decision-making. Higher values on this parameter denote that the participant acts more deterministically according to the MVT leaving rule, whereas lower values indicate that the participant is more likely to divert from this decision rule. The second free parameter, c, is an intercept that estimates participants’ exploitation bias. In this equation, κi refers to the rate at which apples deplete from patches. As in previous research (23), we assumed that participants use a running estimate of the depletion rate, which we calculated through averaging the depletion rate experienced on previous trials in that environment. On each trial, the true depletion rate was calculated as di = si/si−1. We then calculated participants’ running estimate of the average reward rate (ki) through averaging across all values of the depletion rate (di) experienced in the environment. Participants estimate the reward expected on the next trial by multiplying the last-known reward value (si) by their estimate of the depletion rate (κi). This value is compared against the estimate of the average reward rate (pi) multiplied by the opportunity cost associated with exploit decisions (h), which was fixed at 3 s in both environments. As such, the term captures the difference between the reward that participants expect from the next exploit decision and their current estimate of the average reward rate (23). Parameter recovery indicated that all parameters could be identified uniquely without parameter correlations, though we found that the parameter c was less well recovered compared to the other parameters in the model (SI Appendix). We compared this model, which uses only a single learning rate for all outcomes, to a model in which the learning rate was split for better-than-expected and poorer-than-expected outcomes (54). Details about this additional model can be found in SI Appendix.
Supplementary Material
Acknowledgments
We would like to thank the NAPAC, Survivors South West Yorkshire, The Survivor’s Trust, and one anonymous support group for their assistance with recruitment for this study.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. M.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2109373119/-/DCSupplemental.
Data Availability
Anonymized CSV data and analysis code have been deposited in Open Science Framework (https://osf.io/8znyx/) (55).
References
- 1.McNamara J. M., Houston A. I., Optimal foraging and learning. J. Theor. Biol. 117, 231–249 (1985). [Google Scholar]
- 2.Boyce W. T., Ellis B. J., Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 17, 271–301 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Danese A., McEwen B. S., Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 106, 29–39 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Humphreys K. L., et al. , Exploration-exploitation strategy is dependent on early experience. Dev. Psychobiol. 57, 313–321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes K., et al. , The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health 2, e356–e366 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Baglivio M. T., Wolff K. T., Piquero A. R., Epps N., The relationship between adverse childhood experiences (ACE) and juvenile offending trajectories in a juvenile offender sample. J. Crim. Justice 43, 229–241 (2015). [Google Scholar]
- 7.Mersky J. P., Topitzes J., Reynolds A. J., Impacts of adverse childhood experiences on health, mental health, and substance use in early adulthood: A cohort study of an urban, minority sample in the U.S. Child Abuse Negl. 37, 917–925 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis B. J., Del Giudice M., Developmental adaptation to stress: An evolutionary perspective. Annu. Rev. Psychol. 70, 111–139 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Sadeghiyeh H., et al. , Temporal discounting correlates with directed exploration but not with random exploration. Sci. Rep. 10, 4020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spivey J., Barrett D., Padilla E., Gonzalez-Lima F., Mother-infant separation leads to hypoactive behavior in adolescent Holtzman rats. Behav. Processes 79, 59–65 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oshri A., et al. , Socioeconomic hardship and delayed reward discounting: Associations with working memory and emotional reactivity. Dev. Cogn. Neurosci. 37, 100642 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haushofer J., Fehr E., On the psychology of poverty. Science 344, 862–867 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Kollins S. H., Delay discounting is associated with substance use in college students. Addict. Behav. 28, 1167–1173 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Belsky J., L. Steinberg, P. Draper, Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Dev. 62, 647–670 (1991). [DOI] [PubMed]
- 15.Lejuez C. W., et al. , Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART). J. Exp. Psychol. Appl. 8, 75–84 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Miu A. C., Bîlc M. I., Bunea I., Szentágotai-Tătar A., Childhood trauma and sensitivity to reward and punishment: Implications for depressive and anxiety symptoms. Pers. Individ. Dif. 119, 134–140 (2017). [Google Scholar]
- 17.Huh H. J., Baek K., Kwon J.-H., Jeong J., Chae J.-H., Impact of childhood trauma and cognitive emotion regulation strategies on risk-aversive and loss-aversive patterns of decision-making in patients with depression. Cogn. Neuropsychiatry 21, 447–461 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Office of National Statistics. Children Looked after in England (Including Adoptions and Care Leavers): 2019–2020 (Department of Education, 2020). [Google Scholar]
- 19.Bellis M. A., et al. , Adverse Childhood Experiences and Their Impact on Health-harming Behaviours in the Welsh Adult Population: Alcohol Use, Drug Use, Violence, Sexual Behaviour, Incarceration, Smoking and Poor Diet. (Public Health Wales, 2015). [Google Scholar]
- 20.Charnov E. L., Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 9, 129–136 (1976). [DOI] [PubMed] [Google Scholar]
- 21.Stephens D., Krebs J., Foraging Theory (Princeton University Press, 1986). [Google Scholar]
- 22.Bettinger R. L., Grote M. N., Marginal value theorem, patch choice, and human foraging response in varying environments. J. Anthropol. Archaeol. 42, 79–87 (2016). [Google Scholar]
- 23.Constantino S. M., Daw N. D., Learning the opportunity cost of time in a patch-foraging task. Cogn. Affect. Behav. Neurosci. 15, 837–853 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloyd A., McKay R., Sebastian C. L., Balsters J. H., Are adolescents more optimal decision‐makers in novel environments? Examining the benefits of heightened exploration in a patch foraging paradigm. Dev. Sci. 24, e13075 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Novick A. M., et al. , The effects of early life stress on reward processing. J. Psychiatr. Res. 101, 80–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson C. A. III, Gabard-Durnam L. J., Early adversity and critical periods: Neurodevelopmental consequences of violating the expectable environment. Trends Neurosci. 43, 133–143 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross L., Hill E., Childhood unpredictability, schemas for unpredictability, and risk taking. Soc. Behav. Personal. 30, 453–473 (2002). [Google Scholar]
- 28.Jensen P. S., et al. , Evolution and revolution in child psychiatry: ADHD as a disorder of adaptation. J. Am. Acad. Child. Adolesc. Psychiatry 12, 1672–1681 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Behrens T. E. J., Woolrich M. W., Walton M. E., Rushworth M. F. S., Learning the value of information in an uncertain world. Nat. Neurosci. 10, 1214–1221 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Browning M., Behrens T. E., Jocham G., O’Reilly J. X., Bishop S. J., Anxious individuals have difficulty learning the causal statistics of aversive environments. Nat. Neurosci. 18, 590–596 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lloyd A., et al. , Delay discounting and under-valuing of recent information predict poorer adherence to social distancing measures during the COVID-19 pandemic. Sci. Rep. 11, 19237 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutton R., Barto A., Reinforcement Learning: An Introduction (MIT Press, 2018). [Google Scholar]
- 33.Lawson R. P., Mathys C., Rees G., Adults with autism overestimate the volatility of the sensory environment. Nat. Neurosci. 20, 1293–1299 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulcu E., Browning M., The misestimation of uncertainty in affective disorders. Trends Cogn. Sci. 23, 865–875 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Cohen R. A., et al. , Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol. Psychiatry 59, 975–982 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Kolling N., Behrens T. E. J., Mars R. B., Rushworth M. F. S., Neural mechanisms of foraging. Science 336, 95–98 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanchard T. C., Hayden B. Y., Neurons in dorsal anterior cingulate cortex signal postdecisional variables in a foraging task. J. Neurosci. 34, 646–655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shenhav A., Straccia M. A., Cohen J. D., Botvinick M. M., Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nat. Neurosci. 17, 1249–1254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metzler M., Merrick M. T., Klevens J., Ports K. A., Ford D. C., Adverse childhood experiences and life opportunities: Shifting the narrative. Child. Youth Serv. Rev. 72, 141–149 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Heron C., et al. , Dopamine modulates dynamic decision-making during foraging. J. Neurosci. 40, 5273–5282 (2020). [DOI] [PMC free article] [PubMed]
- 41.Romer D., Reyna V. F., Satterthwaite T. D., Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Dev. Cogn. Neurosci. 27, 19–34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsen B., Luna B., Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci. Biobehav. Rev. 94, 179–195 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellis B. J., Boyce W. T., Belsky J., Bakermans-Kranenburg M. J., van Ijzendoorn M. H., Differential susceptibility to the environment: An evolutionary--neurodevelopmental theory. Dev. Psychopathol. 23, 7–28 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Hanson J. L., Hariri A. R., Williamson D. E., Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol. Psychiatry 78, 598–605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pechtel P., Pizzagalli D. A., Disrupted reinforcement learning and maladaptive behavior in women with a history of childhood sexual abuse: A high-density event-related potential study. JAMA Psychiatry 70, 499–507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boecker R., et al. , Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PLoS One 9, e104185 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taswell C. A., Costa V. D., Murray E. A., Averbeck B. B., Ventral striatum’s role in learning from gains and losses. Proc. Natl. Acad. Sci. U.S.A. 115, E12398–E12406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanson J. L., et al. , Early adversity and learning: Implications for typical and atypical behavioral development. J. Child Psychol. Psychiatry 58, 770–778 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLaughlin K. A., et al. , Childhood social environment, emotional reactivity to stress, and mood and anxiety disorders across the life course. Depress. Anxiety 27, 1087–1094 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lenow J. K., Constantino S. M., Daw N. D., Phelps E. A., Chronic and acute stress promote overexploitation in serial decision making. J. Neurosci. 37, 5681–5689 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Felitti V. J., et al. , Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 14, 245–258 (1998). [DOI] [PubMed] [Google Scholar]
- 52.Blais A.-R., Weber E. U., A domain-specific risk-taking (DOSPERT) scale for adult populations. Judgm. Decis. Mak. 1, 15 (2006). [Google Scholar]
- 53.A. L. Anwyl-Irvine, J. Massonnié, A. Flitton, N. Kirkham, J. K. Evershed, . Gorilla in our midst: An online behavioral experiment builder. Behav. Res. Methods. 52, 388–407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garrett N., Daw N. D., Biased belief updating and suboptimal choice in foraging decisions. Nat. Commun. 11, 3417 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.A. Lloyd, R. T. McKay, N. Furl, Adverse Childhood Experiences and patch foraging. Open Science Framework. https://osf.io/8znyx/. Deposited 23 December 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized CSV data and analysis code have been deposited in Open Science Framework (https://osf.io/8znyx/) (55).