Abstract
The worldwide pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to the unprecedented pace of development of multiple vaccines. This review evaluates how adenovirus (Ad) vector platforms have been leveraged in response to this pandemic. Ad vectors have been used in the past for vaccines against other viruses, most notably HIV and Ebola, but they never have been produced, distributed, or administered to humans at such a large scale. Several different serotypes of Ads encoding SARS-CoV-2 Spike have been tested and found to be efficacious against COVID-19. As vaccine rollouts continue and the number of people receiving these vaccines increases, we will continue to learn about this vaccine platform for COVID-19 prevention and control.
Keywords: COVID-19, SARS-CoV-2, vaccine, adenovirus, vector
INTRODUCTION
Coronavirus disease 2019 (COVID-19) was identified in December 2019 in Wuhan, China, and quickly spread around the globe (1, 2). Over the past 18 months, at the time of this writing, over 4.5 million people have died from this disease, and over 210 million people have been infected (3). The sequence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of COVID-19, was reported on January 10, 2020; work immediately began on vaccines to prevent SARS-CoV-2 infection (4). This vaccine development work resulted in the emergency authorization by various national and international organizations of mRNA vaccines (5, 6), inactivated virus vaccines (7, 8), protein subunit vaccines (9), and four adenovirus (Ad)-vectored vaccines (10–13).
The vast majority of COVID-19 vaccines focus on eliciting immune responses, particularly binding and neutralizing antibodies, against the Spike protein of SARS-CoV-2. Spike is a homo-trimeric protein on the virion surface responsible for engaging its receptor, angiotensinconverting enzyme 2 (ACE2), present on host cell plasma membranes, to facilitate viral entry and establishment of viral infection (14–16). Antibodies that bind Spike and prevent ACE2 engagement can neutralize SARS-CoV-2 and protect a host (17–22). Additionally, non-neutralizing antibody responses can protect the host by facilitating immunological control and clearance of the virus (23). Furthermore, virus-specific CD8+ T cells can clear infected cells and control viral replication (24). Thus, vaccines that include Spike can protect against symptomatic COVID-19 and possibly against infection as well (5, 6, 10–12).
Historically, vaccines typically consisted of inactivated or attenuated versions of the pathogen or protein subunits. Years of research into next-generation vaccine platforms for existing and emerging diseases led to the development of nucleic acid and viral vectors as platforms for vaccines, which can be easily adapted from one pathogen to the next by exchanging the gene of interest encoded (25). These gene-based vaccines have been crucial to the vaccine response to the COVID-19 pandemic.
ADENOVIRAL VECTOR HISTORY
Ads are nonenveloped double-stranded DNA viruses with icosahedral capsids between 80 and 100 nm in diameter, first identified in the 1950s (26). There are over 100 described serotypes of human Ads (27, 28). The entire Ad genome has been sequenced, and the mechanisms of assembly and replication are fairly well characterized. Ads are known to infect a variety of cell types, including epithelial and endothelial cells as well as hepatocytes and myoblasts (26). This understanding of basic Ad biology laid the foundation for Ads to become the first in vivo gene expression vectors used for therapeutic gene delivery in the 1990s to treat alpha-1 antitrypsin deficiency and cystic fibrosis (29, 30). However, even though the Ad platform reliably delivered the genes of interest, the vectors also elicited immune responses that prohibited the long-term expression of these therapeutic genes. Ad vectors are highly immunogenic and trigger an inflammatory innate response upon introduction to the host, leading to an adaptive immune response to the Ad capsid (31, 32). Though this immunogenicity limits the potential of Ad vectors for gene therapy, it is attractive for a vaccine platform (33).
The short-term expression of a transgene and inherent immunogenicity of Ad viral particles make Ads a promising vaccine platform that requires no additional adjuvants, unlike many other protein or subunit vaccines. Ad vectors have been studied as a vaccine platform for a variety of different viruses including HIV, Ebola, Zika, and SARS-CoV-2 (11–13, 34–38). Different Ad serotypes have been used in these vaccines, ranging from human Ad5 to the less prevalent human Ad26 to chimpanzee Ads (ChAds) or rhesus Ads (RhAds) (39, 40). A subset of these experimental vaccines has been authorized for emergency use for Ebola and SARS-CoV-2 (36–38).
Ad-vectored vaccines exploit the inherent infectivity of Ads to facilitate in vivo expression of a target vaccine antigen. This is made possible by the deletion of crucial viral replication genes, specifically the E1 and E3 cassettes, and insertion of the coding sequence for a vaccine antigen, rendering the vectors replication incompetent while maintaining the original size of the genome (31) (Figure 1). Ad-vectored vaccines induce an innate immune response in parallel to vaccine antigen expression, leading to an adaptive T cell and B cell response to both the Ad vector itself and the vaccine antigen. The innate response is driven primarily by recognition of the Ad genome in the host cell by cytosolic DNA sensors such as Toll-like receptor 9 (TLR9) and cyclic guanosine monophosphate-AMP synthase (cGAS); empty Ad capsids induce markedly lower innate responses (32). These sensors trigger an inflammatory cascade including interferon (IFN) and other cytokine production as well as recruitment of immune cells to the site of administration. Macrophages and dendritic cells (DCs) are among the first cells recruited, and these cells can both take up the viral particles and subsequently express the vaccine transgene locally and after trafficking to the draining lymph nodes (41). This expression in lymph nodes, as well as uptake of locally expressed antigen after release and lymphatic drainage, enables the production of a robust adaptive immune response. Cognate T and B cells are activated by DCs in lymph nodes; CD8+ T cells are activated so that they may egress from lymph nodes, home to the site of infection, and kill infected cells. Naïve B cells produce initial low-affinity antibodies and also enter germinal center (GC) reactions to affinity mature and eventually produce high-affinity antibodies, and memory B cells that recognize the antigen may also proliferate and differentiate into antibody-secreting cells (41, 42). This process is directed both against the Ad vector itself and, importantly, against the vaccine antigen encoded in the vector.
Figure 1.
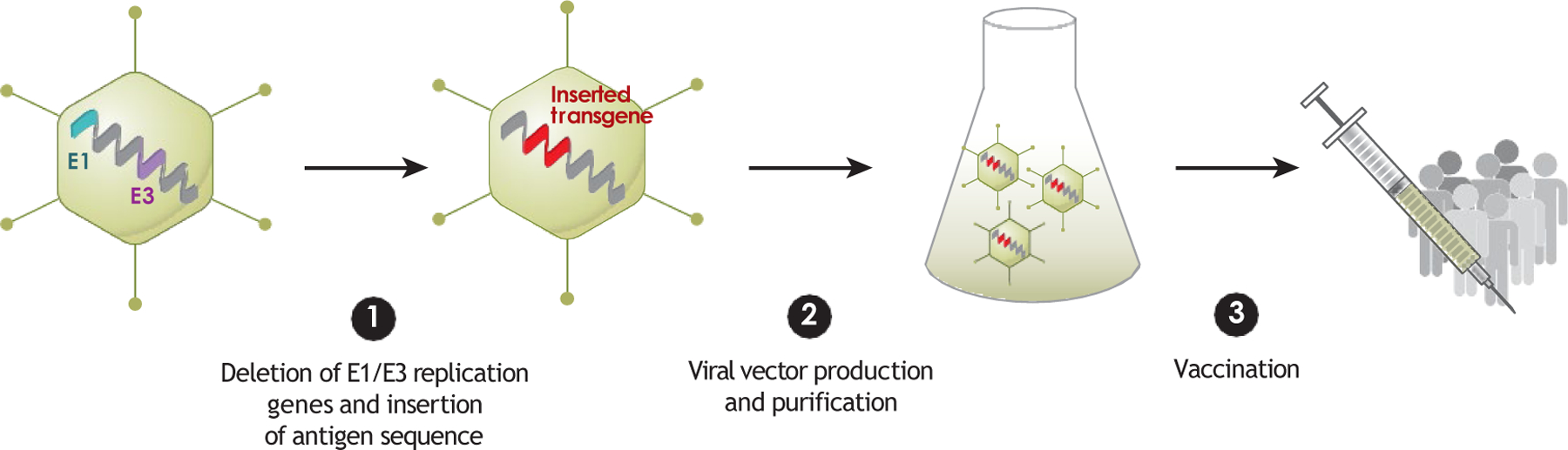
Adenoviral vector vaccine development process. 1 E1/E3 replication genes are first removed from an adenovirus serotype of choice, and the antigen of interest is then inserted into the viral genome. 2 The viral vector is produced in manufacturing cells that support replication of the replication-deficient (E1/E3-deleted) vector, and the vector is purified and then administered. 3 The vector enterscells of the recipient, and the antigen of interest is expressed.
Anti-vector immunity can pose challenges for Ad vaccine strategies. Most of the initial work on Ad vectors centered on Ad5, a well-characterized and highly prevalent Ad (43–45). Given the pervasiveness of Ad5 in humans, much of the world’s population has previously been exposed to Ad5 and has circulating anti-Ad5 antibodies (45). High titers of antibodies against the serotype of vector being used could reduce infectivity of the viral vector, thereby impairing the expression of the vaccine transgene and the desired antivaccine immune response (46, 47).
Though Ad5 vectors are still used today, a major strategy to circumvent this issue of preexisting immunity has been the use of rare Ad serotypes or nonhuman Ads (48). Extensive work in this area has been done to characterize the seroprevalence as well as the immunogenicity of various Ads as vectors, leading to the emergence of Ad26 as a promising candidate (45, 48). In parallel, work has also been conducted to characterize and vectorize Ads from nonhuman primates (NHPs) to which humans have very little to no previous exposure, including ChAds and RhAds (39, 40).
Given its modular nature, an Ad vaccine platform can readily be adapted to include virtually any antigen of interest. This has proven to be crucial to rapid vaccine development in response to the emergence of SARS-CoV-2 and the COVID-19 pandemic.
SPIKE ISOFORMS IN VACCINES
Each of the Ad vector-based vaccines for COVID-19 conditionally approved by the United States Food and Drug Administration (FDA), European Medicines Agency (EMA), Chinese National Medical Products Administration, Russian Ministry of Health, and/or the World Health Organization (WHO) at the time of writing consists of a replication-incompetent Ad vector encoding the full-length Spike protein of SARS-CoV-2 (Table 1). The Ad26.COV2.S vaccine from Janssen/Johnson & Johnson ( JnJ) includes two proline mutations shown to stabilize the Spike, as well as the deletion of the furin cleavage site (49, 50). The ChAdOx1 nCoV-19 vaccine from AstraZeneca and the Ad5-nCoV vaccine from CanSino include the tissue plasminogen activation (tPA) leader sequence before the sequence of the Spike protein (13, 51). The Gam-COVID-Vac vaccine from the Gamaleya Research Institute encodes the SARS-CoV-2 Spike protein as it was first sequenced and reported in January 2020 (1, 2, 12, 13).
Table 1.
Antigens and regimens of approved adenovirus-vectored COVID-19 vaccines
| Company name | Vaccine name | Adenovirus serotype | Exact antigen | Dosage regimen |
|---|---|---|---|---|
| Johnson & Johnson | Ad26.COV2.S | Ad26 | S.PP (furin deleted) | Single-shot regimen; two-shot regimen being studied |
| AstraZeneca | ChAdOx1 nCoV-19 | ChAdOxl | tPA.S | Two-shot regimen |
| CanSino | Ad5-nCoV | Ad5 | tPA.S | Single-shot regimen |
| Gamaleya Research Institute | Gam-COVID-Vac (Sputnik V) | Ad26, Ad5 | S | Two-shot regimen with Ad26 and Ad5 |
PRECLINICAL TRIALS
Each of these Ad vector-based vaccines was evaluated in preclinical and clinical studies. Preclinical models included NHPs, usually rhesus macaques, as well as mice, ferrets, and Syrian golden hamsters. NHPs are a preferred preclinical model for vaccine development because they recapitulate human immune responses and because SARS-CoV-2 infects and replicates in the upper (nose, mouth, throat, etc.) and lower (bronchi and lungs) respiratory tracts of NHPs, as it does in humans (52, 53). However, NHPs usually develop only mild disease when infected with SARS-CoV-2 (52–55). Hamsters provide a stringent model for protection studies as they develop severe clinical disease, including weight loss and pneumonia, as well as replication of SARS-CoV-2 in a variety of tissues including the upper and lower respiratory tracts (56–58). Ferrets, a small animal model widely used in the study of respiratory viruses because infected ferrets can cough and transmit viral particles to other ferrets, can also be infected with SARS-CoV-2 and transmit it to cohoused naïve ferrets (59, 60). SARS-CoV-2 infection in ferrets is restricted to the upper respiratory tract, with little to no virus in the lungs. Additionally, ferrets have no detectable weight loss due to infection, though their body temperatures do rise (59). Mice cannot be infected with SARS-CoV-2 naturally, but transgenic mice expressing human ACE2 (hACE2) can be infected with SARS-CoV-2, and wild-type mice can be infected with mouse-adapted strains of SARS-CoV-2 (61, 62). hACE2 transgenic mice lose weight and have high levels of viral replication in the lower respiratory tract (i.e., the lungs) upon infection with SARS-CoV-2 (61). Mouse-adapted SARS-CoV-2 can replicate in the upper and lower respiratory tracts of wild-type BALB/c mice, causing more severe disease in older mice and more closely recapitulating the human clinical phenotypes of COVID-19 (62). Each of these models provides important insight into the immunogenicity and protective capacity of candidate vaccines while still in preclinical stages.
Ad26.COV2.S Vaccine ( Janssen/Johnson & Johnson)
The single-dose Ad26.COV2.S vaccine from JnJ protected rhesus macaques from SARS-CoV-2 challenge 6 weeks after vaccination (49). The clinical vaccine candidate was one of seven candidate Ad26-based vaccines tested in this study, each expressing different isoforms of SARS-CoV-2 Spike. The vector expressing full-length, di-proline stabilized, furin-cleavage-site-deleted Spike was the most immunogenic and efficacious construct (49). Ad26.COV2.S elicited robust humoral responses including Spike receptor binding domain (RBD) binding antibodies, pseudovirus and live virus neutralizing antibodies, and IFNγ+ CD8+ and CD4+ T cell responses by 4 weeks post vaccination. Protective efficacy was determined by measuring the levels of viral subgenomic mRNA (sgRNA), which is indicative of replicating virus (63). Ad26.COV2.S vaccine afforded complete protection of the lungs, sampled by bronchoalveolar lavage, and near complete protection of the upper respiratory tract, sampled by nasal swab; only one of six NHPs had detectable sgRNA in the nasal swab post challenge (49).
The Ad26.COV2.S vaccine was also tested in the hamster model (64). This model relies mainly on measuring weight loss and sgRNA in tissues to determine the protective capacity of the vaccine against severe clinical manifestations of SARS-CoV-2 infection. Ad26.COV2.S-vaccinated hamsters exhibited no mortality alongside minimal weight loss and pneumonia, whereas unvaccinated controls showed severe weight loss and pneumonia and some mortality (64). The combination of these NHP and hamster data formed the preclinical basis for the advancement of Ad26.COV2.S to clinical trials, which have led to conditional approval (49, 64) by the FDA, EMA, WHO, and other regulatory agencies. Continued preclinical work has demonstrated the efficacy of Ad26.COV2.S against challenge with SARS-CoV-2 variants, specifically B.1.351 (beta), in NHPs (65).
ChAdOx1 nCoV-19 (AstraZeneca)
ChAdOx1 is the replication-deficient vector version of ChAdY25, a nonhuman Ad first isolated from chimpanzee fecal samples (39). Since ChAdY25 does not regularly infect humans, preexisting immunity to the vector is low (39). The ChAdOx1 nCoV-19 vaccine encodes the tPA leader sequence fused to the 5× end of a codon-optimized version of the full-length SARS-CoV-2 Spike sequence (51). ChAdOx1 nCoV-19 was reported to be highly immunogenic in mice, eliciting SARS-CoV-2 Spike-binding and live virus–neutralizing antibodies as well as antigen-specific IFNγ-producing CD8+ and CD4+ T cells (51). This immunogenicity profile was recapitulated in NHPs via either a prime-boost or a prime-only vaccine regimen and was demonstrated to protect the NHPs from viral replication in the lower respiratory tract, but less protection was observed in the upper respiratory tract (51). An additional preprint reports that intranasal administration of ChAdOx1 nCoV-19 to NHPs also protects against pneumonia but does not reduce shedding of the D614G variant of SARS-CoV-2 (66).
In addition to mice and NHPs, the ChAdOx1 nCoV-19 vaccine was also tested in ferrets. In ferrets, the vaccine was reported to induce neutralizing antibodies against both the original Wuhan-Hu-1 strain of SARS-CoV-2 and the D614G variant and reduce viral shedding of SARSCoV-2 following challenge (67).
Though the single-dose intramuscular (IM) vaccination with ChAdOx1 nCoV-19 was sufficient to partially protect NHPs and ferrets, the prime-boost program induced significantly stronger humoral immune responses and this regimen, with two doses 28 days apart, advanced to clinical trials and now to conditional approval by the EMA, WHO, and other regulatory agencies (51).
Ad5-nCoV (CanSino)
The Ad5-nCoV vaccine, similar to the ChAdOx1 nCoV-19 vaccine, encodes the full-length, mammalian-expression-codon-optimized Spike with a tPA signal peptide (68). This vaccine reportedly induced robust Spike-specific binding antibodies as well as neutralizing antibodies via both IM and intranasal (IN) administration and induced IFNγ-, TNFα-, or IL-2-producing CD8+ T cells in mice via IM administration (68). The lungs of both IMand IN-vaccinated mice were completely protected from mouse-adapted SARS-CoV-2 upon evaluation 3 or 5 days post infection. The murine nasal turbinate was completely protected in the IN group, whereas it was only partially protected in the IM group. In ferrets, the Ad5-nCoV vaccine was similarly immunogenic via either IM or IN administration and resulted in complete (IN) or partial (IM) upper respiratory tract protection against infection upon challenge with SARS-CoV-2 virus (68). Both the IN and IM versions of this vaccine were pursued in clinical trials, which have led to conditional approval of the IM-administered vaccine by the Chinese National Medical Products Administration.
Gam-COVID-Vac or Sputnik V (Gamaleya Research Institute)
The Gam-COVID-Vac vaccine, also referred to as Sputnik V, is a two-dose regimen of two different nonreplicating Ad vectors (Ad26 and Ad5) each encoding the full-length Spike gene from SARS-CoV-2 (69). Previous work in NHPs demonstrated that Ad26 induces polyfunctional cellular immune responses, which can be boosted by a subsequent Ad5 immunization. These and other heterologous Ad prime-boost data in NHPs support this regimen (70). No preclinical data have been published for the Gam-COVID-Vac vaccine, although clinical studies reference unpublished preclinical data in NHPs and hamsters, including induction of cellular and humoral immune responses that protected NHPs from infection and immunosuppressed hamsters from death due to SARS-CoV-2 infection (69). These data supported the clinical dosage for the first-in-human and later-stage trials, which led to conditional approval by the Russian Ministry of Health (69).
PHASE I/II: SAFETY AND IMMUNOGENICITY TRIALS
Each of the Ad-vectored vaccines discussed here was evaluated in first-in-human trials (phase I/II) to evaluate its safety, dosage, and immunogenicity.
Ad26.COV2.S ( Janssen/Johnson & Johnson)
Ad26.COV2.S was reported to be safe and immunogenic in September 2020 (37, 38). The phase I/II trial tested two doses, 1 × 1011 and 5 × 1010 viral particles (VP), either as a single-shot or as a two-shot vaccine with the boost 8 weeks later, in healthy individuals aged 18–55 and over 65. In both cohorts, the vaccine was found to induce Spike-specific binding and neutralizing antibodies by day 29 after the initial dose and antigen-specific CD8+ and Th1 CD4+ T cells by day 15 post vaccination (37).
In a 25-participant subset of the 18–55-year-old cohort, Spike and RBD-binding antibodies were detected in vaccine recipients as early as day 8 after the initial vaccine dose, quickly followed by detectable neutralizing antibodies at day 15 (38). These antibodies were shown to be multifunctional via antibody-dependent complement deposition (ADCD), antibody-dependent neutrophil phagocytosis (ADNP), antibody-dependent cellular phagocytosis (ADCP), and antibody dependent natural killer cell activation (ADNKA) in addition to the neutralizing activity and were sustained through at least day 71 post vaccination. The Ad26 vector also elicited neutralizing antibodies against itself in most of the participants after one dose and in all of the participants who received two doses after the second dose (38). These data led to the initiation of a phase III trial to assess the efficacy of one dose at 5 × 1010 VP, since this regimen was robustly immunogenic and minimal differences were seen in the higher-dose or boosted groups (37, 38).
ChAdOx1 nCoV-19 (AstraZeneca)
The ChAdOx1 nCoV-19 vaccine has been studied in the context of a variety of dosage regimens. The first reported phase I/II trial centered on either a single dose of 5 × 1010 VP or a primeboost regimen with a second administration of 5 × 1010 VP at day 28 post initial vaccination. The vaccination induced Spike-binding antibodies and virus-neutralizing antibodies in all the recipients by day 14, with neutralizing antibody titers increasing until measured at day 56 in the single-shot group and peaking at day 35 in the prime-boost group (71). These antibody responses included antibody-dependent neutrophil/monocyte phagocytosis (ADNP, ADCP), ADCD, and ADNKA; these Fc-mediated functions were substantially enhanced in the boosted group (72). Both the regimens also induced robust cellular responses, which were studied in detail for the single-dose group. The cellular response after one dose was found to be Th1 biased in the CD4+ T cell compartment and to also include a robust, cytotoxic CD8+ T cell response (73). These data, including the data demonstrating improved antibody responses post boost, formed the basis of phase III trials to assess the efficacy of two doses of ChAdOX1 nCoV-19.
Ad5-nCoV (CanSino)
A dose-escalation phase I trial of Ad5-nCoV tested three doses (5 × 1010, 1 × 1011, and 1.5 × 1011 VP) in participants aged 18–60 and showed that the vaccine induced RBD-binding and SARS-CoV-2-neutralizing antibodies as well as specific IFNγ-producing T cells (74). Patients with pre-existing Ad5-neutralizing titers ≥1:200 had lower responses on average than those with pre-existing Ad5-neutralizing titers ≤1:200 (74). A phase II trial with a larger participant pool further tested the immunogenicity and safety of the 5 × 1010 VP and 1 × 1011 VP doses, which were both less reactogenic than the 1.5 × 1011 VP dose in the phase I trial (13). The two doses induced comparable levels of humoral responses targeting the RBD, pseudovirus neutralization, and Spike-specific IFNγ-producing T cell response by day 28 post vaccine—and this was observed regardless of pre-existing Ad5-neutralizing titers. The 5 × 1010 VP dose regardless was less reactogenic than and nearly as immunogenic as the 1 × 1011 VP dose and was thus advanced into a phase III efficacy trial (13).
Gam-COVID-Vac (Gamaleya Research Institute)
The heterologous prime-boost regimen consisting of an Ad26 and an Ad5 dose was studied in a phase I/II trial to determine the optimal timing of the second dose as well as the order in which the two different doses should be given (69). Binding and neutralizing antibodies were detected in the participants starting 14 days post vaccination and continued to climb in titer until day 21 post vaccine in the single-dose cohorts (only one shot of either Ad26 or Ad5) and until at least day 42 post first vaccine in the boosted cohorts (69). The single-dose regimens elicited weaker cellular responses, as well as lower levels of antigen-specific CD8+ and CD4+ T cell proliferation and IFNγ release, than the two-dose regimen. The frozen vaccine and lyophilized vaccines performed similarly in each subgroup (69). The regimen selected for the phase III trial consisted of first the Ad26-vectored Spike dose of 1 × 1011 VP and then the Ad5-vectored Spike dose of 1 × 1011 VP 21 days later (12).
PHASE III: SAFETY AND EFFICACY TRIALS
Ad26.COV2.S ( Johnson & Johnson)
The phase III trial for Ad26.COV2.S by JnJ included more than 44,000 participants in Argentina, Brazil, Chile, Colombia, Mexico, Peru, South Africa, and the United States (10). Participants were randomized in a double-blind manner at a 1:1 ratio to receive 5 × 1010 VP Ad26.COV2.S or placebo. The primary endpoints of the study were efficacy against moderate to severe COVID19 at least 14 and 28 days post immunization. The single-shot Ad26.COV2.S vaccine was found to be 66.1% effective in preventing moderate to severe COVID-19 at least 28 days post vaccine across all the sites of the trial. The vaccine was reported to be 72% effective in the United States, 68% effective in Brazil, and 64% effective in South Africa with 95% of viruses identified as the B.1.351 (beta) variant (10). The vaccine provided 85% protection against severe disease and 100% protection against hospitalization and death in all regions studied.
ChAdOx1 nCoV-19 (AstraZeneca)
The ChAdOx1 nCoV-19 phase III trial by AstraZeneca was conducted in the United Kingdom, Brazil, and South Africa (11). More than 23,000 participants were randomized, in a blinded manner and 1:1 ratio, to receive either the ChAdOx1 nCoV-19 vaccine or the placebo as two doses at least 28 days apart. The primary analysis assessed symptomatic COVID-19 in patients more than 14 days after administration of the second dose. Efficacy in patients who received two standard doses was reported to be 62.1%, while efficacy in patients in the United Kingdom who received a low dose followed by a standard dose was higher, likely due to different spacing between the two doses (11). Efficacy against the B.1.351 (beta) variant was found to be negligible in a small study in South Africa, and a study in the United Kingdom observed efficacy against the B.1.1.7 (alpha) variant of 70.4% (75, 76).
Ad5-nCoV (CanSino)
CanSino has reported an efficacy for its Ad5-nCoV vaccine of 65.7% against symptomatic COVID-19 from a double-blind study conducted in Argentina, Chile, Mexico, Pakistan, and the Russian Federation (NCT04526990). Approximately 30,000 participants were randomized 1:1 to the treatment or control group. The primary endpoints assessed were the incidence of PCRpositive COVID-19 cases at least 28 days after vaccination and the incidence of severe adverse events.
Gam-COVID-Vac (Gamaleya Research Institute)
The Gam-COVID-Vac vaccine was tested in a double-blind phase III study at 25 sites in Russia (12). Almost 22,000 participants were enrolled and randomized 3:1 to receive the two-dose vaccine or placebo. The primary outcome was the incidence of polymerase chain reaction (PCR)-positive COVID-19 cases at least 21 days after the first dose of the vaccine, and the efficacy was reported to be 91.6% (12).
Summary
All of the vaccines were reported in the phase III trials to be safe. Following rollout of the vaccines, the AstraZeneca and JnJ vaccines have been associated with rare cases of thrombosis and thrombocytopenia syndrome (TTS), also known as vaccine-induced immune thrombotic thrombocytopenia (VITT), which resembles autoimmune heparin-induced thrombocytopenia with antiplatelet factor 4 antibodies (77–80). These rare events resulted in warning labels, and preclinical and clinical research is currently under way to better understand and ultimately avoid these reactions. Recently, a case fulfilling the definition of TTS/VITT has also been reported with the Moderna mRNA vaccine (81). The AstraZeneca and JnJ vaccines have also recently been associated with rare cases of Guillain-Barré syndrome.
VARIANTS OF CONCERN
Although coronaviruses have RNA proofreading mechanisms for replication, mutations do arise, including in Spike to escape host immune pressure (82, 83). Due to the extremely high number of infections of SARS-CoV-2 since late 2019, it is not surprising that variants of SARS-CoV-2 have developed (1, 2). Mutations in Spike and other viral proteins are associated with SARS-CoV-2 variants of interest and variants of concern (VOCs) (84). Due to the ways in which these mutations alter Spike, they can increase affinity to ACE2 and decrease affinity to antibodies raised against the original Wuhan-Hu-1 Spike either through infection or vaccination, since the vaccines tested and approved thus far were based on this original Spike (85). These alterations include, for example, charge switches or additions (e.g., E484K of the B.1.351 and P.1 variants or L452R of the B.1.617 variant) or addition of large aromatic side chains (e.g., N501Y of the B.1.1.7, B.1.351, and P.1 variants), which abrogate binding of antibodies unable to accommodate these significant changes to the paratope (84, 86). Many reports have shown that certain VOCs, namely B.1.351 (beta), P.1 (gamma), and B.1.617.2 (delta), are less potently neutralized and less tightly bound in vitro by serum from vaccinated individuals (87, 88). Some phase III trial sites were in areas with high VOC rates, and these trials, such as that of Ad26.COV2.S and ChAdOx1 nCoV-19 in South Africa, provide insight into the efficacy of the vaccines expressing Spike from the Wuhan-Hu-1 or WA1/2020 strain against the VOCs. Ad26.COV2.S was found to be 64% efficacious against mild to moderate disease and 82% effective against severe disease in South Africa, where the B.1.351 (beta) variant was dominant (10). The VOCs have sparked the development and production of variant versions of the vaccines currently in use.
LONG-TERM DURABILITY
The COVID-19 pandemic resulted in the most rapid vaccine development in history, and now millions of people have been vaccinated by Ad-vectored vaccines. Past clinical trials of Ad vaccines have reported antibody responses lasting at least 1 year (34, 35). The Ad26.COV2.S vaccine from JnJ has a reported durability of cellular and humoral responses of up to 8 months, including increased breadth of neutralizing antibodies over time, against variants including B.1.351 (beta) and B.1.617.2 (delta) (89). Updates on the durability of the COVID-19 vaccine responses will continue to emerge in real time.
CONCLUSIONS
Ad-vectored vaccines are playing an important role in the global vaccine efforts against COVID-19. The highly immunogenic, easily adaptable, and readily manufactured Ad vectors have proven well suited for pandemic responsiveness: Four Ad-based vaccines obtained regulatory approval in approximately 1 year after the SARS-CoV-2 sequence became available. The Ad26.COV2.S, ChAdOx1 nCov-19, Ad5 nCoV, and Gam-COVID-Vac vaccines have demonstrated protective efficacy against symptomatic COVID-19 disease in humans. Clinical research in academia and industry is continuing on additional Ad-vectored vaccines for SARS-CoV-2. Ad vaccines will also likely be developed against a variety of pathogens in the future.
Table 2.
Efficacy and approval status of adenovirus-vectored COVID-19 vaccines
| Company name | Vaccine name | Adenovirus serotype | Reported efficacy against symptomatic COVID-19 |
|---|---|---|---|
| Johnson & Johnson | Ad26.COV2.S | Ad26 | 66.1%(72% United States, 68% Brazil, 64% South Africa) |
| AstraZeneca | ChAdOx1 nCoV-19 | ChAdOx1 | 76% |
| CanSino | Ad5-nCoV | Ad5 | 65.3% |
| Gamaleya Research Institute | Gam-COVID-Vac or Sputnik V | Ad5, Ad26 | 91.6% |
Footnotes
DISCLOSURE STATEMENT
C.J.-D. and D.H.B. have done laboratory research involving the JnJ vaccine. D.H.B. is a coinventor on provisional vaccine patents (63/121,482; 63/133,969; 63/135,182).
LITERATURE CITED
- 1.Zu F, Zhao S, Yu B, et al. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579:265–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang X-L, Wang X-G, et al. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO (World Health Organ.). 2020. WHO COVID-19 Dashboard https://covid19.who.int/
- 4.Lu R, Zhao X, Li J, et al. 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395:565–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. 2020. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med 383:2603–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden LR, El Sahly HM, Essink B, et al. 2021. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med 384:403–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al Kaabi N, Zhang Y, Xia S, et al. 2021. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA 326(1):35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanriover MD, Doganay HL, Akova M, et al. 2021. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 385:875–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath PT, Galiza EP, Baxter DN, et al. 2021. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N. Engl. J. Med 10.1056/NEJMoa2107659 [DOI] [PMC free article] [PubMed]
- 10.Sadoff J, Gray G, Vandebosch A, et al. 2021. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med 384:2187–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voysey M, Clemens SAC, Madhi SA, et al. 2021. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. 2021. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397:671–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu F-C, Guan X-H, Li Y-H, et al. 2020. Immunogenicity and safety of a recombinant adenovirus type5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 396:479–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wrapp D, Wang N, Corbett KS, et al. 2020. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367:1260–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F 2016. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol 3:237–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosch BJ, Van der Zee R, De Haan CA, Rottier PJ. 2003. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol 77:8801–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouwer PJ, Caniels TG, van der Straten K, et al. 2020. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science 369:643–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju B, Zhang Q, Ge J, et al. 2020. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 584:115–19 [DOI] [PubMed] [Google Scholar]
- 19.Kreer C, Zehner M, Weber T, et al. 2020. Longitudinal isolation of potent near-germline SARS-CoV-2neutralizing antibodies from COVID-19 patients. Cell 182:843–54.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbiani DF, Gaebler C, Muecksch F, et al. 2020. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 584:437–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wec AZ, Wrapp D, Herbert AS, et al. 2020. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science 369:731–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zost SJ, Gilchuk P, Chen RE, et al. 2020. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat. Med 26:1422–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bootz A, Karbach A, Spindler J, et al. 2017. Protective capacity of neutralizing and non-neutralizing antibodies against glycoprotein B of cytomegalovirus. PLOS Pathog 13:e1006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahan K, Yu J, Mercado NB, et al. 2021. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 590:630–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebre MS, Brito LA, Tostanoski LH, et al. 2021. Novel approaches for vaccine development. Cell 184:1589–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginsberg HS. 1984. The Adenoviruses Berlin: Springer [Google Scholar]
- 27.Wold W, Horwitz M. 2007. Adenoviruses. In Fields Virology, ed. Knipe DM, Howley PM, pp. 2395–436. Philadelphia: Lippincott Williams & Wilkins [Google Scholar]
- 28.HAdV Working Group. 2021. HAdV Working Group http://hadvwg.gmu.edu/
- 29.Crystal RG, McElvaney NG, Rosenfeld MA, et al. 1994. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat. Genet 8:42–51 [DOI] [PubMed] [Google Scholar]
- 30.Lemarchand P, Jaffe HA, Danel C, et al. 1992. Adenovirus-mediated transfer of a recombinant human alpha 1-antitrypsin cDNA to human endothelial cells. PNAS 89:6482–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crystal RG. 2014. Adenovirus: the first effective in vivo gene delivery vector. Hum. Gene Ther 25:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iacobelli-Martinez M, Nemerow GR. 2007. Preferential activation of Toll-like receptor nine by CD46utilizing adenoviruses. J. Virol 81:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. 2010. From vaccines to memory and back. Immunity 33:451–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salisch NC, Stephenson KE, Williams K, et al. 2021. A double-blind, randomized, placebo-controlled phase 1 study of Ad26.ZIKV.001, an Ad26-vectored anti–Zika virus vaccine. Ann. Int. Med 174:585–94 [DOI] [PubMed] [Google Scholar]
- 35.Baden LR, Stieh DJ, Sarnecki M, et al. 2020. Safety and immunogenicity of two heterologous HIV vaccine regimens in healthy, HIV-uninfected adults (TRAVERSE): a randomised, parallel-group, placebocontrolled, double-blind, phase 1/2a study. Lancet HIV 7:e688–e698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollard AJ, Launay O, Lelievre J-D, et al. 2021. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis 21:493–506 [DOI] [PubMed] [Google Scholar]
- 37.Sadoff J, Le Gars M, Shukarev G, et al. 2021. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N. Engl. J. Med 384:1824–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephenson KE, Le Gars M, Sadoff J, et al. 2021. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 325:1535–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dicks MD, Spencer AJ, Edwards NJ, et al. 2012. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLOS ONE 7:e40385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbink P, Kirilova M, Boyd M, et al. 2018. Rapid cloning of novel rhesus adenoviral vaccine vectors. J. Virol 92:e01924–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coughlan L 2020. Factors which contribute to the immunogenicity of non-replicating adenoviral vectored vaccines. Front. Immunol 11:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palm A-KE, Henry C. 2019. Remembrance of things past: long-term B cell memory after infection and vaccination. Front. Immunol 10:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danthinne X, Imperiale M. 2000. Production of first generation adenovirus vectors: a review. Gene Ther 7:1707–14 [DOI] [PubMed] [Google Scholar]
- 44.Bett AJ, Prevec L, Graham FL. 1993. Packaging capacity and stability of human adenovirus type 5 vectors. J. Virol 67:5911–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barouch DH, Kik SV, Weverling GJ, et al. 2011. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 29:5203–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saxena M, Van TTH, Baird FJ, et al. 2013. Pre-existing immunity against vaccine vectors—friend or foe? Microbiology 159(Part 1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pichla-Gollon SL, Lin S-W, Hensley SE, et al. 2009. Effect of preexisting immunity on an adenovirus vaccine vector: in vitro neutralization assays fail to predict inhibition by antiviral antibody in vivo. J. Virol 83:5567–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abbink P, Lemckert AA, Ewald BA, et al. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol 81:4654–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mercado NB, Zahn R, Wegmann F, et al. 2020. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586:583–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsieh C-L, Goldsmith JA, Schaub JM, et al. 2020. Structure-based design of prefusion-stabilized SARSCoV-2 spikes. Science 369:1501–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Doremalen N, Lambe T, Spencer A, et al. 2020. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 586:578–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandrashekar A, Liu J, Martinot AJ, et al. 2020. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 369:812–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng W, Bao L, Liu J, et al. 2020. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 369:818–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rockx B, Kuiken T, Herfst S, et al. 2020. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368:1012–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munster VJ, Feldmann F, Williamson BN, et al. 2020. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature 585:268–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan JF-W, Zhang AJ, Yuan S, et al. 2020. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in a golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis 71:2428–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imai M, Iwatsuki-Horimoto K, Hatta M, et al. 2020. Syrian hamsters as a small animal model for SARSCoV-2 infection and countermeasure development. PNAS 117:16587–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sia SF, Yan L-M, Chin AW, et al. 2020. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 583:834–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim Y-I, Kim S-G, Kim S-M, et al. 2020. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 27:704–9.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi J, Wen Z, Zhong G, et al. 2020. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 368:1016–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bao L, Deng W, Huang B, et al. 2020. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583:830–33 [DOI] [PubMed] [Google Scholar]
- 62.Dinnon KH, Leist SR, Schäfer A, et al. 2020. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 586:560–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dagotto G, Mercado NB, Martinez DR, et al. 2021. Comparison of subgenomic and total RNA in SARSCoV-2-challenged rhesus macaques. J. Virol 95:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tostanoski LH, Wegmann F, Martinot AJ, et al. 2020. Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat. Med 26:1694–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu J, Tostanoski LH, Mercado NB, et al. 2021. Protective efficacy of Ad26.COV2.S against SARS-CoV-2 B.1.351 in macaques. Nature 596:423–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Doremalen N, Purushotham J, Schulz J, et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces shedding of SARS-CoV-2 D614G in rhesus macaques. bioRxiv 426058 10.1101/2021.01.09.426058 [DOI]
- 67.Marsh GA, McAuley AJ, Au GG, et al. 2021. ChAdOx1 nCoV-19 (AZD1222) vaccine candidate significantly reduces SARS-CoV-2 shedding in ferrets. NPJ Vaccines 6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu S, Zhong G, Zhang J, et al. 2020. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun 11:4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Logunov DY, Dolzhikova IV, Zubkova OV, et al. 2020. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, nonrandomised phase 1/2 studies from Russia. Lancet 396:887–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J, Ewald BA, Lynch DM, et al. 2008. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol 82:4844–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Folegatti PM, Ewer KJ, Aley PK, et al. 2020. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396:467–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barrett JR, Belij-Rammerstorfer S, Dold C, et al. 2021. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med 27:279–88 [DOI] [PubMed] [Google Scholar]
- 73.Ewer KJ, Barrett JR, Belij-Rammerstorfer S, et al. 2021. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med 27:270–78 [DOI] [PubMed] [Google Scholar]
- 74.Zhu F-C, Li Y-H, Guan X-H, et al. 2020. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-inhuman trial. Lancet 395:1845–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Madhi SA, Baillie V, Cutland CL, et al. 2021. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N. Engl. J. Med 384:1885–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Emary KR, Golubchik T, Aley PK, et al. 2021. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet 397:1351–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muir K-L, Kallam A, Koepsell SA, Gundabolu K. 2021. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N. Engl. J. Med 384:1964–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sadoff J, Davis K, Douoguih M. 2021. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination— response from the manufacturer. N. Engl. J. Med 384:1965–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schultz NH, Sørvoll IH, Michelsen AE, et al. 2021. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med 384:2124–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greinacher A, Thiele T, Warkentin TE, et al. 2021. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med 384:2092–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sangli S, Virani A, Cheronis N, et al. 2021. Thrombosis with thrombocytopenia after the messenger RNA–1273 vaccine. Ann. Int. Med 10.7326/L21-0244 [DOI] [PMC free article] [PubMed]
- 82.Sanjuán R, Nebot MR, Chirico N, et al. 2010. Viral mutation rates. J. Virol 84:9733–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Denison MR, Graham RL, Donaldson EF, et al. 2011. Coronaviruses: an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol 8:270–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.CDC (Cent. Dis. Control Prev.). 2021. SARS-CoV-2 variant classifications and definitions https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html. Accessed Mar. 16, 2021
- 85.Ramanathan M, Ferguson ID, Miao W, Khavari PA. 2021. SARS-CoV-2 B.1.1.7 and B.1.351 Spike variants bind human ACE2 with increased affinity. Lancet Infect. Dis 8:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuan M, Huang D, Lee C-CD, et al. 2021. Structural and functional ramifications of antigenic drift in recent SARS-CoV-2 variants. Science 373:818–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alter G, Yu J, Liu J, et al. 2021. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 596:268–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ikegame S, Siddiquey M, Hung C-T, et al. 2021. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Res. Square 10.21203/rs.3.rs-400230/v1 [DOI] [PMC free article] [PubMed]
- 89.Barouch DH, Stephenson KE, Sadoff J, et al. 2021. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N. Engl. J. Med 385:951–53 [DOI] [PMC free article] [PubMed] [Google Scholar]


