Abstract
Background and Aims
Many plant taxa in the Qinghai–Tibetan Plateau (QTP) and the Hengduan Mountains (HM) radiated rapidly during the Quaternary but with frequent secondary contact between diverging populations. Incomplete lineage sorting and introgressive hybridization might be involved during the rapid radiation, but their effects on phylogeography have not been fully determined.
Methods
We investigated the chloroplast DNA (cpDNA)/internal transcribed spacer (ITS) sequence variations of 611 samples of Rhodiola bupleuroides, R. discolor, R. fastigiata and R. chrysanthemifolia from the QTP and HM to compare the phylogeographic patterns between the four species with different evolutionary histories, geographic ranges and reproductive modes.
Key Results
The divergence times of these species were consistent with the last peak of in situ speciation in the HM. While closely related species exhibited different phylogeographic patterns, they shared several ribotypes and haplotypes in sympatric populations, suggesting introgressive hybridization. A significant phylogenetic discordance between ribotypes and haplotypes was detected in three species, implying incomplete lineage sorting. Rhodiola discolor houses an extraordinary richness of cpDNA haplotypes, and this finding may be attributed to adaptive radiation.
Conclusion
In addition to geographic isolation and climate oscillations during the Quaternary, both introgressive hybridization and incomplete lineage sorting play important roles in species that experienced rapid diversification in the QTP and HM.
Keywords: Rhodiola, hybridization, incomplete lineage sorting, phylogeography
INTRODUCTION
The Qinghai–Tibetan Plateau (QTP) is widely recognized as a biodiversity hotspot, in part because of the extraordinary diversity and endemism of vascular plants (Sun et al., 2017; Cai et al., 2019). The QTP arose after the collisions between the Indian and Eurasian plates approx. 50 million years ago (Mya), followed by the collision of the Indian plate with the present-day QTP at its eastern margins to form the Hengduan Mountains (HM; Royden et al., 2008). The orogenesis of the QTP and HM led to the formation and subsequent intensification of Asian monsoons and to the cycle of droughts in central Asia (Mulch and Chamberlain, 2006; Royden et al., 2008; Favre et al., 2015). This phenomenon resulted in remarkable differences in geographic and climatic histories between the QTP and HM, thus affecting the distributions and phylogeographic patterns of resident organisms. Much attention has focused on the possible effects of these historical geological events in the two regions on biodiversity through phylogeographic approaches (Shen et al., 2019; Wang et al., 2019), whereas less research has been focused on understanding the roles of secondary contact, introgression and incomplete lineage sorting (ILS), in shaping contemporary biodiversity patterns.
In general, we found differentiation between conspecific populations occupying the QTP and HM in many species such as Buddleja crispa, as indicated by gene flow (Yue et al., 2012; Zhang et al., 2015). This pattern can be attributed to various causes. First, it may stem from the recent uplift of the QTP. The evident phylogeographic divisions between the eastern and western sides of the Mekong–Salween Divide for Taxus wallichiana (Liu et al., 2013) and Sinopodophyllum hexandrum (Li et al., 2011) formed during the last major uplift of the QTP. Populations of these species would have persisted on both sides of the Divide during glacial periods, and then expanded to their current distributions but impeded by the geographical barriers of the QTP. Second, such a pattern is strongly influenced by local environmental conditions. For instance, the west–middle–east lineages of Hippophae tibetana are coincident with patterns of precipitation (Wang et al., 2010). Third, the phylogeographic divisions of some species, such as Pedicularis longiflora (Yang et al., 2008) and Tsuga dumosa (Cun and Wang, 2010), formed in the late Pleistocene and were significantly affected by climate oscillations. Populations of both species expanded to the QTP from the HM refugia after the glacial maximum, forming the present west–east divisions, with low genetic diversity within the QTP populations but high diversity within the HM populations.
Significant phylogeographic structure is often apparent in plant species, such as Ligularia tongolensis (Wang et al., 2011) and Pinus yunnanensis (Wang et al., 2013), within the HM itself. Gene flow in these species is restricted by the complex topography, species-specific microenvironments occupied by each taxon, or habitat fragmentation. The complex topography and diverse habitats resulted in an in situ HM speciation rate that is three times higher than that of the QTP (Xing and Ree, 2017; Ding et al., 2020; Lai et al., 2021), contributing to the present biodiversity hotspot (Myers et al., 2000). Rapid speciation rates suggest that ILS may be a pervasive phenomenon for many related species within the HM because of insufficient time for fixation of alternative alleles across loci. Expansion from refugia would have led to secondary contact of previously isolated populations or closely related species in this region. Cross-species introgressive hybridization (Yang et al., 2019) has been frequently detected in the zones of sympatry or parapatry among species (Abbott et al., 2013) and played a role in the diversification of species (Grant et al., 2005). However, the roles of ILS and introgressive hybridization have not been rigorously assessed for plants in the QTP and HM, although the number of phylogeography studies has increased dramatically in recent years.
In the present study, we focused on the comparative phylogeography of Rhodiola species distributed in the QTP and HM. Rhodiola is a perennial herbaceous genus that originated (21 Mya) and diversified (12 Mya) largely coincident with the two extensive uplifts of the QTP (Zhang et al., 2014a). Therefore, the recent evolution of Rhodiola has been coincident with the orogenesis of the QTP and subsequent climate changes. Previous studies on single Rhodiola species such as R. alsia (Q. Gao et al., 2012) and R. dumulosa (Hou and Lou, 2014) have mainly been restricted to regions within the QTP or HM. A recent comparison of R. fastigiata and R. alsia indicated that even species with similar morphology and distribution can have different demographic histories in Quaternary glacial periods (Zhang et al., 2018). In contrast, a phylogeographic study of the Rhodiola sect. Trifida (included seven species) demonstrated that species with similar geographic distribution and ecological niches responded similarly to Quaternary climatic oscillations (Li et al., 2018).
The present study focused on four Rhodiola species belonging to different Rhodiola clades (Zhang et al., 2014a; Ding et al., 2020), including sister species and species from different clades, with varying distributions. We include species that are endemic to either the QTP or HM, span both regions, and both monoecious and dioecious species. Rhodiola bupleuroides and R. discolor are sister species, and the former is mainly distributed in the QTP, growing in thickets, grasslands and rock crevices on slopes at 2400–5700 m above sea level (masl), whereas the latter is restricted to the HM, occurring in forests, grassy slopes and rocky cliffs between 2800 and 4300 masl. Rhodiola fastigiata belongs to the same evolutionary clade consisting of the above-mentioned sister species and has a relatively wide distribution across the QTP and the HM, mainly growing in rocky slopes between 3500 and 5400 masl. Rhodiola chrysanthemifolia is from a distinct clade; it is mainly found in grasslands, rocks, rock crevices between 3200 and 4200 masl and ranges across the QTP and HM. Rhodiola chrysanthemifolia is monoecious, while the other three species are dioecious (Wu and Raven, 2001).
We compared the phylogeographic patterns to determine any significant differences in phylogeographic patterns between species occupying different regions, the driving factors for phylogeographic patterns of Rhodiola and whether ILS or hybridization causes phylogenetic discordance in the focal species.
MATERIALS AND METHODS
Sampling and DNA extraction
To assess the phylogeographic patterns of these four species, we investigated the chloroplast DNA (cpDNA)/internal transcribed spacer (ITS) sequence variations in 611 individuals from 64 populations covering most of their respective geographical ranges, including 13 populations of Rhodiola bupleuroides, 27 populations of R. discolor, 11 populations of R. fastigiata and 13 populations of R. chrysanthemifolia (Fig. 1). Information on sampling localities for each population is summarized in Supplementary data Table S1. To avoid sampling the same individuals twice, we conducted sampling at least 10 m apart. Young leaves were collected and stored in silica gel for drying. Total genomic DNA was extracted from the dried leaves by using the Plant Genomic DNA Kit (TianGen Biotech Co., Ltd). Voucher specimens of all populations are preserved in the herbarium of Fudan University, Shanghai, China.
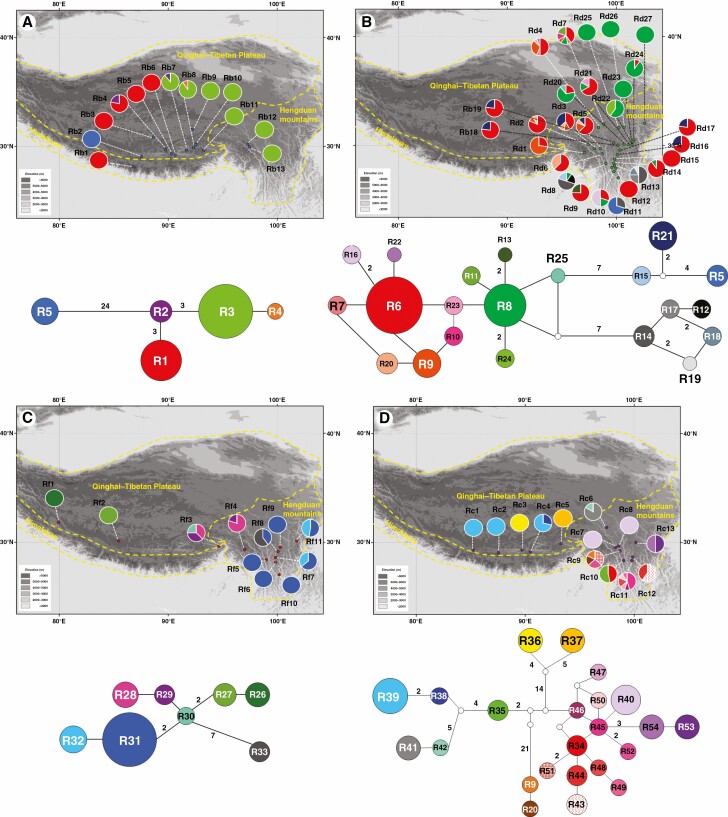
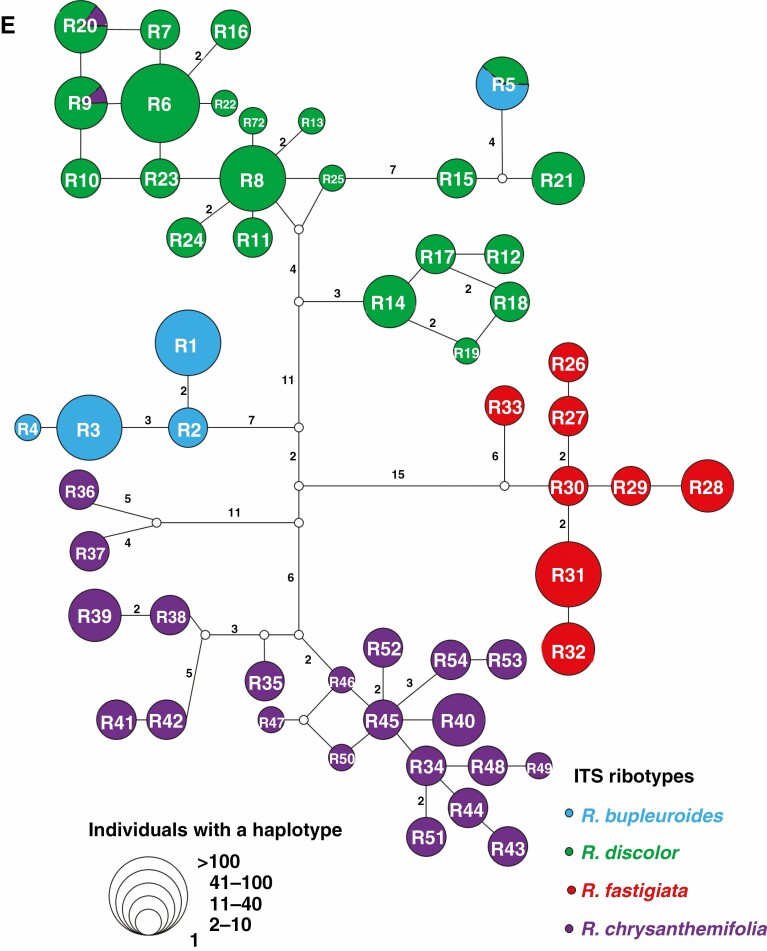
Fig. 1.
Distribution of ITS ribotypes with their respective ribotype networks for (A) Rhodiola bupleuroides, (B) R. discolor, (C) R. fastigiata and (D) R. chrysanthemifolia. (E) ITS ribotype network for all four Rhodiola species combined.
Amplification and sequencing
Two cpDNA intergenic spacers and the ITS fragment were amplified and sequenced for these species. The primers used were c (5′-CGAAATCGGTAGACGCTACG-3′) and f (5′-ATTTGAACTGGTGACACGAG-3′; Taberlet et al., 1991) for trnL-F, trnS (5′-GCCGCTTTAGCTCACTCAGC-3′) and trnG (5′-GAACGAATCACACTTTTACCAC-3′) for trnS-G (Hamilton, 1999), and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) for ITS (Mayuzumi and Ohba, 2004). We used PCR with 30 μL reaction mixture volumes containing 3 μL of 10× buffer, 1.5 μL of each primer, 4.8 μL of dNTP mixture, 0.3 μL of TakaRa Ex Taq (5 U μL–1) and 50 ng of template genomic DNA. The PCR cycling profiles are as follows: initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 53–55 °C (adjust according to different primers) for 45 s, extension at 72 °C for 1–2 min (adjust according to the length of segments) and a final extension at 72 °C for 10 min. PCR products were sequenced in both directions by using a Majorbio 3730 automatic DNA sequencer. To assess the repeatability of the sequencing results, we resequenced 52 individuals with private ribotypes or haplotypes and individuals with shared ITS ribotypes and cpDNA haplotypes at BGI Genomics Co., Ltd. The sequences from both companies were identical, indicating the reliability of our results.
Sequence alignment
All sequences were checked using Chromas 2.32 (http://technelysium.com.au/). Mononucleotide repeat length variations in cpDNA and ITS were excluded because of ambiguous alignment. DNA sequence alignment was performed with Clustal X (Thompson et al., 1997) and checked manually. All ITS and cpDNA fragments were uploaded to NCBI (Supplementary data Appendix 1). For all subsequent analyses, gaps were treated as missing. We did not consider single base mutations with frequencies lower than 1 %, but single base mutation that appeared more than twice within a population were kept. The two cpDNA sequences (trnL-F and trnS-G) were concatenated for all analyses. Both ITS ribotypes and cpDNA haplotypes were resolved using the PHASE algorithm (Stephens et al., 2001; Stephens and Donnelly, 2003) in DnaSP 5.10.01 (Librado and Rozas, 2009) by using default settings for 10 000 iterations.
Genetic diversity and population structure analyses
Arlequin version 3.5 (Excoffier and Lischer, 2010) was used to calculate haplotype diversities (h) and nucleotide diversities (π) for each population. Average gene diversity (HS), total gene diversity (HT) and a comparison between population divergence (GST and NST) with 1000 permutation tests were estimated using PERMUT (Pons and Petit, 1996). A significantly higher NST than GST indicates phylogeographic structure, where closely related ribotypes or haplotypes are often found in the same area. We explored the population genetic structure for the four Rhodiola species as a complex and for each species for ITS and cpDNA by using STRUCTURE v2.3.3 (Pritchard et al., 2000). The program was not provided with prior information on ancestral populations and was run ten times for each value of K ancestral populations, with K varying from 1 to total population number plus 3. The burn-in period and Markov chain Monte Carlo (MCMC) were set to 10 000–100 000 iterations, respectively, and ten replicates per K were run. The most likely value of K was determined using the ΔK statistic of Evanno et al. (2005) calculated in Structure Harvester v.0.6.94 (Earl, 2012). The ITS ribotype and cpDNA haplotype networks were constructed in NETWORK 5 by using median joining (Bandelt et al., 1999) and maximum parsimony (MP) methods (Polzin and Daneshmand, 2003).
Phylogenetic relationships
Phedimus aizoon was chosen as the outgroup for the phylogenetic analysis based on the Rhodiola phylogeny of Zhang et al. (2014a). The ITS, trnL-F and trnS-G sequences of P. aizoon were downloaded from the NCBI (accession numbers: AB088615, KF113788 and KJ570333). We estimated the phylogenetic relationships of ITS ribotypes and cpDNA haplotypes using MP, Maximum likelihood (ML) and Bayesian inference (BI). Parsimony analysis was done using PAUP 4.0b10 (Swofford and Sullivan, 2003), in which all characters were weighed equally, and indels were treated as missing data, by using heuristic searches with MULTREES and TBR branch swapping. PAUP was set to run 1000 replicates each with ten random sequence additions and NNI branch swapping for the bootstrap analysis. ML analysis was performed in RAxML version 7.0.4 (Stamatakis, 2006) by using the general time reversible (GTR) model and gamma rate heterogeneity with 1000 bootstraps. For BI, we used a TrNef+G model for ITS and a TVM+I+G model for cpDNA as determined by the Akaike information criterion in Modeltest version 3.7 (Posada and Buckley, 2004; Posada, 2006). Bayesian analyses were conducted in MrBayes version 3.2.1 (Ronquist and Huelsenbeck, 2003) with the following parameter settings: nst = 6, rates = gamma, pinvar = 0, base = (0.25, 0.25, 0.25, 0.25) for ITS; and nst = 6, rates = gamma, pinvar = 0.6531, base = (0.3642, 0.1570, 0.1623, 0.3165) for cpDNA. Each run was started from a random tree by using four Metropolis-coupled MCMCs with 3 000 000 generations for ITS and 20 000 000 for cpDNA, and sampling was conducted for every 100 generations. We used a burn-in of 25 % and a 50 % majority rule consensus tree generated from the remaining trees. Posterior probabilities were used to assess the robustness of the BI trees.
Historical demography
Neutrality tests with Tajima’s D (Tajima, 1989) and Fu’s Fs (Fu, 1997) and pairwise mismatch distributions (Rogers and Harpending, 1992; Schneider and Excoffier, 1999) were used to test for signatures of demographic expansion by Arlequin version 3.5. The sum of squared differences (SSD) and raggedness index of Harpending (HRag; Harpending, 1994) were employed to test the sudden expansion model (Rogers and Harpending, 1992). For any evidence of expansion, we used the equation t = τ/2u (Rogers and Harpending, 1992; Rogers, 1995) to estimate the time since expansion. The value of u was estimated using the equation u = μ × k × g, where μ is the number of substitutions per site per year (ss–1 year–1), k is the average sequence length and g is the generation time. The substitution rate used to estimate the divergence times was 6.075 × 10–9 ss–1 year–1 for ITS (Richardson et al., 2001; Zhang et al., 2014b, 2018; Li et al., 2018) and 1.52 × 10-9 ss–1 year–1 for cpDNA (Yamane et al., 2006; Zhang et al., 2014b, 2018; Li et al., 2018). The value for g was 10 years based on empirical evidence from previous studies on Rhodiola (Zhang et al., 2014b, 2018; Li et al., 2018).
Lineage divergences
Beast version 1.8.2 (Drummond and Rambaut, 2007) was used to infer the divergence time between lineages based on the ITS and cpDNA dataset. To examine the rate constancy among lineages, we performed a likelihood ratio test by using PAUP version 4.0b10 (Swofford and Sullivan, 2003). A molecular clock was rejected for ITS (2logeLR = 216, d.f. = 53, P < 0.05) and cpDNA (2logeLR = 292, d.f. = 156, P < 0.01). Divergence times and confidence intervals were estimated in BEAST, assuming GTR+GAMMA substitution models, lognormal relaxed clocks and a birth–death process. BEAST was run for 10 000 000 generations for both ITS and cpDNA data, and all parameters were sampled for every 2000 generations. Tracer v1.6.0 (Drummond et al., 2012) was used to evaluate convergence MCMC and ensure that all effective sample sizes for each parameter are >200. Tree annotator v1.6.1 was used to build the maximum clade credibility tree (burn-in 25 %). To estimate divergence times, we used the mean of normal distribution with the 95 % confidence interval of the substitution rate in shrubs and herbs for ITS (6.075 × 10–9 ss–1 year–1, s.d. = 1.590 × 10–9 ss–1 year–1; Richardson et al., 2001; Zhang et al., 2014b, 2018; Li et al., 2018) and the evolutionary rate of the non-coding regions of the cpDNA (1.52 × 10-9 ss–1 year–1, s.d. = 0.06 × 10–9 ss–1 year–1; Yamane et al., 2006; Zhang et al., 2014b, 2018; Li et al., 2018), because no fossils of Rhodiola or its close relatives were found.
RESULTS
ITS sequence variation and ribotype distribution
The length of aligned ITS sequences was 623 bp. The estimated haplotype diversity h and nucleotide diversity π varied across populations, with values of 0–0.810 and 0–17.245×10-3 for the four species, respectively (Supplementary data Table S2). HS values were much lower than HT values for the ITS ribotypes of the four species (Table 1). A total of 54 different ITS ribotypes were recovered, comprising five in R. bupleuroides (R1–R5), 21 in R. discolor (R5–R25), eight in R. fastigiata (R26–R33) and 23 in R. chrysanthemifolia (R9, R20 and R34–R54, Supplementary data Table S1). Two, ten, four and nine populations of R. bupleuroides, R. discolor, R. fastigiata and R. chrysanthemifolia, respectively, harboured private ribotypes (Supplementary data Table S1; Fig. 1). Ribotype R5 occurred in R. bupleuroides population Rb2 at Lhazê (ten individuals) and R. discolor population Rd11 at Lijiang (seven individuals). Ribotypes R9 and R20 were shared by the Markam populations of R. chrysanthemifolia (two individuals each of R9 and R20 in Rc9) and R. discolor (three individuals of R9 and one individual of R20 in Rd5). The shared ribotypes R9 and R20 were also found in other geographically proximate populations of R. discolor (Rd1, Rd2, Rd3, Rd4, Rd6 and Rd7, Supplementary data Table S1; Fig. 1E).
Table 1.
Genetic diversity and differentiation of four Rhodiola species estimated based on ITS and cpDNA sequences
| Species | ITS | cpDNA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H S (s.e.) | H T (s.e.) | π (×10–3) | G ST (s.e.) | N ST (s.e.) | H S (s.e.) | H T (s.e.) | π (×10–3) | G ST (s.e.) | N ST (s.e.) | |
| R. bupleuroides | 0.062 (0.0349) | 0.630 (0.0719) | 0.156 (0.357) | 0.901 (0.0558) | 0.980* (0.0167) | 0.659 (0.0537) | 0.943 (0.0284) | 5.544 (5.063) | 0.301 (0.0451) | 0.674* (0.0628) |
| R. discolor | 0.400 (0.0507) | 0.754 (0.0418) | 1.717 (1.878) | 0.469 (0.0597) | 0.545n.s. (0.0744) | 0.724 (0.0356) | 0.985 (0.0062) | 11.262 (8.842) | 0.264 (0.0360) | 0.282n.s. (0.0381) |
| R. fastigiata | 0.244 (0.0887) | 0.748 (0.1154) | 0.718 (1.6363) | 0.674 (0.1100) | 0.779n.s. (0.1125) | 0.455 (0.0997) | 0.983 (0.0194) | 14.705 (15.2859) | 0.537 (0.1018) | 0.657* (0.1004) |
| R. chrysanthemifolia | 0.301 (0.0880) | 0.956 (0.0389) | 1.811 (4.7220) | 0.685 (0.0866) | 0.807*(0.1006) | 0.241 (0.0826) | 0.973 (0.0175) | 2.568 (3.8444) | 0.752 (0.0870) | 0.836n.s. (0.0696) |
H S, average gene diversity; HT, total gene diversity; π, nucleotide diversity; NST, population divergence for ordered alleles; GST, population divergence for unordered alleles. *NST is significantly larger than GST (P < 0.05), n.s. not significant.
cpDNA sequence variation and haplotype distribution
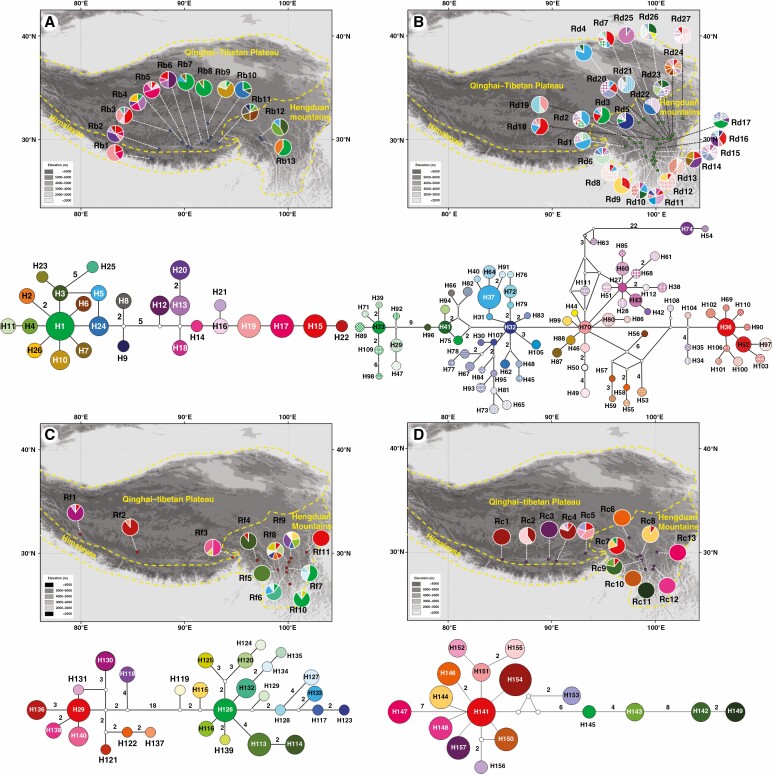
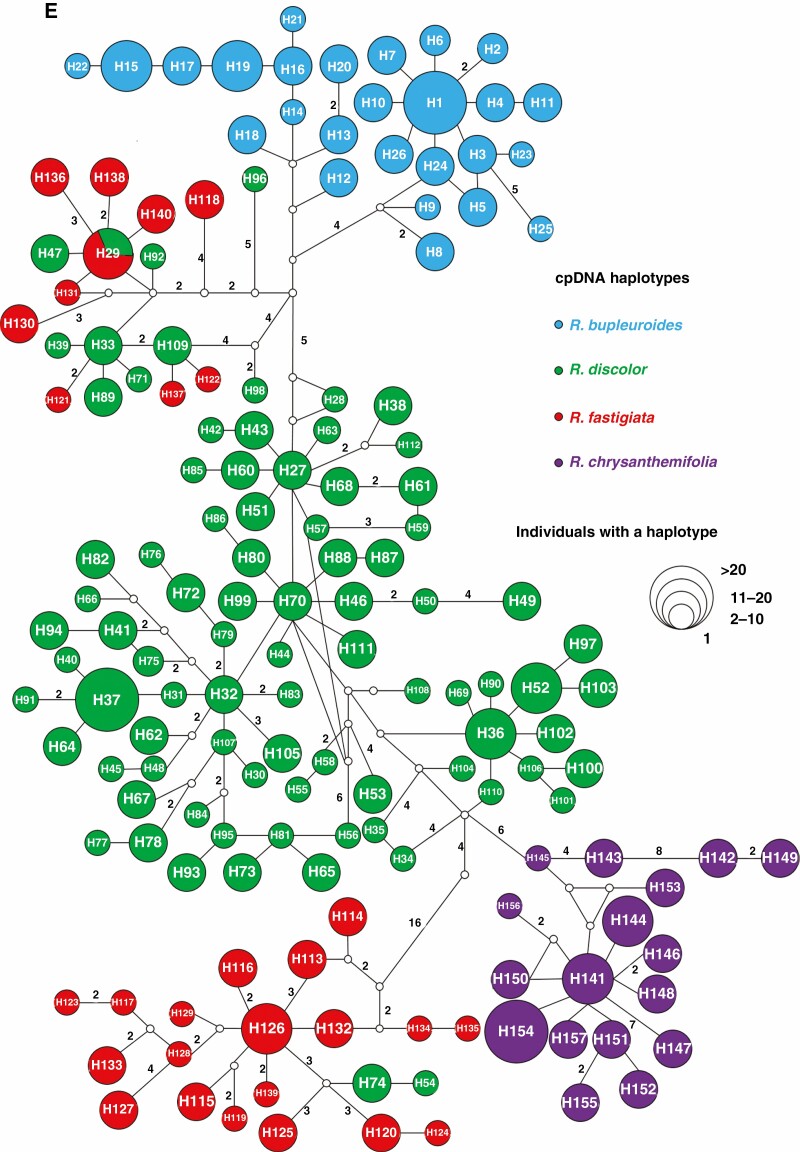
The sequencing of the intergenic spacers trnL-F and trnS-G generated fragments of 941 and 722 bp, respectively. The estimated haplotype diversity h and nucleotide diversity π were 0–0.978 and 0–43.167×10-3 for the four species, respectively (Supplementary data Table S2). HS values were much lower than HT values for the cpDNA haplotypes of the four focal species (Table 1). A total of 157 haplotypes were found, comprising 26 in R. bupleuroides (H1–H26), 86 in R. discolor (H27–H112), 29 in R. fastigiata (H113–H140, H29) and 16 in R. chrysanthemifolia (H141–H157) (Supplementary data Table S1). A total of ten, 24, ten and nine populations of R. bupleuroides, R. discolor, R. fastigiata and R. chrysanthemifolia, respectively, had private haplotypes (Supplementary data Table S1; Fig. 2). The cpDNA haplotype H29 was shared by the Markam R. discolor population Rd6 (six individuals) and four R. fastigiata populations (ten individuals in Rfas11 and one individual each in Rfas1, Rfas4 and Rfas8, Fig. 2). In addition, ten cpDNA haplotypes were detected in R. fastigiata grouped with the clade comprising R. discolor haplotypes, whereas two R. discolor cpDNA haplotypes were embedded with the R. fastigiata clade (Fig. 2).
Fig. 2.
Distribution of cpDNA haplotypes and their respective haplotype network for (A) R. bupleuroides, (B) R. discolor, (C) R. fastigiata and (D) R. chrysanthemifolia. (E) cpDNA haplotype network for all four Rhodiola species combined.
Population structure
The four Rhodiola species each has high among-population genetic divergence, with GST ranging from 0.469 to 0.901 for ITS and 0.264 to 0.752 for cpDNA (Table 1). The NST was significantly larger than GST for R. bupleuroides (both ITS and cpDNA), R. fastigiata (cpDNA) and R. chrysanthemifolia (ITS), implying phylogeographic structuring on genetic diversity (Table 1). The STRUCTURE results showed that K values were 3, 2, 5 and 2 for R. bupleuroides, R. discolor, R. fastigiata and R. chrysanthemifolia, respectively, for ITS, and were 2, 22, 2 and 3, respectively, for cpDNA (Supplementary data Fig. S2). Rhodiola bupleuroides populations were separated into west (QTP) and east lineages (HM) according to the results from our STRUCTURE (Supplementary data Fig. S2a, b), network (Figs 1A and 2A) and phylogenetic analyses (Figs 3 and 4). The STRUCTURE analysis for the Rhodiola complex showed that both ITS and cpDNA variations did differentiate these populations species into two clusters (K = 2), rather than into four groups according to species delimitation (Supplementary data Fig. S2h, i), suggesting reticular relationships between them.
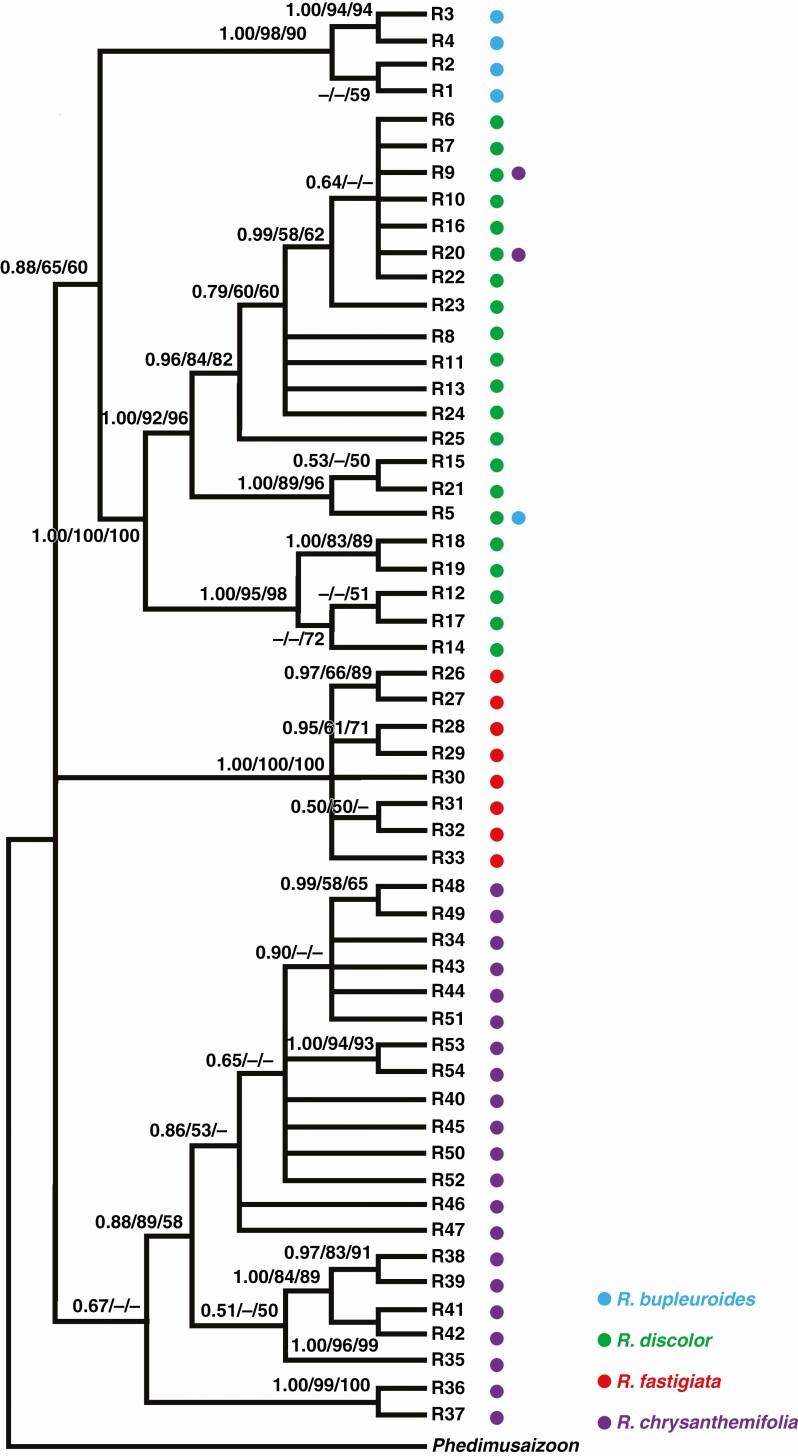
Fig. 3.
Phylogenetic relationships of the four Rhodiola species based on ITS sequences. The numbers associated with each node indicate Bayesian posterior probabilities (left), and MP (middle) and ML (left) bootstrap support values, respectively.
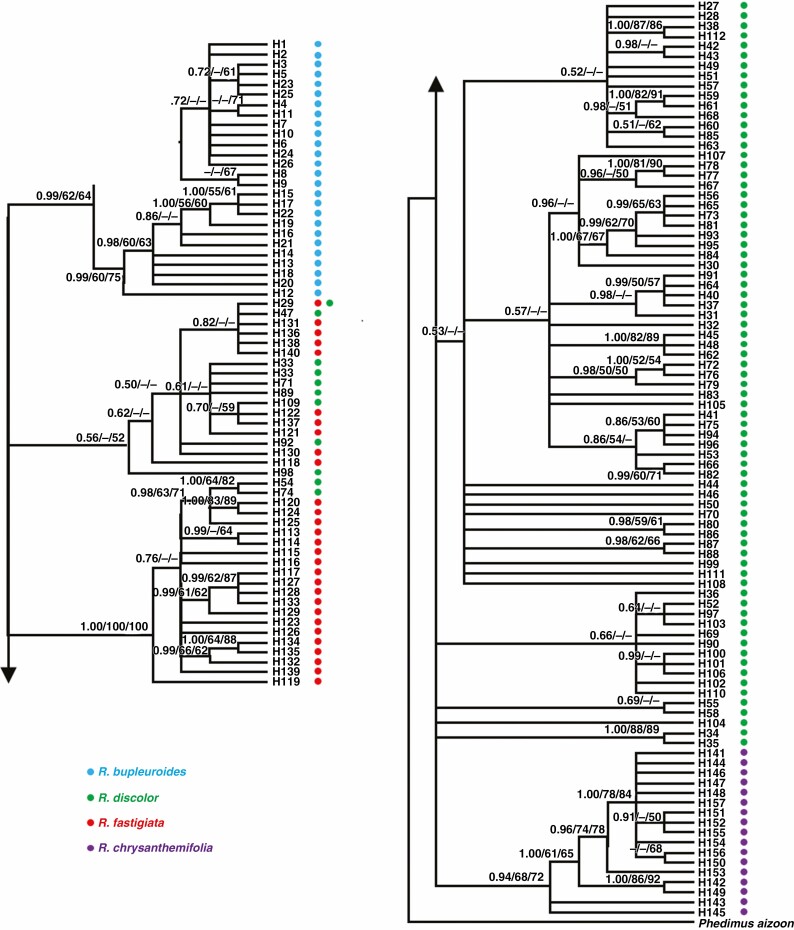
Fig. 4.
Phylogenetic relationships of the four Rhodiola species based on cpDNA sequences. The numbers associated with each node indicate Bayesian posterior probabilities (left), and MP (middle) and ML (left) bootstrap support values, respectively.
Phylogenetic relationships, lineages divergence and demographic history
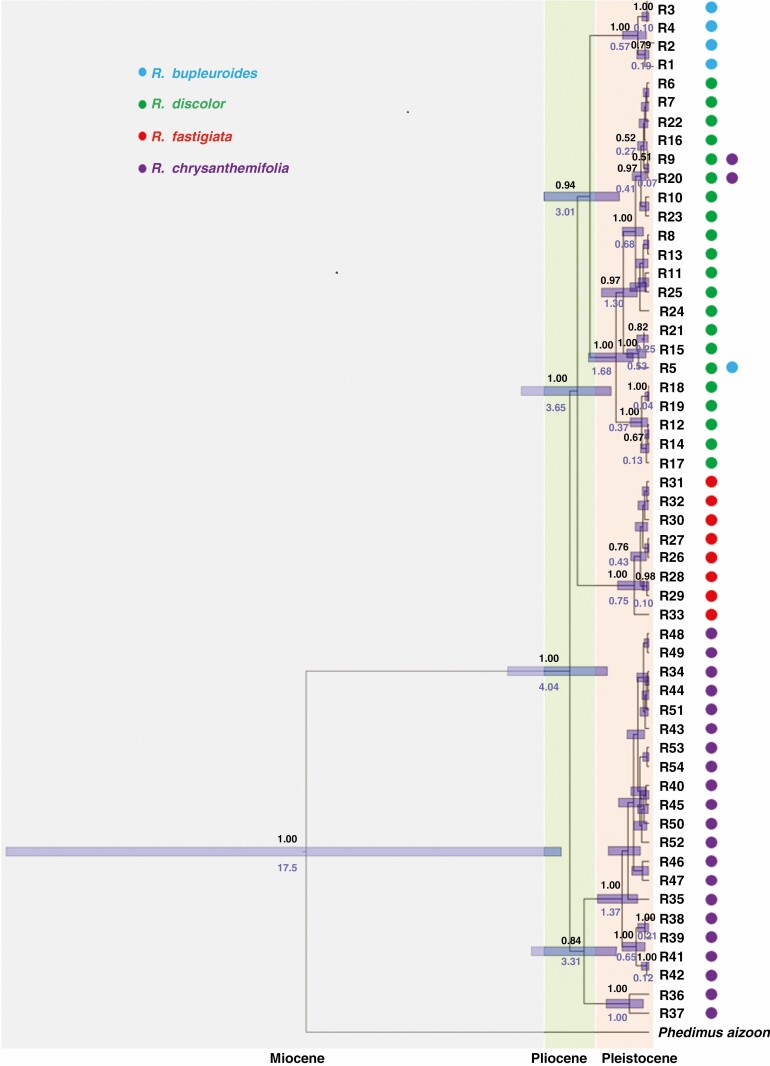
For both ITS and cpDNA, phylogenies based on MP, ML and Bayesian methods were largely congruent (Figs 3 and 4) and consistent with the NETWORK analysis results (Figs 1E and 2E). The phylogeny of ITS ribotypes formed three clades, namely (1) R. bupleuroides and R. discolor, (2) R. fastigiata and (3) R. chrysanthemifolia (Fig. 3). Dating based on ITS suggested the divergence time of 3.01 Mya between R. bupleuroides and R. discolor [95 % highest posterior density (HPD) 1.51–5.36 Mya], 3.65 Mya between their common ancestor and R. fastigiata (95 % HPD 1.93–6.52 Mya) and 4.04 Mya between R. chrysanthemifolia and the common ancestor of the three other species (95 % HPD 2.13–7.21 Mya, Fig. 5). The two ITS clades (eastern and western clades) of R. bupleuroides diverged at 0.57 Mya (95 % HPD 0.13–1.34 Mya). Diversification among the ITS ribotypes occurred mostly within the last 1 million years of the Pleistocene (Fig. 5).
Fig. 5.
Maximum clade credibility tree of four Rhodiola species inferred from ITS data.
The phylogenetic relationships among cpDNA haplotypes showed that R. chrysanthemifolia or R. bupleuroides formed individual clades, but that neither R. fastigiata nor R. discolor comprised their own monophyletic group, showing a distinct pattern from that of the ITS ribotypes (Fig. 4). The analysis of cpDNA data suggested that the divergence time between R. chrysanthemifolia and the three other species was 4.45 Mya (95 % HPD 3.17–6.10 Mya), while that among R. discolor, R. bupleuroides and R. fastigiata occurred within the last 3.9 Mya (95 % HPD 2.79–5.28 Mya, Fig. 6). Most cpDNA haplotypes diverged within the last 1 million years of the Pleistocene (Fig. 6).
Fig. 6.
Maximum clade credibility tree of four Rhodiola species inferred from cpDNA data.
Results from the mismatch distribution analysis for both ITS and cpDNA suggested that no populations of any of the four species underwent rapid expansion (Supplementary data Fig. S1). This finding is supported both by Tajima’s D and Fu’s Fs values (Table 2). Results from the mismatch distribution analysis for the east or west lineage of R. bupleuroides separately implied recent population expansion (for east, ITS and cpDNA data; for west, ITS data) (Supplementary data Fig. S3). This finding is supported by the values of SSD and HRag (Supplementary data Table S3). Population expansion for the west lineage dates to approx. 0.011 Mya based on ITS, and that for the east lineage dates to approx. 0.011 Mya based on ITS and 0.098 Mya based on cpDNA.
Table 2.
Results of mismatch distribution analysis and neutrality tests for four Rhodiola species based on ITS sequences and cpDNA sequences
| Dataset | Species | τ | SSD | P-value | HRag | P-value | Tajima’s D | P-value | Fs | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| ITS | R. bupleuroides | 0.774 | 0.045 | 0.014 | 0.137 | 0.094 | –0.190 | 0.846 | 0.261 | NA |
| R. discolor | 2.502 | 0.086 | 0.140 | 0.321 | 0.296 | 0.393 | 0.761 | 2.002 | NA | |
| R. fastigiata | 0.298 | 0.064 | 0.099 | 0.162 | 0.177 | 0.539 | 0.954 | 0.981 | NA | |
| R. chrysanthemifolia | 3.758 | 0.068 | 0.106 | 0.190 | 0.127 | 0.650 | 0.904 | 1.376 | NA | |
| cpDNA | R. bupleuroides | 8.173 | 0.196 | 0.173 | 0.310 | 0.516 | 0.407 | 0.652 | 2.562 | 0.827 |
| R. discolor | 11.168 | 0.165 | 0.100 | 0.295 | 0.281 | 0.121 | 0.568 | 3.359 | 0.848 | |
| R. fastigiata | 36.903 | 0.215 | 0.031 | 0.345 | 0.404 | –0.378 | 0.515 | 5.376 | NA | |
| R. chrysanthemifolia | 1.524 | 0.125 | 0.036 | 0.182 | 0.267 | –0.177 | 0.720 | 2.093 | NA |
τ, the mode of the mismatch distribution; SSD, the sum of squared deviations; HRag, the raggedness index.
DISCUSSION
Population history
The phylogeny relationships among four Rhodiola species in our study were consistent with the species tree presented in Zhang et al. (2014a) and Ding et al., (2020). Rhodiola chrysanthemifolia diverged earlier than the three other species, R. fastigiata then diverged from R. bupleuroides and R. discolor, and the last two are sister species. Their divergence times are coincident with the last peak of in situ speciation in HM (Ding et al., 2020), indicating that most QTP endemic species originated within the past 6 million years (Lai et al., 2021), and implying an important role for geographic isolation and climate oscillations in speciation in Rhodiola.
The diversification of the ITS ribotypes and cpDNA haplotypes for these four Rhodiola species occurred mostly within the late half of the Pleistocene (Figs 5 and 6). The number of haplotypes also increased rapidly over this same span, especially for cpDNA in R. discolor, where we found 86 haplotypes, of which 69 were private. Phylogeographic studies of other plant species in the QTP and HM also report rapid diversification of cpDNA haplotypes within the last million years, including Hippuris vulgaris (Chen et al., 2013), Primula ovalifolia (Xie et al., 2017), Rhodiola alsia (J. Gao et al., 2012), Sophora davidii (Fan et al., 2013) and Stellera chamaejasme (Zhang et al., 2010). The orogenesis of the QTP ended in the Pleistocene, in which the Asian monsoon cycles also formed (Ding et al., 2020). The phylogeographic patterns for these species are probably not directly related to the orogenesis of the QTP; rather, topographic changes, geographic isolation and climate oscillations during the Pleistocene have driven the present population differentiation.
We found 15.38–69.23 % of private ITS ribotypes and 69.23–90.91 % of private cpDNA haplotypes in most populations across the geographic range of the four species. These private ribotypes and haplotypes also existed in multiple clades of the phylogenetic tree, which diverged earlier than the Last Glacial Maximum (approx. 22 000 BP). The results suggest multiple Plesitocene refugia in the QTP and HM for Rhodiola, in accordance with predictions from ecology niche modelling (You et al., 2018) and studies of other Rhodiola species (Li et al., 2018).
Phylogeographic patterns
Although these four Rhodiola species were closely related, they showed different phylogeographic patterns. We found marked west–east differentiation in R. bupleuroides (Figs 1A and 2A). The divergence between the west and east lineage of R. bupleuroides based on ITS dates to approx. 0.57 Mya (Fig. 5) during the Wangkun glaciation, an extensive glaciation event within the QTP (Cui et al., 2011). The population expansion for both east and west lineages dates to approx. 10 000 years ago based on both ITS and cpDNA data, implying altitudinal shifts in the distribution of this taxon.
We found that both R. bupleuroides and R. discolor, which are distributed in the QTP and HM, respectively, may have experienced isolation by geography, followed by adaptation to different environments and ultimately allopatric speciation. This sequence of events suggests that geographic and climate change remarkably influenced many species with different distributions. The uplift of the QTP created a geographic barrier that isolated populations and species, blocking the warm and moist air from the Indian Ocean (Mulch and Chamberlain, 2006; Royden et al., 2008; Favre et al., 2015). Considering the influence of the westerlies and the central Asia monsoon, the climate in the QTP became cold, and the area drought prone. This climate change resulted in the formation of alpine meadows (Mulch and Chamberlain, 2006; Royden et al., 2008; Favre et al., 2015). Considering that the HM was affected by monsoon circulation and high levels of precipitation, the complex topography and numerous valleys would have created various microhabitats. Therefore, the interaction between topography and climate shaped the phylogeographic patterns of both QTP and HM species.
Divergent phylogeographic patterns among co-distributed species imply that different ecological niches and reproductive strategies also affect genetic population structure. The complex phylogeographic pattern and extraordinarily high number of haplotypes in R. discolor implies rapid diversification within this species. This condition would have been caused by isolation interacting with varied environments within the HM and the broad ecological niche and ability to adapt in this species. Adaptive radiations and rapid diversification are essential in generating biodiversity in the HM, fuelled by niche competition that promotes rapid diversification of species (Hughes and Atchison, 2015; Xing and Ree, 2017). Ecological niche modelling revealed that magnitudes for the bioclimatic variables Precipitation of Wettest Month (BIO13) and Precipitation of Warmest Quarter (BIO18) were higher for HM Rhodiola species than for those distributed in the QTP and elsewhere (You et al., 2018). Therefore, percipitation may be an important driver of diversification among HM species. In summary, the HM has been a cradle for species diversification and adaptive radiations because of the abundant precipitation and varied microhabitats and topography.
Phylogenetic discordance
Clear differences were observed in the phylogenetic relationships among species in cpDNA vs. ITS. We also found shared ribotypes and haplotypes among the four Rhodiola species. These shared ribotypes and haplotypes may have stemmed from introgression between species or by retention of ancestral polymorphisms caused by incomplete lineage sorting (Du et al., 2011). Considering that the shared ribotype (R9 and R20) and haplotype (H29) both occur only in sympatric populations in the respective ranges of these species, introgression rather than incomplete lineage sorting is the most likely explanation. The estimated divergence time between R9 and R20 is approx. 0.07 Mya, implying that introgressive hybridization between R. discolor and R. chrysanthemifolia may have begun in the late Pleistocene driven perhaps by climate oscillations and range shifts. In contrast, ITS ribotype R5 was found in a R. bupleuroides population near Lhazê and a R. discolor population at Lijiang, and these sites are relatively far apart (approx. 1000 km straight-line distance). Considering the large geographic distance between these populations, lineage sorting is likely to explain this pattern rather than hybridization.
Ten cpDNA haplotypes of R. fastigiata were embedded with the R. discolor clade, including the shared haplotype H29. Two cpDNA R. discolor haplotypes are part of the R. fastigiata clade. These recently diverged R. fastigiata cpDNA haplotypes are distributed across the species range, from the west QTP to the HM. Hybridization occurred between R. fastigiata and R. discolor, followed by rapid divergence between the species. In sum, these young Rhodiola species tend to appear polyphyletic or paraphyletic because of both ILS and introgressive hybridization.
However, a couple of caveats need to be pointed out for interpreting the discordance of phylogenies generated with different genetic markers. First, ITS is biparentally inherited, whereas cpDNA is parentally inherited, indicating that the former may experience higher intraspecific gene flow than the latter. According to Currat et al. (2008), introgression at cpDNA markers should be detected in most sympatric populations, whereas ITS introgression is expected only in populations located closer to the front of expansion (Du et al., 2011). Such a prediction seems be verified by the shared cpDNA haplotypes in the sympatric population of R. discolor and R. fastigiata but no ITS ribotype shared by them (Fig. 2). Second, ITS and cpDNA markers differ not only in the rates of gene flow but also in ploidy levels and hence in effective sizes (Ne); therefore, ITS needs a much longer sorting time to form a monophyletic lineage than cpDNA (4Ne generation vs. Ne generation; Du et al., 2011). Thus, ILS signals were detected from ITS variations rather than cpDNA variations in recent radiation taxa, such as Rhodiola species in our study.
The differences of DNA markers used in the rates of gene flow and in sorting times of lineage formation, combined with that of divergence timings and population demography, may inform interspecific gene flow (Du et al., 2011). Considering that R. chrysanthemifolia is a relatively old species, and R. bupleuroides experienced recent population expansion, the direction of introgression of the paternally inherited cpDNA markers is from R. chrysanthemifolia to the other Rhodiola species, and R. bupleuroides is the expanding species introgressed by genetic material from the other species. The shared cpDNA haplotype H29 is widely distributed in the populations of R. fastigiata, but only in one population of R. discolor (Fig. 2; Supplementary data Table S1), suggesting that the direction of introgression is from the former to the latter.
Generality and significance of hybridization in the HM
Hybridization is an important mechanism for generating biodiversity and driving rapid adaptive divergence, and it often occurs between closely related species and those implicated in adaptive radiations (Gourbiere and Mallet, 2010). Hybridization can provide raw genetic material for adaptive divergence or initiate new hybrid lineages, potentially leading to speciation (Abbott et al., 2013). Many Rhodiola species may have originated via hybridization, and many Rhodiola conspecific populations are in the process of speciation. Reticulate evolution may also have occurred in Rhodiola, thus enhancing adaptive radiation by introducing genetic variation that allows emerging populations to take advantage of new ecological opportunities.
The complex topography of the HM and climate oscillations during the Pleistocene created opportunities for secondary contact and population admixture, and strengthened natural interspecific hybridization for species. Many hybridization events and patterns of introgression have been documented in the HM across a range of taxa, including Pinus (J. Gao et al., 2012), Picea (Sun et al., 2014), Ostryopsis (Liu et al., 2014), Ligularia (Zhang et al., 2017), Rhododendron (Zheng et al., 2017), Primula (Xie et al., 2017), Roscoea (Du et al., 2012), Salix (Wu et al., 2015) and Silene (Zhang et al., 2016). These HM hybrid zones would have provided abundant genetic variation for the diversification of the HM flora, contributing to the present elevated diversity with 16 550 species of vascular plants, including 3300 endemic species and 90 endemic genera (Sun et al., 2017). Natural hybridization is implicated in the evolution of 25 % of plant species and facilitates speciation and evolutionary innovation via transfer of adaptive traits by introgression, formation of recombinant forms or allopolyploidization (Yang et al., 2019).
Conclusion
The isolation by geography following the orogenesis of the QTP may underlie speciation in Rhodiola but caused reticulate evolution. The four Rhodiola species studied displayed divergent phylogeographic patterns with different population histories, in which both introgressive hybridization and ILS played prominent roles in shaping contemporary phylogeographic patterns. The complex topography and climate oscillations during the Quaternary after the uplift of the QTP and the formation of the HM might have accelerated rates of population differentiation and resulted in radiation among Rhodiola species. In addition, the complex phylogeographic patterns and extraordinary haplotypic richness of R. discolor imply rapid and recent diversification within this species.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: general information of the sampled populations of Rhodiola bupleuroides, R. discolor, R. fastigiata and R. chrysanthemifolia. Table S2: population genetic diversity of four Rhodiola species. Table S3: results of mismatch distribution analysis and neutrality tests for west and east lineages of R. bupleuroides based on ITS sequences and cpDNA sequences. Figure S1: mismatch distribution for each Rhodiola species based on ITS and cpDNA data. Figure S2: ΔK as a function of K for each Rhodiola species and for the four species as a complex, and their population genetic divergence. Figure S3: mismatch distribution for east and west lineages of R. bupleuroides based on ITS and cpDNA data. Appendix S1: GenBank accession numbers for ribotype/haplotype nucleotide sequences.
ACKNOWLEDGEMENTS
We are grateful to Danhui Qi (Southwest Forestry University, Kunming, China), Hanze Ma, Ding Li, Naiqi Tao and Shanmei Cheng for their help with the field work, and Hanze Ma and Guozhen Fan for laboratory work. ZS, YW, WZ, JY and JYou conceived the study. ZS, JYou, WZ, QL, WL, YZ and FL collected the samples. JYou conducted the PCR assays and the statistical analyses, and wrote the manuscript. ZS, SL and GZ supervised and contributed to this writing, and all authors contributed substantially to revisions.
FUNDING
This study was supported by the National Natural Science Foundation of China (31830009), the National Key Basic Research Program of China (2014CB954100), the Applied Fundamental Research Foundation of Yunnan Province (2014GA003) and the Special Foundation for Basic Research Program of Yunnan Province (202001AU070148). The authors declare no conflict of interest.
LITERATURE CITED
- Abbott R, Albach D, Ansell S, et al. 2013. Hybridization and speciation. Journal of Evolutionary Biology 26: 229–246. [DOI] [PubMed] [Google Scholar]
- Bandelt H, Forster P, Röhl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution 16: 37–48. [DOI] [PubMed] [Google Scholar]
- Cai J, Yu W, Zhang T, Wang H, Li D. 2019. China’s biodiversity hotspots revisited: a treasure chest for plants. Phytokeys 130: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Du Z, Sun S, Gituru RW, Wang Q. 2013. Chloroplast DNA phylogeography reveals repeated range expansion in a widespread aquatic herb Hippuris vulgaris in the Qinghai–Tibetan Plateau and adjacent areas. PLoS One 8: e60948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui ZJ, Chen YX, Zhang W, et al. 2011. Research history, glacial chronology and origins of Quaternary glaciations in China. Quaternary Sciences 31: 749–764. [Google Scholar]
- Cun Y, Wang X. 2010. Plant recolonization in the Himalaya from the southeastern Qinghai–Tibetan Plateau: geographical isolation contributed to high population differentiation. Molecular Phylogenetics and Evolution 56: 972–982. [DOI] [PubMed] [Google Scholar]
- Currat M, , RuediM, , PetitRJ, , ExcoffierL. 2008. The hidden side of invasions: massive introgression by local genes. Evolution 62: 1908–1920. [DOI] [PubMed] [Google Scholar]
- Ding WN, Ree RH, Spicer RA, Xing YW. 2020. Ancient orogenic and monsoon-driven assembly of the world’s richest temperate alpine flora. Science 369: 578–581. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du FK, Peng XL, Liu JQ, Lascoux M, Hu FS, Petit RJ. 2011. Direction and extent of organelle DNA introgression between two spruce species in the Qinghai–Tibetan Plateau. New Phytologist, 192: 1024–1033. [DOI] [PubMed] [Google Scholar]
- Du G, Zhang Z, Li Q. 2012. Morphological and molecular evidence for natural hybridization in sympatric population of Roscoea humeana and R. cautleoides (Zingiberaceae). Journal of Plant Research 125: 595–603. [DOI] [PubMed] [Google Scholar]
- Earl DA. 2012. Structure harvester: a website and program for visualizing structure output and implementing the Evanno method. Conservation Genetics Resources 4: 359–361. [Google Scholar]
- Evanno G, Regnaut S, Goudet J. 2005. Detecting the number of clusters of individuals using the software structure: a simulation study. Molecular Ecology 14: 2611–2620. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer HE. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10: 564–567. [DOI] [PubMed] [Google Scholar]
- Fan D, Yue J, Nie Z, Li Z, Comes HP, Sun H. 2013. Phylogeography of Sophora davidii (Leguminosae) across the ‘Tanaka–Kaiyong Line’, an important phytogeographic boundary in Southwest China. Molecular Ecology 22: 4270–4288. [DOI] [PubMed] [Google Scholar]
- Favre A, Päckert M, Pauls SU, et al. 2015. The role of the uplift of the Qinghai–Tibetan Plateau for the evolution of Tibetan biotas. Biological Reviews 90: 236–253. [DOI] [PubMed] [Google Scholar]
- Fu Y. 1997. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang B, Mao J, Ingvarsson P, Zeng Q, Wang X. 2012. Demography and speciation history of the homoploid hybrid pine Pinus densata on the Tibetan Plateau. Molecular Ecology 21: 4811–4827. [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhang D, Duan Y, et al. 2012. Intraspecific divergences of Rhodiola alsia (Crassulaceae) based on plastid DNA and internal transcribed spacer fragments. Botanical Journal of the Linnean Society 168: 204–215. [Google Scholar]
- Gourbiere S, Mallet J. 2010. Are species real? The shape of the species boundary with exponential failure, reinforcement, and the ‘missing snowball’. Evolution 64: 1–24. [DOI] [PubMed] [Google Scholar]
- Grant PR, Grant BR, Petren K. 2005. Hybridization in the recent past. The American Naturalist 166: 56–67. [DOI] [PubMed] [Google Scholar]
- Hamilton MB. 1999. Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Molecular Ecology 8: 521–523. [PubMed] [Google Scholar]
- Harpending HC. 1994. Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Human Biology 66: 591–600. [PubMed] [Google Scholar]
- Hou Y, Lou A. 2014. Phylogeographical patterns of an alpine plant, Rhodiola dumulosa (Crassulaceae), inferred from chloroplast DNA sequences. Journal of Heredity 105: 101–110. [DOI] [PubMed] [Google Scholar]
- Hughes CE, Atchison GW. 2015. The ubiquity of alpine plant radiations: from the Andes to the Hengduan Mountains. New Phytologist 207: 275–282. [DOI] [PubMed] [Google Scholar]
- Lai YJ, Han Y, Schuiteman A, et al. 2021. Diversification in Qinghai–Tibet Plateau: Orchidinae (Orchidaceae) clades exhibiting pre-adaptations play critical role. Molecular Phylogenetics and Evolution 157: 107062. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhai S, Qiu Y, Guo Y, Ge X, Comes HP. 2011. Glacial survival east and west of the ‘Mekong–Salween Divide’ in the Himalaya–Hengduan Mountains region as revealed by AFLPs and cpDNA sequence variation in Sinopodophyllum hexandrum (Berberidaceae). Molecular Phylogenetics and Evolution 59: 412–424. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhong D, Rao G, Wen J, Ren Y, Zhang J. 2018. Gone with the trees: phylogeography of Rhodiola sect. Trifida (Crassulaceae) reveals multiple refugia on the Qinghai–Tibetan Plateau. Molecular Phylogenetics and Evolution 121: 110–120. [DOI] [PubMed] [Google Scholar]
- Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- Liu B, Abbott RJ, Lu Z, Tian B, Liu J. 2014. Diploid hybrid origin of Ostryopsis intermedia (Betulaceae) in the Qinghai–Tibet Plateau triggered by Quaternary climate change. Molecular Ecology 23: 3013–3027. [DOI] [PubMed] [Google Scholar]
- Liu J, Möller M, Provan J, Gao L, Poudel RC, Li D. 2013. Geological and ecological factors drive cryptic speciation of yews in a biodiversity hotspot. New Phytologist 199: 1093–1108. [DOI] [PubMed] [Google Scholar]
- Mayuzumi S, Ohba H. 2004. The phylogenetic position of eastern Asian Sedoideae (Crassulaceae) inferred from chloroplast and nuclear DNA sequences. Systematic Botany 29: 587–598. [Google Scholar]
- Mulch A, Chamberlain CP. 2006. The rise and growth of Tibet. Nature 439: 670–671. [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853. [DOI] [PubMed] [Google Scholar]
- Polzin T, Daneshmand SV. 2003. On Steiner trees and minimum spanning trees in hypergraphs. Operations Research Letters 31: 12–20. [Google Scholar]
- Pons O, Petit RJ. 1996. Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144: 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D. 2006. ModelTest Server: a web-based tool for the statistical selection of models of nucleotide substitution online. Nucleic Acids Research 34(suppl_2): W700–W703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D, Buckley TR. 2004. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology 53: 793–808. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JE, Pennington RT, Pennington TD, Hollingsworth PM. 2001. Rapid diversification of a species-rich genus of neotropical rain forest trees. Science 293: 2242–2245. [DOI] [PubMed] [Google Scholar]
- Rogers AR. 1995. Genetic evidence for a Pleistocene population explosion. Evolution 49: 608–615. [DOI] [PubMed] [Google Scholar]
- Rogers AR, Harpending H. 1992. Population growth makes waves in the distribution of pairwise genetic differences. Molecular Biology and Evolution 9: 552–569. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Royden LH, Burchfiel BC, van der Hilst RD. 2008. The geological evolution of the Tibetan Plateau. Science 321: 1054–1058. [DOI] [PubMed] [Google Scholar]
- Schneider S, Excoffier L. 1999. Estimation of past demographic parameters from the distribution of pairwise differences when the mutation rates vary among sites: application to human mitochondrial DNA. Genetics 152: 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Ran J, Wang X. 2019. Phylogenomics disentangles the evolutionary history of spruces (Picea) in the Qinghai–Tibetan Plateau: implications for the design of population genetic studies and species delimitation of conifers. Molecular Phylogenetics and Evolution 141: 106612. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. 2003. A comparison of bayesian methods for haplotype reconstruction from population genotype data. The American Journal of Human Genetics 73: 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. 2001. A new statistical method for haplotype reconstruction from population data. The American Journal of Human Genetics 68: 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Zhang J, Deng T, Boufford DE. 2017. Origins and evolution of plant diversity in the Hengduan Mountains, China. Plant Diversity 39: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Abbott RJ, Li L, Li L, Zou J, Liu J. 2014. Evolutionary history of Purple cone spruce (Picea purpurea) in the Qinghai–Tibet Plateau: homoploid hybrid origin and Pleistocene expansion. Molecular Ecology 23: 343–359. [DOI] [PubMed] [Google Scholar]
- Swofford DL, Sullivan J. 2003. Phylogeny inference based on parsimony and other methods using PAUP*. In: Salemi M, Vandamme A-M, eds. The phylogenetic handbook: a practical approach to DNA and protein phylogeny. Cambridge: Cambridge University Press, 160–206. [Google Scholar]
- Taberlet P, Gielly L, Pautou G, Bouvet J. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17: 1105–1109. [DOI] [PubMed] [Google Scholar]
- Tajima F. 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Mao J, Zhao W, Wang X. 2013. Impact of geography and climate on the genetic differentiation of the subtropical pine Pinus yunnanensis. PLoS One 8: e67345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Qiong LA, Sun K, et al. 2010. Phylogeographic structure of Hippophae tibetana (Elaeagnaceae) highlights the highest microrefugia and the rapid uplift of the Qinghai–Tibetan Plateau. Molecular Ecology 19: 2964–2979. [DOI] [PubMed] [Google Scholar]
- Wang J, Pan Y, Gong X, Chiang Y, Kuroda C. 2011. Chloroplast DNA variation and phylogeography of Ligularia tongolensis (Asteraceae), a species endemic to the Hengduan Mountains region of China. Journal of Systematics and Evolution 49: 108–119. [Google Scholar]
- Wang Z, Meng S, Rao G. 2019. Quaternary climate change and habitat preference shaped the genetic differentiation and phylogeography of Rhodiola sect. Prainia in the southern Qinghai–Tibetan Platea. Ecology and Evolution 9: 8305–8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang D, Yang Y, Chen J. 2015. Homoploid hybridization between native Salix cavaleriei and exotic Salix matsudana (Salicaceae). Plant Diversity and Resources 37: 1–10. [Google Scholar]
- Wu Z, Raven PH. 2001. Flora of china. St Louis, MO: Missouri Botanical Garden Press. [Google Scholar]
- Xie Y, Zhu X, Ma Y, Zhao J, Li L, Li Q. 2017. Natural hybridization and reproductive isolation between two Primula species. Journal of Integrative Plant Biology 59: 526–530. [DOI] [PubMed] [Google Scholar]
- Xing Y, Ree RH. 2017. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proceedings of the National Academy of Sciences, USA 114: E3444–E3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K, Yano K, Kawahara T. 2006. Pattern and rate of indel evolution inferred from whole chloroplast intergenic regions in sugarcane, maize and rice. DNA Research 13: 197–204. [DOI] [PubMed] [Google Scholar]
- Yang F, Li Y, Ding X, Wang X. 2008. Extensive population expansion of Pedicularis longiflora (Orobanchaceae) on the Qinghai–Tibetan Plateau and its correlation with the Quaternary climate change. Molecular Ecology 17: 5135–5145. [DOI] [PubMed] [Google Scholar]
- Yang R, Folk R, Zhang N, Gong X. 2019. Homoploid hybridization of plants in the Hengduan mountains region. Ecology and Evolution 9: 8399–8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Qin X, Ranjitkar S, et al. 2018. Response to climate change of montane herbaceous plants in the genus Rhodiola predicted by ecological niche modelling. Scientific Reports 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Chen G, Sun W, Sun H. 2012. Phylogeography of Buddleja crispa (Buddlejaceae) and its correlation with drainage system evolution in southwestern China. American Journal of Botany 99: 1726–1735. [DOI] [PubMed] [Google Scholar]
- Zhang J, Meng S, Allen GA, Wen J, Rao G. 2014a. Rapid radiation and dispersal out of the Qinghai–Tibetan Plateau of an alpine plant lineage Rhodiola (Crassulaceae). Molecular Phylogenetics and Evolution 77: 147–158. [DOI] [PubMed] [Google Scholar]
- Zhang J, Meng S, Rao G. 2014b. Phylogeography of Rhodiola kirilowii (Crassulaceae): a story of Miocene divergence and Quaternary expansion. PLoS One 9: e112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Montgomery BR, Huang S. 2016. Evidence for asymmetrical hybridization despite pre- and post-pollination reproductive barriers between two Silene species. AoB Plants 8: plw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhong D, Song W, Zhu R, Sun W. 2018. Climate is not all: evidence from phylogeography of Rhodiola fastigiata (Crassulaceae) and comparison to its closest relatives. Frontiers in Plant Science 9: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Gong X, Folk R. 2017. Evidence for continual hybridization rather than hybrid speciation between Ligularia duciformis and L. paradoxa (Asteraceae). Peerj 5: e3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen G, Ma Y, Ge J, Sun W. 2015. Genetic diversity and population structure of Buddleja crispa Bentham in the Himalaya–Hengduan Mountains region revealed by AFLP. Biochemical Systematics and Ecology 58: 13–20. [Google Scholar]
- Zhang Y, Volis S, Sun H. 2010. Chloroplast phylogeny and phylogeography of Stellera chamaejasme on the Qinghai–Tibet Plateau and in adjacent regions. Molecular Phylogenetics and Evolution 57: 1162–1172. [DOI] [PubMed] [Google Scholar]
- Zheng S, Tian X, Huang C, Wang L, Feng Y, Zhang J. 2017. Molecular and morphological evidence for natural hybridization between Rhododendron decorum and R. delavayi (Ericaceae). Biodiversity Science 25: 627–637. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.