Abstract
Objective:
To evaluate the relationship between diurnal salivary cortisol patterns and distress from heart palpitations in midlife women.
Methods:
We analyzed baseline data from 293 women who were eligible for a 3×2 factorial trial of exercise or yoga vs. routine activity, and omega-3 fish oil vs. placebo for vasomotor symptoms. Women self-collected salivary cortisol using swabs at four time points over two consecutive days and reported distress from heart racing or pounding during the past two weeks using a single item. Sample description and covariate data included demographics, clinical data, vasomotor symptom frequency from daily diaries, medication use, and validated questionnaires on depression, stress, and insomnia (Patient Health Questionnaire-8, Perceived Stress Scale, and Insomnia Severity index). Data were analyzed using descriptive statistics, chi-square and t-tests, and repeated measure linear regression models.
Results:
Participants were on average 54.6 (SD=3.6) years old, most were white (67%),, most were postmenopausal (84%), and 26% reported distress related to palpitations. In adjusted models, the morning (wake plus 30-minute) geometric mean daily salivary cortisol concentrations were significantly more blunted in those with distress from palpitations compared to those without distress (p≤0.03). When all covariates were controlled, distress from palpitations was the sole significant predictor of wake plus 30-minute cortisol (−0.25 [−0.45 to −0.04], p=0.02).
Conclusions:
Palpitations among midlife women may be associated with blunted morning cortisol, and this relationship is not explained by demographics, clinical variables, vasomotor symptoms, medications, depression, stress, or insomnia.
Keywords: Cortisol, menopause, palpitations, hormone, biomarker
INTRODUCTION
Heart palpitations occurring during the menopause transition are poorly understood. Women report palpitations as skipped, irregular, or exaggerated heartbeats or heart pounding.1, 2 Palpitations are common during the menopause transition. A recently published review concluded that palpitations affect as many as 40% of perimenopausal women and 54% of postmenopausal women.3 Another recent study found 25% of women reported some distress related to palpitations and that distress was related to lower menopausal quality of life.4 Greater understanding of palpitations could further symptom assessment and management during the menopause transition.
Identification of biomarkers associated with palpitations may help elucidate biological mechanisms to further treatment efforts. Biomarkers can be used as risk factors in identifying women who may be most in need of interventions to prevent or manage symptoms. Biomarkers can also help identify protective factors that can help prevent symptoms or prevent severe symptoms.5 There are five types of biomarkers recommended as a minimum dataset for symptom science research: cortisol, inflammatory cytokines, hypothalamic-pituitary-adrenal axis marker, brain derived neurotrophic factor, and genetic polymorphisms.6 Of these, only genetic markers have been studied in relation to palpitations in women during the menopause transition. Two studies showed no associations between palpitations in peri- or postmenopausal women and CYP1B1 Leu432Val7, 8 or CYP17A1rs7435729 genotypes. These genotypes are linked to estrogen/progesterone synthesis or serum concentrations. The only other biomarkers that have been studied in relation to palpitations prevalence during the menopause transition are (1) serum thyroid-stimulating hormone (rho=−0.18, p=0.02) and (2) free thyroxine (rho=0.25, p=0.04).10 In an ongoing literature review, we have found no other studies of biomarkers of palpitations during the menopause transition.
The data repository from the Menopause Strategies Finding Lasting Answers to Symptoms and Health (MsFLASH) trial 03 provides a unique opportunity to evaluate the potential biomarker, cortisol. Cortisol is recommended as a key biomarker of symptoms6 and was previously found to be associated with menopausal vasomotor symptoms.11, 12 In peri- and post-menopausal women, a blunted awakening cortisol response was associated with more frequent11, 12 and severe12 daily diary-recorded vasomotor symptom. Excess cortisol may affect the myocardial tissue directly through cardiomyocyte glucocorticoid signaling and indirectly by heightening catecholamine and renin-angiotensin effects.13 In addition, cortisol may be related to high vasomotor symptom frequency through stress/anxiety/depression, declining serum estrogen concentrations, and/or insomnia.11 Research shows cortisol directly affects the cardiovascular system14 and health outcomes.
Using MsFLASH data from three trials (n=759), we previously identified covariates to consider in research on heart palpitations in women at the menopause transition.4 Smoking (past, current) was associated with lower odds of reporting distress from palpitations, whereas body mass index, depressive symptoms, perceived stress, and insomnia were all positively associated with distress from palpitations.4
The overall purpose of our investigation was to compare daily salivary cortisol patterns between midlife women who did and did not report distress from palpitations in the past two weeks. We anticipated finding a relationship between variations in diurnal cortisol patterns and distress from palpitations, and we compared daily cortisol patterns between women who did and did not report distress from menopausal heart palpitations controlling for stress, depression, insomnia, and other potential covariates.
METHODS
Design and Setting
This was a cross-sectional analysis of baseline data from the MsFLASH trial 03. MsFLASH research sites in Indianapolis, Oakland, and Seattle enrolled participants between 2011 and 2012. Other publications detail trial results.15–19 Briefly, the trial was a 3×2 factorial design of exercise or yoga vs. routine activity and omega-3 fish oil vs. placebo with outcomes assessed at baseline and 12 weeks.
Sample
All participants met common MsFLASH inclusion criteria, which included age 40–62 years, in good general health, being peri- or postmenopausal based on menses or follicle stimulating hormone (FSH) and estradiol values, and reporting 14 or more episodes of bothersome or severe vasomotor symptoms (hot flashes, night sweats) per week during a 3 week screening period. Common and trial specific exclusion criteria were: use of hormones, hormonal contraceptives, or any treatments for vasomotor symptoms in the past month; regular user of omega-3 products; regular user of exercise, yoga, or related activities; contraindications to the study interventions, or major depressive episode in the past 3 months. In addition, for this analysis, women were excluded if they were missing all 8 cortisol samples, palpitations distress information, or data on covariates (e.g., demographics, clinical data, stress, depressive symptoms, insomnia).
The trial did not exclude women taking selective serotonin reuptake inhibitors, selective serotonin norepinephrine inhibitors, monoamine oxidase inhibitors other medications for anxiety/depression/sleep, St. John’s Wort gabapentin, pregabalin, selective estrogen receptor modulators, aromatase inhibitors, or glucocorticoids. To account for the possible impact of these medications on cortisol, we included use of any of these medications in the past 4 weeks (yes, no) in the analysis.
Dataset
The dataset for this analysis consisted of baseline data from questionnaires, data collected during baseline study visits, and salivary cortisol values from self-collected swabs. Distress from heart palpitations was assessed with a single item of experiencing “distress from heart racing or pounding in the past two weeks” with response options of “not at all”, “a little bit”, “moderately”, “quite a bit”, or “extremely”. Similar to another paper,4 responses of “not at all” were coded as “no palpitations distress” and all other responses were coded as “palpitations distress”.
Salivary cortisol values were obtained at 4 time points on each of two baseline days: upon awakening (wake), 30 minutes later (wake+30), early afternoon, and bedtime. As detailed in our prior publication, women were given specific instructions about how to complete the self-collection to ensure quality samples were obtained.11 Women used pre-labeled Salivette swabs (Starstedt AG & Co, Lumbrecht, Rhineland-Palatinate, Germany) and placed them into their home freezer within 4 hours of collection. Within 1–3 weeks of sample collection, women transported the swabs in freezer bags to the clinical sites where the swabs were stored at −70 °C degrees until they were analyzed. Swabs were later shipped to and analyzed by the University of Washington School of Nursing laboratory using high-sensitivity cortisol enzyme immunoassay kits (Salimetrics, State College, PA). The sensitivity is < 0.007 μg and the assay range is 0.012–3.000 μg/dl.
Covariate data included demographics (age, race/ ethnicity, marital status, employment), clinical data [smoking history, menopause status, body mass index (BMI), systolic and diastolic blood pressure, ], medication use, vasomotor symptom frequency from daily diaries, and scores from the Perceived Stress Scale,20 Patient Health Questionnaire,21, 22 and Insomnia Severity index.23
Analysis
The analysis included data from 293 of the 355 women included in the MsFLASH 03 trial. Of the 355 women included in the trial, 46 were excluded for missing baseline cortisol data at all of the 8 collection times, another 2 were excluded for missing palpitations distress data, and another 14 were excluded for missing covariate data.
This analysis followed the analytic strategy used in Reed et al.’s paper on diurnal cortisol and vasomotor symptoms.11 Demographic, clinical, and symptom data were compared between palpitations distress groups (no, yes) using t-tests and chi-square tests. No adjustments were made for multiple comparisons given the exploratory nature of the analysis. The distributions of cortisol values at all 4 time points were skewed, thus the values were log transformed. Geometric means and their corresponding 95% confidence intervals for salivary cortisol at each time point were graphed. Additional calculated values included estimated daytime cortisol exposure (as the mean daily area under the curve over the two days), To evaluate the associations of cortisol and palpitations distress, data from both days of cortisol collection were included as repeated measures in linear regression models of log transformed cortisol values at each time point as a function of palpitation distress (yes/no) and other factors in three adjusted models. Model 1 adjusted day and clinical center. Model 2 adjusted covariates in model 1, age, race/ethnicity, marital status, full-time employment, smoking, menopause status, body mass index, systolic blood pressure, diastolic blood pressure, and vasomotor symptoms. Model 3 adjusted covariates in model 2, medication use in the past 4 weeks (any use of selective serotonin receptor inhibitor, selective serotonin norepinephrine receptor inhibitor, monoamine oxidase inhibitor, anti-anxiety medication (unspecified), tricyclic antidepressants, St. John’s Wort, other medications for anxiety/depression/sleep, gabapentin, pregabalin, selective estrogen receptor modulators, aromatase inhibitors, glucocorticoids), depression, stress, and insomnia. Anxiety was highly correlated with depressive symptoms and was not included in the models. Robust standard errors were calculated via generalized estimating equations to account for correlation between repeated measures from each participant. Analyses were conducted in SAS for Windows Version 9.4 (Cary, NC).
Results
There were 76 women (26%) who reported any degree of distress from palpitations. Characteristics for the sample of 293 women and for palpitations distress groups are shown in Table 1. Groups did not differ on age, race/ethnicity, marital status, employment, smoking history, menopause status, BMI, or systolic or diastolic blood pressure. The majority of participants were white or Black, never smokers, married or partnered, overweight or obese, postmenopausal, and with normal blood pressure. Women reporting palpitations distress reported significantly higher levels of use of one of the aforementioned medications in the past 4 weeks (35.5% vs. 21.2%, p=0.01), vasomotor symptoms (8.4±4.5 vs. 7.3±3.7, p=0.04), perceived stress (17.1±6.2 vs. 12.6±6.9, p<0.001), depressive symptoms (4.0±4.0 vs. 3.5±3.4, p<0.001), and insomnia severity (13.8±5.5 vs. 10.9±5.0, p<0.001) compared to those reporting no distress.
Table 1.
Frequency Statistics for Demographic and Clinical Characteristics by Reported Distress from Heart Palpitations During the Menopause Transition
| Distress from palpitations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n=293) | No (n=217) | Yes (n=76) | |||||||||
| n | % | n | % | M | SD | n | % | M | SD | p a | |
|
| |||||||||||
| Race/Ethnicity | 0.17 | ||||||||||
| White | 196 | 66.9 | 151 | 69.6 | 45 | 59.2 | |||||
| Black | 68 | 23.2 | 48 | 22.1 | 20 | 26.3 | |||||
| Other/Unknownb | 29 | 9.9 | 18 | 8.3 | 11 | 14.5 | |||||
| Married /partnered | 198 | 67.6 | 148 | 68.2 | 50 | 65.8 | 0.70 | ||||
| Employed full-time | 178 | 60.8 | 135 | 62.2 | 43 | 56.6 | 0.39 | ||||
| Smoking | 0.52 | ||||||||||
| Never | 193 | 65.9 | 140 | 64.5 | 53 | 69.7 | |||||
| Past | 76 | 25.9 | 60 | 27.6 | 16 | 21.1 | |||||
| Current | 24 | 8.2 | 17 | 7.8 | 7 | 9.2 | |||||
| Menopause Status | 0.40 | ||||||||||
| Postmenopausal | 245 | 83.6 | 181 | 83.4 | 64 | 84.2 | |||||
| Late transition | 43 | 14.7 | 31 | 14.3 | 12 | 15.8 | |||||
| Early transition | 5 | 1.7 | 5 | 2.3 | 0 | 0.0 | |||||
| BMI (kg/m2) | 0.95 | ||||||||||
| <25 | 107 | 36.5 | 82 | 37.8 | 25 | 32.9 | |||||
| 25 – <30 | 112 | 38.2 | 80 | 36.9 | 32 | 42.1 | |||||
| ≥30 | 74 | 25.3 | 55 | 25.3 | 19 | 25.0 | |||||
| Medicationsc | 73 | 24.9 | 46 | 21.2 | 27 | 35.5 | 0.01 | ||||
| Age at screening (years) | 54.6 | (3.6) | 54.8 | (3.7) | 54.1 | (3.4) | 0.16 | ||||
| Systolic BP (mm Hg) | 117.2 | (13.6) | 116.6 | (13.4) | 118.8 | (14.1) | 0.23 | ||||
| Diastolic BP (mm Hg) | 72.6 | (9.2) | 72.4 | (9.0) | 73.0 | (9.7) | 0.65 | ||||
| VMS frequency | 7.6 | (3.9) | 7.3 | (3.7) | 8.4 | (4.5) | 0.04 | ||||
| PSS Stress | 13.8 | (7.0) | 12.6 | (6.9) | 17.1 | (6.2) | <0.001 | ||||
| PHQ-8 Depression | 4.0 | (3.7) | 3.5 | (3.4) | 4.0 | (4.0) | <0.001 | ||||
| ISI Insomnia | 11.6 | (5.3) | 10.9 | (5.0) | 13.8 | (5.5) | <0.001 | ||||
p-value comparing participants without distress to those endorsing a little bit, moderately, quite a bit, or extremely distressed from racing or pounding heart in the past two weeks based on Chi-square tests for categorical variables and t-tests for continuous variables.
Other/unknown race/ethnicity in the total sample (n=29) includes 5 Hispanic, 5 American Indian, 12 Asian/Pacific Islander, and 7 unknown race/ethnicity participants. The small numbers of participants in each category precluded a detailed comparison of palpitations by these detailed racial/ethnic group and therefore, participants were combined into one category.
Taking at least one of the following in the past 4 weeks: selective serotonin receptor inhibitor, selective serotonin norepinephrine receptor inhibitor, monoamine oxidase inhibitor, anti-anxiety medication (unspecified), tricyclic antidepressants, St. John’s Wort, other medications for anxiety/depression/sleep, gabapentin, pregabalin, selective estrogen receptor modulators, aromatase inhibitors, glucocorticoids.
M=mean; SD=standard deviation; BMI=body mass index; BP=blood pressure; VMS=vasomotor symptoms; PSS=Perceived Stress Scale; PHQ=Patient Health Questionnaire; ISI=Insomnia Severity Index.
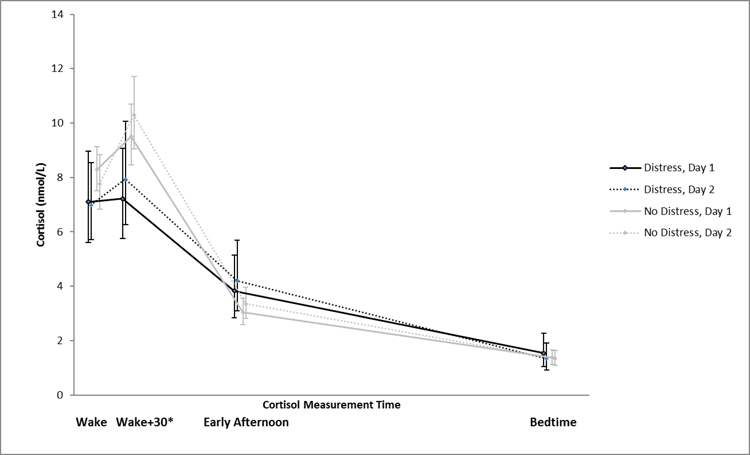
Figure 1 shows mean daily cortisol values by palpitations distress groups at each time point on each of the two assessment days. Both groups demonstrated the expected diurnal variation in salivary cortisol, with higher morning and lower evening values. However, visually, there appeared to be a flattened cortisol awakening response with slightly blunted Wake and Wake+30 cortisol values in the palpitations distress group compared to the no distress group. Afternoon and bedtime cortisol values appeared similar.
Figure 1.
Geometric Mean Salivary Cortisol Concentrations Over Time by Reported Palpitations Distress
Figure 1 shows mean daily cortisol values by palpitations distress groups at each time point on each of the two assessment days. Both groups showed the expected salivary cortisol diurnal variation (higher morning, lower evening). Compared to the no distress group, the palpitations distress group had significantly lower Wake+30 cortisol values. *p< .05
Adjusted models in Table 2 show significantly more blunted Wake+30 cortisol values in the palpitations distress group compared to those without distress (odds ratio [OR] = −0.26 [95% CI, −0.47 to −0.05]; OR = −0.24 [95% CI, −0.45 to −0.02]; OR = −0.25 [95% CI, −0.45 to −0.04]; all p ≤ 0.03). Total area under the curve, wake, afternoon, and bedtime cortisol values were not significantly different between distress groups in the adjusted models.
Table 2.
Adjusted Models of Cortisol Values by Palpitations Distress
| Palpitations Distress | ||||
|---|---|---|---|---|
| No | Yes | |||
|
| ||||
| Model | Estimate (95% CI) | Estimate a (95% CI) | p b | |
| Total AUCc | 1 | 1.00 (ref) | −0.13 (−0.35 to 0.09) | 0.26 |
| 2 | 1.00 (ref) | −0.15 (−0.36 to 0.06) | 0.17 | |
| 3 | 1.00 (ref) | −0.15 (−0.37 to 0.07) | 0.18 | |
|
| ||||
| Wake | 1 | 1.00 (ref) | −0.11 (−0.31 to 0.08) | 0.25 |
| 2 | 1.00 (ref) | −0.12 (−0.32 to 0.07) | 0.21 | |
| 3 | 1.00 (ref) | −0.08 (−0.27 to 0.12) | 0.45 | |
|
| ||||
| Wake + 30 | 1 | 1.00 (ref) | −0.26 (−0.47 to −0.05) | 0.02 |
| 2 | 1.00 (ref) | −0.24 (−0.45 to −0.02) | 0.03 | |
| 3 | 1.00 (ref) | −0.25 (−0.45 to −0.04) | 0.02 | |
|
| ||||
| Early afternoon | 1 | 1.00 (ref) | 0.18 (−0.07 to 0.44) | 0.16 |
| 2 | 1.00 (ref) | 0.17 (−0.07 to 0.41) | 0.17 | |
| 3 | 1.00 (ref) | 0.15 (−0.11 to 0.41) | 0.26 | |
|
| ||||
| Bedtime | 1 | 1.00 (ref) | 0.03 (−0.30 to 0.35) | 0.88 |
| 2 | 1.00 (ref) | 0.02 (−0.29 to 0.33) | 0.91 | |
| 3 | 1.00 (ref) | 0.02 (−0.30 to 0.34) | 0.90 | |
Estimates, 95% confidence intervals, and
p-values from 15 different repeated measure linear regression models (3 models x 5 outcomes) represent the mean log transformed cortisol difference between participants reporting racing heart distress compared with those not reporting it, after adjustment for other factors as described below.
Model 1: Adjusted for day, clinical center
Model 2: Adjusted for factors in Model 1 + age, race, marital status, full-time employment, smoking, menopause status, body mass index, systolic blood pressure, diastolic blood pressure, vasomotor symptoms
Model 3: Adjusted for factors in Models 1 and 2 + medication use, depression (Patient Health Questionnaire-8), stress (Perceived Stress Scale), and insomnia (Insomnia Severity Index)
Total area under the curve is scaled to the median wake to bedtime, 14 hours
AUC = Area under the salivary cortisol concentration versus time curve; CI = Confidence interval; ref = Reference
Details of the significant adjusted model for Wake+30 cortisol values are shown in Table 3. Palpitations distress remained a significant predictor of Wake+30 cortisol (OR = −0.25 [95% CI, −0.45 to −0.04], p = 0.02) after adjusting for day, clinical center, age, race/ethnicity, marital status, employment, smoking, menopause status, BMI, systolic and diastolic blood pressure, vasomotor symptoms, medication use, depressive symptoms, stress, and insomnia. Other cortisol timepoints were not significant.
Table 3.
Factors Associated with Wake + 30 Cortisol Values Based on Fully Adjusted Model
| Variable | Estimate (95% CI)a | p-valuea |
|---|---|---|
| Racing heart distress | −0.25 (−0.45 to −0.04) | 0.02 |
| Age (5-year increase) | −0.03 (−0.16 to 0.10) | 0.67 |
| Race/Ethnicity | 0.57 | |
| White | 1.00 (ref) | |
| African American | −0.13 (−0.37 to 0.12) | |
| Other/Unknownb | 0.02 (−0.22 to 0.26) | |
| Married / living as married | −0.09 (−0.26 to 0.08) | 0.32 |
| Full-time employed | 0.02 (−0.16 to 0.19) | 0.86 |
| Smoking | 0.27 | |
| Never | 1.00 (ref) | |
| Past | 0.04 (−0.15 to 0.22) | |
| Current | −0.33 (−0.76 to 0.11) | |
| Menopause Status | 0.13 | |
| Early transition | −0.99 (−1.61 to −0.37) | |
| Late transition | 0.03 (−0.19 to 0.25) | |
| Postmenopausal | 1.00 (ref) | |
| BMI (5 kg/m2 increase) | 0.07 (−0.06 to 0.19) | 0.30 |
| Systolic BP (10 mmHg increase) | −0.06 (−0.17 to 0.05) | 0.27 |
| Diastolic BP (10 mmHg increase) | 0.03 (−0.13 to 0.19) | 0.68 |
| VMS/wk (5 event increase) | −0.10 (−0.22 to 0.01) | 0.08 |
| Medication usec | 0.03 (−0.18 to 0.23) | 0.79 |
| PHQ-8 Depression (5-point increase) | −0.09 (−0.23 to 0.04) | 0.20 |
| PSS Stress (5-point increase | 0.05 (−0.03 to 0.12) | 0.22 |
| ISI Insomnia (5-point increase) | −0.01 (−0.10 to 0.09) | 0.87 |
Estimates, 95% confidence intervals, and p-values from a repeated measure GEE linear regression model represent the mean log transformed cortisol difference associated with each model variable.
Other/unknown race/ethnicity in the total sample (n=29) includes 5 Hispanic, 5 American Indian, 12 Asian/Pacific Islander, and 7 unknown race/ethnicity participants. The small numbers of participants in each category precluded a detailed comparison of palpitations by these detailed racial/ethnic group and therefore, participants were combined into one category.
Taking at least one of the following in the past 4 weeks: selective serotonin receptor inhibitor, selective serotonin norepinephrine receptor inhibitor, monoamine oxidase inhibitor, anti-anxiety medication (unspecified), tricyclic antidepressants, St. John’s Wort, other medications for anxiety/depression/sleep, gabapentin, pregabalin, selective estrogen receptor modulators, aromatase inhibitors, glucocorticoids.
Models are additionally adjusted for day and clinical center.
BMI = Body mass index; BP = Blood pressure; CI = Confidence intervals; ISI = Insomnia Severity Index; PHQ = Patient health Questionnaire; PSS = Perceived Stress Scale; ref = Reference; VMS = Vasomotor symptoms (e.g., hot flashes, night sweats); wk = week. Higher mean values on VMS, PSS, PHQ-8, and ISI indicate greater symptoms.
DISCUSSION
This report details salivary cortisol patterns in relation to menopausal palpitations. Our goal in conducting this analysis was to begin to characterize biomarkers of heart palpitations in menopausal women, which may help unravel the mechanisms or biological pathways involved and assist in targeting therapies. We had available assayed salivary cortisol swabs and self-reported palpitations distress data along with other carefully collected data on covariates from the MsFLASH network.24 The available dataset was unique in this regard and suitable for addressing the study purpose.
Psychological stress does not seem to explain study findings. Persons experiencing acute psychological stress also exhibit blunted cortisol awakening responses. 25, 26 However, in our sample, the relationship between blunted Wake+30 cortisol and palpitations distress was not explained by perceived stress, depressive symptoms, or other variables including demographics, vasomotor symptoms, or insomnia severity. In addition, there is no evidence that the distress from palpitations is a severe enough stressor to alter cortisol awakening responses.
Whether palpitations, or palpitations distress and the associated blunted morning cortisol response are associated with ECG abnormalities remains unknown. We have found only two published studies combining self-reported palpitations with electrocardiographic (ECG) findings that included women.27, 28 Neither reported menopause status. Other studies done in people with Cushing’s disease suggest that there could be a physiological relationship between a blunted cortisol awakening response and palpitations. People with Cushing’s have blunted cortisol awakening response,29 also exhibit arterial hypertension, and are at high risk of arrhythmias, including atrial fibrillation.30, 31 These arrythmias may be experienced as palpitations. Men with Cushing’s disease exhibit both blunted cortisol awakening response and low serum testosterone concentrations with resulting QT interval prolongation and potentially higher risk of ventricular arrhythymias.32 It does not appear that similar studies have been done in women, though low serum progesterone concentrations have also been associated with QT interval prolongation, though there is not yet evidence to support a higher risk of ventricular arrhythmias.
Disrupted diurnal cortisol patterns, even without palpitations, are associated with cardiovascular mortality.33, 34 In an analysis of 250 patients undergoing coronary artery bypass graft surgery, blunted morning or elevated evening values in salivary cortisol samples taken throughout a single day were related to more major adverse cardiac events and greater mortality at a mean of 2.7 years post-surgery.34 In the CARDIA study, flattened cortisol slopes across 4 time points (e.g., blunted at all time points) were associated with coronary calcification,35 a risk factor for adverse cardiac events. In contrast, in a secondary analysis of the London-community-based Whitehall II study, only elevated evening salivary cortisol concentrations (rather than blunted morning cortisol levels) were significantly associated with cardiovascular mortality.33
The findings have some potential clinical implications. Findings help unravel the mechanisms or biological pathways that might be involved in development of palpitations during the menopause transition. Greater knowledge in this area could help with early detection and/or early prevention. Knowing that women reporting distress from palpitations have blunted morning cortisol levels, which are a marker of cardiovascular disease, is indicative that fully assessing reported palpitations is important.
There were strengths and limitations to this study. In terms of strengths, this was a relatively large sample of peri- and post-menopausal women with a sufficient number reporting palpitations distress. Salivary cortisol values were collected at four time points over two days. Palpitations data were obtained using a self-report item as is common in other studies (see review36). Data on vasomotor symptoms, depressive symptoms, stress, and insomnia severity were obtained using well-validated measurement tools. Body mass index and blood pressure were assessed with standardized procedures during study clinic visits. Limitations were sample exclusion of women without vasomotor symptoms, limited racial and ethnic diversity, and dichotomizing the complex symptom of heart palpitations into a “yes”, “no” variable. Real time bother related to palpitations at the time of cortisol collection was not available. Future studies could improve the data collection methods.
CONCLUSION
This study suggests that palpitations distress was associated with blunted morning salivary cortisol, which has in turn been associated with increased cardiovascular mortality in some populations. The longer-term cardiovascular consequences of the relationship between menopausal palpitations and blunted morning cortisol response in women warrants further study.
Sources of Funding:
This publication was funded by an Indiana University Ethel Clarke Fellowship, and a Collaboration in Translational Research Grant (Carpenter/Tisdale MPI) from the Indiana Clinical and Translational Sciences Institute funded, in part by Grant Number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (Foroud PI). Dr. Sheng was supported as a postdoctoral fellow under 5T32CA117865 (V. Champion, PI). MsFLASH 01-04 were supported by a cooperative agreement issued by the National Institute of Aging (NIA), in collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Research and Women’s Health (ORWH), and grants U01AG032656, U01AG032659, U01AG032669, U01AG032682, U01AG032699, U01AG032700 from the NIA and an administrative supplement for the Seattle site U01 AG032682. In Indiana, the project was supported by the Indiana Clinical and Translational Sciences Institute, funded in part by grant UL1 RR025761 from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award. MsFLASH05 was supported by the National Institutes of Health National Institute on Aging 5R01AG048209. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Disclosures/Conflicts of Interest: Dr. Carpenter reports personal fees from Kappa Santé and the University of Wisconsin Milwaukee. Dr. Thurston reports consulting fees from Astellas Pharma Inc, Pfizer, Proctor & Gamble, and Virtue Health. Dr. Reed receives grant support from Bayer, AG and receives royalties from UpToDate. Dr. Tisdale’s institution receives funding from Indiana Clinical & Translational Sciences Institute, National Heart, Lung, and Blood Institute, Agency for Healthcare Research & Quality, and American Heart Association. Dr. Tisdale receives a stipend for services as Scientific Editor of Pharmacotherapy (journal). Dr. Von Ah receives honorarium for work for NIH/NCI for advisory board membership Palliative and Supportive Care PDQ. Mr. Larson and Drs. Sheng, Chen, Kovacs, and Guthrie have nothing to disclose.
REFERENCES
- 1.Cho L Ever since I started menopause, my heart flutters from time to time. It usually lasts several seconds. Is this normal? Heart Advis. 2006;9(10):8. [PubMed] [Google Scholar]
- 2.Sievert LL, Obermeyer CM. Symptom clusters at midlife: A four-country comparison of checklist and qualitative responses. Menopause. 2012;19(2):133–44. doi: 10.1097/gme.0b013e3182292af3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter JS, Sheng Y, Elomba C, et al. A systematic review of palpitations prevalence by menopausal status. Current Obstetrics and Gynecology Reports. 2021;10:7–13. doi: 10.1007/s13669-020-00302-z [DOI] [Google Scholar]
- 4.Carpenter JS, Tisdale JE, Chen CX, et al. A Menopause Strategies-Finding Lasting Answers for Symptoms and Health (MsFLASH) investigation of self-reported menopausal palpitation distress. J Womens Health (Larchmt). 2020. doi: 10.1089/jwh.2020.8586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miaskowski C, Aouizerat BE. Biomarkers: symptoms, survivorship, and quality of life. Semin Oncol Nurs. 2012;28(2):129–38. doi: 10.1016/j.soncn.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page GG, Corwin EJ, Dorsey SG, et al. Biomarkers as common data elements for symptom and self-management science. J Nurs Scholarsh. 2018;50(3):276–86. doi: 10.1111/jnu.12378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luptakova L, Sivakova D, Sramekova D, Cvicelova M. The association of cytochrome P450 1B1 Leu432Val polymorphism with biological markers of health and menopausal symptoms in Slovak midlife women. Menopause. 2012;19(2):216–24. doi: 10.1097/gme.0b013e3182281b54 [DOI] [PubMed] [Google Scholar]
- 8.Luptáková L, Siváková D, Čerňanová V, Cvičelová M. Menopausal complaints in Slovak midlife women and the impact of CYP1B1 polymorphism on their incidence. Anthropologischer Anzeiger. 2012;69(4):399–415 [PubMed] [Google Scholar]
- 9.Loja-Chango R, Pérez-López FR, Simoncini T, Escobar GS, Chedraui P. Increased mood symptoms in postmenopausal women related to the polymorphism rs743572 of the CYP17 A1 gene. Gynecol Endocrinol. 2016;32(10):827–30. doi: 10.1080/09513590.2016.1177015 [DOI] [PubMed] [Google Scholar]
- 10.Slopien R, Owecki M, Slopien A, Bala G, Meczekalski B. Climacteric symptoms are related to thyroid status in euthyroid menopausal women. Journal of Endocrinological Investigation 2020;43(1):75–80. doi: 10.1007/s40618-019-01078-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed SD, Newton KM, Larson JC, et al. Daily salivary cortisol patterns in midlife women with hot flashes. Clin Endocrinol (Oxf). 2015. doi: 10.1111/cen.12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauer T, Tottenham LS, Ethier A, Gordon JL. Perimenopausal vasomotor symptoms and the cortisol awakening response. Menopause. 2020;27(11):1322–7. doi: 10.1097/GME.0000000000001588 [DOI] [PubMed] [Google Scholar]
- 13.Wolf P, Winhofer Y, Krssak M, Krebs M. Heart, lipids and hormones. Endocr Connect. 2017;6(4):R59–R69. doi: 10.1530/EC-17-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, Zhang TN, Knight JK, Goodwin JE. The Glucocorticoid Receptor in Cardiovascular Health and Disease. Cells. 2019;8(10). doi: 10.3390/cells8101227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed SD, Guthrie KA, Newton KM, et al. Menopausal quality of life: RCT of yoga, exercise, and omega-3 supplements. American journal of obstetrics and gynecology. 2013;210(3):244.e1–11. doi: 10.1016/j.ajog.2013.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sternfeld B, Lacroix A, Caan BJ, et al. Design and methods of a multi-site, multi-behavioral treatment trial for menopausal symptoms: The MsFLASH experience. Contemp Clin Trials. 2013;35(1):25–34. doi: 10.1016/j.cct.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen LS, Joffe H, Guthrie KA, et al. Efficacy of omega-3 for vasomotor symptoms treatment: A randomized controlled trial. Menopause. 2014;21(4):347–54. doi: 10.1097/GME.0b013e31829e40b8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: A randomized controlled trial. Menopause. 2014;21(4):339–46. doi: 10.1097/GME.0b013e31829e4baa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sternfeld B, Guthrie KA, Ensrud KE, et al. Efficacy of exercise for menopausal symptoms: A randomized controlled trial. Menopause. 2014;21(4):330–8. doi: 10.1097/GME.0b013e31829e4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole SR. Assessment of differential item functioning in the Perceived Stress Scale-10. Journal of epidemiology and community health. 1999;53(5):319–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals. 2002;32:509–21 [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307 [DOI] [PubMed] [Google Scholar]
- 24.Newton KM, Carpenter JS, Guthrie KA, et al. Methods for the design of vasomotor symptom trials: the Menopausal Strategies: Finding Lasting Answers to Symptoms and Health network. Menopause. 2014;21(1):45–58. doi: 10.1097/GME.0b013e31829337a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll D, Ginty AT, Whittaker AC, Lovallo WR, de Rooij SR. The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neurosci Biobehav Rev. 2017;77:74–86. doi: 10.1016/j.neubiorev.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips AC, Ginty AT, Hughes BM. The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2013;90(1):1–7. doi: 10.1016/j.ijpsycho.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 27.Weber BE, Kapoor WN. Evaluation and outcomes of patients with palpitations. Am J Med. 1996;100(2):138–48. doi: 10.1016/s0002-9343(97)89451-x [DOI] [PubMed] [Google Scholar]
- 28.Kinlay S, Leitch JW, Neil A, Chapman BL, Hardy DB, Fletcher PJ. Cardiac event recorders yield more diagnoses and are more cost-effective than 48-hour Holter monitoring in patients with palpitations. A controlled clinical trial. Ann Intern Med. 1996;124(1 Pt 1):16–20. doi: 10.7326/0003-4819-124-1_part_1-199601010-00003 [DOI] [PubMed] [Google Scholar]
- 29.Roa SL, Elias PC, Castro M, Moreira AC. The cortisol awakening response is blunted in patients with active Cushing’s disease. Eur J Endocrinol. 2013;168(5):657–64. doi: 10.1530/EJE-12-0982 [DOI] [PubMed] [Google Scholar]
- 30.Schernthaner-Reiter MH, Siess C, Micko A, et al. Acute and life-threatening complications in Cushing’s syndrome: prevalence, predictors and mortality. J Clin Endocrinol Metab. 2021. doi: 10.1210/clinem/dgab058 [DOI] [PubMed] [Google Scholar]
- 31.Koracevic G, Micic S, Stojanovic M, et al. High likelihood for atrial fibrillation in Cushing’s syndrome. Eur Rev Med Pharmacol Sci. 2020;24(3):1391–7. doi: 10.26355/eurrev_202002_20196 [DOI] [PubMed] [Google Scholar]
- 32.Pecori Giraldi F, Toja PM, Michailidis G, et al. High prevalence of prolonged QT interval duration in male patients with Cushing’s disease. Exp Clin Endocrinol Diabetes. 2011;119(4):221–4. doi: 10.1055/s-0031-1271628 [DOI] [PubMed] [Google Scholar]
- 33.Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J Clin Endocrinol Metab. 2011;96(5):1478–85. doi: 10.1210/jc.2010-2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronaldson A, Kidd T, Poole L, Leigh E, Jahangiri M, Steptoe A. Diurnal Cortisol Rhythm Is Associated With Adverse Cardiac Events and Mortality in Coronary Artery Bypass Patients. J Clin Endocrinol Metab. 2015;100(10):3676–82. doi: 10.1210/jc.2015-2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68(5):657–61. doi: 10.1097/01.psy.0000244071.42939.0e [DOI] [PubMed] [Google Scholar]
- 36.Sheng Y, Carpenter JS, Elomba CD, et al. Review of menopausal palpitations measures. Midlife Women’s Health. 2021;7(5):1–12. doi: 10.1186/s40695-021-00063-6 [DOI] [PMC free article] [PubMed] [Google Scholar]