Abstract
Importance
Neurological and neuropsychiatric symptoms that persist or develop three months after the onset of COVID-19 pose a significant threat to the global healthcare system. These symptoms are yet to be synthesized and quantified via meta-analysis.
Objective
To determine the prevalence of neurological and neuropsychiatric symptoms reported 12 weeks (3 months) or more after acute COVID-19 onset in adults.
Data sources
A systematic search of PubMed, EMBASE, Web of Science, Google Scholar and Scopus was conducted for studies published between January 1st, 2020 and August 1st, 2021. The systematic review was guided by Preferred Reporting Items for Systematic Review and Meta-Analyses.
Study selection
Studies were included if the length of follow-up satisfied the National Institute for Healthcare Excellence (NICE) definition of post-COVID-19 syndrome (symptoms that develop or persist ≥3 months after the onset of COVID-19). Additional criteria included the reporting of neurological or neuropsychiatric symptoms in individuals with COVID-19.
Data extraction and synthesis
Two authors independently extracted data on patient characteristics, hospital and/or ICU admission, acute-phase COVID-19 symptoms, length of follow-up, and neurological and neuropsychiatric symptoms.
Main outcome(s) and measure(s)
The primary outcome was the prevalence of neurological and neuropsychiatric symptoms reported ≥3 months post onset of COVID-19. We also compared post-COVID-19 syndrome in hospitalised vs. non-hospitalised patients, with vs. without ICU admission during the acute phase of infection, and with mid-term (3 to 6 months) and long-term (>6 months) follow-up.
Results
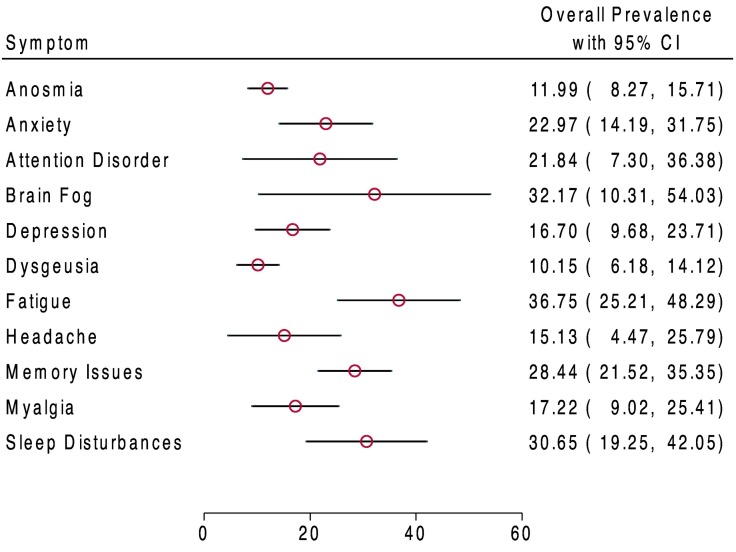
Of 1458 articles, 18 studies, encompassing a total of 10,530 patients, were analysed. Overall prevalence for neurological post-COVID-19 symptoms were: fatigue (37%, 95% CI: 25%–48%), brain fog (32%, 10%–54%), memory issues (28%, 22%–35%), attention disorder (22%, 7%–36%), myalgia (17%, 9%–25%), anosmia (12%, 8%–16%), dysgeusia (10%, 6%–14%) and headache (15%, 4%–26%). Neuropsychiatric conditions included sleep disturbances (31%, 19%–42%), anxiety (23%, 14%–32%) and depression (17%, 10%–24%). Neuropsychiatric symptoms substantially increased in prevalence between mid- and long-term follow-up. Compared to non-hospitalised patients, patients hospitalised for acute COVID-19 had reduced frequency of anosmia, anxiety, depression, dysgeusia, fatigue, headache, myalgia, and sleep disturbance at three (or more) months post-infection. Cohorts with >20% of patients admitted to the ICU during acute COVID-19 experienced higher prevalence of fatigue, anxiety, depression, and sleep disturbances than cohorts with <20% of ICU admission.
Conclusions and relevance
Fatigue, cognitive dysfunction (brain fog, memory issues, attention disorder) and sleep disturbances appear to be key features of post-COVID-19 syndrome. Psychiatric manifestations (sleep disturbances, anxiety, and depression) are common and increase significantly in prevalence over time. Randomised controlled trials are necessary to develop intervention strategy to reduce disease burden.
Keywords: COVID-19, Neuro-COVID-19, Post-COVID-19 syndrome, Post-COVID-19 neurological syndrome, PCNS, Long-Haulers, Long-COVID, SARS-CoV-2, ICU
1. Introduction
Acute coronavirus disease-2019 (COVID-19) continues to overwhelm healthcare systems and is responsible for significant morbidity and mortality. Of equal and urgent concern is the burden associated with COVID-19 symptoms that persist beyond the onset of infection, called COVID-19 long haul (LH) symptoms or post-COVID-19 syndrome. These features have been identified in patients regardless of acute COVID-19 severity [1]. Previous studies have documented residual symptoms that continue (or develop) 4–12 weeks after the onset of acute COVID-19, known as “post-acute or long COVID-19” [2]. However, data on neurological symptoms that persist (or develop) three months or more after acute COVID-19 have not yet been systematically consolidated.
The National Institute for Health and Care Excellence (NICE) guidelines define post-COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks (3 months) and are not explained by an alternative diagnosis [2].”.
Symptoms of acute COVID-19 such as myalgia, dizziness, headache, and impaired consciousness may have a neurological aetiology and persist beyond the acute phase [3,4]. Micro-emboli in brain tissue [5,6], blood-brain barrier dysfunction [7,8], neuro-inflammation [9] leading to coagulopathy, and factors related to hospital admission (mechanical ventilation and medications such as sedatives) may contribute to long-term neurological symptoms. Psychiatric conditions secondary to social isolation, hysteria and the loss of loved ones, are likely features of post-COVID-19 syndrome [10]. Even in the absence of social factors, the nature of hospitalisation and an intensive care unit (ICU) stay, and course of critical illness likely influence the incidence of neuropsychiatric symptoms post infection [8].
Herein, we aimed to evaluate the prevalence of neurological and neuropsychiatric symptoms of post-COVID-19 syndrome three months after the onset of acute COVID-19 infection. We also report the prevalence of these symptoms in outpatient (community), non-ICU hospitalised, and ICU cohorts as well as at two different time points (mid-term: 3–6 months and long-term: > 6 months) after the onset of acute COVID-19 infection. Associations between post-COVID-19 syndrome and hospitalisation during acute phase illness also were assessed.
2. Methods
2.1. Search strategy and selection criteria
PubMed, EMBASE, Web of Science, Google Scholar and Scopus were searched using a priori search criteria for articles published between January 1st, 2020 and August 1st, 2021. An effort was made to account for plurals, acronyms, and synonyms. The search was not limited by language. These results then were reviewed by the research team for eligibility. Covidence©, Cochrane's online systematic review platform, was used to streamline the review process. All articles that met the inclusion criteria were retrieved and the full text reviewed. References of included papers were screened for additional studies. The detailed search strategy can be found in Appendix 1. The study protocol was registered (22/06/2021) with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42021254647).
2.2. Study eligibility: inclusion and exclusion criteria
Studies were eligible if (1) the length of follow-up satisfied the NICE definition of post-COVID-19 syndrome (symptoms that develop or persist ≥3 months after the onset of COVID-19); (2) investigators reported the prevalence of neurological or neuropsychiatric symptoms in COVID-19-confirmed individuals; (3) they had a cohort size >50 patients; and (4) the hospitalisation status (community infection, hospital admission, ICU admission) of all patients (during the acute phase) was reported. We excluded case reports, editorials, commentaries, meta-analysis articles, review articles, animal studies, and articles with a paediatric population (age < 18 years). We excluded articles when no details on neurological or neuropsychiatric symptoms were available.
2.3. Study selection and data extraction
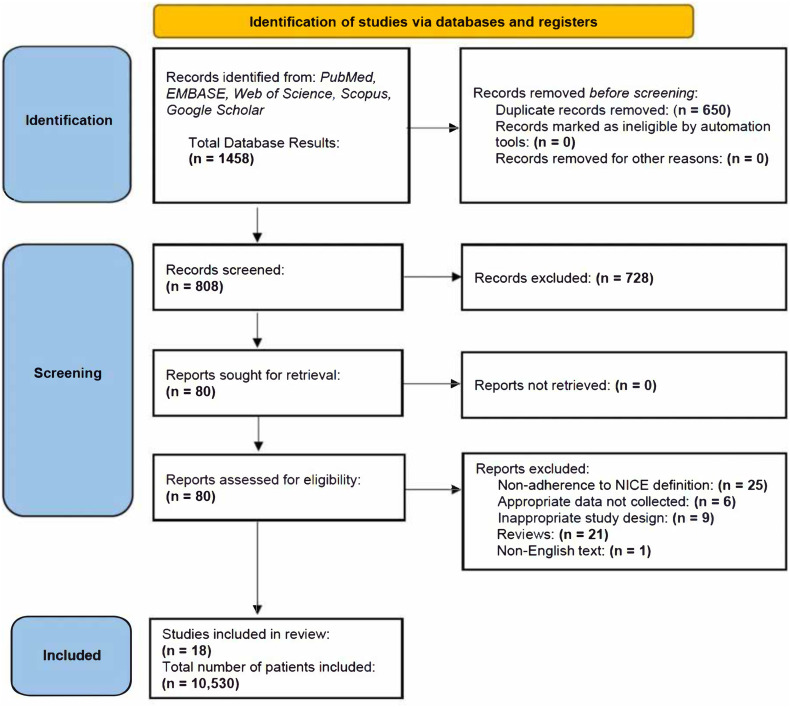
Studies were screened by two authors (L.P, N.V) independently for relevance and adherence to inclusion/exclusion criteria. The full text was then thoroughly assessed to confirm eligibility, emphasising adherence to the NICE definition of post-COVID-19 syndrome. Conflicts were resolved by consensus or referral to a third reviewer (S.M.C). Fig. 1 provides the PRISMA flow-chart for study inclusion/exclusion. Thereafter, data was extracted from eligible studies into a shared Excel spreadsheet (Microsoft, Redmond, WA). Study type, year of publication, baseline patient characteristics, features of the acute phase presentation, length of follow-up, hospitalisation status (ICU admission, length of hospital and/or ICU stay) and neurological and neuropsychiatric outcomes measured were tabulated for each study. The number of patients who experienced each outcome and number of patients in whom each particular outcome was measured were both recorded in the spreadsheet.
Fig. 1.
PRISMA Flow chart, where the total number of patients included refers to those considered in the analysis only.
2.4. Definitions of outcomes
Primary outcomes were both neurological and neuropsychiatric symptoms of post-COVID-19 syndrome. Neurological symptoms included: (1) anosmia [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20]] (2) dysgeusia [[11], [12], [13],[15], [16], [17], [18]] (3) headache [11,[13], [14], [15], [16],18,21,22] (4) any cognitive dysfunction [13,14,16,[18], [19], [20], [21]] (see: Supplemental Table 2) (5) any fatigue [11,13,14,16,18,19,[21], [22], [23], [24], [25]] (fatigue/malaise [11,13,14,16,18,19,[21], [22], [23]], chronic fatigue syndrome [24], post exertional malaise [21]) (6) pain (neuropathic pain [15,23], myalgia [11,12,[14], [15], [16],18,21,22]) and (7) peripheral nervous system symptoms (movement disorders [16,22], paraesthesia [14,22]). Neuropsychiatric features included: (1) anxiety [14,18,19,22,23,[25], [26], [27]] (2) depression [14,18,19,22,23,[25], [26], [27]] (3) sleep disturbances/insomnia [[13], [14], [15],19,21,22,25,27] and (4) post-traumatic stress disorder (PTSD) [14,24]. Again, these outcomes only were recorded if assessed at least 3 months after onset of acute COVID-19. These outcomes were not mutually exclusive and could co-occur.
2.5. Quality assessment/risk of bias
Bias was assessed independently by two investigators (L.P, J.B) using the Newcastle-Ottawa (NOS) bias assessment tool [28] (Appendix 2). NOS scores were based on three domains: patient selection, comparability, and assessment of outcome or exposure. Each study received a score between 0 and 9, where a score < 6 indicates an elevated risk of bias. The cohort chosen ought to represent the average patient diagnosed with COVID-19 who experiences residual symptoms three or more months after acute illness onset. Fully recovered COVID-19 positive patients were used to develop study controls/characteristics. Data were deemed unsatisfactory if >20% of patients were lost to follow-up without explanation. Studies obtained a non-zero score (one-star) for comparability if they distinguished between SARS-CoV-2 positive and COVID-19 patients. Modesti et al.'s adapted NOS scale was used to evaluate the methodological quality of cross-sectional studies using the categories; selection, comparability, and outcome [29] (Appendix 2). Disagreements or discrepancies were resolved in consensus with a third investigator.
2.6. Statistical analysis
This systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines also were adhered to (see Appendix 3). The prevalence of each outcome was calculated for each study based on the number of patients with the specific outcome divided by the total number of COVID-19 patients for which the outcome was assessed. This then was pooled in meta-analysis across all the included studies. For all meta-analyses of prevalence, we used random-effects model (REML) with the inverse variance method. This calculation was performed only when three or more studies had the outcome of interest reported. Sidik-Jonkman estimator was used for tau [30], Hartung-Knapp adjustment for Confidence intervals (CI) [31], and the Freeman-Tukey double arcsine transformation to calculate prevalence rates for each outcome. Heterogeneity was assessed using the Cochrane Q statistic (chi-square test) and the magnitude of heterogeneity with the I2 statistic [32]; I2 quantifies the degree of heterogeneity (from 0% to 100%). Continuous variables, if normally distributed (e.g., age, length of hospital/ICU stay), were reported as means with their standard deviation (SD). In lieu of subgroup analysis, descriptive analysis reported cumulative prevalence (the total number of patients with the outcome divided by the total number among whom the outcome was reported), stratified by hospitalisation status and time of follow-up. These results were presented as a fraction/proportion (%). Reporting 95% confidence intervals (CI) was deemed unsuitable; instead, cohort size (denominator) was used to infer validity. Odds ratios (OR) and 95% CI were calculated to determine whether patients admitted to a hospital experienced more neurological symptoms of post-COVID-19 syndrome than non-admitted patients. All analysis was performed using the statistical software package STATA (StataCorp LLC, College Station, TX).
3. Results
The search identified 1458 studies, among which 80 full-text articles were assessed for eligibility. Of these, 18 studies (3 case-control, 6 cross-sectional, 8 prospective cohort, and 1 retrospective cohort study) were included. A total of 10,530 patients were available for final analysis (Fig. 1).
3.1. Risk of bias assessment
The NOS bias assessment indicated no elevated risk of bias overall (mean score 8, SD: 1) (Appendix 2). Two multi-centre prospective cohort trials were judged to have a high risk of bias, as these investigators failed to distinguish between COVID-19 patients and those who were only SARS-CoV-2 PCR positive. Therefore, these two studies were assigned a zero score for comparability. All the case-control studies were considered of high quality (>7) (Appendix 2). Quality assessment showed that all included cohorts had adequate data on patient characteristics, and neurological and/or neuropsychiatric symptoms, with detail more than sufficient to allow for inferences pertinent to clinical practice.
3.2. Baseline characteristics
Among the 10,530 patients, 59% were female and the mean age was 52 years (SD: 10). Patients in hospitals were significantly older than those in the community (57 years [SD: 7] vs. 46 years [SD: 4]). More than half of all patients were hospitalised during their acute-phase COVID-19 infection (51%) and 13% were admitted to an ICU. Hypertension (23%) and diabetes mellitus (12%) were the most common comorbidities. Not surprisingly, all comorbidities (coronary artery disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, and hypertension) were more common in hospitalised than non-hospitalised patients (Table 1 ).
Table 1.
Descriptive statistics for overall study population, n (%), stratified by hospitalisation.
| All Patients (N=10,530) |
Non-hospitalised (N= 4,747) |
Hospitalised (N=5,783) |
|
|---|---|---|---|
| Demographic Characteristics | |||
| Malea | 4115/10140 (41) | 924/4245 (22) | 2975/5464 (54) |
| Ageb, mean, (SD) | 52 (10) | 46 (4) | 57 (7) |
| Acute COVID-19 Information | |||
| Hospital admission | 6107/10530 (58) | 324/4747 (7) | 5783/5783 (100) |
| Duration of hospital admissionc, days (SD) | 12 (4) | - | 12 (4) |
| ICU admission | 522/4045 (13) | - | 522/4045 (13) |
| Duration of ICU admissiond, days (SD) | 13 (4) | - | 13 (4) |
| Comorbidities | |||
| CAD | 117/4682 (3) | 30/3762 (1) | 87/920 (9) |
| CKD | 232/4088 (6) | - | 232/4088 (6) |
| COPD | 187/8032 (2) | 15/3762 (0) | 172/4270 (4) |
| Diabetes | 998/8217 (12) | 68/3762 (2) | 930/4455 (21) |
| Hypertension | 1885/8217 (23) | 342/3762 (9) | 1543/4455 (35) |
| Acute COVID-19 Symptoms | |||
| Anosmia | 416/818 (51) | 202/353 (57) | 214/465 (46) |
| Confusion | 7/120 (6) | - | 7/120 (6) |
| Dysgeusia | 346/776 (45) | 183/353 (52) | 163/423 (39) |
| Headache | 198/413 (48) | 183/353 (52) | 15/60 (25) |
| Myalgia | 100/538 (19) | - | 100/538 (19) |
| Neurological symptoms of post-COVID-19 syndrome | |||
| Anosmia | 357/3164 (11) | 93/505 (18) | 264/2659 (10) |
| Attention Disorder | 271/1207 (22) | 73/130 (56) | 198/1077 (18) |
| Brain Foge | 1557/4329 (36) | 1515/3914 (39) | 42/415 (10) |
| Confusione | 95/949 (10) | 74/152 (49) | 21/797 (3) |
| Dysgeusia | 246/2703 (9) | 86/505 (17) | 160/2198 (7) |
| Fatigue | 3197/7173 (45) | 2430/4747 (51) | 767/2426 (32) |
| Headache | 1502/7437 (20) | 1398/4267 (33) | 104/3170 (3) |
| Memory Issuese | 1584/5033 (29) | 1311/3892 (34) | 273/1141 (24) |
| Movement Disorder | 28/857 (3) | - | 28/857 (3) |
| Myalgia | 1373/7555 (18) | 1159/4267 (27) | 214/3288 (7) |
| Pain | 582/2086 (28) | 107/350 (31) | 475/1736 (27) |
| Paraesthesia | 78/1218 (6) | - | 78/1218 (6) |
| Neuropsychiatric symptoms of post-COVID-19 syndrome | |||
| Anxiety | 598/3104 (20) | 198/632 (31) | 400/2472 (16) |
| Depression | 480/3104 (15) | 173/632 (27) | 307/2472 (12) |
| PTSD | 135/964 (14) | 35/130 | 100/834 (12) |
| Sleep disturbance | 2411/7993 (30) | 1411/3892 (36) | 1000/4101 (24) |
Non-hospitalised (community) setting refers to the sub-population in which <10% were reported as being admitted to hospital during acute phase COVID-19. Hospital setting refers to the sub-population in which all patients were hospitalised during acute phase COVID-19. Only outcomes reported by more than one study are tabulated. “-“denotes values that were not able to be reported. SD, Standard Deviation, ICU, Intensive Care Unit, CAD, Coronary Artery Disease, COPD, Chronic Obstructive Pulmonary Disease, CAD, Coronary Artery Disease, CKD, Chronic Kidney Disease, PTSD, Post-Traumatic Stress Disorder.
Male non-hospitalised (community) values and hospitalised values do not sum to all patients as the number of males was not always reported for the subgroups.
Age: weighted mean of patient age with standard deviation of mean.
Duration of the hospital admission: weighted mean of hospital admission length with standard deviation of the mean.
Duration of ICU admission: weighted mean of ICU admission length with standard deviation of the mean.
See Supplemental Table 2 for clarification on the reporting of these outcomes.
Anosmia, dysgeusia and headache were the most frequently reported neurological manifestations of acute COVID-19 (Table 1), and all were more common among non-hospitalised than hospitalised patients (57%, 52%, 52% vs. 46%, 39%, 25%, respectively).
3.3. Prevalence of post-COVID-19 syndrome
Upon meta-analysis of all patients, fatigue was the most frequent symptom of neurological post-COVID-19 syndrome (37%, 95% CI: 25%–48%). Brain fog (32%, 10%–54%), sleep disturbances (31%, 19%–42%) and memory issues (28%, 22%–35%) were similarly frequent (Fig. 2 ).
Fig. 2.
Weighted prevalence of neurological and neuropsychiatric symptoms (95% CI) reported in post-COVID-19 syndrome.
Weighted prevalence is presented for outcomes that were reported in at least three studies. REML was then used to determine the weighted frequency (e.g., Supplemental Fig. 2a). Cohort size shows the total number of patients in whom the symptom was assessed. Red dots show weighted frequency. Black lines show 95% confidence intervals.
The prevalence of neurological symptoms was generally lower among hospitalised than non-hospitalised patients. As neuropsychiatric symptoms of post-COVID-19 syndrome, anxiety and depression were more frequently reported in the community than in patients hospitalised during their acute phase of COVID-19 (31% and 27% vs. 16% and 12%, respectively) (Table 1).
3.4. Duration of post-COVID-19 syndrome
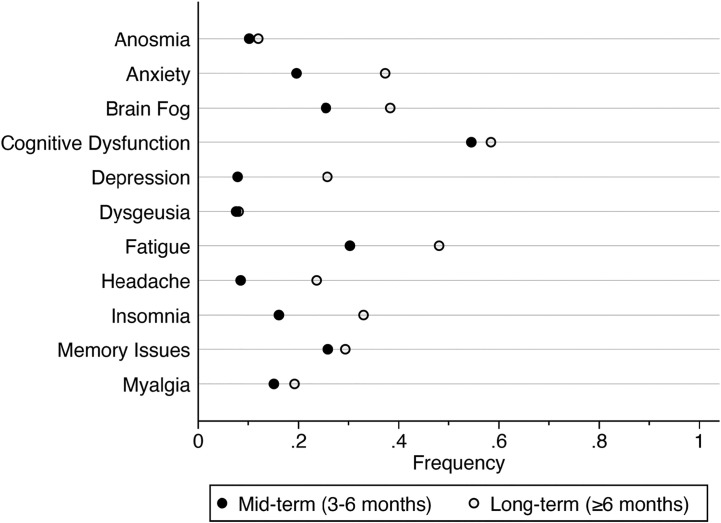
The prevalence of neurological and neuropsychiatric symptoms of post-COVID-19 syndrome were higher when assessed at or beyond six months (long-term) than when assessed between three and six months (mid-term). Anosmia, dysgeusia, myalgia and cognitive dysfunction did not change significantly in prevalence (<5% change) between mid-term and long-term follow-up. Conversely, neuropsychiatric symptoms (anxiety and depression) were substantially increased in prevalence long-term versus mid-term (Fig. 3 ).
Fig. 3.
Frequency (proportion) of symptoms reported in post-COVID-19 syndrome.
At least three studies were required to provide mid-term and long-term follow-up for frequencies to be displayed. Black dots indicate symptom frequency when assessed between three and six months after acute COVID-19 onset. White dots indicate symptom frequency when assessed at six or more months After acute illness onset.
3.5. Hospitalisation and severity of illness
Patients who were hospitalised during acute COVID-19 were less likely to develop anosmia, anxiety, depression, dysgeusia, fatigue, headache, memory issues, myalgia, and sleep disturbances 3 months (or more) after COVID-19 onset than those who remained non-hospitalised throughout their acute infection (Supplemental Fig. 1).
Anxiety and depression were approximately three and two times more prevalent in cohorts among which >20% of participants had been admitted to an ICU (during acute COVID-19) as in cohorts in which <20% underwent ICU admission (Supplemental Table 1).
4. Discussion
In a meta-analysis of over 10,000 patients extracted from 18 published studies, neurological and neuropsychiatric symptoms both were common three months after an acute COVID-19 infection. Fatigue, cognitive dysfunction (brain fog, memory issues, attention disorder) and sleep disturbances were the most prevalent features of neurological/neuropsychiatric post-COVID-19 syndrome, all identified in almost one third of patients three months after the onset of acute COVID-19 illness (Fig. 1). Interestingly, these symptoms persisted and were even more common long-term (six or more months post infection) than when assessed mid-term (three to six months). Thus, post-COVID-19 syndrome poses a significant long-term global public health concern that affects both hospitalised and non-hospitalised patients. There was notable heterogeneity among studies reporting cognitive dysfunction such as memory issues (I2 = 97%). Discrepancies in the definitions of cognitive dysfunction, brain fog, memory issues and attention disorder limit the accuracy of the pooled prevalence estimates within our meta-analysis. Further studies must employ a standardised definition of ‘cognitive dysfunction’ and determine specific deficits (memory, spatial, sensorineural) using quantitative neurological tests.
Although anosmia, dysgeusia, and headache were common neurological manifestations of acute COVID-19, they were not major symptoms of post-COVID-19 syndrome, indicating that these specific symptoms generally resolve. In a retrospective cohort of 3737 patients who reported anosmia and/or dysgeusia during acute COVID-19, 68% recovered their sense of smell and 73% their taste within six weeks of symptom onset [17]. After three months, however, the proportion of patients with recovery decreased, plateauing after roughly 20 weeks. Similarly, our analysis suggests that anosmia and dysgeusia do not persist or develop for the first time beyond three months in most patients, generally being only acute COVID-19 symptoms [10].
Notably, in our sample of patients, symptoms such as anxiety, depression, brain fog, fatigue and insomnia increased in frequency from mid- to long-term follow up, which may indicate that these symptoms are more likely to develop than persist post-infection (Fig. 3) [10,33]. Limited data prevented calculation of uncertainties associated with the prevalence of mid to long-term symptoms however it is likely that: (1) inconsistent definitions of ‘cognitive dysfunction’ augmented findings at both time points (Supplemental Table 2) (2) prevalence of symptoms common in the community even in the absence of COVID-19 (headache and fatigue) are likely overestimated, especially long-term. Still, large retrospective cohort studies that followed patients from mid to long-term confirm similar trends in the neuropsychiatric and neurological symptoms herein reported [10,33].
Persistent symptoms may arise from a combination of biological and psychological mechanisms. For example, SARS-CoV-2 RNA may remain in brain tissue long-term, worsening neuronal loss over time [4,[34], [35], [36]]. Moreover, innate immune cell entry secondary to blood brain barrier dysfunction may prolong neuro-inflammation [34,37]. Social isolation, confinement, trauma during acute-infection, and persistent fatigue are heavily implicated in the development of neuropsychiatric symptoms post-infection, especially sleep disturbances [38].
Surprisingly, hospitalisation did not increase the frequency of neurological post-COVID-19 syndrome relative to the levels observed in non-hospitalised patients. This may be due to patients with COVID-19 in the community generally being younger than those in hospitals [39]. A retrospective analysis of 57,000 non-hospitalised patients identified higher prevalence rates for chronic fatigue syndrome, malaise, and fatigue in patients under 65 years old than in all patients collectively [33]. Recall bias and the lack of objective metrics to assess symptoms also likely augment reporting in the community setting [40]. However, stratification by ICU status showed that symptoms such as fatigue, dysgeusia, paraesthesia, headache, anxiety, and depression of post-COVID-19 syndrome are likely to be associated with acute disease severity [41] (Supplemental Fig. 2). Menges et al. reported that fatigue (OR: 4.63; 95 CI: 1.02–32.88) was significantly associated with ICU stay, but not with hospital stay (OR 1.00; 95% CI: 0.59–1.71) [23]. Our analysis revealed an increased prevalence of fatigue in ICU patients relative to both hospitalised non-ICU and non-hospitalised patients, consistent with prior reports [42]. Further investigations are required to establish if standard disease severity scales, such as WHO disease severity score, can predict the future development of post-COVID-19 syndrome [43].
Symptoms of post-ICU syndrome (PICS) and the symptoms of post-COVID-19 syndrome (in ICU patients) overlap, therefore prevalence of neuropsychiatric symptoms and fatigue may be overestimated. While neurological features of PICS are similar to post-COVID-19 syndrome, movement disorders and PTSD are frequent manifestations of PICS and are largely uncommon in post-COVID-19 syndrome [44,45]. In ICU admitted acute respiratory distress syndrome patients without COVID-19, 12 months post-ICU admission the prevalence of neuropsychiatric symptoms and memory issues were similar to our findings in ICU admitted COVID-19 patients (≥3 months) [44]. ICU admission due to COVID-19 may increase risk of PICS [46]. As such, clinicians must be aware of both conditions.
It is important to highlight that hippocampal [47,48] and cortical atrophy [36], hypoxic-ischemic changes [49] and small vessel disease [50] are neuropathological processes documented to occur secondary to inflammation and oxidative stress during COVID-19 [36,51]. Consequences of these pathological processes may manifest in long-term as cognitive dysfunction (e.g., brain fog, memory issues, and attention disorder). Our study demonstrates that cognitive dysfunction is a prominent feature of post-COVID-19 syndrome and requires immediate attention and interventional strategies.
Limitations of this study include the inability to establish the impact of neurological post-COVID-19 symptoms on quality of life [23]. Similarly, the severity of symptoms in post-COVID-19 syndrome remains to be adequately characterised.
Given the common occurrence of post-COVID-19 syndrome, the development of consistent diagnostic criteria for neurological and neuropsychiatric post-COVID-19 and a standardized approach to the multidisciplinary follow-up of COVID-19 patients may be key to reducing disease burden [52]. Furthermore, increased awareness of neurological and neuropsychiatric post-COVID-19 syndrome and future research on interventional strategies for post-COVID-19 syndrome are necessary to improve long-term outcomes, mitigate disease burden, and improve quality of life.
5. Conclusions
In our analysis, we have demonstrated that neurological and neuropsychiatric symptoms are common components of post-COVID-19 syndrome, with some symptoms present in roughly one third of patients assessed three months after the onset of acute COVID-19 disease. Fatigue and cognitive dysfunction (brain fog, memory issues, attention disorder) were key neurological features. Neuropsychiatric symptoms appear to increase in prevalence over time, rather than resolve. Increased awareness of neurological and neuropsychiatric post-COVID-19 syndrome and research on interventional strategies to combat post-COVID-19 syndrome are necessary to improve quality of life and mitigate disease burden.
Author contributions
Study concept and design: Lavienraj Premraj, Sung-Min Cho, Nivedha V. Kannapadi, Denise Battaglini, Jonathon Fanning.
Search Strategy: Stella M. Seal, Sung-Min Cho, Lavienraj Premraj.
Acquisition, analysis, or interpretation of data: Jack Briggs, Lavienraj Premraj, Nivedha V. Kannapadi.
Statistical analysis: Lavienraj Premraj, Nivedha V. Kannapadi, Jack Briggs.
Tables and figures: Lavienraj Premraj, Nivedha V. Kannapadi, Sung-Min Cho.
First drafting of the manuscript: Lavienraj Premraj, Nivedha V. Kannapadi.
Critical revision for important intellectual content and final approval of the manuscript: Sung-Min Cho, Denise Battaglini, Jonathon Fanning, John Fraser, Chiara Robba.
Source of funding
None.
Conflicts of interest/disclosures
All authors declare that there is nothing to disclose. No conflicts of interests are noted.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jns.2022.120162.
Appendix A. Supplementary data
Supplementary material
References
- 1.Logue J.K., Franko N.M., McCulloch D.J., et al. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw. Open. 2021;4(2):e210830. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesan P. NICE guideline on long COVID. Lancet Respir. Med. 2021;9(2):129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh K.K., Chaubey G., Chen J.Y., Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Phys. Cell Phys. 2020;319(2):C258–c267. doi: 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon L., McNamara C., Gaur P., et al. Cerebral microhaemorrhage in COVID-19: a critical illness related phenomenon? Stroke Vasc. Neurol. 2020;5(4):315–322. doi: 10.1136/svn-2020-000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawlani V., Scotton S., Nader K., et al. COVID-19-related intracranial imaging findings: a large single-centre experience. Clin. Radiol. 2021;76(2):108–116. doi: 10.1016/j.crad.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol. Dis. 2020;146 doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 9.Lee M.-H., Perl D.P., Nair G., et al. Microvascular injury in the brains of patients with Covid-19. N. Engl. J. Med. 2021;384(5):481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Augustin M., Schommers P., Stecher M., et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg. Health - Eur. 2021;6 doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellan M., Soddu D., Balbo P.E., et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw. Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrigues E., Janvier P., Kherabi Y., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Inf. Secur. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group TWCftCS Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325(15):1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet (London, England) 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y., Li X., Geng D., et al. Cerebral micro-structural changes in COVID-19 patients – an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen N.N., Hoang V.T., Lagier J.-C., Raoult D., Gautret P. Long-term persistence of olfactory and gustatory disorders in COVID-19 patients. Clin. Microbiol. Infect. 2021;27(6):931–932. doi: 10.1016/j.cmi.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orrù G., Bertelloni D., Diolaiuti F., et al. Long-COVID syndrome? A study on the persistence of neurological, psychological and physiological symptoms. Healthcare. 2021;9(5):575. doi: 10.3390/healthcare9050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor R.R., Trivedi B., Patel N., et al. Post-COVID symptoms reported at asynchronous virtual review and stratified follow-up after COVID-19 pneumonia. Clin. Med. 2021;21(4):e384–e391. doi: 10.7861/clinmed.2021-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ugurlu B.N., Akdogan O., Yilmaz Y.A., et al. Quantitative evaluation and progress of olfactory dysfunction in COVID-19. Eur. Arch. Otorhinolaryngol. 2021;278(7):2363–2369. doi: 10.1007/s00405-020-06516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis H.E., Assaf G.S., McCorkell L., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. SSRN Electron. J. 2021;38:7–19. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero-Duarte Á., Rivera-Izquierdo M., Guerrero-Fernández de Alba I., et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19(1):129. doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menges D., Ballouz T., Anagnostopoulos A., et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0254523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simani L., Ramezani M., Darazam I.A., et al. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J. Neuro-Oncol. 2021;27(1):154–159. doi: 10.1007/s13365-021-00949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frontera J.A., Yang D., Lewis A., et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021;426 doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González J., Benítez I.D., Carmona P., et al. Pulmonary function and radiologic features in survivors of critical COVID-19. Chest. 2021;160(1):187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Q., Xu M., Li J., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin. Microbiol. Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells G., Shea B., O’Connell J. Vol. 7. Ottawa Health Research Institute Web site; 2014. The Newcastle-Ottawa Scale (NOS) for Assessing The Quality of Nonrandomised Studies in Meta-analyses. [Google Scholar]
- 29.Modesti P.A., Reboldi G., Cappuccio F.P., et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidik K., Jonkman J.N. A comparison of heterogeneity variance estimators in combining results of studies. Stat. Med. 2007;26(9):1964–1981. doi: 10.1002/sim.2688. [DOI] [PubMed] [Google Scholar]
- 31.IntHout J., Ioannidis J.P.A., Borm G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014;14(1):25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 33.Estiri H., Strasser Z.H., Brat G.A., et al. Evolving phenotypes of non-hospitalized patients that indicate long covid. BMC Medicine. 2021;19:1–10. doi: 10.1186/s12916-021-02115-0. 2021.2004.2025.21255923, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Najjar S., Najjar A., Chong D.J., et al. Central nervous system complications associated with SARS-CoV-2 infection: integrative concepts of pathophysiology and case reports. J. Neuroinflammation. 2020;17(1):231. doi: 10.1186/s12974-020-01896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu K., Zou J., Chang H.Y. RNA-GPS predicts SARS-CoV-2 RNA localization to host mitochondria and nucleolus. Cell Systems. 2020;11(1):102–108. doi: 10.1016/j.cels.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Generoso J.S., Barichello De Quevedo J.L., Cattani M., et al. Neurobiology of COVID-19: how can the virus affect the brain? Br. J. Psychiatr. 2021;43(6):650–664. doi: 10.1590/1516-4446-2020-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theoharides T.C., Cholevas C., Polyzoidis K., Politis A. Long-COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. Biofactors. 2021;47(2):232–241. doi: 10.1002/biof.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morin C.M., Bjorvatn B., Chung F., et al. Insomnia, anxiety, and depression during the COVID-19 pandemic: an international collaborative study. Sleep Med. 2021;87:38–45. doi: 10.1016/j.sleep.2021.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goërtz Y.M.J., Van Herck M., Delbressine J.M., et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020:00542–02020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomura S., Yoneoka D., Shi S., et al. An assessment of self-reported COVID-19 related symptoms of 227,898 users of a social networking service in Japan: Has the regional risk changed after the declaration of the state of emergency? Lancet Reg. Health - Western Pacific. 2020;1 doi: 10.1016/j.lanwpc.2020.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zorowitz R.D. ICU-acquired weakness: a rehabilitation perspective of diagnosis, treatment, and functional management. Chest. 2016;150(4):966–971. doi: 10.1016/j.chest.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sudre C.H., Murray B., Varsavsky T., et al. Attributes and predictors of long COVID. Nat. Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikkelsen M.E., Christie J.D., Lanken P.N., et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am. J. Respir. Crit. Care Med. 2012;185(12):1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rawal G., Yadav S., Kumar R. Post-intensive care syndrome: an overview. J. Transl. Int. Med. 2017;5(2):90–92. doi: 10.1515/jtim-2016-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unoki T., Sakuramoto H., Uemura S., et al. Prevalence of and risk factors for post-intensive care syndrome: multicenter study of patients living at home after treatment in 12 Japanese intensive care units, SMAP-HoPe study. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0252167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poloni T.E., Medici V., Moretti M., et al. COVID-19-related neuropathology and microglial activation in elderly with and without dementia. Brain Pathol. 2021;31(5) doi: 10.1111/bpa.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solomon I.H., Normandin E., Bhattacharyya S., et al. Neuropathological features of Covid-19. N. Engl. J. Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radnis C., Qiu S., Jhaveri M., Da Silva I., Szewka A., Koffman L. Radiographic and clinical neurologic manifestations of COVID-19 related hypoxemia. J. Neurol. Sci. 2020;418:117119. doi: 10.1016/j.jns.2020.117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowenstein C.J., Solomon S.D. Severe COVID-19 is a microvascular disease. Circulation. 2020;142(17):1609–1611. doi: 10.1161/CIRCULATIONAHA.120.050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huth S.F., Cho S.-M., Robba C., et al. Neurological manifestations of Coronavirus disease 2019: a comprehensive review and meta-analysis of the First 6 months of pandemic reporting. Front. Neurol. 2021;12:664599. doi: 10.3389/fneur.2021.664599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moghimi N., Di Napoli M., Biller J., et al. The neurological manifestations of post-acute sequelae of SARS-CoV-2 infection. Curr. Neurol. Neurosci. Rep. 2021;21(9):44. doi: 10.1007/s11910-021-01130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material