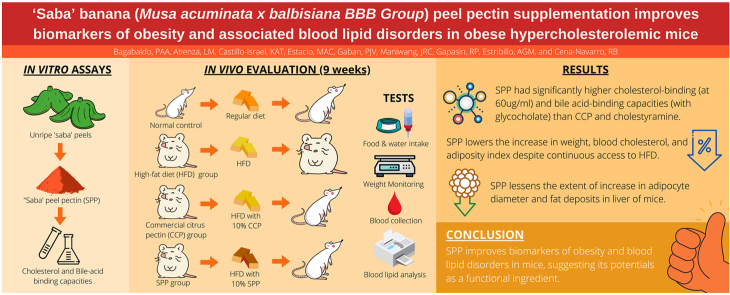
Abstract
‘Saba’ banana peel contains significant amounts of pectin but with very limited commercial use. To increase its value, the present study investigated the effect of ‘saba’ peel pectin (SPP) on biomarkers of obesity and associated blood lipid disorders in vivo and identified its potential mechanism via in vitro lipid lowering assays. ICR male mice were induced with obesity and hypercholesterolemia using 45% high fat diet (HFD) for three weeks. The mice were then randomly allocated to four groups fed various diets ad libitum for nine weeks as follows: (1) normal diet (ND), (2) high-fat diet (HFD), (3) HFD with 10% w/w commercial citrus pectin (HFD-CCP), and (4) HFD with 10% w/w SPP (HFD-SPP). For the in vitro study, lipid lowering assays were carried out using published protocols with some modifications. Results showed that the mean endline body weight of HFD-CCP and HFD-SPP were significantly lower than HFD group despite having comparable feed intake. The pectin-supplemented groups also had lower blood total cholesterol than HFD group. Necropsy results showed no significant treatment-related difference in the relative organ weights, except for the liver of HFD group being pale, enlarged, and heavier than the other mice groups. This is consistent with the microscopic observations of liver sections from HFD-CCP and HFD-SPP which had occasional fat deposits only whereas HFD group showed mild necrosis and fat infiltration. In terms of body fat, the adiposity index was significantly lower among HFD-SPP and HFD-CCP than the HFD group, with both pectin-supplemented groups showing lesser extent of increase in adipocyte diameter. Meanwhile, HFD-CCP and HFD-SPP groups were significantly comparable in terms of body weight, blood lipids, organ and adipose tissue weights, adiposity index, and liver morphology. In vitro assays revealed that SPP had significantly higher cholesterol and bile acid binding capacities at 60 μg/mL and 20 μg/mL, respectively than CCP and bile acid-binding drug, cholestyramine. These showed that SPP supplementation improves biomarkers of obesity and associated blood lipid disorders at par with commercially-available citrus pectin possibly via cholesterol and bile acid binding pathways, suggesting that SPP may be a potential functional ingredient with anti-obesity and anti-hypercholesterolemic properties.
Keywords: Adiposity, Blood lipids, Mice, Obesity, Pectin, ‘Saba’ banana peel
Graphical abstract
Highlights
-
•
Cholesterol binding capacity of ‘saba’ peel pectin (SPP) was highest at 60ug/ml.
-
•
With glycocholate, SPP had higher bile-acid binding capacity than cholestyramine.
-
•
SPP lowers weight gain in mice despite continuous access to high fat diet (HFD).
-
•
SPP lessens the increase in adipocyte diameter and fat deposits in liver of mice.
-
•
SPP group had comparable blood cholesterol and % adiposity index with the control.
1. Introduction
The prevalence of obesity is reaching epidemic proportions worldwide, with 39% of adults aged 18 years and above were overweight in 2016, and 13% were obese (WHO, 2021). In the Philippines, there is also an increasing trend in the prevalence of overweight and obesity in adults from 16.6% in 1993 to 36.6% in 2019, while for children 0–5 years old, 5.1–10 years, and 10.1–19 years old, the prevalence were 3.5%, 10.4%, and 10.7%, respectively (FNRI, 2019). Obesity, especially with visceral fat accumulation, is a serious risk factor for so-called metabolic syndrome, which includes insulin resistance, glucose intolerance, hypertension, and dyslipidemia (Matsuzawa, 2014), suggesting that the body fat is an important contributor to these diseases. Fortunately, there is strong evidence that modest body weight loss of 5–10% significantly reduces the risk of these comorbidities (NAASO, 1998).
There are a variety of effective options for weight loss which consist of three lines of therapy namely lifestyle changes, pharmacotherapy, and surgery. Behavioral (or lifestyle) interventions are considered the cornerstone of obesity treatment designed to produce long-term weight losses through healthy eating and gradual increase in physical activity (Jacob and Isaac, 2012; Looney and Raynor, 2013; and Olson, 2017). However, lifestyle interventions were ineffective because of the difficulty in maintaining sustained lifestyle changes and limited services to support change (Faith et al., 2000; Middleton et al., 2013; and Smith et al., 2014). Pharmacological therapy, on the other hand, is used as an adjunct therapy for those patients who fail to respond to lifestyle modification alone (Nuffer, 2019). These drugs work through the central nervous system (CNS) pathways that reduce appetite, enhance satiety or decrease fat absorption however, side effects were reported including nausea, dry mouth, constipation, diarrhea, vomiting, bloating, among others (Srivastava and Apovian, 2017). Last line of treatment is surgical therapy which has been considered to function as restrictive in which the size of the gastric pouch is greatly reduced leading to malabsorption of nutrients that contributes to weight loss (Wolfe et al., 2016). Potential complications include intestinal obstruction, marginal ulcer, ventral hernia, gallstones, and vitamin and mineral deficiencies (Albaugh and Abumrad, 2018).
The following limitations of these therapies led to a large and growing market for alternative therapies, particularly the use of natural dietary compounds and supplements due to their high nutraceutical value and low rate of reported adverse effects (Astell et al., 2013). Clinical investigations of herbal medicine have been shown to be an effective treatment for obesity, and animal experiments have begun to reveal the potential mechanisms which include increased leptin sensitivity, decreased proinflammatory cytokine expression, reduced insulin resistance, regulation of lipid metabolism, and reduced body weight and food intake (Liu et al., 2017). The use of some natural anti-obesity products could also be considered as a supportive tool to keep obese people achieve weight-loss goals, with less serious side effects and which may give not only anti-obesity effect but also other health benefits, such as anti-diabetic and anti-hyperlipidemic properties (Sun et al., 2016).
One of the dietary constituents reported to have health-promoting properties beyond basic nutrition is pectin. Pectin is a natural soluble complex hetero-polysaccharide which comprises a functionally significant moiety of the primary cell walls of terrestrial plants, and can be found in all fruits and vegetables (McCann and Roberts, 1991). Industrially, it is commonly extracted from citrus peels or apple pomace, and is used as a gelling agent, thickener, water binder, emulsifier and stabilizer in foods (May, 1990). Recent uses of pectin have emerged as it was found to have anti-lipidemia, anti-hyperglycemia, and anti-obesity properties. Kwon et al. (2005), Marounek et al. (2007), Sanchez et al. (2008), Marounek et al. (2010a), Adam et al. (2015a), Zhu et al. (2015), Adam et al. (2016), and Sefcikova and Racek (2016) already showed that supplementation of apple and citrus pectin in the diet of rats exhibited lower body weight, blood cholesterol, blood triglycerides, relative liver weight, and adiposity index as compared with the negative controls despite continuous access to high fat diet. These observed effects could be associated with several mechanisms of action including decreased food intake, increased bile acid excretion and reduced bile acid reabsorption, and production of short chain fatty acids (SCFAs) which may impact cholesterol synthesis and fat metabolism (Anderson et al., 2009; Gunness and Gidley, 2010; and Surampudi et al., 2016).
The effect of pectin on blood cholesterol may also be attributed to the interference of micelle formation in the first stage of lipid metabolism in the small intestine. The rate of diffusion of bile acids and cholesterol-containing micelles are lowered. In turn, the uptake of cholesterol and bile acids are reduced and are excreted through feces (Majee et al., 2018). In this manner, pectin can be expected to have lipid-lowering capacity. However, the exact mechanism for this action is not yet determined (Tyagi et al., 2015).
Interestingly, pectin can be extracted generally from banana peels regardless of variety (Pathak et al., 2016). In particular, peels of two banana varieties from Egypt, namely William and Maghrabi, were reported to contain 12.77% and 13.03% pectin w/w of the sample (Ragab et al., 2016). In the case of unripe ‘saba’ banana peel (SBP), pectin yield was reported to be at 16.54% w/w of sample using an optimized method of extraction with 0.5N HCl for 4 h at 90 °C (Castillo-Israel et al., 2015). It was also found to contain 14.13% moisture content (MC), 13.83% ash content, 1503.16 equivalent weight (EW), 5.25% methoxyl content (MeC), 39.68% AUA, and 75.03% degree of esterification (DE).
In the Philippines, ‘saba’ banana [Musa acuminata x balbisiana (BBB Group)] is the most popular cultivar grown domestically which accounts to 39% of total production, widely processed into chips/crackers (Molina and Roa, 2000). Saba peels comprise 40% waste of the total weight of fresh banana (Nagarajaiah and Prakash, 2011) and it is estimated that there are 220 tons of by-products produced per hectare yearly (Shah et al., 2005). At present, these peels are not being used for any other purposes and are mostly dumped as solid waste at large expense (Ragab et al., 2016). This in turn could cause serious ecological damages since its decomposition in the soil is responsible for the increase in CO2 and methane emitted into the atmosphere (Topp and Pattey, 1997) while its high carbohydrate content can increase the oxygen biochemical demand in rivers (Zhang et al., 2005).
In the present study, mature unripe all green stage ‘saba’ peels were used as the source of pectin to efficiently utilize the waste from banana products manufacturing in the Philippines. SPP was already extracted and characterized and has been shown that it is a potential source of pectin for commercial use (Castillo-Israel et al., 2015). However, being a novel source of pectin, the effect of SPP on obesity biomarkers is currently unknown. This study therefore aims to evaluate the effects of SPP on biomarkers of obesity and associated blood lipid disorders in vivo and to determine its potential mechanisms of action via in vitro lipid lowering assays.
2. Materials and method
All animal experiments performed were approved by the University of the Philippines Los Baños Animal Care and Use Committee (UPLB ACUC) with approval number CHE-2019-001.
2.1. Preparation and extraction of Saba Banana peel pectin
The ‘saba’ banana peel samples were obtained from banana processing industries in Lipa City, Batangas. These were washed initially with tap water to minimize microbial load and remove adhering contaminants such as soil and leaves. These were dried at 55 °C for 18–24h in a cabinet dryer (Memmert, Germany) and powdered using a grinder (Oster, USA) at the Institute of Food Science and Technology – College of Agriculture and Food Science (IFST-CAFS), UPLB. SBP pectin was extracted using food grade citric acid (Chemline Scientific, Philippines) according to the optimized method described by Castillo-Israel et al. (2015). Briefly, the banana peel wastes were subjected to drying and then grinding to become powder. The homogenized banana peel powder was added to 0.50N citric acid, pH 1.7 heated with continuous stirring at 90 ± 5 °C on a stirring hot plate for 4h. The solution was cooled and filtered through an ordinary screen with 1-mm mesh size with two-layer cheesecloth. The filtrate collected was added with twice its volume of absolute ethanol. The precipitates were filtered through a miracloth and then oven dried for 2 days at 55 °C.
2.2. Animals and diet
A total of sixty (60) 6-week old male ICR mice weighing 22.0 ± 2.0g were obtained from the Laboratory Animal Facility of the Research Institute for Tropical Medicine at Alabang, Muntinlupa, Metro Manila, Philippines. Mice were individually housed in commercial polycarbonate cages with stainless steel top with sterile corn cob bedding under 12h: 12h light/dark cycle lights on at 7:00 a.m. and off at 7:00 p.m., 24 ± 2 °C room temperature and 50–60% humidity at the laboratory animal experimental room, Department of Basic Veterinary Sciences, College of Veterinary Medicine, UPLB. The mice were acclimatized for one week given a commercial mouse maintenance diet (Dyets, Pennsylvania, USA) and distilled water ad libitum.
The high fat diet (HFD) was formulated based on the composition of Dyets, Inc. (Pennsylvania, USA) with 45% calories from fat. Briefly, HFD was formulated by heating the agar solution in a microwave for 3 min. Pork lard was added, heated for another minute, then placed in an electric mixer and stirred until it reached 40 °C. All the dry ingredients were then added to the agar-lard solution and mixed until homogenous. For HFD-pectin mixtures, exactly the same procedures were applied but the powdered pectin was added together with all the dry ingredients. The mice diet produced was then transferred in a clean container, covered, labeled, and stored inside a freezer until use. Table 1 shows the composition and caloric content of the formulated diets.
Table 1.
Composition and caloric content of the different diets.
| Ingredients | kcal per g | ND (g) | HFD (g) | HFD-CCP (g) | HFD-SPP (g) |
|---|---|---|---|---|---|
| Casein | 3.58 | 100.0 | 116.54 | 115.77 | 115.55 |
| L-Cystine | 4.0 | 1.5 | 1.75 | 1.75 | 1.75 |
| Sucrose | 4.0 | 50 | 100.69 | 100.69 | 100.69 |
| Cornstarch | 3.6 | 198.7 | 42.42 | 0 | 0 |
| Dyetrose | 3.8 | 66.0 | 58.27 | 57.0 | 57.5 |
| Soybean Oil | 9.0 | 35.0 | 0 | 0 | 0 |
| t-Butylhydroquinone | 0 | 0.007 | 0.003 | 0.003 | 0.003 |
| Cellulose | 0 | 25.0 | 29.13 | 29.13 | 29.13 |
| Mineral Mix #210025 | 0.88 | 17.5 | 0 | 0 | 0 |
| Vitamin Mix #310025 | 3.87 | 5.0 | 0 | 0 | 0 |
| Choline Bitartrate | 0 | 1.25 | 1.17 | 1.17 | 1.17 |
| Salt Mix #210088 | 1.6 | 0 | 5.83 | 5.83 | 5.83 |
| Dicalcium Phosphate | 0 | 0 | 7.57 | 7.57 | 7.57 |
| Calcium Carbonate | 0 | 0 | 3.20 | 3.20 | 3.20 |
| Potasium Citrate H2O | 0 | 0 | 9.61 | 9.61 | 9.61 |
| Vitamin Mix #3000050 | 3.92 | 0 | 5.83 | 5.83 | 5.83 |
| Pork Lard | 9.0 | 0 | 118.00 | 117.72 | 117.35 |
| Commercial citrus pectin | 3.61 | 0 | 0 | 50.0 | 0 |
| Saba banana peel pectin | 3.59 | 0 | 0 | 0 | 50.0 |
| Total | 500.0 | 500.0 | 505.3 | 505.2 | |
| Total Calories | 1880.02 | 2295.23 | 2312.77 | 2309.54 | |
| kcal per gram | 3.76 | 4.59 | 4.58 | 4.57 |
ND– normal diet; HFD– high fat diet; HFD-CCP– HFD w/10% commercial citrus pectin; HFD-SPP– HFD w/10% SPP.
Commercial citrus pectin (CCP) [L# 11612929, Alysons’ Chemical Enterprises, Inc., Quezon City, Philippines] and ‘saba’ peel pectin (SPP) were added to the HFD at 10% w/w with some adjustments to achieve isonitrogenous and isocaloric diets containing approximately 4.60 kcal per gram and carbohydrate-protein-fat composition of 36–18-46%, respectively. This dose was based on the in vivo studies of Kwon et al. (2005), Sanchez et al. (2008), Adam et al. (2015a), and Adam et al. (2016) wherein supplementation of 10% pectin w/w of the diet exhibited significant decrease in body weight, blood cholesterol, blood triglycerides, relative liver weight, and adiposity index as compared with the HFD control.
2.3. In vivo efficacy test of anti-obesity and anti-hyperlipidemic activities of ‘saba’ peel pectin
2.3.1. Induction of obesity and hypercholesterolemia
Throughout induction period, all mice to be induced were taken as one group and were given HFD ad libitum for three (3) weeks to induce obesity and hypercholesterolemia. Mice with more than 20% body weight gain were considered as obese (Wang et al., 2010) while mice with at least 20% increase in blood total cholesterol (TC) were considered hypercholesterolemic (Franciosi et al., 2009). Mice that met the criteria for these conditions were included in the intervention phase.
2.3.2. Intervention phase
The mice were randomly allocated into four groups (n = 10 each group) namely: Group 1: normal diet (Dyets, Inc. (Dyet# 110700, AIN-93G) [ND Group]; Group 2: high fat diet (HFD Group); Group 3: HFD + 10% w/w commercial citrus pectin (HFD-CCP); and Group 4: HFD + 10% w/w SBP pectin (HFD-SPP). The ND group have normal mice serving as normal control whereas the other three groups have obese and hypercholesterolemic mice. Animals received their respective diets ad libitum for nine (9) weeks of supplementation.
2.3.3. Food and water intake and body weight measurement
The feed intake of mice was measured daily. Pre-weighed feeds were given to each mouse and daily refuse was measured using a digital top loading balance. The actual intake for the day was computed by subtracting the refused from the total feeds given. For the water intake, each mouse was given 10 mL of water per day. The actual water intake for the day was computed by subtracting the water refused from the total water given. Meanwhile, the weight of each mouse was measured weekly using a digital top loading balance (Shimadzu, Japan).
2.3.4. Blood lipid measurement
Blood samples were collected from the retro-orbital sinus using heparinized micro hematocrit tubes (NRIS, Denmark). Collected blood samples were placed in a 1 mL microcentrifuge tube (Eppendorf tube, USA), labeled, and then analyzed for TC and TAG using Arkray SpotChem-EZ SP-4430 blood chemistry analyzer (ARKRAY Inc., Japan). Blood TC and TAG were measured at baseline, then every three weeks until sacrifice of mice.
2.3.5. Euthanasia and necropsy of mice
Each mouse was anesthetized via intraperitoneal injection of pentobarbital sodium (Dolethal, Vetoquinol UK Ltd) at a dose of 80 mg/kg. After intracardiac blood collection, the mice organs such as the left and right kidneys, small and large intestines, stomach, and liver were examined for gross pathologic changes. In addition, fat pads including subcutaneous, epididymal, mesenteric, abdominal, and heart fat were also collected. The organs and fat pads were then weighed using an analytical balance (Shimadzu, Japan). The relative organ weights were determined by dividing the weight of the organ (g) by body weight (g) and multiplied by 100. Meanwhile, the total adiposity index was determined by calculating the sum of the subcutaneous, epididymal, mesenteric, abdominal, and heart fat weights (g) divided by body weight (g) and multiplied by 100.
2.3.6. Tissue processing and microscopic examination
Liver and abdominal fat samples were processed using paraffin technique, sectioned with a rotatory microtome, and stained with Hematoxylin and Eosin. All samples were examined under light microscopy (Zeiss Primostar) and evaluated in a blind fashion to avoid bias. Moreover, the mean diameter of abdominal white adipocytes was also calculated in μm to serve as an indicator of adipocyte hypertrophy. A minimum of three white adipocytes per each fat pad was measured in restricted view fields on a computer monitor using an automated image analysis system (Image J).
2.4. In vitro lipid lowering assays
The lipid lowering properties of the SPP compared with CPP were analyzed by measuring the cholesterol binding capacity and bile acid binding capacity.
2.4.1. Cholesterol binding capacity
The assay for cholesterol binding capacity was carried out using the methods of Boungoura et al. (2009) with modifications. Here, cholesterol micellar solution without sample was used as control to calculate recovery.
For sample preparation, pectin powders (0.05 g) were dissolved in 30 mL 15 mM phosphate buffer (UNICHEM, India and Duksan Pure Chemicals Co. Ltd., South Korea) with pH of 7.4 in a 50-mL beaker. The pectin solution was heated to 50 °C with continuous stirring (Witeg, Germany) for 30 min. After heating, the pectin solution was transferred to a 50-mL volumetric flask and added with 15 mM phosphate buffer to a volume of 50 mL. This pectin solution, which has a concentration of 1000 μg/mL was used as the stock solution. Concentrations of 20, 40, 60, 80, and 100 μg/mL were prepared from the stock solution.
The positive control used for this assay was cholestyramine (Sigma-Aldrich, St. Louis, MO, USA). Citrus pectin (0.05g) and cholestyramine (0.05g) were directly dissolved in a 50 mL 15 mM phosphate buffer as the stock solution. Concentrations, as mentioned above, were prepared from the stock solutions.
Reagents used for cholesterol micellar solutions were bought from Sigma-Aldrich (St. Louis, MO, USA) and were analytical reagent grade. Cholesterol micellar solution containing 10 mM sodium taurocholate, 0.4 mM cholesterol, 1 mM oleic acid, 132 mM NaCl, and 15 mM phosphate buffer (pH 7.4) was sonicated for 20 min.
For the in vitro cholesterol micellar solubility inhibition assay, approximately 450 μL of the cholesterol micellar solution was added with 450 μL of sample. The solutions were incubated at 37 °C for 24h in an oven incubator (Memmert, Germany). The reaction was performed in triplicates. Then, the solutions were centrifuged (IEC, USA) for 10min. The supernatant was collected and used for cholesterol concentration determination.
From the supernatant, 80 μL of each sample were pipetted into 96-well microplate in triplicates. Then, 100 μL of glacial acetic acid (Labscan, Thailand) was added followed by slowly adding of 120 μL Zak's reagent (TPC, India; Sigma Aldrich, USA; and Labscan, Thailand). The solutions were mixed well by pipetting the solution up and down. For the control, 80 μL of cholesterol micellar solution was added instead of the sample. The solution was further incubated for 15min at room temperature and absorbance was measured at 560 nm using a microplate reader (Thermo Scientific™ Multiskan™ GO, Waltham, MA, USA).
In preparing for the standard calibration curve, cholesterol standards of glacial acetic acid with the following concentrations were prepared: 20 μg/mL, 25 μg/mL, 30 μg/mL, 35 μg/mL, 40 μg/mL, and 45 μg/mL. Absorbance at 560 nm was recorded and a standard curve was constructed.
2.4.2. Bile acid binding assay
Bile acid binding was performed according to the method of Kongo-Dia-Moukala et al. (2011) with modifications.
For sample preparation, pectin powders (0.002 g) were dissolved in 60 mL 50 mM phosphate buffer with pH of 6.5 in a 50-mL beaker. The pectin solutions were heated to 50 °C with continuous stirring (Witeg, Germany) for 30min. After heating, the pectin solutions were transferred to a 100-mL volumetric flask and added with 50 mM phosphate buffer to a volume of 100 mL. The positive control used for this assay was cholestyramine. Positive control was prepared by directly dissolving 0.002 g of the powder in 100 mL 50 mM phosphate buffer in 100-mL volumetric flasks.
In bile acid solution preparation, the bile acids used for this assay were sodium taurocholate and sodium glycocholate (Sigma-Aldrich, St. Louis, MO, USA). Two micromolar (2 mM) of each bile acid were prepared in a 50 mM phosphate buffer of pH 6.5. The control that was used to calculate bound bile acid was 2 mM bile acid without sample.
For the in vitro bile acid binding assay, triplicates of solutions of 1 mL of 2 mM bile acid solution and 1 mL of each sample were prepared. The solution was incubated for 1h at 37 °C in an oven incubator (Memmert, Germany). After incubation, the solution was centrifuged (IEC, USA) for 20min. Then, the supernatant was collected, filtered with a 0.45-μm filter, and transferred into UPLC vials. Unbound taurocholate and glycocholate after the reaction were measured using Waters Acquity H Class Ultra Performance Liquid Chromatographic system with Photodiode Array detector (Waters, Prague, Czech Republic).
The following concentrations of bile acid solutions were prepared for the standard calibration curve: 0 mM, 0.4 mM, 0.8 mM, 1.2 mM, 1.6 mM, and 2.0 mM. Peak area at 210 nm was recorded using UPLC. Standard reading was performed before and after reading all samples. Bile acid binding assays using taurocholate and glycocholate were performed in separate experiments.
In Ultra-Performance Liquid Chromatography (UPLC), three injections of 3 μL of the sample supernatant or bile acid standard (sodium taurocholate or sodium glycocholate) were performed. The sample was eluted with methanol (Duksan Pure Chemicals Co. Ltd., South Korea): 0.4%KH2PO4 (70:30) at a flow rate of 0.15 mL/min for 3 min. Peak area at 210 nm were recorded and used to calculate concentration of unbound bile acids. Concentration of unbound bile acids was calculated from the standard calibration curve. Percent bound bile acids were calculated using the formula:
where Cc = concentration of bile acid in the control, and
Cs = concentration of bile acid in the samples
2.5. Statistical analysis
Results were expressed as mean ± standard deviation (SD). The data were analyzed using student's t-test for the differences in the in vitro lipid lowering capacities, paired sample t-test for the differences before and after treatment within the mice group, and one-way analysis of variance (ANOVA) for the differences between mice groups with post-hoc analysis Tukey test at p < 0.05 using Statistical Package for the Social Sciences (SPSS) version 20.
3. Results
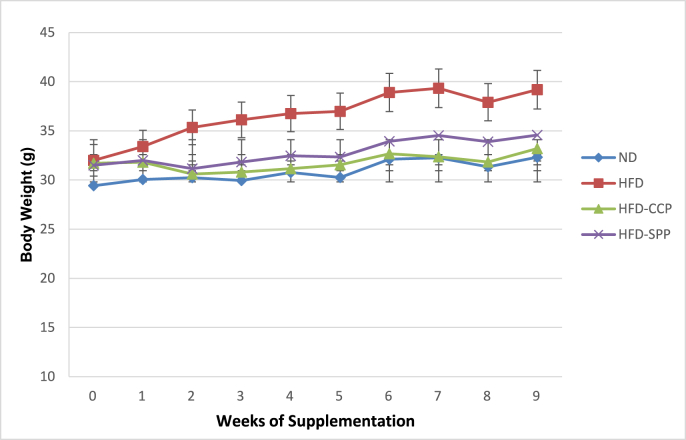
3.1. Body weight
Mean body weight of ND mice were significantly lower compared with the different HFD treatment mice (p < 0.05) at the start of the supplementation period (Fig. 1). Weight gain was noted in all treatment groups throughout the supplementation period although significantly highest gain was observed in the HFD mice. Weight gain of HFD-CCP and HFD-SPP mice was statistically comparable with the control ND mice (p < 0.05).
Fig. 1.
Mean (± SD) body weight of all the mice groups throughout the supplementation period. ND – normal diet; HFD – high fat diet; HFD-CCP – high fat diet with 10% commercial citrus pectin; HFD-SPP – high fat diet with 10% ‘saba’ peel pectin.
3.2. Food and water intake
Mice regardless of treatment had normal to high feed consumption during the supplementation period (Table 2). Nonetheless, mice from the ND group consumed significantly lower amounts of feeds and calories as compared with the HFD, HFD-CCP, and HFD-SPP groups (p < 0.05). There was no significant difference in the feed and caloric intakes among the HFD, HFD-CCP and HFD-SPP groups.
Table 2.
Mean feed and water intake of mice during supplementation period.
| GROUP | MEAN FEED INTAKE |
MEAN WATER INTAKE (mL) | |
|---|---|---|---|
| Amount (g) | Calories (kcal) | ||
| ND | 3.94 ± 0.18a | 14.81 ± 0.68a | 8.97 ± 0.69a |
| HFD | 5.38 ± 0.52b | 24.70 ± 2.38b | 6.79 ± 0.83b |
| HFD-CCP | 5.22 ± 0.49b | 23.91 ± 2.24b | 7.82 ± 0.66bc |
| HFD-SPP | 5.31 ± 0.31b | 24.29 ± 1.44b | 7.95 ± 0.70ac |
ND– normal diet; HFD– high fat diet; HFD-CCP– HFD w/10% commercial citrus pectin; HFD-SPP– HFD w/10% SPP.
Values represent the mean ± SD (n = 7) with post-hoc analysis Tukey test at p < 0.05.
Means in the same column followed by different letter(s) are significantly different at p < 0.05.
In terms of the water intake, the HFD group had a significantly lower mean water intake compared with the ND and HFD-SPP groups (p < 0.05) but was not significantly different with the HFD-CCP (Table 2). No significant difference was seen in water intakes of HFD-CCP and HFD-SPP throughout the supplementation period. Meanwhile, water intakes of the ND and HFD-SPP groups were statistically comparable.
3.3. Blood lipids
It was observed that the blood TC level of ND mice was significantly lower compared with the HFD and HFD-pectin groups (p < 0.05) which had comparably high blood TC levels prior to the 9-week supplementation (Table 3). From week 0 to week 9, there was no significant difference in the blood TC levels of all mice within each group. Although, the pectin-supplemented groups had consistently lower blood TC levels compared with the HFD group. The highest reduction in blood TC levels in the HFD-SPP group was observed during the 6th week of supplementation which was significantly lower than that of the HFD group (p < 0.05). Meanwhile, statistically comparable blood TC levels were observed between the HFD-CCP and HFD-SPP groups throughout supplementation (p > 0.05).
Table 3.
Mean blood total cholesterol levels of mice during supplementation.
| GROUP | MEAN BLOOD TOTAL CHOLESTEROL (mg/dL) |
||||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 3 | Week 6 | Week 9 | % Change (Wk 0 vs. 3) | % Change (Wk 0 vs. 6) | % Change (Wk 0 vs. 9) | |
| ND | 128.57 ± 15.37a | 132.71 ± 16.08a | 144.86 ± 14.22 a | 142.43 ± 18.26a | +3.22%ns | +12.67%s | +10.78%s |
| HFD | 174.90 ± 25.69b | 207.86 ± 23.79b | 223.29 ± 22.15b | 210.71 ± 27.93b | +18.85%ns | +27.67%ns | +20.47%ns |
| HFD-CCP | 174.90 ± 25.69b | 154.29 ± 20.77a | 160.00 ± 30.28a | 152.86 ± 28.04a | −11.78%s | −8.52%ns | −12.60%ns |
| HFD-SPP | 174.90 ± 25.69b | 164.57 ± 24.62a | 161.86 ± 45.26a | 177.00 ± 27.70ab | −5.91%ns | −7.46%ns | +1.20%ns |
ND– normal diet; HFD– high fat diet; HFD-CCP– HFD w/10% commercial citrus pectin; HFD-SPP– HFD w/10% SPP.
Values represent the mean ± SD (n = 7) with post-hoc analysis Tukey test at p < 0.05.
Means in the same column followed by different letter(s) are significantly different at p < 0.05.
- significant; ns - not significant.
On the other hand, the effects of CCP and SPP treatments on the blood TAG levels of mice are shown in Table 4. There was no significant difference in the blood TAG levels of all mice from week 0 to week 9, except for the blood TAGs of the HFD-CCP group at week 9 being statistically significant as compared with the HFD group and with the baseline (p < 0.05).
Table 4.
Mean blood triglyceride levels of mice during supplementation.
| GROUP | MEAN BLOOD TRIGLYCERIDES (mg/dL) |
||||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 3 | Week 6 | Week 9 | % Change (Wk 0 vs. 3) | % Change (Wk 0 vs. 6) | % Change (Wk 0 vs. 9) | |
| ND | 99.71 ± 23.34a | 118.00 ± 41.23a | 116.71 ± 28.48a | 82.71 ± 23.69a | +18.34%ns | +17.05%ns | −17.05%ns |
| HFD | 109.52 ± 43.15a | 95.14 ± 33.40a | 86.00 ± 14.92aab | 131.14 ± 57.86a | −13.13%ns | −21.48%ns | +19.74ns |
| HFD-CCP | 109.52 ± 43.15a | 113.71 ± 48.21a | 77.86 ± 20.64b | 68.57 ± 41.89b | −3.94%ns | −28.91%ns | −37.39%s |
| HFD-SPP | 109.52 ± 43.15a | 87.14 ± 20.99a | 79.71 ± 17.52b | 93.14 ± 59.78a | −21.01%ns | −27.22%ns | −14.96%ns |
ND– normal diet; HFD– high fat diet; HFD-CCP– HFD w/10% commercial citrus pectin; HFD-SPP– HFD w/10% SPP.
Values represent the mean ± SD (n = 7) with post-hoc analysis Tukey test at p < 0.05.
Means in the same column followed by different letter(s) are significantly different at p < 0.05.
– significant; ns – not significant.
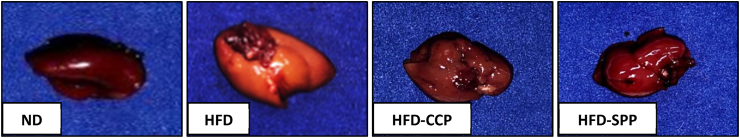
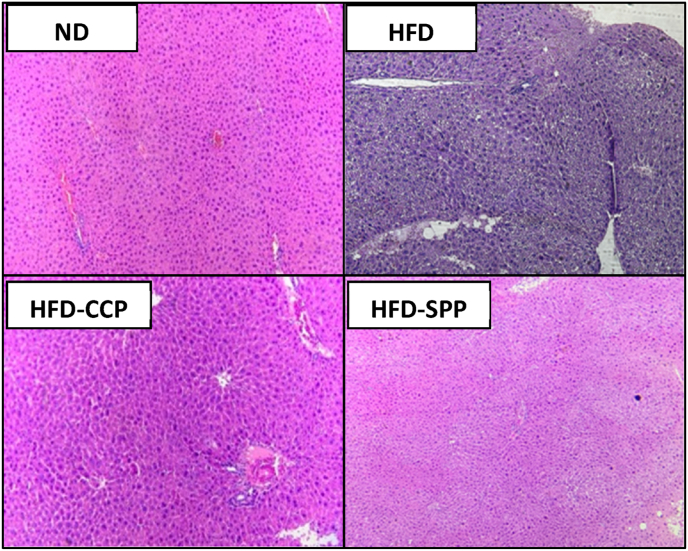
3.4. Macroscopic and microscopic observation of organs
There were no treatment-related gross lesions observed in selected organs of mice, including left and right kidneys, small and large intestines, and stomach, treated with different diets. However, it was observed that the HFD group mice developed pale, soft, and enlarged livers which were absent in other mice groups (Fig. 2). In terms of the other organs, there was no significant treatment-related difference in terms of the relative organ weights among all the four groups of mice, except for the liver (Table 5). The HFD group had relatively higher liver weight than the other mice groups, with the difference with the ND group being statistically significant. This result was consistent with the liver histopathology wherein the HFD group had a mild infiltration of fat deposit in the liver parenchyma and mild necrosis in the hepatocytes which were not found in the ND and found to a lesser extent in the pectin-supplemented groups (Fig. 3). It was also noted that the liver sections from HFD-SPP and HFD-CCP groups did not significantly differ from each other, morphologically.
Fig. 2.
Representative photograph of gross appearance of mice livers per group. ND – normal diet; HFD – high fat diet; HFD-CCP – high fat diet with 10% commercial citrus pectin; HFD-SPP – high fat diet with 10% ‘saba’ peel pectin. The liver from the HFD group was soft, pale, and enlarged. These gross abnormalities of the liver were not found in the livers of the mice in the ND and pectin-supplemented groups.
Table 5.
Organ-to-body weight ratios of selected organs in mice after supplementation.
| GROUP | BODY ORGAN RELATIVE WEIGHTS (%) |
|||||
|---|---|---|---|---|---|---|
| L. Kidney | R. Kidney | S. Intestine | L. Intestine | Stomach | Liver | |
| ND | 0.72 ± 0.10a | 0.75 ± 0.08a | 1.32 ± 0.33a | 0.53 ± 0.14a | 0.55 ± 0.07a | 4.08a ± 0.43a |
| HFD | 0.75 ± 0.11a | 0.83 ± 0.15a | 1.40 ± 0.39a | 0.56 ± 0.21a | 0.49 ± 0.10a | 4.76b ± 0.32b |
| HFD-CCP | 0.71 ± 0.10a | 0.74 ± 0.11a | 1.40 ± 0.44a | 0.69 ± 0.15a | 0.45 ± 0.14a | 4.28 ± 0.42b |
| HFD-SPP | 0.76 ± 0.08a | 0.73 ± 0.14a | 1.11 ± 0.24a | 0.50 ± 0.16a | 0.46 ± 0.09a | 4.22 ± 0.29ab |
ND– normal diet; HFD– high fat diet; HFD-CCP– HFD w/10% commercial citrus pectin; HFD-SPP– HFD w/10% SPP Values represent the mean ± SD (n = 7) with post-hoc analysis Tukey test at p < 0.05.
Means in the same column followed by different letter(s) are significantly different at p < 0.05.
Fig. 3.
Effect of CCP and SPP on histopathological features of the liver. Representative photograph of H&E staining of liver sections from each group of mice: ND – normal diet; HFD – high fat diet; HFD-CCP – high fat diet with 10% commercial citrus pectin; HFD-SPP – high fat diet with 10% ‘saba’ peel pectin. Liver histopathology showed that the HFD-CCP and HFD-SPP groups only had occasional fat deposits as compared with the HFD group which showed mild infiltration of fat deposits in the liver parenchyma and mild necrosis in the hepatocytes. The ND group showed normal appearance of the liver architecture with no fat deposits observed. Liver sections from HFD-SPP and HFD-CCP groups did not significantly differ from each other, morphologically.
3.5. Macroscopic and microscopic observation of adipose tissues
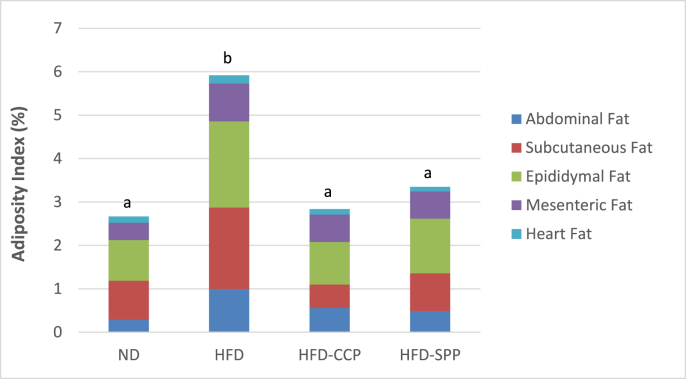
No treatment-related gross lesions were observed in the different adipose tissues namely: abdominal, subcutaneous, epididymal, mesenteric, and heart fat. The HFD group generally had higher weights of all the adipose tissues as compared with the ND and HFD-pectin groups (Fig. 4). Notably, mice in the HFD-CCP group had significantly lower weight of subcutaneous fat while both the HFD-CCP and HFD-SPP groups had significantly lower weights of epididymal fat as compared with the HFD group (p < 0.05). Meanwhile, no significant difference was seen on the weights of all adipose tissues of mice from the HFD-CCP and HFD-SPP groups. In terms of the adiposity index, the HFD group had significantly higher adiposity index as compared with the other groups (p < 0.05) with epididymal fat as the largest contributor (33.54%), followed by the subcutaneous fat (31.59%), by abdominal fat (16.95%), by mesenteric fat (14.73%) and by heart fat (3.18%) [Fig. 4]. On the other hand, the adiposity indexes of the HFD-CCP and HFD-SPP groups were statistically comparable with that of the ND group (p > 0.05).
Fig. 4.
Mean percent adiposity index of mice groups after supplementation period.Post-hoc analysis Tukey test was performed at p < 0.05. Bars with different letter(s) are significantly different at p < 0.05. ND – normal diet; HFD – high fat diet; HFD-CCP – high fat diet with 10% commercial citrus pectin; HFD-SPP – high fat diet with 10% ‘saba’ peel pectin.
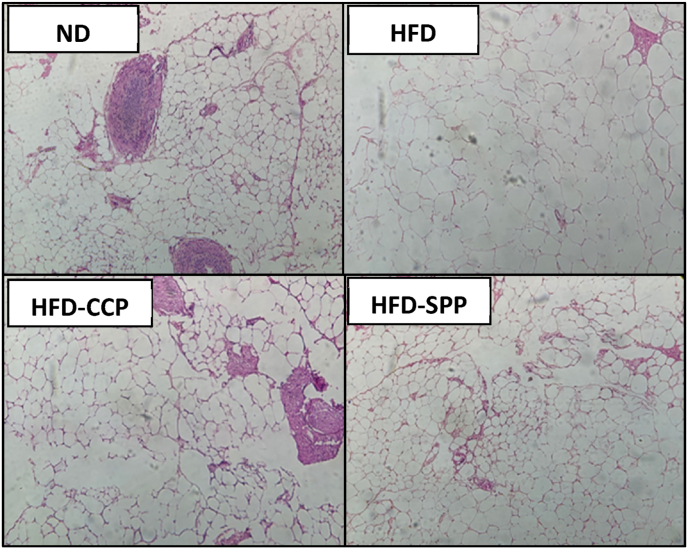
These macroscopic observations agreed with the findings in the histological sections of the abdominal fat (Fig. 5). The HFD group had the highest mean diameter of adipocytes at 31.78 μm which was significantly higher than that of the ND group (17.05 μm) and HFD-CCP group (21.75 μm). It was also observed that the HFD-CCP group had a normal architecture of adipocytes similar to the ND group. On the other hand, the HFD-SPP group exhibited an increase in the diameter of adipocytes but to a lesser extent than the HFD group (29.24 μm).
Fig. 5.
Effect of CCP and SPP on architecture and diameter of adipocyte. Representative photograph of H&E staining of abdominal fat sections from each group of mice: ND – normal diet; HFD – high fat diet; HFD-CCP – high fat diet with 10% commercial citrus pectin; HFD-SPP – high fat diet with 10% saba peel pectin. The HFD group had the highest mean diameter of adipocytes at 31.78 μm which was significantly higher than that of the ND group (17.05 μm) and HFD-CCP group (21.75 μm). It was also observed that the HFD-CCP group had a normal architecture of adipocytes similar to the ND group. On the other hand, the HFD-SPP group exhibited an increase in the diameter of adipocytes but to a lesser extent than the HFD group (29.24 μm).
3.6. In vitro lipid lowering assays
Cholesterol binding capacity of SPP (Table 6) was found to be significantly higher (54.78%) than CCP (49.07%) and cholestyramine (51.65%) at 60 μg/mL (p < 0.05). At 20 and 100 μg/mL, SPP and CCP did not vary significantly from cholestyramine. At 40 μg/mL concentration, SPP and CCP were not significantly different in their cholesterol binding capacities, and values were significantly higher than cholestyramine.
Table 6.
Cholesterol binding capacity of ‘saba’ peel pectin, citrus pectin, and cholestyramine at different concentrations.
| SAMPLE | CONCENTRATION (μg/mL) |
||||
|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | |
| SPP | 52.48a ± 1.10 | 53.27a ± 1.18 | 54.78a ± 0.93 | 55.07a ± 1.31 | 51.36a ± 1.43 |
| CCP | 51.17a ± 0.80 | 53.02a ± 1.00 | 49.07c ± 1.17 | 50.04b ± 3.61 | 49.99a ± 1.80 |
| CH | 48.33a ± 6.43 | 45.65b ± 3.59 | 51.65b ± 2.33 | 48.82b ± 2.38 | 52.43a ± 2.78 |
SPP – saba peel pectin; CCP – commercial citrus pectin; CH – cholestyramine.
Values represent the mean ± SD with post-hoc analysis Tukey test at p < 0.05.
Means in the same column followed by different letter(s) are significantly different at p < 0.05.
Bile acid binding capacities of both the CCP and SPP were analyzed using only one concentration, 20 μg/mL based on a preliminary test that 20 μg/mL, the lowest concentration used, exhibited bile acid binding capacity. Bile acid binding capacity of SPP (Table 7) with glycocholate was significantly higher (27.37%) compared with CCP (21.69%) and cholestyramine (22.95%) (p < 0.05). Binding with taurocholate was however significantly lesser for both SPP (46.10%) and CCP (47.21%) compared with cholestyramine (49.95%).
Table 7.
Bile acid binding capacity of ‘saba’ peel pectin, citrus pectin, and cholestyramine at 20 μg/mL.
| SAMPLE | BILE ACID |
|
|---|---|---|
| Taurocholate | Glycocholate | |
| SPP | 46.10b ± 1.29 | 27.37a ± 1.05 |
| CCP | 47.21b ± 1.07 | 21.69b ± 0.70 |
| CH | 49.95a ± 2.34 | 22.95b ± 1.80 |
SPP – saba peel pectin; CCP – commercial citrus pectin; CH – cholestyramine.
Values represent the mean ± SD with post-hoc analysis Tukey test at p < 0.05.
Means in the same column followed by different letter(s) are significantly different at p < 0.05.
4. Discussion
4.1. Feed intake, water intake, and Body weight
CCP and SPP supplementation at 10% w/w of diet for nine weeks produced anti-obesity effects that resulted in 18.08% and 13.40% lower body weight at the end line as compared with the HFD group, respectively. ANOVA and Tukey test showed that the mean weight gain in the HFD-CCP and HFD-SPP group was 4.3-fold and 2.5-fold lower as compared with the HFD control group, respectively (p < 0.05). Certainly, this reduction in the rate of body weight gain in HFD-CCP and HFD-SPP mice was not caused by reduced food intake since the intake rate was consistent across these three groups. However, pectin can still lower the energy intake by physically impeding nutrients entrapped in the fibrous plant cell walls (Hervik and Svihus, 2019) which can contribute in lower body weight. This result agreed with the in vivo evaluation studies done in apple and citrus pectin resulting to 10.0–15.50% lower body weight as compared with the HFD controls (Kwon et al., 2005; Sanchez et al., 2008; Marounek et al., 2010a; Adam et al., 2015a; Adam et al., 2015b, 2016).
In terms of water intake, HFD-CCP and HFD-SPP groups had 13.17% and 14.59% higher intakes as compared with the HFD group suggesting that pectin supplementation could have an influence on the water consumption especially that same observation was already documented in broiler chicks, wherein the capacity of a pectin to bind water can, at least in part, explain the dose-dependent increase in water intake (Langhout and Schutte, 1996). Higher water intake can increase satiety, but this seems to be not the case in this study given the statistically comparable mean feed intake of HFD and pectin-supplemented mice. Though, the higher water intake could still have contributed to the suppression of body weight since increase in hydration status could promote body weight loss (Thornton, 2016).
4.2. Blood total cholesterol and triglycerides
At the end of supplementation, the HFD-CCP and HFD-SPP groups had 37.85% and 19.05% lower blood TC levels as compared with the HFD group, respectively. The highest reduction in blood TC levels in the HFD-SPP group was observed at 6th week, which was significantly lower than the HFD group (p < 0.05). This reduction in the blood TC levels could be associated with the cholesterol and bile acid-binding properties of SPP as revealed by the vitro experiments. These properties of pectin help in bile acid excretion and reduction in bile acid reabsorption (Anderson et al., 2009; Gunness and Gidley, 2010), leading to lower serum TC and LDL-C concentrations (Wahlström et al., 2016). The observed effect of SPP on blood TC was comparable with the values obtained from hawthorn (Zhu et al., 2015), apple (Sanchez et al., 2008 and Adam et al., 2015a), and citrus pectin (Marounek et al., 2010a and Marounek et al., 2010b). Meanwhile, the observed decreased effect of SPP at 9th week could possibly be due to the aging-induced changes in cholesterol metabolism of mice such as increased adipose over muscle mass, increased insulin resistance, and decreased breakdown of cholesterol to bile acids (Uranga and Keller, 2010). On the other hand, in terms of blood TAG, Tukey test showed no significant difference found between pectin-supplemented groups and HFD group, except for HFD-CCP at 9th week.
These findings suggest more pronounced suppression of CCP than SPP on the increase in both blood TC and TAG brought about by consumption of HFD. This could be associated with fact that the weight- and cholesterol-lowering properties of the viscous fiber pectin depend on its physico-chemical properties (Surampudi et al., 2016), particularly the higher methoxyl content and anhydrouronic acid content, and significantly lower ash content of CCP than SPP (Castillo-Israel et al., 2015) which can readily form gels that are dispersible in water with better quality and gelation properties (May, 1990 and Shaha et al., 2013).
4.3. Adipose tissues and organs
In terms of adipose tissues, notable results were seen in the HFD-CCP and HFD-SPP groups having a 3.2-fold and 2.1-fold lower relative weight of subcutaneous fat and a 2.0-fold and 1.6-fold lower weight of epididymal fat as compared with the HFD group, respectively. Pectin-supplemented groups also had a lower increase in the diameter of adipocytes than the HFD group despite continuous feeding on HFD. Likewise, ANOVA and Tukey test revealed that the adiposity indexes of the HFD-CCP and HFD-SPP groups were 52.03% and 43.41% lower as compared with the HFD group (p < 0.05). These were in agreement with related in vivo studies having a 41.0–52.0% decrease in adiposity index after supplementation of pectin (Kwon et al., 2005; Adam et al., 2015a; and Sefcikova and Racek, 2016).
The livers of HFD mice were found to be soft, pale, and enlarged soft, with fat deposits and necrosis in the hepatocytes. These conditions were not seen among pectin-supplemented groups indicating the protective effect of pectin on liver. Aside from the cholesterol and bile acid-binding capacities of CCP and SPP, this protective effect could be related to production of short-chain fatty acids (SCFAs) upon breakdown of pectin which promotes increased energy expenditure and fuel partitioning in favor of fat oxidation (Gobin et al., 2003; Karra et al., 2009). Meanwhile, no significant effect was observed in terms of gross anatomy and relative weights of the other organs. The lower body fat and suppression of fat deposits in the liver among pectin-supplemented groups could also be associated with the observed higher water intake of mice, possibly promoting increased lipolysis (Moro and Lafontan, 2013) and water-induced thermogenesis (Vij and Joshi, 2013).
4.4. In vitro lipid lowering assays
The mechanism of pectin's property to lower lipid digestion has not yet been fully established though several studies have already proven the cholesterol and bile acid-binding properties of pectin (Tyagi et al., 2015; Majee et al., 2018). However, these properties of pectin may differ depending on the source since chemical characteristics such as degree of esterification, molecular weight, and galacturonic acid content also vary with the source (Edashige et al., 2008).
Cholesterol binding of pectin is affected mainly by pectin source and type, which includes degree of esterification and molecular weight. Moreover, viscosity of pectin also indirectly influences cholesterol binding (Brouns et al., 2012). Concentration of pectin influences its viscosity in solution (Kar and Arslan, 1999), which suggests that increasing concentration should correspond to increased viscosity. In the assay used, pectin was allowed to bind to cholesterol micellar solution (CMS). The capacity of SPP to bind to cholesterol solubilized in CMS is an important parameter in measuring the lipid-lowering capability of SPP. CMS created for the assay mimics the micelle, which is composed of bile acids, fatty acids and monoglycerides that are supposedly liberated from TAG hydrolysis by lipase, and dietary cholesterol (Lehninger et al., 2008). This study showed significant binding of cholesterol, which in turn prevents it from being soluble in the micelle. These micelles are vital to lipid metabolism because these serve as carriers of both dietary cholesterol and fatty acids for absorption in the intestines. Once cholesterol is not solubilized in the micelle, it forms a separate oil phase in the intestinal lumen and becomes unavailable for absorption (Jesch and Carr, 2017). In the digestive system, one possible mechanism of cholesterol lowering property of pectin is the increased lumen viscosity which leads to reduced rate of glucose diffusion and absorption in the small intestine. This effect consequently causes a decrease in insulin production. Reduced insulin then decreases the activity of 3-hydroxy-3-methylglutaryl CoA enzyme, which is responsible for the synthesis of cholesterol (Celus et al., 2018). However, in vitro results from this study suggest that the pectin itself binds to cholesterol by lowering the actual concentration of cholesterol in the micelle solution.
Furthermore, bile acids are important aspects of lipid digestion. They are a group of amphiphilic molecules synthesized from cholesterol, which are naturally produced by the body for their role in digestion, transport, and absorption of dietary lipids in the gastrointestinal tract (Lopez-Pena et al., 2019). In lipid digestion, bile acids adsorb to the surfaces of lipids in GIT breaking it down into smaller sizes with greater areas to act upon by lipases (Euston, 2017). Bile acids are also an integral part of the micelle that solubilize and transport triglyceride hydrolysis products into the epithelium cells (Lopez-Pena et al., 2019). Pectin molecules are negatively charged in neutral pH due to its linear anionic regions of galacturonic acids. Similarly, bile acids are also negatively charged. Nevertheless, both molecules have nonpolar groups which may interact with each other via hydrophobic forces. For instance, the nonpolar region of bile acids may be bound to the nonpolar methyl groups of pectin (Lopez-Pena et al., 2019). Aside from hydrophobic interactions, increase in bile acid excretion in vivo was associated with gel formation and viscosity effects in GIT (Dongowski and Lorenz, 2004). Since pectin was incubated with either sodium taurocholate or sodium glycocholate in vitro, results of this study suggest that SPP was able to bind to the bile acid itself without the influence of GIT environmental conditions.
5. Conclusion
Pectin supplementation as 10% ‘saba’ peel pectin (SPP) and 10% commercial citrus pectin (CCP) fraction of total diet is beneficial in the prevention of weight gain, lowering blood cholesterol, and suppression of fat accumulation in the body and liver of HFD-induced obese and hypercholesterolemic mice, with CCP exerted relatively higher suppression capabilities than SPP. The in vitro assays revealed that the mechanism of lipid lowering by SPP is through cholesterol and bile acid-binding pathways. Thus, the present study suggests that SPP may be a potential anti-obesity and hypocholesterolemic compound that can be used as a functional ingredient in special food applications and value-added products.
CRediT authorship contribution statement
Paul Alteo A. Bagabaldo: Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization. Liezl M. Atienza: Conceptualization, Methodology, Validation, Investigation, Resources, Data curation, Writing – review & editing, Supervision, Project administration, Funding acquisition. Katherine Ann T. Castillo-Israel: Conceptualization, Methodology, Validation, Investigation, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition. Maria Amelita C. Estacio: Conceptualization, Methodology, Validation, Investigation, Resources, Writing – review & editing, Supervision, Project administration. Prince Joseph V. Gaban: Investigation. Jonna Rose C. Maniwang: Investigation, Supervision. Roxanne P. Gapasin: Investigation, Supervision. Abbie Glenn M. Estribillo: Investigation, Formal analysis, Data curation. Rohani B. Cena-Navarro: Data curation, Visualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by the National Research Council of the Philippines – Department of Science and Technology (NRCP-DOST). We also thank all the members of the project team for helping throughout the data collection.
References
- Adam C.L., Thomson L.M., Williams P.A., Ross A.W. Soluble fermentable dietary fibre (pectin) decreases caloric intake, adiposity and lipidaemia in high-fat diet-induced obese rats. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam C.L., Williams P.A., Garden K.E., Thomson L.M., Ross A.W. Dose-dependent effects of a soluble dietary fibre (pectin) on food intake, adiposity, gut hypertrophy and gut satiety hormone secretion in rats. PLoS One. 2015;10(1) doi: 10.1371/journal.pone.0115438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam C.L., Gratz S.W., Peinado D.I., Thomson L.M., Garden K.E., Williams P.A., Richardson A.J., Ross A.W. Effects of dietary fibre (pectin) and/or increased protein (casein or pea) on satiety, body weight, adiposity and caecal fermentation in high fat diet-induced obese rats. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albaugh V.L., Abumrad N.N. Surgical treatment of obesity. 2018;7:F1000Res. doi: 10.12688/f1000research.13515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.W., Baird P., Davis R.H., Ferreri S., Knudtson M., Koraym A., Waters V., Williams C.L. Health benefits of dietary fiber. Nutr. Rev. 2009;67(4):188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- Astell K.J., Mathai M.L., Su X.Q. A review on botanical species and chemical compounds with appetite suppressing properties for body weight control. Plant Foods Hum. Nutr. 2013;68(3):213–221. doi: 10.1007/s11130-013-0361-1. [DOI] [PubMed] [Google Scholar]
- Boungoura M., Wenshui X., Jiali Z. In-vitro binding capacity of cholesterol and bile salts by partially depolymerized chitosans. Academic J. Food Technol. 2009;4(3):126–135. [Google Scholar]
- Brouns F., Theuwissen E., Adam A., Bell M., Berger A., Mensink R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012;66(5):591–599. doi: 10.1038/ejcn.2011.208. [DOI] [PubMed] [Google Scholar]
- Castillo-Israel K.A.T., Baguio S.F., Diasanta M.D.B., Lizardo R.C.M., Dizon E.I., Mejico M.I.F. Extraction and characterization of pectin from Saba banana [Musa'saba'(Musa acuminata x Musa balbisiana)] peel wastes: a preliminary study. Int. Food Res. J. 2015;22(1) [Google Scholar]
- Celus M., Kyomugasho C., Van Loey A.M., Grauwet T., Hendrickx M.E. Influence of pectin structural properties on interactions with divalent cations and its associated functionalities. Compr. Rev. Food Sci. Food Saf. 2018;17(6):1576–1594. doi: 10.1111/1541-4337.12394. [DOI] [PubMed] [Google Scholar]
- Dongowski G., Lorenz A. Intestinal steroids in rats are influenced by the structural parameters of pectin. J. Nutr. Biochem. 2004;15:196–205. doi: 10.1016/S0955-2863(03)00080-9. [DOI] [PubMed] [Google Scholar]
- Edashige Y., Murakami N., Tsujita T. Inhibitory effect of pectin from the segment membrane of citrus fruits on lipase activity. J. Nutr. Sci. Vitaminol. 2008;54(5):409–415. doi: 10.3177/jnsv.54.409. [DOI] [PubMed] [Google Scholar]
- Euston S.R. Molecular simulation of biosurfactants with relevance to food systems. Curr. Opin. Colloid Interface Sci. 2017;28:110–119. [Google Scholar]
- Faith M.S., Fontaine K.R., Cheskin L.J., Allison D.B. Behavioral approaches to the problems of obesity. Behav. Modif. 2000;24(4):459–493. doi: 10.1177/0145445500244001. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Research Institute (FNRI) Expanded national nutrition survey. 2018-2019. http://enutrition.fnri.dost.gov.ph/site/presentation.php?year=2019 from FNRI Website.
- Franciosi S., Sosa M.A.G., English D.F., Oler E., Oung T., Janssen W.G., De Gasperi R., Schmeidler J., Dickstein D.L., Schmitz C., Gandy S. Novel cerebrovascular pathology in mice fed a high cholesterol diet. Mol. Neurodegener. 2009;4(1):42. doi: 10.1186/1750-1326-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin S., Thuillier L., Jogl G., Faye A., Tong L., Chi M., Bonnefont J.P., Girard J., Prip-Buus C. Functional and structural basis of carnitine palmitoyltransferase 1A deficiency. J. Biol. Chem. 2003;278(50):50428–50434. doi: 10.1074/jbc.M310130200. [DOI] [PubMed] [Google Scholar]
- Gunness P., Gidley M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010;1(2):149–155. doi: 10.1039/c0fo00080a. [DOI] [PubMed] [Google Scholar]
- Hervik A.K., Svihus B. The role of fiber in energy balance. J. Nutr. Metab. 2019 doi: 10.1155/2019/4983657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J.J., Isaac R. Behavioral therapy for management of obesity. Indian J. Endocrinol. Metab. 2012;16(1):28. doi: 10.4103/2230-8210.91180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesch E.D., Carr T.P. Food ingredients that inhibit cholesterol absorption. Prev. Nutr. Food Sci. 2017;22(2):67–80. doi: 10.3746/pnf.2017.22.2.67. doi.org/10.3746/pnf.2017.22.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar F., Arslan N. Effect of temperature and concentration on viscosity of orange peel pectin solutions and intrinsic viscosity–molecular weight relationship. Carbohydr. Polym. 1999;40(4):277–284. [Google Scholar]
- Karra E., Chandarana K., Batterham R.L. The role of peptide YY in appetite regulation and obesity. J. Physiol. 2009;587(1):19–25. doi: 10.1113/jphysiol.2008.164269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongo-Dia-Moukala J.U., Zhang H., Irakoze P.C. In-vitro binding capacity of bile acids by defatted corn protein hydrolysate. Int. J. Mol. Sci. 2011;12:1066–1080. doi: 10.3390/ijms12021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J.Y., Ann I.S., Park K.Y., Cheigh H.S., Song Y.O. The beneficial effects of pectin on obesity in vitro and in vivo. J. Korean Soc. Food Sci. Nutr. 2005;34(1):13–20. [Google Scholar]
- Langhout D.J., Schutte J.B. Nutritional implications of pectins in chicks in relation to esterification and origin of pectins. Poultry Sci. 1996;75(10):1236–1242. doi: 10.3382/ps.0751236. [DOI] [PubMed] [Google Scholar]
- Lehninger A.L., Nelson D.L., Cox M.M. fifth ed. W.H. Freeman; New York: 2008. Lehninger Principles of Biochemistry. [Google Scholar]
- Liu Y., Sun M., Yao H., Liu Y., Gao R. Herbal medicine for the treatment of obesity: an overview of scientific evidence from 2007 to 2017. Evid. Based Comple. Alternat. Med. 2017 doi: 10.1155/2017/8943059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney S.M., Raynor H.A. Behavioral lifestyle intervention in the treatment of obesity. Health Serv. Insights. 2013:6 15–31. doi: 10.4137/HSI.S10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Pena C., Arroyo-Maya I.J., Mcclements D.J. Interaction of a bile salt (sodium taurocholate) with cationic (ε-polylysine) and anionic (pectin) biopolymers under simulated gastrointestinal conditions. Food Hydrocolloids. 2019;87:352–359. [Google Scholar]
- Majee S.B., Avlani D., Ghosh P., Biswas G.R. Therapeutic and pharmaceutical benefits of native and modified plant pectin. J. Med. Plants Res. 2018;12(1):1–6. [Google Scholar]
- Marounek M., Volek Z., Skřivanová E., Tůma J. Effects of amidated pectin alone and combined with cholestyramine on cholesterol homeostasis in rats fed a cholesterol-containing diet. Carbohydr. Polym. 2010;80(3):989–992. [Google Scholar]
- Marounek M., Volek Z., Skřivanová E., Tůma J., Dušková D. Comparative effect of amidated pectin and psyllium on cholesterol homeostasis in rats. Cent. Eur. J. Biol. 2010;5(3):299–303. [Google Scholar]
- Marounek M., Volek Z., Synytsya A., Čopíková J. Effect of pectin and amidated pectin on cholesterol homeostasis and cecal metabolism in rats fed a high-cholesterol diet. Physiol. Res. 2007;56(4):433–442. doi: 10.33549/physiolres.930967. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. Obesity and metabolic syndrome: the contribution of visceral fat and adiponectin. Diabetes Manag. 2014;4(4):391–401. [Google Scholar]
- May C.D. Industrial pectins: sources, production and applications. Carbohydr. Polym. 1990;12(1):79–99. [Google Scholar]
- McCann M.C., Roberts K. Academic Press; London, UK: 1991. The Cytoskeletal Basis of Plant Growth and Form; pp. 109–129. [Google Scholar]
- Middleton K.R., Anton S.D., Perri M.G. Long-term adherence to health behavior change. Am. J. Lifestyle Med. 2013;7(6):395–404. doi: 10.1177/1559827613488867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina A.B., Roa V.N., editors. Advancing Banana and Plantain R and D in Asia and the Pacific. National Research, Development and Extension Agenda for Banana; 2000. p. 93. [Google Scholar]
- Moro C., Lafontan M. Natriuretic peptides and cGMP signaling control of energy homeostasis. Am. J. Physiol. Heart Circ. Physiol. 2013;304(3):H358–H368. doi: 10.1152/ajpheart.00704.2012. [DOI] [PubMed] [Google Scholar]
- Nagarajaiah S.B., Prakash J. Chemical composition and antioxidant potential of peels from three varieties of banana. Asian J. Food Agro-Ind. 2011;4(1):31–46. [Google Scholar]
- North American Association for the Study of Obesity (NAASO) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults-the evidence report. Obes. Res. 1998;6(2):51S–209S. [PubMed] [Google Scholar]
- Nuffer W. Nutrition in the Prevention and Treatment of Abdominal Obesity. Academic Press; 2019. Pharmacologic agents chapter for abdominal obesity; pp. 51–66. [Google Scholar]
- Olson K. Behavioral approaches to the treatment of obesity. R. I. Med. J. 2017;100(3):21. [PubMed] [Google Scholar]
- Pathak P.D., Mandavgane S.A., Kulkarni B.D. Valorization of banana peel: a biorefinery approach. Rev. Chem. Eng. 2016;32(6):651–666. [Google Scholar]
- Ragab M., Osman M.F., Khalil M.E., Gouda M.S. Banana (Musa sp.) peels as a source of pectin and some food nutrients. J. Agric. Res. Kafr El-Sheikh Univ. 2016;42(4):88–102. [Google Scholar]
- Sanchez D., Muguerza B., Moulay L., Hernández R., Miguel M., Aleixandre A. Highly methoxylated pectin improves insulin resistance and other cardiometabolic risk factors in Zucker fatty rats. J. Agric. Food Chem. 2008;56(10):3574–3581. doi: 10.1021/jf703598j. [DOI] [PubMed] [Google Scholar]
- Sefcikova Z., Racek L. Effect of pectin feeding on obesity development and duodenal alkaline phosphatase activity in Sprague-Dawley rats fed with high-fat/high-energy diet. Acta Physiol. Hung. 2016;103(2):183–190. doi: 10.1556/036.103.2016.2.5. [DOI] [PubMed] [Google Scholar]
- Shah M.P., Reddy G.V., Banerjee R., Babu P.R., Kothari I.L. Microbial degradation of banana waste under solid state bioprocessing using two lignocellulolytic fungi (Phylosticta spp. MPS-001 and Aspergillus spp. MPS-002) Process Biochem. 2005;40(1):445–451. [Google Scholar]
- Shaha R.K., Punichelvana Y.N.A., Afandi A. Optimized extraction condition and characterization of pectin from kaffir lime (Citrus hystrix) Res. J. Agric. For. Sci. 2013;1(2):1–11. [Google Scholar]
- Smith K.L., Straker L.M., McManus A., Fenner A.A. Barriers and enablers for participation in healthy lifestyle programs by adolescents who are overweight: a qualitative study of the opinions of adolescents, their parents and community stakeholders. BMC Pediatr. 2014;14(1):53. doi: 10.1186/1471-2431-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava G., Apovian C.M. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 2017;14(1):12–24. doi: 10.1038/nrendo.2017.122. [DOI] [PubMed] [Google Scholar]
- Sun N.N., Wu T.Y., Chau C.F. Natural dietary and herbal products in anti-obesity treatment. Molecules. 2016;21(10):1351. doi: 10.3390/molecules21101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surampudi P., Enkhmaa B., Anuurad E., Berglund L. Lipid lowering with soluble dietary fiber. Curr. Atherosclerosis Rep. 2016;18(12):75. doi: 10.1007/s11883-016-0624-z. [DOI] [PubMed] [Google Scholar]
- Thornton S.N. Increased hydration can be associated with weight loss. Front. Nutr. 2016;3(18):1–8. doi: 10.3389/fnut.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp E., Pattey E. Soils as sources and sinks for atmospheric methane. Can. J. Soil Sci. 1997;77(2):167–177. [Google Scholar]
- Tyagi V., Sharma P., Malviya R. Pectin and their role in food and pharmaceutical industry: a review. J. Chronother. Drug Deliv. 2015;6:65–77. [Google Scholar]
- Uranga R.M., Keller J.N. Diet and age interactions with regards to cholesterol regulation and brain pathogenesis. Curr. Gerontol. Geriatr. Res. 2010 doi: 10.1155/2010/219683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij V.A., Joshi A.S. Effect of ‘water induced thermogenesis’ on body weight, body mass index and body composition of overweight subjects. J. Clin. Diagn. Res. 2013;7(9):1894. doi: 10.7860/JCDR/2013/5862.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlström A., Sayin S.I., Marschall H.U., Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metabol. 2016;24(1):41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Wang Y.M., Wang W.P., Wang L.P., Lü Q.H., Zhou X.H. Calorie control increased vaspin levels of serum and periepididymal adipose tissue in diet-induced obese rats in association with serum free fatty acid and tumor necrosis factor alpha. Chin. Med. J. 2010;123(7):936–941. [PubMed] [Google Scholar]
- Wolfe B.M., Kvach E., Eckel R.H. Treatment of obesity: weight loss and bariatric surgery. Circ. Res. 2016;118(11):1844–1855. doi: 10.1161/CIRCRESAHA.116.307591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Obesity and overweight – fact sheet. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (Updated 09 June 2021). Accessed on September 30, 2021 from WHO Website.
- Zhang P., Whistler R.L., Be Miller J.N., Hamaker B.R. Banana starch: production, physicochemical properties, and digestibility—a review. Carbohydr. Polym. 2005;59(4):443–458. [Google Scholar]
- Zhu R.G., Sun Y.D., Li T.P., Chen G., Peng X., Duan W.B., Zheng Z.Z., Shi S.L., Xu J.G., Liu Y.H., Jin X.Y. Comparative effects of hawthorn (Crataegus pinnatifida Bunge) pectin and pectin hydrolyzates on the cholesterol homeostasis of hamsters fed high-cholesterol diets. Chem. Biol. Interact. 2015;238:42–47. doi: 10.1016/j.cbi.2015.06.006. [DOI] [PubMed] [Google Scholar]