To the Editor:
FOR EDITORIAL COMMENT, SEE PAGE 1431
Survivors of COVID-19 face challenges that persist after hospitalization,1 and a growing number of health care systems are developing multidisciplinary clinics to care for patients with postacute sequelae of COVID-19 (PASC).2 Yet, little is known about how care is delivered to patients with COVID-19 after hospital discharge. We sought to characterize postdischarge care delivery for PASC across a large network of US academic and community hospitals.
Methods
We surveyed hospitals participating in the National Heart, Lung, and Blood Institute Clinical Trials Network for the Prevention and Early Treatment of Acute Lung Injury (PETAL Network). The survey focused on the structure of outpatient follow-up for patients discharged after hospitalization for COVID-19 (e-Appendix 1). The survey included 13 questions, mostly closed-ended, with the potential for additional follow-up questions. Electronic survey invitations were sent in July 2021 and were completed over 8 weeks. Data from the 2019 American Hospital Association annual survey database were used to characterize hospitals. The Vanderbilt University Medical Center Institutional Review Board determined this study was exempt from full review.
Results
Of 51 eligible PETAL Network sites, 47 hospitals responded to this survey (92% response rate). Surveys were completed by physicians (n = 29), hospital administration (n = 11), social workers or discharge coordinators (n = 7), research staff (n = 7), or other clinicians (n = 5). PETAL hospitals were urban (100%), mostly public or not-for-profit (87%), teaching hospitals (81%) that were distributed nationally: Midwest (21%), Northeast (26%), South (23%), West (30%). Ten hospitals (21%) had ≥ 20% of their patients insured by Medicaid.
Of the 47 responding hospitals, 37 (79%) provided discharge information to hospitalized patients with COVID-19 that was specific to COVID—70% counseled patients on reasons to return to the hospital, 66% on isolation precautions, and 64% on reasons to call primary care. Only 26% of hospitals provided discharge information that included potential symptoms or impairments of postacute sequelae of COVID-19. Postdischarge contact occurred in some capacity at 30 hospitals (63%). The most common methods of contact were through clinic visits (either in-person or virtual) (43%) or telephone (38%).
Thirty-three hospitals (70%) had a postdischarge outpatient clinic designed specifically for patients with COVID-19 (ie, PASC clinic), with 20 started before August 2020. Compared with hospitals with PASC clinics, hospitals without PASC clinics were more likely to be smaller, for-profit hospitals (Table 1). Hospitals without PASC clinics were also more likely to be in a ZIP code with a median annual income less than $40,000 and have a higher proportion of their patients insured by Medicaid than hospitals with PASC clinics.
Table 1.
Characteristics of Hospitals With and Without PASC Clinicsa
| Characteristic | PASC Clinic (n = 33) | No PASC Clinic (n = 14) |
|---|---|---|
| Maintains clinical registry of patients with COVID | 26 (78.8%) | 13 (92.9%) |
| Has a post-ICU clinic | 13 (39.4%) | 4 (28.6%) |
| Hospital ownership type | ||
| Public | 9 (30.0%) | 5 (35.7%) |
| Not for profit | 21 (70.0%) | 6 (42.9%) |
| For profit | 0 (0.0%) | 3 (21.4%) |
| Hospital size by No. of beds | ||
| < 300 | 0 (0.0%) | 2 (14.3%) |
| 300-399 | 1 (3.3%) | 2 (14.3%) |
| 400+ | 29 (96.7%) | 10 (71.4%) |
| ICU size as proportion of total hospital beds | ||
| < 10% | 4 (13.3%) | 1 (7.1%) |
| 10%+ | 26 (86.7%) | 13 (92.9%) |
| Teaching hospitalb | 27 (90.0%) | 11 (78.6%) |
| Proportion of Medicaid patients served | ||
| < 10% | 13 (43.3%) | 3 (21.4%) |
| 10%-20% | 13 (43.3%) | 5 (35.7%) |
| 20%+ | 4 (13.3%) | 6 (42.9%) |
| Median income by ZIP code | ||
| < $40,000 | 13 (43.3%) | 8 (57.1%) |
| $40,000-$100,000 | 8 (26.7%) | 2 (14.3%) |
| $100,000+ | 9 (30.0%) | 4 (28.6%) |
| Nonrural | 30 (100.0%) | 14 (100.0%) |
| Geographic regions | ||
| Midwest | 4 (13.3%) | 5 (35.7%) |
| Northeast | 8 (26.7%) | 1 (7.1%) |
| South | 6 (20.0%) | 5 (35.7%) |
| West | 12 (40.0%) | 3 (21.4%) |
| No. of PASC clinics within hospital | ||
| One | 19 (61.3%) | … |
| More than onec | 12 (36.4%) | … |
| PASC clinic distinct from post-ICU clinic | 24 (72.7%) | … |
| Sources of PASC clinic referralsd | ||
| Patient/family request | 20 (60.6%) | … |
| Physician discretion | 16 (48.5%) | … |
| Specific referral criteria | 13 (39.4%) | … |
| Automatic | 7 (21.2%) | … |
| PASC clinic formatd | ||
| In-clinic | 31 (93.9%) | … |
| Virtual | 26 (78.8%) | … |
| Telephone | 17 (51.5%) | … |
| At patient’s home | 4 (12.1%) | … |
PASC = postacute sequelae of COVID-19.
Data for three hospitals were not available in the American Hospital Association (AHA) database.
As defined by the AHA database and the Association of American Medical Colleges.
Two sites did not provide responses.
Respondents could select all that apply, so numbers/percentages may not add to 100.
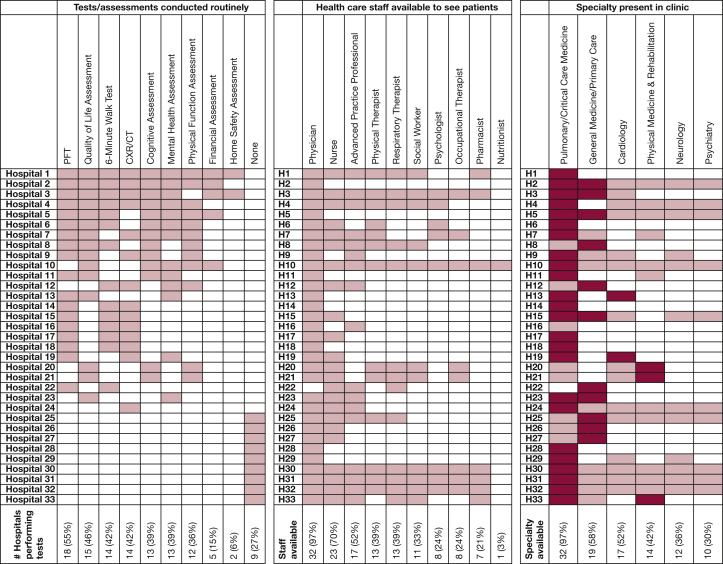
Nearly all hospitals with PASC clinics required a referral for a patient to be seen (n = 32; 97%). Most referrals (70%) relied on physician discretion or patient/family requests; 39% of hospitals used specific criteria for referral; and only 21% of hospitals referred all hospitalized COVID patients. First-time patients in PASC clinics often received a range of testing, such as pulmonary function testing, quality of life assessment, 6-min walk testing, chest radiography, cognitive assessment, mental health assessment, and physical function assessment (Fig 1). Most PASC clinics (73%) were distinct from their hospital’s post-ICU clinic. Of the 14 hospitals that did not have a PASC clinic, only two (14%) had plans to create one.
Figure 1.
Routine testing, available staff, and multidisciplinary presence across PASC clinics. ∗In panel 3 (specialties available to see patients), darker shaded boxes indicate specialties that led the hospital’s PASC clinic. CXR = chest radiography; H = hospital; PASC = postacute sequelae of COVID-19; PFT = pulmonary function testing.
Discussion
Using a diverse network of hospitals across the United States, this study is the first large-scale, multicenter evaluation of care delivery after hospitalization for COVID-19. Our data demonstrate substantial variation in dissemination of PASC symptoms or impairments, posthospital follow-up, and access to PASC clinics nationally. Hospitals without PASC clinics were more likely than hospitals with PASC clinics to be smaller, for profit, and to serve higher proportions of patients insured by Medicaid, which could reflect a paucity of resources at these hospitals. However, it is important to note that although this study provides one of the broadest assessments of PASC care to date and includes a diverse mix of academic tertiary and community hospitals, the survey was administered to hospitals within the PETAL Network, which is composed primarily of higher resourced, urban hospitals.
We identified several key areas for potential improvement in PASC care. First, despite the growing movement toward multidisciplinary PASC care, the effectiveness of these clinics remains unknown. Although multidisciplinary PASC clinics could reduce care fragmentation, they could also promote low-value care through unnecessary testing or divert resources away from care with established benefit. It also remains unclear what testing or assessments might be of high value for patients with PASC. It will be essential to examine the impact of these clinics on patient outcomes and to identify which, if any, aspects of PASC clinics might be beneficial. Second, there is a need to evaluate the extent to which PASC pathophysiology and management differ from sequelae of other infections or critical illnesses (eg, post-intensive care syndrome). Third, if multidisciplinary post-COVID care is found to be beneficial, about one of three hospitals did not have a PASC clinic, which may limit access to patients.3 Finally, most hospitals lacked systematic strategies to identify patients for multidisciplinary PASC care, relying on clinician referrals. This may result in some patients who might benefit from coordinated PASC care being unable to receive it. For example, COVID disproportionately affected Black and Hispanic individuals in the United States,4 who may be less integrated within health care systems and less likely to receive PASC care when referrals are not performed systematically and are instead tied to existing health care relationships.
Many health care systems in the United States urgently developed PASC clinics to face an impending crisis of COVID-19 survivorship. Multidisciplinary PASC clinics may offer opportunities to coordinate care, conduct systematic PASC evaluation, and create an environment for iterative gains in PASC knowledge. Opportunities exist to leverage large networks of PASC clinics to establish (1) longitudinal observational studies to understand the epidemiology of PASC; (2) clinical trials to study therapeutic interventions for patients with PASC; (3) the effectiveness of multidisciplinary models of PASC care; (4) scalable care delivery models; and (5) collaborative quality improvement initiatives across PASC clinics. Future studies should aim to understand the effectiveness and equity of dedicated, multidisciplinary care on improving longer-term, patient-centered outcomes for COVID survivors.
Acknowledgments
Author contributions: T. S. V. had full access to all data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: T. S. V., I. D. P., K. C. V., K. S. M., S. E. J., J. A. P., C. L. H.; acquisition of data: T. S. V., A. S.; analysis and interpretation of data: T. S. V., A. S.; drafting of the manuscript: T. S. V., A. S.; critical revision of the manuscript for important intellectual content: T. S. V., A. S., I. D. P., K. C. V., K. S. M., S. E. J., J. A. P., C. L. H.; statistical analysis: T. S. V.; obtained funding: C. L. H.
Role ofsponsors: The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation or approval of the manuscript
∗NationalHeart, Lung, and Blood Institute Clinical Trials Network for the Prevention and Early Treatment of Acute Lung Injury (PETAL Network) Collaborators:ALIGNE Clinical Center: Baystate Medical Center—Jay S. Steingrub (Principal Investigator), Mark A. Tidswell, Lori-Ann Kozikowski, Cynthia Kardos, Lesley DeSouza; Brigham and Women’s Hospital—Rebecca M. Baron, Mayra Pinilla-Vera, David M. Rubins, Antonio Arciniegas; Maine Medical Center—Richard Riker, Christine Lord; University of Florida—Marie-Carmelle Elie; BOSTON Clinical Center: Beth Israel Deaconess Medical Center—Daniel Talmor (Principal Investigator), Nathan Shapiro (Principal Investigator), Valerie Banner-Goodspeed; Massachusetts General Hospital—Kathryn A. Hibbert, Kelsey Brait, Natalie Pulido; University of Mississippi Medical Center—Alan Jones, James Galbraith, Utsav Nandi, Rebekah Peacock, Jenna Davis; Hennepin County Medical Center—Matthew Prekker, Michael Puskarich, Seth Jones, Anne Roerhl, Audrey Hendrickson; CALIFORNIA Clinical Center: UCSF San Francisco—Michael Matthay (Principal Investigator), Kirsten Kangelaris, Kathleen Liu, Kimberly Yee, Hanjing Zhuo; UCLA Ronald Reagan Medical Center—Gregory Hendey (Principal Investigator), Steven Chang, Nida Qadir, Andrea Tam, Rebecca Beutler, Trisha Agarwal; Stanford University Hospital—Joseph Levitt, Jennifer G. Wilson, Angela Rogers, Jonasel Roque, Rosemary Vojnik; UC Davis—Timothy E. Albertson, James A. Chenoweth, Jason Y. Adams, Brian M. Morrissey, Skyler J. Pearson; UCSF Fresno—Eyad Almasri, Alyssa Hughes; COLORADO Clinical Center: University of Colorado Hospital—Marc Moss (Principal Investigator), Adit Ginde (Principal Investigator), Jeffrey McKeehan, Lani Finck, Michelle Howell, Carrie Higgins; Denver Health Medical Center—Jason Haukoos, Stephanie Gravitz, Carolynn Lyle, Ivor S. Douglas, Terra Hiller, Audrey Goold; National Jewish Health/Saint Joseph’s Hospital—James Finigan; MICHIGAN Clinical Center: University of Michigan Medical Center—Robert Hyzy (Principal Investigator), Pauline Park (Principal Investigator), Michael Sjoding, Stephen Kay, Kristine Nelson, Kelli McDonough; Henry Ford—Namita Jayaprakash, Emanuel P Rivers, Jennifer Swiderek, Jasreen Kaur Gill, Jacqueline Day; Sinai Grace Hospital—Robert Sherwin, James Wooden, Thomas Mazzoco; MONTEFIORE-SINAI Clinical Center: Montefiore Moses—Michelle Ng Gong (Principal Investigator), Michael Aboodi, Ayesha Asghar, Omowunmi Amosu, Hiwet Tzehaie; Montefiore Weiler—Aluko A. Hope, Jen-Ting Chen, Rahul Nair, Brenda Lopez, Obiageli Offor; University of Arizona—Jarrod M. Mosier, Cameron D. Hypes, Elizabeth Salvagio, Christian Bime, Elaine Cristan; Ichan School of Medicine at Mount Sinai—Lynne D. Richardson (Principal Investigator), Kusum S. Mathews, Neha Goel, Patrick Maher, Samuel Acquah, Donald Cardone, Gary Oldenburg, Andrew Dunn; OHIO Clinical Center: University of Cincinnati Medical Center—Duncan Hite (Principal Investigator), Kristin Hudock, Jose Gomez Arroyo, Tammy Roads; Cleveland Clinic Foundation—Abhijit Duggal, Eduardo Mireles-Cabodevila; PACIFIC NORTHWEST Clinical Center: Harborview Medical Center—Bryce R.H. Robinson (Principal Investigator), Nicholas J. Johnson, Stephanie Gundel; University of Washington—Laura Evans; Swedish Medical Center—D. Shane O'Mahony, Julie A. Wallick; Cedars-Sinai Medical Center—Isabel Pedraza; Oregon Health and Science University—Catherine L Hough (Principal Investigator), Akram Khan, Olivia Krol, Milad Karami Jouzestani, Kelly Vranas; PITTSBURGH Clinical Center: UPMC—Donald M. Yealy (Principal Investigator), Derek C. Angus (Principal Investigator), Alexandra Weissman, David T. Huang, Aimee Boeltz-Skrtich; Penn State Hershey Medical Center—Steven Moore; Temple University Hospital—Derek Isenberg; SOUTHEAST Clinical Center: Wake Forest Baptist Health—D. Clark Files (Principal Investigator), Chadwick Miller (Principal Investigator), Kevin Gibbs, Lori Flores, Mary LaRose, Lauren Koehler, Leigha Landreth; University of Kentucky—Peter Morris, Evan Cassity, Jamie Sturgill, Kirby Mayer, Ashley Montgomery-Yates; Virginia Commonwealth University Medical Center—Marjolein de Wit, Jessica Mason; Medical University of South Carolina—Andrew Goodwin, Abigail Grady, Patterson Burch; University of Virginia Health—Kyle B. Enfield, Jeffrey M. Sturek, Mary Marshall; UTAH Clinical Center: Intermountain Medical Center, LDS Hospital, Utah Valley Regional Medical Center, McKayDee Hospital—Joseph R. Bledsoe (Principal Investigator), Samuel M. Brown (Principal Investigator), Ithan D. Peltan, Colin K Grissom, Brent Armbruster; University of Utah Hospital—Estelle Harris; VANDERBILT Clinical Center: Duke University Medical Center—John Eppensteiner, Bria Johnston Hall, Grace L. Hall, Lauren McGowan, Andrew Bouffler, Erica Walker, Samuel Francis, Tedra Porter; Louisiana State University Health Sciences Center—Bennett P deBoisblanc, Matthew R Lammi, David R Janz, Paula Lauto, Connie Romaine, Marie Sandi; Vanderbilt University Medical Center—Todd W Rice, Wesley H. Self; Clinical Coordinating Center: Massachusetts General Hospital Biostatistics Center (CCC)—Nancy Ringwood, Alexander Nagrebetsky, Laura Fitzgerald; Steering Committee Chair: Johns Hopkins Hospital—Roy G. Brower; National Heart, Lung, and Blood Institute: Lora A. Reineck, Neil R. Aggarwal, Karen Bienstock.
Data sharing: Survey data are available through the researchers on request.
Additional information:e-Appendix 1 is available online under “Supplementary Data.”
Footnotes
FUNDING/SUPPORT: This work was supported by the National Institutes of Health (NIH) [3U01HL123009-06S1, U01HL123009, U01HL122998, U01HL123018, U01HL123023, U01HL123008, U01HL123031, U01HL123004, U01HL123027, U01HL123010, U01HL123033, U01HL122989, U01HL123022, and U01HL123020]. The Research Electronic Data Capture data tools used in this study were supported, in part, by the NIH/National Center for Advancing Translational Sciences [UL1TR000445]. T. S. V. was supported by the Agency for Healthcare Research and Quality (AHRQ) [R01HS028038] and the NIH [K23HL140165]. K. S. M. was supported by the NIH [K23HL130648]. C. L. H. was supported by the NIH [K24HL141526].
FINANCIAL/NONFINANCIAL DISCLOSURES: The authors have reported to CHEST the following: T. S. V. reports research funding from the NIH, Agency for Healthcare Research and Quality (AHRQ), American Thoracic Society, and Society of Critical Care Medicine. I. D. P. reports research funding from the National Institutes of Health (NIH), Centers for Disease Control and Prevention, and Janssen Pharmaceuticals and funding to his institution from Asahi Kasei Pharma and Regeneron, all outside the present work. K. S. M. reports funding from the NIH and Roivant/Kinevant Sciences, outside of the submitted work. J. A. P. reports research funding from the NIH. K. C. V. is supported by resources from the VA Portland Health Care System. C. L. H. reports funding from the NIH and American Lung Association. None declared (A. S., S. E. J.)
DISCLAIMER: This article does not necessarily represent the view of the US government or the US Department of Veterans Affairs.
Contributor Information
Thomas S. Valley, Email: valleyt@umich.edu.
National Heart, Lung, and Blood Institute Clinical Trials Network for the Prevention and Early Treatment of Acute Lung Injury (PETAL):
Jay S. Steingrub, Mark A. Tidswell, Lori-Ann Kozikowski, Cynthia Kardos, Lesley DeSouza, Rebecca M. Baron, Mayra Pinilla-Vera, David M. Rubins, Antonio Arciniegas, Richard Riker, Christine Lord, Marie-Carmelle Elie, Daniel Talmor, Nathan Shapiro, Valerie Banner-Goodspeed, Kathryn A. Hibbert, Kelsey Brait, Natalie Pulido, Alan Jones, James Galbraith, Utsav Nandi, Rebekah Peacock, Jenna Davis, Matthew Prekker, Michael Puskarich, Seth Jones, Anne Roerhl, Audrey Hendrickson, Michael Matthay, Kirsten Kangelaris, Kathleen Liu, Kimberly Yee, Hanjing Zhuo, Gregory Hendey, Steven Chang, Nida Qadir, Andrea Tam, Rebecca Beutler, Trisha Agarwal, Joseph Levitt, Jennifer G. Wilson, Angela Rogers, Jonasel Roque, Rosemary Vojnik, Timothy E. Albertson, James A. Chenoweth, Jason Y. Adams, Brian M. Morrissey, Skyler J. Pearson, Eyad Almasri, Alyssa Hughes, Marc Moss, Adit Ginde, Jeffrey McKeehan, Lani Finck, Michelle Howell, Carrie Higgins, Jason Haukoos, Stephanie Gravitz, Carolynn Lyle, Ivor S. Douglas, Terra Hiller, Audrey Goold, James Finigan, Robert Hyzy, Pauline Park, Michael Sjoding, Stephen Kay, Kristine Nelson, Kelli McDonough, Namita Jayaprakash, Emanuel P. Rivers, Jennifer Swiderek, Jasreen Kaur Gill, Jacqueline Day, Robert Sherwin, James Wooden, Thomas Mazzoco, Michelle Ng Gong, Michael Aboodi, Ayesha Asghar, Omowunmi Amosu, Hiwet Tzehaie, Aluko A. Hope, Jen-Ting Chen, Rahul Nair, Brenda Lopez, Obiageli Offor, Jarrod M. Mosier, Cameron D. Hypes, Elizabeth Salvagio, Christian Bime, Elaine Cristan, Lynne D. Richardson, Kusum S. Mathews, Neha Goel, Patrick Maher, Samuel Acquah, Donald Cardone, Gary Oldenburg, Andrew Dunn, Duncan Hite, Kristin Hudock, Jose Gomez Arroyo, Tammy Roads, Abhijit Duggal, Eduardo Mireles-Cabodevila, Bryce R.H. Robinson, Nicholas J. Johnson, Stephanie Gundel, Laura Evans, D. Shane O'Mahony, Julie A. Wallick, Isabel Pedraza, Catherine L. Hough, Akram Khan, Olivia Krol, Milad Karami Jouzestani, Kelly Vranas, Donald M. Yealy, Derek C. Angus, Alexandra Weissman, David T. Huang, Aimee Boeltz-Skrtich, Steven Moore, Derek Isenberg, D. Clark Files, Chadwick Miller, Kevin Gibbs, Lori Flores, Mary LaRose, Lauren Koehler, Leigha Landreth, Peter Morris, Evan Cassity, Jamie Sturgill, Kirby Mayer, Ashley Montgomery-Yates, Marjolein de Wit, Jessica Mason, Andrew Goodwin, Abigail Grady, Patterson Burch, Kyle B. Enfield, Jeffrey M. Sturek, Mary Marshall, Joseph R. Bledsoe, Samuel M. Brown, Ithan D. Peltan, Colin K. Grissom, Brent Armbruster, Estelle Harris, John Eppensteiner, Bria Johnston Hall, Grace L. Hall, Lauren McGowan, Andrew Bouffler, Erica Walker, Samuel Francis, Tedra Porter, Bennett P. deBoisblanc, Matthew R. Lammi, David R. Janz, Paula Lauto, Connie Romaine, Marie Sandi, Todd W. Rice, Wesley H. Self, Nancy Ringwood, Alexander Nagrebetsky, Laura Fitzgerald, Roy G. Brower, Lora A. Reineck, Neil R. Aggarwal, and Karen Bienstock
Supplementary Data
References
- 1.Iwashyna T.J., Kamphuis L.A., Gundel S.J., et al. NHLBI Prevention and Early Treatment of Acute Lung Injury (PETAL) Network. Continuing cardiopulmonary symptoms, disability, and financial toxicity 1 month after hospitalization for third-wave COVID-19: early results from a US nationwide cohort. J Hosp Med. 2021;16(9):531–537. doi: 10.12788/jhm.3660. [DOI] [PubMed] [Google Scholar]
- 2.Parker A.M., Brigham E., Connolly B., et al. Addressing the post-acute sequelae of SARS-CoV-2 infection: a multidisciplinary model of care. Lancet Respir Med. 2021;9(11):1328–1341. doi: 10.1016/S2213-2600(21)00385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chopra V., Flanders S.A., O’Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.