Abstract
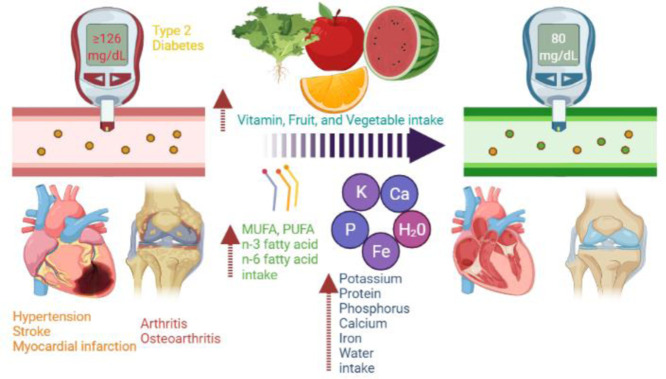
Persons with underlying noncommunicable diseases (NCDs) are more likely to acquire severe coronavirus disease 2019 disease and to die from coronavirus disease 2019. An urgent need for potential therapy to prevent and control NCDs is critical. We hypothesized that higher intakes of multiple individual nutrients, fruits, or vegetables would be linked with a low risk of NCDs in the Korean population. Thus, we aim to explore the association between NCDs, including cardiovascular diseases, type 2 diabetes mellitus (T2DM), arthritis, depression, and dietary factors. A total of 56,462 adults aged 18 years (2009-2019) were included. Dietary factors, including intakes of multiple individual nutrients, fruits, and vegetables, were assessed. Multivariable-adjusted logistic regression models were used to explore the associations between dietary factors and NCDs. Interactions were found between intakes of multiple individual nutrients and sex for T2DM, hypertension, stroke, myocardial infarction, arthritis, and osteoarthritis. Only in women was a 2-fold increase in daily multiple individual nutrient intake (vitamins A, B1, B2, B3, C; potassium, protein; phosphorus; calcium; iron; monounsaturated fatty acid and polyunsaturated fatty acid; n-3 fatty acid and n-6 fatty acid; and water) associated with a lower prevalence of T2DM, hypertension, stroke, myocardial infarction, arthritis, and osteoarthritis. In both women and men, high fruit or vegetable consumption was linked with a lower risk of T2DM, hypertension, dyslipidemia, osteoarthritis, and depression than low consumption. Our findings found higher intakes of fruits, vegetables, and multiple individual nutrients are linked with a lower risk of NCDs in the Korean adult population. Further work is needed to identify whether interactions between intake of multiple individual nutrients, vegetables, and fruits affect the presence of NCDs.
Keywords: NCDs, Vitamins, Nutrients, Vegetables, Fruits
Abbreviations: BMI, body mass index; CI, confidence interval; CV, coefficient of variation; CVD, cardiovascular disease; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; KCDC, Korea Centers for Disease Control and Prevention; KNHANES, Korea National Health and Nutrition Examination Survey; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; MUFA, monounsaturated fatty acid; NCD, noncommunicable disease; OR, odds ratio; PUFA, polyunsaturated fatty acid; RR, relative risk; T2DM, type 2 diabetes mellitus
Graphical Abstract
1. Introduction
Noncommunicable diseases (NCDs) such as cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) account for more than 70% of global deaths each year. Of note, NCDs make up about 85% of premature deaths in low- and middle-income countries [1]. The elderly, adults as well as children, are all vulnerable to the risk factors that contribute to NCDs. However, NCDs disproportionately impact vulnerable and disadvantaged groups in affluent countries [2].
In the past few decades, the consumption of junk and processed foods has continued to rise rapidly in both developing and developed countries [3], [4], [5]. Besides, calories obtained from fiber-rich foods (e.g., pulses, whole grains, roots) have been decreasing, whereas calories obtained from oils, fats, meat, and sugars have been accelerating rapidly globally. Dietary patterns and nutrient intake are influenced by this nutrition transition, which affects the risk of developing NCDs [6]. On the other hand, physical inactivity, exposure to heavy metals (e.g., cadmium, mercury, lead), tobacco smoke, or the harmful use of alcohol also increase the risk of NCDs [3,7]. As a result, prevention and control of NCDs are critical components of the management of NCDs [1].
The benefits of nutrient-dense food-rich diets have been highlighted in terms of longevity, healthy aging, and morbidity [8]. Furthermore, several researchers have attempted to identify the role of diets in NCDs, with the relationships between multiple individual nutrients, fruit, and vegetable intake and NCDs still being controversial. For instance, some studies, for example, have shown that n-3 polyunsaturated fatty acids have beneficial effects on arthritis [9], [10], [11]. However, some studies have found no link between n-3 polyunsaturated fatty acid intake and the risk of arthritis [12,13]. Our recent study also showed thiamine intake was associated with lower risks of NCDs (e.g., hypertension, myocardial infarction or angina, T2DM, depression, dyslipidemia) [4]. These studies have prompted the search for dietary factors that may aid in the prevention and control of NCDs. We hypothesized that higher intakes of multiple individual nutrients, fruits, or vegetables were linked with a low risk of CVD, T2DM, arthritis, and depression in the Korean population. Thus, data from national population-based surveys were used in this study to identify associations between CVD, T2DM, arthritis, and depression and dietary factors such as multiple individual nutrients, fruit, and vegetable intake. Multivariable-adjusted logistic regression models were used to assess the associations between dietary factors and NCDs. We also identify the possible interaction of sex in the association of NCDs with total vitamin dietary factors. To visualize the moderating effects of hypertension and T2DM, marginal effects were employed using the results of logistic regression analysis. With our deeper analysis, our findings may partly explain sex differences in the association between dietary factors and CVDs, which was not explored enough. These findings also confirm the previous findings showing the role of dietary factors in the pathology of CVDs. Taken together, this study may partly contribute to gaining a better understanding of the effects of dietary factors on NCDs in women and men.
2. Methods and Materials
2.1. Design and data collection
Design and data collection methods have been published elsewhere [14,15]. Between 2009 and 2019, 91,394 persons participated in the Korea National Health and Nutrition Examination Survey (KNHANES). Specifically, randomly selected subjects numbered 10,533 (2009), 8958 (2010), 8518 (2011), 8058 (2012), 8018 (2013), 7550 (2014), 7380 (2015), 8150 (2016), 8127 (2017), 7992 (2018), and 8110 (2019). Participants in this study (1) completed all 3 parts of the study, which included a health examination survey, a health interview survey, and a nutrition survey, and (2) provided information on multiple individual nutrients, fruit and vegetable consumption, lifestyle, current medications, and medical and family history [4]. In this study, we excluded 19,000 participants under the age of 18, and 7146 records with missing values for major diseases (5712, hypertension and T2DM; 3, dyslipidemia; 8, arthritis; 8, rheumatoid arthritis; 1315, stroke; 91, myocardial infarction [MI] or angina; 9, depression), dietary factors (7828 missing vitamin B1), and 753 subjects who had an energy intake of more than 5000 Kcal (499) or less than 500 Kcal (254), and 205 who were pregnant or lactating. As a result, the data of 56,462 adults were analyzed. Information on demographic and social characteristics (e.g., age group, education levels, occupation) is defined elsewhere [4]. All individuals were required to provide written informed consent before examinations, which were conducted by the Department of the Korea Centers for Disease Control and Prevention (KCDC). These surveys were conducted with the approval of the institutional review board of the KCDC (2009-01CON-03-2C, 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, 2013-12EXP-03-5C, 2015-01-02-6C, 2018-01-03-P-A). KNHANES was conducted without approval in 2016, 2017, and 2019, according to the opinion of the institutional review board of the KCDC [14].
2.2. Food intake
Daily dietary multiple individual nutrient intakes were measured as mean 24-hour dietary intakes and calculated using the Korean Nutrition Society's Can-Pro 3.0 nutrient intake assessment software [16]. Individuals completed a semiquantitative questionnaire on food frequency, which addressed the intake of 63 food products. From the 63 food items, 3 groups (green vegetables, other vegetables, and fruits) were chosen. The green vegetable group includes radish leaves, spinach, cucumber, and pepper; the other vegetable group includes radish, pumpkin, carrot, sprouts, cabbage, Korean cabbage, and tomato; and the fruit group includes persimmon, pear, watermelon, peach, strawberry, tangerine, banana, apple, grape, and citrus. The participants were divided into 3 groups based on the frequency with which they consumed vegetables and fruits [3].
2.3. Serum levels of vitamins A, D, and E
Serum 25-hydroxyvitamin D levels were measured with a 125I radioimmunoassay kit (DiaSorin Inc., Stillwater, MN, USA) using a γ-counter (1470 Wizard; PerkinElmer, Turku, Finland). Intra- and inter-assay coefficients of variation (CV) were 6.7% to 12.5% and 7.6% to 12.9% for serum 25-hydroxyvitamin D, respectively [16]. A serum 25-hydroxyvitamin D level of 75 nmol/L or higher indicates vitamin D sufficiency [17]. Serum retinol (µmol/L) and serum α-tocopherol (µmol/L) levels were measured with an Agilent1200 (Agilent1200; Agilent, USA) using Chromsystems (Chromsystems; Chromsystems Instruments & Chemicals, Germany) reagents. The reference normal ranges for serum retinol and α-tocopherol in adults are 1.7 to 2.2 µmol/L and 12 to 30 µmol/L, respectively [18,19]. The intra- and inter-assay CVs for serum retinol and serum α-tocopherol were in the range of 3.1% to 7.8% [16].
2.4. Laboratory measurements
During medical examinations, weight, height, waist circumference, and blood pressure were measured using the standard procedures by trained staff. Blood specimens were taken following an 8-hour fast and analyzed at Korea's Neodin Medical Institute. Lipid profiles (high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], total cholesterol, triglycerides), glycated hemoglobin (HbA1c), and fasting glucose levels were measured as previously described [16]. In brief, an enzymatic assay was used to determine triglycerides (mg/dL), total cholesterol (mg/dL), and HDL-C (mg/dL) using Cholest N HDL, Pureauto SCHO-N, and Cholestest LDL agent (Sekisui, Tokyo, Japan) on a Hitachi Automatic Analyzer 7600-210 (Hitachi, Tokyo, Japan). Serum LDL-C (mg/dL) is calculated using the Friedewald equation (serum LDL-C = serum total cholesterol – serum HDL-C – serum triacylglycerol/5) [20]. HbA1c levels (%) were analyzed with high-performance liquid chromatography 723G7 (Tosoh, Tokyo, Japan). Fasting glucose levels (mg/dL) were measured using a Hitachi automatic analyzer 7600. The intra- and inter-assay CVs were <2.40% and <1.60% for triglycerides; <0.62% and <1.22% for total cholesterol; <2.60% and <2.30% for HDL-C; 3.12% and <2.80% for HbA1c; and <2.93% and <2.41% for fasting glucose levels, respectively [16].
2.5. Urinary cotinine and smoking verification
Urinary cotinine levels were measured as previously described [16]. Spot urinary specimens were collected to measure urinary cotinine levels by gas chromatography/mass spectrometry using a PerkinElmer Clarus 600T unit with a detection limit of 1.26 ng/mL. Standard reference materials were used for internal quality assurance and control purposes (ClinChek, RECIPE, Munich, Germany). Participants with a urinary cotinine level of ≥50 ng/mL were defined as cotinine-verified smokers [3,7].
2.6. Parameters
Alcohol consumption was classified as low and high (high-risk drinking was defined as more than 5 drinks per day for more than a month). Subjects who had smoked more than 100 cigarettes in their lifetime and continued to smoke daily or on occasion were classified as current smokers; others were classified as ex-/nonsmokers. Physical activity was dichotomized as either regular or irregular. Regular physical activity was defined as either (1) participation in vigorous physical activity, ≥20 minutes per session ≥3 days a week; (2) or participation in moderate physical activity, ≥30 minutes per session ≥5 days per week; (3) or participation in walking, ≥30 minutes per session ≥5 days a week [21].
Dyslipidemia was defined as having 1 or more of the following conditions: LDL-C ≥ 160 mg/dL, triglycerides ≥ 200 mg/dL, and HDL-C < 40 mg/dL. Hypertension was defined as having a systolic blood pressure of 140 mmHg or a diastolic blood pressure of 90 mmHg or being on antihypertensive medication. T2DM was defined as having a fasting plasma glucose of 126 mg/dL or being on antidiabetic medication, or a HbA1c of 65%. Stroke, MI, MI or angina, arthritis, osteoarthritis, rheumatoid arthritis, and depression were defined as physician diagnosis, current presence or treatment for stroke, angina, MI, MI or angina, arthritis, osteoarthritis, rheumatoid arthritis, and depression. A family history of CVD was defined as having at least 1 parent or sibling with a diagnosis of hypertension, ischemic heart disease, or stroke. A family history of T2DM or hyperlipidemia was defined as having at least 1 parent or sibling with a diagnosis of T2DM or hyperlipidemia [4].
2.7. Statistical analyses
STATA software (version 160; Stata Corp, TX, USA) was used for the statistical analysis. For categorical variables, the baseline characteristics of participants are presented as frequencies and proportions, and for continuous variables, as means and standard deviations or median and interquartile range. Survey sample weights were used in all analyses to generate estimates that were representative of the noninstitutionalized civilian Korean population [4,7,22].
The daily levels of multiple individual nutrients (e.g., B vitamins, vitamin C, protein, iron) were log2 transformed because their distributions were right skewed. The daily levels of multiple individual nutrients were described as the geometric mean and 95% confidence interval (CI) [22].
The associations between NCDs (e.g., T2DM, hypertension, stroke) and daily levels of multiple individual nutrients, and vegetables and fruits were assessed by logistic regression. Potential covariates were obtained from the literature, chosen based on subjective prior knowledge, or shown by univariate analysis to have P values of ≤.25, and these variables were entered into the full model [23]. Potential factors included age group (≤29, 30-39, 40-49, 50-59, 60-69, 70-79, ≥80 years), sex (male, female), monthly household income (<2000, ≥2000 and <4000, ≥4000 and <6000, ≥6000 thousand won), physical activity (not regular, regular), body mass index (BMI) (<18.5, ≥18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine-verified smoker (yes, no), smoking status (non-/ex-smoker, current smoker), occupation (white collar, blue collar, none), education levels (≤middle school, high school, ≥college), energy intake (Kcal), family history of CVDs or T2DM or hyperlipidemia (yes, no). The interactions of NCDs and daily levels of multiple individual nutrients, and vegetables and fruits were also assessed. Because many factors are associated with NCDs, we only predict the risks of hypertension and T2DM through marginal effects. Statistical tests were 2-sided, and P values <.05 were considered statistically significant [4,7,22].
3. Results
A total of 56,462 adults (mean age 51.1 ± 16.1 years, min-max: 18-80) that participated in the KNANES 2009-2019 survey were included. Among women (n = 33,354), there were significantly more who were aged from 40 to 59 years, unemployed, married, living in a rural location, had a low education level, had a low monthly income household, were normal weight (BMI ≥ 18.5 and <25 kg/m2), nonsmokers, had less physical activity, and had a family history of T2DM or dyslipidemia.
Women had a significantly higher prevalence of arthritis and osteoarthritis, and higher waist circumference, total cholesterol, HDL-C, energy intake, and fruit consumption than in men. By contrast, levels of vitamins (B1, B2, B3, C, A, and D), retinol, potassium, sodium, phosphorus, iron, protein, n-3 fatty acid, n-6 fatty acid, monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA), water, and fiber intake were significantly greater in men. Table 1 shows baseline characteristics, cardiometabolic risk factors, and dietary patterns according to gender.
TABLE 1.
Baseline characteristics according to the gender of the study population
| Variables | n | Males n = 23,108 |
Females n = 33,354 |
|---|---|---|---|
| Demographic and social characteristics | |||
| Age (y) | 56,462 | 51.43 ± 17.14 | 51.43 ± 17.14 |
| Age group (%) | 56,462 | ||
| ≤29 | 6886 | 2986 (12.9) | 3900 (11.7) |
| 30-39 | 9262 | 3525 (15.3) | 5737 (17.2) |
| 40-49 | 10,044 | 3923 (17.0) | 6121 (18.4) |
| 50-59 | 10,489 | 4146 (17.9) | 6343 (19.0) |
| 60-69 | 10,007 | 4392 (19.0) | 5615 (16.8) |
| 70-79 | 7678 | 3330 (14.4) | 4348 (13.0) |
| ≥80 | 2096 | 806 (3.5) | 1290 (3.9) |
| Marital status (%) | 56,445 | ||
| Married | 47,977 | 18,826 (81.5) | 29,151 (87.4) |
| Living alone | 8468 | 4274 (18.5) | 4194 (12.6) |
| Residential areas (%) | 56,462 | ||
| Urban | 44,766 | 18,090 (78.3) | 26,676 (78.0) |
| Rural | 11,696 | 5018 (21.7) | 6678 (20.0) |
| Occupation (%) | 56,168 | ||
| White-collar | 18,832 | 8167 (35.6) | 10,665 (32.1) |
| Blue-collar | 13,596 | 8047 (35.1) | 5549 (16.7) |
| None | 23,740 | 6726 (29.3) | 17,014 (51.2) |
| Education level (%) | 56,255 | ||
| ≤Middle school | 19,893 | 6824 (29.6) | 13,069 (39.3) |
| High school | 18,441 | 8077 (35.1) | 10,364 (31.2) |
| ≥College | 17,921 | 8128 (35.3) | 9793 (29.5) |
| Monthly household income (%)a | 56,136 | ||
| <2000 | 17,848 | 7042 (30.7) | 10,806 (32.6) |
| ≥2000 and <4000 | 16,655 | 7029 (30.5) | 9626 (29.0) |
| ≥4000 and <6000 | 11,385 | 4702 (20.5) | 6683 (20.2) |
| ≥6000 | 10,248 | 4204 (18.3) | 6044 (18.2) |
| BMI group (%) | 56,310 | ||
| <18.5 | 2442 | 700 (3.0) | 1742 (5.3) |
| ≥18.5 and <25 | 35,495 | 13,778 (59.8) | 21,717 (65.3) |
| ≥25 and <30 | 15,854 | 7595 (33.0) | 8259 (24.8) |
| ≥30 | 2519 | 976 (4.2) | 1543 (4.6) |
| Smoking status (%) | 55,631 | ||
| Non/ex-smoker | 44,880 | 13,654 (60.1) | 31,226 (94.8) |
| Current smoker | 10,751 | 9054 (39.9) | 1697 (5.2) |
| Cotinine verified smokers (%) | 56,462 | ||
| No | 21,988 | 7598 (32.9) | 14,390 (43.1) |
| Yes | 34,474 | 15,510 (67.1) | 18,964 (56.9) |
| High-risk drinking status (%) | 56,324 | ||
| No | 50,868 | 27,760 (83.6) | 23,108 (100) |
| Yes | 5456 | 5456 (16.4) | 0 (0.0) |
| Physical activity (%) | 56,462 | ||
| Not regular | 42,480 | 17,390 (75.3) | 25,090 (75.2) |
| Regular | 13,982 | 5718 (24.7) | 8264 (24.8) |
| Family history of CVD (%) | 55,999 | ||
| No | 34,084 | 15,332 (65.1) | 18,752 (57.8) |
| Yes | 21,915 | 8,238 (34.9) | 13,677 (42.2) |
| Family history of diabetes (%) | 55,337 | ||
| No | 45,176 | 19,521 (83.6) | 25,655 (80.2) |
| Yes | 10,161 | 3822 (16.4) | 6339 (19.8) |
| Family history of hyperlipidemia (%) | 43,907 | ||
| No | 50,923 | 21,778 (95.6) | 29,145 (93.6) |
| Yes | 2984 | 995 (4.4) | 1989 (6.4) |
| Medical history | |||
| Type 2 diabetes (%) | 56,462 | ||
| No | 51,319 | 20,649 (89.4) | 30,670 (92.0) |
| Yes | 5143 | 2459 (10.6) | 2684 (8.0) |
| Hypertension (%) | 56,462 | ||
| No | 42,944 | 17,284 (74.8) | 25,660 (76.9) |
| Yes | 13,518 | 5824 (25.2) | 7694 (23.1) |
| Dyslipidemia (%) | 56,462 | ||
| No | 48,281 | 20,209 (87.5) | 28,072 (84.2) |
| Yes | 8181 | 2899 (22.5) | 5282 (25.8) |
| Stroke (%) | 56,462 | ||
| No | 55,136 | 22,407 (97.0) | 32,729 (98.1) |
| Yes | 1,326 | 701 (3.0) | 625 (1.9) |
| MI or angina (%) | 56,462 | ||
| No | 54,842 | 22,245 (96.3) | 32,597 (97.7) |
| Yes | 1620 | 863 (3.7) | 757 (2.3) |
| MI (%) | 56,462 | ||
| No | 55,895 | 22,738 (98.4) | 33,157 (99.4) |
| Yes | 567 | 370 (1.6) | 197 (0.6) |
| Arthritis (%) | 56,462 | ||
| No | 48,688 | 21,693 (93.9) | 26,995 (80.9) |
| Yes | 7,774 | 1,415 (6.1) | 6,359 (19.1) |
| Osteoarthritis (%) | 56,462 | ||
| No | 49,549 | 21,879 (94.7) | 27,670 (83.0) |
| Yes | 6913 | 1229 (5.3) | 5684 (17.0) |
| Rheumatoid arthritis (%) | 56,462 | ||
| No | 55,275 | 22,880 (99.0) | 32,395 (97.1) |
| Yes | 1187 | 228 (1.0) | 959 (2.9) |
| Depression (%) | 56,462 | ||
| No | 53,936 | 22,594 (97.8) | 31,342 (94.0) |
| Yes | 2526 | 514 (2.2) | 2012 (6.0) |
| Food intake | |||
| 24-h recall | |||
| Energy intake (Kcal) | 56,462 | 2255.93 ± 828.06 | 1677.02 ± 643.67 |
| Vitamin B1 intake (mg)b | 56,462 | 1.47 (1.46-1.48) | 1.11 (1.10-1.12) |
| Vitamin B2 intake (mg)b | 56,462 | 1.29 (1.28-1.30) | 1.00 (0.99-1.01) |
| Vitamin B3 intake (mg)b | 56,462 | 15.19 (15.08-15.29) | 11.38 (11.32-11.45) |
| Vitamin C intake (mg)b | 56,462 | 61.61 (60.87-62.36) | 57.40 (56.78-58.02) |
| Vitamin A intake (mg)b | 56,462 | 525.69 (519.01-532.45) | 429.08 (424.36-433.94) |
| Vitamin D (ng/mL)‡ | 56,462 | 17.79 (17.67-17.91) | 15.68 (15.59-15.77) |
| Retinol (µg)b | 56,462 | 47.76 (46.67-48.87) | 38.64 (37.86-39.43) |
| Sodium (mg)b | 56,462 | 4019.85 (3988.86-4051.08) | 2825.27 (2805.63-2845.04) |
| Potassium (mg)b | 56,462 | 2923.75 (2906.11- 2941.50) | 2383.88 (2371.06-2396.76) |
| Phosphorus (mg)b | 56,462 | 1137.52 (1131.16-1143.91) | 874.23 (870.07- 878.42) |
| Protein (g)b | 56,462 | 71.66 (71.21-72.11) | 52.47 (52.20-52.75) |
| Iron (mg)b | 56,462 | 13.83 (13.73-13.93) | 10.85 (10.78-10.92) |
| Saturated fatty acid (g)b | 56,462 | 11.01 (10.85- 11.17) | 8.13 (8.03-10.24) |
| MUFA (g)b | 56,462 | 10.89 (10.71-11.06) | 8.05 (7.93-8.16) |
| PUFA (g)b | 56,462 | 9.67 (9.54-9.80) | 7.29 (7.20-7.38) |
| n-3 fatty acid (g)b | 56,462 | 1.35 (1.33-1.37) | 1.05 (1.03-1.06) |
| n-6 fatty acid (g)b | 56,462 | 8.02 (7.91-8.13) | 6.00 (5.93-6.08) |
| Fiber (g)b | 56,462 | 23.54 (23.29-23.78) | 20.07 (19.89-20.26) |
| Water (g)b | 56,462 | 927.09 (919.46-934.79) | 754.54 (749.33-759.78) |
| Food Frequency Questionnairec | |||
| Green vegetables (%) | 15,534 | ||
| Low consumption | 11,597 | 4273 (72.1) | 7324 (76.2) |
| Medium consumption | 3,018 | 1236 (20.9) | 1782 (18.5) |
| High consumption | 919 | 415 (7.0) | 504 (5.3) |
| White vegetables (%) | 15,571 | ||
| Low consumption | 12,132 | 4626 (77.9) | 7506 (78.0) |
| Medium consumption | 2706 | 1033 (17.4) | 1673 (17.4) |
| High consumption | 733 | 283 (4.7) | 450 (4.6) |
| Fruits (%) | 15,573 | ||
| Low consumption | 12,918 | 5246 (88.3) | 7672 (79.7) |
| Medium consumption | 1407 | 344 (5.9) | 1063 (9.3) |
| High consumption | 1248 | 352 (5.8) | 896 (11.0) |
| Laboratory measurements | |||
| Waist circumference (cm)b | 56,462 | 58.41 ± 9.20 | 79.03 ± 9.93 |
| Total cholesterol (mg/dL)b | 56,462 | 187.40 ± 36.78 | 191.39 ± 36.76 |
| LDL-C (mg/dL)b | 56,462 | 113.51 ± 33.08 | 115.21 ± 33.31 |
| Triglyceride (mg/dL)d | 56,462 | 122 (84-185) | 95 (66-141) |
| HDL-C (mg/dL)b | 56,462 | 46.91 ± 11.22 | 53.16 ± 12.39 |
| HbA1c (%) | 56,462 | 5.85 ± 0.94 | 5.76 ± 0.82 |
| Fasting glucose (mg/dL) | 56,462 | 102.45 ± 25.08 | 97.44 ± 21.56 |
| hs-CRP (mg/L)d | 56,462 | 0.61 (0.15-1.23) | 0.51 (0.09-1.01) |
| Hemoglobin (g/dL) | 56,462 | 15.17 ± 1.26 | 13.08 ± 1.15 |
| Systolic blood pressure (mmHg)b | 56,462 | 121.82 ± 15.58 | 117.61 ± 18.12 |
| Diastolic blood pressure (mmHg)b | 56,462 | 77.87 ± 10.61 | 73.81 ± 9.83 |
| AST (IU/L) | 56,462 | 22.90 ± 0.15 | 22.23 ± 0.11 |
| ALT (IU/L) | 56,462 | 23.40 ± 0.22 | 21.15 ± 0.24 |
ALT, alanine aminotransferase; AST, aminotransferase; BMI, body mass index (kg/m2); CVD, cardiovascular disease; hs-CRP, high sensitivity C-reactive protein; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Thousand won.
Median (IQR).
Geometric mean and 95% confidence interval.
Data available from 2012 to 2016.
After adjustment for potential confounders, a 2-fold increase in daily vitamins B1, B2, B3, A, C, and retinol were negatively associated with T2DM. Furthermore, a doubling of daily potassium, protein, iron, MUFA, PUFA, n-3 fatty acid, n-6 fatty acid, and water intake was significantly associated with a decrease in T2DM by 10% (odds ratio [OR] 0.90; 95% CI, 0.85-0.94), 14% (OR 0.86; 95% CI, 0.82-0.90), 6% (OR 0.94; 95% CI, 0.90-0.98), 9% (OR 0.91; 95% CI, 0.88-0.95), 6% (OR 0.94; 95% CI, 0.91-0.98), 5% (OR 0.95; 95% CI, 0.92-0.98), 5% (OR 0.95; 95% CI, 0.91-0.99), and 14% (OR 0.86; 95% CI, 0.82-0.90), respectively. We found that a high intake of other vegetables and fruits was a significant contributor to the reduced prevalence of T2DM. The OR (95% CI) for high-consumption (highest tertile) groups for other vegetable intake was 0.67 (0.52-0.86) in comparison with the low-consumption group. The OR (95% CI) for high-consumption (highest tertile) groups for green vegetable intake was 0.71 (0.56-0.89) in comparison with the low-consumption group. High and moderate fruit intake reduced the risk of T2DM significantly more than the low-consumption group (OR 0.66; 95% CI, 0.48-0.91), and (OR 0.44; 95% CI, 0.30-0.64). Furthermore, smoking and high-risk drinking were positively associated with the prevalence of T2DM. Adjusted ORs (95% CI) for NCD risks are provided in Table 2 .
Table 2.
Association between noncommunicable diseases and vitamin, nutrient, fruit, and vegetable intake, and health-related behaviors
| Variables | Diabetes n = 5143 |
Dyslipidemia n = 8181 |
Hypertension n = 13,518 |
Stroke n = 1326 |
MI n = 567 |
MI or angina n = 1620 |
Arthritis n = 7774 |
Osteoarthritis N = 6913 |
Rheumatoid arthritis n = 1187 |
Depression n = 2566 |
|---|---|---|---|---|---|---|---|---|---|---|
| Vitamins | ||||||||||
| Log2 vitamin B1 | 0.89 (0.85-0.93) | 1.02 (0.98-1.06) | 0.92 (0.88-0.95) | 0.89 (0.82-0.97) | 0.87 (0.76-0.99) | 0.88 (0.82-0.96) | 0.96 (0.93-0.99) | 0.96 (0.93-0.99) | 0.93 (0.87-1.00) | 0.92 (0.88-0.97) |
| Log2 vitamin B2 | 0.87 (0.84-0.91) | 1.11 (0.99-1.14) | 0.91 (0.88-0.94) | 0.85 (0.79-0.92) | 0.87 (0.76-0.99) | 0.94 (0.88-1.01) | 0.98 (0.95-1.01) | 0.98 (0.95-1.01) | 0.98 (0.92-1.05) | 0.97 (0.93-1.02) |
| Log2 vitamin B3 | 0.87 (0.83-0.91) | 0.94 (0.91-0.98) | 0.92 (0.88-0.95) | 0.82 (0.76-0.90) | 0.76 (0.67-0.86) | 0.89 (0.82-0.96) | 0.95 (0.92-0.98) | 0.95 (0.91-0.98) | 0.94 (0.87-1.02) | 0.86 (0.84-0.93) |
| Log2 vitamin C | 0.94 (0.92-0.97) | 0.99 (0.97-1.01) | 0.97 (0.95-0.99) | 0.97 (0.93-1.02) | 0.93 (0.87-1.00) | 0.97 (0.93-1.01) | 0.99 (0.98-1.02) | 0.99 (0.98-1.02) | 0.98 (0.94-1.02) | 0.97 (0.94-0.99) |
| Log2 vitamin A | 0.96 (0.93-0.98) | 1.01 (0.99-1.03) | 0.99 (0.97-1.01) | 0.98 (0.94-1.03) | 0.91 (0.85-0.98) | 0.97 (0.93-1.01) | 0.99 (0.97-1.01) | 0.99 (0.97-1.01) | 0.98 (0.94-1.02) | 0.96 (0.93-0.99) |
| Log2 retinol | 0.97 (0.95-0.98) | 1.02 (0.98-1.03) | 0.98 (0.97-0.99) | 0.96 (0.94-0.98) | 0.96 (0.93-0.99) | 0.99 (0.97-1.01) | 0.99 (0.98-1.01) | 0.99 (0.99-1.01) | 1.00 (0.98-1.02) | 1.01 (0.99-1.03) |
| Log2 serum vitamin A | 1.77 (1.05-2.97) | 3.09 (2.09-4.56) | 2.51 (1.68-3.73) | 2.10 (0.76-5.79) | 4.76 (1.05-20.52) | 2.59 (1.05-6.43) | 0.98 (0.63-1.52) | 0.99 (0.63-1.59) | 1.30 (0.49-3.47) | 2.15 (1.07-3.77) |
| Log2 serum vitamin D | 0.77 (0.65-0.92) | 1.09 (0.94-1.27) | 0.94 (0.82-1.07) | 0.58 (0.41-0.82) | 1.01 (0.57-1.79) | 1.17 (0.86-1.59) | 1.23 (1.09-1.38) | 1.31 (1.16-1.48) | 0.90 (0.70-1.14) | 0.96 (0.81-1.14) |
| Log2 serum vitamin E | 0.44 (0.28-0.70) | 1.66 (1.20-2.30) | 0.94 (0.67-1.32) | 0.51 (0.20-1.32) | 2.48 (0.65-9.48) | 0.33 (0.13-0.80) | 0.97 (0.67-1.40) | 0.88 (0.59-1.31) | 1.34 (0.60-2.99) | 0.63 (0.36-1.09) |
| 24-h recall | ||||||||||
| Log2 potassium | 0.90 (0.85-0.94) | 1.04 (0.99-1.08) | 0.90 (0.87-0.94) | 0.85 (0.78-0.92) | 0.79 (0.69-0.91) | 0.90 (0.83-0.98) | 0.99 (0.95-1.02) | 0.98 (0.95-1.02) | 0.98 (0.91-1.06) | 0.92 (0.87-0.97) |
| Log2 protein | 0.86 (0.82-0.90) | 1.02 (0.98-1.06) | 0.91 (0.86-0.95) | 0.79 (0.72-0.86) | 0.77 (0.67-0.88) | 0.90 (0.82-0.98) | 0.94 (0.91-0.98) | 0.94 (0.90-0.98) | 0.94 (0.87-1.02) | 0.89 (0.84-0.93) |
| Log2 phosphorus | 0.86 (0.76-0.89) | 0.99 (0.93-1.06) | 0.87 (0.82-0.92) | 0.73 (0.63-0.83) | 0.67 (0.55-0.82) | 0.84 (0.74-0.96) | 0.93 (0.88-0.99) | 0.93 (0.88-0.99) | 0.90 (0.79-1.01) | 0.83 (0.76-0.90) |
| Log2 calcium | 0.88 (0.83-0.94) | 1.08 (1.03-1.13) | 0.89 (0.85-0.93) | 0.83 (0.75-0.92) | 0.85 (0.73-1.00) | 0.93 (0.84-1.02) | 0.99 (0.95-1.04) | 1.00 (0.96-1.05) | 0.93 (0.85-1.01) | 0.96 (0.90-1.02) |
| Log2 iron | 0.94 (0.90-0.98) | 1.01 (0.98-1.05) | 0.94 (0.91-0.97) | 0.87 (0.80-0.94) | 0.80 (0.72-0.90) | 0.90 (0.84-0.97) | 0.98 (0.95-1.02) | 0.99 (0.96-1.02) | 0.94 (0.88-1.00) | 0.94 (0.90-0.98) |
| Log2 MUFA | 0.91 (0.88-0.95) | 1.02 (0.99-1.05) | 0.93 (0.90-0.96) | 0.89 (0.84-0.95) | 0.94 (0.86-1.03) | 0.98 (0.93-1.04) | 0.99 (0.96-1.02) | 0.99 (0.96-1.02) | 0.95 (0.89-1.01) | 0.96 (0.92-0.99) |
| Log2 PUFA | 0.94 (0.91-0.98) | 1.03 (0.99-1.06) | 0.95 (0.93-0.99) | 0.90 (0.84-0.96) | 0.87 (0.78-0.96) | 0.97 (0.91-1.04) | 0.96 (0.93-0.99) | 0.96 (0.93-0.99) | 0.95 (0.89-1.02) | 0.92 (0.88-0.96) |
| Log2 n-3 | 0.95 (0.92-0.98) | 1.03 (0.99-1.06) | 0.98 (0.96-1.01) | 0.94 (0.89-0.99) | 0.86 (0.79-0.94) | 0.97 (0.92-1.03) | 0.98 (0.96-1.01) | 0.98 (0.95-1.01) | 0.98 (0.93-1.04) | 0.93 (0.90-0.96) |
| Log2 n-6 | 0.95 (0.91-0.99) | 1.02 (0.99-1.05) | 0.95 (0.92-0.98) | 0.90 (0.84-0.96) | 0.89 (0.80-0.99) | 0.98 (0.91-1.04) | 0.97 (0.93-1.00) | 0.97 (0.93-1.00) | 0.95 (0.88-1.01) | 0.93 (0.88-0.97) |
| Log2 water | 0.86 (0.82-0.90) | 1.02 (0.98-1.06) | 0.91 (0.88-0.95) | 0.79 (0.72-0.86) | 0.77 (0.67-0.88) | 0.90 (0.82-0.98) | 0.94 (0.91-0.98) | 0.94 (0.90-0.98) | 0.94 (0.87-1.02) | 0.86 (0.84-0.93) |
| Log2 fiber | 1.00 (0.91-1.10) | 1.05 (0.98-1.13) | 0.89 (0.83-0.96) | 0.84 (0.70-0.99) | 0.71 (0.55-0.91) | 0.90 (0.76-1.05) | 1.01 (0.94-1.09) | 1.01 (0.93-1.09) | 0.94 (0.80-1.10) | 0.83 (0.74-0.92) |
| FFQ | ||||||||||
| Green vegetables(%) | ||||||||||
| Low consumption | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Moderate consumption | 0.93 (0.64-1.34) | 0.87 (0.75-1.02) | 0.86 (0.65-1.08) | 1.04 (0.48-2.28) | 1.91 (0.66-5.53) | 1.45 (0.75-2.84) | 1.07 (080-1.43) | 0.87 (0.75-1.02) | 0.82 (0.57-1.19) | 0.97 (0.68-1.39) |
| High consumption | 0.71 (0.56-0.89) | 0.67 (0.51-0.89) | 0.84 (0.74-0.99) | 0.64 (0.37-1.12) | 1.18 (0.55-2.52) | 1.15 (0.74-1.78) | 0.89 (0.74-1.06) | 0.87 (0.71-1.06) | 0.56 (0.26-1.20) | 0.74 (0.59-0.94) |
| Other vegetables(%) | ||||||||||
| Low consumption | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Moderate consumption | 1.23 (0.86-1.76) | 0.90 (0.69-1.20) | 0.82 (0.62-1.08) | 0.96 (0.79-2.40) | 1.83 (0.54-6.15) | 1.38 (0.75-2.84) | 0.99 (0.72-1.36) | 1.01 (0.67-1.01) | 0.87 (0.44-1.72) | 0.90 (0.60-1.35) |
| High consumption | 0.67 (0.52-0.86) | 0.89 (0.76-1.04) | 0.81 (0.69-0.95) | 0.79 (0.46-1.36) | 1.60 (0.77-3.36) | 1.23 (0.56-2.69) | 0.83 (0.69-1.00) | 0.87 (0.71-1.42) | 0.80 (0.54-1.17) | 0.83 (0.66-1.05) |
| Fruits(%) | ||||||||||
| Low consumption | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Moderate consumption | 0.66 (0.48-0.91) | 0.89 (0.73-1.10) | 0.90 (0.74-1.10) | 1.13 (0.61-2.09) | 0.52 (0.12-2.20) | 0.78 (0.42-1.47) | 0.82 (0.65-1.03) | 0.86 (0.67-1.10) | 1.10 (0.71-1.70) | 0.83 (0.61-1.12) |
| High consumption | 0.44 (0.30-0.64) | 0.78 (0.63-0.69) | 0.76 (0.61-0.93) | 0.72 (0.35-1.49) | 0.48 (0.11-2.01) | 0.74 (0.38-1.43) | 0.82 (0.65-1.03) | 0.74 (0.57-0.96) | 0.78 (0.48-1.26) | 0.76 (0.51-1.03) |
| Health-related behaviors | ||||||||||
| Physical activity | ||||||||||
| Not regular | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Regular | 0.93 (0.82-1.05) | 0.99 (0.91-1.10) | 1.02 (0.93-1.11) | 0.88 (0.68-1.13) | 0.58 (0.39-0.87) | 0.97 (0.78-1.21) | 1.03 (0.95-1.13) | 1.04 (0.95-1.14) | 0.88 (0.72-1.07) | 0.92 (0.80-1.04) |
| Smoking | ||||||||||
| Non/ex-smoker | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Current smoker | 0.95 (0.72-1.24) | 1.03 (0.83-1.29) | 1.02 (0.83-1.26) | 0.82 (0.50-1.35) | 0.83 (0.41-1.68) | 0.84 (0.53-1.34) | 0.91 (0.76-1.10) | 0.96 (0.79-1.17) | 0.74 (0.50-1.08) | 1.25 (0.98-1.60) |
| Cotinine verified smokers (%) | ||||||||||
| No | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Yes | 1.38 (1.07-1.77) | 1.08 (0.88-1.32) | 1.04 (0.85-1.27) | 1.51 (0.96-2.39) | 1.43 (0.74-2.77) | 1.38 (0.90-2.11) | 1.03 (0.87-1.22) | 0.94 (0.79-1.17) | 1.60 (1.15-2.23) | 1.23 (0.99-1.55) |
| High-risk drinking status (%) | ||||||||||
| No | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Yes | 1.49 (1.23-1.79) | 1.38 (1.17-1.62) | 0.99 (0.84-1.17) | 1.44 (0.82-2.51) | 2.54 (0.62-10.51) | 1.17 (0.71-1.95) | 1.07 (0.93-1.23) | 1.05 (0.90-1.22) | 1.19 (0.88-1.61) | 1.22 (1.02-1.47) |
Diabetes: adjusted for age group (≤29, 30-39, 40-49, 50-59, 60-69, 70-79, ≥80), sex (male, female), monthly household income (<2000, ≥2000 and <4000, ≥4000 and <6000, ≥6000), physical activity (not regular, regular), BMI (<18.5, ≥18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine-verified smoker (yes, no), smoking status (non/ex-smoker, current smoker), occupation (white-collar, blue-collar, none), education levels (≤middle school, high school, ≥college), energy intake (Kcal), family history of diabetes (yes, no).
Dyslipidemia: adjusted for age group (≤29, 30-39, 40-49, 50-59, 60-69, 70-79, ≥80), sex (male, female), monthly household income (<2000, ≥2000 and <4000, ≥4000 and <6000, ≥6000), physical activity (not regular, regular), BMI (<18.5, ≥18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine, physical activity (not regular, regular), BMI (<18.5, ≥ 18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine-verified smoker (yes, no), smoking status (non/ex-smoker, current smoker), occupation (white-collar, blue-collar, none), education levels (≤middle school, high school, ≥college), energy intake (Kcal), family history of dyslipidemia (yes, no).
Hypertension, stroke, MI or angina, and MI: adjusted for age group (≤29, 30-39, 40-49, 50-59, 60-69, 70-79, ≥80), sex (male, female), monthly household income (<2000, ≥2000 and <4000, ≥4000 and <6000, ≥6000), physical activity (not regular, regular), BMI (<18.5, ≥18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine, physical activity (not regular, regular), BMI (<18.5, ≥18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine-verified smoker (yes, no), smoking status (non/ex-smoker, current smoker), occupation (white-collar, blue-collar, none), education levels (≤middle school, high school, ≥college), energy intake (Kcal), family history of diabetes, dyslipidemia, and CVD (yes, no).
Arthritis, rheumatoid arthritis, osteoarthritis and depression: adjusted for age group (≤29, 30-39, 40-49, 50-59, 60-69, 70-79, ≥80), sex (male, female), monthly household income (<2000, ≥2000 and <4000, ≥4000 and <6000, ≥6000), physical activity (not regular, regular), BMI (<18.5, ≥18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine, physical activity (not regular, regular), BMI (<18.5, ≥18.5 and <25, ≥ 25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine-verified smoker (yes, no), smoking status (non/ex-smoker, current smoker), occupation (white-collar, blue-collar, none), education levels (≤middle school, high school, ≥college), energy intake (Kcal).
Health-related behaviors: adjusted for age group (≤29, 30-39, 40-49, 50-59, 60-69, 70-79, ≥80), sex (male, female), monthly household income (<2000, ≥2000 and <4000, ≥4000 and <6000, ≥6000), physical activity (not regular, regular), BMI (<18.5, ≥18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine, BMI (<18.5, ≥18.5 and <25, ≥25 and <30, and ≥30 kg/m2), and smoking status (non/ex-smoker, current smoker), occupation (white-collar, blue-collar, none), education levels (≤middle school, high school, ≥college), energy intake (Kcal), family history of diabetes (yes, no), or high-risk drinking (yes, no) or physical activity (not regular, regular), or cotinine-verified smoker (yes, no).
BMI, body mass index; CVD, cardiovascular disease; FFQ, food frequency questionnaire; MI, myocardial infarction; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; Ref, reference value.
In the interaction model, with a 2-fold increase in daily vitamins B2, C, and A intake in women, the ORs (95% CI) for metabolic equivalents were 0.91 (0.84-0.98), 0.92 (0.88-0.97), and 0.93 (0.89-0.98), respectively. Furthermore, among female adults, a doubling of daily iron, MUFA, PUFA, and n-6 fatty acid intake was significantly associated with a decrease in metabolic equivalents by 8% (OR 0.91; 95% CI, 0.84-0.99), 9% (OR 0.91; 95% CI, 0.84-0.99), 8% (OR 0.92; 95% CI, 0.86-0.98), 8% (OR 0.92; 95% CI, 0.85-0.99), and 91% (OR 0.91; 95% CI, 0.85-0.99), respectively. The interaction of sex in the association between NCDs with daily intakes of multiple individual nutrients is provided in Table 3 .
Table 3.
Interaction of sex in the association between non-communicable diseases with vitamin and nutrient intake
| Variables | Diabetes n = 5143 |
Hypertension n = 13,518 |
Stroke n = 1326 |
MI n = 567 |
Arthritis n = 7774 |
Osteoarthritis n = 6913 |
|---|---|---|---|---|---|---|
| Vitamin | ||||||
| Log2 vitamin B1# sex | – | Log2 vitamin B1# males (Ref) | – | – | Log2 vitamin B1# males (Ref) | Log2 vitamin B1# males (Ref) |
| 0.92 (0.86-0.99) | 0.86 (0.79-0.94) | 0.88 (0.81-0.96) | ||||
| Log2 vitamin B2# sex | Log2 vitamin B2# males (Ref) | Log2 vitamin B2# males (Ref) | – | Log2 vitamin B2# males (Ref) | – | – |
| 0.91 (0.84-0.98) | 0.85 (0.80-0.91) | 0.79 (0.64-0.98) | ||||
| Log2 vitamin B3# sex | – | Log2 vitamin B3# males (Ref) | – | – | Log2 vitamin B3# males (Ref) | – |
| 0.90 (0.84-0.98) | 0.89 (0.82-0.97) | |||||
| Log2 vitamin C# sex | Log2 vitamin C# males (Ref) | Log2 vitamin C# males (Ref) | – | – | – | – |
| 0.92 (0.88-0.97) | 0.96 (0.92-0.99) | |||||
| Log2 vitamin A# sex | Log2 vitamin A# males (Ref) | – | – | – | – | – |
| 0.93 (0.89-0.98) | ||||||
| 24-h recall | ||||||
| Log2 potassium# sex | – | Log2 potassium# males (Ref) | – | – | – | – |
| 0.90 (0.84-0.97) | ||||||
| Log2 protein# sex | – | Log2 protein# males (Ref) | – | – | Log2 protein# males (Ref) | Log2 protein# males (Ref) |
| 0.89 (0.83-0.96) | 0.86 (0.79-0.94) | 0.89 (0.81-0.98) | ||||
| Log2 phosphorus# sex | – | – | – | – | Log2 phosphorus# males (Ref) | |
| 0.86 (0.75-0.98) | ||||||
| Log2 calcium# sex | Log2 calcium# sex (male) | Log2 calcium# sex (male) | – | – | – | – |
| 0.86 (0.77-0.96) | 0.85 (0.78-0.93) | |||||
| Log2 iron# sex | Log2 iron# males (Ref) | – | – | – | – | – |
| 0.91 (0.84-0.99) | ||||||
| Log2 MUFA# sex | Log2 MUFA# males (Ref) | Log2 MUFA# males (Ref) | Log2 MUFA# males (Ref) | – | – | – |
| 0.92 (0.86-0.98) | 0.86 (0.82-0.90) | 0.89 (0.79-0.99) | ||||
| Log2 PUFA# sex | Log2 PUFA# males (Ref) | Log2 PUFA# males (Ref) | Log2 PUFA# males (Ref) | Log2 PUFA# males (Ref) | – | – |
| 0.92 (0.85-0.99) | 0.86 (0.81-0.91) | 0.85 (0.75-0.98) | 0.80 (0.66-0.98) | |||
| Log2 n-3fatty acid # sex | – | – | Log2 n-3 fatty acid# males (Ref) | – | – | – |
| 0.89 (0.80-0.99) | ||||||
| Log2 n-6 fatty acid# sex | Log2 n-6 fatty acid# males (Ref) | Log2 n-6 fatty acid# males (Ref) | Log2 n-6 fatty acid# males (Ref) | Log2 n-6 fatty acid# males (Ref) | – | – |
| 0.91 (0.85-0.99) | 0.85 (0.80-0.90) | 0.86 (0.75-0.98) | 0.78 (0.64-0.96) | |||
| Log2 water# sex | – | Log2 water# males (Ref) | – | – | Log2 water# males (Ref) | Log2 water# males (Ref) |
| 0.89 (0.83-0.96) | 0.86 (0.79-0.94) | 0.88 (0.81-0.98) |
BMI, body mass index; MI, myocardial infarction; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Diabetes: adjusted for age group (≤29, 30-39, 40-49, 50-59, 60-69, 70-79, ≥80), sex (male, female), monthly household income (<2000, ≥2000 and <4000, ≥4000 and <6000, ≥6000), physical activity (not regular, regular), BMI (<18.5, ≥18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine-verified smoker (yes, no), smoking status (non/ex-smoker, current smoker), occupation (white-collar, blue-collar, none), education levels (≤middle school, high school, ≥college), energy intake (Kcal), family history of diabetes (yes, no).
Hypertension, stroke, and MI: adjusted for age group (≤29, 30-39, 40-49, 50-59, 60-69, 70-79, ≥80), sex (male, female), monthly household income (<2000, ≥2000 and <4000, ≥4000 and <6000, ≥6000), physical activity (not regular, regular), BMI (<18.5, ≥18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine-verified smoker (yes, no), smoking status (non/ex-smoker, current smoker), occupation (white-collar, blue-collar, none), education levels (≤middle school, high school, ≥college), energy intake (Kcal), family history of diabetes, dyslipidemia and CVDs (yes, no).
Arthritis and osteoarthritis: adjusted for age group (≤29, 30-39, 40-49, 50-59, 60-69, 70-79, ≥80), sex (male, female), monthly household income (<2000, ≥2000 and <4000, ≥4000 and <6000, ≥6000), physical activity (not regular, regular), BMI (<18.5, ≥ 18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine-verified smoker (yes, no), smoking status (non/ex-smoker, current smoker), occupation (white-collar, blue-collar, none), education levels (≤middle school, high school, ≥college), energy intake (Kcal).#: interaction between two variables.
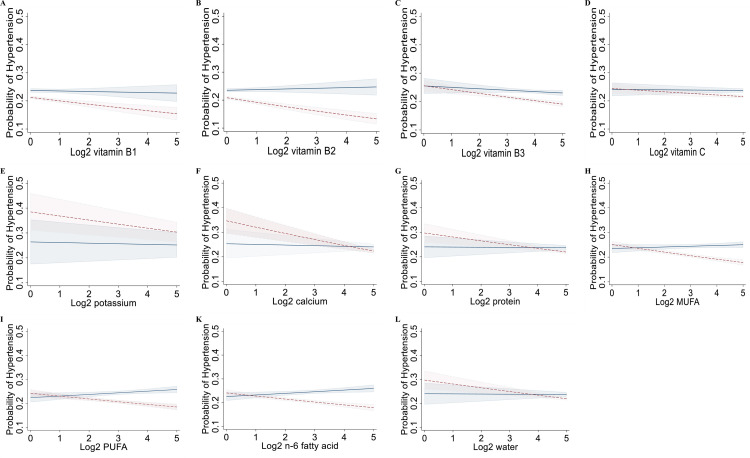
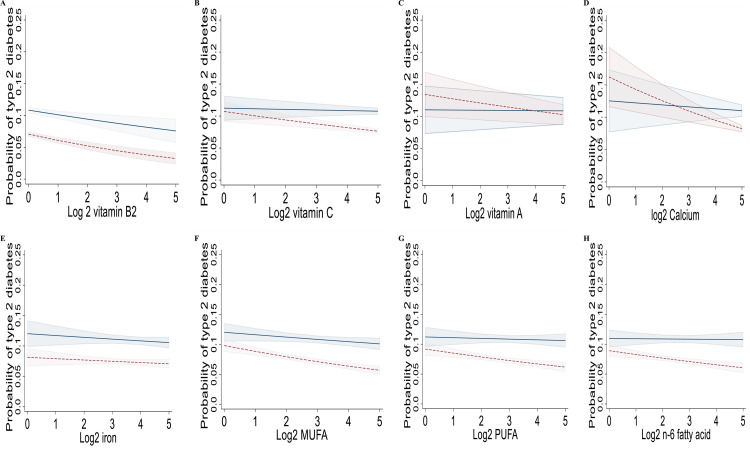
Figure 1A-H and Fig. 1A-L show the marginal effects of daily levels of multiple individual nutrients on hypertension and T2DM by sex in the Korean population after adjustment for potential cofounders. The associations between the intakes of multiple individual nutrients showed similar trends. Intakes of multiple individual nutrients had negative associations with the prevalence of hypertension and T2DM.
Figure 1.
Marginal effects of multiple individual nutrient intakes on hypertension (A-L) by sex after adjustment for potential cofounders in the interaction model in the population (n= 56,462), a cross-sectional study, Korean National Health and Nutrition Examination Survey, Korea, 2009–2019. The margin effect showed higher intakes of multiple individual nutrients (e.g., vitamins B1, B2; C, potassium, calcium, protein, MUFA, PUFA, and n-6 fatty acid) were associated with a lower risk of hypertension in almost all females. Models were adjusted for age group (<29, 30-39, 40-49, 50-59, 60-69, 70-79, >80), sex (male, female), monthly household income (<2000, ≥2000 and <4000, ≥4000 and <6000, ≥6,000), physical activity (not regular, regular), BMI (<18.5, ≥18.5 and <25, ≥25 and <30, and ≥30 kg/m2), high-risk drinking (yes, no), and cotinine-verified smoker (yes, no), smoking status (non/ex-smoker, current smoker), occupation (white-collar, blue-collar, none), education levels (≤middle school, high school, ≥college), energy intake (Kcal), family history of diabetes, dyslipidemia, and CVD (yes, no). BMI, body mass index; CVD, cardiovascular disease; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid. —, males, —-, females.
Figure 2.
Marginal effects of multiple individual nutrient intakes on type 2 diabetes (A-H) by gender after adjustment for potential cofounders in the interaction model in the population (n= 56,462), a cross-sectional study, Korean National Health and Nutrition Examination Survey, Korea, 2009–2019. The margin effect showed higher intakes of multiple individual nutrients (e.g., vitamins B2, C, A; calcium, iron, MUFA, PUFA, and n-6 fatty acid) were associated with a lower risk of type 2 diabetes in almost all females. Models were adjusted for age group (≤29, 30-39, 40-49, 50-59, 60-69, 70-79, ≥80), sex (male, female), monthly household income (<2000, ≥2000 and <4000, ≥4000 and <6000, ≥6000), physical activity (not regular, regular), BMI (<18.5, ≥ 18•5 and < 25, ≥ 25 and < 30, and ≥ 30 Kg/m2), high-risk drinking (yes, no), and cotinine-verified smoker (yes, no), smoking status (non/ex-smoker, current smoker), occupation (white-collar, blue-collar, none), education levels (≤middle school, high school, ≥college), energy intake (Kcal), and family history of diabetes (yes, no). BMI, body mass index; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid. —, males, —-, females.
4. Discussion
Our hypothesis that an increased intake of multiple individual nutrients, fruits, or vegetables, was linked with the lower risk of CVC, T2DM, arthritis, and depression in the Korean population was confirmed by this study. Our study found a negative relationship between intakes of multiple individual nutrients, vegetables, and fruits and CVC, T2DM, arthritis, and depression in adults. An increase in daily vitamin (vitamins A, B1, B2, B3, C) and nutrient (potassium, protein, phosphorus, calcium, iron, MUFA, PUFA, n-3 fatty acid, n-6 fatty acid, water) intakes was associated with a lower prevalence of T2DM, hypertension, stroke, myocardial infarction, arthritis, and osteoarthritis, only in females. High fruit or vegetable consumption was linked with the low risk of cardiovascular diseases, T2DM, arthritis, and depression in both women and men.
Diet is the most important modifiable factor in the prevention and management of NCDs. Evidence has reported an inverse association between NCDs (e.g., CVD, T2DM, arthritis, depression) and dietary patterns rich in multiple individual nutrients, fruits, and vegetables. Our study found that an increase in daily multiple individual nutrients, fruit, and vegetable intake was associated with a decrease in CVDs, which was in line with previous studies. Specifically, a Chinese study showed that consuming fresh fruits daily reduced blood glucose levels by 0.5 mmol/L, systolic blood pressure by 4.0 mmHg, which was negatively related to ischemic stroke (hazard ratio [HR]: 0.75; 95% CI, 0.72-0.79), coronary heart disease (HR: 0.66; 95% CI, 0.58-0.75), and hemorrhagic stroke (HR: 0.64; 95% CI, 0.56-0.74), and the risks of cardiovascular death (HR: 0.60; 95% CI, 0.54-0.67), as compared with subjects who never or rarely consumed fresh fruit [24]. Another cohort study in China also found that higher dietary total fruit and vegetable intake protected against CVD; the HRs for vegetable and fruit intake were 0.94 (95% CI, 0.59-1.50) and 0.62 (95% CI, 0.37-1.03), respectively [25]. Additionally, a Japanese study found that regular consumption of citrus fruit may reduce the risk of CVD, especially stroke and cerebral infarction; the HR for almost daily intake versus infrequent intake of citrus fruit was 0.51 (95% CI, 0.29-0.88) in women and 0.57 (95% CI, 0.33-1.01) in men [26]. Furthermore, 2 cohort studies conducted in England and Australia found that eating fruits decreases the risk of total CVD mortality in women aged 35 to 69 and >70 years [27,28]. In terms of T2DM, our study found that increased intakes of multiple individual nutrients, vegetables, and fruits were linked with the low risk of developing T2DM. A systematic review and meta-analysis of 23 prospective cohort studies also showed that a higher intake of fruit (relative risk [RR] 0.91; 95% CI, 0.87-0.96), and green leafy vegetables (RR 0.87; 95% CI, 0.81-0.93), yellow vegetables (RR 0.72; 95% CI, 0.57-0.90), cruciferous vegetables (RR 0.82; 95% CI, 0.67-0.99) is associated with a lower risk of T2DM compared with the lowest intake [29]. Du et al. found consuming fresh fruit daily reduced blood glucose levels by 0.5 mmol/L, compared with subjects who never or rarely consumed fresh fruit [24].
On the other hand, nutrients play an important role in regulating blood pressure and preventing CVD and T2DM. Our study suggested that increased nutrient intake (e.g., potassium, protein, n-3, n-6, MUFA, PUFA, water, vitamins, iron) reduces the risk of CVD (e.g., hypertension, MI or angina, MI, stroke) and T2DM, which concurs with the findings of previous studies. For instance, Ascherio et al. found that increased daily consumption of protein and potassium may have a blood pressure-lowering effect [30]. A recent meta-analysis of prospective cohort studies reported that intake of plant protein was related to a lower risk of all-cause mortality (pooled effect size 0.92; 95% CI, 0.87-0.97) and CVD mortality (pooled hazard ratio 0.88; 95% CI, 0.80-0.96). Our recent study suggested that increased vitamin B1 and B2 intake can reduce the risk of developing metabolic syndrome, which is the most common cause of NCDs [3,4,7]. A cohort study and 2 meta-analyses of randomized controlled trials showed potential benefits of a high-MUFA and PUFA diet in improving metabolic factors (e.g., serum lipids, blood pressure, glycemic control) among both healthy individuals and T2DM patients [31], [32], [33]. A recent study also reported that dietary n-3 and n-6 fatty acid intake was negatively related to the risk of hypertension in US adults [34]. Sciacqua et al. showed that lower levels of serum phosphorus are associated with impaired glucose tolerance and insulin resistance [35]. In the present study, iron intake was negatively associated with the risk of CVDs and T2DM. A literature review showed the association between iron and an increased risk of CVDs and T2DM [36]. Fernandez-Cao et al. also reported that high dietary intake of heme iron was related to an increased risk of developing type 2 diabetes in a Mediterranean population at high cardiovascular risk [37]. A meta-analysis of 11 prospective studies found dietary total iron, non-heme iron, or supplemental iron intakes were not related to the risk of developing T2DM [38]. Thus, further prospective studies with robust evaluations of dietary and supplement sources of iron intake and CVD and T2DM outcomes are required.
Arthritis is a public health concern that affects a significant majority of the population and is related to a lower quality of life. Our study found that intakes of multiple individual nutrients, fruits, and vegetables were negatively associated with arthritis. This is supported by a previous cross-sectional study of 6588 subjects aged ≥50 years suggesting that a higher consumption of vegetables and fruits was related to a reduced prevalence of severe knee pain (OR 0.59; 95% CI, 0.48-0.73) compared with a lower consumption of vegetables and fruits [39]. The anti-inflammatory function of B vitamins is directly associated with their pain modulatory role [40]. These findings suggested that increased vitamin B1 and B3 intake could affect pain alleviation and inflammation. Jalal et al. reported that vitamin B1 (150 and 200 mg/kg) may reduce thermal hyperalgesia, paw edema, and serum levels of tumor necrosis factor-α and interleukin-1β during complete Freund adjuvant-induced arthritis in rats [40]. On the other hand, we found increased intakes of protein, water, and PUFA were negatively related to the risk of developing arthritis and osteoarthritis. PUFA has been shown to provide therapeutic benefits for arthritis patients due to its anti-inflammatory properties. Thomas et al. suggested that increased consumption of long-chain n-3 fatty-acids (oily fish/fish oil supplements) could improve function and pain in osteoarthritis patients [41]. However, a recent study found no links between n-6 PUFA and the consequences of arthritis [42]. Therefore, more prospective studies with robust assessments of dietary and supplement sources of PUFA intake and arthritis consequences are required.
We also found increased protein intake was negatively related to the risk of developing arthritis and osteoarthritis. This finding disagreed with previous studies. Benito-Garcia et al. reported that there is no association between protein and the risk of rheumatoid arthritis [43]. Besides, Pattiso et al. also showed subjects consuming the highest levels of total protein (OR 2.9; 95% CI, 1.1-7.5) had a higher risk of inflammatory polyarthritis compared with subjects with lower protein intakes [44]. Of note, Zwart et al. reported lower protein intake was related to lower upper leg muscle strength. However, Nicola et al. found high dietary protein intake could increase the risk of falls in older persons [45,46]. These findings show that the role of protein intake in arthritis remains unclear. Thus, further work is needed to explore the association between these relationships.
There has been an increase in research interest in the impact of dietary behavior on mental health outcomes in recent years [22]. Our study found that higher consumption of vitamins, nutrients, and vegetables is associated with a significantly lower risk of depression development, which is consistent with previous studies. A systematic review and meta-analysis of 27 articles including 16 cross-sectional, 9 cohort, and 2 case-control studies on fruit, vegetables, and/or total fruit and vegetable consumption for depression [47]. The results showed that consumption of vegetables was related to a 14% lower risk of depression (overall RR 0.86; 95% CI, 0.75-0.98) in cohort studies and a 25% lower risk of depression (overall RR 0.75; 95% CI, 0.62-0.91) in cross-sectional studies. A higher intake of fruits has reduced the risk of depression by 17% (RR 0.83; 95% CI, 0.71-0.98) in cohort studies and 24% (RR 0.76; 95% CI, 0.63-0.92) in cross-sectional studies [48]. B vitamins are essential in the neurochemical pathways involving dopaminergic, noradrenergic, serotonergic, and cholinergic systems as well as GABA and glutamate neurotransmitter systems. Our recent study found that increased daily vitamin B1 intake can reduce the prevalence of depression [4]. A literature review also showed B vitamins (e.g., vitamins B1, B3, B6, B9, B12) are vital for neuronal function, and insufficiencies have been related to depression [49]. Although the mechanism underlying depression and vitamins A and C is unclear, it is possible to speculate that it is related to oxidative stress and inflammation. A meta-analysis reported that vitamin C deficiency has been related to cognitive impairment and depression [50]. In the present study, we found increased daily vitamin A intake was negatively related to the risk of depression. Our result supports a previous study, in which low serum levels of carotenoids are related to depressive symptoms and increase the risk of developing new depressive symptoms in older persons [51].
There have been a few studies that have identified sex differences in the association between intake of multiple individual nutrients and CVDs. Our study found that intakes of multiple individual nutrients were linked with a lower risk of CVDs (e.g., hypertension, stroke, MI, MI or angina), diabetes and dyslipidemia in women, but not in men. It could be explained that multiple individual nutrients, fruits, and vegetables may modulate molecular events and signaling pathways associated with reducing lipid metabolism disorders, correcting endothelial dysfunction, suppressing platelet function, inhibiting thrombosis, antihypertension, alleviating ischemia/reperfusion injury, reducing oxidative stress, and inhibiting inflammatory responses [51]. Antioxidant vitamins (vitamins C, E, and A), for example, and B vitamins can reduce oxidative stress, which may play a key role in the pathogenesis of atherosclerosis and CVDs [52]. On the other hand, B vitamins have analgesic and anti-inflammatory effects that are related to opioid-like mechanisms and are mediated through the release of nitrite oxide [53]. B vitamins may increase the activity of 5-HT, as well as the effect and/or availability of norepinephrine, which contributes to pain transmission as an inhibitory pain transmitter [54]. Vitamin D can also induce cardioprotective effects and plays an important role in maintenance of cardiovascular health [55]. n-3 and n-6 fatty acids may reduce blood cholesterol and/or triglycerides, esterify cholesterol, reduce blood viscosity, improve blood microcirculation, and reduce platelet aggregation and/or thrombosis [56]. These findings support the current recommendation of increasing multiple individual nutrients, fruit and vegetable intake to lower the risk of NCDs.
We found that health-related behaviors such as smoking and alcohol consumption are related to NCDs. Of note, regular physical activity may reduce the risk of developing MI. Ying et al. suggested that exercise training after MI can improve quality of life, positive effects, circulation function, heart rate, metabolic equivalents, and lower the risk of all-cause mortality [57]. Taken together, exercise should be implemented and widely applied in clinics to help MI patients.
To the best of our knowledge, this large-scale Korean study is the first to report the association between intakes of multiple individual nutrients, fruits, and vegetables, and NCDs at a national level. However, our study has several limitations. First, the cross-sectional method used prevented the assessment of causality between NCDs and multiple individual nutrients, fruit, and vegetable intakes. Second, because no physiological antioxidant status markers were assessed during KNHANES, oxidation status and multiple individual nutrient levels in serum and tissue were not evaluated. Several serum vitamins, including vitamin E and vitamin D, were assessed. Third, intakes of multiple individual nutrients were evaluated based on 24-hour recall, and, therefore, may have been under or overestimated. A 24-hour recall, on the other hand, was discovered to be an effective method of evaluating food intake, and all subjects were instructed to maintain their usual dietary habits before the evaluations. Fourth, data on vegetables and fruits were only available from 2012 to 2016. Fifth, these findings might be less relevant to other ethnicities.
In summary, NCDs have become a serious issue worldwide as well as in Korea. The study shows increased intakes of multiple individual nutrients, fruit, and vegetables were linked with a lower risk of NCDs and may protect the public against the burden of NCDs. These findings may inspire health policymakers and public health practitioners to invest in preventative and control strategies. However, further studies are required to determine whether interactions between multiple individual nutrients, fruit and vegetable intakes influence the prevalence of NCDs.
Author Declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Sources of Support
This work was supported by a Research Promotion Program of Sunchon National University.
Author Contributions
H.D.N. conceived and designed the study. H.D.N., M.S.K., and H.O., contributed to the collection of the data. H.D.N. analyzed, wrote and revised the paper. All authors contributed to approve the final manuscript.
References
- 1.WHO World Health Statistics. 2018 https://www.who.int/docs/default-source/gho-documents/world-health-statistic-reports/6-june-18108-world-health-statistics-2018.pdf Accessed on 18.06.2021. Available at. [Google Scholar]
- 2.WHO Noncommunicable diseases. 2021 https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases Accessed on 18.06.2021. Available at. [Google Scholar]
- 3.Duc HN, Oh H, Kim MS. Effects of antioxidant vitamins, curry consumption, and heavy metal levels on metabolic syndrome with comorbidities: a Korean community-based cross-sectional study. Antioxidants (Basel) 2021:10. doi: 10.3390/antiox10050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duc HN, Oh H, Yoon IM, Kim M-S. Association between levels of thiamine intake, diabetes, cardiovascular diseases and depression in Korea: a national cross-sectional study. J Nutr Sci. 2021;10:e31. doi: 10.1017/jns.2021.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney J. Food consumption trends and drivers. Philos Trans R Soc Lond B Biol Sci. 2010;365:2793–2807. doi: 10.1098/rstb.2010.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alliance WCRFIaTN The link between food, nutrition, diet and non-communicable diseases. 2014 https://www.eldis.org/document/A69772 Accessed on 18.06.2021. Available at. [Google Scholar]
- 7.Nguyen HD, Kim MS. Effects of heavy metal, vitamin, and curry consumption on metabolic syndrome during menopause: a Korean community-based cross-sectional study. Menopause. 2021;28:1. doi: 10.1097/GME.0000000000001825. [DOI] [PubMed] [Google Scholar]
- 8.Iriti M, Varoni EM, Vitalini S. Healthy diets and modifiable risk factors for non-communicable diseases-the European perspective. Foods. 2020;9 doi: 10.3390/foods9070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdulrazaq M, Innes JK, Calder PC. Effect of ω-3 polyunsaturated fatty acids on arthritic pain: a systematic review. Nutrition. 2017;39-40:57–66. doi: 10.1016/j.nut.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Senftleber NK, Nielsen SM, Andersen JR, Bliddal H, Tarp S, Lauritzen L, et al. Marine oil supplements for arthritis pain: a systematic review and meta-analysis of randomized trials. Nutrients. 2017;9 doi: 10.3390/nu9010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kostoglou-Athanassiou I, Athanassiou L, Athanassiou P. The effect of omega-3 fatty acids on rheumatoid arthritis. Mediterr J Rheumatol. 2020;31:190–194. doi: 10.31138/mjr.31.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skoczyńska M, Świerkot J. The role of diet in rheumatoid arthritis. Reumatologia. 2018;56:259–267. doi: 10.5114/reum.2018.77979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felson DT, Misra D, LaValley M, Clancy M, Chen X, Lichtenstein A, et al. Fatty acids and osteoarthritis: the MOST study. Osteoarthritis Cartilage. 2021 doi: 10.1016/j.joca.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.KCDC The National Health and Nutrition Examination Survey. Approval of Research Ethics Review. 2021 Accessed on 18.06.2021. Available at https://knhaneskdcagokr/knhanes/sub03/sub03_06_moddo. [Google Scholar]
- 15.Kweon S, Kim Y, Jang M-j, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES) Int J Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KCDC The National Health and Nutrition Examination Survey. Download data analysis guide. 2021 https://knhanes.kdca.go.kr/knhanes/sub03/sub03_06_02.do Accessed on 18.06.2021. Available at. [Google Scholar]
- 17.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of Vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 18.NCBI Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. 2021 https://www.ncbi.nlm.nih.gov/books/NBK222318/ Accessed on 18.06.2021. Available at. [Google Scholar]
- 19.Péter S, Friedel A, Roos FF, Wyss A, Eggersdorfer M, Hoffmann K, Weber P. A systematic review of global alpha-tocopherol status as assessed by nutritional intake levels and blood serum concentrations. Int J Vitam Nutr Res. Dec 2015;85(5-6):261–281. doi: 10.1024/0300-9831/a000281. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 21.Yun S, Nguyen Duc H, Park JS, Oh C, Kim MS. The association between metabolic syndrome and iron status in pre-and postmenopausal women: KNHANES in 2012. Br J Nutr. 2021:1–23. doi: 10.1017/S0007114521001331. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen HD, Oh H, Hoang NHM, Jo WH, Kim MS. Environmental science and pollution research role of heavy metal concentrations and vitamin intake from food in depression: a national cross-sectional study (2009-2017) Environ Sci Pollut Res Int. 2021:1–13. doi: 10.1007/s11356-021-15986-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosmer DW, Jr, Lemeshow S, Sturdivant RX. John Wiley & Sons; 2013. Applied logistic regression. [DOI] [Google Scholar]
- 24.Du H, Li L, Bennett D, Guo Y, Key TJ, Bian Z, et al. Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med. 2016;374:1332–1343. doi: 10.1056/NEJMoa1501451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu D, Zhang X, Gao YT, Li H, Yang G, Huang J, et al. Fruit and vegetable intake and risk of CHD: results from prospective cohort studies of Chinese adults in Shanghai. Br J Nutr. 2014;111:353–362. doi: 10.1017/S0007114513002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada T, Hayasaka S, Shibata Y, Ojima T, Saegusa T, Gotoh T, et al. Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi Medical School cohort study. J Epidemiol. 2011;21:169–175. doi: 10.2188/jea.je20100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodgson JM, Prince RL, Woodman RJ, Bondonno CP, Ivey KL, Bondonno N, et al. Apple intake is inversely associated with all-cause and disease-specific mortality in elderly women. Br J Nutr. 2016;115:860–867. doi: 10.1017/S0007114515005231. [DOI] [PubMed] [Google Scholar]
- 28.Lai HT, Threapleton DE, Day AJ, Williamson G, Cade JE, Burley VJ. Fruit intake and cardiovascular disease mortality in the UK Women's Cohort Study. Eur J Epidemiol. 2015;30:1035–1048. doi: 10.1007/s10654-015-0050-5. [DOI] [PubMed] [Google Scholar]
- 29.Wang PY, Fang JC, Gao ZH, Zhang C, Xie SY. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: a meta-analysis. J Diabetes Investig. 2016;7:56–69. doi: 10.1111/jdi.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ascherio A, Rimm EB, Hernán MA, Giovannucci EL, Kawachi I, Stampfer MJ, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998;98:1198–1204. doi: 10.1161/01.cir.98.12.1198. [DOI] [PubMed] [Google Scholar]
- 31.Miller M, Sorkin JD, Mastella L, Sutherland A, Rhyne J, Donnelly P, et al. Poly is more effective than monounsaturated fat for dietary management in the metabolic syndrome: the muffin study. J Clin Lipidol. 2016;10:996–1003. doi: 10.1016/j.jacl.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on glycaemic control in patients with abnormal glucose metabolism: a systematic review and meta-analysis. Ann Nutr Metab. 2011;58:290–296. doi: 10.1159/000331214. [DOI] [PubMed] [Google Scholar]
- 33.Qian F, Korat AA, Malik V, Hu FB. Metabolic effects of monounsaturated fatty acid-enriched diets compared with carbohydrate or polyunsaturated fatty acid-enriched diets in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes care. 2016;39:1448–1457. doi: 10.2337/dc16-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Sun B, Zhang D. Association of dietary n3 and n6 fatty acids intake with hypertension: NHANES 2007-2014. Nutrients. 2019;11:1232. doi: 10.3390/nu11061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sciacqua A, Perticone M, Cimellaro A, Tassone EJ, Tripepi G, Andreucci M, et al. Multiplicative effect of serum phosphorus levels and insulin resistance on hypertensive vascular stiffness. Thromb Haemost. 2016;115:227–229. doi: 10.1160/TH15-04-0349. [DOI] [PubMed] [Google Scholar]
- 36.Basuli D, Stevens RG, Torti FM, Torti SV. Epidemiological associations between iron and cardiovascular disease and diabetes. Front Pharmacol. 2014;5:117. doi: 10.3389/fphar.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Cao JC, Arija V, Aranda N, Bullo M, Basora J, Martínez-González MA, et al. Heme iron intake and risk of new-onset diabetes in a Mediterranean population at high risk of cardiovascular disease: an observational cohort analysis. BMC public health. 2013;13:1042. doi: 10.1186/1471-2458-13-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao W, Rong Y, Rong S, Liu L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta-analysis. BMC Med. 2012;10:119. doi: 10.1186/1741-7015-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han HS, Chang CB, Lee DC, Lee JY. Relationship between total fruit and vegetable intake and self-reported knee pain in older adults. J Nutr Health Aging. 2017;21:750–758. doi: 10.1007/s12603-016-0842-7. [DOI] [PubMed] [Google Scholar]
- 40.Zaringhalam J, Akbari A, Zali A, Manaheji H, Nazemian V, Shadnoush M, et al. Long-term treatment by vitamin B(1) and reduction of serum proinflammatory cytokines, hyperalgesia, and paw edema in adjuvant-induced arthritis. Basic Clin Neurosci. 2016;7:331–340. doi: 10.15412/J.BCN.03070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas S, Browne H, Mobasheri A, Rayman MP. What is the evidence for a role for diet and nutrition in osteoarthritis? Rheumatology (Oxford) 2018 May 1;57(suppl_4):iv61–iv74. doi: 10.1093/rheumatology/key011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krok-Schoen JL, Brasky TM, Hunt RP, Rohan TE, Baker TA, Li W, et al. Dietary long-chain n-3 fatty acid intake and arthritis risk in the Women's Health Initiative. J Acad Nutr Diet. 2018;118:2057–2069. doi: 10.1016/j.jand.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benito-Garcia E, Feskanich D, Hu FB, Mandl LA, Karlson EW. Protein, iron, and meat consumption and risk for rheumatoid arthritis: a prospective cohort study. Arthritis Res Ther. 2007;9:R16. doi: 10.1186/ar2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pattison DJ, Symmons DP, Lunt M, Welch A, Luben R, Bingham SA, et al. Dietary risk factors for the development of inflammatory polyarthritis: evidence for a role of high level of red meat consumption. Arthritis Rheum. 2004;50:3804–3812. doi: 10.1002/art.20731. [DOI] [PubMed] [Google Scholar]
- 45.de Zwart AH, van der Leeden M, Roorda LD, Visser M, van der Esch M, Lems WF, et al. Dietary protein intake and upper leg muscle strength in subjects with knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Rheum. 2019;39:277–284. doi: 10.1002/art.20731. [DOI] [PubMed] [Google Scholar]
- 46.Veronese N, Soysal P, Stubbs B, Maggi S, Jackson SE, Demurtas J, et al. Dietary protein intake and falls in older people: longitudinal analyses from the osteoarthritis initiative. J Am Med Dir Assoc. 2019;20:1623–1627. doi: 10.1016/j.jamda.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Saghafian F, Malmir H, Saneei P, Milajerdi A, Larijani B, Esmaillzadeh A. Fruit and vegetable consumption and risk of depression: accumulative evidence from an updated systematic review and meta-analysis of epidemiological studies. Br J Nutr. 2018;119:1087–1101. doi: 10.1017/S0007114518000697. [DOI] [PubMed] [Google Scholar]
- 48.Mikkelsen K, Stojanovska L, Apostolopoulos V. The effects of vitamin B in depression. Curr Med Chem. 2016;23:4317–4337. doi: 10.2174/0929867323666160920110810. [DOI] [PubMed] [Google Scholar]
- 49.Plevin D, Galletly C. The neuropsychiatric effects of vitamin C deficiency: a systematic review. BMC Psychiatry. 2020;20:315. doi: 10.1186/s12888-020-02730-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milaneschi Y, Bandinelli S, Penninx BW, Corsi AM, Lauretani F, Vazzana R, et al. The relationship between plasma carotenoids and depressive symptoms in older persons. World J Biol Psychiatry. 2012;13:588–598. doi: 10.3109/15622975.2011.597876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao CN, Meng X, Li Y, Li S, Liu Q, Tang GY, et al. Fruits for prevention and treatment of cardiovascular diseases. Nutrients. 2017;9 doi: 10.3390/nu9060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaziano JM. Antioxidant vitamins and cardiovascular disease. Proc Assoc Am Physicians. 1999;111:2–9. doi: 10.1046/j.1525-1381.1999.09229.x. [DOI] [PubMed] [Google Scholar]
- 53.Reyes-García G, Castillo-Henkel C, Medina-Santillán R, Terán-Rosales F, Granados-Soto V. Mechanisms of analgesic action of B vitamins in formalin-induced inflammatory pain. Proc West Pharmacol Soc. 2002;45:144–146. [PubMed] [Google Scholar]
- 54.Medina-Santillán R, Pérez-Flores E, Mateos-García E, Reyes-García G, Granados-Soto V, FJJDdr Flores-Murrieta. AB-Vitamin mixture reduces the requirements of diclofenac after tonsillectomy: a double-blind study. Drug Dev Res. 2005;66:36–39. doi: 10.1002/ddr.20036. [DOI] [Google Scholar]
- 55.Mozos I, Marginean O. Links between vitamin D deficiency and cardiovascular diseases. Biomed Res Int. 2015;2015 doi: 10.1155/2015/109275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bird JK, Calder PC, Eggersdorfer M. The role of n-3 long chain polyunsaturated fatty acids in cardiovascular disease prevention, and interactions with statins. Nutrients. 2018;10:775. doi: 10.3390/nu10060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xing Y, Yang S-D, Wang M-M, Feng Y-S, Dong F, Zhang F. The beneficial role of exercise training for myocardial infarction treatment in elderly. Front Physiol. 2020;11:270. doi: 10.3389/fphys.2020.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]