Abstract
Epidemiologic and mechanistic evidence is increasingly supporting the notion that obstructive sleep apnea is a risk factor for dementia. Hence, the identification of patients at risk of cognitive decline due to obstructive sleep apnea may significantly improve preventive strategies and treatment decision-making. Cerebrospinal fluid and blood biomarkers obtained through genomic, proteomic and metabolomic approaches are improving the ability to predict incident dementia. Therefore, fluid biomarkers have the potential to predict vulnerability to neurodegeneration in individuals with obstructive sleep apnea, as well as deepen our understanding of pathophysiological processes linking obstructive sleep apnea and dementia. Many fluid biomarkers linked to Alzheimer’s disease and vascular dementia show abnormal levels in individuals with obstructive sleep apnea, suggesting that these conditions share common underlying mechanisms, including amyloid and tau protein neuropathology, inflammation, oxidative stress, and metabolic disturbances. Markers of these processes include amyloid-β, tau proteins, inflammatory cytokines, acute-phase proteins, antioxydants and oxidized products, homocysteine and clusterin (apolipoprotein J). Thus, these biomarkers may have the ability to identify adults with obstructive sleep apnea at high risk of dementia and provide an opportunity for therapeutic intervention. Large cohort studies are necessary to establish a specific fluid biomarker panel linking obstructive sleep apnea to dementia risk.
Keywords: Sleep-disordered breathing, Alzheimer’s disease, Vascular dementia, Mild cognitive impairment, Cerebrospinal fluid, Proteomics, Metabolomics, Genomics, Inflammation, Oxidative stress
Glossary of terms
All-cause dementia
Presence of dementia regardless of subtypes, such as Alzheimer’s disease, vascular dementia and others.
Mild cognitive impairment
Objective cognitive impairment that is more severe than is expected for normal aging, but has no or only a minimal impact on activities of the daily living. Mild cognitive impairment can be considered a prodromal stage of Alzheimer’s disease, although it can be caused by other conditions such as vascular and psychiatric diseases. Moreover, it can lead to dementias other than Alzheimer’s disease, remain stable or even return to normal cognitive functioning.
Omics
Large-scale evaluation of biomarkers by molecule types, generally in a discovery-based approach, including genomics, proteomics, and metabolomics.
Preclinical and prodromal Alzheimer’s disease
The preclinical stage corresponds to cognitively normal individuals who will later develop Alzheimer’s disease while the prodromal stage corresponds to individuals with mild cognitive impairment who will later develop Alzheimer’s disease. These stages can be identified longitudinally and may also be of interest in other type of dementias. Moreover, recent recommendations suggest that identifying preclinical and prodromal Alzheimer’s disease is possible at a single time point when abnormal biomarkers levels that are highly suggestive of Alzheimer’s disease pathology are present.
Introduction
Approximately 36 million people worldwide have dementia and this number is expected to reach 115 million in 2050 [1]. Alzheimer’s disease (AD) is the most common type of dementia and is characterized by the aggregation of extracellular amyloid-β (Aβ) and intracellular hyperphosphorylation of tau proteins, leading to amyloid plaques and neurofibrillary tangles respectively [2]. Vascular dementia (VaD) is the second most common type of dementia and is caused by cerebrovascular events [3]. About 20% of dementia cases are mixed, with the presence of both AD and VaD pathophysiological processes [4]. Mild cognitive impairment (MCI) is the presence of cognitive decline with no or mild functional impact that can evolve over time towards dementia [5]. MCI that eventually becomes dementia is called prodromal dementia, although novel recommendations suggest that identifying prodromal dementia is possible at a given point when MCI is combined with abnormal biomarkers levels highly suggestive of dementia pathology [5]. Since the neuropathological process starts years before the clinical manifestations of AD and VaD [6], one of the most important targets is preclinical dementia, i.e., cognitively normal individuals who will later develop dementia. Current research efforts aim to identify preclinical dementia in order to eventually apply disease-modifying therapy [5].
Dementia biomarkers that enable the identification of individuals at risk of dementia are receiving increasing attention. They are biological indicators of risk, or progression towards neurodegenerative processes, and are useful because early diagnosis is central to dementia research and clinical screening [7]. Neuroimaging biomarkers that measure brain atrophy, reduced brain glucose metabolism, or amyloid plaques can detect changes up to 15 years before dementia onset [6], but they have limited availability and are expensive. Cognitive assessment is easy and accessible, but cognitive impairment occurs later in the course of the disease [6]. Fluid biomarkers of dementia are a great alternative to neuroimaging and cognitive biomarkers: they are inexpensive and have the potential to be easily implemented in large cohort studies or used for screening dementia risk in older individuals.
Targeting modifiable risk factors are of utmost importance to reduce the occurrence and progression of dementia. An emerging modifiable risk factor for dementia is obstructive sleep apnea (OSA), which is characterized by respiratory obstructions during sleep [8]. Recently, several cohort studies and a meta-analysis have identified OSA as a risk factor for cognitive decline starting at an earlier age, as well as for MCI and all-cause dementia [9–14]. This review will mainly focus on AD and VaD, because they are the two most common causes of dementia. Moreover, epidemiological and mechanistic evidence suggests a relationship between these two types of dementia and OSA. Because continuous positive airway pressure (CPAP) effectively treats OSA [15], it is a modifiable risk factor of dementia. CPAP may delay the onset of cognitive decline [13], although results on the cognitive effects of CPAP in middle-aged individuals are inconsistent [16]. Some studies showed that CPAP improves neuropsychological functioning over a few weeks [17] and slows the rate of cognitive decline over three years [18] in patients with both AD and OSA. CPAP also improved cognition in stroke patients [19]. With a prevalence in the elderly that reaches over 50% in some studies [20], OSA could become an important target to prevent dementia.
Many pathophysiological mechanisms have been proposed to explain the relationship between OSA and dementia (see Fig. 1). First, recent evidence suggests that the consequences of OSA, namely disturbed sleep and intermittent hypoxia, may induce AD neuropathology. Combined Aβ and tau pathology seems to play a causative role in AD [5]. Thus, processes leading to increased Aβ generation and deposition in the brain, reduced Aβ clearance to the cerebrospinal fluid (CSF), as well as tau hyperphosphorylation may play a role in the pathogenesis of AD. Disrupted slow-wave sleep in healthy adults was associated with increased Aβ levels in the CSF [21], suggesting that disturbed sleep may lead to Aβ generation. Moreover, an innovative animal model demonstrated that sleep plays an important role in the Aβ clearance by enlarging the interstitial space [22], which suggests that disturbed sleep in OSA may lead to reduced Aβ clearance. In addition, studies identified multiple pathways linking hypoxia/ischemia with Aβ deposition in the brain (see [23] for a review). Hypoxia/ischemia increases Aβ generation by affecting its precursor, Aβ precursor protein (APP). Hypoxia/ischemia increases APP production and cleavage into Aβ, and reduces the activity of neuroprotective enzymes that act on APP [23]. In addition, a few studies suggest that both sleep disruption and chronic hypoxia increase tau protein levels and their hyperphosphorylation [21,24–26].
Fig. 1. Potential mechanisms linking obstructive sleep apnea (OSA) to the development of dementia.
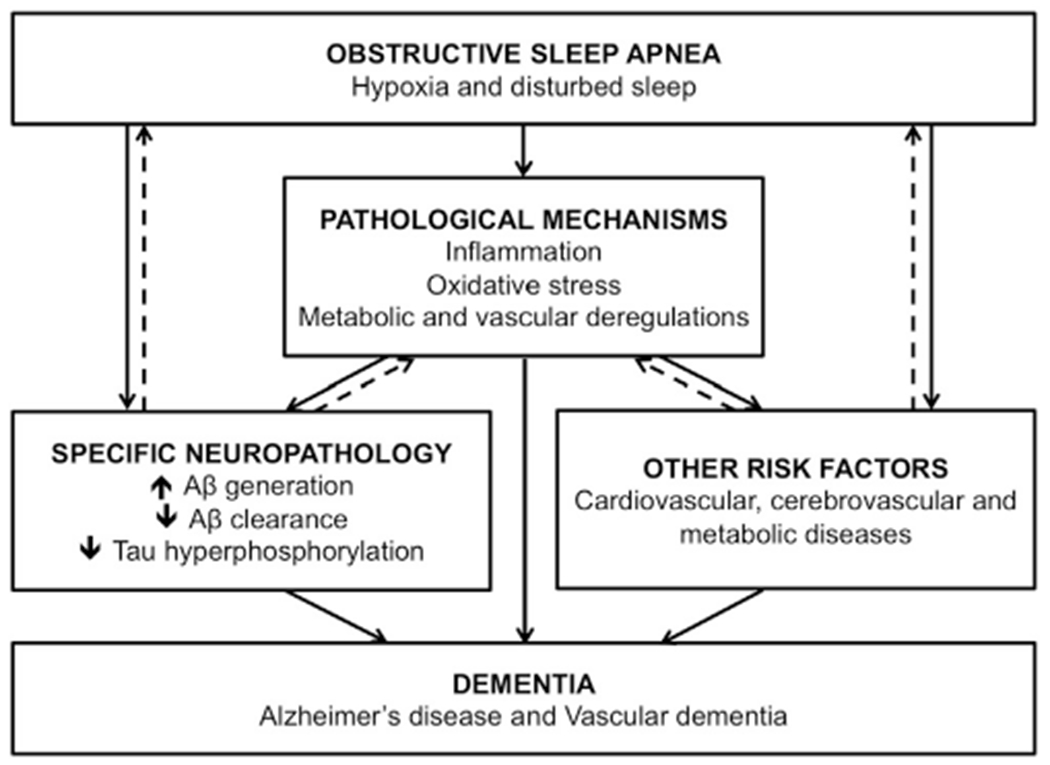
Recent evidence suggests that the hallmarks of OSA, i.e., hypoxia and disturbed sleep, may directly contribute to the development of the neuropathology of Alzheimer’s disease (AD) in the brain. Secondly, OSA may indirectly be involved in the incidence of dementia via its role in the development of other risk factors. Of note, OSA is a risk factor for stroke and cerebrovascular events, which in turn are a core pathophysiological component of vascular dementia. Moreover, OSA may also indirectly contribute to the development of dementia via secondary pathological mechanisms involved in neurodegeneration, such as oxidative stress. These mechanisms may also interact with concomitant AD neuropathology or other risk factors. Dotted arrows represent potential bidirectional relationships. The presence of amyloid-β (Aβ) deposition in the brain has been associated with sleep impairments as well as pathological mechanisms such as inflammation. Other risk factors, such as obesity, are associated with the development of OSA and other pathological mechanisms, such as inflammation.
It is likely that OSA also plays an indirect role in the development of dementia by increasing the incidence of cardiovascular and metabolic diseases, including hypertension, obesity, metabolic syndrome, stroke and diabetes [27], which are themselves independent risk factors of dementia [3,28]. Moreover, sleep fragmentation and intermittent hypoxia in OSA both induce inflammation, oxidative stress, mitochondrial dysfunction, endothelial dysfunction, and metabolic deregulation [29], which have all been proposed to foster the development of AD and VaD [30,31] (see Fig. 1).
This theoretical review aims to identify the most promising fluid biomarkers of dementia that should be included in future large research cohorts of individuals with OSA. Ultimately, a panel of fluid biomarkers could improve direct patient care significantly by identifying adults with OSA at higher risk of developing dementia, and could be useful to unravel the pathophysiological mechanisms linking OSA to dementia. To achieve this goal, we first present the most promising candidate biomarkers of dementia that emerged from the recent literature. We present evidence on CSF and blood biomarkers of AD and VaD, including those from genomics, proteomics, and metabolomics. When possible, we also present the corresponding findings of these biomarkers in the OSA population. Finally, we propose a research agenda for testing fluid biomarkers of dementia in OSA.
Genomics
Genetics of dementia
Usually using blood samples, genetic and genome-wide association studies investigate specific polymorphisms, which are the occurrence of different alleles in the population. The most important and well-established genetic risk factor for sporadic AD is the apolipoprotein E4 (APOE4) allele (see [32] for a review), which was first described in the early 90’s [33]. AD risk is increased by 300% for one APOE4 allele and 1500% for two APOE4 alleles, while the APOE2 allele is protective [32]. APOE is involved in lipid transport and membrane integrity in the brain as well as in Aβ degradation [34].
Although none are associated with AD as strongly as APOE, several other polymorphisms modify the incidence of AD, including clusterin (CLU), sortilin-related receptor-1 (SORL1), complement component receptor 1 (CR1), ATP-binding cassette transporter A member 7 (ABCA7), fermitin family member 2 (FERMT2), major histocompatibility complex class II (HLA-DRB5, HLA-DRB1), bridging integrator 1 (BIN1), and phosphatidylinositol-binding clathrin assembly molecule (PICALM) [32]. These genes affect the risk for AD by about 10–20% each, and they code for proteins involved in Aβ production and clearance, tau toxicity, endosomal vesicle cycling, lipid transport, and the immune response. Recently, polymorphisms of the aquaporin 4 (AQP4) gene were investigated in relationship with AD risk. This gene codes for a water channel involved in the clearance of products via the so-called glymphatic system. AQP4 polymorphisms modified the rate of cognitive decline after AD diagnosis in one study, and moderated the relationship between sleep quality and Aβ burden measured with PET imaging in another [35,36]. In the context of recent discoveries linking sleep quality to Aβ clearance in the interstitial fluid [22], more studies are needed to better define how AQP4 polymorphisms are involved in the relationship between sleep disruption and the incidence of dementia.
Because many polymorphisms are now linked to incident AD, genetic susceptibility to dementia for a given individual can be computed with a polygenic score (PS). The risk of developing AD and the age of onset can be predicted with a PS combining 31 polymorphisms, which correlates with several other biomarkers of AD, including CSF Aβ and tau protein levels [37]. A PS computed with thousands of polymorphisms has high predictive accuracy for AD even when the most common polymorphisms such as APOE are not included [38], suggesting that the genetic susceptibility to AD includes a large number of genes with only a small risk associated to each individually.
VaD risk is increased by the APOE4 allele as well as by other specific genetic polymorphisms, including 5,10-methylenetetrahydrofolate reductase (MTHFR), which is involved in homocysteine conversion, as well as tumor necrosis factor-α (TNF-α) and transforming growth factor β1 (TGF-β1), which are cytokines involved in the immune response (see [39] for a review). Genes increasing the risk of stroke have also the potential to modify the risk of VaD [39]. Ischemic stroke can be predicted with a PS computed with thousands of polymorphisms [40]. Moreover, a PS computed with polymorphisms that are associated with stroke correlated with cognitive abilities in adults without stroke [41].
Dementia genetics factors in OSA
Multiple genes were associated with the presence of OSA and its severity [42]. However, very few of them have been linked to incident dementia. Looking at genes related to dementia risk, recent meta-analyses concluded that there is no association between APOE polymorphism and the presence of OSA [43,44]. However, the APOE4 allele may interact with OSA to worsen cognitive performance: OSA severity was associated with worse memory, attention, executive functioning as well as worse global cognition only in APOE4 carriers [45–48]. Polymorphisms of TNF-α and interleukin-6 (IL-6), which code for inflammatory cytokines, as well as HLA-DRB1 have all been associated with the presence of OSA [43,49–51], although how inflammation may lead to OSA remains to be clarified.
CSF biomarkers
CSF biomarkers of dementia
CSF biomarkers that are directly related to AD neuropathology can be used to diagnose probable AD (see [52,53] for reviews). Low CSF Aβ42 levels and Aβ42/40 ratio are the signature of AD and probably result in the increased brain deposition of Aβ in the form of amyloid plaques [52,53]. Low CSF Aβ42 levels are also observed in the preclinical stages of AD (i.e., cognitively normal adults who will later develop AD) [53], suggesting that Aβ deposition is a very early process. Low CSF Aβ42 levels can also be observed in VaD, but they are less marked than in AD [54]. On the other hand, most reports show no Aβ40 level changes in AD patients [52].
High CSF tau levels also have high sensitivity and specificity for AD and prodromal AD, but were often reported as unchanged in preclinical AD [52]. Increased CSF tau levels may occur later than the Aβ changes in the neurodegenerative process [53], which has been confirmed with recent work using positron emission tomography (PET) markers [55]. While CSF total tau levels are elevated in both AD and VaD, phosphorylated tau levels are more specific to AD and can be useful to discriminate between these two types of dementia [52,53].
Although CSF Aβ42, Aβ42/40 ratio and tau levels are increasingly recognized as established biomarkers for dementia, cut-off values to diagnose AD remain variable across studies and assays [56]. Values for AD diagnosis vary between <480 and 640 pg/ml for Aβ42 levels and between >250 and 375 pg/ml for total tau levels [57]. Regarding the Aβ42/40 ratio, recent studies suggest a cut-off value between <0.05 and 0.06 [58,59].
In addition to biomarkers representing AD neuropathology, novel and promising CSF biomarkers have recently emerged in the literature. Lactate accumulation in the CSF, hypothesized to represent mitochondrial dysfunction, was evidenced in AD patients compared to controls and VaD patients [60]. Chitinase-3-like protein 1 (YKL-40) and visinin-like protein 1 (VILIP-1), which are markers of inflammation and neuronal injury respectively, are other promising CSF biomarkers of AD. Although only YKL-40 is high in MCI and AD patients, high levels of both YKL-40 and VILIP-1 were shown to predict conversion from MCI to AD [61].
CSF biomarkers in OSA
Adults with OSA had low CSF Aβ40, Aβ42 and VILIP-1 levels, but unchanged CSF tau levels were found [62]. In a recent study in which all subjects reported subjective cognitive impairment, those with OSA exhibited lower CSF Aβ42 levels, a higher tau/Aβ42 ratio, a higher lactate level, but no tau level changes when compared to controls and OSA patients treated with CPAP [63]. A case-report of a 57-year-old man with severe OSA and subjective cognitive impairment showed abnormal low CSF levels of Aβ42 and Aβ42/40 (<500 pg/ml and <0.06) and high total tau/Aβ42 ratio levels (>0.52) that were normalized with a one-year treatment with CPAP [64]. In the largest cross-sectional and only longitudinal study to date performed in cognitively normal elderly, OSA severity indices were not associated with CSF Aβ42 levels at cross-section but correlated with annual rates of CSF Aβ42 level decrease over the two-year follow-up [65], which would be consistent with increases in amyloid burden over time. Interestingly, the decrease in the annual rate of CSF Aβ42 in association with OSA severity was larger than what would be predicted by the APOE4 allele alone. Overall, most of these recent reports performed in subjects aged 50–70 years suggest that OSA is associated with low CSF Aβ42 levels as would be expected in the preclinical stages of dementia.
Blood-based proteomic biomarkers of AD neuropathology
Blood-based biomarkers of AD neuropathology in dementia
Some studies found lower blood Aβ42 levels in patients with AD or MCI, but also in cognitively normal and MCI adults who will later develop dementia (see [66] for a review). However, others have failed to replicate these results and even reported higher blood Aβ42 levels in cognitively normal adults in their preclinical stage of AD [66]. A meta-analysis of seven studies showed that higher blood Aβ42 levels were present in subjects with preclinical AD [67]. Blood Aβ42 levels may depend on the stage of the disease: enhanced levels might be present in earlier stages of the disease, and then levels decrease as the cognitive decline progresses [66,67]. This pattern could represent increased Aβ production and reduced clearance at first, with aggregation into plaques later on. Results seem less promising for blood Aβ40 levels, as both increased and decreased levels were reported in AD patients [66]. A meta-analysis indicated high blood Aβ40 levels in the preclinical stage of AD [67], whereas unchanged levels in these early stages were also reported [66]. Overall, mixed results were obtained on blood Aβ40 levels at all stages of the disease. On the other hand, the blood Aβ42/40 ratio yields more promising results. A meta-analysis including over 10 000 subjects concluded that a low blood Aβ42/40 ratio was associated with a high risk of developing all-cause dementia [68]. A low blood Aβ42/40 ratio was indeed reported in subjects with AD, MCI and with preclinical AD [66].
A higher blood tau level is a new marker measured with high-sensitivity techniques that has been associated with the presence of AD and MCI [66]. A recent study with a three-year follow-up found that high tau protein levels were associated with the progression from cognitively normal to MCI, but not from MCI to all-cause dementia [69]. This could be due to the short follow-up period and to the low number of dementia cases observed and thus, other studies are needed to confirm blood tau protein level as a biomarker for dementia and whether they are associated with neurofibrillary tangles in the brain.
Blood-based biomarkers of AD neuropathology in OSA
In young adults, whereas OSA was not associated with blood Aβ42 and Aβ40 levels, moderate-severe OSA was associated with increased blood total tau levels compared to both mild OSA and controls [70]. In another study, middle-aged OSA subjects had significantly higher blood Aβ40 and Aβ42 levels as well as phosphorylated tau levels compared to controls, although unchanged total tau levels were observed [71]. Overall, these first reports suggest that OSA may lead to increased blood tau and Aβ levels.
Blood-based proteomic biomarkers of inflammation
Blood-based inflammatory biomarkers of dementia
Inflammation is hypothesized to play a major role in VaD and AD [30,31,72]. In fact, there may be a bidirectional relationship between inflammation and neurodegeneration: inflammation is caused by Aβ deposition, oxidative stress, and cellular damage; and inflammation is followed by Aβ generation, blood–brain barrier (BBB) pathology, and further oxidative stress. A recent meta-analysis investigating 51 biomarkers in 171 studies concluded that inflammatory biomarkers are increased in AD [73], including IL-1β, IL-2, IL-6, IL-18, chemokine ligand-10, TNF-α converting enzyme and TNF receptors, and interferon gamma (IFN-γ). All of them are cytokines or chemokines involved in the immune response and glial activation [73]. Other elevated blood inflammatory biomarkers in AD patients included α1-antichymotrypsin (ACT) and high-sensitivity c-reactive protein (hsCRP), two acutephase proteins involved in coagulation and lysis, and vascular cell adhesion molecule-1 (VCAM-1), an adhesion molecule which assists other markers to reach the inflammation site [73]. Most significant results were observed for IL-1β, IL-6, ACT, hsCRP and TNF receptors. However, a few studies showed either unchanged or reduced levels in AD patients [73]. As for VaD patients, they also show elevated cytokines compared to controls, including IL-1β, IL-4, IL-5, TNF-α, and IFN-γ [74].
In MCI subjects, despite the fact that most blood inflammatory biomarkers are unchanged, some studies have reported that a few cytokines are upregulated, including IL-1β, TNF-α, intercellular adhesion molecule 1 (ICAM-1), and IFN-α [75]. In six large cohort studies, higher blood levels of hsCRP, ACT and IL-6 were all associated with either a higher risk of cognitive decline or all-cause dementia, AD and VaD (see [76] for a review), even though there are inconsistencies regarding which specific biomarkers were elevated.
Blood-based inflammatory biomarkers investigated in OSA
It is well known that OSA may lead to an inflammatory response [29,77]. In a first meta-analysis of 15 studies, it was reported that blood hsCRP levels are higher in OSA patients compared to controls and that these levels are modulated by obesity and OSA severity [78]. The second meta-analysis of 47 articles found that blood TNF-α levels are also elevated in OSA subjects [79]. Finally, the third meta-analysis of 51 studies showed that blood levels of CRP, TNF-α, IL-6, IL-8 and adhesion molecules (ICAM-1, Selectins and VCAM-1) are increased in OSA subjects [80]. In these meta-analyses, the large majority of studies showed elevated blood inflammatory biomarkers in OSA.
Blood-based proteomic and metabolomic biomarkers of oxidative stress
Blood-based biomarkers of oxidative stress in dementia
Oxidative stress is the production of highly reactive free radicals that damage nucleic acids, proteins and lipids [81]. In dementia, oxidative stress is hypothesized to be an important mechanism leading to tissue damage (see [81] for a review). It occurs following inflammation and Aβ deposition [31], suggesting that oxidative stress may be an effector between dementia pathology and neuronal death. Moreover, oxidative stress leads to atherosclerosis and is involved in VaD pathogenesis [82].
Homocysteine is an amino acid with proprieties that makes it one of the most promising blood biomarkers for all-cause dementia. Indirectly, hyperhomocysteinemia is a marker of B12 deficiency, which can cause cognitive decline [83]. Directly, high levels of homocysteine cause oxidative stress, and thus, it is involved in BBB dysfunction, cardiovascular and cerebrovascular diseases, neuronal death, enhanced Aβ toxicity, Aβ generation and deposition as well as tau protein phosphorylation [83]. In 2002, homocysteine was already shown to be associated with dementia [84], and a meta-analysis of 10 studies concluded that homocysteine blood levels are elevated in AD patients [73]. This result was replicated in both AD and VaD patients in another meta-analysis of 13 studies, with higher levels in VaD than in AD [85]. Overall, many cohort studies showed that elevated blood levels of homocysteine are associated with the incidence of all-cause dementia up to 35 years later [83].
Reduced blood levels of antioxidants were reported in AD and MCI, including superoxide dismutase (SOD), selenium, vitamin E, and total antioxidant capacity [81]. In VaD, blood levels of many antioxidants are also reduced, including vitamins E, C and A as well as SOD [82]. Interestingly, supplements of vitamins C and E in the elderly may protect against the development of VaD, as well as mixed dementia [86], but more studies are needed to set the appropriate intake levels for these vitamins.
Biomarkers of free radicals, damaged proteins, lipid peroxidation and nucleic acid oxidation are increased in both AD and MCI patients compared to controls: hemo-oxygenase-1, protein carbonyls, oxidized protein products, malondialdehyde (MDA), hydroperoxides, 8,12-isoiPF(2alpha)-VI, 8-hydroxy-2-desoxyguanosine (8-OHdg) and 8-oxoguanine [81]. Overall, patients with dementia or at risk show reduced antioxidant capacity combined with increased oxidative stress production and damage.
Blood-based biomarkers of oxidative stress in OSA
OSA produces oxidative stress directly via intermittent hypoxia and mitochondrial dysfunction, as well as indirectly through associated comorbidities, such as obesity and hypertension [87]. Oxidative stress may be an important mechanism by which OSA is associated with neurodegeneration [29]. Two meta-analyses of ten studies concluded that homocysteine levels are elevated in OSA, especially in subjects with severe OSA and in subjects under 50 years of age [88,89]. In fact, all included studies indicated higher homocysteine levels in severe OSA subjects. In another meta-analysis of six studies, blood homocysteine levels were significantly reduced after three months of CPAP [90].
A recent review has comprehensively discussed the involvement of oxidative stress in OSA in relation with altered neurocognitive functioning [87]. Lower antioxidant blood levels were reported in OSA subjects, including SOD and vitamin E [87]. Moreover, OSA subjects exhibited increased blood levels of proteins and metabolites associated with oxidative stress and damage, including oxidized protein products, MDA and 8-OHdg [87]. All of these were correlated with impaired cognition in various cognitive domains, such as attention, memory, language, and executive function [87]. Moreover, higher levels of MDA and oxidized proteins products were found in subjects with both MCI and OSA compared to OSA subjects without MCI [91]. These studies strongly suggest that oxidative stress in OSA is a mechanism underlying cognitive impairment.
Blood-based proteomic and metabolomic biomarkers of lipid metabolism
Blood lipoproteins and lipids as biomarkers of dementia
Clusterin (also named APOJ) is one of the most promising biomarkers of dementia and is involved in cholesterol homeostasis and transport in the brain in partnership with APOE [34]. Moreover, clusterin interacts with Aβ, which gives it both neuroprotective and neurotoxic roles. At relatively low Aβ concentration, clusterin can play a neuroprotective role by binding Aβ and thus, facilitating its clearance and inhibiting its aggregation [92]. On the other hand, it can also play a neurotoxic role by contributing to the formation of Aβ oligomers and by mediating Aβ toxicity through the activation of cytotoxic pathways [92]. Blood clusterin levels are increased in both AD and MCI subjects as compared to controls [93,94], and are associated with the risk of developing AD [95] even though these results were not supported by all groups (see [93] for a review). Moreover, higher clusterin levels predict faster progression of cognitive decline in MCI and AD subjects [94,95]. The relation between blood clusterin levels and cognitive decline seems, however, not linear: higher clusterin levels were associated with a higher risk of all-cause dementia in subjects over 80 years, but with a lower risk of all-cause dementia and stroke in subjects younger than 80 years [96]. This finding supports the neuroprotective/neurotoxic duality of clusterin that appears to be moderated by age.
Because both APOE and high-density lipoprotein cholesterol (HDL-C) have protective functions (i.e., Aβ clearance, metabolite exchanges between neurons and glia, transport of cholesterol to be eliminated and reduction of atherosclerosis), their reduced blood levels in association with dementia may represent reduced neuroprotection [97]. Lower blood APOE levels are generally observed in prodromal AD and AD patients, particularly in APOE4 carriers [98], and are associated with increased risk of developing dementia (see [93] for a review). However, other studies showed surprisingly unchanged blood APOE levels in AD patients [93]. Although many studies were performed on HDL-C, only about half found lower HDL-C levels in AD patients or in non-demented adults who will develop AD [93].
Blood lipoproteins and lipids investigated in OSA
To our knowledge, only one study investigated clusterin blood levels in OSA and found that clusterin levels were elevated and associated with a worse memory performance [99]. Blood APOE levels were also shown to be slightly elevated in middle-aged OSA subjects compared to controls [100]. This could represent compensatory mechanisms in order to protect against atherosclerosis processes. A large meta-analysis including 64 studies that include over 18 000 subjects showed that OSA is associated with abnormal lipid profiles, which include low blood HDL-C levels [101]. The authors hypothesized that oxidative stress and inflammation in OSA might cause dyslipidemia, which could be followed by atherosclerosis. A meta-analysis of six studies reported that CPAP treatment reduces total cholesterol but does not affect HDL-C levels [102].
Proposed research agenda to investigate promising fluid biomarkers of dementia in OSA
Summary of promising fluid biomarkers of dementia that could be used in OSA
OSA is increasingly recognized as a prevalent risk factor for dementia. What makes OSA particularly appealing in the context of preventive dementia strategies is that it can be treated efficiently with CPAP or other interventions. Whether screening and treating OSA could have an impact on the incidence of dementia remains to be investigated. To achieve this goal, epidemiological and mechanistic evidence needs to be drawn from intensive research efforts to properly establish OSA as a modifiable risk factor. Establishing a panel of promising biomarkers linking OSA to dementia has the potential to significantly advance our understanding of the pathophysiological mechanisms involved in neurodegeneration. Moreover, studying large cohorts of older adults with OSA using those biomarkers could also help developing protocols to identify OSA individuals who are at risk of dementia.
While OSA and dementia show different profile in terms of genetic susceptibility, there is important overlap between these two conditions with regards to associated fluid biomarkers, suggesting common pathophysiological mechanisms such as AD neuropathology, inflammation, oxidative stress, and metabolic dysfunctions. In Table 1, we summarized the strongest biomarkers identified in dementia research that are concordant or share similar profiles with OSA. CSF and blood biomarkers of AD neuropathology are only partially concordant in the early stages of dementia and in OSA, but very few studies were performed in OSA. The strongest similarities are for biomarkers of inflammation and oxidative stress, which show a very similar pattern in OSA and both AD and VaD. However, because there are many biomarkers representing inflammation and oxidative stress, it is unclear whether a single one is especially useful compared to the others. However, homocysteine emerged as an important biomarker for both dementia and OSA. Finally, lipoproteins such as APOE and HDL-C are interesting but not consistent in dementia research, and do not seem to be affected by CPAP.
Table 1.
Most promising fluid biomarkers of dementia investigated in OSA.
| Biomarkers | Stages of neurodegeneration |
OSA | |
|---|---|---|---|
| Dementia (all-cause dementia, AD or VaD) | Early stages (MCI, preclinical or prodromal dementia) | ||
| CSF | |||
| Aβ42 | ↓[52–54] | ↓[53] | ↓[62–65] |
| Tau proteins | ↑[52,53] | ↑; unchanged [52,53] | unchanged [62,63] |
| Blood | |||
| Proteomics – AD neuropathology | |||
| Aβ42 | mostly ↓ [66] | ↑; unchanged; ↓[66,67] | ↑; unchanged [70,71] |
| Aβ42/40 ratio | ↓[66] | mostly ↓[66,68] | – |
| Tau proteins | ↑[66] | ↑[66, 69] | ↑[70,71] |
| Proteomics – Inflammation | |||
| Cytokines (IL-6, IL-1β, TNF-α) | mostly ↑[73,74] | ↑; unchanged [75,76] | ↑[79,80] |
| Acute-phase proteins (hsCRP, ACT) | mostly ↑[73] | mostly ↑[76] | ↑[78,80] |
| Proteomics and metabolomics – Oxidative stress | |||
| Homocysteine | ↑[73, 83, 85] | ↑[83, 84] | ↑[88, 89] |
| Superoxide dismutase | mostly ↓[81, 82] | mostly ↓[81] | ↓[87] |
| Vitamin E | ↓[81, 82] | ↓[81] | ↓[87] |
| MDA/8-OHdg | mostly ↑[81] | mostly ↑[81] | ↑[87, 91] |
| Proteomics and metabolomics – Lipid metabolism | |||
| Clusterin | ↑; unchanged [93, 94] | ↑; unchanged; ↓[93–96] | ↑[99] |
AD, Alzheimer’s disease; VaD, vascular dementia; MCI, mild cognitive impairment; OSA, obstructive sleep apnea; CSF, cerebrospinal fluid; Aβ, amyloid-β; IL-6, interleukin-6; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; hsCRP, high-sensitivity c-reactive protein; ACT, α1-antichymotrypsin; MDA, malondialdehyde; 8-OHdg, 8-hydroxy-2-desoxyguanosine.
Because of this important overlap regarding biomarkers findings in dementia and OSA research, we hypothesize that OSA may lead to pathophysiological processes involved in neurodegeneration pathogenesis (see Fig. 1). However, this hypothesis is made on the basis that these mechanisms play a causal role in neurodegeneration, and are not solely markers of its presence. Because dementia is a complex disease that involves several pathological pathways, biomarkers probably represent both cause and effect of neurodegeneration, and thus, longitudinal cohort studies are needed to understand which biomarkers represent pathological mechanisms in OSA and dementia.
Of note, the weight of the evidence presented in the current review is different for biomarkers in dementia and OSA research. Indeed, we constructed this review with the strongest biomarkers in dementia research, and then reviewed whether these specific biomarkers were also investigated in individuals with OSA. Therefore, more studies are needed to assess biomarkers levels in OSA, including Aβ and tau levels in CSF and blood, CSF lactate levels, and clusterin. On the other hand, some biomarkers found in relation to dementia were studied repeatedly in OSA, including inflammatory cytokines and acute-phase proteins, homocysteine, oxidative stress markers and HDL-C. It is, however, important to note that despite the fact that many dementia biomarkers were evaluated in OSA individuals compared to a control group, they were not yet investigated in OSA in relation to the risk of dementia.
In addition to candidate biomarkers that were selected based on the current literature, longitudinal discovery studies investigating hundreds of biomarkers are necessary to identify a specific panel of biomarkers and mechanisms for both OSA and dementia. Combining multiple biomarkers would be a promising approach in OSA-related dementia, since there is little overlap between studies for any single biomarker [103]. For example, the combination of CSF Aβ42 and tau proteins are more reliable than any of them individually to identify MCI individuals who will progress to AD [53]. With the expectation that a biomarker panel is identified and replicated, individuals at higher risk of incident dementia could be regularly screened with this panel combined with the evaluation of their genetic susceptibility measured with a PS.
Limitations of fluid biomarkers
However, fluid biomarkers have their own limitations, which should be considered when designing a cohort study or a clinical protocol. CSF collection is more invasive than blood collection and requires medical expertise, which may be more challenging in some clinical settings, large cohort studies, and longitudinal studies with multiple testing. Thus, blood-based biomarkers are the easiest to perform, but they are limited by the BBB, which restricts the accessibility of neurodegeneration biomarkers via the blood. Nevertheless, brain biomarkers can be cleared from the cerebral extracellular fluid into the blood by multiple normal and pathological mechanisms [104], giving partial access to neurodegenerative biomarkers through blood. Importantly, the BBB starts to break down relatively early in aging and with the progression of dementia [72]. This phenomenon can also be observed in OSA [77]. This suggests that the presence of OSA may allow detecting blood-based biomarkers of dementia even sooner because of the double insult on the BBB.
Another important limitation for blood biomarkers is their non-specificity: many organs other than the brain produce them. For example, Aβ peptides are not exclusive to the brain and can be produced by other peripheral cells such as platelets or muscle cells [105]. Another example is inflammation: many comorbid diseases occurring in the elderly are characterized by organ inflammation that may influence blood biomarkers [106]. The non-specificity of peripheral biomarkers is a limitation when we seek to measure cerebral rather than systemic processes. However, some argue that dementia is a systemic disease: pathological processes in the brain are also present in peripheral cells, such as Aβ production or systemic inflammation, and could also contribute to further neurodegeneration [107,108]. Thus, understanding the potentially bidirectional relationship between peripheral and central pathophysiological mechanisms involved in dementia would clarify how and when blood biomarkers are the most useful in OSA research.
Identifying OSA subjects at risk of dementia with the use of fluid biomarkers
Because all OSA patients probably do not face the same risk of developing dementia, stratified analyses of OSA subjects as a function of incident dementia could help identify which characteristics and their associated biomarker profile best predict dementia. Factors that may modulate the association between OSA and biomarkers of dementia include demographic factors (age, sex, race), lifestyle (cognitive reserve, physical activity), OSA severity (mild, moderate and severe OSA, hypoxemia, sleep fragmentation, daytime sleepiness), genetic polymorphisms and comorbidities (obesity, cardiovascular risk factors and diseases, depression).
We also need to evaluate whether fluid biomarkers are associated with neuroimaging anomalies and neuropsychological deficits in OSA. This would help clarify how fluid biomarkers predict brain changes in terms of structure and function, and thus, further our understanding of underlying mechanisms. The temporal relationship between dementia biomarkers should also be validated within the OSA population to increase our ability to predict the progression to dementia. Aβ deposition occurs first, followed by tau dysfunction, brain atrophy, cognitive decline and finally, clinical presentation [6]. This theoretical model was challenged by data-driven modelling using artificial intelligence where vascular deregulation and inflammation changes significantly precede Aβ and tau alterations in subjects at risk of conversion to dementia [109]. Moreover, assessing whether neurodegenerative processes and OSA effects have an additive or interaction effect on fluid biomarkers would help predict cognitive decline and clinical progression.
Effects of OSA treatment on fluid biomarkers levels
One of the most important questions is whether an effective treatment for OSA positively impacts the levels of fluid biomarkers and slows or even prevents incident dementia. These results could become strong incentives for patients to comply with OSA treatment, and for clinical care professionals and public health policy makers to promote OSA treatment if other studies confirmed them. Indeed, CPAP may delay the age at which clinical manifestations appear [13]. Moreover, a few studies reviewed here suggest that abnormal levels of some fluid biomarkers in OSA subjects are reversible with CPAP [63,64,90]. The incidence of dementia should be assessed in treated OSA subjects compared to those who refused treatment, and the effectiveness of the CPAP treatment could be documented with fluid biomarkers.
In patients with dementia, an effective CPAP treatment in those with OSA improved neuropsychological functions or slowed the rate of cognitive decline [17,18]. These results suggest that treating OSA in demented patients is beneficial, possibly because the OSA-related mechanisms contributing to neurodegeneration are withdrawn. Treatment effects on fluid biomarker levels in demented OSA patients may explain how OSA contributes to clinical neurodegeneration, but also document short and long-term effects of OSA treatment on brain health. Moreover, combining the evaluation of fluid biomarkers with a CPAP treatment could be applied in subjects at earlier stages of neurodegeneration, including MCI subjects, APOE4 carriers, or those with a high genetic susceptibility measured with a PS.
Necessity of large cohort studies to investigate fluid biomarkers of dementia in OSA
In order to establish a specific biomarker panel and underlying mechanisms in OSA that are associated with neurodegeneration, large longitudinal cohort studies are needed. A long follow-up period as well as a large sample size would allow sufficient dementia cases to emerge for a reliable assessment of the relationships. In addition, large sample sizes are necessary to stratify the population according to demographic characteristics and comorbidities to establish a clinical portrait of OSA individuals at risk of developing dementia. In OSA treatment studies, long follow-up periods are also necessary to determine the optimal minimum treatment duration for an effect on fluid biomarkers levels and incident dementia. Because techniques for studying fluid biomarkers are fairly inexpensive, investigation of biomarkers in OSA in association with dementia could be implemented in various large cohort studies. Cohort studies on aging, dementia, and cardiovascular risk factors would necessarily have a significant proportion of individuals presenting OSA given its high prevalence in this portion of the population. Moreover, international collaborations and networks are a promising solution in order to profit from large samples and produce clearer and stronger results. Therefore, we propose that OSA assessment should be included in large cohort studies investigating preclinical dementia using ambulatory devices or polysomnography.
Practice points.
Obstructive sleep apnea is increasingly recognized as a risk factor for dementia and is associated with potential mechanisms that might lead to neurodegeneration;
There is a large overlap in biomarkers that were evaluated independently in dementia and in obstructive sleep apnea. For most of them, the nature and direction of the changes are strikingly similar;
Amyloid-β, tau proteins, cytokines, acute-phase proteins, homocysteine, oxidative stress markers, and clusterin seem to be key candidate fluid biomarkers to predict and understand the development of neurodegeneration in patients with obstructive sleep apnea.
Proposed research agenda.
With large cohort studies and a network approach, the following questions should be investigated:
Which specific fluid biomarker panel is associated with incident dementia in individuals with obstructive sleep apnea?
Which specific biomarker panel is more informative about the mechanisms underlying the link between obstructive sleep apnea and dementia?
What demographic and biomarker pattern characterizes the adults with obstructive sleep apnea who are at higher risk of developing dementia?
Does treatment with continuous positive airway pressure normalize fluid biomarker levels in individuals with obstructive sleep apnea and, if so, does it protect from or delay the incidence of dementia?
Acknowledgements
This theoretical review was funded by the Fonds de la recherche en santé – Québec, the Canadian Institutes of Health Research and the Canadian Sleep and Circadian Network. The authors do not have personal financial interests associated with products described in the manuscript.
Abbreviations
- 8-OHdg
8-hydroxy-2-desoxyguanosine
- Aβ
amyloid-β
- ABCA7
ATP-binding cassette transporter A member 7
- ACT
α1-antichymotrypsin
- AD
Alzheimer’s disease
- APOE
apolipoprotein
- APP
amyloid-β precursor protein
- AQP4
aquaporin 4
- BBB
blood–brain barrier
- BIN1
bridging integrator 1
- CLU (or APOJ)
clusterin
- CPAP
continuous positive airway pressure
- CR1
complement component receptor 1
- CSF
cerebrospinal fluid
- FERMT2
fermitin family member 2
- HDL-C
high-density lipoprotein cholesterol
- HLA
major histocompatibility complex
- hsCRP
high-sensitivity c-reactive protein
- ICAM-1
intercellular adhesion molecule 1
- IFN
interferon
- IL
interleukins
- MCI
mild cognitive impairment
- MDA
malondialdehyde
- MTHFR
5,10-methylenetetrahydrofolate reductase
- OSA
obstructive sleep apnea
- PET
positron emission tomography
- PICALM
phosphatidylinositol-binding clathrin assembly molecule
- PS
polygenic score
- SOD
superoxide dismutase
- SORL1
sortilin-related receptor 1
- TGF-β1
transforming growth factor β1
- TNF-α
tumor necrosis factor α
- VaD
vascular dementia
- VCAM-1
vascular cellular adhesion molecule 1
- VILIP-1
visinin-like protein 1
- YKL-40
chitinase-3-like protein 1
Footnotes
Conflicts of interest
The authors do not have any conflicts of interest to disclose.
References
* The most important references are denoted by an asterisk.
- [1].Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheim Dement 2013;9(1):63–75. e2. [DOI] [PubMed] [Google Scholar]
- [2].Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer’s disease. Lancet 2016;388(10043):505–17. [DOI] [PubMed] [Google Scholar]
- [3].O’Brien JT, Thomas A. Vascular dementia. Lancet 2015;386(10004):1698–706. [DOI] [PubMed] [Google Scholar]
- [4].Custodio N, Montesinos R, Lira D, Herrera-Perez E, Bardales Y, Valeriano-Lorenzo L. Mixed dementia: a review of the evidence. Dement Neuropsychol 2017;11(4):364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheim Dement 2018;14(4):535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 2012;367(9):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Breitner JCS, Poirier J, Etienne PE, Leoutsakos JM. Rationale and structure for a new center for studies on prevention of Alzheimer’s disease (StoPAD). J Prev Alzheim Dis 2016;3(4):236–42. [DOI] [PubMed] [Google Scholar]
- [8].Malhotra A, White DP. Obstructive sleep apnoea. Lancet 2002;360(9328):237–45. [DOI] [PubMed] [Google Scholar]
- [9].Chang WP, Liu ME, Chang WC, Yang AC, Ku YC, Pai JT, et al. Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan. PLoS One 2013;8(10):e78655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ding X, Kryscio RJ, Turner J, Jicha GA, Cooper G, Caban-Holt A, et al. Self-reported sleep apnea and dementia risk: findings from the prevention of Alzheimer’s disease with vitamin E and selenium trial. J Am Geriatr Soc 2016;64(12):2472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[11].Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol 2017;74(10):1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lutsey PL, Misialek JR, Mosley TH, Gottesman RF, Punjabi NM, Shahar E, et al. Sleep characteristics and risk of dementia and Alzheimer’s disease: the atherosclerosis risk in communities study. Alzheim Dement 2018;14(2):157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015;84(19):1964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306(6):613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Calik MW. Treatments for obstructive sleep apnea. J Clin Outcome Manag 2016;23(4):181–92. [PMC free article] [PubMed] [Google Scholar]
- [16].Pan YY, Deng Y, Xu X, Liu YP, Liu HG. Effects of continuous positive airway pressure on cognitive deficits in middle-aged patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Chin Med J (Engl) 2015;128(17):2365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc 2008;56(11):2076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Troussiere AC, Charley CM, Salleron J, Richard F, Delbeuck X, Derambure P, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2013;85(12):1405–8. [DOI] [PubMed] [Google Scholar]
- [19].Aaronson JA, Hofman WF, van Bennekom CA, van Bezeij T, van den Aardweg JG, Groet E, et al. Effects of continuous positive airway pressure on cognitive and functional outcome of stroke patients with obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med 2016;12(4):533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 2017;34:70–81. [DOI] [PubMed] [Google Scholar]
- [21].Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain 2017;140(8):2104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342(6156):373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Salminen A, Kauppinen A, Kaarniranta K. Hypoxia/ischemia activate processing of Amyloid Precursor Protein: impact of vascular dysfunction in the pathogenesis of Alzheimer’s disease. J Neurochem 2017;140(4): 536–49. [DOI] [PubMed] [Google Scholar]
- [24].Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Abeta and pTau in a mouse model of Alzheimer’s disease. Brain Res 2013;1529:200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang CE, Yang X, Li L, Sui X, Tian Q, Wei W, et al. Hypoxia-induced tau phosphorylation and memory deficit in rats. Neurodegener Dis 2014;14(3):107–16. [DOI] [PubMed] [Google Scholar]
- [26].Gao L, Tian S, Gao H, Xu Y. Hypoxia increases Abeta-induced tau phosphorylation by calpain and promotes behavioral consequences in AD transgenic mice. J Mol Neurosci 2013;51(1):138–47. [DOI] [PubMed] [Google Scholar]
- [27].Drager LF, Polotsky VY, O’Donnell CP, Cravo SL, Lorenzi-Filho G, Machado BH. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol 2015;309(7):H1101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheim Dement 2015;11(6):718–26. [DOI] [PubMed] [Google Scholar]
- [29].Polsek D, Gildeh N, Cash D, Winsky-Sommerer R, Williams SCR, Turkheimer F, et al. Obstructive sleep apnoea and Alzheimer’s disease: in search of shared pathomechanisms. Neurosci Biobehav Rev 2018;86:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Calabrese V, Giordano J, Signorile A, Laura Ontario M, Castorina S, De Pasquale C, et al. Major pathogenic mechanisms in vascular dementia: roles of cellular stress response and hormesis in neuroprotection. J Neurosci Res 2016;94(12):1588–603. [DOI] [PubMed] [Google Scholar]
- [31].Candore G, Bulati M, Caruso C, Castiglia L, Colonna-Romano G, Di Bona D, et al. Inflammation, cytokines, immune response, apolipoprotein E, cholesterol, and oxidative stress in Alzheimer disease: therapeutic implications. Rejuvenation Res 2010;13(2–3):301–13. [DOI] [PubMed] [Google Scholar]
- [32].Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med 2016;18(5):421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet 1993;342(8873):697–9. [DOI] [PubMed] [Google Scholar]
- [34].Leduc V, Jasmin-Belanger S, Poirier J. APOE and cholesterol homeostasis in Alzheimer’s disease. Trends Mol Med 2010;16(10):469–77. [DOI] [PubMed] [Google Scholar]
- [35].Burfeind KG, Murchison CF, Westaway SK, Simon MJ, Erten-Lyons D, Kaye JA, et al. The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer’s disease. Alzheim Dement (NY) 2017;3(3):348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rainey-Smith SR, Mazzucchelli GN, Villemagne VL, Brown BM, Porter T, Weinborn M, et al. Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Abeta-amyloid burden. Transl Psychiatry 2018;8(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Desikan RS, Fan CC, Wang Y, Schork AJ, Cabral HJ, Cupples LA, et al. Genetic assessment of age-associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med 2017;14(3):e1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Escott-Price V, Sims R, Bannister C, Harold D, Vronskaya M, Majounie E, et al. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain 2015;138(Pt 12):3673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ikram MA, Bersano A, Manso-Calderon R, Jia JP, Schmidt H, Middleton L, et al. Genetics of vascular dementia - review from the ICVD working group. BMC Med 2017;15(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hachiya T, Kamatani Y, Takahashi A, Hata J, Furukawa R, Shiwa Y, et al. Genetic predisposition to ischemic stroke: a polygenic risk score. Stroke 2017;48(2):253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Harris SE, Malik R, Marioni R, Campbell A, Seshadri S, Worrall BB, et al. Polygenic risk of ischemic stroke is associated with cognitive ability. Neurology 2016;86(7):611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tanizawa K, Chin K. Genetic factors in sleep-disordered breathing. Respir Investig 2018;56(2):111–9. [DOI] [PubMed] [Google Scholar]
- [43].Varvarigou V, Dahabreh IJ, Malhotra A, Kales SN. A review of genetic association studies of obstructive sleep apnea: field synopsis and meta-analysis. Sleep 2011;34(11):1461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xu H, Qian Y, Guan J, Yi H, Yin S. No association between the ApoE epsilon2 and epsilon4 alleles and the risk of obstructive sleep apnea: a systematic review and meta-analysis. Biomed Rep 2015;3(3):313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Johnson DA, Lane J, Wang R, Reid M, Djonlagic I, Fitzpatrick AL, et al. Greater cognitive deficits with sleep-disordered breathing among individuals with genetic susceptibility to Alzheimer disease. The multiethnic study of atherosclerosis. Ann Am Thorac Soc 2017;14(11):1697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nikodemova M, Finn L, Mignot E, Salzieder N, Peppard P. Association of sleep disordered breathing and cognitive deficit in APOE ε4 carriers. Sleep 2013;36(6):873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].O’Hara R, Schröder C, Kraemer H, Kryla N, Cao C, Miller E, et al. Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE e4 carriers. Neurology 2005;65(4):642–4. [DOI] [PubMed] [Google Scholar]
- [48].Spira AP, Blackwell T, Stone KL, Redline S, Cauley JA, Ancoli-Israel S, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc 2008;56(1):45–50. [DOI] [PubMed] [Google Scholar]
- [49].Wu Y, Cao C, Wu Y, Zhang C, Zhu C, Ying S, et al. TNF-alpha-308G/A polymorphism contributes to obstructive sleep apnea syndrome risk: evidence based on 10 case-control studies. PLoS One 2013;9(9):e106183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhong A, Xiong X, Shi M, Xu H. Roles of interleukin (IL)-6 gene polymorphisms, serum IL-6 levels, and treatment in obstructive sleep apnea: a meta-analysis. Sleep Breath 2016;20(2):719–31. [DOI] [PubMed] [Google Scholar]
- [51].Silva L, Lopes J, Ramalheira J, Cunha D, Carvalho C, Bettencourt A, et al. Obstructive sleep apnoea syndrome and HLA in the North of Portugal. Rev Neurol 2015;61(7):301–7. [PubMed] [Google Scholar]
- [52].Humpel C, Hochstrasser T. Cerebrospinal fluid and blood biomarkers in Alzheimer’s disease. World J Psychiatr 2011;1(1):8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[53].Blennow K, Zetterberg H, Fagan AM. Fluid biomarkers in Alzheimer disease. Cold Spring Harb Perspect Med 2012;2(9):a006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Llorens F, Schmitz M, Knipper T, Schmidt C, Lange P, Fischer A, et al. Cerebrospinal fluid biomarkers of Alzheimer’s disease show different but partially overlapping profile compared to vascular dementia. Front Aging Neurosci 2017;9:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].McDade E, Bateman RJ. Tau positron emission tomography in autosomal dominant Alzheimer disease: small windows, big picture. JAMA Neurol 2018;75(5):536–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Blennow K, Zetterberg H. The past and the future of Alzheimer’s disease fluid biomarkers. J Alzheim Dis 2018;62(3):1125–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Park SA, Chae WS, Kim HJ, Shin HS, Kim S, Im JY, et al. Cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease in South Korea. Alzheimer Dis Assoc Disord 2017;31(1):13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lewczuk P, Lelental N, Spitzer P, Maler JM, Kornhuber J. Amyloid-beta 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer’s disease: validation of two novel assays. J Alzheim Dis 2015;43(1):183–91. [DOI] [PubMed] [Google Scholar]
- [59].Sauvee M, Didier Laurent G, Latarche C, Escanye MC, Olivier JL, Malaplate-Armand C. Additional use of Abeta42/Abeta40 ratio with cerebrospinal fluid biomarkers P-tau and Abeta42 increases the level of evidence of Alzheimer’s disease pathophysiological process in routine practice. J Alzheim Dis 2013;41(2):377–86. [DOI] [PubMed] [Google Scholar]
- [60].Liguori C, Stefani A, Sancesario G, Sancesario GM, Marciani MG, Pierantozzi M. CSF lactate levels, tau proteins, cognitive decline: a dynamic relationship in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2015;86(6):655–9. [DOI] [PubMed] [Google Scholar]
- [61].Kester MI, Teunissen CE, Sutphen C, Herries EM, Ladenson JH, Xiong C, et al. Cerebrospinal fluid VILIP-1 and YKL-40, candidate biomarkers to diagnose, predict and monitor Alzheimer’s disease in a memory clinic cohort. Alzheimer’s Res Ther 2015;7(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[62].Ju YE, Finn MB, Sutphen CL, Herries EM, Jerome GM, Ladenson JH, et al. Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol 2016;80(1):154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[63].Liguori C, Mercuri NB, Izzi F, Romigi A, Cordella A, Sancesario G, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep 2017;40(5). [DOI] [PubMed] [Google Scholar]
- [64].Liguori C, Chiaravalloti A, Izzi F, Nuccetelli M, Bernardini S, Schillaci O, et al. Sleep apnoeas may represent a reversible risk factor for amyloid-beta pathology. Brain 2017;140(12):e75. [DOI] [PubMed] [Google Scholar]
- *[65].Sharma RA, Varga AW, Bubu OM, Pirraglia E, Kam K, Parekh A, et al. Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly. A longitudinal study. Am J Respir Crit Care Med 2018;197(7):933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Baird AL, Westwood S, Lovestone S. Blood-based proteomic biomarkers of Alzheimer’s disease pathology. Front Neurol 2015;6:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Song F, Poljak A, Valenzuela M, Mayeux R, Smythe GA, Sachdev PS. Meta-analysis of plasma amyloid-beta levels in Alzheimer’s disease. J Alzheim Dis 2011;26(2):365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma amyloid-beta as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol 2012;69(7):824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mielke MM, Hagen CE, Wennberg AMV, Airey DC, Savica R, Knopman DS, et al. Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the mayo clinic study on aging. JAMA Neurol 2017;74(9):1073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Motamedi V, Kanefsky R, Matsangas P, Mithani S, Jeromin A, Brock MS, et al. Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apnea. Sleep Med 2018;43:71–6. [DOI] [PubMed] [Google Scholar]
- [71].Bu XL, Liu YH, Wang QH, Jiao SS, Zeng F, Yao XQ, et al. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep 2015;5:13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Takeda S, Sato N, Morishita R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front Aging Neurosci 2014;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[73].Lai KSP, Liu CS, Rau A, Lanctot KL, Kohler CA, Pakosh M, et al. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry 2017;88(10): 876–82. [DOI] [PubMed] [Google Scholar]
- [74].Schmitz M, Hermann P, Oikonomou P, Stoeck K, Ebert E, Poliakova T, et al. Cytokine profiles and the role of cellular prion protein in patients with vascular dementia and vascular encephalopathy. Neurobiol Aging 2015;36(9):2597–606. [DOI] [PubMed] [Google Scholar]
- [75].Brosseron F, Krauthausen M, Kummer M, Heneka MT. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol Neurobiol 2014;50(2):534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Dziedzic T Systemic inflammatory markers and risk of dementia. Am J Alzheim Dis Other Demen 2006;21(4):258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lim DC, Pack AI. Obstructive sleep apnea and cognitive impairment: addressing the blood-brain barrier. Sleep Med Rev 2014;18(1):35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li K, Wei P, Qin Y, Wei Y. Is C-reactive protein a marker of obstructive sleep apnea?: A meta-analysis. Medicine (Baltim) 2017;96(19):e6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Li Q, Zheng X. Tumor necrosis factor alpha is a promising circulating biomarker for the development of obstructive sleep apnea syndrome: a meta-analysis. Oncotarget 2017;8(16):27616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[80].Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013;9(10):1003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[81].Garcia-Blanco A, Baquero M, Vento M, Gil E, Bataller L, Chafer-Pericas C. Potential oxidative stress biomarkers of mild cognitive impairment due to Alzheimer disease. J Neurol Sci 2017;373:295–302. [DOI] [PubMed] [Google Scholar]
- [82].Bennett S, Grant MM, Aldred S. Oxidative stress in vascular dementia and Alzheimer’s disease: a common pathology. J Alzheim Dis 2009;17(2):245–57. [DOI] [PubMed] [Google Scholar]
- *[83].Smith AD, Refsum H. Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr 2016;36:211–39. [DOI] [PubMed] [Google Scholar]
- [84].Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 2002;346(7):476–83. [DOI] [PubMed] [Google Scholar]
- [85].Ho RC, Cheung MW, Fu E, Win HH, Zaw MH, Ng A, et al. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. Am J Geriatr Psychiatr 2011;19(7):607–17. [DOI] [PubMed] [Google Scholar]
- [86].Masaki KH, Losonczy KG, Izmirlian G, Foley DJ, Ross GW, Petrovitch H, et al. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology 2000;54(6):1265–72. [DOI] [PubMed] [Google Scholar]
- [87].Zhou L, Chen P, Peng Y, Ouyang R. Role of oxidative stress in the neurocognitive dysfunction of obstructive sleep apnea syndrome. Oxid Med Cell Longev 2016;2016. 9626831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[88].Li K, Zhang J, Qin Y, Wei YX. Association between serum homocysteine level and obstructive sleep apnea: a meta-analysis. BioMed Res Int 2017;2017. 7234528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Niu X, Chen X, Xiao Y, Dong J, Zhang R, Lu M, et al. The differences in homocysteine level between obstructive sleep apnea patients and controls: a meta-analysis. PLoS One 2014;9(4):e95794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chen X, Niu X, Xiao Y, Dong J, Zhang R, Lu M, et al. Effect of continuous positive airway pressure on homocysteine levels in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath 2014;18(4): 687–94. [DOI] [PubMed] [Google Scholar]
- [91].He Y, Chen R, Wang J, Pan W, Sun Y, Han F, et al. Neurocognitive impairment is correlated with oxidative stress in patients with moderate-to-severe obstructive sleep apnea hypopnea syndrome. Respir Med 2016;120:25–30. [DOI] [PubMed] [Google Scholar]
- [92].Li X, Ma Y, Wei X, Li Y, Wu H, Zhuang J, et al. Clusterin in Alzheimer’s disease: a player in the biological behavior of amyloid-beta. Neurosci Bull 2014;30(1):162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Koch M, Jensen MK. HDL-cholesterol and apolipoproteins in relation to dementia. Curr Opin Lipidol 2016;27(1):76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Song F, Poljak A, Crawford J, Kochan NA, Wen W, Cameron B, et al. Plasma apolipoprotein levels are associated with cognitive status and decline in a community cohort of older individuals. PLoS One 2012;7(6):e34078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Jongbloed W, van Dijk KD, Mulder SD, van de Berg WD, Blankenstein MA, van der Flier W, et al. Clusterin levels in plasma predict cognitive decline and progression to Alzheimer’s disease. J Alzheim Dis 2015;46(4):1103–10. [DOI] [PubMed] [Google Scholar]
- [96].Weinstein G, Beiser AS, Preis SR, Courchesne P, Chouraki V, Levy D, et al. Plasma clusterin levels and risk of dementia, Alzheimer’s disease, and stroke. Alzheim Dement (Amst) 2016;3:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein E: from lipid transport to neurobiology. Prog Lipid Res 2011;50(1):62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Poirier J Apolipoprotein E, cholesterol transport and synthesis in sporadic Alzheimer’s disease. Neurobiol Aging 2005;26(3):355–61. [DOI] [PubMed] [Google Scholar]
- [99].Peng Y, Zhou L, Cao Y, Chen P, Chen Y, Zong D, et al. Relation between serum leptin levels, lipid profiles and neurocognitive deficits in Chinese OSAHS patients. Int J Neurosci 2017;127(11):981–7. [DOI] [PubMed] [Google Scholar]
- [100].Xu H, Zheng X, Qian Y, Guan J, Yi H, Zou J, et al. Metabolomics profiling for obstructive sleep apnea and simple snorers. Sci Rep 2016;6:30958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Nadeem R, Singh M, Nida M, Waheed I, Khan A, Ahmed S, et al. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med 2014;10(5):475–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Xu H, Yi H, Guan J, Yin S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Atherosclerosis 2014;234(2):446–53. [DOI] [PubMed] [Google Scholar]
- [103].Kiddle SJ, Sattlecker M, Proitsi P, Simmons A, Westman E, Bazenet C, et al. Candidate blood proteome markers of Alzheimer’s disease onset and progression: a systematic review and replication study. J Alzheim Dis 2014;38(3):515–31. [DOI] [PubMed] [Google Scholar]
- [104].Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 2015;11(8):457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Roher A, Esh C, Kokjohn T, Castano E, Van Vickle G, Kalback W, et al. Aβ peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheim Dement 2009;5(1):18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Henriksen K, O’Bryant SE, Hampel H, Trojanowski JQ, Montine TJ, Jeromin A, et al. The future of blood-based biomarkers for Alzheimer’s disease. Alzheim Dement 2014;10(1):115–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Bu XL, Xiang Y, Jin WS, Wang J, Shen LL, Huang ZL, et al. Blood-derived amyloid-beta protein induces Alzheimer’s disease pathologies. Mol Psychiatr 2017. In press. [DOI] [PubMed] [Google Scholar]
- [108].Le Page A, Dupuis G, Frost EH, Larbi A, Pawelec G, Witkowski JM, et al. Role of the peripheral innate immune system in the development of Alzheimer’s disease. Exp Gerontol 2018;107:59–66. [DOI] [PubMed] [Google Scholar]
- [109].Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC, Alzheimer’s Disease Neuroimaging I. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 2016;7:11934. [DOI] [PMC free article] [PubMed] [Google Scholar]


