ABSTRACT
One hundred percent orange juice (OJ) has no added sugar, naturally contains flavonoids and ascorbic acid, and can modulate the body's oxidative and inflammatory systems. This scoping review, systematic review, and meta-analysis investigated associations between 100% OJ and markers of inflammation or oxidation in healthy adults and those at risk for chronic diseases. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and scoping review extension. Literature in English was searched to July 2021 in Embase and 4 Ovid platform databases. Clinical and observational studies of any duration were eligible. Cochrane Collaboration tools were used to assess the risk of bias in controlled trials. Strength of evidence was determined using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach. The scoping review presents a qualitative synthesis of evidence in summary and results tables. Twenty-one interventional studies (16 controlled trials and 5 before-after studies) conducted in 307 healthy and 327 at-risk participants were included. Six common markers [C-reactive protein (CRP) or high-sensitivity CRP (hs-CRP), IL-6, TNF-α, malondialdehyde (MDA), oxidized LDL (oxLDL), and antioxidant capacity] measured across 16 studies were systematically reviewed, and results were synthesized narratively. Random-effects model meta-analyses were conducted on 10 studies reporting hs-CRP, IL-6, and/or MDA. After consuming 100% OJ, healthy and at-risk participants showed significantly lower IL-6 concentrations (pooled net difference: −1.51 pg/mL; 95% CI: −2.31, −0.70) and lower, but nonsignificant, hs-CRP (pooled net change: −0.58 mg/L; 95% CI: −1.22, 0.05) and MDA (crossover trials pooled net difference: −0.06 μmol/L; 95% CI: −0.19, 0.08). Findings suggest that 100% OJ may reduce inflammation, but results should be interpreted with caution due to moderate risk of bias, very low strength of evidence, and the low number of subjects. This study was registered on PROSPERO (https://www.crd.york.ac.uk/prospero/) as CRD42021235438.
Keywords: 100% juice, orange juice, citrus, fruit and vegetable juices, inflammation, oxidative stress, cytokines, C-reactive protein
Statement of Significance: This is the first systematic review and meta-analysis to assess the impact of 100% orange juice interventions on common markers of inflammation and oxidative stress in healthy adult populations and those at risk for chronic diseases. This review further summarizes current evidence across all reported markers of inflammation and oxidation and highlights gaps in the literature on this topic to indicate possible areas for future research.
Introduction
Fruits and vegetables are a cornerstone of healthy diets and dietary recommendations. Beyond providing basic human nutrition, fruits and vegetables have health-promoting effects including their role in reducing inflammation and their potential to help prevent various chronic diseases. Numerous systematic reviews of intervention studies have indicated that certain fruits and vegetables in the diet, particularly cruciferous vegetables, dark-green leafy vegetables, citrus fruits, and dark-colored berries, have superior effects on biomarkers, surrogate endpoints, and outcomes of chronic disease (1). A recent review found strong scientific evidence for providing health recommendations to increase fruit and vegetable consumption for disease prevention (1), and fittingly, the 2020–2025 Dietary Guidelines for Americans (DGAs) advise that fruits and vegetables constitute half of the plate at each meal (2).
On average, Americans consume only half the recommended fruit servings each day (2) with ∼65% of total fruit consumed as whole fruit and the remainder as 100% fruit juice (3). While the DGAs recommend that at least half of total fruit servings be from whole fruit, including fresh, canned, frozen, and dried forms (2), adding 100% fruit juice to whole-fruit intake increases Americans’ total fruit consumption >50% and brings the population closer to meeting recommended fruit servings. While much of the naturally occurring fiber and vitamin C are lost during processing, 100% fruit juices show similar vitamin and mineral content as equal quantities of whole fruit (4). Importantly, these juices retain much of the antioxidant nutrients and phytochemicals of the whole fruit, which can reduce both inflammation and chronic disease risk and support human health (4, 5). Analyses of NHANES 2003–2006 and 2013–2016 datasets found that, contrary to popular belief, dietary fiber intake among 100% fruit juice consumers was not lower than that in nonconsumers (6, 7). Further modeling analyses of the 2013–2016 data for adults showed that replacing 100% fruit juice in the diet with isocaloric whole-fruit equivalents would increase fiber intake by just 1 g (8). Consumers of 100% orange juice (OJ), the most commonly consumed 100% juice in the United States (9), have shown higher intakes of bioactive flavonoids, lower added sugars, and higher-quality diets overall than nonconsumers of 100% OJ. Specifically, the diets of 100% OJ consumers included more vitamin C, potassium, calcium, vitamin D, flavanones, and total flavonoids (7). Adult consumers of 100% OJ have also shown lower BMI values (7, 10), waist circumferences (7), total cholesterol concentrations, and LDL-cholesterol concentrations compared with non-consumers (10). While this evidence does not imply that 100% OJ consumption causes these differences, it does suggest that 100% OJ can be part of a healthy diet.
Although it has been universally accepted that adequate fruit consumption is protective against inflammation and oxidative stress (9), there is a lack of consensus on recommending 100% juice consumption due to concerns about the higher energy density, higher sugar content, and lower dietary fiber content per serving. Diet may positively or negatively impact the risk of chronic disease by modulating an individual's inflammatory status and, subsequently, levels of oxidative stress. Low-grade inflammation, defined as increased concentrations of inflammatory markers [e.g., C-reactive protein (CRP) and interleukins], has been recognized as a risk factor for several chronic diseases, including type 2 diabetes, cardiovascular disease, and cancer (11). Chronic inflammation increases the risk of cellular damage through an overproduction of reactive oxygen species, which leads to oxidative stress and damage to biomolecules (e.g., proteins, DNA) (12). Studies have suggested that 100% OJ consumption modulates antioxidant and anti-inflammatory properties at both the cellular and molecular level up to 5 h after consumption (13–15). A 2013 narrative review addressed the intake of OJ and markers of inflammation in healthy subjects and included some results related to oxidative stress markers (16); however, no authoritative systematic review currently exists in the peer-reviewed literature, and several clinical studies have been published since this time.
The objectives of this research were to conduct a scoping review to identify gaps in the research on the role of 100% OJ in inflammation and oxidative stress and to conduct a systematic review to examine the effects of 100% OJ on select markers of inflammation and oxidative stress in healthy adults and adults at risk for developing chronic diseases.
Methods
This report includes a scoping review of studies on 100% OJ and any reported markers of inflammation or oxidative stress as well as a systematic review of studies assessing commonly reported markers of inflammation and oxidative stress identified in the scoping review [i.e., CRP or high-sensitivity CRP (hs-CRP), IL-6, TNF-α, malondialdehyde (MDA), oxidized LDL (oxLDL), and antioxidant capacity]. This study was conducted according to the National Academy of Medicine's Standards for Systematic Reviews (17) and the Cochrane Handbook for Systematic Reviews of Interventions, version 6.2 (18). Our reporting follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (19, 20) and the extension for scoping reviews (21). Prior to data extraction, the review protocol was registered on the International Prospective Register of Systematic Reviews, PROSPERO (https://www.crd.york.ac.uk/prospero/; registration number: CRD42021235438).
Data sources and searches
We developed search strategies limited to the English language and human studies that examined the relation of 100% OJ with markers of oxidative stress and inflammation. Search terms included “orange juice” and related terms (e.g., fruit and vegetable juices, beverages), terms related to inflammation (e.g., cytokines, leukocytes) and oxidative stress (e.g., free radicals, lipoxygenases), and study design [e.g., randomized controlled trials (RCTs), prospective studies]. (See the PROSPERO protocol for search details.) We implemented searches in the Embase database (1966 to 24 July 2021) and databases from the Ovid platform including MEDLINE® (1946 to 24 July 2021), Cochrane Central Register of Controlled Trials (1991 to 24 July 2021), Global Health (1910 to 24 July 2021), and CAB Abstracts (1910 to 24 July 2021). We performed reference mining for studies included in relevant systematic reviews (16, 22) and searched for full-text articles related to relevant conference abstracts.
Study selection
Duplicate citations across databases were removed prior to the screening process. Titles and abstracts were screened by 2 independent investigators using the Rayyan application for systematic reviews (23). Full-text articles of included abstracts were retrieved and screened by 1 investigator according to the study eligibility criteria presented in Supplemental Table 1. All rejected articles were reviewed by a second investigator to confirm or refute exclusion. Disagreements between investigators were adjudicated by a third investigator or group consensus.
We considered any intervention study (randomized, nonrandomized, and single- or multiple-arm), prospective cohort study, nested case-control study, and case-cohort study for inclusion regardless of study duration. Eligible populations included adults (≥18 y old) who were healthy, generally healthy (<20% of study population with disease), or considered “at risk” of developing a chronic disease due to having metabolic syndrome, obesity, mild hypercholesterolemia, prediabetes, or hypertension. The intervention or exposure of interest was 100% OJ, where “100%” was defined using the US FDA food-labeling criteria of “Juices directly expressed from a fruit or vegetable (i.e., not concentrated and reconstituted)” (21 CFR §101.30, i) (24) and without added ingredients. In addition to juices described as “100% juice,” we considered these descriptors: fresh, pure, whole, natural, or not from concentrate. Any study specifying interventions with “orange juice” or juice from mandarin oranges, blood oranges, or bergamot oranges was considered for inclusion. Eligible comparators were different quantities of 100% OJ, other foods or beverages (including usual diet), placebos, or no OJ. The outcomes of interest were markers of inflammation and oxidative stress as identified by each study.
Data extraction
Standardized data-extraction forms were created to collect information on characteristics of each study [location, funding source, design, durations (intervention/exposure, washout periods, overall), intervention details (juice description, fresh or commercial preparation, dose, citrus variety), and inflammation/oxidative stress markers assessed] and population [age, sex, health status, average BMI and associated weight status (25), smoking status, and population description reported by the study], as well as study results. Due to the immense variety of oxidative stress and inflammatory markers identified across included studies, with little overlap between studies, study results were extracted in 2 phases. In the first phase, the direction and strength of association with 100% OJ were extracted for all reported markers along with key findings for significant differences or trends between 100% OJ and comparator interventions. In the second phase, controlled trials reporting on the most reported markers of inflammation and oxidative stress were identified for systematic review. Here, specific results for both 100% OJ and comparator study arms were extracted using separate extraction forms for studies with crossover and non-crossover designs. For studies reporting outcome measures at multiple time points, data were extracted from the baseline and longest follow-up periods. One study with a 4-wk intervention reported baseline and final marker results at rest and both 30 min and 24 h after a bout of exhaustive exercise (26). Results were extracted from this study for all follow-up time points. If studies reported multiple analysis models, we used the most adjusted model. WebPlotDigitizer (27) online software (version 4.4) was used to extract data presented in figures, and study authors were contacted for data when published results were not reported in sufficient detail. Data were extracted by 1 investigator and checked by another investigator for agreement. Disagreements were resolved by consensus.
Risk of bias
For all included controlled trials, 2 investigators independently performed risk-of-bias (ROB) assessments at the study level using the Cochrane Collaboration's tool for assessing ROB in randomized trials (28) with additional considerations for crossover trials (29). Studies were rated as low, some concern, or high ROB across 5 domains that assessed bias due to the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported results. An additional domain measuring bias due to period or carryover effects was assessed for crossover trials.
Strength of evidence and meta-analyses
The present systematic review focused on the most reported markers of inflammation or oxidative stress identified in the scoping review, which included CRP or hs-CRP, IL-6, TNF-α, MDA, oxLDL, and antioxidant capacity. These outcomes were synthesized separately as prespecified in analysis plans. For all included studies, results were synthesized narratively in text and presented in summary and results tables. Group comparisons reported with P ≤ 0.05 were considered statistically significant. The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach (30, 31) was used to determine collective strength of evidence (SoE) across studies. For each outcome assessed, SoE profile tables were compiled to report the number and design of studies measuring each outcome as well as the overall limitations, imprecision, inconsistency, indirectness, and publication bias across relevant studies. Summary of findings and SoE grades (i.e., very low, low, moderate, or high) were also reported to indicate the degree of confidence that estimated effects from reviewed evidence were close to the true effect. Antioxidant capacity was excluded from SoE assessments due to the variety of measurement methods used by included studies.
Meta-analyses were limited to markers measured by 3 or more controlled trials with non-100% OJ comparators. Only 1 controlled trial reported on CRP (32), only 1 study reported usable quantitative data for TNF-α (33), and antioxidant capacity was measured by numerous different methods across studies; therefore, these 3 markers were excluded from meta-analyses. Due to heterogeneity in the study designs and health status of study populations, a fixed-effect model meta-analysis was deemed inappropriate, so random-effects model meta-analyses were conducted (34). As prespecified in analysis plans, crossover and parallel trials were analyzed separately, but overall pooled effects were calculated across study designs if measures of effect were identical.
To prepare data for the meta-analyses, several pre-analysis calculations and assumptions were made, and details are reported in the Supplemental Methods. Meta-analysis results were interpreted as statistically significant if the confidence intervals for pooled effect sizes excluded 0. Cochrane's Q statistic was calculated as a measure of heterogeneity, where P ≤ 0.1 was considered significant heterogeneity and I2 values of 25%, 50%, and 75% were interpreted as low, moderate, and high heterogeneity, respectively. Stata SE software (version 15.1; StataCorp) was used for all calculations and meta-analyses.
Results
Figure 1 presents the study search and selection process. Altogether, 1754 citations were identified through database searches and 8 more were identified through reference mining. After duplicates were removed, 1183 citations remained for screening and 1098 were excluded. We retrieved 85 full-text publications, and 22 articles covering 21 studies met inclusion criteria for the scoping review. The 63 excluded articles and exclusion reasons are presented in Supplemental Table 2.
FIGURE 1.
PRISMA flowchart of literature search and study selection. 1Twenty-two articles reporting on 21 studies. hs-CRP, high-sensitivity C-reactive protein; MDA, malondialdehyde; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Sixteen included studies assessing the most reported markers were systematically reviewed and synthesized narratively. Studies deemed ineligible for meta-analysis included 1 study reporting only antioxidant capacity (35) and 5 others with the wrong study design (single-arm or multiple-arm before-after trials) (36–38), a lack of non-100% OJ comparator (39), or indirect measurements (i.e., mRNA expression) of the markers of interest (40). The remaining 10 studies were eligible for meta-analysis (26, 32, 33, 41–47), and from these studies, the outcomes with sufficient data for meta-analysis were hs-CRP, IL-6, and MDA.
Study and participant characteristics
A summary of characteristics for all included studies is presented in Table 1, where potential gaps in the literature are represented by the value, 0 (0%), where no study met the given characteristic. Individual study characteristics are presented in Table 2. The 21 studies included in the scoping review were all interventional studies. No observational studies met the eligibility criteria primarily due to insufficient detail in dietary assessments to identify 100% OJ intake. Included studies comprised 11 crossover RCTs (32, 33, 35, 39, 41–43, 45, 47–49), 1 parallel RCT (46), 4 nonrandomized parallel controlled interventions (26, 40, 44, 50), and 5 before-after studies with a single-arm design (36–38, 51) or multiple study arms (52). A subgroup analysis of one of the crossover RCTs was also included in the review (53). Sample sizes ranged from 2 to 100, with nearly half of the studies (48%) including ≤20 participants. Nine studies (43%) assessed acute interventions where follow-up durations ranged from 2 to 24 h. Other studies lasted 1 to 31 wk.
TABLE 1.
Summary of study characteristics overall and by markers of inflammation or oxidative stress most reported by the 21 included studies1
| Top-reported markers of inflammation and oxidative stress | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Total | CRP or hs-CRP | IL-6 | TNF-α | MDA | OxLDL | Antioxidant capacity |
| n | 21 | 9 | 5 | 5 | 6 | 3 | 7 |
| Design, n (% of studies) | |||||||
| Randomized, crossover | 11 (52%) | 4 (44%) | 4 (80%) | 2 (40%) | 3 (50%) | 2 (67%) | 3 (43%) |
| Randomized, parallel | 1 (5%) | 1 (11%) | 0 (0%) | 0 (0%) | 1 (17%) | 0 (0%) | 1 (14%) |
| Nonrandomized, parallel | 4 (19%) | 1 (11%) | 1 (20%) | 1 (20%) | 1 (17%) | 1 (33%) | 0 (0%) |
| Before-after | 5 (24%) | 3 (33%) | 0 (0%) | 2 (40%) | 1 (17%) | 0 (0%) | 3 (43%) |
| Region, n (% of studies) | |||||||
| North America | 4 (19%) | 0 (0%) | 1 (20%) | 1 (20%) | 0 (0%) | 0 (0%) | 1 (14%) |
| South America | 4 (19%) | 3 (33%) | 1 (20%) | 1 (20%) | 2 (33%) | 0 (0%) | 3 (43%) |
| Europe | 11 (52%) | 5 (56%) | 2 (40%) | 3 (60%) | 3 (50%) | 3 (100%) | 2 (29%) |
| Middle East | 1 (5%) | 1 (11%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Africa | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) | 0 (0%) | 1 (14%) |
| Sample size, n (% of studies) | |||||||
| <10 | 2 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 10–20 | 8 (38%) | 2 (22%) | 1 (20%) | 1 (20%) | 3 (50%) | 1 (33%) | 4 (57%) |
| 21–30 | 4 (19%) | 2 (22%) | 2 (40%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 31–50 | 5 (24%) | 4 (44%) | 2 (40%) | 3 (60%) | 1 (17%) | 1 (33%) | 2 (29%) |
| 51–100 | 2 (10%) | 1 (11%) | 0 (0%) | 0 (0%) | 2 (33%) | 1 (33%) | 1 (14%) |
| Total study duration, n (% of studies) | |||||||
| Acute (≤1 wk) | 2 (10%) | 0 (0%) | 1 (20%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) |
| >1 to 4 wk | 8 (38%) | 1 (11%) | 2 (40%) | 1 (20%) | 2 (33%) | 0 (0%) | 2 (29%) |
| >1 to 3 mo | 10 (48%) | 8 (89%) | 2 (40%) | 3 (60%) | 3 (50%) | 2 (67%) | 5 (71%) |
| >3 to 8 mo | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) | 1 (33%) | 0 (0%) |
| Intervention/arm duration, n (% of studies) | |||||||
| Acute (≤24 h) | 9 (43%) | 1 (11%) | 3 (60%) | 2 (40%) | 1 (17%) | 1 (33%) | 2 (29%) |
| >1 to 7 d | 1 (5%) | 1 (11%) | 1 (20%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) |
| >1 to 4 wk | 6 (29%) | 3 (33%) | 1 (20%) | 0 (0%) | 2 (33%) | 1 (33%) | 1 (14%) |
| >1 to 3 mo | 5 (24%) | 4 (44%) | 0 (0%) | 2 (40%) | 3 (50%) | 1 (33%) | 4 (57%) |
| Mean or median age, n (% of studies) | |||||||
| 18–25 y | 4 (19%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33%) | 0 (0%) | 3 (43%) |
| >25 to 50 y | 15 (71%) | 8 (89%) | 5 (100%) | 5 (100%) | 3 (50%) | 3 (100%) | 4 (57%) |
| >50 y | 2 (10%) | 1 (11%) | 0 (0%) | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
| Health status, weight status,2 n (% of studies) | |||||||
| Healthy, normal weight | 10 (48%) | 2 (22%) | 3 (60%) | 1 (20%) | 2 (33%) | 1 (33%) | 3 (43%) |
| Generally healthy, overweight | 4 (19%) | 2 (22%) | 0 (0%) | 1 (20%) | 2 (33%) | 0 (0%) | 2 (29%) |
| At-risk, overweight | 3 (14%) | 2 (22%) | 1 (20%) | 1 (20%) | 0 (0%) | 1 (33%) | 0 (0%) |
| At-risk, obese | 4 (19%) | 3 (33%) | 1 (20%) | 2 (40%) | 2 (33%) | 1 (33%) | 2 (29%) |
| Comparator intervention,3 n (% of studies) | |||||||
| Water | 4 (19%) | 0 (0%) | 2 (40%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Glucose or sugar beverage | 5 (24%) | 1 (11%) | 3 (60%) | 2 (40%) | 0 (0%) | 1 (33%) | 0 (0%) |
| Non-100% OJ | 4 (19%) | 2 (22%) | 2 (40%) | 1 (20%) | 1 (17%) | 2 (67%) | 0 (0%) |
| Placebo juice | 5 (24%) | 1 (11%) | 1 (20%) | 1 (20%) | 1 (17%) | 0 (0%) | 4 (57%) |
| Other beverage | 2 (10%) | 0 (0%) | 1 (20%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Without OJ | 4 (19%) | 2 (22%) | 0 (0%) | 0 (0%) | 3 (50%) | 1 (33%) | 2 (29%) |
| No comparator | 5 (24%) | 4 (44%) | 0 (0%) | 2 (40%) | 1 (17%) | 0 (0%) | 3 (43%) |
Values of 0 (0%) indicate potential gaps in the literature, where no study in the review met the given characteristic. CRP, C-reactive protein; hs-CRP, high-sensitivity C-reactive protein; MDA, malondialdehyde (a secondary lipid peroxidation marker); OJ, orange juice; OxLDL, oxidized LDL.
Categories for weight status based on BMI (in kg/m2): normal-weight BMI = 18.5–24.9, overweight BMI = 25.0–29.9, obese BMI = ≥30.0 (25).
The sum of each column is greater than the column total n due to multiple comparators for some studies.
TABLE 2.
Study and participant characteristics with results for markers of inflammation or oxidative stress and key findings from included articles1
| Author, year (ref); country | Study design; overall risk-of-bias judgment | Funding source | Total n enrolled; % male | Mean (SD)2 age, y | Health status; smoking status | Mean (SD)2 BMI (kg/m2); weight status3 | Study duration | 100% OJ group(s); orange variety or type | Comparison group(s) | Results for 100% OJ group(s)4 | Key findings for 100% OJ vs. comparison group(s)5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asgary et al., 2014 (41); Iran | Crossover RCT; some concern | Nonprofit | 22; 31.8 | ∼34.5 (11, 12) | 100% healthy; nonsmokers | ∼24.5 (4.3, 5.5); normal or healthy | 10 wk total: 4 wk per intervention and 2-wk washout | 1000 mL/d freshly squeezed OJ; Citrus sinensis | 1000 mL/d commercial OJ from concentrate | Serum VCAM-1: - -Serum E-selectin: - -Serum hs-CRP: - -Serum IL-6: 0 | There were marginally significant differences in serum hs-CRP concentrations after intake of OJ from concentrate (lower hs-CRP at endpoint) and 100% OJ (greater decrease from baseline hs-CRP) |
| Azzini et al., 2017 (36); Italy | Before-after; NA | Government | 20; 0 | 36 (7) | At-risk; nonsmokers | 34.4 (4.8); obese | 12 wk | 500 mL/d commercial pasteurized red OJ; NR | NA | Plasma TAC [FRAP assay]: 0Plasma CRP: 0Plasma TNF-α: 0Plasma leptin: 0 | No comparator |
| Boussetta et al., 2020 (42); Tunisia | Crossover RCT; high | None | 11; NR | 22.5 (0.5) | 100% healthy (soccer players); nonsmokers | 23.2 (0.4); normal or healthy | ∼2 wk total: 2.5 h per intervention, ≥72-h washout, 1 wk between trials (polluted and nonpolluted) | 500 mL fresh blood OJ with no chemical products added; NR | 500 mL placebo juice | [After an intense bout of exercise]Plasma TAC [TAS assay]: ++ (polluted and nonpolluted area)Plasma MDA: ++ (polluted and nonpolluted area) | Compared to placebo juice, consuming 100% OJ before an intense bout of exercise resulted in significantly lower plasma MDA after exercise in both polluted and nonpolluted areas |
| Buscemi et al., 2012 (33); Italy | Crossover RCT; high | None | 21; 52.6 | 48 (13) | At-risk (nondiabetic subjects at increased cardiovascular risk with >2 diagnostic criteria of MetS); some smokers | 32.1 (4.9); obese | ∼2.5 wk total: 1 wk per intervention, 3-d washout | 500 mL/d commercial pasteurized OJ; Citrus sinensis varieties (tarocco, sanguinello, and moro) | 500 mL/d placebo juice with water, orange aroma, colorants (azorubin and tartrazine), sucrose, and citric acid | Serum IL-6: - -Serum TNF-α: - -Serum hs-CRP: - -Plasma protein carbonyl groups: 0 | Compared to placebo juice, consuming 100% OJ significantly reduced inflammation as indicated by lower serum concentrations of IL-6, TNF-α, and hs-CRP |
| Cerletti et al., 2015 (48); Italy | Crossover RCT; some concern | Government | 18; 50 | 36.9 (10.5) | At-risk (≥1 cardiovascular risk factor including 58% with prediabetes); some smokers | 26.8 (4.0); overweight | ∼1 wk total: 2 h per intervention, 7 ± 2-d washout | 1) 1 L commercial pasteurized anthocyanin-rich red OJ; 2) 1 L commercial pasteurized anthocyanin-poor blond OJ; NR | 1 L water | [After consuming a fatty meal]WBC count: 0 (both juices)PMN MPO release: - (blond OJ), - - (red OJ) | Compared to water, consuming blond OJ or red OJ with a fatty meal significantly attenuated rise in WBC counts and moderately attenuated MPO release (P = 0.06 for blond OJ, and P = 0.11 for red OJ vs. water) |
| Chaves et al., 2017 (43); Brazil | Crossover RCT; some concern | Government | 12; 41.7 | NR (NR) | 100% healthy; smoking status NR | Range: 20–25; normal or healthy | ∼2 wk total: 5 h per intervention, ≥1-wk washout between interventions | 500 mL commercial pasteurized 100% OJ; NR | 1) 500 mL water, 2) 500 mL isocaloric beverage (water with glucose) | [After consuming a high-fat, high-carbohydrate meal]Serum IL-4: 0Serum IL-6: decreased (P value NR)Induced PBMC proteins (for 100% OJ): 26S protease regulatory subunit 7, 26S proteasome non-ATPase regulatory subunit 11, serine-protein kinase ATM, de promyelocytic leukemia protein | A high-fat, high-carbohydrate meal with water or the isocaloric beverage increased IL-6, but inflammation was significantly mitigated with 100% OJ which reduced IL-6. Induced PBMC proteins (for all 3 beverages): apolipoprotein A-II, ceruloplasmin, and hemopexin |
| Deopurkar et al., 2010 (40); USA | Nonrandomized parallel controlled intervention; some concern | Government and nonprofit | 48; NR | NR (NR) | 100% healthy; smoking status NR | Range: 21.5–24.4; normal or healthy | 5 h | 300 kcal commercial not from concentrate Florida OJ; NR | 1) 300 kcal glucose, 2) 300 kcal gourmet heavy whipping cream, 3) 300 mL water | TNF-α mRNA: 0IL-1β mRNA: 0IL-6 mRNA: 0NF-κB: 0TLR-2 mRNA or protein: 0TLR-4 mRNA or protein: 0SOCS1 mRNA: 0SOCS3 mRNA or protein: 0SOCS7 mRNA: 0Plasma endotoxin concentrations: 0 (LPS and LBP) | Compared6 to glucose or cream, 100% OJ attenuated acute changes in NF-κB; TNF-α, IL-1β, and SOCS3 mRNA; and SOCS3 protein. Compared6 to cream only, 100% OJ attenuated acute changes in TLR-4 mRNA and protein expressions plus plasma endotoxin concentrations |
| Dong et al., 2016 (32); UK | Crossover RCT; some concern | Industry | 39; 100 | 48 (SEM: 1) | At-risk (≥1 cardiometabolic risk factor including mild hypercholesterolemia); nonsmokers | 28.4 (0.4); overweight | ∼6 wk total: 7 h per intervention, 2-wk washout between interventions | 1) 240 mL commercial pure premium OJ without pulp; 2) 240 mL fresh juice made from lightly blended whole oranges; NR | 1) 240 mL OJ with added orange pomace fiber, and 2) 240 mL isocaloric sugar-matched control | [After consuming a high-fat meal7]Serum CRP: - -Serum IL-6: ++Serum IL-10: -Serum IL-1β: 0Serum TNF-α: 0Plasma oxLDL: 0 | No differences |
| Dourado et al., 2015 (37); Brazil | Before-after; NA | Industry | 50; NR | ∼35.6 (8.6, 10.5) | Generally healthy; smoking status NR | ∼25.9 (1.7, 3.0); overweight | 8 wk | 750 mL/d 100% OJ without added sugars; NR | NA | [Same findings for participants with normal weight and overweight]Lipid peroxidation [TBARS assay] as MDA: - -Serum TAC [DPPH method]: ++Serum hs-CRP: - -Serum IL-4: 0Serum IL-10: 0Serum IL-12: ++Serum TNF-α: 0Serum IFN-γ: 0 | No comparator |
| Ghanim et al., 2010 (50); USA | Nonrandomized parallel controlled intervention; some concern | Government and nonprofit | 30; NR | NR (NR) | 100% healthy; smoking status NR | Range: 20–25; normal or healthy | 5 h | 300 kcal commercial pasteurized not from concentrate OJ; NR | 1) 300 kcal glucose plus 350 mL water, 2) water | [After consuming a high-fat, high-carbohydrate meal]ROS-PMN: ++ROS-MNC: ++p47phox protein: 0TLR2 mRNA or protein: 0TLR4 mRNA or protein: 0SOCS-3 protein: 0SOCS-3 mRNA: only baseline reportedp38 protein: 0MMP-9 mRNA: 0Plasma MMP-9: 0Plasma endotoxins (LPS): 0Phosphorylated p38 protein to p38 ratio: only baseline reported | Compared6 to water and glucose, 100% OJ attenuated acute changes induced by a high-fat, high-carbohydrate meal for these markers: ROS-PMN; MMP-9, TLR2, and TLR4 mRNA expression; SOCS-3, p47phox, p38, and phosphorylated p38 protein expression; plasma MMP-9; plasma endotoxins |
| Guarnieri et al., 2007 (49); Italy | Crossover RCT; some concern | NR | 7; 0 | 26 (2.1) | 100% healthy; nonsmokers | 20.1 (1.4); normal or healthy | ∼4 wk total: 24 h per intervention, 2-wk washout between interventions | 300 mL commercial pasteurized blood OJ; NR | 1) 300 mL vitamin C plus water, 2) 300 mL water with sugars equal to blood OJ content | H2O2-induced MNBC DNA damage (strand breaks): - - | Compared6 to the vitamin C and sugar control beverages, 100% blood OJ attenuated MNBC DNA damage induced by H2O2 |
| Hollands et al., 2018 (39); UK | Crossover RCT; some concern | Government and nonprofit | 45; 48.8 | 52.2 (13.6) | At-risk (all with abdominal obesity based on waist circumference); nonsmokers | 29.0 (5.1); overweight | ∼11 wk total: 4 wk per intervention, ≥3-wk washout | 500 mL/d commercial not from concentrate: 1) standard blond OJ, 2) Sicilian blood OJ; NR | None | Serum hs-CRP: 08 | No difference |
| Johnston et al., 2005 (52); USA | Before-after with multiple arms; NA | Nonprofit | 6; 0 | 36.8 (4.5) | 100% healthy; nonsmokers | 26.8 (3.3); overweight | >2 wk total: 2 h per intervention, 8 d between 100% or comparator OJs 1 and 2, but washout between 100% OJ and comparator trials NR | 237 mL commercial pasteurized not from concentrate 100% pure OJ without pulp: 1) freshly opened, 2) after 8 d of storage; NR | 237 mL OJ reconstituted from frozen concentrate: 1) freshly opened, 2) after 8 d of storage | Plasma LPOs [TBARS assay]: 0 (freshly opened OJ and after 8 days of storage) | Day 1 preparation6: lipid peroxidation (incremental TBARS) decreased with OJ from concentrate and did not change with 100% OJ. Day 8 of storage6: Consumption of both juices increased TBARS. Day 1 and day 8 differences6 were significant for OJ from concentrate and not significant for 100% OJ |
| Perrone et al., 2020 (44); Italy | Nonrandomized parallel controlled intervention; high | NR | 20; 100 | ∼27.3 (2.8, 3.2) | 100% healthy (professional athletes practicing cross-country skiing); nonsmokers | ∼23.4 (2.8, 3.2); normal or healthy | ∼4 wk | Common diet plus 500 mL/d 100% pure bergamot juice; Citrus bergamia | Common diet with no OJ | [After 30 d intensive training]hs-CRP: - -oxLDL: 0 | Compared to no OJ, 100% OJ attenuated increases in oxLDL after 30 d intensive training and significantly improved hs-CRP |
| Pittaluga et al., 2013 (26)9; Italy | Nonrandomized parallel controlled intervention; high | Nonprofit | 22; 0 | ∼68.7 (2.7, 5.1) | Generally healthy; nonsmokers | ∼26.0 (2.1, 3.2); overweight | 4-wk intervention; 24 h per trial with exhaustive exercise | 750 mL/d fresh juice from pigmented red oranges; Citrus sinensis, Sanguinello cultivar | No OJ | [At rest and after a bout of exhaustive exercise9]Plasma GSH: 0 (t0′, t24hr), ++ (t30′)Plasma GSSG: - - (t0′, t30′), 0 (t24hr)GSH/GSSG: 0 (t0′, t24hr), ++ (t30′)Apoptosis of DNA laddering: 0 (t0′, t30′, t24hr)Hemolysis rate: - - (t0′, t30′), 0 (t24hr)Micronuclei rate: 0 (t0′, t30′, t24hr)Serum 8-OHdG: 0 (t0′, t30′, t24hr)Plasma hypoxanthine: 0 (t0′, t24hr), - - (t30′)Plasma xanthine: 0 (t0′, t24hr), - - (t30′)Plasma MDA: 0 (t0′, t24hr), - - (t30′) | Compared6 to no OJ, 100% OJ reduced oxidative stress (GSSG) and hemolysis rate in resting conditions and attenuated acute GSH depletion, lipid oxidation and peroxidation (MDA), and hemolysis rate after a bout of exhaustive exercise. 100% OJ may also attenuate oxypurine concentrations after exhaustive exercise |
| Rangel-Huerta et al., 2015 (45); Spain | Crossover RCT; some concern | Industry | 100; NR | NR (NR) | At-risk (most with alterations in ≥1 clinical sign of metabolic syndrome); nonsmokers | ∼33.2 (0.5, 0.6); obese | 31 wk total: 12 wk per intervention, 7-wk washout | 500 mL/d commercial OJ with normal amount of polyphenols; NR | 500 mL/d commercial OJ enriched with polyphenols extracted from orange albedo and pulp | Plasma GSH: 0Plasma GSSG: 0Urine 8-iso-PGF2α: - -Urine 8-OHdG: - -Plasma oxLDL: 0Plasma LPOs: - -Plasma MDA: 0 | Compared to enriched OJ, consuming 100% OJ resulted in significantly reduced plasma LPO levels and a greater reduction in urine 8-OHdG, but enriched OJ produced significantly lower urine 8-OHdG concentrations |
| Rangel-Huerta et al., 2017 (53)10; Spain | Subgroup analysis of crossover RCT (nonrandom selection of subjects from only sequence 1); NA | Industry | 30; NR | ∼44 (9, 11) | At-risk (most with alterations in ≥1 clinical sign of metabolic syndrome); nonsmokers | ∼32.2 (3.6, 4.2); obese | 12 wk | 500 mL/d commercial OJ with normal amount of polyphenols; NR | 500 mL/d commercial OJ enriched with polyphenols (hesperidin, narirutin, and didymin) obtained from albedo and pulp | 9-HODE + 13-HODE: 0Serum 5-HETE: ++Serum 12-HETE: 0Derivate dihydroxy fatty acids: 0 (12,13-DiHOME), 0 (9,10-DiHOME) | Consuming polyphenol-enriched OJ significantly decreased metabolites 9-HODE + 13-HODE, 12,13-DiHOME, and 9,10-DiHOME; significantly increased 12-HETE; and attenuated increases in 5-HETE seen with 100% OJ.10 Results suggest added protection against oxidative stress and inflammation with polyphenol enriched OJ |
| Ribeiro et al., 2017 (46); Brazil | Parallel RCT; some concern | Industry | 84; 30.8 | 36 (1) | At-risk; smoking status NR | 33 (3); obese | 12 wk | Reduced-calorie diet plus 500 mL/d commercial 100% OJ; Pera Rio oranges | Reduced-calorie diet with no OJ | Serum hs-CRP: - -Serum lipid peroxidation [TBARS assay] as MDA: - -Serum TAC [radical 2,20-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) assay]: 0 | Compared to no OJ, consuming 100% OJ produced significantly lower serum hs-CRP concentrations at 12 wk |
| Riso et al., 2005 (47); Italy | Crossover RCT; high | Government | 16; 0 | NR (NR) | 100% healthy; smoking status NR | Range: 16.0–23.3; normal or healthy | 9–10 wk total: 3–4 wk per intervention (where 1 group had an extra week of juice), 3-wk washout | Standardized diet plus 600 mL/d commercial pasteurized blood OJ; NR | Standardized diet with no OJ | Plasma TAC [reduction by antioxidants, of Cu2+ to Cu+]: 0Plasma MDA: 0Urinary 11-Dehydro-TXB2: 0H2O2-induced DNA damage (% DNA in tail): 0 (21 d juice intake group), - - (28 d juice intake group) | Compared6 to no OJ, consumption of 100% OJ for 28 days (but not 21 d) attenuated H2O2-induced DNA damage |
| Sánchez-Moreno et al., 2003 (51); Spain | Before-after; NA | Government | 12; 50 | 22 (3) | 100% healthy; some smokers | 22.2 (1.6); normal or healthy | 2 wk | 500 mL/d commercial OJ; NR | NA | Plasma 8-epi-PGF2α: - - (men and smokers), 0 (women and nonsmokers) | No comparator |
| Silveira et al., 2015 (38); Brazil | Before-after; NA | Government | 35;54.3 | ∼36 (9.0, 9.4, 10.3, 11.8) | Generally healthy; nonsmokers | ∼26.0 (2, 3); overweight | 8 wk | 500 mL/d frozen, ready-to-drink red OJ; Sanguı ´nea de Mombuca variety | NA | Serum hs-CRP: - - (normal weight and overweight/obese)Serum antioxidant capacity [DPPH method]: ++ (normal weight and overweight/obese) | No comparator |
| Snyder et al., 2011 (35); USA | Crossover RCT; some concern | Nonprofit | 16; NR | 20.1 (NR) | 100% healthy; nonsmokers | 23.8 (range: 20–27.4); normal or healthy | 4 wk total: 3 h per intervention, 1-wk washout between interventions | 591 mL fresh-squeezed OJ; navel oranges | 591 mL placebo juice: 1) with ascorbic acid and sugar mixture (fructose, glucose, and sucrose), 2) comparator juice 1 plus hesperidin, 3) comparator juice 2 plus naringenin, and luteolin | Serum antioxidant capacity [ORAC assay]: - -Serum lipoprotein oxidation: - - (AUC as overall measurement of lipoprotein diene formation), ++ (lag time to onset of diene formation) | Compared6 to the other 3 interventions, consuming 100% OJ resulted in improved serum antioxidant capacity (based on greater ORAC at postprandial 1 h and longer serum lipoprotein oxidation lag time at all time points) and greater oxidative protection (based on lower serum lipoprotein oxidation AUC). Authors point out these results were contrary to 100% OJ having the smallest increase in serum total phenolic content |
CRP, C-reactive protein; Dehydro-TXB2, dehydrothromboxane B2; DPPH, 2,2-diphenyl-1-picrylhydrazyl; FRAP, ferric reducing antioxidant power; hs-CRP, high-sensitivity C-reactive protein; DiHOME, dihydroxyoctadecanoic acid; GSH, reduced glutathione; GSH/GSSG, glutathione redox ratio; GSSG, oxidized glutathione; HETE, hydroxyeicosatetraenoic acid; HODE, hydroxyoctadecadienoic acid; iso-, isoprostane; LBP, lipopolysaccharide-binding protein; LPO, lipid peroxidation; MDA, malondialdehyde (a secondary lipid peroxidation marker); MMP, matrix metallopeptidase; MNBC, mononuclear blood cell; MNC, mononuclear cell; NA, not applicable; NR, not reported; OJ, orange juice; ORAC, oxygen radical absorbance capacity; oxLDL, oxidized LDL; PBMC, peripheral blood mononuclear cell; PGF, prostaglandin F; PMN, polymorphonuclear cell; PMN MPO, intracellular polymorphonuclear leukocyte myeloperoxidase; RCT, randomized controlled trial; ref, reference; ROS, reactive oxygen species; SOCS, suppresser of cytokine signaling; TAC, total antioxidant capacity; TAS, total antioxidant status; TBARS, thiobarbituric acid reactive substance; TLR, Toll-like receptor; VCAM, vascular endothelial adhesion molecule; WBC, white blood cell; 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
If total mean (SD) was not reported for study participants, the table presents means as calculated weighted averages (indicated by the “∼” symbol) and separate group SDs reported in the original study (presented in parentheses separated by a comma).
Categories for weight status based on BMI (kg/m2): normal-weight BMI = 18.5–24.9, overweight BMI = 25.0–29.9, obese BMI = ≥30.0 (25).
Results for 100% OJ group: ++, significant increase (P ≤ 0.05); +, marginally significant increase (0.05 < P < 0.10); 0, no effect; -, marginally significant decrease (0.05 < P < 0.10); - -, significant decrease (P ≤ 0.05). Methods or assays used for measuring antioxidant capacity and lipid peroxidation are included in brackets (e.g., [FRAP assay], [ORAC assay], [TAS assay]).
Key findings exclude markers showing no difference between intervention groups.
Results from statistical tests of treatment arm comparisons with 100% OJ were not reported, so the table presents results of effects as described in each article's text.
Results from 2-factor repeated-measures ANOVA where separate treatment effects were not reported. Significance indicates time effects (i.e., change in the marker from baseline) after both meals irrespective of the treatments given with breakfast.
Within-group differences not reported. Overlap in reported mean ± SD values for before and after measures suggests no statistically significant changes in within-group hs-CRP for either blood or blond 100% OJ treatments.
Blood measures were taken before (t0′) and 30 min (t30′) and 24 h after (t24hr) participants underwent a single bout of exhaustive exercise. In results for 100% OJ, we report comparisons of baseline and post–juice supplementation at each time point (e.g., t30′ baseline compared with t30′ after supplementation).
Statistical significance for metabolites related to oxidative stress and inflammation was reported as calculated q-values (false discovery rates accounting for multiple comparisons and estimating reliability of results). For key findings, q ≤ 0.1 was considered statistically significant.
Participants ranged in age from 18 to 84 y, with over half (57%) of the studies reporting average participant ages in their 20s or 30s. Most study populations were considered healthy with normal weight status (48%) or generally healthy with overweight status (19%). The other 7 study populations were deemed at risk of chronic diseases due to obesity (33, 36, 39, 45, 46) and/or cardiometabolic risk factors such as prediabetes (48) and mild hypercholesterolemia (32). Two studies were conducted exclusively with athletes [soccer players (42) and professional athletes practicing cross-country skiing (44)].
For 100% OJ interventions, most studies (67%) reported using commercial and/or pasteurized preparations, while 4 studies used fresh OJ, 1 study assessed both commercial and fresh preparations, and 2 studies did not report preparation type. Ten studies compared 100% OJ consumption with just 1 comparator or no OJ, while 3 compared different 100% OJs [i.e., red to blond (39) or commercial to fresh (32, 48)], and 7 studies assessed multiple interventions. No study compared 100% OJ with vegetable juices or with whole, unblended fruit. Nine studies assessed the impact of 100% OJ on inflammation or oxidative stress induced by a specified trigger [i.e., high-fat (32, 48) or high-fat, high-carbohydrate meal (43, 50), hydrogen peroxide induced in vitro DNA damage (47, 49), exhaustive exercise (26) or intense training (44), and exercise in polluted vs. nonpolluted environments (42)].
Inflammation and oxidative stress markers assessed
Included articles reported >50 markers of inflammation or oxidative stress, which are presented in Table 3. Half of the articles reported on proteins such as CRP, hs-CRP, NF-κB, proteomes in peripheral blood mononuclear cells, and vascular endothelial adhesion molecule 1 (VCAM-1). Several articles also reported on antioxidants and cytokines. The most reported markers were CRP or hs-CRP (n = 9), antioxidant capacity (n = 7), MDA (n = 6), the proinflammatory cytokines IL-6 (n = 5) and TNF-α (n = 5), and oxLDL (n = 3). All other markers were reported by just 1 or 2 articles, indicating potential gaps in the reviewed literature.
TABLE 3.
All markers of inflammation and oxidative stress reported by 22 included articles1
| Marker type | Articles, % | Specific markers | n |
|---|---|---|---|
| Antioxidants | 41 | Antioxidant capacity | 7 |
| GSH | 2 | ||
| GSSG | 2 | ||
| Cytokine suppressors (mRNA and/or protein expression) | 9 | SOCS-1 | 1 |
| SOCS-3 | 2 | ||
| SOCS-7 | 1 | ||
| Cytokines2 | 32 | IFN-γ* | 1 |
| IL-1β*3 | 2 | ||
| IL-4** | 2 | ||
| IL-6*3 | 5 | ||
| IL-10*** | 2 | ||
| IL-12* | 1 | ||
| TNF-α*3 | 5 | ||
| DNA damage | 18 | 8-OHdG | 2 |
| % DNA in tail | 1 | ||
| DNA strand breaks | 1 | ||
| Hemolysis rate | 1 | ||
| Micronuclei rate | 1 | ||
| Endotoxins | 9 | LPS | 2 |
| Enzymes | 9 | MMP-9 | 1 |
| MPO | 1 | ||
| NADPH oxidase subunit p47phox protein | 1 | ||
| p38 MAP kinase | 1 | ||
| p38, phosphorylated | 1 | ||
| Glycoproteins | 9 | E-selectin | 1 |
| LBP | 1 | ||
| Hormones | 5 | Leptin | 1 |
| Leukocytes | 5 | WBC | 1 |
| Lipid peroxidation | 36 | 8-epi-PGF2α | 1 |
| 8-iso-PGF2α | 1 | ||
| MDA | 6 | ||
| LPOs | 2 | ||
| Lipoproteins, pathological | 14 | OxLDL | 3 |
| Metabolites | 5 | 11-Dehydro-TXB2 | 1 |
| 9,10-DiHOME | 1 | ||
| 12,13-DiHOME | 1 | ||
| 9-HODE | 1 | ||
| 13-HODE | 1 | ||
| 5-HETE | 1 | ||
| 12-HETE | 1 | ||
| Other proteins | 50 | CRP | 2 |
| hs-CRP | 7 | ||
| NF-κB | 1 | ||
| Proteomes in PBMCs | 1 | ||
| VCAM-1 | 1 | ||
| Oxypurines | 5 | Hypoxanthine | 1 |
| Xanthine | 1 | ||
| Protein oxidation | 5 | Plasma protein carbonyl groups | 1 |
| Reactive oxygen species | 5 | ROS-PMN | 1 |
| ROS-MNC | 1 | ||
| Toll-like receptor (mRNA and/or protein expression) | 9 | TLR2 | 2 |
| TLR4 | 2 |
Two articles covering 1 study reported different outcomes. CRP, C-reactive protein; Dehydro-TXB2, Dehydrothromboxane B2; hs-CRP, high-sensitivity C-reactive protein; DiHOME, dihydroxyoctadecanoic acid; GSH, reduced glutathione; GSSG, oxidized glutathione; HETE, hydroxyeicosatetraenoic acid; HODE, hydroxyoctadecadienoic acid; iso-, isoprostane; LBP, lipopolysaccharide-binding protein; LPO, lipid peroxidation; MAP, mitogen activated protein; MDA, malondialdehyde (a secondary lipid peroxidation marker); MMP, matrix metallopeptidase; MNC, mononuclear cell; MPO, myeloperoxidase; OxLDL, oxidized LDL; PBMC, peripheral blood mononuclear cell; PGF, prostaglandin F; PMN, polymorphonuclear cell; ROS, reactive oxygen species; SOCS, suppresser of cytokine signaling; TLR, Toll-like receptor; VCAM, vascular endothelial adhesion molecule; WBC, white blood cell; 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
Cytokines: *proinflammatory, **adaptive immunity, ***anti-inflammatory.
Measured by 1 study as mRNA or protein expression of the marker in mononuclear cells (40).
Risk of bias
ROB assessments were carried out at the study level for all included randomized and nonrandomized controlled trials with parallel (n = 5) or crossover (n = 11) designs, and results are presented in Supplemental Figures 1 and 2. Five studies were rated “high” and 11 were rated “some concern” for overall ROB. All 16 studies were rated “some concern” for bias in reporting due to no prepublished analysis plans.
All 5 parallel trials failed to randomize or report details about randomization and concealment; therefore, 4 studies warranted some concern for bias and 1 study comparing athletes with different levels of baseline fitness (44) was rated high. This same study, as well as another study (26), assigned participants to self-administer juice daily for a month but failed to report on adherence so were rated high for this ROB domain. The remaining 3 studies were rated “low” for adherence to assigned intervention, and all 5 parallel trials were rated low for ROB due to missing outcome data and measurement of the outcomes.
Most crossover studies reported few to no details about the randomization process, and many failed to report baseline characteristics by study sequence. Due to these issues, all but 2 studies (32, 39) showed some concern for bias due to the randomization process. For period and carryover effects, 2 studies with washout periods of <1 wk were rated high, and 4 studies not reporting on period effects were rated as some concern. Although 1 study with technical issues was rated high for adherence to assigned intervention, and another warranted some concern for bias due to missing outcome data, all others were rated low for these domains. All 11 crossover studies were rated low ROB for outcome measurement.
Synthesis of results
Table 2 presents results from all included studies for changes in inflammatory and oxidative stress markers related to 100% OJ intake along with key findings for 100% OJ compared with comparator interventions. Due to the heterogeneity across study designs and populations, few studies reporting on each marker, and small study sample sizes, caution should be used when interpreting results and drawing conclusions. In general, study findings suggested that 100% OJ interventions by themselves improved markers, offered protective effects against induced inflammation and oxidative stress, or had no effect. When 100% OJ was compared with non-100% OJ interventions, studies reported either significantly beneficial effects from 100% OJ (e.g., attenuated increased inflammation; reduced rather than increased oxidative stress) or no difference in markers between interventions. One exception was a crossover RCT that compared 100% OJ with a commercial OJ enriched with polyphenols extracted from oranges. One article covering this study found that 100% OJ resulted in significantly greater within-group reductions in lipid peroxidation (LPO) and urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG; markers of oxidative stress), but that polyphenol-enriched OJ produced lower concentrations of urinary 8-OHdG than 100% OJ at endpoint (45). A secondary analysis conducted in a subgroup of participants from this study found that polyphenol-enriched OJ produced significantly beneficial metabolite responses compared with 100% OJ, suggesting stronger protective effects against oxidative stress (53). It is worth noting that this subgroup was not randomly selected from the study population and that only data from sequence 1 of the crossover trial were used in analyses. Another study of note was a before-after study with multiple arms that assessed the impact of juice made from freshly prepared OJ from frozen concentrate or just-opened commercial 100% OJ compared with these OJs on day 8 of storage (52). This study found that, when participants consumed freshly prepared OJ from concentrate, LPO concentrations decreased, indicating reduced oxidative stress, but just-opened commercial 100% OJ produced no change. The study authors attributed this difference to the higher ascorbic acid content of OJ from frozen concentrate, which contains and retains more naturally occurring vitamin C than chilled commercial OJ due to its different processing and packaging methods. After 8 d of storage, consuming either juice increased participants’ LPO, and this increase was significant only after consuming OJ from frozen concentrate due to the decreased LPO on day 1.
The results of markers reported by 3 or more studies (CRP or hs-CRP, IL-6, TNF-α, MDA, oxLDL, and antioxidant capacity) are synthesized below.
C-reactive protein
Two studies reported on CRP. A before-after study showed no change from baseline concentrations after female participants with obesity consumed 500 mL of red OJ daily for 12 wk (36). An acute (7-h) crossover RCT assigned at-risk males with mild hypercholesterolemia to a high-fat meal with 240 mL of commercial 100% OJ without pulp, fresh OJ, an isocaloric sugar-matched control, or OJ with added orange pomace fiber (32). This study reported lower CRP concentrations with time (postprandial 7 h) but no treatment or interaction effects.
Altogether, 7 studies reported on hs-CRP, and 6 of these reported significantly reduced hs-CRP concentrations in healthy [normal weight (41, 44), overweight (38), or both (37)] and at-risk participants with obesity (33, 46) consuming 500 or 1000 mL of 100% OJ/d for 1 to 12 wk. One other study reported no change from baseline hs-CRP for participants with abdominal obesity who consumed 500 mL/d of 100% standard blond OJ and OJ from Sicilian blood oranges—each over 4 wk (39). Four of the studies reporting on hs-CRP had non-100% OJ comparators. Three found significant improvements in hs-CRP concentrations (P < 0.05) for 100% OJ interventions compared with placebo juice with water, orange aroma, sucrose, and citric acid (33) or no OJ (44, 46). The other study found marginally significant differences between groups, with a greater decrease in mean hs-CRP with 100% OJ intake but lower final hs-CRP concentrations with OJ from concentrate (P = 0.07) (41).
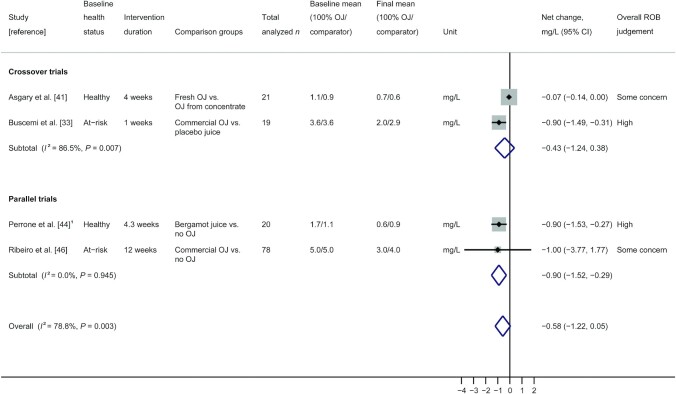
A random-effects model meta-analysis of these latter 4 controlled trials (2 crossover, 1 nonrandomized parallel, and 1 randomized parallel) with 138 total participants is presented in Figure 2. Results showed no difference in net change hs-CRP concentrations (change from baseline) after consuming 100% OJ or a comparator intervention (pooled net change: −0.58 mg/L; 95% CI: −1.22, 0.05) with high and significant heterogeneity (I2 = 78.8%; P = 0.003). This heterogeneity was resolved when 2 studies rated high overall ROB were removed in a sensitivity analysis (I2 = 0.0%; P = 0.51), and overall conclusions remained nonsignificant (pooled net change: −0.07 mg/L; 95% CI: −0.14, 0.00).
FIGURE 2.
Random-effects model meta-analysis of crossover and parallel trials measuring hs-CRP in participants given 100% OJ and non-100% OJ interventions. 1Nonrandomized study; box sizes represent study weight. hs-CRP, high-sensitivity C-reactive protein; OJ, orange juice; ROB, risk of bias.
IL-6
Five studies measuring IL-6 concentrations produced mixed results. Two controlled trials conducted in healthy participants with normal weight reported no change from baseline IL-6 concentration 5 h after consuming 300 kcal of commercial 100% OJ (where IL-6 mRNA expression was measured in mononuclear cells) (40) or 4 wk after consuming 1000 mL fresh OJ daily (where IL-6 was measured in serum) (41). These results were similar for all study comparators [i.e., OJ from concentrate (41) and glucose, cream, or water (40)]. Two crossover RCTs found significant decreases in serum IL-6 compared with baseline, with 100% OJ interventions showing beneficial results over comparators. One of these was conducted in at-risk participants with obesity who consumed 500 mL/d of commercial 100% OJ or placebo juice with water, orange aroma, colorants, sucrose, and citric acid for 1 wk (33). The second was an acute study where IL-6 was measured in healthy participants with normal weight 5 h after consuming a high-fat, high-carbohydrate meal with 500 mL commercial 100% OJ, water, or an isocaloric beverage with water and glucose (43). Last, an acute (7 h) crossover RCT conducted in men with cardiometabolic risk factors (e.g., overweight and mild hypercholesterolemia) found IL-6 to be significantly increased from baseline to 7 h after consuming a high-fat meal with 240 mL of each intervention beverage including commercial 100% OJ, fresh OJ, OJ with orange pomace added, and an isocaloric sugar-matched control (32).
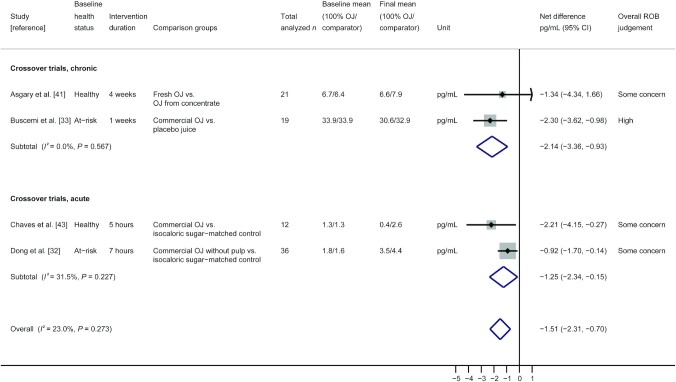
Four of the crossover trials measuring IL-6 (2 acute and 2 chronic trials) were included in a random-effects model meta-analysis, and pooled results are presented in Figure 3 along with results from study duration subgroups. For this analysis, effect measures represent between-group differences at the final postintervention measurement period only (net difference) and not change from baseline IL-6. Pooled results for 88 total participants showed significantly lower IL-6 concentrations after consuming 100% OJ compared with comparators with low heterogeneity, which was not significant (pooled net difference: −1.51 pg/mL; 95% CI: −2.31 pg/mL, -0.70 pg/mL; I2 = 23.0%; P = 0.27). Subgroup analyses showed similar results and conclusions for both acute and chronic studies. When 1 study rated high ROB was removed from the analysis, overall conclusions were similar, but heterogeneity went away (n = 69 participants; pooled net difference: −1.11 pg/mL; 95% CI: −1.81 pg/mL, −0.41 pg/mL; I2 = 0.0%; P = 0.48). Subgroup analyses by juice preparation type are presented in Supplemental Figure 3 and showed similar overall conclusions, with moderate but nonsignificant heterogeneity for the 3 trials using commercial 100% OJ (n = 67; pooled net difference: −1.61 pg/mL; 95% CI: −2.63 pg/mL, −0.59 pg/mL; I2 = 48.6%; P = 0.14).
FIGURE 3.
Random-effects model meta-analysis of crossover trials measuring IL-6 in participants given 100% OJ and non-100% OJ interventions with subgroup analysis by study duration (acute or chronic). Box sizes represent study weight. OJ, orange juice; ROB, risk of bias.
TNF-ɑ
Studies assessing TNF-α reported no change from baseline and/or protective effects with 100% OJ intake. Two before-after studies reporting no change in TNF-α were conducted in healthy participants with normal and/or overweight (750 mL OJ/d for 8 wk) (37) or at-risk females with obesity (500 mL OJ/d for 12 wk) (36). An acute (7-h) crossover RCT in at-risk males showed no change from baseline TNF-α after consuming a high-fat meal plus 240 mL commercial 100% OJ or fresh OJ, suggesting a protective effect (32). However, results were similar after intake of OJ with orange pomace fiber and intake of an isocaloric sugar-matched control. An acute (5-h) nonrandomized parallel controlled intervention with healthy participants of normal weight found no change in TNF-α mRNA expression in mononuclear cells after consumption of water or 300 kcal 100% OJ, suggesting protective effects when compared with increased TNF-α after intake of 300 kcal glucose or cream (40). Last, a crossover RCT in at-risk participants with obesity found significantly lower TNF-α concentrations after 1 wk of 500 mL/d commercial 100% OJ compared with baseline and placebo juice (water, orange aroma, colorants, sucrose, and citric acid) (33).
Malondialdehyde
A secondary product of lipid peroxidation, MDA, was measured in 6 studies reporting beneficial antioxidant effects (lower MDA) or benign results after 100% OJ consumption. One before-after study conducted in generally healthy participants with overweight reported decreased MDA concentrations with intake of 750 mL OJ/d for 8 wk (37). A parallel RCT in at-risk participants with obesity also reported decreased MDA, but results were similar after 12 wk with or without 500 mL/d OJ and a reduced-calorie diet (46). One nonrandomized parallel controlled intervention assigned generally healthy participants with overweight to 750 mL/d of fresh red OJ or no OJ for 4 wk and assessed change in MDA after a single bout of exhaustive exercise (26). This study found no difference in MDA at rest or 24 h post-exercise, but 100% OJ attenuated increases in MDA 30 min after exercise when compared with baseline and the no-OJ group. Two crossover RCTs showed no change from baseline MDA for 100% OJ or comparator interventions. One assigned healthy females with normal weight to a standardized diet with 600 mL/d of 100% blood OJ or no OJ for 3 wk (47). The other assigned at-risk participants with obesity to 500 mL/d 100% OJ or polyphenol-enriched OJ for 12 wk (45). Finally, 1 acute (2.5 h) crossover RCT assigned soccer players to 500 mL of fresh blood OJ or placebo juice before an intense bout of exercise meant to induce oxidative stress (42). While both interventions resulted in significant increases from baseline MDA, 100% OJ significantly attenuated the increase in MDA, suggesting some protection against oxidative stress.
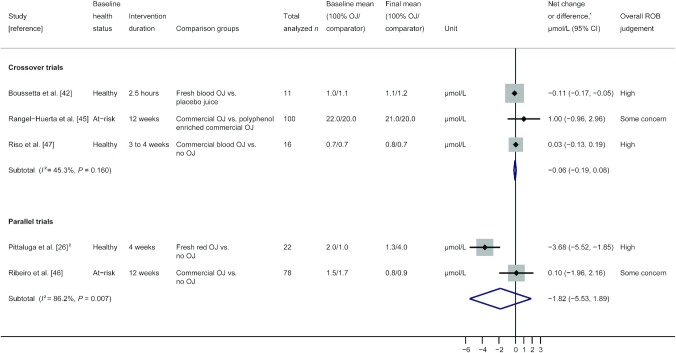
Three crossover trials reporting on MDA were included in a random-effects model meta-analysis, and results are presented in Figure 4. This forest plot also presents individual and pooled results from 1 nonrandomized and 1 randomized parallel trial. In this analysis, crossover trial effect measures are the difference between group MDA concentrations at the final time point (net difference), while parallel trial effect measures represent between-group differences for changes from baseline MDA (net change). Pooled results for the 3 meta-analyzed crossover trials show no effect of 100% OJ on MDA for 127 total participants, with moderate but nonsignificant heterogeneity (pooled net difference: −0.06 μmol/L; 95% CI: −0.19 μmol/L, 0.08 μmol/L; I2 = 45.3%; P = 0.16). Removing studies rated high ROB resulted in insufficient data to perform meta-analyses.
FIGURE 4.
Random-effects model meta-analysis of crossover and parallel trials measuring MDA in participants given 100% OJ and non-100% OJ interventions. 1Different effect measures for parallel (net change) and crossover (net difference) trials. 2Nonrandomized study; Box sizes represent study weight. MDA, malondialdehyde; OJ, orange juice; ROB, risk of bias.
Oxidized LDL
In 3 studies reporting on oxLDL, a measure of lipid oxidation, 100% OJ interventions were shown to attenuate oxidative stress or have no effect. One acute (7-h) crossover RCT conducted in at-risk males with overweight and mild hypercholesterolemia found no change from baseline plasma oxLDL concentrations after consumption of a high-fat meal plus 240 mL of commercial 100% OJ or fresh OJ (32). These results did not differ from comparator interventions including OJ with added orange pomace fiber or an isocaloric sugar-matched control. A nonrandomized parallel controlled intervention assigned male professional athletes to 500 mL fresh bergamot juice or no OJ daily for 30 d with intensive training (44). Here, fresh OJ intake attenuated oxLDL increases seen with the no-OJ group. Finally, a crossover RCT in at-risk participants with obesity showed no change from baseline plasma oxLDL after 12 wk of consuming 500 mL/d of 100% OJ or polyphenol-enriched OJ (45).
Antioxidant capacity
Total antioxidant capacity (TAC) was evaluated using various measures in 2 acute crossover RCTs and 5 multiweek studies. One acute study conducted in healthy participants with normal weight measured serum antioxidant capacity using an oxygen radical absorbance capacity (ORAC) assay 1, 2, and 3 h after participants consumed 591 mL fresh, 100% OJ (35). Average ORAC levels increased significantly from baseline to 1 h after participants consumed the fresh OJ, but levels decreased significantly from baseline to 3 h. The result at postprandial 1 h suggested improved serum antioxidant capacity compared with 3 placebo juices (ascorbic acid and sugar) with or without added flavonoids, but there were no significant differences between groups by hour 3. The other acute study used the total antioxidant status (TAS) assay measuring plasma concentrations and found significantly increased TAS from baseline in healthy soccer players who drank 500 mL 100% blood OJ before an intense bout of exercise; however, change in TAS was no different from what was seen with placebo juice (42).
Three of the multiweek studies found no changes in TAC regardless of 100% OJ or no OJ intake. Two of these studies conducted in at-risk individuals with obesity assigned participants to 12 wk of either 500 mL/d of red OJ [female-only before-after study; plasma TAC measured by ferric reducing antioxidant power (FRAP) assay] (36) or a reduced-calorie diet with or without 500 mL/d 100% OJ from Pera Rio oranges [parallel RCT; TAC measured by radical 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) assay] (46). The other study, a crossover RCT, found a nonsignificant increase from baseline plasma antioxidant capacity in healthy participants with normal weight who consumed a standardized diet with or without 600 mL/d of commercial 100% blood OJ for 3 wk (47). Two before-after studies in generally healthy populations used the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method to measure TAC and found significantly increased levels after 8 wk of 100% OJ consumption compared with baseline. One study assigned participants with overweight to 750 mL/d of 100% OJ (37) while the other assigned participants with normal weight and overweight to 500 mL/d of red OJ (38).
Strength of evidence
Evidence from 14 total studies measuring CRP or hs-CRP, IL-6, TNF-α, MDA, and oxLDL was assessed for SoE. Results can be found in Supplemental Table 3. All 5 markers had very low strength of evidence, primarily due to moderate ROB, small sample sizes and related imprecision, and inconsistency in reported effects across studies. Collective evidence suggested that 100% OJ interventions may produce no effects on CRP, TNF-α, MDA, and oxLDL concentrations but may produce small beneficial effects on hs-CRP and IL-6 concentrations.
Discussion
Summary of evidence
In this study, we aimed to conduct a scoping review, systematic review, and meta-analysis to identify gaps in the literature and summarize available evidence on the impact of 100% OJ on markers of inflammation and oxidative stress in healthy and at-risk adults. The 21 studies identified in this review reported >50 markers of inflammation and oxidative stress. Markers reported by 3 or more studies, including CRP or hs-CRP, IL-6, MDA, TNF-α, oxLDL, and antioxidant capacity, were systematically reviewed, and meta-analyses were conducted on hs-CRP, IL-6, and MDA. Noteworthy gaps in the reviewed literature included the lack of observational studies on this topic and the paucity of studies measuring most markers. Only 2 reviewed studies included >50 participants, no intervention lasted >3 mo, and no studies compared 100% OJ interventions with vegetable juices or whole-fruit equivalents. Importantly, only 2 studies assessed samples with an average age >50 y; yet, incidence of inflammation-related chronic diseases, such as heart disease and diabetes, is known to increase sharply with age and to be more prevalent in those over age 65 (54). Furthermore, few studies were conducted in North and South America where consumption of OJ is high (55) and diseases related to chronic inflammation are prevalent (56–58).
Overall, the impact of 100% OJ interventions alone was either beneficial or null on all markers reported in the included studies. These results remained consistent when 100% OJ was compared with non-100% OJ interventions regardless of which comparators were used (e.g., water, isocaloric sugar-matched controls, no 100% OJ), with the possible exception of a commercial OJ enriched with polyphenols derived from oranges. The meta-analysis for IL-6 concentrations also showed significant improvements in inflammation after 100% OJ interventions compared with non-100% OJ comparators, with no significant heterogeneity between included studies. However, our meta-analyses conducted for hs-CRP and MDA concentrations showed no overall effect after 100% OJ consumption, with some significant heterogeneity that was at least partially explained by ROB among studies measuring hs-CRP. Although results from individual studies and the IL-6 meta-analysis suggested generally improved or attenuated inflammation and oxidative stress following consumption of 100% OJ, the paucity of studies measuring each marker and the very low SoE for commonly reported markers necessitate caution when drawing conclusions regarding effects of 100% OJ on inflammation and oxidative stress.
To our knowledge, no other systematic review has looked at the role of 100% OJ in markers of inflammation and oxidative stress, but 4 reviews published in the last decade considered the impact of fruit and/or juices on inflammation and oxidative stress in healthy or at-risk populations. One very recent systematic review assessed the antioxidant effects of natural foods, including OJ among many other juices and foods, for participants with dyslipidemia (59). Just 1 study using OJ as an intervention in a before-after design was included [a study also included in the present review (37)]. The review authors reported that OJ was among the “natural” foods found to increase plasma antioxidant activity and decrease inflammation and oxidative damage of lipids in dyslipidemias. One older review on the impact of polyphenol-containing fruits and fruit products on inflammation in humans (60) included 2 acute studies on OJ [also reviewed in the present study (40, 50)]. This review concluded that postprandial inflammation induced by Western eating patterns may be stabilized by polyphenol-rich beverages, such as OJ, accompanying the meal. Another older review included 8 studies with OJ interventions (16), 4 of which were included in the present review (33, 40, 50, 51), and investigated their acute and chronic anti-inflammatory properties. The review authors reported that OJ-mediated plasma inflammatory response and related gene expression may be due to the presence of bioactive compounds such as flavonoids. These authors further suggested that fruit juices such as OJ may be useful in preventing and treating chronic disease. This is consistent with the findings from a prior systematic review and meta-analysis that compared incident type 2 diabetes (T2D) risk associated with intake of 100% fruit juice with sugar-sweetened fruit juice in prospective cohort studies (61). Subgroup effects in a stratified meta-analysis showed no effect of 100% fruit juice on incident T2D but a significantly higher risk (28%) of T2D incidence with sugar-sweetened fruit juice intake. It is now well known that several markers of inflammation and oxidative stress are involved in the pathogenesis of diet-related chronic diseases such as T2D (62) and cardiovascular disease (63). While 100% juice has been considered by some to contain too much sugar and not enough fiber to be healthful, evidence uncovered in our present work supports potential benefits of 100% OJ for both healthy and at-risk populations.
Strengths and limitations
One major strength of this study is the inclusion and synthesis of all studies reporting any marker of inflammation or oxidative stress associated with 100% OJ interventions. Although not all study designs included in the scoping review were appropriate for meta-analysis, most studies utilized an RCT design, which is considered the gold standard to assess causality. The addition of before-after studies as well as acute studies provided a comprehensive overview of the literature on this topic. Despite the low number of studies reporting on even the most common markers (CRP or hs-CRP, IL-6, TNF-α, MDA, and oxLDL), the synthesis of available data provides a foundation of understanding for the association between 100% OJ compared with non-100% OJ interventions and inflammation or oxidative stress.
The primary limitations of this study are the low number of articles reporting on each marker of inflammation or oxidative stress, the small sample sizes among included studies, and the very low SoE for commonly reported markers. Consequently, prespecified subgroup analyses by health status and juice preparation type were not possible for most markers of interest, and existing data are insufficient to conduct meta-regression analyses for evaluating potential dose–response relations. Post hoc subgroup analyses by study duration (acute or chronic) were also not possible for most markers given the current evidence, but future reviews could include study duration in analyses to better elucidate the relation between acute postprandial fluctuations and chronic low-grade inflammatory states.
Despite these limitations, 1 aim of this study was to identify gaps in the research, and we believe we were successful in accomplishing this by summarizing the characteristics (especially design, populations, and interventions) of all studies on this topic identified by our search strategies. Furthermore, the breadth of markers, when taken together, provides an indication of overall effects of 100% OJ on the body's inflammatory and oxidation responses. Although the inclusion of crossover trials was a strength of this review, the inappropriate reporting by all included crossover trials negated our ability to capitalize on the power inherent in this study design. One other limitation was the variety of comparators used by studies included in this review. Peluso et al. (64) warned against misinterpreting the effect of juice interventions on postprandial inflammation and oxidative stress and implored the careful consideration of comparator interventions when interpreting effects. In conjunction with the lack of published research on each marker, the variety of reported comparators made it impossible to synthesize results for 100% OJ compared with comparators of similar makeup in this review.
Conclusions
Overall, evidence reviewed in this study suggests that interventions with 100% OJ are not likely to increase inflammatory or oxidative responses in healthy or at-risk adults. Rather, 100% OJ may provide beneficial or null effects on numerous markers of inflammation and oxidative stress in these populations, although experimental data on long-term effects (beyond 3 mo of intake) are still lacking. In particular, 100% OJ may reduce hs-CRP and IL-6 concentrations over time in some healthy individuals and those at risk for chronic disease. Although evidence across >50 markers supports these findings, moderate RoB among controlled trials and very low SoE within the most reported markers urge caution when interpreting results. More large, well-designed studies are needed to increase confidence in conclusions, especially for hs-CRP and IL-6, which may be improved with regular consumption of 100% OJ in both healthy and at-risk individuals.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—TCW: contributed to the conception of the research; MC and KCC: contributed equally to the design of the research; KCC and ARB: contributed equally to the acquisition of the data; KCC: analyzed and interpreted the data; KCC and TCW: drafted the manuscript; and all authors: critically revised the manuscript and read and approved the final manuscript.
Notes
Supported by an unrestricted competitive grant from the Florida Department of Citrus to Think Healthy Group.
Author disclosures: TCW has received scientific consulting fees and research grants from the Produce for Better Health Foundation. The other authors report no conflicts of interest. The sponsor had no role in the study design; the collection, analysis, and interpretation of data; the writing of the manuscript; or the decision where to submit the paper for publication.
Supplemental Methods, Supplemental Figures 1–3, and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CRP, C-reactive protein; DGA, Dietary Guidelines for Americans; hs-CRP, high-sensitivity C-reactive protein; LPO, lipid peroxidation; MDA, malondialdehyde (a secondary lipid peroxidation marker); OJ, orange juice; ORAC, oxygen radical absorbance capacity; oxLDL, oxidized LDL; RCT, randomized controlled trial; SoE, strength of evidence; T2D, type 2 diabetes; TAC, total antioxidant capacity; TAS, total antioxidant status; 8-OHdG, 8-hydroxy-2′-deoxyguanosine.
Contributor Information
Kelly Copeland Cara, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA; Department of Public Health and Community Medicine, School of Medicine, Tufts University, Boston, MA, USA.
Andrew R Beauchesne, School of Medicine, Tufts University, Boston, MA, USA.
Taylor C Wallace, Think Healthy Group, Inc., Washington, DC, USA; Department of Nutrition and Food Studies, George Mason University, Fairfax, VA, USA.
Mei Chung, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA; Department of Public Health and Community Medicine, School of Medicine, Tufts University, Boston, MA, USA.
References
- 1. Wallace TC, Bailey RL, Blumberg JB, Burton-Freeman B, Chen CYO, Crowe-White KM, Drewnowski A, Hooshmand S, Johnson E, Lewis R et al. Fruits, vegetables, and health: a comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit Rev Food Sci Nutr. 2020;60(13):2174–211. [DOI] [PubMed] [Google Scholar]
- 2. US Department of Agriculture; US Department of Health and Human Services . Dietary guidelines for Americans, 2020-2025. 9th ed [Internet]. December 2020. Available from: DietaryGuidelines.gov. [Google Scholar]
- 3. Drewnowski A, Rehm CD. Socioeconomic gradient in consumption of whole fruit and 100% fruit juice among US children and adults. Nutr J. 2015;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clemens R, Drewnowski A, Ferruzzi MG, Toner CD, Welland D. Squeezing fact from fiction about 100% fruit juice. Adv Nutr. 2015;6(2):236S–43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruxton CHS, Gardner EJ, Walker D. Can pure fruit and vegetable juices protect against cancer and cardiovascular disease too? A review of the evidence. Int J Food Sci Nutr. 2006;57(3-4):249–72. [DOI] [PubMed] [Google Scholar]
- 6. O'Neil CE, Nicklas TA, Zanovec M, Kleinman RE, Fulgoni VL. Fruit juice consumption is associated with improved nutrient adequacy in children and adolescents: the National Health and Nutrition Examination Survey (NHANES) 2003–2006. Public Health Nutr. 2012;15(10):1871–78. [DOI] [PubMed] [Google Scholar]
- 7. Maillot M, Vieux F, Rehm C, Drewnowski A. Consumption of 100% orange juice in relation to flavonoid intakes and diet quality among US children and adults: analyses of NHANES 2013-16 data. Front Nutr. 2020;7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agarwal S, Fulgoni VL III, Welland D. Intake of 100% fruit juice is associated with improved diet quality of adults: NHANES 2013-2016 analysis. Nutrients. 2019;11(10):2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Economic Research Service of the US Department of Agriculture . Apples and oranges are America's top fruit choices. [internet]. 2020; [cited 2021 Jul 27]. Available from: https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=58322. [Google Scholar]
- 10. O'Neil C, Nicklas T, Rampersaud G, Fulgoni V. 100% Orange juice consumption is associated with better diet quality, improved nutrient adequacy, decreased risk for obesity, and improved biomarkers of health in adults: National Health and Nutrition Examination Survey, 2003-2006. Nutr J. 2012;11(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–27. [DOI] [PubMed] [Google Scholar]
- 12. Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res. 2014;34(11):907–29. [DOI] [PubMed] [Google Scholar]
- 13. Johnston CS, Dancho CL, Strong GM. Orange juice ingestion and supplemental vitamin C are equally effective at reducing plasma lipid peroxidation in healthy adult women. J Am Coll Nutr. 2003;22(6):519–23. [DOI] [PubMed] [Google Scholar]
- 14. Sánchez-Moreno C, Cano MP, de Ancos B, Plaza L, Olmedilla B, Granado F, Elez-Martínez P, Martín-Belloso O, Martín A. Pulsed electric fields—processed orange juice consumption increases plasma vitamin C and decreases F2-isoprostanes in healthy humans. J Nutr Biochem. 2004;15(10):601–7. [DOI] [PubMed] [Google Scholar]
- 15. Sanchez-Moreno C, Cano MP, de Ancos B, Plaza L, Olmedilla B, Granado F, Martin A. Effect of orange juice intake on vitamin C concentrations and biomarkers of antioxidant status in humans. Am J Clin Nutr. 2003;78(3):454–60. [DOI] [PubMed] [Google Scholar]
- 16. Coelho RC, Hermsdorff HH, Bressan J. Anti-inflammatory properties of orange juice: possible favorable molecular and metabolic effects. Plant Foods Hum Nutr. 2013;68(1):1–10. [DOI] [PubMed] [Google Scholar]
- 17. Institute of Medicine (US) Committee on Standards for Systematic Reviews of Comparative Effectiveness Research . Finding what works in health care: standards for systematic reviews. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 18. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). [Internet]. Cochrane Collaboration; 2021. Available from: www.training.cochrane.org/handbook. [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- 22. Crowe-White K, Parrott JS, Stote KS, Gutschall M, Benson-Davies S, Droke E, O'Neil CE, Wolfram T, Ziegler P. Metabolic impact of 100% fruit juice consumption on antioxidant/oxidant status and lipid profiles of adults: an evidence-based review. Crit Rev Food Sci Nutr. 2017;57(1):152–62. [DOI] [PubMed] [Google Scholar]
- 23. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US FDA . Food labeling: specific food labeling requirements, 21CFR 101.30. 2021 ed [Internet]. Code of Federal Regulations. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.30.
- 25. Centers for Disease Control and Prevention; US Department of Health and Human Services . About adult BMI: how is BMI interpreted for adults. [Internet] ? 2020; [cited 2021 April 11]. Available from: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html#InterpretedAdults.
- 26. Pittaluga M, Sgadari A, Tavazzi B, Fantini C, Sabatini S, Ceci R, Amorini AM, Parisi P, Caporossi D. Exercise-induced oxidative stress in elderly subjects: the effect of red orange supplementation on the biochemical and cellular response to a single bout of intense physical activity. Free Radic Res. 2013;47(3):202–11. [DOI] [PubMed] [Google Scholar]
- 27. Rohatgi A. WebPlotDigitizer. Version 4.4 [software]. Pacifica (CA): Rohatgi, Ankit; 2020. [Google Scholar]
- 28. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 29. RoB 2.0 Working Group on Cross-over Trials, Higgins J, Li T, Altman D, Curtin F, Senn S. Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2). Additional considerations for cross-over trials, preliminary tool version, 18 March 2021. [Internet]. In: Higgins JP, Li T, Sterne J, editors. 2021. Available from: https://drive.google.com/file/d/11LFgCuDpWk5-BvBNbHtNzbJv5-qVpTWb/view.
- 30. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ, Grade Working Group . What is “quality of evidence” and why is it important to clinicians?. BMJ. 2008;336(7651):995–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Grade Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dong H, Rendeiro C, Kristek A, Sargent LJ, Saunders C, Harkness L, Rowland I, Jackson KG, Spencer JPE, Lovegrove JA. Addition of orange pomace to orange juice attenuates the increases in peak glucose and insulin concentrations after sequential meal ingestion in men with elevated cardiometabolic risk. J Nutr. 2016;146(6):1197–203. [DOI] [PubMed] [Google Scholar]
- 33. Buscemi S, Rosafio G, Arcoleo G, Mattina A, Canino B, Montana M, Verga S, Rini G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am J Clin Nutr. 2012;95(5):1089–95. [DOI] [PubMed] [Google Scholar]
- 34. Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196–207. [DOI] [PubMed] [Google Scholar]
- 35. Snyder SM, Reber JD, Freeman BL, Orgad K, Eggett DL, Parker TL. Controlling for sugar and ascorbic acid, a mixture of flavonoids matching navel oranges significantly increases human postprandial serum antioxidant capacity. Nutr Res. 2011;31(7):519–26. [DOI] [PubMed] [Google Scholar]
- 36. Azzini E, Venneria E, Ciarapica D, Foddai MS, Intorre F, Zaccaria M, Maiani F, Palomba L, Barnaba L, Tubili C et al. Effect of red orange juice consumption on body composition and nutritional status in overweight/obese female: a pilot study. Oxid Med Cell Longev. 2017;2017:1672567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dourado G, Cesar TB. Investigation of cytokines, oxidative stress, metabolic, and inflammatory biomarkers after orange juice consumption by normal and overweight subjects. Food Nutr Res. 2015;59:28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Silveira JQ, Dourado GK, Cesar TB. Red-fleshed sweet orange juice improves the risk factors for metabolic syndrome. Int J Food Sci Nutr. 2015;66(7):830–6. [DOI] [PubMed] [Google Scholar]
- 39. Hollands WJ, Armah CN, Doleman JF, Perez-Moral N, Winterbone MS, Kroon PA. 4-Week consumption of anthocyanin-rich blood orange juice does not affect LDL-cholesterol or other biomarkers of CVD risk and glycaemia compared with standard orange juice: a randomised controlled trial. Br J Nutr. 2018;119(4):415–21. [DOI] [PubMed] [Google Scholar]
- 40. Deopurkar R, Ghanim H, Friedman J, Abuaysheh S, Sia CL, Mohanty P, Viswanathan P, Chaudhuri A, Dandona P. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care. 2010;33(5):991–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Asgary S, Keshvari M, Afshani MR, Amiri M, Laher I, Javanmard SH. Effect of fresh orange juice intake on physiological characteristics in healthy volunteers. ISRN Nutr. 2014;2014:405867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boussetta N, Abedelmalek S, Khouloud A, Ben anes A, Souissi N. Does red orange juice supplementation has a protective effect on performance, cardiovascular parameters, muscle damage and oxidative stress markers following the yo-yo intermittent recovery test level-1 under polluted air?. Int J Environ Health Res. 2020;30(6):630–42. [DOI] [PubMed] [Google Scholar]
- 43. Chaves DFS, Carvalho PC, Brasili E, Rogero MM, Hassimotto NA, Diedrich JK, Moresco JJ, Yates JR 3rd, Lajolo FM. Proteomic analysis of peripheral blood mononuclear cells after a high-fat, high-carbohydrate meal with orange juice. J Proteome Res. 2017;16(11):4086–92. [DOI] [PubMed] [Google Scholar]
- 44. Perrone MA, Donatucci B, Pieri M, Salimei C, Marini S, Bernardini S, Iellamo OF. The anti-inflammatory and antioxidant effects of bergamot (citrus bergamia) in professional athletes during endurance training. Acta Medica Mediterranea. 2020;36(4):2491–7. [Google Scholar]
- 45. Rangel-Huerta OD, Aguilera CM, Martin MV, Soto MJ, Rico MC, Vallejo F, Tomas-Barberan F, Perez-de-la-Cruz AJ, Gil A, Mesa MD. Normal or high polyphenol concentration in orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. J Nutr. 2015;145(8):1808–16. [DOI] [PubMed] [Google Scholar]
- 46. Ribeiro C, Dourado G, Cesar T. Orange juice allied to a reduced-calorie diet results in weight loss and ameliorates obesity-related biomarkers: a randomized controlled trial. Nutrition. 2017;38:13–9. [DOI] [PubMed] [Google Scholar]
- 47. Riso P, Visioli F, Gardana C, Grande S, Brusamolino A, Galvano F, Galvano G, Porrini M. Effects of blood orange juice intake on antioxidant bioavailability and on different markers related to oxidative stress. J Agric Food Chem. 2005;53(4):941–7. [DOI] [PubMed] [Google Scholar]
- 48. Cerletti C, Gianfagna F, Tamburrelli C, De Curtis A, D'Imperio M, Coletta W, Giordano L, Lorenzet R, Rapisarda P, Reforgiato Recupero G et al. Orange juice intake during a fatty meal consumption reduces the postprandial low-grade inflammatory response in healthy subjects. Thromb Res. 2015;135(2):255–59. [DOI] [PubMed] [Google Scholar]
- 49. Guarnieri S, Riso P, Porrini M. Orange juice vs vitamin C: effect on hydrogen peroxide-induced DNA damage in mononuclear blood cells. Br J Nutr. 2007;97(4):639–43. [DOI] [PubMed] [Google Scholar]
- 50. Ghanim H, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, Mohanty P, Dandona P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr. 2010;91(4):940–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sánchez-Moreno C, Cano MP, de Ancos B, Plaza L, Olmedilla B, Granado F, Martín A. Effect of orange juice intake on vitamin c concentrations and biomarkers of antioxidant status in humans. Am J Clin Nutr. 2003;78(3):454–60. [DOI] [PubMed] [Google Scholar]
- 52. Johnston CS, Hale JC. Oxidation of ascorbic acid in stored orange juice is associated with reduced plasma vitamin C concentrations and elevated lipid peroxides. J Am Diet Assoc. 2005;105(1):106–9. [DOI] [PubMed] [Google Scholar]
- 53. Rangel-Huerta OD, Aguilera CM, Perez-de-la-Cruz A, Vallejo F, Tomas-Barberan F, Gil A, Mesa MD. A serum metabolomics-driven approach predicts orange juice consumption and its impact on oxidative stress and inflammation in subjects from the BIONAOS study. Mol Nutr Food Res. 2017;61(2):02. [DOI] [PubMed] [Google Scholar]
- 54. Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012;54(Suppl):S29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Neves MF, Trombin VG, Marques VN, Martinez LF. Global orange juice market: a 16-year summary and opportunities for creating value. Trop Plant Pathol. 2020;45(3):166–74. [Google Scholar]
- 56. Pizuorno A, Fierro NA. Latin America and chronic diseases: a perfect storm during the COVID-19 pandemic. Ann Hepatol. 2021;22:100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pahwa R, Goyal A, Bansal P, Jialal I. Chronic inflammation. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 58. Newman D, Tong M, Levine E, Kishore S. Prevalence of multiple chronic conditions by U.S. state and territory, 2017. PLoS One. 2020;15(5):e0232346–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Medina-Vera I, Gómez-de-Regil L, Gutiérrez-Solis AL, Lugo R, Guevara-Cruz M, Pedraza-Chaverri J, Avila-Nava A. Dietary strategies by foods with antioxidant effect on nutritional management of dyslipidemias: a systematic review. Antioxidants (Basel). 2021;10(2):e0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Joseph SV, Edirisinghe I, Burton-Freeman BM. Fruit polyphenols: a review of anti-inflammatory effects in humans. Crit Rev Food Sci Nutr. 2016;56(3):419–44. [DOI] [PubMed] [Google Scholar]
- 61. Xi B, Li S, Liu Z, Tian H, Yin X, Huai P, Tang W, Zhou D, Steffen LM. Intake of fruit juice and incidence of type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2014;9(3):e93471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. [DOI] [PubMed] [Google Scholar]
- 63. Lorenzatti AJ. Anti-inflammatory treatment and cardiovascular outcomes: results of clinical trials. Eur Cardiol. 2021;16:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peluso I, Palmery M. Risks of misinterpretation in the evaluation of the effect of fruit-based drinks in postprandial studies. Gastroenterol Res Pract. 2014;2014:870547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.