Abstract
Neuroinflammation relying on the inflammatory responses of glial cells has emerged as an impactful component of the multifactorial etiology of neurodegeneration in glaucoma. It has become increasingly evident that despite early adaptive and reparative features of glial responses, prolonged reactivity of the resident glia, along with the peripheral immune cells, create widespread toxicity to retinal ganglion cell (RGC) axons, somas, and synapses. As much as the synchronized responses of astrocytes and microglia to glaucoma-related stress or injury, their bi-directional interactions are critical to build and amplify neuroinflammation and to dictate the neurodegenerative outcome. Although distinct molecular programs regulate somatic and axonal degeneration in glaucoma, inhibition of neurodegenerative inflammation can provide a broadly beneficial treatment strategy to rescue RGC integrity and function. Since inflammatory toxicity and mitochondrial dysfunction are converging etiological paths that can boost each other and feed into a vicious cycle, anti-inflammatory treatments may also offer a multi-target potential. This review presents an overview of the current knowledge on neuroinflammation in glaucoma with particular emphasis on the cell-intrinsic and cell-extrinsic factors involved in the reciprocal regulation of glial responses, the interdependence between inflammatory and mitochondrial routes of neurodegeneration, and the research aspects inspiring for prospective immunomodulatory treatments. With the advent of powerful technologies, ongoing research on molecular and functional characteristics of glial responses is expected to accumulate more comprehensive and complementary information and to rapidly move the field forward to safe and effective modulation of the glial pro-inflammatory activities, while restoring or augmenting the glial immune-regulatory and neurosupport functions.
Keywords: glia, glaucoma, immunomodulation, neurodegeneration, neuroinflammation, retinal ganglion cell
1. Introduction
Glaucoma is a major cause of irreversible blindness that currently affects ~80 million individuals worldwide. The number of the people suffering from this blinding disease is predicted to exceed 100 million by the year 2040 (Quigley and Broman, 2006, Tham et al., 2014). A gradual loss of visual function in glaucoma patients corresponds to progressive degeneration of retinal ganglion cells (RGCs), which is characterized by somatic apoptosis in the retina, axonal degeneration in the optic nerve, and synaptic loss at dendrites and axon terminals (recently reviewed (Syc-Mazurek and Libby, 2019, Tezel, 2020, 2021)). On the basis of experimental studies, glaucomatous neurodegeneration is driven by interconnected pathogenic processes that comprise biomechanical, vascular, metabolic, oxidative, and inflammatory components. Among multiple stressors for RGCs in glaucoma, aging and elevated intraocular pressure (IOP) are most prevalent. Currently available treatments for glaucoma therefore depend on the lowering of IOP; however, even efficient control of this modifiable risk factor cannot always prevent disease progression. As increasingly viewed, many other factors beyond IOP affect RGC survival in glaucoma, and glaucomatous neurodegeneration encompasses a complex interplay of multiple triggers, multiple cell types, and multiple molecular pathways. Molecular understanding of glaucomatous neurodegeneration is incomplete; however, distinct molecular programs are seen to concomitantly regulate somatic and axonal degeneration, while genetic, epigenetic, and systemic factors can also modify the risk for initiation and propagation of RGC degeneration.
Emerging evidence illustrates that besides individual susceptibility of RGCs to glaucomatous injury, responses of surrounding glia are also decisive for the fate of these exceptional neurons. Both astrocytes and microglia profoundly respond to glaucoma-related stress, and their intricate interactions determine diverse outcomes of the glia-RGC relationship as being either neurosupportive or neurodestructive. Despite glial resilience to maintain neuronal homeostasis under stressful conditions, these cells may lose their beneficial features and gain a detrimental trait exacerbating neurodegeneration in a chronic course. Likewise, a sustained low-grade inflammation that mainly relies on glial inflammatory responses is recognized to play a crucial role in glaucomatous neurodegeneration (recently reviewed (Baris and Tezel, 2019, Baudouin et al., 2020, Williams et al., 2017c)). Glia-driven neuroinflammation is detectable at multiple injury sites from retina to upper brain centers and at multiple time points from early to latest stages of RGC degeneration. Owing to the immune privileged status of the retina and optic nerve, glia-driven neuroinflammation, as opposed to classical inflammation, is somehow dampened to an intermediate state called parainflammation (Medzhitov, 2008). Even though this state may initially represent a beneficial response to minimize tissue injury and promote its healing, it may afterward convert into a neurodegenerative process.
Finely regulated morphological, molecular, and functional responses of astrocytes and microglia in glaucoma (Lye-Barthel et al., 2013, Seitz et al., 2013, Son et al., 2010) are detectable throughout the RGC projections (You et al., 2019); however, the type and extent of these responses may vary depending on the region, disease stage, and severity (Formichella et al., 2014, Oikawa et al., 2020, Qu and Jakobs, 2013). A topographical spread of glial reactivity along RGC projections may even be manifest in the contralateral normotensive eyes of animal models (Gallego et al., 2012, Ramirez et al., 2020, Ramirez et al., 2010), including the projection sites of RGCs in superior colliculus (Sapienza et al., 2016, Tribble et al., 2021b). Although bilateral spread of astrocyte responses may reflect early metabolic redistribution through gap junction-coupled networks (Cooper et al., 2020), this adaptive situation to promote neuron survival is transient, and prolonged inflammatory responses of glial cells may ultimately worsen neuron injury in the entire visual pathway. With respect to widespread neurodegenerative impacts of neuroinflammation, inhibition of the pro-inflammatory activities can protect RGC somas, axons, and synapses in glaucoma (Baris and Tezel, 2019). Undoubtedly, a thorough understanding of the molecular regulation of neuroinflammation is the key to open the way towards a therapeutic approach by immunomodulation.
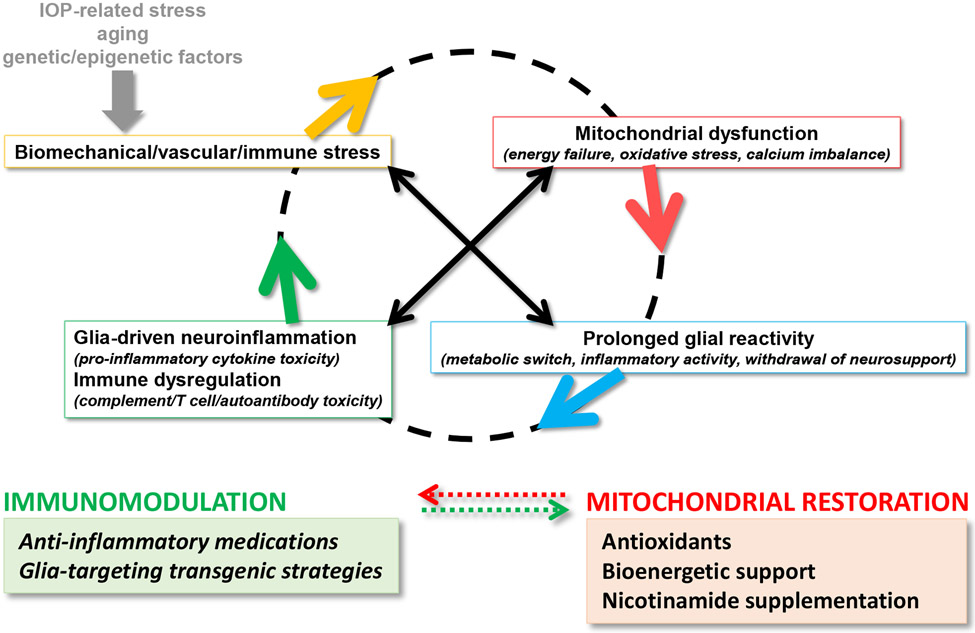
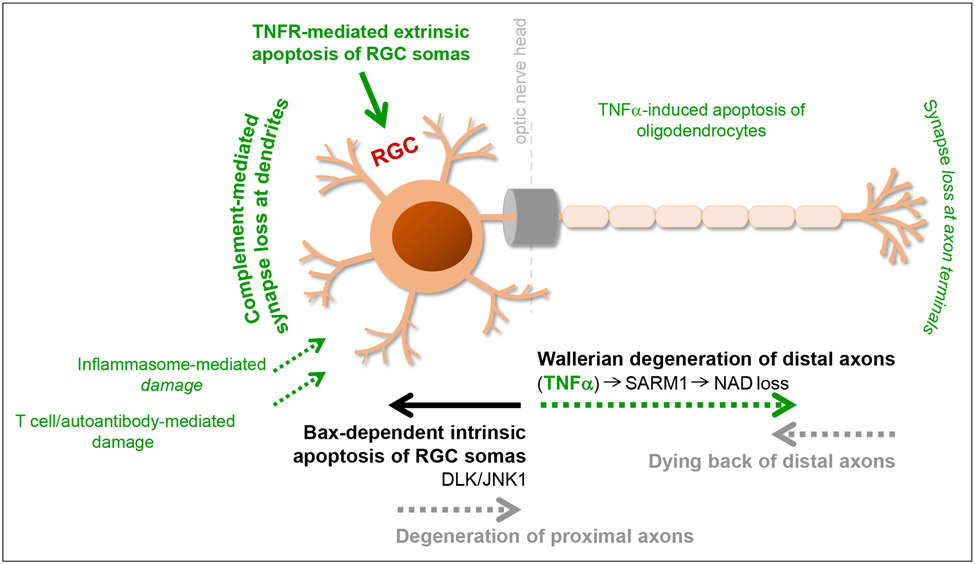
An intriguing aspect comes forward from the substantial roles of mitochondria in neuroinflammation, as mitochondrial dysfunction and inflammatory toxicity present converging neurodegenerative paths that can boost each other’s impacts in glaucoma (recently reviewed (Duarte, 2021, Tezel, 2021)) (Figure 1). While mitochondria are indispensable for RGC health and visual function, structural and functional alterations of the mitochondria impact RGC survival through energy deficits, oxidative stress, and calcium imbalance. As reviewed elsewhere, mitochondria regulate both RGC apoptosis and axon degeneration in glaucoma (Syc-Mazurek and Libby, 2019, Tezel, 2020, 2021). Mitochondria are also critical for regulation of the inflammatory polarization of immune cells, and dysfunctional mitochondria stimulate glial inflammatory responses (Bader and Winklhofer, 2020), as well as rising the susceptibility of RGCs to inflammatory injury. In turn, pro-inflammatory mediators impair mitochondria and amplify the neurodegenerative impacts of mitochondrial dysfunction (van Horssen et al., 2019). This interaction feeding into a vicious cycle is an important area for continued research to explore unified treatment strategies for broader treatment effects in glaucoma (Tezel, 2021). In fact, the best therapeutic benefit for such a multifactorial neurodegenerative disease can be obtained from combination of treatments targeting multiple etiological paths or treatments with the multi-target potential that immunomodulation can offer.
Figure 1. Converging etiological paths of glaucomatous neurodegeneration.
Mitochondrial dysfunction and glia-driven neuroinflammation present interdependent pathogenic processes. Major stressors in glaucoma, including intraocular pressure (IOP) elevation and aging, along with genetic/epigenetic susceptibilities, create biomechanical, vascular, and/or immune stress on RGCs. While dysfunctional mitochondria induce glial inflammatory responses, pro-inflammatory mediators further impair mitochondria, thereby feeding into a vicious cycle that amplifies neurodegeneration in glaucoma. As reviewed herein, preclinical studies explore new strategies for immunomodulation in glaucoma (such as those shown in the green box), which are also expected to protect mitochondria against inflammatory injury. By similarly counting on the interdependence between mitochondrial dysfunction and neuroinflammation, inflammatory outcomes can also be modulated by the treatments targeting mitochondrial dysfunction (such as those shown in the red box, which are tested in clinical studies).
This review highlights recent advances in the molecular understanding of glia-driven neuroinflammation in glaucoma. In this context, particular emphasis is given on the astrocyte-microglia coordination in inflammatory responses, the cell-intrinsic and cell-extrinsic factors regulating neuroinflammation, the interdependence between inflammatory and mitochondrial etiological paths, and the research aspects inspiring for potential immunomodulatory treatments for glaucoma.
2. Glial responses in glaucoma
2.1. Glial subtypes involved in neuroinflammation
Glial cells, including both astrocytes and microglia, are decisively engaged in RGC health and disease. As discussed in the next section, glial cells are highly capable to detect and respond to glaucoma-related stress and to danger signals arising from stressed or damaged RGCs. Although early glial responses encompass adaptive and reparative elements to remove or inhibit stressful and pathogenic factors, this state cannot sustain over prolonged periods. Evidently, persisting tissue stress in glaucoma can prime glial cells for a pro-inflammatory and neurodegenerative shift.
Astrocytes play fundamental roles to retain tissue homeostasis in the retina and optic nerve. Astrocytic processes envelop RGC somas and axons and provide structural support that, in glaucoma, is particularly important to overcome IOP-related biomechanical stress. By removing cellular debris, astrocytes can also contribute to tissue healing, as supported by their increased phagocytic activity in glaucoma (Nguyen et al., 2011). Besides important housekeeping functions, astrocytes are critically involved in the formation, maturation, and maintenance of RGC synapses and synaptic transmission. Along with the retinal Müller glia, astrocytes recycle neurotransmitters and modulate the extracellular glutamate level to maintain synapse function and to prevent glutamate-mediated neurotoxicity to RGCs (Li et al., 1999). By sensing metabolic disturbances, astrocytes, together with the Müller glia and oligodendrocytes, also act to re-establish metabolic homeostasis (Carreras et al., 2015, Saab et al., 2016, Tekkok et al., 2005). They provide neurotrophic, bioenergetic, and antioxidant support, and regulate the extracellular pH, ion, and fluid homeostasis. Moreover, astrocytes are an integral part of the neurovascular unit and regulate neurovascular coupling to meet dynamic metabolic needs of RGCs to preserve synaptic activity. In addition to blood flow regulation, interactions of astrocytes with other components of the neurovascular unit, mainly including vascular endothelium, play a key role in maintaining the tight junctions characteristic of the blood-brain barrier (Sofroniew, 2015, Wareham and Calkins, 2020). These immune-regulatory cells also interact with other resident and peripheral immune cells to sustain the homeostasis of retina and optic nerve tissues.
In response to glaucoma-related stress, astrocytes undergo a significant transformation process known as reactive astrogliosis. Besides astrocytes, retinal Müller glia with remarkable attributes (Bringmann et al., 2006, Lahne et al., 2020, Vecino et al., 2016) also prominently respond in glaucoma (Seitz et al., 2013, Wang et al., 2002). Considering clinical correlations, the retinoschisis detected by retinal OCT imaging in glaucoma patients is highly suggestive of the Müller glia involvement in glaucomatous neurodegeneration (Fortune et al., 2018). However, compared to astrocytes, these critical radial astroglia of the retina remain relatively understudied in the field of glaucoma research.
Reactive astroglia that involve molecularly and functionally heterogonous subpopulations (Escartin et al., 2019) can mobilize opposing activities with neurosupportive or neurodestructive consequences. Both in human donor eyes and animal models with glaucoma, remarkable morphological changes of astrocytes are similarly accompanied by a dynamic spectrum of molecular and functional alterations (Johnson et al., 2007, Nikolskaya et al., 2009, Qu and Jakobs, 2013, Quillen et al., 2020, Sun et al., 2017, Tehrani et al., 2016, Tezel et al., 2003, Tezel et al., 2012b). Although astrocytes primarily function for the goal to preserve tissue homeostasis, limit damage, and optimize neuron survival and visual function, prolonged glial reactivity may turn out to be damaging for RGCs. Many of the beneficial functions of astrocytes also become insufficient, once their reactivity switch is turned on in glaucoma. Glaucomatous astrocytes present upregulated expression of glial fibrillary acidic protein (GFAP), enlarged size of cytoskeleton, and increased extension of cellular processes and migrate into injury sites (Dai et al., 2012, Hernandez et al., 2008, Inman and Horner, 2007, Lye-Barthel et al., 2013, Sun et al., 2013, Tezel et al., 2001a). By becoming hypertrophic, reactive astrocytes maintain the structural integrity and form a physical barrier to insulate healthy neurons and to prevent them from being exposed to neurodegenerative mediators. In the meantime, however, reactive astrocytes activate a self-survival program that may restrict their neurotrophic, bioenergetic, and antioxidant support to RGCs. Along with the deficiency in their neurosupport functions, astrocyte-mediated extracellular matrix remodeling and profibrotic processes eventually amplify the glaucoma-related biomechanical and vascular stress converging at the optic nerve head (Burgoyne, 2011, Burgoyne et al., 2005, Dai et al., 2012, Roberts et al., 2010). As reviewed herein, astrocytes’ immune-regulatory functions also fail in glaucoma, and increased production of neurotoxic mediators promote inflammatory injury to RGCs.
Microglia, on the other hand, are principal innate immune cells of myeloid origin. These resident macrophages are the first responders to injurious insults in the central nervous system. Microglia continuously survey their microenvironment to monitor and keep its homeostasis via scavenging and phagocytosing functions and provide neurotrophic support. Microglia exhibit motility in the surveilling mode, but they can broadly migrate once they receive alerting signals. Similar to astrocyte responses, microglial reactivity and migration into injury sites are evident in experimental models (Bosco et al., 2011, Ebneter et al., 2010, Howell et al., 2011, Qu and Jakobs, 2013, Taylor et al., 2011) and human donor eyes with glaucoma (Neufeld, 1999, Tezel et al., 2003, Yuan and Neufeld, 2001) and are accompanied by a spectrum of neurotoxicities in the glaucomatous retina and optic nerve (Bosco et al., 2008, Bosco et al., 2015). Even though homeostatic roles of microglia are critical for synaptic plasticity by pruning and elimination, this function may also turn into a harmful process in glaucoma (Williams et al., 2016).
Pertaining to glial inflammatory reactivity, innate immune responses of astrocytes and microglia are initially in the favor of optimizing survival, but under persisting stress, they may become active players of inflammatory injury through increased phagocytic activities and increased production of neurotoxic mediators. While early reactivity of microglia are more transient and restricted, astrocyte responses are rapidly produced, broadly manifest, and long-lasting. In this regard, microglia can transmit danger signals to ignite inflammatory responses, and astrocytes with a more dominant quantity (Balaratnasingam et al., 2014) serve as amplifiers of neuroinflammation. Astrocytes organized into syncytial structures by gap junctions also enable long-range signaling for expanded inflammatory responses. Furthermore, owing to their strategic position, dysfunction of reactive astrocytes disturbs the maintenance of vascular barriers, and a leaky barrier allows the entry of peripheral immune cells into the retina and optic nerve (Howell et al., 2012).
Although resident astrocytes and microglia have been the main focus of neuroinflammation, infiltrating immune cells should not be overlooked. Experimental evidence supports that other than resident glia, blood-born monocytes may participate in neuroinflammation as well. While local inflammatory responses facilitate the access of these peripheral immune cells into the retina and optic nerve, their parenchymal infiltration may also amplify local innate immune responses and contribute to the inflammatory injury of RGCs in glaucoma (Howell et al., 2012). Involvement of the blood-borne cells in glaucoma pathophysiology is supported by reduced neurodegeneration after whole-body radiation (Howell et al., 2012) or pharmacological or genetic targeting strategies (Williams et al., 2019) in DBA/2J mice with hereditary glaucoma. Parenchymal infiltration of the circulating immune cells, or non-cellular components of the immune system, is a regulated process involving the interactions between glia and vascular endothelial cells. This process is initiated by the cytokines and chemokines released from resident glia. As the initial signaling leads to loosening of endothelial tight junctions, overexpression of cell adhesion proteins by astrocytes promotes adhesion and migration of immune cells (Howell et al., 2011, Johnson et al., 2011). Further studies of glaucoma models should clarify how other factors, such as help me signals from RGCs, or the cross-talk with Müller glia that are also involved in the regulation of blood-retina barrier, play a role in this process. Optic disc hemorrhages (Uhler and Piltz-Seymour, 2008) and parapapillary chorioretinal atrophy areas (Tezel et al., 2001c), which are commonly detected in patients with glaucoma in association to their increased risk for disease progression (De Moraes et al., 2012), may be viewed supportive of the breaches in vascular barriers. On the other hand, the glial-lymphatic (glymphatic) clearance pathway, a paravascular drainage system existing in the eye and optic nerve (Wostyn et al., 2017), may allow the access of cerebrospinal fluid into optic nerve via the spaces between astrocytic endfeet (Mathieu et al., 2017), as well as allowing the communication with peripheral immune system. Activation of glial inflammatory responses and disruption of vascular barriers may also lead to glymphatic dysfunction, and the accumulated waste molecules may magnify neuroinflammation (Natale et al., 2021, Verheggen et al., 2018).
Myelinating oligodendrocytes that are existent behind the optic nerve head also provide metabolic support to axons, as well as maintaining the axonal integrity (Saab et al., 2016). Oligodendrocytes may not be directly involved in inflammatory responses; however, they may be targeted for inflammatory injury (Ko et al., 2020, Nakazawa et al., 2006, Oikawa et al., 2020, Son et al., 2010). Glia-mediated demyelination may even precede the axon loss via transsynaptic degeneration in glaucoma (You et al., 2019). Oligodendrocytes’ function as a physical barrier for immune cell infiltration into the optic nerve may also be weaken (Dewar et al., 2003, Fitzgerald et al., 2010) after their glaucoma-related injury. Same as Müller glia, oligodendrocytes deserve increased attention of the glaucoma researchers to further investigate their contribution to neurodegeneration and neuroinflammation in glaucoma.
Dendritic cells are another group of resident immune-regulatory cells critical for adaptive immune responses. These CD11c-positive cells existing in the retina (Heuss et al., 2014, Lehmann et al., 2010) have antigen presenting ability (Schlereth et al., 2016), and their major histocompatibility complex (MHC) class-II expression is upregulated after optic nerve crush. Since Gpnmb transmembrane glycoprotein that exhibits mutation in DBA/2J mice is expressed in dendritic cells, the preexisting inflammation in these mice was associated with the altered ocular immune privilege resulting from deficient Gpnmb expression in dendritic cells (Mo et al., 2003). However, the precise role of dendritic cells in antigen presentation to stimulate or maturate adaptive immunity in glaucoma remains unclear.
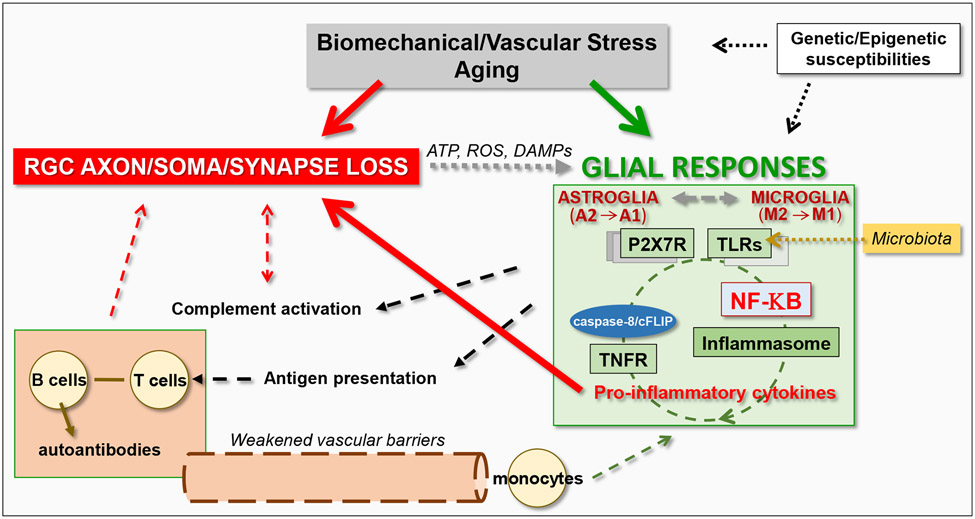
Taken together, heterogeneous responses of glial cells in glaucoma, ranging from neurosupportive to neurodestructive, include prominent inflammatory reactivity that may also support or damage RGCs. As reviewed herein, IOP-related biomechanical or vascular stress, aging, RGC damage, genetic background, and past experiences affecting the epigenetic programs may collaboratively compromise glial immune-regulatory functions over the course of glaucoma. Consequently, a chronic cycle, or recurring waves, of immune stimulation may lead to detrimental rather than beneficial outcomes. The inflammatory circuit initially includes immediate local responses of the resident glia that are followed by the recruitment of peripheral immune cells. By acting as antigen-presenting cells, microglia and astrocytes may also prime lymphocytes for activation of adaptive immune responses (Chidlow et al., 2016, Tezel et al., 2007b, Yang et al., 2001c, Yang et al., 2000). As a result, besides innate neurotoxicity, expanded responses of the immune system may lead to adaptive immunity that may be damaging to RGCs through T lymphocytes and autoantibodies reactive to ocular antigens (Baris and Tezel, 2019, Mac Nair and Nickells, 2015, Williams et al., 2017c). Additionally, complement-mediated processes that are reviewed in section 5 may contribute to the inflammatory phenotype, as well as damaging neurons (Harder et al., 2020b). Thus, neuroinflammation results from the integration of resident glial responses with infiltrating elements of the immune system (Figure 2). Next sections review astrocyte-microglia interactions, initiation of inflammatory responses, amplification cycles, and recruitment of the systemic immune system, which altogether dictate the outcome.
Figure 2. Induction and amplification of glia-driven neuroinflammation in glaucoma.
Glial cells, including both astroglia and microglia, prominently respond to glaucoma-related tissue stress and RGC injury. By sensing the ATP, reactive oxygen species (ROS), and other damage-associated molecular patterns (DAMPs) released from stressed or dying cells, glial cells stimulate inflammation through purinergic receptors (such as P2X7R), pattern-recognition receptors (such as TLRs), and inflammasome activation. The produced pro-inflammatory neurotoxic cytokines contribute to RGC injury. The glia-driven inflammation through multiple pathways are commonly regulated by the NF-κB-mediated transcriptional program. Besides cytokine-mediated neurotoxicity, inflammatory outcomes of complement activation and adaptive immune responses may also contribute to neurodegeneration.
2.2. Astrocyte-microglia interactions
As much as their individual roles in healthy and diseased tissues, a strong partnership between astrocytes and microglia is indispensable to build and amplify neuroinflammation (Kwon and Koh, 2020, Vainchtein and Molofsky, 2020). By releasing various molecules, they establish a mutual communication, besides providing auto-regulatory feedback. Bi-directional interactions of astrocytes and microglia provide a tight modulation of their immune-regulatory or pro-inflammatory fates. Apparently, an early microglial response is followed by astrocyte-mediated expansion of neuroinflammation, and astrocyte-derived factors stimulate microglia for migration and phagocytosis. Regardless of the spatial and temporal diversity of glial responses to perturbations, astrocytes and microglia can function in coordination as one unit. This capacity is derived from distinct but coordinated responses to shared environmental signals, aside from direct molecular conversation. In glaucoma, IOP-related stress and RGC injury appear to be most important to synchronize the astrocyte-microglia unit. Other than the intimate interactions between resident astrocytes and microglia, glia-produced mediators may also promote the parenchymal extravasation of the circulating immune cells that may reinforce neuroinflammation. Recent research advances have empowered to characterize high diversity and subpopulations of glial subtypes in health and disease (Matias et al., 2019, Miller, 2018) and have yield novel molecular clues about the astrocyte-microglia cooperation for neuroinflammation.
The communication between astrocytes and microglia may be mediated through different mechanisms. First of all, both astrocytes and microglia can sense and respond to the same signals in synchrony, and their shared expression of innate immune receptors may coordinate their cross-talk and inflammatory functions. As detailed in section 3.1, these glial cells can sense cellular stress by recognizing the damage-associated molecular patterns (DAMPs). One of the shared signals that astrocytes and microglia commonly respond is the ATP released from stressed or dying RGCs, which can stimulate inflammatory responses through purinergic signaling. Besides purinergic receptors, these glia also express pattern recognition receptors (PRRs), such as toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs). Upon ligation of these receptors by intrinsic DAMPs, an inflammatory response can rapidly be mobilized as well (Krizaj et al., 2014).
Secondly, astrocytes and microglia can directly communicate to amplify or diminish their individual or shared responses. A molecular conversation to promote neuroinflammation may occur through the release of a variety of cytokines, chemokines, or complement molecules. Astrocytes are the major source of many chemokines, while microglia express corresponding chemokine receptors, thereby stimulating microglial motility. Astrocyte-derived cytokines can also regulate the microglial function in synapse engulfment (Vainchtein et al., 2018). Similarly, complement components, such as complement component-3 (C3) expressed by inflammatory astrocytes (Liddelow et al., 2017) can stimulate phagocytosis function of microglia that express complement receptor-3 (CR3). Reciprocal signaling between astrocytes and microglia can also maintain their relative proportions, as microglia control astrocyte numbers by actively engulfing them in the retina (Punal et al., 2019).
As a third cross-talk mechanism between astrocytes and microglia, one cell type receives the signal and relays it onto other cell type. This can be exemplified by the microglia-initiated stimulation of pro-inflammatory and neurotoxic astrocytes (Liddelow et al., 2017). As recently introduced, microglia stimulate the conversion of astrocytes from an anti-inflammatory and neurosupportive status (A2) to a pro-inflammatory and neurotoxic status (A1) in glaucoma (Guttenplan et al., 2020, Liddelow et al., 2017). This conversion is mediated by microglia-derived interleukin-1alpha (IL-1α), tumor necrosis factor alpha (TNFα), and C1q, because triple knockout of these molecules (IL-1−/− TNFα−/− C1q−/−) reduced A1 transformation of astrocytes in ocular hypertensive mouse eyes (Guttenplan et al., 2020).
Before moving further, it is worthwhile to clarify the terminology used for different glial phenotypes. Although astrocytes and microglia present diverse functions in innate immune responses, a traditional simple categorization of macrophages and microglia has included M1 versus M2 phenotypes. These two opposing subpopulations can produce pro-inflammatory or immune-regulatory mediators according to their activation states. The M1 microglia classically activated by LPS and IFNγ produce pro-inflammatory and neurotoxic cytokines (such as TNFα, IL-1β), or oxidants, and raise neurotoxicity. However, the M2 microglia alternatively activated by the release of anti-inflammatory cytokines (IL-10, TGFβ) promote tissue remodeling and repair (Murray et al., 2014, Ransohoff, 2016, Sousa et al., 2017). Following these myeloid-derived cells, astrocytes have also been categorized using a parallel terminology, as A1 versus A2 astrocytes (Escartin et al., 2019, Itoh et al., 2018). Nevertheless, it should be emphasized that this dichotomized classification cannot be fully informative because a combination of different polarization states, ranging from M1 to M2, or A1 to A2, may simultaneously exist. Since some molecular changes may reflect physiological adaptive plasticity, rather than being a reactive response to damaging stimuli, functional outcomes may vary as well. Moreover, by considering the glial reactivity that is elicited in response to tissue stress and injury, which differ depending on the tissue region, disease stage, and severity, the heterogeneity in glial polarization shifts, spatially and temporally. As mentioned above, the astrocyte-microglia communication also continuously shape each other’s inflammatory phenotype. Therefore, polarization of glial cells should be deemed on a dynamic range of molecular and functional parameters, rather than two simplified populations.
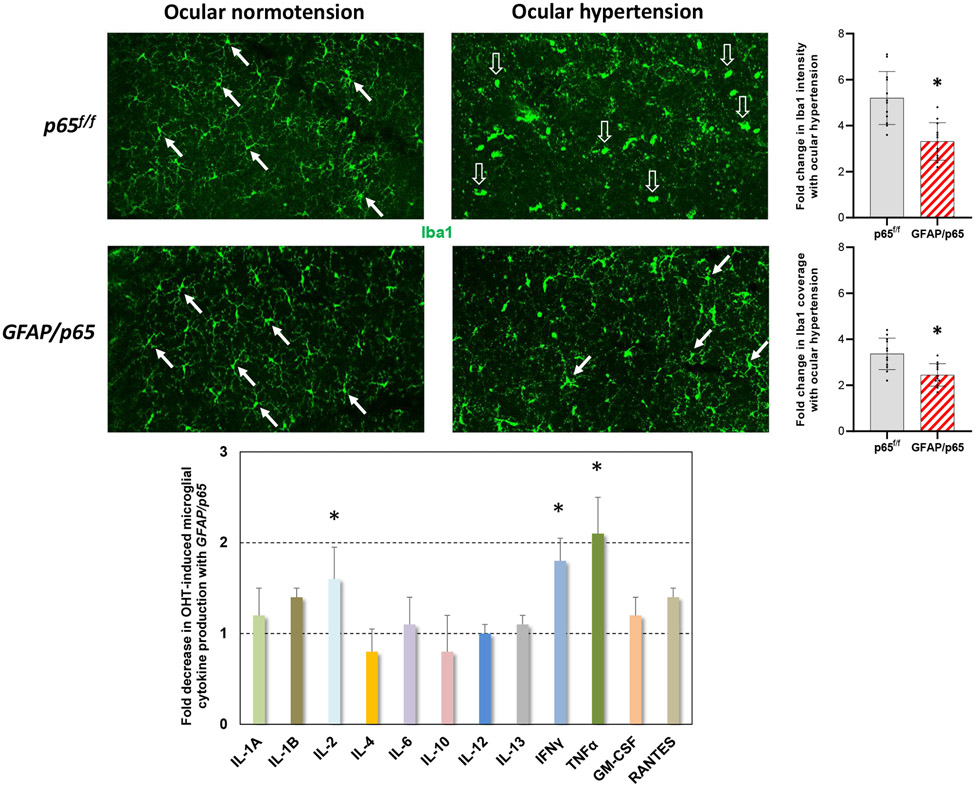
It should also be highlighted that similar to microglia-mediated regulation of astrocyte polarization in glaucoma, astrocyte-derived factors, including NF-κB-regulated pro-inflammatory cytokines, may be involved in shaping the inflammatory responses of microglia as well (Yang et al., 2020). This interaction that was recently noticed in ocular hypertensive mouse eyes (Yang et al., 2020) has also been focused in a more recent pilot study (presented at ARVO 2021, Abstract 3517862; Figure 3). As expected, inhibition of NF-κB activation by the cre/lox-based conditional deletion of astroglial p65 resulted in decreased production of pro-inflammatory cytokines in experimental mouse glaucoma. Intriguingly, besides impeded inflammatory activity of astroglia, some alterations were also detectable in microglia responses after p65 deletion in GFAP-expressing astroglia. On the basis of morphological and molecular analyses, the pro-inflammatory response of microglia was found significantly lower in ocular hypertensive GFAP/p65 mice than ocular hypertensive p65f/f controls. These preliminary observations imply that similar to microglial modulation of astrocyte responses (Liddelow et al., 2017), astrocytes, via NF-κB-regulated mediators, can modulate microglia responses, thereby intensifying neuroinflammation. These observations stimulate further studies of glial interactions in glaucoma.
Figure 3. Astrocyte-microglia communication in neuroinflammation.
Similar to microglia-derived factors stimulating inflammatory activation of astrocytes, astroglia-derived factors may shape the inflammatory responses of microglia in experimental glaucoma. Besides decreased inflammatory activity of astroglia after cre/lox-based conditional deletion of p65 in GFAP-expressing astrocytes, some alterations were also detectable in microglia responses. The morphological response of microglia to ocular hypertension (a shift from ramified morphology, shown by white arrows, to ameboid morphology, shown by translucent arrows) was less prominent, and both the intensity and the coverage of Iba1 labeling were ~30% less in ocular hypertensive eyes of GFAP/p65 mice than ocular hypertensive p65f/f controls (*P<0.001; n= >4 mice/group). As presented in the bar graph (mean±SD), ocular hypertension-induced production of NF-κB-regulated pro-inflammatory cytokines, including IL-2, IFNγ, and TNFα, was significantly lower in the isolated samples of microglia (by immunomagnetic cell selection) from ocular hypertensive GFAP/p65 mice than ocular hypertensive p65f/f controls (*P<0.02; n= >20 mice/group). Intraocular pressure elevation was induced by anterior chamber microbead injections, and the ocular hypertensive mice were followed for 12 weeks. This work has recently been presented at ARVO 2021 Meeting (Abstract 3517862).
In contrast to pro-inflammatory outcomes of the astrocyte-microglia partnership, their communication by immune-regulatory cytokines may restore brain homeostasis after an inflammatory insult. For example, microglial production of IL-10, a regulatory cytokine resolving inflammation, may act through astrocytic IL-10 receptor and stimulate TGFβ secretion that attenuates microglial activation (Norden et al., 2014). Downregulation of purinergic receptors by microglia-derived cytokines may also transform astrocytes to a neuroprotective status (Shinozaki et al., 2017).
Thus, as illustrated in Figures 1 and 2, glial cell responses to glaucoma-related stress and intimate interactions between resident astroglia, microglia, and blood-born components of the immune system control the initiation and amplification of neuroinflammation with neurodegenerative outcomes in glaucoma. Therapeutic modulation of the neurotoxicity generated from innate immunity, complement activation, and adaptive immune responses is therefore regarded as a promising treatment strategy. In the context of immunomodulation, alleviating the pro-inflammatory status of glial cells may prevent or reverse neurotoxic inflammation, whereas enhancing the glial immune-regulatory status may promote neuron survival and repair. Additionally, since neuroinflammation is regulated by microglia-derived and astrocyte-derived signals, which can amplify each other, the therapeutics targeting of one glial subtype may possibly shift the phenotype of other glial subtype as well. Additional studies are strongly warranted to better characterize glial responses in glaucoma; however, distinguishing the individual contributions of astrocytes and microglia to neurodegenerative inflammation seems highly challenging. Not only for their close proximity to each other at the glaucomatous injury sites, but astrocytes and microglia can also synchronically respond to the same stressors and continuously regulate each other’s phenotype during glaucomatous neurodegeneration. Although there may be some sequential differences in glial responses, prolonged tissue stress and the asynchrony in degeneration of individual RGCs during the chronic course of glaucoma may re-amplify inflammatory responses, thereby further complicating the dissection of sequential events. Evidently, inflammation signaling includes many shared components between astrocytes and microglia, which commonly lead to increased production of a similar set of pro-inflammatory cytokines. Despite some differences in their homeostatic functions (such as glutamate clearance or metabolic support predominated by astrocytes, and complement-mediated clearance by microglia), many glial functions overlap between glial subtypes and may become even more similar after glaucoma-related dysregulations. However, recent advances in transcriptomic profiling of astrocytes and microglia have enabled to look at each population individually (Itoh et al., 2018, Sousa et al., 2017), as well as drastically changing the molecular understanding of their communication. Increasing availability of powerful research tools for analysis of molecular responses at the single cell level has similarly changed the focus of glaucoma research. While single-cell RNA sequencing-based transcriptomic profiling and proteomic profiling of the isolated cell types accumulate cell type-specific molecular information, combining the multi-omics data with targeted manipulations in functional studies should rapidly lead new discoveries in the field. An improved comprehension of individual contributions, synchronized responses, and molecular conversation of astrocytes and microglia in neuroinflammation will then aid effective modulation of neurodegenerative inflammation in glaucoma.
3. Inducers and sensors of glia-driven neuroinflammation in glaucoma
3.1. Glaucoma-related stress and injury
Recent studies of transcriptomic (Howell et al., 2011, Johnson et al., 2007, Nikolskaya et al., 2009, Panagis et al., 2010, Steele et al., 2006, Tribble et al., 2020, Yang et al., 2007) or proteomic profiling (Luo et al., 2010, Tezel et al., 2012b, Tezel et al., 2010, Yang et al., 2011) of human donor eyes or animal models with glaucoma have indicated an early upregulation of numerous molecules involved in inflammation pathways. The identified molecules included sensors and inducers (such as pattern recognition receptors, TLRs or NLRs), transducers and regulators (such as MyD88, MAPKs, NF-κB), amplifiers and effectors (such as cytokines and chemokines) of inflammation signaling (Baris and Tezel, 2019, Yang et al., 2011).
Both astrocytes and microglia are exquisitely sensitive to their microenvironment. Apparently, astrocytes can directly sense IOP-related mechanical strain through mechanosensitive ion channels (Beckel et al., 2014, Choi et al., 2015). Stimulation of the inflammatory reactivity may also include a mechanosensitive route mediated by the cooperation of pannexin channels and purinergic receptors (Krizaj et al., 2014). In addition to mechanosensation of IOP-related stress, various other factors, intrinsic to RGCs, or extrinsic, can induce glial inflammatory responses via purinergic receptor or TLR signaling in glaucoma.
As being innate immune cells functioning for the body’s defense, glial cells are equipped to detect extrinsic pathogen-associated molecular patterns (PAMPs). It is separately reviewed in section 3.4 that PAMPs linked to microbiota may likely play an immune-stimulatory role in glaucoma by activating PRRs (Astafurov et al., 2014, Chen et al., 2018). More relevant to glaucoma-related local factors, glial cells can also recognize DAMPs that are intrinsic danger signals of non-microbial origin. The DAMPs can be released, or exposed, from stressed or dying cells. As outlined in section 3.2.1, damaged mitochondria and the mitochondrial DAMPs released after increased membrane permeability can stimulate inflammatory responses in glaucoma. By sensing the DAMPs released from RGCs or glia, glial cells respond by purinergic receptor or TLR signaling, activate inflammasome, produce pro-inflammatory mediators, and recruit additional cells (Krizaj et al., 2014). ATP, as a DAMP, can activate inflammasome, intracellularly, and its mechanosensitive release through pannexin channels (Beckel et al., 2014, Reigada et al., 2008) enables the function of extracellularly transported ATP as an intercellular transmitter via purinergic signaling. Extracellular ATP levels are highly elevated in the glaucomatous retina and optic nerve head in animal models (Lu et al., 2015), and glial cells possess purinergic receptors, including ionotrophic P2X7R (Krizaj et al., 2014, Lu et al., 2015), which are also primed by IOP-related mechanosensitive signaling. Consequently, activation of the ATP-gated purinergic receptors leads to inflammasome activation as detected in the ocular hypertensive animal models (Albalawi et al., 2017, Beckel et al., 2014, Lu et al., 2015, Pronin et al., 2019, Reigada et al., 2008).
The PRRs, including the TLRs located on the cell surface or endosomes can also be activated by DAMPs. In the human donor eyes with glaucoma, different TLR isoforms and their adaptor proteins displayed an early increase in astroglia and microglia (Luo et al., 2010). Experimental animals with induced ocular hypertension (Tezel et al., 2012b) and DBA/2J mice with hereditary glaucoma (Howell et al., 2011) similarly presented upregulation of TLRs. Glaucomatous stress-related intrinsic ligands that can activate TLRs were shown to include Hsps and oxidative stress end-products (Luo et al., 2010). Interestingly, tenascin C that is an extracellular matrix molecule upregulated in the glaucomatous optic nerve head (Johnson et al., 2007, Pena et al., 1999, Wallace et al., 2015) may also serve as another intrinsic ligand for TLRs (Zuliani-Alvarez et al., 2017). This is supported by an experimental study of tenascin deficient mice, in which the pro-inflammatory cytokine response of retinal glia to optic nerve antigen injection was converted to an anti-inflammatory response (Wiemann et al., 2020). After recognizing DAMPs, TLRs recruit toll/interleukin (IL)-1-receptor (TIR) domain-containing adaptors, such as MyD88 (myeloid differentiation primary-response protein 88) or TIR-domain-containing adapter-inducing interferon-β (TRIF). TLR signaling then leads to activation of downstream transcription factors, including NF-κB that induces the production of cytokines and chemokines functioning as amplifiers and effectors of neuroinflammation. Various endogenous signals have been shown to activate innate and adaptive immune responses mediated through TLR signaling in neurodegenerative diseases (Rifkin et al., 2005). Similarly, evidence from experimental models of glaucoma demonstrated that glial TLR signaling, by activating the MyD88-dependent pathway, can lead to NF-κB activation, cytokine production, and T lymphocyte stimulation (Luo et al., 2010).
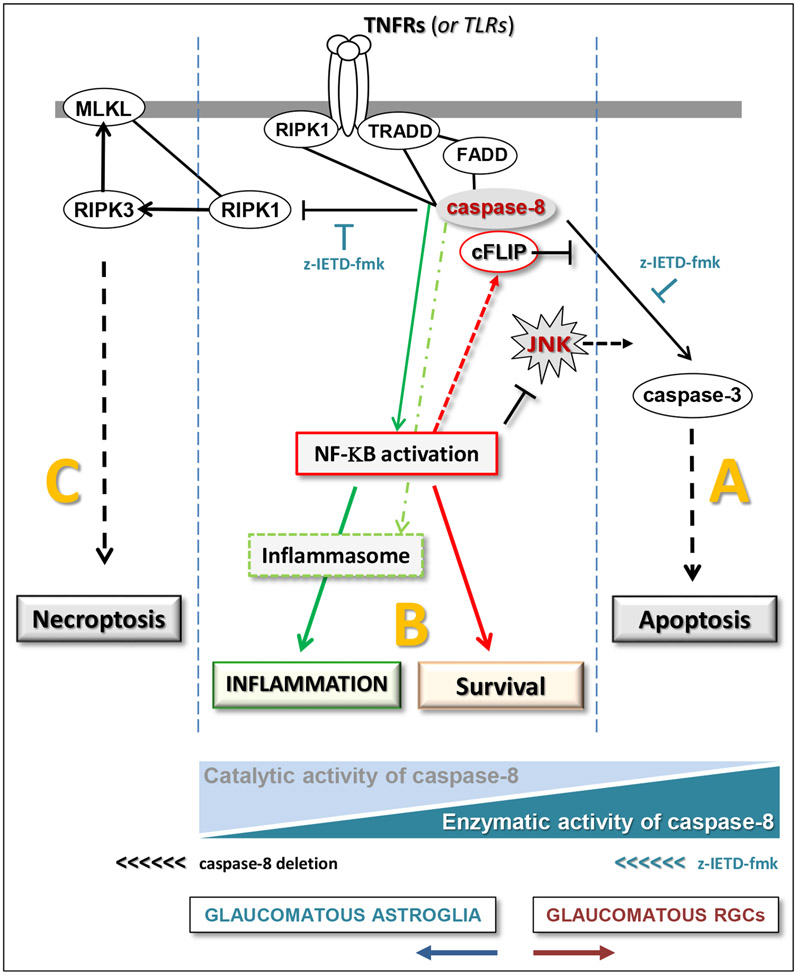
Thus, extracellular DAMPs bind to PRRs and transduce extracellular information to glial cells. As of note, when cells undergo necrosis or necroptosis (that may possibly occur during glaucomatous axon neurodegeneration (Ko et al., 2020)) and lose the integrity of plasma membrane, their intracellular content is released into the extracellular milieu. In the case of apoptosis, however, the cellular content hidden in the cell may be exposed to the immune system when apoptotic cells are engulfed by microglia that can sense the DAMPs sequestered after phagocytosis. Alternatively, if apoptotic cells are not rapidly cleared by phagocytosis, the integrity of their cellular membrane may also be lost, thereby leaking DAMPs (Kono and Rock, 2008). Moreover, intracellular DAMPs can be delivered to the cell surface after injury (Zhu et al., 2011) or may be transported by exosomes as separately outlined in section 3.3.
The NLRs involved in the inflammasome function as PRRs as well. Besides these inflammasome sensors, inflammasomes include pro-caspase-1 and an adaptor protein, apoptosis-associated speck-like protein containing a CARD (ASC). After sensing ATP, or other DAMPs, assembly and activation of this cytosolic multiprotein complex leads to caspase-1-mediated cleavage of pro-interleukins into their active forms and secretion of the cytokines. In support of this inflammatory route in the innate immune responses in glaucoma, ocular hypertensive glia in the retina of human donors (Yang et al., 2011) and experimental animals with glaucoma (Albalawi et al., 2017, Pronin et al., 2019, Tezel et al., 2012b) exhibited upregulation of inflammasome components, including the molecular components of NLR family pyrin domain-containing 3 (NLRP3) inflammasome. Recent studies also showed the contribution of inflammasome to retinal inflammation and RGC death by pyroptosis after induction of acute ocular hypertension. In this model, deletion of caspase-1, pannexin-1 channel (Pronin et al., 2019), or pro-pyroptotic gasdermin D (Chen et al., 2020) ameliorated RGC death. Likewise, NLRP3 knockout mice presented reduced neuroinflammation and delayed RGC demise after optic nerve crush (Puyang et al., 2016).
Hence, as illustrated in Figure 2, cooperative actions of TLRs and inflammasome lead to maturation and secretion of pro-inflammatory cytokines that can further amplify inflammation through NF-κB-mediated transcriptional activation of more cytokines and other immune mediators. As previously mentioned in section 2.2, pro-inflammatory cytokines are involved in astrocyte-microglia communication by inducing their pro-inflammatory states (Liddelow et al., 2017, Yang et al., 2020). While TNFα, an earliest and most abundant pro-inflammatory cytokine released from microglia, can activate A1 astrocytes, the cytokines derived from astrocytes can also provide feedback into this regulatory loop to shape microglia responses (Yang et al., 2020). Besides inducing innate immune responses, glial cytokines empower glial cells for antigen presentation to alert the adaptive immune system as well (Tezel et al., 2007b). In this process, locally generated mediators may also alter the barrier permeability of microvasculature, thereby facilitating the recruitment of circulating immune cells and interaction with the resident glia (Chen et al., 2018, Howell et al., 2012), as pointed out in Section 2.1.
The inflammatory signals arising from damaged RGCs are supported by the attenuation of glial reactivity when the RGC death induced by optic nerve crush was blocked by Bax deletion (Mac Nair et al., 2016). Conversely, in spite of the protection of RGC axons by WldS in DBA/2J mice, glial inflammatory responses persisted (Harder et al., 2017), thereby supporting the independence of inflammatory signals from intrinsic axon degeneration (Ko et al., 2020). On the other hand, deletion of CD11b (Nakazawa et al., 2006), or deactivation of retina and optic nerve head microglia by minocycline (Bordone et al., 2017, Bosco et al., 2008, Levkovitch-Verbin et al., 2006) at early stages of disease delayed neurodegeneration in experimental glaucoma. As reviewed herein, various experimental treatments to modulate glia-driven neuroinflammation have also provided protection to RGCs in ocular hypertensive animals.
Notably, aging is another source for cellular stress in glaucoma, through which the immune system adopts a pro-inflammatory profile, as referred to inflammaging (Franceschi et al., 2007). Indeed, microglial priming in the aged brain has been linked to chronic inflammation in several age-related neurodegenerative diseases. Astrocytes have similarly presented age-related pro-inflammatory changes with important implications in age-related synapse loss and neuroinflammation (Clarke et al., 2018). Such aging-related alterations are likely relevant to neuroinflammation in glaucoma that is an age-related disease, most common in the elderly.
With regard to the vascular stress-related component of neurodegeneration in glaucoma, several studies detected increased expression of endothelins in the retina and optic nerve head of human glaucoma and animal models, and inhibition of the endothelin signaling with an endothelin receptor antagonist provided protection against glaucomatous damage in DBA/2J mice (Howell et al., 2011). Regarding neuroinflammation, intravitreal injection of endothelin-1 in rats induced pro-inflammatory cytokine production in the retina (Nor Arfuzir et al., 2020), as well as causing transient retinal vasoconstriction, hypoxia, and Jun-mediated RGC death (Marola et al., 2020). As a related outcome, increased expression of hypoxia-inducible factor-1 alpha (HIF1α) may also function as a metabolic regulator of neuroinflammation (Corcoran and O'Neill, 2016), which is detailed in section 3.2.3.
An additional stress-related molecule linked to neuroinflammation includes lipocalin-2 that is an acute-phase protein secreted by reactive glia. Diverse cellular processes regulated by lipocalin-2 include iron homeostasis, oxidative stress, and neuroinflammation. As an autocrine promoter of pro-inflammatory responses through NF-κB activity, glial lipocalin-2 was implicated in several neurodegenerative diseases in the brain and retina (Ghosh et al., 2017, Lattke et al., 2017, Naude et al., 2012, Zhao et al., 2019). It was also found upregulated in the glaucomatous retina (Guo et al., 2011, Panagis et al., 2010, Steele et al., 2006, Yang et al., 2007) and induced in vitro neurotoxicity that could be attenuated by iron chelation (Yoneshige et al., 2021).
Thus, various molecular components of the glaucoma-related stress functions as sensors and inducers of glia-driven neuroinflammation in glaucoma. Of note, neuroinflammation in glaucoma may be considered to include the common sensor, transducer, and effector mechanisms associated with various other neurodegenerative diseases. This may imply that as much as the context-dependent signals, other characteristics are also important to define disease-specific pathologies. In the case of glaucoma, specific stressors like increased IOP, unique characteristics of RGCs, and distinctive architecture of the optic nerve head can shape the glial inflammatory responses molding neuroinflammation and neurodegeneration.
3.2. Mitochondrial dysfunction
Mitochondrial dysfunction is placed at the center of an interconnected network of pathogenic processes for RGC degeneration in glaucoma. Glaucoma-related biomechanical and vascular stress, along with aging, can impair mitochondrial structure and function, resulting in energy deficits, oxidative stress, and calcium imbalance. Mitochondria are not only essential for the regulation of RGC apoptosis in glaucoma, but mitochondria-originated neurodegenerative outcomes regulate axon degeneration as well (Casson et al., 2020, Chrysostomou et al., 2013, Tezel, 2020). It has become increasingly evident that the importance of mitochondria goes beyond cellular energy generation or regulation of neuron survival. Multifaceted functions of mitochondria also include the control of inflammatory responses (Bader and Winklhofer, 2020). Dysfunctional mitochondria in glaucoma may similarly stimulate neuroinflammation, aside from increasing the susceptibility of RGCs to inflammatory injury. In addition, aging, also coupled with the impaired mitochondria, may cause a pro-inflammatory profile (Clarke et al., 2018, Franceschi et al., 2007), as well as spoiling the intrinsic mechanisms to cope with inflammation (Mattson and Arumugam, 2018).
As much as a critical role for mitochondrial dysfunction to stimulate inflammation (Bader and Winklhofer, 2020), pro-inflammatory mediators can impair mitochondria and amplify the neurodegenerative impacts of mitochondrial failure (van Horssen et al., 2019). In the same way, mitochondria contribute to inflammasome activation (Dela Cruz and Kang, 2018, Liu et a1., 2018, Yang et al., 2019a) but may also be a targeted by the inflammasome-mediated injury that potentiates mitochondrial ROS production and increased membrane permeability (Yu et al., 2014). It is therefore highly appealing to suggest that the recovery of mitochondrial health may be immunomodulatory, while anti-inflammatory treatments may protect mitochondria against cytokine-mediated injury. There appears to be an intricate interdependence between mitochondria dysfunction and inflammation; however, which of these processes represent the upstream pathology is unclear.
As outlined in the following sections, essential roles for mitochondria at the interface between neurodegeneration and neuroinflammation may be related to inflammatory responses to mitochondrial content, redox-sensitive processes, and the metabolic regulation of glial inflammatory polarization.
3.2.1. Molecular components released from mitochondria
Owing to their proteobacterial origin, mitochondria contain immunogenic molecules that can stimulate inflammation resembling the innate immunity against microbial pathogens (West et al., 2011). Despite immune tolerance mechanisms to prevent innate immunity against healthy mitochondria, such as mitophagy (Nakahira et al., 2011, Zhou et al., 2011), the constituents of damaged mitochondria can initiate a danger response by mimicking the microbial components (Meyer et al., 2018, Rongvaux, 2018). The potential of mitochondria as a target for innate immune responses is supported by the inflammatory reactivity induced by mitochondrial lysates (Wilkins et al., 2015).
Mitochondrial constituents are released after mitochondrial membrane barriers are damaged in response to stress. This leak may be through the mitochondrial permeability transition pore that opens the inner mitochondrial membrane and the Bax/Bak channel that regulates the permeability of outer mitochondrial membrane. After released into cytoplasm or extracellular milieu, these endogenous molecules normally hidden from the immune system act as danger signals and activate innate immune receptors. Mitochondrial DAMPs, such as ATP, mtDNA, cardiolipin, heat shock protein 60 (Hsp60), or reactive oxygen species (ROS), can induce inflammatory responses by engaging the PRRs for transcriptional activation of inflammatory mediators and inflammasome-mediated secretion of cytokines.
Cytosolic mitochondrial DNA (mtDNA), particularly the oxidized mtDNA, can activate inflammasome, which can be released extracellularly and stimulate TLRs too (Shimada et al., 2012, West and Shadel, 2017, Zhong et al., 2018). Cardiolipin, another mitochondrial DAMP, can similarly initiate PRR-mediated inflammation signaling in astrocytes and microglia (Mathew et al., 2012). Hsp60 that displays increased expression in the glaucomatous retina and optic nerve head (Tezel et al., 2000, Yang et al., 2015b) is a mitochondrial chaperone involved in the mitochondrial unfolded protein response. This highly antigenic stress protein associated with various autoimmune diseases also holds important links to autoimmunity in glaucoma (Chen et al., 2018, Wax, 2001, Wax et al., 2008).
Mitochondrial quality control by mitophagy is a critical intrinsic response to prevent inflammation, because removing the damaged mitochondria can eliminate mitochondria-originated inflammatory stimulants. Although mitophagy provides an immune tolerance mechanism towards impaired mitochondria (Nakahira et al., 2011, Zhou et al., 2011), inflammasome activation may inhibit mitophagy (Rawat et al., 2019, Yu et al., 2014). An autophagic activity was detected in DBA/2J glaucoma (Kleesattel et al., 2015); however, mitochondrial recycling by mitophagy was inefficient (Coughlin et al., 2015). Accordingly, restoration of the dysfunctional mitophagy in ocular hypertensive rat (Dai et al., 2018) or mouse eyes (Hass and Barnstable, 2019) improved RGC survival. Suppression of the mammalian target of rapamycin (mTOR) that is a key nutrient sensor, metabolic regulator, and suppressor of autophagy also diminished metabolic failure and neurodegeneration in a recent study of DBA/2J glaucoma (Harder et al., 2020a). Yet, these studies did not evaluate treatment responses on neuroinflammation. In support of the view that autophagic stimulation can eliminate the inflammatory outcomes of damaged mitochondria (Baik et al., 2019, Ip et al., 2017), neuroprotective outcomes of rapamycin in rat eyes with chronic ocular hypertension were attributed to inhibition of microglial activation and NF-κB-regulated pro-inflammatory cytokine production (Su et al., 2014). As reviewed later in section 9.1, optineurin is a critical molecule for the regulation of mitophagy and neuroinflammation in glaucoma.
Mitophagy for mitochondrial recovery coordinates with the mitochondrial unfolded protein response that protects mitochondria through mitochondria-nucleus communication to promote mitochondrial biogenesis and metabolic adaptation (Shpilka and Haynes, 2018). With respect to the inflammatory ability of misfolded proteins in neurodegenerative diseases (Daniele et al., 2015, Kim et al., 2013, Salminen et al., 2020, Ta et al., 2016), the unfolded protein response originated in the mitochondria, or in the endoplasmic reticulum, also appears to be important for immune regulation. However, any contribution of mitochondrial UPR to neuroinflammation remains undefined.
Intriguingly, the inflammatory signals from microglia to astrocytes may be mediated, in part, by fragmented mitochondria that can drive the dissemination of neuroinflammation. This is supported by a recent study, in which the fragmented extracellular mitochondria released from microglia propagated inflammatory neurodegeneration by inducing the activation of A1 astrocytes and cytokine-mediated neurotoxicity in mouse models with neurodegenerative diseases. Selective inhibition of the dynamin-related protein-1/Fis1-mediated excessive mitochondrial fission in the microglia interrupted this pathological cycle between microglia, astrocytes, and neurons (Joshi et al., 2019). This study that yields another example for the astrocyte-microglia interactions in neuroinflammation suggests that the cell debris containing various DAMPs can trigger inflammatory responses. Whether the mitochondrial fragmentation detected in animal models of glaucoma has links to glial inflammatory activity warrants further investigation. Another related question that would also be interesting to answer is whether transcellular mitophagy may represent a similar inflammatory path, through which the mitochondria within RGC axons are taken up by nearby astrocytes for degradation within astrocytic lysosomes (Davis et al., 2014). As previously mentioned, necroptosis, a necrosis-like programmed cell death, may also help the exposure of mitochondrial content to immune cells. This inflammatory mode of cell death (He et al., 2009, Zhang et al., 2009) has recently been implicated in TNFα-induced degeneration of RGC axons in a neuroinflammatory model of glaucoma (Ko et al., 2020).
3.2.2. Oxidative stress
Mitochondria is a major source for free radical generation that can result in oxidative stress when the production of ROS overcomes the intrinsic antioxidant capacity. Oxidative stress constitutes a main route for mitochondria-originated inflammatory responses (Tezel, 2011, Tezel et al., 2007b, Yang et al., 2016), in addition to direct neurodegenerative outcomes that can intensify RGC damage in glaucoma (Chidlow et al., 2017, Chrysostomou et al., 2013, Tezel, 2006). By changing the physiological redox balance, ROS can modulate intracellular signaling and enhance the secretion of pro-inflammatory molecules. For instance, NF-κB, the key activator of the glia-produced inflammatory mediators in glaucoma (Tezel et al., 2012b, Yang et al., 2020) is a redox-sensitive transcription factor. Indeed, antioxidant treatment resulted in decreased activities of this immune regulator molecule in experimental glaucoma (Yang et al., 2016). Similar to various other DAMPs originating from mitochondria, the NLRP3 inflammasome can sense ROS (Zhou et al., 2011). While oxidative stress stimulates pro-inflammatory cytokine production of reactive glia (Tezel et al., 2007b), these cytotoxic molecules may further damage mitochondria (Tezel and Yang, 2004). Oxidative stress also promotes oxidation of cellular macromolecules, including proteins (Tezel et al., 2005). Like other DAMPs, oxidatively modified proteins, or oxidized mtDNA, serve as intrinsic ligands for glial TLRs, as well as activating the inflammasome. Evidently, TLR signaling induces inflammatory responses of astrocytes and microglia and signals for the proliferation and cytokine secretion of T lymphocytes (Luo et al., 2010). Moreover, ROS function as co-stimulatory molecules for glial antigen presentation to T lymphocytes (Tezel et al., 2007b), and protein oxidation (Tezel et al., 2005) modifies the antigenicity of retina and optic nerve proteins to induce autoimmunity against self-molecules (Tezel et al., 2012a). Advanced glycation end-products that are generated through oxidative stress-related processes and accumulate in the glaucomatous tissues (Tezel et al., 2007a) may similarly act as an antigenic stimulus, besides keeping the ability to activate inflammation signaling via specific receptors, RAGE. Additional evidence supports the role for oxidative stress-related modifications to induce complement dysregulation in glaucoma (Tezel et al., 2010). Agreeing with the inflammatory outcomes of oxidative stress, antioxidant treatment alleviated inflammation and improved RGC survival in experimental glaucoma (Yang et al., 2016). When Tempol, a multifunctional antioxidant, was delivered by constant infusion using osmotic mini pumps, glial NF-κB activation and pro-inflammatory cytokine production were reduced, and RGC somas and axons were rescued in ocular hypertensive rat eyes (Yang et al., 2016). Ketogenic diet to modify lipid metabolism, which resolved energy compromise and oxidative stress and protected RGCs against DBA/2J glaucoma (Harun-Or-Rashid et al., 2018), also ameliorated NF-κB-regulated inflammation (Harun-Or-Rashid and Inman, 2018). Many other experimental or clinical studies tested different antioxidant strategies (Garcia-Medina et al., 2020, Tribble et al., 2021a) and detected mild to moderate protection against glaucoma but did not assess treatment responses on inflammatory outcomes.
By insulting the mitochondria, light, especially the short wavelength form, may increase the risk for ROS generation and oxidative damage to energy-depleted RGCs in glaucoma (Osborne et al., 2010, Osborne et al., 2006). A similar potentiation of oxidative stress in the light-exposed retina was implicated in the inflammatory path leading to age-related macular degeneration (Ozawa, 2020). Disruption of mitochondrial iron homeostasis may also synergistically contribute to inflammatory outcomes of mitochondrial dysfunction and oxidative stress. While iron boosts microglial cytokine secretion (Nnah et al., 2020), pro-inflammatory cytokines like TNFα promote iron accumulation through NF-κB signaling and also affect iron homeostasis through transcriptional modification of iron transporters (Holland et al., 2018, McCarthy et al., 2018, Thomsen et al., 2015, Urrutia et al., 2013). As a potent generator of oxidative stress, iron was implicated in retinal neurodegenerative diseases (He et al., 2007). Iron-regulating molecules, such as transferrin, ceruloplasmin, and ferritin, were also found upregulated in the retina and optic nerve head astroglia in human glaucoma and animal models (Chidlow et al., 2017, Farkas et al., 2004, Miyahara et al., 2003, Stasi et al., 2007). Although increased expression of these iron-regulators may argue with the iron accumulation in glaucomatous tissues, iron chelation with oral administration of deferiprone protected RGCs in ocular hypertensive mice (Cui et al., 2020).
3.2.3. Metabolic regulation of glia polarization
Besides oxidative stress, bioenergetic pathways bound mitochondrial dysfunction and neuroinflammation together. Mitochondria are critical for establishment and maintenance of immune responses that require an active metabolism. By controlling the cellular metabolism and signaling pathways, mitochondria occupy a central place in the regulation of inflammatory polarization of immune cells. Evidently, glia-polarizing signals for the regulation of effector activities trigger metabolic shifts. Inflammatory maturation of M1 macrophages is consistent with a metabolic reprogramming for aerobic glycolysis as supported by suppression of mitochondrial oxidative phosphorylation and enhancement of glucose uptake and glycolytic flux. In contrast, M2 macrophages adopt a metabolic program dominated by oxidative phosphorylation. In line with these, inhibition of the M1-like pro-inflammatory phenotype resulted in increased mitochondrial respiration, thereby linking the mitochondrial oxidative metabolism to an anti-inflammatory program (Jha et al., 2015, Langston et al., 2017, Vats et al., 2006). Although metabolism has initially emerged as a key regulator of the peripheral immune cells (Pollizzi and Powell, 2014), the field has quickly expanded to brain microglia. It was then shown that amyloid beta may be sufficient to rapidly switch microglial metabolism from respiratory to glycolytic a crucial step for their pro-inflammatory transformation and increased phagocytosis (Baik et al., 2019).
Most of the evidence for the metabolic programming of anti-inflammatory actions has accumulated from the studies of IL-10. This anti-inflammatory cytokine restricts inflammation by decreasing the pro-inflammatory cytokine production and increasing the anti-inflammatory gene expression through signal transducer and activator of transcription-3 (STAT3) signaling (Lang et al., 2002). In response to inflammatory stimuli, IL-10 regulates the glycolic commitment of macrophages by preserving oxidative phosphorylation via the suppression of nitric oxide. While inflammatory macrophages utilize arginine for nitric oxide production, IL-10 limits the availability of arginine by increasing the arginase expression (Baseler et al., 2016, Corraliza et al., 1995), inhibiting the inducible nitric oxide synthase (iNOS) transcription (Cunha et al., 1992, Huang et al., 2002, Huang et al., 2009), or enhancing the iNOS protein degradation. IL-10 also suppresses mTOR and promotes autophagy to eliminate dysfunctional mitochondria, ROS generation, and inflammasome activation (Ip et al., 2017).
Pertaining to neuroinflammation, pharmacologic suppression of mTOR resulted in a decreased microglial release of pro-inflammatory cytokines, IL-1β and TNFα, thereby implicating mTOR signaling in amyloid beta-induced glycolytic shift in Alzheimer’s disease (Baik et al., 2019). As previously mentioned, rapamycin-mediated inhibition of mTOR that controls cellular metabolism and negatively regulates autophagy reduced microglial activation and pro-inflammatory cytokine production in ocular hypertensive rat eyes (Su et al., 2014). Another study using rapamycin, or pyruvate, detected a metabolic improvement in DBA/2J mice but did not examine inflammatory responses (Harder et al., 2020a).
More recently, mitochondrial isoform of arginase (Arg2) has been highlighted as a downstream mediator of the IL-10-regulated anti-inflammatory responses. This enzyme required for ammonia detoxification in the urea cycle was shown to play an integral role for metabolic regulation of inflammation. The Arg2 that is regulated by the IL-10/miR-155 axis was found essential for metabolic reprogramming to resolve the inflammatory status of macrophages through an IL-10-mediated downregulation of HIF1α and IL-1β, in vitro (Dowling et al., 2021). The arginase pathway has also been linked to various retina and brain injuries (Fouda et al., 2020). Arguing with the Arg2-mediated anti-inflammatory activities, however, Arg2 knockout protected retina structure and function and reduced glial reactivity after retinal ischemia-reperfusion injury and optic nerve crush (Shosha et al., 2016, Xu et al., 2018).
When looking upon HIF1α in the metabolic regulation of neuroinflammation (Corcoran and O'Neill, 2016), its expression was found increased in the retina and optic nerve head of human donor eyes with glaucoma (Tezel and Wax, 2004) and ocular hypertensive mice (Jassim et al., 2021, Jassim and Inman, 2019, Williams et al., 2017b), thereby supporting a hypoxic element of glaucomatous neurodegeneration. A HIF1α-regulated mechanistic background for neuroinflammation also appears to be aligned with the switch from mitochondrial to glycolytic metabolism detected in the ocular hypertensive human retinas (Yang et al., 2015b) or DBA/2J glaucoma (Jassim et al., 2021).
These observations altogether stimulate a new field of research to determine whether metabolic alterations may shift the glial immune-regulatory functions towards neurodegenerative inflammation in glaucoma, and whether therapeutic manipulation of the bioenergetic pathways may help resolve neuroinflammation. Recent experimental treatments to increase metabolic cofactors, such as nicotinamide adenine dinucleotide (NAD), by vitamin 3 supplementation (Tribble et al., 2021c, Williams et al., 2017b), or to provide alternative energy sources by pyruvate supplementation (Harder et al., 2020a), which are now under clinical trial, presented an encouraging trend for neuroprotection in glaucoma; however, their immunomodulatory outcomes remain undefined. More recently, systemic treatment with the NAD precursor nicotinamide riboside similarly provided structural and functional protection to RGCs in mice after optic nerve crush or ocular hypertension-induced injury. Nicotinamide riboside treatment also suppressed astrocyte reactivity after optic nerve crush (Zhang et al., 2021).
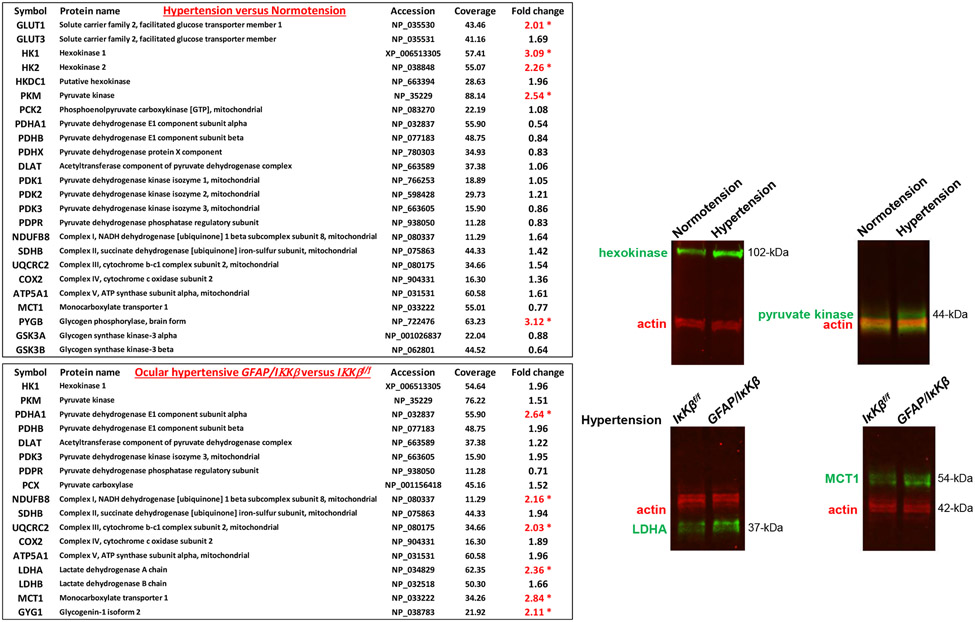
It is also worth mentioning that recent unpublished observations in experimental glaucoma indicated an altered metabolic profile of reactive astrocytes (presented at ARVO Meeting 2019, Abstracts 3788 and 3792). As displayed in Figure 4, analysis of the isolated astrocyte proteome by isotope labeling-based quantitative mass spectrometry detected a glycolytic upregulation as prominent by increased expression of the enzymes that catalyze the rate-limiting steps of glycolysis, including hexokinase and pyruvate kinase. However, unchanged or reduced expression of pyruvate dehydrogenase implied a limited entry of the glycolysis-derived pyruvate into the tricarboxylic acid cycle and oxidative phosphorylation, thereby pointing to aerobic glycolysis. The glycolytic activity was parallel to increased expression of glucose transporter-1 that lifts glucose uptake and increased expression of glycogen phosphorylase that boosts glycogen turnover. Similar to previous observations in DBA/2J glaucoma (Harun-Or-Rashid et al., 2018), glaucomatous astroglia also exhibited a decrease in the expression of monocarboxylate transporter-1 (MCT1). Since the astrocyte-neuron energetic shuttle supplies RGCs with energy substrates to fuel oxidative metabolism (Tekkok et al., 2005), this observation suggests that reactive astrocytes direct the metabolic resources towards their inflammatory activation, rather than metabolite delivery to RGCs. Conversely, transgenic inhibition of inflammation by IκKβ deletion in astrocytes, which provided RGC protection in experimental glaucoma (Yang et al., 2020), reversed this metabolic profile as suggested by increased expression of pyruvate dehydrogenase and MCT1. These observations raise exciting possibilities as to whether neuroinflammation and the altered metabolic programming in astrocytes can amplify the metabolic vulnerability of RGCs in glaucoma, and whether immunomodulation may improve cellular metabolism, besides eliminating the inflammatory toxicity to RGCs. It is tempting to speculate that therapeutic inhibition of the pro-inflammatory activities in astrocytes may tune their altered bioenergetic profile and may alleviate their own energy need, thereby recovering glial metabolic support to RGCs in glaucomatous eyes. This stimulating aspect of immunomodulation also awaits further studies to explore, as much as the other way around.
Figure 4. Switched metabolic profile of reactive astrocytes in experimental mouse glaucoma.
Proteomic analysis of the isolated astrocyte proteins (by immunomagnetic cell selection) by isotope labeling-based quantitative mass spectrometry presented upregulation of glycolysis as evident by increased expression of enzymes catalyzing the rate-limiting steps of glycolysis, including hexokinase and phosphofructokinase (*P<0.05). Alterations in the expression of selected proteins were also validated by Western blot analysis of astrocyte proteins. In support of aerobic glycolysis, unchanged or reduced expression of pyruvate dehydrogenase suggested limited entry of pyruvate into the tricarboxylic acid cycle and oxidative phosphorylation. However, there was an accompanying decrease in the expression of monocarboxylate transporter (MCT1) in the glaucomatous astroglia, suggesting decreased metabolite delivery to stressed RGCs. Inhibition of NF-κB activation by IκKβ deletion in GFAP-expressing astrocytes (GFAP/IκKβ) reversed this metabolic profile of reactive astrocytes in mouse glaucoma, as prominent by an increased expression of pyruvate dehydrogenase and MCT1. Intraocular pressure elevation was induced by anterior chamber microbead injections, and the ocular hypertensive mice were followed for 12 weeks. This work has recently been presented at ARVO 2019 Meeting (Abstracts 3788 and 3792).
3.3. Exosomes
Exosomes, nanoscale extracellular vesicles carrying lipids, proteins, mRNA transcripts, and microRNAs, contribute to intercellular communication in normal and pathological conditions. In response to various stimuli, microglial cells were shown to release exosomes to thereby interact with other glial cells for propagation of inflammatory signals (Paolicelli et al., 2019). Astrocyte-derived exosomes that can similarly transport misfolded proteins and DAMPs, including heat shock proteins, were also implicated in spreading the neuroinflammation in neurodegenerative diseases (Gupta and Pulliam, 2014). On the basis of proteomic profiling, the cargo of the astrocyte-derived nanovesicles presented stimulus-dependent modifications that may be anti-inflammatory or pro-inflammatory (Datta Chaudhuri et al., 2020). In the mouse retina, the exosomes isolated from the microglia exposed to elevated hydrostatic pressure promoted the production of pro-inflammatory mediators and increased the motility, proliferation, and phagocytosis of retinal microglia, in vitro and in vivo (Aires et al., 2020). These secreted transporters were detected in the aqueous humor (Dismuke et al., 2015); however, whether the exosomes loaded with DAMPs are involved in inflammatory stimulation during glaucomatous neurodegeneration is unknown.
It is also important to note that there are alternative ways for cellular communication, not requiring extracellular secretion. As detected in the ocular tissues, including trabecular meshwork (Keller et al., 2017) and retina (Alarcon-Martinez et al., 2020), tunneling nanotubes (TNTs) connect the cytoplasm of adjacent cells and allow the direct transport of their cellular cargo. Cellular communication through TNT-mediated transport of lysosomes, endosomes, mitochondria, and miRNAs has been implicated in homeostatic and pathogenic processes in the eye (Chinnery and Keller, 2020); however, their explicit roles in the regulation of neuroinflammation and neurodegeneration remain elusive. It would also be interesting to determine whether TNTs are involved in astrocytic degradation of the mitochondria taken up from RGC axons (Davis et al., 2014).
3.4. Microbiota
The environmental factors that are thought to influence glial inflammatory responses include chronic subclinical peripheral inflammation. It is of increasing interest that commensal microbiota has the ability to generate a pro-inflammatory microenvironment. As being the conventional ligands of TLRs, PAMPs can stimulate innate immune responses. Depending on the quantity and complexity, these pathogenic components invading the retina may similarly contribute to glial inflammatory reactivity by acting through TLRs. Indeed, subcutaneously applied bacterial lipopolysaccharide that activates TLR4 was shown to aggravate microglial activity and RGC injury in experimental mouse glaucoma (Astafurov et al., 2014). Alternatively, commensal microbiota-induced systemic immune responses may lead to inflammatory neurotoxicity of infiltrating reactive T lymphocytes in ocular hypertensive mice (Chen et al., 2018). The latter is based on the molecular mimicry and cross-reactivity between microbial and human antigens (Romano et al., 1999, Wax, 1998). As implicated in many autoimmune diseases, Hsps, highly conserved from bacteria to human, can induce cross-reactive immune responses (Wax, 1998), as well as functioning as TLR ligands to activate innate and adaptive immunity (Luo et al., 2010). Different subtypes of these stress-response proteins that are upregulated in human glaucoma (Tezel et al., 2000) were linked to neuroinflammatory and neurodegenerative processes (Bell et al., 2018, Chen et al., 2018, Tezel and Wax, 2000b, Wax et al., 2008). Evidently, T lymphocytes cross-reactive with microbial and human Hsps can infiltrate into ocular hypertensive mouse eyes and promote RGC degeneration (Chen et al., 2018). Hsp antibodies at concentrations similar to that detected in the glaucoma patients’ blood can also facilitate RGC death by neutralizing the specific protein function after cellular internalization in the human retina, ex vivo (Tezel and Wax, 2000b). Furthermore, commensal microbiota may potentially modify the susceptibility to neurodegenerative injury by contributing to an epigenetic reprogramming (Nayyar et al., 2020).
4. Systemic immune responses in glaucoma
In addition to innate immunity, glaucoma-related tissue stress and injury, along with the outcomes of mitochondrial dysfunction, can induce adaptive immune responses by stimulating the inflammatory reactivity of glial cells. Apparently, the pro-inflammatory environment in glaucoma, which is enriched by glia-derived cytokines, chemokines, and adhesion molecules, is quite favorable for glia-lymphocyte interactions and antigen presentation for expanded immune responses. Increased expression of MHC molecules on reactive glia in human donor eyes with glaucoma (Gramlich et al., 2013, Yang et al., 2001c) and ocular hypertensive animals (Chidlow et al., 2016, Ebneter et al., 2010, Howell et al., 2011) supports the activated ability of these cells to stimulate adaptive immunity. While reactive T lymphocytes can migrate through the intact blood-retina barrier (Hu et al., 2000, Shechter et al., 2013, Xu et al., 2003), glaucoma-related and age-related alterations in vascular barriers (Chan-Ling et al., 2007, Flammer and Mozaffarieh, 2007, Wareham and Calkins, 2020) may further facilitate their contact with resident glia and the contact of generated autoantibodies with local antigens. Indeed, transient ocular hypertension was sufficient to stimulate retinal infiltration of the T lymphocytes pre-sensitized by commensal microflora (Chen et al., 2018). In both antigen immunization and adoptive transfer experiments, infiltrating reactive T lymphocytes promoted RGC damage by pro-inflammatory cytokine toxicity (Chen et al., 2018, Gramlich et al., 2015, Joachim et al., 2012, Kuehn et al., 2016, Laspas et al., 2011, Wax et al., 2008).
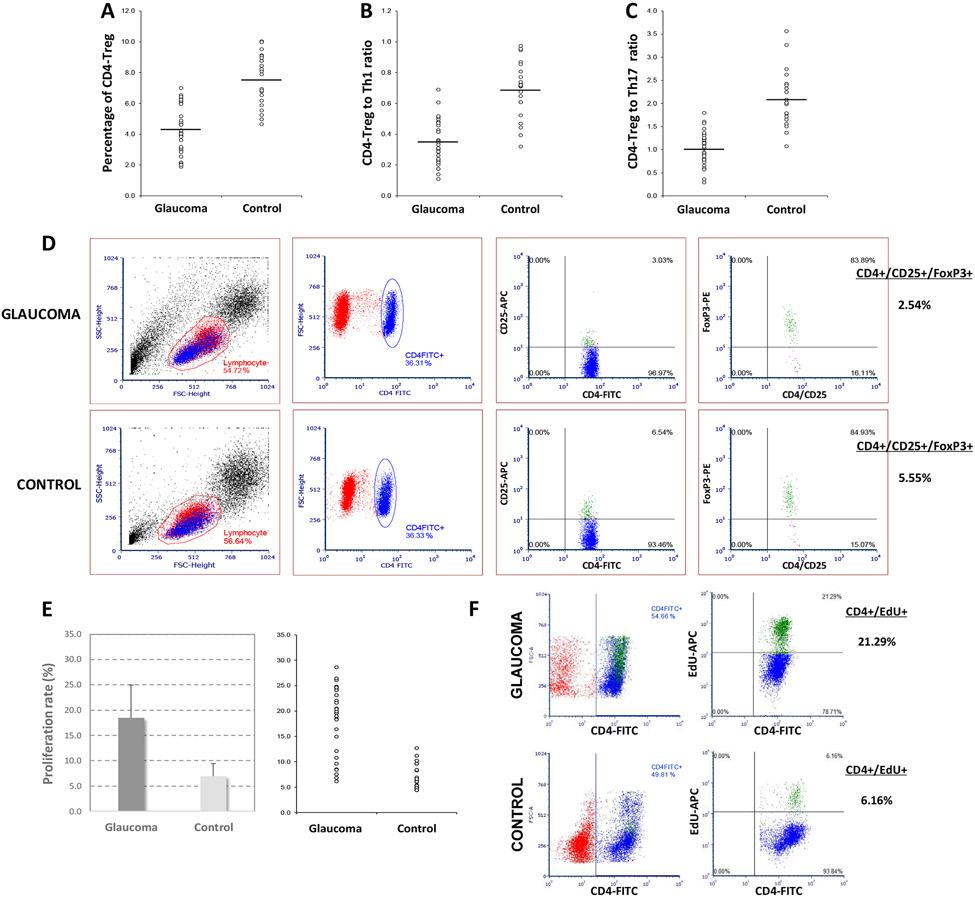
In parallel with the increased titers of pro-inflammatory cytokines in the glaucomatous blood (Huang et al., 2010), studies of isolated lymphocytes indicated a shift in subset distribution of helper T lymphocytes (Th). T lymphocyte profiling detected predomination of Th1 cells that produce pro-inflammatory cytokines over the Th2 subpopulation producing anti-inflammatory cytokines (Guo et al., 2018, Wong et al., 2015, Yang et al., 2001b).
As presented in Figure 5, a more recent study also revealed an imbalance of the regulatory T lymphocytes (Tregs) and a corresponding inflammatory stimulation of Th cells (Yang et al., 2019b). In comparison to non-glaucomatous controls, the T lymphocytes isolated from the glaucoma patients’ blood exhibited a significant increase in in vitro proliferation and pro-inflammatory cytokine production. Interestingly, the inflammatory reactivity of T lymphocytes to glaucomatous retinal antigens was greater than their reactivity to non-glaucomatous retinal antigens (Yang et al., 2019b). These observations are consistent with the increased release of ATP, ROS, and other DAMPs that can prime for innate and adaptive immune responses in glaucoma, as outlined in section 3.1. Since Tregs suppress other Th cells to maintain immune homeostasis and to prevent autoimmunity, the shift detected in T lymphocyte subset distribution suggests that the systemic immune responses may not be counterbalanced by an efficient immune suppression in glaucoma. On the one hand, this imbalance may reflect an adaptive self-tolerance mechanism to promote reparative tissue cleaning in the retina and optic nerve that are typically immune-privileged tissues. On the other, the subset imbalance may point to an increased risk for T lymphocyte-mediated inflammatory injury to RGCs.
Figure 5. Imbalance of T lymphocyte subsets in the glaucoma patients’ blood.
T lymphocyte subset markers were analyzed in the peripheral blood samples collected from patients with glaucoma (n=32) and age-matched non-glaucomatous controls (n=21). The percentage of CD4+/CD25+/FoxP3+ Treg (CD4-Treg) within the entire CD4+ population (A), the Treg to Th1 (B) or Treg to Th17 (C) ratios were significantly lower in the glaucoma group (P<0.001). The bars on univariate scatterplots present the group mean. Panel D shows representative flow cytometry images. Next, the same samples were analyzed for a proliferation marker after in vitro stimulation with retinal antigens. As presented by bar graphs (mean±SD) and univariate scatterplots in panel E, the percentage of CD4+/EdU+ cells was significantly higher in the glaucomatous samples than controls (P<0.001). Panel F shows representative flow cytometry images. This work has recently been published (Yang et al., 2019b).
In addition to mitochondrial DNA mutations in patients with glaucoma (Van Bergen et al., 2015), analysis of the mitochondrial function in isolated lymphocytes detected decreased rates of respiration and ATP production in association with an increased susceptibility for glaucomatous damage (Lascaratos et al., 2015, Lee et al., 2012, Van Bergen et al., 2015). Yet, it remains undefined whether this observation reflects a metabolic basis (Dumitru et al., 2018) for the pro-inflammatory T lymphocyte imbalance in glaucoma, as previously highlighted for the metabolic regulation of glia polarization.
Regarding autoantibodies, multiple evidences suggest a connection of the increased autoantibody production to mitochondrial dysfunction in glaucoma. Apparently, many of the antibodies presenting increased titers in the blood samples of glaucoma patients were found reactive to mitochondrial antigens (Tezel et al., 2012a). One of the most commonly detected autoantibodies in glaucoma was against Hsp60 (Chen et al., 2018, Wax, 2001, Wax et al., 2008) that is a mitochondrial chaperone and a marker of the mitochondrial unfolded protein response. Various mitochondrial enzymes (Beutgen et al., 2019) and cardiolipin (Chauhan et al., 2008) were also shown to induce adaptive, as well as innate, immune responses in glauoma. In addition, immunoproteomic analysis of the autoantibodies isolated from the glaucoma patients’ blood displayed a greater reactivity to oxidized retinal proteins, thereby pointing to mitochondrial dysfunction for oxidative modifications to increase the antigenicity of self-molecules (Tezel et al., 2012a). Autoantibodies reactive to ocular antigens keep the potential to damage RGCs by neutralizing specific protein function (Tezel and Wax, 2000b); however, autoantibody-mediated processes may not produce inflammatory outcomes. In spite of this, the increased antigen presentation that initiates the process leading to autoantibody generation and the facilitated retinal access of autoantibodies that results from weakened vascular barriers are the consequence of glial inflammatory responses.
5. Complement activation in neuroinflammation
Complement activation that presents a link between innate and adaptive immune responses may also contribute to neuroinflammation in glaucoma, as well as RGC degeneration. By functioning as a pattern recognition molecule, C1q tags synapses and dying neurons, thereby initiating the classical complement activation pathway. The complement cascade seems particularly important for synaptic degeneration of RGCs in glaucoma (Williams et al., 2016), a process similar to complement-mediated developmental pruning of the unwanted synapses (Stevens et al., 2007). Since DBA/2J mice are naturally deficient in complement component 5 and prevent downstream formation of the cytolytic membrane attack complex, complement activation in these mice may primarily reflect a targeted process for tissue clearance to limit inflammation (Howell et al., 2013). Even so, this final damaging product of the complement cascade was detected in human donor eyes with glaucoma (Kuehn et al., 2006, Tezel et al., 2010). Moreover, in support of the complement-mediated injury to RGCs, transgenic or pharmacologic treatments inhibiting complement components provided protection in animal models (Howell et al., 2011, Howell et al., 2014, Howell et al., 2013). Gene therapy targeting complement C3 also delayed the progression of neurodegeneration in DBA/2J glaucoma (Bosco et al., 2018). In contrast, at early stages of glaucoma, C3 was found to induce neuroprotective functions of astrocytes via epidermal growth factor receptor (EGFR) signaling (Harder et al., 2017). This discrepancy may be related to the dynamic shift between different activation states and the C3 expression of astrocytes (Liddelow et al., 2017) in early or late stages of glaucoma.
Complement-mediated tissue clearance limits inflammation; however, complement anaphylatoxins, end-products of the complement cascade, may also participate in inflammatory responses. For example, C3a, a C3 cleavage product, may recruit microglia and infiltrating monocytes that express C3a receptor-1 (C3ar1). A recent study (Harder et al., 2020b) showed C3ar1 as a damaging neuroinflammatory factor in DBA/2J glaucoma, as C3ar1 deficiency temporarily lowered the risk for RGC degeneration in ocular hypertensive eyes. While the membrane attack complex induces cell lysis, its sublytic levels may also activate inflammation by triggering intracellular calcium fluxes (Laudisi et al., 2013, Triantafilou et al., 2013). Although resident glia can initiate complement cascades in glaucoma, it is unclear whether blood-derived complement ligands may amplify inflammatory responses in later stages, which may become increasingly available after the breach of vascular barriers.
6. Amplifiers of neuroinflammation and effectors of neurodegeneration in glaucoma
Early reactivity of glial cells in glaucoma is characterized by an increased production of pro-inflammatory cytokines, such as TNFα, IFNγ, IL-1, IL-2, IL-12, and chemokines, such as CCL2, CCL5 (Howell et al., 2011, Howell et al., 2012, Yang et al., 2016, Yang et al., 2011, Yang et al., 2020, Yang et al., 2021). Glia-produced pro-inflammatory cytokines function as amplifiers of the inflammation program and effectors of the inflammatory neurotoxicity. Chemokines, on the other hand, recruit additional immune cells. This process promotes the secretion of other pro-inflammatory molecules, thereby exacerbating neuroinflammation. Additional molecular outcomes, such as ROS or iNOS, also amplify inflammation and cause collateral damage (Yuan and Neufeld, 2001). Meanwhile, several other molecules provide counter-regulation to attenuate the inducers or amplifiers of inflammation (Tezel et al., 2012b, Yang et al., 2011). A relative imbalance in the magnitude and duration of pro-inflammatory cytokine production, as opposed to production of anti-inflammatory cytokines, results in a condition founding neuroinflammation. Parallel alterations in the expression of cytokine receptors are also decisive for the inflammatory outcome (Gregory et al., 2011, Tezel et al., 2001b). Consisting with the increased glial production of pro-inflammatory cytokines in the glaucomatous retina and optic nerve, glaucomatous aqueous humor and blood samples present increased levels of these cytokines as well (Chua et al., 2012, Huang et al., 2010, Kuchtey et al., 2010, Sawada et al., 2010).
When viewing the inflammatory responses of glial cells in glaucoma, TNFα comes forward as a major pro-inflammatory cytokine produced in the glaucomatous retina and optic nerve (Tezel, 2008, Tezel et al., 2001b, Tezel et al., 2012b, Yan et al., 2000, Yang et al., 2011). Besides astrocytes and microglia, TNFα can be produced by Müller glia (Cueva Vargas et al., 2015) and take part in their inflammatory responses (Hu et al., 2020). Not only for considerably strong pro-inflammatory activities, but TNFα is also important for its direct toxicity to RGCs in glaucoma. By initiating the TNFR1-mediated dead receptor signaling, this pro-inflammatory cytokine induces RGC apoptosis, oligodendrocyte death, and axon degeneration (Cueva Vargas et al., 2015, Ko et al., 2020, Nakazawa et al., 2006, Tezel and Wax, 2000a, Tezel et al., 2004). A corresponding increase in the expression of TNFR1 on RGCs (Tezel et al., 2001b) may represent an additional factor increasing the susceptibility of RGCs to TNFα-mediated inflammatory injury in glaucomatous eyes. TNFα-mediated RGC apoptosis is executed via proteolytic caspase activation and mitochondrial dysfunction (Tezel and Wax, 2000a, Tezel and Yang, 2004, Yang et al., 2011), whereas TNFα-induced degeneration of RGC axons is mediated by the sterile alpha and TIR motif containing-1 (SARM1) and NAD-dependent pathway (Ko et al., 2020). Inflammatory injury to glaucomatous RGCs at different subcellular regions is illustrated in Figure 6.
Figure 6. Inflammatory injury to RGCs in glaucoma.
Various outcomes of glia-driven neuroinflammation promote neurotoxicity to RGC axons, somas, and synapses at dendrites and axon terminals in glaucoma. While pro-inflammatory cytokines produced by reactive glia, including TNFα, can induce RGC apoptosis, axon degeneration, and oligodendrocyte death by activating dead receptor signaling (TNFR), complement cascades mediate the loss of RGC synapses.
Activation of SARM1 induces axon degeneration through Wallerian degeneration and dying back that are the pathogenic processes linked to glaucomatous degeneration of distal axons in proximal-to-distal or distal-to-proximal directions, respectively (Beirowski et al., 2008, Crish et al., 2010, Howell et al., 2007, Schlamp et al., 2006). Deletion of SARM1 rescues injured RGC axons (Yang et al., 2015a); however, signaling for RGC soma death by dual leucine zipper kinase (DLK) and jun kinase-1 (JNK1) is SARM1-independent (Fernandes et al., 2018). SARM1 with NADase activity induces NAD decrease, which is a redox cofactor participating in various biochemical reactions essential for mitochondrial function, aging, and neurodegeneration (Lautrup et al., 2019, Verdin, 2015). Likewise, reduced bioavailability of NAD is critical for glaucomatous neurodegeneration, and dietary supplementation of nicotinamide, or overexpression of nicotinamide nucleotide adenylyltransferase 1 (NMNAT1), a key NAD-producing enzyme, can diminish metabolic failure and RGC injury in mouse glaucoma (Williams et al., 2017b, Zhu et al., 2013). TIR domains, which are the signature signaling domains for innate immunity, connect SARM1 to innate immune signaling, including TLR signaling as mentioned in section 3.1 (O'Neill and Bowie, 2007). The recent study of Ko et al. linked TNFα-mediated neuroinflammation to SARM1/NAD-dependent neurodegeneration in glaucoma, as intravitreally injected TNFα induced SARM1-dependent axon degeneration, and subsequent oligodendrocyte loss and RGC soma death (Ko et al., 2020). A non-canonical form of necroptosis was also implicated in the TNFα-mediated axon degeneration. Unlike canonical necroptosis that can be induced when TNFα-mediated apoptosis is suppressed (He et al., 2009, Zhang et al., 2009), mixed lineage kinase domain like pseudokinase (MLKL) is not the final executioner of non-canonical necroptosis that can trigger axon degeneration by inducing the loss of axonal survival factors and activating SARM1 for NAD cleavage and calcium influx (Ko et al., 2020). Age-related or glaucoma-related depletion of NAD may trigger multiple pro-inflammatory responses in glaucoma, since NAD acts as an anti-inflammatory molecule through poly-ADP-ribose polymerase-1 activation and sirtuin inhibition (Yanez et al., 2019). The accompanying mitochondrial dysfunction can also stimulate inflammatory responses, as outlined in section 3.2. The SARM1/NAD axis of the inflammatory neurodegeneration is a motivating area to further study in glaucoma models.
Besides direct neurotoxicity of TNFα/TNFR1 signaling in glaucoma, downstream NF-κB activation leads to transcriptional activation of more immune mediators and feed into an amplification cycle of neuroinflammation (Yang et al., 2020). Many pro-inflammatory cytokines and chemokines exhibiting glial upregulation in glaucoma, including TNFα, IFNγ, IL-1, IL-2, IL-12, IL-17, FasL (Ju et al., 2006, Krizaj et al., 2014, Nikolskaya et al., 2009, Sapienza et al., 2016, Steele et al., 2006, Yang et al., 2011), or iNOS (Neufeld et al., 1999), are NF-κB’s target genes. TNFα can also induce inflammasome by activating NF-κB and caspase-8 (Chi et al., 2014, Gurung and Kanneganti, 2015, Monie and Bryant, 2015). Evidently, TNFα is critically involved in astrocyte-microglia communication for inflammatory stimulation (Liddelow et al., 2017). In addition to upregulation of NF-κB-regulated immunoreceptors, such as MHC molecules (Yang et al., 2001c) and RAGE (Tezel et al., 2007a) on glaucomatous glia, TNFα is an essential cytokine for glia-T lymphocyte interactions, thereby stimulating the effector cells for systemic immune responses as well (Tezel et al., 2007b).
Another pro-inflammatory cytokine in the same family with TNFα, FasL, is also secreted by reactive glia and may induce RGC apoptosis and axon degeneration in experimental glaucoma (Ju et al., 2006, Krishnan et al., 2019). A related regulatory mechanism includes the production of soluble, as opposed to membrane-associated, receptors for FasL, which may prevent the signaling for pro-inflammatory and neurodestructive outcomes (Gregory et al., 2011, Krishnan et al., 2016).
Several studies have tested different strategies to modulate the glial responses molding neuroinflammation in animal models of glaucoma. Blockage of the pro-inflammatory cytokine signaling with TNFα (Roh et al., 2012) or Fas receptor (Krishnan et al., 2019) antagonists reduced RGC death and axon loss in experimental glaucoma. Modulation of glial reactivity by minocycline, a tetracycline derivative (Bordone et al., 2017, Bosco et al., 2008, Levkovitch-Verbin et al., 2006), or ibudilast, a cAMP phosphodiesterase inhibitor (Cueva Vargas et al., 2016) lessened RGC degeneration and improved axonal transport in glaucoma models. Rapamycin treatment that diminishes neurodegeneration in DBA/2J glaucoma (Harder et al., 2020a) was also linked to decreased microglial production of pro-inflammatory cytokines (Su et al., 2014). In addition, caffeine as an antagonist of adenosine receptors was suggested to lower the inflammatory reactivity of retinal microglia and the loss of RGCs in ocular hypertensive rat eyes (Madeira et al., 2016). More recently, modulation of the inflammatory glial phenotype with NLY01, a glucagon-like peptide-1 receptor agonist, protected RGCs against ocular hypertensive injury in mice (Sterling et al., 2020). More research is needed to further validate and expand these experimental observations; however, potential off-target effects on systemic immune defense and glial neurosupport demand alternative strategies for more specific targeting, which are still in exploration as detailed in section 8.
7. Transducers of inflammation signaling
For TLR signaling that leads to production of NF-κB-regulated inflammatory mediators, MyD88 is a critical adaptor (Barton and Medzhitov, 2003, Hoebe et al., 2003, Takeda and Akira, 2004). This adaptor molecule, upregulated in human glaucoma (Luo et al., 2010) and DBA/2J glaucoma (Howell et al., 2011), is engaged in inflammatory responses of retinal microglia and astrocytes. The glial TLR signaling activated by intrinsic stress-related ligands, such as Hsps and oxidative stress end-products, and the secretion of pro-inflammatory cytokines were reduced by pharmacologic inhibition of MyD88 (Luo et al., 2010). Deficiency of another TLR adaptor, TRIF (Yamamoto et al., 2003), also attenuated microglial cytokine production and promoted RGC survival and axon regeneration after optic nerve crush (Lin et al., 2012).
Recruitment of receptor-interacting protein kinase-1 (RIPK1) to TNFR signaling complex, which is expressed in the glaucomatous retina (Yang et al., 2011), can similarly initiate the pro-inflammatory signaling mediated through NF-κB activation. Mitogen-activated protein kinases (MAPKs), expressed by glial cells in glaucoma (Tezel et al., 2003, Tezel et al., 2012b, Yang et al., 2011), mediate NF-κB activation via TLR and TNFR signaling in innate immune cells (Arthur and Ley, 2013, Shi and Sun, 2018). Transcriptional activity of NF-κB depends on the ubiquitin-mediated degradation of an inhibitor molecule, IκB, after its phosphorylation by the inhibitor of kappaB kinase complex (IκK). Within this complex, IκKβ is the main activating kinase for IκB degradation in the canonical pathway with inflammatory outcomes (Delhase et al., 1999, Ghosh and Karin, 2002, Oeckl et al., 2012, van Loo et al., 2006). Indeed, as outlined in section 8, the cre/lox-based conditional deletion of astroglial IκKβ lowered pro-inflammatory cytokine production and rescued RGC survival and function in experimental glaucoma (Yang et al., 2020). For upstream processing of the NF-κB-activating signaling, IκK activation involves MAPKs, p38 and MAP2K/ERK (Henry and Martin, 2017, Peltzer et al., 2016). The MAP3K7 (TAK1), expressed in the glaucomatous tissues (Yang et al., 2011), functions as an upstream kinase in the IκK/NF-κB pathway, and its deficiency attenuates NF-κB activation (Sato et al., 2005). The MAP3K3, expressed by glaucomatous astrocytes (Tezel et al., 2012b), also participates in the NF-κB activation induced by TNFα or IL-1 (Blonska et al., 2005, Yang et al., 2001a).
Regarding JNKs, another member of the MAPKs, diverse pathogenic processes that are implicated in glaucomatous neurodegeneration, including distortion of axonal cytoskeleton, neurotrophin deficiency, mitochondrial dysfunction, metabolic failure, oxidative stress, or endoplasmic reticulum stress, can activate these signaling molecules (Fernandes et al., 2013, Kwong and Caprioli, 2006, Marola et al., 2020, Syc-Mazurek et al., 2017, Tezel et al., 2003, Tezel et al., 2004). JNK1 that plays a major role in the RGC soma death signaled through the intrinsic pathway, which is triggered after early axon injury (Fernandes et al., 2013, Harder et al., 2018, Syc-Mazurek et al., 2017), is also engaged in the RGC apoptosis signaled through the extrinsic pathway, which is triggered after TNFR1 binding (Dvoriantchikova and Ivanov, 2014, Tezel et al., 2004). Besides the evidence for JNK1 involvement in TNFR-mediated or TLR-mediated inflammation signaling, JNK1 can prime inflammasome-mediated inflammation by phosphorylating a critical inflammasome component, NLRP3 (Song et al., 2017). However, more research is necessary to understand the importance of the TLR-JNK1-inflammasome axis for neuroinflammation in glaucoma.
Although NF-κB, the key transcriptional activator of inflammatory mediators, is separately outlined in the next section, various glial pathways acting in parallel also include various other signaling molecules and transcription factors that function to maintain immune homeostasis. As a matter of fact, divergent functions of glial cells are mostly defined by state-dependent transcription factors, including NF-κB being pro-inflammatory and STAT being anti-inflammatory (Liddelow et al., 2017, Liddelow et al., 2020).
STAT3, a member of the Jak-STAT signaling family, is known to regulate astrogliosis and mediate the neuroprotective functions of reactive astrocytes (Ben Haim et al., 2015, Das et al., 2020). Indeed, the anti-inflammatory cytokines regulated by STAT3-dependent pathways (Lang et al., 2002) are activated in A2 astrocytes (Anderson et al., 2016, Liddelow et al., 2020). The astrocytes in human glaucoma (Yang et al., 2011) and animal models (Johnson et al., 2011, Lozano et al., 2019, Sun et al., 2017, Tezel et al., 2012b) exhibit increased STAT3 expression as well. Although STAT3-dependent transcription is mostly considered beneficial, inhibition of STAT3-mediated astrogliosis ameliorated pathological alterations and reduced inflammatory responses in Alzheimer’s disease (Reichenbach et al., 2019). In glaucoma, however, astrocyte-specific knockout of STAT3 impaired astrogliosis in the optic nerve head and exacerbated RGC injury and dysfunction in ocular hypertensive mice (Sun et al., 2017).
The EGFR that is expressed by astrocytes in the optic nerve head (Liu and Neufeld, 2004, 2003) was implicated in the conversion of quiescent astrocytes to reactive astrocytes after optic nerve injury (Liu et al., 2006). The EGFR signaling has recently been associated to early and beneficial immune responses against glaucoma in DBA/2J mice (Harder et al., 2017). In a more recent study of DBA/2J mice, RNA-sequencing on optic nerve head microglia indicated ocular hypertension-induced suppression of the homeostatic gene expression. This study pointed to a microglial immune receptor signaling mediated by triggering receptor expressed on myeloid cells TREM (Krasemann et al., 2017, Neumann and Takahashi, 2007) and tyrosine kinase-binding protein (TYROBP) as a potential regulator mechanism of microglia responses to elevated IOP (Tribble et al., 2020).
Another molecule, decorin that is a small leucine-rich proteoglycan is known to antagonize TGFβ, an immune-regulatory and pro-fibrotic cytokine. The mice lacking this TGFβ-modulating molecule presented ocular hypertension, morphological changes of the glial lamina, and loss of RGC axons as typically detected in glaucoma (Schneider et al., 2021). In primary open-angle glaucoma, decorin presented a dramatic downregulation in the optic nerve head (Kirwan et al., 2009) as well in trabecular meshwork cells (Schneider et al., 2021). However, how these observations translate into immune regulation in glaucoma remains unclear.
Glucocorticoid signaling is also known to suppress glial reactivity and inflammation. Indeed, administration of corticosteroid agonists activated glucocorticoid receptor on Müller glia and improved neuron survival by suppressing the microglial reactivity (Gallina et al., 2015). In contrary, prolonged glucocorticoid therapy is known to cause glucocorticoid-induced ocular hypertension leading to glaucoma. These seemingly conflicting actions of the glucocorticoid receptor appear to be related with transactivation or transrepression of this ligand-activated transcription factor. While many of the anti-inflammatory properties of glucocorticoids are mediated by receptor transrepression, transactivation of the glucocorticoid receptor accounts for ocular hypertension (Patel et al., 2019). Elucidation of the regulatory roles of endogenous glucocorticoids in the inflammatory processes of glaucoma awaits further studies.
8. NF-κB in transcriptional regulation of neuroinflammation in glaucoma
Multiple pathways, including TNFR signaling (Tezel et al., 2012b, Yang et al., 2011), TLR signaling (Howell et al., 2011, Luo et al., 2010, Tezel et al., 2012b), and inflammasome Yang, 2011 #2735;Tezel, 2012 #2858;Albalawi, 2017 #4437;Pronin, 2019 #4623}, cooperate for glia-driven neuroinflammation in glaucoma, which are commonly regulated by NF-κB, the key transcriptional activator of inflammatory mediators (Harari and Liao, 2010). In response to environmental signals, TNFR and TLR signaling leads to transcriptional priming of inflammasome via NF-κB activation that upregulates the expression of inflammasome components and the precursors of inflammasome-activated cytokines, like IL-1β.
Various molecules involved in the NF-κB activation pathway are prominently affected in the glaucomatous human retina (Yang et al., 2011) and in the proteome of astrocytes isolated from ocular hypertensive animals (Tezel et al., 2012b). The importance of NF-κB for neurodegenerative inflammation in glaucoma is not only based on molecular profiling, but it is also supported by functional analysis (Yang et al., 2020). The cre/lox-based conditional deletion of IκKβ in GFAP-expressing astroglia (GFAP/IκKβ) in experimental glaucoma resulted in decreased production of pro-inflammatory cytokines that are transcriptional targets for NF-κB, such as TNFα, IFNγ, IL-1, IL-2. Inhibition of the NF-κB-regulated inflammatory activities of astroglia provided structural and functional protection to RGCs in ocular hypertensive eyes (Figures 7 and 8). Findings of this study also pointed to non-cell-autonomous functions of NF-κB in astrocyte-microglia communication through the secretion of NF-κB-regulated inflammatory mediators (Yang et al., 2020). Previous studies of other neurodegeneration models have similarly supported the pathogenic importance of NF-κB-mediated inflammation (Brambilla et al., 2012, Haenold et al., 2014, Lian et al., 2015). Besides inflammatory stress, other gene targets of NF-κB, such as those linked to astrocyte-mediated extracellular matrix remodeling and profibrotic processes, may possibly participate in the glaucoma-related biomechanical stress as well (Tezel, 2020). This common regulator of multiple inflammation and degeneration pathways therefore appears to be a favorable treatment target for glaucoma. Even so, NF-κB also activates key anti-apoptotic genes (Karin and Lin, 2002, Van Antwerp et al., 1996) and regulates a wide range of processes critical for neuron survival, synapse formation, and plasticity (Boersma et al., 2011, Meffert et al., 2003). Concerning the available NF-κB inhibitors that lack of cell type specificity, glia-targeting manipulation of NF-κB comes forward to avoid off-target effects on neuron survival and function, as well as keeping the systemic immune defense intact. Emerging gene engineering techniques and new delivery tools, including astroglia-specific viral vectors (Biswal et al., 2014, Dalkara et al., 2011, Koerber et al., 2009, Liu et al., 2015, Meng et al., 2015, Prentice et al., 2011, Wu et al., 2020) are expected to rapidly enable glia-targeting strategies for glaucoma treatment. In the meantime, continuously growing knowledge about divergent responses of astrocytes and functional interactions with microglia should be carefully translated to further substantiate and enhance treatment benefits from glial NF-κB inhibition.
Figure 7. NF-κB-regulated activation of neurodegenerative inflammation in glaucoma.
A. Ocular hypertension was experimental induced by anterior chamber microbead injections in mice with or without cre/lox-based conditional deletion of IκKβ in GFAP-expressing astroglia. Optic nerve cross-sections presented well preserved structure of RGC axons (A), and the number of remaining axons was significantly higher (B) in ocular hypertensive GFAP/IκKβ mice compared to ocular hypertensive IκKβf/f controls (***P<0.001). As shown in Panel C, deletion of astroglial IκKβ resulted in an approximately 60% protection of RGC axons in ocular hypertensive eyes over 12 weeks. Similarly, retinal whole mounts labeled for RBPMS showed a visible protection of RGC somas (D), and the number of RBPMS-labeled RGCs was significantly higher (E) in ocular hypertensive GFAP/IκKβ mice compared to ocular hypertensive IκKβf/f controls (***P<0.001). As shown in Panel F, deletion of astroglial IκKβ resulted in an approximately 60% protection of RGC somas in ocular hypertensive eyes over 12 weeks. Presented data (mean±SD) represents a minimum of 16 mice per group. This work has recently been published (Yang et al., 2020).
Figure 8. Functional recovery by inhibition of glia-driven neuroinflammation in glaucoma.
Ocular hypertension was experimental induced by anterior chamber microbead injections in mice with or without cre/lox-based conditional deletion of IκKβ in GFAP-expressing astroglia. Recording of pattern electroretinography (PERG) responses in GFAP/IκKβ mice and IκKβf/f controls with or without experimentally induced ocular hypertension (A) showed preserved PERG amplitude (***P<0.001) in ocular hypertensive GFAP/IκKβ mice compared to ocular hypertensive IκKβf/f controls (B and C). Presented data (mean±SD) represents a minimum of 16 mice per group. This work has recently been published (Yang et al., 2020).
In association with NF-κB, caspase-8 is also coupled with the inflammation pathways activated in glaucoma (Yang et al., 2021). Caspase-8, the initiator caspase in the TNFR-mediated apoptosis pathway, is detected in the apoptotic RGCs in human glaucoma (Yang et al., 2011) and animal models (Huang et al., 2005, McKinnon et al., 2002, Tezel et al., 2012b). However, biological functions of caspase-8 are not limited to its enzymatic activity for the execution of apoptosis, since its catalytic activity can also promote NF-κB-mediated cell survival and inflammation (Tummers and Green, 2017). Besides TNFR signaling, caspase-8 is involved in the inflammatory processes mediated through TLR signaling (Kim and Li, 2013, Lemmers et al., 2007, Philip et al., 2016, Shenderov et al., 2014) and inflammasome (Chi et al., 2015, Chi et al., 2014, Feltham et al., 2017, Gurung and Kanneganti, 2015, Monie and Bryant, 2015). A recent study sought to determine cleavage-dependent versus cleavage-independent functions of caspase-8 in experimental glaucoma (Yang et al., 2021). Pharmacological inhibition of caspase-8 cleavage by z-IETD-fmk treatment protected RGCs against ocular hypertension-induced apoptosis but did not affect the glial inflammatory potential. Instead, the cre/lox-based conditional deletion of astroglial caspase-8 (GFAP/caspase-8) restricted neurodegenerative inflammation and protected RGC structure and function in ocular hypertensive eyes. Additionally, the complete lack of caspase-8 activity in GFAP/caspase-8 mice induced RIPK3 and MLKL-driven necroptosis in astrocytes, because caspase-8 activity is required for RIPK cleavage to prevent necroptotic flow (Yang et al., 2021). This study focused on astroglia; however, caspase-8 has also been linked to microglia-mediated inflammation and neurotoxicity in several neurodegenerative diseases (Burguillos et al., 2011).
The findings of this study also illustrated how precisely regulated parallel pathways can modulate inflammatory signaling to run in opposing directions (Figure 9). Cell type-specific diverse functions of caspase-8, which influence both RGC apoptosis and astroglia-driven neuroinflammation in experimental glaucoma, was connected to FLICE-like inhibitory protein (cFLIP), another gene target for NF-κB (Yang et al., 2021). By interacting with caspase-8, this inactive caspase-8 homolog functions as a molecular switch between cell death, survival, and inflammation signals after TNFR or TLR binding (Chang et al., 2002, Irmler et al., 1997, Oberst et al., 2011, Silke and Strasser, 2013, Vandenabeele and Melino, 2012). This regulator molecule, highly expressed in astrocytes but not in RGCs, inhibits caspase-8-mediated cell death but induces glial pro-inflammatory outcomes (Golks et al., 2006, Philip et al., 2016). The caspase-8/cFLIP interaction therefore appears to be another promising treatment target for targeting the glia to modulate NF-κB-regulated inflammation signaling, while eliminating any undesired effects on NF-κB-regulated protective processes.
Figure 9. Molecular regulation of the parallel pathways modulating inflammation signaling.
As illustrated by the blue triangles at the bottom, different states of caspase-8 function, including its enzymatic or catalytic activities, are critical for the outcome. While the enzymatic activity of caspase-8 induces apoptosis in RGCs (A), its catalytic activity, in the absence of full proteolytic cleavage, signals towards cell survival in astroglia (B). Alternatively, the lack of caspase-8 activity induces necroptosis (C). Caspase-8 deletion in astroglia changes the state from “B to C”, while the inhibition of caspase-8 cleavage by z-IETD-fmk shifts the signal from “A to B” in RGCs. By interacting with caspase-8, cFLIP inhibits caspase-8-mediated cell death but induces pro-inflammatory outcomes. This regulator molecule that is highly expressed in astroglia but not in RGCs functions as a molecular switch between cell death, survival, and inflammation signals. This work has recently been published (Yang et al., 2021).
9. Genetic and epigenetic susceptibilities for immune stress
9.1. Genetic risk factors
Along with the danger signals arising from stressed and injured tissues, genetic and epigenetic factors may also affect the susceptibility to develop neurodegenerative inflammation. Apparently, many risk-associated mutations or polymorphisms detected in glaucoma comprise the genes expressed by glia, which may potentially lead to their dysfunction in immune response, thereby adversely affecting the outcomes. Yet, variable penetrance of identified variations suggests polygenic effects and complex interactions of genetic, epigenetic, and environmental risk factors (Libby et al., 2005a, Wiggs and Pasquale, 2017).
An increasing collection of glaucoma-related genes include those linked to cytokine signaling (Wiggs, 2015). Several gene polymorphisms of TNFα (Xin et al., 2013), other cytokines (Atanasovska Velkovska et al., 2021), or TLR4 (Shibuya et al., 2008, Takano et al., 2012) were also related to glaucoma in different ethnic populations. In addition, the gain-of-function mutation of the pro-inflammatory gene TBK1 (TNFR-associated factor NF-κB activator (TANK)-binding kinase-1) was correlated to increased risk for normal-pressure glaucoma (Fingert et al., 2011). This gene that modulates multiple inflammation pathways and autophagy has also been connected to inflammatory processes in different neurodegenerative diseases (Ahmad et al., 2016).
Among the genetic predispositions identified in glaucoma patients, optineurin (Sarfarazi and Rezaie, 2003) presents particular importance to neuroinflammation. This ubiquitin-binding protein has been implicated in various cellular processes, including selective autophagy to protect from prolonged endoplasmic reticulum stress, regulation of inflammation, and cell death (Slowicka and van Loo, 2018). Optineurin is a negative regulator of NF-κB activation, which competes with NEMO (NF-κB essential modulator) for binding to ubiquitinated RIP (Zhu et al., 2007) and also facilitates the CYLD-dependent deubiquitination of RIP. These mechanisms are abrogated by the glaucoma-associated mutation of optineurin, which cannot prevent TNFα-induced NF-κB activation (Nagabhushana et al., 2011). The TBK1 associated with an increased risk for normal-pressure glaucoma also interacts with the mutant optineurin for NF-κB-regulated inflammation signaling. Another critical role of the mutated optineurin in RGC death has been linked to disruption of cellular iron homeostasis (Sirohi et al., 2013).
TREM2 and apolipoprotein E (APOE) variants that were connected to the inflammatory pathology in the Alzheimer’s Disease were also detected in glaucoma; however, only one APOE variant was associated with a reduced risk for primary open-angle glaucoma, while TREM2 variants were not found to significantly contribute to disease risk (Margeta et al., 2020). On the other hand, findings of a recent study in DBA/2J mice supported a regulator role of the TREM/TYROBP signaling network in microglia responses to elevated IOP (Tribble et al., 2020).
9.2. Epigenetic risk factors
Epigenetic regulation mechanisms, involving DNA methylation, histone modifications, and non-coding RNAs that play important roles in transcriptional and post-transcriptional regulation of gene expression, may also modulate inflammatory responses of astrocytes (Neal and Richardson, 2018, Wahane et al., 2019). Histone deacetylation have been related to early nuclear atrophy and gene silencing in RGCs (Pelzel et al., 2012, Schmitt et al., 2016) and pro-fibrotic processes in the glaucomatous optic nerve head (McDonnell et al., 2014, Park et al., 2017). However, only a few studies, mostly including microRNA expression, searched for the epigenetic factors that may affect the inflammatory phenotype or the susceptibility of RGCs to inflammatory injury. One recent study analyzed the role of epigenetic modulation in individual variations among glaucomatous human donors that presented differences in the retinal expression pattern of TNFα-induced protein-3 (TNFAIP3, A20). As presented in Figure 10, on the basis of bisulfite sequencing, this study linked the transcriptional repression of TNFAIP3 to increased DNA methylation (Yang et al., 2011). By antagonizing the interactions with ubiquitin-conjugating enzymes, this ubiquitin-editing zinc finger protein inhibits both TNFα-mediated inflammation and apoptosis (Vereecke et al., 2009). By negatively regulating TNFα signaling, TNFAIP3 restricts NF-κB-mediated innate and adaptive immune responses in the central nervous system (Mohebiany et al., 2020, Voet et al., 2018). TNFAIP3 knockout results in spontaneous neuroinflammation, while its partial loss is sufficient to cause chronic, spontaneous low-grade brain inflammation (Guedes et al., 2014). Previous genetic studies have also demonstrated mutations in the TNFAIP3 locus as risk alleles for multiple autoimmune diseases (Vereecke et al., 2009). Although increased DNA methylation and transcriptional repression of TNFAIP3 that was detected in glaucoma patients is already a decade-old observation, it is hoped to stimulate further studies to explore the epigenetic control of neuroinflammation. Without a doubt, epigenetic regulation of gene expression by chromatin accessibility and transcription factor binding (Sinha et al., 2021, Wahane et al., 2019) is an exciting area that offers valuable information to glaucoma research. Even more inspiring is the recent progress of high-throughput techniques that can lead to cell type-specific epigenetic information at genome-wide scale, while integrative analysis of the multi-omics data can accumulate more comprehensive and complementary information.
Figure 10. Epigenetic modulation of immune regulation.
A. Quantitative Western blot analysis of retinal proteins detected a prominent variation in TNFAP3 (A20, a negative regulator of NF-κB-regulated inflammation) expression among ten human donor eyes with glaucoma. As presented by the bar graph, four out of ten of these samples presented an over two-fold increased expression over age-matched controls (*P<0.01); however, TNFAIP3 expression was unchanged or decreased in rest of the human glaucoma samples. B. When the cytosine nucleotide methylation in the TNFAIP3 promoter was studied, in the glaucoma samples with low TNFAIP3 expression (corresponding to donors #4, 5, and 9), three CpG (triangle) and ten non-CpG (circle) methylation sites (filled symbols) were found. In the glaucoma samples exhibiting high expression of TNFAIP3 (corresponding to donors #1 and 7), these sites remained unmethylated. Panel C presents two examples of the bisulfite sequencing results. This work has recently been published (Yang et al., 2011).
Several microRNAs, the small non-coding RNAs acting as epigenetic modulators, have also been shown to regulate microglial polarization and microglia-mediated neuroinflammation in in vitro or in vivo models of glaucoma-related stress. Acute IOP elevation led to changes in microRNA expression and upregulation of target genes in the retina, which included those linked to inflammation pathways, such as p38 MAPK, TNFα, and iNOS (Wang et al., 2017). In addition, the miR-93/STAT3 pathway was connected to downregulation of microglia-driven neuroinflammation in the retina and protection of RGCs against acute ocular hypertension (Wang et al., 2021). In a study of experimental glaucoma, downregulation of miR-200a was also found to stimulate FGF7-mediated MAPK signaling and the expression of CD11b and iNOS (Peng et al., 2019).
10. Conclusions
While work continues on the finer details, this review aimed to provide a broad perspective on the current knowledge on neuroinflammation in glaucoma. It is increasingly evident that IOP-related biomechanical or vascular stress, aging, RGC damage, genetic and epigenetic susceptibilities may collectively compromise glial immune-regulatory functions in the glaucomatous retina and optic nerve. Consequently, despite early adaptive and reparative aspects of glial reactivity, prolonged inflammatory responses of the resident glia and peripheral immune cells promote neurodegenerative outcomes. An increasing promise for therapeutic modulation of neuroinflammation rests on the evidence for broad impacts of inflammatory toxicity inflammation on RGC axons, somas, dendritic trees, and brain synapses. Although distinct molecular programs regulate somatic and axonal degeneration in glaucoma, widespread impacts of neuroinflammation offer immunomodulation as a treatment strategy for widespread protection. Besides protecting the integrity of RGC axons, somas, and synapses, immunomodulation may allow functional recovery to RGCs. Indeed, therapeutic immunomodulation by targeting astroglial NF-κB (Yang et al., 2020) or caspase-8 (Yang et al., 2021) provided both structural and functional protection to RGCs in experimental glaucoma. The RGCs rescued in ocular hypertensive mice triple knockout for IL1α, TNFα, and C1q were also electrophysiologically functional (Guttenplan et al., 2020). Consistently, treatment with glial modulators, minocyclin (Bordone et al., 2017, Bosco et al., 2008) or ibudilast (Cueva Vargas et al., 2016), protected axonal transport function. However, RGCs rescued by deletion of Bax that is essential for RGC apoptosis in glaucoma (Donahue et al., 2019, Howell et al., 2007, Libby et al., 2005b), or inhibition of caspases that are final executioners of apoptosis, donot present a remarkable functional improvement. Instead, Bax-depleted RGCs become quiescent, and the surviving neurons may be even more detrimental to the functional circuit (Donahue et al., 2019, Libby et al., 2005b). Similarly, treatments targeting distal axon degeneration have no prominent effect on RGC somas (Beirowski et al., 2008, Howell et al., 2007), unless supplemented with nicotinamide (Williams et al., 2017a). Undoubtedly, the health of RGC axons, somas, and synapses are ultimately dependent on one another, and the visual information can only be transmitted as long as the neuronal connectivity remains intact. However, the time shift of axonal and somatic degeneration processes may not always provide a useful treatment period for the benefit of visual function. By targeting glial responses, immunomodulation offers an alternative treatment strategy to prevent or reverse neurodegenerative inflammation for broad treatment benefits.
Immunomodulation to supplement glaucoma treatment is also appealing for its multi-target potential that may allow a unified treatment modality to manipulate converging neurodegenerative paths in glaucoma. As highlighted in this review, there are intimate interactions of the pathogenic routes with inflammatory or mitochondrial origin. Damaged mitochondria, a key element of glaucomatous neurodegeneration, can stimulate inflammation signaling (Bader and Winklhofer, 2020), while cellular metabolism regulates the function of immune cells. In opposition, inflammatory mediators may impair mitochondria and amplify the neurodegenerative impacts of mitochondrial failure (van Horssen et al., 2019). By counting on the interdependence between neuroinflammation and mitochondrial dysfunction, anti-inflammatory treatments may also improve mitochondrial integrity and tune cellular metabolism, as well as eliminating the inflammatory toxicity to RGCs. While treatments targeting neuroinflammation may protect mitochondria, mitochondrial restoration may also be immunomodulatory, which is another active focus of glaucoma research with high promise to develop new therapeutics.
As reviewed herein, glial cells, particularly astrocytes due to their broadly manifest, long-lasting, and more impactful responses, are promising treatment targets to rebuild immune homeostasis, enhance RGC survival, and improve the outcome of glaucoma. Yet, with respect to bi-directional communication between microglia and astrocytes during neuroinflammation, importance of these glial subtypes cannot be exclusively outweighed. Glia-targeting modulation of neuroinflammation in glaucoma awaits further studies exploring the divergent and dynamic responses of astrocytes and microglia and the intricate network of signals between these glial cells. Recent advances in single-cell technologies and unification of the single-cell multi-omics data and the outcomes of functional studies can provide more comprehensive and complementary insights into molecular regulation of neuroinflammation in glaucoma. The integrated new information can then guide towards new treatments to safely and effectively target glial inflammatory responses, while restoring or augmenting glial homeostatic and defensive functions.
Funding support:
Dr. Tezel’s research is supported by research grants from National Eye Institute (R01EY028153), AR and JR Peacock Trusts, and Homer McK. Rees Scholarship. Her work is also supported in part by a National Eye Institute grant for Core Facilities for Vision Research (P30EY019007) and an unrestricted grant from Research to Prevent Blindness.
Footnotes
Competing interest: The author declares no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad L, Zhang SY, Casanova JL, Sancho-Shimizu V, 2016. Human TBK1: A gatekeeper of neuroinflammation. Trends Mol Med. 22, 511–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aires ID, Ribeiro-Rodrigues T, Boia R, Catarino S, Girao H, Ambrosio AF, Santiago AR, 2020. Exosomes derived from microglia exposed to elevated pressure amplify the neuroinflammatory response in retinal cells. Glia. 68, 2705–2724. [DOI] [PubMed] [Google Scholar]
- Alarcon-Martinez L, Villafranca-Baughman D, Quintero H, et al. , 2020. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature. 585, 91–95. [DOI] [PubMed] [Google Scholar]
- Albalawi F, Lu W, Beckel JM, Lim JC, McCaughey SA, Mitchell CH, 2017. The P2X7 receptor primes IL-1beta and the NLRP3 inflammasome in astrocytes exposed to mechanical strain. Front Cell Neurosci. 11, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, et al. , 2016. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 532, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JS, Ley SC, 2013. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 13, 679–692. [DOI] [PubMed] [Google Scholar]
- Astafurov K, Elhawy E, Ren L, et al. , 2014. Oral microbiome link to neurodegeneration in glaucoma. PLoS One. 9, e104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasovska Velkovska M, Goricar K, Blagus T, Dolzan V, Cvenkel B, 2021. Association of genetic polymorphisms in oxidative stress and inflammation pathways with glaucoma risk and phenotype. J Clin Med. 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader V, Winklhofer KF, 2020. Mitochondria at the interface between neurodegeneration and neuroinflammation. Semin Cell Dev Biol. 99, 163–171. [DOI] [PubMed] [Google Scholar]
- Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI, Mook-Jung I, 2019. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer's Disease. Cell Metab. 30, 493–507 e496. [DOI] [PubMed] [Google Scholar]
- Balaratnasingam C, Kang MH, Yu P, Chan G, Morgan WH, Cringle SJ, Yu DY, 2014. Comparative quantitative study of astrocytes and capillary distribution in optic nerve laminar regions. Exp Eye Res. 121, 11–22. [DOI] [PubMed] [Google Scholar]
- Baris M, Tezel G, 2019. Immunomodulation as a neuroprotective strategy for glaucoma treatment. Curr Ophthalmol Rep. 7, 160–169. [PMC free article] [PubMed] [Google Scholar]
- Barton GM, Medzhitov R, 2003. Toll-like receptor signaling pathways. Science. 300, 1524–1525. [DOI] [PubMed] [Google Scholar]
- Baseler WA, Davies LC, Quigley L, Ridnour LA, Weiss JM, Hussain SP, Wink DA, McVicar DW, 2016. Autocrine IL-10 functions as a rheostat for M1 macrophage glycolytic commitment by tuning nitric oxide production. Redox Biol. 10, 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin C, Kolko M, Melik-Parsadaniantz S, Messmer EM, 2020. Inflammation in glaucoma: From the back to the front of the eye, and beyond. Prog Retin Eye Res. 83, 100916–100941. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Argall AJ, Lim JC, et al. , 2014. Mechanosensitive release of adenosine 5'-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia. 62, 1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Coleman MP, Martin KR, 2008. The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur J Neurosci. 28, 1166–1179. [DOI] [PubMed] [Google Scholar]
- Bell K, Und Hohenstein-Blaul NVT, Teister J, Grus F, 2018. Modulation of the immune system for the treatment of glaucoma. Curr Neuropharmacol. 16, 942–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haim L, Ceyzeriat K, Carrillo-de Sauvage MA, et al. , 2015. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer's and Huntington's diseases. J Neurosci. 35, 2817–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutgen VM, Perumal N, Pfeiffer N, Grus FH, 2019. Autoantibody biomarker discovery in primary open angle glaucoma using serological proteome analysis (SERPA). Front Immunol. 10, 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal MR, Prentice HM, Dorey CK, Blanks JC, 2014. A hypoxia-responsive glial cell-specific gene therapy vector for targeting retinal neovascularization. Invest Ophthalmol Vis Sci. 55, 8044–8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonska M, Shambharkar PB, Kobayashi M, Zhang D, Sakurai H, Su B, Lin X, 2005. TAK1 is recruited to the tumor necrosis factor-alpha (TNF-alpha) receptor 1 complex in a receptor-interacting protein (RIP)-dependent manner and cooperates with MEKK3 leading to NF-kappaB activation. J Biol Chem. 280, 43056–43063. [DOI] [PubMed] [Google Scholar]
- Boersma MC, Dresselhaus EC, De Biase LM, Mihalas AB, Bergles DE, Meffert MK, 2011. A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis. J Neurosci. 31, 5414–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone MP, Gonzalez Fleitas MF, Pasquini LA, Bosco A, Sande PH, Rosenstein RE, Dorfman D, 2017. Involvement of microglia in early axoglial alterations of the optic nerve induced by experimental glaucoma. J Neurochem. 142, 323–337. [DOI] [PubMed] [Google Scholar]
- Bosco A, Anderson SR, Breen KT, et al. , 2018. Complement C3-targeted gene therapy restricts onset and progression of neurodegeneration in chronic mouse glaucoma. Mol Ther. 26, 2379–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Inman DM, Steele MR, et al. , 2008. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 49, 1437–1446. [DOI] [PubMed] [Google Scholar]
- Bosco A, Romero CO, Breen KT, Chagovetz AA, Steele MR, Ambati BK, Vetter ML, 2015. Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis Model Mech. 8, 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Steele MR, Vetter ML, 2011. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol. 519, 599–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Dvoriantchikova G, Barakat D, Ivanov D, Bethea JR, Shestopalov VI, 2012. Transgenic inhibition of astroglial NF-kappaB protects from optic nerve damage and retinal ganglion cell loss in experimental optic neuritis. J Neuroinflammation. 9, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A, 2006. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 25, 397–424. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF, 2011. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp Eye Res. 93, 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT, 2005. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 24, 39–73. [DOI] [PubMed] [Google Scholar]
- Burguillos MA, Deierborg T, Kavanagh E, et al. , 2011. Caspase signalling controls microglia activation and neurotoxicity. Nature. 472, 319–324. [DOI] [PubMed] [Google Scholar]
- Carreras FJ, Aranda CJ, Porcel D, Rodriguez-Hurtado F, Martinez-Agustin O, Zarzuelo A, 2015. Expression of glucose transporters in the prelaminar region of the optic-nerve head of the pig as determined by immunolabeling and tissue culture. PLoS One. 10, e0128516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson RJ, Chidlow G, Crowston JG, Williams PA, Wood JPM, 2020. Retinal energy metabolism in health and glaucoma. Prog Retin Eye Res. 81: 100881–100899. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Hughes S, Baxter L, Rosinova E, McGregor I, Morcos Y, van Nieuwenhuyzen P, Hu P, 2007. Inflammation and breakdown of the blood-retinal barrier during "physiological aging" in the rat retina: a model for CNS aging. Microcirculation. 14, 63–76. [DOI] [PubMed] [Google Scholar]
- Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME, Yang X, 2002. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 21, 3704–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BC, Mikelberg FS, Balaszi AG, LeBlanc RP, Lesk MR, Trope GE, 2008. Canadian glaucoma study: 2. risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 126, 1030–1036. [DOI] [PubMed] [Google Scholar]
- Chen H, Cho KS, Vu THK, et al. , 2018. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat Commun. 9, 3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Deng Y, Gan X, et al. , 2020. NLRP12 collaborates with NLRP3 and NLRC4 to promote pyroptosis inducing ganglion cell death of acute glaucoma. Mol Neurodegener. 15, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Chen H, Li F, Zhu Y, Yin W, Zhuo Y, 2015. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-kappaB pathway in acute glaucoma. J Neuroinflammation. 12, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Li F, Chen H, et al. , 2014. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1beta production in acute glaucoma. Proc Natl Acad Sci U S A. 111, 11181–11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow G, Ebneter A, Wood JP, Casson RJ, 2016. Evidence supporting an association between expression of major histocompatibility complex II by microglia and optic nerve degeneration during experimental glaucoma. J Glaucoma. 25, 681–691. [DOI] [PubMed] [Google Scholar]
- Chidlow G, Wood JPM, Casson RJ, 2017. Investigations into hypoxia and oxidative stress at the optic nerve head in a rat model of glaucoma. Front Neurosci. 11, 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery HR, Keller KE, 2020. Tunneling nanotubes and the eye: Intercellular communication and implications for ocular health and disease. Biomed Res Int. 2020, 7246785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Sun D, Jakobs TC, 2015. Astrocytes in the optic nerve head express putative mechanosensitive channels. Mol Vis. 21, 749–766. [PMC free article] [PubMed] [Google Scholar]
- Chrysostomou V, Rezania F, Trounce IA, Crowston JG, 2013. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr Opin Pharmacol. 13, 12–15. [DOI] [PubMed] [Google Scholar]
- Chua J, Vania M, Cheung CM, Ang M, Chee SP, Yang H, Li J, Wong TT, 2012. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Mol Vis. 18, 431–438. [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA, 2018. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A. 115, E1896–E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, Pasini S, Lambert WS, D'Alessandro KB, Yao V, Risner ML, Calkins DJ, 2020. Redistribution of metabolic resources through astrocyte networks mitigates neurodegenerative stress. Proc Natl Acad Sci U S A. 117, 18810–18821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran SE, O'Neill LA, 2016. HIF1alpha and metabolic reprogramming in inflammation. J Clin Invest. 126, 3699–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corraliza IM, Soler G, Eichmann K, Modolell M, 1995. Arginase induction by suppressors of nitric oxide synthesis (IL-4, IL-10 and PGE2) in murine bone-marrow-derived macrophages. Biochem Biophys Res Commun. 206, 667–673. [DOI] [PubMed] [Google Scholar]
- Coughlin L, Morrison RS, Horner PJ, Inman DM, 2015. Mitochondrial morphology differences and mitophagy deficit in murine glaucomatous optic nerve. Invest Ophthalmol Vis Sci. 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ, 2010. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A. 107, 5196–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva Vargas JL, Belforte N, Di Polo A, 2016. The glial cell modulator ibudilast attenuates neuroinflammation and enhances retinal ganglion cell viability in glaucoma through protein kinase A signaling. Neurobiol Dis. 93, 156–171. [DOI] [PubMed] [Google Scholar]
- Cueva Vargas JL, Osswald IK, Unsain N, Aurousseau MR, Barker PA, Bowie D, Di Polo A, 2015. Soluble tumor necrosis factor alpha promotes retinal ganglion cell death in glaucoma via calcium-permeable AMPA receptor activation. J Neurosci. 35, 12088–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui QN, Bargoud AR, Ross AG, Song Y, Dunaief JL, 2020. Oral administration of the iron chelator deferiprone protects against loss of retinal ganglion cells in a mouse model of glaucoma. Exp Eye Res. 193, 107961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha FQ, Moncada S, Liew FY, 1992. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem Biophys Res Commun. 182, 1155–1159. [DOI] [PubMed] [Google Scholar]
- Dai C, Khaw PT, Yin ZQ, Li D, Raisman G, Li Y, 2012. Structural basis of glaucoma: the fortified astrocytes of the optic nerve head are the target of raised intraocular pressure. Glia. 60, 13–28. [DOI] [PubMed] [Google Scholar]
- Dai Y, Hu X, Sun X, 2018. Overexpression of parkin protects retinal ganglion cells in experimental glaucoma. Cell Death Dis. 9, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara D, Kolstad KD, Guerin KI, Hoffmann NV, Visel M, Klimczak RR, Schaffer DV, Flannery JG, 2011. AAV mediated GDNF secretion from retinal glia slows down retinal degeneration in a rat model of retinitis pigmentosa. Mol Ther. 19, 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele SG, Beraud D, Davenport C, Cheng K, Yin H, Maguire-Zeiss KA, 2015. Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci Signal. 8, ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Li Z, Noori A, Hyman BT, Serrano-Pozo A, 2020. Meta-analysis of mouse transcriptomic studies supports a context-dependent astrocyte reaction in acute CNS injury versus neurodegeneration. J Neuroinflammation. 17, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta Chaudhuri A, Dasgheyb RM, DeVine LR, Bi H, Cole RN, Haughey NJ, 2020. Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia. 68, 128–144. [DOI] [PubMed] [Google Scholar]
- Davis CH, Kim KY, Bushong EA, et al. , 2014. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A. 111, 9633–9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CG, Liebmann JM, Ritch R, 2012. Predictive factors within the optic nerve complex for glaucoma progression: Disc hemorrhage and parapapillary atrophy. Asia Pac J Ophthalmol (Phila). 1, 105–112. [DOI] [PubMed] [Google Scholar]
- Dela Cruz CS, Kang MJ, 2018. Mitochondrial dysfunction and damage associated molecular patterns (DAMPs) in chronic inflammatory diseases. Mitochondrion. 41, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhase M, Hayakawa M, Chen Y, Karin M, 1999. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 284, 309–313. [DOI] [PubMed] [Google Scholar]
- Dewar D, Underhill SM, Goldberg MP, 2003. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 23, 263–274. [DOI] [PubMed] [Google Scholar]
- Dismuke WM, Challa P, Navarro I, Stamer WD, Liu Y, 2015. Human aqueous humor exosomes. Exp Eye Res. 132, 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Maes ME, Grosser JA, Nickells RW, 2019. BAX-depleted retinal ganglion cells survive and become quiescent following optic nerve damage. Mol Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JK, Afzal R, Gearing LJ, et al. , 2021. Mitochondrial arginase-2 is essential for IL-10 metabolic reprogramming of inflammatory macrophages. Nat Commun. 12, 1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte JN, 2021. Neuroinflammatory mechanisms of mitochondrial dysfunction and neurodegeneration in glaucoma. Journal of Ophthalmology. 2021, 4581909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru C, Kabat AM, Maloy KJ, 2018. Metabolic adaptations of CD4(+) T Cells in inflammatory disease. Front Immunol. 9, 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoriantchikova G, Ivanov D, 2014. Tumor necrosis factor-alpha mediates activation of NF-kappaB and JNK signaling cascades in retinal ganglion cells and astrocytes in opposite ways. Eur J Neurosci. 40, 3171–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebneter A, Casson RJ, Wood JP, Chidlow G, 2010. Microglial activation in the visual pathway in experimental glaucoma: spatiotemporal characterization and correlation with axonal injury. Invest Ophthalmol Vis Sci. 51, 6448–6460. [DOI] [PubMed] [Google Scholar]
- Escartin C, Guillemaud O, Carrillo-de Sauvage MA, 2019. Questions and (some) answers on reactive astrocytes. Glia. 67, 2221–2247. [DOI] [PubMed] [Google Scholar]
- Farkas RH, Chowers I, Hackam AS, et al. , 2004. Increased expression of iron-regulating genes in monkey and human glaucoma. Invest Ophthalmol Vis Sci. 45, 1410–1417. [DOI] [PubMed] [Google Scholar]
- Feltham R, Vince JE, Lawlor KE, 2017. Caspase-8: not so silently deadly. Clin Transl Immunology. 6, e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, Harder JM, Kim J, Libby RT, 2013. JUN regulates early transcriptional responses to axonal injury in retinal ganglion cells. Exp Eye Res. 112, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, Mitchell KL, Patel A, Marola OJ, Shrager P, Zack DJ, Libby RT, Welsbie DS, 2018. Role of SARM1 and DR6 in retinal ganglion cell axonal and somal degeneration following axonal injury. Exp Eye Res. 171, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Robin AL, Stone JL, et al. , 2011. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum Mol Genet. 20, 2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Bartlett CA, Harvey AR, Dunlop SA, 2010. Early events of secondary degeneration after partial optic nerve transection: an immunohistochemical study. J Neurotrauma. 27, 439–452. [DOI] [PubMed] [Google Scholar]
- Flammer J, Mozaffarieh M, 2007. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv Ophthalmol. 52 Suppl 2, S162–173. [DOI] [PubMed] [Google Scholar]
- Formichella CR, Abella SK, Sims SM, Cathcart HM, Sappington RM, 2014. Astrocyte reactivity: a biomarker for retinal ganglion cell health in retinal neurodegeneration. J Clin Cell Immunol. 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune B, Ma KN, Gardiner SK, Demirel S, Mansberger SL, 2018. Peripapillary retinoschisis in glaucoma: Association with progression and OCT signs of Muller cell involvement. Invest Ophthalmol Vis Sci. 59, 2818–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouda AY, Eldahshan W, Narayanan SP, Caldwell RW, Caldwell RB, 2020. Arginase pathway in acute retina and brain Injury: Therapeutic opportunities and unexplored avenues. Front Pharmacol. 11, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, et al. , 2007. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 128, 92–105. [DOI] [PubMed] [Google Scholar]
- Gallego BI, Salazar JJ, de Hoz R, et al. , 2012. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J Neuroinflammation. 9, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Zelinka CP, Cebulla CM, Fischer AJ, 2015. Activation of glucocorticoid receptors in Muller glia is protective to retinal neurons and suppresses microglial reactivity. Exp Neurol. 273, 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Medina JJ, Rubio-Velazquez E, Lopez-Bernal MD, Cobo-Martinez A, Zanon-Moreno V, Pinazo-Duran MD, Del-Rio-Vellosillo M, 2020. Glaucoma and antioxidants: Review and update. Antioxidants (Basel). 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M, 2002. Missing pieces in the NF-kappaB puzzle. Cell. 109 Suppl, S81–96. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Shang P, Yazdankhah M, et al. , 2017. Activating the AKT2-nuclear factor-kappaB-lipocalin-2 axis elicits an inflammatory response in age-related macular degeneration. J Pathol. 241, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golks A, Brenner D, Krammer PH, Lavrik IN, 2006. The c-FLIP-NH2 terminus (p22-FLIP) induces NF-kappaB activation. J Exp Med. 203, 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramlich OW, Beck S, von Thun Und Hohenstein-Blaul N, Boehm N, Ziegler A, Vetter JM, Pfeiffer N, Grus FH, 2013. Enhanced insight into the autoimmune component of glaucoma: IgG autoantibody accumulation and pro-inflammatory conditions in human glaucomatous retina. PLoS One. 8, e57557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramlich OW, Ding QJ, Zhu W, Cook A, Anderson MG, Kuehn MH, 2015. Adoptive transfer of immune cells from glaucomatous mice provokes retinal ganglion cell loss in recipients. Acta Neuropathol Commun. 3, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MS, Hackett CG, Abernathy EF, et al. , 2011. Opposing roles for membrane bound and soluble Fas ligand in glaucoma-associated retinal ganglion cell death. PLoS One. 6, e17659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes RP, Csizmadia E, Moll HP, Ma A, Ferran C, da Silva CG, 2014. A20 deficiency causes spontaneous neuroinflammation in mice. J Neuroinflammation. 11, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Wu N, Niu X, Wu Y, Chen D, Guo W, 2018. Comparison of T helper cell patterns in primary open-angle glaucoma and normal-pressure glaucoma. Med Sci Monit. 24, 1988–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Johnson EC, Cepurna WO, Dyck JA, Doser T, Morrison JC, 2011. Early gene expression changes in the retinal ganglion cell layer of a rat glaucoma model. Invest Ophthalmol Vis Sci. 52, 1460–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Pulliam L, 2014. Exosomes as mediators of neuroinflammation. J Neuroinflammation. 11, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Kanneganti TD, 2015. Novel roles for caspase-8 in IL-1beta and inflammasome regulation. Am J Pathol. 185, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenplan KA, Stafford BK, El-Danaf RN, Adler DI, Munch AE, Weigel MK, Huberman AD, Liddelow SA, 2020. Neurotoxic reactive astrocytes drive neuronal death after retinal injury. Cell Rep. 31, 107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenold R, Weih F, Herrmann KH, et al. , 2014. NF-kappaB controls axonal regeneration and degeneration through cell-specific balance of RelA and p50 in the adult CNS. J Cell Sci. 127, 3052–3065. [DOI] [PubMed] [Google Scholar]
- Harari OA, Liao JK, 2010. NF-kappaB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 1207, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Braine CE, Williams PA, et al. , 2017. Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective. Proc Natl Acad Sci U S A. 114, E3839–E3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Guymer C, Wood JPM, et al. , 2020a. Disturbed glucose and pyruvate metabolism in glaucoma with neuroprotection by pyruvate or rapamycin. Proc Natl Acad Sci U S A. 117, 33619–33627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Williams PA, Braine CE, Yang HS, Thomas JM, Foxworth NE, John SWM, Howell GR, 2020b. Complement peptide C3a receptor 1 promotes optic nerve degeneration in DBA/2J mice. J Neuroinflammation. 17, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Williams PA, Soto I, Foxworth NE, Fernandes KA, Freeburg NF, Libby RT, John SWM, 2018. Jnk2 deficiency increases the rate of glaucomatous neurodegeneration in ocular hypertensive DBA/2J mice. Cell Death Dis. 9, 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harun-Or-Rashid M, Inman DM, 2018. Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. J Neuroinflammation. 15, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harun-Or-Rashid M, Pappenhagen N, Palmer PG, Smith MA, Gevorgyan V, Wilson GN, Crish SD, Inman DM, 2018. Structural and functional rescue of chronic metabolically stressed optic nerves through respiration. J Neurosci. 38, 5122–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass DT, Barnstable CJ, 2019. Mitochondrial uncoupling protein 2 knock-out promotes mitophagy to decrease retinal ganglion cell death in a mouse model of glaucoma. J Neurosci. 39, 3582–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X, 2009. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 137, 1100–1111. [DOI] [PubMed] [Google Scholar]
- He X, Hahn P, Iacovelli J, Wong R, King C, Bhisitkul R, Massaro-Giordano M, Dunaief JL, 2007. Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res. 26, 649–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CM, Martin SJ, 2017. Caspase-8 acts in a non-enzymatic role as a scaffold for assembly of a pro-inflammatory "FADDosome" complex upon TRAILs stimulation. Mol Cell. 65, 715–729 e715. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Miao H, Lukas T, 2008. Astrocytes in glaucomatous optic neuropathy. Prog Brain Res. 173, 353–373. [DOI] [PubMed] [Google Scholar]
- Heuss ND, Pierson MJ, Montaniel KR, et al. , 2014. Retinal dendritic cell recruitment, but not function, was inhibited in MyD88 and TRIF deficient mice. J Neuroinflammation. 11, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, Du X, Georgel P, et al. , 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 424, 743–748. [DOI] [PubMed] [Google Scholar]
- Holland R, McIntosh AL, Finucane OM, et al. , 2018. Inflammatory microglia are glycolytic and iron retentive and typify the microglia in APP/PS1 mice. Brain Behav Immun. 68, 183–196. [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, et al. , 2007. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 179, 1523–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Macalinao DG, Sousa GL, et al. , 2011. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 121, 1429–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, MacNicoll KH, Braine CE, Soto I, Macalinao DG, Sousa GL, John SW, 2014. Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma. Neurobiol Dis. 71, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Ryan M, Graham LC, Smith RS, John SW, 2013. Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice. J Neuroinflammation. 10, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, et al. , 2012. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest. 122, 1246–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Pollard JD, Chan-Ling T, 2000. Breakdown of the blood-retinal barrier induced by activated T cells of nonneural specificity. Am J Pathol. 156, 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Xu MX, Zhou H, Cheng S, Li F, Miao Y, Wang Z, 2020. Tumor necrosis factor-alpha aggravates gliosis and inflammation of activated retinal Muller cells. Biochem Biophys Res Commun. 531, 383–389. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Stevens BR, Nielsen RB, Slovin PN, Fang X, Nelson DR, Skimming JW, 2002. Interleukin-10 inhibition of nitric oxide biosynthesis involves suppression of CAT-2 transcription. Nitric Oxide. 6, 79–84. [DOI] [PubMed] [Google Scholar]
- Huang P, Qi Y, Xu YS, Liu J, Liao D, Zhang SS, Zhang C, 2010. Serum cytokine alteration is associated with optic neuropathy in human primary open angle glaucoma. J Glaucoma. 19, 324–330. [DOI] [PubMed] [Google Scholar]
- Huang W, Dobberfuhl A, Filippopoulos T, Ingelsson M, Fileta JB, Poulin NR, Grosskreutz CL, 2005. Transcriptional up-regulation and activation of initiating caspases in experimental glaucoma. Am J Pathol. 167, 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Lin YS, Wang CY, et al. , 2009. Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology. 128, e275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman DM, Horner PJ, 2007. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 55, 942–953. [DOI] [PubMed] [Google Scholar]
- Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R, 2017. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 356, 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, et al. , 1997. Inhibition of death receptor signals by cellular FLIP. Nature. 388, 190–195. [DOI] [PubMed] [Google Scholar]
- Itoh N, Itoh Y, Tassoni A, et al. , 2018. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proc Natl Acad Sci U S A. 115, E302–E309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassim AH, Fan Y, Pappenhagen N, Nsiah NY, Inman DM, 2021. Oxidative stress and hypoxia modify mitochondrial homeostasis during glaucoma. Antioxid Redox Signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassim AH, Inman DM, 2019. Evidence of hypoxic glial cells in a model of ocular hypertension. Invest Ophthalmol Vis Sci. 60, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AK, Huang SC, Sergushichev A, et al. , 2015. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 42, 419–430. [DOI] [PubMed] [Google Scholar]
- Joachim SC, Gramlich OW, Laspas P, et al. , 2012. Retinal ganglion cell loss is accompanied by antibody depositions and increased levels of microglia after immunization with retinal antigens. PLoS One. 7, e40616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Doser TA, Cepurna WO, Dyck JA, Jia L, Guo Y, Lambert WS, Morrison JC, 2011. Cell proliferation and interleukin-6-type cytokine signaling are implicated by gene expression responses in early optic nerve head injury in rat glaucoma. Invest Ophthalmol Vis Sci. 52, 504–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Jia L, Cepurna WO, Doser TA, Morrison JC, 2007. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci. 48, 3161–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AU, Minhas PS, Liddelow SA, Haileselassie B, Andreasson KI, Dorn GW 2nd, Mochly-Rosen D, 2019. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat Neurosci. 22, 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju KR, Kim HS, Kim JH, Lee NY, Park CK, 2006. Retinal glial cell responses and Fas/FasL activation in rats with chronic ocular hypertension. Brain Res. 1122, 209–221. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A, 2002. NF-kappaB at the crossroads of life and death. Nat Immunol. 3, 221–227. [DOI] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Sun YY, Yang YF, Acott TS, 2017. Tunneling nanotubes are novel cellular structures that communicate signals between trabecular meshwork cells. Invest Ophthalmol Vis Sci. 58, 5298–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ho DH, Suk JE, et al. , 2013. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 4, 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Li J, 2013. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. 4, e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan RP, Wordinger RJ, Clark AF, O'Brien CJ, 2009. Differential global and extracellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Mol Vis. 15, 76–88. [PMC free article] [PubMed] [Google Scholar]
- Kleesattel D, Crish SD, Inman DM, 2015. Decreased energy capacity and increased autophagic activity in optic nerve axons with defective anterograde transport. Invest Ophthalmol Vis Sci. 56, 8215–8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko KW, Milbrandt J, DiAntonio A, 2020. SARM1 acts downstream of neuroinflammatory and necroptotic signaling to induce axon degeneration. J Cell Biol. 219, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber JT, Klimczak R, Jang JH, Dalkara D, Flannery JG, Schaffer DV, 2009. Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Mol Ther. 17, 2088–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rock KL, 2008. How dying cells alert the immune system to danger. Nat Rev Immunol. 8, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann S, Madore C, Cialic R, et al. , 2017. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 47, 566–581 e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Fei F, Jones A, Busto P, Marshak-Rothstein A, Ksander BR, Gregory-Ksander M, 2016. Overexpression of soluble Fas ligand following adeno-associated virus gene therapy prevents retinal ganglion cell death in chronic and acute murine models of glaucoma. J Immunol. 197, 4626–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Kocab AJ, Zacks DN, Marshak-Rothstein A, Gregory-Ksander M, 2019. A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma. J Neuroinflammation. 16, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Ryskamp DA, Tian N, Tezel G, Mitchell CH, Slepak VZ, Shestopalov VI, 2014. From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr Eye Res. 39, 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchtey J, Rezaei KA, Jaru-Ampornpan P, Sternberg P Jr., Kuchtey RW, 2010. Multiplex cytokine analysis reveals elevated concentration of interleukin-8 in glaucomatous aqueous humor. Invest Ophthalmol Vis Sci. 51, 6441–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MH, Kim CY, Ostojic J, et al. , 2006. Retinal synthesis and deposition of complement components induced by ocular hypertension. Exp Eye Res. 83, 620–628. [DOI] [PubMed] [Google Scholar]
- Kuehn S, Stellbogen M, Noristani R, Peters M, Dick HB, Joachim SC, 2016. Systemic ocular antigen immunization leads only to a minor secondary immune response. J Neuroimmunol. 293, 114–122. [DOI] [PubMed] [Google Scholar]
- Kwon HS, Koh SH, 2020. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 9, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JM, Caprioli J, 2006. Expression of phosphorylated c-Jun N-terminal protein kinase (JNK) in experimental glaucoma in rats. Exp Eye Res. 82, 576–582. [DOI] [PubMed] [Google Scholar]
- Lahne M, Nagashima M, Hyde DR, Hitchcock PF, 2020. Reprogramming Muller glia to regenerate retinal neurons. Annu Rev Vis Sci. 6, 171–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ, 2002. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 169, 2253–2263. [DOI] [PubMed] [Google Scholar]
- Langston PK, Shibata M, Horng T, 2017. Metabolism supports macrophage activation. Front Immunol. 8, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascaratos G, Chau KY, Zhu H, et al. , 2015. Resistance to the most common optic neuropathy is associated with systemic mitochondrial efficiency. Neurobiol Dis. 82, 78–85. [DOI] [PubMed] [Google Scholar]
- Laspas P, Gramlich OW, Muller HD, et al. , 2011. Autoreactive antibodies and loss of retinal ganglion cells in rats induced by immunization with ocular antigens. Invest Ophthalmol Vis Sci. 52, 8835–8848. [DOI] [PubMed] [Google Scholar]
- Lattke M, Reichel SN, Magnutzki A, et al. , 2017. Transient IKK2 activation in astrocytes initiates selective non-cell-autonomous neurodegeneration. Mol Neurodegener. 12, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudisi F, Spreafico R, Evrard M, et al. , 2013. Cutting edge: the NLRP3 inflammasome links complement-mediated inflammation and IL-1beta release. J Immunol. 191, 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautrup S, Sinclair DA, Mattson MP, Fang EF, 2019. NAD(+) in brain aging and neurodegenerative disorders. Cell Metab. 30, 630–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sheck L, Crowston JG, et al. , 2012. Impaired complex-I-linked respiration and ATP synthesis in primary open-angle glaucoma patient lymphoblasts. Invest Ophthalmol Vis Sci. 53, 2431–2437. [DOI] [PubMed] [Google Scholar]
- Lehmann U, Heuss ND, McPherson SW, Roehrich H, Gregerson DS, 2010. Dendritic cells are early responders to retinal injury. Neurobiol Dis. 40, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers B, Salmena L, Bidere N, et al. , 2007. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem. 282, 7416–7423. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Kalev-Landoy M, Habot-Wilner Z, Melamed S, 2006. Minocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transection. Arch Ophthalmol. 124, 520–526. [DOI] [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Nickells RW, 1999. Experimental induction of retinal ganglion cell death in adult mice. Invest Ophthalmol Vis Sci. 40, 1004–1008. [PubMed] [Google Scholar]
- Lian H, Yang L, Cole A, et al. , 2015. NFkappaB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer's disease. Neuron. 85, 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Gould DB, Anderson MG, John SW, 2005a. Complex genetics of glaucoma susceptibility. Annu Rev Genomics Hum Genet. 6, 15–44. [DOI] [PubMed] [Google Scholar]
- Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, John SW, 2005b. Susceptibility to neurodegeneration in a glaucoma is modified by bax gene dosage. PLoS Genet. 1, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, et al. , 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Marsh SE, Stevens B, 2020. Microglia and astrocytes in disease: Dynamic duo or partners in crime? Trends Immunol. 41, 820–835. [DOI] [PubMed] [Google Scholar]
- Lin S, Liang Y, Zhang J, et al. , 2012. Microglial TIR-domain-containing adapter-inducing interferon-beta (TRIF) deficiency promotes retinal ganglion cell survival and axon regeneration via nuclear factor-kappaB. J Neuroinflammation. 9, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Chen H, Johns TG, Neufeld AH, 2006. Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. J Neurosci. 26, 7532–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Neufeld AH, 2004. Activation of epidermal growth factor receptor causes astrocytes to form cribriform structures. Glia. 46, 153–168. [DOI] [PubMed] [Google Scholar]
- Liu B, Neufeld AH, 2003. Activation of epidermal growth factor receptor signals induction of nitric oxide synthase-2 in human optic nerve head astrocytes in glaucomatous optic neuropathy. Neurobiology of Disease. 13, 109–123. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang D, Hu D, Zhou X, Zhou Y, 2018. The role of mitochondria in NLRP3 inflammasome activation. Mol Immunol. 103, 115–124. [DOI] [PubMed] [Google Scholar]
- Liu Y, Miao Q, Yuan J, et al. , 2015. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J Neurosci. 35, 9336–9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano DC, Choe TE, Cepurna WO, Morrison JC, Johnson EC, 2019. Early optic nerve head glial proliferation and Jak-Stat pathway activation in chronic experimental glaucoma. Invest Ophthalmol Vis Sci. 60, 921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Hu H, Sevigny J, et al. , 2015. Rat, mouse, and primate models of chronic glaucoma show sustained elevation of extracellular ATP and altered purinergic signaling in the posterior eye. Invest Ophthalmol Vis Sci. 56, 3075–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Yang X, Kain AD, Powell DW, Kuehn MH, Tezel G, 2010. Glaucomatous tissue stress and the regulation of immune response through glial toll-like receptor signaling. Invest Ophthalmol Vis Sci. 51, 5697–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye-Barthel M, Sun D, Jakobs TC, 2013. Morphology of astrocytes in a glaucomatous optic nerve. Invest Ophthalmol Vis Sci. 54, 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Nair CE, Nickells RW, 2015. Neuroinflammation in glaucoma and optic nerve damage. Prog Mol Biol Transl Sci. 134, 343–363. [DOI] [PubMed] [Google Scholar]
- Mac Nair CE, Schlamp CL, Montgomery AD, Shestopalov VI, Nickells RW, 2016. Retinal glial responses to optic nerve crush are attenuated in Bax-deficient mice and modulated by purinergic signaling pathways. J Neuroinflammation. 13, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MH, Ortin-Martinez A, Nadal-Nicolas F, Ambrosio AF, Vidal-Sanz M, Agudo-Barriuso M, Santiago AR, 2016. Caffeine administration prevents retinal neuroinflammation and loss of retinal ganglion cells in an animal model of glaucoma. Sci Rep. 6, 27532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta MA, Letcher SM, Igo RP Jr., et al. , 2020. Association of APOE with primary open-angle glaucoma suggests a protective effect for APOE epsilon4. Invest Ophthalmol Vis Sci. 61, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marola OJ, Syc-Mazurek SB, Howell GR, Libby RT, 2020. Endothelin 1-induced retinal ganglion cell death is largely mediated by JUN activation. Cell Death Dis. 11, 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A, Lindsley TA, Sheridan A, Bhoiwala DL, Hushmendy SF, Yager EJ, Ruggiero EA, Crawford DR, 2012. Degraded mitochondrial DNA is a newly identified subtype of the damage associated molecular pattern (DAMP) family and possible trigger of neurodegeneration. J Alzheimers Dis. 30, 617–627. [DOI] [PubMed] [Google Scholar]
- Mathieu E, Gupta N, Ahari A, Zhou X, Hanna J, Yucel YH, 2017. Evidence for cerebrospinal fluid entry into the optic nerve via a glymphatic pathway. Invest Ophthalmol Vis Sci. 58, 4784–4791. [DOI] [PubMed] [Google Scholar]
- Matias I, Morgado J, Gomes FCA, 2019. Astrocyte heterogeneity: impact to brain aging and disease. Front Aging Neurosci. 11, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Arumugam TV, 2018. Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metab. 27, 1176–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy RC, Sosa JC, Gardeck AM, Baez AS, Lee CH, Wessling-Resnick M, 2018. Inflammation-induced iron transport and metabolism by brain microglia. J Biol Chem. 293, 7853–7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell F, O'Brien C, Wallace D, 2014. The role of epigenetics in the fibrotic processes associated with glaucoma. J Ophthalmol. 2014, 750459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, et al. , 2002. Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest Ophthalmol Vis Sci. 43, 1077–1087. [PubMed] [Google Scholar]
- Medzhitov R, 2008. Origin and physiological roles of inflammation. Nature. 454, 428–435. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D, 2003. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 6, 1072–1078. [DOI] [PubMed] [Google Scholar]
- Meng X, Yang F, Ouyang T, Liu B, Wu C, Jiang W, 2015. Specific gene expression in mouse cortical astrocytes is mediated by a 1740bp-GFAP promoter-driven combined adeno-associated virus 2/5/7/8/9. Neurosci Lett. 593, 45–50. [DOI] [PubMed] [Google Scholar]
- Meyer A, Laverny G, Bernardi L, Charles AL, Alsaleh G, Pottecher J, Sibilia J, Geny B, 2018. Mitochondria: An organelle of bacterial origin controlling inflammation. Front Immunol. 9, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SJ, 2018. Astrocyte heterogeneity in the adult central nervous system. Front Cell Neurosci. 12, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara T, Kikuchi T, Akimoto M, Kurokawa T, Shibuki H, Yoshimura N, 2003. Gene microarray analysis of experimental glaucomatous retina from cynomologous monkey. Invest Ophthalmol Vis Sci. 44, 4347–4356. [DOI] [PubMed] [Google Scholar]
- Mo JS, Anderson MG, Gregory M, et al. , 2003. By altering ocular immune privilege, bone marrow-derived cells pathogenically contribute to DBA/2J pigmentary glaucoma. J Exp Med. 197, 1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebiany AN, Ramphal NS, Karram K, et al. , 2020. Microglial A20 protects the brain from CD8 T-cell-mediated immunopathology. Cell Rep. 30, 1585–1597 e1586. [DOI] [PubMed] [Google Scholar]
- Monie TP, Bryant CE, 2015. Caspase-8 functions as a key mediator of inflammation and pro-IL-1beta processing via both canonical and non-canonical pathways. Immunol Rev. 265, 181–193. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, et al. , 2014. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 41, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushana A, Bansal M, Swarup G, 2011. Optineurin is required for CYLD-dependent inhibition of TNFalpha-induced NF-kappaB activation. PLoS One. 6, e17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, et al. , 2011. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 12, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T, Nakazawa C, Matsubara A, et al. , 2006. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 26, 12633–12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale G, Limanaqi F, Busceti CL, Mastroiacovo F, Nicoletti F, Puglisi-Allegra S, Fornai F, 2021. Glymphatic system as a gateway to connect neurodegeneration from periphery to CNS. Front Neurosci. 15, 639140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naude PJ, Nyakas C, Eiden LE, et al. , 2012. Lipocalin 2: novel component of proinflammatory signaling in Alzheimer's disease. FASEB J. 26, 2811–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayyar A, Gindina S, Barron A, Hu Y, Danias J, 2020. Do epigenetic changes caused by commensal microbiota contribute to development of ocular disease? A review of evidence. Hum Genomics. 14, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M, Richardson JR, 2018. Epigenetic regulation of astrocyte function in neuroinflammation and neurodegeneration. Biochim Biophys Acta Mol Basis Dis. 1864, 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld AH, 1999. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch Ophthalmol. 117, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Neufeld AH, Sawada A, Becker B, 1999. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci U S A. 96, 9944–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Takahashi K, 2007. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. 184, 92–99. [DOI] [PubMed] [Google Scholar]
- Nguyen JV, Soto I, Kim KY, et al. , 2011. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci U S A. 108, 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolskaya T, Nikolsky Y, Serebryiskaya T, et al. , 2009. Network analysis of human glaucomatous optic nerve head astrocytes. BMC Med Genomics. 2, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnah IC, Lee CH, Wessling-Resnick M, 2020. Iron potentiates microglial interleukin-1beta secretion induced by amyloid-beta. J Neurochem. 154, 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nor Arfuzir NN, Agarwal R, Iezhitsa I, Agarwal P, Ismail NM, 2020. Magnesium acetyltaurate protects against endothelin-1 induced RGC loss by reducing neuroinflammation in Sprague dawley rats. Exp Eye Res. 194, 107996. [DOI] [PubMed] [Google Scholar]
- Norden DM, Fenn AM, Dugan A, Godbout JP, 2014. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 62, 881–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Bowie AG, 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 7, 353–364. [DOI] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, et al. , 2011. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 471, 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckl P, Lattke M, Wirth T, Baumann B, Ferger B, 2012. Astrocyte-specific IKK2 activation in mice is sufficient to induce neuroinflammation but does not increase susceptibility to MPTP. Neurobiol Dis. 48, 481–487. [DOI] [PubMed] [Google Scholar]
- Oikawa K, Ver Hoeve JN, Teixeira LBC, Snyder KC, Kiland JA, Ellinwood NM, McLellan GJ, 2020. Sub-region-specific optic nerve head glial activation in glaucoma. Mol Neurobiol. 57, 2620–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN, Kamalden TA, Majid AS, del Olmo-Aguado S, Manso AG, Ji D, 2010. Light effects on mitochondrial photosensitizers in relation to retinal degeneration. Neurochem Res. 35, 2027–2034. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Lascaratos G, Bron AJ, Chidlow G, Wood JP, 2006. A hypothesis to suggest that light is a risk factor in glaucoma and the mitochondrial optic neuropathies. Br J Ophthalmol. 90, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa Y, 2020. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox Biol. 37, 101779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagis L, Zhao X, Ge Y, Ren L, Mittag TW, Danias J, 2010. Gene expression changes in areas of focal loss of retinal ganglion cells in the retina of DBA/2J mice. Invest Ophthalmol Vis Sci. 51, 2024–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bergamini G, Rajendran L, 2019. Cell-to-cell communication by extracellular vesicles: Focus on microglia. Neuroscience. 405, 148–157. [DOI] [PubMed] [Google Scholar]
- Park HL, Kim JH, Jung Y, Park CK, 2017. Racial differences in the extracellular matrix and histone acetylation of the lamina cribrosa and peripapillary sclera. Invest Ophthalmol Vis Sci. 58, 4143–4154. [DOI] [PubMed] [Google Scholar]
- Patel GC, Millar JC, Clark AF, 2019. Glucocorticoid receptor transactivation is required for glucocorticoid-induced ocular hypertension and glaucoma. Invest Ophthalmol Vis Sci. 60, 1967–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer N, Darding M, Walczak H, 2016. Holding RIPK1 on the ubiquitin leash in TNFR1 signaling. Trends Cell Biol. 26, 445–461. [DOI] [PubMed] [Google Scholar]
- Pelzel HR, Schlamp CL, Waclawski M, Shaw MK, Nickells RW, 2012. Silencing of Fem1cR3 gene expression in the DBA/2J mouse precedes retinal ganglion cell death and is associated with histone deacetylase activity. Invest Ophthalmol Vis Sci. 53, 1428–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JD, Varela HJ, Ricard CS, Hernandez MR, 1999. Enhanced tenascin expression associated with reactive astrocytes in human optic nerve heads with primary open angle glaucoma. Exp Eye Res. 68, 29–40. [DOI] [PubMed] [Google Scholar]
- Peng H, Sun YB, Hao JL, Lu CW, Bi MC, Song E, 2019. Neuroprotective effects of overexpressed microRNA-200a on activation of glaucoma-related retinal glial cells and apoptosis of ganglion cells via downregulating FGF7-mediated MAPK signaling pathway. Cell Signal. 54, 179–190. [DOI] [PubMed] [Google Scholar]
- Philip NH, DeLaney A, Peterson LW, et al. , 2016. Activity of uncleaved caspase-8 controls anti-bacterial immune defense and TLR-induced cytokine production independent of cell death. PLoS Pathog. 12, e1005910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollizzi KN, Powell JD, 2014. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 14, 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice HM, Biswal MR, Dorey CK, Blanks JC, 2011. Hypoxia-regulated retinal glial cell-specific promoter for potential gene therapy in disease. Invest Ophthalmol Vis Sci. 52, 8562–8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin A, Pham D, An W, et al. , 2019. Inflammasome activation induces pyroptosis in the retina exposed to ocular hypertension injury. Front Mol Neurosci. 12, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punal VM, Paisley CE, Brecha FS, et al. , 2019. Large-scale death of retinal astrocytes during normal development is non-apoptotic and implemented by microglia. PLoS Biol. 17, e3000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyang Z, Feng L, Chen H, Liang P, Troy JB, Liu X, 2016. Retinal ganglion cell loss is delayed following optic nerve crush in NLRP3 knockout mice. Sci Rep. 6, 20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Jakobs TC, 2013. The time course of gene expression during reactive gliosis in the optic nerve. PLoS One. 8, e67094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Broman AT, 2006. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 90, 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillen S, Schaub J, Quigley H, Pease M, Korneva A, Kimball E, 2020. Astrocyte responses to experimental glaucoma in mouse optic nerve head. PLoS One. 15, e0238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AI, de Hoz R, Fernandez-Albarral JA, et al. , 2020. Time course of bilateral microglial activation in a mouse model of laser-induced glaucoma. Sci Rep. 10, 4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AI, Salazar JJ, de Hoz R, et al. , 2010. Quantification of the effect of different levels of IOP in the astroglia of the rat retina ipsilateral and contralateral to experimental glaucoma. Invest Ophthalmol Vis Sci. 51, 5690–5696. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, 2016. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 19, 987–991. [DOI] [PubMed] [Google Scholar]
- Rawat P, Teodorof-Diedrich C, Spector SA, 2019. Human immunodeficiency virus Type-1 single-stranded RNA activates the NLRP3 inflammasome and impairs autophagic clearance of damaged mitochondria in human microglia. Glia. 67, 802–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach N, Delekate A, Plescher M, Schmitt F, Krauss S, Blank N, Halle A, Petzold GC, 2019. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer's disease model. EMBO Mol Med. 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigada D, Lu W, Zhang M, Mitchell CH, 2008. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience. 157, 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A, 2005. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 204, 27–42. [DOI] [PubMed] [Google Scholar]
- Roberts MD, Liang Y, Sigal IA, Grimm J, Reynaud J, Bellezza A, Burgoyne CF, Downs JC, 2010. Correlation between local stress and strain and lamina cribrosa connective tissue volume fraction in normal monkey eyes. Invest Ophthalmol Vis Sci. 51, 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh M, Zhang Y, Murakami Y, Thanos A, Lee SC, Vavvas DG, Benowitz LI, Miller JW, 2012. Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS One. 7, e40065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Li Z, Arendt A, Hargrave PA, Wax MB, 1999. Epitope mapping of anti-rhodopsin antibodies from patients with normal pressure glaucoma. Invest Ophthalmol Vis Sci. 40, 1275–1280. [PubMed] [Google Scholar]
- Rongvaux A, 2018. Innate immunity and tolerance toward mitochondria. Mitochondrion. 41, 14–20. [DOI] [PubMed] [Google Scholar]
- Saab AS, Tzvetavona ID, Trevisiol A, et al. , 2016. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron. 91, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Kauppinen A, 2020. ER stress activates immunosuppressive network: implications for aging and Alzheimer's disease. J Mol Med (Berl). 98, 633–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza A, Raveu AL, Reboussin E, et al. , 2016. Bilateral neuroinflammatory processes in visual pathways induced by unilateral ocular hypertension in the rat. J Neuroinflammation. 13, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfarazi M, Rezaie T, 2003. Optineurin in primary open angle glaucoma. Ophthalmol Clin North Am. 16, 529–541. [DOI] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, et al. , 2005. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 6, 1087–1095. [DOI] [PubMed] [Google Scholar]
- Sawada H, Fukuchi T, Tanaka T, Abe H, 2010. Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma. Invest Ophthalmol Vis Sci. 51, 903–906. [DOI] [PubMed] [Google Scholar]
- Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW, 2006. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 7, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlereth SL, Kremers S, Schrodl F, Cursiefen C, Heindl LM, 2016. Characterization of antigen-presenting macrophages and dendritic cells in the healthy human sclera. Invest Ophthalmol Vis Sci. 57, 4878–4885. [DOI] [PubMed] [Google Scholar]
- Schmitt HM, Schlamp CL, Nickells RW, 2016. Role of HDACs in optic nerve damage-induced nuclear atrophy of retinal ganglion cells. Neurosci Lett. 625, 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Pawlak R, Weber GR, et al. , 2021. A novel ocular function for decorin in the aqueous humor outflow. Matrix Biol. 97, 1–19. [DOI] [PubMed] [Google Scholar]
- Seitz R, Ohlmann A, Tamm ER, 2013. The role of Muller glia and microglia in glaucoma. Cell Tissue Res. 353, 339–345. [DOI] [PubMed] [Google Scholar]
- Shechter R, London A, Schwartz M, 2013. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. 13, 206–218. [DOI] [PubMed] [Google Scholar]
- Shenderov K, Riteau N, Yip R, et al. , 2014. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8-and TRIF-dependent pathway. J Immunol. 192, 2029–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi JH, Sun SC, 2018. Tumor necrosis factor receptor-associated factor regulation of nuclear factor kappaB and mitogen-activated protein kinase pathways. Front Immunol. 9, 1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya E, Meguro A, Ota M, et al. , 2008. Association of Toll-like receptor 4 gene polymorphisms with normal tension glaucoma. Invest Ophthalmol Vis Sci. 49, 4453–4457. [DOI] [PubMed] [Google Scholar]
- Shimada K, Crother TR, Karlin J, et al. , 2012. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 36, 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki Y, Shibata K, Yoshida K, Shigetomi E, Gachet C, Ikenaka K, Tanaka KF, Koizumi S, 2017. Transformation of astrocytes to a neuroprotective phenotype by microglia via P2Y1 receptor downregulation. Cell Rep. 19, 1151–1164. [DOI] [PubMed] [Google Scholar]
- Shosha E, Xu Z, Yokota H, Saul A, Rojas M, Caldwell RW, Caldwell RB, Narayanan SP, 2016. Arginase 2 promotes neurovascular degeneration during ischemia/reperfusion injury. Cell Death Dis. 7, e2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpilka T, Haynes CM, 2018. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol. 19, 109–120. [DOI] [PubMed] [Google Scholar]
- Silke J, Strasser A, 2013. The FLIP Side of Life. Sci Signal. 6, pe2. [DOI] [PubMed] [Google Scholar]
- Sinha S, Satpathy AT, Zhou W, et al. , 2021. Profiling chromatin accessibility at single-cell resolution. Genomics Proteomics Bioinformatics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi K, Chalasani ML, Sudhakar C, Kumari A, Radha V, Swarup G, 2013. M98K-OPTN induces transferrin receptor degradation and RAB12-mediated autophagic death in retinal ganglion cells. Autophagy. 9, 510–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowicka K, van Loo G, 2018. Optineurin functions for optimal immunity. Front Immunol. 9, 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, 2015. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 16, 249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JL, Soto I, Oglesby E, Lopez-Roca T, Pease ME, Quigley HA, Marsh-Armstrong N, 2010. Glaucomatous optic nerve injury involves early astrocyte reactivity and late oligodendrocyte loss. Glia. 58, 780–789. [DOI] [PubMed] [Google Scholar]
- Song N, Liu ZS, Xue W, et al. , 2017. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell. 68, 185–197 e186. [DOI] [PubMed] [Google Scholar]
- Sousa C, Biber K, Michelucci A, 2017. Cellular and molecular characterization of microglia: A unique immune cell population. Front Immunol. 8, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi K, Nagel D, Yang X, Ren L, Mittag T, Danias J, 2007. Ceruloplasmin upregulation in retina of murine and human glaucomatous eyes. Invest Ophthalmol Vis Sci. 48, 727–732. [DOI] [PubMed] [Google Scholar]
- Steele MR, Inman DM, Calkins DJ, Horner PJ, Vetter ML, 2006. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest Ophthalmol Vis Sci. 47, 977–985. [DOI] [PubMed] [Google Scholar]
- Sterling JK, Adetunji MO, Guttha S, Bargoud AR, Uyhazi KE, Ross AG, Dunaief JL, Cui QN, 2020. GLP-1 receptor agonist NLY01 reduces retinal inflammation and neuron death secondary to ocular hypertension. Cell Rep. 33, 108271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, et al. , 2007. The classical complement cascade mediates CNS synapse elimination. Cell. 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Su W, Li Z, Jia Y, Zhuo Y, 2014. Rapamycin is neuroprotective in a rat chronic hypertensive glaucoma model. PLoS One. 9, e99719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Moore S, Jakobs TC, 2017. Optic nerve astrocyte reactivity protects function in experimental glaucoma and other nerve injuries. J Exp Med. 214, 1411–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Qu J, Jakobs TC, 2013. Reversible reactivity by optic nerve astrocytes. Glia. 61, 1218–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syc-Mazurek SB, Fernandes KA, Libby RT, 2017. JUN is important for ocular hypertension-induced retinal ganglion cell degeneration. Cell Death Dis. 8, e2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syc-Mazurek SB, Libby RT, 2019. Axon injury signaling and compartmentalized injury response in glaucoma. Prog Retin Eye Res. 73, 100769–100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta HM, Le TM, Ishii H, et al. , 2016. Atf6alpha deficiency suppresses microglial activation and ameliorates pathology of experimental autoimmune encephalomyelitis. J Neurochem. 139, 1124–1137. [DOI] [PubMed] [Google Scholar]
- Takano Y, Shi D, Shimizu A, et al. , 2012. Association of Toll-like receptor 4 gene polymorphisms in Japanese subjects with primary open-angle, normal-tension, and exfoliation glaucoma. Am J Ophthalmol. 154, 825–832 e821. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S, 2004. TLR signaling pathways. Semin Immunol. 16, 3–9. [DOI] [PubMed] [Google Scholar]
- Taylor S, Calder CJ, Albon J, Erichsen JT, Boulton ME, Morgan JE, 2011. Involvement of the CD200 receptor complex in microglia activation in experimental glaucoma. Exp Eye Res. 92, 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani S, Davis L, Cepurna WO, et al. , 2016. Astrocyte structural and molecular response to elevated intraocular pressure occurs rapidly and precedes axonal tubulin rearrangement within the optic nerve head in a rat model. PLoS One. 11, e0167364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR, 2005. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J Neurosci Res. 81, 644–652. [DOI] [PubMed] [Google Scholar]
- Tezel G, 2020. A broad perspective on the molecular regulation of retinal ganglion cell degeneration in glaucoma. Prog Brain Res. 256, 49–77. [DOI] [PubMed] [Google Scholar]
- Tezel G, 2011. The immune response in glaucoma: a perspective on the roles of oxidative stress. Exp Eye Res. 93, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, 2021. Multifactorial pathogenic processes of retinal ganglion cell degeneration in glaucoma towards multi-target strategies for broader treatment effects. Cells. 10, 1372–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, 2006. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog Retin Eye Res. 25, 490–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, 2008. TNF-alpha signaling in glaucomatous neurodegeneration. Prog Brain Res. 173, 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Chauhan BC, LeBlanc RP, Wax MB, 2003. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci. 44, 3025–3033. [DOI] [PubMed] [Google Scholar]
- Tezel G, Hernandez MR, Wax MB, 2000. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch Ophthalmol. 118, 511–518. [DOI] [PubMed] [Google Scholar]
- Tezel G, Hernandez MR, Wax MB, 2001a. In vitro evaluation of reactive astrocyte migration, a component of tissue remodeling in glaucomatous optic nerve head. Glia. 34, 178–189. [DOI] [PubMed] [Google Scholar]
- Tezel G, Li LY, Patil RV, Wax MB, 2001b. Tumor necrosis factor-alpha and its receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 42, 1787–1794. [PubMed] [Google Scholar]
- Tezel G, Luo C, Yang X, 2007a. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci. 48, 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Siegmund KD, Trinkaus K, Wax MB, Kass MA, Kolker AE, 2001c. Clinical factors associated with progression of glaucomatous optic disc damage in treated patients. Arch Ophthalmol. 119, 813–818. [DOI] [PubMed] [Google Scholar]
- Tezel G, Thornton IL, Tong MG, et al. , 2012a. Immunoproteomic analysis of potential serum biomarker candidates in human glaucoma. Invest Ophthalmol Vis Sci. 53, 8222–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB, 2004. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol. 122, 1348–1356. [DOI] [PubMed] [Google Scholar]
- Tezel G, Wax MB, 2000a. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 20, 8693–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB, 2000b. The mechanisms of hsp27 antibody-mediated apoptosis in retinal neuronal cells. J Neurosci. 20, 3552–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, 2004. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Ophthalmol Vis Sci. 45, 4049–4059. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Cai J, 2005. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 46, 3177–3187. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Cai J, Powell DW, 2012b. An astrocyte-specific proteomic approach to inflammatory responses in experimental rat glaucoma. Invest Ophthalmol Vis Sci. 53, 4220–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Kain AD, Powell DW, Kuehn MH, Kaplan HJ, 2010. Oxidative stress and the regulation of complement activation in human glaucoma. Invest Ophthalmol Vis Sci. 51, 5071–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Peng Y, Sun SL, Sun D, 2007b. Mechanisms of immune system activation in glaucoma: oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Invest Ophthalmol Vis Sci. 48, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Yang J, Wax MB, 2004. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 996, 202–212. [DOI] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY, 2014. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 121, 2081–2090. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Andersen MV, Christoffersen PR, Jensen MD, Lichota J, Moos T, 2015. Neurodegeneration with inflammation is accompanied by accumulation of iron and ferritin in microglia and neurons. Neurobiol Dis. 81, 108–118. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Hughes TR, Triantafilou M, Morgan BP, 2013. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 126, 2903–2913. [DOI] [PubMed] [Google Scholar]
- Tribble JR, Harder JM, Williams PA, John SWM, 2020. Ocular hypertension suppresses homeostatic gene expression in optic nerve head microglia of DBA/2 J mice. Mol Brain. 13, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribble JR, Hui F, Joe M, Bell K, Chrysostomou V, Crowston JG, Williams PA, 2021a. Targeting diet and exercise for neuroprotection and neurorecovery in glaucoma. Cells. 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribble JR, Kokkali E, Otmani A, et al. , 2021b. When is a control not a control? Reactive microglia occur throughout the control contralateral pathway of retinal ganglion cell projections in experimental glaucoma. Transl Vis Sci Technol. 10, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribble JR, Otmani A, Sun S, et al. , 2021c. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol. 43, 101988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummers B, Green DR, 2017. Caspase-8: regulating life and death. Immunol Rev. 277, 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler TA, Piltz-Seymour J, 2008. Optic disc hemorrhages in glaucoma and ocular hypertension: implications and recommendations. Curr Opin Ophthalmol. 19, 89–94. [DOI] [PubMed] [Google Scholar]
- Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, Gonzalez-Billault C, Nunez MT, 2013. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem. 126, 541–549. [DOI] [PubMed] [Google Scholar]
- Vainchtein ID, Chin G, Cho FS, et al. , 2018. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 359, 1269–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein ID, Molofsky AV, 2020. Astrocytes and microglia: in sickness and in health. Trends Neurosci. 43, 144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM, 1996. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 274, 787–789. [DOI] [PubMed] [Google Scholar]
- Van Bergen NJ, Crowston JG, Craig JE, et al. , 2015. Measurement of systemic mitochondrial function in advanced primary open-angle glaucoma and Leber Hereditary Optic Neuropathy. PLoS One. 10, e0140919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Horssen J, van Schaik P, Witte M, 2019. Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci Lett. 710, 132931. [DOI] [PubMed] [Google Scholar]
- van Loo G, De Lorenzi R, Schmidt H, et al. , 2006. Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol. 7, 954–961. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Melino G, 2012. The flick of a switch: which death program to choose? Cell Death Differ. 19, 1093–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats D, Mukundan L, Odegaard JI, et al. , 2006. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 4, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC, 2016. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 51, 1–40. [DOI] [PubMed] [Google Scholar]
- Verdin E, 2015. NAD(+) in aging, metabolism, and neurodegeneration. Science. 350, 1208–1213. [DOI] [PubMed] [Google Scholar]
- Vereecke L, Beyaert R, van Loo G, 2009. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 30, 383–391. [DOI] [PubMed] [Google Scholar]
- Verheggen ICM, Van Boxtel MPJ, Verhey FRJ, Jansen JFA, Backes WH, 2018. Interaction between blood-brain barrier and glymphatic system in solute clearance. Neurosci Biobehav Rev. 90, 26–33. [DOI] [PubMed] [Google Scholar]
- Voet S, Mc Guire C, Hagemeyer N, et al. , 2018. A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat Commun. 9, 2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahane S, Halawani D, Zhou X, Zou H, 2019. Epigenetic regulation of axon regeneration and glial activation in injury responses. Front Genet. 10, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DM, Pokrovskaya O, O'Brien CJ, 2015. The function of matricellular proteins in the lamina cribrosa and trabecular meshwork in glaucoma. J Ocul Pharmacol Ther. 31, 386–395. [DOI] [PubMed] [Google Scholar]
- Wang J, Valiente-Soriano FJ, Nadal-Nicolas FM, et al. , 2017. MicroRNA regulation in an animal model of acute ocular hypertension. Acta Ophthalmol. 95, e10–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cioffi GA, Cull G, Dong J, Fortune B, 2002. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Invest Ophthalmol Vis Sci. 43, 1088–1094. [PubMed] [Google Scholar]
- Wang Y, Chen S, Wang J, et al. , 2021. MicroRNA-93/STAT3 signalling pathway mediates retinal microglial activation and protects retinal ganglion cells in an acute ocular hypertension model. Cell Death Dis. 12, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareham LK, Calkins DJ, 2020. The neurovascular unit in glaucomatous neurodegeneration. Front Cell Dev Biol. 8, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax MB, 1998. Anti-Ro/SS-A positivity and heat shock protein antibodies in patients with normal-pressure glaucoma. J Glaucoma. 125, 145–157. [DOI] [PubMed] [Google Scholar]
- Wax MB, 2001. Serum autoantibodies to heat shock proteins in glaucoma patients from Japan and the United States. Invest Ophthalmol Vis Sci. 108, 296–302. [DOI] [PubMed] [Google Scholar]
- Wax MB, Tezel G, Yang J, Peng G, Patil RV, Agarwal N, Sappington RM, Calkins DJ, 2008. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J Neurosci. 28, 12085–12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Shadel GS, 2017. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol. 17, 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Shadel GS, Ghosh S, 2011. Mitochondria in innate immune responses. Nat Rev Immunol. 11, 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann S, Reinhard J, Reinehr S, Cibir Z, Joachim SC, Faissner A, 2020. Loss of the extracellular matrix molecule tenascin-C leads to absence of reactive gliosis and promotes anti-inflammatory cytokine expression in an autoimmune glaucoma mouse model. Front Immunol. 11, 566279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, 2015. Glaucoma genes and mechanisms. Prog Mol Biol Transl Sci. 134, 315–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Pasquale LR, 2017. Genetics of glaucoma. Hum Mol Genet. 26, R21–R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins HM, Carl SM, Weber SG, Ramanujan SA, Festoff BW, Linseman DA, Swerdlow RH, 2015. Mitochondrial lysates induce inflammation and Alzheimer's disease-relevant changes in microglial and neuronal cells. J Alzheimers Dis. 45, 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Braine CE, Kizhatil K, et al. , 2019. Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma. Mol Neurodegener. 14, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Harder JM, Foxworth NE, Cardozo BH, Cochran KE, John SWM, 2017a. Nicotinamide and WLD(S) act together to prevent neurodegeneration in glaucoma. Front Neurosci. 11, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, Smithies O, John SW, 2017b. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 355, 756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Marsh-Armstrong N, Howell GR, al. e., 2017c. Neuroinflammation in glaucoma: A new opportunity. Exp Eye Res. 157, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Tribble JR, Pepper KW, Cross SD, Morgan BP, Morgan JE, John SW, Howell GR, 2016. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol Neurodegener. 11, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Huang P, Li W, Li Y, Zhang SS, Zhang C, 2015. T-helper1/T-helper2 cytokine imbalance in the iris of patients with glaucoma. PLoS One. 10, e0122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostyn P, De Groot V, Van Dam D, Audenaert K, Killer HE, De Deyn PP, 2017. The glymphatic hypothesis of glaucoma: A unifying concept incorporating vascular, biomechanical, and biochemical sspects of the disease. Biomed Res Int. 2017, 5123148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Parry M, Hou XY, et al. , 2020. Gene therapy conversion of striatal astrocytes into GABAergic neurons in mouse models of Huntington's disease. Nat Commun. 11, 1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Gao L, Wu T, Sun F, 2013. Roles of tumor necrosis factor alpha gene polymorphisms, tumor necrosis factor alpha level in aqueous humor, and the risks of open angle glaucoma: a meta-analysis. Mol Vis. 19, 526–535. [PMC free article] [PubMed] [Google Scholar]
- Xu H, Manivannan A, Liversidge J, Sharp PF, Forrester JV, Crane IJ, 2003. Requirements for passage of T lymphocytes across non-inflamed retinal microvessels. J Neuroimmunol. 142, 47–57. [DOI] [PubMed] [Google Scholar]
- Xu Z, Fouda AY, Lemtalsi T, et al. , 2018. Retinal neuroprotection from optic nerve trauma by deletion of Arginase 2. Front Neurosci. 12, 970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, et al. , 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301, 640–643. [DOI] [PubMed] [Google Scholar]
- Yan X, Tezel G, Wax MB, Edward DP, 2000. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch Ophthalmol. 118, 666–673. [DOI] [PubMed] [Google Scholar]
- Yanez M, Jhanji M, Murphy K, Gower RM, Sajish M, Jabbarzadeh E, 2019. Nicotinamide augments the anti-Inflammatory properties of resveratrol through PARP1 activation. Sci Rep. 9, 10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lin Y, Guo Z, et al. , 2001a. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol. 2, 620–624. [DOI] [PubMed] [Google Scholar]
- Yang J, Patil RV, Yu H, Gordon M, Wax MB, 2001b. T cell subsets and sIL-2R/IL-2 levels in patients with glaucoma. Am J Ophthalmol. 131, 421–426. [DOI] [PubMed] [Google Scholar]
- Yang J, Wu Z, Renier N, et al. , 2015a. Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell. 160, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yang P, Tezel G, Patil RV, Hernandez MR, Wax MB, 2001c. Induction of HLA-DR expression in human lamina cribrosa astrocytes by cytokines and simulated ischemia. Invest Ophthalmol Vis Sci. 42, 365–371. [PubMed] [Google Scholar]
- Yang P, Das PK, Kijlstra A, 2000. Localization and characterization of immunocompetent cells in the human retina. Ocul Immunol Inflamm. 8, 149–157. [PubMed] [Google Scholar]
- Yang Q, Liu R, Yu Q, Bi Y, Liu G, 2019a. Metabolic regulation of inflammasomes in inflammation. Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hondur G, Li M, Cai J, Klein JB, Kuehn MH, Tezel G, 2015b. Proteomics analysis of molecular risk factors in the ocular hypertensive human retina. Invest Ophthalmol Vis Sci. 56, 5816–5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hondur G, Tezel G, 2016. Antioxidant treatment limits neuroinflammation in experimental glaucoma. Invest Ophthalmol Vis Sci. 57, 2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Luo C, Cai J, Powell DW, Yu D, Kuehn MH, Tezel G, 2011. Neurodegenerative and inflammatory pathway components linked to TNF-alpha/TNFR1 signaling in the glaucomatous human retina. Invest Ophthalmol Vis Sci. 52, 8442–8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zeng Q, Baris M, Tezel G, 2020. Transgenic inhibition of astroglial NF-kappaB restrains the neuroinflammatory and neurodegenerative outcomes of experimental mouse glaucoma. J Neuroinflammation. 17, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zeng Q, Goktas E, et al. , 2019b. T-lymphocyte subset distribution and activity in patients with glaucoma. Invest Ophthalmol Vis Sci. 60, 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zeng Q, Tezel G, 2021. Regulation of distinct caspase-8 functions in retinal ganglion cells and astroglia in experimental glaucoma. Neurobiol Dis. 150, 105258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Quigley HA, Pease ME, Yang Y, Qian J, Valenta D, Zack DJ, 2007. Changes in gene expression in experimental glaucoma and optic nerve transection: the equilibrium between protective and detrimental mechanisms. Invest Ophthalmol Vis Sci. 48, 5539–5548. [DOI] [PubMed] [Google Scholar]
- Yoneshige A, Hagiyama M, Takashima Y, Ueno S, Inoue T, Kimura R, Koriyama Y, Ito A, 2021. Elevated hydrostatic pressure causes retinal degeneration through upregulating lipocalin-2. Front Cell Dev Biol. 9, 664327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Joseph C, Wang C, et al. , 2019. Demyelination precedes axonal loss in the transneuronal spread of human neurodegenerative disease. Brain. 142, 426–442. [DOI] [PubMed] [Google Scholar]
- Yu J, Nagasu H, Murakami T, Hoang H, Broderick L, Hoffman HM, Horng T, 2014. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc Natl Acad Sci U S A. 111, 15514–15519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Neufeld AH, 2001. Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res. 64, 523–532. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J, 2009. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 325, 332–336. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang N, Chrenek MA, et al. , 2021. Systemic treatment with nicotinamide riboside is protective in two mouse models of retinal ganglion cell damage. Pharmaceutics. 13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Xu X, Jiang Y, et al. , 2019. Lipocalin-2 may produce damaging effect after cerebral ischemia by inducing astrocytes classical activation. J Neuroinflammation. 16, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Liang S, Sanchez-Lopez E, et al. , 2018. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 560, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J, 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature. 469, 221–225. [DOI] [PubMed] [Google Scholar]
- Zhu G, Wu CJ, Zhao Y, Ashwell JD, 2007. Optineurin negatively regulates TNFalpha-induced NF-kappaB activation by competing with NEMO for ubiquitinated RIP. Curr Biol. 17, 1438–1443. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wang L, Ruan Y, et al. , 2011. An efficient delivery of DAMPs on the cell surface by the unconventional secretion pathway. Biochem Biophys Res Commun. 404, 790–795. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Sasaki Y, Milbrandt J, Gidday JM, 2013. Protection of mouse retinal ganglion cell axons and soma from glaucomatous and ischemic injury by cytoplasmic overexpression of Nmnat1. Invest Ophthalmol Vis Sci. 54, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani-Alvarez L, Marzeda AM, Deligne C, Schwenzer A, McCann FE, Marsden BD, Piccinini AM, Midwood KS, 2017. Mapping tenascin-C interaction with toll-like receptor 4 reveals a new subset of endogenous inflammatory triggers. Nat Commun. 8, 1595. [DOI] [PMC free article] [PubMed] [Google Scholar]