Abstract
Background
Recently, several randomized controlled trials (RCTs) investigated immunotherapy-based regimens versus chemotherapy alone in patients with advanced esophageal squamous cell carcinoma (ESCC). Here we conducted a systematic review and meta-analysis on the efficacy and activity of programmed cell death protein 1 blockade in these patients, with focus on the value of programmed death-ligand 1 combined positive score (CPS) for selecting patients who may benefit the most.
Methods
RCTs investigating treatment with or without immune checkpoint inhibitors for advanced ESCC were selected. The hazard ratio (HR) and the odds ratio were used to compare the treatment effect on survival outcomes and tumor response, respectively, for immunotherapy-based regimens compared with standard chemotherapy, overall and according to geographic region or treatment line. We carried out a subgroup analysis comparing patients with CPS ≥10 or <10 and the evidence for treatment effect was evaluated by interaction test.
Results
A total of 5257 patients and 10 RCTs were included. Overall, the HR for overall survival benefit with immunotherapy-based regimens was 0.71 [95% confidence interval (CI) 0.66-0.76] compared with chemotherapy alone; such effect was independent from geographical region (Asia versus rest of the world) and treatment line (upfront versus second/further lines). The HR for progression-free survival benefit and the odds ratio for overall response rate increase were 0.78 (95% CI 0.66-0.93) and 1.50 (95% CI 1.22-1.83), respectively. The HR for overall survival benefit with immunotherapy-based treatment was 0.60 (95% CI 0.51-0.70) for CPS ≥10 subgroup versus 0.83 (95% CI 0.69-1.00) for CPS <10 (P for interaction 0.009).
Conclusions
Immune checkpoint inhibitors have a consistent benefit in reducing the risk of death for ESCC patients which is dependent on programmed death-ligand 1 CPS status. Further investigations of biomarkers for immunotherapy in the subgroup of patients with CPS <10 are needed.
Key words: esophageal squamous cell carcinoma, immune checkpoint inhibitors, meta-analysis, combined positive score
Highlights
-
•
Programmed cell death protein 1 (PD-1) blockade is confirmed as effective and active in patients with advanced esophageal squamous cell carcinoma.
-
•
The efficacy is independent from geographic region and treatment line.
-
•
The efficacy is dependent on programmed death-ligand 1 (PD-L1) CPS status using the 10 cut-off.
-
•
Additional biomarkers of response to immunotherapy or novel combination strategies are needed in the CPS <10 subgroup.
Introduction
Fluoropyrimidine plus platinum-based chemotherapy has been the mainstay of first-line treatment of patients with advanced esophageal squamous cell cancer (ESCC) for about four decades.1 During this timeframe, there has not been significant improvement in patients' outcomes, that have historically been poor, with a median overall survival (OS) of around 5 months.2 Second-line chemotherapy was supported only by non-randomized trials, overall showing limited activity of taxanes or irinotecan and failure of targeted agents such as gefinitib.3,4 Immune checkpoint inhibitors (ICIs) have recently produced seismic changes in the treatment landscape of patients with ESCC. Several randomized controlled trials (RCTs) have consistently demonstrated a significant survival advantage with the use of an anti-programmed cell death protein 1 (PD-1) agent as compared with second-line chemotherapy in patients with pretreated disease, or with the upfront use of chemo-immunotherapy when compared with chemotherapy alone in patients with newly diagnosed advanced disease.5, 6, 7, 8, 9, 10, 11, 12, 13 More recently, dual cytotoxic T lymphocyte-associated antigen-4 (CTLA-4)/PD-1 blockade with ipilimumab/nivolumab combination emerged as a potentially effective upfront option, mainly for chemotherapy-ineligible patients.14
These recent advances in the field of immuno-oncology for patients with ESCC were made in industry sponsored trials and several open issues need to be addressed to optimize the therapeutic index of ICIs in ESCC. Firstly, since a relevant proportion of patients do not benefit from ICIs, clinical validation of predictive biomarkers may lead to optimize treatment personalization and investigation of new treatment combinations, especially in immune-resistant populations. The most validated biomarker for sensitivity to ICIs in ESCC is programmed death-ligand 1 (PD-L1) expression using combined positive score (CPS). Several RCTs investigated the efficacy of ICIs in the pre-specified subgroup of patients with PD-L1 CPS ≥10, since this subgroup may be enriched with subjects with long-term benefit from treatment. CPS was not a stratification factor in any of the RCTs used to license ICIs in ESCC, however, and no overarching dataset has been analyzed to confirm the predictive role of CPS to inform patients' selection. Secondly, the magnitude of benefit from ICIs may be different in Western versus Eastern patients due to geographic variations in disease etiology and tumor immunity signatures, as has already been shown for gastric cancer.15 With the aim of deriving useful information for clinical practice, we conducted this systematic review and meta-analysis on the efficacy and activity of PD-1 blockade in patients with advanced ESCC.
Methods
Inclusion criteria and search strategy
The objective of this analysis was to assess the outcome associated with the use of anti-PD-(L1) agents with or without standard chemotherapy versus standard chemotherapy alone in patients with advanced ESCC enrolled in randomized trials as first- or subsequent-line therapies.
We reviewed PubMed, Cochrane Library, and EMBASE, for citations from January 2011 to 1 October 2021. The search criteria were limited to articles published in English language and phase III or phase II RCTs that compared treatments with or without ICIs in advanced (recurrent or metastatic) ESCC. The MeSH terms used for the search in PubMed and Cochrane Library were (esophageal or esophagus or oesophageal) and (cancer or carcinoma) and (squamous or epidermoid) and (PD-L1 or PD-1 or CTLA-4 or ‘immune checkpoint inhibitors’). If more than one publication was found for the same trial, the most recent, complete and updated was included in the final analysis. Phase I trials, adenocarcinoma histology, and comparisons including any other experimental drug (except ICIs) were excluded.
Data extraction
Data extraction was conducted independently by two co-authors (AL and FP) according to the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) statement D16; any discrepancy was resolved by consensus with a senior author (FP). The data obtained for each trial were: first author's name, year of publication, trial phase, number of patients assessable, number of arms, drugs used in the experimental and in the control arm, median follow-up, median progression-free survival (PFS) and median OS with the relative hazard ratios (HRs) and 95% confidence intervals (CIs), number of patients who had partial or complete response. Publication bias was calculated according to Cochrane risk of bias tool. Each trial was scored as high, low, or unclear risk of bias on the basis of the following aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome and assessment, incomplete outcome data, selective reporting, and other bias. In our meta-analysis, blinding of participants and personnel was not practical for these trials, and we considered that our main outcomes (OS) were not likely to be influenced by the absence of blinding. Thus, if one trial did not follow the blinding method, we did not judge it as low quality. Trials in which all domains except blinding of patients and clinicians at low risk of bias were considered to have an overall low risk of bias.
Statistical method
HRs for OS and PFS with the relative 95% CIs were extracted from each study. The proportion of patients with overall response rate (ORR) and the derived 95% CIs were calculated for each study. We also estimated the RR and the 95% CIs of events in patients assigned to the experimental treatment, as compared with the control arm in the same study. Summary HRs and RRs were calculated with random- or fixed-effect models depending on the heterogeneity of included studies. When substantial heterogeneity was not observed, the pooled estimate calculated based on the fixed-effects model was reported using the inverse variance method. Statistical heterogeneity between trials included in the meta-analysis was assessed using the chi-square test, and inconsistency was quantified with the I2 statistic [100% × (Q−df/Q)].17 The assumption of homogeneity was considered invalid for P values <0.05. When substantial heterogeneity was observed, the pooled estimate calculated based on the random-effects model was reported using the method used by DerSimonian and Laird,18 which considers both within- and between-study variations. Results according to the fixed- and the random-effect models, as well as observed heterogeneity, are reported in Table 1. Sensitivity analysis was conducted by omitting one study by turn to test the robustness of the primary outcomes. Publication bias was assessed by using the funnel plot with the bias indicator test from Egger et al.
Table 1.
List of included studies with their individual features
| Author/year | Trial/type of study | Ethnicity | No. pts | CPS ≥10/PD-L1 ≥1 | Line of therapy | Histology | Median follow-up (months) | Arms | ORR (%) | PFS (HR, 95%CI) | OS (HR, 95%CI) | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sun/2021 | KEYNOTE590/randomized | Asian and non | 373 versus 376 | 143 versus 143b/NA | 1st | ESCC & ADK | PEMBRO + CT versus CT alone | — | 0.65 (0.54-0.78)b | 0.72 (0.60-0.88)b | Low | |
| Kojima/2020 | KEYNOTE181/randomized | Asian and non | 314 versus 314 | 85 versus 82b/NA | 2nd | ESCC & ADK | PEMBRO versus CT (investigator's choice) | 16.7 versus 7.4b | 0.92 (0.75-1.13)b | 0.75 (0.61-0.93)b | Low | |
| Kato/2019 | ATTRACTION3/randomized | Asian and non | 210 versus 209 | NA/101 versus 102e | 2nd | ESCC | NIVO versus CT (investigator's choice) | 19 versus 22 | 1.08 (0.87-1.34) | 0.77 (0.62-0.96) | Low | |
| Chau/2021 | CHECKMATE648a/randomized | Asian and non | 321 versus 325 versus 324 | NA/158 versus 158 versus 157e | 1st | ESCC | NIVO + CT versus NIVO + IPI versus CT alone | 47 versus 27c 28 versus 27d |
0.81 (0.64-1.04)c 1.26 (1.04-1.52)d |
0.74 (0.58-0.96)c 0.78 (0.62-0.98)d |
Moderate | |
| Shen/2021 | ORIENT15a/randomized | Asian | 327 versus 332 | 188 versus 193/NA | 1st | ESCC | Sintilimab + CT versus CT alone | 66.1 versus 45.5 | 0.55 (0.46-0.67) | 0.62 (0.50-0.77) | Moderate | |
| Xu/2021 | JUPITER06a/randomized | Asian | 257 versus 257 | 115 versus 97/201 versus 200f | 1st | ESCC | Toripalimab + CT versus CT alone | — | 0.58 (0.46-0.74) | 0.58 (0.43-0.78) | Moderate | |
| Huang/2020 | ESCORT/randomized | Asian | 228 versus 220 | NA/26 versus 35e | 2nd | ESCC | Camrelizumab versus CT | 20.2 versus 6.4 | 0.69 (0.56-0.86) | 0.71 (0.57-0.88) | Low | |
| Shen/2021 | RATIONALE302a/randomized | Asian and non | 256 versus 256 | 89 versus 68/NA | 2nd | ESCC | Tislelizumab versus CT | 20.3 versus 9.8 | 0.83 (0.67-1.01) | 0.70 (0.57-0.85) | Moderate | |
| Luo/2021 | ESCORT-1st/randomized | Asian | 298 versus 298 | NA/104 versus 98e | 1st | ESCC | Camrelizumab + CT versus CT alone | 72.1 versus 62.1 | 0.56 (0.46-0.68) | 0.70 (0.56-0.88) | Low | |
| Xu/2020 | ORIENT2a/randomized | Asian | 95 versus 95 | — | 2nd | ESCC | Sintilimab versus CT | 12.6 versus 6.3 | 1.00 (0.77-1.39) | 0.70 (0.50-0.97) | Moderate |
ADK, adenocarcinoma; CI, confidence interval; CPS, combined positive score; CT, chemotherapy; ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; IPI, ipilimumab; NA, not applicable; NIVO, nivolumab; ORR, overall response rate; OS, overall survival; PD-L1, programmed death-ligand 1; PEMBRO, pembrolizumab; PFS, progression-free survival.
Abstract.
ESCC group.
NIVO + CT versus CT.
NIVO + IPI versus CT.
Tumor proportion score.
CPS.
A two-tailed P value <0.05 was considered statistically significant. All data were collected using Microsoft Office Excel 2007; statistical analyses were carried out using RevMan software for meta-analysis (v. 5.4).19
Results
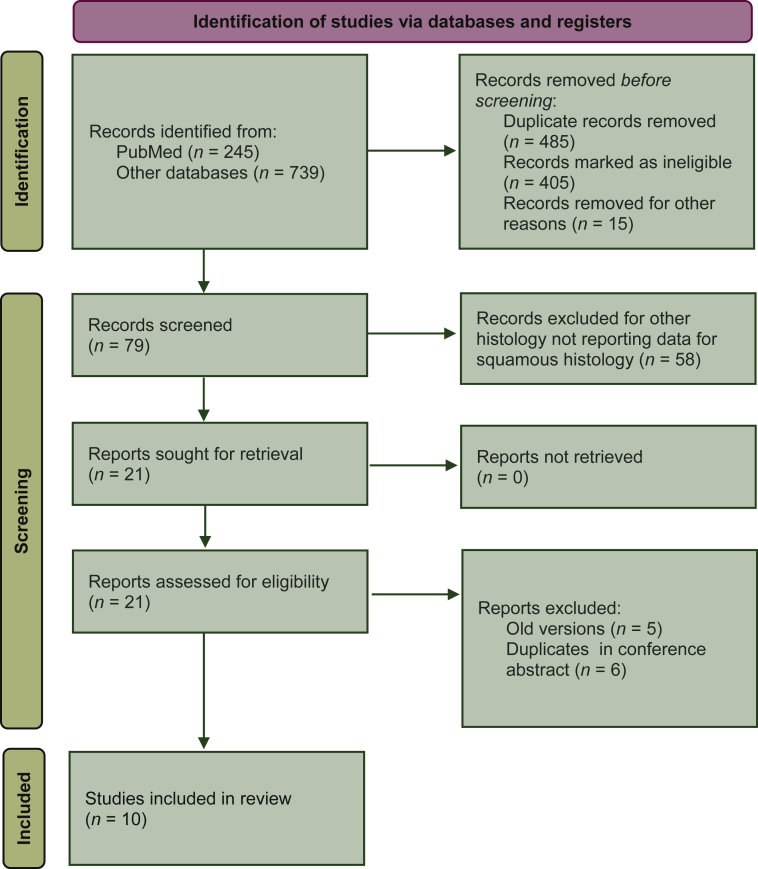
The electronic search revealed a total of 984 citations including conference abstracts. After screening, 905 records were immediately eliminated because they did not match the initial requirements. Afterwards, 79 full-text articles were assessed and 58 were eliminated for the reasons reported in Figure 1. At the end of the review process, 10 studies among 21 potentially eligible were included in the qualitative and quantitative synthesis (Figure 1). Studies' characteristics are shown in Table 1.
Figure 1.
Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) flow diagram of included studies.
As shown in Table 1, a total of nine phase III studies and one randomized phase II study were included, although CheckMate 648 had two evaluable experimental treatment arms. Five studies were conducted in first line and five in second or further line. Eight studies were focused only on ESCC, whereas two studies enrolled patients affected by ESCC or esophageal adenocarcinoma (EAC) with data reported in both subgroups. Four of the 10 trials reported data in subgroups of patients with PD-L1 CPS ≥10 versus <10. Three studies reported OS data according to PD-L1 tumor proportion score (TPS) ≥1% versus <1%. Five studies enrolled only Asian patients and five studies patients from Asia and rest of the world (ROW). Among the latter, only three studies reported data on OS by geographical region. After excluding the patients affected by EAC, the total number of patients randomly assigned in these trials was 5257, with 2789 receiving an immunotherapy-based treatment and 2468 receiving chemotherapy alone.
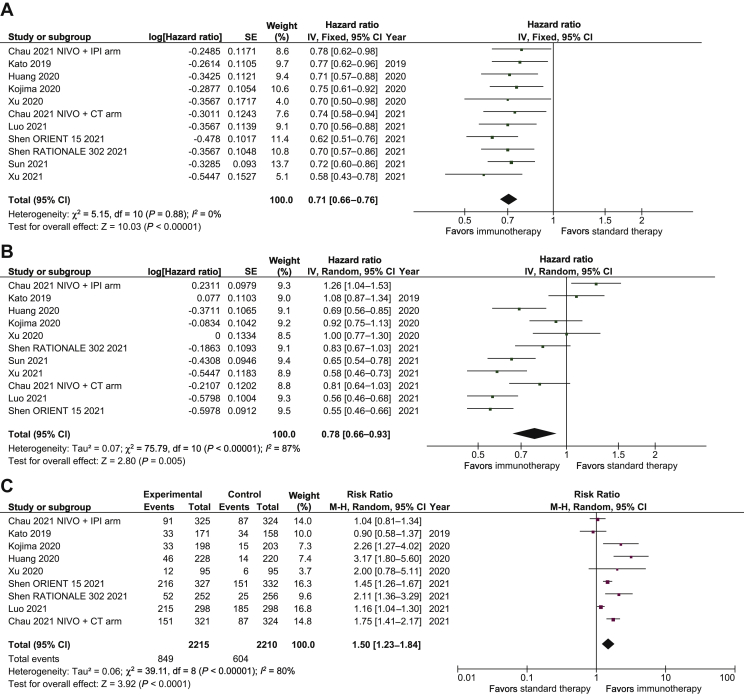
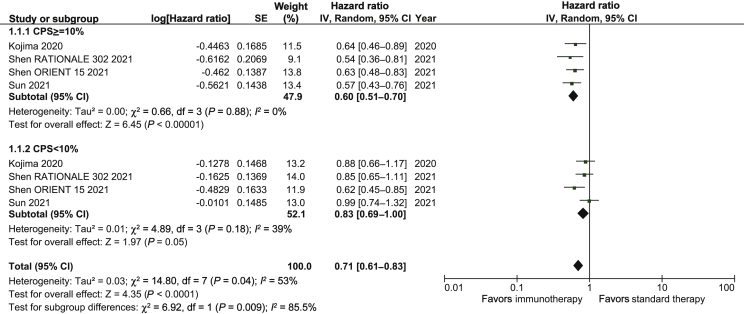
Overall, the HR for OS benefit with immunotherapy-based regimens compared with chemotherapy alone was 0.71 (95% CI 0.66-0.76; P < 0.01; Figure 2A). Results were similar in first-line trials (HR, 0.70; 95% CI 0.64-0.77; P < 0.01) and second or further line trials (HR, 0.71; 95% CI 0.65-0.79; P < 0.01; Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100380). Notably, we demonstrate that treatment effect was significantly different in the two subgroups with CPS ≥10 and CPS <10 (P for interaction = 0.009; Figure 3). The HR for OS benefit in patients with PD-L1 CPS ≥10 was 0.60 (95% CI 0.51-0.70; P < 0.01) compared with 0.83 (95% CI 0.69-1.00; P = 0.05) in patients with CPS <10. The effect of immunotherapy in patients with PD-L1 TPS ≥1% differed significantly from that observed in the PD-L1 TPS <1% subgroup (P for interaction = 0.01; Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100380). The HR for OS benefit in the PD-L1 TPS ≥1% subgroup was 0.61 (95% CI 0.53-0.71; P < 0.01) compared with 0.82 (95% CI 0.69-0.97; P = 0.02) in the PD-L1 TPS <1% subgroup. The treatment effect was not significantly different between Asian countries versus ROW patients (P for interaction, 0.21; Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2021.100380). Immunotherapy improved OS both in the Asian subgroup (HR, 0.69; 95% CI 0.63-0.75; P < 0.01) and the ROW subgroup (HR, 0.79; 95% CI 0.64-0.98; P = 0.03).
Figure 2.
Forest plot of the efficacy of immunotherapy-based regimens compared with chemotherapy in ESCC.
(A) Overall survival. (B) Overall PFS. (C) ORR.
CI, confidence interval; CT, chemotherapy; df, degrees of freedom; ESCC, esophageal squamous cell carcinoma; IPI, ipilimumab; IV, inverse variance; M-H, Mantel-Haenszel; NIVO, nivolumab; OS, overall survival; ORR, overall response rate; PFS, progression-free survival; SE, standard error.
Figure 3.
Subgroup analysis of OS in patients with CPS ≥10 or <10.
CI, confidence interval; CPS, combined positive score; SE standard error.
With respect to PFS, overall, immunotherapy-based regimens were superior to chemotherapy alone (HR, 0.78; 95% CI 0.66-0.93; P < 0.01; Figure 2B). The HRs for PFS were 0.7 (95% CI 0.53-0.91) in first-line trials and 0.88 (95% CI 0.76-1.03) in second or further line trials. With respect to ORR, only eight trials reported overall data: the odds ratio (OR) in patients receiving immunotherapy-based regimens versus chemotherapy alone was 1.50 (95% CI 1.23-1.84; P < 0.01; Figure 2C). The ORs for response were 1.32 (95% CI 1.08-1.62) and 1.88 (95% CI 1.16-3.05) in first- and second/further line trials, respectively. All trials except KEYNOTE 590 to date have not reported data on PFS and ORR endpoints in the CPS <10 subgroup and in the Asia and ROW subgroups, thus impeding testing for interaction with regard to these endpoints.
Publication bias
As shown in Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2021.100380, there was no evidence of publication bias with Begg's test (P = 0.43) or with Egger's test (P = 0.17). After the one-study-removed procedure, HR for OS ranged from 0.70 to 0.72.
Discussion
In ESCC, there have been recent changes to the standard of care for advanced cancers in both the first- and second-line settings based on multiple RCTs. These changes have led to prolonged survival for ESCC patients, but questions remain which are challenging to answer in the context of single trial datasets. With the aim of assessing the precise benefit of immunotherapy-based regimens in patients with advanced ESCC, we conducted this meta-analysis of published trials and have demonstrated an overall 29% reduction of the risk of death and a 50% increase of the chance of tumor response in the experimental immunotherapy arms. The OS benefit of immunotherapy-based regimens was independent from treatment line and from geographic region. We conclude that anti-PD-1 inhibitors have a class effect in ESCC and that the drivers of ESCC (predominantly alcohol and tobacco use) lead to a broadly uniform disease biology which is sensitive to immune checkpoint inhibition. This is in contrast to gastric and gastroesophageal adenocarcinoma where diverse regional factors associated with cancer causation and other patient-based factors like disease burden and body habitus may impact on immunotherapy outcomes.20
Despite a clear reduction in the risk of death for immune sensitive ESCC patients treated with ICIs, a significant subgroup of patients with advanced ESCC do not derive any benefit from immunotherapy. Therefore, biomarkers such as PD-L1 expression may have a potentially important predictive role. In studies of gastroesophageal adenocarcinoma, raising the CPS cut-off from 1 to 5 or 10 maximized the therapeutic index of immunotherapy.20,21 This concept of biomarker-based enrichment was prospectively validated by the CheckMate 649 trial, which met its primary endpoint of OS prolongation in the primary population of adenocarcinoma patients with CPS ≥5.22 We suggest that future trials of immunotherapy for ESCC patients are enriched in the same way to refine the molecular selection of the optimal candidates for immunotherapy.
Reflecting on the licensing status of ICIs in ESCC, for patients with pretreated ESCC, nivolumab was approved by both the European Medicines Agency (EMA) and Food and Drug Administration (FDA) for molecularly unselected patients, whereas pembrolizumab was approved only for ESCC patients with CPS ≥10 (but not for EAC) based on different statistical design and enrolled population of registration studies. Notably, PD-L1 expression according to TPS did show a trend towards affecting the magnitude of benefit from nivolumab in ATTRACTION-3, but no analysis according to PD-L1 CPS is available for nivolumab in that trial, so this approach is reasonable. In the first line, regional differences in licensing approach have become apparent. Pembrolizumab was recently approved by the FDA in the USA for all treatment-naive advanced ESCC patients regardless of CPS, posing a challenge for clinicians regarding potential discrepancies between regulatory approvals and evidence from RCTs. In contrast, EMA has approved pembrolizumab for ESCC only with a PD-L1 CPS ≥10, which is supported by our results.
One limitation of our study is that PD-L1 scoring in ESCC has only recently been adopted, and there is a lack of standardization of the platform and antibody used for evaluation. In contrast to other tumors where PD-L1 is routinely assessed on tumor cells, assessment on tumor and immune cells using CPS is more complex and guidelines for cross platform and antibody validation and pathology quality assurance are lacking.23 Therefore, the absolute cut-off of CPS <10 could be dependent on the method of assessment, the type of specimen analyzed (e.g. surgical sample versus endoscopic biopsy), and subject to interpatient variation. Additionally, PD-L1 expression may be heterogeneous, both spatially and longitudinally, meaning that some patients classified as CPS <10 could clearly derive benefit from ICIs. Finally, these data derive from subgroup analyses of a limited number of trials with available CPS results, and the borderline statistically significant results in the CPS <10 population may be related to a lack of statistical power which could be improved by the incorporation of future studies. Finally, our study showed a statistically significant interaction between treatment and PD-L1 expression assessed by TPS using the 1% cut-off, although limited by the small sample size and number of included studies. Therefore, future studies should focus on a different PD-L1 scoring system to properly evaluate the concordance of their predictive value.
In conclusion, in this meta-analysis of published trials evaluating ICIs in ESCC, we demonstrate that ICIs have a consistent benefit in reducing the risk of death for ESCC patients which is independent of country of origin, but dependent on PD-L1 CPS status. Our results highlight the urgent need to investigate additional biomarkers of response to immunotherapy in the subgroup of patients with CPS <10. Having identified the group of patients who benefit from current ICI-based therapies, however, the next steps for the immune-sensitive subgroup will be to develop upon the solid base provided by the first generation of ICI therapies.
Acknowledgments
Funding
Italian Association for Cancer Research (AIRC) [grant number IG 23624] to FP.
Disclosure
FP reports personal fees from Merck Sharp & Dohme, AstraZeneca, Organon, Amgen, Sanofi, Bayer, Servier, Merck-Serono, Lilly; research grants from Bristol Myers Squibb and AstraZeneca. ES reports personal fees from AstraZeneca, Astellas, Bristol Myers Squibb, Celgene, Five Prime, Gritstone, Merck, Roche, Servier, and Zymworks. The other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Kies M.S., Rosen S.T., Tsang T.K., et al. Cisplatin and 5-fluorouracil in the primary management of squamous esophageal cancer. Cancer. 1987;60(9):2156–2160. doi: 10.1002/1097-0142(19871101)60:9<2156::aid-cncr2820600906>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Njei B., McCarty T.R., Birk J.W. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatol. 2016;31(6):1141–1146. doi: 10.1111/jgh.13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thallinger C.M., Raderer M., Hejna M. Esophageal cancer: a critical evaluation of systemic second-line therapy. J Clin Oncol. 2011;29(35):4709–4714. doi: 10.1200/JCO.2011.36.7599. [DOI] [PubMed] [Google Scholar]
- 4.Petty R.D., Dahle-Smith A., Stevenson D.A.J., et al. Gefitinib and EGFR gene copy number aberrations in esophageal cancer. J Clin Oncol. 2017;35(20):2279–2287. doi: 10.1200/JCO.2016.70.3934. [DOI] [PubMed] [Google Scholar]
- 5.Sun J.M., Shen L., Shah M.A., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 6.Kojima T., Shah M.A., Muro K., et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 7.Kato K., Cho B.C., Takahashi M., et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 8.Shen L., Lu Z., Wang J., et al. Sintilimab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced or metastatic esophageal squamous cell cancer: first results of the phase III ORIENT-15 study. Ann Oncol. 2021;32(suppl_5):S1283–S1346. [Google Scholar]
- 9.Xu R.H., Wang F., Cui C., et al. JUPITER-06: A randomized, double-blind, phase III study of toripalimab versus placebo in combination with first-line chemotherapy for treatment naive advanced or metastatic esophageal squamous cell carcinoma (ESCC) Ann Oncol. 2021;32(suppl 5):S1040–S1075. [Google Scholar]
- 10.Huang J., Xu J., Chen Y., et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 11.Shen L., Kato K., Kim S.-B., et al. RATIONALE 302: Randomized, phase 3 study of tislelizumab versus chemotherapy as second-line treatment for advanced unresectable/metastatic esophageal squamous cell carcinoma. J Clin Oncol. 2021;39(15 suppl):4012. doi: 10.1200/JCO.21.01926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo H., Lu J., Bai Y., et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st Randomized Clinical Trial. J Am Med Assoc. 2021;326(10):916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J., Li Y., Fan Q., et al. Sintilimab in patients with advanced esophageal squamous cell carcinoma refractory to previous chemotherapy: a randomized, open-label phase II trial (ORIENT-2) J Clin Oncol. 2020;38(suppl 15):4511. [Google Scholar]
- 14.Chau I, Doki Y, Ajani JA, et al. Nivolumab plus ipilimumab or nivolumab plus chemotherapy versus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma: First results of the CheckMate 648 study. 2021 ASCO Annual Meeting. Abstract 4001. Presented June 5, 2021.
- 15.Lin S.J., Gagnon-Bartsch J.A., Tan I.B., et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut. 2015;64(11):1721–1731. doi: 10.1136/gutjnl-2014-308252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. 2009;339:b2535. [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Review Manager (RevMan) The Nordic Cochrane Centre; Copenhagen: 2012. The Cochrane Collaboration [Computer program]. Version 5.4. 2012.http://ims.cochrane.org/revman/download Available at. [Google Scholar]
- 20.Shitara K., Van Cutsem E., Bang Y., et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–1580. doi: 10.1001/jamaoncol.2020.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei M., Siemers N.O., Pandya D., et al. Analyses of PD-L1 and inflammatory gene expression association with efficacy of nivolumab ± ipilimumab in gastric cancer/gastroesophageal junction cancer. Clin Cancer Res. 2021;27(14):3926–3935. doi: 10.1158/1078-0432.CCR-20-2790. [DOI] [PubMed] [Google Scholar]
- 22.Janjigian Y.Y., Shitara K., Moehler M., et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn S., Kim K.M. PD-L1 expression in gastric cancer: interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod Pathol. 2021;34:1719–1727. doi: 10.1038/s41379-021-00823-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.