Abstract
Objective
Psoriasis is a chronic inflammatory disease with autoimmune aetiology. A possible link between psoriasis and autoimmune thyroid disease (AITD) has been suggested in some studies with inconsistent findings. This meta-analysis aims to determine the association between psoriasis and AITD.
Design
A meta-analysis of observational studies.
Data sources
PubMed, EMBASE, Scopus and the Cochrane Library were searched up to 1 November 2021.
Eligibility criteria for selecting studies
We included non-randomised studies, each with over 50 cases in every group, focusing on the rate of comorbidity between psoriasis and AITD.
Data extraction and synthesis
Two independent reviewers screened the articles and extracted data. The restricted maximum-likelihood was applied to perform the meta-analysis. OR and 95% CIs were pooled to compare the prevalence of AITD in psoriasis and control groups. Heterogeneity was assessed with I2 statistic. The Newcastle-Ottawa Scale and Agency for Healthcare Research and Quality were applied for quality assessment. The risk of bias was assessed with Risk Of Bias In Non-randomised Studies-of Interventions (ROBINS-I).
Results
Eleven available studies with data on 253 313 patients with psoriasis and 1 376 533 controls were included. Meta-analysis showed that patients with psoriasis had a higher prevalence of AITD (OR 1.76, 95% CI 1.35 to 2.28, Z=4.25, p<0.01), especially loss-of-function disorder of the thyroid gland. Both thyroglobulin antibodies positive rate (OR 1.98, 95% CI 1.27 to 3.10, Z=3.00, p<0.01) and thyroid peroxidase antibodies positive rate (OR 2.15, 95% CI 1.31 to 3.52, Z=3.05, p<0.01) were also increased in the psoriasis group compared with the control group.
Conclusions
Our study indicates that the rate of co-occurring AITD was significantly increased in patients with psoriasis. It suggests that the increased risk of AITD should be concerned in patients with psoriasis.
PROSPERO registration number
CRD42020206005.
Keywords: thyroid disease, immunology, psoriasis
Strengths and limitations of this study.
This is the first meta-analysis focusing on the risk of autoimmune thyroid disease (AITD) for psoriasis patients, which included hypothyroidism, hyperthyroidism, subclinical hypothyroidism, subclinical hyperthyroidism, Hashimoto’s thyroiditis, Graves’ disease, thyroglobulin antibodies positivity and thyroid peroxidase antibodies positivity.
All studies included were of moderate to high quality and were representative. All studies were published in recent years.
The heterogeneity in the pooled data cannot be ignored, and was not improved by subgroup analysis. However, meta-regression analysis identified the differences of sample size and scope of research on AITD among these studies as the potential sources of heterogeneity.
Introduction
Psoriasis is a chronic inflammatory disease with autoimmune aetiology, affecting approximately 125 million people around the world.1 The skin lesions of psoriasis occur mainly on the scalp, trunk and exterior surfaces of the limbs, and manifest as erythema, plaques and scales.2 Apart from the impaired appearance and intense pruritus of the skin lesions, various comorbidities have a significant impact on the quality of life in patients with psoriasis.3 4 Among the comorbidities, autoimmune thyroid disease (AITD) has been characterised in patients with psoriasis. For patients with psoriasis who also develop complications, their management requires extra attention.5 Therefore, understanding the risk of other diseases on psoriasis has important clinical significance.
AITD is an inflammatory disease of the thyroid gland with the presence of thyroid autoantibodies, lymphocytic infiltration of thyroid parenchyma and even thyroid dysfunction.6 Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) are the two main clinical subtypes of AITD. GD is characterised by hyperthyroidism and the presence of thyroid-stimulating hormone receptor antibodies (TRAb) in serum, while HT is characterised by hypothyroidism and the presence of thyroid peroxidase antibodies (TPOAb) or thyroglobulin antibodies (TgAb) in serum.7
Psoriasis and AITD share some common pathophysiological features, such as Th1-predominant adaptive immune reaction.6 Hence, the relationship between AITD and psoriasis has been hypothesised and studied. Antonelli et al first reported that the prevalence of AITD in patients with psoriatic arthritis was significantly higher than in the general population.8 However, the study reported by Tsai et al9 pointed that the association between psoriasis and AITD was limited. In recent years, several observational studies regarding the association between psoriasis and AITD were published in succession, but the results of the studies were inconsistent.10–16 In addition, Karadag et al reported that the commonly administered acitretin treatment for psoriasis system treatment affects the levels of free T4 (thyroid hormone).17 To address this discrepancy, we designed and performed a meta-analysis with the existing evidence to assess the relationship between psoriasis and AITD and provide guidance on the clinical management of psoriasis.
Methods
Search strategy
The literature search was conducted through PubMed, EMBASE, Scopus and the Cochrane Library for relevant studies published before 1 November 2021. Detailed literature-search strategies of the databases are presented in table 1.
Table 1.
Database source and retrieval strategy
| Database | Retrieval strategy |
| PubMed | (((((((((((Thyroid(Title/Abstract)) OR (Thyroiditis(Title/Abstract))) OR (Hashimoto Thyroiditis(Title/Abstract))) OR (Graves Disease(Title/Abstract))) OR (Hyperthyroidism(Title/Abstract))) OR (Hypothyroidism(Title/Abstract))) OR (TgAb(Title/Abstract))) OR (TPOAb(Title/Abstract))) OR (TRAb(Title/Abstract))) OR (Endocrine Comorbidities(Title/Abstract))) OR (Autoimmune diseases(Title/Abstract))) AND ((psoriasis(Title/Abstract)) OR (psoriatic(Title/Abstract))) |
| Embase | (thyroid:ab, ti OR thyroiditis:ab, ti OR 'hashimoto thyroiditis':ab, ti OR 'graves disease':ab, ti OR hyperthyroidism:ab, ti OR hypothyroidism:ab, ti OR tgab:ab, ti OR tpoab:ab, ti OR trab:ab, ti OR 'endocrine comorbidities':ab, ti OR 'autoimmune diseases':ab, ti) AND (psoriasis:ab, ti OR psoriatic:ab, ti) |
| Scopus | ((TITLE-ABS-KEY (thyroiditis) OR TITLE-ABS-KEY (hashimoto AND thyroiditis) OR TITLE-ABS-KEY (graves AND disease) OR TITLE-ABS-KEY (hyperthyroidism) OR TITLE-ABS-KEY (hypothyroidism) OR TITLE-ABS-KEY (tgab) OR TITLE-ABS-KEY (tpoab) OR TITLE-ABS-KEY (trab))) AND (TITLE-ABS-KEY (psoriasis) OR TITLE-ABS-KEY (psoriatic)) |
| Cochrane | (((Psoriasis) OR psoriatic) OR Pustulosis of Palms) AND ((((((((Thyroid) OR Thyroiditis) OR Thyroiditides) OR Hashimoto Disease) OR Graves Disease) OR Hyperthyroidism) OR Hypothyroidism)). And chose literature published up to “2021/11/01”. Then chose “Trials” |
Inclusion and exclusion criteria
The inclusion criteria for the studies included in our analysis were the following: (1) The prevalence of AITD in patients with psoriasis/psoriatic arthritis and non-psoriasis were studied; (2) The study was a cohort study, case–control study or cross-sectional study; (3) The observed indicators were at least one of the following outcomes: the prevalence of hypothyroidism, hyperthyroidism, HT, GD or the positive rate of TPOAb, TgAb or TRAb; (4) The number of patients with psoriasis and the control group should be over 50. Drug-related studies, animal studies, reviews and conference abstracts were excluded from our analysis.
Data extraction
The specific process for analysing the studies generated from the search was as follows: record screening and data extraction were performed by two independent authors (XZ and SZ) according to the above retrieval strategy. When disagreements could not be resolved through consensus by the two authors, these were referred to the third author (RW) and resolved through discussion. The following information in the included studies was extracted: the name of the first author, year of publication, country of origin, study design, sample size, the definition of psoriasis and AITD, gender ratio and mean age.
Quality assessment
The Newcastle-Ottawa Scale (NOS)18 was used to assess the quality of the included cohort and case–control studies. The quality of the study was scored on three dimensions: selection, comparability and exposure/outcome. Studies that achieved 0–3, 4–6, 7–9 scores were considered of low, moderate and high quality respectively. Additionally, the tools recommended by the Agency for Healthcare Research and Quality (AHRQ)19 were used for the cross-sectional studies. Eleven items were included in AHRQ. The study was assigned one point if the answer ‘yes’, otherwise no points were assigned. Studies that achieved 0–3, 4–7 and 8–11 points were considered of low, moderate and high quality, respectively. Moreover, the ROBINS-I (Risk Of Bias In Non-randomised Studies-of Interventions) was used to assess the risk of bias.20 The assessments were carried by two authors (XZ and SZ), and checked by the third author (RW).
The meta-analysis was performed using Stata V.16.0 software. We used ORs and 95% CIs to describe the differences between patients with and without psoriasis. Differences were considered statistically significant when p<0.05. The prediction interval was used to explore the prevalence of AITD in individuals with psoriasis. The I2 statistic was used to evaluate heterogeneity as follows: I2 ≤25%, no heterogeneity; 25% ≤ I2≤50%, mild heterogeneity; 50% <I2≤75%, moderate heterogeneity; I2 >75%, severe heterogeneity. The random effects model was applied throughout the analyses. Publication bias was assessed by funnel plot and Egger’s test (publication bias was considered when p<0.1). Sensitivity analysis was performed to assess the stability of the meta-analysis by omitting one study in each turn. Univariate meta-regression analysis was used to investigate the sources of heterogeneity. The flow chart was drawn in Adobe Illustrator, and the forest plots, funnel plots, and Egger’s test charts were drawn by Stata V.16.0 software.
Patient and public involvement
No patients or members of the public were involved in this review.
Results
Search results
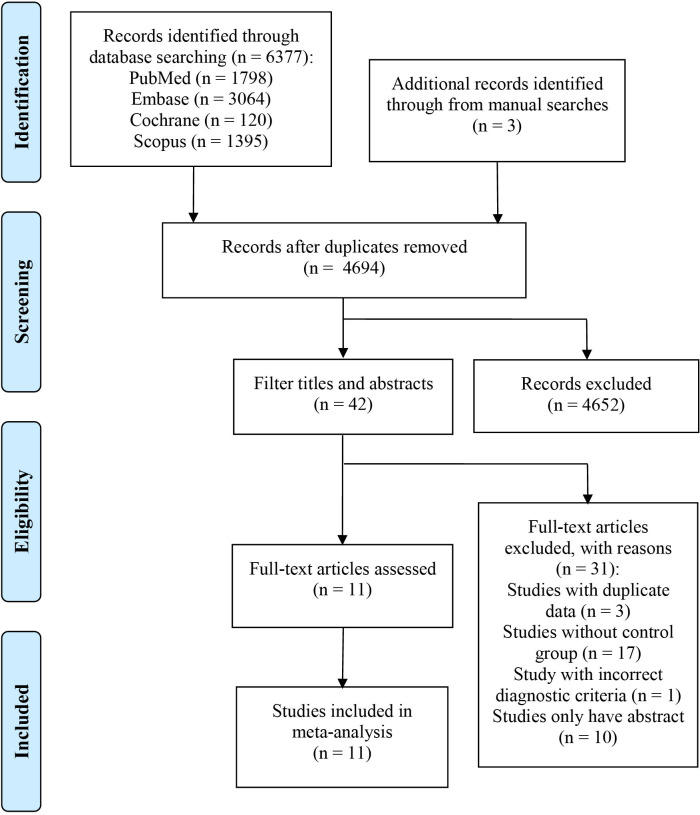
After removing duplicate results, we identified 6380 published studies in the initial search: 6377 studies were included by searching through databases and three studies were harvested by manually searching the references of relevant studies. After screening the titles and abstracts, the remaining 42 studies underwent further full-text screening. Eventually, 11 studies8–16 21 22 that met the inclusion criteria were included in the final analysis (figure 1).
Figure 1.
Flow chart for study screening.
Study characteristics
The basic characteristics of the included studies are shown in (tables 2 and 3). A total of 2 53 313 patients with psoriasis and 1 376 533 control patients were included in the analysis. Two of the studies were cohort studies, eight were case-controlled studies and one was a cross-sectional study.
Table 2.
Characteristics of the included studies
| Study (author) | Year | Country | Study design | No patients | No controls | Patients, % female | Patients, mean age |
| Antonelli et al8 | 2006 | Italy | Case–control | 80 | 400 | 45 | 57 |
| Tsai et al9 | 2011 | China | Case–control | 51 800 | 207 200 | 38.5 | 46.4 |
| Peluso et al21 | 2011 | Italy | Case–control | 108 | 318 | 52.8 | 39.9 |
| Wu et al22 | 2012 | American | Case–control | 25 341 | 126 705 | 48.4 | 48.9 |
| Vassilatou et al15 | 2017 | Greece | Case–control | 114 | 286 | 49.1 | 52.7 |
| Kiguradze et al13 | 2017 | Greece | Cross-sectional | 9654 | 846 961 | NA | NA |
| Haddad et al12 | 2017 | Israel | Case–control | 3161 | 31 610 | 53.4 | 58.4 |
| Fallahi et al11 | 2017 | Italy | Cohort | 97 | 97 | 47.4 | 56 |
| Alidrisi et al10 | 2019 | Iraq | Case–control | 56 | 54 | 58.9 | 43.05 |
| Wang et al16 | 2019 | China | Cohort | 162 842 | 162 842 | 45.5 | 45 |
| Valduga et al14 | 2021 | Valduga | Case–control | 60 | 60 | 66.7 | 54.5 |
Table 3.
Characteristics of the included studies (2)
| Study (Author) | Definition of psoriasis | Definition of AITD | NOS score |
| Antonelli et al8 | The criteria of Vasey and Espinoza | Serum levels of thyroid stimulating hormone and TgAb and TPOAb | 7 |
| Tsai et al9 | ICD-9-CM code 696.0 | ICD-9-CM codes 242.9 x, 244.9 x | 6 |
| Peluso et al21 | Classification of Psoriatic Arthritis study group criteria | Serum levels of TgAb>115 IU/mL or TPOAb>34 IU/mL, and Thyroid ultrasonography | 7 |
| Wu et al22 | ICD-9-CM code 696.0 | ICD-9-CM codes 242.0, 245.2 | 7 |
| Vassilatou et al15 | Moll and Wright criteria | Serum levels of TgAb>115 IU/mL or TPOAb>34 IU/mL | 7 |
| Kiguradze et al13 | ICD-9-CM code 696.0 | ICD-9-CM codes 245.2 | 6* |
| Haddad et al12 | Clinical diagnosis from Clalit Health Services | Clinical diagnosis from Clalit Health Services | 6 |
| Fallahi et al11 | Criteria of Vasey and Espinoza | Serum levels of TgAb or TPOAb>100 IU/mL | 6 |
| Alidrisi et al10 | Clinical diagnosis from Endocrine and Metabolism Centre | Serum levels of TgAb>115 IU/mL or TPOAb>34 IU/mL | 6 |
| Wang et al16 | ICD-9-CM code 696.0 | ICD-9-CM code 242, 242.0, 244, 246 | 8 |
| Valduga et al14 | Clinical diagnosis and measured by Psoriasis Area and Severity Index | Hypothyroidism was detected, or when they need for thyroid hormone replacement therapy or positive of TPOAb with or without TgAb | 6 |
*This was a score based on the AHRQ evaluation.
AHRQ, Agency for Healthcare Research and Quality; AITD, autoimmune thyroid disease; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; NOS, Newcastle-Ottawa Scale; TgAb, thyroglobulin antibodies; TPOAb, thyroid peroxidase antibodies.
Quality of studies
Overall, five studies were of high quality and six of moderate ones (table 3). In the studies checked with NOS (n=10), five studies were considered of moderate quality because the control groups were not from the same community. The only cross-sectional study checked with AHRQ was a moderate quality study as the method for control of confounding was not clear. Based on ROBINS-I, all included studies had moderate risk in overall bias (table 4).
Table 4.
Risk of bias for the 11 included studies based on the ROBINS-I tool
| Study (author) | Year | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
| Antonelli et al8 | 2006 | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk |
| Tsai et al9 | 2011 | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk |
| Peluso et al21 | 2011 | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk |
| Wu et al22 | 2012 | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Moderate risk | Moderate risk |
| Vassilatou et al15 | 2017 | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Kiguradze et al13 | 2017 | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Moderate risk | Moderate risk |
| Haddad et al12 | 2017 | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk |
| Fallahi et al11 | 2017 | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk |
| Alidrisi et al10 | 2019 | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk |
| Wang et al16 | 2019 | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Moderate risk |
| Valduga et al14 | 2021 | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Moderate risk | Moderate risk |
ROBINS-I, Risk Of Bias In Non-randomised Studies-of Interventions.
Prevalence of AITD in patients with psoriasis
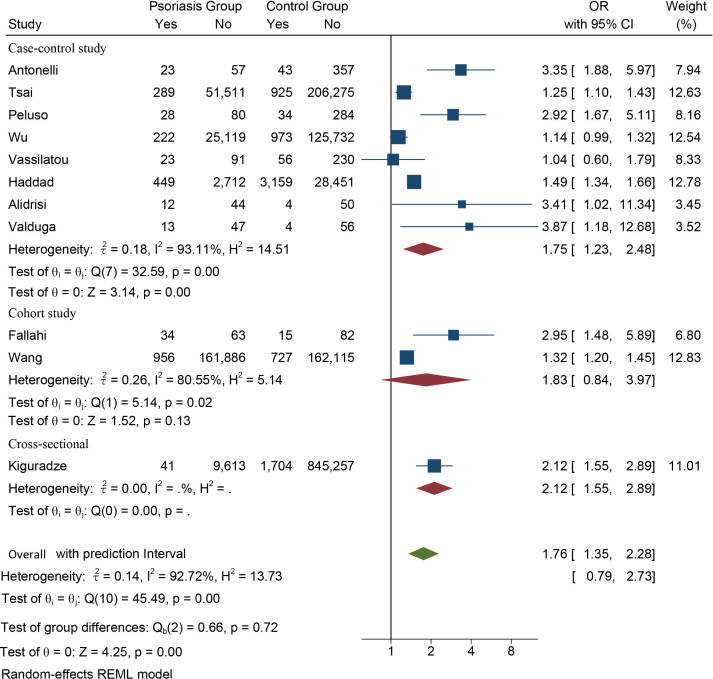
Eleven studies provided available data on the prevalence of AITD in patients with psoriasis. The meta-analysis showed that patients with psoriasis had a higher prevalence of AITD than the controls (OR 1.76, 95% CI 1.35 to 2.28, Z=4.25, p<0.01). The prediction interval ranged from 0.79 to 2.73, and the heterogeneity was severe (I2=92.72%).
Heterogeneity analysis
To investigate potential sources of heterogeneity, we first performed a subgroup analysis by types of study design. The high rate of comorbidity between psoriasis and AITD was also observed in the cross-sectional study strata (OR 2.12, 95% CI 1.55 to 2.89), and in the case–control study strata (OR 1.75, 95% CI 1.23 to 2.48, Z=3.14, p<0.01) but with heterogeneity remaining severe (figure 2), which indicated that inconsistency of the study designs was not the source of high heterogeneity.
Figure 2.
Forest plot of the association between psoriasis and autoimmune thyroid disease.
We further conducted a meta-regression analysis to explore the reason for between-study heterogeneity. Seven variables were included in the regression model, covering average age, sex ratio, nation (China or other counties), race (Caucasian or non-Caucasian), sample size, clinical types of psoriasis (psoriasis vulgaris or psoriatic arthritis), and scope of research on AITD (all studies were divided into two categories: one focusing on the loss-of-function disorder of the thyroid gland alone and the other focusing on both the loss-of-function disorder and hyperfunction disorder of the thyroid gland). When the statistical significance was set as p<0.1, sample size (β=−0.40, SE=0.20, p=0.07) and scope of research on AITD (β=0.45, SE=0.15, p=0.02) were the potential sources of high heterogeneity.
We removed three studies8 13 21 outside the funnel plot and then conducted the meta-analysis again. The results of the reanalysis also showed that patients with psoriasis had a higher prevalence of AITD than the controls (OR 1.34, 95% CI 1.20 to 1.50, Z=5.07, p<0.01), but with moderate heterogeneity (I2=57.30%). The results indicated that these three studies may have contributed to severe heterogeneity in the previous analysis. The source of heterogeneity in the study of Antonelli et al was that all the patients were patients with psoriatic arthritis,8 and the prevalence of AITD in patients with psoriatic arthritis may be higher than that of patients with psoriasis.23 The source of heterogeneity in the study by Peluso et al may have been due to the control group being comprised of hospital staff rather than the general population.21 The source of heterogeneity in the study of Kiguradze et al may lie in the fact that it was a cross-sectional study.13 These three studies were not excluded because they had little effect on the final results of the analysis.
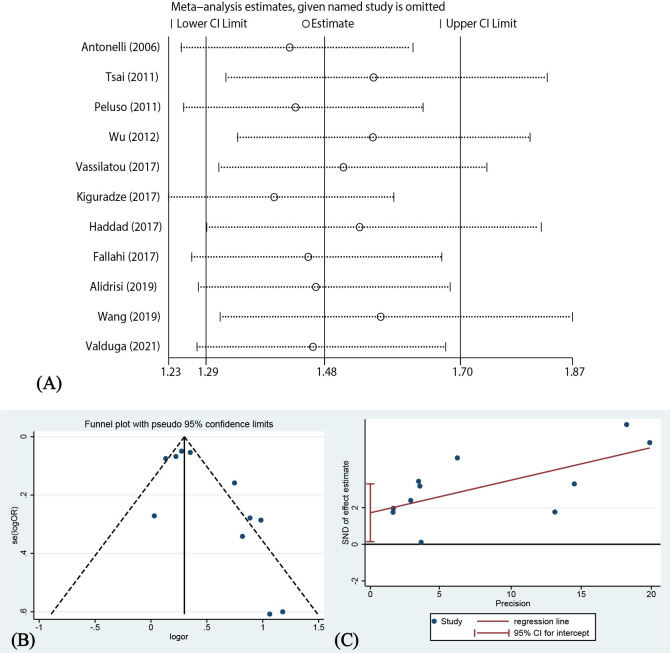
Sensitivity analysis and publication bias
Omission of either of the included studies did not significantly change the CI of the combined effect (figure 3A). Therefore, the results of the analysis were considered reliable and stable. The funnel plot for the publication bias is shown in figure 3B. The results of Egger’s test showed significant publication bias (p=0.036, figure 3C). There was a possibility of exaggerating the association between psoriasis and AITD.
Figure 3.
Sensitivity analysis and publication bias. SND, standard normal deviate.
Psoriasis and thyroid function status
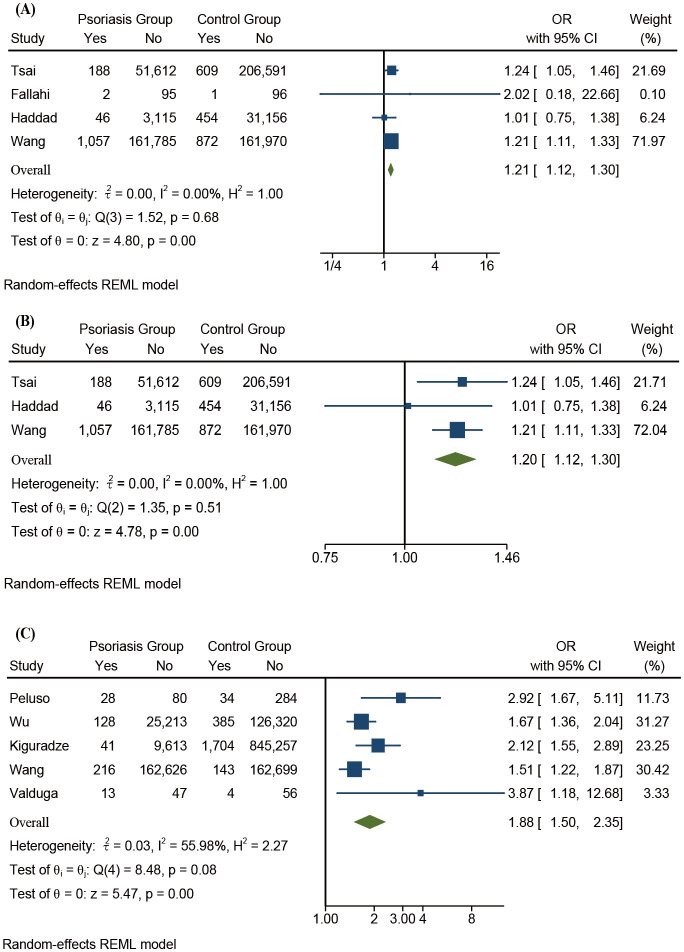
Patients with psoriasis had a higher prevalence of hypothyroidism than the controls (OR 1.21, 95% CI 1.12 to 1.30, Z=4.80, p<0.01) and no significant heterogeneity was observed (I2=0.00%). Patients with psoriasis had a higher prevalence of hyperthyroidism than the controls (OR 1.20, 95% CI 1.12 to 1.30, Z=4.78, p<0.01) and no significant heterogeneity was observed (I2=0.00%, figure 4A, B).
Figure 4.
(A) Forest plots of psoriasis and hypothyroidism. (B) Forest plots of psoriasis and hyperthyroidism. (C) Forest plots of psoriasis and HT. HT, Hashimoto’s thyroiditis; REML, restricted maximum-likelihood.
Additionally, a higher prevalence of subclinical hypothyroidism and subclinical hyperthyroidism was observed in patients with psoriasis compared with the controls (Subclinical hypothyroidism: OR 2.24, 95% CI 0.26 to 19.13, Z=0.74, p=0.46. Subclinical hyperthyroidism: OR 2.66, 95% CI 0.70 to 10.07, Z=1.44, p=0.15). However, the difference was not statistically significant (online supplemental figure 1A, B).
bmjopen-2021-055538supp001.pdf (195.8KB, pdf)
Psoriasis and specific AITD
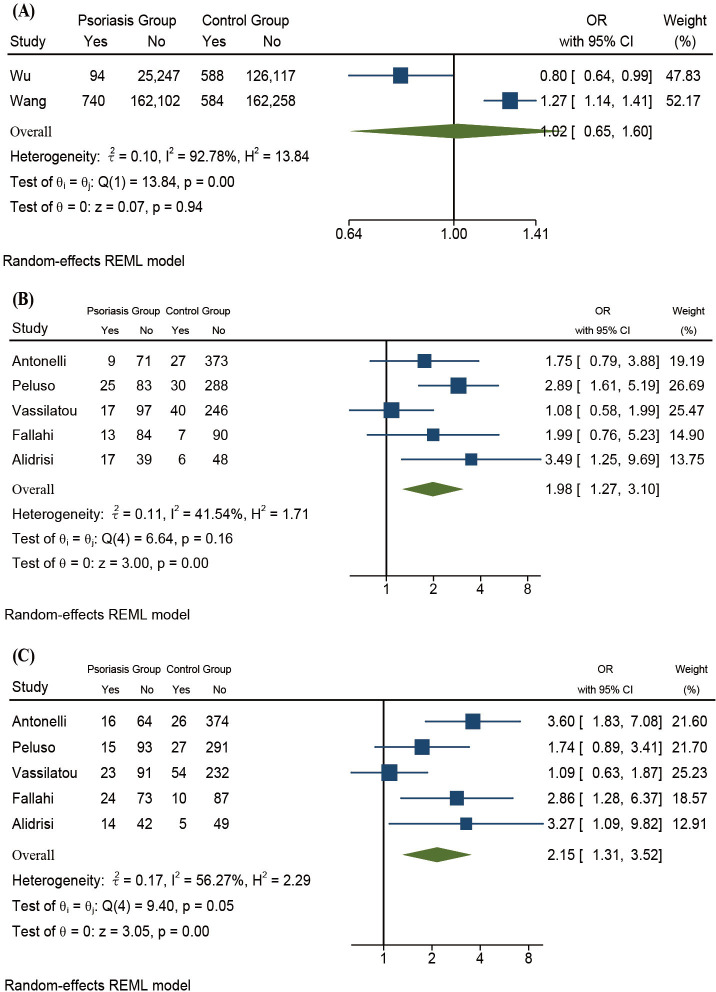
The prevalence of HT in patients with psoriasis and the controls was 0.215% and 0.199%, respectively. In comparison, the prevalence of GD in patients with psoriasis and the controls was 0.443% and 0.405%, respectively. The prevalence of HT was significantly higher in patients with psoriasis than the controls (OR 1.88, 95% CI 1.50 to 2.35, Z=5.47, p<0.01) and the heterogeneity was moderate (I2=55.98%). The prevalence of GD was also higher in patients with psoriasis than the controls (OR 1.02, 95% CI 0.65 to 1.60, Z=0.07, p=0.94) and the heterogeneity was severe (I2=92.78%). However, the difference was not statistically significant (figures 4C and 5A).
Figure 5.
(A) Forest plots of psoriasis and GD. (B) Forest plots of psoriasis and TgAb. (C) Forest plots of psoriasis and TPOAb. GD, Graves’ disease; REML, restricted maximum-likelihood; TgAb, thyroglobulin antibodies; TPOAb, thyroid peroxidase antibodies.
Psoriasis and thyroid serological antibodies
No studies provided data on the positive rate of TRAb in patients with psoriasis, so we only included TgAb and TPOAb in the meta-analysis (figure 5B, C). The positive rate of TgAb was significantly higher in patients with psoriasis than the controls (OR 1.98, 95% CI 1.27 to 3.10, Z=3.00, p<0.01) and the heterogeneity was mild (I2=41.54%). The positive rate of TPOAb was significantly higher in patients with psoriasis than the controls (OR 2.15, 95% CI 1.31 to 3.52, Z=3.05, p<0.01) and the heterogeneity was moderate (I2=56.27%).
Discussion
To our knowledge, this is the first meta-analysis focusing on the risk of AITD for psoriasis patients. The study by Khan et al is the first meta-analysis of studies on the association between AITD and the incidence risk of psoriasis.24 By summarising all available evidence on the association between psoriasis and AITD, we found that the prevalence of AITD, particularly HT, was higher in patients with psoriasis than the control individuals. Additionally, elevated positive rates of TgAb and TPOAb were also observed in patients with psoriasis. As psoriasis is a type of discosmetic dermatosis and therefore likely to be of concern, patients with psoriasis are more likely to be active about seeing a doctor regarding their condition than patients with AITD. As such, we recommend that patients with psoriasis receive a thyroid-related examination when they have suspicious AITD-related symptoms. By promoting early diagnosis and treatment of AITD, patients may be able to avoid thyroid dysfunction.
Main findings
The primary finding of this meta-analysis is that the prevalence of AITD is increased in patients with psoriasis compared with the general population. However, severe heterogeneity was observed. In order to determine whether or not the inconsistency of the study designs was the primary source of heterogeneity, the subgroup analysis based on different study designs was conducted. However, the heterogeneity was not limited by subgroup analysis; hence the heterogeneity in this meta-analysis was not caused by inconsistency of the study design. Through further meta-regression analysis, we found that the differences in sample size and scope of research on AITD among these studies might explain the high level of between-study heterogeneity. Furthermore, the heterogeneity was improved when we focused on the link of psoriasis with specific clinical characters of AITD, such as hypothyroidism, hyperthyroidism and the positivity rate of autoantibodies.
According to the definition of the study designs, an accurate cause–effect relationship can only be demonstrated in cohort studies. This study included three types of study designs, including cohort, case-controlled and cross-sectional studies. Therefore, the results should be interpreted with caution. Additionally, it has been demonstrated that the prevalence of HT in patients with psoriasis is elevated compared with the controls. HT, a main clinical subtype of AITD, is generally accompanied by hypothyroidism. An elevated frequency of hypothyroidism was also observed in patients with psoriasis. Taken together, the current data indicates that psoriasis may be closely associated with the loss-of-function disorder of the thyroid gland.
Common pathogenesis of psoriasis and AITD
Both abnormal immunological reactions and underlying genetic risk can contribute to the pathogenesis of psoriasis and AITD.25 26 These two diseases share some autoimmune processes and susceptibility genes, which may explain the concurrence of psoriasis and AITD. The predominant Th1 immune reaction has been observed in patients with psoriasis,27 28 such as Th1 infiltration in involved tissues, and high serum levels of Th1-prototype chemokines and cytokines (TNF-α, IFN-γ and CXCL10), all of which are present in AITD.29–32 Additionally, Th17-mediated immune disorder has also been observed in psoriasis and AITD.33 34 The two diseases share several predisposing genetic alleles or regions. For example, the genetic data from 265 families with two or more autoimmune disorders have shown that the PTPN22-R620W allele has a remarkable association with HT and a mild association with psoriasis.35 Additionally, other SNP variations in the PTPN22 gene have been demonstrated to be indicators for evaluating the risk of psoriasis.36 37 IL12B has been generally recognised as a psoriasis susceptibility gene,38 an upstream variation of which affects the phenotype of AITD in men.39
Implications for practice
As information relating to patient medications was not provided by in the original research, drug exposure may be a source of residual confounding in this study and a potential risk factor for concurrence of psoriasis and AITD, apart from the reasons mentioned above. β-blocker, used to control thyrotoxicosis-related symptoms, has been implicated in induction or exacerbation of psoriasis.40 41 In addition, it has been reported that the administration of acitretin, a common drug for the treatment of psoriasis, can lead to the reduction of free T4 (thyroid hormone) levels in patients with psoriasis.17 Therefore, once the patients develop suspicious symptoms after these treatments, diagnostic investigation and intervention should be considered as early as possible to avoid the exacerbation. On the other hand, the treatment for AITD and psoriasis can be mutually beneficial. For example, propylthiouracil, a drug used to inhibit thyroid hormone synthesis, has been effective in the treatment of psoriasis.42 Based on the above findings, it is recommended that the treatment options be adjusted once patients with psoriasis are diagnosed with comorbid AITD.
Limitations
There are several limitations to this meta-analysis. First, the meta-analysis included studies with different study designs. Given this, we conducted a subgroup analysis of each study design, which also showed that the patients with psoriasis had an increased prevalence of AITD in the cross-sectional study strata and the case–control study strata. Second, there is considerable heterogeneity in this study. Subgroup analysis and meta-regression analysis helped us to identify the potential sources of the heterogeneity. However, there are likely to be other unknown reasons responsible for the heterogeneity. Third, the lack of information on drug application made drug exposure a confounding factor. Therefore, further large-scale and high-quality prospective studies are still required to validate our findings.
Conclusions
The present meta-analysis revealed that AITD was more prevalent in patients with psoriasis than in the general population, especially loss-of-function disorder of the thyroid gland. Moreover, patients with psoriasis were found to have elevated positive rates of TPOAb and TgAb compared with the control individuals. Accordingly, we recommend that every dermatologist be conscious of this association and suggest necessary examinations and intervention be considered as soon as possible when patients with psoriasis have suspicious AITD-related symptoms.
Supplementary Material
Footnotes
XZ and SZ contributed equally.
Correction notice: This article has been corrected since it was published. PZ has been added as co-corresponding author.
Contributors: XZ and SZ carried out the extraction of reference data, meta-analysis and wrote manuscripts, RW and PZ supported and assisted the manuscripts, YS and SL reviewed and suggested the manuscripts. YS and PZ were responsible for the work and the conduct of the study, controlled the decision to publish. All authors were involved in finalising the manuscript.
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 82173427 to YS), The ‘Xiangya Outstanding Doctor’ Bonus and the Fundamental Research Funds for the Central Universities of Central South University (No. 2019zzts905).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1.Griffiths CEM, van der Walt JM, Ashcroft DM, et al. The global state of psoriasis disease epidemiology: a workshop report. Br J Dermatol 2017;177:e4–7. 10.1111/bjd.15610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langley RGB, Krueger GG, Griffiths CEM. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis 2005;64 Suppl 2:ii18–23. 10.1136/ard.2004.033217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daudén E, Castañeda S, Suárez C, et al. Clinical practice guideline for an integrated approach to comorbidity in patients with psoriasis. J Eur Acad Dermatol Venereol 2013;27:1387–404. 10.1111/jdv.12024 [DOI] [PubMed] [Google Scholar]
- 4.Schaefer CP, Cappelleri JC, Cheng R, et al. Health care resource use, productivity, and costs among patients with moderate to severe plaque psoriasis in the United States. J Am Acad Dermatol 2015;73:585–93. 10.1016/j.jaad.2015.06.049 [DOI] [PubMed] [Google Scholar]
- 5.Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol 2019;80:27–40. 10.1016/j.jaad.2018.06.057 [DOI] [PubMed] [Google Scholar]
- 6.Antonelli A, Ferrari SM, Corrado A, et al. Autoimmune thyroid disorders. Autoimmun Rev 2015;14:174–80. 10.1016/j.autrev.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 7.Hesarghatta Shyamasunder A, Abraham P. Measuring TSH receptor antibody to influence treatment choices in Graves' disease. Clin Endocrinol 2017;86:652–7. 10.1111/cen.13327 [DOI] [PubMed] [Google Scholar]
- 8.Antonelli A, Delle Sedie A, Fallahi P, et al. High prevalence of thyroid autoimmunity and hypothyroidism in patients with psoriatic arthritis. J Rheumatol 2006;33:2026–8. [PubMed] [Google Scholar]
- 9.Tsai T-F, Wang T-S, Hung S-T, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci 2011;63:40–6. 10.1016/j.jdermsci.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 10.Alidrisi HA, Al Hamdi K, Mansour AA. Is there any association between psoriasis and Hashimoto's thyroiditis? Cureus 2019;11:e4269. 10.7759/cureus.4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallahi P, Ferrari SM, Ruffilli I, et al. Increased incidence of autoimmune thyroid disorders in patients with psoriatic arthritis: a longitudinal follow-up study. Immunol Res 2017;65:681–6. 10.1007/s12026-017-8900-8 [DOI] [PubMed] [Google Scholar]
- 12.Haddad A, Ashkenazi RI, Bitterman H, et al. Endocrine comorbidities in patients with psoriatic arthritis: a population-based case-controlled study. J Rheumatol 2017;44:786–90. 10.3899/jrheum.161274 [DOI] [PubMed] [Google Scholar]
- 13.Kiguradze T, Bruins FM, Guido N, et al. Evidence for the association of Hashimoto's thyroiditis with psoriasis: a cross-sectional retrospective study. Int J Dermatol 2017;56:553–6. 10.1111/ijd.13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valduga JAG, Rebeiko LB, Skare TL. Prevalence of Hashimoto's thyroiditis in psoriasis patients. Rev Assoc Med Bras 2021;67:52–7. 10.1590/1806-9282.67.01.20200274 [DOI] [PubMed] [Google Scholar]
- 15.Vassilatou E, Papadavid E, Papastamatakis P, et al. No association of psoriasis with autoimmune thyroiditis. J Eur Acad Dermatol Venereol 2017;31:102–6. 10.1111/jdv.13767 [DOI] [PubMed] [Google Scholar]
- 16.Wang S-H, Wang J, Lin Y-S, et al. Increased risk for incident thyroid diseases in people with psoriatic disease: a cohort study. J Am Acad Dermatol 2019;80:1006–12. 10.1016/j.jaad.2018.11.049 [DOI] [PubMed] [Google Scholar]
- 17.Karadag AS, Ozlu E, Kostek O, et al. Effect of low-dose acitretin treatment on pituitary hormones in psoriasis vulgaris: a retrospective study. Indian J Dermatol Venereol Leprol 2019;85:300–4. 10.4103/ijdvl.IJDVL_628_17 [DOI] [PubMed] [Google Scholar]
- 18.Wells G, Shea B, O'connell D. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in Newcastle-analyses, 2019. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 19.Rostom A, Dubé C, Cranney A. AHRQ evidence reports, 2004. Available: https://www.ncbi.nlm.nih.gov/books/NBK35156/
- 20.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peluso R, Lupoli GA, Del Puente A, et al. Prevalence of thyroid autoimmunity in patients with spondyloarthropathies. J Rheumatol 2011;38:1371–7. 10.3899/jrheum.101012 [DOI] [PubMed] [Google Scholar]
- 22.Wu JJ, Nguyen TU, Poon K-YT, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol 2012;67:924–30. 10.1016/j.jaad.2012.04.039 [DOI] [PubMed] [Google Scholar]
- 23.Vastarella M, Megna M, Lupoli GA, et al. Is there any association between psoriasis, psoriatic arthritis and thyroid autoimmunity? Australas J Dermatol 2021;62:e207-e211. 10.1111/ajd.13484 [DOI] [PubMed] [Google Scholar]
- 24.Khan SR, Bano A, Wakkee M, et al. The association of autoimmune thyroid disease (AITD) with psoriatic disease: a prospective cohort study, systematic review and meta-analysis. Eur J Endocrinol 2017;177:347–59. 10.1530/EJE-17-0397 [DOI] [PubMed] [Google Scholar]
- 25.Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol 2012;7:385–422. 10.1146/annurev-pathol-011811-132448 [DOI] [PubMed] [Google Scholar]
- 26.Simmonds MJ. Gwas in autoimmune thyroid disease: redefining our understanding of pathogenesis. Nat Rev Endocrinol 2013;9:277–87. 10.1038/nrendo.2013.56 [DOI] [PubMed] [Google Scholar]
- 27.Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol 2012;9:302–9. 10.1038/cmi.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol 2014;32:227–55. 10.1146/annurev-immunol-032713-120225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonelli A, Ferrari SM, Giuggioli D, et al. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev 2014;13:272–80. 10.1016/j.autrev.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 30.Ruffilli I, Ragusa F, Benvenga S, et al. Psoriasis, psoriatic arthritis, and thyroid autoimmunity. Front Endocrinol 2017;8:139. 10.3389/fendo.2017.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanba T, Watanabe M, Inoue N, et al. Increases of the Th1/Th2 cell ratio in severe Hashimoto's disease and in the proportion of Th17 cells in intractable Graves' disease. Thyroid 2009;19:495–501. 10.1089/thy.2008.0423 [DOI] [PubMed] [Google Scholar]
- 32.Peng S, Li C, Wang X, et al. Increased Toll-like receptors activity and TLR ligands in patients with autoimmune thyroid diseases. Front Immunol 2016;7:578. 10.3389/fimmu.2016.00578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hänsel A, Günther C, Ingwersen J, et al. Human slan (6-sulfo lacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong TH17/TH1 T-cell responses. J Allergy Clin Immunol 2011;127:787–94. 10.1016/j.jaci.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 34.Vitales-Noyola M, Ramos-Levi AM, Martínez-Hernández R, et al. Pathogenic Th17 and Th22 cells are increased in patients with autoimmune thyroid disorders. Endocrine 2017;57:409–17. 10.1007/s12020-017-1361-y [DOI] [PubMed] [Google Scholar]
- 35.Criswell LA, Pfeiffer KA, Lum RF, et al. Analysis of families in the multiple autoimmune disease genetics Consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet 2005;76:561–71. 10.1086/429096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Liao W, Chang M, et al. Further genetic evidence for three psoriasis-risk genes: ADAM33, CDKAL1, and PTPN22. J Invest Dermatol 2009;129:629–34. 10.1038/jid.2008.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Wang Z, Rani PL, et al. Identification of PTPN22, ST6GAL1 and Jazf1 as psoriasis risk genes demonstrates shared pathogenesis between psoriasis and diabetes. Exp Dermatol 2017;26:1112–7. 10.1111/exd.13393 [DOI] [PubMed] [Google Scholar]
- 38.Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet 2007;80:273–90. 10.1086/511051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh JP, Berry J, Liu S, et al. The clinical presentation of autoimmune thyroid disease in men is associated with IL12B genotype. Clin Endocrinol 2011;74:508–12. 10.1111/j.1365-2265.2010.03970.x [DOI] [PubMed] [Google Scholar]
- 40.Balak DM, Hajdarbegovic E. Drug-Induced psoriasis: clinical perspectives. Psoriasis 2017;7:87–94. 10.2147/PTT.S126727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDermott MT. Hyperthyroidism. Ann Intern Med 2020;172:ITC49. 10.7326/AITC202004070 [DOI] [PubMed] [Google Scholar]
- 42.Gnanaraj P, Malligarjunan H, Dayalan H, et al. Therapeutic efficacy and safety of propylthiouracil in psoriasis: an open-label study. Indian J Dermatol Venereol Leprol 2011;77:673–6. 10.4103/0378-6323.86477 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055538supp001.pdf (195.8KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.