Abstract
Objective
Nasal septal pathologies requiring surgical intervention are common in the population. Additionally, nasal chondrocytes are becoming an important cell source in cartilage tissue engineering strategies for the repair of articular cartilage lesions. These procedures damage the nasal septal cartilage whose healing potential is limited due to its avascular, aneural, and alymphatic nature. Despite the high incidence of various surgical interventions that affect septum cartilage, limited nasal cartilage repair characterizations have been performed to date.
Methods
To evaluate the healing of the nasal septum cartilage perforation, a septal biopsy was performed in 14 sheep. Two and 6 months later, the tissue formed on the place of perforation was explanted and compared with the native tissue. Tissue morphology, protein and gene expression of explanted tissue was determined using histological, immunohistochemical and real-time quantitative polymerase chain reaction analysis.
Results
Tissue formed on the defect site, 2 and 6 months after the biopsy was characterized as mostly connective tissue with the presence of fibroblastic cells. This newly formed tissue contained no glycosaminoglycans and collagen type II but was positively stained for collagen type I. Cartilage-specific genes COL2, AGG, and COMP were significantly decreased in 2- and 6-month samples compared with the native nasal cartilage. Levels of COL1, COL4, and CRABP1 genes specific for perichondrium and connective tissue were higher in both test group samples in comparison with native cartilage.
Conclusions
Newly formed tissue was not cartilage but rather fibrous tissue suggesting the role of perichondrium and mucosa in tissue repair after nasal septum injury.
Keywords: nasal chondrocytes, biopsy, cartilage, healing, sheep
Introduction
The nasal septum cartilage is the key midline support structure of the nose. It separates the left and right airways of the nasal cavity, dividing the 2 nostrils providing the supportive framework for the nose. 1 Nasal septum cartilage, like articular cartilage, is hyaline cartilage made of extracellular matrix (ECM) produced by chondrocytes. ECM predominantly contains collagen type II (COL2A1), hyaluronic acid, chondroitin sulfate proteoglycans like aggrecan (AGG) and link proteins like laminin, fibronectin, and cartilage oligomeric matrix protein (COMP).2,3 Nasal septum cartilage is surrounded by perichondrium, a dense membrane tightly attached to cartilage, composed of fibrous connective tissue.4,5 That is the last area to which nerves, blood, and lymphatic vessels protrude providing oxygen and nutrients to the cartilage. Some authors suggest that the perichondrium consists of 2 separate layers: an inner chondrogenic layer (cambium) containing fibroblast-like cells and cartilage progenitors, and an outer fibrous layer containing fibroblasts and collagen type I.4,6 The inner layer contributes to cartilage production while the outer layer provides mechanical support and protection. The limited healing capacity of the nasal septum as well as other cartilage structures of the head and neck, could be accredited to the dual role of perichondrium—the inner layer provides cells for cartilage formation but the outer layer produces fibrous overgrowth preventing cartilage regeneration. 7 The previous study identified several potential markers of perichondrium such as thrombospondin 2 (Tsp2), galectin-1, dickkopf WNT signaling pathway inhibitor 3 (Dkk3), and transcriptional factor MafB in the inner layer; and undulin (COL14A) and cellular retinoic acid binding protein-I (CRABP-I) in the outer layer. 8 Additionally, Hinton et al. 9 highlighted collagen type IV and XIV as matrix proteins with high expression throughout perichondrium in comparison with cartilage. On the other hand, COL2A1, AGG, and COMP are considered as well-known cartilage markers and are not found in perichondrium. 10
Pathologies of the nasal septum cartilage are common in population as 80% of people suffer from deviated nasal septum conditions. 11 In addition, different infections, inflammatory and systematic diseases, or congenital malformations can damage nasal septum cartilage. 12 Furthermore, nasal septum chondrocytes possess the superior capacity to generate hyaline-like cartilage tissues in comparison with articular chondrocytes so the biopsy of the nasal septum enables quick and easy chondrocyte harvest for articular cartilage regeneration.13,14 It also eliminates donor-site morbidity at the articular harvest site. These medical interventions as well as other aesthetic interventions produce perforation of nasal cartilage tissue. Cartilage, however, has very limited healing capacity due to its avascular, aneural, and alymphatic nature. 15 Despite the high number of patients who undergo different nasal cartilage surgical procedures (biopsies, rhinoplasty revisions), little nasal cartilage healing characterizations have been performed compared with other cartilages.
Therefore, the aim of this study was to characterize the quality and origin of the tissue formed in a place of the nasal septum cartilage defect. To explore the potential of nasal septum cartilage healing, we created nasal septum perforations in a sheep animal model. The tissue formed in the perforation was examined and characterized after 2 and 6 months of healing. Evaluation of tissue morphology was done by histology and immunohistochemistry. The origin of the healing tissue was assessed by gene expression analysis of cartilage markers (COL2, AGG, and COMP) as well as inner (DKK3) and outer (CRABP1) perichondrium markers. Additionally, levels of COL1 as a marker of connective tissue and COL4 found in both cartilage and perichondrium were determined.
Methods
Tissue Samples
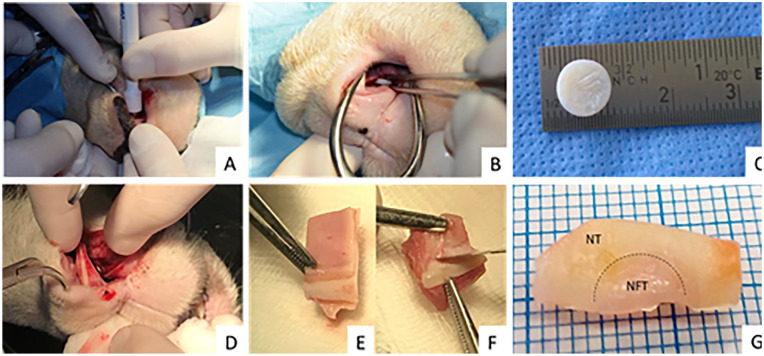
Fourteen mature domestic sheep Ovis aries aries, aged 3 to 5 years underwent a full-thickness, perforative biopsy of the nasal septum cartilage (8 mm in diameter) under general anesthesia. The surgical procedure started with a vertical incision made in the rostral nasal mucous epithelium. Using a periosteal elevator, the mucosal layer was caudally elevated from the nasal cartilage. Full-thickness nasal septum cartilage ( Fig. 1C ) surrounded on both sides by a perichondral layer was harvested with the skin biopsy punch and taken out with forceps ( Fig. 1A and B ). The perforation was covered with septal mucosal layer and vertical incision was sutured using the lactomer 9-1 (Polysorb 3-0; Covidien, Dublin, Ireland). Two or 6 months later, the cartilaginous nasal septum containing the place of the previous biopsy site was explanted ( Fig. 1D ). Explanted tissue was cut in half with one-half taken for histological analysis ( Fig. 1E ) and the other for gene expression analysis ( Fig. 1F ). Samples taken for histological analysis contained layers of mucosa and submucosa along with cartilage and perichondrium layer. Additionally, healthy nasal septum tissue samples from each sheep were explanted for histological analysis and used as positive controls. Mucosa and submucosa layers were stripped down in samples used for the real-time quantitative polymerase chain reaction (qPCR) analysis. These samples were further divided into 2 zones: native nasal cartilage (NC) or control samples, and healing sub samples or newly formed tissue (NFT) ( Fig. 1G ). All surgical procedures were approved by the local ethics committee and were conducted with the employment of 3R (replacement, reduction, and refinement) principles.
Figure 1.
Nasal septum biopsy, tissue explantation, and preparation of samples for analysis. (A and B) Biopsy of nasal septum cartilage using an 8-mm skin biopsy puncher and forceps. (C) Harvested nasal septum cartilage with perichondrium layer. (D) Explantation of the nasal septum containing the place of the previous biopsy and surrounding tissue (2 and 6 months after the biopsy). (E) Half of the explanted tissue (with mucosa and submucosa layer) used for histological analysis. (F) Half of the explanted tissue (without surrounding layers) used for gene expression analysis. (G) Explanted half used for gene expression analysis divided into newly formed tissue (NFT) and native nasal cartilage (NC).
Histological and Immunohistochemical Analysis
Samples were fixed in 4% formaldehyde, embedded in paraffin, and cut in 5-µm thick sections. Tissue sections were stained with hematoxylin/eosin (HE), safranin O, and Alcian blue. Immunohistochemical detection of collagen type I and collagen type II was performed as previously described 16 with several modifications. Anti-collagen type I (1:500, ab90395, Abcam, Cambridge, UK) and anti-collagen type II (1:200, #II-II6B3, DSHB, University of Iowa, USA) primary antibodies were applied. Antigen retrieval for collagen type II was performed with 0.1% pronase and 2.5% hyaluronidase while sodium citrate buffer and proteinase K were used as antigen retrieval in collagen type I detection. Blocking was performed with 10% goat serum (Dako) and 3% hydrogen peroxide. Detection was performed with Dako REAL EnVision Detection system (Agilent Technologies, USA) consisting of goat anti-rabbit/mouse horseradish peroxidase (HRP) conjugated secondary antibody and 3,3′-diaminobenzidine (DAB) solution. The stained sections were scanned with NanoZoomer 2.0-RS (Hamamatsu Photonics K.K., Japan).
RNA Extraction and Real-Time Quantitative PCR
To characterize a NFT in place of the induced lesion, for every sample, both, healthy and healing part were taken for further procedures. Total RNA was isolated from each sample using the TRIzol method for RNA isolation as described by Lee et al. 17 Briefly, tissue samples were embedded in DEPC water (Santa Cruz Biotechnology, Dallas, TX, USA), snap frozen in liquid nitrogen, and cryosectioned into 10-µL sections. During cryosectioning, the first and the last few sections of the healthy or NC samples were discarded to eliminate any perichondrium residue. For healing or NFT samples, no tissue sections were discarded. The obtained tissue sections were stored in 0.5 mL of TRI reagent (Sigma-Aldrich, St. Louis, MO, USA) and RNA isolation procedure was completed according to the manufacturer’s instructions. RNA concentration and quality were estimated with Nanodrop (Thermo Fisher Scientific, USA). Isolated RNA (1 µg) was treated with DNase I (Thermo Scientific, Waltham, MA, USA), while subsequent reverse transcription of total RNA was performed using High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems, Foster City, CA, USA). Samples of cDNA were amplified with the addition of SYBR Green PCR Master Mix (Applied Biosystems) and gene-specific primers (Macrogen, LIGO, Seoul, South Korea) on the 7500 Fast Real-Time PCR System (Applied Biosystems). Amplification steps were as follows: 1 PCR cycle at 95 °C for 10 minutes and 40 PCR cycles at 95 °C for 15 seconds and at 58 °C for 1 minute. Primers were designed using PrimerQuest (IDT SciTools) setup page and analyzed in silico using the AmplifX 1.7. software (N.Julien, CNRS, INP, Inst Neurophysiopathol, Marseille, France). The primer sequences were as follows: collagen type II (COL2A1), 5′GACAAAGGAGAAACTGGAGAG3′(F) and 5′GGATTCCGTTAGCACCATCTT3′(R): aggrecan (AGG), 5′CCCAACTGATGCTTCTATCC3′(F) and 5′CACAGCTTCTGGTCAATCTC3′(R), cartilage oligomeric matrix protein (COMP) 5′GGAAGGACAAGACATCCTAC3′(F) and 5′CTGGGAGAAGCAGAAGACC3′(R), collagen type I (COL1A1), 5′CAGCAGATCGAGAACATCC3′(F) and 5′CTCCATGTTGCAGAAGACC3′(R), collagen type IV (COL4A1), 5′GGCTACTCCTTTGTGATGC3′(F) and 5′GGGCTTCTTGAACATCTCG3′(R), Dickkopf-related protein 3 (DKK3), 5′GAGGTTGAGGAACTGATGG3′(F) and 5′ACCTTGGTTTCTGTGTTGG3′(R), cellular retinoic acid binding protein (CRABP1), 5′CAAGTGCAGGAGCTTACC3′(F) and 5′CATAATCCTCGTGCAGACC3′(R), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′GATTGTCAGCAATGCCTCCT3′(F) and 5′AAGCAGGGATGATGTTTTGG3′(R). Sequences for GAPDH gene primers were acquired from previous study and it represented the reference gene. 18 Gathered data were reviewed and exported using 7500 Software v2.0.6 while further analysis was performed on ΔCt values.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). The significant statistical differences of gene expression levels in healthy native cartilage and healing tissue samples were tested with 1-way analysis of variance and Tukey’s multiple comparison post hoc test. Homogeneity of variances was tested with Bartlett’s test. Data (ΔCt values) were presented as mean ± SD. Differences between tested groups were considered significant when P values <0.05 (*p < 0.0322; ***P < 0.0002; ****P < 0.0001).
Results
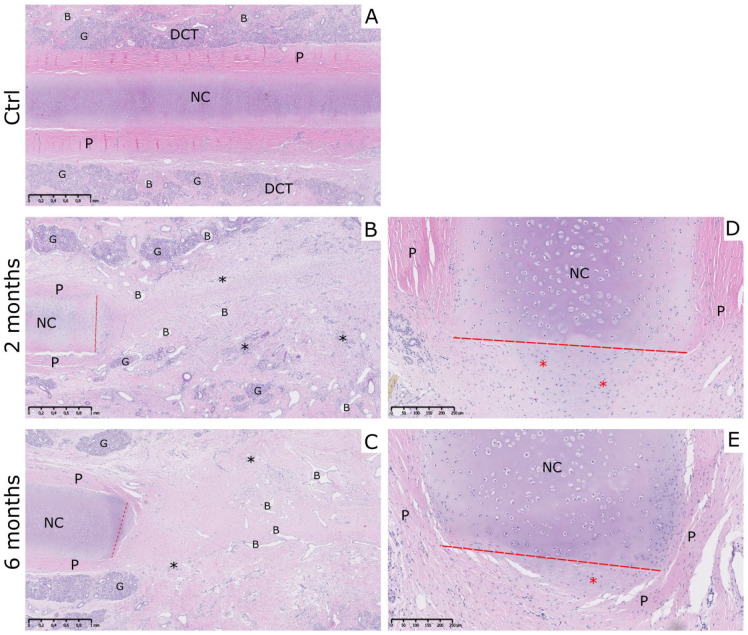
Explantation of nasal septum samples revealed healing of perforations on the place of the previous biopsy site in all 14 sheep at the 2- and 6-month time points. Prior to the explantation, sheep were checked for the presence of functional septum problems when breathing. No nasal or tracheal stridor, bleeding, or nasal discharge were detected in any of the sheep included in the study. To evaluate newly formed healing tissue in a place of septum perforation, tissue samples were stained with basic histological stains HE and safranin O. Analysis of HE-stained control NC samples showed continuous native nasal cartilage surrounded by perichondrium. Around the perichondrium, there was a dense connective tissue (DCT) containing blood vessels and seromucous glands. Native cartilage had common features including uniformly stained ECM with darker territorial areas, lacunae and isogenous groups of chondrocytes observed ( Fig. 2A ). HE-stained sections of 2- and 6-month NFT group showed overall similarity in morphology. In all the sections, the cartilage layer was discontinued with clearly distinguishable incision site (red line) and perichondrium visible by the sides of the cartilage bordering with irregular DCT. In the center of perforation, uniform fibrous-like tissue with the presence of blood vessels and lymphocytes could be seen ( Fig. 2B and C ). Alcian blue staining confirmed the presence of mucosal tissue at the site of perforation in only a few samples of both test groups (data not shown). In most of the 2- and 6-month NFT samples, aggregation of chondrocytes could be noted outgrowing from the border of the incised septal cartilage. These aggregations differed in cellular content, size, and positioning, as well as the ECM appearance when compared to native intact septal cartilage. The protruding cells seemed to be smaller and more numerous, while the ECM appeared to be less hyaline and irregularly oriented. Additionally, edges of native cartilage at the incision site were overgrown by perichondrium cells ( Fig. 2D and E ).
Figure 2.
Morphology of the tissue formed on the place of the defect site. (A) Morphology of the control healthy nasal cartilage (NC) samples revealed the presence of native hyaline nasal cartilage in the center, perichondrium by the sides of the cartilage and submucosal layer with blood vessels, and seromucous glands. (B and C) In 2- and 6-month newly formed tissue (NFT) samples, nasal cartilage tissue was discontinued and the place of incision was clearly visible. Tissue formed on the defect site was characterized as connective tissue with the presence of fibroblastic cells, blood vessels, and lymphocytes. (D and E) Newly formed cell aggrecations at edge of incised septal cartilage were visible in both NFT groups. Also, perichondrium cells overgrew the edges of native cartilage and were found on the site of perforation. Red dashed line indicates the place of incision, black asteriks indicate the presence of lymphocytes (*), newly formed cell aggregations are indicated with red asterisks (*). NC, native nasal cartilage; P, perichondrium; DCT, irregular dense connective tissue; B, blood vessels; G, seromucous glands. Hematoxylin-eosin (HE) staining. Scale on figures A, B, and C represents 1 mm. Scale on figures D and E represents 250 µm.
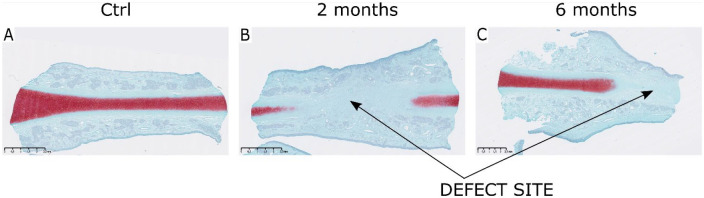
Safranin O–stained control samples showed continuous septum cartilage proteoglycans stained in red ( Fig. 3A ). In 2- and 6-month NFT group safranin O–stained sections, defect site was clearly distinguished by poor content of cartilage proteoglycans ( Fig. 3B and C ).
Figure 3.
Safranin O staining of the explanted tissue samples. (A) Control native nasal cartilage samples stained with safranin O revealed characteristic septal hyaline cartilage rich in glycosaminoglycans (GAGs). (B and C) In comparison, there were no GAGs in 2- and 6-month newly formed tissue (NFT) samples at the place of biopsy and the defect site was visible. Red stain indicates the presence of cartilage-specific proteoglycans. Scale represents 2.5 mm.
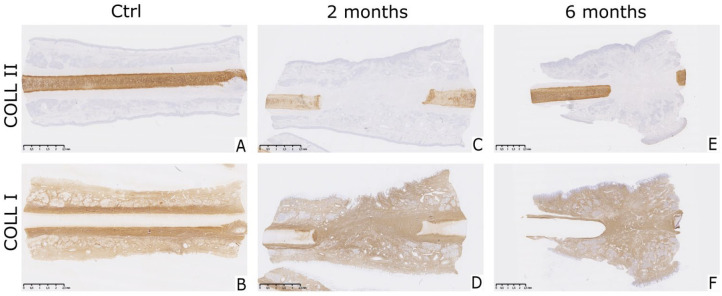
Collagen type II fibers are characteristic for hyaline cartilage, while collagen type I immunostaining marks perichondrium and surrounding connective tissue ( Fig. 4 ). Native NC of all samples was positive ( Fig. 4A ) while NFT in the place of perforation stained negative for collagen type II ( Fig. 4B and C ). Newly formed cell aggregations at edge of incised septal cartilage, noted previously in HE sections, were stained with similar intensity as the native nasal cartilage ( Fig. 4B and C ). NFT at the site of perforation could be associated with perichondrium or DCT through collagen type I immunostaining. In all samples, the perichondrium had a fibrillar appearance and was stained intensively brown. The same looking tissue could be found surrounding the site of the incision as well as throughout the perforation. Surrounding DCT was also positive for collagen type I but with different morphology when compared with perichondrium. Interestingly, previously mentioned cellular aggregations were also stained for collagen type I, presumably indicating that perichondral cells contribute to their formation ( Fig. 4E and F ).
Figure 4.
Expression of collagen type II and I in tissue formed on the defect site. (A and B) Native nasal cartilage was positively stained for collagen type II and negatively for collagen type I in healthy native cartilage control samples. (C, D, E, and F) Collagen type I was abundantly present in the perichondrium, the mucosal and submucosal layers of both control and healing samples. Tissue formed on the place of the perforation was intensively stained brown indicating presence of collagen type I, while no positive staining for collagen type II was visible. Brown stain indicates positive staining. Scale bar represents 2.5 mm.
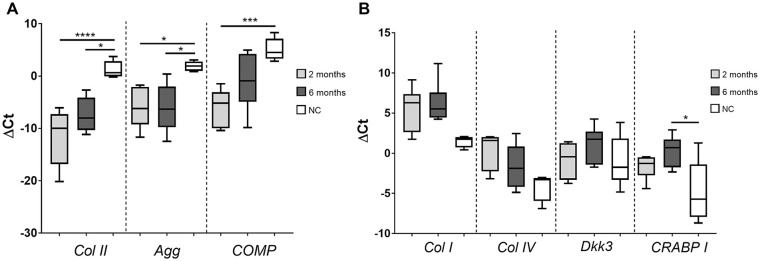
Expression of tissue-specific genes was examined with COL2, AGG, and COMP genes as cartilage-specific, and DKK3 and CRABP1 as perichondrium-specific. Moreover, perichondrium specific genes were selected to distinguish the inner (DKK3) and outer perichondrium layer (CRABP1). COL1 expression represented both perichondrium and surrounding DCT.
Expression of genes in both 2- and 6-month NFT group samples was compared with healthy NC control samples. Cartilage markers COL2 and AGG were significantly lower in both test groups while expression of COMP was found to be significantly lower only in the 2-month group when compared with the NC ( Fig. 5A ). In contrast, COL1, COL4, and CRABP1 had elevated expression levels in both test groups when compared with NC control samples but without a statistically significant difference. CRABP1 was found to be significantly elevated only in the 6-month groups ( Fig. 5B ). Higher expression of COL4 and CRABP1 genes in both 2- and 6-month NFT group indicate the role of the outer perichondrium layer in the formation of new tissue at the site of perforation. DKK3 expression levels in both test groups were similar to native NC, which suggest minor input of the inner perichondrium layer in the formation of new tissue. Low levels of cartilage-specific markers in both test groups indicate low regeneration potential of nasal cartilage after the biopsy and confirms results obtained by immunohistochemistry. The difference in gene expression between samples harvested 2 and 6 months after the biopsy was not statistically significant.
Figure 5.
Relative gene expression in native nasal cartilage, 2-, and 6-month group samples. (A) Cartilage-specific genes (COL II, AGG, and COMP) were significantly decreased in 2- and 6-month newly formed tissue (NFT) group samples compared with the healthy native nasal cartilage (NC). (B) Gene expression levels of COL I, COL IV, DKK3, and CRABP I were higher in 2- and 6-month group samples compared with the NC group but without significant differences. The exception was a higher expression of CRABP I in the 6-month group. Real-time quantitative polymerase chain reaction (qPCR) was performed with specific primers and results were expressed as ΔCt values. Data were presented as mean ± SD (NC, n = 14; 2-month samples, n = 7; 6-month samples, n = 7). COL II, collagen type II; AGG, aggrecan; COMP, cartilage oligomeric matrix protein; COL I, collagen type I; COL IV, collagen type IV; DKK3, Dickkopf WNT signaling pathway inhibitor 3; CRABPI, cellular retinoic acid binding protein-I. One-way analysis of variance and Tukey’s post hoc test, P > 0.0332 (*), P > 0.0002 (***), P > 0.0001 (****).
Discussion
The nasal septum cartilage can be damaged by different nasal pathologies or the harvest of nasal chondrocytes for tissue engineering purposes. However, the quality and structure of the tissue formed after surgical treatments of the nasal septum are not known. Since it is not possible to assess the morphology of the tissue after septal surgical interventions, to address this question, we have analyzed morphology, gene and protein expression of the NFT at the place of nasal septum biopsy in a sheep animal model. Despite the differences between ovine and human nasal anatomy, sheep are recognized as good models for training surgical procedures in otorhinolaryngology.19,20
Our findings suggest that repair tissue was not cartilage but rather fibrous tissue. NFT on the place of perforation contained mostly fibroblastic cells, blood vessels, and collagen type I pointing to the input of perichondrium and/or mucosa and submucosa in tissue repair ( Fig. 2B and C ). Regenerative potential of nasal mucosa and perichondrium was already described and perichondral grafts, mucosal mucoperichondral, and/or mucoperiosteal flaps from the internal nasal cavity are used for the treatment of small to medium septal perforations.21-23 Treatment of septal perforations larger than 2 cm includes an insert of connective scaffolding grafts between mucosal flaps. Nevertheless, it is still challenging for surgeons to ensure complete filling of these large perforations.24,25 In our study, the size of the biopsy was comparable to small-to-medium septum perforations in humans.
Histological and immunohistochemical analysis of 2 and 6-month NFT samples, showed there were no GAGs and collagen type II present at the defect site ( Figs. 3 and 4 ). Round-like cells of similar morphology to chondrocytes were visible only near the incision extending from the native nasal cartilage ( Fig. 2 ). However, the ECM around these cells differed from native NC and was positive for both collagens type I and II ( Fig. 4 ). Both groups, 2- and 6 months after the biopsy, show low or nonexistent expression of cartilage marker genes in NFT obviously disputing chondrogenic activity in this area ( Fig. 5 ). Furthermore, in the NC control group, these genes had elevated expression rates, which is consistent with previous findings.9,26,27 Contrary to our findings, previous studies showed that the formation of neocartilage at the place of septal perforation was possible in a rabbit model. 28 Neocartilage tissue with a characteristic immature appearance was detected in a young rabbit model 4 weeks after lesion introduction. However, this was the case only if perichondrium was lifted from the cartilage and laid back following the cartilage biopsy implying the importance of perichondrium in nasal cartilage regeneration. These observations were later confirmed in the adult rabbit model where the presence of neocartilage with several cartilage proliferation centers was described. 29 Verwoerd-Verhoef et al. 30 described similar looking neocartilage tissue formed after perpendicular cartilage transection in a rabbit model. The neocartilage with multiple mitoses was described as a triangularly shaped tissue covered by perichondrium and separated from the septum cartilage by a demarcation zone of necrotic tissue.
The existence of 2 perichondral layers with distinct features and functions has been described by previous authors. 4 Also, Duynstee et al. 7 confirmed a different role of perichondrium layers in nasal cartilage wound healing. They showed that in the presence of both perichondrium layers, the outer fibrous layer covers the cut edge and subsequently prevents induction of cartilage regeneration by the inner cambium layer. 7 The osteogenic and chondrogenic potential of cells isolated from the perichondrium inner cambium layer was described by Amaral et al. 31 Even though we have removed the perichondral layer during nasal septum biopsy, histological analysis revealed intrusion of fibrous-like perichondrium from the both sides of intact cartilage toward the middle of the incision and further into the perforation in all 2- and 6-month NFT samples. To confirm the presence of perichondral cells in the NFT, we examined the expression of several genes by the qPCR method. While the expression of COL2 in hyaline cartilage, and COL1 in perichondrium were established in many publications.32-34 Detection of more specific perichondrium markers became a more interesting subject due to perichondral cell contamination in tissue engineering procedures. 5 Few perichondrium marker candidates were found,8,9,35 from which COL4, DKK3, and CRABP1 genes were chosen for this study. CRABP1, COL4, and COL1 exhibited significantly elevated expression rates in NFT compared with the native cartilage ( Fig. 5 ). Even though COL4 is present also in cartilage, 36 it is still one of the genes with the highest expression difference when comparing developing perichondrium and cartilage. 35 Furthermore, DKK3 and CRABP1 genes were found to be specifically connected to the inner and outer perichondrium layer, respectively. 8 Although DKK3 was primarily found in the perichondrium, some cells resigning in the cartilage periphery were also found expressing this gene. As the inner perichondrium layer has been proposed to participate in appositional cartilage growth, it was suggested that DKK3 expressing cells migrate from perichondrium and aid cartilage growth. The protein product of CRABP1 gene is a molecule that subsequently results in negative regulation of cartilage growth. 37 Concordantly, CRABP1 expression was noted as elevated in NFT while DKK3 expression was not significantly different from its expression in native NC ( Fig. 5 ). Together, these observations are in concordance with the earlier described role of the outer perichondrium layer in the production of fibrous tissue following cartilage injury. 7 However, it should be noted that some authors consider the septal perichondrium as one continuous layer. 33
By characterizing tissue formed after nasal septum biopsy in a large animal model, our study contributed to better understanding of the role of surrounding connective tissue in nasal septum cartilage healing.
Conclusion
This study for the first time describes NFT after nasal septum biopsy in a large animal preclinical model. Once again, the low regenerative potential of cartilaginous tissue was confirmed as the induced perforation was filled with fibrous-like tissue. Both histology and gene expression analysis of NFT confirmed elevated perichondrium and dense connective tissue markers compared to cartilage-specific markers. Even though the new tissue is not cartilaginous, it still may have sufficient properties to support normal septum function. An interesting follow-up would be to examine the perforation site at a further time point to determine long-term changes in the formed tissue as well as its functional properties and possible morbidity. Further studies remain to be conducted to fully reveal consequences subsequent to nasal septum biopsy.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the European Union’s Horizon 2020 research and innovation program under grant agreement no. 681103, BIO-CHIP.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All surgical procedures were approved by the local ethics committee (class: UP/I-322-01/16-01/04, file No. 525-10/0255-165; class: UP/ I-322-01/17-01/103, file No. 525-10/0255-18-6).
ORCID iDs: Matea Brezak  https://orcid.org/0000-0001-8382-4782
https://orcid.org/0000-0001-8382-4782
Alan Ivković  https://orcid.org/0000-0003-0236-6244
https://orcid.org/0000-0003-0236-6244
Inga Urlić  https://orcid.org/0000-0001-7321-2192
https://orcid.org/0000-0001-7321-2192
References
- 1. Park SS, Becker DG. Rhinological evaluation. In: Laws ER, Lanzino G, editors. Transsphenoidal surgery. WB Saunders; 2010:36-42. [Google Scholar]
- 2. Ustünel I, Cayli S, Güney K, Celik-Ozenci C, Tanriöver G, Sahin Z, et al. Immunohistochemical distribution patterns of collagen type II, chondroitin 4-sulfate, laminin and fibronectin in human nasal septal cartilage. Acta Histochem. 2003;105(2_suppl):109-14. doi: 10.1078/0065-1281-00699 [DOI] [PubMed] [Google Scholar]
- 3. Theocharis AD, Karamanos NK, Papageorgakopoulou N, Tsiganos CP, Theocharis DA. Isolation and characterization of matrix proteoglycans from human nasal cartilage. Compositional and structural comparison between normal and scoliotic tissues. Biochim Biophys Acta. 2002;1569(1-3):117-26. doi: 10.1016/s0304-4165(01)00242-2 [DOI] [PubMed] [Google Scholar]
- 4. Aksoy F, Yildirim YS, Demirhan H, Özturan O, Solakoglu S. Structural characteristics of septal cartilage and mucoperichondrium. J Laryngol Otol. 2012;126(1):38-42. doi: 10.1017/s0022215111002404 [DOI] [PubMed] [Google Scholar]
- 5. Asnaghi MA, Power L, Barbero A, Haug M, Köppl R, Wendt D, et al. Biomarker signatures of quality for engineering nasal chondrocyte-derived cartilage. Front Bioeng Biotechnol. 2020;8:283. doi: 10.3389/fbioe.2020.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bairati A, Comazzi M, Gioria M. A comparative study of perichondrial tissue in mammalian cartilages. Tissue Cell. 1996;28(4):455-68. doi: 10.1016/s0040-8166(96)80031-0 [DOI] [PubMed] [Google Scholar]
- 7. Duynstee ML, Verwoerd-Verhoef HL, Verwoerd CD, Van Osch GJ. The dual role of perichondrium in cartilage wound healing. Plast Reconstr Surg. 2002;110(4):1073-9. doi: 10.1097/01.Prs.0000020991.10201.6c [DOI] [PubMed] [Google Scholar]
- 8. Bandyopadhyay A, Kubilus JK, Crochiere ML, Linsenmayer TF, Tabin CJ. Identification of unique molecular subdomains in the perichondrium and periosteum and their role in regulating gene expression in the underlying chondrocytes. Dev Biol. 2008;321(1):162-74. doi: 10.1016/j.ydbio.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hinton RJ, Serrano M, So S. Differential gene expression in the perichondrium and cartilage of the neonatal mouse temporomandibular joint. Orthod Craniofac Res. 2009;12(3):168-77. doi: 10.1111/j.1601-6343.2009.01450.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Funari VA, Day A, Krakow D, Cohn ZA, Chen Z, Nelson SF, et al. Cartilage-selective genes identified in genome-scale analysis of non-cartilage and cartilage gene expression. BMC Genomics. 2007;8:165. doi: 10.1186/1471-2164-8-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lavernia L, Brown WE, Wong BJF, Hu JC, Athanasiou KA. Toward tissue-engineering of nasal cartilages. Acta Biomater. 2019;88:42-56. doi: 10.1016/j.actbio.2019.02.025 [DOI] [PubMed] [Google Scholar]
- 12. Stumpe MR, Chandra RK. Disorders of the nasal septum. In: Stucker FJ, de Souza C, Kenyon GS, Lian TS, Draf W, Schick B, editors. Rhinology and facial plastic surgery. Springer; 2009. p. 151-63. [Google Scholar]
- 13. Mumme M, Barbero A, Miot S, Wixmerten A, Feliciano S, Wolf F, et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 2016;388(10055):1985-94. doi: 10.1016/s0140-6736(16)31658-0 [DOI] [PubMed] [Google Scholar]
- 14. Pelttari K, Mumme M, Barbero A, Martin I. Nasal chondrocytes as a neural crest-derived cell source for regenerative medicine. Curr Opin Biotechnol. 2017;47:1-6. doi: 10.1016/j.copbio.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 15. Goldberg A, Mitchell K, Soans J, Kim L, Zaidi R. The use of mesenchymal stem cells for cartilage repair and regeneration: a systematic review. J Orthop Surg Res. 2017;12(1):39. doi: 10.1186/s13018-017-0534-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vukasovic A, Asnaghi MA, Kostesic P, Quasnichka H, Cozzolino C, Pusic M, et al. Bioreactor-manufactured cartilage grafts repair acute and chronic osteochondral defects in large animal studies. Cell Prolif. 2019;52(6):e12653. doi: 10.1111/cpr.12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee JTY, Cheung KMC, Leung VYL. Extraction of RNA from tough tissues with high proteoglycan content by cryosection, second phase separation and high salt precipitation. J Biol Methods. 2015;2(2_suppl):e20. doi: 10.14440/jbm.2015.40 [DOI] [Google Scholar]
- 18. Garza-Veloz I, Romero-Diaz VJ, Martinez-Fierro ML, Marino-Martinez IA, Gonzalez-Rodriguez M, Martinez-Rodriguez HG, et al. Analyses of chondrogenic induction of adipose mesenchymal stem cells by combined co-stimulation mediated by adenoviral gene transfer. Arthritis Res Ther. 2013;15(4):R80. doi: 10.1186/ar4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Touska P, Awad Z, Tolley NS. Suitability of the ovine model for simulation training in rhinology. Laryngoscope. 2013;123(7):1598-601. doi: 10.1002/lary.23974 [DOI] [PubMed] [Google Scholar]
- 20. de Oliveira HF, Bollela VR, Anselmo-Lima WT, Costa C, Nakanishi M. A feasible, low-cost, reproducible lamb’s head model for endoscopic sinus surgery training. PLoS One. 2017;12(6):e0180273. doi: 10.1371/journal.pone.0180273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma A, Janus JR, Hamilton GS. Regenerative medicine and nasal surgery. Mayo Clin Proc. 2015;90(1):148-58. doi: 10.1016/j.mayocp.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 22. Eviatar A, Myssiorek D. Repair of nasal septal perforations with tragal cartilage and perichondrium grafts. Otolaryngol Head Neck Surg. 1989;100(4):300-2. doi: 10.1177/019459988910000409 [DOI] [PubMed] [Google Scholar]
- 23. Re M, Paolucci L, Romeo R, Mallardi V. Surgical treatment of nasal septal perforations. Our experience. Acta Otorhinolaryngol Ital. 2006;26(2_suppl):102-9. [PMC free article] [PubMed] [Google Scholar]
- 24. Hwang SM, Lim O, Hwang MK, Kim MW, Lee JS. The clinical analysis of the nasal septal cartilage by measurement using computed tomography. Arch Craniofac Surg. 2016;17(3):140-5. doi: 10.7181/acfs.2016.17.3.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Metzinger SE. Diagnosing and treating nasal septal perforations. Aesthet Surg J. 2005;25(5):524-9. doi: 10.1016/j.asj.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 26. Cohen NP, Foster RJ, Mow VC. Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J Orthop Sports Phys Ther. 1998;28(4):203-15. doi: 10.2519/jospt.1998.28.4.203 [DOI] [PubMed] [Google Scholar]
- 27. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461-8. doi: 10.1177/1941738109350438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Skoog T, Ohlsén L, Sohn SA. Perichondrial potential for cartilagenous regeneration. Scand J Plast Reconstr Surg. 1972;6(2_suppl):123-5. doi: 10.3109/02844317209036711 [DOI] [PubMed] [Google Scholar]
- 29. Kaiser ML, Karam AM, Sepehr A, Wong H, Liaw LH, Vokes DE, et al. Cartilage regeneration in the rabbit nasal septum. Laryngoscope. 2006;116(10):1730-4. doi: 10.1097/01.mlg.0000231430.81255.75 [DOI] [PubMed] [Google Scholar]
- 30. Verwoerd-Verhoef HL, ten Koppel PG, van Osch GJ, Meeuwis CA, Verwoerd CD. Wound healing of cartilage structures in the head and neck region. Int J Pediatr Otorhinolaryngol. 1998;43(3):241-51. doi: 10.1016/s0165-5876(98)00003-2 [DOI] [PubMed] [Google Scholar]
- 31. do Amaral RJ, Pedrosa Cda S, Kochem MC, Silva KR, Aniceto M, Claudio-da-Silva C, et al. Isolation of human nasoseptal chondrogenic cells: a promise for cartilage engineering. Stem Cell Res. 2012;8(2_suppl):292-9. doi: 10.1016/j.scr.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 32. Aigner T, Bertling W, Stöss H, Weseloh G, von der Mark K. Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J Clin Invest. 1993;91(3):829-37. doi: 10.1172/jci116303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bleys RL, Popko M, De Groot JW, Huizing EH. Histological structure of the nasal cartilages and their perichondrial envelope. II. The perichondrial envelope of the septal and lobular cartilage. Rhinology. 2007;45(2_suppl):153-7. [PubMed] [Google Scholar]
- 34. Bairati A, Comazzi M, Gioria M. An ultrastructural study of the perichondrium in cartilages of the chick embryo. Anat Embryol (Berl). 1996;194(2_suppl):155-67. doi: 10.1007/bf00195009 [DOI] [PubMed] [Google Scholar]
- 35. Späth SS, Andrade AC, Chau M, Baroncelli M, Nilsson O. Evidence that rat chondrocytes can differentiate into perichondrial cells. JBMR Plus. 2018;2(6):351-61. doi: 10.1002/jbm4.10056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Foldager CB, Toh WS, Gomoll AH, Olsen BR, Spector M. Distribution of basement membrane molecules, laminin and collagen type IV, in normal and degenerated cartilage tissues. Cartilage. 2014;5(2_suppl):123-32. doi: 10.1177/1947603513518217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eames BF, de la Fuente L, Helms JA. Molecular ontogeny of the skeleton. Birth Defects Res C Embryo Today. 2003;69(2_suppl):93-101. doi: 10.1002/bdrc.10016 [DOI] [PubMed] [Google Scholar]