Abstract
Objective
To investigate GAG-ECM (glycosaminoglycan–extracellular matrix) interactions in different cartilage types. To achieve this, we first aimed to determine protocols for consistent calculation of GAG content between cartilage types.
Design
Auricular cartilage containing both collagen and elastin was used to determine the effect of lyophilization on GAG depletion activity. Bovine articular, auricular, meniscal, and nasal cartilage plugs were treated using different reagents to selectively remove GAGs. Sulfated glycosaminoglycan (sGAG) remaining in the sample after treatment were measured, and sGAG loss was compared between cartilage types.
Results
The results indicate that dry weight of cartilage should be measured prior to cartilage treatment in order to provide a more accurate reference for normalization. Articular, meniscal, and nasal cartilage lost significant amounts of sGAG for all reagents used. However, only hyaluronidase was able to remove significant amount of sGAG from auricular cartilage. Furthermore, hyaluronidase was able to remove over 99% of sGAG from all cartilage types except auricular cartilage where it only removed around 76% of sGAG. The results indicate GAG-specific ECM binding for different cartilage types and locations.
Conclusions
In conclusion, lyophilization can be performed to determine native dry weight for normalization without affecting the degree of GAG treatment. To our knowledge, this is the first study to compare GAG-ECM interactions of different cartilage types using different GAG extraction methods. Degree of GAG depletion not only varied with cartilage type but also the same type from different anatomic locations. This suggests specific structure-function roles for GAG populations found in the tissues.
Keywords: auricular, glycosaminoglycans, meniscus, nasal septal, proteoglycans
Introduction
Proteoglycans (PGs) are soluble macromolecules contributing to the integrity and homeostasis of cartilage. They are composed of negatively charged sulfated glycosaminoglycan (sGAG) chains covalently attached to a protein core. 1 PGs in cartilage can be divided into 2 main groups, aggregating PGs and nonaggregating PGs, based on their ability to aggregate with the unsulfated GAG, hyaluronan (HA). 1 It is known that GAGs in articular cartilage contribute to viscoelastic properties. 2 To investigate the effect of GAG content on mechanical properties of cartilage, GAG can be selectively removed from the extracellular matrix (ECM) and then the depleted tissue compared to native cartilage tissue. 2 Several protocols in literature are used to selectively remove GAGs in cartilage, namely, chondroitinase ABC, guanidine hydrochloride, and hyaluronidase (usually Hyal-4). These 3 reagents facilitate the release of specific GAGs at specific pH levels. Chondroitinase ABC is an enzyme that degrades chondroitin sulfate by cleaving the GAG at disaccharide linkages, while digesting HA slowly at pH 8. 3 Guanidine hydrochloride depletes both aggregating and nonaggregating PGs at pH 4.5. 4 Hyaluronidase cleaves the HA backbone, where PGs are attached, at an optimum pH between 5.0 and 5.5. 5 The degree of GAG depletion from cartilage depends on several factors: shape and size of the cartilage plug, the protocol used for treatment, and molecular and structural interactions between GAGs and other macromolecules in the ECM.
There is literature2,6,7 to suggest that these interactions of GAG with other ECM components may differ according to cartilage type and anatomical location. For example, collagen fiber arrangement and PG content of articular cartilage changes from its surface to the deep zone. 8 Auricular cartilage has collagen and elastin fibers that are arranged in a honeycomb-like structure. 1 Furthermore, elastin fibers have demonstrated specific ultrastructural association with PGs in studies with bovine and chick aortas, possibly due to the positive lysine groups in elastin fibers that can interact with sGAG.9-11 Similar interactions are not mentioned for auricular cartilage.
In previous studies on GAG removal (sulfated and unsulfated) from cartilage,2,6,12 sample wet weights are measured, treated, washed, then lyophilized to obtain dry weight ( Fig. 1A-(1) ). They are then biochemically analyzed to determine sGAG content (using a 1,9-dimethyl methylene blue [DMMB] assay 13 ). Measured sGAG content is normalized by the dry weight determined following treatment (posttreatment dry weight).2,6 However, this posttreatment dry weight may be considerably different from the original dry weight as a significant mass loss can occur during treatment. Control samples are lyophilized and dry weight measured directly (they do not undergo any treatment). We hypothesize that this introduces errors when comparing control and experimental groups as the reference dry weights for normalization are measured at different stages in sample processing. Some studies use wet weight to normalize sGAG content.14,15 However, if different cartilage types are studied, using wet weights to normalize sGAG content introduces other inaccuracies as water content differs between cartilage types.2,16 Determining dry weight of samples prior to treatment (pretreatment dry weight) would ensure consistency of dry weights used for normalizing sGAG content. However, it is not known whether the degree of GAG depletion would be affected by lyophilization, due to possible ECM physicochemical changes caused by removal of water during this process.
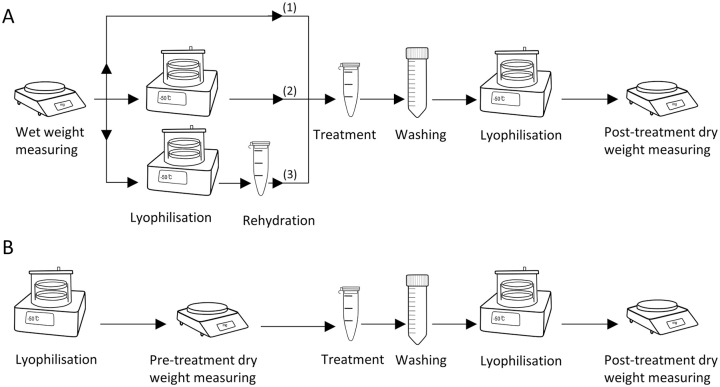
Figure 1.
(A) Three protocols used to investigate the effect of lyophilization on the degree of glycosaminoglycan (GAG) depletion in cartilage. (1) Treatment prior to lyophilization. This is the most commonly used protocol in literature, (2) treatment following lyophilization, and (3) treatment following lyophilization and rehydration. Wet weights of all the samples were measured at the beginning, and posttreatment dry weights were measured at the end. Normalized weights (posttreatment dry weight/wet weight) were compared to see the effect of lyophilization on the degree of GAG depletion in cartilage samples. (B) Protocol used to determine the effect of treatment on mass loss. Pretreatment dry weight and posttreatment dry weights were measured and compared to see the effect of treatment on dry weight of the cartilage samples.
We hypothesize that the interaction of GAGs and other ECM macromolecules vary with cartilage type (i.e., hyaline, fibrous, and elastic) and anatomical location. In this study, we aim to investigate the differences of GAG-ECM interactions in different cartilage types from different anatomical locations using selective GAG depletion with either chondroitinase ABC, guanidine hydrochloride, or hyaluronidase. Specifically, cartilage from tibial plateau (articular hyaline), ear (auricular elastic), meniscus (meniscal fibrocartilage), and nose (nasal septum hyaline) were investigated. To do this we first defined a protocol for cartilage GAG treatment that incorporates the native (or pretreatment) dry weight for sGAG content calculation. We determined whether there is an effect of lyophilization on the degree of GAG depletion, and in turn, determined whether there is a significant mass loss in cartilage samples due to GAG depletion.
Materials and Methods
Materials
Chondroitinase ABC, guanidine hydrochloride (50940), and hyaluronidase IV-S (H3884) were purchased from Sigma Aldrich (Castle Hill, Australia). Protease inhibitor was purchased from Gibco Antibiotic-Antimycotic (Life Technologies, New York). Unless indicated otherwise, all other chemicals were purchased from Sigma Aldrich, Castle Hill, Australia.
Sample Harvesting
To investigate the effect of lyophilization on GAG depletion, auricular cartilage containing both collagen and elastin were used. Bovine ears were obtained from a 2- to 3-year-old animal from a local abattoir. Since all animals were slaughtered for food purposes, ethical permission was not required. Skin and perichondrium were removed. Forty-five bovine auricular cartilage plugs (Ø5 × ~3 mm) were cored and halved for investigating the effect of lyophilization on GAG depletion (n = 90). A further 12 plugs (Ø5 × ~3 mm) were cored for testing mass loss in cartilage samples after GAG depletion (n = 12).
To investigate the effect of selective GAG treatment on different cartilage types, bovine ears and stifle joints were obtained from 6 animals aged 20 to 28 weeks and heads from 6 animals aged 1 to 2 weeks. All samples were obtained fresh at a local abattoir. Articular and meniscal cartilage were obtained by dissecting tissues surrounding the femoral and tibial condyles and the menisci. Nasal cartilage was obtained by dissecting the nasal septum. Perichondrium layers were removed from nasal septum and auricle. Cartilage plugs (Ø8 × ~2 mm) from articular, auricular, meniscal, and nasal cartilage were cored (n = 42 per type). All samples were stored at −80°C until further use.
Preparation of Treatment Solutions
Chondroitinase ABC (0.1 U/mL) was prepared in buffer base (50 mM Tris-base, 60 mM sodium acetate, 0.02% w/v BSA, pH 8.0). A solution of 4 M guanidine hydrochloride buffered in 0.05 M sodium acetate was prepared (pH 4.5 at 4°C), as described previously. 4 Hyaluronidase (2000 U/mL) was prepared by diluting hyaluronidase (4000 U/mL in 20 mM sodium phosphate, 77 mM sodium chloride, 0.01% w/v BSA, pH 7.0) in phosphate buffer (3 M sodium phosphate, pH 5.35 at 37°C) in 1:1 ratio and resulting in a hyaluronidase solution with pH 5.35.
Lyophilization and Treatment
In order to investigate the effect of lyophilization on GAG depletion, wet weight of the cartilage samples (n = 15/group) was measured. Samples were treated with guanidine hydrochloride using 3 different protocols ( Fig. 1A ): (1) treatment prior to lyophilization (protocol generally used in literature), (2) treatment following lyophilization, and (3) treatment following lyophilization and rehydration. For each protocol, control groups were treated with a corresponding blank solution (buffer solutions without the active component).
Treatment was performed by incubating samples with 1 mL of guanidine hydrochloride for 24 hours, at 4°C on a mechanical shaker at 850 rpm. Following treatment, samples were washed for 24 hours in 1% protease inhibitor in deionized water at 4°C to remove any remaining reagent from the sample. Samples were lyophilized over 16 hours at −50°C and 0.005 mbar pressure (freeze dryer 2.5 L, −50°C benchtop model, Labconco, Kanas City, USA) and weighed. Posttreatment dry weight was normalized with wet weight. For protocol 3, rehydration was performed by equilibrating the sample in phosphate buffered saline (PBS) for 24 hours at 4°C.
In order to determine the effect of treatment on mass loss, 12 samples were lyophilized, and pretreatment dry weights were measured ( Fig. 1B ). Cartilage plugs were divided into 2 groups (n = 6 per group) for treatment with guanidine hydrochloride active and blank solutions. After washing, samples were lyophilized, and posttreatment dry weights were measured.
GAG Treatment
To investigate the effect of selective GAG treatment on different cartilage types, cartilage plugs were halved: half for treatment and sGAG measurement, and half for treatment and histology. Samples from each anatomical location were tested resulting in 7 groups per cartilage location (n = 6/group): a control group and an active and blank group for each of 3 chemicals (chondroitinase ABC, guanidine hydrochloride, and hyaluronidase). The optimal protocol of the previous 2 aims was used for sample dry weight determination. Reagents were prepared as described above and treatments performed in 1 mL of solution per sample for 24 hours. Chondroitinase ABC and hyaluronidase treatments were performed at 37°C, while guanidine hydrochloride treatment was performed at 4°C.
Biochemical and Histological Analysis
Following treatment, samples were incubated in 1% protease inhibitor for 24 hours to remove any residue of the treatment solutions. Samples were digested overnight at 60°C by proteolytic enzyme, papain (1 mg/mL papain in 20 mM monosodium phosphate monobasic monohydrate, 5 mM ethylenediaminetetraacetic acid, and 2 mM dithiothreitol; pH 6.8). Sulfated-GAG content was determined by DMMB assay, as described previously. 13 Absorbance was measured at 520 nm using a microplate reader (Multiscan FC 357, Thermo Fisher Scientific Instruments, Shanghai, China). Shark chondroitin sulfate (C4384) was used as standard. Measured sGAG content was normalized with pretreatment dry weights.
For histology, cartilage sections were fixed in neutral buffered formalin (4% formaldehyde, AMBER Scientific, NBF-5L) overnight at room temperature, washed in PBS, and transferred into 70% ethanol. Sections were processed overnight using an automated tissue processor (Sakura Tissue-TekVIP6, Olympus, Australia). Sections were dehydrated in series of ethanol solutions; in 90% (V/V) for an hour, twice in 100% (V/V), 2 hours each and again in 100% (V/V) for an hour. This was followed by clearing (2 exchanges in xylene, 2 hours each), and infiltration of wax at 60°C; 3 exchanges, 1 hour each and 1 exchange of 30 minutes. Samples were then embedded in paraffin. Tissue blocks were sectioned at 5 µm.
Hematoxylin and eosin staining (H&E) and Safranin-O staining were carried out following standard protocols. All histology slides were scanned with a slide scanner (3D Histech, Panoramic SCAN II) with Carl Zeiss Plan-Apochromat.
Statistical Analysis
In order to investigate the effect of lyophilization on GAG depletion, one-way ANOVA was carried out to test significant differences (P < 0.05) between the existing and proposed protocols. To investigate mass loss with treatment, dry weights before and after treatment were tested using a paired Student’s t test (P < 0.05).
To investigate the effect of selective GAG treatment on cartilage types, GAG content of native control and treated groups were compared. A nonparametric statistical test, Wilcoxon paired samples signed rank test, was used to identify significant differences (P < 0.05). All statistics were performed with RStudio (V 4.2.3, R Core Team, Vienna, Austria).
Results
Effect of Lyophilization on GAG Depletion
Investigation of the effect of lyophilization on GAG depletion with 3 protocols showed no significant effect on the normalized weights ( Fig. 2 ). All active groups showed significantly less GAG content compared to the corresponding blank group indicating that the treatment has removed GAG from both lyophilized and fresh samples. This suggests that there is no significant GAG depletion due to lyophilization alone or lyophilization following rehydration when compared to the fresh treatment of the cartilage samples.
Figure 2.
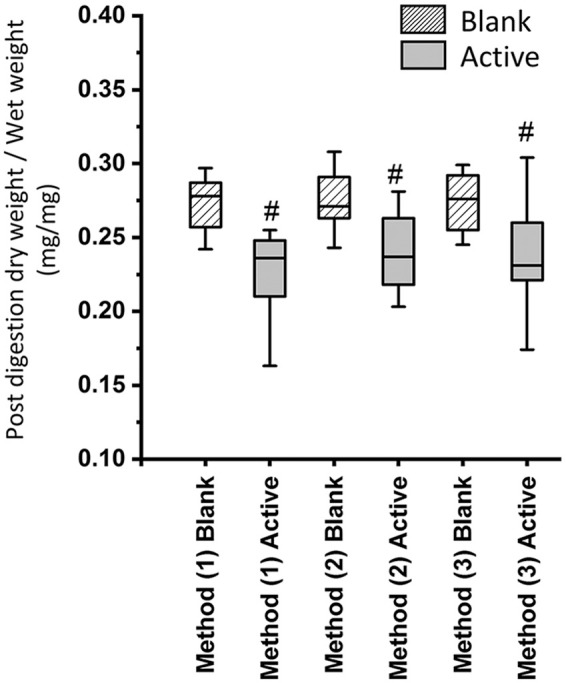
Normalized weights (posttreatment dry weight/wet weight). (1) Treatment prior to lyophilization, (2) treatment following lyophilization, and (3) treatment following lyophilization and rehydration. N = 15 per group, # indicates P < 0.05.
Mass Loss from Treatments and Protocol Selection
The actively treated group had a significantly higher mean pretreatment dry weight (±SD) of 9.12 ± 1.84 mg compared to a posttreatment dry weight of 8.22 ± 1.96 mg. The blank group did not show a significant difference between pre- and posttreatment dry weights (11.27 ± 1.90 mg and 11.13 ± 2.00 mg, respectively). This indicates that removal of GAG changes the dry weight of the cartilage samples significantly and is not an appropriate reference for normalization.
Due to no effect of protocol—that is, protocols 1, 2, and 3 in Figure 1 —on GAG depletion, and a significant mass loss from treatment, the protocol measuring the dry mass of lyophilized samples prior to treatment 2 is recommended. Thus, in the subsequent experiment investigating selective GAG depletion treatment, dry weights were measured prior to treatment to avoid confounding results when comparing controls and treated groups.
Effect of Selective GAG Depletion Treatment on Different Cartilage Types
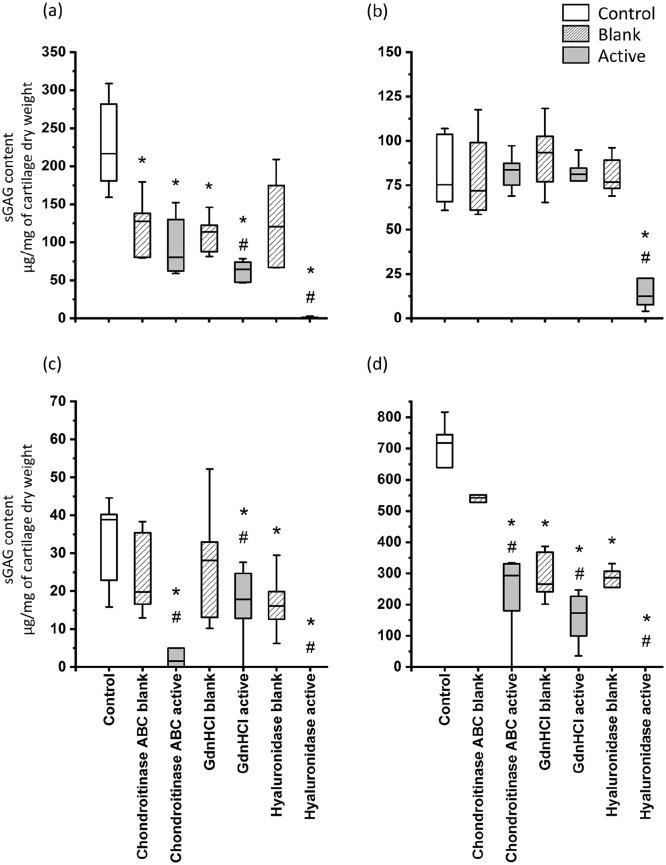
In articular cartilage ( Fig. 3a ), all active groups resulted in lower sGAG content than the control group. However, only the guanidine hydrochloride active and hyaluronidase active groups were statistically significant when compared with the corresponding blank groups. In auricular cartilage ( Fig. 3b ), the hyaluronidase active group showed significantly less sGAG content compared to both blank and control groups. With every other GAG extraction treatment of auricular cartilage, sGAG loss was not significant compared to the control group or corresponding blank group.
Figure 3.
Sulfated glycosaminoglycan (sGAG) content of the bovine (a) articular, (b) auricular, (c) meniscal, and (d) nasal cartilage samples after enzymatic treatments. N = 6/group, * indicates P < 0.05 compared to the control group, # indicates P < 0.05 compared to the corresponding blank group. GdnHCl = guanidine hydrochloride. NB. Y-axis range is different for each plot due to large differences between cartilage types. Pretreatment dry weights were used to normalize the sGAG contents.
In meniscal cartilage, all 3 reagents, chondroitinase ABC, guanidine hydrochloride, and hyaluronidase, resulted in significant loss of sGAG content compared to the control group and corresponding blank groups ( Fig. 3c ). In nasal cartilage ( Fig. 3d ), all active and blank groups lost significant sGAG content compared to the control, except chondroitinase ABC blank group. In addition, every active group showed significantly less sGAG content than the corresponding blank group.
Hyaluronidase resulted in over 99% sGAG loss in all the cartilage types tested except in auricular samples. Auricular samples treated with hyaluronidase contained on average 19 ± 17 µg/mg sGAG (i.e., approximately 24% of the sGAG content of the control group), which indicates that hyaluronidase was not successful in completely removing sGAG.
Histology
No changes to ECM structure were seen on H&E staining in articular, meniscal, and nasal cartilage samples following treatment (Supplementary Fig. S1 A(iii-vii), C(iii-vii), D(iii-vii)) compared to control (Supplementary Fig. S1 A(i, ii), C(i, ii), D(i, ii)) and blank samples (Supplementary Fig. S2 A(i, ii, iii), C(i, ii, iii), D(i, ii, iii)). In auricular cartilage, all active group samples showed ECM discontinuities due to disruption of elastin fibers (Supplementary Fig. S1(iii-vii)).
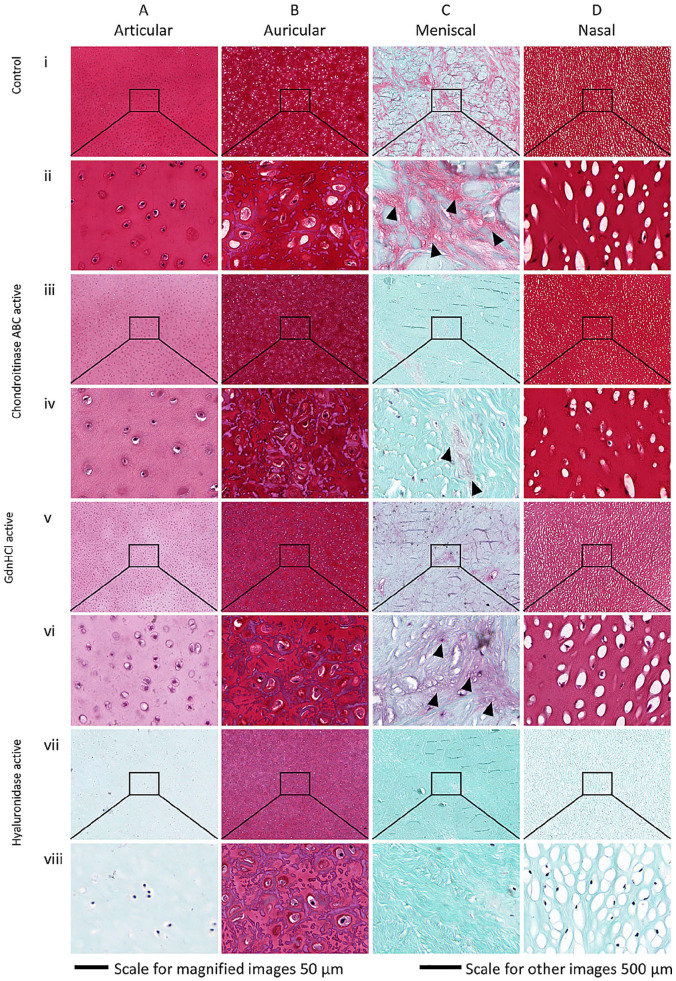
Articular, auricular, and nasal control samples showed intense red Safranin-O staining indicating presence of high GAG content ( Fig. 4A(i, ii) , B(i, ii) , and D(i, ii) ), respectively, which was reduced for samples after treatment ( Fig. 4A(iii-viii) , B(iii-viii) , and D(iii-viii) ). Confirming the high sGAG content measured by DMMB for chondroitinase ABC and guanidine hydrochloride active groups of auricular cartilage, red staining was intense indicating the presence of GAG even after treatment ( Fig. 4A(iii-vi) ). Absence of red staining in articular, meniscal, and nasal samples treated with hyaluronidase indicates complete GAG loss ( Fig. 4A(vii, viii) , C(vii, viii) , D(vii, viii) ). These results mirror the DMMB results for hyaluronidase-treated articular, meniscal, and nasal groups ( Fig. 3a , c , d ), which show almost complete sGAG loss. Some reddish pink color observed in the auricular samples ( Fig. 4B(vii, viii) ) indicate the presence of GAG after active hyaluronidase treatment. The low GAG content in meniscal cartilage measured by DMMB was confirmed by histology, where blank samples have little staining compared to other cartilage types (Fig. S3 C(i-iii)). A light red to pink staining in meniscal samples was concentrated along the collagen fibers ( Fig. 4C(i) ), indicating association of GAG with collagen fibers. This staining was also present after chondroitinase ABC and guanidine hydrochloride treated samples ( Fig. 4C(iii-vi) ). Only hyaluronidase-treated meniscal cartilage did not show any pink staining associated with collagen fibers ( Fig. 4C(vii, viii) ). Overall, GAG loss patterns measured by DMMB were confirmed by histology.
Figure 4.
Histological Safranin-O staining of cartilage ECM (extracellular matrix). Control and actively treated cartilage samples for (A) articular (deep zone), (B) auricular, (C) meniscal, and (D) nasal cartilage indicating presence of sulfated glycosaminoglycan (sGAG; pink/red). Row (i, ii) native control, (iii, iv) chondroitinase ABC active, (v, vi) guanidine hydrochloride active, and (vii, viii) hyaluronidase active. Black arrow heads indicate alignment of sGAG with collagen content in meniscal cartilage.
Discussion
This study aimed to investigate GAG-ECM interactions in different cartilage types from different anatomical locations using selective GAG depletion with chondroitinase ABC, guanidine hydrochloride, or hyaluronidase. To do this we first defined a protocol for cartilage GAG treatment that incorporates native (or pretreatment) dry weight for sGAG content calculation, in order to avoid confounding results when (1) comparing different GAG treatments where some are already known from literature to have an effect on tissue mass and (2) comparing cartilage tissues which are known to have different amount of water. Results showed that the degree of sGAG loss varied according to cartilage type and reagent used. Articular and nasal samples showed similar pattern of sGAG loss for the reagents used, while auricular and meniscal samples were less affected. This protocol was then used for the subsequent GAG treatment study.
To develop this protocol, we demonstrated that lyophilization has no effect on the degree of GAG depletion. In current literature only fresh (or fresh frozen) sample treatment are described, and dry weights are measured after treatment.2,6,12 Furthermore, GAG depletion can cause a significant dry mass loss in samples, and posttreatment dry weight does not reflect the original dry weight of the cartilage. Use of posttreatment dry weight for normalizing results is less accurate than pretreatment dry weight. This affects interpretation of results, particularly when comparing experimental groups with the control which did not undergo treatment. Results showed that the reagent’s ability to deplete the targeted components (GAG in this case) is not affected by initial water content (i.e., whether it is hydrated or lyophilized). Lyophilizing the sample before treatment allows measurement of original dry weight, which is referred to as “pretreatment dry weight.”
Using pretreatment dry weight has an advantage over wet weight, which can be unstable and may vary during sample handling. In addition, water content of different cartilage types varies, 2 making it less accurate for comparing cartilage constituents in different types. We recommend using pretreatment dry weight to improve the precision of the results. For example, dry weight basis may also be used to express the content of other components such as collagen and elastin in addition to sGAG. Additionally, when comparing native and tissue-engineered cartilage constructs, dry weight might be a reliable normalizing parameter as water content in tissue engineering products could vary significantly from native tissues.
Chondroitinase ABC removed twice as much sGAG (with respect to corresponding blank group) from nasal cartilage (~57%) compared to articular cartilage (~23%), which are both hyaline cartilages. For the other 2 reagents (guanidine hydrochloride and hyaluronidase) both articular and nasal cartilage showed similar sGAG percentage losses. This suggests the availability of more chondroitin sGAG chains that decorate the PGs are available in nasal cartilage. However, this may also be due to lower collagen content in nasal cartilage, which allows chondroitinase ABC to reach chondroitin sulfate chains more easily than in articular cartilage. Collagen content of nasal cartilage is reported to be ~25% to 40% of the dry weight, whereas collagen comprises 60% to 80% of dry weight in articular cartilage. 17 In both articular and nasal samples, some sGAG loss was seen even in blank groups, indicating sGAG was easily removed even without an active reagent. This could be due to weak attachment of GAG with the rest of the ECM, allowing them to leach into the buffer solutions. The effect of mechanical shaking during treatment and washing may also exacerbate sGAG loss. However, significant GAG depletion was not seen in blank groups of auricular and meniscal cartilage, which underwent the same protocols.
Hyaluronidase was successful in removing over 99% of sGAG in articular, meniscal, and nasal samples but failed to remove all sGAG from auricular samples. Our results for hyaluronidase treatment of articular and auricular samples match those of Nimeskern et al. 2 This was further validated by Safranin-O staining seen in the respective samples. Hyaluronidase cleaves the hyaluronan backbone where PGs are attached. It also attacks chondroitin sulfate. 18 This behavior of auricular samples could be due to the additional elastin meshwork available in the auricular ECM, as elastin has positive lysine amino groups, enabling it to bind to negatively charged sGAG. 19 Mallinger et al 10 have shown GAG in human auricular cartilage has a higher sulfate content compared to GAG from nasal cartilage. Safranin-O staining results of this study support their results as auricular samples have shown intense red color, Figure 4B(i) . In addition, elastin fibers in bovine and chick aortas have shown specific ultrastructural attachment to the heparan sulfate PG.9,11 This suggests that similar interactions could be expected in auricular cartilage. In fact, the specific PG or GAG expression of auricular cartilage is not available in literature to further explain the results. Therefore, identification of types of sGAG in auricular cartilage is required.
Safranin-O staining results further validated GAG loss patterns observed with DMMB. The intensities of the red color which correspond to the presence of GAGs varied between the groups indicating variable GAG losses. The staining of meniscal samples which aligned with collagen fibers could be due to GAG chains present in aggrecan. Aggrecan is known to be associated with the radial collagen fibers seen in meniscal cartilage. 20 H&E staining did not show any visible structural distortion in articular, meniscal, and nasal samples due to treatments. This indicates that reagents have selectively acted on GAGs in cartilage without distorting the collagen. However, the disruption of elastin fibers was seen in actively treated auricular samples. It is possible that GAG has a specific role in maintaining elastin fiber structure in auricular cartilage. However, such roles of GAGs in auricular cartilage have not been investigated in literature.
The effectiveness of treatment protocols may have been affected due to sample thicknesses and age. It is hard to achieve precise sample thickness. Sample thicknesses in this study were approximately 3 mm. However, 24-hour prolonged treatment with continuous shaking was used to mitigate the effect of the thickness to give enough time for all reagents to diffuse into the ECM. Nasal cartilage samples were from young bovine (1-2 weeks) and other cartilage types were obtained from 20- to 28-week-old animals. It is known that the cartilage ECM composition changes with age. 16 Therefore, these results may not reflect the GAG-ECM interactions of nasal cartilage of similar-aged animals as articular, auricular, and meniscal cartilage. Furthermore, cartilage growth will not have reached maturity and these samples would include growth cartilage. The results observed in this study would likely differ for mature cartilage samples.
Existing literature uses enzymes or other reagents to extract sGAG from cartilage to evaluate sGAG or to evaluate biomechanical behavior of cartilage without sGAG. This is the first study, to our knowledge, where GAG depletion of different cartilages types was compared to explain sGAG-ECM interactions. Currently, GAG types in articular cartilage are well-described.21-23 However, such data are not explicitly available in literature regarding other cartilage types. Moreover, the interaction of sGAG with ECM is largely unknown. Thus, there is a need for investigations focusing on sGAG-ECM macromolecule interactions in different cartilage types to assess their influence on cartilage mechanobiology. The first step in understanding these interactions is to identify GAG types present in each cartilage type and location. Their spatial arrangement can be then studied with fluorescent labelling of GAG types. 24 Understanding these sGAG-ECM interactions in other cartilage types will be beneficial for cartilage tissue engineering, highlighting required biological features in engineered products to improve mechanical performance.
Conclusion
In conclusion, the results of this study show that dry weight of cartilage should be measured prior to cartilage treatment in order to provide reference dry weight for normalization. For future studies where it is required to cleave GAG and various macromolecules from cartilage, lyophilization can be performed initially to determine the native dry weight without affecting the degree of treatment. Degree of GAG depletion not only varied with cartilage type but also cartilage of the same type from different anatomical locations. The variation in degree of GAG depletion among the different types of cartilage using different reagents indicates different interactions in the ECM that are specific to the GAG populations found in these tissues. This suggests specific structure-function roles for both cartilage type and location of GAG populations found in the tissues.
Supplemental Material
Supplemental material, sj-pdf-1-car-10.1177_19476035211000811 for Macromolecular Interactions in Cartilage Extracellular Matrix Vary According to the Cartilage Type and Location by Manula S. B. Rathnayake, Brooke L. Farrugia, Karyna Kulakova, Colet E. M. ter Voert, Gerjo J. V. M. van Osch and Kathryn S. Stok in CARTILAGE
Footnotes
Authors’ Note: This work was carried out in Department of Biomedical Engineering, University of Melbourne, Parkville, Australia.
Acknowledgments and Funding: The authors acknowledge Professor Eleanor J. Mackie and dissection laboratory manager, Brendan Kehoe from the Melbourne Veterinary School at the University of the Melbourne, for helping source bovine samples from a local abattoir, and Mr. Cameron Patrick from the Statistical Consulting Centre at the University of Melbourne, for providing statistical advice on analyzing the results of this study. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The University of Melbourne intramural support.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Since all animals were slaughtered for food purposes, ethical permission was not required.
Animal Welfare: Guidelines for humane animal treatment did not apply to the present study because all animals were slaughtered for food purposes.
ORCID iDs: Manula S. B. Rathnayake  https://orcid.org/0000-0002-2203-0994
https://orcid.org/0000-0002-2203-0994
Karyna Kulakova  https://orcid.org/0000-0001-5680-9501
https://orcid.org/0000-0001-5680-9501
Kathryn S. Stok  https://orcid.org/0000-0002-0522-4180
https://orcid.org/0000-0002-0522-4180
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car.
References
- 1. Culav EM, Clark CH, Merrilees MJ. Connective tissues: matrix composition and its relevance to physical therapy. Phys Ther. 1999;79:308-19. [PubMed] [Google Scholar]
- 2. Nimeskern L, Utomo L, Lehtoviita I, Fessel G, Snedeker JG, van Osch GJVM, et al. Tissue composition regulates distinct viscoelastic responses in auricular and articular cartilage. J Biomech. 2016;49:344-52. [DOI] [PubMed] [Google Scholar]
- 3. Yamagata T, Saito H, Habuchi O, Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968;243:1523-35. [PubMed] [Google Scholar]
- 4. Hardingham TE, Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974;139:565-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gorham SD, Olavesen AH, Dodgson KS. Effect of ionic strength and pH on the properties of purified bovine testicular hyaluronidase. Connect Tissue Res. 1975;3:17-25. [DOI] [PubMed] [Google Scholar]
- 6. Bara JJ, Johnson WE, Caterson B, Roberts S. Articular cartilage glycosaminoglycans inhibit the adhesion of endothelial cells. Connect Tissue Res. 2012;53:220-8. [DOI] [PubMed] [Google Scholar]
- 7. Bayliss MT, Ali SY. Isolation of proteoglycans from human articular cartilage. Biochem J. 1978;169:123-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Izadifar Z, Chen X, Kulyk W. Strategic design and fabrication of engineered scaffolds for articular cartilage repair. J Funct Biomater. 2012;3:799-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehrlich KC, Radhakrishnamurthy B, Berenson GS. Isolation of a chondroitin sulfate–dermatan sulfate proteoglycan from bovine aorta. Arch Biochem Biophys. 1975;171:361-9. [DOI] [PubMed] [Google Scholar]
- 10. Mallinger R, Geleff S, Bock P. Histochemistry of glycosaminoglycans in cartilage ground substance. Alcian-blue staining and lectin-binding affinities in semithin Epon sections. Histochemistry. 1986;85:121-7. [DOI] [PubMed] [Google Scholar]
- 11. Radhakrishnamurthy B, Ruiz HA, Jr, Berenson GS. Isolation and characterization of proteoglycans from bovine aorta. J Biol Chem. 1977;252:4831-41. [PubMed] [Google Scholar]
- 12. Basalo IM, Chen FH, Hung CT, Ateshian GA. Frictional response of bovine articular cartilage under creep loading following proteoglycan digestion with chondroitinase ABC. J Biomech Eng. 2006;128:131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173-7. [DOI] [PubMed] [Google Scholar]
- 14. Keenan KE, Besier TF, Pauly JM, Han E, Rosenberg J, Smith RL, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthritis Cartilage. 2011;19:171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lemke AK, Sandy JD, Voigt H, Dreier R, Lee JH, Grodzinsky AJ, et al. Interleukin-1alpha treatment of meniscal explants stimulates the production and release of aggrecanase-generated, GAG-substituted aggrecan products and also the release of pre-formed, aggrecanase-generated G1 and m-calpain-generated G1-G2. Cell Tissue Res. 2010;340:179-88. [DOI] [PubMed] [Google Scholar]
- 16. Homicz MR, McGowan KB, Lottman LM, Beh G, Sah RL, Watson D. A compositional analysis of human nasal septal cartilage. Arch Facial Plast Surg. 2003;5:53-8. [DOI] [PubMed] [Google Scholar]
- 17. Correro-Shahgaldian MR, Introvigne J, Ghayor C, Weber FE, Gallo LM, Colombo V. Properties and mechanobiological behavior of bovine nasal septum cartilage. Ann Biomed Eng. 2016;44:1821-31. [DOI] [PubMed] [Google Scholar]
- 18. Hoffman P, Meyer K, Linker A. Transglycosylation during the mixed digestion of hyaluronic acid and chondroitin sulfate by testicular hyaluronidase. J Biol Chem. 1956;219:653-63. [PubMed] [Google Scholar]
- 19. Fornieri C. Lysyl oxidase activity and elastin/glycosaminoglycan interactions in growing chick and rat aortas. J Cell Biol. 1987;105:1463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valiyaveettil M, Mort JS, McDevitt CA. The concentration, gene expression, and spatial distribution of aggrecan in canine articular cartilage, meniscus, and anterior and posterior cruciate ligaments: a new molecular distinction between hyaline cartilage and fibrocartilage in the knee joint. Connect Tissue Res. 2005;46:83-91. [DOI] [PubMed] [Google Scholar]
- 21. Melrose J, Hayes AJ, Whitelock JM, Little CB. Perlecan, the “jack of all trades” proteoglycan of cartilaginous weight-bearing connective tissues. Bioessays. 2008;30:457-69. [DOI] [PubMed] [Google Scholar]
- 22. Melrose J, Isaacs MD, Smith SM, Hughes CE, Little CB, Caterson B, et al. Chondroitin sulphate and heparan sulphate sulphation motifs and their proteoglycans are involved in articular cartilage formation during human foetal knee joint development. Histochem Cell Biol. 2012;138:461-75. [DOI] [PubMed] [Google Scholar]
- 23. Melrose J, Smith S, Cake M, Read R, Whitelock J. Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: an ageing study. Histochem Cell Biol. 2005;124:225-35. [DOI] [PubMed] [Google Scholar]
- 24. Hayes AJ, Tudor D, Nowell MA, Caterson B, Hughes CE. Chondroitin sulfate sulfation motifs as putative biomarkers for isolation of articular cartilage progenitor cells. J Histochem Cytochem. 2008;56:125-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-car-10.1177_19476035211000811 for Macromolecular Interactions in Cartilage Extracellular Matrix Vary According to the Cartilage Type and Location by Manula S. B. Rathnayake, Brooke L. Farrugia, Karyna Kulakova, Colet E. M. ter Voert, Gerjo J. V. M. van Osch and Kathryn S. Stok in CARTILAGE