Abstract
Background
High mobility group box 1 (HMGB1) is increased in osteoarthritis (OA) tissue and chondrocytes stimulated with interleukin-1β (IL-1β). Suppression of HMGB1 expression is correlated with reduced inflammatory responses induced by IL-1β. This study aimed to investigate how inhibition of HMGB1 by glycyrrhizin might affect inflammatory responses and viability of OA patient–derived chondrocytes treated with IL-1β.
Design
The amounts of HMGB1 in the cartilage tissue and synovial fluid in patients with OA were assessed by Western blot and enzyme-linked immunosorbent assay (ELISA). Chondrocytes were extracted from OA patients and maintained in culture. The impact of glycyrrhizin on IL-1β-induced cell toxicity and inflammatory mediators and cytokines, including prostaglandin E2 (PGE2), nitric oxide (NO), proinflammatory cytokines, and metalloproteases (MMPs), were assessed by ELISA, Western blot, quantitative real-time polymerase chain reaction, and the Griess reagent assay.
Results
We confirmed that HMGB1 was significantly upregulated in specimens acquired from patients with OA. HMGB1 inhibition by glycyrrhizin improved cell viability of chondrocytes treated with IL-1β. Glycyrrhizin suppressed IL-1β-induced upregulation of HMGB1 and inflammatory mediators and cytokines, including PGE2, NO, proinflammatory cytokines, and MMPs.
Conclusion
Our results indicate that glycyrrhizin may be a potential therapy for OA patients and these promising findings warrant further study for clinical application.
Keywords: osteoarthritis, high mobility group box 1, interleukin-1β, glycyrrhizin, inflammation
Introduction
Osteoarthritis (OA) is the main cause of disability in the elderly and affects millions of individuals around the world. OA is a disorder of joints associated with damage to the protective cartilage, leading to pain and limited mobility. 1 As a critical contributor to the pathogenesis of OA, inflammation is implicated in OA development and progression. Consequently, identification of pharmaceutical drugs targeting inflammation may be a potential OA therapy. 2
Multiple inflammatory modulators have been found to be abnormally expressed in OA tissue. Nitric oxide (NO) and prostaglandin E2 (PGE2) and production is elevated in cartilage tissue of patients with OA. 3 PGE2 is a key catabolic factor of OA 4 and has been shown to regulate proteoglycan degradation of OA cartilage. 5 NO is an important inflammatory mediator that is involved in many physiological and pathophysiological circumstances. NO is produced in large amounts by OA cartilage and the synovial fluid and serum of patients with OA have high levels of nitrite. NO promotes the activities of the metalloproteases (MMPs) that mediate cartilage degeneration and the degradation of extracellular matrix. 6 In fact, expressions of several MMP family members are elevated in osteoarthritis; these MMPs are involved in the degradation of components of the noncollagen matrix of the joint. 7
Proinflammatory cytokines are also implicated in OA pathogenesis. 8 Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) regulate articular cartilage matrix degradation. 8 Treatment of chondrocytes isolated from OA patients with IL-1β can induce inflammatory responses, including production of PGE2, NO, and MMPs, as such it has been used to study the molecular mechanism of OA and develop therapeutic strategies in vitro. 9
Accumulating evidence has suggested that high mobility group box 1 (HMGB1) is involved in the destruction of bone and cartilage of OA patients and is critically involved in OA pathogenesis. The level of HMGB1 is elevated in OA tissues and chondrocytes treated with IL-1β. 10 Reducing HMGB1 expression by overexpression of microRNA MIR-140-5p that targets HMGB1-inhibited apoptosis, MMP expression and inflammation in chondrocytes treated with 1β. 10
Glycyrrhizin is the main bioactive component of a widely used medicinal plant Glycyrrhiza glabra or licorice and has various pharmacological functions, including antiviral and anti-inflammatory functions. 11 In traditional medicine, glycyrrhizin has been used in the treatment of ulcer, jaundice, gastritis, and bronchitis while high doses of glycyrrhizin may cause toxicity such as pseudohyperaldesteronism. 12 Glycyrrhizin is an inhibitor of HMGB1 and has been shown to reduce HMGB1 secretion from RAW 264.7 cells activated by lipopolysaccharide. Glycyrrhizin inhibits the mitogenic and chemotactic activities of HMGB1. 13 We hypothesized that treatment of chondrocytes with glycyrrhizin might suppress IL-1β-induced inflammatory responses by inhibiting HMGB1. In the current study, we investigated the impact of HMGB1 inhibition by the pharmacological inhibitor glycyrrhizin in IL-1β-treated chondrocytes isolated from OA patients addressing the viability of the cells and inflammatory responses.
Materials and Methods
Subjects
This study included 40 patients with primary knee OA. Among the patients, 18 were female and 22 were male and the average age was 63.8 years. Additionally, 30 healthy individuals were also recruited. Among the healthy volunteers, 14 were female and 16 were male, and the average age were 61.3 years. Patients with autoimmune diseases, joint diseases, including polyarthritis and rheumatoid arthritis (RA), other postseptic or posttraumatic arthritis, development dysplasia, or skeletal dysplasia were excluded. Participants in the control group had no previous history of joint disease or arthritis. Normal articular cartilage specimens were acquired from individuals undergoing surgery due to fracture. Articular cartilage tissue and synovial fluid were collected from 30 randomly assigned OA patients and all 30 healthy individuals. The cartilage tissue from the remaining 10 OA patients was used for isolating chondrocytes. All the procedures were approved by the First Affiliated Hospital of Harbin Medical University. Written informed consents were obtained from all participants.
Chondrocyte Isolation and Culture
Chondrocytes were isolated from osteoarthritic patients according to a previously described procedure. 14 Briefly, cartilage tissue from OA patients was homogenized in bacterial collagenase and trypsin containing Hank’s medium at 37°C for 1 hour. Chondrocytes were released in DMEM (Dulbecco’s modified Eagle medium) containing bacterial collagenase and fetal bovine serum (FBS). Isolated cells were resuspended in freezing media (10% DMSO [dimethyl sulfoxide], 90% FBS) and frozen for subsequent experiments. Chondrocytes were cultured in DMEM containing penicillin/streptomycin, 2 mM glutamine, 10% FBS, and 1% vitamin supplements at 37°C in a humidified incubator with 5% CO2.
IL-1β and glycyrrhizin were added to culture media at indicated concentrations when the cells were 80% confluent and remained in the media for 24 hours. Glycyrrhizin was purchased from Sigma-Aldrich and the concentrations used in this study were 0.5 mM, 1 mM, 2 mM, 5 mM, and 10 mM according to a previous study. 15
Si-HMGB1 Transfection in Chondrocytes
To knock down HMGB1 in chondrocytes, cells were transfected with si-HMGB1 (GenePharma Co., Shanghai, China) using Lipofectamine 3000 (Life Technologies, Carlsbad, CA). To determine how HMGB1 knockdown affected IL-1β-induced inflammatory response, cells were transfected with si-HMGB1 first and were stimulated with IL-1β at 6 hours following transfection.
Cell Counting Kit-8 (CCK8) Assay
Viability of chondrocytes following treatments was determined by the CCK8 assay as described previously. 10 Briefly, after indicated stimulations, cells were treated with 10 μL/well CCK8 solution and incubated for 1 hour at 37°C in cell culture incubator. Cell viability of the cells was determined by the optical density at 450 nm.
Western Blot
The levels of HMGB1 in cartilage tissue obtained from the knee joints of the healthy controls and OA patients and in cultured chondrocytes were assessed as described previously. 14 Briefly, the cartilage tissue and chondrocytes were homogenized in lysis buffer containing protease inhibitors. An equivalent amount of proteins was subjected to electrophoresis. The proteins were transferred onto Hybond-P membranes, blocked with Tris-buffered saline (TBS) that was supplemented with 0.1% Tween 20 and 5% fat-free milk, incubated in anti-HMGB1 (1:2000, Abcam) antibody for overnight at 4°C, and in secondary antibodies after washing. The ECL detection kit (Amersham Pharmacia Biotech) was used for membrane development. Protein levels were presented as ratios with regard to the control group.
Enzyme-Linked Immunosorbent Assay
HMGB1 expression in synovial fluid of OA patients and control healthy individuals and isolated chondrocytes of the patients and PGE2, TNF-α, IL-6, and MMP (MMP-1, MMP-3 and MMP-13) concentrations in chondrocytes were assessed using relevant enzyme-linked immunosorbent assay (ELISA) kits (R&D systems, Minneapolis, MN).
Quantitative Real-Time Polymerase Chain Reaction
mRNA levels of relevant genes were assessed by quantitative real-time polymerase chain reaction (qRT-PCR) according to a previous procedure. 10 Total RNA was extracted using a RNeasy Mini kit (Qiagen). The concentration of RNA was measured by the NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA). cDNA was obtained by reverse transcription from 1 μg total RNA. qRT-PCR was completed using the Mini-Opticon qRT-PCR system and SYBR-Green RealMasterMix (Bio-Rad, Hercules, CA). Data were presented relative to controls. The primers used in this study include: TNF-α, forward 5′-ATCTTCTCGAACCCCGAGTGA-3′ and reverse 5′-CGGTTCAGCCACTGGAGCT-3′; IL-6, forward 5′-GGTACATCCTCGACGGCATCT-3′ and reverse 5′-GTGCCTCTTTGCTGCTTTCAC-3′; HMGB1, forward 5′-AAAGCGGACAAGGCCCGTTAT-3′ and reverse 5′-AAGAGGAAGAAGGCCGAAGGAG-3′; β-actin, forward 5′-GGACTTCGAGCAAGAGATGG-3′ and reverse 5′-AGCACTGTGTTGGCGTACAG-3′; MMP-1, forward 5′-CTGGCCACAACTGCCAAATG-3′ and reverse 5′-CTGTCCCTGAACAGCCCAGTACTTA-3′; MMP-3, forward 5′-ATTCCATGGAGCAGGCTTTC-3′ and reverse 5′-CATTTGGGTCAAACTCCAACTGTG-3′; and MMP-13, forward 5′-TCCCAGGAATTGGTGATAAAGTAGA-3′ and reverse 5′-CTGGCATGACGCGAACAATA-3′. β-actin was used as an internal control.
Griess Reagent Assay
Griess regent assay was performed to determine NO production in chondrocytes as described previously. 9 Briefly, the Griess reagent was incubated with culture media for 30min and the optical density at 540 nm was measured.
Statistical Analysis
Data were analyzed by SPSS software. Student t test, one-way analysis of variance analysis and Tukey’s post hoc test was performed for comparisons between groups. Data were presented as mean ± standard deviation (SD). P < 0.05 was considered as statistically significant. Each experiment was repeated for at least 3 times.
Results
Increased HMGB1 Expression in OA Patients
HMGB1 expression was first examined in OA patients. ELISA showed that HMGB1 levels in OA patients’ synovial fluid were significantly elevated compared with healthy individuals ( Fig. 1A ). To further confirm this finding, Western blot analysis of cartilage tissues was performed ( Fig. 1B ). Consistently, HMGB1 protein expression was substantially increased in OA patients compared with healthy individuals ( Fig. 1C ).
Figure 1.
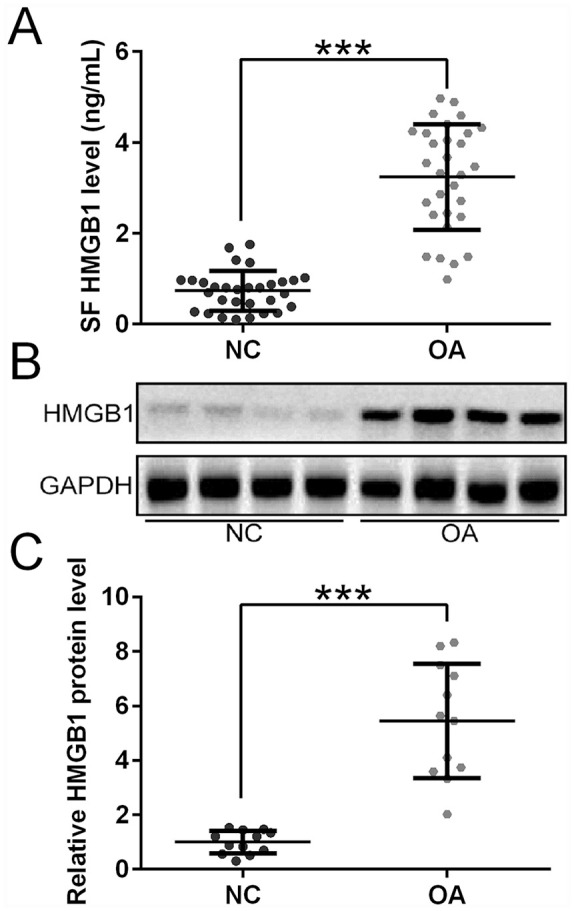
HMGB1 expression in healthy individuals (NC) and patients with osteoarthritis (OA). (A) Measurement of HMGB1 levels in synovial fluid of healthy controls (n = 30) and OA patients (n = 30) by ELISA. (B) Representative Western blots of HMGB1 expression in cartilage tissues from the knee joints of 1 healthy control and 1 OA patient. (C) Quantification of Western blots analysis showing HMGB1 expression in cartilage tissues from the knee joints of healthy controls (n = 12) and OA patients (n = 12). ***P < 0.001.
Glycyrrhizin Improved Chondrocyte Viability in the Presence of IL-1β
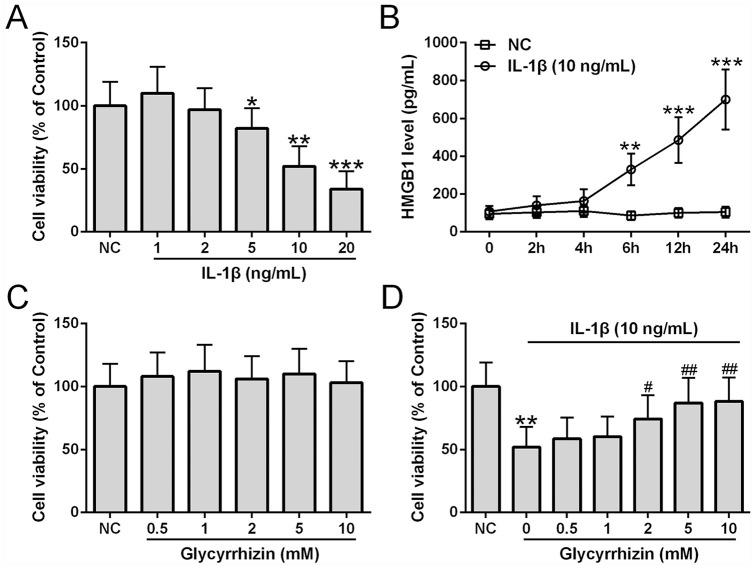
We examined the survival of chondrocytes treated with different doses of IL-1β and found that IL-1β dose-dependently reduced cell viability ( Fig. 2A ). The dose 10 ng/mL was used for subsequent experiments since this concentration not only induced significant viability reduction of chondrocytes isolated from OA patients, but it has also been shown to induce apoptosis in normal human chondrocytes in a previous study. 10 HMGB1 production was also measured from chondrocytes treated with 10 ng/mL IL-1β and found that IL-1β treatment led to an increased HMGB1 production over time ( Fig. 2B ). The effect of HMGB1 inhibition by glycyrrhizin on IL-1β-induced toxicity was assessed in chondrocytes. Glycyrrhizin did not significantly impact viability of untreated chondrocytes at all concentrations examined ( Fig. 2C ). Importantly, glycyrrhizin dose-dependently alleviated IL-1β-induced toxicity at the concentrations of 2 mM, 5 mM, and 10 mM completely suppressed IL-1β-induced cytotoxicity ( Fig. 2D ). Subsequently, glycyrrhizin at 5 mM was used.
Figure 2.
Cell viability of osteoarthritis (OA) patient chondrocytes after treatment with different concentrations of interleukin-1β (IL-1β) for 24 hours (A), n = 6. (B) ELISA was used to measure the HMGB1 levels in chondrocytes stimulated with IL-1β (10 ng/mL) over the time course, n = 6 for each time point. (C) Viability of chondrocytes after treatment with glycyrrhizin at indicated concentrations for 24 hours, n = 6. (D) Viability of chondrocytes after co-treatment of IL-1β (10 ng/mL) and glycyrrhizin at indicated concentrations for 24 hours (n = 6). All the experiments were repeated independently 3 times. #P < 0.05, ##P < 0.01 compared with IL-1β treatment. *P < 0.05, **P < 0.01, ***P < 0.001 compared with NC.
Glycyrrhizin Inhibited HMGB1 Expression in Chondrocytes Treated with IL-1β
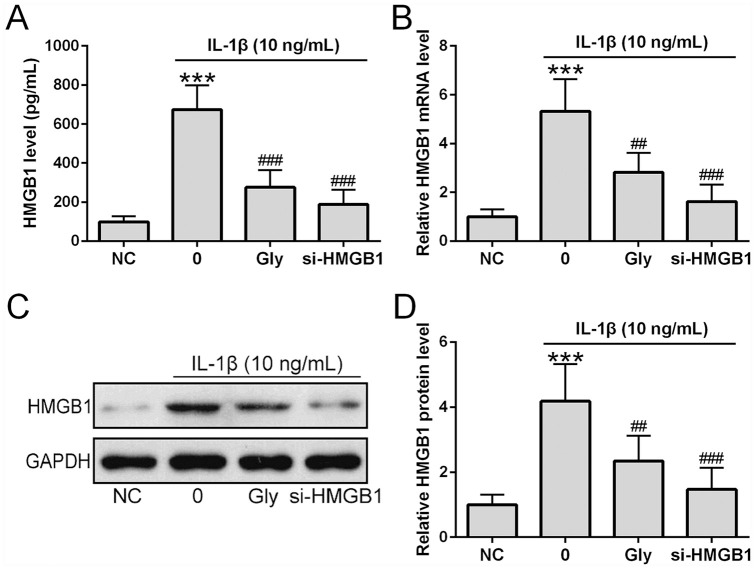
We then determined whether glycyrrhizin affected HMGB1 expression in chondrocytes derived from OA patients treated with IL-1β and compared the effects of the inhibitor with siRNA targeting HMGB1 (si-HMGB1). ELISA showed that both glycyrrhizin and si-HMGB1 significantly reduced HMGB1 levels in culture media of cells stimulated with IL-1β compared with those that were not treated with si-HMGB1 ( Fig. 3A ). qRT-PCR showed that both glycyrrhizin and si-HMGB1 significantly suppressed IL-1β-induced upregulation of HMGB1 mRNA expression ( Fig. 3B ). Consistently, Western blot analysis also showed that IL-1β increased levels of HMGB1 protein in chondrocytes was suppressed by glycyrrhizin and si-HMGB1 ( Fig. 3C and D ).
Figure 3.
Glycyrrhizin inhibited HMGB1 expression in interleukin-1β (IL-1β)-treated chondrocytes. si-HMGB1 was transfected into osteoarthritis patients’ chondrocytes 6 hours before the stimulation. ELISA was used to measure the concentration of HMGB1 from chondrocytes co-treated with 5 mM glycyrrhizin and IL-1β (10 ng/mL) for 24 hours (A) (n = 6). HMGB1 mRNA expression in chondrocytes co-treated with glycyrrhizin (5 mM) and IL-1β (10 ng/mL) for 24 hours (B) (n = 3). Examination of HMGB1 protein expression in chondrocytes co-treated with glycyrrhizin (5 mM) and IL-1β (10 ng/mL) for 24 hours using Western blot (C) with relative expression normalized to the untreated group (n = 3). All the experiments were repeated independently 3 times. ***P < 0.001 compared with normal controls (NC). ##P < 0.01, ###P < 0.001 compared with single IL-1β treatment.
Glycyrrhizin Suppressed IL-1β-Induced PGE2 and Nitrite Production
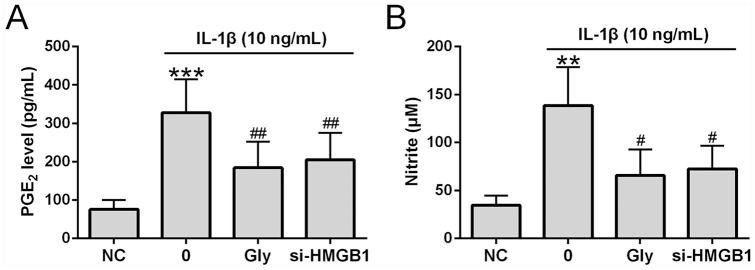
We determined how HMGB1 inhibition affected PEG2 and nitrite production. Similar to previous reports,3,16 IL-1β treatment in chondrocytes with significantly increased PGE2 and NO productions ( Fig. 4A and B ). Importantly, increased PGE2 and NO production was suppressed by glycyrrhizin and si-HMGB1.
Figure 4.
Glycyrrhizin suppressed nitrite and prostaglandin E2 (PGE2) production in chondrocytes. Cells were co-treated with glycyrrhizin (5 mM) and interleukin-1β (IL-1β; 10 ng/mL) for 24 hours. si-HMGB1 was transfected into osteoarthritis patients’ chondrocytes 6 hours before the stimulation. (A) PGE2 levels determined by ELISA (n = 6). (B) Nitrite production (n = 6). All the experiments were repeated independently 3 times. **P < 0.01, ***P < 0.001 compared with normal controls (NC). #P < 0.05, ##P < 0.01 compared with single IL-1β treatment.
Glycyrrhizin Suppressed IL-1β-Induced Inflammatory Responses in Chondrocytes
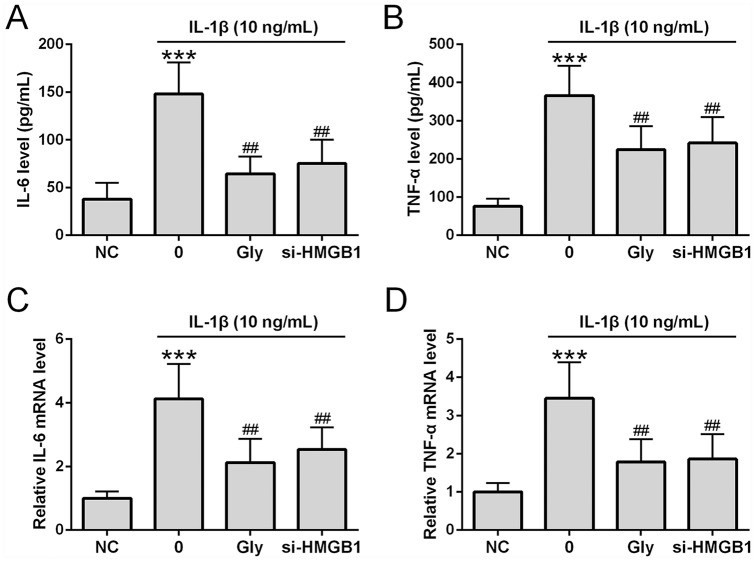
We next determined how HMGB1 inhibition affected IL-1β-induced inflammatory responses. ELISA showed that IL-1β significantly increased IL-6 ( Fig. 5A ) and TNF-α ( Fig. 5B ) release into the culture media. Similarly, qRT-PCR showed that IL-1β significantly increased IL-6 ( Fig. 5C ) and TNF-α ( Fig. 5D ) mRNA expression. Co-treatment of chondrocytes with IL-1β and glycyrrhizin or transfection of si-HMGB1 prior to IL-1β treatment significantly reduced the levels of TNF-α and IL-6 in the media as well as IL-6 and TNF-α mRNA expression in chondrocytes.
Figure 5.
Glycyrrhizin suppressed interleukin-1β (IL-1β)-induced proinflammatory cytokine production in treated chondrocytes. Chondrocytes were co-treated with glycyrrhizin (5 mM) and IL-1β (10 ng/mL) for 24 hours. si-HMGB1 was transfected into osteoarthritis patients’ chondrocytes 6 hours before the stimulation. Levels of IL-6 (A) and tumor necrosis factor-α (TNF-α) (B) were determined by ELISA (n = 6). IL-6 and TNF-α mRNA expression was assessed through qRT-PCR (C and D, respectively) (n = 3). All the experiments were repeated independently 3 times. ***P < 0.001 compared with NC, ##P < 0.01 compared with single IL-1β treatment.
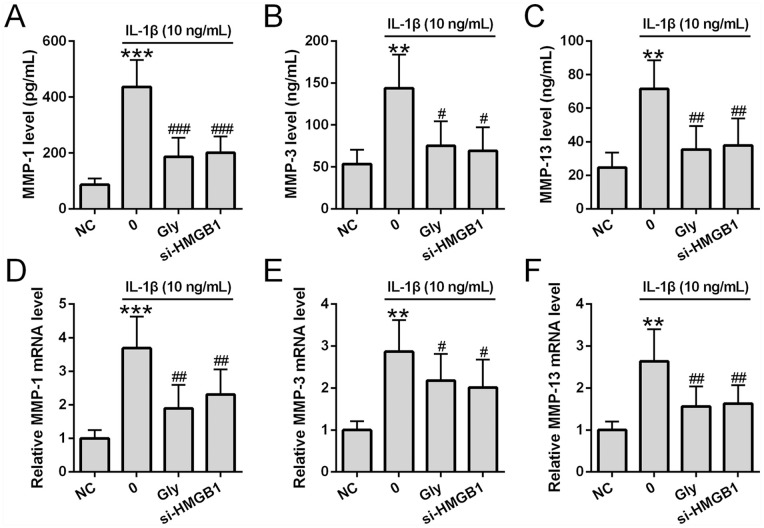
Glycyrrhizin Suppressed IL-1β Induced MMP Production in Chondrocytes
Finally, we investigated how glycyrrhizin affected IL-1β-mediated MMP production. ELISA analysis revealed that MMP-1 ( Fig. 6A ), MMP-3 ( Fig. 6B ), and MMP-13 ( Fig. 6C ) levels were significantly increased in culture media at 24 hours after IL-1β treatment. Similarly, qRT-PCR showed that MMP-1 ( Fig. 6D ), MMP-3 ( Fig. 6E ), and MMP-13 ( Fig. 6F ) mRNA expression was significantly increased in chondrocytes at 24 hours after IL-1β treatment. Importantly, both glycyrrhizin and si-HMGB1 showed similar suppression of IL-1β induced upregulation of MMP levels in cell culture media as well as MMP mRNA expression in chondrocytes.
Figure 6.
Glycyrrhizin suppressed interleukin-1β (IL-1β)-induced matrix metalloproteinase (MMP) expression in chondrocytes. Chondrocytes were co-treated with glycyrrhizin (5 mM) and IL-1β (10 ng/mL) for 24 hours. si-HMGB1 was transfected into chondrocytes 6 hours before the stimulation. MMP-1, MMP-3, and MMP-13 levels were determined by ELISA (A-C) (all n = 6). MMP-1, MMP-3, and MMP-13 mRNA expressions were assessed by qRT-PCR (D-F) (n = 3). All the experiments were also repeated independently 3 times. **P < 0.01, ***P < 0.001 compared with normal controls (NC), #P < 0.05, ##P < 0.01, ###P < 0.001 compared with single IL-1β treatment.
Discussion
OA is a devastating disorder leading to disability in affected individuals. In this study, by determining the effect of an inhibitor of HMGB1, glycyrrhizin, in IL-1β-treated chondrocytes isolated from OA patients, we investigated the potential of glycyrrhizin as a therapeutic strategy in an in vitro OA model. We confirmed that HMGB1 levels were significantly increased in the cartilage tissue and synovial fluid of patients with OA. Treatment of chondrocytes derived from OA tissue with IL-1β resulted in significant suppression of cell viability and elevation of HMGB1 expression levels. Glycyrrhizin improved survival of chondrocytes treated with IL-1β while not affecting the viability of untreated cells, and it suppressed HMGB1 levels released from and in IL-1β treated chondrocytes. We also determined the effects of glycyrrhizin on inflammatory mediators, proinflammatory cytokines and MMPs in IL-1β-treated chondrocytes. Our study showed that inhibition of HMGB1 suppressed PGE2 and nitrite production, reduced proinflammatory cytokine levels including TNF-α and IL-6 and suppressed MMP production.
The nuclear protein HMGB1 can be translocated to the cytoplasm and released from the cell on certain inflammatory stimulation. 17 As a later inflammation mediator, HMGB1 is involved in extended and persistent systemic inflammation in patients with rheumatoid arthritis. 18 Previous studies have shown that stimulation of osteoarthritic synoviocytes leads to release of HMGB1 and potentiates IL-1β-induced inflammation in OA. 19 Staining of OA tissue shows that HMGB1 expression pattern correlated with the severity of OA, with healthy looking cartilage being mainly HMGB1-negative and more severe OA lesions having more HMGB1 in the cytoplasm and pericellular matrix. 20 Similarly, HMGB1 levels in the synovial fluid are also positively correlated with the severity of knee OA. 21 Consistently, our study also showed that compared with healthy individuals, the cartilage tissue and synovial fluid obtained from patients with OA had significantly higher levels HMGB1.
The high expression of HMGB1 in patients with OA and the function of HMGB1 in potentiating inflammatory response have made HMGB1 an ideal target for the development of OA therapeutics. A previous study showed that an HMGB1 targeting microRNA MIR-140-5p in OA was reduced and restoration of this microRNA suppressed HMGB1 expression, which in turn suppressed HMGB1-mediated inflammatory consequences such as MMP expression, apoptosis, and inflammatory cytokines. 10 Efforts have also been devoted to the identification of HMGB1 antagonists, and the natural antiviral and anti-inflammatory tritepene, glycyrrhizin was found as an HMGB1 inhibitor that binds to the protein and suppresses the cytokine activities. 22 The anti-inflammatory effect of glycyrrhizin has been explored in several fields, such as the area of sepsis where HMGB1 is regarded as an important late stage mediator 15 and skin thermal injury where glycyrrhizin was shown to exert a protective effect on remote organs against thermal injury. 23 However, its potential efficacy in OA has not been investigated previously. Our study is the first to determine the impact of glycyrrhizin on IL-1β-mediated cytotoxicity in chondrocytes isolated from patients with OA.
Consistent with a previous study. 9 OA chondrocytes treated with IL-1β showed reduced survival in a cell viability assay, increased production of inflammatory mediators PGE2 and NO, elevated levels of pro-inflammatory cytokines and increased expression of MMPs. Glycyrrhizin at a concentration between 0.5 mM and 10 mM did not change the viability of chondrocytes that were not treated by IL-1β, suggesting that the HMGB1 is not cytotoxic, although a previous study showed reduced survival of RAW264.7 cells when the cells were treated with 10 mM glycyrrhizin. 15 Importantly, our study showed that glycyrrhizin dose-dependently alleviated IL-1β induced cytotoxicity and 5 mM and 10 mM glycyrrhizin completely rescued cell viability in the presence of IL-1β.
Since HMGB1 is a major mediator of inflammation in OA, we explored the anti-inflammatory effect of glycyrrhizin in IL-1β-treated chondrocytes. PGE2 is a major inflammation regulator in OA and reduction of PGE2 production by cyclooxygenase-2 inhibitors has resulted in diminished inflammation. 24 Similarly, NO is also increased in patients with OA and excessive NO production suppresses synthesis of cartilage matrix, leading to its degradation. 3 Our results revealed that IL-1β lead to elevated production of PGE2 and nitrite, which was suppressed by glycyrrhizin treatment in chondrocytes. Additionally, we also showed that glycyrrhizin suppressed IL-1β induced over production of pro-inflammatory cytokines and MMPs. Since MMPs and the pro-inflammatory cytokines are involved in the degradation of OA cartilage tissue, our findings suggest glycyrrhizin may protect cartilage tissue from degradation.
To further confirm that glycyrrhizin mediated inhibition of inflammatory responses in IL-1β-treated chondrocytes was due to HMGB1 but not due to some nonspecific effect. We simultaneously studied the effect of si-HMGB1 in chondrocytes treated with IL-1β. Our study showed that the anti-inflammatory role of glycyrrhizin was recapitulated by si-HMGB1-mediated knocking down of HMGB1 protein, suggesting that the effects were specific to HMGB1 instead of off target events. These findings are consistent with the suppression of HMGB1 by microRNA MIR-140-5p, showing that microRNA mediated reduction of HMGB1 alleviated IL-1β-induced apoptosis and inflammation in chondrocytes.
Conclusions
In summary, our study showed that specific inhibition of HMGB1 in IL-1β-activated chondrocytes derived from OA patients improved cell viability and suppressed inflammatory responses by reducing PGE2, NO, proinflammatory cytokines and MMPs, suggesting that glycyrrhizin is a potential pharmacological treatment of OA. Our promising findings warrant further investigation of the clinical application of glycyrrhizin in OA patients.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All the procedures were approved by the First Affiliated Hospital of Harbin Medical University.
Informed Consent: Written informed consents were obtained from all participants.
Trial Registration: Not applicable.
ORCID iD: Limin Hou  https://orcid.org/0000-0002-6927-4996
https://orcid.org/0000-0002-6927-4996
References
- 1. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745-59. [DOI] [PubMed] [Google Scholar]
- 2. Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amin AR, Dave M, Attur M, Abramson SB. COX-2, NO, and cartilage damage and repair. Curr Rheumatol Rep. 2000;2:447-53. [DOI] [PubMed] [Google Scholar]
- 4. Gosset M, Berenbaum F, Levy A, Pigenet A, Thirion S, Cavadias S, et al. Mechanical stress and prostaglandin E2 synthesis in cartilage. Biorheology. 2008;45:301-20. [PubMed] [Google Scholar]
- 5. Hardy MM, Seibert K, Manning PT, Currie MG, Woerner BM, Edwards D, et al. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002;46:1789-803. [DOI] [PubMed] [Google Scholar]
- 6. Mehana EE, Khafaga AF, El-Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019;234:116786. [DOI] [PubMed] [Google Scholar]
- 7. Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529-43. [DOI] [PubMed] [Google Scholar]
- 8. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33-42. [DOI] [PubMed] [Google Scholar]
- 9. Wang C, Zeng L, Zhang T, Liu J, Wang W. Tenuigenin prevents IL-1β-induced inflammation in human osteoarthritis chondrocytes by suppressing PI3K/AKT/NF-κB signaling pathway. Inflammation. 2016;39:807-12. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Shen S, Li Z, Li W, Weng X. MIR-140-5p affects chondrocyte proliferation, apoptosis, and inflammation by targeting HMGB1 in osteoarthritis. Inflamm Res. 2020;69:63-73. [DOI] [PubMed] [Google Scholar]
- 11. Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBPP. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother Res. 2018;32:2323-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Batiha GE, Beshbishy AM, El-Mleeh A, Abdel-Daim MM, Devkota HP. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules. 2020;10:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li J, Shi J, Sun Y, Zheng F. Glycyrrhizin, a potential drug for autoimmune encephalomyelitis by inhibiting high-mobility group box 1. DNA Cell Biol. 2018;37:941-6. [DOI] [PubMed] [Google Scholar]
- 14. Sartori-Cintra AR, de Mara CS, Argolo DL, Coimbra IB. Regulation of hypoxia-inducible factor-1alpha (HIF-1α) expression by interleukin-1beta (IL-1β), insulin-like growth factors I (IGF-I) and II (IGF-II) in human osteoarthritic chondrocytes. Clinics (Sao Paulo). 2012;67:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim YM, Kim HJ, Chang KC. Glycyrrhizin reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and endotoxemic mice by p38/Nrf2-dependent induction of HO-1. Int Immunopharmacol. 2015;26:112-8. [DOI] [PubMed] [Google Scholar]
- 16. Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang H, Wang H, Chavan SS, Andersson U. High mobility group box protein 1 (HMGB1): the prototypical endogenous danger molecule. Mol Med. 2015;21(Suppl 1):S6-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taniguchi N, Kawakami Y, Maruyama I, Lotz M. HMGB proteins and arthritis. Hum Cell. 2018;31:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia-Arnandis I, Guillen MI, Gomar F, Pelletier JP, Martel-Pelletier J, Alcaraz MJ. High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1β in osteoarthritic synoviocytes. Arthritis Res Ther. 2010;12:R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heinola T, Kouri VP, Clarijs P, Ciferska H, Sukura A, Salo J, et al. High mobility group box-1 (HMGB-1) in osteoarthritic cartilage. Clin Exp Rheumatol. 2010;28:511-8. [PubMed] [Google Scholar]
- 21. Li ZC, Cheng GQ, Hu KZ, Li MQ, Zang WP, Dong YQ, et al. Correlation of synovial fluid HMGB-1 levels with radiographic severity of knee osteoarthritis. Clin Invest Med. 2011;34:E298. [DOI] [PubMed] [Google Scholar]
- 22. Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14:431-41. [DOI] [PubMed] [Google Scholar]
- 23. Shen L, Cui Z, Lin Y, Wang S, Zheng D, Tan Q. Anti-inflammative effect of glycyrrhizin on rat thermal injury via inhibition of high-mobility group box 1 protein. Burns. 2015;41:372-8. [DOI] [PubMed] [Google Scholar]
- 24. Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol. 2006;119:229-40. [DOI] [PubMed] [Google Scholar]