Abstract
Objective
This study aimed to clarify physiological reloading on disuse atrophy of the articular cartilage and bone in the rat knee using the hindlimb suspension model.
Design
Thirty male rats were divided into 3 experimental groups: control group, hindlimb suspension group, and reloading after hindlimb suspension group. Histological changes in the articular cartilage and bone of the tibia were evaluated by histomorphometrical and immunohistochemical analyses at 2 and 4 weeks after reloading.
Results
The thinning and loss of matrix staining in the articular cartilage and the decrease in bone volume induced by hindlimb suspension recovered to the same level as the control group after 2 weeks of reloading. The proportion of the noncalcified and calcified layers of the articular cartilage and the thinning of subchondral bone recovered to the same level as the control group after 4 weeks of reloading.
Conclusions
Disuse atrophy of the articular cartilage and bone induced by hindlimb suspension in the tibia of rats was improved by physiological reloading.
Keywords: reloading, disuse atrophy, cartilage atrophy, articular cartilage, hindlimb suspension
Introduction
Physiological joint loading is one of the essential factors for maintenance of the functional integrity and metabolism of the articular cartilage.1-3 The articular cartilage has a high sensitivity to these mechanical signals and adaptive response to these stimuli, such as dynamic compression, fluid shear, tissue shear, and hydrostatic pressure.3-5 This functional process is referred to as mechanotransduction and chondrocytes quantitatively regulate the rate of matrix synthesis and degradation and change the composition of the extracellular matrix. 3 With moderate loading, the articular cartilage thickens as the surface area increases, and the matrix content increases.5-8 Conversely, many basic studies in animals and humans reported that in the absence of load, the articular cartilage undergoes histological changes, such as thinning, decreased matrix staining, and subchondral bone expansion.1,2,5,6,9-15 Vincent et al. proposed such histological changes due to lack of mechanical stress, such as loading, as cartilage atrophy. 2 In clinical practice, many patients may be affected by articular cartilage atrophy because they often require bed rest, which is temporarily associated with treatment. 11 Our previous study also reported that unloading conditions for 4 weeks by hindlimb suspension caused significant thinning and decreased matrix staining of the articular cartilage in the tibia of the knee using the rat model.16,17 Moreover, in the animal study, disuse atrophy of articular cartilage is a pathological condition that can lead to early-onset and severe osteoarthritis (OA). 17 The incidence of OA is increasing worldwide, making it a major medical and economic problem.18-20 Therefore, the treatment of disuse atrophy of the cartilage is an important issue to be clarified in orthopedics, rehabilitation medicine, and physical therapy.
In the skeletal muscle, it is widely known that inactivity causes disuse atrophy21-23 and that reactivation improves atrophy.24-26 In contrast, there are no previous studies on its treatment and recovery, and the treatment and recovery processes for disuse atrophy of the articular cartilage are unclear. Owing to the absence of blood vessels and dependence on nutrition from the synovial fluid, it is traditionally believed that the articular cartilage has a poor repair capacity.4,5 For example, in both full- and partial-thickness articular cartilage defects, it is considered impossible for the hyaline cartilage to repair the defect spontaneously once it is lost. 27 However, we speculated that disuse atrophy of the articular cartilage could also be recovered by reloading. As a rationale, cartilage thickness changes depending on the load, and some studies showed that it increased with repeated exercise.7,28 Moreover, previous studies have reported that proteoglycan metabolism is relatively fast (half-life, 8 days) and that proteoglycan synthesis increases after injury.29,30 Moreover, our previous study provided potential data to suggest that the thinning of the articular cartilage and loss of matrix staining caused by unloading can be restored by reloading. 17
Based on the abovementioned information, this study aimed to clarify the effect of reloading on disuse cartilage atrophy induced by unloading conditions using a rat hindlimb suspension model. In addition to the articular cartilage, the bone was included in the analysis object because the unloading condition causes histological changes in the subchondral bone, and there is a functional relationship between cartilage, subchondral bone, and bone in response to loading.
Methods
Experimental Animals and Animal Care
The study protocol was approved by the Animal Research Committee of the Graduate School of Medicine of Kanazawa University (Kanazawa, Japan; approval no. 204125) and conducted in accordance with the ARRIVE guidelines31,32 and Guidelines for the Care and Use of Laboratory Animals of Kanazawa University.
Thirty male Wistar rats (8 weeks old) were purchased from Japan SLC (Shizuoka, Japan) and housed under normal conditions for 5 weeks before the start of the experiments to acclimatize the animals to their new environment. One rat was housed per cage in a sanitary ventilated room under controlled temperature and humidity conditions and a 12-hour light–dark cycle with ad libitum access to food and water. The experimental animals were monitored 2 to 3 times per week to control their health status, including general food and water intake, surgical wound condition, gait, and hindlimb suspension. The experimenter cleaned the cages once or twice every 2 weeks to keep the breeding environment clean.
The rats were divided into 3 groups: control group (Con, n = 15, 5 rats each for 4, 6, and 8 weeks), hindlimb suspension group (HS, n = 5), and reloading after hindlimb suspension group (RL, n = 10, 5 rats each for 6 and 8 weeks). In the Con group, the rats were kept in a physiological environment. In the HS and RL groups, the rats were subjected to tail suspension for 4 weeks. Rats subjected to hindlimb suspension were allowed to walk freely in their cages using only their forelimbs. In the HS group, after the hindlimb suspension period, the rats were sacrificed and used to identify the histological changes in disuse of the cartilage due to the unloading environment and confirm the histological condition before reloading in RL group. In the RL group, after hindlimb suspension period, the rats were removed from the device on hindlimb suspension and allowed to walk freely in a physiological environment using all limbs for 2 or 4 weeks.
After starting the experiment, no further interventions, including range of motion exercise, were performed during the experimental period, and no analgesics or anti-inflammatory drugs were administered.
Hindlimb Suspension
In the HS and RL groups, the rats were subjected to tail suspension throughout the experiment. Hindlimb tail suspension was performed according to our previous study.16,17,33,34 Briefly, under inhalation anesthesia with isoflurane, the tail of each rat was disinfected. Then, a sterile steel wire was used to drill into the proximal coccyx in which the wire remained and was shaped into a ring. Moreover, the tail ring was connected with another wire to a track hung above the cage, thereby enabling the animals to move freely on their forelimbs in the cage.
Histological Preparation
As described previously,16,17,34,35 decalcified paraffin sections were prepared for histology. Both knees were excised frontally to evaluate the histological changes in the medial tibiofemoral joints. The right knee was used for histomorphometric analysis, and the left knee was used for immunohistochemical analysis. The specimens were serially sliced to observe the region where the articular cartilage of the femur and tibia could be in direct contact without meniscus involvement. The paraffin sections (3-µm thickness) were stained with hematoxylin–eosin and toluidine blue (0.05%, 15 min) as general staining and with immunohistochemical staining. Finally, the sections were viewed under a light microscope and imaged using a digital camera (BX51 and DP74; Olympus Corporation, Tokyo, Japan) to evaluate the histological changes in the articular cartilage.
Histomorphometrical Analyses of the Articular Cartilage
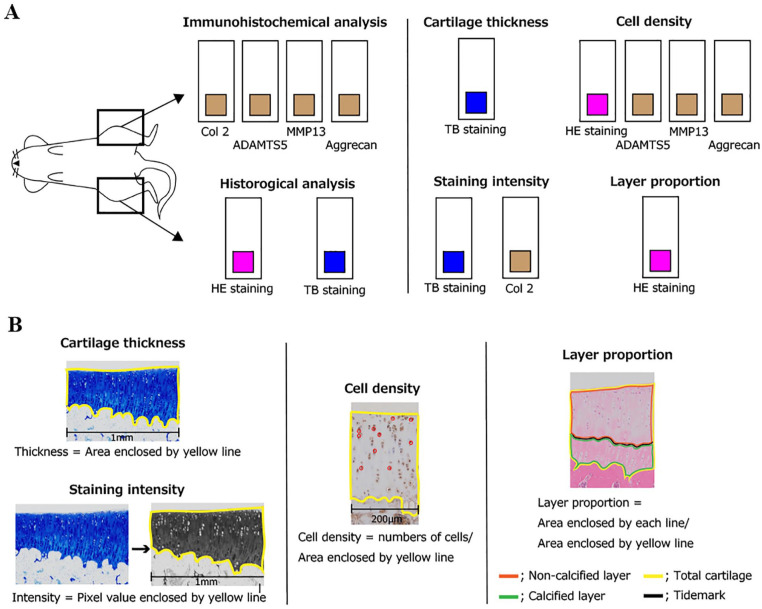
The protocol for histomorphometrical analyses is shown in Figure 1 . As described previously,9,16,34-37 Adobe Photoshop CC imaging software (Adobe Systems, Inc., San Jose, CA, USA) was used to perform histomorphometrical analyses of the articular cartilage to evaluate the following 3 parameters: cartilage thickness, intensity of matrix staining with toluidine blue, and chondrocyte density.
Figure 1.
Schematic of histological analysis for articular cartilage: (A) staining method and analysis parameters and (B) overview of each analysis method. The right knee was used for histological analysis, and the left knee was used in the immunohistochemical analysis. For each staining, cartilage thickness, cell density, staining intensity, and layer proportion were evaluated. For details on these parameters, please refer to the text for evaluation methods. ADAMTS-5 = A disintegrin and metalloproteinase with thrombospondin motifs 5.
HE, hematoxylin–eosin; TB, toluidine blue.
To evaluate the total cartilage thickness, digitized images of the sections stained with toluidine blue were used. Cartilage thickness was defined as the distance between the cartilage surface and osteochondral junction. We used the measured area of the cartilage with a width of 1 mm at the center of the lesion at the tibia in the medial tibiofemoral joint. Moreover, using the images stained with hematoxylin–eosin, the proportion of the noncalcified and calcified layers was evaluated using the tidemark as the indicator. Specifically, with a width of 200 µm, these proportions were calculated by each area of the noncalcified and calcified layers by dividing the total area of the articular cartilage.
To evaluate matrix intensity, digitized images of cartilage stained with toluidine blue were converted to grayscale (white, 255; black, 0) to assess the relative intensity of toluidine blue staining. The average staining intensity was calculated at the same area in the same manner as performed for the measurement of articular cartilage thickness.
To evaluate chondrocyte density, digitized images of the sections stained with hematoxylin–eosin were used. Chondrocyte density was determined as the number of chondrocytes per unit area of cartilage. This unit area was calculated using the same method as that for the abovementioned cartilage thickness, and the width to be measured was set to 200 µm. Chondrocytes with visible nuclei within the area of interest were counted manually.
Histomorphometrical Analyses of the Bone
Bone volume and thickness of the subchondral bone were measured at the tibial medial condyle according to the method of Nagira et al. 38 ( Fig. 2 ). This is a highly reliable measurement method that shows a high correlation with the results from microcomputed tomography. 38 Bone volume (%) was defined as follows: (area of the cancellous bone − area of the bone marrow; trabecular bone)/area of cancellous bone × 100. Subchondral bone thickness was defined as the region between the osteochondral junction and bone marrow cavity.
Figure 2.
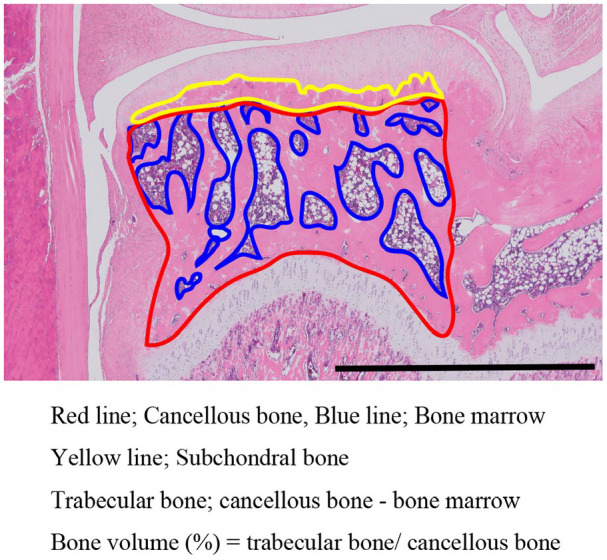
Definition and calculation of bone volume and subchondral bone thickness. The area enclosed by the red line shows the area of the cancellous bone, and the area enclosed by the blue line shows the area of the bone marrow. The area of the cancellous bone minus the area of the bone marrow is the area of the trabecular bone. The area enclosed by the yellow line shows the area of the subchondral bone. The thickness of the subchondral bone was calculated by dividing the area of the subchondral bone by its width. Scale bar = 2 mm.
Immunohistochemical Staining and Analyses
Paraffin sections were immunohistochemically stained using Aggrecan antibody (diluted 1:50, 13880-1-AP; Proteintech, Tokyo, Japan) and type-II collagen (diluted 1:50, ab34712; Abcam, Tokyo, Japan) as parameter of cartilage synthesis and matrix metalloproteinase 13 (MMP 13; diluted 1:50, 18165-1-AP; Proteintech Tokyo, Japan) and A disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5; diluted 1:50, ab41037; Abcam, Tokyo, Japan) as a parameter of cartilage degradation. Moreover, 0.01 mol/L phosphate-buffered saline (PBS, pH 7.4) was used for the washes (5 min for 3 times) performed between each step. After deparaffined by xylenes and dehydrated by alcohols, the sections (3-µm thickness) were antigen activated by heat treatment using hot water bath (citrate buffer, pH 6.0, 70°C, 90 min) for Aggrecan, MMP 13, and ADAMTS-5 and by proteinase K (S3020, 10 min; Dako Japan, Tokyo, Japan) for type-II collagen. After inactivated endogenous peroxidase using methanol with 3% H2O2 for 20 min and blocked nonspecific binding of immunoglobulins using Protein Block Serum-Free (X0909; Dako Japan, Tokyo, Japan) for 15 min, the sections were incubated overnight with the primary antibody at 4°C. Then, sections were rinsed in PBS, and a subsequent reaction was made by the polymer technique using Histofine Simple Stain Rat MAX PO (MULTI) (414191, Nichirei Biosciences Inc., Tokyo, Japan) for 1 h at room temperature. Immunoreactivity was visualized using Histofine Simple Stain MAX PO (MULTI) (725191, Nichirei Biosciences Inc., Tokyo, Japan) for 5 min at room temperature. The sections were counterstained with hematoxylin for 1 min.
The protocol for immunohistochemical analyses is shown in Figure 1 . In this analysis, the staining intensity was calculated for type-II collagen in the same way as histomorphometrical analyses described above. For Aggrecan, MMP13, and ADAMTS-5, the staining positive cells were counted, and the positive cell density was calculated in the same way as histomorphometrical analyses described above.
Statistical Analyses
All statistical analyses were performed using JMP 14 software (SAS Institute, Cary, NC, USA). All data were statistically analyzed as parametric data. The sample size was 5 for each group. Descriptive statistics were calculated as mean with standard deviation for body weight, histomorphometrical data, and immunohistochemical data. All continuous data were assessed for homoscedasticity using the Levene test. To evaluate differences between the Con and HS groups and the Con and RL groups at the same experimental period, unpaired t-test was used. Welch correction was applied for variables with unequal variance.
A P value < .05 indicated statistical significance for all analyses; exact P values are shown in the figures.
In this study, the total number of groups is 6, and a large number of experimental animals are needed to perform multiple comparisons. For the statistical analysis in this study, we selected unpaired t-test and Welch correction between the same experimental period to reduce the number of animals used, based on the 3Rs principle of animal experiment (Reduction, Replacement, and Refinement) and calculated the number of required experimental animals using G*Power and performed the statistical analysis with the minimum number of experimental animals. Sample-size calculation was performed using the sample size and power tool in G*Power 3.1 (free; available at https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower.html),39,40 based on the pilot experimental data of the main parameter, cartilage thickness, and matrix intensity at 4 weeks, including the first 5 rats, in the Con and HS groups. For these parameters, a minimum of 3 and 5 were required in the Con and HS groups, respectively, with a power of .80 and significance level of P < .05. Similarly, sample sizes were calculated for cartilage thickness and matrix intensity obtained from 5 rats in the HS group and 5 rats in the RL group at 2 weeks in the preliminary experiments. As a result, a minimum of 3 rats were required in the HS and RL groups, respectively, with a power of .80 and significance level of .05. Therefore, we set the sample size to 5 rats per group.
Results
General Condition
No experimental animals died by accident or other unexpected causes for the experimental periods. Moreover, no rats developed infections in their tail wounds. Thus, all experimental animals reached the planned experimental period safely, without adverse events. Please refer to supporting information ( S1 Table . Body weight) for data on weight changes in the rats during the experiment.
Histomorphometrical Analyses for the Articular Cartilage
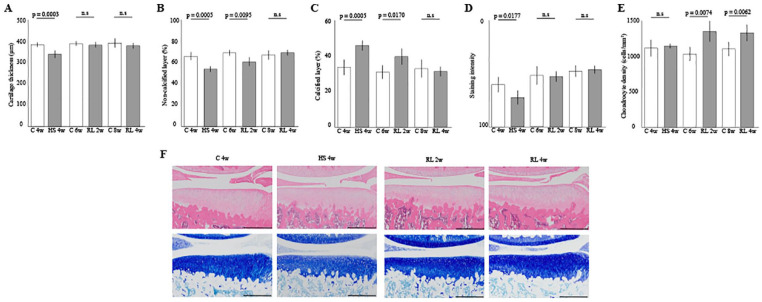
In total cartilage thickness, thickness was decreased for the hindlimb suspension for 4 weeks ( Fig. 3A , Con 4w vs HS 4w, P = .0003). However, the thinning cartilage recovered to the same level as that of the Con group after 2 weeks of reloading ( Fig. 3A , Con 6w vs RL 2w, n.s.). The proportion of the noncalcified layer was significantly decreased for 4 weeks of hindlimb suspension ( Fig. 3B , Con 4w vs HS 4w, P = .0005). The proportion showed an increasing trend after 2 weeks of reloading and recovered to the same level as that of the Con group after 4 weeks of reloading ( Fig. 3B , Con 8w vs RL 4w, n.s.). Similarly, the proportion of the calcified layer was significantly increased for 4 weeks of hindlimb suspension ( Fig. 3C , Con 4w vs HS 4w, P = .0005). The proportion showed a decreasing trend after 2 weeks of reloading and recovered to the same level as that of the Con group after 4 weeks of reloading ( Fig. 3C , Con 8w vs RL 4w, n.s.).
Figure 3.
Histomorphometrical effect of the reloading on cartilage atrophy in the medial tibia: (A) cartilage thickness, (B) and (C) the percentage of the noncalcified and calcified layers in the articular cartilage, (D) matrix intensity of staining by toluidine blue, (E) chondrocyte density, and (F) representative histological findings. Scale bar = 500 µm. The thinning of the articular cartilage and decreases of staining intensity caused by disuse cartilage atrophy was restored by reloading for 2 weeks. Moreover, 4 weeks of reloading was required to restore the proportion of noncalcified and calcified layers. The density of chondrocytes was not affected by disuse atrophy, and significant increase in cell density was observed with reloading. HS = hindlimb suspension; RL = reloading.
In the matrix intensity by toluidine blue staining, the intensity was decreased for the hindlimb suspension for 4 weeks ( Fig. 3D , Con 4w vs HS 4w, P = .0177). After 2 weeks of reloading, the intensity recovered to the same level as that of the Con group ( Fig. 3D , Con 6w vs RL 2w, n.s.).
In chondrocyte density, no significant changes in the cell density were observed after 4 weeks of hindlimb suspension ( Fig. 3E , Con 4w vs HS 4w, n.s). At 2 and 4 weeks after reloading, the density increased significantly ( Fig. 3D , Con 6w vs RL 2w; P = .0074, Con 8w vs RL 4w; P = .0062).
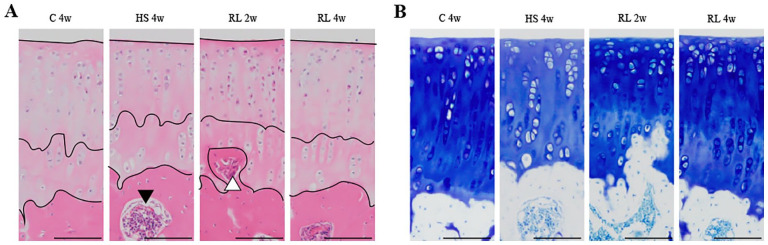
Overviews of the articular cartilage of the tibia are shown in Figure 3F , and high magnification images are shown in Figure 4 . For detailed data on histomorphometrical result in the articular cartilage, please refer to supporting information ( S2 Table . Cartilage results).
Figure 4.
High magnification of representative histological finding of the tibia cartilage. Unloading and reloading did not cause any damage or fibrosis to the articular cartilage surface, which remained smooth and intact (A and B). An island of the bone marrow tissue (black triangle) is observed in the subchondral bone (A). Vascular invasion into the subchondral bone is suggested. Bone tissue invading the subchondral bone into the calcified layer (white triangle) is observed (B). Ossification of cartilage tissue is suggested. Scale bar = 100 µm. HS = hindlimb suspension; RL = reloading.
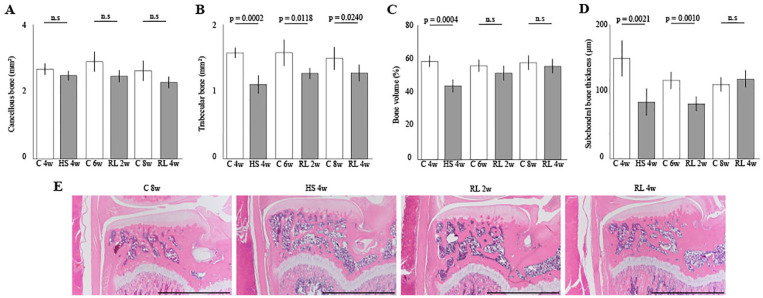
Histomorphometrical Analyses of the Bone
In the area of the cancellous bone, no significant difference was observed in the entire experimental period ( Fig. 5A ). In the area of the trabecular bone, the area in the Con group was significantly wider than that in the HS and RL groups in the entire experimental period ( Fig. 5B ). The bone volume was decreased for the hindlimb suspension for 4 weeks ( Fig. 5C , Con 4w vs HS 4w, P = .0004). However, the decreased area recovered to the same level as the Con group after 2 weeks of reloading ( Fig. 5C , Con 6w vs RL 2w, n.s.).
Figure 5.
Histomorphometrical effect of the reloading on the bone atrophy in medial tibia: (A and B) area of the cancellous and trabecular bones, (C) percentage of bone volume, (D) thickness of subchondral bone, and (E) representative histological findings. Scale bar = 2 mm. The cancellous bone was not affected by unloading or reloading, and trabecular bone was significantly decreased by unloading and showed an increasing trend with reloading. As a result, the bone volume was significantly reduced by unloading and recovered to the same level as that of the Con group after 2 weeks of reloading. Similarly, the thickness of the subchondral bone was also significantly reduced by unloading, but it took 4 weeks to recover to the level of the Con group. HS = hindlimb suspension; RL = reloading.
In the thickness of the subchondral bone, after 4 weeks of hindlimb suspension, the thickness was significantly reduced and remained significantly reduced at 2 weeks of reloading ( Fig. 5D , Con 4w vs HS 4w; P = .0021, Con 6w vs RL 2w; P = .0010). However, the decreased thickness recovered to the same level as that of the Con group after 4 weeks of reloading ( Fig. 5D , Con 8w vs RL 4w, n.s.).
Overviews of the bone and subchondral bone of the tibia are shown in Figure 5E . For detailed data on histomorphometrical result in the bone and subchondral bone, please refer to supporting information ( S3 Table . Bone results).
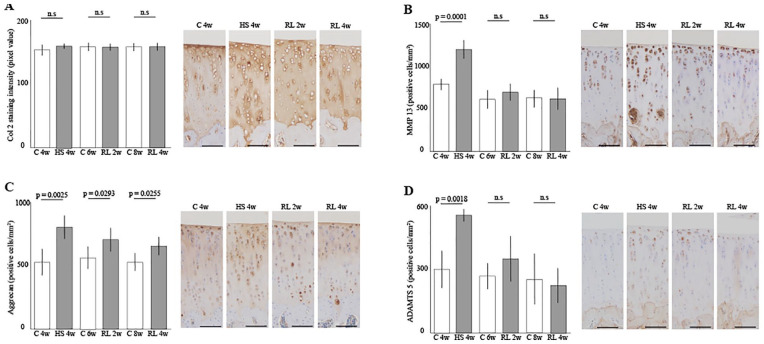
Immunohistochemical Analyses of the Articular Cartilage
The resulting graph and histology are shown in Figure 6 . The staining intensity of type-II collagen was not significantly changed by unloading and reloading ( Fig. 6A ). MP13-positive cell density showed a significant increase with unloading for 4 weeks but was comparable to that of the control group after 2 and 4 weeks of reloading ( Fig. 6B , Con 4w vs HS 4w; P = .0001). Aggrecan-positive cell density was significantly higher than the control group at all unloading and reloading times ( Fig. 6C , Con 4w vs HS 4w; P = .0025, Con 6w vs RL 2w; P = .0293, Con 8w vs RL 4w; P = .0255). ADAMTS-5-positive cell density showed a significant increase with unloading for 4 weeks but was comparable to that of the control group after 2 and 4 weeks of reloading ( Fig. 6B , Con 4w vs HS 4w; P = .0018). For detailed data on immunohistochemical result in the articular cartilage, please refer to supporting information ( S4 Table . IHS results).
Figure 6.
Immunohistochemical effect of the reloading on the cartilage atrophy in the medial tibia: (A) Staining intensity of type-II collagen, (B) MMP13-positive cell density, (C) Aggrecan-positive cell density, And (D) ADAMTS-5-positive cell density. The staining intensity of the collagen did not change significantly during the experiment. MMP13 and ADAMTS-5-positive cell densities were significantly increased by 4 weeks of unloading but returned to a steady state after 2 weeks of reloading. Aggrecan-positive cell density was significantly increased throughout the experiment. Scale bar = 100 µm. ADAMTS-5 = A disintegrin and metalloproteinase with thrombospondin motifs 5; HS = hindlimb suspension; RL = reloading.
Discussion
This study aimed to examine the effect of reloading on the recovery of articular cartilage and bone from disuse atrophy caused by the unloading environment for 4 weeks in the rat knee joint. As a result, 2 weeks was required for recovery of cartilage thinning and decrease of matrix staining, and 4 weeks was required for recovery of the proportion of cartilage layers. In the bone, recovery of the bone mass required 2 weeks, and recovery of subchondral bone thickness required 4 weeks. With regard to the bone, unloading causes bone loss, and reloading improves temporary osteoporosis.14,41 However, in the articular cartilage, there have been no reports of such atrophy and recovery by reloading. Although it was previously recognized that the repair capacity of the articular cartilage, especially in traumatic cartilage injury, is poor, the results of this study clearly show that it has sufficient recovery capacity by reloading for disuse atrophy. This new finding is novel and informative and could have an impact on medicine in articular cartilage. Moreover, it has been reported that the articular cartilage becomes thinner and softer due to the unloading environment, making it more susceptible to damage, and that disuse atrophy of the articular cartilage is a factor in the development and severity of OA. 17 Furthermore, Haapala et al. explained the importance of prevention of articular cartilage atrophy after joint immobilization, since it did not recover sufficiently by remobilization. 42 Therefore, the need for and importance of prevention and treatment of disuse atrophy of the articular cartilage will increase in clinical practice in the future.
In this study, 3 interesting histological results were obtained. First, reloading increased the density of chondrocytes. In this study and previous studies,9,34 disuse atrophy of the articular cartilage results in thinning with no change in density. Thus, the actual number of chondrocytes decreases. We speculate that reloading may have increased the density of chondrocytes due to the need for matrix production to recover atrophic cartilage. This is because Lotz et al. reported that chondrocyte density defines not only the cartilage volume but also its composition and repair capacity, 43 and Eckstein et al. reported that exercise load level correlates with cartilage volume and that articular cartilage has a functional adaptive capacity to change its structure depending on the specific environment. 5
Second, the result on the ratio of the layers of articular cartilage is shown. In the previous studies9,14 and the present study, when the articular cartilage atrophied, the ratio of the noncalcified layer decreased, and the ratio of the calcified layer increased. The noncalcified layer is responsible for friction reduction in the superficial layer and shock absorption of load in the deep layer.4,43-45 We speculate that the unloading environment may have reduced the need for these functions, resulting in a selective decrease of noncalcified layer. Moreover, 4 weeks of reloading restored this ratio to the same level as that in the control group, which may be one reason for this speculation.
Third, the immunohistochemical results indicate a balance between matrix synthesis and destruction. The number of matrix disrupting MMP13- and ADAMTS-5-positive cells increased in the unloading environment and returned to a steady state upon reloading at 2 weeks. Conversely, the collagen did not change significantly with or without loading, while Aggrecan increased significantly throughout the experiment with unloading and reloading. Thus, although MMP13 and collagen and Aggrecan and ADAMTS-5 were paired for synthesis and destruction,6,44,46 they showed different changes, suggesting that the activation of Aggrecan and collagen by reloading may be through different pathways. The different degradation rates, synthesis rates, and half-lives of collagen and Aggrecan may also have an effect. Furthermore, it may be related to the fact that both chondrocyte density and number of Aggrecan-positive cells remain significantly increased after reloading, despite the recovery of cartilage thickness and percentage of layers.
The histological results of this study revealed that disuse atrophy of the articular cartilage can be recovered by reloading. However, the histological changes of this disuse atrophy are minor compared with those of traumatic cartilage damage, OA, and joint contracture,47-49 and the results of this study do not suggest that articular cartilage may recover from these conditions with severe histological changes. Moreover, the animals used in this study were rats, which are rodents. Generally, it has been reported that small animals and humans differ in metabolism, maturation age, thickness, chondrocyte density, and layer proportions.50-53 Therefore, further research is required to apply the recovery of reloading against disuse atrophy of the articular cartilage to humans.
There are 2 major limitations in this study. First, the sample size may be relatively small. We used G*Power to calculate the required sample size to study the statistical size (power of .80 and significance level of P < .05). Then, we used the unpaired t-test and performed statistical analysis. However, multiple comparisons may be more appropriate to account for within- and between-group errors. If multiple comparisons were used, a larger sample size would be required because of the large number of groups to be compared, 6 in our protocol. Second, we have not been able to evaluate the mechanical stresses occurring in the knee joints of rats during the experiment. Although hindlimb suspension can achieve an unloading condition of the knee joint, it does not limit joint movements, such as flexion and extension of the knee joint. It has been reported that such joint motion without pressure from loading promotes repair of the articular cartilage in traumatic cartilage damage and OA.54,55 Therefore, in this study, it is possible that joint motion during the non-loading period had some effect on the recovery of the articular cartilage during reloading.
In conclusion, disuse atrophy of the articular cartilage and bone caused by unloading can be recovered quantitatively and qualitatively after 4 weeks of reloading. This is a useful finding with many implications for basic and clinical medicine and may modify disease and treatment concepts in articular cartilage. Further studies are needed to analyze whether active exercise therapy can further accelerate recovery and whether intermittent loading during unloading periods can prevent disuse atrophy of the articular cartilage.
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_19476035211063857 for Physiological Reloading Recovers Histologically Disuse Atrophy of the Articular Cartilage and Bone by Hindlimb Suspension in Rat Knee Joint by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Supplemental material, sj-docx-2-car-10.1177_19476035211063857 for Physiological Reloading Recovers Histologically Disuse Atrophy of the Articular Cartilage and Bone by Hindlimb Suspension in Rat Knee Joint by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Supplemental material, sj-docx-3-car-10.1177_19476035211063857 for Physiological Reloading Recovers Histologically Disuse Atrophy of the Articular Cartilage and Bone by Hindlimb Suspension in Rat Knee Joint by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Supplemental material, sj-docx-4-car-10.1177_19476035211063857 for Physiological Reloading Recovers Histologically Disuse Atrophy of the Articular Cartilage and Bone by Hindlimb Suspension in Rat Knee Joint by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Footnotes
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
Authors’ Note: The authors alone are responsible for the content and writing of the paper.
Acknowledgments and Funding: The authors thank the members of the Department of Human Pathology at the Kanazawa University Graduate School of Medicine for offering advice regarding the histopathological techniques and performing the immunohistochemical staining.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a JSPS KAKENHI grant-in-aid for Early Career Scientists (grant no: 20K19444).
Author Contributions: All of the authors made substantial contributions to (1) the conception and design of the study, data acquisition, and analysis and interpretation of the data; (2) drafting the article or critically revising it for important intellectual content; and (3) providing final approval of the submitted version of the manuscript.
The authors’ specific contributions were as follows:
(1) Conception and design of the study: IT, TM, HK, and MH
(2) Analysis and interpretation of the data: IT, TM, HK, and MH
(3) Drafting of the article: IT, HK, and MH
(4) Critical revision of the article for important intellectual content: IT, HK, and MH
(5) Final approval of the article: IT, TM, HK, and MH
(6) Obtaining funding: IT, TM, HK, and MH
(7) Collection and assembly of the data: IT, TM, and MH
Ikufumi Takahashi (t_ikuhumi@med.kanazawa-u.ac.jp) is responsible for the integrity of the work as a whole from inception to the finished article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by our institutional review board.
ORCID iD: Ikufumi Takahashi  https://orcid.org/0000-0003-1924-3101
https://orcid.org/0000-0003-1924-3101
References
- 1. Bader DL, Salter DM, Chowdhury TT. Biomechanical influence of cartilage homeostasis in health and disease. Arthritis. 2011;2011:979032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vincent TL, Wann AKT. Mechanoadaptation: articular cartilage through thick and thin. J Physiol. 2019;597(5):1271-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu BY, Jin Y, Ma XH, Wang CY, Guo Y, Zhou D. The potential role of mechanically sensitive ion channels in the physiology, injury, and repair of articular cartilage. J Orthop Surg. 2020;28(3):1-8. [DOI] [PubMed] [Google Scholar]
- 4. Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77-95. [DOI] [PubMed] [Google Scholar]
- 5. Eckstein F, Hudelmaier M, Putz R. The effects of exercise on human articular cartilage. J Anat. 2006;208(4):491-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jørgensen AEM, Kjær M, Heinemeier KM. The effect of aging and mechanical loading on the metabolism of articular cartilage. J Rheumatol. 2017;44(4):410-7. [DOI] [PubMed] [Google Scholar]
- 7. Hamann N, Zaucke F, Heilig J, Oberlander KD, Bruggemann GP, Niehoff A. Effect of different running modes on the morphological, biochemical, and mechanical properties of articular cartilage. Scand J Med Sci Sports. 2014;24(1):179-88. [DOI] [PubMed] [Google Scholar]
- 8. Ni GX, Liu SY, Lei L, Li Z, Zhou YZ, Zhan LQ. Intensity-dependent effect of treadmill running on knee articular cartilage in a rat model. Biomed Res Int. 2013;2013:172392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nomura M, Sakitani N, Iwasawa H, Kohara Y, Takano S, Wakimoto Y, et al. Thinning of articular cartilage after joint unloading or immobilization. An experimental investigation of the pathogenesis in mice. Osteoarthritis Cartilage. 2017; 25(5):727-36. [DOI] [PubMed] [Google Scholar]
- 10. Benichou C, Wirotius JM. Articular cartilage atrophy in lower limb amputees. Arthritis Rheum. 1982;25(1):80-2. [DOI] [PubMed] [Google Scholar]
- 11. Vanwanseele B, Eckstein F, Knecht H, Stussi E, Spaepen A. Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthritis Rheum. 2002;46(8):2073-8. [DOI] [PubMed] [Google Scholar]
- 12. Moriyama H, Yoshimura O, Kawamata S, Takayanagi K, Kurose T, Kubota A, et al. Alteration in articular cartilage of rat knee joints after spinal cord injury. Osteoarthritis Cartilage. 2008;16(3):392-8. [DOI] [PubMed] [Google Scholar]
- 13. O’Connor KM. Unweighting accelerates tidemark advancement in articular cartilage at the knee joint of rats. J Bone Miner Res. 1997;12(4):580-9. [DOI] [PubMed] [Google Scholar]
- 14. Tomiya M, Fujikawa K, Ichimura S, Kikuchi T, Yoshihara Y, Nemoto K. Skeletal unloading induces a full-thickness patellar cartilage defect with increase of urinary collagen II CTx degradation marker in growing rats. Bone. 2009;44(2_suppl):295-305. [DOI] [PubMed] [Google Scholar]
- 15. Luan HQ, Sun LW, Huang YF, Wu X, Niu H, Liu H, et al. Use of micro-computed tomography to evaluate the effects of exercise on preventing the degeneration of articular cartilage in tail-suspended rats. Life Sci Space Res. 2015;6:15-20. [DOI] [PubMed] [Google Scholar]
- 16. Takahashi I, Matsuzaki T, Kuroki H, Hoso M. Disuse histological changes of an unloading environment on joint components in rat knee joints. Osteoarthr Cartil Open. 2019;1: 100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takahashi I, Matsuzaki T, Kuroki H, Hoso M. Disuse atrophy of articular cartilage induced by unloading condition accelerates histological progression of osteoarthritis in a post-traumatic rat model. Cartilage. Epub 2020 Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rahmati M, Nalesso G, Mobasheri A, Mozafari M. Aging and osteoarthritis: central role of the extracellular matrix. Ageing Res Rev. 2017;40:20-30. [DOI] [PubMed] [Google Scholar]
- 19. Tsezou A. Osteoarthritis year in review 2014: genetics and genomics. Osteoarthritis Cartilage. 2014;22(12):2017-24. [DOI] [PubMed] [Google Scholar]
- 20. Salmon JH, Rat AC, Achit H, Ngueyon-Sime W, Gard C, Guillemin F, et al. Health resource use and costs of symptomatic knee and/or hip osteoarthritis. Osteoarthritis Cartilage. 2019;27:1011-7. [DOI] [PubMed] [Google Scholar]
- 21. Yamazaki T, Haida N, Tachino K. Effects of weight bearing intervals on disuse atrophy of rats soleus muscle. J Jpn Phys Ther Assoc. 1998;1:19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamazaki T. Influence of hindlimb unweighting and intermittent weight bearing on dynamics of nuclei in rat soleus muscle. J Jpn Phys Ther Assoc. 2003;6:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamazaki T, Haida N, Tachino K. Influence of the time when weight bearing is started on disuse atrophy in rat soleus muscle. J Jpn Phys Ther Assoc. 2001;4(1):13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Wang X, Feng W. Reloading promotes recovery of disuse muscle loss by inhibiting TGFbeta pathway activation in rats after hind limb suspension. Am J Phys Med Rehabil. 2017;96:430-7. [DOI] [PubMed] [Google Scholar]
- 25. Shimkus KL, Shirazi-Fard Y, Wiggs MP, Ullah ST, Pohlenz C, Gatlin DM, et al. Responses of skeletal muscle size and anabolism are reproducible with multiple periods of unloading/reloading. J Appl Physiol. 2018;125:1456-67. [DOI] [PubMed] [Google Scholar]
- 26. Figueiredo VC, D’Souza RF, Van Pelt DW, Lawrence MM, Zeng N, Markworth JF, et al. Ribosome biogenesis and degradation regulate translational capacity during muscle disuse and reloading. J Cachexia Sarcopenia Muscle. 2021;12(1):130-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hunziker EB, Lippuner K, Keel MJ, Shintani N. An educational review of cartilage repair: precepts & practice—myths & misconceptions—progress & prospects. Osteoarthritis Cartil. 2015;23:334-50. [DOI] [PubMed] [Google Scholar]
- 28. Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52(11):3507-14. [DOI] [PubMed] [Google Scholar]
- 29. Mankin HJ, Lippiello L. The turnover of adult rabbit articular cartilage. J Bone Joint Surg Am. 1969;51(8):1591-600. [PubMed] [Google Scholar]
- 30. Meachim G. The effect of scarification on articular cartilage in the rabbit. J Bone Joint Surg Am. 1963;45-B:150-61. [Google Scholar]
- 31. Percie du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18(7) e3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takahashi I, Matsuzaki T, Yoshida S, Kitade I, Hoso M. Differences in cartilage repair between loading and unloading environments in the rat knee. J Jpn Phys Ther Assoc. 2014;17(1):22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takahashi I, Takeda K, Matsuzaki T, Kuroki H, Hoso M. Reduction of knee joint load suppresses cartilage degeneration, osteophyte formation, and synovitis in early-stage osteoarthritis using a post-traumatic rat model. PLoS One. 2021;16(7):e0254383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takahashi I, Matsuzaki T, Kuroki H, Hoso M. Joint unloading inhibits articular cartilage degeneration in knee joints of a monosodium iodoacetate-induced rat model of osteoarthritis. Osteoarthritis Cartilage. 2019;27(7):1084-93. [DOI] [PubMed] [Google Scholar]
- 36. Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24-34. [DOI] [PubMed] [Google Scholar]
- 37. Iijima H, Aoyama T, Ito A, Tajino J, Nagai M, Zhang X, et al. Immature articular cartilage and subchondral bone covered by menisci are potentially susceptive to mechanical load. BMC Musculoskelet Disord. 2014;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nagira K, Ikuta Y, Shinohara M, Sanada Y, Omoto T, Kanaya H, et al. Histological scoring system for subchondral bone changes in murine models of joint aging and osteoarthritis. Sci Rep. 2020;10:10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2_suppl):175-91. [DOI] [PubMed] [Google Scholar]
- 41. Basso N, Heersche JN. Effects of hind limb unloading and reloading on nitric oxide synthase expression and apoptosis of osteocytes and chondrocytes. Bone. 2006;39(4):807-14. [DOI] [PubMed] [Google Scholar]
- 42. Haapala J, Arokoski J, Pirttimäki J, Lyyra T, Jurvelin J, Tammi M, et al. Incomplete restoration of immobilization induced softening of young beagle knee articular cartilage after 50-week remobilization. Int J Sports Med. 2000;21(1): 76-81. [DOI] [PubMed] [Google Scholar]
- 43. Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51(2_suppl):241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11(3):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eschweiler J, Horn N, Rath B, Betsch M, Baroncini A, Tingart M, et al. The biomechanics of cartilage-an overview. Life. 2021;11:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rezus E, Burlui A, Cardoneanu A, Macovei LA, Tamba BI, Rezus C. From pathogenesis to therapy in knee osteoarthritis: bench-to-bedside. Int J Mol Sci. 2021;22:2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier J-P, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartil. 2006;14:13-29. [DOI] [PubMed] [Google Scholar]
- 48. Watanabe M, Campbell TM, Reilly K, Uhthoff HK, Laneuville O, Trudel G. Bone replaces unloaded articular cartilage during knee immobilization. A longitudinal study in the rat. Bone. 2021;142:115694. [DOI] [PubMed] [Google Scholar]
- 49. Varlet PM, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair. J Bone Joint Surg Am. 2003;85:45-57. [PubMed] [Google Scholar]
- 50. McCoy AM. Animal models of osteoarthritis: comparisons and key considerations. Vet Pathol. 2015;52(5):803-18. [DOI] [PubMed] [Google Scholar]
- 51. Poole R, Blake S, Buschmann M, Goldring S, Laverty S, Lockwood S, et al. Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthritis Cartilage. 2010;18(Suppl 3):S10-6. [DOI] [PubMed] [Google Scholar]
- 52. Aigner T, Cook JL, Gerwin N, Glasson SS, Laverty S, Little CB, et al. Histopathology atlas of animal model systems—overview of guiding principles. Osteoarthritis Cartilage. 2010;18(Suppl 3):S2-6. [DOI] [PubMed] [Google Scholar]
- 53. van der Kraan PM. Factors that influence outcome in experimental osteoarthritis. Osteoarthritis Cartilage. 2017;25(3):369-75. [DOI] [PubMed] [Google Scholar]
- 54. van Valburg AA, van Roy HL, Lafeber FP, Bijlsma JW. Beneficial effects of intermittent fluid pressure of low physiological magnitude on cartilage and inflammation in osteoarthritis. An in vitro study. J Rheumatol. 1998;25(3):515-20. [PubMed] [Google Scholar]
- 55. Salter RB, Simmonds DF, Malcolm BW, Rumble EJ, MacMichael D, Clements ND. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1980;62(8):1232-51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_19476035211063857 for Physiological Reloading Recovers Histologically Disuse Atrophy of the Articular Cartilage and Bone by Hindlimb Suspension in Rat Knee Joint by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Supplemental material, sj-docx-2-car-10.1177_19476035211063857 for Physiological Reloading Recovers Histologically Disuse Atrophy of the Articular Cartilage and Bone by Hindlimb Suspension in Rat Knee Joint by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Supplemental material, sj-docx-3-car-10.1177_19476035211063857 for Physiological Reloading Recovers Histologically Disuse Atrophy of the Articular Cartilage and Bone by Hindlimb Suspension in Rat Knee Joint by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE
Supplemental material, sj-docx-4-car-10.1177_19476035211063857 for Physiological Reloading Recovers Histologically Disuse Atrophy of the Articular Cartilage and Bone by Hindlimb Suspension in Rat Knee Joint by Ikufumi Takahashi, Taro Matsuzaki, Hiroshi Kuroki and Masahiro Hoso in CARTILAGE