Abstract
Objective
This study evaluated the effects of mesenchymal stem cell-extracellular vesicles (MSC-EVs) on chondrocyte proliferation in vitro and on cartilage repair in vivo following bone marrow stimulation (BMS) of focal chondral defects of the knee.
Methods
Six adult Göttingen minipigs received 2 chondral defects in each knee. The pigs were randomized to treatment with either BMS combined with MSC-EVs or BMS combined with phosphate-buffered saline (PBS). Intraarticular injections MSC-EVs or PBS were performed immediately after closure of the surgical incisions, and at 2 and 4 weeks postoperatively. Repair was evaluated after 6 months with gross examination, histology, histomorphometry, immunohistochemistry, and micro-computed tomography (µCT) analysis of the trabecular bone beneath the defect.
Results
Defects treated with MSC-EVs had more bone in the cartilage defect area than the PBS-treated defects (7.9% vs. 1.5%, P = 0.02). Less than 1% of the repair tissue in both groups was hyaline cartilage. International Cartilage and Joint Preservation Society II histological scoring showed that defects treated with MSC-EVs scored lower on “matrix staining” (20.8 vs. 50.0, P = 0.03), “cell morphology” (35.4 vs. 53.8, P = 0.04), and “overall assessment” (30.8 vs. 52.9, P = 0.03). Consistently, defects treated with MSC-EVs had lower collagen II and higher collagen I areal deposition. Defects treated with MSC-EVs had subchondral bone with significantly higher tissue mineral densities than PBS-treated defects (860 mg HA/cm3 vs. 838 mg HA/cm3, P = 0.02).
Conclusion
Intraarticular injections of MSC-EVs in conjunction with BMS led to osseous ingrowth that impaired optimal cartilage repair, while enhancing subchondral bone healing.
Keywords: articular cartilage, cartilage repair, knee, bone marrow stimulation, mesenchymal stem cells
Introduction
Mesenchymal stromal/stem cells (MSCs) are presently the most tested cell type in regenerative medicine against several injuries and diseases (https://www.clinicaltrials.gov/), including cartilage injuries and osteoarthritis (OA).1-3 A recent comparative study of implantation of MSCs and autologous chondrocyte implantation (ACI) reported equivalent clinical outcomes after 10 years, supporting the safety and efficacy of MSCs for use in cartilage repair. 4 However, as with all cell-based therapies, there are logistical and operational costs and challenges in maintaining the cell viability and potency from cell harvest and expansion to storage and delivery to patient.
In recent years, the therapeutic mechanisms of MSCs in tissue repair have been increasingly attributed to their secretion of trophic factors, particularly extracellular vesicles (EVs), which include exosomes. 5 Based on the current state-of-the-art in EV preparation, the isolation of different EV types based on their biogenesis is not possible due to overlapping size range and the lack of specific markers. Consequently, current EV preparations are heterogeneous with different EV types. We previously established that our MSC-EV preparation contains at least 3 EV types, including exosomes derived from endosomes. 6 In view that current EV preparations are heterogeneous, we use the term “MSC-EVs” that is synonymous with MSC exosomes as per recent recommendations.5,7 We have previously performed detailed analyses by mass spectrometry, antibody array, and microarray that revealed MSC-EVs having a complex cargo of nucleic acids, proteins, and lipids, with >850 proteins 8 and >150 miRNAs. 9 These EV proteins and miRNAs are functionally complex and are implicated in many diverse biochemical and cellular processes such as communication, structure and mechanics, inflammation, EV biogenesis, tissue repair and regeneration, and metabolism. 10
To date, MSC-derived EVs including exosomes have been reported to protect against myocardial ischemia reperfusion injury, 11 promote recovery of function after muscle injury, 12 enhance wound healing,13,14 promote hepatic regeneration, 15 ameliorate graft-versus-host-disease, 16 and more recently improve cartilage regeneration.17-19 Several animal studies, including ours, have demonstrated that MSC-EVs can repair osteochondral defects and alleviate OA pain and degeneration, as recently reviewed. 20 However, these studies were mainly performed in rodents and the efficacy of MSC-EVs for cartilage repair remains to be demonstrated in a large animal model relevant for clinical translation.
Bone marrow stimulation (BMS) techniques, such as microfracture, is a first-line treatment option for symptomatic lesions of the knee articular cartilage (<2-3cm2), because of its technical ease and cost-effectiveness. 21 With these techniques, perforations of the subchondral bone allow for migration of endogenous MSCs from the subchondral bone marrow cavity into the cartilage defect site. However, many studies have shown that the repair tissue is predominantly inferior fibrocartilage and deterioration of the clinical outcomes were reported as early as 2 years posttreatment.22,23 In efforts to improve BMS in cartilage repair, numerous adjuvants to BMS have been intensively studied over the last decade. These adjuvants include cells (i.e., MSCs), 24 biologics (i.e., platelet derivatives),25-27 and biomaterials (i.e., collagen or chitosan scaffolds)28-31 have been developed and tested. Indeed, some studies have demonstrated improved cartilage repair with the use of scaffolds as adjuvant following BMS.29,32-40 To date, the use of MSC-EVs as adjuvant to BMS in cartilage repair has yet to be explored.
The purpose of our study was to test the potential of MSC-EVs to enhance cartilage repair following BMS in a minipig model. The biological activity of MSC-EVs was assessed by in vitro chondrocyte proliferation prior to in vivo animal testing. We hypothesized that intraarticular injections of MSC-EVs could augment BMS in cartilage and subchondral bone repair, compared with BMS alone.
Methods
Preparation of MSC-EVs
Isolation and characterization of MSC-EVs were performed as previously described.11,41 Briefly, immortalized E1-MYC 16.3 human embryonic stem cell–derived MSCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, GE Healthcare) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA). 42 To obtain the EVs, cells were grown in a chemically defined medium for 3 days and the conditioned medium was harvested as previously described. 41 The conditioned medium was cleared of cell debris, fractionated, and concentrated 50× by tangential flow filtration using a membrane with a molecular weight cutoff of 100 kDa (Sartorius, Göttingen, Germany). The EVs were assayed for protein concentration using a NanoOrange Protein Quantification Kit (Thermo Fisher Scientific). Every batch of EV preparation was ascertained to have similarly sized particles of 50 to 200 nm and presence of exosome-associated markers including ALIX, TSG101, and CD81. 11 The MSC-EVs were 0.22-µm filtered and stored at −20 °C until use.
Human Chondrocyte Isolation and Culture
Human chondrocytes were obtained from nonarthritic knees as previously described. 43 Cartilage biopsies were collected from the intercondylar groove in the distal femur of 6 healthy patients (mean age: 26.3 years; male: 4; female: 2) undergoing anterior cruciate ligament reconstruction. The biopsies were collected after obtaining the patients’ written consent. The protocol was approved by the local ethics committee under the Danish National Committee on Health Research Ethics (#M-2008-008).
The biopsies were transported to the laboratory in phosphate-buffered saline (PBS) with 10% FBS and 1% penicillin/streptomycin (PS). Each biopsy was cut into smaller pieces followed by digestion with 0.1% collagenase II (Thermo Fisher Scientific) for 18 to 20 hours at 37 °C. The cells were washed in DMEM/F-12 with Glutamax (Sigma-Aldrich, Darmstadt, Germany) and seeded in a 25 cm2 culture dish using standard culture medium composed of DMEM-low glucose (LG), 10% FBS, 4 mM L-glutamine, and 1% PS. Upon confluence, the cells were trypsinized and cryopreserved in standard culture medium supplemented with 5% dimethyl sulfoxide (DMSO). For subsequent experiments, the cryopreserved cells were thawed and seeded at 10,000 cells/cm2 in standard culture medium. Medium change was performed every 3 to 4 days. Upon confluence, the cells were trypsinized and further subcultured to passage 2 cells for experiments.
Chondrocyte Proliferation
Chondrocytes were seeded with 5,000 cells/well in DMEM-LG supplemented with 10% FBS, 4 mM L-glutamine, and 1% PS in 96-well plates and incubated for 5 hours. The medium was then replaced with low serum medium composed of DMEM-LG supplemented with 0.5% FBS, 4 mM L-glutamine, and 1% PS, and the plates were incubated for 19 hours. Subsequently, the medium was replaced with low serum medium containing 1, 5, or 10 µg/mL of MSC-EVs or vehicle control (PBS). After 2, 24, 48, and 72 hours, cell metabolic activity was assessed using Cell Counting Kit-8 (CCK-8) assay kit (Sigma-Aldrich) following the manufacturer’s instructions. Briefly, 10 µL of the CCK-8 solution was added to each well and the cells were incubated for 2 hours. The amount of formazan dye generated by metabolically active cells was measured at 450 nm with a Victor 3 Multilabel Counter (Perkin Elmer, Shelton, CT). Total DNA content that is reflective of cell number was also measured. 44 Briefly, 50 µL CelLytic M Cell Lysis Reagent (Sigma-Aldrich) was added to each well and the DNA concentration was measured using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Fisher Scientific, Roskilde, Denmark) according to manufacturer’s instructions. The fluorescence was measured at excitation 480 nm and emission 520 nm using Victor 3 Multilabel Counter (Perkin Elmer). Microscopic images were taken for cells treated 10 µg/mL of MSC-EVs or PBS after 72 hours using a Zeiss Axio Imager M2 microscope (Carl Zeiss Microscopy GmbH, Germany).
Animals and Surgical Procedure
The study was conducted according to the Danish Law on Animal Experimentation and approved by the Danish Ministry of Justice Ethical Committee (J.nr. 2017-15-0201-01343). Six skeletally mature male Göttingen minipigs (mean body weight 36.0 kg, range 34-38.5 kg; age 24.2 months, range 23.7-24.9 months) were used in the study.
The Göttingen minipig chondral defect model has previously been described by our group.29,45-47 Briefly, after premedication with Zoletil mix 1 mL/10 kg (tiletamin 2.5 mg/mL, zolazepam 2.5 mg/mL, torbugesic 0.5 mg/mL, ketaminol 2.5 mg/mL, and rompun 2.5 mg/mL; Virbac, Denmark), general anesthesia and local analgesia were achieved with Etomidate (Hypnomidate, 0.25 mL/kg; Janssen Pharmaceuticals), sevoflurane (3%; AbbVie, Denmark), fentanyl (0.175 mL/kg/h; Hameln Pharmaceuticals), and lidocaine (Xylocaine 10 mL, 20 mg/mL; Astra Zeneca, Denmark). Preoperatively, prophylactic antibiotics were administered (penicillin procaine, 0.03 mL/kg; Ceva Sante Animale, France).
The knee joint was accessed through the patellar ligament and the trochlea was exposed. Using a skin biopsy punch and a curette, 2 chondral defects (ø = 6 mm) were created in the trochlea of each knee. The defect size of 6 mm is known to be a critical size defect in Göttingen minipigs. 48 One defect was made in the distal, medial trochlea, and another was made in the lateral trochlea, 0.5 to 1 cm proximal to the first defect. The defects were debrided using a curette, and the calcified cartilage layer carefully removed without damaging the subchondral bone. For bone marrow stimulation (BMS), 4 holes (depth 5 mm, diameter 1 mm) were drilled into the subchondral bone, and bleeding from the bone marrow was observed.
After BMS, the patella ligament, subcutaneous tissue, and skin were sutured. The pig was then randomized to receive intraarticular injections of either 1 mg MSC-EVs in 1 mL PBS or 1 mL PBS. Each pig received the same treatment in both knees to avoid possible systemic effects of MSC-EVs on the repair tissue in the contralateral knee. Injections were performed immediately after closure of the surgical incisions, and at 2 and 4 weeks postoperatively. Ultrasound-guided injections were performed at 2 and 4 weeks postsurgery.
Postoperatively, the animals were given subcutaneous lidocaine for pain management. Finadyne 5% (Flunixin meglumin, 1.1 mg/kg, oral paste, Intervet, Denmark) was given for 5 days. Immediate weight-bearing and full-range of motion was allowed postoperatively. Trained animal keepers, supervised by a veterinarian, closely observed the pigs 3 times daily. Six months after surgery, the animals were euthanized using pentobarbital (0.4 mL/kg) under general anesthesia. Osteochondral blocks of approximately 1 cm × 1 cm × 1 cm surrounding the defect were cut for analyses that included µCT, histological staining and scoring, histomorphometry, and immunohistochemistry.
Macroscopic Evaluation
Macroscopic evaluation was performed by an experienced investigator (KTCH) using the International Cartilage and Joint Preservation Society (ICRS) macroscopic evaluation score. All condyles were scored immediately after euthanasia and the lesion was graded for parameters including degree of defect repair, integration to border zone, and macroscopic appearance for cartilage repair, with a total perfect score of 12 for normal cartilage.
Sample Preparation
The samples were prepared as previously described. 49 Briefly, samples were dehydrated in ethanol of increasing concentration (70% to 96%) and cleared in isopropanol and xylene. The samples were then embedded in methyl methacrylate (MMA).
µCT
The µCT was performed as previously described. 29 Briefly, the MMA embedded osteochondral blocks were µCT scanned (Scanco µCT 35; Scanco Medical, Brüttiselen, Switzerland) in high-resolution mode (1,000 projections/180°) using an isotropic voxel size of 10 µm, X-ray voltage of 55 kV, current of 145 µA, and an integration time of 800 ms. A 2-mm-high cylindrical VOI (volume of interest) with a diameter of 6 mm was drawn in the trabecular bone 1.5 mm beneath the defect using a custom-made computer program ( Fig. 1 ). 50 The VOI was imported into the software provided with the scanner (IPL version 6.5, Scanco). In order to remove noise, the 3D data sets were low-pass filtered using a Gaussian filter (σ = 1.3, support = 2) before segmentation with a fixed threshold filter (threshold = 510.3 mg HA/cm3). Analyses included bone volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), connectivity density (CD), structure model index (SMI), and tissue mineral density (TMD).
Figure 1.
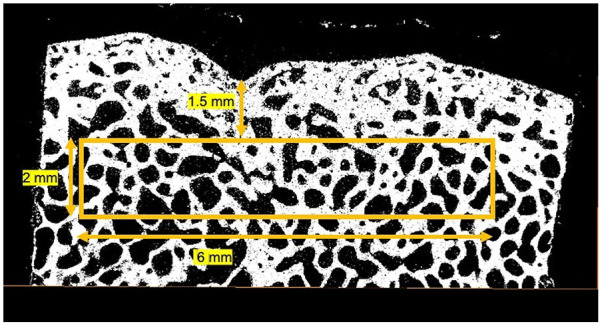
Schematic illustration of the VOI for µCT. A 2-mm-high cylindrical VOI was drawn with a diameter of 6 mm in the trabecular bone 1.5 mm beneath the defect.
Histological Staining
Using a hard tissue microtome (Reichert Jung Polycot), the MMA embedded osteochondral blocks were cut into 7-μm-thick sections. All samples were stained with hematoxylin and eosin, safranin O (counterstain with Fast Green and Weigert’s Iron Hematoxylin), and stained for collagen type I and II by immunohistochemistry.
Histomorphometry
The morphology of the repair tissue was quantitatively evaluated through histomorphometry, as described by Foldager et al. 51 Defects were halved, and sections were cut for every 350 μm, yielding 7 sections per defect. At ×10 magnification, a 5 × 5 point counting grid was superimposed onto each section (newCAST software; Visiopharm) and 50% of each cartilage defect was counted according to tissue type (hyaline cartilage, fibrocartilage, fibrous tissue, bone, or vascular tissue) using hematoxylin and eosin staining with polarized light to accentuate collagen fibers as previously described.29,49 A region of interest (ROI) was drawn from a projected tidemark from the adjacent normal cartilage. The histomorphometric evaluation was performed by a single blinded assessor (KTCH) with experience in evaluation of experimental cartilage repair.
Histological Scoring
A central section of each defect was used for blinded evaluation with the ICRS II histological score. In the ICRS II score, sections were evaluated in 14 categories (tissue morphological characteristics, matrix staining, cell morphological characteristics, chondrocyte clustering, surface architecture, basal integration, formation of a tidemark, subchondral bone abnormalities, inflammation, abnormal calcification, vascularization, surface assessment, deep zone assessment, and overall assessment) with a visual analogue scale from 0 (severely abnormal) to 100 (normal). 45 Safranin O-stained sections were used for the ICRS II evaluation. Semiquantitative evaluation was performed by 2 independent blinded assessors (KTCH and MCL) with experience in experimental cartilage repair.
Immunohistochemistry
Immunohistochemical staining was performed as previously described with polyclonal rabbit antibodies for either collagen type I (Abcam, Ab34710, Cambridge, UK) or collagen type II (Neomarkers, MS 306-P0, Fremont, CA).29,52 Negative staining controls were labelled with rabbit serum (X0902; Dako Universal Staining System, Carpinteria, CA) or mouse IgG isotype control (Thermo Fisher Scientific), respectively. Streptavidin-horseradish peroxidase and aminoethyl carbazole was used for labelling, according to the manufacturer’s instructions (Dako). Counterstaining was performed with Mayer’s hematoxylin. Each slide was evaluated for percentage of positively stained areas for collagen type I and II and categorized in quartiles: 0% to 25%, 25% to 50%, 50% to 75%, or 75% to 100%. 45
Statistical Analyses
For cell culture studies, all quantitative data were reported as mean ± standard error of mean (SEM). Paired t-test was performed for normally distributed data, while a Mann-Whitney test was performed for nonnormally distributed data. A P-value less than 0.05 was considered statistically significant. For the animal study, sample size was determined by power analysis based on the amount of fibrocartilage in the repair tissue with histomorphometry as the primary endpoint. On the basis of recent studies,29,53 we expected that BMS without enhancement would yield 60% fibrocartilage in the repair tissue, and enhanced BMS would yield 80% fibrocartilage. The standard deviation (SD) for the fibrocartilage percentage was expected to be 15 points. Power was set to 80%, α = 0.05, and β = 0.2. Based on these assumptions 10 treatment units per study group was needed. Twelve units were included per group to account for possible animal dropout.
A mixed-effect model was fitted to the data for measures of cartilage repair (histomorphometry and histology [ICRS II score]). In this pig model, knee (left or right) was treated as random effects, and treatment (BMS + MSC-EVs or BMS + PBS) and defect site (proximal/lateral or distal/medial) as fixed effects. Separate analyses were performed for each category of the ICRS II score. The µCT data were analyzed using unpaired t-tests. The number of samples in each quartile of positive collagen staining was analyzed using Fisher’s exact test. Two-tailed P-values <0.05 were considered significant. Interreader correlation coefficients were calculated to assess the reproducibility of the ICRS II score. Pearson correlation coefficients were determined using Prism 8 (GraphPad Software, Inc.). Statistical analysis was performed using STATA version 15.1 (StataCorp, College Station, TX) or Prism 8.
Results
Chondrocyte Proliferation
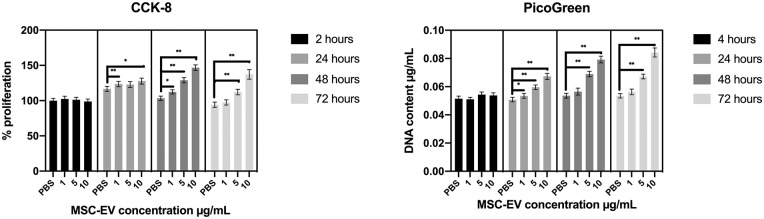
A previous study reported that MSC-EVs enhanced metabolic activity and proliferation of rat chondrocytes in a dose-dependent manner. 18 To ascertain the effects of MSC-EVs on proliferation of human chondrocytes relevant for clinical cartilage repair, we tested the effects of varying concentrations of MSC-EVs on human chondrocytes over 72 hours. Metabolic activity was measured by a CCK-8 assay, while proliferation with increase in cell number was assessed by measurement of total DNA content. MSC-EVs significantly enhanced metabolic activity of human chondrocytes in a dose-dependent manner after only 24 hours at 10 µg/mL (P < 0.001; Fig. 2 ). From 48 to 72 hours, chondrocytes treated with 10 µg/mL MSC-EVs demonstrated at least 1.5-fold higher metabolic activity than the PBS vehicle control (P < 0.001; Fig. 2 ). Consistent with the increase in metabolic activity, DNA content reflective of the cell number also increased with increasing concentrations of MSC-EVs. Similar to effects of MSC-EVs on cell metabolic activity, significant differences in cell number between EV treatment and control were observed with 5 and 10 µg/mL MSC-EVs from 24 hours that persisted to 72 hours ( Fig. 2 ). At 72 hours, chondrocytes treated with 10 µg/mL MSC-EVs demonstrated 1.6-fold higher DNA content than the PBS-treated chondrocytes (P < 0.0001; Fig. 2 ). The increases in metabolic activity and cell number were further evidenced by the increase in number of chondrocytes observed under microscope following EV treatment at 72 hours ( Fig. 3 ).
Figure 2.
Left: CCK-8 metabolic assay showing an MSC-EV concentration- and time-dependent increase in the metabolic activity of the chondrocytes. Bars show proliferation rate relative to chondrocytes cultured with PBS at 2 hours ± SEM (n = 6). Right: PicoGreen assay showing an MSC-EV concentration- and time-dependent increase in DNA content which points toward an increase in chondrocyte proliferation. Bars show DNA content ± SEM (n = 6). *P < 0.001, **P < 0.0001.
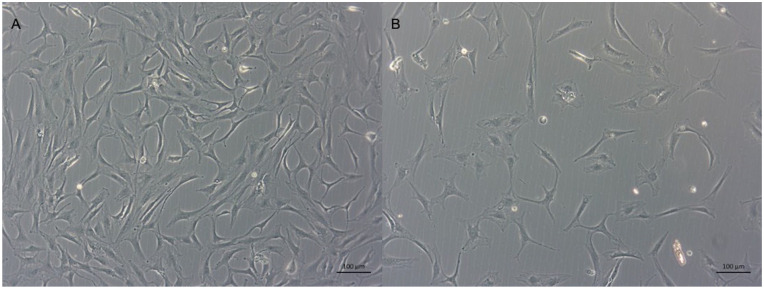
Figure 3.
Representative microscopic images of chondrocytes cultured with 10 μg/mL MSC-EVs (A) or PBS (B) for 72 hours. Scale bar: 100 μm (n = 3).
In Vivo Results
All animals completed the 6-month follow-up without complications. On macroscopic assessment, the repair tissue in both groups was easily distinguishable from the adjacent native cartilage, with regard to color and regularity (Suppl. Fig. 1). The macroscopic appearance did not differ between the 2 groups and both groups had close ICRS macroscopic scores (8.0 vs. 8.0; P = 0.99).
µCT
Defects treated with BMS + MSC-EVs had subchondral bone with significantly higher tissue mineral densities than defects treated with BMS + PBS (860 mg HA/cm3 vs. 838 mg HA/cm3, P = 0.02), pointing toward formation of more mature bone. The other microstructural parameters (bone volume fraction [BV/TV], trabecular number [Tb.N], trabecular thickness, trabecular separation [Tb.Sp], connectivity density [CD], and structure model index [SMI]) did not differ significantly between the 2 groups ( Table 1 ).
Table 1.
Micro-CT data showing significantly higher tissue mineral density in defects treated with BMS+MSC-Evs (p = 0.02).
| Parameter | BMS + MSC-EVs a | BMS + PBS a |
|---|---|---|
| Bone volume fraction (BV/TV) | 0.42 ± 0.02 | 0.39 ± 0.03 |
| Connectivity density (CD) (1/mm3) | 31.8 ± 4.57 | 25.7 ± 1.53 |
| Trabecular number (Tb.N) (1/mm) | 2.97 ± 0.09 | 2.86 ± 0.10 |
| Trabecular thickness (Tb.Th) (mm) | 0.14 ± 0.00 | 0.13 ± 0.01 |
| Trabecular spacing (Tb.Sp) (mm) | 0.30 ± 0.01 | 0.32 ± 0.02 |
| Tissue mineral density (mg HA/cm 3 ) b | 860 ± 5.04 | 838 ± 6.54 |
| Structure model index (SMI) | −1.31 ± 0.11 | −1.15 ± 0.30 |
BMS = bone marrow stimulation; MSC-EV = mesenchymal stem cell-extracellular vesicle; PBS = phosphate-buffered saline.
μCT data: mean ± standard error of the mean.
Bold = parameters with significant differences.
Histomorphometry
Histomorphometric analysis revealed a significantly higher amount of bone in the cartilage defect area in defects treated with BMS + MSC-EVs compared with defects treated with BMS + PBS (7.9% vs. 1.5%; P = 0.02; Figs. 4 - 6 ).
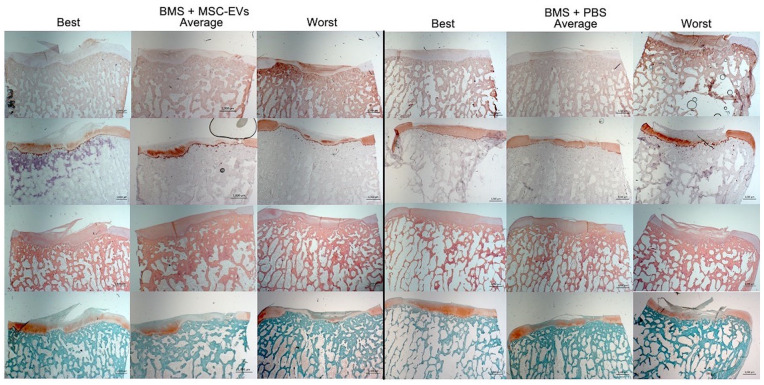
Figure 4.
Examples of best, worst, and average outcome after BMS + MSC-EVs (left) and BMS + PBS (right). Interestingly, the best and worst examples from both groups represent 2 defects within the same knee. Col 1 = collagen type 1 immunostaining; Col 2 = collagen type 2 immunostaining; HE = hematoxylin and eosin; Saf O = Safranin O staining. Magnification: 100×. Scale bar: 1,000 µm.
Figure 5.
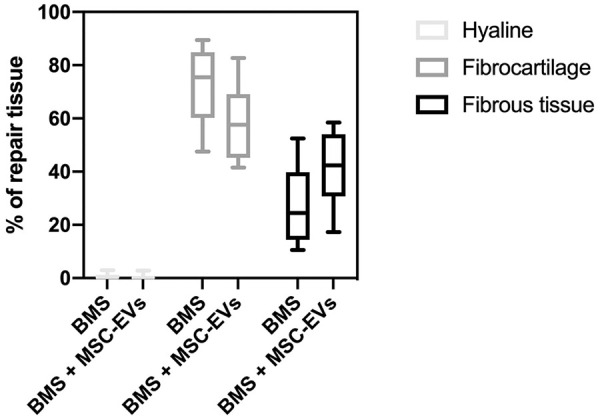
Histomorphometric analysis of cartilage repair tissue. No significant differences are seen between treatments. BMS = bone marrow stimulation.
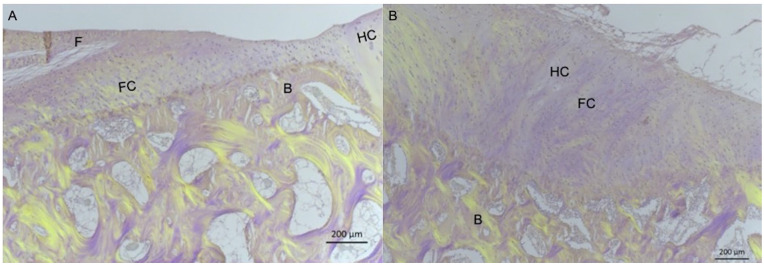
Figure 6.
Representative images of HE-stained specimens viewed with polarized light from defect treated with BMS + MSC-EVs (A) and BMS + PBS (B). On the left, bone is seen above the projected tidemark from the adjacent hyaline cartilage, while a small amount of fibrous tissue is observed superficially. On the right, a small area is seen on the right with round cells in lacunae and no collagen fibers and was scored as hyaline cartilage. The different tissue types are presented: HC = hyaline cartilage; FC = fibrocartilage; F = fibrous tissue; B = bone. Magnification: 40×, scale bar: 200 μm.
After excluding the osseous ingrowth into the cartilage defect area, there was a trend toward less fibrocartilage (58.9% vs. 71.7%, P = 0.10) and more fibrous tissue (40.6% vs. 27.7%, P = 0.09) in defects treated with MSC-EVs, although this did not differ significantly. Less than 1% of the repair tissue in both groups was hyaline cartilage ( Fig. 5 ).
ICRS II Scoring
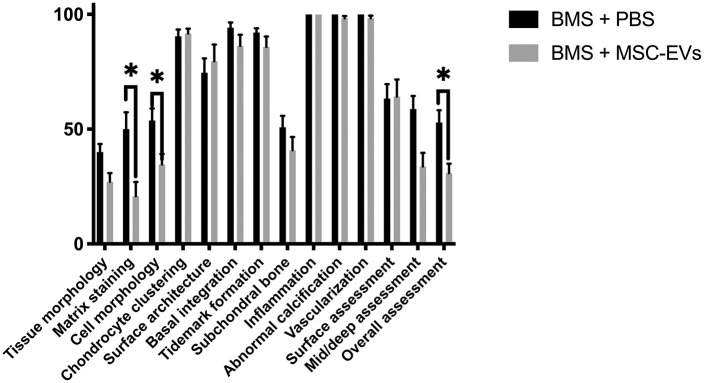
Correlation coefficients between the 2 blinded assessors were good to excellent (r = 0.76-0.96) for all categories. No inflammation or adverse tissue response was seen in any of the defects. Good defect filling was found for all defects in both groups. The defects in the group treated with BMS + MSC-EVs scored significantly lower than the defects in the BMS + PBS group for several parameters including “matrix staining” (20.8 vs. 50.0, P = 0.03), “cell morphology” (35.4 vs. 53.8, P = 0.04), and “overall assessment” (30.8 vs. 52.9, P = 0.03) ( Fig. 7 ). Furthermore, there was a trend toward lower scores in the BMS + MSC-EVs group for “tissue morphology” (27.1 vs. 40.0, P = 0.05) and “mid/deep assessment” (33.8 vs. 58.8, P = 0.06). Three defects treated with BMS + MSC-EVs had a minor amount of abnormal ossification in the repair tissue, while this was not observed in the BMS + PBS group.
Figure 7.
Mean International Cartilage Repair Society (ICRS) II scores (±standard error of mean [SEM]). N = 12 for each group. Higher scores indicate better repair tissue. BMS = bone marrow stimulation. Grey bar = BMS + exosomes, black bar = BMS only. *P < 0.05.
Immunohistochemistry
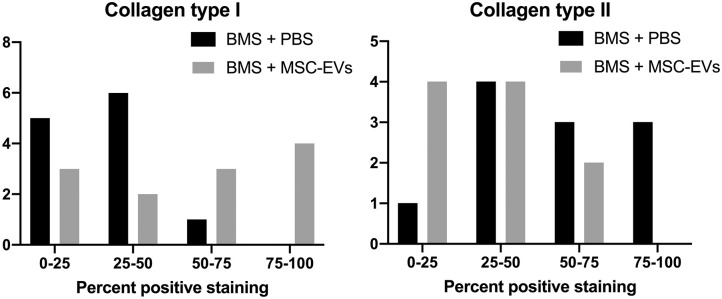
Defects treated with BMS + MSC-EVs had significantly lower collagen II positive staining (P = 0.01) and significantly higher collagen type I positive staining than that of BMS + PBS treated defects (P = 0.002; Fig. 8 ).
Figure 8.
Quartiles of positive collagen I (left) and collagen II (right) staining. Grey bar = BMS + exosomes, black bar = BMS only. The y-axis represents the number of samples with the specific quartile of positive staining. In normal hyaline cartilage low amounts of collagen type I and high amounts of collagen type II is seen. BMS = bone marrow stimulation.
Discussion
The primary finding of this study was that the application of MSC-EVs as an adjuvant to BMS failed to enhance cartilage repair over BMS alone, but instead seemed to promote subchondral bone regeneration in our Göttingen minipig chondral defect model. Consistent with our previous studies that used the same source of human MSC-EVs, no adverse responses from the immunocompetent animals that included mice, rats, rabbits, and pigs were observed.11,17,18,54,55
Previous studies have used MSCs as an adjunct to BMS/microfracture technique to improve articular cartilage repair in animal models24,56,57 and in patients. 58 In an equine study, McIlwraith et al. observed that intraarticular bone marrow MSCs with hyaluronic acid (HA) applied in conjunction with microfracture enhanced matrix deposition of the repair tissue, compared to microfracture with HA alone. However, there were no other significant improvements in either gross, histological, or radiological outcomes with MSC treatment. 24 Hashimoto et al. provided a case series of 11 patients treated with microfracture either with or without autologous bone marrow MSCs and reported improvements in some of the radiological and clinical outcomes with MSC treatment after 48 weeks. 58 As the therapeutic efficacy of MSCs has been increasingly attributed to their secreted EVs including exosomes, we investigated here the efficacy of MSC-EVs as an adjuvant in augmenting cartilage and subchondral bone healing in our established minipig model. 46 To the best of our knowledge, the effects of intraarticular MSC-EVs on augmenting bone marrow stimulation for cartilage and subchondral bone repair has yet to be investigated. We hypothesized that intraarticular injections of MSC-EVs could augment BMS in cartilage and subchondral bone healing.
Contrarily to our hypothesis, we observed that intraarticular MSC-EVs applied in conjunction with BMS resulted in osseous ingrowth, impairing optimal cartilage repair, while enhancing subchondral bone healing. Consequently, while there were little to no hyaline cartilage observed in the defects irrespective of MSC-EV treatment, we observed lesser collagen type II and more collagen type I deposition, and a trend toward less fibrocartilage and more fibrous tissue in defects treated with MSC-EVs as compared to defects treated with PBS. In previous studies with BMS in our minipig model similar proportions of hyaline cartilage, fibrocartilage, and fibrous tissue has been seen.29,53 Likewise, subchondral remodeling is often quite pronounced in minipigs. In a previous study we found up to 5% bone in the cartilage repair tissue area after bone marrow stimulation. 29
The integrity of the subchondral bone is a significant determinant for the success of cartilage repair. 59 The subchondral bone plate and subarticular spongiosa play structural and metabolic roles in supporting the overlying articular cartilage. 60 Structural alterations of the subchondral bone such as subarticular osteophytes, 61 subchondral bone resorption, 62 and cysts 61 have been reported in animals and in patients following bone marrow stimulation of cartilage defects, which are significant drawbacks of this technique. The tissue mineral density assesses the intrinsic density of the bone material, while the bone mineral density quantifies the amount of bone tissue per unit volume. Consequently, the finding of increased tissue mineral density indicates a more mineralized bone tissue and thus an improved maturation of the subchondral bone tissue. 63 However, it should be noted that bone material properties are not linearly related to the mechanical properties. Experimentally, a power relationship has, for example, been established between bone density and bone strength with a power of approximately 2. 64 Consequently, a difference in TMD of 2.6% will have higher impact on the bone mechanical properties than 2.6%. For these reasons, we find the difference in TMD to be of physiological relevance.
Based on our observations, it is plausible that the endogenous MSCs from the subchondral bone marrow had inherent propensity for bone formation, that were further enhanced by MSC-EVs leading to osseous ingrowth into the overlying cartilage. On this note, the ability of MSC-EVs to enhance osteogenic differentiation and mineralization of osteoblasts and MSCs toward bone formation has also been reported in several studies, as recently reviewed. 65
In contrast to the findings of the present study, previous studies that employed critical sized osteochondral defect models without BMS/microfracture have reported therapeutic effects of MSC-EVs in enhancing cartilage and subchondral bone regeneration.18,19,54 In those studies, performed in rats, weekly intraarticular injections of MSC-EVs were therapeutically efficacious in repairing and regenerating critical sized osteochondral defects, achieving hyaline cartilage repair and subchondral bone regeneration by the end of 12 weeks. 19 Of interest, MSC-EVs were found to enhance osteochondral repair through well-coordinated mobilization of appropriate reparative cell types in the cartilage, subchondral bone, and synovium. 18 In the present study, perforations into the subchondral bone marrow created by BMS allowed for rapid migration of mainly bone marrow mononuclear cells including MSCs that could have resulted in rapid ossification and enhanced healing of the subchondral bone instead of the articular cartilage. Collectively, these findings suggest that the therapeutic effects of MSC-EVs are likely influenced by the animal model, defect microenvironment, and the reparative cell types mobilized for repair. It would therefore be necessary to test MSC-EVs in different models of cartilage defects, along with the current surgical interventions, to determine and optimize the efficacy of MSC-EVs for cartilage repair in different clinical scenarios.
As in all studies, there are limitations to the present study. Reduced loading of cartilage defect undergoing repair is highly recommended clinically. 22 However, postoperative immobilization is not an option in Göttingen minipigs.45,66 This may cause overload of the repair tissue, resulting in reduced healing response and possible osseous overgrowth. It is well documented that critical sized defects fill with mainly fibrous tissue in Göttingen minipigs if left untreated,46,48,67 and an untreated control group was therefore not included. The study evaluated the repair after 6 months, which is substantially shorter than in the human clinical scenario, but it is considered sufficient for organized cartilage repair in minipigs.67,68 Nevertheless, other studies have also suggested that the repair tissue may require more than 6 months for maturation.48,69
In summary, we have demonstrated that intraarticular injections of MSC-EVs were effective in enhancing healing of subchondral bone, but not cartilage, partly due to osseous ingrowth into the overlying cartilage, in our Göttingen minipig chondral defect model with BMS. Although earlier studies have demonstrated that MSC-EVs are efficacious in osteochondral repair, it is evident from our present study that their therapeutic effects are influenced by the animal model, defect microenvironment, and the reparative cell types participating in the repair. To determine the efficacy of MSC-EVs for (osteo)chondral repair without BMS in a large animal model, studies utilizing minipigs for (osteo)chondral defect repair without BMS are currently ongoing. Future studies are also required to see whether dose, scaffold, and timing of MSC-EV treatment will further enhance BMS for cartilage repair in addition to subchondral bone regeneration.
Supplemental Material
Supplemental material, sj-pdf-1-car-10.1177_19476035211029707 for Mesenchymal Stem Cell Extracellular Vesicles as Adjuvant to Bone Marrow Stimulation in Chondral Defect Repair in a Minipig Model by Kris T. C. Hede, Bjørn B. Christensen, Morten L. Olesen, Jesper Skovhus Thomsen, Casper B. Foldager, Wei Seong Toh, Sai Kiang Lim and Martin C. Lind in CARTILAGE
Supplemental material, sj-pdf-2-car-10.1177_19476035211029707 for Mesenchymal Stem Cell Extracellular Vesicles as Adjuvant to Bone Marrow Stimulation in Chondral Defect Repair in a Minipig Model by Kris T. C. Hede, Bjørn B. Christensen, Morten L. Olesen, Jesper Skovhus Thomsen, Casper B. Foldager, Wei Seong Toh, Sai Kiang Lim and Martin C. Lind in CARTILAGE
Footnotes
Acknowledgments and Funding: The authors would like to thank laboratory technicians Anna Bay Nielsen and Anette Baatrup at the Orthopaedic Research Laboratory, Aarhus University Hospital, Denmark, for their help and cooperation. The μCT scanner was donated by the VELUX Foundation. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was financially supported by the Danish Rheumatism Association and “Direktør Emil C. Hertz og Hustru Inger Hertz’ Fond.”
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was approved by the Danish Ministry of Justice Ethical Committee (J.nr. 2017-15-0201-01343).
Animal Welfare: The study was approved by the Danish Law on Animal Experimentation.
ORCID iDs: Kris T. C. Hede  https://orcid.org/0000-0002-3309-5320
https://orcid.org/0000-0002-3309-5320
Bjørn B. Christensen  https://orcid.org/0000-0002-3207-4536
https://orcid.org/0000-0002-3207-4536
Jesper Skovhus Thomsen  https://orcid.org/0000-0001-9386-6679
https://orcid.org/0000-0001-9386-6679
Martin C. Lind  https://orcid.org/0000-0002-7204-813X
https://orcid.org/0000-0002-7204-813X
Supplementary material for this article is available on the Cartilage website at https://journals.sagepub.com/home/car.
References
- 1. Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74-9. doi: 10.1002/term.8 [DOI] [PubMed] [Google Scholar]
- 2. Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5:146-50. doi: 10.1002/term.299 [DOI] [PubMed] [Google Scholar]
- 3. Akgun I, Unlu MC, Erdal OA, Ogut T, Erturk M, Ovali E, et al. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135:251-63. doi: 10.1007/s00402-014-2136-z [DOI] [PubMed] [Google Scholar]
- 4. Teo AQA, Wong KL, Shen L, Lim JY, Toh WS, Lee EH, et al. Equivalent 10-year outcomes after implantation of autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation for chondral defects of the knee. Am J Sports Med. 2019;47:2881-7. doi: 10.1177/0363546519867933 [DOI] [PubMed] [Google Scholar]
- 5. Witwer KW, Van Balkom BWM, Bruno S, Choo A, Dominici M, Gimona M, et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8:1609206. doi: 10.1080/20013078.2019.1609206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai RC, Tan SS, Yeo RWY, Choo ABH, Reiner AT, Su Y, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828. doi: 10.3402/jev.v5.29828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, et al. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics. 2012;2012:971907. doi: 10.1155/2012/971907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen TS, Lai RC, Lee MM, Choo ABH, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215-24. doi: 10.1093/nar/gkp857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toh WS, Lai RC, Hoi J, Hui P, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56-64. doi: 10.1016/j.semcdb.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 11. Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214-22. doi: 10.1016/J.SCR.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 12. Iyer SR, Scheiber AL, Yarowsky P, Henn RF, Otsuru S, Lovering RM. Exosomes isolated from platelet-rich plasma and mesenchymal stem cells promote recovery of function after muscle injury. Am J Sports Med. 2020;48:2277-86. doi: 10.1177/0363546520926462 [DOI] [PubMed] [Google Scholar]
- 13. Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33:2158-68. doi: 10.1002/stem.1771 [DOI] [PubMed] [Google Scholar]
- 14. Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8:169-84. doi: 10.7150/thno.21234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76. doi: 10.1186/scrt465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23:1233-44. doi: 10.1089/scd.2013.0479 [DOI] [PubMed] [Google Scholar]
- 17. Zhang S, Ye K, Teo W, Chuah SJ, Lai RC, Lim SK, et al. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. 2019;200:35-47. doi: 10.1016/j.biomaterials.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 18. Zhang S, Chuah SJ, Lai RC, Hoi J, Hui P, Lim SK, et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16-27. doi: 10.1016/j.biomaterials.2017.11.028 [DOI] [PubMed] [Google Scholar]
- 19. Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135-40. [DOI] [PubMed] [Google Scholar]
- 20. Tan SSH, Tjio CKE, Wong JRY, Wong KL, Chew JRJ, Hui JHP, et al. Mesenchymal stem cell exosomes for cartilage regeneration: a systematic review of preclinical in vivo studies. Tissue Eng Part B Rev. 2021;27(1):1-13. doi: 10.1089/ten.teb.2019.0326 [DOI] [PubMed] [Google Scholar]
- 21. Aae TF, Randsborg PH, Lurås H, Årøen A, Lian ØB. Microfracture is more cost-effective than autologous chondrocyte implantation: a review of level 1 and level 2 studies with 5 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2018;26:1044-52. doi: 10.1007/s00167-017-4802-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;(391 Suppl):S362-S369. doi: 10.1097/00003086-200110001-00033 [DOI] [PubMed] [Google Scholar]
- 23. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22:1986-96. doi: 10.1007/s00167-013-2676-8 [DOI] [PubMed] [Google Scholar]
- 24. Mcilwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, et al. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Anthroscopy. 2011;27(11):1552-61. doi: 10.1016/j.arthro.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 25. Hapa O. Does platelet-rich plasma enhance microfracture treatment for chronic focal chondral defects? An in-vivo study performed in a rat model. Acta Orthop Traumatol Turc. 2013;47(3):201-7. doi: 10.3944/AOTT.2013.2928 [DOI] [PubMed] [Google Scholar]
- 26. Huh SW, Shetty AA, Kim J, Kim YJ, Choi NY, Jun YJ, et al. The effect of platelet rich plasma combined with microfracture for the treatment of chondral defect in a rabbit knee. Tissue Eng Regen Med. 2014;11:178-85. doi: 10.1007/s13770-013-1115-8 [DOI] [Google Scholar]
- 27. Milano G, Passino ES, Deriu L, Careddu G, Manunta L, Manunta A, et al. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18(7):971-80. doi: 10.1016/j.joca.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 28. Stanish WD, Mccormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am. 2013;95:1640-50. doi: 10.2106/JBJS.L.01345 [DOI] [PubMed] [Google Scholar]
- 29. Hede K, Christensen BB, Olesen ML, Thomsen JS, Foldager CB, Lind MC. CARGEL Bioscaffold improves cartilage repair tissue after bone marrow stimulation in a minipig model. J Exp Orthop. 2020;7(1):26. doi: 10.1186/S40634-020-00245-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steinwachs MR, Gille J, Volz M, Anders S, Jakob R, De Girolamo L, et al. Systematic review and meta-analysis of the clinical evidence on the use of autologous matrix-induced chondrogenesis in the knee. Cartilage. 2019;1947603519870846. doi: 10.1177/1947603519870846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao L, Orth P, Cucchiarini M, Madry H. Autologous matrix-induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med. 2019;47(1):222-31. doi: 10.1177/0363546517740575 [DOI] [PubMed] [Google Scholar]
- 32. Baba R, Onodera T, Momma D, Matsuoka M, Hontani K, Elmorsy S, et al. A novel bone marrow stimulation technique augmented by administration of ultrapurified alginate gel enhances osteochondral repair in a rabbit model. Tissue Eng Part C Methods. 2015;21:1263-73. 10.1089/ten.TEC.2015.0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoemann CD, Chen G, Marchand C, Tran-Khanh N, Thibault M, Chevrier A, et al. Scaffold-guided subchondral bone repair. Am J Sports Med. 2010;38:1845-56. doi: 10.1177/0363546510369547 [DOI] [PubMed] [Google Scholar]
- 34. Gomoll AH. Microfracture and augments. J Knee Surg. 2012;25:9-16. doi: 10.1055/s-0031-1299654 [DOI] [PubMed] [Google Scholar]
- 35. Shive MS, Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, et al. BST-CarGel® treatment maintains cartilage repair superiority over microfracture at 5 years in a multicenter randomized controlled trial. Cartilage. 2015;6:62-72. doi: 10.1177/1947603514562064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen G, Sun J, Lascau-Coman V, Chevrier A, Marchand C, Hoemann CD. Acute osteoclast activity following subchondral drilling is promoted by chitosan and associated with improved cartilage repair tissue integration. Cartilage. 2011;2:173-85. doi: 10.1177/1947603510381096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Howard D, Wardale J, Guehring H, Henson F. Delivering rhFGF-18 via a bilayer collagen membrane to enhance microfracture treatment of chondral defects in a large animal model. J Orthop Res. 2015;33:1120-7. doi: 10.1002/jor.22882 [DOI] [PubMed] [Google Scholar]
- 38. Power J, Hernandez P, Guehring H, Getgood A, Henson F. Intra-articular injection of rhFGF-18 improves the healing in microfracture treated chondral defects in an ovine model. J Orthop Res. 2014;32:669-76. doi: 10.1002/jor.22580 [DOI] [PubMed] [Google Scholar]
- 39. Baba R, Onodera T, Matsuoka M, Hontani K, Joutoku Z, Matsubara S, et al. Bone marrow stimulation technique augmented by an ultrapurified alginate gel enhances cartilage repair in a canine model. Am J Sports Med. 2018;46:1970-9. doi: 10.1177/0363546518770436 [DOI] [PubMed] [Google Scholar]
- 40. Kim IL, Pfeifer CG, Fisher MB, Saxena V, Meloni GR, Kwon MY, et al. Fibrous scaffolds with varied fiber chemistry and growth factor delivery promote repair in a porcine cartilage defect model. Tissue Eng Part A. 2015;21:2680-90. doi: 10.1089/ten.tea.2015.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lai RC, Yeo RWY, Padmanabhan J, Choo A, De Kleijn DPV, Lim SK, eds. Isolation and characterization of exosome from human embryonic stem cell-derived c-myc-immortalized mesenchymal stem cells. In: Methods in Molecular Biology. Humana Press; 2016:477-94. [DOI] [PubMed] [Google Scholar]
- 42. Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo ABH, et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9:47. doi: 10.1186/1479-5876-9-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Foldager CB, Nielsen AB, Munir S, Ulrich-Vinther M, Søballe K, Bünger C, et al. Combined 3D and hypoxic culture improves cartilage-specific gene expression in human chondrocytes. Acta Orthop. 2011;82:234-40. doi: 10.3109/17453674.2011.566135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toh WS, Lim TC, Kurisawa M, Spector M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials. 2012; 33:3835-45. doi: 10.1016/j.biomaterials.2012.01.065 [DOI] [PubMed] [Google Scholar]
- 45. Christensen BB, Olesen ML, Lind M, Foldager CB. Autologous cartilage chip transplantation improves repair tissue composition compared with marrow stimulation. Am J Sports Med. 2017;45:1490-6. doi: 10.1177/0363546517694617 [DOI] [PubMed] [Google Scholar]
- 46. Christensen BB, Foldager CB, Olesen ML, Vingtoft L, Rölfing JHD, Ringgaard S, et al. Experimental articular cartilage repair in the Göttingen minipig: the influence of multiple defects per knee. J Exp Orthop. 2015;2:13. doi: 10.1186/s40634-015-0031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olesen ML, Christensen BB, Foldager CB, Hede KC, Jørgensen NL, Lind M. No effect of platelet-rich plasma injections as an adjuvant to autologous cartilage chips implantation for the treatment of chondral defects. Cartilage. 2019;194760351986531. doi: 10.1177/1947603519865318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gotterbarm T, Breusch SJ, Schneider U, Jung M. The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: retrospective analysis of 180 defects. Lab Anim. 2008;42:71-82. doi: 10.1258/la.2007.06029e [DOI] [PubMed] [Google Scholar]
- 49. Christensen BB, Foldager CB, Olesen ML, Hede KC, Lind M. Implantation of autologous cartilage chips improves cartilage repair tissue quality in osteochondral defects. Am J Sports Med. 2016;44:1597-604. doi: 10.1177/0363546516630977 [DOI] [PubMed] [Google Scholar]
- 50. Sikjaer T, Rejnmark L, Thomsen JS, Tietze A, Brüel A, Andersen G, et al. Changes in 3-dimensional bone structure indices in hypoparathyroid patients treated with PTH(1-84): a randomized controlled study. J Bone Miner Res. 2012;27:781-8. doi: 10.1002/jbmr.1493 [DOI] [PubMed] [Google Scholar]
- 51. Foldager CB, Nyengaard JR, Lind M, Spector M. A stereological method for the quantitative evaluation of cartilage repair tissue. Cartilage. 2015;6:123-32. doi: 10.1177/1947603514560655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Foldager CB, Toh WS, Gomoll AH, Olsen BR, Spector M. Distribution of basement membrane molecules, laminin and collagen type IV, in normal and degenerated cartilage tissues. Cartilage. 2014;5:123-32. doi: 10.1177/1947603513518217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Olesen ML, Christensen BB, Foldager CB, Hede KC, Bergholt NL, Lind M. No effect of platelet-rich plasma as adjuvant to bone marrow stimulation for the treatment of chondral defects in a large animal model. Arch Orthop Trauma Surg. 2020;140:77-84. doi: 10.1007/s00402-019-03292-7 [DOI] [PubMed] [Google Scholar]
- 54. Wong KL, Zhang S, Wang M, Ren X, Afizah H, Lai RC, et al. Intra-articular injections of mesenchymal stem cell exosomes and hyaluronic acid improve structural and mechanical properties of repaired cartilage in a rabbit model. Arthroscopy. 2020;36(8):2215-28.e2. doi: 10.1016/j.arthro.2020.03.031 [DOI] [PubMed] [Google Scholar]
- 55. Charles CJ, Li RR, Yeung T, Mazlan SMI, Lai RC, de Kleijn DPV, et al. Systemic mesenchymal stem cell-derived exosomes reduce myocardial infarct size: characterization with MRI in a porcine model. Front Cardiovasc Med. 2020;7:601990. doi: 10.3389/fcvm.2020.601990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nishimori M, Deie M, Kanaya A, Exham H, Adachi N, Ochi M. Repair of chronic osteochondral defects in the rat. J Bone Joint Surg Br. 2006;88:1236-44. doi: 10.1302/0301-620X.88B9.17810 [DOI] [PubMed] [Google Scholar]
- 57. Ceylan HH, Bilsel K, Buyukpinarbasili N, Ceylan HH, Tuncay I, Sen C. Can chondral healing be improved following microfracture? The effect of adipocyte tissue derived stem cell therapy. Knee. 2016;23:442-9. doi: 10.1016/j.knee.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 58. Hashimoto Y, Nishida Y, Takahashi S, Nakamura H, Mera H, Kashiwa K, et al. Transplantation of autologous bone marrow-derived mesenchymal stem cells under arthroscopic surgery with microfracture versus microfracture alone for articular cartilage lesions in the knee: a multicenter prospective randomized control clinical trial. Regen Ther. 2019;11:106-13. doi: 10.1016/j.reth.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sport Traumatol Arthrosc. 2010;18:434. doi: 10.1007/S00167-010-1072-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Imhof H, Sulzbacher I, Grampp S, Czerny C, Youssefzadeh S, Kainberger F. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest Radiol. 2000;35(10):581-8. [DOI] [PubMed] [Google Scholar]
- 61. Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, et al. Outcomes after a single-stage procedure for cell-based cartilage repair. Am J Sports Med. 2011; 39:1170-9. doi: 10.1177/0363546511399382 [DOI] [PubMed] [Google Scholar]
- 62. Saris DBF, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(Suppl 1):10S-19S. 10.1177/0363546509350694 [DOI] [PubMed] [Google Scholar]
- 63. Berli M, Borau C, Decco O, Adams G, Cook RB, Aznar JM, et al. Localized tissue mineralization regulated by bone remodelling: a computational approach. PLoS One. 2017; 12:e0173228. doi: 10.1371/journal.pone.0173228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ebbesen EN, Thomsen JS, Beck-Nielsen H, Nepper-Rasmussen HJ, Mosekilde L. Lumbar vertebral body compressive strength evaluated by dual-energy X-ray absorptiometry, quantitative computed tomography, and ashing. Bone. 1999;25:713-24. doi: 10.1016/S8756-3282(99)00216-1 [DOI] [PubMed] [Google Scholar]
- 65. Tan SHS, Wong JRY, Sim SJY, Tjio CKE, Wong KL, Chew JRJ, et al. Mesenchymal stem cell exosomes in bone regenerative strategies—a systematic review of preclinical studies. Mater Today Bio. 2020;7:10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hurst JM, Steadman JR, O’Brien L, Rodkey WG, Acvs D, Briggs KK. Rehabilitation following microfracture for chondral injury in the knee. Clin Sports Med. 2010;29:257-65. doi: 10.1016/j.csm.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 67. Mainil-Varlet P, Rieser F, Grogan S, Mueller W, Saager C, Jakob RP. Articular cartilage repair using a tissue-engineered cartilage-like implant: an animal study. Osteoarthritis Cartilage. 2001;9(Suppl A):S6-S15. doi: 10.1053/joca.2001.0438 [DOI] [PubMed] [Google Scholar]
- 68. Harman BD, Weeden SH, Lichota DK, Brindley GW. Osteochondral autograft transplantation in the porcine knee. Am J Sports Med. 2006;34:913-8. doi: 10.1177/0363546505283257 [DOI] [PubMed] [Google Scholar]
- 69. Petersen JP, Ueblacker P, Goepfert C, Adamietz P, Baumbach K, Stork A, et al. Long term results after implantation of tissue engineered cartilage for the treatment of osteochondral lesions in a minipig model. J Mater Sci Mater Med. 2008;19:2029-38. doi: 10.1007/s10856-007-3291-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-car-10.1177_19476035211029707 for Mesenchymal Stem Cell Extracellular Vesicles as Adjuvant to Bone Marrow Stimulation in Chondral Defect Repair in a Minipig Model by Kris T. C. Hede, Bjørn B. Christensen, Morten L. Olesen, Jesper Skovhus Thomsen, Casper B. Foldager, Wei Seong Toh, Sai Kiang Lim and Martin C. Lind in CARTILAGE
Supplemental material, sj-pdf-2-car-10.1177_19476035211029707 for Mesenchymal Stem Cell Extracellular Vesicles as Adjuvant to Bone Marrow Stimulation in Chondral Defect Repair in a Minipig Model by Kris T. C. Hede, Bjørn B. Christensen, Morten L. Olesen, Jesper Skovhus Thomsen, Casper B. Foldager, Wei Seong Toh, Sai Kiang Lim and Martin C. Lind in CARTILAGE