Abstract
Objective
Inflammation plays a central role in the pathophysiology of rheumatic diseases as well as in osteoarthritis. Temperature, which can be quantified using infrared thermography, provides information about the inflammatory component of joint diseases. This systematic review aims at assessing infrared thermography potential and limitations in these pathologies.
Design
A systematic review was performed on 3 major databases: PubMed, Cochrane library, and Web of Science, on clinical reports of any level of evidence in English language, published from 1990 to May 2021, with infrared thermography used for diagnosis of osteoarthritis and rheumatic diseases, monitoring disease progression, or response to treatment. Relevant data were extracted, collected in a database, and analyzed for the purpose of this systematic review.
Results
Of 718 screened articles 32 were found to be eligible for inclusion, for a total of 2094 patients. Nine studies reported the application to osteoarthritis, 21 to rheumatic diseases, 2 on both. The publication trend showed an increasing interest in the last decade. Seven studies investigated the correlation of temperature changes with osteoarthritis, 16 with rheumatic diseases, and 2 with both, whereas 2 focused on the pre-post evaluation to investigate treatment results in patients with osteoarthritis and 5 in patients with rheumatic diseases. A correlation was shown between thermal findings and disease presence and stage, as well as the clinical assessment of disease activity and response to treatment, supporting infrared thermography role in the study and management of rheumatic diseases and osteoarthritis.
Conclusions
The systematic literature review showed an increasing interest in this technology, with several applications in different joints affected by inflammatory and degenerative pathologies. Infrared thermography proved to be a simple, accurate, noninvasive, and radiation-free method, which could be used in addition to the currently available tools for screening, diagnosis, monitoring of disease progression, and response to medical treatment.
Keywords: infrared thermography, joints, osteoarthritis, rheumatic diseases
Introduction
Inflammatory and degenerative joint diseases are common and progressive clinical entities, often associated with significant pain and functional disability. Among those with the greatest impact on society are osteoarthritis (OA) and rheumatic diseases such as rheumatoid arthritis (RA). 1 Osteoarthritis is considered a degenerative joint disease that affects all articular joints and is associated with degeneration of the joint cartilage and menisci, subchondral sclerosis, and inflammation of the synovial membrane. Osteoarthritis is the most common musculoskeletal disease and its prevalence, currently, around 20% to 30% of the population of the wealthier countries, will increase further with the progressive lengthening of life expectancy.2,3 After cardiovascular diseases, OA is the most frequent cause of disability and a reduction in work activity. In addition, rheumatic diseases severely affect patient quality of life, involving joints, connective tissue, muscles, tendons, and fibrous tissue. In the general population, RA prevalence ranges from 0.5% to 1%,4,5 being higher in women (in a female/male ratio of 2.5/1) in industrialized countries. It can occur at any age, with the peak incidence being between the age of 40 and 70 years. 6 As OA, RA is a major cause of disability, so that at least 50% of those affected are unable to maintain a full-time job within 10 years of disease onset. 7
Inflammation plays a central role in the pathophysiology of these diseases, in particular for RA, with an immune activation and leukocyte infiltration into the normally sparsely populated synovial compartment. Consequently, particularly in the first stages of the disease, tissue edema and fibrin deposition are prominent and can manifest clinically as joint swelling and pain.6,8,9 Moreover, increasing evidence points to an important role of inflammation in OA, which can no longer be simply labeled as a “degenerative” disease. Inflammation promotes damage to joints and bones, causing joint-related functional deficits, especially in the earlier stages. The quantification of the inflammatory component in the disease processes could help to better target treatments to address this pathological aspect and to manage better each patient according to the specific disease phase and manifestation. In this light, the temperature is a key physical property, which can directly reflect the articular inflammatory processes. The increase above normal values is 1 of the 4 classical signs of inflammation, and thermal cameras can detect and quantify these temperature changes.10 -13 Infrared thermography (IRT), a noninvasive and radiation-free method which provides information on the thermal, metabolic, and vascular conditions of the human body, could be therefore used in addition to the currently available tools for diagnosis, monitoring of disease progression, and response to medical treatment, in particular in relation to the inflammatory component of joint diseases.14 -16
This systematic review aims at assessing the potential and limitations of thermography in the study of the inflammatory component of joint inflammatory and degenerative diseases.
Materials and Methods
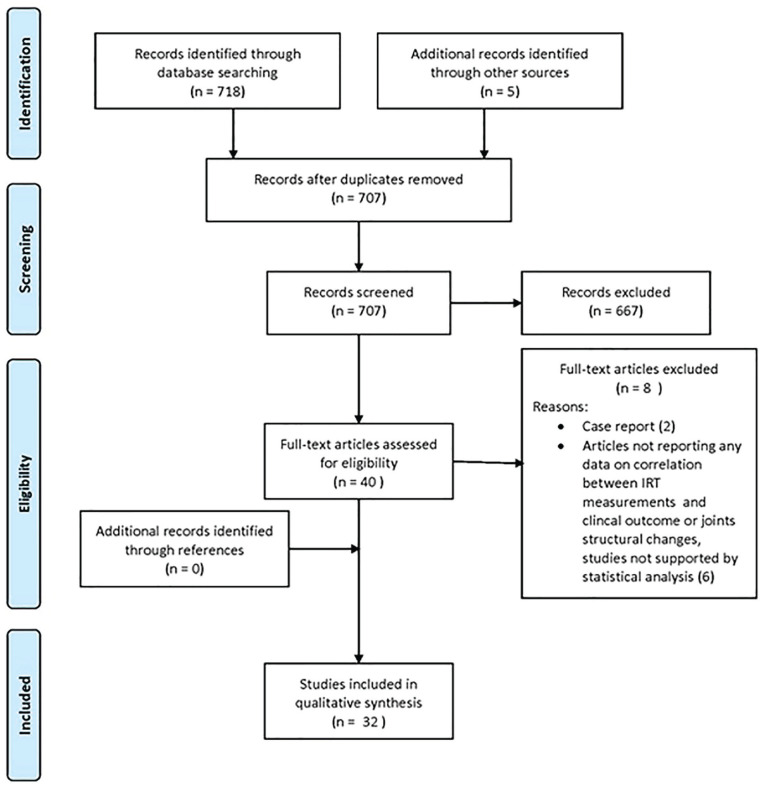
A systematic review of the literature was performed on the articles dealing with inflammatory and degenerative joint diseases evaluated by IRT. The search was performed on May 7, 2021 on PubMed, Cochrane library, and Web of Science databases with the string: (Infrared Camera or Thermography or Infrared Thermography or Thermal Camera or IRT) AND (Arthritis or Osteoarthritis or OA or Rheumatoid Arthritis or RA). The list of references for each selected article was manually searched for more papers of interest. The guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) were used ( Fig. 1 ). 17 Two independent observers (G.S. and G.C.) conducted the screening process and analysis separately. First, the articles were screened by title and abstract. The following inclusion criteria were used during the initial screening of titles and abstracts: clinical reports of any level of evidence, written in the English language, published in the last 30 years (1990-2021), with IRT used for diagnosis of OA and rheumatic diseases, monitoring disease progression or response to medical treatment. Exclusion criteria were articles written in other languages, studies on animals, preclinical studies, reviews, case reports, studies not supported by statistical analysis, or studies analyzing other applications of IRT. In the second step, the full texts of the selected articles were screened, with further exclusions according to the previously described criteria. Afterward, articles not reporting any data on the correlation between IRT measurements and joints structural changes (documented by currently available tools like x-ray, computed tomography, MRI, and ultrasound) or clinical outcome were excluded. Relevant data (publication year, type of study, number of patients, pathology, joints involved, type of IRT system used, treatment assessed, results) were then extracted and collected in a unique database with the consensus of the 2 observers to be analyzed for the purposes of the present article ( Tables 1 and 2 ).
Figure 1.
PRISMA flow chart of the article selection process. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis; IRT = infrared thermography.
Table 1.
Characteristics of the OA Included Studies.
| Author-Year-Journal | No. of patients | Age range | Male/Female | Pathology | Joint | Findings | Technology |
|---|---|---|---|---|---|---|---|
| Brito, C.J. (2020)
18
Journal of Thermal Analysis and Calorimetry |
70 | 69-81 | 24/46 | OA | Knee | Different thermal skin response after an acute training session between OA subjects and control group | FLIR T335 |
| Belcaro, G., (2019)
19
Minerva Ortopedica E Traumatologica |
45 | 44-55 | 45/0 | OA | Knee | IRT detects the response to medical treatment in knee OA | FLIR 440 |
| Bhowmik, M. K. (2016)
20
Thermosense: Thermal Infrared Applications |
15 | 23-75 | 4/11 | OA (/RA) | Knee | Different patterns of heat intensity distribution over affected and normal joints | FLIR T650sc |
| Jin, C. (2012)
21
Journal of Medical and Biological Engineering |
133 | NA | NA | OA | Knee | Screening system of normal and abnormal cases, based on temperature patterns, with an accuracy rate of 85.5%, a sensitivity of 85.7%, and a specificity of 85.5% | FLIR SC620 |
| Borojević, N. (2011)
22
Periodicum Biologorum |
13 | NA | 4/16 | OA (/RA) | Hand | 1. Joints’ absolute temperature differences between pathologic
and control group 2. Different patterns of heat intensity distribution over affected and normal joints |
Thermo Tracer TH7102WL |
| Denoble, A. E. (2010)
23
Clinical Medicine Insights |
30 | 40-45 | 4/30 | OA | Knee | Correlation between thermal patterns and radiological severity grade | Meditherm Med2000™ |
| Mejersjö, C. (2008)
24
Journal of Oral Rehabilitation |
29 | 39-76 | 2/27 | OA | TMJ | 1. Joints’ absolute temperature differences between pathologic
and control group 2. Temperature did not correlate with medical treatment’s response and symptoms |
Exacon MC 8900 |
| Varju, G. (2004)
25
Rheumatology |
91 | 46-87 | 18/73 | OA | Hand | 1. Correlation between thermal patterns and radiological
severity grade 2. KL1 joints are warmer than KL0 joints, whereas KL 2, 3, 4 joints were colder than KL0 joints |
Compix® PC2000e |
| Gratt, B. M. (1994)
26
Journal of Orofacial Pain |
80 | NA | 27/53 | OA | TMJ | Joints’ absolute temperature differences between pathologic and control group | Agema® 870 thermovision unit |
| Gratt, B. M. (1993)
27
Journal of Orofacial Pain |
20 | NA | 4/16 | OA | TMJ | 1. Joints’ absolute temperature differences between pathologic
and control group 2. Different patterns of heat intensity distribution over affected and normal joints |
Draeger® 8000 |
| Vargas ESNCO. (2020)
28
Reumatologia |
25 | 54-78 | 6/19 | OA | Knee | Superficial temperature of the knee was not associated with Kellgren-Lawrence grading scale | NA |
OA = osteoarthritis; IRT = infrared thermography; RA = rheumatoid arthritis; TMJ = temporomandibular joint; KL = Kellgren-Lawrence.
Table 2.
Characteristics of the Rheumatic Diseases Included Studies.
| Author-Year-Journal | No. of patients | Age range | Male/Female | Pathology | Joint | Findings | Technology |
|---|---|---|---|---|---|---|---|
| Umapathy, S. (2020)
29
Biomed Tech |
60 | 35-47 | NA | RA | Knee | 1. Joints’ absolute temperature differences between pathologic
and control group 2. IRT findings correlate with serologic markers of inflammation (ESR and CRP) 3. IRT correlate with CDUS findings |
FLIR T400 |
| Gizińska, M. (2019)
30
Journal of Thermal Analysis and Calorimetry |
120 | 46-68 | NA | RA | Foot | 1. Joints’ absolute temperature differences between pathologic
and control group 2. IRT did not correlate with CDUS findings |
FLIR SC640 |
| Laskari, K. (2019)
31
Rheumatology |
243 | NA | NA | RA | MJ | 1. Joints’ absolute temperature differences between pathologic
and control group 2. IRT findings correlate with clinical score (DAS-28 and HAQ score) 3. IRT correlate with CDUS findings |
RTM-01 RES |
| Pauk, J. (2019)
32
Medical & Biological Engineering & Computing |
30 | 43-69 | NA | RA | Hand | After a cooling test the temperature Tmax-Tmin measured along the fingers line was higher in RA patients compared with healthy participants post-cooling and post-rewarming | FLIR E60bx |
| Jones, B.(2018)
33
Clinical Rheumatology |
79 | 18-70 | 20/59 | RA | Hand | 1. Joints’ absolute temperature differences between pathologic
and control group 2. IRT findings did not associate with clinical measures of disease activity |
FLIR T300 |
| Svaic, V. (2018)
34
Quantitative Infrared Thermography Conference |
9 | 59-83 | NA | RA | Hand | IRT detected the effect of cryotherapy in rheumatic hands | Fluke Ti25 |
| Umapathy, S. (2018)
35
Journal of Medical and Biological Engineering |
60 | 34-56 | 15/45 | RA | Hand | 1. Joints’ absolute temperature differences between pathologic
and control group 2. the best joints to image are the MCPs of the second and third fingers |
FLIR T400 |
| Lerkvaleekul, B (2017)
36
Physiological Measurement |
61 | NA | NA | JIA | Wrist | 1. Joints’ absolute temperature differences between pathologic
and control group 2. IRT findings correlate with clinical outcome (swelling) |
FLIR E60 |
| Snekhalatha, U. (2017)
37
Journal of Engineering in Medicine |
60 | NA | NA | RA | Knee | 1. IRT findings correlate with clinical score (DAS-28 and HAQ
score) 2. IRT findings correlate with serologic markers of inflammation (ESR and CRP) |
FLIR T400 |
| Bhowmik, M. K. (2016)
20
Thermosense: Thermal Infrared Applications |
15 | 23/75 | 0/15 | OA (/RA) | Knee | Difference between contralateral Knees in Ra patients are minor compared with patients with OA, due to the similar spreading of RA inflammation in bilateral joints | FLIR T650 |
| Lasanen, R (2015)
38
Physiological Measurement |
20 | 4-16 | 9/11 | JIA | Knee | Joints’ absolute temperature differences between pathologic and control group | FLIR T630 |
| Capo, A (2015)
39
Microvascular Research Journal |
21 | 38-67 | 11/10 | PsA | Hand | 1. Joints’ absolute temperature differences between pathologic
and control group 2. IRT findings correlate with pain 3. IRT correlate with CDUS findings 4. Different thermal skin response after an acute training session between pathological subjects and control group |
FLIR 660sc |
| Snekhalatha, U. (2015)
40
Journal of Engineering in Medicine |
50 | 30-75 | 12/38 | RA | Hand | 1. Joints’ absolute temperature differences between pathologic
and control group 2. The best joints to image are the MCP, the proximal interphalangeal joints, and the distal interphalangeal joints of the third finger |
FLIR T400 |
| Frize, M. (2012)
41
Studies in Health Technology and Informatics |
52 | NA | NA | RA | MJ | 1. Joints’ absolute temperature differences between pathologic
and control group 2. The best joints to image are the MCPs of the second and thirrd fingers, and the proximal interphalangeal joints of the third finger 3. Automatic system to classify patients into the 3 levels of RA activity with a sensitivity of 96% and a specificity of 92% |
Model NA (320 × 420 pixels, operating in the 7.5 μm-13 μm range) |
| Korman, P. (2012)
42
Rheumatology International |
47 | 46-66 | 8/39 | RA | Hand | IRT detected the effect of cryotherapy in rheumatic hands | FLIR SC2000 |
| Borojević, N. (2011)
22
Periodicum Biologorum |
8 | NA | NA | OA (/RA) | Hand | Joints’ absolute temperature differences between pathologic and control group | Thermo Tracer TH7102WL |
| Frize, M (2011)
43
Sensors |
30 | 19-70 | 14/16 | RA | MJ | 1. Joints’ absolute temperature differences between pathologic
and control group 2. The best joints to image are the MCPs of the second and third fingers and the knees |
Model NA (320 × 420 pixels, operating in the 7.5 μm-13 μm range) |
| Spalding SJ (2008)
44
Arthritis Research & Therapy |
22 | NA | NA | RA/JIA | MJ | The mean ± SD HDI in control joints was lower compared with joints with active rheumatic diseases | Meditherm med2000 |
| Blyth, T. (1998)
45
British Journal of Rheumatology |
82 | NA | 16/66 | RA | Knee | IRT detect the response to 3 different medical treatments in rheumatic knees, revealing improvements compared with baseline, but without finding difference between the 3 groups | NA |
| MacDonald, A. G. (1994)
46
Clinical Rheumatology |
28 | NA | NA | RA | Knee | 1. IRT findings correlate with serologic markers of inflammation
(ESR and CRP) 2. IRT findings correlate with pain 3. IRT detect the response to intra-articular steroid injection in rheumatic knees |
NA |
| Gatt A. (2020)
47
PloS One |
83 | 25-70 | 15/68 | RA | Foot | Patients with RA in clinical and radiological remission exhibit significantly different feet thermographic patterns compared with healthy controls | NA |
| Tan YK. (2020)
48
Clinical radiology |
37 | 43-69 | 9/28 | RA | Hand | Joints in patients with RA have significantly higher temperature readings when ultrasound-detected joint inflammation is present | FLIR T640 |
| Tan YK. (2021)
49
Clinical radiology |
37 | 43-69 | 9/28 | RA | Hand | Novel use of combined thermal and ultrasound imaging in RA shows superiority to either imaging alone in terms of correlation with DAS-28 | FLIR T640 |
RA = rheumatoid arthritis; IRT = infrared thermography; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; CDUS = color Doppler ultrasound; MJ = multiple joints; JIA = juvenile idiopathic arthritis; OA = osteoarthritis; MCP = metacarpophalangeal joints; TMJ = temporomandibular joint; PsA = psoriatic arthritis; DAS = Disease Activity Score; HAD = Health Assessment Questionnaire; HDI = Heat Distribution Index.
Results
The articles screened were 718 and of these 32 articles were found to be eligible for inclusion in the present review. Among these, 9 studies reported IRT application to OA, 21 to rheumatic diseases (17 RA, 2 juvenile arthritis, 1 both RA and juvenile arthritis, 1 psoriatic arthritis), 2 studies reported on both OA and RA ( Tables 1 and 2 ). In total, 2094 patients were studied, 735 women and 308 men (from 18 studies, whereas the other 14 studies did not specify the female/male ratio), with age ranging from 4 to 83 years. The publications with specific reference to disease, location, and patients studied are reported in Figure 2 , which shows an increasing trend with three-fourths of the papers of the last 30 years published in the last decade.
Figure 2.
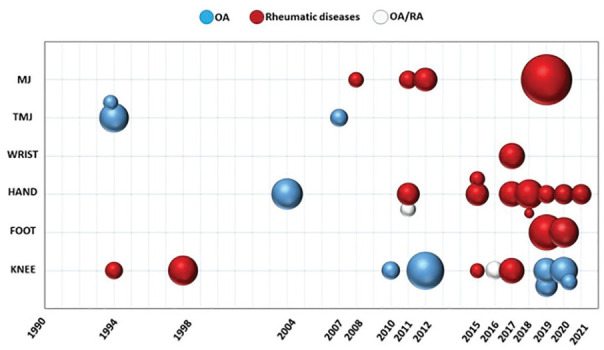
Timeline of the included studies. MJ = multiple joints; TMJ = temporomandibular joint; OA = osteoarthritis; RA = rheumatoid arthritis; sphere size = number of patients included in the study.
Of the 32 articles, retrieved from the literature search, as shown in Tables 1 and 2 , 7 investigated the correlation of temperature changes with OA, 16 the correlation with rheumatic diseases, and 2 with both OA and rheumatic diseases. While 2 focused on the pre-post evaluation to investigate treatment results in patients with OA and 5 in patients with rheumatic diseases (1 study for each group also provided correlation data, between the temperature changes and the presence of OA and rheumatic disease). In the following chapters, results are summarized and detailed, distinguishing for OA and rheumatic diseases; moreover, for each group, correlational and pre-post studies are discussed separately.
Correlational Studies to Investigate OA-Related Temperature Changes
Of the 10 identified studies, 5 treated the knee joint,18,20,21,23,28 3 the temporomandibular joint (TMJ),24,26,27 and 2 the hand22,25 ( Table 1 ). Four of these papers22,24,26,27 reported on IRT used to identify the joints’ absolute temperature differences between patients affected by OA and the control group. All these studies reported significant differences between the 2 groups. Infrared thermography was also used to capture different patterns of heat distribution between healthy and pathological joints. One study 20 highlighted that, due to the difference in inflammation spreading, the mean, standard deviation, and contrast value of intensity distribution over IRT images could discriminate between the affected OA knee and normal knee. Another study 21 also proposed an automated screening system for knee OA based on the different patterns of the temperature detected by IRT: 266 knee thermal images were acquired, and a knee feature extraction algorithm based on patella-centring was performed. The extracted features were fed to a support vector machine (SVM) classifier to perform an automated recognition. The SVM classifier had an accuracy rate of 85.5%, a sensitivity of 85.7%, and a specificity of 85.5% in detecting normal and abnormal cases. The feasibility of using the symmetry/asymmetry distribution of temperatures to discriminate between normal or abnormal thermal patterns, and so between normal or pathological joints, was investigated by 2 other studies,22,27 although without reaching statistical significance.
Infrared thermography was also used in 3 papers to investigate the correlation between thermal patterns and radiological severity grade.23,25,28 Two studies described a statistically significant correlation between temperatures and radiological disease severity based on the Kellgren-Lawrence (KL) classification.23,25,50 Interestingly, one 25 also underlined that the temperature of joints with a radiological diagnosis of KL grade 1 was higher than those of KL 0, whereas temperatures of joints with more advanced OA grades were lower, probably due to the prevalence of degenerative OA features. The third study did not find a correlation between temperatures and KL classification. 28
Finally, one study 18 reported on the difference in terms of lower limb thermal skin response after an acute training session between OA subjects and control group: the exercised knee affected by OA was significantly warmer than controls after exercise.
IRT-Based Studies to Investigate OA Treatment Outcome
Of the 2 identified studies, 1 treated the knee joint 19 and 1 the TMJ 24 ( Table 1 ). These papers used IRT to monitor the response to medical treatments, Movardol in knee OA, 19 and Diclofenac Sodium 24 in TMJ OA. The presence of a diffuse vascularization and inflammation (visible as “redness” and measurable by thermography) was significantly decreased with the supplement of Movardol considering the maximum temperature of the area and the global average temperature of the skin over the affected knee. On the contrary, the use of Diclofenac Sodium in patients with TMJ OA showed an improvement of the clinical outcomes after 3 months, but the skin surface temperature measured over the TMJ showed no difference between patients and controls.
Correlational Studies to Investigate Rheumatic Diseases Related to Temperature Changes
Of the 20 identified studies, 5 treated the knee joint,20,29,37,38,46 8 the joints of the hand,22,32,33,35,39,40,48,49 1 the wrist, 36 2 the joints of the foot,30,47 and 4 multiple joints31,41,43,44 ( Table 2 ). Thirteen of these papers22,29-31,33,35,36,38-41,43,47 reported a statistical difference in joints’ absolute temperature between patients affected by rheumatic diseases and healthy controls. While in these studies the presence of an active inflammation process was detected by IRT as increased temperature, one study 44 used instead of a particular index, the HDI, 11 with the aim to avoid possible biases related to the measurement of the absolute temperature. The authors compared HDI values of 10 control wrists and metacarpophalangeal joints (MCPs) with 18 wrists with active arthritis and 9 MCPs with active arthritis (RA and juvenile arthritis). The HDI in joints with active arthritis was significantly higher compared with control joints. Moreover, they documented that an HDI cut-off of 1.3°C discriminated well between controls and patients with active arthritis. Infrared thermography was also used to capture different patterns of heat distribution between healthy and pathological joints 20 ; however, there was no difference between contralateral joints, due to the similar spreading of inflammation in bilateral joints of patients with RA.
Another approach was proposed by Pauk et al.: 32 the authors found that the baseline temperature of fingers in patients with RA was not significantly different from healthy participants. However, the use of a cooling test allowed for increasing the temperature contrast between the maximum and the minimum temperature (Tmax-Tmin) along the finger’s axis. The temperature Tmax-Tmin measured along the finger’s line was higher in patients with RA compared with healthy participants post-cooling and post-rewarming. The authors attributed this finding to vasculitis, a relevant complication of an advanced stage of RA, which in 90% of patients manifests as focal ischemia of the fingers.
Four papers35,40,41,43 also reported the best joints to image to detect the presence of active disease: the MCPs of the second and third fingers, the proximal interphalangeal joints of the third finger, the distal interphalangeal joints of the third finger, and the knees.
Eight studies reported a statistically significant correlation between joint temperature and clinical assessment of disease activity. In particular, IRT findings correlated with clinical scores (DAS-28 and HAQ score),37,31 serologic markers of inflammation (ESR—erythrocyte sedimentation rate and CRP—C-reactive protein),29,37,46 swelling, 36 and pain.39,46 One study 41 generated a classifier using decision trees (DTs—C5.0) to assess the level of severity, with the objective to separate patients into 3 classes of RA activity (low, medium, and high level): the data sets obtained from the calculations showed high performance of the DT classifier with a sensitivity of 96% and a specificity of 92%. Contrarily, in one paper, 33 the thermographic analysis was not associated with clinical measures of disease activity. There was no statistically significant correlation between joint temperature and clinical assessment of disease activity including DAS-28, swollen joints, ESR, and CRP.
Another important finding is the correlation between higher thermal values on the skin overlying joints and the presence of active inflammation assessed through color Doppler ultrasound (CDUS), which was reported in 6 papers. Among these, 5 studies29,31,39,48,49 documented a statistically significant correlation between IRT and CDUS, in particular one of them affirmed that the use of combined thermal and ultrasound imaging in RA showed superiority to either imaging alone in terms of correlation with DAS-28. 49 Instead, one paper 30 did not find significant results in the comparison of IRT joint average temperatures and CDUS inflammation findings.
Finally, one study 39 investigated the effect of a training session in patients with active psoriatic arthritis. The average baseline temperature was higher in this group compared with healthy control patients, and post-exercise, the authors documented a longer time needed from the end of the exercise to reach the minimum temperature compared with the control group. Accordingly, the authors underlined that, even in the absence of clinical symptoms, IRT can detect inflammatory-related processes resulting in skin temperature recovery alterations induced by exercise.
IRT-Based Studies to Investigate Rheumatic Diseases Treatment Outcome
Of the 4 identified studies, 2 treated the knee joint45,46 and 2 the hand34,42 ( Table 2 ). These papers used IRT to monitor the response of joints affected by RA to medical treatments, in particular, intra-articular steroid injection, 46 steroid associated with other drugs, 45 and cryotherapy.34,42 MacDonald et al. 46 documented the effect of knee intra-articular steroid injections: after treatment, a clear improvement in MTI (a specific index used instead of absolute temperatures) 51 was documented. Interestingly, the authors observed the same effect to a similar degree also in the contralateral not injected knee. Also, Blyth et al., 45 evaluating 3 different treatments for knees affected by RA (triamcinolone hexacetonide—TH, TH with rifampicin, or TH with methotrexate), documented that IRT revealed improvements at 3 months of follow-up compared with baseline, although it could not find the difference among the 3 treatments. Cryotherapy was applied with different cooling agents (ice massage, cold air, and nitrogen vapor), all showing to induce temperature changes in hand’s joints.34,42 Moreover, according to Svaic et al., 34 cryotherapy was an effective physiotherapeutic method not only for lowering the joint temperature, but also for reducing the pain level and improving function in patients with RA, although with data collected only in the immediate phase after treatment.
Discussion
The main finding of this study is that IRT is an effective approach to document the presence of joint inflammation, with correlations to other imaging and clinical features documented for different joints affected both by OA and rheumatic diseases.
The evolution of IRT in the last 30 years led to its use for multiple purposes within human medicine, showing a great potential to improve the medical diagnosis and therapy monitoring within medical fields that include oncology, neurology, urology, rheumatology, and others.52-54 Among these, results in orthopedics revealed that IRT is a sensitive and reliable method for diagnosis, evaluation, and monitoring of OA and rheumatoid diseases of different joints.55,56 This systematic review underlined the correlation between surface skin temperature and joints’ inflammatory and degenerative diseases. Anatomic relations of the skin and circulatory system, together with increased metabolism and a richer blood supply to tissues beneath the surface, represent important factors in thermographic analysis. Tissues with a higher metabolism and a richer blood supply beneath the surface skin, as it happens in the inflammatory process, emit more infrared rays, allowing identifying and monitoring this component of OA and rheumatic diseases by IRT. Moreover, skin temperature can be taken into consideration also to study the physiology of thermoregulation and the thermal dysfunction associated with pain. Indeed, pain plays a main role in the course and treatment of joints’ inflammatory and degenerative diseases, and it is known that in knee OA there is a high prevalence of both nociceptive and neuropathic pain, which are both associated with an alteration of the thermal distribution of the human body in the form of hyperthermic or hypothermic regions. 57
Based on this rationale and the positive IRT results, in the last years, the trend of publications has significantly increased also thanks to the technological advance of the thermal cameras and the software used to generate and analyze the thermal images. In fact, the feasibility and reliability have considerably improved as a result of new standards and protocols in clinical practice. Skin temperature can be affected by many internal and external factors, such as circadian rhythm, metabolic rate, emotional state, physical activities, use of drugs, alcohol, and cigarettes, temperature, humidity, and light of the room in which the thermal images are captured, the distance and angle between the thermal cameras and patient, and other factors.11,58 Accordingly, to minimize the possible biases due to these factors, the International Academy of Clinical Thermology (IACT) has developed guidelines and protocols for the equipment, design, and environmental conditions of the laboratory room, as well as for the management of the patient, which makes IRT more reliable and user-friendly.
The available literature investigated different aspects of the application of IRT in clinical practice. Infrared thermography has been used for diagnosis, monitoring of disease progression, and response to medical treatment mainly in patients with RA or other rheumatic diseases. In fact, inflammation plays a central role in the pathophysiology of these diseases, thus thermography can be easily used to detect the presence of illness, and it can discriminate well between healthy and pathological joints. While the usefulness of IRT in rheumatic diseases could be expected, increasing evidence also underlines the important role of inflammation in OA, which can no longer be simply labeled as a “degenerative” disease, especially in the earlier stages. In fact, many studies found a correlation between skin temperature and OA. The large majority of the studies found a correlation between the presence of illness (both in OA and rheumatic diseases) and either an increase of absolute skin temperature over the affected joints compared with healthy subjects or the presence of disease with different patterns of heat distribution, which reflected the spreading of joint inflammation. In the case of rheumatic diseases, the synovium is typically inflamed. Angiogenesis and vascular reorganization in the synovium are also usually observed patterns in patients with RA. The presence of synovitis may explain why patients with RA have overall warmer joints than healthy controls. As skin temperature rises within increased subcutaneous blood flow, IRT is a possible method for detecting joint inflammation in patients with arthritis. As the spreading of inflammation in bilateral joints is similar, and the feature differences are minor between contralateral joints, the increased values were identified through the comparison with healthy subjects.
On the contrary, in the OA setting, there is a degeneration of the joint surface and the subsequent release of degraded proteoglycans and proteolytic enzymes into the synovial fluid, leading to more degeneration and causing a secondary inflammation, with a growth of blood vessels and nerves into the synovium and deteriorated cartilage. Prostaglandins are important mediators of inflammation and in this case are powerful vasodilators that can also potentiate afferent C fiber sensitization. All of this causes the difference in inflammation spreading, absolute temperatures, and the values of intensity distribution over IRT images that could also discriminate between the affected joints and normal contralateral joints.
The feasibility of using the symmetry/asymmetry distribution of temperatures to discriminate between normal or abnormal thermal patterns, as well as between normal or pathological joints, could be further examined by developments in IRT technologies. In this regard, Jin et al. 21 proposed an automated screening system for knee OA, precisely an effective knee feature extraction algorithm based on patella-centering and analyzed by a supervised machine learning classifier algorithm, an SVM. This SVM works by assigning labels to objects from a set of training examples. This method showed promising results with high accuracy, sensitivity, and specificity in detecting normal and abnormal cases. This system was based on different patterns of temperature. In fact, the absolute temperature may present biases due to the patient and environmental factors, thus specific indexes have been developed, and research in this direction could further increase the reliability of this technology and the ability to detect and distinguish different disease patterns.
In addition to the correlation with other imaging findings (such as x-ray and CDUS) documenting the presence of disease and/or joint inflammation, most of the studies were also able to confirm a correlation between IRT findings and the clinical assessment of disease activity detected by clinical scores, serologic markers, and clinical examination (pain, swelling, and function). In this regard, Frize and Ogungbemile 41 even generated a classifier using IRT images to assess the level of severity in the patient group with a sensitivity of 96% and a specificity of 92%. Finally, 7 studies investigated the usefulness of IRT as a complementary diagnostic tool, showing the potential for monitoring disease progression and response to medical treatments such as steroids, cryotherapy, and exercise. Overall, all joints supported the usefulness of IRT, with some findings suggesting IRT being even more suitable to detect the presence of active disease at the MCPs’ joints, proximal and distal interphalangeal finger joints, and at the knee joints.
The overall literature findings support the potential of using IRT for different joints and for both OA and rheumatic diseases, to identify and classify the inflammatory component of the disease, as well as to monitor the treatment outcome. However, after decades of research, data are still based on a small number of patients for each clinical condition. Accordingly, the literature presents some limitations, which this systematic review reflects a small number of available studies reporting on a small number of patients. In addition, the heterogeneity of diseases treated in the studies and the different districts affected by pathology has hampered the possibility to perform a meta-analysis. The different technologies and protocols employed in the studies have been a further hindrance. In fact, this is an evolving field, starting from studies developing thermographic indexes like the one by Ring et al. in 1974, there are now different authors relying on different patterns, indexes, and technological settings.59-61 However, even though the literature presents heterogeneous studies of low level, this systematic review still offered important indications, showing the feasibility and reliability of IRT as a complementary diagnostic tool in inflammatory and degenerative joints’ diseases. These findings could serve as a base for further studies toward the development of more specific high-level studies in which IRT is used following specific protocols and guidelines to minimize possible bias, evaluate the real potential of this approach, and help to develop and improve IRT technologies.
The evolution of IRT could further change the field, improving the performance of current technologies, and open the possibility for new clinical applications. For example, new-generation systems based on 3-dimensional and thermal imaging can provide measures of joint volume, shape, and thermal patterns that are more reliable than standard thermal measures. 44 The analysis of the available evidence already shows promise, and further technological advances may lead to an even more reliable and broader use of IRT not only in the research setting but also in the clinical practice, to improve the study and management of OA and rheumatic diseases.
Conclusions
Infrared thermography is a simple, accurate, noninvasive, and radiation-free method, which provides information on the thermal, metabolic, and vascular conditions of the human body, and could be therefore used in addition to the currently available tools for screening, diagnosis, monitoring of disease progression, and response to medical treatment. The systematic review of the literature showed an increasing interest in this technology, with several applications in different joints affected by inflammatory and degenerative pathologies. A correlation was shown between thermal findings and disease presence and stage, as well as the clinical assessment of disease activity and response to treatment, supporting the potential role of IRT in the study and management of OA and rheumatic diseases.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Gianluigi Capone  https://orcid.org/0000-0003-0575-703X
https://orcid.org/0000-0003-0575-703X
References
- 1. Fusco M, Skaper SD, Coaccioli S, Varrassi G, Paladini A. Degenerative joint diseases and neuroinflammation. Pain Pract. 2017;17(4):522-32. doi: 10.1111/papr.12551. [DOI] [PubMed] [Google Scholar]
- 2. Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-30. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355-69. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. Global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316-22. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 5. van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32(2_suppl):174-87. doi: 10.1016/j.berh.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 6. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903-11. doi: 10.1016/s0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 7. Langley P, Müller-Schwefe G, Nicolaou A, Liedgens H, Pergolizzi J, Varrassi G. The impact of pain on labor force participation, absenteeism and presenteeism in the European Union. J Med Econ. 2010;13(4):662-72. doi: 10.3111/13696998.2010.529379. [DOI] [PubMed] [Google Scholar]
- 8. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023-38. doi: 10.1016/s0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 9. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094-108. doi: 10.1016/s0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 10. Haberman JD, Ehrlich GE, Levenson C. Thermography in rheumatic diseases. Arch Phys Med Rehabil. 1968;49(4):187-92. [PubMed] [Google Scholar]
- 11. Salisbury RS, Parr G, De Silva M, Hazleman BL, Page-Thomas DP. Heat distribution over normal and abnormal joints: thermal pattern and quantification. Ann Rheum Dis. 1983;42(5):494-9. doi: 10.1136/ard.42.5.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kastberger G, Stachl R. Infrared imaging technology and biological applications. Behav Res Methods Instrum Comput. 2003;35(3):429-39. doi: 10.3758/bf03195520. [DOI] [PubMed] [Google Scholar]
- 13. Ring EF, Dieppe PA, Bacon PA. Thermographic assessment of inflammation and anti-inflammatory drugs in osteoarthritis. Br J Clin Pract. 1981;35(7-8):263-4. [PubMed] [Google Scholar]
- 14. Will RK, Ring EF, Clarke AK, Maddison PJ. Infrared thermography: what is its place in rheumatology in the 1990s? Br J Rheumatol. 1992;31(5):337-44. doi: 10.1093/rheumatology/31.5.337. [DOI] [PubMed] [Google Scholar]
- 15. Brenner M, Braun C, Oster M, Gulko PS. Thermal signature analysis as a novel method for evaluating inflammatory arthritis activity. Ann Rheum Dis. 2006;65(3):306-11. doi: 10.1136/ard.2004.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frize M, Karsh J, Herry C, Adéa C, Aleem I, Payeur P. Preliminary results of severity of illness measures of rheumatoid arthritis using infrared imaging. Proceedings of the 2009 IEEE International Workshop on Medical Measurements and Applications, MeMeA; 2009 May 29-30; Cetraro, Italy. New York: IEEE. doi: 10.1109/MEMEA.2009.5167981. [DOI] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brito CJ, Miarka B, García-Pastor T, Pérez DIV, Marins JCB, Sillero-Quintana M. Osteoarthritis subjects have differentiated lower extremity thermal skin response after the concurrent acute training session. J Therm Anal Calorim. 2021;145:2467-75. [Google Scholar]
- 19. Belcaro G, Feragalli B, Cornelli U, Cotellese R, Hu S, Dugall M, et al. Evaluation of knee periosteal and cartilage morphology in subjects with arthrosis: management with Movardol. Minerva Ortop Traumatol. Epub 2019 Apr 1. [Google Scholar]
- 20. Bhowmik MK, Bardhan S, Das K, Bhattacharjee D, Nath S. Pain related inflammation analysis using infrared images. Int Soc Opt Eng. 2016;1981:986116. [Google Scholar]
- 21. Jin C, Yang Y, Xue Z-J, Liu K-M, Liu J. Automated analysis method for screening knee osteoarthritis using medical infrared thermography. J Med Biol Eng. 2013;33(5):471-7. [Google Scholar]
- 22. Borojević N, Kolarić D, Grazio S, Grubišić F, Antonini S, Nola IA. Thermography hand temperature distribution in rheumatoid arthritis and osteoarthritis. Period Biol. 2011;113(4):445-8. [Google Scholar]
- 23. Denoble AE, Hall N, Pieper CF, Kraus VB. Patellar skin surface temperature by thermography reflects knee osteoarthritis severity. Clin Med Insights Arthritis Musculoskelet Disord. 2010;3:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mejersjö C, Wenneberg B. Diclofenac sodium and occlusal splint therapy in TMJ osteoarthritis: a randomized controlled trial. J Oral Rehabil. 2008;35(10):729-38. [DOI] [PubMed] [Google Scholar]
- 25. Varju G, Pieper CF, Renner JB, Kraus VB. Assessment of hand osteoarthritis: correlation between thermographic and radiographic methods. Rheumatology. 2004;43(7):915-9. [DOI] [PubMed] [Google Scholar]
- 26. Gratt BM, Sickles EA, Ross JB, Wexler CE, Gornbein JA. Thermographic assessment of craniomandibular disorders: diagnostic interpretation versus temperature measurement analysis. J Orofac Pain. 1994;8(3):278-88. [PubMed] [Google Scholar]
- 27. Gratt BM, Sickles EA, Wexler C. Thermographic characterization of osteoarthrosis of the temporomandibular joint. J Orofac Pain. 1993;7(4):345-53. [PubMed] [Google Scholar]
- 28. Silva NCOVE, Dos Anjos RL, Santana MMC, Battistella LR, Marcon Alfieri F. Discordance between radiographic findings, pain, and superficial temperature in knee osteoarthritis. Reumatologia. 2020;58(6):375-80. doi: 10.5114/reum.2020.102002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Umapathy S, Thulasi R, Gupta N, Sivanadhan S. Thermography and colour Doppler ultrasound: a potential complementary diagnostic tool in evaluation of rheumatoid arthritis in the knee region. Biomed Tech Biomed Eng. 2020;65(3):289-99. doi: 10.1515/bmt-2019-0051. [DOI] [PubMed] [Google Scholar]
- 30. Gizińska M, Rutkowski R, Szymczak-Bartz L, Romanowski W, Straburzyńska-Lupa A. Thermal imaging for detecting temperature changes within the rheumatoid foot. J Therm Anal Calorim. 2021;145:77-85. doi: 10.1007/s10973-020-09665-0. [DOI] [Google Scholar]
- 31. Laskari K, Pentazos G, Pitsilka D, Raftakis J, Konstantonis G, Toutouzas K, et al. Joint microwave radiometry for inflammatory arthritis assessment. Rheumatology. 2020;59(4):839-44. [DOI] [PubMed] [Google Scholar]
- 32. Pauk J, Ihnatouski M, Wasilewska A. Detection of inflammation from finger temperature profile in rheumatoid arthritis. Med Biol Eng Comput. 2019;57(12):2629-39. [DOI] [PubMed] [Google Scholar]
- 33. Jones B, Hassan I, Tsuyuki RT, Dos Santos MF, Russell AS, Yacyshyn E. Joints: myth or reality? a thermographic joint assessment of inflammatory arthritis patients. Clin Rheumatol. 2018;37(9):2567-71. doi: 10.1007/s10067-018-4108-0. [DOI] [PubMed] [Google Scholar]
- 34. Svaic V, Zura N. Cryotherapy effects measured by infrared thermography in elderly people with rheumatoid arthritis. Available from: https://www.qirt2018.de/portals/qirt18/doc/Tu.2.B.3.pdf.
- 35. Umapathy S, Vasu S, Gupta N. Computer aided diagnosis based hand thermal image analysis: a potential tool for the evaluation of rheumatoid arthritis. J Med Biol Eng. 2018;38(4):666-77. [Google Scholar]
- 36. Lerkvaleekul B, Jaovisidha S, Sungkarat W, Chitrapazt N, Fuangfa P, Ruangchaijatuporn T, et al. Comparisons between thermography and ultrasonography with physical examination for wrist joint assessment in juvenile idiopathic arthritis. Physiol Meas. 2017;38(5):691-700. doi: 10.1088/1361-6579/aa63d8. [DOI] [PubMed] [Google Scholar]
- 37. Snekhalatha U, Rajalakshmi T, Gopikrishnan M, Gupta N. Computer-based automated analysis of X-ray and thermal imaging of knee region in evaluation of rheumatoid arthritis. Proc Inst Mech Eng H. 2017;231(12):1178-87. doi: 10.1177/0954411917737329. [DOI] [PubMed] [Google Scholar]
- 38. Lasanen R, Piippo-Savolainen E, Remes-Pakarinen T, Kröger L, Heikkilä A, Julkunen P, et al. Thermal imaging in screening of joint inflammation and rheumatoid arthritis in children. Physiol Meas. 2015;36(2_suppl):273-82. doi: 10.1088/0967-3334/36/2/273. [DOI] [PubMed] [Google Scholar]
- 39. Capo A, Ismail E, Cardone D, Celletti E, Auriemma M, Sabatini E, et al. Joint functional impairment and thermal alterations in patients with psoriatic arthritis: a thermal imaging study. Microvasc Res. 2015;102:86-91. doi: 10.1016/j.mvr.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 40. Snekhalatha U, Anburajan M, Sowmiya V, Venkatraman B, Menaka M. Automated hand thermal image segmentation and feature extraction in the evaluation of rheumatoid arthritis. Proc Inst Mech Eng H. 2015;229(4):319-31. doi: 10.1177/0954411915580809. [DOI] [PubMed] [Google Scholar]
- 41. Frize M, Ogungbemile A. Estimating rheumatoid arthritis activity with infrared image analysis. Stud Health Technol Inform. 2012;180:594-8. [PubMed] [Google Scholar]
- 42. Korman P, Straburzyńska-Lupa A, Romanowski W, Trafarski A. Temperature changes in rheumatoid hand treated with nitrogen vapors and cold air. Rheumatol Int. 2012;32(10):2987-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frize M, Adéa C, Payeur P, Gina Di Primio M, Karsh J, Ogungbemile A. Detection of rheumatoid arthritis using infrared imaging. Int Soc Opt Eng. 2011;7962:79620M. [Google Scholar]
- 44. Spalding SJ, Kwoh CK, Boudreau R, Enama J, Lunich J, Huber D, et al. Three-dimensional and thermal surface imaging produces reliable measures of joint shape and temperature: a potential tool for quantifying arthritis. Arthritis Res Ther. 2008;10(1):R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blyth T, Stirling A, Coote J, Land D, Hunter J. Injection of the rheumatoid knee: does intra-articular methotrexate or rifampicin add to the benefits of triamcinolone hexacetonide? Br J Rheumatol. 1998;37(7):770-2. [DOI] [PubMed] [Google Scholar]
- 46. MacDonald AG, Land DV, Sturrock RD. Microwave thermography as a noninvasive assessment of disease activity in inflammatory arthritis. Clin Rheumatol. 1994;13(4):589-92. doi: 10.1007/BF02242999. [DOI] [PubMed] [Google Scholar]
- 47. Gatt A, Mercieca C, Borg A, Grech A, Camilleri L, Gatt C, et al. Thermal characteristics of rheumatoid feet in remission: baseline data. PLoS One. 2020;15(12):e0243078. doi: 10.1371/journal.pone.0243078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tan YK, Hong C, Li H, Allen JC, Jr, Thumboo J. Thermography in rheumatoid arthritis: a comparison with ultrasonography and clinical joint assessment. Clin Radiol. 2020;75(12):963.e17-963.e22. doi: 10.1016/j.crad.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 49. Tan YK, Hong C, Li H, Allen JC, Jr, Thumboo J. A novel use of combined thermal and ultrasound imaging in detecting joint inflammation in rheumatoid arthritis. Eur J Radiol. 2021;134:109421. doi: 10.1016/j.ejrad.2020.109421. [DOI] [PubMed] [Google Scholar]
- 50. Kellgren JH, Lawrence JS. Atlas of standard radiographs. The epidemiology of chronic rheumatism. Vol. 2. Oxford, UK: Blackwell; 1963. [Google Scholar]
- 51. Fraser S, Land D, Sturrock RD. Microwave thermography—an index of inflammatory joint disease. Br J Rheumatol. 1987;26(1):37-9. doi: 10.1093/rheumatology/26.1.37. [DOI] [PubMed] [Google Scholar]
- 52. Lahiri BB, Bagavathiappan S, Jayakumar T, Philip J. Medical applications of infrared thermography: a review. Infrared Phys Technol. 2012;55(4):221-35. doi: 10.1016/j.infrared.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ring EF, Ammer K. Infrared thermal imaging in medicine. Physiol Meas. 2012;33(3):R33-46. doi: 10.1088/0967-3334/33/3/r33. [DOI] [PubMed] [Google Scholar]
- 54. Ring EF. Historical development of thermometry and thermal imaging in medicine. J Med Eng Technol. 2006;30(4):192-8. doi: 10.1080/03091900600711332. [DOI] [PubMed] [Google Scholar]
- 55. Calin MA, Mologhianu G, Savastru R, Calin MR, Brailescu CM. A review of the effectiveness of thermal infrared imaging in the diagnosis and monitoring of knee diseases. Infrared Phys Technol. 2015;69:19-25. doi: 10.1016/j.infrared.2015.01.013. [DOI] [Google Scholar]
- 56. Ring EF. Thermographic and scintigraphic examination of the early phase of inflammatory disease. Scand J Rheumatol Suppl. 1987;65:77-80. doi: 10.3109/03009748709102180. [DOI] [PubMed] [Google Scholar]
- 57. Herry CL, Frize M. Quantitative assessment of pain-related thermal dysfunction through clinical digital infrared thermal imaging. Biomed Eng Online. 2004;3(1):19. doi: 10.1186/1475-925x-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reinberg A. Circadian changes in the temperature of human beings. Bibl Radiol. 1975;6:128-39. [PubMed] [Google Scholar]
- 59. Collins AJ, Ring EF, Cosh JA, Bacon PA. Quantitation of thermography in arthritis using multi-isothermal analysis. I. Thermographic index. Ann Rheum Dis. 1974;33(2_suppl):113-5. doi: 10.1136/ard.33.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ring EF, Collins AJ, Bacon PA, Cosh JA. Quantitation of thermography in arthritis using multi-isothermal analysis. II. Effect of nonsteroidal anti-inflammatory therapy on the thermographic index. Ann Rheum Dis. 1974;33(4):353-6. doi: 10.1136/ard.33.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ring EF, McEvoy H, Jung A, Zuber J, Machin G. Standards for devices used for the measurement of human body temperature. J Med Eng Technol. 2010;34(4):249-53. doi: 10.3109/03091901003663836. [DOI] [PubMed] [Google Scholar]