Abstract
Objective
To analyze the effects of light therapy (LT) on cartilage repair for knee osteoarthritis (OA) treatment.
Design
The PubMed, Embase, Scopus, and Web of Science databases were searched up to August 31, 2020 to identify in vitro and in vivo studies that analyzed the effects of LT on knee cartilage for OA treatment. The study and sample characteristics, LT intervention parameters and posttreatment outcomes were analyzed. Risk of bias was assessed using the Risk of Bias Assessment for Non-randomized Studies (RoBANS) tool.
Results
Three in vitro and 30 in vivo studies were included. Most studies were judged as high risk of performance and detection bias. Biochemical outcomes were analyzed for both in vitro and in vivo studies, and histological and behavioral outcomes were analyzed for in vivo studies. LT reduced extracellular matrix (ECM) degradation, inflammation, and OA progression, promoting ECM synthesis. LT improved pain-like behavior in animal models, having no apparent effect on gait performance. There were conflicting findings of some of the biochemical, histological, and behavioral outcomes.
Conclusion
The included studies presented different strategies and LT parameters. LT resulted in positive effects on cartilage repair and may be an adequate therapy for OA treatment.
Keywords: cartilage, knee, osteoarthritis, light therapy, laser
Introduction
Articular cartilage is composed of highly specialized cells, chondrocytes, that are sparsely distributed and have low replicative ability. The low replicative potential and the absence of vascular and neural support limit the repair process of the damaged cartilage.1,2 Osteoarthritis (OA) is a degenerative joint disease with multifactorial etiology, being age, joint injury, trauma, and obesity the main predisposing risk factors. 3 The increased expression of inflammation mediators alters the cartilage homeostasis by favoring the catabolic activity of chondrocytes, resulting in cartilage matrix disruption and loss.3,4 Contrary to others inflammatory arthritis diseases (e.g., rheumatoid arthritis), OA does not involve chronic systemic inflammation, 3 but has rather a joint-specific mechanism, leading to articular cartilage degeneration, subchondral bone remodeling, synovial thickening, and joint space narrowing. 4
A variety of nonsurgical and surgical treatments are available for the management of OA. Light therapy (LT) is an option of nonsurgical treatment, which aims to promote cartilage tissue regeneration. The cellular mechanisms by which LT stimulates cells include light absorption by cytocrome c oxidase at mitochondria.5,6 The activation of cytochrome c oxidase increases the calcium ions (CA2+), oxygen reactive species, and adenosine triphosphate (ATP) production. 6 These molecules are involved in several intracellular signaling pathways that lead to gene transcriptions related to cell proliferation, protein synthesis, and inflammation decrease.5,6 Despite the growing body of scientific evidence showing beneficial physiological effects,7 -10 LT has not been yet incorporated in clinical practice guidelines of OA treatment.11 -13
A systematic review 14 from 2013 analyzed the effect of LT parameters on animal models, but it failed to comprehensively address the effects on cartilage regeneration. Since then, many in vivo studies have been published and most were included in another recent systematic review. 15 However, their analysis was limited to the grading of cartilage quality, and other important outcomes such as extracellular matrix (ECM) synthesis/degradation, inflammation markers, and behavioral and histological outcomes, were not evaluated. Their evaluation is important for a more comprehensive and in-depth understanding of the efficacy of LT in cartilage repair. This systematic review aims to summarize the cartilage regeneration outcomes of in vitro and in vivo studies after applying LT interventions (isolated and compared with control or other interventions).
Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) guidelines. 16
Search Strategy and Study Selection
The electronic databases PubMed, Embase, Scopus, and Web of Science were searched to identify original in vitro and animal (in vivo) studies that assessed the effects of LT on knee cartilage for OA treatment. Searches were performed from database inception up to August 31, 2020. The search strategy is presented in Supplementary Table S1. The reference list of the most relevant studies was also screened to identify any other potentially eligible studies.
All records were exported to an Excel file (Microsoft Office) and the duplicates were removed by the software filter and then manually verified. Two authors (SO and RA) independently screened all titles and abstracts of initially identified on the search. The full texts of the potentially eligible studies were extracted and evaluated by the same authors to further assess their eligibility. Two other reviewers (AL and OC) were consulted in case of disagreement. The inclusion criteria were (1) in vitro or animal (in vivo) studies, (2) studies that focused on the effects of LT on knee articular cartilage, and (3) studies that included in vitro or in vivo OA models. The exclusion criteria were (1) reviews or meta-analysis, conference proceedings, or case studies; (2) studies not written in English language; (3) studies that did not assess the effects of LT on chondrocytes activity; (4) in vivo studies that analyzed laser irradiation under arthroscopy; or (5) studies that used an inflammatory arthritis model (e.g., rheumatoid arthritis).
Risk of Bias Assessment
The risk of bias of the included studies was assessed using the Risk of Bias Assessment tool for Non-Randomized Studies (RoBANS). The RoBANS is a validated tool to assess the risk of bias of nonrandomized studies. 17 It contains 6 domains for risk of bias comprising the “Selection of Participants,” “Confounding Variables,” “Exposure of Measurement,” “Blinding of Outcome Assessment,” “Incomplete Outcome Data,” and “Selective Outcome Reporting.” The criteria of each domain were adapted to the context of our systematic review, to specifically analyze the risk of bias arising from in vitro and in vivo studies. Two other domains—“Planning and Implementation of Interventions” and “Funding Bias”—were added to analyze other sources of bias that arise specifically from LT interventions. Table 1 describes the criteria used to judge the risk of bias of each domain. Two authors (SO and RA) judged and classified the risk of bias of all included studies as low risk, high risk or unclear.
Table 1.
Domains and Description for the Appraisal of the Risk of Bias for In Vitro and In Vivo Studies Using Risk of Bias Assessment Tool for Non-Randomized Studies (RoBANS).
| Domain | Description for In Vitro and In Vivo Studies |
|---|---|
| Selection of chondrocytes or animals |
Selection bias caused by inadequate selection
of cells and animal participants. In in vitro studies, selection of chondrocytes cells should be performed from commercially available cell lines or from cartilage samples collected from animals. In both cases, chondrocytes should be obtained from hyaline cartilage. Chondrocytes should be isolated from more than one animal with same characteristics (type, race, weight, and age) from the same anatomical site. Ideally, allocation of cells would be randomized. Chondrocytes phenotype after isolation protocol should be confirmed for specific chondrogenic surface markers (e.g., CD44, CD49, CD73, CD90, CD105, CD151, and CD166) and/or for chondrogenic markers (e.g., COL II, ACAN, SOX-9).16 -18 Control and intervention groups should be clearly defined. In in vivo studies, animal participants with same characteristics should be selected. Ideally, allocation of animals would be randomized. Controls and intervention groups should be clearly described. |
| Confounding variables |
Selection bias caused by inadequate
confirmation and consideration of confounding
variables. For in vitro studies, cartilage should be collected from the same anatomical sites and isolated chondrocytes should have the same viability and count among groups. Studies should implement the same isolation protocol and same protocol for establishing the primary cell culture(s). The number of cell passages should be the same for all experimental groups and should not be too high, since chondrocytes lose their phenotype with increasing number of passages. 19 The culture medium volume should be the same for all wells among experimental groups to avoid different radiation scattering between groups. Same experimental conditions should be guaranteed for both control and intervention groups (e.g., humidity, CO2 and temperature conditions). In vivo studies should be consistent regarding the animal model, race, weight and age, ratio of male/female among experimental and control groups and number of animals per group. Animal participants should be housed under the same conditions, light cycles time and temperature. The animal euthanasia and OA induction method should be clearly described and the same among groups. The day of OA induction should be clearly defined and the recovery time before interventions should be performed for all experimental and control groups. |
| Exposure of measurement |
Performance bias caused by inadequate
measurement of exposure. Measurement techniques should be adequate and well-established for the specific outcomes that studies are assessing, and their measurement protocol should be clearly described to allow for replication. Semiquantitative and/or qualitative analysis should be performed by two independent observers to ascertain interoperator reliability. |
| Blinding outcome assessment |
Detection bias caused by inadequate blinding of
outcome assessment. Outcome assessor and/or data analysist not blinded to group (i.e., intervention vs. control). For quantitative analyses, the blinding of outcome assessor and/or data analyst was not considered necessary. Otherwise (semiquantitative and qualitative analyses), blinding was required. |
| Incomplete outcome data |
Attrition bias caused by inadequate handling of
incomplete data outcome. Missing data in >5% of outcome variables. |
| Selective outcome reporting |
Reporting bias caused by selective outcome
reporting. Based on reporting of the collected/assessed outcomes and multiple subgroup analyses. |
| Planning and implementation of interventions* |
Performance bias caused by inadequate planning
and implementation of interventions. LT should be performed by the same operator and parameters should be clearly described to allow for replication. The application mode such as distance to cells/skin, scanning or skin contact method, angle of light source should be clearly described. Type of light source, operating mode (continuous or pulsed) and number of actuators should be defined in each experimental group. LT parameters should be stated, as well as the number of LT sessions and the number of irradiated points. A temperature control should be performed during interventions since LT should not induce a temperature rise in tissues or cells.4,20 Previous calibration and/or power parameters control during experiments should be performed. In in vitro studies, the radiation scattering between wells in same well plate must be considered during irradiation. Blinding of personnel or testing source (cells/animals) is not possible. In these interventions, the LT parameters are pre-determined, the personnel who applies the intervention (LT) cannot change the intervention or affect the outcomes. Thus, we did not judge performance bias related to blinding of personnel or testing source. |
| Funding bias a |
Funding bias caused due to financial sponsoring
or conflict of interest. Conflict of interest from study authors and/or sponsoring of industry. |
CD44, CD49, CD73, CD90, CD105, CD151, and CD166, antigen molecules at cells surface; COL II, collagen type II; ACAN, aggrecan; SOX-9, SRY-box transcription factor 9; OA, osteoarthritis; LT, light therapy.
Domains added to the validated RoBANS tool to adjust to the context of this systematic review.
Data Collection, Extraction, and Statistics
All data were extracted from the included studies into a Microsoft Excel spreadsheet by 1 author (SO) and reviewed by 3 other authors (RA, AL, and OC). The data collected were the following: sample size and characteristics (cell and animal type, in vitro and in vivo OA models, animal race, gender, age, and weight, experimental groups, number of animals per group, and sample collection methods), study characteristics (year, study design, aim, measured variables, limitations, and general remarks), LT parameters (emitter type, wavelength, operating mode, frequency, duty cycle, pulse duration, power, power density, beam spot size, energy per point, total energy, energy density, irradiation time, treatment duration, application technique, irradiation area, and the number of points irradiated), biochemical and histological cartilage response outcomes. The biochemical outcomes describe the chondrocytes activity, ECM synthesis and/or degradation and the inflammatory activity. The histological outcomes comprise the effects on the quality of articular cartilage. In addition, behavioral outcomes, that analyze the pain-like behaviors and comprised the gait performance, weightbearing, and mechanical hyperalgesia analysis were collected.
The median, 25% and 75% percentiles, and minimum and maximum values were calculated for each LT parameter.
Results
Search Strategy
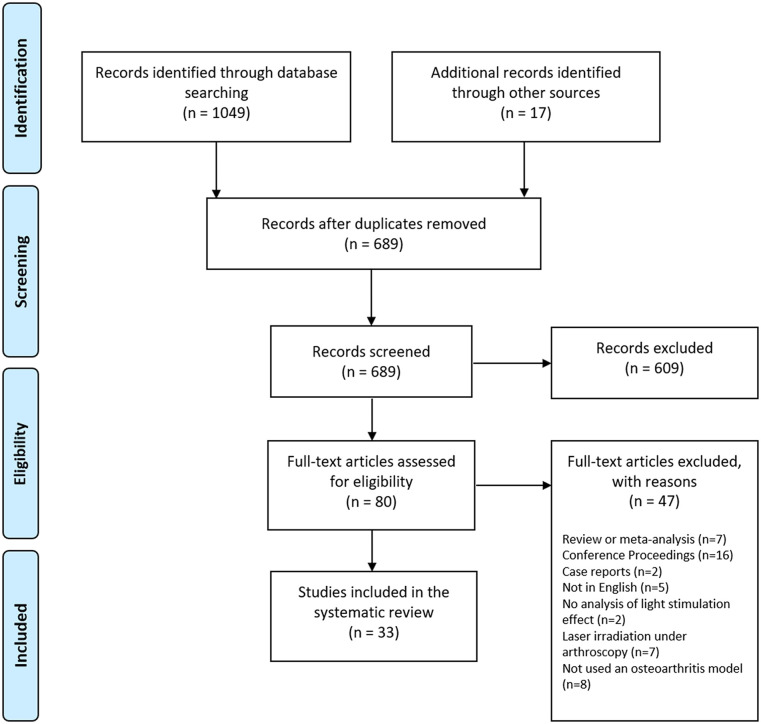
The PRISMA flowchart search can be found in Fig. 1 . From the initial 1049 records, identified 33 studies (3 in vitro18 -20 and 30 in vivo7 -9,21 -47) met the eligibility criteria and were included in this systematic review.
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) flowchart of included and excluded studies.
Risk of Bias
In Vitro Studies
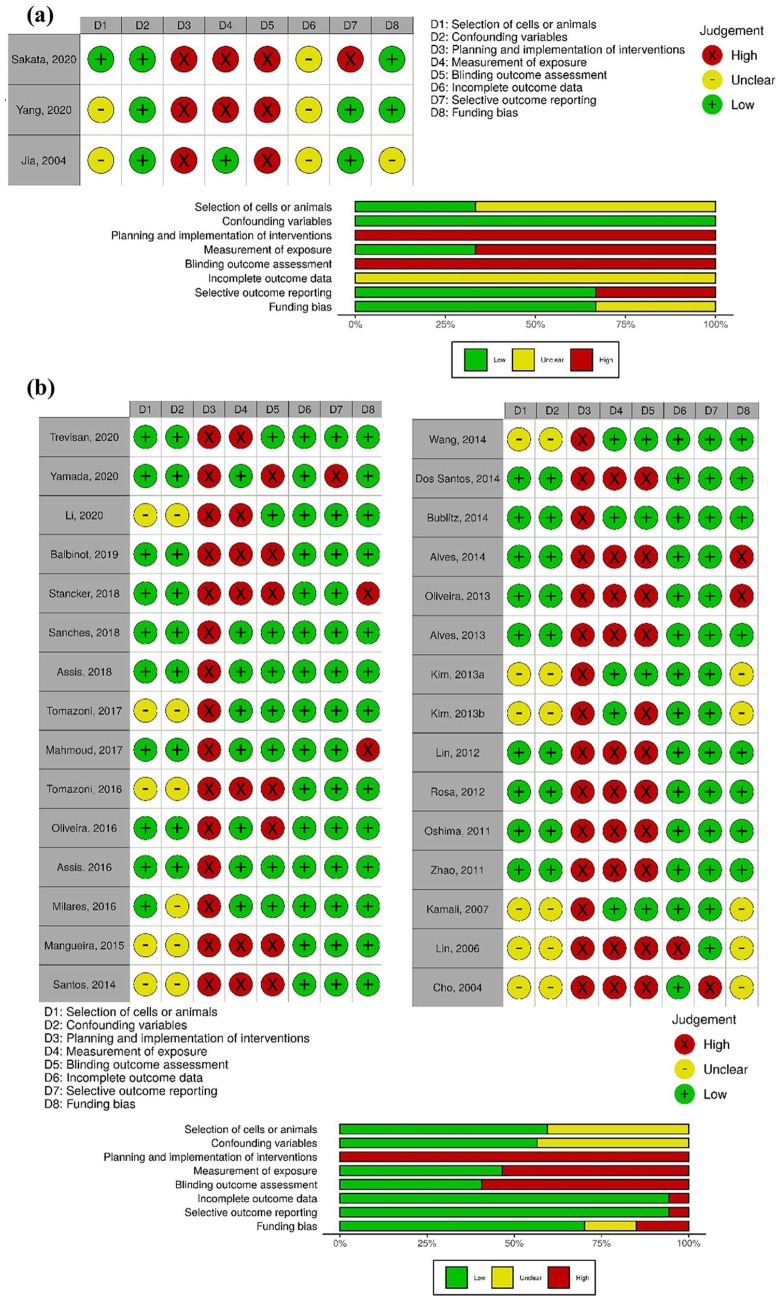
The judgment of risk of bias for each in vitro study and a summary for each domain is displayed in Figure 2a . The “Selection of Cells” domain presented unclear risk of bias in 2 in vitro studies19,20 that did not report the animal characteristics from which the cartilage samples were obtained. The “Confounding Variables” domain was judged as low risk of bias for all in vitro studies. Although, the culture medium volume was not provided in 2 studies19,20 and the influence of the culture medium volume in varying levels of irradiation could not be assessed. The “Planning and Implementation of Interventions,” “Exposure of Measurement,” and “Blinding Outcome Assessment” domains were judged as high risk of bias due to an inadequate measurement of semiquantitative or qualitative outcomes. The “Incomplete Outcome Data” domain was judged as unclear risk for all in vitro studies because these studies did not report the sample size.
Figure 2.
Risk of bias plots. Traffic lights and weight summary plots for (a) in vitro studies and (b) in vivo studies.
In Vivo Studies
The judgment of risk of bias for each in vivo study and a summary for each domain is displayed in Fig. 2b . The “Selection of Animals” domain was judged as unclear risk in 40% of in vivo studies because animal age, sex, or weight were not reported, precluding the evaluation of the potential risk of differences between groups. The “Planning and Implementation of Interventions” domain was judged as high risk of bias for all studies because the studies did not control for the temperature before, during and after the LT intervention. The temperature control is an important factor since the observed outcomes may result from thermic effects and not from the application of light by itself.48,49 All studies also failed in consistently reporting all the LT parameters. The “Exposure of Measurement” domain was judged as high risk of bias in 43% of studies as these studies did not include 2 independent observers for semiquantitative and/or qualitative analysis. In the absence of those independent operators, blinding should have been implemented to avoid detection bias, which was only performed by 53% of studies as represented by “Blinding Outcome Assessment” domain. The “Selective Reporting of Outcomes” domain was judged as high risk of bias in 13% of studies as these studies did not report the results of all measured outcomes. The “Funding Bias” domain was also judged as high risk of bias in 13% of studies due to the lack of reporting of potential conflict of interest.
In Vitro Studies
Study Characteristics
All in vitro studies were based on monocultures experiments. Two studies (67%) used chondrocytes isolated from the knee cartilage of New Zealand white rabbits, which were further expanded in vitro. One study (33%) used human chondrocytes cell lines. Only 1 study (33%) conducted experiments in an in vitro OA model, which consisted in the administration of recombinant human interleukin-1β (IL-1β) to stimulate the inflammatory environment that occurs naturally in knees with OA.
LT Parameters
All studies reported the wavelength, operating mode, power output, energy density, irradiation time, treatment duration, and irradiation area parameters. The light stimulus was used in pulse mode in 1 study (33%) and continuous in the remaining 2 studies (67%). The median laser wavelength was 632.8 nm (range, 632.8-910.0 nm), with a median power output of 7 mW (range, 2.5-10.0 mW), and a median energy density of 4.0 J/cm2 (range, 2.50-5.87 J/cm2) for a median duration of 390 seconds (range, 180-660 seconds) with a median irradiating area of 0.91 cm2 (range, 0.785-9.6 cm2). Supplementary Table S2 reports the LT parameters used in each study. Supplementary Table S3 summarizes the descriptive statistics of the reported LT parameters.
Biochemical Outcomes
The in vitro studies only reported biochemical outcomes (Supplement Table S4), including the chondrocytes activity (67%, k = 2), ECM synthesis and/or degradation (100%, k = 3) and the expression of inflammatory markers (67%, k = 2). Table 2 presents the study design and outcomes reported from each in vitro study.
Table 2.
Study Design and Reported Biochemical Outcomes for In Vitro Studies.
| First Author, Year | Operating Mode and Treatment Duration | Study Design | Biochemical Outcomes | ||
|---|---|---|---|---|---|
| Chondrocytes Proliferation and Activity | ECM Synthesis/Degradation | Inflammatory Markers | |||
| Sakata, 2020 18 | Pulsed mode once for the duration of 4 or 12h | Human articular chondrocyte-knee (NHAC-Kn) cell line treated with recombinant human IL-1β | NA | ↓ MMP-1 and ↓ MMP-3 after both 4 and 8 J/cm2
No effect on MMP-9 and MMP-13 after both 4 and 8 J/cm2 (gene expression by RT-PCR, protein expression by Western Blot and ELISA) |
↓ IL-1β, ↓ IL-6, ↓ TNF-α for both 4 and 8
J/cm2
(gene expression by RT-PCR and protein expression by Western blot) |
| Yang, 2020 19 | Continuous, 8 min daily for 1, 3 and 5 days | Chondrocytes isolated from New Zealand white rabbits’
cartilage and expanded C28/I2 Human Chondrocyte Cell Line for gene expression analysis only |
↑ Chondrocytes viability after 8 min of LT (MTS
assay) No effect after 11 and 13 min of LT exposure ↑ Viable cells (live and dead assay) |
No effects on GAGs production (Alcian blue assay) until
6 days but were higher than control in posttreatment
period ↑ Matrix deposition (safranin O staining) ↓ COL I for all time points and ↑ COL II after 5 days (protein expression by western blot) ↑ ACAN, ↑ COL II, ↑ SOX-9 after 5 days and ↓ COL I after 3 and 5 days (gene expression by RT-PCR) ↓ MMP-13 after IL1-β and LT stimulation (protein expression by western blot and gene expression by RT-PCR) |
↓ IL1-β after LT and ↓ TNF-α after IL1-β and LT
stimulation (protein expression by Western blot and gene expression by RT-PCR) |
| Jia, 2004 20 | Continuous, 3 times at 24-hour intervals. | Chondrocytes isolated from New Zealand rabbits´ cartilage and expanded | ↑ Chondrocytes viability after irradiation at 4
J/cm2
Irradiations at 1, 2, 3 J/cm2 did not improve this outcome After 5 and 6 J/cm2, the viability decreased (XTT assay) |
↑ GAGs expression intensity (Toluidine-blue
staining) ↑ COL II expression intensity (immunocytochemistry) |
NA |
COL I and II, collagen type I and II; MMP, matrix metalloproteinase; ACAN, aggrecan; SOX-9, SRY-box transcription factor 9; GAGs, glycosaminoglycans; IL, interleukin; TNF-α, tumor necrosis factor-α; RT-PCR, reverse transcription polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay; NA, not applicable.
The chondrocyte proliferation and viability measured by MTS 19 [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] and XTT 20 [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] assays were increased after LT, which were time and dosage dependent.
At the ECM, the matrix proteins collagen type II (COL II) and aggrecan (ACAN) expressions were increased after LT treatment measured by polymerase chain reaction (PCR 19 ) and immunocytochemistry. 20 One study 19 reported no significant differences in glycosaminoglycans (GAGs) stained by Alcian blue during the treatment duration but revealed a significant difference at the posttreatment follow-up (until day 12 posttreatment). The same study 19 reported a downregulation of collagen type I (COL I) and an increase in transcription factor SOX-9 expression (measured by PCR and Western blot). 19 After the light exposure, another study 18 demonstrated a significant decline in the expression of matrix metalloproteinase (MMP) MMP-1 and MMP-3, in contrast to the expressions of MMP-9 and MMP-13 that were not significantly altered (measured by PCR and Western blot). 18 Conversely, a significant decrease in the expression of MMP-13 (measured by PCR and Western blot) after LT was reported in other study. 19
The inflammation analyzes revealed a significant downregulation of inflammatory markers such as IL-1β, IL-6, and TNF-α (measured by PCR),18,19 with or without the administration of IL-1β to the cell culture medium. 18
In Vivo Animal Studies
Study Characteristics
A total of 1,400 animals were in 30 in vivo studies, distributed in 2 to 9 experimental groups, containing 5 to 20 animals per group. The most common animal model was rat (80%, k = 24), of which 83% were Wistar rats. Male animals were more commonly used (73%), with only 2 studies including females (7%) and 3 studies including both genders (10%). The rat models (n = 1071) were 1.5 to 3 months old and weighed 150 to 350 g, whereas the rabbit models (n = 267) were 4 to 15 months old and weighed between 2,000 and 4,500 g.
The in vivo studies described different models of experimental OA induction, being the most common the anterior cruciate ligament transection (ACLT) in the knee (k = 11, 37%), followed by intra-articular injections of papain solution (k = 9, 30%) or monoiodoacetate (MIA) (k = 5, 17%). After the animal sacrifice, most studies collected the knee joints (k = 22, 73%). The study design and outcomes reported for in vivo studies are presented in Table 3 .
Table 3.
Study Design and Biochemical, Histological, and Behavioral Outcomes for In Vivo Studies.
| First Author, Year | Operating
Mode Treatment Duration (No. of Sessions) |
Experimental Design | OA Model | Animal
Type Gender Animal Race Age (months) Weight (g) |
Biochemical Outcomes | Histological Outcomes | Behavioral Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ECM Synthesis/Degradation | Inflammatory and Pain Markers | Osteoarthritis Grade | Morphometric Analysis | Cartilage Organization | ||||||
| Trevisan, 2020 21 | Continuous 3 times per week (12) |
G1: OA (n = 10) G2: OA + LT (n = 10) |
ACL transection | 20 Rats Male Wistar 2 months 150 g |
↑ COL II [G2 vs G1]
(immunoexpression) ↑ TGF-β (growth factor) [G2 vs. G1] (immunoexpression) |
NA | ↓ OARSI score [G2 vs. G1] |
NA | Abnormal chondrocyte orientation and proliferation. Few irregularities and fibrillation after LT. | No effect on gait performance |
| Yamada, 2020 9 | Pulsed 3 times per week (8) |
G1: Saline injection (n =
10) G2: Saline + LT at 18 J/cm2 (n = 10) G3: OA (n = 10) G4: OA + LT at 6 J/cm2 (n = 10) G5: OA + LT at 18 J/cm2 (n = 10) |
Intra-articular injection of MIA | 50 Rats Male Wistar 3 months 250 g |
NA | ↓ Lipid oxidation [G4/G5 vs. G2] in spinal
cord and [G4 vs. G2] in blood
serum ↓ Protein carbonyl [G5 vs. G2] in spinal cord ↑ SOD activity [G4/G5 vs. G2] in brainstem ↓ Nonprotein thiol [G4/G5 vs. G2] in blood serum ↑ Nonprotein thiol [G4/G5 vs. G2] in brainstem ↓ MPO activity [G5 vs. G2] in intra-articular lavage ↑ MPO activity [G4/G5 vs. G2] in blood Serum ↓ IL-1β, TNF-α, IL-6 [G5 vs. G2] No differences in IL-10 (protein expression by ELISA) |
NA | NA | NA | ↑ Mechanical Hyperplasia ↑ Spontaneous pain [G5 vs. G2] |
| Li, 2020 8 | NR daily (7) |
G1: Saline injection (n = 8) G2: OA (n = 8) G3: OA + LT (n = 8) G4: OA + sham LT (n = 8) |
Intra-articular injection of MIA | 32
Rats Male Sprague-Dawley NR 220-250 g |
↓ MMP-3 [G3 vs. G2/G4] (immunoexpression) |
↓ TNF-α, ↓ IL-1β, ↓ IL-6 [G3 vs. G2/G4] (protein expression by ELISA) |
↓ OARSI score [G3 vs. G2/G4] |
NA | LT inhibited cartilage destruction and proteoglycan loss. | ↑ Mechanical hyperalgesia ↑ Weightbearing [G3 vs. G2/G4] |
| Balbinot, 2019 7 | Continuous Daily (15) |
G1: OA (n = 9) G2: OA + LT (n = 10) |
Intra-articular injection of MIA | 19 Rats Male Wistar 3 months 355 ± 22 g |
↑ PGs ↑ Chondrocyte content [G2 vs. G1] (toluidine blue staining) |
↓ Reactive astrogliosis in spinal
cord (immunoexpression) [G2 vs. G1] |
NA | ↑ Chondrocyte content [G2 vs. G1] (toluidine blue staining) |
NA | No effect on gait performance; ↑ Weightbearing; ↑ Mechanical Hyperplasia [G2 vs. G1] |
| Stancker, 2018 22 | Continuous Daily (7) |
G1: Culture medium injection (n =
10) G2: OA (n = 10) G3: OA + LT (n = 10) G4: OA + ADSCs (n = 10) G5: OA + ADSCs + LT (n = 10) |
Intra-articular injection of papain solution | 50 Rats Male Wistar 3 months 250-300 g |
↑ COL II [G3/G4 vs. G2] [G5 vs. G2/G4] ↓ MMP-2 and ↓ MMP-13 [G3/G4/G5 vs. G2] No differences among treated groups. (Protein expression by Western blot) ↓ MMP-1, ↓ MMP-2, ↑ TIMP-1 and ↑ TIMP-2 [G3/G4 vs. G2] [G5 vs G2/G3/G4] (Gene expression by PCR) |
↓ IL-1-β and ↓ TNF-α [G3/G4 vs. G2] [G5 vs. G2/G4] ↓ IL-6 [G3/G4 vs. G2] [G5 vs. G2/G3/G4] ↑ IL-10 [G3 vs. G2] [G5 vs. G2/G4] (Gene expression by PCR) |
NA | NA | NA | NA |
| Sanches, 2018 23 | Continuous 3 times a week (29) |
G1: OA (n = 10) G2: OA + LT (n = 10) G3: OA + CS/GL (n = 10) G4: OA + LT + CS/GL (n = 10) |
ACL transection | 40 Rats Male Wistar 2 months 150 g |
↑ COL II [G4 vs. G1]
No differences among treated groups. (immunoexpression) |
↓ IL-1β and ↑ IL-10 [G4 vs. G1]
No differences among treated groups. (immunoexpression) |
↓ OARSI score [G3/G4 vs. G1] No differences among treated groups |
↓ Density of chondrocytes [G2/G3/G4 vs. G1] No differences among treated groups. No difference in cartilage thickness |
Degradation, fibrillation, hypercellularity and chondrocytes disorganization among treated groups. | NA |
| Assis, 2018 24 | Continuous NR |
G1: OA (n = 10) G2: OA + aerobic exercise (n = 10) G3: OA + aquatic exercise (n = 10) G4: OA + aerobic exercise + LT (n = 10) G5: OA + aquatic exercise + LT (n = 10) |
ACL transection | 50 Rats Male Wistar 1.5 months 150 ± 11.2 g |
No effect on COL I ↑ COL II [G2/G3/G4/G5 vs. G1] No differences among treated groups. (immunoexpression) |
↑ IL-10 [G2/G3/G4/G5 vs. G1]
↑ TGF-β [G2/G4 vs. G1] No differences among treated groups. (immunoexpression) |
↓ OARSI score [G2/G3/G4/G5 vs. G1] No differences among treated groups. |
NA | Abnormal chondrocyte orientation and proliferation, few irregularities and fibrillation among treated groups. | NA |
| Tomazoni, 2017 25 | Continuous 3 times a week (24) |
G1: No intervention (n = 6) G2: OA (n = 6) G3: OA + exercise (n = 6) G4: OA + NSAID (n = 6) G5: OA + LT (n = 6) G6: OA + exercise + NSAID (n = 6) G7: OA + exercise + LT (n = 6) G8: OA + NSAID + LT (n = 6) G9: OA + exercise + LT + NSAID (n = 6) |
Intra-articular injection of papain solution | 54
Rats Male Wistar NR 200-250 g |
NA | ↓ IL-1β [G5 vs. G2/G3/G4/G6G9] [G7 vs. G6/G9] No effect on IL-6 and TNF-α (gene expression by PCR) ↓ IL-1β [G4/G5/G7/G8 vs. G2] [G5 vs. G2/G7] [G7 vs. G6/G9] ↓ IL-6 [G3/G4/G5/G6/G7/G8/G9 vs. G2] [G5/G7 vs. G4/G6] ↓ TNF-α [G3/G4/G5/G6/G7/G8/G9 vs. G2] [G5/G7 vs. G3/G6] (Protein expression by ELISA) |
NA | NA | NA | NA |
| Mahmoud, 2017 26 | Pulsed 2-3 times a week (10) |
G1: OA (n = 6) G2: OA + US (n = 6) G3: OA + LT (n = 6) |
Injection of saline solution of Lev | 18 Rats Male Albino 3 months 150-200 g |
NA | ↓ IL-1β [G2/G3 vs. G1]
↓ IL-6 [G2 vs. G1] ↑ IL-10 and ↓ INF-γ [G2 vs. G1/G3] No effect on TNF-α (Protein expression by ELISA) |
NA | ↓ Mankin scores [G2/G3 vs. G1] |
Similar histological evidences among treated groups: smooth surface without irregularities. | ↑ Knee maximum extension [G2/G3 vs. G1] |
| Tomazoni, 2016 28 | Continuous 3 times a week (24) |
G1: No intervention (n = 6) G2: OA (n = 6) G3: OA + LT (n = 6) G4: OA + exercise (n = 6) G5: OA + Diclo (n = 6) G6: OA + exercise + LT (n = 6) G7: OA + Diclo + LT (n = 6) G8: OA + exercise + Diclo (n = 6) G9: OA + exercise + LT + Diclo (n = 6) |
Intra-articular injection of papain solution | 54
Rats Male Wistar NR 200-250 g |
↓ MMP-3 [G3/G5/G7/G9 vs. G2] No differences among treated groups. ↓ MMP-13 [G3 vs. G2/G7/G6] [G4/G5/G8/G9 vs. G2/G7] (gene expression by PCR) |
↓ Inflammatory cells number [G3/G5 vs. G2/G4/G6/G7/G8/G9] (intra-articular wash counting) ↑ MPO activity [G3/G6 vs. G2/G4/G5/G7] (synovial supernatant quantification) |
NA | NA | LT and exercise alone promoted a homogeneous chondrocytes distribution. Remaining treated groups presented chondrocytes distributed heterogeneously. | NA |
| de Oliveira, 2016 27 | Continuous NR |
G1: No intervention (n = 18) G2: OA (n = 18) G3: OA + LT (n = 18) |
Intra-articular injection of papain solution | 54 Rats Male Wistar 3 months 250-300 g |
NA | ↓ TNF-α [G3 vs. G2]
(Protein expression by ELISA) |
NA | NA | NA | ↑ Mechanical hyperalgia [G3 vs. G2] |
| Assis, 2016 29 | Continuous 3 times a week (24) |
G1: No intervention (n = 10) G2: OA (n = 10) G3: OA + aerobic exercise (n = 10) G4: OA + LT (n = 10) G5: OA + aerobic exercise + LT (n = 10) |
ACL transection | 50 Rats Male Wistar 1.5 months 150 g |
↓ MMP-13 [G3/G4/G5 vs. G2]
No differences among treated groups. (immunoexpression) |
↓ IL-1β [G3/G4/G5 vs. G2]
No differences among treated groups. ↓ Caspase-3 [G3/G4 vs. G2] [G5 vs. G2/G3/G4] (immunoexpression) |
↓ OARSI score [G3/G4/G5 vs. G2] No differences among treated groups. |
No differences in chondrocytes density ↑ Cartilage thickness [G3/G4/G5 vs. G2] No differences among treated groups. |
Few signs of fibrillation and irregularities, moderate number of chondrocytes and organization among treated groups. | NA |
| Milares, 2016 30 | Continuous 3 times a week (24) |
G1: OA (n = 10) G2: OA + exercise (n = 10) G3: OA + LT (n = 10) G4: OA + exercise + LT (n = 10) |
ACL transection | 40 Rats Male Wistar 1.5 months 150 g |
↓ MMP-13 [G4 vs. G1]
No differences among treated groups. (immunoexpression) |
↓ IL-1β and ↓ Caspase-3 [G2/G4 vs. G1] No differences among treated groups. (immunoexpression) |
↓ OARSI score [G2/G3/G4 vs. G1] No differences among treated groups. |
↓ Chondrocytes density and ↑ Cartilage
thickness [G2/G3/G4 vs. G1] No differences among treated groups. |
Less tissue degradation, marks of fibrillation and irregularities, and chondrocytes organization among treated groups. | NA |
| Mangueira, 2015 31 | Continuous NR |
G1: Saline injection (n = 9) G2: OA (n = 9) G3: OA + LT at 660 nm (n = 10) G4: OA + LT at 780 nm (n = 10) |
Intra-articular injection of collagenase | 36
Rats Male Wistar NR 220-260 g |
↑ COL III area [G3 vs. G2/G4] ↑ COL II area [G3/G4 vs. G2] (Picrosirius red staining) |
NA | NA | NA | NA | NA |
| Dos Santos, 2014 32 | Continuous NR |
G1: No intervention (n = 5) G2: OA (n = 5) G3: OA + PMT at 2 J (n = 5) G4: OA + LT at 4 J (n = 5) |
Intra-articular injection of papain solution | 20 Rats Male Wistar 3 months 250-300 g |
NA | ↓ Neutrophils and ↓ Macrophages [G3/G4 vs. G2] [G3 vs. G4] (Intra-articular wash counting) ↓ IL-1β and ↑ IL-10 [G3 vs. G2] No differences among treated groups. ↓ TNF-α [G4 vs. G2/G3] [G3 vs. G2] ↓ IL-6 [G4 vs. G3] (Gene expression by PCR) |
NA | NA | Similar histological evidences among treated groups: low intensity acute inflammation and normal articular surface. | NA |
| Wang, 2014 34 | Continuous 3 times a week (6, 12, 18, or 24) |
G1: OA (n = 80) G2: OA + LT (n = 80) |
ACL transection | 160 Rabbits NR New Zealand 6 months 3500± 800 g |
↑ COL II, ↑ ACAN, ↑ TGF-β, ↑ MMP-1 and ↑ MMP-13 after 8
weeks [G2 vs. G1]
↑ TIMP-1 and ↑ MMP-3 after 6 and 8 weeks [G2 vs. G1] No effect on IGF-1, BMP-2 and BMP-7 (gene expression by PCR) |
↓ IL-1β after 6 and 8 weeks [G2 vs. G1] (gene expression by PCR) |
↓ OA score after 6 and 8 weeks [G2 vs. G1] |
NA | LT improved cartilage damage and erosion at all locations. | ↑ Weight bearing after 6 and 8 weeks [G2 vs. G1] |
| Dos Santos, 2014 33 | Continuous 5 times a week (15 or 30) |
G1: Control (n = 20) G2: OA (n = 20) G3: LT at 10 J/cm2 (n = 20) G4: LT 50 J/cm2 (n = 20) |
ACL transection | 80 Rats Male Wistar 3 months 300 ± 20 g |
No differences in COL II organization and
intensity ↓ MMP-13 after 8 weeks [G3/G4 vs G2] No differences among treated groups (immunoexpression) |
No effect on TNF-α and
IL-1β (immunoexpression) |
NA | ↓ Cell number after 8 weeks [G3/G4 vs. G2] No differences among treated groups No effect of cartilage thickness |
G4 showed chondrocytes disorganization and intense presence of cells, while G3 presented slight fibrillation and moderate presence of cells after 8 weeks of LT. | NA |
| Bublitz, 2014 35 | Continuous 5 times per week (15) |
G1: OA (n = 10) G2: LT at 10 J/cm2 (n = 10) G3: LT at 50 J/cm2 (n = 10) |
ACL transection | 30 Rats Male Wistar 3 months 300 ± 20 g |
No effect on MMP-13
expression (immunoexpression) |
No effect on IL-1β
expression (immunoexpression) |
NA | No effect on chondrocytes number. ↓ Cartilage area [G2/G3 vs. G1] ↓ PGs reduction score [G2/G3 vs. G1] (safranin O staining) No differences among treated groups |
G2 presented more tissue and chondrocytes organization with no fibrillation in comparison to G1 and G2. G3 presented tissue disorganization in comparison to G2, but better organized than G1. | NA |
| Alves, 2014 36 | Continuous 3 times a week (4, 7, or 10) |
G1: No intervention (n = 15) G2: OA (n = 15) G3: LT at 50 mW (n = 15) G4: LT at 100 mW (n = 15) |
Intra-articular of papain solution | 60 Rats Male Wistar 3 months 250-300 g |
↓ COL I and ↑ COL II [G3/G4 vs G2] (Picrosirius red staining) ↓ MMP-2 and ↓ MMP-9 after 7 and 14 days [G3/G4 vs G2] ↓ MMP-2 and ↓ MMP-9 after 21 days [G3 vs G2] (Protein expression by Western blot) No differences among treated groups |
NA | NA | NA | After 21 days, G3 showed tissue repair, but fewer fibroblast. G4 presented a thick synovial membrane and tissue repair. | NA |
| Oliveira, 2013 37 | Continuous 5 times a week (15 or 30) |
G1: No intervention (n = 20) G2: OA (n = 20) G3: LT at 10 J/cm2 (n = 20) G4: LT at 50 J/cm2 (n = 20) |
ACL transection | 80 Rats Male Wistar 3 months 300 ± 20 g |
No effect on MMP-13 ↑ COL I after 5 weeks [G3 vs. G2] ↓ COL I after 8 weeks [G3/G4 vs. G2] (immunoexpression) No differences among treated groups |
No differences in TNF-α and
IL-1β (immunoexpression) |
No effect on Mankin score | ↓ Chondrocytes number after 8 weeks [G3/G4 vs. G2] No differences among treated groups No effect on cartilage thickness. |
After 8 weeks, G3 showed a better tissue organization compared with G2, moderate number of cells, slight fibrillation and irregularities. G4 exhibited more disorganized tissue compared to G3, moderate presence of chondrocytes and fibrillation. | NA |
| Alves, 2013 38 | Continuous Once |
G1: No intervention (n = 15) G2: OA (n = 15) G3: LT at 50 mW (n = 15) G4: LT at 100 mW (n = 15) |
Intra-articular injection of papain solution | 60 Rats Male Wistar 3 months 250-300 g |
NA | ↓ IL-1β and ↓ IL-6 [G3 vs. G2]
↓ TNF-α [G4 vs. G2/G3] (gene expression by PCR) ↓ Neutrophils [G3/G4 vs. G2] ↓ Macrophages [G3 vs. G2/G4] (Intra-articular wash counting) |
NA | NA | G3 presented tissue with fibroblast and discrete inflammatory cells, while G4 showed acute inflammatory infiltrate, red blood cells and hyaline material presence. | NA |
| Kim, 2013 39 | Pulsed 5 times per week (15) |
G1: Saline injection (n =
10) G2: OA (n = 10) G3: LT (n = 10) |
Intra-articular injection of MIA | 30
Rats Male Sprague-Dawley NR 150-160 g |
NA | ↓ TNF-α, ↓ IL-1β and ↓ IL-6 [G3 vs. G2] (protein expression in blood by ELISA) |
NA | NA | NA | NA |
| Kim, 2013 40 | Pulsed 5 times per week (15) |
G1: Saline injection (n =
10) G2: OA (n = 10) G3: LT (n = 10) |
Intra-articular injection of MIA | 30
Rats Male Sprague-Dawley NR 150-160 g |
NA | NA | NA | NA | NA | ↑ Mechanical hyperplasia after 14 and 21 days ↑ Weightbearing after 21 days [G3 vs. G2] |
| Lin, 2012 41 | Continuous 5 times a week (10) |
G1: No intervention (n = 8) G2: OA (n = 8) G3: LT (n = 8) |
ACL transection | 24 Rabbits male/female (50%-50%) New Zealand 3 months 2,000-2,500 g |
NA | ↓ Caspase-3 [G3 vs. G2]
No effect on Caspase-8 (immunoexpression) |
↓ Markin score [G3 vs. G2] |
NA | After LT, the tissue presented a smoother surface with slight disorganization. | NA |
| Da Rosa, 2012 42 | Continuous Daily (7, 14, or 21) |
G1: OA (n = 12) G2: LT at 660 nm (n = 12) G3: LT at 808 nm (n = 12) |
Intra-articular injection of papain solution | 36 Rats Male Wistar 3 months 250-300 g |
NA | No effect on the number of inflammatory
cells. (immunoexpression) |
NA | NA | ↑ Newly formed vessels after 7 days [G2 vs. G1] ↓ Fibrosis intensity after 14 days [G2/G3 vs. G1] ↓ Fibrosis intensity after 21 days [G3 vs. G1] G3 presented better results regarding the formation of epithelium and new blood vessels. |
NA |
| Oshima, 2011 43 | Pulsed 5 times a week (25) |
G1: OA (n = 7) G2: LT (n = 7) |
ACL transection | 14 Rabbits Female New Zealand 9-15 months 3,500-4,500 g |
↑ COL II [G2 vs. G1]
(gene expression by PCR) No effect on ACAN, MMP-3, and MMP-13 |
↓ TNF-α [G2 vs. G1]
(gene expression by PCR) No effect on IL-1β |
↓ OA grade [G2 vs. G1] (Gross appearance analysis) |
NA | NA | NA |
| Zhao, 2011 44 | NR Every other day (15) |
G1: OA (n = 10) G2: OA + sham LT (n = 10) G3: Laser 10.6 µm + 650 nm (n = 10) G4: LT at 650 nm (n = 10) G5: Laser 10.6 µm (n = 10) |
Naturally after extreme exercise | 50 Mice male/female (50%-50%) C57 Black 5 months 20-25 g |
NA | NA | ↓ Mankin score [G3 vs. G2] |
NA | NR | NA |
| Kamali, 2007 45 | Pulsed Twice per week (8, 16, or 32) |
G1: Control (n = 6-8) G2: LT (n = 6-8) |
Osteo-chondral defect (5 × 4 mm) | 41 Rabbits NR Dutch White 4 months 2000 ± 300 g |
NA | NA | NA | No differences in cartilage thickness. ↑ Cartilage stiffness (biomechanical analysis) after 8 weeks [G2 vs. G1] |
NA | NA |
| Lin, 2006 46 | Continuous 3 times a week (24) |
G1: Early-stage OA; (n =
3-4) G2: Intermediate-stage OA (n = 3-4) G3: Late-stage OA (n = 3-4) G1s, 2s, 3s: LT (n = 3-4) G1c, 2c, 3c: Control (n = 3-4) |
Intra-articular injection of papain solution | 78
Rats Female Wistar NR 320-350 g |
NA | NA | ↓ OA grade [G1s vs. G1c] [G2s vs. G2c] |
NA | G1s and G1c showed fibrillation. G2s and G2c presented chondrocyte enlargement. G3s and G3c exhibited deep fibrillation and partial to total cartilage loss. | NA |
| Cho, 2004 47 | Pulsed Daily (15 or 30) |
G1: OA (n = 5) G2: LT for 2 weeks (n = 5) G3: LT for 4 weeks (n = 5) G4: OA for 2 weeks without LT (n = 5) G5: OA for 4 weeks without LT (n = 5) |
Intra-articular injection of H2O2 | 28 Rabbits NR New Zealand 10 months 2,500-3,000 g |
NA | NA | NA | NA | After 4 weeks of LT, chondrocytes disorganization was observed. | NA |
NR, not reported; NA, not applicable; G, group; LT, light therapy; OA, osteoarthritis; ACL, anterior cruciate ligament; MIA, monosodium iodoacetate; Lev, levofloxacin; COL I, II, and III, collagen type I, II, and III; MMP, matrix metalloproteinase; TIMP, tissue inhibitors of metalloproteinases; IL, interleukin; TNF-α, tumor necrosis factor-α; INF-γ, interferon gamma; ACAN, aggrecan; PGs, proteoglycans; OARSI, Osteoarthritis Research Society International; PCR, polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay; ADSCs, adipose-derived stem cells; US, ultrasounds; NSAID, nonsteroidal anti-inflammatory drug; Diclo, sodium diclofenac; SOD, superoxide dismutase; MOP, myeloperoxidase.
LT Parameters
The light stimulus was used in continuous mode in more than half of the studies (70%, k = 21). The median wavelength was 808 nm (range, 630-904 nm), at a median power of 50 mW (range, 30-60,000 mW), a median power density of 1,700 mW/cm2 (range, 0.4-3570 mW/cm2), and a median energy density of 50 J/cm2 (range, 2-1,500 J/cm2) for a median irradiation time of 40 seconds (range, 10-900 seconds). Most commonly, the treatment duration was 3 times a week (90%, k = 27) with a median number of sessions of 15 (range, 1-32) by skin contact (83%, k = 25) in a median of 2 (range, 1-2) points within the joint. Irradiation area was the less reported parameter, being only provided in 3 studies (10%). Supplementary Table S2 reports the LT parameters used in each in vivo study and Supplementary Table S3 summarizes the descriptive statistics for the reported LT parameters.
Biochemical Outcomes
The chondrocytes proliferation was not analyzed in any of the in vivo studies, but more than half of the studies evaluated the ECM synthesis/degradation and inflammatory markers (Supplementary Table S4).
The ECM synthesis and/or degradation after LT varied across the included studies. The expressions of COL II (measured by immunoexpression, 21 Western blot, 22 picrosirius red staining,31,36 and PCR34,43), GAGs (measured by toluidine blue staining 7 ), and ACAN (measured by PCR 34 ) increased significantly following LT. However, there was one study that revealed no effect on COL II and ACAN expressions (measured by immunoexpression 32 and PCR, 43 respectively). The MMP-13 expression after LT showed conflicting results; it was significantly downregulated in 5 studies (measured by PCR22,28,34 and immunoexpression29,33) and its expression was not affected in 4 studies (measured by immunoexpression30,35,37 and PCR 43 ). The MMP-3 analysis also showed a significantly decreased expression (measured by immuoexpression 8 and PCR 28 ) or no effect (measured by PCR 43 ) after LT. Other MMPs such as MMP-1, MMP-2, and MMP-9 were significantly reduced following LT (measured by PCR22,34 and Western blot 36 ). The LT also significantly increased the expression of the tissue inhibitors of metalloproteinases TIMP-1 and TIMP-2 (measured by PCR22,34).
The pro-inflammatory markers also showed conflicting evidence across the included studies. The most reported markers were IL-1β and TNF-α. These 2 markers were consistently and significantly decreased after LT (measured by enzyme-linked immunosorbent assay [ELISA]8,9,25 -27,39, PCR,22,25,33,34,38,43 and imunoexpression 29 ). Similar trends were observed for expressions of IL-6 (measured by ELISA8,9,25,39 and PCR22,25,33,38) and caspase-3 (measured by immunoexpression29,41), whereas the anti-inflammatory marker IL-10 significantly increased after light treatment (measured by PCR22,33). In contrast, some studies reported no effect on the expressions of IL-1β (measured by immunoexpression32,35,37 and PCR 43 ), IL-6 (measured by ELISA 26 ), TNF-α (measured by ELISA 26 and immunoexpression32,37), IL-10 (measured by ELISA9,26), and caspase-3 and caspase-8 (both measured by immunoexpression30,41). The LT exposure promoted a significant decrease in total number of inflammatory cells, namely neutrophils and macrophages (quantified by ELISA9,28 and differential cell counting33,38). On the contrary, another study reported no effect on total number of inflammatory cells as measured by immunoexpression. 42 LT also reduced oxidation (measured by lipid oxidation assay 9 ) and astrogliosis (measured by immunoexpression 7 ), which is associated with central inflammation in the spinal cord. 7
Histological Outcomes
The histological outcomes were assessed through the grading of OA, morphometric analysis, and cartilage organization (Supplementary Table S4). The LT significantly decreased the stage of OA, as graded by the Osteoarthritis Research Society International (OARSI) or OARSI modified score,8,21,29,30 Mankin,26,41,44 or other score systems.34,43,46 Only 2 studies23,37 reported no effect on OA grading after LT.
The LT interventions resulted in conflicting morphometric findings. While some studies showed significant decrease in chondrocytes density23,30,32,37 and increase in cartilage thickness,29,30 other studies35,45 reported no statistical differences on these 2 outcomes. The most commonly reported histological effects of LT were the slowing down of chondrocytes proliferation (in number and organization), fewer signs of fibrillation and less irregularities in articular cartilage.8,21,23,24,26,28,29,32,34 -37,41,46 Three studies33,36,38 detected local inflammatory signs after the induction of OA in, and 1 study 42 reported the formation of epithelium and new blood vessels after LT.
Behavioral Outcomes
The pain-like behavior was assessed by gait performance, weight distribution in each hind limb (weightbearing) and mechanical hyperplasia analysis (Supplementary Table S4). The LT did not influence the gait performance7,21 or maximum knee extension. 26 Weightbearing and mechanical hyperplasia were significantly increased after LT.7 -9,34,40,50
Discussion
The main findings of this systematic review are that LT promoted ECM synthesis and lowered pain and inflammation in in vitro and in vivo studies, suggesting a potential to slow down OA and cartilage degeneration.
The majority of the studies were judged as high risk of performance and detection bias. The dosage calculation was inconsistent since some authors considered the beam surface,21,22 while others assumed an irradiated surface area,7,43 and roughly one third of studies8,26,39,41,43-47,51 failed to report all LT parameters. Those factors preclude direct comparisons among studies. Lack of temperature control during stimulation also contributed for the high risk of performance bias. More than half of the studies did not measure the qualitative and/or semiquantitative data adequately, lacking 2 independent observers7,8,18,19,21,22,28,31-33,36-38,41-44,46,47 or blinding of the observers.7,9,20,22,27,28,31-33,36-38,40-44,46,47 These qualitative and semiquantitative analyses are more prone to errors as they estimate the concentrations and do not provide an accurate quantification. The use of distinct techniques may also diagnose differently the outcomes. One study did not find statistically significant differences in gene expression of IL-1β by PCR after LT, but on the protein expression (ELISA assay) analysis differences were detected. 25 Therefore, it is also advisable to complement qualitative analysis with a proper quantitative measurement of the outcomes.8,22,25,26,28,32,34,38,39,43,50
Many research models of OA have been explored to study the disease and its effect on the whole joint. The included in vitro studies used monolayer cultures of chondrocytes from primary sources. Other in vitro models such as cartilage explants, 52 3-dimensional culture 53 or co-culture with other cell types also implicated in the disease 54 were not explored in the context of light stimulation. The application of those models could also contribute with relevant insights about the in vitro effects of LT. In vivo models allow a more in-depth study of OA disease in the whole joint, as well as the effects of time, motion, and weightbearing. The included in vivo studies used small animals, most induced OA by surgical procedures (e.g., ACLT) or by chemical injections (e.g., papain and MIA). The surgical OA models promote joint destabilization which, consequently, results in OA, enabling a more comprehensive study of cartilage degeneration and its progression.21,23,24,29,30,32,34,35,37,41,43 The chemically induced models focus mainly on the inflammation and pain mechanisms.7-9,22,25,27,28,33,36,38-40,42,46 Thereby, in vitro and in vivo research models are significant to elucidate the LT effects on OA, contributing to the growing understanding of this therapy, before translating into clinical studies.
The use of LT resulted in biochemical-induced effects on articular cartilage. The in vitro studies demonstrated that the LT yielded a positive biochemical effect in cartilage, including an increase of ECM synthesis (COL II, ACAN, and GAGs) and a downregulation of ECM proteases (MMPs) and inflammatory markers (IL-1β, IL-6, and TNF-α).18-20 The LT produced a continuing effect on chondrocytes activity that persisted up to 12 days after treatment. 19 The in vivo studies showed that LT stimulated ECM matrix production while inhibiting its degradation.7,8,21,22,28,29,32,34,36,43 There was however 1 study reporting no effect in any of the matrix proteins 32 and4 other studies30,35,37,43 reporting no effect on MMPs expression. The COL I expression, which is commonly seen in fibrocartilage, was unaffected or decreased,24,36,37 suggesting that the LT is promoting a hyaline-like cartilage regeneration. 1 The effects of LT on inflammation markers were inconsistent. While most studies found a significant decrease on the expression of inflammatory markers,8,9,22,25-30,33,34,38,39,41,43 other studies did not find any effect.32,35,37,42 These findings combined suggest that LT can slow down the cartilage degeneration and has a potential to modulate the OA-derived joint inflammation, but the effects are variable.
Cartilage degeneration is characterized by chondrocytes hypertrophic proliferation and differentiation, resulting in chondrocytes apoptosis and cartilage replacement by bone. 55 The use of LT improved cartilage quality as assessed by histological analysis and suggested a deceleration of cartilage degeneration.8,21,24,26,28,34,41 Histologically, the included studies found a decrease of both OA progression8,21,26,29,30,34,41,44 and chondrocytes density23,29,30,32,37 with improved cartilage thickness.29,30 Four studies did not show any effect in any of the histological outcomes, including OA progression, chondrocytes density or cartilage thickness.23,35,37,45 These findings combined suggest that LT seem to have a significant effect in slowing down the OA progression.
The animal behavior after exposure to LT highlighted an analgesic effect as observed by weightbearing readaptation (distribution of weight across the hind paws) and mechanical hyperplasia (paw withdrawal in response to an increasing force).7-9,34,40,50 Although no changes on gait performance were observed, a control group without induction of OA would be needed to confirm if the lack of differences mean the LT had no effect on gait patterns or if the induction OA did not result in gait impairments.7,21 One study 46 used in vivo models with different OA stages and demonstrated that LT stimulated cartilage regeneration only at early and intermediate stages of OA, which suggests that LT might be unable to delay OA progression at more advanced stages.
To interpret outcomes, it is important to understand how variables can interfere in those results and that physiological effects of LT are dose dependent. While a very low energy may not be sufficient to promote a cellular response, an excessively high energy will inhibit those effects.5,6 Dose dependency of LT was investigated in 2 studies,19,20 which confirmed that longer exposure times or energy densities of LT did not result in better cellular viability. The influence of the LT dosages on articular cartilage repair was variable across the studies and remains inconclusive. While 1 study 33 reported that 71.4 J/cm2 at 50 mW was better than 142 J/cm2 at 50 mW in eliciting an anti-inflammatory response, another study 38 concluded that 142 J/cm2 at 50 mW was more efficient in modulating all inflammatory markers than 142 J/cm2 at 100 mW. Other studies32,35,37 also compared lower energy densities (10 and 50 J/cm2 at 30 mW) but no significant effect was observed in the inflammation process. Only 1 study 9 showed that 9 J/cm2 at an average power of 40 mW enhanced the anti-inflammatory response in pulsed mode. The effect of different wavelengths was also investigated. Both in vitro and in vivo studies applied red and near-infrared light (600-1100 nm), which corresponds to a higher absorption by chromophores at cellular mitochondria. 56 However, while an wavelength of 660 nm was better in repairing cartilage than 780 nm, 31 a wavelength of 808 nm was more effective in stimulating angiogenesis than 660 nm. 42 Other studies applied higher wavelength values using carbon dioxide laser (10.6 μm) to stimulate knee “acupoints” for laser acupuncture, but only 2 studies8,44 used this type of laser, limiting the conclusions. Finally, the included studies applied a wide range of other LT parameters (other than energy density, power, and wavelength), which hampers more direct comparisons between the studies and limits the critical rationale about the most appropriate dosage for knee cartilage repair.
The effects of LT combined with other conservative therapies was assessed in a few studies. When the LT was combined with different exercise modalities or topical use of nonsteroidal anti-inflammatory drugs (NSAIDs), there were no additional effects as compared with LT alone.24,25,28-30 On the other hand, when combined with intra-articular injection of stem cells or with chondroitin and glucosamine sulfate, LT showed an enhanced therapeutic effect in the articular cartilage.22,23
The World Association for Laser Therapy (WALT) guidelines recommends minimum dosage values for the application of LT in the knee.57,58 The WALT guidelines recommend a minimum of 4 J ± 50% energy per point, at a power of 5 to 500 mW, for 20 to 300 seconds, at 780 to 860 nm, when using GaAlAs lasers. 57 More than one-fourth of the studies did not follow those recommendations, applying lower values of energy per point—0.3 J and 1.4 J.23,24,29,30,32,35,37 Some of these studies were previously highlighted for not showing any effects on ECM synthesis, 32 downregulation of MMPs30,35,37 and inflammation markers,32,35,37 and lack of histological improvements.23,35,37 The administration of dosage values of LT below the therapeutic window may not be enough to trigger a cellular response. Most of the studies that used lower dosage values only observed significant differences in some of the outcomes (biochemical, histological, or behavioral) after more than 20 sessions of treatment.23,24,29,30,32,37 The number of sessions of treatment appears to be related to energy dose, with lower dosage values requiring more sessions of LT to elicit therapeutic effects. Following the WALT guidelines is of upmost importance to ensure optimized results, to establish direct comparisons among studies and to standardize the LT dosages according to the diagnosis. However, the WALT guidelines are only valid for GaAs and GaAlAs lasers and their recommended values may not be applicable to other type of lasers, such as He-Ne,19,34 InGaAlP, 42 and to LEDs (light-emitting diodes). 21 It is thus paramount and a priority to extend these guidelines to other types of lasers to allow researchers and clinicians to apply recommended dosages regardless of the type of laser utilized.
Some limitations of this systematic review should be highlighted. Our search strategy identified only a small number of in vitro studies, which limits our discussion on the in vitro effects of LT. Only in vivo studies that used OA models were included, excluding inflammatory arthritis and rheumatoid arthritis models, knowing that this may have restricted some studies to this analysis. However, the effect of LT on systemic inflammatory diseases was not within goal of this systematic review. The lack of consistent reporting of the same outcomes under the same testing conditions precluded the performance of a meta-analysis. Performance and detection bias were judged as high risk of bias for all studies, which limits the strength of the conclusions that can be made.
This systematic review aims to provide future directions in LT field. The outcomes tables (Tables 2 and 3, Supplementary Tables S2 and S3) summarized in this systematic review provide a useful source for comparison of different parameters and their findings. Researchers should report clearly all stimulation parameters and follow WALT guidelines to standardize the application of LT and to ensure a minimum therapeutic effect. Further efforts should focus on extending the current guidelines to other laser types and LEDs. The implementation of LT and techniques to measure the outcomes should also be improved in future studies by controlling the temperature during stimulation and complementing their qualitative analyses with quantitative measurements.
Conclusions
There was poor standardization of LT parameters, its application methods, and outcomes measured. Still, the in vitro and in vivo research models suggest that the use of LT may be considered as a nonsurgical treatment option on the management of knee OA, especially on early stages, since positive effects on ECM production, inflammatory response, deceleration of OA progression and pain-like behavior have been demonstrated. In addition, future studies should comprehensively report the LT parameters and comply the WALT guidelines.
Supplemental Material
Supplemental material, sj-pdf-1-car-10.1177_19476035211007902 for In Vitro and In Vivo Effects of Light Therapy on Cartilage Regeneration for Knee Osteoarthritis: A Systematic Review by Sofia Oliveira, Renato Andrade, Betina B. Hinckel, Filipe Silva, João Espregueira-Mendes, Óscar Carvalho and Ana Leal in CARTILAGE
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by FCT with the reference projects UID/EEA/04436/2020 and FunImp – POCI-01-0145-FEDER-030498. The funder was not involved in any aspect of the project, such as the study design and manuscript draft. The funder was not involved in the interpretation or publication of the study results.
Author Contributions: All authors were involved in the idealization of the systematic review and contributed for the design. SO and RA screened the articles and full text articles. Conflicts were resolved by OC and AL. SO extracted data to Microsoft Excel spreadsheet and it was reviewed by RA, BBH, OC, and AL. SO and RA performed risk of bias assessment. S.O drafted the manuscript with input from all authors. BBH, FS, and JEM provided guidance and advice during all steps of the development of the systematic review. All authors have read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Sofia Oliveira  https://orcid.org/0000-0001-9617-5910
https://orcid.org/0000-0001-9617-5910
João Espregueira-Mendes  https://orcid.org/0000-0001-7429-4900
https://orcid.org/0000-0001-7429-4900
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Correa D, Lietman SA. Articular cartilage repair: current needs, methods and research directions. Semin Cell Dev Biol. 2017;62:67-77. doi: 10.1016/j.semcdb.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 2. Roseti L, Desando G, Cavallo C, Petretta M, Grigolo B. Articular cartilage regeneration in osteoarthritis. Cells. 2019;8(11):1305. doi: 10.3390/cells8111305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krishnana Y, Grodzinsky AJ. Cartilage diseases. Matrix Biol. 2018;71-72:51-69. doi: 10.1016/j.matbio.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res. 2018;11:2189-96. doi: 10.2147/JPR.S154002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22(3):7000417. doi: 10.1109/JSTQE.2016.2561201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dompe C, Moncrieff L, Matys J, Grzech-Leśniak K, Kocherova I, Bryja A, et al. Photobiomodulation—underlying mechanism and clinical applications. J Clin Med. 2020;9(6):1724. doi: 10.3390/jcm9061724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balbinot G, Schuch CP, Nascimento PS, Lanferdini FJ, Casanova M, Baroni BM, et al. Photobiomodulation therapy partially restores cartilage integrity and reduces chronic pain behavior in a rat model of osteoarthritis: involvement of spinal glial modulation. Cartilage. 2019;1947603519876338. doi: 10.1177/1947603519876338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Y, Wu F, Wei J, Lao L, Shen X. The effects of laser moxibustion on knee osteoarthritis pain in rats. Photobiomodul Photomed Laser Surg. 2020;38(1):43-50. doi: 10.1089/photob.2019.4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamada EF, Bobinski F, Martins DF, Palandi J, Folmer V, da Silva MD. Photobiomodulation therapy in knee osteoarthritis reduces oxidative stress and inflammatory cytokines in rats. J Biophotonics. 2020;13(1):e201900204. doi: 10.1002/jbio.201900204 [DOI] [PubMed] [Google Scholar]
- 10. Angelova A, Ilieva EM. Effectiveness of high intensity laser therapy for reduction of pain in knee osteoarthritis. Pain Res Manag. 2016;2016:9163618. doi: 10.1155/2016/9163618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geenen R, Overman CL, Christensen R, et al. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77(6):797-807. doi: 10.1136/annrheumdis-2017-212662 [DOI] [PubMed] [Google Scholar]
- 12. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-88. doi: 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 13. Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2_suppl):220-233. doi: 10.1002/art.41142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Araruna Alves AC, de Oliveira Silva AA, de Melo Rambo CS, Leal Júnior ECP, Albertini R, dos Reis dos Santos I, et al. Effects of low-level laser irradiation on cartilage injury in animal models: a systematic review. Med Sci Technol. 2013;54(1):35-42. doi: 10.12659/MST.883868 [DOI] [Google Scholar]
- 15. Xiang A, Deng H, Cheng K, Liu H, Lin L, Qu X, et al. Laser photobiomodulation for cartilage defect in animal models of knee osteoarthritis: a systematic review and meta-analysis. Lasers Med Sci. 2020;35(4):789-96. doi: 10.1007/s10103-019-02937-8 [DOI] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim S, Park J, Lee Y, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408-14. doi: 10.1016/j.jclinepi.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 18. Sakata S, Kunimatsu R, Tsuka Y, Nakatani A, Hiraki T, Gunji H, et al. High-frequency near-infrared diode laser irradiation attenuates IL-1β-induced expression of inflammatory cytokines and matrix metalloproteinases in human primary chondrocytes. J Clin Med. 2020;9(3):881. doi: 10.3390/jcm9030881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang X, Liu TC, Liu S, Zhu W, Li H, Liang P, et al. Promoted viability and differentiated phenotype of cultured chondrocytes with low level laser irradiation potentiate efficacious cells for therapeutics. Front Bioeng Biotechnol. 2020;8:468. doi: 10.3389/fbioe.2020.00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jia YL, Guo ZY. Effect of low-power He-Ne laser irradiation on rabbit articular chondrocytes in vitro. Lasers Surg Med. 2004;34(4):323-8. doi: 10.1002/lsm.20017 [DOI] [PubMed] [Google Scholar]
- 21. Trevisan ES, Martignago CCS, Assis L, Tarocco JC, Salman S, Dos Santos L, et al. Effectiveness of LED photobiomodulation therapy on treatment with knee osteoarthritis: a rat study. Am J Phys Med Rehabil. 2020;99(8):725-32. doi: 10.1097/PHM.0000000000001408 [DOI] [PubMed] [Google Scholar]
- 22. Stancker TG, Vieira SS, Serra AJ, do Nascimento Lima R, Dos Santos Feliciano R, Silva JA, Jr, et al. Can photobiomodulation associated with implantation of mesenchymal adipose-derived stem cells attenuate the expression of MMPs and decrease degradation of type II collagen in an experimental model of osteoarthritis? Lasers Med Sci. 2018;33(5):1073-84. doi: 10.1007/s10103-018-2466-0 [DOI] [PubMed] [Google Scholar]
- 23. Sanches M, Assis L, Criniti C, Fernandes D, Tim C, Renno ACM. Chondroitin sulfate and glucosamine sulfate associated to photobiomodulation prevents degenerative morphological changes in an experimental model of osteoarthritis in rats. Lasers Med Sci. 2018;33(3):549-57. doi: 10.1007/s10103-017-2401-9 [DOI] [PubMed] [Google Scholar]
- 24. Assis L, Tim C, Magri A, Fernandes KR, Vassão PG, Renno ACM. Interleukin-10 and collagen type II immunoexpression are modulated by photobiomodulation associated to aerobic and aquatic exercises in an experimental model of osteoarthritis. Lasers Med Sci. 2018;33(9):1875-82. doi: 10.1007/s10103-018-2541-6 [DOI] [PubMed] [Google Scholar]
- 25. Tomazoni SS, Leal-Junior ECP, Pallotta RC, Teixeira S, de Almeida P, Lopes-Martins RÁB. Effects of photobiomodulation therapy, pharmacological therapy, and physical exercise as single and/or combined treatment on the inflammatory response induced by experimental osteoarthritis. Lasers Med Sci. 2017;32(1):101-8. doi: 10.1007/s10103-016-2091-8 [DOI] [PubMed] [Google Scholar]
- 26. Mahmoud GS, Elbedewey M, Abdelkader AM, Bekheet AB, El-Kossy DM, Sabbahi SA. Effects of ultrasound versus low level laser therapy in levofloxacin-induced rat model of knee osteoarthritis. IOSR J Dent Med Sci. 2017;16(6):78-85. doi: 10.9790/0853-1606017885 [DOI] [Google Scholar]
- 27. de Oliveira VLC, Silva JA, Jr, Serra AJ, Pallotta RC, da Silva EAP, de Farias Marques AC, et al. Photobiomodulation therapy in the modulation of inflammatory mediators and bradykinin receptors in an experimental model of acute osteoarthritis. Lasers Med Sci. 2016;32(1):87-94. doi: 10.1007/s10103-016-2089-2 [DOI] [PubMed] [Google Scholar]
- 28. Tomazoni SS, Leal-Junior ECP, Frigo L, Pallotta RC, Teixeria S, de Almedia P, et al. Isolated and combined effects of photobiomodulation therapy, topical nonsteroidal anti-inflammatory drugs, and physical activity in the treatment of osteoarthritis induced by papain. J Biomed Opt. 2016;21(10):108001. doi: 10.1117/1.jbo.21.10.108001 [DOI] [PubMed] [Google Scholar]
- 29. Assis L, Milares LP, Almeida T, Tim C, Magri A, Fernandes KR, et al. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthritis Cartilage. 2016;24(1):169-77. doi: 10.1016/j.joca.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 30. Milares LP, Assis L, Siqueira A, Claudino V, Domingos H, Almedia T, et al. Effectiveness of an aquatic exercise program and low-level laser therapy on articular cartilage in an experimental model of osteoarthritis in rats. Connect Tissue Res. 2016;57(5):398-407. doi: 10.1080/03008207.2016.1193174 [DOI] [PubMed] [Google Scholar]
- 31. Mangueira NM, Xavier M, De Souza RA, Salgado MAC, Silveira L, Villaverde AB. Effect of low-level laser therapy in an experimental model of osteoarthritis in rats evaluated through Raman spectroscopy. Photomed Laser Surg. 2015; 33(3):145-53. doi: 10.1089/pho.2014.3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dos Santos AA, Oliveira P, Fernandes KR, Rhon L, Tim CR, Vasilceac FA, et al. Effects of low-level laser therapy on cartilage repair in an experimental model of osteoarthritis. Photonics Lasers Med. 2014;3(3):255-64. doi: 10.1515/plm-2013-0063 [DOI] [Google Scholar]
- 33. Dos Santos SA, Alves ACA, Leal-Junior ECP, Albertini R, de Paula Vieria R, Ligeiro AP, et al. Comparative analysis of two low-level laser doses on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Lasers Med Sci. 2014;29(3):1051-58. doi: 10.1007/s10103-013-1467-2 [DOI] [PubMed] [Google Scholar]
- 34. Wang P, Liu C, Yang X, Zhou Y, Wei X, Ji Q, et al. Effects of low-level laser therapy on joint pain, synovitis, anabolic, and catabolic factors in a progressive osteoarthritis rabbit model. Lasers Med Sci. 2014;29(6):1875-85. doi: 10.1007/s10103-014-1600-x [DOI] [PubMed] [Google Scholar]
- 35. Bublitz C, Medalha C, Oliveira P, Assis L, Milares LP, Fernandes KR, et al. Low-level laser therapy prevents degenerative morphological changes in an experimental model of anterior cruciate ligament transection in rats. Lasers Med Sci. 2014;29(5):1669-78. doi: 10.1007/s10103-014-1546-z [DOI] [PubMed] [Google Scholar]
- 36. Alves ACA, Albertini R, Dos Santos SA, Leal-Junior ECP, Santana E, Serra AJ, et al. Effect of low-level laser therapy on metalloproteinase MMP-2 and MMP-9 production and percentage of collagen types I and III in a papain cartilage injury model. Lasers Med Sci. 2014;29(3):911-9. doi: 10.1007/s10103-013-1427-x [DOI] [PubMed] [Google Scholar]
- 37. Oliveira P, Santos AA, Rodrigues T, Tim CR, Pinto KZ, Magri AMP, et al. Effects of phototherapy on cartilage structure and inflammatory markers in an experimental model of osteoarthritis. J Biomed Opt. 2013;18(12):128004. doi: 10.1117/1.jbo.18.12.128004 [DOI] [PubMed] [Google Scholar]
- 38. Alves ACA, Vieira RDP, Leal-Junior ECP, dos Santos S, Ligerio AP, Albertini R, et al. Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res Ther. 2013;15(5):R116. doi: 10.1186/ar4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim G, Kim E. Anti-inflammation effects of low intensity laser therapy on monosodium iodoacetate-induced osteoarthritis in rats. J Phys Ther Sci. 2013;25(2_suppl):173-5. doi: 10.1589/jpts.25.173 [DOI] [Google Scholar]
- 40. Kim G, Kim E. Analgesic efficacy of low intensity laser therapy in a monosodium iodoacetate-induced osteoarthritic rat model. J Phys Ther Sci. 2013;25(3):309-12. doi: 10.1589/jpts.25.309 [DOI] [Google Scholar]
- 41. Lin HD, He CQ, Luo QL, Zhang JL, Zeng DX. The effect of low-level laser to apoptosis of chondrocyte and caspases expression, including caspase-8 and caspase-3 in rabbit surgery-induced model of knee osteoarthritis. Rheumatol Int. 2012;32(3):759-66. doi: 10.1007/s00296-010-1629-5 [DOI] [PubMed] [Google Scholar]
- 42. Da Rosa AS, Dos Santos AF, Da Silva MM, Facco GG, Perrerira DM, Alves ACA, et al. Effects of low-level laser therapy at wavelengths of 660 and 808 nm in experimental model of osteoarthritis. Photochem Photobiol. 2012;88(1):161-6. doi: 10.1111/j.1751-1097.2011.01032.x [DOI] [PubMed] [Google Scholar]
- 43. Oshima Y, Coutts RD, Badlani NM, Healey RM, Kubo T, Amiel D. Effect of light-emitting diode (LED) therapy on the development of osteoarthritis (OA) in a rabbit model. Biomed Pharmacother. 2011;65(3):224-9. doi: 10.1016/j.biopha.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 44. Zhao L, Shen XY, Cao YL, Wang L, Deng H, Zhang H. Effects of laser irradiation on arthritic histopathology and heat shock protein 70 expression in C57 black mice with osteoarthritis. Zhong Xi Yi Jie He Xue Bao. 2011;9(7):761-7. doi: 10.3736/jcim20110710 [DOI] [PubMed] [Google Scholar]
- 45. Kamali F, Bayat M, Torkaman G, Ebrahimi E, Salavati M. The therapeutic effect of low-level laser on repair of osteochondral defects in rabbit knee. J Photochem Photobiol B. 2007;88(1):11-5. doi: 10.1016/j.jphotobiol.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 46. Lin YS, Huang MH, Chai CY. Effects of helium-neon laser on the mucopolysaccharide induction in experimental osteoarthritic cartilage. Osteoarthritis Cartilage. 2006;14(4):377-83. doi: 10.1016/j.joca.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 47. Cho HJ, Lim SC, Kim SG, Kim YS, Kang SS, Choi SH, et al. Effect of low-level laser therapy on osteoarthropathy in rabbit. In Vivo. 2004;18(5):585-92. [PubMed] [Google Scholar]
- 48. Hojo T, Fujioka M, Otsuka G, Inoue S, Kim U, Kubo T. Effect of heat stimulation on viability and proteoglycan metabolism of cultured chondrocytes: preliminary report. J Orthop Sci. 2003;8(3):396-9. doi: 10.1007/s10776-002-0643-2 [DOI] [PubMed] [Google Scholar]
- 49. Ito A, Aoyama T, Tajino J, Nagai M, Yamaguchi S, Iijima H, et al. Effects of the thermal environment on articular chondrocyte metabolism: a fundamental study to facilitate establishment of an effective thermotherapy for osteoarthritis. J Jpn Phys Ther Assoc. 2014;17(1):14-21. doi: 10.1298/jjpta.Vol17_003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Oliveira VLC, Silva JA, Jr, Serra AJ, Pallotta RC, da Silva EAP, de Farias Marques AC, et al. Photobiomodulation therapy in the modulation of inflammatory mediators and bradykinin receptors in an experimental model of acute osteoarthritis. Lasers Med Sci. 2017;32(1):87-94. doi: 10.1007/s10103-016-2089-2 [TNJ: Repeated. Same as 27.] [DOI] [PubMed] [Google Scholar]
- 51. Kim ED, Won YH, Park SH, Seo JH, Kim DS, Ko MH, et al. Efficacy and safety of a stimulator using low-intensity pulsed ultrasound combined with transcutaneous electrical nerve stimulation in patients with painful knee osteoarthritis. Pain Res Manag. 2019;2019:7964897. doi: 10.1155/2019/7964897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haltmayer E, Ribitsch I, Gabner S, Rosser J, Gueltekin S, Peham J, et al. Co-culture of osteochondral explants and synovial membrane as in vitro model for osteoarthritis. PLoS One. 2019;14(4):e0214709. doi: 10.1371/journal.pone.0214709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yeung P, Cheng KH, Yan CH, Chan BP. Collagen microsphere based 3D culture system for human osteoarthritis chondrocytes (hOACs). Sci Rep. 2019;9(1):12453. doi: 10.1038/s41598-019-47946-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mehta S, Akhtar S, Porter RM, Önnerfjord P, Bajpayee AG. Interleukin-1 receptor antagonist (IL-1Ra) is more effective in suppressing cytokine-induced catabolism in cartilage-synovium co-culture than in cartilage monoculture. Arthritis Res Ther. 2019;21(1):238. doi: 10.1186/s13075-019-2003-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis : role in initiation and progression of cartilage degeneration ? Osteoarthritis Cartilage. 2012;20(3):223-32. doi: 10.1016/j.Joca.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 56. Huang YY, Chen ACH, Carroll JD, Hamblin MR. Biphasic dose response in low level lightherapy. Dose Response. 2009;7(4):358-83. doi: 10.2203/dose-response.09-027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. WALT: World Association for Photobiomodulation Therapy. Recommended treatment doses for low level laser therapy. Published 2010. Accessed Mach 24, 2021. https://waltza.co.za/wp-content/uploads/2012/08/Dose_table_780-860nm_for_Low_Level_Laser_Therapy_WALT-2010.pdf
- 58. WALT: World Association for Photobiomodulation Therapy. Recommended treatment doses for low level laser therapy 904 nm wavelength. https://waltza.co.za/wp-content/uploads/2012/08/Dose_table_904nm_for_Low_Level_Laser_Therapy_WALT-2010.pdf. Published 2010. Accessed December 10, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-car-10.1177_19476035211007902 for In Vitro and In Vivo Effects of Light Therapy on Cartilage Regeneration for Knee Osteoarthritis: A Systematic Review by Sofia Oliveira, Renato Andrade, Betina B. Hinckel, Filipe Silva, João Espregueira-Mendes, Óscar Carvalho and Ana Leal in CARTILAGE