Abstract
Study Objectives:
To compare type 2 polysomnography (T2PSG) to the gold standard type 1 in-laboratory polysomnography (T1PSG) for diagnosing obstructive sleep apnea (OSA) in children; validate home T2PSG in children with suspected OSA.
Methods:
Eighty-one participants (ages 6–18) with suspected OSA had simultaneous T1PSG and T2PSG in the sleep laboratory, 47 participants (ages 5–16) had T1PSG in the sleep laboratory and T2PSG performed at home. Sleep scientists staged and scored polysomnography data, and pediatric sleep physicians assigned a diagnosis of normal or OSA. Participant demographics, polysomnography variables, and diagnoses were compared using chi-square and Fisher’s exact tests for nominal variables, t test for continuous variables and Cohen’s kappa to assess concordance.
Results:
Acceptable recordings were obtained for every home T2PSG. When T1PSG and T2PSG were simultaneous, correlation between the number of arousals, respiratory disturbance index, and sleep stages was excellent. T2PSG at home demonstrated less stage 2 sleep, more rapid eye movement sleep, and higher sleep efficiency. Comparison of home T2PSG to T1PSG for diagnosing OSA showed a false-positive rate of 6.6% and false-negative rate of 3% for those performed at home.
Conclusions:
T2PSG in the home is feasible with excellent concordance with T1PSG for the purposes of diagnosing OSA in children aged 5–18 years. Home T2PSG may be more representative of a “normal” night for children and could benefit those suspected of having OSA by reducing waiting times for laboratory PSG, improving access to PSG and possibly reducing costs of investigating and treating OSA.
Citation:
Withers A, Maul J, Rosenheim E, O’Donnell A, Wilson A, Stick S. Comparison of home ambulatory type 2 polysomnography with a portable monitoring device and in-laboratory type 1 polysomnography for the diagnosis of obstructive sleep apnea in children. J Clin Sleep Med. 2022;18(2):393–402.
Keywords: home sleep apnea testing, pediatric obstructive sleep apnea, polysomnography
BRIEF SUMMARY
Current knowledge/Study Rationale: Ambulatory polysomnography performed in the home with a portable monitoring device has potential advantages compared to in-laboratory polysomnography, including increased access and cost benefits. There is very little evidence that type 2 polysomnography in the home is feasible in children and the accuracy compared to the gold standard in-laboratory polysomnography for the purpose of diagnosing obstructive sleep apnea is unknown.
Study Impact: This study demonstrates that polysomnography in the home is feasible in children and accurate for diagnosing obstructive sleep apnea compared to the current gold standard. Results indicate that home polysomnography may be more accurate than in-laboratory polysomnography due to a more “normal” environment for the child.
INTRODUCTION
Obstructive sleep apnea (OSA) is the intermittent partial and/or complete obstruction of the upper airway during sleep. Immediate physiological consequences of upper airway obstruction include tachycardia, hypertension, hypoxia, and hypercapnia.1–4 These physiological consequences of upper airway obstruction cause arousal from sleep, leading to sleep fragmentation.5 In children sleep fragmentation causes daytime symptoms of sleepiness, behavioral problems, poor attention, and irritability.2,6 Untreated OSA is known to lead to serious long-term health consequences, primarily affecting neurocognition and the cardiovascular system.2,6,7 Other serious long-term consequences include impaired somatic growth, pulmonary hypertension, and systemic inflammation.1,5,6
Treatment for simple OSA with adenotonsillectomy is generally effective.8 Although serious surgical complications are rare, they include respiratory compromise and even death, with a large meta-analysis finding respiratory complications occurred more often in children undergoing adenotonsillectomy for OSA than those with recurrent tonsillitis.9 Therefore, accurate diagnosis of OSA is essential to plan perioperative care and avoid unnecessary surgical intervention.
Although up to 10% of children snore,10 the prevalence of OSA in children is estimated to be between 1.2% and 5.7%.5 Sleep study or polysomnography (PSG) is considered the gold standard for diagnosing OSA in children.5,11 There is strong evidence that children and adolescents who snore regularly and have any signs or symptoms of OSA should have a PSG,5 with the American Academy of Pediatrics strongly recommending that all children being investigated for snoring should have a PSG to diagnose OSA, assess perioperative risk, and guide postoperative care.5,12,13
Despite this recommendation less than 10% of children in the United States referred for adenotonsillectomy for suspected OSA have had any form of overnight monitoring prior to surgery, and less than 5% have had a PSG.2,14 There are several reasons for this. Snoring is common in children. The increased recognition of the importance of treating OSA has resulted in increased referrals to pediatric sleep services and a dramatic increase in wait times. Dedicated pediatric sleep laboratories are lacking worldwide, with limited staff skilled in performing and interpreting pediatric PSG a major contributor.15 In addition, performing a PSG is expensive, usually requiring access to an overnight bed in a hospital sleep laboratory.15 Therefore, many children with significant OSA remain undiagnosed and untreated and easily accessible alternatives to PSG are urgently needed. While various methods for identifying OSA in children, such as symptom questionnaires,15–17 oximetry,18–20 video/audio recording,21–23 and nap studies24 have been proposed as alternatives to type 1 PSG (T1PSG), these techniques lack sensitivity and have been shown to be unsuitable, even as screening tools.15
T1PSG is performed in a sleep laboratory with a sleep scientist or technician in attendance and full cardiopulmonary monitoring and recording,25,26 whereas type 2 PSG (T2PSG), type 3 PSG (T3PSG), and type 4 polysomnography are unattended studies performed outside the sleep laboratory with a portable monitoring device (PMD).25,26 Comprehensive monitoring (which may include video monitoring and/or end tidal CO2/transcutaneous CO2) is included for T2PSG, whereas T3PSG generally records 4 variables (such as oxygen saturation, heart rate, respiratory bands, airflow) and type 4 polysomnography records continuous single or double variables, such as oxygen saturations and nasal airflow.25,26 Ambulatory PSGs performed in the home are an attractive alternative to T1PSG as they are readily available, are portable, and do not require the overnight presence of skilled staff. Allowing the patient to sleep in their own bed replicates the normal sleep environment (such as exposure to aeroallergens and tobacco smoke) and potentially improves sleep duration, quality, and comfort.
Although a number of studies in children have indicated that PSG with PMD can be performed successfully27–30 and may afford significant cost savings,31 others report significant artifact in the recordings leading to a high failure rate.32
The validity of PSG performed with PMD in children is questionable as most studies have contained very small numbers of children who also had a T1PSG to allow comparison of results.30,33,34 Direct comparison of type 4 polysomnography (oximetry) and T1PSG has demonstrated poor agreement between the apnea-hypopnea index and desaturation index19 and poor sensitivity for diagnosing OSA as children may have obstructive events without oxygen desaturation.30 Numerous studies in children comparing T3PSG (either at home or in a sleep lab) to T1PSG show that accuracy of T3PSG is variable,30 and underestimation of the apnea-hypopnea index is common.30,33,35 These discrepant results are likely explained by the lack of electroencephalogram (EEG) recording during T3PSG, as events that cause arousal rather than desaturation are not identified30 and staging is not possible, making it difficult to differentiate awake from rapid eye movement (REM) sleep and determine sleep onset accurately.35
Although 2 studies in children that examined T2PSG performed in the home36,37 contained large numbers of children (201 and 162) and demonstrated feasibility, the number of children who had a T1PSG performed for direct comparison was extremely small (4 of 20136 and 5 of 16237). It is therefore difficult to draw any firm conclusions regarding the accuracy of T2PSG based on these results.30
Therefore, despite the potential advantages, practice is currently limited by a lack of evidence that ambulatory PSG in children, particularly T2PSG, is feasible and that results are comparable to those of the gold standard T1PSG. Therefore, the primary aims of this study were to determine the diagnostic concordance for OSA when comparing T2PSG and T1PSG in a sufficiently powered study, assess feasibility, and validate the use of ambulatory T2PSG in children for the purpose of diagnosing OSA. Our secondary aims were to compare polysomnographic parameters such as sleep stages, arousals, and sleep efficiency between T1PSG and ambulatory T2PSG, to compare respiratory disturbance index (RDI) and respiratory event index (REI; for the studies that took place in the home) and to provide a simple cost comparison in the Australian setting. We hypothesized that concordance of diagnoses between T2PSG and T1PSG would be excellent, that polysomnographic parameters would be comparable, and that use of ambulatory T2PSG would be feasible and afford considerable cost savings.
METHODS
Protocol
Ethics approval was granted by the Princess Margaret Children’s Hospital Human Research Ethics Committee. This study was performed at the sleep disorders unit (SDU) in Princess Margaret Children’s Hospital in Perth, Western Australia. Participants were enrolled consecutively when attending for T1PSG. All children who were newly referred for assessment of possible OSA between the ages of 5 and 18 years were eligible for inclusion unless there were anticipated behavioral problems at the time of set-up. This is a clinical decision that influences the type of investigation undertaken by the sleep department. Informed consent was obtained from all parents and informed assent from children when possible.
All participants had a T1PSG and a T2PSG performed for comparison. The T1PSGs were performed in the accredited SDU according to unit protocols with Compumedics Profusion PSG 3 equipment (Compumedics Limited, Victoria, Australia) in accordance with published clinical practice guidelines.5 The T1PSG were attended and managed by an accredited and experienced sleep technologist. The variables measured were EEG, chin and diaphragmatic electromyogram, electrooculogram, airflow thermistor, nasal pressure, respiratory inductance plethysmography, arterial oxygen saturation, body position, time-linked audio-video, transcutaneous carbon dioxide, and electrocardiogram (see Table S1 in the supplemental material).
The T2PSGs were performed with Somté ambulatory sleep study system equipment (Compumedics). They were conducted either simultaneously with T1PSG in the SDU where equipment was applied for both T1PSG and T2PSG at the same time (the “in-laboratory study”) or in the participant’s home on a different night (the “in-home study”). The variables measured were EEG, chin and diaphragmatic electromyogram, electrooculogram, airflow thermistor, nasal pressure, respiratory inductance plethysmography, arterial oxygen saturation, and body position (see Table S1).
The decision to perform T2PSG in the home or simultaneously was based on physical size of the child, parental preference, location of the home, and safety of the home environment. Simultaneous application of equipment was only possible in older, larger children, and in-home T2PSG was chosen if children were too small for simultaneous application. When T1PSG and T2PSG equipment were applied simultaneously, the nasal cannula was shared between setups; however, all other equipment was duplicated, with EEG leads for each setup placed over half of the head instead of the full head. Two oronasal thermistors were able to be placed as a very thin, disposable paper strip thermistor (4140119 Embla BreathSensor airflow thermistor [Natus Medical Incorporated, Pleasanton, CA]; Figure 1) was applied for the T2PSG and fit neatly underneath the nasal prongs and second thermistor. The Inductotrace (Formerly Respitrace), Ambulatory Monitoring Systems, Ardsley, NY, bands are very thin and 2 sets were easily applied.
Figure 1. The Natus Embla BreathSensor airflow thermistor.
The Natus Embla BreathSensor airflow thermistor used for T2PSG in this study (image reproduced with permission from VMedical), New South Wales, Australia. T2PSG = type 2 polysomnography.
In-home T2PSG was not offered if there was significant risk to staff attending the home to set up the PMD (for example a history of domestic violence or dangerous pets). In-home T2PSG was only offered if travel time from the SDU to the participant’s home would be 30 minutes or less. When T2PSG was performed in the participant’s home, it occurred within 2 weeks of the T1PSG. The participants having an in-home T2PSG had equipment applied in their home by a Hospital in the Home nurse who taught an adult to check the equipment at regular intervals and how to replace leads if necessary. An adult was required to sleep in the room with the child. The following morning a Hospital in the Home nurse returned to pick up the equipment and returned it to the SDU for cleaning and data download. When participants had a simultaneous T1PSG and T2PSG in the laboratory, equipment was checked by the sleep technologist at scheduled intervals as would happen by the adult in the child’s home.
Analysis of PSG data
Raw PSG data was downloaded to the SDU computer system. A single sleep scientist used standard rules38,39 to visually analyze and stage sleep and score respiratory events and arousals. Apneas and hypopneas were classified as central, mixed, or obstructive. A randomly allocated pediatric sleep physician examined the data and made a diagnosis of normal or OSA (mild, moderate, or severe). As the primary aim was to examine diagnostic concordance between T1PSG and T2PSG, study failure was defined subjectively by inadequate data recorded to allow a confident diagnosis to be made and/or objectively as loss of signals rendering the study unable to be staged or scored.
Economic comparison
Costs of performing T1PSG and ambulatory T2PSG were calculated by adding fees for staff hours required to travel to the participant’s home to set up the study; retrieve the equipment the next morning; stage, score, and report the study; hospital bed costs; and equipment costs/consumables.
Statistical analysis
For the purpose of comparing T1PSG and T2PSG performed simultaneously, sample size (n = 81) was calculated utilizing the results of a pilot study to detect κ ≥ 0.7 with 80% power at the .05 significance level40,41 using PASS 11 and the Concord Library. The T1PSG and T2PSG data for an individual child was considered a “data pair.” Data pairs were excluded from statistical analysis if there was significant signal loss in 1 or both of a pair whereby a measurement could not be reliably calculated. Statistical analysis was completed in SPSS using chi-square test and Fischer’s exact test for nominal variables, independent sample t tests for continuous variables, and paired-samples correlation for Cohen’s κ. A P-value of < .05 was selected to indicate statistical significance.
RESULTS
Participants
Between December 2007 and November 2011, 2379 T1PSGs were performed in the SDU, of which 1146 were performed in children aged between 5 and 18 years. Of those eligible for inclusion, 128 agreed to participate. All 128 participants had a T1PSG and T2PSG performed, 81 with the in-laboratory study and 47 with the in-home study. There were no significant differences in sex mix (P = .893); however, the in-laboratory cohort was older (P < .001), taller (P < .001), and had higher weights (P < .001), body mass index (P = .001), and body mass index z-scores (P = .033) (see Table S2 and Table S3 in the supplemental material). There were 47 medical comorbidities noted in 43 participants, including trisomy 21, neurofibromatosis, attention deficit hyperactivity disorder, asthma, obesity, and craniofacial anomalies (Table 1). Four participants had 2 comorbidities each (obesity and cardiomyopathy, obesity and attention-deficit hyperactivity disorder, obesity and asthma, Duchenne muscular dystrophy, and type 1 diabetes).
Table 1.
Participant medical comorbidities.
| Condition | In-Laboratory Study | In-Home Study | Total |
|---|---|---|---|
| ADHD | 2 | 1 | 3 |
| Obese/overweight | 16 | 3 | 19 |
| Cardiac* | 1 | 0 | 1 |
| Trisomy 21 | 1 | 0 | 1 |
| DMD | 0 | 1 | 1 |
| Cleft palate | 1 | 1 | 2 |
| Craniofacial disorder† | 2 | 1 | 3 |
| Prader-Willi syndrome | 1 | 0 | 1 |
| Neurofibromatosis | 0 | 2 | 2 |
| Bronchiectasis | 1 | 1 | 2 |
| Asthma | 1 | 3 | 7 |
| Type 1 diabetes | 2 | 1 | 3 |
| Neurological‡ | 1 | 1 | 2 |
| Total | 33 | 15 | 48 |
Includes cardiomyopathy and corrected congenital heart disease. †Includes retrognathia, Pierre-Robin Sequence and craniofacial disorder not otherwise specified. ‡Includes spastic diplegia and epilepsy. ADHD = attention-deficit hyperactivity disorder, DMD = Duchenne Muscular Dystrophy.
In-laboratory study
There were 3 participants for whom a diagnosis was not possible due to poor signal quality in at least 1 of the data pairs. Statistically significant differences between T1PSG and T2PSG were more stage 1 sleep, less stage 2 sleep, and more slow wave sleep in T2PSG (Table 2). As studies were performed simultaneously, a significant difference in sleep efficiency and total sleep time was not observed. Correlation between the number of arousals, respiratory disturbance index, and sleep stages was excellent, with κ ranging from 0.845 to 0.977, all highly statistically significant (Table 3).
Table 2.
Comparison of PSG parameters for in-laboratory study.
| n | T1PSG Mean | T2PSG Mean | Mean Difference | SE | P | CI | |
|---|---|---|---|---|---|---|---|
| Arousals | 80 | 16.7 | 16.5 | 0.18 | 0.4 | .651 | –0.6, 0.9 |
| RDI | 81 | 8.9 | 8.1 | 0.83 | 0.5 | .071 | –0.7, 1.7 |
| Stage 1 | 76 | 9.10 | 10.5 | –1.36 | 0.4 | .003 | –2.2, –0.5 |
| Stage 2 | 76 | 47.3 | 42.9 | 4.36 | 0.6 | < .001 | 3.1, 5.6 |
| SWS | 76 | 26.5 | 29.1 | –2.54 | 0.5 | < .001 | –3.6, –1.5 |
| REM | 78 | 16.9 | 17.2 | –0.29 | 0.4 | .418 | –1.0, 0.4 |
CI = confidence interval, PSG = polysomnography, RDI = respiratory disturbance index, REM = rapid eye movement, SEM = standard error of mean, SWS = slow wave sleep, T1PSG = type 1 polysomnography, T2PSG = type 2 polysomnography.
Table 3.
Correlation between PSG parameters for in-laboratory study.
| PSG Parameter | n | Correlation (κ) | P |
|---|---|---|---|
| Arousals | 80 | 0.977 | < .001 |
| RDI | 81 | 0.995 | < .001 |
| Stage 1 (%) | 76 | 0.860 | < .001 |
| Stage 2 (%) | 76 | 0.845 | < .001 |
| SWS (%) | 76 | 0.851 | < .001 |
| REM (%) | 78 | 0.853 | < .001 |
Correlation between PSG parameters when comparing T1PSG and T2PSG for the in-laboratory study. PSG = polysomnography, RDI = respiratory disturbance index, REM = rapid eye movement, SWS = slow wave sleep, T1PSG = type 1 polysomnography, T2PSG = type 2 polysomnography.
In-home study
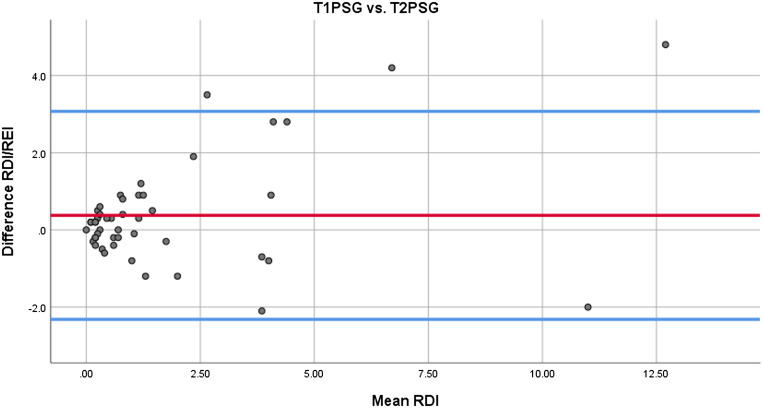
There were no study failures. Statistically significant differences were less stage 2 sleep, more REM sleep, and higher sleep efficiency in T2PSG (Table 4). Correlation between RDI/REI was excellent, stage 1 sleep substantial, stage 2 sleep and slow wave sleep moderate, REM and arousals poor, and no correlation observed for sleep efficiency (Table 5). Concordance between T1PSG and T2PSG for RDI/REI is presented as a Bland-Altman plot (Figure 2), with Bland-Altman plots for stage 1 sleep, stage 2 sleep, slow wave sleep, REM, and arousals in the supplemental material (Figure S1, Figure S2, Figure S3, Figure S4, and Figure S5 in the supplemental material).
Table 4.
Comparison of PSG parameters for in-home study.
| n | T1PSG Mean | T2PSG Mean | Mean Difference | SE | P | CI | |
|---|---|---|---|---|---|---|---|
| Arousals | 47 | 12.9 | 12.7 | 0.22 | 4.9 | .755 | –1.2, 1.6 |
| RDI/REI | 47 | 1.6 | 1.9 | –0.38 | 1.4 | .067 | –0.8, 0.0 |
| Stage 1 | 43 | 8.9 | 8.3 | 0.58 | 3.7 | .306 | –0.5, 1.7 |
| Stage 2 | 43 | 45.5 | 43.7 | 1.76 | 5.4 | .040 | 0.8, 3.4 |
| SWS | 43 | 26.3 | 26.3 | –0.04 | 5.4 | .962 | –1.7, 1.6 |
| REM | 45 | 18.9 | 21.8 | –2.80 | 4.5 | < .001 | –4.2, –1.4 |
| Efficiency | 47 | 85.0 | 90.8 | –5.79 | 1.7 | .001 | –9.1, –2.4 |
Comparison of PSG parameters when comparing T1PSG and T2PSG for in-home study. CI = confidence interval, PSG = polysomnography, RDI = respiratory disturbance index, REI = respiratory event index, REM = rapid eye movement, SE = standard error of mean, SWS = slow wave sleep, T1PSG = type 1 polysomnography, T2PSG = type 2 polysomnography.
Table 5.
Correlation between PSG parameters for in-home study.
| PSG Parameter | n | Correlation (κ) | P |
|---|---|---|---|
| Arousals | 47 | 0.360 | .013 |
| RDI/REI | 47 | 0.887 | < .001 |
| Stage 1 (%) | 43 | 0.734 | < .001 |
| Stage 2 (%) | 43 | 0.615 | < .001 |
| SWS (%) | 43 | 0.594 | < .001 |
| REM (%) | 45 | 0.303 | .043 |
| Efficiency (%) | 47 | –0.122 | .413 |
Correlation between PSG parameters when comparing T1PSG and T2PSG in the in-home study. PSG = polysomnography, RDI = respiratory disturbance index, REI = respiratory event index, REM = rapid eye movement, SWS = slow wave sleep, T1PSG = type 1 polysomnography, T2PSG = type 2 polysomnography.
Figure 2. Concordance for RDI/REI.
Concordance for RDI/REI between T1PSG and T2PSG. Mean is shown in red and the upper and lower limits of agreement (mean ± 1.96 SD) in blue. RDI = respiratory disturbance index, REI = respiratory event index, T1PSG = type 1 polysomnography, T2PSG = type 2 polysomnography.
Diagnosis
For the in-laboratory study (n = 78), there was agreement between diagnosis in 68 of 78 participants (87%), κ = 0.795 (standard error of mean 0.062, P = .000). Where there was disagreement between diagnosis (n = 10), the most frequent disagreement was no OSA found in T1PSG and mild OSA in T2PSG (n = 7) (Table 6). There was no significant difference in diagnostic agreement in those participants with and without a medical comorbidity (27/30, 90% vs 42/51, 86%, P = .734).
Table 6.
Comparison of diagnosis between T1PSG and T2PSG.
| T2PSG Diagnosis | |||||
|---|---|---|---|---|---|
| In-Laboratory Study | No OSA | Mild OSA | Moderate OSA | Severe OSA | Total |
| T1PSG diagnosis | |||||
| No OSA | 34 | 7 | 0 | 0 | 41 |
| Mild OSA | 2 | 22 | 0 | 0 | 24 |
| Moderate OSA | 0 | 1 | 4 | 0 | 5 |
| Severe OSA | 0 | 0 | 0 | 8 | 8 |
| Total | 36 | 30 | 4 | 8 | 78 |
| In-Home Study | No OSA | Mild OSA | Moderate OSA | Severe OSA | Total |
| T1PSG diagnosis | |||||
| No OSA | 31 | 1 | 0 | 0 | 32 |
| Mild OSA | 1 | 10 | 1 | 0 | 12 |
| Moderate OSA | 0 | 0 | 3 | 0 | 3 |
| Severe OSA | 0 | 0 | 0 | 0 | 0 |
| Total | 32 | 11 | 4 | 0 | 47 |
Comparison of diagnosis obtained between T1PSG and T2PSG for in-laboratory study and in-home study, differences in italic. OSA = obstructive sleep apnea, T1PSG = type 1 polysomnography, T2PSG = type 2 polysomnography.
For the in-home study (n = 47), there was agreement between diagnosis in 44 of 47 children (94%), κ = 0.865 (standard error of mean 0.074, P = .000) (Table 6). Disagreement between diagnosis (n = 3) was as follows: no OSA in T2PSG and mild OSA in T1PSG (n = 1), mild OSA in T2PSG and no OSA in T1PSG (n = 1), and moderate OSA in T2PSG and mild OSA in T1PSG (n = 1). There was no significant difference in diagnostic agreement in those participants with and without a medical comorbidity (13/14, 93% vs 31/33, 94%, P < 0.001).
To calculate the false positive and false negative rates for T2PSG compared to the gold-standard T1PSG, 2 × 2 tables were constructed (see Table S4 and Table S5 in the supplemental material). For the in-laboratory study, the false positive rate was 7/42 (17%) and false negative rate 3/26 (5.5%). For the in-home study, the false positive rate was 1/15 (6.6%) and false negative rate 1/32 (3%).
Cost comparison
For a T2PSG, staff time was required for a Hospital in the Home safety interview (35 minutes), travel time (30 minutes), set-up of equipment in the home (60 minutes), and pick-up and return of equipment the next morning (35 minutes), totaling 2.6 hours. For a T1PSG, 8.5 hours of staff time were required for laboratory set-up, monitoring, and performance of the PSG, therefore T1PSG required an extra 5.9 hours of paid staff time to perform. In addition, a T1PSG had a cost for an inpatient hospital bed.
DISCUSSION
Few studies have critically evaluated PMDs, in particular T2PSG, in the home as an alternative to T1PSG in children for the purposes of diagnosing OSA.15,27 Concerns have been raised about the feasibility of using PMDs,32 their reliability in children, and validity of results.33,42 Despite these concerns, ambulatory T2PSG has the potential to significantly increase the access of children with OSA to diagnostic studies and treatment, as well as reduce costs associated with investigating OSA. This study addressed a significant knowledge gap, using an appropriately powered sample to determine diagnostic concordance between the gold standard T1PSG and a T2PSG, and feasibility of T2PSG in the home setting.
The simultaneous recordings performed in the laboratory provide a reasonable estimate of the performance characteristics of the T2PSG using a PMD. Our results clearly demonstrate that ambulatory T2PSG is feasible in children, with a zero-failure rate in the present study. This contrasts with the findings of Poels et al32 where only 29% of home T3PSG in 24 children were deemed successful. Poels et al attribute their high failure rate to tasking the child’s caregiver with placing all of the sensors and starting the recording device. Our failure rate of zero highlights the importance of having a trained staff member set up the equipment in the home, rather than the parent, in agreement with a number of studies.29,34,36,37 In addition, in this study an adult caregiver was required to sleep in the same room as the child, regularly check equipment, and follow clear instructions as to how and when to replace displaced leads. The importance of a motivated adult who is explicitly instructed how to replace leads was highlighted by Marcus et al,36 who found only one third of caregivers attempted to replace a lead if it became displaced. In addition, after a small pilot study at the authors’ center identified poor signal quality as an issue in study failure, a comprehensive multichannel T2PSG system was utilized to reduce signal loss.
Comparing polysomnographic variables between T1PSG and home T2PSG, there was significantly more REM sleep and higher sleep efficiency in the home group. These findings agree with previous studies.42–44 Fröhlich and Lehmkuhl43 demonstrated higher sleep efficiency, longer sleep duration, and reduced sleep onset latency when recording sleep parameters at home, and Jacob et al found that home T3PSG was associated with increased sleep efficiency and reduced arousal index.29 These findings have been replicated in other studies, with some children reporting that they slept even better than usual with the PMD at home.37 Our observed differences in sleep efficiency and architecture between T1PSG and T2PSG in the home may be due to true biological differences or measurement variability from one night to the next; however, an alternative explanation is that children slept much better during the T2PSG because they were at home. Home T2PSG is likely to be more comfortable than T1PSG and more representative of a normal night for the child. For a home T2PSG, the child’s time for going to bed and getting up in the morning is dictated by the child’s normal routine and individual needs whereas in the laboratory this is to some degree dictated by laboratory protocols and staff rostering. As children are often frightened of sleeping in the unfamiliar environment of a sleep laboratory, poor sleep, reduced total sleep time, and a poor representation of a usual night may result, calling into question the validity of the results.
In addition to our findings of higher sleep efficiency during home T2PSG, we found significantly more REM sleep. It is well known that 2 of the characteristics of the “first night effect” are a reduced amount of REM sleep and longer REM latency.45 The increased amount of REM sleep observed during T2PSG in this study may be explained by that lack of or lessened first night effect given the child was sleeping in their own bed in a familiar environment. Therefore, the low correlation (κ = 0.303) found between REM in T1PSG and T2PSG may reflect a true difference caused by reduced first night effect.” An alternative explanation is the lack of video may have made differentiating awake and REM difficult, resulting in an overestimate of the amount of REM sleep. The authors feel this is unlikely given there was a single, very experienced sleep scientist who staged all studies; however, this possibility cannot be completely excluded and is addressed below in the limitations.
Despite differences in polysomnographic values between T1PSG and home T2PSG performed on different nights, comparison of RDI/REI and diagnosis demonstrated excellent concordance. This highlights that even if sleep architecture is significantly different between T1PSG and home T2PSG, the resultant diagnosis is not, so that clinical management will not be affected. If anything, the higher sleep efficiency noted in the children studied at home would argue for greater diagnostic accuracy of a T2PSG with a PMD at home compared to a laboratory study for younger children. In addition, home T2PSG is potentially more accurate for the detection of OSA, as OSA in children is more likely to occur during REM sleep and higher amounts of REM sleep were observed at home. Consequently, the “false positive” diagnoses of OSA obtained during home T2PSG in this study may have in fact been “true positives.”
Discrepant diagnosis could be explained by difficulties in siting equipment simultaneously for the purposes of validating the PMD, compromising quality of signals. This is unlikely to be a clinically significant issue as 2 sets of equipment would not need to be applied in clinical practice. The absence of audio-video recording in T2PSG may have resulted in overscoring of obstructive events due to difficulty distinguishing wake from REM sleep; however, inclusion of EEG monitoring makes this unlikely. The low “false negative” rate of 5.5% (2/36) and the fact that more obstructive events were scored in our cohort during T2PSG despite lack of CO2 monitoring refute concerns that T2PSG may miss clinically significant OSA in children.
The tendency to overdiagnose mild OSA would affect the management of a small number of patients. Based on American Academy of Pediatrics recommendations, adenotonsillectomy for mild OSA would only be recommended by most clinicians if there are clinical features of adenotonsillar hypertrophy or other conservative measures have failed to resolve symptoms and there is evidence of persistent OSA. The latter could be determined by an interval PSG either in the laboratory or in the home using a PMD.
The potential cost-savings from implementing a model of care using ambulatory T2PSG are large. The initial infrastructure costs are significantly lower compared to a sleep laboratory and could be a cost-effective solution for jurisdictions without access to a dedicated pediatric sleep laboratory. We have also estimated the comparative ongoing costs per study, including staff salaries, travel (for home set-up), and consumables. In Australia, ambulatory T2PSG costs approximately 50% of the cost of a T1PSG in an accredited pediatric sleep laboratory.
This study has a number of limitations. Our results are applicable to children between the ages of 5–18 years referred for evaluation of OSA who are able to cooperate with PSG setup. Although our study included at least 3 children with attention deficit hyperactivity disorder, 1 with trisomy 21, and 2 with neurofibromatosis, further study is needed to specifically evaluate whether T2PSG is feasible in children with pervasive developmental disorders, behavioral disorders, or uncooperative behavior. This is of particular importance as it is likely these children would better tolerate home PSG given the familiar environment and reduced disruption to their usual routine. The number of participants in our study who were recorded as having a medical comorbidity is likely to have been significantly underestimated, as medical comorbidities were retrospectively obtained using coding data.
Although we have demonstrated feasibility of T2PSG in school-aged children, it has been suggested that the risk-benefit ratio for T2PSG may be greater for children aged over 13 years, hence this study may have underestimated the clinical utility of T2PSG. Future studies could compare feasibility and accuracy in different age groups to address this knowledge gap.
Not all of the eligible families were willing to participate, emphasizing the fact that success of T2PSG in the home is dependent upon selecting families who are willing and engaged to facilitate the process. We did not collect data examining socioeconomic status or parental level of education; however, careful examination of these factors in future will assist in selection of patients who are most suitable for home PSG.
The lack of video recording during T2PSG may cause difficulty differentiating awake and REM as well as determining sleep onset time, potentially overestimating the amount of REM and total sleep time. In addition, given that the apnea-hypopnea index is less validated in children, the inclusion of video may add significant value to PSG by aiding interpretation of results. This study only examined children referred for investigation of OSA; however, inclusion of video could widen the application of ambulatory PSG to include children with suspected bruxism, parasomnias, and behavioral sleep disorders. It is likely that in future inclusion of video for ambulatory home PSG will significantly expand the clinical utility and improve diagnostic accuracy.
In summary, our results demonstrate that ambulatory T2PSG for the purpose of diagnosing OSA in children is feasible with results comparable to T1PSG. The success of a T2PSG in the home is likely to be enhanced by having a trained staff member set up the equipment in the home with appropriate training of an adult caregiver to monitor and replace equipment. Our results support findings of previous studies that T2PSG performed in the home is more representative of a normal night for children because of improved sleep efficiency and more REM sleep, suggesting that T2PSG may be more accurate than T1PSG for diagnosing OSA in children. Additional monitoring equipment, particularly those allowing assessment of autonomic system activity and hence arousals (for example, peripheral arterial tonometry) could be useful to further improve the diagnostic accuracy of ambulatory home PSG.15 Other potentially useful additions include video, actigraphy, recording of body position, snoring, transcutaneous CO2, and airflow.15 Establishing a diagnostic service based on T2PSG can afford a significant cost saving per patient and is a cost-effective adjunct to established services that need to increase throughput.
The authors propose that conducting ambulatory T2PSG will reduce pressure on sleep laboratory beds, staff, and resources, decrease waiting times for T1PSG, and afford significant cost savings to the health care system. Thus, use of home-based T2PSG can help address the growing demand for a timely and accurate diagnosis of OSA in children and adolescents, allowing treatment to be expedited and avoiding long-term serious health consequences of untreated OSA.
DISCLOSURE STATEMENT
All authors have contributed to, reviewed, and approved this manuscript. Work for this study was performed at Princess Margaret Children’s Hospital, Perth, Western Australia, Australia (Princess Margaret Children’s Hospital closed in June 2018 and reopened at a new site as Perth Children’s Hospital. All authors were employed at Princess Margaret Children’s Hospital when this work was performed). All financial support for this research was provided in-kind by the Department of Respiratory and Sleep Medicine. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors sincerely thank all the families that participated in this research, and Ms. Natasha Bear and Associate Professor Brad Zheng for statistical support.
ABBREVIATIONS
- EEG
electroencephalogram
- OSA
obstructive sleep apnea
- PMD
portable monitoring device
- PSG
polysomnography
- RDI
respiratory disturbance index
- REI
respiratory event index
- REM
rapid eye movement
- SDU
sleep disorders unit
- T1PSG
type 1 polysomnography
- T2PSG
type 2 polysomnography
- T3PSG
type 3 polysomnography
REFERENCES
- 1. O’Brien LM , Gozal D . Principles and Practice of Pediatric Sleep Medicine. Philadelphia: : Saunders (Elsevier) ; 2005. . [Google Scholar]
- 2. Mitchell RB , Pereira KD , Friedman NR . Sleep-disordered breathing in children: survey of current practice . Laryngoscope. 2006. ; 116 ( 6 ): 956 – 958 . [DOI] [PubMed] [Google Scholar]
- 3. Kheirandish L , Gozal D . Neurocognitive dysfunction in children with sleep disorders . Dev Sci. 2006. ; 9 ( 4 ): 388 – 399 . [DOI] [PubMed] [Google Scholar]
- 4. Gozal D . Sleep, sleep disorders and inflammation in children . Sleep Med. 2009. ; 10 ( Suppl 1 ): S12 – S16 .Philadelphia: Saunders (Elsevier); 2005. [DOI] [PubMed] [Google Scholar]
- 5. Marcus CL , Brooks LJ , Draper KA , et al. American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome . Pediatrics. 2012. ; 130 ( 3 ): 576 – 584 . [DOI] [PubMed] [Google Scholar]
- 6. Capdevila OS , Kheirandish-Gozal L , Dayyat E , Gozal D . Pediatric obstructive sleep apnea: complications, .management, and long-term outcomes . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 274 – 282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhattacharjee R , Kheirandish-Gozal L , Pillar G , Gozal D . Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children . Prog Cardiovasc Dis. 2009. ; 51 ( 5 ): 416 – 433 . [DOI] [PubMed] [Google Scholar]
- 8. Ahn YM . Treatment of obstructive sleep apnea in children . Korean J Pediatr. 2010. ; 53 ( 10 ): 872 – 879 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Luca Canto G , Pachêco-Pereira C , Aydinoz S , et al . Adenotonsillectomy complications: a meta-analysis . Pediatrics. 2015. ; 136 ( 4 ): 702 – 718 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel MR , Davidson TM . Home sleep testing in the diagnosis and treatment of sleep disordered breathing . Otolaryngol Clin North Am. 2007. ; 40 ( 4 ): 761 – 784 . [DOI] [PubMed] [Google Scholar]
- 11. Kaditis AG , Alonso Alvarez ML , Boudewyns A , et al . Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management . Eur Respir J. 2016. ; 47 ( 1 ): 69 – 94 . [DOI] [PubMed] [Google Scholar]
- 12. Muzumdar H , Arens R . Diagnostic issues in pediatric obstructive sleep apnea . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 263 – 273 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schechter MS ; Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome . Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome . Pediatrics. 2002. ; 109 ( 4 ): e69 . [DOI] [PubMed] [Google Scholar]
- 14. Weatherly RA , Mai EF , Ruzicka DL , Chervin RD . Identification and evaluation of obstructive sleep apnea prior to adenotonsillectomy in children: a survey of practice patterns . Sleep Med. 2003. ; 4 ( 4 ): 297 – 307 . [DOI] [PubMed] [Google Scholar]
- 15. Gozal D , Kheirandish-Gozal L . New approaches to the diagnosis of sleep-disordered breathing in children . Sleep Med. 2010. ; 11 ( 7 ): 708 – 713 . [DOI] [PubMed] [Google Scholar]
- 16. Chervin RD , Weatherly RA , Garetz SL , et al . Pediatric sleep questionnaire: prediction of sleep apnea and outcomes . Arch Otolaryngol Head Neck Surg. 2007. ; 133 ( 3 ): 216 – 222 . [DOI] [PubMed] [Google Scholar]
- 17. Brouilette R , Hanson D , David R , et al. A diagnostic approach to suspected obstructive sleep apnea in children . J Pediatr. 1984. ; 105 ( 1 ): 10 – 14 . [DOI] [PubMed] [Google Scholar]
- 18. Nixon GM , Kermack AS , Davis GM , Manoukian JJ , Brown KA , Brouillette RT . Planning adenotonsillectomy in children with obstructive sleep apnea: the role of overnight oximetry . Pediatrics. 2004. ; 113 ( 1 ): e19 – e25 . [DOI] [PubMed] [Google Scholar]
- 19. Kirk VG , Bohn SG , Flemons WW , Remmers JE . Comparison of home oximetry monitoring with laboratory polysomnography in children . Chest. 2003. ; 124 ( 5 ): 1702 – 1708 . [DOI] [PubMed] [Google Scholar]
- 20. Malbois M , Giusti V , Suter M , Pellaton C , Vodoz JF , Heinzer R . Oximetry alone versus portable polygraphy for sleep apnea screening before bariatric surgery . Obes Surg. 2010. ; 20 ( 3 ): 326 – 331 . [DOI] [PubMed] [Google Scholar]
- 21. Chau KW , Ng DK , Kwok KL , et al . Application of videotape in the screening of obstructive sleep apnea in children . Sleep Med. 2008. ; 9 ( 4 ): 442 – 445 . [DOI] [PubMed] [Google Scholar]
- 22. Sivan Y , Kornecki A , Schonfeld T . Screening obstructive sleep apnoea syndrome by home videotape recording in children . Eur Respir J. 1996. ; 9 ( 10 ): 2127 – 2131 . [DOI] [PubMed] [Google Scholar]
- 23. Rembold CM , Suratt PM . Children with obstructive sleep-disordered breathing generate high-frequency inspiratory sounds during sleep . Sleep. 2004. ; 27 ( 6 ): 1154 – 1161 . [DOI] [PubMed] [Google Scholar]
- 24. Marcus CL , Keens TG , Ward SL . Comparison of nap and overnight polysomnography in children . Pediatr Pulmonol. 1992. ; 13 ( 1 ): 16 – 21 . [DOI] [PubMed] [Google Scholar]
- 25. Hensley MJ , Hillman DR , McEvoy RD , et al . Guidelines for sleep studies in adults. Australasian Sleep Association and Thoracic Society of Australia and New Zealand; : Sydney: ; 2005. . [Google Scholar]
- 26. Merlin T , Liufu Z , Wang S . Unattended sleep studies in the diagnosis and reassessment of obstructive sleep apnoea; 2010. Adelaide, Australia: Adelaide Health Technology Assessment. http://msac.gov.au/interntet/msac/publidhing.nsf/Content/6D179C512E2170A7CA25801000123B34/$File/1130_MSAC_Report.pdf Accessed October 15, 2021.
- 27. Kirk VG , Flemons WW , Adams C , Rimmer KP , Montgomery MD . Sleep-disordered breathing in Duchenne muscular dystrophy: a preliminary study of the role of portable monitoring . Pediatr Pulmonol. 2000. ; 29 ( 2 ): 135 – 140 . [DOI] [PubMed] [Google Scholar]
- 28. Gruber R , Xi T , Frenette S , Robert M , Vannasinh P , Carrier J . Sleep disturbances in prepubertal children with attention deficit hyperactivity disorder: a home polysomnography study . Sleep. 2009. ; 32 ( 3 ): 343 – 350 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacob SV , Morielli A , Mograss MA , Ducharme FM , Schloss MD , Brouillette RT . Home testing for pediatric obstructive sleep apnea syndrome secondary to adenotonsillar hypertrophy . Pediatr Pulmonol. 1995. ; 20 ( 4 ): 241 – 252 . [DOI] [PubMed] [Google Scholar]
- 30. Tan HL , Kheirandish-Gozal L , Gozal D . Pediatric home sleep apnea testing: slowly getting there! Chest. 2015. ; 148 ( 6 ): 1382 – 1395 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel A , Watson M , Habibi P . Unattended home sleep studies for the evaluation of suspected obstructive sleep apnoea syndrome in children . J Telemed Telecare. 2005. ; 11 ( Suppl 1 ): 100 – 102 . [DOI] [PubMed] [Google Scholar]
- 32. Poels PJ , Schilder AG , van den Berg S , Hoes AW , Joosten KF . Evaluation of a new device for home cardiorespiratory recording in children . Arch Otolaryngol Head Neck Surg. 2003. ; 129 ( 12 ): 1281 – 1284 . [DOI] [PubMed] [Google Scholar]
- 33. Zucconi M , Calori G , Castronovo V , Ferini-Strambi L . Respiratory monitoring by means of an unattended device in children with suspected uncomplicated obstructive sleep apnea: a validation study . Chest. 2003. ; 124 ( 2 ): 602 – 607 . [DOI] [PubMed] [Google Scholar]
- 34. Scalzitti N , Hansen S , Maturo S , Lospinoso J , O’Connor P . Comparison of home sleep apnea testing versus laboratory polysomnography for the diagnosis of obstructive sleep apnea in children . Int J Pediatr Otorhinolaryngol. 2017. ; 100 : 44 – 51 . [DOI] [PubMed] [Google Scholar]
- 35. Tan HL , Gozal D , Ramirez HM , Bandla HP , Kheirandish-Gozal L . Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea . Sleep. 2014. ; 37 ( 2 ): 255 – 260 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marcus CL , Traylor J , Biggs SN , et al . Feasibility of comprehensive, unattended ambulatory polysomnography in school-aged children . J Clin Sleep Med. 2014. ; 10 ( 8 ): 913 – 918 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodwin JL , Enright PL , Kaemingk KL , et al . Feasibility of using unattended polysomnography in children for research—report of the Tucson Children’s Assessment of Sleep Apnea study (TuCASA) . Sleep. 2001. ; 24 ( 8 ): 937 – 944 . [DOI] [PubMed] [Google Scholar]
- 38. Katz ES , Marcus CL . Diagnosis of Obstructed Sleep Apnea Syndrome in Infants and Children . In: Kryger M , Roth T , Dement W , eds. Principles and Practice of Pediatric Sleep Medicine. Philadelphia: : Saunders (Elsevier) ; 2005. . [Google Scholar]
- 39. Iber C , Ancoli-Israel S , Chesson AL Jr , Quan SF ; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: : American Academy of Sleep Medicine; ; 2007. . [Google Scholar]
- 40. Flack VF , Afifi AA , Lachenbruch PA , Schouten HJA . Sample size determinations for the two rater kappa statistic . Psychometrika. 1988. ; 53 ( 3 ): 321 – 325 . [Google Scholar]
- 41. Sim J , Wright CC . The kappa statistic in reliability studies: use, interpretation, and sample size requirements . Phys Ther. 2005. ; 85 ( 3 ): 257 – 268 . [PubMed] [Google Scholar]
- 42. Budhiraja R , Quan SF . Outcomes from the Tucson Children’s Assessment of Sleep Apnea Study . Sleep Med Clin. 2009. ; 4 ( 1 ): 9 – 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frölich J , Lehmkuhl G . Home polysomnography in children—a complementary assessment method of sleep disorders in children . Somnologie (Berl). 2004. ; 8 ( 2 ): 61 – 64 . [Google Scholar]
- 44. Stores G , Crawford C , Selman J , Wiggs L . Home polysomnography norms for children . Technol Health Care. 1998. ; 6 ( 4 ): 231 – 236 . [PubMed] [Google Scholar]
- 45. Agnew HW Jr , Webb WB , Williams RL . The first night effect: an EEG study of sleep . Psychophysiology. 1966. ; 2 ( 3 ): 263 – 266 . [DOI] [PubMed] [Google Scholar]