Abstract
Study Objectives:
The aims of this study were to explore changes in the telomere length (relative telomere repeat copy/single-copy gene [T/S ratio]) and serum neurofilament light chain (sNfL) levels in female patients with chronic insomnia disorder (CID), examine their relationships with emotional abnormalities and cognitive impairment, and determine whether these 2 indicators were independently associated with sleep quality.
Methods:
The CID group contained 80 patients diagnosed with CID, and 51 individuals constituted a healthy control group. Participants completed sleep, emotion, and cognition assessments. Telomere length was detected through quantitative real-time polymerase chain reaction. Enzyme-linked immunosorbent assay was used to determine sNfL concentrations.
Results:
Relative to the healthy control group, the CID group had elevated Pittsburgh Sleep Quality Index, Hamilton Anxiety Scale-14, and Hamilton Depression Rating Scale-17 scores and reduced Montreal Cognitive Assessment scale scores, a decreased T/S ratio, and an increased sNfL concentration. Subgroup analysis according to various CID-associated sleep factors showed that poor sleep performance corresponded to a lower T/S ratio. Higher anxiety levels and more cognitive dysfunction correlated with shorter telomere lengths. The T/S ratio negatively correlated with age, whereas the sNfL concentration positively correlated with age in the CID group. The Pittsburgh Sleep Quality Index score negatively correlated with the T/S ratio but did not correlate with sNfL levels. Multiple linear regression analysis showed that the T/S ratio had a negative and independent effect on Pittsburgh Sleep Quality Index scores.
Conclusions:
The CID group had shorter telomeres and higher sNfL concentrations, and reduced telomere length independently affected sleep quality.
Citation:
Ren C-Y, Liu P-P, Li J, et al. Changes in telomere length and serum neurofilament light chain levels in female patients with chronic insomnia disorder. J Clin Sleep Med. 2022;18(2):383–392.
Keywords: chronic insomnia, age, telomere, neurofilament light chain
BRIEF SUMMARY
Current Knowledge/Study Rationale: The aims of this study were to explore changes in the telomere length (relative telomere repeat copy/single-copy gene [T/S ratio]) and serum neurofilament light chain levels in female patients with chronic insomnia disorder, examine their relationships with emotional abnormalities and cognitive impairment, and determine whether these 2 indicators were independently associated with sleep quality.
Study Impact: The study further identified that females with chronic insomnia disorder had a shorter telomere length, a higher serum neurofilament light chain concentration, and the emotional and cognitive impairments associated with chronic insomnia. Crucially, we found that T/S ratio could be regarded as an independent factor correlated with sleep quality.
INTRODUCTION
Insomnia is defined as a lack of satisfaction with sleep duration or quality, despite appropriate sleep opportunities and environments, associated with an impact on daytime social function.1 The clinical features of insomnia include difficulty falling asleep, poor sleep maintenance, early awakening, decreased sleep quality, reduced total sleep time and associated fatigue, mood disorders, and declines in cognitive function. According to statistics, the global prevalence of insomnia is approximately 30–35%.2 Survey data from 10 countries in 2005 showed that 45.4% of respondents in China reported experiencing varying degrees of insomnia.3 Moreover, insomnia is more prevalent in women than in men. A potential reason for the sex difference in the prevalence of insomnia is gonadal steroid effects.3
Chronic insomnia disorder (CID) refers to the continuous experience of insomnia, characterized by symptoms occurring more than 3 nights per week and a disease duration of more than 3 months.1 CID differs from simple sleep loss, a decline in sleep quality, and acute insomnia and has been associated with irreversible brain dysfunction4 and an increased risk of anxiety and depression5 and age-related diseases, such as hypertension, diabetes, heart disease, and stroke.6
As human longevity continues to be extended, aging has attracted the attention of medical workers and the general public. Studies have shown that early cognitive decline might predict further cognitive impairments and dementia,7 and insomnia might play a role in this process.8 Cognitive impairments are the primary complaints of many patients with insomnia and are reported as the reasons for their first visits to a doctor.4 Age-mismatched cognitive impairment has been observed in patients with insomnia in clinical practice. Numerous studies have also shown that insomnia affects cognitive function and might accelerate the aging process.9,10 Moreover, insomnia has been associated with disorders in the amyloid-β (Aβ) metabolism in cerebrospinal fluid and an increased risk of age-related pathology.11
Telomeres are nucleoprotein structures consisting of repeated TTAGGG DNA sequences found at the end of each chromosome in eukaryotic cells.12 The shortening of telomere length after each cell cycle has been attributed to the incomplete replication of the chromosomal end regions during mitosis.13 Telomere length represents one of the most-recognized markers of cell senescence because telomeres shorten with age, resulting in chromosome instability and cell senescence or apoptosis. Moreover, a number of studies in recent years have suggested that telomere shortening is related to age-related diseases, such as cancer,14 Alzheimer disease (AD),15 cardiovascular diseases,16 diabetes,17 chronic obstructive pulmonary disease,18 and hypertension.19 Telomere shortening has also been associated with chronic stress20 and negative emotions.21
Sleep disorders, such as insomnia, can cause cognitive damage and accelerate aging.9 The relationship between telomere length (a marker of aging) and sleep has been studied in a healthy, older population.22 The results of a study performed in a healthy population (ages ranging from 45 to 77 years) to examine the relationship between self-reported sleep quality and telomere length suggested that good sleep quality could weaken the negative correlation between age and telomere length. Adequate sleep was only positively correlated with telomere length in older individuals, suggesting the existence of age-related sleep effects on telomere length.22
Sleep loss has been associated with increased oxidative stress damage,23 cellular immune abnormalities, stress response, inflammatory response,24 the unfolded protein response,25 and a variety of physiological and pathological processes.26 These mechanisms have also been found to be involved in cellular aging processes. We hypothesized that telomere length might serve as a potential marker linking insomnia with cellular senescence. Previous studies focusing on healthy older individuals demonstrated that high sleep quality corresponded to longer telomere length;22 however, little attention has been paid to changes in telomere length among female patients with CID. Neurofilament light chain (NfL) is thought to serve as a marker of axonal damage and has been associated with CID.4 Neurofilament is a specific cytoskeleton protein expressed by neurons, composed of NfL, neurofilament middle chain, neurofilament heavy chain, and α-internexin.27 Recent studies have suggested that elevated cerebrospinal fluid and serum levels of NfL are associated with age.28–30 Therefore, the relationships between NfL, sleep, and cognition are worth exploring.
In summary, the aims of this study were to assess changes in telomere lengths and NfL levels in female patients with CID; explore their relationships with concomitant CID symptoms, such as emotional abnormalities and cognitive impairment; examine the effects of age on the correlation between these 2 indicators in female patients with CID; and determine whether these 2 indicators were independently associated with sleep quality.
METHODS
Participants
According to strict inclusion and exclusion criteria, 80 female patients with CID were recruited from the Department of Neurology of The First Affiliated Hospital of Anhui University of Science and Technology from June 2018 to October 2019. In addition, 51 female healthy controls (HCs) with background demographics (age and education) similar to those for patients with CID were matched and recruited during the same period at the same hospital.
The case group (CID group) inclusion criteria were as follows:
According to the CID diagnostic criteria established in the third edition of the International Classification of Sleep Disorders (ICSD-3), patients were diagnosed with CID if they reported insomnia symptoms no less than 3 times per week with a duration of no less than 3 months, associated with daytime dysfunction. Insomnia symptoms should not be explained by any other type of sleep disorder.31
Patients aged between 18 and 65 years.
Patients with normal verbal communication abilities and the capacity to complete the scale tests.
Patients who voluntarily participated in the study and signed the informed consent form.
The HC group consisted of healthy people selected to match the CID group according to age, sex, and educational level using the following inclusion criteria: (1) no history of insomnia, (2) no emotional (anxiety and depression) or cognitive disorders, (3) normal verbal communication abilities and the capacity to complete the scale tests, (4) no acute insomnia reported the night before scale evaluation and blood sample collection, and (5) voluntary participation in the study and the signing of informed consent.
Patients experiencing secondary insomnia associated with organic diseases, mental disorders, and nervous system diseases were excluded. Participants with physical diseases, such as heart diseases, lung diseases, liver diseases, kidney diseases, autoimmune diseases, pathogenic microbial infections, tumors, or any psychiatric disorders were excluded. Individuals who reported the ingestion of high levels of caffeine (> 400 mg, 4–5 cups), tea, or alcohol or any history of psychotropic drug use (including hypnosis, sedation, antianxiety, antidepression, and antipsychotic medications) were not eligible for this study.
This study was approved by the Clinical Trial Ethics Committee of Huainan First People’s Hospital, and all participants signed informed consent.
Baseline data collection
Background information
General information (name, age, sex, educational level, and body mass index [BMI]) and clinical data (including medical history; personal history; family history; sleep, mood, and cognitive function assessments; laboratory examination; and imaging data) were collected.
Sleep evaluation
The Pittsburgh Sleep Quality Index (PSQI) can be used in the diagnosis of insomnia and the evaluation of sleep quality among patients with sleep disorders and the general population.32 In the current study, the PSQI was used to assess the sleep status of all participants over the course of 1 month. The 18 self-assessment questions are divided into 7 components (participants answer each component using a range of 0–3 points). The maximum score is 21 points, with a higher score indicating worse quality of sleep. In China, a PSQI score above 7 is considered to indicate insomnia symptoms.33 We used total scores to determine whether the participants experienced insomnia and then assessed sleep quality. The 7 PSQI components of sleep quality, sleep latency, total sleep time, sleep efficiency, sleep disorder, hypnotic drug use, and daytime dysfunction were explored individually and in detail.
The Insomnia Severity Index includes 7 items and assesses the severity and impact of insomnia. All items are rated on a scale of 0–4 (a score of 0 indicates no symptom, and a score of 4 indicates severe symptoms). The total score ranges from 0 to 28, with 0–7 points indicating no insomnia, 8–14 points indicating subthreshold insomnia, 15–21 points indicating moderate insomnia, and 22–28 points indicating severe insomnia.34
Emotional evaluation
The Hamilton Depression Rating Scale (17 items) (HAMD-17) is composed of 17 items, assessing depression, guilt, suicidal tendencies and behaviors, difficulty falling asleep, lack of deep sleep, early awakening, interest in work and other activities, hysteresis, agitation, mental anxiety, physical anxiety, gastrointestinal symptoms, systemic symptoms, sexual symptoms, hypochondria, weight loss, and self-awareness. The maximum total score is 52, and the score positively correlates with depression severity. A score greater than 24 points was assessed as severe depression, 17–24 points as moderate depression, 7–17 points as mild depression, and less than 7 points as no depressive symptoms.35
The Hamilton Anxiety Scale (14 items) (HAMA-14) is used to assess the severity of anxiety, including 14 items that assess anxious mood; tension; fear; insomnia; cognitive function; depression; somatic anxiety; sensory, cardiovascular, respiratory, and urogenital symptoms; gastrointestinal symptoms; autonomic nervous symptoms; and behavioral performance during the scale interview.36 All items are scored on a scale of 0–4, with higher scores indicating increasing severity. The maximum total score is 56. Fewer than 8 points corresponds to no anxiety, 8–13 points correspond to mild anxiety, a score of 14–20 is classified as moderate anxiety, 21–28 is classified as severe anxiety, and greater than 29 points is classified as extremely severe anxiety.
Assessment of cognitive function
The Montreal Cognitive Assessment scale (MoCA) has high reliability and validity for the assessment of cognitive function and the screening of cognitive impairments. The MoCA can assess visual space and executive function, naming, memory, attention, language, abstraction, delayed recall, and orientation.37 Among participants with less than 12 years of education, 1 point should be added to their test results to correct for educational level bias. The maximum score is 30, and scores above 26 are categorized as normal cognitive function, 18–26 as mild cognitive impairment, 10–17 as moderate cognitive impairment, and < 10 as severe cognitive impairment.
Blood sample collection and storage
A 4-mL sample of fasting venous blood was extracted from the elbow between 8:00 am and 10:00 am and placed into a blood collection vessel. The serum and blood cells were separated by centrifugation in a TGL-16B centrifuge (ShangHai Anting Scientific Instrument Factory, China) at 3,000 rpm for 5 minutes. The serum was placed in an Eppendorf tube (1.5 mL), and both serum and blood cells were stored at −80°C until testing. The telomere lengths in peripheral blood leukocytes were detected using a Roche Light Cycler II PCR 480 (Roche, Switzerland) quantitative real-time polymerase chain reaction (qRT-PCR) kit. Enzyme-linked immunosorbent assay (Millipore Corporation; USA) was used to determine serum NfL (sNfL) concentrations.
Statistical analysis
In the current study, the normally distributed data are described as the mean ± SD. Student’s t test was used to compare differences between the 2 groups. Comparisons of differences between multiple groups were performed using 1-way analysis of variance, with Bonferroni correction to perform comparisons between 2 individual groups. For nonparametric data (non–normally distributed), the variables are reported in terms of the 25th, 50th, and 75th percentiles. The Mann–Whitney U test was used to compare differences between 2 groups, and the Kruskal–Wallis H test was used to compare differences between multiple groups, with Bonferroni correction for comparisons between 2 individual groups. Comparisons of categorical variables, such as sex, between 2 groups were performed using the chi-square test. Pearson’s correlation analysis was used to analyze the correlations among age, PSQI scores, relative telomere repeat copy/single-copy gene ratio (T/S ratio), and sNfL concentrations. Correlations between multiple factors and sleep quality were analyzed by multiple linear regression analysis. The level of statistical significance was set at a 2-tailed P value of .05. SPSS version 22.0 statistical software (IBM Corporation, Armonk, NY) was used to process data.
RESULTS
Baseline data
A total of 131 participants were included in this study, including 80 participants in the CID group and 51 participants in the HC group. The baseline data for participants in the 2 groups were reasonably well matched, with no significant differences in age and BMI (P > .05; Table 1).
Table 1.
General data, sleep quality, emotion, cognition, T/S ratio, and sNfL.
| Item | CID (n = 80) | HCs (n = 51) | Statistic | P |
|---|---|---|---|---|
| Age, y | 48.900 ± 10.416 | 47.392 ± 10.088 | t = 0.818 | .415 |
| BMI, kg/m2 | 23.519 ± 3.404 | 23.588 ± 2.957 | t = −0.119 | .905 |
| PSQI score | 14 (13, 16) | 0 (0, 2) | U = 0.000 | <.001 |
| ISI score | 14 (11, 17) | 0 (0, 0) | U = 0.000 | <.001 |
| HAMA-14 score | 12 (8, 16.75) | 1 (1, 2) | U = 34.500 | <.001 |
| HAMD-17 score | 10 (7, 14) | 1 (0, 2) | U = 16.5000 | <.001 |
| MoCA score | 18 (15, 22) | 23 (20, 26) | U = 1094.000 | <.001 |
| T/S ratio | 1.313 ± 0.430 | 2.672 ± 0.373 | t = −18.540 | <.001 |
| sNfL, pg/mL | 72.220 ± 22.763 | 48.456 ± 10.775 | t = 6.967 | <.001 |
Normally distributed variables are expressed as means ± SDs; non–normally distributed variables are expressed as 50th (25th, 75th) percentiles. BMI = body mass index, CID = chronic insomnia disorder, HAMA-14 = Hamilton Anxiety Scale (14 items), HAMD-17 = Hamilton Depression Rating Scale (17 items), HC = healthy control, ISI = Insomnia Severity Index, MoCA = Montreal Cognitive Assessment, PSQI = Pittsburgh Sleep Quality Index, sNfL = serum neurofilament light chain, T/S ratio = relative telomere repeat copy/single-copy gene ratio.
Sleep, negative emotions, and cognitive function assessments
The scores for the PSQI and Insomnia Severity Index in the CID group were higher than those in the HC group (P < .001; Table 1). The CID group had higher HAMA-14 and HAMD-17 scores than those for the HC group (P < .001; Table 1). The mean MoCA score for the CID group was lower than that for the HC group (P < .001; Table 1).
Comparisons of the T/S ratio in peripheral blood leukocytes and sNfL concentrations between the 2 groups
The T/S ratio in peripheral blood leukocytes in the CID group was significantly lower than that in the HC group (P < .001; Table 1). The CID group also had a higher sNfL concentration than that in the HC group (P < .001; Table 1).
Comparison of the T/S ratio in peripheral blood leukocytes between CID subgroups
The comparison of the T/S ratio between the CID subgroups is shown in Table 2. No significant differences in the T/S ratio were found for subgroups according to educational level (illiteracy, primary school, middle school, high school, and university or above), degrees of depression (no depression, mild depression, and moderate-to-severe depression), or sleep latency (< 15 minutes, 16–30 minutes, 31–60 minutes, and > 60 minutes).
Table 2.
T/S ratio comparison of each subgroup in CID.
| Item | Cases, n (%) | T/S Ratio | Statistic | P |
|---|---|---|---|---|
| Education | H = 5.694 | .223 | ||
| Illiteracy | 13 (16.25) | 1.180 (1.015, 1.630) | ||
| Primary school | 26 (32.50) | 1.245 (1.038, 1.490) | ||
| Middle school | 25 (31.25) | 1.190 (0.930, 1.783) | ||
| High school | 7 (8.75) | 1.100 (1.050, 1.420) | ||
| Graduate and above | 9 (11.25) | 1.750 (1.185, 2.190) | ||
| Anxiety | H = 10.303 | .016 | ||
| No symptom | 17 (21.25) | 1.620 (1.220, 1.857) | ||
| Mild symptom | 35 (43.75) | 1.190 (0.970, 1.725) | ||
| Moderate symptom | 21 (26.25) | 1.120 (0.875, 1.315) | ||
| Severe symptom | 7 (8.75) | 1.200 (1.030, 1.570) | ||
| Depression | H = 1.792 | .408 | ||
| No symptom | 12 (15.00) | 1.380 (1.112, 1.721) | ||
| Mild symptom | 63 (78.75) | 1.200 (1.020, 1.620) | ||
| Moderate to severe symptoms | 5 (6.25) | 1.030 (0.955, 1.435) | ||
| Cognition impairment | H = 9.073 | .011 | ||
| No | 8 (10.00) | 1.813 (1.365, 2.310) | ||
| Mild | 34 (42.50) | 1.170 (1.015, 1.553) | ||
| Moderate to severe | 38 (47.50) | 1.205 (1.017, 1.533) | ||
| Sleep quality | H = 14.382 | .001 | ||
| Satisfaction | 5 (6.25) | 1.620 (1.325, 2.140) | ||
| Poor | 46 (57.50) | 1.275 (1.075, 1.731) | ||
| Worse | 29 (36.25) | 1.020 (0.850, 1.310) | ||
| Total sleep time | H = 13.055 | .001 | ||
| <2 hours | 6 (7.50) | 0.865 (0.678, 1.095) | ||
| 2–4 hours | 56 (70.00) | 1.195 (1.020, 1.523) | ||
| 4–6 hours | 18 (22.50) | 1.595 (1.170, 1.981) | ||
| Sleep efficiency | H = 24.345 | .000 | ||
| <40% | 13 (16.25) | 1.020 (0.773, 1.085) | ||
| 40–60% | 50 (62.50) | 1.205 (1.020, 1.493) | ||
| >60% | 17 (21.25) | 1.840 (1.425, 1.998) | ||
| Sleep latency | H = 3.088 | .214 | ||
| <15 minutes | 2 (2.50) | 1.210 (1.000, 1.210) | ||
| 16–30 minutes | 4 (5.00) | 1.718 (1.350, 1.811) | ||
| 31–60 minutes | 6 (7.50) | 1.275 (1.048, 1.574) | ||
| >60 minutes | 68 (85.00) | 1.195 (1.020, 1.608) |
Normally distributed variables are expressed as means ± SDs; non–normally distributed variables are expressed as 50th (25th, 75th) percentiles. CID = chronic insomnia disorder, T/S ratio, relative telomere repeat copy/single-copy gene ratio.
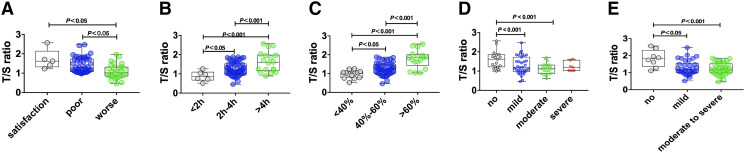
Significant differences in the T/S ratio were found according to sleep quality (satisfactory, poor, and worse; H = 14.382, P = .001). The subgroup with worse sleep quality had a lower T/S ratio than both the poor sleep quality and the satisfactory sleep quality subgroups (Figure 1A). CID subgroups with differences in total sleep time (< 2 hours, 2–4 hours, and 4–6 hours) showed significantly different T/S ratios (F = 13.055, P = .001). The subgroup with a total sleep time of fewer than 2 hours had a lower T/S ratio than the subgroups with a higher total sleep time (Figure 1B). The subgroup with a total sleep time of 2–4 hours had a lower T/S ratio than the subgroup with a total sleep time more than 4 hours (Figure 1B). Significant differences in the T/S ratios were observed according to sleep efficiency (< 40%, 40–60% and > 60%; F = 24.345, P < .001). The subgroup with less than 40% sleep efficiency had a lower T/S ratio than the 2 subgroups with better sleep efficiency (Figure 1C). Compared with the subgroup with the highest sleep efficiency, the subgroup with 40–60% sleep efficiency had a lower T/S ratio (Figure 1C). The T/S ratios differed significantly across 4 CID subgroups with varying degrees of anxiety (no anxiety, mild anxiety, moderate anxiety, and severe anxiety; H = 10.303, P = .016). The subgroup with no anxiety had a higher T/S ratio than those of the mild and moderate anxiety subgroups (Figure 1D). Significant differences in T/S ratios were observed among CID subgroups with varying degrees of cognitive impairments (F = 9.073, P = .011). The T/S ratio in the subgroup with normal cognitive function was higher than those in subgroups with mild and moderate to severe cognitive impairments (Figure 1E). Bonferroni correction was applied to the results of all subgroup comparisons.
Figure 1. Comparison of T/S ratio among CID subgroups.
CID = chronic insomnia disorder, T/S ratio = telomere repeat copy/single-copy gene ratio.
Relationships between age and the T/S ratio and sNfL concentration
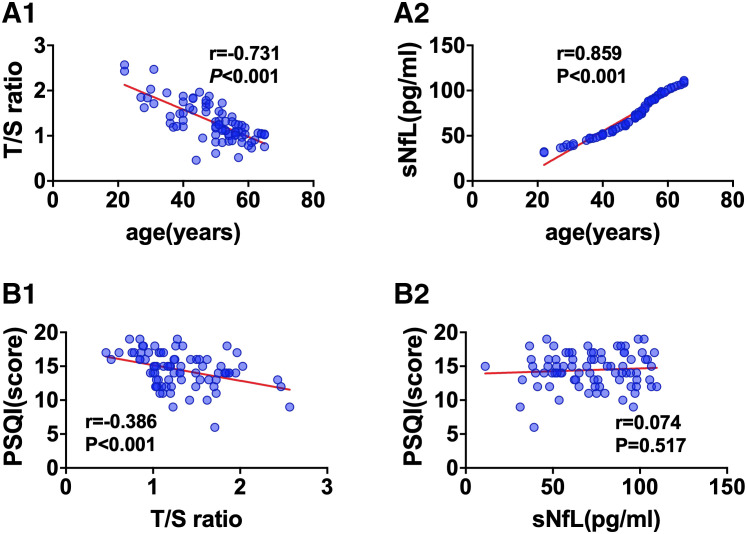
The T/S ratio in peripheral blood leukocytes was negatively correlated with age in the CID group (r = −.731, P < .001; Figure 2A1). A positive correlation between the sNfL concentration and age was also observed in the CID group (r = .859, P < .001; Figure 2A2).
Figure 2. Correlations between age or PSQI and T/S ratio or sNfL in the CID group.
(A1, A2) Correlation between age and T/S ratio or sNfL in the CID group. (B1, B2) Correlation between PSQI and T/S ratio or sNfL in the CID group. CID = chronic insomnia disorder, sNfL = serum neurofilament light chain, T/S ratio = telomere repeat copy/single-copy gene ratio.
Correlations between the PSQI score and the T/S ratio and sNfL concentration
The results of the correlations analysis between PSQI scores and the T/S ratio and sNfL concentration among patients with CID showed that the PSQI score correlated negatively with the T/S ratio (r = −.386, P < .001; Figure 2B1) but did not correlate with the sNfL concentration (Figure 2B2).
Multiple linear regression analysis exploring the effects of multiple factors on self-reported sleep quality
The contributions of multiple factors to PSQI scores were explored by performing multiple linear regression analysis. PSQI scores were incorporated into the model as the dependent variable, Y, and age, educational level, BMI, T/S ratio, and sNfL concentration were incorporated into the model as independent variables, X. The T/S ratio (β = −4.079, P < .001) and sNfL concentrations were independent risk factors for PSQI scores, with both having negative influences on PSQI scores (Table 3).
Table 3.
Multiple linear regression analysis of T/S ratio and sleep factors.
| Independent Variable | β | Std. Error | t | P |
|---|---|---|---|---|
| Constant | 23.040 | 3.807 | 6.051 | .000 |
| Age | −0.054 | 0.059 | −0.915 | .363 |
| Education | 0.170 | 0.267 | 0.636 | .527 |
| BMI | 0.019 | 0.087 | 0.220 | .827 |
| T/S ratio | −4.079 | 0.903 | −4.517 | .000 |
| sNfL | −0.021 | 0.025 | −0.840 | .404 |
BMI = body mass index, sNfL = serum neurofilament light chain, Std. Error = standard error, T/S ratio, relative telomere repeat copy/single-copy gene ratio, β = unstandardized coefficient.s
DISCUSSION
T/S ratio and sNfL concentration in female patients with CID
Telomere length can be considered a marker for aging in individuals. In the current study, the T/S ratio in peripheral blood leukocytes in the CID group was significantly lower than that in the HC group, which suggested that CID might be involved in the aging process and is consistent with previous research. In a study of 126 volunteers comparing those older than 70 years with those aged 60–69 years, insomnia was only associated with shorter telomere lengths in individuals older than 70 years.38 This study suggested that sleep disturbances may increase cellular aging during later years of life. However, the current study involved patients with CID who were younger than 65 years to avoid the effects of older age on telomere length, further supporting that insomnia shortens telomere length. Another difference was that participants in our study were all female patients with CID. But it is also worth mentioning that women’s sleep is susceptible to estrogen, which may be a limitation of this study, and male participants need to be included in future studies. We hypothesized that differences in T/S ratio between men and women might influence the results. Telomere length has been negatively correlated with oxidative stress and cellular DNA damage.39,40 Oxidative stress could lead to DNA damage in circulating blood cells or epigenetic modifications of nuclear DNA, resulting in excessively shortened telomere lengths.40 A previous study showed that insomnia might lead to increased levels of inflammatory cytokines and oxidation, which might also promote the shortening of telomere length.41 To the best of our knowledge, telomerase is the primary protein responsible for the maintenance of telomere structure and length.42 Long-term sleep loss can cause an imbalance in the hypothalamic-pituitary-adrenal axis43 and the abnormal secretion of cortisol,44 which can reduce telomerase activity, resulting in shortened telomeres.45
The CID group had higher sNfL concentrations than the HC group. sNfL is a marker of axonal injury and has been associated with neuron loss.46 Under normal physiological conditions, NfLs are stable in axons.29 However, during some neurodegenerative diseases, such as amyotrophic lateral sclerosis, dementia with Lewy bodies, and Parkinson disease, the reduced expression or hyperphosphorylation of NfL subunit proteins can result in the destruction of the NfL network.47 A previous study showed that functional and structural damage to neurons, axons, and glial cells could be detected in patients with CID, associated with increased NfL protein levels.4
In the current study, to analyze the relationship between the T/S ratio and each specific factor of the PSQI, different CID subgroups were assigned according to sleep quality, sleep time, sleep efficiency, and sleep latency. Comparisons among subgroups divided by other sleep factors indicated that subgroups with better sleep quality, more sleep time, and higher sleep efficiency had longer telomere lengths than those with poor sleep performance, suggesting that sleep quality, time, and efficiency were associated with changes in the T/S ratio; however, no effect on the T/S ratio was observed for sleep latency. The results of comparisons among subgroup differences were consistent with previous studies.48,49 Insomnia, total sleep time in individuals with insomnia, and long sleepers with no insomnia have all been associated with short telomeres.50 In addition, studies in the general population and in children have reported positive correlations between telomere length and sleep duration.48,49 However, another study showed no associations among sleep duration, sleep latency, and telomere length in samples of healthy women.51 The inconsistency of the participants may be the main reason for this difference.
Anxiety, depression, and cognitive impairment were associated with CID and changes in the T/S ratio and sNfL concentration
In the current study, the total scores on the anxiety and depression scales in the CID group were significantly higher than those in the HC group. Increased anxiety and depression (especially mild anxiety and depression, as only 15% and 7%, respectively, were severe) can occur after experiencing symptoms of insomnia. Previous studies have shown that most insomnia cases were accompanied by anxiety and depression.52,53 Among young women, a serious lack of pleasure and daytime anxiety after waking could be attributed to poor sleep quality.54 A limited understanding exists regarding the underlying mechanisms of insomnia and negative emotions. The amygdala, which is located in the medial temporal lobe, plays a key role in emotional processing,55,56 which is associated with major mood disorders, such as schizophrenia, anxiety, major depression, and obsessive-compulsive disorder,57,58 The amygdala receives nerve signals from neurons that regulate the sleep–wake cycle, including β-aminobutyric acid and dopaminergic, serotonergic, and norepinephrinergic neurons associated with the regulation of sleep arousal.59,60 In a clinical study, amygdalar atrophy was observed in patients with CID using magnetic resonance imaging.61 The severity of insomnia was associated with changes in the right medial central amygdala, whereas anxiety was associated with the basolateral nucleus. Insomnia has also been associated with the abnormal secretion of cortisol,62 dysfunction in the hypothalamic-pituitary-adrenal axis,63 and serotonin and dopamine dysfunction,64 which could promote the occurrence of negative emotions.
The results of the present study showed that the total MoCA scores of patients in the CID group were significantly lower than those in the HC group, suggesting cognitive impairment among patients with CID. An earlier study of patients with AD demonstrated that the overexpression of inflammatory cytokines caused by insomnia had an adverse effect on cognitive function.65 The disruption of normal sleep structures in rats caused damage to the hippocampus and resulted in cognitive dysfunction.66 Another mechanism that might link insomnia to cognitive function was the neurodegenerative process associated with Aβ. Cerebrospinal fluid proteins (including Aβ and τ) could be found in the extracellular spaces around brain cells and have been associated with neurodegenerative diseases. The clearance rate of Aβ has been found to increase during sleep, and insomnia led to its accumulation and associated cognitive decline.67
The current study also compared subgroups based on education, anxiety, depression, and cognitive function. No differences in the T/S ratios were observed between subgroups distinguished by educational level and depression, which was consistent with previous studies.21,68 According to these results, the subgroup with no anxiety had a longer T/S ratio than those with mild and moderate anxiety. The T/S ratio in the subgroup with normal cognitive function was higher than those in subgroups with mild and moderate-to-severe cognitive impairments. These differences suggested that anxiety and cognitive impairments in CID were associated with shorter telomere lengths and aging. Telomeres are nucleoprotein structures consisting of repeated DNA fragments that are highly sensitive to oxidative stress. The shortening of the telomere length in leukocytes may be triggered by factors such as oxidative stress and chronic systemic inflammation. Moreover, increased oxidative stress in patients with anxiety may also shorten telomere lengths.21 In the study by Scarabino et al,69 shorter telomere lengths were related to the occurrence of AD. Evidence suggests that amyloid plaques in the brain are accompanied by neuroinflammatory states. In patients with AD and mild cognitive impairment, peripheral immune activation accompanied by a neuroinflammatory state might be associated with shorter telomeres. However, it should be noted that differences in the telomere lengths across the various anxiety and cognitive impairment subgroups may also be caused by insomnia, suggesting that insomnia might affect mood, cognition, and telomere length simultaneously. Additional research remains necessary to explore these links.
T/S ratio independently affected sleep quality
To explore the factors that influence sleep quality, the correlations between the T/S ratio and sNfL concentrations and PSQI scores were analyzed. The results showed that the T/S ratio was negatively correlated with PSQI scores, whereas the sNfL concentration was not correlated with PSQI scores, suggesting that shortened telomere length corresponded to poor sleep quality. Furthermore, the result of the multiple linear regression analysis (including age, educational level, BMI, T/S ratio, and sNfL concentration) also indicated that only the T/S ratio was an independent influencing factor for PSQI scores. In general, the correlation between T/S ratio and sleep quality was independent, implying that the shortening of telomere length and the process of aging were directly associated with sleep quality among patients with CID.
We hypothesized that age-related changes in sNfL levels would play an important role in sleep quality. Specifically, although changes in both indicators among the CID group were associated with changes in sleep quality and age, only changes in the T/S ratio were independently and directly associated with sleep quality, whereas changes in the sNfL levels appeared to be more strongly related to age than to sleep. Previous studies have shown that telomere length and sNfL levels were age related.12,28 Telomere lengths decrease and the sNfL concentrations increase during aging. These phenomena were confirmed by the current study. Pearson’s correlation analysis showed that the T/S ratio in peripheral blood leukocytes in the CID group was significantly negatively correlated with age, whereas sNfL concentration was positively correlated with age. In summary, the correlations between age and these 2 indicators and the results of the multiple linear regression observed in this study indicate that the T/S ratio could be regarded as an independent factor correlated with sleep quality.
CONCLUSIONS
Telomere length and the sNfL level are 2 biological indicators correlated with age. Moreover, their associations with sleep quality have been reported in previous studies of the general population. Our study further identified that female patients with CID had shorter telomere lengths and higher sNfL concentrations, and the emotional and cognitive impairments associated with CID might be associated with these 2 indicators. Crucially, we found that the T/S ratio could be regarded as an independent factor correlated with sleep quality. In this study, a large sample of patients with chronic insomnia was enrolled, but a limitation was that only female patients were enrolled and there was a lack of objective sleep data, mainly due to low willingness to complete polysomnography monitoring. Although a complete sleep history was obtained at the beginning of the study, a weakness of the work was that specific tools to evaluate sleep-disordered breathing, such as the Berlin and STOP BANG questionnaires, were not administered. In view of this, we will focus on expanding the sample, including male participants, completing the assessment of obstructive sleep apnea syndrome (OSAS) scales, and analyzing objective sleep data in the future. In summary, early treatment to avoid the chronic process of insomnia may have significance for the prevention of aging-associated decline, and neurobiological indicators, such as sNfL concentration, may be used to indicate the severity and prognosis of insomnia.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was funded by the Huainan Science and Technology Project (2017A0593), the 2018 Graduate Scientific Research Innovation Program of Bengbu Medical College (Byycx1848), the university-level funded projects of Anhui University of Science and Technology (QN2019124), the National Natural Science Foundation of China (no. 81971483, 81672445), and the Top Talents Program of Disciplines (Majors) in Colleges and Universities of Anhui Province (gxbjZD12). The funders had no role in the study design, data collection, analysis, and decision to publish or preparation of the manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the support and assistance from the Department of Neurology of the First Affiliated Hospital of Anhui University of Science and Technology. Data availability: All data generated or analyzed during this study are included in this article.
ABBREVIATIONS
- AD
Alzheimer disease
- BMI
body mass index
- CID
chronic insomnia disorder
- HAMA-14
Hamilton Anxiety Scale (14 items)
- HAMD-17
Hamilton Depression Rating Scale (17 items)
- HC
healthy control
- MoCA
Montreal Cognitive Assessment
- NfL
neurofilament light chain
- PSQI
Pittsburgh Sleep Quality Index
- sNfL
serum neurofilament light chain
- T/S ratio
telomere repeat copy/single-copy gene ratio
REFERENCES
- 1. Sutton EL . Insomnia . Med Clin North Am. 2014. ; 98 ( 3 ): 565 – 581 . [DOI] [PubMed] [Google Scholar]
- 2. Morin CM , Drake CL , Harvey AG , et al . Insomnia disorder . Nat Rev Dis Primers. 2015. ; 1 ( 1 ): 15026 . [DOI] [PubMed] [Google Scholar]
- 3. Soldatos CR , Allaert FA , Ohta T , Dikeos DG . How do individuals sleep around the world? Results from a single-day survey in ten countries . Sleep Med. 2005. ; 6 ( 1 ): 5 – 13 . [DOI] [PubMed] [Google Scholar]
- 4. Zhang P , Tan CW , Chen GH , et al . Patients with chronic insomnia disorder have increased serum levels of neurofilaments, neuron-specific enolase and S100B: does organic brain damage exist? Sleep Med. 2018. ; 48 : 163 – 171 . [DOI] [PubMed] [Google Scholar]
- 5. Li YI , Starr LR , Wray-Lake L . Insomnia mediates the longitudinal relationship between anxiety and depressive symptoms in a nationally representative sample of adolescents . Depress Anxiety. 2018. ; 35 ( 6 ): 583 – 591 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kay-Stacey M , Attarian H . Advances in the management of chronic insomnia . BMJ. 2016. ; 354 : i2123 . [DOI] [PubMed] [Google Scholar]
- 7. Rabin LA , Smart CM , Amariglio RE . Subjective cognitive decline in preclinical Alzheimer’s disease . Annu Rev Clin Psychol. 2017. ; 13 ( 1 ): 369 – 396 . [DOI] [PubMed] [Google Scholar]
- 8. de Almondes KM , Costa MV , Malloy-Diniz LF , Diniz BS . Insomnia and risk of dementia in older adults: systematic review and meta-analysis . J Psychiatr Res. 2016. ; 77 : 109 – 115 . [DOI] [PubMed] [Google Scholar]
- 9. Sexton CE , Sykara K , Karageorgiou E , et al . Connections between insomnia and cognitive aging . Neurosci Bull. 2020. ; 36 ( 1 ): 77 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dzierzewski JM , Dautovich N , Ravyts S . Sleep and cognition in older adults . Sleep Med Clin. 2018. ; 13 ( 1 ): 93 – 106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen DW , Wang J , Zhang LL , Wang YJ , Gao CY . Cerebrospinal fluid amyloid-β levels are increased in patients with insomnia . J Alzheimers Dis. 2018. ; 61 ( 2 ): 645 – 651 . [DOI] [PubMed] [Google Scholar]
- 12. Turner KJ , Vasu V , Griffin DK . Telomere biology and human phenotype . Cells. 2019. ; 8 ( 1 ): E73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fasching CL . Telomere length measurement as a clinical biomarker of aging and disease . Crit Rev Clin Lab Sci. 2018. ; 55 ( 7 ): 443 – 465 . [DOI] [PubMed] [Google Scholar]
- 14. Okamoto K , Seimiya H . Revisiting telomere shortening in cancer . Cells. 2019. ; 8 ( 2 ): E107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo Y , Yu H . Leukocyte telomere length shortening and Alzheimer’s disease etiology . J Alzheimers Dis. 2019. ; 69 ( 3 ): 881 – 885 . [DOI] [PubMed] [Google Scholar]
- 16. De Meyer T , Nawrot T , Bekaert S , De Buyzere ML , Rietzschel ER , Andrés V . Telomere length as cardiovascular aging biomarker: JACC review topic of the week . J Am Coll Cardiol. 2018. ; 72 ( 7 ): 805 – 813 . [DOI] [PubMed] [Google Scholar]
- 17. Wang J , Dong X , Cao L , et al . Association between telomere length and diabetes mellitus: a meta-analysis . J Int Med Res. 2016. ; 44 ( 6 ): 1156 – 1173 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Córdoba-Lanús E , Cazorla-Rivero S , Espinoza-Jiménez A , et al . Telomere shortening and accelerated aging in COPD: findings from the BODE cohort . Respir Res. 2017. ; 18 ( 1 ): 59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zgheib NK , Sleiman F , Nasreddine L , et al . Short telomere length is associated with aging, central obesity, poor sleep and hypertension in Lebanese individuals . Aging Dis. 2018. ; 9 ( 1 ): 77 – 89 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Esch T , Kream RM , Stefano GB . Chromosomal processes in mind-body medicine: chronic stress, cell aging, and telomere length . Med Sci Monit Basic Res. 2018. ; 24 : 134 – 140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang X , Sundquist K , Hedelius A , Palmér K , Memon AA , Sundquist J . Leukocyte telomere length and depression, anxiety and stress and adjustment disorders in primary health care patients . BMC Psychiatry. 2017. ; 17 ( 1 ): 148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cribbet MR , Carlisle M , Cawthon RM , et al . Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults . Sleep. 2014. ; 37 ( 1 ): 65 – 70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gulec M , Ozkol H , Selvi Y , et al . Oxidative stress in patients with primary insomnia . Prog Neuropsychopharmacol Biol Psychiatry. 2012. ; 37 ( 2 ): 247 – 251 . [DOI] [PubMed] [Google Scholar]
- 24. Syauqy A , Hsu CY , Rau HH , Kurniawan AL , Chao JC . Association of sleep duration and insomnia symptoms with components of metabolic syndrome and inflammation in middle-aged and older adults with metabolic syndrome in Taiwan . Nutrients. 2019. ; 11 ( 8 ): E1848 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anafi RC , Pellegrino R , Shockley KR , Romer M , Tufik S , Pack AI . Sleep is not just for the brain: transcriptional responses to sleep in peripheral tissues . BMC Genomics. 2013. ; 14 ( 1 ): 362 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tufik S , Andersen ML , Bittencourt LR , Mello MT . Paradoxical sleep deprivation: neurochemical, hormonal and behavioral alterations: evidence from 30 years of research . An Acad Bras Cienc. Preprint posted online September 2009. . [DOI] [PubMed] [Google Scholar]
- 27. Gaetani L , Blennow K , Calabresi P , Di Filippo M , Parnetti L , Zetterberg H . Neurofilament light chain as a biomarker in neurological disorders . J Neurol Neurosurg Psychiatry. 2019. ; 90 ( 8 ): 870 – 881 . [DOI] [PubMed] [Google Scholar]
- 28. Mattsson N , Andreasson U , Zetterberg H , Blennow K ; Alzheimer’s Disease Neuroimaging Initiative . Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease . JAMA Neurol. 2017. ; 74 ( 5 ): 557 – 566 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khalil M , Teunissen CE , Otto M , et al . Neurofilaments as biomarkers in neurological disorders . Nat Rev Neurol. 2018. ; 14 ( 10 ): 577 – 589 . [DOI] [PubMed] [Google Scholar]
- 30. Disanto G , Barro C , Benkert P , et al. ; Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis . Ann Neurol. 2017. ; 81 ( 6 ): 857 – 870 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sateia MJ . International Classification of Sleep Disorders-third edition: highlights and modifications . Chest. 2014. ; 146 ( 5 ): 1387 – 1394 . [DOI] [PubMed] [Google Scholar]
- 32. Buysse DJ , Reynolds CF III , Monk TH , Hoch CC , Yeager AL , Kupfer DJ . Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) . Sleep. 1991. ; 14 ( 4 ): 331 – 338 . [PubMed] [Google Scholar]
- 33. Tsai PS , Wang SY , Wang MY , et al . Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects . Qual Life Res. 2005. ; 14 ( 8 ): 1943 – 1952 . [DOI] [PubMed] [Google Scholar]
- 34. Morin CM , Belleville G , Bélanger L , Ivers H . The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response . Sleep. 2011. ; 34 ( 5 ): 601 – 608 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamilton M . A rating scale for depression . J Neurol Neurosurg Psychiatry. 1960. ; 23 ( 1 ): 56 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thompson E . Hamilton Rating Scale for Anxiety (HAM-A) . Occup Med (Lond). 2015. ; 65 ( 7 ): 601 . [DOI] [PubMed] [Google Scholar]
- 37. Nasreddine ZS , Phillips NA , Bédirian V , et al . The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment . J Am Geriatr Soc. 2005. ; 53 ( 4 ): 695 – 699 . [DOI] [PubMed] [Google Scholar]
- 38. Carroll JE , Esquivel S , Goldberg A , et al . Insomnia and telomere length in older adults . Sleep. 2016. ; 39 ( 3 ): 559 – 564 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tasali E , Ip MS . Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 207 – 217 . [DOI] [PubMed] [Google Scholar]
- 40. Kim KS , Kwak JW , Lim SJ , Park YK , Yang HS , Kim HJ . Oxidative stress-induced telomere length shortening of circulating leukocyte in patients with obstructive sleep apnea . Aging Dis. 2016. ; 7 ( 5 ): 604 – 613 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Irwin MR , Olmstead R , Carroll JE . Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation . Biol Psychiatry. 2016. ; 80 ( 1 ): 40 – 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zvereva MI , Shcherbakova DM , Dontsova OA . Telomerase: structure, functions, and activity regulation . Biochemistry (Mosc). 2010. ; 75 ( 13 ): 1563 – 1583 . [DOI] [PubMed] [Google Scholar]
- 43. Vargas I , Vgontzas AN , Abelson JL , Faghih RT , Morales KH , Perlis ML . Altered ultradian cortisol rhythmicity as a potential neurobiologic substrate for chronic insomnia . Sleep Med Rev. 2018. ; 41 : 234 – 243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen GH , Xia L , Wang F , Li XW , Jiao CA . Patients with chronic insomnia have selective impairments in memory that are modulated by cortisol . Psychophysiology. 2016. ; 53 ( 10 ): 1567 – 1576 . [DOI] [PubMed] [Google Scholar]
- 45. Choi J , Fauce SR , Effros RB . Reduced telomerase activity in human T lymphocytes exposed to cortisol . Brain Behav Immun. 2008. ; 22 ( 4 ): 600 – 605 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khalil M , Pirpamer L , Hofer E , et al . Serum neurofilament light levels in normal aging and their association with morphologic brain changes . Nat Commun. 2020. ; 11 ( 1 ): 812 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beck R , Deek J , Safinya CR . Structures and interactions in “bottlebrush” neurofilaments: the role of charged disordered proteins in forming hydrogel networks . Biochem Soc Trans. 2012. ; 40 ( 5 ): 1027 – 1031 . [DOI] [PubMed] [Google Scholar]
- 48. Jackowska M , Hamer M , Carvalho LA , Erusalimsky JD , Butcher L , Steptoe A . Short sleep duration is associated with shorter telomere length in healthy men: findings from the Whitehall II cohort study . PLoS One. 2012. ; 7 ( 10 ): e47292 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. James S , McLanahan S , Brooks-Gunn J , et al . Sleep duration and telomere length in children . J Pediatr. 2017. ; 187 : 247 – 252, e1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tempaku P , Hirotsu C , Mazzotti D , et al . Long sleep duration, insomnia, and insomnia with short objective sleep duration are independently associated with short telomere length . J Clin Sleep Med. 2018. ; 14 ( 12 ): 2037 – 2045 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prather AA , Puterman E , Lin J , et al . Shorter leukocyte telomere length in midlife women with poor sleep quality . J Aging Res. 2011. ; 721390 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cox RC , Sterba SK , Cole DA , Upender RP , Olatunji BO . Time of day effects on the relationship between daily sleep and anxiety: an ecological momentary assessment approach . Behav Res Ther. 2018. ; 111 : 44 – 51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ypsilanti A , Lazuras L , Robson A , Akram U . Anxiety and depression mediate the relationship between self-disgust and insomnia disorder . Sleep Health. 2018. ; 4 ( 4 ): 349 – 351 . [DOI] [PubMed] [Google Scholar]
- 54. Kalmbach DA , Arnedt JT , Swanson LM , Rapier JL , Ciesla JA . Reciprocal dynamics between self-rated sleep and symptoms of depression and anxiety in young adult women: a 14-day diary study . Sleep Med. 2017. ; 33 : 6 – 12 . [DOI] [PubMed] [Google Scholar]
- 55. Sergerie K , Chochol C , Armony JL . The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies . Neurosci Biobehav Rev. 2008. ; 32 ( 4 ): 811 – 830 . [DOI] [PubMed] [Google Scholar]
- 56. Pessoa L . Emotion and cognition and the amygdala: from “what is it?” to “what’s to be done?” Neuropsychologia. 2010. ; 48 ( 12 ): 3416 – 3429 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. He C , Gong L , Yin Y , et al . Amygdala connectivity mediates the association between anxiety and depression in patients with major depressive disorder . Brain Imaging Behav. 2019. ; 13 ( 4 ): 1146 – 1159 . [DOI] [PubMed] [Google Scholar]
- 58. Zhang L , Hu X , Li H , et al . Characteristic alteration of subcortical nuclei shape in medication-free patients with obsessive-compulsive disorder . Neuroimage Clin. 2019. ; 24 : 102040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Benarroch EE . The amygdala: functional organization and involvement in neurologic disorders . Neurology. 2015. ; 84 ( 3 ): 313 – 324 . [DOI] [PubMed] [Google Scholar]
- 60. Sah P , Faber ES , Lopez De Armentia M , Power J . The amygdaloid complex: anatomy and physiology . Physiol Rev. 2003. ; 83 ( 3 ): 803 – 834 . [DOI] [PubMed] [Google Scholar]
- 61. Gong L , Liao T , Liu D , et al . Amygdala changes in chronic insomnia and their association with sleep and anxiety symptoms: insight from shape analysis . Neural Plast. 2019. ; 8549237 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Dalfsen JH , Markus CR . The serotonin transporter polymorphism (5-HTTLPR) and cortisol stress responsiveness: preliminary evidence for a modulating role for sleep quality . Stress. 2018. ; 21 ( 6 ): 503 – 510 . [DOI] [PubMed] [Google Scholar]
- 63. van Dalfsen JH , Markus CR . The influence of sleep on human hypothalamic-pituitary-adrenal (HPA) axis reactivity: a systematic review . Sleep Med Rev. 2018. ; 39 : 187 – 194 . [DOI] [PubMed] [Google Scholar]
- 64. Blake MJ , Trinder JA , Allen NB . Mechanisms underlying the association between insomnia, anxiety, and depression in adolescence: implications for behavioral sleep interventions . Clin Psychol Rev. 2018. ; 63 : 25 – 40 . [DOI] [PubMed] [Google Scholar]
- 65. Irwin MR , Vitiello MV . Implications of sleep disturbance and inflammation for Alzheimer's disease dementia . Lancet Neurol. 2019. ; 18 ( 3 ): 296 – 306 . [DOI] [PubMed] [Google Scholar]
- 66. Tartar JL , Ward CP , McKenna JT , et al . Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation . Eur J Neurosci. 2006. ; 23 ( 10 ): 2739 – 2748 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mander BA , Winer JR , Jagust WJ , Walker MP . Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 2016. ; 39 ( 8 ): 552 – 566 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Verhoeven JE , Révész D , van Oppen P , Epel ES , Wolkowitz OM , Penninx BW . Anxiety disorders and accelerated cellular ageing . Br J Psychiatry. 2015. ; 206 ( 5 ): 371 – 378 . [DOI] [PubMed] [Google Scholar]
- 69. Scarabino D , Broggio E , Gambina G , Corbo RM . Leukocyte telomere length in mild cognitive impairment and Alzheimer’s disease patients . Exp Gerontol. 2017. ; 98 : 143 – 147 . [DOI] [PubMed] [Google Scholar]