ABSTRACT
MicroRNAs exert crucial effects in the drug resistance. The purpose of this research was to investigate the miR-25-3p effects on DDP resistance in NSCLC. We used RT-qPCR to evaluate the expression of miR-25-3p. Cell growth was determined using MTS assay. Cellular bio-activity was analyzed via Colony formation, Annexin V/PI, and Transwell assay. Luciferase reporter assay was used to determine miR-25-3p and PTEN binding. Western blot was used to determine PTEN, PI3K, p-AKT/AKT expression. In-vivo study was used to determine the effects of miR-25-3p on the tumor growth. Expression of miR-25-3p is increased in NSCLC cisplatin resistant A549 and H1299 cells. Furthermore, miR-25-3p mimic enhanced drug resistance, and accelerated cell invasion and metastasis. Moreover, miR-25-3p mimic resulted in the activation of PTEN/PI3K/AKT pathway. However, miR-25-3p inhibitors exhibited the opposite trend. We further identified PTEN as a potential target of miR-25-3p. PTEN knockout promoted cisplatin resistance, while PTEN mimic displayed opposite effects. Interestingly, miR-25-3p further boosted cisplatin resistance cells in vivo, and miR-25-3p inhibitors reduced the in-vivo tumor volume. MiR-25-3p/PTEN/PI3K/AKT axis might accelerate DDP tolerance in NSCLC, which may serve as a potential target for chemotherapy resistance in NSCLC.
KEYWORDS: miR-25-3p, PTEN/PI3K/AKT, cisplatin resistance, NSCLC
Graphical abstract
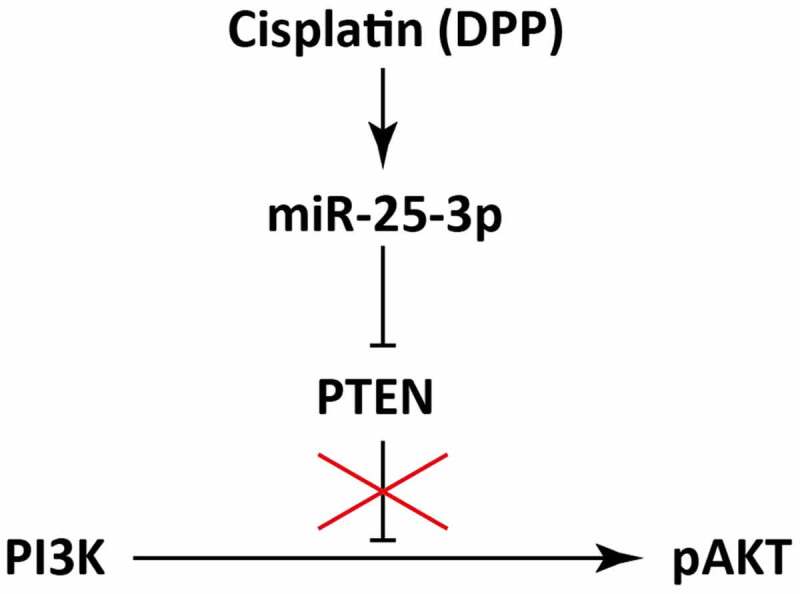
1. Introduction
Non-small cell lung cancer (NSCLC) is regarded as most acute and prevalent cancer [1]. So far, treatment for NSCLC remains chemotherapy-based [2]. Chemotherapy, as the preferred treatment means, exerts an important function in ameliorating the quality of life and prolonging the overall survival among NSCLC patients [3,4].
Cisplatin, also termed as DDP and considered as vulgaris antitumor drug, accelerates apoptosis via DNA damage and changes of cell metabolism [4]. However, it has been reported that cisplatin triggers several acute adverse reactions, including alopecia, vomiting, and myelosuppression [5]. Chemotherapy resistance mainly comprises primary drug resistance and acquired drug resistance [6]. Acquired drug resistance mianly displays tumor cells sensitivity to certain chemotherapeutic drugs decreased after several applications [7,8].
Many recent investigations suggested that PI3K/AKT signaling pathway play critical role in several diseases, including metabolic diseases and tumors by playing critical role in the cell proliferation [9,10]. Therefore, PI3K/AKT pathway inhibits the tumor development, and brings about poor prognosis of many cancers [11]. Several microRNAs (miRNAs), small non-coding RNAs (ncRNAs), have been identified as potential biomarkers for NSCLC [12]. Moreover, these miRNAs regulate the biological and cellular functions in NSCLC, thus playing a role in the pathogenesis of NSCLC [13]. Numerous miRNAs have been identified to be associated with PTEN/PI3K/AKT signaling pathway which play vital role in the survival of cancer patients [14,15]. Interestingly, miRNAs mediate the cisplatin resistance in NSCLC [16,17]. It has been studied that miR-26a level regulates AKT expression to counter DDP resistance [18]. Whereas, miRNA-17/1244 regulates cisplatin resistance in NSCLC via targeting TP53 and βR2 [19,20]. It has been previously investigated that miR-25-3p is upregulated in the cigarette smoke condensate (CSC) [21]. Moreover, miR-25-3p promotes invasion of human non-small cell lung cancer via CDH1 [22]. However, the exact role of miR-25-3p in cisplatin drug resistance mechanism in NSCLC remained unclear.
Based on this, our research attempted to inquire miR-25-3p expression in cisplatin resistance, so as to provide theoretical basis for improving the clinical efficacy of cisplatin chemotherapy in NSCLC.
2. Materials and methods
2.1. Cell culture and cisplatin treatment
A549 and H1299 cells were derived from the American culture library (Tongpai Biotechnology Co., Ltd). The cells were resuspended, and cultivated in RPMI1640 with 10% FBS at 37°C supplemented with 5% CO2. To obtain cisplatin resistant NSCLC cells, different concentrations of cisplatin (2, 4, 6, 8, 16, 32, 64) μmol/L were used for 6 h. Later, cells were used for subsequent assays.
2.2. qRT-PCR analysis
Trizol was used to isolate RNA. Then, monolithic RNA (Shanghai shanran Biotechnology Co., Ltd) kit was used to prepare the cDNA. SYBR Green Master Mix II (Adlai Biotechnology Co., Ltd) was used for RT-qPCR. U6 and GAPDH were used as internal control. 2-ΔΔCT approaches were utilized to analyze data.
2.3. MTS assay
Applied miR-25-3p mimics to transfect A549/DDP cells. Similarly, A549 cells were also treated with miR-25-3p inhibitor or NC inhibitor using liposome 2000 (Beijing wobison Technology Co., Ltd). After 72 h, the cell growth was assessed by MTS (Beijing Jinglei Technology Development Co., Ltd). Absorbance was measured via ELISA reader (Shandong Boke Biological Industry Co., Ltd).
2.4. Luciferase constructs assay
For luciferase assay, we inserted amplified DNA sequence into the psi-check2 vector. Briefly, we prepared the wildtype (WT) and Mutant (MUT) PTEN 3ʹ-UTR luciferase vectors. Then, cells were transfected with these vectors in presence of miR-25-3p mimics or NC, and the luciferase activity was determined.
2.5. Colony formation assay
A total of 400 cells/well were cultured into 6-well plates for 14 d, and treated with miR-25-3p mimics or inhibitors. Then, cells were stained using crystal violet solution (Qingdao jieshikang Biotechnology Co., Ltd). To calculate the data, a cluster of 50 cells was regarded as a colony.
2.6. Transwell assay
First, 100 μL Matt Riegel gel was added into serum-free culture medium. Then, 100 μL of cellular suspension and 200 μL serum-free culture media was mixed in Transwell upper chamber. Transwell lower chamber was filled with 300 μL complete culture medium containing 0.05% fetal bovine serum, and incubated together for 2 d. Later, upper chamber was fixed via paraformaldehyde and dyed via crystal violet. Polyester films were gained from the upper room base. The field was acquired under high microscope (× 400).
2.7. Flow cytometry test
The cells were transfected with miR-25-3p mimics or inhibitors. V-FITC and PI were added into cell suspension, and incubated at 4°C overnight. The flow cytometry was applied to detect apoptosis rate.
2.8. Western blot assays
Total protein was extracted using RIPA buffer, and quantified using BCA kit. Then, protein samples (20 μg) were run using SDS-PAGE. Subsequently, the proteins were transferred to PVDF membrane, blocked with 5% skim milk, and incubated with specific first antibody at 4°C for overnight. Finally, the membranes were washed, and further incubated with the horseradish peroxidase coupled secondary antibody. Finally, the protein bands were identified by using the enhanced chemiluminescence kit (Nanjing novozan Biotechnology Co., Ltd). Β-actin was used as internal control.
2.9. Tumor formation in nude mice
Mice were acquired from animal laboratory. All experiments related to the use of animals were permitted through the ethics committee. 0.5 × 107 cells were inoculated under the soft skin of the right forelimb on the back of nude mice. Cisplatin (3 mg/kg) was injected i.p. to mice once every two weeks. Later, the animals were sacrificed, and the tumor volume and weight were calculated. The animal experiment research protocol was approved by the Ethics Committee of China-Japan Union Hospital of Jilin University and performed in accordance with the ‘Guidelines for the care and use of experimental animals.’
2.10. Statistical analysis
Data were exhibited as X ± S and were analyzed by t-test between different groups. P < 0.05 was considered as statistically significant difference.
3. Results
3.1. Expression of miR-25-3p is increased during cisplatin resistance in NSCLC
It was previously studied that miR-25-3p is upregulated in the cigarette smoke condensate (CSC) [21], and promotes invasion of human non-small cell lung cancer via CDH1 [22]. However, the exact role of miR-25-3p in cisplatin drug resistance mechanism in NSCLC remained unclear. In this study, firstly we detected A549 DDP cells sensitivity to DDP via MTS methods; see Figure 1a for more details. Our outcomes found that A549/H1299 DDP drug-fast was less. Surprisingly, we also found that H1299 cells displayed weaker strength to DDP (Figure 1b). Moreover, the apoptosis rate was decreased after the DDP treatment (Figure 1c). Finally, we used RT-qPCR to analyze the miR-25-3p expression. Our results demonstrated that expression of miR-25-3p was significantly increased in both cell lines after DDP treatment (Figure 1d). This concluded that the expression of miR-25-3p is increased during cisplatin resistance in NSCLC.
Figure 1.
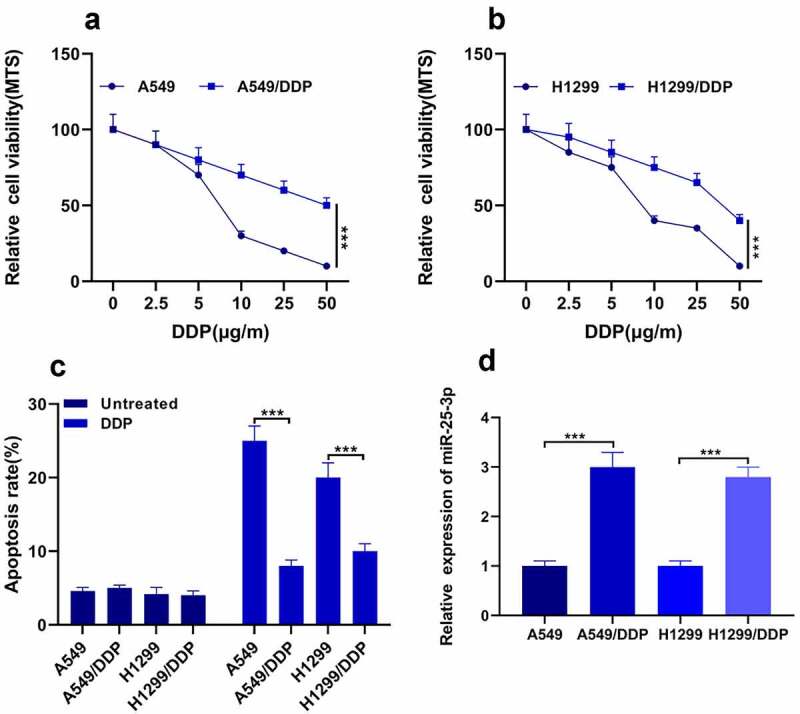
miR-25-3p was highly expressed in NSCLC. (a–b) MTS was utilized for determining two cells’ sensitivity to DDP. (c) Flow cytometry was utilized for measuring apoptosis. (d) RT-PCR found that miR-25-3p expression in two cells was memorably lower. *** P < 0.001
3.2. miR-25-3p mimic promotes cisplatin resistance and migration
Then, we probed whether miR-25-3p mimic could regulate DDP drug-fast and migration in A549 and H1299 cell lines. Our results showed that miR-25-3p mimics increase the cell proliferation and migration. Whereas miR-25-3p inhibitor displayed opposite trend (Figure 2a–d). These results implied that miR-25-3p play a role in promoting cisplatin resistance and NSCLC migration.
Figure 2.
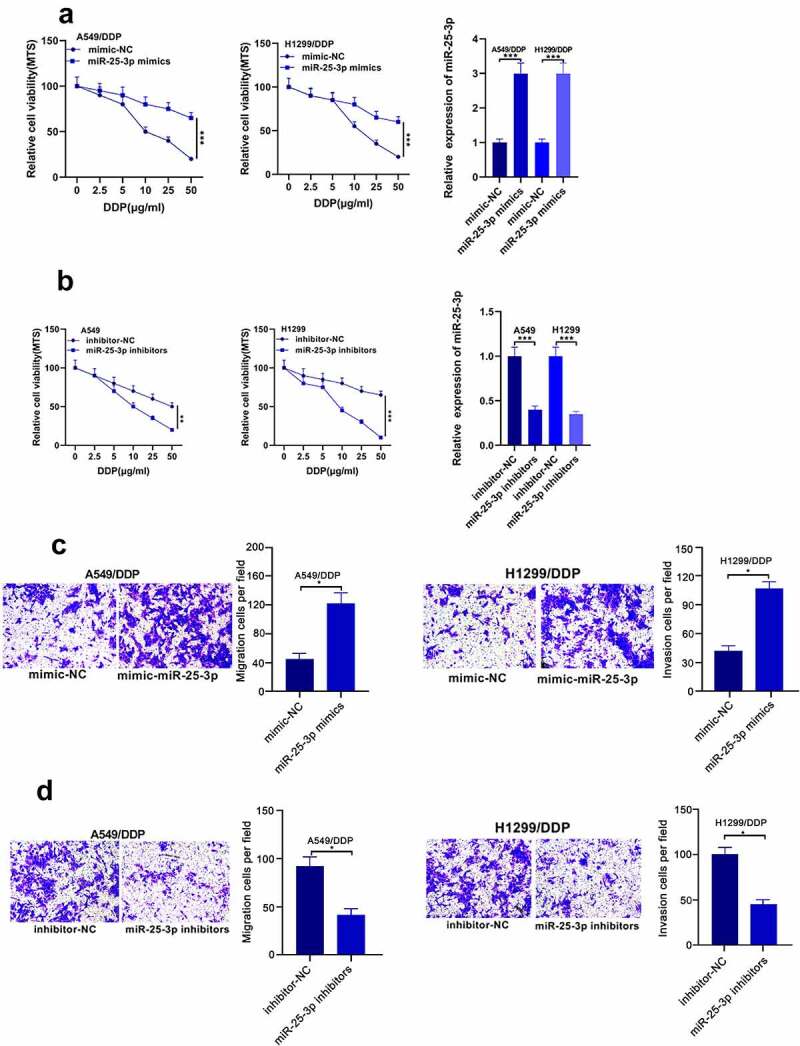
miR-25-3p lowered DDP drug-fast as well as A549/DDP progress. (a–b) MTS was applied to detect miR-25-3p level via miR-25-3p mimic/inhibitor treatment. (c-d) The A549/DDP cells’ movement was determined via miR-25-3p mimic/inhibitor transfection. **/***P < 0.01/0.001
3.3. miR-25-3p mimic restrained DDP led apoptosis
In order to validate the role of miR-25-3p in A549 and H1299 cells apoptosis, we performed the flow cytometry. From the Figure 3a, our team discovered that miR-25-3p over-expression exerted inhibitory effects on the cisplatin-induced apoptosis. On the contrary, miR-25-3p inhibitor showed opposite trends (Figure 3b). This shows that mir-25-3p restrained DDP-induced apoptosis.
Figure 3.
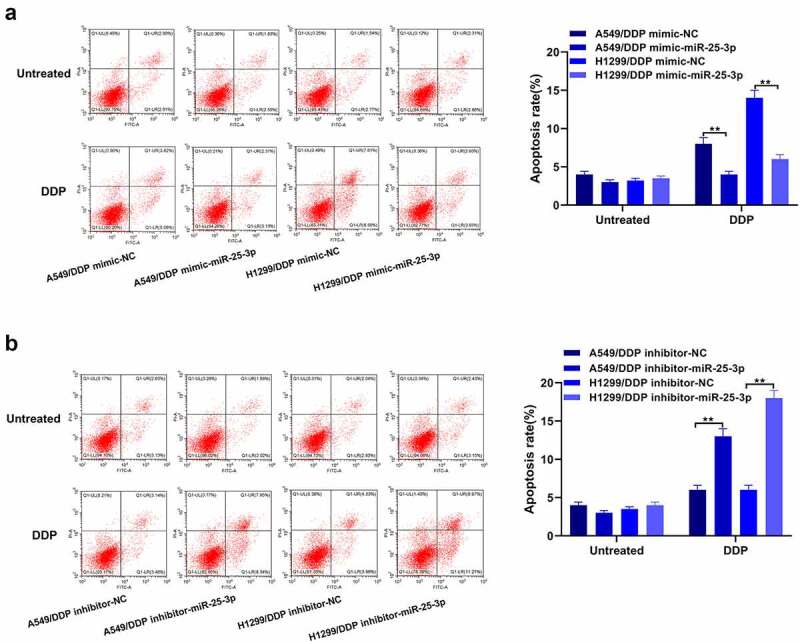
miR-25-3p over-expression controlled apoptosis. (a–b) Flow cytometry was adopted to detect apoptotic via miR-25-3p mimic/restrainer transfection. **P < 0.01
3.4. PTEN deem as a target for miR-25-3p
According to the target prediction tools, PTEN was found as a potential target of miR-25-3p (Figure 4a). Then we used the luciferase assay, and we found that luciferase activity was significantly decreased in the presence of PTEN WT plasmid and miR-25-3p mimics (Figure 4b). However, PTEN-MUT transfection did not show any change in the relative luciferase activity. Moreover, A549/H1299 DDP displayed higher expression of PTEN (Figure 4c). Interestingly, miR-25-3p mimic significantly decreased PTEN expression. Nevertheless, miR-25-3p inhibitors displayed the opposite effect (Figure 4d–e). These results showed that miR-25-3p targets the 3ʹ UTR of PTEN, and regulates PTEN expression.
Figure 4.
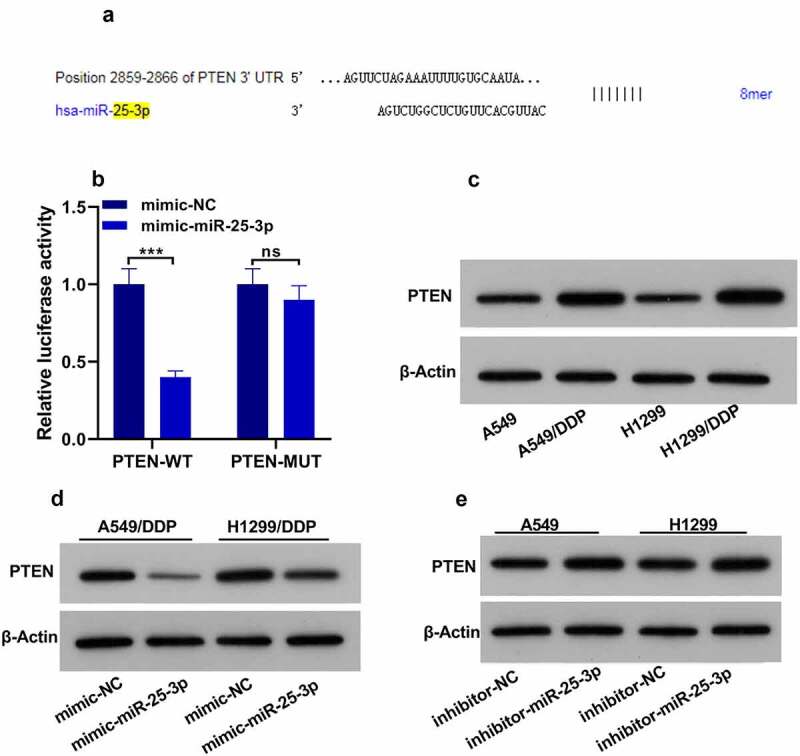
PTEN was called as miR-25-3p spot. (a) The bioinformatics database shows that miR-25-3p contains the conservative binding site of PTEN. (b) The cell activity was detected by fluorescein. (c) Western blot was utilized for measuring PTEN expression. (d–e) The miR-25-3p mimic/restrainer plasmid was infected into two cells for 24 h. Then, PTEN expression was assessed. ** P < 0.01
3.5. miR-25-3p participates in NSCLC drug-fast via PTEN/PI3K/AKT signaling pathway
Our further results showed that miR-25-3p over-expression improved IC50. miR-25-3p high-expression/PTEN co transfection decreased this higher IC50. PTEN-overexpression further boosted miR-25-3p inhibitor effects on IC50 of A549/DDP cells (Figure 5a). Similarly, PTEN-overexpression partially deteriorated the effects of miR-25-3p on the colony forming ability in A549 cells. Surprisingly, miR-25-3p inhibitor/PTEN simultaneous-transfection displayed higher ability to inhibit colony formation (Figure 5b). Furthermore, PTEN overexpression substantially reversed the miR-25-3p resist-apoptotic effects. Besides, it further raised the apoptosis of A549/DDP cells which was induced by miR-25-3p inhibitor (Figure 5c–d). Finally, western blot analysis showed that miR-25-3p boosted PI3K expression as well as p-Akt/Akt proportion (Figure 5e).
Figure 5.
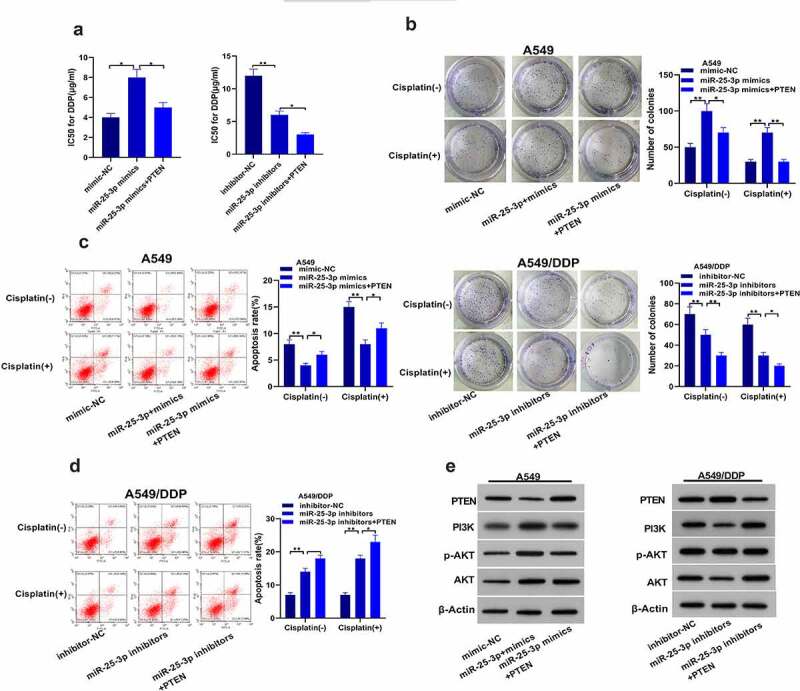
miR-25-3p enhanced chemoresistance of NSCLC cells. (a) miR-25-3p mimics ameliorated IC50 value. (b) Colony forming assay was used to detect miR-25-3p restrainer role on cell multiplication. (c) DDP induced apoptotic of A549 cells infected with miR-25-3p over-expression via flow cytometry. (d) Cisplatin induced apoptotic of A549/DDP cells infected with miR-25-3p inhibitor via flow cytometry. (e) After miR-25-3p mimic/inhibitor/PTEN cotransfected, our crew detected PTEN, PI3K, p-AKT and AKT levels. (-), not treated with cisplatin (+), treated with cisplatin
3.6. miR-25-3p attended DDP drug-fast via animal test
Next, we found that the tumor volume in animals injected with miR-25-3p/A549 group was larger than that of miR-NC/A549 group (Figure 6a). The mean tumor volume in miR-25-3p inhibitors was evidently decreased (Figure 6b). Moreover, miR-25-3p mimics significantly decreased cisplatin-induced PTEN-expression. Besides, it also increased PI3K/p-AKT expression. However, miR-25-3p inhibitors displayed the opposite trend (Figure 6c–d).
Figure 6.
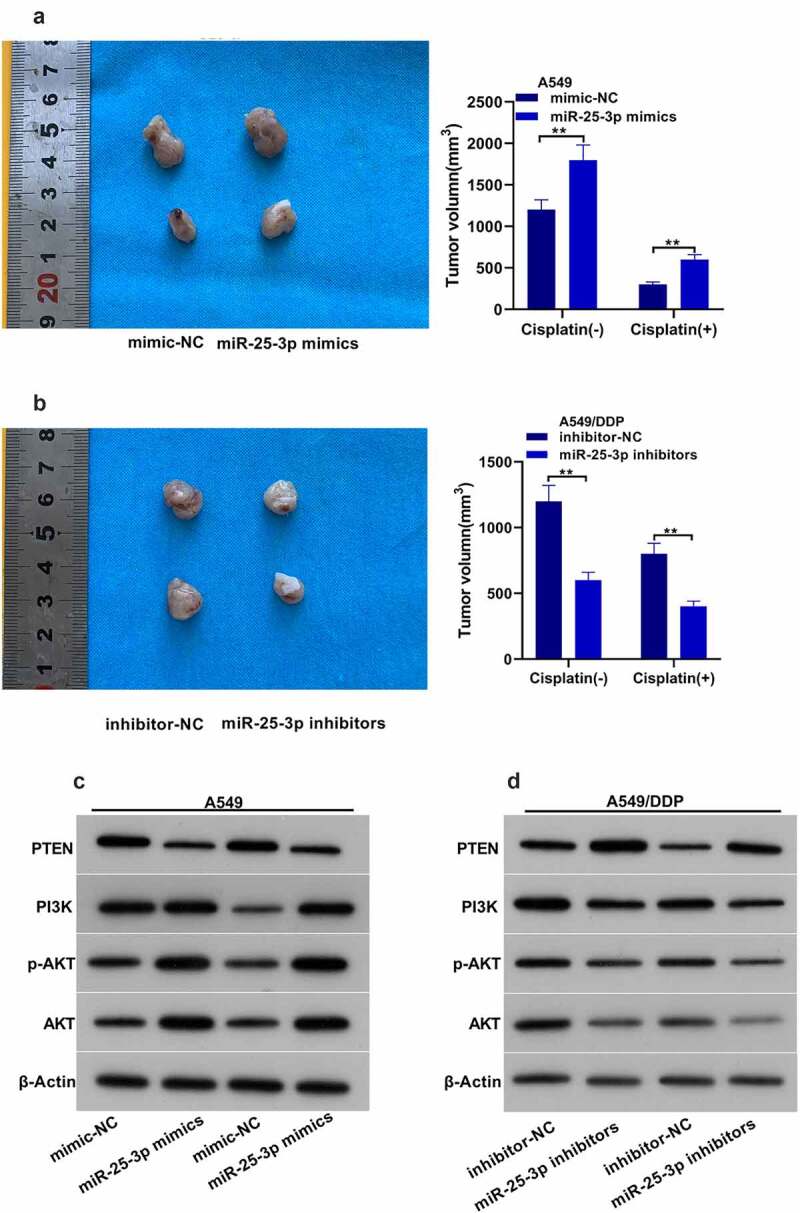
miR-25-3p partook DDP drug-fast via animal assay. (a–b) Mice tumor volume. (c–d) Western blot was used to detect PTEN, PI3K as well as p-AKT/AKT expression via miR-25-3p mimic/inhibitor transfection
4. Discussion
It is well known that with the aggravation of environmental pollution created by industrialization in China, the prevalence of NSCLC has been increased notably [23]. At the moment, cisplatin is regarded as the principal treatment for NSCLC [24]. Recent studies show that some patients may be susceptible to cisplatin during initial stages of treatment, while others may develop resistance gradually [4]. Unexpectedly, higher dose of cisplatin may also bring some serious side effects, and ultimately lower the life expectancy [25]. Therefore, we designed this study to expound DDP drug-fast for tumor to provide the individualized therapeutic strategy for NSCLC.
In this study, we explored that miR-25-3p expression is increased in NSCLC. Furthermore, over expression of miR-25-3p accelerated A549/DDP cell growth and metastasis, and restrained apoptosis.
Recent studies indicated that PTEN/PI3K pathway is intently related to tumor progression [26]. PI3K, which is composed of p85 and P110 subunits, acts as a phosphokinase. Whereas, Akt is known as the cardinal effector protein of PI3K [27]. The activation PI3K/AKT has been linked with the proliferation of cancerous cells and the expression of several tumor genes [28]. Our study validated that down regulation of PTEN reversed DDP drug-fast. Moreover, we also validated that miR-25-3p and PTEN exhibit a binding relationship. Therefore, we further found that miR-25-3p negatively regulates the expression of PTEN, and activates the PI3K/AKT signaling pathway, which might be considered as a latent mechanism for promoting cisplatin resistance in NSCLC.
It was found that PTEN inactivation raises NSCLC invasiveness and growth via PI3K/AKT/NFkB pathway [29]. Moreover, PTEN upregulation can minimize the NSCLC development and invasiveness [30]. Similar to our results, previous studies also identified miR-26 and miR-21 to be involved in the cell proliferation via adjusting PTEN [31,32]. However, in this study, we identified miR-25-3p/PTEN axis which affects NSCLC development in NSCLC cell lines and in-vivo tumor development.
5. Conclusion
In conclusion, our study reported that miR-25-3p expression is upregulated in cisplatin-resistant NSCLC cells. Moreover, overexpression of miR-25-3p enhances DDP drug-fast via regulating PTEN/PI3K/AKT signaling pathway. Therefore, inhibition of miR-25-3p may act as a novel strategy to overcome cisplatin resistance in NSCLC.
Funding Statement
Science and Technology Department of Jilin Province [20200403106SF].
Highlights
Expression of miR-25-3p is upregulated in cisplatin-resistant A549 and H1299 NSCLC cells.
MiR-25-3p regulates the NSCLC proliferation, and migration through PTEN/PI3K/AKT pathway.
Silencing miR-25-3p accelerates DDP tolerance, and decreases the tumor growth in vivo.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Yamamoto N, Goto K, Nishio M, et al. Final overall survival in JO22903, a phase II, open-label study of first-line erlotinib for Japanese patients with EGFR mutation-positive non-small-cell lung cancer. Int J Clin Oncol. 2017;22:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chan BA, Coward JI.. Chemotherapy advances in small-cell lung cancer. J Thorac Dis. 2013;5(Suppl 5):S565–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kelley MJ, Jha G, Shoemaker D, et al. Phase II study of dasatinib in previously treated patients with advanced non-small cell lung cancer. Cancer Invest. 2017;35:32–35. [DOI] [PubMed] [Google Scholar]
- [4].Krzakowski M, Lucas C, Gridelli C.. Fractionated scheme of oral vinorelbine as single-agent therapy or in combination with cisplatin concomitantly with thoracic radiotherapy in stage III non-small-cell lung cancer: dose-escalation phase I trial. Clin Lung Cancer. 2014;15:266–273. [DOI] [PubMed] [Google Scholar]
- [5].Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg Chem. 2019;88:102925. [DOI] [PubMed] [Google Scholar]
- [6].Kentepozidis N, Economopoulou P, Christofyllakis C, et al. Salvage treatment with irinotecan/cisplatin versus pemetrexed/cisplatin in patients with non-small cell lung cancer pre-treated with a non-platinum-based regimen in the first-line setting: a randomized phase II study of the Hellenic Oncology Research Group (HORG). Clin Transl Oncol. 2017;19:317–325. [DOI] [PubMed] [Google Scholar]
- [7].Okamoto K, Okamoto I, Takeda M, et al. A phase I study of split-dose cisplatin and etoposide with concurrent accelerated hyperfractionated thoracic radiotherapy in elderly patients with limited-disease small cell lung cancer. Jpn J Clin Oncol. 2014;44:743–748. [DOI] [PubMed] [Google Scholar]
- [8].Su SF, Li M, Geng YC, et al. Randomized phase II study of pemetrexed-cisplatin or docetaxel-cisplatin plus thoracic intensity-modulated radiation therapy in patients with stage IV lung adenocarcinoma. Am J Cancer Res. 2019;9:1235–1245. [PMC free article] [PubMed] [Google Scholar]
- [9].Zhu Q, Liang X, Dai J, et al. Prostaglandin transporter, SLCO2A1, mediates the invasion and apoptosis of lung cancer cells via PI3K/AKT/mTOR pathway. Int J Clin Exp Pathol. 2015;8:9175–9181. [PMC free article] [PubMed] [Google Scholar]
- [10].Riaz F, Chen Q, Lu K, et al. Inhibition of miR-188-5p alleviates hepatic fibrosis by significantly reducing the activation and proliferation of HSCs through PTEN/PI3K/AKT pathway. J Cell Mol Med. 2021;25:4073–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang R, Zhang Q, Peng X, et al. Stellettin B induces G1 arrest, apoptosis and autophagy in human non-small cell lung cancer A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep. 2016;6:27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Winter J, Jung S, Keller S, et al. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. [DOI] [PubMed] [Google Scholar]
- [13].Ghafouri-Fard S, Shoorei H, Branicki W, et al. Non-coding RNA profile in lung cancer. Exp Mol Pathol. 2020;114:104411. [DOI] [PubMed] [Google Scholar]
- [14].Ghafouri-Fard S, Abak A, Tondro Anamag F, et al. The emerging role of non-coding RNAs in the regulation of PI3K/AKT pathway in the carcinogenesis process. Biomed 2021;137:111279. [DOI] [PubMed] [Google Scholar]
- [15].Ghafouri-Fard S, Abak A, Shoorei H, et al. Regulatory role of microRNAs on PTEN signaling. Biomed 2021;133:110986. [DOI] [PubMed] [Google Scholar]
- [16].Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435:834–838. [DOI] [PubMed] [Google Scholar]
- [17].Taheri M, Shoorei H, Tondro Anamag F, et al. LncRNAs and miRNAs participate in determination of sensitivity of cancer cells to cisplatin. Exp Mol Pathol. 2021;104602. DOI: 10.1016/j.yexmp.2021.104602 [DOI] [PubMed] [Google Scholar]
- [18].Lu J, Zhan Y, Feng J, et al. MicroRNAs associated with therapy of non-small cell lung cancer. Int J Biol Sci. 2018;14:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li W, Wang W, Ding M, et al. MiR-1244 sensitizes the resistance of non-small cell lung cancer A549 cell to cisplatin. Cancer Cell Int. 2016;16:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jiang Z, Yin J, Fu W, et al. MiRNA 17 family regulates cisplatin-resistant and metastasis by targeting TGFbetaR2 in NSCLC. PLoS One. 2014;9:e94639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang J, Bai R, Li M, et al. Excessive miR-25-3p maturation via N6-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu B, Sun X. miR-25 promotes invasion of human non-small cell lung cancer via CDH1. Bioengineered 2019;10:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kwon JH, Kim JH, Lee JA, et al. Phase II study with fractionated schedule of docetaxel and cisplatin in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2010;66:889–897. [DOI] [PubMed] [Google Scholar]
- [24].Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–774. [DOI] [PubMed] [Google Scholar]
- [25].Niho S, Kubota K, Nihei K, et al. Dose-escalation study of thoracic radiotherapy in combination with pemetrexed plus Cisplatin followed by pemetrexed consolidation therapy in Japanese patients with locally advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer. 2013;14:62–69. [DOI] [PubMed] [Google Scholar]
- [26].Qi L, Sun K, Zhuang Y, et al. Study on the association between PI3K/AKT/mTOR signaling pathway gene polymorphism and susceptibility to gastric cancer. J buon. 2017;22:1488–1493. [PubMed] [Google Scholar]
- [27].Feng LM, Wang XF, Huang QX. Thymoquinone induces cytotoxicity and reprogramming of EMT in gastric cancer cells by targeting PI3K/Akt/mTOR pathway. J Biosci. 2017;42:547–554. [DOI] [PubMed] [Google Scholar]
- [28].Wu YJ, Lin SH, Din ZH, et al. Sinulariolide inhibits gastric cancer cell migration and invasion through downregulation of the EMT process and suppression of FAK/PI3K/AKT/mTOR and MAPKs signaling pathways. Mar Drugs. 2019;17(12):668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Akca H, Demiray A, Tokgun O, et al. Invasiveness and anchorage independent growth ability augmented by PTEN inactivation through the PI3K/AKT/NFkB pathway in lung cancer cells. Lung Cancer. 2011;73:302–309. [DOI] [PubMed] [Google Scholar]
- [30].Bi L, Chen J, Yuan X, et al. Salvianolic acid A positively regulates PTEN protein level and inhibits growth of A549 lung cancer cells. Biomed Rep. 2013;1:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Qin X, Yan L, Zhao X, et al. microRNA-21 overexpression contributes to cell proliferation by targeting PTEN in endometrioid endometrial cancer. Oncol Lett. 2012;4:1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huse JT, Brennan C, Hambardzumyan D, et al. N-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23:1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]


