Abstract
PURPOSE
Metastatic breast cancer (MBC) has a heterogeneous clinical course. We sought to develop a prognostic model for overall survival (OS) that incorporated contemporary tumor and clinical factors for estimating individual prognosis.
METHODS
We identified patients with MBC from our institution diagnosed between 1998 and 2017. We developed OS prognostic models by Cox regression using demographic, tumor, and treatment variables. We assessed model predictive accuracy and estimated annual OS probabilities. We evaluated model discrimination and prediction calibration using an external validation data set from the National Comprehensive Cancer Network.
RESULTS
We identified 10,655 patients. A model using age at diagnosis, race or ethnicity, hormone receptor and human epidermal growth factor receptor 2 subtype, de novo versus recurrent MBC categorized by metastasis-free interval, Karnofsky performance status, organ involvement, frontline biotherapy, frontline hormone therapy, and the interaction between variables significantly improved predictive accuracy (C-index, 0.731; 95% CI, 0.724 to 0.739) compared with a model with only hormone receptor and human epidermal growth factor receptor 2 status (C-index, 0.617; 95% CI, 0.609 to 0.626). The extended Cox regression model consisting of six independent models, for < 3, 3-14, 14-20, 20-33, 33-61, and ≥ 61 months, estimated up to 5 years of annual OS probabilities. The selected multifactor model had good discriminative ability but suboptimal calibration in the group of 2,334 National Comprehensive Cancer Network patients. A recalibration model that replaced the baseline survival function with the average of those from the training and validation data improved predictions across both data sets.
CONCLUSION
We have generated and validated a robust prognostic OS model for MBC. This model can be used in clinical decision making and stratification in clinical trials.
Metastatic breast cancer (MBC) is considered incurable, but survival has improved over time.1-4 MBC accounts for 5%-10% of newly diagnosed breast cancers, termed de novo stage IV MBC.2,5,6 Most patients are initially diagnosed with nonmetastatic disease and receive local therapy (breast surgery and radiation therapy) and systemic treatment (chemotherapy, biotherapy, and hormone therapy) but eventually develop recurrent MBC. Patients with de novo MBC tend to have better prognosis compared with those with recurrent MBC7,8; being naïve to treatment, they may respond better to systemic therapy, whereas inherent biologic differences could also explain this phenomenon.8-10 Patients with de novo or recurrent MBC are typically treated with similar systemic therapy. In recent decades, numerous prognostic factors in MBC have been identified.11-28 However, some of these prognostic models are now outdated, and many suffer from methodologic shortcomings such as small sample size, the absence of contemporaneous tumor data, or lack of external validation.
CONTEXT
Key Objective
To develop a prognostic model for overall survival (OS) in patients with newly diagnosed metastatic breast cancer (MBC) that incorporates contemporary tumor and clinical factors with an accompanying online tool that estimates annual OS probabilities.
Knowledge Generated
Using robust statistical methodology applied to two large independent cohorts of patients with MBC, we demonstrated that demographic, contemporary tumor characteristics and treatment variables were important determinants of OS in MBC and that their combinatorial effect further refines survival estimates. The selected multifactor prognostic model that estimates up to 5 years of annual OS probabilities had good discriminative ability, and a recalibration methodology improved prediction across both independent data sets.
Relevance
This prognostic model for OS in MBC can be used in clinical decision making, refining stratification factors for clinical trials, and elucidating biologic factors contributing to metastasis and drug resistance and could also support a novel substaging classification for patients with MBC.
The objective of this study was to develop prognostic modeling for overall survival (OS) in MBC that incorporates contemporary clinical and tumor factors such as hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status. Using robust statistical methodology applied to two large independent cohorts, we sought to demonstrate that clinical and tumor characteristics were important determinants of OS and that their combinatorial effect would further refine survival estimates in prognostic statistical models. Furthermore, we developed an online tool that estimates annual OS probabilities for individual patients with MBC on the basis of clinical and tumor characteristics. Prognostic modeling in MBC could be useful in clinical decision making, refining stratification factors for clinical trials, and elucidating biologic factors contributing to metastasis and drug resistance. Given the heterogeneity of MBC outcomes, the results presented here could also support a novel substaging classification for patients with MBC.29-32
METHODS
Training Cohort
We identified women or men diagnosed with de novo or recurrent MBC between 1998 and 2017 from a prospective database of patients with breast cancer evaluated at The University of Texas MD Anderson Cancer Center. We chose starting in 1998 because since then, our institution has consistently measured HER2 expression33,34 on breast tumors and most patients with HER2-positive MBC have received trastuzumab.35,36 We obtained age at diagnosis of primary breast cancer and of MBC; race or ethnicity; tumor histologic and nuclear grade; estrogen receptor (ER), progesterone receptor (PR), and HER2 expression in the primary breast tumor and metastatic lesion; de novo MBC versus recurrent MBC categorized by metastasis-free interval (MFI, time elapsed between the date of diagnosis of primary localized breast cancer and diagnosis of MBC, < 24 months and ≥ 24 months8,21,22,26,37,38); type and number of organs affected by metastasis; Karnofsky performance status (KPS; categories: 10-60, 70-80, and 90-100) at first presentation with MBC; prior systemic treatment; and frontline treatment (initial systemic therapy given within 90 days of diagnosis of MBC). We obtained tumor grade, ER, PR, and HER2 status from the pathology report and determined the tumor stage at initial diagnosis of breast cancer following the American Joint Committee on Cancer (AJCC) guidelines current at the date of diagnosis.39-41 We used a composite histologic grade; however, if missing, we used nuclear grade as a surrogate (Data Supplement). A tumor was considered HR-positive if either ER or PR was positive, or HR-negative if both ER and PR were negative.42 The combination of HR and HER2 status generated a four-level variable: HR-positive and HER2-negative, HR and HER2-positive, HR-negative and HER2-positive, and HR and HER2-negative (triple-negative). If available, we used the reported HR and HER2 status of a metastatic lesion; otherwise, we used the HR and HER2 status of the primary breast tumor. We categorized the organs involved with metastatic disease as follows: (1) bone-only, (2) nonvisceral (ie, soft tissue, lymphadenopathy, and skin; could include bone), (3) visceral without CNS involvement (non-CNS), and (4) CNS with or without other organ involvement. We obtained approval from the MD Anderson Institutional Review Board, with a waiver of consent given the retrospective nature and minimal patient risk.
Validation Cohort
We used the National Comprehensive Cancer Network (NCCN) Breast Cancer Outcomes database to identify patients with MBC who received treatment at one of the 16 NCCN centers (Data Supplement) between July 1, 1997, and December 31, 2012 (last follow-up date: February 15, 2013), on the basis of data availability. We excluded patients registered at MD Anderson, also an NCCN center, and those who did not have complete data on age at diagnosis, race or ethnicity, tumor stage, tumor grade, de novo versus recurrent MBC by MFI, HR and HER2 status, KPS, organ involvement, or frontline therapy.
Statistical Analysis
The primary end point was OS calculated from the date of diagnosis of MBC to the date of death, while censoring live patients at the date of their last clinic visit. Death was ascertained by the Tumor Registry department of each institution. The cutoff data collection date for the training cohort was September 5, 2017. We first fitted univariate and multivariate Cox proportional hazards (PH) regression models assessing the statistical significance of all variables. We checked the PH assumption by inspecting the smoothed scaled Schoenfeld residuals and the hazard ratios by time intervals and assessed potential nonlinear effects of covariates (eg, age) using spline functions. When the PH assumption was violated, we fitted an extended Cox regression model allowing for time-varying coefficients.43-46 We calculated Harrell's C-index to evaluate the discrimination capacity of each model. A P value < .05 indicated statistical significance. We developed an algorithm to estimate individual prognosis using a Cox regression model to estimate the OS probabilities by including patients who had data on age at diagnosis, race or ethnicity, tumor stage, tumor grade, de novo versus recurrent MBC by MFI, HR and HER2 status, KPS, organs involved with metastasis, and frontline treatment. We defined prognostic index as the weighted sum of the variables in the Cox regression model, where the weights were the regression coefficients. We evaluated the model calibration by comparing the observed and predicted OS probabilities for five risk groups (by partitioning the prognostic index on its 16th, 39th, 62nd, and 84th percentiles) at 1, 2, 3, 4, and 5 years.47 This partitioning generated two smaller groups with the lowest and highest risks of death and three larger central groups with intermediate risks. On a standard normal scale, the 39th and 62nd percentiles correspond to approximately ±1 standard deviation from the mean.
We evaluated internal validity of the selected model by the apparent C-index (the selected model in the training data tested in the training data) and the bootstrap method.48 A Cox regression model was fit in each bootstrap sample of patients selected from the original training data, and we computed the C-index in the bootstrap sample (bootstrap C-index) and in the training data (test C-index). After selecting 100 bootstrap samples, the model performance was estimated by the apparent C-index minus the average of the difference between the bootstrap C-index and the test C-index.49
To assess the external validation, we computed predictions for each patient in the validation cohort using the model fit to the training data set and compared such predictions with the observed outcomes. Because of poor calibration, we conducted a recalibration methodology to improve calibration in the validation cohort yet maintain reasonable calibration in the training data set. A complete description of the statistical methods, including calibration and recalibration methods, is available in the Data Supplement. Statistical analyses were performed using SAS 9.4, R-3.5.2, and S-PLUS 8.2 for Windows software. SAS macro %SURVCSTD50,51 was used to calculate the C-index for survival data with time-dependent covariates.
RESULTS
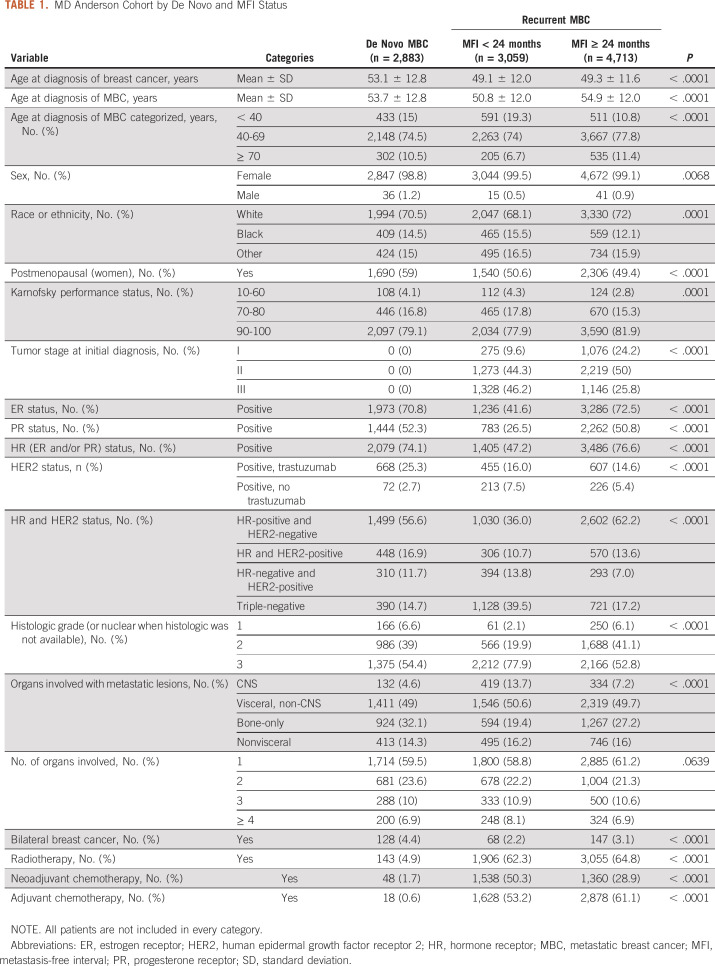
We identified 10,655 patients with MBC, of whom 92 (0.9%) were men, seen at MD Anderson between 1998 and 2017. Table 1 shows patients' characteristics categorized by de novo and recurrent MBC by MFI (< 24 months and ≥ 24 months): 2,883 (27%) had de novo MBC, 3,059 (29%) had recurrent MBC with an MFI of < 24 months, and 4,713 (44%) had recurrent MBC with MFI ≥ 24 months. The median follow-up time from diagnosis of MBC was 56 months (95% CI, 53 to 57; de novo: 58 months; recurrent: 54 months). The median OS was 29 months (95% CI, 28 to 30; de novo: 41 months; recurrent: 25 months). At the cutoff date, 6,712 (63%) patients had died. Among those alive, 51% had a date of last follow-up within 2 years of September 2017, whereas in 15%, such date was > 5 years.
TABLE 1.
MD Anderson Cohort by De Novo and MFI Status
The training cohort was a subset of 7,606 (71%) patients (69 men) with complete data for prognostic model building after excluding 29% of patients for whom one or more variables listed in the Methods section were missing; of note, HER2 status was missing in 9%. The excluded subset did not differ significantly in age of diagnosis, but had more Black patients, more with tumor stage I, fewer with stage III, more with triple-negative tumors, and fewer with bone-only disease.
After comparing several prognostic models, we selected a model (labeled model 1) with the best prognostic accuracy for OS with an integrated area under the curve52 of 0.783 and an apparent C-index of 0.731 (95% CI, 0.724 to 0.738). By contrast, a model with only HR and HER2 had a C-index of 0.617 (95% CI, 0.609 to 0.636). Model 1 contained the covariate age at diagnosis of MBC, race or ethnicity, de novo versus recurrent MBC by MFI, HR and HER2 status, KPS, type and number of organs involved with metastasis, frontline biotherapy, frontline hormone therapy, the interaction between de novo versus recurrent MBC and HR and HER2 status, and the interaction between de novo versus recurrent MBC and frontline hormone therapy. The prognostic model that contained primary tumor stage and tumor grade had similar performance to model 1 (C-index 0.738); however, these variables are not always available at diagnosis of MBC. Prior systemic therapy (neoadjuvant and adjuvant), previous radiotherapy, and frontline chemotherapy (for MBC) did not substantially improve the performance of the model and were therefore excluded. Year of diagnosis of MBC as a continuous variable or a binary one using several cutoff years (2007, 2010, and 2012) did not improve the performance of the model. The multivariate Cox regression analysis on OS with the variables used for model 1 is given in the Data Supplement.
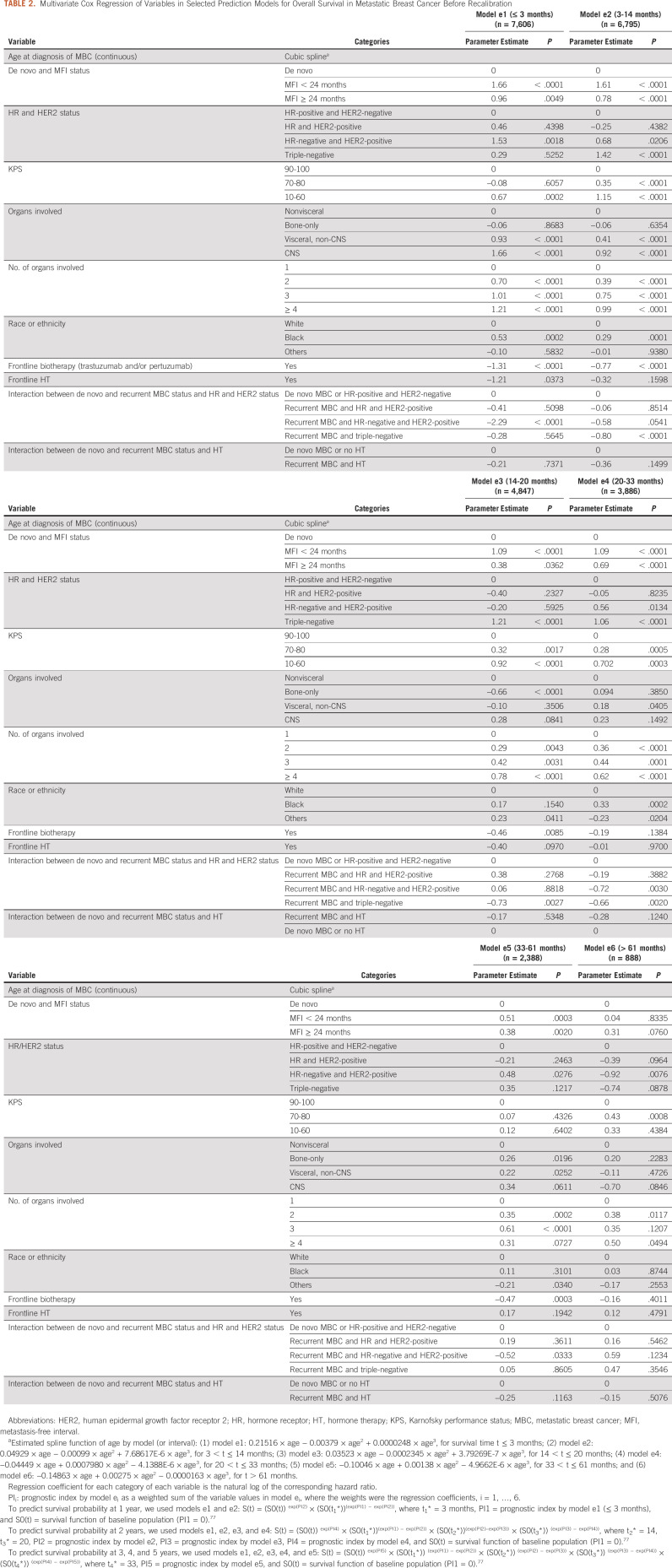
Scaled Schoenfeld residual plots indicated a violation of PH assumption for MFI, HR and HER2 subtype, KPS, type of organs involved, number of organs involved, race or ethnicity, frontline biotherapy, frontline hormone therapy, and the interaction between de novo versus recurrent MBC and HR and HER2 subtype. An extended Cox regression model allowing for time-varying coefficients that included the same covariates as in model 1 was fit using 3, 14, 20, 33, and 61 months as cutoff values to ensure that as many covariates as possible met the PH assumption within each disjoint interval. Model e1 censored all patients at risk after 3 months; models e2 through e5 included patients who were alive at the start of each time interval and censored at the end of each time interval; model e6 included patients who were alive beyond 61 months (Table 2). For models e1-e5, no covariates violated the PH assumption. For model e6, tests suggested persistent time-varying effect for type of organs involved, but none of the remaining covariates violated the PH assumption. After selecting 100 bootstrap samples, the estimate of model performance was 0.734, indicating good predictive performance in the internal validation setting.
TABLE 2.
Multivariate Cox Regression of Variables in Selected Prediction Models for Overall Survival in Metastatic Breast Cancer Before Recalibration
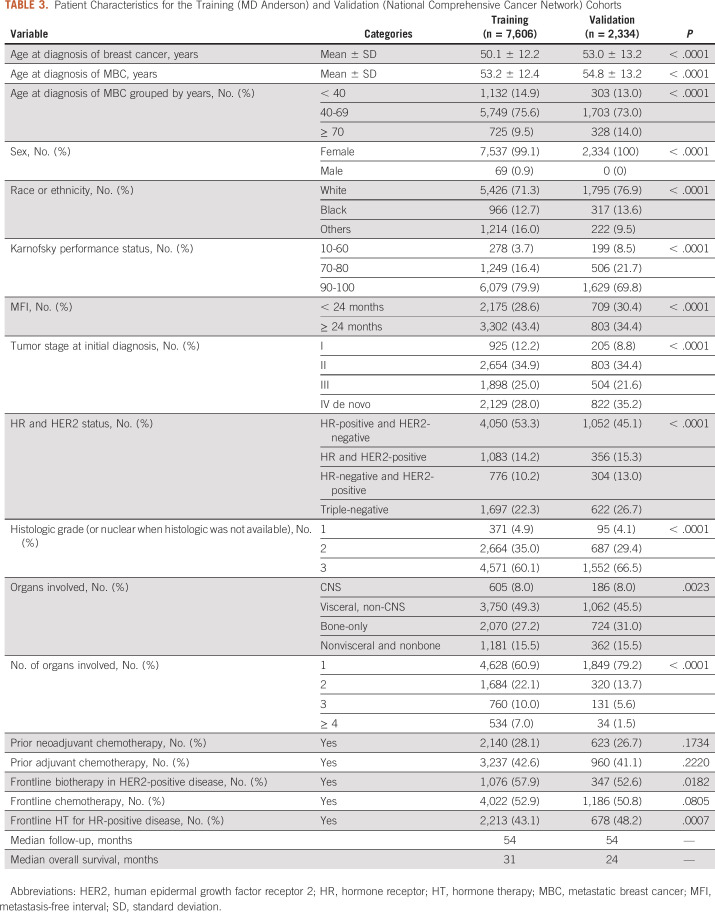
The validation cohort consisted of 2,334 patients after excluding 1,733 MD Anderson patients. Table 3 compares their characteristics with those of the training cohort (n = 7,606). The validation cohort had no men, a slightly older mean age at the diagnosis of MBC (54.8 v 53.2 years) and higher rates of de novo MBC (35.2% v 28.0%), White race or ethnicity (76.9% v 71.3%), triple-negative (26.7% v 22.3%) and HER2-positive (28.3% v 24.4%) disease, and only one organ involved (79.2% v 60.9%). The types of organs involved by metastasis were comparable. For both cohorts, about 8% had CNS involvement and 15% had nonvisceral disease. Bone-only disease was slightly more common in the validation cohort (31% v 27.2%). Visceral non-CNS disease was more frequent in the training cohort (49.3% v 45.5%). The median follow-up time was 54 months for both cohorts. The median OS was 24 months for the validation cohort versus 31 months for the training cohort. Figures 1 and 2 depict the Kaplan-Meier curves by the risk groups defined by the prognostic index on the basis of each model for both the training and validation cohorts, which suggest a clear separation of the risks of death across the groups.
TABLE 3.
Patient Characteristics for the Training (MD Anderson) and Validation (National Comprehensive Cancer Network) Cohorts
FIG 1.
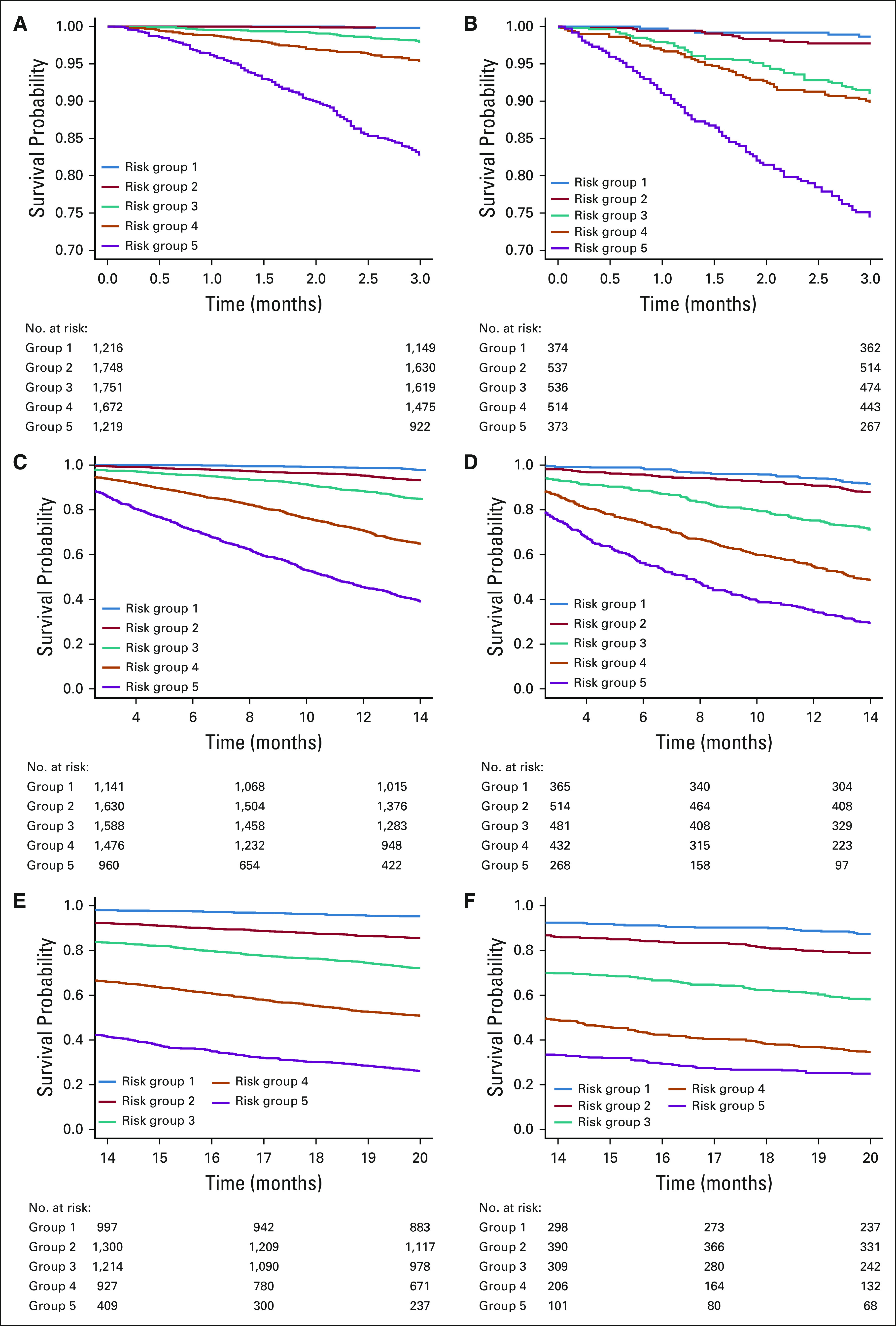
Kaplan-Meier overall survival curves for the training versus validation cohorts by risk groups formed by prognostic index percentiles on the basis of models e1, e2, and e3. (A) Kaplan-Meier curve for training cohort by risk groups formed by prognostic index percentiles on the basis of model e1. Solid blue line indicates risk group 1 (prognostic index ≤ 16th percentiles); solid red line indicates risk group 2 (16th percentiles < prognostic index ≤ 39th percentiles); solid green line indicates risk group 3 (39th percentiles < prognostic index ≤ 62th percentiles); solid orange line indicates risk group 4 (62th percentiles < prognostic index ≤ 84th percentiles); and solid purple line indicates risk group 5 (prognostic index > 84th percentiles); numbers at risk by risk group are presented (by the order of risk group); risk group 1 and risk group 2 are overlapping. (B) Kaplan-Meier curve for validation cohort by risk groups formed by prognostic index percentiles on the basis of model e1. (C) Kaplan-Meier curve for training cohort by risk groups formed by prognostic index percentiles on the basis of model e2. (D) Kaplan-Meier curve for validation cohort by risk groups formed by prognostic index percentiles on the basis of model e2. (E) Kaplan-Meier curve for training cohort by risk groups formed by prognostic index percentiles on the basis of model e3. (F) Kaplan-Meier curve for validation cohort by risk groups formed by prognostic index percentiles on the basis of model e3.
FIG 2.
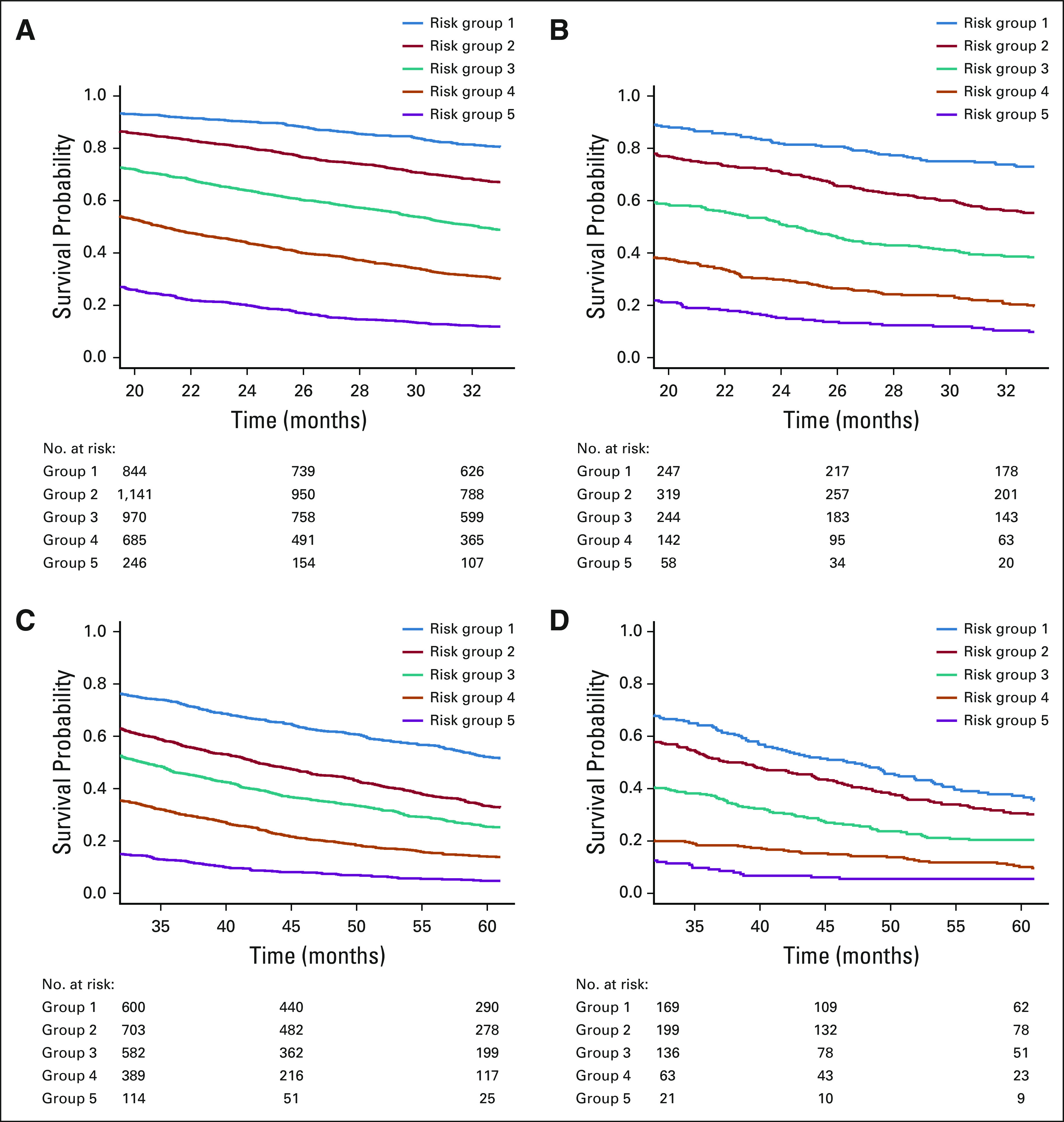
Kaplan-Meier overall survival curves for the training versus validation cohorts by risk groups formed by prognostic index percentiles on the basis of models e4 and e5. (A) Kaplan-Meier curve for training cohort by risk groups formed by prognostic index percentiles on the basis of model e4. Solid blue line indicates risk group 1 (prognostic index < 16th percentiles); solid red line indicates risk group 2 (16th percentiles < prognostic index < 39th percentiles); solid green line indicates risk group 3 (39th percentiles < prognostic index < 62th percentiles); solid orange line indicates risk group 4 (62th percentiles < prognostic index < 84th percentiles); and solid purple line indicates risk group 5 (prognostic index > 84th percentiles); numbers at risk by risk group are presented. (B) Kaplan-Meier curve for validation cohort by risk groups formed by prognostic index percentiles on the basis of model e4. (C) Kaplan-Meier curve for training cohort by risk groups formed by prognostic index percentiles on the basis of model e5. (D) Kaplan-Meier curve for validation cohort by risk groups formed by prognostic index percentiles on the basis of model e5.
Calibration plots from the training cohort using the selected prognostic model (Table 2) and the predicted and observed annual OS probabilities for each of the predetermined five risk groups starting from the date of diagnosis of MBC are shown in the Data Supplement; the plots show good model prediction. The prognostic model was not well-calibrated when applied to the validation cohort, which can be seen in the Data Supplement; the observed annual OS probabilities were lower than the predicted OS probabilities. To improve the calibration, we recalibrated the prediction model by replacing the survival function of the baseline population with the average of the baseline survival functions from the training and validation data without modifying the slope of the prognostic index. The calibration plots after recalibration for the training and validation cohorts are shown in the Data Supplement, respectively. Using the recalibrated model, we developed an online tool that estimates individual annual OS probabilities for up to 5 years and is available at The University of Texas MD Anderson Cancer Center website.53
DISCUSSION
We generated a robust statistical prognostic model for OS of patients with MBC, incorporating contemporary variables commonly available at diagnosis. This prognostic model estimates annual OS probabilities for up to 5 years using the input of clinical and biologic variables and is available as an online tool for the practicing oncologist when discussing prognosis with patients with newly diagnosed MBC. This tool can also estimate event rates in defined patient populations to aid in clinical trial design and sample size calculations.
Several of the variables in our prognostic model have been previously validated. De novo patients with MBC tend to have better outcomes compared with those with recurrent MBC, but less pronounced when the MFI is > 24 months.7,8,38 African American patients with MBC tend to have an increased risk of death compared with White patients, despite receiving similar treatments.54 We believe that the best method to determine prognosis in MBC is by a comprehensive statistical model that combines several of the established prognostic factors.
Several multivariate analyses of prognostic factors in MBC have been reported, including from our institution. The M-bioscore model uses tumor receptor status, low tumor burden, and low nuclear grade as prognostic variables; however, this model did not consider performance status nor age, and the follow-up time was limited (median, 13 months).28 Many of the other analyses are now outdated and do not consider contemporary well-established variables such as receptor status,11-15 or such data were missing in a considerable number of patients.16-19,55 Modalities of treatment and staging have also evolved. Several reports lacked complete data for HER2 status.21-23,26,56-59 Some reports focused on determining prognostic factors in subgroups of patients with MBC with specific organs involved, such as bone,60,61 liver,62 or the CNS,63 or focused on subsets, such as de novo MBC5,25,64-66 or elderly women.67 Although more recent publications consider contemporary variables, these analyses are limited by small sample sizes20,27,65,68,69 or short median follow-up times28 or lack external validation.8,24,38 Circulating tumor cells are well-established as a prognostic factor in MBC,70,71 and circulating tumor DNA is emerging as a novel prognostic factor72-74; however, because of cost and lack of technology availability and standardization, that information is not currently routinely collected.
In the current 8th edition of the AJCC TNM staging system, a single M1 stage covers all MBC. However, MBC is a heterogeneous disease with dissimilar outcomes, and we strongly support the idea of partitioning the M1 stage into substages to reflect this phenomenon. Formal substages could assist the counseling of patients about treatment options and risk versus benefit considerations and could also aid in randomized clinical trial design by enabling better estimation of sample sizes and choice of stratification criteria. As early as 1980, good sites of breast cancer recurrence, such as bone, and bad sites, such as brain, were identified.32 More recent studies have proposed dividing the M1 stage in de novo MBC.29-31 Our findings support modifying the current M1 stage75,76 by classifying patients with de novo MBC into prognostic categories using a combination of clinical and tumor characteristics.
Our study has limitations. First, we used information from a single high-volume cancer center with a particular patient population and referral and practice patterns. Second, the selected prognostic model did not calibrate well when applied to the validation cohort; however, it improved with recalibration procedures. Third, the validation cohort lacked male patients, but the proportion in the training cohort was very small and did not change the discriminative ability or the calibration or recalibration results. Fourth, the study spanned a long period that saw significant diagnostic and therapeutic advances3,4; however, year of diagnosis did not improve the performance of the model. Fifth, the final recalibrated prediction model was not validated using another independent data set. Sixth, certain variables known to be prognostic were not available, like tumor mutation burden. Seventh, certain patient subpopulations were under-represented in the models (ie, Black women). Finally, there could be additional interaction effects with other variables not considered or that were unavailable, such as tumor response to frontline treatment, type of prior breast surgery, and type of axillary nodal evaluation.
In conclusion, we have generated a robust and contemporary prognostic model for OS in patients with MBC that was validated internally and externally using a representative nationwide database. Furthermore, we developed an online tool available for clinicians and useful for clinical trial design. Caution is recommended for the use of this model in under-represented patient populations. Our findings support an update of the current AJCC TNM staging system in which patients with MBC would be classified into separate M1 substage prognostic categories.
ACKNOWLEDGMENT
We acknowledge the NCCN for providing access to their database. Editorial assistance was provided by Sunita Patterson, Editing Services, Research Medical Library, MD Anderson Cancer Center.
Carlos H. Barcenas
Research Funding: Puma Biotechnology
Rashmi K. Murthy
Honoraria: Puma Biotechnology, Genentech, Seattle Genetics, Novartis, AstraZeneca
Consulting or Advisory Role: Genentech/Roche, Puma Biotechnology, Seattle Genetics, AstraZeneca, Novartis
Research Funding: Genentech/Roche, Daiichi Sankyo, Pfizer, EMD Serono, Seattle Genetics, AstraZeneca
Travel, Accommodations, Expenses: Seattle Genetics, Genentech
Robert W. Carlson
Employment: Flatiron Health (I)
Patents, Royalties, Other Intellectual Property: Patents relating to inventions as employee of NCCN
Other Relationship: National Comprehensive Cancer Network
Debu Tripathy
Consulting or Advisory Role: Novartis, Pfizer, GlaxoSmithKline, Genomic Health, AstraZeneca, OncoPep
Research Funding: Novartis, Polyphor
Travel, Accommodations, Expenses: Novartis, AstraZeneca
Gabriel N. Hortobagyi
Consulting or Advisory Role: Novartis
Research Funding: Novartis
Travel, Accommodations, Expenses: Novartis
No other potential conflicts of interest were reported.
SUPPORT
Supported by the Breast Cancer Research Foundation (Grant No. BCRF 15122133 to G.N.H.) and the National Cancer Institute at the National Institutes of Health (award No. P30CA016672; Biostatistics Resource Group and Clinical Trials Office). The MD Anderson database management was supported by the Department of Breast Medical Oncology.
AUTHOR CONTRIBUTIONS
Conception and design: Carlos H. Barcenas, Rashmi K. Murthy, Akshara S. Raghavendra, Yisheng Li, Debu Tripathy, Gabriel N. Hortobagyi
Financial support: Carlos H. Barcenas
Administrative support: Carlos H. Barcenas, Gabriel N. Hortobagyi
Provision of study materials or patients: Carlos H. Barcenas, Robert W. Carlson, Debu Tripathy, Gabriel N. Hortobagyi
Collection and assembly of data: Carlos H. Barcenas, Akshara S. Raghavendra, Yisheng Li, Limin Hsu, Robert W. Carlson, Debu Tripathy, Gabriel N. Hortobagyi
Data analysis and interpretation: Carlos H. Barcenas, Juhee Song, Akshara S. Raghavendra, Yisheng Li, Limin Hsu, Robert W. Carlson, Debu Tripathy
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Carlos H. Barcenas
Research Funding: Puma Biotechnology
Rashmi K. Murthy
Honoraria: Puma Biotechnology, Genentech, Seattle Genetics, Novartis, AstraZeneca
Consulting or Advisory Role: Genentech/Roche, Puma Biotechnology, Seattle Genetics, AstraZeneca, Novartis
Research Funding: Genentech/Roche, Daiichi Sankyo, Pfizer, EMD Serono, Seattle Genetics, AstraZeneca
Travel, Accommodations, Expenses: Seattle Genetics, Genentech
Robert W. Carlson
Employment: Flatiron Health (I)
Patents, Royalties, Other Intellectual Property: Patents relating to inventions as employee of NCCN
Other Relationship: National Comprehensive Cancer Network
Debu Tripathy
Consulting or Advisory Role: Novartis, Pfizer, GlaxoSmithKline, Genomic Health, AstraZeneca, OncoPep
Research Funding: Novartis, Polyphor
Travel, Accommodations, Expenses: Novartis, AstraZeneca
Gabriel N. Hortobagyi
Consulting or Advisory Role: Novartis
Research Funding: Novartis
Travel, Accommodations, Expenses: Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kaklamani VG, Gradishar WJ.Endocrine therapy in the current management of postmenopausal estrogen receptor‐positive metastatic breast cancer Oncologist 22507–5172017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the number of women living with metastatic breast cancer in the United States Cancer Epidemiol Prev Biomarkers 26809–8152017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst MF, van de Poll-Franse LV, Roukema JA, et al. Trends in the prognosis of patients with primary metastatic breast cancer diagnosed between 1975 and 2002 Breast 16344–3512007 [DOI] [PubMed] [Google Scholar]
- 4. Caswell-Jin JL, Plevritis SK, Tian L, et al. Change in survival in metastatic breast cancer with treatment advances: Meta-analysis and systematic review. JNCI Cancer Spectr. 2018;2:pky062. doi: 10.1093/jncics/pky062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertaut A, Mounier M, Desmoulins I, et al. Stage IV breast cancer: A population‐based study about prognostic factors according to HER 2 and HR status Eur J Cancer Care 24920–9282015 [DOI] [PubMed] [Google Scholar]
- 6.Barinoff J, Schmidt M, Schneeweiss A, et al. Primary metastatic breast cancer in the era of targeted therapy—Prognostic impact and the role of breast tumour surgery Eur J Cancer 83116–1242017 [DOI] [PubMed] [Google Scholar]
- 7.Dawood S, Broglio K, Ensor J, et al. Survival differences among women with de novo stage IV and relapsed breast cancer Ann Oncol 212169–21742010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lobbezoo D, Van Kampen R, Voogd A, et al. Prognosis of metastatic breast cancer: Are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer. 2015;112:1445. doi: 10.1038/bjc.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Den Brok WD, Speers CH, Gondara L, et al. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed Breast Cancer Res Treat 161549–5562017 [DOI] [PubMed] [Google Scholar]
- 10.Yardley DA, Kaufman PA, Brufsky A, et al. Treatment patterns and clinical outcomes for patients with de novo versus recurrent HER2-positive metastatic breast cancer Breast Cancer Res Treat 145725–7342014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swenerton KD, Legha SS, Smith T, et al. Prognostic factors in metastatic breast cancer treated with combination chemotherapy Cancer Res 391552–15621979 [PubMed] [Google Scholar]
- 12.Nash CH, III, Jones SE, Moon TE, et al. Prediction of outcome in metastatic breast cancer treated with adriamycin combination chemotherapy Cancer 462380–23881980 [DOI] [PubMed] [Google Scholar]
- 13.Fey MF, Brunner KW, Sonntag RW.Prognostic factors in metastatic breast cancer Cancer Clin Trials 4237–2471981 [PubMed] [Google Scholar]
- 14.Hortobagyi GN, Smith TL, Legha SS, et al. Multivariate analysis of prognostic factors in metastatic breast cancer J Clin Oncol 1776–7861983 [DOI] [PubMed] [Google Scholar]
- 15.Vincent MD, Powles TJ, Skeet R, et al. An analysis of possible prognostic features of long term and short term survivors of metastatic breast cancer Eur J Cancer Clin Oncol 221059–10651986 [DOI] [PubMed] [Google Scholar]
- 16.Mick R, Begg CB, Antman KH, et al. Diverse prognosis in metastatic breast cancer: Who should be offered alternative initial therapies? Breast Cancer Res Treat 1333–381989 [DOI] [PubMed] [Google Scholar]
- 17.Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: Analysis of 439 patients Breast Cancer Res Treat 5667–781999 [DOI] [PubMed] [Google Scholar]
- 18.Kramer JA, Curran D, Piccart M, et al. Identification and interpretation of clinical and quality of life prognostic factors for survival and response to treatment in first-line chemotherapy in advanced breast cancer Eur J Cancer 361498–15062000 [DOI] [PubMed] [Google Scholar]
- 19.Ryberg M, Nielsen D, Osterlind K, et al. Prognostic factors and long-term survival in 585 patients with metastatic breast cancer treated with epirubicin-based chemotherapy Ann Oncol 1281–872001 [DOI] [PubMed] [Google Scholar]
- 20.Chang J, Clark GM, Allred DC, et al. Survival of patients with metastatic breast carcinoma: Importance of prognostic markers of the primary tumor Cancer 97545–5532003 [DOI] [PubMed] [Google Scholar]
- 21.Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer Ann Oncol 192012–20192008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puente J, Lopez-Tarruella S, Ruiz A, et al. Practical prognostic index for patients with metastatic recurrent breast cancer: Retrospective analysis of 2,322 patients from the GEICAM Spanish El Alamo Register Breast Cancer Res Treat 122591–6002010 [DOI] [PubMed] [Google Scholar]
- 23.Planchat E, Durando X, Abrial C, et al. Prognostic value of initial tumor parameters after metastatic relapse Cancer Invest 29635–6432011 [DOI] [PubMed] [Google Scholar]
- 24.Jung SY, Rosenzweig M, Sereika SM, et al. Factors associated with mortality after breast cancer metastasis Cancer Causes Control 23103–1122012 [DOI] [PubMed] [Google Scholar]
- 25.Ren Z, Li Y, Hameed O, et al. Prognostic factors in patients with metastatic breast cancer at the time of diagnosis Pathol Res Pract 210301–3062014 [DOI] [PubMed] [Google Scholar]
- 26.Regierer AC, Wolters R, Ufen MP, et al. An internally and externally validated prognostic score for metastatic breast cancer: Analysis of 2269 patients Ann Oncol 25633–6382014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kontani K, Hashimoto S, Murazawa C, et al. Factors responsible for long-term survival in metastatic breast cancer. World J Surg Oncol. 2014;12:344. doi: 10.1186/1477-7819-12-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdel-Rahman O.M-bioscore: Proposing a new statistical model for prognostic factors in metastatic breast cancer patients J Comp Eff Res 7845–8542018 [DOI] [PubMed] [Google Scholar]
- 29.Plichta JK, Thomas SM, Sergesketter AR, et al. A novel staging system for de novo metastatic breast cancer refines prognostic estimates Ann Surg 2020. doi: 10.1097/SLA.0000000000004231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He ZY, Lian CL, Wang J, et al. Incorporation of biologic factors for the staging of de novo stage IV breast cancer. NPJ Breast Cancer. 2020;6:43. doi: 10.1038/s41523-020-00186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin C, Wu J, Ding S, et al. Subdivision of M1 stage for de novo metastatic breast cancer to better predict prognosis and response to primary tumor surgery J Natl Compr Canc Netw 171521–15282019 [DOI] [PubMed] [Google Scholar]
- 32.Kagan AR, Gilbert HA, Chan P, et al. The need for a staging system for metastasis, with emphasis on breast cancer metastasis Cancer Clin Trials 3281–2831980 [PubMed] [Google Scholar]
- 33.Carlson RW, Moench SJ, Hammond ME, et al. HER2 testing in breast cancer: NCCN Task Force report and recommendations J Natl Compr Canc Netw 4S1–S222006suppl 3quiz S23-S24 [PubMed] [Google Scholar]
- 34.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update J Clin Oncol 313997–40132013 [DOI] [PubMed] [Google Scholar]
- 35.Lobbezoo DJ, van Kampen RJ, Voogd AC, et al. Prognosis of metastatic breast cancer subtypes: The hormone receptor/HER2-positive subtype is associated with the most favorable outcome Breast Cancer Res Treat 141507–5142013 [DOI] [PubMed] [Google Scholar]
- 36.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2 N Engl J Med 344783–7922001 [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto N, Watanabe T, Katsumata N, et al. Construction and validation of a practical prognostic index for patients with metastatic breast cancer J Clin Oncol 162401–24081998 [DOI] [PubMed] [Google Scholar]
- 38.Llombart-Cussac A, Pivot X, Biganzoli L, et al. A prognostic factor index for overall survival in patients receiving first-line chemotherapy for HER2-negative advanced breast cancer: An analysis of the ATHENA trial Breast 23656–6622014 [DOI] [PubMed] [Google Scholar]
- 39.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. ed 7. New York, NY: Springer; 2010. [Google Scholar]
- 40.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. ed 7. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 41.Fleming I, Cooper J, Kenson DE. AJCC Cancer Staging Manual. ed 5. Philadelphia, PA: Lippincott-Raven; 1997. [Google Scholar]
- 42.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer J Clin Oncol 282784–27952010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreau T, Oquigley J, Mesbah M.A global goodness-of-fit statistic for the proportional hazards model Applied Stat 34212–2181985 [Google Scholar]
- 44. Bellera CA, MacGrogan G, Debled M, et al. Variables with time-varying effects and the Cox model: Some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol. 2010;10:20. doi: 10.1186/1471-2288-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gore SM, Pocock SJ, Kerr GR.Regression-models and non-proportional hazards in the analysis of breast-cancer survival Applied Stat 33176–1951984 [Google Scholar]
- 46.Schemper M.Cox analysis of survival-data with nonproportional hazard functions Statistician 41455–4651992 [Google Scholar]
- 47. Royston P, Altman DG. External validation of a Cox prognostic model: Principles and methods. BMC Med Res Methodol. 2013;13:33. doi: 10.1186/1471-2288-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steyerberg EW, Harrell FE., JrPrediction models need appropriate internal, internal-external, and external validation J Clin Epidemiol 69245–2472016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, et al. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis J Clin Epidemiol 54774–7812001 [DOI] [PubMed] [Google Scholar]
- 50.Kremers WK.Concordance for Survival Time Data: Fixed and Time-Dependent Covariates and Possible Ties in Predictor and Time Rochester, MN: Mayo Clinic; 2007. pp 17 [Google Scholar]
- 51.Finney DJ. Probit Analysis. ed 3. New York, NY: Cambridge University Press; 1971. [Google Scholar]
- 52.Uno H, Cai TX, Tian L, et al. Evaluating prediction rules for t-year survivors with censored regression models J Am Stat Assoc 102527–5372007 [Google Scholar]
- 53.https://biostatistics.mdanderson.org/BreastCancerSurvival/Calculator_Stage4.aspx
- 54.Polite BN, Cirrincione C, Fleming GF, et al. Racial differences in clinical outcomes from metastatic breast cancer: A pooled analysis of CALGB 9342 and 9840—Cancer and Leukemia Group B J Clin Oncol 262659–26652008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenberg PA, Hortobagyi GN, Smith TL, et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer J Clin Oncol 142197–22051996 [DOI] [PubMed] [Google Scholar]
- 56.Stuart-Harris R, Shadbolt B, Palmqvist C, et al. The prognostic significance of single hormone receptor positive metastatic breast cancer: An analysis of three randomised phase III trials of aromatase inhibitors Breast 18351–3552009 [DOI] [PubMed] [Google Scholar]
- 57.Liu MT, Huang WT, Wang AY, et al. Prediction of outcome of patients with metastatic breast cancer: Evaluation with prognostic factors and Nottingham prognostic index Support Care Cancer 181553–15642010 [DOI] [PubMed] [Google Scholar]
- 58.Kwast AB, Voogd AC, Menke-Pluijmers MB, et al. Prognostic factors for survival in metastatic breast cancer by hormone receptor status Breast Cancer Res Treat 145503–5112014 [DOI] [PubMed] [Google Scholar]
- 59.Kim HJ, Ahn SG, Lee HM, et al. Metastasis-free interval is closely related to tumor characteristics and has prognostic value in breast cancer patients with distant relapse J Breast Cancer 18371–3772015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobson AF, Shapiro CL, Van den Abbeele AD, et al. Prognostic significance of the number of bone scan abnormalities at the time of initial bone metastatic recurrence in breast carcinoma Cancer 9117–242001 [DOI] [PubMed] [Google Scholar]
- 61.Parkes A, Warneke CL, Clifton K, et al. Prognostic factors in patients with metastatic breast cancer with bone-only metastases Oncologist 231282–12882018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pentheroudakis G, Fountzilas G, Bafaloukos D, et al. Metastatic breast cancer with liver metastases: A registry analysis of clinicopathologic, management and outcome characteristics of 500 women Breast Cancer Res Treat 97237–2442006 [DOI] [PubMed] [Google Scholar]
- 63.Altundag K, Bondy ML, Mirza NQ, et al. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis Cancer 1102640–26472007 [DOI] [PubMed] [Google Scholar]
- 64.Nguyen DH, Truong PT, Walter CV, et al. Limited M1 disease: A significant prognostic factor for stage IV breast cancer Ann Surg Oncol 193028–30342012 [DOI] [PubMed] [Google Scholar]
- 65.Kawano A, Shimizu C, Hashimoto K, et al. Prognostic factors for stage IV hormone receptor-positive primary metastatic breast cancer Breast Cancer 20145–1512013 [DOI] [PubMed] [Google Scholar]
- 66. Miao H, Hartman M, Bhoo-Pathy N, et al. Predicting survival of de novo metastatic breast cancer in Asian women: Systematic review and validation study. PLoS One. 2014;9:e93755. doi: 10.1371/journal.pone.0093755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Follana P, Barriere J, Chamorey E, et al. Prognostic factors in 401 elderly women with metastatic breast cancer Oncology 86143–1512014 [DOI] [PubMed] [Google Scholar]
- 68. Dieci MV, Tsvetkova V, Orvieto E, et al. Immune characterization of breast cancer metastases: Prognostic implications. Breast Cancer Res. 2018;20:62. doi: 10.1186/s13058-018-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guth U, Magaton I, Huang DJ, et al. Primary and secondary distant metastatic breast cancer: Two sides of the same coin Breast 2326–322014 [DOI] [PubMed] [Google Scholar]
- 70.Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer J Clin Oncol 231420–14302005 [DOI] [PubMed] [Google Scholar]
- 71.Dawood S, Broglio K, Valero V, et al. Circulating tumor cells in metastatic breast cancer: From prognostic stratification to modification of the staging system? Cancer 1132422–24302008 [DOI] [PubMed] [Google Scholar]
- 72.Pairawan S, Hess KR, Janku F, et al. Cell-free circulating tumor DNA variant allele frequency associates with survival in metastatic cancer Clin Cancer Res 261924–19312020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stover DG, Parsons HA, Ha G, et al. Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer J Clin Oncol 36543–5532018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng J, Holland-Letz T, Wallwiener M, et al. Circulating free DNA integrity and concentration as independent prognostic markers in metastatic breast cancer Breast Cancer Res Treat 16969–822018 [DOI] [PubMed] [Google Scholar]
- 75.Plichta JK, Ren Y, Thomas SM, et al. Implications for breast cancer restaging based on the 8th edition AJCC staging manual Ann Surg 271169–1762020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giuliano AE, Connolly JL, Edge SB, et al. Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual CA Cancer J Clin 67290–3032017 [DOI] [PubMed] [Google Scholar]
- 77.Thomas L, Reyes EM.Tutorial survival estimation for Cox regression models with time-varying coefficients J Stat Softw 611–232014 [Google Scholar]