Highlights
-
•
The cultural and ethnic differences were taken into consideration in the study design.
-
•
It is the first meta-analysis to evaluate whether protein supplementation exerts additional benefits on sarcopenia in Asian and non-Asian countries.
-
•
Protein supplementation combined with exercise exerts superior benefit on lower-extremity strength in healthy older adults with sarcopenia in Asian countries.
Keywords: Older adults, Sarcopenia, Protein, Exercise, Muscle strength
Abstract
While there is growing research interest in the effects of nutrition and exercise on delaying sarcopenia, the results are inconclusive and there is scarce information on regional patterns. This review evaluated the effects of the combination of protein supplementation and exercise on muscle strength, mass and physical performance, compared to exercise alone or with a placebo, in healthy older adults in Asian and non-Asian countries. Fourteen studies were included in the analysis, involving a total of 888 healthy older adults (>60 years). A significant increase in the lower-extremity strength was observed in the combined intervention group compared to the exercise group in Asian countries (SMD: 0.24, 95% CI [0.00, 0.47], p = 0.048, I2 = 0.0%). No statistical differences were found relating to upper-extremity strength, muscle mass and physical performance. Protein supplementation combined with exercise provides additional benefit on lower-extremity strength in healthy older adults with sarcopenia in Asian countries.
Introduction
Sarcopenia is a chronic disease characterized by adverse muscle changes such as a decline in skeletal muscle mass, strength, and function, contributing to frailty, disability, poor quality of life and increased mortality (Wiedmer et al., 2021). According to the European Working Group on Sarcopenia in Older People (EWGSOP) (Cruz-Jentoft et al., 2018) and the Asian Working Group for Sarcopenia (AWGS) (Chen et al., 2020), diagnostic indicators of sarcopenia include muscle strength, physical performance and appendicular skeletal muscle mass.
Sarcopenia is prevalent among older adults, with an estimated prevalence of 11% in men and 9% in women older than 60 years (Papadopoulou et al., 2020). The global population ageing poses new challenges, with profound implications for various sectors of society including public health and medical cost. Given the serious hazard posed by sarcopenia, the remedial actions of sarcopenia have attracted increasing attention. The treatments of sarcopenia include rehabilitation exercise, nutritional support, drug treatment, and hormone replacement (Zhang et al., 2020). However, the effectiveness of protein supplementation on muscle strength, mass and physical performance in older population with sarcopenia is inconclusive (Labata-Lezaun et al., 2020). Furthermore, it is still unclear whether combined effects of protein supplementation and exercise are superior than those of exercise alone in healthy older adults with sarcopenia.
Many factors may contribute to this discrepancy in the literature. The prevalence of sarcopenia have been reported to vary with race/ethnicity (Du et al., 2018). Notably, differences have been documented in skeletal muscle mass and the age-related decline in skeletal muscle mass between various racial/ethnic groups (Silva et al., 2010). Specifically, Asian populations are reported to experience a more rapid deterioration in muscle strength and functioning, compared to white populations (Shaw et al., 2017). Moreover, ethnic and cultural differences in habitual dietary pattern and method of food preparation may further contribute to various results in the literature and randomized controlled trials (RCTs) conducted in different countries provide inconclusive results (Ganapathy & Nieves, 2020). Importantly, genetic variations in dietary responses to bioactive nutrients (Marcum, 2020) and differential gene expression involved in sarcopenia-related muscular changes (He et al., 2019) may also play an important role in explaining this discrepancy. Therefore, it is important to examine the evidence from different regions separately. Although there is considerable interest in the effects of nutrition and exercise on sarcopenia, there is scarce information on regional patterns.
Hence, the aim of this study is to evaluate if the combination of protein supplementation and exercise is more effective than exercise alone or with placebo in improving muscle strength, muscle mass and physical performance in healthy older adults in Asian and non-Asian countries.
Materials and methods
Protocol and registration
The study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement checklist (Moher et al., 2009). The registered number was INPLASY202180085, with the following DOI number: https://doi.org/10.37766/inplasy2021.8.0085.
Search strategy and selection criteria
Systematic literature search was conducted in Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Web of Science, and ScienceDirect from inception of the database until July 2021. The following terms were included in the literature search: older adults (older or “older people” or “elderly people” or “aging people” or “aged people” or “senior people” or “geriatric people” or “late life adults”), intervention (exercise or “resistance training” or “strength training” or “home training” or “comprehensive training program” or “whole-body electromyostimulation”), intervention (“protein supplement” or “nutritional supplement” or “dietary supplement” or “amino acid” or leucine or HMB or beta-hydroxy-beta-methylbutyrate), and sarcopenia (sarcopenic or “muscle loss” or amyotrophy). The search was restricted to articles published in English. Bibliographic search was conducted by reviewing the reference lists of the included studies or key texts. Moreover, manual searches and lists of references from additional studies were included, and other similar systematic reviews were checked in order to find potential studies that might meet the inclusion criteria.
Studies meeting the following criteria were included: (1) participants were aged 60 years or above; (2) healthy participants with sarcopenia, defined with at least one of the following indicators: muscle mass loss, low muscle strength, or poor physical performance; (3) the intervention group was exercise combined with protein or amino acid supplementation, and the comparison group was exercise alone or with placebo supplementation; (4) study design: RCTs; and (5) outcome: muscle strength, muscle mass and physical performance.
The exclusion criteria were as follows: studies including older adults with a specific health condition such as hip fracture, knee osteoarthritis, stroke, cancer, diabetes, human immunodeficiency virus, chronic obstructive pulmonary disease, chronic kidney disease, liver cirrhosis, other critical illness, and recent transplants were excluded; studies were also excluded if they were published as a protocol, conference abstract, case report, commentary, editorial, or letter to editor.
Study selection and quality evaluation
Data collection process
For each study included in this systematic review, the following data was extracted: (1) region, (2) study (author’s last name and year of publication), (3) country, (4) population (number, gender, age), (5) exercise intervention, (6) protein supplementation intervention, (7) comparison group, (8) outcome, (9) main results.
Risk of bias of individual studies
The Risk of Bias 2 tool (RoB 2) from the Cochrane Collaboration was used to assess the risk of bias of the RCTs included in this systematic review and meta-analysis, with a domain-based evaluation that classifies seven domains from each RCT into “low”, “unclear” or “high” risk of bias (Higgins et al., 2016). The seven domains are based on selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selecting reporting), and other bias (e.g., limitations).
Statistical Analyses
The statistical analysis was conducted using the Stata version 14.0 (College Station, TX77845, USA) and the Review Manager 5.4 software (the Cochrane Collaboration, London, UK, 2020). Mean difference was used to determine the effect size. The standardized mean difference (SMD) was calculated to compare the outcomes, using a random-effects model (DerSimonian-Laird approach (Kelley & Kelley, 2012)). Outcomes were presented as a mean change from baseline in upper-extremity strength, lower-extremity strength, muscle mass, and gait speed. In addition, standard deviations (SDs) of the change were calculated if the data were not stated in the RCTs. The equation was: [SDpre2 + SDpost2-2 × Corr(pre, post) × SDpre × SDpost]0.5. SDpre was the SD before the intervention, SDpost was the SD after the intervention, and Corr(pre, post) was within-participant correlation. The within-participant correlation was set as 0.5 if the correlation was not reported. If necessary, SD was calculated by standard error or confidence interval. Heterogeneity across studies was evaluated by I2 statistics. Heterogeneity was classified as “small”, “moderate”, or “substantial” if I2 was < 25%, 25–75%, and > 75%, respectively (Higgins et al., 2003). The individual influence of each study on the overall result was evaluated by removing them individually. The potential existence of publication bias was determined by the Egger’s test, with visual inspection of funnel plots. When studies did not report specific data (e.g., only graphs), an email was sent to the corresponding author asking for the specific data. If no response was received, the study was removed from the meta-analysis. All statistical results with P value < 0.05 was considered statistically significant.
Results
Study selection
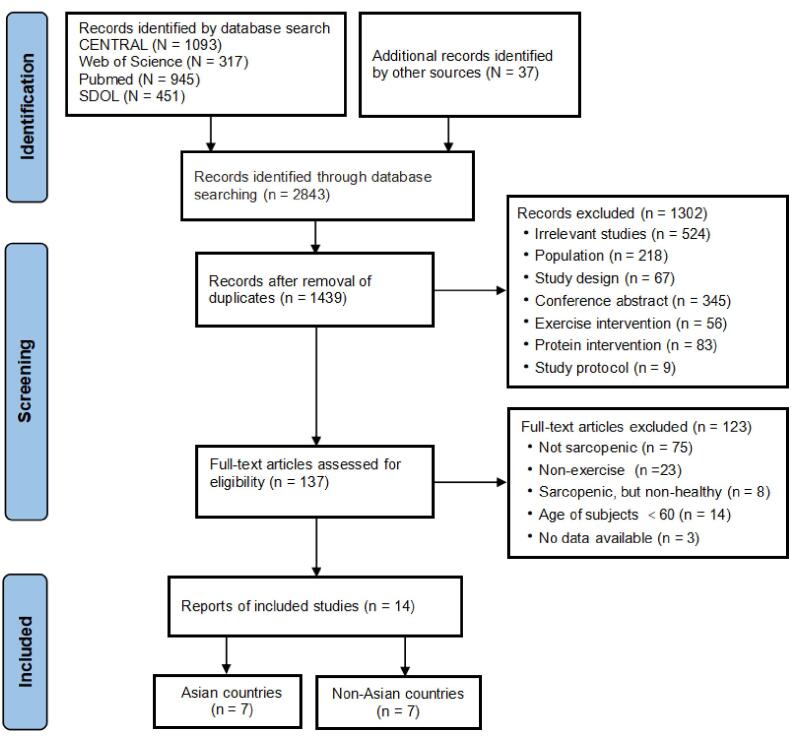
The process of study selection is shown as a PRISMA flow diagram (Fig. 1). A total of 2806 publications were identified by searching the databases and 37 were identified by other sources. The titles and abstracts of all included studies were screened, and 1302 were excluded after this preliminary filter. Subsequently, full text screening was carried out and 14 publications were included in the meta-analysis. Specifically, 7 studies were carried out in Asian countries and 7 studies were carried out in non-Asian countries.
Fig. 1.
Flow diagram of the study selection.
Study characteristics
The characteristics of the included studies are presented in Table 1, involving 14 studies and a total of 888 elderly individuals. Studies included in this systematic review involved participants from 4 continents. Specifically, 7 studies were carried out in Asia, 3 studies in North America, 1 study in South America, and 3 studies in Europe. Among the 7 studies in Asian countries involving a total of 529 elderly individuals, 266 participants were included in the combined intervention group (exercise training + protein supplementation) and 263 participants were included in the exercise group (only exercise training). Among the 7 studies in non-Asian countries involving a total of 359 elderly individuals, 179 participants were included in the combined intervention group (exercise training + protein supplementation) and 180 participants were included in the exercise group (only exercise training).
Table 1.
Characteristics of the studies included in the qualitative analysis.
| Region | Study | Diagnosis of Sarcopenia | Country | Population |
Ex Intervention |
PS Intervention |
CGb | Outcome | Main Results | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (EG/CG) | G (M/F) | Age | Typea | Duration × Frequency | Type | g/d | |||||||
| Asian countries | (Zhu et al., 2018) | Low ASM/Ht2 (DXA) Low HS Low GS |
China | 76 (36/40) | 18/58 | ≥ 65 | 1 | 12 w × 3 s/w | Protein + HMB (VD) |
17.22 + 2.24 g/d | 1 | MS MM PP |
ND UES ↑LES ↑MM ND GS |
| (Kim et al., 2021) | Low SMI (BIA) | Japan | 130 (65/65) | 0/130 | ≥ 65 | 1 | 12 w × 1 s/w | EAA | 3 g/d | 2 | MS MM PP |
ND UES ↑ LES ↑MM ↑GS |
|
| (Kim et al., 2012) | Low ASM/Ht2 (DXA) Low KES Low GS Low BMI |
Japan | 77 (38/39) | 0/77 | ≥ 75 | 1 | 12 w × 2 s/w | EAA (leucine enriched) | 6 g/d | 1 | MS MM PP |
↑ LES ND MM ↑GS | |
| (Kim et al., 2016) | Low SMI (DXA) High BFP Low HS Low GS |
Japan | 71 (36/35) | 0/71 | ≥ 70 | 1 | 12 w × 2 s/w | EAA (catechin) |
3 g/d | 1 | MS MM PP |
ND UES ↑LES ND MM ↑GS |
|
| (Yamada et al., 2019) | Low PP Low MS Low MM (BIA) | Japan | 56 (28/28) | 18/38 | ≥ 65 | 2 | 12 w × 2 s/w | Protein (VD) |
10 g/d |
1 | MS MM PP |
ND UES ↑ LES ↑MM ND GS | |
| (Li et al., 2021) | Low MM (BIA) Low HS Low GS |
China | 85 (48/37) | 36/49 | ≥ 60 | 1 | 12 w × 3 s/w | Whey protein (VD) |
30 g/d |
1 | MS MM |
↑UES ↑MM | |
| (Shahar et al., 2013) | Low SM (BIA) | Malaysia | 34 (15/19) | NR | ≥ 60 | 1 | 12 w × 2 s/w | Protein | M: 20 g/d F: 40 g/d |
1 | MS MM PP |
ND UES ND MM ND GS | |
| Non-Asian countries | (Nabuco et al., 2019) | Low ALST (DXA) High BFM | Brazilian | 26 (13/13) | 0/26 | ≥60 | 2 | 12w × 3 s/w | Whey protein | 35 g/d | 2 | MS MM PP |
↑UES ↑ LES ↑MM ↑GS |
| (Rondanelli et al., 2016) | Low RMM(DXA) | Italy | 130 (69/61) | 55/77 | ≥65 | 1 | 12 w × 5 s/w | Whey protein + EAA (VD) | 22 + 8.9 g/d | 2 | MS MM |
↑UES ↑MM | |
| (Kemmler et al., 2016) | Low SMI (DXA) High BF |
Germany | 50 (25/25) | 0/50 | ≥70 | 3 | 26 w × 1 s/w | Whey protein + l-leucine (VD) | 21 + 2.8 g/d | 1 | MS MM PP |
ND UES ↑MM ND GS | |
| (Rathmacher et al., 2020) | Low BMI(DXA) | USA | 64 (30/34) | 38/26 | ≥60 | 2 | 48 w × 3 s/w | HMB (VD) |
3 g/d | 1 | MM | ↑MM | |
| (Zdzieblik et al., 2015) | Low HS Low MM(DXA) | Germany | 53 (26/27) | 53/0 | 72.2 ± 4.68 | 2 | 12 w × 3 s/w | Collagen peptide | 15 g/d | 2 | MS MM |
↑ LES ↑MM | |
| (Mathieu L. Maltais et al., 2016) | Low ALMI (DXA) | Canada | 18 (8/10) | 18/0 | 65 ± 5 | 2 | 16 w × 3 s/w | Protein + EAA | 12 + 7 g /d | 2 | MM | ↑MM | |
| (Mathieu L Maltais et al., 2016) | Low AMMI Low MMI (DXA) | Canada | 18 (8/10) | 18/0 | ≥60 | 2 | 16 w × 3 s/w | Protein + EAA | 12 + 7 g /d | 2 | MS MM PP |
↑UES ↑MM ND GS | |
EG = experimental group; CG = comparison group; N = number; G = gender; M = male; F = female; Ex = exercise; PS = protein supplementation; ND = no significant differences; ↑= significant increase for experimental group; w = week; d = day; s = session; aType: 1 = mixed exercise; 2 = resistance exercise; 3 = whole-body electromyostimulation; bCG: 1 = exercise alone; 2 = exercise alone with placebo supplementation; ASM/Ht2 = appendicular skeletal muscle mass/height2; DXA = dual energy x-ray absorptiometry; HS = handgrip strength; GS = gait speed; HMB = β-hydroxy-β-methyl-butyrate; VD = vitamin D; MS = muscle strength; MM = muscle mass; PP = physical performance; UES = upper-extremity strength; LES = lower-extremity strength; SMI = skeletal muscle mass index; BIA = bioelectrical impedance analysis; EAA = essential amino acids; KES = knee extension strength; BMI = body mass index; BFP = body fat percent; SM = skeletal muscle; NR = no report; ALST = appendicular lean soft tissue; BFM = body fat mass; RMM = relative muscle mass; BF = body fat; ALMI = appendicular lean mass index; AMMI = appendicular muscle mass index; MMI = muscle mass index.
Among the studies carried out in Asian countries, 3 studies included only female participants whereas other 4 studies included both male and female participants. Sample sizes across studies ranged from 34 (Shahar et al., 2013) to 130 (Kim et al., 2021). Exercise prescriptions included resistance exercise (1 studies) and mixed exercise (6 studies). The intervention period of all studies was 12 weeks, with the most common frequency of 2 s/w (n = 4). Nutritional interventions included essential amino acids (EAA) (3 studies) and protein (4 studies), with the dosage ranging from 3 g/d to 40 g/d.
Among the studies carried out in non-Asian countries, 2 studies included only female participants while 3 studies included only male participants and the other 2 studies included both male and female participants. Sample sizes across studies ranged from 18 (Maltais et al., 2016, Maltais et al., 2016) to 130 (Rondanelli et al., 2016). Exercise prescriptions included resistance exercise (5 studies), mixed exercise (1 study), and whole-body electromyostimulation (1 study). The intervention period ranged from 12 weeks to 48 weeks, with the most common frequency of 3 s/w (n = 5). Nutritional interventions included protein supplementation with or without EAA (5 studies), HMB (1 study), and collagen peptide (1 study), with the dosage ranging from 3 g/d to 35 g/d.
Risk of bias assessment
The RoB 2 tool summary and risk of bias graphs are shown in Appendix A1. Among all the studies, 12studies (86%) had at least four domains with “low risk” and 4 studies (29%) had at least six domains with “low risk”. Meanwhile, 8 studies (57%) had no domains as “high risk”. Among the studies in Asian countries, selection bias (allocation concealment) and reporting bias were 100% with “low risk”. Among the studies in non-Asian countries, selection bias, performance bias and detection bias were 100% with “low risk”.
Outcomes
The effects of combined intervention of protein supplementation and exercise as well as exercise intervention on muscle strength, muscle mass and physical performance in healthy older participants in Asian and non-Asian countries were evaluated as a whole and separately in the present study. Exercise prescriptions included resistance exercise, mixed training, and whole-body electromyostimulation (WB-EMS). Among the exercise interventions included in the present study, mixed training and resistance exercise were most adopted. The primary outcome assessed was muscle strength (upper- and lower-extremity strength). The secondary outcomes included muscle mass and physical performance. Muscle mass measured in these studies included lean body mass, appendicular muscle mass (AMM), and appendicular skeletal muscle mass (ASM). Physical performance was mainly evaluated by using gait speed (GS).
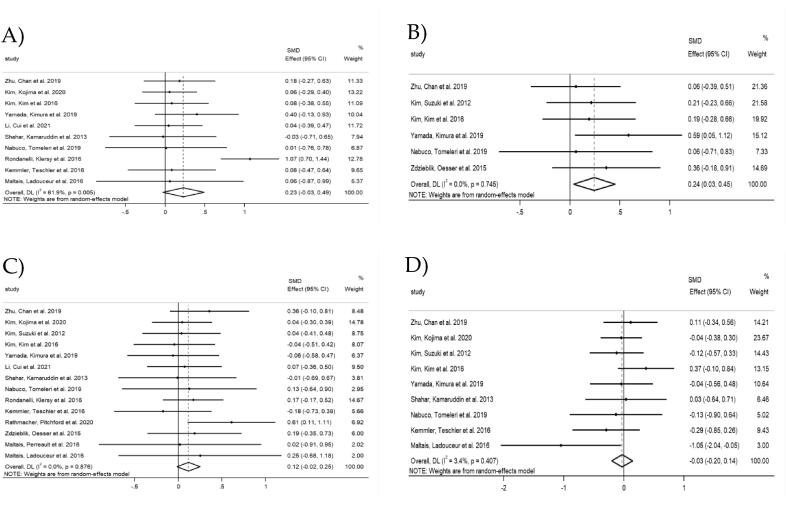
A meta-analysis was conducted on all eligible studies in Asian and non-Asian countries to evaluate the overall effects of combined intervention of protein supplementation and exercise as well as exercise intervention on muscle strength, muscle mass and physical performance in healthy older participants, regardless of the regions (Fig. 2). The results showed that the upper-extremity strength did not differ between combined intervention group and exercise group, with a SMD of 0.23 (95% CI [-0.03, 0.49], p = 0.083) and a substantial heterogeneity (I2 = 61.9%, p = 0.005) (Fig. 2A). To investigate this factor, all studies were removed once from the analysis. When the study by Rondanelli et al. (2016) was removed, the heterogeneity was reduced from 61.9% to 0% (SMD = 0.11, 95% CI [-0.06, 0.27], p = 0.220) (Appendix A2.2). In contrast, a significant increase in the lower-extremity strength was observed in the combined intervention group compared to the exercise group, with a SMD of 0.24 (95% CI [0.03, 0.45], p = 0.022) and a small heterogeneity (I2 = 0.0%, p = 0.745) (Fig. 2B). However, no significant differences in muscle mass were observed between the combined intervention group and the exercise group (SMD: 0.12, 95% CI [-0.02, 0.25], p = 0.084, I2 = 0.0%) (Fig. 2C). Similarly, physical performance measured as gait speed did not differ between combined intervention group and exercise group, with a SMD of −0.03 (95% CI [-0.20, 0.14], p = 0.736) and a small heterogeneity (I2 = 3.4%, p = 0.407) (Fig. 2D).
Fig. 2.
Forest plots of outcomes in Asian and Non-Asian countries: A) upper-extremity strength; B) lower-extremity strength; C) muscle mass; D) gait speed.
Upper-extremity strength and Lower-extremity strength
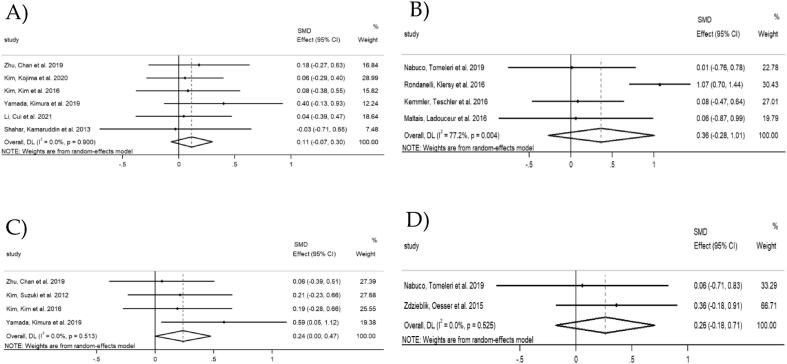
Upper-extremity strength was measured as handgrip strength in 6 studies in Asian countries involving 452 participants (228 in combined intervention group and 224 in exercise group). The results showed that the upper-extremity strength did not differ between combined intervention and exercise group, with a SMD of 0.11 (95% CI [-0.07, 0.30], p = 0.224) and a small heterogeneity (I2 = 0.0%, p = 0.900) (Fig. 3A).
Fig. 3.
Forest plots of upper-extremity strength: A) Asian countries; B) Non-Asian countries. Forest plots of lower-extremity strength: C) Asian countries; D) Non-Asian countries.
Regarding the studies in non-Asian countries, 4 studies provided data on upper-extremity strength involving 224 participants (115 in combined intervention group and 109 in exercise group). Two of them assessed handgrip strength and the other two used chest press or lat pull-down. The results showed that the upper-extremity strength did not differ between combined intervention and the exercise group, with a SMD of 0.36 (95% CI [-0.28, 1.01], p = 0.266) and a substantial heterogeneity (I2 = 77.2%, p = 0.004) (Fig. 3B). To investigate this factor, all studies were removed once from the analysis. When the study by Rondanelli et al. (2016) was removed, the heterogeneity was reduced from 77.2% to 0% (SMD = 0.06, 95% CI [-0.34, 0.47], p = 0.770) (Appendix A3).
Four studies in Asian countries provided data on lower-extremity strength, involving 280 participants (138 in combined intervention group and 142 in exercise group). Lower-extremity strength was assessed using knee extension (n = 3) or leg extension (n = 1). Notably, there was a significant increase in the lower-extremity strength in the combined intervention group compared to the exercise group, with a SMD of 0.24 (95% CI [0.00, 0.47], p = 0.048). The I2 statistic revealed a small heterogeneity (I2 = 0.0%, p = 0.513) (Fig. 3C).
Two studies in non-Asian countries provided data on lower-extremity strength, including 79 participants (39 in combined intervention group and 40 in exercise group). Lower-extremity strength was assessed using knee extension with different units. The SMD between combined intervention and the exercise group was 0.26 (95% CI [ −0.18, 0.71], p = 0.247), with a small heterogeneity (I2 = 0.0%, p = 0.525) (Fig. 3D).
Muscle mass
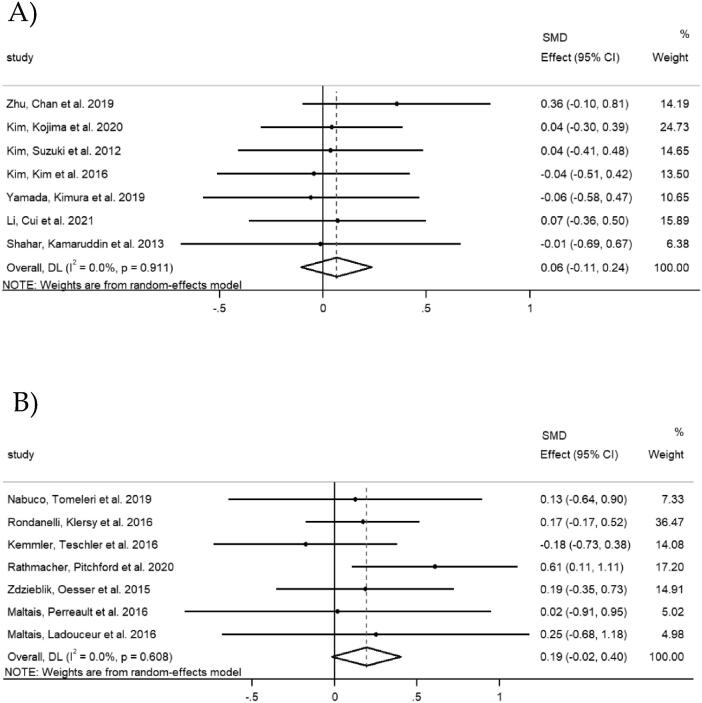
Seven studies in Asian countries investigated the changes in muscle mass. A total of 266 participants were included in the combined intervention group and 263 participants were included in the exercise group. Three studies measured AMM, three studies measured ASM or ASM/height2 (ASM/Ht2) and one study measured muscle mass. The overall SMD was 0.06 (95% CI [ −0.11, 0.24], p = 0.456), with a small heterogeneity (I2 = 0.0%, p = 0.911) (Fig. 4A).
Fig. 4.
Forest plots of muscle mass: A) Asian countries; B) Non-Asian countries.
Seven studies in non-Asian countries provided data on muscle mass. A total of 179 participants were included in the combined intervention group and 180 participants were included in the exercise group. The overall SMD was 0.19 (95% CI [-0.02, 0.40], p = 0.070), with a small heterogeneity (I2 = 0.0%, p = 0.608) (Fig. 4B).
Physical performance
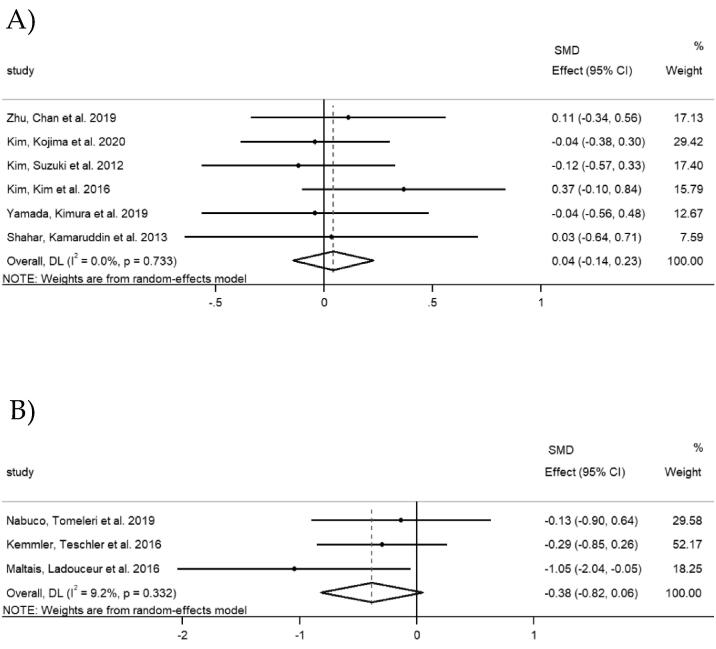
Six studies in Asian countries involving 444 participants (218 in combined intervention group and 226 in exercise group) investigated changes in physical performance. Four studies evaluated gait speed in meters per second and one study in the total number of meters walked in 6 min, therefore higher values implying better physical performance. However, one study assessed it as 5-m comfortable walking time with higher values implying worse physical performance. Hence, the data direction of this study was opposed for the meta-analysis. The results showed that there was no significant increase in the gait speed (SMD = 0.04, 95% CI [-0.14, 0.23], p = 0. 654), with a small heterogeneity (I2 = 0.0%, p = 0.733) (Fig. 5A).
Fig. 5.
Forest plots of gait speed: A) Asian countries; B) Non-Asian countries.
Gait speed was investigated in three studies in non-Asian countries, involving 94 participants (46 in combined intervention group and 48 in exercise group). One study evaluated gait speed in meters per second, with higher values implying better physical performance. However, two studies assessed it as 10-m and 4-m comfortable walking time with higher values implying worse physical performance. Therefore, the data directions of these two studies were opposed for the meta-analysis. The overall SMD was −0.38, with a 95% CI of [-0.82, 0.06] (p = 0.087). The I2 statistic revealed a small heterogeneity (I2 = 9.2%, p = 0.332) (Fig. 5B).
Sensitivity analysis of upper-, lower-extremity strength, muscle mass and physical performance revealed that the overall effect did not change and visual inspection of the funnel plots revealed no evidence of publication bias. Egger’s regression plots did not show any substantial asymmetry and Egger’s test indicated no significant publication bias (Appendix A4-7).
Discussion
Taking into consideration the cultural and ethnic differences in dietary consumption and food preparation, as well as the ethnicity associated differences in the prevalence and development of sarcopenia, the present review aims to summarize the effects of exercise intervention combined with protein supplementation compared to exercise intervention alone or with placebo supplementation on muscle strength, muscle mass and physical performance in the healthy elderly population in Asian and non-Asian countries. To our knowledge, this is the first meta-analysis that compares the combination effects of exercise and protein supplementation with exercise on sarcopenia in healthy older participants in Asian and non-Asian countries.
The age-related decline in muscle strength, muscle mass and physical performance has been associated with falls and fractures in older adults, increasing the risk of physical disability and functional impairment. The present review demonstrated that lower-extremity strength was significantly increased in the combined intervention group compared to the exercise group in the overall analysis of all studies as well as subgroup analysis of Asian studies, indicating the additional benefit of protein supplementation on muscle strength, which is a key characteristic for determining sarcopenia as suggested by the European Working Group on Sarcopenia in Older People (EWGSOP) (Cruz-Jentoft et al., 2018). This is in accord with a previous systematic review and meta-analysis indicating that protein supplementation combined with resistance training was more effective in improving the muscle mass and strength in the elderly, compared to resistance training alone (Hou et al., 2019). Consistent with these findings, protein supplementation incorporated with muscle strengthening exercise were found to be more beneficial in increasing muscle strength and mass, as well as performance in physical mobility in elderly adults, compared with muscle strengthening exercise alone (Liao et al., 2019).
In contrast, the present review reported that no significant differences were observed on lower-extremity strength between the combined intervention group and the exercise group in older adults in non-Asian countries, which might be due to small sample size and large individual variability. There were no statistical differences between the those two groups on upper-extremity strength, muscle mass and physical performance in Asian countries and non-Asian countries. In support of these findings, a recent meta-analysis comparing resistance training plus protein supplementation with resistance training in a healthy elderly population also reported no statistical differences in upper- and lower-limb strength, handgrip strength and physical performance (Labata-Lezaun et al., 2020). Similarly, protein supplementation did not exert superior effects when combined with resistance exercise training on lean body mass, upper body strength, lower extremity muscle strength and physical performance in non-frail community-dwelling older adults (ten Haaf et al., 2018). In addition, a recent systematic review and network meta-analysis also revealed that the combined intervention of exercise and nutrition exerted similar beneficial effects on muscle strength and physical performance when compared with exercise alone (Wu et al., 2021).
Many factors might have contributed to the discrepancy regarding whether protein supplementation provides additional benefit on muscle characteristics, including differences in participant profile (age, baseline muscle characteristics, and health conditions), inter-individual variation, baseline habitual intake of protein, protein supplementation (type, amount and duration), exercise intervention protocols (type, intensity and duration) and different methods employed to evaluate muscle characteristics and physical performance. The mixed results observed in the present study could be due to the heterogeneity between the selected studies, particularly the variability in the exercise protocol and protein supplementation. Furthermore, the differences in the criteria used to identify sarcopenia by the selected studies may also have contributed to this discrepancy.
The present review reported protein supplementation yielded additional improvement of lower-extremity strength in older adults in Asian countries, despite the lack of additional increase in muscle mass. Notably, the correlations of muscle mass with muscle strength and muscle function in older adults are low, suggesting that muscle quality may be an important determinant of muscle strength and physical performance during aging (Goodpaster et al., 2006). As one aspect of muscle quality, skeletal muscle density is more strongly associated with muscle strength and physical performance than muscle size and therefore may become a more clinically meaningful surrogate of muscle performance for the diagnosis of sarcopenia (Wang et al., 2021).
Higher dietary protein intake (≥1.0 g/kg/day) is associated with better physical performance in community-dwelling older adults (Coelho-Júnior et al., 2018). There is emerging evidence suggesting that at least 1.2 g/kg/day of protein intake is recommended to maintain optimal muscle function in the elderly (Nowson & Connell, 2015). Consistent with these findings, 1.5 g/kg/day of whey protein supplementation for 12 weeks significantly increased appendicular skeletal muscle mass, skeletal muscle mass index and gait speed in older adults with mild frailty at risk of malnutrition, compared to the 0.8 and 1.2 g/kg/day groups (Park et al., 2018). Specifically, whole body net protein balance was enhanced with protein intake above 0.8 g/kg/day, primarily due to elevated protein synthesis rates at whole body and muscle levels (Kim et al., 2015). Notably, an even distribution of daily protein intake across meals facilitates optimal protein proteostasis and is independently associated with greater muscle strength in the elderly (Farsijani et al., 2017). It is recommended that each meal should provide 20–30 g protein, to achieve maximum stimulation of muscle protein fractional synthetic rate (Deutz & Wolfe, 2013). Increasing protein intake, especially at breakfast and lunch, contributes to alleviating muscle loss with aging (Smeuninx et al., 2020). Furthermore, apart from stimulating protein synthesis and suppressing protein breakdown, nutritional strategies to improve mitochondrial function and reduce inflammation in support of healthy aging also facilitate the delay of sarcopenia (Cannataro et al., 2021). It is noteworthy that specific amino acids (e.g., leucine (Martínez-Arnau et al., 2020)) and other nutrients (e.g., vitamin D (Uchitomi et al., 2020), long-chain n-3 polyunsaturated fatty acids (Scotto di Palumbo et al., 2021)) also play an important role in the prevention and management of sarcopenia. These nutrients exert biological function through the regulation of miRNAs to facilitate muscle protein anabolism and attenuate muscle atrophy (Yu et al., 2021). Specifically, dairy protein supplementation inhibited the expression of miR-1, −486, −23a, −23b, −26a, −148b, let-7b and −7g involved in key muscle regulatory pathway in adults during lower limb immobilization, demonstrating potential protective effect in mitigating muscle loss (D'Souza et al., 2018). The role of miRNAs in regulating bioactive effects has become evident in recent years (Chao et al., 2019).
Different types of exercise including resistance training, endurance training, mixed training of resistance training and endurance training, and whole-body vibration have been investigated for their promising effects on counteracting sarcopenia. Research on comparative effectiveness of different exercise interventions demonstrates that resistance training is the most effective intervention to enhance muscle strength and physical performance in the elderly, compared to endurance training and whole-body vibration (Lai et al., 2018).
The consistency in results of overall analysis of all 14 studies and subgroup analysis of Asian studies (but not subgroup analysis of non-Asian studies) highlights the importance and necessity of conducting overall analysis of studies regardless of regions as well as performing subgroup analysis of studies according to regions. The effectiveness of protein supplementation on lower-extremity strength in older adults in non-Asian studies needs to be examined in more future well-designed RCTs.
The main limitations of this review are the small number of studies and total sample size, due to the inclusion criteria that focused on the effects of combination intervention of protein supplementation and exercise as well as exercise intervention alone on healthy older adults in Asian countries and non-Asian countries. Differences in the types and doses of protein supplementation as well as variations in the exercise protocol and duration need to be taken into consideration when interpreting those results. Moreover, the included studies did not report the amount of baseline protein intake and total protein intake, making it difficult to determine the effectiveness of protein intake on preventing sarcopenia.
Conclusions
The present review suggests that protein supplementation combined with exercise exerts superior benefit on lower-extremity strength in healthy older adults with sarcopenia in Asian countries, when compared to exercise alone or with a placebo. However, no additional benefits from protein supplementation were observed on upper-extremity strength, muscle mass and physical performance regardless of the regions. More well-designed RCTs with information on baseline and total protein intake for longer follow-up periods are warranted to evaluate the effectiveness of protein supplementation and exercise on the prevention and management of sarcopenia in healthy older adults.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Beijing Technology and Business University Technological Innovation Service Capacity Building-Basic Scientific Research Expenses (PXM2020_014213_000017). The authors are grateful to all the researchers whom we cited in this review for their significant and valuable research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100210.
Contributor Information
Lu Li, Email: lil@btbu.edu.cn.
Xinqi Liu, Email: liuxinqi@btbu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Cannataro R., Carbone L., Petro J.L., Cione E., Vargas S., Angulo H.…Bonilla D.A. Sarcopenia: Etiology, nutritional approaches, and miRNAs. International Journal of Molecular Sciences. 2021;22(18):9724. doi: 10.3390/ijms22189724. https://www.mdpi.com/1422-0067/22/18/9724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Z., Shanshan L., Desheng W., Dan L., Xiaobo Z., Amin I.…Jianbo X. miRNAs as regulators of antidiabetic effects of fucoidans. eFood. 2019;1(1):2–11. doi: 10.2991/efood.k.190822.001. [DOI] [Google Scholar]
- Chen L.-K., Woo J., Assantachai P., Auyeung T.-W., Chou M.-Y., Iijima K., Kim S. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. Journal of the American Medical Directors Association. 2020;21(3):300–307. doi: 10.1016/j.jamda.2019.12.012. e302. [DOI] [PubMed] [Google Scholar]
- Coelho-Júnior H.J., Milano-Teixeira L., Rodrigues B., Bacurau R., Marzetti E., Uchida M. Relative Protein intake and physical function in older adults: A systematic review and meta-analysis of observational studies. Nutrients. 2018;10(9):1330. doi: 10.3390/nu10091330. https://www.mdpi.com/2072-6643/10/9/1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., EWGSOP2, t. E. G. f. Sarcopenia: Revised European consensus on definition and diagnosis. Age and Ageing. 2018;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza R.F., Zeng N., Figueiredo V.C., Markworth J.F., Durainayagam B.R., Mitchell S.M.…Mitchell C.J. Dairy protein supplementation modulates the human skeletal muscle microRNA response to lower limb immobilization. Molecular Nutrition & Food Research. 2018;62(7):1701028. doi: 10.1002/mnfr.201701028. [DOI] [PubMed] [Google Scholar]
- Deutz N.E., Wolfe R.R. Is there a maximal anabolic response to protein intake with a meal? Clinical Nutrition. 2013;32(2):309–313. doi: 10.1016/j.clnu.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K., Goates S., Arensberg M.B., Pereira S., Gaillard T. Prevalence of sarcopenia and sarcopenic obesity vary with race/ethnicity and advancing age. Diversity and Equality in Health and Care. 2018;15(4):175–183. [Google Scholar]
- Farsijani S., Payette H., Morais J.A., Shatenstein B., Gaudreau P., Chevalier S. Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3-y physical function decline, in free-living older adults: The Quebec longitudinal study on Nutrition as a Determinant of Successful Aging (NuAge study) The American Journal of Clinical Nutrition. 2017;106(1):113–124. doi: 10.3945/ajcn.116.146555. [DOI] [PubMed] [Google Scholar]
- Ganapathy A., Nieves J.W. Nutrition and sarcopenia—What do we know? Nutrients. 2020;12(6):1755. doi: 10.3390/nu12061755. https://www.mdpi.com/2072-6643/12/6/1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster B.H., Park S.W., Harris T.B., Kritchevsky S.B., Nevitt M., Schwartz A.V.…Study, f. t. H. A The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. The Journals of Gerontology: Series A. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- He L., Khanal P., Morse C.I., Williams A., Thomis M. Differentially methylated gene patterns between age-matched sarcopenic and non-sarcopenic women. Journal of Cachexia, Sarcopenia and Muscle. 2019;10(6):1295–1306. doi: 10.1002/jcsm.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Sterne J.A., Savovic J., Page M.J., Hróbjartsson A., Boutron I.…Eldridge S. A revised tool for assessing risk of bias in randomized trials. Cochrane Database of Systematic Reviews. 2016;10(Suppl 1):29–31. [Google Scholar]
- Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Lei Y., Li X., Huo C., Jia X., Yang J.…Wang X.-M. Effect of protein supplementation combined with resistance training on muscle mass, strength and function in the elderly: A systematic review and meta-analysis. The Journal of Nutrition, Health & Aging. 2019;23(5):451–458. doi: 10.1007/s12603-019-1181-2. [DOI] [PubMed] [Google Scholar]
- Kelley G.A., Kelley K.S. Statistical models for meta-analysis: A brief tutorial. World Journal of Methodology. 2012;2(4):27–32. doi: 10.5662/wjm.v2.i4.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmler W., Teschler M., Weissenfels A., Bebenek M., von Stengel S., Kohl M.…Engelke K. Whole-body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. Results of the randomized controlled FORMOsA-sarcopenic obesity study. Osteoporosis International. 2016;27(11):3261–3270. doi: 10.1007/s00198-016-3662-z. [DOI] [PubMed] [Google Scholar]
- Kim H., Kim M., Kojima N., Fujino K., Hosoi E., Kobayashi H.…Yoshida H. Exercise and nutritional supplementation on community-dwelling elderly japanese women with sarcopenic obesity: A randomized controlled trial. Journal of the American Medical Directors Association. 2016;17(11):1011–1019. doi: 10.1016/j.jamda.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Kim H., Kojima N., Uchida R., Somekawa S., Inoue N., Kobayashi H., Osuka Y. The additive effects of exercise and essential amino acid on muscle mass and strength in community-dwelling older Japanese women with muscle mass decline, but not weakness and slowness: A randomized controlled and placebo trial. Aging Clinical and Experimental Research. 2021;33(7):1841–1852. doi: 10.1007/s40520-020-01713-x. [DOI] [PubMed] [Google Scholar]
- Kim H.K., Suzuki T., Saito K., Yoshida H., Kobayashi H., Kato H., Katayama M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: A randomized controlled trial. Journal of the American Geriatrics Society. 2012;60(1):16–23. doi: 10.1111/j.1532-5415.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- Kim I.-Y., Schutzler S., Schrader A., Spencer H., Kortebein P., Deutz N.E.P.…Ferrando A.A. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. American Journal of Physiology-Endocrinology and Metabolism. 2015;308(1):E21–E28. doi: 10.1152/ajpendo.00382.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labata-Lezaun N., Llurda-Almuzara L., López-de-Celis C., Rodríguez-Sanz J., González-Rueda V., Hidalgo-García C.…Pérez-Bellmunt A. Effectiveness of protein supplementation combined with resistance training on muscle strength and physical performance in elderly: A systematic review and meta-analysis. Nutrients. 2020;12(9):2607. doi: 10.3390/nu12092607. https://www.mdpi.com/2072-6643/12/9/2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Tu Y.-K., Wang T.-G., Huang Y.-T., Chien K.-L. Effects of resistance training, endurance training and whole-body vibration on lean body mass, muscle strength and physical performance in older people: A systematic review and network meta-analysis. Age and Ageing. 2018;47(3):367–373. doi: 10.1093/ageing/afy009. [DOI] [PubMed] [Google Scholar]
- Li Z., Cui M., Yu K., Zhang X.-W., Li C.-W., Nie X.-D., Wang F. Effects of nutrition supplementation and physical exercise on muscle mass, muscle strength and fat mass among sarcopenic elderly: A randomized controlled trial. Applied Physiology, Nutrition, and Metabolism. 2021;46(5):494–500. doi: 10.1139/apnm-2020-0643. [DOI] [PubMed] [Google Scholar]
- Liao C.-D., Chen H.-C., Huang S.-W., Liou T.-H. The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: A systematic review and meta-regression analysis of randomized trials. Nutrients. 2019;11(8):1713. doi: 10.3390/nu11081713. https://www.mdpi.com/2072-6643/11/8/1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltais M.L., Ladouceur J.P., Dionne I.J. The effect of resistance training and different sources of postexercise protein supplementation on muscle mass and physical capacity in sarcopenic elderly men. Journal of Strength and Conditioning Research. 2016;30(6):1680–1687. doi: 10.1519/JSC.0000000000001255. [DOI] [PubMed] [Google Scholar]
- Maltais M.L., Perreault K., Courchesne-Loyer A., Lagacé J.-C., Barsalani R., Dionne I.J. Effect of resistance training and various sources of protein supplementation on body fat mass and metabolic profile in sarcopenic overweight older adult men: A pilot study. International Journal of Sport Nutrition and Exercise Metabolism. 2016;26(1):71–77. doi: 10.1123/ijsnem.2015-0160. [DOI] [PubMed] [Google Scholar]
- Marcum J.A. Nutrigenetics/nutrigenomics, personalized nutrition, and precision healthcare. Current Nutrition Reports. 2020;9(4):338–345. doi: 10.1007/s13668-020-00327-z. [DOI] [PubMed] [Google Scholar]
- Martínez-Arnau F.M., Fonfría-Vivas R., Buigues C., Castillo Y., Molina P., Hoogland A.J.…Cauli O. Effects of leucine administration in sarcopenia: A randomized and placebo-controlled clinical trial. Nutrients. 2020;12(4):932. doi: 10.3390/nu12040932. https://www.mdpi.com/2072-6643/12/4/932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Grp P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement (Reprinted from Annals of Internal Medicine) Physical Therapy. 2009;89(9):873–880. doi: 10.1093/ptj/89.9.873. [DOI] [PubMed] [Google Scholar]
- Nabuco H.C.G., Tomeleri C.M., Fernandes R.R., Sugihara Junior P., Cavalcante E.F., Cunha P.M.…Cyrino E.S. Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma-metabolism biomarkers in older women with sarcopenic obesity: A randomized, double-blind, placebo-controlled trial. Clinical Nutrition ESPEN. 2019;32:88–95. doi: 10.1016/j.clnesp.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Nowson C., Connell S. Protein requirements and recommendations for older people: A review. Nutrients. 2015;7(8):6874–6899. doi: 10.3390/nu7085311. https://www.mdpi.com/2072-6643/7/8/5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou S.K., Tsintavis P., Potsaki G., Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. The Journal of Nutrition, Health & Aging. 2020;24(1):83–90. doi: 10.1007/s12603-019-1267-x. [DOI] [PubMed] [Google Scholar]
- Park Y., Choi J.-E., Hwang H.-S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. The American Journal of Clinical Nutrition. 2018;108(5):1026–1033. doi: 10.1093/ajcn/nqy214. [DOI] [PubMed] [Google Scholar]
- Rathmacher J.A., Pitchford L.M., Khoo P., Angus H., Lang J., Lowry K.…Sharp R.L. Long-term effects of calcium β-hydroxy-β-methylbutyrate and vitamin D3 supplementation on muscular function in older adults with and without resistance training: a randomized, double-blind, controlled study. The Journals of Gerontology: Series A. 2020;75(11):2089–2097. doi: 10.1093/gerona/glaa218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondanelli M., Klersy C., Terracol G., Talluri J., Maugeri R., Guido D.…Perna S. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. The American Journal of Clinical Nutrition. 2016;103(3):830–840. doi: 10.3945/ajcn.115.113357. [DOI] [PubMed] [Google Scholar]
- Scotto di Palumbo A., McSwiney F.T., Hone M., McMorrow A.M., Lynch G., De Vito G., Egan B. Effects of a long chain n-3 polyunsaturated fatty acid-rich multi-ingredient nutrition supplement on body composition and physical function in older adults with low skeletal muscle mass. Journal of Dietary Supplements. 2021;1–16 doi: 10.1080/19390211.2021.1897057. [DOI] [PubMed] [Google Scholar]
- Shahar S., Kamaruddin N.S., Badrasawi M., Sakian N.I.M., Abd Manaf Z., Yassin Z., Joseph L. Effectiveness of exercise and protein supplementation intervention on body composition, functional fitness, and oxidative stress among elderly Malays with sarcopenia. Clinical Interventions in Aging. 2013;8:1365–1375. doi: 10.2147/CIA.S46826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Dennison E., Cooper C. Epidemiology of sarcopenia: Determinants throughout the lifecourse. Calcified Tissue International. 2017;101(3):229–247. doi: 10.1007/s00223-017-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.M., Shen W., Heo M., Gallagher D., Wang Z., Sardinha L.B., Heymsfield S.B. Ethnicity-related skeletal muscle differences across the lifespan. American Journal of Human Biology. 2010;22(1):76–82. doi: 10.1002/ajhb.20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeuninx B., Greig C.A., Breen L. Amount, source and pattern of dietary protein intake across the adult lifespan: A cross-sectional study. Frontiers Nutrition. 2020;7(25) doi: 10.3389/fnut.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Haaf D.S.M., Nuijten M.A.H., Maessen M.F.H., Horstman A.M.H., Eijsvogels T.M.H., Hopman M.T.E. Effects of protein supplementation on lean body mass, muscle strength, and physical performance in nonfrail community-dwelling older adults: A systematic review and meta-analysis. The American Journal of Clinical Nutrition. 2018;108(5):1043–1059. doi: 10.1093/ajcn/nqy192. [DOI] [PubMed] [Google Scholar]
- Uchitomi R., Oyabu M., Kamei Y. Vitamin D and sarcopenia: Potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients. 2020;12(10):3189. doi: 10.3390/nu12103189. https://www.mdpi.com/2072-6643/12/10/3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Yin L., Zhao Y., Su Y., Sun W., Chen S.…Engelke K. Muscle density, but not size, correlates well with muscle strength and physical performance. Journal of the American Medical Directors Association. 2021;22(4):751–759.e752. doi: 10.1016/j.jamda.2020.06.052. [DOI] [PubMed] [Google Scholar]
- Wiedmer P., Jung T., Castro J.P., Pomatto L.C.D., Sun P.Y., Davies K.J.A., Grune T. Sarcopenia – Molecular mechanisms and open questions. Ageing Research Reviews. 2021;65 doi: 10.1016/j.arr.2020.101200. [DOI] [PubMed] [Google Scholar]
- Wu P.-Y., Huang K.-S., Chen K.-M., Chou C.-P., Tu Y.-K. Exercise, nutrition, and combined exercise and nutrition in older adults with sarcopenia: A systematic review and network meta-analysis. Maturitas. 2021;145:38–48. doi: 10.1016/j.maturitas.2020.12.009. [DOI] [PubMed] [Google Scholar]
- Yamada M., Kimura Y., Ishiyama D., Nishio N., Otobe Y., Tanaka T.…Arai H. Synergistic effect of bodyweight resistance exercise and protein supplementation on skeletal muscle in sarcopenic or dynapenic older adults. Geriatrics & Gerontology International. 2019;19(5):429–437. doi: 10.1111/ggi.13643. [DOI] [PubMed] [Google Scholar]
- Yu Y., Zhang J., Wang J., Sun B. MicroRNAs: The novel mediators for nutrient-modulating biological functions. Trends in Food Science & Technology. 2021;114:167–175. doi: 10.1016/j.tifs.2021.05.028. [DOI] [Google Scholar]
- Zdzieblik D., Oesser S., Baumstark M.W., Gollhofer A., König D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. British Journal of Nutrition. 2015;114(8):1237–1245. doi: 10.1017/S0007114515002810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yu Y., Wang J. Protein nutritional support: The classical and potential new mechanisms in the prevention and therapy of sarcopenia. Journal of Agricultural and Food Chemistry. 2020;68(14):4098–4108. doi: 10.1021/acs.jafc.0c00688. [DOI] [PubMed] [Google Scholar]
- Zhu L.-Y., Chan R., Kwok T., Cheng K.-C.-C., Ha A., Woo J. Effects of exercise and nutrition supplementation in community-dwelling older Chinese people with sarcopenia: A randomized controlled trial. Age and Ageing. 2018;48(2):220–228. doi: 10.1093/ageing/afy179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.