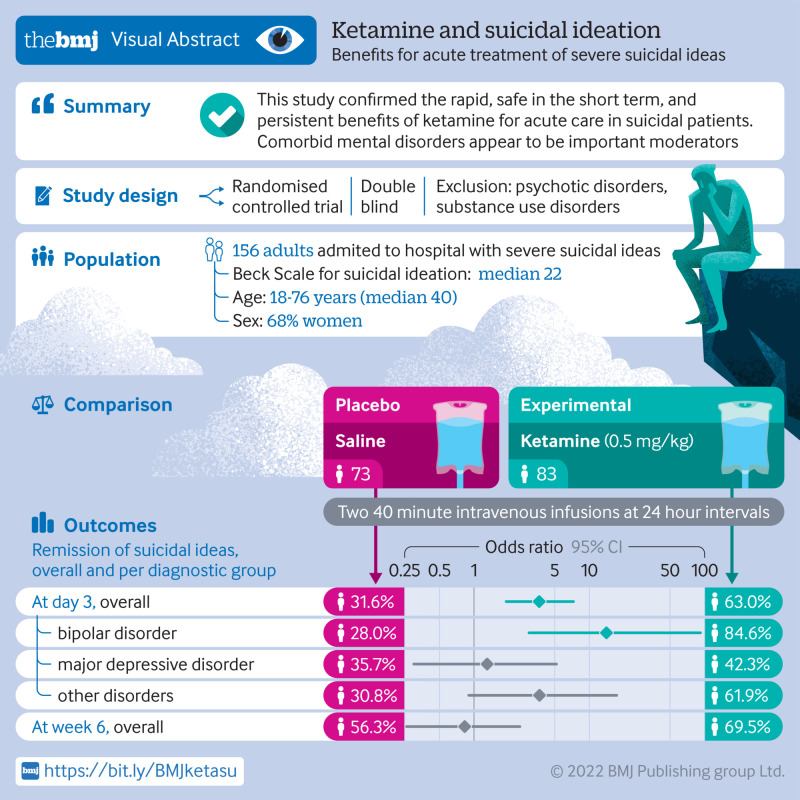
Abstract
Objective
To confirm the rapid onset anti-suicidal benefits of ketamine in the short term and at six weeks, overall and according to diagnostic group.
Design
Prospective, double blind, superiority, randomised placebo controlled trial.
Setting
Seven French teaching hospitals between 13 April 2015 and 12 March 2019.
Eligibility criteria for participants
Aged 18 or older with current suicidal ideation, admitted to hospital voluntarily. Exclusion criteria included a history of schizophrenia or other psychotic disorders, substance dependence, and contraindications for ketamine.
Participants
156 participants were recruited and randomised to placebo (n=83) or ketamine (n=73), stratified by centre and diagnosis: bipolar, depressive, or other disorders.
Intervention
Two 40 minute intravenous infusions of ketamine (0.5 mg/kg) or placebo (saline) were administered at baseline and 24 hours, in addition to usual treatment.
Main outcome measures
The primary outcome was the rate of patients in full suicidal remission at day 3, according to the scale for suicidal ideation total score ≤3. Analyses were conducted on an intention-to-treat basis.
Results
More participants receiving ketamine reached full remission of suicidal ideas at day 3 than those receiving placebo: 46 (63.0%) of 83 participants in the ketamine arm and 25 (31.6%) of 73 in the placebo arm (odds ratio 3.7 (95% confidence interval 1.9 to 7.3), P<0.001). This effect differed according to the diagnosis (treatment: P<0.001; interaction: P=0.02): bipolar (odds ratio 14.1 (95% confidence interval 3.0 to 92.2), P<0.001), depressive (1.3 (0.3 to 5.2), P=0.6), or other disorders (3.7 (0.9 to 17.3, P=0.07)). Side effects were limited. No manic or psychotic symptom was seen. Moreover, a mediating effect of mental pain was found. At week 6, remission in the ketamine arm remained high, although non-significantly versus placebo (69.5% v 56.3%; odds ratio 0.8 (95% confidence interval 0.3 to 2.5), P=0.7).
Conclusions
The findings indicate that ketamine is rapid, safe in the short term, and has persistent benefits for acute care in suicidal patients. Comorbid mental disorders appear to be important moderators. An analgesic effect on mental pain might explain the anti-suicidal effects of ketamine.
Trial registration
ClinicalTrials.gov NCT02299440.
Introduction
Around 700 000 people worldwide die from suicide annually, and 10 to 20 times this number attempt suicide.1 Suicide is the second most important cause of death in adolescents and young adults.2 The 12 month prevalence of suicidal ideas is 2% in the adult population globally, including 0.5% with a suicidal plan.3 Although most suicidal ideas will not lead to a suicidal act, all suicidal acts are preceded by suicidal ideas. Thus rapid resolution of a suicidal crisis before it is acted on might prevent many deaths. Moreover, reducing the intensity of the suicidal pain could facilitate psychosocial intervention.
Only limited evidence based options are available to treat suicidal crises. Antidepressants might reduce the risk of suicide, particularly in individuals aged over 25, but onset of beneficial effects is delayed by several weeks and a trial of several drugs is often necessary.4 Moreover, antidepressants are not recommended for people with bipolar disorders. Similarly, clozapine and lithium might be effective anti-suicidal drugs in schizophrenia and mood disorders, respectively, but not in the short term.5 Psychotherapy takes several sessions to be efficient,6 and the evidence for electroconvulsive therapy remains weak.5 Admission to hospital, anxiolytics, and hypnotics are commonly used despite limited scientific evidence. Moreover, suicide occurs at high rates in psychiatric units during admission and after discharge, questioning the efficacy of this procedure.7
Recently, ketamine has been shown to have a rapid effect on depressive symptoms and suicidal ideation after a single dose.8 9 10 11 A meta-analysis12 showed a beneficial effect on suicidal ideation scores within 4 hours after infusion, lasting for at least the first 72 hours. Of note, a recent review of the literature suggested that intravenous ketamine has a better effect than intranasal esketamine.13 Previous studies, however, were subject to several methodological limitations. Firstly, the suicidal risk was often poorly measured (eg, with one single item of a depression scale). Secondly, response (usually defined as a 50% score reduction on a scale) was the most common outcome rather than remission (that is, complete absence of suicidal ideas). Thirdly, samples were usually small. Fourthly, most studies were conducted in unipolar disorder with limited knowledge about the effect in bipolar disorder, despite the high suicide risk,14 and in non-mood disorders. Finally, the psychophysiological mechanisms of action remain poorly understood. Notably, it is now established that mental pain contributes to an increased risk of suicidal ideas and acts,15 suggesting that suicidal acts aim to put an end to unbearable mental pain. Whether the antalgic effects of ketamine contribute to its anti-suicidal effects remains to be tested.
We aimed in this study to examine full remission of suicidal ideas 72 hours after two infusions of ketamine versus placebo in a large sample. Three groups of patients were a priori selected: those with a bipolar disorder, or a depressive disorder, or another main diagnosis. Furthermore, we tested whether ketamine acted on suicidal ideas through an analgesic effect on mental pain. Finally, we examined the persistence of the effect of ketamine at six weeks. We hypothesised that (a) ketamine will be better than placebo for inducing full suicidal remission at day 3; (b) this effect will vary according to the diagnostic group; (c) this effect will be mediated through alleviating mental pain; (d) this effect will persist over six weeks.
Methods
Study design
This six week, double blind, randomised placebo controlled study (named KETIS) was conducted in seven academic hospitals in metropolitan France. Ethical approval was obtained on 18 July 2014 from the research ethics board “Comité de Protection des Personnes (CPP) Sud Méditerranée III” (ref: 2014.06.03 bis). This study was prospectively registered on 20 November 2014 on https://www.clinicaltrials.gov/ (NCT02299440), and is listed on EudraCT: 2014-001324-30. All patients gave their informed, written, and signed consent before inclusion.
Patients
Participants were recruited during admission to hospital in psychiatry for suicidal ideation. Inclusion criteria were patients aged 18 or older, with a clinician rated scale for suicidal ideation (SSI16) total score >3; voluntarily admitted to hospital; French speaking; able to provide informed consent; insured or beneficiary of a health insurance plan. Exclusion criteria were a history of schizophrenia or other psychotic disorders based on Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria; schizoid or schizotypic personality disorders; presence of psychotic symptoms at initial interview; substance dependence during the preceding month (except nicotine or caffeine); positive urine screening for illicit substances (except cannabis); pregnancy (known or positive at baseline urine test) or breastfeeding; unstable somatic condition; known or suspected contraindication for ketamine, including hypersensitivity to ketamine, hypertension, class IV cardiac insufficiency, history of stroke, hepatic or cutaneous porphyria, history of intracranial hypertension; clinically important anomalies found during clinical examination, biological tests or electrocardiogram; non-stabilised hypertension or hypertension >180/100; concomitant electroconvulsive therapy; current participation or participation within the past three months in another interventional study; patients under judicial protection or guardianship.
Randomisation and masking
Patients were randomised 1:1 to placebo or ketamine after inclusion, before perfusion preparation. Patients were further randomised by blocks of random size stratified by centre and by diagnostic category based on the Mini-International Neuropsychiatric Interview (MINI) 5.0,17 using a programme developed specifically for the study (SAS; Cary, NC). Participants were assigned a unique identification code. The three diagnostic categories were bipolar disorder; major depressive disorder; and any psychiatric disorder with no mention of a bipolar disorder, major depressive disorder, or any exclusion diagnosis (notably psychotic disorders and substance dependence; see exclusion criteria in “Patients” section).
Investigators and patients were blinded to study arm. The perfusion product was prepared within each participating department by a designated nurse, who was the only person with knowledge of randomisation results. This nurse did not participate in other aspects of the study and kept the arm assignments secret. Both ketamine and placebo perfusions were transparent and visually similar.
Procedures
At baseline, a sociodemographic and clinical assessment was conducted comprising the MINI 5.017 for psychiatric diagnoses according to DSM-IV criteria; the SSI (both clinician rated and self-rated versions18); the clinician rated Columbia suicide severity rating scale (CSSRS)19; the self-rated physical and psychological pain-visual analogue scale (PPP-VAS)20; the self-rated Beck hopelessness scale (BHS)21; the 30 item inventory of depressive symptomatology (IDS-C30), clinician rated version22; and the clinical global impression scale (CGI) to assess the global impression according to the clinician.
Then, patients received a first 40 minute intravenous infusion of ketamine (0.5 mg/kg) or placebo 0.9% (saline solution) in addition to their current treatment. This procedure has been used in most previous studies.12 A second administration was performed 24 hours later (including during weekends or bank holidays). The choice of two infusions was based on available data at the time of protocol writing in 2013/2014.8 23 Moreover, at that time, most protocols used a placebo, whereas later studies used midazolam.12 Usual care for these patients included a combination of admission to hospital, regular meetings with the healthcare staff (physicians, nurses), medication, individual and group psychotherapy, and family meetings.
Clinical evaluations were conducted at 40 minutes, 2 hours, 4 hours, day 1 (before the second infusion), day 2, and day 3. They comprised the SSIs, CSSRS, PPP-VAS, BHS, IDS-C30, CGI, and an assessment of safety and side effects with the Young mania rating scale (YMRS),24 patient rated inventory of side effects (PRISE),25 and brief psychiatric rating scale (BPRS).26 Patients were then followed up until the end of week 6, with assessments at day 4, week 2, week 4, and week 6.
Outcomes
The primary outcome was the rate of patients with a clinician rated SSI total score ≤3 (that is, in current full suicidal remission) at day 3 for each treatment arm and in each diagnostic group. SSI is a scale assessing suicidal ideation based on 19 items scored 0 to 2 (maximum score 38).18 Here, we used the level of suicidal ideation on the day of assessment. Secondary outcomes at day 3 were, for each arm and in each diagnostic group: rates of remission at intermediate time points; changes in SSI, BHS, PPP-VAS, IDS-C30, and CGI mean scores between baseline and day 3; rates of suicide attempts (CSSRS) during the three day period; and treatment side effects in each arm (YMRS, PRISE, and BPRS). At week 6, secondary outcomes were the rates of full suicidal remission in both arms.
Protocol amendments
Details of changes to the original protocol are presented in table S1. Briefly, changes were minor, including changes in investigators, biobank procedures (for future studies), extension of the inclusion period to reach the targeted sample size, and stopping the use of the self-rated quick 16 item Inventory of Depressive Symptomatology to save time, in addition to several questionnaires at 40 minutes, 2 hours, and 4 hours to reduce the burden of assessment.
Statistical analysis
This was a superiority trial. Calculation of sample size was done with nQuery software version 8.7.2.0 (table S2). Based on available literature at the time of the study conception, the hypothesis was an absolute difference of 45% resolution of suicidal ideation at day 3 between the two arms in each of the diagnostic groups (60% in the ketamine arm v 15% in the placebo arm). To test this difference with a power of 85%, a two sided α risk of 5% and taking into account the risk of α inflation associated with multiple comparisons (n=3), 52 patients were required for each diagnostic category (26 per arm), for a total of 156. A data monitoring committee oversaw the study.
Quantitative data were expressed as mean and standard deviation or median and interquartile range, according to their distribution. Qualitative data were expressed as absolute number and frequency (%). Comparison between groups used, as appropriate, Student’s t, Wilcoxon’s, Χ2, or Fisher’s tests. The rates of patients with SSI ≤3 at day 3 were compared between the two arms by a logistic regression model to take into account an arm by diagnostic group interaction to test heterogeneity. If the interaction term was significant, the between arm comparison was performed within each of the diagnostic categories. Holm’s corrections were performed to adjust for multiple comparisons. The associated odds ratios were estimated with 95% confidence interval with the profile likelihood method.
Linear mixed models were used to examine the effect of ketamine compared with placebo over time, on the different scores used as secondary endpoints, with patient considered as random effect. Time, drug, and time by drug interaction were tested. Analyses were performed within each of the diagnostic groups when interaction with arm was significant. The associations between treatment, suicidal ideas, and psychological pain were explored with a mediation model. All included patients were analysed according to the intention-to-treat principle. A P value ≤0.05 was considered as statistically significant. Statistical analysis was performed with R 3.5.1 software (R Development Core Team, (2018). R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement
Patients and the public were not involved in the design or conduct of this study, because their involvement in the design of scientific studies is recent in France and was not customary when the study was started in 2013. As the benefits of public involvement are obvious, this approach will be prioritised in our future studies. Moreover, patients will be involved in the discussion and dissemination of the findings of this study.
Results
Patient characteristics
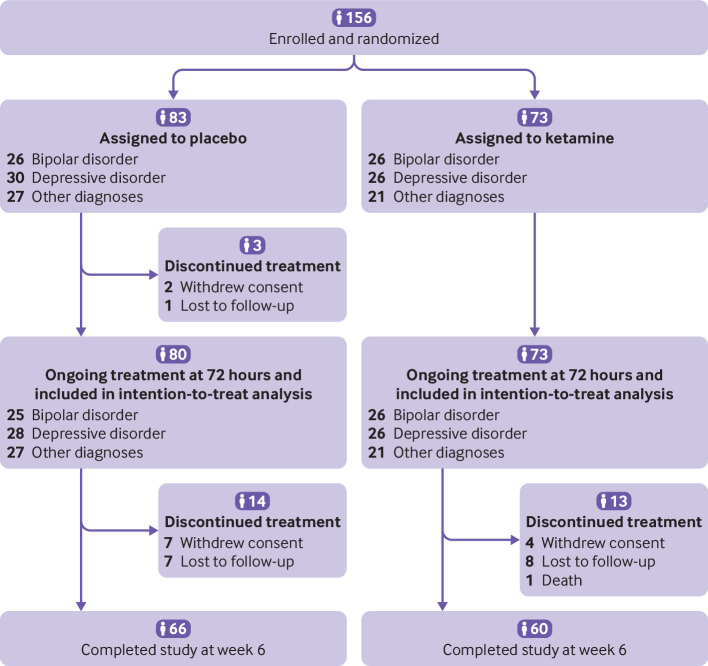
Between 13 April 2015 and 12 March 2019, 156 patients were recruited and randomised to ketamine (n=73) or placebo (n=83), stratified into three groups: bipolar disorder (n=26/26, respectively); depressive disorder (26/30); and other diagnoses (21/27; fig 1). One centre (Centre Hospitalier Universitaire, Nîmes) included 75.0% of all patients.
Fig 1.
Trial profile
Details of the participants’ characteristics are shown in table 1 (and tables S3-S5 for characteristics of each diagnostic group). Of note, patients had a past history of a suicidal act in 67 (93.1%) of 72 participants (data for one patient missing) in the ketamine arm, and in 70 (85.4%) of 82 participants (data for one patient missing) in the placebo arm. At inclusion, patients were severely suicidal in 71 (97.3%) of 73 participants in the ketamine arm and in 71 (86.6%) of 82 participants (data for one patient missing) in the placebo arm, based on the suicidality section of MINI 5.0 (10 or more points on a 32 point scale). All patients remained in hospital at day 3. At weeks 2, 4, and 6, rates of admission to hospital in the placebo and ketamine arms were, respectively 53.4%/41.8%, 34.4%/31.7%, and 22.7%/11.7%.
Table 1.
Baseline characteristics of the intention-to-treat population, by treatment group. Values are numbers (%) unless stated otherwise
| Characteristics | Placebo (n=83) | Ketamine (n=73) |
|---|---|---|
| Sex: | ||
| Male | 31 (37.3) | 19 (26.0) |
| Female | 52 (62.7) | 54 (74.0) |
| Age (years), median (range) | 41 (18-76) | 38 (18-75) |
| Height (cm), mean (SD) | 167.9 (9.0) | 167.2 (9.4) |
| BMI (kg/m2), median (IQR) | 24.2 (21.1-27.4) | 23.9 (21.2-30.1) |
| SSI suicidal ideas score, clinician rated, median (IQR) | 20 (16-24) | 22 (16-26) |
| SSI suicidal ideas score, patient rated, median (IQR) | 17 (14-20) | 17 (13-20) |
| CSSRS suicidal ideation intensity score, median (range) | 19 (11-25) | 20 (11-25) |
| MINI : severe suicidal ideas | 71/82 (86.6) | 71 (97.3) |
| PPP-VAS physical pain score, median (IQR) | 2.0 (0.0-5.0) | 1.9 (0.0-5.3) |
| PPP-VAS psychological pain score, median (IQR) | 8.0 (6.6-9.1) | 8.0 (6.0-8.8) |
| BHS hopelessness score, median (IQR) | 16.0 (12.0-17.0) | 16.0 (13.2-17.0) |
| IDS-C30 depression score, median (IQR) | 37.0 (30.0-43.5) | 37 (29-48) |
| Major depressive episode: current | 38 (45.8) | 32 (43.8) |
| Dysthymia: current | 5 (6.0) | 8 (11.0) |
| Manic episodes: past | 17 (20.5) | 14 (19.2) |
| Hypomanic episodes: past | 10 (12.0) | 11 (15.1) |
| Panic disorder: current | 8/82 (9.8) | 11 (15.1) |
| Agoraphobia: current | 12/82 (14.6) | 11 (15.1) |
| Social phobia: current | 17/82 (20.7) | 13 (17.8) |
| Generalised anxiety: current | 16 (19.3) | 14 (19.2) |
| Obsessive-compulsive disorder: current | 1 (1.2) | 3 (4.1) |
| Post-traumatic stress disorder: current | 11 (13.3) | 12 (16.4) |
| Alcohol dependence: past | 6 (7.2) | 6 (8.2) |
| Substance dependence: past | 6 (7.2) | 4 (5.5) |
| Anorexia nervosa: current | 1 (1.2) | 0 (0) |
| Bulimia nervosa: current | 5 (6.0) | 6 (8.2) |
| Previous history of suicide attempt | 70/82 (85.4) | 67/72 (93.1) |
| Medication at day 3: | ||
| Lithium | 4 (4.8) | 2 (2.7) |
| Antipsychotics | 36 (43.4) | 30 (41.1) |
| Antiepileptics | 12 (14.5) | 5 (6.8) |
| Antidepressants | 28 (33.7) | 18 (24.7) |
| Anxiolytics | 42 (50.6) | 38 (52.1) |
| Hypnotics | 10 (12) | 5 (6.8) |
| Other psychotropic medication | 3 (3.6) | 5 (6.8) |
| Antalgic | 2 (2.4) | 10 (13.7) |
| Other medication | 26 (31.3) | 23 (31.5) |
BHS=Beck hopelessness scale; CSSRS=Columbia severity suicide rating scale; IDS-C30=30 item inventory for depressive symptomatology, clinician rated version; IQR=interquartile range; MINI=Mini-International Neuropsychiatric Interview, seventh version (7.0.2); PPP-VAS=physical and psychological pain-visual analogue scale. SD=standard deviation; SSI=Beck scale for suicidal ideation.
Main outcomes at day 3
By day 3 (primary endpoint), two patients withdrew consent and one was lost to follow-up (after the second infusion) in the placebo arm. On day 3, 46 (63.0%) of 73 patients in the ketamine arm and 25 (31.6%) of 79 patients (data for one patient missing) in the placebo arm had reached full remission of suicidal ideas (odds ratio 3.7 (95% confidence interval 1.9 to 7.3), P<0.001). These results were unchanged after adjustment for sex and presence of severe suicidal ideas (odds ratio 3.9 (2.0 to 7.9), P<0.001) or antalgic use (3.7 (1.9 to 7.6), P<0.001). Change over time in rates of suicidal remission occurred by 40 minutes after infusion and persisted over the three day period (fig 2).
Fig 2.
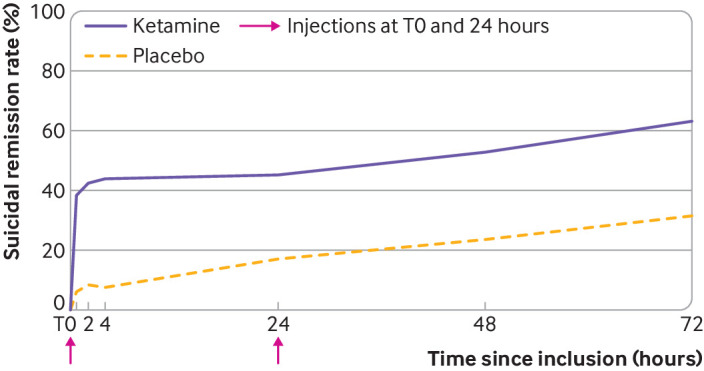
Change in rates of suicidal remission over time within 72 hours of the first injection. Here, suicidal remission corresponds to a score < 3 on the Beck scale for suicidal ideation, clinician rated version
This effect differed according to the diagnostic group, with a significant interaction between arm and group, after adjustment according to sex and presence of severe suicidal ideas (treatment arm: t=14.7; df=1, P<0.001; diagnostic group: t=3.3; df=2; P=0.2; interaction: t=7.1; df=2; P=0.03): 84.6% (n=22) v 28.0% (n=7) in the bipolar disorder group (odds ratio 14.1 (3.0 to 92.2), P<0.001), 42.3% (n=11) v 35.7% (n=10) in the depressive disorder group (1.3 (0.3 to 5.2), P=0.6), and 61.9% (n=13) v 30.8% (n=8) in the other diagnoses group (3.7 (0.9 to 17.3), P=0.07). Adding the main effect of recruitment centre into the analyses did not modify the outcomes.
Secondary outcomes at day 3
Table S6 in the supplemental materials shows information on the detailed outcomes. We found significant differences (after adjustment for centre and diagnostic group) between the ketamine and placebo arms at day 3 in SSI median scores—both for the clinician rated (1.0 (interquartile range 0-8.0) v 8.0 (2.0-15.5), respectively; unstandardised regression coefficient β=−5.0 (95% confidence interval −7.7 to −2.3), P<0.001) and patient rated versions (median score 7.0 (4.0-12.0) v 11.5 (7.0-16.2); β=−2.5 (−4.5 to −0.4), P=0.02)—and in scores of depression (17.4 (standard deviation 12.1) v 24.2 (12.7); β=−6.5 (−10.5 to −2.4), P=0.002), psychological pain (3.7 (interquartile range 0.3-6.3) v 5.0 (2.0-8.0); β=−1.2 (−2.2 to −0.1), P=0.03), hopelessness (9.0 (4.0-15.0) v 13.0 (8.0-17.0); β=-2.5 (−4.4 to −0.6), P=0.01), and global clinical impression, but not physical pain (0.1 (0.0-3.0) v 0.5 (0.0-3.5); β=−0.1 (−1.0 to 0.8), P=0.8). During the first three days, one suicide attempt occurred in the ketamine arm and none in the placebo arm.
No increase in YMRS and BPRS scores was seen in any patient from any arm (table S6). Table 2 reports the main side effects during the first three days. All side effects were rated as minor, and all symptoms reported in table 2 reduced significantly between the first assessment and day 4 (all P<0.05). Seventeen patients (23.3%) experienced at least one side effect in the ketamine group versus seven (8.4%) in the placebo group. The most common side effects in the ketamine group were sedation (11.0%), depersonalisation (9.6%), and nausea (6.8%).
Table 2.
Reports of side effects in each treatment arm within the 72 hour period
| Ketamine (n=73) | N (%) | Placebo (n=83) | N (%) |
|---|---|---|---|
| Sedation | 8 (11.0) | Sedation | 2 (2.4) |
| Depersonalisation/derealisation | 7 (9.6) | Epistaxis | 2 (2.4) |
| Nausea | 5 (6.8) | Dizziness | 2 (2.4) |
| Dizziness | 3 (4.1) | Increased appetite | 1 (1.2) |
| Agitation | 2 (2.7) | Sore muscles | 1 (1.2) |
| Tremor | 2 (2.7) | Nausea | 1 (1.2) |
| Blurred vision | 2 (2.7) | Neck stiffness | 1 (1.2) |
| Anger | 1 (1.4) | Sadness | 1 (1.2) |
| Hallucination | 1 (1.4) | Dry mouth | 1 (1.2) |
| Sweating | 1 (1.4) | Vomiting | 1 (1.2) |
| Hypotension | 1 (1.4) | ||
| Tachycardia | 1 (1.4) | ||
| Vomiting | 1 (1.4) | ||
| Dry mouth | 1 (1.4) | ||
| Diarrhoea | 1 (1.4) | ||
| Vagal syncope | 1 (1.4) |
Finally, we tested the hypothesis that the improvement of suicidal ideation was mediated by its effect on psychological pain, as assessed by the patient rated version of the SSI. The treatment was associated with a significant reduction in SSI scores after 72 hours (t=−1.9, P=0.05, accounting for centre), but this association was weaker (t=−1.8, P=0.07) after accounting for psychological pain, while psychological pain remained highly associated with SSI scores (t=7.2; P<0.001), suggesting a mediation effect.
Outcomes at week 6
Between day 4 and week 6 (study end), seven patients withdrew consent and seven patients were lost to follow-up in the placebo arm. In the ketamine arm, one patient died from suicide (determined by the oversight committee to be unrelated to the intervention), four withdrew consent, and eight were lost to follow-up. Therefore, 60 (82.2%) patients of the 73 in the ketamine group and 66 (79.5%) patients of 83 in the placebo group completed the study.
Over the study, eight patients (9.8%) in the placebo arm (data for one patient missing) and six patients (8.2%) in the ketamine arm attempted suicide (two v zero, respectively, in the bipolar disorder group; one v five in the depressive disorder group; and five v one in the other diagnosis group). Between day 4 and week 6, the ketamine arm continued to have better full suicidal remission than the placebo arm (69.5 v 56.3% at week 6), although this was not significant at week 6 owing to reduced suicidality in the placebo group over time (odds ratio 0.8 (95% confidence interval 0.3 to 2.5), P=0.7; fig 3). Results for each intermediate endpoint are reported in table S6.
Fig 3.
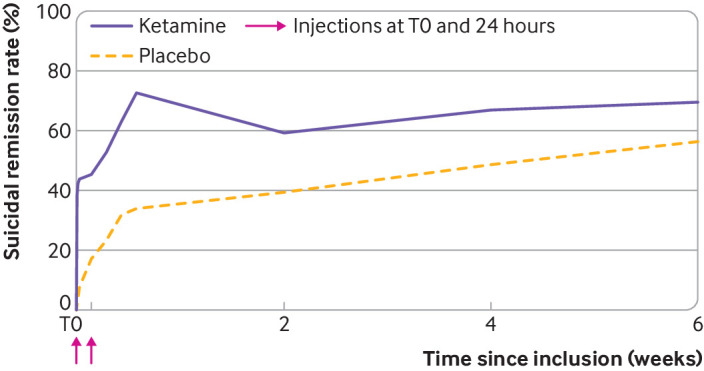
Change in rates of suicidal remission over time within 6 weeks of the first injection. Here, suicidal remission corresponds to a score < 3 on the Beck scale for suicidal ideation, clinician rated version
Discussion
Principal findings
This study confirmed in a large randomised controlled trial that ketamine is a fast acting, efficient treatment of suicidal ideation. In this population at very high suicidal risk, 63.0% reached full remission at three days after two infusions in the ketamine group in comparison with 31.6% in the placebo group. This effect was rapid, with 43.8% remission only two hours after the first infusion versus 7.3% in the placebo group. Ketamine was well tolerated without severe side effects. Main side effects, including sedation, depersonalisation/derealisation, nausea, and dizziness, were of short duration and occurred in around 10% or fewer participants. In addition, the effect persisted at six weeks in 69.5% of individuals treated with ketamine (versus 56.3% in those receiving placebo).
This study highlights a major moderating effect on primary mental disorders. A strong effect of ketamine versus placebo was found in the group with bipolar disorder, whereas the effect was moderate and did not quite reach significance in the group with “other psychiatric disorders,” and was non-significant in major depressive disorders. Results in bipolar disorder—a disorder associated with a high suicidal risk and limited options to treat depression27—are highly encouraging and support two previous small studies.8 28 Notably, no mood switch was seen in the 26 patients with bipolar disorder treated with ketamine. Results in individuals not fulfilling the criteria for a full major depressive episode (whether bipolar or major depressive disorders) also support the observation that ketamine is efficient independently from depressive episodes, as suggested by previous authors.29 This group comprised a substantial number of individuals with post-traumatic stress disorder, dysthymia, and anxiety disorders (panic disorder, agoraphobia, generalised anxiety disorder). These patients would probably also have had personality disorders, although this was not formerly measured.
The non-significant outcomes in depressive disorders are more challenging to interpret. This group showed the highest placebo effect (36% v 28% in bipolar disorder and 31% in other diagnoses). This placebo effect is also higher than that found in a meta-analysis of 10 trials analysing 157 suicidal patients with overall remission rates (clinician rated measures) of around 30% in the control group.29 Moreover, the effect of ketamine (42%) was lower than in the two other groups in our study (84% and 62%) and in the meta-analysis (around 55%).29 Our study, therefore, might have lacked power to detect an effect in this particular group with depressive disorders. Additionally, one study of treatment resistant depression suggests that repeated doses of ketamine might be necessary for some patients to achieve remission of severe suicidal ideas.30 Therefore, this group might be particularly heterogeneous, with both more patients sensitive to a placebo effect and more patients requiring repeated ketamine infusions.
The persistence of the ketamine effect at six weeks of intake is not in line with previous studies10 11 31 and the related meta-analysis,12 but those three studies together analysed only 63 patients. More long term studies are needed.
Over the six week period, 8.2% of patients in the ketamine arm and 9.8% in the placebo arm attempted suicide, including one fatal act. Detailed examination of these events in the intervention group showed that (a) none of them had exacerbated depression or suicidal ideation scores after infusion, suggesting that ketamine had no direct negative effect; (b) all these patients were poor responders to ketamine during the first three days as indicated by depression and suicidal ideation scores; and (c) some of them finally reached remission of suicidal ideas after several days, which might have led to decreased vigilance. It must be remembered that the resolution of a suicidal crisis necessitates more than medication alone. Psychological, social, and family care and support should always be combined with pharmacotherapy. Finally, this study was not designed to assess the benefits of ketamine for prevention of a suicidal act, and larger studies and meta-analyses will be necessary. Of note, a recent review of 15 studies13 suggested that in the short term no more suicidal acts occurred in the ketamine group than in the placebo group.
Overall, the tolerance of ketamine was good, as three quarters of patients had no side effects, and side effects were largely minor and of short duration. This result is in line with a recent review of literature emphasising that tolerance of ketamine is good.32
Additional findings
Our study suggests that the beneficial effect of ketamine on suicidal ideation could be mediated by an effect on psychological pain. Although mental pain does not necessarily lead to suicidal ideas, recent studies suggest that individuals with severe suicidal ideas (notably those with a plan) also have high levels of mental pain.33 Ketamine might therefore exert its effects through analgesic mechanisms that reduce mental pain. Indirect support for this suggestion is the observation that the effects of ketamine on depression might involve the opioid system34 (although this is controversial35), that buprenorphine—a μ opioid partial agonist—is also effective on suicidal ideas,36 and that mental pain has been associated with the nociceptin system.33
Limitations
Results should be interpreted in light of several limitations. Firstly, although this is a large study and sufficiently powered, analyses within diagnostic groups were on smaller samples, which might explain both the large effect size of ketamine in bipolar disorder, and the lack of significant differences in the depressive disorder group. Secondly, as ketamine can induce recognisable effects (depersonalisation, dizziness), masking might have been compromised for both the patients and the investigators, but this was not formally measured. It should, however, be noted that only 9.6% of patients in the ketamine group experienced depersonalisation and 4.1% dizziness (v 2.4% in the placebo group) while other side effects were unspecific and found in the placebo group. Midazolam has been used instead of placebo in a few studies and should be considered as a suitable control in the future. Thirdly, the rapid resolution of suicidal ideas after receiving ketamine does not equate to a reduced risk of suicidal acts, notably after hospital discharge. Indeed, the rates of suicide attempts during follow-up were similar between the groups. Moreover, ketamine is a drug with a potential for abuse. Longer follow-up of larger samples will be necessary to examine benefits on suicidal behaviours and long term risks.
Conclusion
This large trial confirms that ketamine rapidly induces remission of severe suicidal ideation in adults, an effect persisting over six weeks in two thirds of patients. This effect seems to be dependent upon comorbid mental disorders. The tolerance was good. Long term benefits and safety of ketamine must be examined and drugs with different mechanisms of action will have to be investigated for non-responders.
What is already known on this topic
Ketamine is a promising drug to rapidly decrease suicidal ideation within minutes of intake, although evidence is limited
Previous studies were conducted in samples of limited size with questionable methodology, including the way in which suicidal ideas were measured; or the fact that response (that is, a reduction of 50% of symptoms on a scale) was often used in preference to remission (that is, a lack of symptoms)
The influence of comorbid mental disorders on the effect of ketamine for reduction of suicidal ideation is unclear
What this study adds
Ketamine is confirmed as a safe in the short term and a rapidly efficient treatment of a suicidal crisis, particularly in patients with bipolar disorder, who have a high suicidal risk with limited options for treatment
The analgesic effect of ketamine might explain its benefits on the reduction of suicidal ideation
Investigation of other drugs with different pharmacological mechanisms for the short term treatment of a suicidal crisis is warranted
Acknowledgments
We thank Fabrice Boulet, Aurélie Chopin, Jorge Lopez-Castroman, Antoine Giron, and Bérangère Gomaere (CHU Nîmes); Guillaume Pineau, Etienne Kimmel (CH St Anne, Paris); Emilie Olié, Lucille Villain (CHU Montpellier); and Vincent Jardon (CHU Lille) for their participation as investigators. We also thank Léonie Gazel for her role as project manager, Carey Suehs for help drafting the protocol, Stéphanie Salles for data management, and Sarah Kabani for proofreading the manuscript.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: MA conceived the study. MA and PF designed the study and wrote the protocol. All authors provided feedback on protocol before implementation. CD performed the analyses. FJ wrote the first draft. All authors agreed on the final version. MA is the guarantor; he had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: Funded by Programme Hospitalier de Recherche Clinique National (PHRC-N) 2013. The study funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The researchers are independent of the funders and all authors had full access to all the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from PHRC-N 2013 for the submitted work; FJ has no declaration of interests for the past five years. PG received, during the past five years, fees for presentations at congresses or participation in scientific boards from Alcediag-Alcen, Angelini, GSK, Janssen, Lundbeck, Otsuka, SAGE, and Servier. WE-H reports personal fees from EISAI, Janssen, Lundbeck, Otsuka, UCB, and Chugai. PC received speaker and consultation fees from Exeltis, Janssen, and Pfizer. GV is part of a scientific board for Janssen. RG has received compensation as a member of the scientific advisory board of Janssen, Lundbeck, Roche, SOBI, and Takeda; he has served as consultant and/or speaker for Astra Zeneca, Boehringer-Ingelheim, Pierre Fabre, Lilly, Lundbeck, MAPREG, Otsuka, Pileje, SANOFI, Servier, LVMH and received compensation; and he has received research support from Servier; cofounder and stock shareholder: Regstem. P-ML has received compensation as consultant and member of a scientific advisory board for Janssen. LS declares fees for advisory board, travel support activities of consultant and lecturer in the past five years received from Janssen, Lundbeck, and Otsuka; fees for advisory board, travel support activities of consultant, lecturer, and faculty member in the past five years received from Eisai, Janssen, Lundbeck, Otsuka, Sanofi, Teva. MA declares fees from Astra Zeneca and Lundbeck, and has been invited to congresses by Janssen-Cilag, Otsuka, Lundbeck, Servier, and Astra Zeneca.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: As the personal identifying information of participants has been removed from the study dataset, it is not possible to send the results of this study to participants. Findings will be shared with clinicians and patients through national and international conferences (in psychiatry or suicidology), the French Suicidology Association (GEPS), the French national observatory on suicide (which includes professionals, and patient associations), and press release.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Ethical approval was obtained on 18 July 2014 from the research ethics board “Comité de Protection des Personnes (CPP) Sud Méditerranée III” (ref: 2014.06.03 bis). This study was prospectively registered on the 20 November 2014 on https://www.clinicaltrials.gov/ (NCT02299440) and is listed on EudraCT: 2014-001324-30. All patients gave their informed, written and signed consent before inclusion.
Data availability statement
Individuals’ participant data that underlie the results reported in this article (after de-identification) will be available. The study protocol, statistical analysis plan, and informed consent form will also be provided on request. Data will be immediately available after publication, with no end date. Data will be provided to investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose for individual participant data meta-analysis or review. Proposals should be directed to pascale.FABBRO@chu-nimes.fr
References
- 1.World Health Statistics. Monitoring health for sustainable development goals. 2016.
- 2. Mokdad AH, Forouzanfar MH, Daoud F, et al. Global burden of diseases, injuries, and risk factors for young people’s health during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2016;387:2383-401. 10.1016/S0140-6736(16)00648-6 [DOI] [PubMed] [Google Scholar]
- 3. Borges G, Nock MK, Haro Abad JM, et al. Twelve-month prevalence of and risk factors for suicide attempts in the World Health Organization World Mental Health Surveys. J Clin Psychiatry 2010;71:1617-28. 10.4088/JCP.08m04967blu [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kennedy SH, Lam RW, McIntyre RS, et al. CANMAT Depression Work Group . Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Can J Psychiatry 2016;61:540-60. 10.1177/0706743716659417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zalsman G, Hawton K, Wasserman D, et al. Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatry 2016;3:646-59. 10.1016/S2215-0366(16)30030-X [DOI] [PubMed] [Google Scholar]
- 6. Hawton K, Witt KG, Salisbury TLT, et al. Psychosocial interventions following self-harm in adults: a systematic review and meta-analysis. Lancet Psychiatry 2016;3:740-50. 10.1016/S2215-0366(16)30070-0 [DOI] [PubMed] [Google Scholar]
- 7. Chung DT, Ryan CJ, Hadzi-Pavlovic D, Singh SP, Stanton C, Large MM. Suicide Rates After Discharge From Psychiatric Facilities: A Systematic Review and Meta-analysis. JAMA Psychiatry 2017;74:694-702. 10.1001/jamapsychiatry.2017.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 2010;67:793-802. 10.1001/archgenpsychiatry.2010.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiong J, Lipsitz O, Chen-Li D, et al. The acute antisuicidal effects of single-dose intravenous ketamine and intranasal esketamine in individuals with major depression and bipolar disorders: A systematic review and meta-analysis. J Psychiatr Res 2021;134:57-68. 10.1016/j.jpsychires.2020.12.038 [DOI] [PubMed] [Google Scholar]
- 10. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial. Am J Psychiatry 2018;175:327-35. 10.1176/appi.ajp.2017.17060647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grunebaum MF, Ellis SP, Keilp JG, et al. Ketamine versus midazolam in bipolar depression with suicidal thoughts: A pilot midazolam-controlled randomized clinical trial. Bipolar Disord 2017;19:176-83. 10.1111/bdi.12487 [DOI] [PubMed] [Google Scholar]
- 12. Witt K, Potts J, Hubers A, et al. Ketamine for suicidal ideation in adults with psychiatric disorders: A systematic review and meta-analysis of treatment trials. Aust N Z J Psychiatry 2020;54:29-45. 10.1177/0004867419883341 [DOI] [PubMed] [Google Scholar]
- 13. Siegel AN, Di Vincenzo JD, Brietzke E, et al. Antisuicidal and antidepressant effects of ketamine and esketamine in patients with baseline suicidality: A systematic review. J Psychiatr Res 2021;137:426-36. 10.1016/j.jpsychires.2021.03.009 [DOI] [PubMed] [Google Scholar]
- 14. Pompili M, Gonda X, Serafini G, et al. Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Bipolar Disord 2013;15:457-90. 10.1111/bdi.12087 [DOI] [PubMed] [Google Scholar]
- 15. Ducasse D, Holden RR, Boyer L, et al. Psychological Pain in Suicidality: A Meta-Analysis. J Clin Psychiatry 2018;79:16r10732. [DOI] [PubMed] [Google Scholar]
- 16. Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol 1979;47:343-52. 10.1037/0022-006X.47.2.343 [DOI] [PubMed] [Google Scholar]
- 17. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(Suppl 20):22-33, quiz 34-57. [PubMed] [Google Scholar]
- 18. Beck AT, Steer RA, Ranieri WF. Scale for Suicide Ideation: psychometric properties of a self-report version. J Clin Psychol 1988;44:499-505. [DOI] [PubMed] [Google Scholar]
- 19. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266-77. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olié E, Guillaume S, Jaussent I, Courtet P, Jollant F. Higher psychological pain during a major depressive episode may be a factor of vulnerability to suicidal ideation and act. J Affect Disord 2010;120:226-30. 10.1016/j.jad.2009.03.013 [DOI] [PubMed] [Google Scholar]
- 21. Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol 1974;42:861-5. 10.1037/h0037562 [DOI] [PubMed] [Google Scholar]
- 22. Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996;26:477-86. 10.1017/S0033291700035558 [DOI] [PubMed] [Google Scholar]
- 23. Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006;63:856-64. 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
- 24. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978;133:429-35. 10.1192/bjp.133.5.429 [DOI] [PubMed] [Google Scholar]
- 25. Rush AJ, Fava M, Wisniewski SR, et al. STAR*D Investigators Group . Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials 2004;25:119-42. 10.1016/S0197-2456(03)00112-0 [DOI] [PubMed] [Google Scholar]
- 26. Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep 1962;10:799-812. 10.2466/pr0.1962.10.3.799. [DOI] [Google Scholar]
- 27. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord 2018;20:97-170. 10.1111/bdi.12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zarate CA, Jr, Brutsche NE, Ibrahim L, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 2012;71:939-46. 10.1016/j.biopsych.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilkinson ST, Ballard ED, Bloch MH, et al. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. Am J Psychiatry 2018;175:150-8. 10.1176/appi.ajp.2017.17040472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phillips JL, Norris S, Talbot J, et al. Single and repeated ketamine infusions for reduction of suicidal ideation in treatment-resistant depression. Neuropsychopharmacology 2020;45:606-12. 10.1038/s41386-019-0570-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu YD, Xiang YT, Fang JX, et al. Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med 2016;46:623-35. 10.1017/S0033291715002159 [DOI] [PubMed] [Google Scholar]
- 32. McIntyre RS, Rosenblat JD, Nemeroff CB, et al. Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. Am J Psychiatry 2021;178:383-99. 10.1176/appi.ajp.2020.20081251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jollant F, Perreira F, Fiori LM, et al. Neural and molecular correlates of psychological pain during major depression, and its link with suicidal ideas. Prog Neuropsychopharmacol Biol Psychiatry 2020;100:109909. 10.1016/j.pnpbp.2020.109909 [DOI] [PubMed] [Google Scholar]
- 34. Williams NR, Heifets BD, Blasey C, et al. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am J Psychiatry 2018;175:1205-15. 10.1176/appi.ajp.2018.18020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoon G, Petrakis IL, Krystal JH. Association of Combined Naltrexone and Ketamine With Depressive Symptoms in a Case series of Patients With Depression and Alcohol Use Disorder. JAMA Psychiatry 2019;76:337-8. 10.1001/jamapsychiatry.2018.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yovell Y, Bar G, Mashiah M, et al. Ultra-Low-Dose Buprenorphine as a Time-Limited Treatment for Severe Suicidal Ideation: A Randomized Controlled Trial. Am J Psychiatry 2016;173:491-8. 10.1176/appi.ajp.2015.15040535 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Data Availability Statement
Individuals’ participant data that underlie the results reported in this article (after de-identification) will be available. The study protocol, statistical analysis plan, and informed consent form will also be provided on request. Data will be immediately available after publication, with no end date. Data will be provided to investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose for individual participant data meta-analysis or review. Proposals should be directed to pascale.FABBRO@chu-nimes.fr